Abstract
Background
Evidence for the effectiveness of nutritional supplements containing protein and energy, often prescribed for older people, is limited. Malnutrition is more common in this age group and deterioration of nutritional status can occur during illness. It is important to establish whether supplementing the diet is an effective way of improving outcomes for older people at risk from malnutrition.
Objectives
This review examined trials for improvement in nutritional status and clinical outcomes when extra protein and energy were provided, usually as commercial 'sip‐feeds'.
Search methods
We searched The Cochrane Library, MEDLINE, EMBASE, Healthstar, CINAHL, BIOSIS, CAB abstracts. We also hand searched nutrition journals and reference lists and contacted 'sip‐feed' manufacturers.
Selection criteria
Randomised and quasi‐randomised controlled trials of oral protein and energy supplementation in older people, with the exception of groups recovering from cancer treatment or in critical care.
Data collection and analysis
Two reviewers independently assessed trials prior to inclusion and independently extracted data and assessed trial quality. Authors of trials were contacted for further information as necessary.
Main results
Sixty‐two trials with 10,187 randomised participants have been included in the review. Maximum duration of intervention was 18 months. Most included trials had poor study quality. The pooled weighted mean difference (WMD) for percentage weight change showed a benefit of supplementation of 2.2% (95% confidence interval (CI) 1.8 to 2.5) from 42 trials. There was no significant reduction in mortality in the supplemented compared with control groups (relative risk (RR) 0.92, CI 0.81 to 1.04) from 42 trials. Mortality results were statistically significant when limited to trials in which participants (N = 2461) were defined as undernourished (RR 0.79, 95% CI 0.64 to 0.97).
The risk of complications was reduced in 24 trials (RR 0.86, 95% CI 0.75 to 0.99). Few trials were able to suggest any functional benefit from supplementation. The WMD for length of stay from 12 trials also showed no statistically significant effect (‐0.8 days, 95% CI ‐2.8 to 1.3). Adverse effects included nausea or diarrhoea.
Authors' conclusions
Supplementation produces a small but consistent weight gain in older people. Mortality may be reduced in older people who are undernourished. There may also be a beneficial effect on complications which needs to be confirmed. However, this updated review found no evidence of improvement in functional benefit or reduction in length of hospital stay with supplements. Additional data from large‐scale multi‐centre trials are still required.
Plain language summary
Protein and energy supplementation in elderly people at risk from malnutrition
Much emphasis is placed on the importance of good diet, usually in relation to concern about the health risks of obesity. However it has been generally agreed that the risk of undernutrition rather than overnutrition is the main cause for concern in elderly people, particularly those who are hospitalised or institutionalised. Malnutrition has been shown to have important effects on recovery in a broad range of patients and conditions. It has been associated strongly with impaired immune response, impaired muscle and respiratory function, delayed wound healing, overall increased complications, longer rehabilitation, greater length of hospital stay and increased mortality. Oral protein and energy supplements are potentially safer and easier to administer than nasogastric enteral feeds and are therefore particularly suited to elderly people and are also widely used. However, there may be problems with the willingness and ability of older people to consume oral supplements, and supplements may not be used effectively. Even if supplements are prescribed, they may not always be given, or are given but not consumed. In addition to taste, the composition and timing of administration in relation to meals may be important. Efforts also need to be made to provide normal meals and snacks which meet the needs of elderly people and to provide assistance with feeding if required.
A total of 10,187 randomised participants from the 62 trials has been included. Maximum duration of intervention was 18 months. The reviewers suggest that supplementation appears to produce a small but consistent weight gain. There was no evidence in this updated review of a beneficial effect on mortality overall, but there may be a beneficial effect on mortality in people who are undernourished. Supplementation may also reduce the number of complications. The reported acceptance of supplements was variable between trials. Some adverse effects such as nausea or diarrhoea were reported. However, there were problems of study design and quality. More studies are required to confirm the beneficial effect on the number of complications, to establish whether there is a beneficial effect on mortality for undernourished elderly people and to provide evidence about whether protein and energy supplements can improve morbidity and functional status in frail older people.
Background
Description of the condition
Much emphasis is placed on the importance of good diet, usually in relation to concern about the health risks of obesity. However it has been generally agreed that the risk of undernutrition rather than overnutrition is the main cause for concern in elderly people, particularly those who are hospitalised or institutionalised (DoH 1992; Potter 1988). There have been recent UK and international initiatives to improve practice in this area of health care (Council Europe 2002; NHSQIS 2003; NICE 2006).
There is no universally accepted clinical definition of malnutrition or undernutrition (the terms are used interchangeably here). However, Allison 2000 defined undernutrition as "a state of energy, protein or other specific nutrient deficiency which produces a measurable change in body function and is associated with worse outcome from illness as well as being specifically reversed by nutritional support".
Increased length of hospital stay is associated with malnutrition. Studies have reported the presence of malnutrition in a substantial proportion of hospital patients both on admission and during hospital stay in the USA, Norway, Ireland, UK, Sweden, The Netherlands and Australia (Bistrian 1976; Bruun 1999; Corish 2000; Edington 2000; Flodin 2000; Kruizenga 2003; Zador 1987). This is a particular problem for elderly people as a) over 40% of hospital admissions are elderly people who have longer periods of illness and longer hospital stay (HCUP 2002), and b) data show that elderly patients are more at risk of malnutrition than others (Gallager‐Allred 1996; Kruizenga 2003; McWhirter 1994).
Reasons for poor nutritional status in older people are multi‐faceted and include the physiological, psychological and social changes associated with aging which affect food intake and body weight, exacerbated by the presence of illness. Five to ten percent of elderly people in the community may also be malnourished (McCormack 1997). Elderly people who are already malnourished at home may be at a disadvantage if admitted to hospital for treatment. Within Europe and the USA, nutritional status has been shown to decline with hospital stay due to a lack of adequate nutritional intake during hospitalisation (Corish 2000; Larsson 1990; McWhirter 1994; Sullivan 1999). This is because the poor nutritional state of many patients often goes unrecognised and there may be a lack of awareness of malnutrition by health professionals who receive little training on nutritional issues (Elia 2001). Disease or treatment such as surgery may also increase nutritional demands, so patients who have a poor appetite or difficulty eating will lose weight.
Measurement of nutritional status
Commonly used methods to measure nutritional status are body mass index (BMI) (weight in kg / height in m2), anthropometry such as triceps skin fold thickness and arm muscle circumference, and history of recent weight loss. Serum albumin has also been used as a measure of nutritional status as malnutrition causes a decrease in the rate of synthesis of albumin, but serum albumin levels are known to be affected by changes in fluid balance and illness itself. Illness, injury and age may all therefore confound the measurement of nutritional status. Improved tools for nutrition risk screening have been developed, which include subjective measures of recent weight loss and inadequate intake (BAPEN 2003). The difficulty of defining and measuring nutritional status may help explain some of the wide variation in the reported prevalence of malnutrition in hospitalised adults of between 11% and 40% (Corish 2000).
Effects of malnutrition
Malnutrition has been shown to have important effects on recovery in a broad range of patients and conditions. It impacts on both physiological and biochemical systems and has been associated strongly with impaired immune response, impaired muscle and respiratory function, delayed wound healing, overall increased complications, longer rehabilitation, greater length of hospital stay and increased mortality (Kelly 1984; Potter 1995; Robinson 1987; Sullivan 1990; Windsor 1988). Apathy, depression, fatigue and a loss of will to recover have been demonstrated following weight loss in experimental volunteers (Keys 1950).
Costs
The economic consequences of malnutrition are also considerable. In 1992 the economic cost to the United Kingdom National Health Service of preventable malnutrition was estimated to be £266 (297 EUR, 2009 currency translation) million a year, mainly due to increased length of bed occupancy and associated treatment costs (Lennard‐Jones 1992). More recently, it has been estimated that the annual additional health care cost of malnutrition and associated disease is over £5.3 (5,9 EUR, 2009 currency translation) billion in the UK (Elia 2005). However more studies which gather information regarding the cost‐effectiveness of nutritional support are required.
Description of the intervention
Oral supplements are potentially safer and easier to administer than nasogastric enteral feeds and are therefore particularly suited to elderly people and are also widely used. However, there may be problems with the willingness and ability of older people to consume oral supplements, and supplements may not be used effectively. Even if supplements are prescribed, they may not always be given, or are given but not consumed (Peake 1998). In addition to taste, the macronutrient composition and timing of administration in relation to meals may be important (Wilson 2002). Efforts also need to be made to provide normal meals and snacks which meet the needs of elderly people and to provide assistance with feeding if required.
Why it is important to do this review
A systematic review in 1998 examined the effects of oral and enteral protein and energy supplementation in adults from thirty eligible trials which were identified up to the end of 1996 (Potter 1998). Outcomes assessed were change in body weight and arm muscle circumference, and case fatality. There were indications that nutritional supplementation was associated with improvements in outcomes assessed. However, uncertainties remained, because of the poor quality of included trials.
The present Cochrane review of older adults, when last updated and published in January 2005 included 49 trials with 4790 randomised participants. Most trials had poor study quality. Results suggested a beneficial effect of supplementation for percentage weight change from 34 trials (weighted mean difference (WMD) 2.3% (95% confidence interval (CI) 1.9 to 2.7) and a reduced mortality in the supplemented groups compared to the control groups from 32 trials (relative risk (RR) 0.74, 95% confidence interval (CI) 1.9 to 2.7).
Other reviews have included a review of randomised and non randomised studies in different diagnostic groups with chronic non malignant disorders (Akner 2001) suggesting that patients with certain disorders (such as hip fracture) may be more likely to benefit than others. Stratton 2003 and colleagues also extensively reviewed the evidence base for nutritional support in a recently published book, including a review of 166 randomised and non‐randomised trials of oral nutritional support published up to 2002 in all ages, across different disease groups, both in hospital and in the community.
A recent update of the systematic review for the Cochrane Collaboration (Avenell 2006), of nutritional supplementation for hip fracture aftercare in older people, included 21 trials involving 1727 participants. There was some evidence that oral protein and energy feeds (evaluated by eight trials), reduced unfavourable outcome (death or complications), but there was no demonstrable effect on deaths alone in participants recovering from hip fracture. However overall, the evidence was still weak due to defects in the reviewed studies, particularly inadequate size, methodology and outcome assessment.
A Cochrane systematic review of dietary advice for illness‐related malnutrition in adults of all ages has also been carried out (Baldwin 2008). Thirty‐six studies (37 comparisons) met the inclusion criteria with 2714 randomised participants. No comparison showed a significant difference in mortality. There were several significant results for change in weight and other nutritional indices favouring nutritional intervention, but it remains uncertain whether nutritional supplements and dietary advice produce the same effects. There was insufficient evidence to draw conclusions about clinical outcomes and cost. For specific information on dietary advice for illness related malnutrition, the reader is referred to Baldwin 2000.
Elderly people who are ill and malnourished may be expected to benefit more from supplementation. Providing higher energy supplements over a longer duration may also be expected to be associated with greater benefit. The present review includes a more comprehensive search for randomised trials to specifically examine the effectiveness of oral protein and energy supplements for elderly people.
Objectives
To assess the effects and acceptability of oral dietary supplements in both hospitalised elderly people and elderly people in the community, irrespective of setting:
to test the null hypothesis that there was no difference in outcomes between participants who were given oral nutritional supplements compared to those participants who were given no intervention, a placebo, or an alternative supplement with a different amount of calories and protein.
to carry out subgroup analysis in order to assess whether participants who were malnourished, were ill, were aged 75 years or over, were given supplements of 400 kcal or more or who had longer duration (35 days or more) of supplementation showed most benefit.
Methods
Criteria for considering studies for this review
Types of studies
Ideally studies were randomised controlled trials, but we also considered quasi‐randomised controlled trials (for example allocation by day of week, date of birth, alternation). We only accepted trials that had a minimum duration of two weeks (with a minimum duration of intervention of one week).
Types of participants
To be defined as elderly, groups of study participants had to have a minimum average age of 65 years. All groups were included, with the exception of groups exclusively of older people in critical care or recovering from cancer treatment who may have had specific nutritional needs relating to their condition. Mixed groups of patients, where some were recovering from cancer and some had been undergoing critical care were included. Efforts were made to obtain data for the groups of interest from the authors.
Types of interventions
Interventions were aimed at improving the intake of protein and energy using only the normal oral route. Protein was provided together with non‐protein energy sources such as carbohydrate and fat, and with or without added minerals and vitamins. We were interested in supplements in the form of:
commercial sip feeds;
milk based supplements;
via the fortification of normal food sources.
Studies of dietary advice alone were not included in this review. We also excluded studies of specially designed immunomodulatory supplements or supplements of specific amino acids. The comparison intervention was 'usual practice' (for example using no supplement or an alternative supplement with a different amount of calories and protein) or a placebo (for example a low energy drink). Although protein‐only supplementation has occasionally been used for experimental purposes, it would not be considered for routine use and was therefore excluded.
Types of outcome measures
Primary outcomes
(for all participants unless otherwise stated)
all cause mortality;
morbidity, number of people with complications (for example pressure sores, deep vein thrombosis, respiratory and urinary infections);
functional status (for example cognitive functioning, muscle functioning, mobility, ability to perform activities of daily living).
Secondary outcomes
participants' perceived quality of life, ideally using a validated scale;
length of hospital stay (hospital patients only);
number of primary care contacts (non‐hospital participants only);
adverse effects of nutritional supplementation;
level of care and support required;
number of hospital / care home admissions / re admissions;
nutritional status (change in anthropometry, for example percentage weight change, percentage change arm muscle circumference);
percentage change in dietary intake (energy and protein intake from food and supplements);
compliance with intervention (proportion of the supplement provided which is consumed, alone or with assistance);
economic outcomes.
Desired timing of outcome measures
The outcome measurements were evaluated at the last available time point of the studies. Short term outcomes were defined as up to three months, medium term outcomes 3 to 6 months and long term outcomes over six months.
Search methods for identification of studies
Electronic searches
Cochrane Central Register of Controlled Trials (Issue 4, 2007);
MEDLINE (until November 2007);
EMBASE (until December 2007);
Healthstar (until March 2001);
CINAHL (until November 2007);
BIOSIS (until December 2007);
CAB abstracts (until October 2007).
The MEDLINE search strategy was adapted for the other electronic databases searched.
The nutrition search strategy was based on the strategy used in a Cochrane review by one of the authors (Avenell 2004). Some additional terms relating to malnutrition and food sources were also included. Phases one and two of the search strategy for randomised controlled trials developed by the United Kingdom Cochrane Centre were used (Alderson 2004). For a detailed search strategy see Appendix 1.
Databases of registered trials were also searched:
Current Controlled Trials (www.controlled‐trials.com), December 2007.
The results were double‐checked with trials identified by two of the authors for previous systematic reviews: trials of routine protein energy supplementation in adults identified between February 1979 and July 1996 using MEDLINE (Potter 1998), and more recently, trials of nutritional supplementation for hip fracture aftercare in the elderly (Avenell 2004), searching The Cochrane Library, MEDLINE, EMBASE, Healthstar, CINAHL, BIOSIS and CAB abstracts.
Searching other resources
Handsearching
The following journals were hand searched:
Journal of Human Nutrition: Applied Nutrition: Vol 36A(1) 1982 ‐ Vol 41A(6) 1987;
Journal of Human Nutrition: Clinical Nutrition: Vol 36C(1) 1982 ‐ Vol 41C(6) 1987;
Journal of Human Nutrition and Dietetics: Vol 1(1) 1988 ‐ Vol 20(3) 2007;
Clinical Nutrition: Vol 1(1) 1982 ‐ Vol 26(6) 2007;
Journal of Parenteral and Enteral Nutrition: Vol 5(1) 1981 ‐ Vol 31(6) 2007;
Proceedings of the Nutrition Society: Vol 50(2) 1991 ‐ Vol 53(3) 1994 and Vol 57(1) 1998 ‐ Vol 66 2007;
Journal of the American Dietetic Association Vol 90(1) 1990 ‐ Vol 107(7) 2007;
American Journal of Clinical Nutrition Vol 62(10) 1995 ‐ Vol 86(4) 2007;
Australian Journal of Nutrition and Dietetics, which became Nutrition and Dietetics,1989 ‐ Vol 64(4) 2007.
The references of all retrieved studies and reviews were searched for additional trials. Books relating to geriatric medicine and nutrition were searched. Authors of published trials, colleagues, and manufacturers of nutritional supplements were contacted for overlooked, unpublished and ongoing trials.
Data collection and analysis
Selection of studies
For the present update, one reviewer (AA) carried out the search by scanning the titles, abstract sections and keywords of every record retrieved. Full articles were then retrieved for further assessment by two reviewers if the information given suggested that the study:
used random allocation to the comparison groups;
compared a protein and energy supplement with no intervention, a placebo or an alternative supplement;
involved participants who were over 65 years old;
assessed one or more relevant clinical outcome measure.
Articles were also retrieved if there was some doubt about eligibility. If necessary, trialists were contacted for further information on methodology and data. If no clarification had been provided, and there had been disagreement about eligibility for inclusion, the review group editorial base would have been consulted.
Data extraction and management
Information was independently extracted by two reviewers (either AA and AV or AM and JP). All differences in data extraction were resolved by discussion with a third reviewer, referring back to the original article.
Information gathered included:
location;
participant description;
inclusion and exclusion criteria;
details and duration of intervention;
baseline characteristics of the individuals studied;
participant flow;
relevant outcome measures recorded.
If any data were missing in a published report (see data extraction list), trialists were contacted for further information.
Assessment of risk of bias in included studies
Methodological quality was assessed by two reviewers (either AA and AV or AM and JP) all differences were resolved by discussion with a third reviewer if necessary. A sensitivity analysis was carried out based on the quality assessment. The assessment protocol scored each item between nil and two as described below. In addition, risk of pre‐allocation disclosure of assignment was rated A, B or C according to the Cochrane Handbook 1997. The following aspects of internal and external validity was reported and assessed:
a) Was the assigned treatment adequately concealed prior to allocation? 2 = method did not allow disclosure of assignment (A) 1 = chance of disclosure of assignment or states random but no description (B) 0 = quasi‐randomised (C)
b) Were the outcomes of patients who withdrew included in the analysis (intention to treat)? 2 = intention to treat analysis based on all cases randomised possible or carried out 1 = states number and reasons for withdrawal but intention to treat analysis not carried out 0 = withdrawals not mentioned, intention to treat analysis not possible
c) Were the outcome assessors blinded to treatment status? 2 = action taken to blind assessors, or outcomes such that bias was unlikely 1 = chance of unblinding of assessors 0 = not mentioned
d) Were the treatment and control group comparable at entry? 2 = good comparability of groups, or confounding adjusted for in analysis 1 = confounding possible; mentioned but not adjusted for 0 = large potential for confounding, or not discussed
e) Were care programmes, other than the trial options, identical? 2 = care programmes identical 1 = differences in care programmes but unlikely to influence study outcomes 0 = not mentioned or differences in care programmes likely to influence study outcomes
f) Were the inclusion and exclusion criteria clearly defined? 2 = clearly defined 1 = inadequately defined 0 = not defined
g) Were the interventions clearly defined (including estimates of nutritional value)? 2 = clearly defined interventions were applied with a standardised protocol 1 = clearly defined interventions were applied but the application protocol was not standardised 0 = intervention and/or application protocol were poorly or not defined
h) Were the participants blind to assignment status following allocation? 2 = effective action taken to blind subjects 1 = small or moderate chance of unblinding subjects 0 = not mentioned (unless double‐blind), or not done
i) Were the treatment providers blind to assignment status? 2 = effective action taken to blind treatment providers 1 = small or moderate chance of unblinding of treatment providers 0 = not mentioned (unless double‐blind), or not done
j) Was the overall duration of surveillance clinically appropriate? 2 = optimal (six months or more) 1 = adequate (one up to six months) 0 = not defined, or not adequate
Data synthesis
Data were combined for meta‐analysis for dichotomous variables mortality and number of patients with complications as described in the protocol. In those studies where the data were reported as the total number of complications instead of the number of affected patients, it was assumed that there was one outcome per patient. For each study relative risks and 95% confidence limits were calculated, the results were combined using fixed‐effect models and presented with 95% confidence limits. Where there was evidence of heterogeneity a random‐effects model was applied. Heterogeneity between comparable trials was explored using the I2 test (Higgins 2003) using more than 50% as the cut‐off for significant heterogeneity. A funnel plot to assess small study bias for mortality data was also carried out.
Data for length of hospital stay were combined for meta‐analysis as a continuous variable. Data were combined for meta‐analysis from studies which provided length of stay data as mean number of days and standard deviation. If the data were provided as the median and interquartile range, the median was used instead of the mean and the standard deviation estimated from the interquartile range. Weighted mean differences and 95% confidence intervals were calculated using a fixed‐effect model which assumes the same underlying effect in all studies and considers any heterogeneity between trials to be due to random errors. A random‐effects model was also used if there was any evidence of heterogeneity.
Data were also combined for meta‐analysis for percentage weight change and arm muscle circumference (AMC) and as described in the protocol. Weighted mean difference and 95% confidence intervals were calculated using fixed‐ and random‐effects models as appropriate for changes in weight and anthropometry measures. The trials reported body weight and anthropometric measures in several ways. For meta‐analysis the same method was used to standardise data as used previously by Potter 1998. The mean and standard deviation of the percentage change in body weight during the trial period was selected as the measurement of choice because of its clinical relevance (Potter 1998). Where percentage weight change was not available the difference was calculated between the initial and final body weight, expressed as a percentage of baseline weight and a standard deviation of 10% inferred. This standard deviation was conservative, and at the upper limit of any of the observed results. As in Potter 1998, if baseline weight was not reported, a standard value of 60 kg was assumed, which applied to all patients regardless of their nutritional status. Assumptions made regarding standard deviations were checked by restricting the analysis to those trials where no inferences were made. As in Potter 1998, arm muscle circumference (AMC) was chosen as the anthropometry measure as it is both a measure of fat and muscle. Where this was not described in a trial it was derived from the mid‐arm circumference or mid‐upper arm circumference (MAC / MUAC) and triceps skinfold (TSF) using a standard formula (Gurney 1973). Anthropometry data were then pooled as weight data.
Data were also combined for meta‐analysis for change in handgrip strength where this was provided or could be calculated from the data provided. Weighted mean difference and 95% confidence intervals were again calculated using the fixed‐ and random‐effects model as appropriate.
Subgroup analysis and investigation of heterogeneity
Subgroup analysis was carried out on the basis of:
baseline nutritional status (nourished, undernourished);
health status (healthy volunteers or ill patients);
mean age (less than 75 years, 75 years or more);
amount of kilocalories provided in supplement (less than 400 kcal, 400 kcal or more);
duration of intervention (less than 35 days, 35 days or more).
Additional post hoc subgroup analyses
As the result of comments resulting from the previous version of this review, additional subgroup analyses were carried out in order to provide a breakdown of the meta‐analyses on the basis of diagnostic group:
mortality by diagnostic group;
complications by diagnostic group;
length of hospital stay by diagnostic group;
percentage weight change by diagnostic group;
percentage arm muscle circumference change by diagnostic group.
Sensitivity analysis
Sensitivity analyses were carried out in order to explore the influence of the following factors on effect size:
repeating the analysis taking into account of study quality, as specified above.
repeating the analysis excluding a large study to establish how much it dominated the results.
repeating the analysis excluding studies using industry funding.
Results
Description of studies
In addition to the twenty‐one thousand titles / abstracts which were identified using the above database search strategies and through hand searching and reference list searching, in 2001, another twelve thousand titles / abstracts were identified for the 2005 update and an additional 28 studies identified for the present update (20 full reports, one ongoing study and seven abstracts). From the database search, reading the abstracts or reading the full article allowed most to be eliminated because they clearly did not meet the inclusion criteria. This left an additional 18 potentially relevant trials. Two reviewers independently assessed these trials and as a result of mutual agreement, 13 additional trials have been included to date (see Characteristics of included studies). The remaining were excluded (Characteristics of excluded studies) for a variety of reasons (for example no outcomes of interest, did not meet age or intervention criteria).
Contacts with authors
Requests for further information have been made. Of the 62 included trials, information on outcomes of interest and study quality was requested for 31 trials and has been obtained for 15 (Banerjee 1978; Brown 1992; Bruce 2003; Hankey 1993; Hankins 1996; Jensen 1997; Krondl 1999; Kwok 2001; Lauque 2000; MacFie 2000; Payette 2004; Potter 2001; Saudny 1997; Schols 1995; Yamaguchi 1998).
Trial design
Included studies were all randomised or quasi‐randomised controlled trials. The large study by Bourdel 2000 with 672 participants was cluster randomised and has not been included in the meta‐analysis, but the results have been included in the narrative part of the review. Young 2004 is also a cluster randomised crossover trial which following discussion with a statistician has been included in the meta‐analysis. Rosendahl 2006 is included in the meta‐analysis as it is clustered for exercise and individually randomised for supplementation.
Six of the included trials also examined the effects of exercise (Bonnefoy 2003; Daniels 2003; Fiatarone 1994; Meredith 1992; Rosendahl 2006; Schols 1995), with the same exercise component in both the supplemented and control groups. In the study by Bonnefoy 2003 a factorial design was used with participants receiving either exercise or memory training. In the study by Fiatarone 1994 there were sufficient data available to include the no exercise group only. In the studies by Schols 1995 and Tidermark 2004, groups of patients randomised to receive nandrolone decanoate has been excluded from the analysis. In the trial of surgical patients (Jensen 1997), there was an analysis of a subgroup of patients over 75 years, which has been used in this review.
Participants
A total of 10,187 randomised participants from the 62 trials has been included. Studies were carried out in Europe, USA, Canada, Australia and Hong Kong. Approximately 55% of participants were female (no information on gender was provided in seven studies). The mean age reported in studies varied from 65 to 88 years (not reported in seven studies). The number of participants in trials varied greatly between 10 (Brown 1992) and 4023 (FOOD trial 2005), 42 trials had fewer than 100 participants.
Although studies took place in a variety of settings, most participants (71%, 26 studies) were hospitalised in‐patients with acute conditions. Other participants were either in long‐stay / care of the elderly / continuing care wards or nursing homes (14%, 15 studies), or at home in the community (15%, 21 studies). Forty studies (48% participants) included older people with no specified disease or condition, except some trials where some or all patients had Alzheimer's disease. Other studies included patients with hip fracture (Brown 1992; Bruce 2003; Daniels 2003; Delmi 1990; Hankins 1996; Madigan 1994; Stableforth 1986; Tidermark 2004), stroke patients (FOOD trial 2005, Gariballa 1998), patients with congestive heart failure (CHF) (Broqvist 1994), patients with chronic obstructive pulmonary disease (COPD) (Deletter 1991; Knowles 1988; Saudny 1997; Schols 1995; Steiner 2003, Vermeeren 2004), older surgical patients (Jensen 1997; MacFie 2000) and patients at home with diabetic foot ulcer (Eneroth 2004). Apart from diagnosis being considered as a marker of nutritional risk (for example post‐surgery, COPD, hip fracture), 60% of participants in the included trials underwent screening and were then classified as actually being undernourished or at nutritional risk. The definition for this (for example weight, BMI, unable to eat independently) varied between studies and was often not provided, very few studies used weight loss as an indicator of nutritional risk. Separate data were provided for those well nourished and undernourished in two studies (Larsson 1990; Potter 2001) and these have been analysed separately as appropriate in this review.
Interventions
The interventions used in the trials aimed to provide between 175 additional kcal/day and up to a maximum of 1350 additional kcal/day. Additional protein was between 10 g protein/day and 50 g protein/day. Less than 400 kcal/day was provided in 20 trials, 400 kcal/day or more in 32 trials, and the energy supplemented was not known for ten trials.
Thirty‐five trials reported using supplements with at least some vitamins and minerals, or both, one gave equivalent vitamins to the control group (Carver 1995), one also gave extra vitamins to some patients in a factorial design (Vlaming 2001), two trials gave calcium and vitamin D supplements to both the intervention and control group (Hampson 2003; Tidermark 2004), 27 trials did not report vitamin content or it was unclear, although the majority of commercial supplements do provide vitamins and minerals. MacFie 2000 had a control group and three groups receiving supplements (pre‐operative supplements only, post‐operative supplements only, and both pre and post‐operative supplements), those receiving supplements have been grouped together.
When reported, supplements were given twice daily for around 31% of participants, but this could also be between one and four times or any number of times for the remaining participants. Thirty‐nine trials used named commercial supplements, the others did not specify a manufacturer. Commercial supplements may have been provided by the manufacturer free of charge, although this was not often explicitly stated. Extra milk alone was provided for one study (Barr 2000), and low lactose milk powder was used in one study (Kwok 2001).
The minimum time period of the intervention was 10 days, the maximum was 18 months. The length of time of the intervention was less than 35 days for 17 trials, and 35 days or more for 37 trials, from admission to discharge in five trials and the intervention period was unclear for two trials. The duration of follow‐up was generally the same as the duration of the intervention, and varied from one week to 18 months.
Outcomes assessed
Most trials assessed nutritional outcomes, particularly weight change and dietary intake, but also change in anthropometry. Mortality was not the principle outcome for most trials. Morbidity and complications and length of hospital stay were provided in a limited number of trials. The majority of trials also included a measure of functional status, however these were often disease specific and too diverse for meta analysis. Sixteen studies measured the effect of supplementation on quality of life. Only one trial provided data on cost effectiveness (Edington 2004).
Funnel plot
The funnel plot of the comparison "oral protein and energy versus routine care ‐ outcome: mortality" appeared to be symmetrical (Figure 1).
1.
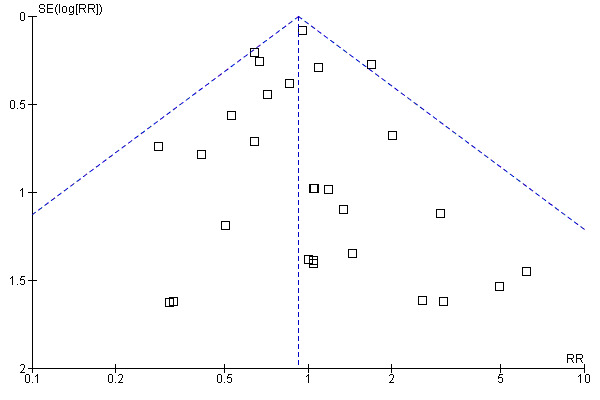
Funnel plot of comparison: 1 Oral protein and energy versus routine care, outcome: 1.1 Mortality.
Risk of bias in included studies
For details see Appendix 2. The method of scoring used is described above (quality assessment of trials). Concealment of allocation was confirmed in only 19 studies which used computer allocation or sealed opaque envelopes. A clear 'intention to treat' analysis was only carried out in 24 studies. The quality was poorest with regard to blinding. Action to ensure blinding of outcome assessors was reported in only 12 studies, action to blind participants in 16 studies, and blinding of treatment providers was reported in only 14 studies.
Effects of interventions
Primary outcomes
Mortality
Deaths were reported in 50 trials. Data from Banerjee 1978; Bonnefoy 2003; Rosendahl 2006; Saudny 1997; Schols 1995; Woo 1994 and Wouters 2005, have been excluded as further clarification from the authors was not received. Data on mortality from Bourdel 2000 have been excluded from the meta‐analysis because the trial was cluster randomised, however Bourdel 2000 found no significant difference in the incidence of death during the 15 day follow‐up: 25 in the nutritional intervention group and 22 in the control group (P = 0.18). The relative risk by the end of follow‐up from the remaining 42 trials (8031 participants) did not show a reduced mortality in supplemented compared with control groups (relative risk (RR) 0.92; 95% confidence interval (CI) 0.81 to 1.04), with no significant statistical heterogeneity (I2 = 0%). The results of fourteen of these trials fall out of the analysis because the effect measure could not be calculated for zero events (no deaths).
Subgroup analyses
The subgroup analyses suggested that the results were statistically significant or approaching statistical significance when limited to trials in which participants (N = 2461) were defined as undernourished (RR 0.79; 95% CI 0.64 to 0.97), and when 400 kcal or more was offered per day in the supplement (N = 7307), (RR 0.89; 95% CI 0.78 to 1.00).
Results were not significant, when analyses were limited to participants who were at least 75 years old (N = 2967), (RR 0.85; 95% CI 0.69 to 1.05), when supplementation was continued for 35 days or more (N = 2454), (RR 0.97, 95% CI 0.77 to 1.24), and when participants were unwell (N = 7636), (RR 0.92; 95% CI 0.81 to 1.04), and when participants were in hospital or in a nursing home (N = 6582), (RR 0.91; 95% CI 0.80 to 1.04), all with evidence of little heterogeneity.
The results of mortality were also not statistically significant when limited to trials when participants were not defined as undernourished (N = 5403), (RR 0.78; 95% CI 0.53 to 1.15 ‐ RR 0.98; 95% CI 0.83 to 1.14), when less than 400 kcal were offered per day in the supplement (N = 858), (RR 1.09; 95% CI 0.59 to 1.98), participants were less than 75 years old (N = 8049 ‐ N = 5082), (RR 0.91; 95% CI 0.80 to 1.03 ‐ RR 0.94; 95% CI 0.80 to 1.11), when supplementation was continued for less than 35 days (N = 5054), (RR 0.92; 95% CI 0.78 to 1.07), when participants had not been defined as unwell (N = 393), (RR 0.98; 95% CI 0.25 to 3.78), and when living in the community (N = 966), (RR 0.99; 95% CI 0.62 to 1.59).
Sensitivity analyses
The results were consistent when analysis was restricted to 15 trials (N = 6604) with clearly concealed randomisation (RR 0.91; 95% CI 0.79 to 1.03).
For six trials, there was co‐authorship with an employee of the manufacturer or full funding of the trial by a manufacturer of oral supplements (Edington 2004; Eneroth 2004; Krondl 1999; Lauque 2000; Lauque 2004; Salas‐Salvado 2005; Vermeeren 2004; Wouters 2002; Wouters 2003; Wouters 2006). The meta‐analysis of mortality data was also therefore carried out with the exclusion of these trials in order to explore the influence on effect size. Results with the remaining 29 trials, suggest that this had no demonstrable effect (RR 0.90; 95% CI 0.79 to 1.03).
Post‐hoc subgroup analyses for mortality based on diagnostic group suggested that the results were statistically significant when including only trials in patients with a variety of geriatric conditions (N = 2701), (RR 0.78; 95% CI 0.62 to 0.98), where most of these participants were in hospital. However, there was no demonstrable benefit for patients with hip fracture (N = 437), (RR 0.91; 95% CI 0.50 to 1.66) from eight small trials. The limited data from other diagnostic groups such as stroke patients and those with chronic obstructive pulmonary disease (COPD) did not suggest a reduction in mortality.
