Abstract
Background and Aim
Abdominal tuberculosis (ATB) in children poses a diagnostic challenge because of its nonspecific clinical features, which often delay the diagnosis. Our aim was to present our real‐world experience and provide an insight into the presentation, pattern of distribution, and diagnosis of the disease.
Methods
A retrospective review was conducted of case records of all children ≤12 years of age diagnosed with ATB from January 2007 to January 2018. Clinical details and investigations were recorded and analyzed.
Results
A total of 218 children (110 boys), with a median age of 10 (0.25–12) years, were included. There was a median delay of 4 (0.5–36) months in establishing the diagnosis. Abdominal pain, fever, and loss of weight were the most common presenting features, with the triad of symptoms present in 54%. Multiple intra‐abdominal sites were involved in 118 (54%) patients, with a combination of the gastrointestinal tract (I) and abdominal lymph nodes (L) being the most common (53/118). Among children with single‐site involvement (n = 100), the most commonly involved was L in 39 (39%), followed by I in 35(35%). Loss of weight was more common in children with involvement of multiple sites (85/118 vs 60/100, P = 0.03). Overall, a confirmed diagnosis was possible in 94 participants (43.1%). Suggestive imaging had the highest diagnostic yield of 85%. Nine (4.1%) patients needed surgical management.
Conclusion
A triad of abdominal pain, fever, and weight loss is suggestive of ATB. Multiple intra‐abdominal sites are frequently involved. Microbiological confirmation is possible in only one‐third of the cases.
Keywords: abdominal tuberculosis, children, presentation
A triad of abdominal pain, fever, and weight loss is suggestive of abdominal tuberculosis in children. Multiple intra‐abdominal sites are frequently involved. Microbiological confirmation is possible in only a third of the cases.
Introduction
The annual incidence of tuberculosis (TB) is nearly 10.1 million, including around 1 million children.1 Despite the advances in diagnosis and treatment, it is estimated that, worldwide, 239 000 children younger than 15 years die from TB in a year.2
TB is endemic in India, and it accounts for about a quarter of the global TB cases, the highest in the world.3 Almost 10% of the cases of TB in India are of pediatric TB, with pulmonary involvement being the most common site of involvement.4
Overall, abdominal TB (ATB) is the sixth most frequent extrapulmonary site.5 It is an uncommon presentation of TB, seen in only 0.3% of pediatric TB.6 It may involve the gastrointestinal (GI) tract, peritoneum, lymph nodes, or solid viscera. The initial symptoms are often nonspecific, and the protean clinical manifestations of ATB are a challenge to physicians, often resulting in a delay in diagnosis.5 Microbiological confirmation of the disease is often difficult, and one has to rely on a strong clinical suspicion, imaging and histopathological findings, and/or response to treatment to conclusively establish the diagnosis.
There is considerable heterogeneity in published literature on pediatric ATB with regard to the clinical presentation, pattern of involvement, and diagnostic yield.6, 7, 8, 9 This is because of the differences in clinical setting (secondary care center vs tertiary referral center), study population characteristics (socioeconomic status, rural vs urban), and department (physician vs surgeon) reporting their data.
Our hospital is one of the largest tertiary public hospitals of India and sees a large number of children with ATB every year. Our aim was to review and present the clinical features, disease site, and diagnostic yield of children with ATB who were evaluated, treated, and followed up in our center.
Methods
We carried out a retrospective review of the medical records of all of our patients ≤12 years of age diagnosed with ATB and registered between January 2007 and January 2018 in the Pediatric Gut Clinic of the Division of Pediatric Gastroenterology, Post Graduate Institute of Medical Education and Research, Chandigarh, India. Ethical approval was obtained from the institutional review board.
ATB was defined as tubercular involvement of the various organs of the abdominal cavity, such as the small or large intestines, peritoneum, lymph nodes, and visceral organs, singularly or in combination.
Diagnosis
Tuberculin skin tests (Mantoux test) were examined 48–72 h after the intradermal injection of five tuberculin units of purified protein derivative. Tests were considered positive if the diameter of induration was ≥15 mm.10
Children diagnosed with ATB were divided into two groups based on their diagnosis.11, 12
“Confirmed case of abdominal tuberculosis”—diagnosis based on the bacteriological identification of Mycobacterium tuberculosis through Ziehl‐Neelsen acid‐fast stain and/or culture and/or polymerase chain reaction (PCR)‐based assays or the presence of caseating granulomas on histology.
