Abstract
Background
Adequate pharmacokinetic and safety data in neonates are lacking for most antiretroviral agents. Raltegravir is a selective HIV-1 integrase strand transfer inhibitor (INSTI) available in a granule formulation suitable for use in neonates and young infants as prophylaxis or treatment of HIV infection.
Methods
IMPAACT P1110 is a phase 1, multicenter non-comparative dose-finding study of raltegravir in infants exposed to HIV-1 infection. A two-cohort adaptive design was utilized where pharmacokinetic data from infants in cohort 1 who received two single doses of raltegravir 3 mg/kg were included in population modeling and simulations to guide selection of a daily dose for infants in cohort 2.
Results
A total of 52 infants enrolled in IMPAACT 1110: cohort 1 (N=16); and cohort 2 (N=36). Using simulations based on population PK modeling incorporating cohort 1 data, the following daily dosing regimen was selected for study: 1.5 mg/kg daily from birth through Day 7; 3 mg/kg twice daily from Days 8 to 28 of life; 6 mg/kg twice daily after 4 weeks of age through 6 weeks of age. The geometric mean protocol exposure targets for AUC, Ctrough, and Cmax were met or slightly exceeded in all infants. The chosen neonatal raltegravir dosing regimen was safe and well tolerated in full-term neonates during treatment over the first 6 weeks of life and follow-up to age 24 weeks.
Conclusions
Raltegravir can be safely administered to full-term infants using the daily dosing regimen studied. This regimen is not recommended for use in premature infants.
Keywords: raltegravir, neonate, pharmacokinetic modeling, HIV, dose determination
Introduction
Limited data exist to provide dosing recommendations for combination antiretroviral regimens to prevent or treat HIV infection in neonates. The Department of Health and Human Services Perinatal Guidelines recommend the administration of a three-drug antiretroviral regimen for empiric treatment of newborns at highest risk of HIV acquisition and for treatment of neonates with documented HIV infection. However, sufficient neonatal pharmacokinetic and safety data with formulations appropriate for use in neonates are available for only a few antiretroviral agents in neonates - zidovudine, lamivudine, and nevirapine at birth plus lopinavir/ritonavir after 2 weeks of age. 1
Raltegravir is a potent and selective HIV-1 integrase strand transfer inhibitor (INSTI) available in a granule formulation suitable for use in neonates and young infants with HIV infection.1 The International Maternal Pediatric Adolescent AIDS Clinical Trials (IMPAACT) P1097 protocol demonstrated that raltegravir readily crosses the placenta and that elimination of transplacentally acquired raltegravir in infants whose mothers received raltegravir during pregnancy is highly variable and prolonged.2 Raltegravir is metabolized by uridine diphosphate glucuronosyltransferase (UGT) 1A1, whose activity is known to be extremely low immediately after birth followed by a dramatic increase over the first weeks of life.3,4 Raltegravir and bilirubin are both metabolized by UGT1A1 and compete for albumin binding sites.5 Neonatal plasma raltegravir concentrations that exceed typical peak raltegravir concentrations of 4500 ng/mL by 50–100 fold could displace sufficient unconjugated bilirubin from albumin to lead to bilirubin induced neurologic dysfunction, including kernicterus, as was seen with sulfisoxazole.5,6
While traditional phase I pharmacokinetic and safety studies are difficult to conduct in neonates, population pharmacokinetic modeling and simulations can be used to inform selection of initial dosing regimens for study in this vulnerable population, improving study safety and efficiency. In this study, an adaptive design with population pharmacokinetic modeling and simulations was used to determine an appropriate raltegravir dose for use in neonates and assess the safety of the dosing regimen selected.
Methods
IMPAACT P1110 is a phase 1, multicenter non-comparative dose-finding study of raltegravir oral granules for suspension in infants exposed to HIV-1 infection. A two-cohort adaptive design was utilized where pharmacokinetic data from infants in cohort 1 who received two singles doses of raltegravir were included in population modeling and simulations to guide selection of a daily raltegravir dose for infants in cohort 2. Full-term infants weighing at least 2 kg at birth and born to mothers living with HIV were eligible to enroll. All infants received raltegravir oral granules for suspension in addition to standard of care antiretroviral agents for prevention of perinatal transmission. Exclusion criteria included elevated bilirubin requiring phototherapy and receipt of disallowed medications including phenytoin, phenobarbital, or rifampin. Local institutional review boards or in-country ethnics committees responsible for oversight of the study granted ethics and regulatory approvals.
Cohort 1 infants received raltegravir administered as a single oral dose within 48 hours of birth followed by a second dose administered at 7– 10 days of life. Raltegravir doses of 3 mg/kg were administered to an initial group of 6 cohort 1 infants whose mothers did not receive raltegravir during pregnancy (raltegravir- naïve). Administration of reduced raltegravir doses to subsequent groups of cohort 1 infants, both raltegravir- naïve and raltegravir-exposed (infants whose mothers received raltegravir within 2–24 hours before delivery) were allowed based on the raltegravir pharmacokinetic results from the initial group of cohort 1 raltegravir-naïve infants. Pharmacokinetic sampling for cohort 1 infants was performed around the initial dose (pre-dose, then 1–2 hours, 4–8 hours, 12 hours, and 24 hours post-dose, followed by a random sample on day 3–4 of life) and the second dose (pre-dose, then 1–2 hours and 24 hours post-dose).
