Abstract
Background
Because of the association of prone positioning with sudden infant death syndrome (SIDS) it is recommended that young infants be placed on their backs (supine). However, the prone position may be a non‐invasive way of increasing oxygenation in participants with acute respiratory distress. Because of substantial differences in respiratory mechanics between adults and children and the risk of SIDS in young infants, a specific review of positioning for infants and young children with acute respiratory distress is warranted.
Objectives
To compare the effects of different body positions in hospitalised infants and children with acute respiratory distress.
Search methods
We searched Cochrane Central Register of Controlled Trials (CENTRAL 2012, Issue 3), which contains the Acute Respiratory Infections Group's Specialised Register, MEDLINE (1966 to April week 1, 2012), EMBASE (2004 to April 2012) and CINAHL (2004 to April 2012).
Selection criteria
Randomised controlled trials (RCTs) or pseudo‐RCTs comparing two or more positions in the management of infants and children hospitalised with acute respiratory distress.
Data collection and analysis
Two review authors independently extracted data from each study. We resolved differences by consensus or referral to a third review author. We analysed bivariate outcomes using an odds ratio and 95% confidence interval (CI). We analysed continuous outcomes using a mean difference and 95% CI. We used a fixed‐effect model unless heterogeneity was significant, in which case we used a random‐effects model.
Main results
We extracted data from 53 studies. We included 24 studies with a total of 581 participants. Three studies used a parallel‐group, randomised design which compared prone and supine positions only. The remaining 21 studies used a randomised cross‐over design. These studies compared prone, supine, lateral, elevated and flat positions.
Prone positioning was significantly more beneficial than supine positioning in terms of oxygen saturation (mean difference (MD) 1.97%, 95% CI 1.18 to 2.77), arterial oxygen (MD 6.24 mm Hg, 95% confidence interval (CI) 2.20 to 10.28), episodes of hypoxaemia (MD ‐3.46, 95% CI ‐4.60 to ‐2.33) and thoracoabdominal synchrony (MD ‐30.76, 95% CI ‐41.39 to ‐20.14). No adverse effects were identified. There were no statistically significant differences between any other positions.
As the majority of studies did not describe how possible biases were addressed, the potential for bias in these findings is unclear.
Authors' conclusions
The prone position was significantly superior to the supine position in terms of oxygenation. However, as most participants were ventilated preterm infants, the benefits of prone positioning may be most relevant to these infants. In addition, although placing infants and children in the prone position may improve respiratory function, the association of SIDS with prone positioning means that infants should only be placed in this position while under continuous cardiorespiratory monitoring.
Plain language summary
Positioning for acute respiratory distress in hospitalised infants and children
Acute respiratory distress is one of the most frequent causes of hospitalisation and death in infants and young children globally. When children with severe respiratory distress are put in hospital, treatment may include additional oxygen or assisted ventilation which may damage the lungs. Infants and children with respiratory distress placed in particular positions may be more comfortable, breathe more easily and have better outcomes. However, different positions may also increase the risk of adverse outcomes. Therefore we searched the literature to identify controlled clinical trials comparing two or more body positions in the management of infants and children hospitalised with acute respiratory distress.
We included 24 studies with a total of 581 participants. Lying on the front (the prone position) was better than lying on the back (the supine position) for oxygenating the blood but the difference was small. The increase in oxygen saturation on average increased by 2%. This finding was based on data from nine studies (195 children, 165 preterm and 95 ventilated) measuring this outcome. The rapid rate of breathing with respiratory distress was slightly lower in the prone position (on average four breaths/minute lower) based on six studies (100 infants aged up to one month, 59 ventilated). No adverse effects were identified. There were no obvious differences with other positions. As most of the 581 children in these studies were preterm babies (60%) and (70%) ventilated by machine, the benefits of prone positioning may be most relevant to these infants.
***It is important to remember that these children were hospitalised. Therefore, given the association of the prone position with sudden infant death syndrome (SIDS), the prone position should not be used for children unless they are in hospital and their breathing is constantly monitored.
As the majority of studies did not describe how possible biases in the design of the study were addressed, the potential for bias in these findings is unclear. Also the findings of this review are limited by the small number of participants in studies, changes in infants and children were only measured for short time periods and the small changes in oxygenation which were seen in this review are not as meaningful as other measures of illness and recovery. Only five studies looked at children older than one year and few studies compared positions other than prone and supine.
Background
Description of the condition
Acute respiratory distress is one of the most frequent causes of hospitalisation and death in young children (Buckmaster 2007; Meurer 2000; Mintegi Raso 2004; Shay 1999) and infants (Ahmed 2004; Caitlin 2008; Chang 2000; Simiyu 2004; Sritipsukho 2007) across the world. While there is no official definition of acute respiratory distress (or breathing difficulty), it is clinically recognised as a presentation of any of the following signs or symptoms: shortness of breath, wheeze, increased breathing rate, increased heart beat, increased chest wall retractions, thoracoabdominal asynchrony, pallor, cyanosis, nasal flare, expiratory grunt and fatigue. Respiratory distress can lead to hypoxaemia with decreased PaO2 (arterial oxygen pressure), increased PaCO2 (arterial carbon dioxide pressure), altered neurological status (confusion) and ultimately to respiratory and/or multiple organ failure and eventually death (Hazinski 1992). Most infants and young children who develop mild to moderate respiratory distress can be managed at home. However, children with more severe respiratory distress require hospitalisation for treatment, which may include supplemental oxygen, intravenous fluids, intravenous antibiotics and possibly assisted ventilation.
Description of the intervention
Positioning participants for therapeutic effect has long been proposed as a mechanism for increasing oxygenation in participants with acute respiratory distress. Body positioning is a non‐invasive intervention which may augment oxygenation while avoiding some of the associated risks (Ball 1999; Bateman 2000; Dean 1996; Lodato 1990). In children, and particularly infants, the risk of injury from oxygen toxicity and mechanical ventilation is greater than in adults as the lungs are going through a period of high growth and development (Bateman 2000; Hazinski 1992). Positioning may reduce the need for such interventions, or at least reduce the length of time they are required, thereby reducing the associated risk of longer‐term lung damage.
How the intervention might work
Numerous studies of both adult and paediatric participants in acute care settings with acute respiratory distress have found the prone position to improve arterial oxygenation when compared to the supine position (Blanch 1997; Bryan 1974; Chatte 1997; Douglas 1977; Fridrich 1996; Gattinoni 1991; Langer 1988; Murdoch 1994; Pappert 1994; Piehl 1976; Pelosi 1998; Stocker 1997; Wagaman 1979). In addition, the use of the prone position is again becoming popular for participants with acute respiratory distress syndrome in intensive care units (Lewandowski 1996). However, the use of prone positioning for infants and young children is controversial as it is linked to sudden infant death syndrome (SIDS) (Ponsonby 1992). Other positions, including lateral (side‐lying) positioning have also been proposed to assist in maintaining optimal ventilation and oxygenation during episodes of respiratory distress (Ball 1999; Dean 1985; Hazinski 1992; Wong 1999).
Structural differences of the respiratory system are evident in infants and young children when compared with adults. Alveolar and bronchiolar growth continues after birth with the alveolar surface area increasing by a factor of 20 as a person reaches adulthood (Hazinski 1992). Supporting airway cartilage, small airway muscles and the intercostal muscles are not fully developed until school age (Adams 1994; Agostoni 1965; Muller 1979) and the chest wall of the infant and young child is much more compliant than the chest wall of an adult (Hazinski 1992). These differences may lead to a relative increase in the infant's or child's respiratory effort during an episode of respiratory distress, which may further compromise the ability to maintain effective ventilation (Adams 1994; Davis 1987; Hazinski 1992; Muller 1979).
Why it is important to do this review
A specific review of positioning for infants and young children with respiratory distress is warranted as the structure and respiratory mechanics of the infant and young child differ from those of the adult. Additionally, infants and young babies differ from adults in their ability to move between positions and to maintain any one particular position. Furthermore, there is the increased risk of SIDS in infants placed in a prone position. Therefore, it is necessary to clarify the benefits and potential risks associated with body positioning in hospitalised infants and children with acute respiratory distress to provide evidence‐based clinical practice.
There have been three reviews without meta‐analysis of positioning in participants with respiratory distress (Ball 1999; Curley 1999; Wong 1999). All of them found that the prone position improves oxygenation. However, most of the participants in the reviewed studies were adult. Several of the studies in these reviews included small numbers of children but neonates were excluded. Nevertheless, the prone position was found to slightly improve oxygenation in a systematic review of 11 trials of neonates undergoing mechanical ventilation (Balaguer 2006).
This review is an update of a systematic review of the effects of positioning on respiratory distress in infants and children (Gillies 2009; Wells 2005).
Objectives
To compare the effects of different body positions on hospitalised infants and children with acute respiratory distress.
Methods
Criteria for considering studies for this review
Types of studies
All randomised controlled trials (RCTs) or pseudo‐RCTs comparing two or more positions in the management of infants and children hospitalised with acute respiratory distress.
Types of participants
Hospitalised infants and children up to 16 years of age with a primary or secondary diagnosis of acute respiratory distress or with an acute exacerbation of a chronic respiratory illness. The studies included the following conditions in infants and children:
acute respiratory failure;
acute respiratory distress syndrome;
acute lung injury;
acute respiratory distress due to lower respiratory tract infections: bronchiolitis, pneumonia (bacterial, viral and atypical);
bronchitis, legionella, whooping cough; chronic neonatal lung disease (bronchopulmonary dysplasia, respiratory distress syndrome of the preterm infant, hyaline membrane disease);
upper respiratory tract infections including croup, epiglottitis (laryngotracheobronchitis);
laryngeal infections; acute episodes of chronic suppurative lung diseases including bronchiectasis and cystic fibrosis; inflammatory respiratory conditions such as asthma;
congenital malformations of the bronchi, lungs, diaphragm and rib cage;
disorders of the pleura (for example pneumothorax, pleural effusion).
The pathophysiology of acute respiratory distress varies substantially. We therefore decided to undertake a subgroup analysis based on the reported cause (and related pathophysiology) of respiratory distress.
We also proposed to include a subgroup analysis of the following age categories:
preterm and neonatal infants (birth to 28 days);
infants (28 days to 12 months);
toddlers and young children (12 months to five years);
school‐age children (five to 16 years).
As there may be greater scope for non‐ventilated participants to benefit from therapeutic body positioning, we also planned a subgroup analysis of ventilated versus non‐ventilated children.
Types of interventions
Body positioning utilised for the management of infants and children with acute respiratory distress included the following:
sitting ‐ erect sitting, lean forward sitting and non‐erect sitting;
prone ‐ prone abdomen free, prone abdomen restricted, semi‐prone, horizontal (flat) and head elevated;
lateral/side‐lying position ‐ horizontal (flat) and head elevated (this position can be good‐lung dependent, where the patient lies on the side of the healthy lung; or good‐lung independent, where the patient lies on the opposite side to the healthy lung);
supine ‐ horizontal (flat) and head elevated;
recumbent/semirecumbent;
kinetic positioning ‐ continuous postural therapy (usually with an automated bed);
body tilting.
We planned a subgroup analysis based on the temporal parameters of the positioning if appropriate.
Types of outcome measures
Primary outcomes
Episodes of apnoea
Mortality ‐ respiratory events
Oxygen saturation (SaO2)
Blood gases (PaCO2 and PaO2)
Oxygenation indices (PaO2/FiO2)
Secondary outcomes
Respiratory rate
Respiratory effort (as defined by study authors)
Heart rate
Per cent inspired oxygen received (FiO2; standardised protocol))
Duration of supplemental oxygenation (standardised protocol)
Intensive care unit (ICU) admission (standardised protocol)
Length of hospital stay
Mortality ‐ all causes
Haemodynamic parameters
Ventilatory parameters
Potential adverse outcomes
Accidental removal and compression of intravenous lines and/or endotracheal tube
Cutaneous damage to the chest wall
Facial oedema
Contractures of the hip and shoulder
Raised intra‐ocular pressure/deterioration in visual acuity
Gastrointestinal event
Any other adverse events reported by study authors
Search methods for identification of studies
Electronic searches
For this update we searched the Cochrane Central Register of Controlled Trials (CENTRAL) 2012, Issue 3, part of The Cochrane Library, www.thecochranelibrary.com (accessed 12 April 2012) which contains the Acute Respiratory Infections Group's Specialised Register, MEDLINE (June 2008 to April week 1, 2012), EMBASE (January 2010 to April 2012) and CINAHL (2008 to April 2012). See Appendix 1 for details of previous search.
We searched MEDLINE and CENTRAL using the following search strategy. We combined the MEDLINE search terms with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE, sensitivity‐ and precision‐maximising version (2008 revision); Ovid format (Lefebvre 2011). The search strategy was adapted to search EMBASE (see Appendix 2) and CINAHL (see Appendix 3).
MEDLINE (Ovid)
1 exp Bronchiolitis/ 2 bronchiolit*.tw. 3 Respiratory Syncytial Virus Infections/ 4 respiratory syncytial viruses/ or respiratory syncytial virus, human/ 5 (respiratory syncytial virus* or rsv).tw. 6 exp Bronchial Diseases/ 7 bronchopneumon*.tw. 8 (acute lung injur* or ali).tw. 9 exp Respiratory Tract Infections/ 10 (respir* adj5 infect*).tw. 11 exp Respiratory Insufficiency/ 12 (respir* adj3 (insufficien* or fail*)).tw. 13 exp Lung Diseases/ 14 pneumon*.tw. 15 ((respir* or bronch*) adj25 distress*).tw. 16 (ards or rds).tw. 17 hyaline membrane disease*.tw. 18 bronchopulmonary dysplasia.tw. 19 chronic neonatal lung disease*.tw. 20 or/1‐18 21 exp Posture/ 22 postur*.tw. 23 supine.tw. 24 prone.tw. 25 (face adj5 down*).tw. 26 (side adj5 (lay or laying or laid or lays or lying or lies)).tw. 27 lateral*.tw. 28 (kinetic adj5 position*).tw. 29 continuous body position*.tw. 30 upright.tw. 31 (high adj5 sitting).tw. 32 (semi‐recumbent or semirecumbent or semi‐recline* or semirecline*).tw. 33 position*.tw. 34 or/21‐33 35 20 and 34
Searching other resources
We made all reasonable efforts to contact recognised experts in the fields of respiratory and intensive care medicine to obtain any additional trials. We manually searched the reference lists of included and excluded studies to identify any other published or unpublished works relevant to the review topic. There were no language or publication restrictions.
Data collection and analysis
Selection of studies
Two review authors independently examined all potentially relevant citations and retrieved in full those thought to fulfil the selection criteria. Where we could not make a judgement based on the citation, or could not achieve consensus, we obtained the full article. Two review authors independently compared each article against the selection criteria to determine which articles to select for data extraction. If differences existed we resolved them either by consensus or by referral to a third review author. We considered non‐English language articles eligible for selection to reduce the risk of publication bias.
Data extraction and management
We used a standardised data extraction form. Two review authors independently extracted data from each study without blinding to authorship or journal publication. Where differences existed we resolved them either by consensus or by referral to a third review author. Where data were missing, or further information was required, we made reasonable attempts to contact the trial authors to obtain the required information. We extracted data from graphs where necessary (Bjornson 1992; Bozynski 1988). Where duplicate publication occurred, the publication with the most data was the primary reference for the review. We kept and managed records of all articles identified from the search strategies, included and excluded, using a reference management system.
Data extraction included the following categories.
Method of allocation
Concealment of allocation
Country and setting where study was performed
Participant details
Inclusion and exclusion criteria
Details of intervention
Outcomes measured
Confounders
Duration of study and frequency of measurements
Numbers enrolled and completing in each group
Baseline characteristics
Results for each group
Assessment of risk of bias in included studies
Two review authors independently assessed the quality of the studies to be included without blinding to authorship or journal of publication. We also assessed the quality of the studies based on blinding to intervention and outcome measurement, and completeness of follow‐up. We resolved differences in terms of allocation of studies into quality categories either by consensus or by referral to a third review author.
Measures of treatment effect
For binary outcomes, we calculated the odds ratio (OR) and 95% confidence interval (CI). For continuous outcomes, we calculated the mean difference (MD) and 95% CI using a fixed‐effect model. If we found heterogeneity we used a random‐effects model (see Assessment of heterogeneity).
Unit of analysis issues
Ideally we would have used first period data from the cross‐over trials and combined these data with data from parallel‐group studies. However, few of the cross‐over trials reported such data. Therefore, we followed the recommendations of Elbourne 2002 that "The results of two or more cross‐over trials might be combined, but with this pooled result kept separate from the data from parallel group trials". Because of incomplete information, we could not correct data for paired analysis in the meta‐analysis of cross‐over trials, which may mean that we over‐estimated the pooled variance. Therefore, significant effects across groups may be less apparent in the meta‐analyses of cross‐over trials compared to the meta‐analyses of parallel‐group studies.
Dealing with missing data
We had planned to use intention‐to‐treat (ITT) data wherever these were reported and to conduct sensitivity analyses between studies that did and did not report ITT data. However, because the majority of studies were cross‐over trials this was not possible.
Assessment of heterogeneity
We interpreted a Mantel‐Haenszel Chi2 value of less than 0.10 and/or an I2 statistic greater than 50% as significant heterogeneity. If heterogeneity was significant we used a random‐effects model.
Assessment of reporting biases
If there had been 10 or more parallel trials reporting the same primary outcome, we would have generated a funnel plot (trial effect against trial size) to investigate the possibility of publication bias but there were not enough data to do this.
Data synthesis
We calculated the odds ratio (OR) and 95% confidence interval (95% CI) for binary outcomes using a fixed‐effect model. We calculated the mean difference (MD) and 95% CI interval for meta‐analysis of continuous outcomes using a fixed‐effect model. If heterogeneity was found (see Assessment of heterogeneity), we used a random‐effects model.
Subgroup analysis and investigation of heterogeneity
We used subgroup analyses to compare findings in different age groups. The only primary outcome for which this could be done was SaO2. There were enough data to compare studies of neonates (up to one month old) and children older than one month but there were not enough data from studies that reported separately for older children, i.e. older than one year. We also conducted a subgroup analysis to compare studies of ventilated participants to studies of infants and children who were not ventilated; and infants and children with varying causes of acute respiratory distress.
Sensitivity analysis
We had planned to undertake a sensitivity analysis based on the level of potential allocation bias. However, there were not enough data to be able to do this.
Results
Description of studies
Results of the search
Following the original search and the first and second updates, 3334 citations were identified; 289 trials were potentially relevant. Based on the title and abstract, we obtained 58 citations reporting 53 studies, commentaries and reviews in full text. Following data extraction, we excluded 29 (31 citations) and included 24 studies (27 citations).
Included studies
Overall, we extracted data from 24 studies. Twenty were randomised controlled cross‐over trials (Bhat 2003; Bjornson 1992; Bozynski 1988; Chang 2002; Crane 1990; Ennis 1978; Fox 1990; Heaf 1983; Jenni 1997; Keene 2000; Kornecki 2001; Levene 1990; Mendoza 1991; Mizuno 1995; Mizuno 1999; Oliveira 2009; Polacek 1992; Sconyers 1987; Schlessel 1993; Wolfson 1992). One study was an alternate allocation cross‐over trial (McEvoy 1997). The remaining three studies (Antunes 2003; Curley 2005; Ibrahim 2007) were parallel randomised trials.
There was a total of 581 participants; 344 (59%) participants were preterm neonates. Two hundred and seventy‐six (80%) of the preterm infants included in the analysis were mechanically ventilated (Antunes 2003; Bhat 2003; Bjornson 1992; Bozynski 1988; Chang 2002; Crane 1990; Fox 1990; Keene 2000; McEvoy 1997; Mendoza 1991; Mizuno 1995; Mizuno 1999; Schlessel 1993; Wolfson 1992) and 66 (19%) of preterm neonates were spontaneously breathing (Jenni 1997; Keene 2000; Oliveira 2009). However, it was not clear whether the remaining 20 preterm infants in the study by Bhat 2003 were ventilated or not. Sixty‐six (11%) of the remaining participants were neonates and infants who were not described as preterm although some preterm infants may have been included (Ennis 1978; Heaf 1983; Levene 1990; Sconyers 1987) and 12 of these infants were mechanically ventilated (Ennis 1978; Heaf 1983). The remaining 169 participants were aged one month to 16 years (Curley 2005; Ibrahim 2007; Kornecki 2001; Polacek 1992) all but 15 of whom (Polacek 1992) were mechanically ventilated.
The inclusion/exclusion criteria were not uniform across included studies. Bjornson 1992 excluded preterm infants with cardiac anomalies other than patent ductus arteriosis (PDA) while other authors (Bozynski 1988; Schlessel 1993) excluded only those infants with significant PDA. Kornecki 2001 excluded two children in a prone versus supine comparison due to physical restrictions preventing them from being placed prone, whilst Antunes 2003, Keene 2000 and Oliveira 2009 excluded infants with any condition which prevented them from being positioned prone or supine. Bozynski 1988 excluded participants with asymmetric lung disease whilst Heaf 1983 only studied participants with unilateral (asymmetric) lung disease. Curley 2005 and Ibrahim 2007 both had extensive exclusion criteria which included cardiac, respiratory and neurological abnormalities. Six studies listed no exclusion criteria (Bhat 2003; Ennis 1978; Fox 1990; Heaf 1983; Jenni 1997; Kornecki 2001).
The interventions included: prone versus supine (Antunes 2003; Bhat 2003; Chang 2002; Curley 2005; Fox 1990; Ibrahim 2007; Keene 2000; Kornecki 2001; Levene 1990; McEvoy 1997; Mendoza 1991; Mizuno 1995; Mizuno 1999; Wolfson 1992); supine versus prone versus lateral (Bjornson 1992; Crane 1990; Heaf 1983); supine versus lateral (Bozynski 1988; Ennis 1978; Schlessel 1993); right lateral versus left lateral (Polacek 1992); prone flat versus prone head elevated (Jenni 1997; Sconyers 1987); and supine flat versus supine head elevated (Sconyers 1987).
The study times also varied greatly, ranging from five minutes after a 15‐minute settling in period in Bozynski 1988 to a median of two days in the study by Antunes 2003.
The outcomes measured and reported also varied across studies. Oxygenation outcomes included arterial oxygen saturation (SaO2), partial pressure of arterial oxygen (PaO2), transcutaneous oxygen pressure (PO2), transcutaneous carbon dioxide pressure (PCO2), ratio of PaO2 to supplemental oxygen (PaO2/FiO2) and hypoxaemia (low SaO2). Ventilatory outcomes included tidal volume, minute volume, dynamic lung compliance, inspiratory resistance, expiratory resistance, total pulmonary resistance, work of breathing and laboured breathing index. Other outcomes were heart rate, respiratory rate, oesophageal pressure and adverse events.
Excluded studies
In the original review, we excluded 27 of the 49 citations selected for review as they did not meet the selection criteria. Nine of these studies stated that only healthy babies or babies without respiratory distress were studied (Avery 1962; Brackbill 1973; Dahl 1972; Heimler 1992; Hutchison 1979; Kurlac 1994; Sahni 1999; Schwartz 1975; Vanderghem 1983). Carlo 1989, on the other hand, is a follow‐up study of babies with a history of respiratory distress which were not hospitalised at the time of the study. In the study by Martin (Martin 1979) there was no evidence of respiratory distress in 11 of 16 infants, while in Maynard 2000, seven of 10 infants showed no evidence of respiratory distress. Nine of the studies were not randomly or systematically allocated (Baird 1991; Itakura 1998; Kishan 1981; Kravitz 1958; Lioy 1988; Lopez‐Herce Cid 2003; Murdoch 1994; Numa 1997; Schrod 1993; Wagaman 1979). In addition, there was no evidence of respiratory distress in two of these studies (Kishan 1981; Kravitz 1958). We excluded Murai 1994 due to bias identified in the study description that participants were weaned from oxygen and assisted ventilation during the study time and that the criteria for weaning was "left to the discretion of the individual attending physicians". We also excluded McKinley 2000 as the positions compared were side‐to‐side turns versus prone‐to‐supine turns, and not prone versus supine versus side lying.
In this update, we identified an additional eight citations for review. However, once we obtained full copies of these citations, we excluded three papers (Anonymous 2005; Branco 2005; Main 2006) as they were commentaries on the study by Curley 2005. In addition, we excluded Sawhney 2005 because only 24% of the children in the study had acute respiratory distress.
Risk of bias in included studies
The overall risk of bias is presented graphically in Figure 1 and summarised in Figure 2.
1.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
2.
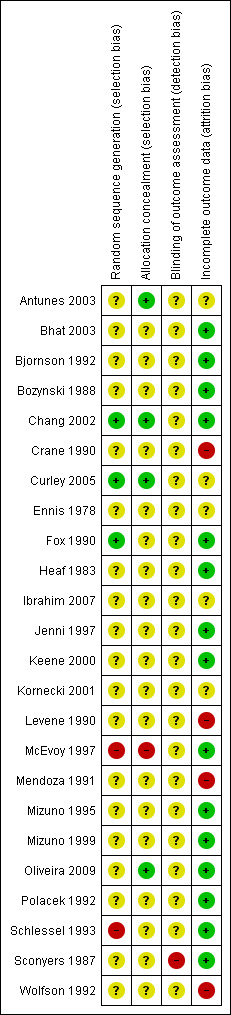
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Only three of the included studies provided an adequate description of the random sequence generation and concealment of allocation (Antunes 2003; Chang 2002; Curley 2005). All of these three studies were at low risk of allocation bias. McEvoy 1997 and Schlessel 1993 were categorised as at high risk of allocation bias. McEvoy 1997 used alternate allocation and in Schlessel 1993 only the lateral positions were randomised. All other studies were considered as unclear risk of bias because sequence generation and allocation concealment was not described.
Blinding
Sconyers 1987 was the only study we considered at high risk of detection bias because bedside observers counted the frequency of breaths and heartbeats. We considered all other studies as an unclear risk because there was inadequate description of the blinding process. In addition, because objective outcomes were used we considered the risk of unblinded observers biasing the outcome unlikely.
Incomplete outcome data
There appeared to be complete follow‐up in the majority of studies which we therefore considered at low risk of attrition bias. We considered five studies as an unclear risk of attrition bias as there was some loss to follow‐up which seemed minimal (Antunes 2003; Curley 2005, Ennis 1978; Ibrahim 2007; Kornecki 2001). However, we considered four studies (Crane 1990; Levene 1990; Mendoza 1991; Wolfson 1992) at high risk of attrition bias. In Crane 1990 data from five of 19 participants were discarded because of "excessive drift or inaccurate or inappropriate calibration" of equipment. Levene 1990 did not collect data from nine infants as the babies did not go to sleep or woke during study time. Mendoza 1991 excluded data for 12 infants from analysis as they did not have any spontaneous or mechanical breaths during the prescribed data collection period and in Wolfson 1992, four of 24 infants were lost to follow‐up though reasons were not given.
Effects of interventions
Parallel studies
Prone versus supine positioning
Oxygenation outcomes
The oxygenation outcomes for the parallel‐group studies comparing prone versus supine positions were the PaO2 and PaCO2 (Curley 2005), PaO2/FiO2 (Curley 2005; Ibrahim 2007), oxygenation index (Curley 2005; Ibrahim 2007) and SaO2 less than 90% (Antunes 2003). The ventilatory outcome tidal volume was also reported in the study by Curley 2005.
There was no difference between prone and supine groups in the percentage of participants experiencing a SaO2 less than 90% at one (odds ratio (OR) 0.13, 95% confidence interval (CI) 0.01 to 1.15; Analysis 1.1) or two days (OR 0.12, 95% CI 0.01 to 2.53; Analysis 1.1). There was also no difference in the PaO2 (mean difference (MD) 2.00, 95% CI ‐5.29 to 9.29; Analysis 1.2), PaCO2 (MD 3.00, 95% CI ‐1.93 to 7.93; Analysis 1.3) or PaO2/FiO2 (MD 28.16, 95% CI ‐9.92 to 66.24; Analysis 1.4). The oxygenation index was significantly improved (MD ‐2.42, 95% CI ‐3.60 to ‐1.25; Analysis 1.5) in the prone group but this was largely due to the greater weighting given to the smaller study by Ibrahim 2007 which reported small standard deviations (SDs). The tidal volume was also significantly lower in the study by Curley 2005 (MD ‐0.60, 95% CI ‐1.05 to ‐0.15; Analysis 1.6).
1.1. Analysis.

