Abstract
Background
Following surgery, surgical wounds can be closed using a variety of devices including sutures (subcuticular or transdermal), staples and tissue adhesives. Subcuticular sutures are intradermal stitches (placed immediately below the epidermal layer). The increased availability of synthetic absorbable filaments (stitches which are absorbed by the body and do not have to be removed) has led to an increased use of subcuticular sutures. However, in non‐obstetric surgery, there is still controversy about whether subcuticular sutures increase the incidence of wound complications.
Objectives
To examine the efficacy and acceptability of subcuticular sutures for skin closure in non‐obstetric surgery.
Search methods
In March 2019, we searched the Cochrane Wounds Specialised Register; the Cochrane Central Register of Controlled Trials (CENTRAL); Ovid MEDLINE (including In‐Process & Other Non‐Indexed Citations); Ovid Embase and EBSCO CINAHL Plus. We also searched clinical trials registries for ongoing and unpublished studies, and scanned reference lists of relevant included studies as well as reviews, meta‐analyses and health technology reports to identify additional studies. There were no restrictions with respect to language, date of publication or study setting.
Selection criteria
All randomised controlled trials which compared subcuticular sutures with any other methods for skin closure in non‐obstetric surgery were included in the review.
Data collection and analysis
Two review authors independently identified the trials, extracted data and carried out risk of bias and GRADE assessment of the certainty of the evidence.
Main results
We included 66 studies (7487 participants); 11 included trials had more than two arms. Most trials had poorly‐reported methodology, meaning that it is unclear whether they were at high risk of bias. Most trials compared subcuticular sutures with transdermal sutures, skin staples or tissue adhesives. Most outcomes prespecified in the review protocol were reported. The certainty of evidence varied from high to very low in the comparisons of subcuticular sutures with transdermal sutures or staples and tissue adhesives; the certainty of the evidence for the comparison with surgical tapes and zippers was low to very low. Most evidence was downgraded for imprecision or risk of bias.
Although the majority of studies enrolled people who underwent CDC class 1 (clean) surgeries, two‐thirds of participants were enrolled in studies which included CDC class 2 to 4 surgeries, such as appendectomies and gastrointestinal surgeries. Most participants were adults in a hospital setting.
Subcuticular sutures versus transdermal sutures
There may be little difference in the incidence of SSI (risk ratio (RR) 1.10; 95% confidence interval (CI) 0.80 to 1.52; 3107 participants; low‐certainty evidence).
It is uncertain whether subcuticular sutures reduce wound complications (RR 0.83; 95% CI 0.40 to 1.71; 1489 participants; very low‐certainty evidence). Subcuticular sutures probably improve patient satisfaction (score from 1 to 10) (at 30 days; MD 1.60, 95% CI 1.32 to 1.88; 290 participants; moderate‐certainty evidence). Wound closure time is probably longer when subcuticular sutures are used (MD 5.81 minutes; 95% CI 5.13 to 6.49 minutes; 585 participants; moderate‐certainty evidence).
Subcuticular sutures versus skin staples
There is moderate‐certainty evidence that, when compared with skin staples, subcuticular sutures probably have little effect on SSI (RR 0.81, 95% CI 0.64 to 1.01; 4163 participants); but probably decrease the incidence of wound complications (RR 0.79, 95% CI 0.64 to 0.98; 2973 participants). Subcuticular sutures are associated with slightly higher patient satisfaction (score from 1 to 5) (MD 0.20, 95% CI 0.10 to 0.30; 1232 participants; high‐certainty evidence). Wound closure time may also be longer compared with staples (MD 0.30 to 5.50 minutes; 1384 participants; low‐certainty evidence).
Subcuticular sutures versus tissue adhesives, surgical tapes and zippers
There is moderate‐certainty evidence showing no clear difference in the incidence of SSI between participants treated with subcuticular sutures and those treated with tissue adhesives (RR 0.77, 95% CI 0.41 to 1.45; 869 participants). There is also no clear difference in the incidence of wound complications (RR 0.62, 95% CI 0.35 to 1.11; 1058 participants; low‐certainty evidence). Subcuticular sutures may also achieve lower patient satisfaction ratings (score from 1 to 10) (MD ‐2.05, 95% CI ‐3.05 to ‐1.05; 131 participants) (low‐certainty evidence). In terms of SSI incidence, the evidence is uncertain when subcuticular sutures are compared with surgical tapes (RR 1.31, 95% CI 0.40 to 4.27; 354 participants; very low‐certainty evidence) or surgical zippers (RR 0.80, 95% CI 0.08 to 8.48; 424 participants; very low‐certainty evidence). There may be little difference in the incidence of wound complications between participants treated with subcuticular sutures and those treated with surgical tapes (RR 0.90, 95% CI 0.61 to 1.34; 492 participants; low‐certainty evidence). It is uncertain whether subcuticular sutures reduce the risk of wound complications compared with surgical zippers (RR 0.55, 95% CI 0.15 to 2.04; 424 participants; very low‐certainty evidence). It is also uncertain whether it takes longer to close a wound with subcuticular sutures compared with tissue adhesives (MD ‐0.34 to 10.39 minutes; 895 participants), surgical tapes (MD 0.74 to 6.36 minutes; 169 participants) or zippers (MD 4.38 to 8.25 minutes; 424 participants) (very low‐certainty evidence). No study reported results for patient satisfaction compared with surgical tapes or zippers.
Authors' conclusions
There is no clear difference in the incidence of SSI for subcuticular sutures in comparison with any other skin closure methods. Subcuticular sutures probably reduce wound complications compared with staples, and probably improve patient satisfaction compared with transdermal sutures or staples. However, tissue adhesives may improve patient satisfaction compared with subcuticular sutures, and transdermal sutures and skin staples may be quicker to apply than subcuticular sutures. The quality of the evidence ranged from high to very low; evidence for almost all comparisons was subject to some limitations. There seems to be no need for additional new trials to explore the comparison with staples because there are high‐quality studies with large sample sizes and some ongoing studies. However, there is a need for studies exploring the comparisons with transdermal sutures, tissue adhesives, tapes and zippers, with high‐quality studies and large sample sizes, including long‐term assessments.
Plain language summary
Stitches that go under the skin for closing wounds after surgery
What is the aim of this review?
The aim of this review was to find out whether subcuticular sutures (stitches placed under the skin) are effective for closing wounds after surgery. We were interested in all types of surgery except obstetric surgery (operations related to childbirth, e.g. caesarean sections). Cochrane researchers collected and analysed all studies related to this question and found 66 relevant randomised controlled trials. Randomised controlled trials are medical studies where patients are chosen at random to receive different treatments. This type of trial provides the most reliable health evidence.
Key messages
In terms of wound infection following surgery, there is no clear difference between stitches that go under the skin and other methods of closing surgical wounds, such as standard stitches that go over the skin, surgical tape, staples, or glue. Stitches that go under the skin probably reduce wound complications compared with staples and improve patient satisfaction compared with stitches that go over the skin or staples. However, glue may improve patient satisfaction, and stitches that go over the skin and staples may be quicker for surgeons.
What was studied in the review?
Surgeons have various options for closing surgical wounds at the end of an operation. Skin closure can be carried out with stitches (sutures) that go under the skin, stitches that go over the skin, staples (clips), tissue adhesives (glue), tapes or other devices. Sutures can be absorbable (the stitches dissolve into the body as part of the healing process and do not need removing) or non‐absorbable (the stitches need removing once the wound has healed).
Surgical site infections are a common problem after surgery and can cause a range of problems for patients. Surgical wounds can also cause unsightly scars if they do not heal correctly. We wanted to find out how stitches that go under the skin compare with other methods of closing surgical wounds in terms of infection, scarring, patient satisfaction, cost, pain, length of hospital stay and quality of life.
What are the main results of the review?
In March 2019, we searched medical databases and identified 66 studies that compared stitches that go under the skin with other methods of skin closure such as standard stitches, skin staples, tissue adhesive, tape, or surgical zippers. Sixty‐four of these studies (involving 7487 participants) were used in our analysis. On average, each study involved 115 people. Most participants were adults (20 to 75 years) undergoing surgery in a hospital setting. Most studies did not state funding sources.
The majority of studies compared stitches that go under the skin with standard stitches, skin staples or tissue adhesives.
The main outcome of interest was whether wounds became infected. There was no clear difference between stitches that go under the skin and other closure methods in the number of people whose wounds became infected.
Compared with stitches that go over the skin, stitches that go under skin probably improve patient satisfaction. There is evidence that stitches that go under the skin probably prevent wound complications and improve patient satisfaction compared with skin staples. Stitches that go under the skin may prevent wound breakdown (skin separation) compared with staples or tissue adhesives, but tissue adhesives may improve patient satisfaction. However, alternative methods may be quicker for surgeons to use than stitches that go under the skin. There was no clear difference between stitches that go under the skin and the alternative closure methods for re‐closure, pain, length of hospital stay and quality of life.
The studies we analysed often involved small numbers of participants and, in many cases, were not reported in a way that meant we could be sure they had been conducted robustly. We cannot, therefore, make conclusive statements about the effectiveness of stitches that go under the skin, and for all comparisons except the comparison with staples, better quality research is needed to form stronger conclusions.
How up to date is this review?
We searched for studies that had been published up to March 2019.
Summary of findings
Background
Description of the condition
Many people undergo surgical procedures in their lifetime. It is estimated that 312.9 million operations are undertaken every year worldwide (95% confidence interval (CI) 266.2 to 359.5; Weiser 2015). Since Weiser 2015 reported that 18.7 million of these are caesarean deliveries, we can estimate that approximately 250 to 300 million of them are non‐obstetric surgeries. In most operations, surgeons make an incision to gain access to the tissue or organs in which the surgery is performed. After the surgical procedure is complete, they close the incision with various wound closure materials (e.g. sutures, tissue adhesives, surgical tapes, staples) and suturing techniques (Regula 2015; Tajirian 2010).
Wound complications such as surgical site infections (SSI) are among the most common issues reported after surgery, and are often very problematic for patients in terms of cosmetic appearance, decreased quality of life, prolonged hospital stays, and increased healthcare costs (De Lissovoy 2009; Perencevich 2003; Zimlichman 2013).
Incidence of wound complications depends on various risk factors including those related to patients (e.g. comorbidities, medications), those related to operations (e.g. the type of surgery, duration of operation and method of wound closure), and preventive measures (Cardo 2004; Gaynes 2001; Kwon 2013; Mangram 1999; Pull ter Gunne 2012; Talbot 2005; Zhang 2014; Zhang 2015).
In the USA, the Centers for Disease Control and Prevention (CDC) provide guidelines and tools for the healthcare community to help prevent SSI, together with resources to help the public understand these infections and take measures to safeguard their own health when possible. Many preventive measures against SSI are recommended and have spread globally. The incidence of SSI varies and depends on the classification of surgical wounds (Garner 1986). The Garner 1986 guideline categorises operative wound sites into four classes (classes 1 to 4) according to the degree of contamination, that is: clean (class 1), clean‐contaminated (class 2), contaminated (class 3), and dirty or infected (class 4) (Garner 1986). This classification is shown in more detail in Appendix 1. CDC recommend taking different preventive approaches according to each class (Mangram 1999).
Description of the intervention
There are many ways to close surgical incisions, for example, using sutures, staples, and other devices (e.g. tissue adhesives, tapes) (Dumville 2014; Regula 2015; Tajirian 2010). Conventional sutures are usually non‐absorbable interrupted sutures (individual stitches, typically placed transdermally) (Pauniaho 2010). Staples are usually non‐absorbable skin closure clips placed transdermally. Other devices for wound closure include tissue adhesives or tapes, but their use is less widespread due to problems with wound dehiscence (breakdown) (Dumville 2014). In addition, costs are increased because of the high price of adhesive compared with that for subcuticular and other sutures. Brown 2009 reported that, for closure of paediatric hernia incisions, material costs related to skin closure were higher for skin adhesive than for suturing (suture materials USD 11.70 versus skin adhesive USD 22.63; P value < 0.001).
Subcuticular suturing was introduced by Carl Thiersch in 1874. The development of the subcuticular suture sprang from concepts for improving wound healing and avoiding infection (Fisher 1980). Subcuticular suturing became known in the field of plastic surgery in the early 1900s through the efforts of Dr Halsted and Dr Davis (Fisher 1980). 'Subcuticular' means intradermal; i.e. within the layer of the skin (immediately below the epidermal layer). Subcuticular sutures can be either absorbable or non‐absorbable. When non‐absorbable filaments are used, the suture ends are not buried in the skin but exposed outside, which can increase the risk of contamination (Stanec 1997). On the other hand, when absorbable sutures are used, they can be completely buried and retained at or near wound ends (La Paudula 1995; Ranaboldo 1992; Singh‐Ranger 2003; Smoot 1998). Synthetic absorbable filaments (e.g. polyglecaprone, polydioxanone, polyglactin) have only recently become available and are now used widely. Prior to this only natural absorbable filaments (e.g. catgut) were available, but they were rarely used for skin closure due to the risk of infection. With subcuticular sutures, no foreign material reaches beyond the epidermis except for the suture ends. This does not leave any mark points (Kobayashi 2015).
Subcuticular sutures were not previously the preferred method of skin closure except in clean surgery, because of the risk of infection. Since the arrival of synthetic absorbable sutures, their use has been spreading rapidly, not only for CDC class 1 (clean) surgery, but also for class 2 and 3 procedures, partly because wound cosmesis (cosmetic appearance) is currently considered more important than it was previously (Tanaka 2014; Taube 1983). The recent development of suture filaments and surgical devices, and the fact that endoscopic surgery is now more widely performed also lie behind the trend.
How the intervention might work
The use of subcuticular sutures for skin closure is an attractive alternative closure method because of the low incidence of wound complications and good cosmetic appearance it produces (Fisher 1980). With subcuticular sutures, no foreign material reaches beyond the epidermis except for the suture ends. This can obviate the need for postoperative suture removal except for the suture ends and does not leave any mark points (Kobayashi 2015).
Common alternatives to subcuticular sutures are conventional transdermal sutures and staples, both of which have to be removed. Staples are attractive because of speed of application (Gatt 1985; Tajirian 2010), however their cost is higher than that of suture filaments in general.
Compared with staples or conventional transdermal sutures, some clinical trials have shown that subcuticular sutures are associated with a lower incidence of wound complications and better cosmetic results after CDC class 1 (clean) surgery such as: orthopaedic procedures (Shetty 2004), cardiovascular surgery (Angelini 1984; Johnson 1997), and obstetric surgery (Ibrahim 2014; Mackeen 2012; Mackeen 2015). For closure of hip wounds, a cost‐effectiveness study showed that subcuticular sutures were significantly better than clips in terms of wound healing and also in terms of cost (Singh 2006). It has also been reported that the cost incurred for closure of sternal (chest bone) and leg incisions in coronary arterial bypass grafting (CABG) patients was significantly greater when skin clips were used for closure than when sutures were used (Angelini 1984; Chughtai 2000; Johnson 1997). Chughtai 2000 reported a cost of USD 4.5 for each wound closed with sutures and USD 15 for each wound closed with staples. In CDC class 2 (clean‐contaminated) surgery such as gastrointestinal procedures, several randomised controlled trials have shown that subcuticular sutures do not increase the incidence of wound complications (Tsujinaka 2013), and that patients prefer this closure technique because it produces better cosmetic results and less pain (Tanaka 2014).
The advantage of subcuticular sutures may be partly attributable to the use of absorbable sutures (Gurusamy 2014); the advantage of absorbable suture materials is that they do not have to be removed later, which saves surgeons time and decreases the anxiety and discomfort of patients (Parell 2003).
Absorbable sutures may, however, lead to an increased inflammatory response (Parell 2003), and it should be noted that the cost of absorbable suture filaments is higher than that for non‐absorbable filaments.
Why it is important to do this review
Two systematic reviews and two meta‐analyses that evaluated subcuticular sutures in cesarean deliveries have been published. One systematic review did not find conclusive evidence about how the skin should be closed (Mackeen 2012), but the others concluded that there was a possible benefit with subcuticular sutures compared with skin staples, because of a lower incidence of wound complications (Clay 2011; Mackeen 2015; Tuuli 2011).
In the field of non‐obstetric surgery however, there is still controversy about whether subcuticular sutures increase the incidence of wound complications, and, to date, no systematic review has been conducted on this important topic.
One related systematic review entitled 'Continuous versus interrupted skin sutures for non‐obstetric surgery' showed that superficial wound dehiscence (wound separation) may be reduced by using continuous subcuticular sutures (Gurusamy 2014). The authors suggested that this difference might depend on whether sutures were absorbable or not, because most of these wound dehiscences were reported in two recent trials in which the continuous skin suture groups received absorbable subcuticular sutures, while the interrupted skin suture groups received non‐absorbable transcutaneous sutures. In this review, we have focussed on investigating the advantages of subcuticular sutures regardless of whether they are continuous or interrupted.
Objectives
To examine the efficacy and acceptability of subcuticular sutures for skin closure in non‐obstetric surgery.
Methods
Criteria for considering studies for this review
Types of studies
We included all relevant published and unpublished RCTs that compared subcuticular sutures with any other sutures or devices for skin closure in non‐obstetric surgery, irrespective of their sample sizes and language of report.
We had planned to include cluster‐randomised trials when effects of clustering were taken into account (however, we found no such cases). We excluded quasi‐randomised controlled trials (in which treatment assignment is decided through methods such as alternate days of the week). No language or publication status restrictions were imposed.
Types of participants
We included patients of any age and sex undergoing non‐obstetric surgery. We included both outpatients and inpatients with any type of disease and with any comorbidities.
We excluded obstetric operations because there is already a Cochrane Review that addresses methods of skin closure after caesarean sections (Mackeen 2012).
Types of interventions
Subcuticular sutures versus any other sutures or devices for skin closure in non‐obstetric surgery. We made a post hoc decision to exclude studies in which tissue adhesives were used in addition to subcuticular sutures as this represented an additional difference between the groups (see Differences between protocol and review).
Experimental interventions
We included studies that used absorbable and non‐absorbable subcuticular sutures for skin closure, irrespective of whether the sutures were continuous or interrupted.
Comparator interventions
We included studies in which a conventional suture (e.g. transdermal interrupted suture) or a device for skin closure (e.g. staples and other skin closure devices) was used as a control intervention.
Types of outcome measures
Primary outcomes
Incidence of surgical site infection (SSI) within 30 days of the operation.
Secondary outcomes
Incidence of wound complications (e.g. haematoma, seroma, skin separation) within 30 days of the operation.
When the data allowed, we also presented the results for specific outcome subcategories, such as complications of higher severity or specific type.
Incidence of wound dehiscence (skin separation). We added this outcome as a post hoc decision. See Differences between protocol and review.
Proportion of re‐closure of the skin incision required within 60 days of the operation.
Incidence of hypertrophic scar at maximal follow‐up.
Incidence of keloid scar at maximal follow‐up.
Wound pain intensity within seven days, and at or after 30 days of the operation (as measured on visual analogue scale, numerical rating scale or other valid instruments).
Length of hospital stay (for inpatient surgery, this included any readmissions for wound‐related complications as defined by the authors for a period of one year).
Cosmesis of scar (as defined by the authors for a minimum follow‐up of six months).
If both self and observer‐rated assessments were available, we gave preference to the latter.
Patient satisfaction as defined by the authors within 30 days, and at or after 60 days of the operation.
Quality of Life (QoL; short‐term and long‐term as defined by the authors).
Wound closure time in the operation (minutes).
Cost at maximal follow‐up (as reported by authors).
If both total cost (including time cost) and material cost per patient were available, we gave preference to the latter.
Search methods for identification of studies
Electronic searches
We searched the following electronic databases to identify reports of relevant clinical trials:
the Cochrane Wounds Specialised Register (searched 26 March 2019);
the Cochrane Central Register of Controlled Trials (CENTRAL; 2019, Issue 2) in the Cochrane Library (searched 26 March 2019);
Ovid MEDLINE including In‐Process & Other Non‐Indexed Citations (1946 to 26 March 2019);
Ovid Embase (1974 to 26 March 2019);
EBSCO CINAHL Plus (Cumulative Index to Nursing and Allied Health Literature; 1937 to 26 March 2019).
The search strategies for the Cochrane Wounds Specialised Register, CENTRAL, Ovid MEDLINE, Ovid Embase and EBSCO CINAHL Plus can be found in Appendix 2. We combined the Ovid MEDLINE search with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity‐ and precision‐maximising version (2008 revision) (Lefebvre 2011). We combined the Embase search with the Ovid Embase filter developed by the UK Cochrane Centre (Lefebvre 2011). We combined the CINAHL Plus searches with the trial filters developed by the Scottish Intercollegiate Guidelines Network (SIGN 2018). There were no restrictions with respect to language, date of publication or study setting.
We also searched the following clinical trials registries:
ClinicalTrials.gov (www.clinicaltrials.gov) (searched 22 March 2019);
World Health Organization (WHO) International Clinical Trials Registry Platform (http://apps.who.int/trialsearch/Default.aspx) (searched 22 March 2019);
EU Clinical Trials Register (https://www.clinicaltrialsregister.eu/ctr‐search/search) (searched 22 March 2019);
University Hospital Medical Information Network Clinical Trials Registry (UMIN‐CTR) (www.umin.ac.jp/ctr/index‐j.htm) (searched 22 March 2019).
Search strategies for clinical trial registries can be found in Appendix 2.
Searching other resources
We searched the bibliographies of all retrieved and relevant publications identified by these strategies for further studies. We checked the reference lists of all included studies and relevant systematic reviews to identify additional studies missed from the original electronic searches.
A citation search was also conducted on the Web of Science to identify articles that cited any of the included studies.
We contacted experts and industry representatives to enquire about unpublished or ongoing studies. We contacted suture manufactures, such as Ethicon and Covidien, but received no response.
Data collection and analysis
Data collection and analysis was carried out according to the methods stated in the published protocol (Goto 2016) which were based on the Cochrane Handbook for Systematic reviews of Interventions (Higgins 2011).
Selection of studies
Two review authors (SG and KH) examined the titles and abstracts of references identified by the electronic search strategies described above to determine which were likely to be relevant. We obtained the full text for each potentially relevant study. These two authors assessed each article independently, and decided whether to include the study in the meta‐analysis. Disagreement between authors was resolved by discussion. Arbitration was provided by a third author (TAF). Agreement between review authors in the study selection was reported. The disagreement in the selection of studies was evaluated by quantifying both the percentage of agreement and Cohen's kappa (k) (Cohen 1960). These are the methods to measure interrater reliability (McHugh 2012). Cohen's kappa gives a score of how much homogeneity or consensus there is in the ratings given by judges. Cohen suggested the Kappa result be interpreted as follows: values ≤ 0 as indicating no agreement and 0.01 to 0.20 as none to slight, 0.21 to 0.40 as fair, 0.41 to 0.60 as moderate, 0.61 to 0.80 as substantial, and 0.81 to 1.00 as almost perfect agreement.
When missing information inhibited the evaluation of a study, we classified the study as a 'study awaiting assessment' and sought further information from the original authors or other possible sources. We described the reasons for exclusion of studies for which we obtained full copies of the text in the 'Characteristics of excluded studies' table. The study selection process is reported in a PRISMA flow diagram to summarise this process (Liberati 2009).
When studies were reported in multiple publications/reports, we obtained all publications. Whilst the study was included only once in the review, we extracted data from all reports to ensure all available relevant data were obtained.
Data extraction and management
Independently, at least two of three review authors (SG and TS or RG) extracted information from the included trials using a structured, pilot‐tested, Excel data extraction form. Any disagreement was resolved either by discussion or by consultation with a fourth author (KH). If necessary, authors of studies were contacted to obtain further clarification. Agreement between the data extractors with regard to the primary outcome was reported. This is one of the methods to measure interrater reliability (McHugh 2012).
This data extraction form included the following items:
general information: title, authors, and year of publication of the first report;
study characteristics including design, setting, country, and duration of the study;
participants: total number; number of each age, sex, and comorbidity; type of surgery; and wound class;
interventions and comparisons: total number of intervention groups, type of interventions, and type of suture materials and suturing method in each arm;
outcomes: definition of outcomes, number of participants allocated to each intervention group, sample size, number of missing participants, number of events (dichotomous outcomes), standard deviation (SD) and mean (continuous outcomes), timing of assessment, and duration of follow‐up;
risk of bias and publication status.
Assessment of risk of bias in included studies
Independently, at least two out of three review authors (SG and TS or RG) assessed the risk of bias of the included studies using the criteria described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). If assessors disagreed, the final rating was made by discussion or with the involvement of an additional assessor (TAF), if necessary. Agreement between the two independent raters in the 'Risk of bias' assessment was reported as percentage agreement and weighted kappa. The following domains were assessed (see Appendix 3):
random sequence generation;
allocation concealment;
blinding of participants and personnel;
blinding of outcome assessment;
incomplete outcome data;
selective outcome reporting;
other bias (distribution of baseline characteristics, industry funding etc.).
We assessed blinding and incomplete outcome data for each of the review outcomes separately. In the 'Risk of bias' table, we have presented the risk of blinding and incomplete outcome data mainly focussing on the short‐term postoperative outcomes including SSI, wound complications and wound dehiscence. For the GRADE assessment for the long‐term outcomes such as cosmesis of scar, patient satisfaction and QOL, we evaluated relevant 'Risk of bias' domains. In this review, we anticipated that blinding of participants and personnel may not be possible. For this reason, the assessment of the risk of blinding focused on whether blinded outcome assessment was reported: blinding of assessment is especially important because assessment of wound outcomes, such as SSI, wound complications and dehiscence, can be subjective. We used blinding of outcome assessment to determine risk of bias from blinding in these instances. Although we recorded risk of bias for blinding of personnel and participants, we did not downgrade the certainty of the evidence for this alone, where the nature of the comparison made it highly likely.
The risk of bias in each domain was assessed and categorised into:
low risk of bias, i.e. plausible bias that is unlikely to alter the results seriously;
high risk of bias, i.e. plausible bias that seriously weakens confidence in the results;
unclear risk of bias, i.e. plausible bias that raises some doubt about the results.
Where inadequate details of randomisation and other characteristics of trials were provided, the risk of bias was classified as unclear, unless further information could be obtained by contacting the authors. We provided a quote from the study report together with a justification for our judgement in the 'Risk of bias' table. We summarised the 'Risk of bias' judgements across different studies for each of the domains listed. Where information on risk of bias related to unpublished data or correspondence with a trialist, we have noted this in the 'Risk of bias' table.
Measures of treatment effect
We used Review Manager 5 (Review Manager 2014) to analyse the data. We identified both dichotomous data and continuous data.
Dichotomous data
For binary outcomes, we presented results as the risk ratio (RR) with 95% CI, because risk is a concept that is more familiar and simpler to understand for clinicians than odds.
Continuous data
Wherever possible, we expressed continuous data as mean difference (MD) with 95% CI. In cases where different scales were used to measure the same or similar construct, we used the standardised mean difference (SMD) with 95% CI for continuous outcomes.
Endpoint versus change data
We used endpoint data, which typically cannot have negative values and are easier to interpret from the clinical point of view. If endpoint data were not available, we had planned to use the change data, however, we found no such cases. We considered this strategy to be less prone to selective reporting.
Time‐to‐event data
For time‐to‐event data, we planned that our primary effect measure would be the hazard ratio (HR) with 95% CI. However, we found no studies which reported this type of data.
Skewed data
To avoid analysing skewed data as normally distributed data, we applied the following standards to all data before inclusion.
We entered data from studies of at least 100 participants into the analysis irrespective of the following rules, because skewed data pose less of a problem in large studies.
-
Endpoint data: when a scale started from the finite number zero, we subtracted the lowest possible value from the mean and divided this by the standard deviation if the data were reported.
If this value was lower than 1.0, it strongly suggested a skew and we excluded the study from meta‐analytic pooling and presented it narratively.
If this ratio was higher than 1.0 but below 2.0, there was suggestion of a skew. We entered the study in the analysis and tested whether its inclusion or exclusion changed the results substantially.
If the ratio was larger than 2.0, the study was included in the analysis because skew was less likely (Altman 1996; Higgins 2011).
When continuous data are presented on a scale that includes the possibility of negative values (such as change data), it is difficult to tell whether data are skewed or not. We had planned to enter such studies into the analysis because change data tend to be less skewed than other data and because excluding studies also leads to bias, as not all the available information is used. However, we found no such cases.
A common way that trialists indicate that they have skewed data is by reporting medians and interquartile ranges. When we encountered this, we noted that the data were skewed and the study was excluded from meta‐analytic pooling and was summarised narratively.
Unit of analysis issues
Cluster‐randomised trials
In cluster‐randomised trials, groups of individuals rather than individuals are randomised to different interventions (Higgins 2011). In this review, no cluster‐randomised trials were identified.
In future versions of this review, when cluster‐randomised trials are analysed as if the randomisation was performed on the individuals rather than the clusters, we will perform approximately correct analyses (Higgins 2011). The idea is to reduce the size of each trial to its 'effective sample size' (Rao 1992). The effective sample size of a single intervention group in a cluster‐randomised trial is its original sample size divided by a quantity called the 'design effect'. The design effect calculated by the equation: 1 + (M – 1) ICC, where M is the average cluster size and ICC is the intra‐cluster correlation coefficient. A common design effect is usually assumed across intervention groups. For dichotomous data, both the number of participants and the number experiencing the event will be divided by the same design effect. For continuous data, only the sample size will be reduced; means and standard deviations should remain unchanged.
Multiple body parts Ⅰ: body parts receive the same intervention
Where studies were randomised at the participant level and outcomes measured at the wound level, we treated the participant as the unit of analysis when the number of incisions (wounds) assessed appeared to be equal to the number of participants (e.g. one wound per person).
In some studies where people were randomised and multiple wounds of the body received the same intervention (e.g. multiple wounds per participant or perhaps only on some participants), a separate outcome judgement was made for each wound, and the number of wounds was used as the denominator in the analysis. Since not all participants had multiple wounds, this was not a cluster trial per se but rather a trial that incorrectly included a mixture of individual and clustered data.
In cases where included studies contained some or all clustered data, we reported this, noting whether data had been (incorrectly) treated as independent. We noted this situation in the other risk of bias of Characteristics of included studies and performed a post hoc sensitivity analysis excluding these studies.
Multiple body parts Ⅱ: body parts receive different interventions
If multiple wounds were randomised to different groups, we had planned to include the trial only if appropriate analysis was undertaken to take within‐subject correlation into account (paired data), or if it was possible to perform such an analysis using the available data. However, it was often not clear whether such analysis had been undertaken. We noted this situation in the 'other risk of bias' of Characteristics of included studies and performed a post hoc sensitivity analysis excluding these studies.
While we accepted the results from trials in which multiple wounds were randomised to different intervention groups (split‐body design), we excluded trials in which a part of the wound was randomised to one intervention and the rest of the wound to another intervention (split‐wound design).
These trials have similarities to cross‐over trials: in cross‐over trials, individuals receive multiple treatments at different times, while in these trials they receive multiple treatments at different sites.
Multiple intervention groups
The studies that compared more than two intervention groups were included in meta‐analysis by making multiple pairwise comparisons between all possible pairs of intervention groups. If two or more interventions were compared with control and were eligible for the same meta‐ analysis, we pooled the intervention arms and compared these with control. We used combined group data, as recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Dealing with missing data
We tried to contact authors of trials to obtain any missing data.
Missing participants
Dichotomous data
All dichotomous data were analysed on the basis of the intention‐to‐treat (ITT) principle. When participants had been withdrawn from a trial and the original authors had not imputed the data appropriately, we assumed that the condition of these participants would have remained unchanged if they had stayed in the trial, or we treated them as treatment failures (a 'worst‐case' scenario). We performed sensitivity analyses to assess how sensitive results were to reasonable changes in the assumptions. We addressed the potential impact of missing data on the findings of the review in the Discussion.
Continuous data
We used continuous data as they were presented by the original authors, without any imputations according to the recommendation in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Whenever ITT data were presented by the authors, we preferred these to 'per protocol or completer' data sets.
Missing data
We contacted investigators or study sponsors in order to obtain numerical outcome data where possible (e.g. when a study was identified as abstract only).
Missing statistics
When only P values or standard error (SE) values were reported, we calculated standard deviations (SDs) (Altman 1996). In the absence of supplemental data after requests to the original authors, we calculated SDs from CIs, T values, or P values as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Otherwise, we had planned to impute them from other studies in the meta‐analysis according to a validated method developed by Furukawa (Furukawa 2000). However, we could not use this method because SDs of wound closure time and length of hospital stays varied greatly by study.
Assessment of heterogeneity
Initially, we investigated heterogeneity by visual inspection of the forest plots. We performed the Chi2 test in order to detect the presence of heterogeneity. We regarded heterogeneity as present if there was a low P value (less than 0.10) in the Chi2 test for heterogeneity. Since the Chi2 test has low power to assess heterogeneity when a small number of participants or studies are included, we set the probability at the 10% level of significance. We also calculated the I2 statistic in order to assess the degree of heterogeneity (Higgins 2002). The I2 statistic is defined as the proportion of total heterogeneity that exceeds what would be expected due to chance (Higgins 2003). It is interpreted as recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), as follows: 0% to 40% may not be important; 30% to 60% may represent moderate heterogeneity; 50% to 90% may represent substantial heterogeneity; 75% to 100% represents considerable heterogeneity.
We also reported Tau2, that is, the between study variance in random‐effects model meta‐analyses.
If apparent upon visual inspection of the forest plots or if there was statistically substantive heterogeneity (I2≥50%), we investigated its potential sources through subgroup and sensitivity analyses.
Assessment of reporting biases
We assessed publication bias by a funnel plot if the number of studies included was 10 or more. We investigated the presence of small study effects for the primary outcome only; along with visual inspection of the plots, we used Egger's test to examine whether the association between estimated intervention effects and the study size was greater than might be expected to occur by chance (Egger 1997).
Data synthesis
We performed meta‐analyses according to the recommendations of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We used a random‐effects model in the analysis because we considered that the different studies would estimate different, yet related, intervention effects (DerSimonian 1986). We used a fixed‐effect model for the sensitivity analysis.
With regard to dichotomous outcomes, risk ratio calculations did not include trials in which no events occurred in either group in the meta‐analysis, whereas risk difference calculations did. We reported the risk difference (RD) if the results using this association measure were different from the risk ratio in terms of statistical significance. However, the risk ratio is the measure that we planned to use to arrive at conclusions, since risk ratios perform better when there are differences in the control event rate (proportion of participants who develop the event in the control groups) (Furukawa 2002).
When there was extreme heterogeneity (I2≥90%), we did not pool the data and presented the results narratively.
Subgroup analysis and investigation of heterogeneity
Subgroup analyses are often exploratory in nature and should be interpreted cautiously. Firstly, because these analyses often involve multiple analyses, they may yield false positive results; secondly, these analyses lack power and are more likely to result in false negative results. With these reservations in mind, we performed the following subgroup analysis for the primary outcome only and only where there were sufficient studies:
absorbable versus non‐absorbable subcuticular sutures;
location of surgery on the body (trunk, extremities, and face) as wound healing rates may be different;
CDC class 1 (clean) versus class 2 (clean‐contaminated) versus class 3 (contaminated) surgery;
continuous versus interrupted skin sutures;
endoscopic (e.g. laparoscopic, thoracoscopic and arthroscopic) versus open surgery.
Sensitivity analysis
The process of undertaking a systematic review and meta‐analyses involves a sequence of decisions that may be somewhat arbitrary or unclear (Higgins 2011). A sensitivity analysis is a repeat of the primary analysis in which alternative decisions or ranges of values are substituted for decisions that were arbitrary or unclear. We performed the following sensitivity analyses for the primary outcome only:
restricted inclusion in the analysis to only those studies that are considered to be at a low risk of selection bias (i.e. adequate allocation sequence generation and adequate allocation concealment). Since it was impossible for both the operators of the procedure and assessors of postoperative short‐term outcomes to be blinded to the intervention, we did not use blinding of personnel and outcome assessment as a marker of trial quality.
examined handling of missing participants firstly by ITT analysis based on the worst‐worst scenario assuming that the dropouts in both the intervention and the control groups had the event of interest, and secondly by the worst‐best scenario, assuming that the dropouts in the intervention had the event of interest while those in the control did not.
excluded studies sponsored by companies that produce suture devices, as they have an inevitable conflict of interest.
for meta‐analyses, use a fixed‐effect model instead of a random‐effects model.
excluded studies that had unit of analysis issues. See 'Differences between protocol and review'.
'Summary of findings' tables and assessment of the quality of the evidence using the GRADE approach
We have presented the main results of the review in 'Summary of findings' tables, which provide key information concerning the quality of evidence, the magnitude of the effect of the interventions examined, and the sum of the available data on the main outcomes, as recommended by Cochrane (Schünemann 2011a). The 'Summary of findings' tables also included an overall grading of the body of evidence related to each of the main outcomes using the GRADE (Grading of Recommendations Assessment, Development and Evaluation) approach (Schünemann 2011b).
The GRADE approach defines the quality of a body of evidence as the extent to which one can be confident that an estimate of effect or association is close to the true quantity of specific interest. The quality of a body of evidence involves consideration of within‐trial risk of bias (methodological quality), directness of evidence, heterogeneity, precision of effect estimates, and risk of publication bias (Schünemann 2011b). We presented the following outcomes in the 'Summary of findings' tables:
incidence of surgical site infection (SSI) within 30 days of the operation;
incidence of wound complications (e.g. haematoma, seroma, skin separation) within 30 days of the operation;
incidence of wound dehiscence (skin separation) (we added this outcome as a post hoc decision);
cosmesis of scar (as defined by the authors for a minimum follow‐up of six months);
patient satisfaction (as defined by the authors, within 30 days, and at, or after, 60 days of the operation);
wound closure time in the operation (minutes);
cost at maximal follow‐up (as reported by authors).
Please see Differences between protocol and review for changes to this section.
For relevant outcomes reported for comparisons not listed above, we presented GRADE assessments narratively within the Results section without inclusion in a 'Summary of findings' table.
In terms of the GRADE assessment, when making decisions for the risk of bias domain, we downgraded only when studies were classed at high risk of bias for one or more domains. We did not downgrade for unclear risk of bias assessments. In assessing the precision of effect estimates, we also followed GRADE guidance (GRADE 2013); we assessed the size of confidence intervals, downgrading twice for imprecision when there were very few events and CIs around effects included both appreciable benefit and appreciable harm.
Results
Description of studies
See Characteristics of included studies; Characteristics of excluded studies; Characteristics of studies awaiting classification and Characteristics of ongoing studies.
Results of the search
The number of references identified by the searches was 1606. Of these, 1480 remained after de‐duplication. We excluded 1321 references after assessment of the titles and abstracts. We retrieved a total of 159 full‐text papers for full inspection. Of these studies, we excluded 64 (65 full‐text articles) with reasons, sixteen were ongoing trials and five presented too little information to be classified. We included the remaining 66 studies (73 full‐text) in the final qualitative analyses; among these, we included 64 in the final quantitative analyses (50 for the primary outcome analyses). See Figure 1 for a PRISMA flow diagram depicting the study selection process. Cohen's weighted kappa among assessors for the selection was 0.963 (percentage of agreement = 96.2%). This is the method to measure interrater reliability (McHugh 2012). It was interpreted as almost perfect agreement.
1.
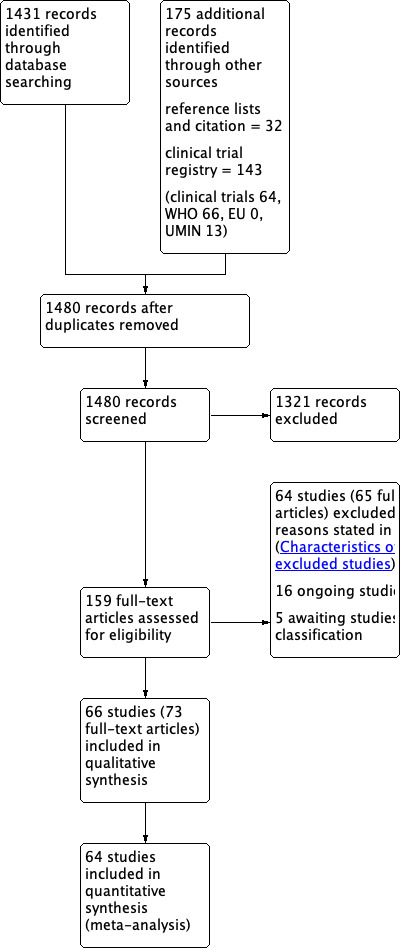
Study flow diagram.
Included studies
We included 66 studies in this review, of which we included 64 in quantitative analyses (50 for the primary outcome analyses). We could not include two studies (Fiennes 1985; Sakka 1995) in quantitative analyses because no data were presented in one study (Fiennes 1985) and the other did not report the number randomised in each group (Sakka 1995); we attempted to contact the authors, but received no reply. The characteristics of the included studies can be summarised as follows (see Characteristics of included studies).
Design
All included studies were randomised controlled trials. Only seven studies had a multicentre design (Imamura 2016; Khan 2006; Kobayashi 2015; Liu 2017; Maartense 2002; Obermair 2007; Tsujinaka 2013). All trials were of parallel group design except for five that had a split‐body design (different wounds on same participant randomised (Anatol 1997; Buchweitz 2005; Rebello 2009; Rosen 1997; Subramanian 2005).
Eleven included trials had more than two arms: eight studies had three arms (Anatol 1997; Barker 1984; Khan 2006; Maartense 2002; Obermair 2007; Rosen 1997; Simpson 1979; Zwart 1989) and three studies had four arms (Eggers 2011; Mullen 1999; Murphy 1995).
Country
Twenty‐four studies were conducted in the United Kingdom. Eight studies were conducted in the United States, five studies were conducted in Japan and the Netherlands, three studies were conducted in Australia, China, Iran and two studies were conducted in Canada, Finland and Nigeria. Other studies were conducted in Denmark, Germany, India, Italy, Malaysia, Mexico, Singapore, Turkey and Trinidad.
Sample sizes
The total sample size varied between eight (50 incisions) (Rebello 2009) and 1264 participants (Kobayashi 2015), with a mean sample size of 115 participants per study. The total number of participants included in the analyses is 7487.
Participants
The majority of studies included only adults, except for nine studies that included adults and children (Anatol 1997; Brown 2009; Hopkinson 1982; Keng 1989; Khajouei 2007; McGreal 2002; Onwuanyi 1990; Tanaka 2016; Taube 1983;) and seven studies that included only children (Ademuyiwa 2009; Grottkau 2010; Ong 2002; Pauniaho 2010; Rebello 2009; Van den Ende 2004; Xu 2014).
Thirty‐seven studies included only CDC class 1 (clean) surgeries such as: orthopaedic procedures (Baek 2009; Eggers 2011; Grottkau 2010; Khan 2006; Rebello 2009; Roolker 2002; Sakka 1995; Shetty 2004; Xu 2014), cardiovascular surgery (Chughtai 2000; Corder 1991; Karabay 2005; Krishnamoorthy 2009; Lazar 2011; Mullen 1999; Tanaka 2016), surgeries through groin crease incision (Ademuyiwa 2009; Anatol 1997; Brown 2009; Keng 1989; Murphy 1995; Ong 2002; Subramanian 2005; Swtizer 2003; Van den Ende 2004), breast surgeries (Barker 1984; Gennari 2004; Steele 1983), port or pacemaker implantation (Chen 2013; Martin 2017; Pitcher 1983), and neck or facial surgeries (Liu 2017; O'Leary 2013; Reed 1997; Selvadurai 1997; Soni 2013; Teoh 2018).
Eleven studies included only CDC class 2 (clean‐contaminated) surgeries such as: gastrointestinal surgeries (Jallali 2004; Kobayashi 2015; Tanaka 2014; Tsujinaka 2013), hepatectomy (Chen 2018), urological surgeries (Sebesta 2004), and gynaecological surgeries (Buchweitz 2005; Jan 2013; Kuroki 2017; Obermair 2007; Rosen 1997).
Ten studies included appendectomies (Andrade 2016; Foster 1977; Ghaderi 2010; Hopkinson 1982; Javadi 2018; Khajouei 2007; Kotaluoto 2012; McGreal 2002; Onwuanyi 1990; Pauniaho 2010). Appendectomies could be classified CDC class 2 to 4. In eight other trials, the contamination level in the surgeries included was variable (Clough 1975; Fiennes 1985; Imamura 2016; Maartense 2002; Ranaboldo 1992; Simpson 1979; Taube 1983; Zwart 1989).
Although the majority of studies enrolled people who underwent CDC class 1 (clean) surgeries, two‐thirds of participants were enrolled in the studies which included CDC class 2 to 4 surgeries such as appendectomies and gastrointestinal surgeries.
Characteristics of interventions
All the included studies used absorbable subcuticular sutures except for eleven studies that used non‐absorbable subcuticular sutures (Ghaderi 2010; Hopkinson 1982; Khajouei 2007; Liu 2017; Onwuanyi 1990; Selvadurai 1997; Soni 2013; Steele 1983; Subramanian 2005; Tanaka 2016; Taube 1983). Two studies used absorbable and non‐absorbable subcuticular sutures (Barker 1984; Simpson 1979). Twenty‐three studies also used subcuticular continuous sutures (Anatol 1997; Andrade 2016; Baek 2009; Chughtai 2000; Eggers 2011; Gennari 2004; Hopkinson 1982; Khan 2006; Kotaluoto 2012; Krishnamoorthy 2009; Lazar 2011; Liu 2017; Martin 2017; McGreal 2002; Murphy 1995; O'Leary 2013; Obermair 2007; Pauniaho 2010; Pitcher 1983; Ranaboldo 1992; Selvadurai 1997; Swtizer 2003; Tanaka 2016) and eight studies used subcuticular absorbable interrupted sutures (Anatol 1997; Chen 2018; Fiennes 1985; Imamura 2016; Kobayashi 2015; Maartense 2002; Tanaka 2014; Tsujinaka 2013). The remaining studies did not report the method of continuous or interrupted sutures. Two studies did not report the nature of the suture material used (Gennari 2004; Mullen 1999).
Twenty‐five of 66 included studies compared subcuticular sutures with transdermal sutures for skin closure (Andrade 2016; Baek 2009; Buchweitz 2005; Chen 2013; Clough 1975; Corder 1991; Fiennes 1985; Foster 1977; Ghaderi 2010; Hopkinson 1982; Javadi 2018; Karabay 2005; Khajouei 2007; Kotaluoto 2012;Liu 2017; McGreal 2002;Murphy 1995; Onwuanyi 1990; Pauniaho 2010; Rosen 1997; Sakka 1995; Simpson 1979; Taube 1983; Tanaka 2014; Zwart 1989). Two of these studies had a third arm using staples (Murphy 1995; Zwart 1989) and one of these studies had a third arm using surgical tapes (Rosen 1997). All studies used non‐absorbable transdermal sutures except for two studies (Buchweitz 2005; Chen 2013). All studies also used transdermal interrupted sutures except for three studies (Murphy 1995; Sakka 1995; Zwart 1989).
Eighteen studies compared subcuticular sutures with staples (Chen 2018; Chughtai 2000; Eggers 2011; Imamura 2016; Khan 2006; Kobayashi 2015; Kuroki 2017; Mullen 1999; Murphy 1995; Obermair 2007; Ranaboldo 1992; Reed 1997; Selvadurai 1997; Shetty 2004; Steele 1983; Subramanian 2005; Tsujinaka 2013; Zwart 1989) and two of these studies also had a third arm using tissue adhesives (Eggers 2011; Khan 2006).
Seventeen trials compared subcuticular sutures with tissue adhesives (Ademuyiwa 2009; Brown 2009; Eggers 2011; Gennari 2004; Jallali 2004; Jan 2013; Khan 2006; Keng 1989; Krishnamoorthy 2009; Maartense 2002; Martin 2017; Ong 2002; Sebesta 2004; Soni 2013; Swtizer 2003; Teoh 2018; Van den Ende 2004) and one of these studies also had a third arm using surgical tapes (Maartense 2002).
Nine studies compared subcuticular sutures with surgical tapes (Anatol 1997; Barker 1984; Grottkau 2010; Lazar 2011; Maartense 2002; O'Leary 2013; Pitcher 1983; Rebello 2009; Rosen 1997). Two of these studies used Steri‐strips® (Maartense 2002; O'Leary 2013; Rosen 1997), three of these studies used Steri‐Strip S® (Grottkau 2010; Lazar 2011; Rebello 2009) and two studies used Opsite®(Barker 1984; Pitcher 1983). Anatol 1997 did not report details of the tapes.
Three studies (Roolker 2002; Tanaka 2016; Xu 2014) compared subcuticular sutures with a new skin closure device, namely surgical zipper.
Funding source
The majority of included studies did not report funding or conflict of interest. Fifteen studies reported the funding of source clearly (Ademuyiwa 2009; Chen 2018; Eggers 2011; Grottkau 2010; Imamura 2016; Jan 2013; Kobayashi 2015; Krishnamoorthy 2009; Kuroki 2017; Lazar 2011; Liu 2017; Martin 2017; Tanaka 2014; Tsujinaka 2013; Xu 2014). Of these studies, four studies received corporate/industry funds (Grottkau 2010; Lazar 2011; Liu 2017; Tsujinaka 2013).
Primary outcome measures
Fifty of the included trials reported incidence of SSI. Agreement between the data extractors with regard to the primary outcome was 97%. The definition of infection varied and the time of postoperative wound examination varied between studies. Only seven trials defined infection according to the CDC criteria for SSI (Chen 2018; Imamura 2016; Kobayashi 2015; Kotaluoto 2012; Kuroki 2017; Tanaka 2014; Tsujinaka 2013), which is considered to be the gold standard definition for wound infection (Mangram 1999). Eleven trials used author‐defined clinical criteria (Andrade 2016; Buchweitz 2005; Chughtai 2000; Eggers 2011; Javadi 2018; Khan 2006; Liu 2017; Maartense 2002; Mullen 1999; Pauniaho 2010; Roolker 2002), and one trial used a self‐devised wound scale to define infection (Karabay 2005). Seven trials defined infection if pus was discharged (Clough 1975; Corder 1991; Foster 1977; Hopkinson 1982: Keng 1989; Ranaboldo 1992; Taube 1983), and three trials required positive wound swabs to define infection (Lazar 2011; Murphy 1995; Shetty 2004). The remaining trials did not record the definition of infection used.
Excluded studies
The characteristics of the excluded studies can be summarised as follows (see Characteristics of excluded studies).
We excluded a total of 65 studies (66 references). Five studies were not RCTs (Bernstein 2001; Navali 2014; Serour 1996; Singh 2006; Watson 1983). Seven studies were quasi‐randomised (Angelini 1984; Cassie 1988; Clayer 1991; Davies 1995; Elliot 1989; Matin 2003; Ralphs 1982). Three references were letters or comments on excluded trials (Cordova 2013; Ries 2016; Watts 1982).
Three studies investigated methods of fascial closure (layers of connective tissue that surround muscles and other structures) (Erel 2001; Greene 1999; Kharwadkar 2005). Five studies investigated methods of closure of other layers of the wound (Cameron 1987; Chan 2017; Leaper 1985; Liang 2015; Nair 1988). Shanahan 1990 investigated methods of dressing. Four studies were not comparisons of subcuticular sutures with any other skin closure methods (Meinke 1996; Nipshagen 2008; Pickford 1983; Plotner 2011). In 15 studies, it was not clear whether the study involved a comparison of subcuticular sutures versus any other skin closure methods (Bernard 2001; Cheng 1997; Eldrup 1981; Gatt 1985; Handschel 2006; Harvey 1986; McLean 1980; Menovsky 2004; Risnes 2001; Sadick 1994; Selo‐Ojeme 2002; Shamiyeh 2001; Sinha 2001; Szabó 2002; Van de Gevel 2010).
Two studies did not assess a relevant wound type (Milone 2014; You 2016), five studies did not assess eligible outcomes of our review, although these studies reported their own outcomes (Alicandri‐Ciufelli 2014; Consorti 2013; Lombardi 2011; Rizvi 2018; Wyles 2016), and three studies were split‐wound design (Johnson 1997; Kerrigan 2010; Richter 2012).
We excluded seven studies because both arms in the study received subcuticular sutures (Blondeel 2014; Buttaro 2015; Park 2015) or dermal sutures (Koonce 2015; Nahas 2004; Parvizi 2013; Rui 2017). We judged that these studies did not focus on subcuticular sutures for skin closure, but on superficial adjunct wound closure methods. In four studies (Glennie 2017; Lalani 2016; Lazar 2008; Mudd 2013), all participants in the intervention group had tissue adhesives (Dermabond) placed. We excluded these studies because they assessed a mixture of subcuticular sutures and tissue adhesives within the same intervention group. See also Differences between protocol and review.