Morbidity
Data on morbidity were available from 28 trials. Data from Wouters 2005 were unsuitable for analysis without additional information from the authors. Data on development of pressure sores from Benati 2001 and Bourdel 2000 were also unsuitable for meta‐analysis. The results from Bourdel 2000 showed an increased risk of developing pressure ulcer in the control group versus the intervention group in 672 elderly patients (RR 0.57; 95% CI 1.03 to 2.38). Data from the remaining 24 trials (N = 6225), have been combined for meta‐analysis (Broqvist 1994; Delmi 1990; Gariballa 1998; Gariballa 2006; Potter 2001; Tidermark 2004 (infective complications); FOOD trial 2005; Hankins 1996; Larsson 1990 (total pressure sores); Hampson 2003; MacFie 2000; Madigan 1994; Saudny 1997; Wouters 2003 (patients too ill to continue used as a measure of complications); Steiner 2003 (exacerbation of COPD); Stableforth 1986 (anaesthetic, surgical infection, gastrointestinal, urinary); Daniels 2003; Lauque 2004; Vermeeren 2004; Young 2004 (hospital readmission); Collins 2005; Eneroth 2004; (incomplete wound healing); Price 2005 (prescription of antibiotics); Salas‐Salvado 2005 (total severe adverse events). Data on complications from Lauque 2000 have not been included because further clarification from the authors is required. The risk of complications by the end of follow‐up in supplemented groups was statistically significantly different from the control groups (RR 0.86; 95% CI 0.75 to 0.99), with no significant statistical heterogeneity. Subgroup analyses for complications based on diagnostic group suggested that there may be a reduced risk of complications with supplementation for hip fracture patients only (RR 0.60; 95% CI 0.40 to 0.91).
Functional status
Functional status measures were very diverse, few were able to suggest any functional benefit with supplementation. One or more of the following measures of mobility were included in 14 studies: number of falls, activity rating, mobility, physical activity, walking, stair climbing, timed up and go (Bonnefoy 2003; Brown 1992; Deletter 1991; Fiatarone 1994; Gray‐Donald 1995; Hampson 2003; Larsson 1990; Madigan 1994; Payette 2004; Schols 1995; Saudny 1997; Steiner 2003; Tidermark 2004; Wouters 2003). In mixed groups of elderly people Gray‐Donald 1995 reported that the number of falls was lower among supplemented participants than controls (0% versus 21%; P = 0.05). Larsson 1990 reported a significant improvement in the activity rating in the supplemented group at eight weeks compared to the control group (P < 0.05) due mainly to improvement in the initially well nourished patients, however the number of patients studied was not clear. Muscle function and mobility were measured by Bonnefoy 2003, there was a short term improvement in quadriceps muscle power at three months with supplementation (56.8%; P = 0.03) but this was not sustained at nine months. Bonnefoy 2003 found no statistically significant effect on six meters walk, five time chair rise, or six stair climb at 3 and 6 months. Wouters 2003 also found no significant effect of supplementation on a timed 'up and go' test (Podsiadlo 1991), although Payette 2004 found a trend towards improvement in this test (P = 0.057).
In patients following hip fracture Madigan 1994 found that patients given supplements did significantly less well. The number of patients unable to reach goal two of physio‐independent mobility was significantly greater in the intervention group (5 versus 1; P < 0.01). Tidermark 2004 also failed to demonstrate any beneficial effect of supplementation on mobility in women with hip fracture. Hampson 2003 found no difference in level of physical activity in elderly community‐living women with osteoporosis given supplements.
Walking distance or velocity was assessed in four studies of patients with COPD, (Deletter 1991; Saudny 1997; Schols 1995; Steiner 2003) with no statistically significant improvement with supplementation reported, however a non significant trend towards improvement in 12 minute walking distance after nine weeks in the supplemented group compared to the control group was noted by Deletter 1991(65 m versus 16 m; P > 0.05). Activities of daily living (ADL) (Katz 1963; Mahoney 1965) was measured in 11 studies (Barr 2000; Bruce 2003; Gariballa 1998; Hankins 1996; Lauque 2004; Potter 2001; Tidermark 2004; Volkert 1996; Woo 1994; Wouters 2002; Wouters 2006). Overall only one study demonstrated a significant improvement at end of follow‐up. Woo 1994 reported a significant difference between the groups with a lower level of functional ability after three months in the control group in patients following chest infection (20 versus 19.5; P < 0.01). Tidermark 2004, however found an improvement at six months (P < 0.05) but no difference at 12 months (number of participants remaining independent 11/16 in the control group versus 14/17 in the intervention group (from graph)). Potter 2001 reported a significant improvement with supplementation only in a subgroup of very malnourished patients (17 versus 11; P < 0.04). Volkert 1996 found an improvement in the ADL score from admission to six months only in the subgroup with good acceptance of the supplement (72% versus 39%; P < 0.05). There was no improvement in frail elderly functional capacity (FEFA) in the study by Manders 2006.
No statistically significant effect of supplementation was reported for hand grip strength in 13 studies (Edington 2004; Gray‐Donald 1995; Kwok 2001; Lauque 2000; MacFie 2000; Manders 2006; Payette 2002; Price 2005; Saudny 1997; Steiner 2003; Tidermark 2004; Wouters 2003; Vermeeren 2004). There was a trend towards improvement in grip strength for COPD patients in the study by Steiner 2003 (0.64 kg force versus ‐0.05; P = 0.06). There was also a trend towards short term improvement in the study by Edington 2004 after eight weeks (1.2 versus ‐0.5; P = 0.055) but with no difference at 24 weeks. Price 2005 reported that the intervention group (when analysed by intention‐to ‐treat) showed a greater increase in handgrip strength over 12 weeks compared to the control group which of borderline statistical significance (P = 0.055). Seven studies (Manders 2006; Payette 2002; Price 2005; Steiner 2003; Tidermark 2004; Wouters 2003; Vermeeren 2004) (535 participants) provided data on the change in handgrip which could be combined for meta‐analysis. Results suggest that supplementation had no demonstrable effect (weighted mean difference (WMD) 0.06; 95% CI ‐0.60 to 0.72).
There was no evidence of an improvement in dynamic strength (maximum weight lifted on a 'thigh‐knee' machine) (Meredith 1992), both the supplemented and control groups gained dynamic strength (P < 0.001) with no effect of diet. There was also no significant effect of the supplement on balance, gait or lower limb strength (Rosendahl 2006). Calf circumference was significantly improved at 24 weeks of supplementation in the study by Manders 2006.
Four studies involving older patients with COPD measured changes in lung function with supplementation. There was a statistically significant improvement in lung function [maximal inspiratory mouth pressure measured in kiloPascals (kPa)] in non tissue depleted patients with COPD between pre and post intervention, which was not seen in the control group (Schols 1995) (0.8 kPa versus 0.5 kPa; P < 0.05), but only in the first four weeks, and in Saudny 1997 forced vital capacity (percentage predicted) improved in the supplemented group as compared with the control group (8.7% versus ‐3.5%; P = 0.015). Deletter 1991 and Vermeeren 2004 however found no evidence of change in ventilatory performance in the supplemented group.
There was no evidence of an improvement in cognitive function between groups with supplementation (Collins 2005; Gariballa 2006; Lauque 2004; Salas‐Salvado 2005; Young 2004).
Secondary outcomes
Health‐related quality of life
Quality of life was ascertained using a variety of measures (general and disease specific well‐being and self perceived health questionnaires (for example SF36, hospital anxiety and depression score (HADS), Nottingham Health Profile (NHP), EQ5D and Self Reported Chronic Respiratory Questionnaire) in 16 studies (Barr 2000; Collins 2005; Edington 2004; FOOD trial 2005; Gariballa 2006; Price 2005; Hampson 2003; Krondl 1999; MacFie 2000; Payette 2002; Saudny 1997; Scorer 1990; Steiner 2003; Tidermark 2004; Woo 1994; Wouters 2002). Collins 2005 was unusable because result between groups was not reported. Gariballa 2006 reported SF36 in a subgroup of patients suggesting significant improvement in physical and social score. Hampson 2003 reported that more women 'felt better' in the supplemented group (48% versus 20%; P = 0.029). Saudny 1997 reported a greater improvement in well‐being in the supplemented group that was not statistically significant (12 points versus ‐10 points; P = 0.07). In the study by Edington 2004, although there was no effect on overall EQ5D score or for the visual analogue scale, the supplemented group reported fewer mobility problems at 24 weeks (P = 0.022). In the study by Krondl 1999, scores for vitality and general health perception increased more from baseline to termination in the supplemented group (P < 0.01), however it was not clear whether these were within or between group differences. No other meaningful between group differences were found.
Length of hospital stay
Length of hospital stay was measured in 12 studies (Brown 1992; Bruce 2003; Delmi 1990; FOOD trial 2005; Gariballa 1998; Gariballa 2006; Hankins 1996; MacFie 2000; Madigan 1994; Potter 2001; Tidermark 2004; Vlaming 2001). Data were analysed separately for three groups in the study by Potter 2001(severely malnourished, moderately malnourished and adequately nourished). Data from Gazzotti 2003 require further clarification from the authors before inclusion. Data were combined for meta‐analysis from studies which provided length of stay data as mean number of days and standard deviation (SD) (Brown 1992; Bruce 2003; FOOD trial 2005; Gariballa 2006; Hankins 1996; Madigan 1994; Vlaming 2001), the SD was assumed to be 10 days in one study (MacFie 2000), based on the SDs for length of stay from the other studies. The median was used instead of the mean and SD estimated from the interquartile range for the four remaining studies (Delmi 1990; Gariballa 1998; Potter 2001; Tidermark 2004). The pooled weighted mean difference for length of stay using a random‐effects model showed no benefit from supplementation ‐0.8 days (‐2.8 to 1.3) with significant heterogeneity (chi‐square 25.53; df 13; P = 0.02; I2 = 49%). Subgroup analyses for length of stay were too limited to suggest any difference between diagnostic groups.
Adverse effects
Eighteen trials discussed adverse effects from supplementation, in most cases no comparison with the control group was performed, six reported no adverse effects (Delmi 1990; McWhirter 1996; Potter 2001; Saudny 1997; Tidermark 2004; Wouters 2002). Problems with tolerance and side‐effects were reported in 12 studies: Eneroth 2004 reported nausea, vomiting and diarrhoea in the intervention group. FOOD trial 2005 reported 28% stopped their supplements before discharge (refusal, weight gain, unwanted, nausea), Gariballa 2006 reported 20% nausea in both groups. Hankins 1996 reported dysphagia, nausea, diarrhoea and fatigue as reasons for drop‐out from the study in four out of 17 patients; Fiatarone 1994 reported diarrhoea in two out of 49 participants; Gazzotti 2003 reported loss of appetite, nausea or diarrhoea in five out of 39 patients; Price 2005 reported intolerance to supplements as reason for withdrawal in 20% of participants and significantly more gastro‐intestinal adverse events in the intervention group. Ovesen 1992 excluded ten out of 37 participants from the study because they refused to continue due to gastro‐intestinal discomfort attributed to the supplements; Vermeeren 2004 reported that nausea caused dropouts in three of 29 patients in the supplemented group and in one out of 27 patients in the control group. Stableforth 1986 stated that "intolerance of the supplements proved to be a handicap in correcting the deficits in many patients".
Nutritional status
Weight change
Measures of weight were converted into percentage weight change to allow data from 42 trials with 3058 participants to be included in the meta‐analysis. Percentage change in body mass index (BMI) was used as a proxy measure for weight in one study (Bonnefoy 2003). A standard deviation of 10% was assumed in 20 studies as described previously.
There was a mean weight loss during the trial period for the supplemented group in seven trials, this contrasts with a mean weight loss in 23 trials for the control group. The pooled weighted mean difference for percentage weight change showed a benefit of supplementation of 2.2% (1.8 to 2.5) with no significant heterogeneity (chi‐square 52.35; df 43; P = 0.16; I2 =17.5%). This would mean an average weight gain of 1.2 kg for a person weighing 55 kg.
Sensitivity analysis of weight change data
When analysis was restricted to 18 trials where no inference was made regarding standard deviations the results remained consistent for weighted mean difference 2.1% (1.7 to 2.5).
Subgroup analyses for weight change
Subgroup analyses for weight change based on diagnostic group confirmed a significant increase in weight for mixed group of patients with geriatric conditions, weighted mean difference 2.7% (2.2 to 3.1), and for patients with chest conditions, weighted mean difference 1.6% (1 to 2.2). This could not be confirmed for other patient groups with the limited data available.
Arm muscle circumference (AMC)
Fifteen trials with 1382 participants reported a measure of AMC which was, or could be calculated from mid‐upper arm circumference and triceps skinfold thickness (Gurney 1973) and combined in a meta‐analysis. The pooled weighted mean difference for percentage AMC change using a fixed‐effect model showed a benefit of supplementation of 1.2% (0.5 to 2), with no significant heterogeneity (chi‐square 20.75; df 15; P = 0.15; I2 = 27.7%).
Subgroup analyses for percentage arm muscle circumference
Subgroup meta‐analyses for percentage AMC change based on diagnostic group were unable to confirm a statistically significant difference in AMC for any diagnostic group where more than one trial could be included.
Intake
Thirty‐two studies using a variety of methods for example dietary recall and weighed intake, reported that supplementation increased daily protein intake and energy intake, or both (Banerjee 1978; Barr 2000; Bourdel 2000; Broqvist 1994; Brown 1992; Deletter 1991; Delmi 1990; Edington 2004; Gariballa 1998; Gazzotti 2003; Hampson 2003 (within group change); Hankey 1993; Hankins 1996; Jensen 1997; Knowles 1988; Krondl 1999; Lauque 2000; MacFie 2000 (pre‐operative only); McWhirter 1996; Meredith 1992; Ovesen 1992; Payette 2002; Payette 2004; Potter 2001; Price 2005; Saudny 1997; Stableforth 1986; Steiner 2003 (within group change); Vermeeren 2004; Woo 1994; Yamaguchi 1998; Young 2004). Wouters 2003 reported no compensation of energy intake from usual diet had occurred with additional supplements. Volkert 1996 found that a statistically significant increase in protein intake during hospital stay was limited to 55% of patients in the intervention group with good acceptance of the supplement. In one study (Fiatarone 1994), supplementation was associated with significant reductions in total energy and protein from habitual diet, and energy intake was only significantly increased in exercising participants who also received nutritional supplementation. Six studies did not find that intake was significantly increased (Gray‐Donald 1995; Kwok 2001; Lauque 2004; Madigan 1994; Salas‐Salvado 2005, Wouters 2006), although reasons for this were not clear. Intake was not reported or not clear in the remaining 21 studies.
Compliance (acceptance of the supplement)
Acceptance was reported to be good in 17 studies although this was often not or variously defined (Barr 2000; Broqvist 1994; Carver 1995; Collins 2005; Delmi 1990; Fiatarone 1994; Gazzotti 2003; Krondl 1999; Kwok 2001; Lauque 2000; Lauque 2004; McWhirter 1996; Potter 2001; Rosendahl 2006; Steiner 2003; Vermeeren 2004; Wouters 2002). Bonnefoy 2003 reported 54% compliance at nine months, FOOD trial 2005 reported 76% compliance, Manders 2006 reported 67% compliance in the 111 out of 176 who completed the study. Payette 2002 found that 55% of participants were compliant at four months, Price 2005 reported 62% compliance overall, Scorer 1990 excluded patients who were unable to consume two cans supplement per day. Vlaming 2001 reported that 63% of 222 participants took 50% or more of the sip‐feed supplement in hospital and Wouters 2003 found no difference in compliance between the intervention and placebo products over six months: 85% (SD 36%) versus 94% (SD 24%) respectively. Bruce 2003 reported that poor compliance in patients after hip fracture had limited the effectiveness of the supplements despite encouragement and strategies offered by the dietitian (mean compliance 20.6/28 cans) due to 'taste problems'. Problems with acceptance were reported in the study by Gray‐Donald 1995, where 36% of potentially eligible participants refused to participate mainly because they did not wish to take a nutritional supplement; of those that did take part, compliance was realised by 68% (measured by counting supplements during a home visit on a weekly basis). Larsson 1990 found that 39 out of 197 patients refused the supplement and were withdrawn from the study and therefore not included in the analysis. Volkert 1996 reported data from 45% of participants who had poor acceptance of the supplements, but stated that "if taken they were well tolerated".
Other outcomes
Insufficient data were provided from these trials to examine other outcomes listed in the protocol, which included number of primary care contacts, level of care and support required. Edington 2004 measured heath care professionals services and social service costs over a period of 24 weeks following hospitalisation with or without eight weeks of supplementation of elderly malnourished patients on discharge from hospital. There was no reduction in health care costs with supplementation.
Discussion
Summary of main results
Supplementation appears to produce a small but consistent weight gain. There was no beneficial effect on mortality overall, but there was a reduction in mortality in undernourished groups and in general geriatric populations which were mainly hospitalised patients. There was a beneficial effect on the number of complications, particularly in hip fracture patients. There was no difference in the length of hospital stay. There was little evidence of benefit to functional outcomes or quality of life.The updated review was dominated by the large international FOOD trial 2005 which tested the hypothesis that adding oral protein‐energy supplements to standard hospital diet until discharge would improve outcome at six months after stroke. The trial contributed 4023 of the 10,187 randomised participants in this review, with only a minority (8%) of the FOOD trial 2005 participants classified as undernourished at baseline. The results of the trial suggest that it is unlikely that routine oral supplementation in well nourished stroke patients is useful. The trial did not answer the question about whether routine oral supplements may have a role to play in the management of undernourished patients.Care must be taken with the inclusion criteria for participants in order to achieve external validity. Trials ideally should have included a representative sample of elderly people who would under normal circumstances be eligible for oral nutrition support. Severely malnourished patients who are likely to benefit most have not been included in many trials for ethical reasons. Furthermore, many trials did not adequately screen participants for nutrition risk or malnutrition. The inclusion of marginal candidates for nutrition support in trials may mask the benefits of treatment (Wolfe 1997).
Mortality
The subgroup comparison of 'undernourished' versus 'not undernourished' was based on a definition of undernourished that was not the same for all trials. Unfortunately there were not enough data provided to stratify the subgroups using a standard measure of nutrition risk such as body mass index or recent weight loss. No single trial was adequately powered or long enough to investigate mortality as a primary outcome.
Morbidity, functional status and quality of life
The definition of complications was also not the same for all trials although most complications were infection related.
Few studies were able to provide data on improvements in functional status or quality of life in general, apart from handgrip data. Measures were too diverse or too limited to combine for meta‐analyses.
Length of stay
The FOOD trial 2005 with a slightly longer average stay for supplemented stroke patients dominated the results for the meta‐analysis of length of hospital stay with supplementation, although non significant, the trend is still towards a slightly shorter stay for supplemented patients.
Nutritional status
The pooled weighted mean difference for percentage weight change in this study showed a small but consistent benefit from supplementation, and there was no evidence that assuming a standard deviation in so many trials biased the results. The evidence from most trials suggested that supplements, if taken, produce weight gain. However we do not know the composition of that weight gain, which could be a gain in fat mass, muscle mass or both. In terms of providing functional benefit, a gain in fat mass may have cosmetic benefit but will not improve muscle strength. Results from Fiatarone 1994 suggested that exercise is also required to produce a significant improvement in muscle strength and function. Trials involving patients living at home provided supplements for periods of between six weeks and six months, with quite modest gains in weight. In the absence of more evidence of benefit for older people in the community, long‐term supplementation at home may not be cost‐effective.
The pooled weighted mean difference for percentage change in arm muscle circumference (which is a measure of both lean and fat tissue) in this study suggested a very small benefit of supplementation.
Intake
It has been suggested that nutritional supplementation may significantly reduce the intake of ordinary diet (Bastow 1983), and this obviously would reduce the effectiveness of supplements. However, the majority of studies reported that supplementation significantly increased total daily protein intake, energy intake or both. It should, however, be remembered that it is very difficult to measure nutrient intake with any degree of accuracy, and few assessors of intake were reported as being blinded to treatment status.
There was one notable exception to the findings above. Results of the trial by Fiatarone 1994 suggested that after 10 weeks of supplementation the supplemented participants did not increase their total energy intake significantly compared to the controls, despite high compliance with the supplements, which was offset by a reduction in normal food intake.
We also do not know yet which component(s) of the supplement may be providing the beneficial effect if any, specifically, whether it is actually the energy and protein that is important or the provision of extra vitamins and minerals from the supplement or all of these. Assessing this was made difficult because it was not clear in many studies whether vitamins and mineral were actually included in the supplements.
At least 10 trials also included some dietary advice as part of the intervention. Furthermore, participants at home received regular phone calls or visits. It is not known what influence this might have had, although it seems likely that this would improve compliance and outcomes. A recent systematic review of dietary advice alone to patients with illness related malnutrition (Baldwin 2008), would suggest that it is difficult to disentangle the different effects of advice and supplements.
Compliance (acceptance of the supplement)
The literature suggests that under normal conditions acceptance of supplements can be a problem for elderly people. This review does not include studies examining only the acceptability in terms of taste of different kinds of supplements for this age group. Problems with acceptance or adverse events such as nausea, gastro‐intestinal discomfort were reported in a few studies. From this review there was no evidence that participants at home were less compliant with supplementation than those in hospital or long term care.
Potential biases in the review process
The review was limited because the quality rating of included trials as reported was poor, particularly for blinding of outcome assessors, participants and treatment providers. Blinding of participants and treatment providers cannot be done without a placebo, and it can be difficult to have an untreated arm for ethical reasons in some trials. Without a placebo group bias may result from supplemented patients receiving a higher standard of care and attention from treatment providers. It should be possible however, to design a trial where the outcome assessors are blinded to the treatment allocation. The fact that this was only done (or at least reported) in 17 studies was a major deficiency of the review and may bias the results towards finding a more beneficial effect.
Analysis of outcomes on an 'intention‐to‐treat basis' was also deficient and this also potentially represents a source of bias. There was inadequate reporting of numbers of participants who were allocated and assessed, and reasons for losses to follow‐up were often not reported. Some patients were excluded from the analysis because they were unable or willing to take the supplements. If the outcomes from all patients had been included in the analysis, then the results would be more representative of the effectiveness of supplements under real life conditions.
The beneficial effect of supplementation on mortality was still evident when only higher quality trials with adequate allocation concealment were included, and also when trials with substantial proprietary involvement were excluded from the analysis. There was therefore no evidence that the commercial trials that did report on mortality, were more likely to report favourable outcomes. But there is a general concern that selective reporting of outcomes such as mortality, could have introduced bias.
Authors' conclusions
Implications for practice.
The results of the present review, which included more than twice the number of patients than the previous version of this review, supports the findings of the previous review in that there is a small weight gain, but no longer supports the finding that there is a beneficial effect on mortality overall. However, mortality in undernourished patients may be reduced. There is more evidence of a reduction in complications than in the previous review. Results however still require to be substantiated as there are doubts due to many included trials having poor study quality.
Although proprietary sip feeds have become a widely accepted means of improving nutritional status, it is not enough to provide supplements and hope for the best. Under normal circumstances patients should have a variety of options for increasing intake. In hospital or long‐term care, at the very least, a choice of attractive and acceptable food should be offered along with dietary advice if required. Furthermore, elderly people may become more malnourished because they do not get assistance with feeding on a busy ward, and encouragement and assistance may be all that they require. There were too few randomised trials that had considered other methods of supplementation such as altering the nutrient density and diversity of the diet, which may be preferable to sip‐feeds for some elderly people, and also few trials which had tried to improve the way that the sip‐feeds were provided in order to improve acceptability and reduce wastage. However proprietary protein and energy supplements used appropriately with nutritionally 'at risk' patients have a useful role to play as part of a raft of measures which should be used to improve the intakes and nutritional status of older people in hospital or long‐term care.
For older people in the community who are at risk, it is also important to consider both dietary ways (for example meals‐on‐wheels) and non‐dietary ways (for example treatment of depression, correction of dental problems and exercise regimes) of improving intakes and nutritional status before they are admitted to hospital. More evidence of benefit from oral nutritional supplements for older people at risk of malnutrition in the community is still required.
Implications for research.
Large scale multi‐centre pragmatic trials of interventions to improve nutritional status of elderly people, particularly malnourished elderly people from clearly defined patient groups (such as patients with hip fracture) are still required. Most individual studies in this review had an intervention time that was too short to have a realistic chance of detecting differences in morbidity, functional status or quality of life. Future trials need to have sufficient statistical power and length of follow‐up to be able to detect any beneficial effects. Future trials of nutritional supplementation should also have properly concealed allocation, blinding and follow‐up of all participants to ensure that those who are not able to consume the supplements, are included (intention‐to‐treat analysis). Trials should also focus more on primary outcomes of relevance to patients such as improvement in function or quality of life measures.
What's new
| Date | Event | Description |
|---|---|---|
| 30 November 2007 | New citation required and conclusions have changed | The last updated and published review in November 2004 included 49 trials with 4790 randomised participants. Most trials had poor study quality. Results suggested a beneficial effect of supplementation for percentage weight change from 34 trials (weighted mean difference (WMD) 2.3% (95% confidence interval (CI) 1.9 to 2.7) and a reduced mortality in the supplemented groups compared to the control groups from 32 trials (relative risk (RR) 0.74, 95% confidence interval (CI) 1.9 to 2.7). Sixty‐two trials with 10,187 randomised participants have been included in the current update. Most included trials had poor study quality. The pooled weighted mean difference (WMD) for percentage weight change showed a benefit of supplementation of 2.2% (95% confidence interval (CI) 1.8 to 2.5) from 42 trials. There was no significant reduction in mortality in the supplemented compared with control groups (relative risk (RR) 0.92, CI 0.81 to 1.04) from 42 trials. Mortality results were statistically significant when limited to trials in which participants (N = 2461) were defined as undernourished (RR 0.79, 95% CI 0.64 to 0.97). The risk of complications was reduced in 24 trials (RR 0.86, 95% CI 0.75 to 0.99). Few trials were able to suggest any functional benefit from supplementation. The WMD for length of stay from 12 trials also showed no statistically significant effect (‐0.8 days, 95% CI ‐2.8 to 1.3). |
History
Protocol first published: Issue 4, 2001 Review first published: Issue 3, 2002
| Date | Event | Description |
|---|---|---|
| 14 October 2004 | New search has been performed | First update: New studies found and included or excluded: 4/1/04 Conclusions changed: 10/9/04 |
Acknowledgements
We would like to thank Christine Baldwin, Professor AK Banerjee, Dr Daniel Bunout, Dr Alison Kretser, Ann Coulston, Dr S Lauque, Diane Palmer, Mr J MacFie, Dr Catherine Hankey, Dr Katrina Brown, Professor Maria Krondl, Professor A Schols, Professor T Kwok, Professor J Woo, Dr MB Jensen, Elizabeth Weekes, Rosemary Price, Helga Saudny‐Unterberger, Jackie Edington, Dr D Bruce, Dr Hélène Payette and Dr Ian Cameron who took time to provide further information about the trials. Magnus McGee, Craig Ramsey and Marion Campbell for statistical advice and Dr Helen Handoll who was involved in the data extraction of trials included in this review from the review by Avenell 2004.
Appendices
Appendix 1. Search strategy
| Search terms |
| Unless otherwise stated, search terms were free text terms; exp = exploded MeSH: Medical subject heading (Medline medical index term); the dollar sign ($) stands for any character(s); the question mark (?) = to substitute for one or no characters; tw = text word; pt = publication type; sh = MeSH: Medical subject heading (Medline medical index term); adj = adjacency.