Comparison 1 Prone versus supine: parallel trials, Outcome 1 Patients with SaO2 < 90%.
1.2. Analysis.

Comparison 1 Prone versus supine: parallel trials, Outcome 2 PaO2.
1.3. Analysis.

Comparison 1 Prone versus supine: parallel trials, Outcome 3 PaCO2.
1.4. Analysis.

Comparison 1 Prone versus supine: parallel trials, Outcome 4 PaO2/FiO2.
1.5. Analysis.

Comparison 1 Prone versus supine: parallel trials, Outcome 5 Oxygenation Index.
1.6. Analysis.

Comparison 1 Prone versus supine: parallel trials, Outcome 6 Tidal volume.
Adverse events
The study by Curley 2005 also reported extubations due to adverse events, obstructions of the endotracheal tube, pressure ulcers and hypercapnia (high levels of arterial carbon dioxide), while Antunes 2003 reported re‐intubations due to adverse events and atelectasis. There was no difference between prone and supine groups for any of these outcomes (Analysis 1.7) except for re‐intubations, which were significantly lower in the prone group (OR 0.10, 95% CI 0.01 to 0.91; Analysis 1.7).
1.7. Analysis.

Comparison 1 Prone versus supine: parallel trials, Outcome 7 Adverse events.
Respiratory rate
Antunes 2003 also reported respiratory rate, but no SDs (Table 11).
1. Median and range data.
| Study | Outcome | Supine n |
Supine (median) |
Supine (range) |
Prone n |
Prone (median) |
Prone (median) |
| Antunes 2003 | Respiratory rate day 1 | 21 | 25 | 20 to 33 | 21 | 20 | 20 to 24 |
| Antunes 2003 | Respiratory rate day 2 | 20 | 20 | 18 to 29 | 20 | 20 | 20 to 22 |
| Curley 2005 | Minute ventilation |
42 | 1.6 | * 1.0 to 3.2 | 42 | 1.6 | 0.6 to 2.8 |
* Interquartile range
Cross‐over studies
Prone versus supine positioning
Oxygenation outcomes
The oxygenation outcomes for the prone versus supine comparison included SaO2, PaO2, transcutaneous PCO2, oxygenation index and SaO2 less than 80%. The ventilatory outcomes were respiratory rate, tidal volume, minute volume, work of breathing, dynamic lung compliance, total pulmonary resistance, inspiratory resistance, expiratory resistance, thoracoabdominal synchrony and laboured breathing index. Additional outcomes included heart rate and oesophageal pressure. Adverse events were not extracted from cross‐over studies because it was not clear which phase of the study, if any, the event could be attributed to.
The results showed a statistically significant improvement in SaO2 for infants positioned in the prone position (MD 1.97%, 95% CI 1.18 to 2.77; Analysis 2.1). Nine studies measured this outcome (Bhat 2003; Bjornson 1992; Chang 2002; Levene 1990; McEvoy 1997; Mendoza 1991; Mizuno 1995; Mizuno 1999; Oliveira 2009) with a total of 195 participants. The 165 participants in the studies by Bhat 2003, Bjornson 1992, Chang 2002, McEvoy 1997, Mizuno 1995, Mizuno 1999, Mendoza 1991 and Oliveira 2009 were preterm infants with respiratory distress (95 of whom were ventilated). The 30 infants in Levene 1990 were aged two to 11 months and were not ventilated. Levene subcategorised the participants into those with upper respiratory tract infection (URTI) and those with lower respiratory tract infection (LRTI). There was no statistically significant difference in SaO2 between the prone or supine position in the URTI group (MD ‐0.50%, 95% CI ‐2.58 to 1.58; Analysis 2.1) or the LRTI group (MD 1.80%, 95% CI ‐0.78 to 4.38; Analysis 2.1).
2.1. Analysis.

Comparison 2 Prone versus supine: cross‐over trials, Outcome 1 SaO2.
Results of analysis for PaO2 showed a statistically significant increase in PaO2 in the prone position (MD 6.24 mm Hg, 95% CI 2.20 to 10.28; Analysis 2.2). One study measured this outcome (Fox 1990). It included 25 participants, all preterm infants intubated and ventilated with intermittent mandatory ventilation or continuous positive airway pressure.
2.2. Analysis.

Comparison 2 Prone versus supine: cross‐over trials, Outcome 2 PaO2.
Results showed no significant change in transcutaneous PCO2 when comparing prone and supine positioning (MD ‐2.53 mm Hg, 95% CI ‐6.06 to 0.99; Analysis 2.3). Three studies measured transcutaneous PCO2 as an outcome (Crane 1990; Mizuno 1995; Mizuno 1999). They included 27 participants in total, all of whom were mechanically ventilated preterm infants.
2.3. Analysis.

