INTRODUCTION:
Race, ethnicity, and socioeconomic status are known to influence staging and survival in colorectal cancer (CRC). It is unclear how these relationships are affected by geographic factors and changes in insurance coverage for CRC screening. We examined the temporal trends in the association between sociodemographic and geographic factors and staging and survival among Medicare beneficiaries.
METHODS:
We identified patients 65 years or older with CRC using the 1991–2010 Surveillance, Epidemiology, and End Results–Medicare database and extracted area-level sociogeographic data. We constructed multinomial logistic regression models and the Cox proportional hazards models to assess factors associated with CRC stage and survival in 4 periods with evolving reimbursement and screening practices: (i) 1991–1997, (ii) 1998–June 2001, (iii) July 2001–2005, and (iv) 2006–2010.
RESULTS:
We observed 327,504 cases and 102,421 CRC deaths. Blacks were 24%–39% more likely to present with distant disease than whites. High-income areas had 7%–12% reduction in distant disease. Compared with whites, blacks had 16%–21% increased mortality, Asians had 32% lower mortality from 1991 to 1997 but only 13% lower mortality from 2006 to 2010, and Hispanics had 20% reduced mortality only from 1991 to 1997. High-education areas had 9%–12% lower mortality, and high-income areas had 5%–6% lower mortality after Medicare began coverage for screening colonoscopy. No consistent temporal trends were observed for the associations between geographic factors and CRC survival.
DISCUSSION:
Disparities in CRC staging and survival persisted over time for blacks and residents from areas of low socioeconomic status. Over time, staging and survival benefits have decreased for Asians and disappeared for Hispanics.
INTRODUCTION
Colorectal cancer (CRC) is the second leading cause of cancer death in the United States and predominantly affects older adults. Prognosis is highly dependent on the stage of disease at diagnosis. The 5-year survival rate is 90% for individuals diagnosed with localized disease but only 14% for those with distant disease (1). Sociodemographic factors, such as race, ethnicity, and socioeconomic status, are known to influence CRC incidence, stage at diagnosis, survival, and mortality (2–6). In a previous case–control study, we showed that sociodemographic disparities in incidence and mortality have persisted over time and that geographic factors were also independently associated with CRC diagnosis and death (7). However, it was unclear whether the increased risk of CRC death for some populations was driven by differences in stage at diagnosis or survival after diagnosis. Understanding at which point disparities arise has important implications for potential solutions because differences in stage at diagnosis indicate a need to improve screening and early detection, whereas differences in survival suggest a need to focus on treatment. Furthermore, the relationships between sociodemographic and geographic factors and CRC staging and survival may change over time with evolving screening and reimbursement practices. The objective of this study was to investigate the temporal trends in CRC staging and survival with respect to sociodemographic and geographic factors in a nationally representative population of older adults.
METHODS
Study design
We conducted a case–case study and time-to-event analysis using the Surveillance, Epidemiology, and End Results (SEER)–Medicare linked database from 1991 to 2010 (8). Individuals 65 years or older who were diagnosed with CRC were identified from the Patient Entitlement and Diagnosis Summary File. Stage at diagnosis was classified based on the SEER summary staging as localized, regional, or distant/metastatic disease. In situ cases were excluded. To examine the predictors of stage at diagnosis, cases of regional and distant cancer were compared with those of localized disease. For the time-to-event analysis, survival was measured from the month of diagnosis to death from CRC. Those who did not die of CRC were censored at death from another cause or in December 2010, whichever was earlier. Both sets of analyses were performed in 4 periods, based on the year of diagnosis, to reflect evolving screening and reimbursement practices: (i) 1991–1997, before Medicare coverage of CRC screening; (ii) 1998–June 2001, during which Medicare covered screening fecal occult blood testing (FOBT) and flexible sigmoidoscopy; (iii) July 2001–2005, when Medicare began covering screening colonoscopy for average-risk individuals; and (iv) 2006–2010, the most recent period with the highest screening uptake in the general population. Race/ethnicity was defined by the Medicare enrollment database categories, which included non-Hispanic white, non-Hispanic black, Asian/Pacific Islander, and Hispanic/Latino categories. Geographic variables were derived using the beneficiary's zip code of residence as listed in the Patient Entitlement and Diagnosis Summary File. Rural/urban status was categorized as urban, large rural, small rural, and isolated small rural as defined by the Rural Urban Community Area codes (9). Local area-level sociodemographic and geographic data, including median household income, educational level, primary care and specialist physician supply, and population density at the Primary Care Service Area (PCSA) level, were extracted from the Dartmouth Atlas of Health Care (10) and categorized into quartiles. Compared with counties, the PCSAs provide more granular and relevant data defined by health utilization rather than arbitrary geopolitical boundaries (11). The PCSA-level specialist supply measures availability of all specialists but does not provide data on particular specialists who might be involved in the diagnosis and treatment of CRC, such as gastroenterologists, surgeons, oncologists, and radiation oncologists. Educational level was measured by the percentage of the population within a PCSA with at least 12 years of education. The PCSA data from the 2000 US Census and 2000–2001 American Medical Association and the American Osteopathic Association Masterfiles were used for the 1991–June 2001 analyses, and data from the 2010 Census and American Medical Association files were used for the July 2001–2010 analyses. This study (protocol #7946) was approved by the Cancer Consortium Institutional Review Board at the Fred Hutchinson Cancer Research Center and University of Washington.
