Abstract
Formation of a pluripotency-specific chromatin network is a critical event in reprogramming somatic cells into pluripotent status. To characterize the regulatory components in this process, we used ‘chromatin RNA in situ reverse transcription sequencing’ (CRIST-seq) to profile RNA components that interact with the pluripotency master gene Oct4. Using this approach, we identified a novel nuclear lncRNA Oplr16 that was closely involved in the initiation of reprogramming. Oplr16 not only interacted with the Oct4 promoter and regulated its activity, but it was also specifically activated during reprogramming to pluripotency. Active expression of Oplr16 was required for optimal maintenance of pluripotency in embryonic stem cells. Oplr16 was also able to enhance reprogramming of fibroblasts into pluripotent cells. RNA reverse transcription-associated trap sequencing (RAT-seq) indicated that Oplr16 interacted with multiple target genes related to stem cell self-renewal. Of note, Oplr16 utilized its 3′-fragment to recruit the chromatin factor SMC1 to orchestrate pluripotency-specific intrachromosomal looping. After binding to the Oct4 promoter, Oplr16 recruited TET2 to induce DNA demethylation and activate Oct4 in fibroblasts, leading to enhanced reprogramming. These data suggest that Oplr16 may act as a pivotal chromatin factor to control stem cell fate by modulating chromatin architecture and DNA demethylation.
INTRODUCTION
Somatic cells can be reprogrammed by defined factors to achieve plasticity and ultimately to convert into induced pluripotent stem cells (iPSCs) (1). During the initiation of this reprogramming process, 3D chromatin matrixes surrounding key stemness genes must be remodeled to form a specific pluripotency-specific architecture in chromosome territories, topologically associated domains and chromatin loops (2–5). These interactions require juxtaposition of DNAs from different domains within a chromosome or from different chromosomes. Physical intrachromosomal interactions between gene promoters and distal enhancer elements of stemness genes play an important role in the transcriptional regulation of these genes, leading to the initiation of pluripotency (6–8). These promoter-enhancer loops are largely organized by mediator and cohesion protein complexes (9). However, it is not clear how mediator and cohesion protein complexes are guided to stemness gene loci to form the pluripotency-specific intrachromosomal looping. Identification of factors that regulate these 3-dimensional structures might improve the efficiency of the reprogramming process.
In addition to chromatin factors, long noncoding RNAs (lncRNAs) have recently been shown to play a major role in gene regulation (10,11). LncRNA are defined as non-coding transcripts that are longer than 200 nucleotides and transcribed by RNA polymerase II; they are frequently 5′-capped, spliced and polyadenylated (12). Some lncRNAs, such as Kcvq1ot1, regulate their target genes by participating in the formation of long distance intrachromosomal loops (13). LncRNAs can regulate the transcriptome of core pluripotency transcription factors (14–18) through epigenetic, transcriptional, and post-transcriptional mechanisms (19). In some cases, lncRNAs may be necessary for the formation of iPSCs from somatic cells (20). It would be important to learn if lncRNAs promote pluripotent reprogramming by controlling the formation of promoter-enhancer chromatin loops in pluripotency-related gene domains.
In this study, we explored the role of lncRNAs in the conversion of fibroblasts into iPSCs. By utilizing a combined ‘chromatin RNA in situ reverse transcription sequencing’ (CRIST-seq) (21) and RAT-seq (22) strategy, we identified Oplr16 (Oct4promoter-interacting long noncoding RNA 16) as a novel nuclear-genome derived lncRNA that is associated with somatic cell reprogramming. Oplr16 epigenetically induces the activation of stem cell core factors by coordinating intrachromosomal looping and recruitment of DNA demethylase TET2. This study highlights the potential role of Oplr16 in the enhancement of reprogramming for regenerative medicine.
MATERIALS AND METHODS
Cell reprogramming
Fibroblasts cultured in six-well plates were infected with Oct4-Sox2-Klf4-c-Myc (OSKM) lentivirus with polybrene (8 μg/ml). Three days later, the cells were collected and transferred on mitomycin C-inactivated MEF feeder cells. The media were replaced with ES medium (DMEM high glucose, 10% FBS, 10% KSR, 1% Glutamax, 1% sodium pyruvate, 1% non-essential amino acids, 0.1% β-mercaptoethanol, 1000 U/ml LIF, 2 μg/ml doxycycline) (23). Both iPSCs and un-reprogrammed cells were collected for further studies (6,24).
RNA-seq to identify differentially expressed lncRNAs in reprogramming
Total RNA was isolated from fibroblasts and iPSCs (6,25) using TRIzol (Invitrogen, Carlsbad, CA, USA). The indexed libraries were prepared using Illumina's TruSeq RNA Sample Prep Kit v2. Paired-end sequencing was performed by Shanghai Biotechnology (Shanghai, PRC) using a HiSeq4000 (Illumina). RNA-seq yielded 145 million raw reads for iPSC and 148 million raw reads for fibroblasts. After Seqtk filtering, a total of 120 million clean reads for mRNAs and 124 million clean reads of lncRNAs were mapped to the mouse genome (genome version: mm10, GRCm38.p4 (ftp://ftp.ensembl.org/pub/release-83/fasta/mus_musculus/dna/Mus_musculus.GRCm38.dna.primary_assembly.fa.gz) using the STAR software (26). Gene counts were normalized to the values of reads per kilobase of transcript per Million mapped reads (RPKM). Cuffdiff was used to calculate the differentially expressed RNAs when the fold-change was >2 and P < 0.05 with an unpaired two-sided t-test (22,27).
Cas9-gRNA guided Oct4 chromatin immunoprecipitation.
A Cas9-guided chromatin immunoprecipitation assay (CRIST-seq) (21) was modified to identify lncRNAs that bind to the Oct4 promoter. The Cas9-Oct4 gRNA vector was constructed by cloning two Oct4 promoter gRNAs (Supplementary Table S1) into the lentiCRISPR-EGFP sgRNA 2 vector (Addgene Plasmid #51761). iPSCs were transfected with the Cas9-gRNAs lentiviruses. After selection by puromycin, cells were collected for immunoprecipitation (28,29). To assay the Oct4 promoter-interacting lncRNA, cells were cross-linked and lysed. Nuclei were collected and reverse transcribed with biotin dNTP (10 mM dNTP with 1:20 biotin-dCTP). After nuclear lysis, the chromatin complex was subjected to sonication, and then the biotinylated lnc-cDNA/Cas9 complex was immunoprecipitated with anti FLAG-Cas9 antibody (F1804, Sigma, MO, USA). After cross-link reversal and proteinase K treatment, the biotin-labeled lnc-cDNAs were further purified from genomic DNAs with M-280 streptavidin beads (Invitrogen 11205D). Second strand cDNA was synthesized by using Stratagene cDNA Synthesis kit (Agilent Technologies, CA, USA) for Illumina lnc-cDNA sequencing. The double-stranded cDNAs were digested by MboI (Thermo Scientific FD0814) and ligated with the NEBNext adaptors (NEBNext® ChIP-seq Library Prep Master Mix Set for Illumina). The library DNAs were subject to Illumina sequencing (Shanghai Biotechnology, Shanghai).
We used a random gRNA (gCT) as control and constructed the control library for sequencing using the same protocol. Meanwhile, we used an anti-IgG antibody as the background control for immunoprecipitation (27).
RNA Extraction and RT-PCR
Total RNA was isolated using TRIzol reagent (Sigma, MO, USA), and then M-MLV (Invitrogen 28025) was used to synthesis cDNA. The RT-PCR reaction was performed with a Bio-Rad Thermol Cycler. All primers utilized are listed in Supplementary Table S1. RT-PCR analyses were performed using FastStart Universal SYBR Green Master (ROX) (Roche 04913914001) on the ABI PRISM 7900HT Sequence Detection System (6,30). Target amplification was performed by PCR of 1 cycle at 95°C for 5 min, 33 cycles at 95°C for 20 s, 62°C for 15 s and 72°C for 15 s and 1 cycle at 72°C for 10 min. The threshold cycle (Ct) values of target genes were assessed and were normalized over the Ct of the β-ACTIN control.
RNA FISH
The RNA FISH protocol was modified and performed as previously described (31,32). Briefly, the probe for RNA FISH was prepared as antisense single strand DNA (ssDNA) by asymmetric PCR and labeled with digoxygenin. After purification, 0.1 μg ssDNA probe and 10 μg salmon sperm DNA (Boehringer, Meylan, France) were precipitated and suspended in 10 μl RNA hybridization buffer (2× SSC, 10% dextran sulfate, 0.2 mg/ml BSA (Invitrogen, CA, USA), 2 mM VCR, 10% formamide). Hybridization was carried out in a moist chamber at 37°C for 12–16 h. The cells were incubated with anti-digoxygenin (Sigma, 11207741910) for 4 h at room temperature. Cells were washed by PBS three times and incubated in DAPI for 5 min at room temperature. Cells were observed with an inverted fluorescence phase contrast microscope (KEYENCE BZ-X710).
Embryoid body differentiation
iPSCs were pre-plated to remove feeder cells, and then diluted to 100 000 cells/ml in the medium without LIF. Cells were adherent to the lid of the Petri dish, while the bottom of the dish is filled with PBS, then the lid was reversed and placed over the bottom. Eight dishes were prepared and cells on two dishes were collected every other day and used for gene expression analysis using quantitative PCR.
Knockdown of Oplr16 lncRNA
Four shRNAs were cloned into two separate pGreenPuro vectors (#SI505A-1, SBI, CA) to knock down Oplr16 (Supplementary Table S1). Vector shOplr16-1 contained shRNAs #1 and #3, and vector shOplr16-2 carried shRNAs #2 and #4. The shRNAs were under the control of the H1 and U6 promoters, separately, in each vector. After lentiviral infection, E14 cells and iPSCs were selected by puromycin and tracked by copGFP reporter in the lentiviral vector. Two random shRNAs (5′-GCAGCAACTGGACACGTGATCTTAA-3′ and 5′- TGAAATGTACTGCGCGTGGAGACTA-3′) were cloned in the same vector to construct the assay control (shCT). The copGFP-positive cells were isolated for quantitative PCR.
The Oct4 promoter-luciferase assay
A DNA fragment of the Oct4 promoter and part of exon 1 was amplified by PCR, and cloned into a pGL3 vector. HEK293T cells were transfected with a mixture of pOct4-luciferase plasmid, pRL-TK-renilla-luciferase plasmid and Oplr16 lentiviral expression plasmid using Lipofectamine 3000 (Invitrogen, CA, USA). The empty lentiviral vector was used as control. After 48 h, cells were collected. Dual-Luciferase Reporter Assay System was used to measure luciferase activity according to the manufacturer's instructions (Promega E1910). All luciferase assays were repeated three times.
