Supplemental Digital Content is available in the text.
Keywords: Blood pressure, Cardiometabolic diseases, Indonesia, Life expectancy, Mortality, Urban health
Abstract
Background:
Evidence on rural–urban differences in adult mortality in low- and middle-income countries (LMICs) is limited and mixed. We examined the size of and factors contributing to rural–urban life expectancy differences among adults in Indonesia, the third most populous LMIC.
Methods:
Data come from the 2000, 2007, and 2014/2015 waves of the Indonesian Family Life Survey, a population-representative longitudinal study with mortality follow-up. We used Poisson regression and life tables to estimate rural–urban differences in life expectancy among 18,867 adult respondents ≥30 years. We then used a novel g-formula–based decomposition to quantify the contribution of rural–urban differences in blood pressure (BP), body mass index (BMI), and smoking to life expectancy differences.
Results:
Compared with urban adults, life expectancy at age 30 was 2.2 (95% confidence interval [CI] = 0.4, 3.9) years higher for rural men and 1.2 (95% CI = −0.4, 2.7) years higher for rural women. Setting the BMI and systolic BP distribution equal in urban and rural adults reduced the urban mortality penalty by 22% for men and 78% for women, with the majority of this reduction coming from the contribution of rural–urban differences in BMI. Smoking did not contribute to the urban mortality penalty for either men or women.
Conclusions:
Adult life expectancy is lower in urban than in rural areas in Indonesia and we estimate that this difference is partly related to differences in BMI and systolic BP.
Urban areas are growing at unprecedented rates in low- and middle-income countries (LMICs).1 For some aspects of health and well-being, urban areas provide substantial improvements over rural areas. Individuals in urban areas tend to have better economic opportunities, availability and access to health care, and standards of living.2–6 However, urban environments also confer greater health risks including lower levels of physical activity, greater access to unhealthy calorie-dense foods, and increased exposure to pollution, road traffic accidents, and population density-driven infectious diseases.7–15 Given that cities are nexuses of both greater opportunity and heightened vulnerability, it remains unclear whether urban residents in LMICs experience higher or lower mortality than their rural counterparts. Although studies find a consistent infant and child mortality advantage in urban compared with rural areas,16–19 the evidence on adult mortality remains mixed20–27 and is limited due to the scarcity of reliable mortality data in many LMICs.
Indonesia is the third most populous LMIC and currently undergoing rapid structural transformation from a predominantly rural, agricultural economy to an urban, services-based economy. Indonesia has been urbanizing more quickly than most other Asian countries, and by 2025, approximately 68% of Indonesia’s population is expected to live in urban areas.28 Rural-to-urban migration is responsible for approximately one-third of the rising share of urban residents in Indonesia, with the remainder coming from natural population growth and the reclassification of rural into urban areas.29 Indonesia is also aging substantially and is now at an advanced stage of the epidemiologic transition: the share of the population ages 50 and older is expected to reach nearly 25% in 2050, and cardiovascular diseases (CVDs) are now estimated to be the leading causes of death.30,31 The importance of CVDs in Indonesia is reflected in its risk factor profile. Indonesia is estimated to have the highest male smoking prevalence in the world (76.2% among men age 15+)32; nearly half of adults over the age of 40 are hypertensive33; and levels of unhealthy weight have rapidly increased to the point where a quarter of adults are now overweight.34
Given rapid urbanization, population aging, and a rising burden of CVD mortality, understanding whether urban Indonesian adults have higher or lower mortality compared with their rural counterparts is an important question. Unfortunately, little is known about the size of and contributors to rural–urban mortality differentials like in many LMICs, the vital statistics system in Indonesia is highly limited in terms of both coverage and quality.35,36 Adults living in urban Indonesia may experience a mortality advantage because urban areas in Indonesia have lower rates of poverty, better access to health care services, and lower rates of child mortality compared with rural areas.37–39 However, there are also reasons to believe an urban mortality penalty may exist. Urbanization has outpaced infrastructure investment in urban Indonesia, resulting in poor access to basic services. In 2009, only 50% of the urban population had access to safe water, sewerage systems existed in only 11 of 98 cities, and only 2% of city residents had access to centralized sanitation systems.40 Lifestyles in urban areas in Indonesia have also changed dramatically, and urban areas are at a more advanced stage of the nutrition transition. Individuals in urban areas consume more processed foods, are less physically active, and are more obese than individuals living in rural areas, suggesting that they may also be at risk of experiencing a greater burden of chronic diseases.41,42
In this study, we investigate whether adult life expectancy is higher or lower in urban, compared with rural, parts of Indonesia. We estimate life expectancy differentials in the absence of vital registration information using data from the Indonesian Family Life Survey (IFLS), a unique data source with both reliable mortality follow-up and measured cardiometabolic risk factor information. We also apply a novel g-formula–based decomposition method to the IFLS data to estimate the contribution of three key cardiometabolic risk factors to rural–urban life expectancy differences.
METHODS
Data
We used data from the 2000, 2007, and 2014/2015 waves of the Indonesian Family Life Survey (IFLS), a multistage cluster stratified panel survey of individuals and households representative of approximately 83% of the Indonesian population. Our main sample consisted of adults ages 30+ interviewed in the 2007 wave with the 2014/2015 wave used to determine mortality follow-up. The 2000 wave was used to measure a single covariate (BMI), which is discussed in the following section. The IFLS is exceptionally well suited to investigating rural–urban mortality differences because it is the only population-representative survey in Indonesia with multiple waves of data, detailed mortality follow-up, and very low levels of attrition (achieving 92% recontact rates by the 2014/2015 wave) due to intensive respondent tracking efforts.43 The IFLS collects measured biomarker and anthropometric data, allowing us to assess the contribution of objectively measured risk factors to rural–urban mortality differentials. We provide detailed information on the IFLS sampling procedures in eAppendix 1 (***). This study was exempt from institutional review board approval because the data are publicly available and deidentified.
