Abstract
Background
Catheter-directed thrombolysis (CDL) is increasingly being used for the treatment of submassive and massive pulmonary embolism. Although this therapy has been shown to be effective at reducing right ventricle strain, the impact on clinical outcomes remains unclear. We therefore aimed to evaluate the outcomes of CDL compared to standard anticoagulation for submassive pulmonary embolism patients in a large cohort.
Methods
We conducted a retrospective observational study of consecutive patients with a primary diagnosis of submassive pulmonary embolism admitted to an intensive care unit within our health system between June 2014 and April 2016. We compared the outcome of patients treated with systemic anticoagulation (medical therapy) vs. catheter-based delivery of tissue plasminogen activator (tPA) (CDL). CDL patients were matched with medical therapy controls using a propensity-score matching algorithm based on the components of the simplified pulmonary embolism severity index (sPESI) score.
Results
Unadjusted mortality rates were 3.0% for CDL vs. 10.4% for medical therapy at 30 days and 8.1% for CDL vs. 22.9% for medical therapy at 1 year. In the propensity-score matched cohort, mortality rates were 3.1% for CDL vs. 6.1% for medical therapy at 30 days and 8.2% for CDL vs. 18.2% for medical therapy at 1 year. Length of stay was significantly shorter in the CDL group. The index admission bleeding and transfusion rates were not increased in the CDL group.
Conclusions
In patients presenting with acute submassive pulmonary embolism who are admitted to an intensive care unit, the group treated with CDL experienced reduced mortality at 30 days and 1 year when compared to medical therapy without increase in bleeding. Further randomized studies are required to confirm these findings.
Keywords: catheter-based therapies, pulmonary embolism
Acute pulmonary embolism (PE) is a common and life-threatening disease, affecting up to 900,000 individuals per year in the US.1,2 The main determinant of acute morbidity in PE is related to the degree of right ventricle (RV) dysfunction, ranging from absent in low risk cases to subclinical in intermediate risk (also categorized as submassive) cases, and hemodynamically significant in massive PEs.3
The therapeutic mainstay for PE is anticoagulation for all patients, with systemic thrombolysis reserved for hemodynamically compromised massive PE.4 The optimal treatment for submassive PE, defined as having evidence of right ventricular strain but without over hemodynamic compromise, is less well defined. Patients with elevated biomarkers of brain natriuretic peptide (BNP) and troponin as well as high PE Score Index (PESI) scores in addition to RV dilatation have been shown to have a high mortality with anticoagulation alone.5,6 Systemic thrombolysis has been investigated in patients with submassive PE, showing that while there was a decrease in hemodynamic collapse, a corresponding increase in the risk of significant hemorrhage was noted, with a resulting net neutral effect on mortality.7 Recent observational studies have shown that the risk of bleeding with systemic thrombolysis remains elevated, with up to a 7% rate of acute hemorrhage and a 4% rate of transfusions following thrombolysis.8
Catheter-directed thrombolysis (CDL) was developed with the goal of achieving the hemodynamic benefit of thrombolysis while minimizing the rate of complications by utilizing lower dosages of thrombolytic agent as well as the localized delivery. However, data on the clinical efficacy of CDL remain limited. The only randomized controlled trial comparing submassive PE patients treated with CDL and anticoagulation with heparin showed there was decreased right ventricular dilation and pulmonary artery pressure by 24 h without increased risk for major bleeding.9 In the single arm registry SEATTLE II registry, CDL was shown to rapidly decrease right ventricular strain as well as decrease in clot burden by pulmonary angiography over the first 48 h of admission.10 This result was also seen in the similarly designed PERFECT trial.11 The impact of CDL on harder clinical endpoints remains unclear at this time, despite the relatively wide spread adoption of this therapy. We have previously compared outcomes of CDL vs. medical therapy (MT) of a large cohort of patients admitted to the intensive care unit with low risk, submassive, and massive PEs.12 There has been no large study comparing CDL to the standard MT with anticoagulation for those patients with submassive PE. We therefore aimed to evaluate the outcomes of CDL compared to standard anticoagulation for submassive PE patients in a large cohort using our health system.
