Abstract
Background and Aim
A number of recent studies have been published evaluating the chemopreventive effect of aspirin against gastric cancer, and an updated meta‐analysis is required to evaluate this relationship further. This study presents a meta‐analysis of studies examining the effect of aspirin on gastric cancer incidence and death.
Methods
The PUBMED and Cochrane Central Registration of Controlled Trials databases were searched for eligible studies published up to December 2018. Pooled risk ratios for gastric cancer incidence and death in aspirin users versus nonusers were determined using fixed‐ and random‐effects models. The influence of the frequency of aspirin use, duration of aspirin use, and geographic location on gastric cancer incidence was evaluated.
Results
The meta‐analysis comprised 33 studies with a total of 1 927 971 patients. The pooled risk ratios for gastric cancer incidence in the fixed‐ and random‐effects models were 0.890 (95% confidence interval, 0.871–0.909) and 0.826 (0.740–0.922), respectively. In Asia and North America, the maximum preventive benefit of aspirin use was observed with weekly or daily use. Aspirin use was most effective for noncardiac gastric cancer. The pooled risk ratios for gastric cancer death in the fixed‐ and random‐effects models were 0.798 (0.749–0.850) and 0.894 (0.780–1.024), respectively. Significant heterogeneity was observed among studies of gastric cancer incidence but not gastric cancer death.
Conclusion
Aspirin use may reduce the risk of gastric cancer incidence and death; however, the relationship may be limited to a specific frequency and duration of aspirin use and geographic location.
Keywords: aspirin, gastric cancer death, gastric cancer incidence
A number of recent studies have been published evaluating the chemopreventive effect of aspirin against gastric cancer, and an updated meta‐analysis is required to evaluate this relationship further. Of 463 studies, we analyzed 33 studies with a total of 1 927 971 patients. The pooled risk ratios for gastric cancer incidence were 0.890 (95% confidence interval, 0.871–0.909) and 0.826 (0.740–0.922) in the fixed‐ and random‐effects models, respectively. The pooled risk ratios for gastric cancer death in the fixed‐ and random‐effects models were 0.798 (0.749–0.850) and 0.894 (0.780–1.024), respectively. Aspirin use may reduce the risk of gastric cancer incidence and death.
Introduction
Gastric cancer, primarily associated with Helicobacter pylori infectious inflammation, is one of the most common fatal cancers worldwide.1, 2 Aspirin exhibits a protective effect in gastrointestinal cancer,3 including gastric cancer development, due to its anti‐inflammatory and antiplatelet functions, including induction of apoptosis4 and inhibition of angiogenesis.5 Previous meta‐analyses have reported a potential relationship between aspirin use and prevention of gastric cancer.6, 7, 8, 9, 10, 11, 12, 13, 14
Previous studies, including a randomized controlled trial (RCT) and our propensity scores‐matched analysis,15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28 did not find a significant association between aspirin use and gastric cancer development.29 The effect of aspirin use on gastric carcinogenesis remains undetermined. There are limited data on whether aspirin use is associated with decreased gastric cancer incidence and death. More than 40 studies on this topic have been published since 2016.7 Further systematic review and meta‐analysis are required to evaluate the protective effects of aspirin use on gastric cancer.
This study provides a comprehensive systematic review and updated meta‐analysis to estimate risk reduction associated with aspirin use on gastric cancer incidence and death.
Methods
Search strategy and eligibility criteria
A search of the PUBMED and Cochrane Central Registration of Controlled Trials database was performed to identify all English‐language studies published that evaluated the association between aspirin use and the risk of gastric cancer incidence or death up to December 2018. Search terms used were “aspirin,” “non‐steroidal anti‐inflammatory agents,” “non‐steroidal anti‐inflammatory drug,” “stomach carcinoma,” “stomach neoplasms,” “stomach cancer,” “stomach tumor,” “stomach adenocarcinoma,” “gastric carcinoma,” “gastric neoplasms,” “gastric cancer,” “gastric tumor,” “gastric adenocarcinoma,” “epidemiology,” “incidence,” and “mortality.” Detailed search queries are provided in Table S1, Supporting information. A manual scan of the bibliographies of relevant articles for additional studies was performed.
Observational and RCT studies of cohorts or case–control studies that met the following criteria were included: written in English, reported on gastric cancer incidence or death, outcomes of aspirin users were compared with nonusers, and studies provided adequate data to enable risk ratio estimation. Aspirin use was defined as nonsteroidal anti‐inflammatory drug (NSAIDs) aspirin use only.
Study selection and data extraction, outcome and variables measured, and study quality assessment
Two researchers (Ryota Niikura and Yoshihiro Hirata) independently reviewed the included studies. Uncertainty about the inclusion of a study was resolved by discussing issues to achieve consensus. Data, including the number of participants, events, or risk ratios for gastric cancer incidence or death; first author's surname; year of publication; type of outcome; study design; patient inclusion criteria; aspirin use frequency, duration, and dose; and age and gender of participants, were independently extracted from the included studies by both researchers and verified for accuracy.
Outcomes included gastric cancer incidence, cardiac gastric cancer incidence, noncardiac gastric cancer incidence, and gastric cancer death.
