Abstract
Background and Purpose:
Circulating levels of sex hormone binding globulin (SHBG) have been inversely linked to obesity, diabetes, and other cardiometabolic disorders. It remains uncertain whether low SHBG is prospectively predictive of stroke risk, particularly in women. We investigated whether SHBG is associated with risk of incident ischemic stroke (IS) among women in the Women’s Health Initiative (WHI).
Methods:
From an observational cohort of 161,808 postmenopausal women enrolled in the WHI at 40 sites across the U.S. from 1993 – 1998, we identified 13,192 participants free of prevalent stroke at baseline who were included in an ancillary study that measured serum SHBG. We used Cox proportional hazards regression, stratified by SHBG measurement assay, to assess IS risk across quintiles of SHBG (Q1 – Q5), adjusting first for demographic variables (Model 1), additionally for body mass index, hypertension, alcohol use, and smoking status (Model 2), and for physical activity and reproductive risk factors (Model 3). In sensitivity analyses, potential mediators (diabetes status, levels of estradiol, testosterone, and CRP) were included.
Results:
Of 13,192 participants (mean age 62.5 years, 67.4% non-Hispanic white, 18.5% black, 7.6% Hispanic, 5.0% Asian), after following for an average of 11.6 years, 768 IS events were adjudicated. Compared to the highest quintile of SHBG levels (referent), women in the lowest SHBG quintile had a higher risk of IS in all three multivariable-models (Model 1: HR 1.88, 95%CI 1.47-2.41, Model 2: HR 1.69, 95%CI 1.30-2.20, Model 3: HR: 1.61, 95%CI 1.19-2.19, trend tests p <0.05 for all models). Including potential mediators such as diabetes, estradiol, and testosterone in the models attenuated but did not eliminate significant inverse associations between SHBG and IS.
Conclusions:
In this prospective cohort of post-menopausal women, there was a statistically significant inverse association between serum SHBG levels and IS risk, which supports the notion that SHBG could be used as a risk stratification tool for predicting IS in women.
Keywords: stroke, sex hormones, sex-specific, Ischemic stroke, cerebrovascular disease/stroke
INTRODUCTION
Age-adjusted risk of ischemic stroke (IS) is generally thought to be lower among women than men;1 however, incidence rates between women and men become similar among older age groups,1 with rates in women over 80 years of age surpassing those of men in some studies.2–4 Due to longer life expectancies, women, overall, have a higher lifetime risk of stroke compared with men.5 Sex differences in the risk of IS across the life course may be related to protective effects of endogenous sex steroids such as estradiol in pre-menopausal women and the lack of these effects among older women,6 though trials of exogenous hormones for the prevention of stroke in post-menopausal women demonstrated an increased risk of stroke with the use of both estrogen and estrogen plus progestin. These increased risks contributed to the termination of the Women’s Health Initiative (WHI) hormone therapy trials.7
A gap exists in understanding the role of endogenous hormones in IS risk, especially among post-menopausal women. Data on endogenous estradiol and risk of cardiovascular disease and/or stroke among women conflict,8,9 though some studies have reported increased stroke risk with higher estradiol levels.9 This presents a need to continue to study the role of sex hormones and their related proteins. Sex hormone-binding globulin (SHBG), a protein that binds to and regulates available testosterone and estradiol, is one potential target. Previous literature has demonstrated inverse associations between SHBG and vascular risk factors (i.e., insulin resistance, inflammation, diabetes, and metabolic syndrome) as well as outcomes, specifically coronary heart disease (CHD) in some studies.8,10–16 The mechanism by which low SHBG levels are related to an increased risk of vascular disease and outcomes is not well understood but likely includes a combination of indirect effects through alterations in the balance between testosterone and estradiol as well direct effects, independent of sex steroids.17
Despite data linking SHBG to vascular risk factors and outcomes such as diabetes and CHD, it remains uncertain whether SHBG is associated with stroke risk, particularly in women. In the current study, our objective was to investigate the association between SHBG and incident IS among post-menopausal women using a subsample from the WHI study.
METHODS
The WHI data can be requested through the Publications and Presentations Committee.
Study Population/Study Design
The WHI is a large, national, prospective cohort study that comprises a group of randomized clinical trials and an observational study. Between 1993 and 1998, 161,808 postmenopausal women, ages 50-79 years at baseline, were enrolled at one of 40 clinical sites in the United States. Follow-up for the main study was through 2005, at which time participants were asked to reconsent to further follow-up through 2010, with a second reconsenting process occurring at that time. Participants had a baseline study visit between 1993 and 1998 followed by annual visits and semi-annual contacts.
Our study sample (Figure 1) is comprised of participants in one of eleven ancillary case control studies in which SHBG was measured as part of the study protocol (Supplemental Table I). Participants were excluded from the analysis if they had a history of any strokes at baseline or if they had missing data on any of the key model covariates. Use of the WHI data for this analysis was approved by the institutional review boards of each principal investigator’s institution.
