Abstract
Immunotherapy has exhibited promising but controversial results in gastric cancer; determining criteria for choosing the appropriate target population is still problematic. Epstein-Barr virus (EBV)-associated gastric carcinoma (EBVaGC) exhibits distinctive genomic aberrations and clinicopathologic features, the positive status of EBV is a potential biomarker. We prospectively recruited 9 patients who were diagnosed with stage-IV EBVaGC, and all of the patients were treated by immune-checkpoint inhibitors. The median age of the patients was 62 years old. The clinicopathologic characteristics demonstrated a male predominance and poor differentiation status of EBVaGC. Lymph nodes were demonstrated to represent the most common metastatic site. Immunochemistry and polymerase chain reaction analysis revealed that all of the patients were proficient mismatch repair, and microsatellite instability-stable and programmed cell death-ligand 1 were detected in 7 patients. Three patients with positive programmed cell death-ligand 1 showed partial response, 5 patients showed stable disease, 1 patient without measurable lesion showed decreasing ascites and tumor marker level after immunotherapy. The longest duration of response was 18 months by the time of the last follow-up. EBVaGC exhibits distinctive clinicopathologic characteristics, and EBV-positive status may be a potential biomarker for gastric cancer immunotherapy.
Key Words: gastric cancer, Epstein-Barr virus, immune-checkpoint inhibitor, biomarker
Despite the administration of cytotoxic chemotherapy and targeted therapy, the prognosis of gastric cancer is still dismal and disappointing.1–3 Immunotherapy for gastric cancer—which primarily consists of programmed cell death-1/programmed cell death-ligand 1 (PD-1/PD-L1) and/or cytotoxic T-lymphocyte–associated antigen-4 inhibitors—has yielded promising but controversial results over recent years. Specifically, the ATTRACTION-2 study demonstrated superior antitumor activity and survival outcomes with nivolumab versus placebo in later-line management of advanced gastric cancer.4 Meanwhile, the KEYNOTE-059 study reported a 60% objective response rate (ORR) in pembrolizumab plus the chemotherapy arm, and pembrolizumab monotherapy showed a similar ORR compared with that of chemotherapy, which was consistent with that of nivolumab.5 Controversially, the KEYNOTE-062 study—which was a phase-III 3-arm clinical trial—indicated that although pembrolizumab plus chemotherapy exhibited a favorable ORR, it did not prolong progression-free survival (PFS) or overall survival (OS). Pembrolizumab monotherapy showed a lower ORR and noninferior OS compared with that of chemotherapy.6 Meanwhile, both KEYNOTE-061 and JAVELIN Gastric-300 studies failed to show the superiority of immunotherapy over chemotherapy.7,8 Because of the low effective rate of immunotherapy and controversial survival outcomes in these previous clinical trials, further studies are needed to better determine the criteria for choosing the appropriate target population among this putatively high heterogenous group, as well as to identify novel biomarkers for improving immunotherapy of gastric cancer.
