Abstract
Anorectal conditions are one of the most common problems evaluated by primary care physicians. Most patients present with rectal pain, rectal bleeding, or purulent drainage per rectum. Colorectal conditions have overlapping symptoms. Thorough history and careful anorectal examination can differentiate common anorectal conditions like hemorrhoids, anorectal abscesses, anal fistula, anal fissure, and anal condyloma. Most of these conditions can be diagnosed and treated without imaging.
Introduction
Anorectal disorders are commonly encountered in primary care practices. There are overlapping symptoms in these disorders causing confusion and misdiagnosis. This paper will review anorectal abscesses, anal fistula, hemorrhoids, anal fissure and anal condyloma.
Anorectal Abscesses
Anorectal abscess results from obstruction of anal glands. Anorectal abscesses are defined by the anatomic space in which they develop. These most commonly involve the perianal and ischiorectal spaces; less often the intersphincteric, supralevator and submucosal spaces.1 The incidence of anorectal abscesses is 8.6–20/100,000, with a male: female ratio of 3:1. They may occur at any age with a peak incidence between 20 and 40 years of age.2
The diagnosis of anorectal abscess is usually based on the patient’s history and physical examination. Perianal pain and swelling are common with superficial abscesses.2 Deeper abscesses also present with pain that is referred to the perineum or buttock but visualized swelling is less common. Inspection of the perineum may reveal superficial erythema and fluctuance with tenderness to palpation. Digital rectal exam commonly elicits severe pain. Imaging is rarely needed.3
The primary treatment of anorectal abscess remains surgical drainage. In general, the incision should be kept as close as possible to the anal verge to minimize the length of a potential fistula. Bedside or in-office drainage with local anesthetic and a scalpel is usually adequate. Relief is immediate. Sometimes, examination under anesthesia is needed to confirm deeper more occult abscesses and to drain more complicated abscesses.4
After drainage, abscess recurrence has been observed in up to 44% of patients, most often within one year of initial treatment. Inadequate drainage (such as using a needle), loculations, horseshoe-type abscess and failure to perform primary fistulotomy have been identified as risk factors for recurrent anorectal abscess.5
Antibiotics should be reserved for patients with anorectal abscess complicated by cellulitis, systemic signs of infection or underlying immunosuppression. The sole treatment of anorectal abscesses with antibiotics is inadequate.
Anal Fistula
A fistula is an abnormal connection between two epithelialized structures. An anal fistula results from chronic infection and epithelialization of prior abscess drainage site. The incidence is 2/100,000/year. Anal fistulae are usually cryptoglandular/abscess-related in over 90% of cases. Blockage of anal crypts/glands leads to infection and abscess formation.6 Following routine abscess drainage, a failure of the tract to heal may lead to fistula formation. Less common causes include trauma and inflammatory bowel disease. Anal fistulae are classified in relationship to sphincter complex as intersphincteric, transphincteric, suprasphincteric and extrasphincteric.7 Intershincteric and low transphicteric are most common.
Patients who present with anal fistula after resolution of the abscess typically report intermittent perianal swelling and drainage. Inspection of the perineum should include a search for surgical scars, signs of perianal Crohn’s disease and the presence of the external opening of the fistula. Probing a fistula during office exam is not necessary. Goodsall’s rule, generally predicts short radial tracts for anterior fistulae close to the anus. Posterior fistulae and those far from the anus are more variable in their course.8
Simple fistulae, in general, do not require diagnostic imaging. If persistent for more than six weeks, examination under anesthesia and treatment is indicated, as they will not resolve spontaneously. Complex and recurrent anal fistula may require imaging such as CT, MRI, ultrasound and fistulogram.
