Abstract
Background
Surgical abortion by vacuum aspiration or dilatation and curettage has been the method of choice for early pregnancy termination since the 1960s. Medical abortion became an alternative method of first trimester pregnancy termination with the availability of prostaglandins in the early 1970s and anti‐progesterones in the 1980s. The most widely researched drugs are prostaglandins (PGs) alone, mifepristone alone, methotrexate alone, mifepristone with prostaglandins and methotrexate with prostaglandins.
Objectives
To compare different medical methods for first trimester abortion.
Search methods
The Cochrane Controlled Trials Register, MEDLINE and Popline were systematically searched. Reference lists of retrieved papers were also searched. Experts in WHO/HRP were contacted.
Selection criteria
Types of studies Randomised controlled trials comparing different medical methods for abortion during first trimester (e.g. single drug, combination) were considered. Trials were assessed and included if they had adequate concealment of allocation, randomisation procedure and follow‐up. Women, pregnant during the first trimester, undergoing medical abortion were the participants. The outcomes were mortality, failure to achieve complete abortion, surgical evacuation, ongoing pregnancy at follow‐up, time until passing of conceptus, blood transfusion, side effects and women's dissatisfaction with the procedure.
Data collection and analysis
Two reviewers independently selected trials for inclusion from the results of the search strategy described previously.The selection of trials for inclusion in the review was performed independently by two reviewers after employing the search strategy described previously. Trials under consideration were evaluated for appropriateness for inclusion and methodological quality without consideration of their results. Data were processed using Revman software.
Main results
Fifty‐eight trials were included in the review. The effectiveness outcomes below refer to 'failure to achieve complete abortion' with the intended method unless otherwise stated. 1) Combined regimen mifepristone/prostaglandin: Mifepristone 600 mg compared to 200 mg shows similar effectiveness in achieving complete abortion (4 trials, RR 1.07, 95% CI 0.87 to 1.32). Misoprostol administered orally is less effective (more failures) than the vaginal route (RR 3.00, 95% CI 1.44 to 6.24) and may be associated with more frequent side effects such as nausea and diarrhoea. Sublingual and buccal routes were similarly effective compared to the vaginal route, but had higher rates of side effects. 2) Mifepristone alone is less effective when compared to the combined regimen mifepristone/prostaglandin (RR 3.76 95% CI 2.30 to 6.15). 3) Five trials compared prostaglandin alone to the combined regimen (mifepristone/prostaglandin). All but one reported higher effectiveness with the combined regimen. The results of these studies could not be combined but the RR of failure with prostaglandin alone is reportedly between 1.4 to 3.75 with the 95% confidence intervals indicating statistical significance. 4) In one trial comparing gemeprost 0.5 mg with misoprostol 800 mcg, misoprostol was more effective (failure with gemeprost: RR 2.86, 95% CI 1.14 to 7.18). 5) There was no difference in effectiveness with use of a divided dose compared to a single dose of prostaglandin. 6) Combined regimen methotrexate/prostaglandin demonstrates similar rates of failure to complete abortion when comparing intramuscular to oral methotrexate administration (RR 2.04, 95% CI 0.51 to 8.07). Similarly, day 3 vs. day 5 administration of prostaglandin following methotrexate administration showed no significant differences (RR 0.72, 95% CI 0.36 to 1.43). One trial compared the effect of tamoxifen vs. methotrexate and no statistically significant differences were observed in effectiveness between the groups.
Authors' conclusions
Safe and effective medical abortion methods are available. Combined regimens are more effective than single agents. In the combined regimen, the dose of mifepristone can be lowered to 200 mg without significantly decreasing the method effectiveness. Vaginal misoprostol is more effective than oral administration, and has less side effects than sublingual or buccal. Some results are limited by the small numbers of participants on which they are based. Almost all trials were conducted in settings with good access to emergency services, which may limit the generalizability of these results.
Plain language summary
Medical methods for early termination of pregnancy can be safe and effective
There are several different surgical techniques for abortion during the first three months. Several drugs can also be prescribed alone or in combination to terminate early pregnancy. This is called medical abortion, and uses the hormones prostaglandins and/or mifepristone (an antiprogesterone often called RU486), and/or methotrexate. This review of trials found that medical methods for abortion in early pregnancy can be safe and effective, with the most evidence of effectiveness for a combination of mifepristone and misoprostol (a prostaglandin). Almost all of the trials were done in well‐resourced settings where women returned for a check‐up.
Background
Up to 42 million abortions are performed each year (Sedgh 2007). Medical abortion has the potential to expand abortion services, where surgical services are limited, and to expand women's choice of abortion method and experience.
Surgical abortion by vacuum aspiration or dilatation and curettage has been the method of choice for early pregnancy termination since the 1960s. Medical abortion became an alternative method of first trimester pregnancy termination with the availability of prostaglandins in the early 1970s followed by the development of an antiprogesterone in the 1980s. Large uncontrolled studies suggested that early medical abortion with mifepristone and a prostaglandin would be an effective method for pregnancy termination (Urquhart 1997).
Various drugs have been used for first trimester medical abortion. The most widely researched are prostaglandins (PGs) alone, mifepristone alone, methotrexate alone, mifepristone with prostaglandins and methotrexate with prostaglandins. Prostaglandins soften the cervix, cause uterine contractions and are used orally or vaginally for ripening of the cervix before surgical or for medical abortion. The most commonly used prostaglandins are gemeprost given vaginally and misoprostol administered either orally (including buccal and sublingual) or vaginally. Misoprostol is a prostaglandin analogue registered for use in nonsteroidal anti‐inflammatory drug (NSAID) induced gastric ulcer prevention and treatment. It has a strong uterotonic effect and is used to induce pregnancy terminations illegally in some parts of the world (Blanchard 1999, Costa 1998) as well as legally, in areas where mifepristone is not available. The reported complete abortion rate for misoprostol alone varies between 61% for single and 93% for repeat doses (Bugalho 1996, Carbonell 1997b). Gemeprost used alone appears to be less effective in inducing complete abortion than when used in combination with mifepristone (Norman 1992).
Mifepristone, an antiprogestogen, blocks the receptors for progesterone and glucocorticosteroid and increases the sensitivity of the uterus to prostaglandins (Bygdeman 1985). This blockage results in the breakdown of maternal capillaries in the decidua, the synthesis of prostaglandins by the epithelium of decidual glands and inhibition of prostaglandin dehydrogenase (WHO 1997).
Mifepristone has been licensed in France and China since 1988, in Great Britain in 1991 and, in the USA and India in 2000 and 2002, respectively. Mifepristone given alone has been shown to result in abortion only in 60‐80% of cases, depending on the gestational age and the dose given (WHO 1997). However, in combination with a prostaglandin at up to 63 days of amenorrhoea, it leads to complete abortion in about 95% of pregnancies (United 1990) or more. The effect of mifepristone develops over a time period of 24‐48 hours; therefore, prostaglandins have usually been administered after 36‐48 hours. Currently, different regimens are in use. The recommended regimen by the manufacturer is mifepristone 600 mg followed by misoprostol (between 400 ‐ 800 mcg) or gemeprost (0.5 ‐ 1 mg vaginally) and is registered for abortion in pregnancies up to 49 days in France and up to 63 days of amenorrhoea in Great Britain. However, a reduced dose of mifepristone combined with a prostaglandin has similar effectiveness and has the advantage of being much less expensive (WHO 1997).
Methotrexate has been used successfully for the treatment of unruptured tubal pregnancy. It is a folic acid antagonist which inhibits purine and pyrimidine synthesis and is cytotoxic to the trophoblast. The use of methotrexate with misoprostol for first trimester abortion was first introduced in 1993 (Creinin 1993, Grimes 1997). This combination was more effective when misoprostol was administered 7 days after methotrexate as compared to 3 days, leading to a complete abortion rate of 98% (Creinin 1995 M800pv).
Side‐effects of medical methods are heavy bleeding, pain, nausea, vomiting and diarrhoea, varying in severity according to the protocols and gestational age (Henshaw 1994). In two randomised controlled trials included in the Cochrane review of the subject, compared to surgical procedures, medical methods are associated with a longer duration of bleeding (Say 2002, updated 2010).
Failed abortion is an infrequent but important complication of medical abortion. Both methotrexate and misoprostol may lead to fetal anomalies if the pregnancy persists, as described by some (Grimes 1997). However, other reports state that none of the malformations reported could be conclusively related to medications used for medical abortion (Wiebe 2006).
Some women prefer medical to surgical abortion. 'More natural', 'being easier', more private', and 'can be done earlier in pregnancy' were reasons to opt for a medical method by some women (Creinin 1996b). Characteristics such as the method being more new, less invasive and the possibility of verifying the expulsion were reported by others (Bachelot 1992).
Medical methods for first trimester abortion are already widely available in some countries and increasingly available throughout the world. It is therefore important to identify the best available agents and regimen for use. Comparison of medical methods with surgical evacuation in the first trimester is the subject of another review: Say 2002, updated 2010.
Objectives
To compare different medical methods for first trimester abortion.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials comparing different medical methods (e.g. single drug, combination), ways of application, or different dose regimens, single or combined, for medical abortion, were considered. Trials were not excluded based on an arbitrary cut‐off limit regarding losses to follow‐up. Trials were excluded if there were unexplained imbalances in different groups at follow‐up and from available outcome data.Trials were assessed and included if they had adequate concealment of allocation, randomisation and follow‐up.
Types of participants
Women, pregnant in the first trimester, undergoing medical abortion.
Types of interventions
Different medical methods used for first trimester abortion, compared with each other or placebo. See 'Search methods for identification of studies' for a list of pharmaceutical preparations.
Types of outcome measures
The main outcome measure was failure to achieve complete abortion. Surgical evacuation (as emergency procedure, non‐emergency procedure, or undefined), ongoing pregnancy at follow‐up, time until passing of conceptus (> 3‐6 hours), blood transfusion, blood loss (measured or clinically relevant drop in haemoglobin), days of bleeding, pain resulting from the procedure (reported by the women or measured by use of analgesics), additional uterotonics used, women's dissatisfaction with the procedure, nausea, vomiting, and diarrhoea were also assessed. Although mortality is considered an important outcome we did not anticipate analyzing abortion‐related mortality within the context of these trials.
Search methods for identification of studies
The Cochrane Controlled Trials Register, MEDLINE and Popline were systematically searched. Reference lists of retrieved papers were also searched. Electronic literature search of MEDLINE (with the Cochrane 3‐stage search strategy)(1966‐2003) and POPLINE (1970‐2003) databases with the following key words: (abortion OR pregnancy termination OR termination of pregnancy) AND (first trimester OR early) AND (mifepristone OR misoprostol OR methotrexate OR dinoprost* OR carboprost OR sulprostone OR gemeprost OR meteneprost OR lilopristone OR onapristone OR epostane OR oxytocin OR RU 486 OR mifegyne). There were no language preferences in the application of the search.
Data collection and analysis
The selection of trials for inclusion in the review was performed independently by two reviewers after employing the search strategy described previously. Trials under consideration were evaluated for appropriateness for inclusion and methodological quality without consideration of their results. A quality score for concealment of allocation has been assigned to each trial, using the criteria described in the Cochrane Handbook:
(A) adequate concealment of the allocation (B) unclear whether adequate concealment of the allocation (C) inadequate concealment of allocation (includes quasi‐randomised studies) (D) allocation concealment not used Only trials scoring A or B were included in the review.
Failure to achieve complete abortion is defined as an abortion which is not completed by the described intended method. Other outcomes are failure of expulsion after 4 ‐ 6 hours, side effects (nausea, vomiting, diarrhoea, abdominal pain), and mean duration of days of bleeding. A further division into early (≤ 49 days of amenorrhoea) and late (> 49 days) gestational age at the time of abortion was made for subgroup analysis. Complications are defined as any serious complication described by the authors and which was not a failure or side effect.
A form was designed to facilitate the process of data extraction which has been performed by two of the reviewers independently. In case of discrepancies between reviewers in either the decision of inclusion/exclusion of studies or in data extraction, this was resolved by consensus. Attempts were made to obtain additional information from authors if required. Whether or not an "intention‐to‐treat" analysis was done in the primary study was examined.
Data were processed using RevMan software. For reasons of clarification some coding was added to some trials included in the meta‐analysis: GP ‐gemeprost, the number next to it ‐ refers to the dose of gemeprost in grams, M ‐ misoprostol, the number next to it ‐ refers to the dose in mcg, MP ‐ minprostin, the number next to it refers to the dose in mg, PGF2 ‐ Prostaglandin F2alpha; PGE1‐ prostaglandin E1 analogue; MI ‐ mifepristone ‐ the number next to it refers to the dose in mg; MT ‐ methotrexate, T ‐ testosterone propionate, TM ‐ tamoxifen; po ‐ oral and pv ‐ vaginal administration.
Results are presented as relative risk and 95% confidence interval (RR; 95%CI) using the fixed effects model. If a large I2 was found in the pooled analysis, a random effects model was applied and the tau 2 value was evaluated for possible heterogeneity and reported if present.
Subgroup analyses were performed where possible for early and late first trimester abortions as the performance of some methods may differ with gestational age: 1) abortion up to 49 days, 2) abortion > 49 days of amenorrhoea. The studies in this field use various combinations of agents, doses, intervals between the antiprogesterone and prostaglandin, and route of administration for prostaglandin. Since all of these variables may affect the outcomes, it was not considered appropriate to combine similar trials into meta‐analysis in many cases. However, it was possible to identify an experimental intervention and a constant (fixed ) intervention which enabled us to group the trials as follows:
Combined regimen mifepristone/prostaglandin:
Intervention: dose of mifepristone (comparison 1)
Intervention: dose of prostaglandin (comparison 2)
Intervention: type of prostaglandin (comparison 3)
Intervention: timing of prostaglandin (comparison 4)
Intervention: misoprostol oral versus vaginal (comparison 5)
Intervention: misoprostol buccal versus vaginal (comparison 6)
Intervention: misoprostol buccal versus oral (comparison 7)
Intervention: misoprostol sublingual versus vaginal (comparison 8)
Intervention: misoprostol sublingual versus oral (comparison 9)
Intervention:single versus split dose prostaglandin (comparison 10)
Intervention: single versus repeated prostaglandin (comparison 11)
Mifepristone alone versus combined regimen mifepristone/prostaglandin (comparison 12)
Prostaglandin alone versus a combined regimen (all) (comparison 13)
Single regimen:
Prostaglandin alone: route of administration (comparison 14)
Mifepristone single regimen ‐ high versus low dose (comparison 15)
Combined regimen methotrexate/prostaglandin:
Intervention: timing of prostaglandin (comparison 16)
Intervention: route of methotrexate: intramuscular versus oral (comparison 17)
Intervention: dose of methotrexate (comparison 18)
Intervention: route of prostaglandin (comparison 19)
Tamoxifen versus methotrexate (combined with prostaglandin):
Intervention: low dose tamoxifen (40 mg)(comparison 20)
Intervention: high dose tamoxifen (160 mg) (comparison 21)
Combined regimen mifepristone/prostaglandin versus mifepristone/prostaglandin plus tamoxifen (comparison 22)
Results
Description of studies
see table: Characteristics of included studies
Risk of bias in included studies
Thirty‐five trials scored adequate allocation concealment (A) and in 23 trials allocation concealment was unclear (B).Two trials used an open‐label design (Schaff 2000 MI200M800, Schaff 2001 M800MI200). Two of the trials mentioned performing an 'intention ‐to ‐treat analysis' (WHO 2000 M400po, WHO 2001 GP1pv).
Effects of interventions
Fifty‐eight trials are included in this review. Due to the many different interventions, trials were grouped into comparisons, as listed below. The main outcome for which the meta‐analyses were performed was failure to achieve complete abortion with the method intended. Data on side‐effects could be combined for some comparisons. Major complications with any of the methods were rarely reported and if so, they are listed in the tables of Characteristics of included studies. Data are presented for different gestational ages where possible (≤ 49 days, > 49 days). One trial presented its data in two different publications (Honkanen 2004, von Hertzen 2003). One trial used 2 different comparisons, and is therefore listed as 2 different trials (Wiebe 1999 and Wiebe 1999 A). Six studies used different regimens/doses/timing of the drugs that could not be combined with any of the other regimens in the compraisons and are therefore listed separately inTable 23 (Wang 2000, Arvidsson 2005, Wiebe 2006, Liao 2004, WHO 1989, WHO 1991).
1. other studies included in the review.
| Study | Intervention | Outcomes | |
| Wang 2000 | Group 1: Day 1: mifepristone 50mg/po 12hourly /2 doses),day 2‐7: mifepristone 25mg po/day Day 3: misoprostol 600mcg/po, day 4‐6: misoporstol 200mcg/day Group 2: Day 1: mifepristone 50mg, followed by 25mg/12hourly/4 times Day 3: misoprostol 600mcg/po |
failure to achieve complete abortion: group 1: 18/1118 group 2: 59/494 ongoing pregnancy: group1:2/1118 group2: 6/494 |
|
| Arvidsson 2005 | Day 1: both groups receive mifepristone 600mg Day 3: group 1: misprostol 400mcg/po group 2: 800mcg/pv |
nausea: group1: 23/48 group2: 17/49 vomiting: group1: 11/48 group2: 5/49 diarrhoea: group1: 3/48 group2: 1/49 women dissatisfied with procedure: group1: 2/48 group2: 1/49 |
|
| Wiebe 2006 | Group 1: methotrexate 50 mg/ m2 followed >/ 72 hours by 400mcg misoprostol vaginal Group 2: misoprostol 400mcg sublingual AND 400mcg misoprostol vaginal |
failure to achieve complete abortion: group1: 62/149 group2: 57/149 nausea: group1: 53/49 group2: 54/149 vomiting: group1: 17/149 group2: 21/149 diarrhoea: group1: 16/149 group2: 41/149 surgical abortion: group1: 9/149 group2: 18/149 |
|
| Liao 2004 | Group 1: mifepristone given: 50 mg, then 12 hrs later 25 mg, then 12 hrs later 50 mg, and finally, 12 hrs later, 25 mg mifepristone. 24 hrs after, 600 mcg misoprostol oral (total: 150mg) Group 2: mifepristone given 30 mg, then 15 mg every 12 hours for 3 doses. 24 hrs after last dose, 600 mcg misoprostol given oral. (total: 75 mg) |
failure to achieve complete abortion: group1: 11/240 group2: 9/240 ongoing pregnancy: group1: 2/240 group2: 1/240 |
|
| WHO 1989 | Group 1: mifepristone 25mg/twice daily for 3 days (total 150 mg) and sulprostone0.25 mg /intramuscular/ on third day a.m. Group 2: mifepristone 25mg /twice daily for 4 days (total 200mg) and sulprostone0.25 mg /intramuscular/ on fourth day a.m. | failure to achieve complete abortion:
group1: 15/125
group2: 13/126 ongoing pregnancy: group1: 3/125 group2: 3/126 |
|
| WHO 1991 | Group 1: mifepristone 25mg/12 hourly/ 5 doses (total 125mg) and gemeprost 1mg/vaginally 60 hours after the start of the treatment Group 2: mifepristone 600mg/single dose and gemeprost 1mg/vaginally 60 hours after the start of the treatment | failure to achieve complete abortion: group1: 12/181 group2: 15/187 |
Our main outcome was failure to achieve complete abortion with the method intended. Fourteen trials used either other definitions (i.e. surgical intervention) or administered additional prostaglandins (Carbonell 1997 M800pv, Creinin 1994 M800&MT, Creinin 1995 M800pv, Creinin 1996 M800pv, Creinin 1997 M800pv, Creinin 2001 MI600 M400, Jain 1999 M800&TM, Ozeren 1999 MP800&MT, Koopersmith 1996, Schaff 2000 MI200M800, Wiebe 1999 A, Wiebe 1999 B, Hamoda 2005). We conducted sensitivity analysis when appropriate to present the results accordingly.
Combined regimen mifepristone/prostaglandin Intervention: dose of mifepristone: (comparison 1;Figure 1)
1.
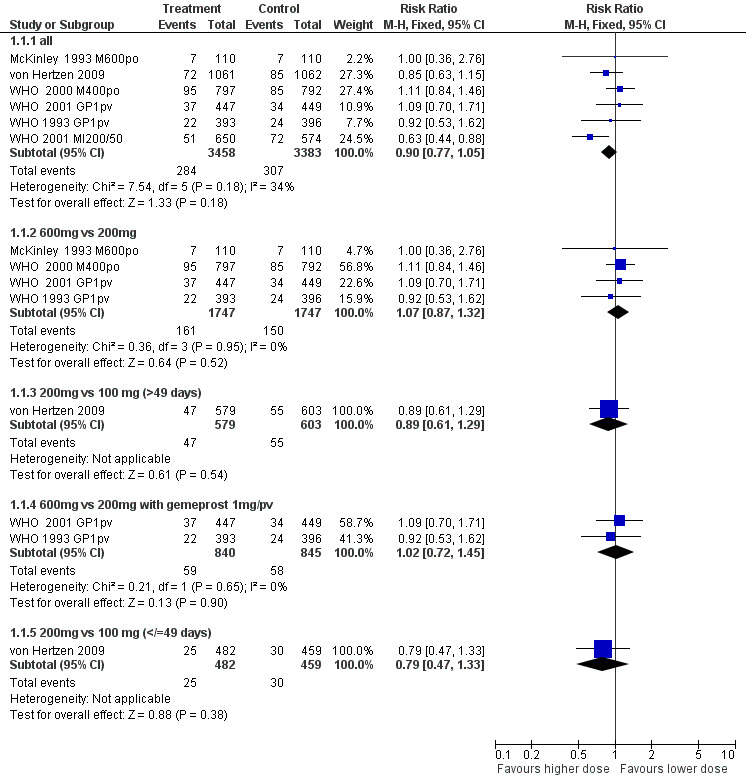
Forest plot of comparison: 1 combined regimen mifepristone/prostaglandin: dose of mifepristone, outcome: 1.1 failure to achieve complete abortion.
There are nine trials included, six are included in the meta‐analysis. The comparisons are 600mg versus 200mg, 200mg versus 100mg, and 200mg versus 50mg of mifepristone (McKinley 1993 M600po, WHO 1993 GP1pvWHO 2000 M400po, WHO 2001 MI200/50, WHO 2001 GP1pv, von Hertzen 2009). Three trials used split doses of mifepristone and are presented in the additional tables (WHO 1989, WHO 1991, Liao 2004).
