Abstract
Background
Methenamine salts are often used as an alternative to antibiotics for the prevention of urinary tract infection (UTI). This review was first published in Issue 1, 2002 and updated in Issue 4, 2007.
Objectives
To assess the benefits and harms of methenamine hippurate in preventing UTI.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL in The Cochrane Library), MEDLINE (from 1950), EMBASE (from 1980), reference lists of articles and abstracts from conference proceedings without language restriction. Manufacturers' of methenamine salts were contacted for unpublished studies and contact was made with known investigators.
Date of last search: June 2012
Selection criteria
Randomised controlled trials (RCT) and quasi‐RCTs of methenamine hippurate used for the prevention of UTIs in all population groups were eligible. A comparison with a control/no treatment group was a prerequisite for selection.
Data collection and analysis
Two authors independently assessed study quality and extracted data. Statistical analyses were performed using the random effects model and the results expressed as risk ratio (RR) for dichotomous outcomes with 95% confidence intervals (CI). An exploration of heterogeneity and a detailed description of results, grouped by population, was undertaken.
Main results
Thirteen studies (2032 participants) were included. Six studies (654 patients) reported symptomatic UTI and eight studies (796 patients) reported bacteriuria. Overall, study quality was mixed. The overall pooled estimates for the major outcome measures were not interpretable because of underlying heterogeneity. Subgroup analyses suggested that methenamine hippurate may have some benefit in patients without renal tract abnormalities (symptomatic UTI: RR 0.24, 95% CI 0.07 to 0.89; bacteriuria: RR 0.56, 95% CI 0.37 to 0.83), but not in patients with known renal tract abnormalities (symptomatic UTI: RR 1.54, 95% CI 0.38 to 6.20; bacteriuria: RR 1.29, 95% CI 0.54 to 3.07). For short‐term treatment duration (1 week or less) there was a significant reduction in symptomatic UTI in those without renal tract abnormalities (RR 0.14, 95% CI 0.05 to 0.38). The rate of adverse events was low.
Authors' conclusions
Methenamine hippurate may be effective for preventing UTI in patients without renal tract abnormalities, particularly when used for short‐term prophylaxis. It does not appear to work in patients with neuropathic bladder or in patients who have renal tract abnormalities. The rate of adverse events was low, but poorly described.
There is a need for further large well‐conducted RCTs to clarify this question, particularly for longer term use for people without neuropathic bladder.
Keywords: Humans; Anti‐Infective Agents, Urinary; Anti‐Infective Agents, Urinary/therapeutic use; Hippurates; Hippurates/therapeutic use; Methenamine; Methenamine/analogs & derivatives; Methenamine/therapeutic use; Randomized Controlled Trials as Topic; Urinary Tract Infections; Urinary Tract Infections/prevention & control
Plain language summary
Methenamine hippurate for preventing urinary tract infections
Bladder and kidney infections (urinary tract infections ‐ UTI) can cause vomiting, pain, dysuria, septicaemia, fever and tiredness, and occasionally kidney damage. Some people are at high risk of repeated UTIs, and they are also more likely to have serious complications (including people with kidney problems, or people who have catheters to release urine). Long‐term use of antibiotics can lead to resistance, so methenamine salts (methenamine or hexamine hippurate) are often used. This review identified 13 studies (2032 participants). Methenamine hippurate may be effective in preventing UTI in patients without renal tract abnormalities particularly when used for short term prophylaxis. It does not appear to be effective for long term prophylaxis in patients who have neuropathic bladder. There were few adverse effects.Additional well controlled randomised controlled trials are necessary in particular to clarify effectiveness for longer term prophylaxis in those without neuropathic bladder.
Background
Description of the condition
Patients at greater risk of UTI include women, those undergoing genito‐urinary procedures, those with abnormal renal tract anatomy or physiology, those with foreign bodies (such as catheters), the immunocompromised, or those with a neuropathic bladder (Macfarlane 1985). One such high risk group is the spinal injured population (with associated neuropathic bladder), where the incidence is estimated at 1.82 UTI episodes/patient/year (Waites 1993). In these high‐risk patients, problems can result from antibiotic resistance and other complications such as autonomic dysreflexia, vesicoureteric reflux, related pyelonephritis, septicaemia, the need for a change in the method of bladder management, or recatheterisation. The end result is that highly susceptible populations often require hospitalisation for what in the general community would be an easily treated condition.
Description of the intervention
Methenamine salts are often used in the prevention of urinary tract infection (UTI). They are frequently used for prolonged periods because, unlike conventional antibiotics, acquired resistance does not appear to develop (Parfitt 1999). Given the problems of increasing antibiotic resistance, methenamine salts may be especially useful in populations susceptible to recurrent UTI.
How the intervention might work
Methenamine salts act via the production of formaldehyde from hexamine, which in turn acts as a bacteriostatic agent (Mayrer 1982). It is uncertain whether urinary acidification and the direct bacteriostatic effect of hippuric acid contribute significantly to its action (Nahata 1982; Wall 1990). Methenamine salts are well tolerated and adverse effects, including minor gastrointestinal upsets, dysuria, abdominal cramps, anorexia, rash and stomatitis (3M 2000; McEvoy 1999), are generally mild (Mayrer 1982).
Why it is important to do this review
It remains highly controversial whether methenamine salts are effective in preventing UTI (Saint 1999). In vitro studies suggest that a urinary pH below 5.5 is needed for bacteriostatic concentrations of free formaldehyde to be generated from methenamine hippurate (Greenwood 1981). There are suggestions that it may be difficult to maintain urinary pH below this level with conventional techniques (typically ascorbic acid) long‐term (Hetey 1990; Wall 1990). It is also suggested that in catheterised patients, the amount of time that any formaldehyde produced remains in the bladder may be insufficient to be clinically effective (Devenport 1984; Saint 1999).
There are two salts of methenamine described in clinical use. It is uncertain whether methenamine hippurate* and methenamine mandelate** are equivalent in effectiveness (Gow 1974; Greenwood 1981; Kasanen 1974). The mandelate salt is not widely available. This review will only consider the effectiveness of methenamine hippurate in preventing UTIs.
Common alternatives to methenamine hippurate in oral urinary prophylaxis include antibiotic therapy (Albert 2004; Niel‐Weise 2005) and cranberry juice or capsules (Jepson 2004).
_____________________________________________________________________ * Methenamine hippurate is the US adopted name (USAN) and recommended international non‐proprietary name modified (rINNM). Other synonyms are: hexamine hippurate ‐ British approved name (BAN) and hexamethylenetramine hippurate. ** Methenamine mandelate is the recommended international non‐proprietary name modified (rINNM). Other synonyms are: hexamine mandelate, hexamine amygdalate, mandelato de metenamina, and hexamethylenetramine mandelate.
Objectives
To determine the benefits and harms of methenamine hippurate is in the prevention of UTI.
-
To determine whether the following may cause variability in observed treatment effects:
The method of bladder management type.
The use of intercurrent urinary acidification in the treatment group.
The effect of short or longer term duration of treatment.
Study quality.
Abnormalities of renal tract function and/or structure.
Methods
Criteria for considering studies for this review
Types of studies
All randomised controlled trials (RCT) (including randomised cross‐over trials or quasi‐RCTs in which allocation to treatment was obtained by alternation, use of alternate medical records, date of birth or other predictable methods) of methenamine hippurate used for the prophylaxis of UTIs were considered. Trials evaluating curative treatment were excluded.
Types of participants
At‐risk populations for UTI, including all bladder management types, sex, age and underlying conditions were eligible for this review.
Types of interventions
Methenamine hippurate versus placebo/no treatment. There were no restrictions based on the dose of drug administered. The treatment group was compared either with a placebo control group or a "no‐treatment" control group. There was no restriction on duration of therapy.
Types of outcome measures
Symptomatic UTI
The primary outcome measure was the proportion of patients with symptomatic UTI according to defined clinical criteria, along with a positive urine culture. This is the most clinically significant outcome measure for populations at long‐term risk of recurrent UTIs. Symptoms of UTI in the general population include: fever, dysuria, frequency, urgency, voiding of small volumes, abrupt onset, suprapubic pain, and loin pain (Stamm 1988). In spinal injured patients, relevant symptoms should be unexplained by other inter‐current pathology and include: fever, autonomic dysreflexia, increased frequency of muscle spasms or spasticity, failure of usual control of urinary incontinence and new abdominal discomfort (NIDRRS 1992).
Quantitative urine culture (irrespective of the presence of symptoms suggestive of UTI)
The number of UTI confirmed by appropriate microbiological criteria. Bacteriuria on quantitative urine analysis of more than 100,000 organisms of a single species per mL is the accepted standard ‐ however, the colony count may vary from 100 to 100,000 depending on the clinical setting (Stamm 1988). Therefore in some situations, (such as a clean suprapubic tap) a colony count of less than 100,000 is acceptable.
Adverse reactions in patients with or without symptoms suggestive of UTI
The proportion of people who reported side effects, and a description of these side effects.
The numbers of people withdrawing from the trials due to side effects, and a description of these side effects.
Search methods for identification of studies
Initial search
Relevant studies were identified by searching electronic databases (Appendix 1‐ Electronic search strategies) and other resources:
Cochrane Central Register of Controlled Trials CENTRAL (in The Cochrane Library).
MEDLINE (from 1966): the search strategy incorporated the Cochrane highly sensitive search strategy for identifying RCTs in MEDLINE (Dickersin 1994).
EMBASE (from 1980): the search strategy incorporated a sensitive strategy for identifying RCTs in EMBASE (Lefebvre 1996).
Reference lists of identified studies and major reviews.
Contact with researchers active in the field and primary authors of identified relevant studies for details of unpublished studies.
Contact with manufacturers' of methenamine salts (3M and Parke Davis) were contacted for unpublished studies.
No language restrictions were applied. Formal translation of selected articles was undertaken through the support of the Royal North Shore Hospital Spinal Unit Research Trust. Letters, abstracts, and unpublished studies were accepted to reduce publication bias. If duplicate publication was suspected, authors were contacted for clarification, and if confirmed, the publication with the most and/or the longest follow‐up data was used for the review.
