Abstract
Background
Transient tachypnoea of the newborn (TTN) results from delayed clearance of lung liquid and is a common cause of admission of full‐term infants to neonatal intensive care units. The condition is particularly common after elective caesarean section. Conventional treatment involves appropriate oxygen administration and continuous positive airway pressure in some cases. Most infants receive antibiotic therapy. Hastening the clearance of lung liquid may shorten the duration of the symptoms and reduce complications.
Objectives
To determine whether diuretic administration reduces the duration of oxygen therapy and respiratory symptoms and shortens hospital stay in term infants presenting with transient tachypnoea of the newborn.
Search methods
An updated search was carried out in September 2015 of the following databases: the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library issue 9, 2015), MEDLINE via Ovid, EMBASE, PubMed, and CINAHL via OVID.
Selection criteria
We included randomised and quasi‐randomised controlled trials that compared the effect of diuretics administration versus placebo or no treatment in infants of less than seven days of age, born at 37 or more weeks of gestation with the clinical picture of transient tachypnoea of the newborn.
Data collection and analysis
We extracted and analysed data according to the methods outlined in the latest Cochrane Handbook for Systematic Reviews of Interventions. Two review authors assessed trial quality in each potentially eligible manuscript and two review authors extracted data.
Main results
Our previous systematic review included two trials enrolling a total of 100 infants with transient tachypnoea of the newborn (Wiswell 1985; Karabayir 2006). The updated search revealed no new trials. Wiswell 1985 randomised 50 infants to receive either oral furosemide (2 mg/kg body weight at time of diagnosis followed by a 1 mg/kg dose 12 hours later if the tachypnoea persisted) or placebo. Karabayir 2006 randomised 50 infants to receive either intravenous furosemide (2 mg/kg body weight) or an equal volume of normal saline placebo. Neither trial reported on the need for respiratory support. Neither trial demonstrated a statistically significant impact of furosemide on transient tachypnoea of the newborn regarding duration of symptoms or length of hospitalisation.
Authors' conclusions
Diuretics cannot be recommended as treatment for transient tachypnoea of the newborn and it should not be used unless additional data become available. This finding suggests that either furosemide is not effective in promoting resorption of lung fluid, or factors other than delayed resorption of this fluid contribute to the pathogenesis of transient tachypnoea of the newborn. The question remains as to whether furosemide given to the infant (or even to the mother before caesarean section) might shorten the duration of the illness. As elective caesarean section continues at a high level, these two interventions might be worthy of trials.
Keywords: Female; Humans; Infant, Newborn; Pregnancy; Administration, Oral; Cesarean Section; Cesarean Section/adverse effects; Diuretics; Diuretics/administration & dosage; Diuretics/therapeutic use; Furosemide; Furosemide/administration & dosage; Furosemide/therapeutic use; Injections, Intravenous; Oxygen Inhalation Therapy; Oxygen Inhalation Therapy/statistics & numerical data; Randomized Controlled Trials as Topic; Transient Tachypnea of the Newborn; Transient Tachypnea of the Newborn/drug therapy
Plain language summary
Diuretics for transient tachypnoea of the newborn (TTN)
Review question: Does diuretic treatment reduce the duration of oxygen therapy and respiratory symptoms and shorten hospital stay in term infants presenting with the clinical syndrome of transient tachypnoea of the newborn?
Background: It is common for full‐term infants born by elective caesarean section to have laboured, rapid breathing (tachypnoea) and to require oxygen for about 48 hours. This transient tachypnoea of the newborn (TTN) is responsible for about half of all cases of neonatal respiratory distress. Although it is transient and not usually serious, the condition requires admission to a neonatal intensive care unit, involves separation of mother and baby, and uses expensive resources. The underlying pathology of TTN is not well understood. The most commonly proposed mechanism is a delay in the resorption of foetal lung fluid after birth. Diuretic medication, including furosemide, may reduce fluid in the lungs.
Studies characteristics: We identified two randomised controlled trials involving 100 babies that compared the effect of furosemide given orally or intravenously versus placebo or no treatment in babies of less than seven days of age, born at 37 or more weeks of gestation with TTN. No new trials were identified in the 2015 update.
Results: Neither trial demonstrated a statistically significant impact of furosemide on transient tachypnoea of the newborn regarding duration of symptoms or length of hospitalisation.
Conclusion: Diuretics cannot be recommended as treatment for transient tachypnoea of the newborn and it should not be used unless additional data become available.
Background
Description of the condition
Transient tachypnoea of the newborn (TTN) was first described by Avery 1966. It is generally considered a benign condition that occurs in about 1% of newborns (Karabayir 2006). In infants born at term, TTN leads to rapid respiration (> 60 bpm), grunting and retraction at, or shortly after, birth. Investigations for infection will be negative and the oxygen requirement usually does not rise above 40%. Chest X‐ray shows streaky interstitial or pleural fluid, prominent interlobar fissures, perihilar vascular markings and sometimes hyperinflation. The symptoms often resolve by 48 hours, occasionally lasting as long as five days. The condition is more common in term infants born by elective caesarean section (Tudehope 1979; Morrison 1995). It may be difficult to distinguish between congenital pneumonia and TTN and many infants receive antibiotics until blood cultures are known to be negative. TTN is regarded as being synonymous with wet lung, benign unexplained respiratory distress in the newborn, neonatal tachypnoea and type 2 respiratory distress syndrome (RDS) (Rennie 1999). The underlying pathology of TTN is not well understood. The most commonly proposed mechanism is a delay in the resorption of foetal lung fluid after birth (Elias 2006). Lung liquid that has been rendered high in protein by either mild asphyxia or amniotic fluid aspiration may contribute to the problem (Avery 1966). In addition, elective caesarean section deprives the foetus of the effect of endogenous catecholamines on resorption of lung liquid.