Ongoing studies and studies awaiting classification
We identified 16 ongoing studies. Eight studies are exploring subcuticular suture versus skin staples (one of these, absorbable staples) (ACTRN12611000399998; CTRI/2018/08/015470; NCT02046239; NCT02936063; NCT03108742; NCT03788239; UMIN000002873; UMIN000003235), five studies are exploring subcuticular suture versus tissue adhesives (CTRI/2018/02/011698; ISRCTN80786695; ISRCTN96030942; NCT01996917; NCT02551510) and three studies are exploring subcuticular suture versus transdermal suture (Maschuw 2014; IRCT20161217031440N1; IRCT20180820040840N1). For further details, see Characteristics of ongoing studies. Five potentially eligible studies have not yet been incorporated into the review. We are awaiting full text for three of these studies (Choudry 1996; Rubio‐Perez 2014; Zhang 2011) and awaiting data for another two studies. In these two studies, we could not use the data for skin closure because they were combined with data for other methods of sutures (Lubowski 1985) and for laceration closure (Singer 2002); we attempted to contact the authors to request data by subgroup, but received no reply. Details of these studies are presented in the table of Characteristics of studies awaiting classification.
Risk of bias in included studies
None of the included studies were at low risk of bias for all domains. All the studies had an unclear or high risk of bias for two or more domains. Given the nature of the intervention, participants and caregivers may not be blinded and therefore almost all of the studies were rated at high or unclear risk of bias for this domain (performance bias). With regard to the other domains where risk of bias could be avoided, there were 32 studies with one or more domains classed at high risk of avoidable bias and we rated seven of these studies as being at high risk of avoidable bias in more than one domain. Most studies had multiple domains which were at unclear risk of bias. For only one domain (reporting bias), we considered the majority of the studies to be at low risk of bias. For details of the 'Risk of bias' judgements for each study, see Characteristics of included studies. A graphical representation of the overall risk of bias in included studies is presented in Figure 2 and Figure 3. The agreement between the two independent raters in the 'Risk of bias' assessment ranged between 62% and 96%, with weighted Kappa between 0.88 to 0.99.
2.
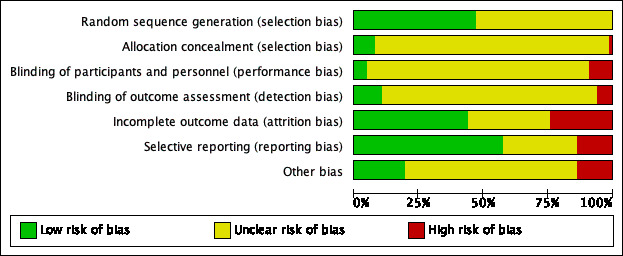
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
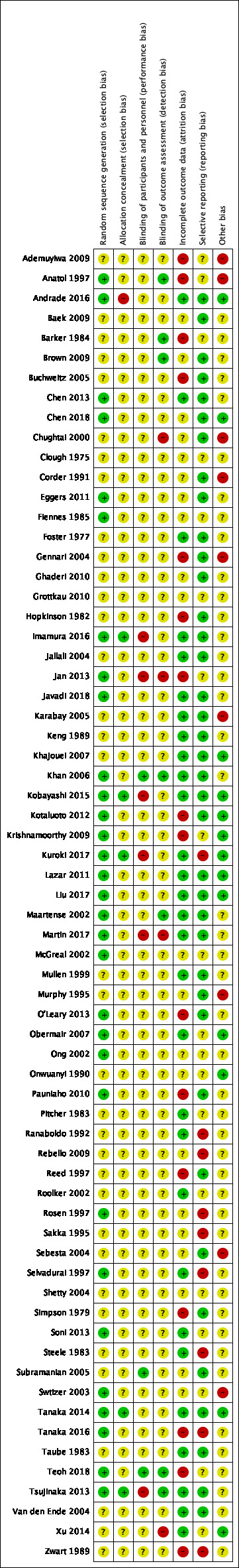
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Sequence generation was performed adequately in 31 studies. Almost all these studies used random sequences generated by computerised randomisation programmes except for Anatol 1997, Chen 2013, Pauniaho 2010, Rosen 1997 and Selvadurai 1997. Anatol 1997 used throwing of a dice, Chen 2013 used envelopes, Pauniaho 2010 used coin tossing, Rosen 1997 used random number draws and Selvadurai 1997 used a table of random numbers. The remaining studies did not report enough information to enable a judgement. We considered these studies to be at unclear risk of bias (sequence generation).
The majority of studies did not report the details about allocation concealment. Some studies gave evidence that they used envelopes that were sealed and opaque, but lacked sufficient evidence that they were sequentially numbered. Many other studies provided no information on allocation of the randomisation sequence at all. We assessed only five studies as being at low risk of bias for both sequence generation and allocation concealment (Imamura 2016; Kobayashi 2015; Kuroki 2017; Tanaka 2014; Tsujinaka 2013). Agreement between the two independent raters in the risk of allocation bias assessment was 96% (weighted Kappa 0.99 for sequence generation and 0.99 for allocation concealment).
Blinding
It is difficult to blind the caregivers, who normally check the wounds every day, and participants, who have them checked every day. Almost all the trials were at high or unclear risk of bias due to lack of blinding of participants and caregivers. However, in three (Khan 2006; Subramanian 2005; Teoh 2018) of 66 trials, the researchers paid particular care to blind the caregivers and the participants by using occlusive dressings. In seven trials, outcome assessors were blinded (Anatol 1997; Barker 1984; Brown 2009; Khan 2006; Maartense 2002; Teoh 2018; Tsujinaka 2013). We considered four studies to be at high risk of detection bias (Chughtai 2000; Jan 2013; Martin 2017; Xu 2014). The remaining trials did not report enough information to enable a judgement. We considered these studies to be at unclear risk of bias. Agreement between the two independent raters in the risk of detection bias assessment was 94% (weighted Kappa 0.99).
Incomplete outcome data
We rated the risk of incomplete outcome reporting for short‐term outcomes. We rated 29 studies as being at low risk of attrition bias and 16 studies as being at high risk of attrition bias. This was often due to high proportional rates of dropout, or unclear reasons for large numbers of dropouts, or both. The remaining 21 studies were judged as being at unclear risk of bias for this domain, as reasons for dropout were not clear, or the extent to which the dropout rates affected the results was unclear. Agreement between the two independent raters in the risk of attrition bias assessment was 62% (weighted Kappa 0.88).
Selective reporting
None of the included trials had a published protocol. We rated 38 studies as being at low risk of selective outcome reporting as they fully reported all the outcomes they had planned to. We rated nine studies as being at high risk, because four studies reported the outcomes they did not pre specify in the methods section (Ranaboldo 1992; Selvadurai 1997; Tanaka 2016; Zwart 1989), four studies reported the important outcomes incompletely (Rebello 2009; Rosen 1997; Sakka 1995; Steele 1983), and one study did not report the outcome prespecified in the trial registry (Kuroki 2017). In the remaining 19 studies, the available information was not enough to make a judgement. Agreement between the two independent raters in the risk of reporting bias assessment was 68% (weighted Kappa 0.88).
Other potential sources of bias
We rated 13 of the included studies as being at low risk of other bias and nine studies as being at high risk. In the remaining 44 studies, the available information was not enough for us to make a judgement. The majority of these studies did not report funding or conflict of interest, thus, we could not judge whether the study had an inappropriate influence of funders. Fifteen studies were identified as having potential unit of analysis issues as it did not appear that paired data or clustered data were accounted for in the analysis (paired data: Anatol 1997; Rebello 2009; Rosen 1997; Subramanian 2005; clustered data: Ademuyiwa 2009; Anatol 1997; Baek 2009; Barker 1984; Chughtai 2000; Corder 1991; Jallali 2004; Jan 2013; Keng 1989; Murphy 1995; Swtizer 2003). We rated these studies as being at unclear or high risk of bias. Agreement between the independent raters in the risk of this bias assessment was 65% (weighted Kappa 0.91).
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5
Summary of findings for the main comparison. Subcuticular sutures compared with transdermal sutures for skin closure in non‐obstetric surgery.
| Subcuticular sutures compared with transdermal sutures for skin closure in non‐obstetric surgery | ||||||
| Patient or population: skin closure in non‐obstetric surgery Setting: hospitals Intervention: subcuticular sutures Comparison: transdermal sutures | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with transdermal sutures | Risk with subcuticular sutures | |||||
|
Surgical site infection (SSI) Incidence of wound infection follow‐up: 7 to 42 days |
71 per 1,000 | 78 per 1,000 (57 to 108) | RR 1.10 (0.80 to 1.52) | 3107 (20 RCTs) | ⊕⊕⊝⊝ Low 1 | There may be little difference between subcuticular and transdermal sutures groups in the incidence of SSI. |
|
Wound complications Incidence of wound complications follow‐up: 5 to 42 days |
102 per 1,000 | 85 per 1,000 (41 to 174) | RR 0.83 (0.40 to 1.71) | 1489 (9 RCTs) | ⊕⊝⊝⊝ Very low 2 | It is uncertain whether subcuticular sutures have an effect on wound complications compared with transdermal sutures. |
|
Wound dehiscence Incidence of wound dehiscence follow‐up: 7 to 42 days |
61 per 1,000 | 21 per 1,000 (5 to 94) | RR 0.35 (0.08 to 1.54) | 866 (6 RCTs) | ⊕⊝⊝⊝ Very low 3 | It is uncertain whether subcuticular sutures reduce the risk of wound dehiscence compared with transdermal sutures (as the certainty of the evidence has been assessed as very low) . |
|
Cosmesis of scar (cosmesis)
assessed with various methods follow‐up: 6 months to 12 months |
Insufficient data reported. We were unable to carry out further analyses. | ‐ | 950 (5 RCTs) | ⊕⊝⊝⊝ Very low 4 | It is uncertain whether subcuticular sutures improve the cosmesis of scar compared with transdermal sutures. | |
| Patient satisfaction (at 30 days) assessed with: score system scale from: 1 to 10 | The mean patient satisfaction score (at 30 days) was 7.4 | The mean patient satisfaction score with subcuticular sutures was 1.6 higher (1.32 to 1.88 higher). | MD 1.60 (1.32 to 1.88) | 290 (1 RCT) | ⊕⊕⊕⊝ Moderate 5 | Patient satisfaction at 30 days is probably higher in subcuticular sutures group compared with transdermal sutures group. |
|
Wound closure time (minutes) |
The mean wound closure time was 5.40 minutes | The mean wound closure time with subcuticular sutures was 5.81 minutes longer (5.13 to 6.49 minutes longer) | MD 5.81 (5.13 to 6.49) | 585 (2 RCTs) | ⊕⊕⊕⊝ Moderate 6 | Wound closure time is probably longer in subcuticular sutures group compared with transdermal sutures group. |
| Cost | The mean cost was 16 Naira | The mean cost with subcuticular sutures was 8 Naira lower (13.05 lower to 2.95 lower). | MD ‐8.00 (‐13.05 to ‐2.95) | 100 (1 RCT) | ⊕⊕⊝⊝ Low 7 | Subcuticular sutures may reduce the cost compared with transdermal sutures. In the study, participants used non‐absorbable (Nylon) subcuticular sutures. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; MD: Mean difference. | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Downgraded two levels: one level due to several trials at high risk of bias in at least one domain (attrition, selection, reporting and other bias); one level for imprecision as the confidence intervals overlapped 1 and 1.25.
2 Downgraded three levels: one level due to high risks of bias across varying domains (attrition, selection, reporting and other bias); one level for imprecision as the confidence intervals overlapped 1 and both 0.75 and 1.25); one level for inconsistency.
3 Downgraded three levels: one level due to risk of bias (attrition and selection bias) and two levels due to imprecision (study 95% CIs are wide).
4 Downgraded three levels: one level for high risk of attrition bias; one level for imprecision (narrative synthesis); one level for inconsistency (two reaching significance and two not).
5 Downgraded one level: one level for imprecision (low numbers of participants).
6 Downgraded one level for inconsistency.
7 Downgraded two levels: one level for risk of bias (the risk of bias in the included single study was unclear in almost every domain); one level for imprecision (low numbers of participants).
Summary of findings 2. Subcuticular sutures compared with skin staples for skin closure in non‐obstetric surgery.
| Subcuticular sutures compared with skin staples for skin closure in non‐obstetric surgery | ||||||
| Patient or population: skin closure in non‐obstetric surgery Setting: hospitals Intervention: subcuticular sutures Comparison: skin staples | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with skin staples | Risk with subcuticular sutures | |||||
|
Surgical site infection Incidence of wound infection follow‐up: 10 to 42 days |
90 per 1,000 | 73 per 1,000 (58 to 91) | RR 0.81 (0.64 to 1.01) | 4163 (14 RCTs) | ⊕⊕⊕⊝ Moderate 1 | There is probably little or no difference between subcuticular sutures and skin staples groups in the incidence of SSI. |
|
Wound complications Incidence of wound complications follow‐up: 10 to 42 days |
110 per 1,000 | 87 per 1,000 (70 to 108) | RR 0.79 (0.64 to 0.98) | 2973 (9 RCTs) | ⊕⊕⊕⊝ Moderate 2 | Subcuticular sutures probably on average decrease wound complications compared with skin staples. |
|
Wound dehiscence Incidence of wound dehiscence follow‐up: 10 to 42 days |
59 per 1,000 | 37 per 1,000 (26 to 56) | RR 0.63 (0.43 to 0.94) | 1984 (7 RCTs) | ⊕⊕⊝⊝ Low 3 | Subcuticular sutures may reduce the risk of wound dehiscence compared with skin staples. |
|
Cosmesis of scar
assessed with: score (using different scales) follow‐up: 6 months to 1 year |
The cosmetic score in the subcuticular sutures group was on average 0.12 SDs (95% CI: 0.11 lower to 0.36 higher) higher in the patients treated with subcuticular sutures than in the patients treated with skin staples. | SMD 0.12 (‐0.11 to 0.35) | 291 (3 RCTs) | ⊕⊕⊝⊝ Low 4 | As a rule of thumb, 0.2 SD represents a small effect, 0.5 a moderate effect, and 0.8 a large effect. There may be little or no difference between subcuticular sutures and skin staples groups in cosmesis of scar. |
|
| Patient satisfaction (at 30 days) assessed with: score system scale from: 1 to 5 | The mean patient satisfaction score (at 30 days) was 4.2 | The mean patient satisfaction score with subcuticular sutures was 0.20 higher (0.10 to 0.30 higher). | MD 0.20 (0.10 to 0.30) | 1232 (1 RCT) | ⊕⊕⊕⊕ High | Patient satisfaction at 30 days after surgery is slightly higher in subcuticular sutures group compared with skin staples group. |
|
Wound closure time (minutes) |
The mean wound closure time ranged from 0.9 to 4.5 minutes | Mean differences ranged between 0.30 and 5.50 minutes across four studies. Further analyses were not undertaken due to statistical heterogeneity in the results. | ‐ | 1384 (4 RCTs) | ⊕⊕⊝⊝ Low 5 | Wound closure time may be a few minutes longer in subcuticular sutures group compared with skin staples group. |
| Cost | Three trials favoured subcuticular sutures. It cost almost 5 to 15 USD lower per participant than staples. Another one favoured staples because most of the cost differential was attributed to procedure times. We were unable to carry out further analyses because of insufficient data. | ‐ | 342 (4 RCTs) | ⊕⊝⊝⊝ Very low 6 | It is uncertain whether subcuticular sutures reduce the cost compared with skin staples. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; MD: Mean difference; SD: Standard deviation; SMD: Standardized mean difference. | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Downgraded one level: one level for imprecision (the confidence intervals overlapped 1 and 0.75).
2 Downgraded one level: one level for imprecision (low numbers of events).
3 Downgraded two levels: one level due to high risks of bias (detection and other bias) in one trial accounting for 24% of the analysis weight; one level for imprecision (low numbers of events).
4 Downgraded two levels: one level due to high risk of bias (attrition and reporting bias); one level for imprecision (the confidence intervals overlapped 0 and minimal clinically important difference).
5 Downgraded two levels: two levels for inconsistency (I2 = 99%).
6 Downgraded three levels: one level for high risk of bias (detection and other bias); one level for imprecision (narrative synthesis); one level for inconsistency.
Summary of findings 3. Subcuticular sutures compared with tissue adhesives for skin closure in non‐obstetric surgery.
| Subcuticular sutures compared with tissue adhesives for skin closure in non‐obstetric surgery | ||||||
| Patient or population: skin closure in non‐obstetric surgery Setting: hospitals Intervention: subcuticular sutures Comparison: tissue adhesives | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with tissue adhesives | Risk with Subcuticular sutures | |||||
|
Surgical site infection Incidence of wound infection follow‐up: 7 to 42 days |
50 per 1,000 | 39 per 1,000 (21 to 73) | RR 0.77 (0.41 to 1.45) | 869 (10 RCTs) | ⊕⊕⊕⊝ Moderate 1 | There is no clear difference in the incidence of SSI between participants treated with subcuticular sutures and those treated with tissue adhesives. Confidence intervals are wide, spanning both appreciable benefits and harms so clear differences between treatments are not apparent. |
|
Wound complications Incidence of wound complications follow‐up: 10 to 42 days |
170 per 1,000 | 106 per 1,000 (60 to 189) | RR 0.62 (0.35 to 1.11) | 1058 (11 RCTs) | ⊕⊕⊝⊝ Low 2 | There is no clear difference in the incidence of wound complications between participants treated with subcuticular sutures and those treated with tissue adhesives. Although the point estimate on the side of a possible benefit, the 95% confidence intervals includes the possibility of both benefit and harm so clear differences between treatments are not apparent. |
|
Wound dehiscence Incidence of wound dehiscence follow‐up: 10 to 42 days |
43 per 1,000 | 10 per 1,000 (3 to 32) | RR 0.23 (0.07 to 0.74) | 1155 (11 RCTs) | ⊕⊕⊝⊝ Low 3 | Subcuticular sutures may decrease wound dehiscence in comparison with tissue adhesives. |
|
Cosmesis of scar assessed with: score system scale from: 1 to 10 (best score) follow up: mean 12 months |
The study reported there were similar outcomes between the two groups (mean score: subcuticular sutures 8.8 vs tissue adhesives 8.8). We were unable to carry out further analyses because of insufficient data. | ‐ | 99 (1 RCT) | ⊕⊕⊝⊝ Low 4 | There may be little or no difference in cosmesis of scar between subcuticular and tissue adhesives groups. | |
|
Patient satisfaction (within 30 days)
assessed with: score system scale from: 1 to 10 follow up: 14 to 21 days |
The mean patient satisfaction score (within 30days) was 9.5 | The mean patient satisfaction score with subcuticular sutures was 2.05 lower (3.05 to 1.05 lower). | MD ‐2.05 (‐3.05 to ‐1.05) | 131 (1 RCT) | ⊕⊕⊝⊝ Low 5 | Patient satisfaction within 30 days after surgery may be lower in subcuticular sutures group compared with tissue adhesives. |
|
Wound closure time (minutes) |
The mean wound closure time ranged from 0.3 to 3.7 minutes | Mean differences ranged between ‐0.34 and 10.39 across 11 studies. Further analyses were not undertaken due to statistical heterogeneity in the results. | ‐ | 895 (11 RCTs) | ⊕⊝⊝⊝ Very low 6 | It is uncertain whether it takes longer time to close a wound with subcuticular sutures than with tissue adhesives (as the certainty of the evidence has been assessed as very low). |
| Cost | The mean cost ranged from 31.96 to 65.1 USD and from 20.3 to 34.01 EUR | Mean differences ranged between ‐57.36 and ‐4.26 USD (‐16.19 and ‐10.30 EUR). Further analyses were not undertaken due to statistical heterogeneity in the results. | ‐ | 422 (4 RCTs) | ⊕⊝⊝⊝ Very low7 | Two studies reported the cost by using USD, the others reported the cost by using EUR. It is uncertain whether subcuticular sutures reduce the cost compared with tissue adhesives (as the certainty of the evidence has been assessed as very low). |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; MD: Mean difference. | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Downgraded one level: one level for imprecision (the confidence intervals overlapped 1 and both 0.75 and 1.25).
2 Downgraded two levels: one level due to high risks of bias across varying domains (detection, attrition and other bias) accounting for 54% of the analysis weight; one level for imprecision (the confidence intervals overlapped 1 and 0.75).
3 Downgraded two levels: downgraded one level due to high risks of bias across varying domains (detection, attrition and other bias) accounting for 45% of the analysis weight and one level for imprecision (low numbers of events).
4 Downgraded two levels: one level due to high risks of bias (attrition and other bias); one level for imprecision (narrative synthesis).
5 Downgraded two levels: one level due to high risks of bias (attrition and other bias); one level for imprecision (low numbers of participants).
6 Downgraded three levels: one level due to high risks of bias across varying domains (detection, attrition and other bias); two levels for inconsistency (I2= 97%).
7 Downgraded three levels: one level due to high risks of bias across varying domains (attrition and other bias); two levels for inconsistency (I2= 96%).
Summary of findings 4. Subcuticular sutures compared with surgical tapes for skin closure in non‐obstetric surgery.
| Subcuticular sutures compared with surgical tapes for skin closure in non‐obstetric surgery | ||||||
| Patient or population: skin closure in non‐obstetric surgery Setting: hospitals Intervention: subcuticular sutures Comparison: surgical tapes | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with surgical tapes | Risk with subcuticular sutures | |||||
|
Surgical site infection Incidence of wound infection follow‐up: 7 to 30 days |
25 per 1,000 | 33 per 1,000 (10 to 107) | RR 1.31 (0.40 to 4.27) | 354 (6 RCTs) | ⊕⊝⊝⊝ Very low1 | It is uncertain whether subcuticular sutures reduce the risk of SSI compared with surgical tapes. |
|
Wound complications Incidence of wound complications follow‐up: 5 to 42 days |
293 per 1,000 | 263 per 1,000 (178 to 392) | RR 0.90 (0.61 to 1.34) | 492 (5 RCTs) | ⊕⊕⊝⊝ Low 2 | There may be little or no difference between subcuticular sutures and surgical tape groups in the incidence of wound complications. |
|
Wound dehiscence Incidence of wound dehiscence follow‐up: 7 to 42 days |
23 per 1,000 | 2 per 1,000 (0 to 33) | RR 0.07 (0.00 to 1.47) | 264 (4 RCTs) | ⊕⊝⊝⊝ Very low3 | It is uncertain whether subcuticular sutures reduce the risk of wound dehiscence compared with surgical tapes (as the certainty of the evidence has been assessed as very low). |
| Cosmesis of scar | One study reported this outcome, but the data of this trial could not be included as it was insufficient. | |||||
| Patient satisfaction | Not reported in any of the studies. | |||||
|
Wound closure time (minutes) |
The mean wound closure time ranged from 1.33 to 5.33 minutes | Mean differences ranged between 0.74 and 6.36 minutes across four studies. Further analyses were not undertaken due to statistical heterogeneity in the results. | ‐ | 169 (4 RCTs) | ⊕⊝⊝⊝ Very low 4 | It is uncertain whether it takes longer time to close a wound with subcuticular sutures than with surgical tapes (as the certainty of the evidence has been assessed as very low). |
| Cost | Two studies reported the cost per participant was 10‐15 USD higher in subcuticular sutures. The other reported the cost was about 30 USD higher in surgical tapes. We were unable to carry out further analyses because of insufficient data. | ‐ | 315 (3 RCTs) | ⊕⊝⊝⊝ Very low 5 | It is uncertain whether subcuticular sutures increase the cost with surgical tapes. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1Downgraded two levels: one level for high risk of bias (attrition bias) and two levels for imprecision (very few events and wide 95% confidence intervals).
2 Downgraded two levels: one level due to high risks of bias across varying domains (attrition, reporting and other bias) accounting for 65% of the analysis weight; one level for imprecision (the confidence intervals overlapped 1 and both 0.75 and 1.25).
3 Downgraded three levels: one level due to high risks of bias across varying domains (attrition and other bias); two levels for imprecision (very few events and wide 95% confidence intervals).
4 Downgraded three levels: one level for imprecision (low numbers of participants); two levels for inconsistency (I2= 90%).
5 Downgraded three levels: one level for high risk of bias (attrition and other bias); one level for imprecision (narrative synthesis); one level for inconsistency (the included studies reported the opposite results, leading to qualitative heterogeneity).
Summary of findings 5. Subcuticular sutures compared with surgical zippers for skin closure in non‐obstetric surgery.
| Subcuticular sutures compared with surgical zippers for skin closure in non‐obstetric surgery | ||||||
| Patient or population: skin closure in non‐obstetric surgery Setting: hospitals Intervention: subcuticular sutures Comparison: surgical zippers | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with surgical zippers | Risk with subcuticular sutures | |||||
|
Surgical site infection Incidence of wound infection follow‐up: 14 to 42 days |
14 per 1,000 | 11 per 1,000 (1 to 117) | RR 0.80 (0.08 to 8.48) | 424 (3 RCTs) | ⊕⊝⊝⊝ Very low 1 | It is uncertain whether subcuticular sutures reduce the risk of SSI compared with surgical zippers. |
|
Wound complications Incidence of wound complications follow‐up: 14 to 42 days |
83 per 1,000 | 45 per 1,000 (12 to 168) | RR 0.55 (0.15 to 2.04) | 424 (3 RCTs) | ⊕⊝⊝⊝ Very low 2 | It is uncertain whether subcuticular sutures reduce the risk of wound complications compared with surgical zippers. |
|
Wound dehiscence Incidence of wound dehiscence follow‐up: 14 to 42 days |
32 per 1,000 | 25 per 1,000 (6 to 101) | RR 0.78 (0.19 to 3.16) | 424 (3 RCTs) | ⊕⊝⊝⊝ Very low 3 | It is uncertain whether subcuticular sutures reduce the risk of wound dehiscence compared with surgical zippers. |
| Cosmesis of scar assessed with: Visual analogue scale at 1 year after surgery Scale from: 0 to 10 | The mean cosmesis of scar (VAS) score was 7.7 | The mean cosmetic VAS score with subcuticular sutures was 0.3 lower (0.72 lower to 0.12 higher). | MD ‐0.3 (‐0.72 to 0.12) | 90 (1 RCT) | ⊕⊕⊝⊝ Low 4 | There may be little or no difference between subcuticular sutures and surgical zippers groups in the cosmesis of scar. |
| Patient satisfaction | Not reported in any of the studies. | |||||
|
Wound closure time (minutes) |
The mean wound closure time was 0.76 to 2.1 minutes | Mean differences ranged between 4.38 and 8.25 minutes across three studies. Further analyses were not undertaken due to statistical heterogeneity in the results. | ‐ | 424 (3 RCTs) | ⊕⊝⊝⊝ Very low 5 | It is uncertain whether it takes longer time to close a wound with subcuticular sutures than with surgical zippers. |
| Cost | The mean cost was 13 USD | The mean cost with subcuticular sutures was 5 USD lower (8.76 lower to 1.26 lower). | MD ‐5.00 (‐8.76 to ‐1.26) | 120 (1 RCT) | ⊕⊕⊝⊝ Low 6 | Subcuticular sutures may reduce the cost compared with surgical zippers. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; MD: Mean difference. | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Downgraded three levels: one level due to high risks of bias (attrition and reporting bias) accounting for 58% of the analysis weight; two levels for imprecision (very low numbers of events and wide 95% confidence intervals).
2 Downgraded three levels: one level due to high risks of bias (detection, attrition and reporting bias) accounting for 62% of the analysis weight; two levels for imprecision (very low numbers of events and wide 95% confidence intervals).
3 Downgraded three levels: one level due to high risks of bias (attrition and reporting bias) accounting for 46% of the analysis weight; two levels for imprecision (very low numbers of events and wide 95% confidence intervals).
4 Downgraded two levels: one level due to high risk of bias in blinding of outcome assessment in the single trial; one level for imprecision (low numbers of participants).
5 Downgraded three levels: one level for high risks of bias (detection, attrition and reporting bias); two levels for inconsistency (I2= 100%).
6 Downgraded two levels: one level for risk of bias (unclear in almost every domain in included single study); one level for imprecision (low numbers of participants).
See: Table 1; Table 2; Table 3; Table 4; Table 5.
Comparison 1. Subcuticular sutures compared with transdermal sutures (23 studies, 3698 participants)
Primary outcome: SSI
Data from 20 trials compared the use of subcuticular sutures with transdermal sutures for SSI. However, as one trial had no cases of infection (Baek 2009), only data from the remaining 19 trials contributed to the meta‐analysis (Andrade 2016; Buchweitz 2005; Chen 2013; Clough 1975; Corder 1991; Foster 1977; Hopkinson 1982; Javadi 2018; Karabay 2005; Khajouei 2007; Kotaluoto 2012; Liu 2017; McGreal 2002; Murphy 1995; Onwuanyi 1990; Pauniaho 2010; Tanaka 2014; Taube 1983; Zwart 1989). Overall, 7.7% (238/3107 participants) developed an SSI. There is no clear difference in the incidence of SSI between the two groups (RR 1.10; 95% CI 0.80 to 1.52; Analysis 1.1) with no important heterogeneity (Tau2= 0.11, I2= 24%). Using risk difference, there were 0 more SSIs per 1000 with subcuticular sutures compared with transdermal sutures (10 fewer to 20 more) (RD 0.00, 95% CI ‐0.01 to 0.02; Tau2= 0, I2= 36%). There may be little difference in the incidence of SSI. No publication bias was evident in the funnel plot (Figure 4) and small size effect was not apparent. The evidence was downgraded to low certainty due to high risk of bias across varying domains (attrition, selection, reporting and other bias affecting 40% of the analysis weight across nine of 19 studies) and imprecision.
1.1. Analysis.
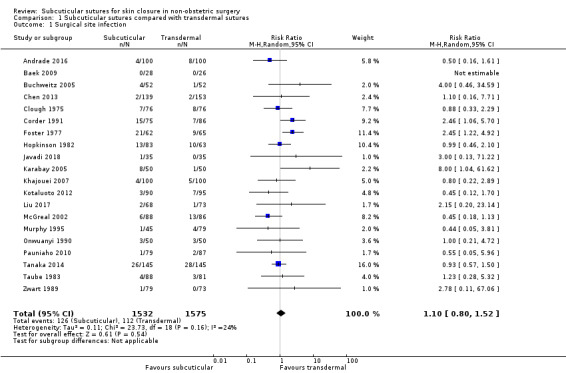
Comparison 1 Subcuticular sutures compared with transdermal sutures, Outcome 1 Surgical site infection.
4.
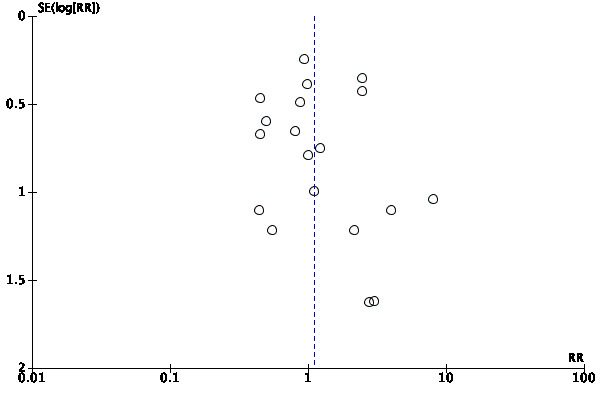
Funnel plot of comparison: 1 Subcuticular sutures compared with transdermal sutures, outcome: 1.1 Surgical site infection.
In the sensitivity analysis, there was no change in the interpretation of results by adopting the fixed‐effects model. We also conducted a sensitivity analysis in which we restricted inclusion in the analysis to only those studies that were considered to be at a low risk of selection bias (Tanaka 2014), there was no change in the interpretation of results (RR 0.93, 95% CI 0.57 to 1.50; Analysis 1.2). Three studies were considered to have potential unit of analysis issues (Baek 2009; Corder 1991; Murphy 1995). We conducted a post hoc sensitivity analysis removing these studies from the meta‐analysis. Again there was no evidence of a difference in the proportion of participants developing infection between groups (RR 1.04, 95% CI 0.75 to 1.42; Analysis 1.2). In an ITT sensitivity analysis based on the worst‐worst scenario, there was also no change in the interpretation of results (RR 1.23, 95% CI 0.97 to 1.55; Analysis 1.2). However, an ITT sensitivity analysis based on the worst‐best scenario showed that the results changed in favour of the transdermal sutures group (RR 1.59, 95% CI 1.05 to 2.42; Analysis 1.2).
1.2. Analysis.
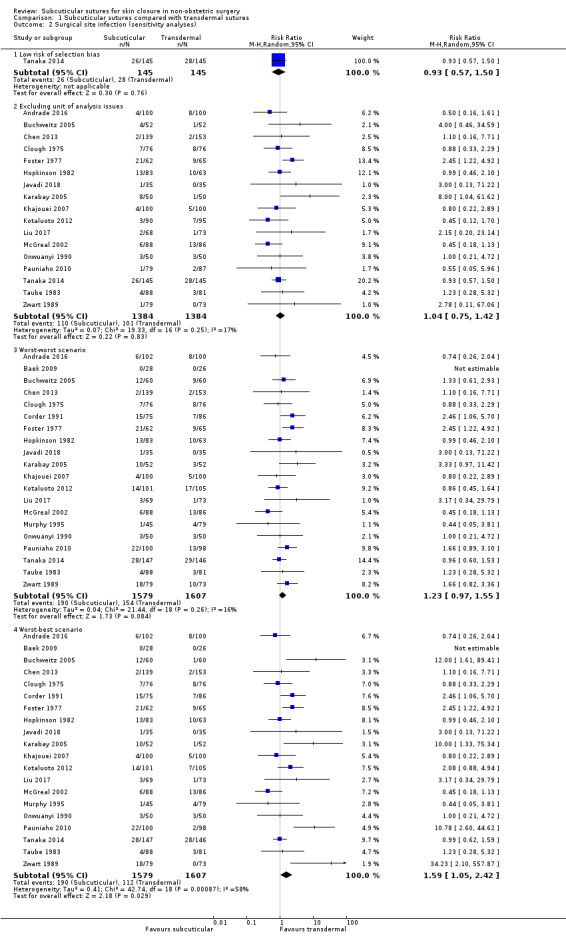
Comparison 1 Subcuticular sutures compared with transdermal sutures, Outcome 2 Surgical site infection (sensitivity analyses).
Secondary outcomes
Wound complications
Data from nine trials compared the use of subcuticular sutures with transdermal sutures for wound complications and contributed to the meta‐analysis (Andrade 2016; Buchweitz 2005; Corder 1991; Ghaderi 2010; Khajouei 2007; Kotaluoto 2012; Onwuanyi 1990; Pauniaho 2010; Rosen 1997). It is uncertain whether subcuticular sutures have an effect on wound complications compared with transdermal sutures because the certainty of the evidence is very low (RR 0.83; 95% CI 0.40 to 1.71; 1489 participants; Tau2= 0.79, I2= 70%) (Analysis 1.3) (very low‐certainty evidence, downgraded once each for risk of bias (variously attrition, selection, reporting and other bias affecting 62% of the analysis weight), for imprecision and for inconsistency).
1.3. Analysis.
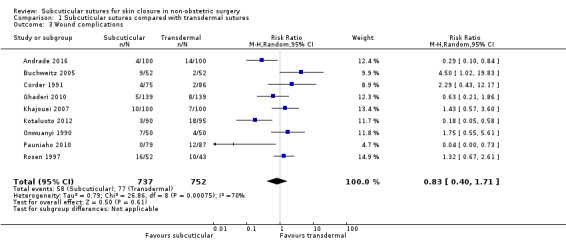
Comparison 1 Subcuticular sutures compared with transdermal sutures, Outcome 3 Wound complications.
Wound dehiscence
Six trials reported wound dehiscence (Andrade 2016; Buchweitz 2005; Javadi 2018; Kotaluoto 2012; Liu 2017; Pauniaho 2010). A total of 33 participants (33/866 (3.8%)) developed wound dehiscence. Of these, 27 participants belonged to non‐absorbable interrupted transdermal sutures group. It is uncertain whether subcuticular sutures reduce the risk of wound dehiscence compared with transdermal sutures because the certainty of the evidence is very low (RR 0.35; 95% CI 0.08 to 1.54; Tau2= 1.59, I2= 49%: Analysis 1.4) (very low‐certainty evidence, downgraded once due to risk of bias (attrition and selection bias) and twice due to imprecision).
1.4. Analysis.
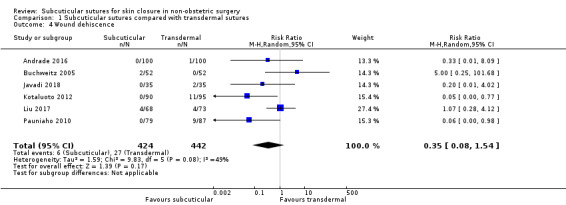
Comparison 1 Subcuticular sutures compared with transdermal sutures, Outcome 4 Wound dehiscence.
Proportion of re‐closure of the skin incision required
Only two trials reported the proportion of re‐closure of the skin incision (Hopkinson 1982; Karabay 2005). It is uncertain whether there is a difference in the proportion of re‐closure between the two groups because the certainty of the evidence is very low (RR 1.16; 95% CI 0.09 to 14.57; Tau2= 1.47, I2= 43%; Analysis 1.5). The evidence was downgraded to very low certainty once due to high risk of bias (attrition and other bias) and twice due to imprecision (only five events in total).
1.5. Analysis.
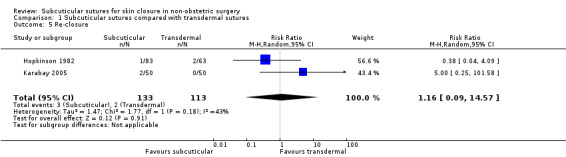
Comparison 1 Subcuticular sutures compared with transdermal sutures, Outcome 5 Re‐closure.
Hypertrophic scar
Only two trials reported this outcome (Onwuanyi 1990; Simpson 1979). A total of 56 participants (56/233 (24%)) developed hypertrophic scar. Of these, 54 participants developed hypertrophic scar in one old trial (Simpson 1979). It is uncertain whether subcuticular sutures increase or reduce the risk of hypertrophic scar compared with transdermal sutures because the certainty of the evidence is very low (RR 0.91; 95% CI 0.25 to 3.39; Tau2= 0.48, I2= 28%; Analysis 1.6) (very low‐certainty evidence, downgraded once due to risk of bias (attrition bias) and twice due to imprecision (wide 95% CI).
1.6. Analysis.
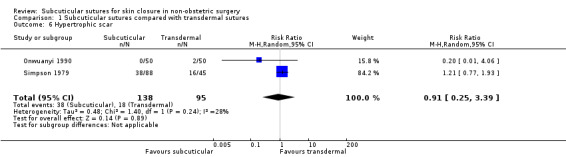
Comparison 1 Subcuticular sutures compared with transdermal sutures, Outcome 6 Hypertrophic scar.
Keloid scar
Clough 1975 (143 participants) reported this outcome, but had no cases with this event. It is uncertain whether subcuticular sutures increase or reduce the risk of keloid scar compared with transdermal sutures because the certainty of the evidence is very low (downgraded once due to risk of bias (unclear in all domains in included single study), and twice due to imprecision (small sample size and no events)).
Wound pain intensity
Four trials (372 participants) reported this outcome (Baek 2009; Javadi 2018; Pauniaho 2010; Rosen 1997). However, the data of three trials could not be combined because of missing data or statistics (see also Characteristics of included studies). In addition, as the data of the other trial (Javadi 2018) was considered to be skewed, we summarised this narratively. Three trials (Baek 2009; Javadi 2018; Pauniaho 2010) reported the mean of severity of pain score using the visual analogue scale (VAS) scored between 0 for the lowest level of pain, and 10 for the most severe pain. Rosen 1997 reported the pain score using VAS scored from 1 (lowest pain) to 5 (most severe pain). All trials reported lower pain scores (range from 0.2 to 1.4) in subcuticular sutures compared with that in transdermal sutures, however, Pauniaho 2010 did not show statistical significance. Javadi 2018 reported the mean scores at postoperative day seven in the subcuticular suture group were significantly lower than the control group (mean 0.86, 95% CI 0.05 to 1.67 (median 1, IQR 0‐1) versus mean 1.40, 95% CI 0.55 to 2.25, (median 1, IQR 1‐2), P value = 0.008). It is uncertain whether subcuticular sutures reduce the pain compared with transdermal sutures because the certainty of the evidence is very low (downgraded due to risk of bias, imprecision and inconsistency).
Length of hospital stay
Four trials reported this outcome (Chen 2013; Karabay 2005; McGreal 2002; Onwuanyi 1990), but the data from three trials (Karabay 2005; McGreal 2002; Onwuanyi 1990) could not be combined in the meta‐analysis because of missing statistics (see also Characteristics of included studies) (374 participants).
All the trials reported there was no significant difference in the length of hospital stay between the two groups. There is probably little or no difference in the length of hospital stay between subcuticular and transdermal sutures groups (MD 0.40 days, 95% CI ‐0.44 to 1.24; 292 participants; Analysis 1.7) because the certainty of the evidence is moderate; downgraded due to imprecision.
1.7. Analysis.
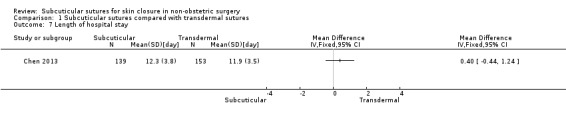
Comparison 1 Subcuticular sutures compared with transdermal sutures, Outcome 7 Length of hospital stay.
Cosmesis of scar (as defined by the authors for a minimum follow‐up of six months)
Five trials (about 950 participants) reported this outcome (Clough 1975; Kotaluoto 2012; Liu 2017; Tanaka 2014; Zwart 1989). Because of the differences in the scale used, we did not conduct meta‐analytic pooling and we presented the results narratively.
Clough 1975 reported surgeon‐assessed cosmetic appearance at three to four years. The cosmetic presence of the wound was classified as good (it was neat and uniform and not more than 3 mm wide) or poor (if it was hypertrophic or greater than 3 mm wide). The proportion of cosmetically poor scars was similar between groups. However, the data could not be used as the group(s) from which 18 participants had dropped out was not clear and the number of participants was imbalanced between two groups.
Kotaluoto 2012 reported cosmesis after a median of 14 months by blinded assessment of photographs. For subjective scar assessment, the Vancouver scar scale, the patient and observer scar assessment scale (POSAS), and a visual analogue scale (VAS) were used. Objective evaluation was carried out by measuring surface area, average width, and estimated concentration change (ECC) of haemoglobin and melanin in the scar using spectrocutometry. Kotaluoto 2012 concluded both objective and subjective analyses showed better cosmetic results for subcuticular suturing. The difference between the two groups was statistically significant as regards POSAS in both patient and observer scales, VAS, surface area, average width, and estimated concentration change (ECC) of melanin. However, there were many dropouts (33%) in the analyses. Liu 2017 reported the cosmetic outcome as assessed by the overall impression on the Dutch Patient and Observer Scar Assessment Scale (POSAS) version 2.0, the POSAS, the 4‐point scale (excellent, good, fair, bad) by the patient and observer, and the measurement with a colorimeter. The cosmetic result was evaluated at three and 12 months after surgery. A observer, blinded to the suturing technique, assessed the scars in person by using the Observer Scar Assessment Scale (OSAS) and the 4‐point scale. At 12 months, no significant differences were found. Tanaka 2014 reported cosmesis using a modified scar assessment scale based on the Hollander Wound Evaluation (Hollander 1995) and POSAS at six months. They showed significantly better cosmetic results for subcuticular suturing as regards to pain, scar vascularity and width. Zwart 1989 reported the objective (assessed by the head nurse) and subjective (assessed by patient) cosmetic results at one, three and six months. The cosmetic presence of the wound was classified as excellent, good, fair or poor. At six months, no statistically significant differences were found between the groups.
Two studies showed there were significant differences in the cosmetic outcomes in favour of subcuticular sutures, and the other studies reported no significant difference. It is uncertain whether subcuticular sutures improve the cosmesis of scar compared with transdermal sutures because the certainty of evidence is very low: downgraded due to high risk of attrition bias, imprecision (narrative synthesis) and inconsistency (two reaching significance and the others not).
Several studies did not contribute to this outcome because, although they evaluated cosmetic appearance, they did so less than six months after surgery (Baek 2009; Buchweitz 2005; Karabay 2005; Taube 1983).
Patient satisfaction
Only two trials reported this outcome (Javadi 2018; Tanaka 2014), but the data from Javadi 2018 could not be combined in the meta‐analysis as they used neither scale or score. Therefore, we presented results narratively. Javadi 2018 (70 participants) reported patient satisfaction with the surgical site scar at 90 days after operation. They concluded that a significantly greater number of patients in the subcuticular suture group were satisfied with their wound healing and scar status compared with the control group (91.42% vs. 71.42%). Tanaka 2014 (290 participants) assessed patient satisfaction at seven days, 30 days, three months and six months using scores on a scale of 1 (lowest) to 10 (best possible score). We used the data at 30 days and six months. Patient satisfaction at 30 days is probably higher in subcuticular sutures group (score 9) compared with transdermal sutures group (score 7.4) with a difference in means of 1.60 (95% CI 1.32 to 1.88; Analysis 1.8). This is moderate‐certainty evidence downgraded once due to imprecision. In addition, patient satisfaction at six months is also probably higher in the subcuticular sutures group (score 9) compared with the transdermal sutures group (score 7.3) with a difference in means of 1.70 (95% CI 1.37 to 2.03; Analysis 1.9) (moderate‐certainty evidence downgraded once due to imprecision).
1.8. Analysis.

Comparison 1 Subcuticular sutures compared with transdermal sutures, Outcome 8 Patient satisfaction (within 30 days).
1.9. Analysis.
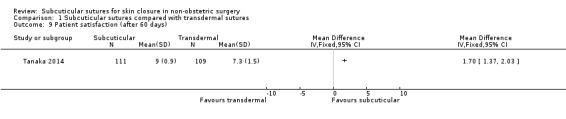
Comparison 1 Subcuticular sutures compared with transdermal sutures, Outcome 9 Patient satisfaction (after 60 days).
Quality of Life
None of the trials reported quality of life.
Wound closure time in the operation (minutes)
Only two trials reported this outcome (Chen 2013; Tanaka 2014). Wound closure time is probably longer in the subcuticular sutures group compared with the transdermal sutures group with a difference in means of 5.81 minutes (95% CI 5.13 to 6.49; Tau2= 0.15, I2= 61%; 585 participants; Analysis 1.10) (moderate‐certainty evidence downgraded once due to inconsistency).
1.10. Analysis.
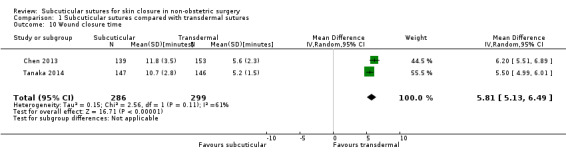
Comparison 1 Subcuticular sutures compared with transdermal sutures, Outcome 10 Wound closure time.
Cost
Only two trials reported cost (Murphy 1995; Onwuanyi 1990), however the data of Murphy 1995 were excluded as they were insufficient because of missing statistics (see also Characteristics of included studies), and only data from Onwuanyi 1990 contributed to the meta‐analysis. In this study, participants used non‐absorbable (Nylon) subcuticular sutures. We calculated the SD from the reported P value. Subcuticular sutures may reduce the cost compared with transdermal sutures. The mean cost was 8 Naira in the subcuticular sutures group compared with 16 Naira in the transdermal sutures group with a difference in means of ‐8.00 Naira (95% CI ‐13.05 to ‐2.95; 100 participants; Analysis 1.11). The evidence was downgraded to low certainty due to risk of bias and imprecision.
1.11. Analysis.
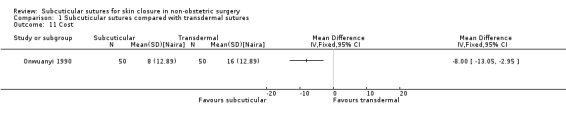
Comparison 1 Subcuticular sutures compared with transdermal sutures, Outcome 11 Cost.
Summary of comparison
Low‐certainty evidence suggests that there may be little difference between subcuticular sutures and transdermal sutures groups in the incidence of SSI. There is very low‐certainty evidence that it is uncertain whether subcuticular sutures have an effect on wound complications and dehiscence compared with transdermal sutures. Moderate‐certainty evidence shows that subcuticular sutures probably improve patient satisfaction compared with transdermal sutures. Additionally, subcuticular sutures may reduce the cost in materials (low‐certainty evidence), which may, however, be offset by increase in costs necessary for longer operations. Wound closure time is probably longer when subcuticular sutures are used (moderate‐certainty evidence). See Table 1.
Comparison 2. Subcuticular sutures compared with skin staples (18 studies, 4428 participants)
None of the trials reported the proportion of re‐closure or the incidence of keloid scar.
Primary outcome: SSI
Fifteen trials compared the use of subcuticular sutures with staples for SSI. However, as one trial had no cases of infection (Selvadurai 1997), only data from the remaining 14 trials contributed to the meta‐analysis (Chen 2018; Chughtai 2000; Eggers 2011; Imamura 2016; Khan 2006; Kobayashi 2015; Kuroki 2017; Mullen 1999; Murphy 1995; Ranaboldo 1992; Shetty 2004; Subramanian 2005; Tsujinaka 2013; Zwart 1989). Overall, 8.1% (337/4163 participants) developed an SSI. There is probably little or no difference in the incidence of SSI between the two groups (RR 0.81, 95% CI 0.64 to 1.01; Analysis 2.1) with no important evidence of heterogeneity (Tau2= 0.01, I2= 5%). No publication bias was evident in the funnel plot (Figure 5) and the small study size effect was not apparent. The evidence was downgraded to moderate certainty due to imprecision. In the sensitivity analysis, there was no change in the interpretation of results by adopting the fixed‐effects model. We also conducted a sensitivity analysis in which we restricted inclusion in the analysis to only those studies that were considered to be at a low risk of selection bias (Imamura 2016; Kobayashi 2015; Kuroki 2017; Tsujinaka 2013), but there was no change in the interpretation of results (RR 0.91, 95% CI 0.72 to 1.16; Analysis 2.2). Three studies were considered to have potential unit of analysis issues (Chughtai 2000; Murphy 1995; Subramanian 2005). We conducted a post hoc sensitivity analysis removing these studies from the meta‐analysis. Again there was no evidence of a difference in the proportion of participants developing infection between the groups (RR 0.81, 95% CI 0.61 to 1.09; Analysis 2.2). ITT sensitivity analyses showed that the results did not change depending on the methods of imputation of the missing data (Analysis 2.2).
2.1. Analysis.
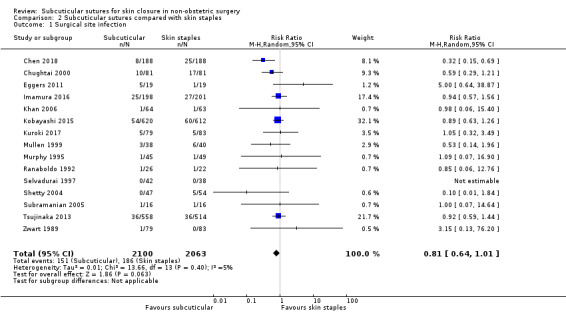
Comparison 2 Subcuticular sutures compared with skin staples, Outcome 1 Surgical site infection.
5.
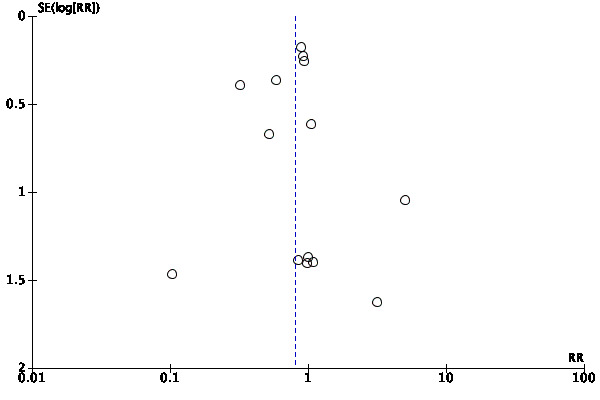
Funnel plot of comparison: 2 Subcuticular sutures compared with skin staples, outcome: 2.1 Surgical site infection.