Comparison 2 Prone versus supine: cross‐over trials, Outcome 3 tcPCO2.
One study measured oxygenation index as an outcome (Kornecki 2001). The participants were 10 children aged two months to 11 years requiring mechanical ventilation for moderate to severe acute respiratory failure. Measurements were taken at 0.5 hours (MD ‐0.83, 95% CI ‐8.04 to 6.38; Analysis 2.4), two hours (MD ‐2.08, 95% CI ‐8.61 to 4.45; Analysis 2.4), four hours (MD ‐4.20, 95% CI ‐9.37 to 0.97; Analysis 2.4), six hours (MD ‐5.13, 95% CI ‐10.13 to ‐0.13; Analysis 2.4), eight hours (MD ‐6.89, 95% CI ‐12.93 to ‐0.85; Analysis 2.4) and 12 hours (MD ‐8.13, 95% CI ‐15.01 to ‐1.25; Analysis 2.4). Measurements demonstrated no statistically significant improvement between prone versus supine positioning until the sixth hour measurement. However, this improvement continued and was maintained up to 12 hours.
2.4. Analysis.

Comparison 2 Prone versus supine: cross‐over trials, Outcome 4 Oxygenation Index.
Meta‐analysis for the number of episodes of SaO2 less than 80% showed a statistically significant decrease in the number of episodes of hypoxaemia, defined as an SaO2 of less than 80%, (MD ‐3.46, 95% CI ‐4.60 to ‐2.33; Analysis 2.5). Two studies measured this outcome (Keene 2000; McEvoy 1997). The alternate allocation cross‐over study which included 55 of the 77 participants achieved a weighting of 91.9% (McEvoy 1997).
2.5. Analysis.

Comparison 2 Prone versus supine: cross‐over trials, Outcome 5 Number of episodes SaO2 < 80%.
The results showed a statistically significant decrease in respiratory rate in infants in a prone position (MD ‐3.84 breaths/min, 95% CI ‐5.93 to ‐1.75; Analysis 2.6). There were six studies which measured this outcome (Mendoza 1991; Mizuno 1995; Mizuno 1999; Oliveira 2009; Sconyers 1987; Wolfson 1992). They included a total of 112 participants who were preterm neonates and infants up to one month of age. Fifty‐nine participants were ventilated and 53 were not ventilated.
2.6. Analysis.

Comparison 2 Prone versus supine: cross‐over trials, Outcome 6 Respiratory rate.
Ventilatory outcomes
There was no significant difference in outcomes between the prone and supine groups: tidal volume (spontaneous breaths: MD 0.33 ml/kg, 95% CI ‐0.20 to 0.87, mechanical breaths: MD 0.40 ml/kg, 95% CI ‐0.58 to 1.38; Analysis 2.7), minute volume (spontaneous breaths: MD ‐1.93 ml/kg/min, 95% CI ‐27.69 to 23.84, mechanical breaths: MD 29.50 ml/kg/min, 95% CI ‐19.78 to 78.78; Analysis 2.8), work of breathing (spontaneous breaths: MD ‐0.38 gm.cm/kg, 95% CI ‐6.36 to 5.60, mechanical breaths: MD ‐2.60 gm.cm/kg, 95% CI ‐15.07 to 9.87; Analysis 2.9), dynamic lung compliance (spontaneous breaths: MD 0.07 ml/cm H2O/kg, 95% CI ‐0.18 to 0.33, mechanical breaths: MD ‐0.01 ml/cm H2O/kg, 95% CI ‐0.15 to 0.13; Analysis 2.10), expiratory resistance (spontaneous breaths: MD ‐22.00 cm H2O/L/S, 95% CI ‐63.74 to 19.74, mechanical breaths: MD ‐31.50 cm H2O/L/S, 95% CI ‐79.08 to 16.08; Analysis 2.13), heart rate less than 90 beats/min (MD ‐0.70 beats/min, 95% CI ‐6.92 to 5.52; Analysis 2.17) and oesophageal pressure (MD 0.51 cm H2O, 95% CI ‐3.17 to 4.19; Analysis 2.18).
2.7. Analysis.

Comparison 2 Prone versus supine: cross‐over trials, Outcome 7 Tidal volume.
2.8. Analysis.

Comparison 2 Prone versus supine: cross‐over trials, Outcome 8 Minute volume.
2.9. Analysis.

Comparison 2 Prone versus supine: cross‐over trials, Outcome 9 Work of breathing.
2.10. Analysis.

Comparison 2 Prone versus supine: cross‐over trials, Outcome 10 Dynamic lung compliance.
2.13. Analysis.

Comparison 2 Prone versus supine: cross‐over trials, Outcome 13 Expiratory resistance.
2.17. Analysis.

Comparison 2 Prone versus supine: cross‐over trials, Outcome 17 Number of episodes of heart rate < 90 bpm.
2.18. Analysis.

Comparison 2 Prone versus supine: cross‐over trials, Outcome 18 Oesophageal pressure.
Heart rate was significantly lower (MD ‐7.05 beats/min, 95% CI ‐13.99 to ‐0.10; Analysis 2.16) in the three studies that measured this outcome but conclusions from these data are limited by the high level of heterogeneity (I2 statistic = 76%).
2.16. Analysis.

Comparison 2 Prone versus supine: cross‐over trials, Outcome 16 Heart rate.
Three studies measured total pulmonary resistance. There was no statistically significant difference in total pulmonary resistance in prone versus supine positioning for spontaneous breaths only, as measured by Mendoza 1991, Mizuno 1999 and Wolfson 1992 (MD ‐17.09 cm H2O/L/S, 95% CI ‐36.20 to 2.02; Analysis 2.11). The studies by Mendoza 1991 and Mizuno 1999 included a total of 33 ventilated preterm infants, with data collected on their spontaneous breaths only. Wolfson 1992, however, included 20 preterm infants who were not intubated or ventilated and were therefore breathing spontaneously. Mendoza 1991 was the only study to provide data for total pulmonary resistance for mechanical breaths only, from 28 ventilated preterm infants. These results showed a statistically significant decrease in total pulmonary resistance for mechanical breaths in ventilated preterm infants in the prone position (MD ‐52.10 cm H2O/L/S, 95% CI ‐88.31 to ‐15.89; Analysis 2.11).
2.11. Analysis.

Comparison 2 Prone versus supine: cross‐over trials, Outcome 11 Total pulmonary resistance.
Mendoza 1991 was the only study to provide data for inspiratory resistance. This study recorded separate data for spontaneous and mechanical breaths. It involved 28 participants who were ventilated preterm infants. There was no statistically significant difference in inspiratory resistance in prone versus supine positioning for spontaneous breaths (MD ‐8.30 cm H2O/L/S, 95% CI ‐33.15 to 16.15; Analysis 2.12). However, there was a statistically significant decrease in inspiratory resistance for mechanical breaths (MD ‐76.60 cm H2O/L/S, 95% CI ‐116.78 to ‐36.42; Analysis 2.12).
2.12. Analysis.

Comparison 2 Prone versus supine: cross‐over trials, Outcome 12 Inspiratory resistance.
Oliveira 2009 and Wolfson 1992 measured thoracoabdominal synchrony as an outcome. There was a significant improvement in thoracoabdominal synchrony in the prone group (MD ‐30.76, 95% CI ‐41.39 to ‐20.14; Analysis 2.14). This analysis included 32 spontaneously breathing infants recovering from respiratory distress.
2.14. Analysis.

Comparison 2 Prone versus supine: cross‐over trials, Outcome 14 Thoracoabdominal synchrony.
Oliveira 2009 also reported the Laboured Breathing Index which was significantly lower when the 12 infants in this study were placed in the prone position (MD ‐0.84, 95% CI ‐1.24 to ‐0.44; Analysis 2.15).
2.15. Analysis.

Comparison 2 Prone versus supine: cross‐over trials, Outcome 15 Laboured Breathing Index.
Lateral versus supine positioning
Oxygenation outcomes
The outcomes for oxygenation measured for the comparison of lateral versus supine positions include: SaO2 (Bjornson 1992), PaO2 (Ennis 1978; Schlessel 1993), PaCO2 (Schlessel 1993), transcutaneous PO2 (Bozynski 1988) and transcutaneous PCO2 (Bozynski 1988; Crane 1990). No statistically significant differences were demonstrated for any of these outcomes: SaO2 (MD 1.42%, 95% CI ‐0.82 to 3.66; Analysis 3.1), PaO2 (right lateral: MD ‐2.41 mm Hg, 95% CI ‐11.77 to 6.95, left lateral: MD ‐3.60 mm Hg, 95% CI ‐13.57 to 6.37; Analysis 3.2), PaCO2 (right lateral: MD ‐1.00 mm Hg, 95% CI ‐3.45 to 1.45, left lateral: MD ‐1.00 mm Hg, 95% CI ‐3.86 to 1.86; Analysis 3.3), transcutaneous PO2 (right lateral: MD 0.55 mm Hg, 95% CI ‐5.46 to 6.56, left lateral: MD 2.67 mm Hg, 95% CI ‐4.51 to 9.85; Analysis 3.4) and transcutaneous PCO2 (right lateral: MD ‐0.63 mm Hg, 95% CI ‐4.66 to 3.41, left lateral: MD ‐0.95, 95% CI ‐5.07 to 3.17; Analysis 3.5).
3.1. Analysis.

Comparison 3 Lateral versus supine: cross‐over trials, Outcome 1 SaO2.
3.2. Analysis.

Comparison 3 Lateral versus supine: cross‐over trials, Outcome 2 PaO2.
3.3. Analysis.

Comparison 3 Lateral versus supine: cross‐over trials, Outcome 3 PaCO2.
3.4. Analysis.

Comparison 3 Lateral versus supine: cross‐over trials, Outcome 4 tcPO2.
3.5. Analysis.

Comparison 3 Lateral versus supine: cross‐over trials, Outcome 5 tcPCO2.
Ventilatory outcomes
Ventilatory outcomes measured included: tidal volume (Schlessel 1993), dynamic lung compliance (Schlessel 1993) and total pulmonary resistance (Schlessel 1993). No statistically significant differences became evident in any of these outcomes for the lateral versus supine comparison: tidal volume (right lateral: MD 0.10 ml/kg, 95% CI ‐0.72 to 0.92, left lateral: MD 0.90, 95% CI ‐0.30 to 2.10; Analysis 3.6), dynamic lung compliance (right lateral: MD ‐0.40 ml/cm H2O/kg, 95% CI ‐1.32 to 0.52, left lateral: MD ‐0.47 ml/cm H2O/kg, 95% CI ‐1.41 to 0.47; Analysis 3.7) and total pulmonary resistance (right lateral: MD ‐1.00 cm H2O/L/S, 95% CI ‐25.60 to 23.60, left lateral: MD 12.00 H2O/L/S, 95% CI ‐16.24 to 40.24; Analysis 3.8).
3.6. Analysis.

Comparison 3 Lateral versus supine: cross‐over trials, Outcome 6 Tidal volume.
3.7. Analysis.

Comparison 3 Lateral versus supine: cross‐over trials, Outcome 7 Dynamic lung compliance.
3.8. Analysis.

Comparison 3 Lateral versus supine: cross‐over trials, Outcome 8 Total pulmonary resistance.
Right lateral versus left lateral positioning
Oxygenation outcomes
The outcomes for oxygenation measured for the comparison of right lateral versus left lateral positions included: PaO2 (Ennis 1978; Schlessel 1993), PaCO2 (Schlessel 1993), transcutaneous PO2 (Bozynski 1988) and transcutaneous PCO2 (Bozynski 1988). No statistically significant differences resulted for any of these outcomes: PaO2 (MD 1.72 mm Hg, 95% CI ‐7.89 to 11.33; Analysis 4.1), PaCO2 (MD 0.00 mm Hg, 95% CI ‐3.14 to 3.14; Analysis 4.2), transcutaneous PO2 (MD ‐2.12 mm Hg, 95% CI ‐8.93 to 4.69; Analysis 4.3) and transcutaneous PCO2 (MD 0.77 mm Hg, 95% CI ‐3.64 to 5.18; Analysis 4.4).
4.1. Analysis.

Comparison 4 Right lateral versus left lateral: cross‐over trials, Outcome 1 PaO2.
4.2. Analysis.

Comparison 4 Right lateral versus left lateral: cross‐over trials, Outcome 2 PaCO2.
4.3. Analysis.

Comparison 4 Right lateral versus left lateral: cross‐over trials, Outcome 3 tcPO2.
4.4. Analysis.

Comparison 4 Right lateral versus left lateral: cross‐over trials, Outcome 4 tcPCO2.
Ventilatory outcomes
Ventilatory outcomes measured included: tidal volume, dynamic lung compliance and total pulmonary resistance (Schlessel 1993). No statistically significant differences emerged for any of these outcomes for the lateral versus supine comparison: tidal volume (MD ‐0.80 ml/kg, 95% CI ‐1.87 to 0.27; Analysis 4.5), dynamic lung compliance (MD 0.07 ml/cm H2O/kg, 95% CI ‐0.71 to 0.85; Analysis 4.6) and total pulmonary resistance (MD ‐13.00 cm H2O/L/S, 95% CI ‐40.93 to 14.93; Analysis 4.7).
4.5. Analysis.

Comparison 4 Right lateral versus left lateral: cross‐over trials, Outcome 5 Tidal volume.
4.6. Analysis.

Comparison 4 Right lateral versus left lateral: cross‐over trials, Outcome 6 Dynamic lung compliance.
4.7. Analysis.

Comparison 4 Right lateral versus left lateral: cross‐over trials, Outcome 7 Total pulmonary resistance.
Elevated versus flat positioning
Two studies compared elevated prone positioning to flat prone positioning (Jenni 1997; Sconyers 1987) and two compared elevated supine positioning to flat supine positioning (Ennis 1978; Sconyers 1987). Ennis 1978 also compared flat versus elevated in the right lateral and left lateral positions.
Oxygenation outcomes
The oxygenation outcomes for the comparison of head elevated versus flat positions included: SaO2 (Jenni 1997), PaO2 (Ennis 1978), SaO2 less than 80% (Jenni 1997), respiratory rate (Sconyers 1987) and the outcomes heart rate (Sconyers 1987) and episodes of heart rate less than 90 beats per minute (Jenni 1997). None of these outcomes pointed to statistically significant differences: SaO2 (MD 0.31%, 95% CI ‐2.23 to 2.85; Analysis 5.1), PaO2 (MD 0.96 mm Hg, 95% CI ‐15.81 to 17.73; Analysis 5.2), SaO2 less than 80% (MD ‐11.75, 95% CI ‐27.46 to 3.96; Analysis 5.3), respiratory rate (MD ‐12.00 breaths/min, 95% CI ‐24.48 to 0.48; Analysis 5.4), heart rate (prone: MD ‐4.00 beats/min, 95% CI ‐13.08 to 5.08, supine: MD ‐1.50 beats/min, 95% CI ‐11.93 to 8.93; Analysis 5.5) and number of episodes of heart rate less than 90 beats per minute (prone: MD ‐2.27, 95% CI ‐11.81 to 7.27; Analysis 5.6).
5.1. Analysis.

Comparison 5 Elevated versus flat: cross‐over trials, Outcome 1 SaO2.
5.2. Analysis.

Comparison 5 Elevated versus flat: cross‐over trials, Outcome 2 PaO2.
5.3. Analysis.

Comparison 5 Elevated versus flat: cross‐over trials, Outcome 3 Number of episodes of SaO2 < 80%.
5.4. Analysis.

Comparison 5 Elevated versus flat: cross‐over trials, Outcome 4 Respiratory rate.
5.5. Analysis.

Comparison 5 Elevated versus flat: cross‐over trials, Outcome 5 Heart rate.
5.6. Analysis.

Comparison 5 Elevated versus flat: cross‐over trials, Outcome 6 Number of episodes heart rate < 90 bpm.
Ventilatory outcomes
The elevated versus flat comparison did not report on ventilatory outcomes.
Supine versus good‐lung dependent positioning
Oxygenation outcomes
The oxygenation outcomes measured for the comparison supine versus good‐lung dependent included: PaO2 (Polacek 1992), transcutaneous PO2 and transcutaneous CO2 (Heaf 1983). There was no significant difference between positions for any of these outcomes: PaO2 (MD ‐3.44 mm Hg, 95% CI ‐30.00 to 23.12; Analysis 6.1), transcutaneous PO2 (MD ‐5.00 mm Hg, 95% CI ‐24.41 to 14.41; Analysis 6.2) and transcutaneous CO2 (MD 0.00 mm Hg, 95% CI ‐6.51 to 6.51; Analysis 6.3).
6.1. Analysis.

Comparison 6 Supine versus good‐lung dependent: cross‐over trials, Outcome 1 PaO2.
6.2. Analysis.

Comparison 6 Supine versus good‐lung dependent: cross‐over trials, Outcome 2 TcPO2.
6.3. Analysis.