The reported prevalence of TTN varies, with some studies attributing up to 40% of neonatal respiratory distress to TTN (Tudehope 1979); and an overall incidence of around 11 per 1000 births. With a tendency to delivery by elective caesarean section for an increasing number of obstetric and foetal indications, the number of infants admitted to neonatal units with TTN is likely to rise.
There are no specific biochemical or haematological markers and the diagnosis is essentially clinical with typical radiological features on chest X‐ray. The natural history is of gradual improvement of respiratory signs as foetal lung fluid is reabsorbed. Treatment is supportive with oxygen to maintain acceptable saturations and occasionally continuous positive airway pressure (CPAP) and even endotracheal ventilation to obtain adequate oxygenation and carbon dioxide clearance. TTN usually settles within 24 hours but may persist for several days and in its more severe forms may be associated with secondary surfactant‐deficient lung disease; and in extreme cases, persistent pulmonary hypertension.
Description of the intervention
Diuretics have been shown to affect fluid dynamics in the lung by both diuretic and non‐diuretic actions (Demling 1978; Belik 1987; Prabhu 1997; Kasap 2008). The diuretic response following intravenous furosemide is more rapid than oral furosemide and injection of furosemide is considered safer in infants with respiratory distress (Kasap 2008).
How the intervention might work
In theory, diuresis should increase the plasma oncotic pressure and draw water from the lungs into the pulmonary vascular bed. This has been shown not to be the case in an adult canine model (Wickerts 1992). It seems more likely that non‐diuretic effects are predominant, with several authors showing improved pulmonary dynamics without demonstrable diuresis (Demling 1978; Prabhu 1997). Given the effects of furosemide on the fluid‐overloaded lung, it is reasonable to hypothesise that it might alter the clinical course of TTN.
Why it is important to do this review
Although TTN is generally a benign self‐limiting condition there is much to be gained from shortening its clinical course provided this can be achieved without side effects. Hastening the clearance of retained foetal lung fluid should improve oxygenation, shorten the clinical course and may reduce complication rates; separation of mothers from their newborn infants is generally undesirable; and furthermore there are significant economic implications of reducing the duration of hospital stay.
Objectives
To determine whether treatment with diuretics reduces the duration of oxygen therapy and respiratory symptoms and shortens hospital stay in term infants presenting with the clinical syndrome of transient tachypnoea of the newborn.
Methods
Criteria for considering studies for this review
Types of studies
Randomised or quasi‐randomised controlled trials.
Types of participants
Infants of less than seven days of age, born at 37 or more completed weeks of gestation with a clinical diagnosis of transient tachypnoea of the newborn.
Types of interventions
Any diuretics compared with placebo or no therapy in the first seven days of life. We accepted any route of administration and any diagnostic criteria. We considered studies looking at single doses as well as multiple doses.
Types of outcome measures
Primary outcomes
Duration of oxygen therapy in hours.
Number of infants receiving CPAP.
Number of infants receiving positive pressure ventilation.
Secondary outcomes
Duration of tachypnoea (> 60 bpm) in hours.
Weight loss within first 24 hours of life.
Patent ductus arteriosus.
Electrolyte disturbances.
Length of hospital stay in hours.
Search methods for identification of studies
We used the standard search methods of the Cochrane Neonatal Review Group.
Published abstracts: we searched the abstracts of the Society for Pediatric Research 1987 to 2015. We used the following keywords in our search: 'furosemide', 'diuretic', 'respiratory distress', 'transient tachypnoea'.
Database of the Cochrane Neonatal Review Group: we screened all publications with the keywords 'furosemide' OR 'frusemide' OR 'diuretic'.
We contacted the authors if data for review were not included in original articles. We approached experts to identify any relevant unpublished material.
Selection process: we selected only randomised controlled trials fulfilling the selection criteria described in the previous sections. The selections were made separately by MK and JA. We resolved any disagreement by discussion.
