Abstract
COVID-19 has rapidly developed into a worldwide pandemic with a significant health and economic burden. There are currently no approved treatments or preventative therapeutic strategies. Hundreds of clinical studies have been registered with the intention of discovering effective treatments. Here, we review currently registered interventional clinical trials for the treatment and prevention of COVID-19 to provide an overall summary and insight into the global response.
Keywords: coronavirus, SARS-CoV-2, COVID-19, 2019-nCoV, pandemic
Race towards a Successful Intervention for Covid-19
Over the past two decades, three novel pathogenic human coronaviruses have emerged from animal reservoirs [1]. These are Middle East respiratory syndrome-related coronavirus (MERS-CoV), severe acute respiratory syndrome coronavirus (SARS-CoV), and, most recently, severe acute respiratory syndrome coronavirus 2 (referred to as COVID-19, SARS-CoV-2, or 2019-nCoV). All three have led to global health emergencies, with significant morbidity and mortality [2]. Before 2020, the largest outbreak was of SARS-CoV in 2003, which affected over 8000 individuals globally and was associated with 774 deaths (case fatality rate of 9.6%)i [3]. The overall cost to the global economy of SARS-CoV was estimated to be between US$30 billion and US$100 billion [4].
Following the first identification in patients with severe pneumonia in Wuhan province, China in November 2019, COVID-19 has spread rapidly and now affects all permanently inhabited continents. This is the greatest pandemic of modern times and has been declared a Public Health Emergency of International Concern by the WHO Director-Generalii. As of 27 March 2020 (date of submission), COVID-19 was affecting 199 countries and territories, with >510 000 confirmed cases globallyiii. It is associated with an estimated mortality of between 1% and 5%iii. Furthermore, human-to-human transmission has continued apace, despite escalating public health measures. Current estimates of the impact on the worldwide economy are US$1 trillion and risingiv.
Currently, there are no approved therapies for either the treatment or prevention of COVID-19. With the predicted number of cases set to rise significantly, this represents a prodigious acute unmet medical need. Several national and international research groups are working collaboratively on a variety of preventative and therapeutic interventions. Potential avenues being explored include vaccine development, convalescent plasma, interferon-based therapies, small-molecule drugs, cell-based therapies, and monoclonal antibodies (mAbs) [5]. However, drug therapy development is a costly and timely process with a high attrition rate [6]. The speed of the normal drug development pathway is unacceptable in the context of the current global emergency. Therefore, there has been considerable interest in repurposing existing drugs and expediting developmental antiviral treatments, such as those for influenza, hepatitis B (HBV), hepatitis C (HCV), and filoviruses, to allow more rapid development [5]. The swift genomic sequencing of COVID-19 has facilitated this process, allowing comparison with MERS-CoV, SARS-CoV, and other morbific viruses [7]. This strategy has identified several genomic regions of interest for therapeutic modulation, specifically the identification of highly conserved regions involving viral enzymes between different pathogenic coronaviruses.
Exploring Current Clinical Trials for Covid-19
Since 2005, it has been recommended by the International Committee of Medical Journal Editors (ICMJE) that all clinical trials should be registered in publicly available domains before they may be considered for publication [8]. The introduction of this requirement and other initiatives to increase clinical trial transparency has contributed to an increasing number of trials being recorded in online registries, such as ClinicalTrials.govv and the International Clinical Trials Registry Platform (ICTRP)vi of the WHO. The logging of trials on registries has vastly facilitated the dissemination of information across several domains, including intervention, methodology, patient group, and outcome measures. Furthermore, in the event of the nonpublication of results, it means that trial information remains freely available for analysis.
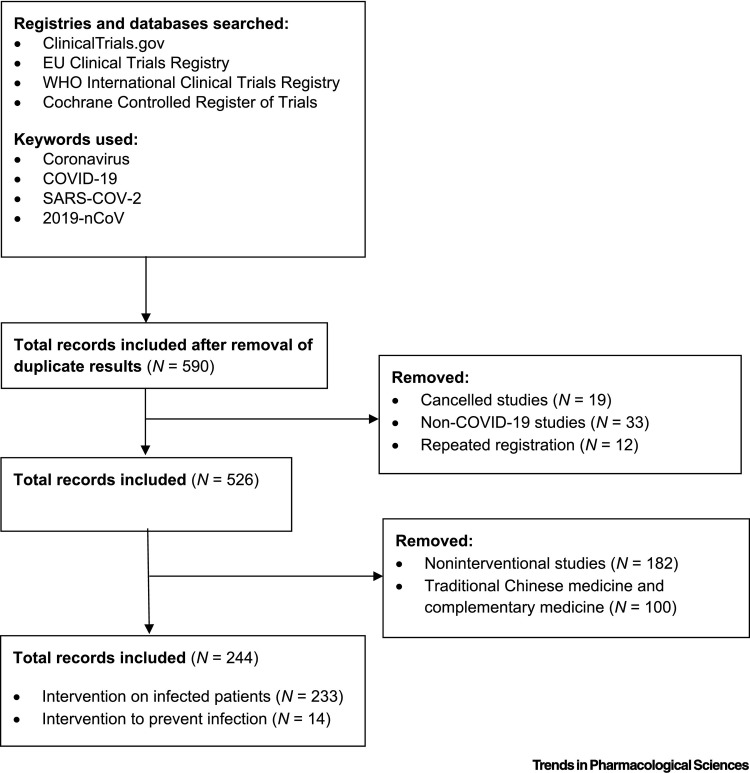
In the context of the current global COVID-19 pandemic, we performed an analysis of online registries (ClinicalTrials.govv, WHO ICTRPvi, EU Clinical Trials Registervii, and Cochrane Central Register of Controlled Trialsviii; Figure 1 ) to collate all registered therapeutic and preventative interventions under clinical investigation. We hope that this will clarify current investigational advances and guide potential future strategies. We identified 344 interventional studies focusing on both preventative strategies and the treatment of patients with COVID-19 (Figure 1) as of 20 March 2020. This search identified 100 studies that focused on forms of traditional Chinese medicine (TCM), including herbal medicines, acupuncture and other forms of complementary medicine. These have not been further analysed due to a lack of scientific rationale, inadequate provision of information regarding active ingredients, and limited applicability to mainstream medical practice. Table 1 (Key Table) shows interventional treatments (Table 1A) and preventative strategies (Table 1B) under clinical investigation for COVID-19.
Figure 1.
Flow Diagram Showing the Study Selection Process of Clinical Trials Discussed in This Article and Listed in Table 1 in the Main Text.
Data in the WHO International Clinical Trials Registry were incorporated from various national registries, including those from Australia, New Zealand, China, The Netherlands, Brazil, India, Cuba, Republic of Korea, Germany, Iran, Japan, Sri Lanka, Thailand, and Peru, and also ClinicalTrials.gov, EU Clinical Trials registry, International Standard Randomised Controlled Trial Number (ISRCTN)xi, and the Pan-African registries. Three studies included treatment for patients with COVID-19 and an intervention to prevention infection in uninfected patients.
Table 1.
Key Table. Ongoing Clinical Trials for the (A) Treatment and (B) Prevention of COVID-19 (Current as of 20 March, 2020)a
| Clinical trial ID (Registry) | Interventionb | Sizec | Randomised | Blinded | Status | Country of origin (pharma sponsor) |
|---|---|---|---|---|---|---|
| (A) Ongoing clinical trials for treatment of COVID-19 | ||||||
| Antiviral | ||||||
| ChiCTR2000029609 (ICTPR) |
Arm A (mild–moderate): chloroquine Arm B (mild–moderate): lopinavir/ritonavir Arm C (mild–moderate): lopinavir/ritonavir + chloroquine Arm D (severe): lopinavir/ritonavir Arm E (severe): chloroquine |
205 | No | No | Recruiting | China |
| ChiCTR2000029600 (ICTPR) |
Arm A: interferon alpha atomisation Arm B lopinavir/ritonavir and interferon alpha atomisation Arm C: favipiravir and interferon alpha atomisation |
90 | No | No | Recruiting | China |
| NCT04261270 (ClinicalTrials.gov) |
Arm A: ASC09 and oseltamivir Arm B: ritonavir and oseltamivir Arm C: oseltamivir |
60 | Yes | Single | Recruiting | China |
| NCT04261907 (ClinicalTrials.gov) |
Arm A: ASC09/ritonavir Arm B: lopinavir/ritonavir |
160 | Yes | No | Recruiting | China (Ascletis Pharm) |
| ChiCTR2000030487 (ICTPR) | Arm A: azvudine | 10 | No | No | Recruiting | China |
| ChiCTR2000030424 (ICTPR) | Arm A: azvudine | 30 | No | No | Not recruiting | China |
| ChiCTR2000030041 (ICTPR) | Arm A: azvudine | 40 | No | No | Not recruiting | China |
| ChiCTR2000029853 (ICTPR) |
Arm A: azvudine Arm B: standard treatment |
20 | Yes | No | Recruiting | China |
| ChiCTR2000029544 (ICTPR) |
Arm A: baloxavir marboxil Arm B: favipiravir Arm C: standard treatment |
30 | Yes | Unspecified | Not recruiting | China |
| ChiCTR2000029548 (ICTPR) |
Arm A: baloxavir marboxil Arm B: favipiravir Arm C: lopinavir/ritonavir |
30 | Yes | No | Not recruiting | China |
| ChiCTR2000030001 (ICTPR) |
Arm A: basic treatment + triazavirin Arm B: basic treatment |
240 | Yes | Yes | Recruiting | China |
| NCT04273763 (ClinicalTrials.gov) |