Identification of the Oplr16 target genes by RNA reverse transcription associated trap sequencing (RAT-seq)
To identify the interacting target genes of Oplr16, a RAT-seq assay was modified (33–35). Cells were cross-linked and lysed. Nuclei were suspended and were reverse transcribed with biotin–dNTP. After nuclear lysis, the chromatin complex was subjected to sonication, and the biotinylated lnc-cDNA/chromatin DNA complex was pulled down with M-280 streptavidin beads (Invitrogen 11205D). After cross-linking reversal and proteinase K treatment, genomic DNAs that interact with Oplr16 were extracted and digested by MboI and ligated with the NEBNext adaptors (NEBNext® ChIP-Seq Library Prep Master Mix Set for Illumina) to construct the library. The library DNAs were subjected to Illumina sequencing (Shanghai Biotechnology, Shanghai) (36). For RAT-seq control, we replaced Oplr16 complementary primers with random primers to perform a RAT assay, and using the same protocol, constructed a control library (22).
After RAT sequencing, clean reads were mapped to the mouse genome (genome version: mm10) using the Bowtie (version:0.12.8) software (37). MACS2 (version: 2.1.1) was used to identify the enriched regions of the genome by comparing the RAT-seq peaks to input samples; a q-value of 0.05 was used as the initial cutoff threshold to minimize peak caller bias (38). To reduce the background, the RAT-seq data were further normalized over the peaks of the control RAT-seq data that were generated by using random oligonucleotide primers in the RAT assay. Differential binding analysis was performed with the DiffBind package using parameters of fold change difference ≥2 and P-value < 0.05, with false discovery rate (FDR) <0.1 (22).
Oplr16 promotes DOX-OSKM reprogramming
Full length Oplr16 cDNA was cloned into a pCMV-RsRed-Puro vector, and the construct was confirmed by DNA sequencing. Meanwhile the empty vector (Vector) and vector with random sequence (LncR-CT) were used as controls. The lentiviruses were packaged in H293 cells. OG2 MEFs were transfected with Oplr16 expression and control lentiviruses and selected by puromycin. MEFs were reprogrammed following the method as previously described (39). Briefly, 20 000 lentivirus-transfected MEFs were seeded in 12-well plates and were cultured in KSR iPSC medium (DMEM high glucose, 10% FBS, 10% KSR, 1% Glutamax, 1% sodium pyruvate, 1% non-essential amino acids, 0.1% β-mercaptoethanol, 1000 U/ml LIF, 2 μg/ml doxycycline). The medium was changed every day. After 10 days, the iPSC colonies were immunostained with Rabbit anti-NANOG Antibody (A300-397A, Bethyl, 1:500 dilution). Positive iPSC colonies per field were recorded (24).
Immunohistochemical staining of stem cell markers
Fluorescent Mouse ES/iPS Cell Characterization kit (#SCR077, Millipore, MA) was used to identify the pluripotency of stem cells following the protocol provided by the manufacturer. Fluorescence images were acquired with a Zeiss AxioCam Camera.
Chromosome conformation capture (3C)
As previously reported (40), 1–5 million cells were crosslinked by 2% formaldehyde at room temperature for 10 min, and glycine was then used for quenching. Lysis buffer was used to lyse cells, followed by MboI (NEB, R0147) digestion at 37°C for at least 2 h. T4 DNA ligase (NEB, M0202) was used for ligation. Proteinase K was used to reverse the crosslinks, and DNA was then purified. The primers for 3C PCR were derived from various regions of Oct4 (5′enhancer, promoter and 3′enhancer). The 3C PCR products were inserted into a pJET Vector (Thermo, K1232) for sequencing. By checking the MboI ligation site, intrachromosomal interactions were confirmed. The 3C ligation products were quantified by using FastStart Universal SYBR Green Master (ROX) (Roche 04913914001) on the ABI PRISM 7900HT Sequence Detection System (41).
SMC1-lncRNA in situ transcription trap sequencing assay
To assay the SMC1 protein interacting lncRNA, cells were cross-linked and lysed. Nuclei were collected and reverse transcribed with biotin dNTP. After nuclear lysis, the chromatin complex was subjected to sonication, then the biotinylated lnc-cDNA/SMC1 complex was immunoprecipitated with anti-SMC1 antibody (Abcam #ab9262). After cross-linking reversal and proteinase K treatment, DNAs were released. The biotin-labeled lnc-cDNAs were further purified from genomic DNAs with M-280 streptavidin beads (Invitrogen 11205D). The second strand cDNA was synthesized by using Stratagene cDNA Synthesis kit (Agilent Technologies, CA, USA) for Illumina lnc-cDNA sequencing. The double-stranded cDNAs were digested by MboI (Thermo Scientific FD0814) and ligated with the NEBNext adaptors (NEBNext® ChIP-Seq Library Prep Master Mix Set for Illumina). The library DNAs were subjected to Illumina sequencing (Shanghai Biotechnology, Shanghai).
Validation of the Oplr16–Oct4 interaction by ChIRP
The Oplr16–Oct4 interaction was also validated by a ChIRP assay (Chromatin Isolation by RNA Purification) (42). E14 cells were cross-linked, lysed and sonicated. Three 3′-end biotinylated oligonucleotide DNA probes were hybridized to the Oplr16 lncRNA. The biotinylated probe/Oplr16/chromatin DNA complex was pulled down with streptavidin C1 beads (Invitrogen 65002). The Oplr16-pulldown chromatin DNA was extracted and quantitated by qPCR for the interacted Oct4.
RNA pull-down
RNAs were transcribed by using T7 RNA polymerase (NEB M0251) and labeled with biotin-CTP. Biotinylated RNAs were incubated with iPSC nuclear extracts, then streptavidin agarose beads (Invitrogen 11205D) were added to each binding reaction and further incubated at RT. Beads were washed and boiled in SDS buffer. Then the retrieved proteins were detected by Western blot.
DNA methylation analysis in the gene promoter
Genomic DNAs were extracted from untreated and treated fibroblasts. EZ DNA Methylation-Gold Kit (Zymo D5005) was used to methylate DNAs. Primers for methylation PCR are listed in Supplementary Table S1. 2× BlueStar (Mclab, BSPM-200) was used for methylation PCR. The amplification process was: 1 cycle at 95°C for 10 min, 32 cycles at 95°C for 20 s, 62°C for 15 s and 72°C for 15 s and 1 cycle at 72°C for 5 min. The PCR products were purified and cloned into pJET vector for sequencing as previously described. After bisulfite treatment, unmethylated cytosines were converted to uracils, while the methylated cytosines remain as cytosines (43,44).
Statistical analysis
The experimental data were prepared and expressed as mean ± SD. Data were analyzed using SPSS software (version 16.0; SPSS, Inc., IL, USA). Student's t-test or one-way ANOVA (Bonferroni test) was performed to compare statistical differences among treatment groups. For every sample, two sets of duplicates were averaged for each of three biological replicates to obtain a final n of three for all statistical analyses. Results were considered statistically significant at P < 0.05. Error bars represent the standard deviation.
RESULTS
Identification of Oplr16 as a pluripotent lncRNA by CRIST-seq
A potential pluripotency-associated lncRNA should meet two criteria. First, it should have the ability to regulate stem cell core factor genes, such as Oct4, Sox2 and Nanog. Second, this lncRNA should be differentially expressed in response to the status of pluripotency. Therefore, we proposed to adopt a novel strategy to identify potential lncRNAs that meet these two criteria (Figure 1A). We used a chromatin RNA in situ reverse transcription sequencing (CRIST-seq) approach to determine which lncRNAs interact with the Oct4 promoter (21), a key transcription factor required for reprogramming somatic cells into iPSCs. Then, conventional RNA transcriptome sequencing (RNA-seq) was used to identify differentially-expressed lncRNAs that may be associated with pluripotent reprogramming (22).
Figure 1.

Identification of Oplr16 as a pluripotent lncRNA. (A) Identification of Oplr16 as a pluripotency-associated lncRNA by integrating RNA-seq and CRIST-seq. RNA-seq was used to identify RNAs that are differentially expressed between fibroblasts and iPSCs collected in the process of reprogramming. In CRIST-seq, the Oct4 promoter was targeted by the expression of the Cas9 Oct4-gRNA. LncRNAs interacting with Oct4 promoter were reverse transcribed in situ into cDNA with biotin-dCTP. After immunoprecipitation, biotin-cDNAs were purified by streptavidin beads for Illumina sequencing. Both sets of databases were integrated to identify the pluripotency-associated lncRNA Oplr16. (B) Reactivation of Oplr16 in reprogramming. Fibroblasts were transfected with a Oct4-Sox2-Kilf4-c-Myc (OSKM) lentivirus. Cells were collected at various stages of reprogramming and expression of Oplr16 was measured by qPCR. E14: mouse embryonic pluripotent stem cell line used as a positive control; iPSC: induced pluripotent stem cells; non-iPSC: un-reprogrammed cells that express four OSKM factors, but fail to complete reprogramming; FBC: fibroblasts. β-Actin was used as the PCR control. ** P < 0.01 as compared with FBC and URC. (C) Oplr16 is predominantly located in the nucleus. Cytoplasmic (cyto) and nuclear RNAs were isolated and reverse transcribed into cDNAs. Location of Oplr16 was determined by PCR. β-Actin: cytoplasm control. U6: nuclear control. (D) Confirmation of nuclear location of Oplr16 by RNA-FISH. Digoxygenin labeled Oplr16 probe was prepared and hybridized in iPSCs and fibroblasts (FBC). DAPI: nuclear control.
Using this strategy, we discovered Oplr16 (NONMMUT101010) as a novel lncRNA that meets the above criteria. First, Oplr16 was highly expressed in iPSCs and E14 embryonic stem cells, but there was no expression in fibroblasts and non-iPSCs (Figure 1B, Supplementary Figure S1A). We isolated RNAs from various tissues, including artery, brain, cerebellum, fat, kidney, liver, lung, spleen and heart and found specific expression of Oplr16 only in E14 embryonic stem cells (Supplementary Figure S1B). Second, the CRIST-seq approach showed that Oplr16 specifically interacted with the Oct4 promoter (Supplementary Figure S2). No interaction signal of Oplr16 was detected in the IgG and random control genes.
We also validated this Oplr16-Oct4 interaction using the previously reported ChIRP (Chromatin Isolation by RNA Purification) (Supplementary Figure S3A) assay (42). LncRNA-chromatin complexes were cross-linked in vivo, sonicated, and hybridized with lncRNA-specific biotinylated antisense oligonucleotide probes. After washing, chromatin complexes that interact with the lncRNA were pulled down using magnetic streptavidin beads for qPCR quantitation. Using this approach, we detected an enriched Oplr16–Oct4 interaction signal in the Oplr16-specifc biotinylated probe group (Supplementary Figure S3B). No interaction signals were detected in the random probe group or in the control group.
