Abstract
Background
Bed rest in hospital or at home is widely recommended for the prevention of preterm birth. This advice is based on the observation that hard work and hard physical activity during pregnancy could be associated with preterm birth and with the idea that bed rest could reduce uterine activity. However, bed rest may have some adverse effects on other outcomes.
Objectives
To evaluate the effect of prescription of bed rest in hospital or at home for preventing preterm birth in pregnant women at high risk of preterm birth.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (18 December 2014), the Cochrane Central Register of Controlled Trials (The Cochrane Library, 2014, Issue 12), MEDLINE (December 2014), EMBASE (December 2014), LILACS (December 2014), and bibliographies of relevant papers.
Selection criteria
Randomized, cluster‐randomized and quasi‐randomized controlled trials with reported data that assess clinical outcomes in women at high risk of spontaneous preterm birth who were prescribed bed rest in hospital or at home for preventing preterm birth, and their babies.
Data collection and analysis
Two review authors independently assessed eligibility, trial quality and extracted data.
Main results
Two studies met the inclusion criteria. One study was not considered for the meta‐analysis, since data combined singleton and multiple pregnancies. No differences in any maternal and perinatal outcomes were reported by the authors. This study was at low risk of selection, performance, detection and attrition bias. Only data from one study were included in the meta‐analysis (1266 women). This study was at unclear risk of bias for most domains due to lack of reporting. Four hundred and thirty‐two women were prescribed bed rest at home and a total of 834 women received a placebo (412) or no intervention (422). Preterm birth before 37 weeks was similar in both groups (7.9% in the intervention group versus 8.5% in the control group; risk ratio (RR) 0.92, 95% confidence interval (CI) 0.62 to 1.37). No other results were reported for any of the other primary or secondary outcomes.
Authors' conclusions
There is no evidence, either supporting or refuting the use of bed rest at home or in hospital, to prevent preterm birth. Although bed rest in hospital or at home is widely used as the first step of treatment, there is no evidence that this practice could be beneficial. Due to the potential adverse effects that bed rest could have on women and their families, and the increased costs for the healthcare system, clinicians should discuss the pros and cons of bed rest to prevent preterm birth. Potential benefits and harms should be discussed with women facing an increased risk of preterm birth. Appropriate research is mandatory. Future trials should evaluate both the effectiveness of bed rest, and the effectiveness of the prescription of bed rest, to prevent preterm birth.
Plain language summary
Bed rest in singleton pregnancies for preventing preterm birth
No evidence to support or refute bed rest in preventing preterm birth.
Although bed rest in hospital or at home is widely used as the first step of treatment, this updated review finds no evidence to support or refute bed rest in preventing preterm birth. The current practice has been based on observational studies that found an association between hard work or hard physical activity and preterm birth. Due to the potential adverse effects that bed rest could have on women and their families, and the increased costs for the healthcare system, the pros and cons of bed rest for preventing preterm birth should be discussed fully.
Background
Description of the condition
Preterm birth, defined as birth occurring prior to 37 weeks of gestation occurs in around 5% to 10% of all pregnancies. In this group, newborns born before 32 weeks account for most neonatal deaths and disorders (Robertson 1992), contributing to at least 75% of neonatal deaths that are not due to congenital malformations (McCormick 1985). Although there are many different therapies available for preventing preterm birth or its neonatal associated morbidity and mortality, very few are proven to be effective and recommended for clinical use (see Cochrane reviews: Han 2013; Flenady 2013; Roberts 2006; Smaill 2007).
Description of the intervention
Bed rest has traditionally been recommended for preventing preterm birth as the first step in treatment and is cited in many obstetrics text books (Crowther 1991; Cunningham 1993; Schwarcz 2005).
How the intervention might work
This advice is based on the observation that hard work and hard physical activity during pregnancy could be associated with preterm birth (Saurel 1985; Teitelman 1990), and with the idea that bed rest could reduce uterine activity (Goldenberg 1994).
On the other hand, bed rest may have some adverse effects on other outcomes. It may increase the likelihood of venous thrombosis (Kovacevich 2000), muscle atrophy and symptoms of musculoskeletal (Maloni 2002) and cardiovascular deconditioning (Gupton 1997; Maloni 1993) and maternal weight loss (Maloni 1993). It may be stressful for women (Gupton 1997; Maloni 1993) and their families, (May 1994; Maloni 2001) inducing ambivalent feelings about the pregnancy, or self‐blame feelings in case of failure to comply with the prescription (Schroeder 1996); it may increase costs for the families, directly because of the expenses for the care of other children, or indirectly through job absenteeism (Maloni 2001; Maloni 2010; Mamelle 1984; McCall 2013). Finally, it may also increase healthcare costs (Allen 1999; Goldenberg 1994).
Why it is important to do this review
It is, therefore, important to assess the effectiveness and safety of bed rest by reviewing the evidence from randomized controlled trials.
Objectives
To evaluate the effect of prescription of bed rest in hospital or at home for preventing preterm birth in pregnant women at high risk of preterm birth.
Methods
Criteria for considering studies for this review
Types of studies
All published, unpublished and ongoing randomized trials with reported data that assess clinical outcomes in women and their babies who where prescribed bed rest in hospital or at home for preventing preterm birth. Randomized, cluster‐randomized and quasi‐randomized controlled trials were all eligible for inclusion.
Types of participants
Pregnant women at high risk of spontaneous preterm birth.
