Abstract
Background
Extracellular vesicles (EVs) are cell-derived vesicles with diverse functions in intercellular communication including disease and infection, and EVs appear to influence HIV-1 pathogenesis. EVs isolated from HIV-1-uninfected semen (SE) but not blood (BE) contain factors that interfere with HIV-1 infection and replication in target cells. The reason for this dichotomy is unknown. Furthermore, the effect of HIV-1 infection and antiretroviral (ARV) drugs on the anti-HIV-1 effects of SE and BE is unknown. Here, we characterize EVs and EV-free plasma isolated from HIV-infected donor semen and blood and their effects on HIV infection.
Methods
EVs and EV-free plasma were purified from autologous blood and semen of HIV-negative, HIV-infected antiretroviral therapy (ART)-naïve, and HIV-infected ART-treated participants. HIV infection was assessed in TZM-bl cell reporter system. ARV concentrations were analyzed using LC-MS/MS.
Results
SE isolated from both HIV-negative and HIV-infected, ART-naïve donors inhibited HIV-1 infection, but BE and semen and blood EV-free plasma did not. In contrast, BE, SE, and EV-free plasma from HIV-infected, ART-treated donors inhibited HIV-1. Importantly, exosomes isolated from ART-treated donors contained concentrations of ARV drugs (ART-EVs) at biologically relevant inhibitory levels.
Conclusions
The HIV-1-inhibitory phenotype of SE is independent of donor HIV-1 or ART status, and ARV drugs and their metabolites are SE- and BE-associated in vivo.
Keywords: Extracellular vesicles, HIV-1, antiretroviral therapy, semen, blood
Introduction
HIV-1 transmission is primarily via semen which leads to ~88% of new infections in the USA1. However, the per-sexual encounter rate of sexual transmission is infrequent, estimated to occur in only one of every 200 to 1000 sexual occurrences1. Transmission rates per encounter depend on HIV-1 VL in semen and the availability and susceptibility of CD4+ cells in the recipient mucosal environment1. Antiretroviral therapy (ART) reduces sexual transmission by decreasing VL in blood and semen in ART-suppressed individuals2,3. Interestingly, extracellular vesicles prepared from semen of HIV-1-negative donors are protective against HIV infection in vitro, suggesting that semen extracellular vesicles (SE) contribute to the low rate of seminal HIV transmission4–7.
Cells release diverse vesicles classified according to defined characteristics. Vesicle populations are heterogenous resulting in non-specific terminology; here we refer to them as extracellular vesicles (EVs)8. EVs are important mediators of disease pathogenesis, including viral infections9. Although similar in physical characteristics to viruses, EVs may facilitate or suppress viral infections8–10. The pathogenic or protective role of EVs during viral infection is mediated by EV composition, cargo, size, and biogenesis conditions8,10, and EVs from tissue culture cells may enhance susceptibility to HIV by delivering HIV co-receptors or viral components to cells, or suppress infection by transferring anti-viral components, altering host cell immune activation, or competing for binding leading to physical blockage9–11.
Body-fluid EVs, including SE and blood extracellular vesicles (BE), are released from a variety of cells in different physiological and pathophysiological states, and thus EVs isolated from different body-fluids may facilitate or suppress viral infections depending on the type of the exosome-originating cells9,10. EVs from body-fluids such as semen, breast milk, and vaginal fluids of HIV-negative donors inhibit HIV-1 infection6,11,12. Although the mechanism(s) of inhibition is incompletely understood, HIV-1-negative SE interfere with different stages of the HIV-1 lifecycle, including reverse transcription, proviral DNA integration, and RNA transcription5–7. The inhibitory mechanisms occur post-entry, as SE had no effect on intracellular p24 or reverse transcriptase activity early post-infection6. SE block HIV replication events at least in part, by targeting HIV-1 Tat, and its regulatory mechanisms including host transcription factor recruitment, transcription initiation and elongation5. SE inhibition is cell- and virus strain-independent as SE inhibited lab adapted and transmitted founder HIV-1 strains in cells of cervical, lymphocytic (including primary blood lymphocytes), and monocytic origin as well as in a murine-AIDS model of infectioin6–8.
The balance between antiviral or proviral effects of EVs varies, and the cellular activation state, HIV infection, and ART treatment influence the overall effect of EVs on HIV replication. To date, the effects of donor HIV status and ART treatment on body-fluid EV function has not been reported. Antiretroviral (ARV) drugs are detected in blood plasma and seminal fluids3, and may modify cell culture EVs13. Thus, ARV drugs may also alter body-fluid exosome composition and function. Although SE inhibit HIV in vitro4–7, exosomes released from HIV-infected cell cultures may contribute to a proviral phenotype14,15. Here, we clarify the effect of SE and BE from HIV-infected ART-naïve and ART-suppressed individuals on HIV infection.
Methods
Ethics:
This study was approved by The University of Iowa and Stony Brook University Institutional Review Boards (IRB). All experiments were completed according to approved University regulations. Participants provided written informed consent and laboratory personnel were blinded to clinical data. Demographics and clinical characteristics were obtained through medical record review. Donors provided both blood and semen samples. Plasma RNA viral load (VL) was measured (Roche Cobas), and CD4+ T-cell counts determined using flow cytometry (University of Iowa Hospitals and Clinics clinical laboratory)16. HIV-1-negative control donors had no history of HIV-1, hepatitis B virus, or hepatitis C virus infection. HIV-1-infected donors were classified as ART-suppressed (VL < 50 copies/ml), or ART-naïve based on treatment history and VL at the time of sample collection.
Purification of extracellular vesicles and extracellular vesicle-free plasma:
Semen and whole blood were processed for EV isolation within 4 hours. Peripheral blood mononuclear cells (PBMCs) were purified using Vacutainer® cellular preparation tubes (CPT) according to manufacturer’s instructions (BD Biosciences, San Jose, CA). EVs were purified from blood and seminal plasma using Exoquick purification as previously described4,6,7,17. Previous studies found that Exoquick provided equivalent HIV-1 functional results with alternative (ultracentrifugation and sucrose gradient ultracentrifugation) EV purification methods6,17. Further, ExoQuick does not affect HIV-1 infectivity5. Blood- or seminal-plasma were mixed with ExoQuick reagent (SBI, Palo Alto, CA) per manufacturer’s instructions and EVs purified by centrifugation. Blood and semen EV pellets were re-suspended in PBS and referred to as BE or SE, while the corresponding EV-free plasma were referred to as EFBP and EFSP, respectively. EV and EV-free plasma protein quantification was determined by NanoDrop absorbance at 280 nm.
Cells and viruses:
TZM-bl cells (NIH AIDS Reagent Program, Germantown, MD), and 293T cells (ATCC) were maintained in complete DMEM (Gibco-BRL/Life Technologies, Carlsbad, CA) containing 5% EV-depleted FBS (Gibco, Carlsbad, CA) as described17. HIV-1 pNL4.3 plasmid (NIH AIDS Reagent Program) was used to generate HIV-1 NL4.3 virus in 293T cells4–6. Virus titers were determined by TZM-bl renilla luciferase units (RLU) and EnzChek Reverse Transcriptase Assay (Life Technologies). Cell viability was determined by MTT assay with three replicates per donor at the time of infection analysis as described4,17.