2.2. Analysis.
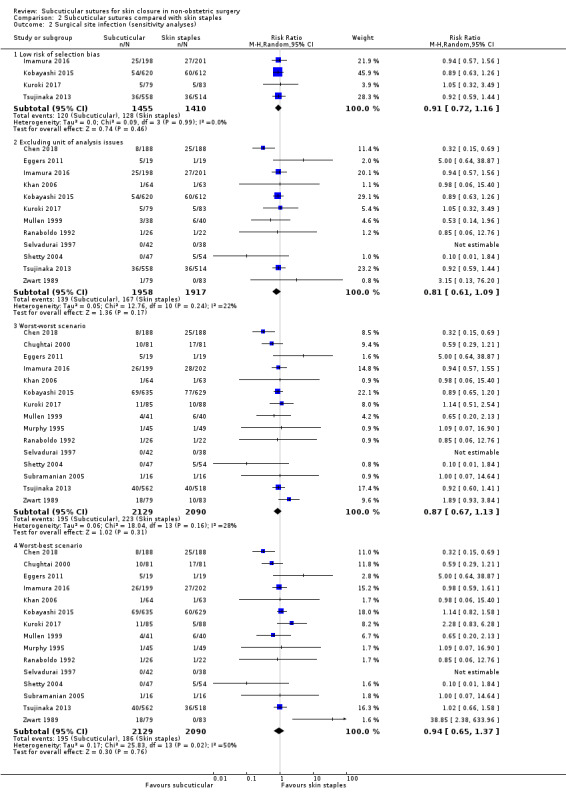
Comparison 2 Subcuticular sutures compared with skin staples, Outcome 2 Surgical site infection (sensitivity analyses).
Secondary outcomes
Wound complications
Nine trials compared the use of subcuticular sutures with staples for wound complications. However, as two trials had no cases of complications (Reed 1997; Selvadurai 1997), only data from the remaining seven trials contributed to the meta‐analysis (Khan 2006; Kobayashi 2015; Kuroki 2017; Obermair 2007; Shetty 2004; Steele 1983; Tsujinaka 2013). A total of 291 participants (291/2973 (9.8%)) developed wound complications. Subcuticular sutures probably on average decrease wound complications in comparison with staples (RR 0.79, 95% CI 0.64 to 0.98; Tau2= 0, I2= 0%; Analysis 2.3) (moderate‐certainty evidence downgraded once due to imprecision).
2.3. Analysis.
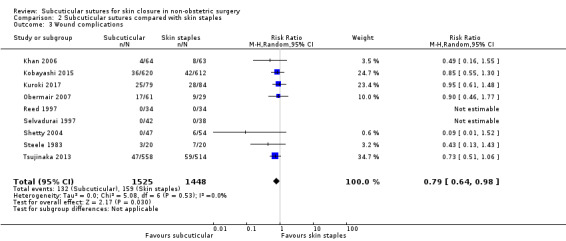
Comparison 2 Subcuticular sutures compared with skin staples, Outcome 3 Wound complications.
Wound dehiscence
Seven trials reported wound dehiscence and contributed to the meta‐analysis (Chen 2018; Chughtai 2000; Eggers 2011; Kuroki 2017; Obermair 2007; Shetty 2004; Tsujinaka 2013). Overall, 4.7% (93/1984 participants) developed wound dehiscence. Subcuticular sutures may reduce the risk of wound dehiscence compared with skin staples (RR 0.63, 95% CI 0.43 to 0.94; Analysis 2.4) with no evidence of heterogeneity (Tau2= 0, I2= 0%). The evidence was downgraded to low certainty due to high risk of bias (detection and other bias affecting 24% of the analysis weight in one (Chughtai 2000) of six studies) and imprecision.
2.4. Analysis.
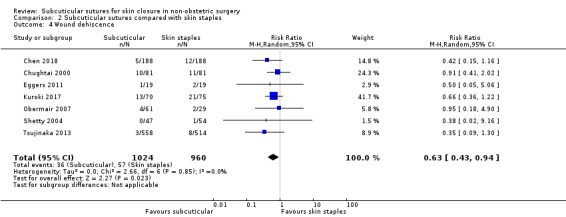
Comparison 2 Subcuticular sutures compared with skin staples, Outcome 4 Wound dehiscence.
Hypertrophic scar
Only three trials reported this outcome (Ranaboldo 1992; Selvadurai 1997; Tsujinaka 2013). However, as one trial had no cases of hypertrophic scar (Ranaboldo 1992), only data from the remaining two trials contributed to the meta‐analysis. A total of 207 participants (207/1195 (17%)) developed hypertrophic scar. Subcuticular sutures probably on average decrease hypertrophic scar in comparison with skin staples (RR 0.77, 95% CI 0.60 to 0.98; Tau2= 0, I2= 0%; Analysis 2.5) (moderate‐certainty evidence downgraded once due to imprecision).
2.5. Analysis.
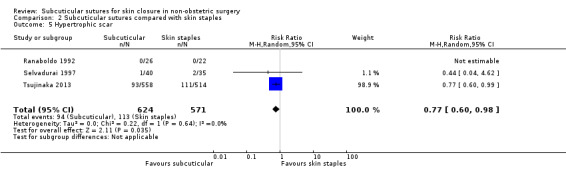
Comparison 2 Subcuticular sutures compared with skin staples, Outcome 5 Hypertrophic scar.
Wound pain intensity
Six trials reported this outcome (Eggers 2011; Obermair 2007; Ranaboldo 1992; Selvadurai 1997; Subramanian 2005; Reed 1997). However, as the data of one trial were insufficiently reported because of missing data (Subramanian 2005), only data from the remaining five trials contributed to the meta‐analysis. Three studies (Obermair 2007; Ranaboldo 1992; Selvadurai 1997) assessed pain intensity using a VAS scale that ran from 0 to 100, where 100 represented maximal pain and two studies (Eggers 2011; Reed 1997) used a VAS scale that ran from 0 to 10, where 10 represented maximal pain. Eggers 2011 reported pain intensity at three and six weeks, Reed 1997 assessed pain intensity by patients about 320 days (mean) after operation, and Obermair 2007 reported pain intensity assessed by patients and surgeons at one and six weeks, and three months. We used the data of pain intensity assessed by patients at one and three months and calculated the SD from the reported 95% CI. Selvadurai 1997 reported pain scores as measured at the first three postoperative days, and we included VAS data at the second postoperative day and calculated SDs from the SEs. In one study (Ranaboldo 1992), we calculated the SD from the reported P value. It is uncertain whether there is a difference in the pain intensity between the two groups because the certainty of the evidence is very low: pain intensity within seven days (scale from 0 to 100, 100 represented the maximum pain) (MD ‐1.86, 95%CI ‐10.37 to 6.65, Tau2= 42.14, I2= 76%; Analysis 2.6) (very low‐certainty evidence, downgraded due to high risk of bias, imprecision (low numbers of participants (218) and wide 95% CI) and inconsistency), pain intensity after 30 days (SMD 0.18, 95%CI ‐0.30 to 0.66, Tau2= 0.11, I2= 61%; Analysis 2.7) (very low‐certainty evidence: downgraded due to high risk of bias (attrition bias affecting 36% of the analysis weight), imprecision (low numbers of participants (196)) and inconsistency).
2.6. Analysis.
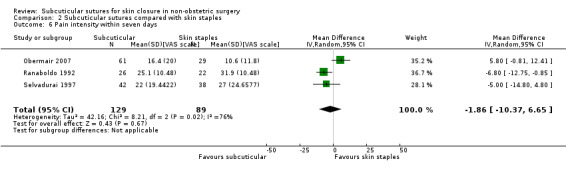
Comparison 2 Subcuticular sutures compared with skin staples, Outcome 6 Pain intensity within seven days.
2.7. Analysis.
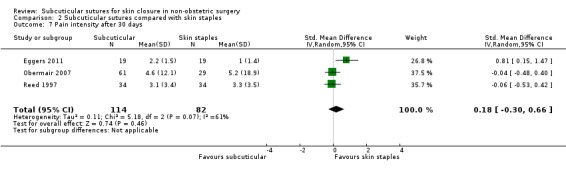
Comparison 2 Subcuticular sutures compared with skin staples, Outcome 7 Pain intensity after 30 days.
Length of hospital stay
Six trials reported this outcome (Chen 2018; Eggers 2011; Khan 2006; Kobayashi 2015; Reed 1997; Tsujinaka 2013). However, as the data of one trial were considered to be skewed (Khan 2006), only data from the remaining five trials contributed to the meta‐analysis. Khan 2006 reported there was no significant difference in this outcome. In the meta‐analysis, it is uncertain whether subcuticular sutures shorten the length of hospital stay compared with skin staples (MD ‐0.58 days, 95% CI ‐1.57 to 0.42, Tau2= 0.78, I2= 77%; 2794 participants; Analysis 2.8) because the certainty of the evidence is very low: downgraded due to high risk of bias (attrition bias affecting 27% of the analysis weight), imprecision and inconsistency.
2.8. Analysis.
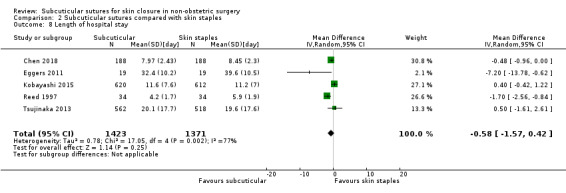
Comparison 2 Subcuticular sutures compared with skin staples, Outcome 8 Length of hospital stay.
Cosmesis of scar (as defined by the authors for a minimum follow‐up of six months)
Only three trials reported this outcome. Reed 1997 assessed cosmesis by patients using a VAS scale that ran from 0 to 10, where 10 represented the best score at about 320 days (mean) after operation. Selvadurai 1997 assessed cosmesis by independent observer using a VAS scale that ran from 0 to 100, where 100 represented maximal score at six months and SD was calculated from SE. Zwart 1989 reported the objective (assessed by the head nurse) and subjective (assessed by patient) cosmetic results at one, three and six months. The cosmetic presence of the wound was classified as excellent, good, fair or poor. We used the objective results and calculated the mean and SD using a scoring scale that ran from 1 (poor) to 4 (excellent). There may be little or no difference in the cosmesis between the two groups (SMD 0.12, 95% CI ‐0.11 to 0.35; Analysis 2.9; 291 participants) with no evidence of heterogeneity (Tau2= 0, I2= 0%). The evidence was downgraded to low certainty due to high risk of bias (attrition and reporting bias) and imprecision.
2.9. Analysis.
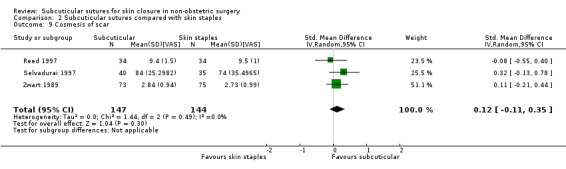
Comparison 2 Subcuticular sutures compared with skin staples, Outcome 9 Cosmesis of scar.
Several studies did not contribute to this outcome because although they evaluated cosmetic appearance they did so less than six months after surgery (Chughtai 2000; Eggers 2011; Khan 2006; Kobayashi 2015; Kuroki 2017; Obermair 2007; Ranaboldo 1992; Steele 1983).
Patient satisfaction
Only three trials reported this outcome (Khan 2006; Kobayashi 2015; Kuroki 2017). However, as the data of two trials were considered to be skewed (Khan 2006; Kuroki 2017), only Kobayashi 2015 contributed to the meta‐analysis. Khan 2006 (127 participants) assessed with a VAS between 0 and 100, where 100 represented maximal satisfaction at eight to 12 weeks after operation and reported there was no significant difference in the patient satisfaction. Kuroki 2017 (163 participants) assessed patient satisfaction with appearance of scar, location of scar and discomfort at scar using scores (represented by per cent, but the details were not available). There were no significant differences in median satisfaction scores of the scar appearance (suture 77% compared with staples 68%, P = 0.11) nor in the satisfaction with the discomfort at the incision site (staples 71% compared with suture 77%, P = 0.20).
Kobayashi 2015 reported patient satisfaction using a scale that ran from 1 to 5, where 5 represented the best score at 30 days after operation. Patient satisfaction at 30 days was slightly higher in the subcuticular sutures group (score 4.4) compared with the skin staples group (score 4.2) with a difference in means of 0.20 (95% CI 0.10 to 0.30; Analysis 2.10; 1232 participants). This is high‐certainty evidence.
2.10. Analysis.
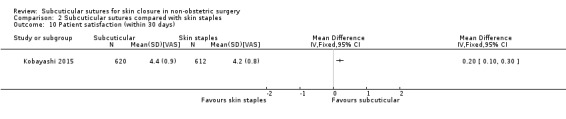
Comparison 2 Subcuticular sutures compared with skin staples, Outcome 10 Patient satisfaction (within 30 days).
Quality of Life
Eggers 2011 reported general health as judged by responses to the SF‐12 v2 (QualityMetric Inc., Lincoln, RI) survey of the physical composite score (PCS) and mental composite score (MCS) at three and six weeks after operation. The scale score (higher better) is norm‐based scoring which is referred to as "50/10" scoring because the score has been standardised so that the general US population has a mean of 50 and SD of 10. We used the data at six weeks. There may be little or no difference between the two groups in the SF‐12 v2 PCS (MD 0.00, 95% CI ‐6.05 to 6.05; Analysis 2.11; 38 participants) and MCS (MD 1.00, 95% CI ‐5.05 to 7.05; Analysis 2.12; 38 participants). The certainty of the evidence is low, downgraded twice for imprecision.
2.11. Analysis.
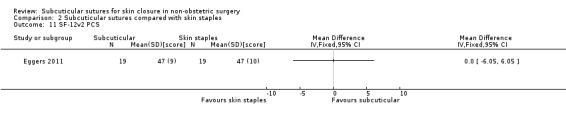
Comparison 2 Subcuticular sutures compared with skin staples, Outcome 11 SF‐12v2 PCS.
2.12. Analysis.
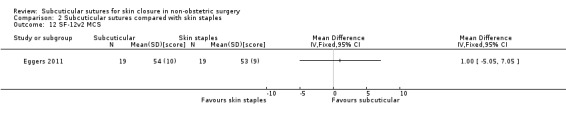
Comparison 2 Subcuticular sutures compared with skin staples, Outcome 12 SF‐12v2 MCS.
Wound closure time in the operation (minutes)
Six studies reported the time taken for closure (Khan 2006; Kobayashi 2015; Ranaboldo 1992; Selvadurai 1997; Steele 1983; Subramanian 2005). Two studies reported wound closure time as speed (rate) seconds/cm (Eggers 2011; Zwart 1989). However, as the data of four trials were insufficient (missing statistics or data) (Eggers 2011; Khan 2006; Ranaboldo 1992; Zwart 1989) (see also Characteristics of included studies), only data from the remaining four trials contributed to the meta‐analysis. As there was extreme heterogeneity (I2= 99%; 1384 participants; Analysis 2.13), we presented the results narratively. The mean wound closure time in the skin staple group ranged from 0.9 to 4.5 minutes. Mean differences ranged between 0.30 and 5.50 minutes across four studies. Further analyses were not undertaken due to statistical heterogeneity in the results. The certainty of the evidence is low, downgraded twice for inconsistency.
2.13. Analysis.
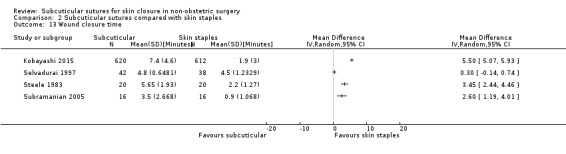
Comparison 2 Subcuticular sutures compared with skin staples, Outcome 13 Wound closure time.
Cost
Four studies (342 participants) reported cost (Chughtai 2000; Eggers 2011; Murphy 1995; Ranaboldo 1992). However as the data of all studies were insufficiently reported because of missing statistics, we presented them narratively. Three of these trials favoured subcuticular sutures (Chughtai 2000; Murphy 1995; Ranaboldo 1992). Chughtai 2000 reported the average cost per case was USD 4.5 for sutures and USD 15 for staples. Murphy 1995 reported the mean cost per patient was USD 4 for sutures and USD 12 for staples. Ranaboldo 1992 reported the mean cost per patient was GBP 1.41 pounds for sutures and GBP 13.99 for staples (including removal cost). Eggers 2011 reported that the total cost associated with surgery for each of the closure groups (including all aspects of surgery associated with materials, labour, and operating room expenses) was USD 1056.3 for sutures and USD 802.8 for staples. They concluded that the staples were to be favoured because most of the cost differential was attributed to procedure times.
It is uncertain whether subcuticular sutures reduce the cost compared with skin staples. The certainty of the evidence is very low downgraded once for high risk of bias (detection and other bias), once for imprecision and once for inconsistency (some studies favoured subcuticular sutures and the other favoured skin staples).
Summary of comparison
Moderate‐certainty evidence shows that subcuticular sutures probably have little effect on SSI; but probably decrease the incidence of wound complications and hypertrophic scars when compared with skin staples. Low‐certainty evidence also shows that subcuticular sutures may reduce the risk of wound dehiscence compared with skin staples. In addition, subcuticular sutures are associated with slightly higher patient satisfaction (high‐certainty evidence). However, wound closure time may be longer compared with staples. See Table 2.
Comparison 3. Subcuticular sutures compared with tissue adhesives (17 studies, 1419 participants)
None of the trials reported the incidence of hypertrophic or keloid scar.
Primary outcome: SSI
Ten trials compared the use of subcuticular sutures with tissue adhesives for SSI. However, as four trials had no cases of infection (Gennari 2004; Keng 1989; Ong 2002; Teoh 2018), only data from the remaining six trials contributed to the meta‐analysis (Eggers 2011; Khan 2006; Maartense 2002; Martin 2017; Sebesta 2004; Van den Ende 2004). Overall, 4.0% (35/869 participants) developed an SSI. There is moderate‐certainty evidence (downgraded once for imprecision) showing no clear difference in the incidence of SSI between participants treated with subcuticular sutures and those treated with tissue adhesives (RR 0.77, 95% CI 0.41 to 1.45; Analysis 3.1). There is no evidence of heterogeneity (Tau2= 0, I2= 0%). Confidence intervals are wide, spanning both appreciable benefits and harms, so clear differences between treatments are not apparent. There are no trials that are considered to be at a low risk of selection bias.
3.1. Analysis.
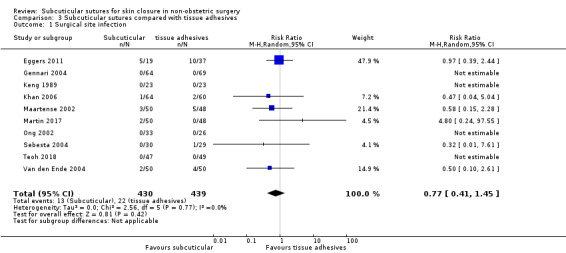
Comparison 3 Subcuticular sutures compared with tissue adhesives, Outcome 1 Surgical site infection.
In the sensitivity analysis, there was no change in the interpretation of results by adopting the fixed‐effects model. One study was considered to have potential unit of analysis issues (Keng 1989). We conducted a post hoc sensitivity analysis removing this study from the meta‐analysis. Again, there was no evidence of a difference in the proportion of participants developing infection between the groups (RR 0.77, 95% CI 0.41 to 1.45; Analysis 3.2). ITT sensitivity analyses showed that the results did not change depending on the methods of imputation of the missing data (Analysis 3.2).
3.2. Analysis.
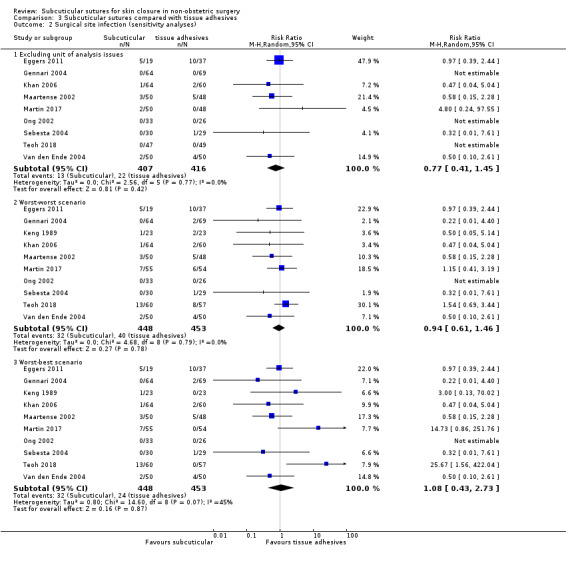
Comparison 3 Subcuticular sutures compared with tissue adhesives, Outcome 2 Surgical site infection (sensitivity analyses).
Secondary outcomes
Wound complications
Eleven trials compared the use of subcuticular sutures with tissue adhesives for wound complications. However, as one trial had no cases of complications (Jallali 2004), only data from the remaining 10 trials contributed to the meta‐analysis (Ademuyiwa 2009; Brown 2009; Jan 2013; Keng 1989; Khan 2006; Krishnamoorthy 2009; Sebesta 2004; Soni 2013; Swtizer 2003; Van den Ende 2004). A total of 152 participants (152/1058 (14.4%)) developed wound complications. There is no clear difference in the incidence of wound complications between participants treated with subcuticular sutures and those treated with tissue adhesives (RR 0.62, 95% CI 0.35 to 1.11; Tau2= 0.32, I2= 46%; Analysis 3.3) (low‐certainty evidence, downgraded once due to high risk of bias across varying domains (detection, attrition and other bias affecting 54% of the analysis weight) and once due to imprecision). Although the point estimate is on the side of a possible benefit, the 95% confidence intervals includes the possibility of both benefit and harm so clear differences between treatments are not apparent. No publication bias was evident in the funnel plot (Figure 6) and the small study size effect was not apparent.
3.3. Analysis.
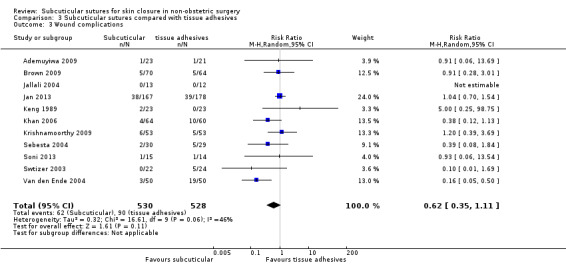
Comparison 3 Subcuticular sutures compared with tissue adhesives, Outcome 3 Wound complications.
6.
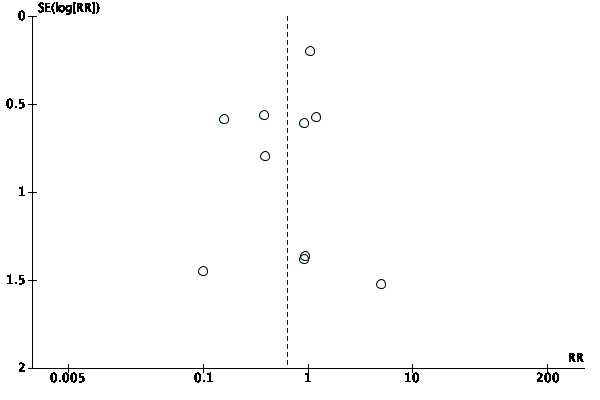
Funnel plot of comparison: 3 Subcuticular sutures compared with tissue adhesives, outcome: 3.3 Wound complications.
Wound dehiscence
Eleven trials reported superficial wound dehiscence. However as five trials had no cases of dehiscence (Brown 2009; Gennari 2004; Martin 2017; Ong 2002; Teoh 2018), only data from the remaining six trials contributed to the meta‐analysis (Eggers 2011; Jan 2013; Sebesta 2004; Soni 2013; Swtizer 2003; Van den Ende 2004). Overall, 2.1% (24/1155 participants) developed wound dehiscence. Subcuticular sutures may decrease wound dehiscence in comparison with tissue adhesives (RR 0.23, 95% CI 0.07 to 0.74; Tau2= 0, I2= 0%; Analysis 3.4). The evidence is low‐certainty, downgraded once due to high risk of bias across varying domains (detection, attrition and other bias affecting 45% of the analysis weight) and once due to imprecision (low numbers of events).
3.4. Analysis.
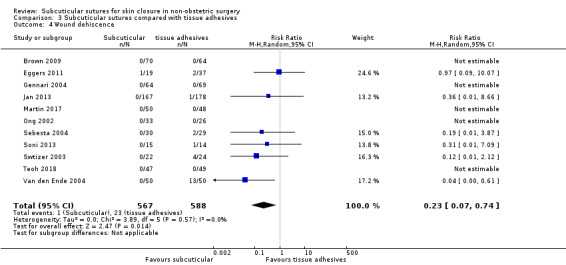
Comparison 3 Subcuticular sutures compared with tissue adhesives, Outcome 4 Wound dehiscence.
Proportion of re‐closure of the skin incision required
Van den Ende 2004 reported the proportion of re‐closure of the skin incision. There is low‐certainty evidence (downgraded twice due to imprecision) showing no clear difference in the proportion of re‐closure between participants treated with subcuticular sutures and those treated with tissue adhesives (RR 0.33; 95% CI 0.01 to 7.99; Analysis 3.5; 100 participants. This comparison was underpowered, with only one event in total.
3.5. Analysis.
Comparison 3 Subcuticular sutures compared with tissue adhesives, Outcome 5 Re‐closure.
Wound pain intensity
Eggers 2011 reported this outcome using a VAS scale that ran from 0 to 10, where 10 represented maximal pain. Eggers 2011 reported pain intensity at three and six weeks. Thus, we included the data at six weeks. There may be little or no difference between the groups in the pain intensity after 30 days (MD 0.55, 95% CI ‐0.35 to 1.45; Analysis 3.6; 56 participants) (low‐certainty evidence, downgraded twice due to imprecision).
3.6. Analysis.
Comparison 3 Subcuticular sutures compared with tissue adhesives, Outcome 6 Pain intensity after 30 days.
Length of hospital stay
Three studies reported this outcome (Eggers 2011; Gennari 2004; Khan 2006). However, as the data of one trial was considered to be skewed (Khan 2006), only data from the remaining two trials contributed to the meta‐analysis. Khan 2006 reported there was no significant difference in this outcome. In the meta‐analysis, there may also be little or no difference between the groups in the length of hospital stay (MD 0.22 days, 95% CI ‐0.39 to 0.84; Tau2= 0, I2= 0%; Analysis 3.7; 189 participants) (low‐certainty evidence, downgraded once due to risk of bias (attrition and other bias) and once due to imprecision).
3.7. Analysis.
Comparison 3 Subcuticular sutures compared with tissue adhesives, Outcome 7 Length of hospital stay.
Cosmesis of scar (as defined by the authors for a minimum follow‐up of six months).
Gennari 2004 reported cosmetic outcomes by blinded surgeon and patient at six months and one year after operation. Because we could not include the data because of missing statistics, we presented it narratively. Gennari 2004 used a score system that ran from 1 to 10, where 10 represented maximal cosmesis. One‐year follow‐up was performed in 97 patients (73%). There were similar outcomes between groups on the wound cosmetic evaluation by both plastic surgeons (tissue adhesive 8.8 versus suture 8.8) and patients (tissue adhesive 8.6 versus suture 8.9) at the 1‐year follow‐up visit. There may be little or no difference in the cosmesis of scar between the two groups. The evidence was downgraded to low certainty due to risk of bias (attrition and other bias) and imprecision. Many studies did not contribute to this outcome because, although they evaluated cosmetic appearance, they did so less than six months after surgery (Ademuyiwa 2009; Brown 2009; Eggers 2011; Jallali 2004; Jan 2013; Keng 1989; Khan 2006; Krishnamoorthy 2009; Maartense 2002; Martin 2017; Sebesta 2004; Soni 2013; Swtizer 2003; Teoh 2018; Van den Ende 2004).
Patient satisfaction
Only two trials (255 participants) reported this outcome (Gennari 2004; Khan 2006). However, as the data of one trial were considered to be skewed (Khan 2006), only the Gennari 2004 trial contributed to the meta‐analysis. Khan 2006 assessed this outcome with a VAS between 0 and 100, where 100 represented maximal satisfaction at 8 to 12 weeks after operation, and reported there was no significant difference in patient satisfaction. Gennari 2004 reported this outcome using a satisfaction score rated by the patients (scale from 1 to 10, where 10 represented best score) in the first three weeks after surgery. We calculated the SD from the reported P value. Tissue adhesives may improve patient satisfaction compared with subcuticular sutures (MD ‐2.05, 95% CI ‐3.05 to ‐1.05; 131 participants; Analysis 3.8). The evidence was downgraded to low certainty due to risk of bias (attrition and other bias) and imprecision.
3.8. Analysis.
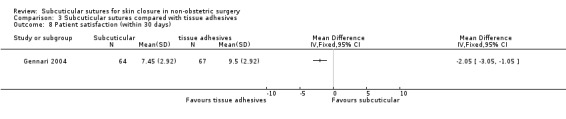
Comparison 3 Subcuticular sutures compared with tissue adhesives, Outcome 8 Patient satisfaction (within 30 days).
Quality of Life
Eggers 2011 (56 participants) reported general health as judged by responses to the SF‐12 v2 (QualityMetric Inc., Lincoln, RI) survey of the physical composite score (PCS) and mental composite score (MCS) at three and six weeks after operation. The scale score (higher better) is norm‐based scoring which is referred to as "50/10" scoring because the score has been standardised so that the general US population has a mean of 50 and SD of 10. We used the data at six weeks. There may be little or no difference between the two groups in the SF‐12 v2 PCS (MD 2.00, 95% CI ‐2.98 to 6.98; Analysis 3.9) and MCS (MD ‐1.50, 95% CI ‐6.78 to 3.78; Analysis 3.10). The certainty of the evidence is low, downgraded twice for imprecision.
3.9. Analysis.
Comparison 3 Subcuticular sutures compared with tissue adhesives, Outcome 9 SF‐12v2 PCS.
3.10. Analysis.
Comparison 3 Subcuticular sutures compared with tissue adhesives, Outcome 10 SF‐12v2 MCS.
Wound closure time in the operation (minutes)
Sixteen studies reported the time taken for closure. However, as the data of two trials were considered skewed (Khan 2006; Krishnamoorthy 2009) and the data of three studies (Eggers 2011; Keng 1989; Teoh 2018) were insufficient (missing data or statistics), only data from the remaining 11 trials (895 participants) contributed to the meta‐analysis. Keng 1989, Khan 2006 and Krishnamoorthy 2009 suggested that subcuticular sutures took significantly more time (about 0.5 to 4 minutes) than tissue adhesives.
In five studies (Jallali 2004; Jan 2013; Maartense 2002; Martin 2017; Van den Ende 2004), we calculated the SD from the reported P values. We also calculated the SD from the reported 95% CI or the SEM (standard error of the mean) in two trials (Gennari 2004; Swtizer 2003).
As there was extreme heterogeneity (I2= 97%; Analysis 3.11), we presented the results narratively. The mean wound closure time in the tissue adhesives group ranged from 0.3 to 3.7 minutes. Mean differences ranged between ‐0.34 and 10.39 minutes across 11 studies. Further analyses were not undertaken due to statistical heterogeneity in the results. The certainty of the evidence is very low downgraded once due to high risk of bias across varying domains (detection, attrition and other bias) and twice for inconsistency (I2= 97% which was mostly quantitative (i.e. most studies agreed in the direction of effect but differed in its magnitude only).
3.11. Analysis.
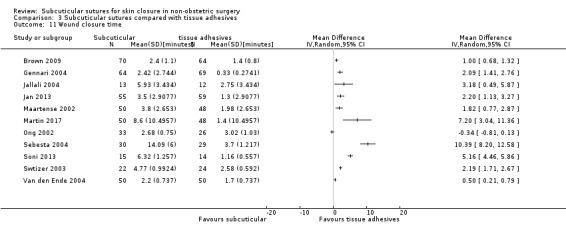
Comparison 3 Subcuticular sutures compared with tissue adhesives, Outcome 11 Wound closure time.
Cost
Seven studies reported the cost. However, as the data of three trials were insufficient (missing data or statistics) (Eggers 2011; Martin 2017; Van den Ende 2004), only data from the remaining four trials contributed to the meta‐analysis. We calculated SDs from the reported P value (Maartense 2002) and from the SEM (Gennari 2004). Two studies (Brown 2009; Sebesta 2004) reported the cost (USD) and the others (Gennari 2004; Maartense 2002) reported the cost (EUR). As there was extreme heterogeneity (I2= 96%; Analysis 3.12), we presented the results narratively. The mean cost in the tissue adhesives group ranged from 31.96 to 65.1 USD and from 20.3 to 34.01 EUR. Mean differences ranged between ‐57.36 and ‐4.26 USD (‐16.19 and ‐10.30 EUR). Further analyses were not undertaken due to statistical heterogeneity in the results. The certainty of the evidence is very low downgraded once due to high risk of bias across varying domains (attrition and other bias) and twice for inconsistency (I2= 96% which was mostly quantitative (i.e. all studies agreed in the direction of effect but differed in its magnitude only).
3.12. Analysis.
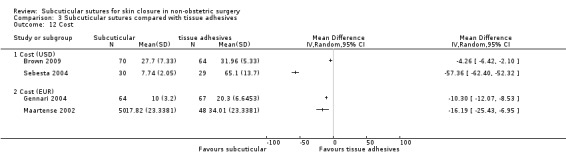
Comparison 3 Subcuticular sutures compared with tissue adhesives, Outcome 12 Cost.
Summary of comparison
There is no clear difference in the incidence of SSI (moderate‐certainty evidence) and wound complications (low‐certainty evidence) between participants treated with subcuticular sutures and those treated with tissue adhesives. Low‐certainty evidence suggests that subcuticular sutures may reduce the risk of wound dehiscence compared with tissue adhesives; but may also achieve lower patient satisfaction ratings. See Table 3.
Comparison 4. Subcuticular sutures compared with surgical tapes (9 studies, 638 participants)
None of the trials reported the proportion of re‐closure, incidence of keloid scar, patient satisfaction or QOL.
Primary outcome: SSI
Data from six trials compared the use of subcuticular sutures with surgical tapes for SSI. However, as two trials had no cases of infection (Barker 1984; Grottkau 2010), only data from the remaining four trials contributed to the meta‐analysis (Lazar 2011; Maartense 2002; O'Leary 2013; Pitcher 1983). Overall, 2.8% (10/354 participants) developed an SSI. There is no clear difference in the incidence of SSI between the two groups (RR 1.31, 95% CI 0.40 to 4.27; Analysis 4.1) with no evidence of heterogeneity (Tau2= 0, I2= 0%). Using risk difference, there were 10 more SSI per 1000 with subcuticular sutures compared with surgical tapes (30 fewer to 40 more) (RD 0.01, 95% CI ‐0.03 to 0.04; Tau2= 0, I2= 0%). It is uncertain whether subcuticular sutures increase or decrease the risk of SSI. The certainty of the evidence is very low, downgraded once for high risk of bias (attrition bias) and twice for imprecision. There are no trials that are considered to be at a low risk of selection bias. In the sensitivity analysis, there was no change in the interpretation of results by adopting the fixed‐effects model. ITT sensitivity analyses showed that the results did not change depending on the methods of the missing data (Analysis 4.2).
4.1. Analysis.
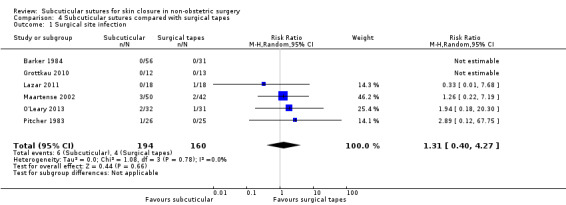
Comparison 4 Subcuticular sutures compared with surgical tapes, Outcome 1 Surgical site infection.
4.2. Analysis.
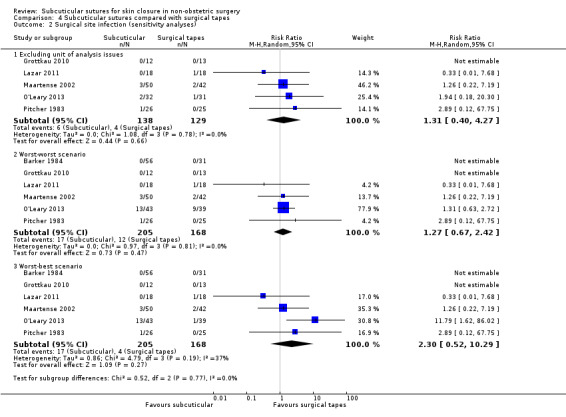
Comparison 4 Subcuticular sutures compared with surgical tapes, Outcome 2 Surgical site infection (sensitivity analyses).
Secondary outcomes
Wound complications
Five trials compared the use of subcuticular sutures with surgical tapes for wound complications and contributed to the meta‐analysis (Anatol 1997; Barker 1984; O'Leary 2013; Pitcher 1983; Rosen 1997). A total of 128 participants (128/492 (26%)) developed wound complications. There may be little or no difference in the incidence of wound complications between the two groups (RR 0.90, 95% CI 0.61 to 1.34 ; Tau2= 0.07, I2= 42%; Analysis 4.3). The certainty of the evidence is low, downgraded once due to high risk of bias (attrition, reporting and other bias in trials accounting for 65% of the analysis weight) and once due to imprecision (the confidence intervals overlapped 1 and both 0.75 and 1.25).
4.3. Analysis.
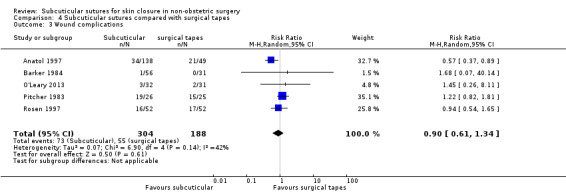
Comparison 4 Subcuticular sutures compared with surgical tapes, Outcome 3 Wound complications.
Wound dehiscence
Four trials reported wound dehiscence. However, as three trials had no cases of dehiscence (Grottkau 2010; Lazar 2011; Rebello 2009), only Anatol 1997 data contributed to the meta‐analysis. All events occurred in the surgical tape group. It is uncertain whether subcuticular sutures reduce the risk of wound dehiscence compared with surgical tapes because the certainty of the evidence is very low (RR 0.07, 95% CI 0.00 to 1.47; Tau2= 0, I2= 0%; Analysis 4.4) (downgraded once due to high risk of bias (attrition and other bias) and twice due to imprecision).
4.4. Analysis.
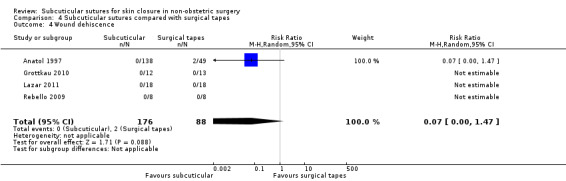
Comparison 4 Subcuticular sutures compared with surgical tapes, Outcome 4 Wound dehiscence.
Hypertrophic scar
Barker 1984 reported this outcome at one year after operation. It is uncertain whether subcuticular sutures increase or reduce the risk of hypertrophic scar compared with surgical tapes because the certainty of the evidence is very low (RR 1.68; 95% CI 0.07 to 40.14; Analysis 4.5). The evidence was downgraded to very low certainty, once due to high risk of bias (attrition bias) and twice due to imprecision.
4.5. Analysis.
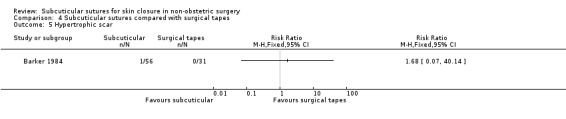
Comparison 4 Subcuticular sutures compared with surgical tapes, Outcome 5 Hypertrophic scar.
Wound pain intensity within seven days
Only three studies reported the wound pain intensity (Lazar 2011; O'Leary 2013; Rosen 1997). However, as the data of one trial were insufficient because of missing statistics (Rosen 1997), only data from the remaining two trials contributed to the meta‐analysis. Both studies assessed this outcome using a satisfaction score rated by the patients (scale, 1‐10, where 10 represented worst score). In one study, we calculated the SD from the reported P value (O'Leary 2013). There may be little or no difference in the pain intensity between the two groups (MD 0.41, 95% CI ‐0.02 to 0.83; Tau2= 0.03, I2= 28%; Analysis 4.6). The evidence was downgraded to low certainty, once due to high risk of bias (attrition bias affecting 56% of the analysis weight) and once due to imprecision (low numbers of participants).
4.6. Analysis.
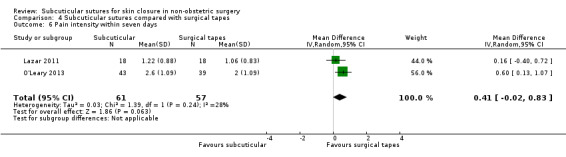
Comparison 4 Subcuticular sutures compared with surgical tapes, Outcome 6 Pain intensity within seven days.
Length of hospital stay
Lazar 2011 reported this outcome. There may be little or no difference between the groups in the length of hospital stay (MD 0.20 days, 95%CI ‐1.25 to 1.65; Analysis 4.7). The certainty of the evidence is low, downgraded twice for imprecision (very few numbers of participants).
4.7. Analysis.
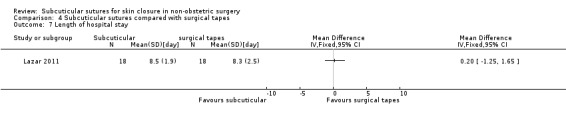
Comparison 4 Subcuticular sutures compared with surgical tapes, Outcome 7 Length of hospital stay.
Cosmesis of scar (as defined by the authors for a minimum follow‐up of six months)
Barker 1984 reported this outcome, but we did not use the data because the dropout numbers were not acceptable (> 30%) and not evenly distributed across the arms (see also Characteristics of included studies). Many studies did not contribute to this outcome because, although they evaluated cosmetic appearance, they did so less than six months after surgery (Grottkau 2010; Lazar 2011; Maartense 2002; O'Leary 2013; Rebello 2009).
Wound closure time in the operation (minutes)
Six studies reported the time taken for closure. However, as the data of two trials were insufficient (missing statistics) (Barker 1984; Pitcher 1983), only data from the remaining four trials contributed to the meta‐analysis (Grottkau 2010; Lazar 2011; Maartense 2002; Rebello 2009). In three studies (Grottkau 2010; Maartense 2002; Rebello 2009), we calculated the SD from the reported P values. As there was considerable heterogeneity (I2= 90%; Analysis 4.8), we presented the results narratively. The mean wound closure time in the surgical tape group ranged from 1.33 to 5.33 minutes. Mean differences ranged between 0.74 and 6.36 minutes across four studies. Further analyses were not undertaken due to statistical heterogeneity in the results. The certainty of the evidence is very low, downgraded once due to imprecision (low numbers of participants (169)) and twice for inconsistency (I2= 90% which was mostly quantitative (i.e. all studies agreed in the direction of effect but differed in its magnitude only)).
4.8. Analysis.
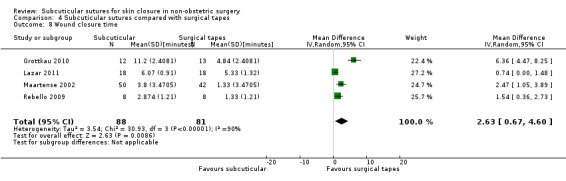
Comparison 4 Subcuticular sutures compared with surgical tapes, Outcome 8 Wound closure time.
Cost
Only three trials reported this outcome (Anatol 1997; Lazar 2011; Maartense 2002). However, as the data of these trials were insufficient (missing statistics), we presented them narratively. Lazar 2011 (36 participants) reported the mean cost per patient was USD 4.36 for sutures and USD 32.91 for surgical tapes using Steri‐Strip S. The other trials favoured surgical tapes (Anatol 1997; Maartense 2002). Anatol 1997 (190 participants) reported the cost per closure was USD 12 to 14 for subcuticular sutures and USD 4.72 for tapes. Maartense 2002 (92 participants) reported the median cost per patient was 17.82€ for sutures and 8.68€ for surgical tapes.
It is uncertain whether subcuticular sutures increase the cost compared with surgical tapes. The certainty of the evidence is very low, downgraded one level for high risk of bias (attrition and other bias), one level for imprecision (narrative synthesis), and one level for inconsistency.
Summary of comparison
Very low‐certainty evidence suggests that it is uncertain whether subcuticular sutures increase or decrease the risk of SSI and wound dehiscence compared with surgical tapes. Low‐certainty evidence shows that there may be little or no difference between subcuticular sutures and surgical tape groups in the incidence of wound complications. See Table 4.
Comparison 5. Subcuticular sutures compared with surgical zippers (3 studies, 447 participants)
None of the studies reported the proportion of re‐closure, incidence of hypertrophic scar or keloid, wound pain intensity, length of hospital stay, patient satisfaction or QOL. Two studies had one or two domains rated at high risk of bias (Tanaka 2016; Xu 2014).
Primary outcome: SSI
Three trials compared the use of subcuticular sutures with surgical zippers for SSI. However, as one trial had no cases of infection (Xu 2014), only data from the remaining two trials contributed to the meta‐analysis (Roolker 2002; Tanaka 2016). Overall, 1.2% (5/424 participants) developed an SSI. It is uncertain whether subcuticular sutures reduce or increase the risk of SSI compared with surgical zippers because the certainty of the evidence is very low (RR 0.80, 95% CI 0.08 to 8.48, Tau2= 1.04, I2= 35%; Analysis 5.1; 424 participants) (very low‐certainty evidence, downgraded once for risk of bias (attrition and reporting bias in trials accounting for 58% of the analysis weight) and twice for imprecision). There were no trials that were considered to be at a low risk of selection bias. In the sensitivity analysis, there was no change in the interpretation of results by adopting the fixed‐effects model and by ITT analyses based on the worst‐worst and the worst‐best scenarios (Analysis 5.8).
5.1. Analysis.
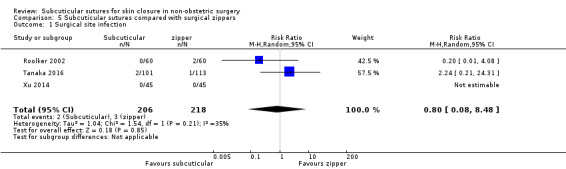
Comparison 5 Subcuticular sutures compared with surgical zippers, Outcome 1 Surgical site infection.
5.8. Analysis.
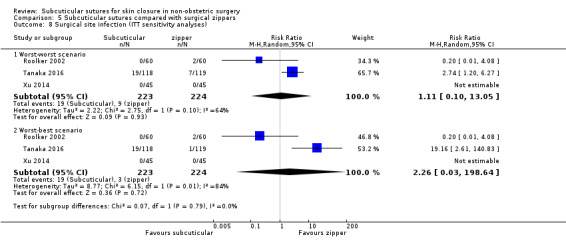
Comparison 5 Subcuticular sutures compared with surgical zippers, Outcome 8 Surgical site infection (ITT sensitivity analyses).
Secondary outcomes
Wound complications
All three studies reported this outcome. It is uncertain whether subcuticular sutures have an effect on wound complications compared with surgical zippers because the certainty of the evidence is very low (RR 0.55, 95% CI 0.15 to 2.04; Tau2= 0.63, I2= 47%; Analysis 5.2; 424 participants) (very low‐certainty evidence, downgraded once for risk of bias (detection, attrition and reporting bias) in trials accounting for 62% of the analysis weight), twice for imprecision).
5.2. Analysis.
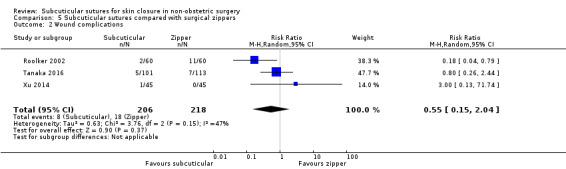
Comparison 5 Subcuticular sutures compared with surgical zippers, Outcome 2 Wound complications.
Wound dehiscence
All three trials reported wound dehiscence. However as one trial had no cases of dehiscence (Xu 2014), only data from the remaining two trials contributed to the meta‐analysis (Roolker 2002; Tanaka 2016). It is uncertain whether subcuticular sutures reduce the risk of wound dehiscence compared with surgical zippers because the certainty of the evidence is very low (RR 0.78, 95% CI 0.19 to 3.16; Tau2= 0.29, I2= 28%; Analysis 5.3; 424 participants) (very low‐certainty evidence, downgraded once for risk of bias (attrition and reporting bias in trials accounting for 46% of the analysis weight) and twice for imprecision).
5.3. Analysis.
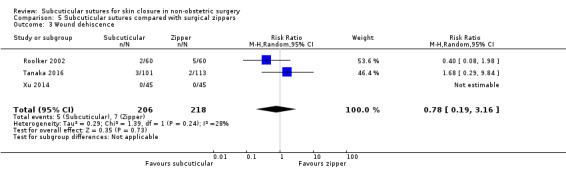
Comparison 5 Subcuticular sutures compared with surgical zippers, Outcome 3 Wound dehiscence.
Cosmesis of scar (as defined by the authors for a minimum follow‐up of six months).
Xu 2014 reported this outcome. Xu 2014 assessed cosmesis by patients using a VAS scale that ran from 0 to 10, where 10 represented the best score, and by one surgeon using the Hollander Incision Evaluation Score (Hollander 1995) at seven days, two weeks, six months, and one year after surgery. We used the data at one year. There maybe little or no difference between the two group in cosmesis using the VAS scale (MD ‐0.3, 95% CI ‐0.72 to 0.12; Analysis 5.4) and using the Hollander Score (MD ‐0.1, 95% CI ‐0.25 to 0.05; Analysis 5.5). The study is considered to be at a high risk of detection bias (Figure 3). The evidence was downgraded to low certainty, once for high risk of bias in blinding of outcome assessment in the single trial and once for imprecision (low numbers of participants; 90 participants).
5.4. Analysis.
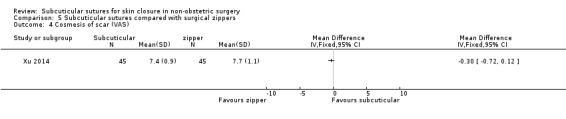
Comparison 5 Subcuticular sutures compared with surgical zippers, Outcome 4 Cosmesis of scar (VAS).
5.5. Analysis.
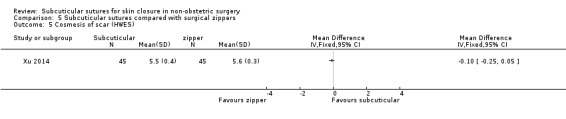
Comparison 5 Subcuticular sutures compared with surgical zippers, Outcome 5 Cosmesis of scar (HWES).
Two studies did not contribute to this outcome because, although they evaluated cosmetic appearance, they did so less than six months after surgery (Roolker 2002; Tanaka 2016).
Wound closure time
All three trials reported this outcome. As there was extreme heterogeneity (I2= 100%; Analysis 5.6), we presented the results narratively. The mean wound closure time in surgical zippers group was 0.76 to 2.1 minutes. All trials suggested that subcuticular sutures took significantly more time than surgical zippers. Mean differences ranged between 4.38 and 8.25 minutes across three studies. Further analyses were not undertaken due to statistical heterogeneity in the results. The evidence was downgraded to very low certainty, once for high risk of bias (detection, attrition and reporting bias) and twice for inconsistency (I2= 100%).
5.6. Analysis.
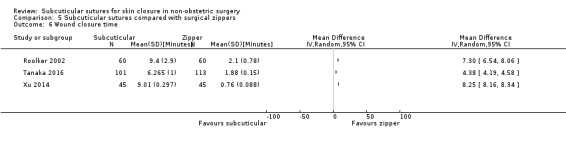
Comparison 5 Subcuticular sutures compared with surgical zippers, Outcome 6 Wound closure time.
Cost
Roolker 2002 reported this outcome. We calculated the SD from the reported P value. Subcuticular sutures may reduce the cost compared with surgical zippers. The mean cost was 8 USD in the subcuticular sutures group compared with 12 USD in the surgical zippers group with a difference in means of ‐5.00 USD (95% CI ‐8.76 to ‐1.26; 120 participants; Analysis 5.7). The evidence was downgraded to low certainty, due to risk of bias and imprecision.
5.7. Analysis.
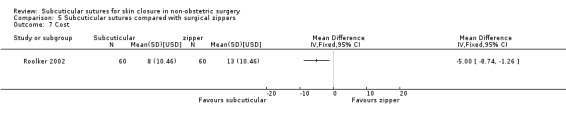
Comparison 5 Subcuticular sutures compared with surgical zippers, Outcome 7 Cost.
Summary of comparison
Very low‐certainty evidence suggests that it is uncertain whether subcuticular sutures increase or decrease the risk of SSI and wound complications and dehiscence compared with surgical zippers. Low‐certainty evidence shows that subcuticular sutures may reduce costs compared to surgical zippers. See Table 5.
Subgroup analyses
The following analyses were aimed at exploring possible sources of heterogeneity for the primary outcome, that is, SSI. We performed subgroup analyses for comparisons which included a sufficient number of studies (comparison 1: subcuticular sutures versus transdermal sutures and comparison 2: subcuticular sutures versus skin staples). We did not have sufficient studies for comparisons 3 through 5. Following the protocol, we divided the included studies into subgroups.
Subgroup analyses for comparison 1 (subcuticular sutures versus transdermal sutures)
1. absorbable versus non‐absorbable subcuticular sutures
There was no heterogeneity between the two subgroups ( I2 = 0%), and there was no change in the interpretation of results (Analysis 1.12).
