Abstract
Background
Desmoid tumors of the extremities often present with pain and functional limitation, but treatment can lead to morbidity and recurrence is common. The impact of treatment with respect to traditional “oncologic” metrics (such as recurrence rate) has been studied extensively, with a shift in recent years away from local therapies as first-line management; however, little is known about the association between treatment modality and long-term functional outcomes for patients with this benign disease.
Questions/purposes
In a retrospective review of consecutive patients treated at two institutions, we asked: (1) Is event-free survival (EFS) different between patients who undergo local treatment and those who do not for primary as well as for recurrent desmoid tumors? (2) What treatment-related factors are associated with worse Patient-reported Outcomes Measurement Information System (PROMIS) function scores at a minimum of 1 year after treatment?
Methods
Between 1991 and 2017, 102 patients with desmoid tumors of the extremities (excluding those of the hands and feet) were treated at two institutions; of those, 85 patients with 90 tumors were followed clinically for at least 1 year (median [range] 59 months follow-up [12 to 293]) and were included in the present analysis. We attempted to contact all patients for administration of PROMIS function (Physical Function Short Form [SF] 10a and Upper Extremity SF v2.0 7a) and Pain Interference (SF 8a) questionnaires. Complete survey data (minimum 1 year follow-up) were available for 46% (39 of 102) of patients with 40 tumors at a median of 125 months follow-up; only these patients were included in PROMIS data analyses. Though there was no formal institutional treatment algorithm in place during the study period, surgical resection typically was the preferred modality for primary tumors; radiation therapy and systemic treatments (including cytotoxic or hormonal agents earlier in the study period, and tyrosine kinase inhibitors later) were often added for recurrent or very symptomatic disease. We coded treatment for each patient into discrete episodes, each defined by a particular treatment strategy: local treatment only (surgery and/or radiation), systemic treatment only, local plus systemic treatment, or observation; treatment episodes rendered at other institutions (that is, before referral) were not included in the analyses. Treatment failure was defined as recurrence after surgical resection, or clinically significant radiologic and/or symptomatic progression after systemic treatment, and EFS was defined as time from treatment initiation to treatment failure or final follow-up. Episodes of treatment for recurrent tumors were analyzed in a pooled fashion, wherein discrete treatment episodes for patients with multiple recurrences were included separately as independent events. We analyzed 56 primary tumors (54 patients), and 101 discrete treatment episodes for recurrent tumors (88 patients). Kaplan-Meier survival curves were constructed separately for the primary and recurrence cohorts, both comparing EFS among patients who received any local treatment (local treatment and local plus systemic treatment groups) versus those who did not (systemic treatment and observation groups). PROMIS function data were analyzed on the bases of patient- and treatment-specific variables, including the PROMIS Pain Interference score as a potential explanatory variable.
Results
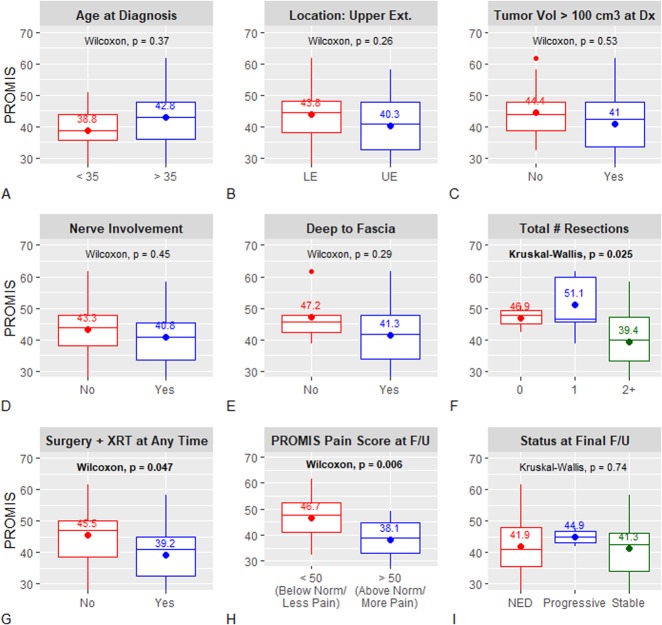
Within both the primary and recurrence cohorts, there were no differences between the local treatment, systemic treatment, and local plus systemic treatment groups with respect to gender, age, axillary/hip girdle location, or tumor volume. Among primary tumors, 5-year EFS was 44% (95% CI 24 to 80) for the systemic-only group versus 15% (95% CI 5 to 44) for the local treatment group (p = 0.087). Within the pooled recurrence treatment episode cohort, 5-year EFS after systemic-only treatment was 70% (95% CI 52 to 94) versus 56% among patients receiving any local treatment (95% CI 44 to 70; p = 0.46). PROMIS function scores were lowest among patients who underwent two or more resections (39 versus 51 versus 47 for ≥2, 1, and 0 resections, respectively; p = 0.025); among those who received both surgery and radiation at any point, either concurrently or in separate treatment episodes, as compared with those who did not (39 versus 46; p = 0.047); and among those with higher levels of pain interference (38 versus 47 for pain interference scores > 50 versus < 50; p = 0.006).
Conclusions
Patients treated with local modalities (surgery and/or radiation, with or without additional systemic therapy) did not experience improved EFS as compared with those treated without local modalities; this was the case for both the primary and the recurrent tumor cohorts. However, PROMIS function scores were lowest among patients who underwent two or more surgical interventions and among those treated with surgery and radiation at any time, suggesting that more aggressive local treatment may be associated with poorer long-term functional outcomes. Prospective collection of patient-reported outcomes data at multiple time points will allow for more direct correlations between treatment modality and impact on function and will help to elucidate the ideal management strategy for these benign but often-symptomatic tumors.
Level of Evidence
Level III, therapeutic study.
Introduction
Desmoid tumors of the extremities often present with pain and functional limitation, but treatment can lead to morbidity and recurrence is common. The array of potential therapeutic options is wide, and substantial variability exists across health care providers and specialties, complicating comparisons of treatment strategies. Historically, management has consisted of aggressive surgical treatment to achieve a cure. Many authors [1, 16, 17, 24, 26]—though not all [8, 21]—have emphasized the need for wide surgical resection with disease-free margins, if possible. Adjuvant radiation therapy was also previously touted as beneficial with respect to local control [1, 7, 9, 17, 22, 24]. More recent reports [3, 5, 19, 21], however, including consensus guidelines from European sarcoma working groups [10, 11], have advocated for a “wait-and-see” observational approach as the first-line desmoid tumor management or for initiation of systemic therapy before surgery or radiation [23].