Figure 1:
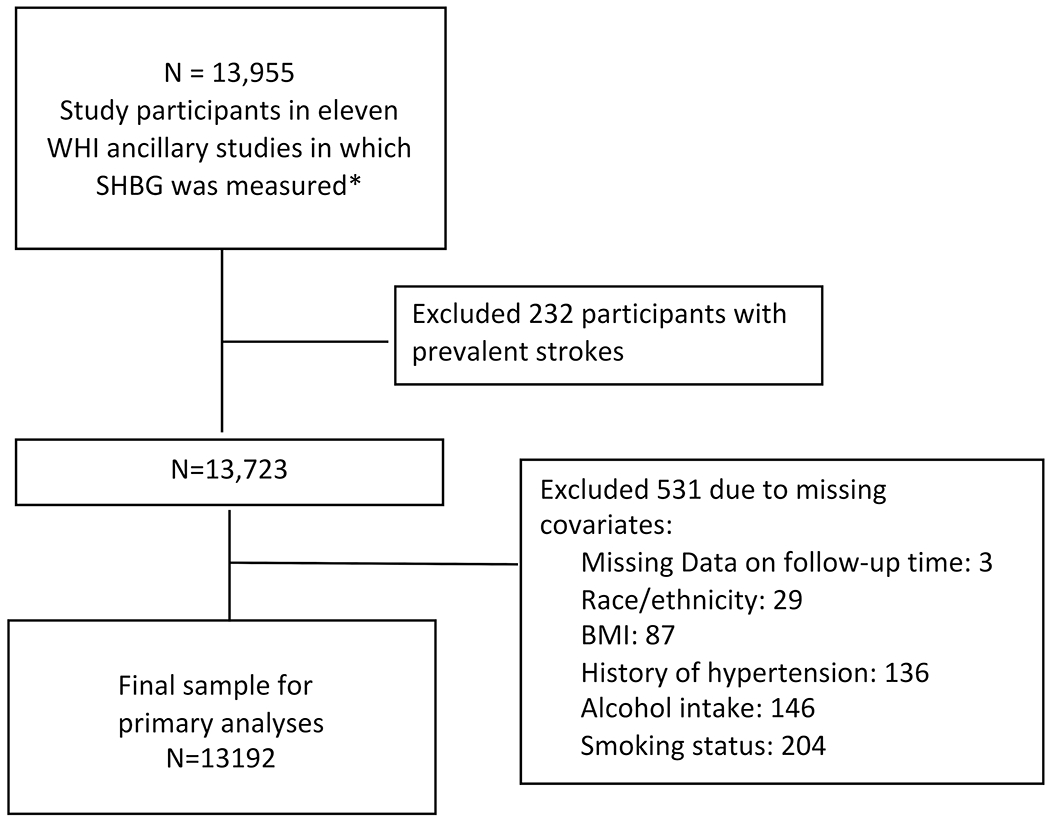
Study population from Women’s Health Initiative (WHI)
*See supplemental appendix for details of ancillary studies; SHBG: Sex hormone binding globulin; BMI: Body mass index
Exposure Measurement
The primary exposure variable was serum SHBG at the time of enrollment. Blood samples were drawn at the initial study visit and stored at −80 degree Celsius. Serum SHBG concentration in nanomoles per liter (nmol/L) was measured at one of three labs (each performing a single type of assay): an electrochemiluminescence immunoassay (Roche Diagnostics) for ancillary study 238 (12.7% of participants), an immunoradiometric assay (Esoterix) for ancillary study W5 (2.2% of participants), and a two-site chemiluminescent immunoassay (Siemens Medical Solutions) for the remaining 9 studies (85.1% of participants). Data on SHBG levels using participants in this group of ancillary studies have been pooled previously, and interassay coefficients of variation between 3.7% and 17.7% were reported.18
Outcome
The outcome of interest was incident IS during the follow-up period. Potential stroke events were initially identified at the time of semi-annual contacts or annual visits using medical history update forms completed by participants. All potential stroke events were then adjudicated by trained study physicians using medical records comprised of physician notes, diagnosis codes, and imaging results. Stroke was defined as the rapid onset of a persistent neurologic deficit lasting at least 24 hours due to cerebrovascular obstruction. To be included as an event, the relevant deficit must have lasted at least 24 hours or have a compatible lesion on CT and/or MRI. Events were considered ischemic (rather than primary hemorrhagic or unknown) if: 1) there was a focal deficit without blood on CT, MRI, or lumbar puncture (if performed); 2) if brain imaging (CT or MRI) showed hypodensities in a tissue pattern compatible with the symptoms; or 3) if there was surgical or autopsy evidence of infarct. Given prior evidence linking SHBG to risk factors for ischemia (i.e., diabetes and insulin resistance) as well as CHD, the focus of this study was on ischemic cerebrovascular events; participants with primary hemorrhage were not included due to concerns for significant differences in pathophysiology and risk factor profiles between stroke subtypes.
Covariates
All covariates included in the analysis were collected at the time of study enrollment. Age, race/ethnicity, and medical comorbidities including history of hypertension and history of ever being treated for diabetes were collected via interview. Social history including alcohol use (≤ 1 drink/month, 1-7 drinks per week, and ≥ 7 drinks per week) and smoking status (never/past/current) were also collected at the time of enrollment via self-report. Body mass index (BMI) (weight in kilograms / (height in meters)2) was collected using baseline height and weight measured at enrollment, and physical activity was reported in met-hours per week. Physical activity, defined as total weekly energy expended through recreational activity, was calculated by combining questions in which women reported frequency and duration of weekly walking, mild, moderate, and strenuous physical activity.
Additionally, reproductive risk factors including age at menarche, age at menopause (as defined as ≥ 1 year without regular periods), history of ever using oral contraceptives, number of full-term pregnancies (≥6 months gestational age), and use of menopausal hormone therapy (MHT) (never/past/current) were collected via self-report at the time of enrollment. MHT was defined as the use of unopposed estrogen or estrogen plus progesterone through a pill or patch.
Biomarker covariates for secondary analyses
Total serum estradiol and total testosterone were measured at baseline visits as part of the ancillary studies described above. Serum C-reactive protein (CRP) was measured at baseline on a sample of participants who were included in the WHI CVI Biomarkers Study. Further detail on sampling schemes and measurement methods can be found on the WHI website.19
Statistical Analysis
To understand the nature of the relationship between SHBG and incident IS, we analyzed SHBG in two ways: first in categories (quintiles) and also as a log-transformed continuous variable due to the right-skewed distribution of the variable. Baseline characteristics of included participants were described using frequencies and proportions for categorical variables and means and standard deviations (SD) (or medians and interquartile ranges (IQR)) for continuous variables as appropriate, in the overall sample and by SHBG quintile.