Comparison 6 Supine versus good‐lung dependent: cross‐over trials, Outcome 3 TcPCO2.
Ventilatory outcomes
The ventilatory outcomes measured included: tidal volume and dynamic lung compliance (Heaf 1983). There was no significant difference between positions for either outcome: tidal volume (MD 0.10 ml/kg, 95% CI ‐5.44 to 5.64; Analysis 6.4) and dynamic lung compliance (MD 0.00 ml/cm H2O/kg, 95% CI ‐1.94 to 1.94; Analysis 6.5).
6.4. Analysis.

Comparison 6 Supine versus good‐lung dependent: cross‐over trials, Outcome 4 Tidal volume.
6.5. Analysis.

Comparison 6 Supine versus good‐lung dependent: cross‐over trials, Outcome 5 Dynamic lung compliance.
Supine versus good‐lung independent positioning
Oxygenation outcomes
The oxygenation outcomes measured for the comparison supine versus good‐lung independent included: PaO2 (Polacek 1992), transcutaneous PO2 and transcutaneous CO2 (Heaf 1983). There was no significant difference between positions for any of these outcomes: PaO2 (MD 2.78 mm Hg, 95% CI ‐23.28 to 28.84; Analysis 7.1), transcutaneous PO2 (MD 4.00 mm Hg, 95% CI ‐15.41 to 23.41; Analysis 7.2) and transcutaneous CO2 (MD 0.00 mm Hg, 95% CI ‐6.79 to 6.79; Analysis 7.3).
7.1. Analysis.

Comparison 7 Supine versus good‐lung independent: cross‐over trials, Outcome 1 PaO2.
7.2. Analysis.

Comparison 7 Supine versus good‐lung independent: cross‐over trials, Outcome 2 tcPO2.
7.3. Analysis.

Comparison 7 Supine versus good‐lung independent: cross‐over trials, Outcome 3 tcPCO2.
Ventilatory outcomes
The ventilatory outcomes measured include: tidal volume and dynamic lung compliance (Heaf 1983). There was no significant difference between positions for either outcome: tidal volume (MD 0.50 ml/kg, 95% CI ‐6.57 to 7.57; Analysis 7.4) and dynamic lung compliance (MD 0.41 ml/cm H2O/kg, 95% CI ‐1.98 to 2.80; Analysis 6.5).
7.4. Analysis.

Comparison 7 Supine versus good‐lung independent: cross‐over trials, Outcome 4 Tidal volume.
Good‐lung dependent versus good‐lung independent positioning
Oxygenation outcomes
The oxygenation outcomes measured for the good‐lung dependent versus good‐lung independent comparison included: PaO2 (Polacek 1992), transcutaneous PO2 and transcutaneous CO2 (Heaf 1983). There was no significant difference between positions for any of these outcomes: PaO2 (MD 6.22 mm Hg, 95% CI ‐21.25 to 33.69; Analysis 8.1), transcutaneous PO2 (MD 9.00 mm Hg, 95% CI ‐10.41 to 28.41; Analysis 8.2) and transcutaneous PCO2 (MD 0.00 mm Hg, 95% CI ‐6.66 to 6.66; Analysis 8.3).
8.1. Analysis.

Comparison 8 Good‐lung dependent versus good lung‐independent: cross‐over trials, Outcome 1 PaO2.
8.2. Analysis.

Comparison 8 Good‐lung dependent versus good lung‐independent: cross‐over trials, Outcome 2 tcPO2.
8.3. Analysis.

Comparison 8 Good‐lung dependent versus good lung‐independent: cross‐over trials, Outcome 3 tcPCO2.
Ventilatory outcomes
The ventilatory outcomes measured included: tidal volume and dynamic lung compliance (Heaf 1983). There was no significant difference between positions for either outcome: tidal volume (MD 0.50 ml/kg, 95% CI ‐6.57 to 7.57; Analysis 8.4) and dynamic lung compliance (MD 0.41 ml/cm H2O/kg, 95% CI ‐1.98 to 2.80; Analysis 8.5).
8.4. Analysis.

Comparison 8 Good‐lung dependent versus good lung‐independent: cross‐over trials, Outcome 4 Tidal volume.
8.5. Analysis.

Comparison 8 Good‐lung dependent versus good lung‐independent: cross‐over trials, Outcome 5 Dynamic lung compliance.
Subgroup analysis
We conducted all subgroup analyses on the meta‐analyses of cross‐over trials of participants placed in the prone and supine positions as these were the only meta‐analyses for which there were enough data to conduct a subgroup analysis.
Ventilated versus non‐ventilated subgroup analysis
For all of these analyses we used data from cross‐over trials comparing prone and supine positions.
SaO2
There was a statistical difference between subgroups of ventilated and non‐ventilated participants in estimates of SaO2 (P = 0.03; Analysis 9.1). However, the SaO2was significantly higher in the prone position compared to the supine position in both subgroups (ventilated infants: MD 2.68%, 95% CI 1.53 to 3.83; unventilated infants: MD 1.23%, 95% CI 0.60 to 1.87; Analysis 9.1) and the overall analysis (MD 2.06%, 95% CI 1.27 to 2.85; Analysis 9.1).
9.1. Analysis.

Comparison 9 Subgroup analysis by ventilation status: cross‐over trials, Outcome 1 SaO2.
Respiratory rate
There was no significant difference between ventilated and non‐ventilated subgroups in respiratory rate (P = 0.65). However, while there was no statistical difference between the prone and supine positions in the non‐ventilated group (MD ‐2.39 breaths/min, 95% CI ‐8.98 to 4.19; Analysis 9.2) there was a statistical difference between the prone and supine positions in the subgroup analysis of ventilated subgroup (MD ‐4.01 breaths/min, 95% CI ‐6.21 to ‐1.80; Analysis 9.2) and the overall analysis (MD ‐3.84 breaths/min, 95% CI ‐5.93 to ‐1.75; Analysis 9.2).
9.2. Analysis.

Comparison 9 Subgroup analysis by ventilation status: cross‐over trials, Outcome 2 Respiratory rate.
Tidal volume
There was no significant difference between subgroups in tidal volume (P = 0.22; Analysis 9.2). There was no difference between the prone and supine position in the ventilated (MD 0.16 ml/kg, 95% CI ‐0.48 to 0.80; Analysis 9.3) and non‐ventilated (MD 0.74 ml/kg, 95% CI ‐0.25 to 1.73; Analysis 9.3) subgroups or the overall analysis (MD 0.33 ml/kg, 95% CI ‐0.20 to 0.87; Analysis 9.3).
9.3. Analysis.

Comparison 9 Subgroup analysis by ventilation status: cross‐over trials, Outcome 3 Tidal volume.
Minute volume
There was no significant difference between subgroups (P = 0.19; Analysis 9.4). There was no difference between the prone and supine position in the subgroup analysis of ventilated infants (MD ‐8.48 ml/kg/min, 95% CI ‐36.07 to 19.11; Analysis 9.4) and non‐ventilated infants (MD 42.74 ml/kg/min, 95% CI ‐29.30 to 114.78; Analysis 9.4) or the overall analysis (MD ‐1.93 ml/kg/min, 95% CI ‐27.69 to 23.84; Analysis 9.4).
9.4. Analysis.

Comparison 9 Subgroup analysis by ventilation status: cross‐over trials, Outcome 4 Minute volume.
Subgroup analysis by age
SaO2
Levene 1990 was the only study that compared SaO2 in the prone and supine positions in infants older than one month. Unlike the pooled estimate of neonatal studies (MD 2.19%, 95% CI 1.35 to 3.04; Analysis 10.1) or the overall estimate (MD 2.06%, 95% CI 1.27 to 2.85; Analysis 10.1) which favoured the prone position, there was no significant difference between prone and supine groups in the Levene 1990 study (MD 0.81%, 95% CI ‐0.91 to 2.53; Analysis 10.1).
10.1. Analysis.

