Abstract
Background
Idiopathic inflammatory myopathies are chronic diseases with significant mortality and morbidity. Whilst immunosuppressive and immunomodulatory therapies are frequently used, the optimal therapeutic regimen remains unclear. This is an update of a review first published in 2005.
Objectives
To assess the effects of immunosuppressants and immunomodulatory treatments for dermatomyositis and polymyositis.
Search methods
We searched the Cochrane Neuromuscular Disease Group Specialized Register (August 2011), the Cochrane Central Register of Controlled Trials (CENTRAL) (Issue 3 2011), MEDLINE (January 1966 to August 2011), EMBASE (January 1980 to August 2011) and clinicaltrials.gov (August 2011). We checked the bibliographies of identified trials and wrote to disease experts.
Selection criteria
We included all randomised controlled trials (RCTs) or quasi‐RCTs involving participants with probable or definite dermatomyositis and polymyositis as defined by the criteria of Bohan and Peter, or definite, probable or mild/early by the criteria of Dalakas. In participants without a classical rash of dermatomyositis, inclusion body myositis should have been excluded by muscle biopsy. We considered any immunosuppressant or immunomodulatory treatment. The two primary outcomes were the change in a function or disability scale measured as the proportion of participants improving one grade, two grades etc, predefined based on the scales used in the studies after at least six months, and a 15% or greater improvement in muscle strength compared with baseline after at least six months. Other outcomes were: the International Myositis Assessment and Clinical Studies Group (IMACS) definition of improvement, number of relapses and time to relapse, remission and time‐to‐remission, cumulative corticosteroid dose and serious adverse effects.
Data collection and analysis
Two authors independently selected papers, extracted data and assessed risk of bias in included studies. They collected adverse event data from the included studies.
Main results
The review authors identified 14 relevant RCTs. They excluded four trials.
The 10 included studies, four of which have been added in this update, included a total of 258 participants. Six studies compared an immunosuppressant or immunomodulator with placebo control, and four studies compared two immunosuppressant regimes with each other. Most of the studies were small (the largest had 62 participants) and many of the reports contained insufficient information to assess risk of bias.
Amongst the six studies comparing immunosuppressant with placebo, one study, investigating intravenous immunoglobulin (IVIg), showed statistically significant improvement in scores of muscle strength in the IVIg group over three months. Another study investigating etanercept showed some evidence of a longer median time to relapse in the etanercept group, a secondary outcome in this review, but no improvement in other assessed outcomes. The other four randomised placebo‐controlled trials assessed either plasma exchange and leukapheresis, eculizumab, infliximab or azathioprine against placebo and all produced negative results.
Three of the four studies comparing two immunosuppressant regimes (azathioprine with methotrexate, ciclosporin with methotrexate, and intramuscular methotrexate with oral methotrexate plus azathioprine) showed no statistically significant difference in efficacy between the treatment regimes. The fourth study comparing pulsed oral dexamethasone with daily oral prednisolone and found that the dexamethasone regime had a shorter median time to relapse but fewer side effects.
Immunosuppressants were associated with significant side effects.
Authors' conclusions
This systematic review highlights the lack of high quality RCTs that assess the efficacy and toxicity of immunosuppressants in inflammatory myositis.
Plain language summary
Drugs that suppress or modify the immune system for dermatomyositis and polymyositis
Dermatomyositis and polymyositis are long‐term inflammatory muscle diseases, causing muscle weakness and disability. For some reason, the body's immune system turns against its own muscles in an autoimmune response. Corticosteroids are the principal treatment but due to side effects, there is a need for additional treatment with drugs that suppress the immune system (immunosuppressants) or modify it (immunomodulatory therapies) to improve patient outcomes. For this review, an update of a review first published in 2005, we found ten randomised trials available, involving 258 participants.
Amongst the six studies comparing immunosuppressant with placebo, one study, investigating intravenous immunoglobulin (IVIg), showed statistically significant improvement in scores of muscle strength in the IVIg group over three months. Another study investigating etanercept showed some evidence of a longer median time to relapse in the etanercept group, a secondary outcome in this review, but no improvement in other assessed outcomes. The other four randomised placebo‐controlled trials assessed either plasma exchange and leukapheresis, eculizumab, infliximab or azathioprine against placebo and all produced negative results.
Three of the four studies comparing two immunosuppressant regimes (azathioprine with methotrexate, ciclosporin with methotrexate, and intramuscular methotrexate with oral methotrexate plus azathioprine) showed no statistically significant difference in efficacy between the treatment regimes. The fourth study comparing pulsed oral dexamethasone with daily oral prednisolone and found that the dexamethasone regime had a shorter median time to relapse but fewer side effects.
Most of the studies were small (the largest had 62 participants) and many of the reports contained insufficient information to assess risk of bias. Immunosuppressants were associated with significant side effects. The small number of RCTs of immunosuppressants and immunomodulatory therapies are inadequate to decide whether these agents are beneficial in dermatomyositis and polymyositis. Two small trials, one of IVIg in dermatomyositis, the other of etanercept in dermatomyositis suggested that they are beneficial. More RCTs are needed.
Background
The inflammatory myopathies include recognised causes of muscle inflammation such as those due to infection by bacteria, viruses and parasites. Idiopathic inflammatory myopathies refer to diseases in which muscle inflammation occurs without a recognised infective cause and these include dermatomyositis and polymyositis. Such diseases are thought to result from an auto‐immune process. Dermatomyositis and polymyositis are characterised by chronic inflammation of skeletal muscle which can result in persisting muscle weakness with significant disability (Dalakas 1991; Dalakas 2001). They may both occur in association with gastrointestinal, pulmonary and cardiac dysfunction, while only dermatomyositis has skin involvement. The prevalence of idiopathic inflammatory myositis is approximately 11 per 100,000 (Ahlstrom 1993). As idiopathic inflammatory myositis is uncommon, optimal therapy has not been adequately defined (Choy 2002).
Corticosteroids are the principal treatment. Both high‐ and low‐dose corticosteroid regimes are used. In many people, long‐term high dose corticosteroids are necessary to control disease and, in a few people, myositis may be refractory to steroid treatment. Therefore, many people with an idiopathic inflammatory myopathy suffer from the side effects of corticosteroids. The mortality and morbidity of inflammatory myositis remains high despite such treatment (Carpenter 1977; Joffe 1993; Riddoch 1975). Thus, there is a frequent need to use additional treatment both to improve the disease response and to reduce the side effects of corticosteroids.
Immunosuppressive agents, especially azathioprine, methotrexate, mycophenolate mofetil and ciclosporin, are commonly used in autoimmune diseases as well as in transplant rejection and chronic inflammatory diseases. They are usually employed as second‐line therapy to corticosteroids for disease refractory to steroid treatment alone. They can also be used as adjuvants to steroid treatment to allow reduction in the dosage of corticosteroids and thereby decrease the risk of long‐term complications. While these treatments are in use for dermatomyositis and polymyositis, the optimal therapeutic regimen remains unclear (Choy 2002). Biological agents, in particular the anti‐TNF agents and the B‐cell depleting agent rituximab, are also presently being assessed as potential therapeutic agents in the inflammatory myopathies.
An alternative approach to improving the treatment of dermatomyositis and polymyositis is the use of immunomodulatory therapy. This includes interferon, intravenous immunoglobulin (IVIg) and plasma exchange which have proven efficacy in various autoimmune disorders. They are gaining attention as possible second‐line treatment for polymyositis and dermatomyositis (Choy 2002; Dalakas 2001).
This is an update of a Cochrane review first published in 2005.
Objectives
To assess the effects of immunosuppressants and immunomodulatory treatments for dermatomyositis and polymyositis.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) or quasi‐RCTs (trials in which allocation is not strictly random but is based, for example, on case record number or date of birth).
Types of participants
People with probable or definite dermatomyositis and polymyositis as defined by the criteria of Bohan and Peter (Bohan 1975 a; Bohan 1975 b) (Table 1) or definite, probable or mild/early by the criteria of Dalakas (Dalakas 1991) (Table 2). In participants without a classical rash of dermatomyositis, inclusion body myositis should have been excluded by muscle biopsy. If no diagnostic criteria were cited, all the assessors would have judged the quality of evidence for correct diagnosis. The study was included in the review only if the assessors agreed that the participants had probable or definite dermatomyositis or polymyositis.
1. Bohan and Peter criteria.
| Features | Polymyositis | Dermatomyositis |
| 1. Symmetrical proximal muscle weakness | Definite: all 1‐4 | Definite: 5 plus any 3 of 1‐4 |
| 2. Muscle biopsy evidence of myositis | Probable: any 3 of 1‐4 | Probable: 5 plus any 2 of 1‐4 |
| 3. Elevation in serum skeletal muscle enzymes | Possible: any 2 of 1‐4 | Possible: 5 plus any 1 of 1‐4 |
| 4. Characteristic electromyographic pattern of myositis | ||
| 5. Typical rash of dermatomyositis |
2. Dalakas criteria (PM: polymyositis; DM: dermatomyositis).
| Features | Definite PM | Probable PM | Definite DM | Mild/early DM |
| Muscle strength | Myopathic muscle weakness | Myopathic muscle weakness | Myopathic muscle weakness | Seemingly normal strength |
| Electromyographic findings | Myopathic | Myopathic | Myopathic | Myopathic or non‐specific |
| Muscle enzymes | Elevated (up to 50‐fold) | Elevated (up to 50‐fold) | Elevated (up to 50‐fold) or normal | Elevated (up to 10‐fold) or normal |
| Muscle‐biopsy findings | Diagnostic for this type of inflammatory myopathy | Non‐specific myopathy without signs of primary inflammation | Diagnostic | Non‐specific or diagnostic |
| Rash or calcinosis | Absent | Absent | Present | Present |
Types of interventions
Any immunosuppressant or immunomodulatory treatment including corticosteroids, azathioprine, methotrexate, ciclosporin, chlorambucil, cyclophosphamide, IVIg, interferon and plasma exchange in dermatomyositis and polymyositis, compared with placebo, no treatment or another immunosuppressant or immunomodulatory treatment.
Types of outcome measures
Primary outcomes
Change in a function or disability scale after at least six months, measured as the proportion of participants improving one grade, two grades etc, predefined based on the scales used in the studies after at least six months. In order to harmonise results we planned to try to convert the results from all studies to either the disability scale that is used in most studies or to one which seemed to us most appropriate.
A 15% or greater improvement in muscle strength compared with baseline after at least six months.
Secondary outcomes
Achieving the International Myositis Assessment and Clinical Studies Group (IMACS) definitions of improvement (DOI) after at least six months. The definitions of improvement use six core set measures among five domains (Oddis 2005; Rider 2004). These core set measures are: the physician global disease activity, parent/patient global disease activity, muscle strength (manual muscle testing (MMT)), physical function assessment, laboratory assessment and extramuscular disease complications. Improvement is defined as occurring if three of any six core set measures improve by 20%, with no more than two worsening by 25% (measures that worsen cannot include manual muscle strength).
Number of relapses and time to relapse.
Remission and time‐to‐remission (remission is modified Rankin score of 0 or 1) (Van Swieten 1988) after at least six months.
Cumulative corticosteroid dose after at least six months.
Serious adverse effects as defined by any untoward medical occurrence that at any dose results in death, is life‐threatening, requires inpatient hospitalisation or prolongation of existing hospitalisation, results in persistent or significant disability/incapacity or is a congenital anomaly/birth defect.
In this update we included 'Summary of findings' tables showing our primary outcomes and the first of our secondary outcomes. 'Summary of findings' tables in future updates of the review will include serious adverse events.
Search methods for identification of studies
We searched the Cochrane Neuromuscular Disease Group Specialized Register (August 2011), the Cochrane Central Register of Controlled Trials (CENTRAL) (Issue 3 2011), MEDLINE (January 1966 to August 2011), EMBASE (January 1980 to August 2011) and clinicaltrials.gov (August 2011) for articles including the terms 'corticosteroids', 'anti‐metabolites' or 'azathioprine' or 'mercaptopurine' or 'methotrexate' or 'ciclosporin' or 'cyclosporin' or 'chlorambucil' or 'cyclophosphamide' or 'immunoglobulin' or 'interferon', 'gamma globulin' or 'plasma exchange' or 'plasmapheresis' or 'immunosuppressant' or 'immunosuppression' and 'dermatomyositis' or 'polymyositis' or 'inflammatory myositis' or 'myositis' and 'randomised controlled trial'. We searched clinicaltrials.gov (completed studies as of August 2011) for completed studies using the terms 'Myositis', 'polymyositis' and 'dermatomyositis'. We undertook a manual search using the bibliographies of trials identified. We also wrote to known disease experts and authors of trials that we discovered, asking them for more information about their trials and whether they knew of trials other than those which we identified.
Electronic database strategies
For detailed search strategies please see: Appendix 1 (CENTRAL), Appendix 2 (MEDLINE), Appendix 3 (EMBASE) and Appendix 4 (Clinicaltrials.gov).
Data collection and analysis
Selection of studies
For the previous version of the review, two review authors (EC and JH) independently selected trials and four authors independently assessed each study.
For the update two review authors (JW and PG) independently selected trials from the Cochrane Neuromuscular Disease Group Specialized Register (August 2011), the Cochrane Central Register of Controlled trials (CENTRAL) (August 2011), clinicaltrials.gov (August 2011), MEDLINE (January 1966 to August 2011) and EMBASE (1980 to August 2011).
Two review authors (JH and PG) independently assessed each study except one, in which JH was an author, which JW and PG independently assessed. The review authors recorded methodological criteria and the results of each study on data extraction forms.
Assessment of risk of bias in included studies
Two review authors (JH and PG) independently assessed the risk of bias for each trial using the domain based 'Risk of bias' tool described in the Cochrane Handbook for Systematic Reviews of Interventions 5.1.0 (Higgins 2011). In one trial JH was an author and therefore PG and JW independently assessed the risk of bias in this study.
We assessed the risk of bias as high, low or unclear based on the following questions, each representing a domain.
• Was the allocation sequence adequately generated? • Was allocation adequately concealed? • Was knowledge of the allocated intervention adequately prevented during the study? • Were incomplete outcome data adequately addressed? • Are reports of the study free of suggestion of selective outcome reporting? • Was the study apparently free of other problems that could put it at a high risk of bias?
We then used the results to create the 'Risk of bias' tables presented in this review. Where the two review authors (JH and PG or PG and JW) could not agree on an domain, this was settled by a third author (JW or JH).
Data synthesis
If sufficient data had been available, we would have performed meta‐analysis using the Cochrane statistical software, Review Manager 5.1 (RevMan) (RevMan 2011). We would have expressed results as risk ratios (RR) and risk differences (RD) with 95% confidence intervals (CI) for dichotomous outcomes and mean difference (MD) and 95% CI for continuous outcomes. We would have carried out tests for homogeneity. If there had been evidence of heterogeneity, we would have performed sensitivity analysis and excluded trials of lowest quality.
Subgroup analysis and investigation of heterogeneity
We would have analysed the following subgroups when possible.
Younger (up to 18 years of age) versus older.
Reason for failure of initial treatment (corticosteroids) in case of second‐line intervention: inadequate response versus unacceptable side effects.
Diagnostic subgroups: polymyositis versus dermatomyositis versus myositis associated with other connective tissue disease versus myositis in the presence of cancer.