We conducted time to event analyses. Participants that did not have an IS during the follow-up period (within 15 years from time of enrollment) were censored due to death, loss to follow-up, or administrative censoring, and length of follow-up in days for each participant is pre-calculated in the WHI dataset. Those participants who were still being followed at 15 years were administratively censored to ensure a standardized length of follow-up across studies.
The association between SHBG and incident IS was first investigated using Kaplan-Meier curves with log rank tests for statistical significance. Next, we used Cox proportional hazards regression to investigate the association of SHBG with IS in sequential models with the highest quintile (Q5) as the referent group, first adjusted for demographics (age, race/ethnicity). Stratification by SHBG measurement assay (with the ‘strata’ statement in SAS) was performed to reduce possible biases relating to the combination of 3 different SHBG assays as described above. Next, we additionally adjusted for BMI, history of hypertension, history of alcohol use, and smoking status. The final model in the sequence was also adjusted for physical activity and risk factors related to participants’ reproductive history (age at menarche, age at menopause, number of full-term pregnancies, history of use of OCPs, and history of MHT use). All potential confounders were chosen based on prior literature or subject knowledge suggesting a potential causal relationship with both the exposure (SHBG) and the outcome (stroke).
Similar sequential Cox proportional hazards regression models were performed with log-transformed SHBG rather than SHBG in quintiles to assess the robustness of our findings and to account for lack of clarity around the appropriate cut-off value of SHBG.
The proportionality assumption was assessed using visualization of Schoenfeld residuals and a test of proportionality when a time x SHBG term was included in each model. For all Cox proportional hazard models, hazard ratios and the corresponding 95% confidence intervals were reported. Statistical significance was assessed using an alpha level of 0.05 (two-sided).
Sensitivity analyses
In a pre-planned sensitivity analysis, models were adjusted for whether participants were a case (vs. control) in any of the ancillary studies to adjust for potential selection bias. Additionally, analyzing SHBG as a log-transformed continuous variable, we stratified Cox models by individual ancillary study to account for potential bias introduced by combining studies. Stratification by ancillary study was not performed with SHBG as quintiles due to very small numbers of stroke events by quintile in individual ancillary studies. Our final sensitivity analysis was adding history of liver disease to the previously adjusted models, given that SHBG Is primarily produced by the liver.
Exploratory Analyses with Potential Mediators
To identify potential mediators in the relationship between SHBG and IS, a variable for each potential mediator was added into the models one at time (along with an SHBG x mediator product term) to evaluate whether inclusion in the models reduced the inverse association between SHBG and IS risk. Only those participants who had valid measurements for mediators were included in each of these models, and due to reduced sample sizes, Cox models were not stratified by SHBG assay with the exception of the DM mediation model which included almost the full sample. The potential mediators included history of diabetes, total serum estradiol and testosterone levels, and C-reactive protein. Once again, hazard ratios with 95% confidence intervals were reported.
All statistical analyses were performed using SAS version 9.4.
RESULTS
Our primary analysis included 13192 unique participants (Figure 1) with 768 IS events. In the entire sample, the mean follow-up time was 11.6 years. Of the total sample, 3.1% were lost to follow-up, 27.5% were censored due to death, and 63.6% were administratively censored.
Baseline characteristics of the participants by SHBG quintiles are displayed in Table 1. The median age of participants ranged between 63.0 (IQR 58.0-69.0) and 67.0 (IQR 61.0-72.0). The age distribution varied by SHBG level (p<0.0001 for Wilcoxon rank sum test across quintiles): participants in the lowest quintile were younger than those in the other quintiles. Non-Hispanic white participants comprised 67.4% of the sample, 18.5% of the overall participants were Black, 7.6% were Hispanic, and 5.0% were Asian. There were no clear trends in race/ethnicity distribution across SHBG levels.
Table 1:
