Abstract
In human history, the paper has long been used as a platform to record and preserve information. However, over the decades, paper has found its application in Biomedical Sciences, too. Both paper-based microfluidic devices (μPADs) and paper-based cultures and scaffolds have shown immense potential to be used as a sensor as well as a supporting material for in vitro tissue engineering. μPADs can be used to perform low-cost and fast biomolecular assays at Point-Of-Care (POC). They are being used to detect various biomarkers like viral proteins, metabolites, oncogenes, and antigens; and conditions like Venous Thromboembolism (VTE). On the other hand, the paper has also been used to develop paper-based 3D cultures and scaffolds to test drugs, and monitor cytotoxic effects in vitro cell microenvironments and also as implantable tissues. In this review, we intend to enumerate the development in the field of μPADs, paper-based cell cultures, and paper-based scaffolds and their plethora of applications over the last decade.
Keywords: μPAD, Paper, Diagnosis, Paper-based-culture, Scaffold
1. Introduction
Historically, paper has been used as a tool to record and preserve information [1], but over time it has found its application in the field of Biomedical Sciences [2]. Paper being a cellulose-based substrate, has a long history to perform biomolecular assays. Due to its affordability, wide availability, and its hydrophilic nature, it is considered to be one of the most promising materials for the fabrication of paper-based biosensors [3]. The first paper-based device for the semi-quantitative detection of glucose in urine was demonstrated in 1956 that further developed into immunochromatographic paper-based lateral flow test strips, with the well-known example of pregnancy test kits [4]. Later on, George Whitesides introduced the method of fabricating microchannels on paper for multiplex analyte detection based on colorimetry; thus giving rise to microfluidic paper-based analytical devices or simply, μPAD [5]. A μPAD can be defined as a paper-based analytical device used to perform biomolecular assays, consisting of a sample-loading zone and a testing zone with varying architecture of channels concerning its application. Thin, light and flexible nature of cellulose paper makes it an excellent raw material to fabricate μPAD with applications ranging from blood-plasma separation to detecting viral, cardiac and oncological biomarkers [6,7]. These low-cost devices can be used in developing economies in Asia and Africa, where deploying sophisticated detection devices like spectrophotometers [85] are not economically viable. Moreover, the population in these economies is higher; thus increasing the chances of the prevalence of epidemics like Ebola and Severe Acute Respiratory Syndrome (SARS) in Africa and China, respectively. Hence, the development of low-cost paper-based analytical devices can help in quarantining those infected by such viruses as well as facilitate early detection of diseases and conditions like Hepatitis-C virus (HCV) infection and Venous Thromboembolism (VTE).
However, μPADs on their own are not efficient enough to perform the biomolecular assay, hence their surfaces are modified to increase the detection of an analyte as well as amplify the signal generated. Surface modification is done using nanomaterial [75,76] like carbon nanotubes (CNT), graphene nanosheets, metal nanoparticles, metal oxide nanoparticles, and their nano-conjugates, which are synthesized using a plethora of methods [8]. For example, arc discharge, laser ablation and chemical vapour deposition in case of CNT or, chemical reduction in case of Graphene Nanosheets and metal nanoparticles [[9], [10], [11]].
On the other hand, paper is also revolutionizing the field of Tissue engineering, viz. paper-based cell cultures and scaffolds [77]. For almost a century, cell cultures were being developed on surfaces of Petri dishes and glass plates in the form of 2D monolayers and tests were performed on them. However, they had major limitations as cell microenvironments were 3D. Hence scientists started to engineer 3D-substrates to make cell cultures. Eventually, they succeeded in doing so using artificial polymers; but the polymers were difficult to fabricate and were mostly non-biodegradable. Thus, the focus shifted to paper-based materials and the substrates developed so far, showed promising potential [12,13]. Similarly, synthetic polymers were used to make scaffolds. Fabrication of these scaffolds was neither convenient nor feasible, but now research is ongoing to replace it with paper-based-scaffolds [14], which are low-cost and can mimic the biological Extra-Cellular-Matrix (ECM). The paper-based scaffolds can provide a valuable platform for basic stem cell research to study material effects and also to perform drug test [[14], [15], [16]]. Generally, for tissue engineering fibrous nature, porosity and flexibility of scaffolds are desired and possessing all these characteristics paper [78] has the potential to replace conventional scaffolding materials like polydimethylsiloxane (PDMS), glass fiber, chitosan, which are difficult to fabricate and are not feasible for low-income economies. For the proper proliferation of cells [83,84] in vitro, a microenvironment that mimics the in vivo characteristics of the body is necessary [17]. However, the challenge with paper is that it is 2D in structure, hence researchers stacked the cell-laden papers on top of one another to make the 3D microenvironments. Using this technique, researchers developed "a beating heart on paper", which was beating for about 3 months; and in another instance, researchers developed a 3D liver model on paper scaffold which showed similar response, like in a real liver in vivo [15,16]. Similar to μPADs, paper substrate used in the fabrication of cultures and scaffolds were surface modified to enhance cellular growth.
This review intends to enumerate the development in the field of μPADs, paper-based cell cultures, and paper-based scaffolds over the last decade. We have explained their structure, composition, surface modification, and functionality. Hopefully, this review will encourage more research in these fields and the development of more commercially viable paper-based devices. Also, may this review be helpful to the new-comers in this field and may demonstrate the importance of such low-cost paper-based analytical devices and scaffolds.
2. Applications of paper as supporting material in Biomedical Sciences
2.1. Biomarker-based disease diagnosis using microfluidics
Biomarkers are indicators of the body’s functioning, and their levels in the blood can indicate the occurrence of certain diseases [18,19]. To estimate the levels of biomarkers in the body, blood plasma is separated from whole blood using techniques such as centrifugation. This procedure is both tedious and requires the help of technicians. Hence, μPADs like those developed by Yang et al can be used to separate blood plasma from whole blood [6]. Before this, researchers devised numerous techniques like electro-osmotic flow, bifurcation (Zweifach-Fung effect), membrane filtration and cross-flow filtration for blood plasma separation [20]. However, these methods come with disadvantages like the requirement of external force for the flow of substances through the microfluidic channel but paper can be used to make microfluidic systems that can work without the requirement of such forces. The μPAD developed by Yang et al used the principle of RBC agglutination induced by anti-bodies. The plasma separation zone in the center was made functionalized by spotting anti-A, B onto it, and the reagents of the colorimetric assay onto the test readout zones in the periphery. To perform the assay, a drop of whole blood was placed at the plasma separation zone, which leads to agglutination of RBC and separation of plasma. The plasma obtained using this process was significantly high to perform the colorimetric assay. Similarly, a year later, Songjaroen et al developed a different method of separating blood plasma from whole blood [20]. They fabricated a μPAD consisting of two types of Paper, i.e. blood filter paper and patterned Whatman No.1 filter paper to separate blood plasma from other components by agglutination of the components and penetration of plasma through the paper by capillary action. By wax dipping method, various layers were incorporated onto the paper for separation of particles greater than 2–3 mm including red cells and platelets from whole blood. Once the plasma is separated from the other components of blood, biomarkers of interest can be analyzed using various biomolecular reactions. In the following paragraphs, we have divided the detection of biomarkers into blood-based and non-blood-based; i.e. biomarkers present in blood and those present in other body fluids like saliva, tears, and urine.
2.1.1. Blood-based detection
In this method, biomarkers present in the blood are analyzed using μPAD. For example, in 2016, Hu et al. developed a μPAD with combined blood serum [73,74] extractor with dual-mode iron health tests: fluorescent analysis for Fe3+ and colorimetric ELISA for ferritin, which is a blood protein containing iron [21]. The μPAD was fabricated by sequentially patterning the filter paper with in situ growth of Carbon-Dots (CDs) and Au-Nano-Particles. For CDs rendered different reaction rates for paper and consequently led to two distinguished regions on paper simultaneously: the fluorescent CDs (F-CDs), for serum iron analysis and the non-fluorescent CDs (nF-CDs) for chemical deposition of Au. The high triggering ability for blood coagulation was shown by the surface, which enabled automatic and highly effective serum extraction on the μPAD. Sensing of serum ferritin was enabled by the bio-modification of AuNPs. Subsequently, μPADs were developed to detect viral, cardiac and oncological biomarkers in blood.
2.1.1.1. Viral biomarkers
According to a report published by Economic Times India, 80% of the population in some villages in the North-Western region of India, especially Punjab, Haryana, and Uttar Pradesh, is suffering from Hepatitis C [22]. Hence early diagnosis is necessary to cure this disease. Generally, the Hepatitis-C virus (HCV) [86] is diagnosed in a two-step process; first, detection of IgG against anti-HCV using enzyme-linked immunosorbent assay (ELISA) and second, recombinant immunoblot assay (RIBA) to confirm the diagnostic result, as ELISA might give a false-positive result. However, in 2014, Mu et al developed a multiplex microfluidic paper-based immunoassay to detect the human IgG antibody against HCV (anti-HCV) [23]. They used a technique called Craft Punch Patterning (CPP) to fabricate the multiple test zones on Nitro-Cellulose paper substrate; as NC is highly flammable. Hence, the developed microfluidic paper-based immunoassay harnessed the 'irreplaceable' merits of paper to integrate the lengthy, costly, and segmented assays rapidly and economically. Then, in 2016, Chen and Xinyu developed a much advance μPAD to detect HCV and Human Immunodeficiency Virus (HIV) together in under 20 min with high accuracy and sensitivity as well as low Limit-Of-Detection (LOD) to existing sensors [24]. They claim to be the first to integrate μPAD for low-cost diagnosis of HIV/HCV co-infection. It had eight electrochemical immunosensors capable of carrying out "ELISAs in parallel and testing multiple serum samples for both HIV and HCV antibody marker”. Moreover, it could transmit the data to a host computer or smartphone via wireless transmission. In another instance, Bedin et al. to develop a wax printed MF1-based-μPAD for the detection of the dengue and Zika NS1 (Non-Structural) viral proteins in blood plasma. The presence of glass fiber enables the separation of plasma from red blood cells [25]. The test zone of the μPAD was functionalized by adding an anti-NS1 antibody. If NS1 is present, it is captured by the anti-NS1 antibody and a blue band appears in the test. If NS1 is absent, there is no signal. The μPAD take 4–6 min to analyze the sample and a smartphone application is used to interpret the results. The μPAD performed better than a commercially available Lateral-Flow-Assay (LFA), both in terms of Limit-Of-Detection (LOD) and of time-to-result.
