Abstract
Targeted memory reactivation (TMR) is a methodology employed to manipulate memory processing during sleep. TMR studies have great potential to advance understanding of sleep-based memory consolidation and corresponding neural mechanisms. Research making use of TMR has developed rapidly, with over 70 articles published in the last decade, yet no quantitative analysis exists to evaluate the overall effects. Here we present the first meta-analysis of sleep TMR, compiled from 91 experiments with 212 effect sizes (N=2,004). Based on multilevel modelling, overall sleep TMR was highly effective [Hedges’ g=0.29, 95% CI: (0.21, 0.38)], with a significant effect for two stages of non-rapid eye movement sleep [Stage NREM 2: Hedges’ g=0.32, 95% CI: (0.04, 0.60); and Slow-Wave Sleep: Hedges’ g=0.27, 95% CI: (0.20, 0.35)]. In contrast, TMR was not effective during REM sleep nor during wakefulness in the present analyses. Several analysis strategies were used to address the potential relevance of publication bias. Additional analyses showed that TMR improved memory across multiple domains, including declarative memory and skill acquisition. Given that TMR can reinforce many types of memory, it could be useful for various educational and clinical applications. Overall, the present meta-analysis provides substantial support for the notion that TMR can influence memory storage during NREM sleep, and that this method can be useful for understanding neurocognitive mechanisms of memory consolidation.
Keywords: sleep, targeted memory reactivation, memory consolidation, meta-analysis
The idea of manipulating memories and thoughts during sleep is fascinating for neuroscientists, psychologists, and the general public. Although the idea may sound like science-fiction, the past decade has witnessed an increasing number of studies wherein memory processing is directly manipulated during sleep. By covertly administering sensory cues while participants are asleep, associated memories from recent learning can be reactivated and modified. This procedure, known as targeted memory reactivation (TMR), gives researchers the ability to noninvasively reactivate specific memories during sleep. More generally, memory reactivation is thought to be a natural feature of sleep that underlies sleep-dependent memory consolidation and the effective preservation of memories (Paller, Mayes, Antony, & Norman, in press).
The use of TMR in various experimental contexts has greatly advanced our understanding of causal relationships between sleep physiology and memory consolidation. TMR research is also attractive because its usefulness could extend beyond the laboratory, with high potential value for enhancing learning via offline memory processing. For example, benefits may be realized for boosting skill and language acquisition, and even enhancing psychotherapeutic effectiveness (for related discussions, see Diekelmann, 2014; Paller, 2017). Despite the influx of publications dedicated to this line of research, two imperative questions remain un-answered: what is the overall effect size aggregating across TMR studies and what are the variables that modulate the effectiveness of TMR? This meta-analysis aims to address these questions, providing quantitative estimates of the overall TMR effect as well as effects under various experimental conditions.
Spontaneous and Targeted Memory Reactivation During Sleep
Memories continue to change, even after initial encoding and between episodes of deliberate rehearsal. Jenkins and Dallenbach (1924) provided initial evidence that offline sleep influenced memory processing: participants showed superior memory retention following sleep versus following an equal period of wakefulness. More recently it has become widely accepted that sleep plays an important role in consolidating and transforming memories (Diekelmann & Born, 2010; Inostroza & Born; 2013; Rasch & Born, 2013; Stickgold & Walker, 2013). For example, it has been reported that sleep can stabilize memories and render them more resistant to retroactive interference (Ellenbogen, Hulbert, Stickgold, Dinges & Thompson-Schill, 2006), and that sleep can promote integration of newly learnt information into existing memory schema (Tamminen, Payne, Stickgold, Wamsley & Gaskell, 2010). Moreover, motivation also shapes sleep-based memory consolidation, given the demonstrated influence of emotion, reward, and future relevance on retention (Fischer & Born, 2011; Payne et al., 2015; Wilhelm, Diekelmann, Molzow, Ayoub, Molle & Born, 2011).
One plausible mechanism supporting sleep-based memory consolidation is that prior learning experiences are spontaneously reactivated during sleep. Techniques such as single-unit recording, scalp electroencephalography (EEG), positron emission tomography (PET), and functional magnetic resonance imaging (fMRI) allow researchers to observe brain activity during post-learning sleep. Specifically, brain activity related to wakeful encoding can spontaneously re-emerge during subsequent sleep, possibly indexing memory reactivation given that the magnitude of such responses can predict post-sleep memory performance (Deuker et al., 2013; Peigneux et al., 2004). These studies relied on spontaneous memory reactivation and did not directly manipulate memory reactivation during sleep. Compelling evidence for causal relationships between sleep-based memory reactivation and improved memory performance could be attained using methods to allow memory reactivation to be externally initiated and guided.
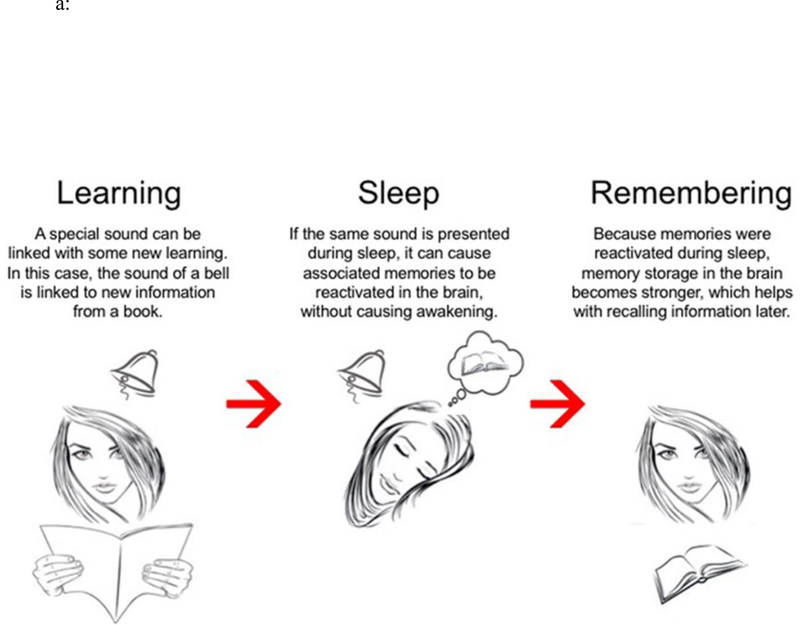
As shown in Figure 1a, TMR paradigms are characterized by three core components: First, specific learning episodes are designed so that strong associations are formed between certain sensory stimuli and learned information. In some cases, the stimuli are the main focus of learning. Secondly, previously learned sensory cues are presented to participants during sleep, usually during specific sleep stages identified by standard polysomnographic methods. Steps are taken to avoid arousal from sleep (e.g., sounds delivered at a low intensity over a white-noise background). Critically, re-exposure to sensory cues is intended to reactivate previously learned information. The last component consists of a post-sleep test upon waking. By comparing performance change scores between reactivated and non-reactivated memories, researchers can isolate the TMR effects due to the reactivation manipulation.
Figure 1.
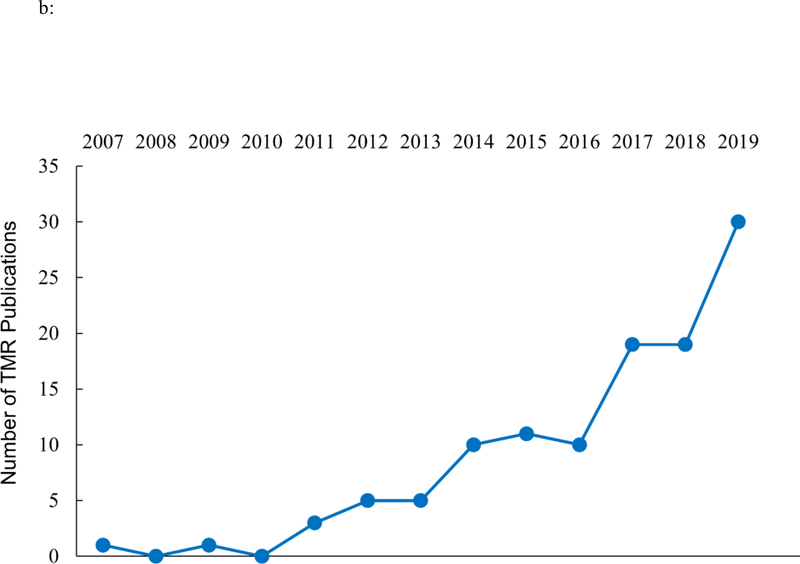
a: Schematic of the typical procedure in a TMR experiment (reprinted from Paller, 2018). 1b: Number of TMR articles (including both human/non-human empirical studies and review articles) published by year since Rasch et al. (2007). The last data point represents the annualized number based on number of articles published from January to June 2019.
Although the term TMR was coined only recently (Oudiette & Paller, 2013), research using memory reminders during sleep was evident since at least the 1950s and has been periodically documented since (e.g., Aarons, 1976; Dillon & Bowles, 1976; Fox & Robbins, 1952; Guerrien et al., 1989; Hars et al., 1985; Hars & Hennevin, 1987; Oswald, Taylor & Treisman, 1960; Tilley et al., 1979; Smith & Weeden, 1990, Wood, Bootzin, Kihlstrom & Schacter, 1992; for a review and discussions of these early studies, see Oudiette & Paller, 2013). These earlier studies not only aimed to reactivate prior learning established during wakefulness, but in some cases also tried to produce novel learning using sensory cues during sleep. Many of these studies were controversial and regularly dismissed on methodological grounds (e.g., Bruce, Evans, Fenwick & Spencer, 1970). However, after Rasch, Buchel, Gais, and Born (2007) and Rudoy, Voss, Westerberg and Paller (2009) published their seminal experiments, this line of research has grown considerably; Figure 1b documents this growth in publications on TMR.
An Overview of TMR Research
In Rasch et al. (2007), the researchers paired an olfactory cue with two learning tasks: a declarative, spatial location task and a procedural, finger-tapping task. Compared with various control conditions, re-exposure of the same olfactory cue during subsequent SWS improved spatial recall, but not finger-tapping performance. Improvement of spatial recall was limited to cueing during SWS, in that cueing during REM or wakefulness did not produce noticeable change. Odor-induced memory reactivation during SWS was additionally supported by fMRI findings showing that exposure to task-relevant odors during SWS elicited hippocampal activity.
Rudoy and colleagues (2009) similarly reactivated spatial memories during SWS but with a set of low-intensity sounds instead of a single odor. These sounds had been presented during learning, each with an image of a semantically related object. Post-sleep results showed that TMR altered memories during SWS, as locations of cued objects were recalled more accurately than were locations of uncued objects. This experiment thus made two unique contributions. First, it demonstrated that reactivation during SWS can be provoked through the auditory modality. Prior thinking was that such auditory input would largely be prevented from reaching the cortex due to gating at the thalamus, whereas olfactory processing does not pass through the thalamus (Zelano & Sobel, 2005). Second, it showed that reactivation with TMR can influence a select subset of specific memories formed during a learning episode.
These and other TMR studies enabled researchers to make strong causal inferences linking offline, sleep-based reactivation to subsequent memory performance. Furthermore, additional insights were provided about the roles of distinct sleep stages and sleep-physiology signals in relation to memory consolidation. Investigating cue-elicited brain activity during sleep can enable researchers to pinpoint neural mechanisms contributing to memory change (Ai et al., 2018; Antony et al., 2018b; Belal et al., 2018; Cairney et al., 2018; Farthouat, Gilson & Peigneux, 2017; Schreiner, Doeller, Jensen, Rasch & Staudigl, 2018; Schreiner, Lehmann & Rasch, 2015; Shanahan et al., 2018). Identifying relevant neural signals (e.g., slow oscillations, spindles, other brain rhythms, and fMRI activations) has now become the target of many creative experimental manipulations. Moreover, oscillatory stimulation can also be used to entrain brain rhythms to shed further light on their roles in memory (e.g., Antony & Paller, 2017; Ngo et al., 2013; for a recent review on different stimulation methods, see Cellini & Mednick, 2018).
Given that translating basic science research to applications outside the lab setting can be advantageous, TMR provides new opportunities to boost learning beyond ordinary sleep (Diekelmann, 2014; Paller, 2017). For example, Diekelmann, Biggel, Rasch and Born (2012) reported that a 40-min sleep with TMR enhanced memory when compared with the same length of sleep without TMR (see also Schönauer, Geisler, & Gais, 2014). Another intriguing possibility is that the benefits of TMR are cumulative and, when applied over longer periods of time, could help those who suffer from more severe memory difficulties such as neurodegenerative diseases (e.g., Westerberg et al., 2012). TMR might also aid approaches in clinical psychotherapy (Oudiette, Antony & Paller, 2014), as using TMR during sleep could reactivate skills from a prior therapy session, helping those who suffer from PTSD, anxiety, depression, among other disorders (Paller, 2017).
To date, TMR research has been studied with many different sorts of learning. As shown in Table 1, this list includes learning paradigms such as word associative learning, visual-spatial memory, emotional memory, skill learning, vocabulary learning, grammar learning, fear conditioning/extinction, and so on. Notably, TMR has also been combined with innovative learning tasks that are not typically studied in memory research, such as phobia-exposure therapy, counter-stereotype learning, multisensory integration, value-based decision making, and so on (e.g., Ai et al., 2018; Honma et al., 2016; Hu et al., 2015; Rihm et al., 2016). Outside of human evidence, TMR has also been conducted with non-human animals including rats, mice, and even with invertebrates such as honeybees (Bender & Wilson, 2012; Purple, Sakurai & Sakaguchi, 2017; Rolls et al., 2013; Rothschild, Eban & Frank, 2017; Zwaka et al., 2015). These cross-species studies provide converging evidence that memory processing can be manipulated during sleep.
Table 1.
Sample, experimental characteristics, and effect sizes information of TMR experiments/tasks included in the meta-analysis.
| References | Sample Characteristics | TMR Experimental Characteristics | Effect Sizes | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample Size | Female Ratio | Age | Country | Cueing Stages | Sleep Length | Memory Task | Learning Type | Focal Analyses | Design | Cueing Modality | Hedges’ g | 95%CI LL | 95% CI UL | Z | p | |
| Dillon & Babor, 1970 | 12 | 0.00 | 20.5 | US | Unspecified | 8 | Word-Word Learning | Declarative | Within | Verbal | 0.40 | −0.17 | 0.97 | 1.388 | .165 | |
| Tilley, 1979 N2 | 8 | NA | NA | US | N2 | 8 | Word-Picture Learning | Declarative | Associative | Within | Verbal | 1.12 | 0.29 | 1.95 | 2.658 | .008 |
| Tilley, 1979 REM | 8 | NA | NA | US | REM | 8 | Word-Picture Learning | Declarative | Within | Verbal | 0.17 | −0.46 | 0.79 | 0.525 | .600 | |
| ^Guerrien et al., 1989 | 10 | 0.00 | 20.0 | US | REM | 8 | Morse Code Learning | Declarative | Between | Nonverbal | 3.47 | 1.58 | 5.36 | 3.601 | < .001 | |
| ^Smith & Weeden, 1990 | 10 | NA | 19.4 | Canada | REM | 8 | Wff n’ Proof Logic Game | Declarative | Between | Nonverbal | 3.17 | 1.39 | 4.95 | 3.482 | < .001 | |
| Rasch et al., 2007 Exp. 1 Spatial | 18 | 0.56 | 24.1 | Germany | SWS | 7.5 | Spatial Location | Declarative | Spatial | Within | Olfactory | 0.69 | 0.19 | 1.18 | 2.717 | .007 |
| Rasch et al., 2007 Exp. 1 Skill | SWS | 7.5 | Finger Sequence Tapping | Skill | Skill | Within | Olfactory | −0.08 | −0.52 | 0.37 | −0.339 | .734 | ||||
| Rasch et al., 2007 Exp. 3 Spatial | 17 | 0.53 | 25.0 | Germany | REM | 7.5 | Spatial Location | Declarative | Within | Olfactory | −0.05 | −0.51 | 0.40 | −0.236 | .813 | |
| Rasch et al., 2007 Exp. 3 Skill | REM | 7.5 | Finger Sequence Tapping | Skill | Within | Olfactory | −0.21 | −0.67 | 0.25 | −0.905 | .366 | |||||
| 1Rasch et al., 2007 Exp. 5 | 12 | 0.50 | 25.4 | Germany | SWS | 1.5 | Spatial Location | Declarative | Spatial | Within | Olfactory | 0.64 | 0.05 | 1.22 | 2.136 | .033 |
| Rudoy et al., 2009 | 12 | 0.83 | 21.5 | US | SWS | 1.5 | Spatial Location | Declarative | Spatial | Within | Nonverbal | 0.48 | −0.08 | 1.04 | 1.671 | .095 |
| Diekelmann et al., 2011 | 12 | 0.17 | 22.3 | Germany | SWS | 0.67 | Spatial Location | Declarative | Spatial | Within | Olfactory | 0.74 | 0.08 | 1.40 | 2.207 | .027 |
| Donohue & Spencer, 2011 | 32 | 0.63 | 20.8 | US | Unspecified | 8 | Word-Word Learning | Declarative | Between | Nonverbal | 0.00 | −0.68 | 0.68 | 0.000 | 1.000 | |
| Antony et al., 2012 | 16 | 0.38 | 21.0 | US | SWS | 1.5 | Finger Sequence Tapping | Skill | Skill | Within | Nonverbal | 0.46 | −0.03 | 0.96 | 1.836 | .066 |
| Ritter et al., 2012 | 30 | 0.87 | 21.3 | Netherlands | Unspecified | 8 | Creative Solutions | Declarative | Between | Olfactory | 1.07 | 0.31 | 1.82 | 2.780 | .005 | |
| van Dongen et al., 2012 | 22 | 0.73 | 22.5 | Netherlands | SWS | 2 | Spatial Location | Declarative | Spatial | Within | Nonverbal | −0.21 | −0.61 | 0.20 | −0.989 | .323 |
