Abstract
The progressive miniaturization of photonic components presents the opportunity to obtain unprecedented microscopic images of colonic polyps in real time during endoscopy. This information has the potential to act as “optical biopsy” to aid clinical decision-making, including the possibility of adopting new paradigms such as a “resect and discard” approach for low-risk lesions. The technologies discussed in this review include confocal laser endomicroscopy, optical coherence tomography, multiphoton microscopy, Raman spectroscopy, and hyperspectral imaging. These are in different stages of development and clinical readiness, but all show the potential to produce reliable in vivo discrimination of different tissue types. A structured literature search of the imaging techniques for colorectal polyps has been conducted. The significant developments in endoscopic imaging were identified for each modality, and the status of current development was discussed. Of the advanced imaging techniques discussed, confocal laser endomicroscopy is in clinical use and, under optimal conditions with an experienced operator, can provide accurate histological assessment of tissue. The remaining techniques show potential for incorporation into endoscopic equipment and practice, although further component development is needed, followed by robust prospective validation of accuracy. Optical coherence tomography illustrates tissue “texture” well and gives good assessment of mucosal thickness and layers. Multiphoton microscopy produces high-resolution images at a subcellular resolution. Raman spectroscopy and hyperspectral imaging are less developed endoscopically but provide a tissue “fingerprint” which can distinguish between tissue types. Molecular imaging may become a powerful adjunct to other techniques, with its ability to precisely label specific molecules within tissue and thereby enhance imaging.
INTRODUCTION
We present a review of the advanced imaging technologies under development in pursuit of real-time diagnosis of colorectal polyps. It is known that high-quality colonoscopy, with polypectomy of detected colorectal adenomas, can arrest the progression of adenoma to carcinoma at an early stage, thereby reducing the incidence of colorectal cancer. The gross morphological appearances of polyps are well described and hold clues to their likely nature, whether hyperplastic or adenomatous with varying degrees of dysplasia (1,2). An emerging generation of endoscopic imaging technologies is beginning to expand beyond the boundaries of visual lesion recognition and generates images of the cellular structure of polyps, often known as “optical biopsy” or “virtual histology” to aid clinical decision-making.
The benefits of real-time assessment of polyp histology could include avoidance of biopsy in low-risk hyperplastic lesions, with associated reduction in complication risks and histological costs. Alternatively, low-risk lesions could be resected and discarded without formal histological analysis. This could also provide early reassurance to patients with benign histology by giving them the result at the time of endoscopy, although it remains to be seen whether optical biopsy will be seen as an acceptable alternative to conventional biopsy by both patients and professional bodies. We review the current technologies showing potential to achieve the goal of accurate optical biopsy but which are not yet in routine or widespread clinical use. These technologies include confocal laser endomicroscopy (CLE), optical coherence tomography (OCT), multiphoton microscopy (MPM), Raman spectroscopy, hyperspectral imaging (HSI), and molecular imaging. This review discusses the endomicroscopic techniques showing the potential to produce virtual histology. Image enhancement techniques such as narrow band imaging or i-scan have not been included. A summary of the status of the included imaging modalities is presented in Table 1.
Table 1.
Summary of the characteristics of the advanced photonic technologies for optical biopsy of colorectal polyps
METHOD
A structured literature search was carried out to identify the current relevant studies. A series of scoping searches were performed, which included each of the discussion areas in this review. The results of these were screened for relevance using the title, then abstract, followed by review of the full text of the article. The Ovid MEDLINE database and the Cochrane Library database were searched in May 2019 for relevant meta-analyses, review articles, clinical trials, and preclinical research. Studies without peer review or not available in English were excluded. The studies included for review were scrutinized for results in humans; ex vivo results and laboratory data were included as appropriate dependant on the available research literature.
Confocal laser endomicroscopy
Of the advanced photonic techniques discussed in this review, CLE has been developed to the greatest degree with commercially available imaging platforms and a body of evidence to support clinical use. CLE obtains very high magnification and resolution images of the mucosal layer of the gastrointestinal (GI) tract by the use of a low-power laser to induce fluorescence or reflectance at a defined plane within tissue. The emitted fluorescent light is then detected; “confocal” refers to the alignment of the illumination and detection systems in the same focal plane. Typically, an exogenous fluorophore such as fluorescein is used (3).
Among the earliest clinical use of CLE was at Showa University in 2003 using the Olympus FluoView instrument, a CLE scanner inserted through the working channel of an endoscope (4). This demonstrated the ability of CLE to visualize colonic mucosal structures in vivo, including pits and goblet cells. Adenomatous tissue was shown to have a different appearance to normal tissue, with greater numbers of visible nuclei.
In 2004, the first endoscope with integrated confocal laser microscopy was demonstrated in Mainz, and the initial capabilities of endoscopic CLE were examined. A high degree of diagnostic accuracy was achieved, up to 99.4% for the prediction of neoplastic histology (5,6). Images could be obtained with a resolution of 0.7 μm and at a variable depth from 0 to 250 μm below the epithelial surface. Fluorescein and acriflavine were used as fluorophores, and fluorescein was found to produce improved labeling of the lamina propria.
The normal appearance of the bowel under CLE was further discussed by Odagi et al., (7) who conducted mapping studies of the small and large intestine from 45 patients and demonstrated good concordance between histological appearances and CLE images. Several studies have since described the features of normal bowel and of polyps seen using CLE (8–10). Adenomatous polyp tissue has been shown to demonstrate several abnormal features, including distorted and elongated glands, crypt budding, fusion of glands, lack of surface maturation, dark irregular epithelium, distorted vascular architecture, and mucin depletion. The transit of fluorescein through adenomatous tissue was also found to be measurably slower than through normal tissue (11). Hyperplastic polyps were shown to have stellate pits with enlarged, branch-like crypts (8). In 2011, the Miami consensus conference drew together a group of expert endoscopists with experience in CLE to define the criteria for diagnosis of different GI lesions, including colonic polyps. Furthermore, the CLE appearance of sessile serrated adenomas has been investigated to assess for characteristic appearances. These include the mucus cap, visible on CLE as a bright, clouded appearance, thin, branched crypts, increased goblet cells, and irregular crypts (12). Descriptions of normal colonic mucosa, hyperplastic polyps, and adenomatous polyps are presented in Figure 1 (13).
Figure 1.