Aspirin use duration was categorized as <5 years, >5 years, or >10 years. Aspirin use frequency and dose were categorized as occasionally, monthly, weekly, or daily use. Geographic location was categorized as Asia, Europe, or North America.
The Newcastle‐Ottawa Scale (NOS) was used to assess the quality of observational studies (range: 0–9), which consisted of each category of selection of the study groups, the comparability of the groups, and the ascertainment of the exposure (case–control studies) or outcome of interest (cohort studies) (Tables S2 and S3).
Statistics
The pooled risk ratios were calculated using both fixed‐effect and random‐effects models. Heterogeneity among studies was evaluated using the Q‐statistic, and the inconsistency I 2 was quantified.30
Subgroup analyses were performed to assess the potential impact of aspirin use duration and frequency, geography, cardiac gastric cancer, and noncardiac gastric cancer on the pooled effects. Publication bias was assessed using funnel plots and the Egger regression test of funnel plot asymmetry.31 In each funnel plot, the standard errors of the estimates were plotted on a vertical reversed scale against the effect estimates on the horizontal scale. The triangle was centered on the pooled estimate and extended to 1.96 times the standard errors on either side. The statistical analyses were performed using the Comprehensive Meta‐Analysis version 3 and SAS version 9.4 (SAS Institute, Cary, NC). A P value <0.05 was considered statistically significant.
Results
A total of 463 studies were identified in the systematic search. After screening the titles and abstracts, 86 studies were considered eligible for analysis. Of these, 53 failed to satisfy inclusion criteria and were excluded. A total of 33 studies were included in the final analysis (Fig. 1).12, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46 Characteristics of the included studies, outcomes, characteristics of the drug exposure, and study quality are provided in Table 1. A total of 1 927 971 patients were analyzed.
Figure 1.
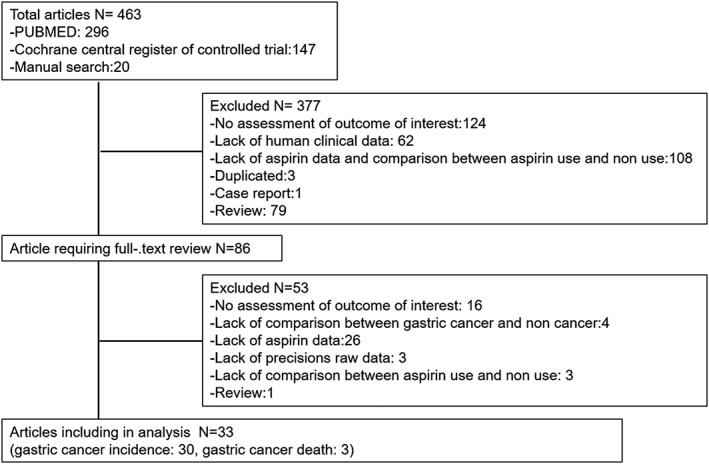
Flow chart for study selection.
Table 1.
Characteristics of studies evaluating aspirin use and gastric cancer incidence and death
| Study. no | Author, year | Geographic area | Outcome, cancer location | Study design | Helicobacter pylori infection rate | Event in aspirin users/nonevents in aspirin users | Event in nonaspirin user/nonevent in nonaspirin user | Aspirin use, frequency and duration | Study quality |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Abnet et al., 200912 | North America |
Incidence Cardia Noncardia |
Cohort | Not reported | 245/227198 | 115/83917 | Not reported | 8 |
| 2 | Bertuccio et al., 201019 | Europe | Incidence | CC | Not reported | 21/229 | 46/543 | <60 months, ≥60 months | 6 |
| 3 | Cheung et al., 201820 | Europe |
Incidence Cardia Noncardia |
CC | High | 25/9045 | 144/54560 | <Monthly, monthly to weekly, weekly to daily, daily <2 years, 2–5 years, ≥5 years | 6 |
| 4 | Coogan et al., 200032 | North America | Incidence | CC | Not reported | 127/3621 | 123/2339 | Regular, nonregular <5 years, >5 years | 6 |
| 5 | Cook et al., 200529 | North America | Incidence | RCT | Not reported | 10/19934 | 10/19942 | Not reported | |
| 6 | Duan et al., 200833 | North America |
Incidence Cardia Noncardia |
CC | Not reported | 139/457 | 575/1612 | 2–7 pills, ≥7 pills/week, <5 years, ≥5 years | 7 |
| 7 | Akre et al., 200134 | Europe |
Incidence Cardia Noncardia |
CC | High | 170/594 | 310/941 | <1 tab, 1–29 tab, ≥30 tab/month | 8 |
| 8 | Farrow et al., 199835 | North America |
Incidence Cardia Noncardia |
CC | Not reported | 159/375 | 453/924 | <1 tab, 1 tab, >1 tab/day <5 years, 5–9 years, ≥10 years | 6 |
| 9 | Figueroa et al., 200922 | North America |
Incidence Cardia Noncardia |