PCR data were available from 2015 onward. The insertion sequence 61 100 was used as a target for PCR amplification in these samples. Culture was performed in the liquid medium MGIT 960
-
2.
“Clinically diagnosed abdominal tuberculosis”—diagnosis based on strong clinical suspicion and exclusion of other diseases, with suggestive features on imaging, histology (noncaseating granulomas and/or chronic inflammatory changes), and biochemistry (elevated ascitic adenosine deaminase >30 U/L) and with a subjective and/or objective response to antitubercular therapy (ATT) with no relapse within 3 months of completion of therapy
All children underwent an abdominal ultrasonogram (USG) and/or computed tomography (CT). Imaging findings considered suggestive of TB included
ascites (free or loculated), high density (on CT) with or without multiple, thin, complete and incomplete septae;
lymphadenopathy (mesenteric, peripancreatic, periportal, and para‐aortic groups of lymph nodes) seen as conglomerate masses and/or as scattered enlarged nodes with hypoechoic or anechoic centers (on USG)/peripheral rim enhancement, nonhomogenous enhancement (on CT);
bowel wall thickening, peritoneal thickening and nodularity, adhesions, mesenteric thickening, and irregular soft tissue densities in the omental area; and
tiny, low‐density foci or multiple low‐attenuation, 1–3 cm round lesions scattered in the liver and/or spleen.
An attempt to obtain tissue for histological diagnosis by radiologically guided, endoscopic, laparoscopic, or surgical means was made in all patients if feasible.
Children who did not fulfill either of the two definitions of “confirmed case” or “clinically diagnosed” as given above were excluded from the analysis.
Children who did not complete treatment or were not followed up for a minimum duration of 3 months after completion of treatment were excluded.
Pattern of involvement
ATB was divided into four patterns of involvement –
Peritoneal—including wet type (characterized by ascites formation), dry fibrotic type (associated with peritoneal thickening, adhesions, omental, and mesenteric thickening with little or no ascites), mixed type (combination of both), and abdominal cocoon (characterized by presence of a membranous sac around the intestinal loops). Involvement based on imaging and/or histology and/or ascitic fluid examination.
Gastrointestinal tract—involvement as found on imaging and/or endoscopy and/or histology
Visceral organs—involvement on imaging and/or histology
Abdominal lymph nodes—involvement as found on imaging and/or histology
Treatment
ATT consisted of four drugs. The intensive phase for the initial 2 months consisted of isoniazid (H), rifampicin (R), pyrazinamide (Z), and ethambutol (E), followed by a maintenance phase of two drugs (HR) for 7–10 months. After 2016, the maintenance phase consisted of three drugs (HRE). ATT‐induced hepatitis was defined as the elevation of alanine aminotransferase >5 times the upper limit of normal or the presence of jaundice. The socioeconomic status of the patient's family was assessed using the revised Kuppuswamy's Socioeconomic Status Scale.13
Statistical analysis
All the data were presented as n (%) or median range. Statistical comparisons were performed using Mann–Whitney's test for two unpaired continuous variables and Fisher's exact test for dichotomous variables. Statistical analysis was performed using the IBM Statistical Package for the Social Sciences v.20.0 (SPSS, IBM Corp, Armonk, NY, USA).
Results
Patient characteristics and clinical presentation
Of a total of 232 patients with an initial diagnosis of ATB, 218 patients (110 girls), with a median age of 10 (0.25–12) years at diagnosis, were included in the analysis. Fourteen patients were excluded (subsequent revision of diagnosis—2 [Crohn's disease—1, GI lymphoma—1], lost to follow up—1, partial response/relapse of symptoms after completion of treatment—2, and currently undergoing treatment—9).
The majority of the patients belonged to the upper lower/lower socioeconomic category (95%, n = 207). A total of 71 (32.5%) patients had a history of contact with a patient suffering from TB. None of the patients were positive for human immunodeficiency virus.