The study of these tumors is further hampered by the limitations of conventional oncologic outcome assessments. Evaluations of survival and recurrence rates alone do not capture long-term functional outcomes; this is a critical “blind spot” in our assessment of treatment for this benign disease. To our knowledge, no prior large study has explicitly examined the associations between treatment and patient-reported function metrics.
Therefore, we asked: (1) Is event-free survival (EFS) different between patients who undergo local treatment and those who do not for primary and for recurrent desmoid tumors? (2) What treatment-related factors are associated with worse PROMIS function scores at a minimum of 1 year after treatment?
Patients and Methods
Study Design and Setting
With the approval of our institutional review board, we retrospectively evaluated data on patients treated for desmoid fibromatosis of the upper and lower extremities between 1991 and 2017 at two affiliated quaternary-care cancer centers. We identified these patients using a registry search tool based on International Classification of Diseases, 9th and 10th Edition codes, as well as free text from pathology reports. Patients with tumors of the axilla and buttock/hip girdle were included and those with tumors of the hand and foot were excluded. Patients with intraperitoneal, chest wall, and abdominal wall tumors were also excluded because tumors in these locations may have a less direct impact on physical function. Additionally, we excluded patients who did not have at least 12 months of oncologic-specific clinical follow-up from the time of initial presentation to our institutions.
Clinical data, including demographics, radiologic variables, treatment details, and outcomes data, were collected via record review. We calculated tumor volumes as the product of tumor dimensions measured with available imaging, or as specified in pathologic and/or radiologic reports. The tumor depth (superficial versus deep to fascia) and direct tumor involvement of major peripheral nerves at the time of presentation to our institution were noted based on operative reports, radiology reports, and direct review of imaging, where appropriate. In patients who underwent surgical resection, microscopically negative margins were considered negative, while all other margins were considered positive.
Definition of Treatment Groups and Episodes
Though there was no formal treatment algorithm in place at our institutions during the study period, surgical resection was typically the preferred modality for primary tumors. Radiation therapy was often added for recurrent disease, particularly in the early portion of the study period. Use of systemic therapy, most often for recurrent or very symptomatic disease, was more likely to include cytotoxic chemotherapy or hormonal agents earlier in the study period, and more likely to include tyrosine kinase inhibitors later.
To answer our first question, we coded treatment and outcomes data for each patient into discrete treatment episodes, each defined by a treatment strategy: local treatment only (surgery and/or radiation), systemic treatment only, or local plus systemic treatment. Patients who were only observed were included in the systemic treatment group for survival analyses.
Treatment success was defined as absence of disease after gross total surgical resection, or radiologically and clinically stable disease after systemic treatment or after radiation as definitive local treatment. Treatment failure was defined as recurrence after gross total surgical resection, or radiologic and/or symptomatic progression after systemic treatment (or definitive radiation), requiring a change in treatment strategy. Retrospective determination of tumor recurrence was based on radiologic findings and the associated documentation of treating physicians; determination of clinically significant radiologic and/or symptomatic progression requiring a change in treatment strategy, for the purpose of assessment of “treatment failure,” relied on review of the contemporaneous assessments of treating physicians, as documented in the record. EFS was defined as the time from treatment initiation to treatment failure, as defined above, with censorship at the final follow-up examination if no failure occurred. For treatment episodes involving systemic therapy, changes in drug selection, drug holidays, or interruptions to drug administration were not considered treatment failure or new treatment episodes, as long as medical therapy was subsequently continued without local treatment. Systemic therapies included prolonged and/or high-dose regimens of NSAIDs (such as sulindac), hormonal agents (including tamoxifen), targeted chemotherapeutics (such as imatinib), and cytotoxic chemotherapeutics. Radiation therapy consisted of preoperative, intraoperative, postoperative, and/or definitive treatments. Surgical biopsies were not considered discrete treatment episodes.
We assessed the outcomes of treatment of recurrent tumors by pooling all such treatment episodes. Treatment episodes for patients who had recurrent tumors at the initial presentation at our institutions were included, as were treatment episodes for recurrence after failure of primary tumor treatment at our institutions. Individual patients with multiple treatment episodes for recurrence, therefore, might be included multiple times.
Acquisition of Patient-reported Functional Data
To answer our second question, we attempted to contact all included patients via direct clinic contact or via postal mail and a minimum of two telephone calls to administer the Patient-reported Outcomes Measurement Information System (PROMIS) Physical Function Short Form 10a, the PROMIS Upper Extremity Short Form version 2.0 7a (for patients with upper extremity tumors), and the PROMIS Pain Interference Short Form 8a. PROMIS instruments are calibrated such that a score of 50 corresponds to the normative mean for the general population, with a SD of 10; higher Upper Extremity and Physical Function scores correlate with better function; higher Pain Interference scores correlate with more severe pain.
Participants
The registry search, as described above, identified 1062 potential desmoid tumor cases in our institutional medical record system; these were subsequently evaluated via a manual record review. We excluded the following: those for which final pathologic analysis was not consistent with a desmoid tumor (91); those for patients treated clinically at another institution: for example, one-time “second opinion” or pathology review-only at our institutions (346); tumors located in the chest wall, abdominal wall, back, or head/neck regions (276); intrathoracic or intraabdominal tumors (224); palmar or plantar tumors (18); and those for patients with insufficient clinical follow-up (17).
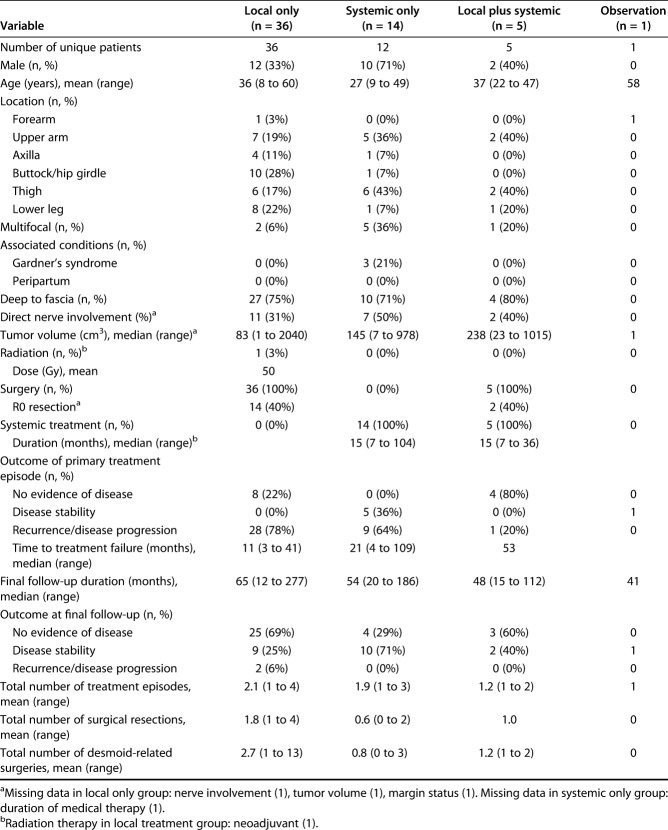
We therefore included 85 patients with 90 tumors, with 59-month median (range) clinical follow-up (12 to 293). Among 56 primary tumors in 54 unique patients, 64% (36 patients) were treated with local treatment only: surgery plus radiation in one patient and surgery alone in the remainder. Twenty-five percent of tumors (14) were treated with systemic treatment alone and 9% (five) were treated with local plus systemic treatment; only one tumor was managed with observation alone (Table 1).