Review update
For this update the Cochrane Renal Group's Specialised Register and CENTRAL was searched. CENTRAL and the Renal Group's specialised register contain the handsearched results of conference proceedings from general and speciality meetings. This is an ongoing activity across the Cochrane Collaboration and is both retrospective and prospective. Please refer to The Cochrane Renal Group's Module in The Cochrane Library for the complete list of nephrology conference proceedings searched.
An update search was carried out in the Cochrane Renal Group's Specialised Register on 16th January 2012.
Data collection and analysis
Selection of studies
Two independent search strategies were conducted by BL and TB. The titles and abstracts were screened by BL. Eligibility of studies for inclusion in this review was determined by BL and TB. A third author JS or JC was consulted to review discrepancies in study inclusion.
Data extraction and management
Data extraction from eligible studies was performed independently by BL and TB. A third author JS or JC was consulted to review discrepancies in data extraction. Decisions of a content or methodological nature were arbitrated by JC and decisions of a statistical nature (such as the episodes to participants discrepancy) were arbitrated by JS.
Assessment of risk of bias in included studies
Allocation concealment
Studies were divided into three groups:
Adequate (A): Central randomisation; randomisation by a pharmacy; use of numbered or coded containers; or sequentially numbered, opaque sealed envelopes.
Inadequate (B): Alternation, reference to case record number or date of birth.
Unclear (C): No allocation concealment was reported at all, or an approach which did not fall into the two previous groups was used.
Generation of randomisation sequence
Studies were divided into three groups:
Adequate: Computer, random number table, shuffled cards, tossed coins or minimisation.
Inadequate: All other methods of sequence generation.
Undefined.
Inclusion in the analysis of all randomised participants
Studies were divided into two groups:
Studies that reported or gave the impression that no subjects had been excluded from the analysis.
Studies that reported having made exclusions.
The reasons for exclusions were documented (e.g. protocol deviations, withdrawals, dropouts and losses to follow‐up).
Intention‐to‐treat analysis
Studies were divided into three groups:
Intention‐to‐treat analysis: Specifically reported by authors that intention‐to‐treat analysis was undertaken and this was confirmed on study assessment, or not stated but confirmed on study assessment.
Not intention‐to‐treat analysis: Not reported and lack of intention‐to‐treat analysis confirmed on study assessment (patients who were randomised were not included in the analysis because they did not receive the study intervention, they withdrew from the study or were not included because of protocol violation); or stated but not confirmed upon study assessment.
Unclear.
Blinding
Blinding of investigators: yes, no.
Blinding of participants: yes, no.
Blinding of outcome assessors: yes, no.
Measures of treatment effect
Where appropriate, pooled analysis was conducted. A random effects model was used for the pooled analysis. This model considers both between and within study variation, and tends to be more conservative than fixed effect modelling (Lau 1998). For dichotomous outcomes, risk ratio (RR) was used as the measure of effect (with 95% confidence interval).
Assessment of heterogeneity
A Cochran "Q" test for heterogeneity and, for the review update, the I² test (Higgins 2003) was performed on all pooled analyses. I² values of 25%, 50% and 75% correspond to low, medium and high levels of heterogeneity.
Assessment of reporting biases
Study assessment was based on the evaluation criteria of Schulz 1995.
Data synthesis
A pooled analysis was attempted for the outcomes symptomatic bacteriuria and bacteriuria using a random‐effects model.
Subgroup analysis and investigation of heterogeneity
Issues of heterogeneity were initially explored by between‐study comparisons. Pre‐specified possible explanations for variations in treatment effect were explored based on interventions, study quality and population. Subgroup analyses were attempted as defined in the objectives. Where studies involved multiple arms, only the methenamine hippurate and control arms were included. A test of interaction was considered to further analyse the subgroups. This approach was not taken due to within strata heterogeneity and sparse data.
Results
Description of studies
Included studies
Thirteen studies (2032 participants) were identified (see ‐ Characteristics of included studies). A variety of methenamine hippurate doses were used (from 1 g to 4 g as the total daily dose). Only two of the studies combined methenamine hippurate with acidification agents (Sander 1976; Thomlinson 1968). The studies involved heterogeneous population groups including; renal tract calculi (Pettersson 1989), women following gynaecological operations (Knoff 1985; Ladehoff 1984; Thomlinson 1968; Tyreman 1986; Schiotz 2002), men undergoing prostate operations (Sander 1976), pregnant women (Furness 1975), patients (predominantly women) with recurrent UTI (Gundersen 1986) post‐menopausal women (Hoivik 1984); pre‐menopausal women (Kasanen 1982) and spinally injured patients (Kuhlemeier 1985; Lee 2007). Six studies involved follow‐up of one month or less (Knoff 1985; Ladehoff 1984; Pettersson 1989; Sander 1976; Thomlinson 1968; Tyreman 1986) and seven studies had follow‐up periods longer than one month (Furness 1975; Gundersen 1986; Hoivik 1984; Kasanen 1982; Kuhlemeier 1985; Lee 2007; Schiotz 2002).
Outcome measures
Symptomatic UTI was measured in eight studies (Furness 1975; Gundersen 1986; Hoivik 1984; Knoff 1985; Lee 2007; Pettersson 1989; Schiotz 2002; Tyreman 1986). None of the studies that included symptomatic UTI used the same criteria. Often the criteria were not adequately described (Knoff 1985; Pettersson 1989), or not described at all (Gundersen 1986; Hoivik 1984; Tyreman 1986). Bacteriological criteria was described by 13 studies and was used as an outcome measure in 12. Most studies used > 100,000 bacteria/mL as the threshold level for significant bacteriuria (Furness 1975; Gundersen 1986; Hoivik 1984; Knoff 1985; Ladehoff 1984; Lee 2007; Thomlinson 1968; Tyreman 1986). Of the other studies, Sander 1976 chose 10,000 bacteria/mL and Kuhlemeier 1985 used 1000 bacteria/mL for catheter specimens and 100,000 bacteria/mL for clean catch samples. Schiotz 2002 used 10,000 bacteria/mL for catheter specimens and 100,000 bacteria/mL for mid‐stream urinary (MSU) samples. Pettersson 1989 did not provide a description of quantitative bacteriological criteria. Unpublished data from two studies (Lee 2007; Schiotz 2002) was used.
Adverse events were poorly described by the majority of studies. The only study to provide a specific methodological design for the collection of adverse events was Furness 1975, who reported birth weight, maturity at delivery and foetal mortality and abnormalities in a study on pregnant women. Five studies collected data at each follow‐up stage (Gundersen 1986; Hoivik 1984; Kasanen 1982; Knoff 1985; Sander 1976). The methodology was not well described and there was no mention of these questions being standardised.
Excluded studies
A total of 32 studies were excluded, usually due to a lack of an untreated control group (see ‐ Characteristics of excluded studies).
Risk of bias in included studies
The overall quality of the studies was poor. Two of the more recent studies demonstrated adequate allocation concealment and blinding (Lee 2007; Schiotz 2002). Those studies with short follow‐up periods were better described. Most studies provided only limited descriptions of comparative baseline characteristics. The baseline characteristics were not described at all in Furness 1975 and Kuhlemeier 1985, and in Gundersen 1986, only age was described. Sander 1976 mentioned a balanced age distribution, but did not provide specific details.
These was a particular problem in four studies (Hoivik 1984; Kuhlemeier 1985; Ladehoff 1984; Thomlinson 1968) where the post‐randomisation exclusions were over 20% (see ‐ Characteristics of included studies). Schiotz 2002, provided unpublished data to address post randomisation exclusions in five participants (from a total of 150).
Effects of interventions
Four studies (Gundersen 1986; Hoivik 1984; Kasanen 1982; Kuhlemeier 1985), counted recurrent events, so that a participant could potentially make multiple contributions to the numerator (see below ‐ episode to participant discrepancy). These studies were not included in the pooled analysis (see below ‐ unpooled studies). Lee 2007 had missing bacteruria data at follow‐up for 34/305 participants. For the numerator in the symptomatic bacteruria analysis, those people with symptoms of UTI and positive bacteriological count were included. As this potentially has an inherent bias towards the null, a sensitivity analysis was performed by repeating the analysis including subjects unconfirmed by positive urine tests (for Lee 2007) (Analysis 1.3).
1.3. Analysis.
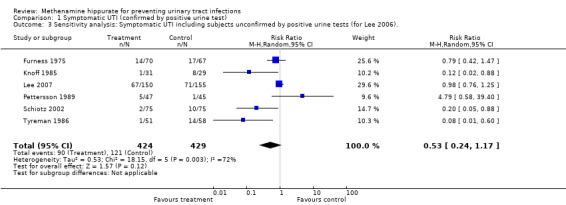
Comparison 1 Symptomatic UTI (confirmed by positive urine test), Outcome 3 Sensitivity analysis: Symptomatic UTI including subjects unconfirmed by positive urine tests (for Lee 2006)..
Symptomatic bacteriuria
This analysis involved six studies (Furness 1975; Knoff 1985; Lee 2007; Pettersson 1989; Schiotz 2002; Tyreman 1986) (Analysis 1.1 (6 studies, 853 participants): RR 0.53, 95% CI 0.24 to 1.18). The tests of heterogeneity was significant (Chi² = 17.74, df = 5, P = 0.003; I² = 71.8%). The sensitivity analysis did not reveal any difference in overall effect when missing urine tests were assumed to be positive (Analysis 1.3 (6 studies, 853 participants): RR 0.53, 95% CI 0.24 to 1.17; I² = 72.4%).
1.1. Analysis.
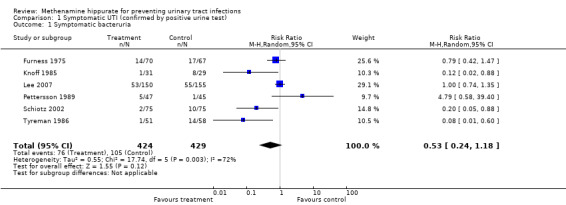
Comparison 1 Symptomatic UTI (confirmed by positive urine test), Outcome 1 Symptomatic bacteruria.