Comparison 10 Subgroup analysis by age: cross‐over trials, Outcome 1 SaO2.
Sensitivity analysis
We had planned to conduct a sensitivity analysis based on quality criteria. However, this was not possible due to inadequate data.
Discussion
Summary of main results
The data from cross‐over studies provides evidence that short and medium‐term prone positioning is beneficial in improving ventilation, oxygenation and chest wall stability in ventilated preterm infants with acutely respiratory distress. No adverse effects were identified.
We observed no other statistically significant effects of any of the other positions.
Overall completeness and applicability of evidence
While there was evidence for a positive effect of prone positioning, the clinical significance is not clear as the effect size tended to be small and the outcomes were relatively transient. There were no data reporting more clinically meaningful outcomes such as mortality, morbidity and recovery variables. In addition, the majority of the included studies compared prone and supine positioning in preterm ventilated neonates. Only three studies reported data for children older than one year of age. Therefore, it was not clear to what extent we can generalise these data in the treatment of other infants and children with acute respiratory distress.
Apart from the prone versus supine comparison, there was simply not enough evidence to draw any conclusions about the effects of other positions in acute respiratory distress.
There were insufficient data to determine if there are any adverse outcomes related to prone or other positioning. None of the studies measured outcomes that may have predisposed participants to adverse outcomes in relation to specific positioning. These might include accidental removal of the endotracheal tube, pressure areas, facial oedema or joint contractures.
The subgroup analysis based on ventilation status showed a significant difference between ventilated and non‐ventilated subgroups although the SaO2 was significantly higher for both subgroups in the prone position. However, these differences between subgroups should not be over‐interpreted because these analyses were based on relatively few data. Similarly, the lack of a significant difference between the prone and supine position in infants and children aged at least one month may have been due to the low number of these children for whom data were available.
Because the pathophysiology of acute respiratory distress varies substantially, we proposed to undertake a subgroup analysis based on the reported cause and related pathophysiology of respiratory distress. However, the only data for subgroups other than preterm neonates came from the one small study by Levene 1990 who reported data from infants with upper and lower respiratory tract infections in the prone and supine positions.
Quality of the evidence
Small participant numbers and short study times are major limitations to the conclusions that we can draw from this review. The majority of studies reported data from fewer than 50 infants or children, with the number of participants ranging in age from four (Bjornson 1992) to 55 (McEvoy 1997). As most of the studies included in this review were of a short duration it is not possible to establish whether any beneficial or adverse effects of positioning become significant over longer periods. Only five studies collected data for more than three hours. Three reported outcomes from 12 to 24 hours (Jenni 1997; Keene 2000; Kornecki 2001) and only two reported data for more than 24 hours (Antunes 2003; Bjornson 1992). The study by Kornecki 2001 highlights the importance of collecting data for longer periods, in that while there was no difference between the prone and supine positions until the sixth hour, oxygenation appears to have steadily increased up to 12 hours.
We observed no other statistically significant effects of any of the other positions. However, this may be due to the low number of identified studies and small participant numbers. Therefore, data from studies with much higher participant numbers are required to draw any conclusions about the effects or lack of effects of the other positions.
Potential biases in the review process
While there were few studies we considered to have a high risk of bias in selection, detection or attrition bias, the majority of studies did not describe how these potential selection or detection biases were addressed. Therefore, there may still be risk of bias in these studies.
Agreements and disagreements with other studies or reviews
The finding from this review that short and medium‐term prone positioning is beneficial in improving ventilation, oxygenation and chest wall stability in acutely respiratory‐distressed ventilated preterm infants was supported by the review of Balaguer 2006, which concluded that the prone position in ventilated preterm neonates resulted in improved oxygenation in the short term. Similarly, reviews of positioning for respiratory‐distressed, ventilated adult participants have also concluded that prone positioning was beneficial in improving oxygenation in the short term (Ball 1999; Curley 1999; Wong 1999).
Authors' conclusions
Implications for practice.
We concluded that prone positioning was significantly more beneficial than other positions in terms of improving ventilation, oxygenation and chest wall stability for ventilated preterm infants hospitalised with acute respiratory distress in the identified cross‐over studies. Whether the benefits of prone positioning may be extrapolated to non‐ventilated infants and children hospitalised with acute respiratory distress is uncertain. At present there are insufficient data in these patient groups to resolve this issue. However, due to the association of the prone position with sudden infant death syndrome (SIDS), all hospitalised infants placed in the prone position must have continuous cardio‐respiratory and SaO2 monitoring.
Implications for research.
Large, international, multicentre, randomised controlled trials are required to better assess the effect of positioning respiratory‐distressed infants and children in the prone position. Any future studies should also collect clinically meaningful data such as mortality, morbidity, recovery variables and adverse effects. Trials also need to determine the optimal frequency and timing of the prone position to gain maximal sustained benefits. As most identified studies reported data from neonates, future studies are needed to determine whether the prone position is also effective for older infants and children. In addition, to investigate whether findings are consistent across a range of participants, future trials should report separate data for subgroups of infants and children based on the age group, the causes of acute respiratory distress, and whether or not they are mechanically ventilated. Further research on the effectiveness of other positions for infants and children with acute respiratory distress is also needed.
What's new
| Date | Event | Description |
|---|---|---|
| 12 April 2012 | New search has been performed | Searches conducted. One new study was identified (Oliveira 2009) but our conclusions remain unchanged. Additional details were added to the Methods and the 'Risk of bias' tables. |
| 26 August 2011 | New citation required but conclusions have not changed | A new author joined the team to update this review. |
History
Protocol first published: Issue 2, 2002 Review first published: Issue 2, 2005
| Date | Event | Description |
|---|---|---|
| 7 August 2008 | New search has been performed | Searches conducted. Two new studies have been included in this updated review. |
| 14 February 2008 | Amended | Converted to new review format. |
| 13 July 2006 | Amended | Plain language summary re‐written. |
| 24 October 2004 | New search has been performed | Searches conducted. |
Acknowledgements
We would like to thank Dominic Fitzgerald, Karen McKay and Narelle Willis for information and advice provided for the draft protocol and Liz Dooley for her help in publishing the original review. We also wish to acknowledge Sonia Smith and Megan Black for their assistance in co‐authoring the protocol published in The Cochrane Library, Issue 2, 2002. We would also like to thank Yusra Badr, Andrew Argent, Terry Neeman and George Swingler for commenting on the draft of this updated review.
Appendices
Appendix 1. Previous search strategy
For this first update we searched the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2008, Issue 3) which contains the Acute Respiratory Infections Group's Specialised Register; MEDLINE (January 1966 to August Week 1, 2008); EMBASE (January 2004 to Week 33, 2008) and CINAHL (January 2004 to August Week 3, 2008).
We combined the MEDLINE search terms with a search strategy used to identify relevant trials (Dickersin 1994). We adapted the search terms to search EMBASE and CINAHL.
TERMS FOR ACUTE RESPIRATORY DISTRESS
1 Exp Bronchiolitis/ 2 Bronchiolitis.tw 3 Exp respiratory syncytial virus infections/ 4 Respiratory syncytial virus.tw 5 RSV.tw 6 Bronchopneumonia.tw 7 Exp bronchial diseases/ [incorporates asthma, bronchopneumonia] 8 Exp respiratory distress syndrome, adult/ 9 ARDS.tw 10 (Acute respiratory distress).tw 11 Acute lung injury.tw 12 ALI.tw 13 Exp respiratory tract infections/ 14 Respiratory distress.tw 15 (Resp$ adj25 distress).tw 16 (Bronch$ adj25 distress).tw 17 Respiratory infection.tw 18 Exp respiratory insufficiency/ [Used for respiratory failure] 19 Respiratory insufficiency.tw 20 Respiratory failure.tw 21 Exp respiratory distress syndrome/ 22 Respiratory distress syndrome.tw 23 Exp pneumonia/ 24 Pneumonia.tw 25 Exp lung diseases/ [incorporates pneumonia, RDS] 26 Exp hyaline membrane disease/ 27 Hyaline membrane disease.tw 28 Exp Bronchopulmonary dysplasia/ 29 Bronchopulmonary dysplasia.tw 30 Chronic neonatal lung disease.tw 31 Or/1‐30
POSITIONING TERMS 32 Exp posture/ [This will capture (exp supine position/) and (exp prone position/)] 33 Postur$.tw 34 Supine.tw 35 Prone.tw 36 Face adj5 down.tw 37 Side adj5 lying.tw 38 Lateral$.tw 39 Kinetic adj5 position$.tw 40 Continuous body position$.tw 41 Upright.tw 42 High adj5 sitting.tw 43 Semirecumbent.tw 44 Position$.tw 45 Or/32‐44 46 31 and 45
Appendix 2. Embase.com search strategy
#28 #24 AND #27 116416 Jan 2011 #27 #25 OR #26 83206316 Jan 2011 #26 random*:ab,ti OR placebo*:ab,ti OR factorial*:ab,ti OR crossover*:ab,ti OR 'cross‐over':ab,ti OR 'cross over':ab,ti OR assign*:ab,ti OR allocat*:ab,ti OR volunteer*:ab,ti OR ((singl* OR doubl*) NEAR/2 (blind* OR mask*)):ab,ti AND [embase]/lim79319116 Jan 2011 #25 'randomized controlled trial'/exp OR 'single blind procedure'/exp OR 'double blind procedure'/exp OR 'crossover procedure'/exp AND [embase]/lim 23497116 Jan 2011 #24 #20 AND #23 1301916 Jan 2011 #23 #21 OR #22 52233216 Jan 2011 #22 postur*:ab,ti OR supine:ab,ti OR prone:ab,ti OR (face NEAR/5 down*):ab,ti OR (side NEAR/5 (lay OR laying OR laid OR lying OR lies OR lays)):ab,ti OR lateral*:ab,ti OR (kinetic NEAR/5 position*):ab,ti OR 'continuous body position':ab,ti OR 'continuous body positions':ab,ti OR upright:ab,ti OR (high NEAR/5 sitting):ab,ti OR 'semi‐recumbent':ab,ti OR semirecumbent:ab,ti OR 'semi‐recline':ab,ti OR semirecline:ab,ti OR position*:ab,ti AND [embase]/lim51802216 Jan 2011 #21 'body posture'/de AND [embase]/lim21003 16 Jan 2011 #20 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 679544 16 Jan 2011 #19 'chronic neonatal lung disease':ab,ti OR 'chronic neonatal lung diseases':ab,ti AND [embase]/lim29 16 Jan 2011 #18 'hyaline membrane disease':ab,ti AND [embase]/lim1319 16 Jan 2011 #17 'hyaline membrane disease'/de AND [embase]/lim2972 16 Jan 2011 #16 ards:ab,ti OR rds:ab,ti AND [embase]/lim9686 16 Jan 2011 #15 (distress* NEAR/2 (respir* OR bronch*)):ab,ti AND [embase]/lim22691 16 Jan 2011 #14 pneumon*:ab,ti AND [embase]/lim99195 16 Jan 2011 #13 'lung disease'/exp AND [embase]/lim513379 16 Jan 2011 #12 (respir* NEAR/3 (insufficien* OR fail*)):ab,ti AND [embase]/lim22834 16 Jan 2011 #11 (respir* NEAR/5 infect*):ab,ti AND [embase]/lim32004 16 Jan 2011 #10 'respiratory tract infection'/exp AND [embase]/lim147424 16 Jan 2011 #9 'acute lung injury':ab,ti OR 'acute lung injuries':ab,ti OR ali:ab,ti AND [embase]/lim 7550 16 Jan 2011 #8 'acute lung injury'/de AND [embase]/lim 3542 16 Jan 2011 #7 bronchopneumon*:ab,ti AND [embase]/lim 2178 16 Jan 2011 #6 'bronchopneumonia'/de AND [embase]/lim 3002 16 Jan 2011 #5 'bronchus disease'/exp AND [embase]/lim 6865616 Jan 2011 #4 'respiratory syncytial virus':ab,ti OR 'respiratory syncytial viruses':ab,ti OR rsv:ab,ti AND [embase]/lim 904616 Jan 2011 #3 'respiratory syncytial virus infection'/de OR 'respiratory syncytial pneumovirus'/de AND [embase]/lim 877916 Jan 2011 #2 bronchiolit*:ab,ti AND [embase]/lim 644716 Jan 2011 #1 'bronchiolitis'/exp AND [embase]/lim 876316 Jan 2011
Appendix 3. CINAHL search strategy
S49 S38 and S48 S48 s39 or S40 or S41 or S42 or S43 or S44 or S45 or S46 or S47 S47 (MH "Quantitative Studies") S46 (MH "Random Assignment") S45 (MH "Placebos") S44 TI placebo* or AB placebo* S43 TI random* or AB random* S42 TI (singl* blind* or doubl* blind* or trebl* blind* or tripl* blind* or singl* mask* or doubl* mask* or trebl* mask* or tripl* mask*) or AB (singl* blind* or doubl* blind* or trebl* blind* or tripl* blind* or singl* mask* or doubl* mask* or trebl* mask* or tripl* mask*) S41 TI clinic* trial* or AB clinic* trial* S40 PT clinical trial S39 (MH "Clinical Trials+") S38 S22 and S37 S37 S23 or S24 or S25 or S26 or S27 or S28 or S29 or S30 or S31 or S32 or S33 or S34 or S35 or S36 S36 TI position* or AB position* S35 TI (semi‐recumbent or semirecumbent or semi‐reclin* or semireclin*) or AB (semi‐recumbent or semirecumbent or semi‐reclin* or semireclin*) S34 TI high N5 sitting or AB high N5 sitting S33 TI upright or AB upright S32 TI continuous body position* or AB continuous body position* S31 TI kinetic N5 position* or AB kinetic N5 position* S30 TI lateral* or AB lateral* S29 TI side N5 laid or AB side N5 laid S28 TI side N5 lying or AB side N5 lying S27 TI side N5 lies or AB side N5 lies S26 TI side N5 lay* or AB side N5 lay* S25 TI face N5 down* or AB face N5 down* S24 TI (supine or prone or postur*) or AB (supine or prone or postur*) S23 (MH "Posture+") S22 S1 or S2 or S3 or S4 or S5 or S7 or S8 or S9 or S10 or S11 or S12 or S13 or S14 or S15 or S16 or S17 or S18 or S19 or S20 or S21 S21 TI chronic neonatal lung disease* or AB chronic neonatal lung disease* S20 TI hyaline membrane disease* or AB hyaline membrane disease* S19 TI (ards or rds) or AB (ards or rds) S18 TI bronch* N25 distress* or AB bronch* N25 distress* S17 TI respir* N25 distress* or AB respir* N25 distress* S16 TI pneumon* or AB pneumon* S15 TI respir* N3 fail* or AB respir* N3 fail* S14 (MH "Bronchial Diseases+") S13 TI respir* N3 insufficien* or AB respir* N3 insufficien* S12 (MH "Lung Diseases+") S11 "respiratory insufficiency" S10 TI respir* N5 infect* or AB respir* N5 infect* S9 (MH "Respiratory Tract Infections+") S8 TI (acute lung injury or ali) or AB (acute lung injury or ali) S7 TI bronchopneumon* or AB bronchopneumon* S6 (MH "Bronchial Diseases") S5 TI ( respiratory syncytial virus* or rsv ) or AB (respiratory syncytial virus* or rsv) S4 (MH "Respiratory Syncytial Virus Infections") S3 (MH "Respiratory Syncytial Viruses") S2 TI bronchiolit* or AB bronchiolit* S1 (MH "Bronchiolitis+")
Data and analyses
Comparison 1. Prone versus supine: parallel trials.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Patients with SaO2 < 90% | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Day 1 | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Day 2 | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 PaO2 | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 PaCO2 | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4 PaO2/FiO2 | 2 | 121 | Mean Difference (IV, Random, 95% CI) | 28.16 [‐9.92, 66.24] |
| 5 Oxygenation Index | 2 | 121 | Mean Difference (IV, Fixed, 95% CI) | ‐2.42 [‐3.60, ‐1.25] |
| 6 Tidal volume | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7 Adverse events | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7.1 Extubations | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 Obstructed endotracheal tube | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.3 Pressure ulcers | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.4 Hypercapnea | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.5 Reintubations | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.6 Atelectasis | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 2. Prone versus supine: cross‐over trials.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 SaO2 | 9 | 390 | Mean Difference (IV, Random, 95% CI) | 1.97 [1.18, 2.77] |
| 1.1 All | 8 | 330 | Mean Difference (IV, Random, 95% CI) | 2.19 [1.35, 3.04] |
| 1.2 URTI | 1 | 26 | Mean Difference (IV, Random, 95% CI) | ‐0.5 [‐2.58, 1.58] |
| 1.3 LRTI | 1 | 34 | Mean Difference (IV, Random, 95% CI) | 1.80 [‐0.78, 4.38] |
| 2 PaO2 | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 tcPCO2 | 3 | 54 | Mean Difference (IV, Fixed, 95% CI) | ‐2.53 [‐6.06, 0.99] |
| 4 Oxygenation Index | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 30 minutes | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 2 hours | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 4 hours | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.4 6 hours | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.5 8 hours | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.6 12 hours | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Number of episodes SaO2 < 80% | 2 | 154 | Mean Difference (IV, Fixed, 95% CI) | ‐3.46 [‐4.60, ‐2.33] |
| 6 Respiratory rate | 6 | 222 | Mean Difference (IV, Fixed, 95% CI) | ‐3.84 [‐5.93, ‐1.75] |
| 7 Tidal volume | 5 | 196 | Mean Difference (IV, Fixed, 95% CI) | 0.35 [‐0.12, 0.82] |
| 7.1 Spontaneous breaths | 5 | 140 | Mean Difference (IV, Fixed, 95% CI) | 0.33 [‐0.20, 0.87] |
| 7.2 Mechanical breaths | 1 | 56 | Mean Difference (IV, Fixed, 95% CI) | 0.40 [‐0.58, 1.38] |
| 8 Minute volume | 5 | 196 | Mean Difference (IV, Fixed, 95% CI) | 4.82 [‐18.01, 27.65] |
| 8.1 Spontaneous breaths | 5 | 140 | Mean Difference (IV, Fixed, 95% CI) | ‐1.93 [‐27.69, 23.84] |
| 8.2 Mechanical breaths | 1 | 56 | Mean Difference (IV, Fixed, 95% CI) | 29.5 [‐19.78, 78.78] |
| 9 Work of breathing | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 9.1 Spontaneous breaths | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.2 Mechanical breaths | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10 Dynamic lung compliance | 2 | 148 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.11, 0.13] |
| 10.1 Spontaneous breaths | 2 | 92 | Mean Difference (IV, Fixed, 95% CI) | 0.07 [‐0.18, 0.33] |
| 10.2 Mechanical breaths | 1 | 56 | Mean Difference (IV, Fixed, 95% CI) | ‐0.01 [‐0.15, 0.13] |
| 11 Total pulmonary resistance | 3 | 162 | Mean Difference (IV, Fixed, 95% CI) | ‐24.72 [‐41.61, ‐7.82] |
| 11.1 Spontaneous breaths | 3 | 106 | Mean Difference (IV, Fixed, 95% CI) | ‐17.09 [‐36.20, 2.02] |
| 11.2 Mechanical breaths | 1 | 56 | Mean Difference (IV, Fixed, 95% CI) | ‐52.1 [‐88.31, ‐15.89] |
| 12 Inspiratory resistance | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 12.1 Spontaneous breaths | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 12.2 Mechanical breaths | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 13 Expiratory resistance | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 13.1 Spontaneous breaths | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 13.2 Mechanical breaths | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 14 Thoracoabdominal synchrony | 2 | 64 | Mean Difference (IV, Fixed, 95% CI) | ‐30.76 [‐41.39, ‐20.14] |
| 15 Laboured Breathing Index | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 16 Heart rate | 3 | 113 | Mean Difference (IV, Random, 95% CI) | ‐7.05 [‐13.99, ‐0.10] |
| 17 Number of episodes of heart rate < 90 bpm | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 18 Oesophageal pressure | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 3. Lateral versus supine: cross‐over trials.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 SaO2 | 1 | 8 | Mean Difference (IV, Fixed, 95% CI) | 1.42 [‐0.82, 3.66] |
| 1.1 Right or left | 1 | 8 | Mean Difference (IV, Fixed, 95% CI) | 1.42 [‐0.82, 3.66] |
| 2 PaO2 | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 Right lateral | 2 | 48 | Mean Difference (IV, Fixed, 95% CI) | ‐2.41 [‐11.77, 6.95] |
| 2.2 Left lateral | 2 | 48 | Mean Difference (IV, Fixed, 95% CI) | ‐3.60 [‐13.57, 6.37] |
| 3 PaCO2 | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 Right lateral | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Left lateral | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 tcPO2 | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 Right lateral | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Left lateral | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 tcPCO2 | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1 Right lateral | 2 | 64 | Mean Difference (IV, Fixed, 95% CI) | ‐0.63 [‐4.66, 3.41] |
| 5.2 Left lateral | 1 | 36 | Mean Difference (IV, Fixed, 95% CI) | ‐0.95 [‐5.07, 3.17] |
| 6 Tidal volume | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.1 Right lateral | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Left lateral | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Dynamic lung compliance | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7.1 Right lateral | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 Left lateral | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Total pulmonary resistance | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8.1 Right lateral | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.2 Left lateral | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 4. Right lateral versus left lateral: cross‐over trials.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 PaO2 | 2 | 48 | Mean Difference (IV, Fixed, 95% CI) | 1.72 [‐7.89, 11.33] |
| 2 PaCO2 | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 tcPO2 | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4 tcPCO2 | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5 Tidal volume | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6 Dynamic lung compliance | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7 Total pulmonary resistance | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 5. Elevated versus flat: cross‐over trials.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 SaO2 | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Prone | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 PaO2 | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Supine | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 All | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Number of episodes of SaO2 < 80% | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 Prone | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Respiratory rate | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 Prone | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Supine | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Heart rate | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 Prone | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Supine | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Number of episodes heart rate < 90 bpm | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.1 Prone | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 6. Supine versus good‐lung dependent: cross‐over trials.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 PaO2 | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 TcPO2 | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 TcPCO2 | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4 Tidal volume | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5 Dynamic lung compliance | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 7. Supine versus good‐lung independent: cross‐over trials.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 PaO2 | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 tcPO2 | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 tcPCO2 | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4 Tidal volume | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5 Dynamic lung compliance | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
7.5. Analysis.

