OBJECTIVES:
Helicobacter pylori may reportedly be associated with extragastric malignancy beyond gastric cancer. The present study aimed to evaluate the association between H. pylori infection and colorectal neoplasia through a systematic review and meta-analysis.
METHODS:
The literature search aimed to retrieve all relevant studies published up to September 2019 that examined the risk for colorectal neoplasia including colorectal adenoma, advanced adenoma, and cancer in patients with H. pylori infection. Meta-analysis was performed to calculate pooled odds ratios (ORs) with corresponding 95% confidence intervals (CIs). If publication bias was observed, the pooled OR was adjusted using the trim-and-fill method.
RESULTS:
Forty-eight studies including 171,045 patients were evaluated, of which 24, 8, and 31 reported H. pylori-associated risk for adenoma, advanced adenoma, and cancer, respectively. H. pylori infection was associated with a significantly higher risk for colorectal adenoma (pooled OR 1.49 [95% CI 1.37–1.62]). H. pylori infection was also associated with a higher risk for advanced colorectal adenoma (pooled OR 1.50 [95% CI 1.28–1.75]). The risk for colorectal cancer in patients with H. pylori infection was also identified (pooled OR 1.44 [95% 1.26–1.65]). Although publication bias was identified in the analysis for colorectal adenoma, the pooled estimate was not significantly changed after adjustment (pooled OR 1.39 [95% CI 1.27–1.52]).
DISCUSSION:
Although this meta-analysis based on the observational studies could not show causality, it demonstrated that colorectal adenoma, advanced adenoma, and cancer were all associated with H. pylori infection.
INTRODUCTION
Approximately one-half of the world's population is infected with Helicobacter pylori, which can be colonized in the stomach through the production of urease (1). H. pylori infection is a well-known risk factor for various gastrointestinal diseases including peptic ulcer and gastric cancer (2). In addition, H. pylori infection is associated with extragastric diseases including immune thrombocytopenic purpura and iron deficiency anemia (3,4).
Interestingly, it has been suggested that H. pylori is also associated with extragastric carcinogenesis including colorectal neoplasia (5). One hypothesis that has been proposed is that H. pylori may facilitate carcinogenesis through hypergastrinemia caused by atrophic gastritis, followed by a decrease in acid secretion (6). The growth-promoting property of gastrin has been postulated to be associated with the development of several tumors including lung cancer and colorectal cancer (CRC). Although the causal relationship between hypergastrinemia and colorectal neoplasia has not yet been fully evaluated, the association between H. pylori infection and colorectal neoplasia has been reported in many recent studies (7–15). In particular, many studies have consistently reported an association between H. pylori infection and colorectal adenoma (11–14). However, there have been conflicting results regarding the association between H. pylori infection and CRC. Several studies have reported a significantly higher proportion of CRCs in patients with H. pylori infection than those without H. pylori infection (7,9), whereas others have not (8,10). Although new studies examining H. pylori infection and CRC continue to be published (16–18), definitive conclusions regarding associations between H. pylori infection and CRC remain elusive.
The insignificant impact of H. pylori infection on CRC reported in several studies may be because of a small number of patients with CRC, which may affect the precision of the effect size (8,10,13). In this situation, a meta-analysis is useful for summarizing the results derived from all relevant studies and provides more precise estimates. Although several meta-analyses investigating this issue have been conducted previously (5,19–21), an updated meta-analysis is needed because many new studies have been published in the interim (10–18,22–25). Here, we aimed to evaluate the association between H. pylori infection and colorectal adenoma, advanced adenoma, and cancer through a systematic review and meta-analysis. It may be helpful to comprehensively understand the risk for colorectal neoplasia in patients with H. pylori infection.
METHODS
Search strategy
All relevant studies published between January 1980 and September 2019 that examined the risk of colorectal neoplasia, including colorectal adenoma, advanced adenoma, and cancer, in patients with H. pylori infection were identified through a literature search using the MEDLINE, Embase, and Cochrane Library databases. The following search string was used: ([Helicobacter] OR [pylori] OR [Campylobacter]) AND ([colorectal] OR [colon] OR [colons] OR [colonic] OR [rectal] OR [rectum] OR [large bowel] OR [large bowels] OR [large intestine] OR [large intestines] OR [large intestinal]) AND ([neoplasm] OR [neoplasms] OR [neoplasia] OR [cancer] OR [cancers] OR [carcinoma] OR [carcinomas] OR [adenoma] OR [adenomas] OR [polyp] OR [polyps] OR [polypoid] OR [tumor] OR [tumors] OR [tumour] OR [tumours]). The following Medline Subject Headings and Embase subject headings were also used for the literature search: Helicobacter, Colorectal neoplasms (for Medline Subject Headings), and Colorectal tumor (for Embase subject headings). The detailed search strategies used in each database are shown in Appendix 1, http://links.lww.com/CTG/A188. In addition, the references of the screened articles were manually searched to identify additional relevant studies. The date of the most recent search update was September 17, 2019.
Study selection
In the first stage of study selection, duplicate articles retrieved using multiple search engines based on the title, abstract, and bibliographic data were discarded. Next, irrelevant articles were excluded by examining titles and abstracts. Finally, the articles were screened by full-text reviews according to the inclusion and exclusion criteria. The inclusion criteria were as follows: patients (individuals who underwent screening colonoscopy for colorectal neoplasia); intervention (H. pylori infection in the stomach); comparator (no H. pylori infection in the stomach); outcome (risk for colorectal neoplasia including colorectal adenoma, advanced adenoma, and cancer in patients with H. pylori infection); and study design (cross-sectional or case-control study). Investigations involving nonhumans, abstracts or unpublished studies, and those published in a language other than English were excluded. If the study population overlapped among studies, the largest study was selected. Two investigators (D.S.C. and C.H.P.) independently evaluated the studies for eligibility; any disagreements were resolved through discussion and consensus. If no agreement could be reached, a third investigator (S.I.S.) determined the eligibility.
The methodological quality of individual studies was evaluated by 2 investigators (D.S.C. and C.H.P.) using the Newcastle-Ottawa scale, whereby the observational studies were scored across 3 categories: selection (4 questions), comparability of the study groups (2 questions), and ascertainment of exposure or outcome (3 questions) (26). Questions regarding the comparability of the study groups were awarded a maximum of 2 points; all other questions were assigned a score of 1 point. Studies with a cumulative score ≥ 7 were considered to be high quality (27–29).