Predictors of interest
We included 9 variables in the model for stage at diagnosis, of which race/ethnicity, median household income, education, and rural vs urban residence were considered the main predictors of interest based on our previous work (7). The same 9 variables, in addition to stage at diagnosis, were included in the model for CRC survival. For each predictor of interest, we examined the temporal trends in these associations across the 4 periods.
Statistical analysis
We developed multivariable multinomial logistic regression models and the Cox proportional hazards models to characterize the association between the predictors of interest for stage at diagnosis and survival, respectively. Multinomial logistic regression is an extension of binary logistic regression that assumes nonperfect separation of the dependent variable choices by the independent variables in the model and independence among the dependent variable choices. The model does not assume normality, linearity, or homoscedasticity. Risk estimates are presented as relative risk ratios (RRRs), which are analogous to odds ratios in binary logistic regression models. The small proportion of cases with a race/ethnicity category other than “white,” “black,” “Asian,” or “Hispanic” and those missing information on the stage of diagnosis were excluded from analysis. The proportional hazards assumption was assessed using time-dependent covariates and Schoenfeld residuals in the Cox model and graphically. The temporal trends were assessed using the Wald tests of interaction terms between a categorical time variable and independent variables as well as pairwise contrasts between consecutive periods for each variable. Results were considered statistically significant if the 2-sided P value was less than 0.05. All data were analyzed using Stata version 13 (StataCorp, College Station, TX).
RESULTS
Study population
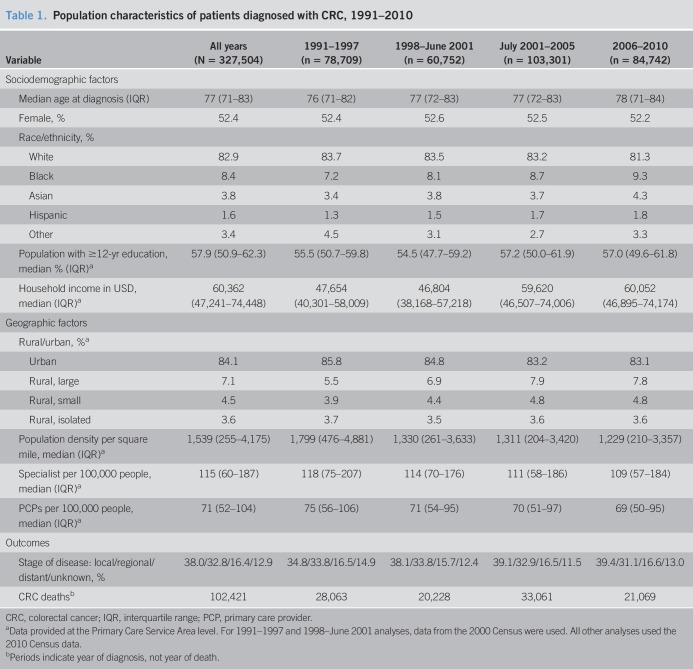
In the 20-year span between 1991 and 2010, 327,504 CRC cases and 102,421 deaths were observed (Table 1). The median age at diagnosis was 77 years. The study population was 52.4% women and 82.9% whites. By the PCSA, the median percentage of the population with at least 12 years of education was 57.9%, and the median household income was 60,362 USD. In terms of geography, 84.1% of individuals lived in urban areas, 7.1% lived in large rural areas, 4.5% lived in small rural areas, and 3.6% lived in small isolated rural areas. The median number of primary care providers and specialists per 100,000 residents in a PCSA were 71 and 115, respectively.