Comparison 7 Supine versus good‐lung independent: cross‐over trials, Outcome 5 Dynamic lung compliance.
Comparison 8. Good‐lung dependent versus good lung‐independent: cross‐over trials.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 PaO2 | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 tcPO2 | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 tcPCO2 | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4 Tidal volume | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5 Dynamic lung compliance | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 9. Subgroup analysis by ventilation status: cross‐over trials.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 SaO2 | 9 | 390 | Mean Difference (IV, Random, 95% CI) | 2.06 [1.27, 2.85] |
| 1.1 Ventilated | 6 | 266 | Mean Difference (IV, Random, 95% CI) | 2.68 [1.53, 3.83] |
| 1.2 Non‐ventilated | 3 | 124 | Mean Difference (IV, Random, 95% CI) | 1.23 [0.60, 1.87] |
| 2 Respiratory rate | 6 | 222 | Mean Difference (IV, Fixed, 95% CI) | ‐3.84 [‐5.93, ‐1.75] |
| 2.1 Ventilated | 3 | 92 | Mean Difference (IV, Fixed, 95% CI) | ‐4.01 [‐6.21, ‐1.80] |
| 2.2 Non‐ventilated | 3 | 130 | Mean Difference (IV, Fixed, 95% CI) | ‐2.39 [‐8.98, 4.19] |
| 3 Tidal volume | 5 | 140 | Mean Difference (IV, Fixed, 95% CI) | 0.33 [‐0.20, 0.87] |
| 3.1 Ventilated | 3 | 78 | Mean Difference (IV, Fixed, 95% CI) | 0.16 [‐0.48, 0.80] |
| 3.2 Non‐ventilated | 2 | 62 | Mean Difference (IV, Fixed, 95% CI) | 0.74 [‐0.25, 1.73] |
| 4 Minute volume | 5 | 140 | Mean Difference (IV, Fixed, 95% CI) | ‐1.93 [‐27.69, 23.84] |
| 4.1 Ventilated | 3 | 78 | Mean Difference (IV, Fixed, 95% CI) | ‐8.48 [‐36.07, 19.11] |
| 4.2 Non‐ventilated | 2 | 62 | Mean Difference (IV, Fixed, 95% CI) | 42.74 [‐29.30, 114.78] |
Comparison 10. Subgroup analysis by age: cross‐over trials.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 SaO2 | 9 | 390 | Mean Difference (IV, Random, 95% CI) | 2.06 [1.27, 2.85] |
| 1.1 Neonates | 8 | 330 | Mean Difference (IV, Random, 95% CI) | 2.19 [1.35, 3.04] |
| 1.2 > One month | 1 | 60 | Mean Difference (IV, Random, 95% CI) | 0.81 [‐0.91, 2.53] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Antunes 2003.
| Methods | Parallel RCT of prone and supine positions | |
| Participants | Included: 43 ventilated preterm infants with a gestational age < 37 weeks and birth weight < 2000 grams and required ventilation for more than 48 hours in the first week of life Excluded: infants with congenital abnormalities or clinical/surgical reasons which made prone positioning impossible Median gestational age: 29 weeks; median birth weight: 1207 g; median age at study: prone group 11 days, supine group 4 days; median FiO2: 0.34 Setting: NICU, Brazil |
|
| Interventions | Infants in the prone group were placed in the supine position from 7 to 10 am each day of the study for washes, physical examination, lab tests and X‐rays; infants in both groups were in the supine position at all other times Median time in study: 2 days for both groups (range: 1 to 10 days) | |
| Outcomes | SaO2 < 90%, SaO2, respiratory rate, positive inspiratory pressure, heart rate | |
| Notes | Median values reported for continuous outcomes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes used for randomisation |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Data from one infant were excluded as the study protocol was not completed |
Bhat 2003.
| Methods | Cross‐over RCT of prone and supine positions | |
| Participants | Included: 20 preterm infants who had respiratory distress syndrome Excluded: not stated Median gestational age: 30 weeks; median birth weight: 1312 g; median age: 4 weeks; oxygen‐dependent: 10 Setting: NICU, children's hospital, UK |
|
| Interventions | Infants were studied on 2 successive days, on each day the study position was maintained for 3 hours | |
| Outcomes | SaO2 | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Appears to be no loss to follow‐up |
Bjornson 1992.
| Methods | Cross‐over RCT of prone, supine and lateral positions | |
| Participants | Included: 4 preterm infants with a gestational age of 24 to 30 weeks and birth weight of 500 to 1500 grams requiring continuing ventilatory assistance after 7 days of life for respiratory distress syndrome Median gestational age: 26.5 weeks; median birth weight: 1003 g; age: 9 to 24 days Excluded: infants with documented infections, congenital defects, cardiac anomalies other than PDA, intraventricular haemorrhage grade III & IV and maternal history of substance abuse Setting: medical centre and general hospital, Washington, US |
|
| Interventions | Infants were placed in each of the positions for 20 minutes each over a 60‐minute session; infants underwent 6 to 9 sessions over 8 to 14 days | |
| Outcomes | SaO2 Data collection began immediately after position change at 1‐minute intervals for 20 minutes each position; data were reported for 6 to 9 sessions |
|
| Notes | One infant completed 7/9 sessions and one completed 6 sessions due to intolerance of handling | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated but decision made by the primary investigator |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Appears to be no loss to follow‐up |
Bozynski 1988.
| Methods | Cross‐over RCT of supine, left lateral and right lateral positions | |
| Participants | Included: 18 ventilated very low birth weight (birth weight 1500 grams or less) of at least 14 days postnatal age with chronic lung disease (characteristic findings on chest radiograph) who had been stable in the preceding 24 hours and normal blood pressure without pharmacologic support
Excluded: infants with thoracostomy tubes, asymmetric lung disease, clinically significant PDA Median gestational age: 27.5 weeks; median birth weight: 970 g; median age: 31 days; median FiO2: 0.32 Setting: US |
|
| Interventions | Infants were settled in position for 15 minutes then measurements recorded every 30 seconds for 5 minutes | |
| Outcomes | tcO2; tcCO2 | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Appears to be no loss to follow‐up |
Chang 2002.
| Methods | Cross‐over RCT of prone and supine positions | |
| Participants | Included: 28 preterm infants, gestational age of 25 to 36 weeks, postnatal age of 7 days or less, mechanically ventilated with intermittent mandatory ventilation and in a stable condition
Excluded: infants treated with sedatives or with known congenital abnormalities Mean gestational age: 30 weeks; mean birth weight: 1378 g; mean age: 30 hours; mean weight: 1335 g; mean FiO2: 0.35 20 had signs of respiratory distress syndrome, 8 did not Setting: 2 tertiary care centres in Taiwan |
|
| Interventions | Infants were settled for 10 minutes in each position and data were to be recorded for 2 hours. However, 10 infants did not complete the 2 hours in both positions therefore the mean time in each position was 104 minutes | |
| Outcomes | SaO2; episodes of low SaO2 (< 90% for > 20 s; duration and frequency) The length of data collection was matched for the shorter position duration |
|
| Notes | Some infants in one position longer than others and later period of data used for comparison in these infants | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomised by a third person |
| Allocation concealment (selection bias) | Low risk | Block randomised by a third person |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | An investigator was present by each bedside so there may have been some bias in data collection, however, outcomes were not subjective |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Appears to be no loss to follow‐up |
Crane 1990.
| Methods | Cross‐over RCT of prone, supine and right lateral positions | |
| Participants | Included: 19 premature infants mechanically ventilated with intermittent mandatory ventilation and PEEP for clinical hyaline membrane disease
Excluded: not stated Mean gestational age: 31 weeks; mean birth weight: 1485 g; age: 20 to 72 hours; mean FiO2: 0.43 Setting: regional NICU, USA |
|
| Interventions | Infants remained in study position from 10 to 85 minutes | |
| Outcomes | tcCO2; PaCO2 Measurements were stabilised for 1 to 4 minutes before data were collected |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Loss to follow‐up 5/19; data were discarded due to excessive drift or inaccurate or inappropriate calibration of equipment |
Curley 2005.
| Methods | Parallel RCT of prone and supine positions | |
| Participants | Included: 102 Intubated and ventilated paediatric participants (2 weeks to 18 years) with a PaO2/FiO2 of less than or equal to 300, bilateral pulmonary infiltrates and no clinical evidence of left atrial hypertension Excluded: less than 42 weeks postconceptual age, unable to tolerate a position change, respiratory failure due to cardiac disease, hypoxaemia without bilateral infiltrates, bone marrow or lung transplant, receiving extracorporeal membrane oxygenation, a non‐pulmonary condition that was exacerbated by the prone position, participated in another trial within previous 30 days, decision to limit life support Median age: 2 years; median FiO2: 0.60; diagnosis: pneumonia 28, bronchiolitis with pneumonia 8, sepsis 7, aspiration 6, other 2 Setting: 7 paediatric ICUs, US |
|
| Interventions | Patients were placed in the prone position 20 hours/day while in the acute phase of their illness up to a maximum of 7 days; the median time in each position (acute phase) was 4 days in the prone group and 5 days in the supine group | |
| Outcomes | OI; PaO2; PaCO2; tidal volume; minute ventilation; FiO2; PaO2/FiO2; PEEP; extubations; obstructed ETT; pressure ulcers; hypercapnia | |
| Notes | Mortality was also reported at 28 days, however, as the intervention continued only up to a maximum of 7 days, we did not include these data in this review | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation performed using a permuted block design |
| Allocation concealment (selection bias) | Low risk | Serial numbered opaque sealed envelopes were used |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Data collection was not blinded but outcomes were not subjective |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Loss to follow‐up 1/102 because consent was withdrawn |
Ennis 1978.
| Methods | Cross‐over RCT of supine, left lateral and right lateral positions | |
| Participants | Included: 9 infants with hyaline membrane disease, all receiving assisted ventilation Excluded: not stated Setting: US |
|
| Interventions | Infants were placed in each position semi‐erect and also lying flat | |
| Outcomes | PaO2 Measurements commenced 5 to 10 minutes after position change or if intervention required, the mean value was calculated from 4 points, i.e. the start and end of recording and 2 equidistant points between |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Loss to follow‐up 1/9 because one infant could not be nursed flat at physicians request |
Fox 1990.
| Methods | Cross‐over RCT of prone and supine positions | |
| Participants | Included: 25 preterm infants with a gestational age of 36 weeks or less, who were receiving intermittent mandatory ventilation and/or CPAP and supplemental oxygen for respiratory distress syndrome, and had an umbilical artery oxygen tension catheter in situ Excluded: infants receiving paralysing drugs Mean gestational age: 31 weeks; mean age: 58 hours; mean weight: 1633 g Setting: level III NICU, US |
|
| Interventions | All in incubators, on water mattress with head elevated 30 degrees, infants were allowed to settle to sleep after positioning before data collection commenced Ventilatory parameters and FiO2 remained unchanged | |
| Outcomes | PaO2 Sampling occurred every 30 seconds for 15 minutes in each position; all data collected within a 1‐hour period |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | First position randomly assigned by coin toss |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Appears to be no loss to follow‐up |
Heaf 1983.
| Methods | Cross‐over RCT of supine and lateral good‐lung dependent, or lateral good‐lung uppermost positions | |
| Participants | Included: 10 infants with unilateral lung disease
Excluded: not stated Mean age: 45 days; mean weight: 3240 g; mean FiO2: 0.33; ventilation: intubated 5, mechanically ventilated 3; diagnosis: post‐diaphragmatic hernia repair 8, hypoplastic right lung 1, atelectasis of the right lung 1 Setting: UK |
|
| Interventions | Only the order of the lateral positions were randomly allocated; both lateral positions were preceded by supine positioning; infants remained in each position for 10 minutes | |
| Outcomes | tcO2; tcCO2 Recorded over the 10 minutes in each position |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Appears to be no loss to follow‐up |
Ibrahim 2007.
| Methods | Parallel RCT of prone and supine positions | |
| Participants | Included: 34 children aged 8 weeks to 10 years on mechanical ventilation for acute respiratory failure Excluded: participants with cardiac or neurological disease, chest or abdominal trauma, neurological surgery, unstable circulatory system or receiving extracorporeal membrane oxygenation Median age: 12 months; FiO2: 0.5; diagnosis: sepsis 13, pneumonia 12, inhalation injury 5, drowning 2 Setting: paediatric ICU, Saudi Arabia |
|
| Interventions | Prone and supine for 20 hours, both groups also received inhaled nitric oxide | |
| Outcomes | OI; PaO2/FiO2 at 1 hour and 20 hours | |
| Notes | Outcome data were also collected at 24 hours, however, as children in the prone group were changed to the supine position at 20 hours, these data were not included | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcome assessment was not blinded but outcomes were not subjective |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Loss to follow‐up 2/34; one because PaO2 improved and the other because of clinical deterioration |
Jenni 1997.
| Methods | Cross‐over RCT of horizontal prone position and prone position with the head elevated | |
| Participants | Included: 12 spontaneously breathing preterm infants with a history of idiopathic recurrent apnoea
Excluded: underlying disease such as anaemia, intracranial haemorrhage, heart disease or infection Mean gestational age: 28 weeks; mean birth weight: 1145 g; mean age; 18 days; mean FiO2: 0.26; other: 8 infants required supplemental oxygen Age: 6 to 38 days Setting: University Children's Hospital, Switzerland |
|
| Interventions | In the elevated position, the infants head was elevated to 15 degrees form the horizontal; each infant spent a total of 24 hours in each position (6 hours each day); all were nursed in an incubator with constantly maintained FiO2 and temperature during the study | |
| Outcomes | SaO2; hypoxaemia (SaO2 < 80%); bradycardia (< 90 beats per minute); thoracic impedance; FiO2 | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Appears to be no loss to follow‐up |
Keene 2000.
| Methods | Cross‐over RCT of prone and supine positions | |
| Participants | Included: 22 infants of less than 34 weeks gestation with symptomatic apnoea or bradycardia at least 24 hours following extubation or discontinuation of CPAP
Excluded: infants with any condition precluding them from being placed prone or supine Mean gestational age: 26.9 weeks; mean birth weight: 865 g; mean postconceptual age: 31.9 weeks; mean weight: 1204 g; diagnosis: respiratory distress syndrome 16, bronchopulmonary dysplasia 7; oxygenated: 13 Setting: USA |
|
| Interventions | Each infant was studied for 24 hours with the position changed every 6 hours | |
| Outcomes | SaO2; hypoxaemia (SaO2 < 80%); apnoea (> 10 secs); bradycardia (< 100 bpm) Most extreme value in each individual and mean duration recorded | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Appears to be no loss to follow‐up |
Kornecki 2001.
| Methods | Cross‐over RCT of prone and supine positions | |
| Participants | Included: 10 children aged 8 weeks to 16 years who required mechanical ventilation for moderate to severe acute respiratory failure Excluded: not stated Mean age: 5 years; mean weight: 22.5 kg; diagnosis: sepsis/acute respiratory distress syndrome 4, pneumonia 3, other 3 Setting: 36‐bed paediatric critical care unit in a tertiary care university‐based children's hospital in the US |
|
| Interventions | Supine first, then random order of first study position; 12 hours in each position | |
| Outcomes | Oxygenation index Data collected at 2, 4, 6, 8, 12 hours for each position |
|
| Notes | Heart rate, PaCO2 and arterial BP were not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Loss to follow‐up 2/12 enrolled, because they could not be placed in prone position due to physical restrictions |
Levene 1990.
| Methods | Cross‐over RCT of prone and supine positions | |
| Participants | Included: 13 infants aged 2 to 11 months with upper respiratory tract infection and nasal discharge and 17 infants with lower respiratory tract infection, or bronchiolitis (as determined clinically by tachypnoea, fever, wheeze, crackles on auscultation) Excluded: infants with SaO2 < 85% in air Setting: UK |
|
| Interventions | After settling in each position for 10 minutes, infants remained in each position for 20 minutes All participants studied during quiet sleep | |
| Outcomes | SaO2 continuously measured over 20 minutes | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Loss to follow‐up 9/39 infants as they did not go to sleep or woke when moved |
McEvoy 1997.
| Methods | Alternately allocated cross‐over trial of prone and supine positions | |
