Abstract
Background
Urinary tract infections, including pyelonephritis, are serious complications that may lead to significant maternal and neonatal morbidity and mortality. There is a large number of drugs, and combination of them, available to treat urinary tract infections, most of them tested in non‐pregnant women. Attempts to define the optimal antibiotic regimen for pregnancy have, therefore, been problematic.
Objectives
The objective of this review was to determine, from the best available evidence from randomised controlled trials, which agent is the most effective for the treatment of symptomatic urinary tract infections during pregnancy in terms of cure rates, recurrent infection, incidence of preterm delivery and premature rupture of membranes, admission to neonatal intensive care unit, need for change of antibiotic, and incidence of prolonged pyrexia.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group Trials Register (November 2009) and reference lists of articles.
Selection criteria
We considered all trials where the intention was to allocate participants randomly to one of at least two alternative treatments for any symptomatic urinary tract infection.
Data collection and analysis
Both review authors assessed trial quality and extracted data.
Main results
We included 10 studies, recruiting a total of 1125 pregnant women. In most of the comparisons there were no significant differences between the treatments under study with regard to cure rates, recurrent infection, incidence of preterm delivery, admission to neonatal intensive care unit, need for change of antibiotic and incidence of prolonged pyrexia. When cefuroxime and cephradine were compared, there were better cure rates (29/49 versus 41/52) and fewer recurrences (20/49 versus 11/52) in the cefuroxime group. There was only one other statistically significant difference when comparing outpatient versus inpatient treatment. Gestational age at birth was greater in women from the outpatient group (38.86 versus 37.21), while birthweight was on average greater in the inpatient group (3120 versus 2659).
Authors' conclusions
Although antibiotic treatment is effective for the cure of urinary tract infections, there are insufficient data to recommend any specific drug regimen for treatment of symptomatic urinary tract infections during pregnancy. All the antibiotics studied were shown to be very effective in decreasing the incidence of the different outcomes. Complications were very rare. All included trials had very small sample sizes to reliably detect important differences between treatments. Future studies should evaluate the most promising antibiotics, in terms of class, timing, dose, acceptability, maternal and neonatal outcomes and costs.
Plain language summary
Treatments for symptomatic urinary tract infections during pregnancy
Antibiotics are very effective at clearing urinary tract infections in pregnancy, and complications are very rare.
Infections in the urinary tract are common in pregnancy. These include infections with no symptoms (asymptomatic bacteriuria), cystitis (bladder infection) and pyelonephritis (kidney infection). Such infections can cause some serious complications for the woman, and may lead to problems for the baby. The review of 10 trials, recruiting a total of 1125 pregnant women, found that several types of antibiotic had very high cure rates of cystitis or pyelonephritis during pregnancy, while complications from treatment were very rare. However, the studies could not show if any particular drug was preferable.
Background
Urinary tract infections (UTIs), including pyelonephritis, are among the most common health problems during pregnancy. They occur in up to 20% of pregnancies in some disadvantaged populations (Mercado 1994) and have been associated with prelabour rupture of membranes, preterm labour and delivery, clinical or subclinical chorioamnionitis, postpartum fever in the mother and neonatal infection, although the causal pathway is unclear. Some studies (Belady 1997; Romero 1988) suggest that microorganisms may produce arachidonic acid, phospholipase A2 and prostaglandins that play an important role in cervical softening and increasing myometrial free calcium content, which stimulates uterine tone and contractions. This may be a possible causal mechanism for preterm labour.
UTIs may lead to serious maternal complications such as septic shock, respiratory insufficiency, fluid balance disorders, chronic renal insufficiency and death. UTIs have been classified as asymptomatic bacteriuria, cystitis and pyelonephritis. The frequency of asymptomatic bacteriuria during pregnancy has been reported to be between 2% to 10% (6% to 7% Botella 1981; 2% to 10% Kaye 1985; 10% Mercado 1994; 2% to 10% Schwarcz 1989; 6% Seebode 1986). If untreated, it is considered that 30% to 50% of these women will develop symptomatic infection later during pregnancy (Seebode 1986). Management of asymptomatic bacteriuria is assessed in another Cochrane review (Smaill 2007). The diagnosis of cystitis is basically clinical, and it is based on symptoms such as disuria, frequency and lower abdominal or supra‐pubic pain, without fever (Block 1987). Pyuria (more than 10 leucocytes per high‐power field) is usually, but not always, present. Pyelonephritis is an acute episode diagnosed in 2% to 4% of pregnant women, when fever, costovertebral tenderness and pyuria are present (Schwarcz 1989; Van Dorsten 1987).
There are many drugs available to treat UTIs. Attempts to define the optimal antibiotic regimen have, therefore, been problematic. The ideal drug would be:
of proven effectiveness in well‐designed prospective, randomised, double‐blind clinical trials;
active against the majority of pathogens likely to be involved;
able to maintain adequate serum and tissue levels throughout the treatment;
not associated with the development of antimicrobial resistance;
inexpensive;
well‐tolerated;
safe for the fetus.
Many different drugs have been used to treat UTIs during pregnancy, including fosfomycin trometamol (De Cecco 1987; Patel 1997; Stein 1998), pipemidic and piromidic acid (De Cecco 1987; Ishigami 1976), nitrofurantoin (Florida 1990; Grischke 1987; Van Dorsten 1987), trimethoprim‐sulphamethoxazole (London 1972; Wren 1972), aztreonam, pivmecillinam (London 1979), ampicillin (Davies 1975; Wren 1969), amoxycillin (Aylesbury 1985; Pedler 1985), carbenicillin (Davies 1975), cephradine (London 1979) and cephalexin (Florida 1990; Pedler 1985). Drugs have been given by oral or intravenous route, in single‐dose or in three‐, seven‐, 10‐ or 15‐day courses, or for the remainder duration of pregnancy. There are, therefore, many options in both the choice of agent and the length of treatment. There is also a wide variation in the antimicrobial spectrum and pharmacokinetic properties of antibiotics. Most of these issues have been studied in non‐pregnant women. Some guidelines recommend multiple doses, but a single dose could also be adequate.
The cure rates after different treatments have been reported as between 70% and 100% (Cunningham 1994; Patel 1997; Seebode 1986).
Objectives
To determine, from the best available evidence from randomised controlled trials (RCTs), which treatment is most effective for symptomatic UTIs during pregnancy in terms of cure rates, recurrent infection, preterm delivery, premature rupture of membranes, admission to neonatal intensive care unit, need for change of antibiotic and incidence of prolonged pyrexia.
Methods
Criteria for considering studies for this review
Types of studies
We considered all trials where the intention was to allocate participants randomly to one of at least two alternative treatments for any symptomatic urinary tract infection. We excluded quasi‐randomised studies. While developing the protocol, we anticipated that there might be both equivalence trials aimed to show that two treatments are similar, but one is either cheaper or easier to administer or with better acceptance, and superior trials aimed to demonstrate that a new regimen is better than the standard. Because of ethical considerations, the relevance of the disease and its impact on maternal and perinatal outcomes, we did not anticipate that there would be placebo‐controlled trials, or trials with a no‐treatment arm.
We also considered cluster randomised trials for inclusion if identified through the search. We proposed to analyse such trials as specified in the section 'Unit of analysis issues'.
Types of participants
Pregnant women with any symptomatic UTI and any degree of severity, receiving antibiotic treatment on an inpatient or outpatient basis.
Types of interventions
We considered trials if at least two different treatments were compared. In addition to the comparison of different antimicrobial agents, we included studies where there was a comparison between the route of administration (e.g. whether oral or intravenous), outpatient versus inpatient regimens, the doses of drug given (if single or multiple doses) and the duration of the treatment (e.g. 1, 3, 7, 10 days or for the remainder of the pregnancy). We conducted analyses separately for these objectives.
We classified antimicrobial agents as follows:
penicillins: e.g. ampicillin;
cephalosporins: e.g. cephazolin, ceftriaxone;
aminoglycosides: e.g. gentamicin;
antimetabolites: e.g. trimethoprim, sulfamethoxazole;
miscellaneous: e.g. nitrofurantoin, fosfomycin trometamol.
Types of outcome measures
Primary outcomes
Cure rates (defined as symptom relief and/or urine clearance by laboratory test);
recurrent infection.
Secondary outcomes
Preterm delivery;
premature rupture of membranes;
admission to neonatal intensive care unit;
need for change of antibiotic;
incidence of prolonged pyrexia;
gestational age at birth (not prespecified);
birthweight (not prespecified).
Regarding the secondary outcomes, we did not pre‐specify gestational age at birth or birthweight in the protocol or the first versions of the review. However, given its importance, the review authors (JCV, EA) considered it relevant to include them as secondary continuous outcomes in this version of the review.
In addition, we collected data (where available) on adverse events of treatment (e.g. allergic and other toxic reactions, diarrhoea associated to the antibiotic, development of bacterial resistance), duration of maternal stay, costs, and other neonatal outcomes.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Pregnancy and Childbirth Group's Trials Register by contacting the Trials Search Co‐ordinator (November 2009).
The Cochrane Pregnancy and Childbirth Group's Trials Register is maintained by the Trials Search Co‐ordinator and contains trials identified from:
quarterly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE;
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL and MEDLINE, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the ‘Specialized Register’ section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are each assigned to a review topic (or topics). The Trials Search Co‐ordinator searches the register for each review using the topic list rather than keywords.
Searching other resources
We searched the reference lists of retrieved articles.
We did not apply any language restrictions.
Data collection and analysis
For methods used when assessing the trials identified in the previous version of this review, seeAppendix 1.
For this update we used the following methods when assessing the trials identified by the updated search (Greece 2007; Tehran 2006).
Selection of studies
Two review authors (JC Vazquez (JCV) and E Abalos (EA)) independently assessed for inclusion all the potential studies we identified as a result of the search strategy. We resolved disagreements by discussion.
Data extraction and management
We designed a form to extract data. For eligible studies, two review authors (JCV, EA) extracted the data using the agreed form. We resolved discrepancies by discussion. We entered data into Review Manager software (RevMan 2008) and checked them for accuracy.
When information regarding any of the above was unclear, we attempted to contact authors of the original reports to provide further details.
Assessment of risk of bias in included studies
Two review authors independently assessed the risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2008). We resolved any disagreement by discussion.
Because of the nature of the intervention, i.e. treatments for a symptomatic acute condition during pregnancy, we did not plan to include crossover trials.
(1) Sequence generation (checking for possible selection bias)
We described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
adequate (any truly random process, e.g. random number table; computer random number generator);
inadequate (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number); or
unclear.
(2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal the allocation sequence in sufficient detail and determine whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
adequate (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
inadequate (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear.
(3) Blinding (checking for possible performance bias)
We described for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We judged studies at low risk of bias if they were blinded, or if we judged that the lack of blinding could not have affected the results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as:
adequate, inadequate or unclear for participants;
adequate, inadequate or unclear for personnel;
adequate, inadequate or unclear for outcome assessors.
However, in some circumstances there may be partial blinding e.g. where outcomes are self‐reported by unblinded participants but they are recorded by blinded personnel without knowledge of group assignment. Where appropriate, we would have evaluated such partial blinding for adequacy when assessing the quality of blinding.
(4) Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations)
We described for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or could be supplied by the trial authors, we re‐included missing data in the analyses which we undertook. We assessed methods as:
adequate: when missing data are less than 20%;
inadequate: when missing data are more or equal than 20%; and
unclear, when no information is provided in the study.
(5) Selective reporting bias
We described for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
adequate (where it is clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
inadequate (where not all the study’s pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
unclear.
(6) Other sources of bias
We described for each included study any important concerns we had about other possible sources of bias (Higgins 2008). For example, if the study:
(a) had a potential source of bias related to the specific study design used; (b) stopped early due to some data‐dependent process (including a formal‐stopping rule); (c) had extreme baseline imbalance; (d) has been claimed to have been fraudulent; or (e) had some other problem likely to influence on the findings.