EV Particle size and concentration:
EV and HIV-1 NL4.3 size and concentration were measured by nanoparticle tracking analysis (NTA) with ZetaView PMX 110 (Particle Metrix, Mebane, NC) and software (v8.04.02). All samples were measured using the same settings and data acquisition was performed in triplicate and each replicate corresponded to 11 positions. The median number (X50) was used to report particle size. Measured concentration was reported per milliliter of donor plasma or virus culture. HIV-1 NL4.3 measurements were completed with formaldehyde-fixed virus.
HIV-1 inhibition:
EVs (100 μg/ml) or EV-free plasma (blood and semen) were simultaneously added with HIV-1 NL4.3 virus (100,000 RLU/100 μl) to TZM-bl indicator cells in complete DMEM containing 5% EV-free FBS. Equivalent volume of PBS was used as vehicle control. Infectivity was determined by RLU after 24 hours using Steady-Glo (Promega, Madison, WI) in triplicate per donor4,17. The optimal concentration of SE (100 μg/ml) used in these studies was previously determined and does not compromise cell viability in multiple cell types6,7. Based on the EV particle concentration (particles/mL) and protein concentration (mg/mL), 100 μg/mL BE corresponded to approximately 1.5×109, 3.5×109, and 9.9×108 particles for HIV-negative (n=6), HIV+ ART-naïve (n=5), and HIV+ ART-suppressed (n=13) subjects, respectively (Supplemental Digital Content Fig. 1A). In HIV-negative subjects, 100 μg/mL SE corresponded to approximately 1.2×109, 5.1×109, and 1.0×109 particles for HIV-negative (n=6), HIV+ ART-naïve (n=5), and HIV+ ART-suppressed (n=13), respectively (Supplemental Digital Content Fig. 1A). There was no significant difference between BE and SE particles/mg for each cohort of donors (Supplemental Digital Content Fig. 1A). It should be noted that because we did not separate cell-free virus from EVs in ART-naïve samples, concentrations reflect virus and EV particle numbers. The HIV-1 pNL4.3 inocula utilized an average of 2.6 × 108 HIV-1 NL4.3 particles per infection. Thus, there were approximately 4–13 BE per HIV-1 particle, and 4–20 SE per HIV-1 particle depending on donor cohort (Supplemental Digital Content Fig. 1B). Based on the semen and blood volumes used to purify SE and BEs, 100 ug EVs represented approximately 50 ul semen and 25 ul blood as the starting volume used in experiments.
ARV LC-MS/MS:
ARV drug concentrations were quantified from 50μg of EV (approximately 5.0×108particles) or EV-free plasma by LC-MS/MS using a Shimadzu Nexera liquid chromatography system and an AB Sciex 6500 triple quadrupole mass spectrometer. Detailed methods for quantification of tenofovir (TFV), emtricitabine (FTC), efavirenz (EFV), dolutegravir (DTG) and intracellular TFV-diphosphate (TFV-DP) and FTC-triphosphate (FTC-TP) were previously published18. ARV concentrations per EV particle (μM/particle) were estimated from particles/mL and μM ARV/50 μg.
ARV-loaded extracellular vesicles formulation and characterization:
Two approaches were used to incorporate ARV into HIV-negative BE. Emtricitabine (50 μg FTC) (NIH AIDS Reagent Program) was Cy3-labeled with Cy3 Fast Conjugation kit (Abcam, Cambridge, UK) according to manufacturer’s instructions. Modifier Reagent (1 μl per 10 μl FTC) was mixed with Cy3 Conjugate. Cy3 Quencher reagent (1 μl per 10 μl FTC) stopped the reaction. First, HIV-negative BE (200 μg) were combined with 50 μg FTC-Cy3 or buffer control for 90 minutes at 37°C before ultracentrifugation at 100,000 × g for 70 minutes. Pellets containing FTC-Cy3 loaded or unlabeled FTC control EVs were resuspended in PBS to the original volume and protein was quantified by Nanodrop absorbance before Cy3 detection and HIV inhibition studies19. Alternatively, 200 μg HIV-negative BE in 50 μl volume were incubated with 50 μg FTC-Cy3 in 20 μl, 10 μl ExoFect reagent (SBI), and 70 μl PBS. Following 10 minute incubation at 37°C, ExoQuick reagent (30 μl) was added. The transfection/ExoQuick solution was placed on ice for 30 minutes prior to centrifugation (13,000 rpm, 3 minutes). Pelleted EVs were resuspended in the original volume of PBS before quantification, Cy3 detection, and HIV inhibition studies20. Both approaches were assessed in three independent donors. 100 μg/ml EVs were used for HIV inhibition studies.
IgG purification:
IgG was depleted from HIV-infected ART-suppressed EVs and plasma by incubation with Protein G-coated magnetic beads (Dynabeads® Protein G, Life Technologies) (2 hours, room temperature [RT], 1 μg protein/1 μl beads) with rotation. IgG was removed from supernatant fluids magnetically as recommended by the manufacturer. IgG-bound beads were exposed to 0.2 M glycine pH 2.0 (20 μl) and incubated at RT for 5 minutes to facilitate IgG elution, and beads removed from IgG magnetically. The eluted IgG was neutralized with 10% volume 1 M phosphate buffer pH 7.5 before IgG quantitation and HIV inhibition studies. IgG quantitation was determined by human IgG antigen ELISA according to manufacturer’s instructions (Molecular Innovations, Novi, MI). IgG studies were repeated with four independent donors.
Statistics:
Data are reported as the mean and standard deviation (SD). Paired two-tailed student’s t test p-value determined statistical significance P<.05=*, P<.01=**, P<.001=***, P<.0001=****, ns=not significant (GraphPad Prism, San Diego, CA).
Results
Extracellular vesicles isolated from semen but not blood of HIV-infected ART-naïve donors inhibit HIV-1 in vitro
Previous studies showed that EVs purified from HIV-negative donor semen inhibit HIV infection whereas blood EVs do not4–7,17. However, the effect of donor HIV-infection on the inhibitory function of body-fluid EVs has not been described. We evaluated SE and BE isolated from HIV-infected ART-naïve donors. To determine if our observations relate to EV-specific effects, the HIV-inhibitory effect of EV-free plasma (EFBP, EFSP) was evaluated. EVs and their cognate EV-free plasma were isolated from blood and semen from donors with and without HIV-1 infection, including HIV-infected ART-naïve viremic, and HIV-infected ART-suppressed. Characteristics of HIV-positive donors are shown in Table 1. As expected, HIV-negative SE inhibited HIV replication in TZM-bl cells while BE did not (Fig. 1A) while HIV-negative EFBP and EFSP did not inhibit HIV-1. EFBP enhanced replication while EFSP had no effect (Fig. 1A). These findings were recapitulated in HIV-infected ART-naïve fractions (Fig. 1A, shaded). The effect of SE and EFBP was independent of cell viability (Supplemental Digital Content Fig. 2A–B). Since HIV replication was inhibited by SE obtained from either HIV-negative or HIV-infected, ART-naïve donors, donor HIV status did not appear to affect the anti-HIV property of SE.