Vital Status
We used the 2014/2015 wave to determine the vital status of all individuals surveyed in the 2007 wave. If an individual died between the 2007 and 2014/2015 waves, a member of their family was asked to report their date of death. For individuals who did not die between waves, we used the date of interview in 2014/2015 as the date of right censoring.
Urban/Rural Classification
We designated individuals as living in urban or rural areas in the baseline 2007 wave using the classification assigned by Badan Pusat Statistik (BPS), the Indonesian Statistics Office. This classification is recorded in the IFLS and is based on population density, the share of households engaged in agricultural labor, and access to facilities such as schools and hospitals.
Mediators
We assessed the contribution of three important cardiometabolic risk factors to rural–urban differences in mortality: smoking, blood pressure, and body mass index (BMI). We chose these three mediators because they were reliably measured in the IFLS and are related to chronic disease development and mortality. Full details on the measurement procedures for these risk factors is provided in eAppendix 1 (***). For smoking, we employed a dichotomous measure of whether an individual reported ever regularly smoking tobacco.
We used direct systolic blood pressure measurements taken by a trained assessor using an Omron HEM-7203 meter.43,44 We averaged the second and third of three total measurements, excluding the first measurement to reduce measurement error from so-called whitecoat effects where the initial blood pressure measurement tends to be artificially high. We focus on systolic blood pressure because diastolic blood pressure tends to level off in midlife, and systolic hypertension is the larger source of blood pressure-related mortality among older adults.45,46
Finally, we considered the role of unhealthy weight using BMI, based on assessor-measured height and weight. Estimating the relationship between BMI and mortality presents a distinct methodologic challenge because individuals tend to lose weight when sick and especially before death. This introduces a form of unobserved confounding that often results in empirical estimates implying that higher BMI is actually protective of mortality.47–49 One way to address this source of confounding is to use BMI measurements taken earlier in an individual’s life. We took advantage of the long-running panel and used this approach, using an individual’s measured BMI from the 2000 wave as the primary measure of weight.
Confounders
Our analyses adjusted for a number of important socioeconomic characteristics (all measured in 2007) including province of residence (entered as province fixed effects), schooling (no schooling, primary schooling, more than primary schooling), household asset–based wealth quintiles, primary occupation (agriculture, manufacturing, retail, service, housework only, retired, unemployed, other), and marital status (never, currently, formerly married). These variables collectively represent a set of social, economic, and environmental characteristics that ultimately influence both the main mediators (smoking, weight, and systolic blood pressure) as well as mortality through other pathways (e.g., health knowledge, healthcare access, and the built environment) and thus capture several sources of confounding that may potentially bias our contribution estimates. We provide more detailed information on all the variables used in our analyses in eAppendix 1 (***).
Missingness and Imputation
eFigure 1 (***) provides detailed information on missingness for our study. Of the 19,243 respondents ages 30+ present in the baseline 2007 wave, none were missing vital status information in 2014/2015. Although confirmed to be alive, 1,107 individuals were not interviewed in 2014/2015, so they were assigned the median date of interview in 2014/2015 as their date of right censoring. Next, among the 19,243 individuals, 376 were missing baseline information on the confounders, an additional 1,847 individuals were missing information on baseline systolic BP and smoking in 2007, and an additional 2,841 were missing information on prior BMI from the 2000 wave (total nonmissing was 14,179/19,243 or 74% of the eligible sample). We used multiple imputation with chained equations to impute the missing values for the three main mediators using all the variables described above in the imputation models for a final imputed sample of 18,867 (we did not impute values for the 376 individuals with missing confounder information).
Methods
We began by converting the data to a person-age format, where we created observations for each age lived between 2007 and either the date of death or date of right censoring in 2014/2015 for each individual (eAppendix 1, ***).
To quantify the magnitude of rural–urban differences in adult mortality, we estimated life expectancy at age 30 (e30) separately by sex and rural/urban residence. To construct e30, we first estimated age- and sex-specific death rates in urban and rural areas by dividing the number of deaths that occurred in each 5-year age group between 30 and 80 between 2007 and 2014/2015 by the total number of person-years lived in that age group. We then used standard period life table techniques to convert these death rates into e3050; this measure is interpreted as the average additional number of years that an individual who survives to age 30 will live if they experience the period mortality rates for the remainder of their lifetime.
Next, we assessed the contribution of the three mediators (cardiometabolic risk factors) to rural–urban differences in e30 using Sudharsanan and Bijlsma’s51 parametric g-formula–based implementation of Jackson and VanderWeele’s52 causal decomposition.51,52 The causal decomposition is a method for evaluating how much of the observed difference in an aggregate outcome between two groups of individuals is due to differences in the distributions of specific causes of that outcome between the two groups. We provide full details on the decomposition in eAppendix 3 (***) and briefly describe the procedure here.
Our specific decomposition question is: “How much of the observed difference in e30 between urban and rural areas is due to differences in the distribution of BMI, smoking, and systolic BP between the two areas?” To answer this question, we began by estimating a parametric model (a Poisson rate regression) for the relationship between all-cause mortality, the main mediators, and the identified confounders. We then applied this model to the observed data to estimate age-specific mortality rates, e30 for both rural and urban areas, and the rural–urban e30 difference. Model-estimated rates using the observed data are often referred to as “natural-course” estimates to distinguish them from non-model-based direct estimates such as those we used to quantify urban–rural differences in e30 described above. Although we do not need to use a parametric model to estimate the observed rural–urban difference, our counterfactual estimates rely on models and therefore a comparison of model-based counterfactual estimates to non-model-based observed estimates of e30 would conflate the contribution of the counterfactual scenario and the influence of the modeling procedures. By using the same parametric model for both the observed and counterfactual scenarios, we can net out the influence of the modeling procedures.