Table 1.
Demographic, tumor-specific and treatment variables for primary tumors
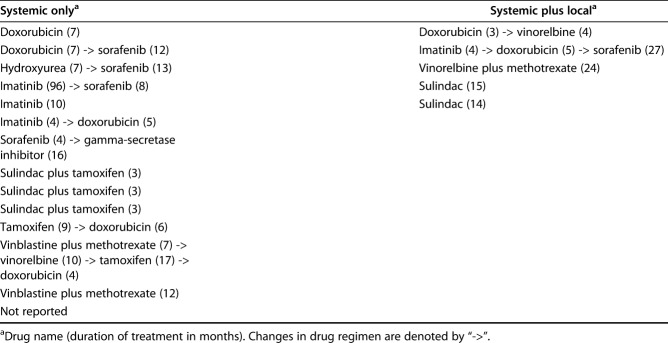
The medications administered to patients in the systemic treatment and local plus systemic treatment groups varied widely, and included traditional cytotoxic chemotherapeutics for six tumors in the systemic treatment group and three tumors in the local plus systemic treatment group; tyrosine-kinase inhibitors (six in the systemic treatment and one in the local plus systemic treatment); aromatase inhibitors (five in the systemic treatment and none in the local plus systemic treatment); and high-dose NSAIDs (three in the systemic treatment and two in the local plus systemic treatment). Many patients were treated with multiple classes of systemic agents (Table 2). The mean numbers of total lifetime desmoid tumor-related surgeries (including resections and surgeries for complications) were 2.7 in the local treatment, 1.2 in the local plus systemic treatment, and 0.8 in the systemic group.
Table 2.
Details of systemic therapy for treatment of primary tumors by treatment group
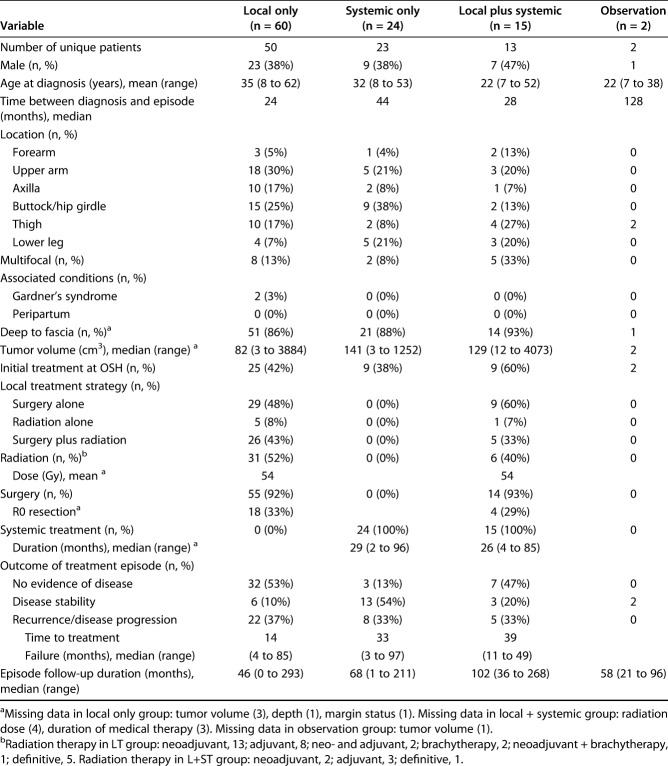
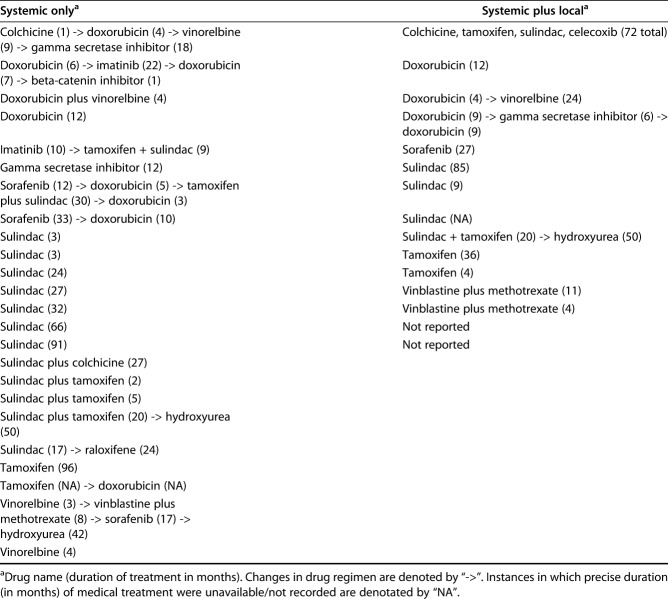
There were 101 discrete treatment episodes for recurrent tumors, including 60 episodes of local treatment, 24 episodes of systemic treatment, 15 episodes of local plus systemic, and two observation episodes (Fig. 1). Concurrent radiation and surgery were used far more frequently in the recurrent tumor local treatment group than in the primary tumor local treatment group: 43% versus 3% (Table 3). As was the case for primary tumors, drug regimens in the systemic treatment and local plus systemic treatment groups were diverse, including traditional cytotoxic chemotherapeutics (nine tumors in the systemic treatment group and five tumors in the local plus systemic treatment groups); tyrosine-kinase inhibitors (seven in the systemic treatment group and two in the local plus systemic group); aromatase inhibitors (eight in the systemic treatment and four in the local plus systemic treatment); and high-dose NSAIDs (14 in the systemic treatment and five in the local plus systemic treatment) (Table 4). All but 29 episodes of treatment for recurrence were initiated within 5 years of the initial diagnosis of desmoid tumor, and all but 11 were initiated within 10 years of diagnosis (Fig. 2).
Fig. 1.