Methods
Study cohort
We performed a retrospective analysis of all patients between June 2014 and April 2016 admitted to an intensive care unit (ICU) with a primary diagnosis of PE within the University of Pittsburgh Medical Center system of 15 hospitals. This project was approved by the University of Pittsburgh Institutional Review Board (PRO16080502), and deemed no informed consent was required. Patients were initially identified for our analysis by ICD 9 and ICD 10 codes for PE, while admission to the ICU was determined by system billing codes. ICU admission was utilized as a surrogate marker to identify high-risk patients from the general PE admission population. From this data list, electronic medical records were reviewed for inclusion into the analysis.
Patients were included if there was a submassive PE on presentation leading to admission to the ICU within the first day of admission. Patients were excluded from the final analysis if they were discharged from an acute care facility within five days preceding the admission with PE, underwent surgical or mechanical thrombectomy, or if they were discharged from the index admission into a hospice program. Patients treated with systemic thrombolysis were included in the analysis if they were classified as a submassive PE and subsequently decompensated to require treatment. Patients treated with systemic thrombolysis as their primary initial therapy were excluded from the analysis. Baseline admission demographics, classification of PE, CT angiography (CTA)-derived RV-to-left ventricle (LV) diameter ratio, and echocardiography-based RV data were extracted from the electronic medical record. Vital signs and laboratory levels reported are the first available values documented in the electronic medical record, and baseline demographics reflect the known patient history at the time of index admission. Number of transfusions and hemorrhages were extrapolated from the electronic medical record utilizing ICD 9 and ICD 10 codes, as well as internal billing codes. All-cause mortality was derived from the social security death index, which is obtained from the updated Social Security Administration Death Master file where our health-care system is certified by the Social Security Administration as an organization that is exempt from the three-year delay.
PE classification
A submassive PE was defined as a patient having an RV-to-LV ratio greater than or equal to one as seen on CTA of the chest3 but without need for vasopressors. All other patients not meeting these criteria were excluded from our analysis. The PE severity index (PESI) score as well as the simplified PESI (sPESI) score were calculated for every patient based on admission vitals and demographic information for further risk stratification.13
Treatment groups
We compared patients that received MT with anticoagulation via IV or oral agents vs. patients that received CDL. The decision to treat with CDL was made by the treating physician and the availability of the technology. CDL was performed utilizing low-dose infusions of tPA (in general, 1 mg/h for up to 12 h on each side) either with ultrasound-assisted catheter-directed thrombolysis or catheter-directed thrombolysis. CDL was only available at 6 out of 15 hospitals included in this study during the time period investigated.
Study endpoints
Our primary endpoint was all-cause mortality. Secondary endpoints included need for transfusion during index admission and within 30 days of admission, and rates of intracranial and gastrointestinal hemorrhage during index admission and within 30 days of admission, as well as length of stay during the index hospitalization. For those patients who expired during their index hospitalization, the date of death served as the date of discharge in regard to the length of stay analysis.
Statistical methods
For descriptive purposes, continuous variables are reported as median (range); categorical variables are reported as frequencies and percentages. Baseline differences between the primary treatment groups were tested using Mann–Whitney tests for continuous variables and chi-squared tests for categorical variables. Propensity-score matching was used to create a cohort of MT controls similar to the patients that received CDL for comparison. The individual components of the sPESI score were used to create the propensity score (yes/no: age > 80 years; active cancer; heart failure or chronic pulmonary disease; heart rate > 110 bpm; systolic BP < 100 mm Hg; arterial oxygenation on presentation < 90%); CDL patients were matched with MT patients using a caliper distance of 0.05, and the cohorts were again compared to establish that there were no significant baseline differences. Survival is reported using Kaplan–Meier curves, with specific point estimates reported at 30 days and 1 year; Cox proportional-hazards models are used to compute hazard ratios for CDL vs. MT for each endpoint. To test for clustering effect between hospitals where CDL was offered as therapy and those where CDL was unavailable, we used two-sample proportion z-test for the mortality rate and Wilcox two-sample test for the length of stay. All statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC).
Results
Between June 2014 and April 2016, 339 patients admitted to an ICU with the primary admission diagnosis of PE were identified meeting the inclusion criteria listed above. A total of 240 patients received MT vs. 99 patients that received CDL. Of note, over that timeframe there were 257 patients admitted to an ICU with RV-to-LV ratio less than one.
Baseline characteristics of the entire unmatched and propensity-score matched cohort are listed in Table 1.
Table 1.