We assessed whether each study was free of other problems that could put it at risk of bias:
yes;
no;
unclear.
(7) Overall risk of bias
We made explicit judgements about whether studies are at high risk of bias, according to the criteria given in the Handbook (Higgins 2008). With reference to (1) to (6) above, we assessed the likely magnitude and direction of the bias and whether we consider it is likely to impact on the findings. We would have explored the impact of the level of bias if we had undertaken sensitivity analyses but it was not possible to conduct sensitivity analysis in the only outcome showing substantial heterogeneity, as the comparison included only two trials.
Measures of treatment effect
Dichotomous data
For dichotomous data, we present results as summary risk ratio with 95% confidence intervals.
Continuous data
For continuous data, we present results as mean differences with 95% confidence intervals. For meta‐analyses, where possible, we used the mean difference if outcomes were measured in the same way between trials. We would use the standardised mean difference to combine trials that measure the same outcome, but use different methods.
For meta‐analyses, we used the fixed‐effect model when heterogeneity was not considered to be statistically significant according to the Chi2, I2 or Tau2 values. Otherwise, we used the random‐effects model.
Unit of analysis issues
Due to the nature of the intervention, we planned to include only individually randomised clinical trials in the review. Therefore, the number of observations in the analysis should match the number of units that were randomised. In the simple parallel group design for a clinical trial, participants are individually randomised to one of two intervention groups, so that we would collect and analyse a single measurement for each outcome from each participant.
For the same reason, it is unlikely to find cluster‐randomised trials dealing with this acute condition. However, it was proposed that if any trial with this type of design were identified and considered eligible for inclusion, we would use special statistical methods to analyse the results, according to the recommendations from the section 16.3 of the Handbook (Higgins 2008) and seek statistical advice, where appropriate.
Cluster‐randomised trials
We found no cluster‐randomised trials for this review.
However, if available in future updates, we will include cluster‐randomised trials in the analyses along with individually randomised trials. We will adjust their sample sizes or standard errors using the methods described in the Handbook (see Section 16.3.4 or 16.3.6 (Higgins 2008) using an estimate of the intracluster correlation co‐efficient (ICC) derived from the trial (if possible), or from another source. If ICCs from other sources are used, we will report this and conduct sensitivity analyses to investigate the effect of variation in the ICC. If we identify both cluster‐randomised trials and individually‐randomised trials, we plan to synthesise the relevant information. We will consider it reasonable to combine the results from both if there is little heterogeneity between the study designs and the interaction between the effect of intervention and the choice of randomisation unit is considered to be unlikely.
We will also acknowledge heterogeneity in the randomisation unit and perform a separate meta‐analysis.
Cross over trials
We did not include crossover designs because they are not appropriate for the topic of this review.
Dealing with missing data
For included studies, we explored levels of attrition. There were no high levels of missing data in the overall assessment of treatment effect.
For all outcomes, we carried out analyses, as far as possible, on an intention‐to‐treat basis, i.e. we attempted to include all participants randomised to each group in the analyses. The denominator for each outcome in each trial was the number randomised minus any participants whose outcomes are known to be missing.
Assessment of heterogeneity
We measured heterogeneity, where appropriate, using I2, Chi2 (for fixed‐effect models) and Tau2 tests (for random‐effects models).
For I² statistics, where the I² estimate was greater than or equal to 50%, we interpreted this as indicating the presence of significant heterogeneity.
In the case of Chi2, we considered a low P value < 0.10 (or a large Chi2 statistic relative to its degree of freedom) as evidence of heterogeneity.
The Tau2 statistic provides an estimate of the between‐study variance in a random‐effects meta‐analysis (Egger 2001).
In the presence of heterogeneity, we considered the potential factors influencing it, and performed the meta‐analysis using the random‐effects model.
Subgroup analyses were not possible as the comparisons with more than one trial included just three studies.
Assessment of reporting biases
If we had suspected reporting bias (see ‘Selective reporting bias’ above), we would have attempted to contact study authors asking them to provide missing outcome data. Where this would not be possible, and the missing data were thought to introduce serious bias, we would have explored the impact of including such studies in the overall assessment of results by a sensitivity analysis.
We planned to explore publication bias using funnel plots, provided that there are at least 10 studies included in the meta‐analysis and all the studies are of different sizes. We planned to interpret results of tests for funnel plot asymmetry in the light of visual inspection of the funnel plot. When there is evidence of small‐study effects, publication bias should be considered as only one of a number of possible explanations, as stated in the Handbook (Higgins 2008).
Data synthesis
We carried out statistical analysis using the Review Manager software (RevMan 2008). We used fixed‐effect inverse variance meta‐analysis for combining data where trials are examining the same intervention, and we judged the trials’ populations and methods sufficiently similar. Where we suspect clinical or methodological heterogeneity between studies sufficient to suggest that treatment effects may differ between trials, we used random‐effects meta‐analysis.
If substantial heterogeneity had been identified in a fixed‐effect meta‐analysis, we would have repeated the analysis using a random‐effects method.
Subgroup analysis and investigation of heterogeneity
We have not planned subgroup analyses for this review, although we will include them in future updates.
Sensitivity analysis
It was not possible to conduct sensitivity analysis in the only outcome showing substantial heterogeneity, as the comparison included only two trials.
Results
Description of studies
We have included 10 trials (14 publications) in this review. Five were conducted in the USA, two in Chile, one in Mexico, one in the Slovak Republic and one in Iran. In most of the trials, treatments were administered under inpatient regimens (Charleston 1996; Florida 1995; Santiago 2000), in one under outpatient regimens (Mexico 1989), and in two the regimens were unknown (Bratislava 2001; Santiago 2001). In four trials the treatments were administrated under both regimens (Los Angeles 1995; Los Angeles 1998; Los Angeles 1999; Tehran 2006). See:Characteristics of included studies. We have excluded eight studies (see Characteristics of excluded studies).
Risk of bias in included studies
Seven studies used an adequate method to generate the allocation sequence (Charleston 1996; Florida 1995; Los Angeles 1995; Los Angeles 1998; Los Angeles 1999; Mexico 1989; Tehran 2006). In the studies Bratislava 2001 and Santiago 2001, the method was unclear, and in Santiago 2000 the allocation was by "strict" admission consecutive order, therefore there is a high possibility of selection bias. This study also reported post‐randomisation exclusion rates of 10.9% and 7.1% for cephradine and cefuroxime, respectively.
Five studies were of good quality in terms of allocation concealment (Florida 1995; Los Angeles 1995; Los Angeles 1998; Los Angeles 1999; Mexico 1989). The three studies conducted in Los Angeles used sealed opaque envelopes, and in the study conducted in Florida the allocation was kept at the hospital pharmacy. The trial conducted in Mexico used a binomial distribution to allocate the participants. In the studies Bratislava 2001, Charleston 1996, Santiago 2001 and Tehran 2006 the method was unclear.
In all of them, sample sizes were insufficient to detect at least a 10% difference in cure rates between 95% and 85%, which was considered the principal outcome for the review. To detect such difference it would have been necessary to recruit at least 137 patients in each group, not taking into account drop‐outs and losses to follow‐up.
Only the Florida 1995 study reported to be double‐blind. In the remainder this domain was either unclear or not done at all.
Overall, the studies were of variable quality; Florida 1995, Los Angeles 1995, Los Angeles 1998, Los Angeles 1999 were better, while Santiago 2000 and Mexico 1989 showed the worst indicators in terms of methodological quality.
The scarcity of data in the reports prevented an appropriate assessment of all the items related to quality and risk of bias, resulting in an uncertainty about some domains, such as selective reporting bias and other potential sources of bias.
The main problem overall was the loss to follow‐up (between 8% and 25%), performance of late microbiological studies and follow‐up samples, and lack of report of important data about pregnancy outcomes.
Effects of interventions
We included 10 studies involving 1125 women.
According to the route of administration (i.e.: oral, intravenous or intramuscular), to whether the treatment were given in hospital or as outpatient regimen, and to whether treatment consisted in single or multiple doses, we have listed comparisons as follows:
1. intravenous plus oral antibiotics versus intravenous only (Charleston 1996);
2. intravenous and oral cephradine versus intravenous and oral cefuroxime (Santiago 2000);
3. intramuscular cephazolin versus intravenous ampicillin plus gentamicin (Los Angeles 1998);
4. intramuscular ceftriaxone versus intravenous ampicillin plus gentamicin (Los Angeles 1998);
5. intramuscular ceftriaxone versus intravenous cephazolin (Los Angeles 1998);
6. oral ampicillin versus oral nitrofurantoin (Mexico 1989);
7. oral fosfomycin trometamol versus oral ceftibuten (Bratislava 2001);
8. outpatient versus inpatient antibiotics (Los Angeles 1995; Los Angeles 1999; Tehran 2006);
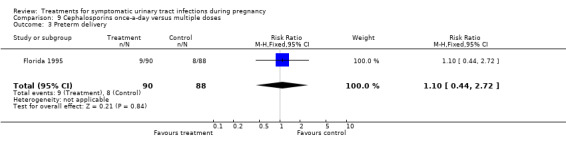
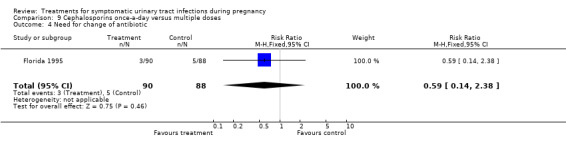
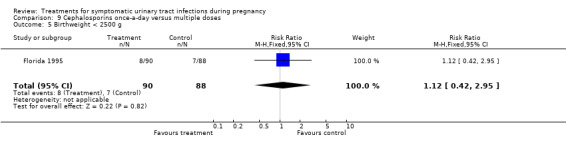
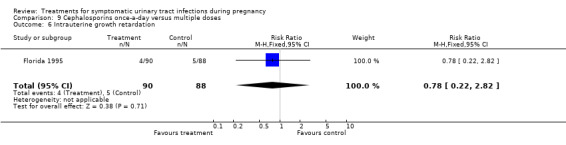
9. cephalosporins once‐a‐day versus multiple doses (Florida 1995);
10. single versus multiple dose of gentamicin (Santiago 2001).
In most of the trials, treatments were administered under inpatient regimens (Charleston 1996; Florida 1995; Santiago 2000), in only one under outpatient regimens (Mexico 1989), and in two the regimens were unknown (Bratislava 2001; Santiago 2001). In three trials the treatments were administered under both regimens (Los Angeles 1998; Los Angeles 1999; Tehran 2006).
We performed four meta‐analyses, all of them within Comparison 8: Outpatient versus inpatient antibiotics. Three meta‐analyses included three studies (Los Angeles 1995; Los Angeles 1999; Tehran 2006) and one included only two studies (Los Angeles 1995; Los Angeles 1999).
Primary outcomes
When cephradine and cefuroxime were compared, the cephradine group had worse cure rates (29/49 versus 41/52; RR 0.75 (95% CI 0.57 to 0.99); one study; 101 participants; P = 0,04; Comparison 2, Analysis 2.1), and the cefuroxime group had fewer recurrent infections (11/52 versus 20/49, one study; RR 1.93 (95% CI 1.03 to 3.60); 101 participants; P = 0,04; Comparison 2, Analysis 2.2).
2.1. Analysis.
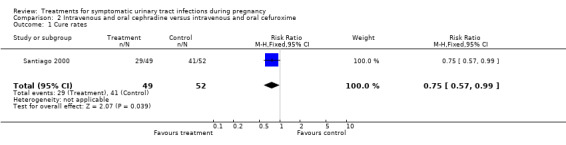
Comparison 2 Intravenous and oral cephradine versus intravenous and oral cefuroxime, Outcome 1 Cure rates.