2.1.1.2. Cardiac biomarkers
According to another article published by Economic Times India, Venous Thromboembolism (VTE) is becoming a leading cause of death along with heart disease, Brain stroke and cancer [26]. It is an under-diagnosed disease among many and most common preventable cause of death in India. VTE leads to long term complications like post-thrombotic syndrome (PTS) and chronic thromboembolic pulmonary hypertension (CTEPH). PTS can cause swelling and pain in the affected leg and CTEPH can cause the right side of the heart to work harder than normal owing to abnormal high-blood pressure. In both the condition, early diagnosis is necessary. It can be achieved by measuring the levels of thrombin or thrombin content in blood serum. The available methodologies to measure thrombin levels require both time and technicians. Recently, Xue et al constructed a Microfluidic paper-based photo-electrochemical device (μPECD) to detect thrombin at point-of-care (POC) [27]. μPECD employs the complete separation of the detection signal and excitation source, thus, providing high sensitivity with a low background signal. The high sensitivity in μPECD can be achieved by the production of large photocurrent signals; which was achieved by incorporating Titanium Dioxide in the paper substrate, due to its good biocompatibility, photoelectric activity, and chemical stability. Again to gain more sensitivity, aptamers were used, instead of antibodies, due to their stability and reusability. To check its real-world feasibility, four human serum samples were tested with the μPECD and ELISA. The results had no significant differences, thus demonstrating the excellent potential practical application of μPECD. However, a year before, Lim et al developed a μPAD for simultaneous detection of multiple cardiac biomarkers, viz. glycogen phosphorylase isoenzyme BB (GPBB) for early diagnosis; and troponin I or T (cTnI or cTnT), creatine kinase-MB (CK−MB) for late diagnosis of Acute Myocardial Infarction (AMI) which is an indicator of Acute Coronary Syndrome (ACS) [28]. Early detection of AMI upon onset of heart attack-like situations is crucial for patient survival. It is generally done by observing elevation of ST-segment in Electro-Cardiogram (ECG), which accounts for 23–40% of misdiagnosed myocardial infarction cases. The developed μPAD could give accurate, reliable and quantitative results.
2.1.1.3. Oncological Biomarker
In the current medical scenario, cancer can be diagnosed using either of the three methods: Medical Imaging, or Endoscopy or Biopsy and Cytology Tests [29]. For early detection of cancer cells, medical Imaging is used which is a non-invasive method involving the use of X-Rays or radionuclides for imaging specific parts of the human body. Endoscopy is used as a diagnostic method for Gastro-Intestinal Oncology related complications. It is used to detect lesions, facilitate macroscopic classification of tumors and enable biopsy [30]. In a biopsy, a tissue sample is taken out from the lump of cells, which might have been found by imaging tests or physical examination [31] and different cytological tests are done to examine the growth of cancerous cells.
However, all the above-mentioned methods are tedious, time-consuming and need expert assistance. Moreover, the methods are not efficient enough to detect cancer cells at an early stage. In the case of imaging tests, it can find large groups of cancer cells but not single cancer cells or even a small cluster of cells [32]. Also, endoscopy, even though being a safe method to visualize the colonic mucosa, however, the “sensitivity of the capsule endoscopy for detecting colonic polyps, advanced adenomas, and colorectal cancer was relatively low” [33]. μPADs being a low-cost, non-invasive device can be a possible solution of detecting cancer cells at an early stage. In 2014, Li et al. proposed a novel idea to detect oncogenes on target DNA on a μPAD in vitro [34]. They developed a porous Au-paper working electrode (Au-PWE) which combined the porosity of paper and high conductivity of Au-nano-particles. The DNA sensor showed tremendous specificity and also had a Limit-Of-Detection (LOD) as low as 8.5 × 10-18 M.
Among the many antigens [79] found in blood, Carcinoembryonic antigen (CEA) can be used as oncological biomarkers which is one the most widely used tumor marker in the clinical diagnosis of colorectal, gastric, pancreatic and cervical carcinomas [35]. CEA levels directly correspond to the stage of the tumor, the outcome of therapy and the prognosis. In 2016, Wang et al developed a highly sensitive label-free paper-based electrochemical immunosensor employing a screen-printed working electrode (SPWE) which was modified with graphene nanocomposite for detection of CEA [35]. It demonstrated high efficiency and high sensitivity to the samples it was tested against. Hence, it could provide a low-cost, sensitive Point-Of-Care diagnosis. However, in 2017, Fan et al demonstrated the detection of Neuron-specific Enolase (NSE), a tumor marker essential for monitoring effect and judging prognosis and diagnosis of small cell lung cancer using a wireless POCT (Point-Of-Care-and-Testing) device comprising of a μPAD-based measuring system [36]. The detection results of NSE were comparable to those measured by a commercial electrochemical workstation. The data generated were hence, transmitted over to the smartphone using Bluetooth and displayed in real-time. Again in 2018, Draz et al. developed a hybrid Paper-Plastic Microchip (PPMC) for flexible and High-Performance Point-Of-Care (POC) Diagnostic device, which was tested for the detection of alpha-fetoprotein (AFP) and CEA as biomarkers of hepatocellular carcinoma (HCC) and colorectal cancer (CRC), respectively [37]. The microchip comprised of three-layer hybrid substrates made of cellulose paper substrate assembled with a transparent plastic sheet using double-sided adhesive. The fabrication process of the microchip took <1h and leveraged the advantages of layer-by-layer assembly. The PPMC had electrodes printed within the cellulose paper, which had better signal resolution compared to the common protocol of screen-printing electrodes on the surface of the chip. The results showed a good correlation between the expected and measured values with a maximum error of <7% (see Fig. 1 ).
Fig. 1.
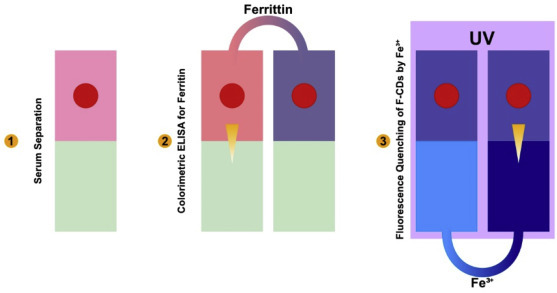
μPAD with combined blood serum extractor with dual-mode iron health tests.
2.1.2. Non-blood-based detection
In non-blood-based detection, biomarkers present in body fluids like, saliva, urine, or tear are used. The advantage that the methods using non-blood-based biomarkers are the fact that there’s no need for any purification methods, unlike blood-based detection where blood plasma needs to be separated from whole blood. Thus, rendering it better than blood-based methods. Nitrates are compounds naturally occurring in the human body and some foods like green leafy vegetables [38]. In the human body, nitrate after absorption in the upper gastrointestinal tract, it reaches the salivary gland via blood circulation and is secreted in the oral cavity where the microflora partially reduces it to nitrite [39]. In 2014, Bhakta et al developed a μPAD for detection of nitrite in saliva, which used Griess reagent for detection, which could indicate chances of developing periodontitis with clinical symptoms like gingival redness, swelling, and bleeding of gums, as it is associated with the concentration of nitrite (NO2 1−) which is an antimicrobial agent produced from nitric oxide released by endothelial NO-synthases in saliva [40]. The selected prototype for the μPADs had a main channel with four identical arms and four round “Testing zones”, designed using black lines and shapes on a white background. The dimensions of the finished μPADs (Fig. 2 ) were 24 mm by 24 mm, with a 2 mm width for the main channel and a 3 mm diameter for the testing zones. The sample was up taken on the μPAD through capillary action and the solution was driven into the branched channels and the testing zone of the μPAD. In an acidic medium and the presence of nitrite, the two components of the Griess reagent led to the formation of a magenta azo compound, in the presence of nitrite. Later, to maximize the color development, the acetic acid of the Griess reagent was replaced by 5% H3PO4. A quick, easy, and inexpensive analytical method of quantifying nitrite levels in human saliva was offered by the optimized configuration. According to the researchers, this methodology could aid research leading to establish a definitive relationship between salivary nitrite levels and periodontitis.
Fig. 2.
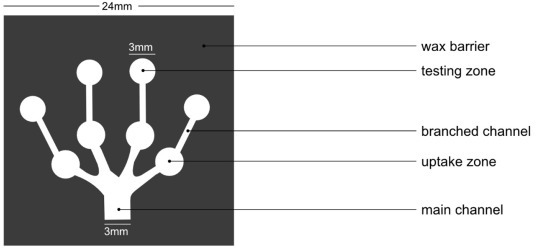
μPAD for the detection of Nitrite in saliva.