Bacteriuria
This analysis involved eight studies (Knoff 1985; Ladehoff 1984; Lee 2007; Pettersson 1989; Sander 1976; Schiotz 2002; Thomlinson 1968; Tyreman 1986) (Analysis 2.1(8 studies, 1114 participants): RR 0.67, 95% CI 0.45 to 0.99). The "Q" test was significant using a random effects model (Chi² = 28.55, df = 7, P = 0.0002; I² = 75.5%), indicating heterogeneity.
2.1. Analysis.
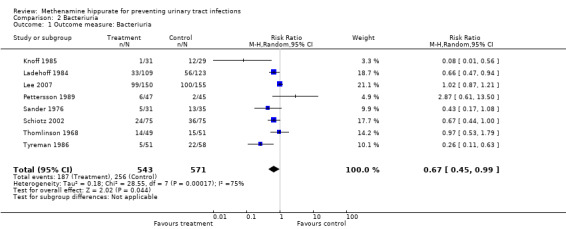
Comparison 2 Bacteriuria, Outcome 1 Outcome measure: Bacteriuria.
Exploration of heterogeneity
Symptomatic bacteriuria
Scrutiny of the forest plot suggests that the study which contributes most to the heterogeneity was Pettersson 1989 which was the only study that did not provide a quantitative threshold value for the confirmatory urinalysis. In addition this study, along with Lee 2007, were the only studies in this group that had a patient population with known renal tract abnormalities. Lee 2007 differed significantly in the underlying population studied (spinal cord injured patients with neuropathic bladder). Furness 1975 had a longer (but ill defined) treatment duration, and used bacteriuria as an inclusion criterion (as opposed to an exclusion criterion in the other studies).
No study included a treatment group with urinary acidification which we could use for subgroup comparison. Four studies used short‐term catheterisation (Knoff 1985; Pettersson 1989; Schiotz 2002; Tyreman 1986), while Furness 1975 did not. Lee 2007 included a mix of bladder management techniques including long term permanent catheterisation. There did not appear to be a trend based on this bladder management subgroup due to the strong influence of Pettersson 1989.
Three studies used higher doses of methenamine hippurate. Pettersson 1989 and Tyreman 1986 used 1 g thrice daily, while Knoff 1985 used 2 g twice daily. The remaining studies (Furness 1975; Lee 2007; Schiotz 2002) used 1 g twice daily. There did not appear to be a consistent effect of methenamine hippurate dose.
Subgroup analysis
A subgroup analysis of studies involving people with and without known renal tract abnormalities was performed (Analysis 1.2). Subgroup analysis suggested that methenamine hippurate may have some efficacy in patients without renal tract abnormalities (Analysis 1.2.1: RR 0.24, 95% CI 0.07 to 0.89; I² = 69.8%) but not with (Analysis 1.2.2: RR 1.54, 95% CI 0.38 to 6.20; I² = 53.4%). The study by Pettersson 1989 included patients with upper renal tract abnormalities, while Lee 2007 also included patients with renal tract abnormalities (15 patients had renal or bladder stones and all participants had neuropathic bladder) and had the longest potential duration of treatment (patients studied up to six months or until they had a UTI). Methenamine hippurate may be ineffective when used in renal tract abnormalities and/or neuropathic bladders (where the outcome is symptomatic UTI).
1.2. Analysis.
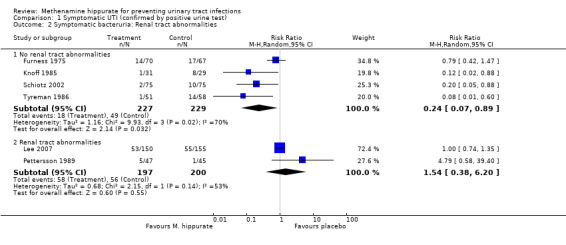
Comparison 1 Symptomatic UTI (confirmed by positive urine test), Outcome 2 Symptomatic bacteruria: Renal tract abnormalities.
Four studies had shorter durations of treatment (≤ 7 days) (Knoff 1985; Pettersson 1989; Schiotz 2002; Tyreman 1986) (Analysis 1.4.1: RR 0.30, 95% CI 0.06 to 1.56; I² = 68.0%). Heterogeneity for this subgroup could be attributed to Pettersson 1989, which included patients with upper renal tract abnormalities. When Pettersson 1989 was removed (Analysis 1.5.1: RR 0.14, 95% CI 0.05 to 0.38; I² = 0%), heterogeneity became not significant. Methenamine hippurate appears to be effective for short duration treatment in those individuals without renal tract abnormalities (where the outcome is symptomatic UTI).
1.4. Analysis.
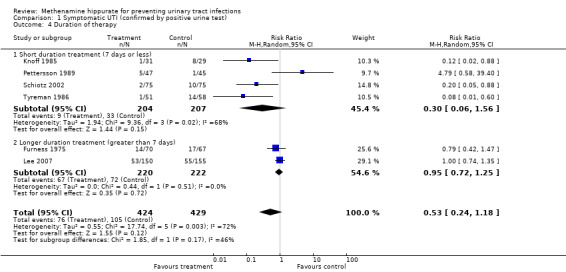
Comparison 1 Symptomatic UTI (confirmed by positive urine test), Outcome 4 Duration of therapy.
1.5. Analysis.
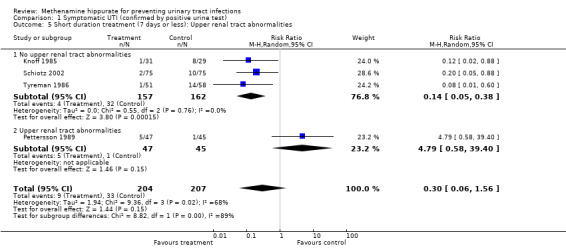
Comparison 1 Symptomatic UTI (confirmed by positive urine test), Outcome 5 Short duration treatment (7 days or less): Upper renal tract abnormalities.
Two studies had longer durations of treatment (≥ 7 days); Furness 1975 (possibly several months) and Lee 2007 (up to six months) (Analysis 1.4.2: RR 0.95, 95% CI 0.72 to 1.25; I² = 0%). The test for heterogeneity was not significant. Methenamine hippurate may be ineffective when used for longer durations.
Bacteriuria
Review of the forest plot (Analysis 2.1) suggests that the study with the largest effect on the heterogeneity was Pettersson 1989. This study (as previously discussed), has a non quantitatively defined outcome measure, and a population group with upper renal tract abnormalities. Lee 2007 and Pettersson 1989 both studied populations which included known renal tract problems. Sander 1976 and Thomlinson 1968 used urinary acidification, but there was no evidence of effect. All studies, with the exception of Lee 2007, used relatively short‐term catheterisation, although this was not specifically defined by Thomlinson 1968. Duration of catheterisation was variable: one day (Pettersson 1989), one to 2.5 days (Schiotz 2002), one to three days (Knoff 1985), usually three days (Tyreman 1986), three to six days (Sander 1976), five to 10 days (Ladehoff 1984) and a mix of bladder management types including permanent catheterisation for up to six months (Lee 2007). Again no consistent effect of bladder management type was found. All studies, with the exception of Lee 2007 and possibly Sander 1976, used short durations of treatment in the study arm. Three of the studies used higher doses of methenamine hippurate (Knoff 1985; Pettersson 1989; Tyreman 1986). No consistent effect with regard to dose was seen.
Subgroup analyses
A subgroup analysis was performed by renal tract abnormality (Analysis 2.2). Subgroup analysis suggested that methenamine hippurate may have some efficacy in patients without renal tract abnormalities (Analysis 2.2.1: RR 0.56, 95% CI 0.37 to 0.83; I² = 56.3%) but not with (Analysis 2.2.2: RR 1.29, 95% CI 0.54 to 3.07; I² = 43.8%).
2.2. Analysis.
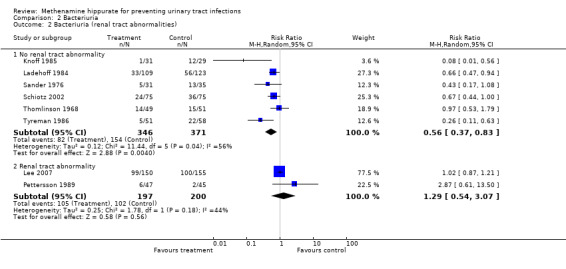
Comparison 2 Bacteriuria, Outcome 2 Bacteriuria (renal tract abnormalities).
Five studies had shorter durations of treatment (≤ 7 days) (Knoff 1985; Pettersson 1989; Schiotz 2002; Thomlinson 1968; Tyreman 1986) (Analysis 2.3.1: RR 0.59, 95% CI 0.29 to 1.22; I² = 71.9%). There was significant heterogeneity for this subgroup however when Pettersson 1989 was removed (Analysis 2.4.1: RR 0.48, 95% CI 0.23 to 0.99; I² = 72.7%), the test for heterogeneity remained significant.
2.3. Analysis.
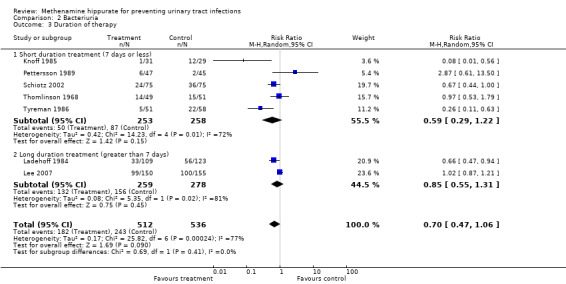
Comparison 2 Bacteriuria, Outcome 3 Duration of therapy.