| Participants | Included: 55 infants with a birth weight less than 1000 g, a diagnosis of respiratory distress syndrome, a FiO2 of more than 0.21 at 28 days or older, and a chest radiograph consistent with chronic lung disease Excluded: infants with multiple congenital anomalies, congenital heart disease, culture proven sepsis, evidence of seizure activity, or grade IV intra‐ventricular haemorrhage Mean gestational age: 26.0; mean birth weight: 775 g; mean age: 42.0 days; mean weight: 1147 g; mean FiO2: 0.30; ventilated: 17 Setting: NICUs, hospital and medical centre, USA |
|
| Interventions | 1 hour in each position; alternatively and consecutively monitored after a 10‐minute stabilisation period in each position before monitoring was commenced; all were quietly asleep | |
| Outcomes | SaO2; heart rate; respiratory rate; hypoxaemia (SaO2 < 80% at 0 to 45 s) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Alternate allocation was used |
| Allocation concealment (selection bias) | High risk | Alternate allocation was used |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Appears to be no loss to follow‐up |
Mendoza 1991.
| Methods | Cross‐over RCT of prone and supine positions | |
| Participants | Included: 33 infants with respiratory distress syndrome and continuing intubation at 2 or more weeks of age; all were ventilated
Excluded: not stated Median gestational age: 27 weeks; median birth weight: 820 g; median age: 28 days; median weight: 1000 g; median FiO2: 0.30 Setting: USA |
|
| Interventions | Study time not stated; feedings stopped at least 30 minutes before study and stomach contents aspirated before pulmonary function testing | |
| Outcomes | SaO2; respiratory rate; heart rate; tidal volume; minute ventilation; compliance; pulmonary resistance; work of breathing | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Loss to follow‐up 7/33 for spontaneous breaths as these infants did not have any spontaneous breaths in one or both positions and 5/33 for computer‐selected breaths as these infants did not have any |
Mizuno 1995.
| Methods | Cross‐over RCT of prone and supine positions | |
| Participants | Included: 6 preterm infants with a birthweight of less than 1000 g with radiographic and clinical evidence of chronic lung disease requiring ventilatory support and a feeding volume of more than 100 ml a day
Excluded: not stated Mean gestational age: 24.9 weeks; mean birth weight: 722.7 g; mean age: 47.5 days; mean weight: 957 g; mean FiO2: 0.33; diagnosis: respiratory distress syndrome 5, dry lung 1 Setting: not stated |
|
| Interventions | The infant was settled into position for at least 1 hour before being fed nasogastrically over 1 hour | |
| Outcomes | SaO2; tcPO2; tidal volume; minute ventilation; heart rate; respiratory rate Measured 10 minutes before and after feeding when infants were quiet and awake |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Appears to be no loss to follow‐up |
Mizuno 1999.
| Methods | Cross‐over RCT of prone and supine positions | |
| Participants | Included: 7 very low birthweight infants with radiographic and clinical evidence of chronic lung disease, requiring ventilatory support and a feeding volume of more than 100 ml a day
Excluded: not stated Mean gestational age: 26.6 weeks; mean birth weight: 845 g; mean age: 57.4 days; mean weight: 1018 g; mean FiO2: 0.24 Setting: not stated |
|
| Interventions | The infant was settled into position for at least 1 hour before being fed nasogastrically over 1 hour | |
| Outcomes | SaO2; tcPCO2; tidal volume; minute ventilation; spontaneous respiratory rate; pulmonary resistance Measured 10 minutes before and during feeding (20 minutes, 40 minutes and end of feeding) when infants were quiet and awake |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Appears to be no loss to follow‐up |
Oliveira 2009.
| Methods | Cross‐over RCT of prone and supine positions | |
| Participants | Included: 12 preterm newborn infants recovering from respiratory distress syndrome with a gestational age of between 28 and 36 weeks; free from congenital malformations; free from clinical or surgical conditions that would prevent positioning from being studied; clinically stable, defined as spontaneous breathing, on room air or oxygen therapy with a FiO2 under 0.40 for more than 72 hours; recovering from respiratory distress syndrome, defined by radiological and clinical criteria; and weight above 1000 g at the time of enrolment Excluded: any factors preventing the infant being put in either the prone or supine position Mean gestational age: 30.8 weeks; mean birth weight: 1316 g; mean postconceptional age: 32.8 weeks; mean weight: 1446 g Setting: NICU, University Hospital, Brazil |
|
| Interventions | After 5 minutes of instrument calibration, infants remained in the study position for 30 minutes while data were recorded | |
| Outcomes | SaO2; tidal volume; minute ventilation; spontaneous respiratory rate; thoracoabdominal asynchrony; laboured breathing index Measured for 30 minutes |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Low risk | Randomly allocated using sealed envelopes |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Appears to be no loss to follow‐up |
Polacek 1992.
| Methods | Cross‐over RCT of supine, lateral atelectatic lung dependent; and lateral atelectatic lung non‐dependent positions | |
| Participants | Included: 25 children, aged from birth to 17 years, and unilateral atelectasis who had undergone heart surgery within the previous 2 weeks, and had an indwelling arterial catheter
Excluded: children with previous underlying disease, presence of pulmonary hypertension, presence of cardiac lesions with persistent intracardiac shunting Median age: 20 months; ventilation: mechanical ventilation 10, supplemental oxygen 9, spontaneously breathing room air 6 Setting: PICU in a tertiary paediatric hospital, USA |
|
| Interventions | All children were placed in the supine position first; only the order of the lateral position were randomised Children were positioned for 15 minutes in each position over a total of 45 minutes | |
| Outcomes | PaO2 Arterial blood gas sampled 15 minutes after position change | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Appears to be no loss to follow‐up |
Schlessel 1993.
| Methods | Cross‐over RCT of supine, left lateral and right lateral positions | |
| Participants | Included: 16 intubated infants of less than 36 weeks gestational age, clinical and radiological findings of respiratory distress syndrome, and decreasing ventilator and oxygen requirements for at least 24 hours prior to the study
Excluded: infants with clinically significant patent ductus arteriosus, asymmetric lung disease, pulmonary interstitial emphysema, pneumothorax Mean gestational age: 33.4 weeks; mean birth weight: 2060 g; mean age: 5 days; FiO2: 0.21 to 0.29 (settings not changed through the study) Setting: USA |
|
| Interventions | Each infant was placed in supine, followed by lateral positioning, them supine again, followed by the other lateral position; only the order of lateral positioning was randomised; each infant was placed for 15 minutes in each position over a total of 1 hour | |
| Outcomes | PaO2; PaCO2; respiratory rate; heart rate; blood pressure; dynamic lung compliance; total pulmonary resistance; inspiratory resistance; expiratory resistance; tidal volume Pulmonary mechanics were measured at 5 and 15 minutes in each position and arterial blood gases at 15 minutes |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | The order of the supine position was not randomised, only the lateral positions were randomised |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Appears to be no loss to follow‐up |
Sconyers 1987.
| Methods | Cross‐over RCT of supine flat, supine elevated, prone flat and prone elevated positions | |
| Participants | Included: 17 infants with tachypnoea of pulmonary aetiology
Patients required to be stable enough not to require respiratory intervention for the 2‐hour study period Excluded: infants with apnoea, bradycardia, multiple chest tubes or significant metabolic acidosis Diagnosis: transient tachypnoea of newborn 6, respiratory distress syndrome 6, bronchopulmonary dysplasia 5 Setting: US |
|
| Interventions | Both supine and prone positions were done while flat and while elevated (20 to 30 degrees above the horizontal) Each infant was placed for 30 minutes in each position | |
| Outcomes | Respiratory rate; heart rate Measured before each position change | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | There was a high potential for bias because the bedside nurse 'counted' the respiratory and heart rates |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Appears to be no loss to follow‐up |
Wolfson 1992.
| Methods | Cross‐over RCT of prone and supine positions | |
| Participants | Included: 24 preterm infants recovering from respiratory distress who had received mechanical ventilation and supplemental oxygen for a minimum of 2 days and who were spontaneously breathing room air or had an FiO2 of less than 0.40 at the time of study
Excluded: those with cardiac involvement or surgical intervention Mean gestational age: 28 weeks; mean birth weight: 1020 g; mean postconceptual age: 33 weeks; mean weight: 1450 g Setting: 2 NICUs in the USA |
|
| Interventions | Study time unclear; minimum 10 minutes in each position and no longer than 3 minutes at a time. Chest wall synchrony was taken over 10 breaths | |
| Outcomes | Tidal volume; peak to peak oesophageal pressure; respiratory rate; minute ventilation; pulmonary resistance; pulmonary compliance; thoracoabdominal synchrony | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Loss to follow‐up 4/24, reasons not given |
BP ‐ blood pressure bpm ‐ beats per minute CPAP ‐ continuous positive airways pressure ETT ‐ endotracheal tube FiO2 ‐ inspired oxygen ICU ‐ intensive care unit NICU ‐ neonatal intensive care unit O2 ‐ oxygen OI ‐ oxygenation index = (mean airway pressure X FiO2% / PaO2) PaCO2 ‐ arterial carbon dioxide tension PaO2 ‐ arterial oxygen tension PDA ‐ patent ductus arteriosis PEEP ‐ positive expiratory end pressure PICU ‐ paediatric intensive care unit RCT ‐ randomised controlled trial SaO2 ‐ oxygen saturation of haemoglobin tcO2 ‐ transcutaneous oxygen level tcCO2 ‐ transcutaneous carbon dioxide level
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Anonymous 2005 | Comment on Curley 2005 |
| Avery 1962 | Healthy infants |
| Baird 1991 | Not randomised |
| Brackbill 1973 | Healthy infants |
| Branco 2005 | Comment on Curley 2005 |
| Carlo 1989 | Not currently hospitalised respiratory‐distressed participants, follow‐up study |
| Dahl 1972 | Healthy infants |
| Heimler 1992 | Healthy preterm infants with apnoea not requiring respiratory support or supplemental oxygen, no evidence of respiratory distress |
| Hutchison 1979 | Healthy infants |
| Itakura 1998 | Not randomised |
| Kavanagh 2005 | Comment on Curley 2005 |
| Kishan 1981 | Does not appear to be randomised, infants do not have respiratory distress |
| Kravitz 1958 | Not randomised, no evidence of respiratory distress |
| Kurlac 1994 | Healthy preterm infants with apnoea, no evidence of respiratory distress, those who required assisted ventilation were excluded |
| Lioy 1988 | Not randomised, no evidence of respiratory distress |
| Lopez‐Herce Cid 2003 | Not randomised |
| Main 2006 | Comment on Curley 2005 |
| Martin 1979 | Not randomised, no evidence of respiratory distress in 11 of 16 infants |
| Maynard 2000 | No evidence of respiratory distress in 7 of 10 infants |
| McKinley 2000 | Comparison side/side turns versus prone/supine turns |
| Murai 1994 | Patients were weaned from oxygen and ventilator during study hence measures/interventions were not consistent |
| Murdoch 1994 | Not randomised |
| Numa 1997 | Not randomised |
| Sahni 1999 | Study of healthy infants |
| Sawhney 2005 | Only 24% of participants had acute respiratory distress |
| Schrod 1993 | Not randomised |
| Schwartz 1975 | Study of healthy babies, no evidence of respiratory distress |
| Vanderghem 1983 | No evidence of respiratory distress |
| Wagaman 1979 | Not randomised |
Contributions of authors
Donna Gillies: protocol development, data extraction, analysis, drafting and finalisation of original and updated reviews. Deborah Wells: protocol development, data extraction, analysis, drafting and finalisation of original review and the 2008 update. Abhishta P Bhandari: data extraction and drafting of the 2011 update.
Sources of support
Internal sources
The Children's Hospital Westmead, Sydney, Australia.
External sources
No sources of support supplied
Declarations of interest
None known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Antunes 2003 {published data only}
- Antunes L, Lugola L, Crocci AJ. Effect of preterm infant position on weaning from mechanical ventilation. Jornal de Pediatria 2003;79(3):239‐44. [PubMed] [Google Scholar]
Bhat 2003 {published data only}
- Bhat RY, Leipala JA, Singh NR, Rafferty GF, Hannam S, Greenough A. Effect of posture on oxygenation, lung volume, and respiratory mechanics in premature infants studied before discharge. Pediatrics 2003;112(1):29‐32. [DOI] [PubMed] [Google Scholar]
Bjornson 1992 {published data only}
- Bjornson KF, Deitz JC, Blackburn S, Billingsley F, Garcia J, Hays R. The effect of body position on the oxygen saturation of ventilated preterm infants. Pediatric Physical Therapy 1992;4(3):109‐15. [Google Scholar]
Bozynski 1988 {published data only}
- Bozynski ME, Naglie RA, Nicks JJ, Burpee B, Johnson RV. Lateral positioning of the stable ventilated very‐low‐birth‐weight infant. Effect on transcutaneous oxygen and carbon dioxide. American Journal of Diseases of Children 1988;142(2):200‐2. [DOI] [PubMed] [Google Scholar]
- Bozynski ME, Nicks JJ, Burpee B. Lateral positioning of mechanically ventilated vlbw infant; effect on tcPO2 and tcCO2. Developmental Medicine & Child Neurology 1986;53(Suppl 28):28. [Google Scholar]
Chang 2002 {published data only}
- Chang YJ. Decreased activity and oxygen desaturation in prone ventilated preterm infants during the first postnatal week. Heart & Lung 2002;31(1):34‐42. [DOI] [PubMed] [Google Scholar]
Crane 1990 {published data only}
- Crane LD, Snyder JE, Knight P, Philips JB, Cassady G. Effects of position changes on transcutaneous carbon dioxide tension in neonates with respiratory distress. Journal of Perinatology 1990;10(1):35‐7. [PubMed] [Google Scholar]
Curley 2005 {published data only}
- Curley MAQ, Arnold JH, Thompson JE, Fackler JC, Grant MJ, Fineman LD, et al. Clinical trial design‐effect of prone positioning on clinical outcomes in infants and children with acute respiratory distress syndrome. Journal of Critical Care 2006;21(1):23‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curley MAQ, Hibberd PL, Fineman LD, Wypij D, Shih MC, Thompson JE, et al. Effect of prone positioning on clinical outcomes in children with acute lung injury: a randomized controlled trial. JAMA 2005;294(2):229‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fineman LD, LaBrecque MA, Shih MC, Curley MAQ. Prone positioning can be safely performed in critically ill infants and children. Critical Care Medicine 2006;7:413‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ennis 1978 {published data only}
- Ennis S, Harris TR. Positioning infants with hyaline membrane disease. American Journal of Nursing 1978;78(3):398‐401. [PubMed] [Google Scholar]
Fox 1990 {published data only}
- Fox MD, Molesky MG. The effects of prone and supine positioning on arterial oxygen pressure. Neonatal Network ‐ Journal of Neonatal Nursing 1990;8(4):25‐9. [PubMed] [Google Scholar]
Heaf 1983 {published data only}
- Heaf DP, Helms P, Gordon I, Turner HM. Postural effects on gas exchange in infants. New England Journal of Medicine 1983;308(25):1505‐8. [DOI] [PubMed] [Google Scholar]
Ibrahim 2007 {published data only}
- Ibrahim TS, El‐Mohamady HS. Inhaled nitric oxide and prone position: how far they can improve oxygenation in pediatric participants with acute respiratory distress syndrome?. Journal of Medical Sciences 2007;7(3):390‐5. [Google Scholar]
Jenni 1997 {published data only}
- Jenni OG, Siebenthal K, Wolf M, Keel M, Duc G, Bucher HU. Effect of nursing in the head elevated tilt position (15 degrees) on the incidence of bradycardic and hypoxemic episodes in preterm infants. Pediatrics 1997;100(4):622‐5. [DOI] [PubMed] [Google Scholar]
Keene 2000 {published data only}
- Keene DJ, Wimmer JE Jr, Mathew OP. Does supine positioning increase apnea, bradycardia, and desaturation in preterm infants?. Journal of Perinatology 2000;20(1):17‐20. [DOI] [PubMed] [Google Scholar]
Kornecki 2001 {published data only}
- Kornecki A, Frndova H, Coates AL, Shemie SD. 4A randomized trial of prolonged prone positioning in children with acute respiratory failure. Chest 2001;119(1):211‐8. [DOI] [PubMed] [Google Scholar]
Levene 1990 {published data only}
- Levene S, McKenzie SA. Transcutaneous oxygen saturation in sleeping infants: prone and supine. Archives of Disease in Childhood 1990;65(5):524‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
McEvoy 1997 {published data only}
- McEvoy C, Mendoza ME, Bowling S, Hewlett V, Sardesai S, Durand M. Prone positioning decreases episodes of hypoxemia in extremely low birth weight infants (1000 grams or less) with chronic lung disease. Journal of Pediatrics 1997;130(2):305‐9. [DOI] [PubMed] [Google Scholar]
Mendoza 1991 {published data only}
- Mendoza JC, Roberts JL, Cook LN. Postural effects on pulmonary function and heart rate of preterm infants with lung disease. Journal of Pediatrics 1991;118(3):445‐8. [DOI] [PubMed] [Google Scholar]
Mizuno 1995 {published data only}
- Mizuno K, Itabashi K, Okuyama K. Effect of body position on the blood gases and ventilation volume of infants with chronic lung disease before and after feeding. American Journal of Perinatology 1995;12(4):275‐7. [DOI] [PubMed] [Google Scholar]
Mizuno 1999 {published data only}