Table 1:
HIV-infected donor clinical characteristics.
| Donor | Blood Plasma (ml) | Seminal Plasma (ml) | Viral load (GE/ml) | ARV Therapy | CD4 count* | ART yrs* |
|---|---|---|---|---|---|---|
| 1 | 3 | 1.0 | 120,000 | Therapy-naïve | 39 | N/A |
| 2 | 3 | 1.0 | 34,000 | Therapy-naïve | 295 | N/A |
| 3 | 3 | 1.0 | 1,318,000 | Therapy-naïve | 6 | N/A |
| 4 | 3 | 0.6 | 35,000 | Therapy-naïve | 453 | N/A |
| 5 | 3 | 0.65 | 16,000 | Therapy-naïve | 489 | N/A |
| 6 | 4 | 1.2 | ND | ABC, 3TC, EFV | 1,037 | > 5 |
| 7 | 4 | 1.2 | ND | ABC, 3TC, DTG | 545 | > 5 |
| 8 | 4 | 0.9 | ND | TAF, FTC, DTG | 944 | > 5 |
| 9 | 3 | 1.0 | ND | ABC, 3TC, EFV | 597 | > 5 |
| 10 | 3 | 0.4 | ND | ABC, 3TC, EFV | 568 | > 5 |
| 11 | 3 | 0.6 | ND | ABC, 3TC, DTG | 564 | > 5 |
| 12 | 3 | 0.6 | 32 | TAF, FTC, DTG | 361 | 1 |
| 13 | 3 | 0.7 | ND | TAF, FTC, DTG | 915 | > 5 |
| 14 | 3 | 0.6 | ND | TDF, FTC, EFV | 721 | > 5 |
| 15 | 3 | 1.0 | ND | TAF, FTC, DRV/rit | 395 | > 5 |
| 16 | 3 | 1.0 | ND | TAF, FTC, DTG | 416 | 0.17 |
| 17 | 3 | 1.0 | ND | TDF, FTC, EFV | 1,159 | > 5 |
| 18 | 3 | 1.0 | ND | ABC, 3TC, DTG | 452 | 0.67 |
GE = genome equivalents; ARV – antiretroviral; CD4 count in cells/mm3; yrs = duration in years; NA = not applicable; ND = below limit of detection; ABC = abacavir; 3TC = lamivudine; EFV = efavirenz, DTG = dolutegravir, TAF = tenofovir alafenamide; FTC = emtrictabine; TDF = tenofovir disoproxil fumarate; DRV/r = ritonavir boosted darunavir
Figure 1: Donor HIV and ART status do not alter HIV-inhibitory function of semen extracellular vesicles.
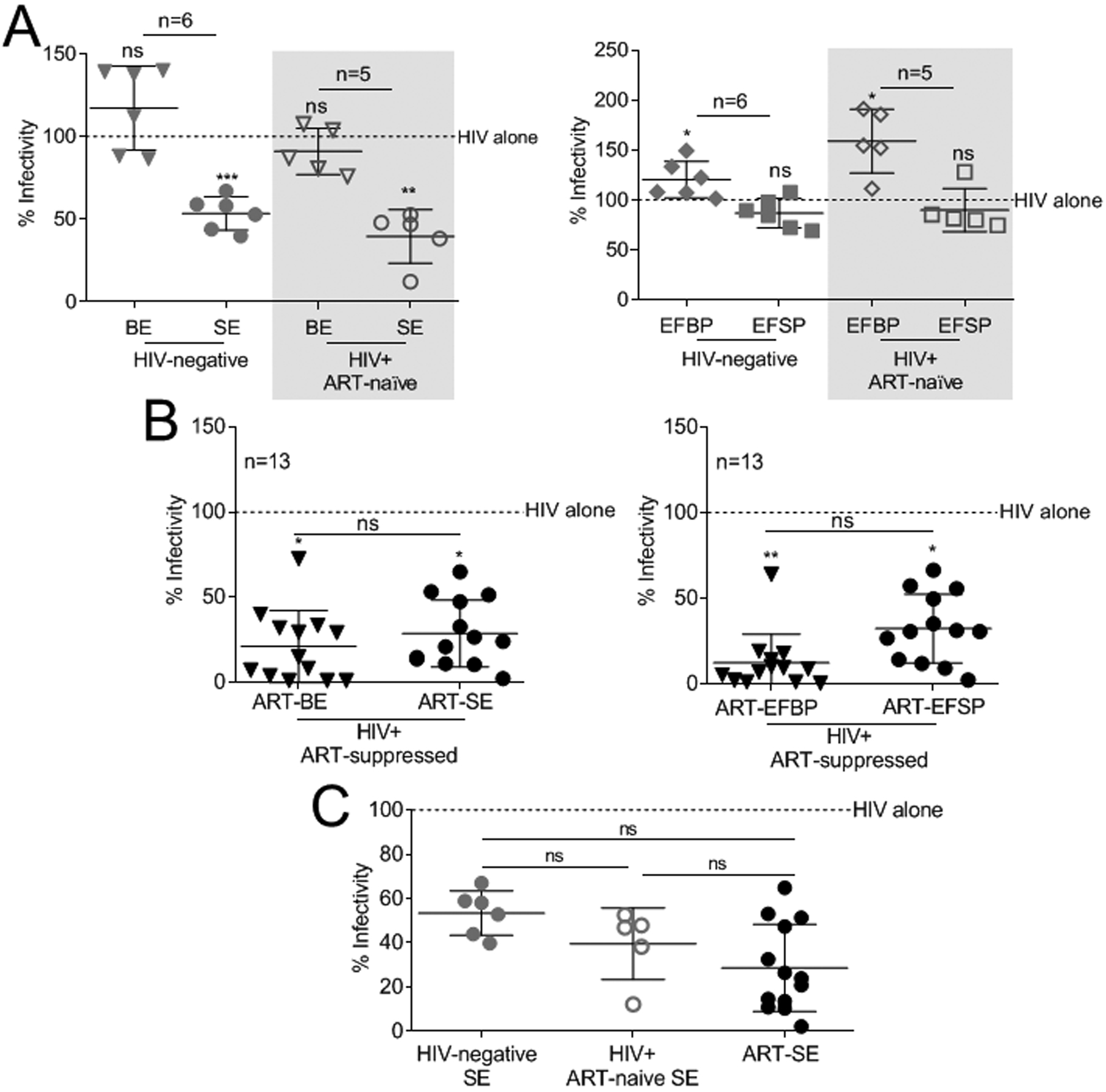
(A-C) Vehicle PBS or 100 μg/ml BE, SE, EFBP, and EFSP isolated from HIV-negative (n=6), HIV-positive ART-naïve (n=5), and HIV-positive ART-suppressed (n=13) donors were added simultaneously with 100,000RLU of HIV-1 NL4.3 virus to TZM-bl indicator cells for 24 h. TZM-bl infectivity was measured by luciferase reporter activity. Vehicle treated cells are set as reference at 100% (broken line). (A) Infectivity of HIV-1 treated with BE, SE, EFBP, and EFSP from HIV-negative and HIV-positive ART-naïve (shaded) donors. (B) Infectivity of HIV-1 treated with BE, SE, EFBP, and EFSP from HIV-positive ART-suppressed donors. Statistics was determined by comparing infectivity values from all donors to vehicle control for each treatment (A-B), and by comparing infectivity values between treatments (B). (C) Infectivity of HIV-1 treated with SE from HIV-negative, HIV-positive ART-naïve, and HIV-positive ART-suppressed donors. Statistics was determined by comparing infectivity values between donor cohorts (C). Significance was determined by student’s t test. *=P<0.05, **=P<0.01, ***=P<0.001. Error bars are SD of biological replicates from the mean of triplicate measurements. ns= not significant.