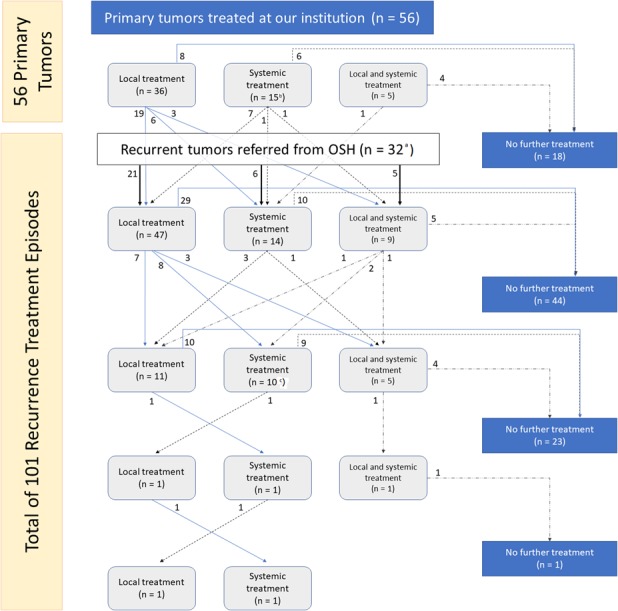
This flowchart demonstrates treatment “pathways” for patients included in the study. OSH = outside hospital. aTwo additional patients referred from OSH for evaluation of reported recurrence were deemed at our institutions not to have recurrent tumors, and therefore were not included in the pooled recurrence treatment episode analysis. bIncluded one patient treated with observation alone. cIncluded two patients treated with observation alone.
Table 3.
Demographic, tumor-specific, and treatment variables for recurrent tumors (pooled treatment episodes)
Table 4.
Details of systemic therapy for treatment of recurrent tumors (pooled treatment episodes) by treatment group
Fig. 2.
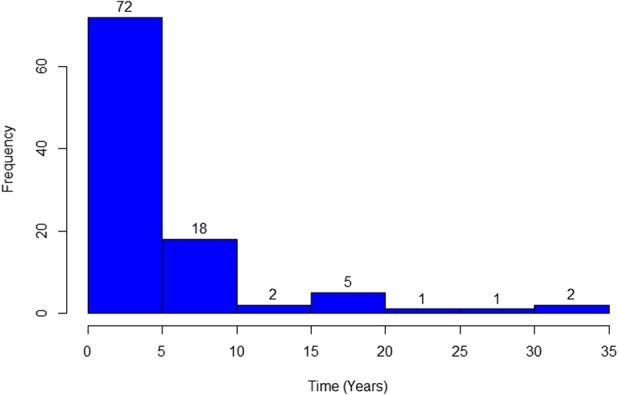
This histogram shows time from initial desmoid diagnosis to initiation of recurrence treatment episode.
Within both the primary and recurrent cohorts, there were no differences between local treatment, systemic treatment, and local plus systemic treatment, respectively, in terms of male gender (primary: 33%, 71%, and 40%; p = 0.05; recurrence: 38%, 38%, and 47%; p = 0.84), age at diagnosis (primary: 36, 27, and 37 years; p = 0.10; recurrence: 35, 32, and 22 years; p = 0.68), axillary/hip girdle tumor location (primary: 39%, 14%, and 0%; p = 0.11; recurrence: 42%, 46%, and 20%; p = 0.24), or tumor volume at treatment (primary: 83, 145, and 238 cm3, p = 0.73; recurrence: 82, 141, and 129 cm3; p = 0.68).
Complete survey data were available for 48% (41 of 85) patients whose records were reviewed; of the 44 patients without complete survey data, three lived outside the United States, one was deceased, one declined to participate, one did not speak English, and the remainder could not be contacted. Survey data for two patients were excluded for survey follow-up less than 12 months, leaving data for 40 tumors in 46% (39) of patients (Table 5). Surveys were completed at a median of 125 months (range 14 to 304) after treatment was initiated at our institutions.
Table 5.
Demographic, tumor-specific, and treatment variables for patients included in the PROMIS questionnaire cohort
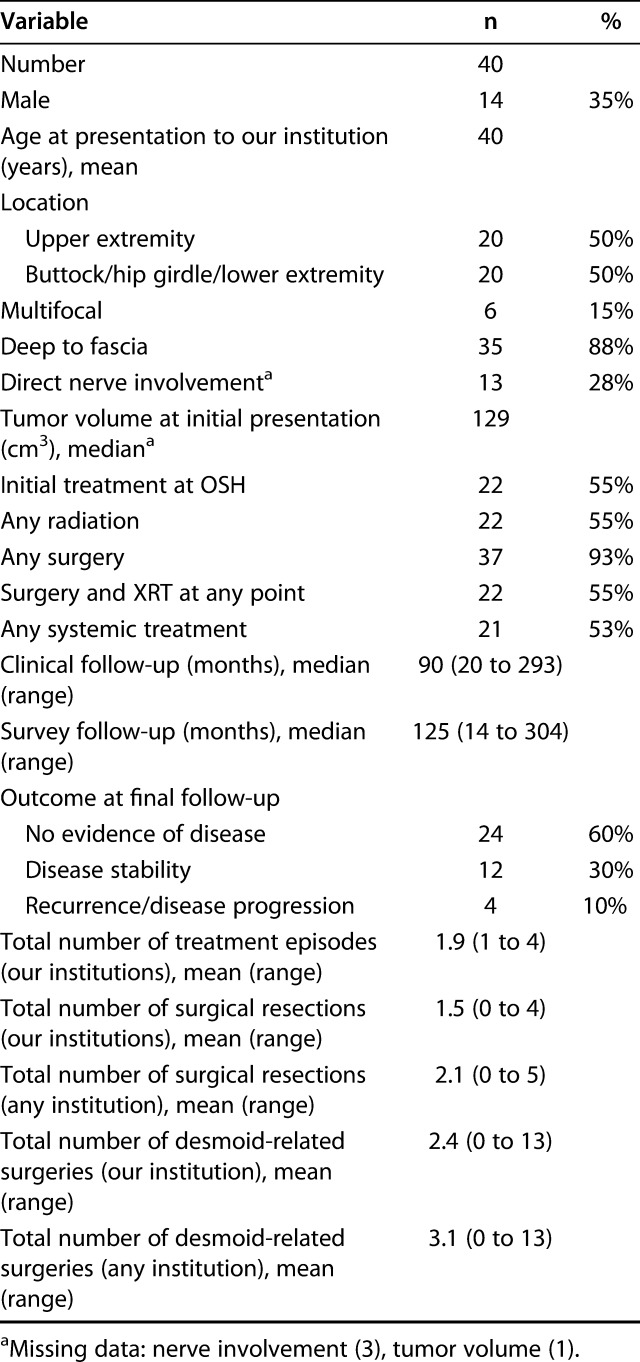
Within the survey-respondent cohort, 93% (37 of 40) underwent at least one surgery, including 55% (22) who also received radiation at some point in their treatment; mean numbers of surgical resections and total desmoid-related surgeries (including those for complications) at any institution were 2.1 and 3.1, respectively. In comparison, among those not included in the survey cohort, 88% (42 of 48) were treated with surgery, including 29% (14) who received radiation as well; mean numbers of surgical resections and total desmoid-related surgeries were 1.6 and 2.0, respectively.