High risk of spontaneous preterm birth can be defined according to:
previous history of preterm birth or second trimester miscarriage;
threatened preterm labour;
positive screening test results, e.g. fetal fibronectin or ultrasound assessment of cervical length;
maternal anthropometric measurements (e.g. attained weight at 24 to 28 weeks, pre‐pregnancy body mass index);
scoring systems based on a combination of different categories of risk factors, including those previously mentioned.
Trials assessing bed rest in women with preterm premature rupture of membranes or multiple pregnancies were not considered (see the related review Crowther 2010).
Types of interventions
As bed rest is an accepted standard initial therapy for women at high risk of preterm birth, it has usually been used as a control intervention in trials evaluating alternative forms of care for preventing preterm birth. However, our intention in this review was to evaluate the effectiveness of bed rest compared with no intervention. Therefore, we considered trials comparing prescription of bed rest at home or in hospital with no intervention. Trials with arms including more than one intervention would also be eligible if arms differed only in the prescription of bed rest (i.e. bed rest and drug versus drug alone). For trials comparing drugs, placebo and bed rest, placebo was considered as no intervention.
Types of outcome measures
Primary outcomes
Preterm birth (less than 37 weeks)
Perinatal mortality
Low birthweight (less than 2500 g)
Neonatal intensive care
Secondary outcomes
Perinatal
Stillbirth
Use of corticosteroids (including incomplete courses of corticosteroids)
Preterm birth less than 32 weeks
Preterm birth less than 28 weeks
Delivery within 24 hours of treatment
Delivery within 48 hours of treatment
Delivery within seven days of treatment
Mean gestational age at birth (in weeks)
Very low birthweight
Neonatal respiratory distress syndrome
Intraventricular hemorrhage
Necrotizing enterocolitis
Bronchopulmonary dysplasia
Surfactant administration
Neonatal care more than 48 hours
Duration of neonatal care
Use of mechanical ventilation
Need of oxygen therapy
Maternal
Maternal mortality
Caesarean section
Thromboembolic events
Maternal infection
Antenatal maternal infection (chorioamnionitis)
Postpartum maternal infection (endometritis)
Dissatisfaction with care
Women views (experience and feeling)
Cost‐effectiveness
Search methods for identification of studies
The following methods section of this review is based on a standard template used by the Cochrane Pregnancy and Childbirth Group.
Electronic searches
We searched the Cochrane Pregnancy and Childbirth Group’s Trials Register by contacting the Trials Search Co‐ordinator (18 December 2014).
The Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co‐ordinator and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE (Ovid);
weekly searches of Embase (Ovid);
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL, MEDLINE and EMBASE, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the ‘Specialized Register’ section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are each assigned to a review topic (or topics). The Trials Search Co‐ordinator searches the register for each review using the topic list rather than keywords.
In addition, we searched CENTRAL (2014, Issue 12) using the terms in Appendix 1.
We also searched MEDLINE (December 2014), EMBASE (December 2014) and LILACS (December 2014) using the terms in Appendix 1.
Searching other resources
We searched the reference lists of retrieved studies.
We did not apply any language or date restrictions.
Data collection and analysis
For methods used in the previous version of this review, see 'Sosa 2004'.
For this update, the following methods were used for assessing the new report identified as a result of the updated search.
The following methods section of this review is based on a standard template used by the Cochrane Pregnancy and Childbirth Group.
Selection of studies
Two review authors (CS and FA) independently assessed for inclusion all the potential studies that were identify as a result of the search strategy. The two review authors resolved any disagreement by consensus or, if necessary, by a third review author (EB or JMB)
Data extraction and management
We designed a form to extract data. For eligible studies, two review authors extracted the data using the agreed form. We resolved discrepancies through discussion or, if required, we consulted a third author. We entered data into Review Manager software (RevMan 2014) and checked for accuracy.
Assessment of risk of bias in included studies
Two review authors independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreement by discussion or by involving a third review author.
(1) Random sequence generation (checking for possible selection bias)
We described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
low risk of bias (e.g. telephone or central randomization; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
It is not possible to blind participants or personnel in these trials the intervention (bed rest or not bed rest). For certain outcomes which could be measured by a blinded outcome assessor, we attempted to assess how such blinding was done.
We assessed the methods as:
unclear risk of bias for participants;
unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We described for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed methods used to blind outcome assessment as:
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We described for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomized participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or could be supplied by the trial authors, we planned to re‐include missing data in the analyses which we undertook.
We have assessed methods as:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomization);
unclear risk of bias.
(5) Selective reporting (checking for reporting bias)
We described for each included study how we investigated the possibility of selective outcome reporting bias and what we found. We assessed the methods as:
low risk of bias (where it is clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
high risk of bias (where not all the study’s pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
We described for each included study any important concerns we had about other possible sources of bias.
(7) Overall risk of bias
We made explicit judgements about whether studies were at high risk of bias, according to the criteria given in the Handbook (Higgins 2011). With reference to (1) to (6) above, we planned to assess the likely magnitude and direction of the bias and whether we considered it is likely to impact on the findings. In future updates, we will explore the impact of the level of bias through undertaking sensitivity analyses ‐ seeSensitivity analysis.
Measures of treatment effect
Dichotomous data
For dichotomous data, the results are presented as summary risk ratio with 95% confidence intervals.
Continuous data
For continuous data, we planned to use the mean difference if outcomes were measured in the same way between trials. No continuous data were analyzed in this update.