2.2. Analysis.
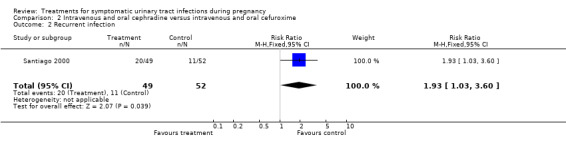
Comparison 2 Intravenous and oral cephradine versus intravenous and oral cefuroxime, Outcome 2 Recurrent infection.
We found no other significant differences among the different antibiotic regimens regarding cure rates and recurrent infections.
All of them were shown to be very effective to achieve high cure rates, and adverse events were reported in few women (see Data collection and analysis).
Secondary outcomes
We found no significant differences for incidence of preterm delivery, admission to neonatal intensive care unit, need for change antibiotic and incidence of prolonged pyrexia, when reported.
The plotted RRs depicted heterogeneity in the outcome need for change antibiotic when comparing outpatient versus inpatient regimes (Comparison 8, outcome 8.5); (pooled RR 0.87, 95% CI 0.31 to 2.44). The I2 statistic for heterogeneity was 84% for this outcome (Chi2 for heterogeneity 6.10, P = 0.01; Tau² = 8.27, df = 1), suggesting a significant heterogeneity between the trials results. Thus, we re‐analysed data using the random‐effects model, but results did not change significantly (pooled RR 0.76, 95% CI 0.01 to 58.95). It should be noted that both studies (Los Angeles 1995; Los Angeles 1999) were conducted by the same group of investigators, in similar settings and with similar populations. Methodology was also very similar, although the antibiotic regimen was slightly different. One should be cautious when interpreting and analysing such heterogeneity because only two trials with a small number of subjects were included in the meta‐analysis (Deeks 2005).
There was only one other statistically significant difference when comparing outpatient versus inpatient treatment. Gestational age at birth was greater in women from the outpatient group (38.86 versus 37.21; MD 1.65 (95% CI 1.25 to 2.05)), while birthweight was on average greater in inpatient group (3120 versus 2659; MD ‐461.22 (95% CI ‐608.33 to ‐314.11)). Such differences were somewhat conflicting. For the outcome gestational age at birth, it is also important to note that standard deviations were quite different (0.21 for outpatient antibiotics group versus 1.62 for inpatient antibiotics group), and such difference is not explained in the text by the authors. Both outcomes were included in the same trial (Tehran 2006) in the abstract, with 64 women in each arm.
Again, because of the small sample size of the studies, and therefore the low statistical power, significant differences in rare adverse outcomes between one regimen and other were also unlikely to be detected.
Discussion
All included trials have very small sample sizes to detect important differences between treatments. It is important to take into account that, in equivalence trials, failure to detect a difference does not imply equivalence between both treatments (Jones 1996). Equivalence trials generally need to be larger than trials aimed to show that one treatment is better than other, because differences are expected to be very small, and therefore, bigger numbers are needed to detect them.
In all of the included studies, sample sizes were insufficient to detect at least a 10% difference in cure rates between 95% and 85%, which was considered the principal outcome for the review. To detect such difference it would have been necessary to recruit at least 137 patients in each group, not taking into account drop‐outs and losses to follow‐up.
The main problem overall was the loss to follow‐up (between 8% and 25%), performance of late microbiological studies and follow‐up samples, as well as reports of important data about final pregnancy outcomes (preterm delivery, birthweight, neonatal morbidities).
Another important problem was the lack of relevant information in the trials to assess appropriately the risk of bias. Most of the studies did not report sufficient details about allocation concealment and blinding; therefore, we could evaluate only four studies as low risk of bias, leaving most of the remaining trials classified as uncertain.
We excluded six studies because symptomatic and asymptomatic pregnant women were analysed together, and it was not possible to perform the analysis for symptomatic women separately. One trial was excluded because of its methodological weaknesses (Florida 1990). Therefore, in most of comparisons there were only one or two trials for each comparison, excepting Comparisons 8.2 and 8.3 (Analysis 8.2 and Analysis 8.3), where we included three studies. Clinically important outcomes such as prelabour rupture of membranes and neonatal complications were not reported in the trials included in the review.
8.2. Analysis.
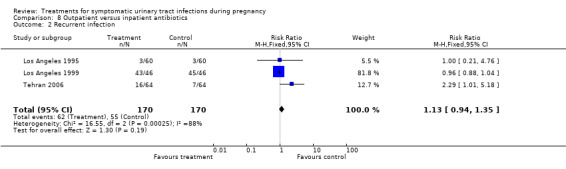
Comparison 8 Outpatient versus inpatient antibiotics, Outcome 2 Recurrent infection.
8.3. Analysis.
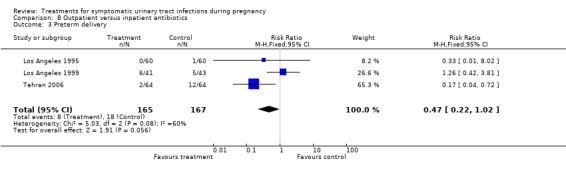
Comparison 8 Outpatient versus inpatient antibiotics, Outcome 3 Preterm delivery.
For antibiotics not contraindicated during pregnancy, data from non‐pregnant women may be useful for decision‐making in the absence of adequate data from pregnant women, but it is very important to take into account the quite different pharmacodynamics of some drugs during pregnancy. The increased renal excretion and therefore greater presence of the antibiotics in the urine may be particularly relevant to the effectiveness of some of them when treating UTIs in pregnancy. The dosing intervals and blood levels to be obtained may also vary markedly. Therefore, one should be cautious when analysing data collected from non‐pregnant women.
In order to gather more information on comparative efficacy of different antibiotics used for symptomatic UTIs, we performed a search of trials and reviews including non‐pregnant women. We identified one review involving 28 controlled trials conducted on women with symptomatic and uncomplicated lower UTIs (Norrby 1990). This review included pregnant and non‐pregnant women treated with antibiotics such as trimethoprim‐sulfamethoxazole, oral cephalosporins and beta‐lactamics. The author concluded that in all the studied antibiotics, a single dose was less efficient than a three‐day, five‐day or more than a five‐day treatment; beta‐lactamics should be administered for five or more days; the optimal treatment duration with trimethoprim/sulphonamide combinations seems to be three days. Finally, when oral cephalosporins are used, adverse reactions are more frequent when treatment is administrated for longer periods of time.
Authors' conclusions
Implications for practice.
At present, because of the lack of primary data with good quality and appropriate sample size, it is not possible to draw reliable conclusions on which is the best class, route or regimen of antibiotic to treat symptomatic UTIs during pregnancy. This review could not show that one treatment regimen is better than another. One possible reason is the insufficient numbers in the included studies.
For all the assessed treatments, cure rates were very high and complications were very rare. It is therefore reasonable to give the simplest and cheapest available treatment and consider giving it to women who will be compliant on an outpatient basis, considering how disruptive it is for a pregnant woman and her family when she is hospitalised.
Implications for research.
Future studies should evaluate the more promising classes of antibiotics, such as nitrofurantoin, trimethoprim‐sulfamethoxazole, cephalosporins and penicillins, in terms of duration (single‐dose or three‐, seven‐, 10‐ or 15‐day doses, or for the remainder of the pregnancy), acceptability (route and side effects), maternal outcomes, neonatal outcomes like premature rupture of membranes, and costs.
It is important to note that two of the trials evaluating outpatient versus inpatient regimens included in this review were conducted in the USA, and the last one was conducted in Iran. The problem may be of particular importance in low‐and middle‐income countries, because of incidence and costs. Therefore, new research in such settings could be very useful to be performed in such settings to determine the best choice of treatment.
Equivalence trials aimed to demonstrate similarity between treatments should have enough power and sample size to detect significant differences, if they do exist.
What's new
| Date | Event | Description |
|---|---|---|
| 31 May 2010 | New search has been performed | Search updated in November 2009. One new trial added (Tehran 2006). |
| 31 May 2010 | New citation required but conclusions have not changed | New author helped update the review. |
History
Protocol first published: Issue 2, 2000 Review first published: Issue 3, 2000
| Date | Event | Description |
|---|---|---|
| 8 September 2009 | Amended | Search updated. Two reports added to Studies awaiting classification (Tehran 2006a; Greece 2007a). |
| 19 June 2008 | Amended | Converted to new review format. |
| 30 January 2006 | New search has been performed | Search updated. One new trial included (Los Angeles 1999). |
Acknowledgements
Prof José Villar, former co‐author of the review, for his work and continued advice and support.
Dr Metin Gulmezoglu, Dr Enrique Ezcurra, HRP/WHO.
Prof Jim Neilson, Mrs Sonja Henderson, Mrs Claire Winterbottom, Ms Lynn Hampson, Cochrane Pregnancy and Childbirth Group, Liverpool Women's Hospital, Liverpool, UK.
Appendices
Appendix 1. Methods used to assess trials included in previous versions of this review
The following methods were used to assess Bratislava 2001; Charleston 1996; Florida 1995; Los Angeles 1995; Los Angeles 1998; Los Angeles 1999; Mexico 1989; Santiago 2000; Santiago 2001; Tehran 2006.
We selected all potential trials for eligibility according to the criteria specified in the protocol. Both review authors independently extracted the data from each publication. We resolved discrepancies by discussion. In addition to the main outcome measures listed above, we collected information on the setting of the study (country, type of population, socioeconomic status), a detailed description of the antibiotic regimen used (drug, dose, route, frequency) and definitions of the outcomes (if provided). Where the numbers were provided, we performed an intention‐to‐treat analysis (where possible). If possible, we would have calculated the number needed to treat from outcomes.
We assessed trials for methodological quality using the standard Cochrane criteria of adequacy of allocation concealment: (A) adequate; (B) unclear; (C) inadequate; (D) allocation concealment was not used.
Information on blinding of outcome assessment and loss to follow up was collected. Power calculations were extracted if available or performed by the authors. This is a very important issue for equivalence trials that require, in general, larger sample size.
We made separate comparisons of different antimicrobial regimens, grouped where appropriate by spectrum of activity, the number of doses given and the route of administration (whether oral, intramuscular or intravenous). We calculated summary odds ratios using a fixed effects model. When statistical heterogeneity was found, we used I‐square statistics (where values above 50% were considered as significant heterogeneity), chi square and P values to determine if such heterogeneity was statistically significant (Deeks 2005). Where possible, we would have performed sensitivity analysis using, e.g. trial quality (A versus B, C, D) and other features.
Data and analyses
Comparison 1. Intravenous + oral antibiotics versus intravenous only.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Cure rates | 1 | 67 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.93, 1.27] |
| 2 Recurrent infection | 1 | 67 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.72 [0.47, 6.32] |
1.1. Analysis.
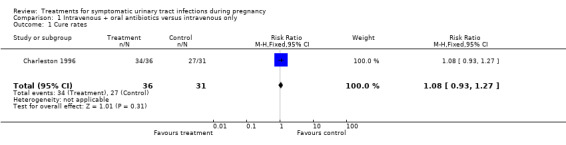
Comparison 1 Intravenous + oral antibiotics versus intravenous only, Outcome 1 Cure rates.
1.2. Analysis.
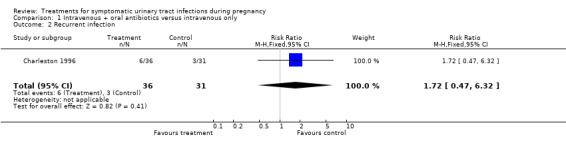
Comparison 1 Intravenous + oral antibiotics versus intravenous only, Outcome 2 Recurrent infection.
