ABSTRACT
Macroautophagy/autophagy is an evolutionarily conserved degradative process with a central role in maintaining cellular homeostasis under conditions of stress, and recent evidence suggests this may occur in part through direct modification of cell signaling. The MET/HGF receptor tyrosine kinase (RTK) signaling axis is an important mediator of cell motility and invasion in normal cell functions and in cancer. We discovered a role for autophagy in regulating ligand-activated MET signaling and cellular responses. When autophagy is induced by starvation, the HGF-activated and internalized MET RTK is selectively recruited for autophagic degradation through complex formation with the MAP1LC3C autophagy protein. Decreased LC3C expression in cancer results in loss of autophagic degradation of MET and enhanced HGF-stimulated cell invasion implicated in metastatic progression. This emerging role for autophagy in selectively regulating signaling proteins has implications for understanding cellular adaptations to stress and the functions of autophagy at different stages of cancer progression.
KEYWORDS: Autophagy, cancer, cell migration, HGF; invasion, LC3C; MET RTK, signaling, signalophagy
Maintaining cellular homeostasis depends on the integration of both intracellular and extracellular cues to modify cellular processes and respond to changes in nutrient availability and metabolic demand. Induction of macroautophagy, hereafter autophagy, promotes catabolic metabolism through engulfment and lysosomal degradation of cellular components, thereby liberating metabolic intermediates for cell maintenance under conditions of nutrient deprivation. Although originally considered a process for bulk cytoplasm degradation, it is now understood that autophagy also selectively targets specific cellular components. Activated tyrosine kinases, including SRC, the RET and KIT receptor tyrosine kinases (RTKs), and the BCR-ABL oncogene, are reported to be among the cargo that can become enriched in autophagosomes. These findings support the idea that autophagy could directly contribute to modulating cell signaling pathways during adaptation to stress, yet mechanisms of selective recruitment of signaling proteins into an autophagic degradative pathway are still poorly understood. We recently reported that starvation-induced autophagy can selectively regulate MET RTK levels to diminish ligand-activated MET-dependent biological responses, including cell motility and invasion [1]. The MET RTK mediates an invasive growth program in development and wound healing, and drives metastatic progression in various cancers. Binding of the MET ligand, HGF, stimulates MET activation and internalization into the endocytic pathway. Recycling of MET back to the plasma membrane controls duration and localization of signaling, such that MET mutations in cancer that diminish MET trafficking to a degradative compartment can be tumorigenic. We report that starvation-induced autophagy can divert HGF-stimulated MET into an autophagic degradative pathway, thereby decreasing MET recycling, signaling, and stability. This mechanism may have important tumor-suppressor functions given that multiple stresses that enhance autophagy are associated with malignant transformation and the tumor microenvironment.
We demonstrate through multiple experimental approaches that the autophagic targeting of MET is a selective process requiring ligand activation and internalization of MET and complex formation of MET with the human Atg8-family protein, MAP1LC3C (hereafter LC3C) (Figure 1A). Similar to other Atg8-family members, LC3C is lipidated and anchored in the membrane of the phagophore, which is the precursor structure where cargo can be recruited for engulfment within autophagosomes. However, we find LC3C in both distinct and overlapping puncta compared to the prototypical Atg8-family member, MAP1LC3B, supporting the idea that there are additional, separate functions for LC3C. Complex formation with MET requires two regions within LC3C, one containing an intact R76, a residue found to mediate a subset of LC3B protein interactions, as well as an intact C-terminal tail extension that is unique to LC3C among the Atg8-family proteins. Under conditions of starvation-induced autophagy, LC3C knockout is sufficient to liberate MET from autophagy-dependent degradation. Autophagy-mediated suppression of MET recycling, signaling, and cell invasion can be rescued by re-expression of LC3C, but not by expression of LC3C mutant proteins that fail to engage in a complex with MET. Negative regulation of HGF-stimulated MET signaling and cell motility by autophagy is observed in multiple established cancer cell lines (BT-549, MDA-231, HeLa) and with multiple gene targets to disrupt different stages of autophagosome biogenesis (ATG5, ATG7, ATG14) or recruitment of MET as an autophagic cargo (LC3C). In cells where MET invasive responses are regulated by autophagy, EGFR-dependent signaling and cell migration are not altered by autophagy, nor is the EGFR diverted to an autophagic compartment under starvation conditions. Hence, our findings support a model where MET signaling and biological responses are selectively abrogated by recruitment of LC3C, promoting MET degradation and signal termination through selective autophagy. We concluded that selective autophagic regulation of MET is likely a widely occurring mechanism in cells containing MET, LC3C, and an intact autophagosome biogenesis pathway.
Figure 1.