Statistical Analysis
Within both the primary and recurrence cohorts, we used Kruskal-Wallis and Fisher’s exact tests, as appropriate, to compare the local treatment, systemic treatment, and local plus systemic treatment groups based on gender, age, axillary/hip girdle location, and tumor volume. We constructed Kaplan-Meier survival curves demonstrating EFS on the bases of broad treatment categories (any local therapy [including local treatment and local plus systemic treatment groups] versus no local therapy [systemic treatment and observation groups]), for both the primary and pooled recurrence cohorts.
We analyzed response data collected through patient-reported outcome instruments with nonparametric tests, as appropriate. We assessed functional outcomes metric data by merging the results of the PROMIS Physical Function and PROMIS Upper Extremity questionnaires. The former were used for patients with lower extremity tumors, while the latter were used for patients with upper extremity tumors. PROMIS Pain Interference data, which have been shown to reflect coping ability and, in turn, impact physical function [4, 13], were analyzed as a potential explanatory variable with respect to function data.
We considered p values below 0.05 to be significant, and performed all statistical analyses using RStudio (version 1.0.153; R Foundation for Statistical Computing, Vienna, Austria).
Results
Event-Free Survival Among Patients Who Undergo Local Treatment and Those Who Do Not, for Primary and for Recurrent Tumors
Five-year EFS did not differ between patients who underwent local treatment (surgery and/or radiation) as compared with those who did not. Within the primary tumor cohort, 5-year EFS was 44% (95% CI 24 to 80) for the systemic-only group versus 15% (95% CI 5 to 44) for the local treatment group (p = 0.087; Fig. 3). Within the pooled recurrence treatment episode cohort, 5-year EFS after systemic-only treatment was 70% (95% CI 52 to 94), while 5-year EFS after treatment involving local modalities was 56% (95% CI 44 to 70; p = 0.46; Fig. 4).
Fig. 3.
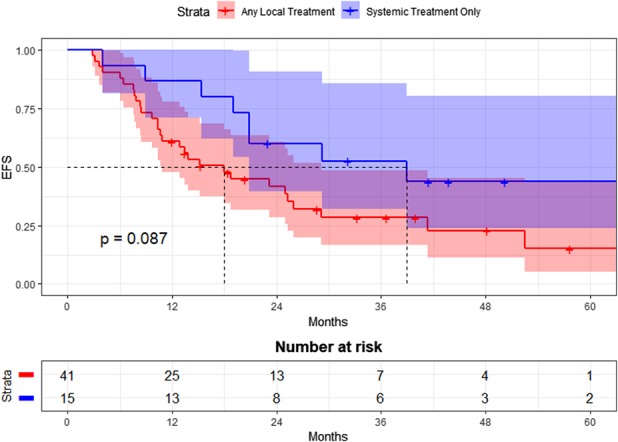
Event-free survival (EFS) after treatment for primary tumors by treatment group is shown here.
Fig. 4.
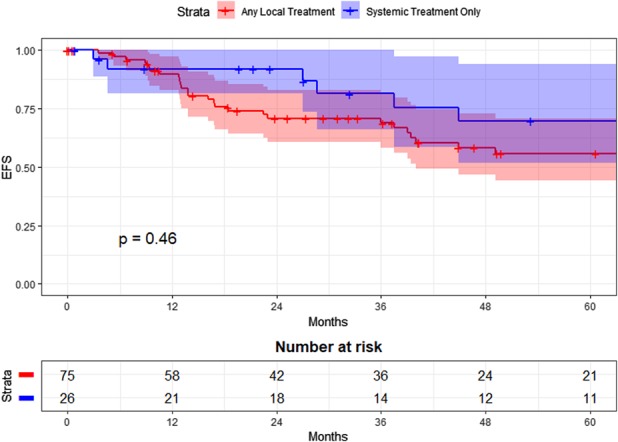
Event-free survival (EFS) after treatment for recurrence by treatment group (pooled treatment episodes) is shown here.
Treatment-Related Factors Associated with Worse PROMIS Function Scores
Mean PROMIS function scores were lowest for patients who underwent two or more surgical resections (39 for ≥2 resections versus 51 for 1 resection versus 47 for 0 resections; p = 0.025) and for those who received both surgery and radiation at any point, either concurrently or in separate treatment episodes, as compared with patients who did not (39 versus 46; p = 0.047; Fig. 5A-I). Additionally, patients with PROMIS pain interference scores below normative population-based means (that is, lower pain levels) had better function scores than those with higher levels of pain interference (47 versus 38, respectively; p = 0.006). There were no differences in PROMIS function scores based on age, location, tumor volume, tumor depth, nerve involvement, or disease status at final oncologic follow-up. Scores for patients whose disease was cured (no evidence of disease at the final follow-up examination) were nearly identical to those of patients with residual but stable disease.
Fig. 5A-I.
PROMIS function outcomes data according to patient- and treatment-specific variables are demonstrated here; by-group mean scores are noted in each sub-plot. LE = lower extremity; UE = upper extremity; Vol = volume; Dx = diagnosis; # = number; XRT = radiation therapy; F/U = final follow-up; NED = no evidence of disease.
Discussion
The unpredictable nature of desmoid tumors, high rates of local recurrence, and general dissatisfaction among both providers and patients has historically resulted in a hodge-podge of treatment options. Though these tumors are treated by orthopaedic, radiation, and medical oncologists, there has been increasing recognition in recent years that management of these very locally aggressive but ultimately benign tumors perhaps ought to be guided by a different set of principles than those governing management of soft tissue sarcomas. In the past, many authors advocated for surgical resection with wide margins [1, 15–17, 24, 26]. More recently, however, management strategies involving less locally aggressive up-front treatments have been advocated, supported by the results from several retrospective series [2, 3, 5, 21], prospective cohorts of adults [18, 20] and children [23], and emphasized by European consensus guidelines [10, 11]. Even so, there exist little or no data reflecting patient-reported functional or symptomatic outcomes to support treatment recommendations. We found that patients treated with local modalities (surgery and/or radiation, with or without additional systemic therapy) did not experience improved EFS as compared with those treated without local modalities; this was the case for both the primary and the recurrent tumor cohorts. However, PROMIS function scores were lowest among patients who underwent two or more surgeries and among those treated with surgery and radiation at any time, suggesting that more aggressive local treatment may have a negative impact on long-term functional outcomes.