Comparison 2. Intravenous and oral cephradine versus intravenous and oral cefuroxime.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Cure rates | 1 | 101 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.57, 0.99] |
| 2 Recurrent infection | 1 | 101 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.93 [1.03, 3.60] |
Comparison 3. Intravenous cephazolin versus intravenous ampicillin + gentamicin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Cure rates | 1 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.93, 1.11] |
| 2 Recurrent infection | 1 | 107 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.52 [0.36, 6.47] |
| 3 Preterm delivery | 1 | 107 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.90 [0.48, 7.55] |
| 4 Admission to neonatal intensive care unit | 1 | 107 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.39 [0.63, 3.09] |
| 5 Need for change of antibiotic | 1 | 118 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.17 [0.25, 105.42] |
| 6 Incidence of prolonged pyrexia | 1 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.21, 2.40] |
3.1. Analysis.
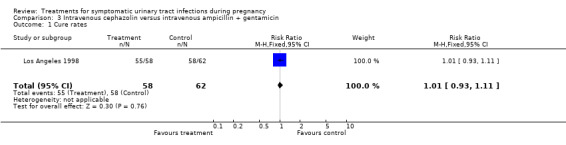
Comparison 3 Intravenous cephazolin versus intravenous ampicillin + gentamicin, Outcome 1 Cure rates.
3.2. Analysis.
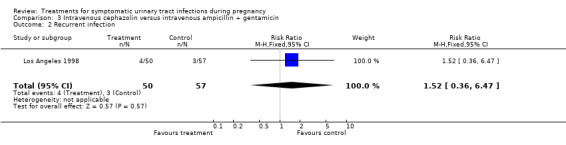
Comparison 3 Intravenous cephazolin versus intravenous ampicillin + gentamicin, Outcome 2 Recurrent infection.
3.3. Analysis.
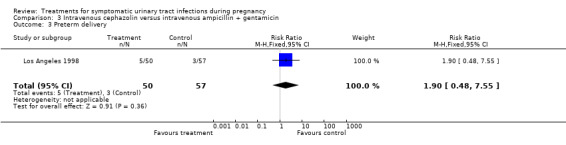
Comparison 3 Intravenous cephazolin versus intravenous ampicillin + gentamicin, Outcome 3 Preterm delivery.
3.4. Analysis.
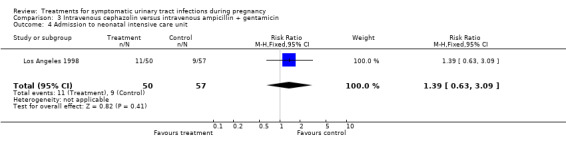
Comparison 3 Intravenous cephazolin versus intravenous ampicillin + gentamicin, Outcome 4 Admission to neonatal intensive care unit.
3.5. Analysis.
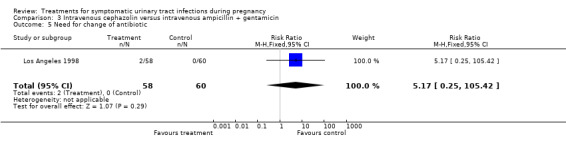
Comparison 3 Intravenous cephazolin versus intravenous ampicillin + gentamicin, Outcome 5 Need for change of antibiotic.
3.6. Analysis.
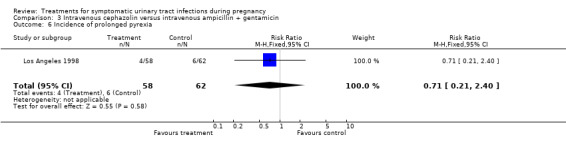
Comparison 3 Intravenous cephazolin versus intravenous ampicillin + gentamicin, Outcome 6 Incidence of prolonged pyrexia.
Comparison 4. Intramuscular ceftriaxone versus intravenous ampicillin + gentamicin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Cure rates | 1 | 121 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.98, 1.13] |
| 2 Recurrent infection | 1 | 109 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.23, 5.19] |
| 3 Preterm delivery | 1 | 109 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.23, 5.19] |
| 4 Admission to neonatal intensive care unit | 1 | 109 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.46 [0.67, 3.18] |
| 5 Need for change of antibiotic | 1 | 121 | Risk Ratio (M‐H, Fixed, 95% CI) | 9.45 [0.52, 171.79] |
| 6 Incidence of prolonged pyrexia | 1 | 121 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.36, 3.08] |
4.1. Analysis.
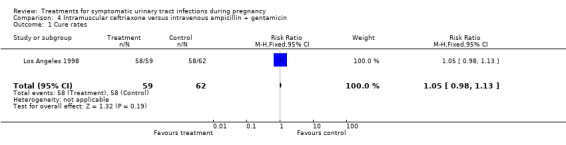
Comparison 4 Intramuscular ceftriaxone versus intravenous ampicillin + gentamicin, Outcome 1 Cure rates.
4.2. Analysis.
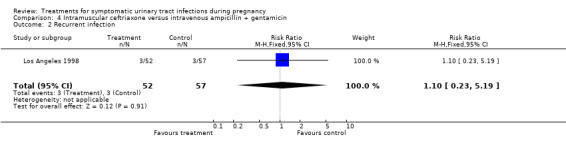
Comparison 4 Intramuscular ceftriaxone versus intravenous ampicillin + gentamicin, Outcome 2 Recurrent infection.
4.3. Analysis.
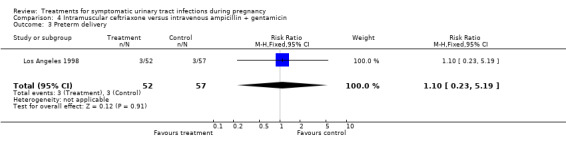
Comparison 4 Intramuscular ceftriaxone versus intravenous ampicillin + gentamicin, Outcome 3 Preterm delivery.
4.4. Analysis.
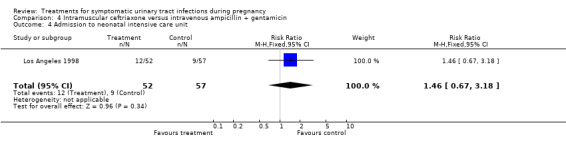
Comparison 4 Intramuscular ceftriaxone versus intravenous ampicillin + gentamicin, Outcome 4 Admission to neonatal intensive care unit.
4.5. Analysis.
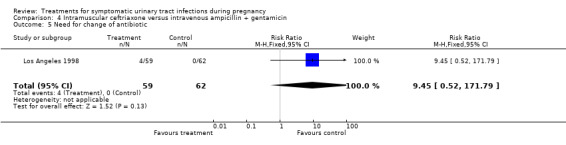
Comparison 4 Intramuscular ceftriaxone versus intravenous ampicillin + gentamicin, Outcome 5 Need for change of antibiotic.
4.6. Analysis.
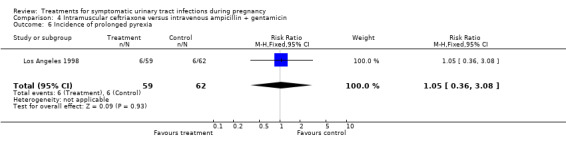
Comparison 4 Intramuscular ceftriaxone versus intravenous ampicillin + gentamicin, Outcome 6 Incidence of prolonged pyrexia.
Comparison 5. Intramuscular ceftriaxone versus intravenous cephazolin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Cure rates | 1 | 117 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.97, 1.11] |
| 2 Recurrent infection | 1 | 102 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.17, 3.06] |
| 3 Preterm delivery | 1 | 102 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.15, 2.29] |
| 4 Admission to neonatal intensive care unit | 1 | 102 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.51, 2.16] |
| 5 Need for change of antibiotic | 1 | 117 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.97 [0.37, 10.32] |
| 6 Indicence of prolonged pyrexia | 1 | 117 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.47 [0.44, 4.96] |
5.1. Analysis.
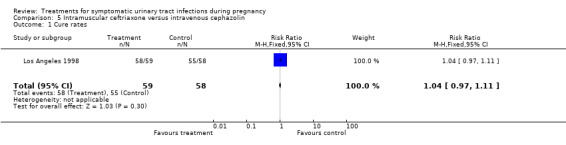
Comparison 5 Intramuscular ceftriaxone versus intravenous cephazolin, Outcome 1 Cure rates.
5.2. Analysis.
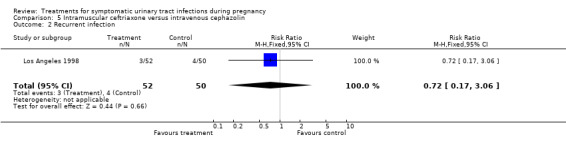
Comparison 5 Intramuscular ceftriaxone versus intravenous cephazolin, Outcome 2 Recurrent infection.
5.3. Analysis.
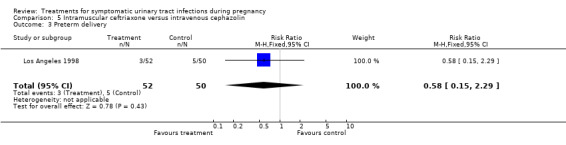
Comparison 5 Intramuscular ceftriaxone versus intravenous cephazolin, Outcome 3 Preterm delivery.
5.4. Analysis.
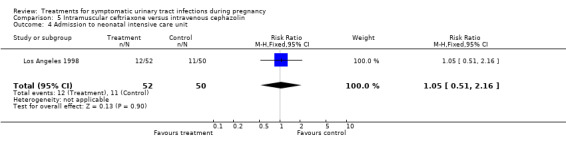
Comparison 5 Intramuscular ceftriaxone versus intravenous cephazolin, Outcome 4 Admission to neonatal intensive care unit.
5.5. Analysis.
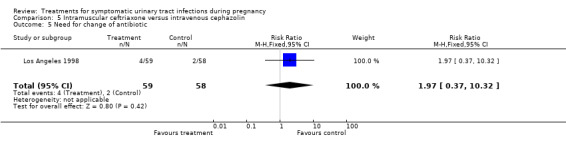
Comparison 5 Intramuscular ceftriaxone versus intravenous cephazolin, Outcome 5 Need for change of antibiotic.
5.6. Analysis.
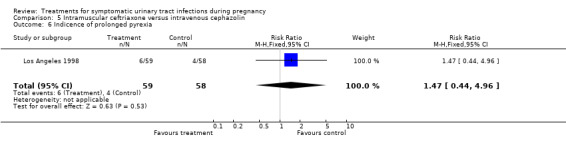
Comparison 5 Intramuscular ceftriaxone versus intravenous cephazolin, Outcome 6 Indicence of prolonged pyrexia.
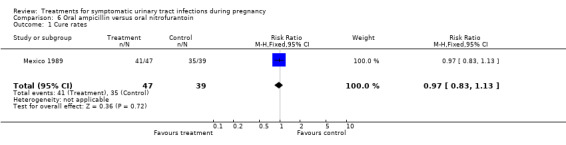
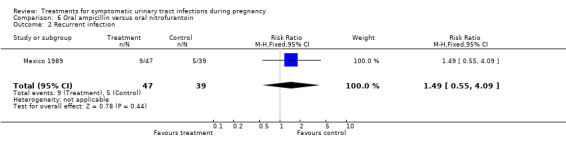
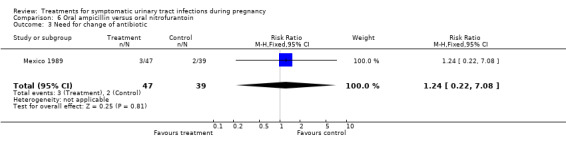
Comparison 6. Oral ampicillin versus oral nitrofurantoin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Cure rates | 1 | 86 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.83, 1.13] |
| 2 Recurrent infection | 1 | 86 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.49 [0.55, 4.09] |
| 3 Need for change of antibiotic | 1 | 86 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.22, 7.08] |
6.1. Analysis.
Comparison 6 Oral ampicillin versus oral nitrofurantoin, Outcome 1 Cure rates.
6.2. Analysis.
Comparison 6 Oral ampicillin versus oral nitrofurantoin, Outcome 2 Recurrent infection.