A daily dosing regimen for cohort 2 infants was selected based on simulations using a population pharmacokinetic model that incorporated raltegravir concentration data from the first 6 cohort 1 infants and from 24 infants and children ages 4 weeks to < 2 years enrolled in IMPAACT P1066, a Phase I/II, multi-center, open-label, non-comparative intensive pharmacokinetic study of raltegravir in infants and children.7,8 Population modeling using PsN/3.7.6, NONMEM/7.3.0 and R/3.1.0 was performed to estimate raltegravir pharmacokinetic parameters, which were then used in simulations of potential daily dosing regimens for evaluation in infants in cohort 2.9 Raltegravir-naïve cohort 2 neonates were started on raltegravir therapy within 48 hours of birth. 9,10 Raltegravir dosing was delayed until 12–60 hours after birth in raltegravir-exposed neonates based on pharmacokinetic modeling and simulations.10 Pharmacokinetic sampling for cohort 2 infants included intensive sampling around the first dose and at two weeks of age with sparse sampling around the time of dose changes according to this schedule: First dose - Pre-dose, 1–2 hours post-dose, 6–10 hours post-dose, and 20–24 hours post-dose; Second dose - 3–6 hours post-dose; Day 6–9 of life - pre-dose; Day 15–18 of life - Pre-dose, 1–2 hours post-dose, 4–6 hours post-dose, 8–12 hours post-dose; Day 28–32 of life - pre-dose; Week 5–6 of life - pre-dose, 3–6 hours post-dose. In cohort 2, the raltegravir-naïve neonate group was fully enrolled prior to enrollment of the raltegravir-exposed neonates.
Raltegravir plasma concentrations were measured using a modified version of a previously published method.11 A simple protein precipitation method using acetonitrile-containing raltegravir internal standard was employed to extract raltegravir from human plasma. The method was fully validated and used isocratic, reverse-phase high-performance liquid chromatography-tandem mass spectrometry on an AB SCIEX 5500. The linear calibration range was 10–10,000 ng/mL from a 10 μL plasma sample.
Pharmacokinetic parameters were estimated for participants in cohorts 1 and 2 using a noncompartmental approach. AUC was estimated using the trapezoidal method as AUC0–24h during once daily dosing and AUC0–12h during twice daily dosing. Ctrough, Cmax and Tmax were determined from inspection of individual raltegravir concentration-time plots. T1/2 was determined as ln(2)/λz, where λz is the elimination rate constant. Oral clearance (CL/F) was determined as dose/AUC. Protocol exposure targets defined from safety and efficacy from studies in older infants, children, and adults were AUC0–24h 12–40 mg*h/L, AUC0–12h 6–20 mg*h/L, Ctrough (C12h or C24h) > 33 ng/mL, and Cmax ≤ 8724 ng/mL.7,12–14 Simulations were used to evaluate potential cohort 2 daily dosing regimens and the regimen that best met the exposure targets for AUC, Ctrough, and Cmax was chosen for evaluation in cohort 2.9,10
The protocol enrollment targets were a minimum of 12 pharmacokinetic evaluable neonates in cohort 1 and 28 pharmacokinetic evaluable neonates (20 raltegravir-naive and 8 raltegravir-exposed) in cohort 2. Pharmacokinetic-evaluable infants were those who were determined by the protocol pharmacologist to provide analyzable data on the primary pharmacokinetic parameters of interest. Participants with unevaluable pharmacokinetic data were excluded from the pharmacokinetic analyses and replaced. Infants were included in the safety analyses if they received at least one dose of raltegravir. Infants were followed with clinical and laboratory safety evaluations through 24 weeks of age. Safety data include death, signs/symptoms, diagnoses and laboratory test results. This includes results of evaluations specified in the protocol and results obtained from laboratory tests performed as part of clinical care. Protocol specified laboratory evaluations included CBC with platelet count, AST, ALT, creatinine, total and direct bilirubin, and HIV nucleic acid test (HIV NAT). Two negative HIV NATs, one collected after the first month of age and the second after 4 months of age were required to determine that an infant was not HIV-infected. Infants who were replaced for pharmacokinetic analysis continued with all study safety follow-up visits and procedures and were included in the safety analyses.
Clinical events and laboratory values were graded according to the DAIDS toxicity table.15 All grade 3 and 4 toxicities were reviewed by the protocol team and followed closely until resolution. The primary safety endpoints were adverse events or suspected adverse drug reactions of grade 3 or 4 severity or infant death. Special management guidelines were provided for total bilirubin exceeding 16.0 mg/dL or need for phototherapy, transfusion therapy or other treatments for hyperbilirubinemia.
To assess genetic polymorphisms that might be associated with elevated bilirubin and raltegravir levels, genetic variants of UGT1A1 (rs5839491), the enzyme primarily responsible for bilirubin and raltegravir metabolism, and SLCO1B3 (rs2117032), an organic acid transporter associated with neonatal hyperbilirubinemia, were evaluated.16–18 Genotyping (optional evaluation for both cohorts) for UGT1A1 and SLCO1B3 polymorphisms was performed on dried blood spots on filter paper that could be obtained with any blood sample. Polymorphisms in UGT1A1 and SLCO1B3 were determined by real-time PCR on DNA extracted from dried blood spots using QIAamp DNA Mini Kit (Qiagen, Valencia, CA). The median raltegravir clearance (CL/F) for the wild type [(TA)6/(TA)6 genotype] versus mutation [heterozygous (TA)6/(TA)7 and (TA)5/(TA)7 or homozygous (TA)5/(TA)5 and (TA)7/(TA)7 genotypes] UGT1A1 groups was analyzed using the Wilcoxon Rank Sum test as previously described.2 Wilcoxon rank sum test was used to compare raltegravir clearance (CL/F) between wild type and mutation UGT1A1 groups for cohort 2 infants.