CC | Not reported | 58/282 | 86/410 | Not reported | 6 |
| 10 | Fortuny et al., 200736 | North America |
Incidence Cardia Noncardia |
CC | Not reported | 314/1902 | 296/1044 | <2 tab, 2–11 tab, >11 tab/week | 6 |
| 11 | Gillies and Skyring, 196828 | Asia | Incidence | CC | Not reported | 2/6 | 23/44 | Not reported | 5 |
| 12 | Gong et al., 201437 | Asia | Incidence | CC | High | 21/81 | 306/573 | Not reported | 8 |
| 13 | Hoyo et al., 200924 | North America |
Incidence Cardia Noncardia |
CC | Not reported | 33/97 | 75/203 | Not reported | 5 |
| 14 | Iqbal et al., 201638 | Asia | Incidence | CC | Not reported | 4158/18416 | 17096/73200 | Not reported | 4 |
| 15 | Kim et al., 201817 | Asia | Incidence | Cohort | Low | 1207/86446 | 4466/375043 | 1–29 days, 30–364 days, 1–2 years 2–3 years, 3–4 years, 4–5 years | 8 |
| 16 | Langman et al., 200023 | Europe | Incidence | CC | Not reported | 148/620 | 465/1830 | 1 tab, 2–6 tab, ≥30 tab/13–24 months 1 tab, 2–6 tab, ≥30 tab/25–36 months 1 tab,2–6 tab, ≥30 tab/13–36 months | 5 |
| 17 | Lee et al., 201239 | Asia | Incidence | CC | Low | 184/799 | 347/636 | Not reported | 7 |
| 18 | Meade, 199855 | Europe | Incidence | CC | Not reported | 5/1268 | 1/1272 | Not reported | 5 |
| 19 | Niikura et al., 201841 | Asia | Incidence | CC | Low | 17/2082 | 8/2082 | Not reported | 6 |
| 20 | Nomura et al., 200342 | North America | Incidence | CC | Not reported | 63/193 | 237/553 | <3 years, >3 years | 5 |
| 21 | Pandeya et al., 201025 | Asia |
Incidence Cardia |
CC | Not reported | 305/1523 | 100/427 | <weekly, ≥weekly | 5 |
| 22 | Sadeghi et al., 200821 | Asia |
Incidence Cardia |
CC | Not reported | 255/1197 | 171/809 | Occasionally, less than weekly, at least weekly | 6 |
| 23 | Schreinemachers et al.,199426 | North America | Incidence | CC | Not reported | 20/6716 | 19/4634 | Not reported | 5 |
| 24 | Suleiman et al., 200043 | Europe |
Incidence Cardia |
CC | Not reported | 35/82 | 24/33 | <1 year, >1 year | 5 |
| 25 | Tsoi et al., 201944 | Asia | Incidence | CC | Not reported | 1385/204170 | 4442/408339 | <7 years, <10 years, <14 years | 4 |
| 26 | Wang et al., 201516 | Asia | Incidence | CC | High | 31/86 | 47/160 | 1–6 tab, ≥7 tab/week <5 years, ≥5 years | 6 |
| 27 | Wu et al., 201045 | Asia | Incidence | CC | Low | 69/25145 | 103/27016 | Not reported | 5 |
| 28 | Kim et al., 201627 | Asia | Incidence | CC | Low | 31/3907 | 86/7808 | 0.5–1 year, 1.1–2 years, 2.1–3 years, >3 years | 4 |
| 29 | Epplein et al., 200918 | North America |
Incidence Cardia Noncardia |
CC | Not reported | 349/86695 | 294/82597 | ≤1 year, 2–5 years, ≥6 years | 4 |
| 30 | Zaridze et al., 199946 | Europe |
Incidence Noncardia |
Cohort | High | 48/135 | 400/923 | Not reported | 9 |
| 31 | Spence et al., 201853 | Europe | Death | Cohort | Not reported | 412/808 | 1980/3025 | <365, ≥365, 1–182, 183–364, 365–547, 548–729, ≥730 tab | 8 |
| 32 | Ratnasinghe et al., 199951 | North America | Death | Cohort | Not reported | 23/14815 | 25/7971 | Not reported | 8 |
| 33 | Thrift et al., 201254 | Asia | Death | Cohort | Not reported | 129/204 | 150/212 | Not reported | 8 |
Aspirin use and risk of gastric cancer incidence
A total of 30 studies on the effect of aspirin use and gastric cancer incidence were included in the analysis. The directions of the estimated risk ratios and 95% confidence intervals (CIs) were not consistent among the studies (Fig. 2). The pooled risk ratios (95% CI) of aspirin users compared with nonusers in the fixed‐ and random‐effects models were 0.890 (0.871–0.909) and 0.826 (0.740–0.922), respectively. Significant heterogeneity was observed among studies in the fixed‐effects model (P < 0.001; I 2 93.2%).
Figure 2.
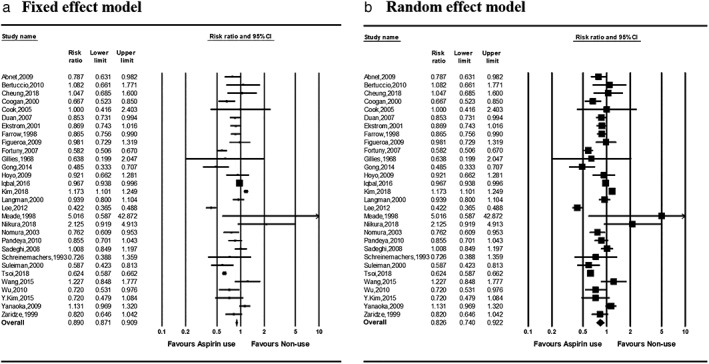
Forest plots of risk ratios for gastric cancer incidence in aspirin users versus nonusers in (a) fixed‐ and (b) random‐effects models. CI, confidence interval.