The primary goal of treatment of anal fistula is fistula resolution without compromising continence. Fistula cause, prior treatments, fistula anatomy, underlying continence, and comorbidities must all be considered when formulating a treatment plan. Regardless, recurrences are common and the potential need for multiple procedures must be discussed preoperatively.9
Fistulotomy (cutting open) is effective treatment for simple anal fistula that results in healing in over 90% of patients and a low chance of continence issues. More complex fistulae involving more sphincter muscle can be treated with rectal advancement flap, ligation of intersphincteric fistula tract (LIFT), fibrin glue, fistula plug, cutting or draining setons (rubber bands).10 The existence of many treatment options reflects that lack of a perfect solution and treatment requires careful counseling and individualization to each patient’s unique presentation. Complicated or multiply recurrent fistulae, fistula in setting of Crohn’s disease, or fistula in patients with poor sphincter function as baseline may even require colostomy.
Hemorrhoids
“Hemorrhoids” represent one of the most common medical and surgical disease processes encountered in the United States. It is estimated that more than 50% of the population over 50 years of age have experienced hemorrhoid problems. Unfortunately, many anorectal conditions, such as cancer and inflammatory bowel disease, are inappropriately attributed to hemorrhoids.11
Hemorrhoids are normally occurring vascular cushions containing blood vessels, smooth muscles, and elastic tissue that serve to maintain closure of anal canal and aid in fecal continence. Symptomatic hemorrhoids develop because of chronic constipation, prolonged straining, poor bowel hygiene, pregnancy, ascites, pelvic mass and pelvic dysfunction that increase abdominal pressure.12
Hemorrhoids are divided into internal and external. Internal hemorrhoids originate proximal to dentate line and external hemorrhoids are located distal to dentate line. Internal hemorrhoidal symptoms typically include painless bright red bleeding, itching, burning, pain, prolapse and swelling.13 The physical examination should typically include visual inspection of the anus and digital examination. Anoscopy can allow direct visualization in the office. Colonoscopy is helpful to rule out other pathology in patients of appropriate age who have not been screened or when reported symptoms are not clearly explained by physical exam.13 Since colorectal cancer can also cause painless anorectal bleedings, the presence of enlarged hemorrhoids does not rule out malignancy.
Internal Hemorrhoids classified into four grades: 1) Prominent internal hemorrhoidal tissue without prolapse; 2) Prolapse with defecation and spontaneous reduction; 3) Prolapse with defecation requiring manual reduction; and 4) Chronically prolapsed and irreducible.1
Dietary modification focusing on adequate fluid intake (64 ounces daily) and fiber intake (approximately 30 grams daily) are primary non-operative strategies for patients with symptomatic hemorrhoid disease. Minimizing straining and time on the commode are also helpful. Most patients with grade I, II, and III hemorrhoid disease, in whom medical treatment fails, may benefit from office-based procedures. These include rubber band ligation, sclerotherapy, and infrared coagulation. Hemorrhoid banding is typically well tolerated is painless, repeatable and requires no alteration in daily activities.12
Surgical hemorrhoidectomy is indicated for patients who fail nonoperative and office-based procedures or have more advanced disease at presentation. Surgical hemorrhoidectomy involves complete removal of all pathologic hemorrhoid tissue, under general or spinal anesthesia. Complications of hemorrhoidectomy include severe pain, bleeding, wound complications and anal stenosis. Significant, lasting fecal incontinence is quite uncommon, urinary retention and rarely infection.14
External hemorrhoids are distal to the dentate line therefore innervated by somatic nerves. Combined internal and external hemorrhoids that are causing symptoms frequently require operative removal as described above. External hemorrhoids thrombosis causes sudden severe anal pain usually precipitated by an episode of constipation or diarrhea. A firm, exquisitely tender lump (often resembling a blueberry) typically accompanies the pain. It rapidly progresses and peaks at 48 hours but can then start to diminish. Bleeding often indicates spontaneous clot drainage.15 Management is directed at pain control with topical anesthetic cream, sitz bath, and oral pain medication. If resolution does not occur or if examined early in its course, simple bedside clot excision with local anesthetic can offer immediate relief. Excision of the thrombosed hemorrhoids in the office or operating room may be required,16 for severe pain presenting in the first few days. If pain is mild and patient presents several days into symptoms, excision is best avoided, as it would likely worsen the pain.