1.12. Analysis.
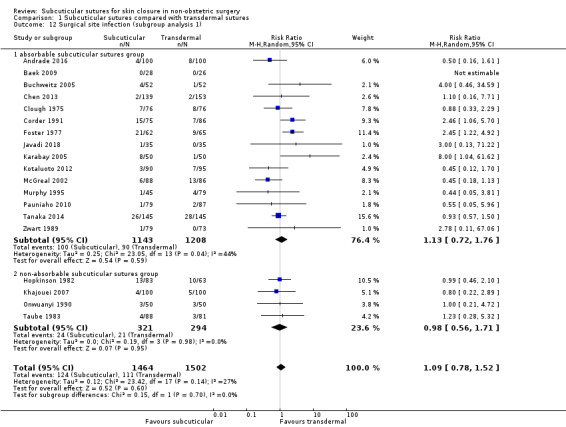
Comparison 1 Subcuticular sutures compared with transdermal sutures, Outcome 12 Surgical site infection (subgroup analysis 1).
2. location of surgery on the body (trunk versus extremities)
Substantial heterogeneity between the two subgroups ( I2 = 74%) was noted and the transdermal sutures were preferable in the extremities subgroup (RR 2.46, 95% CI 1.06 to 5.70; Analysis 1.13). However, this result in the extremities subgroup comprised only one study that was rated as being at high risk of bias in one domain (Corder 1991).
1.13. Analysis.
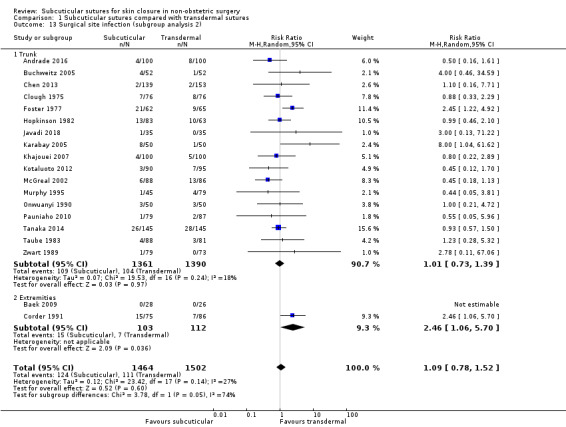
Comparison 1 Subcuticular sutures compared with transdermal sutures, Outcome 13 Surgical site infection (subgroup analysis 2).
3. CDC class 1 (clean) versus class 2 (clean‐contaminated)
We did not include in the analysis studies that were unclear about contamination level (Clough 1975; Taube 1983; Zwart 1989). There was no change in the interpretation of results (Analysis 1.14) and there was moderate heterogeneity between the two subgroups ( I2 = 50.1%).
1.14. Analysis.
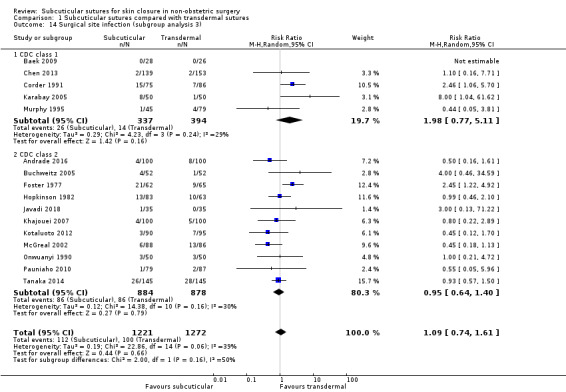
Comparison 1 Subcuticular sutures compared with transdermal sutures, Outcome 14 Surgical site infection (subgroup analysis 3).
4. continuous versus interrupted skin suture
Substantial heterogeneity between the two subgroups ( I2 = 67.8%) was noted and the subcuticular sutures were preferable in the continuous sutures subgroup (RR 0.47, 95% CI 0.26 to 0.84; Analysis 1.15).
1.15. Analysis.
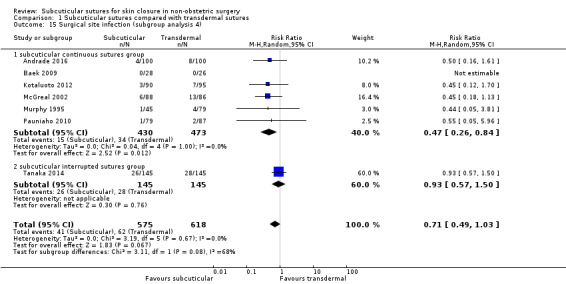
Comparison 1 Subcuticular sutures compared with transdermal sutures, Outcome 15 Surgical site infection (subgroup analysis 4).
5. endoscopic versus open surgery.
There were insufficient studies reporting these data to undertake these analyses.
Subgroup analyses for comparison 2 (subcuticular sutures versus skin staples)
1. absorbable versus non‐absorbable subcuticular sutures
There was no heterogeneity between the two subgroups ( I2 = 0%), and there was no change in the interpretation of results (Analysis 2.14).
2.14. Analysis.
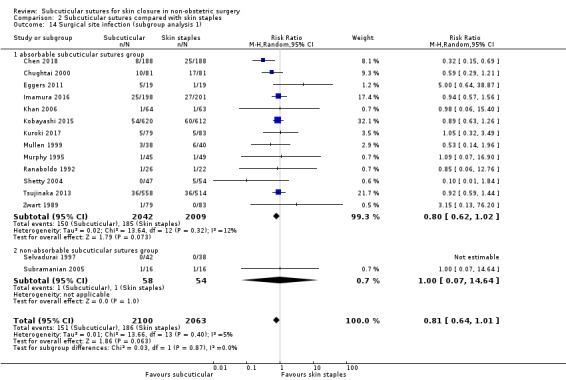
Comparison 2 Subcuticular sutures compared with skin staples, Outcome 14 Surgical site infection (subgroup analysis 1).
2. location of surgery on the body (trunk versus extremities)
There was no heterogeneity between the two subgroups ( I2 = 0%), and there was no change in the interpretation of results (Analysis 2.15).
2.15. Analysis.
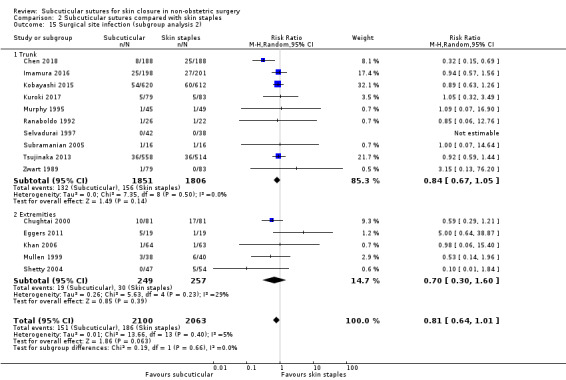
Comparison 2 Subcuticular sutures compared with skin staples, Outcome 15 Surgical site infection (subgroup analysis 2).
3. CDC class 1 (clean) versus class 2 (clean‐contaminated)
We did not include in the analysis studies that were unclear about contamination level (Imamura 2016; Ranaboldo 1992; Zwart 1989). There was no heterogeneity between the two subgroups ( I2 = 0%), and there was no change in the interpretation of results (Analysis 2.16).
2.16. Analysis.
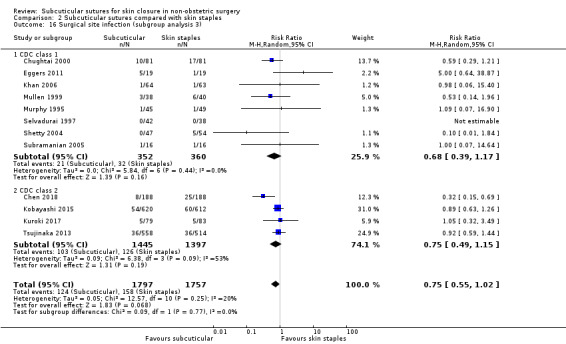
Comparison 2 Subcuticular sutures compared with skin staples, Outcome 16 Surgical site infection (subgroup analysis 3).
4. continuous versus interrupted skin suture
We did not include studies in the analysis that were unclear about the methods of suturing (continuous or interrupted). There was no heterogeneity between the two subgroups ( I2 = 0%), and there was no change in the interpretation of results (Analysis 2.17).
2.17. Analysis.
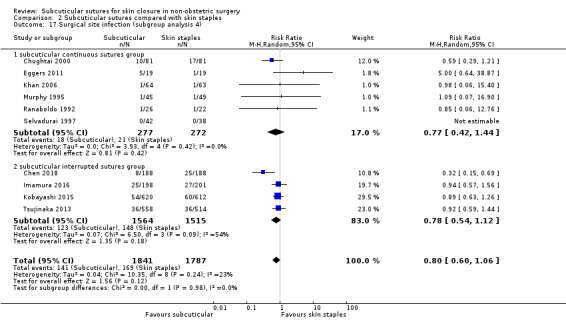
Comparison 2 Subcuticular sutures compared with skin staples, Outcome 17 Surgical site infection (subgroup analysis 4).
5. endoscopic versus open surgery.
There were insufficient studies reporting these data to undertake these analyses.
Discussion
Summary of main results
This review compared subcuticular sutures with any other skin closure methods for participants undergoing non‐obstetric operations. Sixty‐six trials with a total of 7487 participants were included: 25 studies compared subcuticular sutures with transdermal sutures; 18 studies compared subcuticular sutures with skin staples; 17 studies compared subcuticular sutures with tissue adhesives; nine studies compared subcuticular sutures with surgical tapes and three studies compared subcuticular sutures with surgical zippers. Most participants appeared to be adults, although several studies enrolled only children or both adults and children. The majority of studies included only CDC class 1 (clean) surgeries, however, two‐thirds of participants were enrolled in the studies which included CDC class 2 to 4 surgeries. The results are summarised in 'Summary of findings' tables.
Primary outcome: surgical site infection (SSI)
SSI is the most frequent healthcare‐associated infection, accounting for 31% of all healthcare‐associated infections among hospitalised patients (Mangram 1999; Magill 2012). It increases medical costs, prolongs hospital stay, and occasionally leads to mortality. There was no clear difference in the risk reduction of SSI between wounds closed with subcuticular sutures and any of the other skin closure methods reported here because of the small sample sizes or low event rates. The effect estimates are imprecise, which is reflected in the results of our GRADE assessments. The certainty of evidence varied from moderate to very low. Compared with skin staples (data from 4163 participants), subcuticular sutures probably have little or no effect on SSI because the certainty of evidence is moderate. There is moderate‐certainty evidence showing no clear difference in the incidence of SSI between participants treated with subcuticular sutures and those treated with tissue adhesives (data from 869 participants); confidence intervals were wide, spanning both appreciable benefits and harms so clear differences between treatments are not apparent. Compared with transdermal sutures (data from 3107 participants), there may be little difference in the incidence of SSI because the certainty of evidence is low. It is uncertain whether subcuticular sutures reduce or increase the risk of SSI compared with surgical tapes (data from 354 participants) or surgical zippers (data from 424 participants) because the certainty of the evidence is very low. The studies in the comparisons with transdermal sutures, surgical tapes or surgical zippers have some issues with high risk of bias in key domains. This is also reflected in the results of our GRADE assessments.
Compared with transdermal sutures, an ITT sensitivity analysis based on the worst‐best scenario showed that the results changed in favour of the transdermal sutures group. It indicated a high risk of bias due to missing outcome data. The reason may be that the majority of included studies that compared subcuticular sutures with transdermal sutures are old studies that did not report the detail of dropouts.
Secondary outcomes
The incidence of wound complications was reported for all comparisons, although the definition of wound complications varied across the studies. Subcuticular sutures for skin closure probably, on average, decrease the incidence of wound complications compared with skin staples (moderate‐certainty evidence based on 2973 participants). Although we downgraded the GRADE assessment due to imprecision (low number of events), the difference was probably more than the minimal clinically important difference. No other comparison showed evidence for a difference in risk of wound complications. We added a secondary outcome 'wound dehiscence' as a post hoc decision because, contrary to our expectations, many studies reported this outcome separately and we considered it clinically relevant. One notable finding in this review was that a clear difference in risk of wound dehiscence became apparent, with a higher risk of dehiscence associated with tissue adhesives. The difference was greater than the minimal clinically important difference. The certainty of the evidence is low, based on 1155 participants; subcuticular sutures may reduce the risk of wound dehiscence compared with adhesives. In addition, subcuticular sutures may decrease the incidence of wound dehiscence compared with skin staples. The difference was more than the minimal clinically important difference. The certainty of the evidence is low, based on 1984 participants. Many studies were also underpowered, and dehiscence was not used as a basis for sample size calculation. This is reflected in the results of our GRADE assessments. We are uncertain whether subcuticular sutures reduce wound dehiscence compared with the other closure methods (as the certainty of evidence has been assessed as very low), although most trials had lower numbers of dehiscence events in the subcuticular sutures group.
Cosmetic outcome is an important long‐term outcome for the patient and we considered appearance at or beyond six months after surgery to be meaningful. Many studies assessed cosmetic outcomes at less than six months. Therefore, we could not include limited numbers of studies. There may be little or no difference between subcuticular sutures and any of the other skin closure methods in most comparisons; this is low‐certainty evidence.
Patient satisfaction is also important when comparing closure methods provided that SSI, wound complications, dehiscence, hypertrophic/keloid scar and cosmetic appearance are satisfactory. Patient satisfaction may include ratings for cosmesis, overall comfort, ability to shower, dressing changes, tension on wounds, hygiene problems, allergic reactions and overall satisfaction. An important benefit of absorbable subcuticular sutures and tissue adhesives is that they do not need to be removed. Subcuticular sutures probably result in greater patient satisfaction than transdermal sutures (moderate‐certainty evidence based on 290 participants in a single trial) or skin staples (high‐certainty evidence based on 1232 participants in a single trial). The difference between subcuticular sutures and staples was very small (the mean patient satisfaction score (scale from 1 to 5) with subcuticular sutures was 0.20 higher), therefore this might be a less clinically important benefit than other comparisons. In contrast, there may be greater satisfaction with tissue adhesives than with subcuticular sutures (low‐certainty evidence based on 131 participants in a single trial).
The results of wound closure time in the pooled analyses were associated with considerable heterogeneity. It may depend on factors such as wound type and length. Thus, we presented the results narratively in most comparisons except for a comparison with transdermal sutures. There is moderate‐certainty evidence that wound closure time is probably longer in the subcuticular sutures group compared with transdermal sutures. Low‐certainty evidence suggested that it may take longer time to close a wound with subcuticular sutures than with skin staples. However, the difference between groups was only a few minutes (mean difference ranged from 0.3 to 5.81 minutes). The clinical impact of the difference depends on the total operation time of each procedure. The effect estimates for wound closure time suggest that this takes longer when subcuticular sutures are compared with tissue adhesives, surgical tapes or surgical zippers, however, the certainty of this evidence is very low.
Costs were not adequately reported in many studies with a few exceptions. There may be some differences in the cost when subcuticular sutures are compared with transdermal sutures or surgical zippers in favour of subcuticular sutures, but this is low‐certainty evidence. The differences were only USD 5 to USD 10, therefore, they are unlikely to exceed the minimal clinically important difference. It depends on the setting. In addition, the difference may be offset by increases in costs necessary for longer operations when subcuticular sutures are compared with transdermal sutures. We are uncertain whether subcuticular sutures increase or reduce the cost compared with skin staples or surgical tapes (very low‐certainty evidence), although most trials suggest lower costs in the subcuticular sutures groups compared with skin staples.
The proportion of re‐closure of the skin incision, incidence of hypertrophic or keloid scars and QOL were reported for a limited number of comparisons. There is moderate‐certainty evidence based on 1195 participants that skin closure by subcuticular sutures probably, on average, decreases the proportion of hypertrophic scars compared with skin staples. The proportion of re‐closure and the incidence of keloid scar was very low where reported and the effect of subcuticular sutures is very uncertain (low or very low‐certainty evidence). Only one trial with a small number of participants reported QOL. There was no clinically important difference in QOL (low‐certainty evidence).
Wound pain intensity and length of hospital stay were reported for many comparisons. We had planned to include any readmissions for wound‐related complications in the length of hospital stay, but none of the studies reported readmissions. There may be little or no clinically important difference between subcuticular sutures and any other methods of skin closure in most comparisons; this is low‐certainty evidence.
Overall completeness and applicability of evidence
There was no apparent exclusion of particular patient groups from the included studies. Therefore, the results of this review would apply to a wide range of people undergoing non‐obstetric surgery. However, due to the variety of the surgical procedures, the overall effects demonstrated in this review should be interpreted with caution. For example, incisions in areas of high tension were excluded when subcuticular sutures were compared with tissue adhesives and surgical tapes. Tissue adhesives and surgical tapes have not been evaluated in this population. In addition the surgical zipper is a new device for skin closure, and we were able to include only three studies (two: orthopaedic surgery, one: cardiac operation; two of three studies for paediatric populations). Note that a subgroup analysis comparing SSI between the study groups based on degree of contamination was carried out in an attempt to evaluate the effect of wound contamination on infection. However, wound classification was not consistently reported in some of the included studies. Other clinical factors that could affect wound outcomes such as endoscopic versus open surgery were not adequately addressed in the included studies. We encourage readers to also examine if surgical characteristics of the included populations were consistent with their particular interests.
It is also noteworthy that there was notable variation among studies with regard to the detailed suture methods such as nature of materials or continuous/interrupted suture methods. We examined their influences, where possible, with regards to the primary outcome only (for the reasons explained in Subgroup analysis and investigation of heterogeneity). However, the methods of continuous or interrupted suture were not consistently reported in many of the included studies.
In addition, we could not explore the influence of studies sponsored by companies, because funding was not reported in many of the included studies. It should be considered in the future studies.
Quality of the evidence
Five comparisons are presented in the 'Summary of findings' tables (Table 1: subcuticular sutures versus transdermal sutures, Table 2: skin staples, Table 3: tissue adhesives, Table 4: surgical tapes and Table 5: surgical zippers). Following a GRADE assessment, the certainty of evidence was high to very low across the outcomes assessed. The quality of the evidence for comparison 4 (surgical tapes) and comparison 5 (zippers) was low to very low, depending on the outcome.
One of the main factors affecting the quality of evidence was the presence of an unclear or high risk of bias, for many included studies, in more than one important domain. As shown in Figure 2 and Figure 3, the majority of included studies were at unclear risk of selection bias; we found 16 studies to be at high risk of attrition bias and nine studies to be at high risk of other potential bias (unit of analysis issues, baseline imbalance, etc); we also found selective outcome reporting to be present. It may be difficult for both the surgeons and assessors of postoperative short‐term outcomes to be blinded to the intervention. The influence of the post‐randomisation exclusion of participants was tested by imputing outcomes for the missing participants under different scenarios. Compared with transdermal sutures, this showed that the worst‐best scenario resulted in different conclusions, and indicated a high risk of bias due to missing outcome data. We reflected this in our assessment of attrition bias in these individual studies, and also in our overall GRADE criteria. Fourteen studies were identified as having potential unit of analysis issues as it did not appear that paired or clustered data were accounted for in the analysis. We also reflected this in our assessment of other potential sources of bias in these individual studies, and also in our overall GRADE criteria.
The main factor affecting the quality of evidence was the lack of precision of results. Almost all outcome results were imprecise due to small sample size and limited number of outcome events, leading to wide confidence intervals. We downgraded evidence by one level when the confidence interval of the overall effect of an outcome crossed the line of no effect (or 1), in addition to the confidence intervals crossing either 0.75 (appreciable benefit) or 1.25 (appreciable harm) and by two levels when there were very few events and CIs around effects included both appreciable benefit and appreciable harm.
We downgraded for inconsistency when the meta‐analyses had I2 values which suggested substantial or considerable heterogeneity, or the included studies reported qualitatively different results. Such downgrading was necessary for some outcomes.
There was no downgrading for indirectness as the included studies were in agreement with the review question.
The outcomes assessed for publication bias were SSI and wound complications. A visual inspection of the funnel plot revealed no evidence of publication bias, therefore, no results were downgraded for publication bias.
Potential biases in the review process
Although this is the first, large and comprehensive systematic review for subcuticular sutures for skin closure and the funnel plots did not show any apparent asymmetry, there is reason to suspect that the available literature may still be affected by publication bias. Even when a study was published, lack of unified outcome measures across studies, especially in the older trials, led to suspicion of outcome reporting bias. We included only trials in which it was clear that the comparison involved subcuticular sutures versus other methods of skin closure. As mentioned in Excluded studies, 15 studies were excluded because it was not clear whether they involved this comparison. Attempts to contact the authors were unproductive. While the majority of these studies were unlikely to be included in this review, some of the studies may have met the inclusion criteria for this review. Many of the included studies were not ideal in terms of risk of bias as individual studies and did not provide sufficient information on long‐term patient outcomes. Fourteen studies were identified as having potential unit of analysis issues. Of these studies, only four studies were identified as it did not appear that paired data were accounted for in the analysis and the remaining studies were identified as it did not appear that clustered data were accounted for in the analysis. We adopted a pragmatic but conservative post hoc approach to analyses including clustered and paired data because a very small number of participants have more than one wound in almost all of these studies. We included such studies in meta‐analyses where possible (where unadjusted clustered data would produce too narrow CIs and unadjusted paired data too wide CIs). We undertook a post hoc sensitivity analysis to explore the impact of including data that had been inappropriately unadjusted. In all cases, it had little effect on the estimate of effect or the confidence intervals. We are therefore confident that our post hoc approach to data from these trials is unlikely to have affected the findings of the review, and that fully including the data increases the comprehensiveness of the review.
Agreements and disagreements with other studies or reviews
This is the first systematic review on this topic.
One related Cochrane systematic review entitled 'Continuous versus interrupted skin sutures for non‐obstetric surgery' showed that there was no significant difference between the groups in the proportion of participants who developed superficial SSI, but superficial wound dehiscence may be reduced by using continuous subcuticular sutures (Gurusamy 2014). In this review, it must be noted that in most of the included studies the continuous skin suture groups received subcuticular sutures, while the interrupted skin suture groups received non‐absorbable transdermal sutures. The results of our review can be said to be in agreement with Gurusamy 2014 in finding no difference in the incidence of SSI. There was no clear difference in the wound dehiscence in our study possibly due to a low number of events.
In obstetric surgery, four systematic reviews and meta‐analyses that evaluated subcuticular sutures have been published. A Cochrane systematic review did not find conclusive evidence about how the skin should be closed (Mackeen 2012). Staples are associated with similar outcomes in terms of wound infection, pain and cosmesis compared with subcuticular sutures. Mackeen 2012 showed staples are associated with an increased risk of skin separation compared with subcuticular sutures. The results of Mackeen 2012 are generally in agreement with our review. We also show subcuticular sutures may reduce the risk of skin separation (wound dehiscence) compared with skin staples.
The others concluded that there was a possible benefit with subcuticular sutures compared with skin staples, because of a lower incidence of wound complications and faster methods of skin closure (Clay 2011; Mackeen 2015; Tuuli 2011). Clay 2011 and Mackeen 2015 also reported wound separation (dehiscence) was significantly more frequent in the group that received staples. These results are in agreement with our review. Although Mackeen 2015 reported there were no significant differences in pain, patient satisfaction, or cosmesis, we found subcuticular sutures were associated with higher scores of patient satisfaction.
Authors' conclusions
Implications for practice.
Sixty‐six studies, including 7487 participants, compared subcuticular sutures with other methods of skin closure. We found no clear difference in the incidence of surgical site infection when subcuticular sutures were compared with any of the skin closure methods including transdermal sutures, skin staples, tissues adhesives, tapes or zippers. When subcuticular sutures were compared with staples, there was moderate‐certainty evidence of a benefit for using sutures for minimising wound complications and hypertrophic scar and there was low‐certainty evidence of a benefit for reducing wound dehiscence. When subcuticular sutures were compared with tissue adhesives, there was low‐certainty evidence of a benefit for using sutures for reducing wound dehiscence. Although there was low‐certainty evidence that patients may prefer tissue adhesives, there was also evidence that patient satisfaction with subcuticular sutures was higher than for transdermal sutures (moderate‐certainty) and staples (high‐certainty). The clinical implications of this still remain to be re‐evaluated because the difference in patient satisfaction between subcuticular sutures and skin staples is relatively small. Although there was some evidence that alternatives to subcuticular sutures were less time consuming to use, there was also evidence that the cost of subcuticular sutures was lower than others. Because the time and cost for subcuticular sutures differs depending on the type of surgery, clinical application should be determined in each case considering the advantages and the disadvantages of each method. The certainty of the evidence varied from high to very low; almost all of the evidence was subject to some limitations.
Implications for research.
An important finding of this review regards the quality of trials. It is desirable that future trials present more detailed descriptions of the randomisation process and provide information about funding. Since incomplete outcome reporting was an important limitation in this review, future trials should follow the CONSORT statement. The reporting of outcomes such as pain, patient satisfaction and cosmesis should be more standardised and reported at the same time points across studies (with measures of variance) to enable data pooling. The follow‐up duration should be at least one year, in order to measure outcomes such as hypertrophic scar formation, keloid scar formation and cosmetic outcome. Future studies should assess quality of life as outcome and ensure complete reporting of continuous outcomes with measures of variance.
There seems to be no further need to explore the comparison between subcuticular sutures and staples because there are some high‐quality studies with large sample sizes and some ongoing studies. However, there is still a need for studies exploring the other comparisons with transdermal sutures, adhesives, tapes or zippers. Such studies must have high‐quality and large sample sizes, including long‐term assessments.
Acknowledgements
The authors would like to thank the following editors and peer referees who provided comments to improve the protocol: Ros Wade (methodologist), Beryl De Souza and Andrew McKean (specialist referees).
The authors would like to thank the following peer referees who provided comments to improve the review: Beryl De Souza (specialist referee), Janet Yarrow (consumer referee) and one other referee who wishes to remain anonymous.
We would also like to acknowledge the translation assistance of Zhenmi Liu and Ahmad Sofi Mahmudi. The authors would like to thank Cochrane Wounds intern, Laura Chiverton, for her work on the Plain Language Summary and Elizabeth Royle and Anne Lethaby for copy editing the protocol and the review respectively.
Appendices
Appendix 1. Classification of surgical wound
| Class 1: clean | An uninfected operative wound in which no inflammation is encountered and the respiratory, alimentary, genital, or uninfected urinary tract is not entered. In addition, clean wounds are primarily closed and, if necessary, drained with closed drainage. Operative incisional wounds that follow nonpenetrating (blunt) trauma should be included in this category if they meet the criteria. |
|
Class 2: clean‐contaminated |
An operative wound in which the respiratory, alimentary, genital, or urinary tracts are entered under controlled conditions and without unusual contamination. Specifically, operations involving the biliary tract, appendix, vagina, and oropharynx are included in this category, provided no evidence of infection or major break in technique is encountered. |
|
Class 3: contaminated |
Open, fresh, accidental wounds. In addition, operations with major breaks in sterile technique (e.g. open cardiac massage) or gross spillage from the gastrointestinal tract, and incisions in which acute, nonpurulent inflammation is encountered are included in this category. |
|
Class 4: dirty‐infected |
Old traumatic wounds with retained devitalised tissue and those that involve existing clinical infection or perforated viscera. This definition suggests that the organisms causing postoperative infection were present in the operative field before the operation. |
Appendix 2. Search strategies
Cochrane Wounds Specialised Register
1 MESH DESCRIPTOR Subcutaneous Tissue EXPLODE ALL AND INREGISTER
2 (hypoderm* or subderm* or intraderm*) AND INREGISTER
3 (subcutaneous or sub‐cutaneous or subcuticular or sub‐cuticular or buried) AND INREGISTER
4 #1 OR #2 OR #3
5 MESH DESCRIPTOR Sutures EXPLODE ALL AND INREGISTER
6 MESH DESCRIPTOR Wound Closure Techniques EXPLODE ALL AND INREGISTER
7 (sutur* or stitch* or closure or close or closing*) AND INREGISTER
8 (Monocryl or Vicryl or PDS) AND INREGISTER
9 #5 OR #6 OR #7 OR #8 AND INREGISTER
10 #4 AND #9 AND INREGISTER
The Cochrane Central Register of Controlled Clinical Trials (CENTRAL)
#1 MeSH descriptor: [Subcutaneous Tissue] explode all trees
#2 (hypoderm* or subderm* or intraderm*):ti,ab,kw
#3 (subcutaneous or sub‐cutaneous or subcuticular or sub‐cuticular or buried):ti,ab,kw
#4 {or #1‐#3} in Trials
#5 MeSH descriptor: [Sutures] explode all trees
#6 MeSH descriptor: [Wound Closure Techniques] explode all trees
#7 (sutur* or stitch* or closure or close or closing*):ti,ab,kw
#8 (Monocryl or Vicryl or PDS):ti,ab,kw
#9 {or #5‐#8} in Trials
#10 #4 and #9 in Trials
Ovid MEDLINE
1 exp Subcutaneous Tissue/
2 (hypoderm* or subderm* or intraderm*).ti,ab.
3 (subcutaneous or sub‐cutaneous or subcuticular or sub‐cuticular or buried).ti,ab.
4 or/1‐3
5 exp Sutures/
6 exp Wound Closure Techniques/
7 (sutur* or stitch* or closure or close or closing*).ti,ab.
8 (Monocryl or Vicryl or PDS).ti,ab.
9 or/5‐8
10 4 and 9
11 randomised controlled trial.pt.
12 controlled clinical trial.pt.
13 randomi?ed.ab.
14 placebo.ab.
15 clinical trials as topic.sh.
16 randomly.ab.
17 trial.ti.
18 or/11‐17
19 exp animals/ not humans.sh.
20 18 not 19
21 10 and 20
Ovid Embase
1 exp subcutaneous tissue/
2 (hypoderm* or subderm* or intraderm*).ti,ab.
3 (subcutaneous or sub‐cutaneous or subcuticular or sub‐cuticular or buried).ti,ab.
4 or/1‐3
5 exp suture/
6 exp wound closure/
7 (sutur* or stitch* or closure or close or closing*).ti,ab.
8 (Monocryl or Vicryl or PDS).ti,ab.
9 or/5‐8
10 and/4,9
11 Randomized controlled trials/
12 Single‐Blind Method/
13 Double‐Blind Method/
14 Crossover Procedure/
15 (random* or factorial* or crossover* or cross over* or cross‐over* or placebo* or assign* or allocat* or volunteer*).ti,ab.
16 (doubl* adj blind*).ti,ab.
17 (singl* adj blind*).ti,ab.
18 or/11‐17
19 exp animals/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/
20 human/ or human cell/
21 and/19‐20
22 19 not 21
23 18 not 22
24 10 and 23
EBSCO CINAHL Plus
S23 S9 AND S22
S22 S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21
S21 TI allocat* random* or AB allocat* random*
S20 MH "Quantitative Studies"
S19 TI placebo* or AB placebo*
S18 MH "Placebos"
S17 TI random* allocat* or AB random* allocat*
S16 MH "Random Assignment"
S15 TI randomi?ed control* trial* or AB randomi?ed control* trial*
S14 AB ( singl* or doubl* or trebl* or tripl* ) and AB ( blind* or mask* )
S13 TI ( singl* or doubl* or trebl* or tripl* ) and TI ( blind* or mask* )
S12 TI clinic* N1 trial* or AB clinic* N1 trial*
S11 PT Clinical trial
S10 MH "Clinical Trials+"
S9 S3 AND S8
S8 S4 OR S5 OR S6 OR S7
S7 TI (Monocryl or Vicryl or PDS) OR AB (Monocryl or Vicryl or PDS)
S6 TI ((sutur* or stitch* or closure or close or closing*) OR AB (sutur* or stitch* or closure or close or closing*)
S5 (MH "Suture Techniques")
S4 (MH "Sutures")
S3 S1 OR S2
S2 TI (subcutaneous or sub‐cutaneous or subcuticular or sub‐cuticular or buried) OR AB (subcutaneous or sub‐cutaneous or subcuticular or sub‐cuticular or buried)
S1 TI (hypoderm* or subderm* or intraderm*) or AB (hypoderm* or subderm* or intraderm*)
US National Institutes of Health Ongoing Trials Register (ClinicalTrials.gov)
[subcuticular]
World Health Organization International Clinical Trials Registry Platform
[subcuticular]
EU Clinical Trials Register
[subcuticular]
University hospital Medical Information Network Clinical Trials Registry (UMIN‐CTR)
[真皮縫合 OR 真皮埋没縫合]
Appendix 3. Criteria for judging risk of bias in the 'Risk of bias' assessment tool
|
RANDOM SEQUENCE GENERATION Selection bias (biased allocation to interventions) due to inadequate generation of a randomised sequence | |
| Criteria for a judgement of low risk of bias | The investigators describe a random component in the sequence generation process such as:
*Minimisation may be implemented without a random element, and this is considered to be equivalent to being random. |
| Criteria for a judgement of high risk of bias | The investigators describe a non‐random component in the sequence generation process. Usually, the description would involve some systematic, non‐random approach, for example generating the sequence:
Other non‐random approaches happen much less frequently than the systematic approaches mentioned above and tend to be obvious. They usually involve judgement or some method of non‐random categorisation of participants, for example allocation:
|
| Criteria for a judgement of unclear risk of bias | Insufficient information about the sequence generation process is available to permit a judgement of 'low risk' or 'high risk'. |
|
ALLOCATION CONCEALMENT Selection bias (biased allocation to interventions) due to inadequate concealment of allocations prior to assignment | |
| Criteria for a judgement of low risk of bias | Participants and investigators enrolling participants could not foresee assignment because one of the following, or an equivalent method, was used to conceal allocation:
|
| Criteria for a judgement of high risk of bias | Participants or investigators enrolling participants could possibly foresee assignments and thus introduce selection bias, such as allocation based on:
|
| Criteria for a judgement of unclear risk of bias | Insufficient information available to permit a judgement of 'low risk' or 'high risk'. This is usually the case if the method of concealment is not described or not described in sufficient detail to allow a definite judgement – for example, if the use of assignment envelopes is described, but it remains unclear whether envelopes were sequentially numbered, opaque and sealed. |
|
BLINDING OF PARTICIPANTS AND PERSONNEL Performance bias due to knowledge of the allocated interventions by participants and personnel during the study | |
| Criteria for a judgement of low risk of bias | Either of the following:
|
| Criteria for a judgement of high risk of bias | Either of the following:
|
| Criteria for a judgement of unclear risk of bias | Either of the following:
|
|
BLINDING OF OUTCOME ASSESSMENT Detection bias due to knowledge of the allocated interventions by outcome assessors | |
| Criteria for a judgement of low risk of bias | Either of the following:
|
| Criteria for a judgement of high risk of bias | Either of the following:
|
| Criteria for a judgement of unclear risk of bias | Either of the following:
|
|
INCOMPLETE OUTCOME DATA Attrition bias due to amount, nature or handling of incomplete outcome data | |
| Criteria for a judgement of low risk of bias | Any one of the following:
|
| Criteria for a judgement of high risk of bias | Any one of the following:
|
| Criteria for a judgement of unclear risk of bias | Either of the following:
|
|
SELECTIVE REPORTING Reporting bias due to selective outcome reporting | |
| Criteria for a judgement of low risk of bias | Either of the following:
|
| Criteria for a judgement of high risk of bias | Any one of the following:
|
| Criteria for a judgement of unclear risk of bias | Insufficient information available to permit a judgement of 'low risk' or 'high risk'. It is likely that the majority of studies will fall into this category. |
|
OTHER BIAS Bias due to problems not covered elsewhere in this table | |
| Criteria for a judgement of low risk of bias | The study appears to be free of other sources of bias. |
| Criteria for a judgement of high risk of bias | There is at least one important risk of bias. For example, the study:
Or in cluster‐randomised trials there is:
|
| Criteria for a judgement of unclear risk of bias | There may be a risk of bias, but there is either:
|
Data and analyses
Comparison 1. Subcuticular sutures compared with transdermal sutures.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Surgical site infection | 20 | 3107 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.80, 1.52] |
| 2 Surgical site infection (sensitivity analyses) | 20 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Low risk of selection bias | 1 | 290 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.57, 1.50] |
| 2.2 Excluding unit of analysis issues | 17 | 2768 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.75, 1.42] |
| 2.3 Worst‐worst scenario | 20 | 3186 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [0.97, 1.55] |
| 2.4 Worst‐best scenario | 20 | 3186 | Risk Ratio (M‐H, Random, 95% CI) | 1.59 [1.05, 2.42] |
| 3 Wound complications | 9 | 1489 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.40, 1.71] |
| 4 Wound dehiscence | 6 | 866 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.08, 1.54] |
| 5 Re‐closure | 2 | 246 | Risk Ratio (M‐H, Random, 95% CI) | 1.16 [0.09, 14.57] |
| 6 Hypertrophic scar | 2 | 233 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.25, 3.39] |
| 7 Length of hospital stay | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8 Patient satisfaction (within 30 days) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 9 Patient satisfaction (after 60 days) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 10 Wound closure time | 2 | 585 | Mean Difference (IV, Random, 95% CI) | 5.81 [5.13, 6.49] |
| 11 Cost | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 12 Surgical site infection (subgroup analysis 1) | 19 | 2966 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.78, 1.52] |
| 12.1 absorbable subcuticular sutures group | 15 | 2351 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.72, 1.76] |
| 12.2 non‐absorbable subcuticular sutures group | 4 | 615 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.56, 1.71] |
| 13 Surgical site infection (subgroup analysis 2) | 19 | 2966 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.78, 1.52] |
| 13.1 Trunk | 17 | 2751 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.73, 1.39] |
| 13.2 Extremities | 2 | 215 | Risk Ratio (M‐H, Random, 95% CI) | 2.46 [1.06, 5.70] |
| 14 Surgical site infection (subgroup analysis 3) | 16 | 2493 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.74, 1.61] |
| 14.1 CDC class 1 | 5 | 731 | Risk Ratio (M‐H, Random, 95% CI) | 1.98 [0.77, 5.11] |
| 14.2 CDC class 2 | 11 | 1762 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.64, 1.40] |
| 15 Surgical site infection (subgroup analysis 4) | 7 | 1193 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.49, 1.03] |
| 15.1 subcuticular continuous sutures group | 6 | 903 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.26, 0.84] |
| 15.2 subcuticular interrupted sutures group | 1 | 290 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.57, 1.50] |
Comparison 2. Subcuticular sutures compared with skin staples.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Surgical site infection | 15 | 4163 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.64, 1.01] |
| 2 Surgical site infection (sensitivity analyses) | 15 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Low risk of selection bias | 4 | 2865 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.72, 1.16] |
| 2.2 Excluding unit of analysis issues | 12 | 3875 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.61, 1.09] |
| 2.3 Worst‐worst scenario | 15 | 4219 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.67, 1.13] |
| 2.4 Worst‐best scenario | 15 | 4219 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.65, 1.37] |
| 3 Wound complications | 9 | 2973 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.64, 0.98] |
| 4 Wound dehiscence | 7 | 1984 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.43, 0.94] |
| 5 Hypertrophic scar | 3 | 1195 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.60, 0.98] |
| 6 Pain intensity within seven days | 3 | 218 | Mean Difference (IV, Random, 95% CI) | ‐1.86 [‐10.37, 6.65] |
| 7 Pain intensity after 30 days | 3 | 196 | Std. Mean Difference (IV, Random, 95% CI) | 0.18 [‐0.30, 0.66] |
| 8 Length of hospital stay | 5 | 2794 | Mean Difference (IV, Random, 95% CI) | ‐0.58 [‐1.57, 0.42] |
| 9 Cosmesis of scar | 3 | 291 | Std. Mean Difference (IV, Random, 95% CI) | 0.12 [‐0.11, 0.35] |
| 10 Patient satisfaction (within 30 days) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 11 SF‐12v2 PCS | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 12 SF‐12v2 MCS | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 13 Wound closure time | 4 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 14 Surgical site infection (subgroup analysis 1) | 15 | 4163 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.64, 1.01] |
| 14.1 absorbable subcuticular sutures group | 13 | 4051 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.62, 1.02] |
| 14.2 non‐absorbable subcuticular sutures group | 2 | 112 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.07, 14.64] |
| 15 Surgical site infection (subgroup analysis 2) | 15 | 4163 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.64, 1.01] |
| 15.1 Trunk | 10 | 3657 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.67, 1.05] |
| 15.2 Extremities | 5 | 506 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.30, 1.60] |
| 16 Surgical site infection (subgroup analysis 3) | 12 | 3554 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.55, 1.02] |
| 16.1 CDC class 1 | 8 | 712 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.39, 1.17] |
| 16.2 CDC class 2 | 4 | 2842 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.49, 1.15] |
| 17 Surgical site infection (subgroup analysis 4) | 10 | 3628 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.60, 1.06] |
| 17.1 subcuticular continuous sutures group | 6 | 549 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.42, 1.44] |
| 17.2 subcuticular interrupted sutures group | 4 | 3079 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.54, 1.12] |
Comparison 3. Subcuticular sutures compared with tissue adhesives.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Surgical site infection | 10 | 869 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.41, 1.45] |
| 2 Surgical site infection (sensitivity analyses) | 10 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Excluding unit of analysis issues | 9 | 823 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.41, 1.45] |
| 2.2 Worst‐worst scenario | 10 | 901 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.61, 1.46] |
| 2.3 Worst‐best scenario | 10 | 901 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.43, 2.73] |
| 3 Wound complications | 11 | 1058 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.35, 1.11] |
| 4 Wound dehiscence | 11 | 1155 | Risk Ratio (M‐H, Random, 95% CI) | 0.23 [0.07, 0.74] |
| 5 Re‐closure | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6 Pain intensity after 30 days | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7 Length of hospital stay | 2 | 189 | Mean Difference (IV, Random, 95% CI) | 0.22 [‐0.39, 0.84] |
| 8 Patient satisfaction (within 30 days) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 9 SF‐12v2 PCS | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 10 SF‐12v2 MCS | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 11 Wound closure time | 11 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 12 Cost | 4 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 12.1 Cost (USD) | 2 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 12.2 Cost (EUR) | 2 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 4. Subcuticular sutures compared with surgical tapes.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Surgical site infection | 6 | 354 | Risk Ratio (M‐H, Random, 95% CI) | 1.31 [0.40, 4.27] |
| 2 Surgical site infection (sensitivity analyses) | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Excluding unit of analysis issues | 5 | 267 | Risk Ratio (M‐H, Random, 95% CI) | 1.31 [0.40, 4.27] |
| 2.2 Worst‐worst scenario | 6 | 373 | Risk Ratio (M‐H, Random, 95% CI) | 1.27 [0.67, 2.42] |
| 2.3 Worst‐best scenario | 6 | 373 | Risk Ratio (M‐H, Random, 95% CI) | 2.30 [0.52, 10.29] |
| 3 Wound complications | 5 | 492 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.61, 1.34] |
| 4 Wound dehiscence | 4 | 264 | Risk Ratio (M‐H, Random, 95% CI) | 0.07 [0.00, 1.47] |
| 5 Hypertrophic scar | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6 Pain intensity within seven days | 2 | 118 | Mean Difference (IV, Random, 95% CI) | 0.41 [‐0.02, 0.83] |
| 7 Length of hospital stay | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8 Wound closure time | 4 | 169 | Mean Difference (IV, Random, 95% CI) | 2.63 [0.67, 4.60] |
Comparison 5. Subcuticular sutures compared with surgical zippers.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Surgical site infection | 3 | 424 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.08, 8.48] |
| 2 Wound complications | 3 | 424 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.15, 2.04] |
| 3 Wound dehiscence | 3 | 424 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.19, 3.16] |
| 4 Cosmesis of scar (VAS) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5 Cosmesis of scar (HWES) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6 Wound closure time | 3 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 7 Cost | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8 Surgical site infection (ITT sensitivity analyses) | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 8.1 Worst‐worst scenario | 3 | 447 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.10, 13.05] |
| 8.2 Worst‐best scenario | 3 | 447 | Risk Ratio (M‐H, Random, 95% CI) | 2.26 [0.03, 198.64] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ademuyiwa 2009.
| Methods | Randomised controlled trial | |
| Participants | Country: Nigeria Number randomised: 45 (52 wounds) Post‐randomisation dropout: 1 vs 0 Mean age (years): not stated Number of males: 44 Duration of follow‐up: 12 weeks Surgery: herniotomy |
|
| Interventions | Participants randomly assigned to 2 groups: Group 1: subcuticular suture (n = 21 + 2), using a 0000 polyglycolic acid (Dexon®) Group 2: skin adhesives (n = 16 + 5), using 2‐Octylcyanoacrylate (Dermabond® manufactured by Ethicon) |
|
| Outcomes | Wound complications (erythema) | |
| Notes | Cosmetic outcome as measured at < 6 months so not included An outcome that study authors referred to as a 'patient satisfaction' score was also measured, however, this seemed to focus on satisfaction of cosmetic appearance so was deemed a cosmetic evaluation; as it was collected at 3 months after surgery, it was not reported here. Source of support: nil COI: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "selection was based on a stratified randomised sampling technique. Patients were stratified into ... Patients falling into each group were thereafter randomly chosen by tossing a coin with..." |
| Allocation concealment (selection bias) | Unclear risk | No direct quotation about whether allocation concealment was done |
| Blinding of participants and personnel (performance bias) Surgical site infection | Unclear risk | No direct quotation about whether the participants or personnel were blinded |
| Blinding of outcome assessment (detection bias) Surgical site infection | Unclear risk | Quote: "assessment of cosmetic appearance based on the Hollander Wound Evaluation Scale and Visual Analogue Scale ... was done by the Plastic Surgeon (who was blinded to the method of wound closure) and the parents respectively." Comment: no direct quotation about whether the outcome assessors of wound infection were blinded |
| Incomplete outcome data (attrition bias) Short‐term outcomes | High risk | Quote: "a patient in the subcuticular suturing group with surgical site infection was excluded from the study as this could affect the eventual outcome of the scar." Comment: a case with SSI which is a relevant outcome to this review was excluded. |
| Selective reporting (reporting bias) | Unclear risk | Study protocol was unavailable, and no specific quote but outcomes other than "wound edge complication" were not prespecified so it is unclear whether all outcomes assessed were fully reported. |
| Other bias | High risk | A case with SSI was excluded from the analysis because of an inappropriate reason, which means that this study did not assess SSI strictly. Randomisation was conducted at a participant level but the analysis was carried out at the level of the wound; it did not appear that clustered data (multiple wounds from individual participants) were accounted for in the analysis. |
Anatol 1997.
| Methods | Randomised controlled trial (3‐arm) Randomised at a wound level (not a participant level) |
|
| Participants | Country: Trinidad
Number randomised: 190 (wounds)
Post‐randomisation dropouts: 3 wounds (at 1 week), 45 wounds (at 6 weeks)
Average age: not stated
Male:female ratio: not stated Duration of study: 27 months Duration of follow‐up: 11 months Inclusion criteria: children under the age of 15 years requiring groin crease incision |
|
| Interventions | Wounds randomly assigned to 3 groups:
Group 1: subcuticular continuous sutures (n = 76), using 3‐0 vicryl subcuticular
Group 2: subcuticular interrupted sutures (n = 62), using: 3‐0 plain catgut Group 3: skin tapes (n = 52) For this review, the subcuticular data (Group 1 and 2) have been combined. No subcutaneous sutures were used. |
|
| Outcomes | Wound complications and dehiscence | |
| Notes | Cost was not used (missing SD) We attempted to contact the authors in July 2017. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "wounds were assigned to skin closure by one of the three methods under assessment, by the throw of a dice". |
| Allocation concealment (selection bias) | Unclear risk | Quote: "wounds were assigned to skin closure by one of the three methods under assessment, by the throw of a dice". |
| Blinding of participants and personnel (performance bias) Surgical site infection | Unclear risk | This information was not available. |
| Blinding of outcome assessment (detection bias) Surgical site infection | Low risk | Quote: "post‐operatively, inspection of the wound was undertaken by an independent observer, a trained nurse (GSH), who was unaware of the method of skin closure used". |
| Incomplete outcome data (attrition bias) Short‐term outcomes | High risk | The rate of dropouts was relatively high (45/190 ≒ 24% at postoperative 6 weeks) and the reasons for missing data were not described. |
| Selective reporting (reporting bias) | Unclear risk | Study protocol unavailable, and it was unclear whether wound complications included the wound infection. |
| Other bias | High risk | Randomisation was conducted at a wound (not a participant level), and the appropriate analysis for paired or clustered data was not carried out. |
Andrade 2016.
| Methods | Randomised controlled trial | |
| Participants | Country: Mexico Number randomised: 202 Post‐randomisation dropout: 2 (intradermal suture group) Mean age (years): 22.89 vs 27.84 Number of males: 54 vs 52 Duration of study: 6 months Duration of follow‐up: 30 days Surgery: open appendectomy |
|
| Interventions | Participants randomly assigned to 2 groups: Group 1: intradermal sutures (n = 100), using polyglactine 910 #000 absorbable continuous suture Group 2: nylon #000 separated non‐absorbable stitches (n = 100) |
|
| Outcomes | Surgical site infection (superficial 2 vs 0 and abscess) Wound complications and wound dehiscence |
|
| Notes | Funding: not reported in the paper Instituto Mexicano del Seguro Social (information from trial registry) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomised list was generated by Microsoft Excel 2011 for the Mac IOS 7.0 program. |
| Allocation concealment (selection bias) | High risk | Quote: "according to a randomized list created by authors and generated by Microsoft Excel 2011 for the Mac IOS 7.0 program, patients were assigned to one of the two study groups." Comment: investigators could possibly foresee assignments. |
| Blinding of participants and personnel (performance bias) Surgical site infection | Unclear risk | Quote: "it was a 4‐phase interventional study, with parallel assignment for intention to treat, 2 arms and double‐blinded randomized, controlled trial." Comment: insufficient information available to permit a judgement |
| Blinding of outcome assessment (detection bias) Surgical site infection | Unclear risk | Quote: "it was a 4‐phase interventional study, with parallel assignment for intention to treat, 2 arms and double‐blinded randomized, controlled trial." Comment: insufficient information available to permit a judgement |
| Incomplete outcome data (attrition bias) Short‐term outcomes | Low risk | The reasons of dropouts were described. |
| Selective reporting (reporting bias) | Low risk | The published reports included all expected outcomes (trial registry: NCT02625987). |
| Other bias | Low risk | The study appeared to be free of other sources of bias. |
Baek 2009.
| Methods | Randomised controlled trial | |
| Participants | Country: Denmark Number randomised: it was not clear; 50 (58 incisions) or 47 (54 incisions) Post‐randomisation dropout: it was not clear; 4 or 1 Mean age (years): 48 Number of males: 14/50 Duration of study: 7 months Duration of follow‐up: 3 months Surgery: endoscopic release of the carpal tunnel |
|
| Interventions | Participants randomly assigned to 2 groups: Group 1: subcuticular continuous sutures (n = 28) with 4/0 absorbable monofilament polyglytone 6211 (Caprosyn®) Group 2: interrupted sutures (n = 26) with nonabsorbable 5/0 monofilament polybutester (Novafil®) Transcutanous sutures were removed in the 14th day postoperatively. |
|
| Outcomes | Wound infection Pain intensity |
|
| Notes | Pain intensity was reported as figure. Cosmetic outcome as measured at < 6 months so not included Funding: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients were randomised to have their wounds closed with either interrupted non‐absorbable sutures or subcuticular continuous absorbable sutures." Comment: insufficient information provided |
| Allocation concealment (selection bias) | Unclear risk | Quote: "patients were randomised to have their wounds closed with either interrupted non‐absorbable sutures or subcuticular continuous absorbable sutures." Comment: insufficient information provided |
| Blinding of participants and personnel (performance bias) Surgical site infection | Unclear risk | No information provided |
| Blinding of outcome assessment (detection bias) Surgical site infection | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) Short‐term outcomes | Unclear risk | Quote: "one patient was withdrawn from the study because the operation was cancelled as the symptoms had spontaneously regressed. Two patients were withdrawn because their operation was converted to an open release, and one patient dropped out after operation because of her inability to attend the follow‐up appointments. Comment: reasons for withdrawal or dropout of four patients provided but insufficient reporting of its distribution among the two groups |
| Selective reporting (reporting bias) | Low risk | Protocol was not available, but all the outcomes they planned to do in the methods section were fully reported. |
| Other bias | Unclear risk | Number randomised was not clear. The study had a potential source of bias related to the specific study design; randomisation was probably conducted at a participant level but the analysis was carried out at the level of the wound; clustered data (multiple wounds from individual participants) were not accounted for in the analysis. |
Barker 1984.