Oplr16 is a 629 bp lncRNA gene located in the olfactory receptor family gene cluster on mouse chromosome 17 (Supplementary Figure S4). By alignment, an Oplr16 homolog was located at chromosome 6 in rat (Supplementary Figure S5). However, no homologous sequence was matched in the human genome. These data suggest that Oplr16 is a pluripotency-associated lncRNA that is specifically transcribed in mouse pluripotent stem cells, but it is not conserved in the human genome.
In a subcellular fractionation assay, we isolated cytoplasmic and nuclear RNAs. RT-PCR showed that Oplr16 was predominantly located in the nucleus (Figure 1C). An RNA-FISH assay also validated its nuclear localization in iPSCs (Figure 1D). Together, these data suggest that Oplr16 is a mouse nuclear lncRNA that is activated during somatic reprogramming.
Oplr16 is required for the maintenance of stem cell pluripotency
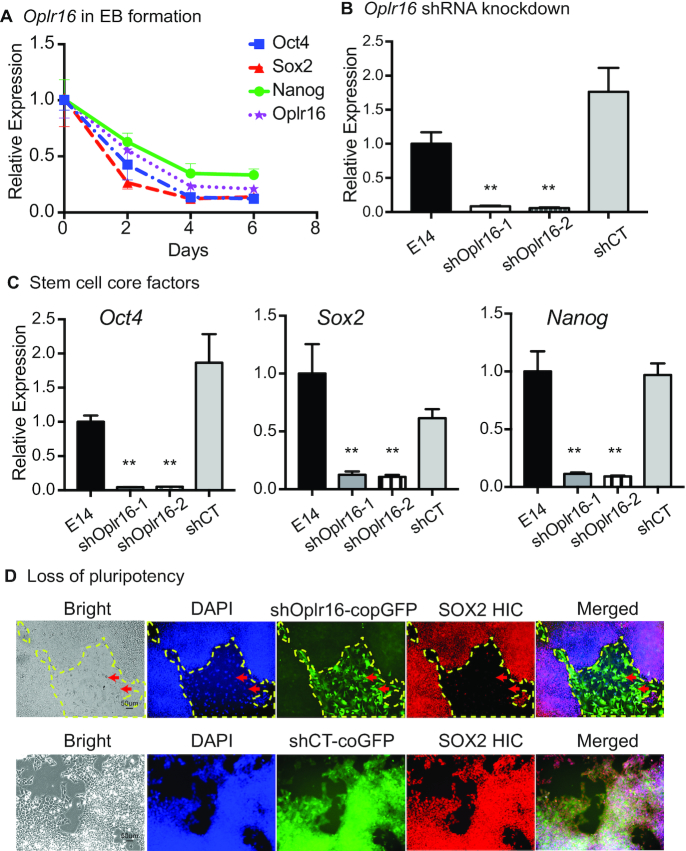
To examine the role of Oplr16 in pluripotency, we collected cells at different days during embryoid body differentiation. Using qPCR, we showed that Oplr16 was positively associated with the status of pluripotency, sharing a similar expression pattern with Oct4, Sox2 and Nanog during embryoid body differentiation (Figure 2A).
Figure 2.
Oplr16 is required for the maintenance of stem cell pluripotency. (A) Expression of Oplr16 decreased during embryoid body differentiation. iPSCs were collected at Days 2, 4, 6 after EB formation and qPCR was used to quantitate the expression of Oplr16 in relation to that of stem cell core factor genes Oct4, Sox2 and Nanog. (B) Knockdown of Oplr16 by shRNAs in E14 cells. shOplr16-1 and shOplr16-2: shRNAs that target Oplr16; shCT: random shRNA control. ** P < 0.01 as compared with iPSC and shCT. (C) Downregulation of stem cell core factor genes Oct4, Sox2 and Nanog by Oplr16 knockdown. After transfection with shOplr16-1 and shOplr16-2 lentiviruses, cells were collected for quantitative PCR. ** P < 0.01 as compared with iPSC and shCT. (D) Knockdown of Oplr16 induces loss of pluripotency. Oplr16 was knocked down by shOplr16 lentiviruses in E14 cells. The shCT lentiviruses were used as control. Lentivirus-transfected cells were tracked by copGFP (green). The pluripotency was examined by histoimmunochemical (HIC) staining of stem cell marker SOX2 (red). ShOplr16-transfected E14 cells showed the loss of pluripotent marker SOX2. Cell morphology was also changed. As the control, the shCT treatment did not alter stem cell pluripotency.
We then studied the function of Oplr16 by knocking down its expression using two separate shRNA lentiviruses (shOplr16-1, shOplr16-2). Each lentiviral vector carried two shRNAs that target Oplr16 (Supplementary Table S1). E14 cells were transfected with shOplr16 lentiviruses. Lentiviruses carrying random shRNAs were used as the control (shCT). After puromycin selection, we collected the copGFP-positive cells for quantitative PCR. Treatment with both lentiviral shRNAs knocked down Oplr16 equally in E14 cells (Figure 2B), leading to a significant downregulation of the three stem cell core factors Oct4, Sox2 and Nanog (Figure 2C).
After Oplr16 knockdown, we examined the pluripotency of transfected cells by histoimmunochemical (HIC) staining of stem cell marker SOX2 (red). We found that the shOplr16-1 transfected cells lost the pluripotent marker SOX2 (Figure 2D, top panels 3–4, copGFP-positive cells). We also observed a change in morphology in the shOplr16-1 transfected cells. The random control shCT-transfected cells did not undergo a change in cellular morphology. After Oplr16 knockdown, E14 cells became very fragile, and they could not proliferate after passage. The cell senescence pathway was activated in Oplr16 knockdown cells (Supplementary Figure S6). Notably, Oplr16-knockdown cells lost the ability of self-renewal, and they failed to form teratomas in nude mice. These data confirmed the requirement of Oplr16 for the maintenance of pluripotency in E14 cells.
Oplr16 promotes pluripotent reprogramming
We then performed a series of assays to examine the role of increased expression of Oplr16 in stem cells. Using a luciferase assay, we amplified the DNA fragment containing the Oct4 promoter and a part of exon 1, and cloned it into a pGL3 vector to construct a pOct4-luciferase plasmid (Supplementary Figure S7A). The full length Oplr16 was also cloned into a pCMV-RsRed-Puro vector. An empty vector and a lncRNA control were used as the assay controls. HEK293T cells were co-transfected with a mixture of the pOct4-luciferase plasmid, the pRL-TK-renilla-luciferase plasmid, and the Oplr16 expression plasmid or control plasmid DNAs. The luciferase reporter assay demonstrated that Oplr16 activated the Oct4 promoter (Supplementary Figure S7B). Future studies are needed to examine the potential mechanisms for the activation in this in vitro system that lacks the ability to form intrachromosomal looping, like the R-loop formation and the recruitment of other transcription factors.
Then, we examined if Oplr16 was able to activate endogenous stem cells core factors in fibroblasts, a critical step needed to initiate pluripotent reprogramming. After Oplr16 lentiviral infection and puromycin selection, fibroblasts were collected for quantitative PCR. Oplr16 was highly expressed in the transfected fibroblasts (Figure 3A) as was Oct4, while the expression of Sox2 and Nanog showed no significant change (Figure 3B).
Figure 3.
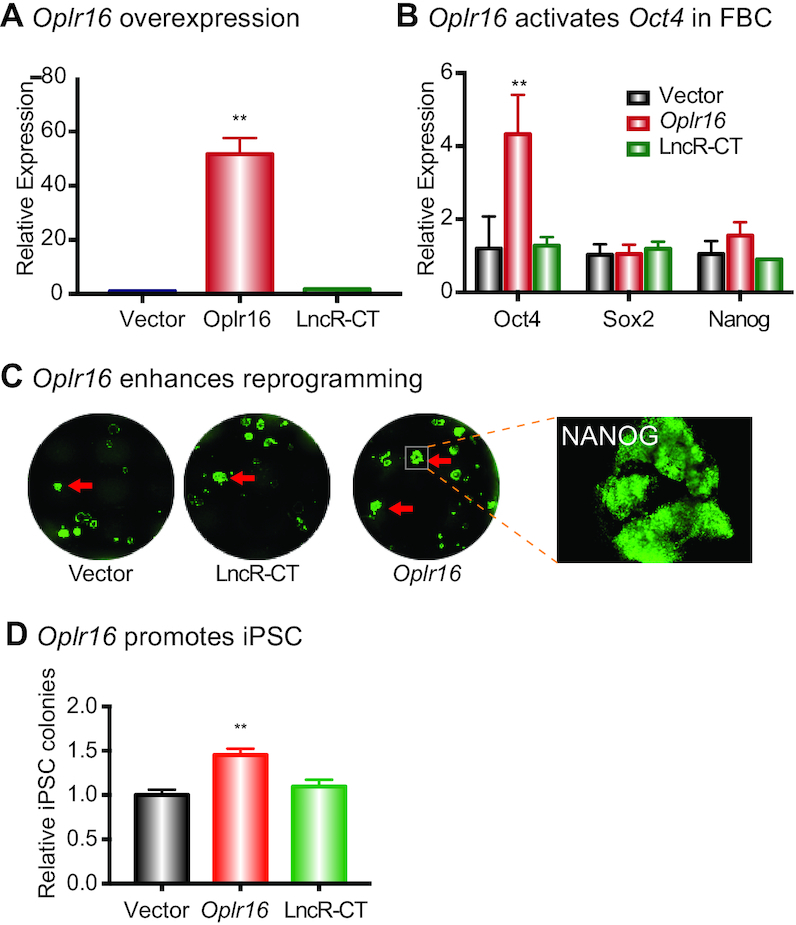
Oplr16 promotes Reprogramming. (A) Overexpression of Oplr16 in fibroblasts. Fibroblasts were transfected by Oplr16 lentiviruses and control lentiviruses. After selection by puromycin, stable cells were collected for qPCR quantitation. (B) Oplr16 activated the endogenous Oct4 in transfected fibroblasts. However, the expression of Sox2 and Nanog show no significant change as compared with Vector and LncR-CT controls. (C) Oplr16 enhanced reprogramming. DOX-inducible MEF cells were transfected with lentiviruses carrying the empty vector, random lncRNA control, and Oplr16. After 10 days of doxycycline (DOX) induction, iPSC colonies were immunostained using an antibody against pluripotency maker NANOG (green). (D) Oplr16 promoted iPSC formation. After DOX induction, the number of NANOG positive iPSC colonies was quantitated. ** P < 0.01 as compared with the controls.