Clinical features are presented in Table 1. The most common symptoms were abdominal pain, fever, and loss of weight. This triad was present in 118 (54%) patients.
Table 1.
Clinical features of children with abdominal tuberculosis (n = 218)
| Symptoms | n (%) | Signs | n (%) |
|---|---|---|---|
| Pain | 177 (81) | Pallor | 168 (77) |
| Fever | 166 (76) | Peripheral lymphadenopathy | 48 (22) |
| Loss of weight | 162 (74) | Clubbing | 27 (12) |
| Nausea/vomiting | 96 (44) | Pedal edema | 17 (8) |
| Anorexia | 145 (66) | Anasarca | 5 (2) |
| Diarrhea | 45 (21) | Jaundice | 3 (1) |
| Hematochezia/bleed per rectum | 11 (5) | Abdominal distension | 94 (43) |
| Abdominal lump | 13 (6) | Doughy abdomen | 60 (27) |
| Constipation | 27 (12) | Abdominal tenderness | 58 (27) |
| Intestinal obstruction | 36 (16) | Palpable lump | 18 (8) |
| Abdominal distension | 79 (36) | Hepatomegaly | 71 (32) |
| Umbilical discharge | 2 (1) | Splenomegaly | 34 (15) |
| Mass per rectum | 1 (0.5) | Ascites | 57 (26) |
| Extragastrointestinal features | 47 (21) | — | — |
| Cough | 35 | — | — |
| Neck swelling | 7 | — | — |
| Headache/seizure | 3 | — | — |
| Erythema nodosum | 1 | — | — |
| Uveitis | 1 | — | — |
There was a median delay of 4 (0.5–36) months from the onset of symptoms to the diagnosis.
Disease distribution
In 145 patients, the disease was localized to the abdomen, while 73 (33%) patients demonstrated disease at other sites—pulmonary—55, Central Nervous System—7, bone marrow—1, cervical/axillary lymph nodal—7, genitourinary—2, and ocular—1.
In 100 (46%) patients, a single abdominal site (SS) was involved, while in the remaining patients, multiple sites (MS) were involved. The sites of abdominal involvement are depicted in Figure 1. Comparing children in whom MS were involved with those with SS, we found that children with MS involvement were more likely to have loss of weight at presentation (Table 2). The likelihood of being a bacteriologically confirmed case was also more likely in such children.
Figure 1.
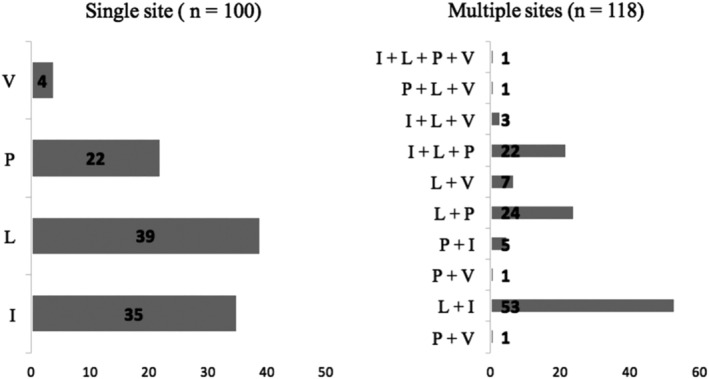
Disease distribution in children with abdominal tuberculosis. V, Visceral organs; P, peritoneal cavity; L, abdominal lymph nodes; I, gastrointestinal tract.
Table 2.
Comparison between children with multiple‐site and single‐site infection
| Multiple site (n = 118) | Single site (n = 100) | P | |
|---|---|---|---|
| Age | 10 (0.25–12) years | 9 (1–12) years | 0.69 |
| Gender | 56 | 52 | 0.78 |
| Duration of symptoms | 3 (0.25–48) months | 4 (0.25–36) months | 0.80 |
| h/o contact | 41 | 30 | 0.38 |
| Clinical features | |||
| Fever | 91 | 75 | 0.42 |
| Abdominal Pain | 92 | 85 | 0.49 |
| Loss of weight | 85 | 60 | 0.03 |
| Nausea/vomiting | 58 | 38 | 0.07 |
| ATB Triad† | 65 | 53 | 0.58 |
| Extra‐GI involvement | 35 | 37 | 0.38 |
| Tuberculin test positivity | 78 | 72 | 0.66 |
| Bacteriological confirmation | 43 | 24 | 0.03 |
The abdominal tuberculosis (ATB) triad comprises of fever, abdominal pain, and loss of weight.