Nitrite (NO3 1−) is one of the metabolic wastes excreted out with urine [41]. The detection of Nitrite (NO2 1−) in urine analysis indicates some Urinary Tract Infection (UTI). It is due to the conversion of nitrate to nitrite by gram-negative bacteria species like Escherichia coli, that Nitrite is detected in urine analysis [42]. Using this concept, Chen and Dong developed a peel-able μPAD attached to diaper for elderly patients, in which the urine sample is collected and analyzed [24]. The μPAD was made of filter paper with patterned wax with varying concentrations of nitrite in eight segregated chemical reagent zones. A color change from white to red-violet can be interpreted as a positive result. The intensity determines the concentration of nitrite in the sample.
In urine, other compounds such as Creatinine (CRN) and uric acid are also released by the body. Creatinine is spontaneously produced in the metabolism of muscle contraction from creatine and phosphocreatine and is excreted by the kidneys. It is an important clinical marker for renal function since the rate of excretion of creatinine is relatively constant. Similar to this design developed by Bhakta et al, Edwardo et al, constructed a μPAD and developed two optimized methods for the simultaneous quantification of two biomarkers in urine, namely creatinine and uric acid, using Jaffe’s method and a Phenanthroline method respectively [43]. The proposed analytical device can be used to evaluate renal function and help prevent kidney failure. The analytical device used was 19 × 19 mm, with 4 mm width for the main channel, 2 mm width for the arms, and 4 mm diameters for the testing and uptake zones. Using a wax printer, the design was printed on a Whatman No. 1 filter paper with wax toner following the description given by Carrilho et al [44].
Then, in 2016, Gabriel et al. showed that tears can also be used for glucose and uric acid estimation. They used Sensitive colorimetric detection for chitosan incorporated paper surface resulting in better solid support to adsorb enzymes ensuring a more effective reactive area in the whole detection zone [45]. These devices were used for the quantitative analysis of glucose and uric acid which yielded an accuracy at the range of 87 to 114%. The remarkable improvements in analytical sensitivity allowed the colorimetric detection of glucose in human tear samples. No statistical difference was found at a confidence level of 95%, in the performance of chitosan-modified μPADs for the detection of glucose when compared to the analysis carried out by the spectrophotometry technique, thus proving the analytical reliability for quantitative analysis. In another instance, Jia et al developed a μPAD for direct detection of glucose from saliva (artificial) using a smartphone-based colorimetric assay, having a LOD of 0.02 mM [46]. They modified the cellulose fiber of the paper with Graphene-Oxide prohibiting any possible enzyme cross-examination, and also enhanced reagent absorptivity, reactive efficiency, and homogeneity of color distribution. They claimed it to be universally applicable as the modification of the μPAD with Graphene-Oxide can be achieved without the use of any linker, binder or retention aid. Thus, the developed device was disposable and could perform rapid and reliable analysis of samples, when compared with other paper-based sensors, owing to automatic smartphone image analysis. Table 1 summarizes the above applications in chronological order.
Table 1.
Development of μPAD to detect different biomarkers (Blood-based, and Non-Blood-based) over the last decade.
| DEVICE DESCRIPTION/ARCHITECTURE | SENSING MECHANISM |
TARGET MOLECULE | ANALYTICAL PERFORMANCE | REAL SAMPLE | REFERENCE |
|---|---|---|---|---|---|
Plasma separation μPAD
|
Colorimetric Assay. Colour change of potassium iodide starch (from colorless to brown) in the presence of hydrogen peroxide was produced by oxidation of glucose by glucose oxidase. |
Plasma glucose | NR | Venous blood | [6] |
Plasma Separation μPAD
|
Plasma separation simultaneously with a colorimetric assay. Protein determination based on Bromocresol Green (BCG) method was performed. |
Plasma Protein | LR 1.6–5.3 g dL-1 |
Blood | ( [20] |
Nitrite detecting μPAD (in saliva)
|
Colorimetric Assay. Griess reagent was used, which lead to the formation of a magenta-azo-compound whose color intensity relates to the concentration of nitrite |
Nitrite | LR 10–1000 μmol/L LOD 10 μmol/L |
Saliva | [40] |
|
Electro-chemiluminescence. Signals related to the target DNA (s2) concentrations were measured from the Paper Working Electrode (PWE). |
DNA | LR 4.0 × 10-17 – 5.0 × 10-17 M LOD 8.5 × 10-18 M |
Human Serum | [34] |
|
Antigen-Antibody reaction. Based on the quantitative detection of mouse IgG, qualitative detection of anti HCV by using an equal mixture of individual recombinant HCV antigens, including Core, NS4, and NS5 (except NS3) was demonstrated. |
Anti-HCV (Core, NS5, AND NS4) | LOQ ∼40 pg LOD ∼1 pg |
Human Serum | [23] |
UTI biomarker detecting μPAD
|
Colorimetric Assay. Sample reacted with 50 mm sulfanilamide,10 mm n-(1-naphthyl)-ethylenediamine 330 mM citric acid in methanol to produce a red-violet color |
Urinary Tract Infection biomarkers. (NITRITE) | NR | Urine | [24] |
A handheld,eight-channel potentiostat
|
Indirect ELISA. Indirect ELISA of antibodies to HIV p24 and HCV core antigens were realized on an E-μPIA handheld potentiostat with a Bluetooth module that was developed for readout of output signals from the device. |
HIV AND HCV antibodies | LOD 300 pg/mL, for HIV 750 pg/mL, for HCV |
Mouse Serum | [24] |
| μPAD for sensitive electrochemical detection of carcinoembryonic antigen | Electrochemical Sensing | Carcinogenic agents | LR 50–500 pg/mL LOD 10 pg/mL |
Human Serum | [6] |
Glucose detecting μPAD
|
Colorimetric Assay. Performed with the help of a selected chromogenic agent (4-AAP/DHBS, KI and TMB FOR glucose detection and 4-AAP/DHBS and OD for uric acid) with an office scanner using 600 dpi resolution. The recorded images were converted to the RGB scale and analyzed. |
Glucose and Uric Acid | LOD: 23 μM, for Glucose 37 μM, for Uric Acid |
Artificial Serum Sample Human Tear Sample |
[45] |
Ketamine detecting E-μPAD
|
Electrochemical Sensing. Electro-oxidation of drugs amplified by the use of Nano-crystalline Zeo-GO (Zeolite-graphene oxide) |
Ketamine (Anaesthetic) | LR 0.001–5 nM/mL DL 0.001nM/mL |
Ketamine samples | [64] |
Blood Iron analyzing μPAD
|
For Fe3+: 5 ml of ascorbic acid was added to 1 ml OF human blood sample mixed with 2 mL of red blood cell lysate buffer and incubated at room temperature for 5mins. A fluorescence quenching test was done on the supernatant of this mixture. For Ferritin: Colorimetric ELISA For Ferritin Analysis On AuNPS/NF-CDs region was done. |
Fe3+ and Ferritin | NR | Blood | [21] |
The μ-PAD had a pattern
|
Antigen-Antibody Reaction. P122 and P82 polyclonal antibodies were used to detect specific antigens. |
Dengue and Zika NS1 Detection | LOD (whole blood) 20 ng/mL, for Dengue and ZIKV |
Whole Blood | [25] |
NSE detecting μPAD
|
Electrochemical Sensing | Neuron Specific Enolase | LR 1–500 ng/mL LOD 10 pg/mL |
Serum | [36] |
Creatinine detecting μpad
|
FOR CREATININE: 2 μL aliquot of a solution of 0.1 mol/l picric acid in 1 mol/l NaOH was spotted onto the uptake zone. For Uric Acid 2 μL aliquot of a solution containing 1,10-phenanthroline(0.06 mol/l)and acetate (0.53 mol/l)was added to the uptake zone and a 2 μl aliquot of a solution of fe3+(0.45 mol/l)in h2so4(0.25mol/l)was spotted onto the testing zone |
Creatinine Uric Acid |
LR 50–600 mg/L, for Creatinine 50–500 mg/L, for Uric Acid LOD 15.7 mg/L, for Creatinine 16.5 mg/L, for Uric Acid |
Urine | [43] |
A paper plastic microchip (PPMC)
|
FOR ZIKV DETECTION, Viral lysate prepared using a 1% Tritonx-100 solution was used after capturing the ZIKV particles using magnetic beads modified with anti-ZIKV envelope monoclonal antibody(anti-ZIKV mab), followed by impedance testing with different virus concentrations. (with the increase in viral concentrations, the impedance decreased; which could be attributed to the increase in charged particles released during the lysis step. FOR HPV, Loop-mediated isothermal amplification was used, which resulted in the loop like amplicons for both HPV16 and 18. FOR MULTIPLEXED DETECTION OF ALPHA FETOPROTEIN AND CARCINOEMBRYONIC ANTIGEN (CEA). PPMC designed with two semi-circular electrodes and two detection zones modified with biomarker specific monoclonal antibodies were used (the increase in concentrations resulted in a decrease in impedance) |
Zika Virus, HPV, Alpha-Fetoprotein And Carcinoembryonic Antigen (CEA) | LOD For cancer protein biomarkers, 100 ng/mL For viral particles, 1000 particle/mL For HPV nucleic acid, 100 copies/mL |
Serum | [37] |
| A μPAD for multiplex detection of cardiac biomarkers with three reaction zones and one sample zone at the center. | Colorimetric detection of multiplex cardiac markers by using only an available phone camera or desktop scanner for quantification. For GPBB detection. The target analyte was sandwiched between a capturing antibody and a detecting antibody and color signals were observed from the labeled nanoparticles conjugate –detecting antibody. (yellow for GPBB, purple for CK-MB and red for cTnT) For a particular analyte. Three different colored NPS (gold, silver and gold urchin) were used as detecting indicators. |
GPBB (Glycogen Phosphorylase Isoenzyme BB) with CK-MB (Creatine Kinase-MB)And cTnT Cardiac Troponin T | LOD For GPBB, 0.5 ng/mL For CK-MB, 0.5 ng/mL For cTnT, 0.05 ng/mL |
SERUM | [28] |
| GO-modified μpads coupled with smartphone-based colorimetric detection for direct quantification Fabricated by methods of photolithography |
Graphene Oxide enhanced colorimetric assay | Glucose | LOD 0.02 mM LR 0∼1 mM |
Artificial Saliva | [46] |
Paper-based electrochemical sensing platform μPEC for Thrombin detection
|
Paper-based TiO2 Nano-sheets (PTNs) and CeO2 were employed as an electron transporting material and photoactive material respectively. Upon illumination, the photogenerated electrons of CeO2 promptly transferred into PTNs increasing in photocurrent intensity. By regulation of electron-transfer tunneling distance between PTNs and CeO2 sensitive detection of Thrombin was realized. |
Thrombin | LR 0.02pM- 100 pM DL 6.7 fM |
Human serum | [27] |
NR - not reported | LOD – Limit OF Detection | LR – Linear Range | LOQ – Limit Of Quantification | DL – Detection Limit.