Arm A: bromhexine (mucolytic), umifenovir, interferon a2b, and favipiravir Arm B: umifenovir and interferon a2b |
60 | Yes | No | Recruiting | China (WanBangDe Pharm. Group) |
| ChiCTR2000030002 (ICTPR) |
Arm A: conventional treatment Arm B: conventional treatment + tranilast |
60 | Yes | No | Recruiting | China |
| ChiCTR2000030472 (ICTPR) |
Arm A: danoprevir/ritonavir Arm B: standard treatment |
20 | Unspecified | No | Recruiting | China |
| ChiCTR2000030259 (ICTPR) |
Arm A: danoprevir/ritonavir Arm B: standard treatment |
60 | Yes | Unspecified | Recruiting | China |
| ChiCTR2000030000 (ICTPR) |
Arm A: danoprevir/ritonavir Arm B: Pegasys Arm C: Novaferon Arm D: Coriolus Arm E: standard treatment |
50 | Unspecified | No | Recruiting | China |
| NCT04252274 (ClinicalTrials.gov) |
Arm A: darunavir and cobicistat Arm B: standard treatment |
30 | Yes | No | Recruiting | China |
| NCT04304053 (ClinicalTrials.gov) |
Arm A: darunavir/cobicistat Arm B: isolation |
3040 | Yes | No | Recruiting | Spain |
| ChiCTR2000029541 (ICTPR) |
Arm A: darunavir/cobicistat and thymosin Arm B: lopinavir/ritonavir and thymosin Arm C: thymosin |
100 | Yes | No | Not recruiting | China |
| NCT04291729 (ClinicalTrials.gov) |
Arm A: darunavir/ritonavir and atomised interferon Arm B: peginterferon a2 Arm C: interferon alpha (Novaferon) Arm D: lopinavir/ritonavir Arm E: atomised interferon + Chinese medicine (unspecified) |
50 | No | No | Recruiting | China (Ascletis Pharmaceutical) |
| ChiCTR2000030535 (ICTPR) |
Arm A: ebastine and interferon alpha inhalation and lopinavir Arm B: interferon alpha inhalation and lopinavir |
100 | Yes | Single | Recruiting | China |
| ChiCTR2000030113 (ICTPR) |
Arm A: favipiravir Arm B: ritonavir |
20 | Yes | No | Recruiting | China |
| ChiCTR2000030254 (ICTPR) |
Arm A: favipiravir Arm B: umifenovir |
240 | Yes | No | Recruiting | China |
| ChiCTR2000030987 (ICTPR) |
Arm A: favipiravir and chloroquine Arm B: favipiravir Arm C: placebo |
150 | Yes | Unspecified | Recruiting | China |
| NCT04310228 (ClinicalTrials.gov) |
Arm A: favipiravir and tocilizumab Arm B: favipiravir Arm C: tocilizumab |
150 | Yes | No | Recruiting | China |
| ChiCTR2000029895 (ICTPR) | Arm A: GD31 | 160 | No | Unspecified | Recruiting | China |
| IRCT20100228003449N27 (ICTPR) |
Arm A: hydroxychloroquine, lopinavir/ritonavir, and interferon beta 1b Arm B: hydroxychloroquine and lopinavir/ritonavir |
30 | Yes | No | Recruiting | Iran |
| IRCT20100228003449N28 (ICTPR) |
Arm A: hydroxychloroquine, lopinavir/ritonavir, and interferon beta 1a Arm B: hydroxychloroquine and lopinavir/ritonavir |
30 | Yes | No | Recruiting | Iran |
| IRCT20100228003449N29 (ICTPR) |
Arm A: hydroxychloroquine, lopinavir/ritonavir, and sofosbuvir/ledipasvir Arm B: hydroxychloroquine and lopinavir/ritonavir |
50 | Yes | No | Recruiting | Iran |
| JPRN-jRCTs041190120 (ICTPR) |
Arm A: immediate favipiravir (Day 1–10) Arm B: delayed favipiravir (Day 6–15) |
86 | Yes | No | Recruiting | Japan |
| 2020-001023-14 (EU-CTR) |
Arm A: inhaled interferon alpha 1b Arm B: placebo |
400 | Yes | Double | Recruiting | UK (Synairgen Ltd) |
| ChiCTR2000029989 (ICTPR) |
Arm A: interferon a1b eye drops Arm B: placebo eye drops |
300 | Yes | Unspecified | Not recruiting | China |
| NCT04293887 (ClinicalTrials.gov) |
Arm A: interferon a1b nebulised Arm B: standard treatment |
328 | Yes | No | Not recruiting | China |
| ChiCTR2000030922 (ICTPR) |
Arm A: interferon alpha 2a and ribavirin Arm B: umifenovir and ribavirin |
30 | Yes | Unspecified | Recruiting | China |
| ChiCTR2000029308 (ICTPR) [11] |
Arm A: lopinavir/ritonavir Arm B standard treatment |
160 | Yes | No | Recruiting | China |
| NCT04307693 (ClinicalTrials.gov) |
Arm A: lopinavir/ritonavir Arm B: hydroxychloroquine Arm C: no intervention |
150 | Yes | No | Recruiting | South Korea |
| ChiCTR2000030187 (ICTPR) |
Arm A: lopinavir/ritonavir Arm B: standard of care |
60 | Yes | Unspecified | Recruiting | China |
| 2020-001113-21 (EU-CTR) |
Arm A: lopinavir/ritonavir Arm B: dexamethasone Arm C: interferon beta 1a Arm D: placebo |
2000 | Yes | No | Recruiting | UK |
| 2020-000936-23 (EU-CTR) |
Arm A: lopinavir/ritonavir Arm B: interferon beta 1a Arm C: remdesivir |
3000 | Yes | No | Recruiting | France |
| NCT04251871 (ClinicalTrials.gov) |
Arm A: lopinavir/ritonavir and interferon alpha inhalation and traditional Chinese medicine Arm B: lopinavir/ritonavir and interferon alpha inhalation |
150 | Yes | No | Recruiting | China |
| ChiCTR2000029468 (ICTPR) |
Arm A: lopinavir/ritonavir and emtricitabine/tenofovir Arm B: lopinavir/ritonavir |
120 | Unspecified | Unspecified | Not recruiting | China |
| JPRN-jRCTs031190227 (ICTPR) | Arm A: lopinavir/ritonavir and hydroxychloroquine | 50 | Unspecified | Unspecified | Not recruiting | Japan |
| ChiCTR2000030166 (ICTPR) |
Arm A: lopinavir/ritonavir and interferon alpha 2b and Qing-Wen Bai-Du-Yin granules Arm B: lopinavir/ritonavir and interferon alpha 2b |
20 | Yes | No | Not recruiting | China |
| ChiCTR2000030218 (ICTPR) |
Arm A: lopinavir/ritonavir and Xiyanping injection Arm B: ritonavir |
80 | Unspecified | Unspecified | Recruiting | China |
| NCT04252885 (ClinicalTrials.gov) |
Arm A: lopinavir/ritonavir + basic treatment (unspecified) Arm B: umifenovir + basic treatment (unspecified) Arm C: basic treatment (unspecified) |
125 | Yes | No | Recruiting | China |
| NCT04276688 (ClinicalTrials.gov) |
Arm A: lopinavir/ritonavir + ribavirin + interferon beta 1b Arm B: lopinavir/ritonavir |
70 | Yes | No | Recruiting | Hong Kong |
| ChiCTR2000029539 (ICTPR) |
Arm A: lopinavir/ritonavir Arm B: standard treatment |
328 | Yes | No | Recruiting | China |
| ChiCTR2000029996 (ICTPR) |
Arm A: low-dose favipiravir Arm B: medium-dose favipiravir Arm C: high-dose favipiravir |
60 | Yes | No | Recruiting | China |
| ChiCTR2000029638 (ICTPR) |
Arm A: nebulised rSIFN-co Arm B: nebulised interferon alpha |
100 | Yes | Yes | Recruiting | China |
| ChiCTR2000029496 (ICTPR) |
Arm A: Novaferon atomisation inhalation Arm B: lopinavir/ritonavir Arm C: Novaferon and lopinavir/ritonavir |
90 | Yes | No | Recruiting | China |
| NCT04303299 (ClinicalTrials.gov) |
Arm A: oseltamivir and chloroquine Arm B: lopinavir/ritonavir and favipiravir Arm C: lopinavir/ritonavir and oseltamivir Arm D: lopinavir/ritonavir and oseltamivir Arm E: favipiravir and lopinavir/ritonavir Arm F: darunavir/ritonavir, oseltamivir, and chloroquine Arm G: standard treatment |
80 | Yes | No | Not recruiting | Thailand |
| NCT04302766 (ClinicalTrials.gov) | Arm A: remdesivir | Unspecified | Unspecified | Unspecified | Available | USA |
| NCT04292899 (ClinicalTrials.gov) |
Arm A: remdesivir Arm B: standard treatment |
400 | Yes | No | Recruiting | USA and Asia (Gilead) |
| NCT04292730 (ClinicalTrials.gov) |
Arm A: remdesivir Arm B: standard treatment |
600 | Yes | No | Recruiting | USA and Asia (Gilead) |
| NCT04280705 (ClinicalTrials.gov) |
Arm A: remdesivir Arm B: placebo |
394 | Yes | Double | Recruiting | USA and South Korea |
| 2020-000841-15 (EU-CTR) |
Arm A: remdesivir Arm B: standard treatment |
400 | Yes | No | Recruiting | Worldwide (Gilead) |
| 2020-000842-32 (EU-CTR) |
Arm A: remdesivir Arm B: standard treatment |
600 | Yes | No | Recruiting | Worldwide (Gilead) |
| NCT04252664 (ClinicalTrials.gov) |
Arm A: remdesivir Arm B: placebo |
308 | Yes | Quadruple | Recruiting | China |
| NCT04257656 (ClinicalTrials.gov) |
Arm A: remdesivir Arm B: placebo |
453 | Yes | Quadruple | Recruiting | China |
| NCT04315948 (ClinicalTrials.gov) |
Arm A: remdesivir Arm B: lopinavir/ritonavir Arm C: lopinavir/ritonavir and interferon beta 1a Arm D: hydroxychloroquine Arm E: standard treatment |
3100 | Yes | No | Recruiting | France |
| ChiCTR2000029387 (ICTPR) |
Arm A: ribavirin and interferon alpha-1b Arm B: lopinavir/ritonavir, and interferon alpha-1b Arm C: ribavirin, lopinavir/ritonavir, and interferon alpha-1b |
108 | Unspecified | Unspecified | Recruiting | China |
| IRCT20200128046294N2 (ICTPR) |
Arm A: sofosbuvir/daclatasvir Arm B: standard treatment |
70 | Yes | Single | Recruiting | Iran |
| ChiCTR2000029400 (ICTPR) |