GI, gastro – intestinal.
Considering individual disease sites, the disease distribution among children with involvement of the GI tract (n = 119) was as follows: ileocolonic—90 (75.6%), isolated small bowel—20 (16.8%), and isolated colonic involvement—9 (7.5%). Among children with peritoneal cavity involvement (n = 77), 41 (55.5%) had a wet ascitic form, 12 (15.5%) had dry fibrotic disease, and 24 (31.1%) had mixed involvement. Visceral involvement (n = 18) was in the form of hepatic—10 (55.5%), splenic—1 (5.5%), and hepatosplenic—7 (38.8%) involvement.
Strength of diagnosis
A total of 94 (43%) children were confirmed cases, while the remaining patients were clinically diagnosed. Among the confirmed cases, 67 (31%) were bacteriologically confirmed. Children with visceral involvement had the highest bacteriological confirmation rate—8 of 18 (44%). In the others, it was 46 (38.7%) for the GI tract, 23 (30%) for the peritoneal cavity, and 44 (29.3%) in children with abdominal lymph nodes.
The tuberculin (Mantoux) test was positive in 78 (35.7%) patients. Suggestive imaging was seen in 185 (84.8%) patients. TB PCR/Xpert MTB reports were available in 62 patients, of which 9 were positive (14.5%). Culture reports were available for 34 patients, of which 1 (3%) was positive for MTB.
The yields of various investigations for diagnosis are summarized in Table 3.
Table 3.
Yield of various investigations for the diagnosis of abdominal tuberculosis
| Yield | ||||
|---|---|---|---|---|
| Investigation | Gastrointestinal tract (n = 119) | Abdominal lymph nodes (n = 150) | Peritoneal cavity (n = 77) | Visceral Organs (n = 18) |
| AFB positivity | 43 (36.1%) | 44 (29.3%) | 23 (29.8%) | 8 (44.4%) |
| Granuloma | 65 (54.7%) | 68 (45.3%) | 26 (72.2%)† | 13 (72%) |
| Ascitic ADA (>30 U/L) | — | — | 35(53.8%)‡ | — |
| Imaging (USG/CT) | 97 (81.5%) | 134 (89.3%) | 71 (92.2%) | 18 (100%) |
| Tuberculin (Mantoux) | 77 (64.7%) | 103 (68.7%) | 52 (67.5%) | 13 (72%) |
Obtained from biopsy from the omentum from patients (n = 36) with dry type (n = 12) or mixed (n = 24) involvement.
Patients (n = 65) with wet ascitic (n = 41) or mixed (n = 24) form of peritoneal involvement.
— Not applicable; ADA, Adenosine Deaminase; AFB, Acid Fast Bacilli; CT, computed tomography; USG, ultrasonogram.
Treatment
Nine patients underwent surgery (perforation peritonitis—6 and intestinal obstruction [including abdominal cocoon in 2]—3). The endoscopic dilatation of intestinal (ileal—1, descending colon—1) strictures were performed in two patients. All patients received ATT for a median duration of 12 (9–18) months. Fifteen (6.8%) patients developed ATT‐induced hepatitis and required modification of treatment. The median duration of follow up after completion of treatment was 9 (3–108) months. There was no relapse in any patient included in this analysis.
Atypical cases
Some of our patients had an unusual presentation.
Six patients presented with perforation peritonitis. One of these patients, who had a pyoperitoneum, was also diagnosed to have secondary Budd–Chiari syndrome.
Two patients (6 year/male and 5 year/male) presented with a tubercular abdominal cocoon (encapsulating sclerosing peritonitis). One of these patients also had an enterocutaneous fistula. Both these patients were managed surgically.
An 8‐year‐old boy presented with a spontaneous tubercular enteroumbilical fistula, which was managed conservatively with ATT.
An 11‐year‐old girl was found to have tubercular involvement of the duodenum, which is an unusual site. She responded to ATT.