All trials: Failure to achieve complete abortion was similar between higher versus the lower dose mifepristone groups (0.90 95%CI 0.77 to 1.05). Analysis 1.1
1.1. Analysis.
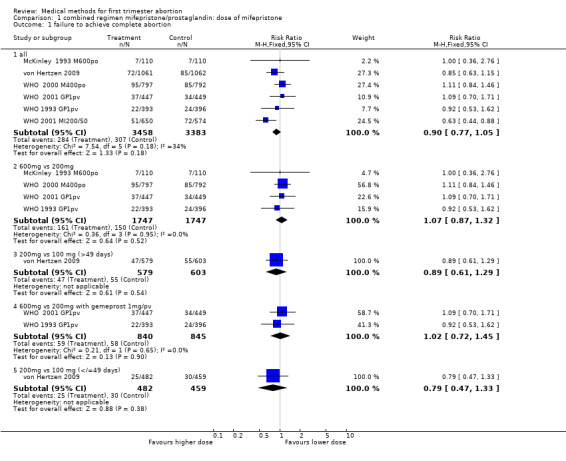
Comparison 1 combined regimen mifepristone/prostaglandin: dose of mifepristone, Outcome 1 failure to achieve complete abortion.
600mg versus 200 mg: There are 6 ( McKinley 1993 M600po; WHO 1989, WHO 1991, WHO 1993 GP1pv, WHO 2000 M400po, WHO 2001 GP1pv) trials included in the review, of which data from 4 trials with overall 3482 women were included in the meta‐analysis (McKinley 1993 M600po; WHO 1993 GP1pv, WHO 2000 M400po, WHO 2001 GP1pv). McKinley used misoprostol 600mcg/po, WHO trials (WHO 1993 GP1pv and WHO 2001 GP1pv) used gemeprost 1mg/pv or misoprostol 400mcg/po (WHO 2000 M400po). There was no difference in failure to achieve complete abortion between 200 mg and 600 mg of mifepristone (RR 1.07 95% CI 0.87 ‐ 1.32). The pooled analysis of the two trials using the same dose and type of prostaglandin (gemeprost 1mg) showed no difference for failure rates (RR 1.02 95%CI 0.72 to 1.45). Time until passing of conceptus >3‐6 hours was similar for the two groups in the three trials reporting on it. The four trials reporting on ongoing pregnancy at follow ‐up
(Liao 2004, McKinley 1993 M600po, von Hertzen 2009, WHO 1993 GP1pv) showed no statistically significant difference between the two groups. These trials used different types and doses of misoprostol and the results are therefore presented for each trial individually. Side effects were similar between the two groups.
200mg versus 100 mg: One trial was included in this comparison (von Hertzen 2009.) This was a four‐arm trial, comparing 100 vs 200 mg of mifepristone followed by 800mcg misoprostol/pv after 24 or 48 hours. Failure rates were similar between the groups.
200mg versus 50 mg: WHO (WHO 2001 MI200/50) used 200 or 50 mg followed by 0.5 or 1 mg of gemeprost/pv. The group receiving mifepristone 50mg and gemeprost 0.5 mg was discontinued after 249 participants were enrolled because the complete abortion rate was below the pre‐determined cut off. Women receiving 200 mg of mifepristone were less likely to have failure in achieving complete abortion (RR 0.63 95%CI 0.44 to 0.8) and had fewer ongoing pregnancies at follow‐up (RR 0.20 95%CI 0.07 to 0.58). Combined regimen mifepristone/prostaglandin Intervention: dose of prostaglandin (comparison 2,Analysis 2.1) Six trials are included in the review, the data from four of them could be included in the meta‐analysis. Two of these trials (Rodger 1989 MI600, WHO 2001 MI200/50) compared gemeprost 1 mg versus gemeprost 0.5 mg in 1284 women. There were fewer failures with the 1 mg dose but the difference did not reach statistical significance (RR 0.75, 95% CI 0.54 to 1.05). The largest trial in this comparison (WHO 2001 MI200/50) used a factorial design (mifepristone 50/200 mg and gemeprost 1/0.5 mg). Looking at the group with mifepristone 200 mg only, the difference between the two doses of gemeprost is less significant (RR 0.81, 95% CI 0.45 to 1.43). The arm with the smallest dose (mifepristone 50 mg and gemeprost 0.5 mg) was stopped prematurely after 249 women were enrolled, as the effectiveness was below the predetermined cut‐off point. Rodger (Rodger 1989 MI600) included 120 women in the study. The first 60 women were not randomised; therefore only data for the second 60 women are included in this review.
2.1. Analysis.
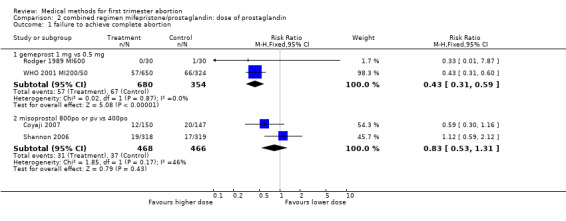
Comparison 2 combined regimen mifepristone/prostaglandin: dose of prostaglandin, Outcome 1 failure to achieve complete abortion.
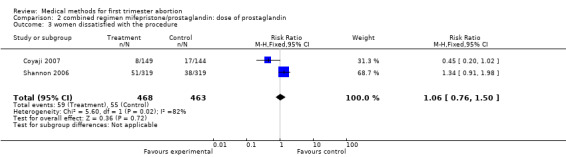
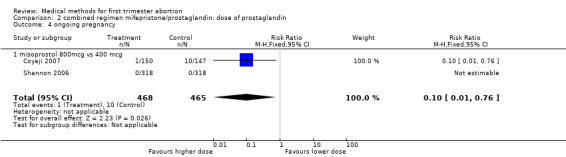
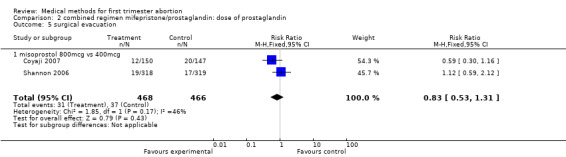
Two trials compared different doses of oral misoprostol after 200 mg of mifepristone. Coyaji 2007 compared misoprostol 400mcg to 800mcg (given orally; 800mcg was administered as a repeat dose of 400mcg after 3 hours). Shannon 2006 used 3 groups, comparing misoprostol 400mcg, 600mcg and 800mcg. Data from the 400mcg and 800mcg groups were included in the review. The failure rates and side effects were similar between the groups. There were fewer ongoing pregnancies in the 800mcg compared to the 400mcg group (0.10 95%CI 0.01 to 0.76). Side effects were similar between the groups.
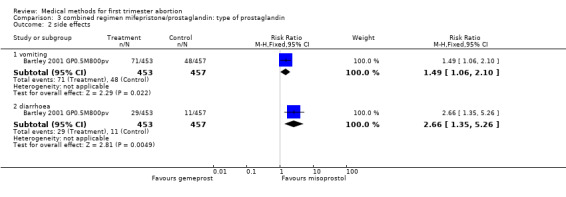
Combined regimen mifepristone/prostaglandin Intervention: type of prostaglandin (comparison 3,Analysis 3.1) 1)gemeprost versus misoprostol Two trials are included (Baird 1995 GP0.5 M600po, Bartley 2001 GP0.5M800pv) using different doses of misoprostol and different routes of administration. Therefore the results were not combined in a meta‐analysis. However, when misoprostol is used at a higher dose (800 mcg) and administered vaginally, it appears to be more effective than gemeprost 0.5 mg (RR 2.86 95%CI 1.14 to 7.18), according to data from a single trial (Bartley 2001 GP0.5M800pv). Vomiting and diarrhoea were more common with misoprostol compared to gemeprost (RR 1.49 95%CI 1.06 to 2.10; RR 2.66 95%CI 1.35 to 5.26). There was no difference for other outcomes, such as ongoing pregnancy and time until passing of conceptus > 3‐6 hours between the groups.
3.1. Analysis.
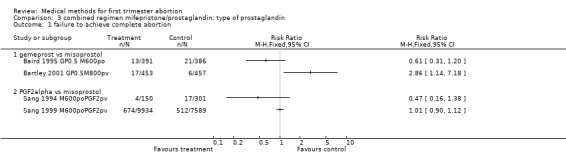
Comparison 3 combined regimen mifepristone/prostaglandin: type of prostaglandin, Outcome 1 failure to achieve complete abortion.
2)PGF2 alpha versus misoprostol There was no difference in efficacy when comparing PGF2 alpha to misoprostol 600 mcg orally (Sang 1994 M600poPGF2pv, Sang 1999 M600poPGF2pv).
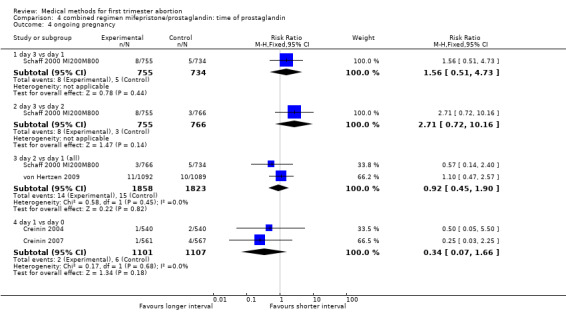
Combined regimen mifepristone/prostaglandin Intervention: timing of prostaglandin (comparison 4,Analysis 4.1) There are six trials included for this comparison. Three trials used different dose regimens as well as time intervals; therefore, the results are presented for each trial separately. Misoprostol administered on day 3 following mifepristone seems to be less effective in achieving complete abortion when compared to day 1 in the one trial reporting on it (Schaff 2000 MI200M800). The follow‐up for all women was on day 8 after mifepristone. There were 53 women in the sample who received additional misoprostol if the gestational sac was present at the first follow‐up visit. It is not clear how these women were distributed by treatment group. There was no difference between the groups with regard to need for surgical evacuation, ongoing pregnancy or women's dissatisfaction with the method. No difference regarding failure rate was shown in one trial when comparing day 3 versus day 2 (Schaff 2000 MI200M800). Two trials compared misoprostol on 2 versus day 0 (Creinin 2001 MI600 M400; Guest 2007). Creinin used mifepristone 600mg followed by misoprostol 400mcg; Guest used mifepristone 200mg followed by misoprostol 800mcg. Failure to achieve complete abortion was lower when misoprostol was administered 36 ‐48 hours compared to 6 hours after mifepristone (RR 0.39 95%CI 0.24 to 0.65). There was no difference in the occurrence of side effects (nausea, vomiting, diarrhoea) between the 2 groups. Two trials (Creinin 2004, Creinin 2007) used the same dose and route. Mifepristone 200mg followed by misoprostol 800mcg pv administered on day 1 was more effective than administration ≤ 6h later (RR 0.65 95%CI 0.46 to 0.92). In the comparison of misoprostol day 2 versus day 1, failure to achieve complete abortion rates were similar when combining results for gestational ages up to 63 days. However, failure rates were higher with misoprostol administered on day 2 compared to day 1 in women > 49 days of gestation based on one trial (von Hertzen 2009) (RR 1.62 95%CI 1.11 to 2.38), not in three studies, when all days of gestation were considered (Sandstrom 1999 MI600GP1pv, Schaff 2000 MI200M800, von Hertzen 2009).
4.1. Analysis.
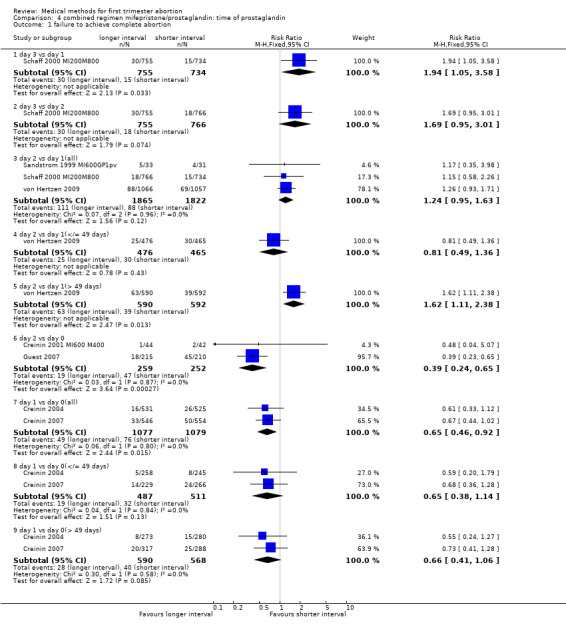
Comparison 4 combined regimen mifepristone/prostaglandin: time of prostaglandin, Outcome 1 failure to achieve complete abortion.
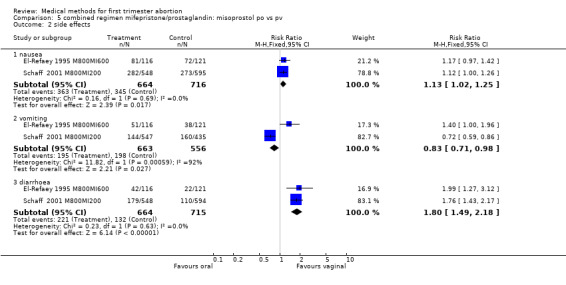
Combined regimen mifepristone/prostaglandin: route of administration for misoprostol Intervention : misoprostol oral versus vaginal (comparison 5,Analysis 5.1) Six trials are included in the review, 2 trials with a total of 1407 women are included in the meta‐analysis (El‐Refaey 1995 M800MI600; Schaff 2000 MI200M800). El‐Refaey used mifepristone 600mg and Schaff used mifepristone 200mg. Both used misoprostol 800mcg orally or vaginally after 48 hours (El‐Refaey) and at least 24 hours (Schaff) after mifepristone. A statistically significant higher number of women had failure to achieve complete abortion when misoprostol was administered orally (RR 3.05 95% CI 1.98 to 4.70). Nausea and diarrhoea occurred more often in the group receiving misoprostol orally (RR 1.13 95% CI 1.02 to1.25; RR 1.80 95% CI 1.49 to 12.18, respectively). Unexpectedly, vomiting occurred more often in the vaginal group in one trial (Schaff 2001 M800MI200), and reporting error cannot be excluded. Three trials used different doses orally and vaginally and were therefore not included in the meta‐analysis (Creinin 2001 and Shannon 2006, Arvidsson 2005). In one trial (Shannon 2006), failure to achieve complete abortion was similar among those who recieved a lower dose (400 mcg) of oral misoprostol than those who received 800 mcg of vaginal misoprostol; however, women were instructed to repeat their misoprostol dose at home one day following the first misoprostol dose in case of scant bleeding, and 28% did so. In 2005, Arvidsson (Arvidsson 2005) reported only on side effects and women's satisfaction (data included in additional tables) following use of either oral or vaginal misoprostol. Tang (Tang 2002) used a combined regimen oral/vaginal in one group and repeated oral misoprostol doses in another group, and these data were therefore not included in the meta‐analysis.
5.1. Analysis.
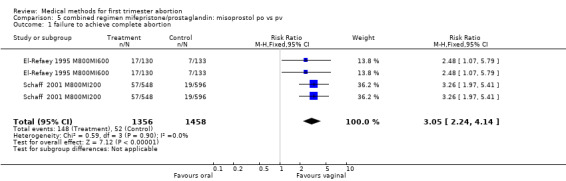
Comparison 5 combined regimen mifepristone/prostaglandin: misoprostol po vs pv, Outcome 1 failure to achieve complete abortion.
Intervention : misoprostol buccal versus vaginal (comparison 6,Analysis 6.1)
6.1. Analysis.
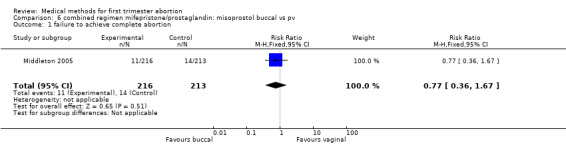
Comparison 6 combined regimen mifepristone/prostaglandin: misoprostol buccal vs pv, Outcome 1 failure to achieve complete abortion.
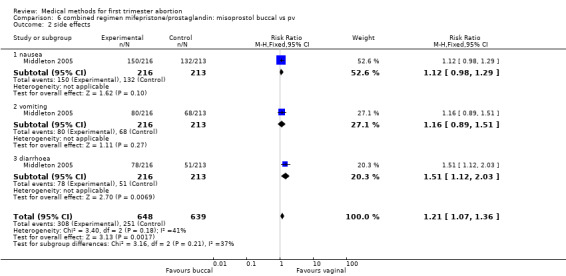
One trial (Middleton 2005) was included for this comparison. Failure to achieve complete abortion was similar in both groups. There were statistically significantly more women with diarrhoea in the buccal compared to the vaginal group (RR 1.51 95%CI 1.12 to 2.03).
Intervention : misoprostol buccal versus oral (comparison 7,Analysis 7.1)
7.1. Analysis.
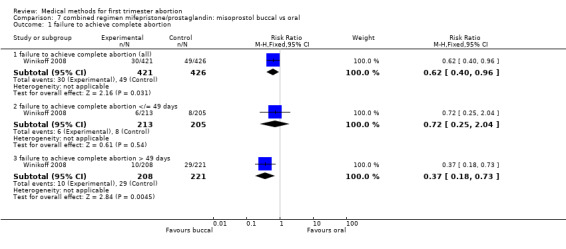
Comparison 7 combined regimen mifepristone/prostaglandin: misoprostol buccal vs oral, Outcome 1 failure to achieve complete abortion.
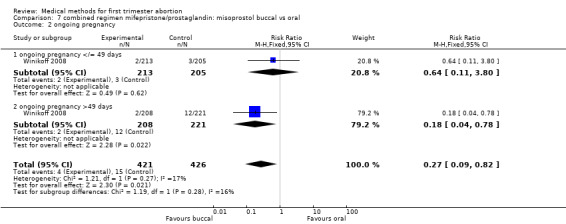
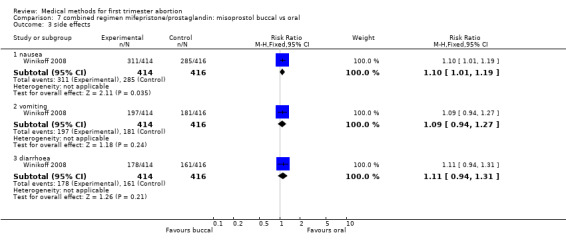
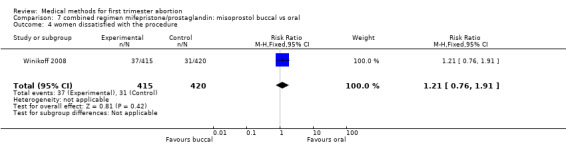
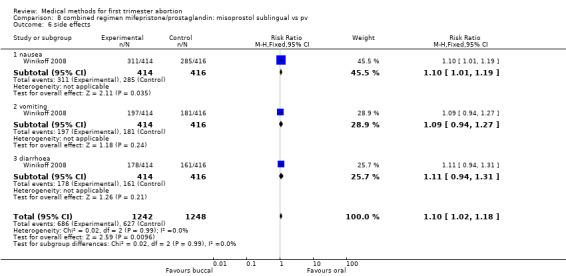
One trial (Winikoff 2008) is included in this comparison. The failure rate was lower in the buccal group (0.45 95%CI 0.25 to 0.79) for all gestational ages and for women with > 49 days of gestation (RR 0.37 95%CI 0.18 to 0.73). The failure rates were similar between the two groups for women ≤ 49 days. Overall ongoing pregnancy rate was lower in the buccal group (RR 0.27 95%CI 0.09 to 0.82) and for women > 49 days of gestation (RR 0.18 95% CI 0.04 to 0.78), while rates were similar for women with gestations ≤ 49 days. Fewer women in the oral group had nausea compared to the buccal group (RR 1.10 95% CI 1.01 to 1.19). Women reported similar rates of satisfaction between the two groups.
Intervention : misoprostol sublingual versus vaginal (comparison 8,Analysis 8.1)
8.1. Analysis.
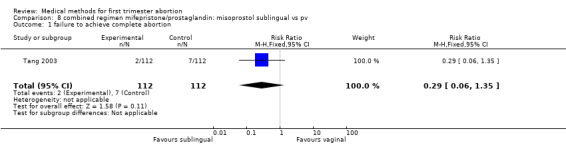
Comparison 8 combined regimen mifepristone/prostaglandin: misoprostol sublingual vs pv, Outcome 1 failure to achieve complete abortion.
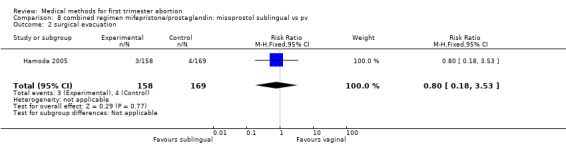
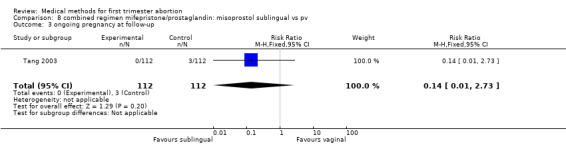
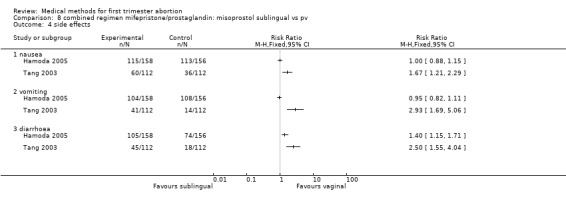
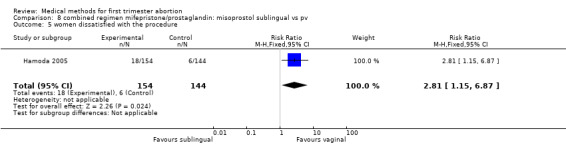
Two trials were included in this comparison (Hamoda 2005, Tang 2003). There was no difference in failure rates or in number of needed surgical evacuations. In one trial (Hamoda 2005) women received additional doses of misoprostol if abortion was incomplete at follow‐up and the results were not presented for the intended method used and were therefore not totaled. Tang 2003 reported that significantly more women in the sublingual group experienced side‐effects: nausea (RR 1.67 95%CI 1.21 to 2.29), vomiting (RR 2.93 95% CI 1.69 to 5.06), diarrhoea (RR 2.5 95%CI 1.55 to 4.04). More women were dissatisfied with the method in the one trial reporting on it (Hamoda 2005)(RR 2.81 95%CI 1.15 to 6.87) compared to the vaginal group. Hamoda did not use an intention to treat analysis; loss to follow up was identical in both groups (n=13).