Baseline Characteristics of Participants by Sex Hormone Binding Globulin (SHBG) Level
| Baseline variables | SHBG Q1 (n=2639) | SHBG Q2 (n=2601) | SHBG Q3 (n=2675) | SHBG Q4 (n=2639) | SHBG Q5 (n=2639) | p-value |
|---|---|---|---|---|---|---|
| SHBG (Median (IQR)) | 23.3 (19.1-26.7) | 35.3 (32.6-38.0) | 47.0 (43.8-50.6) | 64.0 (58.6-69.8) | 107 (88.5-141.0) | n/a |
| Age (Median (IQR)) | 63.0 (58.0-69.0) | 66.0 (60.0-71.0) | 67.0 (60.0-72.0) | 67.0 (61.0-72.0) | 66.0 (59.0-71.0) | <0.0001 |
| Race/ethnicity, N (%) | ||||||
| American Indian/Alaskan | 36 (1.4) | 24 (0.9) | 27 (1.0) | 15 (0.6) | 15 (0.6) | |
| Native | <0.0001 | |||||
| Asian/Pacific Islander | 123 (4.7) | 99 (3.8) | 118 (4.4) | 117 (4.4) | 193 (7.3) | |
| Black/African-American | 534 (20.2) | 459 (17.6) | 458 (17.1) | 440 (16.7) | 547 (20.7) | |
| Hispanic/Latino | 216 (8.2) | 203 (7.8) | 173 (6.5) | 168 (6.4) | 240 (9.1) | |
| Non-Hispanic white | 1712 (64.9) | 1794 (69.0) | 1879 (70.2) | 1885 (71.4) | 1620 (61.4) | |
| Other | 18 (0.7) | 22 (0.8) | 20 (0.7) | 14 (0.5) | 24 (0.9) | |
| Body Mass Index (Mean (SD)) | 32.2 (6.0) | 30.2 (5.9) | 28.7 (5.9) | 27.0 (5.6) | 26.2 (5.5) | <0.0001 |
| History of Hypertension, N (%) | 1289 (48.84) | 1080 (41.52) | 1017 (38.02) | 927 (35.13) | 858 (32.51) | <.0001 |
| History of Diabetes, N (%) | 308 (11.7) | 168 (6.5) | 124 (4.6) | 82 (3.1) | 64 (2.4) | <0.0001 |
| Physical Activity (Met-hours/week), Median (IQR) | 4.5 (0.5-11.8) | 6.0 (1.5-14.1) | 7.5 (1.9-16.7) | 8.3 (2.5-18.3) | 8.3 (2.2-18.3) | <0.0001 |
| Alcohol Use, N (%) | ||||||
| ≤1 drink/month | 1501 (56.9) | 1309 (50.3) | 1314 (49.1) | 1243 (47.1) | 1328 (50.3) | <0.0001 |
| 1-7 drinks/per week | 922 (34.9) | 1024 (39.4) | 1076 (40.2) | 1111 (42.1) | 1082 (41.0) | |
| ≥ 7 drinks/week | 216 (8.2) | 268 (10.3) | 285 (10.6) | 285 (10.8) | 229 (8.7) | |
| Smoking Status, N (%) | ||||||
| Never | 1368 (51.84%) | 1378 (52.98%) | 1445 (54.02%) | 1372 (51.99%) | 1418 (53.73%) | 0.002 |
| Past | 1087 (41.19%) | 1001 (38.49%) | 1000 (37.38%) | 1010 (38.27%) | 968 (36.68%) | |
| Current | 184 (6.97%) | 222 (8.54%) | 230 (8.60%) | 257 (9.74%) | 253 (9.59%) | |
| Age at menopause (Mean, SD) | 47.8 (6.7) | 47.9 (6.8) | 48.0 (6.6) | 48.5 (6.3) | 48.1 (6.5) | 0.004 |
| Age at menarche, N (%) | ||||||
| ≤ 10 | 97 (4.4) | 97 (4.4) | 88 (3.8) | 65 (2.9) | 84 (3.8) | |
| 11 | 268 (12.2) | 234 (10.7) | 251 (11.0) | 192 (8.9) | 198 (9.0) | 0.001 |
| 12 (ref) | 464 (21.1) | 446 (20.4) | 491 (21.4) | 476 (21.5) | 426 (19.3) | |
| 13 | 541 (24.5) | 582 (26.6) | 565 (24.7) | 568 (25.7) | 570 (25.6) | |
| 14 | 348 (15.8) | 374 (17.1) | 388 (16.9) | 414 (18.7) | 385 (17.5) | |
| ≥ 15 | 486 (22.1) | 458 (20.9) | 508 (22.2) | 497 (22.5) | 540 (24.5) | |
| Oral contraceptive use ever, N (%) | 1039 (39.4) | 911 (35.0) | 854 (31.9) | 829 (31.4) | 876 (33.2) | <.0001 |
| Number of full-term pregnancies, N (%) | ||||||
| 0 | 304 (11.6) | 275 (10.6) | 313 (11.8) | 315 (12.0) | 306 (11.7) | 0.0010 |
| 1 | 230 (8.8) | 223 (8.6) | 233 (8.9) | 252 (9.6) | 271 (10.3) | |
| 2-4 | 1550 (59.0) | 1542 (59.3) | 1615 (60.8) | 1604 (61.3) | 1590 (60.7) | |
| ≥5 | 541 (20.6) | 559 (21.5) | 494 (18.6) | 445 (17.0) | 452 (17.3) | |
| Use of MHT at baseline, N (%) | ||||||
| Never | 1904 (72.1) | 1869 (71.9) | 1839 (68.8) | 1814 (68.7) | 1518 (57.6) | |
| Past | 623 (23.6) | 611 (23.5) | 686 (25.6) | 622 (23.6) | 552 (20.9) | <0.0001 |
| Current | 112 (4.2) | 120 (4.6) | 149 (5.6) | 203 (7.7) | 565 (21.4) | |
SHBG: Sex hormone binding globulin; IQR: interquartile range; SD: standard deviation; MHT: menopausal hormone therapy; Chi-square tests, one-way analysis of variance, or Wilcoxon rank sum tests were used to compare proportions/frequencies, means, and medians as appropriate.
Risk factor profiles differed across SHBG quintiles. Compared with participants in the highest SHBG quintile, those in the lowest SHBG quintile had a higher mean BMI (32.2(SD 6.0) vs. 26.2(SD 5.5), respectively p<0.0001) and had higher proportions of hypertension (48.8% vs. 32.5%, respectively, p<0.0001) and diabetes at baseline (11.7% vs. 2.4%, respectively, p<0.0001). With respect to reproductive risk factors, mean age at menopause was similar across SHBG quintiles. Compared with those in the highest quintile, those in the lowest quintile of SHBG were more likely to report current use of MHT (4.2% vs. 21.4%, respectively, p<0.0001).
Unadjusted, a greater proportion of participants in the lowest quintile had IS (6.9%) compared with the highest quintile (3.8%), p<0.0001. Accounting for length of follow-up time using Kaplan-Meier curves with log-rank tests, results were similar (p<0.0001, Figure 2).