2.2. Tissue engineering applications
Cells are best cultured in microenvironments in vitro, which can mimic the original environment where the cells grow. Previously, cell cultures were done on 2D substrates monolayers of cells on a glass plate [47,48] but had limitations as cell microenvironments were 3-Dimensional (3D). 3D microenvironments can bridge the gap between animal models and monolayer cell cultures [49] Hence, to develop 3D microenvironments with functional phenotype were developed using micro-engineering techniques like photolithography, replica modeling and micro-contact printing [[50], [51], [52]]. The material used in these techniques was synthetic polymers which were limited and required complicated fabrication processes and expensive instruments [52]. To prepare cell culture scaffolds, smooth polymers are used, but to develop such scaffolds with topographical features, time-consuming methods such as hot embossing and soft lithography had to be used. One such example of the 3D microenvironment is spheroids. Spheroids are hydrogel scaffolds that are widely used to assess therapeutic efficacies and toxicities. These microenvironments mimicked the poorly vascularized tumor tissue due to the presence of chemical gradients. These chemical gradients formed zones viz., necrotic cells at the core, quiescent cells in the interior and proliferating cells along the exterior [49]. However, spheroids had certain limitations: Firstly, histological sections had to be made to study these spheroids; Secondly, they offered limited experimental control over the formed chemical gradients; and lastly, only a small subset of cell lines are capable of forming aggregates [80,81,82]. Hence, paper-based-cultures (PBCs) emerged as a promising alternative to polymer-based-cultures. Fig. 3 shows a pictorial representation of the different zones of cells in tumors, spheroids, and Cells-in-Gels-in-Paper (see Fig. 4 ).
Fig. 3.
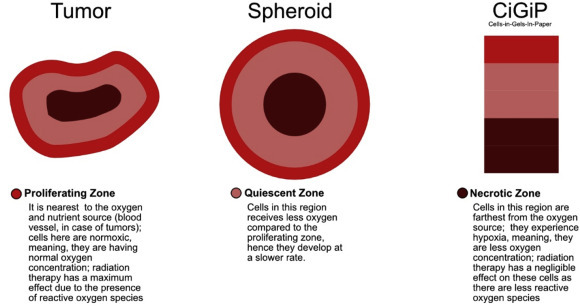
Different zones of cells in a tumor, spheroids, and Cells-In-Gels-In-Paper.
Fig. 4.

Timeline of CiGiP-based 3D cultures.
2.2.1. Paper-based cultures
Paper is a flexible, porous, low-cost, biocompatible biomaterial offered as a good choice for making scaffolds [14]. Due to the flexible nature of paper [53], rapid and profitable methods like roll to roll fabrication methods, like printing can be used for applying chemicals (i.e. pharmaceutical ingredients or Toxic chemicals) in a controlled way and on a large scale. Paper is composed of a cellulose network that acts like the Extra-Cellular-Matrix (ECM). Paper is a 2D substrate, hence they are stacked on top of one another to create a 3D cell microenvironment. In 2009, Derda et al developed a technique of “stacking and de-stacking layers of paper impregnated with suspensions of cells in extracellular matrix hydrogel making it possible to control oxygen and nutrient gradients in 3D and to analyze molecular and genetic responses” called ‘Cells-in-Gels-in-Paper’ or CiGiP [54]. Using the same technique, in 2011, a group demonstrated that rapid generation and analysis of 3D cultures is achievable by stacking sheets of paper that contain 96 cell-containing zones [55]. Before this technique, the majority of 3D cultures based on high throughput assays which were only able to detect the average behavior of cells present in the culture. However, using CiGiP, it was easier to culture arrays of thin planar sections of tissues, either alone or stacked to create complex tissue models and thus, it was simpler to isolate cells from a specific region of the culture and analyze their behavior. This opened new possibilities where paper can be used as a substrate-material to mimic cell microenvironments. For instance, in 2014 Frederique et al developed a similar 96-well cell culture using CiGiP to examine the cytotoxic effects of apoptosis inducer, Phenyl-Arsine-Oxide (PAO) and alkylating agent, Cyclo-Phospho-Amide (CPA) on human breast cancer cells [56].
Again in 2016, Simon et al developed a CiGiP 3D culture system to evaluate the metabolic response of lung cancer cells to ionizing radiation within gradients of oxygen and other nutrients [12]. These cell-laden scaffolds composed of the paper-plastic composite were placed in an acrylic holder that enabled the exchange of fresh medium with the stack and generate a decreasing gradient of oxygen. This arrangement mimicked the environment of a poorly-vascularized environment of tissues like that of cancerous tumors: top stack was well-oxygenated whereas the bottom stacks were experiencing hypoxic conditions and an increase in expression of HIF-1α and CAIX. A similar model was used to study the chemoresistance of colorectal carcinoma cells [49]. Herein, stainless steel holders were used instead of an acrylic frame which ensured the scaffolds were in conformal contact throughout the experiment. In 2018, Wang and his group developed a multi-layer CiGiP to analyze tumor cell migration in 3D cultures [16]. To monitor the progression of malignant tumors, the motility of the cells is measured. The developed multi-layered 3D culture was seeded with prostate cancer cell in the middle layer and their heterogeneous motility to the top and bottom layers was measured. Fig 4shows a timeline of the development of CiGiP-based paper cultures. Research Labs, across the globe, uses Polystyrene (PS) Dishes to prepare 2D cell cultures [57].
However, in 2013, Juvonen, et al. developed a 2-dimensional printed paper-based array, which was successfully used for 2-D cell cultures and performed better than PS-dishes. Hydrophobic polydimethylsiloxane (PDMS) ink was used to print patterned structures on paper substrates for the preparation of arrays used for two-dimensional cell culture and a lipophilic staining agent was preferred for cell imaging [53]. They considered four different types of coated paper substrates with characteristic roughness and surface energy for analyzing the substrate surface factors that influence the 2-D cell culture. The main components in the top coating layer of the paper Substrates were: (P-1) precipitated calcium carbonate pigments and Latex binder; (P-2) a mixture of two different kaolin pigments and Latex binder; and (P-3) kaolin pigments and a latex binder. The sample P-4 was coated with an aqueous latex blend consisting of a 3:2 wt ratio of low-Tg film-forming (‘‘soft’’) styrene-butadiene and high-Tg non-film-forming (‘‘hard’’) polystyrene. It was found that the PDMS film had the lowest surface energy whereas the Polystyrene (PS) dish had the highest surface energy. Thus, paper proved to be a better alternative to prepare 2D cell cultures, too.
2.2.2. Organ-on-chip
Other than using paper to make paper-based-cultures to monitor chemoresistance of cells or metabolic responses; it has also be used to make 3D cell cultures that not only mimic the tissue environment but also develop working implantable tissues. Moreover, they showed similar activity and response to drugs and other chemicals. For instance, in 2014, Park et al. demonstrated the feasibility of paper materials for stem cell culture, differentiation, and implantation to repair tissue defects [14]. They developed a paper scaffold made of Weighing Paper (WP) which significantly enhanced osteogenic differentiation and in vivo bone [71, 72] regeneration of human-Adipose-Derived-Stem-Cells (hADSCs). In their research, Papers laden with osteogenically differentiated hADSCs and human endothelial cells, when stacked together led to vascularized bone formation in vivo. Fabricating such a paper-based scaffold was met with challenges as papers can be easily damaged by heat or organic chemicals when applied to modify the surface. Hence, they used a method called initiated-Chemical-Vapour-Deposition (iCVD), in which the conformal coating of functional polymer films can be applied over the paper substrate without any damage. Similarly, in 2015, Petersen et al. investigated the applicability of single layer paper-based scaffolds for the three-dimensional (3D) growth and osteogenic differentiation of equine adipose-derived stem cells (EADSC) and achieved a viable culture EADSC [58]. Likewise, Kim et al developed a paper scaffold, whose surface was conformally modified with poly(styrene-co-maleic anhydride) layer supported the prolonged culture of chondrocytes. It was versatile and allowed area-selective cell seeding. When transplanted properly into a three-ring defect trachea in rabbits [87,88], it replaced the native trachea without stenosis after 4 weeks and provided a tight seal without the need for sutures [59]. In the same year, Li Wang and his group claim to have made a ‘heart on a paper’. It was the first attempt to fabricate a paper-based-array for the culture, proliferation, and direct differentiation of human-induced-Pluripotent-Stem-Cells (hiPSCs) into functional beating cardiac tissue. The hiPSC-derived cardiac tissue was able to maintain a long-term spontaneous beating activity of about 3 months [15]. A year later, Whitesides et al. showed the use of paper to guide the deposition of minerals like Calcium Phosphates by Osteoblasts. The porous and fibrous structure of paper enables the transport of nutrients and oxygen across the entire thickness of the paper, which thereby allows cellular remodeling, proliferation, and differentiation of cells [60] (see Fig. 5 ).