Intervention : misoprostol sublingual versus oral (comparison 9,Analysis 9.1)
9.1. Analysis.
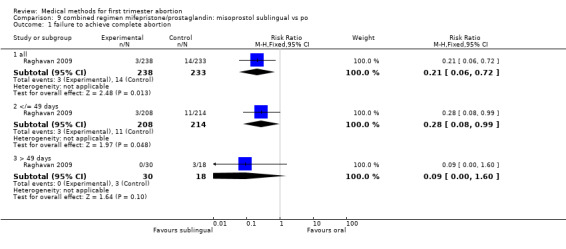
Comparison 9 combined regimen mifepristone/prostaglandin: misoprostol sublingual vs po, Outcome 1 failure to achieve complete abortion.
One trial was included in this comparison (Raghavan 2009). Women in the sublingual group were less likely to fail to achieve complete abortion compared with the oral group (RR 0.21 95%CI 0.06 to 0.72). More women were dissatisfied with the procedure in the sublingual group; however, this difference did not reach statistical significance (RR 1.96 95%CI 0.94 to 4.09). Side effects were similar among the two groups.
Combined regimen: mifepristone/prostaglandin Intervention: single versus split dose of prostaglandin (comparison 10,Analysis 10.1) One trial was included in this comparison (El‐Refaey 1994). There was no statistically or clinically significant difference between administration of 800 mcg of misoprostol as a single dose or by 2 doses of 400 mcg, 2 hours apart (RR 0.70 95% CI 0.21 ‐ 2.39) regarding failure rates. The side‐effects tended to favour the split‐dose group but were not statistically significant different between the 2 groups.
10.1. Analysis.
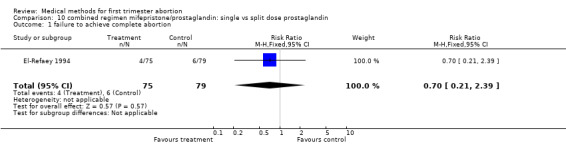
Comparison 10 combined regimen mifepristone/prostaglandin: single vs split dose prostaglandin, Outcome 1 failure to achieve complete abortion.
Intervention: single versus continuous misoprostol (comparison 11,Analysis 11.1)
11.1. Analysis.
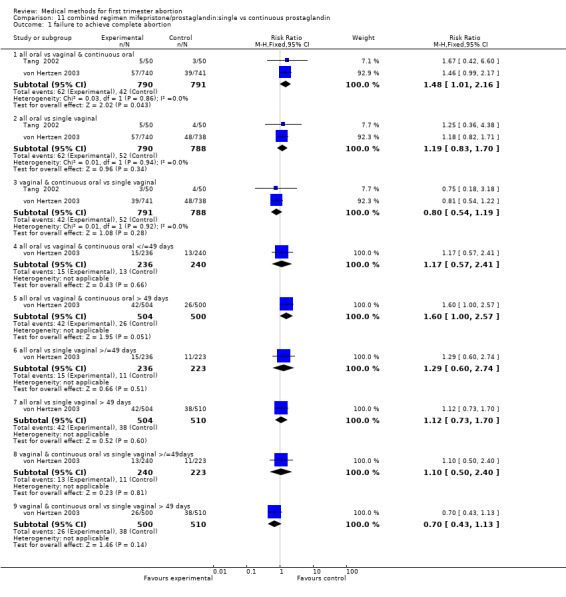
Comparison 11 combined regimen mifepristone/prostaglandin:single vs continuous prostaglandin, Outcome 1 failure to achieve complete abortion.
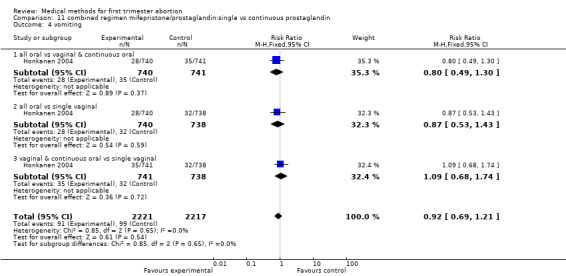
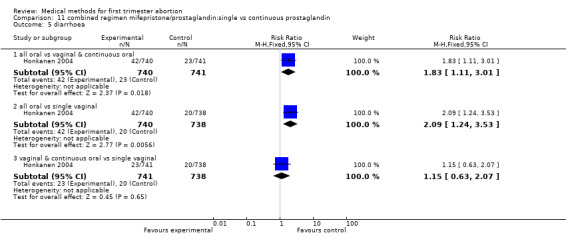
Two trials are included (Tang 2002, von Hertzen 2003). Honkanen 2004 reports on the same trial as von Hertzen 2003, but on different outcomes. Both trials compared oral misoprostol 400 mcg twice daily continued for 7 days after either an initial oral (group A) or vaginal 800mcg (group B) and single vaginal dose (group C) among 150 women. All women had received mifepristone 200mg 48 hours prior to misoprostol. More women failed to achieve complete abortion in the all oral group (A) compared to the vaginal and continuous oral misoprostol group (B) (RR 1.60 95%CI 1.00 to 2.57). When analysed by subgroups of gestational age, the difference was present in women > 49 days of gestation (RR 1.48 95%CI 1.01 to 2.16) but not in women ≤ 49 days. More women in the all oral group (A) had diarrhoea compared to the vaginal & continuous oral group and single vaginal group (RR 1.83 95%CI 1.11 to 3.01 group B and RR 2.09 95%Ci 1.24 to 3.53 group C). There was no difference with regard to nausea or vomiting and number of days of bleeding, reported as median and range (Tang 2002): group A: 16 (8‐107), group B:15 (8‐65), group C:16( 8‐74) and as median: 13 days (group A), 12 days (group B) and 12 days (group C) (Honkanen 2004).
Intervention: Mifepristone alone versus mifepristone/prostaglandin (comparison 12,Analysis 12.1) Three trials were included in this comparison: compared to the combination regimen, mifepristone alone was significantly less effective (RR of failure 3.76 95% CI 2.30 ‐ 6.15) (Cameron 1986 MI600GP1pv, Swahn 1989 MI200MP1po, Zheng 1989 MI600PGF2pv).
12.1. Analysis.
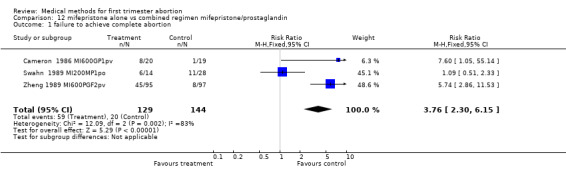
Comparison 12 mifepristone alone vs combined regimen mifepristone/prostaglandin, Outcome 1 failure to achieve complete abortion.
Prostaglandin alone versus a combined regimen (all) (comparison 13, Analysis 13.1) Six trials were included in this comparison (Cheng 1994 PGE1&T, Creinin 1994 M800&MT, Jain 1999 M800&TM, Jain 2002 M800&MI, Ozeren 1999 MP800&MT, Wiebe 2006). Wiebe 2006 compared methotrexate combined with 400mcg misoprostol vaginal or misoprostol 400mcg sublingual or 400mcg vaginal and was not included in the meta‐analysis, but data are presented in the additional table. One trial used additional doses of prostaglandin and did not specify which women received them (Jain 1999 M800&TM). The studies consistently demonstrate that compared to a combination regimen, misoprostol alone was significantly less effective in achieving complete abortion (2.50 95%CI 1.89 to 3.32). The analysis, excluding the Jain 1999 M800&TM trial showed similar results (RR 2.40 95%CI 1.79 to 3.20). There was less nausea with misoprostol only compared to the combined regimen in the 3 trials reporting on it (nausea RR 0.71 95%CI 0.56 ‐ 0.88) (Creinin 1994 M800&MT, Ozeren 1999 MP800&MT, Jain 2002 M800&MI).
13.1. Analysis.
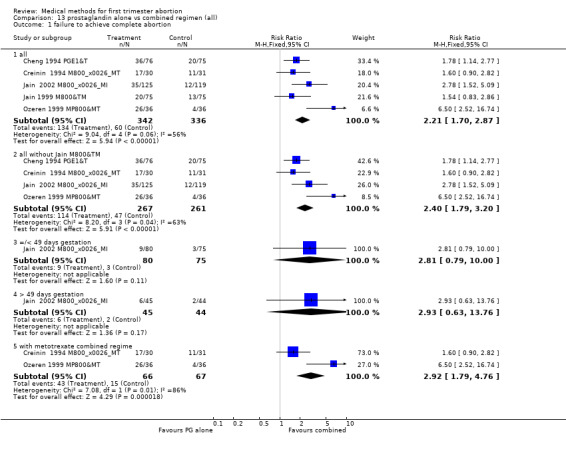
Comparison 13 prostaglandin alone vs combined regimen (all), Outcome 1 failure to achieve complete abortion.
Prostaglandin alone: route of administration (comparison 14, Analysis 14.1)
14.1. Analysis.
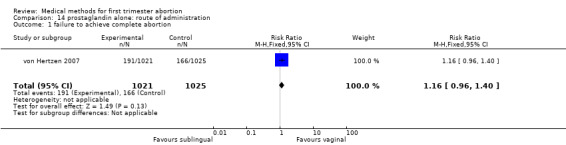
Comparison 14 prostaglandin alone: route of administration, Outcome 1 failure to achieve complete abortion.
One trial was included, comparing misprostol sublingual versus vaginal application, given in three doses each of 800mcg either 3 or 12 hourly. There was no difference in failure to achieve complete abortion between the groups. More women in the sublingual group had vomiting and diarrhoea compared to the vaginal group (RR1.54 95%CI 1.14 to 2.08 and RR 1.53 95%CI 1.33 to 1.76).
Mifepristone single ‐ high versus low dose (comparison 15,Analysis 15.1) One trial was included in this comparison (Birgerson 1988). No difference between a low (140 mg) and high (700 mg) dose of mifepristone was found regarding the failure rate.
15.1. Analysis.
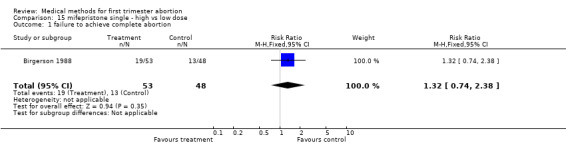
Comparison 15 mifepristone single ‐ high vs low dose, Outcome 1 failure to achieve complete abortion.
Combined regimen: methotrexate/prostaglandin Timing of prostaglandin (comparison 16,Analysis 16.1) Three trials are included in the review (Carbonell 1997 M800pv, Carbonell 1998 M800pv, Creinin 1995 M800pv) and data from 2 trials are included in the meta‐analysis (Carbonell 1997 M800pv, Carbonell 1998 M800pv).There was no significant difference in failure to achieve complete abortion between misoprostol given on day 5 compared to day 3 (RR 0.72 95% CI 0.36‐1.43) or on day 5 compared to day 4 (RR 0.73 95% CI 0.37‐1.48) following methotrexate.
16.1. Analysis.
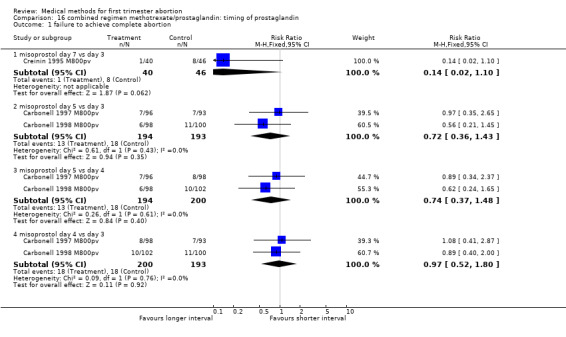
Comparison 16 combined regimen methotrexate/prostaglandin: timing of prostaglandin, Outcome 1 failure to achieve complete abortion.
Route of methotrexate: intramuscular versus oral (comparison 17,Analysis 17.1) One trial compared intramuscular versus oral administration of methotrexate (Wiebe 1999 B). There was no difference regarding the failure rate (RR 2.04 95% CI 0.51‐8.07) or side effects (nausea: RR 0.52 95% CI 0.22‐1.25; vomiting: RR 4.89 95% CI 0.57‐42.21; diarrhoea: RR 1.22 95% CI 0.18‐8.34).
17.1. Analysis.
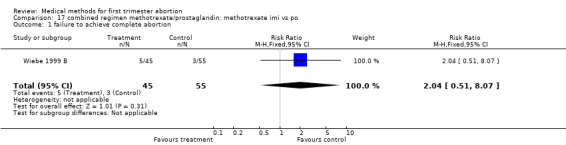
Comparison 17 combined regimen methotrexate/prostaglandin: methotrexate imi vs po, Outcome 1 failure to achieve complete abortion.
Dose of methotrexate (comparison 18,Analysis 18.1) Two trials were eligible to be included in the review (Creinin 1996 M800pv, Creinin 1997 M800pv). Both trials had a very small sample size (10 women in each group); they used differently dosed regimens and are therefore presented separately.
18.1. Analysis.
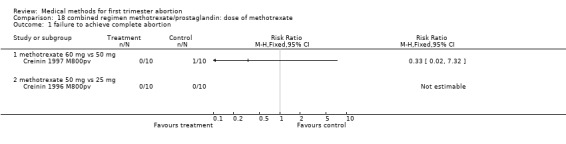
Comparison 18 combined regimen methotrexate/prostaglandin: dose of methotrexate, Outcome 1 failure to achieve complete abortion.
Route of prostaglandin (misoprostol) (comparison 19,Analysis 19.1) One trial (Wiebe 2004) compared buccal versus vaginal administration of misoprostol 3‐6 days after methotrexate. Women received additional misoprostol; it is unclear how many or in which treatment group. The vaginal route was more effective in achieving complete abortion (RR 1.43 95%CI 1.08 to 1.90). There was no difference regarding occurrence of side‐effects between the groups.
19.1. Analysis.
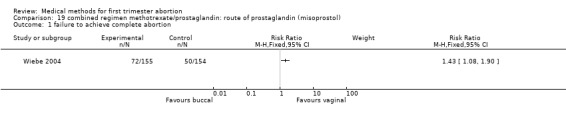
Comparison 19 combined regimen methotrexate/prostaglandin: route of prostaglandin (misoprostol), Outcome 1 failure to achieve complete abortion.
Tamoxifen versus methotrexate (combined with prostaglandin): Wiebe compared methotrexate to tamoxifen, both followed by misoprostol. The trial was conducted in 2 phases: phase 1 used low‐dose tamoxifen (40 mg) and phase 2 used high‐ dose (160 mg). This trial has therefore been referred to as Wiebe 1999 (low dose) and Wiebe 1999 A (high dose).
Intervention: low dose tamoxifen (40 mg)(comparison 20,Analysis 20.1) There was no statistically significant difference regarding failure rates between the groups (RR 2.04 95% CI 0.86‐4.84) and side‐ effects (nausea: RR 0.56 95% CI 0.33‐0.971; vomiting: RR 1.70 95% CI 0.42‐6.92; diarrhoea: RR 1.53 95% CI 0.26‐8.96) in the one trial included (Wiebe 1999).
20.1. Analysis.
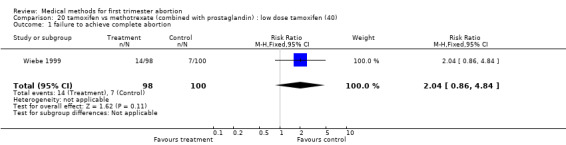
Comparison 20 tamoxifen vs methotrexate (combined with prostaglandin) : low dose tamoxifen (40), Outcome 1 failure to achieve complete abortion.
Intervention:high dose tamoxifen (160 mg) (comparison 21,Analysis 21.1) There was no statistically significant difference regarding failure rates between the 2 groups (RR 1.96 95% CI 0.93‐4.15) or side‐ effects (nausea: RR 0.78 95% CI 0.54‐1.10; vomiting: RR 0.65 95% CI 0.28‐1.53; diarrhoea: RR 1.23 95% CI 0.34‐4.43).
21.1. Analysis.
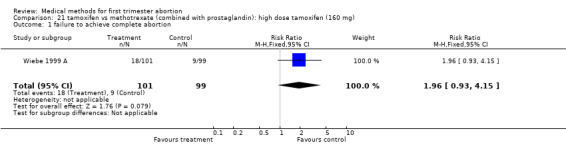
Comparison 21 tamoxifen vs methotrexate (combined with prostaglandin): high dose tamoxifen (160 mg), Outcome 1 failure to achieve complete abortion.
Combined regimen mifepristone/prostaglandin versus mifepristone/prostaglandin plus tamoxifen (comparison 22, Analysis 22.1) One trial was included (Wu 1993); no statistically significant difference between the 2 groups regarding failure to achieve complete abortion was found (RR 1.29 95% CI 0.82 ‐ 2.02).
22.1. Analysis.
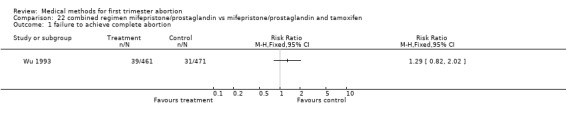
Comparison 22 combined regimen mifepristone/prostaglandin vs mifepristone/prostaglandin and tamoxifen, Outcome 1 failure to achieve complete abortion.
Other comparisons: Wang (Wang 2000) compared mifepristone 25mg/day over 7 days (total dose of 250mg) followed by oral misoprostol 200mg /day over 3 days (total dose of 1200mcg) to mifepristone 150mg on day 1 followed by oral misoprostol 600mcg on day 3. The doses and regimens in the two groups make it difficult to make any meaningful conclusion from this comparison. Koopersmith (Koopersmith 1996) compared misoprostol alone to misoprostol/tamoxifen and misoprostol/ laminaria. The sample size was very small which preclude makinge any meaningful conclusions from this study. These 2 trials are included in the additional tables. Additionally, Blanchard 2005 used various doses, routes and time of misoprostol administered alone in a very small sample of women.
Discussion
The literature on different medical abortion methods is vast, but contains relatively few randomised controlled trials comparing the different regimens. The trials included were all conducted after the mifepristone/misoprostol regimen was licensed for sale in Great Britain and France and rather sought to determine if a lower dose and less costly regimen could be as effective as the licensed one. Grimes (Grimes 1997) and Bygdeman (Bygdeman 2002) in their reviews mentioned the different aspects to be considered when using medical abortion methods. Medical methods used are mostly combined regimens and many different types of combinations are described. To facilitate synthesising the data, trials were grouped into comparisons, as listed above. The objective of this approach was to enable the evaluation of the experimental intervention being studied trying to avoid getting lost in the endless permutations of the combinations of different components. The focus was mainly on primary outcomes, such as effectiveness, complications, side‐effects and acceptability. Meta‐analysis was complicated by the use of different pharmaceutical agents, different doses and different routes of application; therefore, most meta‐analyses contain only a small number of reasonably comparable trials. The review focused on the primary outcome of effectiveness; firm conclusions on associated side‐effects or relatively uncommon complications, such as continuing pregnancy or haemorrhage.
These data support that the most common combined regimen (mifepristone/misoprostol) is an effective and safe method for pregnancy termination in the first trimester. The effect of mifepristone is not decreased by lowering the dose from previously recommended 600 mg to 200 mg when combined with at least 400 mcg of misoprostol. In earlier studies, it was demonstrated that the linear dose‐response effect of mifepristone does not occur in doses above 100 mg (Beaulieu 1997). A combination regimen with a prostaglandin is more effective than use of prostaglandin alone. Similarly, mifepristone alone is less effective than when combined with a prostaglandin.
Different prostaglandins have been used for medical abortion, but misoprostol has superior attributes; misoprostol is at least as effective as gemeprost and is less costly, does not require refrigeration and offers different routes of administration. Of the different routes of misoprostol administration, vaginal appears to be superior to oral administration in terms of efficacy in the meta‐analysis and majority of trials, and has fewer side effects when compared to oral and sublingual routes.
In regards to the role of gestational age, when comparing abortion at ≤ 7 weeks to those at 9 weeks or more, at least a doubling in the rate of failure was reported in one study (WHO 2000 M400po). There was not sufficient data to confirm these findings in this review.
Methotrexate, combined with a prostaglandin, has been used in some studies with an effectiveness of mostly > 90%. No trial comparing mifepristone/prostaglandin with methotrexate/prostaglandin was identified.
An important aspect of this review is the overall very low rate of major complications reported among the various medical abortion regimens. The most common complication is the need for blood transfusion (about 0.2% ) (see table 'characteristics of included studies'). The reported self‐limiting side‐effects of medical abortion regimens are mainly due to the prostaglandins (nausea, vomiting, diarrhoea). The dose, route and type of prostaglandin used may influence the occurrence of side effects, as higher doses and oral administration are associated with an increase in nausea and vomiting.
The generalizability of these results to some settings may be limited, as most trials considered in the review had inclusion criteria which were strict: intrauterine pregnancy was confirmed by ultrasound, emergency back‐up facilities were available and follow‐up was high. Fortunately, an increasing number of studies are focusing on the provision of medical abortion outside these particular constructs, although they were not the focus of this review. Additional barriers to introduction of medical abortion may include the relatively high cost and need for registration of mifepristone.
Acceptibility with medical abortion methods is often associated with the success of the abortion, and may decrease with higher gestational ages (Honkanen 2002; Winikoff 1997; Honkanen 2004). Whether acceptability of different application routes are linked to age, parity or cultural differences is not well established. The difference in time intervals between mifepristone and methotrexate and the administration of prostaglandin, or their use outside the health‐care setting may also play a role in the acceptability of one method over the other.
Other comparisons, such as tamoxifen/prostaglandin combination have not been evaluated extensively enough to draw firm conclusions. Some outcomes such as number of days of bleeding with the procedure, pain, time to return of menstruation or acceptability have not been assessed sufficiently.
Authors' conclusions
Implications for practice.
The available data from this review demonstrates that the combination mifepristone/misoprostol is a safe and effective abortion method in the first trimester up to 63 days. The effectiveness is not reduced by lowering the currently licensed dose of 600 mg of mifepristone to 200mg. Data on methotrexate/prostaglandin regimen is scarce. This review does not address introducing medical abortion where back‐up facilities are not available and women are less likely to attend for the follow up.
Implications for research.
Methotrexate in combination with a prostaglandin may be an alternative to the mifepristone/prostaglandin regimen in places where mifepristone is either unaffordable or unavailable. However, further research should be conducted to compare the methotrexate/prostaglandin combination regimen with the standard mifepristone/prostaglandin regimen. There is scarce data on issues such as which method is preferable when in addressing specific side‐effects, bleeding patterns, acceptability or financial impact of the different methods. Good quality acceptability studies are important to investigate the components of medical abortion regimens that affect acceptability in different settings.