The role of Oplr16 in pluripotency was further examined using a DOX-inducible reprogramming system (Supplementary Figure S8). The Oplr16 lentiviruses were transfected into OG2 MEF cells that carry DOX-inducible OSKM genes. After puromycin selection, doxycycline (DOX) was added to initiate pluripotent reprogramming. After 10 days, iPSC colonies started to form in the Oplr16-expressed group (Supplementary Figure S8C). Using immunostaining for the pluripotency marker NANOG (green), we observed more iPSC colonies in Oplr16-transfected cells than that in control cells (Figure 3C and D). Cell senescence is a critical barrier to cell reprogramming (45). In the Oplr16-treated group, we noted the downregulation of senescence marker genes (Supplementary Figure S9), in parallel with the enhancement of reprogramming.
We further examined the function of Oplr16 in a ‘rescue’ assay in the LIF-withdrawal cells. E14 cells were transfected with Oplr16 lentiviruses and seeded at very low density in a medium that lacks LIF. After LIF withdrawal, E14 cells lost pluripotency and became differentiated at very low density. We showed that Oplr16 partially rescued the defects caused by LIF-withdrawal, including the stem cell spheroid formation and the downregulation of stem cell core factor genes (Supplementary Figure S10).
Oplr16 specifically binds to the Oct4 promoter
We then used a RNA reverse transcription-associated trap sequencing (RAT-seq) approach (38) to map the genome-wide targets for Oplr16 (Figure 4A). E14 cells were collected and Oplr16 lncRNAs was labeled in situ with biotin-dCTP using stringent reverse transcription with three Oplr16-specific complementary primers. Random primers were used as the assay control (RAT-CT). The biotin–Oplr16 cDNA chromatin complex was isolated by streptavidin beads for Illumina library sequencing.
Figure 4.
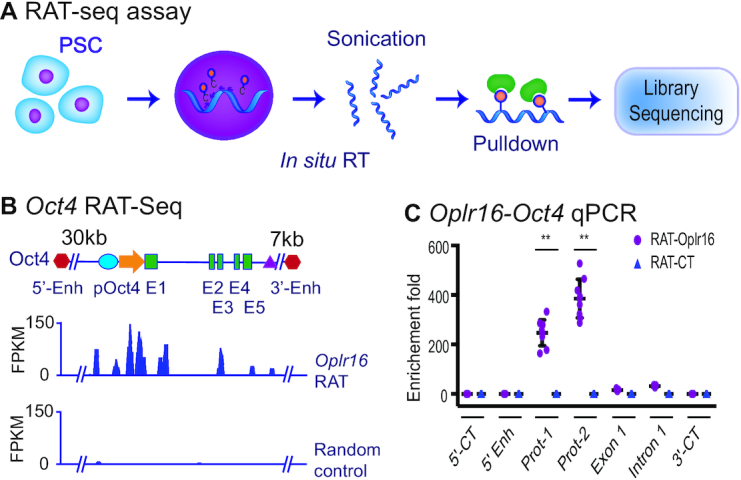
Oplr16 binds to the Oct4 promoter. (A) Schematic diagram of the RNA reverse transcription-associated trap sequencing (RAT-seq) assay. Oplr16 lncRNA was in situ reverse transcribed using three Oplr16-specific complementary primers with biotin-dCTP. The random primers were used as the negative control (RAT-CT). After nuclear lysis, the bitoin-Oplr16 cDNA chromatin complex was isolated by streptavidin beads and the Oplr16-interacting target DNAs were isolated for Illumina library sequencing. (B) The IGV analysis of Oplr16 binding signals in the Oct4 locus. 5′-Enh, 3′-Enh: the Oct4 5′- and 3′-enhancers; pOct4: Oct4 promoter; E1-E5: Oct4 exons. (C) Quantitative PCR mapping of Oplr16 binding in the Oct4 locus. The RAT pulldown complex was used to map the Oplr16 binding. 5′-CT, 3′-CT: the RAT control sites in the Oct4 locus. Note the enrichment of the Oplr16 binding signals in the promoter region (Prot-1 and Prot-2) (N = 9, ** P < 0.001 as compared with the RAT control).
IGV analysis showed enrichment of Oplr16 at the Oct4 locus, with a peak in the promoter area. The control RAT showed no binding peaks at that locus (Figure 4B). Quantitative PCR also showed enrichment of Oplr16 in the Oct4 promoter area as compared to the RAT-CT control (Figure 4C).
We also examined the binding to another core stem cell factor Sox2, which was not activated by Oplr16 (Supplementary Figures S11A and B). The Oplr16 RAT IGV analysis showed no peak at the Sox2 promoter (Supplementary Figures S11C-S11D). Gene ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) analyses revealed that Oplr16 is involved in many signal pathways that are related to development and cell differentiation (Supplementary Figures S12-S13). The top listed target genes were associated with embryonic development and several signal pathways (Supplementary Figure S14).
Oplr16 plays an important role in the chromatin looping
We previously discovered a promoter-enhancer intrachromosomal loop that is critical for the initiation and maintenance of stem cell pluripotency (6). We used a chromosome conformation capture (3C) approach (36,41) to examine if Oplr16 is involved in this intrachromosomal looping. E14 cells were crosslinked and lysed, followed by MboI digestion and T4 DNA ligation. After proteinase K treatment, DNA was purified for 3C PCR using primers from different regions of Oct4, including the 5′-enhancer, promoter and 3′-enhancer (Figure 5A). As previously reported (6), we detected pluripotency-associated 3C loops between the enhancers and promoters in E14 cells. However, knockdown of Oplr16 abolished these intrachromosomal loops in treated cells (Figure 5B), while the treatment of E14 cells with the shRNA control (shCT) did not interfere with intrachromosomal looping.
Figure 5.
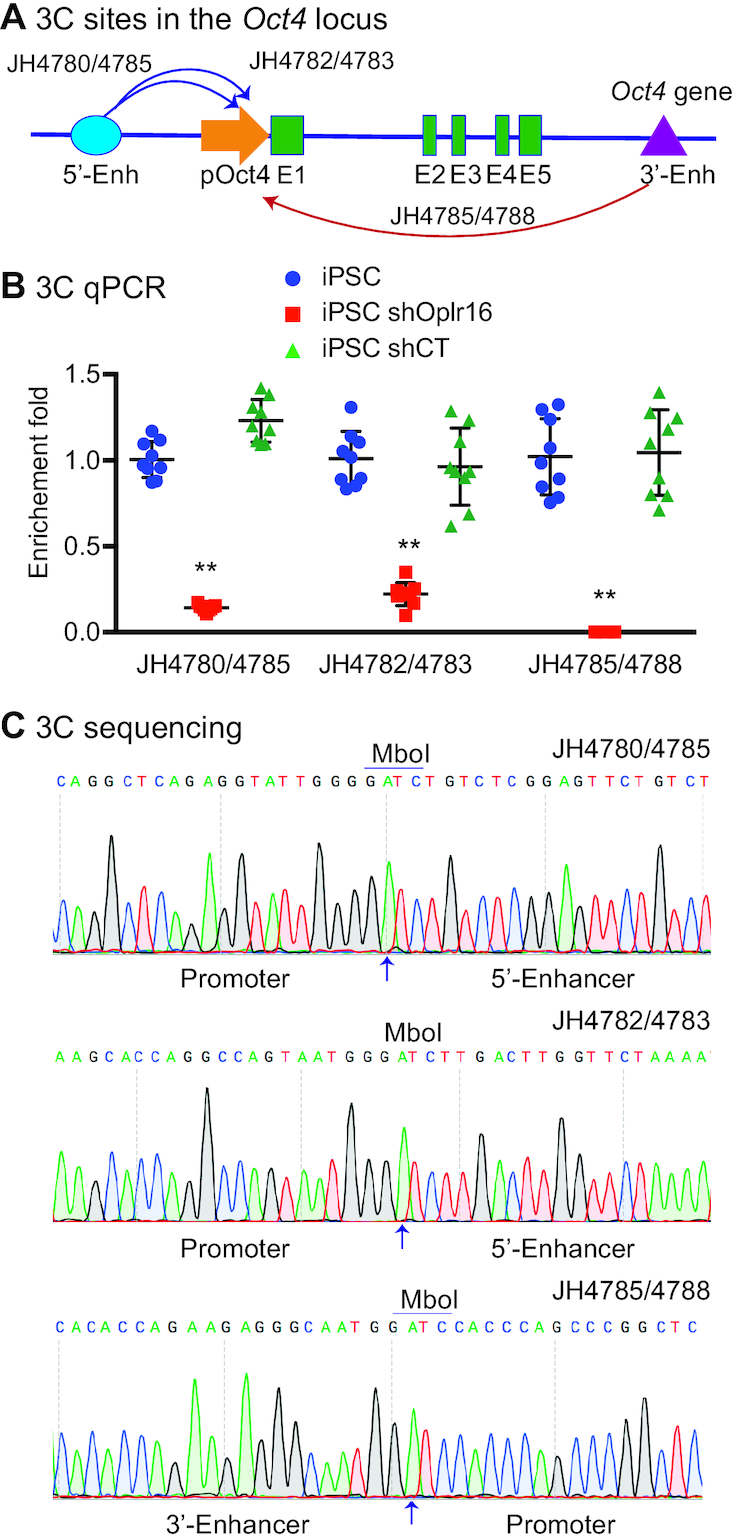
Oplr16 is essential for the maintenance of intrachromosomal looping in the Oct4 locus. (A) The PCR sites in the Oct4 locus. Intrachromosomal interactions were quantitated by 3C (chromatin conformation capture) qPCR. 5′-Enh: 5′-enhancers; pOct4: Oct4 promoter; E1-E5: Exons; 3′-Enh: 3′-Enhancer. Arrows: intrachromosomal interactions. (B) Knockdown of Oplr16 abolished intrachromosomal interaction loops. The 3C interaction was detected by qPCR. For comparison, the relative 3C interaction was calculated by setting the 5′- and 3′- controls as 1 (N = 9, ** P < 0.001 as compared with the shCT and iPSC controls). (C) Sequencing of the Oct4 intrachromosomal loop products. Arrows: the 3C ligation product containing the MboI site that is flanked by the promoter and the enhancer sequences.
We validated these intrachromosomal loops by DNA sequencing of 3C PCR products. As expected, we observed that the MboI ligation site was flanked by the sequences of the Oct4 promoter and enhancers (Figure 5C). These data suggest that Oplr16 is important for the formation and/or maintenance of these specific intrachromosomal interactions. Knockdown of Oplr16 abolished the 3D chromatin structures required for the maintenance of pluripotency.
Oplr16 recruits SMC1 to maintain chromatin looping
The cohesion-complex gene SMC1 is critical for intrachromosomal interactions (6). In order to determine if Oplr16 maintains intrachromosomal looping through SMC1, we used an SMC1 RNA-chromatin immunoprecipitation (RIP) assay and showed that Oplr16 interacted with SMC1 (Figure 6A and B).
Figure 6.