Next, we estimated what e30 for the urban population would be if they had the same distribution of the mediators as the rural population. To do this, we first fit a set of models for each of the three mediators as a function of the same covariates used to estimate the mortality outcomes. We then used these regressions to estimate the distribution of the three mediators within each of the covariate strata. We then set the urban population to have the same distribution of the mediators as the rural population by drawing new values of the mediators for each individual in the urban population from the estimated distribution of the mediators of rural individuals in the same covariate strata. Next, we used the original mortality model coefficients with the updated mediator data, leaving the urban population unchanged on all the confounders, to predict a set of counterfactual age-specific mortality rates for the urban population. We then used these counterfactual mortality rates to form counterfactual estimates of e30 in urban areas (because the mediators are unchanged in rural areas, we do not need to form a new counterfactual e30). Finally, we compared the natural-course rural–urban difference in e30 to the counterfactual difference. Because we equalized the distributions of the three mediators between urban and rural areas, any change in the rural–urban e30 difference between the counterfactual and natural-course scenarios reflects the contribution of differences in the distribution of the mediators to the observed e30 difference.
We used a bootstrap procedure with 500 replications to account for the sampling variability in the life expectancy and decomposition estimates. We specified 95% confidence intervals based on the 2.5th and 97.5th percentiles of the 500 bootstrap estimates.
RESULTS
Descriptive Characteristics of the Sample
For both men and women (men: N = 9,211, 49% of the total sample; women: N = 9,656, 51% of the total sample), the sample is around 47 years old on average and split evenly between urban and rural areas, with the majority of individuals residing in Java (Table). Most individuals are currently married, although the share of formerly married women is higher than among men (23% compared with 5.4%). A lack of formal education is also more common among women than men (18% compared with 6.9%). Men and women have similar mean levels of systolic blood pressure (132.4 for men and 133.6 for women) and BMI (21.4 for men and 22.6 for women) but having ever smoked is far higher for men (75% compared with 5.6%).
TABLE.
Descriptive Characteristics of the Sample in the Baseline Wave
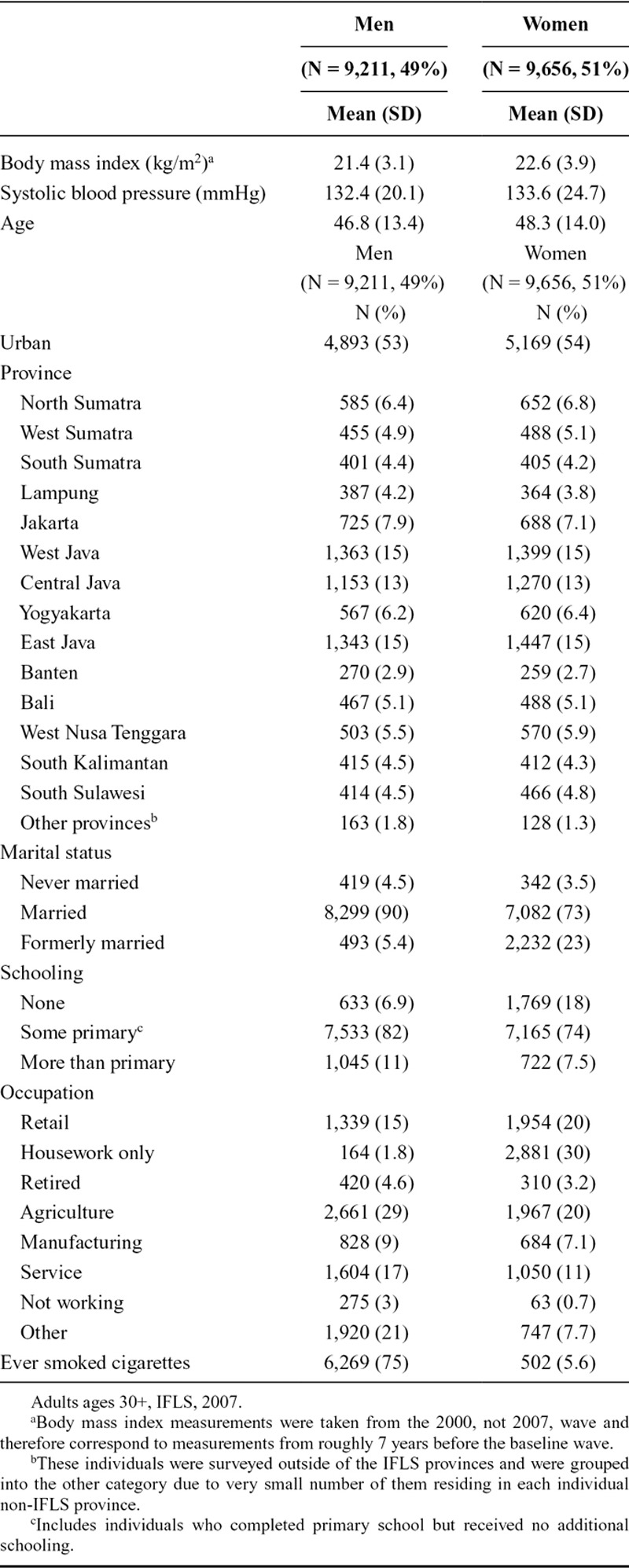
Rural–Urban Differences in Life Expectancy at Age 30
Life expectancy at age 30 (e30) is higher in rural, compared with urban, areas for both men and women (Figure 1). For men, e30 is 41.5 years (95% CI = 40.4, 42.6) in urban areas compared with 43.7 years (95% CI = 42.4, 44.9) in rural areas (difference: 2.2, 95% CI = 0.4, 3.9). The magnitude of this difference is smaller for women but still favors rural areas. Life expectancy at age 30 for women is 44.2 years (95% CI = 43.2, 45.3) in urban areas compared with 45.4 years (95% CI = 44.2, 46.5) in rural areas (difference: 1.2, 95% CI = −0.4, 2.7).