Baseline demographics of sample population, both for the unadjusted and propensity matched groups.
| Unadjusted |
Propensity matched |
|||||
|---|---|---|---|---|---|---|
| Medical | CDL | p-Value | Medical | CDL | p-Value | |
| 240 | 99 | 99 | 99 | |||
| Demographics and clinical characteristics | ||||||
| Gender | 0.51 | 0.13 | ||||
| Male | 121 (50.42%) | 46 (46.46%) | 57 (57.58%) | 46 (46.46%) | ||
| Female | 119 (49.58%) | 53 (53.54%) | 42 (42.42%) | 53 (53.54%) | ||
| Age | 66 (18.0–100) | 60.0 (19.0–92.0) | 0.143 | 59 (18.0–84) | 58 (19.0–92.0) | 0.89 |
| History of cancer | 72 (30.83%) | 12 (12.12%) | 0.0003 | 15 (15.15%) | 12 (12.12%) | 0.53 |
| CHF | 20 (8.33%) | 4 (4.04%) | 0.161 | 2 (2.02%) | 4 (4.04%) | 0.41 |
| CAD | 34 (14.17%) | 10 (10.1%) | 0.31 | 13 (13.13%) | 10 (10.10%) | 0.51 |
| Chronic lung disorder | 55 (22.92%) | 12 (12.12%) | 0.023 | 22 (22.22%) | 12 (12.12%) | 0.06 |
| Pregnant/postpartum (8 wk) | 0 (0.0%) | 0 (0.0%) | * | 0 (0.0%) | 0 (0.0%) | * |
| Hypercoaguable condition | 5 (2.56%) | 3 (8.11%) | 0.0984 | 2 (2.44%) | 3 (8.11%) | 0.15 |
| HIT | 2 (0.83%) | 0 (0.0%) | 0.36 | 1 (0.%) | 0 (0.0%) | 0.316 |
| Prior DVT/PE | 39 (19.50%) | 14 (19.18%) | 0.95 | 23 (27.06%) | 14 (19.18%) | 0.24 |
| AC as outpatient | 15 (7.89%) | 2(5.41%) | 0.59 | 8 (9.76%) | 2 (5.41%) | 0.43 |
| Vitals | ||||||
| HR | 105 (58.0–166) | 107 (10.0–155) | 0.70 | 107(62–148) | 107 (10.0–155) | 0.77 |
| SBP | 129.0 (18–211) | 129.5 (82.0–180) | 0.97 | 131 (84–211) | 130 (82.0–180) | 0.83 |
| DBP | 79.0 (30.0–149) | 78.0 (21.0–1031) | 0.63 | 79.0 (43.0–149) | 78 (21.0–1031) | 0.59 |
| O2 SAT | 94.0 (70.0–100) | 95.0 (9.0–100) | 0.58 | 95.0 (70.0–100) | 95.0 (9.0–100) | 0.97 |
| RR | 20.0 (7.0–96.0) | 22.0 (2.0–200) | 0.031 | 20.0 (7.0–96.0) | 22.0 (2.0–200) | 0.04 |
| Temp. < 36℃ (96.8°F) | 8 (3.33%) | 5 (5.05%) | 0.45 | 2 (2.02%) | 5 (5.05%) | 0.25 |
| Altered mental status | 61 (25.42%) | 17 (17.17%) | 0.101 | 26 (26.26%) | 17 (17.17%) | 0.12 |
| sPESI | 1 (0–5) | 1 (0–4) | 0.025 | 0 (0–4) | 1 (0–4) | 0.15 |
| Laboratory | ||||||
| Creatinine (mg/dL) | 1.0 (0.35–6.7) | 0.96 (0.46–9.81) | 0.15 | 1.0 (0.39–6.28) | 0.965 (0.46–9.81) | 0.20 |
| Troponin (ng/mL, EN set to 0) | 0.26 (0.036–8.3) | 0.43 (0.04–9.8) | 0.165 | 0.25 (0.054–7.05) | 0.44 (0.04–9.8) | 0.13 |
| BNP (pg/mL) | 154.5 (5.0–4266) | 223 (16–2077) | 0.009 | 123 (5.0–3250) | 223 (16–2077) | 0.04 |
| Hemodynamics | ||||||
| EF | 55.0 (27.0–71.0) | 55 (40.0–60) | 0.364 | 55.0 (27.0–71.0) | 55.0 (40.0–60.0) | 0.312 |
| PASP | 44.0 (8.0–132.0) | 42.0 (8.0–100.0) | 0.57 | 45.0 (18–76.0) | 42.0 (8.0–100.0) | 0.31 |
| RV dilated by TTE | 136 (62.39%) | 76 (80.85%) | 0.0054 | 49 (60.23%) | 76 (80.85%) | 0.01 |
| RV/LV ratio by CTA | 1.283 (1.0–4.27) | 1.54 (1.0–3.31) | <0.0014 | 1.22 (1.0–2.64) | 1.54 (1.0–3.31) | 0.01 |