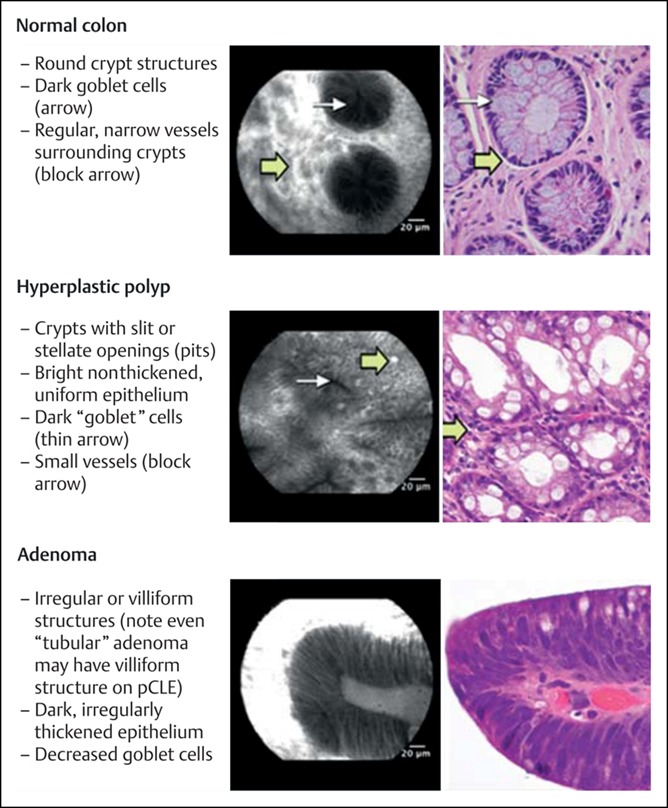
The Miami classification for probe-based confocal laser endomicroscopy.
To date, 7 studies have attempted to assess the diagnostic accuracy of CLE for the in vivo diagnosis of adenomatous polyps vs nonadenomatous tissue (5,14–19). These include diagnoses made in real time during endoscopy and diagnoses made “off line” using the images obtained during endoscopy. The most recent meta-analysis in 2016 included 410 patients, who had a total of 1,074 lesions. This concluded that CLE demonstrated a pooled sensitivity of 83%, for the diagnosis of adenoma in a detected polyp, and specificity of 90% (20). It should be remembered that these diagnostic accuracies are produced by experts, familiar with CLE.
Other roles of CLE in the assessment of colonic polyps include assessments of lesion margins, depth, and residual tissue after polypectomy. Research in these areas is ongoing (19,21–23). In common with many of the optical biopsy techniques, CLE presents endoscopists with the challenge of interpreting the microscopic images. This skill is typically not frequently used by endoscopists, and the effects of training and experience on the accuracy of diagnosis are not fully understood. However, the learning curve of performing CLE and interpreting images does appear to be fast (24,25).
Optical coherence tomography
In clinical use since the 1990s for examination of the retina, the images acquired by OCT may be described as conceptually similar to ultrasound images, although using a measurement of reflected light rather than sound energy (26). OCT images can demonstrate subsurface tissue layers and structures with a depth of up to approximately 2 mm and resolution of 1–2 μm, suggesting the potential for assessment of mucosal thickness and lesion characterization (27,28). With advances in miniaturization of optical components, endoscopic OCT probes have become possible and have been produced either as forward-scanning or rotary-scanning devices (29). A rotary-scanning probe typically scans at 90° to the shaft of the endoscope and can be withdrawn or inserted to scan an area of mucosa. A forward-scanning probe directs the scanning beam in the direction of view, much like a forward-viewing endoscope. Typically, an optical fiber within the scanning tip is oscillated by a piezoelectric transducer to form a scanning pattern such as a spiral or grid (30). Both designs have been studied for potential application for polyp diagnosis in the colon.
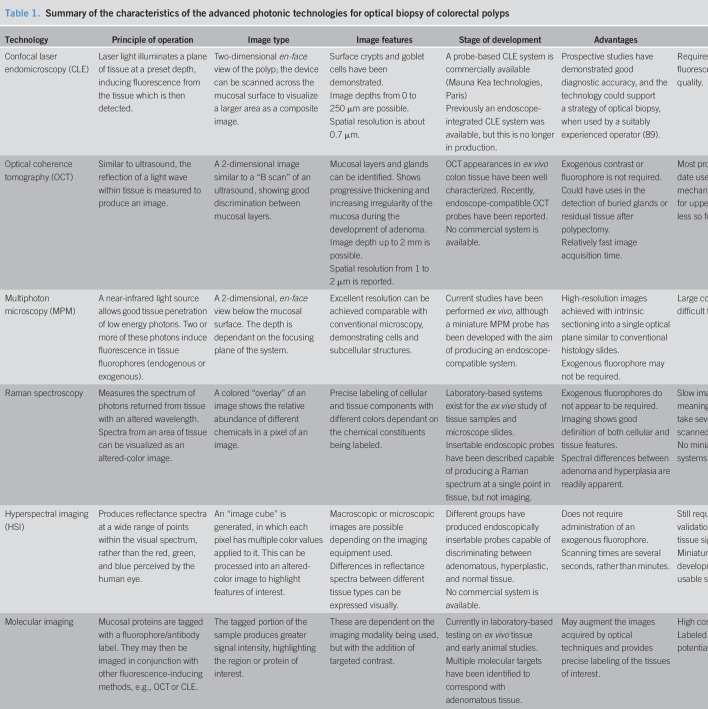
Studies to define the capabilities of OCT have been successful in imaging murine and human colonic mucosa and demonstrated that the light-scattering properties of hyperplastic polyps and adenomatous polyps differed from healthy tissue (31–33). The layers of the colon have been well demonstrated in a rat model, showing clear demarcation of the mucosa, submucosa, and the muscularis propria. The appearances were also shown to vary, depending on the degree of pressure placed on the tissue (34). Studies of OCT for assessing adenomatous tissue have also demonstrated visible changes; in a rat model of adenoma and colorectal cancer development, serial OCT imaging shows a progressive mucosal thickening and attenuation of tissue boundaries as adenoma progresses (Figure 2) (35,36). Other studies in a mouse model have found that OCT can detect foci of abnormal crypts even in grossly normal mucosa (37). Further studies in humans by Alder et al. (38) have helped to define the OCT appearances of healthy human colonic tissue and in a variety of disease conditions, including ulcerative colitis and radiation proctitis. A spiral-scanning probe was used to acquire a three-dimensional image dataset, showing detail of the crypt patterns and close resemblance to traditional histology. This approach is a further refinement of the OCT technology using near-infrared light, volumetric laser endomicroscopy. Recent studies of volumetric laser endomicroscopy probes have demonstrated the ability to image colorectal polyps immediately after resection, showing definition of the mucosal layers and abnormal glandular architecture in dysplastic tissue (39).
Figure 2.