Unit of analysis issues
Cluster‐randomized trials
We planned to include cluster‐randomized trials in the analyses along with individually‐randomized trials. We planned to adjust their sample sizes using the methods described in the Handbook using an estimate of the intracluster correlation co‐efficient (ICC) derived from the trial (if possible). if we used ICCs from other sources, we planned to report this and conduct sensitivity analyses to investigate the effect of variation in the ICC. In future updates, if we identify both cluster‐randomized trials and individually‐randomized trials, we plan to synthesize the relevant information. We will consider it reasonable to combine the results from both if there is little heterogeneity between the study designs and the interaction between the effect of intervention and the choice of randomization unit is considered to be unlikely.
We will also acknowledge heterogeneity in the randomization unit and perform a sensitivity analysis to investigate the effects of the randomization unit.
One of the included studies compared different interventions in prenatal clinics (unit of analysis: clinics). However, within each intervention cluster they randomized pregnant woman to five interventions (unit of analysis: pregnant women). Therefore, no cluster analysis was needed to be performed.
Cross‐over trials
Cross‐over designs are not a valid study design for this review.
Other unit of analysis issues
In trials with more than two treatment groups, to overcome unit‐of‐analysis errors, we combined groups to create single pair‐wise comparisons.
Dealing with missing data
For included studies, we noted levels of attrition. Had we found high levels of missing data, we planned to explore the impact of including studies in the overall assessment of treatment effect by using sensitivity analysis.
For all outcomes, we carried out analyses on an intention‐to‐treat basis (i.e. we included all participants randomized to each group in the analyses, and all participants were analyzed in the group to which they were allocated, regardless of whether or not they received the allocated intervention). The denominator for each outcome in each trial was the number randomized minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the Tau², I² and Chi² statistics. We regarded heterogeneity as substantial if the Tau² was greater than zero and either an I² was greater than 30% or there was a low P value (< 0.10) in the Chi² test for heterogeneity.
Assessment of reporting biases
In future updates, if there are 10 or more studies in the meta‐analysis, we will investigate reporting biases (such as publication bias) using funnel plots. We will assess funnel plot asymmetry visually. If asymmetry is suggested by a visual assessment, we will perform exploratory analyses to investigate it.
Data synthesis
We carried out statistical analysis using the Review Manager software (RevMan 2014). We planned to use fixed‐effect meta‐analysis for combining data where it was reasonable to assume that studies were estimating the same underlying treatment effect and the trials’ populations and methods were judged sufficiently similar. If there was clinical heterogeneity sufficient to expect that the underlying treatment effects differ between trials, or if substantial statistical heterogeneity was detected, we planned to use random‐effects meta‐analysis to produce an overall summary, if an average treatment effect across trials was considered clinically meaningful. If we finally used random‐effects analyses, the results would be presented as the average treatment effect with 95% confidence intervals, and the estimates of Tau² and I².
Subgroup analysis and investigation of heterogeneity
If we identified substantial heterogeneity, we planned to investigate it using subgroup analysis. We would have considered whether an overall summary was meaningful, and if it was, used random‐effects analysis to produce it.
There was not enough data included in analyses to carry out subgroup analysis.
In future updates, we will carry out the following subgroup analyses:
(1) Subgroups of participants according to method of risk assessment based on:
previous obstetric history;
threatened preterm labour;
positive screening test results;
maternal anthropometric measurements;
selection by scoring systems.
(2) Subgroups of interventions:
prescription of bed rest at home versus prescription of bed rest in hospital.
Subgroup analysis will be restricted to the review’s primary outcomes:
preterm birth (less than 37 weeks);
perinatal mortality;
low birthweight (less than 2500 g);
neonatal intensive care.
We will assess subgroup differences by interaction tests available within RevMan (RevMan 2014). We will report the results of subgroup analyses quoting the Chi² statistic and P value, and the interaction test I² value.
Sensitivity analysis
In future updates, if more data are available, we will carry out sensitivity analyses to explore the effect of trial quality assessed by concealment of allocation, high attrition rates, or both, with poor quality studies being excluded from the analyses in order to assess whether this makes any difference to the overall result.
Results
Description of studies
Results of the search
Our search identified eight reports of six trials that met the initial criteria for hard copy scrutiny. Two studies were included (Elliott 2005; Hobel 1994) and four were excluded (Brun 2011; Larsen 1980; Latorre 2014; Ma 1992).
Included studies
The first report (Hobel 1994) reported a cluster‐randomized controlled trial designed to evaluate a program for prevention of preterm birth that included an educational intervention plus increased clinic visits. Eight hospitals were randomized to either intervention (5) or control units (3). The intervention hospitals had to apply the prevention program to all high‐risk pregnant women identified through a scoring system. Besides the prevention program, women in intervention hospitals were randomized to receive one of five interventions: bed rest, psychosocial support by social worker, progestins, placebo or no intervention. This study is included in the current review. Due to the fact that individual randomization was used within each clinic to deliver the five interventions, no sample sizes or standard errors adjustments were required.
One report published in 2005 (Elliott 2005) was a randomized multicenter study that examined the impact of activity restriction on the preterm birth rate among women experiencing threatened preterm labour with negative fetal fibronectin. From a total of 246 eligible women, 73 women (singleton and multiple pregnancies) were included. The authors compared "Activity restriction" (consisted of bed rest with the exception of bathroom and showering privileges and being able to travel to their physician appointments) versus "No activity restriction" (consisted of resuming normal activities, including home and work responsibilities). This is the second study included in this review.