MEDLINE 1. nutrition [MeSH, all subheadings included] 2. nutri* (textword) 3. maln* (textword) 4. undernutr* (textword) 5. under‐nutr* (textword) 6. undernourish* (textword) 7. under‐nourish* (textword) 8. protein‐energy malnutrition [MeSH, all subheadings included] 9. protein‐energy malnutrition (textword) 10. nutritional status [MeSH, all subheadings included] 11. nutrition disorders [MeSH, all subheadings included] 12. food,fortified [MeSH, all subheadings included] 13. food,formulated [MeSH, all subheadings included] 14. diet [MeSH, all subheadings included] 15. diet therap* (textword) 16. dietary supplements [MeSH, all subheadings included] 17. (diet* or nutri*) near supplement* (textword) 18. enteral nutrition [MeSH, all subheadings included] 19. dietary proteins [MeSH, all subheadings included] 20. energy intake [MeSH, all subheadings included] 21. randomized controlled trial.pt. 22. controlled clinical trial.pt. 23. randomized controlled trials.sh. 24. random allocation.sh. 25. double‐blind method.sh. 26. single‐blind method.sh. 27. or/1‐26 28. limit 27 to animal 29. limit 27 to human 30. 28 not 29 31. 27 not 30 32. clinical trial.pt. 33. exp clinical trials/ 34. (clinic$ adj25 trial$).tw. 35. ((singl$ or doubl$ or trebl$ or tripl$) adj (mask$ or blind$)).tw. 36. placebos.sh. 37. placebo$.tw. 38. random$.tw. 39. research design.sh. 40. (latin adj square).tw. 41. or/32‐40 42. limit 41 to animal 43. limit 41 to human 44. 42 not 43 45. 41 not 44 46. comparative study.sh. 47. exp evaluation studies/ 48. follow‐up studies.sh. 49. prospective studies.sh. 50. (control$ or prospectiv$ or volunteer$).tw. 51. cross‐over studies.sh. 52. or/46‐51 53. limit 52 to animal 54. limit 52 to human 55. 53 not 54 56. 52 not 55 57. 31 or 45 or 56 58. obesity [MeSH, all subheadings included] 59. critical care [MeSH, all subheadings included] 60. 58 or 59 61. or/1‐20 62. 61 not 60 63. 62 and 57 64. limit 63 to (newborn infant or infant <1 to 23 months> or preschool child <2 to 5 years> or child <6 to 12 years> or adolescence <13 to 18 years> or adult <19 to 44 years>) 65. 63 not 64 |
Appendix 2. Risk of bias
| Study | a) A/C b) ITT | c) assessor blind | d) comparability | e) identical care | f) in/exclusion | g) interventions | h) participant blind | i) carers blind | j) duration |
| Banerjee 1978 | 1 2 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 1 |
| Barr 2000 | 1 1 | 0 | 2 | 1 | 2 | 0 | 0 | 0 | 0 |
| Benati 2001 | 1 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Bonnefoy 2003 | 2 1 | 0 | 1 | 2 | 1 | 2 | 2 | 2 | 2 |
| Bourdel 2000 | 1 2 | 1 | 2 | 0 | 1 | 1 | 0 | 0 | 0 |
| Broqvist 1994 | 1 2 | 2 | 1 | 0 | 2 | 1 | 2 | 2 | 1 |
| Brown 1992 | 0 2 | 2 | 1 | 0 | 2 | 0 | 0 | 0 | 0 |
| Bruce 2003 | 0 1 | 0 | 2 | 1 | 2 | 1 | 0 | 0 | 2 |
| Carver 1995 | 0 1 | 1 | 2 | 2 | 2 | 2 | 2 | 0 | 1 |
| Collins 2005 | 1 0 | 1 | 2 | 2 | 2 | 2 | 2 | 2 | 1 |
| Daniels 2003 | 2 2 | 1 | 2 | 0 | 2 | 1 | 0 | 0 | 1 |
| Deletter 1991 | 1 2 | 0 | 2 | 0 | 2 | 0 | 0 | 0 | 1 |
| Delmi 1990 | 1 1 | 0 | 2 | 0 | 2 | 2 | 0 | 0 | 2 |
| Edington 2004 | 2 1 | 0 | 0 | 0 | 2 | 2 | 0 | 0 | 1 |
| Eneroth 2004 | 1 1 | 2 | 0 | 2 | 2 | 0 | 2 | 2 | 2 |
| Fiatarone 1994 | 1 2 | 0 | 2 | 2 | 2 | 2 | 2 | 2 | 1 |
| FOOD 2005 | 2 2 | 0 | 2 | 0 | 2 | 1 | 0 | 0 | 2 |
| Gariballa 1998 | 2 2 | 1 | 1 | 2 | 1 | 0 | 0 | 0 | 1 |
| Gariballa 2006 | 2 2 | 2 | 2 | 0 | 2 | 2 | 2 | 2 | 2 |
| Gazzotti 2003 | 1 1 | 0 | 2 | 0 | 2 | 0 | 0 | 0 | 1 |
| Gegerle 1986 | 1 2 | 0 | 2 | 0 | 0 | 2 | 0 | 0 | 0 |
| Gray‐Donald 1995 | 1 2 | 1 | 2 | 0 | 2 | 1 | 0 | 0 | 1 |
| Hankey 1993 | 1 0 | 0 | 0 | 2 | 1 | 1 | 0 | 0 | 1 |
| Hankins 1996 | 2 2 | 0 | 2 | 1 | 2 | 2 | 0 | 0 | 1 |
| Hampston 2003 | 2 1 | 0 | 1 | 0 | 2 | 1 | 0 | 0 | 2 |
| Hubsch 1992 | 1 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Jensen 1997 | 2 1 | 1 | 1 | 0 | 2 | 1 | 0 | 0 | 1 |
| Knowles 1988 | 1 2 | 2 | 1 | 0 | 2 | 1 | 0 | 0 | 1 |
| Krondl 1999 | 0 0 | 0 | 1 | 2 | 2 | 2 | 0 | 0 | 1 |
| Kwok 2001 | 0 1 | 2 | 0 | 2 | 2 | 2 | 0 | 0 | 1 |
| Larsson 1990 | 2 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 1 |
| Lauque 2000 | 1 1 | 0 | 2 | 0 | 2 | 2 | 0 | 0 | 1 |
| Lauque 2004 | 1 1 | 0 | 2 | 0 | 2 | 1 | 0 | 0 | 2 |
| MacFie 2000 | 2 1 | 0 | 2 | 2 | 2 | 0 | 0 | 0 | 1 |
| Manders 2006 | 1 1 | 2 | 2 | 0 | 2 | 2 | 2 | 2 | 1 |
| McEvoy 1982 | 1 2 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 |
| McWhirter 1996 | 1 1 | 0 | 2 | 0 | 1 | 2 | 0 | 0 | 1 |
| Madigan 1994 | 1 0 | 0 | 0 | 0 | 2 | 1 | 0 | 0 | 1 |
| Meredith 1992 | 1 1 | 0 | 2 | 2 | 2 | 1 | 0 | 0 | 1 |
| Ovesen 1992 | 1 1 | 1 | 0 | 2 | 2 | 1 | 2 | 2 | 0 |
| Payette 2002 | 1 2 | 1 | 2 | 0 | 2 | 0 | 0 | 0 | 1 |
| Payette 2004 | 1 0 | 1 | 0 | 0 | 2 | 1 | 0 | 0 | 1 |
| Potter 2001 | 2 2 | 1 | 1 | 2 | 2 | 2 | 0 | 0 | 0 |
| Price 2005 | 2 2 | 0 | 1 | 0 | 2 | 1 | 0 | 0 | 1 |
| Rosendahl 2006 | 2 2 | 2 | 2 | 1 | 2 | 2 | 2 | 2 | 2 |
| Salas‐Salvado 2005 | 1 1 | 0 | 2 | 0 | 2 | 1 | 0 | 0 | 1 |
| Saudny 1997 | 2 1 | 1 | 1 | 0 | 2 | 1 | 0 | 0 | 1 |
| Schols 1995 | 1 1 | 2 | 0 | 2 | 2 | 2 | 0 | 0 | 1 |
| Scorer 1990 | 1 0 | 0 | 2 | 0 | 2 | 0 | 1 | 0 | 1 |
| Stableforth 1986 | 1 1 | 0 | 0 | 2 | 2 | 1 | 0 | 0 | 0 |
| Steiner 2003 | 2 2 | 1 | 2 | 2 | 2 | 1 | 2 | 2 | 1 |
| Tidermark 2004 | 2 2 | 1 | 2 | 2 | 2 | 0 | 0 | 0 | 2 |
| Vermeeren 2004 | 1 1 | 1 | 2 | 2 | 2 | 0 | 2 | 2 | 1 |
| Vlaming 2001 | 2 2 | 1 | 2 | 0 | 2 | 2 | 2 | 2 | 0 |
| Volkert 1996 | 1 1 | 0 | 2 | 0 | 2 | 2 | 0 | 0 | 2 |
| Woo 1994 | 1 2 | 2 | 2 | 2 | 2 | 0 | 0 | 0 | 1 |
| Wouters 2002 | 1 1 | 0 | 1 | 2 | 2 | 2 | 2 | 2 | 1 |
| Wouters 2003 | 0 1 | 0 | 1 | 2 | 2 | 2 | 2 | 2 | 2 |
| Wouters 2005 | 2 1 | 2 | 2 | 0 | 2 | 2 | 2 | 2 | 1 |
| Wouters 2006 | 1 1 | 0 | 2 | 0 | 2 | 1 | 0 | 0 | 1 |
| Yamaguchi 1998 | 2 0 | 0 | 2 | 2 | 1 | 1 | 2 | 0 | 2 |
| Young 2004 | 2 2 | 0 | 1 | 0 | 2 | 2 | 0 | 0 | 1 |
| Footnotes: allocation concealment: A ‐ adeequate, B ‐ unclear, C ‐ not adequate; ITT: intention‐to‐treat; 0 = worst, 2 = best | |||||||||
Data and analyses
Comparison 1. Oral protein and energy versus routine care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 42 | 8031 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.81, 1.04] |
| 2 Mortality: Subgroup analysis for nutritional status | 40 | 7869 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.80, 1.03] |
| 2.1 Undernourished | 25 | 2466 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.64, 0.97] |
| 2.2 Nourished | 16 | 5403 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.83, 1.14] |
| 3 Mortality: Subgroup analysis for kcal offered per day | 38 | 8165 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.79, 1.01] |
| 3.1 400 kcal or more/day | 24 | 7307 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.78, 1.00] |
| 3.2 <400 kcal/day | 14 | 858 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.59, 1.98] |
| 4 Mortality: Subgroup analysis for age category | 40 | 8049 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.80, 1.03] |
| 4.1 Mean age 75 years or more | 30 | 2967 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.69, 1.05] |
| 4.2 Mean age <75 years | 12 | 5082 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.80, 1.11] |
| 5 Mortality: Subgroup analysis for period of supplementation | 38 | 7608 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.82, 1.06] |
| 5.1 <35 days supplementation | 12 | 5154 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.78, 1.07] |
| 5.2 35 days or more of supplementation | 26 | 2454 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.77, 1.24] |
| 5.3 Mean age <75 years | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Mortality: Subgroup analysis for wellness | 41 | 8029 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.81, 1.04] |
| 6.1 Well | 6 | 393 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.25, 3.78] |
| 6.2 Unwell | 35 | 7636 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.81, 1.04] |
| 7 Mortality: subgroup analysis for hospital or community | 35 | 7548 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.81, 1.04] |
| 7.1 In‐patients | 21 | 6582 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.80, 1.04] |
| 7.2 Community | 14 | 966 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.62, 1.59] |
| 8 Mortality: Sensitivity analysis | 41 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 Category 'A' concealment of allocation | 15 | 6604 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.79, 1.03] |
| 8.2 Exclusion of Larsson 1990 | 40 | 7584 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.84, 1.09] |
| 8.3 Exclusion of trials with known commmercial involvement | 29 | 7190 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.79, 1.03] |
| 9 Mortality: Subgroup analysis by diagnostic group | 38 | 7496 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.78, 1.01] |
| 9.1 Geriatric conditions | 23 | 2701 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.62, 0.98] |
| 9.2 Hip fracture | 8 | 437 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.50, 1.66] |
| 9.3 Congestive heart failure | 1 | 22 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.44 [0.10, 20.21] |
| 9.4 Chest conditions | 2 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.07 [0.13, 73.30] |
| 9.5 Perioperative | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.16, 11.38] |
| 9.6 Stroke | 2 | 4063 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.79, 1.10] |
| 9.7 Diabetic conditions | 1 | 53 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.07, 15.75] |
| 10 Participants with complications | 24 | 6225 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.75, 0.99] |
| 11 Participants with complications: Subgroup analysis by diagnostic group | 22 | 5727 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.76, 1.00] |
| 11.1 Geriatric conditions | 8 | 1026 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.77, 1.08] |
| 11.2 Hip fracture | 6 | 298 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.40, 0.91] |
| 11.3 Chest conditions | 3 | 165 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.58, 2.73] |
| 11.4 Perioperative | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.11 [0.68, 6.54] |
| 11.5 Stroke patients | 2 | 4063 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.40, 1.03] |
| 11.6 Congestive heart failure | 1 | 22 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.0 [0.38, 130.56] |
| 11.7 Diabetic conditions | 1 | 53 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.54, 1.35] |
| 12 % Weight change | 45 | 3058 | Mean Difference (IV, Fixed, 95% CI) | 2.15 [1.80, 2.49] |
| 13 % Weight change: Subgroup analysis by diagnostic group | 44 | 3056 | Mean Difference (IV, Fixed, 95% CI) | 2.15 [1.80, 2.49] |
| 13.1 Geriatric conditions | 32 | 2387 | Mean Difference (IV, Fixed, 95% CI) | 2.65 [2.19, 3.10] |
| 13.2 Hip fracture | 4 | 235 | Mean Difference (IV, Fixed, 95% CI) | 0.58 [‐1.04, 2.19] |
| 13.3 Chest conditions | 5 | 284 | Mean Difference (IV, Fixed, 95% CI) | 1.58 [0.99, 2.17] |
| 13.4 Perioperative | 1 | 100 | Mean Difference (IV, Fixed, 95% CI) | ‐1.90 [‐6.43, 2.63] |
| 13.5 Stroke patients | 1 | 31 | Mean Difference (IV, Fixed, 95% CI) | 1.58 [‐5.55, 8.71] |
| 13.6 Congestive heart failure | 1 | 19 | Mean Difference (IV, Fixed, 95% CI) | 1.43 [‐7.89, 10.75] |
| 14 % Weight change sensitivity analysis | 19 | 1599 | Mean Difference (IV, Fixed, 95% CI) | 2.07 [1.68, 2.46] |
| 14.1 No inferences required | 19 | 1599 | Mean Difference (IV, Fixed, 95% CI) | 2.07 [1.68, 2.46] |
| 15 % Arm muscle circumference change | 16 | 1382 | Mean Difference (IV, Fixed, 95% CI) | 1.20 [0.45, 1.96] |
| 16 % Arm muscle circumference change: Subgroup analysis by diagnostic group | 16 | 1382 | Mean Difference (IV, Random, 95% CI) | 1.25 [0.22, 2.28] |
| 16.1 Geriatric conditions | 11 | 1216 | Mean Difference (IV, Random, 95% CI) | 1.00 [‐0.20, 2.21] |
| 16.2 Hip fracture | 1 | 10 | Mean Difference (IV, Random, 95% CI) | 2.92 [0.16, 5.68] |
| 16.3 Chest conditions | 2 | 106 | Mean Difference (IV, Random, 95% CI) | 3.46 [‐3.40, 10.32] |
| 16.4 Perioperative | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16.5 Stroke patients | 1 | 31 | Mean Difference (IV, Random, 95% CI) | 0.86 [‐6.27, 7.99] |
| 16.6 Congestive heart failure | 1 | 19 | Mean Difference (IV, Random, 95% CI) | ‐0.39 [‐9.71, 8.93] |
| 17 Length of Stay | 14 | 5735 | Mean Difference (IV, Random, 95% CI) | ‐0.75 [‐2.84, 1.34] |
| 18 Length of stay: Subgroup analysis by diagnostic group | 13 | 5290 | Mean Difference (IV, Random, 95% CI) | ‐1.17 [‐3.90, 1.57] |
| 18.1 Geriatric conditions | 4 | 875 | Mean Difference (IV, Random, 95% CI) | ‐0.80 [‐6.49, 4.89] |
| 18.2 Hip fracture | 6 | 263 | Mean Difference (IV, Random, 95% CI) | ‐2.14 [‐7.71, 3.42] |
| 18.3 Chest conditions mean age >65 years | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18.4 Perioperative mean age >65 years | 1 | 100 | Mean Difference (IV, Random, 95% CI) | ‐2.0 [‐6.53, 2.53] |
| 18.5 Stroke patients mean age >65 years | 2 | 4052 | Mean Difference (IV, Random, 95% CI) | ‐6.50 [‐25.88, 12.88] |
| 18.6 Congestive heart failure mean >65 years | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Handgrip | 7 | 535 | Mean Difference (IV, Fixed, 95% CI) | 0.06 [‐0.60, 0.72] |
1.1. Analysis.
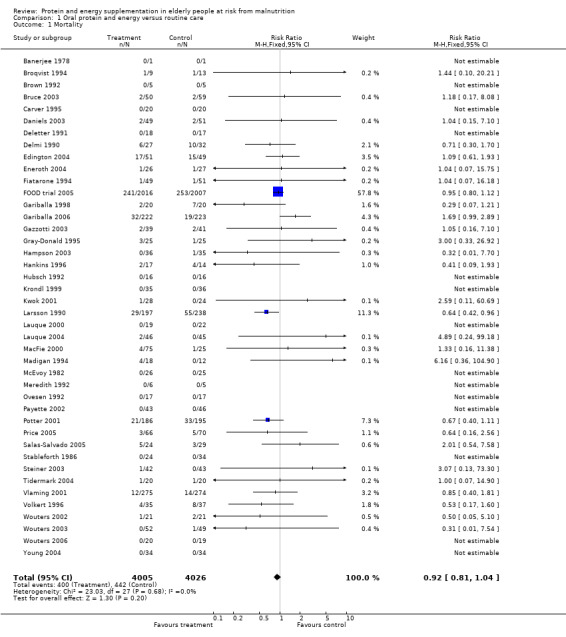
Comparison 1 Oral protein and energy versus routine care, Outcome 1 Mortality.
1.2. Analysis.
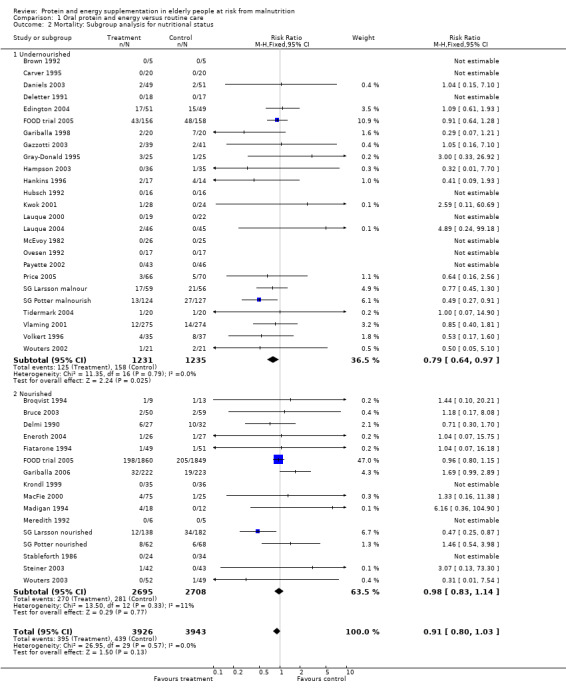
Comparison 1 Oral protein and energy versus routine care, Outcome 2 Mortality: Subgroup analysis for nutritional status.
1.3. Analysis.
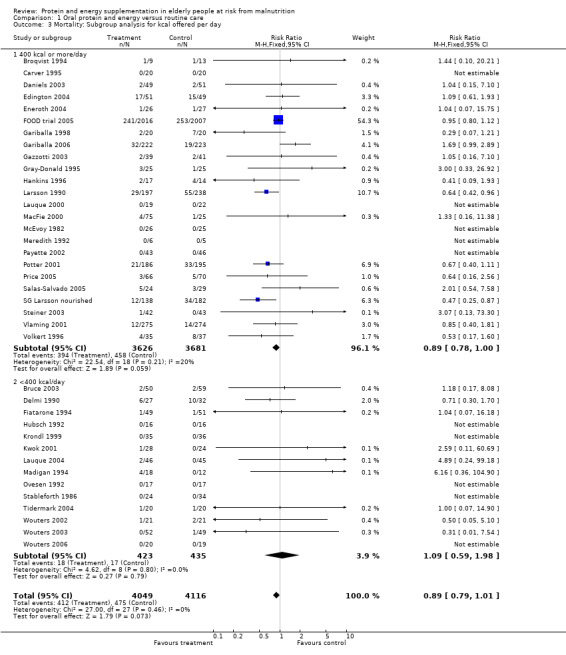
Comparison 1 Oral protein and energy versus routine care, Outcome 3 Mortality: Subgroup analysis for kcal offered per day.
1.4. Analysis.
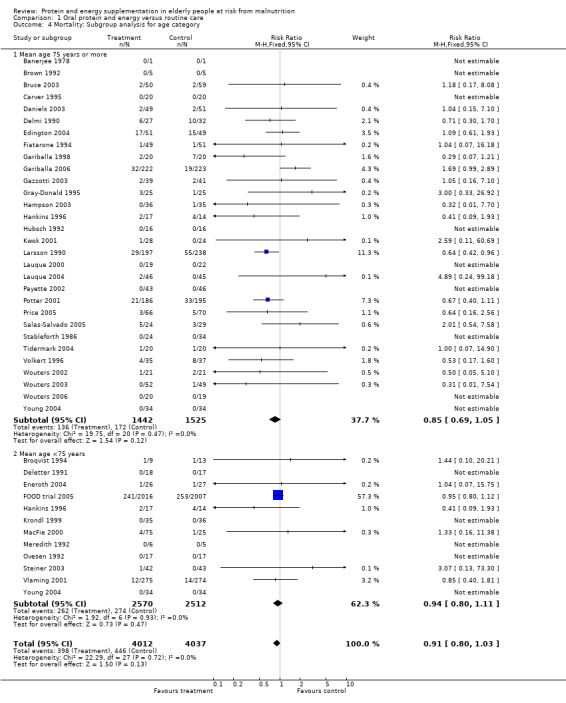
Comparison 1 Oral protein and energy versus routine care, Outcome 4 Mortality: Subgroup analysis for age category.
1.5. Analysis.
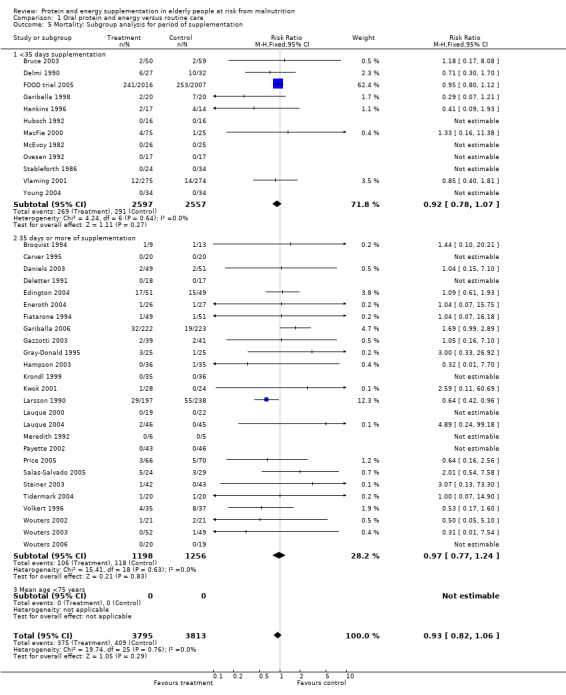
Comparison 1 Oral protein and energy versus routine care, Outcome 5 Mortality: Subgroup analysis for period of supplementation.
1.6. Analysis.
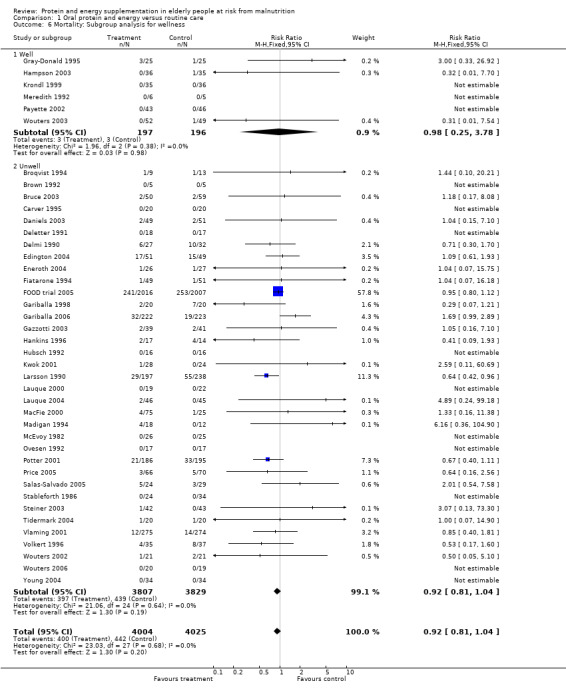
Comparison 1 Oral protein and energy versus routine care, Outcome 6 Mortality: Subgroup analysis for wellness.
1.7. Analysis.
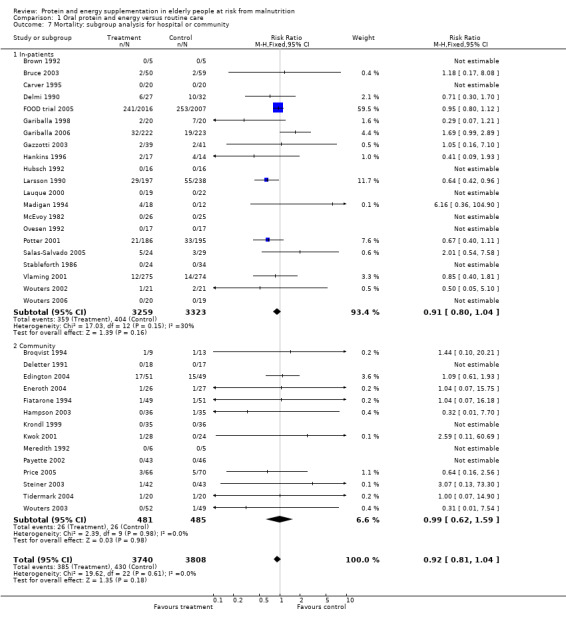
Comparison 1 Oral protein and energy versus routine care, Outcome 7 Mortality: subgroup analysis for hospital or community.
1.8. Analysis.
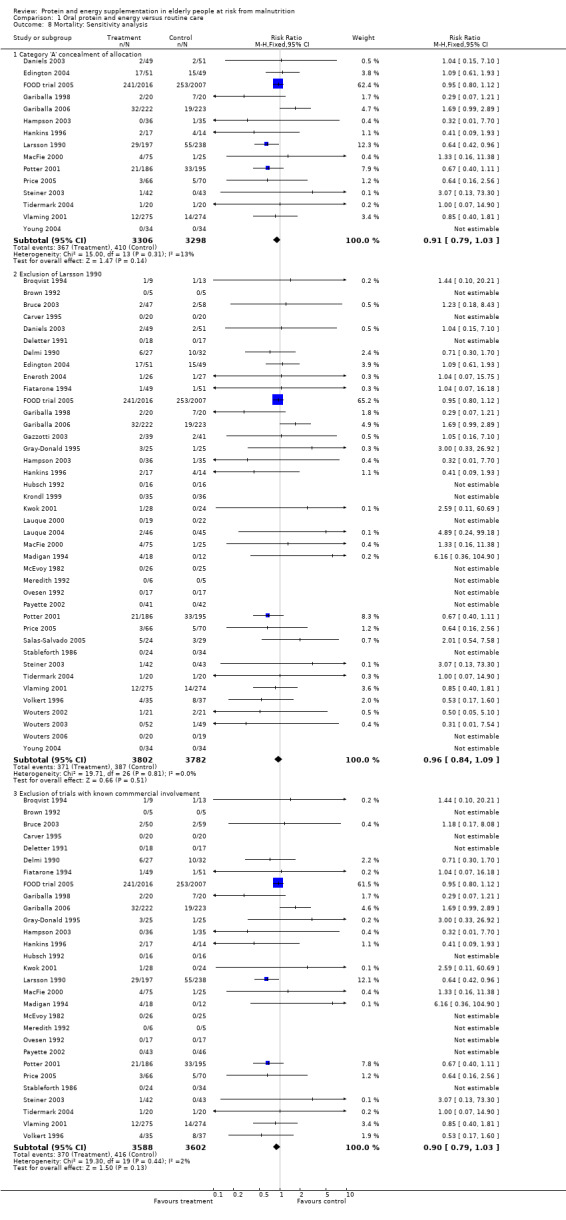
Comparison 1 Oral protein and energy versus routine care, Outcome 8 Mortality: Sensitivity analysis.
1.9. Analysis.
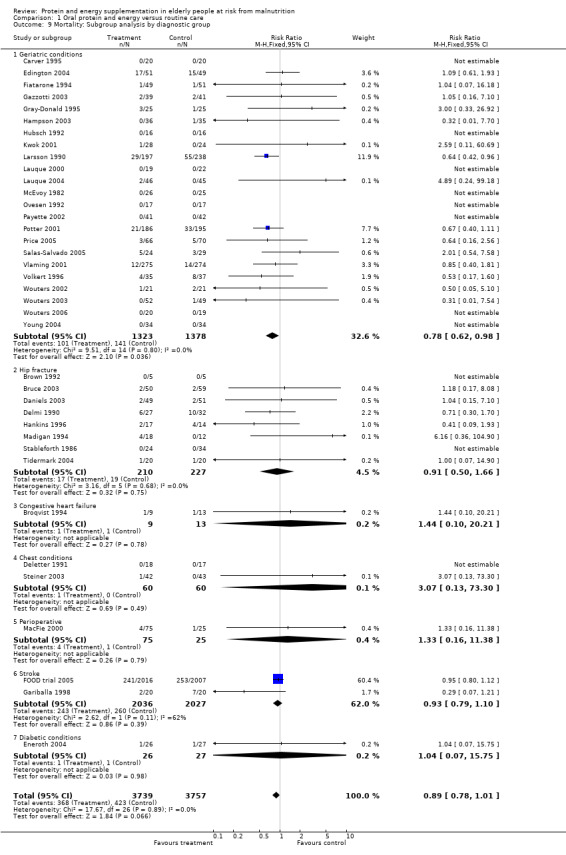
Comparison 1 Oral protein and energy versus routine care, Outcome 9 Mortality: Subgroup analysis by diagnostic group.
1.10. Analysis.
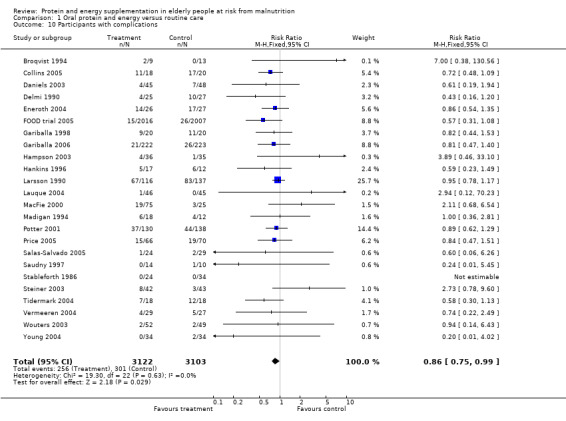
Comparison 1 Oral protein and energy versus routine care, Outcome 10 Participants with complications.
1.11. Analysis.
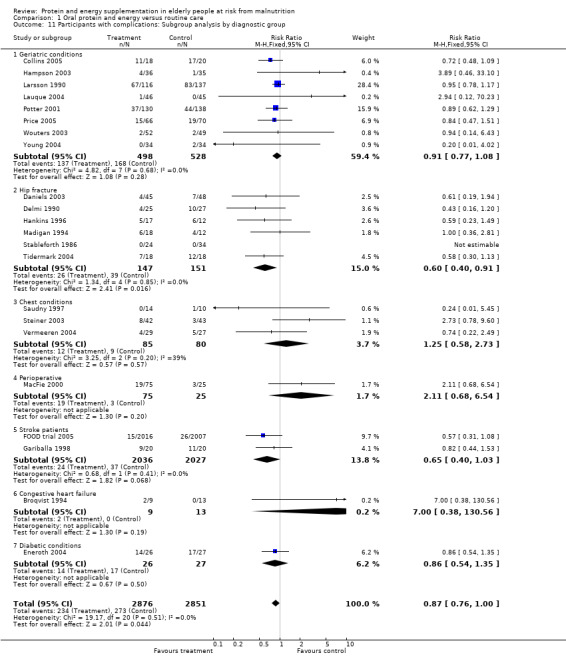
Comparison 1 Oral protein and energy versus routine care, Outcome 11 Participants with complications: Subgroup analysis by diagnostic group.
1.12. Analysis.
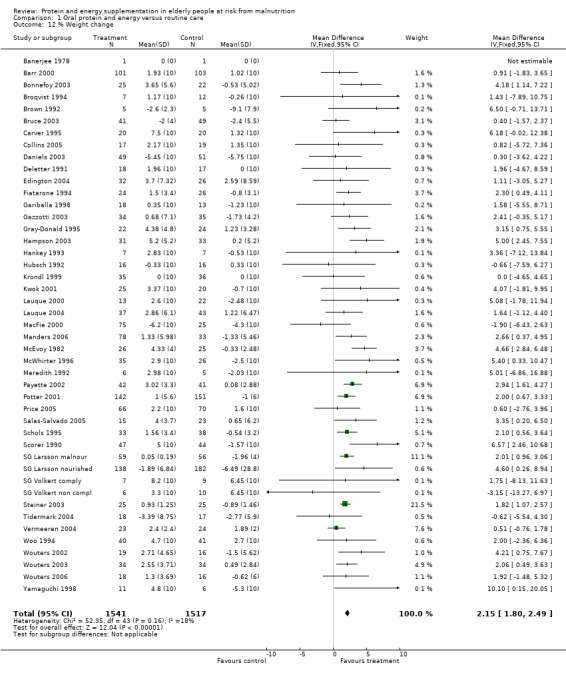
Comparison 1 Oral protein and energy versus routine care, Outcome 12 % Weight change.
1.13. Analysis.
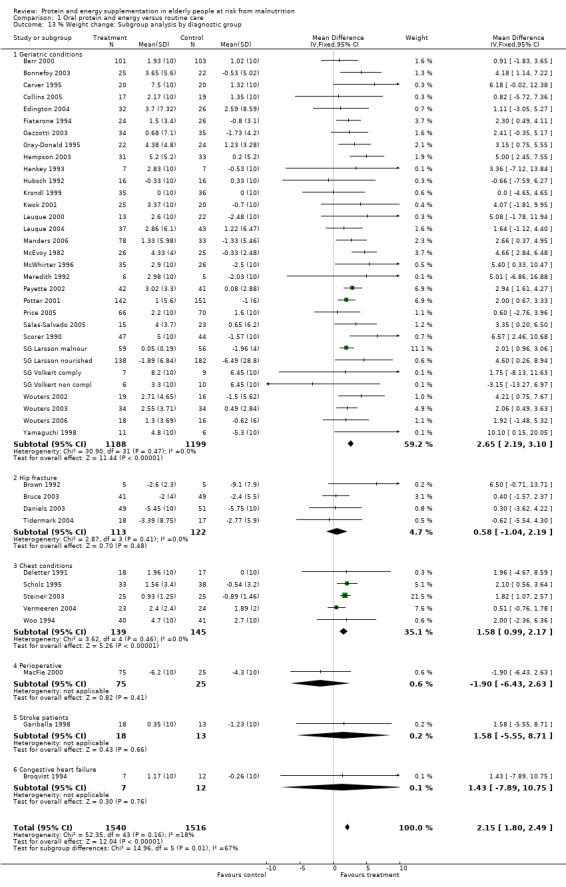
Comparison 1 Oral protein and energy versus routine care, Outcome 13 % Weight change: Subgroup analysis by diagnostic group.
1.14. Analysis.
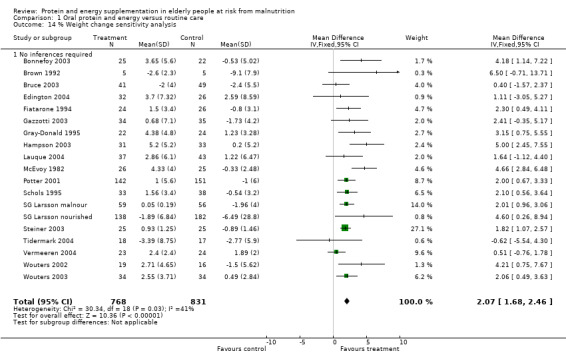
Comparison 1 Oral protein and energy versus routine care, Outcome 14 % Weight change sensitivity analysis.
1.15. Analysis.
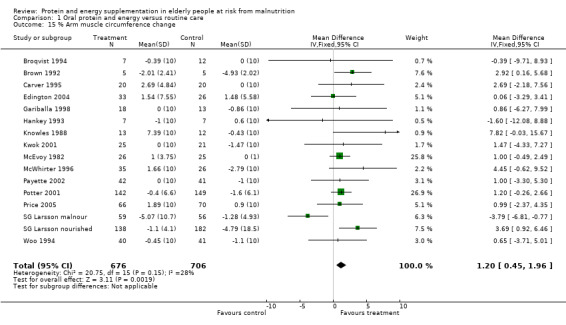
Comparison 1 Oral protein and energy versus routine care, Outcome 15 % Arm muscle circumference change.
1.16. Analysis.
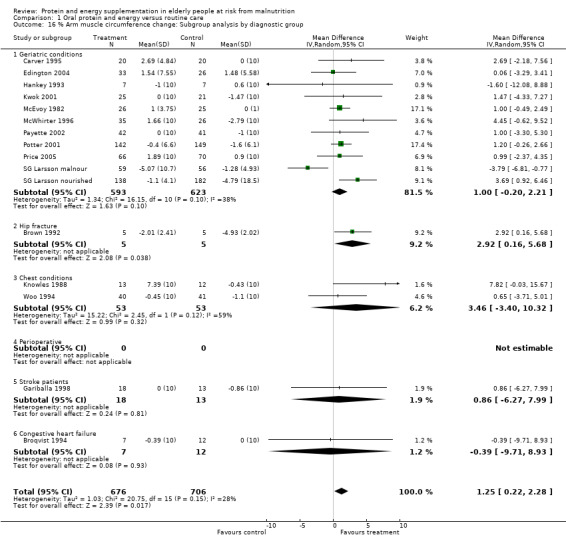
Comparison 1 Oral protein and energy versus routine care, Outcome 16 % Arm muscle circumference change: Subgroup analysis by diagnostic group.
1.17. Analysis.
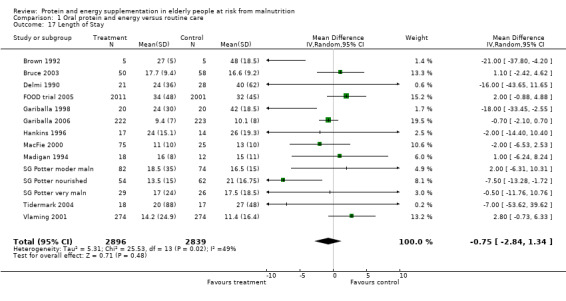
Comparison 1 Oral protein and energy versus routine care, Outcome 17 Length of Stay.
1.18. Analysis.
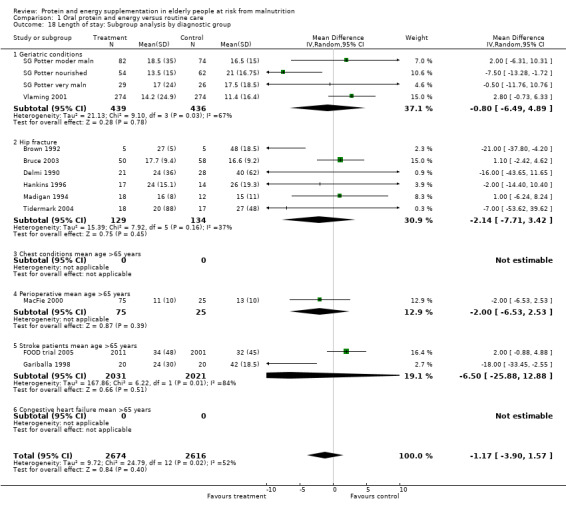
Comparison 1 Oral protein and energy versus routine care, Outcome 18 Length of stay: Subgroup analysis by diagnostic group.