Data extraction and study endpoint
Using a data extraction form that was developed in advance, the 2 reviewers (D.S.C. and C.H.P.) independently extracted the following information: first author; year of publication; study design; country in which the study was conducted; study period; study population; testing for H. pylori infection; and risk for colorectal adenoma, advanced adenoma, and cancer with H. pylori infection. Advanced adenoma was defined by the presence of one of the following features in accordance with the current guideline (30): villous component, ≥10 mm in size, and high-grade dysplasia.
The primary endpoint of this meta-analysis was the overall risk for colorectal neoplasia including colorectal adenoma and cancer. The secondary endpoint was the risk for colorectal neoplasia according to the testing method used to diagnose H. pylori infection and study design.
Statistical analysis
A meta-analysis was performed to calculate pooled odds ratios (ORs) with corresponding 95% confidence intervals (CIs). A random-effects model was used in this meta-analysis. Study heterogeneity was assessed using 2 methods: Cochran Q test, which was considered to be statistically significant for heterogeneity if P was <0.1, and the I2 statistic, with values > 50% suggestive of significant heterogeneity (31). Publication bias was assessed using the Egger regression test (32). Heterogeneity was also visually examined using funnel plots of the logarithmic ORs vs their SEs (33). If publication bias was observed, pooled ORs were adjusted using the Duval and Tweedie trim-and-fill method (34). All P-values were 2-tailed, and P < 0.05 was considered to be statistically significant in all tests (except for heterogeneity, and the Egger regression test). In addition, subgroup analyses were conducted according to the inclusion of immunoglobulin [Ig] G anti-H. pylori antibody as a test for H. pylori infection because IgG anti-H. pylori antibody represents past or current infection of H. pylori, whereas other tests such as rapid urease test, histologic examination, or culture identify only current H. pylori infection. Individual studies that used IgG anti-H. pylori antibody and other tests were classified into the IgG anti-H. pylori antibody subgroup, whereas those that used H. pylori tests other than IgG anti-H. pylori antibody were classified into other-than-IgG anti-H. pylori antibody subgroup. Subgroup analyses were further performed according to the study design (cross-sectional vs case-control) and population (hospital-based vs community-based). Sensitivity analysis was also performed after excluding individual studies with a low-quality score or outlier.
Analysis and reporting were performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines (35). All statistical procedures were performed using Review Manager version 5.3.5 (Cochrane Collaboration, Copenhagen, Denmark), with the exception of publication bias analysis, which was performed using R (version 3.6.0; R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Study selection and characteristics
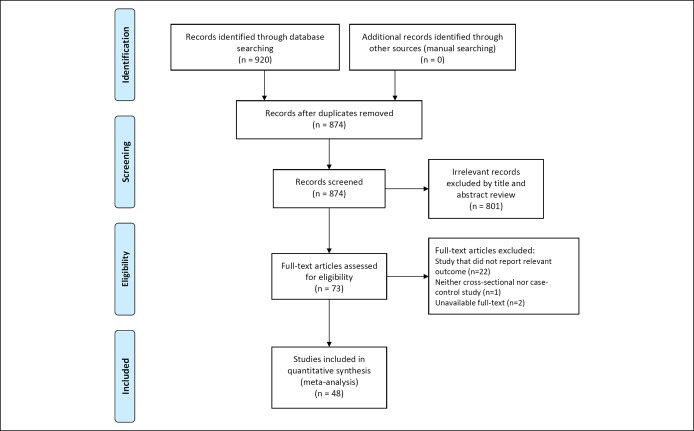
A flow diagram of study selection is shown in Figure 1. A total of 920 studies were identified in the literature search. Among the 920 studies, 46 duplicates across the multiple search engines were discarded. An additional 801 irrelevant studies were excluded based on their titles and abstracts. After full-text review of these 73 studies, 25 were excluded for the following reasons: did not report relevant outcomes (n = 22), neither cross-sectional nor case-control study (n = 1), and unavailable full-text (n = 2).
Figure 1.
Flow diagram of studies included in the meta-analysis.
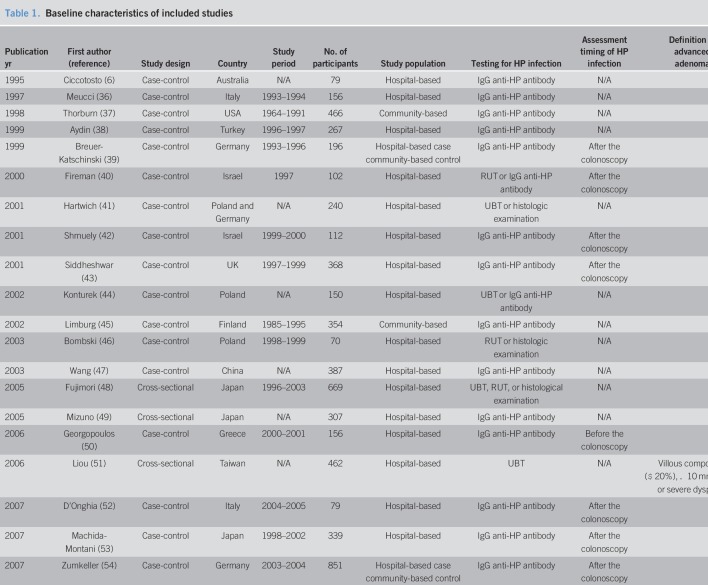
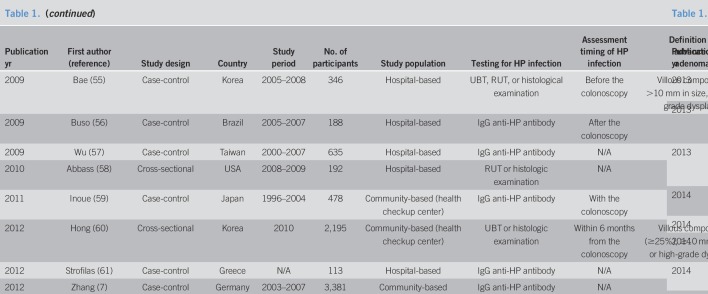
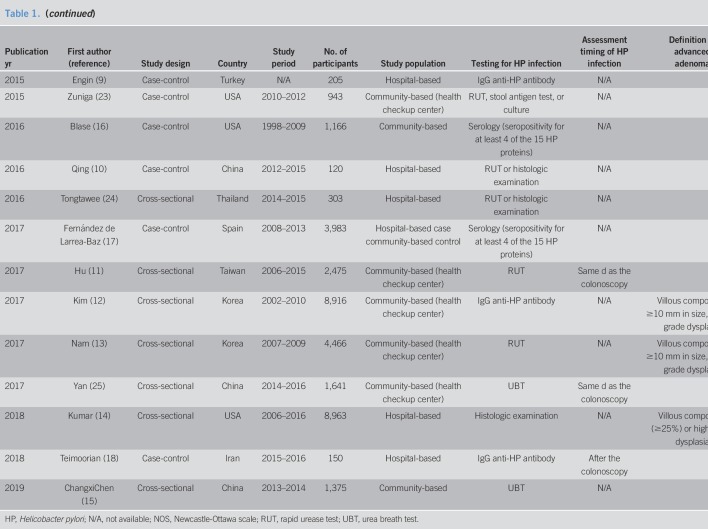
Ultimately, 48 studies involving a total of 171,045 patients were included in the meta-analysis (6–18, 22–25, 36–66). The characteristics of the included studies are summarized in Table 1 . These studies were published between 1995 and 2019, and their enrollment periods ranged from 1964 to 2016 . Of the 44 studies, 18 were cross-sectional and the remaining 30 were case-control. Serological testing including IgG anti-H. pylori antibody was used in 31 studies to diagnose H. pylori infection. The urea breath test, rapid urease test, and histological examination were used in 8, 10, and 12 individual studies, respectively.