Figure 2:
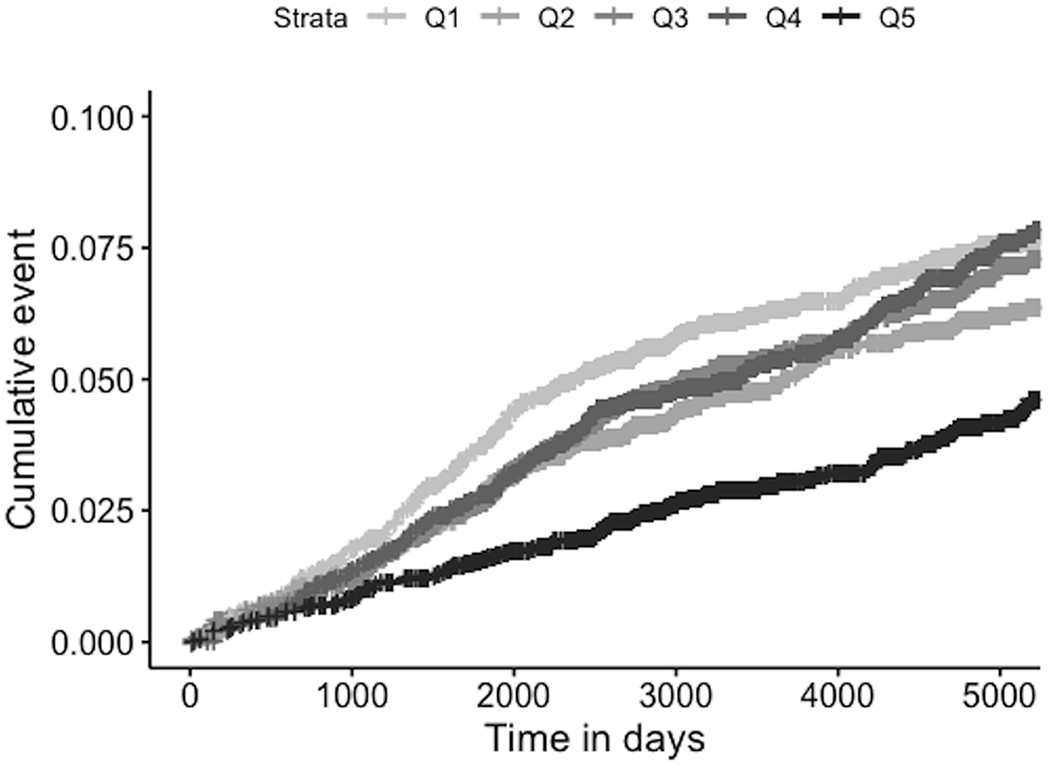
Cumulative Hazards of Incident Ischemic Stroke, Unadjusted, by SHBG Quintile
Q1: Lowest SHBG quartile, Q5: Highest SHBG Quartile; Log-rank test p<0.0001
Results of sequential Cox proportional hazard models are displayed in Table 2 and Figure 3. Stratified by SHBG assay and adjusted for age and race, those in the lowest quintile (Q1) of SHBG had 88% higher hazards (95% CI 1.47-2.41) of IS compared with those in the highest reference group (Q5) (trend test p<0.0001). Adjustment for BMI, history of hypertension, alcohol use, and smoking status resulted in somewhat attenuated hazard ratios (Q1 vs Q5 (ref), 1.69, 95% CI 1.30-2.20, trend test p=0.004). The addition of weekly reported physical activity and reproductive risk factors (age at menopause, number of full term pregnancies, MHT use at baseline, history of OC use, and age at menarche) resulted in a HR of 1.61, 95%CI 1.19-2.19, trend test p=0.04). Hazard ratios for Q2, Q3, and Q4 were smaller but still demonstrate an inverse association with IS risk compared with Q5 (highest quintile and reference group) (Table 2).
Table 2:
Hazards of Incident Ischemic Stroke in the Women’s Health Initiative by Sex Hormone Binding Globulin Quintile
| Model 1* | Model 2** | Model 3† | |
|---|---|---|---|
| SHBG | HR (95%CI) | HR (95%CI) | HR (95%CI) |
| Q1 | 1.88 (1.47-2.41) | 1.69 (1.30-2.20) | 1.61 (1.19-2.19) |
| Q2 | 1.34 (1.03-1.73) | 1.27 (0.98-1.65) | 1.24 (0.91-1.68) |
| Q3 | 1.44 (1.13-1.85) | 1.40 (1.09-1.80) | 1.44 (1.08-1.92) |
| Q4 | 1.49 (1.16-1.91) | 1.46 (1.14-1.87) | 1.49 (1.12-1.98) |
| Q5 | Reference | Reference | Reference |
Q1: Lowest quintile; Q5: Highest quintile;
Adjusted for age, race/ethnicity, SHBG assay as strata variable, p <0.0001 (test for trend);
Adjusted for Model 1 and body mass index, history of hypertension, alcohol use, and smoking status, p=0.004 (test for trend);
Adjusted for Model 2 and physical activity, age at menopause, parity, use of menopausal hormone therapy at baseline, history of using oral contraceptives, age at menarche, p=0.04 (for trend test). Due to missing data for covariates, sample size for Model 3 is 9688.
Figure 3:
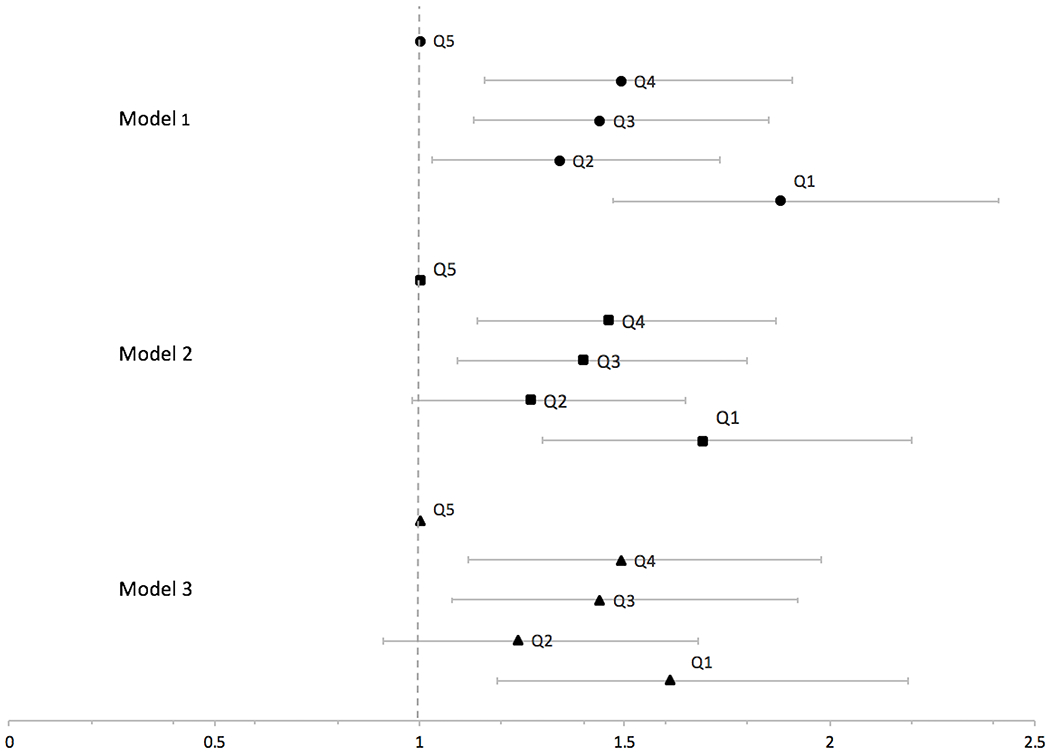
Hazard Ratios (95% CI) of Incident Ischemic Stroke in the Women’s Health Initiative by Sex Hormone Binding Globulin Quintile
Q1 is the lowest SHBG quintile; Q5 is the highest SHBG quintile. Model 1 is adjusted for age and race/ethnicity, Model 2 adjusted for Model 1 plus body mass index, history of hypertension, alcohol use, and smoking status. Model 3 adjusted for Model 2 plus physical activity, age at menopause, parity, use of menopausal hormone therapy at baseline, history of using oral contraceptives, age at menarche. All models are stratified by SHBG measurement assay.