Electronic searches
Two review authors (MK, JA) independently performed the electronic database searches of: the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library issue 9, 2015); MEDLINE via Ovid; EMBASE up to 18 September 2015; CINAHL via OVID; PubMed. We considered articles in any language as long as there was an abstract in English indicating content. We considered that the following were synonymous with transient tachypnoea of the newborn: 'wet lung', 'benign unexplained respiratory distress in the newborn', 'neonatal tachypnoea' and 'type 2 RDS'. The following keywords were used: 'furosemide', 'frusemide', 'diuretics', 'tachypnoea', 'respiratory distress', 'wet lung', 'type 2 RDS' and 'type II RDS'. Subject heading: 'infant, newborn'. We used truncation symbols, when appropriate, to capture variations in spelling and the endings of the search terms. Search Terms: (furosemide OR frusemid* OR diuretics) AND ((transient tachypnoea) OR (transient tachypnea) OR (wet lung) OR (respiratory distress) OR (type 2 RDS) OR (type II RDS)) AND Plus the following database‐specific terms: PubMed: ((infant, newborn[MeSH] OR newborn OR neonate OR neonatal OR premature OR low birth weight OR VLBW OR LBW or infan* or neonat*) AND (randomized controlled trial [pt] OR controlled clinical trial [pt] OR Clinical Trial[ptyp] OR randomized [tiab] OR placebo [tiab] OR clinical trials as topic [mesh: noexp] OR randomly [tiab] OR trial [ti]) NOT (animals [mh] NOT humans [mh])). EMBASE: (infant, newborn or newborn or neonate or neonatal or premature or very low birth weight or low birth weight or VLBW or LBW or Newborn or infan* or neonat*) AND (human not animal) AND (randomized controlled trial or controlled clinical trial or randomized or placebo or clinical trials as topic or randomly or trial or clinical trial). CINAHL: (infant, newborn OR newborn OR neonate OR neonatal OR premature OR low birth weight OR VLBW OR LBW or Newborn or infan* or neonat*) AND (randomized controlled trial OR controlled clinical trial OR randomized OR placebo OR clinical trials as topic OR randomly OR trial OR PT clinical trial). Cochrane Library: (infant or newborn or neonate or neonatal or premature or very low birth weight or low birth weight or VLBW or LBW).
Searching other resources
We also searched symposia, clinical trial registries, proceedings of related conferences, previous reviews, cross‐references, abstracts, relevant bibliographies, expert informants, and we handsearched relevant journals.
Data collection and analysis
We used the standard methods of the Cochrane Neonatal Review Group for data collection. No eligible study was identified for this 2015 update. All trials were independently assessed by each review author. Each review author assessed the methodological quality with respect to: i) masking of allocation; ii) masking of intervention; iii) complete follow‐up; iv) blinding of outcome measure. Each review author extracted data separately and resolved any disparity. The intention was to perform subgroup analysis for route of administration (oral, intravenous or nebulised). However, since only two studies were identified (Wiswell 1985; Karabayir 2006), this was not possible. We used the standard methods of the Cochrane Neonatal Review Group for data analysis. We planned to analyse treatment effects on categorical outcomes using risk ratio (RR), risk difference (RD), number needed to treat to benefit (NNTB), and the number needed to treat for an additional harmful outcome (NNTH). We used mean difference (MD) for treatment effects measured on a continuous scale. We calculated 95% confidence intervals (CIs).
Selection of studies
The selection of studies was performed independently by two review authors as follows:
we merged search results using reference management software thereby removing duplicate records of the same reports;
we examined titles and abstracts to remove irrelevant reports;
we retrieved the full text of the potentially relevant reports;
we linked multiple reports of the same study together;
we examined full‐text reports for compliance of the studies with eligibility criteria;
we corresponded with trial investigators, when appropriate, to clarify study eligibility;
we noted reasons for inclusion and exclusion of articles at all stages;
we resolved disagreements through consensus when needed;
we made final decisions on study inclusion and proceeded to data collection;
we resolved all discrepancies through a consensus process.
Data extraction and management
Two review authors independently extracted data from the included studies using a pre‐designed data collection form and entered data electronically with appropriate version control. Each review author independently completed a comparison of extracted data. Any disagreements were discussed with a third review author and the decisions documented. Where there were missing data, we planned to contact the study authors using every means available (e‐mail, formal letter, facsimile, phone call). All relevant data were entered into Review Manager 5 (RevMan 2014) by one review author (MK) and re‐checked for accuracy by the second review author (JA). The reliability of data extraction and data entry was examined throughout the process.
The data extracted were as follows:
general study information such as title, authors, contact address, publication source, publication year;
characteristics of the study: design, study setting, inclusion and exclusion criteria, and quality criteria (e.g. randomisation method; allocation procedure; blinding of patients, caregivers and outcome assessors; withdrawals and dropouts; overall sample size; sample size per group; number of groups; painful procedure; number of interventions);
characteristics of the study population and baseline characteristics of the intervention and control groups (age, sex etc.) with numbers in each group;
characteristics of the interventions, such as treatment comparators, dose (volume), method of administration, and frequency of administration;
outcome measures (such as changes in tachypnoea);
results for the intention‐to‐treat population (where possible); outcome measures at the end of the placebo phase; any summary measures with standard deviations, confidence intervals and P values were given; dropout rate; and reasons for withdrawal;
any adverse events.
Assessment of risk of bias in included studies
We assessed the risk of bias of the included studies using the method described by Cochrane (Higgins 2011). Each study was assessed under the following six domains:
Random sequence generation (selection bias);
Allocation concealment (selection bias);
Blinding of participants and personnel (performance bias); and blinding of outcome assessment (detection bias);
Incomplete outcome data (attrition bias);
Selective outcome reporting (reporting bias);
Other sources of bias.