Table 1.
Population characteristics of patients diagnosed with CRC, 1991–2010
Stage at diagnosis
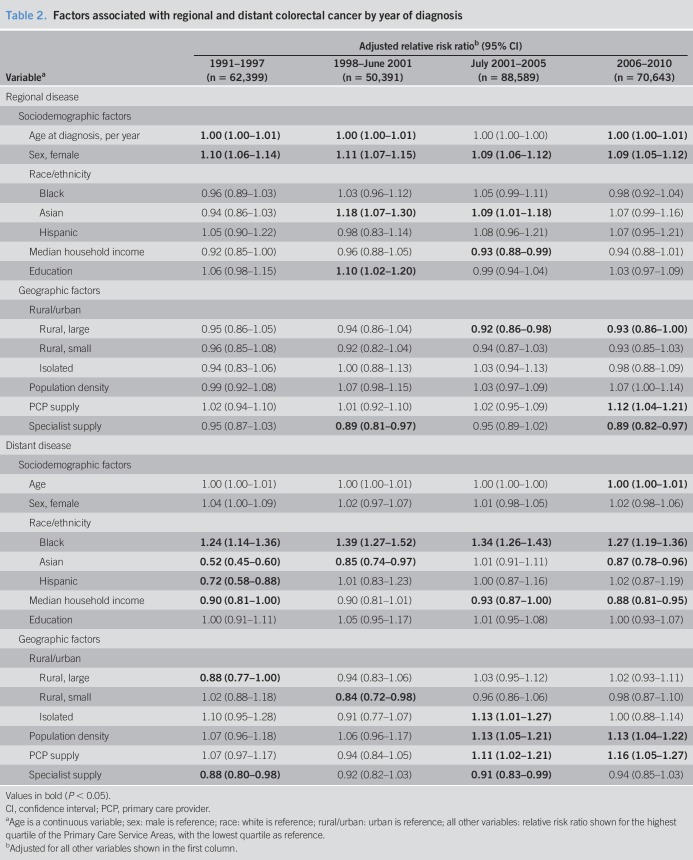
Over the entire study period and excluding cases with unknown staging, the distribution of stage at diagnosis was 43.6% localized, 37.6% regional, and 18.8% distant/metastatic disease (Table 1). Women were 9%–11% more likely than men to be diagnosed with regional disease, but there was no difference for distant disease. Among the 4 racial/ethnic groups, blacks had the highest risk of metastatic CRC and had 24%–39% higher risk than whites (Table 2). Before Medicare coverage for CRC screening, from 1991 to 1997, blacks had a 24% higher risk of distant CRC than whites (RRR 1.24; 95% confidence interval [CI] 1.14–1.36). The risk peaked during the period that screening FOBT and flexible sigmoidoscopy became covered under Medicare (1998–June 2001: RRR 1.39; 95% CI, 1.27–1.52; P = 0.04, for trend). After the coverage of screening colonoscopy began in 2001, the risk of being diagnosed with distant disease decreased for blacks but remained statistically higher compared with whites (2006–2010: RRR 1.27; 95% CI, 1.19–1.36; P < 0.01, for trend from July 2001–2005 to 2006–2010). Conversely, Asians had the lowest risk of metastatic CRC. Compared with whites, Asians had a 23%–48% lower risk of distant disease in 3 of the 4 periods (1991–1997: RRR 0.52; 95% CI, 0.45–0.60; 1998–2001: RRR 0.85; 95% CI, 0.74–0.97; and 2006–2010: RRR 0.87; 95% CI, 0.78–0.96). With the exception of the 2001–2005 period, during which Asians and whites had an equivalent risk of metastatic CRC, the relative protection against metastatic disease observed in Asians declined in each of the other 3 periods (P < 0.001 for trend over the study period). Similarly, Hispanics had a 28% lower risk of being diagnosed with distant disease compared with whites in 1991–1997 (RRR 0.72; 95% CI, 0.58–0.88), but after 1998, the difference between Hispanics and whites disappeared (P = 0.02 for trend over the study period). For socioeconomic factors, individuals residing in areas with the highest quartile of median household income had a statistically significant 7%–12% lower risk of metastatic CRC in 3 of the 4 periods (1991–1997: RRR 0.90; 95% CI, 0.81–1.00; 2001–2005: RRR 0.93; 95% CI, 0.87–1.00; and 2006–2010: RRR 0.88; 95% CI, 0.81–0.95); the risk estimate from 1998 to 2001 was consistent with that of the other 3 periods but did not reach statistical significance. No differences by educational level were detected. In terms of rural vs urban residence, no statistically significant trends were observed over time or between the different rural categories within each period.