Results
Cohort 1: Two Single Raltegravir Doses
Sixteen mother-infant pairs enrolled in cohort 1 from participating IMPAACT sites in three countries.(Table 1) There were 10 infants born to mothers who did not receive raltegravir prior to delivery (raltegravir-naïve) and 6 infants born to mothers who received raltegravir prior to and during delivery (raltegravir-exposed). There were 8 females and 8 males with mean (range) gestational age 39 weeks (37–40), birth weight 3.05 kg (2.3–4.20). One infant was excluded from the pharmacokinetic analysis due to a possible switch of collected samples. Evaluable pharmacokinetic results are available for 15 of the16 infants and all were considered evaluable for safety.
Table 1.
Demographics of Infants in Cohorts 1 and 2
| Variable | Cohort 1 RAL-naïve n=10 | Cohort 1 RAL-exposed n=6 | Cohort 2 RAL-naïve n=26 | Cohort 2 RAL-exposed n=10 | Total Cohorts 1 and 2 n=52 |
|---|---|---|---|---|---|
| Sex | |||||
| Male | 4 (40%) | 4 (67%) | 14 (54%) | 6 (60%) | 28 (54%) |
| Female | 6 (60%) | 2 (33%) | 12 (46%) | 4 (40%) | 24 (46%) |
| Race | |||||
| Black or African American | 9 (90%) | 2 (33%) | 18 (69%) | 2 (20%) | 31 (60%) |
| White | 0 (0%) | 2 (33%) | 3 (12%) | 2 (20%) | 7 (13%) |
| Multiracial | 1 (10%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (2%) |
| Other | 0 (0%) | 1 (17%) | 5 (19%) | 2 (20%) | 8 (15%) |
| Unknown | 0 (0%) | 1 (17%) | 0 (0%) | 0 (0%) | 1 (2%) |
| Asian | 0 (0%) | 0 (0%) | 0 (0%) | 4 (40%) | 4 (8%) |
| Ethnicity | |||||
| Hispanic or Latino | 2 (20%) | 3 (50%) | 19 (73%) | 5 (50%) | 29 (56%) |
| Not Hispanic or Latino | 7 (70%) | 2 (33%) | 7 (27%) | 4 (40%) | 20 (38%) |
| More than one ethnicity | 1 (10%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (2%) |
| Unknown | 0 (0%) | 1 (17%) | 0 (0%) | 1 (10%) | 2 (4%) |
| Birth Weight (kg) [mean and range] | 3.14 (2.4–4.2) | 2.90 (2.3–3.4) | 2.95 (2.4–3.7) | 3.04 (2.1–4.1) | 3.00 (2.1–4.2) |
| Gestational age at birth (weeks) [mean and range] | 39 (38–40) | 38 (37–40) | 38 (37–41) | 39 (38–41) | 39 (37–41) |
| Mode of Delivery | |||||
| Spontaneous vaginal | 3 (30%) | 0 (0%) | 5 (19%) | 3 (30%) | 11 (21%) |
| Caesarean | 7 (70%) | 6 (100%) | 21 (81%) | 7 (70%) | 41 (79%) |
| Country | |||||
| USA | 7 (70%) | 4 (67%) | 6 (23%) | 1 (10%) | 18 (35%) |
| Brazil | 2 (20%) | 2 (33%) | 17 (65%) | 4 (40%) | 25 (48%) |
| South Africa | 1 (10%) | 0 (0%) | 3 (12%) | 1 (10%) | 5 (10%) |
| Thailand | 0 (0%) | 0 (0%) | 0 (0%) | 4 (40%) | 4 (7%) |
| Evaluable for Pharmacokinetic Analysis | 9 (90%) | 6 (100%) | 25 (96%) | 10 (100%) | 50 (96%) |
The first 6 raltegravir-naïve infants received 3 mg/kg initial doses. While none of the 6 infants exceeded the Cmax upper limit, four of the 6 infants exceeded the AUC0–24h upper limit of 40 mg*h/L. The initial dose was reduced after interim analysis for subsequent enrollments: raltegravir-naïve infants received 2 mg/kg while raltegravir-exposed infants received 1.5 mg/kg for first dose. All infants regardless of maternal raltegravir usage received 3 mg/kg for the second dose at 7–10 days of life. Raltegravir pharmacokinetic parameters for cohort 1 following initial doses are included in Table 2.
Table 2.
Cohort 1 Raltegravir Pharmacokinetic Parameters After First Dose for Raltegravir-Naive and Raltegravir-Exposed Neonates
| PK Parameter | RAL-Naïve 3 mg/kg Single Dose (N = 6) | RAL-Naïve 2 mg/kg Single Dose (N = 3) | RAL-Exposed 1.5 mg/kg Single Dose (N = 6) | |||
|---|---|---|---|---|---|---|
| Geometric Mean (CV%) | Target | Geometric Mean (CV%) | Target | Geometric Mean (CV%) | Target | |
| AUC0–24h (mg*h/L)a | 53.9 (34.6%) | Met: 2 | 44.3 (71.9%) | Met: 1 | 37.4 (92.7%) | Met: 1 |
| Above: 4 | Above: 2 | Above: 4 | ||||
| Below: 0 | Below: 0 | Below: 1 | ||||
| C24h trough (ng/mL)b | 1444 (63.1%) | Met: 6 | 806 (246.3%) | Met: 3 | 906 (209.5%) | Met: 6 |
| Below: 0 | Below: 0 | Below: 0 | ||||
| Cmax (ng/mL)c | 3361 (35.5%) | Met: 6 | 3405 (38.1%) | Met: 3 | 2189 (73.3%) | Met: 6 |
| Above: 0 | Above: 0 | Above: 0 | ||||
| Tmax (hours) | 6.5 (76.5%) | N/A | 4.4 (4.4%) | N/A | 5.2 (85.9%) | N/A |
| T1/2 (hours) | 11.8 (27.2%) | N/A | 17.2 (107.2%) | N/A | 12.6 (48.7%) | N/A |
AUC0–24h target 12–40 mg*h/L.