We made an overall assessment based on the findings of the six domains. Two review authors assessed each domain according to preset criteria and judged it as either 'low risk of bias', 'high risk of bias", or 'unclear' (uncertain risk of bias). We planned to resolve discrepancies in judgements by discussion. Review authors were not blinded to the study authors, locations of the studies, author funding, or study acknowledgements.
Measures of treatment effect
We planned to calculate risk ratio (RR), risk difference (RD), the number needed to treat to benefit (NNTB), and the number needed to treat to harm (NNTH) along with the 95% CI for dichotomous outcomes. We expressed the treatment effect as mean difference (MD) along with 95% CIs for continuous outcomes.
Unit of analysis issues
We only considered parallel studies for this review.
Dealing with missing data
We planned to contact the primary author of a study to provide additional data in cases where data were missing. Two review authors planned to estimate the values from the graphs in studies if results were presented graphically and it was not possible to reach authors, or they were contacted and did not provide original data. We planned to present the results as descriptive data in the Results section if the numbers were not similar. If median, range, and sample size had been reported, the mean and standard deviation would have been estimated using established methods. A sensitivity analysis would have been conducted to determine the impact of imputed data.
Assessment of heterogeneity
We planned to assess between‐study heterogeneity using the I² and Chi² statistics (Higgins 2003). I² values were categorised in the following manner: 0% to 40% — might not be important; 30% to 60% — may represent moderate heterogeneity; 50% to 90% — may represent substantial heterogeneity; 75% to 100% — considerable heterogeneity (Higgins 2011). If I² values were greater than 40%, the magnitude and accompanying P value would have been considered in the overall interpretation. In addition, at least two review authors would reassess the included studies to determine if there were qualitative differences leading to heterogeneity which would prevent combining them. If present, heterogeneity would be explored according to a priori subgroup analysis described below.
Assessment of reporting biases
We described how we investigated the possibility of selective outcome reporting bias and what we found for each included study. We assessed the methods as:
adequate (where it is clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
inadequate (where not all the study’s pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
unclear.
Other sources of bias: For each included study, we described any important concerns we had about other possible sources of bias (for example, whether there was a potential source of bias related to the specific study design or whether the trial was stopped early due to some data‐dependent process). We assessed whether each study was free of other problems that could put it at risk of bias as 'yes'; 'no'; or 'unclear'. If needed, we planned to explore the impact of the level of bias through undertaking sensitivity analyses.
Data synthesis
Where appropriate, we performed meta‐analysis of pooled data using a fixed‐effect model. Review Manager 5 software was used for statistical analysis (RevMan 2014). We planned to use the Mantel‐Haenszel method for estimates of typical RR and RD. We planned to use the inverse variance method for measured quantities.
Subgroup analysis and investigation of heterogeneity
Issues for subgroup analysis were planned as follows:
route of administration (oral, intravenous (IV), inhalation);
furosemide dose;
number of dosages (frequency);
characteristics of participants: age (months); weight of infant (kg).
Sensitivity analysis
In future updates of this review if sufficient studies are identified, we will conduct sensitivity analyses for the following: studies determined to be at high risk of bias compared with those at low risk of bias and unclear risk of bias.
Results
Description of studies
Results of the search
Our search yielded 14 potential citations. Two trials met our inclusion criteria (Wiswell 1985; Karabayir 2006). An additional ongoing trial was identified from www.clinicaltrials.gov (NCT01407848).
Included studies
Two published RCTs with a total of 100 participants (experimental and control) were deemed appropriate for inclusion: Wiswell 1985 (n = 50); Karabayir 2006 (n = 50). See table 'Characteristics of included studies'.
Wiswell 1985 investigated 50 consecutive admissions to a single neonatal unit with transient tachypnoea of the newborn as defined by:
onset of tachypnoea (respiratory rate > 60 per minute) within six hours after birth;
persistence of tachypnoea for at least 12 hours;
chest roentgenogram indicating abnormalities characteristic of transient tachypnoea of the newborn (hyperaeration; vascular congestion; and excessive interstitial and/or pleural fluid);
absence of other disorders likely to cause tachypnoea (polycythaemia, air dissection syndromes, hypoglycaemia, pulmonary haemorrhage, aspiration syndromes, congenital heart disease, respiratory distress syndrome and pneumonitis).
After informed consent and within six hours of birth, the infants were randomised to receive either oral furosemide (2 mg/kg body weight at time of diagnosis followed by a 1 mg/kg dose 12 hours later if the tachypnoea persisted); or placebo given in the form of an equal volume of coloured sterile water indistinguishable from the furosemide. They measured response to treatment in terms of:
weight loss as a percentage of birth weight at 24 hours of age and at discharge;
duration of hospitalisation;
duration of tachypnoea (respiratory rate > 60 per minute).
Karabayir 2006 investigated 50 consecutive admissions to a single neonatal unit with transient tachypnoea of the newborn as defined by:
onset of tachypnoea within six hours after birth;
persistence of tachypnoea for at least 12 hours;
chest roentgenogram consistent with TTN. Infants were excluded if they had: a history of meconium aspiration or premature rupture of membranes; other disorders likely to cause tachypnoea (polycythaemia, hypoglycaemia, sepsis, pneumonia, respiratory distress syndrome and aspiration syndromes);
a cardiac murmur on physical examination.