| Fuentemilla et al., 2013 | 9 | 0.67 | 41.0 | Spain | SWS | 8 | Sound-Word Learning | Declarative | Associative | Within | Nonverbal | 0.70 | 0.03 | 1.38 | 2.045 | .041 |
| Hauner et al., 2013 | 15 | 0.53 | 24.5 | US | SWS | 1.5 | Fear Extinction | Conditioning | Fear | Within | Olfactory | 0.51 | 0.00 | 1.03 | 1.968 | .049 |
| Oudiette et al., 2013 | 15 | 0.60 | 20.7 | US | SWS | 1.5 | Spatial Location | Declarative | Spatial | Within | Nonverbal | 0.24 | −0.25 | 0.72 | 0.962 | .336 |
| Cairney et al., 2014 Neutral | 15 | 0.80 | 20.4 | UK | SWS | 1.5 | Spatial Location Neutral Picture | Declarative | Spatial | Within | Nonverbal | 0.08 | −0.40 | 0.56 | 0.325 | .745 |
| Cairney et al., 2014 Negative | SWS | 1.5 | Spatial Location Negative Picture | Declarative | Emotion | Within | Nonverbal | 0.19 | −0.30 | 0.67 | 0.758 | .449 | ||||
| Cordi et al., 2014 | 16 | 0.63 | 23.9 | Switzerland | REM | 4 | Spatial Location | Declarative | Within | Olfactory | 0.08 | −0.38 | 0.55 | 0.346 | .729 | |
| Cousins et al., 2014 | 16 | 0.50 | 24.8 | UK | SWS | 8 | SRTT | Skill | Skill | Within | Nonverbal | 0.87 | 0.31 | 1.43 | 3.064 | .002 |
| Cox et al., 2014 | 28 | 0.89 | 20.1 | Netherlands | SWS | 2 | Spatial Location | Declarative | Spatial | Within | Olfactory | 0.03 | −0.33 | 0.39 | 0.137 | .891 |
| Rihm et al., 2014 Spatial | 21 | 0.71 | 23.4 | Switzerland | SWS | 7.5 | Spatial Location | Declarative | Spatial | Between | Olfactory | 0.95 | 0.08 | 1.82 | 2.134 | .033 |
| Rihm et al., 2014 unpublished False memories | SWS | 7.5 | DRM | Declarative | False Memories | Between | Olfactory | 0.10 | −0.72 | 0.93 | 0.245 | .807 | ||||
| Schönauner et al., 2014 | 28 | NA | 22.3 | Germany | SWS | 3 | Finger Sequence Tapping | Skill | Skill | Within | Nonverbal | 0.47 | 0.09 | 0.85 | 2.432 | .015 |
| Sterpenich et al., 2014 N2 | 28 | 0.50 | 21.6 | Belgium | N2 | 8 | Sound-Negative/Neutral Picture Learning | Declarative | Emotion | Between | Nonverbal | 0.26 | −0.46 | 0.98 | 0.703 | .482 |
| Sterpenich et al., 2014 REM | 28 | 0.50 | 21.6 | Belgium | REM | 8 | Sound-Negative/Neutral Picture Learning | Declarative | Between | Nonverbal | −1.01 | −1.78 | −0.24 | −2.586 | .010 | |
| Ai et al., 2015 | 46 | 0.59 | 22.0 | China | SWS | 1.5 | Fear Extinction | Conditioning | Fear | Between | Nonverbal | −0.71 | −1.30 | −0.12 | −2.370 | .018 |
| Creery et al., 2015 | 20 | 0.60 | 21.0 | US | SWS | 1.5 | Spatial Location | Declarative | Spatial | Within | Nonverbal | 0.25 | −0.18 | 0.68 | 1.157 | .247 |
| ^He et al., 2015 3 mins | 35 | 0.49 | 23.8 | China | SWS | 4 | Fear Extinction | Conditioning | Fear | Between | Nonverbal | 1.94 | 1.15 | 2.73 | 4.805 | < .001 |
| ^He et al., 2015 10 mins | 31 | 0.55 | 23.6 | China | SWS | 4 | Fear Extinction | Conditioning | Fear | Between | Nonverbal | 4.83 | 3.44 | 6.21 | 6.838 | < .001 |
| Hu et al., 2015 | 38 | 0.50 | 21.8 | US | SWS | 1.5 | Counter-bias Learning | Others | Within | Nonverbal | 0.51 | 0.18 | 0.84 | 3.011 | .003 | |
| Rihm & Rasch. 2015 N2 | 14 | 1.00 | 23.3 | Switzerland | N2 | 4 | Evaluative Conditioning | Conditioning | Emotion | Within | Nonverbal | −0.11 | −0.61 | 0.39 | −0.445 | .656 |
| Rihm & Rasch. 2015 REM | 16 | 1.00 | 23.3 | Switzerland | REM | 4.5 | Evaluative Conditioning | Conditioning | Within | Nonverbal | 0.07 | −0.41 | 0.55 | 0.287 | .774 | |
| Schreiner & Rasch, 2015 | 15 | 0.53 | 25.1 | Switzerland | SWS | 3 | Word-Word Learning (Foreign) | Declarative | Language | Within | Verbal | 0.50 | −0.04 | 1.05 | 1.821 | .069 |
| Schreiner, Lehmann et al., 2015 Control | 16 | 0.63 | 23.3 | Switzerland | SWS | 3 | Word-Word Learning (Foreign) | Declarative | Language | Within | Verbal | 0.82 | 0.28 | 1.37 | 2.962 | .003 |
| Schreiner, Lehmann et al., 2015 Correct | 14 | 0.71 | 22.7 | Switzerland | SWS | 3 | Word-Word Learning (Foreign) | Declarative | Language | Within | Verbal | 0.77 | 0.20 | 1.34 | 2.659 | .008 |
| Schreiner, Lehmann et al., 2015 False | 13 | 0.69 | 21.2 | Switzerland | SWS | 3 | Word-Word Learning (Foreign) | Declarative | Language | Within | Verbal | 0.95 | 0.32 | 1.57 | 2.964 | .003 |
| Cairney et al., 2016 Direct | 30 | 0.53 | 19.9 | UK | SWS | 1.5 | Spatial Location | Declarative | Spatial | Within | Nonverbal | 0.42 | 0.05 | 0.78 | 2.247 | .025 |
| Cairney et al., 2016 Indirect | SWS | 1.5 | Picture-Word Learning | Declarative | Associative | Within | Nonverbal | −0.03 | −0.38 | 0.32 | −0.167 | .867 | ||||
| Cousins et al., 2016 | 22 | 0.36 | 23.5 | UK | SWS | 8 | SRTT | Skill | Skill | Within | Nonverbal | 0.40 | −0.03 | 0.83 | 1.837 | .066 |
| Diekelmann et al., 2016 | 36 | 0.53 | 21.9 | Germany | SWS | 8 | SRTT | Skill | Skill | Between | Olfactory | 0.35 | −0.31 | 1.00 | 1.044 | .297 |
| Groch et al., 2016 Adolescent | 21 | 0.29 | 12.3 | Switzerland | SWS | 8 | CBM | Declarative | CBM | Within | Verbal | 0.31 | −0.11 | 0.74 | 1.440 | .150 |
| Groch et al., 2016 Adult | 19 | 0.74 | 22.2 | Switzerland | SWS | 8 | CBM | Declarative | CBM | Within | Verbal | 0.30 | −0.15 | 0.75 | 1.300 | .193 |
| Honma et al., 2016 | 16 | 0.75 | 20.1 | US | SWS | 8 | Rubber Hand Illusion | Others | Within | Nonverbal | 0.98 | 0.38 | 1.58 | 3.209 | .001 | |
| Laventure et al., 2016 N2 | 39 | 0.41 | 25.1 | Canada | N2 | 8 | Finger Sequence Tapping | Skill | Skill | Between | Olfactory | 0.94 | 0.27 | 1.61 | 2.734 | .006 |
| Laventure et al., 2016 REM | 37 | 0.41 | 24.7 | Canada | REM | 8 | Finger Sequence Tapping | Skill | Between | Olfactory | 0.27 | −0.38 | 0.93 | 0.825 | .409 | |
| Lehmann et al., 2016 SWS Emotion | 21 | 0.76 | 22.1 | Switzerland | SWS | 3 | Word-Emotional Picture Learning | Declarative | Emotion | Within | Verbal | 0.54 | 0.10 | 0.98 | 2.394 | .017 |
| Lehmann et al., 2016 SWS Neutral | SWS | 3 | Word-Neutral Picture Learning | Declarative | Associative | Within | Verbal | 0.16 | −0.25 | 0.57 | 0.756 | .450 | ||||
| Lehmann et al., 2016 REM Emotion | 20 | 0.80 | 22.3 | Switzerland | REM | 6 | Word-Emotional Picture Learning | Declarative | Within | Verbal | 0.21 | −0.22 | 0.63 | 0.944 | .345 | |
| Lehmann et al., 2016 REM Neutral | REM | 6 | Word-Neutral Picture Learning | Declarative | Within | Verbal | −0.25 | −0.68 | 0.18 | −1.150 | .250 | |||||
| Rihm et al., 2016 | 36 | 0.89 | 25.7 | Switzerland | SWS | 1.5 | Phobia Exposure Therapy | Others | Fear | Between | Olfactory | 0.21 | −0.43 | 0.85 | 0.650 | .516 |
| Batterink & Paller, 2017 | 35 | 0.66 | 22.4 | US | SWS | 1.5 | Artificial Language Learning | Declarative | Language | Between | Verbal | 0.76 | 0.09 | 1.44 | 2.231 | .026 |
| Batterink et al., 2017 Nonverbal | 16 | 0.54 | 20.7 | US | SWS | 1.5 | Sound-Word Learning (Foreign) | Declarative | Language | Within | Nonverbal | −0.07 | −0.54 | 0.39 | −0.307 | .759 |
| Batterink et al., 2017 Verbal | 10 | 0.54 | 20.7 | US | SWS | 1.5 | Word-Word Learning (Foreign) | Declarative | Language | Within | Verbal | −0.03 | −0.60 | 0.53 | −0.118 | .906 |
| Cairney et al., 2017 Exp. 1 Nonverbal | 28 | 0.00 | 20.3 | UK | SWS | 8 | Sound-Word Learning | Declarative | Associative | Within | Nonverbal | 0.47 | 0.09 | 0.85 | 2.428 | .015 |
| Cairney et al., 2017 Exp. 1 Verbal | SWS | 8 | Speech-Word Learning | Declarative | Associative | Within | Verbal | 0.55 | 0.17 | 0.94 | 2.798 | .005 | ||||
| Cairney et al., 2017 Exp. 2 Nonverbal | 23 | 0.00 | 21.0 | UK | SWS | 8 | Sound-Word Learning | Declarative | Associative | Within | Nonverbal | 0.54 | 0.11 | 0.96 | 2.479 | .013 |
| Cairney et al., 2017 Exp. 2 Verbal | SWS | 8 | Speech-Word Learning | Declarative | Associative | Within | Verbal | −0.18 | −0.58 | 0.21 | −0.905 | .365 | ||||
| Farthouat et al., 2017 | 14 | 0.64 | 22.4 | Belgium | SWS | 1.5 | Word-Word Learning | Declarative | Associative | Within | Verbal | 0.26 | −0.26 | 0.77 | 0.980 | .327 |
| Groch et al., 2017 a Control | 13 | 0.31 | 13.2 | Switzerland | SWS | 8 | CBM | Declarative | CBM | Within | Verbal | 0.03 | −0.49 | 0.56 | 0.120 | .904 |
| Groch et al., 2017 a Social Anxiety | 13 | 0.62 | 13.4 | Switzerland | SWS | 8 | CBM | Declarative | CBM | Within | Verbal | 0.16 | −0.36 | 0.68 | 0.604 | .546 |
| Groch et al., 2017b | 16 | 0.69 | 20.3 | Switzerland | SWS | 8 | Picture-Word Learning | Declarative | Associative | Within | Verbal | 0.14 | −0.36 | 0.63 | 0.534 | .594 |
| Hennies et al., 2017 | 28 | 0.39 | 22.7 | UK | SWS | 8 | Memory Abstraction | Declarative | Between | Nonverbal | −1.00 | −1.77 | −0.24 | −2.570 | .010 | |
| Oyarzún et al.,2017 Contiguous | 22 | 0.73 | 23.2 | Spain | SWS | 1 | Spatial Location | Declarative | Spatial | Within | Nonverbal | 0.24 | −0.16 | 0.65 | 1.171 | .242 |
| Oyarzún et al.,2017 Delayed | 28 | 0.71 | 23.2 | Spain | SWS | 1 | Spatial Location | Declarative | Spatial | Within | Nonverbal | −0.44 | −0.82 | −0.06 | −2.285 | .022 |
| Pereira et al., 2017 | 29 | 0.62 | 24.2 | Brazil | N2 | 1.5 | Finger Sequence Tapping | Skill | Skill | Between | Tactile | −0.57 | −1.30 | 0.16 | −1.541 | .123 |
| Tamminen et al., 2017 | 20 | 0.80 | 19.3 | UK | SWS | 1.5 | Word-Word Learning | Declarative | Associative | Within | Verbal | −0.13 | −0.56 | 0.29 | −0.622 | .534 |
| Ai et al., 2018 | 47 | 0.85 | 23.4 | China | N2 | 1.5 | Auction decision making | Others | Within | Verbal | 0.66 | 0.35 | 0.97 | 4.124 | < .001 | |
| Antony et al., 2018a Separate Spatial | 30 | 0.73 | 26.5 | US | SWS | 1.5 | Spatial Location | Declarative | Spatial | Within | Nonverbal | 0.37 | 0.01 | 0.73 | 2.007 | .045 |
| Antony et al., 2018a Separate Associative | SWS | 1.5 | Sound-Picture Learning | Declarative | Associative | Within | Nonverbal | −0.04 | −0.38 | 0.31 | −0.200 | .842 | ||||
| Antony et al., 2018a Competitive Spatial | 30 | 0.70 | 26.5 | US | SWS | 1.5 | Spatial Location | Declarative | Spatial | Within | Nonverbal | −0.17 | −0.52 | 0.18 | −0.949 | .343 |
| Antony et al., 2018a Competitive Associative | SWS | 1.5 | Sound-Picture Learning | Declarative | Associative | Within | Nonverbal | −0.09 | −0.44 | 0.26 | −0.493 | .622 | ||||
| Antony et al., 2018b Spatial | 18 | 0.50 | 21.8 | US | SWS | 1.5 | Spatial Location | Declarative | Spatial | Within | Nonverbal | 0.54 | 0.06 | 1.01 | 2.223 | .026 |
| Antony et al., 2018b Associative | SWS | 1.5 | Sound-Picture Learning | Declarative | Associative | Within | Nonverbal | 0.10 | −0.34 | 0.54 | 0.443 | .658 | ||||
| Ashton et al., 2018 Negative Associative | 19 | 0.68 | 22.0 | UK | SWS | 1.5 | Sound-Negative Picture Learning | Declarative | Emotion | Within | Nonverbal | 0.03 | −0.40 | 0.46 | 0.150 | .881 |
| Ashton et al., 2018 Neutral Associative | SWS | 1.5 | Sound-Neutral Picture Learning | Declarative | Associative | Within | Nonverbal | 0.10 | −0.33 | 0.53 | 0.447 | .655 | ||||
| Ashton et al., 2018 Negative Spatial | SWS | 1.5 | Spatial Location_ Negative Picture | Declarative | Emotion | Within | Nonverbal | −0.33 | −0.77 | 0.11 | −1.457 | .145 | ||||
| Ashton et al., 2018 Neutral Spatial | SWS | 1.5 | Spatial Location_ Neutral Picture | Declarative | Spatial | Within | Nonverbal | −0.22 | −0.66 | 0.22 | −0.987 | .324 | ||||
| Cairney et al., 2018 | 27 | 0.70 | 19.7 | UK | SWS | 1.5 | Word-Picture Learning | Declarative | Associative | Within | Verbal | 0.50 | 0.09 | 0.90 | 2.420 | .016 |
| Cordi et al., 2018 | 23 | 0.65 | 71.0 | Switzerland | SWS | 3 | Word-Word Learning (Foreign) | Declarative | Language | Within | Verbal | 0.06 | −0.33 | 0.46 | 0.309 | .758 |
| Johnson et al., 2018 | 9 | 0.56 | 27.9 | US | SWS | 8 | Target Throwing | Skill | Skill | Between | Nonverbal | 1.85 | 0.36 | 3.34 | 2.441 | .015 |
| Klinzing et al., 2018 Acetycholine | 15 | 0.00 | 23.9 | Germany | SWS | 0.67 | Spatial Location | Declarative | Spatial | Within | Olfactory | 0.42 | −0.08 | 0.92 | 1.628 | .103 |
| Klinzing et al., 2018 Control | 14 | 0.00 | 23.9 | Germany | SWS | 0.67 | Spatial Location | Declarative | Spatial | Within | Olfactory | 0.98 | 0.36 | 1.59 | 3.128 | .002 |
| Seibold et al., 2018 | 19 | 0.63 | 22.1 | Germany | SWS | 0.67 | Spatial Location | Declarative | Spatial | Within | Olfactory | 0.22 | −0.22 | 0.66 | 0.969 | .333 |
| Shanahan et al., 2018 | 18 | 0.61 | 25.1 | US | SWS | 1.25 | Spatial Location | Declarative | Spatial | Within | Olfactory | 0.33 | −0.15 | 0.81 | 1.355 | .175 |
| Shimizu et al., 2018 | 37 | 0.43 | 25.1 | US | SWS | 1.5 | Spatial Navigation | Declarative | Spatial | Between | Nonverbal | 1.34 | 0.64 | 2.05 | 3.750 | < .001 |
| Simon et al., 2018 | 18 | 0.78 | 20.2 | US | SWS | 8 | Directed Forgetting | Declarative | Within | Nonverbal | 0.74 | 0.24 | 1.25 | 2.891 | .004 | |
| 2Strachan et al., 2018, preprint | 23 | 0.52 | 21.3 | UK | SWS | 1.5 | Trust Learning | Others | Within | Nonverbal | 0.12 | −0.28 | 0.52 | 0.584 | .559 | |
| Göldi & Rasch, 2019 | 66 | NA | 21.9 | Switzerland | Unspecified | 8 | Word-Word Learning | Declarative | Within | Verbal | −0.09 | −0.33 | 0.15 | −0.752 | .452 | |
| Göldi et al., 2019 | 16 | 0.81 | 20.9 | Switzerland | SWS | 3 | Word-Word Learning (Foreign) | Declarative | Language | Within | Verbal | 0.52 | 0.02 | 1.02 | 2.030 | .042 |
| Humiston & Wamsley 2019 | 31 | 0.52 | 19.5 | US | SWS | 1.5 | Counter-bias Learning | Others | Within | Nonverbal | −0.06 | −0.41 | 0.29 | −0.333 | .739 | |
| Johnson et al., 2019 | 25 | 0.52 | 26.0 | US | SWS | 1 | Target Throwing | Skill | Skill | Between | Nonverbal | 0.82 | 0.00 | 1.63 | 1.966 | .049 |
| Vargas et al., 2019 | 24 | NA | 21 | US | SWS | 1.5 | Spatial Location | Declarative | Spatial | Within | Nonverbal | 0.24 | −0.17 | 0.64 | 1.148 | .251 |
| Wang, Antony et al., 2019 | 24 | 0.58 | 22.3 | US | SWS | 1.5 | Spatial Location | Declarative | Spatial | Within | Nonverbal | 0.30 | −0.10 | 0.69 | 1.466 | .143 |
| Bar et al., 2019, preprint | 19 | 0.58 | 27.4 | Israel | SWS | 2 | Spatial Location | Declarative | Spatial | Within | Olfactory | 0.21 | −0.23 | 0.64 | 0.934 | .350 |
| Cheng et al., unpublished | 20 | 0.70 | 20.5 | US | SWS | 1.5 | Motor Learning | Skill | Skill | Within | Nonverbal | 0.53 | 0.08 | 0.99 | 2.280 | .023 |
| Gao et al., 2019 abstract | 41 | 0.70 | 21.2 | US | SWS | 8 | Lecture Learning | Declarative | Between | Nonverbal | 0.39 | −0.30 | 1.07 | 1.110 | .267 | |
| Schechtman et al., 2019, preprint | 31 | 0.68 | 20.8 | US | SWS | 1.5 | Spatial Location | Declarative | Spatial | Within | Nonverbal | 0.53 | 0.16 | 0.89 | 2.809 | .005 |
| Cousins, 2014, Dissertation Chapter 5, Exp. 1 | 15 | 0.73 | 20.3 | UK | SWS | 8 | DRM | Declarative | False Memories | Within | Nonverbal | 0.13 | −0.35 | 0.61 | 0.513 | .608 |
| Cousins, 2014, Dissertation Chapter 5, Exp. 2 | 16 | 0.44 | 23.9 | UK | SWS | 8 | DRM | Declarative | False Memories | Within | Verbal | −0.20 | −0.67 | 0.27 | −0.831 | .406 |
| Konrad, 2014, Dissertation Exp. 3 | 11 | 0.00 | 22.5 | Germany | SWS | 1 | Method of Loci | Declarative | Associative | Within | Nonverbal | 0.10 | −0.44 | 0.65 | 0.369 | .712 |
| Vargas, 2016, Dissertation Exp. 1 | 14 | NA | 25.5 | US | SWS | 1.5 | Reality-Monitoring | Declarative | False Memories | Within | Nonverbal | 0.04 | −0.46 | 0.53 | 0.139 | .889 |
| Vargas, 2016, Dissertation Exp. 2 | 16 | NA | 22.5 | US | SWS | 1.5 | Word-Picture Learning | Declarative | Associative | Within | Verbal | −0.03 | −0.50 | 0.43 | −0.138 | .890 |
Notes: NA: not available. REM: rapid eye movement, SWS: slow-wave sleep. Sleep length is given in hours. SRTT: serial reaction time task; DRM: Deese-Roediger-McDermott false memory task; CBM: cognitive bias modification. CI: confidence interval. UL and LL refer to the upper and lower limit of the 95% confidence interval of effect sizes. When age range instead of mean age was reported, we calculated the midpoint of the age range as an estimate of the sample’s mean age.