Table 1.
Baseline characteristics of included studies
Table 1-A.
Baseline characteristics of included studies (continued)
Table 1-B.
Baseline characteristics of included studies (continued)
The Newcastle-Ottawa scale quality scores of the included studies are shown in Table 1, with scores ranging from 4 to 9. Of the 44 included studies, 33 (68.8%) were rated as high quality.
The risk for colorectal adenoma
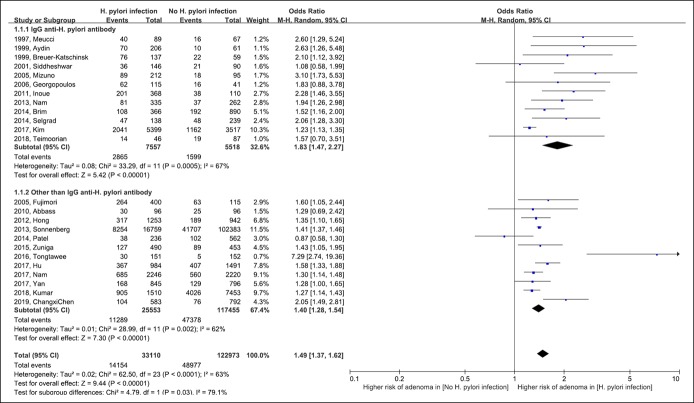
The risk for colorectal adenoma in patients with H. pylori infection is shown in Figure 2. Overall, H. pylori infection was associated with a significantly higher risk for colorectal adenoma (pooled OR 1.49 [95% CI 1.37–1.62]; degrees of freedom [df] = 23; P < 0.001; I2 = 63%). In the subgroup analysis of studies that used IgG anti-H. pylori antibody to diagnose H. pylori infection, H. pylori infection was associated with an increased risk for colorectal adenoma (pooled OR 1.83 [95% CI 1.47–2.27]; df = 11; P < 0.001; I2 = 67%). H. pylori infection was also associated with a high risk for colorectal adenoma in a subgroup analysis of studies in which H. pylori testing, other than anti-H. pylori antibody, was used (pooled OR 1.40 [95% CI 1.28–1.54]; df = 11; P = 0.002; I2 = 62%). The risk for colorectal adenoma appeared to be higher in the IgG anti-H. pylori antibody subgroup than the other subgroup (test for subgroup difference, df = 1; P = 0.03; I2 = 79%).
Figure 2.
Forest plot of the risk for colorectal adenoma with Helicobacter pylori infection. CI, confidence interval; M-H, Mantel-Haenszel.
The risk for advanced adenoma
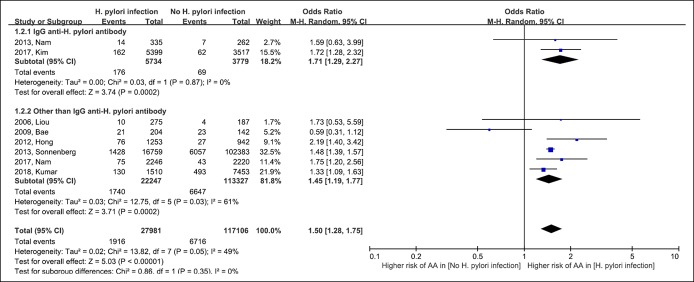
The risk for advanced adenoma was reported in 8 included studies (12–14,51,55,60,63,64). As shown in Table 1, the studies used the definition of advanced adenoma similar to those proposed in the guideline (30). Figure 3 shows the risk for advanced adenoma in patients with H. pylori infection. The pooled OR for H. pylori infection for advanced adenoma was similar to that for colorectal adenoma (pooled OR 1.50 [95% CI 1.28–1.75]; df = 7; P = 0.05; I2 = 49%). The pooled OR was 1.71 (95% CI 1.29–2.27) in the IgG anti-H. pylori antibody group and 1.45 (95% CI 1.19–1.77) in the other-than-IgG anti-H. pylori antibody group. The risk for advanced colorectal adenoma did not differ between the subgroups (test for subgroup difference, df = 1; P = 0.35; I2 = 0%).
Figure 3.
Forest plot of the risk for advanced colorectal adenoma with Helicobacter pylori infection. AA, advanced adenoma; CI, confidence interval; M-H, Mantel-Haenszel.
The risk for colorectal cancer
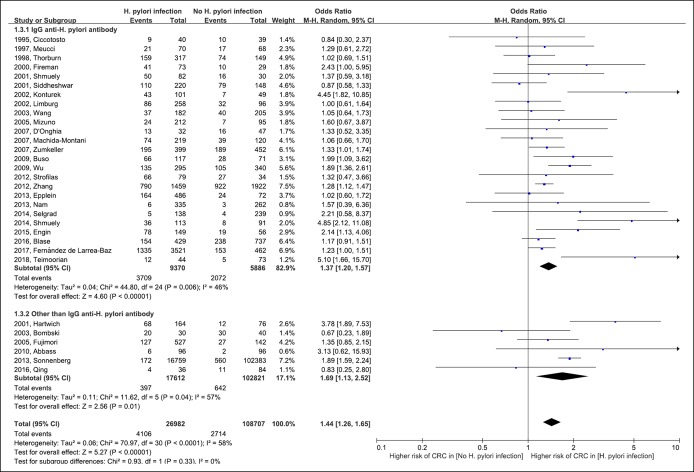
Figure 4 shows the risk for CRC in patients with H. pylori infection. Although 21 of 31 included studies did not report statistically significant results, the results of meta-analysis revealed a significant association between H. pylori infection and CRC (pooled OR 1.44 [95% CI 1.13–2.52]; df = 30; P < 0.001; I2 = 58%). The results of subgroup analysis according to the H. pylori test method did not differ between the subgroups (IgG anti-H. pylori antibody subgroup: pooled OR 1.37 [95% CI 1.20–1.57] vs other-than-IgG anti-H. pylori antibody subgroup: pooled OR 1.69 [95% CI 1.13–2.52]; test for subgroup difference: df = 1; P = 0.93; I2 = 0%).