Myositis‐specific autoantibodies: participants with autoantibodies versus participants without autoantibodies (Bunch 1980).
Sensitivity analysis
We would have carried out sensitivity analysis to assess the effect of using different diagnostic criteria on outcome: probable and definite versus definite only (Bohan 1975 a; Bohan 1975 b) versus Dalakas 1991 versus non‐specified.
Results
Description of studies
The number of papers found by the new, current strategies are: MEDLINE 774; EMBASE 1880; Cochrane Neuromuscular Disease Group Specialized Register 30 (14 new papers); CENTRAL 43; and clinicaltrials.gov 77. From the searches we identified fourteen potentially relevant RCTs (Bunch 1980; Bunch 1981; Chung 2007; Coyle 2008; Dalakas 1993; Donov 1995; Fries 1973; Miller 1992; Miller 2002; Muscle Study Group 2011; Takada 2002; Vencovsky 2000; Van de Vlekkert 2010; Villalba 1998). Ten are full publications in peer reviewed journals, four are only published as abstracts (Coyle 2008; Donov 1995; Miller 2002; Takada 2002). Two of the studies included authors who were also authors of this review (Miller 2002 (JW) and Van de Vlekkert 2010 (JH)). We excluded four trials (see Characteristics of excluded studies). We excluded one as it was an open unblinded follow‐up of another study included in this review, and one because the agent being investigated was not felt to be immunosuppressive or immunomodulatory. We excluded the third because we could not confirm that the participants had polymyositis on the basis of the inclusion criteria. We excluded the fourth study as there was no evidence of randomisation or blinding.
The characteristics of the ten selected studies are listed in Characteristics of included studies.
We identified 10 ongoing studies, which are described in Characteristics of ongoing studies.
Study designs
Six studies compared immunosuppressant or immunomodulatory therapy with placebo (Bunch 1980; Coyle 2008; Dalakas 1993; Miller 1992; Muscle Study Group 2011; Takada 2002), four trials compared one immunosuppressant regime with another: one compared ciclosporin with methotrexate (Vencovsky 2000), one methotrexate with azathioprine (Miller 2002), one oral daily prednisolone with pulsed oral dexamethasone (Van de Vlekkert 2010) and the fourth trial compared intravenous methotrexate with oral methotrexate plus azathioprine (Villalba 1998). Three trials were cross‐over studies (Coyle 2008; Dalakas 1993; Villalba 1998).
Participants
All trials recruited adults over 18 years of age. SIx trials included participants with either polymyositis or dermatomyositis (Coyle 2008; Miller 1992; Miller 2002; Vencovsky 2000; Villalba 1998; Van de Vlekkert 2010), one trial studied polymyositis participants only (Bunch 1980) while the other three only included dermatomyositis participants (Dalakas 1993; Muscle Study Group 2011; Takada 2002). The Bohan and Peter diagnostic criteria (Bohan 1975 a; Bohan 1975 b) were the most frequently used. Muscle biopsies were performed in five studies (Bunch 1980; Coyle 2008; Dalakas 1993; Miller 1992; Vencovsky 2000). Three of the six trials that included participants with polymyositis specifically stated exclusion of inclusion body myositis (Miller 1992; Vencovsky 2000; Villalba 1998). A fourth, reported in abstract, is known to have excluded inclusion body myositis (Miller 2002). A fifth excluded participants who had three or more three rimmed vacuoles per 1000 muscle fibers on muscle biopsy (Van de Vlekkert 2010).
Interventions
The interventions studied included the following.
Monthly infusions of 2 g/kg of immunoglobulin or placebo for three months (Dalakas 1993).
Plasma exchange, leukapheresis or sham apheresis with twelve treatments given over a one month period (Miller 1992).
Azathioprine 2 mg/kg/day or placebo for three months in addition to 60 mg of prednisolone daily (Bunch 1980).
Prednisolone and either low‐dose methotrexate (15 mg weekly) or azathioprine (2.5 mg/kg daily) for one year (Miller 2002).
Methotrexate 7.5 to 15 mg (mostly 10 mg) orally weekly or ciclosporin 3.0 to 3.5 mg/kg/day for at least six months (Vencovsky 2000).
Oral methotrexate up to 25 mg weekly with azathioprine 150 mg daily for six months or intravenous methotrexate 500 mg/m2 every two weeks for 12 treatments each with leucovorin rescue (50 mg/m2 every six hours for four doses) (Villalba 1998).
Infliximab 5 mg/kg at weeks 0, 2, 6 and 14 or placebo (Coyle 2008).
Eculizumab (a humanised monoclonal antibody to C5 that inhibits cleavage of C5) 8 mg/kg weekly for five weeks then two‐weekly for a further two doses or placebo (Takada 2002).
Oral dexamethasone pulse therapy or oral daily prednisolone (Van de Vlekkert 2010).
Etanercept (50mg subcutaneously weekly) or placebo for 52 weeks (Muscle Study Group 2011).
Outcomes
Function or disability was assessed in eight trials using the modified Convery Assessment Scale (Miller 1992; Villalba 1998), the modified Rankin scale (Van de Vlekkert 2010), the Health Assessment Questionnaire (HAQ) (Muscle Study Group 2011), the Short Form 36 Health Survey (SF‐36) (Van de Vlekkert 2010), ad hoc scales (Dalakas 1993; Vencovsky 2000), time to arise from a chair and time to walk 30 feet (Muscle Study Group 2011), the Neuromuscular Symptom Score (NSS) (Dalakas 1993; Van de Vlekkert 2010), the individualised neuromuscular quality of life questionnaire (Muscle Study Group 2011) or timed walk (Miller 2002).
Improvement in MMT by 15% or more was used as a defined outcome in one study (Coyle 2008) and could be inferred from another (Dalakas 1993).
Assessment of muscle strength was done by MMT in eight of the selected trials. Two used the standard six point MRC scale, (Miller 1992; Villalba 1998), one the five point MRC scale (Van de Vlekkert 2010) and two expanded MRC scales, one an eight point scale (Dalakas 1993) and one an expanded 13 point scale (Muscle Study Group 2011). One study used a non‐standard scale (with 0 being normal and ‐4 being no movement) (Bunch 1980). Two, published in abstract form only, did not define the MMT scale used (Coyle 2008; Takada 2002). The studies also varied in the number of muscle groups assessed. One used 26 muscle groups (Muscle Study Group 2011), two used 18 muscle groups (Bunch 1980; Dalakas 1993), two used 16 muscle groups (Miller 1992; Villalba 1998) but one of these did not specify the muscle groups used (Miller 1992) and one used 15 muscle groups (Van de Vlekkert 2010). Two published in abstract form only did not include the number of muscle groups used (Coyle 2008; Takada 2002).
In the majority of studies the MMT results were expressed as a sum score, this being the addition of all the scores from all the muscles tested, and maximum sum scores were therefore 80 (Miller 1992; Villalba 1998), 90 (Dalakas 1993), and 140 (Van de Vlekkert 2010). The maximum sum score for one non‐standard strength scale was not given (Bunch 1980) and another quoted a maximum score of 160 (Coyle 2008). One study reported the mean manual muscle strength of all the muscles tested (Muscle Study Group 2011). One trial assessed muscle endurance using repetitive testing with a 1 kg weight on a range of muscle groups that were stated. The maximum score in this test was 56 but it was not stated how this score was obtained (Vencovsky 2000). One trial used myometry of nine muscle groups and hand grip measurements to assess muscle strength (Miller 2002).
The IMACS definition of improvement was used as an outcome in two studies (Coyle 2008; Muscle Study Group 2011), in a modified form in one case (Muscle Study Group 2011).
Two studies assessed time to relapse or treatment failure as an outcome (Muscle Study Group 2011; Van de Vlekkert 2010).
The number of participants in remission and time‐to‐remission was an outcome measure in one study, which compared dexamethasone therapy to prednisolone therapy (Van de Vlekkert 2010).
Cumulative steroid dose was an outcome measure in one 52‐week study comparing etanercept to placebo (Muscle Study Group 2011).
Risk of bias in included studies
Two trials were open label studies (Vencovsky 2000; Villalba 1998). One study had a randomised period followed by an open label cross‐over period which were not reported separately (Coyle 2008). One study included the SF‐36 in the protocol; however, in the abstract the trial authors did not report the result (Takada 2002). Due to limited information many of the 'Risk of bias' domains for the studies were reported as 'unclear' (see 'Risk of bias' tables in the section Characteristics of included studies and Figure 1).
1.
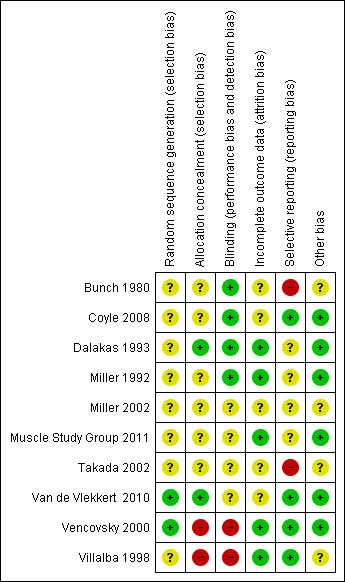
Methodological quality summary: review authors' judgments about each methodological quality item for each included study.
Effects of interventions
Primary outcome measure
Change in function or disability scale at six months
IVIg
In Dalakas 1993 (15 participants), although an activities of daily living (ADL) score was assessed, the results in the two groups were not reported systematically and statistical comparison between the two groups was not reported. A significant improvement in the NSS (measured in 13 participants) was reported for IVIg (44.1 (SD 8.2) pretreatment and 51.4 (SD 6.0) at three months) but not for the placebo group (45.9 (SD 9.0) pretreatment and 45.7 (SD 11.3) at three months). The NSS is a score based on 20 activities, each scored from zero to three, where three signifies no impairment and zero severe impairment.
Azathioprine
One azathioprine trial did not have functional measures as an outcome (Bunch 1980). In another trial (28 participants), the abstract stated there was no significant difference between azathioprine and low dose methotrexate using timed walk as the outcome measure (data not available for analysis) (Miller 2002). In a trial which included participants on azathioprine and methotrexate, ADL score was used as an outcome but the results were only reported in participants who improved according to the trial definition (Villalba 1998).
Plasma exchange or apheresis
In one study the ADL scale measured after just one month of plasma exchange, leukopheresis or placebo was reported as not showing any significant change (Miller 1992, 39 participants, data not supplied).
Methotrexate
In Vencovsky 2000 (36 participants), significant improvement at 6 months in a composite score of muscle endurance and function (maximum score 56) was found in those taking methotrexate, from 24.1 (SD 14.9) to 40 (SD 9.3). Subgroup analysis showed that dermatomyositis and polymyositis participants showed similar changes in the composite score. There was also significant improvement over six months in the 'clinical assessment' score, a composite score of disease manifestations and function, from 12.0 (SD 6.7) to 5.6 (SD 4.6), and the global patient's assessment, the patient's subjective scoring of disability from 0 to 10, from 4.7 (SD 2.0) to 7.3 (SD 2.3).
The comparative arm was those treated with ciclosporin, with no statistical difference noted at six months between methotrexate and ciclosporin for the composite score of muscle endurance and function (MD 6.80, 95% CI ‐1.65 to 15.25) (Analysis 5.1), the 'clinical assessment' score (MD 1.90, 95% CI ‐1.83 to 5.63) (Analysis 5.2) or the global patient's assessment (MD 0.30, 95% CI ‐1.20 to 1.80) (Analysis 5.3).
5.1. Analysis.
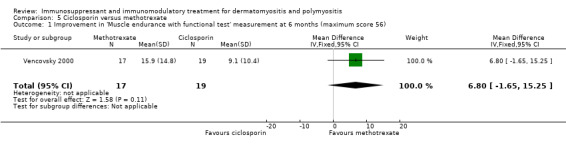
Comparison 5 Ciclosporin versus methotrexate, Outcome 1 Improvement in 'Muscle endurance with functional test' measurement at 6 months (maximum score 56).
5.2. Analysis.
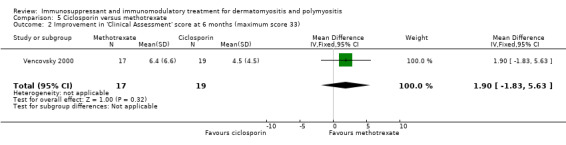
Comparison 5 Ciclosporin versus methotrexate, Outcome 2 Improvement in 'Clinical Assessment' score at 6 months (maximum score 33).
5.3. Analysis.
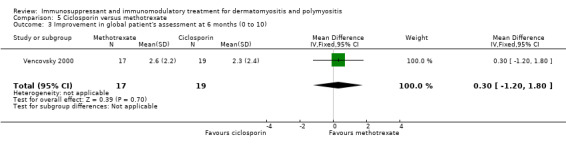
Comparison 5 Ciclosporin versus methotrexate, Outcome 3 Improvement in global patient's assessment at 6 months (0 to 10).
In Villalba 1998, which compared a combination of oral azathioprine and methotrexate to intravenous methotrexate, activities of daily living score was used as an outcome but the results were only reported in participants who improved according to the trial definition.
Ciclosporin
In Vencovsky 2000 (36 participants), significant improvement in a composite score of muscle endurance and function (maximum score was 56) was found in those taking ciclosporin, from 30.5 (SD 12.8) to 39.6 (SD 14.6) at six months. There was also significant improvement over six months in the 'clinical assessment' score, a composite score of disease manifestations and function, from 11.3 (SD 5.5) to 6.8 (SD 5.1), and the global patient's assessment, the patient's subjective scoring of disability from 0 to 10, from 4.5 (SD 2.0) to 6.8 (SD 2.4).
The comparative arm was those treated with methotrexate, with no statistical difference seen between methotrexate and ciclosporin at 6 months for the composite score of muscle endurance and function (MD 6.20, 95% CI ‐2.25 to 14.65) (Analysis 5.1), the 'clinical assessment' score (MD 1.90, 95% CI ‐1.83 to 5.630) (Analysis 5.2) or the global patient's assessment (MD 0.30, 95% CI ‐1.20 to 1.80) (Analysis 5.3).
Infliximab
Beyond the IMACS definitions of improvement, change in function or disability scale was not reported in this study (Coyle 2008).
Etanercept
In a 52‐week pilot study of etanercept compared to placebo (16 participants), no statistically significant differences between treatment groups were found for time to walk 30 feet (s) (MD 1.10, 95% CI ‐6.57 to 8.77) (Analysis 7.6), time to rise from a chair (MD 1.17, 95% CI ‐1.37 to 3.71) (Analysis 7.7), HAQ (MD ‐0.02, 95% CI ‐0.55 to 0.51) (Analysis 7.1), individualized Neuromuscular Quality of Life (INQoL) (MD ‐5.40, 95% CI ‐14.19 to 3.39) (Analysis 7.9) or physical component summary of the SF‐36 at 52 weeks (MD 6.4, 95% CI 1.98 to 10.81) (Analysis 7.10) (Muscle Study Group 2011).
7.6. Analysis.
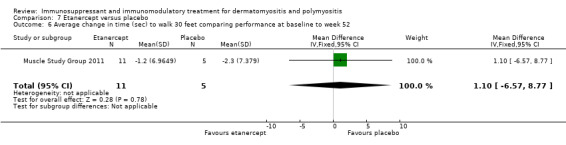
Comparison 7 Etanercept versus placebo, Outcome 6 Average change in time (sec) to walk 30 feet comparing performance at baseline to week 52.