Indicates statistical outliers based on studentized residuals. For details of outlier and influence case analyses, please see SOM.
Rasch et al. (2007) Exp. 5, the effect size was calculated based on the comparison between sleep- and wake-TMR.
Strachan et al., 2018, preprint was coded as “unpublished” in the analyses as we finished the literature search in June, 2019. This paper was subsequently published in July, 2019, and was updated in the reference list as Strachan et al. (2019).
A Quantitative Assessment of TMR
To date, over 90 TMR experiments have been performed on humans. These studies can inform our current understanding of what domains of learning are especially amenable to benefit from sleep reactivation. In addition, certain experimental factors may influence the effectiveness of TMR, including sleep stage when sensory cues are presented (SWS vs. REM, Lehmann et al., 2016; Rasch et al., 2007; N2 vs. REM, Laventure et al., 2016; Sterpenich et al., 2014; N2 vs. SWS, Belal et al. 2018), memory strength prior to sleep (Cairney, Lindsay, Sobczak, Paller & Gaskell, 2016; Creery et al., 2015), amount of prior knowledge (Groch, Schreiner, Rasch, Huber & Wilhelm, 2017), and degree of competition between memories (Antony et al., 2018a; Oyarzún et al., 2017). Review articles by Oudiette and Paller (2013), Schouten and colleagues (2017), Cellini and Capuozzo (2018), and Paller and colleagues (in press) have aptly summarized the breadth of topics investigated using the procedure, yet no quantitative summary of experimental effects exists. Narrative reviews typically adopt a vote-counting approach in summarizing existing evidence, taking TMR results as either significant or not (Cellini & Capuozzo, 2018, Table 1; Schouten et al., 2017; Tables 2–4). Despite its appealing simplicity, this vote-counting approach can be misleading because null results and inconsistent findings are attributed to sampling errors or procedural variations in a descriptive rather than in a quantitative manner (Siddaway, Wood & Hedges, 2018). In contrast, meta-analytic approaches synthesize all available effect sizes, while taking statistical power and precision of estimates into consideration to quantitatively estimate the effectiveness of specific procedures. Moreover, by partitioning effect sizes into different categories, moderator analyses in a meta-analysis can advance theoretical understanding of how experimental factors may influence memory consolidation, such as sleep stages (NREM vs. REM), learning types (declarative vs. skill learning), and how learning outcomes are measured (recall vs. recognition etc.).
Here, we aggregated all available datasets to provide evidence relevant for assessing the effect size of memory benefits produced by TMR. First, we aimed to provide an overall estimate of the TMR effect. We then planned a series of moderator analyses to address the aforementioned questions. Our foremost research question concerns whether TMR is specific to certain cueing stages, such as N2, SWS, REM, and wakeful states. Another potentially important question never directly examined in any single study is whether TMR effectiveness varies as a function of sleep duration (ranging from 0.67 hours to 8 hours). This variable can be examined in a meta-analysis because it aggregates studies with different sleep durations.
We compared effects on different types of learning, based on current theorizing in memory research. Learning tasks were categorized into either declarative memory, skill acquisition, conditioning, or other types of learning. The last category includes studies that cannot easily be grouped into conventional categories, such as phobia-exposure therapy, social learning, multisensory integration, value-based decision making, etc. In addition to learning tasks, we coded how TMR may differentially influence various outcome measurements such as 1) recall that relies on cued or free recall testing, 2) recognition in discriminating old and new items, 3) behavioral performance when memory is not explicitly probed, such as speed and accuracy during RT-based tasks, or problem solving, 4) subjective ratings when participants are asked to self-report how they feel and think regarding mnemonic materials, and 5) skin conductance response, SCR.
In another analysis, we investigated whether TMR effects varied as a function of within- versus between-subject designs, and whether TMR effectiveness differed as a function of sensory stimulation modality (auditory_verbal, auditory_nonverbal, or olfactory cues). Our hope is that the results from these analyses will serve as a resource for future parameter selection and lessen ambiguity concerning boundary conditions of effective TMR application.
Lastly, acknowledging that learning tasks vary, we conducted focal analyses to examine subsets of studies with homogeneous learning tasks combined with NREM TMR. We identified the following topics: spatial learning, associative learning, language acquisition, false memories, and skill learning. We additionally investigated cognitive bias modifications, emotional memories, and fearful memories, given the potential clinical benefit of improving symptoms associated with mood- and trauma-related disorders. For example, because TMR can reactivate and bias memories regarding potential interpretation of ambiguous scenes (Groch et al., 2016; Groch et al., 2017), it may be useful for reducing habitual negative biases observed in depressive and anxiety disorders (Hallion & Ruscio, 2011). Compared with overall analyses that span a range of different tasks and conditions, focal analyses with relatively homogenous procedures can be advantageous because estimated effect sizes can help guide future research on similar topics.
Method
We relied on two meta-analysis handbooks, Lipsey and Wilson (2001) and Borenstein, Hedges, Higgins and Rothstein (2009), as our primary references in each stage of implementing the meta-analysis. We also followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement of Moher et al. (2009) and their 27-item meta-analysis checklist to guide our meta-analysis and preparation of the manuscript (see supplementary online materials SOM for the PRISMA statement).
Literature Search
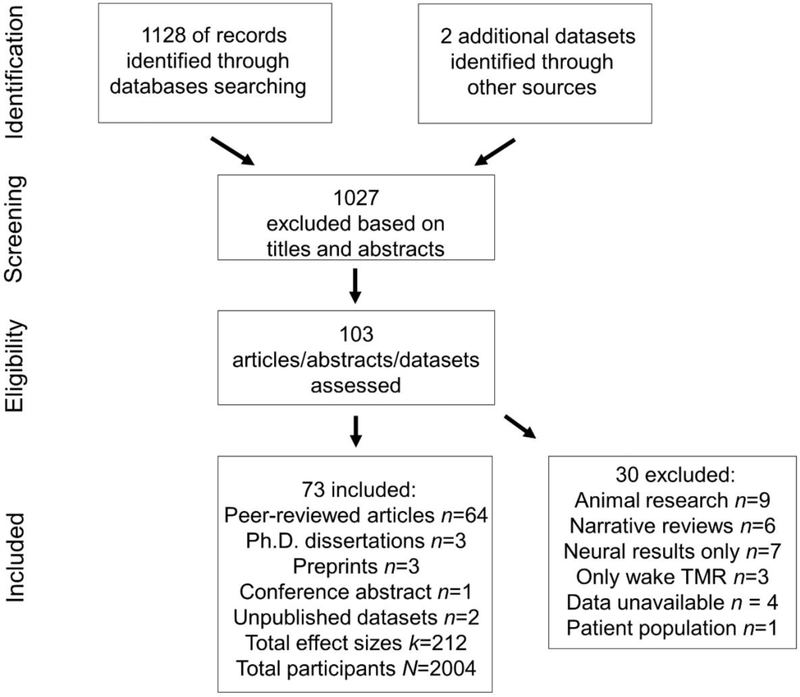
Figure 2 depicts a PRISMA flowchart of the literature search. To strive for an exhaustive list of datasets, we followed three steps. First, we conducted searches with online databases including Web of Science, PsycINFO (via ProQuest, including journals/books/dissertations/theses), PubMed, and bioRxiv/PsyArxiv through June, 2019 with key words referring to memory reactivation and sleep. Exact key words using Boolean operators are (targeted memory reactivation OR memory reactivation OR memory cueing OR memory replay) AND (sleep OR N2 OR slow-wave sleep OR SWS OR NREM OR REM). In this way, we collected (1) peer-reviewed published and in-press research articles, (2) unpublished dissertations/theses, and (3) preprints uploaded to repositories (i.e., bioRxiv, PsyArxiv). Unpublished dissertations and preprints were included to attempt to weigh against publication bias. In the second step, we contacted researchers who had previously published on TMR or on sleep and memory consolidation to solicit unpublished datasets and under-review manuscripts. We included these identified unpublished datasets and manuscripts in the meta-analysis (some of the manuscripts were either subsequently published or overlapping with unpublished dissertations identified earlier). In Step 3, we checked the reference sections from related review articles to identify missing references (Aarons, 1976; Cellini & Capuozzo, 2018; Diekelmann & Born, 2010; Oudiette & Paller, 2013; Rasch & Born, 2013; Schouten, Pereira, Tops & Louzada, 2017; Stickgold & Walker, 2013). All authors checked and agreed on the final reference list.
Figure 2:
A PRISMA flow chart of literature search and inclusion.
Inclusion/Exclusion Criteria
We applied the following inclusion/exclusion criteria to select studies for this meta-analysis. First, sensory stimulation must have been applied to reactivate prior learning instead of inducing novel learning or EEG activity change (e.g., Arzi et al., 2012; Arzi et al., 2014; Antony & Paller, 2017; Dillon &Bowles, 1976; Ngo et al., 2013; Züst, Ruch, Wiest & Henke, 2019). Second, given that our primary research question concerns sleep TMR, we excluded articles that only examined wake TMR (Alm, Ngo & Olson, 2019; Schreiner & Rasch, 2015; Tambini, Berners-Lee & Davachi, 2017). Third, we only included studies that used human participants, excluding the few nonhuman animal TMR studies that have been published (e.g., Barnes & Wilson, 2014; Bender & Wilson, 2012; Purple, Sakurai & Sakaguchi, 2017; Rolls et al., 2013). Fourth, studies must have reported behavioral effects, excluding articles that only examined neural mechanisms of TMR (e.g., Batterink, Creery & Paller, 2016). Lastly, sufficient statistical details must have been available to extract relevant effect sizes (means, SD, F, and t). When statistical details were not reported in the text, we either contacted corresponding authors to request relevant data or extracted needed data from published figures in the article using “metaDigitise” (Pick, Nakagawa & Noble, 2018).
Coding of Study Characteristics
Coding was conducted by the first author and double-checked by the second author. Disagreements were resolved through discussions. Interrater reliability was calculated with Cohen’s Kappa coefficient (Cohen, 1960), using “ICC” package in R (Wolak, 2015). In general, raters showed high consistency, with a range of κ from 0.94 to 1.00. We coded each experiment based on three aspects: publication status, sample characteristics, and experimental design characteristics. For publication status, we coded each experiment with 1) publication year, 2) publication type (peer-reviewed journal article, dissertation, conference abstract, preprint, and unpublished dataset), and 3) publication status (journal articles coded as published, with all remaining coded as unpublished). Regarding sample characteristics, we coded each experiment with 1) sample size, 2) gender ratio, 3) mean age, and 4) country of origin.
Regarding experimental design characteristics, we first coded each experiment based on TMR cueing stages, such that whether TMR was administered during N2, SWS, REM, unspecified (i.e., when TMR was administered without EEG monitoring), or wakefulness. If cues were delivered during both N2 and SWS, the study was coded as SWS, and all N2 and SWS TMR studies were further combined as NREM. We then coded sleep duration as a continuous variable on how long participants were given to sleep, ranging from 0.67 to 8 hours.
Learning tasks used in each experiment were categorized as declarative memory, skill learning, conditioning, and other types of learning. We then examined each outcome measurement, and coded them into one of five categories: recall, recognition, behavioral performance, subjective ratings, and SCR.
Lastly, we coded whether TMR was administered using a between- or a within-subject design, and which sensory modality was used in TMR cueing, including auditory_nonverbal vs. auditory_verbal vs. olfactory cues.
Following moderator analyses, we conducted focal analyses based on tasks and experimental conditions of interest, as opposed to the all-inclusive nature of the main analyses. Specifically, we selected TMR studies focusing on spatial learning that used spatial object-location tasks and navigation tasks (e.g., Rasch et al., 2007; Rudoy et al., 2009; Shanahan et al., 2018; Shimizu et al., 2018). A second topic covered associative learning tasks in which participants learned stimuli pairings (e.g., spoken words/sounds to be paired with words/pictures, e.g., Cairney, Sobczak, Lindsay & Gaskell, 2017; Cairney et al., 2018; Fuentemilla et al., 2013). A third topic included TMR studies that examined language learning, including foreign vocabulary acquisition, grammatical learning, and generalization (e.g., Batterink & Paller, 2017; Cordi, Schreiner & Rasch, 2018; Schreiner & Rasch, 2015a, 2017). For false memories, identified tasks typically used either Deese-Roediger-McDermott procedures or reality monitoring tasks (Cousins, 2014, unpublished dissertation; Rihm, Diekelmann, Born & Rasch, unpublished dataset; Vargas, 2018 unpublished dissertation). In addition to these analyses focused on declarative memories, we examined studies involving skill learning because of their implications in enhancing motor performance and thus motor rehabilitation. We planned to focus on performance measures of reaction speed and accuracy (e.g. Antony et al., 2012; Cousins et al., 2016; Laventure et al., 2016), as well as explicit knowledge of motor sequences in skill learning (e.g., Cousins et al., 2014; Diekelmann et al., 2016). Lastly, we synthesized effect sizes from studies with translational implications in clinical settings, namely cognitive bias modification (e.g., Groch et al., 2016; Groch et al., 2017), emotional memories (e.g., Ashton et al., 2018; Cairney et al., 2014; Lehmann et al., 2016; Rihm & Rasch, 2015), and fearful memories (e.g., Ai et a., 2015; Hauner et al., 2013; He et al., 2015). Coding of study characteristics and categorization of focal analyses can be found in Table 1 and in SOM.
Effect Size Calculation
To calculate effect sizes, we used equations recommended in Dunlap, Cortina, Vaslow and Burke (1996), Lakens’s (2013, with spreadsheet available at https://osf.io/vbdah/), and Morris and DeShon (2002). In TMR research, effect sizes are best captured by comparing post-minus-pre-sleep performance changes between cued versus uncued conditions in terms of standardized mean differences (i.e., the Cohen’s d family). For both within- and between-subject designs, we calculated effect sizes based on mean and SDs as a common metric to (a) allow direct comparisons and moderator analyses across within- and between-subject designs and (b) avoid the risk of inflated effect sizes and false-positive rates (Dunlap et al., 1996; Lakens, 2013, Table 1; Morris & DeShon, 2002). Across the whole sleep TMR dataset, 96.7% (205 out of 212) of effect sizes were calculated based on means and SDs.
In a within-subject TMR study, participants receive both cued and uncued treatments within a single sleep session (e.g., Rudoy et al., 2009), or in two sleep sessions if the design calls for counterbalanced sleep manipulations (Rasch et al., 2007). For within-subject designs, we searched for post- minus pre-sleep memory change scores for cued and uncued conditions and their associated SDs, respectively. Means and associated SDs for cued and uncued conditions’ change scores were used to calculate the TMR cueing effect in terms of Cohen’s dav, as recommended for meta-analyses (Lakens, 2013, Formula 10 and Table 1). If means and S.D.s (or S.E.s) were not reported nor available, then we searched for statistical tests that examined the effects. Such statistical tests can be reported in one of the three following forms: 1) a within-subject ANOVA that reported a 2 (pre- vs. post-sleep) by 2 (cued vs. uncued) interaction; 2) a paired-sample t-test that compared changes in memory scores (over sleep) for cued and uncued items; or 3) a paired-sample t-test that compared cued vs. uncued post-sleep memory scores (in these cases, the post-sleep memory performance was scaled to the corresponding pre-sleep memory performance, see Rasch et al., 2007). Based on these statistics, we transformed the reported F-values from the two-way interaction (with one-degree of freedom tests), or the t-values from the paired-sample t-tests to Cohen’s dz (see Lakens, 2013, Formula 7; Morries & DeShon, 2002, p118, Formula 28).
When a between-subject design was used, participants in the experimental TMR group received sensory cues to reactivate prior learning, whereas participants in the control group received learning-incongruent sensory cues or no cues at all (e.g., He et al., 2015; Rihm, Diekelmann, Born & Rasch, 2014; Sterpenich et al., 2014). Here, to calculate TMR effect sizes, we preferentially chose the incongruent cue control group over the no-stimulation group to make sensory stimulation constant between groups. The no-stimulation group was used when this was the only control group available, or when there were multiple TMR experiments and thus multiple control groups were needed (as in Sterpenich et al., 2014, when both N2 and REM TMR were examined). For between-subject TMR studies, we searched for the pre- vs. post-sleep memory change scores from the experimental and control group and their associated S.D.s. The change scores and the associated S.D.s for experimental and control groups were used to calculate effect size in terms of Cohen’s ds (Lakens, 2013, Formula 1). When means and S.D.s/S.E.s were not reported in the article, we again searched for key statistical tests that examined TMR effects. Here, the effect could be tested in a mixed 2 (between-subject variable: TMR vs. control groups) by 2 (within-subject variable, pre- vs. post-sleep) ANOVA. Alternatively, the TMR effect could be derived from an independent sample t-test comparing post-sleep memory performance between the experimental and control groups, or comparing pre- vs. post-sleep memory change scores between the two groups. We then transformed the F- and the t-values from these statistical tests to calculate effect sizes in Cohen’s ds (Lakens, 2013, Formula 2; Morries & DeShon, 2002, p118, Formula 27).
Lastly, as effect sizes in Cohen’s d are upward biased with small samples (Cummings, 2012; Lakens, 2013, p.5), we employed Hedges’ g correction function to all individual effect sizes: Hedges’ g = Cohen’s d * (1-(3/(4*df-1))), where df denotes degree of freedom reported in the statistical test (Hedges, 1981, see also Borenstein et al., 2009; Formula 4.22).
Publication Bias Analyses
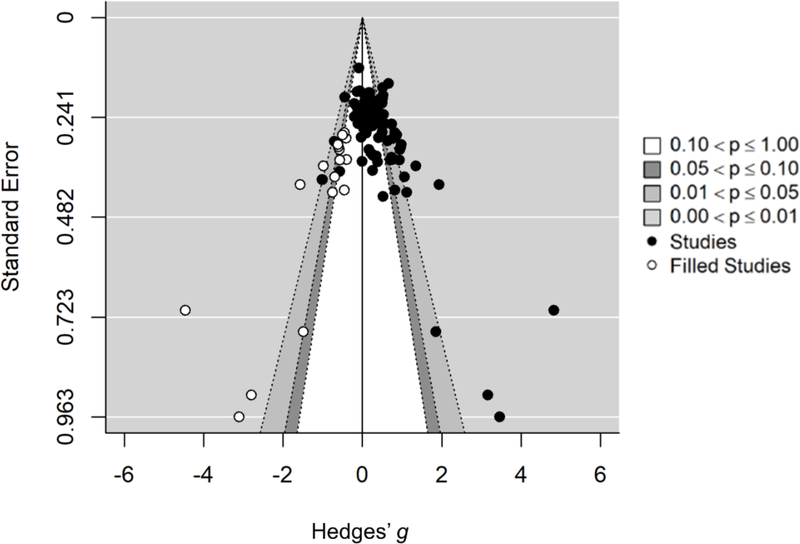
We employed a variety of methods to investigate how publication bias may influence the estimated effect sizes from sleep TMR research. We first used a funnel plot to display effect sizes against their standard errors. According to Egger and colleagues (Egger, Smith, Schneider & Minder, 1997), existence of publication bias can be detected through an asymmetric funnel plot because low-powered positive findings are more likely to be published than equally powered negative findings.
Second, we employed the Trim-Fill method (Duval & Tweedie, 2000), which imputes artificial effect sizes to make the funnel plot symmetric, and then calculated corrected effect sizes. Third, we used publication status (published vs. unpublished) as a categorical moderator to assess whether published studies have significantly larger effect sizes than unpublished studies.
Fourth, we chose the three-parameter likelihood selection model (Iyengar & Greenhouse, 1988), which extends the original selection model proposed by Hedges (1984) in estimating and correcting publication bias. The three-parameter model includes not only the synthesized effect size as a parameter, but also considers the heterogeneity across effect sizes, and the probability of nonsignificant studies to be published calculated by the maximum likelihood function. In the current study, the three-parameter selection model was set as a one-tailed model with the probability of publishing nonsignificant studies with a step function cut-off at p = .025 by maximum likelihood, following the assumption that directionally consistent and statistically significant studies are more likely to be published. Notably, this three-parameter selection model shows promising performance to adjust effect size in conditions varying in the synthesized effect size, heterogeneity, sample size, and the extent of publication bias across different simulation studies (Carter et al., 2019; McShane, Böckenholt, & Hansen, 2016).
