OBJECTIVES:
To summarize the literature on the influence of exercise on the gut microbiota of healthy adults.
METHODS:
A systematic and comprehensive search in electronic database, including SciELO, Scopus, PubMed, and Web of Science up to July 5, 2019. Eligibility criterion was original studies conducted on healthy humans including exercise interventions or interventions involving any type of physical activity.
RESULTS:
The initial search retrieved 619 articles of which 18 met the inclusion criteria, 9 were observational, 4 reported very short-term exercise interventions, and 5 reported medium/long-term exercise interventions. Higher levels of physical activity or cardiorespiratory fitness were positively associated with fecal bacterial alpha diversity. Contrasting associations were detected between both the level of physical activity and cardiorespiratory fitness and fecal counts for the phyla Firmicutes, Bacteroidetes, and Proteobacteria. Higher levels of physical activity and cardiorespiratory fitness were positively associated with the fecal concentration of short-chain fatty acids. Reports on the effects of very short-term and medium/long-term exercise interventions on the composition of the gut microbiota were inconsistent.
DISCUSSION:
Higher levels of physical activity and cardiorespiratory fitness are associated with higher fecal bacterial alpha diversity and with the increased representation of some phyla and certain short-chain fatty acids in the feces of healthy adults. Very short-term and medium/long-term exercise interventions seem to influence the fecal counts of some phyla. However, the heterogeneity between studies hampers any strong conclusions from being drawn. Better-designed studies are needed to unravel the possible mechanisms through which exercise might influence the composition and activity of the human gut microbiota.
INTRODUCTION
Humans live in symbiosis with different microorganisms present on the skin and in the oral cavity, vagina, and gut (1). These microorganisms affect host nutrition, metabolic function, gut development, and the maturation of the immune system and epithelial cells (2). The gut microbiota refers to the microorganisms (approximately 100 trillion of them) (3) that colonize the gastrointestinal tract (4). Five phyla representing ∼160 species can be detected in the large intestine alone (5). The most representative phyla are Firmicutes (60%–65%), Bacteroidetes (20%–25%), and Proteobacteria (5%–10%), although this may vary widely between one person and another (6).
Eubiosis, which is associated with good health status (7), requires the intestinal ecosystem to be in good microbial equilibrium; dysbiosis is any change in this equilibrium (7). Dysbiosis has been strongly linked to obesity, type 2 diabetes (8), inflammatory bowel disease (9), colon cancer, and autism (10). Some studies have shown that restoring eubiosis in the gut of obese mice improves their metabolic profile (11–13) and reduces insulin resistance.
Physical activity is characterized by any movement of the skeletal muscles that demands energy expenditure, whereas exercise is a structured, planned, and repetitive physical activity, the purpose of which is to improve or maintain physical fitness (14). Several studies report that increasing the amount of physical activity undertaken improves the physical and mental health of persons of any age (15). Exercise can be included in the treatment of many chronic diseases (15,16). In animal models, exercise seems to restore eubiosis in the gut (17–19), although the mechanisms involved remain unknown (20,21).
The influence of exercise on the gut microbiota of healthy humans is poorly understood (1,22,23). Three systematic reviews on the subject are available, but they suffer from 2 major limitations (24–26): (i) they omit information on several key studies (27–40) and (ii) they focus on both healthy and unhealthy human subjects alike (because the effect of exercise on the gut microbiota in healthy humans is unclear, studying the effect of exercise on the gut microbiota in unhealthy humans hampers the interpretation of the results; it is impossible to know whether any changes are caused by exercise or the disease itself). The present work focuses on the influence of exercise on the gut microbiota of healthy adults.
MATERIAL AND METHODS
This systematic review was conducted adhering to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis statement (41) and was registered with the International Prospective Register of Systematic Reviews (PROSPERO registration number: CRD42018114664).
Search strategy
A literature search was conducted across the SciELO, Scopus, PubMed, and the Web of Science databases, taking into account the reports published up until July 5, 2019. The following search strategies were followed: for SciELO, (gut) AND (microbiota) (see Table S1, Supplementary Digital Content 1, http://links.lww.com/CTG/A166); for Scopus, (gut) AND (microbiota) AND (exercise) AND (human) AND (humans) (see Table S2, Supplementary Digital Content 1, http://links.lww.com/CTG/A166), and for PubMed ((((((((((((((((((“Gastrointestinal Microbiome”) OR (((“Fecal Microbiota”) OR “Cecal Microbiota”) OR “Fecal Microbiota”)))))) AND (((Exercises) OR Training))))) AND Human) NOT (((((((((((((((((((“Mice”[Medical Subject Heading (MeSH)]) OR “Rats”[MeSH]) OR “Animal Experimentation”[MeSH]) OR “Models, Animal”[MeSH])) OR (“rats” OR “mouse”))) OR “mice”)) OR “rat”)))))))))))))))) NOT Review. When exploring PubMed, MeSH terms were included to increase the power of the search (see Table S3, Supplementary Digital Content 1, http://links.lww.com/CTG/A166). A slightly different search strategy was used for the Web of Science database because it does not include MeSH terms: ((((((((((((((((((Gut) OR Intestinal) OR Gastrointestinal) OR Fecal) OR Cecal) OR Faecal)) AND (((((Flora) OR Microflora) OR Microbiotas) OR Microbiome) OR Microbiomes)))) AND (((Exercises) OR Training)) AND Human))) NOT (Mice OR Rat* OR (Experiment* AND Animal*) OR (Research* AND Animal*) OR mouse OR (model* AND animal*))))))). For further details see Table S4 (Supplementary Digital Content 1, http://links.lww.com/CTG/A166).
Selection criteria
The inclusion criteria were: (i) observational and intervention studies, (ii) studies including exercise interventions (either very short-term or medium/long-term) or interventions involving any type of physical activity, (iii) studies including effects on gut microbiota as an outcome. Case-control studies were included, but only those that reported data for the healthy controls. The exclusion criteria were: (i) studies written in languages other than English or Spanish, (ii) studies including unhealthy people, (iii) reviews, and (iv) studies in animal models. No restrictions were placed on subject age or body composition.
After removing duplicates, eligibility was finally assessed by (i) reading the title and abstract and (ii) reading the full text if still potentially eligible.
Data extraction
The following information was collected from each included study: (i) the authors' names and bibliographic references; (ii) the number of subjects and their sex, age, and body mass index (BMI); (iii) exercise outcomes; (iv) control diet type (standardized diet and/or adjusting the results for nutritional intake); (v) fecal sample collection; (vi) the technique used for gut microbiota analysis; and (vii) the main findings. Two authors (L.O.-A. and H.X.) conducted the literature search and data extraction independently; disagreements were resolved by consensus. The articles selected were classified according to the type of study (observational, very short-term exercise interventions, and medium/long-term exercise interventions).