Medical history was taken from admission to reflect known history on presentation. Vital signs and laboratory values reflect initial admission values. Undetectable troponin and BNP values were set to zero. Baseline imaging findings on admission imaging: transthoracic echocardiograms (TTE) and CT angiography (CTA). Patients treated with CDL had higher rates of RV dilation as evidenced on TTE as well as by RV to LV dilation on CTA. CHF: congestive heart failure; CAD: coronary artery disease; HIT: heparin induced thrombocytopenia; DVT: deep venous thrombosis; PE: pulomonary embolism; AC: anticoagulation; HR: heart rate; SBP: systolic blood pressure; DBP; diastolic blood pressure: SAT: saturation; sPESI: simplified pulmonary embolism severity index; BNP: Brain natriuretic peptide; EF: ejection fraction; PASP: pulmonary artery systolic pressure; RV/LV: right ventricle/left ventricle. *reflects no statistics able to be calculated as there were no patients who were pregnant or postpartum in our cohort.
Six patients within the MT group were failures of MT and were treated with systemic thrombolysis and/or vasopressors. Within the full study population, patients receiving MT had a greater prevalence of a history of malignancy and chronic lung disorder as well as presenting with altered mental status, which did result in patients with MT showing slightly higher PE severity (PESI) scores than patients that received CDL. The other components of the PESI score – age, gender, history of heart failure, history of chronic lung disorders, heart rate, systolic blood pressure, and altered mental status – were similar between the two groups. There was a difference in the respiratory rate between the CDL and MT groups, albeit of only two breaths per minute. The CDL group did have significantly higher RV/LV ratio as seen on CTA of the chest. The Pearson correlation coefficient was calculated between PESI scores and the mortality, with r = 0.92.
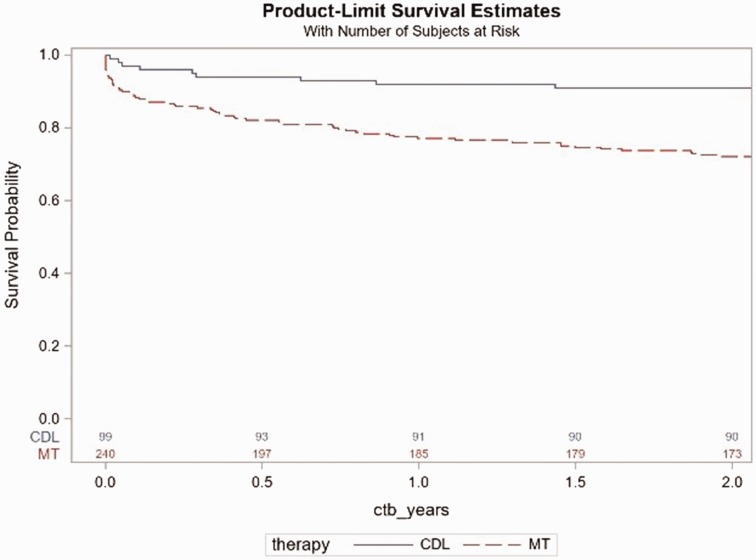
Survival after submassive PE in the full study population is presented in Fig. 1.
Fig. 1.
Kaplan–Meier survival by treatment group from admission day in the full study population cohort. There were 76 deaths (67 medical patients, 9 CDL patients) over the two-year follow-up with a HR for CDL vs. medical therapy of 0.3, 95% CI 0.146–0.587, p = 0.0005.
Overall, mortality was lower in the patients that received CDL vs. those that received MT (hazard ratio (HR) = 0.3, 95% confidence interval (CI) 0.146–0.587, p = 0.005). When assessing specific time points of interest, mortality was significantly lower in the CDL treatment compared to MT at 30 days (3.0% for CDL vs. 10.4% for MT) and one year: (8.1% for CDL vs. 22.9% for MT). In order to test for a clustering effect between hospitals with and without CDL, two-sample proportion test showed no significant difference in their mortality rates (p = 0.70) for medically treated patients. Wilcox two-sample test also showed no statistically significant difference in their length of stay (p = 0.375) between the hospitals with and without CDL.