OCT time series images of the development of adenoma in an azoxymethane mouse model of colonic adenoma showing (a) mucosal thickening at 8 weeks post treatment, (b), moderate mucosal protrusion at 14 weeks, (c) further mucosal thickening at 22 weeks, (d) signal attenuation and absence of tissue boundaries at adenoma, 26 weeks, and (e) is the corresponding histological section, containing adenoma. Reproduced with permission from Dr Jennifer K. Barton (35). OCT, optical coherence tomography.
The spiral-scanning probes for OCT allow the survey of large areas of mucosa and have been studied in the esophagus and biliary tree but may not be as applicable in the irregular lumen of the colon (40). Two groups have recently reported the development of forward-viewing OCT probes with rapid image acquisition times which could allow immediate assessment of visualized mucosa (41,42).
The images obtained by OCT require some interpretation, but this process could be simplified by the application of image analysis algorithms. Qi et al. (43) have demonstrated the ability to virtually reconstruct the OCT images into a 3-dimensional model of the mucosa, which illustrates crypt architecture and allows identification of aberrant foci. The role of OCT in the diagnosis of polyps remains an area of ongoing research interest. It is an attractive prospect for the generation of “virtual histology” because it does not necessarily require exogenous fluorophores or other markers and provides images at a useful level of tissue penetration and spatial resolution. OCT in the esophagus is being studied as a potential tool for Barrett's esophagus screening, using a spiral scanner (44). This approach seems less applicable to the colon, with its many folds and flexures. It seems more probable that the role of OCT could be for precise scanning of a detected lesion to assess the crypt architecture and mucosal layers. It could also have a role in margin and depth assessments of a larger polyp or to assess for the presence of residual tissue after polypectomy. An early clinically usable system is reported by Ding et al. (45) using a near-patient OCT microscopy system to image unprepared polyp tissue immediately after resection during colonoscopy before histological analysis. A high accuracy of prediction is achieved, with a short learning curve. Future developments may include insertable OCT probes suitable for colonoscopy or eventually integrated into the colonoscope.
Multiphoton microscopy
MPM relies on the absorption by a tissue fluorophore of 2 or more photons, followed by emission of a single photon. The fluorophore molecule may be endogenous, such as collagen, or exogenous, such as fluorescein. The technique demonstrates the ability to allow real-time observation of living tissue at a cellular level, including functional information implied by the fluorescence from metabolically active molecules (46). Typically a near-infrared laser light source is used; the photons are relatively of low energy and longer wavelength, and so are absorbed less in tissue than photons of bluer light, with consequent deeper tissue penetration (47).
MPM in the GI tract has been limited by the large size of many of the components, including lasers and scanning devices. In 2008, a miniaturized MPM probe was reported by Rogart et al. (48) and used to image rat colonic tissue ex vivo, as well as colonic tissue samples collected from volunteers. Collagen autofluorescence produced high-resolution images of cells and subcellular structures, comparable with conventional histological images.
A miniaturized forward-viewing probe was reported in 2012, using an oscillating optical fiber and capable of scanning a 110 × 110 μm field of view. The scanning device has a diameter of 3 mm, has a rigid length of 40 mm, and could be developed to allow insertion through the working channel of an endoscope for in vivo tissue imaging (49). This system has been used to produce reference images of the mouse GI tract, as well as to study the progression of adenoma to carcinoma in a mouse model of carcinogenesis. This demonstrated the ability of MPM to show progressive development of aberrant crypt foci and elongated, crowded nuclei (50). Other studies of adenoma progression have shown an increase in the volume of submucosal collagen and changes in its organization (51).
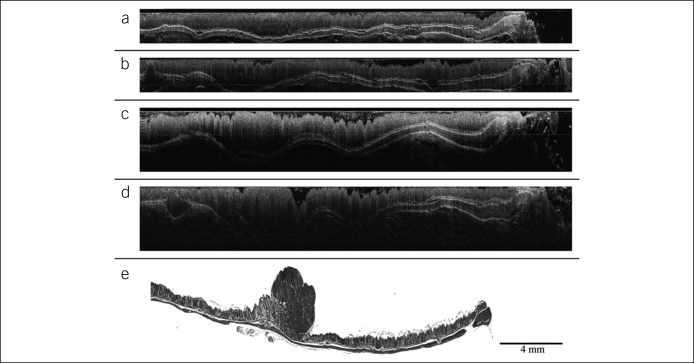
Other groups have performed comparison of human colonic mucosa against rectal adenocarcinoma in unstained resected tissue, demonstrating significant morphological differences between healthy mucosa and cancerous tissue under MPM and again showing images comparable with histological slides (Figure 3) (52). To date, these studies have been performed ex vivo, and no system capable of in vivo MPM is currently available (53–55).
Figure 3.
Ex vivo images of fresh biopsies from (a) healthy colonic mucosa, (b) adenomatous polyp, and (c) adenocarcinoma at a depth of 30 μm from tissue surface. Scale bar is 10 μm. Reproduced with permission from Dr Riccardo Cicchi (52).
MPM is an exciting imaging modality, allowing microscopic images to be produced from tissue, without the need for biopsy. In addition to structural information, the functional information obtained from fluorescence of metabolites has the potential to provide diagnostic information. A further interesting development is the report of a dual OCT and MPM imaging system, which could combine the information of structural and mucosal layers from OCT with the precise cellular imaging of MPT (56,57).
Raman spectroscopy
Raman spectroscopy relies on observing the interactions between photons and matter during light scattering. Using a Raman spectrometer, it can be observed that a tiny proportion of light reflected from an object has an altered color because of photons interacting with molecular vibrations and consequently shifting their wavelength. This is known as the Raman shift, and measurement of this altered spectrum can provide information about the composition of the reflecting material (58). Raman spectral information may be combined with optical microscopy to produce an altered-color image of tissue, demonstrating cellular and subcellular structures labeled by their chemical constituents (59).
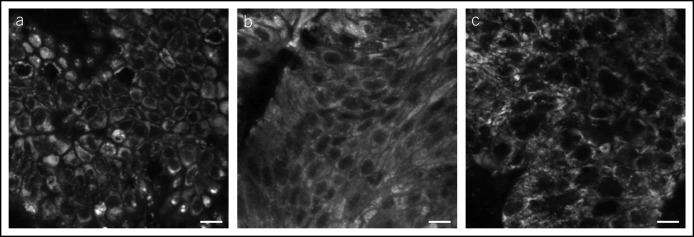
Early studies of Raman spectroscopy in the GI tract have shown the ability to produce Raman spectra from human GI mucosa, both ex vivo (60–63) and in vivo (64–66). The in vivo spectra were obtained by passing a fiber-optic probe through the working channel of an endoscope and placing the tip into contact with the mucosa. An insertable probe has been developed, capable of obtaining a Raman spectrum with an acquisition time of 1 second. Differences were observed between the spectral signal generated from hyperplastic and adenomatous polyps (Figure 4) (67). The spectra associated with each type of polyp can be distinguished by statistical analysis, increasing the prospect of automated or computer-aided diagnosis of polyp histology before resection (68).