| Methods | Randomised controlled trial (3 arms) | |
| Participants | Country: UK Number randomised: 100 (102 biopsies) Post‐randomisation dropouts: 15 (14: biopsy proved malignant, 1: lost follow‐up after one week) Average age: not stated Male:female ratio: not stated Duration of follow‐up: 1 year Surgery: breast biopsy | |
| Interventions | Group 1: subcuticular sutures (n = 27), using Dexon
Group 2: subcuticular sutures (n = 29), using Prolene Group 3: surgical tapes, using Op‐site adhesive membrane (n = 31) For this review, the subcuticular data (groups 1 and 2) have been combined. Prolene suture or Op‐site membrane was removed by author. Only in the Group 3, subcutanous sutures were used. |
|
| Outcomes | Wound infection Wound complications (haematoma), hypertrophic scar, cosmesis (at 6 months and 1 year), and wound closure time |
|
| Notes | Objective and subjective cosmesis was assessed at 6 months and 1 year. However, we did not use the data because dropouts were not acceptable in number (> 30% of the original sample) and not evenly distributed across treatment arms. Wound closure time was not used (missing SD). Fund: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "one hundred consecutive patients (102 biopsies) were randomly allocated by sealed envelope to wound closure with either Op‐Site skin closure, subcuticular Prolene or subcuticular Dexon." Comment: insufficient information provided |
| Allocation concealment (selection bias) | Unclear risk | Quote: "one hundred consecutive patients (102 biopsies) were randomly allocated by sealed envelope to wound closure with either Op‐Site skin closure, subcuticular Prolene or subcuticular Dexon." Comment: insufficient information provided |
| Blinding of participants and personnel (performance bias) Surgical site infection | Unclear risk | Insufficient information provided |
| Blinding of outcome assessment (detection bias) Surgical site infection | Low risk | Quote: "at this point an independent assessor (outpatient sister) with no knowledge of the method closure was called to inspect the wounds, and with the patients filled in a questionnaire." Comment: probably done |
| Incomplete outcome data (attrition bias) Short‐term outcomes | High risk | Quote: "fourteen of the biopsies proved malignant and these were withdrawn from follow up after one week. One patient in the subcuticular Dexon group never returned for follow up. Of the remainder, 27 were in the Dexon group, 29 in the Prolene group and 31 in the Op‐Site group". Comment: The number of withdrawals was relatively high in total and it was not reported which trial groups these participants were from. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no direct quote but the outcomes to be assessed were not prespecified in the methods so it was unclear whether they were fully reported. |
| Other bias | Unclear risk | Randomisation was conducted at a participant level but the analysis was carried out at the level of the wound; it did not appear that clustered data (multiple wounds from individual participants) were accounted for in the analysis. Funding not reported |
Brown 2009.
| Methods | Randomised controlled trial | |
| Participants | Country: US Number randomised: 134 Post‐randomisation dropout: not stated Mean age (years): 3.3 vs 4.4 Number of males: 59 vs 51 Duration of study: 1 year Duration of follow‐up: 6 weeks Surgery: paediatric herniorrhaphy |
|
| Interventions | Participants randomly assigned to 2 groups: Group 1: subcuticular suture (n = 70), using a 5‐0 Monocryl Group 2: skin adhesives (n = 64), using 2‐Octylcyanoacrylate (Dermabond® manufactured by Ethicon Inc, a Johnson & Johnson company, Somerville, New Jersey, USA) |
|
| Outcomes | Wound complications, wound dehiscence, wound closure time and cost | |
| Notes | Cosmetic outcome as measured at < 6 months so not included Funding: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "just before wound closure, a sealed envelope indicating randomization to skin adhesive or suture closure was revealed." Comment: no information about how the randomisation sequence was generated |
| Allocation concealment (selection bias) | Unclear risk | Quote: "just before wound closure, a sealed envelope indicating randomization to skin adhesive or suture closure was revealed." Comment: no information about whether envelopes were opaque and sequential |
| Blinding of participants and personnel (performance bias) Surgical site infection | Unclear risk | Quote: "masking as to skin adhesive vs suture closure was not possible for the operating surgeon because of the nature of the intervention." Comment: no blinding of surgeons, but it was not clear whether there was blinding of patients and healthcare providers. |
| Blinding of outcome assessment (detection bias) Surgical site infection | Low risk | Quote: "however, subsequent interviewers were masked as to group during assessment of cosmetic outcome measures, as well as those related to efficiency, cost, and complications of wound closure." |
| Incomplete outcome data (attrition bias) Short‐term outcomes | Unclear risk | No report of any loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | Comment: the study protocol was not available, but the important outcome measures stated in the methods section were reported in the results. |
| Other bias | Unclear risk | Insufficient information (funding and COI) |
Buchweitz 2005.
| Methods | Randomised controlled trial (split‐body design) | |
| Participants | Country: Germany Number randomised: 60 Post‐randomisation dropout: 8 Mean age (years): 33 Duration of study: 12 months Duration of follow‐up: 3 months Surgery: laparoscopic surgery (with at least two 5‐mm ports in the lower abdomen) for gynecologic indications |
|
| Interventions | Wounds randomly assigned to 2 groups: Group 1: subcuticular absorbable sutures (n = 52), using 4‐0 polyglatin 910 Group 2 (second incision): interrupted transcutanous absorbable sutures (n = 52), using 4‐0 polyglatin 910 If necessary, a third port was placed and closure of this wound was performed by applying adhesive paper tape (n = 23) (Steri‐Strip; 3M Health Care, St. Paul, MN, USA). We excluded this group for the review. |
|
| Outcomes | Wound infection Wound complications and wound dehiscence |
|
| Notes | Cosmetic outcome as measured at < 6 months so not included Funding: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients were randomised by means of a blind envelope system just before surgery." Comment: insufficient information provided |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information provided |
| Blinding of participants and personnel (performance bias) Surgical site infection | Unclear risk | No information provided |
| Blinding of outcome assessment (detection bias) Surgical site infection | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) Short‐term outcomes | High risk | Quote: "of 60 eligible patients, 52 returned the questionnaire (86.7%)". Comment: many post‐randomisation dropouts |
| Selective reporting (reporting bias) | Low risk | Quote: "postoperatively in‐hospital complications regarding wound healing were recorded along with hospital stay." Quote: "adverse wound outcomes (wound infections, wound dehiscence, and persistent rubor) were also observed most frequently in subcuticular sutured port sites compared to transcutaneous closure (P = 0.039)". Comment: protocol was not available, but all the outcomes in the methods section were fully reported. |
| Other bias | Unclear risk | Funding not reported |
Chen 2013.
| Methods | Randomised controlled trial | |
| Participants | Country: China Number randomised: 292 Post‐randomisation dropout: not stated Mean age (years): 73.7 ± 9.8 vs 69.3 ± 11.5 Number of males: 89 vs 97 Duration of study: 4 years Duration of follow‐up: 6 months Surgery: cardiac pacemaker implantation |
|
| Interventions | Participants randomly assigned to 2 groups: Group 1: intradermal sutures group (n = 153), using ETHICON absorbable suture filaments Group 2: traditional (transdermal interrupted) sutures group (n = 139), using absorbable suture filaments |
|
| Outcomes | Wound infection Length of hospital stay and wound closure time |
|
| Notes | Funding: not reported in the paper This study published in Chinese, so a Chinese translator (Zhenmi Liu) helped the data extraction and assessment of ROB. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Using envelopes |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information provided |
| Blinding of participants and personnel (performance bias) Surgical site infection | Unclear risk | No information provided |
| Blinding of outcome assessment (detection bias) Surgical site infection | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) Short‐term outcomes | Low risk | Based on the data analysis |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available but it was clear that the published reports included all expected outcomes (including wound infection), including those that were prespecified. |
| Other bias | Unclear risk | Funding was not reported. There was baseline imbalance. |
Chen 2018.
| Methods | Randomised controlled trial | |
| Participants | Country: China Number randomised: 376 Post‐randomisation dropout: not stated Mean age (years): 52.33 vs 52.19 Number of males: 135 vs 131 Duration of study: 5 years Duration of follow‐up: 30 days Surgery: hepatectomy (HCC) |
|
| Interventions | Participants randomly assigned to 2 groups: Group 1: subcuticular sutures group (n = 188), using interrupted subcuticular sutures technique with a 4‐0 monofilament absorbable polydioxanone suture (PDS‐II, Ethicon). The suture interval was 15–25 mm, and suture bite length was 15–25 mm from the edge of the skin. Group 2: skin staples group (n = 188); metallic skin staples were applied 10–15 mm apart The same ERAS program was applied to both groups of patients. |
|
| Outcomes | Postoperative length of hospital stay (PLOS), wound infection (CDC criteria), and wound separation | |
| Notes | This research was supported by the National Science and Technology Major Special Project (2012ZX10002010001009) and the Guangxi University of Science and Technology Research Fund (KY2015LX056). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The 376 patients were randomized into 8 blocks, which were then allocated randomly to receive subcuticular sutures or staples during hepatectomy (188 patients per group). Block andomization allowed us to eliminate effects of differences in admission time and ensure that the two intervention groups would have the same size." |
| Allocation concealment (selection bias) | Unclear risk | Insufficient data provided |
| Blinding of participants and personnel (performance bias) Surgical site infection | Unclear risk | No information provided |
| Blinding of outcome assessment (detection bias) Surgical site infection | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) Short‐term outcomes | Unclear risk | No report of any loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available but it was clear that the published reports included all expected outcomes (including wound infection), including those that were prespecified. |
| Other bias | Low risk | The study appeared to be free of other sources of bias. |
Chughtai 2000.
| Methods | Randomised controlled trial | |
| Participants | Country: Canada Number randomised: 162 Post‐randomisation dropout: not stated Mean age (years): 64 vs 65.8 Number of males: 67 vs 55 Duration of study: 36 months Duration of follow‐up: 3‐6 weeks Surgery: closure of sternal and leg incisions in coronary artery bypass grafting (CABG) patients |
|
| Interventions | Participants randomly assigned to 2 groups: Group 1: subcuticular sutures (n = 81), using 4‐0 Monocryl running suture Group 2: stainless steel skin clips (n = 81), using Proximate Plus MD (Ethicon, USA) |
|
| Outcomes | Surgical site infection (wound infection and mediastinitis) Wound dehiscence and cost |
|
| Notes | Cost was not used (missing SD). Cosmetic outcome as measured at < 6 months so not included Funding: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients were preoperatively prospectively randomly placed to have their sternal and leg incisions ..." Comment: insufficient information provided |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information provided |
| Blinding of participants and personnel (performance bias) Surgical site infection | Unclear risk | No information provided |
| Blinding of outcome assessment (detection bias) Surgical site infection | High risk | Quote: "all assessments were perfomed by the same surgeon in a nonblinded fashion". Comment: no blinding assessment and the outcome (Infection) measurement was likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) Short‐term outcomes | Unclear risk | Insufficient information provided |
| Selective reporting (reporting bias) | Low risk | Quote: "incisions were assessed for the presence or absence of leakage, inflammation, infection". Quote: "there was a significantly greater rate of infection of the sternal wound when closed with clips (P = 0.05) than with subcuticular". Comment: protocol was not available, but all the outcomes they planned to do were reported. |
| Other bias | High risk | Randomisation was conducted at a participant level but the analysis was carried out at the level of the wound; it did not appear that clustered data (multiple wounds from individual participants) were accounted for in the analysis. Possible issues with clustering for infection outcome data Funding not reported Baseline imbalance: sex and diabetes |
Clough 1975.
| Methods | Randomised controlled trial | |
| Participants | Country: UK Number randomised: 161 Post‐randomisation dropout: 9 Age, number of males: not stated Duration of study: 4 months Duration of follow‐up: 4 weeks Surgery: surgery having abdominal or groin incision |
|
| Interventions | Participants randomly assigned to 2 groups: Group 1: subcuticular suture (n = 76), using a 3/0 atraumatic straight polyglycolic‐acid (PGA) suture Group 2: conventional interrupted suture (n = 76), using a 2/0 Silk Subcuticular suture do not have to be removed. |
|
| Outcomes | Wound infection Keloid scar and cosmesis |
|
| Notes | No wound was keloid, so it was not included. Funding: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients were randomly allocated into two groups". Comment: insufficient information provided |
| Allocation concealment (selection bias) | Unclear risk | Quote: "patients were randomly allocated into two groups". Comment: insufficient information provided |
| Blinding of participants and personnel (performance bias) Surgical site infection | Unclear risk | No information provided |
| Blinding of outcome assessment (detection bias) Surgical site infection | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) Short‐term outcomes | Unclear risk | Quote: "161 patients were entered for the trial in four months, 152 were followed for the required week period ‐ 76 sutured with P.G.A and 76 sutured with silk." Comment: insufficient information provided about reasons for missing data and its distribution among groups |
| Selective reporting (reporting bias) | Unclear risk | Quote: "wounds were finally categorised as no wound infection, non‐suppurative wound infection, or suppurative wound infection ... the length of the wound and the time taken for it to be sutured were measured". Comment: study protocol not available. The important outcomes in Methods were reported but it was unclear whether all outcomes assessed were fully reported. |
| Other bias | Unclear risk | Funding not reported Not sufficient information provided about baseline balance although there may be imbalance between groups, for example, in wound contamination (8 participants contaminated for PGA, 15 for silk), and race (according to the report by Arabi Y (Clough 1975)). |
Corder 1991.
| Methods | Randomised controlled trial | |
| Participants | Country: UK
Number randomised: 126 (161 wounds)
Post‐randomisation dropouts: 3
Median age: 43 vs 45
Male:female ratio: 20 (27%) vs 32 (37%) Duration of study: 1 year Duration of follow‐up: 6 weeks Inclusion criteria: patients undergoing high saphenous ligation |
|
| Interventions | Wounds randomly assigned to 2 groups:
Group 1: subcuticular sutures (n = 75), using 3‐0 polyglycolic acid (PGA)
Group 2: interrupted mattress sutures (n = 86), using monofilament nylon Nylon sutures were removed on the seventh postoperative day. |
|
| Outcomes | Wound infection Wound complications (wound sinus) |
|
| Notes | One patient undergoing bilateral surgery was wrongly randomised using two unilateral cards. Funding: not reported We attempted to contact the authors in July 2017. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients undergoing unilateral and bilateral surgery were randomized separately using random numbers and cards kept in the theatre suite". Comment: insufficient information provided |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information provided |
| Blinding of participants and personnel (performance bias) Surgical site infection | Unclear risk | No direct quotations about whether the participants or personnel were blinded |
| Blinding of outcome assessment (detection bias) Surgical site infection | Unclear risk | Quote: "at this time patients were asked if there had been a discharge of pus from the wound(s), and if this was the case a wound infection was said to have occurred". Comment: in this study, patients were the outcome assessors, but there was no description whether they were blinded. |
| Incomplete outcome data (attrition bias) Short‐term outcomes | Unclear risk | It was reported that the number of dropouts was three, but it was not reported which trial groups these participants were from. |
| Selective reporting (reporting bias) | Low risk | Study protocol was unavailable, but all the outcomes they planned to do were fully reported. |
| Other bias | High risk | Randomisation was conducted at a participant level but the analysis was carried out at the level of the wound; it did not appear that clustered data (multiple wounds from individual participants) were accounted for in the analysis. Although one case was allocated in an incorrect way, the case was taken into analysis. In addition, the patients were the outcome assessors. |
Eggers 2011.
| Methods | Randomised controlled trial (4‐arm) | |
| Participants | Country: US Number randomised: 90 (or 75?) Post‐randomisation dropout: 15 (it was not reported which trial groups these participants were from) Mean age (years): 68 Number of males: not stated Duration of follow‐up: 6 weeks Surgery: total knee arthroplasty (minimally invasive technique) |
|
| Interventions | Group 1 (n = 19): subcutaneous closure method: sutures at 1.5/cm; skin closure method: 2‐octylcyanoacrylate (Dermabond®) tissue adhesive (Ethicon Inc, a Johnson & Johnson
company, Somerville, New Jersey, USA)
Group 2 (n = 18): subcutaneous closure method: sutures at 1.5/cm; skin closure method: butylcyanoacrylate tissue adhesive (Histoacryl Blue tissue adhesive B Braun Corp)
Group 3 (n = 19): subcutaneous closure method: sutures at 1.0/cm; skin closure method: staples (Visistat 35W Stapler (Teleflex Corp) Group 4 (n = 19): subcutaneous closure method: sutures at 1.0/cm; skin closure method: 4‐0 Monocryl suture (poliglecaprone 25; Ethicon) for cutanous closure We note there were slight differences to the procedures in each group for the method of closure of the subcutaneous layer. Details for subcutaneous closure methods and skin closure methods are provided above. For this review, the tissue adhesives data (groups 1 and 2) have been combined |
|
| Outcomes | Wound infection (infection not defined) Wound dehiscence, pain intensity, length of hospital stay, quality of life, wound closure time and cost We have taken the total number of events reported over the 6‐week period, however, it was not clear whether some participants reported more than 1 event as the number of infections was reported rather than number of people having an infection. |
|
| Notes | Wound closure time was not used because only speed (sec/cm) was reported. Cost was not used (missing SD). Pain intensity and quality of life assessed at 3 and 6 weeks; we used the data at 6 weeks. Cosmetic appearance data not used as measured at < 6 months Funding: Foundation for Southwest Orthopedic Research |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "once an informed consent was obtained, the eligible subjects were randomly categorized (via a pseudorandom number generator algorithm) into 1 of 4 cohorts". |
| Allocation concealment (selection bias) | Unclear risk | No direct quotation about whether allocation concealment was done |
| Blinding of participants and personnel (performance bias) Surgical site infection | Unclear risk | No direct quotation about whether the participants or personnel were blinded |
| Blinding of outcome assessment (detection bias) Surgical site infection | Unclear risk | Insufficient information available to permit a judgement of how high the risk was |
| Incomplete outcome data (attrition bias) Short‐term outcomes | Unclear risk | Quote: "of the 90 subjects recruited, 15 were excluded because of screen failure; 6 were diagnosed with arthrofibrosis after surgery, 4 failed to follow preferred physical therapy, and 5 sustained unrelated co‐morbidities preventing study completion." "all 75 eligible subjects returned for all postoperative visits with the exception of 2 subjects who provided survey information via telephone during the sixth week visit". Comment: data on 15 participants were excluded from analysis data. It seemed that these participants were excluded post‐randomisation, but it was not clear. |
| Selective reporting (reporting bias) | Low risk | Study protocol was unavailable, but all the outcomes they planned to do were fully reported. |
| Other bias | Unclear risk | 4‐arm design |
Fiennes 1985.
| Methods | Randomised controlled trial | |
| Participants | Country: UK Number randomised: not stated (maybe 43 participants) Post‐randomisation dropout: not stated Age, number of males: not stated Duration of study: 4 months Duration of follow‐up: 12 weeks Surgery: hernia repair and cholecystectomy |
|
| Interventions | Participants randomly assigned to 2 groups Group 1: subcuticular suture (n = 21) using 3/0 polyglactin interrupted inverted subcuticular mattresses Group 2: conventional suture (n = 22) using 3/0 nylon interrupted mattresses |
|
| Outcomes | Wound healing problems (details were not defined), surgical time, cost All outcomes were not defined and specific data was not reported. There was no difference in wound healing problems. Surgical time was twice as long in the subcuticular technique than in the conventional suture technique. Cost was related to the unit cost of each type of material. |
|
| Notes | Funding: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients were assigned to nylon or Vicryl by lot drawing. " |
| Allocation concealment (selection bias) | Unclear risk | Quote: "patients were assigned to nylon or Vicryl by lot drawing. " Comment: insufficient information provided |
| Blinding of participants and personnel (performance bias) Surgical site infection | Unclear risk | Insufficient information provided |
| Blinding of outcome assessment (detection bias) Surgical site infection | Unclear risk | Insufficient information provided |
| Incomplete outcome data (attrition bias) Short‐term outcomes | Unclear risk | Insufficient information provided |
| Selective reporting (reporting bias) | Unclear risk | Quote: "there was no difference in the incidence of wound healing problems. The subcuticular technique took twice as much surgical time and the cost was related only to the unit cost of each type of material." Comment: study protocol not available. It was unclear whether all outcomes assessed were fully reported. |
| Other bias | Unclear risk | Funding not reported |
Foster 1977.
| Methods | Randomised controlled trial | |
| Participants | Country: UK Number randomised: 127 Post‐randomisation dropout: 0 Age, Number of males: not stated Duration of study: 16 months Duration of follow‐up: one month Surgery: appendicectomy through a right iliac fossa incision |
|
| Interventions | Participants randomly assigned to 2 groups: Group 1: subcuticular suture (n = 62), using a polyglycolic‐acid suture Group 2: interrupted suture (n = 65), using a 00 nylon Subcuticular sutures do not have to be removed. |
|
| Outcomes | Wound infection | |
| Notes | Funding: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients were randomly allocated into two groups at the start of the operation." Comment: insufficient information provided |
| Allocation concealment (selection bias) | Unclear risk | Quote: "patients were randomly allocated into two groups at the start of the operation." Comment: insufficient information provided |
| Blinding of participants and personnel (performance bias) Surgical site infection | Unclear risk | No information provided |
| Blinding of outcome assessment (detection bias) Surgical site infection | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) Short‐term outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | Quote: "an infection was defined as an obvious discharge of pus either spontaneous or after incision". Quote: "the frequency of a subsequent wound infection was examined in relation to the degree of appendicular inflammation (table II)". Comment: the important outcome (SSI) prespecified in Methods was reported and all the outcomes they planned to do were reported. |
| Other bias | Unclear risk | Funding not reported Insufficient information provided about baseline balance |
Gennari 2004.
| Methods | Randomised controlled trial | |
| Participants | Country: Italy Number randomised: 133 Post‐randomisation dropout: 0 vs 2 (early: 10 days), 15 vs 21 (1 year) Mean age (years): 51 vs 48.7 Number of males: 0 vs 1 Duration of study: 6 months Duration of follow‐up: 1 year Surgery: breast surgery |
|
| Interventions | Participants randomly assigned to 2 groups: Group 1: subcuticular running sutures (n = 64: 85 incisions), using 4‐0 or 5‐0 monofilament Group 2: 2‐octylcyanoacrylate (OCA) (n = 69: 87 incisions), using Dermabond (Ethicon Inc, Somerville, NJ) For all participants, subcutaneous sutures were applied. |
|
| Outcomes | Wound infection Wound dehiscence, length of hospital stay, patient satisfaction, cosmesis (at 6 months and 1 year), wound closure time and cost |
|
| Notes | SD of time and cost was calculated from SEM. SD of satisfaction was calculated from P value. Cosmesis: missing statistics Funding: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "one hundred and thirty‐three patients were randomised into 2 groups to receive either SWC (n = 64; 48.1%) or closure with OCA (n = 69; 51.9%)". Comment: insufficient information provided |
| Allocation concealment (selection bias) | Unclear risk | Quote: "one hundred and thirty‐three patients were randomised into 2 groups to receive either SWC (n = 64; 48.1%) or closure with OCA (n = 69; 51.9%)" Comment: insufficient information provided |
| Blinding of participants and personnel (performance bias) Surgical site infection | Unclear risk | No information provided |
| Blinding of outcome assessment (detection bias) Surgical site infection | Unclear risk | Quote: "cosmetic outcome with blind assessment" Comment: no information provided about the primary outcome assessment (infection) though cosmesis was assessed by a blinded surgeon. |
| Incomplete outcome data (attrition bias) Short‐term outcomes | High risk | Comment: number of dropouts was low (two patients in the adhesive group), but inappropriate exclusion (bleeding) |
| Selective reporting (reporting bias) | Low risk | Quote: "at each postoperative visit, the wound was examined for infection, inflammation, wound dehiscence or separation, and scarring". Quote: "there were no instances of wound dehiscence, hematoma, or infection in either group". Comment: all the outcomes they planned to do were fully reported. |
| Other bias | High risk | Randomisation was conducted at a participant level but some data were presented at the level of the wound (the data were not correctly analysed for clustered data). Baseline imbalance: mean length 103.9 (suture group) versus 85.6 (OCA group), although there was no significant difference. No information provided about funding |
Ghaderi 2010.
| Methods | Randomised controlled trial | |
| Participants | Country: Iran Number randomised: 278 Post‐randomisation dropout: not stated Mean age (years): 24.8 vs 25.3 Number of males: 170 Duration of study: 4 years Duration of follow‐up: 6 months Surgery: appendectomy Exclusion criteria: complicated appendicitis (gangrenous, perforated, and peritoneal) |
|
| Interventions | Participants randomly assigned to 2 groups: Group 1: subcuticular sutures group (n = 139), using 3‐0 Nylon, cut, SUPPA Corporation, Iran Group 2: interrupted sutures group (n = 139), using 3‐0 Nylon, cut, SUPPA Corporation, Iran |
|
| Outcomes | Wound complications (Including pus discharge, enduration, surgical site tenderness, open drainage, fever, haematoma) |
|
| Notes | Funding: not reported in the paper This study was published in Persian, so a Persian translator (Ahmad Sofi Mahmudi) helped the data extraction and assessment of ROB. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) Surgical site infection | Unclear risk | Insufficient information |
| Blinding of outcome assessment (detection bias) Surgical site infection | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) Short‐term outcomes | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | Low risk | Study protocol not available but it was clear that the published reports included all expected outcomes, including those that were prespecified. |
| Other bias | Unclear risk | Insufficient information |
Grottkau 2010.
| Methods | Randomised controlled trial | |
| Participants | Country: US Number randomised: 25 Post‐randomisation dropout: not stated Mean age (years): 14.1 Number of males: 7 Duration of follow‐up: 3 months Surgery: posterior instrumented spinal fusion (children) |
|
| Interventions | Participants randomly assigned to 2 groups: Group 1 (n = 12): subcuticular suture (3‐0 Monocryl suture) with conventional Steri‐Strip Group 2 (n = 13): surgical tapes, using coaptive film (Steri‐Strip S; 3M company, 3M Center St. Paul,MN) The subcutaneous tissue was closed in both groups. |
|
| Outcomes | Wound infection Wound dehiscence and wound closure time |
|
| Notes | SD of closure time was calculated from P value. Cosmetic appearance measured at < 6 months so not included Corporate/Industry funds were received in support of this work. No benefits in any form have been or will be received from a commercial party related directly or indirectly to the subject of this manuscript. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "skin closure in each patient was randomised to skin closure with either coaptive film or subcuticular sutures." Comment: insufficient information provided |
| Allocation concealment (selection bias) | Unclear risk | Quote: "skin closure in each patient was randomised to skin closure with either coaptive film or subcuticular sutures." Comment: insufficient information provided |
| Blinding of participants and personnel (performance bias) Surgical site infection | Unclear risk | No information provided |
| Blinding of outcome assessment (detection bias) Surgical site infection | Unclear risk | Quote: "cosmetic differences were assessed by a blinded plastic surgeon using a 0 to 10 visual analogue scale (10 is the highest score)". Comment: no information provided about the primary outcome assessment (infection) though cosmesis was assessed by a blinded surgeon. |
| Incomplete outcome data (attrition bias) Short‐term outcomes | Unclear risk | No information provided |
| Selective reporting (reporting bias) | Unclear risk | Quote: "there were no instances of infection or wound dehiscence using either technique". Comment: the outcomes to be assessed were not defined in the methods section so it was not clear whether all planned outcomes were fully reported. |
| Other bias | Unclear risk | Comment: no information provided about baseline imbalance |
Hopkinson 1982.
| Methods | Randomised controlled trial | |
| Participants | Country: UK Number randomised: 184 Post‐randomisation dropout: 38 Mean age: 19.9 vs 20.9 Numbe of males: not stated Duration of follow‐up: 2 weeks Surgery: appendicectomy through a right iliac fossa incision |
|
| Interventions | Participants randomly assigned to 2 groups: Group 1: subcuticular continuous suture (n = 83), using Prolene Group 2: interrupted suture (n = 63), using Prolene Skin sutures were removed between five and seven days after operation. |
|
| Outcomes | Wound infection Re‐closure |
|
| Notes | Funding: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "after closure of the muscle layer a randomly selected sealed envelope was opened to determine the choice of skin suture". Comment: insufficient information provided |
| Allocation concealment (selection bias) | Unclear risk | Quote: "after closure of the muscle layer a randomly selected sealed envelope was opened to determine the choice of skin suture". Comment: insufficient information provided. |
| Blinding of participants and personnel (performance bias) Surgical site infection | Unclear risk | No information provided |
| Blinding of outcome assessment (detection bias) Surgical site infection | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) Short‐term outcomes | High risk | Quote: "since 33 patients failed to attend for follow‐up and five were excluded because of protocol violations, 146 cases were evaluated". Comment: multiple post‐randomisation dropouts and the reasons for missing outcome data which are likely to be related to the primary outcome of the review were not provided. |
| Selective reporting (reporting bias) | Low risk | Quote: "all wounds were examined daily and again two weeks after the patient left hospital; those with a discharge were considered to be infected". Quote: "wound closed with interrupted sutures and those closed with a subcuticular suture became infected in 15.9% and 15.7% respectively". Comment: the important outcome (infection) prespecified in Methods was reported although Methods and Results sections were not divided. All the outcomes they planned to do were reported. |
| Other bias | Unclear risk | Funding not reported Insufficient information provided about baseline balance |
Imamura 2016.
| Methods | Multicentre randomised controlled trial | |
| Participants | Country: Japan Number randomised: 401 Post‐randomisation dropout: 8 vs 7 Median age (years): 72 vs 73 Number of males: 125 vs 129 Duration of study: 5 years Duration of follow‐up: 30 days Surgery: open laparotomy |
|
| Interventions | Participants randomly assigned to 2 groups: Group 1: subcuticular sutures (n = 191), using 4‐0 monofilament absorbable interrupted suture (polydioxanone; PDS‐II, Ethicon, Tokyo) Group 2: metallic skin staples (n = 195) |
|
| Outcomes | Surgical site infection Lengh of hospital stay |
|
| Notes | Length of hospital stay was reported median (IQR), so we judged that the data were skewed. Funding: research Grant from Tokyo Metropolitan Government |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "after being screened for the inclusion and exclusion criteria, eligible patients were enrolled through a web‐based system established for this trial and randomized by a computer‐generalized permuted‐block sequence". |
| Allocation concealment (selection bias) | Low risk | A web‐based system was used. |
| Blinding of participants and personnel (performance bias) Surgical site infection | High risk | Quote: "patients and investigators were not masked to group assignment". Comment: no blinding, and the outcome was likely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) Surgical site infection | Unclear risk | Quote: "the principal surgeons were also asked to check for the presence of superficial SSIs during hospitalization and in the outpatient clinic until 30 days after surgery". Comment: insufficient information provided about whether outcome assessors were blinded |
| Incomplete outcome data (attrition bias) Short‐term outcomes | Low risk | Dropouts were low in number and evenly distributed. Therefore, the proportion of missing outcomes was not enough to have a clinically relevant impact on the intervention effect estimate. |
| Selective reporting (reporting bias) | Low risk | Study protocol was not available but all the study's prespecified outcomes in UMIN‐CTR (trial registry) have been reported. |
| Other bias | Unclear risk | Study protocol changed after the study had started, but it was not clear how strongly the change influenced the outcome. |
Jallali 2004.
| Methods | Randomised controlled trial | |
| Participants | Country: UK Number randomised: 25 (99 incisions) Post‐randomisation dropout: 0 Median age (years): 56 vs 36 Number of males: 43 Duration of follow‐up: 8 weeks Surgery: laparoscopic cholecystectomy |
|
| Interventions | Participants randomly assigned to 2 groups: Group 1: (13 participants; 51 wounds): subcuticular 3‐0 polydiaxonone (Vicryl) suture Group 2: (12 participants; 48 wounds): 2‐octylcyanoacrylate (Dermabond) tissue adhesive (Ethicon) The suture group required a Mepore (Molnlycke Health Care, Dunstable, UK) dressing |
|
| Outcomes | Wound complications and wound closure time (not clear if these data were collected for multiple wounds on the same person) | |
| Notes | Outcome reporting was unclear, so not sure if results were reported for a reference wound for each participant or if outcome data from multiple wounds were collected SD of wound closure time was calculated from P value Cosmetic outcome as measured at < 6 months so not included Funding: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomization was performed by asking the patient to select an envelope out of a hat". Comment: although it seemed efforts were in place to randomise patients, it was not wholly clear that a truly randomised sequence was generated. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "randomization was performed by asking the patient to select an envelope out of a hat". Comment: no description of envelopes used |
| Blinding of participants and personnel (performance bias) Surgical site infection | Unclear risk | No direct quotations about whether the participants or personnel were blinded |
| Blinding of outcome assessment (detection bias) Surgical site infection | Unclear risk | Quote: "these photograph of the wounds were then rated ... by a plastic surgeon who was blinded to the treatment method." Comment: cosmetic outcome was assessed by a blinded assessor, however, the outcome reported here relevant to the review was wound complications and time to skin closure. It was not clear if this was assessed by a blinded assessor. |
| Incomplete outcome data (attrition bias) Short‐term outcomes | Low risk | Quote: "all patients were followed up" |
| Selective reporting (reporting bias) | Low risk | Comment: the study protocol was not available, but the important outcome measures stated in the methods section were reported in the results. |
| Other bias | Unclear risk | Baseline imbalance: age Based on information collected it was not clear whether the data were correctly analysed for clustered data. |
Jan 2013.
| Methods | Randomised controlled trial | |
| Participants | Country: UK Number randomised: not stated (152?), 114 analysis Post‐randomisation dropout: 38? (it was not reported which trial groups these participants were from) Mean age (years): 45.4 vs 41.7 Number of males: 0 Duration of study: 32 months Duration of follow‐up: 2 weeks Surgery: female laparoscopic surgery |
|
| Interventions | Participants randomly assigned to 2 groups: Group 1: subcuticular suture (n = 55), using 3‐0 VicrylTM Group 2: skin adhesives (n = 59), using LiquiBand® Surgical S (Advanced Medical Solutions, Plymouth, UK) is a new formulation with a blend of monomeric n‐butyl and 2‐octyl cyanoacrylates. |
|
| Outcomes | Wound complications, wound dehiscence and wound closure time | |
| Notes | Cosmetic outcome as measured at < 6 months so not included An outcome that study authors referred to as a 'patient satisfaction' score was also measured, however, this seemed to focus on satisfaction of cosmetic appearance so was deemed a cosmetic evaluation. Wound closure time (missing SD), so SD was calculated from P value. Funding provided by Advanced Medical Solutions Ltd (Plymouth, UK), who also funded the presentation of the paper at a national conference. The authors reported no other conflicts of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "a random number generator determined the assignment to either LBSS or sutures at time of surgery". |
| Allocation concealment (selection bias) | Unclear risk | No direct quotation about whether allocation concealment was done |
| Blinding of participants and personnel (performance bias) Surgical site infection | High risk | Quote: "due to the inherent differences between the two closure methods, it was not possible for the surgeon, study subjects, or evaluator to be masked form the knowledge of the randomised treatment assignments." Comment: no blinding, and the outcome was likely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) Surgical site infection | High risk | No blinding, and the outcome was likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) Short‐term outcomes | High risk | Quote: "a total of 152 subjects were enrolled in this study ... Twenty‐six subjects did not complete the study and were terminated due to voluntary withdrawal, not meeting inclusion/exclusion criteria, or being lost to follow‐up. Twelve subjects were omitted from data analysis due to missing or incomplete data points, resulting in a total of 114 subjects used in the data analysis." Comment: subjects that should have been analysed originally were excluded from the analysis. It could affect the outcome significantly. |
| Selective reporting (reporting bias) | Unclear risk | Study protocol unavailable, and it was not clear whether wound complications included the wound infection. |
| Other bias | Unclear risk | Randomisation was probably conducted at a participant level but the analysis was partly carried out at the level of the wound; it did not appear that clustered data (multiple wounds from individual participants) were accounted for in the analysis. |
Javadi 2018.
| Methods | Randomised controlled trial | |
| Participants | Country: Iran Number randomised: 70 Post‐randomisation dropout: 0 Mean age (years): 22.4 vs 24 Number of males: 20 vs 22 Duration of study: not reported Duration of follow‐up: 90 days Surgery: open appendectomy |
|
| Interventions | Participants randomly assigned to 2 groups: Group 1: subcuticular sutures (n = 35), using 4/0 monofilament monocryl absorbable suture supported by 3 Steri‐StripsTM Group 2: interrupted mattress sutures (n = 35), using 4/0 nylon |
|
| Outcomes | Severity of pain, wound infection, subcutaneous abscess and patient satisfaction | |
| Notes | Funding: not reported in the paper Not prospective registration (according to ICTRP) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A total of 70 patients were randomly assigned into 2 groups including case and control groups using simple randomization method by the computerized random number table." |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information provided |
| Blinding of participants and personnel (performance bias) Surgical site infection | Unclear risk | Quote: "We conducted this randomized single‐blinded controlled study..." Comment: no direct quotation about whether the participants or personnel were blinded |
| Blinding of outcome assessment (detection bias) Surgical site infection | Unclear risk | Quote: "All patients were visited by the same surgeon". Comment: no direct quotation about whether the outcome assessors were blinded |
| Incomplete outcome data (attrition bias) Short‐term outcomes | Low risk | There was no dropout. |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available but it was clear that the published reports included all expected outcomes (including wound infection), including those that were prespecified. |
| Other bias | Unclear risk | Insufficient information |
Karabay 2005.
| Methods | Randomised controlled trial | |
| Participants | Country: Turkey Number randomised: 100 Post‐randomisation dropout: 2 vs 2 Mean age (years): 61.1 vs 64.8 Number of males: 38 vs 31 Duration of study: 18 months Duration of follow‐up: 6 weeks Surgery: open‐cardiac operations through a median sternotomy |
|
| Interventions | Participants randomly assigned to 2 groups: Group 1: intracutaneous closure (IC) (n = 50) with polycaprolate absorbable polyfilament (Dexon 4.0, United States Surgical Corporation; Norwalk, Conn) Group 2: transcutaneous closure (TC) (n = 50) with nonabsorbable monofilament (Prolene 4.0, Ethicon, Inc., Somerville, NJ) Transcutanous sutures were removed on the 14th day postoperatively. |
|
| Outcomes | Surgical site infection Proportion of re‐closure |
|
| Notes | Length of hospital stay: missing SD Cosmetic outcome as measured at < 6 months so not included Funding: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote : "we selected at random 50 patients to undergo IC and 50 patients to undergo TC". Comment: insufficient information provided |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information provided |
| Blinding of participants and personnel (performance bias) Surgical site infection | Unclear risk | Insufficient information provided |
| Blinding of outcome assessment (detection bias) Surgical site infection | Unclear risk | Insufficient information provided |
| Incomplete outcome data (attrition bias) Short‐term outcomes | Low risk | Quote: "follow‐up was completed in 100% of the 100 patients". Comment: two early deaths excluded but unlikely to introduce bias |
| Selective reporting (reporting bias) | Low risk | Quote: "wound infections were evaluated according to the specific wound site evaluation scheme". Quote: "total incidence of early wound infection for the 6‐week follow‐up period was 2% for the TC group and 16% for the IC group (P = 0.016). No late wound infections were observed in either group". Comment: protocol not available, but the important outcome data (infection) prespecified in Methods was reported and all the outcomes they stated in methods were fully reported. |
| Other bias | High risk | Baseline imbalance; sex and diabetes Funding not reported |
Keng 1989.
| Methods | Randomised controlled trial | |
| Participants | Country: UK Number randomised: 43 (46 incisions) Post‐randomisation dropout: 1 vs 2 Mean age (years): 49.7 vs 53.4 Number of males: 35 Duration of study: 7 months Duration of follow‐up: one month Surgery: minor operations with groin incisions (inguinal hernia, femoral hernia, saphenous ligations, testicular operations and lymph node biopsies) |
|
| Interventions | Participants randomly assigned to 2 groups: Group 1: (n = 23 incisions): subcuticular running 3‐0 Dexon suture Group 2: (n = 23 incisions): Histoacryl‐Blue tissue adhesives (Histoacryl) |
|
| Outcomes | Wound infection Wound complications (haematoma) and wound closure time |
|
| Notes | Wound closure time was not used (missing statistics). Cosmetic outcome as measured at < 6 months so not included Funding: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients were randomised just prior to skin closure into two groups. Even numbers were closed with Dexon subcuticular suture (Dexon group) and odd numbers were closed with Histoacryl‐Blue tissue adhesive (Histoacryl)". Comment: whilst use of odd and even numbers was detailed in terms of how the randomisation was implemented, there was no detail about how the sequence was generated. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "even numbers were closed with Dexon subcuticular suture (Dexon group) and odd numbers were closed with Histoacryl‐Blue tissue adhesive (Histoacryl)". Comment: no indication that this sequence was concealed from surgeons |
| Blinding of participants and personnel (performance bias) Surgical site infection | Unclear risk | No information provided |
| Blinding of outcome assessment (detection bias) Surgical site infection | Unclear risk | Quote: "cosmesis was assessed by an independent observer (the experienced clinic sister) on a scale of one to five ... " Comment: blinding of outcome assessment achieved for cosmetic appearance, but this was not used in this review. Blinding not clear with regard to the other outcomes |
| Incomplete outcome data (attrition bias) Short‐term outcomes | Low risk | Comment: 3/43 participants were lost to follow‐up. It was considered at low risk of bias |
| Selective reporting (reporting bias) | Low risk | Quote: "infection (defined as the presence of pus or opened wound)" Quote: "there were no infections or excessive inflammation in any of the wounds assessed". Comment: protocol not available, but the important outcome data (infection) prespecified in Methods was reported and all the outcomes they planned to do were fully reported. |
| Other bias | Unclear risk | Randomisation was conducted at a participant level but the analysis was carried out at the level of the wound; it did not appear that clustered data (multiple wounds from individual participants) were accounted for in the analysis. Funding: not reported |
Khajouei 2007.
| Methods | Randomised controlled trial | |
| Participants | Country: Iran Number randomised: 200 Post‐randomisation dropout: 0 Mean age (years): 21.3 vs 23.6 Number of males: 77 vs 84 Duration of study: 14 months Duration of follow‐up: 2 weeks Surgery: emergency appendectomy |
|
| Interventions | Participants randomly assigned to 2 groups: Group 1: subcuticular sutures (n = 100), using 4‐0 nylon Group 2: a few stitches of 3/0 nylon with horizontal mattress technique (n = 100) |
|
| Outcomes | Wound infection Wound complications (erythema or swelling) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "in this prospective randomized study conducted on 200 patients with suppurative or gangrenous appendicitis" Comment: no direct quotation about how the allocation sequence was generated |
| Allocation concealment (selection bias) | Unclear risk | No direct quotation about whether allocation concealment was done |
| Blinding of participants and personnel (performance bias) Surgical site infection | Unclear risk | No direct quotation about whether the participants or personnel were blinded |
| Blinding of outcome assessment (detection bias) Surgical site infection | Unclear risk | No direct quotation about whether the outcome assessors were blinded |
| Incomplete outcome data (attrition bias) Short‐term outcomes | Low risk | No direct quotation about the number of dropouts, but it is inferred that there was no dropout. |
| Selective reporting (reporting bias) | Low risk | Study protocol not available, but in the introduction and methods section, SSI was defined as the main outcome in this study and all the outcomes they planned to do were fully reported. |
| Other bias | Low risk | Study appeared to be free of other sources of bias. |
Khan 2006.
| Methods | Multicentre randomised controlled trial (3 arms) | |
| Participants | Country: Australia Number randomised: 187 Surgery: total hip (n = 102) and total knee arthroplasty (n = 85) Post‐randomisation dropout: 0 Median age (years): Hip; 69 vs 71 vs 69, knee; 73 vs 70 vs 66 Number of males: 33 vs 24 vs 30 Duration of study: 7 months Duration of follow‐up: 12 weeks |
|
| Interventions | Participants randomly assigned to 3 groups: Group 1: subcuticular sutures (n = 64), using 3‐0 absorbable poliglecaprone suture (Monocryl, Johnson and Johnson) Group 2: tissue adhesives (n = 60), using OCA (Dermabond, Johnson and Johnson, New Brunswick, New Jersey) Group 3: skin clips (n = 63) |
|
| Outcomes | Surgical site infection (early) Wound complications (early), patient satisfaction, length of hospital stay and wound closure time |
|
| Notes | Cosmetic outcome as measured at < 6 months so not included Infection and complications data collected at 2 time points ‐ early (inpatients; about one week) and late (between 8 and 12 weeks after operation) Patient satisfaction, length of hospital stay and wound closure time were reported by medians and interquartile ranges. So we judged that the data were skewed. Funding: not reported COI: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients were randomised using a computer generated method stored in sealed identical opaque envelopes". |
| Allocation concealment (selection bias) | Unclear risk | Quote: "patients were randomised using a computer generated method stored in sealed identical opaque envelopes. Allocation took place in the operating theatre after closure of the deep layers". Comment: no information whether envelopes were sequentially numbered |
| Blinding of participants and personnel (performance bias) Surgical site infection | Low risk | Quote: "patients and assessors remained blinded to the treatment allocated until the dressings were changed, prior to discharge. At follow‐up, the assessors were not informed of the technique of closure". |
| Blinding of outcome assessment (detection bias) Surgical site infection | Low risk | Quote: "patients and assessors remained blinded to the treatment allocated until the dressings were changed, prior to discharge. At follow‐up, the assessors were not informed of the technique of closure." |
| Incomplete outcome data (attrition bias) Short‐term outcomes | Low risk | No direct quotation, but no account of a loss to follow‐up, therefore judged as low risk |
| Selective reporting (reporting bias) | Low risk | Quote: "primary outcome measure was the development of complications in the wound, either as an inpatient ('early') or after discharge ('late')." Quote: "primary outcomes are listed in Table II. With THR there was no significant difference between the groups in terms of the number of patients with complications either when in hospital or after discharge". Comment: protocol not available, but primary outcome data (infection) prespecified in Methods was reported and all the outcomes they planned to do were fully reported. |
| Other bias | Unclear risk | Funding not reported |
Kobayashi 2015.
| Methods | Multicentre randomised controlled trial | |
| Participants | Country: Japan Number randomised: 1264 Post‐randomisation dropout: 32 (15 vs 17) Median age (years): 65 vs 67 Number of males: 335 vs 335 Duration of study: 21 months Duration of follow‐up: 30 days Surgery: elective colorectal cancer surgery |
|
| Interventions | Participants randomly assigned to 2 groups: Group 1: subcuticular sutures (n = 620), using 4‐0 or 5‐0 monofilament absorbable interrupted suture Group 2: staples (n = 612) |
|
| Outcomes | Surgical site infection Wound complications, length of hospital stay, patient satisfaction and wound closure time |
|
| Notes | Funding: Grant‐in‐Aid for Cancer Research from the Ministry of Health, Labour and Welfare of Japan We contacted the authors in July 2017 and got additional data (randomised method and mean/SD of outcomes). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients were recruited by participating surgeons and enrolled before surgery. Randomization was done in a 1 : 1 allocation ratio to balance treatment over the following factors: institution, type of surgery (open versus laparoscopic) and tumour location (colon versus rectum). " "surgeons were notified of the allocation by telephone before surgery. ... These processes were managed by the data centre located at National Cancer Centre Hospital East, Kashiwa, Japan." Comment: no direct quotation, but using a computer random number generator managed by the data center (author's response) |
| Allocation concealment (selection bias) | Low risk | Central allocation (telephone method) |
| Blinding of participants and personnel (performance bias) Surgical site infection | High risk | Quote: "neither the patient nor the investigators were blinded to the allocation". Comment: no blinding, and the outcome was likely to have been influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) Surgical site infection | Unclear risk | No direct quotation about whether the outcome assessors were blinded |
| Incomplete outcome data (attrition bias) Short‐term outcomes | Low risk | Quote: "thirty‐two patients were excluded owing to: reoperation (staples, 16; subcuticular sutures, 8), major protocol violation (subcuticular sutures, 3), cancelled operation (staples, 1; subcuticular sutures, 2), withdrawn consent (subcuticular sutures, 1) or ineligibility (subcuticular sutures, 1)". Comment: rate of dropouts relatively low (32/1264 = 2.5%) and evenly distributed Therefore, the proportion of missing outcomes was not enough to have a clinically relevant impact on the intervention effect estimate. |
| Selective reporting (reporting bias) | Low risk | Study protocol not available but all the study's prespecified outcomes in UMIN‐CTR (trial registry) that are of interest in the review have been reported. |
| Other bias | Low risk | Quote: "this work was supported by a Grant‐in‐Aid for Cancer Research from the Ministry of Health, Labour and Welfare of Japan.