Extracellular vesicles and extracellular vesicle-free plasma isolated from HIV-infected ARV-suppressed donors inhibit HIV
ARV drugs alter cell-culture EV content in HIV infected cell supernatants13. To determine the effect of ARV treatment on anti-HIV properties of EVs, BE and SE were isolated from HIV-infected ART-suppressed donors (Table 1; ART therapy average > 5 years, Supplemental Digital Content Table 1). BE and SE from these subjects are referred to as ART-extracellular vesicles (ART-BE, ART-SE), and their EV-free plasma referred to as ART-EFBP and ART-EFSP. All fractions (ART-BE, ART-SE, ART-EFBP, ART-EFSP) robustly inhibited HIV infection without affecting cell viability (Fig. 1B, Supplemental Digital Content Fig. 2C). There were no discernable size or concentration difference in EVs between the three clinical study groups, though donor-dependent variation was observed (Supplemental Digital Content Fig. 3). Thus, differences in HIV inhibition did not appear to be related to the physical characteristics of the EVs. Since the cell environment and EV function may differ amongst the three clinical groups, the HIV inhibitory potency of SE was assessed. Direct comparison of SE activity revealed no significant differences in inhibition efficacy (46.5% HIV-negative SE, 59.6% ART-naïve SE, and 69.2% ART-SE) (Fig. 1C).
HIV-specific immunoglobulin (IgG) does not mediate HIV inhibition by ART-BE or ART-SE
HIV-specific IgG antibodies may limit HIV infection under some circumstances21. It is unknown whether HIV-neutralizing IgG are EV-associated or if they play a role in EV-mediated inhibition of HIV. IgG was co-precipitated from ART-BE and ART-SE (Fig. 2A, 2B), and ART-EFBP and ART-EFSP (Fig. 2B). IgG purified from BE and SE (ART-BE-IgG, ART-SE-IgG) or ART-EFBP and ART-EFSP did not inhibit HIV infection compared to IgG-depleted BE and SE (Fig. 2C, shaded). As expected, IgG treatments did not alter cell viability (Supplemental Digital Content Fig. 4). Of note, IgG neutralizing antibodies from human specimens are donor HIV strain-specific. Thus, human-derived IgG may not neutralize the laboratory-adapted HIV strain used in these studies. Nonetheless, the data suggests that IgG present in ART-extracellular vesicles is not responsible for the observed inhibitory phenotype.
Figure 2: HIV-1-specific IgG does not contribute to HIV inhibition.
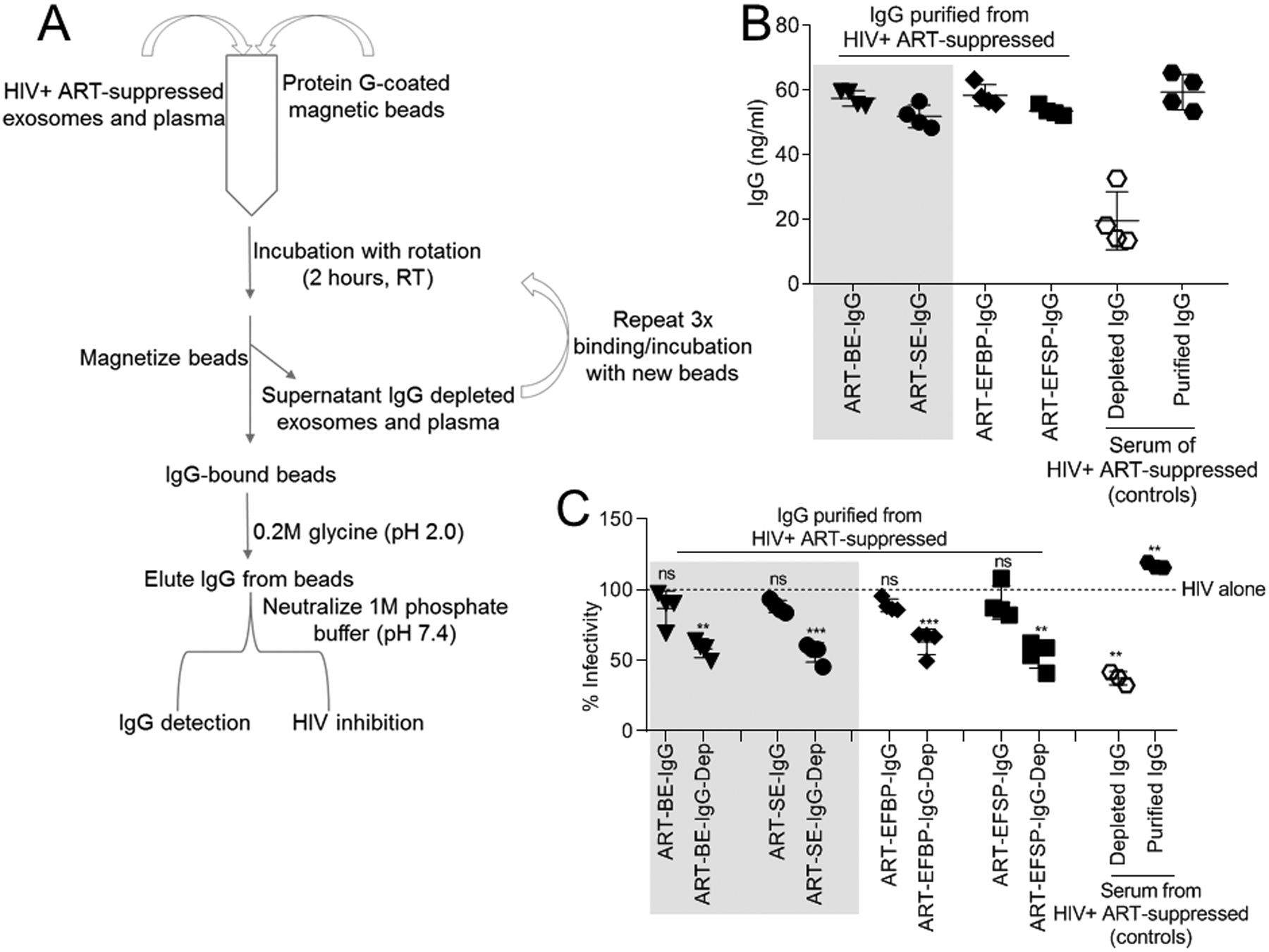
(A) A series of immuno-capture techniques purified IgG from HIV-infected ART-suppressed EV-free plasma and EVs (n=4). (B) Concentration of purified IgG was measured by IgG-specific ELISA. IgG purification from HIV-infected ART-suppressed unfractionated serum was used as controls. (C) HIV-1 infectivity measured by incubation of 100,000RLU HIV-1 NL4.3 virus with purified and depleted IgG fractions, IgG controls, or vehicle PBS on TZM-bl cells for 24 h. Infectivity was measured by TZM-bl luciferase reporter activity. Vehicle treated cells are set as reference at 100% for infectivity (broken line). Statistics was determined by comparing infectivity values from all donors to vehicle control for each IgG fraction. Significance was determined by student’s t test. *=P<0.05, **=P<0.01, ***=P<0.001. Error bars are SD of biological replicates from the mean of triplicate measurements. ns= not significant.