Fig. 5.
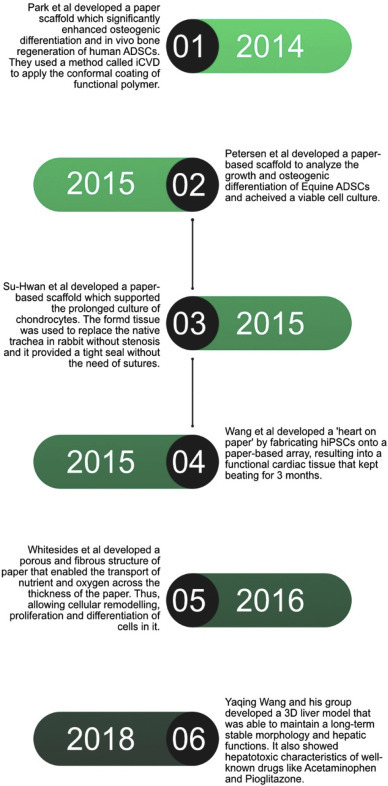
Timeline of the development of Organ-On-Paper.
In another model, Yaqing Wang and his group developed a 3D liver model (Fig. 6 ), which was formed by the co-cultures of human-induced-Hepatocytes (hiHEPs) and Human-Umbilical-Vein-Endothelial-Cells (HUVECs) on the paper scaffold. It was able to maintain a long-term stable morphology and hepatic functions assessed by secretion of albumin, synthesis of urea and CYP450 gene expression which is responsible for the oxidation of steroids, fatty acids, and xenobiotics, for 60 days. Also, the 3D culture showed hepatotoxic characteristics of well-known drugs like Acetaminophen (APAP) and Pioglitazone [16].
Fig. 6.
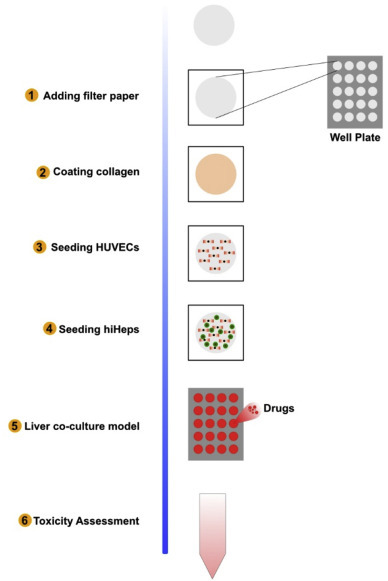
Schematic diagram depicting the procedure of fabricating the paper-based array in the well-plate and establishing the liver co-culture model.
3. Discussion and challenges
Paper being a thin, light and flexible material, has long been used as a substrate to perform biomolecular assays using the conventional technique of Lateral Flow Immunoassay (LFA) [3,61,62]. However, the devices were only able to give the user, a qualitative response; rather than a quantitative one; i.e. a yes or no, rather than an estimation of the concentration of the analyte. Microfluidic- Paper-based-Analytical-Device (μPAD) sensors bought a paradigm shift in the field of biosensors and showed its potential to revolutionize the diagnosis [21,24,25] and therapeutics [14,59] sectors of Biomedical Sciences. These sensors were not only able to give a colorimetric response but also give an estimation of the concentration of the analyte. On the other hand, the paper has also found its applications in developing paper-based-cultures and scaffolds which mimicked the 3D cellular environment of tissues. Both these technologies have to overcome many hurdles to become a mainstream technology to be used as biosensors and tissue scaffolds. Also, μPADs are being used as sensors to test milk quality, or to test the presence of drugs like Ketamine or to detect and quantify nucleic acid [[63], [64], [65]]. In this review, we examined the progress done in the last 10 years in both these technologies.
A major challenge with paper-based sensors is the composition of paper, which is mostly cellulose and hemicellulose. It is inherently, a non-conducting material; hence paper was primarily used for colorimetric tests. Hence, modification was done in the paper substrate using carbon nanotubes, graphene nanosheets, metal nanoparticles, metal oxide nanoparticles, and their nano-conjugates, to fabricate μPADs, like those of Electrochemical-μPAD (E− μPAD); thus improving the performance of the sensor system [66]. E− μPADs are being given prime attention due to its ability to provide low cost, and precise estimation of target molecules. Another challenge was that of amplifying the signals detected using μPADs. Hence, in 2018 Wu et al. developed a μPAD with ultra-sensitive and high-throughput detection of cancer biomarkers by introducing graphene onto the surface of the immune-device which efficiently accelerated the electron transfer and enhanced the detection signal [7]. Moreover, they provided a solution for accomplishing controlled radical polymerization reaction, Activator Generated Electron Transfer for Atom Transfer Radical (AGET ATRP), on a μPAD.
However, μPADs face other difficulties like complexity of cells migration from loading zone to testing zone [67], lack of proper adhesion sites [68] lack sensitivity to amount of analytes and complexity in integration of functional reagents to substrates [21].To tackle the problems different scientist groups proposed different solutions. To tackle the problem of transporting suspensions of discrete objects like cells or microparticles laser micromachined hybrid open/paper microfluidic chips were developed in which the liquid carrier was transported in a Mylar microchannel but driven by a paper channel, with quantitative flow control [67]. Then, Ng et al. developed the ES-PCL/FP (Electro-spin-PCL over Filter Paper) which showed incredible cell adhesion of human-Foetal-Osteoblastic cells (hFOB). Lack of sensitivity was tackled by concentrating targets from a larger volume by speeding up intrinsic thermodynamic partitioning in aqueous two-phase systems [67]. Lastly, the integration of functional groups or surface modification can be controlled by using techniques like initiated-Chemical-Vapour-Deposition (iCVDs). Also, these paper-based-sensors are sensitive to temperature and humidity, as the detection zones are dependent on the temperature and relative humidity of the environment [69]. Even though the rise in temperature improves enzyme activity, they also evaporate the water from the spots faster and even denature enzymes. The thermal stability can be increased by adsorption of enzymes on paper with conjunction with a humidifier substance.
On the other hand, paper on its own is not an optimal material for culturing cells, as it lacks cell adhesion moieties [68]. To develop the ‘heart on paper’, Li Wang and his group cultured the hiPSCs on the paper array pre-coated with gelatine and Matrigel to facilitate the proper proliferation of cells. Similarly, Yaqing Wang and his group pre-coated their paper-substrates with a thin layer of 100 μg/ml rat tail collagen type I for better cell proliferation in the substrate. Hence, optimum surface modification was required for proper cell adhesion and proliferation. In a recent study, Ng et al compared three paper-substrate modification techniques, namely dip coating, hydrogel modification, and electro-spinning over Filter Paper (FP) (Whatman grade 114) using human foetal osteoblast (hFOB) [68]. Dip coating provided the least favorable cellular response, also resulted in reduced porosity; thus decreasing the number of anchoring spots for the cells and limiting the overall cell penetration. In the case of hydrogels; the high molecular weight polymers did not obstruct cell penetration and distribution within the scaffold. But, Electro-spin- Polycaprolactone coating over FP (ES-PCL/FP) proved to be superior in cell viability and mechanical properties due to better surface morphology and higher porosity of ES-PCL/FP. PCL and paper complemented each other, resulting in the highest metabolic and ALP activity. Also, these culture systems must be disassembled before analysis, thus preventing the taking of measurements as gradients formed. To this, a potential solution was developed Matthew et al to measure spatial and temporal information about oxygen gradients, without disassembling the layers by using a luminescent thin-film sensor [13]. Oxygen readily diffused into these films resulting in changes in luminescence intensity with changes in the surrounding oxygen tension.
Lastly, a challenge that remains with paper-based scaffolds is that of imaging them to check cell adhesions, proliferation or interaction with chemicals. Currently, such scaffolds are unfolded layer-by-layer and then imaged [70]. It is both laborious and can give conflicting results. Again, most laboratory techniques are for testing and evaluating drug responses on 2D cultures, hence new methods have to be invented to overcome the same [17] Hence, more research is required to improve paper-based sensors and scaffolds.
4. Conclusion and future directions
Paper has been used as supporting material in Biomedical Sciences and has shown immense potential for the development of biosensors and scaffolding-material in the field of Biosensors and Tissue Engineering, respectively. This literature review enumerated the applications of paper as a supporting material over a decade and the research and development going on in the field of Biomedical Sciences. Earlier, LFAs were used for diagnostic purposes but they gave only qualitative results; but with further research paper-based devices were prepared which were able to give qualitative as well as quantitative results. For diagnosis purposes using paper, various biomarkers are considered, viz. viral proteins, metabolites viz. NO3, NO, Creatinine, Uric Acid which can be detected using a μPAD. Moreover, paper has also been used for oncological diagnosis and prognosis and proved to be efficient and easy to handle platform which gave an edge to cancer therapy.
