INTRODUCTION:
Monitoring of disease activity is essential in patients with inflammatory bowel disease. Although endoscopic remission is the ideal therapeutic goal, noninvasive biomarkers (blood and fecal) are more acceptable to patients and are less costly. We evaluated the performance of combinations of fecal and blood markers on the detection of endoscopically active disease.
METHODS:
Patients with ulcerative colitis (UC) or Crohn's disease (CD) on stable medications were recruited. Blood markers included C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), albumin, platelet count (PLT), and hemoglobin. Fecal biomarkers included fecal calprotectin (FCT) and fecal immunochemical test (FIT). These markers were compared with the endoscopic Mayo score for UC and the Simple Endoscopic Score for CD.
RESULTS:
One hundred thirteen patients (mean age 44.7 years, 63.7% men, 54.9% patients with UC and 45.1% patients with CD) were recruited. FCT correlated well with FIT (r = 0.58), CRP (r = 0.56), ESR (r = 0.40), albumin (r = −0.54), PLT (r = 0.61), and hemoglobin (r = −0.35; all Ps < 0.001). Among 66 patients with endoscopic evaluation, 39.4% with endoscopically active disease had higher FCT, FIT, CRP, ESR, PLT, lower albumin, and hemoglobin compared with those in endoscopic remission (all Ps < 0.01). All 7 markers demonstrated good area under receiver operating characteristics (>0.7), with FCT being the best (0.91) for endoscopically active disease. Combining FCT and FIT improved the specificity to 95%, but the sensitivity decreased to 65.4%. In the subgroup analysis of UC, adding PLT to FIT improved the sensitivity and specificity to 100% and 90.9%, respectively.
DISCUSSION:
The combined use of fecal biomarkers and blood indexes is superior to the use of fecal biomarkers alone in identifying endoscopically active disease.
INTRODUCTION
Inflammatory bowel disease (IBD) is a chronic relapsing condition that is characterized by recurrent or persistent intestinal inflammation of the gastrointestinal tract (GIT). Appropriate use of medical therapy can suppress inflammation and induce remission, preventing complications and reducing mortality (1,2). Monitoring of disease activity is essential to decide on the best treatment strategy for patients with IBD, particularly in the increasing popular treat-to-target approach (3,4). Clinical activity scores, such as the Simple Clinical Colitis Activity Index (SCCAI) for ulcerative colitis (UC) and the Harvey–Bradshaw Index (HBI) for Crohn's disease (CD), have been developed and widely used, which unfortunately correlate poorly with objective measures of disease activity (5,6). Endoscopic assessment of mucosal healing is considered the most direct and widely accepted standard of evaluation, as a surrogate for histological remission, which is associated with improved clinical outcomes, including reduced risk of surgery and hospitalization (7–10). Ileocolonoscopy is the procedure for assessment of mucosal healing in patients with UC and ileocolonic/colonic involvement in patients with CD. However, colonoscopy is an invasive procedure and can be costly, rendering it impractical to be repeated frequently for monitoring of disease activity.
Multiple noninvasive biomarkers have been developed as surrogate markers of endoscopic activity. Among them, fecal calprotectin (FCT) and fecal immunochemical test (FIT) are the 2 widely applied stool biomarkers. FCT is a calcium- and zinc-binding cytosolic protein in the cytosol of inflammatory cells (e.g., neutrophils) and is a marker for intestinal inflammation. It has been extensively evaluated as a biomarker for predicting inflammation in patients with IBD (11,12). Although the FIT was initially used in colorectal cancer screening, emerging data suggest its potential role in monitoring disease activity in patients with IBD (13,14). Although FCT has been directly compared with the FIT showing similar predictive accuracies for endoscopic remission or mucosal healing (15,16), it is unknown whether there are any additional benefits for the combined use of FCT and FIT. A recent study from Canada reported the feasibility of combination use of FCT, FIT, and clinical activity scores in the identification of endoscopic remission (17). In daily clinical practice, the approach of evaluating a combination of parameters is widely practiced. In particular, blood parameters are commonly used as adjunctive biomarkers for IBD disease activity, including C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), serum albumin, platelet count, and hemoglobin levels. In this study, we aimed to evaluate the role of combining commonly used fecal and blood parameters in the identification of endoscopically active IBD.
MATERIALS AND METHODS
Ethical considerations
The study was conducted in accordance with the Declaration of Helsinki. The study protocol was approved by the Institutional Review Board/Ethics Committee of the University of Hong Kong and the Hong Kong West Cluster of Hospital Authority (UW 16-109).