Arm A: traditional Chinese medicine Arm B: lopinavir/ritonavir Arm C: traditional Chinese medicine and lopinavir/ritonavir |
60 | Unspecified | Unspecified | Recruiting | China |
| ChiCTR2000030262 (ICTPR) |
Arm A: type 1 interferon and TFF2 dose 1 Arm B: type 1 interferon and TFF2 dose 2 Arm C: standard treatment |
30 | Yes | Unspecified | Recruiting | China |
| ChiCTR2000029573 (ICTPR) |
Arm A: umifenovir Arm B: Novaferon and umifenovir Arm C: lopinavir/ritonavir Arm D: umifenovir Arm E: novaferon and lopinavir/ritonavir Arm F: novaferon and umifenovir |
480 | Yes | No | Not recruiting | China |
| ChiCTR2000029621 (ICTPR) |
Arm A: umifenovir Arm B: standard treatment |
380 | Yes | No | Recruiting | China |
| NCT04254874 (ClinicalTrials.gov) |
Arm A: umifenovir Arm B: umifenovir and pegylated interferon alpha 2b |
100 | Yes | Single | Recruiting | China |
| NCT04255017 (ClinicalTrials.gov) |
Arm A: umifenovir Arm B: oseltamivir Arm C: lopinavir/ritonavir |
400 | Yes | Single | Recruiting | China |
| ChiCTR2000029993 (ICTPR) |
Arm A: umifenovir and Liushen capsule Arm B: standard treatment |
40 | Yes | No | Recruiting | China |
| NCT04275388 (ClinicalTrials.gov) |
Arm A: Xiyanping injection, lopinavir/ritonavir and interferon alpha nebulisation Arm B: lopinavir/ritonavir and interfer n alpha nebulisation |
348 | Yes | No | Not recruiting | China (Jiangxi Qingfeng Pharmaceutical) |
| Antimalarial | ||||||
| ChiCTR2000030031 (ICTPR) |
Arm A: chloroquine Arm B: placebo |
120 | Yes | Double | Recruiting | China |
| ChiCTR2000029988 (ICTPR) |
Arm A: chloroquine Arm B: standard treatment |
80 | Unspecified | Unspecified | Recruiting | China |
| ChiCTR2000029975 (ICTPR) | Arm A: chloroquine | 10 | No | Unspecified | Not recruiting | China |
| ChiCTR2000029939 (ICTPR) |
Arm A: chloroquine Arm B: standard treatment |
100 | Yes | Single | Recruiting | China |
| ChiCTR2000029935 (ICTPR) | Arm A: chloroquine | 100 | No | Unspecified | Recruiting | China |
| ChiCTR2000029837 (ICTPR) |
Arm A: chloroquine Arm B: placebo |
120 | Yes | Double | Not recruiting | China |
| ChiCTR2000029826 (ICTPR) |
Arm A: chloroquine Arm B: placebo |
45 | Yes | Double | Not recruiting | China |
| ChiCTR2000029542 (ICTPR) |
Arm A: chloroquine Arm B: standard treatment |
20 | Unspecified | Unspecified | Recruiting | China |
| ChiCTR2000029741 (ICTPR) |
Arm A: chloroquine Arm B: lopinavir/ritonavir |
112 | Yes | No | Recruiting | China |
| ChiCTR2000030718 (ICTPR) |
Arm A: chloroquine Arm B: standard treatment |
80 | Yes | No | Recruiting | China |
| ChiCTR2000029992 (ICTPR) |
Arm A: chloroquine and hydroxychloroquine Arm B: standard treatment |
100 | Yes | No | Not recruiting | China |
| ChiCTR2000030417 (ICTPR) |
Arm A: chloroquine aerosol inhalation Arm B water aerosol inhalation |
30 | Unspecified | Unspecified | Not recruiting | China |
| ChiCTR2000030082 (ICTPR) |
Arm A: dihydroartemisinin/piperaquine tablets combined with antiviral treatment (presumed alpha-interferon + umifenovir) Arm B: alpha-interferon + umifenovir |
40 | Yes | No | Suspended | China |
| ChiCTR2000029898 (ICTPR) |
Arm A: hydroxychloroquine Arm B: chloroquine |
100 | Yes | No | Recruiting | China |
| NCT04261517 (ClinicalTrials.gov) |
Arm A: hydroxychloroquine Arm B: standard of care |
30 | Yes | No | Recruiting | China |
| ChiCTR2000030054 (ICTPR) |
Arm A: hydroxychloroquine Arm B: standard treatment |
100 | Yes | No | Not recruiting | China |
| ChiCTR2000029868 (ICTPR) |
Arm A: hydroxychloroquine Arm B: standard treatment |
200 | Yes | Unspecified. | Recruiting | China |
| ChiCTR2000029740 (ICTPR) |
Arm A: hydroxychloroquine Arm B: standard treatment |
78 | Yes | No | Recruiting | China |
| ChiCTR2000029559 (ICTPR) |
Arm A: hydroxychloroquine Arm B: hydroxychloroquine Arm C: placebo |
300 | Unspecified | Unspecified | Recruiting | China |
| 2020-000890-25 (EU-CTR) [17] | Arm A: hydroxychloroquine | 25 | No | No | Recruiting | France |
| ChiCTR2000029899 (ICTPR) |
Arm A: hydroxychloroquine Arm B: chloroquine |
100 | Yes | No | Recruiting | China |
| NCT04315896 (ClinicalTrials.gov) |
Arm A: hydroxychloroquine Arm B: placebo |
500 | Yes | Quadruple | Not recruiting | Mexico |
| NCT04316377 (ClinicalTrials.gov) |
Arm A: hydroxychloroquine Arm B: standard treatment |
202 | Yes | No | Not recruiting | Norway |
| Immunosuppressants | ||||||
| NCT04263402 (ClinicalTrials.gov) |
Arm A: methylprednisolone (<40 mg/day) Arm B: methylprednisolone (40–80 mg/day) |
100 | Yes | Single | Recruiting | China |
| ChiCTR2000030089 (ICTPR) |
Arm A: conventional treatment + adalimumab Arm B: conventional treatment |
60 | Yes | No | Not yet recruiting | China |
| ChiCTR2000030481 (ICTPR) |
Arm A: early corticosteroid intervention Arm B: middle–late corticosteroid intervention Arm C: standard care |
200 | Yes | No | Recruiting | China |
| NCT04288713 (ClinicalTrials.gov) | Arm A: eculizumab | Unspecified | Unspecified | Unspecified | Available | USA |
| NCT04280588 (ClinicalTrials.gov) |
Arm A: fingolimod Arm B: standard treatment |
30 | No | No | Recruiting | China |
| ChiCTR2000030703 (ICTPR) |
Arm A: ixekizumab and antiviral therapy Arm B: antiviral therapy |
40 | Yes | Single | Recruiting | China |
| NCT04275245 (ClinicalTrials.gov) [20] | Arm A: meplazumab | 20 | No | No | Recruiting | China |
| NCT04273321 (ClinicalTrials.gov) |
Arm A: methylprednisolone Arm B: standard treatment |
400 | Yes | No | Recruiting | China |
| NCT04244591 (ClinicalTrials.gov) |
Arm A: methylprednisolone Arm B: standard treatment |
80 | Yes | No | Recruiting | China |
| ChiCTR2000029656 (ICTPR) |
Arm A: methylprednisolone Arm B: standard treatment |
100 | Yes | No | Not recruiting | China |
| ChiCTR2000029386 (ICTPR) |
Arm A: methylprednisolone Arm B: standard treatment |
48 | Yes | Unspecified | Recruiting | China |
| NCT04315298 (ClinicalTrials.gov) |
Arm A: sarilumab high dose Arm B: sarilumab low dose Arm C: placebo |
400 | Yes | Quadruple | Recruiting | USA (Regeneron Pharmaceuticals) |
| ChiCTR2000030058 (ICTPR) |
Arm A: standard treatment + leflunomide Arm B: standard treatment + placebo |
200 | Yes | Yes | Not yet recruiting | China |
| ChiCTR2000030196 (ICTPR) | Arm A: tocilizumab | 60 | No | No | Not recruiting | China |
| ChiCTR2000029765 (ICTPR) |
Arm A: tocilizumab Arm B: standard treatment |
188 | Yes | Unspecified | Recruiting | China |
| NCT04315480 (ClinicalTrials.gov) | Arm A: tocilizumab | 30 | No | No | Not recruiting | France |
| NCT04317092 (ClinicalTrials.gov) | Arm A: tocilizumab | 330 | No | No | Recruiting | Italy |
| ChiCTR2000030442 (ICTPR) | Arm A: tocilizumab, IVIG, and CCRT | 100 | No | Unspecified | Not recruiting | China |
| ChiCTR2000030580 (ICTPR) |
Arm A: tozumabd and adalimumab Arm B: standard treatment |
60 | Yes | Unspecified | Recruiting | China |
| Immune modulators | ||||||
| NCT04317040 (ClinicalTrials.gov) |
Arm A: CD24Fc Arm B: placebo |
230 | Yes | Quadruple | Not recruiting | USA (OncoImmune) |
| ChiCTR2000029776 (ICTPR) |
Arm A: conventional treatment + polyinosinic-polycytidylic acid Arm B: conventional treatment |
40 | Yes | No | Recruiting | China |
| NCT04299724 (ICTPR) | Arm A: Covid-19/aAPC vaccine | 100 | No | No | Recruiting | China |
| ChiCTR2000030939 (ICTPR) | Arm A: CSA0001 | 10 | Yes | Unspecified | Recruiting | China |
| ChiCTR2000030016 (ICTPR) |
Arm A: inhaled inactive Mycobacterium vaccine Arm B: inhaled physiological saline |
60 | Yes | Yes | Recruiting | China |
| ChiCTR2000030167 (ICTPR) |
Arm A: interleukin-2 Arm B: standard treatment |
80 | Yes | Unspecified | Not recruiting | China |
| NCT04261426 (ClinicalTrials.gov) |
Arm A: IVIG Arm B: standard treatment |
80 | Yes | No | Not recruiting | China |
| NCT04276896 (ICTPR) | Arm A: LV-SMENP-DC vaccine and antigen specific cytotoxic T cells | 100 | No | No | Recruiting | China |
| NCT04268537 (ClinicalTrials.gov) |
Arm A: PD-1-blocking Ab Arm B: thymosin Arm C: standard treatment |
120 | Yes | Single | Not recruiting | China |
| ChiCTR2000030028 (ICTPR) |
Arm A: PD-1 mAb + standard treatment Arm B: standard treatment |
40 | Yes | No | Not yet recruiting | China |
| NCT04312997 (ClinicalTrials.gov) |
Arm A: PUL-042 nebuliser Arm B: sterile saline inhaler |