6.3. Analysis.
Comparison 6 Oral ampicillin versus oral nitrofurantoin, Outcome 3 Need for change of antibiotic.
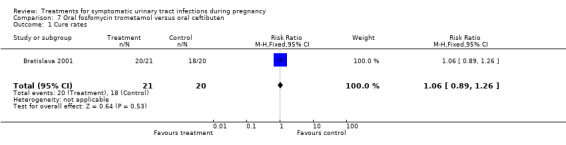
Comparison 7. Oral fosfomycin trometamol versus oral ceftibuten.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Cure rates | 1 | 41 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.89, 1.26] |
7.1. Analysis.
Comparison 7 Oral fosfomycin trometamol versus oral ceftibuten, Outcome 1 Cure rates.
Comparison 8. Outpatient versus inpatient antibiotics.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Cure rates | 3 | 340 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [1.00, 1.14] |
| 2 Recurrent infection | 3 | 340 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.94, 1.35] |
| 3 Preterm delivery | 3 | 332 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.22, 1.02] |
| 4 Admission to neonatal intensive care unit | 1 | 84 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.26, 2.17] |
| 5 Need for change of antibiotic | 2 | 212 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.01, 58.95] |
| 6 Incidence of prolonged pyrexia | 1 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.01, 2.02] |
| 7 Gestational age at birth | 1 | 128 | Mean Difference (IV, Fixed, 95% CI) | 1.65 [1.25, 2.05] |
| 8 Birthweight | 1 | 128 | Mean Difference (IV, Fixed, 95% CI) | ‐461.22 [‐608.33, ‐314.11] |
8.1. Analysis.
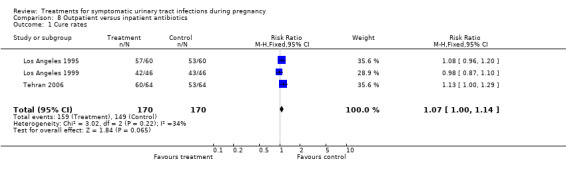
Comparison 8 Outpatient versus inpatient antibiotics, Outcome 1 Cure rates.
8.4. Analysis.
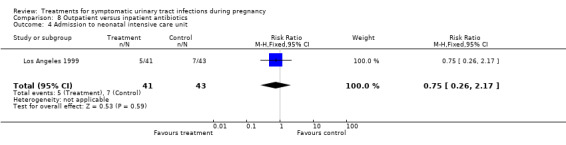
Comparison 8 Outpatient versus inpatient antibiotics, Outcome 4 Admission to neonatal intensive care unit.
8.5. Analysis.
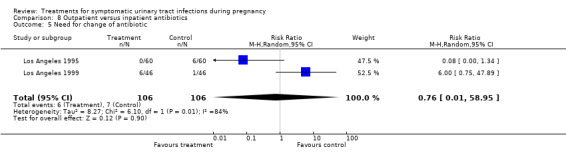
Comparison 8 Outpatient versus inpatient antibiotics, Outcome 5 Need for change of antibiotic.
8.6. Analysis.
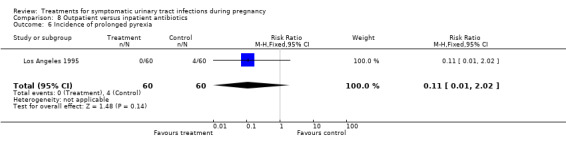
Comparison 8 Outpatient versus inpatient antibiotics, Outcome 6 Incidence of prolonged pyrexia.
8.7. Analysis.
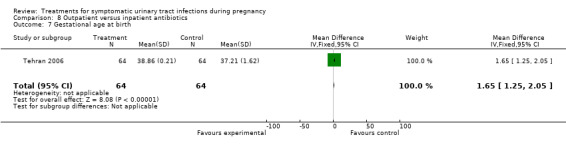
Comparison 8 Outpatient versus inpatient antibiotics, Outcome 7 Gestational age at birth.
8.8. Analysis.
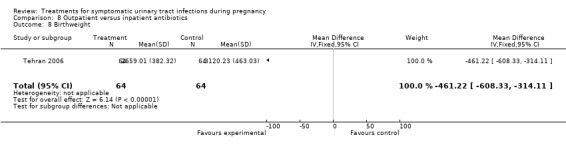
Comparison 8 Outpatient versus inpatient antibiotics, Outcome 8 Birthweight.
Comparison 9. Cephalosporins once‐a‐day versus multiple doses.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Cure rates | 1 | 178 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.96, 1.09] |
| 2 Recurrent infection | 1 | 122 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.17, 3.11] |
| 3 Preterm delivery | 1 | 178 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.1 [0.44, 2.72] |
| 4 Need for change of antibiotic | 1 | 178 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.14, 2.38] |
| 5 Birthweight < 2500 g | 1 | 178 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.42, 2.95] |
| 6 Intrauterine growth retardation | 1 | 178 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.22, 2.82] |
9.1. Analysis.
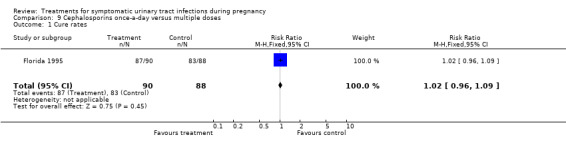
Comparison 9 Cephalosporins once‐a‐day versus multiple doses, Outcome 1 Cure rates.
9.2. Analysis.
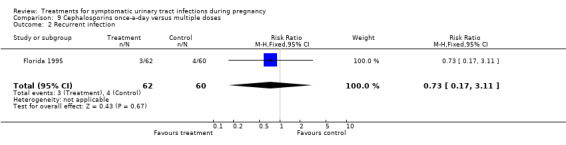
Comparison 9 Cephalosporins once‐a‐day versus multiple doses, Outcome 2 Recurrent infection.
9.3. Analysis.
Comparison 9 Cephalosporins once‐a‐day versus multiple doses, Outcome 3 Preterm delivery.
9.4. Analysis.
Comparison 9 Cephalosporins once‐a‐day versus multiple doses, Outcome 4 Need for change of antibiotic.
9.5. Analysis.
Comparison 9 Cephalosporins once‐a‐day versus multiple doses, Outcome 5 Birthweight < 2500 g.
9.6. Analysis.
Comparison 9 Cephalosporins once‐a‐day versus multiple doses, Outcome 6 Intrauterine growth retardation.
Comparison 10. Single versus multiple dose of gentamicin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Cure rates | 1 | 106 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.91, 1.03] |
10.1. Analysis.
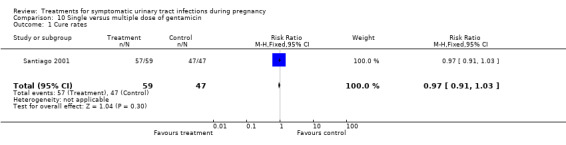
Comparison 10 Single versus multiple dose of gentamicin, Outcome 1 Cure rates.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bratislava 2001.
| Methods | Prospective, multicenter clinical study. Women were randomised to receive two different treatments. No further details. | |
| Participants | 41 pregnant women were included in the study. Inclusion criteria: pregnant women above 18 years of age with typical symptoms of lower UTI (dysuria, urgency, frequency, suprapubic pain), pyuria (> 10 leucocytes/ HPF x 400) and significant bacteriuria (>= 10 3 CFU/ml of midstream urine). Exclusion criteria: women with signs and symptoms of upper UTI (loin pain, fever, etc), asymptomatic bacteriuria, having known or presumed allergy towards the agents used, presence of urinary tract abnormalities or other complicating factors and patients unwilling to participate or comply with the protocol requirements. | |
| Interventions | Group 1 (n = 21): single 3 g oral dose of FT. Group 2 (n = 20): 3‐day course of 400 mg oral CB once daily. Two follow‐up visits were scheduled for the days 7‐10 and 28‐42. | |
| Outcomes | Cure rate (sterile urine or growth < 10 3 CFU/ml) (20/21 (95.2%) vs 18/20 (90.0%). Persistence (growth of original bacterial strain >= 10 3 CFU/ml) (1/21 (4.76%) vs 2/20 (10%). | |
| Notes | Comenius University School of Medicine, Bratislava, Slovak Republic. Population characteristics not stated. According to the authors, both treatment groups were comparable regarding age, weight and renal function. Authors concluded that the treatment of acute cystitis in pregnant women with a single‐dose of fosfomycin trometamol and with a 3‐day course of ceftibuten achieved comparable clinical and bacteriological cure rates and were well tolerated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Patients were randomised, no further details provided. |
Charleston 1996.
| Methods | Allocation by a coin‐flip table established at the beginning of the study. | |
| Participants | Women admitted for antepartum pyelonephritis, with an estimated average gestational age of 22 weeks at admission. The groups were well matched for age, race and temperature. Inclusion criteria: admission oral temperature of 38°C or greater, costovertebral angle tenderness, and a positive urine culture with 100,000 colony‐forming units/ml. Exclusion criteria: evidence of renal abscess, a prior episode of pyelonephritis during the index pregnancy, and women not exhibiting all the inclusion criteria. | |
| Interventions | Both groups received intravenous cephazolin only or cephazolin plus gentamicin or cephazolin plus other antibiotic or ampicillin plus gentamicin or other antibiotic (the initial antibiotic regimen was determined by the attending physician). In addition, the study group (n = 36) received nitrofurantoin 100 mg q.i.d. to complete 10 days of antibiotic therapy (intravenous followed by oral therapy). The control group (n = 31) received no further oral antibiotic therapy. No long‐term suppressive therapy was used in any of the participants. Women were removed from the study at the time of any positive urine culture or episode of recurrent pyelonephritis. | |
| Outcomes | Cure rates (intravenous antibiotics plus nitrofurantoin 34/36, intravenous antibiotics only 27/31); recurrent infection (intravenous antibiotics plus nitrofurantoin 6/36, intravenous antibiotics only 3/31). | |
| Notes | Charleston, North Carolina, USA. August 1990 to December 1994. Women were from a lower socioeconomic clinic population with about 1/3 of the women enrolled in the study not returning for their 2‐week culture check (9 women in the no oral therapy group and 8 women in the oral therapy group). Authors considered that this fact would call into question the compliance of women in the oral antibiotic treatment group, and that some of the early recurrent infections in the oral therapy group could represent women who were non‐compliant in completing their course of nitrofurantoin. However, the number of enrolled women that did not return was similar in both groups. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Coin‐flip table |
| Incomplete outcome data addressed? All outcomes | Low risk | |
Florida 1995.
| Methods | Allocation by a computer‐generated randomisation schedule. The randomisation schedule was kept in the hospital pharmacy and was not accessible to the clinicians. | |
| Participants | All women admitted to the hospital with clinical signs and symptoms of acute pyelonephritis. The diagnosis was made in febrile women (temperature >= 100.4°F), who met any two of the following criteria: (1) chills, (2) costovertebral angle tenderness, (3) urinalysis showing bacteria and white blood cells. Exclusion criteria: history of an acute allergic reaction to cephalosporins or penicillins and recent use of antibiotics. | |
| Interventions | Study group (n = 90): three doses, one being ceftriaxone, 1 g IV every day; the other two doses were placebo solutions of normal saline solution. Control group (n = 88): cephazolin, 2 g IV every 8 hours. IV treatment was continued until the woman was non‐febrile (temperature < 100.4°F) for at least 48 hours. Women who failed to respond completely (with resolving clinical signs and symptoms) within 3 days received IV gentamicin (120 mg bolus and 1.5 mg/kg maintenance every 8 hours) in addition to the original antibiotics. Once non‐febrile, with complete clinical resolution, participants were discharged on a 10‐day course of an oral antibiotic consistent with isolated sensitivity findings. Most women received 500 mg of either cephalexin or cephradine four times daily. | |
| Outcomes | Cure rates (ceftriaxone 87/90, cefazolin 83/88); recurrent pyelonephritis (ceftriaxone 3/62, cephazolin 4/60); preterm delivery (ceftriaxone 9/90, cephazolin 8/88); antibiotic change required (ceftriaxone 3/90, cephazolin 5/88); birthweight less than 2500 g (ceftriaxone 8/90, cephazolin 5/88); intrauterine growth retardation (ceftriaxone 4/90, cephazolin 5/88). | |
| Notes | Florida, USA. October 1990 through December 1992. University Medical Center of Jacksonville, University of Florida's urban campus. Obstetric patients are cared by resident physicians in obstetrics and gynaecology and nurse‐midwives supervised by a full‐time faculty obstetrician‐gynaecologist. The authors consider that although a test of cure was not performed in 17% of study patients, this should not have influenced their findings, because the randomisation produced similar treatment groups in all measured respects. They also comment that the mean total cost for ceftriaxone (a single 1 g dose) was 3202 USD less than that for cephazolin (2 g intravenous every 8 hours). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Computer‐generated randomisation schedule. |
| Allocation concealment? | Low risk | The randomisation schedule was kept in the hospital pharmacy and was not accessible to the clinicians. |
| Blinding? All outcomes | Low risk | Double‐blind. |
| Incomplete outcome data addressed? All outcomes | Low risk | |
| Free of other bias? | Low risk | |
Los Angeles 1995.