Excluded studies
One article (Larsen 1980) reported a trial comparing the prescription of ritodrine and bed rest versus bed rest alone. As both arms considered bed rest, the study was excluded from this review. A second article (Hesseldahl 1979) was a previous report of the same data of Larsen's trial; hence it was not considered.
A third article (Ma 1992) published in a Chinese journal reported a trial comparing magnesium sulfate versus barbiturates, salbutamol sulfate and bed rest. Due to the fact that there were no groups that differed only in the prescription of bed rest, the study was excluded from this review.
A fourth study (Brun 2011) was a pilot study in which the authors compared bed‐rest exercise (muscle‐conditioning exercises such as technique, breathing, perceived exertion) versus bed‐rest plus music. Since both arms considered bed‐rest ‐hospitalized for activity restriction ‐ the study was excluded.
Finally, two reports of the same study (Latorre 2014) described the comparison of ambulatory management with hospitalized management of pregnant women with threatened preterm labor and cervical length > 25 mm. In both arms (ambulatory and hospitalized management) bed rest was included.
Risk of bias in included studies
See Figure 1 and Figure 2 for a summary of 'Risk of bias' assessments.
1.
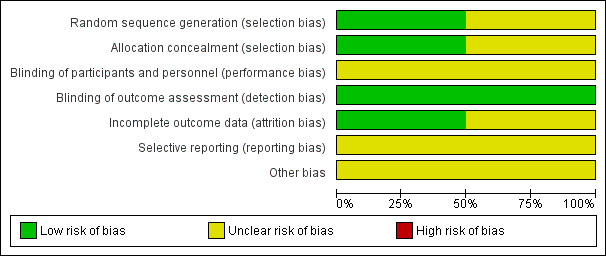
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
2.
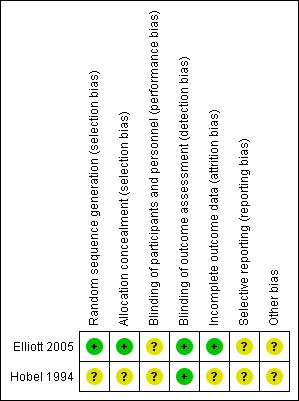
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
In the Elliot study (Elliott 2005), there is low risk of selection bias since a computer‐generated randomization schedule and a third party not involved with the study operations were used. Details about methods of randomization and allocation concealment were not described for Hobel 1994.
Blinding
It is not possible to blind participants or personnel in these trials to the intervention (bed rest or no bed rest). For certain outcomes that could be measured by a blinded outcome assessor, we attempted to assess how such blinding was done. However, we found that no study mentioned how such assessors were blinded. In Hobel 1994, the only reported outcome is preterm birth rate and it is unlikely that this outcome was influenced by lack of blinding. In Elliott 2005, caregivers were not blinded to group assignment but the evaluated outcome was not likely to be influenced by lack of binding.
Incomplete outcome data
In Elliott 2005, there were no missing data, therefore, the risk of attrition bias was low. This study was not included in the meta‐analysis since the trial included twin pregnancies and it was not possible to obtain data for singleton pregnancies. We have asked the authors for the data relating to singleton pregnancies but the data set that they used for the analysis is currently not available. In Hobel 1994, completeness of follow‐up was not described. Moreover, the number of women originally included in the intervention hospitals does not match the numbers included in the table of results. Although an explanation for this disagreement is included in the text, there are still differences that cannot be explained.
Selective reporting
There was Insufficient information to permit judgement of high or low risk and so selective reporting remains unclear.
Other potential sources of bias
We did not consider the overall quality of the Hobel study (Hobel 1994). We only considered the comparison within the intervention hospitals, in which individual women were randomized to one of five interventions, including bed rest. Few details on the methods used in this secondary trial are included in the report, preventing us from evaluating the internal validity. Neither is there a description of baseline characteristics of randomized women. There was insufficient information within the Elliott 2005 study.
Effects of interventions
Two studies met the criteria for inclusion in this review (Elliott 2005; Hobel 1994).
One of the studies (Elliott 2005) was not considered for the meta‐analysis since data for singleton pregnancies are not available.
From the second study (Hobel 1994), a total of 1774 women were randomized in five hospitals: 432 to prescription of bed rest; 411 to progestin; 407 to social support; 412 to placebo and 422 to no intervention. In this analysis, we compared the results in women assigned to bed rest (432) versus women assigned to placebo and no intervention (834). Both placebo and no intervention groups were combined and considered as controls (Hrobjartsson 2001).
Primary outcomes
Elliott 2005 reported on the following primary outcomes, preterm birth, low birthweight, very low birthweight, NICU admission, but the data for singleton pregnancies were not available for analysis. The authors did not find differences between bed rest and non activity restriction for any maternal and perinatal outcomes.
In Hobel 1994, preterm birth before 37 weeks was similar in both groups (7.9% in the intervention group versus 8.5% in the control group; risk ratio (RR) 0.92, 95% confidence interval (CI) 0.62 to 1.37.), Analysis 1.1. No other primary outcomes were reported in this study.
1.1. Analysis.
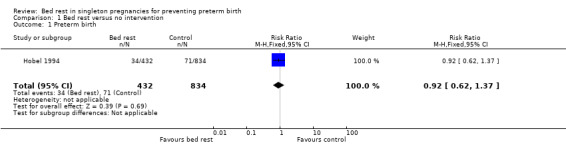
Comparison 1 Bed rest versus no intervention, Outcome 1 Preterm birth.