2.4. Analysis.
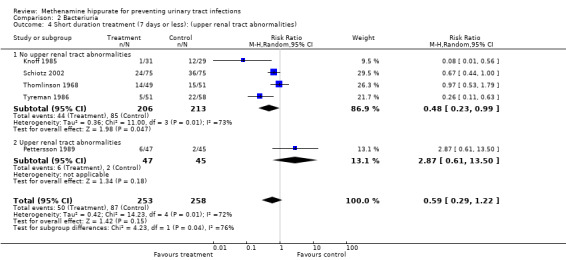
Comparison 2 Bacteriuria, Outcome 4 Short duration treatment (7 days or less): (upper renal tract abnormalities).
Two studies had longer durations of treatment (≥ 7 days) Ladehoff 1984 (8 to 13 days); Lee 2007 (up to 6 months) (Analysis 2.3.2: RR 0.85, 95% CI 0.55 to 1.31; I² = 81.3%). The test for heterogeneity was significant.
Lee 2007 was the only pooled study which included (spinal cord injured) patients with neuropathic bladder. The results showed a null result with tight confidence intervals.
Unpooled studies
Four studies were excluded from the pooled analysis. The study populations were: recurrent UTI (Kasanen 1982), male spinal cord injury (Kuhlemeier 1985), post‐menopausal (Gundersen 1986) and pre‐menopausal (Hoivik 1984) women. All of these studies used a design where a participant could potentially make multiple contributions to the total number of positive tests (the numerator), and possibly the total number of tests carried out (the denominator). We have called this an "episode to participant discrepancy" (described below) and excluded these studies from the pooled analysis because of the lack of independence of repeated observations in the same individual. In addition, there were excessive post‐allocation losses in Kuhlemeier 1985 (38%). The overall direction of three of these studies (Gundersen 1986; Hoivik 1984; Kasanen 1982) was towards a treatment effect from methenamine hippurate. The results of Kuhlemeier 1985 were difficult to interpret in view of the high post‐allocation losses. Given the methodological problems with these studies, it is possible that any estimate of effect size may be exaggerated by these studies.
Adverse events
All the studies that reported adverse events showed low rates, although the methodology for most of these studies was poor with regard to this outcome measure (Table 1 ‐ Population and adverse events). Nausea was the most common symptom and was noted in 12 patients from a total of six studies (Gundersen 1986; Kasanen 1982; Kuhlemeier 1985; Ladehoff 1984; Lee 2007; Schiotz 2002). In pregnancy, no obvious differences in birth weight, maturity at delivery, foetal abnormality or abortion were noted between treated and untreated patients (Furness 1975). Constipation was described once by Gundersen 1986 and twice by Lee 2007. Rash was described in single instances by Kasanen 1982 and Schiotz 2002 and twice by Lee 2007. Diarrhoea (Lee 2007), sore throat (Knoff 1985) and stinging in the bladder (Hoivik 1984) were all described in single instances.
1. Population and adverse events.
| Studies | Adverse events | Population |
| Furness 1975 | No significant difference in birth weight or maturity at delivery between the treatments. There was one major foetal abnormality (anencephaly), which occurred in the control group. Abortion: 1 occurred in the control group, and 1 in another study arm (not methenamine hippurate). Three intra‐uterine deaths are mentioned, but they are not assigned to specific treatment arms. |
Pregnant and post‐natal women. |
| Gundersen 1986 | One patient with nausea/rash and 1 death (apoplexia cerebri: presumed unrelated) in the 15 patients in the methenamine hippurate group, and 1 episode of constipation and 1 person leaving the study due to lack of effect in the 15 patients in the placebo group | Post‐menopausal women. |
| Hoivik 1984 | Six patients leaving the study early from 28 patients in the methenamine hippurate 1 g twice daily group (21%), 1 from 12 in the methenamine hippurate 1 g daily group (8%) and 4 from 12 in the control group (33%). Three episodes of side‐effects are described in the 1 g twice daily group (1 episode of intense stinging in the bladder region leading to the patient leaving the trial, and 2 unspecified). Two episodes of side effects are mentioned in the methenamine hippurate 1 g daily group and 3 in the placebo group, but these are only described as "minor". |
Pre‐menopausal women. |
| Kasanen 1982 | Three episodes of side effects in the methenamine hippurate 1 g daily group (1 episode of rash and 2 of nausea), leading to 2 withdrawals from the study (from 73 participants). The placebo group in comparison suffered 2 episodes of side effects (1 episode of nausea and 1 of abdominal pain), leading to 2 withdrawals. |
Recurrent UTI. |
| Knoff 1985 | 1/31 in the methenamine hippurate 2 g twice daily group with a sore throat and 1/29 in the placebo group. | Women post gynaecological operations. |
| Kuhlemeier 1985 | Two patients in the methenamine hippurate group suffering nausea. | Male spinal cord‐injured patients. |
| Ladehoff 1984 | One patient left the study due to complications on methenamine hippurate 500 mg twice daily (nausea). | Women post gynaecological operations. |
| Lee 2007 | Two patients in the methenamine hippurate group suffered constipation, diarrhoea or rash respectively. One suffered nausea (unpublished data). | Spinal Cord Injured Patients with neuropathic bladders. |
| Thomlinson 1968 | No reported side effects | Women post gynaecological operations. |
| Tyreman 1986 | No reported side effects | Women post gynaecological operations. |
| Sander 1976 | No reported side effects | Men undergoing prostate operations. |
| Schiotz 2002 | Eleven patients (7.6%) had an adverse event. Eight had nausea (5 in the treatment group and 3 in the placebo group). Three participants had a rash (1 methenamine versus 2 placebo). Two withdrawals (one from each group). | Women undergoing gynaecological laparotomy or vaginal plastic surgery. |
| Pettersson 1989 | No mention of side effects | Patients undergoing extracorporeal shockwave lithotripsy. |
Episodes to participant discrepancy
Four studies (Gundersen 1986; Hoivik 1984; Kasanen 1982; Kuhlemeier 1985) used a design where a participant could potentially make multiple contributions to the total number of positive tests (the numerator), and possibly the total number of tests carried out (the denominator).
Gundersen 1986: Six patients in the experimental group reported seven times with symptoms of UTI, while ten patients in the control reported 29 times. Follow‐up was every month for six months.
Hoivik 1984: Nineteen symptomatic recurrences were documented in the 28 patients in the full dose experimental group, four recurrences were documented in the 12 patients in the half dose experimental group and 26 recurrences were documented in the 12 patients in the placebo group. Follow‐up took place at seven specified intervals over one year.
Kasanen 1982: Eighteen (bacteriuria) recurrences were documented in the 72 patients in the experimental group, 43 recurrences were documented in the 68 patients in the placebo group. Urine investigations were performed at two‐month intervals, up to one year.
Kuhlemeier 1985: Two hundred and two studies were performed in 161 patients.
Three studies (Gundersen 1986; Hoivik 1984; Kasanen 1982) used repeated measures (regular urine tests), which led to individuals potentially contributing several times to the number of positive tests. It may be possible, after access to primary study data, for some of these studies to be included in the pooled analysis. Kuhlemeier 1985 allowed patients to pass through the study process more than once, hence allowing them to contribute multiply to both the total number of positive tests, and disproportionately to the number of tests carried out. This was one of a number of serious methodological problems found in this study (see ‐ Characteristics of included studies).
The standard significance tests used in this meta‐analysis assume that a series of observations are selected randomly from the population (Armitage 1994). The result of an "episode to participant discrepancy" is that these tests may not be valid, as they do not take into account the possible dependence among repeated observations in the same individual.
In order to address this issue, clarification has been sought from each of the primary authors involved. Until the discrepancy is resolved with primary study data, these studies will not be included in the pooled analysis. It is possible that any estimate of effect size may be exaggerated by these studies.
Discussion
Summary of main results
Symptomatic UTIs
The outcome measure of most clinical significance is symptomatic UTI. Unfortunately, there was no consistency in definition and most were poorly defined. Eight studies addressed this outcome, from six different populations. Although six of the eight studies suggested a study direction in favour of treatment with methenamine hippurate, the significant methodological problems discussed above suggest that this should be treated cautiously. Two studies that dealt with longer term use of methenamine hippurate unfortunately had to be excluded because they treated recurrent events as independent. Exploration of heterogeneity among the six studies included in the pooled analysis provided a further look at between‐study differences. The subgroup analysis by renal tract abnormality revealed continued heterogeneity between the four studies of patients without renal tract abnormality (Furness 1975; Knoff 1985; Schiotz 2002; Tyreman 1986).
A subgroup analysis of four studies that used short duration (≤ 7 days) treatment with methenamine hippurate also showed heterogeneity. When Pettersson 1989 was removed (analysis 01.05.01), the heterogeneity became non‐significant. It may be that methenamine hippurate is more effective for short duration treatment in those individuals without renal tract abnormalities (where the outcome is symptomatic UTI). Combining the two studies with longer duration treatment (Furness 1975; Lee 2007) suggested that methenamine hippurate may be ineffective when used for longer durations, although this tentative conclusion should be treated with extreme caution as it is based on one of several subgroup analyses and a subgroup containing only two very different studies. Lee 2007 was the largest clinical study with the tightest confidence intervals and for the spinal cord injured group with neuropathic bladder showed no treatment effect when used (predominantly) for community prophylaxis. A test for modification of the treatment effect by urinary acidification, bladder management type or dose through formal subgroup analysis was not possible due to insufficient studies.
Bacteriuria
Bacteriuria as an outcome measure was much better defined. Unfortunately it is also of less clinical significance in population groups who experience chronic infections (NIDRRS 1992). Twelve studies addressed this outcome measure. Again, the majority reported results in favour of treatment. Unfortunately, counting of recurrent events was again a problem. Excluding these studies and conducting a pooled analysis of eight studies revealed an overall direction in favour of treatment but in the presence of significant heterogeneity. The study that showed a treatment effect in the opposite direction was Pettersson 1989. Heterogeneity was explored by looking at differences among the eight studies in the pooled analysis. A subgroup analysis of six studies of patients without underlying renal tract abnormality still showed heterogeneity. The direction of the pooled estimate for this subgroup favoured treatment with methenamine hippurate. It is possible that methenamine hippurate is more effective in patients without underlying renal tract abnormality, although this information would need to be treated cautiously, given the persisting heterogeneity. A subgroup analysis of five studies with short treatment duration also demonstrated heterogeneity. Exclusion of the Pettersson 1989 outlier explained much but not all of the underlying heterogeneity. It is possible that short duration treatment is more effective in people without underlying renal tract abnormality, but again this possibility would need to be treated very cautiously.