1.19. Analysis.
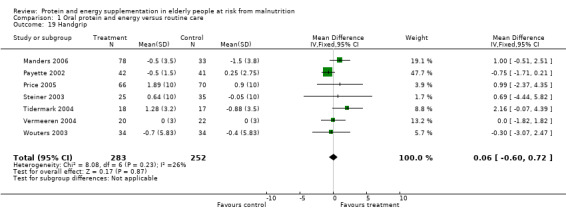
Comparison 1 Oral protein and energy versus routine care, Outcome 19 Handgrip.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Banerjee 1978.
| Methods | Method of randomisation: not stated Assessor blinding: dietary assessment not blinded Intention to treat: carried out Lost to follow‐up: intervention 2 withdrawn because refused to continue,1 withdrawn due to increased blood urea; control 0 Timing of intervention: 14 weeks | |
| Participants | Location: long‐stay wards University Hospital, South Manchester 63 patients Inclusion criteria: informed consent, not receiving Complan for 3 months prior to study Exclusion criteria: failure to gain consent Sex: 42 female, 21 male Age: mean age 81y | |
| Interventions | a: two drinks Complan: 265 kcal, 18.6g protein, 26.4g carbohydrate, 9.6 g fat per day in addition to normal food intake b: normal food intake Allocated: 33/30 Assessed: 26/24 | |
| Outcomes | Main outcomes: Mortality Additional outcomes: Measures of nutritional status ‐ changes in skinfold thickness from the first non‐supplemented to the second (supplemented) period Measures of dietary intake ‐ changes in mean food intake from the first (non‐supplemented) period to the second (supplemented) period compared in the supplemented and control groups (measured over 5 days) | |
| Notes | Further details (method of randomisation, blinding, similarity of care programmes) obtained from trialist 18/4/01 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Barr 2000.
| Methods | Method of randomisation: not stated Assessor blinding: only reported for blood pressure Intention to treat: 204 randomised, results presented for 200, intention to treat analysis not possible Lost to follow‐up: intervention 3; control 1 Timing of intervention: 12 weeks | |
| Participants | Location: 6 medical centres, USA 204 patients Inclusion criteria: Adult men and women between 55 and 85 years and in good health BMI between 16 and 36, 5 years post menopause, consumption of 1.5 servings or fewer per day of dairy foods, willingness to consume additional 3x8oz milk/day Exclusion criteria: Unstable hypertension or dyslipidemia within last month, unstable hormone therapy use within last year, chronic or life threatening diseases, serious abnormality that would interfere with study participation, substance or alcohol abuse, no calcium supplementation for more than 4 weeks before enrolment in the study, diabetes, blood pressure over systolic or 95 mm Hg diastolic, total blood cholesterol greater than 6.75mmol/L, fasting blood glucose level greater than 7.8mmol/L Sex: 35‐36% male, 64‐65% female Age: mean age 65y Living at home | |
| Interventions | a: Participants added 3 x 8 oz servings of fluid milk daily (low fat or fat free) to their usual consumption of dairy products b: Participants maintained their usual diets Allocated: 101/103 Assessed: 98/102 for all outcomes | |
| Outcomes | Main outcomes: Additional outcomes: Anthropometric indices ‐ weight change Functional status ‐ Barthel index, mental health inventory, general perceived health scale, work activity scale Measures of dietary intake ‐ change in energy and protein intake | |
| Notes | No further information required | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Benati 2001.
| Methods | Method of randomisation: not stated ‐ allocation concealment: B Assessor blinding: not mentioned Intention to treat: unclear Lost to follow‐up: unclear Timing of the intervention: 2 weeks Length of follow‐up: 2 weeks | |
| Participants | Location: Department of Geriatric Medicine, Forli, Italy 10 patients Inclusion criteria: Inpatients with severe cognitive impairment. MMSE 15 or less (maximum 30), and pressure ulcers Exclusion criteria: Unlikely to benefit from nutritional supplementation Sex: 3 female 7 male Age: 71‐91 years | |
| Interventions | a: Normal hospital diet and 2 x 200ml/day of high protein and calorie supplementary feeding (500 kcal, 37g protein approx) b: Normal hospital diet Allocated: 5?/5? Assessed: 5/5 | |
| Outcomes | Main outcomes: pressure sore status Additional outcomes: Measures of nutritional status | |
| Notes | Third arm received supplement enriched with arginine | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Bonnefoy 2003.
| Methods | Method of randomisation: remote allocation ‐ allocation concealment: A Assessor blinding: not mentioned Intention to treat: unclear for one participant Lost to follow‐up: unclear which arm 5 clinical events, 6 retracted consent, 3 dropped out Timing of the intervention: Twice daily between meals Length of follow‐up: 9 months | |
| Participants | Location: 16 retirement homes in Lyon, France 57 patients Inclusion criteria: Multiple diagnoses, length of stay at least 3 years in retirement homes, over 72 years, defined as frail by GP Exclusion criteria: Uncontrolled or rapidly evolving diseases, dementia, type 1 diabetes, severe renal insufficiency, functional handicap preventing exercising, long‐term corticosteroid therapy with receipt of vitamins before the study Sex: 50 female 7 male Age: 83 (?SEM 0.91), 83 (1.24) | |
| Interventions | a: 2 x 200 ml, total intake 1686 kJ (400 kcal) 30% Prot, 50% CHO, 20% Fat, providing approximately 50% of the RDA for vitamins and minerals, four different flavours in unmarked containers, twice daily at 10.00 and 16.00 b: 4 different flavours in unmarked containers, neither energy, protein, vitamins or minerals Allocated: 28/29 Assessed: 22/20 | |
| Outcomes | Main outcomes: mobility and muscle power Additional outcomes: compliance to supplements Measures of nutritional status‐ BMI, Fat free mass | |
| Notes | Factorial design half participants also had exercise programme and half memory training. One death unclear from which group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Bourdel 2000.
| Methods | Method of randomisation: Cluster randomised Assessor blinding: no Intention to treat: carried out Lost to follow‐up: intervention 0; control 0 Timing of the intervention: 15 days or less if discharged Length of follow‐up: 15 days | |
| Participants | Location: Bordeaux, France 672 patients Inclusion criteria: Ward had >40% of patients >65years, patients in an acute phase of critical illness, unable to move by themselves, unable to eat independently at admission Exclusion criteria: patients with pressure ulcers on admission Sex: 437 female, 235 male Age: 83.6 (SD7.3), 83.0 (7.1) | |
| Interventions | a: 2 x 200 kcal commercial supplements with breakfast and mid afternoon (400 kcal, 30g protein) and assistance with meals b: usual nutritional care Allocated: 295/377 (Cluster randomised) Assessed: 295/377 | |
| Outcomes | Main outcomes: mortality, pressure ulcers Additional outcomes: Measures of dietary intake ‐energy and protein intake | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Broqvist 1994.
| Methods | Method of randomisation: not stated Assessor blinding: not reported Intention to treat: carried out, Lost to follow‐up: one unable to continue because of illness, 2 died Timing of intervention: 8 weeks | |
| Participants | Location: not given, presumably University Hospital, Linkoping, Sweden 19 patients Inclusion criteria: Patients with severe congestive heart failure (NewYork Heart Association functional class III‐IV) Exclusion criteria: Diabetes mellitus, severe liver or renal insufficiency Sex: 3 female, 19 male Age: mean age 72y | |
| Interventions | a: 500 ml oral nutritional supplement (Biosorb 1500 (Pharmacia, Germany) 750 kcal, 30 g protein) b: 1:10 diluted version of intervention as placebo (7.5 kcal, 3 g protein) Allocated: 9/13 Assessed: 7/12 | |
| Outcomes | Main outcomes: Mortality Morbidity and complications ‐NYHA (New York Heart Association) functional class, complications (renal failure and diabetic coma), malnourished after 8 weeks Additional outcomes: Measures of nutritional status ‐ weight, triceps skinfold (mm), arm muscle circumference (cm) Measures of dietary intake ‐ energy and protein intake | |
| Notes | Request for further details sent 11/5/01 on inpatient or outpatient status, location of trial and further details regarding composition of supplement and how it was provided | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Brown 1992.
| Methods | Method of randomisation: alternating number Assessor blinding: blinded Intention to treat: carried out Lost to follow‐up: no losses to follow‐up Timing of intervention: from second day of admission until discharge | |
| Participants | Location: hospital, Ipswich, UK 10 patients Inclusion criteria: thin (based on weight for height, triceps skinfold, mid‐arm circumference ‐ two out of three more than one standard deviation below reference mean), elderly, females with hip fracture Exclusion criteria: malignant disease, mental illness, renal or hepatic failure, neurological disorder, stroke, diabetes Sex: all female Age: not given, but "elderly" | |
| Interventions | a: Patient offered oral nutritional supplement Fresubin (Fresenius) calculated to make up deficit between intake from normal hospital diet and requirement. Fresubin provides 4.2 kJ or 1kcal/ml, as 15% protein energy, 30% fat energy and 55% carbohydrate energy b: Normal hospital diet Allocated: 5/5 Assessed: 5/5 | |
| Outcomes | Main outcomes: Mortality Morbidity and complications ‐ pressure sore Length of stay ‐ days to discharge from orthopaedic surgeon Postoperative functional status ‐ two stage walking goals Additional outcomes: Measures of nutritional status ‐ percentage losses in weight, triceps skinfold, midarm circumference, arm muscle circumference Measures of dietary intake | |
| Notes | Author provided protocol of trial and information on method of randomisation and outcome assessment. Request for further details (other outcomes, period of follow‐up) sent 19/5/99, resent 3/2/00 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
Bruce 2003.
| Methods | Method of randomisation: quasi‐randomised by year of birth Assessor blinding: not reported Intention to treat: unclear Lost to follow‐up: intervention 3; control 1 Timing of intervention: started within 2 to 3 days after surgery, for 28 days Length of follow‐up: 6 months | |
| Participants | Location: Royal Perth hospital, Freemantle, Australia 109 patients Inclusion criteria: female patients with hip fracture, consent given Exclusion criteria: BMI <20 or >30 kg/m2, nursing home resident, resident outside metropolitan Perth (preventing follow‐up), diseases expected to influence nutritional intake (malignancy, severe organ failure), diabetes (to avoid potential hyperglycaemia), fracture due to major trauma Sex: 109 female Age: mean 84 years | |
| Interventions | a: One 235 ml can of Sustagen Plus daily (Mead Johnston), providing 352 kcal or 1.47 MJ, 17.6 g protein, 11.8 g fat, 44.2 g carbohydrate, vitamins and minerals; chocolate and vanilla flavours. Dietitian carried out preliminary taste test and offered encouragement and strategies to help with compliance, e.g. ways to alter taste and timing of supplement. And routine care b: Routine care Allocated: 50/59 Assessed: 47/58 (mortality) | |
| Outcomes | Main outcomes: mortality Length of stay ‐ hospital Postoperative functional status ‐ % with fall in Katz score, level of care and extent of support required after discharge ‐ % discharged home, % home at 6 months Additional outcomes: Anthropometric indices ‐ weight Nutritional indicators in blood ‐ albumin Patient compliance ‐ consumption of cans of supplement | |
| Notes | More information requested and received August 2003 Classified as nourished, acute admission and hospitalised. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
Carver 1995.
| Methods | Method of randomisation: not stated Assessor blinding: unclear whether all or some outcomes Intention to treat: not carried out, those unable to consume supplements not included in the analysis Lost to follow‐up: intervention 2 withdrawn due to reluctance to take extra drinks, 1 withdrawn due to objection to being weighed and measured; control 1 withdrawn due to reluctance to take extra drinks, 2 withdrawn due to objection to being weighed and measured Timing of intervention: 12 weeks, twice daily | |
| Participants | Location: All residents of care of the elderly wards at a large psychiatric teaching hospital, Edinburgh 46 patients Inclusion criteria: diagnosed as having some degree of senile dementia, BMI 15.1‐19.9 Exclusion criteria: physical pathology, likely to be discharged during the study period, no consent from relatives Sex: 36 female, 10 male Age: men: mean age 68‐69y, women: 79‐80y Health Status: malnourished, senile dementia | |
| Interventions | a: 200 ml oral supplement Fortisip twice daily providing 600 kcal/day in addition to normal meals b: 200 ml oral vitamin preparation twice daily providing the same vitamins as Fortisip, but virtually no macronutrients, in addition to normal meals Allocated: 23/23 Assessed: 20/20 | |
| Outcomes | Main outcomes: Mortality Additional outcomes: Measures of nutritional status ‐ weight change, body mass index, mid upper arm circumference, triceps skinfold Patient compliance ‐ numbers consuming all drinks offered | |
| Notes | Request for further details sent 18/9/01 on method of randomisation, blinding of outcome assessors and treatment providers and further details of anthropometry outcomes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
Collins 2005.
| Methods | Method of randomisation: not stated Assessor blinding: only study nurse and participants blinded not research dietitian or investigator Lost to follow‐up: unclear Intention to treat: very poor detail Lost to follow‐up: unclear Timing of the intervention: given for 4 weeks 3 times/day with meals Length of follow‐up: 4 weeks | |
| Participants | Location: elderly home nursed patients referred for wound management, Australia 38 patients Inclusion criteria: over 60 years old, informed consent, all types of wounds‐ skin grafts, lacerations, skin tears, ulcers, pressure ulcers, post surgical wounds Exclusion criteria: allergy or intolerance to milk based products Sex: 55.3% male Age: 79.2 (SD 6.3), 81.0 (SD 9.5) | |
| Interventions | a: 237 ml of 8 kJ/ml oral nutritional supplement b: 237 ml of 4 kJ/ml oral nutritional supplement Allocated: 18/20 Assessed: unclear | |
| Outcomes | Main outcomes: Dietary intake, cognitive function, quality of life, wound healing Additional outcomes: | |
| Notes | Insufficient description of allocation concealment | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Daniels 2003.
| Methods | Method of randomisation: pharmacy maintained, computer generated, opaque envelopes concealment: A Assessor blinding: yes Intention to treat: yes Lost to follow‐up: intervention 2 withdrawn; control 1 withdrawn Timing of the intervention: from day 7 post surgery. From discharge to 6 weeks Length of follow‐up: 12 weeks | |
| Participants | Location: Elderly people post lower limb fracture 100 patients Inclusion criteria: Nutritionally at risk (MAC 25th percentile or less) Exclusion criteria: did not reside within southern Adelaide, unable to comprehend instructions relating to positioning of upper arm for eligibility assessment, unable to fully weight bear on the side of injury for more than 7 days post admission, not independently mobile post fracture, medically unstable more than 7 days post admission, suffering from cancer, chronic renal failure, unstable angina or unstable diabetes Sex: 38/41 female Age:83y/84y | |
| Interventions | a: 1.5 kcal/ml sip feed for 6 weeks, individually prescribed, home visits 3 x week from discharge to 6 weeks b: home visits 3 x week from discharge to 6 weeks Allocated: 49/51 Assessed: 45/48 | |
| Outcomes | Main outcomes: mortality. mobility and muscle power Additional outcomes: compliance to supplements Measures of nutritional status‐ % weight change | |
| Notes | Allocated to nutrition alone or nutrition plus resistance training exercise, resistance training exercise alone, or neither | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Deletter 1991.
| Methods | Method of randomisation: not stated Assessor blinding: not mentioned Intention to treat: carried out Lost to follow‐up: intervention 0; control 0 Timing of intervention: 9 weeks | |
| Participants | Location:Veterans Affairs Medical Center‐ Lexington 37 patients Inclusion criteria: outpatients, over 55 years old, irreversible airway obstruction, no COPD exacerbation within 4 weeks of enrolment, less than 90% ideal body weight, ambulatory, English speaking, able to communicate verbally and in writing Exclusion criteria: muscular discomfort of chest wall, pain on inspiration, diabetes, thyroid disease, neoplastic disease, serious heart disease, alcoholism, hepatic failure, renal failure, malabsorption, surgery within 3 months of the study. Sex: all male Age: mean age 67y | |
| Interventions | a: high fat, low carbohydrate formula (Pulmocare, Ross laboratories, Columbus, OH) 1 can/day, 16.7% protein, 28% carbohydrate, 55% fat b: normal dietary routines Allocated: 37 Assessed: 18/17 | |
| Outcomes | Main outcomes: Lung function, 6 and 12 minute walking distance Additional outcomes: Measures of nutritional status‐weight, changes in skinfold thickness from baseline to the end of the supplemented period Measures of dietary intake‐ energy and protein intake | |
| Notes | Further details: Request for further details (energy content and volume of supplement, blinding) sent 18/10/01 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Delmi 1990.
| Methods | Method of randomisation: not stated Assessor blinding: not reported Intention to treat: appears intention to treat, but denominators unclear Lost to follow‐up: deaths reported, but unclear if other losses to follow‐up Timing of intervention: from admission to orthopaedic unit to end of stay in second (recovery) hospital,given once daily for a mean period of 32 days Length of follow‐up: 6 months | |
| Participants | Location: orthopaedic unit in hospital and recovery hospital, Geneva, Switzerland 59 patients Inclusion criteria: femoral neck fracture after an accidental fall, aged over 60y Exclusion criteria: fracture from violent external trauma, pathological fracture due to tumour or non‐osteoporotic osteopathy; overt dementia; renal, hepatic, or endocrine disease; gastrectomy or malabsorption; taking phenytoin, steroids, barbiturates, fluoride or calcitonin Sex: 53 female, 6 male Age: mean age 82y | |
| Interventions | a: 250 ml oral nutritional supplement (1.06 MJ or 254 kcal, 20.4 g protein, 29.5 g carbohydrate, 5.8 g lipid, 525 mg calcium, 750 IU vitamin A, 25 IU vitamin D3, nicotinamide, folate, calcium pantothenate, biotin, minerals; and vitamins E, B1, B2, B6, B12, C) and standard hospital diet b: Standard hospital diet Allocated: 27/32 Assessed: unclear/unclear at 6 months | |
| Outcomes | Main outcomes: Mortality Morbidity and complications ‐ complications (total, bedsore, severe anaemia, cardiac failure, infection, gastrointestinal ulcer, other) Side effects of treatment ‐ favourable clinical course (excludes death, major complication, or two or more minor complications) Length of stay ‐ orthopaedic unit and recovery hospital Additional outcomes: Measures of dietary intake ‐ energy and protein intake | |
| Notes | Numbers of complications unclear, request for further details sent 24/5/99, resent 7/2/00 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Edington 2004.
| Methods | Method of randomisation: envelope prepared by statistician‐ allocation concealment: A Assessor blinding: not reported Intention to treat: for some outcomes Lost to follow‐up: 42 withdrawn (unclear which arm) Timing of intervention: 8 weeks from baseline visit within 7 days after discharge from hospital Length of follow‐up: 6 months | |
| Participants | Location: North Staffordshire Hospital NHS trust, Hammersmith NHS Trust and Newcastle upon Tyne Hospital NHS Trust (4 Hospitals) 100 patients Inclusion criteria: female elderly malnourished patients newly discharged from hospital, aged 65 years or older, BMI<20, or <25 with documented evidence of weight loss of at least 10% of body weight in the last 6 months prior to the study period or 5% of more in the last 3 months prior to the study period. Score of 7 or more on the abbreviated mental test. Exclusion criteria: Incapable of taking supplements to provide a minimum of 600 kcal/day, not able to be weighed standing, intolerant of any of the ingredients in the study supplements, history of diabetes, hyperglycaemia or chronic renal failure, requiring Parenteral Nutrition or Enteral feeds as a sole source of nutrition or had been prescribed supplements during the last week of their hospital stay. Informed consent. Sex: 55 female, 25 male | |
| Interventions | a: The choice of one or more nutritional supplements: Ensure Plus tetrapak, Enlive tetrapak, Formance pudding or Ensure Bar, Abbott Laboratories Ltd. Supplements 600‐1000 kcal aim to increase to ideal body weight 0.5kg/ week based on Schofield equations b: Routine care Allocated: 51/49 Assessed: 32/26 (weight) | |
| Outcomes | Main outcomes: mortality Functional status ‐ Handgrip, health related quality of life, requirement for health and social care services, health care costs Additional outcomes: Anthropometric indices ‐ weight change, %AMC change, dietary intake change | |
| Notes | Classified as malnourished, unwell, acute admission and in the community Commercial trial | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Eneroth 2004.
| Methods | Method of randomisation: states randomisation but no description Assessor blinding: yes Intention to treat: withdrew patients if hospitalised or non‐compliant Lost to follow‐up: intervention 8 (withdrawn excluding 1 death); control 3 (withdrawn excluding 1 death) Timing of the intervention: daily for 6 months taken between meals Length of follow‐up: 2 years | |
| Participants | Location: Dept of Internal Medicine, Lund University Hospital, Sweden 53 patients Inclusion criteria: patients over 60 years with diabetes mellitus and a Wagner grade or 2 foot ulcer over 4 weeks duration, distal blood pressure measured in the last 3 months | |
| Interventions | a: 400 ml Fortimel (1 kcal/ml Nutricia AB, Netherlands) b: 400 ml placebo Allocated: 26/27 Assessed: 17/23 | |
| Outcomes | Main outcomes: Mortality, wound healing, amputations, nutritional status, compliance Additional outcomes: wound healed at 6 months, amputations | |
| Notes | No description of allocation concealment | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Fiatarone 1994.
| Methods | Method of randomisation: not stated Assessor blinding: not reported Intention to treat: carried out Lost to follow‐up: intervention 1 dropped out due to lack of interest, 1 lost to follow‐up (excluding death); control 1 dropped out due to lack of interest, 3 lost to follow‐up (excluding death) Timing of interventions: once per day in the evening for 10 weeks, administered in blinded containers | |
| Participants | Location: Hebrew Rehabilitation Center for the Aged, Boston 50 patients no exercise, 50 patients with exercise Inclusion criteria: residents in long‐term care, over 70y, ability to walk 6 metres unaided Exclusion criteria: severe cognitive impairment, rapidly progressive terminal illness, acute illness, unstable chronic illness, myocardial infarction or fracture within 6 months, IDDM, weight loss diet, undergoing resistance training, musculoskeletal cardiovascular abnormality, BMI over 32 Sex: 31 female, 19 male (no exercise), 32 female, 18 male (exercise) Age: 86/89 (no exercise) Health Status: 'healthy' residents of long term care, undernourished analysed separately | |
| Interventions | a: usual diet plus Exceed (Ross laboratories) 240 ml: 360 kcal, 60% carbohydrate, 23% fat, 17% protein b: usual diet plus Crystal light (4 kcal) Allocated: no exercise 24/26, exercise 25/25 Assessed: unclear | |
| Outcomes | Main outcomes: Mortality Functional status ‐ stair climbing, physical activity, gait Side effects of treatment ‐ diarrhoea Additional outcomes: Measures of nutritional status ‐ weight change, BMI, skinfold thickness, change in lean body mass Measures of dietary intake ‐ energy and protein intake | |
| Notes | Data from non exercise group only, request for further details regarding numbers of side effects, weight denominators and intake denominators for all groups, blinding of outcome assessors sent 18/9/01 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
FOOD trial 2005.
| Methods | Method of randomisation: remote phone call to gain computer randomisation Assessor blinding: 6 months follow‐up may have been blinded Intention to treat: carried out Lost to follow‐up: intervention 7; control 4 Timing of the intervention: from randomisation until discharge Length of follow‐up: 6 months | |
| Participants | Location: 125 hospitals in 15 countries 4023 patients Inclusion criteria: able to swallow, uncertainty of clinician about need to use supplements, recent stroke (first or recurrent no more than 7 days before admission), patient or relative consent within 30 days of admission or within 30 days of a stroke occurring in hospital Exclusion criteria: subarachnoid haemorrhage Sex: 2149 male, 1874 female Age: 71 (SD 12), 71 (SD 13) | |
| Interventions | a: protein energy supplement 360 ml (2.26MJ, 22.5g Pr), suitable commercial supplements used in most centres e.g. liquid, yoghurt, pudding. Prescribed on drug charts b: normal hospital diet Allocated: 2016/2007 Assessed: 2012/2000 | |
| Outcomes | Main outcomes: Mortality or poor outcome, length of stay, quality of life, complications, residence at follow‐up Additional outcomes: | |
| Notes | Adequate description of allocation concealment | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Gariballa 1998.
| Methods | Method of randomisation: telephone assignment, balanced in blocks of four Assessor blinding: action taken to blind assessors but some outcome measures may have involved unblinded assessors (ADL, dietary intake) Intention to treat: carried out Lost to follow‐up: intervention 1; control 1 Timing of intervention: up to four weeks duration or until death or discharge Length of follow‐up: 3 months | |
| Participants | Location: Leicester General Infirmary, UK 42 patients Inclusion criteria: Acute ischemic stroke patients (WHO criteria), impaired nutritional status (TSF and MAC greater than or equal to 1SD below the mean), no difficulty swallowing one week after stroke, conscious during the first week after stroke onset Exclusion criteria: Difficulty swallowing one week after stroke, cerebral subarachnoid haemorrhage, active gastrointestinal disease, gastric surgery, biochemical evidence of hepatic or renal impairment, uncontrolled heart failure, diagnosed malignancy, sepsis, persistent swallowing difficulty, malignancy, chronic renal failure, hepatic disease, no consent from patient or next of kin, did not reside locally, not notified of admission, unstable diabetes, failure to gain consent, severe mental impairment, refusal to participate Sex: 21 female, 21 male Age: mean age 79y | |
| Interventions | a: up to 400 ml oral nutritional supplement (Fortisip) providing 600 kcal/day, 20 g protein/day b: standard hospital diet Allocated: 21 /21 Assessed: 18/13 (at 3 months) | |
| Outcomes | Main outcomes: Mortality Morbidity and complications ‐ number of infective complications requiring systemic antibiotics (chest infections, urinary tract infections, septicaemias) Length of acute hospital stay Discharge destination within 3 months Functional status ‐ Barthel Index Additional outcomes: Measures of nutritional status ‐ percentage losses in weight, mid upper arm circumference, triceps skinfold Measures of dietary intake ‐ energy and protein intake | |
| Notes | Request for further details sent 11/5/01 regarding nutritional composition of supplement, how much was provided per day, criteria for evidence of malnutrition, control group access to supplements, whether patients were given assistance with supplements, whether patients who died were included in the calculation for length of stay, infective complications and total complications | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Gariballa 2006.
| Methods | Method of randomisation: generated by trial statistician, sequentially numbered opaque envelopes kept in different city, phoned for randomisation Assessor blinding: action taken to blind assessors through use of identical placebo Intention to treat: carried out Lost to follow‐up: intervention 0; control 0 Timing of intervention: up to four weeks duration or until death or discharge Length of follow‐up: 6 months | |
| Participants | Location: university hospital, UK 445 patients Inclusion criteria: acutely ill hospitalised patients aged 65y or more, able to swallow, able to sign consent Exclusion criteria: gastric surgery, malabsorption, BMI > 40kg/m2, coma, severe dementia (Abbreviated Mental Test score < 6), malignancy, living in an institution, already taking supplements Sex: 234 female, 211 male Age: mean age 77y | |
| Interventions | Bottles of supplement given at 08.00 and 12.00 for 6 weeks, including in the community if out of hospital a: 2 bottles of 200ml each, providing in total 995kcal, 50g protein and 100% of reference nutrient intake for vitamins and minerals, and standard hospital diet b: 2 placebo bottles of 200ml each, providing in total 60kcal and no protein or micronutrients, and standard hospital diet Allocated: 223/222 Assessed: 223/222 (at 6 months) | |
| Outcomes | Main outcomes: Mortality Morbidity and complications ‐ infections Length of acute hospital stay Readmissions Functional status ‐ Barthel Index, cognitive function in subgroup Additional outcomes: Measures of nutritional status ‐ weight, mid upper arm circumference, triceps skinfold Measures of dietary intake ‐ energy and protein intake | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Gazzotti 2003.
| Methods | Method of randomisation: unmarked envelope Assessor blinding: no mentioned Intention to treat: appears so for mortality, LOS, destination Lost to follow‐up: none Timing of the intervention: for 8 weeks Length of follow‐up: 60 days | |
| Participants | Location: Geriatric ward of Centre Hospitalier de la Citradelle, Liege, Belgium 80 patients Inclusion criteria: all patients aged 75y or over, admitted for acute conditions, short form MNA<11 within 72 hours of admission, followed by full MNA total score 17‐23.5 (at risk of malnutrition) Exclusion criteria: medical condition preventing oral feeding, end‐of‐life patients, severe dementia, presenting clinical signs of dehydration or heart failure, diseases requiring special dietary treatment Sex: 61 female, 19 male Age: 81.5 (SD 7.6), 78.8 (SD 6.1) | |
| Interventions | a: 1 Clinutren soup (1kcal/ml) and 1 Clinutren (1.5 kcal/ml), 500 kcal, 21g protein/day plus standard diet b: standard diet Allocated: 39/41 Assessed: 39/41 | |
| Outcomes | Main outcomes: mortality, length of hospital stay, Additional outcomes: side‐effects Measures of nutritional status‐ MNA, weight Measures of dietary intake ‐energy and protein intake | |
| Notes | Classified as acute admission, undernourished, hospitalised | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Gegerle 1986.
| Methods | Method of randomisation: not stated Assessor blinding: not mentioned Intention to treat: carried out Lost to follow‐up: intervention 0; control 0 Timing of intervention: as soon as eating post‐op, for duration of hospitalisation, given at 8pm Length of follow‐up: 12 weeks | |
| Participants | Location: hospital, Geneva, Switzerland 16 patients Inclusion criteria: hip fracture post surgery Sex: 13 female, 3 male Age: mean age 77y | |
| Interventions | a: 250 ml drink daily providing 254 kcal, 20 g protein b: normal dietary routines Allocated: 7/9 Assessed: unclear | |
| Outcomes | Main outcomes: Additional outcomes: Measures of nutritional status‐ Measures of dietary intake‐ energy and protein intake | |
| Notes | Request for further details sent 18/10/01 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Gray‐Donald 1995.
| Methods | Method of randomisation: not stated Assessor blinding: carried out apart from nutrition data Intention to treat: carried out Lost to follow‐up: intervention 0; control 0 Timing of intervention: 12 weeks | |
| Participants | Location: Sherbrooke, Quebec, Canada 48 patients Inclusion criteria: over 60y receiving long‐term publicly financed home help services (housework, personal hygiene or food preparation), defined as at nutritional risk according to the following criteria a) involuntary weight loss of greater than 5% of body weight in the last month, 7.5% in the last 3 months or greater than 10% in the last 6 months and BMI less than 27 or b) BMI less than 24 Exclusion criteria: receiving palliative care, alcoholic, active cancer, illness requiring a therapeutic diet incompatible with supplementation Sex: 34 female, 14 male Age: 76/79 Health Status: living at home, nutritionally at risk | |
| Interventions | a: each subject provided with 2x235 mL cans per day of a commercial liquid formula (Ensure, Ensure Plus, or Enrich, Ross Laboratories, Canada) chosen by themselves in order to improve compliance. Providing between 1045‐1480 kJ per can. Ensure 1045 kJ, 8.74 g protein, Enrich with fibre 1085 kJ, 9.4 g protein, Ensure Plus 1480 kJ, 13.0 g protein b: no treatment provided, but visited every week and given encouragement and suggestions to improve the quality of their diets Allocated: 25/25 Assessed: 22/24 | |
| Outcomes | Main outcomes: Mortality Functional status ‐ general well being score, number of falls, self‐perceived health, hand grip strength Additional outcomes: Measures of nutritional status ‐ anthropometric indices ‐ change in weight, triceps, supra iliac, subscapular skinfolds Measures of dietary intake ‐ energy intake Patient compliance ‐ 7 or more cans of supplement per week | |
| Notes | No other information required | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Hampson 2003.
| Methods | Method of randomisation: unmarked envelope Assessor blinding: not mentioned Intention to treat: unclear Lost to follow‐up: none Timing of the intervention: for 8 weeks Length of follow‐up: 1 year | |
| Participants | Location: London, UK 71 patients Inclusion criteria: community dwelling elderly women, recruited through GPs, aged over 70y, BMI less than or = to 21kg/m2 and osteoporosis at femora; neck and/or total hip Exclusion criteria: progressive wasting disease, severe renal impairment, severe cardiorespiratory disease, endocrine diseases, therapy with drugs interfering with bone metabolism, cognitive impairment Sex: all female Age: 76 (SD 4.2), 76.7 (SD 5.7) | |
| Interventions | a: 1g calcium and 800iu cholecalciferol/day and advice and supplements to increase BMI by 1 kg/m2 or more over 6 months Fortisip and/or Fortijuice one or two cartons (200ml carton provides 300 kcal, 12g protein, 11g fat, 36.8g carbohydrate, vitamins, minerals and trace elements b: 1g calcium and 800iu cholecalciferol and asked not to change standard diet Allocated: 36/35 Assessed: 31/33 | |
| Outcomes | Main outcomes: mortality Additional outcomes: physical activity and well‐being Measures of nutritional status‐ weight, Measures of dietary intake ‐energy, protein and fat intake | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Hankey 1993.
| Methods | Method of randomisation: not stated Assessor blinding: not reported Intention to treat: not possible Lost to follow‐up: 4 (unclear which arm) Timing of intervention: 8 weeks, offered with routine drug prescription, mid am and mid pm, maxijul (glucose polymer, SHS) put into all drinks at nurse's discretion | |
| Participants | Location: Sanderson Rehabilitation Hospital, Newcastle 14 patients Inclusion criteria: over 75y, continuing care elderly, plasma albumin less than 40g/L Exclusion criteria: non given Sex: 11 female, 3 male Age: 88/87 Health Status: long‐term care, at nutritional risk | |
| Interventions | a: normal hospital food plus Build‐up (Nestlé, Clinitec), unclear kcal/day, unclear g protein/day and unclear kcal/day b: normal hospital diet Allocated: 10/10 Assessed: 7/7 | |
| Outcomes | Main outcomes: none Additional outcomes: Measures of nutritional status‐ Anthropometric indices ‐ change in weight, mid upper arm circumference, triceps skinfold Measures of dietary intake ‐ energy and protein intake | |
| Notes | Request for further information sent 18/9/01. Reply received 18/9/01 with information regarding how many had acute illness, whether all outcomes were measured at 8 weeks, method of randomisation, blinding of outcome assessors, whether care programmes were identical, inclusion and exclusion criteria | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Hankins 1996.
| Methods | Method of randomisation: sealed, opaque envelopes in blocks of 10, appears stratified by place of residence Assessor blinding: not done Intention to treat: carried out Lost to follow‐up: intervention 0; control 1 Timing of intervention: started within 5 days of surgery, given once in the morning and once in the evening for 30 days, served on meal tray in hospital by nurses, given by family or self‐administered out of hospital Length of follow‐up: 2 months | |
| Participants | Location: acute care in Hornsby Ku‐ring‐gia Hospital and rehabilitation hospitals, Sydney, Australia 32 patients Inclusion criteria: fractured neck of femur after accidental fall; admitted from home, hostel or nursing home; age 65y or older; mid upper arm circumference less than or equal to 25th centile for sex and age Exclusion criteria: malignancy, chronic renal failure, hepatic disease, no consent from patient or next of kin, did not reside locally, not notified of admission, unstable diabetes Sex: 27 female, 5 male Age: mean 86y | |
| Interventions | a: Oral supplement of 250 ml Sustagen twice daily (total daily intake 22.5 g protein, 10 g fat, 60 g carbohydrate, 1.71 MJ or 409 kcal energy, 500 µg vitamin A, 6.6 µg vitamin D, 50.8 mg vitamin C, 1.2 mg thiamin, 1.15 mg riboflavin, 13 mg niacin, 1.3 µg vitamin B12, 825 mg calcium, 670 mg phosphorus, 8 mg iron, 66 µg iodine, 1.2g potassium, 370 mg sodium) plus standard hospital diet b: Standard hospital diet Allocated: 17/15 Assessed: 17/14 | |
| Outcomes | Main outcomes: Mortality Morbidity and complications ‐ complications (total, infection, pressure sores, pulmonary embolism, delirium, anaemia, cardiac failure, acute renal failure) Side effects of treatment ‐ favourable clinical course (excludes death, major complication, or two or more minor complications) Length of stay ‐ acute hospital, rehabilitation hospital, and total stay Postoperative functional status ‐ Barthel Index Care required after discharge ‐ place of residence at two months Additional outcomes: Measures of nutritional status ‐ self‐reported weight, mid upper arm circumference, Measures of dietary intake ‐ energy and protein intakes from food and supplement Patient compliance ‐ numbers completing full 30 days of supplement | |
| Notes | Request for further details (blinding of outcome assessors, details of supplement administration, further information on outcomes) sent. Reply from trialist (11/6/99) gave details of outcome assessor blinding, supplement administration and outcomes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Hubsch 1992.
| Methods | Method of randomisation: not stated Assessor blinding: not mentioned Intention to treat: carried out Lost to follow‐up: none Timing of intervention: during hospital stay, mean 25 days | |
| Participants | Location: Heidelberg, Germany 32 patients Inclusion criteria: undernourished geriatric patients aged 75y and older Sex: all female Age: over 75y | |
| Interventions | a: 250 ml (238 kcal, 20 g protein) supplement daily in addition to normal hospital diet b: normal hospital diet Allocated: 16/16 Assessed: 16/16 | |
| Outcomes | Main outcomes: mortality Additional outcomes: Measures of nutritional status‐weight | |
| Notes | Request for further details sent 20/04/01 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Jensen 1997.