Study quality
With the purpose of evaluating the quality of the studies included, we used Physiotherapy Evidence Database methodological quality scale (42). This tool consists of 11 items assessing the interpretability of studies, internal and external validity, and it is able to detect potential bias with good reliability (42,43). The total score was obtained by adding of the scores for items 2–11. Scores of ≤3 were viewed as describing studies of low methodological quality, 4–6 those of moderate quality, and ≥7 those of high methodological quality.
RESULTS
General overview
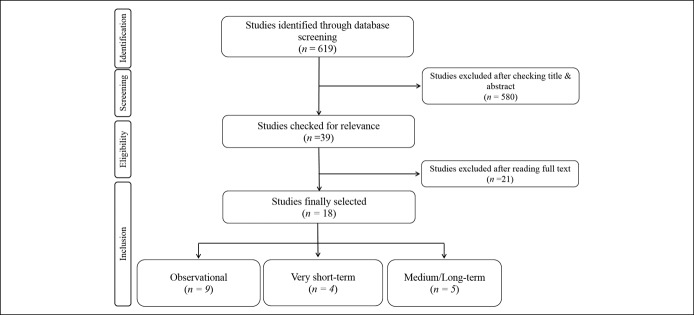
Figure 1 shows the preferred reporting items for systematic reviews and meta-analysis consort diagram for the search strategy. A total of 619 studies were found across the 4 databases examined (no eligible studies were detected in the SciELO and Scopus databases), 580 of which were excluded after reading the title and abstract. Thus, 18 studies met the inclusion criteria, 9 of which were observational (i.e., 3 cross-sectional (35,36,44) and 6 case-control studies (27,28,33,34,45,46)) (see Table S5, Supplementary Digital Content 1, http://links.lww.com/CTG/A166), 4 of which were very short-term exercise intervention studies (2 was a case-control study (32,37)) (see Table S6, Supplementary Digital Content 1, http://links.lww.com/CTG/A166), and 5 of which were medium/long-term exercise intervention studies (one was a case-control study (47)) (see Table S7, Supplementary Digital Content 1, http://links.lww.com/CTG/A166). All studies were published between 2014 and 2019.
Figure 1.
Flowchart showing the literature search and article selection process.
The sample size of 17 of these 18 studies ranged from 3 to 88 subjects (27–40,45–47), and one had a sample size of 1,493 subjects (44). Five of the 18 studies involved only women (29,30,34,36,46), 3 involved only men (28,31,45), 8 involved both men and women (27,32,33,35,38–40,47), and 2 studies did not report subject sex (37,44). The subject age ranged from 18 to 77 years; one study did not report the subject age (44). Four studies included sedentary subjects (29,30,40,47), 3 focused on active participants (27,31,39), and 9 involved both sedentary and recreationally active subjects (28,32–36,44–46). Two studies did not report the levels of physical activity practiced (37,38). Physical activity was assessed using a questionnaire in 5 studies (27,28,34,44,45) and using an accelerometry-based method in one (46). Four studies measured cardiorespiratory fitness through the maximum oxygen consumption (VO2max) test (33,35–37).
Eight studies did not record the participants' diet before assessments were made (28,33–35,37,40,44,45); 6 studies (27,29,30,32,36,46) examined the diet by the means of food records or food frequency questionnaires and adjusted the results accordingly. Four studies established a fixed diet for some days before the collection of feces (31,38,39,47).
The bacterial alpha diversity was reported in 12 studies (n = 66·6%) (28,29,31,34,35,38–40,44–47) (Figure 2, see Tables S5 to S7, Supplementary Digital Content 1, http://links.lww.com/CTG/A166) and beta diversity in 7 (n = 38·8%) (29,34,35,40,44,46,47) (see Tables S5, S6, and S7, Supplementary Digital Content 1, http://links.lww.com/CTG/A166). The studies reported on the gut microbiota data using different taxonomic ranks (see Tables S3, S5, S6, and S7, Supplementary Digital Content 1, http://links.lww.com/CTG/A166); 2 reported at the phylum level (36,37), 7 at the genus level (27,29,32,35,38,45,47), and 5 at the species level (30,31,39,44,46). Only one study reported the ratio Firmicutes/Bacteroidetes (33), and only 3 reported the concentration of short-chain fatty acids (SCFAs) in the feces (28,35,47) (Figure 3). Such a heterogeneity hampers comparisons; no meta-analysis was performed. However, the gut microbiota data reported by the different studies could all be converted to the phylum level, making comparisons and interpretations somewhat easier (Figures 4 and 5 and Table S8 see Supplementary Digital Content 1, http://links.lww.com/CTG/A166).
Figure 2.
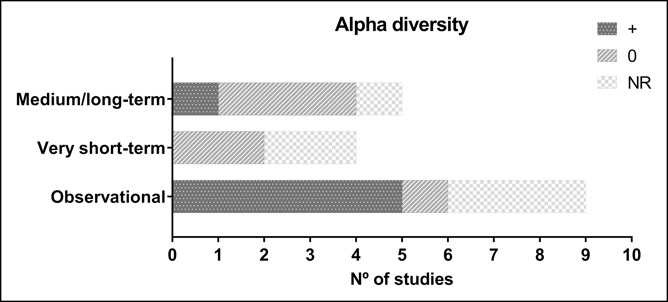
Influence of exercise on alpha diversity by study design. The dark grey bars (+) indicate the number of studies showing a positive association between physical activity or cardiorespiratory fitness and bacterial alpha diversity. The light grey bars (0) represent the number of studies showing no influence of physical activity or cardiorespiratory fitness on alpha diversity. The light grey and white bars (“NR”) indicate the number of studies that reported no results in this respect. NR, no results.
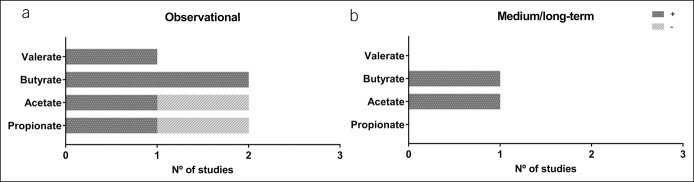
Figure 3.