C24h trough concentration >33 ng/mL.
Cmax <8724 ng/mL.
Key to Acronyms: AUC = area under the curve; Cmax = maximum concentration; CV = coefficient of variation; PK = pharmacokinetic; RAL = raltegravir; T1/2 = half-life; Tmax = time to reach maximum concentration
Cohort 1 infants also received zidovudine (n=15), nevirapine (n=11), lamivudine (n=6), nelfinavir (n=2) or lopinavir/ritonavir (n=1). All cohort 1 infants received 2 single doses of raltegravir. Raltegravir was well tolerated. None of the infants died, or had total bilirubin exceeding 16.0 mg/dL, or received phototherapy, transfusion therapy or other therapies for hyperbilirubinemia. Four (25%) cohort 1 infants had Grade 3 or 4 adverse events: Grade 4 decreased ANC (N=1), Grade 3 neonatal hypertension (N=1), Grade 3 decreased blood glucose (N=1), and Grade 4 anemia (N=1). The Grade 4 decreased ANC, which resolved to Grade 1 five weeks later, was thought to be possibly treatment related to raltegravir. None of the other adverse events was thought to be related to raltegravir.
Cohort 2: Daily Raltegravir Dosing Through 6 Weeks of Life
Thirty-six mother-infant pairs (26 raltegravir-naïve and 10 raltegravir-exposed) enrolled in cohort 2 from participating IMPAACT sites in 4 countries. (Table 1) There were 20 males and 16 females with mean (range) gestational age 39 weeks (37–41), birth weight 2.98 kg (2.1–4.1). One raltegravir-naïve infant received one dose of raltegravir followed by the mother withdrawing consent on the day of enrollment after the first 2 pharmacokinetic samples were obtained. Raltegravir was prematurely discontinued in 2 other infants: 1 infant due to weight loss later assessed as not related to raltegravir and 1 infant due to parental concern about infant blood sampling. Cohort 2 infants also received nevirapine (n=28), zidovudine (n=33), and lamivudine (n=4).
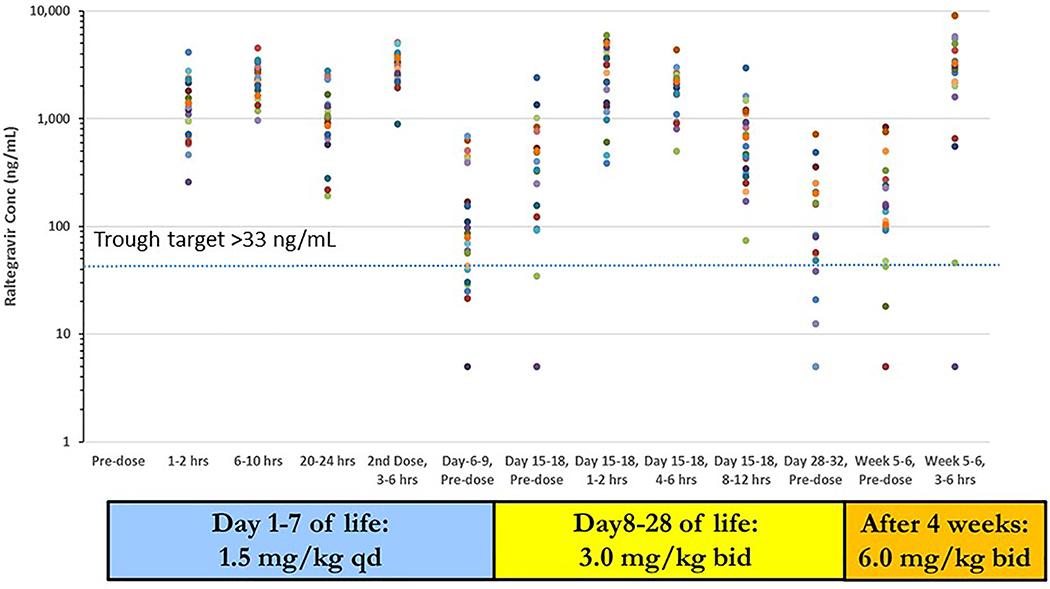
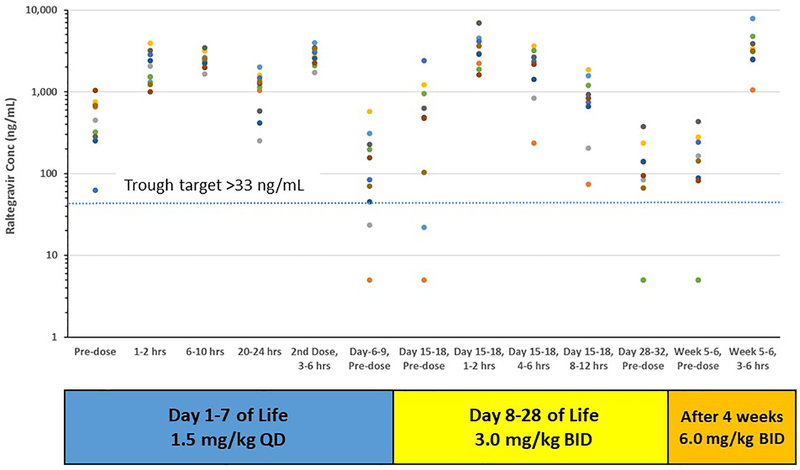
The raltegravir daily dosing regimen selected for evaluation in cohort 2 was 1.5 mg/kg once daily through day 7; 3 mg/kg twice daily from days 8 to 28 of life; 6 mg/kg twice daily after 4 weeks of age.9,10 Raltegravir- naïve infants were first dosed at a median of 36.5 hours of age and raltegravir- exposed infants were first dosed at a median of 24.4 hours of age. Figure 1 (raltegravir-naïve infants) and Figure 2 (raltegravir-exposed infants) present the raltegravir concentration data over the 6 weeks of study. Raltegravir pharmacokinetic parameters estimated during the two intensive sampling visits for cohort 2 raltegravir-naïve and raltegravir-exposed infants are presented in Table 3.
