Abstract
Background
Substantial evidence exists to show the positive effects of radialextracorporeal shock wave therapy (ESWT) on bone formation. However, it is unknown whether rESWT can act locally at the growth plate level to stimulate linear bone growth. One way to achieve this is to stimulate chondrogenesis in the growth plate without depending on circulating systemic growth factors. We wished to see whether rESWT would stimulate metatarsal rat growth plates in the absence of vascularity and associated systemic growth factors.
Questions/purposes
To study the direct effects of rESWT on growth plate chondrogenesis, we asked: (1) Does rESWT stimulate longitudinal bone growth of ex vivo cultured bones? (2) Does rESWT cause any morphological changes in the growth plate? (3) Does rESWT locally activate proteins specific to growth plate chondrogenesis?
Methods
Metatarsal bones from rat fetuses were untreated (controls: n = 15) or exposed to a single application of rESWT at a low dose (500 impulses, 5 Hz, 90 mJ; n = 15), mid-dose (500 impulses, 5 Hz, 120 mJ; n = 14) or high dose (500 impulses, 10 Hz, 180 mJ; n = 34) and cultured for 14 days. Bone lengths were measured on Days 0, 4, 7, and 14. After 14 days of culturing, growth plate morphology was assessed with a histomorphometric analysis in which hypertrophic cell size (> 7 µm) and hypertrophic zone height were measured (n = 6 bones each). Immunostaining for specific regulatory proteins involved in chondrogenesis and corresponding staining were quantitated digitally by a single observer using the automated threshold method in ImageJ software (n = 6 bones per group). A p value < 0.05 indicated a significant difference.
Results
The bone length in the high-dose rESWT group was increased compared with that in untreated controls (4.46 mm ± 0.75 mm; 95% confidence interval, 3.28-3.71 and control: 3.50 mm ± 0.38 mm; 95% CI, 4.19-4.72; p = 0.01). Mechanistic studies of the growth plate’s cartilage revealed that high-dose rESWT increased the number of proliferative chondrocytes compared with untreated control bones (1363 ± 393 immunopositive cells per bone and 500 ± 413 immunopositive cells per bone, respectively; p = 0.04) and increased the diameter of hypertrophic chondrocytes (18 ± 3 µm and 13 ± 3 µm, respectively; p < 0.001). This was accompanied by activation of insulin-like growth factor-1 (1015 ± 322 immunopositive cells per bone and 270 ± 121 immunopositive cells per bone, respectively; p = 0.043) and nuclear factor-kappa beta signaling (1029 ± 262 immunopositive cells per bone and 350 ± 60 immunopositive cells per bone, respectively; p = 0.01) and increased levels of the anti-apoptotic proteins B-cell lymphoma 2 (718 ± 86 immunopositive cells per bone and 35 ± 11 immunopositive cells per bone, respectively; p < 0.001) and B-cell lymphoma-extra-large (107 ± 7 immunopositive cells per bone and 34 ± 6 immunopositive cells per bone, respectively; p < 0.001).
Conclusion
In a model of cultured fetal rat metatarsals, rESWT increased longitudinal bone growth by locally inducing chondrogenesis. To verify whether rESWT can also stimulate bone growth in the presence of systemic circulatory factors, further studies are needed.
Clinical Relevance
This preclinical proof-of-concept study shows that high-dose rESWT can stimulate longitudinal bone growth and growth plate chondrogenesis in cultured fetal rat metatarsal bones. A confirmatory in vivo study in skeletally immature animals must be performed before any clinical studies.
Introduction
Longitudinal bone growth occurs in the epiphyseal growth plate, where the chondrocytes proliferate, hypertrophy, and die, leaving a cartilaginous matrix that is vascularized and replaced by osteoids deposited by osteoblasts. This process, also known as endochondral ossification, may be regulated by local and hormonal factors [32]. Indian hedgehog and parathyroid hormone-related proteins are well-known to intrinsically regulate chondrocyte proliferation and hypertrophy by forming a negative feedback loop in the growth plate [19]. Other recognized modulators of bone growth are growth hormone and insulin-like growth factor-1, both known to stimulate chondrocyte proliferation. The latter also stimulates cell proliferation and differentiation [30]. Growth plate chondrogenesis is also regulated by apoptotic activity, which directs the fate of terminal hypertrophic chondrocytes [7].
The human growth plate is easily affected by trauma, infection, or inflammation, resulting in stimulation, deceleration, or cessation of growth that further leads to deformities and limb-length inequalities [1]. Clinical management often includes surgical interventions such as epiphyseodesis [4] and limb lengthening [15], which are known to have potential side effects including incomplete growth arrest or soft-tissue complications [29]. A noninvasive technique is therefore desirable to effectively and selectively modulate bone growth with minimal side effects.
Extracorporeal shock wave therapy (ESWT), a noninvasive modality, can generate focused shock waves or defocused shock waves based on their coupling sources. Focused extracorporeal shock waves are generated by electrohydraulic, electromagnetic, and piezoelectric devices, while defocused shock waves generated by pneumatic generators produce radial extracorporeal shock waves. The physical properties of radial shock waves differ from those of focused shock waves in terms of linear pressure, and radial shock waves have relatively low energy and velocity of propagation, as well as a short rise time. In contrast to shock waves generated by focal devices, the energy produced by radial shock waves is the highest at the skin interface, deviating and fading as it penetrates deeper [18]. Focused ESWT has a well-defined penetration depth and is used to treat delayed union or nonunion of bone fractures in humans [33], while very few studies have attempted to study the effect of rESWT on the induction of new bone formation [13], which is primarily mediated through upregulation of angiogenic factors, cell proliferation markers, and bone morphogenic proteins [6, 37]. Whether rESWT has the capacity to modulate longitudinal bone growth if applied locally without systemic factors has not yet been evaluated. We hypothesized that locally applied rESWT may trigger cellular changes in the cartilage of the growth plate, thereby modulating linear bone growth, depending on the energy and frequency used. To elucidate this, we applied different doses of rESWT to ex vivo cultured fetal rat metatarsal bones, which allowed us to study the direct effect of rESWT on chondrocyte proliferation and hypertrophy. We asked the following questions: (1) Does rESWT stimulate longitudinal bone growth of ex vivo cultured bones? (2) Does rESWT cause any morphological changes in the growth plate? (3) Does rESWT locally activate proteins specific to growth plate chondrogenesis?