The pooled risk ratios of the groups of patients with aspirin use duration <5 years, >5 years, and >10 years were 0.996 (0.953–1.040), 0.850 (0.732–0.987), and 0.826 (0.659–1.035), respectively, in the fixed‐effects model and 0.857 (0.764–0.960), 0.823 (0.607–1.117), and 0.826 (0.484–1.409), respectively, in the random‐effects model (Fig. 3).
Figure 3.
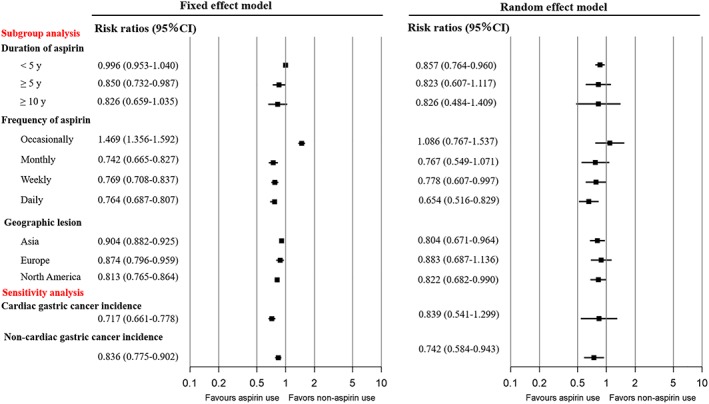
Relative risks for the subgroup analyses. CI, confidence interval.
With regard to the frequency of aspirin use, the pooled risk ratios for the occasional, monthly, weekly, and daily users were 1.469 (1.356–1.592), 0.742 (0.665–0.827), 0.769 (0.708–0.837), and 0.744 (0.687–0.807), respectively, in the fixed‐effects model. The random‐effects model had similar results, with the exception of the pooled risk ratios in the occasional and monthly use subgroups of 1.086 (0.767–1.537) and 0.767 (0.549–1.071), respectively (Fig. 3).
The pooled risk ratios in Asia, Europe, and North America were 0.904 (0.882–0.925), 0.874 (0.796–0.959), and 0.813 (0.765–0.864), respectively, in the fixed‐effects model and 0.804 (0.671–0.964), 0.883 (0.687–1.136), and 0.822 (0.682–0.990), respectively, in the random‐effects model (Fig. 3).
Aspirin use and risk of cardiac and noncardiac gastric cancer incidence
The relationship between aspirin use and the risk of cardiac and noncardiac gastric cancer incidence was examined in 12 and 11 studies, respectively. Pooled risk ratios for cardiac gastric cancer and noncardiac gastric cancer patients were 0.717 (0.661–0.778) and 0.836 (0.775–0.902) in the fixed‐effects model and 0.839 (0.541–1.299) and 0.742 (0.584–0.943) in the random‐effects model (Fig. 3).
Publication bias
The funnel plot is presented in Figure 5a. Of a total of 30 data points, 9 lay outside the triangle, and 13 lay on the right side of the triangle altitude. No significant publication bias was observed by the Egger regression test (P = 0.284).
Figure 5.
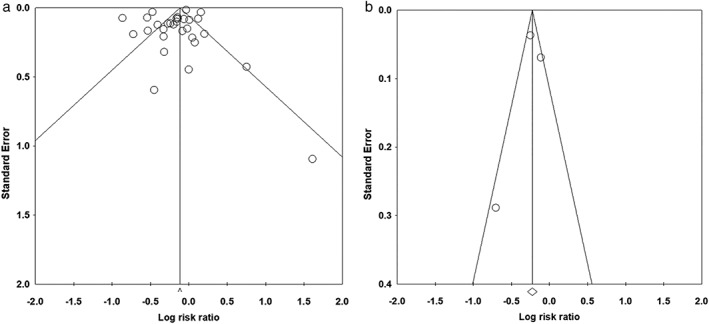
Funnel plot of possible publication bias of the effect of aspirin use on (a) gastric cancer incidence and (b) gastric cancer death.
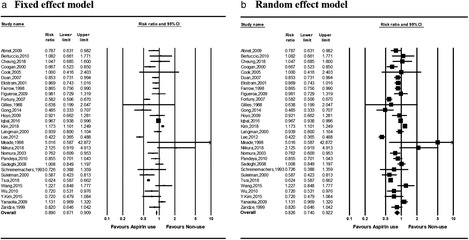
Aspirin use and risk of gastric cancer death
Three studies examined the effect of aspirin use on gastric cancer death. The direction of the risk ratios was not consistent between the studies (Fig. 4). The summary risk ratios in aspirin users compared with nonusers were 0.798 (0.749–0.850) and 0.894 (0.780–1.024) in the fixed‐ and random‐effects models, respectively. No significant heterogeneity was observed among studies in the fixed‐effects model (P = 0.054). No significant publication bias was observed based on the Egger regression test of funnel asymmetry (P = 0.805; Fig. 5b).