Paper-based scaffolds have shown the immense capability to revolutionize the field of Tissue engineering. It has been used to develop ‘heart on a paper’ and co-cultures of Liver cells where drug testing showed promising results. The porous, and fibrous structure of paper enabled the transport of nutrients and oxygen across the entire thickness of the paper, which thereby allows cellular remodeling, proliferation, and differentiation of cells. The scaffolds developed were also used as an implantable material on rabbits to replace a damaged trachea. However, out of the various laboratory experiments and researches on μPADs, presented in this paper, none have found commercial utility till now. Therefore, current and future researches need to be more market-oriented and be commercially viable to the masses. For that, the researchers have to overcome the aforementioned challenges in the writing of the paper.
A future can be imagined in which people use a paper for diagnosing malicious diseases like cancer at home in a simple kit, similar to the pregnancy test kits available in today’s market. Moreover, these devices will be cheap compared to the ones available now, and also easily disposable. On the other hand, scaffolds used in Tissue Engineering can be used to test drugs using cells obtained from the person requiring the drug. Drugs then will be patient-specific with negligible side effects. Also, the development and deployment of these drugs to the market will be faster compared to today’s standards, as clinical trials could be performed faster using the cells from the patients. Thus, reducing the development time and cost at the same time.
In conclusion, more research is required to access the change in analytical performance of the various surface modification done on the paper substrate; the effect of temperature and other environmental factors on the biosensors and non-enzymatic approaches must be explored in near future. In the field of paper-based cultures and scaffolds, more research in cell viability in the cultures and scaffolds must be done. Also, more research is required to compare the available cytotoxic results done on 2D cell cultures with the new 3D cell cultures.
Declaration of competing interest
No conflicts of interest.
Biographies

Bimalendu Deka is doing his Bachelor's in Technology in Biomedical Engineering from the School of Technology, North-Eastern Hill University. His areas of interest are Nanomedicine, Biosensors, Microfluidics, Tissue Engineering, and Bio-MEMS.

Rima Kalita is doing her Bachelor's in Technology in Biomedical Engineering from the School of Technology, North-Eastern Hill University. Her areas of interest are Nanomedicine, Microfluidics, Tissue Engineering, and Bioelectronics.

Dinesh Bhatia received his Ph.D. in the field of bio-rehabilitation engineering from Motilal Nehru National Institute of Technology (MNNIT), Allahabad. His specific interests lie in the development of bio-rehabilitative devices and the study of EMG signals. He is currently an Associate Professor in the Department of Biomedical Engineering, North Eastern Hill University, Shillong, Meghalaya, India. He was the recipient of the BOYSCAST Fellowship, INAE Fellowship and was also the International Young Biomedical Scientist ICMR award. He has several publications in reputed journals, books and members of different engineering and life sciences societies.

Animesh Mishra received his Post-Doctoral Degree DM Cardiology from G.B.Pant Hospital and associated MAMC, New Delhi, Delhi University in 2004. His specific interests lie in the Interventional Cardiology, particularly Heart Failure Management. He is currently a Professor in the Department of Cardiology, NEIGRIHMS, Shillong, Meghalaya, India with teaching and research experience of more than 23 years. He was the recipient of the Indian Society of Cardiology Fellowship (2011), and many other fellowships. He has several publications in reputed journals, books and members of different Medical and professional bodies.
References
- 1.Authority i. A. The leon valley dead sea scrolls. 2019, October 1. https://www.deadseascrolls.org.il/learn-about-the-scrolls/introduction?locale=en_US Retrieved from Dead Sea Scrolls.
- 2.history T.C. A timeline of pregnancy testing. 2019, November 11. https://history.nih.gov/exhibits/thinblueline/timeline.html Retrieved from National Institutes of Health (NIH)
- 3.Martin A.J., Synge R.L. A new form of chromatogram employing two liquid phases: a theory of chromatography. 2. Application to the micro-determination of the higher monoamino-acids in proteins. Biochem. J. 1941;35(12):1358–1368. doi: 10.1042/bj0351358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Landmark N.H. Al and helen free and the development of diagnostic test strips. 2010, May 1. https://www.acs.org/content/acs/en/education/whatischemistry/landmarks/diagnosticteststrips.html Retrieved from American Chemical Society Chemistry For Life.
- 5.Martinez A.W., Phillips S.T., Butte M.J., Whitesides G.M. Patterned paper as a platform for inexpensive, low-volume, portable bioassays. Angew Chem. Int. Ed. Engl. 2007;46(8):1318–1320. doi: 10.1002/anie.200603817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang X., Forouzan O., Brown T.P., Shevkoplyas S.S. Integrated separation of blood plasma from whole blood for microfluidic paper-based analytical devices. Lab Chip. 2012;12(2):274–280. doi: 10.1039/c1lc20803a. [DOI] [PubMed] [Google Scholar]
- 7.Wu Y., Xue P., Hui K.M., Kang Y. A paper-based microfluidic electrochemical immunodevice integrated with amplification-by-polymerization for the ultrasensitive multiplexed detection of cancer biomarkers. Biosens. Bioelectron. 2014;52:180–187. doi: 10.1016/j.bios.2013.08.039. [DOI] [PubMed] [Google Scholar]
- 8.Kumar A., Purohit B., Mahato K., Chandra P. In: Immunosensors. Minhaz U.A., Mohammed Z., Eiichi T., editors. Royal Society of Chemistry; 2019. Advance engineered nanomaterials in point-of-care immunosensing for biomedical diagnostics. [Google Scholar]
- 9.Jan P., Jana D., Jana C., Jaromir H., Ondrej J., Vojtech A., Rene K. Methods for carbon nanotubes synthesis—review. J. Mater. Chem. 2011;21(40):15872–15884. [Google Scholar]
- 10.Zhang X., Li K., Li H., Lu J., Fu Q., Chu Y. Graphene nanosheets synthesis via chemical reduction of graphene oxide using sodium acetate trihydrate solution. Synth. Met. 2014;193:132–138. [Google Scholar]
- 11.Carla D.D., Beatriz R.N., Maria E.C. Review of the methodologies used in the synthesis gold nanoparticles by chemical reduction. J. Alloys Compd. 2019;798:714–715. [Google Scholar]
- 12.Simon K.A., Mosadegh B., Minn K.T., et al. Metabolic response of lung cancer cells to radiation in a paper-based 3D cell culture system. Biomaterials. 2016;95:47–59. doi: 10.1016/j.biomaterials.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 13.Boyce M.W., Kenney R.M., Truong A.S., Lockett M.R. Quantifying oxygen in paper-based cell cultures with luminescent thin film sensors. Anal. Bioanal. Chem. 2016;408(11):2985–2992. doi: 10.1007/s00216-015-9189-x. [DOI] [PubMed] [Google Scholar]
- 14.Park H.J., Yu S.J., Yang K., et al. Paper-based bioactive scaffolds for stem cell-mediated bone tissue engineering. Biomaterials. 2014;35(37):9811–9823. doi: 10.1016/j.biomaterials.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 15.Wang L., Xu C., Zhu Y., et al. Human induced pluripotent stem cell-derived beating cardiac tissues on paper. Lab Chip. 2015;15(22):4283–4290. doi: 10.1039/c5lc00919g. [DOI] [PubMed] [Google Scholar]
- 16.Wang L.X., Zhou Y., Fu J.J., Lu Z., Yu L. Separation and characterization of prostate cancer cell subtype according to their motility using a multi-layer CiGiP culture. Micromachines (Basel) 2018;9(12):660. doi: 10.3390/mi9120660. Published 2018 Dec 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cramer S.M., Larson T.S., Lockett M.R. Tissue papers: leveraging paper-based microfluidics for the next generation of 3D tissue models. Anal. Chem. 2019;91(17):10916–10926. doi: 10.1021/acs.analchem.9b02102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abdelhak A., Huss A., Kassubek J., Tumani H., Otto M. Serum GFAP as a biomarker for disease severity in multiple sclerosis. Sci. Rep. 2018;8:14798. doi: 10.1038/s41598-018-33158-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pandey J.K., Agarwal D. Biomarkers: a potential prognostic tool for silicosis. Indian J. Occup. Environ. Med. 2012;16(3):101–107. doi: 10.4103/0019-5278.111746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Songjaroen T., Dungchai W., Chailapakul O., Henry C.S., Laiwattanapaisal W. Blood separation on microfluidic paper-based analytical devices. Lab Chip. 2012;12(18):3392–3398. doi: 10.1039/c2lc21299d. [DOI] [PubMed] [Google Scholar]
- 21.Hu S.W., Qiao S., Xu B.Y., Peng X., Xu J.J., Chen H.Y. Dual-functional carbon dots pattern on paper chips for Fe3+ and ferritin analysis in whole blood. Anal. Chem. 2017;89(3):2131–2137. doi: 10.1021/acs.analchem.6b04891. [DOI] [PubMed] [Google Scholar]
- 22.Akhter S. Some of the places in India, almost 80% of the population is suffering from Hep C: dr. Yogesh Batra. 2018, July 28. https://health.economictimes.indiatimes.com/news/diagnostics/in-some-of-the-places-in-india-almost-80-of-the-population-is-suffering-from-hep-c-dr-yogesh-batra/65172694 Retrieved from ETHealthWorld.