Materials and Methods
The study was approved by the institutional review board (IRB Min. no. 8513) and institutional animal ethics committee (IAEC no. 25/2013) of Christian Medical College, Vellore, India. The methods were performed in accordance with experimental animal care guidelines.
Metatarsal Bone Harvest, Culture, and Shock Wave Treatment
The metatarsal bone culture model allows for an investigation of local growth plate chondrogenesis by separating circulatory and systemic growth factors. The length of the bones can be monitored in real time, allowing for an exploration of chondrocyte proliferation and hypertrophy with a histomorphometric analysis [22].
All reagents were purchased from Sigma Aldrich (St. Louis, MO, USA), unless otherwise specified.
Experimental Overview
Pregnant Sprague-Dawley rats were euthanized, and laparotomy was performed to surgically remove fetuses from the uterus on Day 20 of gestation. The hind paws of the fetuses were collected in gentamycin-supplemented Dulbecco’s modified medium nutrient mixture/F12 on ice. The metatarsals were dissected per a published protocol [8]. The bones were divided into four experimental groups and cultured in 24-well plates. On the day after harvesting, bones in the treatment groups were exposed to rESWT once; untreated bones served as controls (n = 13). Briefly, multiple bones were put in sterile 1.5-ml Eppendorf tubes filled with a complete medium containing Dulbecco’s modified medium nutrient mixture/F12 supplemented with 50 µg/mL of ascorbic acid, 1 mM of β-glycerophosphate, 0.2% bovine serum albumin, and 20 µg/mL of gentamycin (Nitin Lifesciences Ltd., Himachal Pradesh, India). Radial shock waves (Radial Spec., Medi Spec., Gaithersburg, MD, USA) were applied to the bones (Fig. 1A). Each bone in Groups 1, 2, and 3 received 500 impulses at 5 Hz/90 mJ (n = 15), 5 Hz/120 mJ (n = 15), and 10 Hz/180 mJ (n = 34), respectively. The doses in the three groups represent doses that are used clinically for other orthopaedic indications, while a dose higher than the clinical dose was chosen to see whether it positively or negatively affected growth. After treatment, bones were cultured individually in a 24-well plate for up to 14 days (Fig. 1B).
Fig. 1.
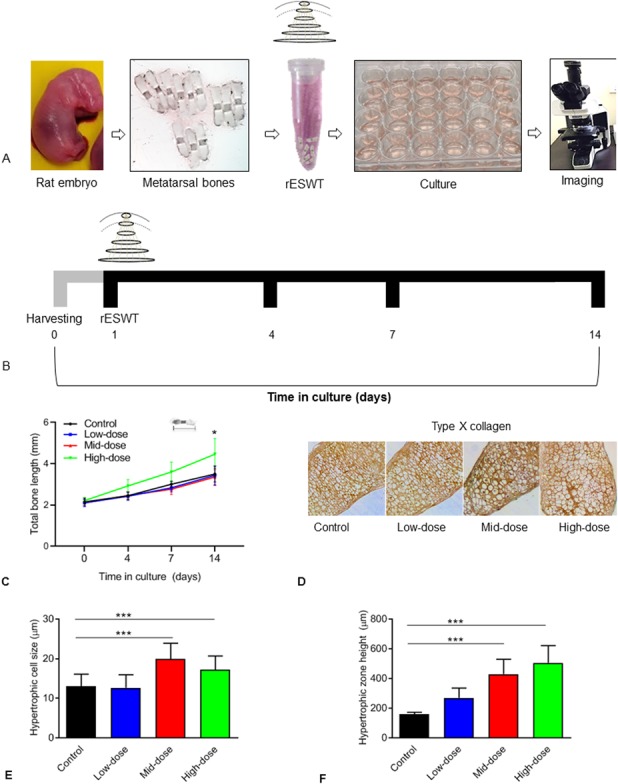
(A) This shows an overview of the experiment, in which rat metatarsals were harvested from embryos on Day 20 of gestation and subjected to different doses of rESWT. Bones were individually cultured and monitored for bone length. (B) This shows the culturing protocol of rat metatarsal bones treated with or without rESWT (single administration on Day 1) and monitored for bone length up to Day 14. (C) This figure shows the total bone length (mm) on different days of culturing in bones exposed to low-dose, mid-dose, or high-dose rESWT. An overall effect on bone length was observed on Day 14 in high-dose rESWT-treated rat bones (green line) compared with control bones (black line). Five-micrometer-thick sections cut along the longitudinal axis (proximal to distal) of the metatarsal bones were used for a histomorphometric analysis. (D) This figure shows Type X collagen immunostaining (n = 6), and (E) shows the mean diameter of hypertrophic chondrocytes (n = 6; control: 13 µm ± 3.06 µm, high-dose: 18 µm ± 3.46 µm; p < 0.001 versus control). (F) This figure shows the mean height of the hypertrophic zone in increased high-dose rESWT-treated bones compared with control bones (n = 6; control: 159 µm ± 8 µm; high-dose: 505 µm ± 59 µm; p < 0.001 versus control).
Bone Length Measurement
Digital images of cultured bones were captured on Days 0, 4, 7, and 14 using an inverted microscope (Leica Microsystems Inc., Wetzlar, Germany) connected to a digital camera (HD M170) and a computer. One of the investigators (SR), who was blinded to the nature of the group, measured the bone length using an inbuilt measurement tool in the Leica software. The intra-assay coefficient of variation was 16.7%. The total bone length at different time points was expressed in millimeters. Bones that were bowed during later stages of growth were measured in two parts and the measurements were added together.