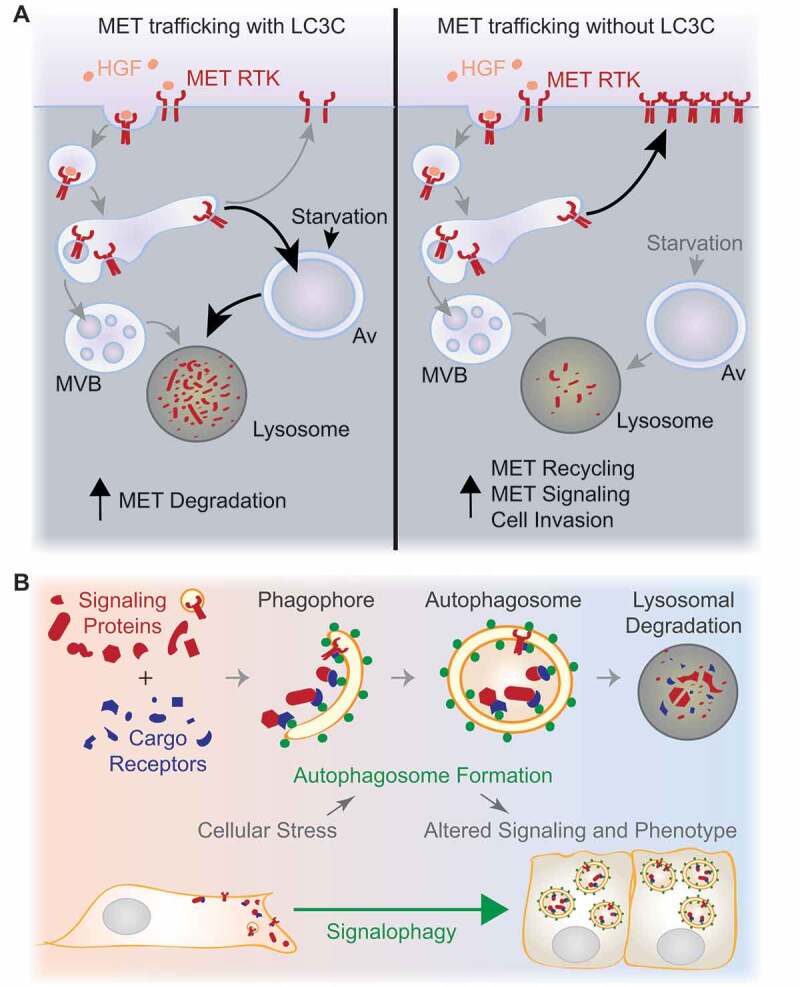
Regulation of cell signaling by autophagy. (A) If LC3C is present (left), localization of the HGF-activated MET RTK to an autophagic degradative pathway is enhanced by starvation-induced upregulation of autophagy. In cancers where LC3C expression is suppressed (right), MET is not targeted for autophagic degradation, resulting in increased MET recycling, prolonged signaling, and enhanced HGF-stimulated migration and invasion. MVB, multivesicular body; Av, autophagosome. (B) Increased autophagosome formation is induced in response to diverse cellular stresses, and can promote homeostasis through degradation of cellular components, including damaged organelles and pathogens. Autophagy also selectively degrades proteins recruited through binding to cargo receptors that interact with Atg8-family proteins (green circles), such as LC3C. The selective sequestration and lysosomal degradation of signaling proteins, termed signalophagy, could allow autophagy to participate in stress-responsive selective remodeling of cell signaling. This regulatory function for autophagy could be an important contributor to cellular adaptation to stress.
Because MET signaling is pro-tumorigenic, we hypothesized that loss of autophagic regulation of MET may occur in some cancers and contribute to tumor progression. In support of this, LC3C expression is decreased in several cancers and we confirmed, as previously demonstrated, that loss of the VHL tumor suppressor in clear cell renal cell carcinoma (ccRCC) results in diminished LC3C, while LC3B-dependent autophagy remains intact. Decrease in LC3C uncouples MET from autophagic regulation, thereby preventing autophagic inhibition of MET-dependent invasion, which is rescued by LC3C re-expression in ccRCC cells. The decreased expression of LC3C associated with VHL loss may be mediated by stabilization of HIF (hypoxia inducible factor), which also occurs in hypoxic regions of tumors. Thus, uncoupling of MET from autophagic regulation could be a widespread occurrence, but this requires additional investigation. Autophagy has both tumor-promoting and tumor-suppressive roles. Together our results support the view that the context-dependent functions of autophagy may be due in part to the composition of the autophagy network mediating selective regulation of specific cargo in different cell types or different stages of tumor progression.
Autophagy cargo are typically recruited through cargo receptors that bind to Atg8-family proteins. As the list of proteins found to be capable of serving as cargo receptors grows, it appears increasingly likely that many signaling proteins can be regulated by selective autophagy. It is attractive to speculate that altering expression of Atg8-family proteins can be used to switch cells from one regulatory program to another and that different Atg8-family members may be essential in particular tissues or at particular stages in development. Future studies of selective regulation of signaling proteins by autophagy, termed signalophagy, may address whether the type of stress inducing autophagy influences cargo selection. If selective autophagy of signaling proteins evolved as a means to promote cellular homeostasis, it would be reasonable to expect that, for example, autophagosomes induced by DNA damage could have different selective cargo than those induced by energetic stress, as adaptations necessary for cell survival differ under these different conditions. This could allow the various stresses that induce autophagy to result in alteration of cellular signaling and functions, providing a potentially rapid and tuneable mechanism for adaptation to stress (Figure 1B).
Funding Statement
This work was supported by the CIHR Foundation Grant [242529] to M. Park.
Disclosure statement
The authors have no potential conflicts of interest to report.
Reference
- [1].Bell ES, Coelho PP, Ratcliffe CDH, et al. LC3C-mediated autophagy selectively regulates the Met RTK and HGF-stimulated migration and invasion. Cell Rep. 2019;29(12):4053–4068. [DOI] [PubMed] [Google Scholar]