Fifth, we employed a selection model with a priori weight functions that could model four different scenarios of publication biases: moderate one-tailed selection, severe one-tailed selection, moderate two-tailed selection, and severe two-tailed selection (Vevea & Woods, 2005). This analysis is advantageous because it shows how estimated effect size may change based on the different magnitudes of publication biases. The specification of priori weights follows the implementation of Vevea and Woods (2005).
Meta-analytic Procedure
We chose a three-level random-effects model over a fixed-effects model. This choice of model is based on the following reasoning.
First, TMR research is characterized by experimental procedures with particular memory tasks administered in conjunction with TMR during different sleep stages. Therefore, we expected considerable heterogeneity across studies.
Second, a random-effects model assumes heterogeneity due to systematic variance among studies, above and beyond sampling error. A random-effects model will thus generate larger standard errors than fixed-effects models, which will lead to more conservative findings and reduced false positives in both overall effect-size estimates and moderator analyses.
Third and most importantly, many TMR experiments have reported more than one measure of memory performance, which violates the key assumption of data independence in typical random-effect models (Borenstein et al., 2011; Lipsey & Wilson, 2001). As an extension of the random-effects model, multilevel modelling can model both within- and between-study variance and thus can address the issue of dependencies (Van den Noortagte & Onghena, 2003). In short, we employed the multilevel modelling to model three levels of variance: 1) variances due to sampling error, 2) within-study variances among multiple effect sizes from the same experiment, and 3) between-study variances among different experiments.
Meta-analytical Computation
Individual effect sizes and corresponding variance measures at an outcome level were calculated in the Comprehensive Meta-analysis software Version 3.3.070 (Biostate, Englewood, NJ, 2014) in Hedges’ g. These values were then fed into the multilevel modelling using R package “metaphor” (Viechtbauer, 2010). To examine how much effect sizes varied from each other in the multilevel modelling, we used Cochran’s Q statistic to test whether individual effect size would vary significantly across the whole dataset (i.e., heterogeneity, Borenstein et al., 2011; Cheung, 2014). A significant Q statistic indicates significant heterogeneity across studies that cannot be explained by sampling error. We report between-studies I2 that denotes that among observed variance across the whole dataset, how much variance in proportional terms is due to differences in true effect sizes between studies rather than sampling error (Higgins & Thompson, 2002). We report τ2 that denotes the variance of estimated effect sizes at an experiment level, with τ indicates standard deviation.
Results
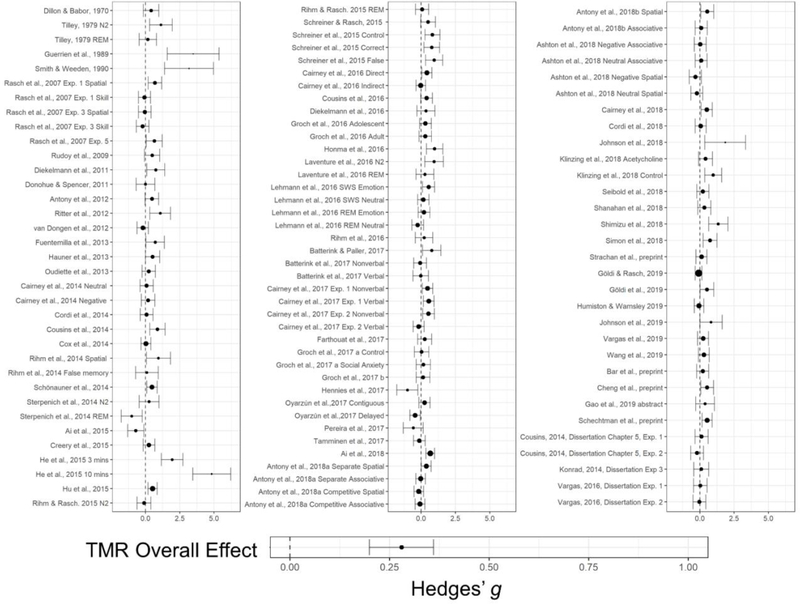
The search and selection process of applicable datasets are shown in the Figure 2 PRISMA flowchart. Included articles can be found in the reference section and are marked with asterisks. Sample and experimental characteristics of included experiments are shown in Table 1, with corresponding effect sizes provided in both Table 1 and Figure 3. All study information and the associated effect size at an outcome level are available in SOM. All data and analysis code can be found in https://osf.io/kg8y3/.
Figure 3:
A forest plot displaying sleep TMR effect sizes calculated from each experiment at a task level, matching descriptive from Table 1. The overall TMR effect was presented, calculated from a random effects model using task-level effect sizes from the forest plot and Table 1.
Study and Sample Characteristics
We collected 73 articles/abstracts/datasets, which contain n=91 experiments with 111 independent samples. The total number of participants was 2004. This dataset contributed k=212 effect sizes to the meta-analysis, with each experiment contributing 2.33 effect sizes on average. Across the whole dataset, the mean sample size for each experiment was 22, with an average age of 23 years old. The mean age within single experiments ranged from 13- to 71-year-old populations, thus covering adolescent, adult, and aging populations. Of these experiments, 51 were conducted in Europe, 31 in North America, 5 in Asia, and 1 in South America. Neither age (β=−0.003, 95% CI: [−0.020, 0.015], p=.747) nor female: male ratio (β=−0.443, 95% CI: [−0.940, 0.055], p=.081) had a significant impact on TMR effects.
Overall Sleep TMR Effects and Publication Bias Analyses
Across all TMR sleep experiments/conditions, sleep TMR showed a significant effect influencing learning with Hedges’ g= 0.29, 95% CI: [0.21, 0.38], Z=6.711, p<.001. Despite this significant TMR effect, there was considerable heterogeneity across effect sizes as revealed by heterogeneity analysis, Q(211)= 588, I2=71%, p<.001, with τ2 =0.112 at an experimental level (i.e., between-experiment, level-3), τ2=0.031 at an outcome level (i.e., within-experiment, level-2). This heterogeneity across studies, and the finding that 71% of variances reflects true differences across effect sizes instead of sampling errors, strongly suggests that TMR effects must be compared across experimental conditions.
Regarding publication biases, Egger’s test showed that the funnel-plot was significantly asymmetric, Z= 8.489, p<.001, indicating the existence of publication biases (Figure 4). With the Trim-and-Fill method, 17 artificial effect sizes were imputed to adjust for potential biases. For the overall sleep TMR effect, the adjusted effect size was still significantly above zero, Hedges’ g=0.18, 95% CI: [0.06, 0.30], Z=2.944, p=.003.
Figure 4:
A contour-enhanced funnel plot displaying all effect sizes at experiment levels (solid circles) from sleep TMR research. Y-axis indicates standard errors of effect sizes, x-axis indicates magnitudes of effect sizes in terms of Hedges’ g. Imputed effect sizes calculated from the Trim-and-Fill analysis are displayed in open circles.
When publication status (yes vs. no) was examined in the moderator analysis, we found that publication status did not significantly influence effect sizes Q(1)=1.005, p=.316, with unpublished studies (k=26) associated with a positive yet nonsignificant effect size, Hedges’ g=0.18, 95% CI: [−0.06, 0.42], Z=1.447, p=.148, while published studies (k=186) had a significant effect size, Hedges’ g=0.31, 95% CI: [0.22, 0.41], Z=6.563, p<.001.
Results from the three-parameter selection model again showed a significant adjusted effect size, with Hedges’ g= 0.13, 95% CI: [0.06, 0.21], Z= 3.472, p<.001. Lastly, employing the selection models with a priori weight functions to model different magnitudes of publication selection processes (Vevea & Woods, 2005), we found that sleep TMR appeared smaller, but remained significant under various scenarios of publication biases: Hedges’ g=0.21 for moderate two-tailed selection; g=0.17 for severe two-tailed selection; g=0.15 moderate one-tailed selection, except in the severe one-tailed selection: g=−0.05.
Moderator Analyses
Because moderator and focal analyses will have fewer effect sizes available, potential outliers and influential cases may significantly influence results. We thus excluded data designated as statistical outliers (studentized residuals smaller or larger than 3, k=4, with 2 from SWS TMRs and 2 from REM TMRs, with all outliers’ studentized residuals larger than 3, i.e. significantly larger TMR effects). We then conducted influential case analyses to identify effect sizes that exert considerable influence on the analyses (see Vechtbauer & Cheung, 2010). Influential cases (k=2) matched those designated as statistical outliers. This left 208 effect sizes in the sleep TMR analysis. In wake TMR, two influential cases were identified and were excluded from the subsequent analyses. Outliers and influential case analyses can be found in SOM.
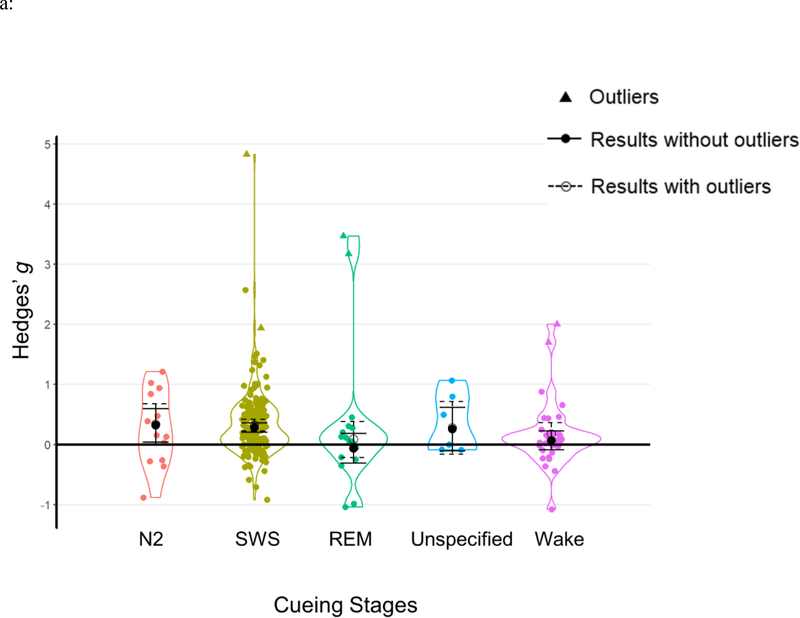
TMR cueing stage
Our first question concerns whether the TMR effect was specific to certain cueing stages. As described in the Methods section (see also Table 1), we coded TMR cueing stages into five categorical moderators: N2 (k=13), SWS (k=174), REM (k=15), unspecified (k=6), and wake (k=30). Results show that cueing stage had a significant influence on TMR effects Q(4)=10.744, p=.03. Specifically, TMR was only significant during the two NREM stages: N2 and SWS. In contrast, TMR was ineffective when cueing was administered during REM, or when TMR was not supervised by EEG monitoring, or during wakefulness (see Table 2a, Figure 5a).
Table 2a.
Statistics from cueing stages moderator analyses.
| Moderators | n(N) | k | Hedges’ g | 95% CI | QB | Z | p |
|---|---|---|---|---|---|---|---|
| Cueing Stages | 10.744 | .030 | |||||
| N2 | 6 (165) | 13 | 0.32 | [0.04, 0.60] | 2.232 | .026 | |
| SWS | 70 (1471) | 174 | 0.27 | [0.20, 0.35] | 6.934 | <.001 | |
| REM | 7 (142) | 15 | −0.06 | [−0.31, 0.18] | −0.501 | .616 | |
| Unspecified | 4 (140) | 6 | 0.26 | [−0.11, 0.62] | 1.383 | .167 | |
| Wake | 18 (366) | 30 | 0.07 | [−0.09, 0.23] | 0.853 | .394 |
Figures 5:
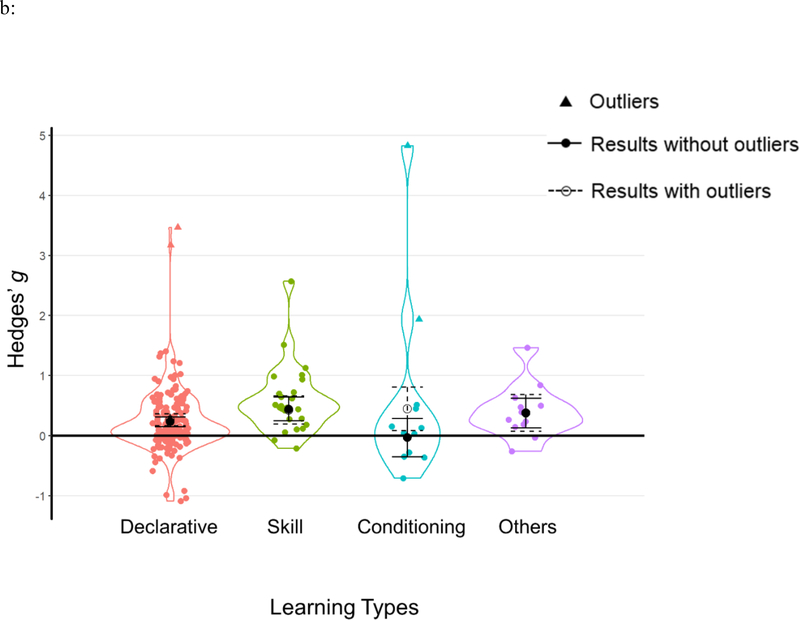
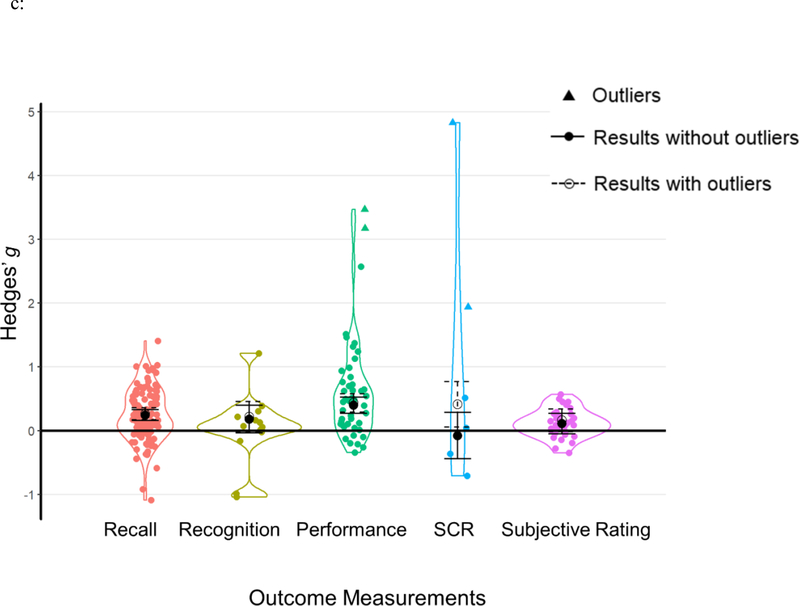
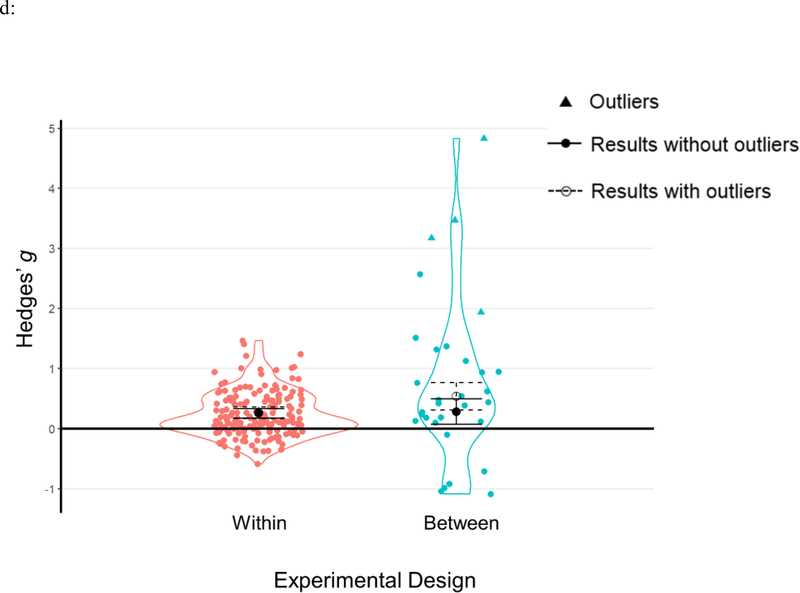
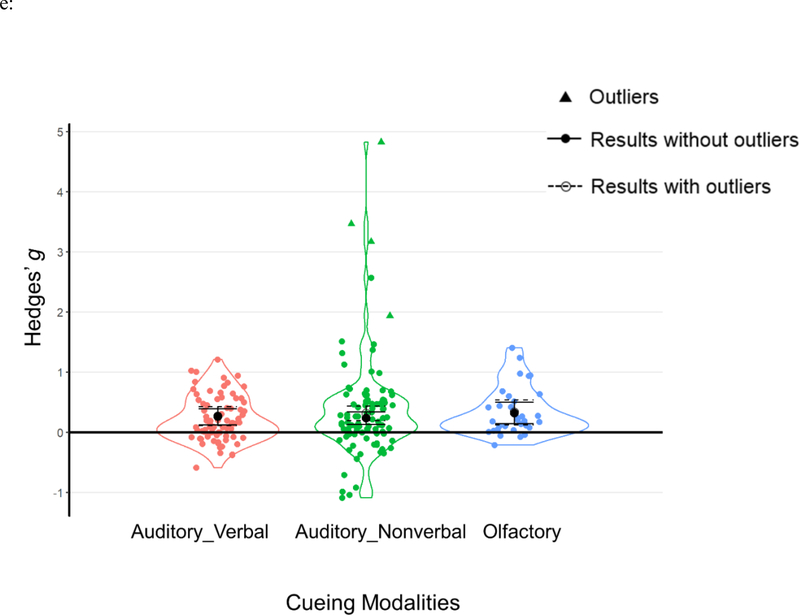
Results of moderator analyses from a) cueing stages; b) learning types; c) outcome measurements; d) experimental designs and e) cueing modalities. Each data point represents an individual effect size at an outcome level. Statistical outliers are the same as those indicated in Table 1 and are marked as triangles. The figure displays aggregated effect sizes from each moderator analyses, with error bars representing 95% CIs. The figure displays both results without outliers (solid lines with solid circles) and results including all data points (dashed lines with open circles).
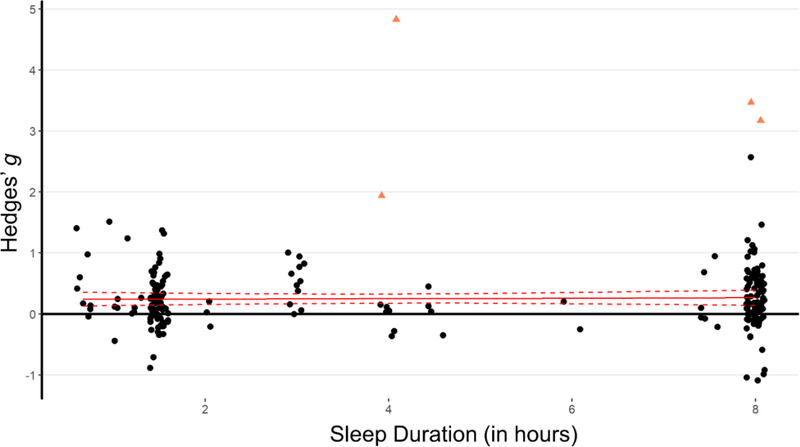
Sleep duration
We then coded sleep duration as a continuous variable, ranging from 0.67 hours’ nap to 8 hours’ overnight sleep. We entered sleep duration as a predictor, with TMR effect as the dependent variable in a meta-regression model. Results showed that sleep duration did not significantly influence TMR effects, β=0.003, 95% CI [−0.022, 0.028], p=.795 (see Figure 6).