Quantitative Histology
Five-mm sections cut along the longitudinal axis (proximal to distal) of the metatarsal bones were stained with Alcian blue (Cyagen Biosciences, Santa Clara, CA, USA). One of the investigators (SR), who was blinded to the nature of the group, performed all histologic measurements using an inverted microscope (Leica Microsystems Inc., Wetzlar, Germany). Hypertrophic cells were defined by a height along the longitudinal axis greater than 7 µm. Twenty-five hypertrophic chondrocytes from the epiphyseal and metaphyseal sides were measured. The intra-assay coefficient of variation was 20.1%. The height of the hypertrophic zone was calculated as the average of five measurements per bone. The intra-assay coefficient of variation was 23.2%. For cell morphometric analysis and immunohistochemistry, six bones per group were analyzed. Only untreated (control) and high-dose-treated bones (Group 3) were selected for further immunostaining, based on growth data at the end of the study period.
Terminal Deoxynucleotidyl Transferase-mediated Deoxyuridine triphosphate- Nick and Labelling
Apoptotic cells were detected using terminal deoxynucleotidyl transferase-mediated deoxy-uridine triphosphate nick end-labelling in situ, using a terminal deoxynucleotidyl transferase FragEL DNA fragmentation kit (Merck Millipore, Burlington, MA, USA). The experiment was performed according to the manufacturer’s protocol. Briefly, sections were incubated in 5 μg/ml of proteinase K for 10 minutes; DNA incorporation of biotin-labelling nucleotides was detected with a streptavidin-horseradish peroxidase conjugate. As a negative control for cell labelling, distilled water was used instead of terminal deoxynucleotidyl transferase. The number of terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick end-labelling-positive cells was calculated using ImageJ software (National Institutes of Mental Health, Bethesda, MD, USA) using a digital automated threshold. For each treatment group, five bones were analyzed.
Immunohistochemistry
Rat metatarsals were fixed in 4% paraformaldehyde and embedded in paraffin, and 5 μm-thick sections were prepared. Immunostaining was performed as described previously. The primary antibodies (1:50 dilution) were as follows: proliferating cell nuclear antigen rabbit polyclonal (Abcam, Cambridge, MA, USA), collagen-X rabbit polyclonal (Abcam, Cambridge, MA, USA), parathyroid hormone-related protein rabbit polyclonal (Merck Millipore, Burlington, MA, USA), B-cell lymphoma-2 rabbit polyclonal (Santa Cruz Biotechnology, Dallas, TX, USA), B-cell lymphoma-extra-large rabbit polyclonal (Santa Cruz Biotechnology, Dallas, TX, USA), Indian hedgehog mouse monoclonal (Santa Cruz Biotechnology, Dallas, TX, USA), Glioma-associated oncogene-1 rabbit polyclonal (Abcam, Cambridge, MA, USA), nuclear factor (NF)-ĸB rabbit polyclonal (Santa Cruz Biotechnology, Dallas, TX, USA), insulin-like growth factor-1 mouse monoclonal (Santa Cruz Biotechnology, Dallas, TX, USA), and FLK1 mouse monoclonal (Santa Cruz Biotechnology, Dallas, TX, USA). A secondary polyclonal anti-mouse or anti-rabbit biotinylated antibody (DakoCytomation, Glostrup, Denmark) was used at a dilution of 1:500. Six bones per group were analyzed. To quantify immunostaining, ImageJ software (National Institutes of Mental Health, Bethesda, MD, USA) was used, and the number of immunopositive cells per bone was calculated digitally by adjusting the automatic threshold. The output remained the same, regardless of the observer.
Statistical Methods
Statistical analyses were performed using GraphPad Prism 8.01 (GraphPad Software, Inc., La Jolla, CA, USA). Quantitative data are expressed as the mean ± standard deviation with 95% confidence intervals. The results were checked for normality with the Shapiro-Wilk test. Normality was observed; thus, the results were compared among all groups using repeated-measures one-way analysis of variance, followed by Holm-Sidak’s multiple comparison test. A two-tailed t-test was used to compare two groups. Probability values < 0.05 indicated a significant difference. All experiments were repeated three times.
Results
First, we asked whether rESWT stimulates longitudinal bone growth of ex vivo cultured bones. When measured during a 14-day-period of culturing after rESWT administration, bones treated with high-dose rESWT were longer than those of untreated controls (4.46 mm ± 0.75 mm; 95% CI, 3.28-3.71 and control: 3.50 mm ± 0.38 mm; 95% CI, 4.19-4.72; p = 0.01). The length of bones exposed to low-dose and mid-dose rESWT did not differ from that of untreated controls (3.43 mm ± 0.44 mm, 2.09 mm ± 0.22 mm, and 1.90 mm ± 0.12 mm, respectively) (Fig. 1C).
Second, we asked whether rESWT causes any morphological changes in the growth plate. To study this, immunostaining for type X collagen was performed, which showed no change in any of the rESWT-treated groups compared with untreated controls (Fig. 1D). The mistomorphometric analysis revealed the size of hypertrophic chondrocytes was larger in the mid-dose and high-dose rESWT-treated groups than in untreated controls (20 µm ± 4 µm, 18 µm ± 3 µm, and 13 µm ± 3 µm, respectively; p < 0.001 versus control for both the mid-dose and high-dose groups) while it was unaffected in the low-dose group (13 µm ± 3 µm) (Fig. 1E). The mean total height of the hypertrophic zone was dose-dependently increased by rESWT in the low-dose, mid-dose, and high-dose groups compared with untreated controls (269 µm ± 65 µm, 429 µm ± 100 µm, 505 µm ± 117 µm, and 159 µm ± 13 µm, respectively; p < 0.001 for mid-dose and high-dose rESWT versus control) (Fig. 1F).