Anal Fissure
Anal fissures are common and often present advertised as “hemorrhoids”. Patients frequently have bright red bleeding and severe tearing/stabbing pain made worse with bowel movements. They generally represent a traumatic tear, usually caused by passage of a hard stool or conversely, diarrhea. They are almost always in the midline, most often posteriorly. The fissures cause severe spasm of the internal sphincter, which leads to an elevated resting pressure and tissue hypoxia, which prevents healing.17
Fissures that are deeper lateral or multiple in number are considered atypical fissures. The differential diagnosis of an atypical fissure includes Crohn’s disease, hematologic malignancies, HIV/AIDS, sexually transmitted diseases, dermatologic conditions, tuberculosis and anal carcinoma.18 Further workup under these circumstances is warranted.
An acute anal fissure can be diagnosed with gentle buttock spreading and inspection. They are often quite small and difficult to see unless the provider has seen many of them. Digital exam causes severe pain and should be avoided if fissure is suggested by symptoms and identified by simple inspection. A chronic anal fissure may be associated with an enlarged anal papilla proximally, sentinel tag or pile distally (which causes many to be misdiagnosed as hemorrhoids). Internal sphincter muscle fibers are frequently visible in the base of the fissure.18
Many acute fissures heal spontaneously but are considered chronic if present greater than six to eight weeks. Medical treatment for an acute anal fissure focuses on improving the bowel function. Resolving the causative constipation with increased water intake and fiber is paramount.19 Treating the underlying cause for a diarrheal illness is recommended. Topical anesthetic such, as lidocaine, may be used for symptomatic relief. Warm sitz baths help decrease sphincter spasm.
Highly symptomatic acute and chronic anal fissures will need more specific treatment, which usually focuses on decreasing sphincter tone. Topical nitroglycerin (0.2–0.4%) can be used to relax the internal sphincter. Rectiv (0.4% nitroglycerin) is commercially available. Headache caused by the nitroglycerin limits its use. Do not use 2% nitroglycerin intended for cardiac disease. Topical calcium channel blockers, such as nifedipine (0.2–2%) and diltiazem (2%) also cause relaxation of the internal sphincter. These medications must be prepared at a compounding pharmacy and do not cause headaches, making them better choice for primary use. It is frequently mixed with lidocaine and other agents for additional symptom relief. Topical therapy combined with stool softening with fiber is effective in up to 80% of cases.20
Surgical management is indicated with failed medical treatment, associated infection, and concern for other pathology like malignancy. Lateral internal sphincterotomy is the gold standard for surgical management and over 95% effective. Many surgeons try to avoid sphincterotomy in women of childbearing age due to the possibility of obstetrical sphincter injury. Sphincterotomy carries with it a risk of incontinence of 8–30%.20 Botulinum toxin can achieve sphincter relaxation of medium duration to allow fissure healing. It has a higher long-term fissure recurrence rate but much lower long-term incontinence risk.20 Other surgical options include fissurectomy and anal flap advancement procedures. The decision of what surgical procedure to use requires discussion between the surgeon and patient.
Anal Condyloma
Anal condyloma are caused by human papillomavirus (HPV). The condyloma or “warts” can be on the perianal skin as well as within the anal canal. It is very important that the treating physician is comfortable with anoscopy so the extent of intra-anal disease can be identified. Anal condyloma are sexually transmitted by both heterosexual and homosexual intercourse. Transmission is most common with unprotected anal receptive intercourse but can occur without. Condoms may not completely prevent transmission of HPV.21
There are more than 120 subtypes of HPV. Subtypes 6 and 11 have a low malignant potential. Other, more oncogenic, subtypes of HPV may affect the anal region. HPV is the leading cause of squamous cell carcinoma of the anus. Patients with HIV/AIDS and other immunosuppressed conditions are at higher risk for HPV disease and development of malignancies.21
The patient generally complains about the presence of lesions. Itching, bleeding, and pain may be associated. Difficulty with perineal hygiene is common with a large volume of disease.22 Condyloma may be mistaken as hemorrhoids by the patient.