FIGURE 1.
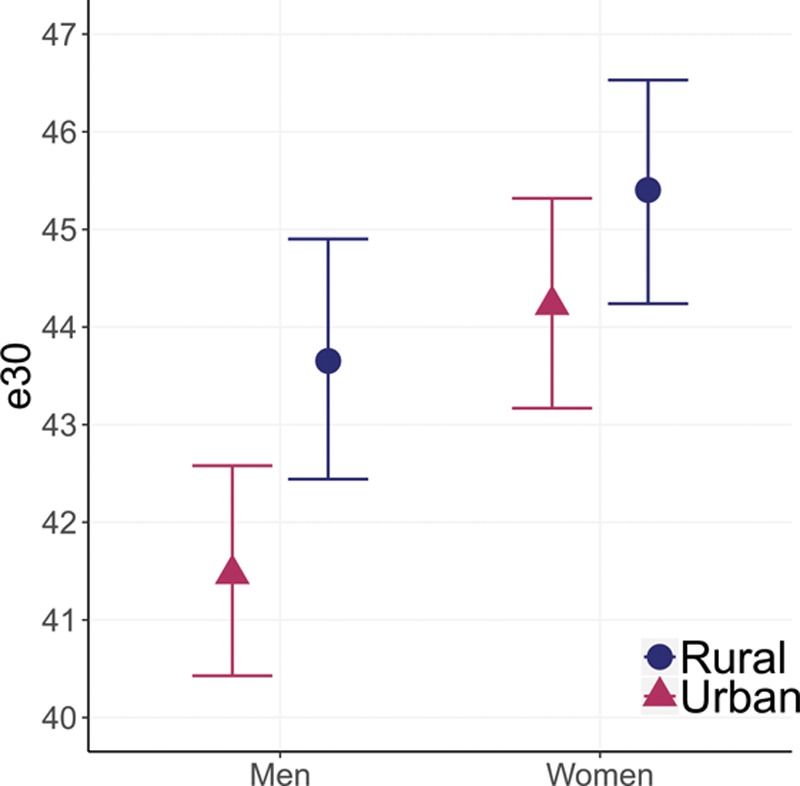
Rural–urban differences in life expectancy at age 30 (e30), adults ages 30+, Indonesian Family Life Survey, 2007–2014/2015. Error bars represent 95% CIs. Male rural–urban difference (95% CI): 2.2 (0.4, 3.9); female rural–urban difference (95% CI): 1.2 (−0.4, 2.7).
Rural–Urban Differences in Smoking, BMI, and Systolic BP
Rural–urban differences in smoking, BMI, and systolic BP are mixed. Men and women living in urban areas have a slightly higher blood pressure at most ages compared with their rural counterparts, although there is substantial overlap in the confidence intervals at most ages. For BMI, however, we observe a clear urban disadvantage. Urban dwellers have substantially higher levels of BMI at nearly every age for both men and women. The relation between ever smoking and urban residence differs by sex and, among women, by age. For men, the prevalence of smoking is approximately 10 percentage points higher in rural areas at nearly every age. For women, rates of smoking are nearly 0 for both urban and rural population between ages 30 and 55. Beyond age 55, there are substantially higher levels of smoking among rural women (Figure 2).
FIGURE 2.
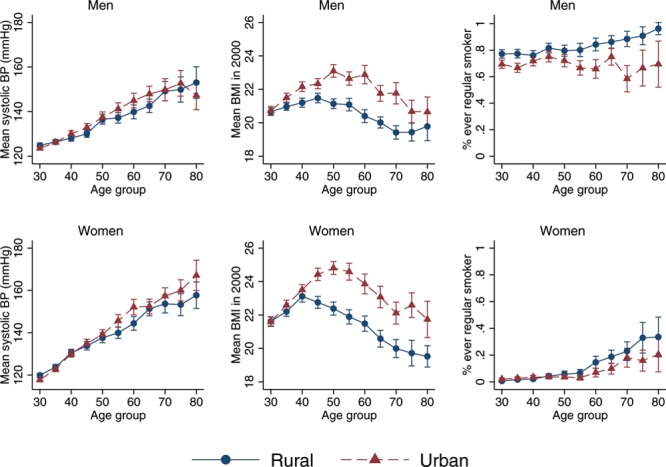
Rural–urban differences in ever smoking, systolic blood pressure, and BMI by age group, adults ages 30+, Indonesian Family Life Survey, 2007–2014/2015. Error bars represent 95% confidence intervals.
Decomposition of Rural–Urban Differences in Life Expectancy at Age 30
Figure 3 presents five estimates of rural–urban life expectancy differences: the natural-course unadjusted difference in life expectancy at age 30 between rural and urban areas, followed by four counterfactual estimates of the difference where the distribution of the three mediators in urban areas is set to the corresponding distribution in rural areas individually and when systolic BP and BMI are set to the rural distribution jointly. We find moderate contributions of two of the three risk factors to the overall rural–urban difference in adult life expectancy for men. BMI is the largest contributor to the rural–urban mortality difference: after equalizing the distribution of BMI between urban and rural areas, the life expectancy difference reduces by 17%, from 2.3 years (95% CI = 0.5, 3.9) to 1.9 years (95% CI = 0.1, 3.5). Systolic BP difference makes a smaller contribution (9%), reducing the difference to 2.1 years (95% CI = 0.4, 3.8). When considered jointly, systolic BP and BMI account for 22% of the urban adult mortality penalty, reducing the difference down to 1.8 years (95% CI = 0.0, 3.4). Due to the higher prevalence of smoking in rural areas, setting the urban smoking distribution to the rural smoking distribution has the opposite effect and widens the rural–urban difference to 2.6 years (95% CI = 0.7, 4.2).