Sex, gestation, birthweight, mode of delivery and time of presentation of the patients, and history of asphyxia were recorded. Initial evaluation of all infants included: chest roentgenogram; capillary blood gases; complete blood count with differential; serum electrolytes, blood urea nitrogen, glucose.
After informed consent, the infants were randomised to receive either intravenous furosemide (2 mg/kg body weight) at time of diagnosis, or an equal volume of normal saline placebo. They measured response to treatment in terms of:
weight loss in the first 24 hours of life and at discharge;
duration of oxygen requirements;
duration of hospitalisation;
duration of tachypnoea (respiratory rate > 60 per minute).
Excluded studies
None identified.
Risk of bias in included studies
See table 'Characteristics of included studies'. Figure 1
1.
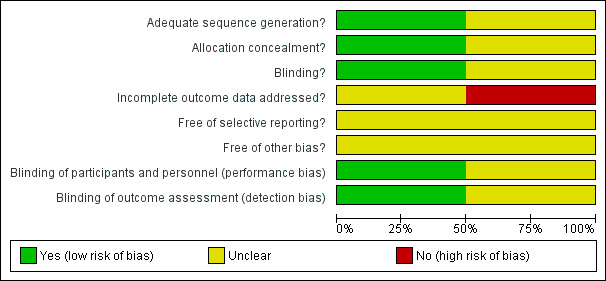
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Both studies did not report the method of concealment of randomisation (Wiswell 1985; Karabayir 2006). However, Wiswell 1985 did respond that he used randomised opaque envelopes to allocate treatment.
Blinding
The placebo preparation (coloured sterile water) was indistinguishable from the oral preparation of furosemide. The investigators also remained blinded.
Incomplete outcome data
Follow‐up was complete and the investigators remained blinded until the study was complete.
Selective reporting
There were no protocols to access so we did not know the original planned outcomes for either of the included studies (Wiswell 1985; Karabayir 2006).
Other potential sources of bias
Karabayir 2006 and Wiswell 1985 have a risk for bias due to unclear reporting of whether a sample size calculation was undertaken or because of the small sample size.
Effects of interventions
Furosemide versus placebo (comparison 1)
Primary outcomes
Duration of oxygen therapy in hours (Outcome 1.1):
Karabayir 2006 found no evidence of effect on the duration of oxygen therapy with mean difference (MD) 0.90 (95% confidence interval (CI) −18.50 to 20.30) (Analysis 1.1).
1.1. Analysis.

Comparison 1 Furosemide versus placebo, Outcome 1 Duration of oxygen requirements in hours.
Number of infants receiving CPAP
Not reported.
Number of infants receiving positive pressure ventilation
Not reported.
Secondary outcomes
Duration of tachypnoea in hours (Outcome 1.2)
Pooled data shows no evidence of effect with MD −1.28 hours (95% CI −13.00 to 10.45) (Analysis 1.2).
1.2. Analysis.

Comparison 1 Furosemide versus placebo, Outcome 2 Duration of tachypnoea in hours.
Weight loss in the first 24 hours of life (per cent of body weight) (Outcome 1.3)
Both Karabayir 2006 and Wiswell 1985 found that the study group lost significantly more weight in the first 24 hours after birth compared with the control group MD 2.73% of body weight (95% CI 1.68 to 3.78) (Analysis 1.3).
1.3. Analysis.

Comparison 1 Furosemide versus placebo, Outcome 3 Weight loss in the first 24 h of life (percent of body weight).
Weight loss on discharge (per cent of body weight) (Outcome 1.4)
Both Karabayir 2006 and Wiswell 1985 found no evidence of effect with MD 1.14% of body weight (95% CI −0.24 to 2.51) (Analysis 1.4).
1.4. Analysis.

Comparison 1 Furosemide versus placebo, Outcome 4 Weight loss at discharge (percent of body weight).
Patent ductus arteriosus
Not reported.
Electrolyte disturbance in the first 24 hours (Outcomes 1.5 to 1.8)
Karabayir 2006 found no evidence of effect on sodium (Na) and potassium (K) levels.
Serum level of Na in the first day of life (mg/dL) (Outcome 1.5): MD −1.50 (95% CI −2.72 to −0.28) (Analysis 1.5).
1.5. Analysis.

Comparison 1 Furosemide versus placebo, Outcome 5 Serum level of Na in the first day of life (mg/dL).
Serum level of Na in the second day of life (mg/dL) (Outcome 1.6): MD −1.50 (95% CI −2.66 to 0.34) (Analysis 1.6).
1.6. Analysis.

Comparison 1 Furosemide versus placebo, Outcome 6 Serum level of Na in the second day of life (mg/dL).
Serum level of K at the first day of life (mg/dL) (Outcome 1.7): MD −0.20 (95% CI −0.48 to 0.08) (Analysis 1.7).
1.7. Analysis.

Comparison 1 Furosemide versus placebo, Outcome 7 Serum level of K at the first day of life (mg/dL).
Serum level of K at the second day of life (mg/dL) (Outcome 1.8): MD −0.20 (95% CI −0.38 to −0.02) (Analysis 1.8).
1.8. Analysis.

Comparison 1 Furosemide versus placebo, Outcome 8 Serum level of K at the second day of life (mg/dL).