PD-L1 expression, microsatellite instability (MSI) status, tumor mutation burden, and Epstein-Barr virus (EBV) positivity have subsequently been proposed as potential biomarkers in gastric cancer immunotherapy.9 In the KEYNOTE-059 cohort 1, pembrolizumab monotherapy showed an ORR of 22.7% [95% confidence interval (CI), 13.8%–33.8%] and 8.6% (95% CI, 2.9%–19.0%) in PD-L1-positive and PD-L1-negative groups, respectively.10 The KEYNOTE-059 cohort 2 showed a similar superiority when comparing PD-L1-positive and PD-L1-negative subgroups in previous untreated gastric cancer patients.5 However, in the KEYNOTE-061 and JAVELIN Gastric-300 studies, no improvement was observed in the PD-L1-positive group.7,8 In contrast, microsatellite instability-high (MSI-H) correlated with a large number of neoantigens and tumor-infiltration lymphocytes. In the KEYNOTE-059 cohort 1, the ORRs were 57.1% (95% CI, 18.4%–90.1%) and 9.0% (95% CI, 5.1%–14.4%) in the MSI-H and non–MSI-H groups respectively.10 Kim et al9 reported that 6 of 7 MSI-H patients showed objective responses, with 3 having complete response and 3 having partial response (PR). In the same study, 8 patients showed a high mutational load—with 6 patients exhibiting MSI-H and 1 patient being EBV-positive—and the ORR reached 88.9%.9
Epstein-Barr virus–associated gastric carcinoma (EBVaGC), which is mutually exclusive with MSI-H, comprises ∼9% of gastric cancers and exhibits distinctive genomic aberrations and clinicopathologic features.11 These aberrations and features include recurrent PIK3CA and ARIDIA mutations, overexpression of PD-L1/PD-L2, and a high prevalence of DNA hypermethylation. Activation of JAK2, immune cell signaling, and mitotic pathways has also been detected in cancer cells.12 However, clinical information on EBVaGC immunotherapy is lacking. Kim and colleagues reported that 6 EBVaGC patients all reached PR, with a median duration of response of 8.5 months. In the 2019 American Society of Clinical Oncology (ASCO), Xu et al13 reported that 1 of 4 patients who were EBV-positive showed PR after immunotherapy. Hence, the validation of the effectiveness of immunotherapy in EBVaGC is urgently needed. Our present study aimed to prospectively demonstrate biomarker identities of the EBV-positive status for gastric cancer immunotherapy.
METHODS
Population
We prospectively recruited patients diagnosed with stage-IV EBVaGC patients at Beijing Cancer Hospital from October 2015 to June 2019. Patients were treated with immunotherapy alone or combined with chemotherapy. Patients recruited in other clinical trials were also eligible in our study. Patients with an ECOG score >2 were excluded. Clinicopathologic information regarding patient age, sex, location, tumor stage, and immunochemical staining was obtained from the Gastrointestinal Oncology Department at Beijing Cancer Hospital. The American Joint Committee on Cancer (AJCC), seventh edition, was used for staging. We used RECIST 1.1 to evaluate responses to treatment. Patients were followed up with phone calls until the end of the study, or until death. The collection of survival data was still in progress at the end of the study. Informed consent was signed by each patient.
Treatment
Nivolumab was administrated every 2 weeks at a dose of 3 mg/kg (60 min intravenous infusion), and ipilimumab was administrated at 1 mg/kg (90 min infusion). Other immune-checkpoint inhibitors were administrated according to the protocols of previously published clinical trials. Dosage adjustments of immunotherapy were not permitted, and administration was suspended if severe adverse effects related to treatment occurred. Immunotherapy was discontinued permanently if any of the following occurred: grade-2 immune-related adverse effects (irAEs) were not recovered after treatment; grade-3 irAE lasted over 7 days; grade-4 irAE was observed; and/or immunotherapy was suspended over 6 weeks. There were no protocol-specific chemotherapy regimens. Fluoropyrimidine (S1 or capecitabine) and platinum were used for combined immunotherapy.
Histology
Tumor specimens were obtained from endoscopic biopsies or surgeries and were fixed with formalin. Tumor sections were subsequently stained with hematoxylin and eosin. Two experienced pathologists from Beijing Cancer Hospital evaluated the tumor specimens and sections. Histologic phenotyping of tumors was performed according to World Health Organization (WHO) guidelines. Lymphoepithelioma-like carcinomas were detected during the inspection, and lymphovascular and perineural invasions were noted when present.
Immunohistochemical staining directed against MLH1, MSH2, MSH6, and PMS2 were performed to evaluate mismatch repair (MMR) deficiency. The antibodies used in the procedure are summarized in Table 1. Tumors with loss of staining in tumor cell nuclei were interpreted to be deficient MMR, and tumors with retained nuclear staining for all MMR proteins were deemed proficient mismatch repair (pMMR).