Figure 4.
Average differences in vivo between the Raman spectra of hyperplastic (red dashed curve) and tubular adenoma (black curve) compared with normal tissue.
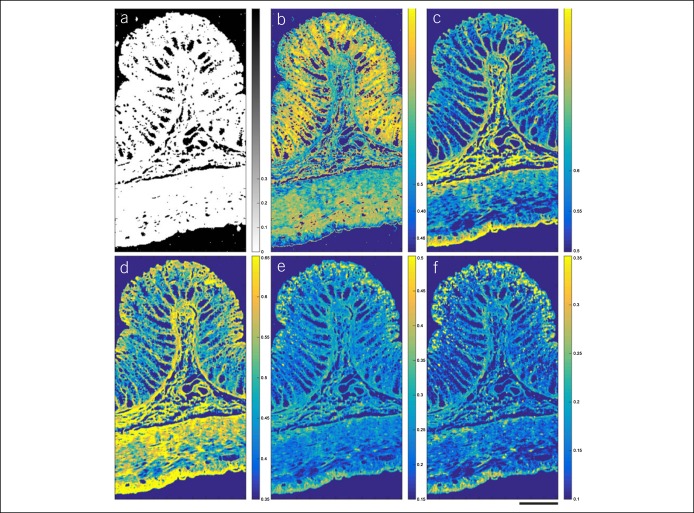
Further developments in Raman spectroscopic imaging include the ex vivo scanning of normal colonic tissue and adenocarcinoma tissue. A Raman spectrometer attached to a scanning microscope has been used to produce pseudocolor maps of tissue sections, highlighting areas of collagen, protein, mucus and lipids, in normal and malignant tissue (69). A similar spectral microscope has demonstrated high resolution discrimination of tissue components in paraffin-embedded microscope slides (Figure 5) (70).
Figure 5.
Maps of Raman spectral similarity in paraffin-fixed colonic tissue. Intensity indicates the similarity with the reference spectra of molecules anticipated to be present, including (a) background mask, (b) DNA, (c) collagen, (d) muscle acetone powder, (e) phosphatidylcholine, and (f) paraffin wax. Scale line is 200 μm.
Raman endoscopic imaging is in its infancy but shows great potential for use in polyp discrimination. Limiting factors include the lack of miniaturized scanning devices and the long image acquisition times, up to several minutes (71).
Hyperspectral imaging
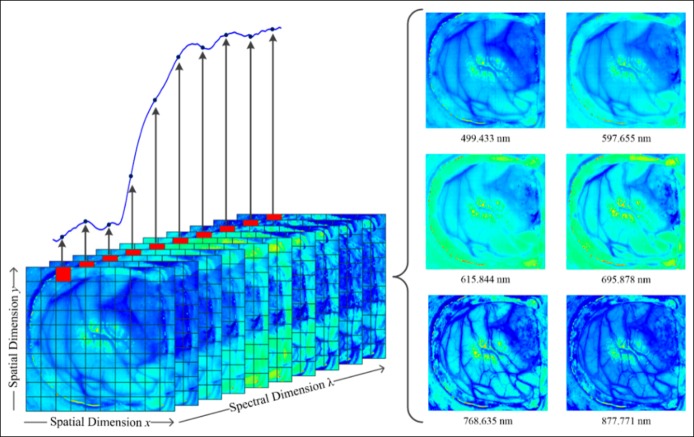
Originally developed for use in satellite imaging and geological surveys, HSI is an emerging field of medical diagnostic imaging, combining imaging with reflectance spectroscopy (72). Each pixel of an image contains information for many wavelength bands of light, rather than the red, green, and blue bands perceived by the human eye. This is expressed in Figure 6 (73). The additional spectral information may then be processed into a clinically useful format. Some current generation endoscopes (Fujifilm) include spectral estimation techniques (flexible spectral imaging color enhancement) which attempt to produce a high-contrast image from a white-light endoscopic image by enhancing the wavelengths of light associated with vascular structures and inflammation (74), True HIS endoscopy platforms are under development, and HSI has been studied in other medical applications including assessment of burns, melanoma, and cervical lesions.
Figure 6.
Illustrative example of the composition of a hyperspectral image (from the surface of the brain). The spectrum shown is obtained from the pixel highlighted in red. Multiple images will be obtained at different wavelengths (right) to compose the hyperspectral datacube. Reproduced with permission from Dr Anastasios Koulaouzidis.
Initial in-vitro studies of HSI and colonic polyp tissue demonstrated the ability of a hyperspectral microscope to distinguish between nuclei, cytoplasm, and lamina propria based on their spectral signatures; the resultant images were analyzed and could accurately distinguish between normal colonic mucosa, adenoma, and adenocarcinoma (75). More recently, there has been renewed interest in developing an endoscopic platform for HSI. One such system was described by Kumashiro et al. in 2016, using a fiber-optic “baby scope” through a conventional colonoscope and a hyperspectral camera to examine the resected polyp tissue. This identified significant spectral differences between normal mucosa and adenomatous tissue (76). The group also performed in vivo imaging and was able to produce an altered-color image, delineating areas of adenoma against normal tissue.
Another HSI imaging system described in 2016 also used a fiber-optic bundle to transmit the spectral data, inserted into the working channel of a colonoscope by a sterile sheath. This system combines the ability to record hyperspectral data and to induce tissue autofluorescence and was used to obtain images of small bowel in a porcine model, showing altered-color images of tissue surface structures, as well as a map of tissue oxygen saturation (77). Subsequently, a pilot study was undertaken in patients with colonic polyps, demonstrating the ability of the system to acquire real-time information during endoscopy. The clinical validation of this approach is ongoing.
Molecular imaging
Molecular Imaging is an emerging technique which uses fluorescently labeled antibodies to allow visualization of the biochemical processes taking place within tissue at a cellular level. This has the potential to provide precise, real-time diagnostic information about the detected lesions.
For the purposes of evaluating polyps, it is desirable to use antibodies directed against markers present on dysplastic tissue or against factors expressed by such tissues (78). For colonic polyps, epitopes of mutations in antigen-presenting complex, K-RAS, and p53 genes can be labeled (79) as can upregulated factors including cathepsin B, epidermal growth factor receptor (EGFR), vascular endothelial growth factor (VEGF), and carcinoembryonic antigen (CEA) (80–84).