Patients
Patients with IBD who were diagnosed with UC or CD and attended the IBD Clinic of the Queen Mary Hospital of Hong Kong, which is a major regional hospital and the teaching hospital of the University of Hong Kong, were identified. All patients were on stable IBD medications (including aminosalicylates, corticosteroids, thiopurines, and biologics) for 12 months before the study recruitment. Patients were excluded if they had previous total colectomy or active colorectal malignancy. All patients provided written informed consent.
The disease extent (for UC) or location and behavior (for CD) was classified according to the Montreal Classification (18). The use of IBD medications, including thiopurines, corticosteroids, biologics, and aminosalicylates, was documented.
Assessment of disease activity
Clinical activity scores, including the SCCAI and HBI, were recorded for patients with UC and CD, respectively, at recruitment. Clinical remission was defined as SCCAI ≤2 for UC or HBI ≤5 for CD.
Blood samples were taken within 14 days from recruitment to measure serum CRP, ESR, platelet count, hemoglobin, and albumin levels. For fecal biomarkers, FCT was measured using the quantitative enzyme-linked immunosorbent assay Quantum Blue Calprotectin Extended (Buhlmann, Basel, Switzerland), with an extended range from 30 to 1,000 μg/g. The FIT was measured using the QuikRead go iFOBT (Orion Diagnostica, Espoo, Finland), which quantifies stool hemoglobin, with ranges from 15 to 200 μg/g. The stool collection tubes were distributed to the patients at recruitment, and patients were instructed to withhold nonsteroidal anti-inflammatory agents or proton-pump inhibitors for 2 weeks before stool sampling because these drugs were associated with elevated FCT levels (19).
Endoscopic disease activity was evaluated with validated scoring systems. For UC, the endoscopic Mayo score was used (20). For CD, the Simple Endoscopic Score for Crohn's Disease (SES-CD) was used to evaluate each segment (terminal ileum, right colon, transverse colon, sigmoid colon, and rectum) for ulceration size, proportion of ulcerated surface, proportion of affected surface, and presence of narrowing (21). All colonoscopies were performed by specialists with advanced training in endoscopic assessment of IBD disease activity. The endoscopists were masked to the results of fecal biomarkers. Endoscopically active disease was defined as endoscopic Mayo score ≥2 for UC (7) or SES-CD ≥4 for CD (22–24). Only colonoscopic examinations with good quality of bowel preparation were included for the analysis.
Statistical analysis
Baseline characteristics of patients were analyzed using standard descriptive statistics. Continuous variables were reported in mean ± SD. Categorical variables were reported in percentages. The Spearman rank correlation test was used to analyze the correlation between serum indexes, fecal biomarkers, and endoscopic scores. Receiver operating characteristic (ROC) curves were constructed for assessment of the accuracy of various blood indexes and fecal biomarkers in the identification of active endoscopic disease. The best cutoff level for each blood index or fecal biomarker was calculated by maximizing the Youden index. A 2-sided P value <0.05 was considered statistically significant. All analyses were performed using SPSS version 25 (Chicago, IL) or R version 3.2.3 (R Foundation for Statistical Computing) statistical software.
RESULTS
Patient characteristics
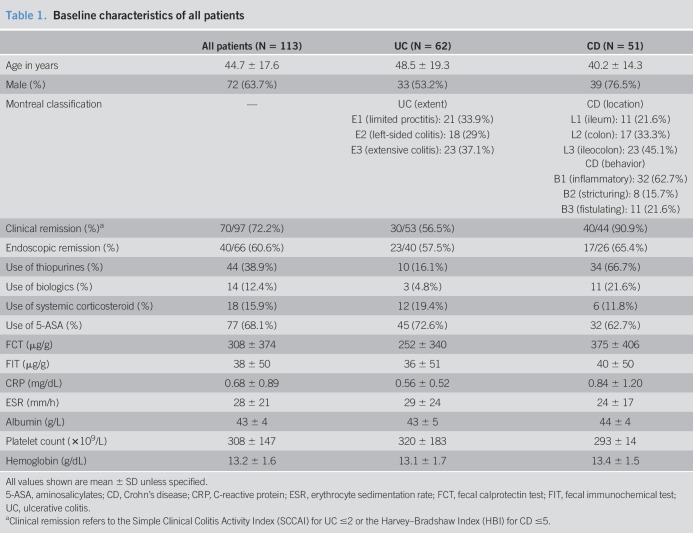
A total of 113 patients (mean age 44.7 ± 17.6 years, 63.7% men, 62 patients with UC [54.9%] and 51 patients with CD [45.1%]) were recruited. The patient characteristics are shown in Table 1. For UC, 37.1% patients had extensive colitis (Montreal Classification E3). The most commonly affected location of patients with CD was the ileocolon (L3, 45.1%), and the major disease behavior was nonstricturing and nonpenetrating (B1, 62.7%). At recruitment, 72.2% patients were in clinical remission. Most (68.1%) of them were on aminosalicylates, and 38.9% were on thiopurine therapy. Biologics and corticosteroids were used in 12.4% and 15.9% of patients, respectively.