The youngest child in our cohort (3 months/male) presented with fever and hepatosplenomegaly and was diagnosed with congenital TB. Large necrotic mesenteric lymph nodes were identified on imaging. Acid Fast Bacilli (AFB) was identified from specimens from the lymph nodes and liver biopsy. He also developed secondary hemophagocytic lymphohistiocytosis (HLH) during the course of the hospital stay. He responded to ATT alone.
Two patients had chylous ascites. In addition to ATT, they received a medium‐chain triglyceride (MCT)‐containing diet. One of the children (8 year/female) also developed portal vein thrombosis was also given octrotide.
Nine patients had an underlying chronic illness [celiac disease—3, chronic liver disease (autoimmune liver disease—1, cryptogenic—2)—3, dilated cardiomyopathy—1, Gaucher disease—1, and partial anomalous pulmonary venous connection—1]
Discussion
The clinical features of ATB are often nonspecific, resulting in diagnostic delays and diagnostic challenges for physicians. There was a median delay of 4 months in establishing the diagnosis of ATB in our patients. Abdominal pain, fever, and weight loss were the most frequent findings on presentation, and a triad of these symptoms was present in more than half of our patients. This triad has been the most commonly reported group of symptoms from centers across the developing14, 15 and developed6 world, and we believe that this triad should be called the “triad of ATB”. In any child who presents with these symptoms, clinicians should strongly consider the diagnosis of ATB.
In this study, various combinations of intestinal, nodal, visceral, and peritoneal involvement were seen in more than half of our patients. This frequency is higher than the reported frequency of 0–32% in published literature,7, 8, 16 which we believe to be an underrepresentation. In an autopsy series of children with ATB, it was found that, in 14 of 17 cases of intestinal TB, the intestinal lesion extended to the peritoneum with adhesions between the bowel loops, while 20 of 21 children with peritoneal TB on autopsy had evidence of disease arising from other sites, suggesting the frequent coexistence of MS of abdominal TB.17 We found that children with MS involvement present with loss of weight more frequently compared to those with SS involvement. This may be related to a higher bacillary load and disease burden in MS patients. Among the children with SS involvement, abdominal lymph nodes were the most commonly involved site.
In our cohort of patients, 33% had simultaneous involvement of TB at other extra‐GI sites as well. In literature, it has been reported in 21–100% patients.8, 18 Pulmonary involvement is most commonly seen and was present in almost a quarter of all our patients. A third of these patients did not have pulmonary symptoms and were detected incidentally on a chest radiograph. This stresses the need for routine chest X‐ ray as a part of the evaluation in children with suspected ATB.
Six patients presented with perforation peritonitis, which is an unusual mode of presentation of ATB in the pediatric age group.19 There is only a handful of patients who have been described to present in such a manner, and they generally have a poor prognosis. In a series of 123 children with perforating peritonitis, 19 (15%) had tubercular enteritis, with 12 of 19 dying in the postoperative period.20 On the contrary, all our patients survived.
Tubercular abdominal cocoon was present in two of our children. It has rarely been reported in children.21 Both of our patients were managed surgically, although there are reports from adults which suggest that a subset of these patients may be managed conservatively.22 Spontaneous tubercular enteroumbilical fistula is an extremely rare manifestation of ATB and was managed conservatively. This is similar to the report by Rao et al., who managed four of five children with such a presentation with ATT alone.23 We had a child with TB‐associated HLH, which is quite uncommon. Only 63 cases have been reported worldwide till date, and mortality is high.24 Our child responded to ATT.
An abnormal abdominal imaging played a pivotal role in the diagnosis of our patients and was suggestive in ~ 85% of them. It is important to try and obtain microbiological evidence in all cases as ATB may mimic other diseases such as Crohn's disease and abdominal lymphoma. Unfortunately, the diagnostic yield is low due to its paucibacillary nature in children. We were able to demonstrate acid‐fast bacilli in only a third of our patients, which is similar to the 32–35% yield reported in literature.6 Even in adults in whom the disease is multibacillary, the rate of bacterial isolation is only 45%.25 Histopathological evidence of TB, that is, the presence of a granuloma with caseous necrosis is more commonly seen and was present in around 43% patients overall, with the best yield coming from the omentum (72.2%). Overall, as the diagnostic yield is poor, if there is a strong clinical suspicion and other causes have been excluded, it is prudent to start treatment with a close follow up for the resolution of symptoms, signs, and features seen on imaging and/or endoscopy.