Figure 4.
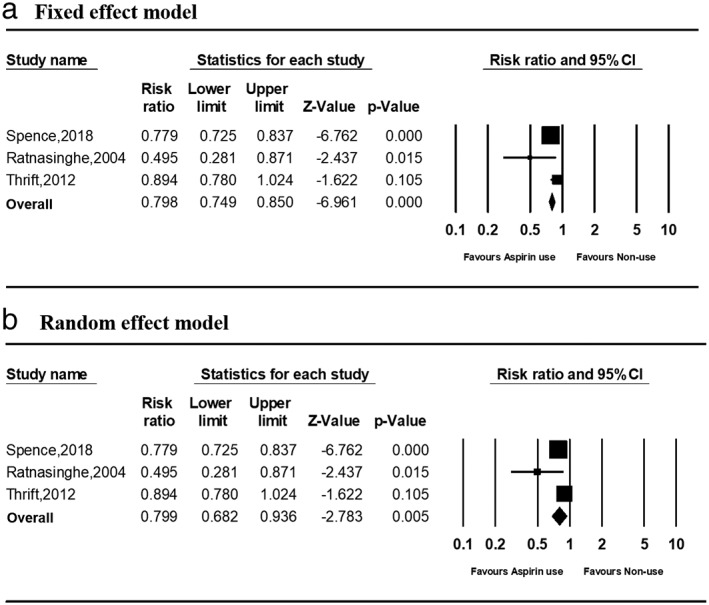
Forest plots of risk ratios for gastric cancer death in aspirin users versus nonusers in (a) fixed‐ and (b) random‐effect models. CI, confidence interval.
Discussion
Aspirin use was associated with the decreased risk of gastric cancer incidence and death. A chemopreventive effect was observed with high‐frequency aspirin use, Asian and North American populations, and noncardiac gastric cancer. No significant chemopreventive effect was observed in long‐term aspirin users or European populations.
Aspirin use contributed to a significant reduction in gastric cancer incidence, which is in agreement with previous meta‐analyses.6, 7, 8, 9, 10, 11, 12, 13, 14 Cancer angiogenesis inhibition and NF‐kappa B activation‐induced tumor apoptosis may inhibit gastric cancer incidence,47, 48 which may lead to a decrease in gastric cancer death. A dose‐dependent inverse association between aspirin use and gastric cancer incidence was confirmed. The risk of gastric cancer incidence was reduced by approximately 20% with weekly or daily aspirin use.
The subgroup analysis of geographic location indicated a stronger chemoprotective effect of aspirin in Asia compared to Europe. Asian populations have a higher H. pylori infection rate, and the observed effect of aspirin may have been confounded by H. pylori infection. Several studies have adjusted for the risk of H. pylori; however, the influence of H. pylori has not been adequately studied. In addition, differences in bacterial virulence genes, such as cytotoxin‐associated gene A (CagA), may have an effect. Previous meta‐ and sensitivity analyses (Table S4) have observed a preventive effect of aspirin on gastric cancer in H. pylori‐infected populations. Aspirin may suppress H. pylori‐associated inflammation, resulting in gastric cancer prevention. Other unmeasured confounding factors may also be involved; for example, aspirin users may receive more H. pylori eradication therapy opportunities than nonusers.
The subgroup analysis indicated that aspirin use for a duration of <5 years had a chemopreventive effect, while aspirin for >5 years did not. These nonlinear associations are consistent with previous meta‐analyses. The majority of studies evaluated aspirin use durations of <10 years. The sample size of patients on long‐term aspirin use may be insufficient to evaluate the association between aspirin use and risk of gastric cancer incidence. The underlying mechanisms are not well understood, and further research is required to evaluate the link between the duration of aspirin use and prevention of carcinogenesis.
The subgroup analysis demonstrated an association between aspirin use and reduced noncardiac gastric cancer incidence; however, no effects were observed on cardiac gastric cancer. This may be due to different pathology and disease progression between cardiac and noncardiac gastric cancers.18, 49, 50 For example, cyclooxygenase‐2 overexpression is generally lower in cardiac gastric cancer than noncardiac gastric cancer.51 It is also possible that noncardiac gastric cancer is associated with H. pylori, while cardiac cancer is not.52
This updated meta‐analysis is the first to demonstrate a preventive effect of aspirin on gastric cancer incidence and death. Several subgroup analyses reviewed the effect of frequency and duration of aspirin use, location of cancer, and geographic location. These results are useful in helping to identify the optimal aspirin use for reducing gastric cancer incidence. This study was limited by the fact that most studies were observational rather than RCTs. In addition, detailed study information from the RCTs (e.g. chemotherapy trials) was not available from the published data. Observational studies may include biased data and unmeasured confounding factors. No publication bias was observed for gastric cancer incidence and death. The placebo effect of aspirin use must also be considered. A high‐quality RCT, with gastric cancer incidence or death as the primary outcome, is required to investigate the chemopreventive effect of aspirin further.
This study found aspirin use to be associated with reduced gastric cancer incidence and death. Greater chemopreventive effects were observed with weekly and daily aspirin use, <5 years duration of use, and Asian and North American populations, particularly for noncardiac gastric cancer.