Inhibitory levels of ARVs are detected in ART-extracellular vesicles
The finding that HIV was inhibited by all ART-BE, -SE, -EFBP, and -EFSP fractions (Fig. 2C) suggests that ARV drugs may be present in all compartments, explaining the inhibition by all fractions (Fig. 2C). Although ARV drug concentrations are reported for semen, testicular tissues, and blood in HIV-infected donors3,22–24, previous studies have not evaluated body-fluid EVs for ARVs. As expected, ARVs were detectable in ART-EFBP and ART-EFSP (Supplemental Digital Content Table 2). Interestingly, ARVs were also detected in ART-BE and ART-SE (Supplemental Digital Content Table 2, Fig. 3A–D). TFV (given as disoproxil fumarate and alafenamide; TDF, TAF) and FTC were included in the ARV regimen of all donors, and were detected in all compartments from all donors. The active metabolites TFV-DP and FTC-TP were also detected in ART-BE and ART-SE, although there were donor-specific differences (Supplemental Digital Content Table 2). Detection of EV-associated TFV-DP and FTC-TP was significant, since intracellular phosphorylation traps these metabolites in the cell lipid membrane, making them susceptible to degradation by phosphatases in the extracellular environment25. Encasement of these ARVs in EVs may allow an evasion mechanism of the extracellular phosphatases although it is also possible that the TFV-DP and FTC-TP detection results from cellular disruption during sample isolation and processing. EFV and DTG were detected in all fractions obtained from subjects taking the medication (Supplemental Digital Content Table 2, Fig. 3C–D).
Figure 3: Extracellular vesicles from HIV-infected ART-suppressed donors contain ARV drugs.
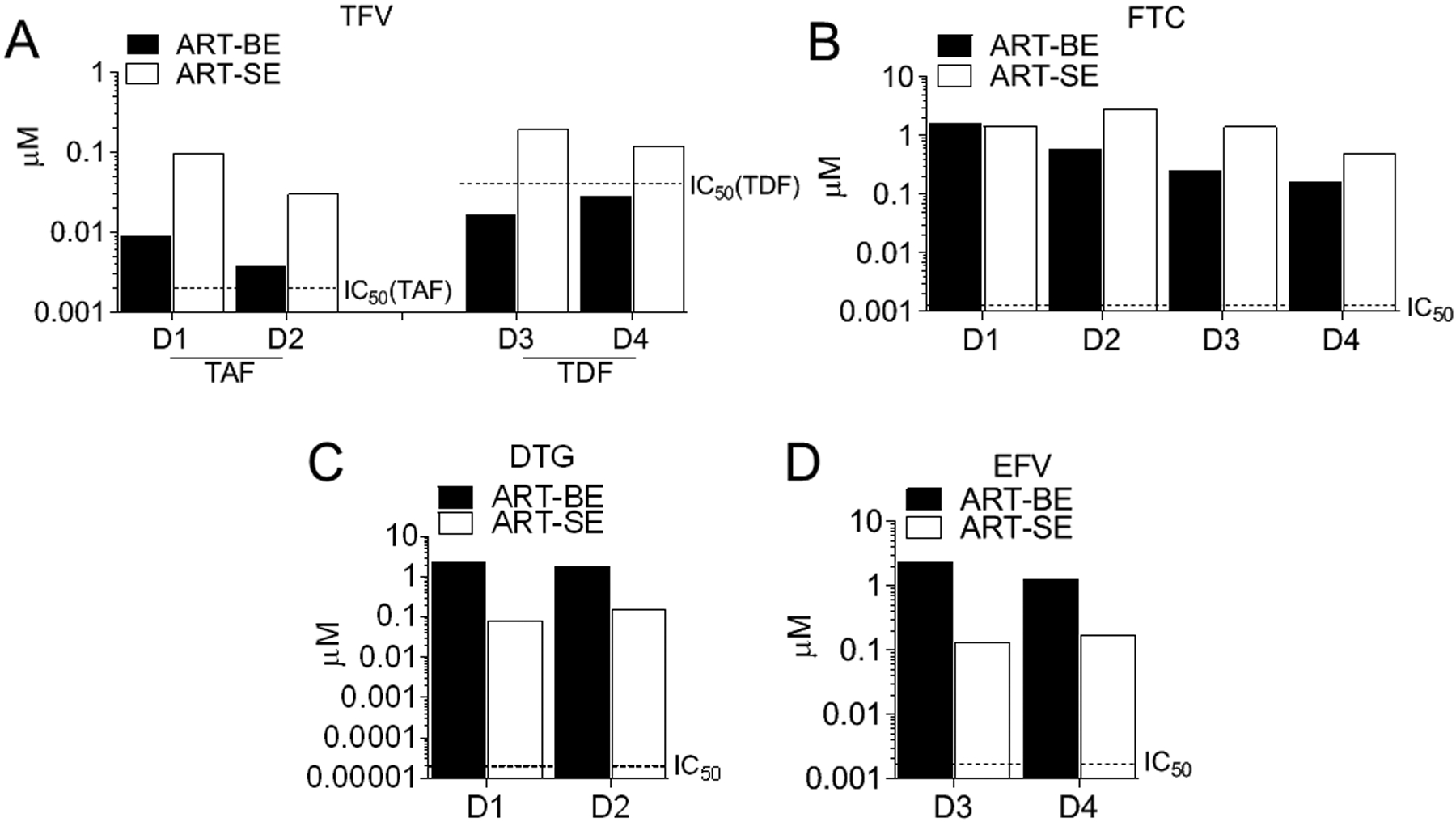
EVs were isolated from HIV-positive ART-suppressed donors’ plasma (ART-BE) and semen (ART-SE). Drug concentrations were measured by LC-MS/MS from 4 donors with similar drug regimen. ARVs are denoted as (A) TFV (tenofovir), (B) FTC (emtricitabine), (C) DTG (dolutegravir), (D) EFV (efavirenz). Broken lines indicate FDA-half maximal inhibitory concentrations (IC50) for HIV-1.