| Methods | Allocation by a table of random numbers with random permuted blocks, with a block size of six. Indicator cards in sealed, opaque, sequentially‐numbered envelopes kept by the principal investigator who was not involved in the randomisation. Once a participant consented, the resident in the emergency room telephoned the principal investigator. | |
| Participants | Pregnant women with an estimated gestational age of less than 24 weeks, had one or more symptoms or signs of upper urinary tract infection (temperature greater than 38.4°C, flank pain, or costovertebral angle tenderness), and had a urinalysis suspicious for urinary tract infection. Exclusion criteria: clinical signs of sepsis, respiratory insufficiency, an initial temperature greater than 39.8°C, blood pressure less than 90/50, pulse greater than 140 beats per minute (sustained), creatinine greater than 1.4 mg/dl, white blood cell count greater than 20 per 10^9/l, a known allergy to cephalosporins, a history of anaphylactic reaction to penicillins, inability to tolerate oral intake, inability to follow instructions, or serious underlying medical illness, including a known renal or urological problem, and subjects who had received antibiotic therapy within two weeks of presentation. | |
| Interventions | Outpatients (study group) (n = 60) received ceftriaxone 1 g IM in the emergency room during the observation period. A home health nurse evaluated the participants 18‐36 hours after discharge and administered a second injection of ceftriaxone 1 g. Participants then completed a 10‐day course of oral cephalexin, 500 mg four times a day. Inpatients (control group) (n = 60) received 1 g IV every 8 hours until they were non‐febrile for 48 hours. All the participants received acetaminophen, cooling measures, and IV fluids while hospitalised. Antibiotic therapy was changed for women with a worsening clinical picture or for those whose did not have clinical improvement at 72 hours. | |
| Outcomes | Cure rates (colony counts in urine of less than 100,000 colonies/ml) (outpatients 57/60, inpatients 53/60); recurrent pyelonephritis (inpatients 3/60, outpatients 3/60); preterm delivery (outpatients 0/60, inpatients 1/60); need for change antibiotic (outpatients 0/60, inpatients 6/60); incidence of prolonged pyrexia (outpatients 0/60, inpatients 4/60). | |
| Notes | Los Angeles, California, USA. April 1991 to July 1993. Authors educated participants who were candidates for outpatient treatment about pyelonephritis and instructed them on the warning signs of septic shock and respiratory insufficiency. The education was continued by visiting nurses who followed up the participants for the first 3 days after discharge. Authors conclude that the majority of patients with pyelonephritis in early pregnancy may be candidates for outpatient therapy. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Allocation by a table of random numbers with random permuted blocks, with a block size of six. |
| Allocation concealment? | Low risk | Indicator cards in sealed, opaque, sequentially‐numbered envelopes kept by the principal investigator who was not involved in the randomisation. |
Los Angeles 1998.
| Methods | Allocation by a computer‐generated random table. Assignments were placed in opaque, sealed envelopes. After a participant gave informed consent, the next envelope was opened to determine the treatment allocation. | |
| Participants | Pregnant women recruited from the emergency department of the hospital earlier than 24 weeks' gestation. Inclusion criteria: one or more symptoms of UTI (temperature at least 100.4°F, flank pain, costovertebral angle tenderness) and urinalysis suspicious for UTI (7‐10 white blood cells per high‐powered field, 20 bacteria per high‐powered field, white blood cell casts, positive nitrites on urinary dipstick). Exclusion criteria: women with history of allergy to the antibiotics being studied, had received antibiotic treatment within 2 weeks of enrolment, recurrent pyelonephritis, medical or other concurrent conditions that precluded enrolment, obvious sepsis, history of substance abuse or concurrent incarceration, nonviable or unwanted pregnancies, threatened abortion, inability to follow up because of geographic restrictions, or refusal to participate in the study. | |
| Interventions | Three groups. First group (n = 62): 2 g ampicillin IV every 4 hours and gentamicin 1.75 mg/kg IV every 8 hours after an initial dose of 2 mg/kg IV gentamicin. Second group (n = 58): 1 g IV cephazolin every 8 hours. Third group (n = 59): two 1 g doses of ceftriaxone IM 24 hours apart, followed by 500 mg of oral cephalexin every 6 hours. All participants received antibiotics and were hospitalised until they were non‐febrile for 48 hours. At discharge, all women were given 10‐day course of cephalexin 500 mg four times a day. Participants then were given nitrofurantoin 100 mg to take once a day for the remainder of their pregnancy and 6 weeks postpartum. Women who failed to demonstrate significant improvement after 72 hours of therapy were reassessed. Decisions to change antimicrobial agents were made on individual basis. | |
| Outcomes | Cure rates (ampicillin plus gentamicin 58/62, cephazolin 55/58, ceftriaxone 1/59); recurrent pyelonephritis (ampicillin plus gentamicin 3/57, cephazolin 4/50, ceftriaxone 3/52); preterm delivery (ampicillin plus gentamicin 3/57, cephazolin 5/50, ceftriaxone 3/52); NICU admission (ampicillin plus gentamicin 9/57, cephazolin 11/50, ceftriaxone 12/52); antibiotic change required (ampicillin plus gentamicin 0/62, cephazolin 2/58, ceftriaxone 4/59); incidence of prolonged pyrexia (ampicillin plus gentamicin 6/62, cephazolin 4/58, ceftriaxone 6/59). | |
| Notes | Los Angeles, California, USA. October 1994 through May 1997. 189 participants (88.8%) returned for follow‐up examination. Urine cultures were obtained from 149 of these subjects. Authors concluded that IM ceftriaxone is as effective as IV ampicillin‐gentamicin and IV cephazolin in treating pyelonephritis in pregnancy before 24 weeks' gestation. The costs for the antibiotics would be 150.00 USD for ampicillin‐gentamicin and 75.00 USD for both cephazolin and ceftriaxone, but ceftriaxone can be given intramuscularly on an outpatient basis, and it could translate into substantial cost savings. Authors also noticed a low rate of preterm birth (6.9%) compared with the institutional and national rates (about 11%). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Computer‐generated random table. |
| Allocation concealment? | Low risk | Opaque, sealed envelopes. |
| Blinding? All outcomes | High risk | Open‐label. |
| Incomplete outcome data addressed? All outcomes | Low risk | |
| Free of other bias? | Low risk | |
Los Angeles 1999.
| Methods | Allocation by a predetermined randomisation schedule using a computer‐generated random table. Assignments were placed in opaque, sealed envelopes. After a participant gave informed consent, the next envelope determined the treatment allocation. | |
| Participants | Pregnant women recruited from the emergency department of the hospital at more than 24 weeks' gestation. Inclusion criteria: one or more symptoms of urinary tract infection (temperature at least 38°C, flank pain, costovertebral angle tenderness) and urinalysis suspicious for UTI (7‐10 white blood cells per high‐powered field, 20 bacteria per high‐powered field, white blood cell casts, nitrites on urinary dipstick). Exclusion criteria: women with history of allergy to the antibiotics being studied, had received antibiotic treatment within 2 weeks of enrolment, signs of sepsis (hypotension with blood pressure of less than 90/50 mmHg, initial temperature more than 39.8°C, or sustained tachycardia of 110 beats per minute, or showing signs of adult respiratory distress syndrome. Other exclusion criteria were serious underlying medical disorders, previously diagnosed renal or urologic disorders, substance abuse, women who could not tolerate oral intake or follow instructions, women with leukocytosis in excess of 20000/mm3, renal insufficiency, with diagnosis of preterm labour. | |
| Interventions | All participants received ceftriaxone as two 1 g doses IM 24 hours apart and had continuous toco‐dynamometry during the initial 24 hours. Intravenous hydration was initiated at the discretion of managing physicians. All subjects were treated subsequently with cephalexin 500 mg by mouth four times a day for 10 days. All participants were counselled on their disease process, treatment plan and potential complications. Outpatients (study group) (n = 46) were discharged to their homes after 24 hours of hospital observation if they were stable. They monitored their temperatures every 4 hours while awake for the next 48 hours, took acetaminophen 625 mg by mouth every 4 hours if temperature over 37.8°C and took fluids. Inpatients (control group) (n = 46) received oral cephalexin until they were non‐febrile for 48 hours, when they were discharged from hospital. Participants were seen 5‐14 days after ceftriaxone and cephalexin therapy for physical examination and midstream collection of urine for urinalysis and culture. | |
| Outcomes | Cure rates (colony counts in urine of less than 100,000 colonies/ml) (outpatients 42/46, inpatients 43/46); recurrent pyelonephritis (inpatients 43/46, outpatients 45/46); preterm delivery (outpatients 6/41, inpatients 5/43); need for change antibiotic (outpatients 6/46, inpatients 1/46); admission to NICU (outpatients 5/41, inpatients 7/43). | |
| Notes | Los Angeles, California, USA. October 1995 through August 1998. Open‐label, two‐centre study. Most of participants (82%) were Hispanic. 154 patients out of 246 pregnant women were not eligible because different reasons. Another 25 were not offered participating for unknown reasons. 81 participants (88%) returned for follow‐up examination within two weeks of initial treatment. Urine cultures were obtained from 76 of these subjects. Authors conclude that in a very selected group of subjects, outpatient treatment with intramuscular ceftriaxone for acute pyelonephritis in pregnancy after 24 weeks' gestation appears as effective as conventional inpatient therapy. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Computer‐generated random table. |
| Allocation concealment? | Low risk | Opaque, sealed envelopes. |
| Blinding? All outcomes | High risk | Open‐label. |
| Incomplete outcome data addressed? All outcomes | Low risk | |
| Free of other bias? | Low risk | |
Mexico 1989.