Figure 6:
A meta-regression analysis revealed no relationship between sleep length and TMR effects. Statistical outliers are the same as those indicated in Table 1 and are marked as triangles. The regression line (the solid line) and its 95% confidence intervals (the dashed lines) were calculated from the meta-regression model without outliers.
In the following moderator analyses, we further excluded 1) unspecified TMR experiments because procedurally, this line of research deviates significantly from other TMR experiments during which sleep is monitored by EEGs (k=6, Dillon & Babor, 1970; Donohue & Spencer, 2011; Göldi & Rasch, 2019; Ritter, Strick, Bos, van Baaren & Dijksterhuis, 2012), and 2) one tactile stimulation TMR study (k=2, Pereira et al., 2017) because it is the only tactile TMR study available, which limits conclusions concerning comparisons with other TMR studies.
Learning types
Following current theories regarding memory systems, we categorized learning tasks into four categories: declarative memory (k=153), skill learning (k=25), conditioning (k=10), and the other types of learning (k=12). Descriptions of memory tasks and their assigned categories can be found in Table 1. Results showed that TMR effects varied significantly among different learning types; Q(3)=8.056, p=.045. Specifically, TMR influenced all types of learning except for conditioning (see Table 2b, Figure 5b).
Table 2b.
Statistics from learning and outcome measurements moderator analyses.
| Moderators | n(N) | k | Hedges’ g | 95% CI | QB | Z | p |
|---|---|---|---|---|---|---|---|
| Learning Type | 8.056 | .045 | |||||
| Declarative | 62 (1219) | 153 | 0.23 | [0.15, 0.31] | 5.563 | <.001 | |
| Skill | 12 (283) | 25 | 0.44 | [0.25, 0.64] | 4.438 | <.001 | |
| Conditioning | 4 (91) | 10 | −0.03 | [−0.35, 0.29] | −0.200 | .841 | |
| Others | 6 (191) | 12 | 0.38 | [0.13, 0.62] | 2.991 | .003 | |
| Outcome Measurements | 11.132 | .025 | |||||
| Recall | 61 (1137) | 103 | 0.24 | [0.16, 0.33] | 5.676 | <.001 | |
| Recognition | 9 (157) | 14 | 0.18 | [−0.04, 0.40] | 1.619 | .105 | |
| Performance | 27 (673) | 46 | 0.40 | [0.27, 0.53] | 6.103 | <.001 | |
| SCR | 4 (91) | 4 | −0.08 | [−0.44, 0.28] | −0.423 | .672 | |
| Subjective Rating | 8 (135) | 33 | 0.11 | [−0.05, 0.27] | 1.355 | .175 |
Outcome measurements
Based on how TMR research measured behavioral outcomes, we categorized each outcome into the following categories: recall (k=103), recognition (k=14), performance (k=46), SCR (k=4), and subjective ratings (k=33). Specific outcomes and their assigned categories can be found in the SOM. Results showed that TMR effects varied significantly depending on how outcomes were assessed, Q(4)=11.132, p=.025. Specifically, TMR had a significant effect on recall and performance measurements, while it had a nonsignificant effect on recognition, SCR, and subjective ratings (see Table 2b, Figure 5c).
TMR design
There was no significant difference between these two types of design, Q(1)=0.055, p=.814. Both between- and within-subject designs were associated with significant and highly comparable TMR effects (see Table 2c, Figure 5d).
Table 2c.
Statistics from experimental designs and cueing modalities moderator analyses.
| Moderators | n(N) | k | Hedges’ g | 95% CI | QB | Z | p |
|---|---|---|---|---|---|---|---|
| Experimental Design | 0.055 | .814 | |||||
| Within | 68 (1303) | 173 | 0.25 | [0.17, 0.34] | 6.192 | <.001 | |
| Between | 14 (446) | 27 | 0.28 | [0.07, 0.50] | 2.595 | .009 | |
| Cueing Modality | 0.688 | .709 | |||||
| Auditory_Verbal | 25 (472) | 74 | 0.26 | [0.13, 0.39] | 3.825 | <.001 | |
| Auditory_Nonverbal | 42 (956) | 94 | 0.23 | [0.13, 0.34] | 4.365 | <.001 | |
| Olfactory | 17 (372) | 32 | 0.32 | [0.15, 0.50] | 3.566 | <.001 |
Notes: n, number of experiments/datasets; N, number of participants; k, number of effect sizes.
Cueing modality
All three TMR cueing modalities—auditory_nonverbal, auditory_verbal, and olfactory cues—were associated with significant and comparable TMR effects: Q(2)=0.688, p=.709 (see Table 2c, Figure 5e).
Focal Analyses
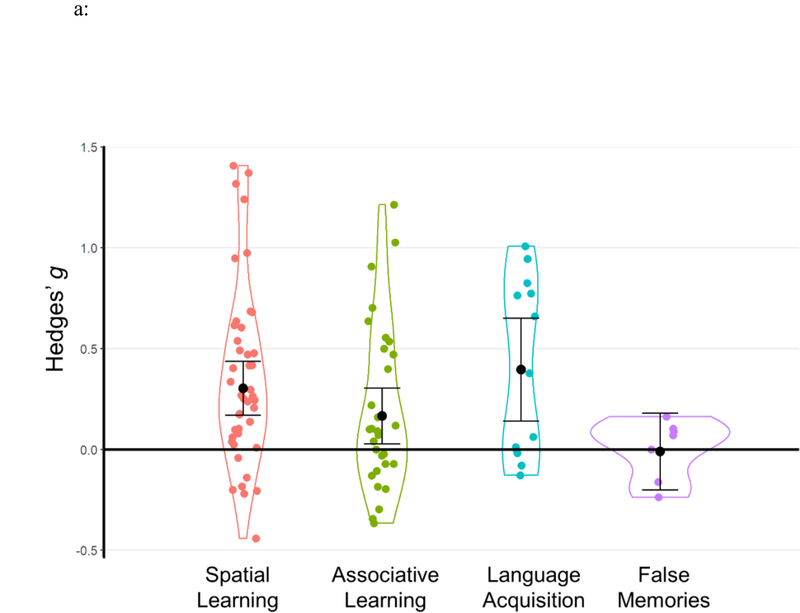
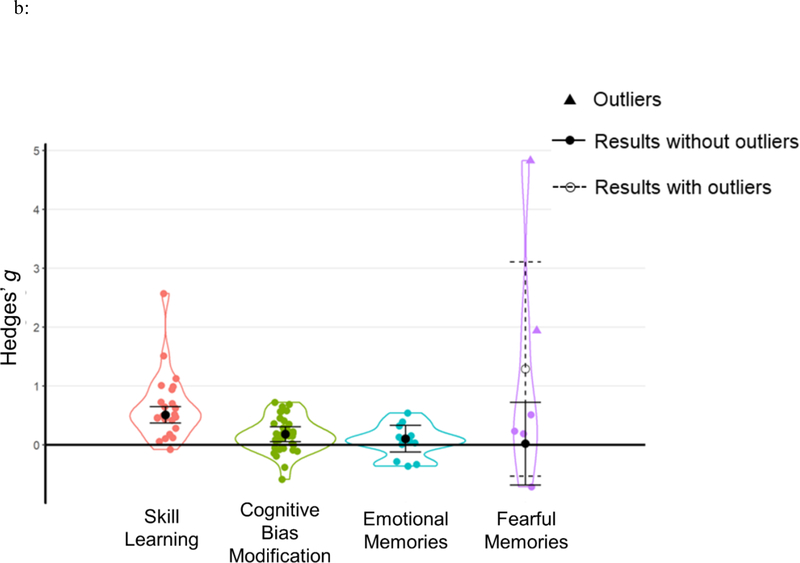
In this section, we present a set of analyses that segregated subsets of relatively homogenous TMR studies in terms of sleep cueing stages and memory tasks. Because only N2 and SWS TMR effects were significant, we combined these experiments as NREM TMR (note that the two outliers from NREM TMR and the tactile N2 TMR study was not included in focal analyses). Focal analyses includes the following categories: spatial learning (k=43), associative learning (k=30), language acquisition (k=13), false memories (k=7), skill learning (k=23), cognitive bias modification (k=36), emotional memories (k=12), and fearful memories (k=4). Results are displayed in Figure 7. Studies included can be found in Table 1, with effect sizes at an outcome level reported in SOM. We present these analyses in a descriptive manner rather than making strong conclusions.
Figure 7:
Results of focal analyses. Each data point represents an individual effect size at an outcome level. Statistical outliers are the same as those indicated in Table 1 and are marked as triangles. The figure displays aggregated effect sizes based on each focal analysis, with error bars representing 95% CIs. For fearful memories, the figure displays both result without outliers (the solid line with a solid circle) and result including all data points (the dashed line with an open circle).
Spatial memories
In spatial learning tasks, participants learned spatial locations of objects on a 2-D grid and practiced placing the objects on the grid followed by feedback (e.g., Rasch et al., 2007; Rudoy et al., 2009). We identified 26 experiments and 43 effect sizes. For this category, TMR during NREM significantly enhanced spatial memories, Hedges’ g=0.30, 95% CI [0.17, 0.44], Z=4.439, p<.001 (see Table 3, Figure 7a).
Table 3.
Statistics from focal analyses.
| Focal analyses | n(N) | k | Hedges’ g | 95% CI | Z | p |
|---|---|---|---|---|---|---|
| Spatial Learning | 26 (553) | 43 | 0.30 | [0.17, 0.44] | 4.439 | <.001 |
| Associative Learning | 16 (320) | 30 | 0.17 | [0.03, 0.30] | 2.354 | .019 |
| Language Acquisition | 9 (158) | 13 | 0.40 | [0.14, 0.65] | 3.046 | .002 |
| False Memories | 4 (66) | 7 | −0.01 | [−0.20, 0.18] | −0.103 | .918 |
| Skill Learning | 10 (229) | 23 | 0.51 | [0.37, 0.65] | 7.108 | <.001 |
| Cognitive Bias Modification | 4 (66) | 36 | 0.18 | [0.06, 0.31] | 2.832 | .005 |
| Emotional Memories | 5 (97) | 12 | 0.10 | [−0.12, 0.33] | 0.905 | .366 |
| Fearful Memories | 3 (97) | 4 | 0.02 | [−0.68, 0.72] | 0.059 | .953 |
Notes: n, number of experiments/datasets; N, number of participants; k, number of effect sizes.
Associative learning
Associative learning tasks involve learning associations between two stimuli (e.g., word/sound-word/picture pairings). Participants memorized associations between two stimuli and then attempted to recall the second member of a pair given the first (Cairney et al., 2018; Fuentemilla et al., 2013). We found that TMR during NREM sleep significantly improved associative learning in these tasks, Hedges’ g=0.17, 95% CI [0.03, 0.30], Z=2.354, p=.019 (see Table 3, Figure 7a).
Language acquisition
This analysis included two lines of research. For vocabulary acquisition, participants memorized novel words (e.g., from a second language) that were paired with words from participants’ native language. During sleep, the second-language words were presented to reactivate the associated memories (e.g., Batterink et al., 2017; Cordi et al., 2018; Schreiner & Rasch, 2015; Schreiner et al., 2015). For grammatical learning and generalization, participants extracted grammatical regularities by learning nonword sequences based on feedback (Batterink & Paller, 2017). Eight experiments reported nine effect sizes, and results suggest that TMR can significantly promote language acquisition in these circumstances, Hedges’ g=0.40, 95% CI [0.14, 0.65], Z=3.046, p=.002 (see Table 3, Figure 7a).
False memories
For this category, TMR was used during sleep to determine whether cues could enhance false memories. We identified four experiments that examined this type of question (Rihm et al., 2014, unpublished dataset; Cousins, 2014, unpublished dissertation, Chapter 5, Experiments 1 and 2; Vargas, 2016, unpublished dissertation, Experiment 1). None of the single studies found a significant impact of TMR on false memories. Overall, TMR failed to influence false memories during sleep, Hedges’ g = −0.01, 95% CI [−0.20, 0.18], Z=−0.103, p=.918 (see Table 3, Figure 7a).
Skill learning
Studies typically included in the skill-learning category are included in this analysis. We focused on measures that sometimes are indicative of implicit performance, namely speed and accuracy, but the range of designs used does not permit any general claims about whether learning was implicit or explicit. Generally, a positive TMR effect would indicate faster or more accurate performance in a motor task. With 18 effect sizes, TMR during NREM enhanced motor performance with a Hedges’ g=0.54, 95% CI [0.38, 0.69], Z=6.782, p<.001. For comparison purposes, we also analysed TMR’s impact on explicit knowledge of skill learning as assessed by explicit memory of motor sequence. With five effect sizes, TMR significantly improved conscious recall of motor sequences with a Hedges’ g=0.41, 95% CI [0.04, 0.78], Z=2.156, p=.031 (see Table 3, Figure 7b).
Cognitive bias modifications
Employing a picture-word learning task in which words could be used to disambiguate interpretation of an ambiguous picture, Groch et al. (2016) investigated whether memories of positive or negative words could be reactivated during sleep, aiming to change interpretations of the ambiguous scenes. This procedure has been used in adolescents and adults, those who are healthy, and those with social anxiety (Groch et al., 2016; Groch et al, 2017). With 36 effect sizes, we found that TMR during NREM significantly changed participants’ memory biases with Hedges’ g = 0.18, 95% CI [0.06, 0.31], Z=2.832, p=.005 (see Table 3, Figure 7b).
Emotional memories
TMR has been used to influence consolidation of emotional memories in both associative learning and spatial learning paradigms. In the current analyses, we did not find an overall effect of TMR on emotional memories: Hedges’ g = 0.10, 95% CI [−0.12, 0.33], Z=0.905, p=.366 (see Table 3, Figure 7b).
Fearful memories
Researchers have employed TMR to modulate fear memories during NREM sleep. For example, TMR was applied to aid in fear extinction (Ai et al., 2015; Hauner et al., 2013) and exposure therapy for phobia (Rihm et al., 2016). In the current analyses, TMR did not induce fear extinction during sleep: Hedges’ g=0.02, 95% CI [−0.68, 0.72], Z=0.059, p=.953(see Table 3, Figure 7b). Given that sleep could potentially influence fear learning either by strengthening associations or enhancing extinction, we also ran an analysis considering TMR effects irrespective of directions. Results showed that TMR significantly modulates fearful memories (Hedges’ g=0.44, Z=2.911, p=.004).
Discussion
Forming enduring memories may depend critically on brain mechanisms whereby learned information is spontaneously reactivated, such as during subsequent sleep (Paller et al., in press). Although spontaneous memory reactivation has been indirectly observed during human sleep (e.g., Deuker et al., 2013; Peigneux et al., 2004), methods to directly manipulate this reactivation should be utilized to promote further understanding, both in human and nonhuman experiments. The method of targeted memory reactivation (TMR), by altering memory processing during sleep, may not only advance our understanding of sleep-based memory consolidation, but may also bear significant translational implications for enhancing various types of learning. For the first time, by collecting a comprehensive dataset of studies and conducting a multilevel random-effects meta-analysis, we have provided an overall assessment of TMR’s effectiveness. In addition, because this dataset comprised studies using a variety of experimental manipulations, we were able to provide additional information by evaluating the influence of factors such as sleep cueing stages and learning types.
TMR Effect as a Function of Sleep Stage
First, sleep TMR was effective in influencing learning and was associated with a small-to-moderate effect size: Hedges’ g= 0.29. TMR effects are likely not the same in sleep versus wake, as effect sizes from N2 and SWS TMR studies were significantly larger than those from REM and wake TMR studies. On the other hand, there are some reports of significant findings from REM and wake TMR (e.g., Oudiette et al., wake TMR group; Sterpenich et al., 2014 REM TMR group). Given the small number of these studies, additional research is likely to produce modified conclusions with respect to TMR during these two conditions.
Because a meta-analysis aggregates multiple TMR studies, we can investigate questions that would be difficult for a single study to address, such as whether sleep duration may differentially influence TMR effects. We found that sleep duration did not influence TMR effects; TMR benefits memory with cues presented during either afternoon or nocturnal NREM. Some have theorized that SWS followed by REM is helpful (see Batterink et al., 2017; Tamminen et al., 2017), but further data are needed to substantiate this idea.
Because our primary research question concerns TMR during sleep, wake TMR conditions in the present analysis were selected from the identified sleep TMR studies, and they were typically matched with sleep TMR in experimental design features such as timing of cueing and time of testing. It should be noted that only a tiny proportion of the huge number of possible wake conditions have been studied: participants in wake TMR could concurrently perform a working memory task, read a book, watch a movie, rest while mind-wandering, or engage in numerous other activities during wakeful cueing periods. Furthermore, cueing could be followed by new interfering information, as in reconsolidation research that also involves memory reactivation (Forcato, Fernandez & Pedreira., 2014; Nader, Schafe & Le Doux, 2000; Nader & Hardt, 2009; Schiller et al., 2010). Another complication is to account for different types of memory that have been emphasized in such studies, from simple conditioning to complex episodic memory paradigms. All these factors pose challenges in generalizing about wake TMR results. In short, given that different experimental procedures with wake TMR can influence memory results (e.g., Tambini et al., 2017), it would be inappropriate to generalize from the small number of wake TMR findings included in this meta-analysis.
TMR’s Impact on Learning
Sleep has been implicated in many types of learning and memory, within both the declarative and nondeclarative categories (Korman et al., 2007; Plihal & Born, 1997, for comprehensive reviews see Diekelmann & Born, 2010; Rasch & Born, 2013; Walker & Stickgold, 2013). Accordingly, it may not be surprising that TMR during sleep can also influence multiple types of learning. However, individual studies varied greatly and many studies reported null or contradictory findings. For example, TMR failed to have a positive impact on sequential finger tapping when olfactory cues were applied during SWS or REM sleep (Rasch et al., 2007). In contrast with these results, subsequent studies found that reactivating motor learning using auditory cues during N2 or SWS could improve performance (Antony et al., 2012, Cousins et al., 2014; Cousins et al., 2016, Laventure et al., 2016; Schönauer et al., 2014). Importantly, different tasks were examined in these different studies, and further work is needed to clarify the relevance of various task factors.
By synthesizing available evidence from different learning tasks, the current meta-analysis shows that TMR can be effective across many types of learning including tasks of declarative memory, skill learning, other types of learning, but not with conditioning. The present meta-analysis also showed that TMR effects depend on how memories are assessed: TMR effects were significant in recall and performance measures, but appeared less effective using recognition, SCR, and subjective ratings. Subsequent focal analyses showed that TMR during NREM significantly influenced associative learning, spatial memories, language acquisition, cognitive bias modification, and skill learning. In contrast, TMR has not had a clear influence on false memories. In the current dataset, the Deese-Roediger-McDermott and reality-monitoring paradigms were used to induce false memories. Both paradigms are well-established in inducing false memories during wakefulness (Gallo, 2010; Gonsalves & Paller, 2000; Gonsalves et al., 2004). However, the role of sleep in influencing false memories remains unclear, as sleep either enhanced or reduced false memories, with effects moderated by pre-sleep encoding quality and retrieval task (e.g., recognition vs. recall, Diekelmann, Landolt, Lahl, Born & Wagner, 2008; Fenn, Gallo, Margoliash, Roediger & Nusbaum, 2009; Pardilla-Delgado & Payne, 2017; Payne et al., 2009). By reactivating learning episodes during NREM, TMR might be expected to provide some clarification on this question. However, the experiments we included in the meta-analysis failed to further influence false memories during sleep.
The apparent inability of TMR to trigger false memory reactivation may be related to an emphasis on SWS. Previous reports suggested that SWS may play a detrimental role in the formation of false memories (Pardilla-Dalgardo & Payne, 2017; Payne et al., 2009), but on the other hand, Vargas (2016, Experiment 1) found a positive correlation between time in SWS and false memory performance. Whether false memories can be modulated by TMR during REM is currently unknown.
Notably, one recent study used TMR to alter memories to reverse prior learning (Simon et al. 2018), which is in some ways akin to a false memory. A tone associated with forgetting was presented during sleep in conjunction with other sounds such that the associated object memories were weakened. In this way, TMR can be used to induce forgetting for specific memories formed previously.