Table 2.
Factors associated with regional and distant colorectal cancer by year of diagnosis
Survival and mortality
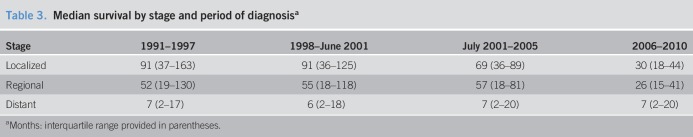
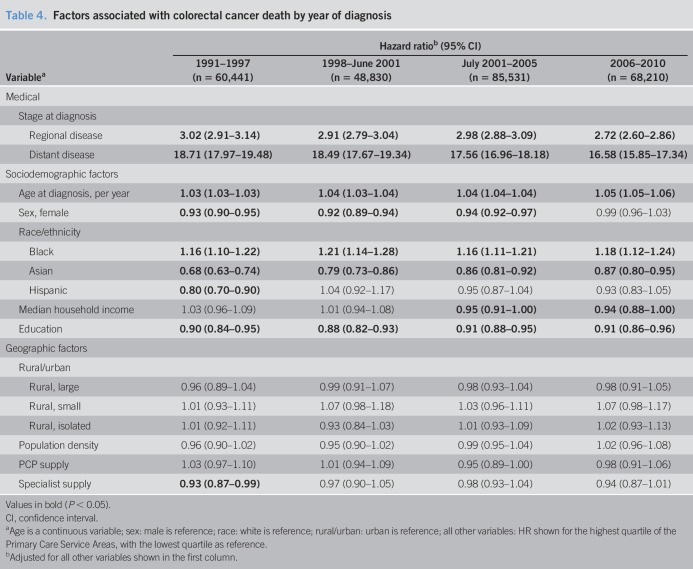
The median survival for localized disease was 91 months for cancers diagnosed between 1991 and 2001, and the apparent shorter survival seen after 2001 is explained by censoring at the end of the study period (Table 3). Individuals diagnosed with regional CRC saw a steady improvement in the median survival from 52 months in 1991–1997 to 57 months in 2001–2005. Again, a short follow-up explains the lower survival observed in 2006–2010. By contrast, the median survival for those diagnosed with distant disease remained consistently at 6–7 months from 1991 to 2010. As suspected, stage at diagnosis was the strongest negative predictor of survival. Mortality in patients diagnosed with regional and distant diseases was 3 and 17–19 times higher than that in those with localized disease, respectively (Table 4). For each additional year in age, there was a 3%–5% increase in CRC-specific mortality. Women had a 6%–8% lower risk of mortality than men from 1991 to 2005, although no difference was seen in 2006–2010.
Table 3.
Median survival by stage and period of diagnosisa
Table 4.
Factors associated with colorectal cancer death by year of diagnosis
Mortality results by race/ethnicity were comparable with findings for stage at diagnosis, with blacks having the worst outcomes, Asians having the best outcomes, and Hispanics having the most similar outcomes relative to whites. Blacks had a 16%–21% increased risk of death over the study period, but there was no clear directional trend over time (1991–1997: hazard ratio [HR] 1.16; 95% CI, 1.10–1.22; and 2006–2010: HR 1.18; 95% CI, 1.12–1.24; P = 0.81, for trend over the study period). Asians had a 32% lower risk of CRC death in 1991–1997 (HR 0.68; 95% CI, 0.63–0.74), but by 2006–2010, there was only a 13% relative risk reduction (HR 0.87; 95% CI, 0.80–0.95; P < 0.001, for trend over the study period). Finally, a 20% lower risk of mortality was observed for Hispanics in 1991–1997 (HR 0.80; 95% CI, 0.70–0.90), but after 1998, no difference in mortality was seen (P = 0.02 for trend from 1991–1997 to 1998–June 2001). Statistically significant associations for both socioeconomic variables were found. Individuals living in areas with the highest quartile of median household income had a 5%–6% lower risk of death in 2001–2010, which corresponds to the period that screening colonoscopy gained Medicare coverage (2001–2005: HR 0.95; 95% CI, 0.91–1.00; and 2006–2010: HR 0.94; 95% CI, 0.88–1.00). By contrast, residence in areas with the highest quartile of educational attainment was consistently associated with a 9%–12% lower risk of death throughout the study period (1991–1997: HR 0.90; 95% CI, 0.84–0.95; and 2006–2010: HR 0.91; 95% CI, 0.86–0.96). Similar to the staging results, we found no consistent and statistically significant association between rural vs urban residence and CRC mortality. In a sensitivity analysis, we examined the associations when stage at diagnosis was removed from the model. The association between race/ethnicity and mortality was strengthened in the unadjusted model, indicating that racial/ethnic differences in survival are partly explained by stage at diagnosis (see Table 1, Supplementary Digital Content 1, http://links.lww.com/CTG/A237).