TABLE 1.
Antibodies Used for Immunochemical Studies
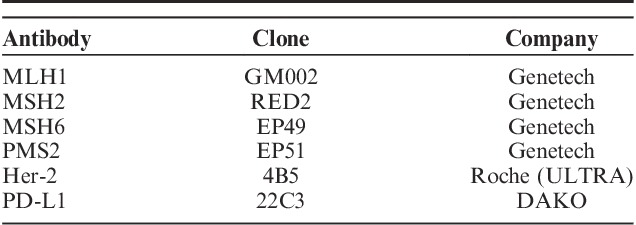
Immunochemical staining for human epidermal growth factor receptor 2 (Her-2) was evaluated using standard criteria, and the extent of overall staining or membranous staining was recorded. PD-L1 staining was performed to assess interactions between tumor cells and lymphocytes. PD-L1 in both tumor cells and stroma were recorded.
The following 5 MSI markers were used for analysis: BAT-25, BAT-26, NR-21, NR-24, and MONO-27. Instabilities in ≥2 microsatellite loci were categorized as MSI-H, an instability in a single loci was categorized as MSI-low, and an absence of MSI in all the 5 markers was categorized as microsatellite instability-stable (MSS; GENTRON).
EBV Detection
EBV status was evaluated by chromogenic EBV-encoded RNA in situ hybridization (Leica Biosystems), which was performed on unstained slides cut from paraffin-embedded tumor blocks. Strong signals in tumor cell nuclei with negative signals in surrounding lymphocytes and normal tissue were considered to be positive results.
RESULTS
The Clinicopathologic Characteristics of EBVaGC Patients
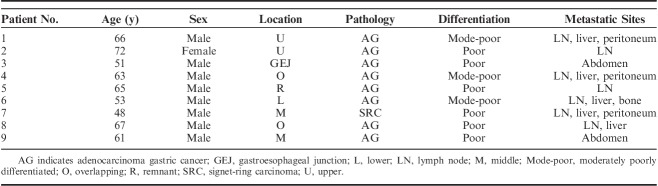
The clinicopathologic characteristics of EBVaGC patients are summarized in Table 2. Nine patients diagnosed with EBVaGC were included in the analysis. The median age was 62 years old. Only one of the patients was female, and the male to female ratio was 8:1. Six of the patients had a positive smoking history, and 4 of them had a positive drinking history. All of the patients were diagnosed with advanced or metastatic EBVaGC. Two of the patients showed overlapping lesions, and 1 lesion was detected in the remnant stomach. Most of the tumors (8/9, 88.9%) were classified as adenocarcinoma; partial signet-ring carcinoma was found in one of the patients. Overall, 67% of the tumors were poorly differentiated, and the rest of the tumors (3/9, 33.3%) were moderately poorly differentiated. More than half of the patients had at least 2 metastatic sites, with lymph node metastasis being the most common site (7/9, 77.8%). In addition, 2 patients (2/9, 22.2%) exhibited lymphovascular or perineural invasion after the inspection.
TABLE 2.
The Clinicopathologic Characteristics of Epstein-Barr Virus–associated Gastric Carcinoma Patients
Immunochemical and In Situ Hybridization Results of EBVaGC Patients
The results of the immunochemical staining and EBV status of the EBVaGC patients are summarized in Table 3. EBV-encoded RNA was detected in all of the patients. For patient no. 2 and no. 3 who underwent surgery, the microscopy inspection demonstrated massive lymphocytic infiltration (Fig. 1). None of them exhibited Her-2 overexpression. PD-L1 was detected in 7 of 9 patients. All of the patients were pMMR, and aside from the patients without available data, all of them were also MSS.
TABLE 3.