7.7. Analysis.
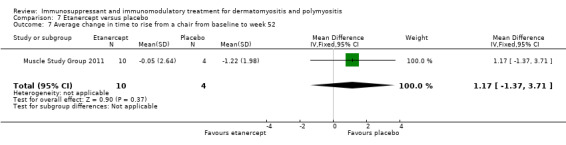
Comparison 7 Etanercept versus placebo, Outcome 7 Average change in time to rise from a chair from baseline to week 52 .
7.1. Analysis.
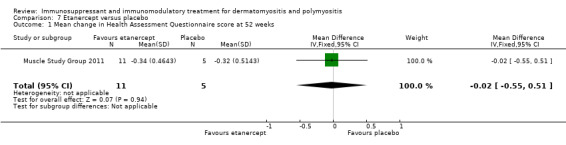
Comparison 7 Etanercept versus placebo, Outcome 1 Mean change in Health Assessment Questionnaire score at 52 weeks.
7.9. Analysis.
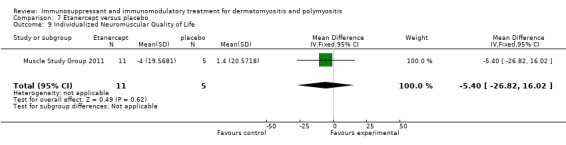
Comparison 7 Etanercept versus placebo, Outcome 9 Individualized Neuromuscular Quality of Life.
7.10. Analysis.
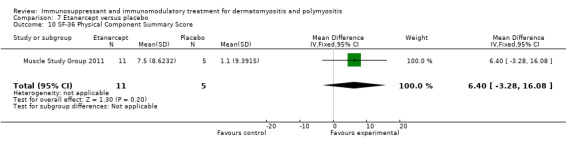
Comparison 7 Etanercept versus placebo, Outcome 10 SF‐36 Physical Component Summary Score.
Eculizumab
In a pilot study of eculizumab compared to placebo, the SF‐36 was included in the study protocol but not reported in the published abstract (Takada 2002).
Dexamethasone
In a study comparing pulsed oral dexamethasone to oral daily prednisolone (62 participants), the NSS showed no significant differences between the two groups at 18 months (MD ‐5.00, 95% CI ‐11.48 to 1.48) (Analysis 8.1) (Van de Vlekkert 2010). We decided not to impute a correlation to calculate the SD of the difference between groups for change from baseline scores as the difference in the means at follow‐up was almost the same as at baseline. The physical function component of the SF‐36 was also reported as showing no significant differences between the two groups at 18 months (full data not supplied).
8.1. Analysis.
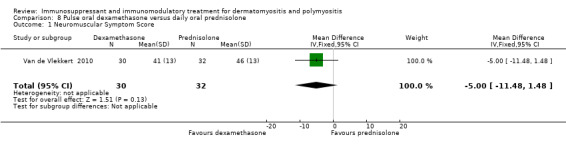
Comparison 8 Pulse oral dexamethasone versus daily oral prednisolone, Outcome 1 Neuromuscular Symptom Score.
15% or greater improvement in muscle strength at six months
Only four trials measured muscle strength at six months or more, using MMT in three (Villalba 1998; Van de Vlekkert 2010; Muscle Study Group 2011) and limited to hand grip in another (Miller 2002); the proportion of participants having a 15% improvement in muscle strength was not an outcome in any of these studies. Only one study used the outcome 15% or greater improvement in muscle strength (Coyle 2008) but this study assessed it at 16 weeks rather than six months.
IVIg
The only trial of IVIg (15 participants) measured muscle strength after just three months (Dalakas 1993). It found a statistically significant improvement in scores of muscle strength (maximum score 90) from 76.6 to 84.6 in the IVIg group but no change in the placebo group. The MD in improvement in muscle strength between the active and placebo group was 9.50 (95% CI 4.33 to 14.67). Using data derived from the figures in the paper, two of the eight participants treated with IVIg achieved ≥15% improvement in muscle strength at three months compared to none of the seven participants in the placebo group (RR 4.44, 95% CI 0.25 to 79.42) (Analysis 1.1).
1.1. Analysis.
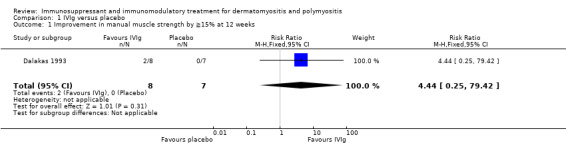
Comparison 1 IVIg versus placebo, Outcome 1 Improvement in manual muscle strength by ≧15% at 12 weeks.
Azathioprine
In a trial of 16 participants with polymyositis, after three months of treatment, muscle strength (maximum score 0, minimum ‐140) increased by 6.5 (SD 23.5) in the azathioprine group compared with 1.1 (SD 12.6) in the placebo group (Bunch 1980). The MD in improvement in muscle strength between the azathioprine group and the placebo group was 5.40 (95% CI ‐13.08 to 23.88) (Analysis 2.1). However, the difference was not statistically significant. In another trial of 28 participants, using change in hand held myometry readings as the primary outcome, the authors reported that azathioprine had equivalent efficacy to methotrexate (Miller 2002), although no specific data were given in the abstract. In a third trial, data on muscle strength were only given in those who improved according to the trial definition (Villalba 1998).
2.1. Analysis.
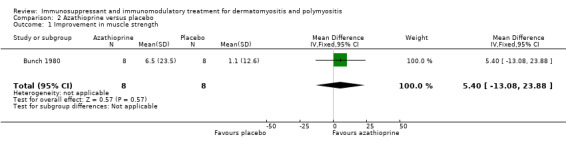
Comparison 2 Azathioprine versus placebo, Outcome 1 Improvement in muscle strength.
Plasma exchange or leukapheresis
The trial of plasma exchange or leukapheresis versus placebo with 39 participants lasted only one month. During this time the RR of muscle strength improvement was not significantly different, being 1.0 (95% CI 0.3 to 3.37) in the active compared with the placebo treatment of the group (Miller 1992) (Analysis 3.1).
3.1. Analysis.
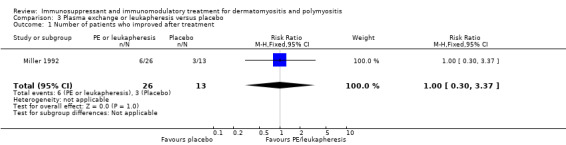
Comparison 3 Plasma exchange or leukapheresis versus placebo, Outcome 1 Number of patients who improved after treatment.
Methotrexate
One trial (28 participants) the investigators reported that hand grip strength after one year did not improve any more with oral methotrexate than with azathioprine (Miller 2002). In a trial of oral methotrexate and azathioprine versus intravenous methotrexate including 30 participants (Villalba 1998), the authors reported no significant difference in muscle strength (maximum score 80) between the two groups at six months (P = 0.50, data not supplied).
Ciclosporin
Muscle strength was not tested for this intervention (Vencovsky 2000).
Infliximab
In a cross‐over study that was unblinded at 16 weeks (when participants on placebo were moved to the active arm), three of the 12 participants achieved a 15% or greater improvement in muscle strength after 16 weeks therapy with infliximab 5 mg/kg compared to none of the six participants during placebo therapy (RR 3.77, 95% CI 0.23 to 63.05) (Analysis 4.1). This difference was not statistically significant (Coyle 2008).
4.1. Analysis.
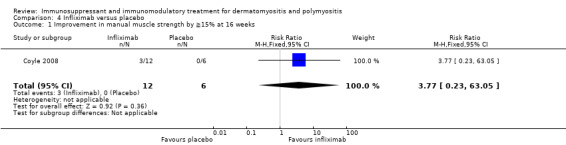
Comparison 4 Infliximab versus placebo, Outcome 1 Improvement in manual muscle strength by ≧15% at 16 weeks.
Etanercept
In a 52‐week randomised, double‐blind, placebo‐controlled trial involving 16 participants, there was no significant difference in the improvement in muscle strength as assessed by MMT (MD 0.06, 95% CI ‐0.15 to 0.27) and quantitative myometry utilizing maximum voluntary isometric contraction testing (MVICT) (MD 0.67, 95% CI ‐0.30 to 2.78) between the treatment groups at 52 weeks. Our primary outcome, a 15% improvement in muscle strength, is a component of the IMACS DOI, but was not reported as individual item in this study (Muscle Study Group 2011).
Eculizumab
In a pilot study, MMT improved by an average of 6% in the actively treated arm (10 participants) after nine weeks of therapy compared to an average of 26% in those who received placebo (three participants) (Takada 2002). These data were from an abstract provided by the manufacturer. No raw data were available for analysis.
Dexamethasone
In an 18‐month placebo‐controlled, double‐blind, randomised trial (62 participants), the mean MMT scores (maximum 140) were 136 (SD 5) in the dexamethasone group and 135 (SD 6) in the prednisolone group at 18 months (Van de Vlekkert 2010).
Secondary outcome measures
Achieving the International Myositis Assessment and Clinical Studies Group (IMACS) definitions of improvement
Only two studies assessed this outcome (Coyle 2008; Muscle Study Group 2011).
In one, which compared infliximab therapy to placebo, seven out of 12 participants improved by the IMACS definition after 16 weeks of therapy with infliximab compared to two out of six participants treated with placebo (RR 1.75, 95% CI 0.51 to 5.98) (Analysis 4.2) (Coyle 2008).
4.2. Analysis.
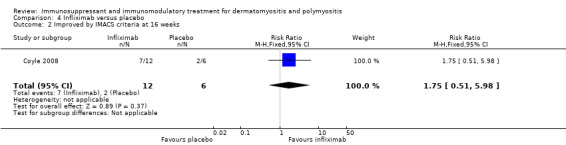
Comparison 4 Infliximab versus placebo, Outcome 2 Improved by IMACS criteria at 16 weeks.
The second, comparing etanercept to placebo, used a modified form of the IMACs DOI in that the average percent of predicted normal MVICT score in addition to the MMT score was used for muscle strength testing (Muscle Study Group 2011). At 24 weeks nine of the 11 participants in the etanercept group achieved this definition of improvement compared to two of the five placebo‐treated participants (RR 2.05, 95% CI 0.67 to 6.20) (Analysis 7.3). At 52 weeks, six of the 11 participants in the etanercept group achieved this definition of improvement compared to three of the five placebo‐treated participants (RR 0.91, 95% CI 0.37 to 2.23) (Analysis 7.4).There was no significant difference between the groups at either of these time points.
7.3. Analysis.
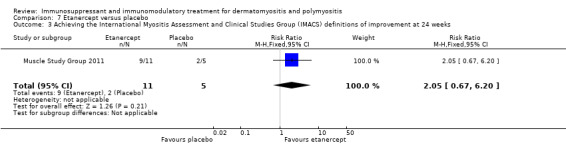
Comparison 7 Etanercept versus placebo, Outcome 3 Achieving the International Myositis Assessment and Clinical Studies Group (IMACS) definitions of improvement at 24 weeks.
7.4. Analysis.
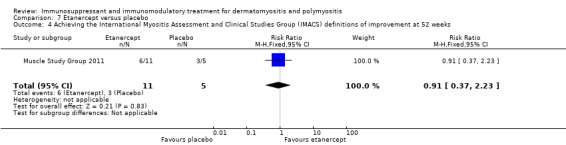
Comparison 7 Etanercept versus placebo, Outcome 4 Achieving the International Myositis Assessment and Clinical Studies Group (IMACS) definitions of improvement at 52 weeks.
Number of relapses and time to relapse
Only two studies assessed time to relapse or treatment failure as an outcome (Muscle Study Group 2011; Van de Vlekkert 2010).
One study compared dexamethasone to prednisolone (Van de Vlekkert 2010). Relapse was defined as 1. a decrease in MRC sum score by four points or more (maximum score 140), or 2. a reduction in the Rankin score by one or more, or 3. a greater than two‐fold increase in serum creatine kinase associated with a reduction in strength or function. At 18 months, 14 of the 30 (47%) dexamethasone‐treated participants had relapsed compared to 12 of the 32 (38%) prednisolone‐treated participants (RR 1.24, 95% CI 0.69 to 2.24) (Analysis 8.3). Median time to relapse was 44 weeks (standard error (SE) 4.7) in the dexamethasone group compare to 60 (SE 2.9) in the prednisolone treated group (log rank test P = 0.03).
8.3. Analysis.
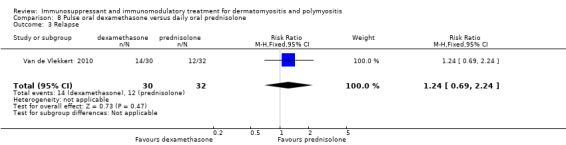
Comparison 8 Pulse oral dexamethasone versus daily oral prednisolone, Outcome 3 Relapse.
The other study compared etanercept to placebo (Muscle Study Group 2011). Treatment failure was said to have occurred if the study physicians felt it necessary to increase the participant's prednisolone and/or change to another agent, or if any one of the following five criteria were fulfilled:1. reduction in the physician global disease activity assessment visual analogue scale by 2 cm or more; 2. worsening of MMT composite score by 20% or more; 3. worsening of oropharyngeal muscle weakness sufficient to compromise nutrition or cause a risk of aspiration; 4. 20% worsening of forced vital capacity or diffusion capacity; 5. no improvement in muscle strength after 12 weeks.
Six of 11 participants in the etanercept arm were treatment failures. All five participants receiving placebo were treatment failures (RR 0.59, 95% CI 0.33 to 1.05) (Analysis 7.8). Median time to treatment failure was 148 days in the placebo arm versus 358 days in the etanercept arm (P = 0.0002).
7.8. Analysis.
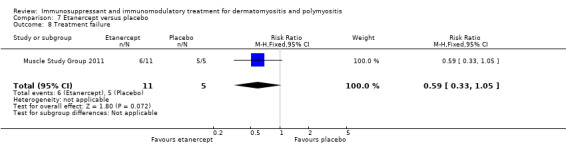
Comparison 7 Etanercept versus placebo, Outcome 8 Treatment failure.
Number of participants in remission, and time‐to‐remission after at least six months
This was an outcome measure in one study, which compared dexamethasone therapy to prednisolone therapy (Van de Vlekkert 2010), and defined remission as a Rankin score of zero or one. At 18 months, five of 30 (17%) dexamethasone‐treated participants and nine of 32 (28%) prednisolone‐treated participants were in remission (RR 0.59, 95% CI 0.22 to 1.57) (Analysis 8.2). Mean time to remission was 58.8 (SE 5.1) weeks in the dexamethasone‐treated group and 58.8 (SE 4.6) weeks in the prednisolone‐treated group (log‐rank test P = 0.73).
8.2. Analysis.
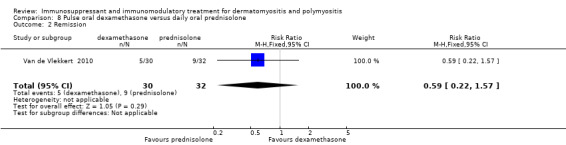
Comparison 8 Pulse oral dexamethasone versus daily oral prednisolone, Outcome 2 Remission.