Adverse events
Adverse events were poorly described, with data of insufficient quality to perform formal statistical evaluation. Overall, the frequency of reported side‐effects was low compared to control.
Alternative oral prophylactic agents
This meta‐analysis provides the suggestion that methenamine hippurate may be more effective for short duration treatment in those individuals without renal tract abnormalities (where the outcome is symptomatic UTI). Caution needs to be taken when interpreting this result due to underlying heterogeneity, the small sample and the subgroup analysis involved. Participants likely to benefit in this short‐duration treatment group are likely to have access to treatment alternatives which are potentially more effective, in particular oral antibiotics, where a recent Cochrane review has demonstrated (weak) evidence that prophylactic antibiotic use reduces symptomatic UTI rates in females undergoing abdominal surgery and short‐term bladder catheterisation (Niel‐Weise 2005). Another Cochrane review demonstrates that cranberry juice may reduce UTI over a 12 month period in women without abnormal renal tracts (Jepson 2004). Unfortunately, such alternative treatments are less demonstrable for those with abnormal renal tracts (including neuropathic bladder) who have high rates of antibiotic resistance (Thom 1999; Waites 2000) and are arguably most in need of non‐antibiotic prophylaxis.
Quality of the evidence
Overall, the studies were of poor quality, although more recent studies were better. The two most recent studies (Lee 2007; Schiotz 2002) demonstrated adequate allocation concealment while the others did not. The majority of the other studies were unblinded. These study characteristics are associated with exaggerated treatment effects (Schulz 1995). Publication bias cannot be completely excluded, as the number of studies available for the pooled analysis was too small to be interpreted using methods such as "funnel plots" (NHMRC 1999). "Episodes to participant discrepancy" problems in four studies (Gundersen 1986; Hoivik 1984; Kasanen 1982; Kuhlemeier 1985) reduced the number of studies available for the pooled analysis.
Authors' conclusions
Implications for practice.
An exploration of heterogeneity raises the (hypothesis generating) possibility that methenamine hippurate may have some efficacy in patients without (but not in patients with) known renal tract abnormality. Short duration therapy (≤ 7 days) in people without renal tract abnormalities may be effective, but this group also have effective clinical alternatives such as oral antibiotic therapy available. Methenamine hippurate appears to be ineffective when used in spinal cord injured patients with neuropathic bladder. The rate of adverse events reported by the studies was low, which suggests that current usage is unlikely to be causing significant harm. There is a need for further large RCTs in particular to explore long duration therapy for patients without neuropathic bladder.
Implications for research.
Additional well controlled RCTs are necessary, in particular to further clarify longer term prophylaxis in those without neuropathic bladder. There needs to be better definition of symptomatic bacteruria outcomes in these clinical studies.
What's new
| Date | Event | Description |
|---|---|---|
| 20 September 2012 | Review declared as stable | As of June 2012 this Cochrane Review is no longer being updated. There have been no new studies published on this topic in the past five years and there are currently no registered ongoing studies. |
History
Protocol first published: Issue 4, 2001 Review first published: Issue 1, 2002
| Date | Event | Description |
|---|---|---|
| 7 June 2012 | New citation required but conclusions have not changed | New search performed, no new studies identified |
| 18 March 2010 | Amended | Contact details updated. |
| 14 October 2008 | Amended | Converted to new review format. |
| 1 August 2007 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
Dr Michelle Chew assisted in screening and translating the Danish and Norwegian articles
Dr Lianne Hunt assisted in translating the French and Belgian articles
Dr Gerrit Fialla and Ms Gudrun Hofmann assisted in translating the German articles
The Royal North Shore Hospital Spinal Unit funded the formal translation of the Norwegian and Danish Articles (Trine).
The referees for their comments and feedback during the preparation and updating of this review.
Thanks to all.
Appendices
Appendix 1. Electronic search strategies
| Databases | Search terms |
| CENTRAL | #1 CYSTITIS MeSH #2 PYELONEPHRITIS MeSH #3 uti* #4 (urinary near infection*) #5 cystitis #6 pyelonephritis #7 bacteriuria #8 (#1 or #2 or #3 or #4 or #5 or #6 or #7 or #8) #9 METHENAMINE MeSH #10 HIPPURATES MeSH #11 MANDELIC ACIDS MeSH #12 hiprex #13 (hippuric next acid) #14 hexamine #15 methenamine #16 hexamethylenetetramine #17 (mandelato near metenamina) #18 (#10 or #11 or #12 or #13 or #14 or #15 or #16 or #16 or #17 or #18) #19 (#9 and #19) |
| MEDLINE | 1. exp Urinary Tract Infections/ 2. exp Cystitis/ 3. exp Pyelonephritis/ 4. (uti or utis).tw. 5. (urin$ adj5 infection$).tw. 6. cystitis.tw. 7. pyelonephritis.tw. 8. bacteriuria.tw. 9. pyuria.tw. 10. or/1‐9 11. Methenamine/ 12. exp Hippurates/ 13. exp Mandelic Acids/ 14. hiprex.tw. 15. hippuric acid.tw. 16. hexamine.tw. 17. methenamine.tw. 18. hexamethylenetetramine.tw. 19. mandelato de metenamina.tw. 20. or/11‐19 21. and/10,20 |
Data and analyses
Comparison 1. Symptomatic UTI (confirmed by positive urine test).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Symptomatic bacteruria | 6 | 853 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.24, 1.18] |
| 2 Symptomatic bacteruria: Renal tract abnormalities | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 No renal tract abnormalities | 4 | 456 | Risk Ratio (M‐H, Random, 95% CI) | 0.24 [0.07, 0.89] |
| 2.2 Renal tract abnormalities | 2 | 397 | Risk Ratio (M‐H, Random, 95% CI) | 1.54 [0.38, 6.20] |
| 3 Sensitivity analysis: Symptomatic UTI including subjects unconfirmed by positive urine tests (for Lee 2006). | 6 | 853 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.24, 1.17] |
| 4 Duration of therapy | 6 | 853 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.24, 1.18] |
| 4.1 Short duration treatment (7 days or less) | 4 | 411 | Risk Ratio (M‐H, Random, 95% CI) | 0.30 [0.06, 1.56] |
| 4.2 Longer duration treatment (greater than 7 days) | 2 | 442 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.72, 1.25] |
| 5 Short duration treatment (7 days or less): Upper renal tract abnormalities | 4 | 411 | Risk Ratio (M‐H, Random, 95% CI) | 0.30 [0.06, 1.56] |
| 5.1 No upper renal tract abnormalities | 3 | 319 | Risk Ratio (M‐H, Random, 95% CI) | 0.14 [0.05, 0.38] |
| 5.2 Upper renal tract abnormalities | 1 | 92 | Risk Ratio (M‐H, Random, 95% CI) | 4.79 [0.58, 39.40] |
Comparison 2. Bacteriuria.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Outcome measure: Bacteriuria | 8 | 1114 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.45, 0.99] |
| 2 Bacteriuria (renal tract abnormalities) | 8 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 No renal tract abnormality | 6 | 717 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.37, 0.83] |
| 2.2 Renal tract abnormality | 2 | 397 | Risk Ratio (M‐H, Random, 95% CI) | 1.29 [0.54, 3.07] |
| 3 Duration of therapy | 7 | 1048 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.47, 1.06] |
| 3.1 Short duration treatment (7 days or less) | 5 | 511 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.29, 1.22] |
| 3.2 Long duration treatment (greater than 7 days) | 2 | 537 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.55, 1.31] |
| 4 Short duration treatment (7 days or less): (upper renal tract abnormalities) | 5 | 511 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.29, 1.22] |
| 4.1 No upper renal tract abnormalities | 4 | 419 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.23, 0.99] |
| 4.2 Upper renal tract abnormalities | 1 | 92 | Risk Ratio (M‐H, Random, 95% CI) | 2.87 [0.61, 13.50] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Furness 1975.
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | The inclusion criteria for this study was asymptomatic bacteriuria, which differed from the majority of studies analysed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Post‐randomisation exclusions were 12% |
| Intention‐to‐treat analysis | Unclear risk | Unclear |
Gundersen 1986.
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | There is an "Episodes of Participant discrepancy" problem with this study (see discussion) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No exclusions post‐randomisation; 3 losses, all accounted for |
| Intention‐to‐treat analysis | Low risk | ITT performed |
Hoivik 1984.
| Methods |
|
|
| Participants |
Exclusion criteria
|
|
| Interventions |
|
|
| Outcomes | All bacteriological examinations were done on morning urine (midstream and wash test). Uricult was used as a screening method. When bacteria was > 105/mL, urine was sent away for resistance testing.
|
|
| Notes | The outcomes of this study had an "episodes of participant discrepancy" problem (see discussion). Side effects were relatively well described compared to the other studies | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated (this was clearly not centrally randomised). The centres had different treatments to randomise (see participants) |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Losses post‐randomisation: 11/52 |
| Intention‐to‐treat analysis | Unclear risk | Not stated |
Kasanen 1982.
| Methods |
|
|
| Participants |
|
|
| Interventions | The patients urine was first rendered sterile by antibiotic treatment. The patients were then allocated into 4 groups
|
|
| Outcomes |
|
|
| Notes | "Episodes of participant discrepancy" (see discussion) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Single blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Withdrawals are described as low (0% placebo, 1.4% in the hippurate group) however, it is not clear from the methodology whether all patients successfully completed all parts of this protocol. |
| Intention‐to‐treat analysis | Unclear risk | Unclear |
Knoff 1985.
| Methods |
|
|
| Participants |
|
|
| Interventions |
Treatment continued for 7 days post surgery |
|
| Outcomes |
|
|
| Notes | Notes: The Authors have been contacted to account for the 4 post randomisation exclusions and to clarify outcome measures | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Post randomisation exclusions: 6%. |
| Intention‐to‐treat analysis | Unclear risk | Unclear |
Kuhlemeier 1985.
