OBJECTIVES:
The diagnostic value of different noninvasive diagnostic modalities and the endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) reliability of duodenal gastrointestinal stromal tumors (GISTs) are ambiguous in the present studies.
METHODS:
Patients with a histopathological diagnosis of the primary duodenal GISTs between the years 2008 and 2018 were analyzed. Data on the treatment and clinicopathological features were recorded. Furthermore, the computed tomography (CT)/magnetic resonance imaging (MRI), EUS, and EUS-FNA results were collected and compared.
RESULTS:
A total of 142 patients were enrolled into the study. In all patients, the most common symptom was gastrointestinal bleeding (44.4%), followed by abdominal pain and bloating (27.5%). Duodenal GISTs were mostly located in the second duodenal portion (52.1%), followed by the first portion (19.0%). EUS had significantly higher sensitivity and positive predictive values than CT or MRI (P = 0.047 and P = 0.005, respectively). The EUS-FNA sensitivity of duodenal GISTs was also significantly higher than the conventional endoscopic biopsy (73.3% vs 33.3%, P = 0.006). A total of 131 patients underwent surgery, including limited resection or pancreaticoduodenectomy. The tumor size and postoperative complication rates were higher in patients who underwent pancreaticoduodenectomy (P = 0.001 and P < 0.001, respectively).
DISCUSSION:
The diagnostic value of EUS is significantly higher than that of CT and MRI for duodenal GISTs. The EUS-FNA can provide a histological diagnosis of duodenal GISTs in most cases.
INTRODUCTION
Gastrointestinal stromal tumors (GISTs) are the most common mesenchymal tumors of the gastrointestinal tract with unique histological characteristics (1). These tumors, generally defined as KIT (CD117) positive and derived from the interstitial cells of Cajal or their precursors, can occur anywhere in the GI tract. The stomach is the most common site, followed by the jejunum and ileum. However, the duodenum is a relatively rare site accounting for approximately 4%–5% (2). The general incidence and prevalence of GISTs are estimated to be approximately 1–1.5 per 100,000 individuals per year and 13 per 100,000, respectively (3). As the understanding of the clinical and molecular features of GISTs deepens, its diagnosis and treatment have been standardized. Many clinical practice guidelines and consensus were published in different parts of the world, such as the United States, Europe, China, Japan, and Korea (4–8).
With the widespread use of auxiliary inspection, many duodenal GISTs that appear as submucosal tumors or nodules are detected by using endoscopy or computed tomography (CT)/magnetic resonance imaging (MRI). Some studies have reviewed and summarized the characteristics of duodenal GISTs of endoscopic ultrasonography (EUS) or CT/MRI (9–12). However, few studies have compared the diagnostic values between different diagnostic modalities.
As submucosal tumors, specimens obtained by routine endoscopic biopsy are difficult to provide an accurate pathological diagnosis, and the pathological diagnosis is often obtained after surgery. However, with the rapid EUS development, the EUS-guided fine-needle aspiration (EUS-FNA) is used to obtain a histological diagnosis before surgery (13,14). However, the EUS-FNA reliability of duodenal GISTs is controversial in the current study.
Surgery is an important treatment strategy because only the complete removal of the primary GISTs can cure it. The modified National Institutes of Health (NIH) consensus classification system, tumor size, mitotic activity, and anatomic site can help predict disease prognosis. Because of the special duodenal location, different surgical procedures, including limited resection (LR) and pancreaticoduodenectomy (PD), are available (15–22).
The main purpose of this study was to evaluate the clinical characteristics, diagnostic value of different noninvasive diagnostic modalities, and EUS-FNA reliability of duodenal GISTs.
METHODS
Patients and design
Patients with a histopathological diagnosis of the primary duodenal GISTs at the Zhejiang University School of Medicine First Affiliated Hospital, between the years 2008 and 2018, were included in this study. Histopathological evaluation and immunohistochemistry for CD117, PDGFRA, CD34, smooth muscle actin, desmin, S-100, and Ki67 were used to diagnose all patients after EUS-FNA or surgery. Surgical approaches included PD and LR. The surgery type was selected based on the tumor size and its position relative to the duodenal papilla. The tumor size and mitotic index were recorded according to the NIH consensus classification system, which was modified by Joensuu in 2008 (23). The study was approved by the Ethics Committee of the Zhejiang University School of Medicine First Affiliated Hospital.