Secondary outcomes
Elliott 2005 reported on gestational age at delivery, but the data for singleton pregnancies were not available for analysis. No secondary outcomes were reported in Hobel 1994.
Discussion
Summary of main results
Two studies met the inclusion criteria but we have only one study that was considered for the meta‐analysis (1266 women). Four hundred and thirty‐two women were prescribed bed rest at home and a total of 834 women received either a placebo (412) or no intervention (422). Preterm birth before 37 weeks was similar in both groups (7.9% versus 8.5%). No other results were reported for any of the other primary or secondary outcomes.
Quality of the evidence
The only trial included in the meta‐analysis has uncertain methodological quality due to lack of reporting. Thus, the validity of the results cannot be supported. It is worth mentioning that in this trial the evaluation of bed rest was a secondary objective among others.
Potential biases in the review process
We sought published and unpublished trials, irrespective of languages. We performed literature searches in different databases. At least two review authors independently assessed the trials for inclusion in the review. Only one trial was considered for the meta‐analysis (Hobel 1994) since data for singleton pregnancies were not obtained from the second trial (Elliott 2005).
Agreements and disagreements with other studies or reviews
Previous observational studies have suggested that hard work and hard physical activity during pregnancy could be associated with preterm birth (Saurel 1985; Teitelman 1990). However, there are no epidemiological studies that have shown that the prescription of bed rest improves the outcomes in high‐risk pregnant women. Based on our meta‐analysis there is no evidence, either supporting or refuting the use of bed rest at home or in hospital, to prevent preterm birth. In addition, the second study considered in the systematic review, but not included in the meta‐analysis, found that bed rest did not decrease preterm birth.
Authors' conclusions
Implications for practice.
Although bed rest is widely used as the first step of treatment, there is no evidence either supporting or refuting its use at home or in hospital to prevent preterm birth. Due to the potential adverse effects that bed rest could have on women and their families, and the increased costs for the healthcare system, the pros and cons of bed rest for preventing preterm birth should be discussed fully. Health providers should discuss the potential benefits and harms of bed rest with women facing an increased risk of preterm birth, and allow them to decide if they should do it or not. Also, if they decide to opt for bed rest then they should also decide how often and for how long. A similar finding regarding hospitalization for bed rest in twin pregnancies at high risk of preterm birth is reported in another review (Crowther 2010).
Implications for research.
Bed rest is one of the most commonly prescribed interventions for women with high‐risk pregnancies, but is one of the less evaluated. Appropriate research is mandatory for those who believe that bed rest may result in a worthwhile reduction in preterm birth and neonatal morbidity. The trials should evaluate both the efficacy of bed rest and the effectiveness of prescribing bed rest to prevent preterm birth.
What's new
| Date | Event | Description |
|---|---|---|
| 18 December 2014 | New search has been performed | Four studies studies identified from updated search. One study included (Elliott 2005); three studies excluded (Brun 2011; Ma 1992; Latorre 2014). |
| 18 December 2014 | New citation required but conclusions have not changed | One new study included. Similar conclusions. |
History
Protocol first published: Issue 2, 2002 Review first published: Issue 1, 2004
| Date | Event | Description |
|---|---|---|
| 1 October 2009 | Amended | Search updated. One report added to Studies awaiting classification (Elliott 2005a). |
| 1 September 2008 | Amended | Converted to new review format. |
Acknowledgements
To Lynn Hampson, Trials Search Co‐ordinator of the Cochrane Pregnancy and Childbirth Group. We would also like to thank Judith A Maloni for her advice and support through the protocol and review process.
Appendices
Appendix 1. Search Terms
Authors wrote and ran the following:
CENTRAL
#1 PREGNANCY (MeSH)
#2 PREGNAN*
#3 PERINATOLOGY (MeSH)
#4 PERINATOLOGY
#5 LABOR‐PREMATURE (MeSH)
#6 PREMATURE
#7 PRETERM
#8 BED‐REST (MeSH)
#9 (BED next REST)
#10 REST*
#11 BED REST
#12 ((((((#1 or #2) or #3) or #4) or #5) or #6) or #7)
#13 (((#8 or #9) or #10) or #11)
#14 (#12 and #13)
MEDLINE SEARCH
#1 Premature Birth
#2 Obstetric Labor premature
#3 Perinatology
#4 Pregnancy
#5 "Premature Birth"[MeSH]
#6 "Obstetric Labor, Premature"[MeSH]
#7 "Perinatology"[MeSH])
#8 "Pregnancy"[MeSH]
#9 (#1 or #2 or #3 or #4 or #5 or #6 or #7 or #8
#10 bed rest
#11 "Bed Rest"[MeSH]
#12 (#10 or #11)
#13 (#9 and #12)
EMBASE SEARCH
#1 'premature'/exp OR premature
#2 'preterm baby'
#3 preterm AND labor
#4 premature AND labor
#5 bed AND rest
#6 rest
#7 (((#1 or #2) or #3) or #4)
#8 (#5 or #6)
#9 (#7 and #8)
LILACS SEARCH
#1 "PREMATURE BIRTH"
#2 "PRETERM LABOR"
#3 "PRETERM"
#4 “REST”
#5 "REST‐ACTIVITY"
#6 "REST‐EXERCISE"
#7 “BED REST”
#8 ((#1 or #2) or #3)
#9 (((#4 or #5) or #6) or #7)
#10 (#8 and #9)
Appendix 2. Methods used to assess trials included in previous versions of this review
The following methods were used to assess Hobel 1994.