ARV levels in 50 μg ART-BE and ART-SE (approximately 5.0×108 particles) were greater than FDA-half maximal HIV inhibitory concentrations (IC50) (DTG=0.02 to 2.14 nM, FTC=0.0013 to 0.64 μM, EFV= 1.7 to 25 nM, TAF=2.0–14.7 nM) (Supplemental Digital Content Table 2, Fig. 3B–D)26–29. TDF levels were above the IC50 (0.04–8.5 μM)30 in SE obtained from all donors, but below the IC50 in BE (Supplemental Digital Content Table 2, Fig. 3A). Estimated ARV levels per individual ART-BE and ART-SE particle did not reach IC50 levels, thus individual EV particles carry very small amounts of ARV drugs; however, EVs may accumulate in cells and directly deliver ARVs (Supplemental Digital Content Fig. 5). Although limited by sample size, trends of ARV compartmentalization in EVs are suggested. TDF/TAF and FTC accumulated more in SE than BE while EFV and DTG appeared higher in BE compared to SE (Supplemental Digital Content Table 2, Fig. 3A–D) consistent with findings that nucleoside and nucleotide reverse transcriptase inhibitor concentrations are higher in semen than blood, while non-nucleoside reverse transcriptase inhibitors and protease inhibitors are not31–36. These results are the first to show that ARVs are body-fluid EV-associated and suggest that ARVs may be selectively compartmentalized into different body-fluid fractions. The data also suggest that the HIV-inhibitory phenotype of ART-extracellular vesicles and ART-EV-free plasma are likely due to the presence of ARVs.
Extracellular vesicle-associated ARV drugs protect against HIV
Unlike SE whose anti-HIV activity is conserved and independent of donor HIV and ART status (Figure 1C), BE do not inhibit HIV replication unless the subject is receiving ART (79.3% inhibition) (Fig. 1A–B). To validate that BE may carry ARVs, we incorporated ARV into BE in vitro, and examined if this inhibited HIV infection. Two independent methods were assessed for incorporating fluorescently labeled FTC into HIV-negative BE (Fig. 4A) and both methods incorporated FTC into BE (Fig. 4B), and led to HIV-1 inhibition (Fig. 4C). Control, HIV-negative BE or HIV-negative BE loaded with the fluorescent marker did not inhibit infection (Fig. 4C) or cell viability (Supplemental Digital Content Fig. 6). These results support the conclusion that donor-ART contributes to the inhibitory phenotype of ART-BE and demonstrate that non-inhibitory BE can deliver functional ARV therapy to inhibit HIV infection.
Figure 4: BE functionalized with ART inhibit HIV-1 infection.
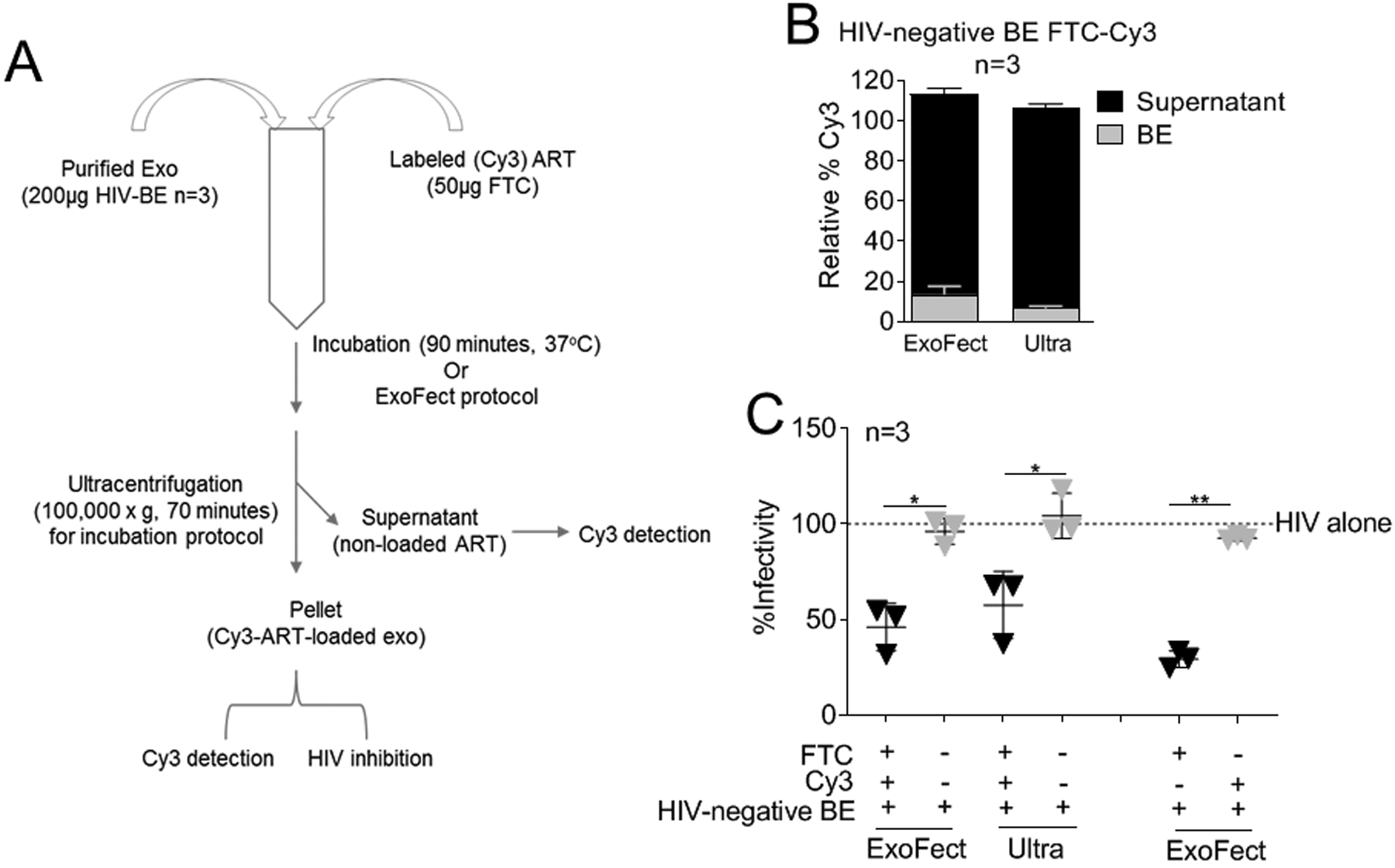
(A) 200 μg BE from HIV-negative donors (n=3) were loaded with 50 μg Cy3 labeled emtricitabine (FTC) by two methods (ExoFect vs. Ultra). (B) Cy3 was detected in the EV pellet and supernatant to determine loading efficiency. Supernatant Cy3 was set at 100% for each donor and loading method. (C) HIV-1 infectivity of 100,000RLU HIV-1 NL4.3 virus in the presence of 100 μg/ml FTC-loaded BE from (A-B), BE-loading controls, or vehicle PBS control on TZM-bl indicator cells for 24 h. TZM-bl infectivity was measured by luciferase reporter activity. Vehicle treated cells are set as reference at 100% (broken line).Statistics was determined by comparing infectivity values of FTC-loaded BE to relevant controls. Significance was determined by student’s t test. *=P<0.05, **=P<0.01, ***=P<0.001. Error bars are SD of biological replicates from the mean of triplicate measurements. ns= not significant.