TMR has also been used to enhance the rubber-hand illusion (Honma, Plass, Brang, Florczak, Grabowecky & Paller, 2016), whereby subjective ownership and proprioceptive drift of a rubber hand was impacted, likely via the integration of multisensory information. This phenomenon is similar to a false memory in the sense that both are illusory. In this case, integration between visual input of the rubber hand and tactile input to participants’ real hand was influenced by TMR during sleep.
Methodological Implications, Statistical Power, and Publication Bias
The TMR methodology provides several advantages for understanding sleep-based memory consolidation. For example, previous research on sleep and memory emphasized comparisons between sleep and wakeful retention intervals to draw inferences about sleep’s influence. The waking condition could consist of an ordinary period of wakefulness or a night of total sleep deprivation. In either case, the waking condition does not provide an ideal contrast for the sleep condition because the two conditions can differ in circadian rhythms, sleep drive, and pre-/post-encoding interference (e.g., Pan & Rickard, 2015). In contrast, TMR’s key experimental manipulation occurs during a specific sleep stage while all other factors are held constant across cued and uncued conditions, including circadian influences and amount of pre- and post-encoding interference.
Furthermore, TMR can be advantageous in terms of statistical power given that memory reactivation can be manipulated during a single sleep session on a within-subject basis. Here, we presented analyses regarding experimental design and cueing modality factors. Regarding between- and within-subject designs, our meta-analysis revealed that both designs were associated with comparable effect sizes in influencing memory with TMR. On average, a between-subject design study would recruit 30 participants, whereas a within-subject design study would recruit 20 participants. This difference in sample size is in keeping with the general rule that within-subject designs provide greater statistical power than do between-subject designs.
Regarding cueing modality, we categorized stimulation into three types: auditory_nonverbal, auditory_verbal, and olfactory. Consistent with individual reports in which verbal and nonverbal cues were directly compared (e.g., Cairney et al., 2017; Batterink et al., 2017), cues from all modalities could impact learning. Interestingly, nonverbal and verbal cues tend to have the similar effect sizes: 0.26 vs. 0.23. The use of verbal cues may greatly expand TMR’s applicability in future studies.
Despite robust sleep TMR benefits across different memory types and experimental paradigms, inspection of the full dataset revealed that more than half of the reported results did not reach statistical significance at the conventional .05 false-positive rate: 72 of 212 sleep TMR effect sizes were significant based on Hedges’ g and the associated 95% CIs. When constraining analyses to NREM TMR studies, 68 out of 189 effect sizes were significant. Given significant TMR effects for sleep and NREM conditions, and in moderator analyses, null results from individual studies can best be attributed to either moderator choices (e.g., cueing during REM, or when recognition or subjective rating was used) or low statistical power in single studies. In order for evidence to accumulate and guide future research effectively, we recommend that studies be designed with relatively high statistical power based on results provided in the current meta-analysis.
Publication bias can arise when significant findings consistent with researchers’ hypotheses are more likely to be published than nonsignificant findings, which poses threats for accurately estimating effect sizes (Rosenthal, 1979). In addition to our efforts to include unpublished datasets, we employed a variety of publication bias adjustment analyses (trim-and-fill, 3-parameter selection model, and the a priori weight functions model) to evaluate the possibility of overestimated effect sizes. Adjusted effect sizes across these analyses remained significant, except for the most extreme case (i.e., the severe, one-tail selection model). These results, when evaluated holistically, suggest that sleep TMR effects are robust against publication biases. However, as recent simulation studies on publication-bias analyses suggested (Carter et al., 2019; McShane et al., 2016), each analysis has limitations and relies on some assumptions. Given significant heterogeneity across effect sizes and the typical sample sizes involved in TMR research, we urge a continued evaluation of possible publication bias. Moreover, to accurately assess TMR in the future, high statistical power and pre-registration strategies are recommended. Lastly, all members of a scientific community, including researchers, reviewers, and journal editors, could work together to combat publication biases by encouraging publication of relevant nonsignificant findings.
Practical Implications
An intriguing possibility for sleep TMR is to complement wakeful learning to enhance cognition and performance (Diekelmann, 2014; Paller, 2017). Among the TMR studies reviewed here, a few topics bear high translational implications in educational and clinical settings. Boosting language acquisition can be particularly meaningful in educational settings. Our dataset included two different types of language studies: vocabulary learning (Batterink et al., 2017; Göldi & Rasch, 2019; Schreiner & Rasch, 2015a; Schreiner et al., 2015) and grammatical learning (Batterink & Paller, 2017). In vocabulary learning, participants associated a foreign or a novel word with its translation in the participants’ native language. Newly learned words were subsequently replayed during sleep to reactive their associated meanings. In grammatical learning, participants viewed nonsense phrases and gradually acquired the underlying grammatical rules via trial-and-error. Generalization was assessed when participants were required to generate correct sequences with new nonsense words (Batterink & Paller, 2017). Overall, TMR during NREM sleep boosted language acquisition, with an effect size of 0.40. Future research could include different age groups, such as young children who are just beginning to gain competence in their native language and sleep a lot.
TMR’s effectiveness in facilitating skill learning has intriguing implications for motor rehabilitation. Individual studies reported that TMR could enhance participants’ speed and/or accuracy (Antony et al, 2012; Laventure et al., 2016), with explicit knowledge of motor sequences in some cases (Cousins et al., 2014; Diekelmann et al., 2016). Importantly, when all effect sizes were considered in the meta-analysis, TMR appeared effective in influencing both performance and knowledge of the learned motor sequences. Future studies can test TMR’s potential for facilitating motor or cognitive rehabilitation among patient populations in clinical settings.
TMR may also hold promise for complementing psychotherapy. In the present meta-analysis, we found that TMR was effective in changing memories with respect to ambiguous scenes (e.g., Groch et al., 2016), but did not influence emotional memories or weaken fearful memories. However, evidence on whether TMR may influence emotional memories and fear extinction was highly mixed (Ai et al., 2015; Ashton et al., 2018; Hauner et al., 2013; He et al., 2015; Lehmann et al., 2016). Results have also been mixed in TMR fear extinction studies in rodents (Barnes & Wilson, 2014; Purple et al., 2017; Rolls et al., 2013). Given the potential clinical relevance, future TMR studies with these types of memory are warranted.
Neural Mechanisms and Theoretical Implications
Investigating TMR-elicited neural activity with EEG and fMRI can help researchers delineate neural mechanisms of memory reactivation and consolidation during sleep. By employing time-frequency analyses to decompose EEG responses, researchers have produced evidence implicating theta rhythms and thalamo-cortical spindle oscillations in memory reactivation and consolidation (e.g., Antony et al., 2018b; Belal et al., 2018; Cairney et al., 2018; Cox et al., 2014; Farthouat et al., 2017; Groch et al., 2017; Laventure et al., 2016; Schreiner et al., 2015; Schreiner et al., 2018; Wang et al., 2019). In particular, decoding cue-elicited brain activities during both wakeful learning and sleep TMR suggests that TMR involves neural patterns resembling prior, wakeful learning content (Belal et al., 2018; Schreiner et al., 2018; Shanahan et al., 2018;). During sleep, TMR-related neural activity could distinguish between distinctive memory representations at a categorical level, with such activity predicting post-sleep memory improvement (Cairney et al., 2018; Wang et al., 2019).
In addition to examining neural activity during sleep (Berkers et al., 2018; Diekelmann, Büchel, Born, & Rasch, 2011; Rasch et al., 2007; Shanahan et al., 2018, von Dongen et al., 2012), researchers also investigated task-related neural activity following sleep TMR. For example, reactivating motor learning during SWS enhanced functional connectivity between caudate nucleus and hippocampus when participants were re-tested on the motor task (Cousins et al., 2016).
In short, beyond behavioral results obtained from the current meta-analysis, neural results can provide additional evidence that TMR promotes consolidation via reactivating prior learning experiences, as described in the active system consolidation hypothesis (Rasch & Born, 2013). Specifically, during NREM sleep, characterized by cortical slow oscillations and thalamocortical spindles, covert memory reactivation can transform newly acquired, hippocampus-dependent learning such that neocortical representations become more stable and resistant to interference.
More research is needed to understand why memory reactivation during sleep is associated with consolidation. Some intriguing clues about relevant neural mechanisms have been obtained to date. For example, TMR cues were found to be more effective when delivered just after spindle refractory periods (Antony et al., 2018b), and less effective when cues were presented closely together (Farthouat et al., 2017; Schreiner et al., 2015). Regarding REM’s role in memory consolidation, although the present meta-analysis did not find a significant REM TMR effect, it remains possible that REM may aid consolidation following reactivation during NREM (Batterink et al., 2017; Tamminen et al. 2017), as proposed in the two-stage sequential processing account (Giuditta, 2014). REM sleep may play an important role for specific types of processing, such as with distant associations, information integration, and emotional memories (Cai et al., 2009; Sterpenich et al., 2014; Tamminen et al., 2017; Wassing, Lakbila-Kamal, Ramautar, Stoffers, Schalkwijk & Van Someren, 2019). Additional studies are warranted to explore the impact of REM sleep on memory processing.
TMR and Reconsolidation
Wake TMR studies resemble reconsolidation studies as both procedures involve encoding, presentation of memory reminders during wakefulness, and subsequent testing. On the other hand, there are notable differences. First, in wake TMR studies memories are typically reactivated shortly after encoding (e.g., within minutes or hours), whereas reconsolidation paradigms tend to reactivate memories following longer delays. Second, wake TMR studies often aim to test whether reactivation during wakefulness stabilizes memories, whereas reconsolidation designs introduce interfering information to modify original memories (Elsey, Van Ast, & Kindt, 2018; Forcato et al., 2014; Kredlow, Unger, & Otto, 2016; Nader et al., 2000; Nader & Hardt, 2009; Schiller et al., 2010). For both types of studies, it is of course essential to consider whether results vary depending on the type of memory examined (e.g., declarative memory vs. conditioning).
Although reactivation could render memories labile and make them susceptible to interfering information, caution must be exercised before inferring that memories were made labile by the experimental manipulation. This issue may be particularly relevant for declarative memories, which may remain modifiable indefinitely (Dudai, 2012). As a case in point, Diekelmann and colleagues (2011) studied TMR followed by interference and found memory impairment after wake TMR, in contrast to the usual memory strengthening effect after NREM TMR. An alternative interpretation for the memory impairment, however, is that wake TMR in this study functioned to blur the temporal distinctiveness of the original information versus the interference information, as odor presentation bridged the two task periods. If the original and interfering information were less temporally distinct in this condition, poorer memory performance would be expected. Therefore, such results do not necessarily provide support for the idea of converting declarative memories into a labile form or for conventional reconsolidation models. Nevertheless, further integration between TMR and reconsolidation research could deepen our understanding of the mechanisms of memory processing, including reactivation, consolidation, and updating.
Limitations and Ethical Concerns
Meta-analysis provides a powerful tool to quantitatively estimate the strength of experimental manipulations, but results that aggregate and summarize a diverse set of paradigms may not be adequate for guiding specific research questions. To overcome this limitation, in addition to presenting syntheses of TMR effects across all experiments and broadly defined topics, we included focal analyses based on selected homogenous manipulations (e.g., NREM only) and learning topics (e.g., associative learning, spatial learning, false memory). These focal analyses could be valuable for providing effect-size estimates pertaining to specific research questions.
Another limitation relates to the way memory tasks were categorized in the learning-type moderator analyses. Many tasks used in TMR studies could not be unambiguously categorized (e.g., trust learning, counter-stereotype learning, multisensory integration, value-based decision making). Furthermore, some tasks placed in one learning category may engage processing that depends on multiple memory systems operative concurrently. For example, artificial grammar learning and other types of statistical learning may involve both implicit learning and declarative memory (e.g., Batterink, Reber, Neville, & Paller, 2015). Some skill learning may engender explicit remembering of motor sequences (i.e., declarative memories) and may engage hippocampal contributions (e.g., Antony et al., 2012; Cousins et al., 2016). It is possible that the combination of explicit and implicit learning of motor sequences makes them more susceptible to memory reactivation during SWS, with corresponding changes in hippocampal-striatal networks (Albouy et al., 2008; Cousins et al., 2016; Walker, Stickgold, Alsop, Gaab & Schlaug, 2005). Acknowledging this limitation, we present individual effect size and variance data for currently available TMR studies with which researchers can re-analyze the data based on any categorization.
TMR may be applicable for many beneficial purposes, but can it also be employed maliciously for mind control? Here it is important to distinguish between new learning and prior learning. People may be able to acquire new information while asleep, but perhaps only in restricted circumstances (e.g., Andrillon et al., 2017; Arzi et al., 2012; Züst et al., 2019). In the case of conditioning during sleep, the idea of introducing new associations without the individual’s awareness parallels the idea of subliminal conditioning while awake. Although the term “sleep learning” usually has the connotation of acquiring new information, the typical process of learning that begins during wakefulness may continue during sleep, in which case sleep is indeed relevant for learning. The effectiveness of TMR is generally contingent on prior learning and associations made with specific cue stimuli (Cairney et al., 2016; Creery et al., 2015). When the learning occurs with a person’s full knowledge and compliance, concerns about mind control are mitigated. However, variants of TMR could be used in future research to attempt to selectively weaken memories (as in Simon et al., 2018), or to change memories, perhaps to the point of creating a false memory or providing a conditioned association that was not present during waking. We thus advocate for the continuing evaluation of ethical concerns as research in this area continues to expand.
Unanswered Questions and Future Directions
Although our results showed that TMR could significantly modify memory processing during NREM sleep, effect sizes varied across studies and tasks, as evidenced by observed heterogeneity. The overall effect size was small to moderate in Cohen’s terms (Cohen, 1988). Thus, one future direction is to investigate how to improve TMR effects. Recent findings indicate that the timing of cue presentation relative to spindles and to the phase of slow oscillations can be critical to the degree of reactivation and consolidation (Antony et al., 2018b; Batterink et al., 2016; Göldi et al., 2019; Shimizu et al., 2018). Thus, a promising research direction will be to test the timing of cueing in relation with slow oscillations and/or spindles via techniques such as closed-loop stimulation.
One intriguing yet unanswered question regards whether targeted and spontaneous memory reactivation entail the same or qualitatively different neural mechanisms. Cueing may simply bias spontaneous reactivation (e.g., Bendor & Wilson, 2012), but there may be important differences. Because neural signals that completely and unequivocally indicate memory reactivation during sleep have not yet been established, this question remains open. Future research could address this question by comparing neural activity associated with targeted versus spontaneous reactivation using various memory paradigms.
Another question about TMR is whether it impacts other learning. That is, if TMR improves memory for cued information, does it harm memory consolidation for information acquired via other recent learning? If some information is reactivated, other information may be less likely to be reactivated. Investigations are limited in that they cannot measure all memories that people recently acquired that might be influenced by TMR. One sense in which memory reactivation can have additional effects is in terms of interrelationships among memories. That is, memory storage may normally involve competition, such that enhanced storage of some information would be expected to have repercussions (Norman, Newman, & Detre, 2007; Paller et al., in press). In this regard, TMR research has begun to examine how competing memories interact during sleep (Antony et al, 2018a; Oyarzún et al. 2017), with evidence showing that competition may weaken memories that are tightly interrelated with cued information.
Many other questions remain to be tested in relation to potential applications of TMR outside the laboratory. One recent study investigated TMR for vocabulary learning in a naturalistic home sleep setting (i.e., unsupervised TMR) using auditory cues presented without EEG monitoring (Göldi & Rasch, 2019). TMR benefits were achieved only among participants for whom sleep was not disturbed by the cues. These results underscore the importance of avoiding arousal from sleep for memory improvement to be observed. Finally, whereas lab TMR studies generally include only one period of sleep with TMR, it will be important to determine whether TMR can have cumulative effects across multiple sleep sessions.
Conclusion
To conclude, by aggregating effect sizes across a comprehensive dataset of TMR research, we present the first quantitative synthesis of the effectiveness of TMR under various conditions. Despite some inconsistent results from single studies, meta-analytical results provide compelling evidence that applying sensory cues during NREM sleep can reactivate associated memories and promote memory consolidation. TMR effects are found across a range of learning domains, including but not limited to declarative memory and skill learning. Whether TMR can be meaningfully beneficial in educational and clinical settings can only be answered via future studies in such settings. We hope this review and meta-analysis will facilitate new studies to advance this exciting field.
Supplementary Material
Public Significance Statements.
Sensory cues can be used to reactivate associated memories during sleep and thus promote memory consolidation. This meta-analysis shows that targeted memory reactivation during sleep can improve memory performance with a small to moderate effect, and that this effect is most clearly evident when memories are reactivated during stages 2 and 3 of non-rapid-eye-movement (NREM) sleep.
Acknowledgements
We thank all investigators who contributed data to the project. The project is supported by the National Natural Science Foundation of China (No. 31700953), Early Career Schema (No. 27610617), and General Research Fund (No. 17601318) of Hong Kong Research Grants Council to X.H., and by the US National Science Foundation grants BCS-1461088 and BCS-1829414, NIH/NINDS grant R01-NS112942, and the Mind Science Foundation.
References
Articles included in the meta-analysis are marked with an asterisk.