Limitations
This study had several limitations. Perhaps most importantly, the definition of treatment failure was broad, and based primarily on the impressions of treating providers as documented in the medical record, as well as on radiologic findings. This is, however, pragmatic and reflective of clinical practice, in which the identification of disease progression and need for alteration in treatment is largely dependent on physician and patient perception. This point also underscores the ways in which the assessment of outcomes of desmoid tumor treatment differs from that of true malignancies, in which more concrete, traditional “oncologic” metrics such as local recurrence and mortality are more relevant. The definition of systemic failure may have been particularly problematic because specific medication changes (such as transition from doxorubicin to imatinib or brief medication holidays) were not considered new treatment episodes as long as systemic therapy was not interrupted by new decisions for surgery or radiation. This certainly biased the analyses of time-to-failure in favor of systemic management because many patients were administered a series of different drugs. Again, however, we feel that this analysis was pragmatic. Especially with the introduction of relatively well-tolerated, orally administered, targeted chemotherapeutics, prolonged episodes of medical management with intermittent regimen modifications are quite reasonable.
The heterogeneity of treatments included in our cohort is substantial; certain modalities—in particular, cytotoxic chemotherapeutics, high-dose radiation, and wide/radical surgical resection—were employed at our institutions far more commonly in the early years of the study period than they are now. Midway through the study period, for instance, the use of conventional cytotoxic chemotherapeutics (such as doxorubicin and vinorelbine) gradually gave way to greater use of targeted agents such as imatinib [12, 20] and gamma secretase inhibitors [14]; sorafenib, another tyrosine kinase inhibitor, has recently been shown to prolong progression-free survival in a randomized controlled trial of advanced and refractory tumors [6]. Additionally, the broad treatment philosophy employed at our institutions (as outlined in the Patients and Methods section) shifted somewhat throughout the study period, and continues to do so into the present. The data presented here are therefore insufficient to support specific treatment regimens. In acknowledging this, we have avoided more granular survival analyses (such as with respect to type of local or systemic treatment).
In our “pooled” recurrence treatment episode cohort, patients with multiply-recurrent tumors (a minority) were included multiple times in the analyses, with each recurrence episode treated as an independent event. Although this may have resulted in the inappropriate statistical “over-representation” of certain individual patients, we feel that this allowed for a more granular (and therefore accurate) analysis of their complex clinical courses. In the absence of a clear, a priori understanding of the ways in which one earlier treatment modality may impact a subsequent one, we feel this analytic framework is the best one.
Our cohort, although sizeable compared with other cohorts of patients with extremity desmoid tumors, was relatively small, restricting the granularity with which specific treatment analyses could be performed. We chose to limit our study only to patients with extremity tumors, excluding those of the hands and feet, as we feel that this cohort represented patients at particular risk of functional deficit after treatment. Patient self-reported data were available for just under half of the studied patients, and therefore were potentially subject to transfer bias. The majority of surveyed patients were initially treated at outside institutions, and therefore had experienced, at minimum, one recurrence. Additionally, a greater proportion of surveyed versus non-surveyed patients were treated with both radiation and surgery, and had higher mean numbers of lifetime surgical resections and total desmoid-related surgeries. The PROMIS functional outcomes data, therefore, reflect outcomes of a subset of the overall cohort that received more aggressive treatment, either because of more challenging tumors or related to patient- and physician-decision making, or both. In light of the PROMIS results, it is possible that surveyed patients experienced worse functional outcomes as compared with non-surveyed patients. We feel this is unlikely to nullify the finding that more aggressive local treatment, especially involving both surgery and radiation, is associated with long-term functional deficits; what we are incompletely able to answer with these data, however, is the extent to which systemic treatment (or even observation) alone impacts function. This will require prospective collection of function data in a larger cohort of patients treated without local therapies.
Event-Free Survival among Patients who Undergo Local Treatment and those who Do Not, for Primary and for Recurrent Tumors
The results of this study suggest that treatment involving local modalities (surgery and/or radiation) was not associated with improved EFS; this was the case in separate analyses of primary and recurrent tumors. As noted in the limitations section above, the heterogeneity of treatment modalities represented in the current study is such that we are limited in our ability to make granular treatment recommendations. However, we feel that the results of the survival analyses at least support the feasibility of systemic-only treatment (or treatment involving less aggressive local approaches), and help to contextualize the results of the patient-reported functional outcome results.
These results are in line with those from other recent reports. A retrospective study published in 2008 of 112 adults with desmoid tumors found equivalent event-free survivals for patients treated with microscopically-complete surgery and those whose tumors were managed medically or with observation alone (roughly 65%) [2]. Likewise, in a 2017 nationwide, prospective cohort of 771 patients with intra- or extra-abdominal desmoid tumors, no difference in 2-year EFS was observed between surgical resection and “wait-and-see” as initial treatment strategies (53% versus 58%, respectively) [19]. Interestingly, however, 2-year EFS was worse (25%) in patients treated with initial surgical resection for tumors in “unfavorable” locations, including the upper extremity; this may speak to the relatively low EFS observed after treatment for primary tumors in our cohort of extremity-only tumors. Among pediatric and adolescent patients enrolled in prospective trials in Germany, 5-year EFS was 59% versus 35%, respectively, after initial treatment with surgery versus systemic therapy; furthermore, significant functional deficiencies were noted in 20% of patients managed surgically, albeit without more detailed characterization [23].
Treatment-Related Factors Associated with Worse PROMIS Function Scores
Among patients who underwent more than one desmoid resection, and among those treated with both surgery and radiation at any point, PROMIS function scores were more than one full SD below normative means. These results were perhaps moderated, in part, by issues of ongoing pain, as function scores were also lower in patients with pain interference levels above normative means. Of course, treatment decisions are made in response to tumor-specific variables, and it is impossible to know whether these findings were because of more aggressive disease, more aggressive treatment modalities, or both. We note, however, that for many patients in our study, surgery was not necessarily a “one-time event.” Among patients with primary tumors that were initially treated with local treatment, the mean number of lifetime resections was 1.8 and the mean number of total desmoid tumor-related surgeries, including those for complications, was 2.7, perhaps speaking to the long-term ramifications of the initial treatment decision. It should be noted that we report the PROMIS function scores of patients who received both surgery and radiation at any point, not necessarily concurrently within the same treatment “episode”; this allowed us to capture the cumulative (and potentially additive) effects of surgery and radiation on long-term functional outcomes. These data suggest that treatment with both surgery and radiation has a substantial effect on long-term function.
A recent focus-group study identified significant psychologic stressors and associated qualitative impact on health-related quality of life among patients with desmoid tumors [25], and 2017 consensus guidelines emphasized the need for “physical, psychologic and social support” for these patients [11]. There is, however, a paucity of data quantifying the functional outcomes after extremity desmoid treatment; to our knowledge, the present study is the first to do so with PROMIS (or other validated patient-reported outcomes) metrics.