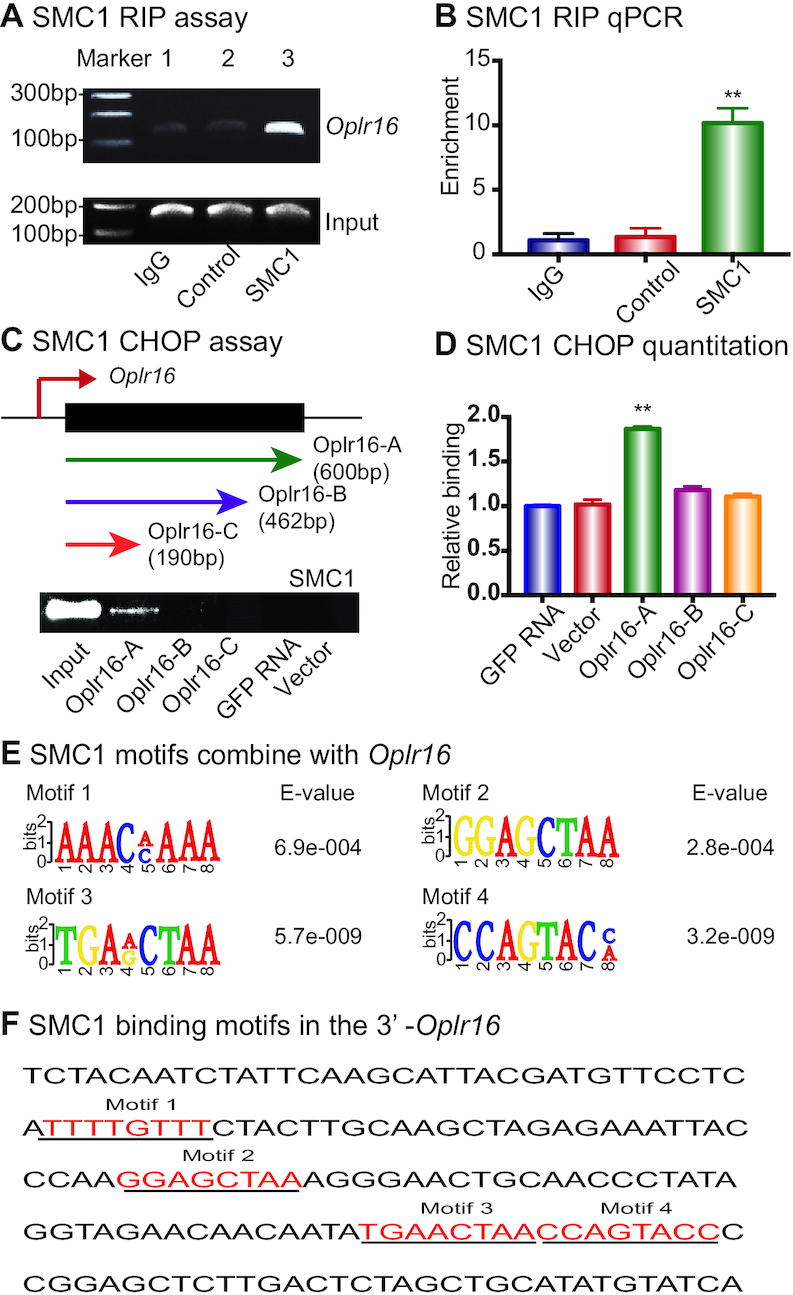
Oplr16 interacts with chromatin factor SMC1. (A) Interaction of Oplr16 with SMC1 by RNA-chromatin immunoprecipitation (RIP). The SMC1–lncRNA chromatin complex was immunoprecipitated with an antibody against SMC1. The immunoprecipitated RNAs were reverse transcribed and the SMC1-interacting Oplr16 was measured by PCR. IgG was used as the antibody control. Input: aliquot DNAs collected during the RIP assay. (B) Quantitative RIP PCR of the Oplr16-SMC1 interaction. (C) Identification of the SMC1 binding fragment in Oplr16. The chromatin oligo affinity precipitation (CHOP) assay was used to determine the specific fragment of Oplr16 that interacts with SMC1. Top panel: Schematic diagram of CHOP mapping. Three different sizes of biotin-labeled Oplr16 fragments were synthesized and bound to streptavidin agarose beads. Recombinant SMC1 proteins were incubated with biotin-Oplr16 streptavidin beads. After elution, the Oplr16-interacted SMC1 proteins were analyzed by Western immunoblotting. Only the full-length Oplr16 showed the interaction with SMC1 (Oplr16-A). GFP RNA was used as the negative control. Input: SMC1 protein. (D) Quantitation of the interaction of SMC1 with Oplr16 fragments. (E) Consensus RNA binding motifs of SMC1 by RIP-seq. (F) The presence of SMC1 motifs in the Oplr16 3′-fragment.
We then used an SMC1 CHOP assay to identify the Oplr16 fragment that binds to SMC1. Three different sizes of biotin-labeled Oplr16 were synthesized, purified by streptavidin agarose beads, and incubated with SMC1 recombinant proteins (Figure 6C). After washing, the Oplr16-interacting SMC1 was eluted and analyzed by immunoblotting. Full-length Oplr16 bound to SMC1 (Figure 6D), while the two fragments that lack the 3′-end did not interact with SMC1. We also performed SMC1 RIP sequencing to identify the conserved RNA binding motifs (Figure 6E). Analysis of lncRNA Oplr16 showed that its 3′-fragment contained at least four SMC1 binding motifs (Figure 6F). Collectively, these data suggest that the 3′-fragment of Oplr16 binds with SMC1.
Oplr16 induces DNA demethylation in fibroblasts
Successful initiation of pluripotent reprogramming requires CpG DNA demethylation in stem cell core factor genes, like Oct4. To examine if Oplr16 activates Oct4 by inducing promoter DNA demethylation, we collected Oplr16 lentivirus-transfected fibroblasts, extracted genomic DNA, and used a EZ DNA Methylation-Gold Kit to map DNA methylation in the Oct4 promoter (Figure 7A). Ectopic expression of Oplr16 induced extensive CpG DNA demethylation in transfected fibroblasts, particularly at the CpG 5 site (Figure 7B). These data suggest that Oplr16 may promote reprogramming by inducing DNA demethylation in the Oct4 promoter.
Figure 7.
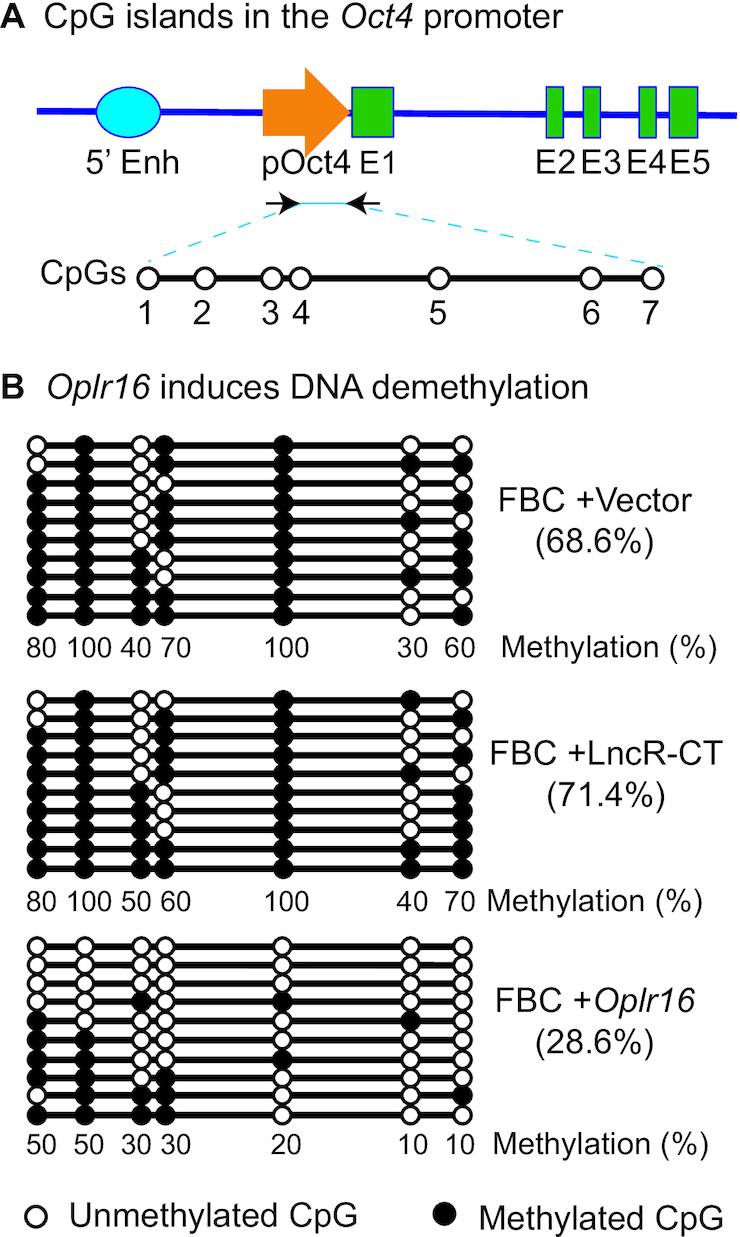
Oplr16 induces DNA demethylation in fibroblasts. (A) CpG islands in the Oct4 promoter. 5′-Enh: 5′-enhancers; pOct4: Oct4 promoter; E1–E5: exons. (B) Oplr16 induces DNA demethylation. Fibroblasts (FBC) were transfected with control vector, lncRNA control (LncR-CT) and Oplr16. Cells were collected for measurement of DNA methylation by sodium bisulfite sequencing. Solid dot: methylated CpG islands; open dot: unmethylated CpG islands. Numbers under each CpG site: the percentage of methylated CpGs for the CpG site. Numbers in the parenthesis: the percentage of total methylated CpGs over the CpGs in the sequencing. Each line represents the sequence for one clone. A total of 10 clones were sequenced for each group.
Oplr16 recruits TET2 to promote demethylation
We further explored the mechanism by which Oplr16 initiates DNA demethylation. We were interested to know if the virally-expressed Oplr16 altered the abundance of the DNA demethylase family of genes Tet1, Tet2 and Tet3, and DNA methyltransferase Dnmt1. Overexpression of Oplr16 upregulated Tet2 in transfected cells (Figure 8A) but had no effect on Tet1, Tet3 and Dnmt1 (Supplementary Figure S15).
Figure 8.