When log-transformed and treated as a continuous variable, a one-unit increase in log-SHBG was associated with an inverse association with IS (HR 0.77 (95%CI 0.67-89), adjusted for age, race/ethnicity, BMI, history of hypertension, alcohol use, smoking, and stratified by SHBG assay.
Sensitivity Analyses
Next, we adjusted for whether participants were a case in any one of the ancillary studies to account for potential selection bias; effect estimates were similar to previous models (Q1 vs. Q5, HR 1.74 (95%CI 1.34-2.25), adjusted for age, race/ethnicity, BMI, history of hypertension, alcohol use, and smoking status.
Additionally, stratifying by individual ancillary study, and adjusting for age, race/ethnicity, BMI, history of hypertension, alcohol, and smoking, the HR for IS per unit increase in log SHBG was 0.86 (0.74-0.99). The small number of stroke events (<10) in some of the individual ancillary studies, however, limit the interpretability of this result.
Finally, adding history of liver disease to the models did not change our effect estimates (Supplemental Table II).
Results of Secondary Analyses
Finally, in subsets of the sample, Table 3 demonstrates results of our exploratory analyses of potential mediators in the relationship between SHBG and incident IS. The diabetes, estradiol/testosterone, and CRP models demonstrated attenuation of the effect estimates compared with hazard ratios in Table 2. Interaction terms for each of the potential mediators (history of diabetes x SHBG, estradiol level x SHBG, testosterone level x SHBG, and CRP level x SHBG) were all non-significant (p>0.05).
Table 3:
Secondary Analyses: Hazards of Incident Ischemic Stroke Across Sex Hormone Binding Globulin Levels, Adjusted for Potential Mediators
| Model 1 with history of diabetes* (N=13184) | Model 2 with total estradiol** (n=10725) | Model 3 with total estradiol and testosterone† (n=5595) | Model 4 with CRP‡ (n=5287) | |
|---|---|---|---|---|
| SHBG | HR (95%CI) | HR (95%CI) | HR (95%CI) | HR (95%CI) |
| Q1 | 1.63 (1.26-2.12) | 1.99 (1.49-2.66) | 1.56 (0.90-2.70) | 1.52 (1.07-2.17) |
| Q2 | 1.25 (0.96-1.62) | 1.61 (1.20-2.15) | 2.15 (1.31-3.51) | 1.32 (0.93-1.89) |
| Q3 | 1.40 (1.09-1.80) | 1.58 (1.19-2.11) | 1.65 (1.00-2.73) | 1.48 (1.05-2.09) |
| Q4 | 1.46 (1.14-1.88) | 1.63 (1.23-2.16) | 2.06 (1.29-3.31) | 1.40 (1.00-1.98) |
| Q5 | Ref | Ref | Ref | Ref |
Q1: Lowest quintile; Q5: Highest quintile; All models are adjusted for age, race/ethnicity, body mass index, history of hypertension, alcohol use, and smoking status, along with potential mediators as noted.
Model 1 also stratified by SHBG assay; p=0.01 (test for trend);
p<0.0001 (test for trend);
p=0.09 (test for trend);
p=0.07 (test for trend)
DISCUSSION
In this prospective cohort of post-menopausal women, there was a statistically significant inverse association between SHBG level and IS risk. Those in the lowest SHBG quintile had less favorable cardiometabolic profiles compared with participants in higher quintiles, and the risk of IS was between 1.6 and 1.9 times higher among those in the lowest SHBG quintile compared with those in the highest SHBG quintile. Though these findings are novel with respect to the evaluation of stroke as the primary outcome, our findings are supported by studies showing an inverse association between SHBG and cardiovascular disease.8,20 Further, while some previous prospective studies of SHBG and CVD outcomes have reported that the association is almost completely explained by other risk factors such as BMI,8 our findings of an inverse association between SHBG level and IS risk were robust to adjustment for demographic factors, BMI, hypertension, smoking, alcohol, and reproductive risk factors including age at menopause and history of MHT use. Other previous data have demonstrated that low SHBG levels are linked to greater odds of having significant carotid atherosclerosis, which may point to a possible mechanism between low SHBG and stroke.21 Though our findings help to establish an epidemiologic link between SHBG and IS, it is important to consider that our data cannot demonstrate biologic plausibility. Though purely speculative, hypotheses for the biologic mechanisms include factors such as large vessel atherosclerosis or changes in thrombogenesis among those with embolic stroke.