Diagnosis is by inspection of the perianal skin as well as anoscopy to visualize the anoderm. Condyloma may vary in number and size of lesions. Screening for other sexually transmitted diseases should be part of the initial evaluation. A complete genitourinary examination is important to try to identify other sites of infection.23
After diagnosis, a treatment plan is established. If there is no intra-anal disease, topical therapies may be used. These include imiquimod (Aldara), podophyllotoxin (Condylox) and sinecatechins (Veregen). Physician administered treatments include cryotherapy, trichloroacetic acid application, surgical excision, electrosurgical fulguration and laser ablative therapy. Imiquimod (Aldara) may be used following complete excision to decrease recurrence.24 More extensive disease mandates frequent trips to the operating room for surgical treatment. It is important to obtain a biopsy, especially in high-risk individuals. Condyloma may have varying degrees of dysplasia, carcinoma in situ and invasive carcinoma.25
Treatment, whether patient applied or physician administered, requires extensive follow-up up. Recurrence is common regardless of treatment choice. Vaccination for HPV should be discussed and screening for sexual partners is recommended.
Conclusion
Though anorectal conditions have overlapping symptoms, careful history and good physical examination can usually confirm diagnosis. If a patient mentions a symptom, they require examination. If a patient fails their initial treatment regimen or if the underlying diagnosis is unclear, referral to a colorectal surgeon is recommended.
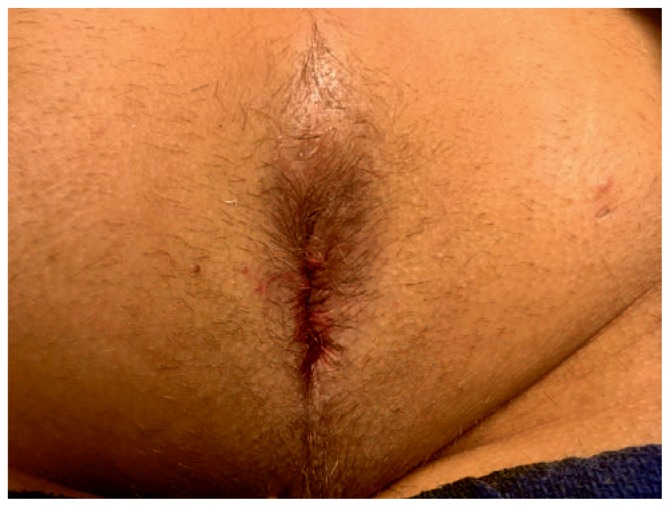
Figure 1.
Perianal abscess
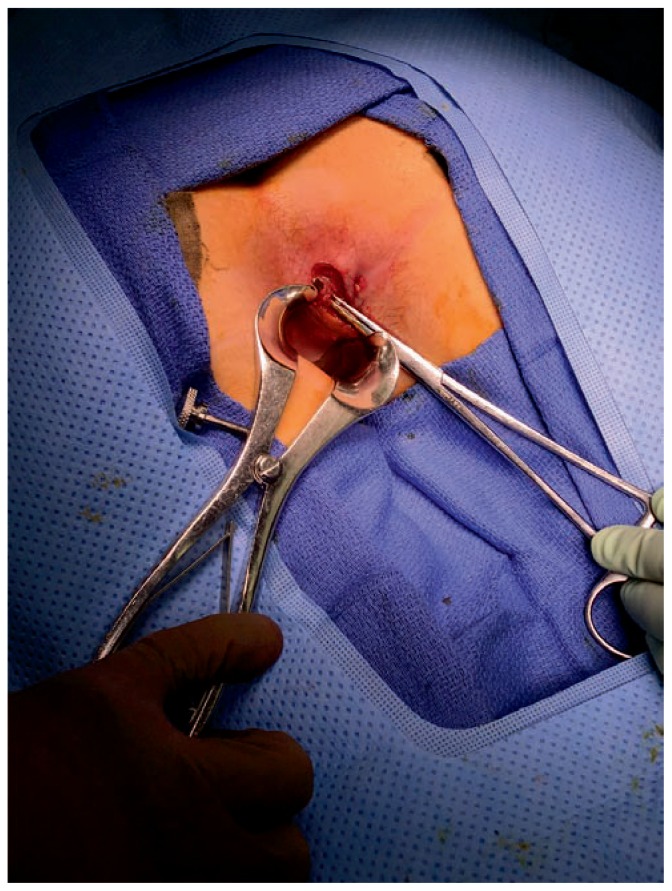
Figure 2.