Table 1.
Baseline characteristics of all patients
The results of the fecal biomarkers and blood indexes are shown in Table 1. There were no significant differences in the levels of any fecal biomarkers or blood indexes between patients with UC and CD (all Ps > 0.05). Sixty-six patients (58.4%) had endoscopic evaluation, and 40 (60.6%) of them were in endoscopic remission.
Correlation between blood indexes, fecal biomarkers, and endoscopic scores
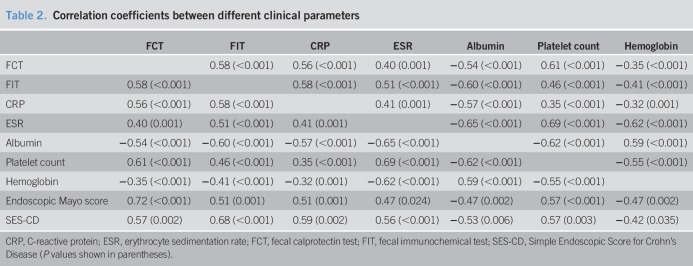
Among all noninvasive markers, FCT correlated best with platelet count (r = 0.61, P < 0.001). FCT also moderately correlated with FIT (r = 0.58), CRP (r = 0.56), albumin (r = −0.54), and ESR (r = 0.40) and weakly with hemoglobin (r = −0.35) (all Ps ≤ 0.001). There were weak to modest correlations among the 5 blood indexes (correlation coefficients 0.32–0.69) (Table 2).
Table 2.
Correlation coefficients between different clinical parameters
Regarding endoscopic scoring, the endoscopic Mayo score showed a strong correlation with FCT (r = 0.72, P < 0.001) and modest correlation with the FIT (r = 0.51, P = 0.001). However, SES-CD showed stronger correlation with the FIT (r = 0.68, P < 0.001) than FCT (r = 0.57, P = 0.002). The correlation coefficients are shown in Table 2, and the corresponding levels of each marker for different endoscopic scores are shown in Tables 1 and 2, Supplementary Digital Content 1, http://links.lww.com/CTG/A216. For SES-CD, because there is no well-established cutoff for severity of endoscopic activity, in this study, it was arbitrarily defined as follows: 0–3 was defined as endoscopic remission, 4–6 was defined as mild activity, and ≥7 was defined as moderate to severe activity (22–25).
Comparison between patients with active endoscopic disease and those in endoscopic remission
Compared with patients in endoscopic remission, those with active disease were significantly younger (47.2 ± 14.2 vs 31.7 ± 22.2 years, P = 0.005), with higher proportion of biologics (5% vs 23.1%, P = 0.04) and steroid use (7.5% vs 50%, P < 0.001). All 7 fecal and blood markers were significantly different between the 2 groups. For patients with active disease, FCT (764 ± 352 vs 182 ± 282 μg/g), FIT (83 ± 68 vs 23 ± 30 μg/g), CRP (1.48 ± 1.56 vs 0.44 ± 0.28 mg/dL), ESR (41 ± 25 vs 19 ± 13 mm/hour), and platelet count (473 ± 213 vs 254 ± 59 × 109/L) were significantly higher, and the serum albumin (39 ± 6 vs 45 ± 3 g/L) and hemoglobin (12.7 ± 1.9 vs 13.7 ± 1.2 g/dL) levels were significantly lower than those in patients in endoscopic remission (P < 0.01 for all 7 comparisons) (see Table 3, Supplementary Digital Content 1, http://links.lww.com/CTG/A216).
Performance of blood indexes and fecal biomarkers
ROC curves were constructed, with the area under receiver operating characteristic (AUROC) values, for all 7 markers to evaluate their individual performance in the identification of endoscopically active disease (Figure 1). FCT demonstrated the highest AUROC (0.91), followed by platelet count (0.87), FIT (0.80), ESR (0.76), CRP (0.74), hemoglobin (0.75), and albumin (0.73).
Figure 1.
Receiver operating characteristic curves of various markers in the identification of endoscopically active inflammatory bowel disease.