DISCUSSION
In this 1991–2010 SEER–Medicare analysis that spans 2 decades of changing screening and reimbursement practices, we found striking differences in CRC stage at diagnosis and survival by race/ethnicity and area-level socioeconomic status. For both distant stage at diagnosis and survival, blacks had the poorest outcomes compared with whites, whereas Asians had the most favorable outcomes. Hispanics had better outcomes than whites initially but lost their protective advantage after Medicare coverage for CRC screening began. Higher area-level income was associated with better outcomes for both stage at diagnosis and survival, whereas area-level education was only associated with improved survival. Rural or urban residence did not appear to influence CRC stage or survival.
Race/ethnicity
To the best of our knowledge, our study is the first to compare stage and survival across periods of varying access to CRC screening. For blacks vs whites, we found that the relative risk for distant CRC increased after Medicare began covering FOBT and sigmoidoscopy. The disparity decreased thereafter but still remained higher in the most recent period than during the era before screening was widely covered. A similar pattern for the black–white disparity in survival was observed, but these trends were not statistically significant. A number of recent studies have examined black–white disparities using the SEER registries (6,12–15), other population-based cancer registries (5,16), and hospital-based cohorts (17). Most data agree with our results and show that blacks are more likely to present with advanced-stage CRC and have worse survival compared with whites, although there are notable exceptions. An analysis of patients with nonmetastatic CRC from 2001 to 2006 in the California Cancer Registry showed no racial disparity in survival within an integrated healthcare system, but blacks still had worse survival for most patients in nonintegrated care settings (16). A SEER analysis from 1975 to 2012 found that the black–white disparity in late-stage presentation disappeared by 2010, but the investigators defined late-stage disease as both regional and distant diseases and did not adjust for sociodemographic factors (15), whereas we separated these 2 nonlocalized stages and adjusted for both sociodemographic and geographic factors. We found black–white disparities only for distant disease and not for regional disease presentation, which were consistent throughout the study period from 1991 to 2010 (Table 2). The reason for the observed racial difference in regional and distant CRC cases is unclear, but it is likely the primary explanation for the difference in our results.
Compared with the literature that focused on CRC disparities in the black population, few studies have examined disparities in Asians and Hispanics. One community-based study found no difference in advanced-stage disease between Asians or Hispanics relative to whites, although this was a single institution study with a relatively small sample size (17). However, a study using the Arizona Cancer Registry found a 3.8% and 3.5% higher absolute rate of advanced-stage CRC for Asians and Hispanics, respectively (5). Whereas these studies considered Asians and Hispanics as single large groups, investigations that examined disaggregated data for Asian and Hispanic subgroups show a more nuanced picture (3,18,19). A SEER analysis from 1988 to 1994 focused on 3 large US Asian subgroups and found that Japanese individuals had a higher rate of early stage disease and 5-year survival compared with Chinese and Filipinos (19). Another SEER study by Chien et al. (3) analyzed 18 different racial and ethnic groups and found that Chinese, Filipinos, and Koreans had a 20%–50% increased risk of presenting with stage III or IV CRC compared with non-Hispanic whites, whereas Japanese were 20% less likely to present with stage IV disease. For Hispanic subgroups, Mexicans, South/Central Americans, and Puerto Ricans had a 30%–40% elevated risk for stage III or IV disease. Stage-adjusted survival was better for Asians (specifically Japanese, Chinese, and Indian/Pakistani) and worse for Hispanics (specifically Mexicans) compared with non-Hispanic whites. Our study extends these earlier works by demonstrating shifting patterns in CRC stage at diagnosis and survival for Asians and Hispanics over time. Similar to what was seen for blacks, the relative risk for distant disease and mortality worsened for Asians and Hispanics after 1998. For Asians, who had the lowest risk of advanced disease and mortality of any racial/ethnic group, CRC stage and survival benefit was attenuated after the widespread coverage of screening. Hispanics also had a stage and survival benefit compared with whites before 1998, but this benefit disappeared as access to screening increased. These trends are concerning because a recent analysis of population-based cancer registries in the United States showed that although CRC is the third most common cancer for most racial and ethnic groups, it is the second most frequent cancer for Hispanic men and women (6). In addition, although Asian women have the lowest baseline death