LIFT (ligation of intersphincteric fistula tract) procedure for anal fistula
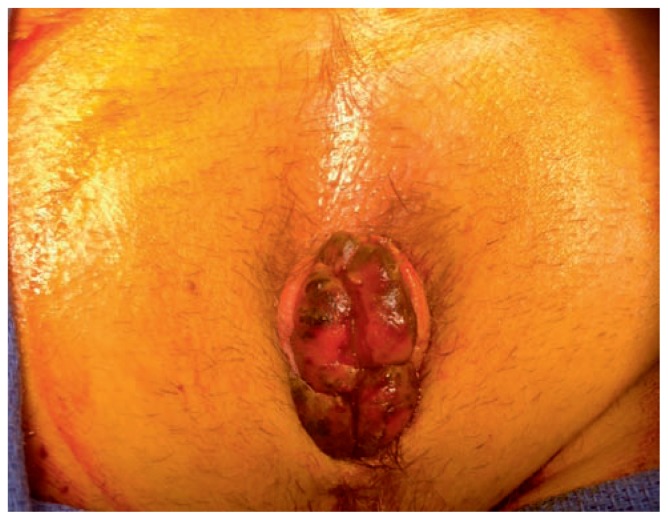
Figure 3.
Thombosed external hemorrhoids
Figure 4.
Posterior midline anal fissure
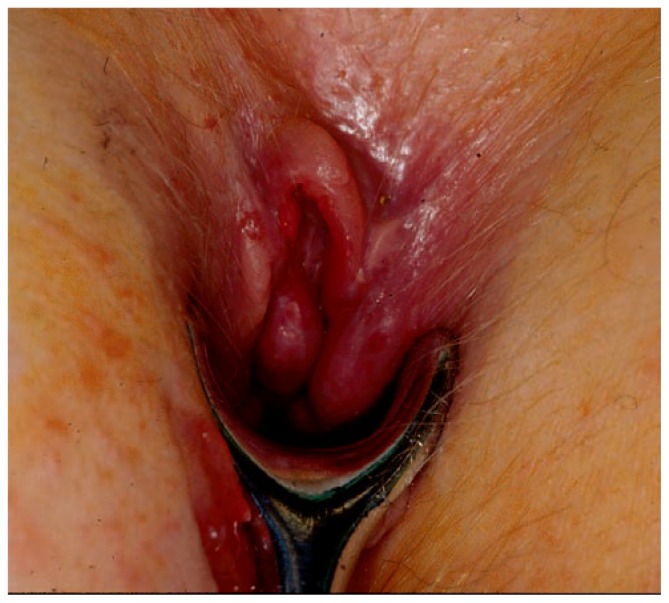
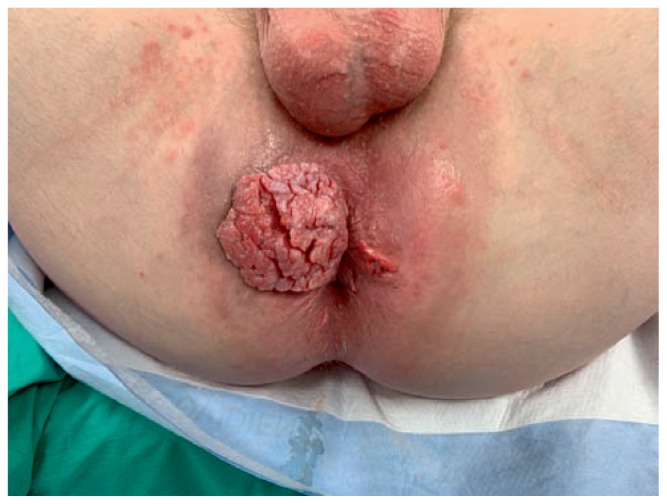
Figure 5.