The subgroup analysis for the AUROC was performed separately for UC and CD. The AUROC for each marker in the corresponding subgroup is shown in Figure 1, Supplementary Digital Content 1, http://links.lww.com/CTG/A216, and there were no significant differences between UC and CD.
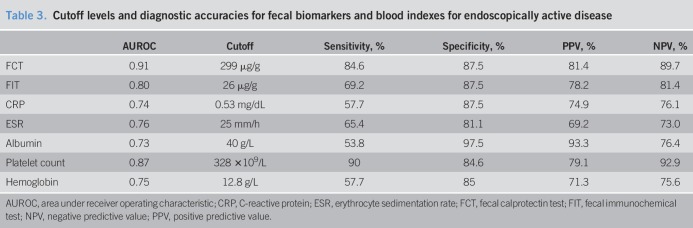
By maximizing the Youden index, the cutoff levels for FCT and FIT were 299 and 26 μg/g, respectively. The respective sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) for each marker are shown in Table 3. FCT alone already identified 84.6% patients with endoscopically active disease, with a specificity of 87.5%. The FIT alone has a low sensitivity for endoscopically active disease of 69.2% only. The presence of elevated FCT or FIT slightly improves the sensitivity to 88.5% but decreases the specificity to 80% (Figure 2). Elevation of both FCT and FIT increased the specificity to 95%, but the sensitivity decreased to 65.4%. The sensitivities and specificities of each blood index are shown in Figure 2, Supplementary Digital Content 1, http://links.lww.com/CTG/A216.
Table 3.
Cutoff levels and diagnostic accuracies for fecal biomarkers and blood indexes for endoscopically active disease
Figure 2.
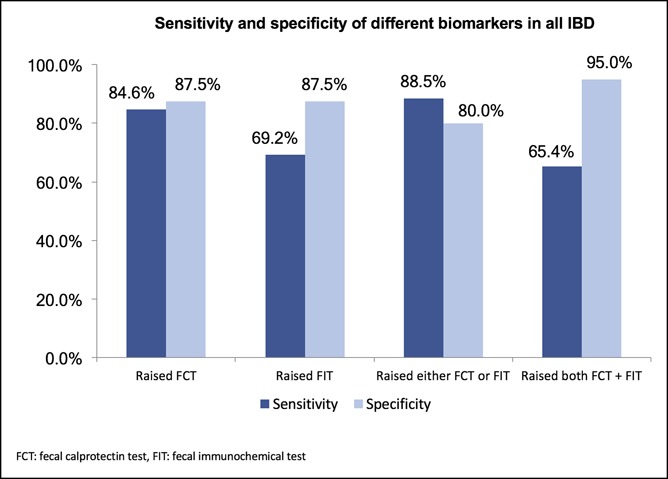
Sensitivity and specificity of different combinations of fecal biomarkers for endoscopically active IBD. IBD, inflammatory bowel disease.
Combination of fecal biomarkers and blood indexes in detecting endoscopically active IBD
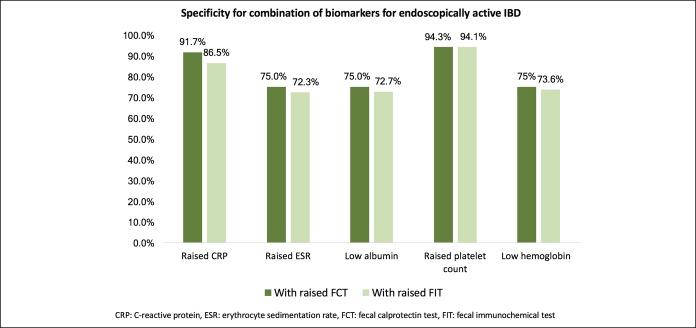
Because combining the 2 fecal biomarkers (FCT and FIT) could only improve the specificity but not the sensitivity for endoscopically active IBD, the role of addition of blood indexes to fecal biomarker was explored. The cutoff levels for each blood index are defined by the derived Youden index, as shown in Table 3. Each blood index was combined with either elevated FCT or FIT, and the respective sensitivities are shown in Figure 3. Addition of ESR, albumin, platelet count, or hemoglobin to FCT improved the overall sensitivity to >90%. However, the addition of CRP to FCT did not further improve the sensitivity (82.4% compared with 84.6% for FCT alone). However, addition of any of the blood indexes of CRP, ESR, serum albumin, platelet count, or hemoglobin to FIT improved the overall sensitivity to >85%. The corresponding specificities of each blood index in combination with elevated FCT or FIT are shown in Figure 4. By adding platelet count to either FCT or FIT, the specificity improved from 87.5% to >94%. Adding CRP to FCT also improved the sensitivity from 87.5% to 91.7%. The other combinations of blood and fecal markers did not improve the specificity. Overall, the best combination was by using increased FIT and increased platelet count, which detected 94.1% of endoscopically active IBD, with 94.1% specificity.