Conclusions
Our results suggest that desmoid tumor treatment strategies involving local modalities may not be associated with improved EFS as compared with those involving systemic treatment only, and that more aggressive local treatment may be associated with greater long-term functional limitations. Though we acknowledge that the data presented here explicitly do not describe the natural history of desmoid tumors, we suspect that desmoid tumors may tend to “burn out,” regardless of treatment modality. Indeed, although the natural history of desmoid tumors is incompletely understood, the wait-and-see approach has been reported to result in disease regression or stability in half (or more) of patients whose tumors were managed with observation alone [1, 3, 5, 18]. Of the 101 treatment episodes for recurrent tumors presented here, nearly 75% occurred within 5 years of the initial diagnosis and more than 90% occurred within 10 years of diagnosis. It should also be noted that EFS rates were, perhaps counterintuitively, higher in the recurrence cohort as compared with the primary cohort. While this may reflect more liberal use of systemic modalities in multiply-recurrent patients, it may also reflect the possibility that disease stability is the standard, not the exception, once multiple years have elapsed. To that end, current practice at our institutions involves evaluation of all new desmoid tumor patients in a multidisciplinary clinic, by a surgeon, a radiation oncologist, and a medical oncologist. More so than was the case early in the study period, we are most likely now to shy away from up-front local therapy, favoring instead systemic options aimed at curbing symptoms and tumor progression. We feel that this practice is supported by the data presented here.
To our knowledge, this is the first study using PROMIS metrics to assess functional outcomes after treatment for desmoid tumors, and represents our attempt to introduce the quantification of functional outcomes in the assessment of desmoid treatment. Of critical importance in the future will be the prospective, in-clinic collection of patient-reported outcomes data at multiple time points throughout the treatment process, ideally integrated into routine clinical follow-up. This will allow clinicians and researchers to evaluate the impact of treatment on function and dimensions of health-related quality of life in a more granular and time-dependent manner. The management of this benign but symptomatic condition must be increasingly guided not by traditional oncologic metrics but by impact on patient symptoms and function.
Acknowledgments
None.
Footnotes
Each author certifies that neither he, nor any member of his immediate family, has funding or commercial associations (consultancies, stock ownership, equity interest, patent/licensing arrangements, etc.) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research® editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research® neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA approval status, of any drug or device before clinical use.
Each author certifies that his institution approved the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
This work was performed at the Massachusetts General Hospital and Brigham and Women’s Hospital, Boston, MA, USA.
References
- 1.Ballo BMT, Zagars GK, Pollack A, Pisters PWT, Pollock RA. Desmoid tumor: prognostic factors and outcome after surgery, radiation therapy, or combined surgery and radiation therapy. J Clin Oncol . 1999;17:158–167. [DOI] [PubMed] [Google Scholar]
- 2.Bonvalot S, Eldweny H, Haddad V, Rimareix F, Missenard G, Oberlin O, Vanel D, Terrier P, Blay JY, Le Cesne A, Le Péchoux C. Extra-abdominal primary fibromatosis: Aggressive management could be avoided in a subgroup of patients. Eur J Surg Oncol . 2008;34:462–468. [DOI] [PubMed] [Google Scholar]
- 3.Briand S, Barbier O, Biau D, Bertrand-Vasseur A, Larousserie F, Anract P, Gouin F. Wait-and-see policy as a first-line management for extra-abdominal desmoid tumors. J Bone Joint Surg Am. 2014;96:631–638. [DOI] [PubMed] [Google Scholar]
- 4.Crijns TJ, Bernstein DN, Ring D, Gonzalez RM, Wilbur DM, Hammert WC. Depression and pain interference correlate with physical function in patients recovering from hand surgery. Hand [Published online adhead of print May 1, 2018]. DOI: 10.1177/1558944718777814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fiore M, Rimareix F, Mariani L, Domont J, Collini P, Le Péchoux C, Casali PG, Le Cesne A, Gronchi A, Bonvalot S. Desmoid-type fibromatosis: A front-line conservative approach to select patients for surgical treatment. Ann Surg Oncol. 2009;16:2587–2593. [DOI] [PubMed] [Google Scholar]
- 6.Gounder MM, Mahoney MR, Van Tine BA, Ravi V, Attia S, Deshpande HA, Gupta AA, Milhem MM, Conry RM, Movva S, Pishvaian MJ, Riedel RF, Sabagh T, Tap WD, Horvat N, Basch E, Schwartz LH, Maki RG, Agaram NP, Lefkowitz RA, Mazaheri Y, Yamashita R, Wright JJ, Dueck AC, Schwartz GK. Sorafenib for Advanced and Refractory Desmoid tumors. N Engl J Med . 2018;379:2417–2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goy BW, Lee SP, Eilber F, Dorey F, Eckardt J, Fu YS, Juillard GJF, Selch MT. The role of adjuvant radiotherapy in the treatment of resectable desmoid tumors. Int J Radiat Oncol Biol Phys . 1997;39:659–665. [DOI] [PubMed] [Google Scholar]
- 8.Gronchi A, Casali PG, Mariani L, Lo Vullo S, Colecchia M, Lozza L, Bertulli R, Fiore M, Olmi P, Santinami M, Rosai J. Quality of surgery and outcome in extra-abdominal aggressive fibromatosis: a series of patients surgically treated at a single institution. J Clin Oncol . 2003;21:1390–1397. [DOI] [PubMed] [Google Scholar]
- 9.Janssen ML, van Broekhoven DLM, Cates JMM, Bramer WM, Nuyttens JJ, Gronchi A, Salas S, Bonvalot S, Grünhagen DJ, Verhoef C. Meta-analysis of the influence of surgical margin and adjuvant radiotherapy on local recurrence after resection of sporadic desmoid-type fibromatosis. Br J Surg . 2017;104:347–357. [DOI] [PubMed] [Google Scholar]