Immunochemical Staining and EBV Status of Epstein-Barr Virus–associated Gastric Carcinoma Patients
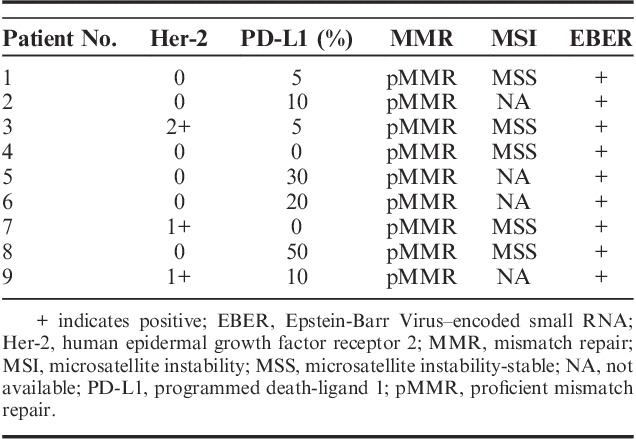
FIGURE 1.
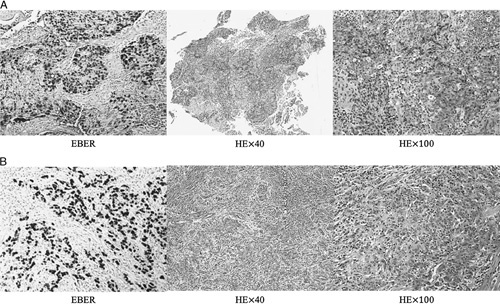
A, The EBER in situ hybridization and HE staining results of patient no. 2. B, The EBER in situ hybridization and HE staining results of patient no. 3. Both of them showed massive lymphocytic infiltration. EBER indicates Epstein-Barr Virus–encoded small RNA; HE, hematoxylin and eosin.
Therapeutic Information and Prognosis of EBVaGC Patients
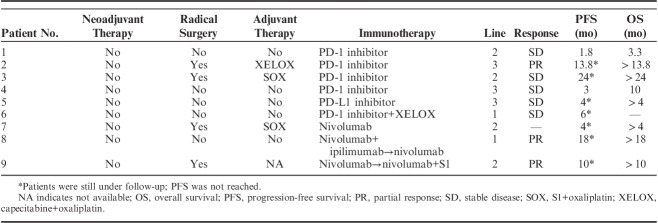
None of the patients underwent neoadjuvant therapy. Radical surgery was conducted in 4 patients (4/9, 44.4%). The adjuvant chemotherapy was chosen based on the National Comprehensive Cancer Network (NCCN) guidelines, which recommend fluoropyrimidine and platinum as the most commonly used approach. However, recurrence was observed within a year among all of these patients. Immunotherapy was used as the first-, second-, and third-line therapy in 2 (2/9, 22.2%), 4 (4/9, 44.4%), and 3 (3/9, 33.3%) of the patients, respectively (Table 4). Three patients (no. 6, no. 8, and no. 9) were administrated combined immunotherapy. One patient (no. 8) exhibited severe irAEs after the first cycle, and nivolumab alone was administrated subsequently.
TABLE 4.
Therapeutic and Survival Information of Epstein-Barr Virus–associated Gastric Carcinoma Patients
Three (3/9, 33.3%) and 5 (5/9, 55.6%) patients showed PR and stable disease after immunotherapy, respectively. It is interesting to note that, all of the patients who showed PR had a positive PD-L1 expression. One patient (no. 7) who was diagnosed with peritoneum metastasis and did not have measurable lesion showed decreasing ascites and tumor markers level after immunotherapy for 4 months. The waterfall plot representing the tumor reduction for each patient with the measurable lesion is shown in Figure 2. PFS and OS were reached in only 2 patients. However, 3 patients (no. 2, no. 8, and no. 9) had durations of responses of 13.8, 18, and 10 months at the time of the last follow-up.
FIGURE 2.