100 | Yes | Quadruple | Not recruiting | USA (Pulmotect) |
| ChiCTR2000030750 (ICTPR) |
Arm A: recombinant chimeric DC vaccine Arm B: normal saline |
120 | Yes | Unspecified | Not recruiting | China |
| ChiCTR2000030007 (ICTPR) |
Arm A: standard treatment + rhG-CSF Arm B: standard treatment |
200 | Yes | No | Not yet recruiting | China |
| ChiCTR2000029636 (ICTPR) | Arm A: standard treatment and vMIP atomised inhalation | 40 | No | No | Recruiting | China |
| ChiCTR2000029806 (ICTPR) |
Arm A: subcutaneous thymosin Arm B: camrelizumab infusion Arm C: conventional treatment |
120 | Yes | No | Recruiting | China |
| ChiCTR2000030779 (ICTPR) |
Arm A: ulinastatin (trypsin inhibitor) Arm B: standard treatment |
100 | Yes | No | Recruiting | China |
| Cytokine removal | ||||||
| ChiCTR2000030475 (ICTPR) | Arm A: CytoSorb cytokine removal | 19 | No | No | Not recruiting | China |
| ChiCTR2000030477 (ICTPR) | Arm A: oXiris membrane | 19 | No | No | Not recruiting | China |
| ChiCTR2000030265 (ICTPR) |
Arm A: oXiris membrane Arm B: standard treatment |
30 | Unspecified | Unspecified | Not recruiting | China |
| ChiCTR2000030835 (ICTPR) |
Arm A: high-dose MSCs Arm B: low-dose MSCs |
20 | No | Unspecified | Recruiting | China |
| ChiCTR2000029817 (ICTPR) |
Arm A: high-dose NK cells and MSCs Arm B: conventional-dose NK cells and MSCs Arm C: preventive-dose NK cells and MSCs |
60 | Unspecified | Unspecified | Not recruiting | China (Guangchou Reborn Health Management Co) |
| ChiCTR2000029606 (ICTPR) |
Arm A: menstrual blood-derived stem cells Arm B: artificial liver therapy Arm C: artificial liver therapy and menstrual blood-derived stem cells Arm D: standard treatment |
73 | Unspecified | Unspecified | Recruiting | China |
| NCT04315987 (ClinicalTrials.gov) | Arm A: MSCs | 24 | No | No | Not recruiting | Brazil (Cellavita Pesquisa Cientifica Ltd) |
| NCT04276987 (ClinicalTrials.gov) | Arm A: MSC-derived exosomes | 30 | No | No | Not recruiting | China (Cellular Biomedicine Group) |
| NCT04288102 (ClinicalTrials.gov) |
Arm A: MSCs Arm B: placebo |
60 | Yes | Quadruple | Recruiting | China |
| NCT04252118 (ClinicalTrials.gov) |
Arm A: MSCs Arm B: standard treatment |
20 | No | No | Recruiting | China (IPM, Vcanbio Cell and Gene Engineering) |
| ChiCTR2000030300 (ICTPR) | Arm A: MSCs | 9 | No | Unspecified | Recruiting | China |
| ChiCTR2000030224 (ICTPR) |
Arm A: MSCs Arm B: normal saline |
32 | Unspecified | Unspecified | Not recruiting | China |
| ChiCTR2000030173 (ICTPR) |
Arm A: MSCs Arm B: standard treatment |
60 | Unspecified | Unspecified | Not recruiting | China (Hunan yuanpin Cell Biotech) |
| ChiCTR2000030020 (ICTPR) | Arm A: MSCs | 20 | No | No | Recruiting | China |
| ChiCTR2000029990 (ICTPR) [22] |
Arm A: MSCs Arm B: saline |
120 | Yes | Unspecified | Recruiting | China |
| ChiCTR2000030261 (ICTPR) |
Arm A: MSC-derived exosomes Arm B: standard treatment |
26 | Unspecified | Unspecified | Not recruiting | China |
| NCT04280224 (ClinicalTrials.gov) |
Arm A: NK cells Arm B: standard treatment |
30 | Yes | No | Recruiting | China |
| ChiCTR2000030509 (ICTPR) |
Arm A: NK cells Arm B: electrolyte injection |
40 | Unspecified | Unspecified | Not recruiting | China |
| ChiCTR2000030944 (ICTPR) |
Arm A: NK cells and MSC Arm B: standard treatment |
20 | Yes | No | Not recruiting | China |
| NCT04302519 (ClinicalTrials.gov) | Arm A: pulp MSCs | 24 | No | No | Not recruiting | China (CAR-T Biotechnology Co, Ltd) |
| ChiCTR2000029580 (ICTPR) |
Arm A: ruxolitinib and MSCs Arm B: standard treatment |
70 | Yes | Single | Recruiting | China |
| NCT04299152 (ClinicalTrials.gov) |
Arm A: stem cell educator therapy Arm B: standard treatment |
20 | Yes | Single | Not recruiting | USA (Tianhe Stem Cell Biotechnologies Inc) |
| ChiCTR2000030329 (ICTPR) |
Arm A: umbilical cord blood CIK cells Arm B umbilical cord NK cells Arm C: standard treatment |
90 | Unspecified | Unspecified | Not recruiting | China |
| ChiCTR2000029812 (ICTPR) |
Arm A: umbilical cord blood mononuclear cell preparations Arm B: standard treatment |
60 | Unspecified | Unspecified | Not recruiting | China (Guangzhou Reborn Health Management Consultation Co) |
| ChiCTR2000029572 (ICTPR) |
Arm A: umbilical cord blood mononuclear cells Arm B: standard treatment |
30 | Yes | Unspecified | Recruiting | China |
| ChiCTR2000029818 (ICTPR) |
Arm A: umbilical cord blood plasma preparations Arm B: standard treatment |
60 | Unspecified | Unspecified | Not recruiting | China (Guangzhou Reborn Health Management Consultation Co) |
| NCT04293692 (ClinicalTrials.gov) |
Arm A: umbilical cord MSCs Arm B: placebo |
48 | Yes | Triple | Withdrawn | China (Wuhan Hamilton Biotechnology) |
| NCT04273646 (ClinicalTrials.gov) |
Arm A: umbilical cord MSCs Arm B: placebo |
48 | Yes | No | Not recruiting | China (Wuhan Biotechnology) |
| NCT04269525 (ClinicalTrials.gov) | Arm A: umbilical cord MSCs | 10 | No | No | Recruiting | China (Tuohua Biological Technology Co) |
| ChiCTR2000030138 (ICTPR) |
Arm A: umbilical cord MSCs Arm B: placebo |
60 | Yes | Double | Not recruiting | China |
| ChiCTR2000030484 (ICTPR) |
Arm A: umbilical cord MSCs Arm B: umbilical cord MSCs and derived exosomes Arm C: placebo |
120 | Unspecified | Unspecified | Not recruiting | China |
| ChiCTR2000030116 (ICTPR) |
Arm A: umbilical cord MSCs dose A Arm B umbilical cord MSCs dose B |
16 | Yes | Unspecified | Recruiting | China |
| ChiCTR2000029816 (ICTPR) |
Arm A: umbilical cord MSCs Arm B: standard treatment |
60 | Yes | No | Not recruiting | China (Guangzhou Reborn Health Management) |
| NCT04313322 (ClinicalTrials.gov) | Arm A: Wharton jelly MSCs | 5 | No | No | Recruiting | Jordan (Stem Cells Arabia) |
| ChiCTR2000030088 (ICTPR) |
Arm A: Wharton jelly MSCs Arm B: saline |
20 | Yes | Unspecified | Not recruiting | China |
| Plasma-based therapy | ||||||
| ChiCTR2000030702 (ICTPR) |
Arm A: convalescent plasma therapy Arm B: standard treatment |
50 | Yes | No | Recruiting | China |
| ChiCTR2000030046 (ICTPR) | Arm A: anti-2019-nCoV virus inactivated plasma | 10 | No | No | Recruiting | China |
| ChiCTR2000030381 (ICTPR) |
Arm A: anti-SARS-CoV-2 inactivated convalescent plasma Arm B: ordinary plasma |
40 | Yes | No | Not recruiting | China |
| ChiCTR2000030010 (ICTPR) |
Arm A: anti-SARS-CoV-2 virus inactivated plasma Arm B: ordinary plasma |
100 | Yes | Double | Not recruiting | China |
| ChiCTR2000030841 (ICTPR) |
Arm A: convalescent immunoglobulin Arm B: gamma-globulin |
10 | No | No | Recruiting | China |
| NCT04264858 (ClinicalTrials.gov) |
Arm A: convalescent immunoglobulin Arm B: gamma globulin |
10 | No | No | Not recruiting | China |
| ChiCTR2000030039 (ICTPR) |
Arm A: convalescent plasma Arm B: standard treatment |
90 | No | No | Recruiting | China |
| ChiCTR2000029850 (ICTPR) |
Arm A: convalescent plasma Arm B: standard treatment |
20 | No | Unspecified | Recruiting | China |
| ChiCTR2000030627 (ICTPR) |
Arm A: convalescent plasma therapy Arm B: standard treatment |
30 | Yes | Unspecified | Recruiting | China |
| ChiCTR2000029757 (ICTPR) |
Arm A: convalescent plasma therapy Arm B: standard treatment |
200 | Yes | No | Recruiting | China |
| ChiCTR2000030929 (ICTPR) |
Arm A: convalescent plasma therapy Arm B: control plasma |
60 | Yes | Double | Not recruiting | China |
| ChiCTR2000030179 (ICTPR) |
Arm A: plasma treatment Arm B: standard treatment |
100 | Yes | Unspecified | Recruiting | China |
| Inhaled gas | ||||||
| ChiCTR2000030258 (ICTPR) |
Arm A: hydrogen inhalatione Arm B: standard treatment |
60 | Yes | No | Not recruiting | China |
| ChiCTR2000029739 (ICTPR) |
Arm A: hydrogen–oxygen nebuliser Arm B: oxygen |
440 | Yes | Unspecified | Recruiting | China |
| NCT04290871 (ClinicalTrials.gov) |
Arm A: inhaled nitric oxide Arm B: no intervention |
104 | Yes | Yes | Not yet recruiting | China |
| NCT04306393 (ClinicalTrials.gov) |
Arm A: inhaled nitric oxide Arm B: no intervention |
200 | Yes | Yes | Not yet recruiting | USA |
| NCT04305457 (ClinicalTrials.gov) |
Arm A: inhaled nitric oxide Arm B: no intervention |
240 | Yes | No | Not yet recruiting | USA |
| NCT04290858 (ClinicalTrials.gov) |
Arm A: inhaled nitric oxide Arm B: no intervention |