Length of hospital stay in hours (Outcome 1.9)
Pooled data shows no evidence of effect with MD −4.95 hours (95% CI −18.54 to 8.64) (Analysis 1.9).
1.9. Analysis.

Comparison 1 Furosemide versus placebo, Outcome 9 Length of hospital stay in hours.
Discussion
Only two trials were found to be eligible for this review (Wiswell 1985; Karabayir 2006). The results failed to show a statistically significant impact of oral and intravenous furosemide on the duration of oxygen requirement in transient tachypnoea of the newborn. The methodological quality of the two studies was satisfactory. Although no adverse effects were reported in either study, it should be noted that the studies were not powered to look for potential side effects. Both oral and intravenous furosemide have been shown not to be appropriate in infants with an acute respiratory disturbance and both have no effect on the clinical progression of TTN.
Summary of main results
The results demonstrate that 2 mg/kg of oral and intravenous furosemide had no effect on the clinical progression of TTN. This finding suggests that furosemide is not effective in the resorption of the fluid in the lungs; or factors other than delayed resorption of the fluid in the lungs, for example surfactant deficiency may contribute to the pathogenesis of TTN. However, there is a need to conduct more studies with larger sample sizes. Therefore, the results have to be interpreted with caution. No significant difference was found between oral and intravenous furosemide in relation to improvement of any of the mentioned outcomes such as tachypnoea. We were unable to identify any possible side effects or any possible long‐term effects. Therefore, further large randomised controlled trials are needed.
Overall completeness and applicability of evidence
The available evidence is applicable, but only two small trials using different study outcome measures could be included in this review.
Quality of the evidence
The quality of the evidence of included studies in this review was reasonable. The two studies adequately reported sequence generation; however, concealment of allocation was insufficiently reported. Blinding of interventions was achieved; however, incomplete outcome data were not addressed. There was risk of 'other' bias in the studies, due to unclear reporting of whether a sample size calculation was undertaken or because of the small sample size in the included studies in this review.
Potential biases in the review process
None known. The methods of the review were designed to minimise the introduction of additional bias. Two review authors independently completed data screening, data extraction and 'risk of bias' rating (MK and JA).
Agreements and disagreements with other studies or reviews
No other reviews known.
Authors' conclusions
Implications for practice.
Based on the available evidence the routine use of furosemide in infants with transient tachypnoea of the newborn cannot be recommended.
Implications for research.
It is plausible that with the increasing rates of elective caesarean section (Khor 2000), the incidence of transient tachypnoea of the newborn will also increase. Although not usually seriously ill, infants with transient tachypnoea of the newborn may develop secondary surfactant‐deficient lung disease and require mechanical ventilation. No group has to date systematically investigated the role of intravenous furosemide or any other diuretic in transient tachypnoea of the newborn. If shown to reduce length of stay or requirement for oxygen or both, then diuretics could have a significant economic benefit. Such a study would almost certainly have to be multicentre in design. Furosemide has been shown to pass freely across the placenta (Beermann 1978). Intravenous administration of furosemide to the mother before elective caesarean section might also be worthy of investigation as a way of assisting clearance of foetal lung liquid and reducing the duration of transient tachypnoea.
What's new
| Date | Event | Description |
|---|---|---|
| 1 October 2015 | New citation required but conclusions have not changed | No new trials. No change to conclusion. |
| 18 September 2015 | New search has been performed | This updates the review "Furosemide for transient tachypnoea of the newborn" (Kassab 2013). Title changed to "Diuretics for transient tachypnoea of the newborn". New authorship. |
History
Protocol first published: Issue 2, 2001 Review first published: Issue 1, 2002
| Date | Event | Description |
|---|---|---|
| 30 April 2013 | New search has been performed | This updates the review "Furosemide for transient tachypnoea of the newborn" (Lewis 2002). New authorship. |
| 15 January 2013 | New citation required but conclusions have not changed | Updated search in January 2013 identified one new trial for inclusion in this review (Karabayir 2006) and one ongoing study (NCT01407848). No change to conclusions. |
| 27 October 2008 | Amended | Converted to new review format. |
Notes
None known.
Acknowledgements
We gratefully acknowledge the contributions of Dr V Lewis and Prof A Whitelaw, the authors of the previous version of this Cochrane review (Lewis 2002).
Data and analyses
Comparison 1. Furosemide versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Duration of oxygen requirements in hours | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | 0.90 [‐18.50, 20.30] |
| 2 Duration of tachypnoea in hours | 2 | 100 | Mean Difference (IV, Fixed, 95% CI) | ‐1.28 [‐13.00, 10.45] |
| 3 Weight loss in the first 24 h of life (percent of body weight) | 2 | 100 | Mean Difference (IV, Fixed, 95% CI) | 2.73 [1.68, 3.78] |
| 4 Weight loss at discharge (percent of body weight) | 2 | 100 | Mean Difference (IV, Fixed, 95% CI) | 1.14 [‐0.24, 2.51] |
| 5 Serum level of Na in the first day of life (mg/dL) | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | ‐1.5 [‐2.72, ‐0.28] |
| 6 Serum level of Na in the second day of life (mg/dL) | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | ‐1.5 [‐2.66, ‐0.34] |
| 7 Serum level of K at the first day of life (mg/dL) | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐0.48, 0.08] |
| 8 Serum level of K at the second day of life (mg/dL) | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐0.38, ‐0.02] |
| 9 Length of hospital stay in hours | 2 | 100 | Mean Difference (IV, Fixed, 95% CI) | ‐4.95 [‐18.54, 8.64] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Karabayir 2006.