Summary of the main findings of the present review with respect to short-chain fatty acids (SCFAs), by study design. Panel (a) represents the main results of all observational studies (n = 2). Panel (b) shows the main results of the medium/long-term studies (n = 1). The dark grey bars (+) indicate the number of studies showing a positive association between levels of physical activity or cardiorespiratory fitness and SCFAs, or a positive effect for an exercise intervention. The light grey bars (−) indicate a negative association between levels of physical activity or cardiorespiratory fitness and SCFAs.
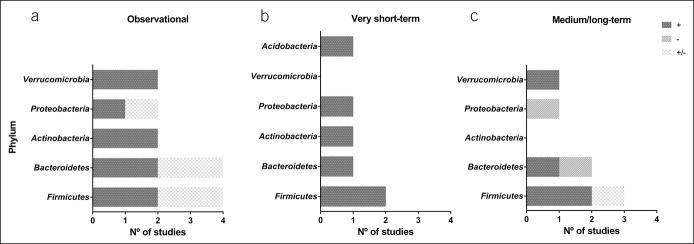
Figure 4.
Summary of the main results of the present review by study design. Panel (a) represents the main findings of all observational studies. Panel (b) represents the main findings of all very short-term intervention studies. Panel (c) represents the main findings of all medium/long-term intervention studies. The dark grey (+) indicate the number of studies that showed a positive influence of physical activity or cardiorespiratory fitness on the different phyla, or a positive effect for an exercise intervention. The light grey bars (−) indicate the number of studies that showed a negative effect for an exercise intervention. The light grey and white bars (+/−) represent the number of studies showing positive and negative findings within the same phyla. These findings come from the observational studies of Clarke et al. (45), and Bressa et al. (46), and from the medium/long-term exercise intervention study of Morita et al. (30).
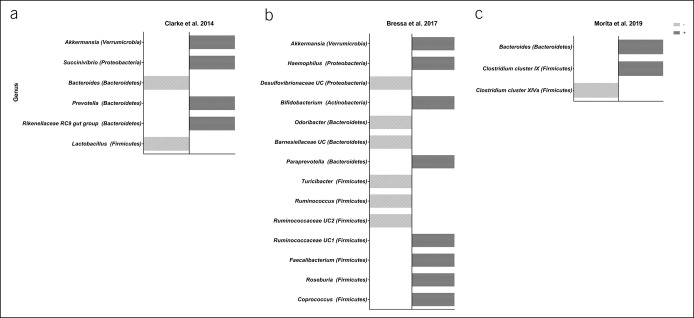
Figure 5.
Summary of the main findings of the observational studies of Clarke et al. (45) (a) and Bressa et al. (46) (b), and the medium/long-term intervention study of Morita et al. (30) (c). The dark grey bars (+) represents a positive association between physical activity and the gut microbiota at the genus level, or a positive effect of an exercise intervention. The light grey bars (−) represent a negative association between physical activity and gut microbiota at the genus level, or a null effect of an exercise intervention.
Methodological quality of clinical trials
Based on Physiotherapy Evidence Database scale criteria, all studies 100% (n = 18) (27–40,44–47) were of medium quality (see Table S9, Supplementary Digital Content 1, http://links.lww.com/CTG/A166). Criteria 2 and 3 (regarding allocation) and 5, 6, and 7 (regarding the blinding process) were the least satisfied by the different studies, whereas criteria 4, 8, 9, 10, and 11 (regarding the design of randomization and the data displayed) were the best satisfied.
Observational studies—detailed examination
Of the 9 observational studies detected, 4 reported a positive association between the level of physical activity and bacterial alpha diversity (28,34,44,45), although one study observed no such association (46). One study reported a positive association between cardiorespiratory fitness and bacterial alpha diversity (35). Three studies did not report bacterial alpha diversity at all (27,33,36). Beta diversity was reported by 4 of the above 9 studies (34,35,44,46), but only one (34) reported physical activity to be associated with it.
McFadzean (44) recorded the self-reported physical activity level (undertaken never, rarely, occasionally, regularly, or daily) of 1,493 people and observed people who undertook physical activity occasionally, regularly, and daily to return higher bacterial alpha diversity values than those who undertook physical activity rarely. They also reported the highest fecal Faecalibacterium prausnitzii (Firmicutes phylum) counts for those who undertook daily physical activity (see Table S5, Supplementary Digital Content 1, http://links.lww.com/CTG/A166). Petersen et al. (27) reported the frequency of training in cyclists to correlate positively with fecal Prevotella (phylum Bacteroidetes) counts, independent of race category (professional or amateur), and after taking into account nutritional intake (see Table S5, Supplementary Digital Content 1, http://links.lww.com/CTG/A166).
Clarke et al. (45), Mörkl et al. (34), and Barton et al. (28) reported bacterial alpha diversity to be higher in athletes compared with healthy, sedentary controls. Clarke et al. (45) observed phylum Proteobacteria and Verrucomicrobia and genus Prevotella (phylum Bacteroidetes) fecal counts to be higher, and those for genus Bacteroides (phylum Bacteroidetes) to be lower, in athletes than in sedentary controls (see Table S5, Supplementary Digital Content 1, http://links.lww.com/CTG/A166 and Figure 5). Barton et al. (28) reported a positive association between physical activity level and fecal SCFA (butyrate, propionate, acetate, and valerate) concentration (see Table S5, Supplementary Digital Content 1, http://links.lww.com/CTG/A166).
Bressa et al. (46) observed no association between objectively measured physical activity levels and bacterial alpha diversity in premenopausal women (see Table S5, Supplementary Digital Content 1, http://links.lww.com/CTG/A166). They did, however, report a positive association between physical activity and fecal counts for genus Bifidobacterium (phylum Actinobacteria) and Akkermansia muciniphila (phylum Verrucomicrobia) (see Table S5, Supplementary Digital Content 1, http://links.lww.com/CTG/A166). They also reported that within the phyla Firmicutes, Bacteroidetes, and Proteobacteria, some genera belonging to the same phylum were present in different amounts in active women compared with controls (see Table S5, Supplementary Digital Content 1, http://links.lww.com/CTG/A166 and Figure 5). It should be noted that these authors adjusted their results for subject diet, as assessed by a food frequency questionnaire.