| Methods | Method of randomisation: sealed envelopes Assessor blinding: blinded Intention to treat: appears intention to treat, but denominators not clear Lost to follow‐up: intervention 5 no intake before discharge; control 7 no intake before discharge Timing of intervention: from time of discharge 110 days elective patients (from 10 days post‐operatively), acute patients from discharge to 110 days post‐discharge | |
| Participants | Location: Surgical Department L, University Hospital of Aarhus, Denmark 34 patients over 75y Inclusion criteria: convalescence after hospital discharge following gastrointestinal surgery, elective patients admitted for colorectal surgery randomised before operation and acute patients randomised prior to discharge operated on due to ileus or peritonitis or had gastric or intestinal surgery performed Exclusion criteria: appendicitis, disseminated malignant disease, inflammatory bowel disease, malabsorption, or dementia Sex: 20 female, 14 male Age: elective 78/82, acute 79/77 Health Status: post‐surgical, not necessarily malnourished | |
| Interventions | a: Advice aimed at a protein intake of 1.5g/kg body weight, milk, quark drink, "Top up special" a complete low fat supplement, and "Plus one" a protein and energy supplement containing no fat (Ferrosan, Soborg, Denmark) (latter 2 offered free). Variety of flavours. Choice of supplement based on patient's taste preference and the recommendations of the dietitian based on estimated requirements. Advice also provided during hospital visits and if felt necessary for compliance, during a home visit. b: Discharged without dietetic advice Allocated: unclear Assessed: 14/20 | |
| Outcomes | Main outcomes: Additional outcomes: Measures of nutritional status‐ Anthropometric indices ‐ weight change, LBM Measures of dietary intake ‐ energy and protein intake | |
| Notes | Request for further information sent 25/10/01. Reply with further information received 9/11/01 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Knowles 1988.
| Methods | Method of randomisation: crossover study unmarked envelope Assessor blinding: carried out Intention to treat: carried out Lost to follow‐up: intervention 0; control 0 Timing of the intervention: for 8 weeks Length of follow‐up: 8 weeks | |
| Participants | Location: Pulmonary Research Laboratory, British Columbia 25 patients Inclusion criteria: ambulatory patients with severe COPD, FEV1 <50% of predicted, stable phase of disease. Did not have acute exacerbation in 3 months prior to study or during study Exclusion criteria: known eating disorder, infectious process, pulmonary disease other than COPD, lactose intolerance, other medical illness, intolerance of nutritional supplement Sex: 4 female, 21 male Age: 68(SD11), 70(SD11) | |
| Interventions | a: Sustacal 24% protein, 22% fat, 54% carbohydrate, aimed to increase calorie intake by 50% above normal level b: usual nutritional care crossover trial Allocated: 13/12 Assessed: 13/12 | |
| Outcomes | Main outcomes: mortality, lung function Additional outcomes: Measures of nutritional status‐ TSF, MAMC weight | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Krondl 1999.
| Methods | Method of randomisation: not stated Assessor blinding: not reported Intention to treat: 4 withdrew ? before or after randomisation Lost to follow‐up: 4 withdrew ? before or after randomisation Timing of intervention: 16 weeks, 24 cans delivered every 28 days, 234ml each, no time of day or daily limit specified, encouraged to spread over days of the week | |
| Participants | Location: Southern Ontario, Canada ‐ community living volunteers 71 patients Inclusion criteria: white, North American/European identity, ability to speak English, independent living, free of major illnesses, not requiring a special diet, free from uncontrolled major disease or infection, avoidance of nutritional supplements at least 30 days prior to study, less than 4 servings of fruit or vegetables per day Exclusion criteria: none given Sex: 54 female, 16 male Age: mean age 70y Health Status: healthy elderly volunteers living at home | |
| Interventions | a: self selected diet plus Boost (Mead Johnson Nutritionals), 234 ml oral nutritional supplement 235 kcal, 11.75 g protein, 5.4 g fat, 35.25 g carbohydrate, 263 mg calcium, 3.8 mg, magnesium 106 mg, potassium 491 mg, phosphorus 261 mg, zinc 3.5 mg, copper 0.5 mg, manganese 0.67 mg, vitamin A 376 RE, vitamin D 1.175 ug, vitamin E 3.52 mg,vitamin C 15 mg, thiamin 0.4 mg, riboflavin 0.47 mg, niacin 5.9 NE, panthothenic acid 1.9 mg, vitamin B‐6 0.59 mg, biotin 14 µg, folate 70 µg, vitamin B‐12 0.9 µg b: self selected diet, no supplements Allocated: 35/36 Assessed: 35/36 | |
| Outcomes | Main outcomes: Mortality Functional status ‐ SF‐36, general well‐being Measures of nutritional status ‐ BMI Measures of dietary intake ‐ energy and protein intake | |
| Notes | Request for information regarding whether the 4 people withdrew before or after randomisation, supplemented group table 3 why 36 people not 35, sent 18/9/01. Reply received 15/10/01 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
Kwok 2001.
| Methods | Method of randomisation: quasi‐randomised
Assessor blinding: not mentioned
Intention to treat: not possible
Lost to follow‐up: intervention 2 dropped out, 1 moved out; control 2 dropped out, 1 moved out Timing of intervention: 7 weeks |
|
| Participants | Location: 2 medium sized private nursing homes Hong Kong 52 patients Inclusion criteria: Exclusion criteria: BMI>27kg/m2 Resident less than 6 months, known wasting disease e.g. cancer or thyrotoxicosis, hospital admission or attendance in accident and emergency department in past month, diabetes mellitus requiring regular medication, regular refusal of milk, milk or oral supplement more than once daily, informed consent from subjects or guardians Sex: 28 female, 19 male Age: mean age 80y | |
| Interventions | a: 4 spoonfuls of milk supplement diluted in warm water twice daily b: no supplement Allocated: 28/24 Assessed: 25/20 | |
| Outcomes | Main outcomes: mortality, grip strength Additional outcomes: measures of nutritional status‐weight, changes in triceps skinfold and mid‐upper arm circumference measures of dietary intake‐ energy and protein intake, side‐effects | |
| Notes | Further details: Request for further details (energy content and volume of supplement, blinding) sent 18/10/01. Reply received 30/10/01 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
Larsson 1990.
| Methods | Method of randomisation: sealed envelopes Assessor blinding: not reported Intention to treat: not done Lost to follow‐up: unclear Timing of intervention: up to 8 weeks, served in the morning and afternoon, when other patients were getting drinks | |
| Participants | Location: 19 wards, University Hospital, Linköping, Sweden 435 patients Inclusion criteria: newly admitted to long‐term medical care, consent to participate, remains hospitalised for over 3 weeks Exclusion criteria: none given Sex: 263 female, 167 male (430) Age: 81y female, 78y male Health Status: long‐term care, 115 with protein‐energy malnutrition, 320 not with protein‐energy malnutrition | |
| Interventions | a: standard hospital diet plus Biosorb drink (Kabi Nutrition, Sweden) 2x200 ml/day providing 400 kcal, 16 g Protein, 16 g Fat, 44.2 g CHO b: standard hospital diet Allocated: 197/238 Assessed: unclear | |
| Outcomes | Main outcomes: Mortality Morbidity and complications ‐ total number pressure sores, number of sores healed Functional status ‐ improvement in modified Norton scale, activity rating | |
| Notes | Request for information sent 21/08/01 regarding vitamin content of supplement, denominators pressure sores, Norton scale, actual patient weights, actual measures of TSF and AMC, resent 14/7/01 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Lauque 2000.
| Methods | Method of randomisation: not stated Assessor blinding: not reported Intention to treat: 6 withdrew from intervention group, intention to treat analysis not carried out Lost to follow‐up: intervention 6 withdrew consent or admitted to hospital; control 0 Timing of intervention: 60 days, strong encouragement was given to consume the entire amount offered | |
| Participants | Location: 8 privately run 80 bed nursing homes in Toulouse, France 35 patients Inclusion criteria: patients aged 65y and over, informed consent from subjects or legal guardian, at risk of malnutrition (MNA 17‐23.5) Exclusion criteria: acute disease, uncertain life expectancy, undergoing chemotherapy, impaired digestion or absorption Sex: 30 female, 5 male Age: mean age 84y Health Status: long‐term care, nutritionally at risk | |
| Interventions | a: nutritional supplements of 300‐500 kcal in addition to regular meals. Four oral supplementation products (Clinutren, Nestle), soup, fruit or dessert each containing 120‐200 kcal, 7.5‐15 g protein and enriched with vitamins and minerals b: no details Allocated: 19/22 Assessed: 13/22 | |
| Outcomes | Main outcomes: Mortality Morbidity and complications ‐ illness Functional status ‐ handgrip Additional outcomes: Measures of nutritional status ‐ mini nutritional assessment score Anthropometric indices ‐ weight change, BMI Measures of dietary intake ‐ energy and protein intake | |
| Notes | Data from groups B and C only (RCT part). Request for more information regarding blinding of outcome assessors, content and administration of supplements and mini nutrition assessment scores sent 18/9/01. Reply received 26/9/01 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Lauque 2004.
| Methods | Method of randomisation: randomised by drawing numbers with sealed envelopes Assessor blinding: the dietitian was the only assessor aware of group allocation Intention to treat: no Lost to follow‐up: intervention 7 excluded (did not comply), 1 lost to follow‐up (hospitalised), 1 withdrawn; control 2 withdrawn Timing of the intervention: for 3 months Length of follow‐up: 6 months | |
| Participants | Location: Geriatric wards and day centres, Toulouse area, France 91 patients Inclusion criteria: Patients with Alzheimer's disease aged 65 years and older, and at risk of undernutrition (MNA score = 23.5) | |
| Interventions | a: Clinutren (Nestlé Clinical Nutrition, Noisiel, France) ranging between 300 and 500 kcal/d in addition to the patients' spontaneous food intake. b: usual care Allocated: 46/45 Assessed: unclear | |
| Outcomes | Main outcomes: Mortality, functional status, weight, body composition, dietary intake Additional outcomes: Hospitalization, no denominators provided for fractures or pressure ulcers | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
MacFie 2000.
| Methods | Method of randomisation: sealed envelopes Assessor blinding: not blinded Intention to treat: carried out Lost to follow‐up: apparently none Timing of intervention: patients were instructed to drink the supplements in addition to and not in place of their normal diet and continued up until the day before surgery, pre op minimum 10 days mean 15d (5‐59), post op minimum 7 days mean 8d (0‐20), post op supps as soon as oral fluids were permitted up until 4 weeks after discharge. 4 groups; preop supps, postop supps, both, neither | |
| Participants | Location: preoperative outpatient phase and postoperative inpatient phase, combined gastroenterology unit at Scarborough hospital 100 patients Inclusion criteria: patients requiring major elective gastrointestinal surgery. Exclusion criteria: dementia, major concurrent, metabolic problems, such as uncontrolled diabetes, advanced liver disease, or uraemia, those requiring emergency surgery. Patients were withdrawn if any member of the clinical or nutrition team considered that adjuvant parenteral or enteral support was indicated or whether it was deemed clinically appropriate to proceed with the surgery within 10 days. Sex: 54 female, 46 male Age: mean ages 63, 68, 66, 64y Health Status: patients receiving gastrointestinal surgery, most patients well nourished | |
| Interventions | a: 2 x 200 mL cartons (Fortisip, Nutricia Ltd) in a variety of flavours providing 1.5 kcal, 0.05 g protein and 0.18 g carbohydrate per ml. A fruit flavoured supplement (fortijuice, Nutritia Ltd) was available as an alternative, providing 1.25 kcal, 0.025 g protein and 0.285 g carbohydrate per ml b: usual diet Allocated: 24/24/27/25 Assessed: 24/24/27/25 | |
| Outcomes | Main outcomes: Mortality Morbidity and complications ‐ complications (total, septic) Length of stay ‐ post operative stay Side effects of treatment ‐ nausea with supplements Functional status ‐ hand grip, postoperative anxiety and depression Additional outcomes: Measures of nutritional status‐ Anthropometric indices ‐ perioperative weight loss, mid upper arm circumference Measures of dietary intake ‐ energy intake | |
| Notes | Analysed 75/25, request for information regarding how many measured for outcomes, why the supplements were stopped, the number of women in group 2 and when the patients died, sent 18/9/01. Reply received 1/10/01 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Madigan 1994.
| Methods | Method of randomisation: not stated Assessor blinding: not reported Intention to treat: not carried out, results presented for 30 patients, 34 randomised, results from the two supplemented groups were combined Lost to follow‐up: unclear Timing of intervention: started on admission for 10 days, once daily after evening meal Length of follow‐up: 3 months post‐discharge | |
| Participants | Location: Illawarra Regional Hospital, Port Kembla Campus, Woolongong, Australia 34 patients Inclusion criteria: femoral neck fracture resulting from an accidental fall, age over 60y, informed consent Exclusion criteria: pathological fracture due to tumour; fracture due to violent external trauma; elective total hip replacement; renal, hepatic, metastatic or endocrine (affecting skeletal metabolism) disease; admitted from nursing home; failure to gain consent; transferred to another hospital for surgery Sex: 22 female, 8 male, of 30 Age: all over 60y | |
| Interventions | a: 250 ml oral supplement prepared by dietitian from ProMod (protein powder) and Polyjoule (glucose polymer) providing 1.30 MJ or 310 kcal; 16 g protein, 41.4g carbohydrate, 9.2 g fat, 0.19 mg riboflavin, 245 mg calcium, phosphorus 171 mg, and standard hospital diet. b: One multivitamin/mineral tablet daily ((ELEVIT RDI, Roche) providing 750 µg vitamin A, 1.1 mg thiamin, 1.7 mg riboflavin, 20 mg nicotinamide, 7 mg pantothenic acid, 1.9 mg pyridoxine, 2 µg vitamin B12, 200 µg biotin, 200 µg folic acid, 30 mg vitamin C, 200 IU vitamin D3, 15 IU vitamin E, 125 mg calcium, 100 mg magnesium, 125 mg phosphorus, 5 mg iron, 1mg copper, 1mg manganese, 7.5 mg zinc 250 ml), plus oral supplement as above, and standard hospital diet c: Standard hospital diet Allocated: unclear Assessed: 18/12 (a+b/c) | |
| Outcomes | Main outcomes: Mortality Morbidity and complications ‐ numbers of complications (urinary infections, wound infections/delayed healing, pressure sores, pneumonia, deep venous thrombosis, sepsis Length of stay ‐ acute hospital Postoperative functional status ‐ number transferred to rehabilitation hospital, days to reach partial or full weight bearing with support, days to reach independent mobility Care required after discharge ‐ discharge to home, hostel, nursing home, number of subjects returning to pre‐morbid mobility Additional outcomes: Measures of nutritional status ‐ mid upper arm circumference (*nr), triceps skinfold Measures of dietary intake ‐ energy and protein intakes from food and supplements Patient compliance ‐ number taking protein supplement for only 7 days | |
| Notes | In the trial report, the two supplemented groups were combined for analysis for comparison with control group. Three subjects eliminated post‐randomisation from analysis because only took protein supplement for 7 days, and one eliminated for developing diabetes. Numbers of patients assigned/assessed not always clear. Request for further details sent 4/2/00 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Manders 2006.
| Methods | Method of randomisation: states randomised but no further details Assessor blinding: double‐blind placebo‐controlled trial Intention to treat: not undertaken Lost to follow‐up: 65 (withdrawn, excluded after randomisation or lost to follow‐up)‐ unclear which arm Timing of intervention: 24 weeks Length of follow‐up: 24 weeks | |
| Participants | Location: homes for elderly or sheltered housing, The Netherlands 176 participants Inclusion criteria: 60 years or older, BMI 30kg/m2 or less Exclusion criteria: serious morbidity (cancer, severe infection, parenteral or tube feeding, gastrointestinal disorders, terminal care); interfering co‐interventions (medications or supplements with effect on safe administration of intervention) Sex: 122 female, 54 male Age: mean age 83y | |
| Interventions | a: 2 x 125ml daily oral liquid supplements with total of 250kcal and 8.75g protein, providing 25‐175% of Dutch RDA of micronutrients b: placebo with water, sweetener, cloudifier, thickening, flavouring, colour, non‐caloric sweetener Allocated: 119/57 Assessed: 78/33 | |
| Outcomes | Main outcomes: Mortality Morbidity and complications ‐ number developing illness Functional status ‐ Barthel Index, Frail Elderly Functional Capacity, Berkhov feeding scale, cognitive function Additional outcomes: Measures of nutritional status ‐ weight change Measures of dietary intake ‐ energy and protein intake | |
| Notes | Emailed Dr Manders on 10/01/2008 to ask for death and illness information according to allocation. No information received 16 March 2008 therefore not included | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
McEvoy 1982.
| Methods | Method of randomisation: not stated Assessor blinding: not reported Intention to treat: apparently no withdrawals Lost to follow‐up: none Timing of intervention: 4 weeks, patients with malabsorption in both groups received any necessary supplements such as calcium, vitamin D and haematinics, not given encouragement to eat extra food | |
| Participants | Location: acute geriatric ward, Freeman Hospital, Newcastle upon Tyne 51 patients Inclusion criteria: elderly patients admitted to an acute geriatric ward. Poorly nourished according to at least two of the following criteria; weight below 85% of ideal weight for height, triceps skinfold thickness below 85% of standard values, serum albumin less than 34g/l. Exclusion criteria: malignant conditions, metabolic disease such as thyrotoxicosis or diabetes Sex: not given Age: not given Health Status: acute geriatric ward, poorly nourished | |
| Interventions | a: in addition to normal hospital diet, two sachets of Build‐up daily providing 36.4 g Protein and 644 kcal, states consumption ensured by the nursing staff b: normal hospital diet Allocated: 26/25 Assessed: 26/25 | |
| Outcomes | Main outcomes: Mortality Additional outcomes: Measures of nutritional status‐ Anthropometric indices ‐ weight change, mid upper arm circumference, triceps skinfold, arm muscle circumference | |
| Notes | More information requested regarding baseline characteristics of study population, care programmes, blinding of outcome assessment, details of supplement sent 14/7/01 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
McWhirter 1996.
| Methods | Method of randomisation: not stated Assessor blinding: not mentioned Intention to treat: states ITT but not clear Lost to follow‐up: 3 withdrawals (unclear which arm) Timing of the intervention: minimum of 7 days Length of follow‐up: until discharge or stopped | |
| Participants | Location: Ninewells Hospital, Dundee 61 patients Inclusion criteria: patients admitted to the medical unit identified as malnourished using anthropometry, BMI < 20 TSF and/or MAMC < 5th percentile Exclusion criteria: none given Sex: not given Age: 69 / 74y | |
| Interventions | a: Tonexis, (Clintec Nutrition Ltd)100 kcal, 3.75g protein, 3.33g fat, 13.8g CHO according to energy requirements and corrected for stress and activity b: no supplement Allocated: ?/? Assessed: 35/26 | |
| Outcomes | Main outcomes: none Additional outcomes: Measures of nutritional status ‐% change in weight and AMC | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Meredith 1992.
| Methods | Method of randomisation: not stated Assessor blinding: not reported Intention to treat: not carried out, 1 excluded for protocol violation Lost to follow‐up: details given Timing of intervention: exercise sessions and diet intervention conducted three times per week over the 12 week period for both groups | |
| Participants | Location: Human Physiology Laboratory, Boston, Massachusetts 11 participants Inclusion criteria: healthy out‐patient volunteers recruited by advertisement, sedentary, non obese men over 60y Exclusion criteria: a wide range of cardiac related conditions, recent embolism, high dose of phenothiazine agents, uncontrolled metabolic disease, clinically severe hypertension (diastolic above 110), severe anaemia, marked obesity (BMI above 30kg/m2), renal hepatic or other metabolic insufficiency, overt psychoneurotic disturbances, moderate to severe pulmonary disease, coagulation defects, neuromuscular disease, connective tissue diseases Sex: all male Age: 68/65y Health Status: very healthy, non obese, living at home and in the research unit | |
| Interventions | a: ad libitum diet plus complete nutrient mixture (Two Cal HN, Ross Laboratories, Ohio) per 100 ml: 200 kcal, 8.3 g protein, 21.9 g carbohydrate, 8.9 g fat, vitamins and minerals, designed to provide an additional 8 kcal and 0.33 g protein per kg of ideal body weight over and above normal ad libitum diet. The vitamin and mineral content was between 25 and 75% of the RDA. The supplement was consumed as two drinks of about 120 ml each, served cold and in a variety of flavours about 10 am and 10 pm every day b: ad libitum diet with no placebo Allocated: 6/6 Assessed: 6/5 | |
| Outcomes | Main outcomes: Mortality Functional status ‐ dynamic strength Additional outcomes: Measures of nutritional status‐ Anthropometric indices ‐ weight change, fat‐free mass, sum of six skinfolds Measures of dietary intake ‐ energy and protein intake | |
| Notes | All participants received strength training and have been included Request for information regarding blinding of outcome assessors and mineral and vitamin content of supplement sent 14/7/01 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Ovesen 1992.
| Methods | Method of randomisation: not stated Assessor blinding: blinded Intention to treat: not carried out, 10 refused to complete due to gastro‐intestinal discomfort ascribed to the sip‐feeds Lost to follow‐up: details given Timing of intervention: 10 days, both interventions have same energy distribution of protein fat and carbohydrate, both served in 200ml tetrabriks without labelling, 10 days supplementation, offered a minimum of 4 x 200ml daily between meals | |
| Participants | 24 patients Inclusion criteria: elderly patients, non infectious chronic disease, hospitalisation expected for at least 10 days, weight loss (within 3 months) of more than 10% of their usual body weight or a plasma albumin of less than 0.4 mmol/l, daily energy intake of less than 1.5 x basal energy expenditure, or a protein intake of less than 1g/kg during the first 48 h of admission Exclusion criteria: patients on special diets, unable to participate in taste testing Sex: 17 female, 7 male Age: 74/75y Health Status: inpatients, poor appetite and intake | |
| Interventions | a: liquid supplement containing 6.3 kJ and 0.06 g protein per ml (Nutrison Energirig, Nutricia, DK), nutritionally complete and lactose free, patients chose either vanilla or orange flavour b: liquid supplement containing 4.2 kJ and 0.04 g protein per ml (Nutrison Standard (Nutricia, DK), nutritionally complete and lactose free, patients chose either vanilla or orange flavour Allocated: 17/17 Assessed: 10/14 | |
| Outcomes | Main outcomes: Mortality Side effects of treatment ‐ gastrointestinal discomfort Additional outcomes: Measures of dietary intake ‐ energy and protein intake Patient compliance ‐ numbers completing 10 days of supplement | |
| Notes | Request for further information regarding method of randomisation, content of supplement, whether outcome assessors were blinded to treatment status, whether other outcomes were measured sent 19/9/01 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Payette 2002.
| Methods | Method of randomisation: not stated Assessor blinding: not blinding for all outcomes Intention to treat: for deaths only Lost to follow‐up: details provided Timing of the intervention: for 16 weeks Length of follow‐up: 16 weeks | |
| Participants | Location: Sherbrooke, Quebec, Canada (17 community service centres) 83 patients Inclusion criteria: receiving long‐term home help services, Older than 65 years, higher nutritional risk a) involuntary weight loss > 5% of weight in past month, or weight loss > 7.5% in past 3 months or > 10% in past 6 months and BMI < 27 or b) BMI < 24 Exclusion criteria: Palliative care, alcoholic, active cancer, illness requiring therapeutic diet incompatible with supplementation Sex: 59 female 24 male Age: 81.6 (SD 7.5), 78.6 (SD 6.1) | |
| Interventions | a: 2x 235ml cans/day Ensure or Ensure plus aim to gain 0.5 kg/ week b: none Allocated: 43/46 Assessed: 41/42 | |
| Outcomes | Main outcomes: mortality, functional status, quality of life Additional outcomes: bed disability days, complications, compliance Measures of nutritional status‐ weight, arm muscle circumference Measures of dietary intake ‐energy and protein intake | |
| Notes | Classified as undernourished, at home and well, need confirmation of denominators | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Payette 2004.
| Methods | Method of randomisation: sealed envelopes, insufficient detail to confirm concealed allocation concealment: A Assessor blinding: not all outcomes blinded Intention to treat: unclear Lost to follow‐up: unclear Timing of the intervention: 24 weeks Length of follow‐up: 24 weeks | |
| Participants | Location: Sherbrooke, Quebec, Canada Inclusion criteria: Recruited from Meals‐on Wheels programmes offered by 7 community‐based volunteer agencies. Over 65y, received at least two meals per week, orientated to time and place, at risk of malnutrition (MNA score greater than or equal to 17 and less than 24‐ modified MNA) Exclusion criteria: Palliative care, alcoholic, active cancer, illness requiring therapeutic diet incompatible with supplement, wheel‐chair bound, BMI >30 Sex: 77 female, 22 male Age: 79.4 (SD 6.1) | |
| Interventions | a: Professional counselling to consume food or supplement to achieve at least 100% Canadian nutritional recommendations for energy, protein and all nutrients. Encouraged to take at least one 250 ml can of NuBasics Plus Complete Nutrition Drink (Nestle) for 24 weeks. Dietitian adjusted supplement according to tolerance. Monthly visits for 6 months b: Monthly contact be phone or visit Allocated: 54(50)/51(49)? Assessed: 50/49 | |
| Outcomes | Main outcomes: muscle strength, functional outcomes, timed up and go, no others yet reported Additional outcomes: Measures of nutritional status‐ Anthropometric indices ‐ triceps skinfold, weight change Measures of dietary intake ‐ change in energy and protein intake | |
| Notes | Reply received regarding details of study 7/04/2004. Further information requested regarding number of participants and randomisation method. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Potter 2001.
| Methods | Method of randomisation: block randomisation with sealed envelopes Assessor blinding: anthropometry and clinical outcome blinded, ADL unlikely to be blinded Intention to treat: carried out Lost to follow‐up: none, all patients accounted for Timing of intervention: from within 48 hours of admission until the time of discharge home, death or referral for institutional placement at 0800, 1400 and 1800 hours | |
| Participants | Location: Medicine for the Elderly Unit, Glasgow, UK 381 patients Inclusion criteria: no known malignancy, able to swallow, non obese (BMI <75th percentile), able to gain consent from patients or relatives Sex: not given Age: not given, but elderly | |
| Interventions | a: 120ml oral nutritional supplement (Entera Frusenius) three times daily intended to provide 540 kcal/day, 22.5 g protein/day b: usual practice Allocated:186/195 Assessed:165/162 | |
| Outcomes | Main outcomes: Mortality Complications (sepsis) Length of stay ‐ Acute hospital Discharge destination Functional status ‐ Barthel Index Additional outcomes: Measures of nutritional status‐arm muscle circumference, percentage weight change Measures of dietary intake ‐ energy intake (random sample of one in three patients on day 3) | |
| Notes | Further information received from author regarding: Date study began and ended, timing of baseline data collection and outcome assessments (weight and AMC), length of follow‐up, whether length of stay included those who died, whether care programmes were identical, further details on complications (sepsis) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Price 2005.
| Methods | Method of randomisation: sealed opaque envelopes, prepared by another individual from computer generated random number table Assessor blinding: open trial Intention to treat: yes Lost to follow‐up: details provided Timing of the intervention: daily for 8 weeks after discharge from hospital Length of follow‐up: 6 months | |
| Participants | Location: Dundee area, Scotland recruited from Ninewells or Royal Victoria Hospital 76 patients Inclusion criteria: recruited from acute general medical wards and medicine for the elderly assessment and discharged back to the community (own home, with relatives, sheltered housing or residential homes) evidence of undernutrition defined by admission BMI = 24 with MAMC or TSF < 10th centile and or 5% or more loss in body weight during hospital stay. Sex: 101 female, 35 male Age: 83.7 (SD 5.2), 85.4 (SD 5.4) | |
| Interventions | a: 2 cartons (400 ml, 600 kcal, 24g protein, 72g carbohydrate Fortisip or Fortifresh, Nutricia, UK) in a variety of flavours b: no supplement provided Allocated: 66/70 Assessed: 35/41 | |
| Outcomes | Main outcomes: Mortality, complications, functional status, anthropometry, weight, dietary intake Additional outcomes: Unplanned readmissions, one or more prescription of antibiotics, new antidepressant prescriptions, introduction of other new medication, out patient or day hospital attendance, respite admission, falls, planned admissions, admissions to residential home | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Rosendahl 2006.
| Methods | Method of randomisation: stratified cluster randomised controlled trial with exercise and nutritional intervention and placebo in 2x2 factorial model Assessor blinding: all assessors blinded Intention to treat: yes Lost to follow‐up: recorded Timing of intervention: 3 months Length of follow‐up: 6 months | |
| Participants | Location: residential care facilities in Emea, Sweden
Inclusion criteria: >65years, dependent of assistance in one or more personal ADL activities on KATZ index, able to stand from a chair with arms with help of only 1 person or less, MMSE of >10, physician approval Exclusion criteria: not detailed Sex: 139 female, 52male Age: mean 84.5years |
|
| Interventions | A: semi protein energy supplement 200ml 7.4g protein and 408kJ /100g B: 200ml drink 0.2g protein and 191kJ per 100g Allocated: 96, 95 Assessed: 82, 81 at 6 months | |
| Outcomes | Main outcomes: Mortality
Berg balance scale
Gait speed self paced
Gait speed maximum
1 RM lower limb strength Others, death recorded |
|
| Notes | Mortality data not included in meta‐analysis as cluster randomised and don't have the intra‐class correlation coefficient. Exercise randomised also for each cohort | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Salas‐Salvado 2005.
| Methods | Method of randomisation: centralised and both groups stratified by initial BMI Assessor blinding: no information given re attempts to blind assessors as intervention very obvious presume unblinded Intention to treat: no Lost to follow‐up: not recorded but numbers do not add up Timing of intervention: 3 months Length of follow‐up: 3 months | |
| Participants | Location: 6 geriatric institutions Catalonia spain, 53 patients
Inclusion criteria: Alzheimers disease DSM IV criteria 3 or above, requiring semi solid or liquid diet, and present weight loss or higher than 5% weight loss in the previous year Exclusion criteria: terminal care, sever acute illness, cancer or history of in last 5years, sever GI disease or any other acute illness able to affect nutritional status during study, significant hepatic or renal disease, enteral or parenteral nutritional support at time of study, chronic treatment with steroids antineoplastic drugs or antibiotics, diabetic patients on insulin, use of nutritional supplements 15days prior to study. Sex: 44 female, 9 male Age: mean 84.7years |
|
| Interventions | A: semi solid foods plus dietetic advice, 3 x 451.52kcals per day plus other normal intake B: dietetic advice plus access to above foods as an extra Allocated: 24, 29 Assessed: 15, 23 at 3 months | |
| Outcomes | Weight change Dietetic intake Functional rating scale | |
| Notes | Numbers don't add up in outcomes section | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Saudny 1997.
| Methods | Method of randomisation: sealed envelopes Assessor blinding: all strength measurements blinded Intention to treat: not carried out, results presented for 24 patients, 33 randomised Lost to follow‐up: intervention 1 refused to come back or could not be located, 1 refused to continue or data could not be used; control 3 refused to come back or could not be located, 1 refused to continue or data could not be used, 1 too ill to continue Timing of intervention: 14 days | |
| Participants | Location: Montreal Chest Institute 24 patients Inclusion criteria: consecutive patients 40‐85y with diagnosis of COPD and a FEV1 that was less than or equal to 60% of the predicted value Exclusion criteria: patients who required mechanical ventilation, had a gastrointestinal disorder, had active cancer or the condition predisposing to weight loss, were terminally ill, were unable to communicate in English or French, suffered from mental confusion or followed a special diet Sex: 9 female, 15 male Age: mean age 69y Health Status: patients hospitalised with an exacerbation of COPD, not necessarily malnourished | |
| Interventions | a: food and beverages ordered by the participants from the hospital menu, additional oral supplements (Ensure, Ensure plus, or a variety of puddings or extra snacks) to assure a caloric intake of at least 1.5 x REE if their BMI was normal (20‐27) and at least 1.7 X REE if their BMI was below 20 b: food and beverages ordered by the participants from the hospital menu Allocated: 17/16 Assessed: 14/10 | |
| Outcomes | Main outcomes: Mortality Morbidity and complications ‐ to ill to continue Length of stay ‐ rehabilitation hospital Functional status ‐ lung function, general well being, grip strength, 6 minute walk test Additional outcomes: Measures of nutritional status‐ Anthropometric indices ‐ weight change Measures of dietary intake ‐ energy and protein intake | |
| Notes | Request for information sent regarding when did deaths occur, were care programmes identical and information about amount of supplement provided sent 14/7/01. Reply with more information received 19/11/01 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Schols 1995.
| Methods | Method of randomisation: not stated Assessor blinding: not reported Intention to treat: carried out, but results not ITT analysis Lost to follow‐up: incomplete report of drop‐outs A group of patients randomised to receive nandrolone decanoate have been excluded from this analysis. All patients included also undertook the standard physical training programme over the period of the study. Length of follow‐up: 8 weeks | |
| Participants | Location: Rehabilitation Centre, University Hospital, Maastricht, the Netherlands 135 patients Inclusion criteria: patients with moderate to severe COPD consecutively admitted to an intensive inpatient pulmonary rehabilitation programme, stable clinical condition, patients who had an acute exacerbation during the study and who were effectively treated with glucocorticosteriods and/or antibiotics remained in the study Exclusion criteria: unstable COPD or greater than 10% increase in FEV of the predicted baseline value after administration of 400ug salbutamol, obesity, malignancies, ischemic heart disease or other cardiac impairment, renal, hepatic, gastrointestinal or endocrine disease, surgery within last 2 months Sex: not given Age: mean age 65y Health Status: patients admitted to an intensive inpatient pulmonary rehabilitation programme with moderate to severe COPD, stratified into two groups : depleted group: less than 90% ideal body weight or fat‐free mass less than 67% (men) 63% women, and non depleted | |
| Interventions | a: meal size and composition were chosen by all the patients themselves and registered daily, patients encouraged to eat their regular meals. In addition, one high calorie drink supplement daily, early evening between 7pm and 9pm for at least 8 weeks: 200 ml, 420 kcal, 51% fat, 35% carbohydrate, 14% protein, containing Nutridrink, Protifar, Fantomalt and oil, 7 flavours b: standard hospital diet, patients encouraged to eat their regular meals Allocated: 72/63 Assessed: 72/63 (33 depleted, 39 non depleted/38 depleted, 25 nondepleted) | |
| Outcomes | Main outcomes: Mortality Functional status ‐ lung function, walking distance Additional outcomes: Measures of nutritional status‐ Anthropometric indices ‐ weight change, mid‐arm muscle circumference, skinfold thickness (4 sites), change in FFM Measures of dietary intake ‐ energy intake Side effects of treatment | |
| Notes | Further information requested regarding randomisation, blinding of outcome assessors, vitamin and mineral content of supplement, denominators for mortality data sent 20/10/01. Reply received 1/11/01 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Scorer 1990.