In addition, patients treated with CDL had decreased length of stay during the initial admission (4 vs. 6 days, p < 0.001). Interestingly, there was a significantly increased rate of transfusion in those patients treated with MT (9.2%) compared to CDL (1.8%) during the index admission (Table 2).
Table 2.
Hospital events.
| Unadjusted |
Propensity matched |
|||||
|---|---|---|---|---|---|---|
| Medical | CDL | p-Value | Medical | CDL | p-Value | |
| 240 | 99 | 99 | 99 | |||
| Index hospitalization events | ||||||
| Length of stay (days) | 6.0 (0.0–45.0) | 4.0 (1.0–16.0) | <0.001 | 6.0 (0.0–45.0) | 4.0 (1.0–16.0) | <0.001 |
| Patients with transfusion after PE | 22 (9.17%) | 2 (2.02%) | 0.02 | 14 (14.14%) | 2 (2.02%) | 0.018 |
| Patients with hemorrhages after PE | 5 (2%) | 3 (3%) | 0.39 | 5 (5%) | 3 (3.1%) | 0.21 |
| Types of hemorrhages | ||||||
| GI | 2 (0.83%) | 1 (1%) | 0.71 | 0 (0.0%) | 1 (1%) | 0.57 |
| Intracranial | 1 (0.41%) | 1 (1%) | 0.60 | 1 (1%) | 1 (1%) | 1 |
| Other | 2 (0.83%) | 2 (2%) | 0.77 | 1(1%) | 2 (2%) | 0.5 |
There was a decreased length of stay in patients treated with CDL compared to standard medical therapy. There was no significant increase in rates of transfusions or hemorrhages during the index admission for those treated with CDL. GI: gastrointestinal; PE: pulmonary embolism.
Despite this difference, the rates and types of documented hemorrhage were not different between the groups.
To create a cohort of CDL patients matched with MT controls with similar risk, we calculated a propensity score comprised of the individual components of the PESI score. Baseline characteristics for the propensity-matched cohort are listed in Table 1 and show no significant differences between the two groups, indicating a good-quality match (see supplemental figures showing the distribution of the age, heart rate, systolic blood pressure, respiratory rate, BNP level, and troponin level in the matched cohort). The RV/LV ratio remained slightly higher in the CDL group relative to MT.
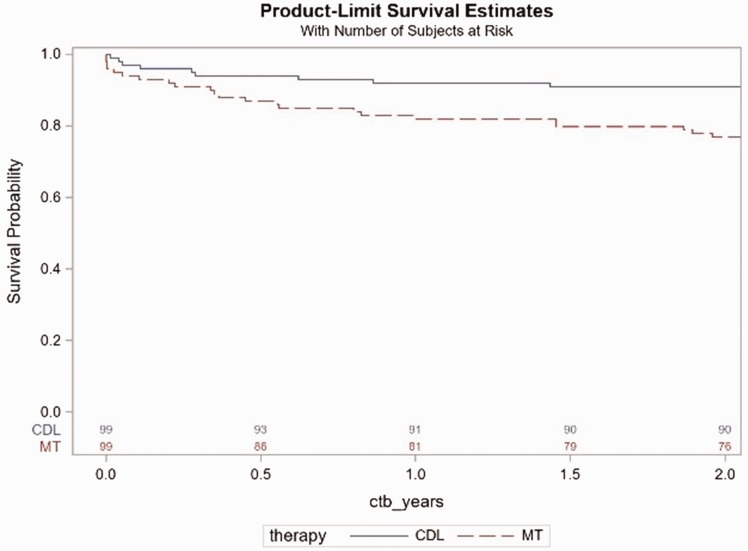
The results in the propensity-matched cohort mirrored results in the full cohort, with decreased overall mortality in the CDL compared to the MT group (30 day 3.0% for CDL vs. 6.1% for MT; one year 8.1% for CDL vs. 18.2% for MT; HR = 0.4, 95% CI 0.169–0.792, p = 0.011, Fig. 2).
Fig. 2.
Kaplan–Meier survival by treatment group in the propensity-score matched cohort from admission day. There were 32 deaths (23 medical patients, 9 CDL patients) with a hazard ratio of 0.4, 95% CI 0.169–0.792, p = 0.011.