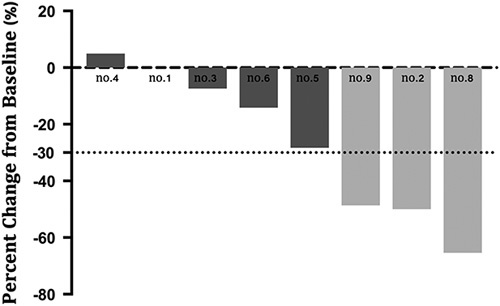
Waterfall plot of response to immunotherapy. Patient no. 7 was skipped because of lack of measurable lesion.
DISCUSSION
In this prospective observational study, we found that EBVaGC patients treated with immunotherapy showed favorable responses and that an EBV-positive status may represent a promising biomarker for gastric cancer immunotherapy. In addition, the included EBVaGC patients exhibited typical clinicopathologic characteristics regarding patient sex, tumor location, PD-L1 expression, MMR, MSI, and differentiation.
The favorable outcomes of EBVaGC patients in our study may be interpreted by several theories. First, immune evasion, which is an evident feature of EBVaGC, reveals the immune-active status of the tumor and may correlate with better responses to immunotherapy. T-cell infiltration is significant in EBVaGC, as demonstrated by the high proliferation and cytotoxicity of CD8+ T cells.14 In addition, CD4+ T lymphocytes, B cells, and natural killer cells also exist in the tumor microenvironment.15 It has been demonstrated by the Cancer Genome Atlas (TCGA) that immune cell signaling is activated in EBVaGC cells.12 Although infiltrating T lymphocytes play a bidirectional role in antitumor processes, we have proposed that the administration of PD-1/PD-L1 inhibitors breaks the microenvironment equilibrium and refuels antitumor activity.16 Immune evasion itself has been demonstrated to correlate with a better prognosis and less lymph node involvement in clinical studies.17,18 In addition, the pathogenesis underlying immune invasion has been thought to correlate with interleukin-1β and interferon-γ overexpression, LMP2A mutations, and EBNA1 repeats and polymorphisms.19–21 A second interpretation regarding the favorable outcomes of EBVaGC patients in our study involves PD-L1 overexpression in EBVaGC. The results from TCGA revealed a PD-L1 overexpression pattern in EBVaGC, which was confirmed by other clinical studies.12,22,23 Overexpression of PD-L1 is thought to be a strategy for tumor cells to elude the host immune environment. The conjunction of PD-1 and PD-L1 leads to the inhibition of T-cell proliferation and cytotoxicity. Hence, the blockade by PD-1/L1 axis inhibitors may boost local immunity and eradicate tumor cells. However, it remains uncertain as to whether PD-L1 is a biomarker of gastric cancer immunotherapy. The KEYNOTE-062 study revealed that in patients with Combined Positive Score ≥10, pembrolizumab yielded a superior OS over chemotherapy. In addition, despite the KEYNOTE-061 pembrolizumab failing to show superiority over paclitaxel, post hoc analysis revealed that OS was significantly improved in patients whose tumors overexpressed PD-L1 Combined Positive Score ≥10 (hazard ratio=0.64, 95% CI, 0.41–1.02).7 Hence, the cutoff value of PD-L1 plays an important role in predicting immunotherapy outcomes. As such, the threshold of PD-L1 should be confirmed afterward.
Several clinical trials have demonstrated the efficacy of immunotherapy in the management of gastric cancer in recent years. Nivolumab and pembrolizumab showed an ORR of 11.2% (95% CI, 7.7%–15.6%) and 15.5% (95% CI, 10.1%–22.4%), respectively.4,10 However, few clinical studies have focused on determining the effectiveness of immunotherapy in EBVaGC patients. Only Kim et al9 reported that all of their 6 recruited EBVaGC patients exhibited a PR after immunotherapy. In addition, Xu and colleagues reported a small sample size of immunotherapy in the management of EBVaGC, with an ORR of 25%. In the present study, we reported that 16.7% of patients showed a PR in monotherapy, 66.7% of patients showed a PR after combined immunotherapy, and 33.3% of patients overall showed a PR. On the basis of the data from the ATTRACTION-2 and KEYNOTE-059 studies, EBVaGC patients showed favorable responses to immunotherapy and EBV-positive status was suggested to be a potential biomarker for gastric cancer immunotherapy. In the present study, 3 of the patients who showed PR were PD-L1-positive, and their durations of responses were 13.8, 18, and 10 months at the time of the last follow-ups; this finding highlighted the long-lasting property of immunotherapy. We conclude that EBV combined with PD-L1 may be a more accurate biomarker combination for determining the efficacy of immunotherapy in gastric cancer.