- Aarons L (1976). Sleep-assisted instruction. Psychological Bulletin, 83(1), 1–40. 10.1037/0033-2909.83.1.1 [DOI] [PubMed] [Google Scholar]
- *Ai SZ, Chen J, Liu JF, He J, Xue YX, Bao YP, … Shi J (2015). Exposure to extinction-associated contextual tone during slow-wave sleep and wakefulness differentially modulates fear expression. Neurobiology of Learning and Memory, 23, 159–167. doi: 10.1016/j.nlm.2015.06.005 [DOI] [PubMed] [Google Scholar]
- *Ai SZ, Yin Y, Chen Y, Wang C, Sun Y, Tang X, … & Shi J (2018). Promoting subjective preferences in simple economic choices during nap. eLife, 7, e40583. doi: 10.7554/eLife.40583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albouy G, Sterpenich V, Balteau E, Vandewalle G, Desseilles M, Dang-Vu T, … & Peigneux P (2008). Both the hippocampus and striatum are involved in consolidation of motor sequence memory. Neuron, 58(2), 261–272. 10.1016/j.neuron.2008.02.008 [DOI] [PubMed] [Google Scholar]
- Alm KH, Ngo CT, & Olson IR (2019). Hippocampal signatures of awake targeted memory reactivation. Brain Structure and Function, 224(2), 713–726. 10.1007/s00429-018-1790-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrillon T, Pressnitzer D, Léger D, & Kouider S (2017). Formation and suppression of acoustic memories during human sleep. Nature Communications, 8(1), 179 10.1038/s41467-017-00071-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Antony JW (2015). Mechanisms of memory reactivation during sleep. (Unpublished doctoral dissertation). Northwestern University, Evanston, Illinois, U.S.A. [Google Scholar]
- *Antony JW, Cheng LY, Brooks PP, Paller KA, & Norman KA (2018a). Competitive learning modulates memory consolidation during sleep. Neurobiology of Learning and Memory, 155, 216–230. doi: 10.1016/j.nlm.2018.08.007 [DOI] [PubMed] [Google Scholar]
- *Antony JW, Gobel EW, O’Hare JK, Reber PJ, & Paller KA (2012). Cued memory reactivation during sleep influences skill learning. Nature Neuroscience, 15(8), 1114–1116. doi: 10.1038/nn.3152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antony JW, & Paller KA (2017). Hippocampal contributions to declarative memory consolidation during sleep In Hannula D & Duff M (Eds.), The Hippocampus from Cells to Systems (pp. 245–280): Springer. doi: 10.1007/978-3-319-50406-3_9 [DOI] [Google Scholar]
- *Antony JW, Piloto L, Wang M, Pacheco P, Norman KA, & Paller KA (2018b). Sleep spindle refractoriness segregates periods of memory reactivation. Current Biology, 28(11), 1736–1743 e1734. doi: 10.1016/j.cub.2018.04.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arzi A, Holtzman Y, Samnon P, Eshel N, Harel E, & Sobel N (2014). Olfactory aversive conditioning during sleep reduces cigarette-smoking behavior. Journal of Neuroscience, 34(46), 15382–15393. doi: 10.1523/JNEUROSCI.2291-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arzi A, Shedlesky L, Ben-Shaul M, Nasser K, Oksenberg A, Hairston IS, & Sobel N (2012). Humans can learn new information during sleep. Nature Neuroscience, 15(10), 1460–1465. doi: 10.1038/nn.3193 [DOI] [PubMed] [Google Scholar]
- *Ashton JE, Cairney SA, & Gaskell MG (2018). No effect of targeted memory reactivation during slow‐wave sleep on emotional recognition memory. Journal of Sleep Research, 27(1), 129–137. doi: 10.1111/jsr.12542 [DOI] [PubMed] [Google Scholar]
- *Bar E, Arzi A, Perl O, Livne E, Sobel N, Dudai Y, & Nir Y (2019). Local Targeted Memory Reactivation in Human Sleep. bioRxiv, 539114 10.1101/539114 [DOI] [PubMed] [Google Scholar]
- Barnes DC, & Wilson DA (2014). Slow-wave sleep-imposed replay modulates both strength and precision of memory. Journal of Neuroscience, 34(15), 5134–5142. 10.1523/JNEUROSCI.5274-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batterink LJ, Creery JD, & Paller KA (2016). Phase of spontaneous slow oscillations during sleep influences memory-related processing of auditory cues. Journal of Neuroscience, 36(4), 1401–1409. doi: 10.1523/JNEUROSCI.3175-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Batterink LJ, & Paller KA (2017). Sleep-based memory processing facilitates grammatical generalization: Evidence from targeted memory reactivation. Brain and Language, 167, 83–93. doi: 10.1016/j.bandl.2015.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batterink LJ, Reber PJ, Neville HJ, & Paller KA (2015). Implicit and explicit contributions to statistical learning. Journal of Memory and Language, 83, 62–78. doi: 10.1016/j.jml.2015.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Batterink LJ, Westerberg CE, & Paller KA (2017). Vocabulary learning benefits from REM after slow-wave sleep. Neurobiology of Learning and Memory, 144, 102–113. doi: 10.1016/j.nlm.2017.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belal S, Cousins J, El-Deredy W, Parkes L, Schneider J, Tsujimura H, … Lewis P (2018). Identification of memory reactivation during sleep by EEG classification. Neuroimage, 176, 203–214. doi: 10.1016/j.neuroimage.2018.04.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendor D, & Wilson MA (2012). Biasing the content of hippocampal replay during sleep. Nature Neuroscience, 15(10), 1439–1444. doi: 10.1038/nn.3203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkers RM, Ekman M, van Dongen EV, Takashima A, Barth M, Paller KA, & Fernández G (2018). Cued reactivation during slow-wave sleep induces brain connectivity changes related to memory stabilization. Scientific Reports, 8(1), 16958. doi: 10.1038/s41598-018-35287-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borenstein M, Hedges LV, Higgins JP, & Rothstein HR (2011). Introduction to meta-analysis: John Wiley & Sons., Ltd. [Google Scholar]
- Bruce DJ, Evans CR, Fenwick PB, & Spencer V (1970). Effect of presenting novel verbal material during slow-wave sleep. Nature, 225(5235), 873–874. [DOI] [PubMed] [Google Scholar]
- *Cairney SA, Durrant SJ, Hulleman J, & Lewis PA (2014). Targeted memory reactivation during slow wave sleep facilitates emotional memory consolidation. Sleep, 37(4), 701–707. 10.5665/sleep.3572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Cairney SA, Guttesen AAV, El Marj N, & Staresina BP (2018). Memory consolidation is linked to spindle-mediated information processing during sleep. Current Biology, 28(6), 948–954. doi: 10.1016/j.cub.2018.01.087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Cairney SA, Lindsay S, Sobczak JM, Paller KA, & Gaskell MG (2016). The benefits of targeted memory reactivation for consolidation in sleep are contingent on memory accuracy and direct cue-memory associations. Sleep, 39(5), 1139–1150. doi: 10.5665/sleep.5772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Cairney SA, Sobczak JM, Lindsay S, & Gaskell MG (2017). Mechanisms of memory retrieval in slow-wave sleep. Sleep, 40(9). doi: 10.1093/sleep/zsx114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter EC, Schönbrodt FD, Gervais WM, & Hilgard J (2019). Correcting for bias in psychology: A comparison of meta-analytic methods. Advances in Methods and Practices in Psychological Science, 2(2), 115–144. 10.1177/2515245919847196 [DOI] [Google Scholar]
- Cellini N, & Capuozzo A (2018). Shaping memory consolidation via targeted memory reactivation during sleep. Annals of The New York Academy of Sciences, 1426, 52–71, doi: 10.1111/nyas.13855 [DOI] [PubMed] [Google Scholar]
- Cellini N, & Mednick SC (2019). Stimulating the sleeping brain: Current approaches to modulating memory-related sleep physiology. Journal of Neuroscience Methods, 316, 125–136. 10.1016/j.jneumeth.2018.11.011 [DOI] [PubMed] [Google Scholar]
- Cheng LY, Che T, Tomic G, Slutzky MW, & Paller KA Practice in your sleep: Targeted memory reactivation enhances execution of continuous motor skill. Unpublished dataset.
- Cheung MW (2014). Modeling dependent effect sizes with three-level meta-analyses: a structural equation modeling approach. Psychological Methods, 19(2), 211–229. doi: 10.1037/a0032968 [DOI] [PubMed] [Google Scholar]
- Cohen J (1960). A coefficient of agreement for nominal scales. Educational and Psychological Measurement, 20, 37–46. 10.1177/001316446002000104 [DOI] [Google Scholar]
- *Cordi MJ, Diekelmann S, Born J, & Rasch B (2014). No effect of odor-induced memory reactivation during REM sleep on declarative memory stability. Frontiers in Systems Neuroscience, 8, 157 10.3389/fnsys.2014.00157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Cordi MJ, Schreiner T, & Rasch B (2018). No effect of vocabulary reactivation in older adults. Neuropsychologia, 119, 253–261. 10.1016/j.neuropsychologia.2018.08.021 [DOI] [PubMed] [Google Scholar]
- *Cousins JN (2014). The role of post-learning reactivation in memory consolidation. (Unpublished doctoral dissertation). The University of Manchester, Manchester, UK. [Google Scholar]
- *Cousins JN, El-Deredy W, Parkes LM, Hennies N, & Lewis PA (2014). Cued memory reactivation during slow-wave sleep promotes explicit knowledge of a motor sequence. Journal of Neuroscience, 34(48), 15870–15876. doi: 10.1523/JNEUROSCI.1011-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Cousins JN, El-Deredy W, Parkes LM, Hennies N, & Lewis PA (2016). Cued reactivation of motor learning during sleep leads to overnight changes in functional brain activity and connectivity. PLOS Biology, 14(5), e1002451. doi: 10.1371/journal.pbio.1002451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Cox R, Hofman WF, de Boer M, & Talamini LM (2014). Local sleep spindle modulations in relation to specific memory cues. Neuroimage, 99, 103–110. 10.1016/j.neuroimage.2014.05.028 [DOI] [PubMed] [Google Scholar]
- *Creery JD, Oudiette D, Antony JW, & Paller KA (2015). Targeted memory reactivation during sleep depends on prior learning. Sleep, 38(5), 755–763. doi: 10.5665/sleep.4670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deuker L, Olligs J, Fell J, Kranz TA, Mormann F, Montag C, … & Axmacher N (2013). Memory consolidation by replay of stimulus-specific neural activity. Journal of Neuroscience, 33(49), 19373–19383. doi: 10.1523/JNEUROSCI.0414-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diekelmann S (2014). Sleep for cognitive enhancement. Frontiers in System Neuroscience, 8, 46. doi: 10.3389/fnsys.2014.00046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diekelmann S, Biggel S, Rasch B, & Born J (2012). Offline consolidation of memory varies with time in slow wave sleep and can be accelerated by cuing memory reactivations. Neurobiology of Learning and Memory, 98(2), 103–111. doi: 10.1016/j.nlm.2012.07.002 [DOI] [PubMed] [Google Scholar]
- Diekelmann S, & Born J (2010). The memory function of sleep. Nature Reviews Neuroscience, 11(2), 114–126. doi: 10.1038/nrn2762 [DOI] [PubMed] [Google Scholar]
- *Diekelmann S, Born J, & Rasch B (2016). Increasing explicit sequence knowledge by odor cueing during sleep in men but not women. Frontiers in Behavioral Neuroscience, 10, 74. doi: 10.3389/fnbeh.2016.00074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Diekelmann S, Büchel C, Born J, & Rasch B (2011). Labile or stable: opposing consequences for memory when reactivated during waking and sleep. Nature Neuroscience, 14(3), 381–386. doi: 10.1038/nn.2744. [DOI] [PubMed] [Google Scholar]
- Diekelmann S, Landolt HP, Lahl O, Born J, & Wagner U (2008). Sleep loss produces false memories. PLOS One, 3(10), e3512 10.1371/journal.pone.0003512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Dillon DJ, & Babor TF (1970). Intervening activity and the retention of meaningful verbal material. Psychonomic Science, 19(6), 369–370. https://link.springer.com/article/10.3758%2FBF03328864 [Google Scholar]
- Dillon DJ, & Bowles EG (1976). Learning of meaningful verbal material following pre-presentation during either REM or Non-REM sleep previous night. Research Communications in Psychology Psychiatry and Behavior, 1(2), 315–326. [Google Scholar]
- *Donohue KC, & Spencer RM (2011). Continuous re-exposure to environmental sound cues during sleep does not improve memory for semantically unrelated word pairs. Journal of Cognitive Education and Psychology, 10(2), 167–177. doi: 10.1891/1945-8959.10.2.167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudai Y (2012). The restless engram: consolidations never end. Annual Review of Neuroscience, 35, 227–247. 10.1146/annurev-neuro-062111-150500 [DOI] [PubMed] [Google Scholar]
- Dunlap WP, Cortina JM, Vaslow JB, & Burke MJ (1996). Meta-analysis of experiments with matched groups or repeated measures designs. Psychological Methods, 1(2), 170–177. 10.1037/1082-989X.1.2.170 [DOI] [Google Scholar]
- Duval S, & Tweedie R (2000). Trim and fill: A simple funnel plot based method of testing and adjusting for publication bias in meta-analysis. Biometrics, 56(2), 455–463. 10.1111/j.0006-341X.2000.00455.x [DOI] [PubMed] [Google Scholar]
- Egger M, Davey Smith G, Schneider M, & Minder C (1997). Bias in meta-analysis detected by a simple, graphical test. British Medical Journal BMJ, 315(7109), 629–634. doi: 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellenbogen JM, Hulbert JC, Stickgold R, Dinges DF, & Thompson-Schill SL (2006). Interfering with theories of sleep and memory: sleep, declarative memory, and associative interference. Current Biology, 16(13), 1290–1294. 10.1016/j.cub.2006.05.024 [DOI] [PubMed] [Google Scholar]
- Elsey JW, Van Ast VA, & Kindt M (2018). Human memory reconsolidation: A guiding framework and critical review of the evidence. Psychological Bulletin, 144(8), 797–848. 10.1037/bul0000152 [DOI] [PubMed] [Google Scholar]
- *Farthouat J, Gilson M, & Peigneux P (2017). New evidence for the necessity of a silent plastic period during sleep for a memory benefit of targeted memory reactivation. Sleep Spindles & Cortical Up States, 1(1), 14–26. 10.1556/2053.1.2016.002 [DOI] [Google Scholar]
- Faul F, Erdfelder E, Lang A-G, & Buchner A (2007). G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavior Research Methods, 39, 175–191. [DOI] [PubMed] [Google Scholar]
- Fenn KM, Gallo DA, Margoliash D, Roediger HL, & Nusbaum HC (2009). Reduced false memory after sleep. Learning & Memory, 16(9), 509–513. doi: 10.1101/lm.1500808 [DOI] [PubMed] [Google Scholar]
- Fischer S, & Born J (2009). Anticipated reward enhances offline learning during sleep. Journal of Experimental Psychology: Learning, Memory, and Cognition, 35(6), 1586–1593. 10.1037/a0017256 [DOI] [PubMed] [Google Scholar]
- Forcato C, Fernandez RS, & Pedreira ME (2014). Strengthening a consolidated memory: the key role of the reconsolidation process. Journal of Physiology-Paris, 108(4–6), 323–333. 10.1016/j.jphysparis.2014.09.001 [DOI] [PubMed] [Google Scholar]
- Fox BH, & Robbin JS (1952). The retention of material presented during sleep. Journal of Experimental Psychology, 43(1), 75–79. 10.1037/h0057555 [DOI] [PubMed] [Google Scholar]
- *Fuentemilla L, Miró J, Ripollés P, Vilà-Balló A, Juncadella M, Castañer S, … & Rodríguez-Fornells A (2013). Hippocampus-dependent strengthening of targeted memories via reactivation during sleep in humans. Current Biology, 23(18), 1769–1775. 10.1016/j.cub.2013.07.006 [DOI] [PubMed] [Google Scholar]
- Gallo DA (2010). False memories and fantastic beliefs: 15 years of the DRM illusion. Memory & Cognition, 38(7), 833–848. doi: 10.3758/MC.38.7.833. [DOI] [PubMed] [Google Scholar]
- *Gao C, Chapagain N, Terlizzese T, Zeter D, Fillmore P, & Scullin MK (2019). Classical music during slow wave sleep facilitates educational learning: A targeted memory reactivation experiment with immediate and 9-month follow-up testing. Sleep, 42(Supplement_1), A39–A39. [Google Scholar]
- Giuditta A (2014). Sleep memory processing: the sequential hypothesis. Frontiers in Systems Neuroscience, 8, 219 10.3389/fnsys.2014.00219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonsalves B, & Paller KA (2000). Neural events that underlie remembering something that never happened. Nature Neuroscience, 3(12), 1316–1321. [DOI] [PubMed] [Google Scholar]
- Gonsalves B, Reber PJ, Gitelman DR, Parrish TB, Mesulam M-M, & Paller KA (2004). Neural evidence that vivid imagining can lead to false remembering. Psychological Science, 15(10), 655–660. 10.1111/j.0956-7976.2004.00736.x [DOI] [PubMed] [Google Scholar]
- *Göldi M, van Poppel EAM, Rasch B, & Schreiner T (2019). Increased neuronal signatures of targeted memory reactivation during slow-wave up states. Scientific Reports, 9, 2715, 10.1038/s41598-019-39178-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Groch S, McMakin D, Guggenbuhl P, Rasch B, Huber R, & Wilhelm I (2016). Memory cueing during sleep modifies the interpretation of ambiguous scenes in adolescents and adults. Developmental Cognitive Neuroscience, 17, 10–18. doi: 10.1016/j.dcn.2015.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Groch S, Preiss A, McMakin DL, Rasch B, Walitza S, Huber R, & Wilhelm I (2017a). Targeted reactivation during sleep differentially affects negative memories in socially anxious and healthy children and adolescents. Journal of Neuroscience, 37(9), 2425–2434. doi: 10.1523/JNEUROSCI.1912-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Groch S, Schreiner T, Rasch B, Huber R, & Wilhelm I (2017b). Prior knowledge is essential for the beneficial effect of targeted memory reactivation during sleep. Scientific Reports, 7, 39763. doi: 10.1038/srep39763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Guerrien A, Dujardin K, Mandai O, Sockeel P, & Leconte P (1989). Enhancement of memory by auditory stimulation during postlearning REM sleep in humans. Physiology and Behavior, 45(5), 947–950. 10.1016/0031-9384(89)90219-9 [DOI] [PubMed] [Google Scholar]
- Hallion LS, & Ruscio AM (2011). A meta-analysis of the effect of cognitive bias modification on anxiety and depression. Psychological Bulletin, 137(6), 940–958. 10.1037/a0024355 [DOI] [PubMed] [Google Scholar]
- Hars B, & Hennevin E (1987). Impairment of learning by cueing during post-learning slow-wave sleep in rats. Neuroscience Letters, 79(3), 290–294. 10.1016/0304-3940(87)90446-0 [DOI] [PubMed] [Google Scholar]
- Hars B, Hennevin E, & Pasques P (1985). Improvement of learning by cueing during postlearning paradoxical sleep. Behavioral Brain Research, 18(3), 241–250. [DOI] [PubMed] [Google Scholar]
- *Hauner KK, Howard JD, Zelano C, & Gottfried JA (2013). Stimulus-specific enhancement of fear extinction during slow-wave sleep. Nature Neuroscience, 16(11), 1553–1555. doi: 10.1038/nn.3527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *He J, Sun HQ, Li SX, Zhang WH, Shi J, Ai SZ, … Lu L (2015). Effect of conditioned stimulus exposure during slow wave sleep on fear memory extinction in humans. Sleep, 38(3), 423–431. doi: 10.5665/sleep.4502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedges LV (1984). Estimation of effect size under nonrandom sampling: The effects of censoring studies yielding statistically insignificant mean differences. Journal of Educational and Behavioral Statistics, 9, 61–85. 10.3102/10769986009001061 [DOI] [Google Scholar]
- *Hennies N, Lambon Ralph MA, Durrant SJ, Cousins JN, & Lewis PA (2017). Cued memory reactivation during SWS abolishes the beneficial effect of sleep on abstraction. Sleep, 40(8), zsx102 10.1093/sleep/zsx102 [DOI] [PubMed] [Google Scholar]
- *Honma M, Plass J, Brang D, Florczak SM, Grabowecky M, & Paller KA (2016). Sleeping on the rubber-hand illusion: memory reactivation during sleep facilitates multisensory recalibration. Neuroscience of Consciousness, 1, 10.1093/nc/niw020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedges LV (1981). Distribution theory for Glass’s estimator of effect size and related estimators. Journal of Educational Statistics, 6(2), 107–128. 10.3102/10769986006002107 [DOI] [Google Scholar]
- Hedges LV, & Olkin I (1985). Statistical models for meta-analysis. Orlando, FL: Academic Press. [Google Scholar]
- Higgins JP, & Thompson SG (2002). Quantifying heterogeneity in a meta-analysis. Statistics in Medicine, 21(11), 1539–1558. doi: 10.1002/sim.1186 [DOI] [PubMed] [Google Scholar]
- *Hu X, Antony JW, Creery JD, Vargas IM, Bodenhausen GV, & Paller KA (2015). Unlearning implicit social biases during sleep. Science, 348(6238), 1013–1015. doi: 10.1126/science.aaa3841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Humiston GB, & Wamsley EJ (2019). Unlearning implicit social biases during sleep: A failure to replicate, 14(1), e0211416 10.1371/journal.pone.0211416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inostroza M, & Born J (2013). Sleep for preserving and transforming episodic memory. Annual Review of Neuroscience, 36, 79–102. doi: 10.1146/annurev-neuro-062012-170429 [DOI] [PubMed] [Google Scholar]