Supporting information
Table S1 Search queries for PUBMED.
Table S2 Assessment of the case–control studies using the Newcastle‐Ottawa scale.
Table S3 Assessment of the Cohort studies using the Newcastle‐Ottawa scale.
Table S4 Subgroup of H. pylori infectious states of relative risk for gastric cancer incidence.
Declaration of conflict of interest: All authors declare no conflicts of interest in this study.
Financial support: This study was supported by KAKENHI Grants‐in‐Aid for Scientific Research (grant numbers 17K15928 [Ryota Niikura], 17K09326, 18KT0067 [Yoshihiro Hirata]), the Asahi Life Foundation (Ryota Niikura), and Manpei Suzuki Diabetes Foundation (Yoshihiro Hirata). The funding agents had no role in the design of the study, data collection and analyses, decision to publish, or preparation of the manuscript.
References
- 1. Hayakawa Y, Sethi N, Sepulveda AR, Bass AJ, Wang TC. Oesophageal adenocarcinoma and gastric cancer: should we mind the gap? Nat. Rev. Cancer. 2016; 16: 305–18. [DOI] [PubMed] [Google Scholar]
- 2. Hayakawa Y, Fox JG, Wang TC. The origins of gastric cancer from gastric stem cells: lessons from mouse models. Cell. Mol. Gastroenterol. Hepatol. 2017; 3: 331–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chan AT, Giovannucci EL, Meyerhardt JA, Schernhammer ES, Curhan GC, Fuchs CS. Long‐term use of aspirin and nonsteroidal anti‐inflammatory drugs and risk of colorectal cancer. JAMA. 2005; 294: 914–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shiff SJ, Rigas B. Nonsteroidal anti‐inflammatory drugs and colorectal cancer: evolving concepts of their chemopreventive actions. Gastroenterology. 1997; 113: 1992–8. [DOI] [PubMed] [Google Scholar]
- 5. Jones MK, Wang H, Peskar BM et al Inhibition of angiogenesis by nonsteroidal anti‐inflammatory drugs: insight into mechanisms and implications for cancer growth and ulcer healing. Nat. Med. 1999; 5: 1418–23. [DOI] [PubMed] [Google Scholar]
- 6. Qiao Y, Yang T, Gan Y et al Associations between aspirin use and the risk of cancers: a meta‐analysis of observational studies. BMC Cancer. 2018; 18: 288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kong P, Wu R, Liu X et al The effects of anti‐inflammatory drug treatment in gastric cancer prevention: an update of a meta‐analysis. J. Cancer. 2016; 7: 2247–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ye X, Fu J, Yang Y, Gao Y, Liu L, Chen S. Frequency‐risk and duration‐risk relationships between aspirin use and gastric cancer: a systematic review and meta‐analysis. PLoS One. 2013; 8: e71522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Algra AM, Rothwell PM. Effects of regular aspirin on long‐term cancer incidence and metastasis: a systematic comparison of evidence from observational studies versus randomised trials. Lancet Oncol. 2012; 13: 518–27. [DOI] [PubMed] [Google Scholar]
- 10. Liao LM, Vaughan TL, Corley DA et al Nonsteroidal anti‐inflammatory drug use reduces risk of adenocarcinomas of the esophagus and esophagogastric junction in a pooled analysis. Gastroenterology. 2012; 142: 442.e5–52.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yang P, Zhou Y, Chen B et al Aspirin use and the risk of gastric cancer: a meta‐analysis. Dig. Dis. Sci. 2010; 55: 1533–9. [DOI] [PubMed] [Google Scholar]
- 12. Abnet CC, Freedman ND, Kamangar F, Leitzmann MF, Hollenbeck AR, Schatzkin A. Non‐steroidal anti‐inflammatory drugs and risk of gastric and oesophageal adenocarcinomas: results from a cohort study and a meta‐analysis. Br. J. Cancer. 2009; 100: 551–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wang WH, Huang JQ, Zheng GF, Lam SK, Karlberg J, Wong BC. Non‐steroidal anti‐inflammatory drug use and the risk of gastric cancer: a systematic review and meta‐analysis. J. Natl. Cancer Inst. 2003; 95: 1784–91. [DOI] [PubMed] [Google Scholar]
- 14. Huang XZ, Chen Y, Wu J et al Aspirin and non‐steroidal anti‐inflammatory drugs use reduce gastric cancer risk: a dose‐response meta‐analysis. Oncotarget. 2017; 8: 4781–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rothwell PM, Wilson M, Elwin CE et al Long‐term effect of aspirin on colorectal cancer incidence and mortality: 20‐year follow‐up of five randomised trials. Lancet. 2010; 376: 1741–50. [DOI] [PubMed] [Google Scholar]
- 16. Wang Y, Shen C, Ge J, Duan H. Regular aspirin use and stomach cancer risk in China. Eur. J. Surg. Oncol. 2015; 41: 801–4. [DOI] [PubMed] [Google Scholar]