Cumulative corticosteroid dose after at least six months
This was an outcome measure in one study comparing etanercept to placebo (Muscle Study Group 2011), although the data were not presented in the paper, they were published on the clinicaltrials.gov website. The mean (SD) cumulative prednisolone dose in g over the one year study period was 5.90709 (3.48285) in the etanercept group and 9.91765 (4.76209) in the placebo group (MD ‐4.01, 95% CI ‐8.66 to 0.64) (Analysis 7.5, no significant difference). The median prednisolone dose from weeks 25 to 52 was significantly higher in the placebo group (median 29.2 mg/day, range 9.9 to 62.6 mg/day) than the etanercept group (median 1.2 mg/day, range 0.0 to 31.1 mg/day) (P = 0.02).
7.5. Analysis.
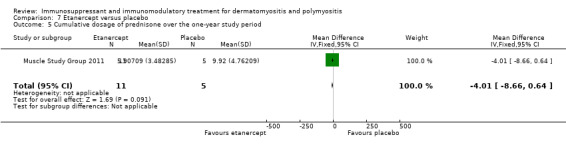
Comparison 7 Etanercept versus placebo, Outcome 5 Cumulative dosage of prednisone over the one‐year study period .
Serious adverse effects
IVIg
In the study of IVIg for dermatomyositis no serious adverse events were reported (Dalakas 1993). In two participants a severe headache occurred with each infusion, requiring treatment with narcotics.
Azathioprine
In the study of azathioprine in polymyositis (Bunch 1980) only 16 of 23 participants completed the study. The other participants failed to complete the study either because they were lost to follow‐up (three), failed to respond to treatment (one on placebo) or experienced adverse effects (three). Two of the three subjects withdrawn from the study due to adverse effects were on azathioprine with one having severe nausea and vomiting and the other developing pneumonitis after one month of treatment. One subject on placebo developed acute diverticulitis and needed surgery. A further subject on azathioprine developed significant leukopenia at the end of the study but this was judged to be unrelated to the study as she was later found to have cyclic neutropenia. Azathioprine was also part of the oral regime in another trial (Villalba 1998) (see under methotrexate).
Plasma exchange or apheresis
Adverse events on apheresis were common in the study comparing plasma exchange, leukapheresis and sham apheresis (Miller 1992). Nine out of 39 participants required placement of a central venous catheter to maintain venous access. Three participants had major vasovagal episodes. Two participants had clinically important citrate reactions; and one participant receiving sham treatments required red cell transfusion for an apheresis‐related decline in haemoglobin. In the plasma exchange group, one participant developed acute transient respiratory distress and one developed herpes zoster.
Methotrexate
Four out of 17 participants receiving oral methotrexate withdrew prematurely from one trial (Vencovsky 2000) because of pancytopenia, gut perforation, acute alveolitis or withdrawal of consent after suffering petechiae. Seven participants had minor adverse events including hypertension and rash which were not sufficient to stop their treatment. Assessment of the adverse effects of methotrexate in another trial (Villalba 1998) is complicated by the fact that oral methotrexate was given in combination with azathioprine and by the cross‐over study design. A total of 28 participants had oral combination therapy (13 of whom had crossed over from the intravenous methotrexate arm). Of these, six had their oral treatment curtailed due to gastrointestinal side effects, and there was one fatality due to Pneumocystis carinii pneumonia. A total of 26 participants had intravenous methotrexate (11 having had prior oral treatment) of whom four had adverse events including gastrointestinal intolerance, abscesses, pseudomonas skin infection and legionella pneumonia. Liver enzyme elevations, infections and gastrointestinal intolerance were common side effects with both oral and intravenous methotrexate (Villalba 1998).
Ciclosporin
In one study two out of 19 subjects taking ciclosporin were withdrawn prematurely due to side effects which were creatinine elevation (one participant) and pneumonia (one participant); a third participant was withdrawn due to the adverse event of non‐compliance (Vencovsky 2000). A further five had minor side effects including hypertension (three participants), bronchitis (one) and bronchopneumonia (one), that did not necessitate withdrawal from the trial (Vencovsky 2000).
Infliximab
Two undisclosed severe adverse events, reported as unrelated to infliximab, occurred in this study. In addition, one participant experienced an infusion reaction and an undisclosed number of mild adverse events occurred (Coyle 2008).
Etanercept
In a small study of 16 participants with dermatomyositis, six severe adverse events were reported in the etanercept group and three in the placebo group (Muscle Study Group 2011). In the etanercept group, the six serious adverse events occurred in three participants, comprising pregnancy and miscarriage in a partner; hospitalization for a urinary tract infection and fever of unknown origin; postherpetic neuralgia and two admissions for psychosis. Two participants in the etanercept group developed positive antinuclear antibodies compared to one of the placebo group (Muscle Study Group 2011). Five participants in the etanercept‐treated group compared to one in the placebo group had worsening of their skin disease.
Eculizumab
In a pilot study of eculizumab compared to placebo the numbers of adverse events was not significantly different between the two groups. The nature of these adverse events was not disclosed. There were no serious adverse events in either group (Takada 2002).
Because the eight trials used different interventions and variable outcome measures, no meta‐analysis was possible.
Dexamethasone
In a study comparing pulsed oral dexamethasone to oral daily prednisolone, there was a high rate of discontinuation of both treatments: 21 out of 30 in the dexamethasone group and 17 out of 32 in the prednisolone group (Van de Vlekkert 2010). The main reasons for discontinuation were relapse at less than six months, no improvement and serious side effects. The dexamethasone‐treated participants had fewer side effects in total, with 22 (79%) suffering any side effect in the dexamethasone group compared to 29 (97%) in the prednisolone‐treated group. The prednisolone group had a greater incidence of mood changes, diabetes mellitus, mean weight gain of more than 5 kg, cushingoid features, skin thinning, gastric symptoms, impaired wound healing, hair loss, infections, acne, hirsutism, hypertension, cataract formation and striae. One adverse event occurred with greater frequency in the dexamethasone group, renal crisis, which occurred in two participants, both in the dexamethasone group.
Subgroup analyses
Although we intended to analyse subgroups, this proved impossible. All the studies examined adult participants, therefore the effect of immunosuppressants on participants under the age of 18 could not be assessed. Participants with and without autoantibodies were not separated into different subgroups when analysing response to treatment. Reason for failed response to corticosteroids was rarely reported and subsequent analyses did not separate participants with inadequate response to corticosteroids versus those who had unacceptable side effects.
Comparison with excluded studies as sensitivity analysis
In contrast to the selected studies, the excluded studies predominantly reported positive results.
Bunch 1981 reported one‐ and three‐year follow‐up data from the 1980 RCT comparing azathioprine with placebo discussed above (Bunch 1980). Muscle strength was not reported but the improvement in functional disability (graded from one to six, one being normal, six unable to walk without assistance) in the azathioprine group (from 4.5 (SD 0.9) to 2.1 (SD 0.6)) was greater than that achieved in the prednisolone only group (from 4 (SD 0.8) to 3 (SD 0.6)). Mean change was 2.4 (SD 1.1) and 1 (SD 0.6) respectively.
Chung and colleagues reported a double‐blind, randomised, placebo‐controlled trial of creatine supplements in participants with established dermatomyositis or polymyositis undergoing a home exercise programme (Chung 2007). There was a significantly greater reduction in the primary outcome, the percentage change in the aggregate functional performance time, in the creatine arm (P = 0.029) at six months (Chung 2007). The aggregate functional performance time consisted of four timed functional activities: a 50 foot timed walk; the 'get up and go test'; a 19‐step stair ascent test; and a 19‐step stair descent test. Muscle strength was reported for individual muscle groups and only showed a significant difference between the two groups at six months, favouring the creatine‐treated arm in four of the ten muscle groups assessed.
Fries 1973 reported a randomised open‐label study comparing 16 weeks of cyclophosphamide therapy with high dose oral prednisolone. The cyclophosphamide dose was titrated to maintain a white cell count of 3500 to 4000 cells/cu mm, this group received no prednisolone. The actual cyclophosphamide dose given averaged 125 mg daily. Where initial white counts permitted, participants in the cyclophosphamide group also received an initial infusion of nitrogen mustard at a dose of 0.4 mg/kg. The prednisolone group were commenced on a dose of 1 mg/kg or greater. Only eight polymyositis subjects were included, with five receiving initial cyclophosphamide therapy and three receiving prednisolone therapy. The trial authors stated that the muscle enzyme and sedimentation rates normalised in the prednisolone group but not the cyclophosphamide group, with a difference that was significant at the five per cent level. Although not stated, as the initial treatment period was 16 weeks it is likely that these data refer to this time period.
Donov 1995 performed a trial of plasmapheresis in 30 children with juvenile dermatomyositis. There was no evidence of randomisation or blinding in the abstract. Four participants received sham plasmapheresis and 26 active therapy. The therapeutic regime consisted of plasmapheresis with a subsequent methylprednisolone dose one to three times a week. Therapy duration varied fom one to 10 weeks. No improvement was seen in the sham plasmapheresis subjects but complete remission seen in 24 participants in the active arm with 'considerably decreased' disease activity in the other two cases. No definitions of complete remission or objective measures of disease activity were given.
Discussion
This systematic review highlights the lack of high quality RCTs that assess the efficacy and toxicity of immunosuppressants in inflammatory myositis. Ten trials were included in the review with only one agent, IVIg demonstrating statistically significant superior efficacy against control, and a second agent, etanercept showing a possible steroid‐sparing effect. Therefore, we have to conclude that there is insufficient evidence from available RCTs to confirm the value of immunosuppressants in inflammatory myositis. For 'Summary of findings' tables see Table 3; Table 4; Table 5; Table 6; Table 7; Table 8; Table 9; Table 10; Table 11; and Table 12.
3. Intravenous immunoglobulin (IVIg) versus placebo for dermatomyositis.
| IVIg versus placebo for dermatomyositis | ||||||
| Patient or population: patients with dermatomyositis and polymyositis Settings: Intervention: IVIg versus placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | IVIg | |||||
| 15% or greater improvement in muscle strength compared with baseline after at least six months | See comment | See comment | Not estimable | ‐ | See comment | No data available, only one study of three months duration |
| Achieving the International Myositis Assessment and Clinical Studies Group (IMACS) definitions of improvement | See comment | See comment | Not estimable | ‐ | See comment | Not measured |
| Change in function or disability scale at six months | See comment | See comment | Not estimable | ‐ | See comment | Although an activities of daily living score was assessed, the results in the 2 groups were not reported systematically and statistical comparison between the 2 groups was not reported. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; IVIg: intravenous immunoglobilin | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
4. Azathioprine versus placebo for dermatomyositis and polymyositis.
| Azathioprine versus placebo for dermatomyositis and polymyositis | ||||||
| Patient or population: patients with dermatomyositis and polymyositis Settings: Intervention: azathioprine | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Azathioprine | |||||
| 15% or greater improvement in muscle strength compared with baseline after at least six months. ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | No data as study only lasted three months |
| Achieving the International Myositis Assessment and Clinical Studies Group (IMACS) definitions of improvement ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | Not measured |
| Change in function or disability scale at six months ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | Not measured |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
5. Azathioprine plus prednisolone versus methotrexate plus prednisolone.
| Azathioprine plus prednisolone compared with methotrexate plus prednisolone for polymyositis or dermatomyositis | ||||||
|
Patient or population: patients with polymyositis or dermatomyositis Settings: Intervention: prednisolone plus either azathioprine 2.5 mg per kg daily Comparison: prednisolone plus methotrexate 15 mg weekly for 1 year | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Low dose methotrexate | Azathioprine | |||||
| 15% or greater improvement in muscle strength compared with baseline after at least 6 months ‐ not reported | See comment | See comment | Not estimable | ‐ | ‐ | Hand‐held myometry used but data not provided |
| Achieving the International Myositis Assessment and Clinical Studies Group (IMACS) definitions of improvement at 52 weeks ‐ not measured | See comment | See comment | Not estimable | ‐ | ‐ | Not measured |
|
Change in function or disability scale at 6 months ‐ not reported |
See comment | See comment | Not estimable | ‐ | ‐ | Timed walk was measured but data not available |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
6. Plasma exchange or leukapheresis versus placebo for dermatomyositis and polymyositis.
| Plasma exchange or leukapheresis versus placebo for dermatomyositis and polymyositis | ||||||
| Patient or population: patients with dermatomyositis and polymyositis Settings: Intervention: plasma exchange or leukapheresis versus placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Plasma exchange or leukapheresis | |||||
| 15% or greater improvement in muscle strength compared with baseline after at least six months ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | One study of only one month duration. |
| Achieving the International Myositis Assessment and Clinical Studies Group (IMACS) definitions of improvement ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | Not measured |
| Change in function or disability scale at six months1 ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | One study of only 1 month duration. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
7. Oral methotrexate with azathioprine versus intravenous methotrexate with leucovorin rescue for dermatomyositis and polymyositis.
| Oral methotrexate with azathioprine versus intravenous methotrexate with leucovorin rescue for dermatomyositis and polymyositis | ||||||
| Patient or population: patients with dermatomyositis and polymyositis Settings: Intervention: oral methotrexate with azathioprine Comparison: intravenous methotrexate with leucovorin rescue | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Intravenous methotrexate with leucovorin rescue | Oral methotrexate with azathioprine | |||||
| 15% or greater improvement in muscle strength compared with baseline after at least six months ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | One study of 1 month's duration |
| Achieving the International Myositis Assessment and Clinical Studies Group (IMACS) definitions of improvement ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | No data |
| Change in function or disability scale at six months ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | No data available. Activities of daily living score was measured but the results were only presented in participants who improved according to the trial definition |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
8. Methotrexate versus ciclosporin for dermatomyositis and polymyositis.
| Methotrexate versus ciclosporin for dermatomyositis and polymyositis | ||||||
| Patient or population: patients with dermatomyositis and polymyositis Settings: Intervention: methotrexate Comparison: ciclosporin | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Ciclosporin | Methotrexate | |||||
| 15% or greater improvement in muscle strength compared with baseline after at least six months ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | Muscle strength data not available. |
| Achieving the International Myositis Assessment and Clinical Studies Group (IMACS) definitions of improvement ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | Not an outcome in this study |
| Change in function or disability scale at six months ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | Function measured but not reported separately from composite score of 'muscle endurance with function'. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
9. Infliximab versus placebo for dermatomyositis and polymyositis.
| Infliximab versus placebo for dermatomyositis and polymyositis | ||||||
| Patient or population: patients with dermatomyositis and polymyositis Settings: Intervention: infliximab versus placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Infliximab | |||||
| 15% or greater improvement in muscle strength compared with baseline after at least six months ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | No data |
| Achieving the International Myositis Assessment and Clinical Studies Group (IMACS) definitions of improvement Follow‐up: 16 weeks | 333 per 1000 | 1000 per 1000 (77 to 1000) | RR 3.77 (0.23 to 63.05) | 18 (1 study) | ⊕⊝⊝⊝ very low1 | ‐ |
| Change in function or disability scale at six months ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | Not measured |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Extremely small numbers and includes open follow‐up.