What's new
| Date | Event | Description |
|---|---|---|
| 3 October 2011 | New citation required but conclusions have not changed | New author Nathalie Kapp helped updating this review and 19 new studies were added |
History
Protocol first published: Issue 4, 2000 Review first published: Issue 1, 2004
| Date | Event | Description |
|---|---|---|
| 15 April 2008 | Amended | Converted to new review format. |
| 17 October 2003 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
None
Data and analyses
Comparison 1. combined regimen mifepristone/prostaglandin: dose of mifepristone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 failure to achieve complete abortion | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 all | 6 | 6841 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.77, 1.05] |
| 1.2 600mg vs 200mg | 4 | 3494 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.87, 1.32] |
| 1.3 200mg vs 100 mg (>49 days) | 1 | 1182 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.61, 1.29] |
| 1.4 600mg vs 200mg with gemeprost 1mg/pv | 2 | 1685 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.72, 1.45] |
| 1.5 200mg vs 100 mg (</=49 days) | 1 | 941 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.47, 1.33] |
| 2 side effects | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 nausea | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 vomiting | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 diarrhoea | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 time until passing of conceptus > 3‐6 hours | 2 | 1116 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.74, 1.07] |
| 4 ongoing pregnancy | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5 surgical evacuation | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
1.2. Analysis.
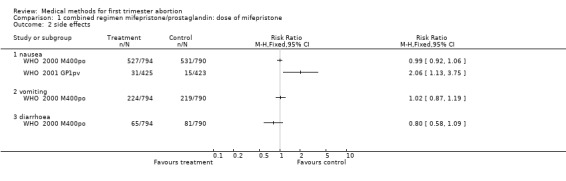
Comparison 1 combined regimen mifepristone/prostaglandin: dose of mifepristone, Outcome 2 side effects.
1.3. Analysis.
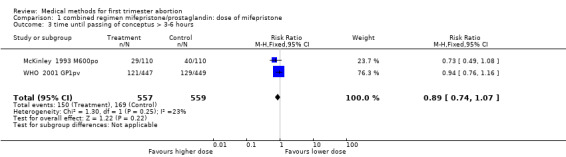
Comparison 1 combined regimen mifepristone/prostaglandin: dose of mifepristone, Outcome 3 time until passing of conceptus > 3‐6 hours.
1.4. Analysis.
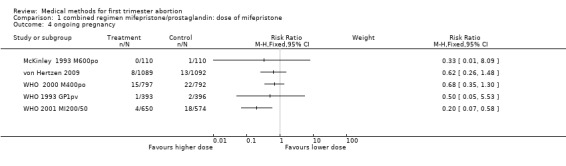
Comparison 1 combined regimen mifepristone/prostaglandin: dose of mifepristone, Outcome 4 ongoing pregnancy.
1.5. Analysis.
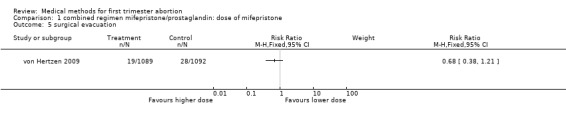
Comparison 1 combined regimen mifepristone/prostaglandin: dose of mifepristone, Outcome 5 surgical evacuation.
Comparison 2. combined regimen mifepristone/prostaglandin: dose of prostaglandin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 failure to achieve complete abortion | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 gemeprost 1 mg vs 0.5 mg | 2 | 1034 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.31, 0.59] |
| 1.2 misoprostol 800po or pv vs 400po | 2 | 934 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.53, 1.31] |
| 2 side effects | 2 | 2802 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.95, 1.27] |
| 2.1 nausea | 2 | 934 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.85, 1.25] |
| 2.2 vomiting | 2 | 934 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.90, 1.64] |
| 2.3 diarrhoea | 2 | 934 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.81, 1.56] |
| 3 women dissatisfied with the procedure | 2 | 931 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.76, 1.50] |
| 4 ongoing pregnancy | 2 | 933 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.10 [0.01, 0.76] |
| 4.1 misoprostol 800mcg vs 400 mcg | 2 | 933 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.10 [0.01, 0.76] |
| 5 surgical evacuation | 2 | 934 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.53, 1.31] |
| 5.1 misoprostol 800mcg vs 400mcg | 2 | 934 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.53, 1.31] |
2.2. Analysis.
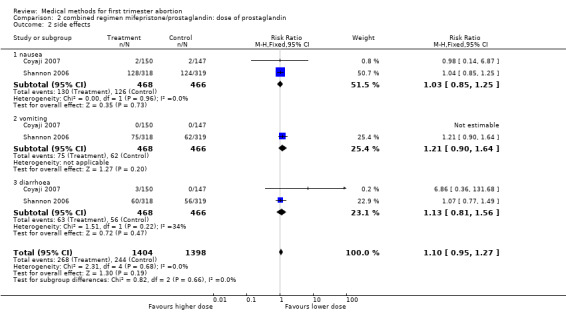
Comparison 2 combined regimen mifepristone/prostaglandin: dose of prostaglandin, Outcome 2 side effects.
2.3. Analysis.
Comparison 2 combined regimen mifepristone/prostaglandin: dose of prostaglandin, Outcome 3 women dissatisfied with the procedure.
2.4. Analysis.
Comparison 2 combined regimen mifepristone/prostaglandin: dose of prostaglandin, Outcome 4 ongoing pregnancy.
2.5. Analysis.
Comparison 2 combined regimen mifepristone/prostaglandin: dose of prostaglandin, Outcome 5 surgical evacuation.
Comparison 3. combined regimen mifepristone/prostaglandin: type of prostaglandin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 failure to achieve complete abortion | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 gemeprost vs misoprostol | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 PGF2alpha vs misoprostol | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 side effects | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 vomiting | 1 | 910 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.49 [1.06, 2.10] |
| 2.2 diarrhoea | 1 | 910 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.66 [1.35, 5.26] |
| 3 ongoing pregnancy | 2 | 1687 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.28, 1.48] |
| 4 time until passing of conceptus > 3‐6 hours | 1 | 910 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.77, 1.23] |
3.2. Analysis.
Comparison 3 combined regimen mifepristone/prostaglandin: type of prostaglandin, Outcome 2 side effects.
3.3. Analysis.
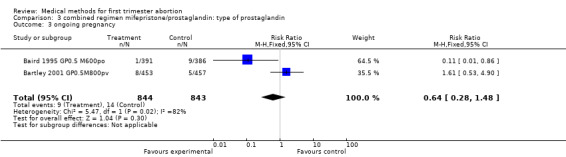
Comparison 3 combined regimen mifepristone/prostaglandin: type of prostaglandin, Outcome 3 ongoing pregnancy.
3.4. Analysis.
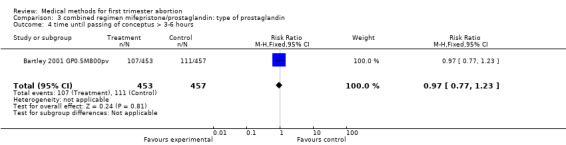
Comparison 3 combined regimen mifepristone/prostaglandin: type of prostaglandin, Outcome 4 time until passing of conceptus > 3‐6 hours.
Comparison 4. combined regimen mifepristone/prostaglandin: time of prostaglandin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 failure to achieve complete abortion | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 day 3 vs day 1 | 1 | 1489 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.94 [1.05, 3.58] |
| 1.2 day 3 vs day 2 | 1 | 1521 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.69 [0.95, 3.01] |
| 1.3 day 2 vs day 1(all) | 3 | 3687 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.95, 1.63] |
| 1.4 day 2 vs day 1(</= 49 days) | 1 | 941 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.49, 1.36] |
| 1.5 day 2 vs day 1(> 49 days) | 1 | 1182 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.62 [1.11, 2.38] |
| 1.6 day 2 vs day 0 | 2 | 511 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.24, 0.65] |
| 1.7 day 1 vs day 0(all) | 2 | 2156 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.46, 0.92] |
| 1.8 day 1 vs day 0(</= 49 days) | 2 | 998 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.38, 1.14] |
| 1.9 day 1 vs day 0(> 49 days) | 2 | 1158 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.41, 1.06] |
| 2 side effects | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 nausea day 3 vs day 1 | 1 | 1358 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.96, 1.14] |
| 2.2 nausea day 3 vs day 2 | 1 | 1384 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.91, 1.06] |
| 2.3 nausea day 2 vs day 1 | 1 | 1434 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.98, 1.16] |
| 2.4 nausea day 2 vs day 0 | 2 | 444 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.58, 1.11] |
| 2.5 vomiting day 3 vs day 1 | 1 | 1358 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.86, 1.19] |
| 2.6 vomiting day 3 vs day 2 | 1 | 1384 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.83, 1.13] |
| 2.7 vomiting day 2 vs day 1 | 1 | 1434 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.90, 1.22] |
| 2.8 vomiting day 2 vs day 0 | 2 | 444 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.59, 1.36] |
| 2.9 diarrhoea day 3 vs day 1 | 1 | 1358 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.99, 1.48] |
| 2.10 diarrhoea day 3 vs day 2 | 1 | 1384 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.95, 1.42] |
| 2.11 diarrhoea day 2 vs day 1 | 1 | 1434 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.85, 1.28] |
| 2.12 diarrhoea day 2 vs day 0 | 2 | 444 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.41, 1.03] |
| 2.13 nausea day 1 vs day 0 | 2 | 2137 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.78, 1.31] |
| 2.14 vomiting day 1 vs day 0 | 2 | 2137 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.79, 1.62] |
| 2.15 diarrhoea day 1 vs day 0 | 2 | 2137 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.58, 1.18] |
| 3 surgical evacuation | 5 | 8330 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.76, 1.27] |
| 3.1 day 3 vs day 1 | 1 | 1489 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.49 [0.78, 2.83] |
| 3.2 day 3 vs day 2 | 1 | 1521 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.80 [0.92, 3.52] |
| 3.3 day 2 vs day 1 | 2 | 3681 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.68, 1.67] |
| 3.4 day 2 vs day 0 | 2 | 511 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.19, 0.85] |
| 3.5 day 1 vs day 0 | 1 | 1128 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.36, 1.20] |
| 4 ongoing pregnancy | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 day 3 vs day 1 | 1 | 1489 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.56 [0.51, 4.73] |
| 4.2 day 3 vs day 2 | 1 | 1521 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.71 [0.72, 10.16] |
| 4.3 day 2 vs day 1 (all) | 2 | 3681 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.45, 1.90] |
| 4.4 day 1 vs day 0 | 2 | 2208 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.07, 1.66] |
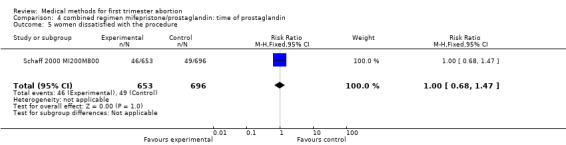
| 5 women dissatisfied with the procedure | 1 | 1349 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.68, 1.47] |
4.2. Analysis.
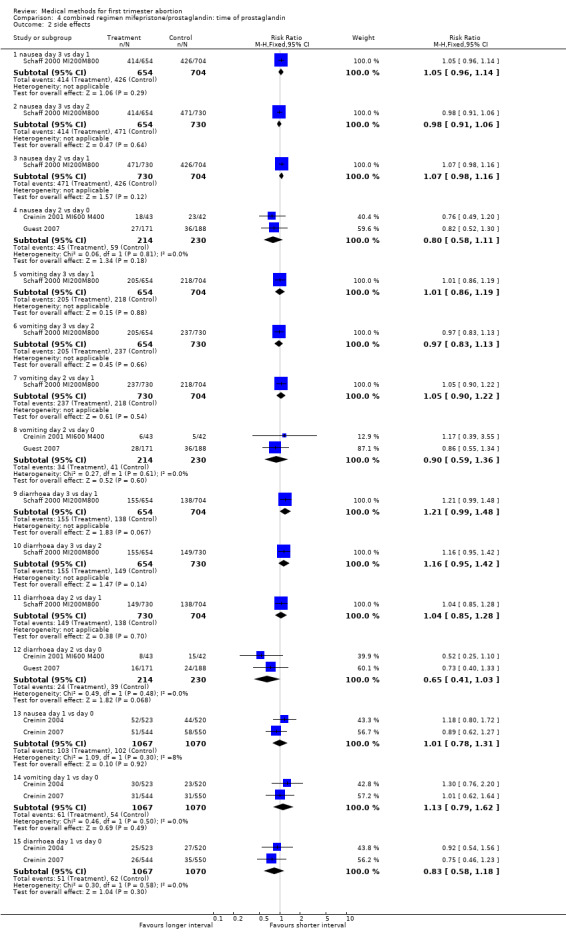
Comparison 4 combined regimen mifepristone/prostaglandin: time of prostaglandin, Outcome 2 side effects.
4.3. Analysis.
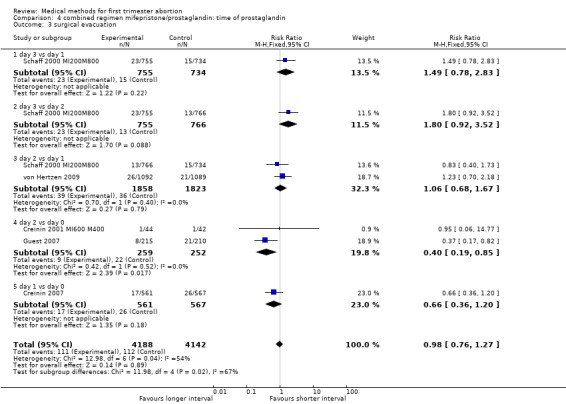
Comparison 4 combined regimen mifepristone/prostaglandin: time of prostaglandin, Outcome 3 surgical evacuation.
4.4. Analysis.
Comparison 4 combined regimen mifepristone/prostaglandin: time of prostaglandin, Outcome 4 ongoing pregnancy.
4.5. Analysis.
Comparison 4 combined regimen mifepristone/prostaglandin: time of prostaglandin, Outcome 5 women dissatisfied with the procedure.
Comparison 5. combined regimen mifepristone/prostaglandin: misoprostol po vs pv.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 failure to achieve complete abortion | 2 | 2814 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.05 [2.24, 4.14] |
| 2 side effects | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 nausea | 2 | 1380 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [1.02, 1.25] |
| 2.2 vomiting | 2 | 1219 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.71, 0.98] |
| 2.3 diarrhoea | 2 | 1379 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.80 [1.49, 2.18] |
5.2. Analysis.
Comparison 5 combined regimen mifepristone/prostaglandin: misoprostol po vs pv, Outcome 2 side effects.
Comparison 6. combined regimen mifepristone/prostaglandin: misoprostol buccal vs pv.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 failure to achieve complete abortion | 1 | 429 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.36, 1.67] |
| 2 side effects | 1 | 1287 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [1.07, 1.36] |
| 2.1 nausea | 1 | 429 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.98, 1.29] |
| 2.2 vomiting | 1 | 429 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.89, 1.51] |
| 2.3 diarrhoea | 1 | 429 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.51 [1.12, 2.03] |
6.2. Analysis.
Comparison 6 combined regimen mifepristone/prostaglandin: misoprostol buccal vs pv, Outcome 2 side effects.
Comparison 7. combined regimen mifepristone/prostaglandin: misoprostol buccal vs oral.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 failure to achieve complete abortion | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 failure to achieve complete abortion (all) | 1 | 847 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.40, 0.96] |
| 1.2 failure to achieve complete abortion </= 49 days | 1 | 418 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.25, 2.04] |
| 1.3 failure to achieve complete abortion > 49 days | 1 | 429 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.18, 0.73] |
| 2 ongoing pregnancy | 1 | 847 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.27 [0.09, 0.82] |
| 2.1 ongoing pregnancy </= 49 days | 1 | 418 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.11, 3.80] |
| 2.2 ongoing pregnancy >49 days | 1 | 429 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.18 [0.04, 0.78] |
| 3 side effects | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 nausea | 1 | 830 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [1.01, 1.19] |
| 3.2 vomiting | 1 | 830 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.94, 1.27] |
| 3.3 diarrhoea | 1 | 830 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.94, 1.31] |
| 4 women dissatisfied with the procedure | 1 | 835 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.76, 1.91] |
7.2. Analysis.
Comparison 7 combined regimen mifepristone/prostaglandin: misoprostol buccal vs oral, Outcome 2 ongoing pregnancy.
7.3. Analysis.
Comparison 7 combined regimen mifepristone/prostaglandin: misoprostol buccal vs oral, Outcome 3 side effects.
7.4. Analysis.
Comparison 7 combined regimen mifepristone/prostaglandin: misoprostol buccal vs oral, Outcome 4 women dissatisfied with the procedure.
Comparison 8. combined regimen mifepristone/prostaglandin: misoprostol sublingual vs pv.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 failure to achieve complete abortion | 1 | 224 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.06, 1.35] |
| 2 surgical evacuation | 1 | 327 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.18, 3.53] |
| 3 ongoing pregnancy at follow‐up | 1 | 224 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.01, 2.73] |
| 4 side effects | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.1 nausea | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 vomiting | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 diarrhoea | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 women dissatisfied with the procedure | 1 | 298 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.81 [1.15, 6.87] |
| 6 side effects | 1 | 2490 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [1.02, 1.18] |
| 6.1 nausea | 1 | 830 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [1.01, 1.19] |
| 6.2 vomiting | 1 | 830 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.94, 1.27] |
| 6.3 diarrhoea | 1 | 830 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.94, 1.31] |
8.2. Analysis.
Comparison 8 combined regimen mifepristone/prostaglandin: misoprostol sublingual vs pv, Outcome 2 surgical evacuation.
8.3. Analysis.
Comparison 8 combined regimen mifepristone/prostaglandin: misoprostol sublingual vs pv, Outcome 3 ongoing pregnancy at follow‐up.
8.4. Analysis.
Comparison 8 combined regimen mifepristone/prostaglandin: misoprostol sublingual vs pv, Outcome 4 side effects.
8.5. Analysis.
Comparison 8 combined regimen mifepristone/prostaglandin: misoprostol sublingual vs pv, Outcome 5 women dissatisfied with the procedure.
8.6. Analysis.
Comparison 8 combined regimen mifepristone/prostaglandin: misoprostol sublingual vs pv, Outcome 6 side effects.
Comparison 9. combined regimen mifepristone/prostaglandin: misoprostol sublingual vs po.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 failure to achieve complete abortion | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 all | 1 | 471 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.06, 0.72] |
| 1.2 </= 49 days | 1 | 422 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.28 [0.08, 0.99] |
| 1.3 > 49 days | 1 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.00, 1.60] |
| 2 side effects | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 nausea | 1 | 471 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.73, 1.04] |
| 2.2 vomiting | 1 | 471 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.59, 1.33] |
| 3 women dissatisfied with the procedure | 1 | 471 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.96 [0.94, 4.09] |
9.2. Analysis.
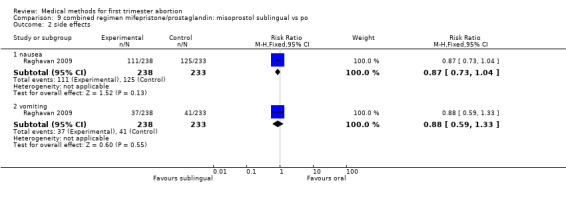
Comparison 9 combined regimen mifepristone/prostaglandin: misoprostol sublingual vs po, Outcome 2 side effects.
9.3. Analysis.
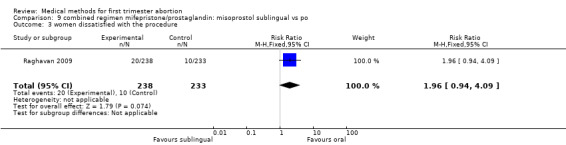
Comparison 9 combined regimen mifepristone/prostaglandin: misoprostol sublingual vs po, Outcome 3 women dissatisfied with the procedure.
Comparison 10. combined regimen mifepristone/prostaglandin: single vs split dose prostaglandin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 failure to achieve complete abortion | 1 | 154 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.21, 2.39] |
| 2 side effects | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 nausea | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 vomiting | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 diarrhoea | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
10.2. Analysis.
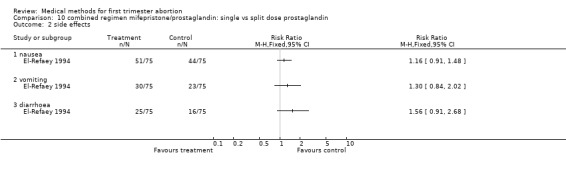
Comparison 10 combined regimen mifepristone/prostaglandin: single vs split dose prostaglandin, Outcome 2 side effects.
Comparison 11. combined regimen mifepristone/prostaglandin:single vs continuous prostaglandin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 failure to achieve complete abortion | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 all oral vs vaginal & continuous oral | 2 | 1581 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.48 [1.01, 2.16] |
| 1.2 all oral vs single vaginal | 2 | 1578 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.83, 1.70] |
| 1.3 vaginal & continuous oral vs single vaginal | 2 | 1579 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.54, 1.19] |
| 1.4 all oral vs vaginal & continuous oral </=49 days | 1 | 476 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.57, 2.41] |
| 1.5 all oral vs vaginal & continuous oral > 49 days | 1 | 1004 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.60 [1.00, 2.57] |
| 1.6 all oral vs single vaginal >/=49 days | 1 | 459 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.60, 2.74] |
| 1.7 all oral vs single vaginal > 49 days | 1 | 1014 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.73, 1.70] |
| 1.8 vaginal & continuous oral vs single vaginal >/=49days | 1 | 463 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.50, 2.40] |
| 1.9 vaginal & continuous oral vs single vaginal > 49 days | 1 | 1010 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.43, 1.13] |
| 2 ongoing pregnancy at follow‐up | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 all oral vs vaginal & continuous oral | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 all oral vs single vaginal | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 vaginal & continuous oral vs single vaginal | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 nausea | 1 | 4438 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.83, 1.12] |
| 3.1 all oral vs vaginal & continuous oral | 1 | 1481 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.65, 1.09] |
| 3.2 all oral vs single vaginal | 1 | 1478 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.72, 1.24] |
| 3.3 vaginal & continuous oral vs single vaginal | 1 | 1479 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.87, 1.45] |
| 4 vomiting | 1 | 4438 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.69, 1.21] |
| 4.1 all oral vs vaginal & continuous oral | 1 | 1481 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.49, 1.30] |
| 4.2 all oral vs single vaginal | 1 | 1478 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.53, 1.43] |
| 4.3 vaginal & continuous oral vs single vaginal | 1 | 1479 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.68, 1.74] |
| 5 diarrhoea | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 all oral vs vaginal & continuous oral | 1 | 1481 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.83 [1.11, 3.01] |
| 5.2 all oral vs single vaginal | 1 | 1478 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.09 [1.24, 3.53] |
| 5.3 vaginal & continuous oral vs single vaginal | 1 | 1479 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.63, 2.07] |
11.2. Analysis.
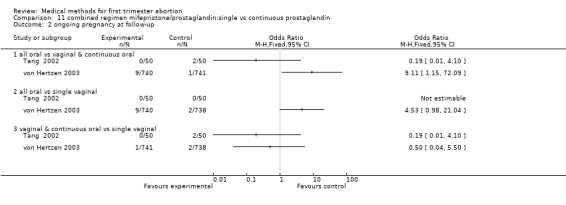
Comparison 11 combined regimen mifepristone/prostaglandin:single vs continuous prostaglandin, Outcome 2 ongoing pregnancy at follow‐up.