It was reported that ∼10% of gastric cancer patients showed hyperprogressive disease (HPD) after immune-checkpoint inhibitor, exhibiting special tumor growth kinetics.24,25 It is interesting to note that, no patient showed PD or HPD in our study, which may due to the small sample size and the special tumor microenvironment of EBVaGC. According to recent studies, primary resistance to immune-checkpoint inhibitors may associated with intrinsic or extrinsic mechanisms, such as signaling pathways alternation, lack antigen mutation, alterations of antigen processing, T-cell phenotype changes or suppressive cell populations.26 And Kamada et al24 recently found that regulatory T cells (Tregs) plays an important role in the HPD mechanism. For EBVaGC, however, massive CD8+ T-cell infiltrate the tumor microenvironment, which means a relative lower proportion of Treg and other suppressive cells. The tumor-infiltration lymphocyte pattern and PD-L1 expression on Treg in EBVaGC should be investigated in the future.
Our present study revealed distinctive clinicopathologic characteristics of EBVaGC patients, such as male predominance and poor differentiation status. Similar observations have been documented in previous studies.18,27 Although van Beek et al18 reported that EBVaGC had a less common lymph node involvement with a significantly lower N stage compared with that of EBVnGC, our present study revealed that distant lymph node metastasis was still the most common site.
It has been suggested by Cristescu et al28 that EBV-positive status is associated with MSS and P53 positivity; similarly, we found that all of the patients were pMMR and MSS in our study. Her-2 is proposed to be a therapeutic target in 10%–20% of gastric cancer patients and in up to 30% of patients with gastroesophageal junction adenocarcinomas.29 In the present study, we found that none of the patients exhibited Her-2 overexpression. The PD-L1-positive rate in different studies shows great discrepancies, ranging from 14.3% to 69.4% according to a meta-analysis.30 The high variability of PD-L1 expression may due to the differences in cutoff values used in different studies. In the present study, 7 of 9 patients showed positive PD-L1 expression, which could be interpreted by the small sample size of our study.
A limitation of our study was its limited sample size. Although EBVaGC comprises ∼9% of gastric cancers, late-stage EBVaGC patients receiving treatments only accounts for 3% of gastric cancer cases. Thus, the efficacy of immunotherapy in EBVaGC patients may require further validation. In addition, these 9 patients were administrated with different immune-checkpoint inhibitors, and some of them were treated with combined chemotherapy. However, there is currently no evidence indicating a difference among different immune-checkpoint inhibitors. Only 2 patients reached a PFS and OS in our present study, which may be due to the long-lasting responses and stable statuses of these patients. In addition, the collection of survival information was still in progress at the end of this study. Another limitation of our study was that immunotherapy was administrated in different lines of palliative care for gastric cancer patients, which may affect responses to immunotherapy.
Our findings demonstrated favorable outcomes of immunotherapy in the management of EBVaGC and distinctive clinicopathologic characteristics of EBVaGC patients. An EBV-positive status may be a potential biomarker for immunotherapy in gastric cancer, which requires further large-scale clinical trials for validation. There are currently some ongoing clinical trials investigating the efficacy of immunotherapy in the palliative care for EBVaGC. Further investigations should elucidate whether immunotherapy can be used as a first-line treatment for EBVaGC and whether immunotherapy is best combined with chemotherapy or targeted therapy.