10. Etanercept versus placebo.
| Etanercept versus placebo | ||||||
| Patient or population: patients with dermatomyositis, either 1. treatment naïve (prednisolone < 2 months) or 2. refractory to therapy with prednisolone > 2 months and either methotrexate (stable dose ≥1 month or more) or intravenous immunoglobulin ≥ 3 months Settings: Intervention: etanercept versus placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Etanercept | |||||
| 15% or greater improvement in muscle strength compared with baseline after at least 6 months ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | Not an outcome in this study |
| Achieving the International Myositis Assessment and Clinical Studies Group (IMACS) definitions of improvement at 24 weeks | 400 per 1000 | 820 per 1000 (268 to 1000) | RR 2.05 (0.67 to 6.2) | 16 (1 study) |
⊕⊕⊕⊝ moderate1 | ‐ |
| Change in function or disability score Various measures Follow‐up: 24 weeks | See comment | See comment | Not estimable | 16 (1 study) | See comment | No significant difference between control and etanercept in any measure of function or disability (final values) (see text) |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Pilot study with small sample size of 16 participants. Wide 95% CI of RR (0.75 to 2.45).
11. Eculizumab versus placebo for dermatomyositis and polymyositis.
| Eculizumab versus placebo for dermatomyositis and polymyositis | ||||||
| Patient or population: patients with dermatomyositis and polymyositis Settings: Intervention: eculizumab versus placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Eculizumab | |||||
| 15% or greater improvement in muscle strength compared with baseline after at least six months ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | Single study of only 9 weeks' duration. |
| Achieving the International Myositis Assessment and Clinical Studies Group (IMACS) definitions of improvement ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | Not an outcome in this study |
| Change in function or disability scale at six months ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | Single study of only 9 weeks' duration. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
12. Pulse oral dexamethasone versus daily oral prednisolone for dermatomyositis and polymyositis.
| Pulse oral dexamethasone versus daily oral prednisolone for dermatomyositis and polymyositis | ||||||
| Patient or population: patients with dermatomyositis and polymyositis, non‐specific myositis, myositis with a co‐existing connective tissue disease or with cancer within 2 years before onset of myositis, disease of less than one year duration. On no more than 20 mg prednisolone and no other immunosuppressant or immunomodulatory therapy Settings: Intervention: pulse oral dexamethasone versus daily oral prednisolone | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Daily oral prednisolone | Pulse oral dexamethasone | |||||
| 15% or greater improvement in muscle strength compared with baseline after at least 6 months ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | Not an outcome in this study |
| Achieving the International Myositis Assessment and Clinical Studies Group (IMACS) definitions of improvement at 52 weeks ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | Not an outcome in this study |
|
Change in function or disability scale
Neuromuscular Symptom Score Scale from: 0 to 60 Follow‐up: 18 months |
The mean function or disability scale score in the control group was 461 | The mean function or disability scale score in the intervention groups was 5 lower (11.48 lower to 1.48 higher) | ‐ | 62 (1 study) | ⊕⊕⊕⊝ moderate2 | ‐ |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Mean final NSS in placebo group.
2 Only one study of 62 participants with 61% discontinuing the study early.
This conclusion appears to contradict the experience of many clinicians. However, the lack of good evidence is not the same as no evidence. The lack of RCTs in inflammatory myositis is typical of the problem faced by evidence‐based medicine. Conducting high quality RCTs in rare diseases is extremely difficult. Yet without data from high quality RCTs, it is impossible for clinicians to assess the benefit/risk ratio of immunosuppressants in myositis accurately. The trial of plasma exchange and leukapheresis is a good example of where a treatment is ineffective but has significant side effects. Indeed, this review found that immunosuppressants are associated with significant side effects. Clearly, it is important for physicians and participants to appreciate the precise benefit and risk of immunosuppressants in inflammatory myositis. Participants with inflammatory myositis are managed by different specialists: neurologists, rheumatologists, dermatologists or general physicians. Since high quality RCTs require sufficient sample sizes to deliver the necessary statistical power, individual clinicians rarely have a sufficient number of potential participants under their care to conduct trials. Therefore, multicentre trials with collaboration between all these specialists are the only means to study this rare disease. In recent years new collaborative efforts such as the International Myositis Assessment and Clinical Studies Group (IMACS), the European Myositis Network (EUMYONET) and the United Kingdom Myositis Network (UK MYONET) have formed to foster such collaboration.
Another major obstacle in evidence‐based medicine in inflammatory myositis has been the lack of international consensus on outcome measures and how data should be presented in publications. However, in recent years the International Myositis Assessment and Clinical Studies Group (IMACS) has developed international multidisciplinary outcome measures (Isenberg 2004; Oddis 2005; Rider 2004). These include the 'definitions of improvement' which comprise six core set measures among five domains (Oddis 2005; Rider 2004). These core set measures are: the physician global disease activity, parent/patient global disease activity, muscle strength (MMT), physical function assessment, laboratory assessment and extramuscular disease complications. Improvement is defined as occurring if three of any six core set measures improve by 20%, with no more than two worsening by 25% (measures that worsen cannot include manual muscle strength).
Most of the studies included in this review used the Bohan and Peters diagnostic criteria (Bohan 1975 a; Bohan 1975 b) which were published well over thirty years ago. An international workshop on clinical trials in inflammatory myositis highlighted the deficiency of the Bohan and Peters criteria in the diagnosis of polymyositis (Hoogendijk 2004). Many of these participants in fact had diagnoses other than polymyositis, especially inclusion body myositis and occasionally dystrophies with associated inflammatory features.
In the absence of adequate RCTs to address the question of which immunosuppressant treatment might be best to use in these inflammatory myopathies, it would be reasonable to look at any non‐randomised trials that might help. However, Van de Vlekkert 2004 undertook a MEDLINE and EMBASE search from 1966 to 2001 for French, German, or English reports of treatment in dermatomyositis and polymyositis. They found 92 eligible papers describing a total of 915 participants (92 of whom were duplicated) in 74 single case reports and 18 case series. These reports were reviewed for 10 standards that were thought important to allow any reader to recognise their own participant, copy the treatment and have some idea of the treatment effect. The authors concluded that the majority of the reports were of dubious quality and thus a meaningful systematic review of case reports was unrealistic.
Authors' conclusions
Implications for practice.
The small number of randomised trials of immunosuppressants and immunomodulatory therapies are inadequate to decide whether these agents are beneficial in dermatomyositis and polymyositis. Two small trials in dermatomyositis one of intravenous immunoglobulin and one of etancercept suggest that these agents may be beneficial.
Implications for research.
More research is needed to investigate the efficacy of immunosuppressant and immunomodulatory agents in dermatomyositis and polymyositis.
What's new
| Date | Event | Description |
|---|---|---|
| 16 August 2016 | Review declared as stable | This review is no longer being updated. It will be replaced by two new reviews. See Published notes. |
History
Protocol first published: Issue 2, 2002 Review first published: Issue 3, 2005
| Date | Event | Description |
|---|---|---|
| 13 August 2012 | Amended | Minor corrections to wording in abstract and objectives |
| 7 March 2012 | New citation required and conclusions have changed | New studies included with new interventions. Conclusions changed. |
| 22 August 2011 | New search has been performed | Searches were updated to August 2011 and four new trials were included. 'Risk of bias' and 'Summary of findings' tables were added. No new authors but change in order of listing. |
| 29 May 2008 | Amended | Converted to new review format. |
| 10 March 2005 | New citation required and conclusions have changed | Substantive amendment |
Notes
This review is no longer being updated. It will be replaced by two Cochrane reviews, Biologics for dermatomyositis and polymyositis, and Immunosuppressant and immunomodulatory treatment other than biologics for dermatomyositis and polymyositis, planned for 2016 and 2017.
Acknowledgements
Bryan Lecky withdrew from authorship of this updated review due to time constraints. However, in the previous version of the review, as an author, he searched for trials, assessed methodological quality and extracted data.
The editorial base of the Cochrane Neuromuscular Disease Group is supported by the MRC Centre for Neuromuscular Diseases and the Muscular Dystrophy Campaign.
Appendices
Appendix 1. CENTRAL search strategy
#1"ADRENAL CORTEX HORMONES" OR CORTICOSTEROID* OR ANTIMETABOLITES OR ANTI‐METABOLITES #2AZATHIOPRINE OR MERCAPTOPURINE OR METHOTREXATE OR CICLOSPORIN* OR CYCLOSPORIN* OR CHLORAMBUCIL OR CYCLOPHOSPHAMIDE #3IMMUNOGLOBULIN* OR INTERFERON* OR "GAMMA GLOBULIN*" #4"PLASMA EXCHANGE" OR PLASMAPHERESIS OR "IMMUNOSUPPRESSIVE AGENT*" OR ADJUVANTS NEXT IMMUNOLOGIC OR IMMUNOMODULAT* #5(#1 OR #2 OR #3 OR #4) #6DERMATOMYOSITIS OR POLYMYOSITIS OR MYOSITIS #7(#5 AND #6)
Appendix 2. MEDLINE (OvidSP) search strategy
1 Adrenal Cortex Hormones/ 2 corticosteroid$.mp. 3 anti‐metabolites.mp. 4 Antimetabolites/ 5 antimetabolites.mp. 6 Azathioprine/ 7 azathioprine.mp. 8 6‐Mercaptopurine/ 9 Mercaptopurine.mp. 10 Methotrexate/ 11 methotrexate.mp. 12 ciclosporin.mp. 13 Cyclosporine/ 14 cyclosporin.mp. 15 Chlorambucil/ 16 chlorambucil.mp. 17 Cyclophosphamide/ 18 cyclophosphamide.mp. 19 Immunoglobulins/ 20 immunoglobulin$.mp. 21 Interferons/ 22 interferon$.mp. 23 gamma‐Globulins/ 24 gamma Globulin$.mp. 25 Plasma Exchange/ 26 plasma exchange$.mp. 27 Plasmapheresis/ 28 Plasmapheresis.mp. 29 Immunosuppressive Agents/ 30 immunosuppress$.mp. 31 Adjuvants, immunologic/ 32 immunomodulat$.mp. 33 or/1‐30 34 Dermatomyositis/ 35 dermatomyositis.mp. 36 Polymyositis/ 37 polymyositis.mp. 38 Myositis/ 39 myositis.mp. 40 or/34‐39 41 33 and 40 42 randomized controlled trial.pt. 43 controlled clinical trial.pt. 44 randomized controlled trials/ 45 random allocation/ 46 double‐blind method/ 47 single‐blind method/ 48 or/42‐47 49 animals/ not humans/ 50 48 not 49 51 clinical trial.pt. 52 exp clinical trial/ 53 (clin$ adj25 trial$).ti,ab. 54 ((singl$ or doubl$ or tripl$ or trebl$) adj25 (blind$ or mask$)).ti,ab. 55 placebos/ 56 placebo$.ti,ab. 57 random$.ti,ab. 58 research design/ 59 or/51‐58 60 59 not 49 61 60 not 50 62 comparative study/ 63 exp evaluation studies/ 64 follow up studies/ 65 prospective studies/ 66 (control$ or prospectiv$ or volunteer$).ti,ab. 67 or/62‐66 68 67 not 49 69 68 not (50 or 61) 70 50 or 61 or 69 71 41 and 70
Appendix 3. EMBASE (OvidSP) search strategy
1 Randomized Controlled Trial/ 2 Clinical Trial/ 3 Multicenter Study/ 4 Controlled Study/ 5 Crossover Procedure/ 6 Double Blind Procedure/ 7 Single Blind Procedure/ 8 exp RANDOMIZATION/ 9 Major Clinical Study/ 10 PLACEBO/ 11 Meta Analysis/ 12 phase 2 clinical trial/ or phase 3 clinical trial/ or phase 4 clinical trial/ 13 (clin$ adj25 trial$).tw. 14 ((singl$ or doubl$ or tripl$ or trebl$) adj25 (blind$ or mask$)).tw. 15 placebo$.tw. 16 random$.tw. 17 control$.tw. 18 (meta?analys$ or systematic review$).tw. 19 (cross?over or factorial or sham? or dummy).tw. 20 ABAB design$.tw. 21 or/1‐20 22 human/ 23 nonhuman/ 24 22 or 23 25 21 not 24 26 21 and 22 27 25 or 26 28 exp Corticosteroid/ 29 adrenal cortex hormone$1.mp. 30 Antimetabolite/ 31 (antimetabolites or anti‐metabolites).mp. 32 Azathioprine/ 33 azathioprine.mp. 34 Mercaptopurine/ 35 mercaptopurine.mp. 36 METHOTREXATE/ 37 methotrexate.mp. 38 Cyclosporin/ 39 cyclosporin.mp. 40 CHLORAMBUCIL/ 41 chlorambucil.mp. 42 ciclosporin.mp. 43 CYCLOPHOSPHAMIDE/ 44 cyclophosphamide.mp. 45 Immunoglobulin/ 46 immunoglobulin$1.mp. 47 INTERFERON/ 48 interferon$1.mp. 49 gamma‐globulin.mp. or Immunoglobulin/ 50 plasma exchange.mp. or Plasmapheresis/ 51 (plasma exchange or plasmapheresis).mp. 52 Immunosuppressive Agent/ 53 immunosuppressive agent$1.mp. 54 Immunological Adjuvant/ 55 (immunologic adj1 adjuvant$1).mp. 56 Immunomodulation/ 57 immunomodulat$.mp. 58 or/28‐57 59 Dermatomyositis/ 60 dermatomyositis.mp. 61 POLYMYOSITIS/ 62 polymyositis.mp. 63 MYOSITIS/ or myositis.mp. 64 or/59‐63 65 58 and 64 66 27 and 65
Appendix 4. clinicaltrials.gov search strategy
1. Dermatomyositis
2. Polymyositis
3. Myositis
Data and analyses
Comparison 1. IVIg versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Improvement in manual muscle strength by ≧15% at 12 weeks | 1 | 15 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.44 [0.25, 79.42] |
Comparison 2. Azathioprine versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Improvement in muscle strength | 1 | 16 | Mean Difference (IV, Fixed, 95% CI) | 5.4 [‐13.08, 23.88] |
Comparison 3. Plasma exchange or leukapheresis versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of patients who improved after treatment | 1 | 39 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.30, 3.37] |
Comparison 4. Infliximab versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Improvement in manual muscle strength by ≧15% at 16 weeks | 1 | 18 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.77 [0.23, 63.05] |
| 2 Improved by IMACS criteria at 16 weeks | 1 | 18 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.75 [0.51, 5.98] |
Comparison 5. Ciclosporin versus methotrexate.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Improvement in 'Muscle endurance with functional test' measurement at 6 months (maximum score 56) | 1 | 36 | Mean Difference (IV, Fixed, 95% CI) | 6.80 [‐1.65, 15.25] |
| 2 Improvement in 'Clinical Assessment' score at 6 months (maximum score 33) | 1 | 36 | Mean Difference (IV, Fixed, 95% CI) | 1.90 [‐1.83, 5.63] |
| 3 Improvement in global patient's assessment at 6 months (0 to 10) | 1 | 36 | Mean Difference (IV, Fixed, 95% CI) | 0.30 [‐1.20, 1.80] |
Comparison 6. Oral methotrexate with azathioprine versus intravenous methotrexate with leucovorin rescue.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Improvement as defined by 'combined evaluation of strength and function' tool | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.67 [0.87, 8.15] |
6.1. Analysis.
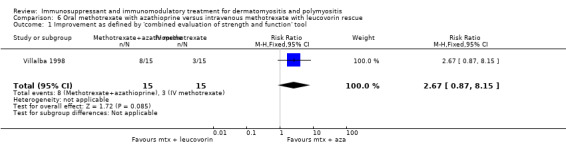
Comparison 6 Oral methotrexate with azathioprine versus intravenous methotrexate with leucovorin rescue, Outcome 1 Improvement as defined by 'combined evaluation of strength and function' tool.