Two reviewers independently assessed the trials for inclusion and methodological quality. The two reviewers resolved any disagreement by consensus or, if necessary, by a third reviewer.
We assessed the methodological quality of included trials using the methods described in the Cochrane Reviewers' Handbook (Clarke 2000).
Allocation concealment was categorised as: (a) adequate; (b) uncertain; or (c) inadequate.
Blinding and completeness of follow‐up were assessed for each outcome using the following criteria: for completeness of follow‐up: (a) less than 3% of participants excluded, (b) 3% to 9.9% of participants excluded, (c) 10% to 19.9% of participants excluded or (d) 20% or more of participants excluded. For blinding of outcome assessment: (a) single blinding, (b) no blinding or blinding not mentioned.
We extracted the data independently using a previously prepared data extraction form. The results were expressed as relative risks for dichotomous outcomes or weighted mean difference for continuous variables, and included 95% confidence intervals using the Cochrane Review Manager software (RevMan 2000).
We included studies irrespective of their methodological quality. In the case of significant heterogeneity among study outcomes, we performed a sensitivity analysis and based our conclusions on the results of studies with the best methodological quality.
Data and analyses
Comparison 1. Bed rest versus no intervention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Preterm birth | 1 | 1266 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.62, 1.37] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Elliott 2005.
| Methods | Randomized controlled trial in 4 hospitals in the southwestern United States. | |
| Participants | Women presenting threatened preterm labour and treated with intravenous magnesium sulfate were screened with fFN. If they were fFN negative, > 14 years of age, intact membranes, documented uterine contractions of > 5/hour at admission, 23 0/7 to 33 6/7 weeks' gestation, < 4 cm dilatation and stabilized with MgSO4, they were invited to participate. | |
| Interventions | AR: consisted of bed rest with the exception of bathroom and showering privileges and being able to travel to their physician appointments (36 patients). NAR: consisted of resuming normal activities (including home and work responsibilities) (37 patients). |
|
| Outcomes | Preterm birth, gestational age at delivery, delivery < 7 days after fFN, low birthweight, very low birthweight, NICU admission, NICU days. | |
| Notes | This trial included twin pregnancies. The authors reported a total of 6 multiple pregnancies. However, from the manuscript it was not possible to obtain data only for singleton pregnancies since the authors reported pooled data. We have asked the author for the data but the data set was not available at the time of the current update. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomized using a computer‐generated randomization schedule. |
| Allocation concealment (selection bias) | Low risk | Study co‐ordinators utilized an 800 number where a third party not involved with study operations opened an opaque, sealed randomization envelope and reported group assignment to the study co‐ordinator at the respective site. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | It is not possible to blind participants or personnel in these trials to the intervention (bed rest or no bed rest). |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Caregivers were not blinded to group assignment however, the evaluated outcomes were not likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement of "Yes" or "No". |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias exists. |
Hobel 1994.
| Methods | Cluster‐randomized trial designed to evaluate an educational intervention. Eight hospitals were randomized to intervention (5) and control (8). Women in the intervention units were again randomized (pregnant woman) to 1 of 5 interventions. | |
| Participants | High‐risk pregnant women evaluated by risk scoring system. 1774 high‐risk pregnant women in the 5 hospitals of the intervention group and 880 pregnant women in the 3 hospitals of the control group. | |
| Interventions | Intervention hospitals carried out an educational intervention consisting of identification of preterm labour, steps to take if signs of preterm labour occurred, and prevention strategies. Besides this intervention, women were randomized to 1 of 5 interventions:
bed rest at home (432);
placebo (412);
progestin (411);
social support (407);
no intervention (422). groups used in this review. |
|
| Outcomes | Preterm birth rate (< 37 weeks). | |
| Notes | For this review we only took into account women individually randomized in the intervention group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Clinics randomized with a restricted block randomized scheme. Insuficient information to permit judgement about individual randomization. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement of "Yes" or "No". |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | It is not possible to blind participants or personnel in these trials to the intervention (bed rest or no bed rest). |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome measurement (preterm birth) is not likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient reporting of attrition/exclusions to permit judgement. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement of "Yes" or "No". |
| Other bias | Unclear risk | Insufficient information. |
AR: activity restriction fFN: fetal fibronectin MgSO4: magnesium sulfate NAR: no activity restriction NICU: neonatal intensive care unit
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Brun 2011 | The main objective was to compare bed‐rest exercise (muscle‐conditioning exercises such as technique, breathing, perceived exertion) versus bed rest plus music. Since both arms considered bed rest ‐hospitalized for activity restriction ‐ the study was excluded. |
| Larsen 1980 | The main objective was to evaluate ritodrine for preventing preterm birth. The compared interventions were ritrodrine and bed rest versus bed rest alone. |
| Latorre 2014 | The main objective was to compare ambulatory management with hospitalized management of pregnant women with threatened preterm labor and cervical length > 25 mm. In both arms (ambulatory and hospitalized management) bed rest was included. |
| Ma 1992 | The main objective was to evaluate the efficacy of magnesium sulfate in the treatment of preterm labour. The authors compared magnesium sulfate versus barbiturates, salbutamol sulfate or bed rest. |
Differences between protocol and review
Methods updated to current Pregnancy and Childbirth Group standard text.
Contributions of authors
Claudio Sosa: design of protocol, writing of protocol and final review. Review of articles. Updating the review. Fernando Althabe: design of protocol, writing of protocol and review. Review of articles. Updating the review. Jose Belizan: design of protocol, writing of protocol and review. Revisions of protocol and final review. Eduardo Bergel: design of protocol, writing of protocol and review. Revisions of protocol and final review.