... The authors declare no conflict of interest". Comment: study appeared to be free of other sources of bias. |
Kotaluoto 2012.
| Methods | Randomised controlled trial | |
| Participants | Country: Finland Number randomised: 206 Post‐randomisation dropout: 11 vs 10 (10.2%) at 21 days Per‐protocol population: 185 Average age: 40.6 vs 40.5 years Male: 50% vs 63% Duration of follow‐up: 3 weeks; cosmesis: 14 months (n = 69 vs 68) Surgery: open appendectomy in adult participants (over 18 years old) |
|
| Interventions | Participants randomly assigned to 2 groups: Group 1: subcuticular continuous sutures (n = 95), using 4‐0 Monocryl (Monocryl, Ethicon Inc.,Johnson & Johnson, Piscataway, NJ, USA; Monosyn,B. Braun Melsungen AG, Melsungen, Germany) Group 2: interrupted sutures (n = 90), using 4‐0 Monosoph (Ethilon, Ethicon Inc.; Monosof, Covidien, Dublin, Ireland) |
|
| Outcomes | Wound infection Wound complications, wound dehiscence and cosmesis (mean 14 months) |
|
| Notes | Funding: not reported COI: none known |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients were then randomized into two wound closure groups by computer‐produced random numbers". |
| Allocation concealment (selection bias) | Unclear risk | No direct quotation about whether allocation concealment was done |
| Blinding of participants and personnel (performance bias) Surgical site infection | Unclear risk | No direct quotation about whether the participants or personnel were blinded |
| Blinding of outcome assessment (detection bias) Surgical site infection | Unclear risk | Quote: "patients were interviewed over the telephone at an average of 21 days post‐operatively, and the same questions as in the evaluation form were asked. The patients also were asked about other possible postoperative problems". |
| Incomplete outcome data (attrition bias) Short‐term outcomes | High risk | There were many post‐randomisation dropouts (21/206) at 21 days postoperatively. |
| Selective reporting (reporting bias) | Low risk | Study protocol was unavailable, but referring to the registry, SSI was defined as the primary outcome of the study and was reported and all the outcomes they planned to do were fully reported. |
| Other bias | Low risk | The study appeared to be free of other sources of bias. |
Krishnamoorthy 2009.
| Methods | Randomised controlled trial | |
| Participants | Country: UK Number randomised: 106 Post‐randomisation dropout: 12 Mean age (years): not stated Number of males: 77% vs 85% Duration of follow‐up: 6 weeks Surgery: coronary artery bypass grafting with endoscopic saphenous vein harvesting |
|
| Interventions | Participants randomly assigned to 2 groups: Group 1: subcuticular suture (n = 53), using a monofilament synthetic absorbable Biosyn 3‐0 (Covidien PLC, Dublin, Ireland) Group 2: skin adhesive (n = 53), using Dermabond (2‐octylcyanoacrylate; Ethicon UK, Edinburgh, United Kingdom) In the suture group, the wound was dressed with Mepore dressing (Mölnlycke Health Care, Manchester, United Kingdom), and a pressure bandage was applied for 48 h. In the adhesive group, a pressure bandage and Steri‐Strips (3M, St Paul,MN) were applied to hold the edges together for 24 hours. |
|
| Outcomes | Wound complications | |
| Notes | Cosmetic outcome as measured at < 6 months so not included An outcome that study authors referred to as a 'patient satisfaction' score was also measured, however, this seemed to focus on satisfaction of cosmetic appearance so was deemed a cosmetic evaluation; as it was collected at 6 weeks after surgery, it was not reported here. Mean and SD of wound closure time were not reported (median was reported). So we judged the data were skewed. Funding: University Hospital of South Manchester NHS Foundation Trust Endowment under the control of Nizar Yonan |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "a computerized randomization system was used to place patients into two groups of 53 each". |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information provided |
| Blinding of participants and personnel (performance bias) Surgical site infection | Unclear risk | Insufficient information provided |
| Blinding of outcome assessment (detection bias) Surgical site infection | Unclear risk | Insufficient information provided |
| Incomplete outcome data (attrition bias) Short‐term outcomes | High risk | Rate of dropouts was relatively high (12/106 ≒ 11% at postoperative 6 weeks) and it was not reported which trial groups these participants were from. |
| Selective reporting (reporting bias) | Unclear risk | Study protocol was unavailable. No specific quote but outcomes other than "wound complication" were not prespecified and patient satisfaction was reported by only P values, so it was unclear whether all outcomes assessed were fully reported. |
| Other bias | Low risk | Quote: "This study was funded by a University Hospital of South Manchester NHS Foundation Trust endowment under the control of Nizar Yonan." |
Kuroki 2017.
| Methods | Parallel randomised controlled trial | |
| Participants | Country: US Number randomised: 173 (85 vs 88) Post‐randomisation dropout: 6 vs 4 at 8 weeks Mean age: 57.1 vs 58.5 years Duration of follow‐up: 8 weeks Duration of study: 33 months Surgery: Women undergoing gynaecologic cancer surgery (body mass index ≥ 30) |
|
| Interventions | Participants randomly assigned to 2 groups: Group 1: subcuticular sutures (n = 79), using an absorbable 4‐0 monofilament suture Group 2: staples (n = 84) had their incision closed with 35‐mm wide stainless steel staples using a Proximate Skin Stapler. Staples were removed at an outpatient appointment 10–14 days postoperatively or sooner if warranted. |
|
| Outcomes | Wound infection Wound dehiscence, wound complications, patient satisfaction and wound closure time The prespecified outcome in the trial registry "Analog Pain Score on Postoperative Days 3‐4" was not reported in the published report. |
|
| Notes | Cosmetic outcome as measured at < 6 months so not included Patient satisfaction and wound closure time were reported by medians and interquartile ranges. So we judged that the data were skewed. Supported by the Washington University Institute of Clinical and Translational Sciences (ICTS) grant UL1 TR000448 from the National Center for Advancing Translational Sciences of the National Institutes of Health (NIH). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. Dr. Bradley Evanoff is the Principal Investigator for the Clinical and Translational Science Award that supports all Washington University ICTS and Clinical Research Training Center activities (L.M.K., A.R.H.). The research was also supported by a NIH/Paul Calabresi Career Development Award for Clinical Oncology (K12) 5K12CA132783‐08 (A.P.N.). The Siteman Cancer Center is supported in part by a National Cancer Institute Cancer Center Support Grant # P30 CA091842 (Eberlein, Principal Investigator). The authors did not report any potential conflicts of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "a computer‐generated one‐to‐one simple randomization scheme was used". |
| Allocation concealment (selection bias) | Low risk | Quote: "skin closure groups were centrally assigned by our study coordinator on telephone verification of the correctness of inclusion criteria. Neither clinicians nor patients were masked to closure type". |
| Blinding of participants and personnel (performance bias) Surgical site infection | High risk | Quote: "lack of blinding among the participants and health care providers could potentially have introduced bias". |
| Blinding of outcome assessment (detection bias) Surgical site infection | Unclear risk | No direct quotation about whether the outcome assessors were blinded |
| Incomplete outcome data (attrition bias) Short‐term outcomes | Low risk | Dropouts were low in number and evenly distributed. Therefore, the proportion of missing outcomes was not enough to have a clinically relevant impact on the intervention effect estimate. |
| Selective reporting (reporting bias) | High risk | The prespecified outcome in the trial registry "Analog Pain Score on Postoperative Days 3‐4" was not reported in the published report. |
| Other bias | Low risk | The study appeared to be free of other sources of bias. |
Lazar 2011.
| Methods | Randomised controlled trial | |
| Participants | Country: US Number randomised: 36 Post‐randomisation dropout: 0 Mean age (years): 65.9 vs 67.3 Number of males: 15 vs 15 Duration of follow‐up: 21 days Surgery: mediansternotomy for a cardiac surgical procedure |
|
| Interventions | Participants randomly assigned to 2 groups: Group 1 (n = 18): subcuticular suture, using running 4–0 polyglactin suture (Vicryl, Ethicon, Inc.) Group 2 (n = 18): surgical tapes, using the Steri‐strip S Surgical Skin closure system (3M HealthCare, St. Paul, MN, USA) using an average of three packages of 100 mm, 3 1/8 strips The subcutaneous tissue was closed in both groups. |
|
| Outcomes | Wound infection Wound dehiscence, pain intensity (7 and 21 days), length of hospital stay, wound closure time and cost |
|
| Notes | Pain intensity at 7 days was used for the review. Cosmetic appearance measured at < 6 months so not included Research funding for this study was provided from a grant by 3M HealthCare, St. Paul, MN, who also donated the Steri‐Strip S Surgical Skin closures used in the study. The authors had full control of the design of the study, methods used, outcome measurements, analysis of data, and production of the written report. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomization was performed using a computer‐generated blocked schedule". |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information provided |
| Blinding of participants and personnel (performance bias) Surgical site infection | Unclear risk | Insufficient information provided |
| Blinding of outcome assessment (detection bias) Surgical site infection | Unclear risk | Quote: "all wounds were inspected daily. Parameters of wound healing were measured on postoperative days 7 and 21 by a nurse clinician." Comment: insufficient information was available as to whether outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) Short‐term outcomes | Low risk | There was no dropout. |
| Selective reporting (reporting bias) | Low risk | According to the study protocol registry, SSI was defined as the secondary outcome and was reported. All the outcomes they planned to do were fully reported. |
| Other bias | Low risk | The study appeared to be free of other sources of bias. |
Liu 2017.
| Methods | Multicentre randomised controlled trial | |
| Participants | Country: Netherlands Number randomised: 142 Post‐randomisation dropout: 1 vs 0 (at one week), 7 vs 6 (at 12 months) Mean age (years): 67 vs 67 Number of males: 47 vs 45 Duration of follow‐up: 12 months Duration of study: one year Surgery: conventional excision or Mohs micrographic surgery (MMS) on the face with an expected primary closure of a defect > 4 mm were approached |
|
| Interventions | Participants randomly assigned to 2 groups: Group 1 (n = 69): running subcuticular sutures (RSS) Group 2 (n = 73): simple interrupted sutures (SIS) All wounds were sutured in layers; for tension relieving deep sutures, absorbable, synthetic braided or monofilament material was used. The skin was closed with nonabsorbable monofilament sutures. The brand of suturing material was dependent upon availability at the department. Sutured wounds were supported by adhesive closure strips and a clean pressure dressing. No occlusive dressing was used. Both SIS and RSS were removed 1 week after surgery. A high sun protection factor sunscreen (Daylong Actinica, Galderma SA, Lausanne, Switzerland) was offered to all patients that needed to be applied onto the scar daily for 3 months after suture removal to standardise postsurgical cosmetics usage. |
|
| Outcomes | Surgical site infection Wound dehiscence, cosmesis (at 3 and 12 months) |
|
| Notes | We included cosmetic appearance at 12 months. Funding sources: supported by Galderma Pharma SA Conflicts of interest: none declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "computer generated list, created using random permuted blocks of 6, that was stratified by hospital was used for randomization". |
| Allocation concealment (selection bias) | Unclear risk | Quote: "the allocation configuration was generated and concealed until interventions were assigned by a secretary not involved in the trial". Comment: insufficient information provided |
| Blinding of participants and personnel (performance bias) Surgical site infection | Unclear risk | Comment: insufficient information provided |
| Blinding of outcome assessment (detection bias) Surgical site infection | Unclear risk | Quote: "the observer of cosmetic outcome was blinded to treatment assignment". Comment: other outcomes (including wound complications and patient assessment of cosmetic outcome) were probably not blinded. But it was uncertain whether the outcome measurement was likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) Short‐term outcomes | Low risk | Dropouts were low in number and the reasons were described. |
| Selective reporting (reporting bias) | Low risk | Study protocol unavailable, but in the methods section, SSI was defined as the secondary outcome and was reported and all the outcomes they planned to do were fully reported. |
| Other bias | Low risk | The study appeared to be free of other sources of bias. |
Maartense 2002.
| Methods | Multicentre (two centres) randomised controlled trial | |
| Participants | Country: Netherland Number randomised: 140 Post‐randomisation dropout: 0 There were no withdrawals, however, 7 patients treated with paper tape and 3 with tissue adhesive were converted to the suture group. Mean age (years): 52 vs 54 vs 57 Number of males: 23 vs 23 vs 26 Duration of study: 17 months Duration of follow‐up: 10‐14 days and 3 months Surgery: elective laparoscopic surgery (CDC class 1or 2) |
|
| Interventions | Participants randomly assigned to 3 groups: Group 1 (n = 50): intracutaneous poliglecaprone (Monocryl®, 4/0, Johnson&Johnson), interrupted sutures Group 2 (n = 48): tissue adhesives, using octylcyanoacrylate (Dermabond®, Johnson&Johnson, Amersfoot, the Netherlands) Group 3 (n = 42): 76 mm x 6 mm adhesive paper tape (SteriStrip® Bioplasty/Uroplasty, Geleen, the Netherlands) |
|
| Outcomes | Wound infection Wound closure time and cost |
|
| Notes | SD of wound closure time and cost were calculated from P value. Cosmetic outcome as measured at < 6 months so not included Funding: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients were allocated to one of the three groups using a computer randomization." |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information provided |
| Blinding of participants and personnel (performance bias) Surgical site infection | Unclear risk | Insufficient information provided |
| Blinding of outcome assessment (detection bias) Surgical site infection | Low risk | Quote: "surgical residents scored wound infections and cosmetic results; they were blinded to the method used for wound closure". |
| Incomplete outcome data (attrition bias) Short‐term outcomes | Low risk | There was no dropout. |
| Selective reporting (reporting bias) | Low risk | Study protocol unavailable, but in the methods section, SSI was defined as the secondary outcome and was reported and all the outcomes they planned to do were fully reported. |
| Other bias | Unclear risk | Funding was not reported. |
Martin 2017.
| Methods | Randomised controlled trial | |
| Participants | Country: US Number randomised: 109 Post‐randomisation dropout: 2 (it was not reported which trial groups these participants were from at 1 month), 5 vs 6 (at 3 months) Mean age (years): 59.0 vs 58.3 Number of males: 9 vs 19 Duration of study: 7 months Duration of follow‐up: 3 months Surgery: implantable venous port placement |
|
| Interventions | Participants randomly assigned to 2 groups: Group 1: subcuticular suture (n = 50), using a running 4‐0 braided synthetic absorbable suture (Polysorb) Group 2: skin adhesives (n = 48), using octyl cyanoacrylate skin adhesive (Sure+Close II; Chemence Medical, Alpharetta, Georgia) |
|
| Outcomes | Infectious complications Wound dehiscence and wound closure time |
|
| Notes | Cosmetic outcome as measured at < 6 months so not included SD of wound closure time was calculated from P value. Cost was reported per package, so not used. Funding: Duke University Department of Radiology |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "using a random number generator" |
| Allocation concealment (selection bias) | Unclear risk | Quote: "the randomization results were placed in sealed envelopes before the start of the procedure". Comment: not specified that opaque envelopes were used |
| Blinding of participants and personnel (performance bias) Surgical site infection | High risk | No blinding, and the outcome was likely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) Surgical site infection | High risk | Quote: "a study member examined the port site 1 and 3 months after the procedure to assess wound integrity and for signs or symptoms of wound infection". Comment: no blinding, and the outcome was likely to be influenced by lack of blinding because a study member assessed outcome. |
| Incomplete outcome data (attrition bias) Short‐term outcomes | Low risk | Dropouts were low in number and the reasons were described. |
| Selective reporting (reporting bias) | Low risk | Study protocol not available but all the study's prespecified outcomes in trial registry have been reported. |
| Other bias | Unclear risk | The study had baseline imbalance in terms of numbers of male vs female participants. |
McGreal 2002.
| Methods | Randomised controlled trial | |
| Participants | Country: UK Number randomised: 181 Post‐randomisation dropout: 7 (it was not reported which trial groups these participants were from) Mean age: 23 vs 20.5 Numbe of males: 47 vs 48 Duration of study: 12 months Duration of follow‐up: 4 weeks Surgery: appendectomy through a Lanz incision |
|
| Interventions | Participants randomly assigned to 2 groups: Group 1: subcuticular continuous suture (n = 88), using a 4/0 polyglycolate (Dexon) Group 2: interrupted suture (n = 86), using a Nylon with wound wicks soaked in 1% povidone‐iodine Wound wicks were removed on the 4th postoperative day |
|
| Outcomes | Surgical site infection (wound infection and abscess) Length of hospital stay |
|
| Notes | Length of hospital stay: missing SD Funding: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients were then randomized by opening a sealed envelope ... a computer had previously carried out the randomization." |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information provided |
| Blinding of participants and personnel (performance bias) Surgical site infection | Unclear risk | Insufficient information provided |
| Blinding of outcome assessment (detection bias) Surgical site infection | Unclear risk | Insufficient information provided |
| Incomplete outcome data (attrition bias) Short‐term outcomes | Unclear risk | Comment: seven post‐randomisation dropouts, but the reasons of dropouts and which trial groups these participants were from were not reported. |
| Selective reporting (reporting bias) | Unclear risk | Quote: "all wound were inspected daily for evidence of wound infection by the same investigator." "Wound width was measured with vernier calipers." Quote: "the overall infection rate was 15 of 174 (8.6%). In patients with wound wicks, it was 10 of 86 (11.6%) compared with 5 of 88 (5.6%) in those closed by subcuticular sutures (P = NS). " Comment: protocol not available. The primary outcome data (infection) prespecified in Methods was reported, but it was unclear whether all outcomes assessed were fully reported. |
| Other bias | Unclear risk | Funding not reported |
Mullen 1999.
| Methods | Randomised controlled trial (4 arms) | |
| Participants | Country: Canada Number randomised: 80 Post‐randomisation dropout: 3 (one: no leg incision, one: reoperation and the other one: patient with IABP, but no infection) vs 0 Mean age (years): 65.5 vs 63.5 Number of males: 31 vs 33 Duration of follow‐up: 6‐8 weeks Surgery: closure of leg incisions in elective coronary artery bypass grafting (CABG) patients |
|
| Interventions | Group 1: subcuticular sutures (n = 38) Group 2: staples (n = 40) For this review, 'skin closure immediately' group and 'closure after protamine administration' group have been combined. |
|
| Outcomes | Wound infection | |
| Notes | Funding: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "each [of the] patients were initially enrolled in the study and randomly assigned to one of four leg wound closure methods". Comment: insufficient information provided |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information provided |
| Blinding of participants and personnel (performance bias) Surgical site infection | Unclear risk | No information provided |
| Blinding of outcome assessment (detection bias) Surgical site infection | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) Short‐term outcomes | Low risk | Dropouts were low in number and the reasons were stated. Therefore, the proportion of missing outcomes was not enough to have a clinically relevant impact on the intervention effect estimate. |
| Selective reporting (reporting bias) | Low risk | Quote: "patients were monitored for infection during their hospital stay by telephone follow‐up and in the patient follow‐up clinic six to eight weeks after surgery. Quote: "leg wound infections occurred in nine patients (11.7%) ‐ seven (9.1%) minor infections and two (2.6%) major infections." Comment: protocol not available, but all the outcome data (infection) prespecified in Methods were reported. |
| Other bias | Unclear risk | Funding not reported Wound lengths and depths were imbalanced among the groups. 4‐arm design |
Murphy 1995.
| Methods | Randomised controlled trial (4 arms) | |
| Participants | Country: UK Number randomised: 114 (173 incisions) Post‐randomisation dropouts: not stated Average age: 67 years Number of males: 70 Duration of follow‐up: 2 weeks Surgery: bypass surgery with a groin incision | |
| Interventions | Group 1: subcuticular sutures (n = 45), using Maxon (Davis and Geck, Gosport, Great Britain)
Group 2: continuous Nylon sutures (n = 38) using a simple over‐and‐over technique Group 3: interrupted Nylon sutures (n= 41) Group 4: clips (n = 49) For this review, the Nylon sutures data (groups 2 and 3) have been combined. Subcutanous sutures were used with two‐layer closure. |
|
| Outcomes | Surgical site infection Wound complications (erythema, serous discharge and hematoma) and cost |
|
| Notes | Wound complications were reported, however, which trial groups these participants were from was not reported. So not included Cost was not used (missing SD). Fund: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients were then randomly assigned to skin closure with either subcuticular Maxon (SC), continuous nylon (CN)". Comment: insufficient information provided |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information provided |
| Blinding of participants and personnel (performance bias) Surgical site infection | Unclear risk | Insufficient information provided |
| Blinding of outcome assessment (detection bias) Surgical site infection | Unclear risk | Insufficient information provided |
| Incomplete outcome data (attrition bias) Short‐term outcomes | Unclear risk | Insufficient information provided |
| Selective reporting (reporting bias) | Low risk | Quote: "infection was defined as a positive culture". Quote: "infection occurred in five (2.9%) of the 173 wounds studied with no significant differences among treatment groups (Table 11)". Comment: protocol not available, but primary outcome data (infection) prespecified in Methods was reported. All the outcomes they planned to do were fully reported. |
| Other bias | High risk | Randomisation was conducted at a participant level but the analysis was carried out at the level of the wound; it did not appear that clustered data (multiple wounds from individual participants) were accounted for in the analysis. |
O'Leary 2013.
| Methods | Randomised controlled trial | |
| Participants | Country: UK Number randomised: 82 Post‐randomisation dropout: 11 vs 8 (at 6 weeks) Mean age (years): 53 vs 54 Number of males: 6 vs 7 Duration of study: 25 months Duration of follow‐up: 6 weeks Surgery: thyroidectomy/parathyroidectomy |
|
| Interventions | Participants randomly assigned to 2 groups: Group 1: subcuticular running suture (n = 43), using an absorbable suture (4–0 Monocryl; Ethicon, Inc) Group 2: surgical tapes (n = 39), using adhesive strips (3M Steri‐Strips; 3M, Minneapolis, MN) Both groups had closure of the deep subcutaneous layer with an absorbable suture (2–0 Vicryl; Ethicon, Inc, Somerville, NJ). Steri‐Strips were were left in situ until they fell off (typically 7–14 days). |
|
| Outcomes | Wound infection Wound complications, pain intensity (at day 1 and 6 weeks) and cost |
|
| Notes | Data of pain intensity on day 1 was used. SD of pain intensity on day 1 was calculated from P value. Cosmetic outcome as measured at < 6 months so not included Cost per package (not per participant) was reported, so not included. Funding: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomization was performed using computer‐generated code to either of the 2 groups." |
| Allocation concealment (selection bias) | Unclear risk | Quote: "a sealed envelope was sent with the patient to the operating room and was opened just before epidermal closure." Comment: not specified that opaque envelopes were used |
| Blinding of participants and personnel (performance bias) Surgical site infection | Unclear risk | No direct quotation about whether the participants or personnel were blinded |
| Blinding of outcome assessment (detection bias) Surgical site infection | Unclear risk | Insufficient information available to permit a judgement of how high the risk was |
| Incomplete outcome data (attrition bias) Short‐term outcomes | High risk | Quote: "nineteen patients withdrew during the study by failing to have the final wound assessment at 6 weeks ...wound analysis at 6 weeks was performed on the remaining 63 patients". Comment: number of dropouts was not small and the reasons were not described. The proportion of missing outcomes would probably be enough to have a clinically relevant impact on the intervention effect estimate. |
| Selective reporting (reporting bias) | Low risk | Study protocol unavailable, but SSI was defined in the outcome of the study in the methods section and was reported and all the outcomes they planned to do were fully reported. |
| Other bias | Unclear risk | Funding: not reported Insufficient information available to permit a judgement of how high the risk was |
Obermair 2007.
| Methods | Multicentre randomised controlled trial (3 arms) | |
| Participants | Country: Australia Number randomised: 90 Post‐randomisation dropout: 0 Mean age (years): 59.8 vs 59.6 Duration of study: 7 months Duration of follow‐up: 3 months Surgery: gynaecologic laparotomy |
|
| Interventions | Group 1: subcuticular continuous sutures, using two absorbable monofilament sutures Polyglecaprone 25, Thicon, Monocryl; Ethicon, Somerville, NJ, USA) (n = 31) Poliglecaprone 6211 (Caprosyn; United States Surgical Corporation) (n = 30) For this review, sutures data have been combined. Group 2: skin staples (n = 29) (AutoSuture; United States Surgical Corporation, Norwalk, CT, USA) Staples were removed from day 5 postoperatively. |
|
| Outcomes | Wound complications (incisional hernia, wound dehiscence, wound discharge, wound infection, wound inflammation, suture protrusion or other wound complication), wound dehiscence and pain intensity | |
| Notes | We used the data of pain intensity by patients at 1 week and 3 months. SD of pain intensity was calculated from 95% confidence intervals. Cosmetic outcome as measured at < 6 months so not included Sponsors and Collaborators: Queensland Centre for Gynaecological Cancer |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "a computer block randomization list (block size of 60) was compiled by an independent researcher stratified by hospital". Comment: probably done |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information provided |
| Blinding of participants and personnel (performance bias) Surgical site infection | Unclear risk | Insufficient information provided |
| Blinding of outcome assessment (detection bias) Surgical site infection | Unclear risk | Insufficient information provided |
| Incomplete outcome data (attrition bias) Short‐term outcomes | Low risk | No direct quotation, but no account of a loss to follow‐up, therefore judged as low risk |
| Selective reporting (reporting bias) | Unclear risk | Quote: "if any wound complications including incisional hernia, wound dehiscence, wound discharge, wound infection, wound inflammation, suture protrusion or other wound complication at any time between surgery and 3 month follow up occurred, these were reported on the case report forms". Quote: "there was no difference in the number of patients assigned to the three wound closure groups who experienced one or more wound complications (Table 1). Six patients experienced wound dehiscence, two assigned to staples, three assigned to Caprosyn and 1 assigned to Monocryl." Comment: primary outcome (infection) prespecified in Methods was reported as wound complications, but the incidence of wound infection was not reported. |
| Other bias | Low risk | Quote: "there was a trend for patients in the Monocryl group to have received surgery for a malignant compared with a benign condition more frequently compared with the other two groups (P = 0.06)". Comment: there might be baseline imbalance but multivariate analysis conducted to adjust for confounders |
Ong 2002.
| Methods | Randomised controlled trial | |
| Participants | Country: Singapore Number randomised: 59 Post‐randomisation dropout: not reported (early), 50 (late: 3 months) Mean age (years): 4.5 vs 4.5 % male: 81.8 vs 88.4 Duration of follow‐up: 3 months Surgery: unilateral or bilateral herniotomy (children) |
|
| Interventions | Participants randomly assigned to 2 groups: Group 1: (n = 33): subcuticular polyglecaprone (Monocryl) suture Group 2: (n = 26): 2‐octylcyanoacrylate (Dermabond) For bilateral herniotomy, only the symptomatic side was recorded. |
|
| Outcomes | Wound infection Wound dehiscence, and wound closure time |
|
| Notes | An outcome that study authors referred to as a 'parent satisfaction with cosmesis' score was also measured, however, this seemed to focus on satisfaction of cosmetic appearance so was deemed a cosmetic evaluation. Cosmetic appearance measured at < 6 months so not included Funding: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "all enrolled patients were allocated to glue or suture by opening serial sealed envelopes prepared with computerised randomisation". |
| Allocation concealment (selection bias) | Unclear risk | Quote: "all enrolled patients were allocated to glue or suture by opening serial sealed envelopes prepared with computerised randomisation". Comment: unclear whether envelopes opaque or not |
| Blinding of participants and personnel (performance bias) Surgical site infection | Unclear risk | No information provided |
| Blinding of outcome assessment (detection bias) Surgical site infection | Unclear risk | Quote: "assessment was done by an independent, blinded observer (staff nurse) using a previously validated score [The Hollander score] ... Parent satisfaction with wound cosmesis was recorded at the same time on a 100 mm visual analogue scale (VAS)". Comment: reasonable to deduce that this represented a low risk of detection bias for nurse‐assessed cosmetic outcome. But, not clear if other outcomes such as wound infection or dehiscence were collected via blinded assessment |
| Incomplete outcome data (attrition bias) Short‐term outcomes | Unclear risk | Insufficient information provided during early follow‐up period |
| Selective reporting (reporting bias) | Unclear risk | Quote: "looking at outcome measures of time efficiency, cosmesis, and wound complications" Quote: "none of the patients reported any rash, wound infection, or dehiscence". Comment: primary outcome (infection) was reported, but it was not clear whether wound complications prespecified in Methods included infections. |
| Other bias | Unclear risk | Funding not reported |
Onwuanyi 1990.
| Methods | Randomised controlled trial | |
| Participants | Country: Nigeria Number randomised: 100 Post‐randomisation dropout: 0 (at 2 weeks), 48 vs 50 (at 6 weeks) Age (years): 8 to 58 (range) Number of males: 28 vs 30 Duration of study: over 18 months Duration of follow‐up: 6 months Surgery: appendectomy |
|
| Interventions | Participants randomly assigned to 2 groups: Group 1: subcuticular suture (n = 50), using a 2/0 Nylon Group 2: interrupted transdermal suture (n = 50), using a 2/0 Nylon |
|
| Outcomes | Wound infection Wound complications, hypertrophic scar and cost |
|
| Notes | Length of hospital stay was not used because of missing statistics. SD of cost was calculated from P value. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "for skin closure the patients were randomly allocated to two groups". Comment: no direct quotation about how the allocation sequence was generated |
| Allocation concealment (selection bias) | Unclear risk | No direct quotation about whether allocation concealment was done |
| Blinding of participants and personnel (performance bias) Surgical site infection | Unclear risk | No direct quotation about whether the participants or personnel were blinded |
| Blinding of outcome assessment (detection bias) Surgical site infection | Unclear risk | No direct quotation about whether the outcome assessors were blinded |
| Incomplete outcome data (attrition bias) Short‐term outcomes | Unclear risk | Reasons for dropouts not described, but it was unclear whether the dropouts might have influenced the outcome significantly. |
| Selective reporting (reporting bias) | Unclear risk | Primary outcome (infection) was reported, but it was not clear whether wound complications prespecified in Methods included infections. |
| Other bias | Low risk | Study appeared to be free of other sources of bias. |
Pauniaho 2010.
| Methods | Randomised controlled trial | |
| Participants | Country: Finland
Number randomised: 198
Post‐randomisation dropouts: 21 vs 11
Per‐protocol population: 166
Average age: 12.7 vs 12.7 years
Number of boys: 57 vs 54 Duration of study: 53 months Duration of follow‐up: 7‐9 days Inclusion criteria: children and adolescents up to 18 years of age with suspected appendicitis |
|
| Interventions | Participants randomly assigned to 2 groups:
Group 1: continuous intradermal sutures (n = 100), using absorbable 4‐0 polyglactin 910 subcuticular with sterile tapes
Group 2: interrupted sutures (n = 98), using 4‐0 nylon Nylon sutures were removed on 7‐9 days postoperatively. |
|
| Outcomes | Wound infection Wound complications, wound dehiscence and pain intensity |
|
| Notes | Pain intensity was not used (missing statistics). Funding: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "the randomization of the wound closure was performed by the circulating nurse at the time of wound closure by tossing a 20 cent coin". |
| Allocation concealment (selection bias) | Unclear risk | Quote: "the randomization of the wound closure was performed by the circulating nurse at the time of wound closure by tossing a 20 cent coin". |
| Blinding of participants and personnel (performance bias) Surgical site infection | Unclear risk | Information not available |
| Blinding of outcome assessment (detection bias) Surgical site infection | Unclear risk | Quote: "the evaluation of the wound was done by a surgeon other than the one having performed the surgery, or by a staff nurse". Comment: insufficient information regarding blinding |
| Incomplete outcome data (attrition bias) Short‐term outcomes | High risk | There were many post‐randomisation dropouts and imbalance between the two groups. |
| Selective reporting (reporting bias) | Low risk | Study protocol unavailable, but SSI was defined as the primary outcome of the study in the methods section and was reported in the results section, and all the outcomes they planned to do were fully reported. |
| Other bias | Unclear risk | Insufficient information was available to permit a judgement. |
Pitcher 1983.
| Methods | Randomised controlled trial | |
| Participants | Country: UK Number randomised: 51 Post‐randomisation dropouts: 0 Average age: 74.6 vs 73.6 Number of males: 28 Duration of follow‐up: one month Surgery: pacemaker implantation | |
| Interventions | Group 1: subcuticular sutures (n = 26), using continuous absorbable 2‐0 Dexon sutures
Group 2: surgical tapes, using Op‐site adhesive membrane (n = 25) Subcutanous tissues were closed with 2 layers of continuous plain catgut suture. |
|
| Outcomes | Surgical site infection Wound complications (erythema, haematoma, serous discharge and skin overlap), and wound closure time |
|
| Notes | Wound closure time was not used (insufficient data and missing statistics). Fund: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients were allocated to either sutureless skin closures or subcuticular sutures according to a predetermined randomization." Comment: insufficient information provided |
| Allocation concealment (selection bias) | Unclear risk | Quote: "patients were allocated to either sutureless skin closures or subcuticular sutures according to a predetermined randomization." Comment: insufficient information provided |
| Blinding of participants and personnel (performance bias) Surgical site infection | Unclear risk | No information provided |
| Blinding of outcome assessment (detection bias) Surgical site infection | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) Short‐term outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Unclear risk | Quote: "the length of the incision was measured and the size of any surrounding inflammation was also measured." "Any further symptoms or residual signs of inflammation or infection were noted". Quote: "one infection occurred: a patient in the suture group developed fever and distension of the pacemaker pocket 3 days after pacemaker implantation despite apparently good skin healing". Comment: study protocol unavailable. The primary outcome (infection) prespecified in Methods was reported, but it was unclear whether all outcomes assessed were fully reported. |
| Other bias | Unclear risk | Funding not reported |
Ranaboldo 1992.
| Methods | Randomised controlled trial | |
| Participants | Country: UK Number randomised: 48 Post‐randomisation dropout: 0 Mean age (years): 66 vs 64 Number of males: 10 vs 12 Duration of follow‐up: one month (hypertrophic scar at one year) Surgery: laparotomy through a midline incision |
|
| Interventions | Participants randomly assigned to 2 groups: Group 1: subcuticular sutures (n = 26), using 3‐0 polydioxanone (PDS; Ethicon, Edinburgh, UK) continuous suture Group 2: staples (n = 22), using Weck (Swindon, UK) |
|
| Outcomes | Wound infection Hypertrophic scar, pain intensity, wound closure time and cost |
|
| Notes | SD calculated from P value (pain intensity) Wound closure time was not used (missing statistics). Cosmetic outcome as measured at < 6 months so not included Funding: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "a total of 48 patients (22 men, 26 women; age 27‐89 (mean 65) years) undergoing laparotomy through a midline incision were randomised to skin closure using an automatic skin stapling device (Weck, Swindon, UK) or continuous subcuticular undyed 3/0 polydioxanone (PDS; Ethicon, Edinburg, UK)." Comment: insufficient information provided |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information provided |
| Blinding of participants and personnel (performance bias) Surgical site infection | Unclear risk | No information provided |
| Blinding of outcome assessment (detection bias) Surgical site infection | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) Short‐term outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | High risk | Quote: "one wound infection with the discharge of pus occurred in each group". Comment: important outcome data (infection) was not prespecified in Methods but reported in Results. |
| Other bias | Unclear risk | Funding not reported |
Rebello 2009.
| Methods | Randomised controlled trial Randomised at a limb level (not a participant level); split‐body design |
|
| Participants | Country: US Number randomised: 8 (50 incisions) Post‐randomisation dropout: not stated Mean age (years): 14.5 Number of males: 4 Duration of study: 20 months Duration of follow‐up: 3 months Surgery: bilateral limb surgery for children with cerebral palsy |
|
| Interventions | Limbs randomly assigned to 2 groups: Group 1: subcuticular suture (n = 25 incisions), using 4‐0 monocryl Group 2: surgical tapes (n = 25 incisions), using coaptive film (Steri Strip S; 3M company, 3M Center St Paul, MN) Deep fascia and subcutaneous tissue were closed with interrupted vicryl |
|
| Outcomes | Wound dehiscence and wound closure time (SD was calculated from P value) | |
| Notes | No events with respect to complications and dehiscence Cosmetic outcome as measured at < 6 months so not included |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "choice of skin closure technique was randomised to limbs". Comment: insufficient information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) Surgical site infection | Unclear risk | No information provided |
| Blinding of outcome assessment (detection bias) Surgical site infection | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) Short‐term outcomes | Unclear risk | No sufficient information provided |
| Selective reporting (reporting bias) | High risk | Quote: "there were no complications or experiences of wound dehiscence". Comment: study protocol unavailable, and the important outcomes (infection or wound complications) were not prespecified in Methods and were not clearly reported. |
| Other bias | Unclear risk | Randomisation was conducted at a participants' limb level but the analysis was carried out at the level of the incision; it did not appear that paired data were accounted for in the analysis. Funding reported, insufficient information regarding baseline balance and possibly inappropriate design and analysis |
Reed 1997.
| Methods | Randomised controlled trial | |
| Participants | Country: UK Number randomised: 87 Post‐randomisation dropout: 19 (it was not reported which trial groups these participants were from) Mean age (years): 47.6 vs 49.6 Number of males: 8 vs 5 Duration of study: over 21 months Duration of follow‐up: not stated Surgery: neck surgery |
|
| Interventions | Participants randomly assigned to 2 groups: Group 1: subcuticular sutures (n = 34), using polydioxanone (PDS; Ethicon, Edinburgh, UK) Group 2: Michel (Downs Aesculop, Braintree, UK) clips (n = 34) |
|
| Outcomes | Wound complication, length of hospital stay, wound pain intensity (VAS) and cosmetic outcome | |
| Notes | Cosmetic outcome as measured about 320 days (mean) after operation, so included Funding: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "each closure was randomised to Michelle clips (Downs Aesculop,Braintree, UK) or subcuticular polydioxanone (PDS; Ethicon, Edinburgh, UK)". Comment: insufficient information provided |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information provided |
| Blinding of participants and personnel (performance bias) Surgical site infection | Unclear risk | No information provided |
| Blinding of outcome assessment (detection bias) Surgical site infection | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) Short‐term outcomes | High risk | Quote: "seventy‐three questionnaires were returned, of which 68 were assessable: 34 for polydioxanone and 34 for clips". Comment: multiple post‐randomisation dropouts (19/87) and it was not reported which trial groups these participants were from. |
| Selective reporting (reporting bias) | Low risk | Quote: "there were no postoperative complications". Comment: the study protocol was not available, but the important outcome measures stated in the methods section were reported in the results. |
| Other bias | Unclear risk | Funding not reported |
Roolker 2002.
| Methods | Randomised controlled trial | |
| Participants | Country: Netherlands Number randomised: 120 Post‐randomisation dropout: 0 Mean age (years): 49.1 vs 44.9 Number of males: 20 vs 25 Duration of study: 16 months Duration of follow‐up: 6 weeks Surgery: orthopaedic surgery (hip, knee and spine) |
|
| Interventions | Participants randomly assigned to 2 groups: Group 1: intracutaneous sutures (n = 60), using PDS (polydioxanone) suture Group 2: Medzip (ATRAX Medical Group, Bermuda) surgical zipper system (n = 60) Zippers or the intracutaneous suture were removed no sooner than postoperative day 10 and before day 14. |
|
| Outcomes | Surgical site infection Wound complications, wound dehiscence, wound closure time and cost |
|
| Notes | Cosmetic outcome as measured at < 6 months so not included Funding: not reported SD of cost was calculated from P value. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "prospective, randomised study was performed to compare intracutaneous skin closure with the zipper. " Comment: no direct quotation about how the allocation sequence was generated |
| Allocation concealment (selection bias) | Unclear risk | No direct quotation about how the allocation sequence was generated |
| Blinding of participants and personnel (performance bias) Surgical site infection | Unclear risk | No direct quotation about whether the participants or personnel were blinded |
| Blinding of outcome assessment (detection bias) Surgical site infection | Unclear risk | No direct quotation about whether the outcome assessors were blinded |
| Incomplete outcome data (attrition bias) Short‐term outcomes | Low risk | No dropouts |
| Selective reporting (reporting bias) | Unclear risk | Quote: "procedure‐related data included handling, wound healing, application time, length of the wound (cm), complications and scar result" Comment: primary outcome (infection) was reported, but it was not clear whether wound healing or complications prespecified in Methods included infections. |
| Other bias | Unclear risk | Funding: not reported Comment: insufficient information available to permit a judgement of how high the risk was |
Rosen 1997.
| Methods | Randomised controlled trial (split‐body design) | |
| Participants | Country: Australia Number randomised: 54 Post‐randomisation dropout: 4 Mean age: not stated Numbe of males: 0 Duration of follow‐up: 4 weeks Surgery: gynaecologic laparoscopy |
|
| Interventions | Participants randomly assigned to receive two of three types of skin closures, one on each side of body and one in umbilicus: Group 1: subcuticular suture (n = 52 incisions), using a 3/0 undyed polyglactin 910 (Ethicon, Somerville, NJ) Group 2: transcutaneous mattress suture (n = 43 incisions), using a 3/0 monofilament nylon suture Group 3: all other wounds were closed with adhesive strips (n = 52 incisions) (3M,Minneapolis, MN). Each woman had to act as her own control. |
|
| Outcomes | Wound complications and pain intensity | |
| Notes | Pain intensity was not included (missing SD). Cosmetic outcome as measured at < 6 months so not included We attempted to contact the authors in August 2017. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "the patients was allocated to a particular combination of closure techniques by random number draw". Comment: probably done |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information provided |
| Blinding of participants and personnel (performance bias) Surgical site infection | Unclear risk | Insufficient information provided |
| Blinding of outcome assessment (detection bias) Surgical site infection | Unclear risk | Insufficient information provided |
| Incomplete outcome data (attrition bias) Short‐term outcomes | Unclear risk | Insufficient information provided |
| Selective reporting (reporting bias) | High risk | The protocol was not available and the published trial did not report the important outcomes (details of complications) defined in the Methods section. |
| Other bias | Unclear risk | Numbers randomised to each group were not reported. Split‐body design. The analysis was carried out at the level of the wound; it did not appear that paired data were accounted for in the analysis. |
Sakka 1995.
| Methods | Randomised controlled trial | |
| Participants | Country: UK Number randomised: 80 (94 operations) Post‐randomisation dropout: 6 (five died and one with revision surgery); it was not reported which trial groups these participants were from. Mean age: 71.4 years Number of males: 32 Duration of study: 33 months Duration of follow‐up: 8 weeks Surgery: hip operation |
|
| Interventions | Participants randomly assigned to 2 groups: Group 1: subcuticular suture (n = unknown), using a 3/0 Dexon completed with steristrips Group 2: continuous transdermal blanket suture (n = unknown), using a non absorbable 3/0 silk The silk sutures were removed at two weeks. |
|
| Outcomes | Wound infection, patient satisfaction | |
| Notes | Number randomised in each group was not reported, so not included We attempted to contact the authors, but received no reply. Cosmetic outcome as measured at < 6 months so not included Funding: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients who ...of the femur had randomised wound closure". Comment: insufficient information provided |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information provided |
| Blinding of participants and personnel (performance bias) Surgical site infection | Unclear risk | No information provided |
| Blinding of outcome assessment (detection bias) Surgical site infection | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) Short‐term outcomes | Unclear risk | There were six post‐randomisation dropouts, but it was not reported which trial groups these participants were from. |
| Selective reporting (reporting bias) | High risk | Comment: protocol not available, and the data could not be entered in the review because the number allocated to each group was not provided (the outcomes of interest in the review were reported incompletely so that they could not be entered in a meta‐analysis). |
| Other bias | Unclear risk | Funding not reported |
Sebesta 2004.
| Methods | Randomised controlled trial | |
| Participants | Country: US Number randomised: 59 (228 wounds) Post‐randomisation dropout: not reported Duration of follow‐up: 2 weeks Surgery: laparoscopic surgery (urology) |
|
| Interventions | Participants randomly assigned to 2 groups: Group 1: (30 participants; 118 incisions): subcuticular suture (4‐0 absorbable sutures, either Vicryl or Monocryl) Group 2: (29 participants; 110 incisions): 2‐octylcyanoacrylate (Dermabond, Ethicon, Somerville, NJ) Those who received subcuticular sutures had their wounds dressed with steri‐strips, a gauze pad, and tape or a Tegaderm dressing. No dressings were applied to the octylcyanoacrylate‐closed wounds. |
|
| Outcomes | Surgical site infection Wound complications, wound dehiscence, wound closure time and cost (material and total) |
|
| Notes | Material cost was used for analysis. Cosmetic appearance measured at < 6 months so not included Funding: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "all patients undergoing laparoscopic surgery by one surgeon (JTB) were randomised to receive skin closure with either subcuticular suture of octylcyanoacrylate". Comment: insufficient information provided |
| Allocation concealment (selection bias) | Unclear risk | Quote: "all patients undergoing laparoscopic surgery by one surgeon (JTB) were randomised to receive skin closure with either subcuticular suture of octylcyanoacrylate". Comment: Not sufficient information provided |
| Blinding of participants and personnel (performance bias) Surgical site infection | Unclear risk | This information was not available. |
| Blinding of outcome assessment (detection bias) Surgical site infection | Unclear risk | Quote: "patients were evaluated 2 weeks postoperatively for evidence of infection, dehiscence, seroma, and general cosmetic appearance". Comment: unclear whether this evaluation was undertaken by a blinded assessor |
| Incomplete outcome data (attrition bias) Short‐term outcomes | Unclear risk | This information was not available. |
| Selective reporting (reporting bias) | Low risk | Quote: "patients were evaluated 2 weeks postoperatively for evidence of infection, dehiscence, seroma, and general cosmetic appearance." Quote: "one patient had a minor wound infection at one incision site treated with oral antibiotics." Comment: protocol not available, but the primary outcome data (infection) prespecified in Methods was reported and all the outcomes they planned to do were fully reported. |
| Other bias | High risk | Duplicate publication. There were many data input errors. |
Selvadurai 1997.
| Methods | Randomised controlled trial | |
| Participants | Country: UK Number randomised: 80 Post‐randomisation dropout: 2 vs 1 (at 3 months), 2 vs 3 (at 6 months) Mean age (years): 52 vs 51 Number of males: 6 vs 7 Duration of follow‐up: 6 months Surgery: thyroid and parathyroid surgery |
|
| Interventions | Participants randomly assigned to 2 groups: Group 1: subcuticular sutures (n = 42), using 3‐0 polypropylene (Prolene) continuous suture which was secured at either end using nylon beads Group 2: metal clips (n = 38), using Michel. 3‐0 Nylon stay sutures were placed at either end and in the centre of the wound Sutures and clips were removed on the second postoperative morning. |
|
| Outcomes | Wound infection Wound complication (haematoma), hypertrophic scar, wound pain intensity (verval response and VAS), cosmesis (verval response and VAS) and wound closure time |
|
| Notes | SD was calculated from SE. Pain scores as measured at the first 3 postoperative days. We included VAS data at second postoperative day. Cosmetic outcome as measured at 3 and 6 months. We included the VAS data assessed by independent observer at 6 months. Funding: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "the randomisation code was generated using a table of random numbers and a sealed envelope system was used". Comment: probably done |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information provided |
| Blinding of participants and personnel (performance bias) Surgical site infection | Unclear risk | No information provided |
| Blinding of outcome assessment (detection bias) Surgical site infection | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) Short‐term outcomes | Low risk | Dropouts were low in number and evenly distributed. Therefore, the proportion of missing outcomes was not enough to have a clinically relevant impact on the intervention effect estimate. |
| Selective reporting (reporting bias) | High risk | Quote: "There were no deep cervical hematomas or wound infections". Comment: study protocol unavailable, and primary outcome data (infection) was not prespecified in Methods but reported in Results. |
| Other bias | Unclear risk | Funding not reported |
Shetty 2004.
| Methods | Randomised controlled trial | |
| Participants | Country: UK Number randomised: 110 Post‐randomisation dropout: 9 Mean age (years): 81.7 vs 83.5 Number of males: 7 vs 13 Duration of study: 4 months Duration of follow‐up: 10 days Surgery: surgery for femoral fractures (hip wound) |
|
| Interventions | Participants randomly assigned to 2 groups: Group 1: subcuticular sutures (n = 47), using 3‐0 Vicryl (Ethicon, Edinburgh, UK) and steri‐strips Group 2: metallic skin staples (n = 54), using Reflex, Delasco [IA], US |
|
| Outcomes | Surperficial wound infection Wound complications, wound dehiscence and re‐closure |
|
| Notes | Funding: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients were randomised into 2 groups with respect to wound closure. A sealed envelope method was used." Comment: no direct quotation about how the allocation sequence was generated |
| Allocation concealment (selection bias) | Unclear risk | No information about whether envelopes were opaque and sequential |
| Blinding of participants and personnel (performance bias) Surgical site infection | Unclear risk | No direct quotation about whether the participants or personnel were blinded |
| Blinding of outcome assessment (detection bias) Surgical site infection | Unclear risk | No direct quotation about whether the outcome assessors were blinded |
| Incomplete outcome data (attrition bias) Short‐term outcomes | Unclear risk | Quote: "nine patients died in the immediate postoperative period, leaving 101 patients in the study." Comment: the reasons for these nine deaths and the trial groups of these participants were not reported. |
| Selective reporting (reporting bias) | Unclear risk | Study protocol was unavailable. Wound infection was defined in the outcome of the study in the Methods section and was reported in the Results section, but it was unclear whether all outcomes assessed were fully reported. |
| Other bias | Unclear risk | Funding: not reported Insufficient information was available to permit a judgement of how high the risk was. |
Simpson 1979.
| Methods | Randomised controlled trial (3 arms) | |
| Participants | Country: UK Number randomised: 152 Post‐randomisation dropout: 19 (13%) Mean age: 48 vs 40 vs 45 Number of males: 28 vs 22 vs 28 Duration of follow‐up: 12 months Surgery: surgery for inguinal hernia, gallstone disease or benign peptic ulcer |
|
| Interventions | Participants randomly assigned to 3 groups: Group 1: subcuticular suture (n = 48), using an absorbable polyglycolic‐acid suture (2‐0 Dexon) Group 2: subcuticular suture (n = 40), using a 0 Nylon Group 3: interrupted suture (n = 45), using a 0 Nylon For this review, the subcuticular data (groups 1 and 2) have been combined. |
|
| Outcomes | Hypertrophic scar | |
| Notes | Funding: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "a consecutive series of 152 patients undergoing surgery for inguinal hernia, gallstone disease or benign peptic ulceration gave informed consent to be randomly allocated to one of the three skin closure with or without a plastic spray dressing." Comment: insufficient information provided |
| Allocation concealment (selection bias) | Unclear risk | Quote: "a consecutive series of 152 patients undergoing surgery for inguinal hernia, gallstone disease or benign peptic ulceration gave informed consent to be randomly allocated to one of the three skin closure with or without a plastic spray dressing." Comment: insufficient information provided |
| Blinding of participants and personnel (performance bias) Surgical site infection | Unclear risk | No information provided |
| Blinding of outcome assessment (detection bias) Surgical site infection | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) Short‐term outcomes | High risk | Multiple post‐randomisation dropouts and the reasons for missing outcome data were reported without its distribution among the groups, which was likely to be related to the primary outcome of the review. |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available, but the outcomes measures stated in the methods section were reported in the results. |
| Other bias | Unclear risk | Funding not reported. Three‐arm design |
Soni 2013.
| Methods | Randomised controlled trial | |
| Participants | Country: India Number randomised: 29 Post‐randomisation dropout: 0 vs 1 (at 3 months) Mean age (years): 32.9 vs 29.7 Number of males: 13 vs 13 Duration of study: 18 months Duration of follow‐up: 3 months Surgery: maxillofacial incisions |
|
| Interventions | Participants randomly assigned to 2 groups: Group 1: subcuticular suture (n = 15), using a 5‐0 monofilament synthetic polypropylene Group 2: skin adhesive (n = 14), using octyl‐2‐cyanoacrylate (2‐OCA; Dermabond; Ethicon, Inc, Somerville, NJ) |
|
| Outcomes | Wound complications, wound dehiscence and wound closure time | |
| Notes | Quote: "for all wounds, subcutaneous or deep dermal sutures (3‐0, 4‐0 Vicryl) were applied to aid in the apposition of the wound edge margins, relieve tension, ensure adequate skin edge eversion, and prevent deposition of 2‐OCA into the wound". The reviewers viewed 'deep dermal suture' as unnecessary and it was similar to subcuticular suture. Cosmetic outcome as measured at < 6 months so not included An outcome that study authors referred to as a 'patient satisfaction' score was also measured, however, this seemed to focus on satisfaction of cosmetic appearance so was deemed a cosmetic evaluation; as it was collected at 3 months after surgery, it was not reported here. Funding: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomly allocated to a suture or a tissue adhesive group using a block randomization system" |
| Allocation concealment (selection bias) | Unclear risk | No direct quotation about whether allocation concealment was done |
| Blinding of participants and personnel (performance bias) Surgical site infection | Unclear risk | No direct quotation about whether the participants or personnel were blinded |
| Blinding of outcome assessment (detection bias) Surgical site infection | Unclear risk | No direct quotation about whether the outcome assessors were blinded |
| Incomplete outcome data (attrition bias) Short‐term outcomes | Low risk | Quote: "one patient in the tissue adhesive group was lost at the 3‐ month follow‐up". Comment: only one dropout, and this did not affect the outcome significantly. |
| Selective reporting (reporting bias) | Unclear risk | No direct quote but the outcome (optimal score based on a validated modified Hollander Wound Evaluation Scale) to be assessed was not prespecified in the methods so it was unclear whether they were fully reported. |
| Other bias | Unclear risk | Funding: not reported |
Steele 1983.
| Methods | Randomised controlled trial | |
| Participants | Country: UK Number randomised: 40 Post‐randomisation dropout: 0 (at 10th day), not stated (at 3 months) Mean age (years), number of males: not stated Duration of follow‐up: 3 months Surgery: total mastectomy |
|
| Interventions | Participants randomly assigned to 2 groups: Group 1: subcuticular sutures (n = 20), using polypropylene suture Group 2: stainless steel staples (n = 20) |
|
| Outcomes | Wound closure time and wound complications (marked redness, necrosis, discharge or pus) | |
| Notes | Number of satisfactory wounds was considered as number of no wound complications. Cosmetic outcome as measured at < 6 months so not included Funding: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "40 consecutive patients undergoing total mastectomy were randomly selected to have their wounds closed by stainless steel staples or subcuticular polypropylen suture". Comment: insufficient information provided |
| Allocation concealment (selection bias) | Unclear risk | Quote: "40 consecutive patients undergoing total mastectomy were randomly selected to have their wounds closed by stainless steel staples or subcuticular polypropylen suture". Comment: insufficient information provided |
| Blinding of participants and personnel (performance bias) Surgical site infection | Unclear risk | No information provided |
| Blinding of outcome assessment (detection bias) Surgical site infection | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) Short‐term outcomes | Low risk | No missing data at 10th postoperative day |
| Selective reporting (reporting bias) | High risk | Quote: "in the final assessment the wound were scored for colour, width, hypertrophy, infection, crosshatching, and overall result." Quote: "the mean composite scores for the 3‐month assessment are shown in Table 3". Comment: protocol not available, and the important outcomes prespecified in Methods was reported incompletely so that it could not be entered in the review. |
| Other bias | Unclear risk | Funding: not reported |
Subramanian 2005.
| Methods | Randomised controlled trial (split‐body design) | |
| Participants | Country: UK Number randomised: 16 Post‐randomisation dropout: not stated Age (years), number of males: not stated Duration of follow‐up: 4 weeks Surgery: bilateral inguinal hernia repairs |
|
| Interventions | Group 1 (one incision): subcuticular sutures (n = 16), using Prolene® (Ethicon) suture Group 2 (the other incision): staples (WECK Visistat®) (n = 16) Sutures and staples were removed at ten days. |
|
| Outcomes | Wound infection Wound pain intensity and wound closure time |
|
| Notes | Pain intensity was reported as figure (missing data). Funding: not reported We attempted to contact the authors in July 2017. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "each patient was randomised into having one incision closed using staples (WECK Visistat®) and the other closed using subcuticular Prolene® (Ethicon™)". Comment: insufficient information provided |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information provided |
| Blinding of participants and personnel (performance bias) Surgical site infection | Low risk | Quote: "postoperatively, both wounds were covered with a padded occlusive Mepore® dressing which prevented the patient or the investigator from directly seeing the wounds, thereby blinding them as to which side was sutured or stapled. This dressing was removed at one week by an independent assessor". Comment: probably done |
| Blinding of outcome assessment (detection bias) Surgical site infection | Unclear risk | Quote: "an independent assessor examined the wound after the dressings were removed at 1 week for the presence of any cutaneous signs of infection". Comment: insufficient information provided |
| Incomplete outcome data (attrition bias) Short‐term outcomes | Unclear risk | No information provided |
| Selective reporting (reporting bias) | Low risk | Quote: "an independent assessor examined the wound after the dressings were removed at 1 week for the presence of any cutaneous signs of infection". Quote: "one cutaneous would infection occurred with each of the two closure methods". Comment: protocol not available, but primary outcome data (infection) prespecified in Methods was reported and all the outcomes they planned to do were fully reported. |
| Other bias | Unclear risk | Split‐body design. Funding was not reported. Insufficient information was available to permit a judgement of how high the risk was. |
Swtizer 2003.