Figure 3.
Sensitivity for endoscopically active IBD with different profiles of fecal biomarkers and blood indexes. IBD, inflammatory bowel disease.
Figure 4.
Specificity for endoscopically active IBD with different profiles of fecal biomarkers and blood indexes. IBD, inflammatory bowel disease.
Subgroup analysis of the blood and fecal markers alone and in combination in patients with UC and CD
Patients with UC and CD were separately analyzed for each biomarker and the combination of a blood index with a fecal marker in detecting endoscopically active disease. In general, compared with patients with CD, the biomarkers alone or in combination had higher sensitivities in patients with UC, but the specificities for individual biomarker were similar for patients with UC and CD.
For UC, increased FCT and low albumin were 100% sensitive for endoscopically active disease, but the specificities were not optimal (85.2% and 74.2%, respectively) (see Figure 3, Supplementary Digital Content 1, http://links.lww.com/CTG/A216). Although adding CRP to FCT did not improve either the sensitivity or specificity, adding platelet count to FCT improved the specificity from 85.2% to 91.3%. Increased FIT alone has 91.7% sensitivity and 78.6% specificity, and adding platelet count to FIT improved the sensitivity and specificity to 100% and 90.9%, respectively (see Figures 4 and 5, Supplementary Digital Content 1, http://links.lww.com/CTG/A216).
For CD, increased FCT or FIT alone is specific (100% and 86.7%, respectively) but not sensitive (64.3% and 63.6%, respectively) for endoscopically active disease (see Figure 6, Supplementary Digital Content 1, http://links.lww.com/CTG/A216). Adding ESR or albumin to FCT improved the sensitivities to 83.3% and 83.3% but compromised the specificities to 76.5% and 80%, respectively. Adding albumin or hemoglobin to FIT improved the sensitivities to 100% but again compromising the specificities to 77.3% and 73.9%, respectively (see Figures 7 and 8, Supplementary Digital Content 1, http://links.lww.com/CTG/A216).
DISCUSSION
This study demonstrated that fecal biomarkers showed good performance characteristics in identifying endoscopically active inflammation in patients with IBD. The AUROCs for FCT and FIT were 0.91 and 0.80, respectively. Using a cutoff level of 299 μg/g for FCT, the sensitivity, specificity, PPV, and NPV were >80%. For a level ≥26 μg/g for the FIT, the sensitivity was lower at 69.2%, whereas the specificity, PPV, and NPV were approximately 80%. Increased FCT alone was able to identify 84.6% patients with active disease, although combining FCT with FIT improved the specificity to 95% but compromising the sensitivity to 65.4%.
On the other hand, combining routinely measured blood indexes (CRP, ESR, albumin, platelet count, and hemoglobin) with fecal biomarkers is another feasible strategy to further enhance the sensitivity (>90%) of endoscopically active IBD. The best combination was increased FIT and increased platelet count with a sensitivity of 94.1% and a specificity of 94.1%. Increased FIT combining with low albumin was 100% sensitive but only 72.7% specific for endoscopically active disease.
In fact, similar strategies using a combination of serum index and fecal biomarkers have been reported in the CALM study, where patients with CD assigned to the tight control management group received treatment escalation if FCT ≥250 μg/g and/or CRP ≥5 mg/L (on top of increase in clinical activity scores or steroid use, which were the only monitoring tools for the comparative arm) (26). The cutoff level for CRP in the current study was consistent with the defined level in the CALM study. In the current study, the combination of CRP and FCT did not further improve the sensitivity for endoscopically active IBD (84.6%–82.4%). However, adding CRP to FIT enhanced the sensitivity from 69.2% to 85.7%, supporting that the FIT may be a cheaper alternative to FCT when used in combination with CRP.
This is the first report to evaluate the combined use of platelet count, albumin, or hemoglobin with fecal biomarkers on the identification of endoscopically active diseases. Unlike FCT, measurement of these blood indexes is simple and routinely performed in most IBD clinics. The advantages of these markers over conventional ileocolonoscopy include safety, acceptability by patients, convenience, rapidity, and reproducibility. Thrombocytosis, in the absence of iron deficiency anemia, is a well-recognized phenomenon in patients with active IBD as a reactive process to chronic inflammation (27,28). The use of platelet count as a surrogate marker for disease activity in IBD is seldom reported (29). In this study, the AUROC of increased platelet count (>328 × 109/L) was 0.865, with 90% sensitivity and 84.6% specificity for active IBD. Combining thrombocytosis with increased FIT further improved the sensitivity to 94.1%.