| Methods | Method of randomisation: not stated ‐ allocation concealment B Assessor blinding: not mentioned Intention to treat: not mentioned Lost to follow‐up: 10 withdrawn (3 insufficient data, 7 weight loss less than 2 kg in run‐in) ‐ unclear which arm Timing of the intervention: 12 weeks Length of follow‐up: 12 weeks | |
| Participants | Location: 53 General Practices, UK carried out by Abbott UK Medical Period of study: 130 patients Inclusion criteria: community dwelling women, 65 years or over, undernourished as defined by GP Exclusion criteria: active neoplastic disease, active metabolic disease, history of malabsorption, inflammatory bowel disease, significant cardiovascular disease, receiving diuretics, corticosteroids or anabolic drugs, institutionalised patients Sex: 85 female 45 male Age: 76/77y | |
| Interventions | a: 3 cans /day Ensure per day between meals and home visits b: carbonated water (330ml can) Allocated: ?/? Assessed: 48/46 | |
| Outcomes | Main outcomes: none Additional outcomes: Nottingham Health Profile, compliance Measures of nutritional status‐ weight | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
SG Larsson malnour.
| Methods | Subgroup of initially malnourished patients | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
SG Larsson nourished.
| Methods | Subgroup of well nourished patients | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
SG Potter malnourish.
| Methods | Subgroup of moderately and severely malnourished patients | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
SG Potter moder maln.
| Methods | Subgroup of moderately malnourished patients | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
SG Potter nourished.
| Methods | Subgroup of adequately nourished patients | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
SG Potter very maln.
| Methods | Subgroup of severely malnourished patients | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
SG Volkert comply.
| Methods | Subgroup of patients with good acceptance of the supplement (one or nearly one portion per day) | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
SG Volkert non compl.
| Methods | Subgroup of patients with poor acceptance of the supplement (one portion every two days or less) | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Stableforth 1986.
| Methods | Method of randomisation: not stated Assessor blinding: not reported Intention to treat: 3 excluded, intention to treat analysis not possible Lost to follow‐up: none Timing of intervention: started after surgery and 24‐36h of crystalloid intravenous fluids. Intervention provided during waking hours for 10 days Length of follow‐up: 4 weeks | |
| Participants | 61 patients randomised Inclusion criteria: hip fracture patients within 12h of fracture, women over 65y Exclusion criteria: none given Sex: all female Age: Mean 81.8y, range 65‐96y | |
| Interventions | a: Encouraged to drink flavoured, Carnation Instant Breakfast in 300ml milk (1.34 MJ or 320 kcal, 18.5 g protein, 11g fat, 40 g carbohydrate, vitamins and minerals) plus ward diet b: Ward diet alone Allocated: unclear Assessed: unclear | |
| Outcomes | Main outcomes: Mortality ‐ all causes Morbidity and complications ‐ anaesthetic, surgical infection, gastrointestinal, urinary Additional outcomes: Measures of nutritional status ‐ weight Measures of dietary intake ‐ energy balance, nitrogen balance | |
| Notes | Limited functional outcomes. Request for further details, especially on longer term follow‐up, sent 13/4/99, resent 7/2/00 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Steiner 2003.
| Methods | Method of randomisation: allocated and dispensed by pharmacy‐ allocation concealment: A Assessor blinding: unclear if investigators knew allocation when analysing the results Intention to treat: yes Lost to follow‐up: intervention 15 (plus 2 withdrawn); control 7 (plus 1 withdrawn) Timing of intervention: 7 weeks, dispensed each week when participant attended outpatient rehabilitation clinic each week Length of follow‐up: 7 weeks | |
| Participants | Location: Glenfield Hospital, Leicester, UK 85 patients Inclusion criteria: Referred to pulmonary rehab programme with clinical and spirometric criteria for COPD, stable at recruitment, optimised medical treatment Exclusion criteria: Unsuitable for exercise‐ cardiac, neuropsychiatric or musculoskeletal disorders, diabetes, glucose intolerance, BMI >30 Sex: 32 female, 53 male Age: 66.0 (SD 9.0)/ 68.0 (SD 8.0) | |
| Interventions | a: 125 ml Respifor (Nutricia, Netherlands)‐ 570 kcal/day (CHO 60%, fat 20%, protein 20%), 3 times daily for duration of rehab. b: Non‐nutritive placebo‐ same packaging and flavouring, 3 times daily for duration of rehab. Allocated: 42/43 Assessed: 25/35 | |
| Outcomes | Main outcomes: mortality, COPD complications, functional outcomes‐ handgrip strength, muscle strength walking, self reported chronic respiratory questionnaire Additional outcomes: Anthropometric indices ‐ weight change, BMI change, dietary intake change, self reported compliance | |
| Notes | Classified as nourished, unwell, and at home Non commercial trial | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Tidermark 2004.
| Methods | Method of randomisation: opaque sealed envelope‐ allocation concealment: A Assessor blinding: unclear for some outcomes Intention to treat: carried out Lost to follow‐up: intervention 1; control 2 Timing of the intervention: 6 months | |
| Participants | Location: Stockholm, Sweden Period of study: Prior to October 2002 Number of patients: 40 Inclusion criteria: Females with acute femoral fracture, > 70 years, BMI less than or equal to 24 kg/m2, absence of severe cognitive disfunction, independent living status and independent walking capability with or without walking aids Exclusion criteria: Patients with fractures not suitable for internal fixation and patients with rheumatoid arthritis or radiographic osteoarthritis Sex: 40 female Age: 83.5 (SD 6.1), 84.1 (SD 4.3) | |
| Interventions | a: Fortimel 200 ml/day, 20 g Protein, plus 1g Calcium and 400 IU Vitamin D b: usual nutritional care plus1g Calcium and 400 IU Vitamin D Allocated: 20/20 Assessed: 18/17 | |
| Outcomes | Length of follow‐up: 12 months Main outcomes: mortality, length of hospital stay during first year after surgery, complications, mobility, activities of daily living, hand grip strength, adverse effects, quality of life Additional outcomes: compliance Measures of nutritional status‐ weight, LBM | |
| Notes | Also looked at the effects of nandrolone in a third arm of the trial Classified as undernourished, acute admission and living in the community. Further details required regarding details of intervention | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Vermeeren 2004.
| Methods | Method of randomisation: no description Assessor blinding: states double blind but no details Intention to treat: states number and reasons for withdrawal but intention to treat not possible Lost to follow‐up: intervention 3 (nausea); control 1 (nausea) ‐ plus 4 withdrawn due to medical problem and 1 alcohol problem (unclear which arm) Timing of the intervention: from first day of hospitalisation 3 times a day between meals Length of follow‐up: unclear | |
| Participants | Location: University hospital Maastricht and regional hospital 'MaximaMedical Centre' in Veldhaven, Netherlands 56 patients Inclusion criteria: Acutely admitted to hospital for exacerbation of COPD (diagnosis based on GOLD criteria). Recent increase in breathlessness, cough and sputum sufficient for admission judged by independent physician. Sex: 32 male, 65 female Age: 66 (SD 8), 65 (SD 10) | |
| Interventions | a: 125 ml Respifor 3 times day (Nutricia, Zoelemeer, The Netherlands), (2.38 MJ/day) b: 125 ml vanilla flavoured water (0 MJ/day) Allocated: 29/27 Assessed: 23/24 | |
| Outcomes | Main outcomes: weight, complications, functional status, dietary intake Additional outcomes: readmissions, side‐effects | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Vlaming 2001.
| Methods | Method of randomisation: block randomisation of 100 by pharmacy, sealed envelopes, remote site Assessor blinding: yes Intention to treat: done Lost to follow‐up: intervention 0; control 0 (all patients accounted for) Timing of the intervention: whenever their clinical team allowed Length of follow‐up: mean 14.2 (SD24.9), 11.4 (SD16.4) days | |
| Participants | Location: The Royal London Hospital and St Bartholomew's Hospital, London 549 patients Inclusion criteria: patients admitted though acute services ‐ general medical, surgical or orthopaedic, thin but not seriously undernourished ‐ BMI 18‐22, or unintentional weight loss greater than or equal to 5% Exclusion criteria: planned admissions to medical or orthopaedic wards, <18y, mental illness, already routine treatment with water soluble vitamins, admission clearly for 2 days or less, previously taken part in trial, BMI <18, unintentional weight loss >10%, therapeutic diets, unable to swallow liquids, randomisation clinically unacceptable, unable to gain consent Sex: 314 female, 235 male Age: 67/66 | |
| Interventions | a: 2 x 200ml Ensure plus, 600 kcal, 25g protein, 80.8g carbohydrate, 19.6g fat plus vitamins and minerals plus or minus a vitamin supplement b: 400ml placebo drink 100kcal, 25g carbohydrate plus or minus a vitamin supplement Allocated: 275/274 Assessed: 271/274 | |
| Outcomes | Main outcomes: mortality, length of hospital stay Additional outcomes: compliance | |
| Notes | Length of follow‐up: 12 months Main outcomes: mortality, length of hospital stay during first year after surgery, complications, mobility, activities of daily living, hand grip strength, adverse effects, quality of life Additional outcomes: compliance Measures of nutritional status‐ weight, LBM | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Volkert 1996.
| Methods | Method of randomisation: states randomised by drawing lots but unclear if those who assigned were blinded Assessor blinding: not reported Intention to treat: not carried out, split analysis on the basis of compliance Lost to follow‐up: intervention 11; control 3 Timing of intervention: during hospital stay and after discharge for 6 months | |
| Participants | Location: during hospitalisation in an Acute Care ward of the Geriatric Centre Bethanien in Heidelberg, Germany, and after discharge 46 patients Inclusion criteria: female, aged 75y or older without malignant disease or need for tube feeding or parenteral nutrition, undernourished by clinical judgement of the examining physician, (BMI used to confirm clinical judgement when data on body weight and height were available), expected hospital stay at least 3 weeks, presumed actual life expectancy of more than 6 months, consent from participants or care givers Exclusion criteria: none given Sex: all female Age: mean age 85y Health Status: long term care, nutritionally 'at risk' | |
| Interventions | a: in addition to the standard hospital diet 2 portions x 200ml of a liquid supplement during hospital stay (200ml soup mid‐morning and 200ml sweet drink in the afternoon daily), different brands but with similar composition but different flavours were used in order to increase variety and patient acceptance. On average 2 portions provided 500 kcal and 30 g protein/day, providing 50 ‐ 150% of the recommendation for most micronutrients. After discharge, 1 daily portion of the sweet supplement was provided. Patients were strongly encouraged to consume the entire amount offered. Patients were initially visited at home to provide new supplements every week, and then every two weeks b: usual care without supplements Allocated: 35/37 Assessed: 20/26 | |
| Outcomes | Main outcomes: Mortality Functional status ‐ Barthel Index Additional outcomes: Measures of nutritional status‐ Anthropometric indices ‐ weight change, LBM Measures of dietary intake ‐ energy and protein intake Patient acceptance ‐ poor acceptance = one portion every two days or less | |
| Notes | Further information requested regarding method of randomisation, blinding of treatment status, denominators for mortality sent 20/10/01 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Woo 1994.
| Methods | Method of randomisation: computer randomised Assessor blinding: blinded apart from dietary assessment Intention to treat: not carried out Lost to follow‐up: incomplete report of drop‐outs Participants in both groups able to return for medical consultation if they felt unwell in any way. Length of follow‐up: 3 months | |
| Participants | Location: Prince of Wales Hospital, a District General hospital, Hong Kong 81 patients Inclusion criteria: patients consecutively admitted to acute medical wards of a district general hospital not catering for chronic disabled or demented patients, primary diagnosis of chest infection (purulent sputum, increasing shortness of breath, pyrexia, elevated white cell count, with or without radiological changes on chest radiography (75% had underlying chronic lung disease) Exclusion criteria: those with heart failure, renal or hepatic failure, stroke, malignancies or bedridden subjects who could not feed themselves, patients who did not give informed consent Sex: 30 female, 51 male Age: mean age 72/74y Health Status: discharged home following acute hospital stay with chest infection, not necessarily malnourished | |
| Interventions | a: 1 month of supplementation, 500 ml Ensure liquid (Abbott Laboratories Ltd) daily, 500 kcal, 14% protein, 32% fat, 54% carbohydrate, minerals and vitamins. Instructed to take it between meals or before bedtime b: no supplement Allocated: 40/41 Assessed: variable | |
| Outcomes | Main outcomes: Mortality Functional status ‐ Barthel Index, appetite, mental test score Additional outcomes: Measures of nutritional status‐ Anthropometric indices ‐ change in BMI, arm muscle circumference, total body fat, FFM Measures of dietary intake ‐ energy and protein intake | |
| Notes | Request for more information regarding computer randomised assignment, numbers and denominators for mortality, details about the provision of supplements, blinding of treatment providers, recruitment of consecutively admitted patients sent 14/7/01. Reply received 23/10/01, more information awaited | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Wouters 2002.
| Methods | Method of randomisation: not stated ‐ allocation concealment: B Assessor blinding: not mentioned Intention to treat: not possible Lost to follow‐up: 3 (2‐taste, 1 consent) ‐ unclear which arm Timing of the intervention: 3 months Length of follow‐up: 3 months | |
| Participants | Location: Two psycho‐geriatric nursing homes in the Netherlands 42 patients Inclusion criteria: 60 years or older, resident at least 2 months in nursing home, diagnosed with dementia syndrome, BMI less than 23 kg/m2 for men and 25 kg/m2 for women Exclusion criteria: Cancer, terminal care, acute illness, severe gastrointestinal disorders, need for parenteral or enteral nutrition, therapeutic diet incompatible with supplementation Sex: 31 female 4 male Age: 85.3 (SD 8.4)/ 78.7 (SD 8.8) | |
| Interventions | a: Two 125ml tetrapaks, twice daily in morning and afternoon between meals 250 kcal/day 8.5g protein, 39.6g carbohydrate, 8.9g fat, vitamins and minerals b: Two 125ml tetrapaks placebo containing water, cloudifier, flavourant, non‐caloric sweetener to resemble supplement in taste and appearance Allocated: 21/21 Assessed: 19/16 | |
| Outcomes | Main outcomes: mortality, activities of daily living Additional outcomes: side‐effects of supplement, compliance Measures of nutritional status‐ weight compliance Measures of dietary intake ‐energy and protein intake | |
| Notes | Classified as in long‐term care, malnourished, unwell, in‐patients | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Wouters 2003.
| Methods | Method of randomisation: quasi‐randomised Assessor blinding: not mentioned Intention to treat: not possible Lost to follow‐up: intervention 2 ill, 11 did not like taste; 5 too much effort to complete; control 2 ill, 7 did not like taste, 5 too much effort to complete Timing of the intervention: 6 months Length of follow‐up: 6 months | |
| Participants | Location: Residences for the elderly or sheltered housing, Wageningen, The Netherlands 68 patients Inclusion criteria: 65 years or older, BMI less than or = to 25 kg/m2, residence in home for elderly or sheltered housing Exclusion criteria: Cancer, chronic gastrointestinal disorders, diet incompatible with supplementation, mentally unable to answer study questions or to remember taking supplement Sex: 39 female 29 male Age: 81.0 (SD 6.9) | |
| Interventions | a: two flavours in 125ml tetrapaks, twice daily in morning and afternoon between meals 250 kcal/day 8.75g protein, 28.5g carbohydrate, 11.25g fat, vitamins and minerals b: placebo Allocated: 52/49 Assessed: 34/34 | |
| Outcomes | Main outcomes: mortality, functional variables, quality of life Additional outcomes: complications (cancer) Measures of nutritional status‐ weight compliance Measures of dietary intake ‐energy and protein intake | |
| Notes | Classified as at home, nourished, well, living in the community | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
Wouters 2005.
| Methods | Method of randomisation: states randomised by person not involved in study, in blocks of 4 based on body mass index Assessor blinding: double‐blind placebo‐controlled trial Intention to treat: carried out but no results given Lost to follow‐up: intervention 5 dropped out; control 5 dropped out ‐ plus 13/10 withdrawn or excluded (unclear which arm) Timing of intervention: 6 months Length of follow‐up: 6 months | |
| Participants | Location: homes for elderly or sheltered housing, The Netherlands 101 participants Inclusion criteria: 65 years or older, BMI 25kg/m2 or less Exclusion criteria: cancer, gastrointestinal disease, therapeutic diet incompatible with supplementation, unable to respond to questionnaires or take supplement Sex: 38 female, 29 male (completers) Age: mean age 83y | |
| Interventions | a: 2 x 125ml daily oral liquid supplements with total of 250kcal and 8.75g protein, providing 30‐160% of US RDA of micronutrients b: 2 x 125ml daily placebo with water, sweetener, cloudifier, thickening, flavouring, colour Allocated: 52/49 Assessed: 34/34 | |
| Outcomes | Main outcomes: Deaths and illness (combined) Functional status ‐ cognitive function | |
| Notes | Emailed for separate illness and death data by allocation 09/01/2008, no response by 16 March 2008 so illness and death data not included | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Wouters 2006.
| Methods | Method of randomisation: states randomised but no further details Assessor blinding: not stated Intention to treat: carried out but no results given Lost to follow‐up: intervention 2; control 3 Timing of intervention: 5 weeks Length of follow‐up: 5 weeks | |
| Participants | Location: nursing home for psychogeriatric residents, The Netherlands 39 patients Inclusion criteria: > 65 years, in nursing home for at least 2 months, acute infection requiring antibiotics Exclusion criteria: cancer, rheumatoid arthritis, insulin dependent diabetes, morbid obesity, need for terminal care, therapeutic diet incompatible with supplementation Sex: 29 female, 5 male (completers) Age: mean age 82.7y | |
| Interventions | a: daily oral liquid supplement with 309kcal and 11.2g protein, vitamins and minerals b: resident referred to dietitian for care, if detected by physician for weight loss, loss of appetite or low intake, usually provided enrichment of food, often energy and protein enriched desserts or drinks Allocated: 20/19 Assessed: 18/16 | |
| Outcomes | Main outcomes: Functional status ‐ Zorg Index Geriatrie (ZIG) Additional outcomes: Measures of nutritional status ‐ weight change, triceps skinfold, mid upper arm circumference, arm muscle circumference Measures of dietary intake ‐ energy and protein intake | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Yamaguchi 1998.
| Methods | Method of randomisation: Assessor blinding: not mentioned Intention to treat: no Lost to follow‐up: details not given Timing of intervention: 18 months | |
| Participants | Location: community living elderly, USA Inclusion criteria: elderly homebound men and women starting to receive meals‐on‐wheels, at nutritional risk Exclusion criteria: Sex: 35 female, 27 male Age: mean age about 78y | |
| Interventions | a: liquid nutrition supplement aimed to provide per day 600 kcal, 30 g protein, 80 g carbohydrate, 18g fat, 760 µg vitamin A, 30 mg vitamin C, 1mg vitamin B6, 3 mg vitamin B12, 200 µg folate, 700 mg calcium, 9 mg iron, 200 mg magnesium, 10.6 mg zinc b: fruit flavoured beverage aimed to provide 105 kcal, 25.5 g carbohydrate, 15 mg calcium, 9 mg magnesium Allocated: 32/30 Assessed: 11/6 | |
| Outcomes | Main outcomes: Additional outcomes: measures of nutritional status‐weight measures of dietary intake‐ energy and protein intake | |
| Notes | Further details requested 15/04/01. Reply received 10/05/01 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Young 2004.
| Methods | Method of randomisation: cluster randomised by unit or dining room using opaque envelopes, allocation concealment A. Assessor blinding: action taken to blind assessors for some of the cognitive function assessment, but not all. Unblinded assessors (dietary intake) Intention to treat: carried out Lost to follow‐up: intervention 2 withdrawn; control 1 withdrawn Timing of intervention: 21 days Length of follow‐up: 21days for each part of crossover design | |
| Participants | Location: academic geriatric care facility, Toronto, Canada 34 patients Inclusion criteria: residents of Alzheimer's disease units, consent from participants' families or legal guardians. Diagnosis of probable Alzheimer's disease made by qualified clinician, ability to self‐feed or requiring only minimal levels of assistance, e.g. opening containers Exclusion criteria: disease requiring nutritional intervention, e.g. type 1 diabetes, prescription of energy‐restricted diet, swallowing difficulties requiring texture‐modified diet, acute illness Sex: 26 female, 5 male (completers) Age: mean age 88y | |
| Interventions | a: nutritional bar with or without sugar and margarine, Ensure, or food providing 250‐258kcal/d and 9‐11g protein/d, given at 10am about one hour after breakfast b: usual care Allocated: crossover design, 34 allocated Assessed: 31 completed two intervention periods and washout | |
| Outcomes | Main outcomes: Morbidity and complications ‐ acute hospital admission Functional status ‐ Severe Impairment Battery, Global Deterioration Scale, Neuropsychiatric Inventory Nursing Home version, London Psychogeriatric Rating Scale Additional outcomes: Measures of nutritional status ‐ weight change Measures of dietary intake ‐ energy and protein intake | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bachrach 2000 | Randomised controlled trial of two different surgical methods following femoral neck fracture, oral supplement intervention not randomised |
| Barateau 1998 | Not randomised controlled trial, multicentred randomised survey |
| Barton 2000 | Randomised controlled trial of normal versus fortified menu, intervention had more energy but less protein than control |
| Bastow 1983 | Randomised controlled trial of nasogastric feeding |
| Bean 1994 | Randomised controlled trial of ornithine alpha‐ketoglutarate versus a defined peptide oral supplement |
| Beattie 2000 | Randomised controlled trial of oral protein and energy supplement, controls mean age 62 years, treatment group 54.4 years |
| Beier 1996 | Randomised controlled trial of nasogastric feeding, controls median age 61.5 years, treatment group 66.5 years |
| Bernstein 2002 | Randomised controlled trial of nutrition education versus exercise |
| Borum 2000 | Prospective cohort study of artificial nutritional support in patients over 18 years |
| Bos 2000 | Not randomised controlled trial, oral protein and energy supplement |
| Breslow 1993 | Non randomised controlled trial, high or low protein, nasogastric or meal supplement |
| Brocker 1994 | Randomised controlled trial of oral ornithine oxoglutarate |
| Brown 1995 | Randomised controlled trial of early or late feeding after percutaneous endoscopic gastrostomy insertion |
| Bunker 1994 | Not randomised controlled trial, oral protein and energy supplement, matched control group, aged 70 to 85 years |
| Bunout 1989 | Randomised controlled trial, oral protein and energy supplement, control group mean age 48 years, intervention group mean age 50 years |
| Bunout 2001 | Free‐living elders half received nutrition supplementation, randomised to resistance training or not. |
| Bunout 2004 | Free‐ living elders half received nutritional supplementation not randomised, randomised to receive resistance training or not. |
| Cabre 1990 | Randomised controlled trial of nasogastric feeding, patients with cirrhosis, no age given |
| Caglar 2002 | Not randomised study of oral nutritional supplementation for hemodialysis patients |
| Calvey 1985 | Randomised controlled trial of normal diet versus normal diet plus carbohydrate and protein versus normal diet plus branched chain amino acid protein, mean age all groups 49 years |
| Campbell 1995 | Randomised controlled trial of exercise and two levels of protein intake, mean age 65 years |
| Carr 1996 | Randomised controlled trial of immediate nasogastric feeding versus intravenous fluids, controls mean age 51years, treatment group mean age 60 years |
| Chee 2003 | Randomised controlled trial of the effect of milk supplementation on bone density. Mean age 58.7 and 59.0 years |
| Creutzberg 2000 | Not randomised controlled trial, oral high caloric supplements in patients with COPD |
| Daly 1992 | Randomised controlled trial in cancer patients of nasogastric immunomodulatory diet |
| Danhof 1982 | Not randomised controlled trial, no control group, oral protein and energy supplementation study of nursing home patients |
| de Jong 2000 | Randomised controlled trial of micronutrient supplementation in frail elderly people |
| Devine 1996 | Randomised trial of calcium versus skimmed milk powder, mean age 63 years |
| Duncan 2006 | Randomised controlled trial of additional personal care from dietetic assistants to improve the outcome of hip fracture patients |
| Efthimou 1988 | Randomised controlled trial of oral protein and energy supplementation, mean age 62 years |
| Elmstahl 1987 | Randomised controlled trial of three oral supplements for elderly patients, mean age 85 years with different levels of protein but same energy content |
| Eneroth 1997 | Not randomised controlled trial, controls matched from another hospital |
| Eneroth 2006 | Randomised controlled trial in hip fracture patients, intervention includes both intravenous and oral supplementation |
| Espaulella 2000 | Randomised controlled trial of isocaloric supplement with and without protein in patients with fractured femur |
| Eyer 1993 | Randomised controlled trial of early versus late nasogastric feeding, mean age under 45 years |
| Forli 2001 | Randomised controlled trial of energy rich diet versus normal hospital diet in lung transplantation candidates |
| Fuenzalida 1990 | Randomised controlled trial of oral protein and energy supplement, mean age 62 years with COPD |
| Gall 1998 | Not randomised controlled trial, fortified food and snacks |
| Gallager 1992 | Randomised controlled trial of supplementary overnight nasogastric feeding |
| Ganzoni 1994 | Randomised controlled trial of dietary advice alone |
| Goris 2003 | Randomised controlled trial of oral protein and energy supplements, mean aged 62 years. |
| Harrington 2004 | Randomised controlled trial of high‐protein, high sodium vs adequate protein low sodium, probably too young |
| Hartgrink 1998 | Randomised controlled trial of supplementary overnight nasogastric feeding |
| Hickson 2004 | Randomised controlled trial of intensive feeding support from health care assistants |
| Hogarth 1996 | Randomised controlled trial, oral supplement glucose and vitamins, no protein |
| Houwing 2003 | Randomised controlled trial, oral supplement enriched with arginine versus placebo for hip fracture patients |
| Hurson 1995 | Randomised controlled trial of oral supplement arginine |
| Jamieson 1997 | Not randomised controlled trial, no information on age, wide range of nutrition interventions |
| Johansen 2004 | Randomised controlled trial of specialised nutrition team versus standard regime, average age 62 years. |
| Johansson 2002 | Randomised controlled trial of internal fixation or cemented primary total hip arthroplasty, a non‐randomised subgroup of patients were included in a study of nutrition support |
| Keane 1998 | Randomised controlled trial of vitamin D‐fortified milk versus unfortified milk |
| Keele 1997 | Randomised controlled trial of oral protein and energy supplement versus normal diet (2 phase), control group mean age 60 years, intervention group mean age 65 years |
| Kemen 1991 | Randomised controlled trial of intact versus hydrolysed protein via jejunostomy, no age given |
| Kerrigan 1996 | Not randomised controlled trial of calorifically dense supplement, no control group, nursing home residents, no age given |
| Kiel 1992 | Not randomised controlled trial, fortification and added nutrients at meal times versus supplementation between meals, no age given |
| Kretser 2000 | Not randomised controlled trial, 21 meals per week intensive meals on wheels programme |
| Kumagai 1999 | Not randomised controlled trial, dietary consultation only |
| Lau 2001 | Randomised controlled trial of calcium supplemented milk powder, control group mean age 56.8 years, intervention group mean age 57.1 years. |
| Lawson 2000 | Cluster randomised by ward to protein and energy supplement or control group, post‐operative orthopaedic patients. Too young |
| Lewis 1987 | Randomised controlled trial in patients with chronic pulmonary disease of protein and energy sip feeds, control group mean age 59 years, treatment group mean age 65 years |
| Neumann 2004 | Randomised controlled trial of 2 oral supplements containing protein and energy in patients recovering from hip fracture‐ intervention group additional protein only |
| Nickerson 1998 | Randomised controlled trial of oral amino acid arginine supplementation |
| Olin 1998 | Not randomised controlled trial, of regular meals versus enriched meals in matched groups of elderly nursing home residents |
| Otte 1989 | Randomised controlled trial of oral protein and energy supplementation of patients with pulmonary emphysema, mean age 56 years and 54 years |
| Pardy 1986 | Not randomised controlled trial, all patients given protein and energy oral supplement, no age given |
| Paton 2004 | Randomised controlled trial of protein and energy supplementation, mean age 38.4 years and 39.5 years |
| Planas 2005 | Randomised controlled trial of protein and energy supplementation, mean age 60 years |
| Price 2006 | Non randomised trial of personalized snack based intervention for hip fracture patients |
| Pupim 2002 | Randomised controlled trial of oral nutritional supplementation, mean age 45.6 years and 50.0 years |
| Rana 1992 | Randomised controlled trial of post‐operative oral protein and energy supplements, control group mean age 64 years, treatment group mean age 58 years |
| Remsburg 2001 | Randomised controlled trial of buffet style dining program versus conventional tray style meal in nursing home residents |
| Rogers 1992 | Randomised controlled trial of increased energy and protein feeding, control group mean age 64 years, treatment group mean age 64 years |
| Schurch 1998 | Randomised controlled trial of isocaloric supplement, with or without protein, patients with fractured femur, mean age 80 years |
| Seri 1984 | Randomised controlled trial of early nutritional support via jejunostomy versus conventional care, average age under 34 years |
| Seven 2003 | Randomised controlled trial early versus late feeding in laryngectomized patients with malignant tumour, mean age 55 years and 56 years |
| Slodkowski 1994 | Dietary study, mean age 54 years |
| Smedley 2004 | Randomised controlled trial of the effects of preoperative and postoperative supplements in patients following GI surgery. Too young |
| Stauffer 1986 | Not randomised controlled trial, oral protein and energy supplementation of patients with chronic pulmonary disease, patients used as own controls |
| Storm 1998 | Randomised controlled trial of supplementation with milk versus calcium carbonate versus placebo, no outcomes of interest |
| Sullivan 1998 | Randomised controlled trial of supplementary overnight nasogastric feeding |
| Tkatch 1992 | Randomised control trial of supplements with versus without extra protein for patients with fractured femur |
| Tschepe 1985 | Randomised controlled trial of oral supplementation with branched‐chain amino‐acids, patients with liver cirrhosis, age not given |
| Vargas 1995 | Randomised controlled trial of supplementary nasogastric feeding, mean age 64 years |
| Vermeeren 2001 | Randomised controlled trial of effects of acute dose of nutritional supplement on metabolism and exercise capacity, supplemented for <1day, mean age part 1 65years, part 2 62 years |
| Von Meyenfeldt 1990 | Randomised controlled trial of pre‐operative parenteral nutrition versus nasogastric nutrition, patients with cancer, mean age over 61years |
| Wachtler 1995 | Randomised controlled trial of oral immunomodulatory diet, mean age 65 years |
| Wara 1985 | Not randomised controlled trial, early liquid diet versus conventional treatment and food |
| Williams 1989 | Controlled trial of protein and energy supplementation in elderly orthopaedic patients, problems with randomisation proceedure |
| Wilson 1986 | Not randomised controlled trial, protein and energy supplementation of patients with emphysema, mean age 60 years |
| Wisten 2005 | Patients in geriatric hospital rehabilitation wards randomised into an intervention group (porridge group) and a control group (standard diet without porridge). Unclear if additional protein and energy provided. No outcomes of interest |
| Wong 2004 | A randomised controlled trial of dietary counselling only |
| Yeh 2000 | Randomised controlled trial, megestrol acetate versus placebo in geriatric nursing home patients |
Characteristics of ongoing studies [ordered by study ID]
Cameron 2003.
| Trial name or title | Effectiveness of oral supplementation for older women with hip and other fractures (EONS) |
| Methods | |
| Participants | 43 older women with hip or other fractures |
| Interventions | (a) Oral nutritional supplementation: 235ml (1.5kcal/ml) daily for 40 days. (b) Usual care. |
| Outcomes | follow‐up: 1 and 4 months post fracture. Outcomes: ADL function, nutritional status and medical complications. |
| Starting date | Started April 2000, follow‐up completed. |
| Contact information | Prof Ian Cameron Rehabilitation Studies Unit University of Sydney PO Box 6 Ryde New South Wales Australia NSW 1680 ianc@mail.usyd.ed.au |
| Notes | Study completion confirmed bu Ian Cameron in October 2004. |
CENEX 2007.
| Trial name or title | CENEX study ‐ cluster randomised trial through 28 community health centres in Santiago, Chile |
| Methods | |
| Participants | Age 65.0 to 67.9 years |
| Interventions | Factorial design of protein and energy supplement and/or exercise programme, or neither |
| Outcomes | follow‐up: 24 months Outcomes: pneumonia, walking capacity, BMI, acute respiratory infection, quality of life (SF36), depression, chronic diseases, physical and functional limitations, productive activity, falls, fracture, blood pressure, anthropometry, timed up‐and‐go, cardiovascular risk factors. |
| Starting date | 2005 |
| Contact information | Alan D Dangour alan.dangour@lshtm.ac.uk |
| Notes |
Elia 2007.
| Trial name or title | Community nutrition support trial |
| Methods | |
| Participants | Elderly individuals in care homes |
| Interventions | (a) Dietary advice (b) Oral nutritional supplements |
| Outcomes | follow‐up: 6 months Outcome: quality of life |
| Starting date | 2007‐2010 |
| Contact information | Prof Marinos Elia University of Southampton England elia@soton.ac.uk |
| Notes |
Kvamme 2007.
| Trial name or title | Nutritional intervention in malnourished elderly patients |
| Methods | |
| Participants | |
| Interventions | (a) Fresubin protein energy drink and advice (b) Advice |
| Outcomes | follow‐up: 6, 12 weeks Outcomes: function, activities of daily living, hand grip, timed up and go, quality of life (SF36), depression, anthropometry |
| Starting date | 2004‐2008 |
| Contact information | Jan M Kvamme University Hospital of Northern Norway jan.magnus.kvamme@unn.no |
| Notes |
Contributions of authors
JAN POTTER: Data from previous review, assistance with selection of studies and data extraction, co‐drafting of the protocol and review.
ANNE MILNE: Literature search, contacting trialists, selection of studies, data extraction, first drafts of protocol and review, data analysis and data presentation.
ANGELA VIVANTI: Assistance with selection of studies and data extraction, co‐drafting of the review.
ALISON AVENELL: Project supervisor, assistance with selection of studies and data extraction, co‐drafting of the protocol and review, assisting with devising data analysis and methodological support.
Sources of support
Internal sources
University of Aberdeen, UK.
External sources
Medical Research Council, UK.
Chief Scientist Office, UK.
Student Awards Agency for Scotland, UK.
Declarations of interest
One reviewer is also the author of an eligible trial (Potter 2001).