| Methods | Allocation by a binomial distribution to assign each enrolled participant to one or another treatment. | |
| Participants | Pregnant women who attend the Antenatal Clinic's External Services. 863 women were screened and 103 were enrolled (12%). Inclusion criteria: (a) dysuria, frequency, suprapelvic pain or tenderness; (b) women who had not received antibiotic treatment during the last 2 weeks; (c) history of UTIs; (d) signed consent to participate and follow‐up. Exclusion criteria: (a) active disease other than cystourethritis; (b) women with criteria of pyelonephritis (fever, chills, anaemia, costovertebral angle tenderness, hypertension); (c) women with asymptomatic bacteriuria; (d) women who had received antibiotic treatment during the last 2 weeks; (e) subjects lost to follow‐up; (f) women who delivered in some other institution; (g) women with some complication who needed to be admitted. | |
| Interventions | Nitrofurantoin (MN) group (n = 49): oral nitrofurantoin 100 mg in the morning and 100 mg in the night during five days. Ampicillin (AM) group (n = 54): oral ampicillin 500 mg in the morning and 500 mg in the night during 5 days. If the treatment failed, a specific antibiotic was indicated according to the sensitivity. | |
| Outcomes | Cure rates (AM group 41/47, MN group 35/39); recurrent cystitis: (AM group 9/47, MN group 5/39); need for change antibiotic (AM group 3/47, MN group 2/39). | |
| Notes | Instituto Nacional de Perinatologia (National Institute of Perinatology), Mexico D.F., Mexico. May 1985 to June 1987. Population not specified. 16.5% participants excluded from the analysis (after treatment) because of loss to follow‐up. Postpartum outcomes from each group were not presented. Such outcomes were given for women who developed urinary complications after treatment, for the remainder of the pregnancy. Authors conclude that both regimens showed similar effectiveness with regard to cure rates, recurrence and treatment failure. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Allocation by a binomial distribution |
| Allocation concealment? | High risk | |
| Blinding? All outcomes | High risk | |
| Incomplete outcome data addressed? All outcomes | Low risk | |
Santiago 2000.
| Methods | Allocation by alternation, according to a "strict" admission order to received one or another treatment. Specific method of allocation not stated. | |
| Participants | Pregnant women between 12 and 34 weeks of gestation attending the Department of Obstetrics, Gynecology and Neonatology. Participants were admitted only during the morning of weekdays because of the availability of microbiological studies. 375 women with diagnosis of pyelonephritis were screened and 111 were enrolled (29.6%). Inclusion criteria: acute pyelonephritis (fever greater then 38°C, chills, low back pain and urinary test with bacteria and leukocytes). A urine culture was used to confirm the diagnosis. Gestational age was determined by the menstrual history and the fetal biometric scan before 24 weeks of gestation. Exclusion criteria: use of antibiotics within 30 days before the start of the trial, another important disease, hypersensitivity and microorganism resistance to the antibiotic, impairment of renal function, clinical signs of sepsis and/or respiratory distress and fetal malformation. | |
| Interventions | CPD group 1 (n = 55): 1 g IV every 6 hours by 72 hours. Then 500 mg oral every 8 hours by 11 days to complete 14 days. CFX group 2 (n = 56) 750 mg IV every 8 hours by 72 hours, dissolved in glucose 5% solution 100 cc, perfunded in 20 minutes. CFX acetyl 250 mg oral was continued by 11 days to complete 14 days. If fever, bacteriemia at admission and/or vomiting persisted, IV treatment was prolonged by 5 days. If microorganism resistance or hypersensitivity to the drugs, the schedule was changed according to the sensitivity. After randomisation, absolute bed rest was prescribed, IV hydration, and control of main parameters and uterine contraction. | |
| Outcomes | Microbiological cure rate, clinical cure rate, recurrent infection, bacteriological failure rate, clinical cure failure. | |
| Notes | Department of Obstetrics, Gynecology and Neonatology, Santiago de Chile, Chile. April 1996 to February 1999. Six women (10.9%) were excluded from group 1 (CPD) and 4 participants (7.1%) from group 2 (CFX) after randomisation. 49 participants (89%) completed the protocol in group 1 and 52 (92.8%) in group 2. Reasons for exclusion: three loss to follow‐up, three because microbiological resistance to cephradine who were assigned to this group, one lethal fetal malformation, one failed to accomplish with treatment (no reasons stated), one low urinary tract infection, one dermatological reaction (rash) to cefuroxime. Intention to treat analysis not performed. Socio‐economic status of the population not stated. Authors conclude that, according the results of this study, CFX is a more efficient therapy that CPD, with similar costs. Funded by Glaxo‐Wellcome, manufacturer of Curocef (R). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | High risk | Not done. |
| Allocation concealment? | High risk | Allocation by alternation, according to a "strict" admission order. |
| Blinding? All outcomes | High risk | |
| Incomplete outcome data addressed? All outcomes | Low risk | |
Santiago 2001.
| Methods | Published only as an abstract. Participants prospectively randomised (no details). | |
| Participants | 106 participants with acute pyelonephritis. Inclusion criteria: fever, low urinary tract symptoms, costovertebral angle tenderness, and a compatible urine analysis. Exclusion criteria: renal function impairment, septic shock, hypersensitivity to gentamicin, as well as other diseases that might affect renal function. | |
| Interventions | Single (S) regimen (n = 59): 3 mg/kg once. Multiple (M) regimen (n = 47): 3 mg/kg 3 times a day for 7 days. | |
| Outcomes | Clinical remission at 72 hours (57/59 (96.6%) vs 47/47 (100%), P > 0.05); microbiologic resolution (59/59 (100%) vs 45/47 (95.7%), P > 0.05); days of hospitalisation (3.5 +‐ 0.9 vs 3.6 +‐ 1.4 days), and nephrotoxicity (serum creatinine > 1.1 mg/dl) (2/59 (3.4%) vs 1/47 (2.1%), P > 0.05). | |
| Notes | CEDIP, Sotero del Rio Hospital, Obstetrics and Gynaecology, University Catolica de Chile, Puente Alto, Santiago, Chile 2001. Published only as an abstract. No other data. Authors stated that demographic background was similar between groups. Authors concluded that in women with acute pyelonephritis during pregnancy, a single or multiple doses of gentamicin results in comparable clinical efficacy. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Prospective randomisation. |
Tehran 2006.
| Methods | Randomised controlled trial. Random blocks (no further details). | |
| Participants | 128 pregnant women past 24 weeks' gestation with acute pyelonephritis. | |
| Interventions | Outpatient therapy (n = 64): two 1‐g doses IM ceftriaxone at 24 hours interval while hospitalised. Discharged and re‐evaluated within 48‐72 hours. Inpatient therapy (n = 64): two 1‐g doses IM ceftriaxone at 24 hours interval while hospitalised. Remained hospitalised until non‐febrile for 48 hours. All patients completed a 14‐day course of oral cephalexin. Urine cultures on admission and 10‐14 days after therapy. |
|
| Outcomes | Maternal outcomes: persistent bacteriuria (inpatient 30/64 (46.88%), outpatient 11/64 (17.19%), P = 0.001); no response to initial treatment (inpatient 9/64 (14.06%), outpatient 4/64 (6.25%), P = NS); recurrent bacteriuria (inpatient 14/64 (21.8%), outpatient 34/64 (53.1%), P = 0.013); recurrent pyelonephritis (Inpatient 7/64 (10.9%), outpatient 16/64 (25%), P = NS). Neonatal outcomes: gestational age at delivery (inpatient 37.21 +‐ 1.62, outpatient 38.86 +‐ 0.21, P = NS); preterm delivery (inpatient 12/64 (18.75%), outpatient 2/64 (3.13%), P = 0.03); birth weight (inpatient 3120 +‐ 463.03, outpatient 2659 +‐ 382.32; P = 0.04). | |
| Notes | Imam Khomeini hospital, Tehran and Sahid Dr Bahonar hospital, Kerman, Tehran, Iran. Published only as an abstract. No other data. Authors stated that the two groups were similar regarding age, parity, temperature, estimated gestational age, initial white blood cell count, and incidence of bacteriemia. Authors concluded that in selected pregnant women with pyelonephritis outpatient antibiotic therapy is effective and safe. The neonatal outcomes were better in outpatients and the maternal outcomes in inpatients. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Random blocks. |
AM: ampicillin CB: ceftibuten CFU: colony‐forming units CFX: cefuroxime CPD: cephradine FT: fosfomycin trometamol HPF: high‐power field IM: intramuscular IV: intravenous MN: nitrofurantoin NICU: neonatal intensive care unit q.i.d: four times a day USD: United States dollars UTI: urinary tract infection VS: versus
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Aylesbury 1985 | The study includes asymptomatic and symptomatic pregnant women and it is not possible to analyse them separately. |
| Florida 1990 | Oral treatment (cephalexin 1 g followed by 500 mg every 6 hours, or ampicillin 500 mg every 6 hours) or intravenous treatment (cephalotin 1 g every 6 hours or ampicillin 1 g every 6 hours) was given randomly to 90 participants, 45 in each group. 12 participants (11.8%) were excluded from the randomisation because of high‐risk pre‐existing medical conditions and 13 participants (14.4%) were excluded before treatment, but after randomisation, because of positive blood cultures. Relevant and interpretable data are not clearly presented, as well as the data from each group separately. Cure rates (oral 3/32, IV 3/39) and recurrent pyelonephritis (oral 3/35, IV 5/42) were presented; preterm delivery rates are presented in a way that is impossible to analyse. |
| Greece 2007 | The study includes asymptomatic and symptomatic pregnant women and it is not possible to analyse them separately. |
| London 1972 | This study includes three different groups of participants: pregnant women, participants from general practice and hospital patients who acquired infection after admission. It included 18 male participants. The groups are not analysed separately, including symptomatic versus asymptomatic pregnant women. |
| London 1979 | The study includes both asymptomatic and symptomatic pregnant women, and it is not possible to analyse them separately. |
| New York 1992 | Patients with symptoms of pyelonephritis were excluded. Patients with asymptomatic bacteriuria and cystitis were included, but data are not separated. |
| Providence 1990 | The study includes asymptomatic and symptomatic pregnant women and it is not possible to analyse them separately. |
| Victoria 1965 | The study includes asymptomatic and symptomatic pregnant women and it is not possible to analyse them separately. |
IV: intravenous
Contributions of authors
Juan C Vazquez: protocol development, abstract form development, abstraction, data table development, data entry, data analysis, writing of review and the 2010 update. Edgardo Abalos, who joined the review team to update the review in 2010: data table development, data analysis, and writing of review. José Villar: protocol development, data analysis, and writing of the first published version.
Sources of support
Internal sources
America Arias Hospital, Havana, Cuba.
Instituto Nacional de Endocrinologia, Havana, Cuba.
External sources
HRP/WHO, Geneva, Switzerland.