11.3. Analysis.
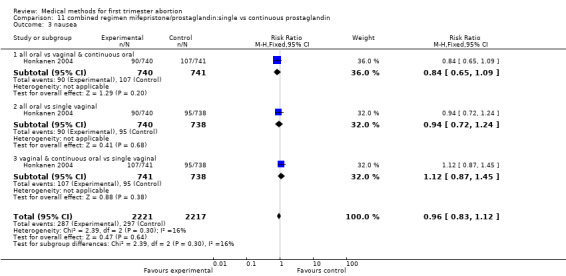
Comparison 11 combined regimen mifepristone/prostaglandin:single vs continuous prostaglandin, Outcome 3 nausea.
11.4. Analysis.
Comparison 11 combined regimen mifepristone/prostaglandin:single vs continuous prostaglandin, Outcome 4 vomiting.
11.5. Analysis.
Comparison 11 combined regimen mifepristone/prostaglandin:single vs continuous prostaglandin, Outcome 5 diarrhoea.
Comparison 12. mifepristone alone vs combined regimen mifepristone/prostaglandin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 failure to achieve complete abortion | 3 | 273 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.76 [2.30, 6.15] |
Comparison 13. prostaglandin alone vs combined regimen (all).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 failure to achieve complete abortion | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 all | 5 | 678 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.21 [1.70, 2.87] |
| 1.2 all without Jain M800&TM | 4 | 528 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.40 [1.79, 3.20] |
| 1.3 =/< 49 days gestation | 1 | 155 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.81 [0.79, 10.00] |
| 1.4 > 49 days gestation | 1 | 89 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.93 [0.63, 13.76] |
| 1.5 with metotrexate combined regime | 2 | 133 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.92 [1.79, 4.76] |
| 2 side effects | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 nausea | 3 | 377 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.56, 0.88] |
| 2.2 vomiting | 3 | 466 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.55, 1.00] |
| 2.3 diarrhoea | 4 | 527 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.95, 1.59] |
13.2. Analysis.
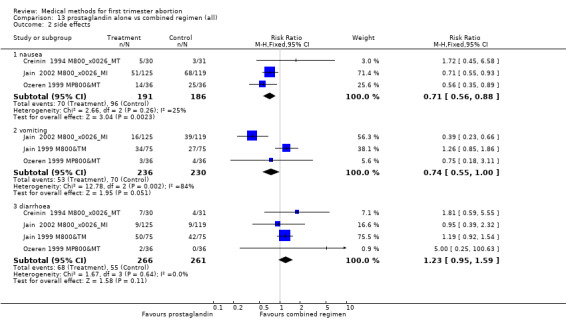
Comparison 13 prostaglandin alone vs combined regimen (all), Outcome 2 side effects.
Comparison 14. prostaglandin alone: route of administration.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 failure to achieve complete abortion | 1 | 2046 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.96, 1.40] |
| 2 side effects | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 nausea | 1 | 2066 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.90, 1.20] |
| 2.2 vomiting | 1 | 2066 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.54 [1.14, 2.08] |
| 2.3 diarrhoea | 1 | 2066 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.53 [1.33, 1.76] |
14.2. Analysis.
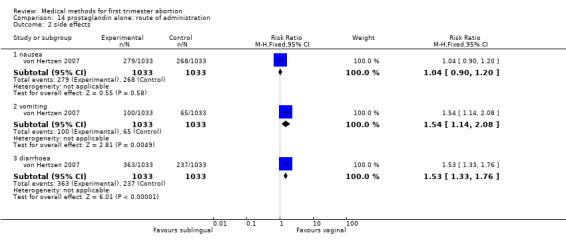
Comparison 14 prostaglandin alone: route of administration, Outcome 2 side effects.
Comparison 15. mifepristone single ‐ high vs low dose.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 failure to achieve complete abortion | 1 | 101 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [0.74, 2.38] |
| 2 side effects | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 nausea | 1 | 101 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.19, 0.84] |
| 2.2 vomiting | 1 | 101 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.07, 1.78] |
15.2. Analysis.
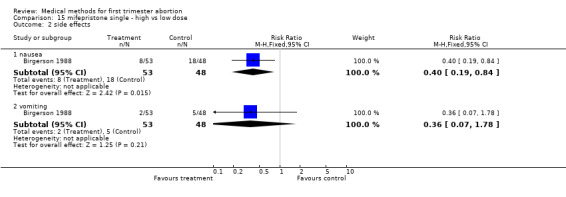
Comparison 15 mifepristone single ‐ high vs low dose, Outcome 2 side effects.
Comparison 16. combined regimen methotrexate/prostaglandin: timing of prostaglandin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 failure to achieve complete abortion | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 misoprostol day 7 vs day 3 | 1 | 86 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.02, 1.10] |
| 1.2 misoprostol day 5 vs day 3 | 2 | 387 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.36, 1.43] |
| 1.3 misoprostol day 5 vs day 4 | 2 | 394 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.37, 1.48] |
| 1.4 misoprostol day 4 vs day 3 | 2 | 393 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.52, 1.80] |
Comparison 17. combined regimen methotrexate/prostaglandin: methotrexate imi vs po.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 failure to achieve complete abortion | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.04 [0.51, 8.07] |
| 2 Side effects | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 nausea | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.22, 1.25] |
| 2.2 vomiting | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.89 [0.57, 42.21] |
| 2.3 diarrhoea | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.18, 8.34] |
17.2. Analysis.
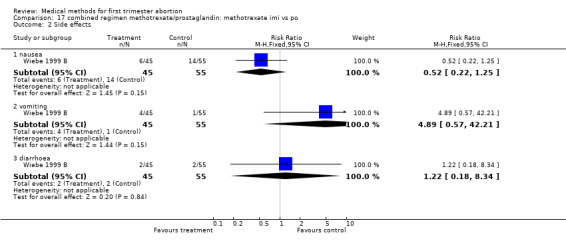
Comparison 17 combined regimen methotrexate/prostaglandin: methotrexate imi vs po, Outcome 2 Side effects.
Comparison 18. combined regimen methotrexate/prostaglandin: dose of methotrexate.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 failure to achieve complete abortion | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 methotrexate 60 mg vs 50 mg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 methotrexate 50 mg vs 25 mg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 19. combined regimen methotrexate/prostaglandin: route of prostaglandin (misoprostol).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 failure to achieve complete abortion | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2 side effects | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 nausea | 1 | 309 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.84, 1.42] |
| 2.2 vomiting | 1 | 309 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.67, 1.56] |
| 2.3 diarrhoea | 1 | 309 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.40 [0.94, 2.07] |
19.2. Analysis.
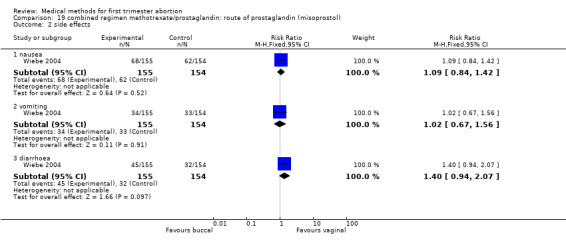
Comparison 19 combined regimen methotrexate/prostaglandin: route of prostaglandin (misoprostol), Outcome 2 side effects.
Comparison 20. tamoxifen vs methotrexate (combined with prostaglandin) : low dose tamoxifen (40).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 failure to achieve complete abortion | 1 | 198 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.04 [0.86, 4.84] |
| 2 side effects | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 nausea | 1 | 198 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.33, 0.97] |
| 2.2 vomiting | 1 | 198 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.70 [0.42, 6.92] |
| 2.3 diarrhoea | 1 | 198 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.53 [0.26, 8.96] |
20.2. Analysis.
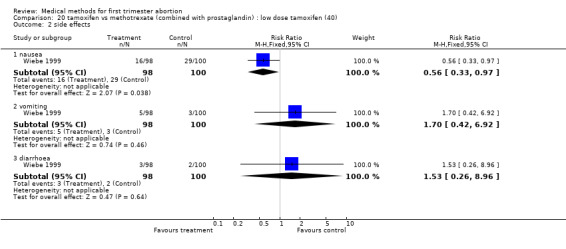
Comparison 20 tamoxifen vs methotrexate (combined with prostaglandin) : low dose tamoxifen (40), Outcome 2 side effects.
Comparison 21. tamoxifen vs methotrexate (combined with prostaglandin): high dose tamoxifen (160 mg).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 failure to achieve complete abortion | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.96 [0.93, 4.15] |
| 2 side effects | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 nausea | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.54, 1.10] |
| 2.2 vomiting | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.28, 1.53] |
| 2.3 diarrhoea | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.34, 4.43] |
21.2. Analysis.
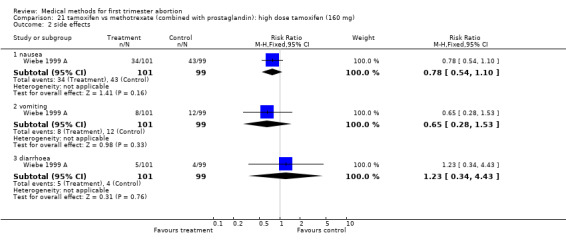
Comparison 21 tamoxifen vs methotrexate (combined with prostaglandin): high dose tamoxifen (160 mg), Outcome 2 side effects.
Comparison 22. combined regimen mifepristone/prostaglandin vs mifepristone/prostaglandin and tamoxifen.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 failure to achieve complete abortion | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Arvidsson 2005.
| Methods | computer randomisation | |
| Participants | 100 women randomised; age and gestational ages averages not given; included gestational age up to 49 days confirmed by ultrasound; exclusion criteria: contraindications for medical abortion.Setting: Karolinska Hospital, Sweden | |
| Interventions | mifepristone 600mg (all) followed 36‐48 hrs later by: group1) misoprostol 400mcg oral group 2) misoprostol 800mcg vaginal |
|
| Outcomes | experience of pain, occurrence of side‐effects, duration of bleeding | |
| Notes | 10 women could not be reached by phone 3‐7 weeks after abortion; 30 women did not agree or weren't asked to be called during this time frame | |
Baird 1995 GP0.5 M600po.
| Methods | computer generated random numbers for the first 300 women, envelopes were shuffled in batches of 20 and numbered consecutively for the reminders no blinding for clinical staff | |
| Participants | 800 pregnant women </= 63 days of amenorrhoea in Edinburgh/Scotland | |
| Interventions | mifepristone 200mg (all) followed by: group 1: gemeprost 0.5mg vaginal and 3 tabs placebo after 48 hours group 2: misoprostol 600mcg oral and vaginal examination after 48 hours | |
| Outcomes | complete, incomplete and missed abortion ongoing pregnancy side effects | |
| Notes | power calculation (80% to detect 5% difference) placebos were not identical to misoprostol 1 woman needed blood transfusion (group 2) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Bartley 2001 GP0.5M800pv.
| Methods | computer generated random numbers | |
| Participants | 999 pregnant women, < 63 days of gestation, confirmed by ultrasound if necessary, at the Royal Infirmary Hospital, Edinburgh Inclusion criteria: aged =/> 16 years, available for follow‐up within 2 weeks Exclusion criteria: ectopic pregnancy, active asthma, liver or renal disease, adrenal insufficiency, anaemia, haemolytic disease, treatment with anticoagulants, smoking > 20 cigarettes/day | |
| Interventions | mifepristone 200mg (all) followed by: group 1: gemeprost 0.5mg/vaginal group 2: misoprostol 800mcg/vaginal | |
| Outcomes | complete, incomplete abortion, ongoing pregnancy, duration of bleeding, side effects | |
| Notes | single blinded 2 women required blood transfusions (1 in each group) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Birgerson 1988.
| Methods | random allocation, not specified | |
| Participants | 153 women, ≤ 49 days of amenorrhoea, confirmed by positive pregnancy test and pelvic examination, Uppsala, Sweden | |
| Interventions | group 1: mifepristone 10mg / twice daily for 7 days group 2: mifepristone 25mg / twice daily for 7 days group 3: mifepristone 50mg / twice daily for 7 days (group 1 vs group 3) | |
| Outcomes | complete, incomplete abortion ongoing pregnancy bleeding pattern side effects | |
| Notes | no mentioning of major complications | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Blanchard 2005.
| Methods | random numbers generated in SPSS; numbered opaque envelopes | |
| Participants | >18 years old in good general health and willing
to return for follow‐up and living <1 hr from clinic; ≤ 56 days of gestation, exclusion: less than 18 years
old, suspected ectopic pregnancy. Study conducted in India and Vietnam |
|
| Interventions | misoprostol only: g1) 4X400mcg/3h/oral; g2) 2X800mcg/oral; g3) 1X600mcg/vaginal; g4) 2X800mcg/3h/oral; g5) 1X800mcg/vaginal | |
| Outcomes | complete/incomplete abortion, ongoing pregnancy | |
| Notes | Initially, women were randomised between the first three regimens. Subsequent review of their low efficacy resulted changing the regimens, and from that point on, women were randomized to treatment group 4 or 5. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Cameron 1986 MI600GP1pv.
| Methods | random allocation, not specified | |
| Participants | 45 pregnant women < 56 days amenorrhoea, confirmed by pregnancy test, pelvic examination and ultrasound Exclusion criteria: multiple pregnancy, spontaneous abortion, cardiovascular or pulmonary disease, allergy, epilepsy | |
| Interventions | group 1: mifepristone 150mg / daily for 4 days group 2: mifepristone 150mg and gemeprost 1‐2 mg vaginal after 48 hours | |
| Outcomes | complete abortion, treatment failure, complications, side effects, pain, bleeding pattern | |
| Notes | 5 women receiving gemeprost 2 mg were excluded from the analysis 1 woman received blood transfusion (group 1); 1 woman had emergency evacuation due to heavy bleeding (group 1) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Carbonell 1997 M800pv.
| Methods | computer randomisation; sealed, opaque envelopes were numbered by a by a person unrelated to the study | |
| Participants | 300 pregnant women, ≤ 63 days of amenorrhoea confirmed by ultrasound Exclusion criteria: previous use of vitamins/folates, white blood cell count <3000/uL, platelet count <100 000/uL, haemoglobin <10.0 mg/dL, aspartate aminotransferase >2 times normal or active liver disease, serum creatinine >1.5 mg/dL or active renal disease, inflammatory bowel disease, intolerance to the medication | |
| Interventions | methotrexate 50mg/m2 intramuscular on recruitment day and misoprostol800 mcg vaginal (self administered) on: group 1: day 3 group 2: day 4 group 3: day 5 additional 800mcg misoprostol in 48 hours interval (up to 4 doses) | |
| Outcomes | complete, incomplete abortion (complete expulsion with additional doses of misoprostol), treatment failure, bleeding pattern, blood parameters, side effects | |
| Notes | power calculation (85% power, significance level of 0.05) no major complications occurred | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Carbonell 1998 M800pv.
| Methods | computer randomisation; sealed, opaque envelopes were numbered by a by a person unrelated to the study | |
| Participants | 315 pregnant women, ≤ 63 days of amenorrhoea confirmed by ultrasound Exclusion criteria: previous use of vitamins/folates, white blood cell count <3000/uL, platelet count <100 000/uL, haemoglobin <10.0 mg/dL, aspartate aminotransferase >2 times normal or active liver disease, serum creatinine >1.5 mg/dL or active renal disease, inflammatory bowel disease, intolerance to the medication | |
| Interventions | methotrexate 50mg oral on recruitment day and misoprostol 800mcg vaginal (self administered) on: group 1: day 3 group 2: day 4 group 3: day 5 additional 800mcg misoprostol in 48 hours interval (up to 4 doses) | |
| Outcomes | complete, incomplete abortion (complete expulsion with additional doses of misoprostol), treatment failure, bleeding pattern, blood parameters, side effects | |
| Notes | power calculation (80% power, significance level of 0.05) no major complications occurred | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Cheng 1994 PGE1&T.
| Methods | double blind, randomisation generated centrally; sealed, opaque envelopes | |
| Participants | 151 women, ≤ 49 days of amenorrhoea confirmed by ultrasound at Shanghai Medical University without medical disorders, contraindication for the study medication or IUD in situ | |
| Interventions | group 1: day 1‐3: testosterone propionate 100mg/imi/day day 4: PGE1 ester (ONO 802) 1mg/pv/6 hourly for a maximum of 4 doses group2: day 1‐3: placebo injections day 4: PGE1 ester (ONO 802) 1mg/pv/6 hourly for a maximum of 4 doses | |
| Outcomes | complete, incomplete abortion, ongoing pregnancy, blood transfusion, duration of bleeding | |
| Notes | no major complications were reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Coyaji 2007.
| Methods | computer generated random sequence; consecutive numbered opaque envelopes | |
| Participants | 18 years or older; 300 women randomised; gestational age less than 8 weeks; study conducted between january 2004 ‐ june 2005; no contraindications to study medication
lived or worked within 1 hour of the study site, agreed to provide an address and telephone number and return for a follow‐up visit study site: India (Pune and Mumbai) |
|
| Interventions | mifepristone 200mg followed after 48 hours by: group 1) 400mcg oral misoprostol and placebo 3h later group 2) 400mg misoprostol repeated once 3 hours later |
|
| Outcomes | complete abortion, side effcets | |
| Notes | ITT done | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Creinin 1994 M800&MT.
| Methods | randomisation according to computer‐generated random number table numbered sealed, opaque envelopes | |
| Participants | 63 pregnant women, ≤ 56 days of amenorrhoea, confirmed by ultrasound, San Francisco General Hospital Exclusion criteria: Exclusion criteria: previous use of vitamins/folates, hematocrit ≤ 0.30, white blood cell count <3000/uL, platelet count <100 000/uL, haemoglobin <10.0 mg/dL, aspartate aminotransferase >2 times normal or active liver disease, serum creatinine >1.5 mg/dL or active renal disease, inflammatory bowel disease, asthma, intolerance to the medication | |
| Interventions | group 1: methotrexate 50mg/m2 intramuscular and misoprostol 800mcg/vaginal after 3 days group 2: misoprostol 800mcg/vaginal | |
| Outcomes | complete abortion, duration of vaginal bleeding, side effects, change in beta‐HCG levels | |
| Notes | power calculation (80% power, significance level of 0.05) based on 95% success with methotrexate and 75% success with misoprostol alone. The required sample size was 98. no mentioning of major complications | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Creinin 1995 M800pv.
| Methods | randomisation according to computer‐generated random number table numbered sealed, opaque envelopes no blinding | |
| Participants | 86 pregnant women, ≤ 56 days of amenorrhoea, confirmed by ultrasound, San Francisco General Hospital Exclusion criteria: previous use of vitamins/folates, hematocrit ≤ 0.30, white blood cell count <3000/uL, platelet count <100 000/uL, haemoglobin <10.0 mg/dL, aspartate aminotransferase >2 times normal or active liver disease, serum creatinine >1.5 mg/dL or active renal disease, inflammatory bowel disease, asthma, intolerance to the medication | |
| Interventions | methotrexate 50mg/m2 intramuscular followed by: group 1: misoprostol 800mcg/vaginal after 3 days group 2: misoprostol 800mcg/vaginal after 7 days | |
| Outcomes | complete abortion, duration of vaginal bleeding, side effects, change in beta‐HCG levels | |
| Notes | power calculation (80% power, significance level of 0.05) no major complications occurred | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Creinin 1996 M800pv.
| Methods | randomisation according to random number tables sealed, opaque envelopes were numbered by a by a person unrelated to the study no blinding | |
| Participants | 20 pregnant women, ≤49 days, confirmed by ultrasound, Magee‐Women's Hospital, Pennsylvania, USA Exclusion criteria: previous use of vitamins/folates, haemoglobin <10.0 mg/dL, aspartate aminotransferase >2 times normal or active liver disease, serum creatinine >1.5 mg/dL or active renal disease, inflammatory bowel disease, intolerance to the medication | |
| Interventions | group 1: methotrexate 25mg/orally followed by misoprostol 800mcg/vaginal after 7 days group 2: methotrexate 50mg/orally followed by misoprostol 800mcg/vaginal after 7 days | |
| Outcomes | complete abortion, duration of vaginal bleeding, side effects, change in haemoglobin/aspartate transferase | |
| Notes | no major complications occurred | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Creinin 1997 M800pv.
| Methods | randomisation according to computer‐generated random number table numbered sealed, opaque envelopes prepared by a person unrelated to the study no blinding | |
| Participants | 20 pregnant women, ≤49 days, confirmed by ultrasound, Magee‐Women's Hospital, Pennsylvania, USA Exclusion criteria: previous use of vitamins/folates, hematocrit < 37%, white blood cell count <3000/uL, platelet count <100 000/uL, haemoglobin <10.0 mg/dL, aspartate aminotransferase >2 times normal or active liver disease, serum creatinine >1.5 mg/dL or active renal disease, inflammatory bowel disease, asthma, intolerance to the medication | |
| Interventions | group 1: methotrexate 50mg/m2 followed by misoprostol 800mcg/vaginal after 7 days group 2: methotrexate 60mg/m2 followed by misoprostol 800mcg/vaginal after 7 days | |
| Outcomes | complete abortion, time to passing of conceptus, side effects, methotrexate levels,change in haemoglobin/aspartate transferase | |
| Notes | no blinding no major complications were reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Creinin 2001.
| Methods | random number tables in blocs of ten, sealed opaque envelopes prepared by person not involved in the trial | |
| Participants | 80 pregnant women, ≤ 49 days pregnant, single pregnancy, confirmed by ultrasound, at the University hospital Pittsburgh, USA; exclusion criteria: contraindication to mifepristone/misoprostol administration, haemoglobin < 10 gm/dL, cardiovascular disease, coagulopathies, IUCD in situ, breast feeding | |
| Interventions | mifepristone 100mg (all) after 2 days, home administration: group 1: misoprostol 400mcg oral group 2: misoprostol 800mcg vaginal | |
| Outcomes | complete abortion, onset of bleeding &cramping, duration of bleeding, side effects | |
| Notes | power calculation power calculation (80% power, significance level of 0.05) no major complications were reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Creinin 2001 MI600 M400.