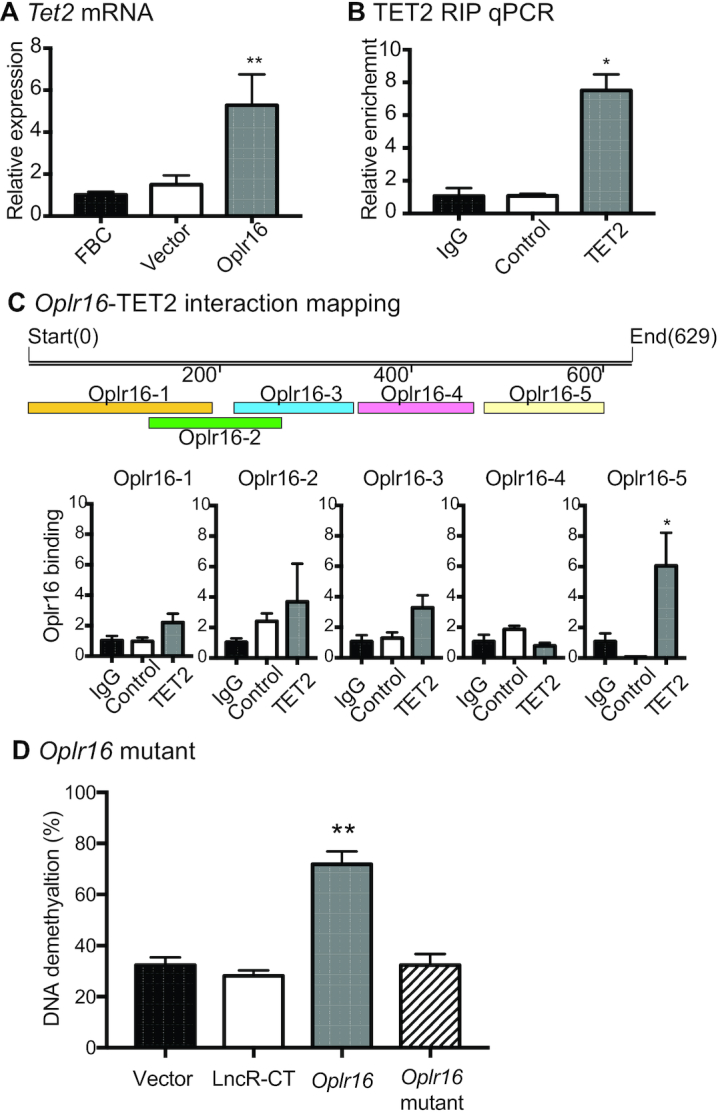
Oplr16 recruits TET2 to induce DNA demethylation. (A) After lentivirus transfection, fibroblasts were collected and quantitative PCR was used to determine the expression of the Tet2 gene. In Oplr16 transfected fibroblasts, Tet2 was upregulated compared to untreated fibroblasts and control vector transfected fibroblasts. (B) Interaction of Oplr16 with TET2 enzyme by RNA-chromatin immunoprecipitation (RIP). The TET2-Oplr16 lncRNA chromatin complex was immunoprecipitated with an antibody against TET2. After de-crosslinking, the immunoprecipitated RNAs were reverse transcribed. The TET2-interacting Oplr16 was measured by quantitative PCR. IgG was used as the antibody control. Input: aliquot DNAs collected during the RIP assay. (C) Identification of the TET2 binding fragment by RIP mapping. After immunoprecipitation with a TET2 antibody, the TET2-interacting Oplr16 lncRNA fragments were mapped by quantitative PCR using overlapping primers (color panels). The Oplr16-5 (3′fragment) showed strong binding to TET2. For comparison, the value of the IgG control was set as 1. Bottom panel: Schematic diagram of RIP mapping. (D) Deletion of the 3′-fragment abolishes the ability of Oplr16 to induce DNA demethylation. Fibroblasts were transfected with lentiviruses carrying the Oplr16 mutant that lack the 3′-fragment. After puromycin selection, cells were collected for detecting DNA methylation status. Compared with the full length Oplr16, the 3′-fragment-deleted mutant failed to induce DNA demethylation in the Oct4 promoter. * P < 0.05, ** P < 0.01 as compared with controls.
We then used RNA immunoprecipitation (RIP) assay to determine if Oplr16 interacts with DNA demethylase TET2. The TET2-RNA chromatin complex was immunoprecipitated with an antibody against TET2 and the anti-TET2 immunoprecipitated RNAs were reverse transcribed to quantitate the TET2-interacting lncRNA. There was a significant interaction between Oplr16 and TET2 as compared with the anti-IgG and lncRNA controls (Figure 8B).
We then mapped the specific fragment that interacts with TET2 using overlapping primers covering the Oplr16 lncRNA. We found the 3′-fragment of Oplr16 (Oplr16-5) showed the highest interaction with TET2 (Figure 8C). Further mapping showed that all the 3′ sub-fragments had similar binding capacity with TET2 (Supplementary Figure S16).
Finally, we studied the function of Oplr16 by deleting its 3′-fragment. The Oplr16 mutant was compared with the wild type Oplr16 for its ability to induce DNA methylation in the Oct4 promoter. Fibroblasts were transfected with lentiviruses carrying the mutant and wild type Oplr16. After puromycin selection, we examined the status of DNA methylation in transfected cells and found that deletion of the 3′-fragment abolished its ability to induce demethylation in the Oct4 promoter (Figure 8D, Supplementary Figure S17). These data suggest that after binding to the Oct4 promoter, Oplr16 may use its 3′-fragment to recruit TET2 and induce DNA demethylation for the initiation of pluripotent reprogramming. The overlapping binding of TET2, SMC1, Oplr16, and histone epigenetic modifications at the Oct4 promoter is shown in Supplementary Figure S18.
DISCUSSION
Despite our success in reprograming somatic cells into a pluripotent state, key questions remain unanswered regarding the underlying molecular mechanisms and key factors that control this plasticity switch. In this study, we identified a novel lncRNA, Oplr16, that functions as a pivotal factor to regulate pluripotent reprogramming. Oplr16 is a critical chromatin component of the Oct4 promoter complex. By binding to the Oct4 locus, it helps maintain a pluripotency-specific enhancer-promoter intrachromosomal loop and induces DNA demethylation in the gene promoter. Through these two epigenetic mechanisms, Oplr16 induces the activation of Oct4, a core stem cell factor gene, as an essential step to initiate pluripotent reprogramming. Knockdown of Oplr16 interrupts the intrachromosomal looping and induces the loss of pluripotency in E14 cells. Oplr16 is closely associated with the status of pluripotency. Oplr16 is specifically expressed in pluripotent stem cells, including iPSCs and E14 embryonic stem cells. It is not transcribed in fibroblasts and differentiated cells, nor is it expressed in any adult tissues examined so far. However, the Oplr16 gene switches on during the reprogramming progress in response to pluripotent inducers. Its expression status parallels that of core stem cell factor genes Oct4, Sox2, and Nanog during the process of reprogramming or embryoid body differentiation. Ectopic expression of Oplr16 enhances the efficiency of fibroblast reprogramming into iPSCs. Collectively, this study demonstrates that Oplr16 is a unique chromatin lncRNA factor that plays a pivotal role in coordinating a pluripotency-associated topological network and epigenotype that are necessary to initiate reprogramming.
A critical event in the initiation of pluripotent reprogramming is the removal of DNA methylation to activate pluripotency-regulated genes (46–48). However, the mechanisms by which DNA demethylation is initiated specifically in these gene promoters during reprogramming are not fully understood. In this study, we provide the first evidence that Oplr16 may function as an epigenetic messenger to help induce DNA demethylation in the Oct4 promoter. Oplr16 does not encode any chromatin factors. However, we show that ectopic expression of this lncRNA in fibroblasts induces upregulation of Tet2, one of the key DNA demethylase family members that are required for the initiation of reprogramming (49). Additionally, lncRNA Oplr16 binds to the Oct4 promoter, where it recruits DNA demethylase TET2 and induces DNA demethylation, initiating gene transcription.
We provide further evidence that suggests the critical role of the 3′-fragment of Oplr16 in DNA demethylation. The 3′-fragment of Oplr16 contains four consensus SMC1-binding motifs. Genetic deletion of the 3′-fragment abolishes the ability of Oplr16 to induce DNA demethylation in the Oct4 promoter. Thus, Oplr16 may act as a special lncRNA molecule that binds to the Oct4 promoter and guides de novo DNA demethylation to activate the gene for reprogramming. However, we cannot exclude the possibility that other epigenotype writers, like Dnmt1, Tet1 and Tet3, are also involved in the regulation of Oct4 and whether the Oplr16 3′-end has the ability to bind both SMC1 and TET2 in in vitro assays. Similarly, it is still unknown how Oplr16-SMC1-TET1 coordinate with each other in chromosomal looping and DNA demethylation.
Another important finding of this study is that Oplr16 acts like a chromatin factor that helps the formation of pluripotency-associated intrachromosomal looping. Chromatin looping between core promoter elements and distal enhancer elements controls the transcriptional activity of genes (50–52). At the Oct4 gene locus, for example, the intrachromosomal loops between the gene promoter and enhancer regulatory regions are specific for pluripotent stem cells. This topological structure helps bring distal regulatory elements, like enhancers, into physical proximity with gene target promoters, thereby activating them to initiate cellular reprogramming (53). In this study, we show that Oplr16 is enriched in the Oct4 promoter and is essential for the maintenance of this intrachromosomal looping. In E14 embryonic stem cells, knockdown of Oplr16 abolishes this intrachromosomal long-range interaction, in parallel with the exit of E14 cells from pluripotency.
It is known that Mediator and Cohesin physically and functionally connect enhancers and core promoters of active genes in embryonic stem cells (54). Mediator, a transcriptional coactivator, forms a complex with Cohesin, which can form rings that connect two DNA segments (8,55). We show that by binding to the Oct4 promoter, Oplr16 may recruit SMC1 and help orchestrate the promoter-enhancer loops. Oplr16 is actively transcribed during reprogramming and acts in concert with other chromatin factors to coordinate a pluripotency-associated epigenotype that is necessary to initiate reprogramming. We have demonstrated the functional importance of the 3′ region of Oplr16 for its interaction with both SMC1 and TET2. The blast search against the mouse reference genome, however, identifies multiple high homology sequences within the 3′ region of Oplr16, suggesting that this region may be highly conserved among different lncRNAs. Future studies are needed to address if they share a common mechanism in gene regulation. Additionally, it is still unclear if the intrachromosomal looping is a primary or secondary effect of the Oplr16-SMC1 interaction.
The role of the cohesion complex SMC1 in orchestrating intrachromosomal interactions, particularly in the regulation of pluripotent genes, has been elucidated using different approaches. For example, knockdown of SMC1with shRNA abolishes the intrachromosomal looping in Oct4, and induces the loss of self-renewal in human H14 ESCs (56). However, we still do not know how Oplr16 coordinates with SMC1 in the looping process. Multiple stem cell core factors and other chromatin factors are also involved in the loop formation. We cannot rule out the possibility that the downregulation of these factors in the Oplr16 knockdown cells may also trigger the loss of intrachromosomal looping.