In 2002, Keller et al. (81) described the topical application during colonoscopy of a fluorescein-labeled anti-CEA antibody and demonstrated that it was possible to induce fluorescence from adenomas. There have not, to date, been follow-up studies examining the diagnostic accuracy or use of this approach. Another target for cellular labeling is cathepsin B, a protease known to be upregulated in adenomatous tissue. This was investigated in 2002 by Marten et al., (80) who demonstrated that adenomas had high uptake of a labeled cathepsin B fluorophore, which could therefore be used to fluorescently label adenomatous tissue.
Further studies of molecular imaging have typically used fluorescent labeling in conjunction with a high-resolution photonics imaging techniques, such as OCT or CLE. A study by Yuan et al. (82) used a carbohydrate (α-l-fucose)-based fluorophore and performed OCT on adenomas in resected tissue from a mouse model. This demonstrated that fluorescent labeling of polyps in tissue is feasible and has the capability to produce high-resolution images with greater contrast than unlabeled OCT. Iftimia et al. (83) developed a labeled antibody to the integrin receptor, which is overexpressed in adenoma cells. This was administered topically to colon tissue in a mouse polyposis model, and the resected colons were examined using OCT. This approach also demonstrated increased fluorescence from abnormal tissue, with the potential to produce an enhanced OCT image.
The growth factors implicated in tumor progression are also potential targets for fluorescent labeling, and in 2010, Winkler et al. (84) produced a study in live mice, investigating the OCT images of polyps labeled with a VEGF antibody. This was delivered topically by colonic lavage, and the areas of high fluorescence correlated with histologically proven adenoma. In the same year, Foersch et al. (85) also studied VEGF molecular targeting using CLE to examine tissue from mouse polyposis models, xenograft models, and in surgical resections containing colorectal cancer. Strong fluorescent signal was achieved from areas of the polyp. These studies further demonstrate the potential for molecular imaging as an adjunct to advanced photonics microscopy techniques.
Another growth factor under active investigation is the EGFR, which may be overexpressed in human colonic neoplasms. Goetz et al. (86) have studied the anti-EGFR antibody cetuximab and shown EGFR-specific fluorescence under CLE in tumors, correlating with the degree of EGFR expression. The same group has also demonstrated correlation between the intensity of fluorescence detected in mouse models and the progression of tumors (87).
In-vivo molecular-labeled imaging is a potential method of highlighting abnormal tissues, which could have great implications for the polyp detection. Early animal studies have demonstrated that labeled anti-CEA produces bright fluorescence from pancreatic and colonic tumors. The effect appears within 30 minutes of administration and could represent a useful adjunct to endoscopic polyp detection (88).
The role of molecular imaging is still emerging. It is an exciting technology, which may enable the development of a “molecular beacon” to detect and diagnose abnormal tissues in the colon. Potential limitations of the technology include its cost, a long half-life after injection of the molecular probe, and concerns about the potential for the labeled antibodies to induce immunogenicity.
DISCUSSION
An exciting era of endoscopy is developing with the emergence of several advanced optical technologies which show the potential for making accurate tissue diagnosis in vivo. This raises the possibility of an accurate optical biopsy, which could fundamentally change the way in which small colonic polyps are managed. Current recommendations suggest that a suitably accurate diagnostic method could allow the consideration of a “resect and discard” approach, in which low-risk polyps are diagnosed in situ and discarded without undergoing full histological analysis (89). This approach relies on a high sensitivity for adverse histology to avoid the possibility of misdiagnosing a diminutive cancer (90).
The technologies discussed in this article are relatively new and are at different stages of development; therefore, little analysis of their clinical effectiveness exists. CLE was reviewed by the ASGE as part of its review of real-time endoscopic prediction of polyp histology and 6 studies were considered informative. The variability and heterogeneity of these studies meant that meta-analysis could not be performed, although it is noted that CLE does show potential for accurate characterization of polyps (89). To date, the other advanced imaging techniques discussed in this article have not been widely studied in humans, but the animal model studies, the ex vivo human studies, and the early in vivo human studies demonstrate huge potential for near-histological quality images of tissue to be obtained. Further advances in miniaturization and the production of endoscopically compatible systems will allow studies to include real-time analysis of tissue in patients recruited prospectively, using histology as the reference standard. Robust studies are required, but this approach will ultimately characterize the clinical role of novel endoscopic imaging techniques.
Challenges facing the progress of virtual histology include the potential procedure delays associated with scanning of polyps; any system would have to be simple and easy to use to gain widespread acceptance from clinicians. A further consideration is the interpretation of virtual histology images. Although the learning curve of image interpretation has been shown to be fast, it is a new and unfamiliar skill to most endoscopists (24). The growing field of computer-aided diagnosis could help overcome this limitation by suggesting the correct interpretation of an image (52), although for accurate diagnostic results the image recognition algorithms require greater amounts of image data than are currently available for these new technologies.
The intended benefits of adopting an optical biopsy strategy include reduction of histology costs and laboratory burden, as well as avoiding the harm associated with unnecessary polypectomy, and allowing the patient to be reassured about low-risk polyps at the time of their endoscopy, rather than waiting for results. The technologies discussed above show the potential to achieve these aims. Further studies defining suitable conditions for their use will allow them to make a significant contribution to endoscopic practice.
CONFLICTS OF INTEREST
Guarantor of the article: Ben Glover, BM, BSc, MRCP.
Specific author contributions: B.G. and N.P. have planned and initiated the review article. B.G. has conducted the literature searches and proposed the drafts. N.P. and Professor J.T. have written and edited the drafts.
Financial support: There are no direct funding sources for this publication. B.G. receives a salary as part of the EU Horizon 2020 initiative.
Potential competing interests: None to report.