Figure 1:
Raltegravir concentrations for raltegravir-naïve cohort 2 infants plotted against collection time. Plasma concentration vs. time points for each infant are represented with a different color. Dotted line represents trough concentration target of 33 ng/mL. Concentrations below the lower limit of quantitation (10 ng/mL) are plotted at 5 ng/mL.
Figure 2:
Raltegravir concentrations for raltegravir-exposed cohort 2 infants plotted against collection time. Plasma concentration vs. time points for each infant are represented with a different color. Dotted line represents trough concentration target of 33 ng/mL. Concentrations below the lower limit of quantitation (10 ng/mL) are plotted at 5 ng/mL.
Table 3.
Cohort 2 Raltegravir Pharmacokinetic Parameters for Raltegravir-Naive and Raltegravir-Exposed Neonates
| PK Parameter | Initial Dose: 1.5 mg/kg Once Daily RAL-Naive (N = 25)d | Initial Dose: 1.5 mg/kg Once Daily RAL-Exposed (N = 10) | Days 15–18: 3.0 mg/kg Twice Daily RAL-Naive (N = 24)e | Days 15–18: 3.0 mg/kg Twice Daily RAL-Exposed (N = 10)f | ||||
|---|---|---|---|---|---|---|---|---|
| Geometric Mean (CV%) | Target | Geometric Mean (CV%) | Target | Geometric Mean (CV%) | Target | Geometric Mean (CV%) | Target | |
| AUC0–24h (mg*h/L)a | 38.2 (42.0%) | Met: 13 | 42.9 (25.3%) | Met: 4 | --------- | ------------- | --------- | ------------ |
| Above: 11 | Above: 6 | |||||||
| Below: 0 | Below: 0 | |||||||
| AUC0–12h (mg*h/L) | ------- | --------- | --------- | --------- | 14.3 (49.5%) | Met: 14 | 18.3 (62.8%) | Met: 3 |
| Above: 8 | Above: 5 | |||||||
| Below: 1 | Below: 1 | |||||||
| Ctrough (ng/mL)b | 948 (84.0%) | Met: 25 | 946 (74.0%) | Met: 10 | 176 (162.1%) | Met: 22 | 274 (176.4%) | Met: 8 |
| Below: 0 | Below: 0 | Below: 2 | Below: 2 | |||||
| Cmax(ng/mL)c | 2350 (36.5%) | Met: 25 | 2565 (23.1%) | Met: 10 | 2849 (47.5%) | Met: 24 | 3667 (46.3%) | Met: 9 |
| Above: 0 | Above: 0 | Above: 0 | Above: 0 | |||||
| Tmax (hours) | 5.4 (71.5%) | N/A | 3.8 (88.8%) | N/A | 2.3 (77.1%) | N/A | 1.9 (52.3%) | N/A |
| T1/2 (hours) | 15.8 (101.4%) | N/A | 14.4 (69.5%) | N/A | 2.5 (34.1%) | N/A | 2.9 (20.7%) | N/A |
AUC targets: AUC0–24h 12–40 mg*h/L and AUC0–12h 6–20 mg*h/L.
Ctrough concentration >33 ng/mL. Note: For initial dose, Clast collected at 24 hours was used. For Days 15–18, C12 was estimated when the 12 hours post dose sample was collected earlier than 12 hours (protocol specified sample collection time of 8–12 hours post dose)
Cmax <8724 ng/mL.
AUC0–24h could not be estimated for one infant.
AUC0–12h could not be estimated for one infant with delayed absorption.
AUC0–12h and Cmax could not be estimated for one infant with incomplete sample collection.
Key to Acronyms: AUC = area under the curve; Cmax = maximum concentration; CV = coefficient of variation; PK = pharmacokinetic; RAL = raltegravir; T1/2 = half-life; Tmax = time to reach maximum concentration
After the initial dose of 1.5 mg/kg, the geometric mean pharmacokinetic target AUC0–24h of 12–40 mg*h/L for both raltegravir-naïve and raltegravir-exposed infants was met or exceeded in all infants. All infants met the Ctrough target of > 33 ng/mL and Cmax target of < 8724 ng/mL after the initial dose. On day 15–18 at the second intensive pharmacokinetic sampling on 3 mg/kg twice daily dosing, the estimated AUC0–12h met or exceeded the geometric mean pharmacokinetic target of 6–20 mg*h/L in 30 of 32 infants. The Cmax target was met in all infants and 30 of 34 (88%) met the Ctrough target. After an observed 6 mg/kg dose was administered at 5–6 weeks of age, all but 2 infants met the Cmax target. The raltegravir plasma concentrations observed in those infants were slightly above the Cmax at 9067 ng/mL (3 hours post-dose) and 9009 ng/mL (4 hours post-dose).