Figure 4.
Forest plot of the risk for colorectal cancer with Helicobacter pylori infection. CRC, colorectal cancer; CI, confidence interval; M-H, Mantel-Haenszel.
Publication bias
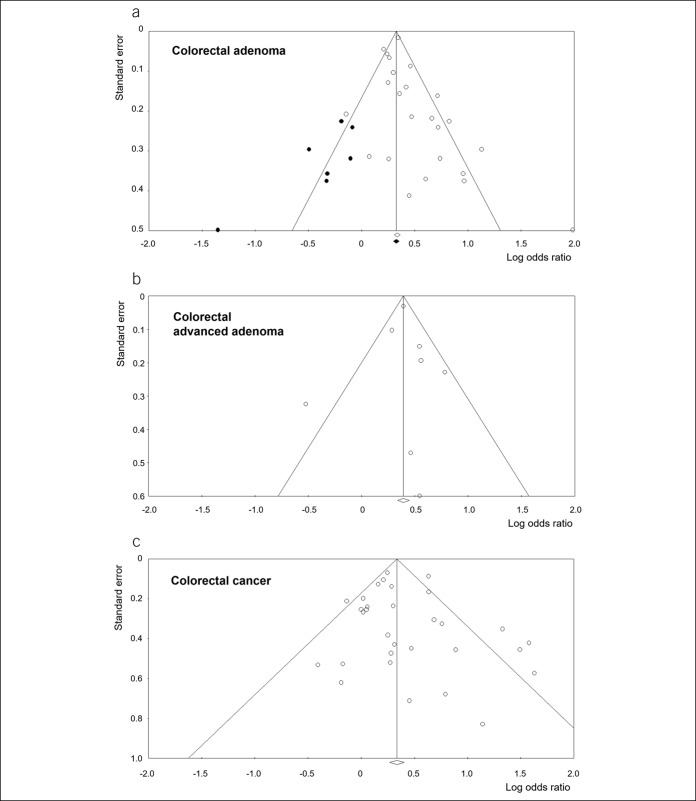
Publication bias was assessed for risk for colorectal adenoma, advanced adenoma, and cancer (Figure 5). There was an asymmetry in the funnel plot for colorectal adenoma. Because significant publication bias was also identified in the Egger regression test for colorectal adenoma (P = 0.046), the Duval and Tweedie trim-and-fill method was applied to the sensitivity analysis. After this adjustment, the significance of the association between H. pylori infection and colorectal adenoma was maintained (pooled OR 1.39 [95% 1.27–1.52]). Meanwhile, publication bias was not identified for advanced adenoma and CRC (P = 0.980 and P = 0.352, respectively [Egger regression test]).
Figure 5.
Funnel plots for the analysis of publication bias. (a) Colorectal adenoma, (b) colorectal adenoma, and (c) colorectal cancer. The white diamond represents the pooled logarithmic odds ratio and corresponding 95% confidence interval, among studies. The black diamond represents the pooled logarithmic odds ratio and corresponding 95% confidence interval, among the observed and imputed studies.
Subgroup analysis according to study design and population
Additional subgroup analyses according to the design of the included studies (i.e., cross-sectional and case-control) were performed (see Figure S1, Supplementary Digital Content 1, http://links.lww.com/CTG/A182, http://links.lww.com/CTG/A183, http://links.lww.com/CTG/A184). Overall, the results of subgroup analyses were similar to the original analysis. The pooled OR for H. pylori infection for colorectal adenoma was 1.44 (95% CI 1.32–1.57) in the cross-sectional studies and 1.78 (95% CI 1.43–2.20) in the case-control study.
Regarding advanced adenoma, the pooled OR was 1.49 (95% CI 1.41–1.58) in the cross-sectional studies. The pooled OR in the 1 case-control study was 0.59 (95% CI 0.31–1.12); however, it is important to note that there was only a single study included in this subgroup analysis. In addition, the pooled OR for H. pylori infection for CRC was 1.91 (95% CI 1.46–2.48) in the cross-sectional studies and 1.34 (95% CI 1.17–1.54) in the case-control studies.
In the subgroup analyses according to the study population (see Figure S2, Supplementary Digital Content 1, http://links.lww.com/CTG/A185, http://links.lww.com/CTG/A186, http://links.lww.com/CTG/A187), the pooled OR for H. pylori infection for colorectal adenoma was 1.71 (95% CI 1.38–2.11) in the hospital-based population and 1.43 (95% CI 1.37–1.62) in the community-based population. Regarding advanced adenoma, although the pooled OR tended to be lower in the hospital-based population (1.07 [95% CI 0.59–1.94]) than in the community-based population (1.55 [95% CI 1.40–1.71]), there was no significant difference. The pooled OR of H. pylori infection for CRC was 1.57 (95% CI 1.30–1.89) in the hospital-based population and 1.27 (95% CI 1.26–1.65) in the community-based population.
Sensitivity analysis
Sensitivity analyses excluding the 15 low-quality studies were performed to assess the robustness of the meta-analyses. Overall, the results of sensitivity analysis were similar to those of the original analysis. Even in only high-quality studies, H. pylori infection was associated with an increased risk for colorectal adenoma (pooled OR 1.45 [95% CI 1.34–1.57]), advanced adenoma (pooled OR 1.49 [95% CI 1.27–1.76]), and cancer (pooled OR 1.46 [95% CI 1.25–1.71]).
Additional sensitivity analysis was performed after excluding the study by Tongtawee et al. (24) which had an outlier in the analysis of colorectal adenoma. As a result, the pooled OR of H. pylori infection for colorectal adenoma was 1.46 (95% CI 1.35–1.57).