| Methods | random number tables, sealed opaque envelopes | |
| Participants | 86 pregnant women, =/> 18 years, ≤ 49 days pregnant, single pregnancy, at the University hospital Pittsburgh, USA exclusion criteria: contraindication to mifepristone/misoprostol administration, haemoglobin < 10 gm/dL, cardiovascular disease, coagulopathies, IUCD in situ, breastfeeding | |
| Interventions | mifepristone 600mg (all) group 1: misoprostol 400mcg after 6‐8 hours/oral group 2: misoprostol 400mcg after 48 hours/oral | |
| Outcomes | complete abortion, onset and duration of bleeding, side effects | |
| Notes | no blinding no major complications were reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Creinin 2004.
| Methods | computer generated randomisation, permuted blocs, stratified by centre; sequentially numbered opaque envelopes | |
| Participants | 26 years old; 1080 women randomized no more than 63 days gestation confirmed by ultrasound; average gestational age of 51 days; willing to have surgical procedure and had a telephone; conducted in 2002‐2003 in USA MAgee‐Women's Hospital in Pittsburgh, Pennsylvania, columbia University, NY, Boston University,Massachusetts University of Rochester, NY | |
| Interventions | mifepristone 200mg followed by misoprostol 800mcg vaginal: group1: administered 6‐8 hours after mifepristone group 2: administered 23‐25 hours after mifepristone |
|
| Outcomes | Complete abortion, side‐effects, bleeding, acceptability | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Creinin 2007.
| Methods | computer generated random numbers, randomisation centrally, permuted block design with varying block sizes; randomisation after taking mifepristone ; no blinding; opaque envelopes | |
| Participants | 1128 women enrolled;mean age 27 years, women with no more than 63 days gestation (mean gestational age 51‐52 days gestation) and willing to follow‐up and with access to a telephone. Exclusion criteria: contraindications to mifepristone or misoprostol, Hbg< 10, IUD in place, on anticoagulants or with coagulopathy, active cervicits or currently breastfeeding. Gestational age confirmed by US. 4 academic centers in the USA; University of Pittsburgh, Oregon Health and Science University, Northwestern University, University of Southern California |
|
| Interventions | mifepristone 200mg followed by: group 1: within 15 minutes, 800mcg misoprostol vaginal group 2: 23‐25 hours later, 800mcg misoprostol vaginal |
|
| Outcomes | complete abortion; side‐effect; bleeding; acceptibility | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
El‐Refaey 1994.
| Methods | sealed, opaque envelopes random assignment before misoprostol administration | |
| Participants | 150 pregnant women </= 56 days of amenorrhoea, confirmed by ultrasound | |
| Interventions | group 1: mifepristone 200mg and misoprostol 800mcg/oral after 48 hours group 2: mifepristone 200mg and misoprostol 400mcg after 48 hours plus 400mcg 2 hours later/oral | |
| Outcomes | changes in blood pressure, pulse rate and temperature complete and incomplete abortion ongoing pregnancy side effects bleeding pattern | |
| Notes | power calculation (5% significance level to detect a 20% reduction in incidence of side effects) no mentioning of major complications | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
El‐Refaey 1995 M800MI600.
| Methods | computer generated random assignment before misoprostol administration, sealed opaque envelopes | |
| Participants | 270 women ≤ 63 days of amenorrhoea, confirmed by ultrasound Exclusion criteria: contraindication for the use of mifepristone and/or misoprostol | |
| Interventions | group 1: mifepristone 600mg and misoprostol 800mcg/orally after 48 hours group 2: mifepristone 600mg and misoprostol 800mcg/vaginally (self‐administration) after 48 hours | |
| Outcomes | complete, incomplete and missed abortion ongoing pregnancy expulsion within 4 hours expulsion without need for surgery side effects | |
| Notes | power calculation (5% significance level to detect difference of 10% in the incidence of women aborting within 4 hours vaginal misoprostol by self administration 1 woman received a blood transfusion (group 2) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Guest 2007.
| Methods | computer generated fixed blocks of 20; 1:1 randomisation; sealed opaque envelopes | |
| Participants | 450 women aged 24‐26 years; no more than 63 days gestation confirmed by US (average 51 days of gestation); exclusion criteria: contraindications for study medication, breastfeeding, Hbg<10, coagulopathy or treatment with anticoagulants, IUD in situ, presence of cardiovascular disease, ectopic pregnancy; study conducted between September 2003 ‐ March 2005 | |
| Interventions | mifepristone 200mg followed by: group 1: 800mcg vaginal misoprostol after 6 hours group 2: 800mcg of vaginal misoprostol after 36‐48 hrs later |
|
| Outcomes | complete abortion, side effcets, acceptability | |
| Notes | ITT analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Hamoda 2005.
| Methods | random number tables; sealed opaque envelopes | |
| Participants | 340 women, average age 24 years randomised; average gestational age 65‐68 days. Exclusion criteria: <16 years, severe asthma,haemorrhagic disorders and treatment with anticoagulants, known allergy to prostaglandins, history of cardiac disease, smoking over the age of 35 years with ECG abnormalities, breastfeeding. study conducted at Aberdeen Royal Infermary, UK, from July 2002 ‐ October 2003 |
|
| Interventions | mifepristone 200 mg, followed 36‐48 hours after by: group 1: 600mcg sublingual misoprostol, followed 3 hours later by 400mcg (if 9‐13 weeks gestation, a third dose of 400mcg was administered) group 2: 800mcg vaginal misoprostol, followed 3 hours later by 400mcg (if 9‐13 weeks gestation, a third dose of 400mcg was administered) |
|
| Outcomes | complete/incomplete abortion, missed abortion, continuing pregnancy | |
| Notes | No ITT; LTFU identical (13) in each group (total 26) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ adequate |
Honkanen 2004.
| Methods | computer generated random sequence; packing company prepared bags containing the medication according to the randomisation sequence | |
| Participants | 2219 women; mean age 27 years; </= 63 days of amenorrhoea; Inclusion criteria:single intrauterine pregnancies, haemoglobin > 100 g/L . Exclusion criteria: medical contraindications or allergy for either mifepristone or misoprostol; past or present thromboembolism; liver disease, pruritus of pregnancy; previous surgery of uterine cervix; presence of an intrauterine device; suspected or proven ectopic pregnancy; smoking > 10 cigarettes/day; risk factor for cardiovascular disease; breastfeeding; Study was conducted from October 1998 ‐ Decembre 2000 in 15 cities in 11 countries, including developed and developing countries: Beijing, Hong Kong and Shanghai ‐ China; Chandigarh, Mumbai and New Delhi ‐ India; Helsinki ‐ Finland; Ho Chi Minh City ‐ Viet Nam; Ljubljana ‐Slovenia; Oslo ‐ Norway; Singapore ‐ Singapore; Stockholm ‐ Sweden; Szeged ‐ Hungary; Targu Mures ‐ Romania; and Ulaanbaatar ‐ Mongolia. |
|
| Interventions | mifepristone 200mg followed 36‐48 hours later by: group 1: misoprostol 800mcg orally followed by misoprostol 400mcg twice/day for 6 days oral group 2: misoprostol 800mcg vaginally followed by misoprostol 400mcg twice/day for 6 days oral group 3: misoprostol 800mcg vaginally followed by placebo tablets twice/day for 6 days oral |
|
| Outcomes | side‐effects and acceptability | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ adequate |
Jain 2002 M800&MI.
| Methods | computer generated random table, opaque vials | |
| Participants | 250 healthy women, </=56 days of amenorrhoea, confirmed by ultrasound, Exclusion criteria: evidence of threatened spontaneous abortion, uterine infection, anaemia, bleeding disorders, cardiovascular or cerebrovascular disease, uterine leiomyomata, allergy against the study medication. | |
| Interventions | group 1: mifepristone 200mg, misoprostol 800mcg/pv on day 3, repeated on day 4 if gestational sac present group 2: Placebo, misoprostol 800mcg/pv on day 3, repeated on day 4 if gestational sac present | |
| Outcomes | successful abortion, side effects | |
| Notes | Placebos were vitamin C tablets (not identical); opaque vials were used to blind the investigator power calculation (5% significance level to detect a 5% difference in success rates between the 2 study groups) no major complications were reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Jain 1999 M800&TM.
| Methods | randomisation by using random number tables | |
| Participants | 150 women pregnant ≤ 56 days confirmed by ultrasound exclusion criteria: cervical dilatation, anaemia, pelvic inflammatory disease, uterine bleeding, uterine leiomyomata, serious medical problems, allergy or contraindications to the study medication | |
| Interventions | group 1: tamoxifen 20mg/twice daily and misoprostol 800mcg/pv after 48 hours group 2: placebo twice daily and misoprostol 800mcg/pv after 48 hours | |
| Outcomes | complete/incomplete abortion, ongoing pregnancy, complications, side effects | |
| Notes | treatment and placebo were placed in identical capsules no major complications were reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A‐ Adequate |
Koopersmith 1996.
| Methods | randomisation into 3 groups randomisation procedure not stated | |
| Participants | 58 women, pregnant ≤ 10 weeks, confirmed by ultrasound, University Hospital Los Angeles, USA Exclusion criteria: uterine infection, prior uterine bleeding, cervical dilatation, anaemia, cardiovascular or cerebral disease, allergy to misoprostol | |
| Interventions | group A: misoprostol 100mcg/vaginally/ 8 hourly to a maximum of 6 doses group B: misoprostol 100mcg/vaginally/ 8 hourly to a maximum of 6 doses and tamoxifen 10mg/orally after the first dose of misoprostol group C: misoprostol 100mcg/vaginally/ 8 hourly to a maximum of 6 doses and laminaria/intracervical immediately before the first dose of misoprostol the dose of misoprostol was increased after the success rate was unsatisfactory after the first 26 women | |
| Outcomes | complete abortion, failure rate, side effects, mean number of doses of misoprostol used, time until passing of conceptus | |
| Notes | no mentioning of major complications | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Liao 2004.
| Methods | computer random table; use of identical appearing packages and capsules/ tablets from pharmacy; identical placebo tablets | |
| Participants | 480 women,average age 26 years; </= 49 days gestation confirmed by ultrsound; Exclusion criteria: abnormal menses, IUD in situ, contraindications for use of study medication; study conducted between November 2001 to June 2002 in 3 hospitals affiliated to University of Beijing, China | |
| Interventions | group 1: mifepristone: 50mg, then 12 hrs later 25mg, then 12 hrs later 50mg, and finally, 12 hrs later, 25mg (total: 150mg). 24 hrs after last dose 600mcg misoprostol orally group 2: mifepristone 30mg, then 15mg every 12 hours for 3 doses (total: 75mg). 24 hrs after last dose, 600mcg misoprostol orally. |
|
| Outcomes | complete abortion | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ adequate |
McKinley 1993 M600po.
| Methods | identical envelopes, shuffled and numbered consecutively | |
| Participants | 220 pregnant women, ≤ 63 days of amenorrhoea, University hospital Edinburgh, Scotland | |
| Interventions | group 1: mifepristone 200mg and misoprostol 600mcg/orally after 48 hours group 2: mifepristone 600mg and misoprostol 600mcg/orally after 48 hours | |
| Outcomes | complete and incomplete abortion, time until passing of conceptus, side effects, bleeding pattern, analgesia use | |
| Notes | blinding for outcome assessment no major complications were reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Middleton 2005.
| Methods | computer generated randomisation in blocs of 8; sealed envelopes | |
| Participants | 442 women < 56 days randomised;mean age 26 years; mean gestaional age 47 days; study conducted between December 2001‐ June 2004 at two clinics at University of Rochester; USA | |
| Interventions | 1‐2 days after mifepristone 200mg: group 1: misoprostol 800mcg buccal group 2: misoprostol 800mcg vaginal buccal: 2 tablets placed inside each cheek and remainders swallowed after 30 minutes; vaginal: all 4 tablets placed profond into the vagina with 1 finger |
|
| Outcomes | complete abortion, side effects, acceptability | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B‐unclear |
Ozeren 1999 MP800&MT.
| Methods | random number tables; sealed opaque envelopes, sequentially numbered | |
| Participants | 108 women ≤ 63 days of amenorrhoea confirmed by ultrasound, University hospital Trabzon, Turkey exclusion criteria: haemoglobin < 100 g/L, leucocytaemie, active liver disease, active renal disease, inflammatory bowel disease, history of methotrexate/ misoprostol intolerance | |
| Interventions | group 1. methotrexate 50mg/m2/imi group 2: misoprostol 800mcg/pv group 3: methotrexate 50mg/m2/imi and misoprostol 800mcg/pv after 3 days | |
| Outcomes | complete abortions, ongoing pregnancies, side effects | |
| Notes | no major complications were reported; 10/36 women in the misoprostol only group received additional misoprostol on day 4 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Raghavan 2009.
| Methods | random code generated in blocs of 10; sequentially numbered, sealed envelopes | |
| Participants | 480 women; </= 63 days gestation. gestational age confirmed by ultrasound if needed; exclusion criteria:ectopic pregnancy, contraindications to study medication, treatment with anticoagulants, lived more than 1 hour away from hospital; study conducted between July 2005 to November 2006 atUniversity hospital Chisinau, Moldova | |
| Interventions | mifepristone 200mg followed 24 hrs later by: group 1: misoprostol 400mcg sublingual group 2: misoprostol 400mcg oral for sublingual: tablet for 30 min under the tongue and swallow rest after; no repeat doses of misoprostol offered |
|
| Outcomes | complete abortion, side effects, acceptibility | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B‐unclear |
Rodger 1989 MI600.
| Methods | randomisation not stated | |
| Participants | 120 pregnant women, <56 days of amenorrhoea, Gynaecological Out‐Patient Department, Royal Infirmary Hospital, Edinburgh, Scotland | |
| Interventions | mifepristone 600mg (all) group 1: gemeprost 0.5mg/pv after 48 hours group 2: gemeprost 1mg/pv after 48 hours | |
| Outcomes | complete, incomplete abortion, onset and duration of bleeding, side effects, haemoglobin levels | |
| Notes | 1 woman received blood transfusion (group 2) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Sandstrom 1999 MI600GP1pv.
| Methods | randomly allocated; using sealed envelopes | |
| Participants | 64 pregnant women, ≤ 56 days, Hillerod Hospital, Denmark Exclusion criteria: previous uterine surgery, previous abnormal vaginal bleeding, ocncomitant medication, IUD in situ, contraindication to one of teh study drugs | |
| Interventions | all: mifepristone 600mg group1: gemeprost 1mg/pv after 24 hours group 2: gemeprost 1mg/pv after 48 hours | |
| Outcomes | complete, incomplete abortion, side effects | |
| Notes | 1 woman needed blood transfusion, not mentioned what group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Sang 1994 M600poPGF2pv.
| Methods | random number tables | |
| Participants | 600 women , ≤ 49 days of pregnancy, multicentre trial in 5 hospitals in Shanghai, China; pregnancy confirmed by gynaecological examination, urine pregnancy test or ultrasound; women were included if there was no history of medical disorders, no IUCD in situ and no contraindication for the study medication | |
| Interventions | group 1: mifepristone 150mg divided into 5 doses, orally, within 3 days; misoprostol 600mcg orally 36‐48 hours later group 2: mifepristone 150mg divided into 5 doses /po, within 3 days; PGF2alpha /pv 36‐48 hours later group 3: mifepristone 200mg po; misoprostol 600mcg/po after 36‐48 hours | |
| Outcomes | complete, incomplete abortion, duration of bleeding, time of resuming of menses, side effects | |
| Notes | no mentioning of major complications | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Sang 1999 M600poPGF2pv.
| Methods | randomisation was generated centrally and women were randomised within centres; sealed opaque envelopes | |
| Participants | multicentre trial, 78 hospitals and family planning clinics from 8 provinces in China; 17542 pregnant women, ≤ 49 days of amenorrhoea, pregnancy confirmed by gynaecological examination, urine pregnancy test or ultrasound; women were included if there was no history of medical disorders, no IUCD in situ and no contraindication for the study medication | |
| Interventions | mifepristone 150mg divided into 5 doses taken orally within 3 days group 1: prostaglandin F2alpha 1mg/pv 36‐48 h after first dose of mifepristone group 2: misoprostol 600mcg/po 36‐48 h after first dose of mifepristone | |
| Outcomes | complete, incomplete abortion, duration of vaginal bleeding, time to resume menses, side effects, women's satisfaction with the procedure | |
| Notes | 1 woman had an allergic shock after misoprostol (group 2) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Schaff 2001 M800MI200.
| Methods | computer generated random assignment, open‐label | |
| Participants | multicentre trial at 15 sites in the USA, incl. hospitals, non‐profit abortion facilities, private family practice and gynaecologist offices 1168 women, ≤ 63 days pregnant confirmed by ultrasound, without clinical or haematological abnormalities or contraindication to the trial medication | |
| Interventions | all women received mifepristone 200mg on day 1 group 1: 800mcg misoprostol/po minimum 24 hours after at home group 2: misoprostol 800mcg/pv minimum 24 hours after at home | |
| Outcomes | complete, incomplete abortion, time to bleeding, side effects | |
| Notes | open ‐ labelled study, power calculation to detect a 5 % difference from 95% to 90% efficacy no hospitalisations and no blood transfusions | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Schaff 2000 MI200M800.
| Methods | computer generated random assignment, allocation, randomisation stratified by sites, allocation was 'concealed'; 53 women used repeat dose of misoprostol ‐ not described teh number of women per group receiveing additional misoprostol | |
| Participants | multicentre trial (16 centres), 2295 women with pregnancies ≤ 56 days confirmed by ultrasound; from 16 US primary care and referral abortion facilities; routine inclusion and exclusion criteria | |
| Interventions | all women received mifepristone 200mg on day 1 group 1: misoprostol 800mcg/pv next day at home group 2: misoprostol 800mcg/pv 2 days later at home group 3: misoprostol 800mcg/pv 3 days later at home | |
| Outcomes | complete abortion, acceptability, adverse effects | |
| Notes | 2 women received blood transfusion (not mentioned which group) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Shannon 2006.
| Methods | computer generated rondom numbers in group of 15; misoprostol tablets were providede in sealed, opaque envelopes after administration of mifepristone. | |
| Participants | 971 women, mean age 28 years, < 56 days of gestation; mean gestational age of 44 days. Exclusion criteria: haemoglobin < 9.5
g/dl, active hepatic or renal disease, type I diabetes mellitus, adrenal insufficiency, glaucoma, sickle cell
anaemia, coagulopathy, uncontrolled seizure disorder, severe cardiovascular disease, allergy or intolerance to study medication, use of chronic oral steroid medications or anticoagulants Study conducted in 2001 at University of British Columbia; University of Sherbrooke; Laval University; University of Toronto; Canada |
|
| Interventions | Mifepristone 200mg followed 24‐28 hours later by: group 1: misoprostol 400mcg oral group 2: misoprostol 600mcg oral group 3: 800mcg misoprostol vaginal. Misoprostol self administered at home. Participants were advised to take a second dose of misoprostol in case bleeding was less than normal menstruation. Ultrasound after 7 days ‐ if ongoing pregnancy: misoprostol 800mcg vaginally |
|
| Outcomes | complete abortion, acceptability, side effects | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐adequate |
Swahn 1989 MI200MP1po.
| Methods | randomly allocated | |
| Participants | 42 pregnant women, ≤ 49 days of amenorrhoea, confirmed by ultrasound | |
| Interventions | all: mifepristone 25mg/twice daily/ for 4 days (=200mg in total) and: group 1: 1 placebo a.m. and p.m./orally group 2: PGE2 (minprostin) 1mg/a.m. and placebo /p.m. /orally group 3: PGE2 1mg/ a.m. and p.m. /orally | |
| Outcomes | complete, incomplete abortion failures, complaints, hormone levels (E2 prostaglandin, beta‐HCG, prolactin) bleeding pattern | |
| Notes | originally planned sample size was 120: study was discontinued due to interim analysis which showed no difference between placebo and PGE2 in the complete abortion rate no major complications were reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Tang 2002.
| Methods | computer generated random table | |
| Participants | 150 pregnant women , </= 63 days of amenorrhoea, confirmed by ultrasound at the University Hospital Hong Kong inclusion criteria: good health, willing to use barrier methods for contraception until first menses after termination, haemoglobin level >110g/L exclusion criteria: significant past or present illness, allergy/contraindication towards study medication, intrauterine device, heavy smoker, breast feeding | |
| Interventions | Mifepristone 200 mg for all women group A: misoprostol 800mcg/po and misoprostol 400mcg/X2/day/po for day 4‐10 group B: misoprostol 800mcg/pv on day 3 and misoprostol 400mcg/X2/day/po for day 4 ‐10 group C: misoprostol 800mcg/pv on day 3 and placebo tablets on day 4‐10 | |
| Outcomes | complete, incomplete, missed abortion, ongoing pregnancy, blood loss, haemoglobin levels | |
| Notes | no major complications were reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Tang 2003.
| Methods | computer generated random numbers; double blinded: women received placebo for vaginal application in the sublingual group; and placebo tablets for sublingual application in the vaginal group | |
| Participants | 224 women, average age 23 years, </= 9 weeks of gestation; average gestational age 7.7 weeks; gestational age confirmed by ultrasound; exclusion criteria: using prescription drugs regularly, IUD in situ, breatsfeeding, multiple pregnancies and heavy smoking. Study conducted at University of Hong Kong. | |
| Interventions | mifepristone 200mg followed 48 hours later by: group1: misoprostol 800mcg sublingual group 2:misoprostol 800mcg vaginal |
|
| Outcomes | complete/incomplete abortion, ongoing pregnancy; haemoglobin concentration; days of bleeding; induction‐abortion intervall, side effects | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A‐adequate |
von Hertzen 2003.
| Methods | see Honkanen 2004 | |
| Participants | ||
| Interventions | ||
| Outcomes | complete, incomplete abortion | |
| Notes | ||
von Hertzen 2007.
| Methods | centrally; random permuted blocs of 10;sealed, sequentially labeled envelopes | |
| Participants | 2066 women; average age 27 years; inclusion criteria: haemoglobin > 95g/L, gestational age </= 63 days, willing to have surgical procedure in case of failure, no serious illnesses, no contraindications for use of study medication, no uterine or cervical scars, no: uncontrolled asthma, hypertension, valvular heart disease, IUD in situ, history of thromboembolism or hemolytic disease, sickle cell anemia or liver disease. Gestational age confirmed by ultrasound. Study conducted at 11 obstetrics and gynaecologic teaching departments in 6 coutries (Armenia, Cuba, Georgia, India, Mongolia, Viet Nam) | |
| Interventions | Misoprostol 3 doses of 800mcg each, in the manner of one of the following: group 1:sublingual every 3 h group 2: sublingual every 12 h group 3: vaginal every 3 h group 4: vaginal every 12 h |
|
| Outcomes | complete, incomplete abortion, side effects | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ adequate |
von Hertzen 2009.