Estaki et al. (35) reported cardiorespiratory fitness to be positively associated with bacterial alpha diversity. Moreover, they reported a positive association between cardiorespiratory fitness and fecal counts for order Clostridiales family Lachnospiraceae, family Erysipelotrichaceae, genus Coprococcus, genus Roseburia (all phylum Firmicutes), and genus Adlercreutzia (phylum Actinobacteria) (see Table S5, Supplementary Digital Content 1, http://links.lww.com/CTG/A166). In addition, they observed cardiorespiratory fitness to be positively associated with fecal butyrate and to be negatively associated with other SCFAs such as propionate and acetate (see Table S5, Supplementary Digital Content 1, http://links.lww.com/CTG/A166). Yang et al. (36) observed that premenopausal women with high cardiorespiratory fitness levels to have higher fecal counts of phylum Bacteroidetes after adjusting for nutritional intake. Finally, Durk et al. (33) reported a positive association between cardiorespiratory fitness and the Firmicutes/Bacteroidetes fecal count ratio in healthy young adults (see Table S5, Supplementary Digital Content 1, http://links.lww.com/CTG/A166).
Very short-term exercise intervention studies—detailed examination
Over 2 independent days, Lundgren-Kownacki et al. (38) assessed how water rehydration affected the gut microbiota after a single load of physical work (a combination of different exercises). They only collected one fecal sample after each of 2 exercise days, but reported no change in the composition of the gut microbiota between these 2 days (the diet having been standardized before assessment) (see Table S6, Supplementary Digital Content 1, http://links.lww.com/CTG/A166). Zhao et al. (39) investigated the composition of the gut microbiota in 20 runners before and after a half-marathon (21.1 km) and found the bacterial alpha diversity not to have changed after the race. However, they did observe an increase in fecal counts of class Coriobacteriia, order Coriobacteriales, family Coriobacteriaceae, genus Collinsella, and Collinsella aerofaciens (all phylum Actinobacteria) after the race. Different families, genera, and species belonging to the phyla Proteobacteria and Firmicutes also showed increased counts (see Table S6, Supplementary Digital Content 1, http://links.lww.com/CTG/A166). It should be noted that the study subjects received the same meal before each fecal collection. Shukla et al. (37), who performed a cycling test and collected fecal samples at baseline, 48 hour, and 72 hour postexercise, observed higher fecal counts for Bacteroidetes and other phyla (data not reported) at 72 hour compared with baseline (see Table S6, Supplementary Digital Content 1, http://links.lww.com/CTG/A166). Finally, Scheiman et al. (32) evaluated how a marathon affected the gut microbiota after a marathon in 15 subjects. They observed that the fecal counts of genus Veillonella significantly increased after the marathon. Later, they confirmed the findings in a separate cohort of 11 ultramarathoners and Olympic rowers after a single bout of exercise. No study analyzed the effect of very short-term exercise on fecal SCFA concentrations (Figure 3).
Medium/long-term exercise intervention studies—detailed examination
Cronin et al. (40) ran an 8-week concurrent training intervention (3 days per week) and observed the bacterial beta diversity, but not the alpha diversity, to have changed at the end of the study period. Similarly, Munukka et al. (29) ran a 6-week endurance intervention (cycling; 3 days per week) study involving women only and observed bacterial beta diversity to have increased by the end of the study, whereas the alpha diversity remained unchanged (see Table S7, Supplementary Digital Content 1, http://links.lww.com/CTG/A166). However, after the intervention, fecal counts for phylum Verrucomicrobia, specifically family Verrucomicrobiaceae and genus Akkermansia, increased after adjusting for nutritional intake. By contrast, counts for phylum Proteobacteria had fallen (see Table S7, Supplementary Digital Content 1, http://links.lww.com/CTG/A166). Morita et al. (30) ran 2 exercise interventions of 12 weeks duration, endurance training (1 hour of walking every day), or resistance training (1 hour training per week). Fecal counts for the genus Bacteroides increased after endurance training, whereas those for Clostridium subcluster XIVa (phylum Firmicutes) decreased. By contrast, Clostridium cluster IX (phylum Firmicutes) increased after the resistance intervention (see Table S7, Supplementary Digital Content 1, http://links.lww.com/CTG/A166 and Figure 5). Keohane et al. (31) studied the effect of a long boat race (4 weeks) on the gut microbiota of 3 men. They collected 4 fecal samples (at baseline, mid-race, just before the race finished, and 3 months postrace) and showed bacterial alpha diversity to have changed from the middle of the race until the end. All participants returned increased fecal counts for the genus Subdoligranulum unclassified, Dorea longicatena, and Roseburia hominis and reduced counts for Bacteroides finegoldii. In one athlete, counts for Prevotella copri remained increased from the middle of the race until 3 months postrace. It should be noted that all athletes had the same diet during the race (see Table S7, Supplementary Digital Content 1, http://links.lww.com/CTG/A166). Allen et al. (47) conducted a 6-week endurance intervention (3 days of endurance training per week) and collected fecal samples at baseline, just after the intervention, and again 6 weeks later. The bacterial alpha and beta diversities did not change, either after the intervention or after the 6-week washout period. However, fecal counts for the order Clostridiales, genus Roseburia, genus Lachnospira, genus Faecalibacterium, and genus Lachnospiraceae unclassified (all phylum Firmicutes) increased after the intervention. The endurance training also increased some fecal SCFA concentrations (such as acetate and butyrate), but only in normal weight individuals. It should be noted that all the subjects in the latter study also followed an energy intake restriction diet during the intervention period (see Table S7, Supplementary Digital Content 1, http://links.lww.com/CTG/A166).
DISCUSSION
The present review indicates both physical activity level and cardiorespiratory fitness to be positively associated with bacterial alpha diversity in healthy humans, whereas the exercise interventions (either very short-term or medium/long-term) had little or no effect on bacterial alpha and beta diversities. Contrasting findings were detected regarding the effect of exercise interventions on fecal counts for the phyla Firmicutes, Bacteroidetes, and Proteobacteria. In addition, some studies reported a positive association between physical activity/cardiorespiratory fitness and fecal SCFA concentration (28,35), this agrees with the findings of one medium/long-term exercise intervention study (47). Physical activity or exercise appears to have different effects on different species belonging to the same phylum. It is unclear whether diet has any bearing on these findings. Taking the examined results together, it would appear that exercise could influence the composition of the gut microbiota in healthy adults, but the heterogeneity of the available studies precludes any firm conclusions from being drawn.
How might exercise exert an effect on the gut microbiota?