240 | Yes | No | Not yet recruiting | China |
| Antifibrotic | ||||||
| NCT04282902 (ClinicalTrials.gov) |
Arm A: pirfenidone Arm B: standard treatment |
294 | Yes | No | Recruiting | China |
| ChiCTR2000030892 (ICTPR) |
Arm A: pirfenidone Arm B: standard treatment |
20 | Yes | No | Recruiting | China |
| ChiCTR2000030333 (ICTPR) |
Arm A: pirfenidone Arm B: standard treatment |
292 | Yes | No | Recruiting | China |
| Antiangiogenic | ||||||
| NCT04275414 (ClinicalTrials.gov) | Arm A: bevacizumab | 20 | No | No | Recruiting | China |
| NCT04305106 (ClinicalTrials.gov) |
Arm A: bevacizumab Arm B: standard treatment |
118 | Yes | Triple | Recruiting | China |
| NCT04273581 (ClinicalTrials.gov) |
Arm A: thalidomide Arm B: placebo |
40 | Yes | Quadruple | Not recruiting | China |
| NCT04273529 (ClinicalTrials.gov) |
Arm A: thalidomide Arm B: placebo |
100 | Yes | Quadruple | Not recruiting | China |
| Antimicrobial | ||||||
| ChiCTR2000030539 (ICTPR) |
Arm A: 3% hydrogen peroxide gargle Arm B: standard treatment |
40 | Unspecified | Unspecified | Not recruiting | China |
| ChiCTR2000029867 (ICTPR) |
Arm A: carrimycin Arm B: lopinavir/ritonavir |
520 | Yes | No | Recruiting | China |
| NCT04286503 (ClinicalTrials.gov) |
Arm A: carrimycin + basic treatment (unspecified) Arm B: lopinavir/ritonavir or umifenovir or chloroquine phosphate + basic treatment (unspecified) |
520 | Yes | No | Recruiting | China (Shenyang Tonglian Group) |
| ChiCTR2000030029 (ICTPR) | Arm A: suramin | 20 | No | No | Not yet recruiting | China |
| Antioxidants | ||||||
| ChiCTR2000029851 (ICTPR) |
Arm A: alpha lipoic acid Arm B: placebo |
68 | Yes | Unspecified | Recruiting | China |
| ChiCTR2000030471 (ICTPR) |
Arm A: lipoic acid injection Arm B: standard treatment |
384 | Yes | Single | Recruiting | China |
| Microbiome | ||||||
| ChiCTR2000030897 (ICTPR) |
Arm A: Newgen beta-gluten probiotic Arm B: standard treatment |
20 | Yes | Unspecified | Recruiting | China |
| ChiCTR2000029999 (ICTPR) |
Arm A: probiotics Arm B: probiotics |
60 | No | No | Not recruiting | China |
| ChiCTR2000029974 (ICTPR) |
Arm A: probiotics Arm B: standard treatment |
300 | Yes | No | Recruiting | China (Qingdao East Sea Pham.) |
| ChiCTR2000029849 (ICTPR) |
Arm A: Unspecified intestinal flora intervention Arm B: standard treatment |
60 | Yes | Unspecified | Recruiting | China |
| NCT04251767 (ClinicalTrials.gov) |
Arm A: washed microbiota transplant Arm B: placebo |
40 | Yes | Quadruple | Enrolling by invitation | China |
| Organ support | ||||||
| ChiCTR2000030503 (ICTPR) |
Arm A: artificial liver system Arm B: standard treatment |
60 | No | No | Recruiting | China |
| ChiCTR2000030540 (ICTPR) |
Arm A: CRRT Arm B: CRRT only for emergency indication |
152 | Unspecified | Unspecified | Not recruiting | China |
| ChiCTR2000030761 (ICTPR) | Arm A: CRRT | 20 | No | No | Not recruiting | China |
| ChiCTR2000030744 (ICTPR) |
Arm A: ECMO Arm B: standard treatment |
30 | No | No | Recruiting | China |
| ChiCTR2000030855 (ICTPR) | Arm A: external diaphragmatic pacing | 200 | No | No | Not recruiting | China |
| ChiCTR2000030773 (ICTPR) | Arm A: Unspecified blood purification | 20 | No | No | Recruiting | China |
| Therapy interventions | ||||||
| ChiCTR2000030260 (ICTPR) |
Arm A: enteral nutrition emulsion Arm B: standard treatment |
20 | Yes | No | Not recruiting | China |
| ChiCTR2000030198 (ICTPR) |
Arm A: health education and pulmonary rehabilitation Arm B: health education |
60 | Unspecified | Unspecified | Not recruiting | China |
| ChiCTR2000030418 (ICTPR) |
Arm A: lung rehabilitation Arm B: usual activity |
80 | Unspecified | Unspecified | Recruiting | China |
| ChiCTR2000030578 (ICTPR) |
Arm A: lung rehabilitation training Arm B: standard treatment |
40 | Unspecified | Unspecified | Not recruiting | China |
| NCT04283825 (ClinicalTrials.gov) |
Arm A: psychological and physical rehabilitation Arm B: standard treatment |
100 | No | No | Not recruiting | China |
| ChiCTR2000030084 (ICTPR) |
Arm A: psychological intervention Arm B: standard treatment |
180 | Unspecified | Unspecified | Recruiting | China |
| ChiCTR2000030467 (ICTPR) |
Arm A: psychological intervention and traditional Chinese medicine Arm B: psychological intervention, traditional Chinese medicine, and traditional Chinese medicine psychological intervention |
60 | Unspecified | Unspecified | Not recruiting | China |
| ChiCTR2000029459 (ICTPR) |
Arm A: pulmonary rehabilitation Arm B: standard treatment |
50 | Unspecified | Unspecified | Not recruiting | China |
| ChiCTR2000030433 (ICTPR) | Arm A: rehabilitation and lung eight-segment exercisef | 80 | No | No | Not recruiting | China |
| ChiCTR2000029460 (ICTPR) |
Arm A: shadowboxing rehabilitation Arm B: standard treatment |
100 | Yes | No | Not recruiting | China |
| Ozonated autohemotherapy | ||||||
| ChiCTR2000030165 (ICTPR) |
Arm A: conventional treatment Arm B (mild): conventional treatment + ozonated autohemotherapy Arm C (severe): conventional treatment + ozonated autohemotherapy |
60 | No | No | Recruiting | China |
| ChiCTR2000030102 (ICTPR) |
Arm A: conventional treatment Arm B: conventional treatment + ozone therapy Arm C (severe): conventional treatment + ozone therapy Arm D (severe): conventional treatment Arm E (critical): conventional treatment + ozone therapy Arm F (critical): conventional treatment |
180 | Yes | No | Recruiting | China |
| ChiCTR2000030006 (ICTPR) |
Arm A: ozonated autohemotherapy Arm B: standard medical treatment |
60 | Yes | No | Recruiting | China |
| Other | ||||||
| ChiCTR2000029742 (ICTPR) |
Arm A: (general): normal treatment Arm B (general): normal treatment + sodium aescinate Arm C (severe): normal treatment + hormonotherapy (presumed glucocorticoids) Arm D (severe): lopinavir/ritonavir Arm E (severe): normal treatment + sodium aescinate |
90 | Yes | No | Recruiting | China |
| ChiCTR2000030328 (ICTPR) |
Arm A: acetylcysteine inhalation (mucolytic effect) via tracheal tube Arm B: saline inhalation via tracheal tube |
60 | Yes | Unspecified | Not recruiting | China |
| ChiCTR2000030398 (ICTPR) |
Arm A: bismuth Arm B: placebo |
340 | Yes | Double | Not recruiting | China |
| ChiCTR2000030055 (ICTPR) |
Arm A: conventional treatment Arm B: conventional treatment + dipyridamole |
460 | Yes | No | Recruiting | China |
| ChiCTR2000030853 (ICTPR) | Arm A: dexmedetomidine | 200 | No | No | Not recruiting | China |
| ChiCTR2000030700 (ICTPR) |
Arm A: enoxaparin sodium Arm B: standard treatment |
60 | Yes | No | Not recruiting | China |
| ChiCTR2000030135 (ICTPR) |
Arm A: high-dose vitamin C Arm B: standard treatment |
39 | Yes | Unspecified | Not recruiting | China |
| NCT04311697 (ClinicalTrials.gov) |
Arm A: intravenous aviptadil followed by nebulised in 48 h if required Arm B: aviptadil nebuliser followed by intravenous in 48 h if required |
20 | Yes | Single | Not recruiting | USA and Israel (NeuroRx) |
| ChiCTR2000030170 (ICTPR) | Arm A: jakotinibg | 8 | Unspecified | Unspecified | Recruiting | China |
| NCT04312009 (ClinicalTrials.gov) |
Arm A: losartan Arm B: placebo |
200 | Yes | Quadruple | Not recruiting | USA |
| NCT04311177 (ClinicalTrials.gov) |
Arm A: losartan Arm B: placebo |
478 | Yes | Quadruple | Not recruiting | USA |
| ChiCTR2000030946 (ICTPR) |
Arm A: low-molecular-weight heparin Arm B: mechanical prevention |
120 | Yes | Unspecified | Recruiting | China |
| NCT04304313 (ClinicalTrials.gov) | Arm A: sildenafil | 10 | No | No | Recruiting | China |
| NCT04308317 (ClinicalTrials.gov) |
Arm A: tetrandrine Arm B: standard treatment |
60 | Yes | No | Enrolling by invitation | China |
| NCT04264533 (ClinicalTrials.gov) |
Arm A: vitamin C Arm B: sterile water for injection |
140 | Yes | Triple | Recruiting | China |
| (B) Ongoing clinical trials for prevention of COVID-19 | ||||||
| Vaccine | ||||||
| NCT04299724 (ClinicalTrials.gov) | Arm A: Covid-19/aAPC vaccine | 100 | No | No | Recruiting | China |
| NCT04313127 (ClinicalTrials.gov) |
Arm A: low-dose Ad5-nCoV Arm B: middle-dose Ad5-nCoV Arm C: high-dose Ad5-nCoV |
108 | No | No | Not recruiting | China (CanSino Biologics) |
| NCT04283461 (ClinicalTrials.gov) |