REFERENCES
- 1.Kudo S, Tamura S, Nakajima T, et al. Diagnosis of colorectal tumorous lesions by magnifying endoscopy. Gastrointest Endosc 1996;44(1):8–14. [DOI] [PubMed] [Google Scholar]
- 2.Hewett DG, Kaltenbach T, Sano Y, et al. Validation of a simple classification system for endoscopic diagnosis of small colorectal polyps using narrow-band imaging. Gastroenterology 2012;143(3):599–607 e1. [DOI] [PubMed] [Google Scholar]
- 3.Committee AT. Confocal laser endomicroscopy. Gastrointest Endosc 2014;80(6):928–38. [DOI] [PubMed] [Google Scholar]
- 4.Sakashita M, Inoue H, Kashida H, et al. Virtual histology of colorectal lesions using laser-scanning confocal microscopy. Endoscopy 2003;35(12):1033–8. [DOI] [PubMed] [Google Scholar]
- 5.Kiesslich R, Burg J, Vieth M, et al. Confocal laser endoscopy for diagnosing intraepithelial neoplasias and colorectal cancer in vivo. Gastroenterology 2004;127(3):706–13. [DOI] [PubMed] [Google Scholar]
- 6.Polglase AL, McLaren WJ, Skinner SA, et al. A fluorescence confocal endomicroscope for in vivo microscopy of the upper- and the lower-GI tract. Gastrointest Endosc 2005;62(5):686–95. [DOI] [PubMed] [Google Scholar]
- 7.Odagi I, Kato T, Imazu H, et al. Examination of normal intestine using confocal endomicroscopy. J Gastroenterol Hepatol 2007;22(5):658–62. [DOI] [PubMed] [Google Scholar]
- 8.Sanduleanu S, Driessen A, Gomez-Garcia E, et al. In vivo diagnosis and classification of colorectal neoplasia by chromoendoscopy-guided confocal laser endomicroscopy. Clin Gastroenterol Hepatol 2010;8(4):371–8. [DOI] [PubMed] [Google Scholar]
- 9.Hurlstone DP, Baraza W, Brown S, et al. In vivo real-time confocal laser scanning endomicroscopic colonoscopy for the detection and characterization of colorectal neoplasia. Br J Surg 2008;95(5):636–45. [DOI] [PubMed] [Google Scholar]
- 10.Wallace MB, Fockens P. Probe-based confocal laser endomicroscopy. Gastroenterology 2009;136(5):1509–13. [DOI] [PubMed] [Google Scholar]
- 11.Wang TD, Friedland S, Sahbaie P, et al. Functional imaging of colonic mucosa with a fibered confocal microscope for real-time in vivo pathology. Clin Gastroenterol Hepatol 2007;5(11):1300–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parikh ND, Gibson J, Nagar A, et al. Confocal laser endomicroscopy features of sessile serrated adenomas/polyps. United European Gastroenterol J 2016;4(4):599–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wallace M, Lauwers GY, Chen Y, et al. Miami classification for probe-based confocal laser endomicroscopy. Endoscopy 2011;43(10):882–91. [DOI] [PubMed] [Google Scholar]
- 14.De Palma GD. Confocal laser endomicroscopy in the “in vivo” histological diagnosis of the gastrointestinal tract. World J Gastroenterol 2009;15(46):5770–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gomez V, Buchner AM, Dekker E, et al. Interobserver agreement and accuracy among international experts with probe-based confocal laser endomicroscopy in predicting colorectal neoplasia. Endoscopy 2010;42(4):286–91. [DOI] [PubMed] [Google Scholar]
- 16.Shahid MW, Buchner AM, Heckman MG, et al. Diagnostic accuracy of probe-based confocal laser endomicroscopy and narrow band imaging for small colorectal polyps: A feasibility study. Am J Gastroenterol 2012;107(2):231–9. [DOI] [PubMed] [Google Scholar]
- 17.Shahid MW, Buchner AM, Raimondo M, et al. Accuracy of real-time vs. blinded offline diagnosis of neoplastic colorectal polyps using probe-based confocal laser endomicroscopy: A pilot study. Endoscopy 2012;44(4):343–8. [DOI] [PubMed] [Google Scholar]
- 18.Gomez V, Shahid MW, Krishna M, et al. Classification criteria for advanced adenomas of the colon by using probe-based confocal laser endomicroscopy: A preliminary study. Dis Colon Rectum 2013;56(8):967–73. [DOI] [PubMed] [Google Scholar]
- 19.Liu C, Li CQ, Zuo XL, et al. Confocal laser endomicroscopy for the diagnosis of colorectal cancer in vivo. J Dig Dis 2013;14(5):259–65. [DOI] [PubMed] [Google Scholar]
- 20.Fugazza A, Gaiani F, Carra MC, et al. Confocal laser endomicroscopy in gastrointestinal and pancreatobiliary diseases: A systematic review and meta-analysis. Biomed Res Int 2016;2016:4638683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abe S, Saito Y, Oono Y, et al. Pilot study on probe-based confocal laser endomicroscopy for colorectal neoplasms: An initial experience in Japan. Int J Colorectal Dis 2018;33(8):1071–8. [DOI] [PubMed] [Google Scholar]
- 22.Kim B, Kim YH, Park SJ, et al. Probe-based confocal laser endomicroscopy for evaluating the submucosal invasion of colorectal neoplasms. Surg Endosc 2017;31(2):594–601. [DOI] [PubMed] [Google Scholar]
- 23.Wijsmuller AR, Ghnassia JP, Varatharajah S, et al. Prospective trial on probe-based confocal laser endomicroscopy for the identification of the distal limit in rectal adenocarcinoma. Surg Innov 2018;25(4):313–22. [DOI] [PubMed] [Google Scholar]
- 24.Buchner AM, Shahid MW, Wallace MB. The learning curve of probe based confocal laser endomicroscopy (pCLE) for detection neoplasia in colon polyps. Gastroenterology 2010;138(5):S95–S. [DOI] [PubMed] [Google Scholar]
- 25.Yuan XM, Li Z, Ji R, et al. Minimal influence of expertise on the evaluation of colorectal neoplastic lesions by confocal laser endomicroscopy. J Gastroenterol Hepatol 2014;29(1):91–5. [DOI] [PubMed] [Google Scholar]
- 26.Tsai TH, Fujimoto JG, Mashimo H. Endoscopic optical coherence tomography for clinical gastroenterology. Diagnostics (Basel) 2014;4(2):57–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.DaCosta RS, Wilson BC, Marcon NE. Optical techniques for the endoscopic detection of dysplastic colonic lesions. Curr Opin Gastroenterol 2005;21(1):70–9. [PubMed] [Google Scholar]
- 28.Wessels R, De Bruin DM, Faber DJ, et al. Optical biopsy of epithelial cancers by optical coherence tomography (OCT). Lasers Med Sci 2014;29(3):1297–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gora MJ, Suter MJ, Tearney GJ, et al. Endoscopic optical coherence tomography: Technologies and clinical applications [invited]. Biomed Opt Express 2017;8(5):2405–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tsai T-H, Potsaid BM, Kraus M, et al., editors. Piezoelectric transducer based miniature catheter for ultrahigh speed endoscopic optical coherence tomography. Optical Coherence Tomography and Coherence Domain Optical Methods in Biomedicine XV; 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pfau PR, Sivak MV, Jr, Chak A, et al. Criteria for the diagnosis of dysplasia by endoscopic optical coherence tomography. Gastrointest Endosc 2003;58(2):196–202. [DOI] [PubMed] [Google Scholar]