Acknowledgments
CONFLICTS OF INTEREST/FINANCIAL DISCLOSURES
Supported by the National Key Research and Development Program of China (No. 2017YFC1308900), National Natural Science Foundation of China (No. 81602057), Clinical Medicine Plus X—Young Scholars Project of Peking University and the Fundamental Research Funds for the Central Universities (No. 20171102).
All authors have declared that there are no financial conflicts of interest with regard to this work.
REFERENCES
- 1.Dank M, Zaluski J, Barone C, et al. Randomized phase III study comparing irinotecan combined with 5-fluorouracil and folinic acid to cisplatin combined with 5-fluorouracil in chemotherapy naive patients with advanced adenocarcinoma of the stomach or esophagogastric junction. Ann Oncol. 2008;19:1450–1457. [DOI] [PubMed] [Google Scholar]
- 2.Ajani JA, Rodriguez W, Bodoky G, et al. Multicenter phase III comparison of cisplatin/S-1 with cisplatin/infusional fluorouracil in advanced gastric or gastroesophageal adenocarcinoma study: the FLAGS trial. J Clin Oncol. 2010;28:1547–1553. [DOI] [PubMed] [Google Scholar]
- 3.Fuchs CS, Tomasek J, Yong CJ, et al. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet. 2014;383:31–39. [DOI] [PubMed] [Google Scholar]
- 4.Kang Y-K, Boku N, Satoh T, et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;390:2461–2471. [DOI] [PubMed] [Google Scholar]
- 5.Bang YJ, Kang YK, Catenacci DV, et al. Pembrolizumab alone or in combination with chemotherapy as first-line therapy for patients with advanced gastric or gastroesophageal junction adenocarcinoma: results from the phase II nonrandomized KEYNOTE-059 study. Gastric Cancer. 2019;22:828–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Josep Tabernero EVC, Bang Y-J, Fuchs CS, et al. Pembrolizumab with or without chemotherapy versus chemotherapy for advanced gastric or gastroesophageal junction (G/GEJ) adenocarcinoma: the phase III KEYNOTE-062 study. J Clin Oncol. 2019;37(suppl):LBA4007. [Google Scholar]
- 7.Shitara K, Ozguroglu M, Bang Y-J, et al. Pembrolizumab versus paclitaxel for previously treated, advanced gastric or gastro-oesophageal junction cancer (KEYNOTE-061): a randomised, open-label, controlled, phase 3 trial. Lancet. 2018;392:123–133. [DOI] [PubMed] [Google Scholar]
- 8.Bang YJ, Ruiz EY, Van Cutsem E, et al. Phase III, randomised trial of avelumab versus physician’s choice of chemotherapy as third-line treatment of patients with advanced gastric or gastro-oesophageal junction cancer: primary analysis of JAVELIN Gastric 300. Ann Oncol. 2018;29:2052–2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim ST, Cristescu R, Bass AJ, et al. Comprehensive molecular characterization of clinical responses to PD-1 inhibition in metastatic gastric cancer. Nat Med. 2018;24:1449–1458. [DOI] [PubMed] [Google Scholar]
- 10.Fuchs CS, Doi T, Jang RW, et al. Safety and efficacy of pembrolizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction cancer: phase 2 Clinical KEYNOTE-059 Trial. JAMA Oncol. 2018;4:e180013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang SC, Ng KF, Chen KH, et al. Prognostic factors in Epstein-Barr virus-associated stage I-III gastric carcinoma: implications for a unique type of carcinogenesis. Oncol Rep. 2014;32:530–538. [DOI] [PubMed] [Google Scholar]
- 12.Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513:202–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu RH, Fang W, Wei XL, et al. Tumor mutational burden identifies chemorefractory gastric cancer with overall survival advantage after receiving toripalimab, a PD-1 antibody. J Clin Oncol. 2019;15(suppl):4021. [Google Scholar]