| Methods | computer generated, central randomisation, random permutation in groups of 8, stratified by gestational age; sealed, opaque sequentially numbered envelopes | |
| Participants | 2181 women; gestational age </=63 days, inclusion criteria: haemoglobin >100g/L, willing to have surgical abortion for failure, agreed to return for follow‐up. exclusion criteria: ill health, contraindications to study medication, severe uncontrolled asthma, porphyria, valvular heart disease, smoking and another risk for CV disease, glaucoma, thromboembolism, liver disease, IUD in situ, breastfeeding, haemolytic disorders. Gestational age confirmed by ultrasound. Study conducted between 2003‐2005 at 13 departments of obstetrics and gynaecology in nine countries (China, Hungary, India, Mongolia, Romania, Slovenia, South Africa, Viet Nam, Serbia) | |
| Interventions | group 1: mifepristone 100 mg and 24 h later misoprostol 800 mcg vaginal group 2: mifepristone 100 mg and 48 h later misoprostol 800 mcg vaginal group 3: mifepristone 200 mg and 24 h later misoprostol 800mcg vaginal group 4: mifepristone 200 mg and 48 h later misoprostol 800mcg vaginal follow up at 2 and 6 weeks |
|
| Outcomes | complete , incomplete, missed abortion, side effects | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A‐adequate |
Wang 2000.
| Methods | women were randomly divided into 2 groups by 2:1 ratio | |
| Participants | Multicentre trial in 9 hospitals in Hebei,China; 1612 pregnant women ≤ 49 days of amenorrhoea, confirmed by ultrasound; without clinical or haematological abnormalities,contraindication for the study medication or IUD in situ. | |
| Interventions | group 1: day 1: mifepristone 50 mg/po 12 hours apart (= total of 100 mg) day 2 to day 7: mifepristone 25 mg/po daily (= total of 250 mg) day 3: misoprostol 600 mcg/po day 4 to day 6: misoprostol 200 mcg daily (= total of 600 mcg) group 2: day 1: mifepristone 50 mg/po then 25 mg/12 hourly/4 times (= total of 150 mg) day 3: misoprostol 600 mcg/po | |
| Outcomes | complete/incomplete abortion, duration of bleeding, resuming of menses, side effects | |
| Notes | post‐randomisation exclusion, protocol deviation, loss to follow‐up not mentioned no mentioning of major complications | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
WHO 2000 M400po.
| Methods | computer generated random numbers, | |
| Participants | multicentre trial: Beijing, Havana, Helsinki, Ho Chi Min City, Hong Kong, Ljubljana, Melbourne, Moscow, Mumbai, Shanghai, Stockholm, St Petersburg, Szeged, Tbilisi, Tianjin, Tunis, Yerevan, 1589 women ≤ 63 days of amenorrhoea, with positive pregnancy test and uterine size consistent with menstrual history exclusion criteria: contraindications for study drug use, history of thromboembolism, liver disease, regular use of prescription drugs, intrauterine device, suspected ectopic pregnancy, heavy cigarette smoking, breastfeeding, irregular menses | |
| Interventions | group 1: mifepristone 200 mg/po group 2: mifepristone 600 mg/po both groups received misoprostol 400 mcg/po after 48 hours | |
| Outcomes | complete/incomplete/missed/unclassified failed abortion, side effects | |
| Notes | identical placebos, identical pill bottles; power calculation (90% power, significance level of 0.05) no major complications were reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
WHO 2001 GP1pv.
| Methods | computer generated sequence of random numbers in block of ten, identical placebo tablets | |
| Participants | multicentre trial, 10 centres: Chandigarh, Edinburgh, Havana, Hong Kong, Ljubljana, Shanghai, Stockholm, Szeged, Tbilisi, Tianjin 896 women, at 57 to 63 days of gestation with regular menstrual cycles, pregnancy confirmed clinically or by ultrasound exclusion criteria: contraindication to the study drugs, chronic respiratory, digestive, endocrine, genito‐urinary, neurological or cardio‐vascular disease, severe liver disease, history of thrombo‐embolism, IUCD in situ, breastfeeding | |
| Interventions | group 1: mifepristone 200 mg group 2: mifepristone 600 mg and gemeprost 1 mg after 48 hours (all) | |
| Outcomes | complete, incomplete, missed abortion, time to onset of bleeding, duration of bleeding, time to return to menses, bleeding before gemeprost, time of expulsion | |
| Notes | power calculation ( 80% power at a significant level of 0.05 ) intention ‐to ‐treat analysis 2 women received blood transfusion, not mentioned which group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
WHO 1989.
| Methods | randomly allocated 10/261 post‐randomisation exclusions: 2: cycle length < 25 days 6: > 49 days pregnant 1: pregnancy not confirmed 1: wrongly randomised 1 woman was lost to follow‐up (group 2) | |
| Participants | Multicentre, Hospitals in Aberdeen, Milan, New Delhi, Shanghai, Singapore, Stockholm, Szeged 261 pregnant women, ≤ 35 years, ≤ 49 days of amenorrhoea confirmed by ultrasound and beta‐HCG if US inconclusive inclusion criteria: regular cycles (25‐35 days) for last 3 months exclusion criteria: unsure about dates, intrauterine device in situ, hormonal contraception during last cycle and intention to start hormonal contraception before first period after abortion | |
| Interventions | group 1: mifepristone 25mg/twice daily for 3 days and sulprostone0.25 mg /intramuscular/ on third day a.m. group 2: mifepristone 25mg /twice daily for 4 days and sulprostone0.25 mg /intramuscular/ on fourth day a.m. | |
| Outcomes | complete, and incomplete abortion failure (intact amniotic sac on follow‐up at 2 weeks) undetermined outcome hormone levels (beta‐HCG, estradiol, prolactin, cortisol, prostaglandin) | |
| Notes | 2 women received blood transfusion; not mentioned which group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
WHO 1991.
| Methods | randomisation at WHO, using random permutation block technique with block size of 8, random numbers were provided to each centre in a sealed envelope | |
| Participants | multicentre; 10 mostly academic hospitals: Aberdeen, Havana, Hong Kong, Ljubljana, Milan, Shanghai, Singapore, Stockholm, Szeged, Wuhan. 385 women were randomised. inclusion criteria: amenorrhoea ≤ 49 days, regular cycles (25‐35 days) for last 3 months exclusion criteria: unsure about dates, intrauterine device in situ, hormonal contraception during last cycle and intention to start hormonal contraception before first period after abortion |
|
| Interventions | group 1: mifepristone 25mg/12 hourly/ 5 doses and gemeprost 1mg/vaginally 60 hours after the start of the treatment group 2: mifepristone 600mg/single dose and gemeprost 1mg/vaginally 60 hours after the start of the treatment | |
| Outcomes | complete, incomplete, missed abortion, continuing pregnancy, side effects, bleeding pattern, haemoglobin and hormone levels | |
| Notes | 1 woman received blood transfusion; not mentioned which group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
WHO 1993 GP1pv.
| Methods | randomisation at WHO, using random permutation block technique with block size of 9, tablets were disposed into labelled bottles, placebos were added to women receiving the lower dose so that all received 3 tablets) | |
| Participants | multicentre, Hospitals in Aberdeen, Edinburgh, Havana, Hong Kong, Ljubljana, Milan, Shanghai, Stockholm, Szeged, Tianjin, Wuhan 1182 pregnant women with a menstrual delay of 7‐28 days inclusion criteria: regular cycles (25‐35 days) for last 3 months, pregnancy confirmed by ultrasound exclusion criteria: unsure about dates, intrauterine device in situ, hormonal contraception during last cycle and intention to start hormonal contraception before first period after abortion, contraindication to mifepristone/misoprostol, regular use of prescribed drugs | |
| Interventions | group 1: mifepristone 200mg/oral group 2: mifepristone 400mg/oral group 3: mifepristone 600mg/oral and prostaglandin 1mg/vaginally after 48 hours (all) | |
| Outcomes | complete, incomplete, missed abortion, continuing pregnancy, side effects, haemoglobin levels, side effects | |
| Notes | 3 women received blood transfusion; not mentioned which group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
WHO 2001 MI200/50.
| Methods | computer generated number sequence | |
| Participants | multicentre trial, 13 centres: Aberdeen, Chandigarh, Edinburgh, Havana, Hong Kong, Ljubljana, Lusaka, Shanghai, Singapore, Stockholm, Szeged, Tbilisi, Tianjin 1224 women <57 days pregnant inclusion criteria: regular cycles, no hormonal contraception or IUD use before first menses after abortion exclusion criteria: medical contraindication for the study medication, history of thromboembolism, liver disease, pruritus in pregnancy, IUD in situ, breastfeeding, heavy smokers | |
| Interventions | group 1: mifepristone 50mg/po and gemeprost 0.5mg/pv on day 3 group 2: mifepristone 50mg/po and gemeprost 1.0mg/pv on day 3 group 3: mifepristone 200mg/po and gemeprost 0.5mg/pv on day 3 group 4: mifepristone 200mg/po and gemeprost 1.0mg/pv on day 3 | |
| Outcomes | complete /incomplete/missed abortion, side effects | |
| Notes | group 1: was discontinued as interim analysis showed below cut‐off results. no blinding for gemeprost 7 women received blood transfusion (2 group 1, 2 group 2, 1 group 3, 2 group4) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Wiebe 1999.
| Methods | computer generated list of random numbers, sealed, opaque envelopes | |
| Participants | 398 women, ≤ 7 weeks pregnant confirmed by ultrasound, University Hospital Vancouver, Canada exclusion criteria: abnormal haematologic parameters | |
| Interventions | Phase 1: group 1: Tamoxifen 40mg/po and 800mcg misoprostol/pv > 48 hours group 2: Methotrexate 50mg/m2 and misoprostol 800mcg/pv >96 hours Phase 2: group 1: Tamoxifen 40 mg/day for 4 days (= total dose of 160mg) and misoprostol 800mcg/pv > 48 hours group 2: Methotrexate 50 mg/m2 and misoprostol 800mcg/pv >96 hours | |
| Outcomes | failure rate, side effects, women's preference | |
| Notes | no major complications were reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Wiebe 1999 A.
| Methods | see Wiebe 1999 | |
| Participants | see Wiebe 1999 | |
| Interventions | Phase 2: group 1: Tamoxifen 40 mg/day for 4 days (= total dose of 160mg) and misoprostol 800 mcg/pv > 48 hours group 2: Methotrexate 50 mg/m2 and misoprostol 800mcg/pv >96 hours | |
| Outcomes | see Wiebe 1999 | |
| Notes | see Wiebe 1999 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Wiebe 1999 B.
| Methods | computer generated list of random numbers, sealed, opaque envelopes | |
| Participants | 100 women, ≤ 7 weeks pregnant confirmed by ultrasound, University Hospital Vancouver, Canada exclusion criteria: abnormal haematologic parameters, systemic disease, intolerance to study medication | |
| Interventions | group 1: methotrexate 50 mg/m2/po and misoprostol 600mcg/pv > 96 hours group 2: methotrexate 50 mg/m2/imi and misoprostol 600mcg/pv > 96 hours | |
| Outcomes | complete, incomplete abortion, side effects | |
| Notes | only data from phase 1 are included, phase 2 was non‐random no major complications were reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Wiebe 2004.
| Methods | computer generated random list; sealed opaque envelopes | |
| Participants | 309 women at </=7 weeks of gestation confirmed by ultrasound; average age 27 years; average gestational age 42 days. Exclusion criteria: haemoglobin<9.5 g/L, seizure disease ,active liver disease, renal insufficiency, allergy/ intolerance to study medication. Study conducted at University of British Colombia, Canada |
|
| Interventions | methotrexate 50 mg/m2 followed 72‐ 144 hours later by: group 1: misoprostol 600mcg buccal (insert between their cheeks and leave for 1 h) group2: misoprostol 600mcg vaginal both groups instructed to repeat the dose 24 hours later if no heavy bleeding had occurred |
|
| Outcomes | successful abortion; side‐effects; acceptability | |
| Notes | women with ongoing pregnancy at day 8 follow‐up received 1‐2 more doses of misoprostol | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ adequate |
Wiebe 2006.
| Methods | 'randomised' | |
| Participants | 300 women with</= 7 weeks gestation confirmed by ultrasound. Patient characteristics not reported ('similar between groups') | |
| Interventions | group 1: methotrexate 50mg/ m2 followed >/ 72 hours by misoprostol 400mcg vaginally group 2: misoprostol 400mcg sublingual AND 400 mcg vaginal |
|
| Outcomes | complete abortion, side effects, acceptability | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Winikoff 2008.
| Methods | computer generated random assignment; random blocs of eight | |
| Participants | 966 women > 18 years old were enrolled. no contraindication to study medication, ≤ 63 days since LMP, access to telephone and emergency transportation. Geatational age confirmed by ultrasound if needed. Between September 2006 ‐ May 2007. Study conducted at seven family planning centres: New York, Chicago, Pittsburgh, Waco, Austin, Boston; USA. | |
| Interventions | mifepristone 200mcg followed 24‐26 hours later at home by: 1) misoprostol 800mg orally, 2) 800mg misoprostol buccal (2X200mg in each cheek to keep for 30 minutes and swallow the remnants) | |
| Outcomes | ||
| Notes | results presented as per protocol analysis; successful abortion was defined as: without need for surgical intervention, regardless of how many doses of misoprostol needed. 14 women in the bucacal group and 13 in the oral group received a second dose of misoprostol. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ sealed opaque envelopes |
Wu 1993.
| Methods | randomisation sequence generated centrally | |
| Participants | multicentre trial in 5 hospitals in Beijing, China 990 women ≤ 49 days of amenorrhoea, pregnancy confirmed by ultrasound, without medical disorders, contraindication for the study medication and IUD in situ | |
| Interventions | group 1: day 1: mifepristone 200mg and tamoxifen 40 mg/po day 2: tamoxifen 40mg/po day 3: PGF2alpha /pv group 2: day 1: mifepristone 200mg and placebo/po day 2: placebo /po day 3: PGF2 alpha/vaginally | |
| Outcomes | complete, incomplete abortion, duration of bleeding, resuming of menses, side effects | |
| Notes | 58/990 women were excluded post‐randomisation due to protocol violation no major complications were reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Zheng 1989 MI600PGF2pv.
| Methods | publication includes 4 studies, 1 of them is a randomised trial, randomisation procedure not stated. | |
| Participants | 192 women, ≤ 49 days of pregnancy seeking abortion in China inclusion/exclusion criteria not stated Follow‐up on day 8 or day 14 | |
| Interventions | group 1: mifepristone 600mg group 2: mifepristone 600mg and prostaglandin F2alpha 1mg/pv | |
| Outcomes | complete and incomplete abortion, ongoing pregnancy, time until passing of conceptus | |
| Notes | only data from trial 4 are included no mentioning of major complications | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ashok 2002 | single cohort, no comparison group |
| Aubeny 2000 | randomisation by day of admission |
| Cheng 1999 | women up to 16 weeks of gestation are included |
| Creinin 1996 A | single cohort, no comparison group |
| Davis 1999 | Data for one group (Methotrexate) was reported for all (randomised and non‐randomised) women together |
| De Nonno 2000 | not RCT |
| ICMR 2000 | allocation concealment and randomisation not stated |
| Jacobson 1990 | This study was not designed to achieve abortion: only to test an existing regimen for treatment of ulcer and its effect on early pregnancy |
| Martin 1998 | intervention not in the scope of the review (oral contraceptives or methotrexate to shorten the duration of bleeding) |
| Ngai 2000 | intervention not in the scope of the review (water and misoprostol compared to misoprostol alone) |
| Norman 1992 | non‐randomised and randomised outcomes presented together |
| Swahn 1994 | single cohort, no comparison group |
| Tang 1999 | intervention not in the scope of the review (oral contraceptives vs palcebo for effectiveness, bleeding duration) |
| Wiebe 2001 | review |
Contributions of authors
RK had the idea and wrote the review. RK and NK did the data extraction. NK, AMG, GJH, CLN and AC reviewed and contributed substantially in all aspects of the review.
Sources of support
Internal sources
Effective Care Research Unit, University of the Witwatersrand, South Africa.
HRP‐ UNDP/UNFPA/WHO/WORLD BANK Special Programme in Human Reproduction, Geneva, Switzerland.