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes | Urine cultures were obtained weekly until the urine showed significant bacterial colonisation (≥ 1000 bacteria/mL for a catheter obtained specimen or 100,000 colonies/mL for a clean catch sample).
|
|
| Notes | Episodes of participant discrepancy (see discussion) Significant problems with the outcome measures, follow‐up and losses Unpublished data regarding quasi randomised assignment | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Assignment to the groups was sequential: first, seventh patient to group 1, second, eighth to group 2 etc (unpublished data) |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Single blinded (unpublished data) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | High risk | There were between 38% to 20% losses to follow‐up depending on outcome measure |
| Intention‐to‐treat analysis | Unclear risk | Unclear |
Ladehoff 1984.
| Methods |
|
|
| Participants |
|
|
| Interventions |
Treatment duration 8 to 13 days |
|
| Outcomes |
|
|
| Notes | The higher number of hysterectomies in the control group may bias towards a treatment effect. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Post randomisation exclusions: 23% |
| Intention‐to‐treat analysis | High risk | No ITT |
Lee 2007.
| Methods |
|
|
| Participants |
Exclusion criteria
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Centralised randomisation, 4 groups factorial design |
| Allocation concealment (selection bias) | Low risk | Adequate |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Yes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Yes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up |
| Intention‐to‐treat analysis | Low risk | Yes |
Pettersson 1989.
| Methods |
|
|
| Participants |
|
|
| Interventions |
Treatment continued for 7 days post surgery |
|
| Outcomes |
|
|
| Notes | The poorly defined outcome measures are a major problem with this study The authors have been contacted for clarification | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Adequate |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | no blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not well described (Between 94‐100%completed follow‐up). No post randomisation exclusions. |
| Intention‐to‐treat analysis | Unclear risk | Unclear |
Sander 1976.
| Methods |
|
|
| Participants |
|
|
| Interventions |
Duration of treatment not explicitly stated |
|
| Outcomes |
|
|
| Notes | The duration of exposure is not clear from this paper There are suggestions in the paper that treatment may have continued for the entire 8‐week follow‐up period, but these have not been explicitly stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | quasi‐RCT |
| Allocation concealment (selection bias) | Unclear risk | not stated |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No post randomisation exclusions |
| Intention‐to‐treat analysis | Unclear risk | Unclear |
Schiotz 2002.
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
The following is unpublished data: All patients had an outpatient appointment after 4‐6 weeks, but not with urinary cultures if there had been no symptoms of UTI. Patients discharged with bacteriuria were instructed to contact the study team if symptoms of infection occurred. Two patients failed to do so, but details of their symptoms and cultures were obtained from their general practitioner and the laboratory and the patients were interviewed by the primary author |
|
| Notes | Notes: 150 participants randomised but only 145 analysed. 5 patients were excluded: 3 because of pre‐operative antibiotic treatment and 2 were not catheterised. additional detail regarding the randomisation group and the outcomes of these patients were sought from the authors who provided the allocation groups and the outcomes for these patients. This data (unpublished) is included in the analysis. The nature follow‐up and how the symptomatic UTI diagnosis was collected has also been provided from the authors | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Adequate |
| Allocation concealment (selection bias) | Low risk | Adequate |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Post randomisation exclusions: unclear |
| Intention‐to‐treat analysis | Unclear risk | Unclear |
Thomlinson 1968.
| Methods |
|
|
| Participants |
|
|
| Interventions |
Treatment continued for 7 days post surgery. |
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | High risk | Alternate allocation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | not stated |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Post allocation exclusions: 23% |
| Intention‐to‐treat analysis | High risk | No ITT |
Tyreman 1986.
| Methods |
|
|
| Participants |
|
|
| Interventions |
Treatment duration: continued for 5 days post surgery |
|
| Outcomes |
|
|
| Notes | No clarification of the methodology used to collect side effect data is given | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Post randomisation exclusions: 14% |
| Intention‐to‐treat analysis | Unclear risk | Unclear |
NS ‐ not stated; SCI ‐ spinal cord injury; UTI ‐ urinary tract infection
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Banovac 1991 | No mention of randomised or quasi randomised control. Marked differences were evident on a comparison between the treatment and control groups with regards to sex and level of spinal injury. ACC |
| Brumfitt 1981 | RCT involving women with a frequent history of UTI did not include a "no treatment" control group. The comparison "control" group was nitrofurantoin. |
| Brumfitt 1983 | RCT involving women with a frequent history of UTI did not include a "no treatment" control group. The comparison "control" group was frequent perineal povidone iodine application (for ethical reasons, as stated by the authors). |
| Casselman 1966 | Probably a before and after study. No control (no treatment) group. |
| Chrapowicki 1975 | No control. All participants were given vitamin C and cranberry juice as well (from German). |
| Christopher 1969 | This (alternate allocation) study, compared methenamine hippurate to sulphamethizole in pregnant women. The study did not include a "no treatment" control group. |
| Cronberg 1987 | Randomised double blind cross over design. This study could potentially be included if data at the first 6 month cross over could be provided. 33% drop out at 1 year but no drop out rate at 6 months. (ACC) |
| Elo 1978 | Not RCT or quasi‐RCT. No control (no treatment) group. |
| Genster 1970 | This study on UTIs following transurethral prostatectomy did not mention using a randomised or quasi randomised control design. ACC |
| Gerstein 1968 | Not RCT or quasi‐RCT. It did not include the use of a "no treatment" control. |
| Gow 1974 | This trial compared methenamine hippurate to methenamine mandelate, without the use of a "non treatment" control. |
| Horcickova 1986 | This study compared methenamine hippurate with methenamine mandelate without a "no treatment" control group. |
| Kasanen 1974a | Comparison of long‐term, low dosage nitrofurantoin, methenamine hippurate, trimethoprim and trimethoprim‐sulphamethoxazole. Absence of a "no treatment" control group. |
| Kasanen 1974b | The main outcome measure is the urinary formaldehyde concentration, which is not an outcome measure specified by our review. |
| Klein 1976 | No control group (from Germany) |
| Kostiala 1982 | No mention of randomised or quasi randomised control. The method of allocation was not specified and there was no mention of randomisation in the methodology. ACC |
| Krechnakova 1979 | 14 patients. Chronic, relapsing pyelonephritis treated with methenamine hippurate on a long term basis. No control stated in this study. |
| LeBlanc 1964 | Mandelamine not methenamine hippurate studied. |
| Murray 1977 | A comparison of hexamine hippurate with sulphamethizole, a "no treatment" control comparison was not provided. |
| Nilsson 1975 | This study is a 16 month cohort study on 24 patients given methenamine hippurate. It does not meet the inclusion criteria of this review, but may provide useful side effect data. |
| Norberg‐A 1980 | RCT, double blind study. Outcome measure was catheter life (not an outcome measure specified in this review). Incidentally, there appears to be an episodes to participants issue with this trial. |
| Norberg‐B 1979a | Not RCT. The study involves the treatment of patients with infected urine rather than the prevention of UTI |
| Norberg‐B 1979b | Non randomised crossover trial. The outcome measure is a quantified sediment of the urine from Geriatric patients with indwelling catheters. This does not match our predefined outcome measures. |
| Nyren 1981 | No mention of randomised or quasi‐randomised control. ACC |
| Olsen 1983 | The comparison "control group" was cefotaxime, not "no treatment". |
| Parkhede 1982 | RCT. The outcome measure is a reduction in urine viscosity. Although this may have an association with catheter blockage, it does not address directly the study question of this meta‐analysis as defined by pre‐study outcome measures. |
| Parvio 1976 | Non randomised crossover trial. Of the original 52 patients, 12 did not complete the 6 month treatment course. |
| Pedersen 1977 | Double blind, parallel experiment comparing methenamine hippurate and "phenylsalicylate" only. (lack of "no treatment" control). |
| Scetbon 1973 | Not RCT or quasi‐RCT (from France) |
| Stover 1980 | This RCT did not provide a "no treatment" control group . The interventions included methenamine hippurate 1 g by mouth twice/day, or ascorbic acid 1 g by mouth 4 times/day. Other problems are a lack of blinding, lack of intention‐to‐treat analysis and in particular no clear description of the number and group of excluded patients. |
| Vainrub 1977 | No mention of RCT or quasi‐RCT. ACC |
| Wibell 1980 | No mention of RCT or quasi‐RCT. Non randomised cross over for the control (no treatment) group. |
ACC ‐ authors contacted for clarification
Contributions of authors
-
Dr Bonne Lee was involved in the following aspects of this project:
Design of the research question, formulation of the study protocol, design and repeated updates of the literature search, selection of articles and extraction of information, arranging translation of articles as appropriate, data analysis, interpretation and write up.
A/Prof Judy Simpson was involved in protocol development, data analysis, statistical support, interpretation, document update and manuscript editing.
A/Prof Jonathan Craig was involved in protocol development, data analysis, interpretation, document update and manuscript editing.
Dr Tushar Bhuta was co‐author, and was involved in literature searching, data selection, extraction and interpretation.
Sources of support
Internal sources
Royal North Shore Hospital Spinal Medicine Department (initial protocol and review), Australia.
External sources
No sources of support supplied
Declarations of interest
Dr Lee is the lead investigator on the NHMRC Project Grant Application: 630448 Probiotic Prophylaxis of Spinal Cord Injury Urinary Tract‐Infection TherapeUtic‐Trial (ProSCIUTTU), which is an Australian government sponsored study looking at the effectiveness of Probiotic therapy in preventing Urinary tract infection and multi drug resistance (Lee 2007). This study was independently assessed by the Cochrane Renal Group's Review Group Coordinator to eliminate potential bias in the assessment of this study.