Data collection
Baseline demographic and clinical characteristics were collected, including the clinical symptoms, gender, age at diagnosis, accuracy of preoperative diagnosis with EUS and CT/MRI, the EUS-FNA diagnostic efficiency and conventional endoscopic biopsy, tumor location (first, second, third, and fourth duodenal portions), surgical approach type, postoperative complications, tumor size, mitotic index, and adjuvant imatinib therapy. Moreover, we collected data of patients whose lesions were pathologically confirmed as non-GISTs but misdiagnosed as duodenal GISTs by EUS or CT/MRI between the years 2008 and 2018. The sensitivity and positive predictive values of EUS and CT/MRI of duodenal GISTs were fully compared.
Statistical analysis
Statistical analysis was performed using SPSS 21.0 software. Categorical variables were expressed in frequencies and percentages and continuous variables as mean and SD. The T-test or χ2 analysis was used to evaluate different variables.
RESULTS
Clinical characteristics of patients
In this study, duodenal GISTs were pathologically diagnosed in 142 patients. The disease incidence was slightly higher in men, with a mean age of 55.9 years. In all patients, the most common symptom was gastrointestinal bleeding (44.4%), followed by abdominal pain and bloating (27.5%). Duodenal GISTs were mostly located in the second duodenal portion (52.1%), followed by the first portion (19.0%) (Table 1).
Table 1.
Clinicopathological characteristics of the patients

Comparison of EUS diagnostic values against CT and MRI
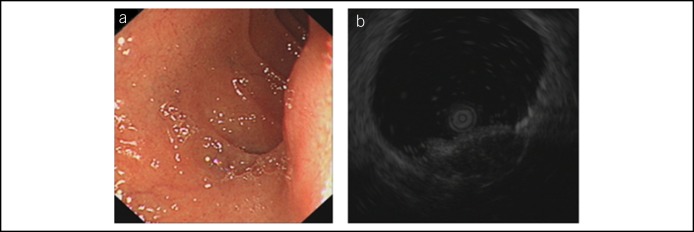
Figure 1 shows the different auxiliary inspection images from the same patient with a huge duodenal GIST. Duodenal GISTs were generally large, well-defined, heterogeneously enhanced, and hypervascular masses with prominent mixed growth pattern on the CT images (Figure 1a). On MRI, the solid components of the tumors appeared as low signal on the T1-weighted images, high signal on the T2-weighted images, and enhanced following gadolinium (Figure 1b). The diagnostic sensitivities in CT and MRI were 66.7% and 61.9%, respectively. Furthermore, the positive predictive values in CT and MRI were 68.8% and 71.1%, respectively. CT/MRI had a high misdiagnosis rate for GISTs and malignant and benign tumors and ectopic pancreas.
Figure 1.
Different auxiliary inspection images from the same patient with a huge duodenal GIST. (a) CT image of duodenal GIST. (b) MRI image of duodenal GIST. (c) Endoscopic image of duodenal GIST. (d), EUS image of duodenal GIST. CT, computed tomography; EUS, endoscopic ultrasonography; GIST, gastrointestinal stromal tumor; MRI, magnetic resonance imaging.
Most duodenal GISTs presented as hypoechoic masses arose from the muscularis propria in EUS (Figure 1c,d). Using contrast-enhanced EUS (CE-EUS) and EUS elastography could further improve the diagnostic accuracy (Figure 2a–c). The EUS diagnostic sensitivity was 82.0%, and the positive predictive value was 90.9%. EUS had a lower misdiagnosis rate for GISTs and malignant and benign tumors and ectopic pancreas than CT/MRI. Moreover, the misdiagnosis by EUS was most common between GIST and heterotopic pancreas (Figure 3).
Figure 2.
(a) Endoscopic image of duodenal GIST. (b) CE-EUS image of duodenal GIST. (c) EUS-EG image of duodenal GIST. (d–f) Operational processes of the EUS-FNA for duodenal GIST. CE-EUS, contrast-enhanced endoscopic ultrasound; EUS-EG, endoscopic ultrasound-guided elastography; EUS-FNA, endoscopic ultrasound-guided fine-needle aspiration; GIST, gastrointestinal stromal tumor.
Figure 3.
Duodenal heterotopic pancreas misdiagnosed as GIST by EUS. EUS, endoscopic ultrasonography; GIST, gastrointestinal stromal tumor.
In general, the EUS diagnostic sensitivity was significantly higher than that of CT and MRI (82.0% vs 66.7% vs 61.9%, P = 0.047). In addition, the positive predictive value of EUS was significantly higher than that of CT and MRI (90.9% vs 68.8% vs 71.1%, P = 0.005) (Table 2).