With respect to the pattern of the relationship between SHBG and incident IS, the largest effect sizes were seen between the top and bottom quintiles (Q5 and Q1). The middle groups (Q2-Q4) all had hazards of stroke > 1 but did show a clear dose response relationship, suggesting the need for further work to identify a cutoff point for SHBG at which stroke risk may increase.
Though the current study was not designed to evaluate either a causal relationship or the biologic mechanism between low SHBG levels and IS, our secondary analyses of potential mediating variables provide some hypotheses-generating data about possible mechanisms. For example, the model that included diabetes as a covariate showed an attenuation of effect estimates, suggesting that some but not all of the association between low SHBG and stroke risk could be due to development of insulin resistance and clinical diabetes. This is in line with several previous studies demonstrating evidence for a causal relationship between low SHBG and both insulin resistance and clinical diabetes.10,11,14 Other secondary analyses that included estradiol, testosterone, and CRP are limited by small sample sizes, but inclusion of testosterone and CRP in the models also attenuated effect estimates, supporting the possibility that some of the relationship between low SHBG and IS may be related to either increasing free testosterone with downstream pro-androgenic effects8 and/or inflammatory pathways.12,22 These secondary analyses, however, are limited by data availability, smaller sample sizes, and a lack of dose response patterns; formal mediation analyses could be performed in future studies.
Our findings of the association between low SHBG and stroke risk have potential implications for the way in which we predict stroke risk in post-menopausal women. For example, the association between low SHBG and IS persisted despite adjustment for many of the classically defined stroke risk factors (i.e., hypertension), suggesting that a measure of endogenous sex hormones like SHBG could possibly improve the performance of commonly used prediction tools. Expert guidelines on the topic of stroke prevention have called for the need to incorporate sex-specific risk factors into current prediction tools;23 in the future, this could include hormonal biomarkers as well as aspects of reproductive history. CHA2DS2-VASc is one tool that incorporates patient sex to better predict stroke in the setting of atrial fibrillation;24 studying the addition of hormonal biomarkers to this rule for both sexes might be one potential future research direction. It is also unclear what an optimal SHBG value would be to use for clinical risk prediction; this could also be assessed in future studies.
Our findings also have potential implications for novel risk factor modification strategies. Not only might SHBG be used in the future as way to improve prediction of stroke risk, but it could serve as a therapeutic target. This is especially appealing given the association between low SHBG and overall poor cardiometabolic health.16 Previous studies have shown positive associations between modifiable lifestyle factors like exercise,18 diet25 and SHBG; whether SHBG could be predictably modified by changes in lifestyle is unknown.
Strengths and Limitations
Our paper has several strengths and limitations that should be noted. A clear strength is the large sample size with racial and ethnic diversity along with the prospective study design of the WHI. There is, however, potential selection bias related to the combination of case control studies which may be present despite our statistical methods including adjustment for case vs. control status in the regression model. In addition, though we were able to adjust for a large number of potential confounding variables, our study was not designed to test for a causal relationship between low SHBG and stroke but only to test associations. We performed exploratory analyses to assess the effect of including potential mediators on the association between low SHBG and incident IS. Since data on exposure variables and mediators were both obtained at baseline, though, the temporal relationship between SHBG and potential mediators cannot be truly determined. Though cross sectional in nature, these secondary analyses could be used to guide future formal mediation analyses. Finally, our results may not be generalizable to hemorrhagic strokes.
CONCLUSIONS
In this prospective cohort of post-menopausal women, there was a significant inverse association between SHBG levels and IS risk, suggesting that that SHBG could improve risk stratification for predicting IS in post-menopausal women. Future research is needed on the nature of the relationship between SHBG and stroke (causal or not), the potential mechanisms, and the ability to use SHBG to improve our current methods of stroke prediction and prevention in women.
Supplementary Material
Acknowledgments
SOURCES OF FUNDING
This work is funded by the National Heart, Lung, and Blood Institute (NHLBI) (K23 HL140081) and the Robert Leet Patterson Clara Guthrie Patterson Trust Mentored Research Award.
The WHI program is funded by the NHLBI, National Institutes of Health, U.S. Department of Health and Human Services through contracts HHSN268201600018C, HHSN268201600001C, HHSN268201600002C, HHSN268201600003C, and HHSN268201600004C.
Footnotes
DISCLOSURES
The authors have no relevant conflicts of interest.
Contributor Information
Tracy E. Madsen, Department of Emergency Medicine, Alpert Medical School of Brown University, Providence, RI.
Xi Luo, Department of Biostatistics and Data Science, The University of Texas Health Science Center at Houston, Houston, TX.
Mengna Huang, Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital, Boston, MA.
Ki E. Park, Department of Medicine, University of Florida, Gainesville, FL.
Marcia L . Stefanick, Departments of Medicine and Obstetrics & Gynecology, Stanford University School of Medicine Stanford, CA.
JoAnn E. Manson, Department of Medicine, Division of Preventive Medicine, Brigham and Women’s Hospital/Harvard Medical School, Boston, MA.
Simin Liu, Department of Epidemiology, Brown University School of Public Health, Deptartments of Medicine and Surgery, Alpert Medical School of Brown University.