Tushar Bhuta: none known
Judy M Simpson: none known
Jonathan C Craig: none known
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
Furness 1975 {published data only}
- Furness ET, McDonald PJ, Beasley NV. Urinary antiseptics in asymptomatic bacteriuria of pregnancy. New Zealand Medical Journal 1975;81(539):417‐9. [MEDLINE: ] [PubMed] [Google Scholar]
Gundersen 1986 {published data only}
- Gundersen R, Hoivik HO, Osmundsen K. Frequent cystitis in elderly women. A double‐blind comparison of hiprex and placebo in general practice [Hyppig forekommende cystitter hos eldre kvinner]. Tidsskrift for Den Norske Laegeforening 1986;106(25):2048‐9. [MEDLINE: ] [PubMed] [Google Scholar]
Hoivik 1984 {published data only}
- Hoivik H. O, Gundersen R, Osmundsen K, Halvorsen P, Hjortdahl P, Stokke JG. Prevention of recurrent cystitis in fertile women. A double‐blind comparison of hiprex and placebo in general practice. Tidsskrift for Den Norske Laegeforening 1984;104(16):1150‐2. [MEDLINE: ] [PubMed] [Google Scholar]
Kasanen 1982 {published data only}
- Kasanen A, Junnila SY, Kaarsalo E, Hajba A, Sundquist H. Secondary prevention of recurrent urinary tract infections. Comparison of the effect of placebo, methenamine hippurate, nitrofurantoin and trimethoprim alone. Scandinavian Journal of Infectious Diseases 1982;14(4):293‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Knoff 1985 {published data only}
- Knoff T. Methenamine hippurate. Short‐term catheterization in gynecologic surgery. A double‐blind comparison of hiprex and placebo. Tidsskrift for Den Norske Laegeforening 1985;105(7):498‐9. [MEDLINE: ] [PubMed] [Google Scholar]
Kuhlemeier 1985 {published data only}
- Kuhlemeier KV, Stover SL, Lloyd LK. Prophylactic antibacterial therapy for preventing urinary tract infections in spinal cord injury patients. Journal of Urology 1985;134(3):514‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ladehoff 1984 {published data only}
- Ladehoff P, Jacobsen JC, Olsen H, Pedersen GT, Sorensen T. The preventive effect of methenamine hippurate (haiprex) on urinary infections after short‐term catheterization. A clinical study [Metenaminhippurats (Haiprex) profylaktiske virkning over for urinvejsinfektion ved korttidskateterisation]. Ugeskrift for Laeger 1984;146(19):1433‐4. [MEDLINE: ] [PubMed] [Google Scholar]
Lee 2007 {published and unpublished data}
- Lee BB, Haran MJ, Hunt LM, Simpson JM, Marial O, Rutkowski SB, et al. Spinal‐injured neuropathic bladder antisepsis (SINBA) trial. Spinal Cord 2007;45(8):542‐50. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Pettersson 1989 {published data only}
- Pettersson B, Tiselius HG. Are prophylactic antibiotics necessary during extracorporeal shockwave lithotripsy?. British Journal of Urology 1989;63(5):449‐52. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sander 1976 {published data only}
- Sander S, Jakobsen A Jr. Preventive hiprex in urinary tract operations. Tidsskrift for Den Norske Laegeforening 1976;96(3):167‐9. [MEDLINE: ] [PubMed] [Google Scholar]
Schiotz 2002 {published data only}
- Schiotz HA. Personal communication 15 October 2006.
- Schiotz HA, Guttu K. Value of urinary prophylaxis with methenamine in gynecologic surgery. Acta Obstetricia et Gynecologica Scandinavica 2002;81(8):743‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Thomlinson 1968 {published data only}
- Thomlinson J, Williams JD, Cope E. Persistence of bacteriuria following gynaecological surgery: A trial of methenamine hippurate. British Journal of Urology 1968;40(4):479‐82. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Tyreman 1986 {published data only}
- Tyreman NO, Andersson PO, Kroon L, Orstam S. Urinary tract infection after vaginal surgery. Effect of prophylactic treatment with methenamine hippurate. Acta Obstetricia et Gynecologica Scandinavica 1986;65(7):731‐3. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Banovac 1991 {published data only}
- Banovac K, Wade N, Gonzalez F, Walsh B, Rhamy RK. Decreased incidence of urinary tract infections in patients with spinal cord injury: effect of methenamine. Journal of the American Paraplegia Society 1991;14(2):52‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Brumfitt 1981 {published data only}
- Brumfitt W, Cooper J, Hamilton‐Miller JM. Prevention of recurrent urinary infections in women: a comparative trial between nitrofurantoin and methenamine hippurate. Journal of Urology 1981;126(1):71‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Brumfitt 1983 {published data only}
- Brumfitt W, Hamilton‐Miller JM, Gargan RA, Cooper J, Smith GW. Long‐term prophylaxis of urinary infections in women: comparative trial of trimethoprim, methenamine hippurate and topical povidone‐iodine. Journal of Urology 1983;130(6):1110‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Casselman 1966 {published data only}
- Casselman J. Hexamethylene‐tetramine hippurate in the treatment of urinary infections. Acta Urologica Belgica 1966;34(3):354‐8. [MEDLINE: ] [PubMed] [Google Scholar]
Chrapowicki 1975 {published data only}
- Chrapowicki T, Krzyzanowska‐Rogozinska T, Kurowska D. Treatment of acute and chronic urinary tract infections in children with an urinary chemotherapeutic agent. Zeitschrift fur Allgemeinmedizin 1975;51(27):1215‐9. [MEDLINE: ] [PubMed] [Google Scholar]
Christopher 1969 {published data only}
- Christopher LJ, Thompson GR. A trial of hippramine in the treatment of bacteriuria of pregnancy. Irish Journal of Medical Science 1969;8(7):331‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Cronberg 1987 {published data only}
- Cronberg S, Welin CO, Henriksson L, Hellsten S, Persson KM, Stenberg P. Prevention of recurrent acute cystitis by methenamine hippurate: double blind controlled crossover long term study. British Medical Journal Clinical Research Ed 1987;294(6586):1507‐8. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Elo 1978 {published data only}
- Elo J, Sarna S, Ahava K, Lepo A. Methenamine hippurate in urinary tract infections in children: prophylaxis, treatment and side effects. Journal of Antimicrobial Chemotherapy 1978;4(4):355‐65. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Genster 1970 {published data only}
- Genster HG, Madsen PO. Urinary tract infections following transurethral prostatectomy: with special reference to the use of antimicrobials. Journal of Urology 1970;104(1):163‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Gerstein 1968 {published data only}
- Gerstein AR, Okun R, Gonick HC, Wilner HI, Kleeman CR, Maxwell MH. The prolonged use of methenamine hippurate in the treatment of chronic urinary tract infection. Journal of Urology 1968;100(6):767‐71. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Gow 1974 {published data only}
- Gow JG. A comparative trial of hexamine hippurate and hexamine mandelate in prevention of recurrent infection of the urinary tract. Practitioner 1974;213(1273):97‐101. [MEDLINE: ] [PubMed] [Google Scholar]
Horcickova 1986 {published data only}
- Horcickova M, Prat V, Hatala M, Nezadalova E, Milotova Z. Methenamine hippurate VUFB and mandelamine VUFB in the prevention of recurrent dysuria in women. Casopis Lekaru Ceskych 1986;125(27):849‐53. [MEDLINE: ] [PubMed] [Google Scholar]
Kasanen 1974a {published data only}
- Kasanen A, Kaarsalo E, Hiltunen R, Soini V. Comparison of long‐term, low‐dosage nitrofurantoin, methenamine hippurate, trimethoprim and trimethoprim‐sulphamethoxazole on the control of recurrent urinary tract infection. Annals of Clinical Research 1974;6(5):285‐9. [MEDLINE: ] [PubMed] [Google Scholar]
Kasanen 1974b {published data only}
- Kasanen A, Mustakallio E, Koskinen EH, Soini V. Methenamine hippurate in the treatment of urinary tract infections. Annals of Clinical Research 1974;6(5):279‐84. [MEDLINE: ] [PubMed] [Google Scholar]
Klein 1976 {published data only}
- Klein D, Puchegger R. Methenamine hippurate in urinary tract infections. Zfa ‐ Zeitschrift Fur Allgemeinmedizin 1976;52(21):1110‐4. [MEDLINE: ] [PubMed] [Google Scholar]
Kostiala 1982 {published data only}
- Kostiala AA, Nyren P, Runeberg L. Effect of nitrofurantoin and methenamine hippurate prophylaxis on bacteria and yeasts in the urine of patients with an indwelling catheter. Journal of Hospital Infection 1982;3(4):357‐64. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Krechnakova 1979 {published data only}
- Krechnakova A, Dzurik R. Prevention of chronic pyelonephritis relapses using metenamine hipuran (author's transl). Casopis Lekaru Ceskych 1979;118(13):402‐4. [MEDLINE: ] [PubMed] [Google Scholar]
LeBlanc 1964 {published data only}
- LeBlanc AQL, McGanity WJ. The impact of bacteriuria in pregnancy ‐ a survey of 1300 pregnant patients. Texas Reports on Biology & Medicine 1964;22:336‐47. [MEDLINE: ] [PubMed] [Google Scholar]
Murray 1977 {published data only}
- Murray CP, Feeney J. A comparison of hexamine hippurate and sulphamethizolein patients with indwelling catheters following pelvic‐floor surgery. Irish Medical Journal 1977;69(4):97‐8. [MEDLINE: ] [PubMed] [Google Scholar]
Nilsson 1975 {published data only}
- Nilsson S. Long‐term treatment with methenamine hippurate in recurrent urinary tract infection. Acta Medica Scandinavica 1975;198(1‐2):81‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Norberg‐A 1980 {published data only}
- Norberg A, Norberg B, Parkhede U, Gippert H, Lundbeck K. Randomized double‐blind study of prophylactic methenamine hippurate treatment of patients with indwelling catheters. European Journal of Clinical Pharmacology 1980;18(6):497‐500. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Norberg‐B 1979a {published data only}