Declarations of interest
None known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Bratislava 2001 {published data only}
- Krcmery S, Hromec J, Demesova D. Treatment of lower urinary tract infection in pregnancy. International Journal of Antimicrobial Agents 2001;17:279‐82. [DOI] [PubMed] [Google Scholar]
Charleston 1996 {published data only}
- Brost BC, Campbell B, Stramm S, Eller D, Newman RB. Randomized clinical trial of antibiotics therapy for antenatal pyelonephritis. Infectious Diseases in Obstetrics and Gynecology 1996;4:294‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Florida 1995 {published data only}
- McAlpine KJ, Sanchez‐Ramos L. Pyelonephritis in pregnancy: once‐a‐day versus multiple dose therapy. American Journal of Obstetrics and Gynecology 1993;168:426. [DOI] [PubMed] [Google Scholar]
- Sanchez‐Ramos L, McAlpine KJ, Adair CD, Kaunitz AM, Delke I, Briones DK. Pyelonephritis in pregnancy: once‐a‐day ceftriaxone vs multiple doses of cefazolin. American Journal of Obstetrics and Gynecology 1995;172:129‐33. [DOI] [PubMed] [Google Scholar]
Los Angeles 1995 {published data only}
- Beckman CRB. Outpatient treatment of pyelonephritis in pregnancy: a randomized controlled trial [letter]. Obstetrics & Gynecology 1996;87:479. [DOI] [PubMed] [Google Scholar]
- Millar L, Wing D, Paul R, Grimes D. Outpatient treatment of pyelonephritis in pregnancy. American Journal of Obstetrics and Gynecology 1994;170:297. [DOI] [PubMed] [Google Scholar]
- Millar LK, Wing DA, Paul RH, Grimes DA. Outpatient treatment of pyelonephritis in pregnancy: a randomized controlled trial. Obstetrics & Gynecology 1995;86:560‐4. [DOI] [PubMed] [Google Scholar]
Los Angeles 1998 {published data only}
- Wing DA, Hendershott CM, Debuque L, Millar LK. A randomized trial of three antibiotic regimens for the treatment of pyelonephritis in pregnancy. Obstetrics & Gynecology 1998;92:249‐53. [DOI] [PubMed] [Google Scholar]
- Wing DA, Hendershott CM, Paul RH, Millar LK. A randomized trial of three antibiotic regimens for the treatment of pyelonephritis in pregnancy. American Journal of Obstetrics and Gynecology 1998;178:S211. [DOI] [PubMed] [Google Scholar]
Los Angeles 1999 {published data only}
- Wing DA, Hendershott CM, Debuque L, Millar LK. Outpatient treatment of acute pyelonephritis in pregnancy after 24 weeks. Obstetrics & Gynecology 1999;94(5 Pt 1):683‐8. [DOI] [PubMed] [Google Scholar]
Mexico 1989 {published data only}
- Calderon‐Jaimes J, Arredondo‐Garcia JL, Olvera‐Salinas J, Echaniz‐Aviles G, Conde‐Gonzalez C, Hernandez‐Nevarez P. Acute cystourethritis during pregnancy [Cistouretritis aguda durante la gestacion]. Ginecologia y Obstetricia de Mexico 1989;57:57‐63. [PubMed] [Google Scholar]
Santiago 2000 {published data only}
- Ovalle A, Martinez MA, Wolff M, Cona ET, Valderrama OC, Villablanca EO, et al. Efficacy, safety and cost of cefuroxime compared with cephradine in the treatment of acute pyelonephritis during pregnancy [Estudio prospectivo, randomizado, comparativo de la eficacia, seguridad y costos de cefuroxima vs cefradina en la pielonefritis aguda del embarazo]. Revista Medica de Chile 2000;128:749‐57. [PubMed] [Google Scholar]
Santiago 2001 {published data only}
- Nien JK, Medina L, Gomez R, Yamamoto M, Gonzalez R, Carstens M, et al. Single versus multiple doses of gentamicin in the treatment of pyelonephritis during pregnancy: a randomized controlled trial. American Journal of Obstetrics and Gynecology 2001;184(1):S193. [Google Scholar]
Tehran 2006 {published data only}
- Ahmadinejad Z, Hantooshzadeh S. Outpatient treatment of acute pyelonephritis in pregnancy after 24 weeks. A randomised controlled trial. 16th European Congress of Clinical Microbiology and Infectious Diseases (ECCMID); 2006 April 1‐4; Nice, France 2006:Abstract no: P1702.
References to studies excluded from this review
Aylesbury 1985 {published data only}
- Masterton RG, Evans DC, Strike PW. Single‐dose amoxycillin in the treatment of bacteriuria in pregnancy and the puerperium ‐ a controlled clinical trial. British Journal of Obstetrics and Gynaecology 1985;92:498‐505. [DOI] [PubMed] [Google Scholar]
Florida 1990 {published data only}
- Angel JL, O'Brien WF, Finan MA, Morales WJ, Lake M, Knuppel RA. Acute pyelonephritis in pregnancy: a prospective study of oral versus intravenous antibiotic therapy. Obstetrics & Gynecology 1990;76:28‐32. [PubMed] [Google Scholar]
- Sanchez‐Ramos L, Wears RL. Acute pyelonephritis in pregnancy: a prospective study of oral versus intravenous antibiotic therapy. Obstetrics & Gynecology 1990;76:891. [PubMed] [Google Scholar]
Greece 2007 {published data only}
- Stamatiou K, Alevizos A, Petrakos G, Lentzas I, Papathanasiou M, Mariolis A, et al. Study on the efficacy of cefaclor for the treatment of asymptomatic bacteriuria and lower urinary tract infections in pregnant women with a history of hypersensitivity to penicillin. Clinical and Experimental Obstetrics and Gynecology 2007;34(2):85‐7. [PubMed] [Google Scholar]
London 1972 {published data only}
- Brumfitt W, Pursell R. Double‐blind trial to compare ampicillin, cephalexin, co‐trimoxazole, and trimethoprim in treatment of urinary infection. British Medical Journal 1972;2:673‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brumfitt W, Pursell R. Trimethoprim‐sulphamethoxazole in the treatment of urinary infection. Medical Journal of Australia Special Supplement 1973;1:44.8. [DOI] [PubMed] [Google Scholar]
London 1979 {published data only}
- Brumfitt W, Franklin I, Hamilton‐Miller J, Anderson F. Comparison of pivmecillinam and cephradine in bacteriuria in pregnancy and in acute urinary tract infection. Scandinavian Journal of Infectious Diseases 1979;11:275‐9. [DOI] [PubMed] [Google Scholar]
New York 1992 {published data only}
- Adelson MD, Graves WL, Osborne NG. Treatment of urinary infections in pregnancy using single versus 10‐day dosing. Journal of the National Medical Association 1992;84:73‐5. [PMC free article] [PubMed] [Google Scholar]
Providence 1990 {published data only}
- Zinner S. Fosfomycin trometamol versus pipemidic acid in the treatment of bacteriuria in pregnancy. Chemotherapy 1990;36:50‐2. [DOI] [PubMed] [Google Scholar]
- Zinner SH, Kass EH. Long term (10 to 14 years) follow‐up of bacteriuria of pregnancy. New England Journal of Medicine 1971;285:820‐4. [DOI] [PubMed] [Google Scholar]
Victoria 1965 {published data only}
- Kincaid‐Smith P, Bullen M. Bacteriuria in pregnancy. Lancet 1965;1:1382‐7. [DOI] [PubMed] [Google Scholar]
Additional references
Belady 1997
- Belady PH, Farkou LJ, Gibbs RS. Intraamniotic infection and premature rupture of membranes. Clinics in Perinatology 1997;24:43‐57. [PubMed] [Google Scholar]
Block 1987
- Block JM, Walstad RA, Bjertnaes A, Holfstad PE, Holte M, Ottemo I, et al. Ofloxacin versus trimethoprim‐sulphamethoxazole in acute cystitis. Drugs 1987;34 Suppl 1:100‐6. [DOI] [PubMed] [Google Scholar]
Botella 1981
- Botella J, Clavero JA. Enfermedades que complican la gestacion. In: Botella J, Clavero JA editor(s). Tratado de ginecologia. Barcelona: Cientifico Medica, 1981:99‐109. [Google Scholar]
Cunningham 1994
- Cunningham FG, Lucas MJ. Urinary tract infections complicating pregnancy. Baillieres Clinical Obstetrics and Gynaecology 1994;8:353‐73. [DOI] [PubMed] [Google Scholar]
Davies 1975
- Davies BL, Mummery RV, Brumfitt W. Ampicillin, carbenicillin indamyl ester, and nifuratel in domiciliary practice. British Journal of Urology 1975;47:335‐41. [DOI] [PubMed] [Google Scholar]
De Cecco 1987
- Cecco L, Ragni N. Urinary tract infections in pregnancy: monuril single‐dose treatment versus traditional therapy. European Urology 1987;Suppl 1:108‐13. [DOI] [PubMed] [Google Scholar]
Deeks 2005
- Deeks JJ, Higgins JPT, Altman DG, editors. Analysing and presenting results. In: Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions 4.2.5 [updated May 2005]; Section 8. In: The Cochrane Library, Issue 3, 2005. Chichester, UK: John Wiley & sons, Ltd.
Egger 2001
- Egger M, Smith GD, Altman DG. Systematic reviews in health care: meta‐analyses in context . Second Edition. London : BMJ , 2001 . [Google Scholar]
Grischke 1987
- Grischke EM, Ruttgers H. Treatment of bacterial infections of the female urinary tract by immunization of the patients. Urologia Internationalis 1987;42:338‐41. [DOI] [PubMed] [Google Scholar]
Higgins 2008
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.1 [updated September 2008]. The Cochrane Collaboration, 2008. Available from www.cochrane‐handbook.org.
Ishigami 1976
- Ishigami J, Mita T, Kataoka N, Miyazaki S, Kaneda K. Comparative clinical experiment by double‐blind method in acute lower urinary tract infections with pipemidic acid and piromidic acid. Japanese Journal of Antibiotics 1976;29:167‐77. [PubMed] [Google Scholar]
Jones 1996
- Jones B, Jarvis P, Lewis JA, Ebbutt AF. Trials to assess equivalence: the importance of rigorous methods. BMJ 1996;313:36‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kaye 1985
- Kaye D. Infecciones del aparato urinario. In: Moniff GRG editor(s). Enfermedades infecciosas en obstetricia y ginecologia. La Habana: Cientifico‐Tecnica, 1985:379‐401. [Google Scholar]
Mercado 1994
- Mercado A. Enfermedad renal y embarazo. In: Cifuentes R editor(s). Obstetricia de alto riesgo. Cali: Aspromedica, 1994:665‐87. [Google Scholar]
Norrby 1990
- Norrby SR. Short‐term treatment of uncomplicated lower urinary tract infections in women. Reviews of Infectious Diseases 1990;12:458‐67. [DOI] [PubMed] [Google Scholar]
Patel 1997
- Patel SS, Balfour JA, Bryson HM. Fosfomicin tromethamine. A review of its antibacterial activity, pharmacokinetic properties and therapeutic efficacy as a single‐dose oral treatment for acute uncomplicated lower urinary tract infections. Drugs 1997;53:637‐56. [DOI] [PubMed] [Google Scholar]
Pedler 1985
- Pedler SJ, Bint AJ. Comparative study of amoxycillin‐clavulanic acid and cephalexin in the treatment of bacteriuria during pregnancy. Antimicrobial Agents and Chemotherapy 1985;27:508‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 2008 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.0. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2008.
Romero 1988
- Romero R, Mazor M. Infection and preterm labour. Clinical Obstetrics and Gynecology 1988;2:553‐84. [DOI] [PubMed] [Google Scholar]
Schwarcz 1989
- Schwarcz LS, Duverges CA, Diaz AG, Fescina RH. Enfermedades del Aparato Urinario. In: Schwarcz LS, Duverges CA, Diaz AG, Fescina RH editor(s). Obstetricia. Buenos Aires: El Ateneo, 1989:269‐75. [Google Scholar]
Seebode 1986
- Seebode JJ, Kamat MH, Apuzzio J. Infecciones de las vias urinarias en el embarazo. In: Iffy L, Kaminetzky HA editor(s). Obstetricia y perinatologia. Buenos Aires: Panamericana, 1986:1086‐90. [Google Scholar]
Smaill 2007
- Smaill FM, Vazquez JC. Antibiotics for asymptomatic bacteriuria in pregnancy. Cochrane Database of Systematic Reviews 2007, Issue 2. [DOI: 10.1002/14651858.CD000490.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Stein 1998
- Stein GE. Single‐dose treatment of acute cystitis with fosfomicin tromethamine. Annals of Pharmacotherapy 1998;32:215‐9. [DOI] [PubMed] [Google Scholar]
Van Dorsten 1987
- Dorsten JP, Lenke RR, Schifrin BS. Pyelonephritis in pregnancy: the role of in‐hospital management and nitrofurantoin suppression. Journal of Reproductive Medicine 1987;32:895‐900. [PubMed] [Google Scholar]
Wren 1969
- Wren BG. Subclinical renal infection and prematurity. Medical Journal of Australia 1969;2(12):596‐600. [DOI] [PubMed] [Google Scholar]
Wren 1972
- Wren BG. Double‐blind trial comparing trimethoprim‐sulphamethoxazole (bactrim) with ampicillin in treating urinary infections. Medical Journal of Australia 1972;1:261‐3. [PubMed] [Google Scholar]
References to other published versions of this review
Vazquez 2003
- Vazquez JC, Villar J. Treatments for symptomatic urinary tract infections during pregnancy. Cochrane Database of Systematic Reviews 2003, Issue 4. [Art. No.: CD002256. DOI: 10.1002/14651858.CD002256] [DOI] [PubMed] [Google Scholar]