The transfusion pattern noticed in the full patient population persisted in the propensity-matched cohort. Again there was no significant difference in the rate of hemorrhage in the propensity-matched groups. The length of stay was again significantly shorter in the CDL group when compared to the MT group (Table 2).
On review of the 34 patients who died in the propensity-matched cohort, we were only able to identify the cause of death in 15 patients. Of those identified patients, 12 patients (80%) died due to PE associated deaths, which we defined as death due to respiratory failure, cardiac arrest, heart failure, or recurrent PE. This definition is consistent with that established in the PEITHO trial.7 Of these 12 patients, 8 patients were in the MT group and 4 in the CDL group, with 4 and 1 patients, respectively, passing away within 30 days of admission. Of note, there were no in-hospital deaths secondary to hemorrhage or other procedure-related complications in the patients within the CDL-treated group. The other four patients with identified causes of mortality died mainly from either progression of underlying malignancy or transfer to comfort measures only care, with one patient dying in a motor vehicle accident.
There were two major procedure-related complications in the CDL group. One patient had hemopericardium following CDL necessitating sternotomy for repair. Another patient experienced a small intracranial hemorrhage which was treated conservatively; of note is that this patient was concomitantly of dual antiplatelet therapy. Both patients survived through the follow-up period.
Discussion
In our retrospective observational analysis, we found a reduced overall mortality in submassive PE patients admitted to the ICU who received catheter-directed thrombolysis when compared to those treated with standard MT. This result was consistent in the initial unadjusted analysis as well as within our propensity-matched cohort. There were no differences in the rates of hemorrhages between the two groups, with only 2.0% of patients treated with CDL requiring a transfusion or diagnosed with a hemorrhage. The CDL group had a shorter length of stay than the MT group.
The current ICD 9 and 10 codes do not have enough detail to select PE based on severity (low risk, submassive, and massive). Therefore, to select submassive patients for this analysis, we used ICU admission in the first 24 h as the main screening criteria in this analysis. Within that group of patients, we selected the patients with submassive PE based on RV/LV ratio greater than or equal to one on imaging. We only included patients with a primary admission diagnosis of PE. Patients who were in an ICU with an alternative diagnosis as well as patients who had a PE immediately within 24 h post-op were not included in this study. This methodology allowed us to select a higher risk PE patient population as depicted in their high mortality.
The PESI score did correlate with mortality in our study cohort, as evidenced by our Pearson correlation coefficient of 0.92. Although on average the patients in this study were PESI class III, the overall death rate in the MT arm was closer to PESI class IV.14 This could be due to the differences in the severity of co-morbidities not captured with enough granularity in the original PESI score, such as evidence of RV strain as seen on imaging or with elevated cardiac biomarkers.15 The mortality in our study however is comparable to the one recently reported by other US centers.16
A number of prior retrospective studies compared CDL with systemic lysis.14,17,18 However, CDL is used primarily in patients with submassive PE, which are rarely treated with systemic lysis. Thus, for treatment of submassive PE, the more relevant clinical question is whether primary CDL has a benefit over anticoagulation alone. Studies comparing systemic thrombolysis vs. anticoagulation alone did find a decreased PE-related death and hemodynamic decompensation, but at the cost of increased bleeding.7 Within the significant limitations of a retrospective analysis, our data suggest improved mortality with CDL in a selected group of submassive PE patients warranting ICU admission. We have recently demonstrated that relatively large number of submassive PE patients’ invasive hemodynamics at the time of the CDL are consistent with cardiogenic shock despite apparent clinical stability.19 Our mortality trends are consistent with prior retrospective data comparing outcomes for CDL with systemic thrombolysis showed acute in-hospital benefit in regard to mortality and bleeding complications.14
Importantly, our analysis did not show a significant difference in the rates of hemorrhage during the index admission compared between the CDL and MT groups. The largest retrospective review of CDL showed decreased bleeding when compared to systemic thrombolysis.14 Meta-analysis of 860 patients in total treated with CDL revealed low rates of intracranial and fatal hemorrhages (0.35% and 0.1%, respectively) and an overall hemorrhage rate of 4.65%.20 Our rates of transfusion were significantly lower in the CDL-treated arm compared to the MT arm during the index admission, which suggests that CDL is at least as safe as MT. The observed rates of transfusion in the CDL are comparable to prior published results based on nationwide databases (9.22%14 and 4.6%20 vs. our observed 1.8%), although we did note a lower rate of diagnosed hemorrhages in our cohort. This suggests that the reduced dose tPA received by patients undergoing CDL is safe and does not lead to increased risk for serious bleeding complications or need for transfusions. Our data are consistent with a pooled analysis of the published data that supports the notion that the bleeding with CDL has been reported to be relatively low when utilized in appropriately selected patients, although ICH is not completely absent.14,20,21
When evaluated in both the full study patient and propensity-matched cohorts, we found that patients treated with CDL had a decreased length of admission compared to those treated with MT. This is likely due to the in part to more rapid improvement in right ventricular hemodynamics associated with CDL as shown in the ULTIMA, SEATTLE II, and PERFECT trials.9–11 The cost-benefit impact of this observation deserves further investigation in dedicated cost-benefit studies in the future.