- 17. Kim MH, Chang J, Kim WJ, Banerjee S, Park SM. Cumulative dose threshold for the chemopreventive effect of aspirin against gastric cancer. Am. J. Gastroenterol. 2018; 113: 845–54. [DOI] [PubMed] [Google Scholar]
- 18. Epplein M, Nomura AM, Wilkens LR, Henderson BE, Kolonel LN. Nonsteroidal antiinflammatory drugs and risk of gastric adenocarcinoma: the multiethnic cohort study. Am. J. Epidemiol. 2009; 170: 507–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bertuccio P, Bravi F, Bosetti C, Negri E, La Vecchia C. Aspirin and gastric cancer risk. Eur. J. Cancer Prev. 2010; 19: 426–7. [DOI] [PubMed] [Google Scholar]
- 20. Cheung KS, Chan EW, Wong AYS et al Aspirin and risk of gastric cancer after Helicobacter pylori eradication: a territory‐wide study. J. Natl. Cancer Inst. 2018; 110: 743–9. [DOI] [PubMed] [Google Scholar]
- 21. Sadeghi S, Bain CJ, Pandeya N, Webb PM, Green AC, Whiteman DC. Aspirin, nonsteroidal anti‐inflammatory drugs, and the risks of cancers of the esophagus. Cancer Epidemiol. Biomarkers Prev. 2008; 17: 1169–78. [DOI] [PubMed] [Google Scholar]
- 22. Figueroa JD, Terry MB, Gammon MD et al Cigarette smoking, body mass index, gastro‐esophageal reflux disease, and non‐steroidal anti‐inflammatory drug use and risk of subtypes of esophageal and gastric cancers by P53 overexpression. Cancer Causes Control. 2009; 20: 361–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Langman MJ, Cheng KK, Gilman EA, Lancashire RJ. Effect of anti‐inflammatory drugs on overall risk of common cancer: case‐control study in general practice research database. BMJ. 2000; 320: 1642–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hoyo C, Schildkraut JM, Murphy SK et al IGF2R polymorphisms and risk of esophageal and gastric adenocarcinomas. Int. J. Cancer. 2009; 125: 2673–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pandeya N, Webb PM, Sadeghi S, Green AC, Whiteman DC; Australian Cancer Study. Gastro‐oesophageal reflux symptoms and the risks of oesophageal cancer: are the effects modified by smoking, NSAIDs or acid suppressants? Gut. 2010; 59: 31–8. [DOI] [PubMed] [Google Scholar]
- 26. Schreinemachers DM, Everson RB. Aspirin use and lung, colon, and breast cancer incidence in a prospective study. Epidemiology. 1994; 5: 138–46. [DOI] [PubMed] [Google Scholar]
- 27. Kim YI, Kim SY, Kim JH et al Long‐term low‐dose aspirin use reduces gastric cancer incidence: a nationwide cohort study. Cancer. Res. Treat. 2016; 48: 798–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gillies M, Skyring A. Gastric ulcer, duodenal ulcer and gastric carcinoma: a case‐control study of certain social and environmental factors. Med. J. Aust. 1968; 2: 1132–6. [DOI] [PubMed] [Google Scholar]
- 29. Cook NR, Lee IM, Gaziano JM et al Low‐dose aspirin in the primary prevention of cancer: the women's health study: a randomized controlled trial. JAMA. 2005; 294: 47–55. [DOI] [PubMed] [Google Scholar]
- 30. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Stat. Med. 2002; 21: 1539–58. [DOI] [PubMed] [Google Scholar]
- 31. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ. 1997; 315: 629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Coogan PF, Rosenberg L, Palmer JR et al Nonsteroidal anti‐inflammatory drugs and risk of digestive cancers at sites other than the large bowel. Cancer Epidemiol. Biomarkers Prev. 2000; 9: 119–23. [PubMed] [Google Scholar]
- 33. Duan L, Wu AH, Sullivan‐Halley J, Bernstein L. Nonsteroidal anti‐inflammatory drugs and risk of esophageal and gastric adenocarcinomas in Los Angeles county. Cancer Epidemiol. Biomarkers Prev. 2008; 17: 126–34. [DOI] [PubMed] [Google Scholar]
- 34. Akre K, Ekstrom AM, Signorello LB, Hansson LE, Nyren O. Aspirin and risk for gastric cancer: a population‐based case‐control study in Sweden. Br. J. Cancer. 2001; 84: 965–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Farrow DC, Vaughan TL, Hansten PD et al Use of aspirin and other nonsteroidal anti‐inflammatory drugs and risk of esophageal and gastric cancer. Cancer Epidemiol. Biomarkers Prev. 1998; 7: 97–102. [PubMed] [Google Scholar]
- 36. Fortuny J, Johnson CC, Bohlke K et al Use of anti‐inflammatory drugs and lower esophageal sphincter‐relaxing drugs and risk of esophageal and gastric cancers. Clin. Gastroenterol. Hepatol. 2007; 5: 1154.e3–9.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gong EJ, Ahn JY, Jung HY et al Risk factors and clinical outcomes of gastric cancer identified by screening endoscopy: a case‐control study. J. Gastroenterol. Hepatol. 2014; 29: 301–9. [DOI] [PubMed] [Google Scholar]