Pre-dose plasma concentrations were obtained at the time of dose increases at one week of age and 4 weeks of age. For samples collected at one week of age, 26 of 33 (79%) were above the Ctrough target. At one month of age, 23 of 33 (70%) were above the Ctrough target.
For infants exposed to raltegravir in utero, the initial dose of raltegravir was administered 12–60 hours after delivery. The pre-dose raltegravir concentrations ranged from 63–1045 ng/mL and none of the 10 infants had excessive raltegravir concentrations after initial dosing. (Figure 2) While there was a trend to higher AUC0–24h in the raltegravir-exposed infants compared to raltegravir-naïve infants, there were no significant differences observed in pharmacokinetic parameters between these two groups of infants. (Table 3)
Thirty-five of the 36 cohort 2 infants were included in the safety analyses. Raltegravir was well tolerated in these infants. Of the 35 infants included in the safety analyses, 11(31%) and 15 (43%) had Grade 3 or 4 adverse events observed through 6 and 24 weeks of life, respectively. The most common Grade 3 or 4 adverse events were decreased hemoglobin (n=4), decreased absolute neutrophil count (n=4), increased bilirubin (n=3) and dyspnea (n=2). All infant adverse events were assessed as not related to raltegravir. There were no significant clinical events, including infant death, total bilirubin exceeding 16.0 mg/dL, or receipt of phototherapy, transfusion therapy or other therapies for hyperbilirubinemia. There were no infants in either cohort diagnosed with HIV-1 infection during the conduct of the study.
Genotype Results for Cohorts 1 and 2
Genotyping consent was obtained for 14 infants enrolled in cohort 1. Of the 14 infants with UGT1A1 genotyping, 6 infants (43%) were (TA)6/(TA)6 homozygotes, 7 (50%) were (TA)6/(TA)7 heterozygotes, 1(7 %) was a (TA)5/(TA)6; CL/F was available for 13 of these infants. There were no significant differences in median raltegravir clearance (CL/F) for the (TA)6/(TA)6 versus (TA)6/(TA)7 genotypes. SLCO1B3 results for infants in cohort 1: 8 (62%) C/C and 5 (38%) C/T.
Among cohort 2 infants, genotyping consent was obtained for 29 infants. Results for both raltegravir-naïve and raltegravir-exposed were combined for the analyses. There were 15 infants (52%) who were (TA)6/(TA)6 normal homozygotes; 14 (48%) had UGT1A1 mutations including 8 (28%) were (TA)6/(TA)7 heterozygotes, 3 (10 %) were ((TA)5/(TA)5, 1 (3%) were (TA)5/(TA)6, 1 (3%) was (TA)5/(TA)7, and 1 (3.%) was (TA)7/(TA)7. Among cohort 2 infants, SLCO1B3 results included 12 (41%) C/C, 9 (31%) C/T, and 8 (28%) T/T. No significant difference was observed in median raltegravir clearance (CL/F) between wild type and mutation UGT1A1 groups (p-values of 0.37 and 0.98 for Cohort 2 infants at first raltegravir dosing and at 2 weeks of life, respectively). Overall median (range) raltegravir clearances were 0.1(0, 0.2) L/hr and 0.5(0.3, 1.9) L/hr at first raltegravir dosing and at 2 weeks of life, respectively. No cohort 1 or cohort 2 infants with genotype data experienced hyperbilirubinemia and sample size was insufficient to allow an assessment of the relationship between UGT1A1 and SLCO1B3 genotypes and bilirubin concentrations.
Discussion
The study of neonatal raltegravir pharmacology poses special challenges due to the rapid changes in raltegravir metabolism in the first weeks of life and the potential for serious toxicity with overdosing during this period. An adaptive design with population analysis and simulations was used to safely but efficiently delineate neonatal raltegravir pharmacokinetics and determine an age-appropriate dosing regimen for full-term neonates. The study enrolled an initial cohort of infants who received two single doses of raltegravir in the first 48 hours after birth and at 7–10 days of life with pharmacokinetic sampling. This provided data describing raltegravir disposition in neonates while not exposing these infants to the risks of accumulation of potentially toxic raltegravir exposures. The data from these infants was pooled with data from older infants for population analysis, followed by simulations of potential daily dosing regimens for use in the first 6 weeks of life.9,10 The regimen that best met the pharmacokinetic targets was chosen for investigation in a second cohort of infants who received daily dosing of raltegravir through 6 weeks of life. Geometric mean protocol exposure targets for AUC, Ctrough and Cmax were met in these infants. In some individuals, AUC following the initial dose or a subsequent dose was slightly above the target range, but these elevations were well below potentially dangerous concentrations and will be transient given the rapid increase in raltegravir metabolism during the first weeks of life. The chosen neonatal raltegravir dosing regimen was safe and well tolerated in full-term neonates during treatment over the first 6 weeks of life and follow-up to age 24 weeks. This regimen was developed by studying a relatively small number of neonates and avoiding the risks associated with administration of excessive raltegravir doses.9 When the population PK model was updated to include the additional cohort 1 data, no modifications to the selected daily dosing regimen were necessary.