Discussion
SE from HIV-negative humans inhibit HIV replication in vitro4–7; however, no prior studies examined the impact of HIV infection on the HIV-inhibitory effect of SE in the presence or absence of ARVs. Using autologous BE and SE and paired EFBP and EFSP, we found that donor HIV infection did not alter SE anti-HIV function, and that ARV drugs are associated with circulating BE and SE. Further, since IgG purified from ART-SE and ART-BE was not inhibitory, the anti-HIV effect of EVs does not appear to be due to virus neutralization by IgG antibodies. The observation that BE isolated from HIV-negative and HIV-infected ART-naïve donors do not inhibit while SE from the same donors inhibit HIV replication indicates that donor HIV status does not influence SE or BE HIV inhibitory properties. This further supports the hypothesis that SE may function to reduce the efficiency of HIV sexual transmission in vivo. Although amyloid fibrils in semen enhance HIV infectivity in vitro37,38, our data and those published previously indicate that semen-derived EVs inhibit replication of HIV and another enveloped virus (Zika) in vitro4–7,39. Of note, the factor(s) contributing to SE-mediated HIV-1 inhibition are not yet identified, and virions and other cellular products may be present in the EV preparation. Thus, we are cautious to attribute these effects exclusively to EVs. Nevertheless, SE purified by gradient centrifugation methods demonstrated the same inhibitory effect, and residual purification reagent (ExoQuick) had no effect on HIV-1, indicating a purification-independent inhibitory factor5,6. Identification of the inhibitory component(s) is needed, and proteomic studies to identify potential factors are underway.
In contrast, BE from individuals treated with ART inhibited HIV, unlike BE from HIV negative and HIV-infected ART-naïve donors. Donor ARV use did not alter ART-SE-inhibitory potential, suggesting that the inhibition by SE in these individuals was not solely dependent on ART. It is notable that ART-BE and ART-SE contained therapeutic levels of ARV and were able to inhibit HIV infection. This is the first report to show an association of body-fluid EVs with ARVs. The levels of DTG, FTC, EFV, and TAF in 50 μg (approximately 5.0×108 particles) BE and SE reached accepted IC50 concentrations for HIV26–29. SE-TFV (from TDF or TAF administration) reached IC50 values (TDF=0.04–8.5 μM, TAF=2.0–14.7 nM) for all donors while BE-TFV (from TDF/TAF) from two donors reached these IC50 values29,30. Together, these data provide strong evidence that EVs can carry and deliver ARV drugs to mediate protection against infection. It is not known whether EV-associated ARVs are surface-associated or enwrapped as luminal cargo, nonetheless, the ART-extracellular vesicles are functionally active.
It appears that ARV are preferentially compartmentalized in ART-BE and ART-SE, although the sample size precludes confidence in this observation. Although TFV (TDF or TAF) and FTC were present in both ART-BE and ART-SE, the concentration was more abundant in ART-SE compared to ART-BE. In contrast, EFV and DTG levels were higher in ART-BE than ART-SE. There were donor differences in the detection and concentrations of the active metabolites TFV-DP and FTC-TP (Supplemental Digital Content Table 2), suggesting EV-specific differential ARV bio-distribution and/or half-life (Figures 2C and 4D). Although our findings are supported by previous reports showing higher levels of NRTIs in semen31,33–36, further studies with increased sample size, different ARV drugs, and controlled experimentation in model systems are needed to determine if TFV and FTC are preferentially concentrated in genitourinary system EVs, and to evaluate the significance of such accumulation to HIV transmission and ARV-toxicities40–43. Although there are few data on EV and ART association13, nanoparticle-encapsulated ARVs are being studied as vehicles to deliver ARV therapy44. In our study, we demonstrated for the first time that body-fluid EVs deliver ARVs at concentrations sufficient to inhibit HIV replication, and that BE can carry ARVs in functionally relevant concentrations.
In summary, these results confirm that SEs inhibit HIV replication, and show that this is independent of donor HIV-infection status5–7,9–12. This is mediated through multiple mechanisms5–7,11,12, and identification of the inhibitory components in SE may inform interventions to mitigate HIV replication and sexual transmission. Further, BE-loaded with ARVs were capable of carrying ARVs at HIV-inhibitory concentrations. These findings suggest that ART-extracellular vesicles may deliver therapy to specific sites in vivo. Taken together, our data highlight the importance of understanding the role of EVs and EV-associated ARVs during HIV infection.
Supplementary Material
Acknowledgements
This work was supported by the National Institute on Drug Abuse (NIDA) [1R01DA042348-01 to CMO], National Institute of Allergy and Infectious Diseases (NIAID) [1RO1AI124965-03 to CVF], VA Merit Review and National Institutes of Health (NIH) [BX000207, NIH 5T32AI343 to JTS], and NIH [5T32AI007533-18 to JLW].
We thank the semen and blood donors for providing samples. We also thank the staff of the University of Iowa Hospitals and Clinics Virology (HIV/AIDS) clinic and Ryan White Program Staff, Dr. Amy Sparks, Ryan Brumm, Jennifer Jagnow of the University of Iowa Reproductive Testing laboratories for assistance with specimen collection, and the Antiviral Pharmacology Laboratory at The University of Nebraska Medical Center for mass spectrometry analysis of antiretroviral drug concentrations.
Footnotes
Conflict of Interest
The authors declare no conflicts of interest.
These data were presented in part at the Conference on Retroviruses and Opportunistic Infections (CROI) March 4–7, 2019 in Seattle, WA.