rate from CRC of any racial or ethnic group, they were also one of the few demographic groups that did not experience a reduction in the death rate from 2010 to 2014. Higher CRC survival among Asian Americans on aggregate has been attributed to factors such as higher socioeconomic status and more favorable tumor characteristics (12). There is no indication that either the biologic or socioeconomic characteristics of Asians in the United States have changed appreciably in the past 2 decades, and we also adjusted for area-level income and educational attainment in our analysis. Therefore, we hypothesize that the reason for the shrinking stage and survival benefit for Asians over time is due to lower rates of screening relative to whites. Similarly, Hispanics had a smaller baseline protective advantage than Asians, and this has vanished since the start of widespread population-level screening in 1998. Recent statistics confirm that Asians and Hispanics have the lowest CRC screening rates in the United States by race and ethnicity. In the 2015 National Health Interview Survey, 52% of Asians and 47% of Hispanics received up-to-date screening, which were both substantially lower than the 62% national average (20).
Socioeconomic status
We found that residence in areas with the highest income was associated with an approximately 10% reduction in metastatic CRC throughout the study period, even after adjusting for multiple other sociodemographic and geographic factors. However, education was not associated with stage, which suggests that income may be the primary socioeconomic determinant of screening and early detection. Our results are consistent with a recent population-based British Cancer Registry study, which found that from 2000 to 2012, individuals with the highest level of deprivation were 26% more likely to present with stage IV CRC compared with the most affluent individuals (21). With respect to survival, we found that residence in areas with the highest educational attainment was associated with an approximately 10% improvement in survival throughout the study period, and high-income areas were associated with an approximately 5% improvement in survival after Medicare coverage for screening colonoscopy. Another recent analysis examined individual-level socioeconomic status using the National Longitudinal Mortality Study and found that between 1979–1998 and 2003–2011, the relative risk of CRC mortality for individuals who did not complete high school compared with those who completed college increased from 1.16 to 2.20 for women and decreased from 1.53 to 1.42 for men (22). Similarly, the relative risk for those under the poverty threshold compared with those with income >600% above the poverty threshold increased from 1.29 to 1.47 for women and decreased from 1.24 to 1.02 for men. In a sex-stratified analysis, we did not observe any differences in the association between income, education, and CRC survival for women and men from 1991–1997 to 2006–2010. We used local-level measures rather than individual-level measures of socioeconomic status and adjusted for a number of variables in addition to age, which may explain the stability of our risk estimates over time. Last, our previous work and those of others have shown a reversal in the relationship between area-level socioeconomic status and CRC mortality over time, in which high socioeconomic status only became protective against CRC mortality after the late 1990s (7,23). We did not see a similar trend reversal in the present analysis, which suggests that the relationship had already begun to transition during the earliest period (1991–1997) we examined.
Geography
In a previous SEER–Medicare analysis from 1973 to 2010, we found that residence in small rural areas was strongly associated with both incident CRC and lethal CRC, but that these associations attenuated over time (7). Our present analysis did not find any consistent association between rural vs urban residence and CRC stage or survival from 1991 to 2010. Together, these results suggest rural residents are more likely to be diagnosed with CRC than urban residents, but they are not more likely to be diagnosed with advanced-stage cancer or have shorter survival after adjusting for stage and relevant sociodemographic factors. This implies that rural vs urban disparities may exist with respect to factors that influence CRC incidence, but treatment outcomes after diagnosis appear to be similar. It should be noted that we classified the rural/urban spectrum using the Rural Urban Community Area codes, which assess both population and commuting patterns at the zip code level. Using an alternative set of definitions for “rural” and “urban” may yield slightly different results. For example, a previous SEER study that divided counties into 5 categories based on the population found a J-shaped relationship for both CRC stage at diagnosis and survival, and the least and most populated categories at either extreme had the worst outcomes (24). Further research is needed to determine whether disaggregating the “urban” category would yield additional insights into the relationship between geography and CRC outcomes.