It should also be emphasized that Oplr16 is not the sole factor that regulates the Oct4 gene in reprogramming. Overexpression of Oplr16 in fibroblasts did not translate into the full activation of Oct4. Other factors, including stem cell core factors, chromatin factors, and other Oct4-interacting lncRNAs, may be also required to coordinate the reprogramming process. Future studies are needed to examine if Oplr16 is able to corporate with other factors in enhancing reprogramming, and if Oplr16 can replace one or more factors in the conventional Oct4-Sox2-Klf4-c-Myc cocktail. Hopefully, a knockout mouse model will be able to further address the role of Oplr16 in embryo development.
In a DOX-inducible Oct4 ES cell model, Niwa et al. found that Oct4 was required to sustain pluripotency and self-renewal. Ectopic expression of Oct4 alone, however, was not sufficient to maintain undifferentiated phenotypes induced by LIF-withdrawal (57). In our study, however, we found that the Oct4-interacting lncRNA Oplr16 alone was able to delay LIF withdrawal-induced differentiation. Thus, epigenetic regulation of the Oct4 promoter alone may not completely account for the roles of Oplr16. The genome-wide target mapping by RAT-seq shows that Oplr16 targets multiple signal pathways in development and cell differentiation. Thus, it is possible that Oplr16 may control pluripotency through multiple mechanisms in addition to the Oct4 pathway.
In summary, this study identifies Oplr16 as a novel pluripotency-associated lncRNA in the mouse. Genetic depletion of Oplr16 causes E14 cells to differentiate and lose pluripotency. Oplr16 maintains stem cell pluripotency through multiple epigenetic mechanisms, including the activation of endogenous stem cell core factor genes, coordination of intrachromosomal looping, regulation of the Tet2 gene, and recruitment of TET2 to induce DNA demethylation. Thus, this lncRNA functions as a chromatin modulator in the regulatory network of stem cell pluripotency and reprogramming of somatic cells. Further studies are needed to explore its potential for use in regenerative therapies. In addition to Oplr16, the CRIST-seq assay has identified dozens of lncRNAs that interact with the Oct4 and/or Sox2 promoters. It would be interesting to learn how these lncRNAs cooperate each other in the maintenance of pluripotency. For example, Peblr20 (Pou5F1enhancer binding lncRNA 20) binds to Oct4 enhancers, where it recruits TET2 demethylase, leading to the activation of the enhancer RNA (eRNA) pathway (58). Similarly, the genome-wide interaction target mapping by RAT-seq shows that Oplr16 binds to many targets involved in multiple signal pathways that are related to development and cell differentiation. It is well known that suppression of cell cycle regulator and senescence pathway genes, like p16, p21 and p53, promotes pluripotent reprogramming (45,59,60). Valproic acid, a histone deacetylase inhibitor, enhances reprograming partially through the suppression of the senescence pathway (25,61). In this study, we also observed that Oplr16 downregulates the senescence pathway in MEF cells. On the other hand, knockdown of Oplr16 upregulates the expression of p16, p21, and p53. Clearly, Oplr16 may control pluripotency through multiple mechanisms. Epigenetic regulation of the Oct4 promoter may just be a portion of the roles of Oplr16. Future studies are needed to detail these Oplr16 non-Oct4 pathways in stem cells and to discover how they work coordinately in the regulation of DNA methylation, histone modifications, and 3D chromatin structures.
DATA AVAILABILITY
As previously reported (21,22), the CRIST-seq, RAT-seq and RNA-seq data generated in this study have been deposited in NIH GEO databases under accession numbers GSE107945, GSE101765 and GSE116605, respectively. The datasets are available at https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE107945; https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE101765; and https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE116605.
Supplementary Material
SUPPLEMENTARY DATA
Supplementary Data are available at NAR Online.
FUNDING
National Key Research and Development Program of China [2018YFA0106902]; National Natural Science Foundation of China [31430021, 81874052, 81672275, 31871297, 81670143, 81900701]; Key Project of Chinese Ministry of Education [311015]; National Basic Research Program of China (973 Program) [2015CB943303]; Nation Key Research and Development Program of China [2016YFC13038000]; Research on Chronic Noncommunicable Diseases Prevention and Control of National Ministry of Science and Technology [2016YFC1303804]; National Health Development Planning Commission Major Disease Prevention and Control of Science and Technology Plan of Action, Cancer Prevention and Control [ZX-07-C2016004]; Natural Science Foundation of Jilin Province [20150101176JC, 20180101117JC, 20130413010GH]; Provincial Science Fund of Jilin Province Development and Reform Commission [2014N147 and 2017C022]; California Institute of Regenerative Medicine (CIRM) grant [RT2-01942]; Merit Review from the United States (U.S.) Department of Veterans Affairs, Biomedical Laboratory Research and Development Service [BX002905].
Conflict of interest statement. None declared.
REFERENCES
- 1. Takahashi K., Yamanaka S.. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006; 126:663–676. [DOI] [PubMed] [Google Scholar]
- 2. Andrey G., Mundlos S.. The three-dimensional genome: regulating gene expression during pluripotency and development. Development. 2017; 144:3646–3658. [DOI] [PubMed] [Google Scholar]
- 3. de Wit E., Bouwman B.A., Zhu Y., Klous P., Splinter E., Verstegen M.J., Krijger P.H., Festuccia N., Nora E.P., Welling M. et al.. The pluripotent genome in three dimensions is shaped around pluripotency factors. Nature. 2013; 501:227–231. [DOI] [PubMed] [Google Scholar]
- 4. Li M., Liu G.H., Izpisua Belmonte J.C.. Navigating the epigenetic landscape of pluripotent stem cells. Nat. Rev. Mol. Cell Biol. 2012; 13:524–535. [DOI] [PubMed] [Google Scholar]
- 5. Shchuka V.M., Malek-Gilani N., Singh G., Langroudi L., Dhaliwal N.K., Moorthy S.D., Davidson S., Macpherson N.N., Mitchell J.A.. Chromatin dynamics in lineage commitment and cellular reprogramming. Genes (Basel). 2015; 6:641–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhang H., Jiao W., Sun L., Fan J., Chen M., Wang H., Xu X., Shen A., Li T., Niu B. et al.. Intrachromosomal looping is required for activation of endogenous pluripotency genes during reprogramming. Cell Stem Cell. 2013; 13:30–35. [DOI] [PubMed] [Google Scholar]
- 7. Hu J.F., Hoffman A.R.. Chromatin looping is needed for iPSC induction. Cell Cycle. 2014; 13:1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kagey M.H., Newman J.J., Bilodeau S., Zhan Y., Orlando D.A., van Berkum N.L., Ebmeier C.C., Goossens J., Rahl P.B., Levine S.S. et al.. Mediator and cohesin connect gene expression and chromatin architecture. Nature. 2010; 467:430–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kim Y.J., Cecchini K.R., Kim T.H.. Conserved, developmentally regulated mechanism couples chromosomal looping and heterochromatin barrier activity at the homeobox gene A locus. PNAS. 2011; 108:7391–7396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yoon J.H., Abdelmohsen K., Gorospe M.. Posttranscriptional gene regulation by long noncoding RNA. J. Mol. Biol. 2012; 425:3723–3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhao Y., Sun L., Wang R.R., Hu J.F., Cui J.. The effects of mitochondria-associated long noncoding RNAs in cancer mitochondria: New players in an old arena. Crit. Rev. Oncol. Hematol. 2018; 131:76–82. [DOI] [PubMed] [Google Scholar]
- 12. Guttman M., Amit I., Garber M., French C., Lin M.F., Feldser D., Huarte M., Zuk O., Carey B.W., Cassady J.P. et al.. Chromatin signature reveals over a thousand highly conserved large non-coding RNAs in mammals. Nature. 2009; 458:223–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhang H., Zeitz M.J., Wang H., Niu B., Ge S., Li W., Cui J., Wang G., Qian G., Higgins M.J. et al.. Long noncoding RNA-mediated intrachromosomal interactions promote imprinting at the Kcnq1 locus. J. Cell Biol. 2014; 204:61–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Perry R.B., Ulitsky I.. The functions of long noncoding RNAs in development and stem cells. Development. 2016; 143:3882–3894. [DOI] [PubMed] [Google Scholar]
- 15. Lee J.T., Bartolomei M.S.. X-inactivation, imprinting, and long noncoding RNAs in health and disease. Cell. 2013; 152:1308–1323. [DOI] [PubMed] [Google Scholar]
- 16. Bao X., Wu H., Zhu X., Guo X., Hutchins A.P., Luo Z., Song H., Chen Y., Lai K., Yin M. et al.. The p53-induced lincRNA-p21 derails somatic cell reprogramming by sustaining H3K9me3 and CpG methylation at pluripotency gene promoters. Cell Res. 2015; 25:80–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sheik Mohamed J., Gaughwin P.M., Lim B., Robson P., Lipovich L.. Conserved long noncoding RNAs transcriptionally regulated by Oct4 and Nanog modulate pluripotency in mouse embryonic stem cells. RNA. 2010; 16:324–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chen R., Liu Y., Zhuang H., Yang B., Hei K., Xiao M., Hou C., Gao H., Zhang X., Jia C. et al.. Quantitative proteomics reveals that long non-coding RNA MALAT1 interacts with DBC1 to regulate p53 acetylation. Nucleic Acids Res. 2017; 45:9947–9959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Quinn J.J., Chang H.Y.. Unique features of long non-coding RNA biogenesis and function. Nat. Rev. Genet. 2016; 17:47–62. [DOI] [PubMed] [Google Scholar]