- 23.Mu X., Zhang L., Chang S., Cui W., Zheng Z. Multiplex microfluidic paper-based immunoassay for the diagnosis of hepatitis C virus infection. Anal. Chem. 2014;86(11):5338–5344. doi: 10.1021/ac500247f. published correction appears in Anal Chem. 2015 Aug 4;87(15):8033. [DOI] [PubMed] [Google Scholar]
- 24.Chen C., Dong T. 2015 37th Annual International Conference of the IEEE Engineering in Medicine and Ciology Society. IEEE; Milan, Italy: 2015. Microfluidic paper-based analytical devices for colorimetric detection of urinary tract infection biomarkers on adult diapers; pp. 5892–5893. [DOI] [PubMed] [Google Scholar]
- 25.Bedin F., Boulet L., Voilin E., Theillet G., Rubens A., Rozand C. Paper-based point-of-care testing for cost-effective diagnosis of acute flavivirus infections. J. Med. Virol. 2017;89(9):1520–1527. doi: 10.1002/jmv.24806. [DOI] [PubMed] [Google Scholar]
- 26.Akhter S. Venous Thromboembolism is becoming a leading cause of disability and death in India : dr. Suviraj John. 2016, November 02. https://health.economictimes.indiatimes.com/news/industry/venous-thromboembolism-is-becoming-a-leading-cause-of-disability-and-death-in-india-dr-suviraj-john/55200566 Retrieved from Economic Times Health World.
- 27.Xue J., Zhang L., Gao C., Zhu P., Yu J. Microfluidic paper-based photoelectrochemical sensing platform with electron-transfer tunneling distance regulation strategy for thrombin detection. Biosens. Bioelectron. 2019;133:1–7. doi: 10.1016/j.bios.2019.03.022. [DOI] [PubMed] [Google Scholar]
- 28.Lim W.Y., Thevarajah T.M., Goh B.T., Khor S.M. Paper microfluidic device for early diagnosis and prognosis of acute myocardial infarction via quantitative multiplex cardiac biomarker detection [published correction appears in Biosens Bioelectron. 2019 Mar 15;129:299. Biosens. Bioelectron. 2019;128:176–185. doi: 10.1016/j.bios.2018.12.049. [DOI] [PubMed] [Google Scholar]
- 29.Society A.C. Tests to find and diagnose cancer. 2019, November 17. https://www.cancer.org/healthy/find-cancer-early/tests-to-find-and-diagnose-cancer.html Retrieved from cancer.org.
- 30.Costa A. Endoscopy in gastrointestinal oncology. 2016, April 16. https://cancerworld.net/e-grandround/endoscopy-in-gastrointestinal-oncology/ Retrieved from Cancer World.
- 31.Society A.C. How is cancer diagnosed? 2015, July 30. https://www.cancer.org/treatment/understanding-your-diagnosis/tests/testing-biopsy-and-cytology-specimens-for-cancer/how-is-cancer-diagnosed.html Retrieved from cancer.org.
- 32.Society A.C. Imaging (radiology) tests for cancer. 2015, November 30. https://www.cancer.org/treatment/understanding-your-diagnosis/tests/imaging-radiology-tests-for-cancer.html Retrieved from cancer.org.
- 33.Van Gossum A., Munoz-Navas M., Fernandez-Urien I., et al. Capsule endoscopy versus colonoscopy for the detection of polyps and cancer. N. Engl. J. Med. 2009;361(3):264–270. doi: 10.1056/NEJMoa0806347. [published correction appears in N Engl J Med. 2009 Sep 17;361(12):1220. Navas, Miguel Munoz [corrected to Munoz-Navas, Miguel]] [DOI] [PubMed] [Google Scholar]
- 34.Li M., Wang Y., Zhang Y., Yu J., Ge S., Yan M. Graphene functionalized porous Au-paper based electrochemiluminescence device for detection of DNA using luminescent silver nanoparticles coated calcium carbonate/carboxymethyl chitosan hybrid microspheres as labels. Biosens. Bioelectron. 2014;59:307–313. doi: 10.1016/j.bios.2014.03.072. [DOI] [PubMed] [Google Scholar]
- 35.Wang Y., Xu H., Luo J., et al. A novel label-free microfluidic paper-based immunosensor for highly sensitive electrochemical detection of carcinoembryonic antigen. Biosens. Bioelectron. 2016;83:319–326. doi: 10.1016/j.bios.2016.04.062. [DOI] [PubMed] [Google Scholar]
- 36.Fan Y., Liu J., Wang Y., et al. A wireless point-of-care testing system for the detection of neuron-specific enolase with microfluidic paper-based analytical devices. Biosens. Bioelectron. 2017;95:60–66. doi: 10.1016/j.bios.2017.04.003. [DOI] [PubMed] [Google Scholar]
- 37.Draz M.S., Moazeni M., Venkataramani M., et al. Hybrid paper-plastic microchip for flexible and high-performance point-of-care diagnostics. Adv. Funct. Mater. 2018;28(26):1707161. doi: 10.1002/adfm.201707161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kris G. Are nitrates and nitrites in foods harmful? 2020, February 10. https://www.healthline.com/nutrition/are-nitrates-and-nitrites-harmful Retrieved from Healthline.
- 39.Eisenbrand G., Spiegelhalder B., Preussmann R. Nitrate and nitrite in saliva. Oncology. 1980;37(4):227–231. doi: 10.1159/000225441. [DOI] [PubMed] [Google Scholar]
- 40.Bhakta S.A., Borba R., Taba M., Jr., Garcia C.D., Carrilho E. Determination of nitrite in saliva using microfluidic paper-based analytical devices. Anal. Chim. Acta. 2014;809:117–122. doi: 10.1016/j.aca.2013.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nnate D.A., Achi N.K. Nitrate metabolism: a curse or blessing to humanity? J. Sci. Res.Rep. 2016;1 [Google Scholar]
- 42.Honrado C., Dong T. A capacitive touch screen sensor for detection of urinary tract infections in portable biomedical devices. Sensors (Basel) 2014;14(8):13851–13862. doi: 10.3390/s140813851. Published 2014 Jul 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rossini E.L., Milani M.I., Carrilho E., Pezza L., Pezza H.R. Simultaneous determination of renal function biomarkers in urine using a validated paper-based microfluidic analytical device. Anal. Chim. Acta. 2018;997:16–23. doi: 10.1016/j.aca.2017.10.018. [DOI] [PubMed] [Google Scholar]
- 44.Carrilho E., Martinez A.W., Whitesides G.M. Understanding wax printing: a simple micropatterning process for paper-based microfluidics. Anal. Chem. 2009;81(16):7091–7095. doi: 10.1021/ac901071p. [DOI] [PubMed] [Google Scholar]
- 45.Gabriel E.F., Garcia P.T., Cardoso T.M., Lopes F.M., Martins F.T., Coltro W.K. Highly sensitive colorimetric detection of glucose and uric acid in biological fluids using chitosan-modified paper microfluidic devices. Analyst. 2016;141(15):4749–4756. doi: 10.1039/c6an00430j. [DOI] [PubMed] [Google Scholar]
- 46.Jia Y., Sun H., Li X., et al. Paper-based graphene oxide biosensor coupled with smartphone for the quantification of glucose in oral fluid. Biomed. Microdevices. 2018;20(4):89. doi: 10.1007/s10544-018-0332-2. Published 2018 Oct 12. [DOI] [PubMed] [Google Scholar]
- 47.Yap A.S., Manley S.W. Thyrotropin inhibits the intrinsic locomotility of thyroid cells organized as follicles in primary culture. Exp. Cell Res. 1994;214(1):408–417. doi: 10.1006/excr.1994.1274. [DOI] [PubMed] [Google Scholar]
- 48.Ollis C.A., Davies R., Munro D.S., Tomlinson S. Relationship between growth and function of human thyroid cells in culture. J. Endocrinol. 1986;108(3):393–398. doi: 10.1677/joe.0.1080393. [DOI] [PubMed] [Google Scholar]
- 49.Boyce M.W., LaBonia G.J., Hummon A.B., Lockett M.R. Assessing chemotherapeutic effectiveness using a paper-based tumor model. Analyst. 2017;142(15):2819–2827. doi: 10.1039/c7an00806f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ye H., Zhang K., Kai D., Li Z., Loh X.J. Polyester elastomers for soft tissue engineering. Chem. Soc. Rev. 2018;47(12):4545–4580. doi: 10.1039/c8cs00161h. [DOI] [PubMed] [Google Scholar]
- 51.Baino F., Fiume E. Elastic mechanical properties of 45S5-based bioactive glass-ceramic scaffolds. Materials (Basel, Switzerland) 2019;12(19):3244. doi: 10.3390/ma12193244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tanaka N., Ota H., Fukumori K., Miyake J., Yamato M., Okano T. Micro-patterned cell-sheets fabricated with stamping-force-controlled micro-contact printing [published correction appears in Biomaterials. 2015 Jan;38:109] Biomaterials. 2014;35(37):9802–9810. doi: 10.1016/j.biomaterials.2014.08.043. [DOI] [PubMed] [Google Scholar]
- 53.Juvonen H., Määttänen A., Laurén P., et al. Biocompatibility of printed paper-based arrays for 2-D cell cultures. Acta Biomater. 2013;9(5):6704–6710. doi: 10.1016/j.actbio.2013.01.033. [DOI] [PubMed] [Google Scholar]