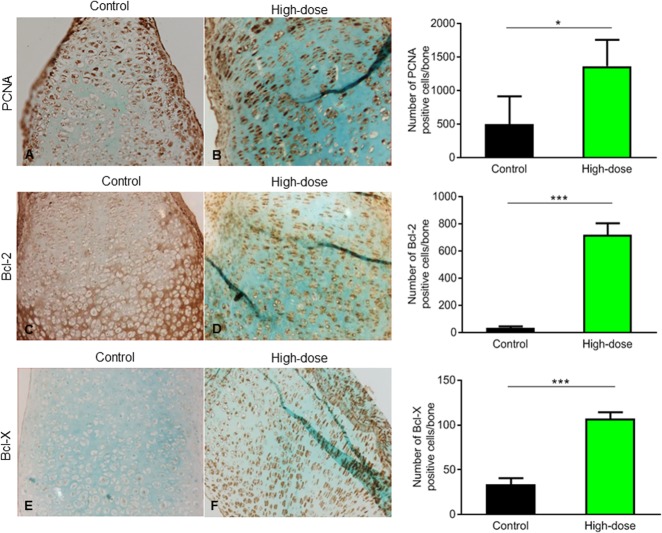
Third, we asked whether rESWT locally activates proteins specific to growth plate chondrogenesis. To explore this, we studied biomarkers of chondrocyte proliferation, apoptosis, and differentiation. As determined by proliferating cell nuclear antigen staining, more proliferative cells were found in the growth plate of bones exposed to high-dose rESWT than in that of controls (1363 ± 393 cells and 500 ± 412 cells; p = 0.04) (Fig. 2A, B). For low-dose and mid-dose rESWT, no effect on cell proliferation was observed. As determined by the terminal deoxynucleotidyl transferase-mediated deoxy-uridine triphosphate nick end-labelling assay, the level of apoptosis was unaffected by rESWT (see Fig. 1A, Supplemental Digital Content 1, http://links.lww.com/CORR/A267). Furthermore, rESWT did not affect caspase-3 and murine double-minute 2 staining, further supporting that rESWT did not induce chondrocyte apoptosis (see Fig. 1B and C, Supplemental Digital Content 1, http://links.lww.com/CORR/A267). In contrast, staining for the anti-apoptotic proteins B-cell lymphoma 2 (718 ± 86 immunopositive cells per bone and 35 ± 11 immunopositive cells per bone, respectively; p < 0.001) (Fig. 2C, D) and B-cell lymphoma-extra-large (107 ± 7 cells and 34 ± 6 cells, respectively; p < 0.001) (Fig. 2E, F) showed more immunopositive cells in bones exposed to high-dose rESWT than in controls, which may explain why they were protected from apoptosis when exposed to high-dose rESWT.
Fig. 2.
(A, B) Proliferating cell nuclear antigen immunostaining showed increased expression in bones treated with high-dose rESWT compared with controls (high dose: 1363 ± 393.1 cells and control: 500 ± 412.6 cells; p = 0.04) counterstained with Alcian blue (magnification x 20). Immunostaining was quantified using ImageJ software (n = 6). (C, D) There was an increased expression of B-cell lymphoma-2 in the proliferative zone in the high-dose rESWT bones compared with controls (high dose: 718 ± 86 immunopositive cells per bone and control: 35 ± 11 immunopositive cells per bone; p < 0.001), (E, F) as well as increased B-cell lymphoma-extra-large in the resting and proliferative zone (high dose: 107 ± 7 immunopositive cells per bone and control: 34 ± 7 immunopositive cells per bone; p < 0.001) of high-dose rESWT bones compared with control; immunostaining was quantified using ImageJ software (n = 6).
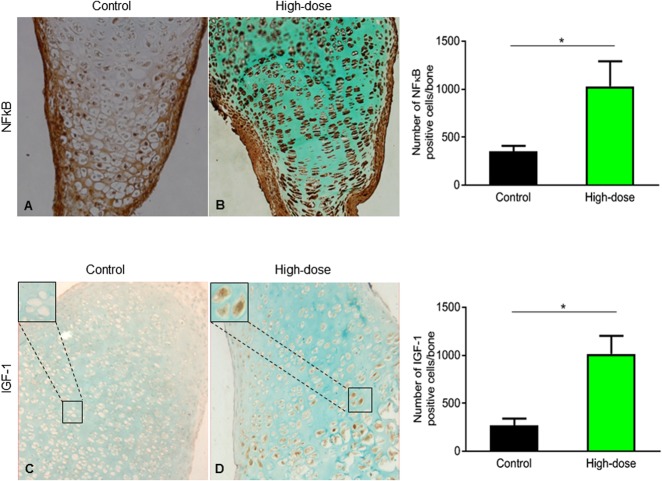
Finally, the effect of rESWT on the expression levels of important growth plate regulatory proteins was studied. NF-ĸB, a key regulator of chondrogenesis, was upregulated in high-dose rESWT-treated bones compared with controls (1029 ± 262 immunopositive cells per bone and 350 ± 60 immunopositive cells per bone, respectively; p = 0.01) (Fig. 3A, B). Insulin-like growth factor-1, a known stimulator of chondrocyte hypertrophy, was also upregulated in high-dose rESWT-treated bones compared with controls (1015 ± 322 immunopositive cells per bone and 270 ± 121 immunopositive cells per bone, respectively; p = 0.043) (Fig. 3C, D).
Fig. 3.
(A, B) These images show the expression of NF-ĸB throughout the growth plate in cultured metatarsals exposed to high-dose rESWT compared with controls (control: 350 ± 60 immunopositive cells per bone; high dose: 1029 ± 262 immunopositive cells per bone; p = 0.01), counterstained with Alcian blue (magnification x 20) and quantified using ImageJ software (n = 6). (C, D) These images show increased insulin-like growth factor 1 immunostaining in high-dose rESWT-treated rat bones (proliferating zone) compared with controls (control: 270 ± 121 immunopositive cells per bone; high dose: 1015 ± 322 immunopositive cells per bone; p = 0.043) counterstained with Alcian blue (magnification x 20). Images were quantified using ImageJ software (n = 6).