Anal condyloma
Footnotes
Rakesh Hegde, MD, (above), John M. Trombold, MD & José M. Dominguez, MD, MSMA member since 1996, are in the Department of Colon and Rectal Surgery, Ferrell-Duncan Clinic, CoxHealth, Springfield, Missouri.
Contact: Rakesh.Hegde@coxhealth.com
Disclosure
None reported.
This article is Part I of a three-part mini-series on Colorectal Surgery.
References
- 1.Eisenhammer S. The internal anal sphincter and the anorectal abscess. Surg Gynecol Obstet. 1956;103:501–506. [PubMed] [Google Scholar]
- 2.Cox SW, Senagore AJ, Luchtefeld MA, Mazier WP. Outcome after incision and drainage with fistulotomy for ischiorectal abscess. Am Surg. 1997;63:686–689. [PubMed] [Google Scholar]
- 3.Gosselink MP, Van Onkelen RS, Schouten WR. The cryptoglandular theory revisited. Colorectal Dis. 2015;17:1041–1043. doi: 10.1111/codi.13161. [DOI] [PubMed] [Google Scholar]
- 4.Ommer A, Herold A, Berg E, Fürst A, Schiedeck T, Sailer M. Germans 3-Guideline: rectovaginal fistula. Ger Med Sci. 2012;10 doi: 10.3205/000166. Doc15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nelson R. Anorectal abscess fistula: what do we know? Surg Clin North Am. 2002;82:1139–51. doi: 10.1016/s0039-6109(02)00063-4. [DOI] [PubMed] [Google Scholar]
- 6.Sainio P. Fistula-in-ano in a defined population. Incidence and epidemiological aspects. Ann Chir Gynaecol. 1984;73:219–224. [PubMed] [Google Scholar]
- 7.Hall JF, Bordeianou L, Hyman N. Outcomes after operations for anal fistula: results of a prospective, multicenter, regional study. Dis Colon Rectum. 2014;57:1304–1308. doi: 10.1097/DCR.0000000000000216. [DOI] [PubMed] [Google Scholar]
- 8.Abramowitz L, Soudan D, Souffran M Groupe de Recher-che en Proctologie de la Société Nationale Française de Colo-Proctologie and the Club de Réflexion des Cabinets et Groupe d’Hépato-Gastroentérologie. The outcome of fistulotomy for anal fistula at 1 year: a prospective multicentre French study. Colorectal Dis. 2016;18:279–285. doi: 10.1111/codi.13121. [DOI] [PubMed] [Google Scholar]
- 9.Garcia-Aguilar J, Belmonte C, Wong WD, Goldberg SM, Madoff RD. Anal fistula surgery. Factors associated with recurrence and incontinence. Dis Colon Rectum. 1996;39:723–729. doi: 10.1007/BF02054434. [DOI] [PubMed] [Google Scholar]
- 10.Rojanasakul, et al. Total anal sphincter saving technique for fistula-in-ano. J Med ASS. 2007:90581–6. [PubMed] [Google Scholar]
- 11.Muldoon JP. The completely closed hemorrhoidectomy: a reliable and trusted friend for 25 years. Dis Colon and Rectum. 1981;24(3):211–14. doi: 10.1007/BF02962338. [DOI] [PubMed] [Google Scholar]
- 12.Cataldo P, Ellis CN, Gregorcyk S, Hyman N, Buie WD, Church J, Chen J, Fleshner P. Practice parameters for the management of hemorrhoids (revised) Dis Colon Rectum. 2005;48:189–194. doi: 10.1007/s10350-004-0921-4. [DOI] [PubMed] [Google Scholar]