- Mizuno K, Aizawa M. Effects of body position on blood gases and lung mechanics of infants with chronic lung disease during tube feeding. Pediatrics International 1999;41(6):609‐14. [DOI] [PubMed] [Google Scholar]
Oliveira 2009 {published data only}
- Oliveira T, Britto R, Franca D, Pereira N, Vaz L, Parreira V. Prone or supine: which is the most beneficial position for thoracoabdominal motion of preterm newborn recovering from RDS?. European Respiratory Society Annual Congress. Berlin, Germany, 2008:E1771.
- Oliveira TG, Rego MA, Pereira NC, Vaz LO, Franca DC, Vieira DS, et al. Prone position and reduced thoracoabdominal asynchrony in preterm newborns. Jornal de Pediatria 2009;85(5):443‐8. [DOI] [PubMed] [Google Scholar]
Polacek 1992 {published data only}
- Polacek TL, Barth L, Mestad P, Lacey‐Haun L, Mills N. The effect of positioning on arterial oxygenation in children with atelectasis after cardiac surgery. Heart & Lung 1992;21(5):457‐62. [PubMed] [Google Scholar]
Schlessel 1993 {published data only}
- Rappa HA, Lesser M, Harper RG. Pulmonary mechanics and gas exchange: effect of lateral positioning during recovery from respiratory distress syndrome. Pediatric Pulmonology 1993;15(1):36‐40. [DOI] [PubMed] [Google Scholar]
Sconyers 1987 {published data only}
- Sconyers SM, Ogden BE, Goldberg HS. The effect of body position on the respiratory rate of infants with tachypnea. Journal of Perinatology 1987;7(2):118‐21. [PubMed] [Google Scholar]
Wolfson 1992 {published data only}
- Wolfson MR, Greenspan JS, Deoras KS, Allen JL, Shaffer TH. Effect of position on the mechanical interaction between the rib cage and abdomen in preterm infants. Journal of Applied Physiology 1992;72(3):1032‐8. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Anonymous 2005 {published data only}
- Anonymous. Prone positioning may not help children with acute lung injury. Nursing 2005;35(11):32cc7‐8. [Google Scholar]
Avery 1962 {published data only}
- Avery ME. Effects of body‐tilting on the resting and expiratory position of newborn infants. Pediatrics 1962;29:255. [Google Scholar]
Baird 1991 {published data only}
- Baird TM, Paton JB, Fisher DE. Improved oxygenation with prone positioning in neonates: stability of increased transcutaneous PO2. Journal of Perinatology 1991;11(4):315‐8. [PubMed] [Google Scholar]
Brackbill 1973 {published data only}
- Brackbill Y, Douthitt TC, West H. Psychophysiologic effects in the neonate of prone versus supine placement. Journal of Pediatrics 1973;82(1):82‐4. [DOI] [PubMed] [Google Scholar]
Branco 2005 {published data only}
- Branco RG, Garcia PC. Prone positioning in children with acute lung injury. JAMA 2005;294(18):2297‐8. [DOI] [PubMed] [Google Scholar]
Carlo 1989 {published data only}
- Carlo WA, Beoglos A, Siner BS, Martin RJ. Neck and body position effects on pulmonary mechanics in infants. Pediatrics 1989;84(4):670‐4. [PubMed] [Google Scholar]
Dahl 1972 {published data only}
- Dahl M, Valimaki I. Postural effect on respiration and heart rate of newborn infants. An impedance pneumographic study. Biology of the Neonate 1972;20(3):161‐9. [DOI] [PubMed] [Google Scholar]
Heimler 1992 {published data only}
- Heimler R, Langlois J, Hodel DJ, Nelin LD, Sasidharan P. Effect of positioning on the breathing pattern of preterm infants. Archives of Disease in Childhood 1992;67(3):312‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hutchison 1979 {published data only}
- Hutchison AA, Ross KR, Russell G. The effect of posture on ventilation and lung mechanics in preterm and light‐for‐date infants. Pediatrics 1979;64(4):429‐32. [PubMed] [Google Scholar]
Itakura 1998 {published data only}
- Itakura Y, Ogawa Y. Effect of body position on tidal volume and minute ventilation in very low birthweight infants. Acta Paediatrica Japonica 1998;40(6):555‐7. [DOI] [PubMed] [Google Scholar]
Kavanagh 2005 {published data only}
- Kavanagh BP. Prone positioning in children with ARDS: positive reflections on a negative clinical trial. JAMA 2005;294(2):248‐50. [DOI] [PubMed] [Google Scholar]
Kishan 1981 {published data only}
- Kishan J, Bhargava SK, Rehman F. Effect of posture on arterial oxygen tension in preterm infants. Indian Pediatrics 1981;18(10):701‐4. [PubMed] [Google Scholar]
Kravitz 1958 {published data only}
- Kravitz H. The effect of the position on the respiratory rate of premature and mature newborn infants. Pediatrics 1958;22:432‐5. [PubMed] [Google Scholar]
Kurlac 1994 {published data only}
- Kurlak LO, Ruggins NR, Stephenson TJ. Effect of nursing position on incidence, type, and duration of clinically significant apnoea in preterm infants. Archives of Disease in Childhood: Fetal & Neonatal Edition 1994;71(1):F16‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lioy 1988 {published data only}
- Lioy J, Manginello FP. A comparison of prone and supine positioning in the immediate postextubation period of neonates. Journal of Pediatrics 1988;112(6):982‐4. [DOI] [PubMed] [Google Scholar]
Lopez‐Herce Cid 2003 {published data only}
- Lopez‐Herce Cid J, Garcia Sanchez E, Garcia Sanz C, Ruperez Lucas M, Alcaraz Romero A, Carrillo Alvarez A. Effects of prone position, inhaled nitric oxide and surfactant in children with hypoxemic pulmonary disease. Anales de Pediatria 2003;58(2):106‐14. [DOI] [PubMed] [Google Scholar]
Main 2006 {published data only}
- Main E. Prone positioning does not reduce the ventilation period or mortality in paediatric acute lung injury. Australian Journal of Physiotherapy 2006;52(1):63. [DOI] [PubMed] [Google Scholar]
Martin 1979 {published data only}
- Martin RJ, Herrell N, Rubin D, Fanaroff A. Effect of supine and prone positions on arterial oxygen tension in the preterm infant. Pediatrics 1979;63(4):528‐31. [PubMed] [Google Scholar]
Maynard 2000 {published data only}
- Maynard V, Bignall S, Kitchen S. Effect of positioning on respiratory synchrony in non‐ventilated pre‐term infants. Physiotherapy Research International 2000;5(2):96‐110. [DOI] [PubMed] [Google Scholar]
- Maynard V, Bignall S, Kitchen S. The effect of positioning on the stability of oxygenation and respiratory synchrony in non‐ventilated pre‐term infants. Journal of Clinical Nursing 1999;8(4):479‐81. [DOI] [PubMed] [Google Scholar]
McKinley 2000 {published data only}
- McKinley D, Hutchinson A, Butt W. Prone body positioning of ventilated children with respiratory illness does not increase complications. 7th Paediatric and Neonatal Intensive Care Conference ‐ Abstract Paper. 2000.
Murai 1994 {published data only}
- Murai DT, Grant JW. Continuous oscillation therapy improves the pulmonary outcome of intubated newborns: results of a prospective, randomized, controlled trial. Critical Care Medicine 1994;22(7):1147‐54. [DOI] [PubMed] [Google Scholar]
Murdoch 1994 {published data only}
- Murdoch IA, Storman MO. Improved arterial oxygenation in children with the adult respiratory distress syndrome: the prone position. Acta Paediatrica 1994;83(10):1043‐6. [DOI] [PubMed] [Google Scholar]
Numa 1997 {published data only}
- Numa AH, Hammer J, Newth CJ. Effect of prone and supine positions on functional residual capacity, oxygenation, and respiratory mechanics in ventilated infants and children. American Journal of Respiratory and Critical Care Medicine 1997;156(4):1185‐9. [DOI] [PubMed] [Google Scholar]
Sahni 1999 {published data only}
- Sahni R, Schulze KF, Kashyap S, Ohira Kist K, Myers MM, Fifer WP. Body position, sleep states, and cardiorespiratory activity in developing low birth weight infants. Early Human Development 1999;54(3):197‐206. [DOI] [PubMed] [Google Scholar]
Sawhney 2005 {published data only}
- Sawhney A, Kumar N, Sreenivas V, Gupta S, Tyagi V, Puliyel JM. Prone versus supine position in mechanically ventilated children: a pilot study. Medical Science Monitor 2005;11(5):CR235‐40. [PubMed] [Google Scholar]
Schrod 1993 {published data only}
- Schrod L. Effect of body position and positioning changes on lung function of ventilated premature and newborn infants. Klinische Pädiatrie 1993;205(3):145‐9. [DOI] [PubMed] [Google Scholar]
Schwartz 1975 {published data only}
- Schwartz FC, Fenner A, Wolfsdorf J. The influence of body position on pulmonary function in low birthweight babies. South African Medical Journal 1975;49(3):79‐81. [PubMed] [Google Scholar]
Vanderghem 1983 {published data only}
- Vanderghem A, Beardsmore C, Silverman M. Postural variations in pulmonary resistance, dynamic compliance, and esophageal pressure in neonates. Critical Care Medicine 1983;11(6):424‐7. [DOI] [PubMed] [Google Scholar]
Wagaman 1979 {published data only}
- Wagaman MJ, Shutack JG, Moomjian AS, Shaffer TH, Schwartz JG, Fox WW. Improved oxygenation and lung compliance with prone positioning of neonates. Journal of Pediatrics 1979;94(5):787‐91. [DOI] [PubMed] [Google Scholar]
- Wagaman MJ, Shutack JG, Moomjian AS, Shaffer TH, Schwartz JG, Fox WW. The effects of different body positions on pulmonary function in neonates recovering from respiratory disease. Pediatric Research 1978;12(4):571. [Google Scholar]
Additional references
Adams 1994
- Adams JA, Zabaleta IA, Sackner MA. Comparison of supine and prone noninvasive measurements of breathing patterns in full term newborns. Pediatric Pulmonology 1994;18(1):8‐12. [DOI] [PubMed] [Google Scholar]
Agostoni 1965
- Agostoni E. Relation between changes of ribcage circumference and lung volume. Journal of Applied Physiology 1965;20:1179. [Google Scholar]
Ahmed 2004
- Ahmed SH, Sarkis NN, Fikry SI. A study of neonatal morbidity and mortality at Damanhour Teaching Hospital Newborn Unit. Journal of the Egyptian Public Health Association 2004;79(5‐6):399‐414. [PubMed] [Google Scholar]
Balaguer 2006
- Balaguer A, Escribano J, Roqué I, Figuls M. Infant position in neonates receiving mechanical ventilation. Cochrane Database of Systematic Reviews 2006, Issue 4. [DOI: 10.1002/14651858.CD003668.pub2] [DOI] [PubMed] [Google Scholar]
Ball 1999
- Ball C. Use of the prone position in the management of acute respiratory distress syndrome. Clinical Effectiveness in Nursing 1999;3:36‐46. [DOI] [PubMed] [Google Scholar]
Bateman 2000
- Bateman S, Arnold J. Acute respiratory failure in children. Current Opinion in Pediatrics 2000;12:233‐7. [DOI] [PubMed] [Google Scholar]
Blanch 1997
- Blanch L, Mancebo J, Perez M, Martinez M, Mas A, Betbese AJ. Short‐term effects of prone position in critically ill participants with acute respiratory distress syndrome. Intensive Care Medicine 1997;23(10):1033‐9. [DOI] [PubMed] [Google Scholar]
Bryan 1974
- Bryan AC. Conference on the scientific basis of respiratory therapy. Pulmonary physiotherapy in the pediatric age group. Comments of a devil's advocate. American Review of Respiratory Disease 1974;110(6 Part 2):143‐4. [DOI] [PubMed] [Google Scholar]
Buckmaster 2007
- Buckmaster AG, Wright IM, Arnolda G, Henderson‐Smart DJ. Practice variation in initial management and transfer thresholds for infants with respiratory distress in Australian hospitals. Who should write the guidelines?. Journal of Paediatrics and Child Health 2007;43(6):469‐75. [DOI] [PubMed] [Google Scholar]
Caitlin 2008
- Catlin A. Extremely long hospitalizations of newborns in the United States: data, descriptions, dilemmas. Advances in Neonatal Care 2008;8(2):125‐32. [DOI] [PubMed] [Google Scholar]
Chang 2000
- Chang SC, Lin CH, Lin YJ, Yeh TF. Mortality, morbidity, length and cost of hospitalization in very‐low‐birth‐weight infants in the era of National Health Insurance in Taiwan: a medical center's experience. Acta Paediatrica Taiwanica 2000;41(6):308‐12. [PubMed] [Google Scholar]
Chatte 1997
- Chatte G, Sab JM, Dubois JM, Sirodot M, Gaussorgues P, Robert D. Prone position in mechanically ventilated participants with severe acute respiratory failure. American Journal of Respiratory and Critical Care Medicine 1997;155(2):473‐8. [DOI] [PubMed] [Google Scholar]
Curley 1999
- Curley, MA. Prone positioning of patients with acute respiratory distress syndrome: a systematic review. American Journal of Critical Care 1999;8(6):397‐405. [PubMed] [Google Scholar]
Davis 1987
- Davis GM, Bureau MA. Pulmonary mechanics in newborn respiratory control. Clinical Perinatology 1987;14:551. [PubMed] [Google Scholar]
Dean 1985
- Dean E. Effect of body position on pulmonary function. Physical Therapy 1985;65:613‐8. [DOI] [PubMed] [Google Scholar]
Dean 1996
- Dean E. Body positioning. In: Frownfelter D, Dean E editor(s). Principles and Practice of Cardiopulmonary Physical Therapy. 3rd Edition. St Louis: Mosby, 1996:299‐320. [Google Scholar]
Dickersin 1994
- Dickersin K, Scherer R, Lefebvre C. Identifying relevant studies for systematic reviews. BMJ 1994;309:1286‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Douglas 1977
- Douglas W, Rheder K, Froukje M, Sessler A, Marsh H. Improved oxygenation in participants with acute respiratory failure: the prone position. American Review of Respiratory Disease 1977;115:559‐66. [DOI] [PubMed] [Google Scholar]
Elbourne 2002
- Elbourne DR, Altman DG, Higgins JPT, Curtin F, Worthington HV, Vail A. Meta‐analyses involving cross‐over trials: methodological issues. International Journal of Epidemiology 2002;31:140‐9. [DOI] [PubMed] [Google Scholar]
Fridrich 1996
- Fridrich P, Krafft P, Hochleuthner H, Mauritz W. The effects of long‐term prone positioning in participants with trauma‐induced adult respiratory distress syndrome. Anesthesia & Analgesia 1996;83(6):1206‐11. [DOI] [PubMed] [Google Scholar]
Gattinoni 1991
- Gattinoni L, Pelosi P, Vitale G, Pesenti A, D'Andrea L, Mascheroni D. Body position changes redistribute lung computed‐tomographic density in participants with acute respiratory failure. Anesthesiology 1991;74(1):15‐23. [DOI] [PubMed] [Google Scholar]
Hazinski 1992
- Hazinski MF. Children are Different Chapter 1 and Pulmonary Disorders Chapter 6. In: Hazinski MF editor(s). Nursing Care of The Critically Ill Child. 2nd Edition. St Louis: Mosby Year Book Inc, 1992. [Google Scholar]
Langer 1988
- Langer M, Mascheroni D, Marcolin R, Gattinoni L. The prone position in ARDS participants. A clinical study. Chest 1988;94(1):103‐7. [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration. Available from www.cochrane‐handbook.org.. Chichester, UK: Wiley‐Blackwell, 2011. [Google Scholar]
Lewandowski 1996
- Lewandowski K, Pappert D, Kuhlen R, Rossaint R, Gerlach H, Falke K. Clinical aspects of acute lung failure in adults (ARDS) [Klinische Aspekte des akuten Lungenversagens des Erwachsenen (ARDS)]. Anaesthetist 1996;45(1):1‐18. [DOI] [PubMed] [Google Scholar]
Lodato 1990
- Lodato RF. Oxygen toxicity. Critical Care Clinics 1990;6:749. [PubMed] [Google Scholar]
Meurer 2000
- Meurer JR, George V, Subichin S, Yauck J, Layde P. Asthma severity among children hospitalized in 1990 and 1995. Archives of Pediatrics and Adolescent Medicine 2000;154(2):143‐9. [DOI] [PubMed] [Google Scholar]
Mintegi Raso 2004
- Mintegi Raso S, Benito Fernandez J, Garcia Gonzalez S, Corrales Fernandez A, Bartolome Albistegui MJ, Trebolazabala Quirante N. Patient demand and management in a hospital pediatric emergency setting. Anales de Pediatria 2004;61(2):156‐61. [DOI] [PubMed] [Google Scholar]
Muller 1979
- Muller NL, Bryan AC. Chest wall mechanics and respiratory muscles in infants. Pediatric Clinics North America 1979;26(3):503‐16. [DOI] [PubMed] [Google Scholar]
Pappert 1994
- Pappert D, Rossaint R, Slama K, Gruning T, Falke KJ. Influence of positioning on ventilation‐perfusion relationships in severe adult respiratory distress syndrome. Chest 1994;106(5):1511‐6. [DOI] [PubMed] [Google Scholar]
Pelosi 1998
- Pelosi P, Tubiolo D, Mascheroni D, Vicardi P, Crotti S, Valenza F, et al. Effects of the prone position on respiratory mechanics and gas exchange during acute lung injury. American Journal of Respiratory and Critical Care Medicine 1998;157(2):387‐93. [DOI] [PubMed] [Google Scholar]
Piehl 1976
- Piehl MA, Brown RS. Use of extreme position changes in acute respiratory failure. Critical Care Medicine 1976;4(1):13‐4. [DOI] [PubMed] [Google Scholar]
Ponsonby 1992
- Ponsonby AL, Dwyer T, Gibbons LE, Cochrane JA, Wang YG. Factors potentiating the risk of sudden infant death syndrome associated with the prone position. New England Journal of Medicine 1993;329(6):377‐82. [DOI] [PubMed] [Google Scholar]
Shay 1999
- Shay DK, Holman RC, Newman RD, Liu LL, Stout JW, Anderson LJ. Bronchiolitis‐associated hospitalizations among US children, 1980‐1996. JAMA 1999;282(15):1440‐6. [DOI] [PubMed] [Google Scholar]
Simiyu 2004
- Simiyu DE. Morbidity and mortality of low birth weight infants in the new born unit of Kenyatta National Hospital, Nairobi. East African Medical Journal 2004;81(7):367‐74. [DOI] [PubMed] [Google Scholar]
Sritipsukho 2007
- Sritipsukho S, Suarod T, Sritipsukho P. Survival and outcome of very low birth weight infants born in a university hospital with level II NICU. Journal of the Medical Association of Thailand 2007;90(7):1323‐9. [PubMed] [Google Scholar]
Stocker 1997
- Stocker R, Neff T, Stein S, Ecknauer E, Trentz O, Russi E. Prone positioning and low‐volume pressure‐limited ventilation improve survival in participants with severe ARDS. Chest 1997;111(4):1008‐17. [DOI] [PubMed] [Google Scholar]
Wong 1999
- Wong W. Use of body positioning in the mechanically ventilated patient with acute respiratory failure: application of Sackett's rules of evidence. Physiotherapy Theory and Practice 1999;15:25‐41. [Google Scholar]
References to other published versions of this review
Gillies 2009
- Gillies D, Wells D. Positioning for acute respiratory distress in hospitalised infants and children. Cochrane Database of Systematic Reviews 2009, Issue 3. [DOI: 10.1002/14651858.CD003645.pub2] [DOI] [PubMed] [Google Scholar]
Wells 2005
- Wells DA, Gillies D, Fitzgerald DA. Positioning for acute respiratory distress in hospitalised infants and children. Cochrane Database of Systematic Reviews 2005, Issue 2. [DOI: 10.1002/14651858.CD003645] [DOI] [PubMed] [Google Scholar]