- 20. Luginbuhl J., Sivaraman D.M., Shin J.W.. The essentiality of non-coding RNAs in cell reprogramming. Noncoding RNA Res. 2017; 2:74–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhang S., Wang Y., Jia L., Du Z., Wen X., Wang C., Hao Y., Yu D., Zhou L., Chen N. et al.. Profiling the long noncoding RNA interaction network in the regulatory elements of target genes by chromatin in situ reverse transcription sequencing. Genome Res. 2019; 29:1521–1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Du Z., Jia L., Wang Y., Wang C., Wen X., Chen J., Zhu Y., Yu D., Zhou L., Chen N. et al.. Combined RNA-seq and RAT-seq mapping of long noncoding RNAs in pluripotent reprogramming. Sci Data. 2018; 5:180255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhu X.Q., Pan X.H., Wang W., Chen Q., Pang R.Q., Cai X.M., Hoffman A.R., Hu J.F.. Transient in vitro epigenetic reprogramming of skin fibroblasts into multipotent cells. Biomaterials. 2009; 31:2779–2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chen M., Zhang H., Wu J., Xu L., Xu D., Sun J., He Y., Zhou X., Wang Z., Wu L. et al.. Promotion of the induction of cell pluripotency through metabolic remodeling by thyroid hormone triiodothyronine-activated PI3K/AKT signal pathway. Biomaterials. 2012; 33:5514–5523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhai Y., Chen X., Yu D., Li T., Cui J., Wang G., Hu J.F., Li W.. Histone deacetylase inhibitor valproic acid promotes the induction of pluripotency in mouse fibroblasts by suppressing reprogramming-induced senescence stress. Exp. Cell Res. 2015; 337:61–67. [DOI] [PubMed] [Google Scholar]
- 26. Dobin A., Davis C.A., Schlesinger F., Drenkow J., Zaleski C., Jha S., Batut P., Chaisson M., Gingeras T.R.. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013; 29:15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ahmad S., Mu X., Yang F., Greenwald E., Park J.W., Jacob E., Zhang C.Z., Hur S.. Breaching Self-Tolerance to alu duplex RNA underlies MDA5-Mediated inflammation. Cell. 2018; 172:797–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Li T., Hu J.F., Qiu X., Ling J., Chen H., Wang S., Hou A., Vu T.H., Hoffman A.R.. CTCF regulates allelic expression of Igf2 by orchestrating a promoter-polycomb repressive complex-2 intrachromosomal loop. Mol. Cell Biol. 2008; 28:6473–6482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhang S., Zhong B., Chen M., Yang G., Li Y., Wang H., Wang G., Li W., Cui J., Hoffman A.R. et al.. Epigenetic reprogramming reverses the malignant epigenotype of the MMP/TIMP axis genes in tumor cells. Int. J. Cancer. 2014; 134:1583–1594. [DOI] [PubMed] [Google Scholar]
- 30. Li T., Chen H., Li W., Cui J., Wang G., Hu X., Hoffman A.R., Hu J.. Promoter histone H3K27 methylation in the control of IGF2 imprinting in human tumor cell lines. Hum. Mol. Genet. 2014; 23:117–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhao Y., Liu S., Li1 X., Zhou L., Meng Y., Li Y., Li L., Jiao B., Bai L., Yu Y. et al.. Aberrant shuttling of long noncoding RNAs during the mitochondria-nuclear crosstalk in hepatocellular carcinoma cells. Am. J. Cancer Res. 2019; 9:999–1008. [PMC free article] [PubMed] [Google Scholar]
- 32. Bonev B., Cavalli G.. Organization and function of the 3D genome. Nat. Rev. Genet. 2016; 17:661–678. [DOI] [PubMed] [Google Scholar]
- 33. Sun J., Li W., Sun Y., Yu D., Wen X., Wang H., Cui J., Wang G., Hoffman A.R., Hu J.F.. A novel antisense long noncoding RNA within the IGF1R gene locus is imprinted in hematopoietic malignancies. Nucleic Acids Res. 2014; 42:9588–9601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wang H., Li W., Guo R., Sun J., Cui J., Wang G., Hoffman A.R., Hu J.F.. An intragenic long noncoding RNA interacts epigenetically with the RUNX1 promoter and enhancer chromatin DNA in hematopoietic malignancies. Int. J. Cancer. 2014; 135:2783–2794. [DOI] [PubMed] [Google Scholar]
- 35. Li X., Chen N., Zhou L., Wang C., Wen X., Jia L., Cui J., Hoffman A.R., Hu J.F., Li W.. Genome-wide target interactome profiling reveals a novel EEF1A1 epigenetic pathway for oncogenic lncRNA MALAT1 in breast cancer. Am. J. Cancer Res. 2019; 9:714–729. [PMC free article] [PubMed] [Google Scholar]
- 36. Chen N., Yan X., Zhao G., Lv Z., Yin H., Zhang S., Song W., Li X., Li L., Du Z. et al.. A novel FLI1 exonic circular RNA promotes metastasis in breast cancer by coordinately regulating TET1 and DNMT1. Genome Biol. 2018; 19:218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Langmead B., Trapnell C., Pop M., Salzberg S.L.. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 2009; 10:R25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhang Y., Liu T., Meyer C.A., Eeckhoute J., Johnson D.S., Bernstein B.E., Nusbaum C., Myers R.M., Brown M., Li W. et al.. Model-based analysis of ChIP-Seq (MACS). Genome Biol. 2008; 9:R137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zhuang Q., Li W., Benda C., Huang Z., Ahmed T., Liu P., Guo X., Ibanez D.P., Luo Z., Zhang M. et al.. NCoR/SMRT co-repressors cooperate with c-MYC to create an epigenetic barrier to somatic cell reprogramming. Nat. Cell Biol. 2018; 20:400–412. [DOI] [PubMed] [Google Scholar]
- 40. Pian L., Wen X., Kang L., Li Z., Nie Y., Du Z., Yu D., Zhou L., Jia L., Chen N. et al.. Targeting the IGF1R pathway in breast cancer using antisense lncRNA-Mediated promoter cis competition. Mol. Ther. Nucleic Acids. 2018; 12:105–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Morgan S.L., Mariano N.C., Bermudez A., Arruda N.L., Wu F., Luo Y., Shankar G., Jia L., Chen H., Hu J.F. et al.. Manipulation of nuclear architecture through CRISPR-mediated chromosomal looping. Nat. Commun. 2017; 8:15993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chu C., Qu K., Zhong F.L., Artandi S.E., Chang H.Y.. Genomic maps of long noncoding RNA occupancy reveal principles of RNA-chromatin interactions. Mol. Cell. 2011; 44:667–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ma A.N., Wang H., Guo R., Wang Y.X., Li W., Cui J., Wang G., Hoffman A.R., Hu J.F.. Targeted gene suppression by inducing de novo DNA methylation in the gene promoter. Epigenet. Chromatin. 2014; 7:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zhao X., Liu X., Wang G., Wen X., Zhang X., Hoffman A.R., Li W., Hu J.F., Cui J.. Loss of insulin-like growth factor II imprinting is a hallmark associated with enhanced chemo/radiotherapy resistance in cancer stem cells. Oncotarget. 2016; 7:51349–51364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Banito A., Rashid S.T., Acosta J.C., Li S., Pereira C.F., Geti I., Pinho S., Silva J.C., Azuara V., Walsh M. et al.. Senescence impairs successful reprogramming to pluripotent stem cells. Genes Dev. 2009; 23:2134–2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kim M., Costello J.. DNA methylation: an epigenetic mark of cellular memory. Exp. Mol. Med. 2017; 49:e322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Nishino K., Umezawa A.. DNA methylation dynamics in human induced pluripotent stem cells. Hum. Cell. 2016; 29:97–100. [DOI] [PubMed] [Google Scholar]
- 48. Apostolou E., Stadtfeld M.. Cellular trajectories and molecular mechanisms of iPSC reprogramming. Curr. Opin. Genet. Dev. 2018; 52:77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sardina J.L., Collombet S., Tian T.V., Gomez A., Di Stefano B., Berenguer C., Brumbaugh J., Stadhouders R., Segura-Morales C., Gut M. et al.. Transcription factors drive Tet2-Mediated enhancer demethylation to reprogram cell fate. Cell Stem Cell. 2018; 23:727–741. [DOI] [PubMed] [Google Scholar]
- 50. Engel J.R., Smallwood A., Harper A., Higgins M.J., Oshimura M., Reik W., Schofield P.N., Maher E.R.. Epigenotype-phenotype correlations in Beckwith-Wiedemann syndrome. J. Med. Genet. 2000; 37:921–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Abe S., Funato T., Takahashi S., Yokoyama H., Yamamoto J., Tomiya Y., Yamada-Fujiwara M., Ishizawa K., Kameoka J., Kaku M. et al.. Increased expression of insulin-like growth factor i is associated with Ara-C resistance in leukemia. Tohoku J. Exp. Med. 2006; 209:217–228. [DOI] [PubMed] [Google Scholar]
- 52. Huang S.L., Wu Y., Yu H., Zhang P., Zhang X.Q., Ying L., Zhao H.F.. Inhibition of Bcl-2 expression by a novel tumor-specific RNA interference system increases chemosensitivity to 5-fluorouracil in Hela cells. Acta Pharmacol. Sin. 2006; 27:242–248. [DOI] [PubMed] [Google Scholar]
- 53. Bikard D., Jiang W., Samai P., Hochschild A., Zhang F., Marraffini L.A.. Programmable repression and activation of bacterial gene expression using an engineered CRISPR-Cas system. Nucleic Acids Res. 2013; 41:7429–7437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Gupta P., Lavagnolli T., Mira-Bontenbal H., Fisher A.G., Merkenschlager M.. Cohesin's role in pluripotency and reprogramming. Cell Cycle. 2016; 15:324–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ohlsson R., Lobanenkov V., Klenova E.. Does CTCF mediate between nuclear organization and gene expression. Bioessays. 2009; 32:37–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Zhang H., Niu B., Hu J.F., Ge S., Wang H., Li T., Ling J., Steelman B.N., Qian G., Hoffman A.R.. Interruption of intrachromosomal looping by CCCTC binding factor decoy proteins abrogates genomic imprinting of human insulin-like growth factor II. J. Cell Biol. 2011; 193:475–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Niwa H., Miyazaki J., Smith A.G.. Quantitative expression of Oct-3/4 defines differentiation, dedifferentiation or self-renewal of ES cells. Nat. Genet. 2000; 24:372–376. [DOI] [PubMed] [Google Scholar]
- 58. Wang C., Jia L., Wang Y., Du Z., Zhou L., Wen X., Li H., Zhang S., Chen H., Chen N. et al.. Genome-wide interaction target profiling reveals a novel Peblr20-eRNA activation pathway to control stem cell pluripotency. Theranostics. 2020; 10:353–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kawamura T., Suzuki J., Wang Y.V., Menendez S., Morera L.B., Raya A., Wahl G.M., Belmonte J.C.. Linking the p53 tumour suppressor pathway to somatic cell reprogramming. Nature. 2009; 460:1140–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Utikal J., Polo J.M., Stadtfeld M., Maherali N., Kulalert W., Walsh R.M., Khalil A., Rheinwald J.G., Hochedlinger K.. Immortalization eliminates a roadblock during cellular reprogramming into iPS cells. Nature. 2009; 460:1145–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Chen X., Zhai Y., Yu D., Cui J., Hu J.F., Li W.. Valproic acid enhances iPSC induction from human bone marrow-derived cells through the suppression of reprogramming-induced senescence. J. Cell. Physiol. 2016; 231:1719–1727. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
As previously reported (21,22), the CRIST-seq, RAT-seq and RNA-seq data generated in this study have been deposited in NIH GEO databases under accession numbers GSE107945, GSE101765 and GSE116605, respectively. The datasets are available at https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE107945; https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE101765; and https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE116605.