- 38. Iqbal U, Yang HC, Jian WS, Yen Y, Li YJ. Does aspirin use reduce the risk for cancer? J. Invest. Med. 2017; 65: 391–2. [DOI] [PubMed] [Google Scholar]
- 39. Lee J, Lee SH, Hur KY, Woo SY, Kim SW, Kang WK. Statins and the risk of gastric cancer in diabetes patients. BMC Cancer. 2012; 12: 596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Thrombosis prevention trial: randomised trial of low‐intensity oral anticoagulation with warfarin and low‐dose aspirin in the primary prevention of ischaemic heart disease in men at increased risk. The Medical Research Council's general practice research framework. Lancet. 1998; 351: 233–41. [PubMed] [Google Scholar]
- 41. Niikura R, Hayakawa Y, Hirata Y et al Distinct chemopreventive effects of aspirin in diffuse and intestinal‐type gastric cancer. Cancer Prev. Res. (Phila.). 2018; 11: 279–86. [DOI] [PubMed] [Google Scholar]
- 42. Nomura AM, Hankin JH, Kolonel LN, Wilkens LR, Goodman MT, Stemmermann GN. Case‐control study of diet and other risk factors for gastric cancer in Hawaii (United States). Cancer Causes Control. 2003; 14: 547–58. [DOI] [PubMed] [Google Scholar]
- 43. Suleiman UL, Harrison M, Britton A, McPherson K, Bates T. H2‐receptor antagonists may increase the risk of cardio‐oesophageal adenocarcinoma: a case‐control study. Eur. J. Cancer Prev. 2000; 9: 185–91. [PubMed] [Google Scholar]
- 44. Tsoi KKF, Ho JMW, Chan FCH, Sung JJY. Long‐term use of low‐dose aspirin for cancer prevention: a 10‐year population cohort study in Hong Kong. Int. J. Cancer. 2019; 145: 267–73. [DOI] [PubMed] [Google Scholar]
- 45. Wu CY, Wu MS, Kuo KN, Wang CB, Chen YJ, Lin JT. Effective reduction of gastric cancer risk with regular use of nonsteroidal anti‐inflammatory drugs in Helicobacter pylori‐infected patients. J. Clin. Oncol. 2010; 28: 2952–7. [DOI] [PubMed] [Google Scholar]
- 46. Zaridze D, Borisova E, Maximovitch D, Chkhikvadze V. Aspirin protects against gastric cancer: results of a case‐control study from Moscow, Russia. Int. J. Cancer. 1999; 82: 473–6. [DOI] [PubMed] [Google Scholar]
- 47. Stark LA, Reid K, Sansom OJ et al Aspirin activates the NF‐kappaB signalling pathway and induces apoptosis in intestinal neoplasia in two in vivo models of human colorectal cancer. Carcinogenesis. 2007; 28: 968–76. [DOI] [PubMed] [Google Scholar]
- 48. Din FV, Dunlop MG, Stark LA. Evidence for colorectal cancer cell specificity of aspirin effects on NF kappa B signalling and apoptosis. Br. J. Cancer. 2004; 91: 381–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Marrelli D, Pedrazzani C, Corso G et al Different pathological features and prognosis in gastric cancer patients coming from high‐risk and low‐risk areas of Italy. Ann. Surg. 2009; 250: 43–50. [DOI] [PubMed] [Google Scholar]
- 50. Kamangar F, Dawsey SM, Blaser MJ et al Opposing risks of gastric cardia and noncardia gastric adenocarcinomas associated with Helicobacter pylori seropositivity. J. Natl. Cancer Inst. 2006; 98: 1445–52. [DOI] [PubMed] [Google Scholar]
- 51. Ratnasinghe D, Tangrea JA, Roth MJ et al Expression of cyclooxygenase‐2 in human adenocarcinomas of the gastric cardia and corpus. Oncol. Rep. 1999; 6: 965–8. [DOI] [PubMed] [Google Scholar]
- 52. Kang JM, Kim N, Shin CM et al Predictive factors for improvement of atrophic gastritis and intestinal metaplasia after Helicobacter pylori eradication: a three‐year follow‐up study in Korea. Helicobacter. 2012; 17: 86–95. [DOI] [PubMed] [Google Scholar]
- 53. Spence AD, Busby J, Johnston BT et al Low‐Dose Aspirin Use Does Not Increase Survival in 2 Independent Population‐Based Cohorts of Patients With Esophageal or Gastric Cancer. Gastroenterology. 2018; 154: 849–60. [DOI] [PubMed] [Google Scholar]
- 54. Thrift AP, Nagle CM, Fahey PP, Smithers BM, Watson DI, Whiteman DC. Predictors of survival among patients diagnosed with adenocarcinoma of the esophagus and gastroesophageal junction. Cancer Causes Control. 2012; 23: 555–64. [DOI] [PubMed] [Google Scholar]
- 55. Thrombosis prevention trial: randomised trial of low‐intensity oralanticoagulation with warfarin and low‐dose aspirin in the primary prevention ofischaemic heart disease in men at increased risk . The Medical Research Council's General Practice Research Framework. Lancet 1998; 351: 233–41. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Search queries for PUBMED.
Table S2 Assessment of the case–control studies using the Newcastle‐Ottawa scale.
Table S3 Assessment of the Cohort studies using the Newcastle‐Ottawa scale.
Table S4 Subgroup of H. pylori infectious states of relative risk for gastric cancer incidence.