| Methods | Double‐blind, placebo‐controlled study. January 2004 to June 2004. |
|
| Participants | 50 term infants with TTN. Inclusion criteria:
Infants were excluded if they had:
|
|
| Interventions | IV furosemide 2 mg/kg or saline placebo. | |
| Outcomes | Duration of supplemental oxygen requirement; the period of tachypnoea; time to discharge from hospital; and weight loss in the first 24 h of life and before discharge. | |
| Notes | SSK, Bakirköy Maternity and Child Disease Education Hospital, Istanbul, Turkey. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Randomised controlled study design. Information reported insufficient for a judgment to be made. |
| Allocation concealment? | Unclear risk | Not stated. Information reported insufficient for a judgment to be made. |
| Blinding? All outcomes | Low risk | Double‐blind intervention. |
| Incomplete outcome data addressed? All outcomes | High risk | Not reported clearly in the text. |
| Free of selective reporting? | Unclear risk | There is no protocol to access so we do not know the original planned outcomes. |
| Free of other bias? | Unclear risk | Not mentioned clearly |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Infants are unaware of study interventions and they were randomly assigned in a double‐blind way to study groups. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of assessors was not mentioned clearly in the study report but as the authors stated it is a double‐blind study, that suggests personnel and assessors were blinded. |
Wiswell 1985.
| Methods | Randomised, double‐blind, placebo‐controlled trial.
Single neonatal unit. Tripler Army Medical Center, Honolulu. |
|
| Participants | 50 consecutive admissions with transient tachypnoea of the newborn to neonatal unit. Inclusion criteria: transient tachypnoea of the newborn as defined by:
|
|
| Interventions | Oral furosemide 2 mg/kg body weight at time of diagnosis; followed by a 1 mg/kg dose 12 h later if the tachypnoea persisted. Placebo: an equal volume of sterile water coloured with two drops of MVI solution (USV Pharmaceutical Corp, Tuckahoe, NY) indistinguishable from the furosemide. | |
| Outcomes | Outcomes: 1) Weight loss as a % of birth weight at 24 h of age and at discharge, 2) Duration of hospitalisation, and 3) Duration of tachypnoea. | |
| Notes | Information on randomisation obtained from authors. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Numbered opaque sealed envelopes. |
| Allocation concealment? | Low risk | Adequtely performed. |
| Blinding? All outcomes | Unclear risk | Not mentioned clearly. |
| Incomplete outcome data addressed? All outcomes | Unclear risk | Not mentioned clearly. |
| Free of selective reporting? | Unclear risk | Not mentioned clearly. |
| Free of other bias? | Unclear risk | Not mentioned clearly. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned clearly. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of assessors was not mentioned clearly in the study report. |
Information on concealment of allocation was obtained by contact with Dr Wiswell.
h: hour(s) IV: intravenous MVI: multi‐vitamin additive RDS: respiratory distress syndrome TTN: transient tachypnoea of the newborn
Characteristics of ongoing studies [ordered by study ID]
NCT01407848.
| Trial name or title | Trial on treatment with inhaled furosemide of preterm and term neonates with transient tachypnoea |
| Methods | Estimated enrolment: 20 Randomised, double‐blind trial Parallel assignment Inclusion criteria: Neonates with 35 + 0 − 39 + 0 GA on the first day of life with the clinical diagnosis of transient tachypnoea; the need for CPAP > 6 h to obtain the oxygen saturation > 92%; written informed consent of parent/guardian Exclusion Criteria: Systemic infection; intubation and mechanical ventilation before Inclusion in the trial; malformation and any other of several diseases with respiratory disturbance; participants involved in other clinical trials |
| Participants | Preterm and term neonates with transient tachypnoea |
| Interventions | Patients received nebulised Furosemide IV solution 1 mg/kg (4x/d) for max. 3 consecutive days versus nebulised 0.9% saline 4x/d for max. 3 days |
| Outcomes | Primary outcome: Reduction of the Silverman score Secondary outcome: Oxygen supplementation, a need for secondary intubation and mechanical ventilation, body weight, CPAP‐time, blood electrolytes, and blood gas |
| Starting date | January 2012 |
| Contact information | Prof. Bernhard Roth Children's Hospital University of Cologne Cologne, NRW, Germany, 50931 +49221478 ext 5064 bernd.roth@uk‐koeln.de |
| Notes | NCT01407848 |
CPAP: continuous positive airway pressure GA: gestational age h: hour(s)
Differences between protocol and review
None
Contributions of authors
Initial version 2002
Dr Vaughan Lewis had the idea for the first review and carried out the search 2002. JA collaborated on the structure of the first review conducted in 2002.
Review update 2013
Manal Kassab (MK): undertook the update of the review, study searching, study retrieval, study selection, data extraction, data entry, statistical analyses (if needed), and report writing.
Wadah Khriesat: review drafting and editing the final review.