Edited (conclusions changed)
References
References to studies included in this review
Banerjee 1978 {published and unpublished data}
- Banerjee AK. Nutritional status of long‐stay geriatric inpatients: effects of a food supplement (Complan). Age & Ageing 1978;7:237‐43. [DOI] [PubMed] [Google Scholar]
Barr 2000 {published data only}
- Barr SI, McCarron DA, Heaney RP, Dawson‐Hughes B, Berga SL, Stern JS, et al. Effects of increased consumption of fluid milk on energy and nutrient intake, body weight and cardiovascular risk factors in healthy older adults. Journal of the American Dietetic Association 2000;7:810‐7. [DOI] [PubMed] [Google Scholar]
- Heaney RP, McCarron DA, Dawson‐Hughes B, Oparil S, Berga SL, Stern JS, et al. Dietary changes favorably affect bone remodeling in older adults. Journal of the American Dietetic Association 1999;99(10):1228‐33. [DOI] [PubMed] [Google Scholar]
Benati 2001 {published data only}
- Benati G, Delvecchio S, Cilla D, Pedone V. Impact on pressure ulcer healing of an arginine‐enriched nutritional solution in patients with severe cognitive impairment. Archives of gerontology and geriatrics 2001;33 Suppl 1:43‐7. [DOI] [PubMed] [Google Scholar]
Bonnefoy 2003 {published data only}
- Bonnefoy M, Cornu C, Normand S, Boutitie F, Bugnard F, Rahmani A, et al. The effects of exercise and protein‐energy supplements on body composition and muscle function in frail elderly individuals: a long‐term controlled randomised study. The British Journal of Nutrition 2003;89(5):731‐9. [DOI] [PubMed] [Google Scholar]
Bourdel 2000 {published data only}
- Bourdel‐Marchasson I, Barateau M, Rondeau V, Dequae‐Merchadou L, Salles‐Montaudon N, Emeriau JP, et al. A multi‐center trial of the effects of oral nutritional supplementation in critically ill older inpatients. GAGE Group. Groupe Aquitaine Geriatrique d'Evaluation. Nutrition 2000;16(1):1‐5. [DOI] [PubMed] [Google Scholar]
Broqvist 1994 {published data only}
- Broqvist M, Arnqvist H, Dahlstrom U, Larsson J, Nylander E, Permert J. Nutritional assessment and muscle energy metabolism in severe chronic congestive heart failure ‐ effects of long term dietary supplementation. European Heart Journal 1994;15(12):1641‐50. [DOI] [PubMed] [Google Scholar]
Brown 1992 {published and unpublished data}
- Brown KM, Seabrook NA. Effect of nutrition on recovery after fractured femur. Medical Audit News 1992;2(1):10‐12. [Google Scholar]
- Brown KM, Seabrook NA. Nutritional influences on recovery and length of hospital stay in elderly women following femoral fracture. Proceedings of the Nutrition Society 1992;51(2):132A. [Google Scholar]
Bruce 2003 {published and unpublished data}
- Bruce D, Laurance I, McGuiness M, Ridley M, Goldswain P. Nutritional supplements after hip fracture: poor compliance limits effectiveness. Clinical Nutrition 2003;22(5):497‐500. [DOI] [PubMed] [Google Scholar]
Carver 1995 {published data only}
- Carver AD, Dobson AM. Effect of dietary supplementation of elderly demented hospital residents. Journal of Human Nutrition and Dietetics 1995;8:389‐94. [Google Scholar]
Collins 2005 {published data only}
Daniels 2003 {published data only}
- Daniels LA, Miller M, Bannerman E, Whitehead C, Crotty M. Weight loss post lower limb fracture despite an intensive oral nutrition and exercise intervention. Clinical Nutrition 2003;22 Suppl:S86. [Google Scholar]
- Daniels LA, Miller MD, Bannerman E, Crotty M. [Adherence to nutritional supplements amongst orthopedic patients: an important clinical and study design issue. Clinical Nutrition 2005;24:535].
- Miller M, Bannerman E, Daniels L, Crotty M. Lower limb fracture, cognitive impairment and risk of subsequent malnutrition: a prospective evaluation of dietary energy and protein intake on an orthopedic ward. European Journal Clinical Nutrition 2006, 60(7):853‐61. [DOI] [PubMed]
- Miller M, Crotty M, Whitehead C, Bannerman E, Daniels L. Nutritional supplementation and resistance training in nutritionally at risk older adults following lower limb fracture. Clinical Rehabilitation 2006, 20(4):311‐23. [DOI] [PubMed]
- Miller M, Daniels L, Bannerman E, Crotty M. Adherence to nutritional supplements among patients with a fall‐related lower limb fracture. Nutrition in Clinical Practice 2005, 20(5):569‐78. [DOI] [PubMed]
Deletter 1991 {unpublished data only}
- Deletter M C. A nutritional intervention for persons with chronic airflow limitation [PhD thesis]. Kentucky: University of Kentucky, 1991. [Google Scholar]
Delmi 1990 {published data only}
- Bonjour JP, Schurch MA, Chevalley P, Ammann P, Rizzoli R. Protein intake, IGF‐1 and osteoporosis. Osteoporosis International 1997;7 Suppl 3:S36‐42. [DOI] [PubMed] [Google Scholar]
- Bonjour JP, Schurch MA, Rizzoli R. Proteins and bone health. Pathologie‐biologie 1997;45(1):57‐9. [PubMed] [Google Scholar]
- Delmi M, Rapin CH, Bengoa J.M, Delmas PD, Vasey H, Bonjour JP. Dietary supplementation in elderly patients with fractured neck of the femur. Lancet 1990;335:1013‐6. [DOI] [PubMed] [Google Scholar]
Edington 2004 {published data only}
- Edington J, Barnes R, Bryan F, Dupree E, Frost G, Hickson M, et al. A prospective randomised controlled trial of nutritional supplementation in malnourished elderly in the community: clinical and health economic outcomes. Proceedings of the Nutrition Society 2004;63:9A. [DOI] [PubMed] [Google Scholar]
- Edington J, Barnes R, Bryan F, Dupree E, Frost G, Hickson M, et al. A prospective randomised controlled trial of nutritional supplementation in malnourished elderly in the community: clinical and health economic outcomes. Clinical Nutrition 2004;23:195‐204. [DOI] [PubMed] [Google Scholar]
Eneroth 2004 {published data only}
Fiatarone 1994 {published data only}
- Fiatarone MA, O'Neill EF, Doyle N, Clements KM, Roberts SB, Kehayias JJ. The Boston FICSIT study: the effects of resistance training and nutritional supplementation of physical frailty in the oldest old. Journal of the American Geriatrics Society 1993;41(3):333‐7. [DOI] [PubMed] [Google Scholar]
- Fiatarone MA, O'Neill EF, Ryan ND, Clements KM, Solares GR, Nelson ME. Exercise training and nutritional supplementation for physical frailty in very elderly people. New England Journal of Medicine 1994;330(25):1769‐75. [DOI] [PubMed] [Google Scholar]
- Fiatarone Singh MA, Bernstein MA, Ryan AD, O'Neill EF, Clements KM, Evans WJ. The effect of oral nutritional supplements on habitual dietary quality and quantity in frail elders. Journal of Nutrition, Health and Aging 2000;4(1):5‐12. [PubMed] [Google Scholar]
- Singh MA, Ding W, Manfredi TJ, Solares GS, O'Neill EF, Clements KM, et al. Insulin‐like growth factor I in skeletal muscle after weight‐lifting exercise in frail elders. American Journal of Physiology 1999;277(1):E135‐43. [DOI] [PubMed] [Google Scholar]
FOOD trial 2005 {published data only}
Gariballa 1998 {published data only}
- Gariballa SE, Parker SG, Castleden CM. A randomised controlled trial of nutritional supplementation after stroke. Age & Ageing 1998;27 Suppl 1:P66. [Google Scholar]
- Gariballa SE, Parker SG, Castleden CM. A randomised controlled trial of nutritional supplements following acute stroke. Age & Ageing 1997;26 Suppl 1:42. [Google Scholar]
- Gariballa SE, Parker SG, Taub N, Castleden CM. A randomized controlled a single‐blind trial of nutritional supplementation after acute stroke. Jpen: Journal of Parenteral & Enteral Nutrition 1998;22(5):315‐9. [DOI] [PubMed] [Google Scholar]
Gariballa 2006 {published data only}
- Gariballa S, Forster S. Associations between underlying disease and nutritional status following acute illness in older people. Clinical Nutrition 2007;26:466‐473. [DOI] [PubMed] [Google Scholar]
- Gariballa S, Forster S. Dietary supplementation and quality of life of older patients: a randomized, double‐blind, placebo‐controlled trial. Journal of the American Geriatrics Society 2007;55:2030‐2034. [DOI] [PubMed] [Google Scholar]
- Gariballa S, Forster S. Effects of acute‐phase response on nutritional status and clinical outcome of hospitalized patients. Nutrition 2006;22:750‐757. [DOI] [PubMed] [Google Scholar]
- Gariballa S, Forster S. Effects of dietary supplements on depressive symptoms in older patients: a randomised double‐blind placebo‐controlled trial. Clinical Nutrition 2007;26:545‐551. [DOI] [PubMed] [Google Scholar]
- Gariballa S, Forster S. Malnutrition is an independent predictor of 1‐year mortality following acute illness. British Journal of Nutrition 2007;98:332‐336. [DOI] [PubMed] [Google Scholar]
- Gariballa S, Forster S, Walters S, Powers H. A randomized, double‐blind, placebo‐controlled trial of nutritional supplementation during acute illness. American Journal of Medicine 2006;119:693‐699. [DOI] [PubMed] [Google Scholar]
Gazzotti 2003 {published data only}
- Gazzotti C, Arnaud‐Battandier F, Parello M, Farine S, Seidel L, Albert A, et al. Prevention of malnutrition in older people during and after hospitalisation: results from a randomised controlled clinical trial. Age and Ageing 2003;32(3):321‐5. [DOI] [PubMed] [Google Scholar]
Gegerle 1986 {published data only}
- Gegerle P, Bengoa JM, Delmi M, Rapin CH, Loizeau E, Vasey H. Dietary survey on the effect of an oral nutritional supplement after femoral neck fracture [Enquete alimentaire apres fracture du col du femur: effet d'un supplement dietetique sur les apports nutritonnels]. Schweizerishe Rundschau fur Medizin Praxis 1986;75(32):933‐5. [PubMed] [Google Scholar]
Gray‐Donald 1995 {published data only}
- Gray‐Donald K, Payette H, Boutier V. Randomized clinical trial of nutritional supplementation shows little effect on functional status among free‐living frail elderly. Journal of Nutrition 1995;25(12):2965‐71. [DOI] [PubMed] [Google Scholar]
- Payette H, Gray‐Donald K, Cyr R, Boutier V. Predictors of dietary intake in a functionally dependent elderly population in the community. American Journal of Public Health 1995;85(5):677‐83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hampson 2003 {published data only}
- Hampson G, Martin FC, Moffat K, Vaja S, Sankaralingam S, Cheung J, et al. Effects of dietary improvement on bone metabolism in elderly underweight women with osteoporosis: a randomised controlled trial. Osteoporosis International 2003;14(9):750‐6. [DOI] [PubMed] [Google Scholar]
Hankey 1993 {published and unpublished data}
- Hankey CR, Summerbell J, Wynne HA. The effect of dietary supplementation in continuing care elderly people: nutritional anthropometric and biochemical parameters. Journal of Human Nutrition and Dietetics 1993;6:317‐22. [Google Scholar]
- Summerbell J, Wynne H, Hankey CR, Williams FM. The effect of age and frailty upon blood esterase activities and their response to dietary supplementation. British Journal of Clinical Pharmacology 1993;36(5):399‐404. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hankins 1996 {unpublished data only}
- Hankins C. Dietary supplementation with sustagen in elderly patients with fractured neck of femur (PhD Thesis). Sydney University. Sydney University, 1996.
Hubsch 1992 {published data only}
- Hubsch S, Volkert D, Oster P, Schlierf G. Effect of dietary supplementation on weight and protein status in undernourished geriatric patients. Clinical Nutrition 1992;11:97. [Google Scholar]
Jensen 1997 {published and unpublished data}
- Jensen MB, Hessov I. Dietary supplementation at home improves the regain of lean body mass after surgery. Nutrition 1997;13(5):422‐30. [DOI] [PubMed] [Google Scholar]
- Jensen MB, Hessov I. Randomization to nutritional intervention at home did not improve postoperative function fatigue or well being. British Journal of Surgery 1997;84(1):113‐8. [PubMed] [Google Scholar]
Knowles 1988 {published data only}
- Knowles JB, Fairbarn MS, Wiggs BJ, Chan YC, Pardy RL. Dietary supplementation and respiratory muscle performance in patients with COPD. Chest 1988;93(5):977‐83. [DOI] [PubMed] [Google Scholar]
Krondl 1999 {published and unpublished data}
- Krondl M, Coleman PH, Bradley CL, Lau D, Ryan N. Subjectively healthy elderly consuming a liquid nutrition supplement maintained body mass index and improved some nutritional parameters and perceived well‐being. Journal of the American Dietetic Association 1999;99(12):1542‐8. [DOI] [PubMed] [Google Scholar]
Kwok 2001 {published and unpublished data}
- Kwok T, Woo J, Kwan M. Does low lactose milk powder improve the nutritional intake and nutritional status of frail older Chinese people living in nursing homes?. Journal of Nutrition, Health and Aging 2001;5(1):17‐21. [PubMed] [Google Scholar]
Larsson 1990 {published data only}
- Ek AC, Larsson J, Schenck H, Thorslund S, Unosson M, Bjurulf P. The correlation between anergy, malnutrition and clinical outcome in an elderly hospital population. Clinical Nutrition 1990;9:185‐9. [DOI] [PubMed] [Google Scholar]
- Ek AC, Unosson M, Larsson J, Schenck H, Bjurulf P. The development and healing of pressure sores related to the nutritional state. Clinical Nutrition 1991;10(5):245‐50. [DOI] [PubMed] [Google Scholar]
- Larsson J, Unosson M, Ek AC, Nilsson L, Thorslund S, Bjurulf P. Effect of dietary supplementation on nutritional status and clinical outcome in 501 geriatric patients ‐ a randomised study. Clinical Nutrition 1990;9:179‐84. [DOI] [PubMed] [Google Scholar]
- Unosson M, Larsson J, EK AC, Bjurulf P. Effects of dietary supplementation on functional condition and clinical outcome measured with a modified Norton scale. Clinical Nutrition 1992;11:134‐9. [DOI] [PubMed] [Google Scholar]
Lauque 2000 {published and unpublished data}
- Lauque S, Arnaud‐Battandier F, Mansourian R, Guigoz Y, Paintin M. Protein‐energy oral supplementation in malnourished nursing‐home residents. A controlled trial. Age and Ageing 2000;29(1):51‐6. [DOI] [PubMed] [Google Scholar]
Lauque 2004 {published data only}
- Lauque S, Arnaud BF, Gillette S, Plaze J, Andrieu S, Cantet C, Vellas B. Improvement of weight and fat‐free mass with oral nutritional supplementation in patients with Alzheimer's disease at risk of malnutrition: A prospective randomized study. Journal of the American Geriatrics Society 2004;52:1702‐1707. [DOI] [PubMed] [Google Scholar]
MacFie 2000 {published and unpublished data}
- MacFie J, Palmer D. Oral dietary supplements before and after surgery. Nutrition 2001;17(2):186. [DOI] [PubMed] [Google Scholar]
- MacFie J, Woodcock NP, Palmer MD, Walker A, Townsend S, Mitchell CJ. Oral dietary supplements in pre‐ and postoperative surgical patients: a prospective and randomized clinical trial. Nutrition 2000;16(9):723‐8. [DOI] [PubMed] [Google Scholar]
Madigan 1994 {unpublished data only}
- Madigan C. Benefits of dietary supplementation in elderly patients with fractured neck of femur (MSc dissertation). MSc dissertation. Sydney (Australia): Sydney Univ., 1994.
Manders 2006 {published data only}
- Manders M. Nutritional care in old age: the effect of supplementation on nutritional status and performance. PhD thesis, Wageningen University, The Netherlands 2006.
McEvoy 1982 {published data only}
- McEvoy AW, James OF. The effect of a dietary supplement (Build‐up) on nutritional status in hospitalized elderly patients. Human Nutrition: Applied Nutrition 1982;36A:374‐6. [PubMed] [Google Scholar]
McWhirter 1996 {published data only}
- McWhirter JP, Pennington CR. A comparison between oral and enteral nutritional supplements in malnourished patients. Nutrition 1996;12:503‐6. [DOI] [PubMed] [Google Scholar]
- McWhirter JP, Pennington CR. The influence of supplemental feeding and a comparison of methods in malnourished hospital patients. Jpen: Journal of Enteral and Parenteral Nutrition 1996;20(1):73. [Google Scholar]
Meredith 1992 {published data only}
- Meredith CN, Frontera WR, O'Reilly KP, Evans WJ. Body composition in elderly men: effect of dietary modification during strength training. Journal of the American Geriatrics Society 1992;40:155‐62. [DOI] [PubMed] [Google Scholar]
Ovesen 1992 {published data only}
- Ovesen L. Palatability and intake of two commercial liquid diets in patients with poor appetite. European Journal of Clinical Nutrition 1991;45(5):273‐5. [PubMed] [Google Scholar]
- Ovesen L. The effect of a supplement which is nutrient dense compared to standard concentration on the total nutritional intake of anorectic patients. Clinical Nutrition 1992;11:154‐67. [DOI] [PubMed] [Google Scholar]
Payette 2002 {published data only}
- Payette H, Boutier V, Coulombe C, Gray DK. Benefits of nutritional supplementation in free‐living, frail, undernoursihed elderly people: a prospective randomized community trial. Journal of the American Dietetic Association 2002;102(8):1088‐95. [PubMed] [Google Scholar]
Payette 2004 {published and unpublished data}
- Payette H, Boutier V, Coulombe C. Efficacy of nutritional intervention in the free‐living frail elderly. American Journal of Clinical Nutrition 2002;75(2):340S. [Google Scholar]
Potter 2001 {published and unpublished data}
- Potter JM, Roberts MA, McColl JH, Reily JJ. Protein energy supplements in unwell elderly patients ‐ a randomised controlled trial. Jpen: Journal of Parenteral and Enteral Nutrition 2001;25(6):323‐9. [DOI] [PubMed] [Google Scholar]
- Potter JM, Roberts MA, Reilly JJ, et al. Randomised controlled trial of protein energy supplementation in elderly emergency admissions (abstract). Age and Ageing 1998;27 Suppl 2:39. [Google Scholar]
- Potter JM, Roberts MA, Reilly JJ, McColl JH. A evaluation of protein energy supplementation in medically ill admissions to a geriatric unit. Proceedings of the Nutrition Society 1998;57(3):88A. [Google Scholar]
- Roberts M, Potter J, McColl J, Reilly J. Can prescription of sip‐feed supplements increase energy intake in hospitalised older people with medical problems. British Journal of Nutrition 2003;90:425‐9. [DOI] [PubMed] [Google Scholar]
Price 2005 {published and unpublished data}
Rosendahl 2006 {published data only}
- Rosendahl E, Lindelof N, Littbrand H, Yifter‐Lindgren E, Lundin‐Olsson L, Haglin L, et al. High‐intensity functional exercise program and protein‐enriched energy supplement for older persons dependent in activities in daily living: a randomised controlled trial. Australian Journal of Physiotherapy 2006;52:105‐113. [DOI] [PubMed] [Google Scholar]
Salas‐Salvado 2005 {published data only}
- Salas J, Planas M, Altimir S, Pagan C, Gonzalez M, Johnston S, et al. Effect of a whole formula diet on nutritional status in institutionalized patients with alzheimer disease. Clinical Nutrition 2004;23(4):769. [DOI] [PubMed] [Google Scholar]
- Salas‐Salvado J, Torres M, Planas M, Altimir S, Pagan C, Gonzalez ME, et al. Effect of oral administration of a whole formula diet on nutritional and cognitive status in patients with Alzheimer's disease. Clinical Nutrition 2005;24:390‐397. [DOI] [PubMed] [Google Scholar]
Saudny 1997 {published and unpublished data}
- Saudny‐Unterberger H, Martin JG, Gray‐Donald K. Impact of nutritional support on functional status during acute exacerbation of chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 1997;156:794‐9. [DOI] [PubMed] [Google Scholar]
Schols 1995 {published and unpublished data}
- Schols A M, Slangen J, Volovics L, Wouters E F. Weight loss is a reversible factor in the prognosis of chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 1998;157(6 Pt 1):1791‐7. [DOI] [PubMed] [Google Scholar]
- Schols AM, Soeters PB, Mostert R, Pluymers RJ, Wouters EF. Physiologic effects of nutritional support and anabolic steroids in patients with chronic obstructive pulmonary disease. A placebo‐controlled randomized trial. American Journal of Respiratory and Critical Care Medicine 1995;152(4 Pt 1):1268‐74. [DOI] [PubMed] [Google Scholar]
Scorer 1990 {unpublished data only}
- Scorer HJM. The effect of nutritional supplementation with Ensure on the general health of undernourished elderly patients in the community. Abbott UK Medical 1990.
SG Larsson malnour {published data only}
- Subgroup of malnourished patients.
SG Larsson nourished {published data only}
- Subgroup of normally nourished patients.
SG Potter malnourish {published and unpublished data}
- Subgroup malnourished.
SG Potter moder maln {published data only}
- Subgroup moderately undernourished.
SG Potter nourished {published and unpublished data}
- Subgroup not malnourished.
SG Potter very maln {published data only}
- Subgroup severely undernourished.
SG Volkert comply {published data only}
- Subgroup of supplemented patients with good acceptance of supplements.
SG Volkert non compl {published data only}
- Subgroup of supplemented patients with poor acceptance of supplements.
Stableforth 1986 {published data only}
- Stableforth PG. Supplement feeds and nitrogen and calorie balance following femoral neck fracture. British Journal of Surgery 1986;73:651‐5. [DOI] [PubMed] [Google Scholar]
Steiner 2003 {published data only}
- Steiner MC, Barton RL, Singh SJ, Morgan MD. Nutritional enhancement of exercise performance in chronic obstructive pulmonary disease: a randomised controlled trial. Thorax 2003;58(9):745‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tidermark 2004 {published data only}
- Ponzer S, Tidermark J, Brismar K, Söderqvist A, Cederholm T. Nutritional status, insulin‐like growth factor‐1 and quality of life in elderly women with hip fractures. Clinical Nutrition 1999;18(4):241‐6. [DOI] [PubMed] [Google Scholar]
- Tengstrand B, Cederholm T, Soderqvist A, Tidermark J. Effects of protein‐rich supplementation and nandrolone on bone tissure after a hip fracture. Clinical Nutrition 2007;26:460‐465. [DOI] [PubMed] [Google Scholar]
- Tidermark J. Quality of life and femoral neck fractures. Acta Orthopaedica Scandinavica ‐ Supplementum 2003;74(309):1‐42. [PubMed] [Google Scholar]
- Tidermark J, Ponzer S, Carlsson P, Söderqvist A, Brismar K, Tengstrand B, et al. Effects of protein‐rich supplementation and nandrolone in lean elderly women with femoral neck fractures. Clinical Nutrition 2004;23(4):587‐90. [DOI] [PubMed] [Google Scholar]
- Tidermark J, Ponzer S, Tengstrand B, Cederholm T. Liquid supplementation and nandrolone to elderly women. Clinical Nutrition 2002;21 Suppl 1:40. [DOI] [PubMed] [Google Scholar]
Vermeeren 2004 {published data only}
Vlaming 2001 {published data only}
- Vlaming S, Biehling A, Hennessey EM, Jamieson CP, Chattophadhyay S, Obeid OA, et al. Should the food intake of patients admitted to acute hospital services be routinely supplemented? A randomized placebo controlled trial. Clinical Nutrition 2001;20:517‐26. [DOI] [PubMed] [Google Scholar]
Volkert 1996 {published data only}
- Volkert D, Hubsch S, Oster P, Schlierf G. Nutritional support and functional status in undernourished geriatric patients during hospitalisation and 6‐month follow‐up. Aging Clinical & Experimental Research (Milano) 1996;8(6):386‐95. [DOI] [PubMed] [Google Scholar]
Woo 1994 {published and unpublished data}
- Woo J, Ho SC, Mak YT, Law LK, Cheung A. Nutritional status of elderly patients during recovery from chest infection. Age & Ageing 1994;23(1):40‐8. [DOI] [PubMed] [Google Scholar]
Wouters 2002 {published data only}
- Wouters‐Wesseling W, Wouters AE, Kleijer CN, Bindels JG, Groot CP, Staveren WA. Study of the effect of a liquid nutrition supplement on the nutritional status of psycho‐geriatric nursing home patients. European Journal of Clinical Nutrition 2002;56(3):245‐51. [DOI] [PubMed] [Google Scholar]
- Wouters‐Wessling W, Wouters AE, Kleijer CN, Groot CP, Staveren WA. Study on the use of a nutrient‐enriched food supplement in psycho‐geriatric nursing home patients. Clinical Nutrition 1999;18 Suppl 1:19. [Google Scholar]
Wouters 2003 {published data only}
- Wouters‐Wesseling W, Hooijdonk C, Wagenaar L, Bindels J, Groot L, Staveren W. The effect of a liquid nutrition supplement on body composition and physical functioning in elderly people. Clinical Nutrition 2003;22(4):371‐7. [DOI] [PubMed] [Google Scholar]
Wouters 2005 {published data only}
- Wouters‐Wesseling W, Vos AP, Hal M, Groot LC, Staverens WA, Bindels JG. The effect of supplementation with an enriched drink on indices of immune function in frail elderly. The Journal of Nutrition, Health and Aging 2005;9(4):281‐286. [PubMed] [Google Scholar]
- Wouters‐Wesseling W, Wagenaar LW, Rozendaal M, Berend Deijen J, groot LC, Bindels JG, et al. Effect of an enriched drink on cognitive function in frail elderly persons. The Journals of Gerontology 2005;60A(2):265‐270. [DOI] [PubMed] [Google Scholar]
Wouters 2006 {published data only}
- Wouters‐Wesseling W, Slump E, Kleijer CN, Groot LC, Staveren WA. Early nutritional supplementation immediately after diagnosis of infectious disease improves body weight in psychogeriatric nursing home residents. Aging Clinical and Experimental Research 2006;18(1):70‐74. [DOI] [PubMed] [Google Scholar]
Yamaguchi 1998 {published and unpublished data}
- D'Antoni GB, Sucher KP, Coulston AM. Dietary intake of at‐risk, free‐living, elderly subjects receiving meals on wheels and a dietary supplement. Journal of the American Dietetic Association 1996;96(9):A79. [Google Scholar]
- Yamaguchi LY, Coulston AM, Lu NC, Dixon LB, Craig LD. Improvement in nutrient intake by elderly meals‐on‐wheels participants receiving a liquid nutrition supplement. Nutrition Today 1998;33(1):37‐44. [Google Scholar]
Young 2004 {published data only}
- Parrott MD, Young KW, Greenwood CE. Energy‐containing nutritional supplements can affect usual energy intake postsupplementation in institutionalized seniors with probable Alzheimer's disease. Journal of the American Geriatrics Society 2006;54:1382‐1387. [DOI] [PubMed] [Google Scholar]
- Young KW, Greenwood CE, Reekum R, Binns MA. Providing nutrition supplements to institutionalized seniors with probable Alzheimer's disease is least beneficial to those with low body weight status. Journal of the American Geriatrics Society 2004;52:1305‐1312. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Bachrach 2000 {published data only}
- Bachrach‐Lindstrom M, Johansson T, Unosson M, Ek AC, Wahlstrom O. Nutritional status and functional capacity after femoral neck fractures: A prospective randomized one‐year follow‐up study. Aging‐Clinical & Experimental Research 2000;12(5):366‐74. [DOI] [PubMed] [Google Scholar]
Barateau 1998 {published data only}
- Barateau M, Corompt A, Soulan J, Bourdel‐Marchasson I. Multicenter nursing study on the importance of nutritional support for the prevention of bedsores in the elderly at risk [Etude multicentrique infirmiere evaluant l'interet d'un soutien nutritionnel dans la prevention des escarres chez la personne agee a risque]. Recherche En Soins Infirmiers 1998;55:42‐9. [PubMed] [Google Scholar]
Barton 2000 {published data only}
- Barton AD, Beigg CL, MacDonald IA, Allison SP. A recipe for improving intakes of elderly hospitalized patients. Clinical Nutrition 2000;19(6):451‐4. [DOI] [PubMed] [Google Scholar]
- Barton AD, Beigg CL, Macdonald IA, Allison SP. High food wastage and low nutritional intakes in hospital patients. Clinical Nutrition 2000;19(6):445‐9. [DOI] [PubMed] [Google Scholar]
Bastow 1983 {published data only}
- Bastow MD, Rawlings J, Allison SP. Benefits of supplementary tube feeding after fractured neck of femur: a randomised controlled trial. British Medical Journal Clinical Research Edition 1983;287:1589‐92. [PMC free article] [PubMed] [Google Scholar]
Bean 1994 {published data only}
- Bean N, Redden J, Goode H, Grimble G, Allison SP. Double‐blind pilot trial in elderly women with fractured femur of ornithine alpha‐ketoglutarate versus a defined formula peptide oral supplement. Proceedings of the Nutrition Society 1994;53:203A. [Google Scholar]
Beattie 2000 {published data only}
- Beattie AH, Prach AT, Baxter JP, Pennington CR. A randomised controlled trial evaluating the use of enteral nutritional supplements postoperatively in malnourished surgical patients. Gut 2000;46(6):813‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Beier 1996 {published data only}
- Beier‐Holgersen R, Boesby S. Influence of postoperative enteral nutrition on postsurgical infections. Gut 1996;39(6):833‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beier‐Holgersen R, Brandstrup B. Influence of early postoperative enteral nutrition versus placebo on cell‐mediated immunity, as measured with the Multitest(R) CMI. Scandinavian Journal of Gastroenterology 1999;34(1):98‐102. [DOI] [PubMed] [Google Scholar]
Bernstein 2002 {published data only}
- Bernstein MA, Nelson MA, Tucker KL, Layne J, Johnson E, Nuernberger A, et al. A home based nutrition intervention to increase consumption of fruits, vegetables, and calcium rich foods in community dwelling elders. Journal of the American Dietetic Association 2002;102(10):1421‐7. [DOI] [PubMed] [Google Scholar]
Borum 2000 {published data only}
- Borum ML, Lynn J, Zhong Z, Roth K, Connors AF, Desbiens N, et al. The effect of nutritional supplementation on survival in seriously ill hospitalized adults. Journal of the American Geriatrics Society 2000;48(5 Suppl):S33‐8. [DOI] [PubMed] [Google Scholar]
Bos 2000 {published data only}
- Bos C, Benamouzig R, Bruhat A, Roux C, Mahe S, Valensi P, et al. Short‐term protein and energy supplementation activates nitrogen kinetics and accretion in poorly nourished elderly subjects. American Journal of Clinical Nutrition 2000;71:1129‐37. [DOI] [PubMed] [Google Scholar]
Breslow 1993 {published data only}
- Breslow RA, Hallfrisch J, Guy DG, Crawley B, Goldberg AP. The importance of dietary protein in healing pressure ulcers. Journal of the American Geriatrics Society 1993;41(4):357‐62. [DOI] [PubMed] [Google Scholar]
Brocker 1994 {published data only}
- Brocker P, Vellas B, Albarede JL, Poynard T. A two‐centre, randomized, double‐blind trial of ornithine oxoglutarate in 194 elderly, ambulatory, convalescent subjects. Age & Ageing 1994;23(4):303‐6. [DOI] [PubMed] [Google Scholar]
Brown 1995 {published data only}
- Brown DN, Miedema BW, King PD, Marshall JB. Safety of early feeding after percutaneous endoscopic gastrostomy. Journal of Clinical Gastroenterology 1995;21(4):330‐1. [DOI] [PubMed] [Google Scholar]
Bunker 1994 {published data only}
- Bunker VW, Stansfield MF, Deacon Smith R, Marzil RA, Hounslow A, Clayton BE. Dietary supplementation and immunocompetence in housebound elderly subjects. British Journal of Biomedical Science 1994;51(2):128‐35. [PubMed] [Google Scholar]
Bunout 1989 {published data only}
- Bunout D, Aicardi V, Hirsch S. Nutritional support in hospitalized patients with alcoholic liver disease. European Journal of Clinical Nutrition 1989;43(9):615‐21. [PubMed] [Google Scholar]
Bunout 2001 {published data only}
- Bunout D, Barrera G, MP, Avendano M, Gattas V, Petermann M, et al. The impact of nutritional supplementation and resistance training on the health functioning of free‐living Chilean elders: results of 18 months of follow‐up. Journal of Nutrition 2001;131(9):2441S‐6S. [DOI] [PubMed] [Google Scholar]
Bunout 2004 {published data only}
- Bunout D, Barrerra, Maza P, Avendano M, Gattas V, Petermann M, Hirsch S. Effects of nutritional supplementation and resistance training on muscle strength in free living elders. Results of one year follow. Journal of Nutrition, Health and Aging 2004;8(2):68‐75. [PubMed] [Google Scholar]
Cabre 1990 {published data only}
- Cabre E, Gonzalez‐Huix F, Abad‐Lacruz A, Esteve M, Acero D, Fernandez‐Banares F, et al. Effect of total enteral nutrition on the short‐term outcome of severely malnourished cirrhotics. A randomized controlled trial. Gastroenterology 1990;98(3):715‐20. [DOI] [PubMed] [Google Scholar]
Caglar 2002 {published data only}
- Carlar K, Fedje L, Dimmitt R, Hakim RM, Shyr Y, Ikizler TA. Therapeutic effects of oral nutritional supplementation during haemodialysis. Kidney International 2002;62:1054‐9. [DOI] [PubMed] [Google Scholar]
Calvey 1985 {published data only}
- Calvey H, Davis M, Williams R. Controlled trial of nutritional supplementation, with and without branched chain amino acid enrichment, in treatment of acute alcoholic hepatitis. Journal of Hepatology 1985;1(2):141‐51. [DOI] [PubMed] [Google Scholar]
Campbell 1995 {published data only}
- Campbell W W, Crim MC, Young V R, Joseph L J, Evans WJ. Dietary protein intake influences resistance training‐induced changes in whole‐body protein turnover. FASEB: Federation of American societies for experimental biology 1994;8(4‐5):A428. [Google Scholar]
- Campbell WW, Crim MC, Dallal GE, Young VR, Evans WJ. Increased protein requirements in elderly people: new data and retrospective reassessments. American Journal of Clinical Nutrition 1994;60(4):501‐9. [DOI] [PubMed] [Google Scholar]
- Campbell WW, Crim MC, Young VR, Evans WJ. Increased energy requirements and changes in body composition with resistance training in older adults. American Journal of Clinical Nutrition 1994;60(2):167‐75. [DOI] [PubMed] [Google Scholar]
- Campbell WW, Crim MC, Young VR, Joseph LJ, Evans WJ. Effects of resistance training and dietary protein intake on protein metabolism in older adults. American Journal of Physiology 1995;268(6 Pt 1):E1143‐53. [DOI] [PubMed] [Google Scholar]
Carr 1996 {published data only}
- Carr CS, Ling KD, Boulos P, Singer M. Randomised trial of safety and efficacy of immediate postoperative enteral feeding in patients undergoing gastrointestinal resection. BMJ 1996;312(7035):869‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chee 2003 {published data only}
- Chee WSS, Suriah AR, Chan SP, Zaitun Y, Chan YM. The effect of milk supplementation on bone mineral density in postmenopausal Chinese women in Malaysia. Osteoporosis International 2003;14:828‐834. [DOI] [PubMed] [Google Scholar]
Creutzberg 2000 {published data only}
- Creutzberg EC, Schols AMWJ, Weling Scheepers CAPM, Buurman WA, Wouters EF. Characterisation of non response to high caloric oral nutrition therapy in depleted patients with COPD. American Journal of Respiratory and Critical Care Medicine 2000;161:745‐52. [DOI] [PubMed] [Google Scholar]
Daly 1992 {published data only}
- Daly JM, Lieberman MD, Goldfine J, Shou J, Weintraub F, Rosato EF, et al. Enteral nutrition with supplemental arginine, RNA, and omega‐3 fatty acids in patients after operation: immunologic, metabolic, and clinical outcome. Surgery 1992;112(1):56‐67. [PubMed] [Google Scholar]
Danhof 1982 {published data only}
- Danhof IE, Huston RL. Nutritional support of geriatric patients. Jpen: Journal of Parenteral & Enteral Nutrition 1982;6(6):588. [Google Scholar]
de Jong 2000 {published data only}
- Paw MJ, Jong N, Pallast EG, Kloek GC, Schouten EG, Kok FJ. Immunity in frail elderly: A randomized controlled trial of exercise and enriched foods. Medicine & Science in Sports & Exercise 2000;32(12):2005‐11. [DOI] [PubMed] [Google Scholar]
- Jong N, Paw MJ, Graaf C, Staveren WA. Effect of dietary supplements and physical exercise on sensory perception. British Journal of Nutrition 2000;83(6):605‐13. [DOI] [PubMed] [Google Scholar]
- Jong N, Paw MJ, Groot LC, Hiddink GJ, Staveren WA. Dietary supplements and physical exercise affecting bone and body composition. American Journal of Public Health 2000;90(6):947‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jong N, Paw MJ, Groot LC, Graaf C, Kok F, Staveren WA. Functional biochemical and nutrient indices in frail elderly people are partly affected by dietary supplements but not by exercise. Journal of Nutrition 1999;129(11):2028‐36. [DOI] [PubMed] [Google Scholar]
Devine 1996 {published data only}
- Devine A, Prince RL, Bell R. Nutritional effect of calcium supplementation by skim milk powder or by calcium tablets on total nutrient intake in postmenopausal women. American Journal of Clinical Nutrition 1996;64:731‐7. [DOI] [PubMed] [Google Scholar]
Duncan 2006 {published data only}
- Duncan DG, Beck SJ, Hood K, Johansen A. Using dietetic assistants to improve the outcome of hip fracture ‐ a randomised controlled trial of nutritional support in an acute trauma ward. Age and Ageing 2006;35(2):148‐53. [DOI] [PubMed] [Google Scholar]
Efthimou 1988 {published data only}
- Efthimiou J, Fleming J, Gomes C, Spiro SG. The effect of supplementary oral nutrition in poorly nourished patients with chronic obstructive pulmonary disease. American Review of Respiratory Disease 1988;137(5):1075‐82. [DOI] [PubMed] [Google Scholar]
Elmstahl 1987 {published data only}
- Elmstahl S, Steen B. Hospital nutrition in geriatric long‐term care in medicine: II. Effects of dietary supplements. Age & Ageing 1987;16:73‐80. [DOI] [PubMed] [Google Scholar]
Eneroth 1997 {published data only}
- Eneroth M, Apelqvist J, Larsson J, Persson BM. Improved wound healing in transtibial amputees receiving supplementary nutrition. International Orthopaedics 1997;21(2):104‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Eneroth 2006 {published data only}
- Eneroth M, Olsson UB, Thorngren KG. Nutritional supplementation decreases hip fracture‐related complications. Clinical Orthopaedics and Related Research 2006;451:212‐217. [DOI] [PubMed] [Google Scholar]
Espaulella 2000 {published data only}
- Espaulella J, Guyer H, Diaz‐Escriu F, Mellado‐Navas JA, Castells M, Pladevall M. Nutritional supplementation of elderly hip fracture patients. A randomized double‐blind placebo‐controlled trial. Age & Ageing 2000;29:425‐31. [DOI] [PubMed] [Google Scholar]
Eyer 1993 {published data only}
- Eyer SD, Micon LT, Konstantinides FN, Edlund DA, Rooney KA, Luxenberg MG. Early enteral feeding does not attenuate metabolic response after blunt trauma. Journal of Trauma‐Injury Infection & Critical Care 1993;34(5):639‐43. [DOI] [PubMed] [Google Scholar]
Forli 2001 {published data only}
- Forli L, Pedersen JI, Bjortuft O, Vatn M, Boe J. Dietary support to underweight patients with end‐stage pulmonary disease assessed for lung transplantation. Respiration 2001;68(1):51‐7. [DOI] [PubMed] [Google Scholar]
Fuenzalida 1990 {published data only}
- Fuenzalida CE, Petty TL, Jones ML, Jarett S, Harbeck RJ, Terry RW. The immune response to short‐term nutritional intervention in advanced chronic obstructive pulmonary disease. American Review of Respiratory Disease 1990;142(1):49‐56. [DOI] [PubMed] [Google Scholar]
Gall 1998 {published data only}
- Gall MJ, Grimble GK, Reeve NJ, Thomas SJ. Effect of providing fortified meals and between‐meal snacks on energy and protein intake of hospital patients. Clinical Nutrition 1998;17(6):259‐64. [DOI] [PubMed] [Google Scholar]
Gallager 1992 {published data only}
- Gallagher J, Schermbeck J, Dixon L, Labbe‐Bell M. Aggressive early management of malnutrition in hip fracture patients. Journal of Parenteral & Enteral Nutrition 1992;16(1):19S. [Google Scholar]
Ganzoni 1994 {published data only}
- Ganzoni A, Heilig P, Schonenberger K, Hugli O Fitting J W, Brandli O. High‐caloric nutrition in chronic obstructive lung disease [Hochkalorische Ernährung bei chronischer obstrukiver Lungenkrankheit]. Schweizerische Rundschau für Medizin Praxis 1994;83(1):13‐6. [PubMed] [Google Scholar]
Goris 2003 {published data only}
- Goris AM, Vermeeren MA, Wouters EF, Schols AM, Westerterp KR. Energy balance in depleted ambulatory patients with chronic obstructive pulmonary disease: the effect of physical activity and oral nutritional supplementation. British Journal of Nutrition 2003;89:725‐729. [DOI] [PubMed] [Google Scholar]
Harrington 2004 {published data only}
- Harrington M, Bennett T, Jakobsen J, Ovesen L, Brot C, Flynn A, et al. The effect of a high‐protein, high sodium diet on calcium and bone metabolism in post‐menopausal women and its interaction with vitamin D receptor genotype. British Journal of Nutrition 2004;91(1):41‐51. [DOI] [PubMed] [Google Scholar]
Hartgrink 1998 {published data only}
- Hartgrink HH, Wille J, Konig P, Hermans J, Breslau PJ. Pressure sores and tube feeding in patients with a fracture of the hip: a randomized clinical trial. Clinical Nutrition 1998;17(6):287‐92. [DOI] [PubMed] [Google Scholar]
Hickson 2004 {published data only}
- Hickson M, Bulpitt C, Nunes M, Peters R, Cooke J, Nicholl C, Frost G. Does additional feeding support provided by health care assistants improve nutritional status and outcome in acutely ill older in‐patients? ‐ A randomised control trial. Clinical Nutrition 2004;23(1):69‐77. [DOI] [PubMed] [Google Scholar]
Hogarth 1996 {published data only}
- Hogarth MB, Marshall P, Lovat LB, Palmer AJ, Frost CG, Fletcher AE, et al. Nutritional supplementation in elderly medical in‐patients: a double‐blind placebo‐controlled trial. Age & Ageing 1996;25:453‐57. [DOI] [PubMed] [Google Scholar]
Houwing 2003 {published data only}
- Houwing RH, Rozendaal M, Wouters‐Wesseling W, Beulens JW, Buskens E, Haalboom JR. A randomised, double‐blind assessment of the effect of nutritional supplementation on the prevention of pressure ulcers in hip‐fracture patients. Clinical Nutrition 2003;22(4):401‐5. [DOI] [PubMed] [Google Scholar]
Hurson 1995 {published data only}
- Hurson M, Regan MC, Kirk SJ, Wasserkrug HL, Barbul A. Metabolic effects of arginine in a healthy elderly population. Journal of Parenteral & Enteral Nutrition 1995;19(3):227‐30. [DOI] [PubMed] [Google Scholar]
Jamieson 1997 {published data only}
- Jamieson CP, Norton B, Day T, Lakeman M, Powell‐Tuck J. The quantitative effect of nutrition support on quality of life in outpatients. Clinical Nutrition 1997;16(1):25‐8. [Google Scholar]
Johansen 2004 {published data only}
- Johansen NN, Kondrup J, Plum LM, Bak L, Norregaard P, Bunch E, et al. Effect of nutritional support on clinical outcome in patients at risk. Clinical Nutrition 2004;23(4):539‐50. [DOI] [PubMed] [Google Scholar]
Johansson 2002 {unpublished data only}
- Johansson T. Displaced femoral neck fractures: A prospective randomized study of clinical outcome, nutrition and costs. Linkoping University Medical Dissertations No 731 2002.