Table 2.
Comparison of diagnostic values among EUS, CT, and MRI
EUS-FNA reliability of duodenal GISTs
In this study, 15 patients were treated with the EUS-FNA. Except for the 4 false-negative results, 11 patients received a histopathological diagnosis of GISTs after the EUS-FNA. The EUS-FNA sensitivity of duodenal GISTs was 73.3%. The operational processes are shown in Figure 2d–f.
At the same time, 51 patients underwent routine endoscopic biopsy. A total of 17 patients received a histopathological diagnosis of GISTs, whereas the remaining 34 patients received false-negative results. The routine endoscopic biopsy sensitivity of duodenal GISTs was 33.3%, which was significantly lower than the EUS-FNA (P = 0.006). Furthermore, most lesions with a positive histopathological diagnosis could be observed with erosion or deep ulcer on the surface (16 of 17, 94.1%).
Endoscopic treatment and surgery comparison
Three patients attempted the endoscopic treatment before surgery. All the endoscopic treatments were suspended because of the perforation risk in one patient and endoscopic loop ligation failure in 2 patients. Furthermore, 128 patients directly accepted surgery. All surgeries were successful. The tumor size in patients with PD was higher than that in patients with LR (6.4 vs 3.7 cm, P = 0.001). The postoperative complications were also more common in patients with PD (32.5% vs 7.7%, P < 0.001).
DISCUSSION
To date, there are many preliminary studies aimed at duodenal GISTs. They concluded the clinical features and radiological characteristics of duodenal GISTs. Some studies also compared the long-term outcomes of different surgical approaches, including LR and PD. However, most are case reports or small sample studies, and the results are controversial among different studies. In our study, the clinicopathological features and treatment data of 142 patients with duodenal GISTs from a single institution were retrospectively analyzed. Aside from summarizing the clinical features, we compared the diagnostic values of various diagnostic modalities.
We found that the disease incidence was slightly higher in men, with a mean age of 55.9 years. Although duodenal GISTs were mainly located in the second duodenal portion, symptoms caused by the periampullary structure obstruction, such as obstructive jaundice and cholangitis, were rare. Gastrointestinal bleeding, including melena, hematemesis, and abdominal discomfort, was the common symptom. These clinical features were consistent with the previous studies (24–26).
We also summarized the CT and MRI features of duodenal GISTs. Consistent with other studies (11,12), GISTs presented as heterogeneously enhanced round or oval hypervascular masses after an enhancement scan in our research. However, these imaging manifestations were similar to the features of duodenal adenocarcinoma, neuroendocrine tumors, and heterotopic pancreas (27,28). Thus, duodenal GISTs were easily misdiagnosed by CT and MRI. Compared with CT/MRI, EUS can additionally provide information on the mucosal surface and lesion location of the intestinal layer. In addition, the EUS sensitivity and sharpness are also better than CT/MRI, especially in small lesions. Duodenal GISTs often presented as hypoechoic masses arose from the muscularis propria in EUS (29), whereas duodenal adenocarcinoma and neuroendocrine tumor were usually derived from the mucus and submucosal layers, respectively. As a result, except for few heterotopic pancreas and duodenal neuroendocrine tumors derived from the muscularis propria, which might lead to a misdiagnosis, most GISTs could be accurately diagnosed by EUS. Therefore, the EUS diagnostic value was significantly higher than that of CT and MRI in our study.
Several studies had evaluated the EUS-FNA sensitivity for the GIST diagnosis (13,14). However, most previous EUS-FNA evaluations mainly centered on the stomach. The sample size of duodenal GISTs was very small, which was less than 5 in most studies. In our study, 15 patients were treated with the EUS-FNA, and the sensitivity was 73.3%. It confirmed that the EUS-FNA could provide relatively reliable results for duodenal GISTs. Except for the EUS-FNA, routine endoscopic biopsy can be used when patients accept EUS. Although the sensitivity could only achieve 33.3%, it can be regarded as a kind of effective measure to obtain an exact pathological diagnosis before surgery, especially for the lesions with deep ulcer or exposed tumor tissue.