FIGURE 3.
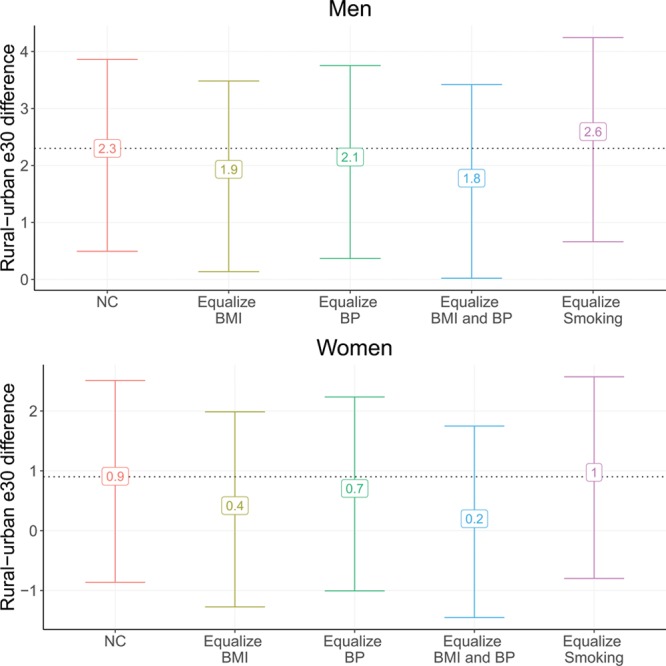
Decomposition analysis of rural–urban difference in life expectancy at age 30 (e30) by ever smoking, systolic BP, and BMI, adults ages 30+, Indonesian Family Life Survey, 2007–2014/2015. Error bars represent 95% confidence intervals. NC indicates natural course estimates.
Rural–urban differences in the three mediators make larger contributions for women, although the observed rural–urban difference is smaller for women relative to men and the size of these differences is small relative to the estimated confidence intervals. Among women, BMI differences also have the largest impact on the rural–urban mortality difference, followed by systolic BP differences. After matching the BMI distributions, the rural–urban life expectancy difference reduces from 0.9 years (95% CI = −0.8, 2.5) to 0.4 years (95% CI = −1.3, 2.0), corresponding to a 56% contribution. When considered jointly, BMI and systolic BP have a 78% contribution to the rural-difference in life expectancy, reducing the difference down to just 0.2 years (95% CI = −1.5, 1.8). In contrast to men, smoking differences between urban and rural areas among women do not contribute to the life expectancy difference.
DISCUSSION
Our study estimates the size of and contributors to rural–urban differences in adult life expectancy in Indonesia, the third most populous LMIC and the fourth most populous country overall. We demonstrate that adult life expectancy is actually lower in urban than in rural areas. This is surprising in the context of two observations. First, infant mortality is lower and child health indicators tend to be much better in urban than rural Indonesia.19,38 Second, rates of poverty are lower and access to health services is better in urban areas.37,39 It is unclear, however, whether this surprising urban adult life expectancy penalty is unique to Indonesia or common across many LMICs. Studies of adult mortality differences in LMICs are mixed and tend to be based on selected causes of death. For example, life expectancy at age 30 (e30) is higher in urban areas for both men and women in every Indian state20; however, in Bangladesh, there is no rural–urban difference in e30 for men, and for women, e30 is higher in rural areas.22 Studies in China have also been mixed and focused on specific, nonrepresentative regions and select causes of death.21,23,25,53 Stroke mortality, for example, was initially higher in some urban parts of China but then reversed over time and became higher in rural areas.25 Similarly, other studies find higher cardiovascular disease and injury mortality in rural China.23,24 Evidence from other countries also shows a mixed picture, with higher overall adult mortality in urban Costa Rica,26 higher stroke mortality in urban Tanzania,27 and higher motor vehicle mortality in rural South Africa.54
Based on a novel causal decomposition method,51,52 we find that differences in the distributions of BMI and systolic BP explain moderate proportions of the urban adult life expectancy penalty. Differences in BMI and systolic BP jointly account for nearly one-fourth of the rural–urban difference in e30 for men, and over three-fourths of the difference for women. These results raise the question of whether the urban mortality penalty is the result of a direct causal effect of urban environments on adult health. One way to evaluate this is to compare rural residents with individuals who moved into urban environments from rural areas. Based on a review of such studies, urban migrants have higher levels of body fat, blood pressure, and blood cholesterol compared with rural nonmigrants across several LMICs.55 However, these differences may be due to systematic differences in the background characteristics between individuals who do and do not migrate. Studies that attempt to adjust for this bias by comparing rural nonmigrant and urban-migrant sibling pairs still find higher rates of obesity, hypertension, and diabetes among the urban migrant-siblings. Importantly, this difference becomes even more pronounced with longer durations in urban areas.9,56 These findings suggest that part of the urban mortality penalty in Indonesia may be driven by a direct effect of urban environments on cardiovascular disease risk factors such as BMI.