Sources of support
Internal sources
-
University of Uruguay, Uruguay.
Manuscript update was done in the Department of Obstetrics and Gynecology, School of Medicine, University of Uruguay
External sources
None, Other.
Declarations of interest
None known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Elliott 2005 {published data only}
- Elliott JP, Miller HS, Coleman S, Rhea D, Abril D, Hallbauer K, et al. A randomized multicenter study to determine the efficacy of activity restriction for preterm labor management in patients testing negative for fetal fibronectin. Journal of Perinatology 2005;25(10):626‐30. [DOI] [PubMed] [Google Scholar]
Hobel 1994 {published data only}
- Hobel C, Ross M, Bemis R, Bragonier J, Nessim S, Sandhu M, et al. The West Los Angeles preterm birth prevention project: I. Program impact on high‐risk women. American Journal of Obstetrics and Gynecology 1994;170:54‐62. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Brun 2011 {published data only}
- Brun CR, Shoemaker JK, Bocking A, Hammond JA, Poole M, Mottola MF. Bed‐rest exercise, activity restriction, and high‐risk pregnancies: a feasibility study. Applied Physiology, Nutrition, and Metabolism 2011;36(4):577‐82. [DOI] [PubMed] [Google Scholar]
Larsen 1980 {published data only}
- Hesseldahl H. A Danish multicenter study on ritodrine in the treatment of pre‐term labour. Danish Medical Bulletin 1979; Vol. 26, issue 3:116‐8. [PubMed]
- Larsen J, Hansen M, Hesseldahl H, Kristoffersen K, Larsen P, Osler M, et al. Ritodrine in the treatment of preterm labour. A clinical trial to compare a standard treatment with three regimens involving the use of ritrodine. British Journal of Obstetrics and Gynaecology 1980;87(11):949‐57. [DOI] [PubMed] [Google Scholar]
Latorre 2014 {published data only}
- Latorre R, Gomez R, Pais F, Avila F, Valenzuela A, Carrillo J, et al. Ambulatory versus hospitalized management of patients with threatened preterm labor and a cervical length > 25mm: a randomized clinical trial. Journal of Maternal‐Fetal & Neonatal Medicine 2014;27(Suppl 1):368. [Google Scholar]
- Latorre R, Walker Labarca B, Gomez R, Pais F, Avila F, Valenzuela A, et al. Ambulatory versus hospitalised management of patients with threatened preterm labour and a cervical length >25mm: a randomised clinical trial. Ultrasound in Obstetrics & Gynecology 2014;44(Suppl 1):1. [Google Scholar]
Ma 1992 {published data only}
- Ma L. Magnesium sulfate in prevention of preterm labor. Chung Hua I Hsueh Tsa Chih Taipei 1992;72(3):158‐61. [PubMed] [Google Scholar]
Additional references
Allen 1999
- Allen C, Glasziou P, Mar C. Bed rest: a potentially harmful treatment needing more careful evaluation. Lancet 1999;354:1229‐33. [DOI] [PubMed] [Google Scholar]
Clarke 2000
- Clarke M, Oxman AD, (editors). Cochrane Reviewers' Handbook 4.1 [updated June 2000]. In: Review Manager (RevMan) [Computer program]. Version 4.1. Oxford, England: The Cochrane Collaboration, 2000.
Crowther 1991
- Crowther C, Chalmers I. Bed rest and hospitalization during pregnancy. In: Chalmers I, Enkin M, Keirse MJNC editor(s). Effective Care in Pregnancy and Childbirth. New York: Oxford University Press, 1991:625. [Google Scholar]
Crowther 2010
- Crowther CA, Han S. Hospitalisation and bed rest for multiple pregnancy. Cochrane Database of Systematic Reviews 2010, Issue 7. [DOI: 10.1002/14651858.CD000110] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cunningham 1993
- Cunningham FG, MacDonald PC, Leveno KJ, Gant NF, Gilstrap III LG. Williams Obstetrics. 19th Edition. Norwalk, Connecticut: Appleton & Lange, 1993:868. [Google Scholar]
Flenady 2013
- Flenady V, Hawley G, Stock OM, Kenyon S, Badawi N. Prophylactic antibiotics for inhibiting preterm labour with intact membranes. Cochrane Database of Systematic Reviews 2013, Issue 12. [DOI: 10.1002/14651858.CD000246.pub2] [DOI] [PubMed] [Google Scholar]
Goldenberg 1994
- Goldenberg RL, Cliver SP, Bronstein J, Cutter GR, Andrews WW, Mennemeyer ST. Bed rest in pregnancy. Obstetrics and Gynecology 1994;84:131‐6. [PubMed] [Google Scholar]
Gupton 1997
- Gupton A, Heaman M, Ashcroft T. Bed rest from the perspective of the high‐risk pregnant woman. Journal of Obstetric, Gynecologic and Neonatal Nursing 1997;26(4):423‐30. [DOI] [PubMed] [Google Scholar]
Han 2013
- Han S, Crowther CA, Moore V. Magnesium maintenance therapy for preventing preterm birth after threatened preterm labour. Cochrane Database of Systematic Reviews 2013, Issue 5. [DOI: 10.1002/14651858.CD000940.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hesseldahl 1979
- Hesseldahl H. A Danish multicenter study on ritodrine in the treatment of pre‐term labour. Danish Medical Bulletin 1979; Vol. 26, issue 3:116‐8. [PubMed]