| Methods | Randomised controlled trial | |
| Participants | Country: US Number randomised: 45 (46 incisions) Post‐randomisation dropout: not stated Mean age (years): 50.2 vs 42.8 Number of males: 43 Duration of follow‐up: 4 weeks Surgery: paediatric herniorrhaphy |
|
| Interventions | Participants randomly assigned to 2 groups: Group 1: (n = 22): subcuticular running 4‐0 polyglecaprone (Monocryl®, Ethicon, Inc) suture Group 2: (n = 24): 2‐octylcyanoacrylate (Dermabond®) tissue adhesive (Ethicon Inc, a Johnson & Johnson company, Somerville, New Jersey, USA) |
|
| Outcomes | Wound complications, wound closure time (SD was calculated from 95% CI) | |
| Notes | Cosmetic outcome as measured at < 6 months so not included Funding: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients were then randomised with the use of a computerized random number generator to receive either 2‐octylcyanoacrylate tissue adhesive or subcuticular running 4‐0 poliglecaprone 25 (Monocryl, Ethicon, Inc) skin closure". Comment: computerised random generator was used. |
| Allocation concealment (selection bias) | Unclear risk | No direct quotation about whether allocation concealment was done. |
| Blinding of participants and personnel (performance bias) Surgical site infection | Unclear risk | No direct quotation about whether the participants or personnel were blinded. |
| Blinding of outcome assessment (detection bias) Surgical site infection | Unclear risk | Quote: "photographs were then scored for cosmesis by four staff surgeons who were blinded to the patients' group identity". Comment: cosmetic outcome was assessed by four blinded assessors, but it was not clear if the outcome about the wound infection was assessed by a blinded assessor, which is relevant to the review. |
| Incomplete outcome data (attrition bias) Short‐term outcomes | Unclear risk | There was one dropout, but the reason for this was not described. |
| Selective reporting (reporting bias) | Unclear risk | Quote: "five wound complications (four wound separations and one draining sinus) were observed in the Dermabond group. There were no wound complications in the suture group". Comment: study protocol unavailable, and it was not defined whether the wound complications included the wound infection. |
| Other bias | High risk | There was a letter pointing out that the P value reported in this study was wrong. Randomisation probably was conducted at a participant level but the analysis was carried out at the level of the wound; it did not appear that clustered data (multiple wounds from individual participants) were accounted for in the analysis. |
Tanaka 2014.
| Methods | Randomised controlled trial | |
| Participants | Country: Japan Number randomised: 293 Post‐randomisation dropout: 2 vs 1 (at 30 days after operation) Median age (years): 66.9 vs 66.7 Number of males: 76 (51.7%) vs 86 (59.6%) Duration of study: 4 years Duration of follow‐up: 6 months Surgery: elective colon cancer surgery |
|
| Interventions | Participants randomly assigned to 2 groups: Group 1: subcuticular sutures (n = 145), using 4‐0 monofilament absorbable interrupted suture (polydioxanone; PDS‐ II Ethicon) Group 2: interrupted transdermal 3‐0 Nylon sutures (n = 145) Nylon suture was removed at 6 days postoperatively. No fat stitch was used. |
|
| Outcomes | Surgical site infection (incisional and organ/space) Cosmesis of scar (111 vs 109 participants), patient satisfaction (within 30 days,after 60 days), cosmesis of scar and wound closure time |
|
| Notes | Funding: nothing We contacted the authors and got additional data (total number of SSI). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "a minimization method was incorporated in the randomization to adjust baseline imbalance between the two groups in age (< 65 and ≥ 65 years) and tumor locations (proximal, distal, and bilateral colons)". |
| Allocation concealment (selection bias) | Low risk | Referring to the UMIN registration, study authors noted that the method of the central registration was used. |
| Blinding of participants and personnel (performance bias) Surgical site infection | Unclear risk | No direct quotation about whether the participants or personnel were blinded |
| Blinding of outcome assessment (detection bias) Surgical site infection | Unclear risk | Quote: "wound complications were assessed by one of the staff surgeons in the colorectal surgery department on day 7 during hospitalization. After the patient was discharged ... after surgery. . ." Comment: no direct quotation about whether the outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) Short‐term outcomes | Low risk | Dropouts were low in number and evenly distributed. Therefore, the proportion of missing outcomes was not enough to have a clinically relevant impact on the intervention effect estimate. |
| Selective reporting (reporting bias) | Low risk | Study protocol not available but all the study's prespecified outcomes in UMIN‐CTR (trial registry) that are of interest in the review have been reported. |
| Other bias | Low risk | The study appeared to be free of other source of bias. |
Tanaka 2016.
| Methods | Randomised controlled trial | |
| Participants | Country: Japan Number randomised: 237 Post‐randomisation dropout: 17 vs 6 Mean age (years): first operation group 16.8 vs 18.6, reoperation group 21.6 vs 30.5 Number of males: 55 vs 58 Duration of study: 22 months Duration of follow‐up: 12 weeks Surgery: cardiac operations through a median sternotomy |
|
| Interventions | Participants randomly assigned to 2 groups: Group 1: subcuticular sutures (n = 101), using 5‐0 Prolene continuous sutures (Ethicon) with the surgical clip on the end of a suture Group 2: zip surgical skin closure device (ZipLine Medical, Campbell, CA) (n = 113) |
|
| Outcomes | Surgical site infection Wound complications, wound dehiscence, and wound closure time |
|
| Notes | Cosmetic outcome as measured at < 6 months so not included Funding: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "enrolled patients were randomly assigned to a Zip surgical group (suture group) using computed simple randomization and were studied prospectively." |
| Allocation concealment (selection bias) | Unclear risk | No direct quotation about whether allocation concealment was done |
| Blinding of participants and personnel (performance bias) Surgical site infection | Unclear risk | No direct quotation about whether the participants or personnel were blinded |
| Blinding of outcome assessment (detection bias) Surgical site infection | Unclear risk | Quote: "follow‐up and complications data were collected by 3 cardiovascular surgeons at 1, 2, 4, 8, and 12 weeks postoperatively ... assessment of cosmetic outcomes was performed by 2 plastic surgeons 3 months after the operation who were blinded to the type of skin closure used". Comment: cosmetic outcome was assessed by blinded assessors, but it was not clear if the outcome about the wound infection was assessed by a blinded assessor, which is relevant to the review. |
| Incomplete outcome data (attrition bias) Short‐term outcomes | High risk | The reasons for dropouts were described, but there was imbalance in the number of dropouts between allocation groups. |
| Selective reporting (reporting bias) | High risk | Quote: "the outcomes analyzed in this study included cosmetic appearance, skin closure time, and complication rate". Comment: study protocol or trial registration unavailable, and outcome data (infection) was not prespecified in the methods section but reported in the results. |
| Other bias | Unclear risk | Funding was not reported. Insufficient information was available to permit a judgement of how high the risk was. |
Taube 1983.
| Methods | Randomised controlled trial | |
| Participants | Country: UK Number randomised: 169 Post‐randomisation dropout: 0 Age: 15‐78 years Numbe of males: 51 vs 52 Duration of follow‐up: 4 weeks Surgery: acute appendicectomy, inguinal herniorrhaphy or saphenofemoral ligation |
|
| Interventions | Participants randomly assigned to 2 groups: Group 1: subcuticular continuous suture (n = 88), using a 2/0 Prolene and sterile Micropore tape Group 2: interrupted suture (n = 81), using a 2/0 Prolene Skin sutures were removed on the seventh day. |
|
| Outcomes | Wound infection | |
| Notes | Cosmetic outcome as measured at < 6 months so not included Funding: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "each patient was allocated at random to one of two groups". Comment: insufficient information provided |
| Allocation concealment (selection bias) | Unclear risk | Quote (Methods): "Each patient was allocated at random to one of two groups." Comment: insufficient information provided |
| Blinding of participants and personnel (performance bias) Surgical site infection | Unclear risk | No information provided |
| Blinding of outcome assessment (detection bias) Surgical site infection | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) Short‐term outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Low risk | Quote: "the semi‐transparent nature of the Micropore allowed recognition of complications (for example, infection), which were recorded". Quote: "in 7 cases the wounds became infected, 2 after herniorrhaphy (1 Group A, 1 Group B) and 5 after appendicectomy (3 Group A, 2 Group B)". Comment: study protocol not available, but the primary outcome (infection) prespecified in Methods was reported and all the outcomes they planned to do were fully reported. |
| Other bias | Unclear risk | Funding not reported Possibility of co‐intervention effect by sterile Micropore tape combination in subcuticular suture group |
Teoh 2018.
| Methods | Randomised controlled trial (2 arms) | |
| Participants | Country: Malaysia Number randomised: 140 Post‐randomisation dropouts: 44 Average age: not stated (median age: 52) Number of males: 8:17 Duration of follow‐up: 3 months Surgery: total thyroidectomy, hemithyroidectomy or parathyroidectomy | |
| Interventions | Group 1: subcuticular sutures (n = 60 (analyse 47)), using braided polyglycolic aced 4/0 suture Group 2: tissue adhesive glue (Leukosan Adhesive; synergy of both octyl‐2‐cyanoacrylate and n‐2‐ butylcyanoacrylate) (n = 57 (analyse data 49)) | |
| Outcomes | Wound infection and dehiscence Cosmesis (POSAS score, SBSES score) Duration time of closing |
|
| Notes | Objective and subjective cosmesis was assessed < 6 months after the surgery, so we could not include the data. The data for duration time of closing was insufficient, so we could not include them. The study was not supported by any funding. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Block randomisation using sealed and numbered envelopes was applied using an online randomisation application 'Sealed Envelope'." |
| Allocation concealment (selection bias) | Unclear risk | Quote: "The envelopes were randomly selected by the patients during admission, and were brought into the operation theatre and revealed only upon the commencement of skin closure." Comment: No information about whether envelopes were opaque and sequential |
| Blinding of participants and personnel (performance bias) Surgical site infection | Low risk | Quote: "Patients and the medical officer who were assigned to assess the wound were blinded to the intervention." |
| Blinding of outcome assessment (detection bias) Surgical site infection | Low risk | Quote: "This was a double‐blinded study involving the patients and an independent observer." |
| Incomplete outcome data (attrition bias) Short‐term outcomes | High risk | Comment: A relatively high number of patients (44/140) were excluded or lost after randomisation. |
| Selective reporting (reporting bias) | Unclear risk | Comment: Protocol was not available. One outcome, "time duration of closing" was not stated in the methods section, but was reported in the results section. |
| Other bias | Unclear risk | Comment: There was a significant difference in the gender between two groups. |
Tsujinaka 2013.
| Methods | Multicentre randomised controlled trial | |
| Participants | Country: Japan Number randomised: 1080 Post‐randomisation dropout: 8 (4 vs 4) Median age (years): 68 vs 68 Number of males: 388 (69.0%) vs 365 (70.5%) Duration of study: 38 months Duration of follow‐up: 6 months Surgery: open gastrointestinal surgery |
|
| Interventions | Participants randomly assigned to 2 groups: Group 1: subcuticular sutures (n = 562), using 3‐0 or 4‐0 monofilament absorbable interrupted suture (polydioxanone; PDS‐ II Ethicon,Tokyo,Japan) Group 2: metallic skin staples (n = 518) |
|
| Outcomes | Surgical site infection Wound complications, hypertrophic scar and length of hospital stay |
|
| Notes | Funding: Johnson & Johnson We contacted the authors in July 2017 and got additional data regarding hospital stay. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "enrolment was done through a web‐based system established for this trial and randomisation by a computer‐generated permuted‐block sequence". |
| Allocation concealment (selection bias) | Low risk | Quote: "enrolment was done through a web‐based system established for this trial and randomisation by a computer‐generated permuted‐block sequence." Comment: central allocation |
| Blinding of participants and personnel (performance bias) Surgical site infection | High risk | Quote: "patients and investigators were not masked to group assignment". Comment: no blinding, and the outcome was likely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) Surgical site infection | Low risk | Quote: "assessment of surgical site infections was done by infection control personnel at the participating institutions who did not have roles in trial design or conduct. Detection of other wound complications was based on whether some treatment (dressing or surgical intervention) for wound management was documented in the medical record, which could minimise bias". Comment: no blinding of outcome assessment, but the review authors judged that the outcome was not likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) Short‐term outcomes | Low risk | Quote: "assessment of case report forms showed that four patients in each group were ineligible for inclusion, and thus the modified intention‐to‐treat population comprised 558 patients in the subcuticular sutures group and 514 in the staples group". Comment: dropouts were low in number and evenly distributed. Therefore, the proportion of missing outcomes was not enough to have a clinically relevant impact on the intervention effect estimate. |
| Selective reporting (reporting bias) | Low risk | Study protocol was not available but all the study's prespecified outcomes in UMIN‐CTR (trial registry) that are of interest in the review have been reported. |
| Other bias | Unclear risk | Imbalanced number between allocation groups, but there was insufficient evidence that an identified problem introduced bias. |
Van den Ende 2004.
| Methods | Randomised controlled trial | |
| Participants | Country: Netherland Number randomised: 100 Post‐randomisation dropout: 0 Mean age (years): 3.0 vs 2.5 Number of males: 82% vs 74% Duration of study: 8 months Duration of follow‐up: 6 weeks Surgery: groin surgery (children) |
|
| Interventions | Participants randomly assigned to 2 groups: Group 1: suture intracutaneously (n = 50), using a 5‐0 Polyglatin (Vicryl) Group 2: skin adhesives (n = 50), using N‐butylcyanoacrylate (Indermil, Loctite Corp, 's‐Hertogen‐bosch, The Netherlands) |
|
| Outcomes | Infection Wound complications, wound dehiscence, re‐closure, wound closure time and cost (€) |
|
| Notes | Cosmetic outcome as measured at < 6 months so not included SD of wound closure time was calculated from P value (0.001). Cost/per package, so not used Funding: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients were selected randomly to receive wound adhesive or suture on the basis of 100 previously prepared and sealed envelopes containing slips for either suture closure or the use of Indermil". Comment: no direct quotation about how the allocation sequence was generated |
| Allocation concealment (selection bias) | Unclear risk | No information about whether envelopes were opaque and sequential |
| Blinding of participants and personnel (performance bias) Surgical site infection | Unclear risk | No direct quotation about whether the participants or personnel were blinded |
| Blinding of outcome assessment (detection bias) Surgical site infection | Unclear risk | No direct quotation about whether the outcome assessors were blinded |
| Incomplete outcome data (attrition bias) Short‐term outcomes | Low risk | There was no dropout. |
| Selective reporting (reporting bias) | Low risk | Quote: "wounds were evaluated for hematoma, evidence of infection, dehiscence, or formation of granuloma". Comment: study protocol unavailable, but the primary outcome data (infection) prespecified in Methods was reported and all the outcomes they planned to do were reported. |
| Other bias | Unclear risk | It was not clear that bilateral wounds from individual participants (paired data) were accounted for in the analysis. |
Xu 2014.
| Methods | Randomised controlled trial | |
| Participants | Country: China Number randomised: 90 Post‐randomisation dropout: 0 Mean age (years): 13.5 vs 13.2 Number of males: 0 Duration of study: 1 year Duration of follow‐up: 1 year Surgery: adolescents with idiopathic scoliosis undergoing posterior spinal fusion surgery |
|
| Interventions | Participants randomly assigned to 2 groups: Group 1: subcuticular sutures (n = 45), using 4‐0 Monocryl absorbable sutures Group 2: surgical zipper (Surgizip; MediTech Healthcare Inc., Singapore) (n = 45) |
|
| Outcomes | Surgical site infection Wound complications, wound dehiscence, cosmesis of scar and wound closure time |
|
| Notes | We used cosmetic outcome data at one year. Funding: National Public Health Benefit Research Foundation, China (Grant no.201002018) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "a randomized and controlled clinical study was prospectively performed". Comment: no direct quotation about how the allocation sequence was generated |
| Allocation concealment (selection bias) | Unclear risk | Quote: "enclosed in sealed identical opaque envelopes, allocation of patients remained" Comment: it was not clear whether sequentially numbered envelopes were used. |
| Blinding of participants and personnel (performance bias) Surgical site infection | Unclear risk | No direct quotation about whether the participants or personnel were blinded |
| Blinding of outcome assessment (detection bias) Surgical site infection | High risk | Quote: "the incision outcome was evaluated at 7 days, 2 weeks, 6 months, and 1 year after surgery by the same assessor (X.L.)." Comment: no direct quotation about whether the outcome assessor was blinded, and the outcome was likely to be influenced by lack of blinding because a study member (the first author) assessed outcome. |
| Incomplete outcome data (attrition bias) Short‐term outcomes | Low risk | There was no dropout. |
| Selective reporting (reporting bias) | Unclear risk | The primary outcome (infection) was reported, but it was not clear whether wound complications prespecified in Methods included infections. |
| Other bias | Low risk | The funding source of this study was clearly described. The study appeared to be free of other source of bias. |
Zwart 1989.
| Methods | Randomised controlled trial (3‐arm) | |
| Participants | Country: Netherlands Number randomised: 235 Post‐randomisation dropout: 17 vs 10 vs 20 (after one month) Mean age (years): 51.9 vs 54.3 vs 57.8 Number of males: 49 vs 53 vs 58 Duration of follow‐up: 6 months Surgery: surgery for elective intra‐abdominal or inguinal hernia |
|
| Interventions | Participants randomly assigned to 3 groups: Group 1: subcuticular sutures (n = 79), using 3‐0 monofilament polydioxanone (PDS) sutures Group 2: continuous 3‐0 monofilament poly‐amide (Ethylon) (n = 73) Group 3: proximate staples (n = 83) |
|
| Outcomes | Wound infection Cosmesis of scar (objective and subjective) and wound closure time |
|
| Notes | Wound closure time was not used (missing statistics). Data of cost was reported per piece and insufficient, so we did not include the outcome. Fund: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomization was performed by drawing a number in the operating room (sealed envelope method)". Comment: no further description about the number used in this study |
| Allocation concealment (selection bias) | Unclear risk | No mention that opaque envelopes were used |
| Blinding of participants and personnel (performance bias) Surgical site infection | Unclear risk | No direct quotation about whether the participants or personnel were blinded |
| Blinding of outcome assessment (detection bias) Surgical site infection | Unclear risk | Quote: "upon discharge and after one and six months the head nurse of the out‐patient department appraised the scar. She had no knowledge of the employed method of wound closure." Comment: cosmetic outcome was assessed by a blinded assessor, but it was not clear if the outcome about the wound infection was assessed by a blinded assessor, which is relevant to the review. |
| Incomplete outcome data (attrition bias) Short‐term outcomes | High risk | Quote: "the follow‐up compliance at one month after discharge was 80.4 percent." Comment: there were many post‐randomisation dropouts. |
| Selective reporting (reporting bias) | High risk | Study protocol unavailable, but outcome data (infection, skin closure speed and cost) was not prespecified in the methods section but reported in the result. |
| Other bias | Unclear risk | Insufficient information available to permit a judgement of how high the risk was |
CABG: coronary artery bypass grafting CDC: Centers for Disease Control and Prevention CI: confidence interval COI: conflict of interest CN: continuous nylon ERAS: enhanced recovery after surgery HCC: hepatocellular carcinoma IABP: intra‐aortic balloon pump IC: intracutaneous closure ICTRP: International Clinical Trials Registry Platform IQR: interquartile range LBSS:LiquiBand® Surgical S MS: Mohs micrographic surgery OCA: octyl‐2‐cyanoacrylate PDS: polydioxanone PGA: polyglycolic acid PLOS: postoperative length of hospital stay POSAS: Patient and Observer Scar Assessment Scale ROB: risk of bias RSS: running subcuticular sutures SBSES: Stony Brook Scar Evaluation Score SC: subcuticular Maxon SD: standard deviation SE: standard error SEM: standard error of the mean SIS: simple interrupted sutures SSI: surgical site infection SWC: standard wound closure TC: transcutaneous closure THR: total hip replacement VAS: Visual Analogue Scale
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Alicandri‐Ciufelli 2014 | Not relevant outcomes (cosmetic data as measured at < 6 months) |
| Angelini 1984 | Quasi‐randomised study (allocation by serial number) |
| Bernard 2001 | It was not clear whether this was a comparison of subcuticular sutures versus others |
| Bernstein 2001 | Not a randomised controlled trial |
| Blondeel 2014 | Quote: "the subcutis was then closed with interrupted suturing. Placement of subcutaneous sutures could extend into the lower dermis to provide close approximation of the skin layers". Both groups received subcuticular sutures. |
| Buttaro 2015 | In the skin staple group, subcuticular suture was performed before skin staples. |
| Cameron 1987 | Not a comparison of skin closure (a comparison of mass closure or no closer) |
| Cassie 1988 | Quasi‐randomised study (allocation by data of birth) |
| Chan 2017 | Not a comparison of skin closure |
| Cheng 1997 | It was not clear whether this was a comparison of subcuticular sutures versus others |
| Clayer 1991 | Quasi‐randomised study (allocation by unit record number) |
| Consorti 2013 | Not relevant outcomes (cosmetic data as measured at < 6 months) |
| Cordova 2013 | Letters. Not a randomised controlled trial |
| Davies 1995 | Quasi‐randomised study (allocation by hospital registration number) |
| Eldrup 1981 | It was not clear whether this was a comparison of subcuticular sutures versus others |
| Elliot 1989 | Quasi‐randomised study (allocation into each group sequentially) |
| Erel 2001 | Not a comparison of skin closure (fascial closure) |
| Gatt 1985 | It was not clear whether this was a comparison of subcuticular sutures versus others |
| Glennie 2017 | All participants in the intervention group had tissue adhesives (Dermabond) placed. They assessed a mixture of subcuticular sutures and tissue adhesives within the same intervention group, thus we excluded this study. |
| Greene 1999 | Not a comparison of skin closure (fascial closure) |
| Handschel 2006 | It was not clear whether this was a comparison of subcuticular sutures versus others. |
| Harvey 1986 | It was not clear whether this was a comparison of subcuticular sutures versus others. |
| Johnson 1997 | Trial in which a part of the surgical incision was randomised (split‐wound design). |
| Kerrigan 2010 | Trial in which a part of the surgical incision was randomised (split‐wound design). |
| Kharwadkar 2005 | Not a comparison of skin closure (fascial closure). All participants had a subcuticular suture placed. |
| Koonce 2015 | Quote: "the deep layer of all surgical wounds was closed using standard deep dermal buried 3‐0 vicryl suture (Ethicon)". Both groups received deep dermal sutures. This study focused on superficial skin closure. |
| Lalani 2016 | All participants in the intervention group had tissue adhesives (Dermabond) placed. They assessed a mixture of subcuticular sutures and tissue adhesives within the same intervention group, thus we excluded this study. |
| Lazar 2008 | All participants in the intervention group had tissue adhesives (Dermabond) placed. They assessed a mixture of subcuticular sutures and tissue adhesives within the same intervention group, thus we excluded this study. |
| Leaper 1985 | Not a comparison of skin closure (a comparison of mass closure or no closure) |
| Liang 2015 | Not a comparison of skin closure (subcutaneous layer suture) |
| Lombardi 2011 | Not relevant outcomes (cosmetic data as measured at < 6 months) |
| Matin 2003 | Quasi‐randomised study (allocation by numbered week of study) |
| McLean 1980 | It was not clear whether this was a comparison of subcuticular sutures versus others |
| Meinke 1996 | Not a comparison of subcuticular sutures versus others (subcuticular pins; instrument) |
| Menovsky 2004 | It was not clear whether this was a comparison of subcuticular sutures versus others. |
| Milone 2014 | Not relevant wound type (not surgical wound) |
| Mudd 2013 | All participants in the intervention group had tissue adhesives (Dermabond) placed. They assessed a mixture of subcuticular sutures and tissue adhesives within the same intervention group, thus we excluded this study. |
| Nahas 2004 | Quote: "inverted subdermal suture was used to approximate the skin edges of the incisions". Both groups received subdermal suture. The trial focused on superficial skin sutures. |
| Nair 1988 | Not a comparison of skin closure (subcutaneous layer suture) |
| Navali 2014 | Not a randomised controlled trial |
| Nipshagen 2008 | Not a comparison of subcuticular sutures versus others |
| Park 2015 | Both groups received subcuticular sutures. |
| Parvizi 2013 | Quote: "closure of subcutaneous fat, the wound edges were approximated with interrupted, buried, resorbable intradermal sutures of 2‐0 Vicryl (Ethicon). To achieve an even distribution of tension along the wound edges, buried sutures at 0.5–1.0‐cm intervals and a total of four 2‐0 Vicryl sutures (70‐cm suture length) are necessary". Both groups received intradermal (subcuticular) sutures. This study focused on superficial skin closure. |
| Pickford 1983 | Not a comparison of subcuticular sutures versus others |
| Plotner 2011 | Not a comparison of subcuticular sutures versus others (trial focused on superficial sutures) |
| Ralphs 1982 | Quasi‐randomised study (allocation by the last figure of their hospital registration number) |
| Richter 2012 | Trial in which a part of the surgical incision was randomised (split‐wound design) |
| Ries 2016 | Comment about an excluded trial (Wyles 2016) |
| Risnes 2001 | It was not clear whether this was a comparison of subcuticular sutures versus others. Both groups received subcutaneous sutures. |
| Rizvi 2018 | No relevant outcomes (cosmetic data as measured at < 6 months) |
| Rui 2017 | Quote: "all patients received ... interrupted suture with 2‐0 absorbable Vicryl sutures (Ethicon Inc.) for superficial fascia and deep dermal layer in order to reduce skin tension and align the wound edges". Both groups received intradermal (subcuticular) sutures. This study focused on superficial skin closure. |
| Sadick 1994 | It was not clear whether this was a comparison of subcuticular sutures versus others. |
| Selo‐Ojeme 2002 | It was not clear whether this was a comparison of subcuticular sutures versus others. |
| Serour 1996 | Not a randomised controlled trial |
| Shamiyeh 2001 | It was not clear whether this was a comparison of subcuticular sutures versus others. |
| Shanahan 1990 | Not a comparison of skin closure (comparison of dressing) |
| Singh 2006 | Not a randomised controlled trial |
| Sinha 2001 | It was not clear whether this was a comparison of subcuticular sutures versus others. |
| Szabó 2002 | It was not clear whether this was a comparison of subcuticular sutures versus others. Both groups received subcutaneous sutures. |
| Van de Gevel 2010 | It was not clear whether this was a comparison of subcuticular sutures versus others. Both groups received subcutaneous sutures. |
| Watson 1983 | Quote: "for less common procedures the methods of skin closure was taken in strict rotation". Not considered randomised controlled study |
| Watts 1982 | Letter to Hopkinson 1982. Not a randomised controlled trial |
| Wyles 2016 | No relevant outcomes |
| You 2016 | No relevant wound type (not surgical wound) |
Characteristics of studies awaiting assessment [ordered by study ID]
Choudry 1996.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Awaiting full text |
Lubowski 1985.
| Methods | Randomised controlled trial (4‐arm) |
| Participants | Country: Australia Number randomised: 57 Post‐randomisation dropout: not stated Age , Number of males: not stated Duration of study: not stated Duration of follow‐up: 6 months Surgery: abdominal surgery |
| Interventions | Each two groups of participants were randomly assigned to 2 groups respectively. Group 1: usual suture (n = 20) of transverse wounds Group 2: staples (n = 12) of transverse wounds Group 3: usual suture (n = 11) of vertical wounds Group 4: staples (n = 14) of vertical wounds |
| Outcomes | Cosmetic score, time of closure |
| Notes | The reported data was the combination for other methods of sutures. No reply after trying to contact the author in August 2017 |
Rubio‐Perez 2014.
| Methods | Parallel randomised controlled trial |
| Participants | Colorectal surgery patients |
| Interventions | Subcuticular continuous suture versus skin staples |
| Outcomes | Primary outcome measures: incidence of surgical site infection within 30 days after surgery, as by CDC definition Secondary outcome measures: prolongation of hospitalisation |
| Notes | Only abstract. We contacted the author in August 2017. Study completed and now authors preparing the paper |
Singer 2002.
| Methods | Randomised controlled trial |
| Participants | Country: US Number randomised: 814 Post‐randomisation dropout: not stated Mean age (years): 31.9 ± 21.1 vs 30.7 ± 21.1 Number of males: 432 Duration of study: 6 months Duration of follow‐up: 12 weeks Surgery: not stated |
| Interventions | Participants randomly assigned to 2 groups. Participants with wounds that required subcutaneous sutures in addition to percutaneous closure were randomised separately from those not requiring deep closure. Group 1: wound closure (n = 406) using 2‐octylcyanoacrylate (Dermabond, Ethicon, Inc, Somerville, NJ) Group 2: standard wound closure methods (sutures, adhesive tapes, or staples) (n = 408) |
| Outcomes | Primary outcome measures: long‐term cosmetic appearance at 3 months after wound repair Secondary outcome : rates of wound infection, rates of wound dehiscence, mean time to skin closure |
| Notes | The reported data was the combination for laceration closure. No reply after trying to contact the author in August 2017 |
Zhang 2011.
| Methods | Parallel randomised controlled trial |
| Participants | Patients who were undergoing breast cancer surgery |
| Interventions | Chinese silk transdermal suture versus VICRYL Plus subcuticular suture |
| Outcomes | Primary Outcome Measures: outcome measures: mean score on cosmetic outcome visual analogue scale (VAS) at 30 days (+/‐ 5) postoperative (photographs by an independent blinded central assessor using a validated 100 mm visual analogue scale, with 0 representing the worst possible scar and 100 representing the best possible scar) Secondary outcome measures: mean cosmetic outcome score on modified Hollander scale at 12 and 30 days, mean surgical site infection score on modified ASEPSIS scale at day 3 to 90 |
| Notes | Awaiting full text No reply after trying to contact the author at the available email addresses |
CDC: Centers for Disease Control and Prevention VAS: Visual Analogue Scale
Characteristics of ongoing studies [ordered by study ID]
ACTRN12611000399998.
| Trial name or title | A randomised study comparing skin closure in benign gynaecologic surgery: staple versus subcuticular suture |
| Methods | Parallel randomised controlled trial |
| Participants | Women undergoing benign gynaecologic surgery (Pfannenstiel incision) |
| Interventions | Staple versus subcuticular suture |
| Outcomes | Primary outcome: postoperative pain by using visual analogue scale (VAS) at two months after randomisation Secondary outcomes: wound infection by medical record at 30 days after the procedure and satisfaction of patient by using Patient Satisfaction Questionnaire (PSQ)at one month after randomisation |
| Starting date | April 2011 |
| Contact information | No reply after trying to contact the author at the available email addresses |
| Notes | Only trial registration available. Status of study is unknown (not recruiting). |
CTRI/2018/02/011698.
| Trial name or title | Comparing two methods for closing the skin after thyroid surgery which are stitching and sticking them with gum |
| Methods | Parallel randomised controlled trial |
| Participants | Patients undergoing thyroid surgery in department of general surgery |
| Interventions | Octyl 2‐cyanoacrylate versus 3‐0 round bodied monocryl suture (used for subcuticular skin suturing for thyroid skin closure) |
| Outcomes | Primary outcome: skin closure time Secondary outcomes: Manchester scar scale (time point: on 30th day and on 90th day); postoperative pain using visual analogue scale (time point: at 24 hours postoperative period) |
| Starting date | February 2016 |
| Contact information | No reply after trying to contact the author at the available email addresses |
| Notes | Trial registered retrospectively Only trial registration available. Study probably completed but not yet published |
CTRI/2018/08/015470.
| Trial name or title | A clinical trial to study the effects of two types of sutures, stapler and under‐the‐skin suture, on skin closure in patients undergoing surgery for hernia in the groin |
| Methods | Parallel randomised controlled study |
| Participants | Adult patients with inguinal hernia undergoing elective open Lichtenstein tension free mesh repair |
| Interventions | Interrupted subcuticular suture with 3‐0 or 4‐0 absorbable monofilament versus metallic skin staple |
| Outcomes | Primary outcome measure: Wound cosmesis (time point: 15 days and 30 days after discharge) Secondary outcome measures: 1. number of analgesic doses required (time point: 24 hours postop) 2. postoperative wound pain (time point: at 6 hours, 12 hours and 24 hours postoperation) 3. rate of wound complications and SSI (time point: prior to discharge, 15 days and 30 days after discharge) 4. time taken for closure proportionate to the length of the wound (time point: intraoperative) |
| Starting date | August 2018 |
| Contact information | |
| Notes |
IRCT20161217031440N1.
| Trial name or title | Comparison of subcuticular and transdermal appendectomy repairs; a randomised clinical trial |
| Methods | Parallel randomised controlled trial |
| Participants | Patients undergoing acute appendicitis surgery |
| Interventions | Subcuticular 3‐0 nylon stitches versus cuticular 3‐0 nylon stitches |
| Outcomes | 1. Level of pain on verbal rating scale at 1 week after surgery 2. Local infection at 1 week after surgery 3. Scar thickness at 1 month after surgery 4. Wound dehiscence at 1 week after surgery |
| Starting date | March 2017 |
| Contact information | |
| Notes | Trial registered retrospectively |
IRCT20180820040840N1.
| Trial name or title | Comparison of the complications of two wound repair methods (subcuticular and mattress) in patients undergoing appendectomy |
| Methods | Parallel randomised controlled trial |
| Participants | Patients undergoing appendectomy |
| Interventions | Subcuticular suture versus mattress suture for skin incision |
| Outcomes | 1. Postoperation pain severity on the Visual Analogue Scale at 0, 6, 12 and 24 hours after surgery 2. Surgical site infection during the patient’s hospitalisation period |
| Starting date | September 2018 |
| Contact information | |
| Notes |
ISRCTN80786695.
| Trial name or title | A comparison of patient satisfaction and complications following keyhole surgery wounds closed with tissue glue or stitches |
| Methods | Parallel randomised controlled trial |
| Participants | Patients undergoing laparoscopic general surgery |
| Interventions | Laparoscopic wound closure with 2‐cyanoacrylate tissue adhesive versus laparoscopic wound closure with subcuticular stitches |
| Outcomes | Primary outcome measures Patient satisfaction in terms of wound appearance and wound closure technique. Measured at six weeks postoperatively Secondary outcome measures 1. wound closure time, measured at the time of operation 2. wound complications (dehiscence, infection, resuturing), measured at six weeks postoperatively 3. wound pain, measured at six weeks postoperatively 4. antibiotic usage, measured at the time of operation 5. unscheduled medical review, measured at six weeks postoperatively |
| Starting date | January 2007 |
| Contact information | No reply after trying to contact the author at the available email addresses |
| Notes | Only trial registration available. Study probably completed but not yet published |
ISRCTN96030942.
| Trial name or title | A randomised trial comparing octyl‐cyanoacrylate and subcuticular sutures for post‐auricular wound cosmesis |
| Methods | Parallel randomised controlled trial |
| Participants | Patients undergoing ear surgery |
| Interventions | Subcuticular 4‐0 vicryl sutures versus octyl‐cyanoacrylate (Dermabond) |
| Outcomes | Primary outcome measures Cosmetic appearance of post‐auricular wounds as judged by a visual analogue scale assessment of photographs by assessors blinded to treatment allocation Secondary outcome measures 1. time taken for skin closure (seconds) 2. early wound complication (infection, dehiscence) assessed at 3 weeks 3. cosmetic appearance of post‐auricular wounds as judged by the Hollander Wound Evaluation Scale assessed at 3 months |
| Starting date | April 2006 |
| Contact information | No reply after trying to contact the author at the available email addresses |
| Notes | Only trial registration available. Study probably completed but not yet published |
Maschuw 2014.
| Trial name or title | Intracutaneous suture versus transcutaneous skin stapling for closure of midline or horizontal skin incision in elective abdominal surgery and their outcome on superficial surgical site infections ‐ INTRANS: study protocol for a randomised controlled trial |
| Methods | Prospective randomised controlled single centre trial in a parallel design |
| Participants | Patients scheduled for any elective abdominal surgery requiring midline or horizontal laparotomy |
| Interventions | Intracutaneous suture in the intervention group and transcutaneous skin stapling in the control group |
| Outcomes | The rate of superficial surgical site infections is defined as the primary endpoint. Secondary endpoints are time for skin closure, satisfaction with the cosmetic outcome 30 days after surgery, prolongation of hospital stay, and duration of sick‐leave due to surgical site infections. |
| Starting date | Enrolment started on 4th March 2013. |
| Contact information | No reply after trying to contact the author at the available email addresses |
| Notes | Only trial registration available. Study probably completed but not yet published |
NCT01996917.
| Trial name or title | Use of Prineo in breast reduction surgery (Prineo) |
| Methods | Parallel randomised controlled trial |
| Participants | Patients undergoing breast reduction surgery |
| Interventions | Prineo versus subcuticular sutures for final skin closure |
| Outcomes | Primary outcome measures: operative time to closure of final skin layer Secondary outcome measures: score on Patient Observer Scar Assessment Scale (POSAS) up to 1 year Other outcome measures: score on the Vancouver Scar Scale (VSS) up to 1 year |
| Starting date | August 2014 |
| Contact information | |
| Notes | Only trial registration available. Study probably completed but not yet published |
NCT02046239.
| Trial name or title | Suture vs staples for skin closure after liver resection |
| Methods | Parallel randomised controlled trial |
| Participants | Patients undergoing liver surgery |
| Interventions | Subcuticular sutures vs staples |
| Outcomes | Primary outcome measures: postoperative infection time (time frame: 1 year) Secondary outcome measures: time taken for skin closure (time frame: 12 months) |
| Starting date | March 2013 |
| Contact information | No reply after trying to contact the author at the available email addresses |
| Notes | Only trial registration available. Study probably completed but not yet published |
NCT02551510.
| Trial name or title | Comparison of skin adhesive to subcuticular suture wound closure after port placement (PWC) |
| Methods | Parallel randomised controlled trial |
| Participants | Patients who undergo a subcutaneous venous port implant procedure |
| Interventions | Topical skin adhesive Histoacryl® Flexible (n‐Butyl‐2‐Cyanoacrylate Monomer) versus subcuticular sutures for skin closure |
| Outcomes | Primary outcome measures: cosmetic outcome after wound healing at 8 weeks: wound assessment by 3 independent evaluators (surgeons) and patient himself based on photo documentation Cosmetic outcome after wound healing: patient and observer scar assessment scale (POSAS) Cosmetic outcome after wound healing: life quality questionnaire EQ5D Secondary outcome measures: infection rate; assessment of adverse events through AE documentation based on the adverse impact of surgical site infections (German: CDC Kriterien); time to wound closure |
| Starting date | August 2015 |
| Contact information | |
| Notes | This study is currently recruiting participants. Completion expected by November 2017. |
NCT02936063.
| Trial name or title | Outcomes comparing different methods of skin closure in patients undergoing head and neck surgery |
| Methods | Parallel randomised controlled trial |
| Participants | Patients undergoing head and neck surgery |
| Interventions | Skin staples versus subcuticular sutures for skin closure |
| Outcomes | Primary outcome measures: Patient and Observer Scar Assessment Survey (POSAS) at 2 years postoperative; survey score ‐ to assess for scar cosmesis outcome Secondary outcome measures: patient satisfaction survey at 2 years postoperative Wound dehiscence at 2 years postoperative Wound infection at 2 years postoperative |
| Starting date | November 2016 |
| Contact information | |
| Notes | Completion expected by August 2018. But the study was withdrawn because principal investigator left the institution before any participants were enrolled. |
NCT03108742.
| Trial name or title | Randomized control study of dermal staples vs subcuticular sutures on postoperative scar after thyroidectomy |
| Methods | Parallel randomised controlled trial |
| Participants | Patient who will have thyroid surgery |
| Interventions | INSORB® (absorbable staple) versus classic intradermal suture |
| Outcomes | Evaluate scar and pains at 6 months |
| Starting date | October 1, 2016 |
| Contact information | |
| Notes | The study was completed, and final data are being collected (January 28, 2019). |
NCT03788239.
| Trial name or title | Wound closure after total knee replacement |
| Methods | Parallel randomised controlled trial |
| Participants | Patient undergoing primary total knee replacements for osteoarthritis or post‐traumatic arthritis (diagnosed on X‐rays and history) Bilateral knee surgeries |
| Interventions | Right (left) knee wound closure by staples and Left (right) knee wound closure by sutures |
| Outcomes | Primary outcome measures: Wound healing [Time frame: surgery till 1 year postop] (wound healing will be assessed using Hollander Score) Complications [Time frame: surgery till 1 year postop] |
| Starting date | December 2018 |
| Contact information | |
| Notes | Recruiting |
UMIN000002873.
| Trial name or title | A randomised comparison between dermal suture with synthetic absorbable sutures and skin staplers |
| Methods | Parallel randomised controlled trial |
| Participants | Patients with oesophageal disease, gastric disease, or colon disease |
| Interventions | Sutures with synthetic absorbable sutures or skin staplers |
| Outcomes | Primary: degree of wound pain Secondary: incidence of surgical site infection |
| Starting date | December 2009 |
| Contact information | We attempted to contact the author in August 2017 at the available email addresses. |
| Notes | Enrolling by invitation |
UMIN000003235.
| Trial name or title | Influence of surgical site infection in hepato‐biliary‐pancreatic diseases |
| Methods | Parallel randomised controlled trial |
| Participants | Surgery: hepato‐biliary‐pancreatic diseases |
| Interventions | Interrupted subcuticular sutures versus skin staples |
| Outcomes | Primary: incidence of surgical site infection Secondary: incidence of surgical site complication and participants satisfaction |
| Starting date | November 2009 |
| Contact information | No reply after trying to contact the author at the available email addresses |
| Notes | Only trial registration available. Study probably completed but not yet published. |
AE: adverse event
CDC: Centers for Disease Control and Prevention
EQ5D: EuroQol 5 Dimension
POSAS: Patient and Observer Scar Assessment Scale
PSQ: Patient Satisfaction Questionnaire
PWC: port wound closure
SSI: surgical site infection
VAS: Visual Analogue Scale
VSS: Vancouver Scar Scale
Differences between protocol and review
In several studies, all participants in the intervention group had tissue adhesives (Dermabond) placed. We excluded these studies as a post hoc decision because they assessed a mixture of subcuticular sutures and tissue adhesives within the same intervention group.
We added a secondary outcome 'wound dehiscence' as a post hoc decision and have presented this outcome in 'Summary of findings' tables because, contrary to our expectations, many studies reported this outcome separately and we considered it clinically relevant. We removed 'Quality of life' from the 'Summary of findings' tables so that we adhered to guidance on the number of outcomes to include in these tables and prioritised patient satisfaction, which is a straightforward patient‐reported outcome measure (quality of life was reported in the only one small study).
Several studies had potential unit of analysis issues that we had not expected. Thus, we performed the post hoc sensitivity analyses excluding these studies.
Contributions of authors
Saori Goto: conceived the review; designed the review; coordinated the review; extracted data; checked quality of data extraction; analysed or interpreted data; undertook quality assessment; checked quality assessment; performed statistical analysis; produced the first draft of the review; contributed to writing or editing the review; wrote to study author/experts/companies; provided data; approved final review prior to submission; is a guarantor of the review.
Takashi Sakamoto: extracted data; checked quality of data extraction; analysed or interpreted data; undertook quality assessment; checked quality assessment; performed statistical analysis; contributed to writing or editing the review; approved final review prior to submission.
Riki Ganeko: extracted data; checked quality of data extraction; analysed or interpreted data; undertook quality assessment; checked quality assessment; contributed to writing or editing the review; approved final review prior to submission.
Koya Hida: designed the review; extracted data; analysed or interpreted data; checked quality of statistical analysis; contributed to writing or editing the review; advised on the review; approved final review prior to submission.
Toshi Furukawa: commented on all methodological content; designed the review; coordinated the review; checked quality of data extraction; analysed or interpreted data; checked quality assessment; checked quality of statistical analysis; contributed to writing or editing the review; advised on the review; approved final review prior to submission.
Yoshiharu Sakai: commented on all surgical content; analysed or interpreted data; contributed to writing or editing the review; advised on the review; secured funding; approved final review prior to submission; is a guarantor of the review.
Contributions of the editorial base
Gill Norman (Editor): edited the review; advised on methodology, interpretation and review content; approved the final review prior to submission.
Gill Rizzello (Managing Editor): coordinated the editorial process; advised on content; edited the protocol and the review.
Naomi Shaw and Sophie Bishop (Information Specialists): designed the search strategy, ran the searches and edited the search methods section.
Ursula Gonthier and Tom Patterson (Editorial Assistants): edited the reference sections of the review.
Sources of support
Internal sources
No sources of support supplied
External sources
This project was supported by the National Institute for Health Research via Cochrane Infrastructure funding to Cochrane Wounds. The views and opinions expressed are those of the authors and do not necessarily reflect those of the NIHR, NHS or the Department of Health and Social Care, UK.
Declarations of interest
Saori Goto: none known.
Takahashi Sakamoto: none known.
Riki Ganeko: none known.
Koya Hida: received grants for research into the treatment of colorectal cancer from the Japanese Foundation for Research and Promotion of Endoscopy, the Mitsubishi Foundation and the Kondo Memorial Foundation in 2017 and 2018.
Toshi Furukawa: receives consultancy fees from Sekisui Chemicals for advising psychologists. He received lecture fees from Meiji, MitsubishiTanabe Pharma, MSD, Pfizer and Shionogi Inc in 2017, 2018 and 2019. He received royalties from Igaku‐Shoin and Nihon Bunka Kagaku‐sha publishers from 2016 to 2018. He has a patent pending for using AI in smart phone cognitive therapy apps. Kyoto University received a grant from MitsubishiTanabe Pharma for research into cognitive behavioural therapy in 2018.
Yoshiharu Sakai: received lecture fees from Olympus, Johnson & Johnson, Covidien Japan Inc, Stryker Japan, Chugai, Taiho Pharmaceutical, Takeda Pharmaceutical, Tsumura & Co and Terumo in 2017, 2018 and 2019. Kyoto University received grants or research support from Tsumura & Co, Daiichi Sankyo, Chugai, Taiho Pharmaceutical, Yakult, Shionogi Inc, Otsuka, and Sanofi in 2017 and 2018.
