Abstract
A novel approach modifying cells to express viral markers to elicit protective immunity responses (decoy cellular vaccination) in the prevention of COVID-19 disease is currently being explored. Our approach entails utilizing SARS-CoV-2 Spike antigen-expressing, non-replicating cells as carriers and presenters of immunogenic antigens, so called “I-cells”. By using irradiated cells as presenting vehicles of SARS-CoV-2 viral antigens(s) in a cellular context, these presented viral proteins can be recognized by the host immune system, thus, an efficient protective immune response might be elicited. Another advantage of this strategy is that the manufacturing process is scalable and yields uniform cell products allowing for “off-the-shelf” frozen supply availability. To prevent engraftment and proliferation of the cells after administration, the cells will be irradiated post-harvesting abolishing in vivo replication potential.
Specifically, immunoreactive Spike-1 proteins from SARS-CoV-2 are expressed on the surface of irradiated target I-cells. Utilizing this innovative strategy, these viral antigen-displaying decoy cells will be developed as a vaccine to protect against COVID-19 disease.
Keywords: COVID-19, SARS-CoV-2, Vaccine, Coronavirus, Antigen, Transgenic cell vaccination
Highlights
-
•
A novel decoy cellular vaccination approach being developed to fight against COVID-19 disease.
-
•
Viral antigen-expressing, non-replicating cells used as both a carrier and antigen-presenting vehicle.
-
•
Scalable manufacturing could allow rapid “off-the-shelf” supply availability.
Enabling the delivery and presentation of pathogen-specific proteins expressed in their native conformation to the host immune system, overcoming the known delivery and conformational inefficiencies associated with nucleic acid and recombinant protein-based approaches is a critical component of successful vaccine development.
The development pathway of I-cells, i.e. utilizing modified “decoy cells” to carry and present target antigens to the host immune system, is depicted in Fig. 1 . Engineered Spike-1 protein is expressed on the surface of K562 human myelogenous leukemia cells via introduction of expression constructs into the cellular genome allowing for stable expression of the transgene. Stable-modified K562 clones are selected, profiled for Spike-1 expression as well as overall immunogenic potency, and prepared as GMP master cell bank to be used for large scale manufacturing. Irradiated cells are formulated as vaccine product and administered via intramuscular or subcutaneous injection.
Fig. 1.
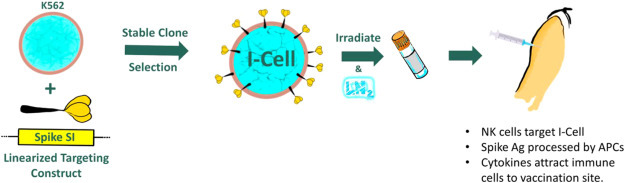
Targeted expression of SARS-CoV-2 Spike immunogen and resulting I-cell vaccine candidate.
The K562 cell line is HLA-negative and, as such, exquisitely sensitive to NK-mediated killing [1,2]. Expression of SARS-CoV-2-encoded proteins in the context of K562 or other NK-sensitive cell lines provides a means of targeting and activating an innate driver of the host adaptive immune response against the immunogen and thus, virally infected host cells expressing the antigen. Cancer vaccine treatments utilizing lethally irradiated K562 cells expressing granulocyte-macrophage colony-stimulating factor (GM-CSF) (GM-K562) have been previously demonstrated to be well tolerated in human clinical trials [3,4]. Doses up to 1 × 108 GM-K562 cells have been administered by multiple parenteral routes and in treatment regimens including up to nine dose administrations. GM-CSF expression from GM-K562, in combination with co-delivered autologous tumor antigen have been shown to elicit a robust cellular and humoral anti-tumor immune response in treated patients [5].
This novel virus decoy cellular vaccine is designed to initiate NK cell-driven immune activation with the ultimate goal of inducing protective adaptive host immunity. NK cell-induced I-cell death should result in cytokine release, recruitment of antigen presenting cells (APCs), and maturation of elicited dendritic cells (DCs) at the site of vaccination. Presentation of SARS-CoV-2 Spike protein-derived peptides by recruited APCs will drive adaptive host immune responses against SARS-CoV-2. Interleukin 12 secreted from mature DCs in combination with NK-secreted GM-CSF and interferon-γ (IFNγ) can combine to recruit T lymphocytes and may induce Th1 cell polarization, thereby inducing development of a potent cytotoxic T cell response and clearance of SARS-CoV-2-infected cells. Confirmation of Th1 polarization vs. a mixed Th1/Th2 or predominantly Th2 response is currently underway in preclinical immunization models. Previous efforts to develop respiratory virus vaccines to protect against Respiratory Syncytial Virus (RSV) and SARS-related disease have demonstrated the potential clinical benefits of eliciting a Th1 adaptive immune response over the disease-exacerbating effects of a Th2 polarized response [3,4]. Immunization studies in mice with four candidate SARS vaccines (VLP, whole virus, and an rDNA-produced Spike protein) led to pulmonary immunopathology upon challenge with SARS virus, an effect that was signified by Th2 polarization in mice immunized with each candidate vaccine [4]. The decoy cell vaccine can drive the host cellular immune response toward Th1, generating both potent cytotoxic T cell immunity against the major determinant of SARS-CoV-2 cellular entry and pathogenesis (Fig. 2 ).