DISCUSSION
Several meta-analyses examining the relationship between H. pylori infection and colorectal neoplasia have been published (5,19–21). However, these meta-analyses included 9–28 individual studies, which represented only a portion of all relevant studies published to date. Furthermore, some of these meta-analyses included individual studies examining H. pylori infection in colorectal tissues, not intragastric H. pylori infection (5,21). Through a systematic review, we included only studies that evaluated the association between intragastric H. pylori infection and colorectal neoplasia. In addition, we identified 14 new individual studies and included them among the 48 studies in the present meta-analysis. Our large-scale meta-analysis provides reliable pooled estimates with high precision. It also evaluated the impact of diagnostic tools or study design on the association between H. pylori infection and colorectal neoplasia through subgroup analyses.
In this study, the risks for colorectal adenoma, advanced colorectal adenoma, and CRC were similar (OR [95% CI]: adenoma vs advanced adenoma vs CRC, 1.49 [1.37–1.62] vs 1.50 [1.28–1.75] vs 1.44 [1.26–1.65]). This finding implied that H. pylori infection may be associated with the early stages of colorectal carcinogenesis. If H. pylori infection is associated with only the advanced stage of colorectal carcinogenesis (such as advanced adenoma or CRC), the risk for colorectal adenoma may be lowered. Of course, we cannot conclude that H. pylori infection facilitates colorectal carcinogenesis because a causal relationship cannot be determined through analysis of cross-sectional or case-control studies. However, H. pylori infection usually occurs when individuals are young and continues until old age if not treated (67). Therefore, it is likely that colorectal neoplasia occurred in patients with H. pylori infection, rather than the possibility of new H. pylori infection in patients who had colorectal neoplasia. In addition, a previous cohort study demonstrated that H. pylori-infected individuals had a 73% higher risk for CRC development during the follow-up period compared with noninfected individuals (68).
There are several hypotheses that support the relationship between H. pylori infection and colorectal neoplasia, one of the most plausible of which is hypergastrinemia in patients with chronic H. pylori infection. Chronic gastritis by H. pylori infection can cause gastric mucosal atrophy, followed by intestinal metaplasia, which results in a decrease in gastric acid secretion from the parietal cells and an increase in intragastric pH. As a result, serum gastrin level is increased by a negative feedback mechanism. Hypergastrinemia may facilitate proliferation of colorectal mucosa, making the colon and rectum more susceptible to carcinogenesis (37,41).
Another hypothesis suggesting the association between H. pylori infection and colorectal neoplasia is the dysbiosis of the gut microbiome in patients with H. pylori infection. In healthy individuals, gastric acid acts as a barrier to various environmental and oral microorganisms. If the gastric acid secretion is decreased, various bacteria can colonize the stomach and gut dysbiosis can be induced (69–71). Although various pathogenetic mechanisms involve colorectal carcinogenesis, changes in the gut microbiome play a very important role in colorectal carcinogenesis (72,73).
The direct carcinogenic effect of H. pylori may also be considered as a cause of high risk for colorectal neoplasia in patients with H. pylori infection. A previous study reported that H. pylori reside in the colorectum and are associated with colorectal neoplasia (74). In that study, the prevalence of H. pylori was higher in both colorectal adenoma tissues and CRC tissues than in normal colorectal tissues. Although the study did not evaluate causal relationships, the results suggested an association between colorectal neoplasia and H. pylori colonization in the colorectum as well as intragastric H. pylori infection.
Our meta-analysis now supports the association between H. pylori infection and CRC; however, this was not previously clear. In fact, of the 31 studies that reported a risk for CRC in patients with H. pylori infection, only 10 demonstrated a significant difference. Twelve studies reported a tendency toward increased risk for CRC without statistical significance in patients who had H. pylori infection. This may be because of the small sample sizes in individual studies examining CRC. By contrast, most individual studies demonstrated a significantly increased risk for adenoma in patients with H. pylori infection. Because the prevalence of colorectal adenoma is higher than that of CRC, it would have been easier to demonstrate statistically significant results.
One other issue in determining the association between H. pylori infection and colorectal neoplasia is the method used to diagnose H. pylori infection. Although serological testing, including IgG anti-H. pylori antibody, is a popular screen for H. pylori infection, it has a limitation in that it does not guarantee current H. pylori infection (75). Therefore, there is a possibility that the impact of H. pylori infection on the risk for colorectal neoplasia may depend on the methods used to diagnose H. pylori infection—IgG anti-H. pylori antibody or other tests. In our subgroup analysis, the risk for colorectal adenoma was higher when H. pylori infection was determined using IgG anti-H. pylori antibody compared with other tests. It may be because studies that used IgG anti-H. pylori antibody included more patients with severe atrophy and intestinal metaplasia. Patients with past H. pylori infections, who usually exhibit severe atrophy and intestinal metaplasia, may be positive for IgG anti-H. pylori antibody, but negative results in other tests including urea breath test and rapid urease test. For now, however, it can be somewhat difficult to draw definitive conclusions because the risk for CRC did not appear to be influenced by the diagnostic methods in our subgroup analysis.
Although we demonstrated an increased risk for colorectal neoplasia in patients with H. pylori infection, whether H. pylori eradication has a beneficial effect on colorectal neoplasia is another issue. Generally, it has been known that H. pylori eradication lowers the risk for gastric cancer by 35% in the general population and by 50% in high-risk populations (76–78). Although it is not clear whether H. pylori eradication prevents the development of colorectal neoplasia, we cautiously speculate that H. pylori eradication may reduce the risk for CRC to a similar extent as gastric cancer.
Although this study was the largest meta-analysis to date and provided precise estimates, it had several limitations. First, all included studies were observational in nature. Although most studies included consecutive patients during the study period, potential selection bias may be a concern. Moreover, 15 of the 48 included studies were assessed to be of low quality. Although sensitivity analysis including only high-quality studies revealed that the pooled estimates were similar to the original results, the meta-analysis findings should be cautiously interpreted. Second, significant heterogeneity was identified in the meta-analyses. This may have been caused by some deviation in the studies from the pooled estimates. However, the direction of effect size was consistent among most of the individual studies. Third, there was a publication bias in the analysis for colorectal adenoma. Small studies tended to report relatively high effect size. This is an obvious limitation to our meta-analysis; however, pooled estimates were not significantly changed after adjusting missing studies by the trim-and-fill method. Fourth, we could not adjust for potentially confounding variables in the meta-analysis because not all included studies provided adjusted ORs.