The narrow duodenal cavity limits endoscopy's operating space. The intestinal wall is thin with abundant blood supply, and duodenal GISTs always arise from the muscularis propria. As a result, the perforation and bleeding risk are a bit high during the process of endoscopic treatment. Hence, surgery is a better choice for duodenal GISTs. LR or PD can be chosen according to the size and location of the tumor. However, the postoperative complications were more common in patients with PD with a larger size in our study. The long-term outcomes of different surgical procedures are contradictory in the present studies. It might be due to the extreme variation of the baseline data and small sample size. In some studies, tumor biology features were similar between patients with LR and those with PD, and the disease-free survival was also close between different patients. Nevertheless, in other studies, disease-free survival of patients with PD was significantly lower, which might be due to the higher tumor size and/or mitotic index in patients with PD.
In conclusion, the diagnostic value of EUS is significantly higher than that of CT and MRI for duodenal GISTs. The EUS-FNA can provide a histological diagnosis of duodenal GISTs in most cases.
CONFLICTS OF INTEREST
Guarantor of the article: Guoqiang Xu, MD, and Hongtan Chen, MD.
Specific author contributions: H.D., L.N., S.L., and X.L. designed the research; H.C. and G.S. treated patients and collected material and clinical data from patients; F.H. and F.Z. analyzed data, and H.D. and G.X. contributed to drafting the manuscript and revising.
Financial support: This work was supported by the Zhejiang Medical Innovation Subject Program (grant number: 15-CX13) and Zhejiang Province University Scientific Research Project (grant number: Y201430629) in China.
Potential competing interests: None to report.
Study Highlights.
WHAT IS KNOWN
✓ EUS, CT and MRI have been widely used in the diagnosis of duodenal GISTs.
✓ With the rapid EUS development, the EUS-FNA is applied to obtain a histological diagnosis before surgery.
WHAT IS NEW HERE
✓ After comparing the diagnostic value of different diagnostic modalities, it is found that the diagnostic value of EUS is significantly higher than CT and MRI for duodenal GISTs.
✓ Compared to routine endoscopic biopsy with low positive rate, the EUS-FNA can provide a histologic diagnosis for duodenal GISTs in a majority of cases.
TRANSLATIONAL IMPACT
✓ The research contributes to formulation of a reasonable treatment plan.
References
- 1.Joensuu H, Fletcher C, Dimitrijevic S, et al. Management of malignant gastrointestinal stromal tumours. Lancet Oncol 2002;3(11):655–64. [DOI] [PubMed] [Google Scholar]
- 2.Miettinen M, Lasota J. Gastrointestinal stromal tumors: Pathology and prognosis at different sites. Semin Diagn Pathol 2006;23(2):70–83. [DOI] [PubMed] [Google Scholar]
- 3.Nilsson B, Bümming P, Meis-Kindblom JM, et al. Gastrointestinal stromal tumors: The incidence, prevalence, clinical course, and prognostication in the preimatinib mesylate era—A population-based study in western Sweden. Cancer 2005;103(4):821–9. [DOI] [PubMed] [Google Scholar]
- 4.von Mehren M, Randall RL, Benjamin RS, et al. Gastrointestinal stromal tumors, version 2.2014. J Natl Compr Canc Netw 2014;12(6):853–62. [DOI] [PubMed] [Google Scholar]
- 5.ESMO/European Sarcoma Network Working Group. Gastrointestinal stromal tumours: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2014;25(Suppl 3):iii21–6. [DOI] [PubMed] [Google Scholar]
- 6.Shen L. Interpretation on 2013 updated Chinese consensus on the diagnosis and treatment of gastrointestinal stromal tumors. [Chinese.] Zhonghua Wei Chang Wai Ke Za Zhi 2014;17(4):305–8. [PubMed] [Google Scholar]
- 7.Nishida T, Hirota S, Yanagisawa A, et al. Clinical practice guideline for gastrointestinal stromal tumor (GIST) in Japan. Int J Clin Oncol 2008;13:416–30. [DOI] [PubMed] [Google Scholar]
- 8.Kang YK, Kang HJ, Kim KM, et al. Clinical practice guideline for accurate diagnosis and effective treatment of gastrointestinal stromal tumor in Korea. Cancer Res Treat 2012;44:85–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hwang JH, Kimmey MB. The incidental upper gastrointestinal subepithelial mass. Gastroenterology 2004;126(1):301–7. [DOI] [PubMed] [Google Scholar]