- 13.Wexner SD. The quest for painless surgical treatment of hemorrhoids continues. J Am Coll Surg. 2001;193:174–178. doi: 10.1016/s1072-7515(01)00997-8. [DOI] [PubMed] [Google Scholar]
- 14.Parks AG. The surgical treatment of hemorrhoids. Br J Surg. 1956;43:337–351. doi: 10.1002/bjs.18004318002. [DOI] [PubMed] [Google Scholar]
- 15.Todd IP, Fielding LP, editors. Colon, Rectum and Anus. Fourth ed. Vol. 3. London: Butterworths; 1983. Rob & Smith’s Operative Surgery Alimentary Tract and Abdominal Wall; pp. 480–488. [Google Scholar]
- 16.Sharif HI, Lee L, Alexander-Williams J. Diathermy hemorrhoidectomy. Int J Colorectal Dis. 1991;6:217–219. doi: 10.1007/BF00341395. [DOI] [PubMed] [Google Scholar]
- 17.Gough MJ, Lewis A. The conservative treatment of fissure-in- ano. Br J Surg. 1983;70:175–176. doi: 10.1002/bjs.1800700312. [DOI] [PubMed] [Google Scholar]
- 18.Jensen SL. Treatment of first episodes of acute anal fissure: prospective randomised study of lignocaine ointment versus hydrocortisone ointment or warm sitz baths plus bran. BMJ. 1986;292:1167–1169. doi: 10.1136/bmj.292.6529.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shub HA, Salvati EP, Rubin RJ. Conservative treatment of anal fissure: an unselected, retrospective and continuous study. Dis Colon Rectum. 1978;21:582–583. doi: 10.1007/BF02586401. [DOI] [PubMed] [Google Scholar]
- 20.Jones OM, Ramalingam T, Merrie A. Randomized clinical trial of botulinum toxin plus glyceryl trinitrate vs. botulinum toxin alone for medically resistant chronic anal fissure: overall poor healing rates. Dis Colon Rectum. 2006;49:1574–1580. doi: 10.1007/s10350-006-0679-y. [DOI] [PubMed] [Google Scholar]
- 21.Welton ML, Sharkey FE, Kahlenberg MS. The etiology and epidemiology of anal cancer. Surg Oncol Clin N Am. 2004;13:263–75. doi: 10.1016/j.soc.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 22.Zbar AP, Fenger C, Efron J, Beer-Gabel M, Wexner SD. The pathology and molecular biology of anal intraepithelial neoplasia: comparisons with cervical and vulvar intraepithelial carcinoma. Int J Colorectal Dis. 2002;17:203–15. doi: 10.1007/s00384-001-0369-0. [DOI] [PubMed] [Google Scholar]
- 23.DeToma G, Cavallaro G, Bitonti A, Polistena A, Onesti MG, Scuderi N. Surgical management of perianal giant condyloma accuminatum. Eur Surg Res. 2006;38:418–22. doi: 10.1159/000094979. [DOI] [PubMed] [Google Scholar]
- 24.Wieland U, Brockmeyer NJ, Weissenborn SJ, Hochdorfer B, Stucker M, Swoboda J, Altmeyer P, Pfister H, Kreuter A. Imiquimod treatment of anal intraepithelial neoplasia in HIV-positive men. Arch Dermatol. 2006;142:1438–44. doi: 10.1001/archderm.142.11.1438. [DOI] [PubMed] [Google Scholar]
- 25.Shepherd N. Anal intraepithelial neoplasia and other neoplastic precursor lesions of the anal canal and perianal region. Gastroenterology Clinic North Am. 2007;36:969–87. doi: 10.1016/j.gtc.2007.08.001. [DOI] [PubMed] [Google Scholar]