All the patients included in this series showed a response to treatment. It is worth noting that, of the 36 children who presented with clinical features of partial intestinal obstruction, only 3 needed an intervention (surgical—1, endoscopic—2). The others responded to conservative management and ATT.
The optimal duration of treatment for ATB has been a matter of debate. A shorter duration of treatment may increase compliance and decrease the risk of ATT toxicity. However, it also poses a risk for relapses. Even though a review of three randomised controlled trials comprising 328 adult participants found a 6‐month regimen to be efficacious, we believe it cannot be extrapolated to children.26 Apart from including only adults, two of the three studies included only those with intestinal involvement, which may not be applicable to children where the majority has involvement of multiple abdominal sites. Moreover, the authors of the review have conceded that the quality of evidence regarding the relapse estimate is very low, which is a big concern.26 Hence, till some robust pediatric data are available, we plan to continue treatment for 9–12 months.
Our study has some limitations. First, it is a retrospective study. Second, we did not have data on TB‐ PCR/Xpert MTB and culture for all the patients, leading to a small number of “confirmed cases”; however, as we only included patients in whom the response to ATT was unequivocal (clinical response and/or endoscopic/imaging resolution), with no relapse on completion of therapy, we believe all our cases to be “true” cases.27 Moreover, the role of Xpert MTB in the diagnosis of ATB has not yet been conclusively established, with literature suggesting that one in five patients are missed when evaluating a specimen from a lymph node.11
The strength of our study is that it presents the real‐world experience of a large number of patients who come from a diverse geographical area of the country. We work in close liaison with our surgeons, general pediatricians, and other pediatric subspecialties; hence, our data include a mix of cases with varying sites and extents of GI and extra‐GI involvement. An attempt to establish a tissue diagnosis was made in all patients, providing the true yield of various investigations.
To conclude, a triad of abdominal pain, fever, and loss of weight is suggestive of ATB. Multiple intra‐abdominal sites are commonly involved together. Microbiological yield is low, and one often has to start therapy based on a strong clinical suspicion. A close follow up for the resolution of clinical and imaging features is warranted.
V Jagadeesh Menon is currently working as a Consultant in Dr Rela Institute and Medical Centre, Chennai.
Declaration of conflict of interest: None.
References
- 1. GBD Tuberculosis Collaborators . The global burden of tuberculosis: results from the Global Burden of Disease Study 2015. Lancet Infect. Dis. 2018; 18: 261–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dodd PJ, Yuen CM, Sismanidis C, Seddon JA, Jenkins HE. The global burden of tuberculosis mortality in children: a mathematical modelling study. Lancet Glob. Health. 2017; 5: e898–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. World Health Organization . Global Tuberculosis Report 2016 Geneva: The Organization, 2016;1–192. Cited 20 May 2019. Available from URL: https://apps.who.int/medicinedocs/en/d/Js23098en/
- 4. Mandal A, Singh A. Recent changes in tuberculosis guidelines for children. Mycobact. Dis. 2017; 7: 237. [Google Scholar]
- 5. Sharma MP, Bhatia V. Abdominal tuberculosis. Indian J. Med. Res. 2004; 120: 305–15. [PubMed] [Google Scholar]
- 6. Delisle M, Seguin J, Zeilinski D, Moore DL. Paediatric abdominal tuberculosis in developed countries: case series and literature review. Arch. Dis. Child. 2016; 101: 253–8. [DOI] [PubMed] [Google Scholar]