Comparison 7. Etanercept versus placebo.
7.2. Analysis.
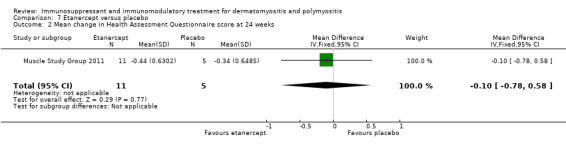
Comparison 7 Etanercept versus placebo, Outcome 2 Mean change in Health Assessment Questionnaire score at 24 weeks.
Comparison 8. Pulse oral dexamethasone versus daily oral prednisolone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Neuromuscular Symptom Score | 1 | 62 | Mean Difference (IV, Fixed, 95% CI) | ‐5.0 [‐11.48, 1.48] |
| 2 Remission | 1 | 62 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.22, 1.57] |
| 3 Relapse | 1 | 62 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.69, 2.24] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bunch 1980.
| Methods | Double‐blind placebo‐controlled trial | |
| Participants | 23 participants with previously untreated polymyositis, but only the 16 participants completing the study were included in final analysis Age in years (SD) of 16 completers: azathioprine group, 38.3 (11.8); placebo group, 40.9 (10.6) Sex of 16 completers: azathioprine group, 5 females, 3 males; placebo group, 2 males, 6 females Mean disease duration (SD) of completers: azathioprine group, 8.6 (6) months, placebo group, 9.6 (9) months Established criteria not quoted but inclusion criteria should satisfy the Bowan and Peter criteria for definitive polymyositis |
|
| Interventions | 60 mg of prednisolone per day plus either azathioprine (2 mg/kg/day) or placebo for 3 months | |
| Outcomes | MMT, CK, muscle biopsy after 3 months | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Methodology for producing random sequence not reported |
| Allocation concealment (selection bias) | Unclear risk | Methods for concealing allocation sequence not described beyond the study being double‐blind, placebo‐controlled |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blinded study with placebo medication for control group |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Significant attrition: 7 participants, of whom 3 were lost to follow up, 4 withdrew due to due side‐effects, failure of therapy or intercurrent illness |
| Selective reporting (reporting bias) | High risk | 7 participants who failed to complete the study were excluded from the final analysis |
| Other bias | Unclear risk | Not clear |
Coyle 2008.
| Methods | 16‐week randomised, double‐blind, placebo‐controlled trial with subsequent open label cross‐over of placebo participants to active arm and active participants to dose escalation | |
| Participants | 11 participants with polymyositis and 1 with dermatomyositis
Age in years (SD): 45.4 (10.9)
Sex: 11 female and 1 male
Mean disease duration (SD): 5.6 (4.0) years Probable or definite refractory dermatomyositis or polymyositis by Bohan and Peter criteria Previously failed or had intolerance to immunosuppressive therapy. Baseline MMT score 80 to 120 (normal 160). Stable prednisolone (≤ 0.5 mg/kg/day) and immunosuppressive dose for at least 4 weeks |
|
| Interventions | Active arm: infliximab 5 mg/kg at weeks 0, 2, 6 and 14. Non‐responders based on IMACS criteria then increased to open label infliximab 7.5 mg/kg at weeks 22, 30 and 38 Placebo arm: placebo at weeks 0, 2, 6 and 14. Non‐responders based on IMACS criteria then given infliximab 5 mg/kg at weeks 16, 18, 22, 30 and 38 |
|
| Outcomes | ≥15% MMT improvement from baseline Improvement as defined by IMACS |
|
| Notes | Data from the open label and double‐blind sections of the study presented together | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Methodology for producing random sequence not reported in abstract |
| Allocation concealment (selection bias) | Unclear risk | Insufficient detail reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Blinded period lasted 16 weeks, then open follow‐up |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Attritions and exclusions not reported in abstract |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Low risk | No evidence to suggest other bias |
Dalakas 1993.
| Methods | Double‐blind RCT | |
| Participants | 15 participants with dermatomyositis: 8 in IVIg group, 7 in placebo group
Age in years: mean 36, range 18 to 55 years
Sex: 10 females, 5 males
Mean disease duration: IVIg group 3.9 years, placebo group 3.8 years No classification criteria used but participants quoted to have all had a diagnostic muscle biopsy, rash and progressive muscle weakness Participants had dermatomyositis that "had become unresponsive or poorly responsive to high‐dose prednisolone or therapeutic doses of another immunosuppressant (methotrexate, azathioprine or cyclophosphamide) given for at least four to six months." Nine participants were taking concomitant immunosuppressive agents (azathioprine (5), methotrexate (3) or cyclophosphamide (1)) during the study |
|
| Interventions | IVIg (2 g/kg) or placebo per month for 3 months | |
| Outcomes | MMT, NSS, ADL Score (based on Barthel index), CK, photographs of rashes, muscle biopsy after 3 months of treatment | |
| Notes | IVIg was beneficial | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Block‐randomisation used but further methodology on randomisation not provided |
| Allocation concealment (selection bias) | Low risk | Randomisation in pharmacy, code not broken until completion of the study |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Medication covered by opaque plastic to hide medication from participants and investigators. Stated that no inadvertent disclosure of randomisation but that all but 1 participant correctly identified the therapy they were on |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Results for all recruited participants reported, although no clear statement on attrition or exclusions made |
| Selective reporting (reporting bias) | Unclear risk | One of the secondary outcomes, ADL score was not reported systematically and statistical comparison between the two groups was not reported |
| Other bias | Low risk | No evidence to suggest other bias |
Miller 1992.
| Methods | Double‐blind RCT | |
| Participants | 39 participants: 19 with polymyositis, 16 with dermatomyositis and 4 with overlap syndromes (42 originally recruited, 3 excluded from analysis: 2 on later muscle biopsy had IBM, 1 withdrew for personal reasons after 1 week of therapy) Plasma exchange group:
Leukapheresis group:
Sham apheresis group:
Participants had had "an incomplete response to high‐dose prednisone therapy (≥ 1mg per kilogram of body weight per day for at least one month), the need for a prednisone dose of at least 0.25 mg per kilogram per day, or the occurrence of unacceptable side effects during the administration of the dose of corticosteroid needed to control disease". "Twenty‐nine of the patients had previously not responded to at least one adequate trial of cytotoxic therapy" Bohan and Peter criteria used |
|
| Interventions | Plasma exchange, leukapherisis or sham apherisis 12 treatments over a 1‐month period | |
| Outcomes | MMT, ADL Score, CK at 1 month | |
| Notes | No benefit | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | During apheresis evaluators not present, cell separator device and attached tubing and component bags shielded from view |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3 participants excluded from analysis. 1 withdrew after 1st week due to personal reasons. 2 excluded as later biopsy showed IBM |
| Selective reporting (reporting bias) | Unclear risk | 2 outcomes described in the methods but other measures including change in CK and change in lymphocyte count reported in results |
| Other bias | Low risk | No evidence to suggest other bias |
Miller 2002.
| Methods | Double‐blind RCT | |
| Participants | 28 participants with polymyositis or dermatomyositis Data on age, sex and disease duration not given |
|
| Interventions | Prednisolone plus either azathioprine 2.5 mg per kg daily or methotrexate 15 mg weekly for 1 year | |
| Outcomes | Muscle strength measured by hand held myometry, timed walks, final steroid dose and side effects | |
| Notes | Equivalent efficacy but methotrexate was better tolerated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported in abstract |
| Allocation concealment (selection bias) | Unclear risk | Not reported in abstract |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No details reported beyond study randomised, double‐blind, placebo‐controlled trial |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
Muscle Study Group 2011.
| Methods | Randomised, double‐blind, placebo‐controlled trial | |
| Participants | 16 participants with adult dermatomyositis.
Mean age in years (range): 43.63 (21 to 61)
Sex:10 female and 6 male
Definite dermatomyositis by Bohan and Peter criteria In active arm 3 newly diagnosed, treatment‐naïve patients and 8 refractory patients with a mean baseline prednisone dosage of 45 mg/day. Mean disease duration 1.1 years, SD 0.8 In placebo arm, 2 newly diagnosed, treatment naïve patients and 3 refractory patients with a mean baseline prednisone dosage of 39 mg/day. Mean disease duration 2.2 years, SD 3.4 |
|
| Interventions | New patients were started on prednisone 60 mg daily. Refractory patients remained their maintenance prednisolone dosage for 2 months or had the dose increased to 60 mg at the discretion of their treating physician After treatment with prednisolone for 2 months, participants received etanercept 50 mg/week or placebo for 52 weeks. This was followed in all participants by tapering of the prednisolone over 24 weeks starting after first treatment with etanercept or placebo |
|
| Outcomes |
Outcome Measures in clinicaltrials.gov summarised
Primary
Occurrence of at least one adverse event (at each visit during the 12 month study)
Tolerability (at any point between baseline (week 0) and the end of the study (week 52)
Average change in each of the following from baseline to week 52 (at baseline (week 0) and week 52): oral temperature, respiration rate, systolic BP, diastolic BP, pulse, body weight in kg
Frequency of subjects with treatment emergent, clinically significant, abnormal laboratory values from baseline to week 52 (at screening, baseline (week 0), weeks 4, 8, 12, 16, 20, 24, 32, 40, and 52) in the following: CK, alanine aminotransferase, gamma‐glutamyl transpeptidase, aldolase, glucose, potassium, white blood cell count, haemoglobin, haematocrit, platelet counts, urine leukocyte values, urine protein laboratory values, urine glucose, urine ketone
Frequency of subjects with treatment emergent, clinically significant, abnormal serum 25‐hydroxyvitamin D laboratory values from the screening visit to week 52 (screening visit and week 52)
Frequency of subjects with treatment emergent, clinically significant, abnormal antinuclear antibody test (ANA) values from the screening visit to week 52 (at screening, weeks 12, 24, 40, and 52)
Frequency of subjects with treatment emergent, clinically significant, abnormal monoclonal protein detection by serum protein electrophoresis from the screening visit to week 52 (screening visit, weeks 12, 24, 40, and 52)
Average cumulative dosage of prednisone over the 1 year study period (baseline until week 52)
Secondary
Average prednisone dosage after week 24 (from week 24 to 52)
Average daily dose of prednisone from baseline to week 52 (baseline through week 52)
‘Other Pre‐specified’
The number of participants who were classified as treatment failures (at any point during the 52 week study)
From baseline to week 52 (at baseline (week 0) and week 52):
•change in the average MMT Score
•average change in time to rise from a chair
•average change in time (s) to walk 30 feet
•average change in Z‐score for DEXA of the femur
•average change in Z‐score for DEXA of the lumbar spine
•average change in physician global activity assessment
•average change in patient global activity assessment score
•average change in Cutaneous Disease Activity and Severity Index (CDASI) score
•change in pruritis rating
•change in HAQ score
•forced vital capacity (FVC) average change in percent predicted
•average change in percent predicted forced expiratory volume in 1 s (FEV1)
•average change in percent predicted diffusion capacity (DLCO) Serious adverse events Other adverse events Outcome measures in published paper Primary Adverse events "Time from randomization to treatment failure" "Average prednisolone dosage after week 24" Secondary Cumulative and average MMT scores Composite maximum voluntary isometric contraction testing (MVICT) scores Myositis Intention to Treat Activity Index (MITAX) Myositis Disease Activity Assessment Visual Analogue Scales (MYOACT) "Subject and physician assessments of global disease activity utilizing Likert and visual analogue scales (VAS)" "Time to arise from a chair and walk 30 feet" HAQ "A modified cutaneous disease activity score index" Patient VAS for pruritis "Relevant components of the MYOACT and MITAX" SF‐36 Individualized Neuromuscular Quality of Life Questionnaire IMACS‐recommended definitions of improvement |
|
| Notes | Initially designed to enrol 40 newly diagnosed patients. Some slight but not significant differences in results data between published study and results section on clinicaltrials.gov. Modified form of the IMACS DOI used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only 2 participants withdrew from the study, one lost to follow up in the etanercept group, 1 due to lack of perceived effect in the placebo group |
| Selective reporting (reporting bias) | Unclear risk | Many of the numerous prespecified outcomes on the clinicaltrials.gov website are not reported in the paper. However, these results are readily available on the clinicaltrials.gov website. Notably average prednisone dosage after week 24, a secondary outcome on the clinicaltrial.gov website, is reported in the paper but the primary outcome, average cumulative dosage of prednisone over the 1 year study period, is not. Several of the outcomes reported in the published paper were not amongst the primary or secondary outcomes published on clinicaltrials.gov website. Some of these are reported in the results section of clinicaltrials.gov as pre‐specified outcomes. Notably, the missing outcomes would be not be possible to measure retrospectively |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Takada 2002.
| Methods | Double‐blind RCT | |
| Participants | 13 participants with dermatomyositis: 10 treated with eclulizumab, 3 treated with placebo Data on age and sex not given Disease duration: ≥ 6 months Eligibility criteria required included: a MMT score ≤136 out of 170, "Patients with persistent disease (defined as active rash plus CK greater than or equal to 2 times ULN, or rapidly progressive disease, or response to steroids with inability to taper dose, or unacceptable side effects of steroids." Participants could be on a stable dose of methotrexate or azathioprine |
|
| Interventions | Eclulizumab 8 mg/kg weekly for 5 doses then 2‐weekly for a further 2 doses. For a total of 10 weeks therapy or placebo | |
| Outcomes | Adverse events MMT score Muscle enzyme level MRI findings SF‐36 Skin findings and skin biopsy |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported beyond study double‐blind and placebo‐controlled |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported beyond study double‐blind and placebo‐controlled |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No attrition reported |
| Selective reporting (reporting bias) | High risk | SF‐36 assessed but not reported in published abstract |
| Other bias | Unclear risk | Insufficient information |
Van de Vlekkert 2010.
| Methods | Double‐blind RCT | |
| Participants | 62 adult participants with treatment‐naive inflammatory myopathies (sporadic IBM excluded) | |
| Interventions | 28‐day cycles of oral high‐dose dexamethasone or daily high‐dose prednisolone Dexamethasone: 6 cycles, 40 mg/day for 4 consecutive days at 28‐day intervals Prednisolone: 70 mg/day starting dose (body weight < 70 kg) or 90 mg/day (body weight ≥ 70 kg) for 28 days. Then slow tapering for 44 or 52 weeks, depending on the starting dose. Dosage decreased every week by 5 mg every other day |
|
| Outcomes |
Primary outcome measures 7‐point composite score of 6 outcomes (Time to) remission and relapse Secondary outcome measures Treatment failure Adverse events Serum CK activity Rankin score (0 to 5) MRC sum score Presence and VAS for pain (0 to 100) Dysphagia Skin changes Presence of arthralgia or Raynaud's NSS (maximum score of 60) SF‐36 |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomisation was performed by the pharmacist of one of the organising hospitals (University Medical Center Utrecht) with a computer‐generated randomisation list, applying stratification by diagnosis." |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomisation was performed by the pharmacist of one of the organising hospitals (University Medical Center Utrecht)" |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Participants, investigator and treating physican blinded to treatment and placebo used. However, reported in paper that 69% of participants guessed their allocation correctly and the first author guessed the allocation correctly in 68% of cases. Allocation unintentially revealed in one participant |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | All participants followed but 61% discontinued allocated medication before end of trial at 18 months. Some differences in the reasons for discontinuation between the groups |
| Selective reporting (reporting bias) | Low risk | No suggestion of selective reporting of outcomes |
| Other bias | Low risk | Stopped early due to rarity of patient group. Free from other problems |
Vencovsky 2000.