Despite these limitations, our study findings provide a better understanding of the risk for colorectal neoplasia, including colorectal adenoma and CRC, in patients with H. pylori infection. Although our meta-analysis based on the observational studies could not show causality, it demonstrates that colorectal adenoma, advanced adenoma, and cancer were all associated with H. pylori infection.
CONFLICTS OF INTEREST
Guarantor of the article: Chan Hyuk Park, MD, PhD.
Specific author contributions: Conception and design: C.H.P., acquisition of data: D.S.C. and C.H.P., analysis and interpretation of data: D.S.C., S.S.I., and C.H.P., statistical analysis: C.H.P., drafting manuscript: D.S.C., critical revision of the article for important intellectual content: S.I.S. and W.G.S., and final approval of the article: all authors.
Financial support: This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HI19C0143).
Potential competing interests: None to report.
Study Highlights.
WHAT IS KNOWN
✓ Helicobacter pylori infection is a well-known risk factor for various gastrointestinal diseases including peptic ulcer and gastric cancer.
✓ It has been suggested that H. pylori is associated with extragastric malignancies.
WHAT IS NEW HERE
✓ The meta-analysis demonstrated the association, rather than causality, between H. pylori infection and colorectal neoplasms including colorectal adenoma, advanced adenoma, and cancer.
✓ The relative risks for colorectal adenoma, advanced adenoma, and cancer in patients with H. pylori infection were similar.
TRANSLATIONAL IMPACT
✓ This meta-analysis may be the basis of study on prevention of colorectal neoplasms through the treatment of H. pylori infection.
Supplementary Material
Footnotes
SUPPLEMENTARY MATERIAL accompanies this paper at http://links.lww.com/CTG/A188, http://links.lww.com/CTG/A182, http://links.lww.com/CTG/A183, http://links.lww.com/CTG/A184, http://links.lww.com/CTG/A185, http://links.lww.com/CTG/A186, http://links.lww.com/CTG/A187
REFERENCES
- 1.Hunt RH, Xiao SD, Megraud F, et al. Helicobacter pylori in developing countries. World Gastroenterology Organisation Global Guideline. J Gastrointestin Liver Dis 2011;20:299–304. [PubMed] [Google Scholar]
- 2.Dooley CP, Cohen H, Fitzgibbons PL, et al. Prevalence of Helicobacter pylori infection and histologic gastritis in asymptomatic persons. N Engl J Med 1989;321:1562–6. [DOI] [PubMed] [Google Scholar]
- 3.Rostami N, Keshtkar-Jahromi M, Rahnavardi M, et al. Effect of eradication of Helicobacter pylori on platelet recovery in patients with chronic idiopathic thrombocytopenic purpura: A controlled trial. Am J Hematol 2008;83:376–81. [DOI] [PubMed] [Google Scholar]
- 4.DuBois S, Kearney DJ. Iron-deficiency anemia and Helicobacter pylori infection: A review of the evidence. Am J Gastroenterol 2005;100:453–9. [DOI] [PubMed] [Google Scholar]
- 5.Wu Q, Yang ZP, Xu P, et al. Association between Helicobacter pylori infection and the risk of colorectal neoplasia: A systematic review and meta-analysis. Colorectal Dis 2013;15:e352–64. [DOI] [PubMed] [Google Scholar]
- 6.Ciccotosto GD, McLeish A, Hardy KJ, et al. Expression, processing, and secretion of gastrin in patients with colorectal carcinoma. Gastroenterology 1995;109:1142–53. [DOI] [PubMed] [Google Scholar]
- 7.Zhang Y, Hoffmeister M, Weck MN, et al. Helicobacter pylori infection and colorectal cancer risk: Evidence from a large population-based case-control study in Germany. Am J Epidemiol 2012;175:441–50. [DOI] [PubMed] [Google Scholar]
- 8.Selgrad M, Bornschein J, Kandulski A, et al. Helicobacter pylori but not gastrin is associated with the development of colonic neoplasms. Int J Cancer 2014;135:1127–31. [DOI] [PubMed] [Google Scholar]
- 9.Engin AB, Karahalil B, Karakaya AE, et al. Helicobacter pylori and serum kynurenine-tryptophan ratio in patients with colorectal cancer. World J Gastroenterol 2015;21:3636–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qing Y, Wang M, Lin YM, et al. Correlation between Helicobacter pylori-associated gastric diseases and colorectal neoplasia. World J Gastroenterol 2016;22:4576–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hu K, Wu M, Chu C, et al. Synergistic effect of hyperglycemia and helicobacterpylori infection status on colorectal adenoma risk. J Clin Endocrinol Metab 2017;102:2744–50. [DOI] [PubMed] [Google Scholar]
- 12.Kim TJ, Kim ER, Chang DK, et al. Helicobacter pylori infection is an independent risk factor of early and advanced colorectal neoplasm. Helicobacter 2017;22:e12377. [DOI] [PubMed] [Google Scholar]
- 13.Nam JH, Hong CW, Kim BC, et al. Helicobacter pylori infection is an independent risk factor for colonic adenomatous neoplasms. Cancer Causes Control 2017;28:107–15. [DOI] [PubMed] [Google Scholar]
- 14.Kumar A, Kim M, Lukin DJ. Helicobacter pylori is associated with increased risk of serrated colonic polyps: Analysis of serrated polyp risk factors. Indian J Gastroenterol 2018;37:235–42. [DOI] [PubMed] [Google Scholar]
- 15.ChangxiChen, Mao Y, Du J, et al. Helicobacter pylori infection associated with an increased risk of colorectal adenomatous polyps in the Chinese population. BMC Gastroenterol 2019;19:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blase JL, Campbell PT, Gapstur SM, et al. Prediagnostic Helicobacter pylori antibodies and colorectal cancer risk in an elderly, caucasian population. Helicobacter 2016;21:488–92. [DOI] [PubMed] [Google Scholar]
- 17.Fernandez de Larrea-Baz N, Michel A, Romero B, et al. Helicobacter pylori antibody reactivities and colorectal cancer risk in a case-control study in Spain. Front Microbiol 2017;8:888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Teimoorian F, Ranaei M, Hajian Tilaki K, et al. Association of Helicobacter pylori infection with colon cancer and adenomatous polyps. Iran J Pathol 2018;13:325–32. [PMC free article] [PubMed] [Google Scholar]