Exercise affects several physiological systems (14), including the skeletal muscle system (23). Several studies have suggested bidirectional crosstalk to exist between the skeletal muscles and the gut—the so-called muscle-gut (20) or gut-muscle (48) axis (Figure 6). This existence of this axis is based on the fact that the contraction of skeletal muscle during exercise has an anti-inflammatory effect because of the release of myokines (49). Recently, Hamasaki (50) reported in a review that some myokines seem to play a role in mediating the secretion of glucagon-like peptide-1 (GLP-1, a key incretin involved in whole-body metabolism) in the gut during exercise (11). Certainly, interleukin-6 is involved in the secretion of GLP-1 by the L-cells in the ileum (51). Further evidence for the existence of the gut-muscle axis lies in the gut microbiota producing SCFAs—key mediators of energy metabolism in the mitochondria of skeletal muscles (52) that in turn help regulate whole-body glucose metabolism (53). Moreover, SCFAs interact with specific G-protein-coupled receptors (GPR41 and GPR43) on the intestinal L-cells (54), stimulating the secretion of GLP-1 (55). Several bacteria are SCFA producers; Bifidobacterium (phylum Actinobacteria) produces acetate that can be transformed into butyrate (56), whereas Akkermansia (phylum Verrucomicrobia) produces propionate and acetate (57). Acetate and butyrate both enhance muscle fat oxidation, changing the oxidative status of muscle fibers (58). Thus, acetate and butyrate enhance metabolic flexibility by improving the capacity to use and switch between lipid and carbohydrate fuels (58). Butyrate also inhibits histone deacetylase, protecting against muscle protein catabolism and therefore preventing age-related muscle mass loss (59). A recent study showed that the daily treatment of Akkermansia munichipila (Verrucomicrobia phylum), during 3 months, in obese individuals with metabolic syndrome is able to improve glucose and lipid metabolism, as well as body weight (60). Curiously, work discussed in the present review suggests that fecal counts of the phyla Actinobacteria and Verrucomicrobia are increased after very short-term (39) and medium/long-term (29) exercise interventions, respectively, and that their numbers are related to physical activity (45,46) and cardiorespiratory fitness (35). Recently, Scheiman et al. (32) demonstrated in mice that Veillonella atypica is able to metabolize lactate into acetate and propionate improving exercise performance, although this assumption remains to be demonstrated in humans. Moreover, Ehrenpreis et al. (61) showed exercise could make that SCFAs were more biologically available to colonic bacteria. We observed that SCFAs appear to increase after medium/long-term exercise interventions (47) and are positively associated with physical activity level (28) and cardiorespiratory fitness (35), suggesting exercise to play a key role in the secretion of SCFAs, that stimulate the muscle-gut axis.
Figure 6.
Main characteristics of the gut microbiota of a long-term sedentary-behavior subject: dysbiosis, low bacterial alpha diversity, and low concentrations of SCFAs. It may be that after an exercise intervention, the gut microbiota becomes more eubiotic with a greater alpha diversity; SCFAs concentrations may also increase. The gold lines represent the possible cross-talk between the gut and the skeletal muscle (gut-muscle axis). The blue lines represent the possible cross-talk between the skeletal muscle and the gut (muscle-gut axis). However, most of the physiological mechanisms that explain these pathways remain unknown, or at least are not well understood; this is highlighted through the use of a question mark. SCFA, short-chain fatty acids.
The diversity of microbes within the human body can be described in their richness and evenness, i.e., by the number of species regarding species abundance (alpha diversity). Several studies have shown that the greater the species diversity, the healthier the phenotype (6,62). The reviewed results suggest that greater physical activity and cardiorespiratory fitness are associated with higher bacterial alpha diversity in healthy adults (28,34,35,44,45). However, very short-term and medium/long-term exercise interventions (<8 weeks of endurance exercise) appear to have little effect on alpha diversity (29,38–40,47) (although after just 4 weeks of endurance exercise an effect was recorded in 3 athletes (31)). This suggests that human bacterial alpha diversity is affected only by longer exercise interventions. However, whether long-term exercise really does increase bacterial alpha diversity, and the prevalence of species able to produce SCFAs (and therefore improve metabolic flexibility) remains a mystery. Some studies (63,64) suggest that long-term exercise interventions can indeed improve metabolic flexibility in humans, but the mechanisms that might explain why are unknown. Further studies are needed to address these questions.
General limitations of the studies included in the present review
The cohorts included in the reviewed studies were quite heterogeneous. No comparisons between sexes or BMI categories could be made because the studies did not report the same kinds of data.
Most of the studies used self-reported questionnaires to determine physical activity (27,28,34,44,45), with 3 (28,34,45) using validated questionnaires. However, it is well known that the estimation of physical activity by questionnaire (which is easy and inexpensive) is less accurate than more objective methods (65). Bressa et al. (46) and Morita et al. (30) used an accelerometry-based method, which offers a potential solution to the problems associated with self-reported data (65). Thus, the lack of observational studies that have used objective measurements of physical activity hinders drawing any reliable conclusions on the effect of physical activity on the gut microbiota.
Currently, all human and mouse studies that have addressed the effect of exercise on the gut microbiota have involved endurance interventions (running or cycling); less attention has been paid to resistance training (30,40), which has a different physiological effect in humans (66–68). Cronin et al. (40) made use of concurrent training (endurance + resistance exercises), although with this type of training it is impossible to know whether the endurance or resistance components have different effects. Morita et al. (30) ran endurance and resistance interventions in elderly adults, but these were dissimilar in training time and intensity, making it impossible to know whether the effects on the gut microbiota were driven by the different types of exercise or the different total training times.
It should be remembered that gut microbiota is affected by the diet (45,69–74). Four studies (31,38,39,47) used a standardized diet with their subjects before and during the intervention period, but this makes it impossible to know whether the changes seen in the gut microbiota were caused by the exercise intervention or the change in diet. New studies including a control group could solve this problem. Moreover, 8 (44.4%) of the studies reviewed (28,33–35,37,40,44,45) did not take diet into account at all; the 7 that did (27,29,30,32,36,46,47) monitored intake through food frequency questionnaires or 24 hours diary records before collecting the fecal samples.
Eleven studies (61·1%) (27–29,31,32,35,39,40,44,46,47) used the advanced Illumina platform to sequence the gut microbiota, whereas 7 used older techniques (30,33,34,36–38,45). For assigning a taxonomic identity to the sequences, the most accurate platform is that of the Ribosomal Database Project (annotation error rate ∼10%). This was used in only 4 studies (27,37,39,47), whereas the Silva and Greengenes databases (error rate ∼17%) (75) were used in 2 (29,45) and 3 (35,44,46), respectively. The remaining studies (n = 9) (28,30–34,36,38,40) used other databases. Moreover, the studies examined in the present review reported their data at different taxonomic levels, making comparisons difficult.