| Methods | RCT | |
| Participants | 20 participants with dermatomyositis and 16 with polymyositis
Ciclosporin (n = 17); methotrexate (n = 19)
Age in years (SD): ciclosporin group 42.6 (12), methotrexate group 38.4 (22.2)
Sex: 23 females and 13 males
Disease duration (SD): ciclosporin group 28 (35) months, methotrexate group 30 (72) months All participants fulfilled Bohan and Peter's definite criteria 30 participants had newly diagnosed disease, 6 participants relapse of previously controlled disease. All participants had weakness on MMT of at least degree 3 in at least 2 muscle groups and "severe and active" disease as judged by a physician |
|
| Interventions | Ciclosporin 3.0 to 3.5 mg/kg/day versus oral methotrexate 7.5 to 15 mg/weekly for 6 months. All participants were treated with prednisone at a dose 0.5 to 1.0 mg/kg/day | |
| Outcomes | Muscle endurance and functional test. Maximum score 56. Improvement defined as an increase of at least 6 compared to baseline, or if original score < 36, an increase to 42 Clinical assessment: clinical composite score. Maximum score 33. Improvement defined as an increase of at least 40% compared to baseline Global patient’s assessment, VAS 0 to 10. Improvement defined as an increase of 2 points or more compared to baseline Muscle MRI as study entry and 3 months Laboratory features including: CK, myoglobin, serum interleukin‐1 receptor antagonist and c‐reactive protein |
|
| Notes | No statistically significant difference between the two groups | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation using a sequence of numbers generated by a computer program |
| Allocation concealment (selection bias) | High risk | Open label trial design |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open label trial design |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 7 participants, 4 in the methotrexate group and 3 in the ciclosporin group, discontinued the study due to side effects. Those who discontinued after the 12 week evaluation were included in the semiquantitative evaluation |
| Selective reporting (reporting bias) | Low risk | Assessments described in the methods were all reported in the results |
| Other bias | Low risk | No evidence to suggest other bias |
Villalba 1998.
| Methods | Randomised, cross‐over, open‐label trial | |
| Participants | 30 participants, 11 with dermatomyositis, 18 with polymyositis and 1 overlap with systemic lupus erythematosus IV methotrexate group: Age in years (SD): 40.1 (9.5) Sex: 12 females, 3 males Disease duration (SD): 34.9 (12.3) months Oral methotrexate and azathioprine group: Age in years (SD): 41.5 (15.4) Sex: 12 females and 3 males Disease duration (SD): 45.1 (45.9) Diagnosis of definite polymyositis or dermatomyositis as defined by Bohan and Peter Inclusion required weakness on MMT of at least 3/5 in at least two muscle groups, a functional deficit of at least 1 level in at least 1 area of ADL, refractory disease (persistent disease despite 1 mg/kg/day prednisone, inability to taper prednisone below 0.25 mg/kg/day or unacceptable side effects) and active disease requiring further immunosuppression as judged by the evaluating physician 25 participants had had failed previous trials of methotrexate and/or azathioprine |
|
| Interventions | IV methotrexate 500 mg/m2 every 2 weeks for 12 treatments followed by leucovorin rescue (50 mg/m2 every 6 hours for 4 doses) versus oral methotrexate plus azathioprine (up to 25 mg/week and 150 mg/day) for 6 months | |
| Outcomes | Primary oucome was 'improvement' as defined by a combine evaluation of strength assessed by MMT (maximum score 80) and function assessed by ADL score (maximum 91) Improvement if net increase of a least 1 grade of muscle strength in at least 2 muscle groups on MMT and an increase in at least 1 functional level in one or more areas of function Assessed at 3 and 6 months |
|
| Notes | No statistically significant difference between the 2 treatments but trend favours combination therapy | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | High risk | No allocation concealment |
| Blinding (performance bias and detection bias) All outcomes | High risk | No blinding performed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 participants withdrawn from the study in each arm. One due to worsening respiratory symptoms and one at own request |
| Selective reporting (reporting bias) | Low risk | Outcomes reported as outlined in methods section |
| Other bias | Unclear risk | Not clear |
ADL: activities of daily living BP: blood pressure CK: creatine kinase DEXA: dual‐emission X‐ray absorptiometry IMACS: International Myositis Assessment and Clinical Studies Group IBM: inclusion body myositis IV: intravenous IVIg: intravenous immunoglobulin MMT: manual muscle testing NSS: Neuromuscular Symptom Score RCT: randomised controlled trial SD: standard deviation SF‐36: Short Form‐36 Health Survey ULN: upper limit of normal VAS: visual analog score
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bunch 1981 | Follow‐up study of RCT by Bunch et al. (Bunch 1980). Participants and observer not blinded to treatment |
| Chung 2007 | Assessing creatine supplementation as a therapy for dermatomyositis and polymyositis. This supplement is not known to be immunosuppressive or immunomodulatory |
| Donov 1995 | No evidence of randomisation or blinding |
| Fries 1973 | Participants could not be confirmed to have polymyositis on the basis of the inclusion criteria |
Characteristics of ongoing studies [ordered by study ID]
ISRCTN40085050.
| Trial name or title | Second line agents in myositis (SELAM trial) |
| Methods | Randomised, double‐blind, placebo‐controlled trial |
| Participants | 90 participants with inflammatory myositis fulfilling the Bohan and Peter criteria, on corticosteroids with active disease |
| Interventions | Participants will be randomised into 4 therapy arms:
Treatments given for 56 weeks |
| Outcomes |
Primary outcome measures MMT by standard MMT (modified MRC) of 16 muscle groups Timed 30 m walk Secondary outcome measures Modified Amyotrophic Lateral Sclerosis Functional Rating Scale (ALSFRS) Generic health status measured by the SF‐36 The acute phase response measured by Westergren erythrocyte sedimentation rate Continuing muscle damage measured by plasma CK The cumulative and change in oral steroid dosage Adverse events |
| Starting date | 2001 |
| Contact information | Academic department of Rheumatology, GKT School of Medicine, London, UK |
| Notes | Study completed but not published. Preliminary data presented at the British Society for Rheumatology 2010 Annual Conference. ISRCTN40085050 |
NCT00001261.
| Trial name or title | Intravenous immunoglobulin (IVIg) for the treatment of inflammatory myopathies |
| Methods | Double‐blind, randomised, placebo‐controlled cross‐over study in which participants will receive IVIg (2 gm/kg over two days each month) or placebo for 3 months and then will cross over to the alternate therapy for another period of 3 months |
| Participants | 30 participants with dermatomyositis, polymyositis or inclusion body myositis, active disease and previous unsuccessful therapy with prednisolone and one immunosuppressive drug |
| Interventions | IVIg or placebo |
| Outcomes | Greater than 15% improvement in baseline muscle strength |
| Starting date | 1999 |
| Contact information | National Institutes of Health Clinical Center (CC) |
| Notes | Study completed according to clinicaltrials.gov website, last updated March 2008 |
NCT00035958.
| Trial name or title | Understanding the pathogenesis and treatment of childhood onset dermatomyositis |
| Methods | Randomised, open‐label, multicentered trial comparing 3 treatments: oral prednisone, oral prednisone and methotrexate, and oral prednisone and etanercept. Treament duration 24 months |
| Participants | 75 participants with definite juvenile dermatomyositis |
| Interventions | Oral prednisone, oral prednisone and methotrexate, or oral prednisone and etanercept |
| Outcomes |
Primary outcome measure Mean duration of steroid therapy and manual muscle strength Secondary outcome measures Disability in daily function and height and weight growth velocity (steroid toxicity measures) |
| Starting date | 2002 |
| Contact information | Daniel J. Lovell, MD, MPH Children's Hospital Medical Center, Cincinnati |
| Notes | Study terminated 'Incorporating the recommendations of the NIH‐formed DSMB in the study procedures would make the project budget over the limit for this funding mechanism.' |
NCT00106184.
| Trial name or title | Rituximab for the treatment of refractory adult and juvenile dermatomyositis (DM) and adult polymyositis (PM) |
| Methods | A randomised, double‐blind, placebo‐controlled delayed therapy trial Group A will receive rituximab 750 mg/m2 body surface area up to a maximum dose of 1 g at weeks 0 and 1 and placebo at weeks 8 and 9 Group B will receive placebo at weeks 0 and 1 and rituximab 750 mg/m2 body surface area up to a maximum dose of 1 g at weeks 8 and 9 |
| Participants | Participants with dermatomyositis, polymyositis or juvenile dermatomyositis (> 5 years old) and refractory disease Intolerance or inadequate response to therapy with corticosteroids plus at least 1 other immunosuppressive agent |
| Interventions | Rituximab 750 mg/m2 body surface area up to a maximum dose of 1 g given at weeks 0 and 1 or weeks 8 and 9 Placebo infusions given at weeks 0 and 1 or weeks 8 and 9 |
| Outcomes |
Primary outcome measure Comparison between the 2 groups of participants in their time to achieve improvement Secondary Outcome Measures Response rates (proportion of improved participants) between Groups A (rituximab‐treated) and B (placebo‐treated) at week 8 20% improvement in MMT over baseline on 2 consecutive time points |
| Starting date | March 2006 |
| Contact information | National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) |
| Notes | Study completed but not published. Preliminary data presented at the American college of Rheumatology 2010 Annual Conference. |
NCT00323960.
| Trial name or title | Five‐year actively controlled clinical trial in new onset juvenile dermatomyositis |
| Methods | Randomised, open label, active control, parallel assignment study |
| Participants | Newly diagnosed and untreated children with probable or definite diagnosis of JDM according to the Bohan and Peter criteria, aged between 1 and 18 years |
| Interventions | Prednisolone, prednisolone plus ciclosporin (5 mg/kg in 2 oral doses daily) or prednisolone plus methotrexate (15 to 20 mg/m2 once per week) |
| Outcomes |
Primary outcome measure 20% improvement in at least 3 core set variables with no more than 1 of the remaining variables (muscle strength excluded) worsened by > 30%. Secondary outcome measures Change over time in the individual components of the JDM core set of variables: a) time to muscle enzymes normalisation; b) frequency of drop‐out of suggested steroids use; c) frequency of drop‐out for inefficacy of treatment. |
| Starting date | May 2006 |
| Contact information | Nicolino Ruperto, MD, MPH 0039‐010‐382854 nicolaruperto@ospedale‐gaslini.ge.it Simona Angioloni, B.A. 0039‐010‐393425 simonaangioloni@ospedale‐gaslini.ge.it |
| Notes | Study currently recruiting according to clinicaltrials.gov website, last updated February 2011 |
NCT00335985.
| Trial name or title | Efficacy and safety study of GB‐0998 for treatment of steroid‐resistant polymyositis and dermatomyositis (PM/DM) |
| Methods | Randomised, double‐blind, placebo‐controlled study |
| Participants | Steroid‐resistant polymyositis and dermatomyositis |
| Interventions | High‐dose IVIg (400 mg of GB‐0998/kg per day) or placebo daily for 5 successive days |
| Outcomes |
Primary outcome measure Change in MMT scores Secondary outcome measures Change in CK and total activity of daily living scores. Adverse events and laboratory tests 'Time frame 8 weeks' |
| Starting date | 2006 |
| Contact information | Professor Nobuyuki Miyasaka, Tokyo Medical and Dental University |
| Notes | Study completed according to clinicaltrials.gov website, last updated July 2009 |
NCT00533091.
| Trial name or title | A phase 1B, randomised, double‐blind, placebo‐controlled, multicenter study to evaluate safety of multiple‐dose, intravenously administered MEDI‐545, a fully human anti interferon‐alpha monoclonal antibody, in adult patients with dermatomyositis or polymyositis |
| Methods | Randomised, double‐blind, placebo‐controlled study 40 participants with a positive gene signature randomised in a 3:1 ratio to initially receive MEDI‐545 (0.3, 1.0, 3.0, or 10.0 mg/kg) 2‐weekly for 12 weeks or placebo. In each dose cohort, 6 participants to receive MEDI‐545 and 2 participants placebo In addition, 8 participants with negative gene signature randomised in a 3:1 ratio to receive MEDI‐545 (3.0 mg/kg) or placebo every 2 weeks for 12 weeks. Thereafter, participants initially randomised to MEDI‐545 to receive the same dose of MEDI‐545 every 2 weeks for 12 weeks and participants initially randomised to placebo to receive MEDI‐545 every 2 weeks. |
| Participants | 40 adult participants with dermatomyositis or polymyositis and a positive gene signature 8 adult participants with dermatomyositis or polymyositis and a negative gene signature |
| Interventions | MEDI‐545 (0.3, 1.0, 3.0, or 10.0 mg/kg) via infusion 2‐weekly, or placebo. |
| Outcomes |
Primary outcome measures The primary endpoints of the study are safety and tolerability of multiple IV doses of MEDI‐545 in adult participants with dermatomyositis or polymyositis Secondary outcome measures The secondary endpoints of the study are the PK and IM of multiple IV doses of MEDI‐545 The third endpoint of the study are the evaluations of disease activities |
| Starting date | April 2007 |
| Contact information | Mick G. Ribeiro 301‐398‐4202 ribeirom@medimmune.com Lisa Farace 301‐398‐4991 faracel@medimunne.com |
| Notes | Study completed according to clinicaltrials.gov website, last updated May 2011 |
NCT00651040.
| Trial name or title | Combined treatment of methotrexate + glucocorticoids versus glucocorticoids alone in patients with polymyositis and dermatomyositis (Prometheus) |
| Methods | Randomised, open‐label, assessor‐blind trial |
| Participants | 50 participants with previously untreated polymyositis or dermatomyositis |
| Interventions | Prednisolone at an initial dose of 1.0 mg/kg/day either alone or with methotrexate Methotrexate arm to receive methotrexate at an initial dose of 10 mg/wk escalating up to 20 to 25 mg/week according to clinical need with folic acid 10 mg given 24 hours after each methotrexate dose Treatment duration of 12 months with a further 12 month follow‐up |
| Outcomes |
Primary outcome measure Total dose of glucocorticoids administered between baseline and the end of treatment Secondary outcome measures Assessment of disease activity and damage, muscle strength and endurance, enzyme levels, glucocorticoid side‐effects, dose, HAQ, SF‐36, treatment failures |
| Starting date | May 2008 |
| Contact information | Jiri Vencovsky, prof. MD. +420234075340 venc@revma.cz Jana Tomasova, MD. PhD. +420234075340 jtomasova@yahoo.com |
| Notes | Study currently recruiting according to clinicaltrials.gov website, last verified July 2011 |
NCT01148810.
| Trial name or title | Efficacy and tolerability of BAF312 in patients with polymyositis and dermatomyositis |
| Methods | Randomised, double‐blind, placebo‐controlled trial of BAF312 or placebo for 12 weeks followed by a further 12 weeks where all participants recieve BAF312 |
| Participants | Polymyositis or dermatomyositis unresponsive to at least 3 months of therapy with corticosteroids with or without disease modifying antirheumatic drugs |
| Interventions | BAF312 |
| Outcomes | IMACS core set measures |
| Starting date | 2010 |
| Contact information | Novartis Pharmaceuticals +41‐61‐324‐1111 |
| Notes | Study currently recruiting according to clinicaltrials.gov website, last verified April 2011 |
NCT01315938.
| Trial name or title | Abatacept treatment in polymyositis and dermatomyositis (ARTEMIS) |
| Methods | Randomised, single‐blind (outcome assessor blinded), delayed treatment study |
| Participants | 20 participants with active dermatomyositis (10) or polmyositis (10) despite therapy with steroids and either methotrexate or azathioprine for at least 3 months |
| Interventions | Participants will be randomised to receive abatacept at time 0 then after 2, 4, 8, 12, 16 and 20 weeks, or to have this treatment delayed for 3 months |
| Outcomes | Primary outcome: The number of responders, defined as improved according to the IMACs criteria, after treatment with abatacept for 6 months Secondary outcomes: The change in the individual components of the IMACS core set measures for disease activity at 3 and 6 months |
| Starting date | January 2011 |
| Contact information | Ingrid E Lundberg, MD, PhD +46851770000 ext 6087 Ingrid.Lundberg@ki.se Jiri Vencovsky, MD, PhD +420224914469 venc@revma.cz Patrick Gordon, MBBS, PhD +44203 299 1735 patrick.gordon2@nhs.net |
| Notes | Study currently recruiting |
ADL: activities of daily living CK: creatine kinase HAQ: Health Assessment Questionnaire IV: intravenous MMT: manual muscle testing SF‐36: Short Form 36 Health Survey
Contributions of authors
Ernest Choy drafted the original review, searched for trials, assessed methodological quality, extracted data and revised the review following peer review. Patrick Gordon drafted the updated review, searched for trials, assessed methodological quality, extracted data and revised the updated review following peer and editorial review. Jessica Hoogendijk and John Winer searched for trials, assessed methodological quality and extracted data.