- 7. Basu S, Ganguly S, Chandra PK, Basu S. Clinical profile and outcome of abdominal tuberculosis in Indian children. Singapore Med. J. 2007; 48: 900–5. [PubMed] [Google Scholar]
- 8. Malik R, Srivastava A, Yachha SK, Poddar U, Lal R. Childhood abdominal tuberculosis: disease patterns, diagnosis, and drug resistance. Indian J. Gastroenterol. 2015; 34: 418–25. [DOI] [PubMed] [Google Scholar]
- 9. Sharma AK, Agarwal LD, Sharma CS, Sarin YK. Abdominal tuberculosis in children experience over a decade. Indian Pediatr. 1993; 30: 1149–53. [PubMed] [Google Scholar]
- 10. Nayak S, Acharjya B. Mantoux test and its interpretation. Indian Dermatol. Online J. 2012; 3: 2–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sharma SK, Ryan H, Khaparde S et al Index‐TB guidelines: guidelines on extrapulmonary tuberculosis for India. Indian J. Med. Res. 2017; 145: 448–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sharma V, Mandavdhare HS, Lamoria S, Singh H, Kumar A. Serial C‐reactive protein measurements in patients treated for suspected abdominal tuberculosis. Dig. Liver Dis. 2018; 50: 559–62. [DOI] [PubMed] [Google Scholar]
- 13. Sharma R. Revised Kuppuswamy's socioeconomic status scale: explained and updated. Indian Pediatr. 2017; 54: 867–70. [PubMed] [Google Scholar]
- 14. Shah I, Uppuluri R. Clinical profile of abdominal tuberculosis in children. Indian J. Med. Sci. 2010; 64: 204–9. [PubMed] [Google Scholar]
- 15. Tinsa F, Essaddam L, Fitouri Z et al Abdominal tuberculosis in children. J. Pediatr. Gastroenterol. Nutr. 2010; 50: 634–8. [DOI] [PubMed] [Google Scholar]
- 16. Kılıç Ö, Somer A, Hançerli Törün S et al Assessment of 35 children with abdominal tuberculosis. Turk. J. Gastroenterol. 2015; 26: 128–32. [DOI] [PubMed] [Google Scholar]
- 17. Ridaura‐Sanz C, López‐Corella E, Lopez‐Ridaura R. Intestinal/Peritoneal tuberculosis in children: an analysis of autopsy cases. Tuberc. Res. Treat. 2012; 2012: 230814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lin YS, Huang YC, Lin TY. Abdominal tuberculosis in children: a diagnostic challenge. J. Microbiol. Immunol. Infect. 2010; 43: 188–93. [DOI] [PubMed] [Google Scholar]
- 19. Dinler G, Sensoy G, Helek D. Tuberculous peritonitis in children: report of nine patients and review of the literature. World J. Gastroenterol. 2008; 14: 7235–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dhar A, Bagga D, Taneja SB. Perforated tubercular enteritis of childhood: a ten year study. Indian J. Pediatr. 1990; 57: 713–6. [DOI] [PubMed] [Google Scholar]
- 21. Singal R, Satyashree B, Mittal A et al Tubercular abdominal cocoon in children ‐ a single centre study in remote area of northern India. Clujul Med. 2017; 90: 179–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sharma V, Mandavdhare HS, Rana SS, Singh H, Kumar A, Gupta R. Role of conservative management in tubercular abdominal cocoon: a case series. Infection. 2017; 45: 601–6. [DOI] [PubMed] [Google Scholar]
- 23. Rao PL, Mitra SK, Pathak IC. Spontaneous tuberculous enteroumbilical fistulas. Am. J. Gastroenterol. 1979; 72: 671–5. [PubMed] [Google Scholar]
- 24. Padhi S, Ravichandran K, Sahoo J, Varghese RG, Basheer A. Hemophagocytic lymphohistiocytosis: an unusual complication in disseminated Mycobacterium tuberculosis . Lung India. 2015; 32: 593–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Radhika S, Rajwanshi A, Kochhar R, Kochhar S, Dey P, Roy P. Abdominal tuberculosis. Diagnosis by fine needle aspiration cytology. Acta Cytol. 1993; 37: 673–8. [PubMed] [Google Scholar]
- 26. Jullien S, Jain S, Ryan H, Ahuja V. Six‐month therapy for abdominal tuberculosis. Cochrane Database Syst. Rev. 2016; 11: CD012163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pratap Mouli V, Munot K, Ananthakrishnan A et al Endoscopic and clinical responses to anti‐tubercular therapy can differentiate intestinal tuberculosis from Crohn's disease. Aliment. Pharmacol. Ther. 2017; 45: 27–36. [DOI] [PubMed] [Google Scholar]