- 19.Rokkas T, Sechopoulos P, Pistiolas D, et al. The relationship of Helicobacter pylori infection and colon neoplasia, on the basis of meta-analysis. Eur J Gastroenterol Hepatol 2013;25:1286–94. [DOI] [PubMed] [Google Scholar]
- 20.Guo Y, Li HY. Association between Helicobacter pylori infection and colorectal neoplasm risk: A meta-analysis based on east Asian population. J Cancer Res Ther 2014;(10 Suppl):263–6. [DOI] [PubMed] [Google Scholar]
- 21.Wang F, Sun MY, Shi SL, et al. Helicobacter pylori infection and normal colorectal mucosa-adenomatous polyp-adenocarcinoma sequence: A meta-analysis of 27 case-control studies. Colorectal Dis 2014;16:246–52. [DOI] [PubMed] [Google Scholar]
- 22.Patel S, Lipka S, Shen H, et al. The association of H. pylori and colorectal adenoma: Does it exist in the US hispanic population? J Gastrointest Oncol 2014;5:463–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zuniga R, Bautista J, Sapra K, et al. Combination of triple therapy and chronic PPI use may decrease risk of colonic adenomatous polyps in Helicobacter pylori infection. Gastroenterol Res Pract 2015;2015:638547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tongtawee T, Kaewpitoon S, Kaewpitoon N, et al. Helicobacter pylori associated gastritis increases risk of colorectal polyps: A hospital based-cross-sectional study in Nakhon ratchasima Province, Northeastern Thailand. Asian Pac J Cancer Prev 2016;17:341–5. [DOI] [PubMed] [Google Scholar]
- 25.Yan Y, Chen YN, Zhao Q, et al. Helicobacter pylori infection with intestinal metaplasia: An independent risk factor for colorectal adenomas. World J Gastroenterol 2017;23:1443–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603–5. [DOI] [PubMed] [Google Scholar]
- 27.Castillo JJ, Mull N, Reagan JL, et al. Increased incidence of non-hodgkin lymphoma, leukemia, and myeloma in patients with diabetes mellitus type 2: A meta-analysis of observational studies. Blood 2012;119:4845–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Singh S, Singh PP, Singh AG, et al. Statins are associated with a reduced risk of hepatocellular cancer: A systematic review and meta-analysis. Gastroenterology 2013;144:323–32. [DOI] [PubMed] [Google Scholar]
- 29.Kim EH, Park SW, Nam E, et al. Role of second-look endoscopy and prophylactic hemostasis after gastric endoscopic submucosal dissection: A systematic review and meta-analysis. J Gastroenterol Hepatol 2017;32:756–68. [DOI] [PubMed] [Google Scholar]
- 30.Lieberman DA, Rex DK, Winawer SJ, et al. Guidelines for colonoscopy surveillance after screening and polypectomy: A consensus update by the US Multi-Society task Force on colorectal cancer. Gastroenterology 2012;143:844–57. [DOI] [PubMed] [Google Scholar]
- 31.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Easterbrook PJ, Berlin JA, Gopalan R, et al. Publication bias in clinical research. Lancet 1991;337:867–72. [DOI] [PubMed] [Google Scholar]
- 34.Duval S, Tweedie R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 2000;56:455–63. [DOI] [PubMed] [Google Scholar]
- 35.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. J Clin Epidemiol 2009;62:e1–34. [DOI] [PubMed] [Google Scholar]
- 36.Meucci G, Tatarella M, Vecchi M, et al. High prevalence of Helicobacter pylori infection in patients with colonic adenomas and carcinomas. J Clin Gastroenterol 1997;25:605–7. [DOI] [PubMed] [Google Scholar]
- 37.Thorburn CM, Friedman GD, Dickinson CJ, et al. Gastrin and colorectal cancer: A prospective study. Gastroenterology 1998;115:275–80. [DOI] [PubMed] [Google Scholar]
- 38.Aydin A, Karasu Z, Zeytinoglu A, et al. Colorectal adenomateous polyps and Helicobacter pylori infection. Am J Gastroenterol 1999;94:1121–2. [DOI] [PubMed] [Google Scholar]
- 39.Breuer-Katschinski B, Nemes K, Marr A, et al. Helicobacter pylori and the risk of colonic adenomas. Colorectal Adenoma Study Group. Digestion 1999;60:210–5. [DOI] [PubMed] [Google Scholar]
- 40.Fireman Z, Trost L, Kopelman Y, et al. Helicobacter pylori: Seroprevalence and colorectal cancer. Isr Med Assoc J 2000;2:6–9. [PubMed] [Google Scholar]
- 41.Hartwich A, Konturek SJ, Pierzchalski P, et al. Helicobacter pylori infection, gastrin, cyclooxygenase-2, and apoptosis in colorectal cancer. Int J Colorectal Dis 2001;16:202–10. [DOI] [PubMed] [Google Scholar]
- 42.Shmuely H, Passaro D, Figer A, et al. Relationship between Helicobacter pylori CagA status and colorectal cancer. Am J Gastroenterol 2001;96:3406–10. [DOI] [PubMed] [Google Scholar]
- 43.Siddheshwar RK, Muhammad KB, Gray JC, et al. Seroprevalence of Helicobacter pylori in patients with colorectal polyps and colorectal carcinoma. Am J Gastroenterol 2001;96:84–8. [DOI] [PubMed] [Google Scholar]
- 44.Konturek PC, Bielanski W, Konturek SJ, et al. Progastrin and cyclooxygenase-2 in colorectal cancer. Dig Dis Sci 2002;47:1984–91. [DOI] [PubMed] [Google Scholar]
- 45.Limburg PJ, Stolzenberg-Solomon RZ, Colbert LH, et al. Helicobacter pylori seropositivity and colorectal cancer risk: A prospective study of male smokers. Cancer Epidemiol Biomarkers Prev 2002;11:1095–9. [PubMed] [Google Scholar]
- 46.Bombski G, Gasiorowska A, Orszulak-Michalak D, et al. Elevated plasma gastrin, CEA, and CA 19-9 levels decrease after colorectal cancer resection. Int J Colorectal Dis 2003;18:148–52. [DOI] [PubMed] [Google Scholar]
- 47.Wang KX, Wang XF, Peng JL, et al. Detection of serum anti-Helicobacter pylori immunoglobulin G in patients with different digestive malignant tumors. World J Gastroenterol 2003;9:2501–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fujimori S, Kishida T, Kobayashi T, et al. Helicobacter pylori infection increases the risk of colorectal adenoma and adenocarcinoma, especially in women. J Gastroenterol 2005;40:887–93. [DOI] [PubMed] [Google Scholar]
- 49.Mizuno S, Morita Y, Inui T, et al. Helicobacter pylori infection is associated with colon adenomatous polyps detected by high-resolution colonoscopy. Int J Cancer 2005;117:1058–9. [DOI] [PubMed] [Google Scholar]