Arm A: mRNA-1273 (25 μg) Arm B: mRNA-1273 (100 μg) Arm C: mRNA-1273 (250 μg) |
45 | No | No | Recruiting | USA (ModernaTX) |
| Antiviral | ||||||
| NCT04304053 (ClinicalTrials.gov) |
Arm A: darunavir/cobicistat Arm B: isolation |
3040 | Yes | No | Recruiting | Spain |
| ChiCTR2000030013 (ICTPR) |
Arm A: interferon a1b Arm B: no intervention |
450 | Unspecified | Unspecified | Not recruiting | China |
| ChiCTR2000029592 (ICTPR) |
Arm A: umifenovir Arm B: without umifenovir |
1000 | Unspecified | No | Not recruiting | China |
| Antimalarial | ||||||
| NCT04303507 (ClinicalTrials.gov) |
Arm A: chloroquine Arm B: placebo |
10000 | Yes | Double | Not recruiting | UK |
| NCT04308668 (ClinicalTrials.gov) |
Arm A: hydroxychloroquine Arm B: placebo |
1500 | Yes | Quadruple | Recruiting | USA |
| ChiCTR2000029803 (ICTPR) |
Arm A: hydroxychloroquine (low dose) Arm B: hydroxychloroquine – high dose Arm C: umifenovir – low dose Arm D: umifenovir – high dose |
320 | Yes | No | Not recruiting | China |
| Personal protective equipment | ||||||
| ChiCTR2000030317 (ICTPR) |
Arm A: gastroscope mask Arm B: without mask |
300 | Yes | No | Not recruiting | China |
| NCT04296643 (ClinicalTrials.gov) |
Arm A: medical masks Arm B: N95 respirators |
676 | Yes | Single | Not recruiting | USA |
| Other | ||||||
| NCT04312243 (ClinicalTrials.gov) |
Arm A: nitric oxide Arm B: no treatment |
460 | No | No | Not recruiting | USA |
| NCT04313023 (ClinicalTrials.gov) |
Arm A: PUL-042 Arm B: normal saline |
200 | Yes | Quadruple | Not yet recruiting | USA (Pulmotect) |
| ChiCTR2000030432 (ICTPR) |
Arm A: rehabilitation and lung eight-segment exercises Arm B: normal activity |
80 | Yes | No | Not recruiting | China |
Abbreviations: Ad5, adenovirus type 5; APC, antigen-presenting cells; CIK cells, cytokine-induced killer cells; CRRT, continuous renal replacement therapy; DC, dendritic cell; ECMO, extracorporeal membrane oxygenation; IVIG, intravenous immunoglobulin; MSCs, mesenchymal stem cells; NK cells, natural killer cells; rhG-CSF, recombinant human granulocyte colony-stimulating factor; rSFIN-co, recombinant supercompound interferon; TFF2, Trefoil factor 2; vMIP, viral macrophage inflammatory protein.
For part (B), this column indicates the intervention to prevent infection.
Participant size as stated in registry entry.
No literature outside trial protocol; likely tocilizumab.
Hydrogen inhalation has shown evidence of antioxidant and anti-inflammatory effects in ischaemia–reperfusion injury.
No literature outside trial protocol; likely a form of lung rehabilitation.
No literature outside trial protocol; possible Janus kinase inhibitor.
Treatment Strategies
Antiviral Treatments
As briefly mentioned earlier, many studies have focused on repurposing established antiviral therapies, especially those that showed prior efficacy against SARS-CoV and MERS-CoV. The combination of lopinavir/ritonavir is the most common exploratory antiviral, appearing in 34 investigational studies (Table 1A: Antivirals). Both drugs function as protease inhibitors and are used extensively in the management of HIV-1 [9]. However, lopinavir has insufficient oral bioavailability for significant therapeutic activity, due to rapid catabolism by the cytochrome P450 enzyme system (specifically 3A4 isoenzyme) [9]. Thus, ritonavir is given concomitantly to inhibit this, significantly boosting the half-life of lopinavir. Lopinavir/ritonavir was investigated for efficacy against SARS-CoV in 2004 and found to be effective compared with a historical control [10]. However, efficacy was not seen in a randomised open-label study (see Glossary) (lopinavir/ritonavir versus standard care) in 199 patients with COVID-19 (Clinical Trial Number: ChiCTR2000029308, recruitment target stated as 160 participants in the registry; Table 1). No significant benefit was seen in either overall mortality or reduction in viral load [11]. The authors highlighted several limitations, including a lack of treatment blinding, with study participants and investigators being aware of treatment assignments, thus reducing study objectivity. While there are multiple other ongoing studies exploring lopinavir/ritonavir in COVID-19, none utilises a double-blind methodology to address this limitation.
Remdesivir is a novel nucleotide analogue antiviral, initially developed for the management of the Ebola and Marburg viruses [12,13]. However, it has efficacy against a range of pathogenic viruses, including both SARS-CoV and MERS-CoV in in vitro and in vivo models [12,14]. There has been much interest in this molecule, following treatment of the first COVID-19 case, and subsequent recovery, in the USA [15]. There are currently ten registered trials taking place globally to investigate efficacy for COVID-19 (Table 1A: Antivirals).
Several other antiviral drugs are being investigated, predominately those with activity against various influenza subtypes and other RNA viruses. These include favipiravir (T-705, Avigan), umifenovir (Arbidol), triazavirin (TZV), and baloxavir marboxil (Xofluza). Many trials are focusing on drugs typically used in the management of RNA viruses, such as HCV and HIV. These include danoprevir/ritonavir, azvudine, sofosbuvir/ledipasvir, sofosbuvir/daclatasvir, darunavir/cobicistat, and emtricitabine/ tenofovir (Table 1A: Antivirals). Additionally, there are 26 studies investigating the utility of antiviral interferon-based treatments, interestingly also looking at various different routes of administration (e.g., nasal).
Antimalarial Treatments
Thirty-five trials are now investigating the use of the antimalarial drugs chloroquine and hydroxychloroquine against COVID-19 (Table 1A: Antimalarials). Chloroquine was found to have significant inhibitory effects on viral cell entry and replication in vitro [12]. An early report of clinical experience in 100 patients with COVID-19 reported both beneficial clinical and virological outcomes with chloroquine treatment [16]. More recently, a nonrandomised open-label study examining the effect of hydroxychloroquine (EU Clinical Trial Numbervii: 2020-000890-25; recruitment target stated as 25 participants in the registry) reported on a cohort of 36 patients [17]. It reported a significant reduction in nasopharyngeal swab viral positivity 6 days after inclusion in the hydroxychloroquine group compared with control. However, in a deviation from their registry-described protocol, 16 patients were designated as controls and six patients received concurrent treatment with azithromycin to prevent bacterial superinfection. Selection of patients receiving azithromycin was based on clinical judgement. The subgroup receiving azithromycin all had negative viral swabs after 6 days compared with 57% (8/14) of hydroxychloroquine alone and 12.5% (2/16) of control [17]. This study is limited by its lack of randomisation and blinding, and small sample size. There is much interest in chloroquine or hydroxychloroquine for the treatment of COVID-19, with a further 34 studies registered (Table 1A: Antimalarials); however, only four report using a robust double-blind randomised controlled protocol to investigate efficacy.
Immunosuppressants/Immunomodulators
There is evidence that a hyperinflammatory response significantly contributes to mortality in COVID-19 infections [18]. Corticosteroids were previously trialled in SARS-CoV; however, the results were inconclusive and adverse effects were associated [19]. Seven registered studies are evaluating the effect of corticosteroids in COVID-19 (Table 1A: Immunosuppressants). There is also interest in the anti-IL-6 drug, tocilizumab (used in the treatment of rheumatoid arthritis), with seven registered trials. Other immunosuppressants being investigated include adalimumab (anti-TNF), eculizumab (anti-C5), sarilumab (anti-IL-6), ixekizumab (anti-17A), and fingolimod (sphingosine-1-phosphate receptor modulator, used against multiple sclerosis). Meplazumab (anti-CD147) inhibits not only T cell chemotaxis, but also virus cell entry [20]. A preprint of a study of 17 patients compared with 11 controls (NCT04275245, original recruitment target 20) reported improved clinical and virological outcomes [20].