Future research needs
Currently, we are not close to understanding the effect of exercise on the human gut microbiota. Future studies should bear in mind the shortcomings highlighted by the present review:
Data homogeneity. Future studies should report information with respect to age, BMI, and gender. It needs to be clear whether the effect of exercise on the gut microbiota is different in these respects.
Physical activity. Most of the observational studies (55%) discussed in the present review used self-reported questionnaires to determine physical activity levels. Objectively measured physical activity (e.g., using accelerometry), would improve the reliability of any associations seen with changes in the gut microbiota.
Type of exercise. When designing future studies, the importance of the type of exercise (endurance vs resistance), intensity (moderate vs vigorous), and subjects status (untrained vs trained individuals) should be understood.
Diet: The gut microbiota is easily affected by changes in food intake (45,69–74). Future studies should control at least what their participants eat during the exercise interventions and before stool collection. Reporting these data as descriptive values will help reveal the general effects of exercise.
Quantification of the gut microbiota. Interest in the human gut microbiota is growing fast, and the technology needed to examine it is advancing rapidly. Future studies should use state-of-the-art technologies, such as the Illumina platform and Ribosomal Database Project annotation. They should also focus on the effect of exercise interventions at all taxonomical levels. Some of the studies discussed in the present work (36,37) reported information at the phylum level, but species within the same phylum were seen to be affected differently by exercise. Reporting at the species level would be a great step forward.
Mechanistic studies. Mouse and human experiments should focus on elucidating the possible mechanisms through which exercise might influence the gut microbiota or vice versa. Basically, the unknown mechanistic pathways behind the gut-muscle and muscle gut axes need to be investigated (Figure 6).
Publication bias. Negative results in science are just as important as positive results (76). In the present review, 11 (61·1%) studies reported only positive results; they did not report whether they observed any negative or null effects. This type of information is crucial.
Based on the 18 studies included in the present review, physical activity and cardiorespiratory fitness seem to be positively associated with bacterial alpha diversity, fecal counts for certain bacterial phyla, and fecal SCFA concentrations in healthy adults. Exercise interventions seem to influence fecal counts for certain phyla. However, the heterogeneity of the examined studies precludes any stronger conclusions from being drawn. Thus, although the current evidence points toward exercise having an effect on the human gut microbiota, more and better designed studies are needed if this is to be confirmed, and the mechanisms involved are to be understood.
CONFLICTS OF INTEREST
Guarantor of the article: Huiwen Xu, MSc, Lourdes Ortiz-Alvarez, MSc, and Borja Martinez-Tellez, PhD.
Specific author contributions: L.O.-A. and H.X. equally contributed to the literature search and disagreements were resolved by consensus. L.O.-A., H.X. and B.M.-T. contributed to the manuscript preparation.
Financial support: The study was funded by the Spanish Ministry of Economy and Competitiveness via the Fondo de Investigación Sanitaria del Instituto de Salud Carlos III (PI13/01393), Retos de la Sociedad (DEP2016-79512-R), and European Regional Development Funds (ERDF), by the Spanish Ministry of Education (FPU 16/05159 and FPU17/01523), the Fundación Iberoamericana de Nutrición (FINUT), the Redes Temáticas De Investigación Cooperativa RETIC (Red SAMID RD16/0022), AstraZeneca HealthCare Foundation, the University of Granada Plan Propio de Investigación 2016 (Excellence actions: Unit of Excellence on Exercise and Health [UCEES]), and by the Junta de Andalucía, Consejería de Conocimiento, Investigación y Universidades (ERDF, SOMM17/6107/UGR). B.M.-T. is supported by individual postdoctoral grants from the Fundación Alfonso Martin Escudero.
Potential competing interests: None to report.
Supplementary Material
ACKNOWLEDGEMENTS
This study is part of a PhD thesis conducted within the Biomedicine Doctoral Studies Program of the University of Granada, Spain. The study was funded by the Spanish Ministry of Economy and Competitiveness by the Fondo de Investigación Sanitaria del Instituto de Salud Carlos III (PI13/01393), Retos de la Sociedad (DEP2016-79512-R), and European Regional Development Funds (ERDF), by the Spanish Ministry of Education (FPU 16/05159 and FPU17/01523), the Fundación Iberoamericana de Nutrición (FINUT), the Redes Temáticas De Investigación Cooperativa RETIC (Red SAMID RD16/0022), AstraZeneca HealthCare Foundation, the University of Granada Plan Propio de Investigación 2016 (Excellence actions: Unit of Excellence on Exercise and Health [UCEES]), and by the Junta de Andalucía, Consejería de Conocimiento, Investigación y Universidades (ERDF, SOMM17/6107/UGR).
Footnotes
Joint first authors.
SUPPLEMENTARY MATERIAL accompanies this paper at http://links.lww.com/CTG/A166
References
- 1.Campbell SC, Wisniewski PJ. Exercise is a novel promoter of intestinal health and microbial diversity. Exerc Sport Sci Rev 2017;45:41–7. [DOI] [PubMed] [Google Scholar]
- 2.Hooper LV, Gordon JI. Commensal host-bacterial relationships in the gut. Science 2001;292:1115–8. [DOI] [PubMed] [Google Scholar]
- 3.Rosenbaum M, Knight R, Leibel RL. The gut microbiota in human energy homeostasis and obesity. Trends Endocrinol Metab 2015;26:493–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eberl G. A new vision of immunity: Homeostasis of the superorganism. Mucosal Immunol 2010;3:450–60. [DOI] [PubMed] [Google Scholar]
- 5.Rajilić-Stojanović M, de Vos WM. The first 1000 cultured species of the human gastrointestinal microbiota. FEMS Microbiol Rev 2014;38:996–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Consortium THMP, Huttenhower C, Gevers D, et al. Structure, function and diversity of the healthy human microbiome. Nature 2012;486:207–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iebba V, Totino V, Gagliardi A, et al. Eubiosis and dysbiosis: The two sides of the microbiota SuMMAry. New Microbiol 2016;39:1–12. [PubMed] [Google Scholar]
- 8.Knip M, Siljander H. The role of the intestinal microbiota in type 1 diabetes mellitus. Nat Rev Endocrinol 2016;12:154–67. [DOI] [PubMed] [Google Scholar]