Pharmacokinetic studies in adults and older children have demonstrated considerable intrasubject and intersubject variability in PK parameters. Current PK targets for Ctrough are based on results from a clinical trial in adults (QDMRK) in which treatment-naive patients with HIV were randomized to receive raltegravir 800 mg once daily or raltegravir 400 mg twice daily. After 48 weeks of treatment, the percentage of patients who achieved HIV RNA viral loads <50 copies/mL was 83% in the once-daily group compared to 89% in the twice-daily group. Patients in the once-daily arm with Ctrough concentrations below 45 nM (20 ng/mL) were at the greatest risk of experiencing treatment failure.8,14 Overall drug exposures were similar in both groups, but the association between higher risk of treatment failure and lower Ctrough concentrations suggests that maintaining raltegravir trough plasma concentrations above 45 nM (20 ng/mL) is important for efficacy.8,14 The Ctrough target of > 33 ng/mL was chosen to be above that associated with efficacy from the adult QDMRK study. While there were several infants with trough concentrations below the target at the time of dose changes (end of week 1 and week 4), this is unavoidable in a population in which there is such a rapid increase in drug metabolism. An 8-fold increase in raltegravir total daily dose is required over the first 4 weeks of life to maintain adequate drug concentrations.
Raltegravir is metabolized by UGT1A1, which also metabolizes bilirubin and is known to have low activity immediately at birth followed by a rapid increase during the first weeks of life.3,4,9 An in vitro study demonstrated that raltegravir competes with bilirubin for albumin binding sites and that plasma raltegravir concentrations that greatly exceed those needed to treat HIV infection pose a risk of displacement of unconjugated bilirubin from albumin leading to bilirubin induced neurologic dysfunction.5 The recommended raltegravir dosing regimen for full-term neonates reflects the rapid maturation of UGT1A1 activity in the weeks after birth. The dosing regimen incorporates a large increase in the daily raltegravir dose from 1.5 mg/kg once daily in the first week of life to 6 mg/kg twice daily at 4 weeks of life. Despite the eight-fold increase in the total daily dose from 1.5 mg/kg to 12 mg/kg per day over this short period of time, raltegravir exposures remained relatively constant and well below potentially neurotoxic levels, demonstrating that the increase in total daily dose incorporated in our regimen matched the typical neonatal postnatal increase in UGT1A1 activity. In the current study, no infants experienced hyperbilirubinemia (defined as total bilirubin exceeding 16.0 mg/dL, or received phototherapy, transfusion therapy or other therapies for hyperbilirubinemia) so we could not assess the relationship between UGT1A1 and SLCO1B3 genetic variants and hyperbilirubinemia in our population. We observed no relationship between UGT1A1 genotype and raltegravir clearance, suggesting that genetic variability in the activity of this metabolic pathway does not impact raltegravir clearance in neonates.
The regimen we developed is complicated and poses challenges for implementation in clinical settings, but this level of complexity is required during this period of rapid change in UGT1A1 activity to balance the need to maintain adequate raltegravir exposure to suppress HIV replication while avoiding accumulation of potentially toxic raltegravir plasma concentrations. Acceptability and feasibility of oral granules for suspension in low resource setting has recently been studied in a family clinic in South Africa. With proper training by health care personnel, caregivers were able to prepare the suspension safely and accurately. 19
In November 2017, the study data and analyses formed the basis for approval by the FDA of the use of raltegravir oral granules for suspension in HIV-1 exposed full-term neonates from birth to 4 weeks of age.20 This approval was the first of a new antiretroviral for use in neonates since emtricitabine in 2006. This approval is limited to full term neonates. Premature infants have lower levels of UGT1A1 activity at birth with a slower rate of increase and are more susceptible to bilirubin neurotoxicity, suggesting that the raltegravir regimen studied in these full-term infants is likely to be excessive and potentially dangerous in preterm neonates. This hypothesis is supported by several case reports of delayed clearance of raltegravir in premature neonates.21–23
Conclusions
Raltegravir oral granules for suspension can be safely administered to full-term infants using the following dosing regimen: 1.5 mg/kg daily from birth through Day 7; 3 mg/kg twice daily from Days 8 to 28 of life; 6 mg/kg twice daily after 4 weeks of age through 6 weeks of age. For infants born to mothers who received raltegravir 2–24 hours prior to delivery, the neonate’s first dose should be delayed until 24–48 hours after birth. This regimen is not recommended for use in premature neonates.
Acknowledgments
Conflicts of Interest and Source of Funding
AC and HT are employees of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA (MSD) [license holder Isentress®]. JL is a paid consultant for Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA (MSD). MM has research support from Gilead Sciences, Merck and Co., and ViiV Healthcare. DC and EA have research support from Merck and Co. and ViiV Healthcare. For the remaining authors none were declared.
Overall support for the International Maternal Pediatric Adolescent AIDS Clinical Trials Network (IMPAACT) was provided by the National Institute of Allergy and Infectious Diseases (NIAID) with co-funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) and the National Institute of Mental Health (NIMH), all components of the National Institutes of Health (NIH), under Award Numbers UM1AI068632 (IMPAACT LOC), UM1AI068616 (IMPAACT SDMC) and UM1AI106716 (IMPAACT LC), and by NICHD contract number HHSN275201800001I. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
References
- 1.Panel on Antiretroviral Therapy and Medical Management of Children Living with HIV. Guidelines for the Use of Antiretroviral Agents in Pediatric HIV Infection. Available at http://aidsinfo.nih.gov/contentfiles/lvguidelines/pediatricguidelines.pdf. Accessed (November 18, 2018).