Although the presence of an urban mortality penalty in Indonesia is consistent with the historical experience of high-income countries, there are important differences in the underlying causes of higher urban mortality. Historical urban mortality penalties were driven predominantly by higher infectious disease mortality among infants and children due to poor conditions in cities, such as overcrowding, inadequate water and sewage systems, and contamination of food and water.57,58 In contrast, our results suggest that the urban mortality penalty in contemporary Indonesia is concentrated at the adult ages and may be related to a faster progression of the nutrition transition and changing lifestyles in urban than rural areas. These problems are compounded by a weak health care system and lack of effective management of cardiometabolic risk factors.34,39,59–63
Our study has several important strengths. First, we were able to use high-quality longitudinal data to estimate information on mortality in Indonesia in the absence of a comprehensive vital registration system. Second, the IFLS also contains measured biomarker information, which allowed us to estimate the contribution of objectively measured CVD risk factors to mortality differences. Despite these strengths, there are still a number of important limitations. While the initial IFLS survey was representative of 83% of the population, our data come from the 2007 wave and thus may no longer be representative due to between-wave attrition. Therefore, our estimates may be biased if individuals surveyed in 2007 are systematically different from the overall population. We also have to rely on next-of-kin reports of date and cause of death due to the lack of a death registration system. Our decomposition estimates rely on adjusting for all sources of confounding of the mediator-mortality relation and may be biased by unobserved confounders. However, we adjust for a fairly extensive set of confounders using detailed socioeconomic data available in the IFLS.
Our results may be biased by rural-to-urban migration or urban reclassification between 2007 and 2014/2015 since we classify individuals based on their rural–urban status in 2007 and assign all deaths that occurred over the intersurvey period to that classification. Based on the prior 2000 and 2007 waves of the IFLS, 11% of rural individuals either migrated or had their areas reclassified as urban by 2007. If this trend continued between 2007 and 2014/2015, some of the deaths and person-years we attributed to the rural population actually occurred among individuals who subsequently moved to urban areas. Because we do not have information on the timing of between-wave migration for individuals who died, we were not able to correct for this bias. However, this bias would lead to artificially high rural—and consequently artificially low—urban death rates, making our main conclusions regarding the urban mortality penalty conservative.
A related issue is whether our “interventions” to equalize levels of blood pressure, BMI, and smoking between rural and urban areas satisfy the consistency assumption and thus can be justified as causal effects. First, there are multiple ways to reduce blood pressure and BMI, each with potentially different impacts on mortality. Our estimate, however, does not correspond to any one specific route and therefore may not represent the true effect of lowering blood pressure and BMI using specific interventions. Second, the levels of the risk factors we observe are the result of life-course exposures that likely also vary between urban and rural areas. By equalizing the levels of the three risk factors at a single point in time using data that are the result of a longitudinal process, our estimates may not correspond to an intervention to equalize the life course histories of the risk factors nor an intervention to equalize the risk factors at a single point in time, ignoring past histories—both of which would likely have different mortality implications. For these two reasons, our decomposition estimates are better seen as a thought experiment: what would happen if urban populations never reached the levels of each risk factor they currently have but rather attained the lower levels of blood pressure and BMI and the higher levels of smoking found in rural areas?
Our findings point to a higher burden of cardiometabolic disease in urban compared with rural Indonesia. As Indonesia continues to age, urbanize, and undergo the nutrition transition, this burden is likely to increase substantially.64 Urban populations are considered to be at the forefront of the epidemiologic and nutrition transitions in Indonesia and in most LMICs. The importance of cardiometabolic risk factors indicates current priorities but also acts as a signal of future health needs in the wider population. Access to and the quality of health care in Indonesia are fairly low39,61 and the system is not well equipped to address the management of cardiometabolic diseases.60
Supplementary Material
Footnotes
Supported by the National Institutes of Health, Eunice Kennedy Shriver National Institute of Child Health and Human Development, Grant number R00HD083519.
The authors report no conflicts of interest.
Supplemental digital content is available through direct URL citations in the HTML and PDF versions of this article (www.epidem.com).
Data are free and publicly available at https://www.rand.org/labor/FLS/IFLS.html. Source code is available on the corresponding author’s website (https://sites.google.com/view/nikkilsud/code?authuser=0).
REFERENCES
- 1.United Nations Department of Economic and Social Affairs, Population Division. World Urbanization Prospects: The 2018 Revision. 2018.
- 2.Young A. Inequality, the urban-rural gap, and migration. Q J Econ. 2013;128:1727–1785. [Google Scholar]
- 3.Rama M, Béteille T, Li Y, Mitra PK, Newman JL. Addressing Inequality in South Asia. 2014The World Bank. [Google Scholar]
- 4.Organization WH, others. Meeting the MDG drinking water and sanitation target: the urban and rural challenge of the decade. 2006.