Fig. 2.
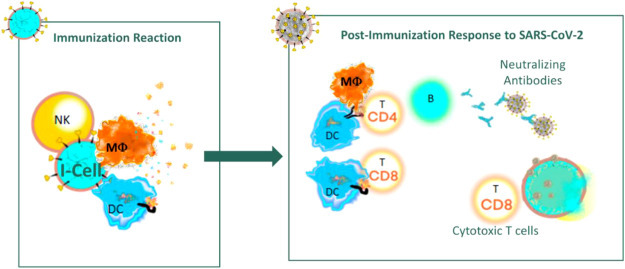
Elicitation of humoral and cellular immune responses following virus decoy cell vaccination.
Utilizing pathogen-derived cellular immunogens (virus-encoded antigens) as initiators of NK-mediated immune responses might provide an exciting method to produce novel vaccine candidates. It is important to highlight the flexibility of the decoy cell vaccination strategy as additional cell vaccines can be generated in an expeditious manner once new antigens of interest have been identified. Thus, successful demonstration of the efficacy of this current anti-SARS-CoV-2 approach in clinical trials would pave the way for use of the decoy cell platform to address a large number of unmet medical needs in the clinic as we seek to protect populations from viral, parasitic, fungal and bacterial diseases.
Funding source
Sorrento Therapeutics is funding the discovery, development and clinical supply manufacturing of the vaccine platform mentioned in this communication.
CRediT authorship contribution statement
Henry Ji: Conceptualization. Ying Yan: Conceptualization. Beibei Ding: Conceptualization. Wenzhong Guo: Conceptualization. Mark Brunswick: Writing - review & editing. Andreas Niethammer: Original writing. Williams SooHoo: Visualization. Robin Smith: Writing - review & editing. Alexis Nahama: Writing - review & editing. Yanliang Zhang: Conceptualization.
Declaration of competing interest
The authors are employed (or Directors) of Sorrento Therapeutics, Inc. The current discovery strategy is being implemented for vaccine development under the I-Cell™ label and within Sorrento's cGMP manufacturing facilities.
References
- 1.Klein E., Ben-Bassat H., Neumann H., Ralph P., Zeuthen J., Polliack A. Properties of the K562 cell line, derived from a patient with chronic myeloid leukemia. Int J Cancer. 1976 Oct 15;18(4):421–431. doi: 10.1002/ijc.2910180405. PubMed PMID: 789258. [DOI] [PubMed] [Google Scholar]
- 2.Nishimura M., Mitsunaga S., Akaza T., Mitomi Y., Tadokoro K., Juji T. Protection against natural killer cells by interferon-gamma treatment of K562 cells cannot be explained by augmented major histocompatibility complex class I expression. Immunology. 1994;83(1):75–80. [PMC free article] [PubMed] [Google Scholar]
- 3.Mangodt T., Van Herck M., Nullens S. The role of Th17 and Treg responses in the pathogenesis of RSV infection. Pediatr Res. 2015;78:483–491. doi: 10.1038/pr.2015.143. [DOI] [PubMed] [Google Scholar]
- 4.Tseng C.-T., Sbrana E., Iwata-Yoshikawa N., Newman P.C., Garron T., Atmar R.L. Correction: immunization with SARS coronavirus vaccines leads to pulmonary immunopathology on challenge with the SARS virus. PLoS ONE. 2012;7(8) doi: 10.1371/annotation/2965cfae-b77d-4014-8b7b-236e01a35492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Curry Cancer therapy trial. Vaccination with lethally irradiated glioma cells mixed with GM-K562 cells in patients undergoing craniotomy for recurrent tumor. 2016. https://clinicaltrials.gov/ct2/show/NCT00694330?term=Curry&draw=2&rank=9