Strengths and limitations
Our study has 2 major strengths. First, we used a large nationally representative sample of Medicare beneficiaries, which increases the generalizability of our results. Second, the periods we selected for analysis correspond to distinct eras of Medicare reimbursement or screening uptake, which provide the relevant policy context for interpreting the findings. Several limitations should also be noted. First, it is unclear whether results from a SEER–Medicare study are generalizable to patients younger than 65 years. Second, patients who were diagnosed with CRC between 2006 and 2010 all had fewer than 5 years of follow-up. However, the recent national report on cancer published by the American Cancer Society and several government organizations found that the 5-year survival for blacks, Asians, and Hispanics from 2006 to 2012 was consistent with our survival analysis from 2006 to 2010 in both the direction and magnitude of association (6). This suggests that CRC outcomes in the Medicare population are comparable with those in the general population and that having less than 5 years of follow-up for survival analysis does not substantively affect this particular result. Third, there are many Asian and Hispanic subgroups with differences in various CRC-related risk factors. Unfortunately, disaggregated racial/ethnic data were not available for this analysis. Fourth, potentially important lifestyle and cultural factors, such as diet, exercise, and health behavior, are not captured in this data set. Last, although we used the PCSA-level contextual data to characterize income and education at the most granular level possible, these remain area-level data and are not directly attributable to individuals.
From 1991 to 2010, during which CRC screening was widely introduced in the United States, we observed persistent disparities in CRC stage at diagnosis and survival for blacks and individuals living in areas with lower socioeconomic status. In addition, stage and survival benefits observed at the beginning of the period decreased for Asians and disappeared for Hispanics over time.
CONFLICTS OF INTEREST
Guarantor of the article: Cynthia W. Ko, MD, MS.
Specific author contributions: P.S.L. and C.W.K. had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: P.S.L., J.D.M., J.W., and C.W.K.; acquisition, analysis, or interpretation of the data: P.S.L., J.D.M., J.W., and C.W.K.; drafting of the manuscript: P.S.L. and C.W.K.; critical revision of the manuscript for important intellectual content: P.S.L., J.D.M., J.W., C.T.-S., S.C.K., S.E.S., and C.W.K.; statistical analysis: P.S.L.; obtained funding: C.W.K.; and administrative, technical, or material support and study supervision: C.W.K.
Financial support: This work is supported, in part, by the National Institutes of Health (National Institute of Diabetes and Digestive and Kidney Diseases grant T32DK007742 and the National Cancer Institute grant K08CA230162 for P.S.L., the National Cancer Institute grant R01CA095994 to J.W., and the National Institute on Minority Health and Health Disparities grant U54MD000538 and the National Center for Advancing Translational Sciences grant U54UL1TR001445 to C.T.-S. and S.C.K.) with resources and the use of facilities at the VA New York Harbor Healthcare System. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies.
Potential competing interests: None to report.
Study Highlights.
WHAT IS KNOWN
✓ Racial and socioeconomic disparities exist in CRC staging and survival.
✓ Screening practices have evolved with changes in reimbursement.
WHAT IS NEW HERE
✓ Over 3 decades, blacks have had persistently worse outcomes than whites for staging and survival.
✓ Asians and Hispanics had better staging and survival outcomes than whites, but over time, this advantage has decreased for Asians and disappeared for Hispanics.
✓ Residents from high-income areas are less likely to be diagnosed with metastatic disease.
✓ Residents from both high-income and high-education areas are less likely to die of CRC.
TRANSLATIONAL IMPACT
✓ These findings demonstrate a continued need to monitor and improve CRC outcomes for racial/ethnic minorities and socioeconomically disadvantaged groups.
Supplementary Material
ACKNOWLEDGMENT
We thank Sanya Anand for her assistance with the literature review.
Footnotes
SUPPLEMENTARY MATERIAL accompanies this paper at http://links.lww.com/CTG/A237
REFERENCES
- 1.National Cancer Institute. Cancer Stat Facts: Colorectal Cancer. Surveillance, Epidemiology, and End Results Program (seer.cancer.gov/statfacts/html/colorect.html). Accessed January 16, 2020.