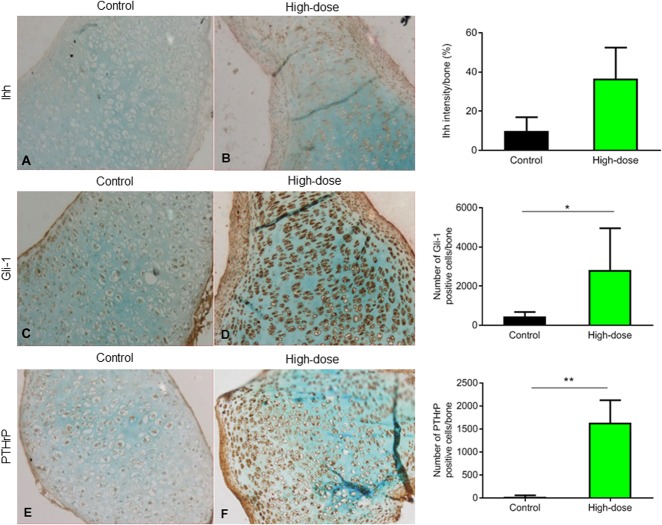
Next, the important regulatory parathyroid hormone-related protein/Indian hedgehog signaling cascade was studied. Although Indian hedgehog signaling in high-dose rESWT-treated bones did not differ from that of controls (Fig. 4A, B), Glioma-associated oncogene-1, a downstream transcription factor of Indian hedgehog signaling, was upregulated in high-dose rESWT-treated bones compared with controls (2812 ± 2139 immunopositive cells per bone and 442 ± 235 immunopositive cells per bone, respectively; p = 0.02) (Fig. 4C, D). Parathyroid hormone-related protein, mainly detected in the resting and proliferating zone, was also increased in the high-dose rESWT-treated bones (1632 ± 493 immunopositive cells per bone and 21 ± 35 immunopositive cells per bone, respectively; p = 0.0048) (Fig. 4E, F), which further supports an effect of rESWT on this important regulatory cascade.
Fig. 4.
Immunostaining (magnification x 20) showed (A, B) no change in the expression of Indian hedgehog signaling in calcified cartilage and increased expression of (C, D) Gli-1 (high dose: 2812 ± 2139 immunopositive cells per bone and control: 442 ± 235 immunopositive cells per bone, p = 0.016; n = 6) and (E, F) parathyroid hormone-related protein in the resting and proliferation zone of high-dose rESWT bones compared with controls (high dose: 1632 ± 493 immunopositive cells per bone and control: 21 ± 36 immunopositive cells per bone; p = 0.029; n = 6). Images were quantified using ImageJ software.
Discussion
Focal modulation of growth is highly desirable and can lead to innovative management of deformities and leg-length discrepancies in growing individuals [14]. ESWT is used clinically to treat non-union fractures [41]. Previous in vivo and in vitro studies have demonstrated that ESWT can increase osteogenesis by stimulating the mechanotransduction pathway [9]. However, the potential for ESWT to modulate bone growth and growth plate chondrogenesis remains unclear. To address this gap in knowledge, we used an ex vivo model of cultured fetal metatarsal bones that allowed us to closely monitor bone growth over time and perform a cellular and molecular analysis of key regulatory proteins involved in growth plate chondrogenesis. Our primary finding was that a single application of high-dose rESWT stimulated the growth of cultured fetal rat metatarsal bones. The assessment of the growth plate’s structure and growth factor expression by immunostaining helped us to understand the effect of rESWT on chondrocytes. Because we observed bone growth in rESWT-treated bones, we wanted to see how rESWT affects different zones of the growth plate when there are no growth factors in the culture. The effect was associated with increased chondrocyte proliferation and hypertrophy, accompanied by local activation of important regulatory proteins including insulin-like growth factor-1 and NF-ĸB. Furthermore, rESWT-treated bones were protected from undergoing apoptosis, which could be linked to upregulation of anti-apoptotic proteins. Altogether, our data suggest that rESWT may stimulate bone growth and chondrogenesis by acting locally at the growth plate level, modulating the expression of key regulatory proteins.
This study has several limitations. First, our results were obtained in an ex vivo organ culture of metatarsal bones and should be considered as a proof-of-concept and not be extrapolated clinically, mainly because the model lacks vascularity. However, in contrast to in vitro primary chondrocyte culturing, this is an excellent model to study bone growth. The pattern of growth observed in response to shock wave treatment may differ when human postnatal bones are used [3]. Further studies are needed to verify whether the observed growth stimulatory effect of rESWT can be reproduced in a disease model with disturbed growth plate function.
Next, the actual energy and frequency of rESWT delivered to the growth plate in this ex vivo model may be difficult to reproduce in humans, considering that shock waves reaching the cartilage are not easily quantifiable [16]. A high-energy dose of rESWT must undergo rigorous preclinical testing. Although we initially had multiple bones per group, some of the bones did not remain intact because of excess mechanical vibration after rESWT, limiting the power of the study. Our measurements, although documented by a single observer, had good repeatability. Another limitation of our study is that the culture medium used in the study contained no growth factors or serum [24], which may have influenced the effects of rESWT. Nevertheless, rESWT stimulated local longitudinal bone growth, showing that this effect can be achieved independent of systemic factors.
Our data provide the first evidence that rESWT can locally stimulate chondrocyte proliferation, hypertrophy, and bone growth in ex vivo cultured fetal rat metatarsal bones. One of the first reports showed bone shortening in a rat model after ESWT [42], while another study showed that the treatment did not affect the femur’s length in immature rabbits [36]. Ozturk et al. [28] have previously demonstrated an increase in the thickness of rabbit physeal cartilage with no change in the final bone length after ESWT, while other studies in rats [27] and rabbits [12, 21, 25] have shown no effect on growth plates. One study in rabbits reported stimulated femoral growth after piezoelectric ESWT by a minimum of 1% [31]. We do not know whether the observed effect depends on local or systemic growth factors. We attempted to address the effect of local rESWT (which differs in terms of energy level, frequency, and depth of penetration), using an ex vivo bone organ culturing system devoid of growth factors or serum, and found that bone growth was stimulated. Our study is different from previously published studies in two ways. First, we investigated the direct effects of shock waves on bone growth in the absence of systemic growth factors. Second, we used a radial shock wave machine that has never been used to investigate any local effects on longitudinal bone growth.