Conversely, several studies are investigating immune stimulation. These include the anti-PD-1 antibody camrelizumab, recombinant IL-2, CSA0001 (LL-37 antiviral peptide with immunomodulatory functions), CD24FC [fusion protein that prevents Toll-like receptor (TLR) activation and activates immunosuppressive Siglec signalling] and recombinant human granulocyte colony-stimulating factor (rhG-CSF) (Table 1A: Immune Modulators). Three studies (NCT04299724, NCT04276896, and ChiCTR2000030750) examine the efficacy of experimental vaccines in infected patients. Three further studies are investigating nonpharmaceutical interventions to modulate the immune system using cytokine filtration devices, such as oXiris and CytoSorb, to reduce circulating cytokines and inflammatory mediators (Table 1A: Cytokine Removal).
Cell and Plasma-Based Therapy
Twenty-four registered studies plan to investigate the role of mesenchymal stem cells (MSCs) (Table 1A: Cell-Based Therapies). MSCs have immunomodulatory and tissue repair effects through the secretion of cytokines and growth factors. They have previously been examined in a Phase I trial in Adult Respiratory Distress Syndrome (ARDS) [21]. Given that most of the deaths in COVID-19 are from respiratory failure, MSCs are postulated to have a beneficial effect. So far, one study of MSCs (ChiCTR2000029990, recruitment target stated as 120 participants in the registry) has reported results in seven patients with COVID-19, showing improvement in both clinical and inflammatory outcome compared with three control patients treated with saline [22]. This study plans to recruit 120 participants with 60 patients in each of the treatment (MSC) and control (saline) arms.
Use of plasma from patients who have recovered from COVID-19 has the potential benefit of providing disease-specific neutralising antibodies, before targeted therapies can be developed. During the Ebola outbreak in 2014, the WHO advised the use of convalescent plasma or whole-blood therapies. However, a nonrandomised comparative study in 84 patients with Ebola found no associated improvement in survival [23]. There are currently 12 registered trials to investigate convalescent plasma or immunoglobulins in COVID-19 (Table 1A: Plasma-Based Therapies).
Alternative Treatment Strategies
Various other treatment strategies are currently under investigation, including the antifibrotic/inflammatory agent pirfenidone (used in treatment of idiopathic pulmonary fibrosis), and the antiangiogenic agents: bevacizumab (anti-VEGF) and thalidomide (Table 1A: Antifibrotics and Antiangiogenics). A further five studies aim to assess the therapeutic utility of modifying the gut microbiome (Table 1A: Microbiome), although the mechanisms by which this is performed are not explicit in the trial registers. Ten other studies are investigating holistic approaches, including physiotherapy, psychology, and nutritional intervention, on disease outcome (Table 1A: Therapy Interventions).
Preventative Strategies
No effective vaccine or antiviral therapeutic agent for postexposure prophylaxis has been approved for preventing COVID-19 infection or any other human coronavirus. The development of vaccines is a complex, time-consuming process with a high attrition rate. Success in generating a vaccine in the recent 2009 flu pandemic (H1N1/09) has fuelled optimism towards one for COVID-19 [24]. Furthermore, both the rapid genomic sequencing of COVID-19 and insights gleaned during vaccine exploration for both MERS-CoV and SARS-CoV (both terminated due to successful disease containment) has allowed preclinical and animal work to advance rapidly [7].
Over 50 novel vaccines are estimated to be in development; however, only three vaccine studies are registered for Phase I evaluation (Table 1B: Vaccines). Two studies are actively recruiting in the USA and China, and a further study is newly registered (initial set-up). A modified mRNA vaccine (mRNA-1273) that encodes the COVID-19 viral spike protein has progressed rapidly through preclinical development to human testing (42 days from sequence identification), developed by Moderna, Inc and the National Institute of Allergy and Infectious Diseases (NIAID). However, such rapid development has prompted safety concerns from some experienced virologists [25]. Other current investigational vaccines being tested in humans include a replicative-defective adenovirus type 5 (Ad5)-nCoV that expresses COVID-19 viral proteins and a lentiviral vector system to express viral proteins and immunomodulatory genes to modify antigen-presenting cells (aAPC) (Table 1B: Vaccines).
Furthermore, postexposure prophylaxis is an attractive strategy for both healthcare workers and household contacts exposed to COVID-19. Currently, six studies are looking at the use of antivirals, such as umifenovir, antimalarials, such as hydroxychloroquine and chloroquine, and the use of recombinant human interferon alpha (a)1b spray for the prevention of infection (Table 1B: Antiviral and Antimalarial).
Global Response
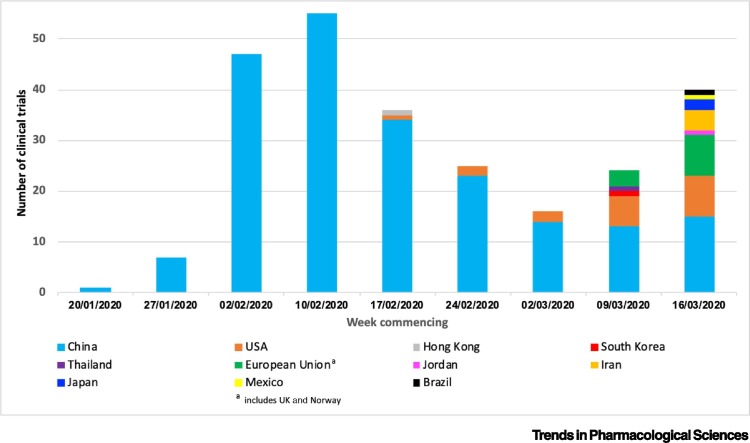
Over 85% of the clinical trials (excluding TCM) for either the prevention and/or treatment of COVID-19 have been registered in China, which is not surprising given that the country saw the outbreak of the disease first. The first clinical trials were registered within 1 month of COVID-19 identification and rapidly expanded after that (Figure 2 ). Public health initiatives have thus far successfully curtailed the previously exponential growth of COVID-19 cases in China. This has reduced the number of potential participants for clinical trials in China and the registration of new clinical trials has since declined. Furthermore, several studies have also been withdrawn or suspended (e.g., NCT04293692 and ChiCTR2000030082).
Figure 2.
First Recording (Week Commencing) of Clinical Trials for COVID-19 in Registry by Country (Primary Sponsor/Principal Investigator Origin).
Data are from registries in Australia, New Zealand, China, The Netherlands, Brazil, India, Cuba, Republic of Korea, Germany, Iran, Japan, Sri Lanka, Thailand, and Peru, and also ClinicalTrials.gov, EU Clinical Trials registry, International Standard Randomised Controlled Trial Number (ISRCTN), and the Pan-Africa registries.
The wider global community has been slower to react. The first case of COVID-19 outside of Asia was reported in late January 2020iii. Subsequently, the incidence of COVID-19 has increased dramatically. The WHO has now declared that Europe has become the new disease epicentre, with 40% and rising of the total number of casesix. However, until recently, <5% of clinical trials for COVID-19 were registered in Europe (Figure 2). The rapid escalation of trial registrations in response to increasing disease incidence seen in China has unfortunately not occurred in Europe. Despite this, there are now encouraging signs. Initiatives focused on pan-European collaboration are being championed by the European Union with a priority on larger patient studies compared with the smaller studies registered in Chinax. Consequently, the median number of participants in European registered studies is 1200 participants, compared with 60 and 394 in China and USA, respectively. An example is NCT04303507 (chloroquine postexposure prophylaxis), which plans to recruit 10 000 participants (Table 1B). However, this may in part reflect a higher proportion of preventative studies currently being carried out that include large numbers of participants. Hopefully, larger studies will provide higher quality evidence, although may take longer to generate results in the context of this escalating public health crisis.
With an increasing number of COVID-19 cases reported in North America, there has also been an increase in clinical trial registrations in the USA. The NIAID registered the first USA-led global trial in mid-February 2020, utilising 50 sites across Asia and USA (Figure 2). Studies registered in the USA have generally placed an emphasis on larger participant numbers than China (Table 1) and on an adaptive trial design for both the treatment and prevention of COVID-19.
Concluding Remarks
The COVID-19 pandemic represents the gravest global public health threat seen since the 1918 influenza outbreak and has rapidly become a global healthcare emergency. Clinical trials need to produce high-quality data that can be used to objectively assess potentials therapies for both the treatment and prevention of this global emergency. It is imperative to plough international resources into high-quality design clinical trials with robust scientific rationale and vigorous statistical rigor. Increasing international collaboration and the globalisation of clinical trials with large patient numbers should be the way forward to provide significant and definitive results.
Disclaimer Statement
M.P.L. received an educational travel grant from Bayer.
Glossary
- Adalimumab
mAb targeted against TNF-α; an immunosuppressant commonly used in inflammatory conditions.
- Anti-PD-1 antibody
antibody against Programmed Cell Death Protein 1 (PD-1); inhibition of PD-1 can reverse immune exhaustion; used in oncology treatment (e.g., melanoma).
- ASC09
HIV protease inhibitor; under development by Ascletis Pharmaceuticals.
- Aviptadil
a vasodilator and short-acting alpha-adrenoreceptor antagonist.
- Azvudine
nucleoside reverse transcriptase inhibitor with efficacy against HCV and HIV.
- Baloxavir marboxil
polymerase acidic endonuclease inhibitor approved for influenza.
- Bevacizumab
mAb targeting vascular endothelial growth factor (VEGF).
- Bismuth
oral medication used in treatment of Helicobacter pylori; some evidence of inhibition of SARS coronavirus helicase ATPase.
- Blinding
experimental procedure in which the participant, investigator, care provider, or outcome assessor in a clinical trial are unaware of which treatment arm the participant is receiving. Studies can be described as the number of roles that are blinded (i.e., single, double or quadruple-blinded study). Blinding reduces the risk of bias in the outcome of a trial.