- 5.World Health Organization. Tracking Universal Health Coverage: First Global Monitoring Report. 2015World Health Organization. [Google Scholar]
- 6.Leslie HH, Spiegelman D, Zhou X, Kruk ME. Service readiness of health facilities in Bangladesh, Haiti, Kenya, Malawi, Namibia, Nepal, Rwanda, Senegal, Uganda and the United Republic of Tanzania. Bull World Health Organ. 2017;95:738–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kinra S. Commentary: beyond urban-rural comparisons: towards a life course approach to understanding health effects of urbanization. Int J Epidemiol. 2004;33:777–778. [DOI] [PubMed] [Google Scholar]
- 8.Yadav K, Krishnan A. Changing patterns of diet, physical activity and obesity among urban, rural and slum populations in north India. Obes Rev. 2008;9:400–408. [DOI] [PubMed] [Google Scholar]
- 9.Kinra S, Andersen E, Ben-Shlomo Y, et al. ; Indian Migration Study Group. Association between urban life-years and cardiometabolic risk: the Indian migration study. Am J Epidemiol. 2011;174:154–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sobngwi E, Mbanya JC, Unwin NC, et al. Exposure over the life course to an urban environment and its relation with obesity, diabetes, and hypertension in rural and urban Cameroon. Int J Epidemiol. 2004;33:769–776. [DOI] [PubMed] [Google Scholar]
- 11.Samuel P, Antonisamy B, Raghupathy P, Richard J, Fall CH. Socio-economic status and cardiovascular risk factors in rural and urban areas of Vellore, Tamilnadu, South India. Int J Epidemiol. 2012;41:1315–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao J, Tu EJ, McMurray C, Sleigh A. Rising mortality from injury in urban China: demographic burden, underlying causes and policy implications. Bull World Health Organ. 2012;90:461–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McGranahan G. Air Pollution and Health in Rapidly Developing Countries. 2012Earthscan. [Google Scholar]
- 14.Nantulya VM, Reich MR. The neglected epidemic: road traffic injuries in developing countries. BMJ. 2002;324:1139–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dye C, Williams BG. The population dynamics and control of tuberculosis. Science. 2010;328:856–861. [DOI] [PubMed] [Google Scholar]
- 16.Van de Poel E, O’Donnell O, Van Doorslaer E. Are urban children really healthier? Evidence from 47 developing countries. Soc Sci Med. 2007;65:1986–2003. [DOI] [PubMed] [Google Scholar]
- 17.Van de Poel E, O’donnell O, Van Doorslaer E. What explains the rural–urban gap in infant mortality: household or community characteristics? Demography. 2009;46:827–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sastry N. What explains rural-urban differentials in child mortality in Brazil? Soc Sci Med. 1997;44:989–1002. [DOI] [PubMed] [Google Scholar]
- 19.The DHS Program. STATCompiler [Internet]. [cited 2019 Jul 8]. Available at: https://www.statcompiler.com/en/.
- 20.Office of the Registrar General and Census Comissioner. SRS Based Life Table 2012-16 [Internet]. Ministry of Home Affairs, Government of India; Available at: http://www.censusindia.gov.in/Vital_Statistics/SRS_Life_Table/Srs_life_Table_2012-16.html.
- 21.Zimmer Z, Kaneda T, Spess L. An examination of urban versus rural mortality in China using community and individual data. J Gerontol B Psychol Sci Soc Sci. 2007;62:S349–S357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Islam MS, Tareque MI, Mondal MNI, Fazle Rabbi AM, Khan HTA, Begum S. Urban-rural differences in disability-free life expectancy in Bangladesh using the 2010 HIES data. PLoS One. 2017;12:e0179987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu Q, Zhang L, Li J, et al. The gap in injury mortality rates between urban and rural residents of Hubei Province, China. BMC Public Health. 2012;12:180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheng L, Tan L, Zhang L, et al. Chronic disease mortality in rural and urban residents in Hubei Province, China, 2008-2010. BMC Public Health. 2013;13:713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang XH, Guan T, Mao J, Liu L. Disparity and its time trends in stroke mortality between urban and rural populations in China 1987 to 2001: changing patterns and their implications for public health policy. Stroke. 2007;38:3139–3144. [DOI] [PubMed] [Google Scholar]
- 26.Rosero-Bixby L, Dow WH. Surprising SES Gradients in mortality, health, and biomarkers in a Latin American population of adults. J Gerontol B Psychol Sci Soc Sci. 2009;64:105–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Walker RW, McLarty DG, Kitange HM, et al. Stroke mortality in urban and rural Tanzania. Adult morbidity and mortality project. Lancet. 2000;355:1684–1687. [DOI] [PubMed] [Google Scholar]
- 28.The World Bank. Indonesia’s Urban Story. 2016.
- 29.Wahyu M. Rural–Urban Linkages: Indonesia Case Study. 2014(Santiago, Chile: RIMISP; Working Paper Series). [Google Scholar]
- 30.United Nations Department of Economic and Social Affairs. 2017World Population Prospects: The 2017 Revision. [Google Scholar]
- 31.Roth GA, Abate D, Abate KH, Abay SM, Abbafati C, Abbasi N, et al. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the Global Burden of Disease Study 2017. The Lancet. 2018;392:1736–1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Organization WH, others. WHO Global Report on Trends in Prevalence of Tobacco Smoking 2015. 2015World Health Organization. [Google Scholar]
- 33.Sudharsanan N. Population-level mortality benefits of improved blood pressure control in Indonesia: a modelling study. Int J Epidemiol. 2019;48:954–965. [DOI] [PubMed] [Google Scholar]
- 34.Witoelar F, Strauss J, Sikoki B. Socioeconomic success and health in later life: evidence from the Indonesia family life survey. In: Aging in Asia: Findings From New and Emerging Data Initiatives. 2012National Academies Press (US). [PubMed] [Google Scholar]
- 35.Mahapatra P, Shibuya K, Lopez AD, et al. ; Monitoring Vital Events. Civil registration systems and vital statistics: successes and missed opportunities. Lancet. 2007;370:1653–1663. [DOI] [PubMed] [Google Scholar]