New
References
References to studies included in this review
Ademuyiwa 2009 {published data only}
- Ademuyiwa AO, Sowande OA, Adejuyigbe O, Usang UE, Bakare TI, Anyanwu LJ. Evaluation of cosmetic appearance of herniotomy wound scars in African children: comparison of tissue glue and subcuticular suturing. Indian Journal of Plastic Surgery 2009;42(2):199‐203. [DOI] [PMC free article] [PubMed] [Google Scholar]
Anatol 1997 {published data only}
- Anatol TI, Roopchand R, Holder Y, Shing‐Hon G. A comparison of the use of plain catgut, skin tapes and polyglactin sutures for skin closure: a prospective clinical trial. Journal of the Royal College of Surgeons of Edinburgh 1997;42(2):124‐7. [PubMed] [Google Scholar]
Andrade 2016 {published data only}
- Andrade LA, Munoz FY, Baez MV, Collazos SS, Angeles Martinez Ferretiz M, Ruiz B, et al. Appendectomy skin closure technique, randomized controlled trial: changing paradigms (ASC). World Journal of Surgery 2016;40:2603‐10. [DOI] [PubMed] [Google Scholar]
Baek 2009 {published data only}
- Baek Hansen T, Kirkeby L, Fisker H, Larsen K. Randomised controlled study of two different techniques of skin suture in endoscopic release of carpal tunnel. Scandinavian Journal of Plastic and Reconstructive Surgery and Hand Surgery 2009;43(6):335‐8. [DOI] [PubMed] [Google Scholar]
Barker 1984 {published data only}
- Barker P. Breast biopsy: long term follow up of three methods of skin closure. Annals of the Royal College of Surgeons of England 1984;66(5):367‐8. [PMC free article] [PubMed] [Google Scholar]
Brown 2009 {published data only}
- Brown JK, Campbell BT, Drongowski RA, Alderman AK, Geiger JD, Teitelbaum DH, et al. A prospective, randomized comparison of skin adhesive and subcuticular suture for closure of pediatric hernia incisions: cost and cosmetic considerations. Journal of Pediatric Surgery 2009;44(7):1418‐22. [DOI] [PubMed] [Google Scholar]
Buchweitz 2005 {published data only}
- Buchweitz O, Wülfing P, Kiesel L. A prospective randomized trial of closing laparoscopic trocar wounds by transcutaneous versus subcuticular suture or adhesive papertape. Surgical Endoscopy 2005;19(1):148‐51. [DOI] [PubMed] [Google Scholar]
Chen 2013 {published data only}
- Chen C‐J, Xu J‐F, Chen Y‐G, Dong J, Gui D‐M, Zhang DD. Comparison of different suture procedures for closure of skin incision in performing cardiac pacemaker implantation. Journal of Interventional Radiology 2013;22(8):625‐8. [Google Scholar]
Chen 2018 {published data only}
- Chen ZS, Zhu SL, Qi LN, Li LQ. A combination of subcuticular suture and enhanced recovery after surgery reduces wound complications in patients undergoing hepatectomy for hepatocellular carcinoma. Scientific Reports 2018;8:12942. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chughtai 2000 {published data only}
- Chughtai T, Chen L‐Q, Salasidis G, Nguyen D, Tchervenkov C, Morin JF. Clips versus suture technique: is there a difference?. Canadian Journal of Cardiology 2000;16(11):1403‐7. [PubMed] [Google Scholar]
Clough 1975 {published data only}
- Arabi Y, Alexander‐Williams J. Hypertrophic scarring after subcuticular polyglycolic‐acid suture. Lancet 1978;1(8066):724. [DOI] [PubMed] [Google Scholar]
- Clough JV, Alexander‐Williams J. Surgical and economic advantages of polyglycolic‐acid suture material in skin closure. Lancet 1975;1(7900):194‐5. [DOI] [PubMed] [Google Scholar]
Corder 1991 {published data only}
- Corder AP, Schache DJ, Farquharson SM, Tristram S. Wound infection following high saphenous ligation. A trial comparing two skin closure techniques: subcuticular polyglycolic acid and interrupted monofilament nylon mattress sutures. Journal of the Royal College of Surgeons of Edinburgh 1991;36(2):100‐2. [PubMed] [Google Scholar]
Eggers 2011 {published data only}
- Eggers MD, Fang L, Lionberger DR. A comparison of wound closure techniques for total knee arthroplasty. Journal of Arthroplasty 2011;26(8):1251‐8. [DOI] [PubMed] [Google Scholar]
Fiennes 1985 {published data only}
- Fiennes AG. Interrupted subcuticular polyglactin sutures for abdominal wounds. Annals of The Royal College of Surgeons of England 1985;67(2):121. [PMC free article] [PubMed] [Google Scholar]
Foster 1977 {published data only}
- Foster GE, Hardy EG, Hardcastle JD. Subcuticular suturing after appendicectomy. Lancet 1977;1(8022):1128‐9. [DOI] [PubMed] [Google Scholar]
Gennari 2004 {published data only}
- Gennari R, Rotmensz N, Ballardini B, Scevola S, Perego E, Zanini V, et al. A prospective, randomized, controlled clinical trial of tissue adhesive (2‐octylcyanoacrylate) versus standard wound closure in breast surgery. Surgery 2004;136(3):593‐9. [DOI] [PubMed] [Google Scholar]
Ghaderi 2010 {published data only}
- Ghaderi H, Shamimi K, Moazzami F, Razavi SH, Aminian A, Jalali SM. A new look at an old dogma: wound complications in two methods of skin closure in uncomplicated appendicitis. Tehran University Medical Journal 2010;68(1):54‐8. [Google Scholar]
Grottkau 2010 {published data only}
- Grottkau BE, Rebello G, Merlin G, Winograd JM. Coaptive film versus subcuticular suture: comparing skin closure time after posterior spinal instrumented fusion in pediatric patients with spinal deformity. Spine 2010;35(23):2027‐9. [DOI] [PubMed] [Google Scholar]
Hopkinson 1982 {published data only}
- Hopkinson GB, Bullen BR. Removable subcuticular skin suture in acute appendicitis: a prospective comparative clinical trial. British Medical Journal 1982;284(6319):869. [DOI] [PMC free article] [PubMed] [Google Scholar]
Imamura 2016 {published data only}
- Imamura K, Adachi K, Sasaki R, Monma S, Shioiri S, Seyama Y, et al. Randomized comparison of subcuticular sutures versus staples for skin closure after open abdominal surgery: a multicenter open‐label randomized controlled trial. Journal of Gastrointestinal Surgery 2016;20:2083‐92. [DOI] [PubMed] [Google Scholar]
Jallali 2004 {published data only}
- Jallali N, Haji A, Watson CJ. A prospective randomized trial comparing 2‐octyl cyanoacrylate to conventional suturing in closure of laparoscopic cholecystectomy incisions. Journal of Laparoendoscopic & Advanced Surgical Techniques. Part A 2004;0(4):209‐11. [DOI] [PubMed] [Google Scholar]
Jan 2013 {published data only}
- Jan H, Waters N, Haines P, Kent A. LiquiBand Surgical S topical adhesive versus sutures for the closure of laparoscopic wounds. A randomized controlled trial. Gynecological Surgery 2013;10(4):247‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Javadi 2018 {published data only}
- Javadi SM, Kasraianfard A, Ghaderzadeh P, Khorshidi HR, Moein A, Makarchian HR, et al. Comparison of subcuticular and interrupted suturing methods for skin closure after appendectomy: a randomized controlled trial. Iranian Red Crescent Medical Journal 2018;20:e14469. [Google Scholar]
Karabay 2005 {published data only}
- Karabay O, Fermanci E, Silistreli E, Aykut K, Yurekli I, Catalyurek H, et al. Intracutaneous versus transcutaneous suture techniques: comparison of sternal wound infection rates in open‐heart surgery patients. Texas Heart Institute Journal 2005;32(3):277‐82. [PMC free article] [PubMed] [Google Scholar]
Keng 1989 {published data only}
- Keng TM, Bucknall TE. A clinical trial of tissue adhesive (histoacryl) in skin closure of groin wounds. Medical Journal of Malaysia 1989;44(2):122‐8. [PubMed] [Google Scholar]
Khajouei 2007 {published data only}
- Khajouei Kermani H, Afsharfard A, Zeynalzadeh M, Najafbeigi A, Yavari P, Kalantar Motamedi MR. Cosmetic surgical repair of contaminated wounds versus traditional loose approximation: does it increase the rate of wound infections?. Medical Journal of the Islamic Republic of Iran 2007;20(4):158‐60. [Google Scholar]
Khan 2006 {published data only}
- Khan RJ, Fick D, Yao F, Tang K, Hurworth M, Nivbrant B, et al. A comparison of three methods of wound closure following arthroplasty: a prospective, randomised, controlled trial. Journal of Bone and Joint Surgery. British Volume 2006;88(2):238‐42. [DOI] [PubMed] [Google Scholar]
Kobayashi 2015 {published and unpublished data}
- Kobayashi S, Ito M, Yamamoto S, Kinugasa Y, Kotake M, Saida Y, et al. Randomized clinical trial of skin closure by subcuticular suture or skin stapling after elective colorectal cancer surgery. British Journal of Surgery 2015;102(5):495‐500. [DOI] [PubMed] [Google Scholar]
- Yamaguchi T, Kotake M, Saito N, Moriya Y, Kusachi S, Kubo Y, et al. A randomized phase III trial of skin closure by subcuticular suture versus skin stapler to prevent incisional surgical site infection after elective colorectal cancer surgery: results of the subcuticular suture against infection (SSI) study. Journal of the American College of Surgeons 2014;219(4 (Suppl)):e7. [Google Scholar]
Kotaluoto 2012 {published data only}
- Koskela A, Kotaluoto S, Kaartinen I, Pauniaho S‐L, Rantanen T, Kuokkanen H. Continuous absorbable intradermal sutures yield better cosmetic results than nonabsorbable interrupted sutures in open appendectomy wounds: a prospective, randomized trial. World Journal of Surgery 2014;38(5):1044‐50. [DOI] [PubMed] [Google Scholar]
- Kotaluoto S, Pauniaho SL, Helminen M, Kuokkanen H, Rantanen T. Wound healing after open appendectomies in adult patients: a prospective, randomised trial comparing two methods of wound closure. World Journal of Surgery 2012;36(10):2305‐10. [DOI] [PubMed] [Google Scholar]
Krishnamoorthy 2009 {published data only}
- Krishnamoorthy B, Najam O, Khan UA, Waterworth P, Fildes JE, Yonan N. Randomized prospective study comparing conventional subcuticular skin closure with Dermabond skin glue after saphenous vein harvesting. Annals of Thoracic Surgery 2009;88(5):1445‐9. [DOI] [PubMed] [Google Scholar]
Kuroki 2017 {published data only}
- Kuroki LM, Mullen MM, Massad LS, Wu N, Liu J, Mutch DG, et al. Wound complication rates after staples or suture for midline vertical skin closure in obese women: a randomized controlled trial. Obstetrics and Gynecology 2017;130(1):91‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroki LM, Mullen MM, Massad LS, Wu N, Liu J, Powell MA, et al. A randomized controlled trial comparing skin closure in obese gynecologic patients: staples vs monofilament suture. Gynecologic Oncology 2017;145(Suppl 1):54‐5. [Google Scholar]
Lazar 2011 {published data only}
- Lazar HL, McCann J, Fitzgerald CA, Cabral HJ. Adhesive strips versus subcuticular suture for median sternotomy wound closure. Journal of Cardiac Surgery 2011;26(4):344‐7. [DOI] [PubMed] [Google Scholar]
Liu 2017 {published data only}
- Liu X, Nelemans PJ, Frenk LD, Sengers H, Tuinder SM, Steijlen PM, et al. Aesthetic outcome and complications of simple interrupted versus running subcuticular sutures in facial surgery: a randomized controlled trial. Journal of the American Academy of Dermatology 2017;77(5):911‐9. [DOI] [PubMed] [Google Scholar]
Maartense 2002 {published data only}
- Maartense S, Bemelman WA, Dunker MS, Lint C, Pierik E, Busch OR, et al. Randomized study of the effectiveness of closing laparoscopic trocar wounds with octylcyanoacrylate, adhesive papertape or poliglecaprone. British Journal of Surgery 2002;89(11):1370‐5. [DOI] [PubMed] [Google Scholar]
Martin 2017 {published data only}
- Martin J, Hollenbeck S, Makar R, Pabon‐Ramos W, Suhocki P, Miller M, et al. Randomized controlled trial of skin adhesive versus subcuticular suture for skin closure after implantable venous port placement. Journal of Vascular & Interventional Radiology 2016;27(3):S198. [DOI] [PubMed] [Google Scholar]
- Martin JG, Hollenbeck ST, Janas G, Makar RA, Pabon‐Ramos WM, Suhocki PV, et al. Randomized controlled trial of octyl cyanoacrylate skin adhesive versus subcuticular suture for skin closure after implantable venous port placement. Journal of Vascular & Interventional Radiology 2017;28:111‐6. [DOI] [PubMed] [Google Scholar]
McGreal 2002 {published data only}
- McGreal GT, Joy A, Manning B, Kelly JL, O'Donnell JA, Kirwan WW, et al. Antiseptic wick: does it reduce the incidence of wound infection following appendectomy?. World Journal of Surgery 2002;26(5):631‐4. [DOI] [PubMed] [Google Scholar]
Mullen 1999 {published data only}
- Mullen JC, Bentley MJ, Mong K, Karmy‐Jones R, Lemermeyer G, Gelfand ET, et al. Reduction of leg wound infections following coronary artery bypass surgery. Canadian Journal of Cardiology 1999;15(1):65‐8. [PubMed] [Google Scholar]
Murphy 1995 {published data only}
- Murphy PG, Tadros E, Cross S, Hehir D, Burke PE, Kent P, et al. Skin closure and the incidence of groin wound infection: a prospective study. Annals of Vascular Surgery 1995;9(5):480‐2. [DOI] [PubMed] [Google Scholar]
O'Leary 2013 {published data only}
- O'Leary DP, Clover AJ, Galbraith JG, Mushtaq M, Shafiq A, Redmond HP. Adhesive strip wound closure after thyroidectomy/parathyroidectomy: a prospective, randomized controlled trial. Surgery 2013;153(3):408‐12. [DOI] [PubMed] [Google Scholar]
Obermair 2007 {published data only}
- Obermair A, Crandon A, Perrin L, Walsh T, Carrazo M, Nicklin J. Randomized trial of skin closure after laparotomy for gynaecological surgery. Australian and New Zealand Journal of Surgery 2007;77(6):460‐3. [DOI] [PubMed] [Google Scholar]
Ong 2002 {published data only}
- Ong CC, Jacobsen AS, Joseph VT. Comparing wound closure using tissue glue versus subcuticular suture for pediatric surgical incisions: a prospective, randomised trial. Paediatric Surgery International 2002;18(5):553‐5. [DOI] [PubMed] [Google Scholar]
Onwuanyi 1990 {published data only}
- Onwuanyi ON, Evbuomwan I. Skin closure during appendicectomy: a controlled clinical trial of subcuticular and interrupted transdermal suture techniques. Journal of the Royal College of Surgeons of Edinburgh 1990;35(6):353‐5. [PubMed] [Google Scholar]
Pauniaho 2010 {published data only}
- Pauniaho S‐L, Lahdes‐Vasama T, Helminen MT, Iber T, Mäkelä E, Pajulo O. Non‐absorbable interrupted versus absorbable continuous skin closure in pediatric appendectomies. Scandinavian Journal of Surgery 2010;99(3):142‐6. [DOI] [PubMed] [Google Scholar]
Pitcher 1983 {published data only}
- Pitcher D. Sutureless skin closure for pacemaker implantation: comparison with subcuticular suture. Postgraduate Medical Journal 1983;59(688):83‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ranaboldo 1992 {published data only}
- Ranaboldo CJ, Rowe‐Jones DC. Closure of laparotomy wounds: skin staples versus sutures. British Journal of Surgery 1992;79(11):1172‐3. [DOI] [PubMed] [Google Scholar]
Rebello 2009 {published data only}
- Rebello G, Parikh R, Grottkau B. Coaptive film versus subcuticular suture: comparing skin closure time following identical, single‐session, bilateral limb surgery in children. Journal of Pediatric Orthopaedics 2009;29(6):626‐8. [DOI] [PubMed] [Google Scholar]
Reed 1997 {published data only}
- Krukowski ZH, Matheson NA. Prospective randomized trial of clips versus subcuticular polydioxanone for neck wound closure. British Journal of Surgery 1997;84(7):1027. [DOI] [PubMed] [Google Scholar]
- Reed MR, Lennard TW. Prospective randomized trial of clips versus subcuticular polydioxanone for neck wound closure. British Journal of Surgery 1997;84(1):118. [DOI] [PubMed] [Google Scholar]
Roolker 2002 {published data only}
- Roolker W, Kraaneveld E, Been HD, Marti RK. Results of a prospective randomised study comparing a non‐invasive surgical zipper versus intracutaneous sutures for wound closure. Archives of Orthopaedic and Trauma Surgery 2002;122(1):2‐4. [DOI] [PubMed] [Google Scholar]
Rosen 1997 {published data only}
- Rosen DM, Carlton MA. Skin closure at laparoscopy. Journal of the American Association of Gynecologic Laparoscopists 1997;4(3):347‐51. [DOI] [PubMed] [Google Scholar]
Sakka 1995 {published data only}
- Sakka S, Graham K, Abdula A. Wound closure in hip surgery. Comparative study of subcuticular vs interrupted suture. A randomised prospective study. Journal of Bone and Joint Surgery. British Volume 1995;77:232. [Google Scholar]
- Sakka SA, Graham K, Abdulah A. Skin closure in hip surgery: subcuticular versus transdermal. A prospective randomized study. Acta Orthopaedica Belgica 1995;61(4):331‐6. [PubMed] [Google Scholar]
Sebesta 2004 {published data only}
- Sebesta MJ, Bishoff JT. Octylcyanoacrylate skin closure in laparoscopy. Journal of the Society of Laparoendoscopic Surgeons 2004;8(1):9‐14. [PMC free article] [PubMed] [Google Scholar]
Selvadurai 1997 {published data only}
- Porter GC, Jayaraj SM, Patel KS. Randomised trial of subcuticular suture versus metal clips for wound closure after thyroid and parathyroid surgery. Annals of the Royal College of Surgeons of England 1997;79(6):469. [PMC free article] [PubMed] [Google Scholar]
- Selvadurai D, Wildin C, Treharne G, Choksy SA, Heywood MM, Nicholson ML. Randomised trial of subcuticular suture versus metal clips for wound closure after thyroid and parathyroid surgery. Annals of the Royal College of Surgeons of England 1997;79(4):303‐6. [PMC free article] [PubMed] [Google Scholar]
Shetty 2004 {published data only}
- Shetty AA, Kumar VS, Morgan‐Hough C, Georgeu GA, James KD, Nicholl JE. Comparing wound complication rates following closure of hip wounds with metallic skin staples or subcuticular vicryl suture: a prospective randomised trial. Journal of Orthopaedic Surgery 2004;12(2):191‐3. [DOI] [PubMed] [Google Scholar]
Simpson 1979 {published data only}
- Simpson JE, Ornstein M, Spicer CC, Cox AG. Hypertrophic scarring: dexon suture in a randomized trial. British Journal of Surgery 1979;66(4):281‐2. [DOI] [PubMed] [Google Scholar]
Soni 2013 {published data only}
- Soni A, Narula R, Kumar A, Parmar M, Sahore M, Chandel M. Comparing cyanoacrylate tissue adhesive and conventional subcuticular skin sutures for maxillofacial incisions: a prospective randomized trial considering closure time, wound morbidity, and cosmetic outcome. Journal of Oral and Maxillofacial Surgery 2013;71(12):2152. [DOI] [PubMed] [Google Scholar]
Steele 1983 {published data only}
- Steele RJ, Chetty U, Forrest AP. Staples or sutures for mastectomy wounds? A randomised trial. Journal of the Royal College of Surgeons of Edinburgh 1983;28(1):17‐8. [PubMed] [Google Scholar]
Subramanian 2005 {published data only}
- Subramanian A, Hayes A, Eze N, Sains P, Jarrett PE. A prospective randomised controlled trial comparing the use of skin staples with subcuticular Prolene suture in inguinal hernia incisions. Journal of One Day Surgery 2005;15(3):61‐3. [Google Scholar]
Swtizer 2003 {published data only}
- Patel B, Sumeray M. Subcuticular closure versus dermabond: a prospective randomized trial. American Surgeon 2004;70(4):369; author reply 370‐1. [PubMed] [Google Scholar]
- Swtizer E, Dinsmore R, North J. Subcuticular closure versus dermabond: a prospective randomised trial. American Surgeon 2003;69:434‐6. [PubMed] [Google Scholar]
Tanaka 2014 {published and unpublished data}
- Tanaka A, Sadahiro S, Suzuki T, Okada K, Saito G. Randomized controlled trial comparing subcuticular absorbable suture with conventional interrupted suture for wound closure at elective operation of colon cancer. Surgery 2014;155(3):486‐92. [DOI] [PubMed] [Google Scholar]
Tanaka 2016 {published data only}
- Tanaka Y, Miyamoto T, Naito Y, Yoshitake S, Sasahara A, Miyaji K. Randomized study of a new noninvasive skin closure device for use after congenital heart operations. Annals of Thoracic Surgery 2016;102:1368‐74. [DOI] [PubMed] [Google Scholar]
Taube 1983 {published data only}
- Taube M, Porter RJ, Lord PH. A combination of subcuticular suture and sterile Micropore tape compared with conventional interrupted sutures for skin closure. A controlled trial. Annals of the Royal College of Surgeons of England 1983;65(3):164‐7. [PMC free article] [PubMed] [Google Scholar]
Teoh 2018 {published data only}
- Teoh LY, Chong SS, Hoh SY, Teoh MS, Ng KL. A comparison of aesthetic outcome between tissue adhesive and subcuticular suture in thyroidectomy wound closure in a multiracial country: a randomized controlled trial. Asian Journal of Surgery 2018;13:13. [DOI] [PubMed] [Google Scholar]
Tsujinaka 2013 {published and unpublished data}
- Tsujinaka T, Yamamoto K, Fujita J, Endo S, Kawada J, Nakahira S, et al. Subcuticular sutures versus staples for skin closure after open gastrointestinal surgery: a phase 3, multicentre, open‐label, randomised controlled trial. Lancet 2013;382(9898):1105‐12. [DOI] [PubMed] [Google Scholar]
- Vo H, Kin C. Randomised controlled trial: study shows insufficient decrease in wound complications with sutured versus stapled skin closure in gastrointestinal operations. Evidence‐Based Medicine 2014;19(3):100. [DOI] [PubMed] [Google Scholar]
Van den Ende 2004 {published data only}
- Ende ED, Vriens PW, Allema JH, Breslau PJ. Adhesive bonds or percutaneous absorbable suture for closure of surgical wounds in children. Results of a prospective randomized trial. Journal of Pediatric Surgery 2004;39(8):1249‐51. [DOI] [PubMed] [Google Scholar]
Xu 2014 {published data only}
- Xu L, Zhu F, Zhu Z, Liu Z, Sun X, Qiao J, et al. Comparison of 2 methods of incision closure in patients with adolescent idiopathic scoliosis undergoing posterior spinal fusion surgery. Spine 2014;39(8):E481‐5. [DOI] [PubMed] [Google Scholar]
Zwart 1989 {published data only}
- Zwart HJ, Ruiter P. Subcuticular, continuous and mechanical skin closure: cosmetic results of a prospective randomized trial. Netherlands Journal of Surgery 1989;41(3):57‐60. [PubMed] [Google Scholar]
References to studies excluded from this review
Alicandri‐Ciufelli 2014 {published data only}
- Alicandri‐Ciufelli M, Piccinini A, Grammatica A, Molteni G, Spaggiari A, Matteo S, et al. Aesthetic comparison between synthetic glue and subcuticular sutures in thyroid and parathyroid surgery: a single‐blinded randomised clinical trial. Acta Otorhinolaryngologica Italica 2014;34:406‐11. [PMC free article] [PubMed] [Google Scholar]
Angelini 1984 {published data only}
- Angelini GD, Butchart EG, Armistead SH, Breckenridge IM. Comparative study of leg wound skin closure in coronary artery bypass graft operations. Thorax 1984;39(12):942‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bernard 2001 {published data only}
- Bernard L, Doyle J, Friedlander SF, Eichenfield LF, Gibbs NF, Cunningham BB. A prospective comparison of octyl cyanoacrylate tissue adhesive (dermabond) and suture for the closure of excisional wounds in children and adolescents. Archives of Dermatology 2001;137(9):1177‐80. [DOI] [PubMed] [Google Scholar]
Bernstein 2001 {published data only}
- Bernstein RM, Rassman WR, Rashid N. A new suture for hair transplantation: poliglecaprone 25. Dermatologic Surgery 2001;27(1):5‐11. [PubMed] [Google Scholar]
Blondeel 2014 {published data only}
- Blondeel PN, Richter D, Stoff A, Exner K, Jernbeck J, Ramakrishnan V. Evaluation of a new skin closure device in surgical incisions associated with breast procedures. Annals of Plastic Surgery 2014;73(6):631‐7. [DOI] [PubMed] [Google Scholar]
Buttaro 2015 {published data only}
- Buttaro MA, Quinteros M, Martorell G, Zanotti G, Comba F, Piccaluga F. Skin staples versus intradermal wound closure following primary hip arthroplasty: a prospective, randomised trial including 231 cases. Hip International 2015;25(6):563‐7. [DOI] [PubMed] [Google Scholar]
Cameron 1987 {published data only}
- Cameron AE, Parker CJ, Field ES, Gray RC, Wyatt AP. A randomised comparison of polydioxanone (PDS) and polypropylene (Prolene) for abdominal wound closure. Annals of the Royal College of Surgeons of England 1987;69(3):113‐5. [PMC free article] [PubMed] [Google Scholar]
Cassie 1988 {published data only}
- Cassie AB, Chatterjee AK, Mehta S, Haworth JM. Pain quantum and wound healing: a comparison of interrupted inversion PDS and standard nylon sutures in abdominal skin closure. Annals of the Royal College of Surgeons of England 1988;70(6):339‐42. [PMC free article] [PubMed] [Google Scholar]
Chan 2017 {published data only}
- Chan VW, Chan PK, Chiu KY, Yan CH, Ng FY. Does barbed suture lower cost and improve outcome in total knee arthroplasty? A randomized controlled trial. Journal of Arthroplasty 2017;32:1474‐7. [DOI] [PubMed] [Google Scholar]
Cheng 1997 {published data only}
- Cheng W, Saing H. A prospective randomized study of wound approximation with tissue glue in circumcision in children. Journal of Paediatrics and Child Health 1997;33(6):515‐6. [DOI] [PubMed] [Google Scholar]
Clayer 1991 {published data only}
- Clayer M, Southwood RT. Comparative study of skin closure in hip surgery. Australian and New Zealand Journal of Surgery 1991;61(5):363‐5. [DOI] [PubMed] [Google Scholar]
Consorti 2013 {published data only}
- Consorti F, Mancuso R, Piccolo A, Pretore E, Antonaci A. Quality of scar after total thyroidectomy: a single blinded randomized trial comparing octyl‐cyanoacrylate and subcuticular absorbable suture. ISRN Surgery 2013;2013:270953. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cordova 2013 {published data only}
- Cordova K, Sweeney S, Jellinek NJ. The elegant ellipse: running subcuticular closures. Dermatologic Surgery 2013;39(5):804‐7. [DOI] [PubMed] [Google Scholar]
Davies 1995 {published data only}
- Davies N, Wheeler RA, Griffiths DM, Burge DM. Opsite skin closure in day case paediatric surgery: is a subcuticular suture necessary?. Journal of The Royal College of Surgeons of Edinburgh 1995;40(6):386‐7. [PubMed] [Google Scholar]
Eldrup 1981 {published data only}
- Eldrup J, Wied U, Andersen B. Randomised trial comparing Proximate stapler with conventional skin closure. Acta Chirurgica Scandinavica 1981;147(7):501‐2. [PubMed] [Google Scholar]
Elliot 1989 {published data only}
- Elliot D, Mahaffey PJ. The stretched scar: the benefit of prolonged dermal support. British Journal of Plastic Surgery 1989;42(1):74‐8. [DOI] [PubMed] [Google Scholar]
Erel 2001 {published data only}
- Erel E, Pleasance PI, Ahmed O, Hart NB. Absorbable versus non‐absorbable suture in carpal tunnel decompression. Journal of Hand Surgery. British and European Volume 2001;26(2):157‐8. [DOI] [PubMed] [Google Scholar]
Gatt 1985 {published data only}
- Gatt D, Quick CR, Owen‐Smith MS. Staples for wound closure: a controlled trial. Annals of the Royal College of Surgeons of England 1985;67(5):318‐20. [PMC free article] [PubMed] [Google Scholar]
Glennie 2017 {published data only}
- Glennie RA, Korczak A, Naudie DD, Bryant DM, Howard JL. Monocryl and Dermabond vs staples in total hip arthroplasty performed through a lateral skin incision: a randomized controlled trial using a patient‐centered assessment tool. Journal of Arthroplasty 2017;24:24. [DOI] [PubMed] [Google Scholar]
Greene 1999 {published data only}
- Greene D, Koch RJ, Goode RL. Efficacy of octyl‐2‐cyanoacrylate tissue glue in blepharoplasty. A prospective controlled study of wound‐healing characteristics. Archives of Facial Plastic Surgery 1999;1(4):292‐6. [DOI] [PubMed] [Google Scholar]
Handschel 2006 {published data only}
- Handschel JG, Depprich RA, Dirksen D, Runte C, Zimmermann A, Kubler NR. A prospective comparison of octyl‐2‐cyanoacrylate and suture in standardized facial wounds. International Journal of Oral and Maxillofacial Surgery 2006;35(4):318‐23. [DOI] [PubMed] [Google Scholar]
Harvey 1986 {published data only}
- Harvey CF, Hume Logan CJ. A prospective trial of skin staples and sutures in skin closure. Irish Journal of Medical Science 1986;155(6):194‐6. [DOI] [PubMed] [Google Scholar]
Johnson 1997 {published data only}
- Johnson RG, Cohn WE, Thurer RL, McCarthy JR, Sirois CA, Weintraub RM. Cutaneous closure after cardiac operations: a controlled, randomized, prospective comparison of intradermal versus staple closures. Annals of Surgery 1997;226(5):606‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kerrigan 2010 {published data only}
- Kerrigan CL, Homa K. Evaluation of a new wound closure device for linear surgical incisions: 3M steri‐strip S surgical skin closure versus subcuticular closure. Plastic and Reconstructive Surgery 2010;125(1):186‐94. [DOI] [PubMed] [Google Scholar]
Kharwadkar 2005 {published data only}
- Kharwadkar N, Naique S, Molitor PJ. Prospective randomized trial comparing absorbable and non‐absorbable sutures in open carpal tunnel release. Journal of Hand Surgery. British and European Volume 2005;30B(1):92‐5. [DOI] [PubMed] [Google Scholar]
Koonce 2015 {published data only}
- Koonce SL, Eck DL, Shaddix KK, Perdikis G. A prospective randomized controlled trial comparing N‐butyl‐2 cyanoacrylate (Histoacryl), octyl cyanoacrylate (Dermabond), and subcuticular suture for closure of surgical incisions. Annals of Plastic Surgery 2015;74(1):107‐10. [DOI] [PubMed] [Google Scholar]
Lalani 2016 {published data only}
- Lalani GG, Schricker AA, Salcedo J, Hebsur S, Hsu J, Feld G, et al. Cardiac device implant skin closure with a novel adjustable, coaptive tape‐based device. Pacing & Clinical Electrophysiology 2016;39:1077‐82. [DOI] [PubMed] [Google Scholar]
Lazar 2008 {published data only}
- Lazar HL, McCann J, Fitzgerald CA, Thompson J, Bao Y, Cabral HJ. Novel adhesive skin closures improve wound healing following saphenous vein harvesting. Journal of Cardiac Surgery 2008;23(2):152‐5. [DOI] [PubMed] [Google Scholar]
Leaper 1985 {published data only}
- Leaper DJ, Allan A, May RE, Corfield AP, Kennedy RH. Abdominal wound closure: a controlled trial of polyamide (nylon) and polydioxanone suture (PDS). Annals of the Royal College of Surgeons of England 1985;67(5):273‐5. [PMC free article] [PubMed] [Google Scholar]
Liang 2015 {published data only}
- Liang X, Han‐Xin Z, Chang‐E J, Chao W, Juan H, Guo‐Quan G. Subcutaneous suture can accelerate wound healing of lower midline incision: a randomized controlled trial. American Surgeon 2015;81(1):23‐30 (8). [PubMed] [Google Scholar]
Lombardi 2011 {published data only}
- Lombardi CP, Bracaglia R, Revelli L, Insalaco C, Pennestri F, Bellantone R, et al. Aesthetic result of thyroidectomy: evaluation of different kinds of skin suture. Annali Italiani di Chirurgia 2011;82(6):449‐56. [PubMed] [Google Scholar]
Matin 2003 {published data only}
- Matin SF. Prospective randomized trial of skin adhesive versus sutures for closure of 217 laparoscopic port‐site incisions. Journal of the American College of Surgeons 2003;196(6):845‐53. [DOI] [PubMed] [Google Scholar]
McLean 1980 {published data only}
- McLean NR, Fyfe AH, Flint EF, Irvine BH, Calvert MH. Comparison of skin closure using continuous and interrupted nylon sutures. British Journal of Surgery 1980;67(9):633‐5. [DOI] [PubMed] [Google Scholar]
Meinke 1996 {published data only}
- Meinke AK. An initial evaluation of subcuticular skin closure with absorbable intradermal pins. Connecticut Medicine 1996;60(4):199‐202. [PubMed] [Google Scholar]
Menovsky 2004 {published data only}
- Menovsky T, Bartels RH, Lindert EL, Grotenhuis JA. Skin closure in carpal tunnel surgery: a prospective comparative study between nylon, polyglactin 910 and stainless steel sutures. Hand Surgery 2004;9(1):35‐8. [DOI] [PubMed] [Google Scholar]
Milone 2014 {published data only}
- Milone M, Musella M, Maietta P, Bianco P, Taffuri C, Salvatore G, et al. Intradermal absorbable sutures to close pilonidal sinus wounds: a safe closure method?. Surgery Today 2014;44(9):1638‐42. [DOI] [PubMed] [Google Scholar]
Mudd 2013 {published data only}
- Mudd CD, Boudreau JA, Moed BR. A prospective randomized comparison of two skin closure techniques in acetabular fracture surgery. Journal of Orthopaedics and Traumatology 2013;15(3):189‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nahas 2004 {published data only}
- Nahas FX, Solia D, Ferreira LM, Novo NF. The use of tissue adhesive for skin closure in body contouring surgery. Aesthetic Plastic Surgery 2004;28(3):165‐9. [DOI] [PubMed] [Google Scholar]
Nair 1988 {published data only}
- Nair UR, Griffiths G, Lawson RA. Postoperative neuralgia in the leg after saphenous vein coronary artery bypass graft: a prospective study. Thorax 1988;43(1):41‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Navali 2014 {published data only}
- Navali AM, Tabrizi A. Comparison of three skin closure methods in knee mid‐anterior incisions. Archives of Bone & Joint Surgery 2014;2(2):98‐102. [PMC free article] [PubMed] [Google Scholar]
Nipshagen 2008 {published data only}
- Nipshagen MD, Hage JJ, Beekman WH. Use of 2‐octyl‐cyanoacrylate skin adhesive (Dermabond) for wound closure following reduction mammaplasty: a prospective, randomized intervention study. Plastic and Reconstructive Surgery 2008;122(1):10‐8. [DOI] [PubMed] [Google Scholar]
Park 2015 {published data only}
- Park SY, Kim KH, Yuk J‐S, Ji HY, Lee JH. Skin closure methods after single port laparoscopic surgery: a randomized clinical trial. European Journal of Obstetrics, Gynecology, and Reproductive Biology 2015;189:8‐12. [DOI] [PubMed] [Google Scholar]
Parvizi 2013 {published data only}
- Parvizi D, Friedl H, Schintler MV, Rappl T, Laback C, Wiedner M, et al. Use of 2‐octyl cyanoacrylate together with a self‐adhering mesh (DermabondTM PrineoTM) for skin closure following abdominoplasty: an open, prospective, controlled, randomized, clinical study. Aesthetic Plastic Surgery 2013;37(3):529‐37. [DOI] [PubMed] [Google Scholar]
Pickford 1983 {published data only}
- Pickford IR, Brennan SS, Evans M, Pollock AV. Two methods of skin closure in abdominal operations: a controlled clinical trial. British Journal of Surgery 1983;70(4):226‐8. [DOI] [PubMed] [Google Scholar]
Plotner 2011 {published data only}
- Plotner AN, Mailler‐Savage E, Adams B, Gloster HM Jr. Layered closure versus buried sutures and adhesive strips for cheek defect repair after cutaneous malignancy excision. Journal of the American Academy of Dermatology 2011;64(6):1115‐8. [DOI] [PubMed] [Google Scholar]
Ralphs 1982 {published data only}
- Ralphs DN, Cannon SR, Bolton JP. Skin closure of inguinal herniorrhaphy wounds in short‐stay patients. British Journal of Surgery 1982;69(6):341‐2. [DOI] [PubMed] [Google Scholar]
Richter 2012 {published data only}
- Richter D, Stoff A, Ramakrishnan V, Exner K, Jernbeck J, Blondeel PN. A comparison of a new skin closure device and intradermal sutures in the closure of full‐thickness surgical incisions. Plastic and Reconstructive Surgery 2012;130(4):843‐50. [DOI] [PubMed] [Google Scholar]
Ries 2016 {published data only}
- Ries MD. CORR Insights®: The Chitranjan Ranawat Award: running subcuticular closure enables the most robust perfusion after TKA: a randomized clinical trial. Clinical Orthopaedics and Related Research 2016;474(1):57‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Risnes 2001 {published data only}
- Risnes I, Abdelnoor M, Baksaas ST, Lundblad R, Svennevig JL. Sternal wound infections in patients undergoing open heart surgery: randomized study comparing intracutaneous and transcutaneous suture techniques. Annals of Thoracic Surgery 2001;72(5):1587‐91. [DOI] [PubMed] [Google Scholar]
Rizvi 2018 {published data only}
- Rizvi SAR, Sabah M, Saquib M, Mahmood F, Alam MS. A prospective comparative study between subcuticular and sutureless technique of skin closure following external dacryocystorhinostomy. Ophthalmic Plastic & Reconstructive Surgery 2018;07:07. [DOI] [PubMed] [Google Scholar]
Rui 2017 {published data only}
- Rui M, Zheng X, Sun SS, Li CY, Zhang XC, Guo KJ, et al. A prospective randomised comparison of 2 skin closure techniques in primary total hip arthroplasty surgery. Hip International 2017;28(1):101‐5. [DOI] [PubMed] [Google Scholar]
Sadick 1994 {published data only}
- Sadick NS, D'Amelio DL, Weinstein C. The modified buried vertical mattress suture. A new technique of buried absorbable wound closure associated with excellent cosmesis for wounds under tension. Journal of Dermatologic Surgery and Oncology 1994;20(11):735‐9. [DOI] [PubMed] [Google Scholar]
Selo‐Ojeme 2002 {published data only}
- Selo‐Ojeme DO, Lim KB. Randomised clinical trial of suture compared with adhesive strip for skin closure after HRT implant. British Journal of Obstetrics and Gynaecology 2002;109(10):1178‐80. [DOI] [PubMed] [Google Scholar]
Serour 1996 {published data only}
- Serour F, Efrati Y, Klin B, Barr J, Gorenstein A, Vinograd I. Subcuticular skin closure as a standard approach to emergency appendectomy in children: prospective clinical trial. World Journal of Surgery 1996;20(1):38‐42. [DOI] [PubMed] [Google Scholar]
Shamiyeh 2001 {published data only}
- Shamiyeh A, Schrenk P, Stelzer T, Wayand WU. Prospective randomized blind controlled trial comparing sutures, tape, and octylcyanoacrylate tissue adhesive for skin closure after phlebectomy. Dermatologic Surgery 2001;27(10):877‐80. [DOI] [PubMed] [Google Scholar]
Shanahan 1990 {published data only}
- Shanahan D, Chester J, Southam JA, Lord PH. Micropore tape dressing: a cheap, effective alternative dressing with subcuticular sutures. Annals of the Royal College of Surgeons of England 1990;72(3):206. [PMC free article] [PubMed] [Google Scholar]
Singh 2006 {published data only}
- Singh B, Mowbray M, Nunn G, Mearns S. Closure of hip wound ‐ clips or subcuticular sutures?. British Orthopaedic Association Annual Congress; 2006 September 26‐29; Glasgow (UK). 2006. [DOI] [PubMed]
- Singh B, Mowbray MA, Nunn G, Mearns S. Closure of hip wound, clips or subcuticular sutures: does it make a difference?. European Journal of Orthopaedic Surgery & Traumatology 2006;16(2):124‐9. [DOI] [PubMed] [Google Scholar]
Sinha 2001 {published data only}
- Sinha S, Naik M, Wright V, Timmons J, Campbell AC. A single blind, prospective, randomized trial comparing n‐butyl 2‐cyanoacrylate tissue adhesive (Indermil) and sutures for skin closure in hand surgery. Journal of Hand Surgery 2001;26(3):264‐5. [DOI] [PubMed] [Google Scholar]
Szabó 2002 {published data only}
- Szabó S, István G. Skin closure in inguinal hernia repair with rapidly absorbing Polyglactin 910/370 (Vicryl‐Rapide) suture material. Magyar Sebeszet 2002;55(2):77‐80. [PubMed] [Google Scholar]
Van de Gevel 2010 {published data only}
- Gevel DF, Hamad MA, Elenbaas TW, Ostertag JU, Schonberger JP. Is the use of Steri‐StripTM S for wound closure after coronary artery bypass grafting better than intracuticular suture?. Interactive Cardiovascular and Thoracic Surgery 2010;10(4):561‐4. [DOI] [PubMed] [Google Scholar]
Watson 1983 {published data only}
- Watson GM, Anders CJ, Glover JR. Op‐Site skin closure: a comparison with subcuticular and interrupted sutures. Annals of The Royal College of Surgeons of England 1983;65(2):83‐4. [PMC free article] [PubMed] [Google Scholar]
Watts 1982 {published data only}
- Watts GT. Removable subcuticular skin suture in acute appendicitis: a prospective comparable clinical trial. British Medical Journal 1982;284(6323):1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wyles 2016 {published data only}
- Wyles CC, Jacobson SR, Houdek MT, Larson DR, Taunton MJ, Sim FH, et al. The Chitranjan Ranawat Award: running subcuticular closure enables the most robust perfusion after TKA: a randomized clinical trial. Clinical Orthopaedics and Related Research 2016;474(1):47‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
You 2016 {published data only}
- You HJ, Son ST, Kim DY, Han SK. A prospective randomized study comparing fibrin sealant versus suture for conjunctival wound closure in orbital wall fracture surgery. International Journal of Oral & Maxillofacial Surgery 2016;45:1418‐23. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Choudry 1996 {published data only}
- Choudry ZA, Arif MT, Akhtar S. Subcuticular versus interrupted skin closure in appendicectomy. Pakistan Journal of Surgery 1996;12(2):61‐3. [Google Scholar]
Lubowski 1985 {published data only}
- Lubowski D, Hunt D. Abdominal wound closure comparing the proximate stapler with sutures. Australian and New Zealand Journal of Surgery 1985;55(4):405‐6. [DOI] [PubMed] [Google Scholar]
Rubio‐Perez 2014 {published data only}
- Rubio‐Perez I, Leon M, Cantero R. Sis‐e fellowship project 'subcuticular continuous suture versus skin staples to reduce surgical site infections in colorectal surgery patients': current status of the investigation. Surgical Infections 2014;15(3):A8. [Google Scholar]
Singer 2002 {published data only}
- Singer AJ, Quinn JV, Clark RE, Hollander JE, TraumaSeal Study Group. Closure of lacerations and incisions with octylcyanoacrylate: a multicenter randomized controlled trial. Surgery 2002;131(3):270‐6. [DOI] [PubMed] [Google Scholar]
Zhang 2011 {published data only}
- Zhang ZT, Zhang HW, Fang XD, Wang LM, Li XX, Li YF, et al. Cosmetic outcome and surgical site infection rates of antibacterial absorbable (Polyglactin 910) suture compared to Chinese silk suture in breast cancer surgery: a randomized pilot research. Chinese Medical Journal 2011;124(5):719‐24. [PubMed] [Google Scholar]
References to ongoing studies
ACTRN12611000399998 {published data only}
- ACTRN12611000399998. A randomized study comparing skin closure in benign gynecologic surgery: staple versus subcuticular suture [A randomized study comparing skin closure in benign gynecologic surgery: staple versus subcuticular suture for postoperative pain]. anzctr.org.au/Trial/Registration/TrialReview.aspx?id=336565 (first received 29 March 2011).
CTRI/2018/02/011698 {published data only}
- CTRI/2018/02/011698. Comparing two methods for closing the skin after thyroid surgery which are stitching and sticking them with gum [Comparison of tissue adhesive glue with subcuticular absorbable suture for skin closure following thyroid surgery ‐ a blinded randomized controlled trial]. ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=22138 (first received 5 February 2018).
CTRI/2018/08/015470 {published data only}
- CTRI/2018/08/015470. A clinical trial to study the effects of two types of sutures, stapler and under‐the‐skin suture, on skin closure in patients undergoing surgery for hernia in the groin [Comparison of skin closure technique with stapler and subcuticular suture in patients with inguinal hernia undergoing Lichtenstein tension‐free mesh repair – an open‐label randomized controlled trial]. ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=27946 (first received 24 August 2018).
IRCT20161217031440N1 {published data only}
- IRCT20161217031440N1. Comparison of subcuticular and transdermal appendectomy repairs; a randomized clinical trial [Comparison of subcuticular and transdermal appendectomy repairs in appendectomy patients; a randomized clinical trial]. en.irct.ir/trial/33020 (first received 27 September 2018).
IRCT20180820040840N1 {published data only}
- IRCT20180820040840N1. Comparison the complications of two wound repair methods (subcuticular and mattress) in patients undergoing appendectomy. en.irct.ir/trial/33709 (first received 10 September 2018).
ISRCTN80786695 {published data only}
- ISRCTN80786695. A comparison of patient satisfaction and complications following keyhole surgery wounds closed with tissue glue or stitches [Patient satisfaction and complications following laparoscopic wound closure with 2‐octylcyanoacrylate or vicryl: a single blind randomised controlled trial]. isrctn.com/ISRCTN80786695 (first received 27 November 2009).
ISRCTN96030942 {published data only}
- ISRCTN96030942. A randomized trial comparing octyl‐cyanoacrylate and subcuticular sutures for post‐auricular wound cosmesis. isrctn.com/ISRCTN96030942 (first received 27 September 2007).
Maschuw 2014 {published data only}
- Maschuw K, Heinz C, Maurer E, Reuss A, Schade‐Brittinger C, Bartsch Detlef K. Intracutaneous suture versus transcutaneous skin stapling for closure of midline or horizontal skin incision in elective abdominal surgery and their outcome on superficial surgical site infections ‐ INTRANS: study protocol for a randomized controlled trial. Trials 2014;15(25):no pagination. [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT01996917 {published data only}
- NCT01996917. Use of prineo in breast reduction surgery (prineo) [Use of prineo for wound closure in breast reduction surgery]. clinicaltrials.gov/ct2/show/NCT01996917 (first received 27 November 2013).
NCT02046239 {published data only}
- NCT02046239. Suture vs staples for skin closure after liver resection [A randomised controlled trial to compare subcuticular suture vs staples for skin closure after liver resection]. clinicaltrials.gov/ct2/show/NCT02046239 (first received 27 January 2014).
NCT02551510 {published data only}
- NCT02551510. Comparison of skin adhesive to subcuticular suture wound closure after port placement (PWC) [Open, randomized, controlled non‐inferiority trial to compare synthetic tissue adhesive and skin suture after port catheter implantation with reference to cosmetic result and economy of time at equal risk for wound infection]. clinicaltrials.gov/ct2/show/NCT02551510 (first received 16 September 2015).
NCT02936063 {published data only}
- NCT02936063. Outcomes comparing different methods of skin closure in patients undergoing head and neck surgery. clinicaltrials.gov/ct2/show/NCT02936063 (first received 18 October 2016).
NCT03108742 {published data only}
- NCT03108742. Randomized control study of dermal staples vs subcuticular sutures on postoperative scar after thyroidectomy. clinicaltrials.gov/ct2/show/NCT03108742 (first received 11 April 2017).
NCT03788239 {published data only}
- NCT03788239. Wound closure after total knee replacement. clinicaltrials.gov/ct2/show/NCT03788239 (first received 27 December, 2018).
UMIN000002873 {published data only}
- UMIN000002873. A randomized comparison between dermal suture with synthetic absorbable sutures and skinstaplers. upload.umin.ac.jp/cgi‐open‐bin/ctr_e/ctr_view.cgi?recptno=R000003509 (first received 10 December 2009).
UMIN000003235 {published data only}
- UMIN000003235. Influence of surgical site infection in hepato‐biliary‐pancreatic diseases. upload.umin.ac.jp/cgi‐open‐bin/ctr_e/ctr_view.cgi?recptno=R000003916 (first received 22 February 2010).
Additional references
Altman 1996
- Altman DG, Bland JM. Detecting skewness from summary information. BMJ (Clinical Research Edition) 1996;313(7066):1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cardo 2004
- Cardo D, Horan T, Andrus M, Dembinski M, Edwards J, Peavy G, et al. National Nosocomial Infections Surveillance (NNIS) System Report, data summary from January 1992 through June 2004, issued October 2004. American Journal of Infection Control 2004;32(8):470‐85. [DOI] [PubMed] [Google Scholar]
Clay 2011
- Clay FS, Walsh CA, Walsh SR. Staples versus subcuticular sutures for skin closure at cesarean delivery: a metaanalysis of randomized controlled trials. American Journal of Obstetrics and Gynecology 2011;204(5):378‐83. [DOI] [PubMed] [Google Scholar]
Cohen 1960
- Cohen J. A coefficient of agreement for nominal scales. Educational and Psychological Measurement 1960;20:37‐46. [DOI: 10.1177/001316446002000104] [DOI] [Google Scholar]
De Lissovoy 2009
- Lissovoy G, Fraeman K, Hutchins V, Murphy D, Song D, Vaughn BB. Surgical site infection: incidence and impact on hospital utilization and treatment costs. American Journal of Infection Control 2009;37(5):387‐97. [DOI] [PubMed] [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177‐88. [DOI] [PubMed] [Google Scholar]
Dumville 2014
- Dumville JC, Coulthard P, Worthington HV, Riley P, Patel N, Darcey J, et al. Tissue adhesives for closure of surgical incisions. Cochrane Database of Systematic Reviews 2014, Issue 11. [DOI: 10.1002/14651858.CD004287.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Smith GD, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ (Clinical Research Edition) 1997;315(7109):629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fisher 1980
- Fisher GT, Fisher JB, Stark RB. Origin of the use of subcuticular sutures. Annals of Plastic Surgery 1980;4:144‐8. [DOI] [PubMed] [Google Scholar]
Furukawa 2000
- Furukawa TA, Barbui C, Cipriani A, Brambilla P, Watanabe N. Imputing missing standard deviations in meta‐analyses can provide accurate results. Journal of Clinical Epidemiology 2000;59:7‐10. [DOI] [PubMed] [Google Scholar]
Furukawa 2002
- Furukawa TA, Guyatt GH, Griffith LE. Can we individualize the 'number needed to treat'? An empirical study of summary effect measures in meta‐analyses. International Journal of Epidemiology 2002;31(1):72‐6. [DOI] [PubMed] [Google Scholar]
Garner 1986
- Garner JS. CDC guideline for prevention of surgical wound infections 1985. Infection Control 1986;7(3):193‐200. [DOI] [PubMed] [Google Scholar]
Gaynes 2001
- Gaynes RP, Culver DH, Horan TC, Edwards JR, Richards C, Tolson JS, et al. Surgical site infection (SSI) rates in the United States, 1992‐1998: the National Nosocomial Infections Surveillance System basic SSI risk index. Clinical Infectious Diseases 2001;33:S69‐77. [DOI] [PubMed] [Google Scholar]
GRADE 2013
- Schünemann H, Brozek J, Guyatt G, Oxman A, editor(s). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). gdt.guidelinedevelopment.org/app/handbook/handbook.html (accessed prior to 12 February 2020).
Gurusamy 2014
- Gurusamy KS, Toon CD, Allen VB, Davidson BR. Continuous versus interrupted skin sutures for non‐obstetric surgery. Cochrane Database of Systematic Reviews 2014, Issue 2. [DOI: 10.1002/14651858.CD010365.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21:1539‐58. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ (Clinical Research Edition) 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hollander 1995
- Hollander JE, Singer AJ, Valentine S, Henry MC. Wound registry: development and validation. Annals of Emergency Medicine 1995;25(5):675–85. [DOI] [PubMed] [Google Scholar]
Ibrahim 2014
- Ibrahim MI, Moustafa GF, Al‐Hamid AS, Hussein MR. Superficial incisional surgical site infection rate after cesarean section in obese women: a randomized controlled trial of subcuticular versus interrupted skin suturing. Archives of Gynecology and Obstetrics 2014;289(5):981‐6. [DOI] [PubMed] [Google Scholar]
Kwon 2013
- Kwon S, Thompson R, Dellinger P, Yanez D, Farrohki E, Flum D. Importance of perioperative glycemic control in general surgery: a report from the Surgical Care and Outcomes Assessment Program. Annals of Surgery 2013;257(1):8‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
La Paudula 1995
- Paudula A. A new technique to secure an entirely buried subcuticular suture. Plastic and Reconstructive Surgery 1995;95:423‐4. [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J, Cochrane Information Retrieval Methods Group. Chapter 6: Searching for studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Liberati 2009
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate health care interventions: explanation and elaboration. PLOS Medicine 2009;6:e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mackeen 2012
- Mackeen AD, Berghella V, Larsen ML. Techniques and materials for skin closure in caesarean section. Cochrane Database of Systematic Reviews 2012, Issue 11. [DOI: 10.1002/14651858.CD003577.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mackeen 2015
- Mackeen AD, Schuster M, Berghella V. Suture versus staples for skin closure after cesarean: a metaanalysis. American Journal of Obstetrics and Gynecology 2015;212(5):621.e1‐10. [DOI] [PubMed] [Google Scholar]
Magill 2012
- Magill SS, Hellinger W, Cohen J, Kay R, Bailey C, Boland B, et al. Prevalence of healthcare‐associated infections in acute care hospitals in Jacksonville, Florida. Infection Control and Hospital Epidemiology 2012;33(3):283‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mangram 1999
- Mangram AJ, Horan TC, Pearson ML, Silver LC, Jarvis WR, Hospital Infection Control Practices Advisory Committee. Guideline for prevention of surgical site infection 1999. Infection Control and Hospital Epidemiology 1999;20(4):250‐78. [DOI] [PubMed] [Google Scholar]
McHugh 2012
- McHugh ML. Interrater reliability: the kappa statistic. Biochemia Medica 2012;22(3):276‐82. [PMC free article] [PubMed] [Google Scholar]
Parell 2003
- Parell GJ, Becker GD. Comparison of absorbable with nonabsorbable sutures in closure of facial skin wounds. Archives of Facial Plastic Surgery 2003;5(6):488‐90. [DOI] [PubMed] [Google Scholar]
Perencevich 2003
- Perencevich EN, Sands KE, Cosgrove SE, Guadagnoli E, Meara E, Platt R. Health and economic impact of surgical site infections diagnosed after hospital discharge. Emerging Infectious Diseases 2003;9(2):196‐203. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pull ter Gunne 2012
- Pull ter Gunne AF, Hosman AJ, Cohen DB, Schuetz M, Habil D, Laarhoven CJ, et al. A methodological systematic review on surgical site infections following spinal surgery: part 1: risk factors. Spine 2012;37(24):2017‐33. [DOI] [PubMed] [Google Scholar]
Rao 1992
- Rao JN, Scott AJ. A simple method for the analysis of clustered binary data. Biometrics 1992;48:577‐85. [PubMed] [Google Scholar]
Regula 2015
- Regula CG, Yag‐Howard C. Suture products and techniques: what to use, where, and why. Dermatologic Surgery 2015;41(Suppl 10):S187‐200. [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Schünemann 2011a
- Schünemann HJ, Oxman AD, Higgins JP, Vist GE, Glasziou P, Guyatt GH, Cochrane Applicability and Recommendations Methods Group and the Cochrane Statistical Methods Group. Chapter 11: Presenting results and 'Summary of findings' tables. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Schünemann 2011b
- Schünemann HJ, Oxman AD, Vist GE, Higgins JP, Deeks JJ, Glasziou P, Cochrane Applicability and Recommendations Methods Group. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
SIGN 2018
- Scottish Intercollegiate Guidelines Network (SIGN). Search filters. www.sign.ac.uk/search‐filters.html (accessed 22 June 2018).
Singh‐Ranger 2003
- Singh‐Ranger D. A simple technique for the retention of a subcuticular suture. Surgeon 2003;1:149‐51. [DOI] [PubMed] [Google Scholar]
Smoot 1998
- Smoot EC. Method for a securing subcuticular suture with minimal buried knot. Plastic and Reconstructive Surgery 1998;102(7):2447‐9. [DOI] [PubMed] [Google Scholar]
Stanec 1997
- Stanec Z, Delimar D, Stanec S. Simple securing of subcuticular running suture. Annals of Plastic Surgery 1997;39(3):332. [DOI] [PubMed] [Google Scholar]
Tajirian 2010
- Tajirian AL, Goldberg DJ. A review of sutures and other skin closure materials. Journal of Cosmetic and Laser Therapy 2010;12(6):296‐302. [DOI] [PubMed] [Google Scholar]
Talbot 2005
- Talbot TR. Diabetes mellitus and cardiothoracic surgical site infections. American Journal of Infection Control 2005;33(6):353‐9. [DOI] [PubMed] [Google Scholar]
Tuuli 2011
- Tuuli MG, Rampersad RM, Carbone JF, Stamilio D, Macones GA, Odibo AO. Staples compared with subcuticular suture for skin closure after cesarean delivery: a systematic review and meta‐analysis. Obstetrics and Gynecology 2011;117(3):682‐90. [DOI] [PubMed] [Google Scholar]
Weiser 2015
- Weiser TG, Haynes AB, Molina G, Lipsitz SR, Esquivel MM, Uribe‐Leitz T, et al. Estimate of the global volume of surgery in 2012: an assessment supporting improved health outcomes. Lancet 2015;385(Suppl 2):S11. [DOI] [PubMed] [Google Scholar]
Zhang 2014
- Zhang Y, Dong J, Qiao Y, He J, Wang T, Ma S. Efficacy and safety profile of antibiotic prophylaxis usage in clean and clean‐contaminated plastic and reconstructive surgery: a meta‐analysis of randomized controlled trials. Annals of Plastic Surgery 2014;72(1):121‐30. [DOI] [PubMed] [Google Scholar]
Zhang 2015
- Zhang Y, Zheng QJ, Wang S, Zeng SX, Zhang YP, Bai XJ, et al. Diabetes mellitus is associated with increased risk of surgical site infections: a meta‐analysis of prospective cohort studies. American Journal of Infection Control 2015;43(8):810‐5. [DOI] [PubMed] [Google Scholar]
Zimlichman 2013
- Zimlichman E, Henderson D, Tamir O, Franz C, Song P, Yamin CK, et al. Healthcare‐associated infections: a meta‐analysis of costs and financial impact on the US health care system. JAMA Internal Medicine 2013;173(22):2039‐46. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Goto 2016
- Goto S, Hida K, Furukawa TA, Sakai Y. Subcuticular sutures for skin closure in non‐obstetric surgery. Cochrane Database of Systematic Reviews 2016, Issue 3. [DOI: 10.1002/14651858.CD012124] [DOI] [PMC free article] [PubMed] [Google Scholar]