When evaluating UC and CD separately, the performances of each biomarker alone and in combination varied significantly in each disease. In patients with UC, the best strategy would be a combination of either fecal marker to platelet count, yielding 100% sensitivity and >90% specificity. By contrast, for patients with CD, there is not a single combination that optimizes both sensitivity and specificity at the same time. Each marker is relatively specific but not sensitive for endoscopically active CD. Other strategy of assessment, e.g., combining ≥2 blood indexes with a fecal marker, may be required.
Regarding subgroup differences between UC and CD, the FIT was reported to be a better marker in UC than in CD in some other studies (13,17), possibly explained by the fact that small bowel involvement of CD may not be readily detected by fecal blood. In the current study, the FIT performed equally well in UC and CD (AUROC 0.80 vs 0.84, respectively). Hemoglobin performed better for active disease in UC than in CD, which was a consistent finding with other reports in the literature (30,31). This observation was likely because of the fact that not all active CD would develop intestinal hemorrhage as opposed to active UC because CD is a transmural process and some may manifest as fistulating or stricturing disease rather than bleeding. By contrast, CRP and albumin performed better in CD than in UC. These 2 markers are acute phase reactants and reflect inflammation, which is usually more pronounced in active CD with transmural involvement than in active UC. Nevertheless, the numerical differences of each AUROC value between UC and CD were not statistically significant. A larger sample size may be necessary to discern the difference between UC and CD.
In this cohort, 42.9% patients with endoscopic active IBD were in apparent clinical remission, whereas 31.6% patients in endoscopic remission were not in clinical remission. This again highlighted the fact that clinical symptoms in patients with IBD should be interpreted with caution and treatment strategies should be based on more objective assessment of disease activity. Because this is a cross-sectional study, the observation of a higher proportion of biologics and steroid use in the active disease group reflected the higher baseline disease activity rather than a causal relationship of the drugs onto the disease activity.
There are limitations in the current study. First, this was a cross-sectional study, and there were no data on subsequent risk of flare or remission. Second, only a single stool sample was evaluated instead of multiple samples, which may subject to intraindividual variability of FCT. It was, however, believed to be insignificant when the cutoff chosen was >250 μg/g (32). Third, there was no concurrent upper GIT and small bowel evaluation for some patients with CD because fecal biomarkers reflect not only colonic disease but also the upper GIT and small bowel disease activity. It is believed to exert a minor effect on the overall results of this study because most patients with CD (78.4%) in this cohort had colonic or ileocolonic disease. Further evaluation of the upper GIT and small bowel, such as imaging and capsule endoscopy, will be beneficial.
In conclusion, we demonstrated that a combination of increased FCT and FIT is very specific for endoscopically active disease, but combining fecal biomarkers with serum indexes further enhances the sensitivity for detection of endoscopically active colonic disease in Asian patients with IBD. In particular, for patients with UC, the combined use of increased FIT and high platelet count is 100% sensitive and 90.9% specific for endoscopically active disease. This simple and economical strategy may be particularly useful in the Asian setting, where resources are limited and FCT is still not widely available. Future studies are required to validate this finding in the prospective management of patients with IBD on prediction of disease flare.
CONFLICTS OF INTEREST
Guarantor of the article: Wai K. Leung, MD.
Specific author contributions: L.-Y.M. was responsible for study concept, design, data acquisition and analysis, and drafting of the manuscript. T.S.M.T. was involved in subject recruitment and data acquisition. K.-S.C. was involved in statistical analysis. L.-J.C. was involved in data acquisition. K.-L.L. was involved in statistical analysis. K.-S.L. was involved at performing laboratory tests and data collection. W.K.L. was involved in study concept and design, analysis and interpretation of data, critical revision of the manuscript, and overall study supervision. All authors have approved the final draft submitted.
Funding support: The fecal calprotectin tests used in this study were supported by the S K Yee Medical Foundation.
Potential competing interests: None to report.
Study Highlights.
WHAT IS KNOWN
✓ Assessing the activity of inflammatory bowel disease is best achieved with endoscopy.
✓ Noninvasive serum or fecal markers are the potential surrogate indicators of disease activity.
WHAT IS NEW HERE
✓ Combining elevated fecal calprotectin (FCT >299 μg/g) and fecal hemoglobin (FIT >26 μg/g) improved the specificity of identifying active inflammatory bowel disease to 95%, yet compromising the sensitivity to 65.4%.