- 2.Clarke DF, Acosta EP, Rizk ML, et al. Raltegravir pharmacokinetics in neonates following maternal dosing. Journal of Acquired Immune Deficiency Syndromes. November 1 2014;67(3):310–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kawade N, Onishi S. The prenatal and postnatal development of UDP-glucuronyltransferase activity towards bilirubin and the effect of premature birth on this activity in the human liver. Biochem J. April 15 1981;196(1):257–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krekels EH, Danhof M, Tibboel D, Knibbe CA. Ontogeny of hepatic glucuronidation; methods and results. Curr Drug Metab. July 2012;13(6):728–743. [DOI] [PubMed] [Google Scholar]
- 5.Clarke DF, Wong RJ, Wenning L, Stevenson DK, Mirochnick M. Raltegravir in vitro effect on bilirubin binding. Pediatr Infect Dis J. September 2013;32(9):978–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andersen DH, Blanc WA, Crozier DN, Silverman WA. A difference in mortality rate and incidence of kernicterus among premature infants allotted to two prophylactic antibacterial regimens. Pediatrics. October 1956;18(4):614–625. [PubMed] [Google Scholar]
- 7.Nachman S, Alvero C, Acosta EP, et al. Pharmacokinetics and 48-Week Safety and Efficacy of Raltegravir for Oral Suspension in Human Immunodeficiency Virus Type-1-Infected Children 4 Weeks to 2 Years of Age. Journal of the Pediatric Infectious Diseases Society. December 2015;4(4):e76–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rizk ML, Du L, Bennetto-Hood C, et al. Population pharmacokinetic analysis of raltegravir pediatric formulations in HIV-infected children 4 weeks to 18 years of age. J Clin Pharmacol. July 2015;55(7):748–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clarke DF, Mirochnick M, Acosta EP, et al. Use of Modeling and Simulations to Determine Raltegravir Dosing in Neonates: A Model for Safely and Efficiently Determining Appropriate Neonatal Dosing Regimens: IMPAACT P1110. Journal of Acquired Immune Deficiency Syndromes. December 1 2019;82(4):392–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lommerse J, Clarke D, Kerbusch T, et al. Maternal-Neonatal Raltegravir Population Pharmacokinetics Modeling: Implications for Initial Neonatal Dosing. CPT Pharmacometrics Syst Pharmacol. September 2019;8(9):643–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Long MC, Bennetto-Hood C, Acosta EP. A sensitive HPLC-MS-MS method for the determination of raltegravir in human plasma. J Chromatogr B Analyt Technol Biomed Life Sci. May 15 2008;867(2):165–171. [DOI] [PubMed] [Google Scholar]
- 12.Nachman S, Zheng N, Acosta EP, et al. Pharmacokinetics, safety, and 48-week efficacy of oral raltegravir in HIV-1-infected children aged 2 through 18 years. Clin Infect Dis. February 2014;58(3):413–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Steigbigel RT, Cooper DA, Teppler H, et al. Long-term efficacy and safety of Raltegravir combined with optimized background therapy in treatment-experienced patients with drug-resistant HIV infection: week 96 results of the BENCHMRK 1 and 2 Phase III trials. Clin Infect Dis. February 15 2010;50(4):605–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rizk ML, Hang Y, Luo WL, et al. Pharmacokinetics and pharmacodynamics of once-daily versus twice-daily raltegravir in treatment-naive HIV-infected patients. Antimicrob Agents Chemother. June 2012;56(6):3101–3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Division of AIDS Table for Grading the Severity of Adult and Pediatric Adverse Events Version 1.0, December, 2004; Clarification August 2009 Accessed November 17, 2019 at https://rsc.niaid.nih.gov/sites/default/files/table-for-grading-severity-of-adult-pediatric-adverse-events.pdf.
- 16.Yang H, Wang Q, Zheng L, et al. Multiple Genetic Modifiers of Bilirubin Metabolism Involvement in Significant Neonatal Hyperbilirubinemia in Patients of Chinese Descent. PLoS One. 2015;10(7):e0132034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alencastro de Azevedo L, Reverbel da Silveira T, Carvalho CG, Martins de Castro S, Giugliani R, Matte U. UGT1A1, SLCO1B1, and SLCO1B3 polymorphisms vs. neonatal hyperbilirubinemia: is there an association? Pediatr Res. August 2012;72(2):169–173. [DOI] [PubMed] [Google Scholar]
- 18.Yueh MF, Chen S, Nguyen N, Tukey RH. Developmental, Genetic, Dietary, and Xenobiotic Influences on Neonatal Hyperbilirubinemia. Mol Pharmacol. May 2017;91(5):545–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Archary M, Zanoni B, Lallemant M, Suwannaprom P, Clarke D, Penazzato M. Acceptability and feasibility of using raltegravir oral granules for suspension for the treatment of neonates in a low resource setting”. Pediatr Infect Dis J, in press February 2020. [DOI] [PubMed] [Google Scholar]
- 20.Isentress package insert. Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc. Whitehouse Station, NJ 08889, USA: January 2019. [Google Scholar]
- 21.Kreutzwiser D, Sheehan N, Dayneka N, et al. Therapeutic drug monitoring guided raltegravir dosing for prevention of vertical transmission in a premature neonate born to a woman living with perinatally acquired HIV. Antiviral Therapy. 2017;22(6):545–549. [DOI] [PubMed] [Google Scholar]
- 22.Clavel-Osorio C, Cazassus F, Stegmann S, Huc-Anais P, Lecam D, Peytavin G. One-month transplacental pharmacokinetics of raltegravir in a premature newborn after short-course treatment of the HIV-1-infected mother. Antimicrobial Agents and Chemotherapy. December 2013;57(12):6393–6394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hegazi A, Mc Keown D, Doerholt K, Donaghy S, Sadiq ST, Hay P. Raltegravir in the prevention of mother-to-child transmission of HIV-1: effective transplacental transfer and delayed plasma clearance observed in preterm neonates. AIDS. November 28 2012;26(18):2421–2423. [DOI] [PubMed] [Google Scholar]