- 54.Derda R., Laromaine A., Mammoto A., et al. Paper-supported 3D cell culture for tissue-based bioassays. Proc. Natl. Acad. Sci. U. S. A. 2009;106(44):18457–18462. doi: 10.1073/pnas.0910666106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Derda R., Tang S.K., Laromaine A., et al. Multizone paper platform for 3D cell cultures. PloS One. 2011;6(5) doi: 10.1371/journal.pone.0018940. Published 2011 May 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Deiss F., Mazzeo A., Hong E., Ingber D.E., Derda R., Whitesides G.M. Platform for high-throughput testing of the effect of soluble compounds on 3D cell cultures. Anal. Chem. 2013;85(17):8085–8094. doi: 10.1021/ac400161j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lerman M.J., Lembong J., Muramoto S., Gillen G., Fisher J.P. The evolution of Polystyrene as a cell culture. Material. Tissue Eng Part B Rev. 2018;24(5):359–372. doi: 10.1089/ten.TEB.2018.0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Petersen G.F., Hilbert B.J., Trope G.D., Kalle W.H., Strappe P.M. A paper-based scaffold for enhanced osteogenic differentiation of equine adipose-derived stem cells. Biotechnol. Lett. 2015;37(11):2321–2331. doi: 10.1007/s10529-015-1898-x. [DOI] [PubMed] [Google Scholar]
- 59.Kim S.H., Lee H.R., Yu S.J., et al. Hydrogel-laden paper scaffold system for origami-based tissue engineering. Proc. Natl. Acad. Sci. U. S. A. 2015;112(50):15426–15431. doi: 10.1073/pnas.1504745112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Camci-Unal G., Laromaine A., Hong E., Derda R., Whitesides G.M. Biomineralization guided by paper templates. Sci. Rep. 2016;6:27693. doi: 10.1038/srep27693. Published 2016 Jun 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bogdanovic J., Koets M., Sander I., et al. Rapid detection of fungal alpha-amylase in the work environment with a lateral flow immunoassay. J. Allergy Clin. Immunol. 2006;118(5):1157–1163. doi: 10.1016/j.jaci.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 62.Verheijen R., Stouten P., Cazemier G., Haasnoot W. Development of a one step strip test for the detection of sulfadimidine residues. Analyst. 1998;123(12):2437–2441. doi: 10.1039/a804695f. [DOI] [PubMed] [Google Scholar]
- 63.Mahato K., Chandra P. Paper-based miniaturized immunosensor for naked eye ALP detection based on digital image colorimetry integrated with smartphone. Biosens. Bioelectron. 2019;128:9–16. doi: 10.1016/j.bios.2018.12.006. [DOI] [PubMed] [Google Scholar]
- 64.Narang J., Malhotra N., Singhal C., et al. Point of care with micro fluidic paper based device integrated with nano zeolite-graphene oxide nanoflakes for electrochemical sensing of ketamine. Biosens. Bioelectron. 2017;88:249–257. doi: 10.1016/j.bios.2016.08.043. [DOI] [PubMed] [Google Scholar]
- 65.Roy S., Mohd-Naim N.F., Safavieh M., Ahmed M.U. Colorimetric nucleic acid detection on paper microchip using loop mediated isothermal amplification and crystal violet dye. ACS Sens. 2017;2(11):1713–1720. doi: 10.1021/acssensors.7b00671. [DOI] [PubMed] [Google Scholar]
- 66.Kumar A., Purohit B., Maurya P.K., Pandey M.L., Chandra P. Engineered nanomaterial assisted signal-amplification strategies for enhancing analytical performance of electrochemical biosensors. Electroanalysis. 2019;31(9):1615–1622. [Google Scholar]
- 67.Wu Chueh-Yu, Adeyiga Oladunni, Lin Jonathan, Di Carlo, Dino Research highlights: increasing paper possibilities. Lab Chip. 2014;14(17) doi: 10.1039/C4LC90067G. [DOI] [PubMed] [Google Scholar]
- 68.Ng K., Azari P., Nam H.Y., Xu F., Pingguan-Murphy B. Electrospin-coating of paper: a natural extracellular matrix inspired design of scaffold. Polymers (Basel) 2019;11(4):650. doi: 10.3390/polym11040650. Published 2019 Apr 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Morbioli G.G., Mazzu-Nascimento T., Stockton A.M., Carrilho E. Technical aspects and challenges of colorimetric detection with microfluidic paper-based analytical devices (μPADs) - a review. Anal. Chim. Acta. 2017;970:1–22. doi: 10.1016/j.aca.2017.03.037. [DOI] [PubMed] [Google Scholar]
- 70.Rodenhizer D., Gaude E., Cojocari D., et al. A three-dimensional engineered tumour for spatial snapshot analysis of cell metabolism and phenotype in hypoxic gradients [published correction appears in Nat Mater. 2016 Feb;15(2):244] Nat. Mater. 2016;15(2):227–234. doi: 10.1038/nmat4482. [DOI] [PubMed] [Google Scholar]
- 71.Bhatia D. Bone-Cement: the new medical quick fix. Chronicles Young Sci. 2010;1 [Google Scholar]
- 72.Bhatia D., Rajak B., Gupta M., Mukherjee A. Application of micro-electro-mechanical systems as neural interface. J.Adv. Biomed. Eng. Technol. 2015;2:1–2. [Google Scholar]
- 73.Bowler R.G. The determination of thiocyanate in blood serum. Biochem. J. 1944;38(5):385–388. doi: 10.1042/bj0380385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Carlson L.A., Wadstrom L.B. Determination of glycerides in blood serum. Clin. Chim. Acta. 1959;4(2):197–205. doi: 10.1016/0009-8981(59)90130-5. [DOI] [PubMed] [Google Scholar]
- 75.Chandra P., Segal E. Institution of Engineering and Technology; 2016. Nanobiosensors for Personalized and Onsite Biomedical Diagnosis. [Google Scholar]
- 76.Evans S.S., Lee D.B., Han T., Tomasi T.B., Evans R.L. Monoclonal antibody to the interferon-inducible protein Leu-13 triggers aggregation and inhibits proliferation of leukemic B cells. Blood. 1990;76(12):2583–2593. [PubMed] [Google Scholar]
- 77.O'Brien F.J. Biomaterials & scaffolds for tissue engineering Mater. Today Off. 2011;14:88–95. doi: 10.1016/S1369-7021(11)70058-X. [DOI] [Google Scholar]
- 78.Mahato K., Srivastava A., Chandra P. Paper based diagnostics for personalized health care: emerging technologies and commercial aspects. Biosens. Bioelectron. 2017;96:246–259. doi: 10.1016/j.bios.2017.05.001. [DOI] [PubMed] [Google Scholar]
- 79.Motoyama T., Watanabe H., Takeuchi S., Watanabe T., Gotoh S., Okazaki E. Cancer; 1990. Cancer Antigen 125, Carcinoembryonic Antigen, and Carbohydrate Determinant 19-9 in Ovarian Tumors; pp. 2628–2629. [DOI] [PubMed] [Google Scholar]
- 80.Navaraj T.W., Heidari H., Polishchuk A., Shakthivel D., Bhatia D., Dahiya R. IEEE Sensors. Busan, South Korea: IEEE. 2016. Upper limb prosthetic control using toe gesture sensors. [Google Scholar]
- 81.Ozhikandathil J., Badilescu S., Packirisamy M. A brief review on microfluidic platforms for hormones detection. J. Neural. Transm. 2017;124(1):47–55. doi: 10.1007/s00702-016-1610-x. [DOI] [PubMed] [Google Scholar]
- 82.Rajak L.B., Gupta M., Bhatia D. Growth and advancements in neural control of limb. Biomedical Science and Engineering. 2015 doi: 10.12691/bse-3-3-1. [DOI] [Google Scholar]
- 83.Tullis J.L., Tinch R.J., Gibson J.G., 2nd, Baudanza P. A simplified centrifuge for the separation and processing of blood cells. Transfusion. 1967;7(3):232–242. [PubMed] [Google Scholar]
- 84.Wang Y., Su W., Wang L., et al. Paper supported long-term 3D liver co-culture model for the assessment of hepatotoxic drugs. Toxicol Res (Camb) 2017;7(1):13–21. doi: 10.1039/c7tx00209b. Published 2017 Sep. 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Willis J.B. The determination of metals in blood serum by atomic absorption spectroscopy—I: Calcium. Spectrochim. Acta. 1960:259–261. doi: 10.1038/186249a0. [DOI] [PubMed] [Google Scholar]
- 86.Zhao C., Liu X. A portable paper-based microfluidic platform for multiplexed electrochemical detection of human immunodeficiency virus and hepatitis C virus antibodies in serum. Biomicrofluidics. 2016;10(2) doi: 10.1063/1.4945311. Published 2016 Apr 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhao P., Liu S., Bai Y., et al. hWJECM-derived oriented scaffolds with autologous chondrocytes for rabbit cartilage defect repairing. Tissue Eng Part A. 2018;24(11–12):905–914. doi: 10.1089/ten.TEA.2017.0223. [DOI] [PubMed] [Google Scholar]
- 88.Zhao P., Liu S., Bai Y., et al. hWJECM-derived oriented scaffolds with autologous chondrocytes for rabbit cartilage defect repairing. Tissue Eng Part A. 2018;24(11–12):905–914. doi: 10.1089/ten.TEA.2017.0223. [DOI] [PubMed] [Google Scholar]