- 10.Aydin A, Tekin F, Günşar F, et al. Value of endoscopic ultrasonography for upper gastrointestinal stromal tumors: A single center experience. Turk J Gastroenterol 2004;15(4):233–7. [PubMed] [Google Scholar]
- 11.Cai PQ, Lv XF, Tian L, et al. CT characterization of duodenal gastrointestinal stromal tumors. AJR Am J Roentgenol 2015;204(5):988–93. [DOI] [PubMed] [Google Scholar]
- 12.O'Sullivan PJ, Harris AC, Ho SG, et al. The imaging features of gastrointestinal stromal tumors. Eur J Radiol 2006;60:431–8. [DOI] [PubMed] [Google Scholar]
- 13.Sepe PS, Moparty B, Pitman MB, et al. EUS-guided FNA for the diagnosis of GI stromal cell tumors: Sensitivity and cytologic yield. Gastrointest Endosc 2009;70(2):254–61. [DOI] [PubMed] [Google Scholar]
- 14.Sekine M, Imaoka H, Mizuno N, et al. Clinical course of gastrointestinal stromal tumor diagnosed by endoscopic ultrasound-guided fine-needle aspiration. Dig Endosc 2015;27(1):44–52. [DOI] [PubMed] [Google Scholar]
- 15.Buchs NC, Bucher P, Gervaz P, et al. Segmental duodenectomy for gastrointestinal stromal tumor of the duodenum. World J Gastroenterol 2010;16(22):2788–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee SY, Goh BK, Sadot E, et al. Surgical strategy and outcomes in duodenal gastrointestinal stromal tumor. Ann Surg Oncol 2017;24(1):202–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sugase T, Takahashi T, Nakajima K, et al. Clinicopathological characteristics, surgery and survival outcomes of patients with duodenal gastrointestinal stromal tumors. Digestion 2016;94(1):30–6. [DOI] [PubMed] [Google Scholar]
- 18.Goh BK, Chow PK, Kesavan S, et al. Outcome after surgical treatment of suspected gastrointestinal stromal tumors involving the duodenum: Is limited resection appropriate? J Surg Oncol 2008;97(5):388–91. [DOI] [PubMed] [Google Scholar]
- 19.Beham A, Schaefer IM, Cameron S, et al. Duodenal GIST: A single center experience. Int J Colorectal Dis 2013;28(4):581–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen P, Song T, Wang X, et al. Surgery for duodenal gastrointestinal stromal tumors: A single-center experience. Dig Dis Sci 2017;62(11):3167–76. [DOI] [PubMed] [Google Scholar]
- 21.Crown A, Biehl TR, Rocha FG. Local resection for duodenal gastrointestinal stromal tumors. Am J Surg 2016;211(5):867–70. [DOI] [PubMed] [Google Scholar]
- 22.Bourgouin S, Hornez E, Guiramand J, et al. Duodenal gastrointestinal stromal tumors (GISTs): Arguments for conservative surgery. J Gastrointest Surg 2013;17(3):482–7. [DOI] [PubMed] [Google Scholar]
- 23.Joensuu H. Risk stratification of patients diagnosed with gastrointestinal stromal tumor. Hum Pathol 2008;39:1411–9. [DOI] [PubMed] [Google Scholar]
- 24.Ma GL, Murphy JD, Martinez ME, et al. Epidemiology of gastrointestinal stromal tumors in the era of histology codes: Results of a population-based study. Cancer Epidemiol Biomarkers Prev 2015;24:298–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sandvik OM, Soreide K, Kvaloy JT, et al. Epidemiology of gastrointestinal stromal tumors: Single-institution experience and clinical presentation over three decades. Cancer Epidemiol 2011;35:515–20. [DOI] [PubMed] [Google Scholar]
- 26.Miettinen M, Kopczynski J, Makhlouf HR, et al. Gastrointestinal stromal tumors, intramural leiomyomas, and leiomyosarcomasin the duodenum: A clinicopathologic, immunohistochemical, and molecular genetic study of 167 cases. Am J Surg Pathol 2003;27:625–41. [DOI] [PubMed] [Google Scholar]
- 27.Tsai SD, Kawamoto S, Wolfgang CL, et al. Duodenal neuroendocrine tumors: Retrospective evaluation of CT imaging features and pattern of metastatic disease on dual-phase MDCT with pathologic correlation. Abdom Imaging 2015;40(5):1121–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim JY, Lee JM, Kim KW, et al. Ectopic pancreas: CT findings with emphasis on differentiation from small gastrointestinal stromal tumor and leiomyoma. Radiology 2009;252(1):92–100. [DOI] [PubMed] [Google Scholar]
- 29.Hwang JH, Kimmey MB. The incidental upper gastrointestinal subepithelial mass. Gastroenterology 2004;126(1):301–7. [DOI] [PubMed] [Google Scholar]