Our ex vivo data showed that rESWT induced morphological changes by increasing chondrocyte proliferation and cellular hypertrophy that together likely contributed to the observed increase in bone length and chondrogenesis. rESWT has shown to increase osteogenic differentiation and proliferation of human mesenchymal stem cells in vitro and in vivo by activating purinergic signaling and p38 mitogen-activated protein kinases via upregulation of adenosine triphosphate [35, 38]. This is also in agreement with a previous report in which high-energy ESWT induced chondrogenesis in a rat model [23]. Furthermore, our data are supported by reports showing that cellular hypertrophy is a major contributor to bone elongation by controlling the shape and size of chondrocytes in the growth plate [5, 17]. It remains to be elucidated whether the observed increase in cellular hypertrophy may interfere with longitudinal bone growth when rESWT is applied in a clinical setting. We also found that rESWT did not induce cell death, likely explained by the fact that the anti-apoptotic proteins were upregulated. In our study, we found increased chondrocyte proliferation; it is well-known that growth occurs when cell proliferation supersedes cell death [7].
Our mechanistic studies revealed that rESWT increased the expression of parathyroid hormone-related protein and B-cell lymphoma-2 in resting- and proliferative-zone chondrocytes. Parathyroid hormone-related protein is an important regulator to maintain the proliferative zone. Mutations in this receptor have been associated with Blomstrand chondrodysplasia [11], while the absence of B-cell lymphoma 2 is associated with short limbs and skeletal dysplasia in mutated mice [2]. This is in line with previous in vitro and in vivo studies in which upregulation of parathyroid hormone-related protein led to an increase in the expression of B-cell lymphoma-2, suggesting that the latter is a downstream target of the parathyroid hormone-related protein signaling pathway [2, 8]. We also found strong upregulation of NF-ĸB when cultured bones were exposed to high-dose rESWT. This information is particularly interesting because mutations impeding the activation of NF-ĸB have been associated with growth failure and growth hormone insensitivity in children [10]. Furthermore, in vitro studies using cultured rat metatarsal bones reported impaired chondrocyte proliferation and hypertrophy after silencing of NF-ĸB [40]. Although there are many therapeutic strategies to inhibit NF-ĸB [20], rESWT could be a novel activator of NF-ĸB, leading to the development of a new treatment regimen to stimulate the growth of specific physes in clinical conditions caused by suppressed activation of NF-ĸB. Activation of NF-ĸB has been reported to be associated with the increased expression of insulin-like growth factor-1, suggesting that NF-ĸB is a downstream target of insulin-like growth factor-1 in the progression of growth plate chondrogenesis [39]. We confirmed that activation of NF-ĸB was associated with an increased expression of insulin-like growth factor-1 in ex vivo high-dose rESWT-treated bones, further supporting earlier reports of a synergistic effect between NF-ĸB and insulin-like growth factor-1 in stimulating longitudinal bone growth [39, 40]. Furthermore, we found increased expression of parathyroid hormone-related protein and Glioma-associated oncogene-1 in bones exposed to rESWT. During the development of growth plate cartilage, pre-hypertrophic and hypertrophic chondrocytes express Indian hedgehog signaling, while Glioma-associated oncogene-1, a downstream target of Indian hedgehog signaling, is mainly expressed by proliferating chondrocytes, perichondral cells, and primary spongiosa [26]. Activation of Indian hedgehog signaling has been reported to increase cellular proliferation, differentiation, and bone growth [34].
In summary, we demonstrated that rESWT can stimulate chondrocyte proliferation, hypertrophy, and longitudinal bone growth in the growth plate when applied locally to cultured rat bones, even in the absence of mechanical forces and circulating growth factors normally present in vivo (Fig. 5). Our study paves the way for similar experiments to verify the effects of high-energy rESWT in an in vivo animal model.
Fig. 5.
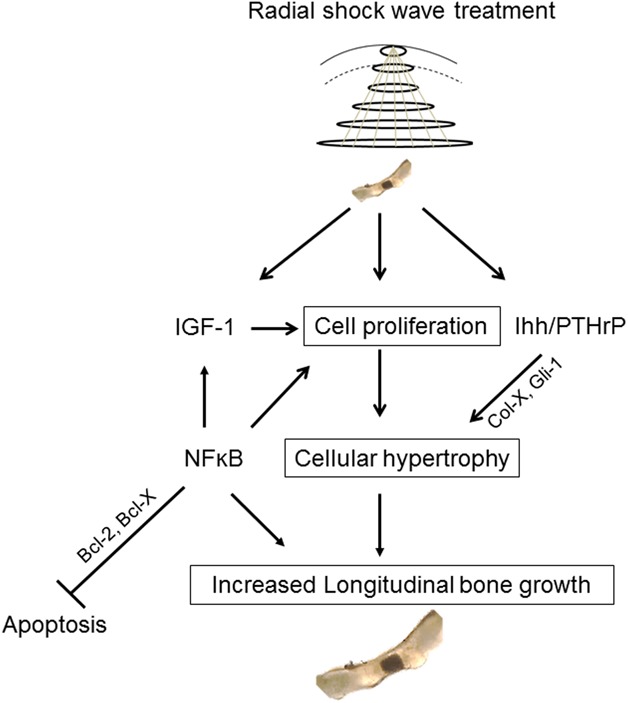
This figure shows the proposed mechanism of action of high-dose rESWT in stimulating growth plate chondrogenesis and local bone growth in cultured fetal rat metatarsal bones.
Acknowledgements
We thank Medispec, USA and Medix Medical Systems Pvt. Ltd., Bengaluru, for their technical assistance during the experiments. We thank Mr. Bijesh Yadav Kumar, Senior Demonstrator, Department of Biostatistics, Christian Medical College, Vellore, for his assistance in statistical analysis. SR personally thanks Dr. Noel Malcolm Walter, MD (Path), FRC Path, for teaching interpretation of growth plate histology and Dr. Sanjay K Chilbule, MS Orth, for having taught dissection of fetal rat metatarsal cultures initially.