- 32.Tumlinson AR, Hariri LP, Utzinger U, et al. Miniature endoscope for simultaneous optical coherence tomography and laser-induced fluorescence measurement. Appl Opt 2004;43(1):113–21. [DOI] [PubMed] [Google Scholar]
- 33.Wang C, Zhang Q, Wu X, et al. Quantitative diagnosis of colorectal polyps by spectral domain optical coherence tomography. Biomed Res Int 2014;2014:570629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Westphal V, Rollins AM, Willis J, et al. Correlation of endoscopic optical coherence tomography with histology in the lower-GI tract. Gastrointest Endosc 2005;61(4):537–46. [DOI] [PubMed] [Google Scholar]
- 35.Hariri LP, Qiu Z, Tumlinson AR, et al. Serial endoscopy in azoxymethane treated mice using ultra-high resolution optical coherence tomography. Cancer Biol Ther 2007;6(11):1753–62. [DOI] [PubMed] [Google Scholar]
- 36.Hariri LP, Tumlinson AR, Besselsen DG, et al. Endoscopic optical coherence tomography and laser-induced fluorescence spectroscopy in a murine colon cancer model. Lasers Surg Med 2006;38(4):305–13. [DOI] [PubMed] [Google Scholar]
- 37.Keenan MR, Leung SJ, Rice PS, et al. Dual optical modality endoscopic imaging of cancer development in the mouse colon. Lasers Surg Med 2015;47(1):30–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Adler DC, Zhou C, Tsai TH, et al. Three-dimensional endomicroscopy of the human colon using optical coherence tomography. Opt Express 2009;17(2):784–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Trindade AJ, Rishi A, Hirten R, et al. Identification of volumetric laser endomicroscopy features of colon polyps with histologic correlation. Gastrointest Endosc 2018;87(6):1558–64. [DOI] [PubMed] [Google Scholar]
- 40.Konda VJ, Koons A, Siddiqui UD, et al. Optical biopsy approaches in Barrett's esophagus with next-generation optical coherence tomography. Gastrointest Endosc 2014;80(3):516–7. [DOI] [PubMed] [Google Scholar]
- 41.Liang K, Ahsen OO, Wang Z, et al. Endoscopic forward-viewing optical coherence tomography and angiography with MHz swept source. Opt Lett 2017;42(16):3193–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Welge WA, Barton JK. In vivo endoscopic Doppler optical coherence tomography imaging of the colon. Lasers Surg Med 2017;49(3):249–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Qi X, Pan Y, Hu Z, et al. Automated quantification of colonic crypt morphology using integrated microscopy and optical coherence tomography. J Biomed Opt 2008;13(5):054055. [DOI] [PubMed] [Google Scholar]
- 44.Lightdale CJ. Optical coherence tomography in Barrett's esophagus. Gastrointest Endosc Clin N Am 2013;23(3):549–63. [DOI] [PubMed] [Google Scholar]
- 45.Ding Q, Deng Y, Yu X, et al. Rapid, high-resolution, label-free, and 3-dimensional imaging to differentiate colorectal adenomas and non-neoplastic polyps with micro-optical coherence tomography. Clin Transl Gastroenterol 2019;10:e00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cho HJ, Chun HJ, Kim ES, et al. Multiphoton microscopy: An introduction to gastroenterologists. World J Gastroenterol 2011;17(40):4456–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Crosignani V, Dvornikov A, Aguilar JS, et al. Deep tissue fluorescence imaging andin vivobiological applications. J Biomed Opt 2012;17(11):116023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rogart JN, Nagata J, Loeser CS, et al. Multiphoton imaging can be used for microscopic examination of intact human gastrointestinal mucosa ex vivo. Clin Gastroenterol Hepatol 2008;6(1):95–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rivera DR, Brown CM, Ouzounov DG, et al. Compact and flexible raster scanning multiphoton endoscope capable of imaging unstained tissue. Proc Natl Acad Sci USA 2011;108(43):17598–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Makino T, Jain M, Montrose DC, et al. Multiphoton tomographic imaging: A potential optical biopsy tool for detecting gastrointestinal inflammation and neoplasia. Cancer Prev Res (Phila) 2012;5(11):1280–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bianchi M, Adur J, Ruff SY, et al. Mouse colorectal cancer an early detection approach using nonlinear microscopy. Biomed Mater Eng 2014;24(6):3419–26. [DOI] [PubMed] [Google Scholar]
- 52.Cicchi R, Sturiale A, Nesi G, et al. Multiphoton morpho-functional imaging of healthy colon mucosa, adenomatous polyp and adenocarcinoma. Biomed Opt Express 2013;4(7):1204–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ying M, Zhuo S, Chen G, et al. Real-time noninvasive optical diagnosis for colorectal cancer using multiphoton microscopy. Scanning 2012;34(3):181–5. [DOI] [PubMed] [Google Scholar]
- 54.Liu N, Chen J, Xu R, et al. Label-free imaging characteristics of colonic mucinous adenocarcinoma using multiphoton microscopy. Scanning 2013;35(4):277–82. [DOI] [PubMed] [Google Scholar]
- 55.Matsui T, Mizuno H, Sudo T, et al. Non-labeling multiphoton excitation microscopy as a novel diagnostic tool for discriminating normal tissue and colorectal cancer lesions. Sci Rep 2017;7(1):6959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang T, Li Q, Xiao P, et al. Gradient index lens based combined two-photon microscopy and optical coherence tomography. Opt Express 2014;22(11):12962–70. [DOI] [PubMed] [Google Scholar]
- 57.Duan X, Li H, Qiu Z, et al. MEMS-based multiphoton endomicroscope for repetitive imaging of mouse colon. Biomed Opt Express 2015;6(8):3074–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zoubir A. Raman Imaging: Techniques and Applications. Springer: Berlin, Heidelburg, New York, 2012, pp 386. [Google Scholar]
- 59.Krafft C, Dietzek B, Popp J. Raman and CARS microspectroscopy of cells and tissues. Analyst 2009;134(6):1046–57. [DOI] [PubMed] [Google Scholar]
- 60.Chowdary MV, Kumar KK, Thakur K, et al. Discrimination of normal and malignant mucosal tissues of the colon by Raman spectroscopy. Photomed Laser Surg 2007;25(4):269–74. [DOI] [PubMed] [Google Scholar]
- 61.Lopes PC, Moreira JA, Almeida A, et al. Discriminating adenocarcinoma from normal colonic mucosa through deconvolution of Raman spectra. J Biomed Opt 2011;16(12):127001. [DOI] [PubMed] [Google Scholar]