✓ To improve the sensitivity, a combination of one fecal marker with one serum marker is a feasible strategy.
✓ Adding elevated platelet count (>328 × 109/L) to fecal hemoglobin is 100% sensitive and 90.9% specific in detecting active ulcerative colitis.
✓ Fecal biomarkers are specific but not sensitive for endoscopically active Crohn's disease. Adding serum marker to fecal marker may improve the sensitivity but compromise the specificity.
TRANSLATIONAL IMPACT
✓ Combination of 1 fecal and 1 serum marker is a useful noninvasive strategy to identify active IBD.
Supplementary Material
Footnotes
SUPPLEMENTARY MATERIAL accompanies this paper at http://links.lww.com/CTG/A216
REFERENCES
- 1.Harbord M, Eliakim R, Bettenworth D, et al. Third European evidence-based consensus on diagnosis and management of ulcerative colitis. Part 2: Current management. J Crohns Colitis 2017;11:769–84. [DOI] [PubMed] [Google Scholar]
- 2.Gomollon F, Dignass A, Annese V, et al. 3rd European evidence-based consensus on the diagnosis and management of Crohn's disease 2016: Part 1: Diagnosis and medical management. J Crohns Colitis 2017;11:3–25. [DOI] [PubMed] [Google Scholar]
- 3.Peyrin-Biroulet L, Sandborn W, Sands BE, et al. Selecting therapeutic targets in inflammatory bowel disease (STRIDE): Determining therapeutic goals for treat-to-target. Am J Gastroenterol 2015;110:1324–38. [DOI] [PubMed] [Google Scholar]
- 4.Colombel JF, D'Haens G, Lee WJ, et al. Outcomes and strategies to support a treat-to-target approach in inflammatory bowel disease: A systematic review. J Crohns Colitis 2020;14:254–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Colombel JF, Keir ME, Scherl A, et al. Discrepancies between patient-reported outcomes, and endoscopic and histological appearance in UC. Gut 2017;66:2063–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jharap B, Sandborn WJ, Reinisch W, et al. Randomised clinical study: Discrepancies between patient-reported outcomes and endoscopic appearance in moderate to severe ulcerative colitis. Aliment Pharmacol Ther 2015;42:1082–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Colombel JF, Rutgeerts P, Reinisch W, et al. Early mucosal healing with infliximab is associated with improved long-term clinical outcomes in ulcerative colitis. Gastroenterology 2011;141:1194–201. [DOI] [PubMed] [Google Scholar]
- 8.Schnitzler F, Fidder H, Ferrante M, et al. Mucosal healing predicts long-term outcome of maintenance therapy with infliximab in Crohn's disease. Inflamm Bowel Dis 2009;15:1295–301. [DOI] [PubMed] [Google Scholar]
- 9.Reinink AR, Lee TC, Higgins PD. Endoscopic mucosal healing predicts favorable clinical outcomes in inflammatory bowel disease: A meta-analysis. Inflamm Bowel Dis 2016;22:1859–69. [DOI] [PubMed] [Google Scholar]
- 10.Bryant RV, Burger DC, Delo J, et al. Beyond endoscopic mucosal healing in UC: Histological remission better predicts corticosteroid use and hospitalisation over 6 years of follow-up. Gut 2016;65:408–14. [DOI] [PubMed] [Google Scholar]
- 11.Schoepfer AM, Beglinger C, Straumann A, et al. Ulcerative colitis: Correlation of the Rachmilewitz endoscopic activity index with fecal calprotectin, clinical activity, C-reactive protein, and blood leukocytes. Inflamm Bowel Dis 2009;15:1851–8. [DOI] [PubMed] [Google Scholar]
- 12.D'Haens G, Ferrante M, Vermeire S, et al. Fecal calprotectin is a surrogate marker for endoscopic lesions in inflammatory bowel disease. Inflamm Bowel Dis 2012;18:2218–24. [DOI] [PubMed] [Google Scholar]
- 13.Inokuchi T, Kato J, Hiraoka S, et al. Fecal immunochemical test versus fecal calprotectin for prediction of mucosal healing in crohn's disease. Inflamm Bowel Dis 2016;22:1078–85. [DOI] [PubMed] [Google Scholar]
- 14.Nakarai A, Kato J, Hiraoka S, et al. Evaluation of mucosal healing of ulcerative colitis by a quantitative fecal immunochemical test. Am J Gastroenterol 2013;108:83–9. [DOI] [PubMed] [Google Scholar]
