Figure 3.
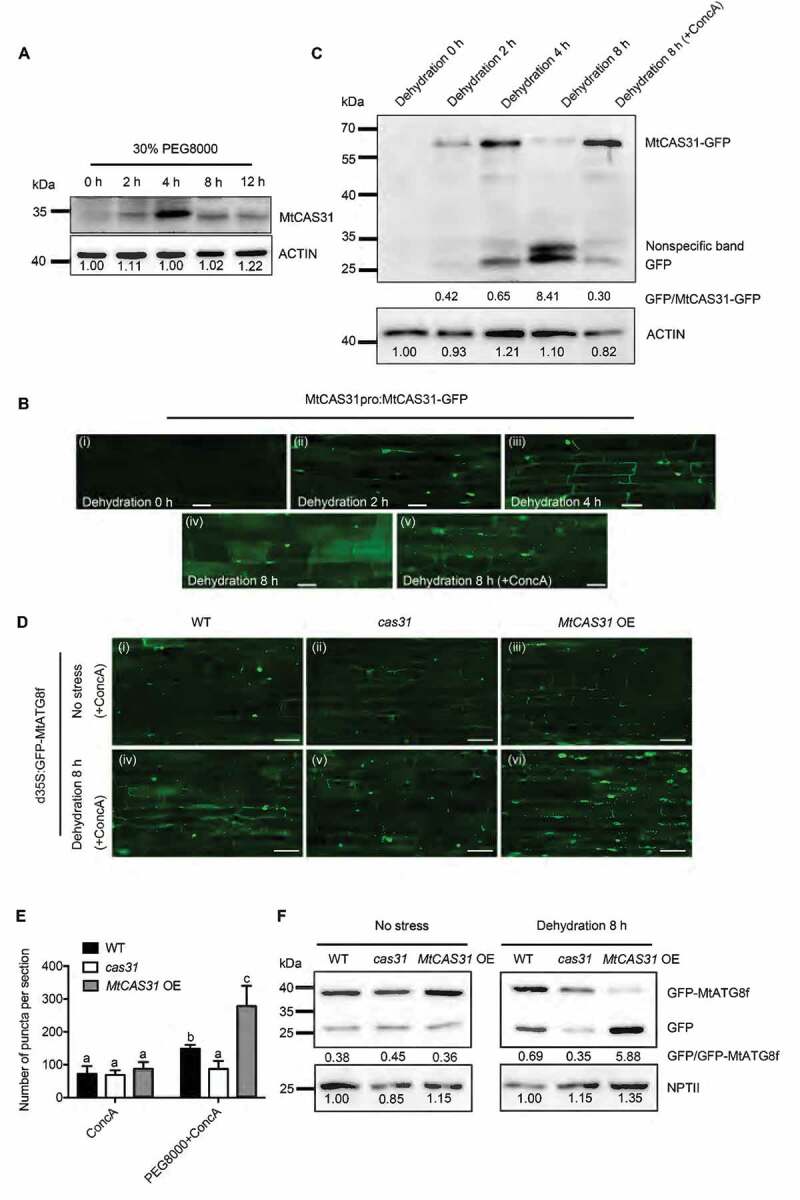
MtCAS31 promotes autophagic degradation under drought stress. (A) Immunoblot analysis of MtCAS31 under dehydration at different PEG 8000 treatment timepoints. (B) M. truncatula hairy roots expressing MtCAS31pro:MtCAS31-GFP were evaluated by confocal laser scanning microscopy in the absence of stress (dehydration for 0 h) (i), under dehydration treatment for 2 h (ii), under dehydration treatment for 4 h (iii), under dehydration treatment for 8 h (iv) and a under a combined 4 h ConcA with 8-h dehydration treatment (v). Bar: 35 μm. No fluorescence signal was detected in the absence of stress, probably because MtCAS31-GFP was driven by the MtCAS31 native promoter, which is induced by dehydration. (C) Immunoblotting analysis of MtCAS31-GFP and free GFP under dehydration treatment at different PEG 8000 treatment timepoints (0, 2, 4 and 8 h) and a combined 4 h ConcA with 8-h dehydration treatment using anti-GFP. (D) Transgenic hairy roots expressing MtATG8f-GFP driven by CaMV35S were detected in wild-type, cas31 mutant and MtCAS31OE plants under no stress and dehydration with ConcA combination treatment. Fluorescence signals were detected by confocal laser scanning microscopy at a 488 nm excitation. Bar: 75 μm. (E) Significance analysis of the number of GFP-MtATG8f puncta per section in Figure 3D. Three sections were used for analysis. Significant differences, indicated by letters, were determined by one-way ANOVA and LSD. (F) Immunoblot analysis of free GFP and GFP-MtATG8f in wild-type, cas31 mutant and MtCAS3OE plants under no stress and 8-h dehydration treatment. NPTII was employed for quantification.