Footnotes
Grants were received from the International Division, Department of Biotechnology, Government of India (BT/IN/Denmark/02/PDN/2011); an internal grant was received from Christian Medical College, Vellore, and the Centre for Stem Cell Research, the Swedish Research Council, and Karolinska Institutet, Sweden. SR was supported by the DBT, Government of India, and Sällskapet Barnavård, Sweden. LS was supported by the Swedish Research Council and Karolinska Institutet, Sweden. FZ was supported by the Swedish Childhood Cancer Research Foundation, Sweden.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research® editors and board members are on file with the publication and can be viewed on request.
Each author certifies that his or her institution approved the animal protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
This work was performed at Christian Medical College, Vellore, India and Karolinska Institutet, Solna, Sweden.
References
- 1.Accadbled F, Foster BK. Management of growth plate injuries. In: Benson M, Fixsen J, Macnicol M, Parsch K, eds. Children's Orthopaedics and Fractures . Springer: London; 2010:687-699. [Google Scholar]
- 2.Amling M, Neff L, Tanaka S, Inoue D, Kuida K, Weir E, Philbrick WM, Broadus AE, Baron R. Bcl-2 lies downstream of parathyroid hormone–related peptide in a signaling pathway that regulates chondrocyte maturation during skeletal development. J Cell Biol. 1997;136:205-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andrade AC, Chrysis D, Audi L, Nilsson O. Methods to study cartilage and bone Development. In: Camacho-Hübner C, Nilsson O, Sävendahl L, eds. Cartilage and Bone Development and Its Disorders. Karger Publishers: Basel; 2011:52-66. [DOI] [PubMed] [Google Scholar]
- 4.Benyi E, Berner M, Bjernekull I, Boman A, Chrysis D, Nilsson O, Waehre A, Wehtje H, Sävendahl L. Efficacy and safety of percutaneous epiphysiodesis operation around the knee to reduce adult height in extremely tall adolescent girls and boys. Int J Pediatr Endocrinol. 2010;2010:740629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Breur G, VanEnkevort B, Farnum C, Wilsman N. Linear relationship between the volume of hypertrophic chondrocytes and the rate of longitudinal bone growth in growth plates. J Orthop Res. 1991;9:348-359. [DOI] [PubMed] [Google Scholar]
- 6.Chen YJ, Wurtz T, Wang CJ, Kuo YR, Yang KD, Huang HC, Wang FS. Recruitment of mesenchymal stem cells and expression of TGF‐β1 and VEGF in the early stage of shock wave‐promoted bone regeneration of segmental defect in rats. J Orthop Res. 2004;22:526-534. [DOI] [PubMed] [Google Scholar]
- 7.Chrysis D, Nilsson O, Ritzen EM, Sävendahl L. Apoptosis is developmentally regulated in rat growth plate. Endocrine. 2002;18:271-278. [DOI] [PubMed] [Google Scholar]
- 8.Chrysis D, Ritzen E, Savendahl L. Growth retardation induced by dexamethasone is associated with increased apoptosis of the growth plate chondrocytes. J Endocrinol. 2003;176:331-337. [DOI] [PubMed] [Google Scholar]
- 9.d'Agostino MC, Craig K, Tibalt E, Respizzi S. Shock wave as biological therapeutic tool: from mechanical stimulation to recovery and healing, through mechanotransduction. Int J Surg. 2015;24:147-153. [DOI] [PubMed] [Google Scholar]
- 10.De Luca F. Role of nuclear factor kappa B (NF-κB) in growth plate chondrogenesis. Pediatr Endocrinol Rev: 2016;13:720-730. [PubMed] [Google Scholar]
- 11.Duchatelet S, Ostergaard E, Cortes D, Lemainque A, Julier C. Recessive mutations in PTHR1 cause contrasting skeletal dysplasias in Eiken and Blomstrand syndromes. Hum Mol Genet 2004;14:1-5. [DOI] [PubMed] [Google Scholar]
- 12.Giusti G, Penteado FT, Santos JB, Alves MT, Faloppa F. Effect of shock waves upon the growth plate in rabbits. Acta Ortop Bras. 2005;13:31-34. [Google Scholar]
- 13.Gollwitzer H, Gloeck T, Roessner M, Langer R, Horn C, Gerdesmeyer L, Diehl P. Radial extracorporeal shock wave therapy (rESWT) induces new bone formation in vivo: results of an animal study in rabbits. Ultrasound Med Biol. 2013;39:126-133. [DOI] [PubMed] [Google Scholar]
- 14.Gottliebsen M, Shiguetomi-Medina JM, Rahbek O, Møller-Madsen B. Guided growth: mechanism and reversibility of modulation. J Child Orthop. 2016;10:471-477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hasler CC, Krieg AH. Current concepts of leg lengthening. J Child Orthop. 2012;6:89-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holfeld J, Tepeköylü C, Kozaryn R, Mathes W, Grimm M, Paulus P. Shock wave application to cell cultures. J Vis Exp. 2014;86:51076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hunziker E, Schenk R. Physiological mechanisms adopted by chondrocytes in regulating longitudinal bone growth in rats. J Physiol. 1989;414:55-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kertzman P, Császár NB, Furia JP, Schmitz C. Radial extracorporeal shock wave therapy is efficient and safe in the treatment of fracture nonunions of superficial bones: a retrospective case series. J Orthop Surg Res. 2017;12:164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kindblom J, Nilsson O, Hurme T, Ohlsson C, Savendahl L. Expression and localization of Indian hedgehog (Ihh) and parathyroid hormone related protein (PTHrP) in the human growth plate during pubertal development. J Endocrinol. 2002;174:R1-R6. [DOI] [PubMed] [Google Scholar]