- 14.Saiki Y, Ohtani H, Naito Y, et al. Immunophenotypic characterization of Epstein-Barr virus-associated gastric carcinoma: massive infiltration by proliferating CD8+ T-lymphocytes. Lab Invest. 1996;75:67–76. [PubMed] [Google Scholar]
- 15.Chen JN, He D, Tang F, et al. Epstein-Barr virus-associated gastric carcinoma: a newly defined entity. J Clin Gastroenterol. 2012;46:262–271. [DOI] [PubMed] [Google Scholar]
- 16.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science. 2011;331:1565–1570. [DOI] [PubMed] [Google Scholar]
- 17.Song HJ, Srivastava A, Lee J, et al. Host inflammatory response predicts survival of patients with Epstein-Barr virus-associated gastric carcinoma. Gastroenterology. 2010;139:84.e2–92.e2. [DOI] [PubMed] [Google Scholar]
- 18.van Beek J, zur Hausen A, Klein Kranenbarg E, et al. EBV-positive gastric adenocarcinomas: a distinct clinicopathologic entity with a low frequency of lymph node involvement. J Clin Oncol. 2004;22:664–670. [DOI] [PubMed] [Google Scholar]
- 19.Cho J, Kang MS, Kim KM. Epstein-Barr virus-associated gastric carcinoma and specific features of the accompanying immune response. J Gastric Cancer. 2016;16:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheng N, Hui DY, Liu Y, et al. Is gastric lymphoepithelioma-like carcinoma a special subtype of EBV-associated gastric carcinoma? New insight based on clinicopathological features and EBV genome polymorphisms. Gastric Cancer. 2015;18:246–255. [DOI] [PubMed] [Google Scholar]
- 21.Chen JN, Jiang Y, Li HG, et al. Epstein-Barr virus genome polymorphisms of Epstein-Barr virus-associated gastric carcinoma in gastric remnant carcinoma in Guangzhou, southern China, an endemic area of nasopharyngeal carcinoma. Virus Res. 2011;160:191–199. [DOI] [PubMed] [Google Scholar]
- 22.Saito R, Abe H, Kunita A, et al. Overexpression and gene amplification of PD-L1 in cancer cells and PD-L1(+) immune cells in Epstein-Barr virus-associated gastric cancer: the prognostic implications. Mod Pathol. 2017;30:427–439. [DOI] [PubMed] [Google Scholar]
- 23.Li Z, Lai Y, Sun L, et al. PD-L1 expression is associated with massive lymphocyte infiltration and histology in gastric cancer. Hum Pathol. 2016;55:182–189. [DOI] [PubMed] [Google Scholar]
- 24.Kamada T, Togashi Y, Tay C, et al. PD-1(+) regulatory T cells amplified by PD-1 blockade promote hyperprogression of cancer. Proc Natl Acad Sci USA. 2019;116:9999–10008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ji Z, Peng Z, Gong J, et al. Hyperprogression after immunotherapy in patients with malignant tumors of digestive system. BMC Cancer. 2019;19:705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sharma P, Hu-Lieskovan S, Wargo JA, et al. Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell. 2017;168:707–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ryan JL, Morgan DR, Dominguez RL, et al. High levels of Epstein-Barr virus DNA in latently infected gastric adenocarcinoma. Lab Invest. 2009;89:80–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cristescu R, Lee J, Nebozhyn M, et al. Molecular analysis of gastric cancer identifies subtypes associated with distinct clinical outcomes. Nat Med. 2015;21:449–456. [DOI] [PubMed] [Google Scholar]
- 29.Ishii T, Kawazoe A, Shitara K. Dawn of precision medicine on gastric cancer. Int J Clin Oncol. 2019;24:779–788. [DOI] [PubMed] [Google Scholar]
- 30.Gu L, Chen M, Guo D, et al. PD-L1 and gastric cancer prognosis: a systematic review and meta-analysis. PLoS One. 2017;12:e0182692. [DOI] [PMC free article] [PubMed] [Google Scholar]