- 9.Huttenhower C, Kostic AD, Xavier RJ. Inflammatory bowel disease as a model for translating the microbiome. Immunity 2014;40:843–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ahn J, Sinha R, Pei Z, et al. Human gut microbiome and risk for colorectal cancer. J Natl Cancer Inst 2013;105:1907–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grasset E, Puel A, Charpentier J, et al. A specific gut microbiota dysbiosis of type 2 diabetic mice induces GLP-1 resistance through an enteric NO-dependent and gut-brain axis mechanism. Cell Metab 2017;25:1075–90.e5. [DOI] [PubMed] [Google Scholar]
- 12.Fabbiano S, Suárez-Zamorano N, Chevalier C, et al. Functional gut microbiota remodeling contributes to the caloric restriction-induced metabolic improvements. Cell Metab 2018;28:907–21.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zimmermann M, Zimmermann-Kogadeeva M, Wegmann R, et al. Mapping human microbiome drug metabolism by gut bacteria and their genes. Nature 2019;570:462–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fiuza-Luces C, Santos-Lozano A, Joyner M, et al. Exercise benefits in cardiovascular disease: Beyond attenuation of traditional risk factors. Nat Rev Cardiol 2018;15:731–43. [DOI] [PubMed] [Google Scholar]
- 15.Pedersen BK, Saltin B. Exercise as medicine—Evidence for prescribing exercise as therapy in 26 different chronic diseases. Scand J Med Sci Sports 2015;25:1–72. [DOI] [PubMed] [Google Scholar]
- 16.Fiuza-Luces C, Garatachea N, Berger NA, et al. Exercise is the real polypill. Physiology 2013;28:330–58. [DOI] [PubMed] [Google Scholar]
- 17.Choi JJ, Eum SY, Rampersaud E, et al. Exercise attenuates PCB-induced changes in the mouse gut microbiome. Environ Health Perspect 2013;121:725–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lambert JE, Myslicki JP, Bomhof MR, et al. Exercise training modifies gut microbiota in normal and diabetic mice. Appl Physiol Nutr Metab 2015;40:749–52. [DOI] [PubMed] [Google Scholar]
- 19.Evans CC, LePard KJ, Kwak JW, et al. Exercise prevents weight gain and alters the gut microbiota in a mouse model of high fat diet-induced obesity. PLoS One 2014;9:e92193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Sire R, Rizzatti G, Ingravalle F, et al. Skeletal muscle-gut axis: Emerging mechanisms of sarcopenia for intestinal and extra intestinal diseases. Minerva Gastroenterol Dietol 2018;64:351–62. [DOI] [PubMed] [Google Scholar]
- 21.Cerdá B, Pérez M, Pérez-Santiago JD, et al. Gut microbiota modification: Another piece in the puzzle of the benefits of physical exercise in health? Front Physiol 2016;7:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen J, Guo Y, Gui Y, et al. Physical exercise, gut, gut microbiota, and atherosclerotic cardiovascular diseases. Lipids Health Dis 2018;17:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Monda V, Villano I, Messina A, et al. Exercise modifies the gut microbiota with positive health effects. Oxid Med Cell Longev 2017;2017:3831972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Codella R, Luzi L, Terruzzi I. Exercise has the guts: How physical activity may positively modulate gut microbiota in chronic and immune-based diseases. Dig Liver Dis 2018;50:331–41. [DOI] [PubMed] [Google Scholar]
- 25.Mach N, Fuster-Botella D. Review: Endurance exercise and gut microbiota: A review. J Sport Heal Sci 2016;6:179–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mitchell CM, Davy BM, Hulver MW, et al. Does exercise alter gut microbial composition? A systematic review. Med Sci Sport Exerc 2018;51:160–7. [DOI] [PubMed] [Google Scholar]
- 27.Petersen LM, Bautista EJ, Nguyen H, et al. Community characteristics of the gut microbiomes of competitive cyclists. Microbiome 2017;5:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barton W, Penney NC, Cronin O, et al. The microbiome of professional athletes differs from that of more sedentary subjects in composition and particularly at the functional metabolic level. Gut 2018;67:625–33. [DOI] [PubMed] [Google Scholar]
- 29.Munukka E, Ahtiainen JP, Puigbó P, et al. Six-week endurance exercise alters gut metagenome that is not reflected in systemic metabolism in over-weight women. Front Microbiol 2018;9:2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morita E, Yokoyama H, Imai D, et al. Aerobic exercise training with brisk walking increases intestinal bacteroides in healthy elderly women. Nutrients 2019;11:868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Keohane DM, Woods T, O'Connor P, et al. Four men in a boat: Ultra-endurance exercise alters the gut microbiome. J Sci Med Sport 2019;22:1059–64. [DOI] [PubMed] [Google Scholar]
- 32.Scheiman J, Luber JM, Chavkin TA, et al. Meta-omics analysis of elite athletes identifies a performance-enhancing microbe that functions via lactate metabolism. Nat Med 2019;25:1104–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Durk RP, Castillo E, Márquez-Magaña L, et al. Gut microbiota composition is related to cardiorespiratory fitness in healthy young adults. Int J Sport Nutr Exerc Metab 2018;29:249–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mörkl S, Lackner S, Müller W, et al. Gut microbiota and body composition in anorexia nervosa inpatients in comparison to athletes, overweight, obese, and normal weight controls. Int J Eat Disord 2017;50:1421–31. [DOI] [PubMed] [Google Scholar]
- 35.Estaki M, Pither J, Baumeister P, et al. Cardiorespiratory fitness as a predictor of intestinal microbial diversity and distinct metagenomic functions. Microbiome 2016;4:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang Y, Shi Y, Wiklund P, et al. The association between cardiorespiratory fitness and gut microbiota composition in premenopausal women. Nutrients 2017;9:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shukla SK, Cook D, Meyer J, et al. Changes in gut and plasma microbiome following exercise challenge in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). PLoS One 2015;10:e0145453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lundgren-Kownacki K, Dahl M, Gao C, et al. Exploring how a traditional diluted yoghurt drink may mitigate heat strain during medium-intensity intermittent work: A multidisciplinary study of occupational heat strain. Ind Health 2017;56:106–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhao X, Zhang Z, Hu B, et al. Response of gut microbiota to metabolite changes induced by endurance exercise. Front Microbiol 2018;9:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cronin O, Barton W, Skuse P, et al. A prospective metagenomic and metabolomic analysis of the impact of exercise and/or whey protein supplementation on the gut microbiome of sedentary adults. mSystems 2018;3:e00044–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ 2009;339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.de Morton NA. The PEDro scale is a valid measure of the methodological quality of clinical trials: A demographic study. Aust J Physiother 2009;55:129–33. [DOI] [PubMed] [Google Scholar]