Keane 1998 {published data only}
- Keane EM, Healy M, O'Moore R, Coakley D, Walsh JB. Vitamin D‐fortified liquid milk: benefits for the elderly community‐based population. Calcified Tissue International 1998;62(4):300‐2. [DOI] [PubMed] [Google Scholar]
Keele 1997 {published data only}
- Keele AM, Bray MJ, Emery PW, Duncan HD, Silk DBA. Two phase randomised controlled trial of post operative dietary supplements in surgical patients. Gut 1997;40:393‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keele AM, Bray MJ, Emery PW, Silk DBA. A randomised controlled clinical trial of post operative oral dietary supplements in surgical patients. Journal of Parenteral and Enteral Nutrition 1995;19 Suppl 1:21S. [Google Scholar]
Kemen 1991 {published data only}
- Kemen M, Homan HH, Mumme A, Zumtobel V. Is intact protein superior for postoperative enteral nutrition than hydrolyzed protein?. Clinical Nutrition 1991;Supp 1:37. [Google Scholar]
Kerrigan 1996 {published data only}
- Kerrigan ER, Maxwell J, Siegel C. Dispensing of a calorically dense oral supplement with medication pass: a creative approach to oral supplementation. Journal of the American Dietetic Society 1996;96(9):A32. [Google Scholar]
Kiel 1992 {published data only}
- Kiel ML, Gilbert SK, James KD, Powell MS. Enhancing weight gain in long‐term care residents through a protein‐calorie supplemented mechanically altered diet. Journal of the American Dietetic Association 1992;92 Suppl:A91. [Google Scholar]
Kretser 2000 {published and unpublished data}
- Kretser A, Kerr W, Voss T, Ackman A, Squires M, Edison NJ, et al. Improving nutritional and functional outcomes in homebound elders via an aggressive 21 meal per week home delivery program. Journal of the American Dietetic Association 2000;100(9):A91. [Google Scholar]
Kumagai 1999 {published data only}
- Kumagai S, Shibata H, Watanabe S, Suzuki T, Haga H, Osada H, et al. An intervention trial to postpone aging in competent elderly. Trial of nutritional improvement in the retirement home. Nippon Koshu Eisei Zasshi ‐ Japanese Journal of Public Health 1999;46(11):1003‐12. [PubMed] [Google Scholar]
Lau 2001 {published data only}
- Lau EM, Woo J, Lam V, Hong A. Milk supplementation of the diet of postmenopausal Chinese women on a low calcium intake retards bone loss. Journal of Bone Mineral Research 2001;16(9):1704‐9. [DOI] [PubMed] [Google Scholar]
Lawson 2000 {published data only}
- Doshi MK, Lawson R, Ingoe LE, Colligan JM, Barton JR, Cobden I. Effect of nutritional supplementation on clinical outcome in post‐operative orthopaedic patients. Clinical Nutrition 1998;17 Suppl 1:30. [Google Scholar]
- Lawson RM, Doshi MK, Ingoe LE, Colligan JM, Barton JR, Cobden I. Compliance of orthopaedic patients with post operative oral nutritional supplementation. Clinical Nutrition 2000;19(3):171‐5. [DOI] [PubMed] [Google Scholar]
Lewis 1987 {published data only}
- Lewis MI, Belman MJ, Dorr‐Uyemura L. Nutritional supplementation in ambulatory patients with chronic obstructive pulmonary disease. American Review of Respiratory Disease 1987;135(5):1062‐68. [DOI] [PubMed] [Google Scholar]
Neumann 2004 {published and unpublished data}
- Jensen G. personal communication. 6 Jan 2005.
- Neumann M, Friedmann J, Roy MA, Jensen GL. Provision of high‐protein supplement for patients recovering from hip fracture. Nutrition 2004;20:415‐419. [DOI] [PubMed] [Google Scholar]
- Neumann MM, Friedmann JM, Jensen GL. [Provision of high protein supplement for patients recovering from hip fracture]. Journal of Parenteral and Enteral Nutrition 2004;28(Suppl 1):S30. [DOI] [PubMed] [Google Scholar]
Nickerson 1998 {published data only}
- Nickerson JA, Langkamp‐Henken B, Stechmiller JK, Bley LL, Greishaw S, Herrlinger‐Garcia KA. Oral and metabolic tolerance of supplemental arginine in elderly nursing home residents with pressure ulcers. Journal of Parenteral & Enteral Nutrition 1998;22 Suppl:75. [Google Scholar]
Olin 1998 {published data only}
- Odlund Olin A, Armyr I, Soop M, Ljungqvist E, Jerstrom S, Classon I, et al. Energy enriched meals improve energy intake in elderly residents in a nursing home. Clinical Nutrition 1998;17 Suppl1:31. [DOI] [PubMed] [Google Scholar]
Otte 1989 {published data only}
- Otte KE, Ahlburg P, D'Amore F, Stellfeld M. Nutrition repletion in malnourished patients with emphysema. Jpen: Journal of Parenteral & Enteral Nutrition 1989;13(2):152‐6. [DOI] [PubMed] [Google Scholar]
Pardy 1986 {published data only}
- Pardy RL, Knowles JL, Fairbarn MS, Chan‐Yan C. Supplemental nutrition does not alter respiratory muscle strength (RMS) or endurance (RME) in ambulatory COPD. American Review of Respiratory Disease 1986;133:A204. [Google Scholar]
Paton 2004 {published data only}
- Paton NI, Chua Y, Earnest A, Chee CBA. Randomised controlled trial of nutritional supplementation in patients with newly diagnosed tuberculosis and wasting. American Journal of Clinical Nutrition 2004;80(2):460‐5. [DOI] [PubMed] [Google Scholar]
Planas 2005 {published data only}
- Planas M, Alvarez J, Garcia‐Peris PA, Cuerda C, Lucas P, Castella M, et al. Nutritional support and quality of life in stable chronic obstructive pulmonary disease (COPD) patients. Clinical Nutrition 2005;24:433‐441. [DOI] [PubMed] [Google Scholar]
Price 2006 {published data only}
- Price RJ, McMurdo ME, Anderson AS. A personalized snack‐based intervention for hip fracture patients: development, feasibility and acceptability. Journal of Human Nutrition and Dietetics 2006;19(2):139‐145. [DOI] [PubMed] [Google Scholar]
Pupim 2002 {published data only}
- Pupim LCB, Ribeiro C de B, Salgueiro NCS, Yamazaki WM, Ramalho HJ, Barberato JB, et al. [Bioquimicos e de composicao corporal de pacientes em hemodialise]. Review of Brasilian Medicine 2002;59(1/2):55‐62. [Google Scholar]
Rana 1992 {published data only}
- Rana SK, Bray J, Menzies‐Gow N, Jameson J, James JJP, Frost P, et al. Short term benefits of post‐operative oral dietary supplements in surgical patients. Clinical Nutrition 1992;11(6):337‐44. [DOI] [PubMed] [Google Scholar]
Remsburg 2001 {published data only}
- Remsburg RE, Luking A, Baran P, Radu C, Pineda D, Bennett RG, et al. Impact of a buffet‐style dining program on weight and biochemical indicators of nutritional status in nursing home residents: A pilot study. Journal of the American Dietetic Association 2001;101(12):1460‐3. [DOI] [PubMed] [Google Scholar]
Rogers 1992 {published data only}
- Rogers RM, Donahoe M, Constantino J. Physiologic effects of oral supplemental feeding in malnourished patients with chronic obstructive pulmonary disease: a randomised control study. American Review of Respiratory Disease 1992;146:1511‐7. [DOI] [PubMed] [Google Scholar]
Schurch 1998 {published data only}
- Bonjour JP, Schurch MA, Chevalley P, Ammann P, Rizzoli R. Protein Intake, IGF‐1 and osteoporosis. Osteoporosis International 1997;7 Suppl 3:S36‐42. [DOI] [PubMed] [Google Scholar]
- Rizzoli R, Schurch MA, Bonjour JP. Protein supplements after osteoporotic hip fracture [letter]. Annals of Internal Medicine 1998;129:1076. [DOI] [PubMed] [Google Scholar]
- Schurch MA, Rizzoli R, Slosman D, Bonjour JP. Protein supplements increase serum IGF‐1 and decrease proximal femur bone loss in patients with recent hip fracture. In: Papapoulos SE, Lips P, Pols HA, Johnston CC, Delmas PD editor(s). Osteoporosis. Amsterdam: Elsevier, 1996:327‐9. [Google Scholar]
- Schurch MA, Rizzoli R, Slosman D, Vadas L, Vergnaud P, Bonjour JP. Protein supplements increase serum insulin‐like growth factor‐I levels and attenuate proximal femur bone loss in patients with recent hip fracture. A randomized double‐blind placebo‐controlled trial. Annals of Internal Medicine 1998;128(10):801‐9. [DOI] [PubMed] [Google Scholar]
Seri 1984 {published data only}
- Seri S, Aquilio E. Effects of early nutritional support in patients with abdominal trauma. Italian Journal of Surgical Sciences 1984;14(3):223‐7. [PubMed] [Google Scholar]
Seven 2003 {published data only}
- Seven H, Calis AB, Turgot S. A randomised controlled trial of early oral feeding in laryngectomized patients. Laryngoscope 2003;113:1076‐9. [DOI] [PubMed] [Google Scholar]
Slodkowski 1994 {published data only}
- Slodkowski M, Pawlowski W, Pertkiewics M, Majewska K, Cebulski W, Szczygiel B. Clinical evaluation of the manufactured Terapin II diet. Polski Tygodnik Lekarski 1994;49(23‐24):526‐9. [PubMed] [Google Scholar]
Smedley 2004 {published data only}
- Smedley F, Bowling T, James M, Stokes E, Goodger C, O'Connor O, et al. Randomised clinical trial of the effects of pre‐operative and postoperative oral nutritional supplements on clinical course and cost of care. British Journal of Surgery 2004;91(8):983‐90. [DOI] [PubMed] [Google Scholar]
Stauffer 1986 {published data only}
- Stauffer JL, Carbone JE, Bendoski MT. Effects of diet supplementation on anthropometric and laboratory nutritional parameters in malnourished ambulatory patients with severe chronic obstructive pulmonary disease (COPD). American Review of Respiratory Disease 1986;133:A204. [Google Scholar]
Storm 1998 {published data only}
- Storm D, Eslin R, Porter ES, Musgrave K, Vereault D, Patton C, et al. Calcium supplementation prevents seasonal bone loss and changes in biochemical markers of bone turnover in elderly New England women: a randomized placebo‐controlled trial. Journal of Clinical Endocrinology & Metabolism 1998;83(11):3817‐25. [DOI] [PubMed] [Google Scholar]
Sullivan 1998 {published data only}
- Sullivan DH, Nelson CL, Bopp MM, Puskarich‐May CL, Walls RC. Nightly enteral nutrition support of elderly fracture patients: a phase I trial. Journal of the American College of Nutrition 1998;17(1):155‐61. [DOI] [PubMed] [Google Scholar]
Tkatch 1992 {published data only}
- Bonjour JP, Rapin CH, Rizzoli R, Tkatch L, Delmi M, Chevalley T, et al. Hip fracture, femoral bone mineral density, and protein supply in elderly patients. In: Munro H, Schlierf editor(s). Nutrition of the elderly. Nestle nutrition workshop series. Vol. 29, New York: Raven Press, 1992:151‐9. [Google Scholar]
- Tkatch L, Rapin CH, Rizzoli R, SlosmanD, Nydegger V, Vasey H, et al. Benefits of oral protein supplementation in elderly patients with fracture of the proximal femur. Journal of the American College of Nutrition 1992;11(5):519‐25. [DOI] [PubMed] [Google Scholar]
Tschepe 1985 {published data only}
- Tschepe A, Holm E, Leweling H, Staedt U, Weber K. Oral administration of branched chain amino acids (BCAA) in patients with liver cirrhosis. A double‐blind, randomized crossover study. Clinical Nutrition 1985;4 Suppl:40. [Google Scholar]
Vargas 1995 {published data only}
- Vargas M, Puig A, Maza M, Morales P, Vargas D, Bunout D, et al. Effects of an inspiratory muscle training program and nutritional support in patients with chronic obstructive lung disease. Revista Medica De Chile 1995;123(10):1225‐34. [PubMed] [Google Scholar]
Vermeeren 2001 {published data only}
- Vermeeren MAP, Wouters EF, Nelissen LH, Lier A, Hofman Z, Schols AM. Acute effect of different nutritional supplements on symptoms and functional capacity in patients with chronic obstructive pulmonary disease. American Journal of Clinical Nutrition 2001;73:295‐301. [DOI] [PubMed] [Google Scholar]
Von Meyenfeldt 1990 {published data only}
- Meyenfeldt MF, Soeters PB, Vente JP, Berlo CL, Rouflart MM, Jong KP, et al. Effect of branched chain amino acid enrichment of total parenteral nutrition on nitrogen sparing and clinical outcome of sepsis and trauma: a prospective randomized double blind trial. British Journal of Surgery 1990;77(8):924‐9. [DOI] [PubMed] [Google Scholar]
Wachtler 1995 {published data only}
- Wachtler P, Axel Hilger RA, Konig W, Bauer KH, Kemen M, Koller M. Influence of a pre‐operative enteral supplement on functional activities of peripheral leukocytes from patients with major surgery. Clinical Nutrition 1995;14(5):275‐82. [DOI] [PubMed] [Google Scholar]
Wara 1985 {published data only}
- Wara P, Hessov I. Nutritional intake after colorectal surgery: a comparison of a traditional and new post operative regime. Clinical Nutrition 1985;4:225. [DOI] [PubMed] [Google Scholar]
Williams 1989 {published data only}
- Driver L. Evaluation of supplemental nutrition in elderly orthopaedic patients [PhD thesis]. Surrey, UK: Univ. of Surrey, 1994. [Google Scholar]
- Driver LT, Lumbers M, Older J, Williams CM. A controlled trial of sip‐feed supplements and compliance. Proceedings of the Nutrition Society 1990;49:173A. [Google Scholar]
- Williams CM, Driver L, Older J, Dickerson JW. The use of a nutritional risk score in identifying patients who may benefit from sip‐feed supplementation in hospital. Proceedings of the Nutrition Society 1988;47:135A. [Google Scholar]
- Williams CM, Driver LT, Older J, Dickerson JW. A controlled trial of sip‐feed supplements in elderly orthopaedic patients. European Journal of Clinical Nutrition 1989;43(4):267‐74. [PubMed] [Google Scholar]
Wilson 1986 {published data only}
- Wilson DO, Rogers RM, Sanders MH, Pennock BE, Reilly JJ. Nutritional intervention in malnourished patients with emphysema. American Review of Respiratory Disease 1986;134(4):672‐7. [DOI] [PubMed] [Google Scholar]
Wisten 2005 {published data only}
- Wisten A, Messner T. Fruit and fibre (Pajala porridge) in the prevention of constipation. Scandanavian Journal of Caring Sciences 2005;19:71‐76. [DOI] [PubMed] [Google Scholar]
Wong 2004 {published data only}
- Wong S Y, Lau E M, Lau W W, Lynn H S. Is dietary counselling effective in increasing dietary calcium, protein and energy intake in patients with osteoporotic fractures? A randomized controlled clinical trial. Journal of Human Nutrition & Dietetics 2004;17:359‐364. [DOI] [PubMed] [Google Scholar]
Yeh 2000 {published data only}
- Yeh SS, Wu SY, Lee TP, Olson JS, Stevens MR, Dixon T, et al. Improvement in quality‐of‐life measures and stimulation of weight gain after treatment with megestrol acetate oral suspension in geriatric cachexia: results of a double‐blind, placebo‐controlled study. Journal of the American Geriatrics Society 2000;48(5):485‐92. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
Cameron 2003 {published data only}
- Au L, Cameron I, Kurrle S, Uy C. Effectiveness of oral nutritional supplementation for older women with hip and other fractures. Australian Society of Geriatric Medicine Annual Meeting. 2003 June 16‐18.
CENEX 2007 {published data only}
- Dangour AD, Albala C, Aedo C, Elbourne D, Grundy E, Walker D, et al. A factorial‐design cluster randomised controlled trial investigating the cost‐effectiveness of a nutrition supplement and an exercise programme on pneumonia incidence, walking capacity and body mass index in older people living in Santiago, Chile: the CENEX study protocol. Nutrition Journal 2007;6(14). [DOI] [PMC free article] [PubMed] [Google Scholar]
Elia 2007 {published data only}
- Elia M. Community nutrition support trial. http://www.controlled‐trials.com/mrct/trial/276549/nutrition 11th December 2007.
Kvamme 2007 {published data only}
- Kvamme JM. Nutritional intervention in malnourished elderly patients. http://www.controlled‐trials.com/mrct/trial/253861/nutrition 11th December 2007.
Additional references
Akner 2001
- Akner G, Cederholm T. Treatment of protein‐energy malnutrition in chronic nonmalignant disorders. American Journal of Clinical Nutrition 2001;74(1):6‐24. [DOI] [PubMed] [Google Scholar]
Alderson 2004
- Alderson P, Green S, Higgins JPT. Medline highly sensitive search strategies for identifying reports of randomized controlled trials in Medline. Cochrane Reviewers Handbook 4.2.2 [updated March 2004]; Appendix 5B. In: The Cochrane Library. Chichester, UK: John Wiley & Sons Ltd., 2004. [Google Scholar]
Allison 2000
- Allison SP. Malnutrition, disease and outcome. Nutrition 2000;16(7/8):590‐3. [DOI] [PubMed] [Google Scholar]
Avenell 2004
- Avenell A, Handoll HHE. Nutritional supplementation for hip fracture aftercare in the elderly. (Cochrane Review). Cochrane Database of Systematic Reviews 2004, Issue 1. [DOI] [PubMed] [Google Scholar]
Avenell 2006
Baldwin 2000
- Baldwin C, Parsons T, Logan S. Dietary advice and oral nutritional supplements for illness‐related malnutrition in adults (Cochrane Review). Cochrane Database of Systematic Reviews 2000, Issue 4. [Google Scholar]
Baldwin 2008
BAPEN 2003
- Malnutrition Advisory Group (MAG). Malnutrition Universal Screening Tool (MUST). BAPEN 2003.
Bistrian 1976
- Bistrian BR, Blackburn GL, Vitale J, Cochran D, Naylor J. Prevalence of malnutrition in general medical patients. Journal of the American Medical Association 1976;253:1567‐70. [PubMed] [Google Scholar]
Bruun 1999
- Bruun LI, Bosaeus I, Bergstad K, Nygaard K. Prevalence of malnutrition in surgical patients: evaluation of nutritional support and documentation. Clinical Nutrition 1999;18(3):141‐7. [DOI] [PubMed] [Google Scholar]
Corish 2000
- Corish CA, Flood P, Mulligan S, Kennedy NP. Apparent low frequency of undernutrition in Dublin hospital in‐patients: should we review the anthropometric thresholds for clinical practice?. British Journal of Nutrition 2000;84:325‐35. [PubMed] [Google Scholar]
Council Europe 2002
- Council of Europe. Food and nutritional care in hospitals. How to prevent undernutrition. www.coe.fr/soc‐sp (accessed 30 Sep 2004).
DoH 1992
- Department of Health. The nutrition of elderly people. Report on health and social subjects No 43. London: HMSO, 1992. [Google Scholar]
Edington 2000
- Edington J, Boorman J, Durrant ER, Perkins A, Giffin CV, James R, et al. Prevalence of malnutrition on admission to four hospitals in England. Clinical Nutrition 2000;19(3):191‐5. [DOI] [PubMed] [Google Scholar]
Elia 2001
- Elia M. The Malnutrition Advisory Group consensus guidelines for the detection and management of malnutrition in the community. Nutrition Bulletin 2001;26:81‐3. [Google Scholar]
Elia 2005
- Elia M, Stratton R, Russell C, Green C, Pang F. The cost of disease‐related malnutrition in the UK and economic considerations for the use of oral nutritional supplements (ONS) in adults. BAPEN, 2005. [Google Scholar]
Flodin 2000
- Flodin L. Svensson S, Cederholm T. Body Mass Index as a predictor of 1 year mortality in geriatric patients. Clinical Nutrition 2000;19(2):121‐5. [DOI] [PubMed] [Google Scholar]
Gallager‐Allred 1996
- Gallager‐Allred CR, Voss AC, Finn SC, McCamish MA. Malnutrition and clinical outcomes: the case for medical nutrition therapy. Journal of the American Dietetic Association 1996;96(4):361‐6. [DOI] [PubMed] [Google Scholar]
Gurney 1973
- Gurney JM, Jelliffe DB. Arm anthropometry in nutritional assessment: nomogram for rapid calculation of muscle circumference and cross‐sectional muscle and fat areas. American Journal of Clinical Nutrition 1973;26(9):912‐5. [DOI] [PubMed] [Google Scholar]
HCUP 2002
- Agency for Healthcare Research and Quality. HCUPnet: A tool for identifying, tracking, and analyzing national hospital statistics 2002. http://hcup.ahrq.gov/HCUPNet.asp (accessed 30th Sept 2004).
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring consistency in meta‐analysis. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Katz 1963
- Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of illness in the aged. The index of ADL: a standard measure of biological and physical function. Journal of the American Medical Association 1963;185:94‐9. [DOI] [PubMed] [Google Scholar]
Kelly 1984
- Kelly SM, Rossa A, Field S, Coughlin M, Schizgal HM, Macklem PT. Respiratory muscle strength and body composition in patients receiving total parenteral nutrition therapy. American Review of Respiratory Disease 1984;130:33‐7. [DOI] [PubMed] [Google Scholar]
Keys 1950
- Keys A, Brozek J, Henschel A, Micklesen O, Taylor HL. The biology of human starvation. Minneapolis: University of Minnesota, 1950:767‐918. [Google Scholar]
Kruizenga 2003
- Kruizenga HM, Wierdsma NJ, Bokhorst MAE, Schueren DE, Hollander HJ, Jonkers‐Schuitema CF, et al. Screening of nutritional status in The Netherlands. Clinical Nutrition 2003;22(2):147‐52. [DOI] [PubMed] [Google Scholar]
Lennard‐Jones 1992
- Lennard‐Jones JE. A positive approach to nutrition as treatment. London: King's Fund Centre, 1992. [Google Scholar]
Mahoney 1965
- Mahoney FI, Barthel D. Functional evaluation: The Barthel Index. Maryland State Medical Journal 1965;14:56‐61. [PubMed] [Google Scholar]
McCormack 1997
- McCormack P. Undernutrition in the elderly population living at home in the community: a review of the literature. Journal of Advanced Nursing 1997;26:856‐63. [DOI] [PubMed] [Google Scholar]
McWhirter 1994
- McWhirter JP, Pennington CR. The incidence and recognition of malnutrition in hospital. BMJ 1994;308:945‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
NHSQIS 2003
- NHS Quality Improvement Scotland (NHSQIS). The National Standards for Food, Fluid and Nutritional Care in Hospitals. www.nhshealthquality.org/nhsqis/qis (accessed September 2004).
NICE 2006
- National Collaborating Centre for Acute Care. Nutrition support in adults. Oral nutrition support, enteral tube feeding and parenteral nutrition. UK National Institute for Health and Clinical Excellence (NICE), 2006. [PubMed] [Google Scholar]
Peake 1998
- Peake HJ, Evans S, Chambers A, Riches C, Frost CG. Nutritional supplementation: how much do people drink?. Proceedings of the Nutrition Society 1998;57:94A. [Google Scholar]
Podsiadlo 1991
- Podsiadlo D, Richardson S. The timed 'Up & Go' : a test of basic functional mobilitty for frail elderly persons. Journal of the American Geriatrics Society 1991;39:148‐8. [DOI] [PubMed] [Google Scholar]
Potter 1988
- Potter JF, Schafer DF, Bohi RL. In‐hospital mortality as a function of body mass index: An age dependant variable. Journal of Gerontology 1988;43(3):59‐63. [DOI] [PubMed] [Google Scholar]
Potter 1995
- Potter J, Klipstein K, Reilly JJ, Roberts M. The nutritional status and clinical course of acute admissions to a geriatric unit. Age & Ageing 1995;24:131‐6. [DOI] [PubMed] [Google Scholar]
Potter 1998
- Potter J, Langhorne P, Roberts M. Routine protein energy supplementation in adults: systematic review. BMJ 1998;317:495‐501. [DOI] [PMC free article] [PubMed] [Google Scholar]
Robinson 1987
- Robinson G, Goldstein M, Levine GM. Impact of nutritional status on DRG length of stay. Jpen: Journal of Parenteral & Enteral Nutrition 1987;11(1):49‐51. [DOI] [PubMed] [Google Scholar]
Stratton 2003
- Stratton RJ, Green CJ, Elia M. Disease‐related malnutrition: an evidence based approach to treatment. Nutricia Healthcare. CABI International, 2003. [Google Scholar]
Sullivan 1990
- Sullivan DH, Patch GA, Walls RC, Lipschitz DA. Impact of nutritional status on morbidity and mortality in a select population of geriatric rehabilitation patients. The American journal of clinical nutrition 1990;51:749‐58. [DOI] [PubMed] [Google Scholar]
Sullivan 1999
- Sullivan DH, Sun S, Walls RC. Protein‐energy undernutrition among elderly hospitalised patients: a prospective study. Journal of the American Medical Association 1999;281:2013‐9. [DOI] [PubMed] [Google Scholar]
Wilson 2002
- Wilson MMG, Purushothaman R, Morley JE. Effect of liquid dietary supplements on energy intake in the elderly. The American journal of clinical nutrition 2002;75:944‐7. [DOI] [PubMed] [Google Scholar]
Windsor 1988
- Windsor JA, Graham MB, Hill GL. Risk factors for post operative pneumonia. Annals of Surgery 1988;208:209‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wolfe 1997
- Wolfe BM, Mathiesen KA. Clinical practice guidelines in nutrition support: can they be based on randomized clinical trials?. Journal of Parenteral & Enteral Nutrition 1997;21(1):1‐6. [DOI] [PubMed] [Google Scholar]
Zador 1987
- Zador DA, Truswell AS. Nutritional status on admission to a general surgical ward in a Sydney hospital. Australia and New Zealand Journal of Medicine 1987;17:234‐40. [DOI] [PubMed] [Google Scholar]