- 62.Li S, Chen G, Zhang Y, et al. Identification and characterization of colorectal cancer using Raman spectroscopy and feature selection techniques. Opt Express 2014;22(21):25895–908. [DOI] [PubMed] [Google Scholar]
- 63.Wood JJ, Kendall C, Hutchings J, et al. Evaluation of a confocal Raman probe for pathological diagnosis during colonoscopy. Colorectal Dis 2014;16(9):732–8. [DOI] [PubMed] [Google Scholar]
- 64.Shim MG, Song LM, Marcon NE, et al. In vivo near-infrared Raman spectroscopy: Demonstration of feasibility during clinical gastrointestinal endoscopy. Photochem Photobiol 2000;72(1):146–50. [DOI] [PubMed] [Google Scholar]
- 65.Molckovsky A, Song LM, Shim MG, et al. Diagnostic potential of near-infrared Raman spectroscopy in the colon: Differentiating adenomatous from hyperplastic polyps. Gastrointest Endosc 2003;57(3):396–402. [DOI] [PubMed] [Google Scholar]
- 66.Bergholt MS, Lin K, Wang J, et al. Simultaneous fingerprint and high-wavenumber fiber-optic Raman spectroscopy enhances real-time in vivo diagnosis of adenomatous polyps during colonoscopy. J Biophotonics 2016;9(4):333–42. [DOI] [PubMed] [Google Scholar]
- 67.Short MA, Wang W, Tai IT, et al. Development and in vivo testing of a high frequency endoscopic Raman spectroscopy system for potential applications in the detection of early colonic neoplasia. J Biophotonics 2016;9(1–2):44–8. [DOI] [PubMed] [Google Scholar]
- 68.Vogler N, Bocklitz T, Subhi Salah F, et al. Systematic evaluation of the biological variance within the Raman based colorectal tissue diagnostics. J Biophotonics 2016;9(5):533–41. [DOI] [PubMed] [Google Scholar]
- 69.Beljebbar A, Bouche O, Diebold MD, et al. Identification of Raman spectroscopic markers for the characterization of normal and adenocarcinomatous colonic tissues. Crit Rev Oncol Hematol 2009;72(3):255–64. [DOI] [PubMed] [Google Scholar]
- 70.Gaifulina R, Maher AT, Kendall C, et al. Label-free Raman spectroscopic imaging to extract morphological and chemical information from a formalin-fixed, paraffin-embedded rat colon tissue section. Int J Exp Pathol 2016;97(4):337–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Krafft C, Dietzek B, Schmitt M, et al. Raman and coherent anti-Stokes Raman scattering microspectroscopy for biomedical applications. J Biomed Opt 2012;17(4):040801. [DOI] [PubMed] [Google Scholar]
- 72.Lu G, Fei B. Medical hyperspectral imaging: A review. J Biomed Opt 2014;19(1):10901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ortega S, Fabelo H, Iakovidis DK, et al. Use of hyperspectral/multispectral imaging in gastroenterology. Shedding Some(-)Different(-)Light into the dark. J Clin Med 2019;8(1):36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Osawa H, Yamamoto H. Present and future status of flexible spectral imaging color enhancement and blue laser imaging technology. Dig Endosc 2014;26(Suppl 1):105–15. [DOI] [PubMed] [Google Scholar]
- 75.Maggioni M, Katz A, Davis GL, et al. Hyperspectral microscopic analysis of normal, benign and carcinoma microarray tissue sections. Opt Biopsy VI 2006;6091:60910I. [Google Scholar]
- 76.Kumashiro R, Konishi K, Chiba T, et al. Integrated endoscopic system based on optical imaging and hyperspectral data analysis for colorectal cancer detection. Anticancer Res 2016;36(8):3925–32. [PubMed] [Google Scholar]
- 77.Elson DS, Hanna GB, Teare J, et al. Flexible multimode endoscope for tissue reflectance and autofluorescence hyperspectral imaging. Biomed Opt 2016:paper OTh2C3. [Google Scholar]
- 78.Muguruma N, Miyamoto H, Okahisa T, et al. Endoscopic molecular imaging: Status and future perspective. Clin Endosc 2013;46(6):603–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hsiung PL, Hardy J, Friedland S, et al. Detection of colonic dysplasia in vivo using a targeted heptapeptide and confocal microendoscopy. Nat Med 2008;14(4):454–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Marten K, Bremer C, Khazaie K, et al. Detection of dysplastic intestinal adenomas using enzyme-sensing molecular beacons in mice. Gastroenterology 2002;122(2):406–14. [DOI] [PubMed] [Google Scholar]
- 81.Keller R, Winde G, Terpe HJ, et al. Fluorescence endoscopy using a fluorescein-labeled monoclonal antibody against carcinoembryonic antigen in patients with colorectal carcinoma and adenoma. Endoscopy 2002;34(10):801–7. [DOI] [PubMed] [Google Scholar]
- 82.Yuan S, Roney CA, Wierwille J, et al. Co-registered optical coherence tomography and fluorescence molecular imaging for simultaneous morphological and molecular imaging. Phys Med Biol 2010;55(1):191–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Iftimia N, Iyer AK, Hammer DX, et al. Fluorescence-guided optical coherence tomography imaging for colon cancer screening: A preliminary mouse study. Biomed Opt Express 2012;3:178–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Winkler AM, Rice PF, Weichsel J, et al. In vivo, dual-modality OCT/LIF imaging using a novel VEGF receptor-targeted NIR fluorescent probe in the AOM-treated mouse model. Mol Imaging Biol 2011;13(6):1173–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Foersch S, Kiesslich R, Waldner MJ, et al. Molecular imaging of VEGF in gastrointestinal cancer in vivo using confocal laser endomicroscopy. Gut 2010;59(8):1046–55. [DOI] [PubMed] [Google Scholar]
- 86.Goetz M, Ziebart A, Foersch S, et al. In vivo molecular imaging of colorectal cancer with confocal endomicroscopy by targeting epidermal growth factor receptor. Gastroenterology 2010;138(2):435–46. [DOI] [PubMed] [Google Scholar]
- 87.Goetz M, Hoetker MS, Diken M, et al. In vivo molecular imaging with cetuximab, an anti-EGFR antibody, for prediction of response in xenograft models of human colorectal cancer. Endoscopy 2013;45(6):469–77. [DOI] [PubMed] [Google Scholar]
- 88.Kaushal S, McElroy MK, Luiken GA, et al. Fluorophore-conjugated anti-CEA antibody for the intraoperative imaging of pancreatic and colorectal cancer. J Gastrointest Surg 2008;12(11):1938–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Committee AT, Abu Dayyeh BK, Thosani N, et al. ASGE Technology Committee systematic review and meta-analysis assessing the ASGE PIVI thresholds for adopting real-time endoscopic assessment of the histology of diminutive colorectal polyps. Gastrointest Endosc 2015;81(3):502.e1–e16. [DOI] [PubMed] [Google Scholar]
- 90.Wang LM, East JE. Diminutive polyp cancers and the DISCARD strategy: Much ado about nothing or the end of the affair? Gastrointest Endosc 2015;82(2):385–8. [DOI] [PubMed] [Google Scholar]