External sources
No sources of support supplied
Declarations of interest
None
Edited (no change to conclusions)
References
References to studies included in this review
Arvidsson 2005 {published data only}
- Arvidsson C, Hellborg M, Gemzell‐Daniellson K. Preference and acceptability of oral versus vaginal administrationof misoprostol in medical abortion with mifepristone. European Journal of Obstetrics & Gynecology and Reproductive Biology 2005;123:87‐91. [DOI] [PubMed] [Google Scholar]
Baird 1995 GP0.5 M600po {published data only}
- Baird DT, Sukcharoen N, Thong KJ. Randomized trial of misoprostol and cervagem in combination with a reduced dose of mifepristone for induction of abortion. Human Reproduction 1995;10(6):1521‐1527. [DOI] [PubMed] [Google Scholar]
Bartley 2001 GP0.5M800pv {published data only}
- Bartley J, Brown A, Elton R, Baird DT. Double‐blind randomized trial of mifepristone in combination with vaginal gemeprost or misoprostol for induction of abortion up to 63 days gestation. Human Reproduction 2001;16(10):2098‐2102. [DOI] [PubMed] [Google Scholar]
Birgerson 1988 {published data only}
- Birgerson L, Odlind V. The antiprogestational agent RU 486 as an abortifacient in early human pregnancy: a comparison of three dose regimens. Contraception 1988;38(4):391‐400. [DOI] [PubMed] [Google Scholar]
Blanchard 2005 {published data only}
- Blanchard K, Tara Shochet T, Coyajic K, Ngoc N, Winikoff B. Misoprostol alone for early abortion: an evaluation of seven potential regimens. Contraception 2005;72:91‐97. [DOI] [PubMed] [Google Scholar]
Cameron 1986 MI600GP1pv {published data only}
- Cameron IT, Michie AF, Baird DT. Therapeutic abortion in early pregnancy with antiprogestogen RU 486 alone or in combination with prostaglandin analogue (gemeprost). Contraception 1986;34(5):459‐468. [DOI] [PubMed] [Google Scholar]
Carbonell 1997 M800pv {published data only}
- Carbonell JL, Velazco A, Varela L, Cabezas E, Fernandez C, Sanchez C. Misoprostol 3,4, or 5 days after methotrexate for early abortion. Contraception 1997;56:169‐174. [DOI] [PubMed] [Google Scholar]
Carbonell 1998 M800pv {published data only}
- Carbonell JLL, Varela L, Velazco A, Cabezas E, Fernandez C, Sanchez C. Oral methotrexate and vaginal misoprostol for early abortion. Contraception 1998;57:83‐88. [DOI] [PubMed] [Google Scholar]
Cheng 1994 PGE1&T {published data only}
- Cheng LN, Zhou YF, Song JY, Li H, Chen JK. A randomized clinical trial in comparison of termination of early pregnancy by 16,16‐dimethyl‐trans PGE1 ester alone or in combination with testosterone propionate. Sheng zhi yu bi yun (Reproduction and Contraception) 1994;1:29‐33. [Google Scholar]
Coyaji 2007 {published data only}
- Coyaji K, Krishna U, Ambardekar S, Bracken H, Raote V, Mandlekar A, Winikoff B. Are two doses of misoprostol after mifepristone for early abortion better than one?. BJOG 2007;114:271–278. [DOI] [PubMed] [Google Scholar]
Creinin 1994 M800&MT {published data only}
- Creinin DM, Vittinghoff E. Methotrexate and misoprostol vs misoprostol alone for early abortion. JAMA 1994;272(15):1190‐1195. [PubMed] [Google Scholar]
Creinin 1995 M800pv {published data only}
- Creinin MD, Vittinghoff E, Galbraith S, Klaisle C. A randomized trial comparing misoprostol three and seven days after methotrexate for early abortion. American Journal of Obstetrics and Gynecology 1995;173:1578‐1584. [DOI] [PubMed] [Google Scholar]
Creinin 1996 M800pv {published data only}
- Creinin MD. Oral methotrexate and vaginal misoprostol for early abortion. Contraception 1996;54:15‐18. [DOI] [PubMed] [Google Scholar]
Creinin 1997 M800pv {published data only}
- Creinin MD, Krohn MA. Methotrexate pharmacokinetics and effects in women receiving methotrexate 50 mg and 60 mg per square meter for early abortion. American Journal of Obstetrics and Gynecology 1997;177:1444‐1449. [DOI] [PubMed] [Google Scholar]
Creinin 2001 {published data only}
- Creinin MD, Pymar HC, Schwartz JL. Mifepristone 100 mg in abortion regimens. Obstetrics and Gynecology 2001;98(3):434‐439. [DOI] [PubMed] [Google Scholar]
Creinin 2001 MI600 M400 {published data only}
- Creinin MD, Schwartz JL, Pymar HC, Fink W. Efficacy of mifepristone followed on the same day by misoprostol for early termination of pregnancy: report of a randomised trial. British Journal of Obstetrics and Gynaecology 2001;108:469‐473. [DOI] [PubMed] [Google Scholar]
Creinin 2004 {published data only}
- Creinin MD, Fox MC, Teal S, Chen A, Schaff EA, Meyn LA. A randomized comparison of misoprostol 6 to 8 hours versus 24 hours after mifepristone for abortion. Obstetrics and Gynecology 2004;103:851‐859. [DOI] [PubMed] [Google Scholar]
Creinin 2007 {published data only}
- Creinin MD, Schreiber CA, Bednarek P, Lintu H, Wagner MS, Meyn LA. Mifepristone and misoprostol administered simultaneously versus 24 hours apart for abortion. Obstetrics and Gynecology 2007;109:885‐894. [DOI] [PubMed] [Google Scholar]
El‐Refaey 1994 {published data only}
- El‐Refaey H, Templeton A. Early abortion induction by a combination of mifepristone and oral misoprostol: a comparison between two dose regimens of misoprostol and their effect on blood pressure. British Journal of Obstetrics and Gynaecology 1994;101:792‐796. [DOI] [PubMed] [Google Scholar]
El‐Refaey 1995 M800MI600 {published data only}
- El‐Refaey H, Rajasekar D, Abdalla M, Calder L, Templeton A. Induction of abortion with mifepristone (RU 486) and oral or vaginal misoprostol. New England Journal of Medicine 1995;332:983‐987. [DOI] [PubMed] [Google Scholar]
Guest 2007 {published data only}
- Guest J, Chien PFW, Thomson MAR, Kosseim ML. Randomised controlled trial comparing the efficacy of same‐day administration ofmifepristone and misoprostol for termination of pregnancy with the standard 36 to48 hour protocol. BJOG An International Journal of Obstetrics and Gynaecology 2007;114:207‐215. [DOI] [PubMed] [Google Scholar]
Hamoda 2005 {published data only}
- Hamoda H, Ashok PW, Flett GMM, Templeton A. A randomised controlled trial of mifepristone in combination with misoprostol administered sublingually or vaginally for medical abortion up to 13 weeks of gestation. BJOG: an International Journal of Obstetrics and Gynaecology 2005;112:1102–1108. [DOI] [PubMed] [Google Scholar]
Honkanen 2004 {published data only}
- Honkanen H, Piaggio G, Hertzen H, Bartfai G, Erdenetungalag R, Gemzell‐Danielsson K, Gopalan S, Horga M, Jerve F, Mittal S, Thi Nhu Ngoc N, Peregoudov A, Prasad R, Pretnar‐Darovec A, Shah R, Song S, Tang O, Wu S. WHO multinational study of three misoprostol regimens after mifepristone for early medical abortion.II: Side effects and women’s perceptions. BJOG: an International Journal of Obstetrics and Gynaecology 2004;111:715‐725. [DOI] [PubMed] [Google Scholar]
Jain 2002 M800&MI {published data only}
- Jain JK, Dutton C, Harwood B, Meckstroth KR, Mishell DR. A prospective randomized, double‐blinded, placebo‐controlled trial comparing mifepristone and vaginal misoprostol to vaginal misoprostol alone for elective termination of early pegnancy. Human Reproduction 2002;17(6):1477‐1482. [DOI] [PubMed] [Google Scholar]
Jain 1999 M800&TM {published data only}
- Jain JK, Meckstroth KR, Park M, Mishell DR Jr. A comparison of tamoxifen and misoprostol to misoprostol alone for early pregnancy termination. Contraception 1999;60(6):353‐356. [DOI] [PubMed] [Google Scholar]
Koopersmith 1996 {published data only}
- Koopersmith TB, Mishell DR. The use of misoprostol for termination of early pregnancy. Contraception 1996;53:237‐242. [DOI] [PubMed] [Google Scholar]
Liao 2004 {published data only}
- Liao A, Han XJ, Wu SY, Xiao DZ, Xiong CL, Wu XR. Randomized, double‐blind, controlled trial of mifepristone incapsule versus tablet form followed by misoprostol for early medical abortion. European Journal of Obstetrics & Gynecology and Reproductive Biology 2004;116:211‐216. [DOI] [PubMed] [Google Scholar]
McKinley 1993 M600po {published data only}
- McKinley C, Thong KJ, Baird DT. The effect of dose of mifepristone and gestation on the efficacy of medical abortion with mifepristone and misoprostol. Human Reproduction 1993;8(9):1502‐1505. [DOI] [PubMed] [Google Scholar]
Middleton 2005 {published data only}
- Middleton T, Schaff E, . Fielding SL, Scahill M, Shannon C, Westheimer E, Wilkinson T, Winikoff B. Randomized trial of mifepristone and buccal or vaginal misoprostol for abortion through 56 days of last menstrual period. Contraception 2005;72:328‐332. [DOI] [PubMed] [Google Scholar]
Ozeren 1999 MP800&MT {published data only}
- Ozeren M, Bilekli C, Aydemir V, Bozkaya H. Methotrexate and misoprostol used alone or in combination for early abortion. Contraception 1999;59:389‐394. [DOI] [PubMed] [Google Scholar]
Raghavan 2009 {published data only}
- Raghavana S, Comendant R, Digol I, Ungureanu S, Friptu V, Bracken H, Winikoff B. Two‐pill regimens of misoprostol after mifepristone medical abortion through 63 days' gestational age: a randomized controlled trial of sublingual and oral misoprostol. Contraception 2009;79:84‐90. [DOI] [PubMed] [Google Scholar]
Rodger 1989 MI600 {published data only}
- Rodger MW, Logan AF, Baird DT. Induction of early abortion with mifepristone (RU 486) and two different doses of prostaglandin pessary (gemeprost). Contraception 1989;39(5):497‐502. [DOI] [PubMed] [Google Scholar]
Sandstrom 1999 MI600GP1pv {published data only}
- Sandstrom O, Brooks L, Schantz A, Grinsted J, Grinsted L, Jacobsen JD, Nielsen SP. Interruption of early pregnancy with mifepristone in combination with gemeprost. Acta Obstet Gynecol Scand 1999;78:806‐809. [PubMed] [Google Scholar]
Sang 1994 M600poPGF2pv {published data only}
- Sang GW, Weng LJ, Shao QX, Du MK, Wu XZ, Lu YL, Cheng LN. Termination of early pregnancy by two regimens of mifepristone with misoprostol and mifepristone with PG05 ‐ a multicentre randomized clinical trial in China. Contraception 1994;50:501‐510. [DOI] [PubMed] [Google Scholar]
Sang 1999 M600poPGF2pv {published data only}
- Sang GW, He CH, Shao QX, Zhuang LQ, Weng LJ, Wu BX, Gao ES, Jiang HY, Mei QM. A large scale introductory trial on termination of early pregnancy by mifepristone in combination with different prostaglandins. Chinese Journal for Clinical Pharmacology 1999;15(5):323‐329. [Google Scholar]
Schaff 2001 M800MI200 {published data only}
- Schaff EA, Fielding SL, Westhoff C. Randomized trial of oral versus vaginal misoprostol at one day after mifepristone for early medical abortion. Contraception 2001;64(2):81‐85. [DOI] [PubMed] [Google Scholar]
Schaff 2000 MI200M800 {published data only}
- Schaff EA, Fielding SL, Westhoff C, Ellertson C, Eisinger SH, Stadalius LS, Fuller L. Vaginal misoprostol administered 1,2 or 3 days after mifepristone for early medical abortion. JAMA 2000;284(15):1948‐1953. [DOI] [PubMed] [Google Scholar]
Shannon 2006 {published data only}
- Shannon C, Wiebe E, Jacot F, Guilbert E, Dunn S, Sheldon W, Winikoff B. Regimens of misoprostol with mifepristone for early medical abortion: a randomised trial.. BJOG 2006;113:621–628. [DOI] [PubMed] [Google Scholar]
Swahn 1989 MI200MP1po {published data only}
- Swahn ML, Ugocsai G, Bygdeman M, Kovacs L, Belsey EM, Look PFA. Effect of oral prostaglandin E2 on uterine contractility and outcome of treatment in women receiving RU 486 (mifepristone) for termination of early pregnancy. Human Reproduction 1989;4(1):21‐28. [DOI] [PubMed] [Google Scholar]
Tang 2002 {published data only}
- Tang OS, Lee SWH, Ho PC. A prospective randomized study on the measured blood loss in medical termination of early pergnancy by three different misoprostol regimens after pretreatment with mifepristone. Human Reproduction 2002;17(11):2865‐2868. [DOI] [PubMed] [Google Scholar]
Tang 2003 {published data only}
- Tang OS, Chan CCW, Ng EHY, Lee SWH, Ho PC. A prospective, randomized, placebo‐controlled trial on the use of mifepristone with sublingual or vaginal misoprostol for medical abortions of less than 9 weeks gestation. Human Reproduction 2003;18:2315‐2318. [DOI] [PubMed] [Google Scholar]
von Hertzen 2003 {published data only}
- Hertzen H, Honkanen H, Piaggio G, Bartfai G, Erdenetungalag R, Gemzell‐Danielsson K, Gopalan S, Horga M, Jerve F, Mittal S, Thi Nhu Ngoc N, Peregoudov A, Prasad R, Pretnar‐Darovec A, Shah R, Song S, Tang O, Wu S. WHO multinational study of three misoprostol regimens after mifepristone for early medical abortion. I: Efficacy. BJOG: an International Journal of Obstetrics and Gynaecology 2003;110:808‐818. [DOI] [PubMed] [Google Scholar]
von Hertzen 2007 {published data only}
- Hertzen H, Piaggio G, Huong N, Arustamyan K, Cabezas E, Gomez M, Khomassuridze A, Shah R, Mittal S, Nair R, Erdenetungalag R, Huong T, Vy N, Phuong N, Tuyet H, Peregoudov A, on behalf of the WHO Research Group on Postovulatory Methods of Fertility Regulation. Efficacy of two intervals and two routes of administration of misoprostol for termination of early pregnancy:a randomised controlled equivalence trial. Lancet 2007;369:1938‐1946. [DOI] [PubMed] [Google Scholar]
von Hertzen 2009 {published data only}
- Hertzen H, Piaggio G, Wojdyla D, Marions L, My Huong NT, Tang OS, Fang AH, Wu SC, Kalmar L, Mittal S, Erdenetungalag R, Horga M, Pretnar‐Darovec A, Kapamadzija A, Dickson K, Anh ND, Tai NV, Tuyet HTD, Peregoudov A for the WHO Research Group on Post‐ovulatory Methods of FertilityRegulation. Two mifepristone doses and two intervals of misoprostol administration for termination of early pregnancy: a randomised factorial controlled equivalence trial. BJOG 2009;116:381–389. [DOI] [PubMed] [Google Scholar]
Wang 2000 {published data only}
- Wang ZH. Prevent from bleeding after medical abortion through prolonged application of mifepristone with misoprostol for terminating early pregnancy. Zhonghua Fu Chan Ke Za Zhi 2000;35(9):554‐557. [PubMed] [Google Scholar]
WHO 2000 M400po {published data only}
- WHO Task Force on Post‐ovulatory Methods of Fertility Regulation. Comparison of two doses of mifepristone in combination with misoprostol for early medical abortion: a randomised trial. British Journal of Obstetrics and Gynaecology 2000;107:524‐530. [DOI] [PubMed] [Google Scholar]
WHO 2001 GP1pv {published data only}
- World Health Organization Task Force on Post‐ovulatory Methods of Fertility Regulation. Medical abortion at 57 to 63 days' gestation with a lower dose of mifepristone and gemeprost. Acta Obstetrica et Gynecologica Scandinavica 2001;80:447‐451. [PubMed] [Google Scholar]
WHO 1989 {published data only}
- WHO Task Force on Post‐Ovulatory Methods for Fertility Regulation. Termination of early human pregnancy with RU 486 (mifepristone) and the prostaglandin analogue sulprostone: a multi‐centre, randomized comparison between two treatment regimens. Human Reproduction 1989;4(6):718‐725. [DOI] [PubMed] [Google Scholar]
WHO 1991 {published data only}
- WHO Task Force on Post‐Ovulatory Methods for Fertility Regulation. Pregnancy termination with mifepristone and gemeprost: a multicenter comparison between repeated doses and a single dose of mifepristone. Fertility and Sterility 1991;56:32‐40. [DOI] [PubMed] [Google Scholar]
WHO 1993 GP1pv {published data only}
- WHO Task Force on Post‐Ovulatory Methods of Fertility Regulation. Termination of Pregnancy with reduced doses of mifepristone. British Medical Journal 1993;307:532‐537. [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 2001 MI200/50 {published data only}
- WHO Task Force on Post‐ovulatory Methods for Fertility Regulation. Lowering the doses of mifepristone and gemeprost for early abortion: a randomised controlled trial. British Journal of Obstetrics and Gynaecology 2001;108:738‐742. [PubMed] [Google Scholar]
Wiebe 1999 {published data only}
- Wiebe ER. Tamoxifen compared to methotrexate when used with misoprostol for abortion. Contraception 1999;59:265‐270. [DOI] [PubMed] [Google Scholar]
Wiebe 1999 A {published data only}
- Wiebe ER. Tamoxifen compared to methotrexate when used with misoprostol for abortion. Contraception 1999;59:265‐270. [DOI] [PubMed] [Google Scholar]
Wiebe 1999 B {published data only}
- Wiebe ER. Oral methotrexate compared with injected methotrexate when used with misoprostol for abortion. Am J Obstet Gynecol 1999;181:149‐152. [DOI] [PubMed] [Google Scholar]
Wiebe 2004 {published data only}
- Wiebe ER, Trouton K. Comparing vaginal and buccal misoprostol when used after methotrexate for early abortion. Contraception 2004;70:463‐466. [DOI] [PubMed] [Google Scholar]
Wiebe 2006 {published data only}
- Wiebe ER, Trouton KJ, Lima R. Misoprostol alone vs. methotrexate followed by misoprostol for early abortion. International Journal of Gynecology and Obstetrics 2006;95:286‐287. [DOI] [PubMed] [Google Scholar]
Winikoff 2008 {published data only}
- Winikoff B, Dzuba IG, Creinin MD, Crowden WA, Goldberg AB, Gonzales J, Howe M, Moskowitz J, Prine L, Shannon CS. Two distinct oral routes of misoprostol inmifepristone medical abortion: a randomized controlled trial. Obstet Gynecol 2008;112(6):1303‐1310. [DOI] [PubMed] [Google Scholar]
Wu 1993 {published data only}
- Wu YM, Li Y, Fan HM, Zhu XJ, Zheng S, Wang S, Liu B, Yan J, Lin H, Fang J. Clinical study of mifepristone in combination with tamoxifen and 15‐methyl‐PGF2alpha methylester for termination of early pregnancy. Sheng Zhi Yi Xue Za Zhi (Journal of reproductive medicine). 1993; Vol. 4:224‐227.
Zheng 1989 MI600PGF2pv {published data only}
- Zheng SR. RU486 (mifepristone): clinical trials in China. Acta Obstet Gynecol Scand Suppl 1989;149:19‐23. [PubMed] [Google Scholar]
References to studies excluded from this review
Ashok 2002 {published data only}
- Ashok PW, Templeton A, Wagaarchchi PT, Flett GM. Factors affecting the outcome of early medical abortion: a review of 4132 consecutive cases. BJOG 2002;109(11):1281‐1289. [DOI] [PubMed] [Google Scholar]
Aubeny 2000 {published data only}
- Aubeny E, Chatellier G. A randomized comparison of mifepristone and self‐administered oral or vaginal misoprostol for early abortion. European Journal of Contraception and Reproductive Health Care 2000;5(3):171‐176. [DOI] [PubMed] [Google Scholar]
Cheng 1999 {published data only}
- Cheng L. Termination of 10‐16 weeks' gestation with mifepristone plus misoprostol: a multicentre randomized clinical trial. Zhonghua Fu Chan Ke Za Zhi 1999;34(5):268‐271. [PubMed] [Google Scholar]
Creinin 1996 A {published data only}
- Creinin MD, Burke AE. Methotrexate and misoprostol for early abortion: a multicenter trial. Acceptability. Contraception 1996;54:19‐22. [DOI] [PubMed] [Google Scholar]
Davis 1999 {published data only}
- Davis AR, Miller L, Tamimi H, Gown A. Methotrexate compared with mercaptopurine for early induced abortion. Obstetrics & Gynecology 1999;93:904‐909. [DOI] [PubMed] [Google Scholar]
De Nonno 2000 {published data only}
- Nonno LS, Westhoff C, Fielding S, Schaff E. Timing of pain and bleeding after mifepristone induced abortion. Contraception 2000;62(6):305‐309. [DOI] [PubMed] [Google Scholar]
ICMR 2000 {published data only}
- Indian Council of Medical Research Task Force. A multicentre randomized comparative clinical trial of 200 mg RU486 (mifepristone) single dose followed by either 5 mg 9‐methylene PGE2 Gel (meteneprost) or 600 mcg oral PGE1 (misoprostol) for termination of early pregnancy within 28 days of missed menstrual period. Contraception 2000;62:125‐130. [PubMed] [Google Scholar]
Jacobson 1990 {published data only}
- Jacobson J, Bergquist C, Rydnert J, Bokstroem H, Huovinen K. No abortion‐inducing effect of the ulcer‐healing dose of the synthetic prostaglandin E2 analogue enprostil in first trimester. Acta Obstetrica Gynecologica Scandinavia 1990;69:135‐138. [DOI] [PubMed] [Google Scholar]
Martin 1998 {published data only}
- Martin CW, Brown AH, Baird DT. A pilot study of the effect of methotrexate or combined oral contraceptive on bleeding patterns after induction of abortion with mifepristone and a prostaglandin pessary. Contraception 1998;58(2):99‐103. [DOI] [PubMed] [Google Scholar]
Ngai 2000 {published data only}
- Ngai SW, Tang OS, Chan YM, Ho PC. Vaginal misoprostol alone for medical abortion up to 9 weeks of gestation: efficacy and acceptability. Human Reproduction 2000;15(5):1159‐1162. [DOI] [PubMed] [Google Scholar]
Norman 1992 {published data only}
- Norman JE, Thong KJ, Rodger MW, Baird DT. Medical abortion in women of <56 days amenorrhoea: a comparison between gemeprost (a PGE1 analogue) alone and mifepristone and gemeprost. British Journal of Obstetrics and Gynaecology 1992;99:601‐606. [DOI] [PubMed] [Google Scholar]
Swahn 1994 {published data only}
- Swahn ML, Kovacs L, Cekan SZ, Aedo AR, Westlund P. Termination of early pregnancy with ZK 98,734: pharmacokinetic behaviour and clinical effect. Human Reproduction 1994;9(1):57‐63. [DOI] [PubMed] [Google Scholar]
Tang 1999 {published data only}
- Tang OS, Gao PP, Cheng L, Lee SW, Ho PC. A randomized double‐blind placebo‐controlled study to assess the effect of oral contraceptive pills on the outcome of medical abortion with mifepristone and misoprostol. Human Reproduction 1999;14(3):722‐725. [DOI] [PubMed] [Google Scholar]
Wiebe 2001 {published data only}
- Wiebe ER. Misoprostol administration in medical abortion. A comparison of 3 regimens. Journal of Reproductive Medicine 2001;46(2):125‐129. [PubMed] [Google Scholar]
Additional references
Bachelot 1992
- Bachelot A, Cludy L, Spira A. Conditions for chosing between drug‐induced and surgical abortions. Contraception 1992;45:547‐559. [DOI] [PubMed] [Google Scholar]
Beaulieu 1997
- Beaulieu EE. RU 486 (Mifepristone) ‐ A short overview of its mechanisms of action and clinical uses at the end of 1996. Annals New York Academy of Sciences. New York Academy of Sciences, 1997:47‐58. [DOI] [PubMed] [Google Scholar]
Blanchard 1999
- Blanchard K, Winikoff B, Ellertson C. Misoprostol used alone for the termination of early pregnancy. Contraception 1999;59:209‐217. [DOI] [PubMed] [Google Scholar]
Bugalho 1996
- Bugalho A, Faundes A, Jamisse L, Usfa M, Maria E, Bique C. Evaluation of the effectiveness of vaginal misoprostol to induce first trimester abortion. Contraception 1996;53:243‐246. [DOI] [PubMed] [Google Scholar]
Bygdeman 1985
- Bygdeman M, Swahn ML. Progesterone receptor blockage. Effect on uterine contractility and early pregnancy. Contraception 1985;32:45‐51. [DOI] [PubMed] [Google Scholar]
Bygdeman 2002
- Bygdeman M, Danielsson KG. Options for early therapeutic abortion. Drugs 2002;62(17):2459‐2470. [DOI] [PubMed] [Google Scholar]
Carbonell 1997b
- Carbonell JLL, Varela L, Velazco A, Fernandez C. The use of misoprostol for termination of early pregnancy. Contraception 1997;55:165‐168. [DOI] [PubMed] [Google Scholar]
Costa 1998
- Costa SH. Commercial availability od misoprostol and induced abortion in Brazil. International Journal of Gynaecology and Obstetrics 1998;63 (suppl 1):S:131‐139. [DOI] [PubMed] [Google Scholar]
Creinin 1993
- Creinin MD, Darney PD. Methotrexate and misoprostol for early abortion. Contraception 1993;48:339‐348. [DOI] [PubMed] [Google Scholar]
Creinin 1996b
- Creinin MD, Burke AE. Methotrexate and misoprostol for early abortion: a multicenter trial. Acceptability. Contraception 1996;54:19‐22. [DOI] [PubMed] [Google Scholar]
Grimes 1997
- Grimes DA. Medical abortion in early pregnancy: a review of the evidence. Obstetrics Gynecology 1997;89:790‐796. [DOI] [PubMed] [Google Scholar]
Henshaw 1994
- Henshaw RC, Naji SA, Russell IT, Templeton AA. A comparison of medical abortion (using mifepristone and gemeprost) with surgical vacuum aspiration: efficacy and early medical sequelae. Human Reproduction 1994;9(11):2167‐2172. [DOI] [PubMed] [Google Scholar]
Honkanen 2002
- Honkanen H, Hertzen H. Users' perspectives on medical abortion in Finland. Contraception 2002;65(6):419‐23. [DOI] [PubMed] [Google Scholar]
Say 2002, updated 2010
- Say L, Brahmi D, Kulier R, Campana A, Gulmezoglu AM. Medical versus surgical methods for first trimester termination of pregnancy. Cochrane Database of Systematic Reviews 2002, Issue 4. [DOI: 10.1002/14651858.CD003037.pub2.] [DOI] [PubMed] [Google Scholar]
Sedgh 2007
- Sedgh G, Henshaw SK, Singh S, Bankole A, Drescher J. Legal abortion worldwide: incidence and recent trends. Int Fam Plan Perspect 2007;33(3):106‐16. [DOI] [PubMed] [Google Scholar]
United 1990
- United Kingdom Multicentre Trial. The efficacy and tolerance of mifepristone and prostaglandin in first trimester termination of pregnancy. Br J Obstet Gynaecol 1990;97:480. [DOI] [PubMed] [Google Scholar]
Urquhart 1997
- Urquhart DR, Templeton AA, Shinewi F, Chapman M, Hawkins K, McGarry J. The efficacy and tolerance of mifepristone and prostaglandin in termination of pregnancy of less than 63 days gestation: UK Multicentre Study ‐ final results. Contraception 1997;55:1‐5. [DOI] [PubMed] [Google Scholar]
WHO 1997
- WHO Scientific Group. WHO Technical Report Series. WHO. Vol. 871, Geneva: WHO, 1997. [Google Scholar]
Winikoff 1997
- Winikoff B, Irving S, Coyaji K, Caberas E, Bilian X, Sujuan G, Ming‐ Kun D, Krishna U, Eschen A, Ellertson C. Safety, efficacy and acceptability of medical abortion in China, Cuba and India: a comparative trial of mifepristone ‐ misoprostol versus surgical abortion. American Journal of Obstetrics and Gynecology 1997;176:431‐437. [DOI] [PubMed] [Google Scholar]