- 2.Albano JD, Ward E, Jemal A, et al. Cancer mortality in the United States by education level and race. J Natl Cancer Inst 2007;99:1384–94. [DOI] [PubMed] [Google Scholar]
- 3.Chien C, Morimoto LM, Tom J, et al. Differences in colorectal carcinoma stage and survival by race and ethnicity. Cancer 2005;104:629–39. [DOI] [PubMed] [Google Scholar]
- 4.Tawk R, Abner A, Ashford A, et al. Differences in colorectal cancer outcomes by race and insurance. Int J Environ Res Public Health 2015;13:ijerph13010048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jandova J, Ohlson E, Torres B S MR, et al. Racial disparities and socioeconomic status in the incidence of colorectal cancer in Arizona. Am J Surg 2016;212:485–92. [DOI] [PubMed] [Google Scholar]
- 6.Jemal A, Ward EM, Johnson CJ, et al. Annual report to the nation on the status of cancer, 1975-2014, featuring survival. J Natl Cancer Inst 2017;109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liang PS, Mayer JD, Wakefield J, et al. Temporal trends in geographic and sociodemographic disparities in colorectal cancer among Medicare patients, 1973-2010. J Rural Health 2017;33:361–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.National Cancer Institute. SEER-Medicare Linked Database (http://healthservices.cancer.gov/seermedicare/). Accessed October 1, 2015.
- 9.WWAMI Rural Health Research Center. Using RUCA Data (http://depts.washington.edu/uwruca/ruca-uses.php). Accessed March 18, 2015.
- 10.The Dartmouth Institute for Health Policy and Clinical Practice. The Dartmouth atlas of health care (http://www.dartmouthatlas.org/tools/downloads.aspx?tab=42). Accessed February 9, 2015.
- 11.Goodman DC, Mick SS, Bott D, et al. Primary care service areas: A new tool for the evaluation of primary care services. Health Serv Res 2003;38:287–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin J, Qiu M, Xu R, et al. Comparison of survival and clinicopathologic features in colorectal cancer among African American, Caucasian, and Chinese patients treated in the United States: Results from the surveillance epidemiology and end results (SEER) database. Oncotarget 2015;6:33935–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Valeri L, Chen JT, Garcia-Albeniz X, et al. The role of stage at diagnosis in colorectal cancer black-white survival disparities: A counterfactual causal inference approach. Cancer Epidemiol Biomarkers Prev 2016;25:83–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lai Y, Wang C, Civan JM, et al. Effects of cancer stage and treatment differences on racial disparities in survival from colon cancer: A United States population-based study. Gastroenterology 2016;150:1135–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.May FP, Glenn BA, Crespi CM, et al. Decreasing black-white disparities in colorectal cancer incidence and stage at presentation in the United States. Cancer Epidemiol Biomarkers Prev 2017;26:762–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rhoads KF, Patel MI, Ma Y, et al. How do integrated health care systems address racial and ethnic disparities in colon cancer? J Clin Oncol 2015;33:854–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chan C, Lopez A, Castaneda G, et al. Black patients with colorectal cancer have more advanced cancer stage at time of diagnosis: A community-based safety-net hospital experience. J Community Health 2017;42:724–9. [DOI] [PubMed] [Google Scholar]
- 18.Torre LA, Sauer AMG, Chen MS, et al. Cancer statistics for Asian Americans, Native Hawaiians, and Pacific Islanders, 2016: Converging incidence in males and females: Cancer statistics for Asian Americans, Native Hawaiians, and Pacific Islanders, 2016. CA Cancer J Clin 2016;66:182–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin SS, Clarke CA, Prehn AW, et al. Survival differences among Asian subpopulations in the United States after prostate, colorectal, breast, and cervical carcinomas. Cancer 2002;94:1175–82. [PubMed] [Google Scholar]
- 20.White A, Thompson TD, White MC, et al. Cancer screening test use—United States, 2015. MMWR Morb Mortal Wkly Rep 2017;66:201–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Askari A, Nachiappan S, Currie A, et al. The relationship between ethnicity, social deprivation and late presentation of colorectal cancer. Cancer Epidemiol 2017;47:88–93. [DOI] [PubMed] [Google Scholar]
- 22.Singh GK, Jemal A. Socioeconomic and racial/ethnic disparities in cancer mortality, incidence, and survival in the United States, 1950-2014: Over six decades of changing patterns and widening inequalities. J Environ Public Health 2017;2017:2819372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Breen N, Lewis DR, Gibson JT, et al. Assessing disparities in colorectal cancer mortality by socioeconomic status using new tools: Health disparities calculator and socioeconomic quintiles. Cancer Causes Control 2017;28:117–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fitzgerald TL, Lea CS, Brinkley J, et al. Colorectal cancer outcome inequalities: Association between population density, race, and socioeconomic status. Rural Remote Health 2014;14:2668. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.