Hiba Bawady: review drafting and editing the final review.
Jasim Anabeer (JA): provided expertise in conducting systematic reviews, assisted with update development, study searching, study retrieval, study selection, and report writing.
Review update 2015
MK: undertook the update of the review, assisted with update development, study searching, study retrieval, study selection, and report writing.
JA: provided expertise in conducting systematic reviews, assisted with update development, study searching, study retrieval, study selection, and report writing.
Sources of support
Internal sources
No sources of support supplied
External sources
-
Eunice Kennedy Shriver National Institute of Child Health and Human Development National Institutes of Health, Department of Health and Human Services, USA.
Editorial support of the Cochrane Neonatal Review Group has been funded with Federal funds from the Eunice Kennedy Shriver National Institute of Child Health and Human Development National Institutes of Health, Department of Health and Human Services, USA, under Contract No. HHSN275201100016C.
Declarations of interest
None
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Karabayir 2006 {published data only}
- Karabayir N, Kavuncuoglu S. Intravenous frusemide for transient tachypnoea of the newborn: a randomised controlled trial. Journal of Paediatrics and Child Health 2006;42(10):640‐2. [DOI] [PubMed] [Google Scholar]
Wiswell 1985 {published data only}
- Wiswell TE, Rawlings MC, Smith MC, Goo ED. Effect of furosemide on the clinical course of transient tachypnea of the newborn. Pediatrics 1985;75(5):908‐10. [PubMed] [Google Scholar]
References to ongoing studies
NCT01407848 {published data only}
- NCT01407848. Trial on treatment with inhaled furosemide of preterm and term neonates with transient tachypnoea. https://clinicaltrials.gov/ct2/show/NCT01407848 (accessed 18 September 2015).
Additional references
Avery 1966
- Avery ME, Gatewood OB, Brumley G. Transient tachypnea of the newborn. Possible delayed resorption of fluid at birth. American Journal of Diseases of Children 1966;111(4):380‐5. [DOI] [PubMed] [Google Scholar]
Beermann 1978
- Beermann B, Groschinsky‐Grind M, Fahraeus L, Lindstrom B. Placental transfer of furosemide. Clinical Pharmacology and Therapeutics 1978;24(5):560‐2. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Belik 1987
- Belik J, Spitzer AR, Clark BJ, Gewitz MH, Fox WW. Effect of early furosemide administration in neonates with respiratory distress syndrome. Pediatric Pulmonology 1987;3(4):219‐25. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Demling 1978
- Demling RH, Will JA. The effect of furosemide on the pulmonary transvascular filtration rate. Critical Care Medicine 1978;6(5):317‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Elias 2006
- Elias N, O'Brodovich H. Clearance of fluid from airspaces of newborns and infants. American Academy of Pediatrics 2006;7:e88‐e94. [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. [DOI]
Kasap 2008
- Kasap B, Duman N, Ozer E, Tatli M, Kumral A, Ozkan H. Transient tachypnea of the newborn: Predictive factor for prolonged tachypnea. Pediatrics International 2008;50(1):81‐4. [DOI] [PubMed] [Google Scholar]
Khor 2000
- Khor LJ, Jeskins G, Cooper GM, Paterson‐Brown S. National obstetric anaesthetic practice in the UK 1997/1998. Anaesthesia 2000;55(12):1168‐72. [DOI] [PubMed] [Google Scholar]
Morrison 1995
- Morrison JJ, Rennie JM, Milton PJ. Neonatal respiratory morbidity and mode of delivery at term: influence of timing of elective caesarean section. British Journal of Obstetrics and Gynaecology 1995;102(2):101‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Prabhu 1997
- Prabhu VG, Keszler M, Dhanireddy R. Pulmonary function changes after nebulised and intravenous frusemide in ventilated premature infants. Archives of Disease in Childhood. Fetal and Neonatal Edition 1997;77(1):F32‐5. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rennie 1999
- Rennie JM, Roberton NRC. Textbook of Neonatology. 3rd Edition. Edinburgh: Churchill Livingstone, 1999. [0443 055416] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre. The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre. The Cochrane Collaboration, 2014.
Tudehope 1979
- Tudehope DI, Smyth MH. Is "transient tachypnoea of the newborn" always a benign disease? Report of 6 babies requiring mechanical ventilation. Australian Paediatric Journal 1979;15(3):160‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Wickerts 1992
- Wickerts CJ, Berg B, Frostell C, Schmidt J, Blomqvist H, Rösblad PG, et al. Influence of hypertonic‐hyperoncotic solution and furosemide on canine hydrostatic pulmonary oedema resorption. Journal of Physiology 1992;458:425‐38. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Kassab 2013
- Kassab M, Khriesat WM, Bawadi H, Anabrees J. Furosemide for transient tachypnoea of the newborn. Cochrane Database of Systematic Reviews 5 June 2013, Issue 6. [DOI: 10.1002/14651858.CD003064.pub2] [DOI] [PubMed] [Google Scholar]
Lewis 2002
- Lewis V, Whitelaw A. Furosemide for transient tachypnea of the newborn. Cochrane Database of Systematic Reviews 2002, Issue 1. [DOI: 10.1002/14651858.CD003064] [DOI] [PubMed] [Google Scholar]