Sources of support
Internal sources
New Source of support, Other.
External sources
No sources of support supplied
Declarations of interest
Professor Ernest Choy has received research grants, and served as member of advisory boards and speaker bureaus of Abbott Laboratories, Allergan, AstraZeneca, Boehringer Ingelheim, Chelsea Therapeutics, Chugai Pharma, Eli Lilly, Ferring Pharmacuetical, GSK, Jazz Pharmaceuticals, MedImmune, Merrimack Pharmaceutical, MSD, Pfizer, Pierre Fabre Medicament, Roche, Schering Plough, Synovate, UCB Celltech and Wyeth.
Dr Patrick Gordon's institution has received grants for three of the studies in this review. One was an excluded study (Chung 2007), funded by various non‐commercial grant‐giving bodies and two are ongoing studies, one funded by Bristol Myers Squibb and the Myositis Support Group (NCT01315938) and the other by the Arthritis Research Campaign (SELAM, ISRCTN40085050). Dr John Winer is a collaborator in the SELAM trial. Bristol Myers Squibb funded Dr Gordon's attendance at EULAR 2011 and 2012 (money paid to institution).
Dr Jessica Hoogendijk is co‐author of a RCT in this review (Van de Vlekkert 2010). She has no other known conflicts of interest.
Dr John Winer's institution undertook an audit of IVIg side effects and received payment for travel and equipment from CSL Behring. He has been involved in an, as yet, unpublished trial of methotrexate and azathioprine for polymyositis and dermatomyositis (Miller 2002).
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
Bunch 1980 {published data only}
- Bunch TW, Worthington JW, Combs JJ, Ilstrup MS, Engel AG. Azathioprine with prednisone for polymyositis. Annals of Internal Medicine 1980;92(3):365‐9. [PUBMED: 6986827] [DOI] [PubMed] [Google Scholar]
Coyle 2008 {published data only}
- Coyle K, Pokrovnichka A, French K, Joe G, Shrader J, Swan L, et al. A randomized, double‐blind, placebo‐controlled trial of infliximab in patients with polymyositis and dermatomyositis. Arthritis and Rheumatism 2008;58(Suppl):Abstract No: 2058. [Google Scholar]
Dalakas 1993 {published data only}
- Dalakas MC, Illa I, Dambrosia JM, Soueidan SA, Stein DP, Otero C, et al. A controlled trial of high‐dose intravenous immune globulin infusions as treatment for dermatomyositis. New England Journal of Medicine 1993;329(27):1993‐2000. [PUBMED: 8247075] [DOI] [PubMed] [Google Scholar]
Miller 1992 {published data only}
- Miller FW, Leitman SF, Cronin ME, Hicks JE, Leff RL, Wesley R, et al. Controlled trial of plasma exchange and leukapheresis in polymyositis and dermatomyositis. New England Journal of Medicine 1992;326(21):1380‐4. [PUBMED: 1472183] [DOI] [PubMed] [Google Scholar]
Miller 2002 {published and unpublished data}
- Miller J, Walsh Y, Saminaden S, Lecky BRF, Winer JB. Randomised double blind controlled trial of methotrexate and steroids compared with azathioprine and steroids in the treatment of idiopathic inflammatory myopathy. Journal of the Neurological Sciences 2002;199(Suppl 1):S53. [Google Scholar]
Muscle Study Group 2011 {published data only}
- Muscle Study Group. A randomized, pilot trial of etanercept in dermatomyositis. Annals of Neurology 2011;70(3):427‐36. [PUBMED: 21688301] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT00112385. A pilot study of etanercept in dermatomyositis. http://clinicaltrials.gov/ct2/show/results/NCT00112385 (accessed 5 July 2012).
Takada 2002 {published data only}
- Takada K, Bookbinder S, Furie R, Oddis C, Mojcik C, Bombara M, et al. A pilot study of eculizumab in patients with dermatomyositis. Arthritis and Rheumatism 2002;46 (Suppl):S489. [Google Scholar]
Van de Vlekkert 2010 {published data only}
- Vlekkert J, Hoogendijk JE, Haan RJ, Algra A, Tweel I, Pol WL, et al. Oral dexamethasone pulse therapy versus daily prednisolone in sub‐acute onset myositis, a randomised clinical trial. Neuromuscular Disorders 2010;20(6):382‐9. [PUBMED: 20423755] [DOI] [PubMed] [Google Scholar]
Vencovsky 2000 {published data only}
- Vencovský J, Jarosová K, Machácek S, Studýnková J, Kafková J, Bartůnková J, et al. Cyclosporine A versus methotrexate in the treatment of polymyositis and dermatomyositis. Scandinavian Journal of Rheumatology 2000;29(2):95‐102. [PUBMED: 10777122] [DOI] [PubMed] [Google Scholar]
Villalba 1998 {published data only}
- Villalba L, Hicks JE, Adams EM, Sherman JB, Gourley MF, Leff RL, et al. Treatment of refractory myositis: a randomized crossover study of two new cytotoxic regimens. Arthritis and Rheumatism 1998;41(3):392‐9. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Bunch 1981 {published data only}
- Bunch TW. Prednisone and azathioprine for polymyositis: long‐term follow up. Arthritis and Rheumatism 1981;24(1):45‐8. [DOI] [PubMed] [Google Scholar]
Chung 2007 {published data only}
- Chung Y, Alexanderson H, Pipitone N, Morrison C, Dastmalchi M, Ståhl‐Hallengren C, et al. Creatine supplements in patients with idiopathic inflammatory myopathies who are clinically weak after conventional pharmacologic treatment: Six‐month, double‐blind, randomized, placebo‐controlled trial. Arthritis and Rheumatism 2007;57:694‐702. [DOI] [PubMed] [Google Scholar]
Donov 1995 {published data only}
- Donov G, Kartasheva V, Fomenko T. Experimental use of plasmapheresis in juvenile dermatomyositis therapy. Clinical & Experimental Rheumatology 1995;13 Suppl 13:561. [Google Scholar]
Fries 1973 {published data only}
- Fries JF, Sharp GC, McDevitt HO, Holman HR. Cyclophosphamide therapy in systemic lupus erythematosus and polymyositis. Arthritis and Rheumatism 1973;16(2):154‐62. [PUBMED: 4716431] [DOI] [PubMed] [Google Scholar]
References to ongoing studies
ISRCTN40085050 {unpublished data only}
- ISRCTN40085050. Second line agents in myositis. http://www.controlled‐trials.com/ISRCTN40085050 (accessed 16 March 2012).
NCT00001261 {published data only}
- NCT00001261. Intravenousimmunoglobulin (IVIg) for the treatment of inflammatory myopathies. http://clinicaltrials.gov/ct2/show/NCT00001261 (accessed 16 March 2012).
NCT00035958 {published data only}
- NCT00035958. Understanding the pathogenesis and treatment of childhood onset dermatomyositis. http://clinicaltrials.gov/ct2/show/NCT00035958 (accessed 16 March 2012).
NCT00106184 {published data only}
- NCT00106184. Rituximab for the treatment of refractory adult and juvenile dermatomyositis (DM) and adult polymyositis (PM). http://clinicaltrials.gov/ct2/show/NCT00106184 (accessed 16 March 2012).
NCT00323960 {published data only}
- NCT00323960. Five‐year actively controlled clinical trial in new onset juvenile dermatomyositis (PRINTOJDMTR). http://clinicaltrials.gov/ct2/show/NCT00323960 (accessed 16 March 2012).
NCT00335985 {published data only}
- NCT00335985. Efficacy and safety study of GB‐0998 for treatment of steroid‐resistant polymyositis and dermatomyositis (PM/DM). http://clinicaltrials.gov/ct2/show/NCT00335985 (accessed 16 March 2012).
NCT00533091 {published data only}
- NCT00533091. A study to evaluate safety of multi‐dose MEDI‐545 in adult patients with dermatomyositis or polymyositis. http://clinicaltrials.gov/ct2/show/NCT00533091 (accessed 16 March 2012).
NCT00651040 {published data only}
- NCT00651040. Combined treatment of methotrexate + glucocorticoids versus glucocorticoids alone in patients with polymyositis and dermatomyositis (Prometheus). http://clinicaltrials.gov/ct2/show/NCT00651040 (accessed 16 March 2012).
NCT01148810 {published data only}
- NCT01148810. Efficacy and tolerability of BAF312 in patients with polymyositis and dermatomyositis. http://clinicaltrials.gov/ct2/show/NCT01148810 (accessed 16 March 2012).
NCT01315938 {published data only}
- NCT01315938. Abatacept treatment in polymyositis and dermatomyositis (ARTEMIS). http://clinicaltrials.gov/ct2/show/NCT01315938 (accessed 16 March 2012).
Additional references
Ahlstrom 1993
- Ahlstrom G, Gunnarsson LG, Leissner P, Sjoden PO. Epidemiology of neuromuscular diseases, including the postpolio sequelae, in a Swedish county. Neuroepidemiology 1993;12(5):262‐9. [DOI] [PubMed] [Google Scholar]
Bohan 1975 a
- Bohan A, Peter JB. Polymyositis and dermatomyositis (first of two parts). New England Journal of Medicine 1975;292(7):344‐7. [DOI] [PubMed] [Google Scholar]
Bohan 1975 b
- Bohan A, Peter JB. Polymyositis and dermatomyositis (second of two parts). New England Journal of Medicine 1975;292(8):403‐7. [DOI] [PubMed] [Google Scholar]
Carpenter 1977
- Carpenter JR, Bunch TW, Engel AG, O'Brien PC. Survival in polymyositis: corticosteroids and risk factors. Journal of Rheumatology 1977;4(2):207‐14. [PubMed] [Google Scholar]
Choy 2002
- Choy EH, Isenberg DA. Treatment of dermatomyositis and polymyositis. Rheumatology 2002;41(1):7‐13. [DOI] [PubMed] [Google Scholar]
Dalakas 1991
- Dalakas MC. Polymyositis, dermatomyositis, and inclusion‐body myositis. New England Journal of Medicine 1991;325(21):1487‐98. [DOI] [PubMed] [Google Scholar]
Dalakas 2001
- Dalakas MC. Progress in inflammatory myopathies: good but not good enough. Journal of Neurology, Neurosurgery and Psychiatry 2001;70(5):569‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hoogendijk 2004
- Hoogendijk JE, Amato AA, Lecky BR, Choy EH, Lundberg IE, Rose MR, et al. 119th ENMC international workshop: trial design in adult idiopathic inflammatory myopathies, with the exception of inclusion body myositis, 10‐12 October 2003, Naarden, The Netherlands. Neuromuscular Disorders 2004;14(5):337‐45. [DOI] [PubMed] [Google Scholar]
Isenberg 2004
- Isenberg D A, Allen E, Farewell V, Ehrenstein MR, Hanna M G, Lundberg IE, et al for the International Myositis and Clinical Studies Group (IMACS). International consensus outcome measures for patients with idiopathic inflammatory myopathies. Development and initial validation of myositis activity and damage indices in patients with adult onset disease. Rheumatology 2004;43(1):49‐54. [DOI] [PubMed] [Google Scholar]
Joffe 1993
- Joffe MM, Love L, Leff RL, Fraser DD, Targoff IN, Hicks JE, et al. Drug therapy of the idiopathic inflammatory myopathies: predictors of response to prednisone, azathioprine and methotrexate and a comparison of their efficacy. American Journal of Medicine 1993;94(4):379‐87. [DOI] [PubMed] [Google Scholar]
Oddis 2005
- Oddis CV, Rider LG, Reed AM, Ruperto N, Brunner HI, Koneru B, et al. International Myositis Assessment and Clinical Studies Group. International consensus guidelines for trials of therapies in the idiopathic inflammatory myopathies. Arthritis and Rheumatism 2005;52(9):2607‐15. [DOI] [PubMed] [Google Scholar]
RevMan 2011 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Riddoch 1975
- Riddoch D, Morgan‐Hughes JA. Prognosis in adult polymyositis. Journal of the Neurological Sciences 1975;26(1):71‐80. [DOI] [PubMed] [Google Scholar]
Rider 2004
- Rider LG, Giannini EH, Brunner HI, Ruperto N, James‐Newton L, Reed AM, et al. International Myositis Assessment and Clinical Studies Group. International consensus on preliminary definitions of improvement in adult and juvenile myositis. Arthritis and Rheumatism 2004;50(7):2281‐90. [DOI] [PubMed] [Google Scholar]
Van de Vlekkert 2004
- Vlekkert J, Tjin‐A‐Ton ML, Hoogendijk JE. Quality of myositis case reports open to improvement. Arthritis and Rheumatism 2004;51(1):148‐50. [DOI] [PubMed] [Google Scholar]
Van Swieten 1988
- Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke 1988;19(5):604‐7. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Choy 2005
- Choy EHS, Winer J, Lecky B, Hoogendik J. Immunosuppressant and immunomodulatory treatment for dermatomyositis and polymyositis. Cochrane Database of Systematic Reviews 2005, Issue 2. [DOI: 10.1002/14651858.CD003643] [DOI] [PubMed] [Google Scholar]
Choy 2005b
- Choy EHS, Hoogendijk JE, Lecky B, Winer JB. Immunosuppressant and immunomodulatory treatment for dermatomyositis and polymyositis. Cochrane Database of Systematic Reviews 2005, Issue 3. [DOI: 10.1002/14651858.CD003643.pub2] [DOI] [PubMed] [Google Scholar]
Choy 2009
- Choy EHS, Hoogendijk JE, Lecky B, Winer JB, Gordon P. Immunosuppressant and immunomodulatory treatment for dermatomyositis and polymyositis. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD003643.pub3] [DOI] [PubMed] [Google Scholar]