- 43.Maher CG, Sherrington C, Herbert RD, et al. Reliability of the PEDro scale for rating quality of randomized controlled trials. Phys Ther 2003;83:713–21. [PubMed] [Google Scholar]
- 44.McFadzean R. Exercise can help modulate human gut microbiota. Undergraduate Honors Theses 2014; Paper 155. [Google Scholar]
- 45.Clarke SF, Murphy EF, O'Sullivan O, et al. Exercise and associated dietary extremes impact on gut microbial diversity. Gut 2014;63:1913–20. [DOI] [PubMed] [Google Scholar]
- 46.Bressa C, Bailén-Andrino M, Pérez-Santiago J, et al. Differences in gut microbiota profile between women with active lifestyle and sedentary women. PLoS One 2017;12:e0171352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Allen JM, Mailing LJ, Niemiro GM, et al. Exercise alters gut microbiota composition and function in lean and obese humans. Med Sci Sports Exerc 2018;50:747–57. [DOI] [PubMed] [Google Scholar]
- 48.Ticinesi A, Lauretani F, Tana C, et al. Exercise and immune system as modulators of intestinal microbiome: Implications for the gut-muscle axis hypothesis. Exerc Immunol Rev 2019;25:84–95. [PubMed] [Google Scholar]
- 49.Pedersen BK. The diseasome of physical inactivity—and the role of myokines in muscle—fat cross talk. J Physiol (Lond) 2009;587:5559–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hamasaki H. Exercise and glucagon-like peptide-1: Does exercise potentiate the effect of treatment? World J Diabetes 2018;9:138–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Steinbacher P, Eckl P. Impact of oxidative stress on exercising skeletal muscle. Biomolecules 2015;5:356–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Clark A, Mach N. The crosstalk between the gut microbiota and mitochondria during exercise. Front Physiol 2017;8:319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kimura I, Inoue D, Hirano K, et al. The SCFA receptor GPR43 and energy metabolism. Front Endocrinol (Lausanne) 2014;5:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tan J, McKenzie C, Potamitis M, et al. The role of short-chain fatty acids in health and disease. Adv Immunol 2014;121:91–119. [DOI] [PubMed] [Google Scholar]
- 55.Christiansen CB, Gabe MBN, Svendsen B, et al. The impact of short-chain fatty acids on GLP-1 and PYY secretion from the isolated perfused rat colon. Am J Physiol Liver Physiol 2018;315:G53–65. [DOI] [PubMed] [Google Scholar]
- 56.Rivière A, Selak M, Lantin D, et al. Bifidobacteria and butyrate-producing colon bacteria: Importance and strategies for their stimulation in the human gut. Front Microbiol 2016;7:979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Derrien M, Belzer C, de Vos WM. Akkermansia muciniphila and its role in regulating host functions. Microb Pathog 2017;106:171–81. [DOI] [PubMed] [Google Scholar]
- 58.Canfora EE, Jocken JW, Blaak EE. Short-chain fatty acids in control of body weight and insulin sensitivity. Nat Rev Endocrinol 2015;11:577–91. [DOI] [PubMed] [Google Scholar]
- 59.Walsh ME, Bhattacharya A, Sataranatarajan K, et al. The histone deacetylase inhibitor butyrate improves metabolism and reduces muscle atrophy during aging. Aging Cell 2015;14:957–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Depommier C, Everard A, Druart C, et al. Supplementation with Akkermansia muciniphila in overweight and obese human volunteers: A proof-of-concept exploratory study. Nat Med 2019;25:1096–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ehrenpreis ED, Swamy RS, Zaitman D, et al. Short duration exercise increases breath hydrogen excretion after lactulose ingestion: Description of a new phenomenon. Am J Gastroenterol 2002;97:2798–802. [DOI] [PubMed] [Google Scholar]
- 62.Lozupone CA, Stombaugh JI, Gordon JI, et al. Diversity, stability and resilience of the human gut microbiota. Nature 2012;489:220–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Battaglia GM, Zheng D, Hickner RC, et al. Effect of exercise training on metabolic flexibility in response to a high-fat diet in obese individuals. Am J Physiol Endocrinol Metab 2012;303:E1440–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fernández-Verdejo R, Bajpeyi S, Ravussin E, et al. Metabolic flexibility to lipid availability during exercise is enhanced in individuals with high insulin sensitivity. Am J Physiol Metab 2018;315:E715–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Prince SA, Adamo KB, Hamel M, et al. A comparison of direct versus self-report measures for assessing physical activity in adults: A systematic review. Int J Behav Nutr Phys Act 2008;5:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kraemer WJ, Deschenes MR, Fleck SJ. Physiological adaptations to resistance exercise: Implications for athletic conditioning. Sport Med An Int J Appl Med Sci Sport Exerc 1988;6:246–56. [DOI] [PubMed] [Google Scholar]
- 67.Welinder C, Ekblad L, Aarsland A, et al. Physiological adaptations to resistance exercise as a function of age. J Proteome Res 2017;10:1416–9. [Google Scholar]
- 68.Rustaden AM, Gjestvang C, Bø K, et al. BodyPump versus traditional heavy load resistance training on changes in resting metabolic rate in overweight untrained women. J Sports Med Phys Fitness 2018;58:1304–1. [DOI] [PubMed] [Google Scholar]
- 69.David LA, Maurice CF, Carmody RN, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014;505:559–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Reddy BS, Weisburger JH, Wynder EL. Effects of high risk and low risk diets for colon carcinogenesis on fecal Microflora and steroids in man. J Nutr 1975;105:878–84. [DOI] [PubMed] [Google Scholar]
- 71.Cotillard A, Kennedy SP, Kong LC, et al. Dietary intervention impact on gut microbial gene richness. Nature 2013;500:585–8. [DOI] [PubMed] [Google Scholar]
- 72.Fava F, Gitau R, Griffin BA, et al. The type and quantity of dietary fat and carbohydrate alter faecal microbiome and short-chain fatty acid excretion in a metabolic syndrome “at-risk” population. Int J Obes (Lond) 2013;37:216–23. [DOI] [PubMed] [Google Scholar]
- 73.Caesar R, Tremaroli V, Kovatcheva-Datchary P, et al. Crosstalk between gut microbiota and dietary lipids aggravates WAT inflammation through TLR signaling. Cell Metab 2015;22:658–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Eid N, Enani S, Walton G, et al. The impact of date palm fruits and their component polyphenols, on gut microbial ecology, bacterial metabolites and colon cancer cell proliferation. J Nutr Sci 2014;3:e46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Edgar R. Taxonomy annotation and guide tree errors in 16S rRNA databases. PeerJ 2018;6:e5030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rewarding negative results keeps science on track. Nature 2017;551:414. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.