- 36.Mikkelsen L, Phillips DE, AbouZahr C, et al. A global assessment of civil registration and vital statistics systems: monitoring data quality and progress. Lancet. 2015;386:1395–1406. [DOI] [PubMed] [Google Scholar]
- 37.Suryahadi A, Suryadarma D, Sumarto S. The effects of location and sectoral components of economic growth on poverty: evidence from Indonesia. J Dev Econ. 2009;89:109–117. [Google Scholar]
- 38.Houweling TA. Mortality inequalities in times of economic growth: time trends in socioeconomic and regional inequalities in under 5 mortality in Indonesia, 1982-1997. J Epidemiol Community Health. 2006;60:62–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mahendradhata Y, Trisnantoro L, Listyadewi S, Soewondo P, Marthias T, Harimurti P, et al. The Republic of Indonesia Health System Review. In: Asia Pacific Observatory on Health Systems and Policies. 2017. (Health Systems in Transition). [Google Scholar]
- 40.World Bank. Indonesia: Avoiding the Trap. 2014. (Jakarta: World Bank; Indonesia Development Policy Review). [Google Scholar]
- 41.Kosaka S, Suda K, Gunawan B, Raksanagara A, Watanabe C, Umezaki M. Urban-rural difference in the determinants of dietary and energy intake patterns: a case study in West Java, Indonesia. PLoS One. 2018;13:e0197626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reardon T, Tschirley D, Dolislager M, Snyder J, Hu C, White S. Urbanization, Diet Change, and Transformation of Food Supply Chains in Asia. 2014; p. Michigan State Unversity; 46. [Google Scholar]
- 43.Strauss J, Witoelar F, Sikoki B. The Fifth Wave of the Indonesia Family Life Survey: Overview and Field Report: Volume 1 [Internet]. 2016. RAND Corporation; [cited 2019 Jul 9]. Available at: http://www.rand.org/pubs/working_papers/WR1143z1.html. [Google Scholar]
- 44.Strauss J, Witoelar F, Sikoki B, Wattie AM. The fourth wave of the Indonesian Family Life Survey (IFLS4): overview and field report. 2009RAND Corp. [Google Scholar]
- 45.Bundy JD, Li C, Stuchlik P, et al. Systolic blood pressure reduction and risk of cardiovascular disease and mortality: a systematic review and network meta-analysis. JAMA Cardiol. 2017;2:775–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Franklin SS, Gustin W, 4th, Wong ND, et al. Hemodynamic patterns of age-related changes in blood pressure. The Framingham Heart Study. Circulation. 1997;96:308–315. [DOI] [PubMed] [Google Scholar]
- 47.Banack HR, Kaufman JS. The “obesity paradox” explained. Epidemiology. 2013;24:461–462. [DOI] [PubMed] [Google Scholar]
- 48.Lavie CJ, Milani RV, Ventura HO. Obesity and cardiovascular disease: risk factor, paradox, and impact of weight loss. J Am Coll Cardiol. 2009;53:1925–1932. [DOI] [PubMed] [Google Scholar]
- 49.Preston SH, Stokes A. Obesity paradox: conditioning on disease enhances biases in estimating the mortality risks of obesity. Epidemiology. 2014;25:454–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Preston SH, Heuveline P, Guillot M. Demography Measuring and Modeling Population Processes. 2000. [Google Scholar]
- 51.Sudharsanan N, Bijlsma MJ. A generalized counterfactual approach to decomposing differences between populations. MPIDR Working Paper. 2019;40. [Google Scholar]
- 52.Jackson JW, VanderWeele TJ. Decomposition analysis to identify intervention targets for reducing disparities. Epidemiology. 2018;29:825–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cheng LW, Tan L, Zhang L, Wei S, Liu L, Long L. Chronic disease mortality in rural and urban residents in Hubei Province, China, 2008–2010. BMC Public Health. 2013;13:713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sherriff B, MacKenzie S, Swart LA, Seedat MA, Bangdiwala SI, Ngude R. A comparison of urban-rural injury mortality rates across two South African provinces, 2007. Int J Inj Contr Saf Promot. 2015;22:75–85. [DOI] [PubMed] [Google Scholar]
- 55.Hernández AV, Pasupuleti V, Deshpande A, Bernabé-Ortiz A, Miranda JJ. Effect of rural-to-urban within-country migration on cardiovascular risk factors in low- and middle-income countries: a systematic review. Heart. 2012;98:185–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ebrahim S, Kinra S, Bowen L, et al. ; Indian Migration Study group. The effect of rural-to-urban migration on obesity and diabetes in India: a cross-sectional study. PLoS Med. 2010;7:e1000268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Haines MR. The urban mortality transition in the United States, 1800-1940. In: Annales De Démographie Historique. 2001. p. Belin; 33–64. [Google Scholar]
- 58.Condran GA, Crimmins E. Mortality differentials between rural and urban areas of states in the Northeastern United States 1890-1900. J Hist Geogr. 1980;6:179–202. [DOI] [PubMed] [Google Scholar]
- 59.Helble MC, Aizawa T. Urbanization and Inequality in Hypertension Diagnosis and Medication in Indonesia. 2016. (Tokyo: Asian Development Bank Institute; ADBI Working Paper 556). [Google Scholar]
- 60.Schröders J, Wall S, Hakimi M, et al. How is Indonesia coping with its epidemic of chronic noncommunicable diseases? A systematic review with meta-analysis. PLoS One. 2017;12:e0179186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Soewondo P, Ferrario A, Tahapary DL. Challenges in diabetes management in Indonesia: a literature review. Global Health. 2013;9:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sohn K. Sick but unaware: hypertension in Indonesia. Biodemography Soc Biol. 2015;61:298–318. [DOI] [PubMed] [Google Scholar]
- 63.Barber SL, Gertler PJ, Harimurti P. The contribution of human resources for health to the quality of care in Indonesia. Health Aff (Millwood). 2007;26:w367–w379. [DOI] [PubMed] [Google Scholar]
- 64.Sudharsanan N, Geldsetzer P. Impact of coming demographic changes on the number of adults in need of care for hypertension in Brazil, China, India, Indonesia, Mexico, and South Africa. Hypertension. 2019;73:770–776. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