References
- 1.Kiessling AA. Retroviruses and reproduction revisited. J Assist Reprod Genet. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ortblad KF, Lozano R, Murray CJ. The burden of HIV: insights from the Global Burden of Disease Study 2010. AIDS. 2013;27(13):2003–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kashuba AD, Dyer JR, Kramer LM, Raasch RH, Eron JJ, Cohen MS. Antiretroviral-drug concentrations in semen: implications for sexual transmission of human immunodeficiency virus type 1. Antimicrob Agents Chemother. 1999;43(8):1817–1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Welch JL, Madison MN, Margolick JB, et al. Effect of prolonged freezing of semen on exosome recovery and biologic activity. Sci Rep. 2017;7:45034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Welch JL, Kaddour H, Schlievert PM, Stapleton JT, Okeoma CM. Semen exosomes promote transcriptional silencing of HIV-1 by disrupting NF-kB/Sp1/Tat circuitry. J Virol. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Madison MN, Roller RJ, Okeoma CM. Human semen contains exosomes with potent anti-HIV-1 activity. Retrovirology. 2014;11:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Madison MN, Jones PH, Okeoma CM. Exosomes in human semen restrict HIV-1 transmission by vaginal cells and block intravaginal replication of LP-BM5 murine AIDS virus complex. Virology. 2015;482:189–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Welch JL, Stapleton JT, Okeoma CM. Vehicles of intercellular communication: exosomes and HIV-1. J Gen Virol. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Madison MN, Okeoma CM. Exosomes: Implications in HIV-1 Pathogenesis. Viruses. 2015;7(7):4093–4118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nolte-’t Hoen E, Cremer T, Gallo RC, Margolis LB. Extracellular vesicles and viruses: Are they close relatives? Proc Natl Acad Sci U S A. 2016;113(33):9155–9161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Naslund TI, Paquin-Proulx D, Paredes PT, Vallhov H, Sandberg JK, Gabrielsson S. Exosomes from breast milk inhibit HIV-1 infection of dendritic cells and subsequent viral transfer to CD4+ T cells. AIDS. 2014;28(2):171–180. [DOI] [PubMed] [Google Scholar]
- 12.Smith JA, Daniel R. Human vaginal fluid contains exosomes that have an inhibitory effect on an early step of the HIV-1 life cycle. AIDS. 2016;30(17):2611–2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DeMarino C, Pleet ML, Cowen M, et al. Antiretroviral Drugs Alter the Content of Extracellular Vesicles from HIV-1-Infected Cells. Sci Rep. 2018;8(1):7653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wiley RD, Gummuluru S. Immature dendritic cell-derived exosomes can mediate HIV-1 trans infection. Proc Natl Acad Sci U S A. 2006;103(3):738–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kadiu I, Narayanasamy P, Dash PK, Zhang W, Gendelman HE. Biochemical and biologic characterization of exosomes and microvesicles as facilitators of HIV-1 infection in macrophages. J Immunol. 2012;189(2):744–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rydze RT, Bhattarai N, Stapleton JT. GB virus C infection is associated with a reduced rate of reactivation of latent HIV and protection against activation-induced T-cell death. Antivir Ther. 2012;17(7):1271–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Madison MN, Welch JL, Okeoma CM. Isolation of Exosomes from Semen for in vitro Uptake and HIV-1 Infection Assays. Bio Protoc. 2017;7(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Couturier J, Winchester LC, Suliburk JW, et al. Adipocytes impair efficacy of antiretroviral therapy. Antiviral Res. 2018;154:140–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Didiot MC, Hall LM, Coles AH, et al. Exosome-mediated Delivery of Hydrophobically Modified siRNA for Huntingtin mRNA Silencing. Mol Ther. 2016;24(10):1836–1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhu Z, Zhang D, Lee H, et al. Macrophage-derived apoptotic bodies promote the proliferation of the recipient cells via shuttling microRNA-221/222. J Leukoc Biol. 2017;101(6):1349–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.French MA, Tjiam MC, Abudulai LN, Fernandez S. Antiviral Functions of Human Immunodeficiency Virus Type 1 (HIV-1)-Specific IgG Antibodies: Effects of Antiretroviral Therapy and Implications for Therapeutic HIV-1 Vaccine Design. Front Immunol. 2017;8:780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moyer TP, Temesgen Z, Enger R, et al. Drug monitoring of antiretroviral therapy for HIV-1 infection: method validation and results of a pilot study. Clin Chem. 1999;45(9):1465–1476. [PubMed] [Google Scholar]
- 23.Anderson PL, Noormohamed SE, Henry K, Brundage RC, Balfour HH Jr., Fletcher CV. Semen and serum pharmacokinetics of zidovudine and zidovudine-glucuronide in men with HIV-1 infection. Pharmacotherapy. 2000;20(8):917–922. [DOI] [PubMed] [Google Scholar]
- 24.Huang Y, Hoque MT, Jenabian MA, et al. Antiretroviral drug transporters and metabolic enzymes in human testicular tissue: potential contribution to HIV-1 sanctuary site. J Antimicrob Chemother. 2016;71(7):1954–1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schauer AP, Sykes C, Cottrell ML, Prince H, Kashuba ADM. Validation of an LC-MS/MS assay to simultaneously monitor the intracellular active metabolites of tenofovir, emtricitabine, and lamivudine in dried blood spots. J Pharm Biomed Anal. 2018;149:40–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dolutegravir. https://aidsinfo.nih.gov/drugs/509/dolutegravir/0/patient.
- 27.Emtricitabine. https://aidsinfo.nih.gov/drugs/208/emtricitabine/23/professional.
- 28.Efavirenz. https://aidsinfo.nih.gov/drugs/269/efavirenz/15/professional#Section_12.4.
- 29.Tenofovir alafenamide. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2016/208464Orig1s000MicroR.pdf.
- 30.Tenofovir Disoproxil Fumarate. https://aidsinfo.nih.gov/drugs/290/tenofovir-disoproxil-fumarate/26/professional.
- 31.Avery LB, Bakshi RP, Cao YJ, Hendrix CW. The male genital tract is not a pharmacological sanctuary from efavirenz. Clin Pharmacol Ther. 2011;90(1):151–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Avery LB, VanAusdall JL, Hendrix CW, Bumpus NN. Compartmentalization and antiviral effect of efavirenz metabolites in blood plasma, seminal plasma, and cerebrospinal fluid. Drug Metab Dispos. 2013;41(2):422–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reddy YS, Gotzkowsky SK, Eron JJ, et al. Pharmacokinetic and pharmacodynamic investigation of efavirenz in the semen and blood of human immunodeficiency virus type 1-infected men. J Infect Dis. 2002;186(9):1339–1343. [DOI] [PubMed] [Google Scholar]
- 34.Taylor S, Pereira AS. Antiretroviral drug concentrations in semen of HIV-1 infected men. Sex Transm Infect. 2001;77(1):4–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vourvahis M, Tappouni HL, Patterson KB, et al. The pharmacokinetics and viral activity of tenofovir in the male genital tract. J Acquir Immune Defic Syndr. 2008;47(3):329–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thompson CG, Cohen MS, Kashuba AD. Antiretroviral pharmacology in mucosal tissues. J Acquir Immune Defic Syndr. 2013;63 Suppl 2:S240–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zirafi O, Kim KA, Roan NR, et al. Semen enhances HIV infectivity and impairs the antiviral efficacy of microbicides. Sci Transl Med. 2014;6(262):262ra157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Munch J, Rucker E, Standker L, et al. Semen-derived amyloid fibrils drastically enhance HIV infection. Cell. 2007;131(6):1059–1071. [DOI] [PubMed] [Google Scholar]
- 39.Muller JA, Harms M, Kruger F, et al. Semen inhibits Zika virus infection of cells and tissues from the anogenital region. Nat Commun. 2018;9(1):2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martin-Carbonero L, Nunez M, Gonzalez-Lahoz J, Soriano V. Incidence of liver injury after beginning antiretroviral therapy with efavirenz or nevirapine. HIV Clin Trials. 2003;4(2):115–120. [DOI] [PubMed] [Google Scholar]
- 41.Sulkowski MS, Thomas DL, Mehta SH, Chaisson RE, Moore RD. Hepatotoxicity associated with nevirapine or efavirenz-containing antiretroviral therapy: role of hepatitis C and B infections. Hepatology. 2002;35(1):182–189. [DOI] [PubMed] [Google Scholar]
- 42.van Leeuwen E, Wit FW, Repping S, et al. Effects of antiretroviral therapy on semen quality. AIDS. 2008;22(5):637–642. [DOI] [PubMed] [Google Scholar]
- 43.Lambert-Niclot S, Poirot C, Tubiana R, et al. Effect of antiretroviral drugs on the quality of semen. J Med Virol. 2011;83(8):1391–1394. [DOI] [PubMed] [Google Scholar]
- 44.Mandal S, Belshan M, Holec A, Zhou Y, Destache CJ. An Enhanced Emtricitabine-Loaded Long-Acting Nanoformulation for Prevention or Treatment of HIV Infection. Antimicrob Agents Chemother. 2017;61(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