- Norberg B, Norberg A, Parkhede U, Gippert H. Effect of short‐term high‐dose treatment with methenamine hippurate on urinary infection in geriatric patients with an indwelling catheter. IV. Clinical evaluation. European Journal of Clinical Pharmacology 1979;15(5):357‐61. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Norberg‐B 1979b {published data only}
- Norberg B, Norberg A, Parkhede U, Gippert H, Akerman M. The effect of short‐term high‐dose treatment with methenamine hippurate of urinary infection in geriatric patients with indwelling catheters. I. The preparation and morphology of a quantified urine sediment. Upsala Journal of Medical Sciences 1979;84(1):67‐74. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Nyren 1981 {published data only}
- Nyren P, Runeberg L, Kostiala AI, Renkonen OV, Roine R. Prophylactic methenamine hippurate or nitrofurantoin in patients with an indwelling urinary catheter. Annals of Clinical Research 1981;13(1):16‐21. [MEDLINE: ] [PubMed] [Google Scholar]
Olsen 1983 {published data only}
- Olsen JH, Friis‐Moller A, Jensen SK, Korner B, Hvidt V. Cefotaxime for prevention of infectious complications in bacteriuric men undergoing transurethral prostatic resection. A controlled comparison with methenamine. Scandinavian Journal of Urology & Nephrology 1983;17(3):299‐301. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Parkhede 1982 {published data only}
- Parkhede U, Norberg B, Norberg A, Eriksson S. The effect of treatment with methenamine hippurate of urinary tract infection in long‐stay geriatric in‐patients with indwelling catheters: III. Evaluation by urine viscosimetry. Journal of Clinical & Experimental Gerontology 1982;4(1):71‐81. [EMBASE: 1982226624] [Google Scholar]
Parvio 1976 {published data only}
- Parvio S. Methenamine hippurate ('hiprex') in the treatment of chronic urinary tract infections: a trial in a geriatric hospital. Journal of International Medical Research 1976;4(2):111‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Pedersen 1977 {published data only}
- Pedersen FB, Korner B. Urinary tract infection after prostate surgery. A controlled, clinical‐bacteriological comparison between methenamine hippurate (haiprex) and phenylsalicylate methenamine preparations. Ugeskrift for Laeger 1977;139(23):1350‐2. [MEDLINE: ] [PubMed] [Google Scholar]
Scetbon 1973 {published data only}
- Scetbon V, Peycelon‐de la Selle MF. Value of mandelamine in the prolonged treatment of urinary infections caused by escherichia coli. Therapeutique 1973;49(4):283‐6. [MEDLINE: ] [PubMed] [Google Scholar]
Stover 1980 {published data only}
- Stover SL, Fleming WC. Recurrent bacteriuria in complete spinal cord injury patients on external condom drainage. Archives of Physical Medicine & Rehabilitation 1980;61(4):178‐82. [MEDLINE: ] [PubMed] [Google Scholar]
Vainrub 1977 {published data only}
- Vainrub B, Musher DM. Lack of effect of methenamine in suppression of, or prophylaxis against, chronic urinary infection. Antimicrobial Agents & Chemotherapy 1977;12(5):625‐9. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wibell 1980 {published data only}
- Wibell L, Scheynius A, Norrman K. Methenamine‐hippurate and bacteriuria in the geriatric patient with a catheter. Acta Medica Scandinavica 1980;207(6):469‐73. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Additional references
3M 2000
- 3M Pharmaceuticals Pty Ltd. MIMS Full prescribing information for hiprex. In: Caswell A editor(s). Mims online version 1.1. Vol. refer: MIMS OTC 1999 p. 249, MIMS Australia, Jan ‐ Mar 2000. [Google Scholar]
Albert 2004
- Albert X, Huertas I, Pereiro I, Sanfelix J, Gosalbes V, Perrota C. Antibiotics for preventing recurrent urinary tract infection in non‐pregnant women. Cochrane Database of Systematic Reviews 2004, Issue 3. [DOI: 10.1002/14651858.CD001209.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Armitage 1994
- Armitage G, Berry G. Statistical inference. Statistical methods in medical research. 3rd Edition. Oxford: Blackwell Scientific Publications, 1994:93‐5. [Google Scholar]
Devenport 1984
- Devenport JK, Swenson JR, Dukes GE Jr, Sonsalla PK. Formaldehyde generation from methenamine salts in spinal cord injury. Archives of Physical Medicine & Rehabilitation 1984;65(5):257‐9. [MEDLINE: ] [PubMed] [Google Scholar]
Dickersin 1994
- Dickersin K, Scherer R, Lefebvre C. Identifying relevant studies for systematic reviews. BMJ 1994;309(6964):1286‐91. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Greenwood 1981
- Greenwood D, Slack RC. The antibacterial activity of hexamine (methenamine), hexamine hippurate and hexamine mandelate. Infection 1981;9(5):223‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hetey 1990
- Hetey SK. Effect of ascorbic acid on urine pH in patients with injured spinal cords. American Journal of Hospital Pharmacy 1990;37(2):235‐7. [MEDLINE: ] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jepson 2004
- Jepson RG, Mihaljevic L, Craig JC. Cranberries for preventing urinary tract infections. Cochrane Database of Systematic Reviews 2004, Issue 2. [DOI: 10.1002/14651858.CD001321.pub3] [DOI] [PubMed] [Google Scholar]
Kasanen 1974
- Kasanen A, Mustakallio E, Koskinen EH, Soini V. Methenamine hippurate in the treatment of urinary tract infections. Annals of Clinical Research 1974;6(5):279‐84. [MEDLINE: ] [PubMed] [Google Scholar]
Lau 1998
- Lau J, Ioannidis JPA, Schmid CH. Summing up evidence: One answer is not always enough. Lancet 1998;351(9096):123‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lefebvre 1996
- Lefebvre C, McDonald S. Development of a sensitive search strategy for reports of randomized controlled trials in EMBASE. Fourth International Cochrane Colloquium, Adelaide Australia, 20‐24 October. 1996.
Macfarlane 1985
- Macfarlane DE. Prevention and treatment of catheter‐associated urinary tract infections. Journal of Infection 1985;10(2):96‐106. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mayrer 1982
- Mayrer AR, Andriole VT. Urinary tract antiseptics. Medical Clinics of North America 1982;66(1):199‐208. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
McEvoy 1999
- Gerald K. McEvoy (Ed). AHFS Drug Information 1999. Bethesda: American Society of Hospital Pharmacists, 1999. [Google Scholar]
Nahata 1982
- Nahata MC, Cummins BA, McLeod DC, Schondelmeyer SW, Butler R. Effect of urinary acidifiers on formaldehyde concentration and efficacy with methenamine therapy. European Journal of Clinical Pharmacology 1982;22(3):281‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
NHMRC 1999
- Anonymous. Summarising and synthesising the studies. How to review the evidence: systematic identification and review of the scientific literature. Canberra: NHMRC, Nov 1999:27‐36. [Google Scholar]
NIDRRS 1992
- National Institute on Disability and Rehabilitation Research Statement (NIDRRS). The prevention and management of urinary tract infections amongst people with spinal cord injuries. Journal of American Paraplegia Society 1992;15(3):194‐204. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Niel‐Weise 2005
- Niel‐Weise BS, Brock PJ. Antibiotic policies for short‐term catheter bladder drainage in adults. Cochrane Database of Systematic Reviews 2005, Issue 3. [DOI: 10.1002/14651858.CD005428] [DOI] [PubMed] [Google Scholar]
Parfitt 1999
- Kathleen Parfitt (ed). Martindale. London: Pharmaceutical Press, 1999. [Google Scholar]
Saint 1999
- Saint S, Lipsky BA. Preventing catheter‐related bacteriuria: should we? Can we? How?. Archives of Internal Medicine 1999;159(8):800‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273(5):408‐12. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Stamm 1988
- Stamm WE. Protocol for diagnosis of urinary tract infection: Reconsidering the criterion for significant bacteriuria. Urology 1988;32(2 Suppl):6‐10. [MEDLINE: ] [PubMed] [Google Scholar]
Thom 1999
- Thom JD, Wolfe V, Perkash I, Lin VW. Methicillin‐resistant Staphylococcus aureus in patients with spinal cord injury. Journal of Spinal Cord Medicine 1999;22(2):125‐31. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Waites 1993
- Waites KB, Canupp KC, Devivo MJ. Epidemiology and risk factors for urinary tract infection following spinal cord injury. Archives of Physical Medicine & Rehabilitation 1993;74(7):691‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Waites 2000
- Waites KB, Chen Y, DeVivo MJ, Canupp KC, Moser SA. Antimicrobial resistance in gram‐negative bacteria isolated from the urinary tract in community‐residing persons with spinal cord injury. Archives of Physical Medicine & Rehabilitation 2000;81(6):764‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Wall 1990
- Wall I, Tiselius HG. Long‐term acidification of urine in patients treated for infected renal stones. Urologia Internationalis 1990;45(6):336‐41. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Lee 2001
- Lee B, Bhuta T, Craig J, Simpson J. Methenamine hippurate for preventing urinary tract infections. Cochrane Database of Systematic Reviews 2001, Issue 4. [DOI: 10.1002/14651858.CD003265] [DOI] [PubMed] [Google Scholar]
Lee 2002
- Lee B, Bhuta T, Craig J, Simpson J. Methenamine hippurate for preventing urinary tract infections. Cochrane Database of Systematic Reviews 2002, Issue 1. [DOI: 10.1002/14651858.CD003265] [DOI] [PubMed] [Google Scholar]
Lee 2007
- Lee BSB, Simpson JM, Craig JC, Bhuta T. Methenamine hippurate for preventing urinary tract infections. Cochrane Database of Systematic Reviews 2007, Issue 4. [DOI: 10.1002/14651858.CD003265.pub2] [DOI] [PubMed] [Google Scholar]