- 20.Lin TH, Pajarinen J, Lu L, Nabeshima A, Cordova L, Yao Z, Goodman S. NF-κB as a therapeutic target in inflammatory-associated bone diseases. Adv Protein Chem Struct Biol. 2017:117-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lüssenhop S, Seemann D, Hahn M, Meiss L. The influence of shock waves on epiphysial growth plates: first results of an in-vivo study with rabbits. In: Siebert W, Buch M, eds. Extracorporeal Shock Waves in Orthopaedics. Springer: Berlin, Heidelberg; 2010:687-699. [Google Scholar]
- 22.Marino S, Staines KA, Brown G, Howard-Jones RA, Adamczyk M. Models of ex vivo explant cultures: applications in bone research. Bonekey Rep. 2016;5:818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mayer‐Wagner S, Ernst J, Maier M, Chiquet M, Joos H, Müller PE, Jansson V, Sievers B, Hausdorf J. The effect of high‐energy extracorporeal shock waves on hyaline cartilage of adult rats in vivo. J Orthop Res. 2010;28:1050-1056. [DOI] [PubMed] [Google Scholar]
- 24.Minkin C, James SS, Tao HH, Yu XH, Pockwinse S, MaCkay C, Marks SC., Jr Skeletal development and formation of osteoclast-like cells from in situ progenitors in fetal mouse metatarsals cultured in chemically defined medium. Bone Miner. 1991;12:141-155. [DOI] [PubMed] [Google Scholar]
- 25.Nassenstein K, Nassenstein I, Schleberger R. Effects of high-energy shock waves on the structure of the immature epiphysis--a histomorphological study. Z Orthop Ihre Grenzgeb. 2004;143:652-655. [DOI] [PubMed] [Google Scholar]
- 26.Ohba S. Hedgehog signaling in endochondral ossification. J Dev Biol. 2016;4:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oztemur Z, Ozturk H, Ozyurek S, Kaloglu C, Golge UH, Bulut O. The long-term effects of extracorporeal shock waves on the epiphysis of the adolescent rat. J Orthop Sci. 2013;18:159-164. [DOI] [PubMed] [Google Scholar]
- 28.Ozturk H, Bulut O, Oztemur Z, Kaloglu C, Kol IO. Effect of high-energy extracorporeal shock waves on the immature epiphysis in a rabbit model. Arch Orthop Trauma Surg. 2008;128:627-631. [DOI] [PubMed] [Google Scholar]
- 29.Paley D. Problems, obstacles, and complications of limb lengthening by the Ilizarov technique. Clin Orthop and Relat Res. 1990;250:81-104. [PubMed] [Google Scholar]
- 30.Reinecke M, Schmid AC, Heyberger-Meyer B, Hunziker EB, Zapf J. Effect of growth hormone and insulin-like growth factor I (IGF-I) on the expression of IGF-I messenger ribonucleic acid and peptide in rat tibial growth plate and articular chondrocytes in vivo. Endocrinology. 2000;141:2847-2853. [DOI] [PubMed] [Google Scholar]
- 31.Saisu T, Takahashi K, Kamegaya M, Mitsuhashi S, Wada Y, Moriya H. Effects of extracorporeal shock waves on immature rabbit femurs. J Pediatr Orthop B. 2004;13:176-183. [DOI] [PubMed] [Google Scholar]
- 32.Sävendahl L. Hormonal regulation of growth plate cartilage. Horm Res Paediatr. 2005;64:94-97. [DOI] [PubMed] [Google Scholar]
- 33.Schaden W, Mittermayr R, Haffner N, Smolen D, Gerdesmeyer L, Wang CJ. Extracorporeal shockwave therapy (ESWT)–First choice treatment of fracture non-unions? IntJ Surg. 2015;24:179-183. [DOI] [PubMed] [Google Scholar]
- 34.St-Jacques B, Hammerschmidt M, McMahon AP. Indian hedgehog signaling regulates proliferation and differentiation of chondrocytes and is essential for bone formation. Genes Dev. 1999;13:2072-2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sun D, Junger WG, Yuan C, Zhang W, Bao Y, Qin D, Wang C, Tan L, Qi B, Zhu D. Shockwaves induce osteogenic differentiation of human mesenchymal stem cells through ATP release and activation of P2X7 receptors. Stem Cells. 2013;31:1170-1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Van Arsdalen KN, Kurzweil S, Smith J, Levin RM. Effect of lithotripsy on immature rabbit bone and kidney development. J Urol. 1991;146:213-216. [DOI] [PubMed] [Google Scholar]
- 37.Wang F, Yang K, Chen R, Wang C, Sheen-Chen S-M. Extracorporeal shock wave promotes growth and differentiation of bone-marrow stromal cells towards osteoprogenitors associated with induction of TGF-β1. J Bone Joint Surg Br. 2002;84:457-461. [DOI] [PubMed] [Google Scholar]
- 38.Weihs AM, Fuchs C, Teuschl AH, Hartinger J, Slezak P, Mittermayr R, Redl H, Junger WG, Sitte HH, Rünzler D. Shockwave treatment enhances cell proliferation and improves wound healing by ATP release coupled extracellular signal-regulated kinase (ERK) activation. J Biol Chem. 2014: M114 580936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu S, Fadoju D, Rezvani G, De Luca F. Stimulatory effects of insulin-like growth factor-I on growth plate chondrogenesis are mediated by nuclear factor-κB p65. J Biol Chem. 2008;283:34037-34044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu S, Flint JK, Rezvani G, De Luca F. Nuclear factor-κB p65 facilitates longitudinal bone growth by inducing growth plate chondrocyte proliferation and differentiation and by preventing apoptosis. J Biol Chem. 2007;282:33698-33706. [DOI] [PubMed] [Google Scholar]
- 41.Xu ZH, Jiang Q, Chen DY, Xiong J, Shi DQ, Yuan T, Zhu XL. Extracorporeal shock wave treatment in nonunions of long bone fractures. Int Orthop. 2009;33:789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yeaman LD, Jerome CP, McCullough DL. Effects of shock waves on the structure and growth of the immature rat epiphysis. J Urol. 1989;141:670-674. [DOI] [PubMed] [Google Scholar]