- 50.Georgopoulos SD, Polymeros D, Triantafyllou K, et al. Hypergastrinemia is associated with increased risk of distal colon adenomas. Digestion 2006;74:42–6. [DOI] [PubMed] [Google Scholar]
- 51.Liou JM, Lin JW, Huang SP, et al. Helicobacter pylori infection is not associated with increased risk of colorectal polyps in Taiwanese. Int J Cancer 2006;119:1999–2000. [DOI] [PubMed] [Google Scholar]
- 52.D'Onghia V, Leoncini R, Carli R, et al. Circulating gastrin and ghrelin levels in patients with colorectal cancer: Correlation with tumour stage, Helicobacter pylori infection and BMI. Biomed Pharmacother 2007;61:137–41. [DOI] [PubMed] [Google Scholar]
- 53.Machida-Montani A, Sasazuki S, Inoue M, et al. Atrophic gastritis, Helicobacter pylori, and colorectal cancer risk: A case-control study. Helicobacter 2007;12:328–32. [DOI] [PubMed] [Google Scholar]
- 54.Zumkeller N, Brenner H, Chang-Claude J, et al. Helicobacter pylori infection, interleukin-1 gene polymorphisms and the risk of colorectal cancer: Evidence from a case-control study in Germany. Eur J Cancer 2007;43:1283–9. [DOI] [PubMed] [Google Scholar]
- 55.Bae RC, Jeon SW, Cho HJ, et al. Gastric dysplasia may be an independent risk factor of an advanced colorectal neoplasm. World J Gastroenterol 2009;15:5722–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Buso AG, Rocha HL, Diogo DM, et al. Seroprevalence of Helicobacter pylori in patients with colon adenomas in a Brazilian university hospital. Arq Gastroenterol 2009;46:97–101. [DOI] [PubMed] [Google Scholar]
- 57.Wu IC, Wu DC, Yu FJ, et al. Association between Helicobacter pylori seropositivity and digestive tract cancers. World J Gastroenterol 2009;15:5465–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Abbass K, Gul W, Beck G, et al. Association of Helicobacter pylori infection with the development of colorectal polyps and colorectal carcinoma. South Med J 2011;104:473–6. [DOI] [PubMed] [Google Scholar]
- 59.Inoue I, Mukoubayashi C, Yoshimura N, et al. Elevated risk of colorectal adenoma with Helicobacter pylori-related chronic gastritis: A population-based case-control study. Int J Cancer 2011;129:2704–11. [DOI] [PubMed] [Google Scholar]
- 60.Hong SN, Lee SM, Kim JH, et al. Helicobacter pylori infection increases the risk of colorectal adenomas: Cross-sectional study and meta-analysis. Dig Dis Sci 2012;57:2184–94. [DOI] [PubMed] [Google Scholar]
- 61.Strofilas A, Lagoudianakis EE, Seretis C, et al. Association of helicobacter pylori infection and colon cancer. J Clin Med Res 2012;4:172–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Epplein M, Pawlita M, Michel A, et al. Helicobacter pylori protein-specific antibodies and risk of colorectal cancer. Cancer Epidemiol Biomarkers Prev 2013;22:1964–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nam KW, Baeg MK, Kwon JH, et al. Helicobacter pylori seropositivity is positively associated with colorectal neoplasms. Korean J Gastroenterol 2013;61:259–64. [DOI] [PubMed] [Google Scholar]
- 64.Sonnenberg A, Genta RM. Helicobacter pylori is a risk factor for colonic neoplasms. Am J Gastroenterol 2013;108:208–15. [DOI] [PubMed] [Google Scholar]
- 65.Brim H, Zahaf M, Laiyemo AO, et al. Gastric Helicobacter pylori infection associates with an increased risk of colorectal polyps in African Americans. BMC Cancer 2014;14:296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shmuely H, Melzer E, Braverman M, et al. Helicobacter pylori infection is associated with advanced colorectal neoplasia. Scand J Gastroenterol 2014;49:35–42. [DOI] [PubMed] [Google Scholar]
- 67.Sherman PM. Appropriate strategies for testing and treating Helicobacter pylori in children: When and how? Am J Med 2004;117(Suppl 5A):30S–5S. [DOI] [PubMed] [Google Scholar]
- 68.Hsu W, Lin C, Sung F, et al. The relationship between Helicobacter pylori and cancer risk. Eur J Intern Med (Leicester) 2014;25:235–40. [DOI] [PubMed] [Google Scholar]
- 69.Park CH, Lee AR, Lee YR, et al. Evaluation of gastric microbiome and metagenomic function in patients with intestinal metaplasia using 16S rRNA gene sequencing. Helicobacter 2019;24:e12547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Imhann F, Bonder MJ, Vich Vila A, et al. Proton pump inhibitors affect the gut microbiome. Gut 2016;65:740–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jackson MA, Goodrich JK, Maxan ME, et al. Proton pump inhibitors alter the composition of the gut microbiota. Gut 2016;65:749–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Park CH, Eun CS, Han DS. Intestinal microbiota, chronic inflammation, and colorectal cancer. Intestinal Res 2018;16:338–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Park CH, Han DS, Oh YH, et al. Role of Fusobacteria in the serrated pathway of colorectal carcinogenesis. Sci Rep 2016;6:25271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jones M, Helliwell P, Pritchard C, et al. Helicobacter pylori in colorectal neoplasms: Is there an aetiological relationship? World J Surg Oncol 2007;5:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sabbagh P, Mohammadnia-Afrouzi M, Javanian M, et al. Diagnostic methods for Helicobacter pylori infection: Ideals, options, and limitations. Eur J Clin Microbiol Infect Dis 2019;38:55–66. [DOI] [PubMed] [Google Scholar]
- 76.Ford AC, Forman D, Hunt RH, et al. Helicobacter pylori eradication therapy to prevent gastric cancer in healthy asymptomatic infected individuals: Systematic review and meta-analysis of randomised controlled trials. BMJ 2014;348:g3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lee YC, Chiang TH, Chou CK, et al. Association between Helicobacter pylori eradication and gastric cancer incidence: A systematic review and meta-analysis. Gastroenterology 2016;150:1113–24 e5. [DOI] [PubMed] [Google Scholar]
- 78.Choi IJ, Kook MC, Kim YI, et al. Helicobacter pylori therapy for the prevention of Metachronous gastric cancer. N Engl J Med 2018;378:1085–95. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.