- Iyengar S, & Greenhouse JB (1988). Selection models and the file drawer problem. Statistical Science, 3, 109–117. [Google Scholar]
- Jenkins JG, & Dallenbach KM (1924). Obliviscence during sleep and waking. The American Journal of Psychology, 35(4), 605–612. 10.2307/1414040 [DOI] [Google Scholar]
- Ji D, & Wilson MA (2007). Coordinated memory replay in the visual cortex and hippocampus during sleep. Nature Neuroscience, 10(1), 100–107. [DOI] [PubMed] [Google Scholar]
- *Johnson BP, Scharf SM, & Westlake KP (2018). Targeted memory reactivation during sleep, but not wake, enhances sensorimotor skill performance: A pilot study. Journal of Motor Behavior, 50(2), 202–209. doi: 10.1080/00222895.2017.1327411 [DOI] [PubMed] [Google Scholar]
- *Klinzing JG, Kugler S, Soekadar SR, Rasch B, Born J, & Diekelmann S (2018). Odor cueing during slow-wave sleep benefits memory independently of low cholinergic tone. Psychopharmacology, 235(1), 291–299. 10.1007/s00213-017-4768-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korman M, Doyon J, Doljansky J, Carrier J, Dagan Y, & Karni A (2007). Daytime sleep condenses the time course of motor memory consolidation. Nature Neuroscience, 10(9), 1206–1213. doi: 10.1038/nn1959 [DOI] [PubMed] [Google Scholar]
- Kredlow MA, Unger LD, & Otto MW (2016). Harnessing reconsolidation to weaken fear and appetitive memories: A meta-analysis of post-retrieval extinction effects. Psychological Bulletin, 142(3), 314–336. doi: 10.1037/bul0000034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakens D (2013). Calculating and reporting effect sizes to facilitate cumulative science: a practical primer for t-tests and ANOVAs. Frontiers in Psychology, 4, 863. doi: 10.3389/fpsyg.2013.00863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau J, Ioannidis JP, Terrin N, Schmid CH, & Olkin I (2006). Evidence based medicine: The case of the misleading funnel plot. BMJ: British Medical Journal, 333(7568), 597. doi: 10.1136/bmj.333.7568.597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Laventure S, Fogel S, Lungu O, Albouy G, Sevigny-Dupont P, Vien C, … Doyon J (2016). NREM2 and sleep spindles are instrumental to the consolidation of motor sequence memories. PLOS Biology, 14(3), e1002429. doi: 10.1371/journal.pbio.1002429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Lehmann M, Schreiner T, Seifritz E, & Rasch B (2016). Emotional arousal modulates oscillatory correlates of targeted memory reactivation during NREM, but not REM sleep. Scientific Reports, 6, 39229. doi: 10.1038/srep39229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis PA, & Durrant SJ (2011). Overlapping memory replay during sleep builds cognitive schemata. Trends in Cognitive Sciences, 15(8), 343–351. 10.1016/j.tics.2011.06.004 [DOI] [PubMed] [Google Scholar]
- Lipsey MW, & Wilson DB (2001). Practical meta-analysis. Sage Publications, Inc. [Google Scholar]
- McShane BB, Böckenholt U, & Hansen KT (2016). Adjusting for publication bias in meta-analysis: An evaluation of selection methods and some cautionary notes. Perspectives on Psychological Science, 11(5), 730–749. 10.1177/1745691616662243 [DOI] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, & Altman DG (2009). Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Annals of Internal Medicine, 151(4), 264–269. doi: 10.1371/journal.pmed.1000097 [DOI] [PubMed] [Google Scholar]
- Morris SB, & DeShon RP (2002). Combining effect size estimates in meta-analysis with repeated measures and independent-groups designs. Psychological Methods, 7(1), 105–125. 10.1037/1082-989X.7.1.105 [DOI] [PubMed] [Google Scholar]
- Ngo HV, Martinetz T, Born J, & Molle M (2013). Auditory closed-loop stimulation of the sleep slow oscillation enhances memory. Neuron, 78(3), 545–553. doi: 10.1016/j.neuron.2013.03.006 [DOI] [PubMed] [Google Scholar]
- Nader K, & Hardt O (2009). A single standard for memory: the case for reconsolidation. Nature Reviews Neuroscience, 10(3), 224–234. DOI: 10.1038/nrn2590 [DOI] [PubMed] [Google Scholar]
- Nader K, Schafe GE, & Le Doux JE (2000). Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature, 406(6797), 722–726. doi: 10.1038/35021052 [DOI] [PubMed] [Google Scholar]
- Norman KA, Newman EL, & Detre G (2007). A neural network model of retrievalinduced forgetting. Psychological Review, 114(4), 887–953. 10.1037/0033-295X.114.4.887 [DOI] [PubMed] [Google Scholar]
- Oswald I, Taylor AM, & Treisman M (1960). Discriminative responses to stimulation during human sleep. Brain, 83, 440–453. 10.1093/brain/83.3.440 [DOI] [PubMed] [Google Scholar]
- Oudiette D, Antony JW, & Paller KA (2014). Fear not: Manipulating sleep might help you forget. Trends in Cognitive Sciences, 18(1), 3–4. doi: 10.1016/j.tics.2013.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Oudiette D, Antony JW, Creery JD, & Paller KA (2013). The role of memory reactivation during wakefulness and sleep in determining which memories endure. Journal of Neuroscience, 33(15), 6672–6678. doi: 10.1523/JNEUROSCI.5497-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oudiette D, & Paller KA (2013). Upgrading the sleeping brain with targeted memory reactivation. Trends in Cognitive Sciences, 17(3), 142–149. doi: 10.1016/j.tics.2013.01.006 [DOI] [PubMed] [Google Scholar]
- *Oyarzún JP, Morís J, Luque D, de Diego-Balaguer R, & Fuentemilla L (2017). Targeted memory reactivation during sleep adaptively promotes the strengthening or weakening of overlapping memories. Journal of Neuroscience, 37, 3537–3516. doi: 10.1523/JNEUROSCI.3537-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paller KA (2017). Sleeping in a brave new world: Opportunities for improving learning and clinical outcomes through targeted memory reactivation. Current Directions in Psychological Science, 26, 532–537. doi: 10.1177/0963721417716928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paller KA (2018). Do house-elves clean your brain while you sleep? Frontiers for Young Minds, 6, 23. doi: 10.3389/frym.2018.00023 [DOI] [Google Scholar]
- Paller KA, Mayes AR, Antony JW, & Norman KA (in press). Replay-based consolidation governs enduring memory storage In The Cognitive Neurosciences, 6th Edition (pp. 265–276), Gazzaniga MS, Mangun GR, & Poeppel D (Eds.), MIT Press. [Google Scholar]
- Pan SC, & Rickard TC (2015). Sleep and motor learning: is there room for consolidation? Psychological Bulletin, 141(4), 812–834. doi: 10.1037/bul0000009 [DOI] [PubMed] [Google Scholar]
- Pardilla-Delgado E, & Payne JD (2017). The impact of sleep on true and false memory across long delays. Neurobiology of Learning and Memory, 137, 123–133. doi: 10.1016/j.nlm.2016.11.016 [DOI] [PubMed] [Google Scholar]
- Payne JD, Kensinger EA, Wamsley EJ, Spreng RN, Alger SE, Gibler K, … & Stickgold R (2015). Napping and the selective consolidation of negative aspects of scenes. Emotion, 15(2), 176–186. doi: 10.1037/a0038683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne JD, Schacter DL, Propper RE, Huang LW, Wamsley EJ, Tucker MA, … & Stickgold R (2009). The role of sleep in false memory formation. Neurobiology of Learning and Memory, 92(3), 327–334. doi: 10.1016/j.nlm.2009.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peigneux P, Laureys S, Fuchs S, Collette F, Perrin F, Reggers J, … Maquet P (2004). Are spatial memories strengthened in the human hippocampus during slow wave sleep? Neuron, 44(3), 535–545. doi: 10.1016/j.neuron.2004.10.007 [DOI] [PubMed] [Google Scholar]
- *Pereira SIR, Beijamini F, Weber FD, Vincenzi RA, da Silva FAC, & Louzada FM (2017). Tactile stimulation during sleep alters slow oscillation and spindle densities but not motor skill. Physiology & Behavior, 169, 59–68. doi: 10.1016/j.physbeh.2016.11.024 [DOI] [PubMed] [Google Scholar]
- Pick JL, Nakagawa S, & Noble DW (2019). Reproducible, flexible and high-throughput data extraction from primary literature: The metaDigitise r package. Methods in Ecology and Evolution, 10(3), 426–431. 10.1111/2041-210X.13118 [DOI] [Google Scholar]
- Plihal W, & Born J (1997). Effects of early and late nocturnal sleep on declarative and procedural memory. Journal of Cognitive Neuroscience, 9(4), 534–547. doi: 10.1162/jocn.1997.9.4.534 [DOI] [PubMed] [Google Scholar]
- Purple RJ, Sakurai T, & Sakaguchi M (2017). Auditory conditioned stimulus presentation during NREM sleep impairs fear memory in mice. Scientific Reports, 7, 46247. doi: 10.1038/srep46247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasch B, & Born J (2013). About sleep’s role in memory. Physiology Review, 93(2), 681–766. doi: 10.1152/physrev.00032.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Rasch B, Buchel C, Gais S, & Born J (2007). Odor cues during slow-wave sleep prompt declarative memory consolidation. Science, 315(5817), 1426–1429. doi: 10.1126/science.1138581 [DOI] [PubMed] [Google Scholar]
- *Rihm JS, Diekelmann S, Born J, & Rasch B (2014). Reactivating memories during sleep by odors: odor specificity and associated changes in sleep oscillations. Journal of Cognitive Neuroscience, 26(8), 1806–1818. doi: 10.1162/jocn_a_00579 [DOI] [PubMed] [Google Scholar]
- *Rihm JS, & Rasch B (2015). Replay of conditioned stimuli during late REM and stage N2 sleep influences affective tone rather than emotional memory strength. Neurobiology of Learning and Memory, 122, 142–151. 10.1016/j.nlm.2015.04.008 [DOI] [PubMed] [Google Scholar]
- *Rihm JS, Sollberger SB, Soravia LM, & Rasch B (2016). Re-presentation of olfactory exposure therapy success cues during non-rapid eye movement sleep did not increase therapy outcome but increased sleep spindles. Frontiers in Human Neuroscience, 10, 340. doi: 10.3389/fnhum.2016.00340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Ritter SM, Strick M, Bos MW, Van Baaren RB, & Dijksterhuis AP (2012). Good morning creativity: task reactivation during sleep enhances beneficial effect of sleep on creative performance. Journal of Sleep Research, 21(6), 643–647. 10.1111/j.1365-2869.2012.01006.x [DOI] [PubMed] [Google Scholar]
- Rolls A, Makam M, Kroeger D, Colas D, de Lecea L, & Heller HC (2013). Sleep to forget: interference of fear memories during sleep. Molecular Psychiatry, 18(11), 1166–1170. doi: 10.1038/mp.2013.121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothschild G, Eban E, & Frank LM (2017). A cortical-hippocampal-cortical loop of information processing during memory consolidation. Nature Neuroscience, 20, 251–259. 10.1038/nn.4457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal R (1979). The file drawer problem and tolerance for null results. Psychological Bulletin, 86(3), 638–641. 10.1037/0033-2909.86.3.638 [DOI] [Google Scholar]
- *Rudoy JD, Voss JL, Westerberg CE, & Paller KA (2009). Strengthening individual memories by reactivating them during sleep. Science, 326(5956), 1079–1079. doi: 10.1126/science.1179013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Schechtman E, Antony JW, Lampe A, Wilson BJ, Norman KA, & Paller KA (2019). Multiple memories can be simultaneously reactivated during sleep as effectively as a single memory. bioRxiv, 662015 10.1101/662015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller D, Monfils MH, Raio CM, Johnson DC, LeDoux JE, & Phelps EA (2010). Preventing the return of fear in humans using reconsolidation update mechanisms. Nature, 463(7277), 49–53. doi: 10.1038/nature08637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Schönauer M, Geisler T, & Gais S (2014). Strengthening procedural memories by reactivation in sleep. Journal of Cognitive Neuroscience, 26(1), 143–153. doi: 10.1162/jocn_a_00471 [DOI] [PubMed] [Google Scholar]
- Schouten DI, Pereira SI, Tops M, & Louzada FM (2017). State of the art on targeted memory reactivation: Sleep your way to enhanced cognition. Sleep Medicine Review, 32, 123–131. doi: 10.1016/j.smrv.2016.04.002 [DOI] [PubMed] [Google Scholar]
- Schreiner T, Doeller CF, Jensen O, Rasch B, & Staudigl T (2018). Theta phasecoordinated memory reactivation reoccurs in a slow-oscillatory rhythm during NREM sleep. Cell Reports, 25(2), 296–301. doi: 10.1016/j.celrep.2018.09.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Schreiner T, Lehmann M, & Rasch B (2015). Auditory feedback blocks memory benefits of cueing during sleep. Nature Communications, 6, 8729. doi: 10.1038/ncomms9729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Schreiner T, & Rasch B (2015a). Boosting vocabulary learning by verbal cueing during sleep. Cerebral Cortex, 25(11), 4169–4179. 10.1093/cercor/bhu139 [DOI] [PubMed] [Google Scholar]
- Schreiner T, & Rasch B (2015b). Cueing vocabulary in awake subjects during the day has no effect on memory. Somnologie-Schlafforschung und Schlafmedizin 19(2), 133–140. [Google Scholar]
- Schreiner T, & Rasch B (2017). The beneficial role of memory reactivation for language learning during sleep: A review. Brain and Language, 167, 94–105. doi: 10.1016/j.bandl.2016.02.005 [DOI] [PubMed] [Google Scholar]
- *Seibold M, Rasch B, Born J, & Diekelmann S (2018). Reactivation of interference during sleep does not impair ongoing memory consolidation. Memory, 26(3), 377–384. 10.1080/09658211.2017.1329442 [DOI] [PubMed] [Google Scholar]
- *Shanahan LK, Gjorgieva E, Paller KA, Kahnt T, & Gottfried JA (2018). Odor-evoked category reactivation in human ventromedial prefrontal cortex during sleep promotes memory consolidation. eLife, 7, e39681 10.7554/eLife.39681.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Shimizu RE, Connolly PM, Cellini N, Armstrong DM, Hernandez LT, Estrada R, … & Simons SB (2018). Closed-loop targeted memory reactivation during sleep improves spatial navigation. Frontiers in Human Neuroscience, 12, 28 10.3389/fnhum.2018.00028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Simon KC, Gómez RL, & Nadel L (2018). Losing memories during sleep after targeted memory reactivation. Neurobiology of Learning and Memory, 151, 10–17. 10.1016/j.nlm.2018.03.003 [DOI] [PubMed] [Google Scholar]
- Siddaway AP, Wood AM, & Hedges LV (2019). How to do a systematic review: A best practice guide for conducting and reporting narrative reviews, metaanalyses, and meta-syntheses. Annual Review of Psychology, 70, 747–770, 10.1146/annurev-psych-010418-102803 [DOI] [PubMed] [Google Scholar]
- *Smith C, & Weeden K (1990). Post training REMs coincident auditory stimulation enhances memory in humans. Psychiatric Journal of the University of Ottawa, 15(2), 85–90. [PubMed] [Google Scholar]
- Steriade M (2003) The corticothalamic system in sleep. Frontiers in Bioscience, 8, d878–d899. [DOI] [PubMed] [Google Scholar]
- *Sterpenich V, Schmidt C, Albouy G, Matarazzo L, Vanhaudenhuyse A, Boveroux P, … Maquet P (2014). Memory reactivation during rapid eye movement sleep promotes its generalization and integration in cortical stores. Sleep, 37(6), 1061–1075, doi: 10.5665/sleep.3762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stickgold R, & Walker MP (2013). Sleep-dependent memory triage: evolving generalization through selective processing. Nature Neuroscience, 16(2), 139–145. doi: 10.1038/nn.3303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Strachan J, Guttesen AÁV, Smith AK, Gaskell MG, Tipper SP, & Cairney SA (2019). Investigating the formation and consolidation of incidentally learned trust. Journal of Experimental Psychology: Learning, Memory, and Cognition. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tambini A, Berners-Lee A, & Davachi L (2017). Brief targeted memory reactivation during the awake state enhances memory stability and benefits the weakest memories. Scientific Reports, 7(1), 15325 10.1038/s41598-017-15608-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Tamminen J, Lambon Ralph MA, & Lewis PA (2017). Targeted memory reactivation of newly learned words during sleep triggers REM-mediated integration of new memories and existing knowledge. Neurobiology of Learning and Memory, 137, 77–82. doi: 10.1016/j.nlm.2016.11.012 [DOI] [PubMed] [Google Scholar]
- Tamminen J, Payne JD, Stickgold R, Wamsley EJ, & Gaskell MG (2010). Sleep spindle activity is associated with the integration of new memories and existing knowledge. Journal of Neuroscience, 30(43), 14356–14360. 10.1523/JNEUROSCI.3028-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Tilley AJ (1979). Sleep learning during stage 2 and REM sleep. Biological Psychology, 9(3), 155–161. 10.1016/0301-0511(79)90035-8 [DOI] [PubMed] [Google Scholar]
- *van Dongen EV, Takashima A, Barth M, Zapp J, Schad LR, Paller KA, & Fernandez G (2012). Memory stabilization with targeted reactivation during human slow-wave sleep. Proceedings of the National Academy of Sciences USA, 109(26), 10575–10580. doi: 10.1073/pnas.1201072109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Vargas IM (2016). Induction and reduction of false memory during sleep using targeted memory reactivation. (Unpublished doctoral dissertation). Northwestern University, Evanston, Illinois, U.S.A. [Google Scholar]
- *Vargas IM, Schechtman E, & Paller KA (2019). Targeted memory reactivation during sleep to strengthen memory for arbitrary pairings. Neuropsychologia, 124, 144–150. 10.1016/j.neuropsychologia.2018.12.017 [DOI] [PubMed] [Google Scholar]
- Vevea JL, & Woods CM (2005). Publication bias in research synthesis: sensitivity analysis using a priori weight functions. Psychological Methods, 10(4), 428–443. 10.1037/1082-989X.10.4.428 [DOI] [PubMed] [Google Scholar]
- Viechtbauer W (2010). Conducting meta-analyses in R with the metafor package. Journal of Statistical Software, 36(3). 1–48. 10.18637/jss.v036.i03 [DOI] [Google Scholar]
- Walker MP, Stickgold R, Alsop D, Gaab N, & Schlaug G (2005). Sleep-dependent motor memory plasticity in the human brain. Neuroscience, 133(4), 911–917. 10.1016/j.neuroscience.2005.04.007 [DOI] [PubMed] [Google Scholar]
- *Wang B, Antony JW, Lurie S, Brooks PP, Paller KA, & Norman KA (2019). Targeted memory reactivation during sleep elicits neural signals related to learning content. Journal of Neuroscience, 10.1523/JNEUROSCI.2798-18.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassing R, Lakbila-Kamal O, Ramautar JR, Stoffers D, Schalkwijk F, & Van Someren EJ (2019). Restless REM sleep impedes overnight amygdala adaptation. Current Biology, 29, 2351–2358.e4, 10.1016/j.cub.2019.06.034 [DOI] [PubMed] [Google Scholar]
- Westerberg CE, Mander BA, Florczak SM, Weintraub S, Mesulam M-M, Zee PC, & Paller KA (2012). Concurrent impairments in sleep and memory in amnestic mild cognitive impairment. Journal of the International Neuropsychological Society, 18, 490–500. doi: 10.1017/S135561771200001X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm I, Diekelmann S, Molzow I, Ayoub A, Mölle M, & Born J (2011). Sleep selectively enhances memory expected to be of future relevance. Journal of Neuroscience, 31(5), 1563–1569. 10.1523/JNEUROSCI.3575-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson MA, & McNaughton BL (1994). Reactivation of hippocampal ensemble memories during sleep. Science, 265(5172), 676–679. doi: 10.1126/science.8036517 [DOI] [PubMed] [Google Scholar]
- Wolak MM. 2015. Package “ICC”. Facilitating Estimation of the Intraclass Correlation Coefficient. [Google Scholar]
- Wood JM, Bootzin RR, Kihlstrom JF, & Schacter DL (1992). Implicit and explicit memory for verbal information presented during sleep. Psychological Science, 3(4), 236–240. 10.1111/j.1467-9280.1992.tb00035.x [DOI] [Google Scholar]
- Zelano C, & Sobel N (2005). Humans as an animal model for systems-level organization of olfaction. Neuron, 48(3), 431–454. 10.1016/j.neuron.2005.10.009 [DOI] [PubMed] [Google Scholar]
- Züst MA, Ruch S, Wiest R, & Henke K (2019). Implicit vocabulary learning during sleep is bound to slow-wave peaks. Current Biology, 29(4), 541–553. 10.1016/j.cub.2018.12.038 [DOI] [PubMed] [Google Scholar]
- Zwaka H, Bartels R, Gora J, Franck V, Culo A, Gotsch M, & Menzel R (2015). Context odor presentation during sleep enhances memory in honeybees. Current Biology, 25(21), 2869–2874. doi: 10.1016/j.cub.2015.09.069 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.