Our results must be interpreted in context of the study limitations. This was a relatively small retrospective observational study encompassing patients admitted to a large health system, which included patients admitted to small community hospitals as well as large academic referral centers. As the study identified patients for review via ICD codes as well as billing codes, there is a potential for missed patients due to coding errors. There is also potential for factors which were not captured in our analysis to have affected patient selection and subsequent outcomes, such as inability for transfer of a patient to a CDL site within our health system. On the other hand, CDL was only available to some patient pools and was not protocolized, thus minimizing a potential therapy selection bias.
In this retrospective analysis of clinical outcomes, patients with submassive PE that were treated with CDL experienced lower mortality as well as a decreased length of stay as compared to standard anticoagulation, without a significant increase in overall rates of hemorrhage or transfusions. Randomized trials are necessary to confirm the causality of these findings.
Supplemental Material
Supplemental material, PUL898368 Supplemental Material for Outcomes of catheter-directed thrombolysis vs. standard medical therapy in patients with acute submassive pulmonary embolism by Stephen D’Auria, Ahmet Sezer, Floyd Thoma, Michael Sharbaugh, Jeffrey McKibben, Robert Maholic, Efthymios D. Avgerinos, Belinda N. Rivera-Lebron and Catalin Toma in Pulmonary Circulation
Acknowledgements
None.
Ethical approval
This project was approved by the University of Pittsburgh Institutional Review Board (PRO16080502), and deemed no informed consent was required.
Guarantor
None.
Contributorship
SD performed chart review and image analysis. AS, FT, JM, performed statistical support. BNR serves as the leader of the PE response team and is responsible for triaging patients. EDA, RM, and CT performed CDL at individual sites and served on local facilities PE response teams. All authors read and assisted with the writing and editing of the manuscript.
Conflicting of interest
SD, AS, FT, MS, JM, EDA, BNR report no competing interests. RM reports competing interests of consultant and speaker for EKOS, national primary investigator for KNOCKOUT trial. CT reports competing interests of national primary investigator for the FLASH registry.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iD
Stephen D’Auria https://orcid.org/0000-0002-4948-278X
Supplemental material
Supplemental material for this article is available online.
References
- 1.Blood clots: a serious but preventable medical condition what you need to know to protect yourself, www.cdc.gov/ncbddd/dvt/documents/blood-clots-fact-sheet.pdf (2018, accessed 2 July 2019).