- Carrimycin
macrolide antibiotic.
- Cytokine-induced killer cells (CIK cell)
CD8+ T cells expanded from ex vivo stimulation of lymphocytes; used in experimental immunotherapy.
- Cobicistat
CYP3A inhibitor licensed for use in HIV; potentiates action of other antiviral medication.
- Danoprevir
NS3/4A protease inhibitor used in treatment of HCV.
- Darunavir
HIV protease inhibitor.
- Dexmedetomidine
sedative α2-adrenergic receptor agonist.
- Dihydroartemisinin/piperaquine
combination antimalarial medication.
- Dipyridamole
antiplatelet medication that is a phosphodiesterase inhibitor; exerts antiviral effects via inhibition of nucleoside uptake.
- Double-blind
where two groups within a study, typically the participant and the outcome assessor, are blinded to the treatment received by the participant.
- Ebastine
H1 receptor antagonist.
- Eculizumab
mAb that inhibits activation of complement protein C5; used in thrombotic microangiopathy.
- Emtricitabine/tenofovir
combination nucleoside reverse transcriptase inhibitor used in the treatment of HIV-1.
- Enoxaparin
low-molecular-weight heparin, an anticoagulant.
- Favipiravir
RNA-dependent RNA polymerase inhibitor, investigated against RNA viruses, such as Influenza, Ebola and Marburg viruses.
- GD31
described within the trial report as novel nucleoside analogue.
- Interferon alpha
cytokine used in the treatment of chronic viral infections, such as HBV and HCV.
- Interferon beta 1b
cytokine used in the treatment of multiple sclerosis.
- Leflunomide
immunosuppressive used in the treatment of rheumatoid arthritis.
- Lipoic acid
antioxidant.
- Losartan
angiotensin-II receptor antagonist.
- Novaferon
recombinant interferon-like protein; in vitro and in vivo model evidence of more potent activity compared with interferon.
- Open-label
a study in which the treatment received by the participant is known to both the participant and investigators.
- Oseltamivir
neuraminidase inhibitor; licenced for influenza A and B treatment.
- Pegasys
pegylated interferon alpha 2a.
- Polyinosinic-polycytidylic acid
immunostimulant; TLR3 agonist.
- PUL-042
immunostimulant; TLR2/6/9 agonist.
- Randomised study
a trial in which the treatment or intervention is randomly allocated to a participant. Randomisation reduces the risk of bias in a trial outcome.
- Recombinant IL-2
cytokine used in cancer immunotherapy treatment (e.g., melanoma).
- Ribavirin
guanosine analogue; antiviral agent used against a range of morbific viral infections (e.g., HCV, human respiratory syncytial virus, and Lassa virus).
- Ruxolitinib
selective inhibitor of Janus Kinase type 1 and 2; used within haematology against polycythaemia vera and myelofibrosis.
- Sildenafil
phosphodiesterase type 5 inhibitor; vasodilator used commonly for erectile dysfunction and pulmonary arterial hypertension.
- Sodium aescinate
saponin extract of Aesculus hippocastanum seeds; investigated for use in lung injury.
- Sofosbuvir/daclatasvir
combination mediation used in treatment of HCV. Sofosbuvir is a nucleotide prodrug and acts as an inhibitor of HCV NS5B RNA-dependant RNA polymerase. Daclatasvir is an HCV NS5A inhibitor.
- Sofosbuvir/ledipasvir
combination mediation used in treatment of HCV; Ledipasvir is an inhibitor of HCV NS5A protein.
- Stem cell educator therapy
circulation of patient blood through a cell separator followed by brief co-culture of immune cells with cord-blood stem cells and return of the educated immune cells to the patient’s circulation.
- Suramin
antitrypanosomal drug used in treatment of African trypanosomiasis.
- Tetrandrine
bisbenzylisoquinoline alkaloid; a calcium channel blocker with anti-inflammatory and immunosuppressant properties.
- Thalidomide
antiangiogenic and immunomodulator used against a range of haematological malignancies, including multiple myeloma. Teratogenic antiemetic causing range of birth defects, such as phocomelia.
- Thymosin
thymus hormones that stimulate development of T cells.
- Tranilast
antiallergic analogue of a tryptophan metabolite; NLRP3 inflammasome inhibitor.
- Triazavirin
guanine nucleotide analogue with broad-spectrum antiviral effects.
- Umifenovir (Arbidol)
non-nucleoside antiviral membrane fusion inhibitor; licensed in Russia for the treatment of influenza.
Resources
iwww.who.int/csr/sars/country/table2004_04_21/en/iiwww.euro.who.int/en/health-topics/health-emergencies/coronavirus-covid-19iiiwww.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reportsivhttps://unctad.org/en/pages/newsdetails.aspx?OriginalVersionID=2300vhttps://clinicaltrials.govviwww.who.int/ictrp/en/viiwww.clinicaltrialsregister.eu/viiiwww.cochranelibrary.com/central/about-centralixwww.euro.who.int/en/health-topics/health-emergencies/coronavirus-covid-19/news/news/2020/3/global-solidarity-across-countries-and-continents-needed-to-fight-covid-19xwww.bioworld.com/articles/433824-eu-boosts-funding-for-covid-19-epidemic-encourages-clinical-trial-cooperationxiwww.isrctn.com/?gclid=Cj0KCQjwjoH0BRD6ARIsAEWO9Dt7ppI5xmcUMgabefiiRnPVSbsoH3CtwieB5maS2z4gzAyZ1nNjd8MaAjEREALw_wcBReferences
- 1.Paules C.I. Coronavirus infections: more than just the common cold. JAMA. 2020;323:707–708. doi: 10.1001/jama.2020.0757. [DOI] [PubMed] [Google Scholar]
- 2.Song Z. From SARS to MERS, thrusting coronaviruses into the spotlight. Viruses. 2019;11:59. doi: 10.3390/v11010059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Wit E. SARS and MERS: recent insights into emerging coronaviruses. Nat. Rev. Microbiol. 2016;14:523–534. doi: 10.1038/nrmicro.2016.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Keogh-Brown M.R., Smith R.D. The economic impact of SARS: how does the reality match the predictions? Health Policy. 2008;88:110–120. doi: 10.1016/j.healthpol.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li G., de Clercq E. Therapeutic options for the 2019 novel coronavirus (2019-nCoV) Nat. Rev. Drug Discov. 2020;19:149–150. doi: 10.1038/d41573-020-00016-0. [DOI] [PubMed] [Google Scholar]
- 6.Lythgoe M.P. Why drugs fail in clinical trials in pulmonary arterial hypertension, and strategies to succeed in the future. Pharmacol. Ther. 2016;164:195–203. doi: 10.1016/j.pharmthera.2016.04.012. [DOI] [PubMed] [Google Scholar]
- 7.Lu R. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Angelis C. Clinical trial registration: a statement from the International Committee of Medical Journal Editors. Lancet. 2004;364:911–912. doi: 10.1016/S0140-6736(04)17034-7. [DOI] [PubMed] [Google Scholar]
- 9.Sham H.L. ABT-378, a highly potent inhibitor of the human immunodeficiency virus protease. Antimicrob. Agents Chemother. 1998;42:3218–3224. doi: 10.1128/aac.42.12.3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chu C.M. Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings. Thorax. 2004;59:252–256. doi: 10.1136/thorax.2003.012658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cao B. A trial of lopinavir–ritonavir in adults hospitalized with severe Covid-19. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2001282. Published online March 18. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang M. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cihlar T. Therapeutic efficacy of the small molecule GS-5734 against Ebola virus in rhesus monkeys. Nature. 2016;531:381–385. doi: 10.1038/nature17180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sheahan T.P. Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic coronaviruses. Sci. Transl. Med. 2017;9 doi: 10.1126/scitranslmed.aal3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holshue M.L. First case of 2019 novel coronavirus in the United States. N. Engl. J. Med. 2020;382:929–936. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gao J. Breakthrough: chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci. Trends. 2020;14:72–73. doi: 10.5582/bst.2020.01047. [DOI] [PubMed] [Google Scholar]
- 17.Gautret P. Hydroxychloroquine and azithromycin as a treatment of COVID-19: preliminary results of an open-label non-randomized clinical trial. Int. J. Antimicrob. Agents. 2020 doi: 10.1016/j.ijantimicag.2020.105949. Published online March 20, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18.Ruan Q. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020 doi: 10.1007/s00134-020-05991-x. Published online March 3, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stockman L.J. SARS: systematic review of treatment effects. PLoS Med. 2006;3:1525–1531. doi: 10.1371/journal.pmed.0030343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bian H. Meplazumab treats COVID-19 pneumonia: an open-labelled, concurrent controlled add-on clinical trial. medRxiv. 2020 doi: 10.1101/2020.03.21.20040691. Published online March 24, 2020. [DOI] [Google Scholar]
- 21.Wilson J.G. Mesenchymal stem (stromal) cells for treatment of ARDS: a phase 1 clinical trial. Lancet Respir. Med. 2015;3:24–32. doi: 10.1016/S2213-2600(14)70291-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leng Z. Transplantation of ACE2-mesenchymal stem cells improves the outcome of patients with COVID-19 pneumonia. Aging Dis. 2020;11:216. doi: 10.14336/AD.2020.0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Griensven J. Evaluation of convalescent plasma for Ebola virus disease in Guinea. N. Engl. J. Med. 2016;374:33–42. doi: 10.1056/NEJMoa1511812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen Z. Generation of live attenuated novel influenza virus A/California/7/09 (H1N1) vaccines with high yield in embryonated chicken eggs. J. Virol. 2010;84:44–51. doi: 10.1128/JVI.02106-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jiang S. Don’t rush to deploy COVID-19 vaccines and drugs without sufficient safety guarantees. Nature. 2020;579:321. doi: 10.1038/d41586-020-00751-9. [DOI] [PubMed] [Google Scholar]