Higgins 2011
- Higgins JPT, Green S, (editors). Cochrane Handbook for Systematic Reviews of Interventions 5.1.0 [updated March 2011]. In: Review Manager (RevMan) [Computer program]. Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Hrobjartsson 2001
- Hrobjartsson A, Gotzsche PC. Is the placebo powerless?. New England Journal of Medicine 2001;344(21):1594‐602. [DOI] [PubMed] [Google Scholar]
Kovacevich 2000
- Kovacevich GJ, Gaich SA, Lavin JP, Hopkins MP, Crane SS, Stewart J, et al. The prevalence of thromboembolic events among women with extended bed rest prescribed as part of the treatment for premature labor or preterm premature rupture of membranes. American Journal of Obstetrics and Gynecology 2000;182(5):1089‐92. [DOI] [PubMed] [Google Scholar]
Maloni 1993
- Maloni JA, Chance B, Zhang C, Cohen AW, Betts D, Gange SJ. Physical and psychosocial side effects of antepartum hospital bed rest. Nursing Research 1993;42(4):197‐203. [PubMed] [Google Scholar]
Maloni 2001
- Maloni JA, Brezinski‐Tomasi JE, Johnson LA. Antepartum bed rest: effect upon the family. Journal of Obstetric, Gynecologic and Neonatal Nursing 2001;30(2):165‐73. [DOI] [PubMed] [Google Scholar]
Maloni 2002
- Maloni JA, Kane JH, Suen LJ, Wang KK. Dysphoria among high‐risk pregnant hospitalized women on bed rest: a longitudinal study. Nursing Research 2002;51(2):92‐9. [DOI] [PubMed] [Google Scholar]
Maloni 2010
- Maloni JA. Antepartum bed rest for pregnancy complications: efficacy and safety for preventing preterm birth. Biological Research for Nursing 2010;2(12):106‐24. [DOI] [PubMed] [Google Scholar]
Mamelle 1984
- Mamelle N, Laumon B, Lazar P. Prematurity and occupational activity during pregnancy. American Journal of Epidemiology 1984;119(3):309‐22. [DOI] [PubMed] [Google Scholar]
May 1994
- May KA. Impact of maternal activity restriction for preterm labor on the expectant father. Journal of Obstetrics, Gynecology and Neonatal Nursing 1994;23(3):246‐51. [DOI] [PubMed] [Google Scholar]
McCall 2013
- McCall CA, Grimes DA, Lyerly AD. "Therapeutic" bed rest in pregnancy: unethical and unsupported by data. Obstetrics and Gynecology 2013;121(6):1305‐8. [DOI] [PubMed] [Google Scholar]
McCormick 1985
- McCormick MC. The contribution of low birth weight to infant mortality and childhood morbidity. New England Journal of Medicine 1966;312:82‐90. [DOI] [PubMed] [Google Scholar]
RevMan 2000 [Computer program]
- The Cochrane Collaboration. Review Manager (RevMan). Version 4.1 for Windows. Oxford, England: The Cochrane Collaboration, 2000.
RevMan 2014 [Computer program]
- The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen, The Nordic Cochrane Centre: The Cochrane Collaboration, 2014.
Roberts 2006
- Roberts D, Dalziel S. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database of Systematic Reviews 2006, Issue 3. [DOI: 10.1002/14651858.CD004454.pub2] [DOI] [PubMed] [Google Scholar]
Robertson 1992
- Robertson PA, Sniderman SH, Laros RK Jr, Cowan R, Heilbran D, Goldenberg RL, et al. Neonatal morbidity according to gestational age and birth weight from tertiary care centers in the United States, 1983 through 1986. American Journal of Obstetrics and Gynecology 1992;166:1629‐41. [DOI] [PubMed] [Google Scholar]
Saurel 1985
- Saurel‐Cubizolles MJ, Kaminski M, Llado‐Arkhipoff J, Du Mazaubrun C, Estryn‐Behar M, Berthier C, et al. Pregnancy and its outcome among hospital personnel according to occupation and working conditions. Journal of Epidemiology and Community Health 1985;39(2):129‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schroeder 1996
- Schroeder CA. Women's experience of bed rest in high‐risk pregnancy. Image ‐ The Journal of Nursing Scholarship 1996;28(3):253‐8. [DOI] [PubMed] [Google Scholar]
Schwarcz 2005
- Schwarcz R, Fescina R, Duverges C. Anomalias de la duración del embarazo. Obstetricia. 6th Edition. Buenos Aires: El Ateneo, 2005:253‐70. [Google Scholar]
Smaill 2007
- Smaill F, Vazquez JC. Antibiotics for asymptomatic bacteriuria in pregnancy. Cochrane Database of Systematic Reviews 2007, Issue 2. [DOI: 10.1002/14651858.CD000490.pub2] [DOI] [PubMed] [Google Scholar]
Teitelman 1990
- Teitelman AM, Welch LS, Hellenbrand KG, Bracken MB. Effect of maternal work activity on preterm birth and low birth weight. American Journal of Epidemiology 1990;131:104‐13. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Sosa 2004
- Sosa C, Althabe F, Belizán JM, Bergel E. Bed rest in singleton pregnancies for preventing preterm birth. Cochrane Database of Systematic Reviews 2004, Issue 1. [DOI: 10.1002/14651858.CD003581.pub2] [DOI] [PubMed] [Google Scholar]