- 10.Kasper B, Baumgarten C, Bonvalot S, Haas R, Haller F, Hohenberger P, Moreau G, Van Der Graaf WTA, Gronchi A. Management of sporadic desmoid-type fibromatosis: A European consensus approach based on patients’ and professionals’ expertise - A Sarcoma Patients EuroNet and European Organisation for Research and Treatment of Cancer/Soft Tissue and Bone Sarcoma Group. Eur J Cancer. 2015;51:127–136. [DOI] [PubMed] [Google Scholar]
- 11.Kasper B, Baumgarten C, Garcia J, Bonvalot S, Haas R, Haller F, Hohenberger P, Penel N, Messiou C, van der Graaf WT, Gronchi A, Bauer S, Blay JY, van Coevorden F, Dileo P, Dürr HR, Fiore M, Grünwald V, Jones R, Judson I, Kettelhack C, Kopeckova K, Lazar A, Lindner LH, Martin-Broto J, Rutkowski P, Stacchiotti S, Stoeckle E, Valverde C, Verhoef K, Wardelmann E, Wartenberg M. An update on the management of sporadic desmoid-type fibromatosis: A European Consensus Initiative between Sarcoma PAtients EuroNet (SPAEN) and European Organization for Research and Treatment of Cancer (EORTC)/Soft Tissue and Bone Sarcoma Group (STBSG). Ann Oncol . 2017;28:2399–2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kasper B, Gruenwald V, Reichardt P, Bauer S, Rauch G, Limprecht R, Sommer M, Dimitrakopoulou-Strauss A, Pilz L, Haller F, Hohenberger P. Imatinib induces sustained progression arrest in RECIST progressive desmoid tumours: Final results of a phase II study of the German Interdisciplinary Sarcoma Group (GISG). Eur J Cancer. 2017;76:60–67. [DOI] [PubMed] [Google Scholar]
- 13.Kortlever JT, Janssen SJ, van Berckel MM, Ring D, Vranceanu AM. What is the most useful questionnaire for measurment of coping strategies in response to nociception? Clin Orthop Relat Res . 2015;473:3511–3518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kummar S, Coyne GOS, Do KT, Turkbey B, Meltzer PS, Polley E, Choyke PL, Meehan R, Vilimas R, Horneffer Y, Juwara L, Lih A, Choudhary A, Mitchell SA, Helman LJ, Doroshow JH, Chen AP. Clinical activity of the γ-secretase inhibitor PF-03084014 in adults with desmoid tumors (aggressive fibromatosis). J Clin Oncol . 2017;35:1561–1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Merchant NB, Lewis JJ, Woodruff JM, Leung DHY, Brennan MF. Extremity and trunk desmoid tumors: A multifactorial analysis of outcome. Cancer. 1999;86:2045–2052. [PubMed] [Google Scholar]
- 16.Mullen JT, DeLaney TF, Kobayashi WK, Szymonifka J, Yeap BY, Chen YL, Rosenberg AE, Harmon DC, Choy E, Yoon SS, Raskin KA, Nielsen GP, Hornicek FJ. Desmoid tumor: Analysis of prognostic factors and outcomes in a surgical series. Ann Surg Oncol . 2012;19:4028–4035. [DOI] [PubMed] [Google Scholar]
- 17.Nuyttens JJ, Rust PF, Thomas CR, Turrisi a T. Surgery versus radiation therapy for patients with aggressive fibromatosis or desmoid tumors: A comparative review of 22 articles. Cancer. 2000;88:1517–1523. [PubMed] [Google Scholar]
- 18.Penel N, Le Cesne A, Bonvalot S, Giraud A, Bompas E, Rios M, Salas S, Isambert N, Boudou-Rouquette P, Honore C, Italiano A, Ray-Coquard I, Piperno-Neumann S, Gouin F, Bertucci F, Ryckewaert T, Kurtz J-E, Coindre J-M, Blay J-Y. Surgical versus non-surgical approach in primary desmoid-type fibromatosis patients: A nationwide prospective cohort from the French Sarcoma Group. Eur J Cancer. 2017;83:125–131. [DOI] [PubMed] [Google Scholar]
- 19.Penel N, Le Cesne A, Bonvalot S, Giraud A, Bompas E, Rios M, Salas S, Isambert N, Boudou-Rouquette P, Honore C, Italiano A, Ray-Coquard I, Piperno-Neumann S, Gouin F, Bertucci F, Ryckewaert T, Kurtz JE, Ducimetiere F, Coindre JM, Blay JY. Surgical versus non-surgical approach in primary desmoid-type fibromatosis patients: A nationwide prospective cohort from the French Sarcoma Group. Eur J Cancer. 2017;83:125–131. [DOI] [PubMed] [Google Scholar]
- 20.Penel N, Le Cesne A, Bui BN, Perol D, Brain EG, Ray-Coquard I, Guillemet C, Chevreau C, Cupissol D, Chabaud S, Jimenez M, Duffaud F, Piperno-Neumann S, Mignot L, Blay J-Y. Imatinib for progressive and recurrent aggressive fibromatosis (desmoid tumors): an FNCLCC/French Sarcoma Group phase II trial with a long-term follow-up. Ann Oncol . 2011;22:452–7. [DOI] [PubMed] [Google Scholar]
- 21.Salas S, Dufresne A, Bui B, Blay JY, Terrier P, Ranchere-Vince D, Bonvalot S, Stoeckle E, Guillou L, Le Cesne A, Oberlin O, Brouste V, Coindre JM. Prognostic factors influencing progression-free survival determined from a series of sporadic desmoid tumors: A wait-and-see policy according to tumor presentation. J Clin Oncol . 2011;29:3553–3558. [DOI] [PubMed] [Google Scholar]
- 22.Santti K, Beule A, Tuomikoski L, Rönty M, Jääskeläinen A-S, Saarilahti K, Ihalainen H, Tarkkanen M, Blomqvist C. Radiotherapy in desmoid tumors. Strahlentherapie und Onkol . 2017;193:269–275. [DOI] [PubMed] [Google Scholar]
- 23.Sparber-Sauer M, Seitz G, von Kalle T, Vokuhl C, Leuschner I, Scheer M, Münter M, Ljungman G, Bielack SS, Niggli F, Ladenstein R, Klingebiel T, Fuchs J, Koscielniak E. Systemic therapy of aggressive fibromatosis in children and adolescents: Report of the Cooperative Weichteilsarkom Studiengruppe (CWS). Pediatr Blood Cancer. 2018;65:1–9. [DOI] [PubMed] [Google Scholar]
- 24.Spear MA, Jennings LC, Mankin HJ, Spiro IRAJ, Springfield DS, Gebhardt MC, Efird JT, Suit HD. Individualizing management of aggressive fibromatoses. Int J Radiat Oncol Biol Phys . 1998;40:637–645. [DOI] [PubMed] [Google Scholar]
- 25.Timbergen MJM, Franse LVVDP, Grünhagen DJ, Graaf WT Van Der. Identification and assessment of health-related quality of life issues in patients with sporadic desmoid-type fibromatosis : a literature review and focus group study. Qual Life Res . 2018;27:3097-3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsagozis P, Stevenson JD, Grimer R, Carter S. Outcome of surgery for primary and recurrent desmoid-type fibromatosis. A retrospective case series of 174 patients. Ann Med Surg . 2017;17:14–19. [DOI] [PMC free article] [PubMed] [Google Scholar]