REFERENCES
- 1.Howard VJ, Madsen TE, Kleindorfer D, Judd SE, Rhodes JD, Soliman EZ, et al. Sex and race differences in incident ischemic stroke and risk factors. Jama Neurol. 2019;76:179–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reeves MJ, Bushnell CD, Howard G, Gargano JW, Duncan PW, Lynch G, et al. Sex differences in stroke: epidemiology, clinical presentation, medical care, and outcomes. Lancet Neurol. 2008;7:915–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haast R a M, Gustafson DR, Kiliaan AJ. Sex differences in stroke. J. Cereb. Blood Flow Metab 2012;32:2100–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Appelros P, Stegmayr B, Terent A. Sex differences in stroke epidemiology: A systematic review. Stroke. 2009;40:1082–1090. [DOI] [PubMed] [Google Scholar]
- 5.Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S, et al. Heart disease and stroke statistics - 2018 update: A report from the American Heart Association. Circulation. 2018;137:E67–E492. [DOI] [PubMed] [Google Scholar]
- 6.Koellhoffer EC, McCullough LD. The Effects of Estrogen in Ischemic Stroke. Transl. Stroke Res 2013;4:390–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wassertheil-Smoller S, Hendrix SL, Limacher M, Heiss G, Kooperberg C, Baird A, et al. Effect of estrogen plus progestin on stroke in postmenopausal women: the Women’s Health Initiative: a randomized trial. JAMA. 2003;289:2673–84. [DOI] [PubMed] [Google Scholar]
- 8.Rexrode KM, Manson JE, Lee I, Ridker PM, Sluss PM, Cook NR, et al. Sex Hormone Levels and Risk of Cardiovascular Events in Postmenopausal Women. Baseline. 2003;1688–1693. [DOI] [PubMed] [Google Scholar]
- 9.Lee JS, Yaffe K, Lui L- Y, Cauley J, Taylor B, Browner W, et al. Prospective study of endogenous circulating estradiol and risk of stroke in older women. Arch. Neurol 2010;67:195–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ding EL, Song Y, Malik VS, Liu S. Sex differences of endogenous sex hormones and risk of type 2 diabetes: a systematic review and meta-analysis. JAMA. 2006;295:1288–99. [DOI] [PubMed] [Google Scholar]
- 11.Chen BH, Brennan K, Goto A, Song Y, Aziz N, You NY, et al. Sex hormone-binding globulin and risk of clinical diabetes in American black, Hispanic, and Asian/Pacific Islander postmenopausal women. Clin. Chem 2012;58:1457–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Joffe H, Ridker PM, Manson JE, Cook NR, Buring JE, Rexrode KM, et al. Sex hormone-binding globulin and serum testosterone are inversely associated with C-reactive protein levels in postmenopausal women at high risk for cardiovascular disease. Ann. Epidemiol 2006;16:105–112. [DOI] [PubMed] [Google Scholar]
- 13.Liu S, Sun Q. Sex differences, endogenous sex-hormone hormones, sex-hormone binding globulin, and exogenous disruptors in diabetes and related metabolic outcomes. J. Diabetes 2017;1–14. [DOI] [PubMed] [Google Scholar]
- 14.Ding EL, Song Y, Manson JE, Hunter DJ, Lee CC, Rifai N, et al. Sex hormone-binding globulin and risk of type 2 diabetes in women and men. N. Engl. J. Med 2009;361:1152–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chaoyang L, Ford ES, Benyi L, Giles WH, Liu S. Association of Testosterone and Sex Hormone – Binding Globulin With Metabolic Syndrome and Insulin Resistance in Men. Diabetes Care. 2010;33:1618–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jaspers L, Dhana K, Muka T, Meun C, Kiefte-de Jong JC, Hofman A, et al. Sex Steroids, Sex Hormone-Binding Globulin and Cardiovascular Health in Men and Postmenopausal Women: The Rotterdam Study. J Clin Endocrinol Metab. 2016;101:2844–2852. [DOI] [PubMed] [Google Scholar]
- 17.Rosner W, Hryb DJ, Kahn SM, Nakhla AM, Romas NA. Interactions of sex hormone-binding globulin with target cells. Mol. Cell. Endocrinol 2010;316:79–85. [DOI] [PubMed] [Google Scholar]
- 18.Goto A, Chen BH, Song Y, Cauley J, Cummings SR, Farhat GN, et al. Age, body mass, usage of exogenous estrogen, and lifestyle factors in relation to circulating sex hormone-binding globulin concentrations in postmenopausal women. Clin. Chem 2014; [DOI] [PubMed] [Google Scholar]
- 19.Women’s Health Initiative Specimen Results [Internet] [cited 2019 Dec 1];Available from: https://www.whi.org/researchers/_layouts/15/WopiFrame.aspx?sourcedoc=/researchers/Documents/WHIExtensionStudyCVDBiomarkerLabMethods.pdf&action=default
- 20.Lapidus L, Lindstedt G, Lundberg PA, Bengtsson C, Gredmark T. Concentrations of sex-hormone binding globulin and corticosteroid binding globulin in serum in relation to cardiovascular risk factors and to 12-year incidence of cardiovascular disease and overall mortality in postmenopausal women. Clin. Chem 1986;32:146–152. [PubMed] [Google Scholar]
- 21.Golden SH, Maguire A, Ding J, Crouse JR, Cauley JA, Zacur H, et al. Endogenous postmenopausal hormones and carotid atherosclerosis: A case-control study of the atherosclerosis risk in communities cohort. Am. J. Epidemiol 2002;155:437–445. [DOI] [PubMed] [Google Scholar]
- 22.Crandall C, Palla S, Reboussin B, Hu P, Barrett-Connor E, Reuben D et al. Cross-Sectional Association between Markers of Inflammation and Serum Sex Steroid Levels in the Postmenopausal Estrogen/Progestin Interventions Trial. J Womens Health. 2006;15:14–23. [DOI] [PubMed] [Google Scholar]
- 23.Bushnell C, McCullough LD, Awad I a, Chireau MV, Fedder WN, Furie KL, et al. Guidelines for the prevention of stroke in women: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45:1545–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abraham JM, Larson J, Chung MK, Curtis AB, Lakshminarayan K, Newman JD, et al. Does CHA2DS2-VASc improve stroke risk stratification in postmenopausal women with atrial fibrillation? Am. J. Med. 2013;126:1143.e1–1143.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang M, Liu J, Lin X, Goto A, Song Y, Tinker LF, et al. Relation of Dietary Carbohydrates Inake to Circulating Sex Hormone-binding Globulin Levels in Postmenopausal Women. J. Diabetes 2017;In Press. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