- 15.Mooiweer E, Fidder HH, Siersema PD, et al. Fecal hemoglobin and calprotectin are equally effective in identifying patients with inflammatory bowel disease with active endoscopic inflammation. Inflamm Bowel Dis 2014;20:307–14. [DOI] [PubMed] [Google Scholar]
- 16.Takashima S, Kato J, Hiraoka S, et al. Evaluation of mucosal healing in ulcerative colitis by fecal calprotectin vs. Fecal immunochemical test. Am J Gastroenterol 2015;110:873–80. [DOI] [PubMed] [Google Scholar]
- 17.Ma C, Lumb R, Walker EV, et al. Noninvasive fecal immunochemical testing and fecal calprotectin predict mucosal healing in inflammatory bowel disease: A prospective cohort study. Inflamm Bowel Dis 2017;23:1643–9. [DOI] [PubMed] [Google Scholar]
- 18.Silverberg MS, Satsangi J, Ahmad T, et al. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: Report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can J Gastroenterol 2005;19(Suppl A):5–36A. [DOI] [PubMed] [Google Scholar]
- 19.Lundgren D, Eklof V, Palmqvist R, et al. Proton pump inhibitor use is associated with elevated faecal calprotectin levels. A cross-sectional study on subjects referred for colonoscopy. Scand J Gastroenterol 2019;54:152–7. [DOI] [PubMed] [Google Scholar]
- 20.Schroeder KW, Tremaine WJ, Ilstrup DM. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. N Engl J Med 1987;317:1625–9. [DOI] [PubMed] [Google Scholar]
- 21.Daperno M, D'Haens G, Van Assche G, et al. Development and validation of a new, simplified endoscopic activity score for crohn's disease: The SES-CD. Gastrointest Endosc 2004;60:505–12. [DOI] [PubMed] [Google Scholar]
- 22.Sipponen T, Karkkainen P, Savilahti E, et al. Correlation of faecal calprotectin and lactoferrin with an endoscopic score for Crohn's disease and histological findings. Aliment Pharmacol Ther 2008;28:1221–9. [DOI] [PubMed] [Google Scholar]
- 23.Schaffer T, Schoepfer AM, Seibold F; Swiss IBDCSG. Serum ficolin-2 correlates worse than fecal calprotectin and CRP with endoscopic Crohn's disease activity. J Crohns Colitis 2014;8:1125–32. [DOI] [PubMed] [Google Scholar]
- 24.Schoepfer AM, Beglinger C, Straumann A, et al. Fecal calprotectin correlates more closely with the Simple Endoscopic Score for Crohn's Disease (SES-CD) than CRP, blood leukocytes, and the CDAI. Am J Gastroenterol 2010;105:162–9. [DOI] [PubMed] [Google Scholar]
- 25.Peyrin-Biroulet L, Panes J, Sandborn WJ, et al. Defining disease severity in inflammatory bowel diseases: Current and future directions. Clin Gastroenterol Hepatol 2016;14:348–54 e317. [DOI] [PubMed] [Google Scholar]
- 26.Colombel JF, Panaccione R, Bossuyt P, et al. Effect of tight control management on Crohn's disease (CALM): A multicentre, randomised, controlled phase 3 trial. Lancet 2018;390:2779–89. [DOI] [PubMed] [Google Scholar]
- 27.Morowitz DA, Allen LW, Kirsner JB. Thrombocytosis in chronic inflammatory bowel disease. Ann Intern Med 1968;68:1013–21. [DOI] [PubMed] [Google Scholar]
- 28.Collins CE, Rampton DS. Review article: Platelets in inflammatory bowel disease—pathogenetic role and therapeutic implications. Aliment Pharmacol Ther 1997;11:237–47. [DOI] [PubMed] [Google Scholar]
- 29.Harries AD, Fitzsimons E, Fifield R, et al. Platelet count: A simple measure of activity in crohn's disease. Br Med J (Clin Res Ed) 1983;286:1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koutroubakis IE, Ramos-Rivers C, Regueiro M, et al. Persistent or recurrent anemia is associated with severe and disabling inflammatory bowel disease. Clin Gastroenterol Hepatol 2015;13:1760–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Antunes CV, Hallack Neto AE, Nascimento CR, et al. Anemia in inflammatory bowel disease outpatients: Prevalence, risk factors, and etiology. Biomed Res Int 2015;2015:728925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Du L, Foshaug R, Huang VW, et al. Within-stool and within-day sample variability of fecal calprotectin in patients with inflammatory bowel disease: A prospective observational study. J Clin Gastroenterol 2018;52:235–40. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.