- 2.DHHS. The surgeon general’s call to action to prevent deep vein thrombosis and pulmonary embolism, Rockville: Office of the Surgeon General, 2008. [PubMed] [Google Scholar]
- 3.Jaff M, McMurtry S, Archer S, et al. Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension: a scientific satement from the Americal Heart Association. Circulation 2011; 123: 1788–1830. [DOI] [PubMed] [Google Scholar]
- 4.Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest 2016; 149: 315–352. [DOI] [PubMed] [Google Scholar]
- 5.Bajaj A, Rathor P, Sehgal V, et al. Prognostic value of biomarkers in acute non-massive pulmonary embolism: a systematic review and meta-analysis. Lung 2015; 193: 639–651. [DOI] [PubMed] [Google Scholar]
- 6.Binder L, Pieske B, Olschewski M, et al. N-terminal pro-brain natriuretic peptide or troponin testing followed by echocardiography for risk stratification of acute pulmonary embolism. Circulation 2005; 112: 1573–1579. [DOI] [PubMed] [Google Scholar]
- 7.Meyer G, Vicaut E, Danays T, et al. Fibrinolysis for patients with intermediate-risk pulmonary embolism. N Engl J Med 2014; 370: 1402–1411. [DOI] [PubMed] [Google Scholar]
- 8.Kiser TH, Burnham EL, Clark B, et al. Half-dose versus full-dose alteplase for treatment of pulmonary embolism. Crit Care Med 2018; 46: 1617–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kucher N, Boekstegers P, Müller OJ, et al. Randomized, controlled trial of ultrasound-assisted catheter-directed thrombolysis for acute intermediate-risk pulmonary embolism. Circulation 2014; 129: 479–486. [DOI] [PubMed] [Google Scholar]
- 10.Piazza G, Hohlfelder B, Jaff MR, et al. A prospective, single-arm, multicenter trial of ultrasound-facilitated, catheter-directed, low-dose fibrinolysis for acute massive and submassive pulmonary embolism: the SEATTLE II study. JACC Cardiovasc Interv 2015; 8: 1382–1392. [DOI] [PubMed] [Google Scholar]
- 11.Kuo WT, Banerjee A, Kim PS, et al. Pulmonary embolism response to fragmentation, embolectomy, and catheter thrombolysis (PERFECT): initial results from a prospective multicenter registry. Chest 2015; 148: 667–673. [DOI] [PubMed] [Google Scholar]
- 12.D’Auria S, Althouse A, Thoma F, et al. Outcomes of catheter-directed thrombolysis versus standard medical therapy in patients admitted to intensive care units with acute pulmonary embolism. Eur Heart J 2018; 39: 55–56. [Google Scholar]
- 13.Aujesky D, Obrosky DS, Stone RA, et al. Derivation and validation of a prognostic model for pulmonary embolism. Am J Respir Crit Care Med 2005; 172: 1041–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arora S, Panaich SS, Ainani N, et al. Comparison of in-hospital outcomes and readmission rates in acute pulmonary embolism between systemic and catheter-directed thrombolysis (from the national readmission database). Am J Cardiol 2017; 120: 1653–1661. [DOI] [PubMed] [Google Scholar]
- 15.Konstantinides SV, Torbicki A, Agnelli G, et al. 2014 ESC Guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J 2014; 35: 3033–3080. [DOI] [PubMed] [Google Scholar]
- 16.Secemsky E, Chang Y, Jain CC, et al. Contemporary management and outcomes of pateints with massive and submassive pulmonary embolism. Am J Med 2018; 131: 1506–1514. [DOI] [PubMed] [Google Scholar]
- 17.Patel N, Patel NJ, Agnihotri K, et al. Utilization of catheter-directed thrombolysis in pulmonary embolism and outcome difference between systemic thrombolysis and catheter-directed thrombolysis. Catheter Cardiovasc Interv 2015; 86: 1219–1227. [DOI] [PubMed] [Google Scholar]
- 18.Liang NL, Avgerinos ED, Singh MJ, et al. Systemic thrombolysis increases hemorrhagic stroke risk without survival benefit compared to catheter-directed intervention for the treatment of acute pulmonary embolism. J Vasc Surg Venous Lymphat Disord 2017; 5: 171–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khandhar SJ, Mehta M, Cilia L, et al. Invasive hemodynamic assessment of patients with submassive pulmonary embolism. Catheter Cardiovasc Interv 2019; 95: 1–6. [DOI] [PubMed] [Google Scholar]
- 20.Bloomer TL, El-Hayek GE, McDaniel MC, et al. Safety of catheter-directed thrombolysis for massive and submassive pulmonary embolism: results of a multicenter registry and meta-analysis. Catheter Cardiovasc Interv 2017; 89: 754–760. [DOI] [PubMed] [Google Scholar]
- 21.Lou B-H, Wang L-H, Chen Y. A meta-analysis of efficacy and safety of catheter-directed interventions in submassive pulmonary embolism. Eur Rev Med Pharmacol Sci 2017; 21: 84–198. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, PUL898368 Supplemental Material for Outcomes of catheter-directed thrombolysis vs. standard medical therapy in patients with acute submassive pulmonary embolism by Stephen D’Auria, Ahmet Sezer, Floyd Thoma, Michael Sharbaugh, Jeffrey McKibben, Robert Maholic, Efthymios D. Avgerinos, Belinda N. Rivera-Lebron and Catalin Toma in Pulmonary Circulation