Abstract
Background
Centralization of cancer care to high-volume facilities has been shown to improve the overall survival of patients with soft-tissue sarcomas. Current evidence regarding the impact of increased hospital volume on treatment patterns and survival rates for patients with primary malignant bone tumors remains limited. Understanding the facility volume-outcome relationship for primary malignant bone tumors will further discussion on ways to promote delivery of quality cancer care across the nation.
Questions/purposes
(1) Is there a difference in overall survival for patients with primary malignant bone tumors undergoing treatment at a high-volume facility (at least 20 patients per year) versus those treated at a low-volume facility (less than 20 patients per year)? (2) Do surgical treatment patterns (limb-salvage versus amputation) and margin status (positive versus negative) vary between high-volume and low-volume facilities?
Methods
The 2004 to 2015 National Cancer Database was queried using International Classification of Disease for Oncology topographical codes to identify patients undergoing treatment (surgery, chemotherapy, and/or radiation therapy) for primary malignant bone tumors of the extremities (C40.0-C40.3, C40.8, and C40.9) or pelvis (C41.4). Histologic codes were used to group the tumors into the following categories: osteosarcomas, Ewing’s sarcomas, chondrosarcomas, chordomas, and other or unspecified. Patients who did not receive any treatment (surgery, chemotherapy, and/or radiotherapy) at the reporting facility were excluded from the study. Facility volume was calculated based on the average number of patients per year for the entire study period. A preliminary stratified Cox regression model was used to identify evidence-based thresholds or cutoffs for high-volume and low-volume facilities, while adjusting for differences in patient, tumor, and treatment characteristics. We identified high-volume facilities as those treating at least 20 patients per year and low-volume facilities as those treating fewer than 20 patients per year. A Kaplan-Meier survival analysis was used to report overall unadjusted 5-year survival rates at high-volume and low-volume facilities. Multivariate Cox regression analyses were used to assess whether undergoing treatment at a high-volume facility was associated with a lower risk of overall mortality, after controlling for differences in baseline demographics, tumor presentation, and treatment characteristics. For patients undergoing surgery, multivariate regression models were used to evaluate whether patients receiving care in a high-volume facility were more likely to receive resections with limb salvage surgery than to receive amputation and whether facility volume was associated with a patient’s likelihood of having a positive or negative surgical margin.
Results
A total of 14,039 patients were included, 15% (2115) of whom underwent treatment in a high-volume facility. Patients undergoing treatment at a high-volume facility were more likely to be white, have tumors involving the pelvis, have larger tumor sizes, and have a higher tumor grade at presentation than those undergoing treatment at a low-volume facility. Unadjusted 5-year overall survival rates were greater for high-volume facilities than for low-volume facilities (65% versus 61%; p = 0.003). After controlling for differences in patient demographics, tumor characteristics (including histologic type, grade, stage, size, and location) and treatment factors, we found that patients treated at high-volume facilities had a slightly lower overall mortality risk than those treated at low-volume facilities (hazard ratio 0.85 [95% CI 0.77 to 0.93]; p < 0.001). Patients treated at high-volume facilities were also slightly more likely to undergo resection with limb-salvage surgery to than to undergo amputation (odds ratio 1.34 [95% CI 1.14 to 1.59]; p = 0.001). Patients undergoing surgical treatment at high-volume facilities also had a lower odds of having positive resection margins than those undergoing treatment at low-volume facilities (OR 0.56 [95% CI 0.44 to 0.72]; p < 0.001).
Conclusions
Patients undergoing treatment for primary malignant bone tumors at high-volume facilities experience a slightly better overall survival than those receiving treatment at low-volume facilities. Further research questioning the value of care at high-volume facilities is required before sweeping changes in regionalization can be considered.
Level of Evidence
Level III, therapeutic study.
Introduction
Evidence has shown an improvement in short-term and long-term patient complications and/or readmissions when patients receive care in a high-volume facility or hospital. This so-called volume-outcome relationship has been demonstrated for various surgical procedures, including total joint arthroplasty [14, 26, 28, 38], spine surgery [3], hip fracture care [23], coronary artery bypass grafting [21], and cancer surgery [13, 15, 18]. For oncologic operations specifically, health policy makers in the United States often have debated the need for centralization of these procedures to high-volume centers to ensure delivery of quality care [31]. However, most evidence advocating for the centralization of cancer care has revolved around abdominal oncologic procedures, such as those of the pancreas [17], liver [34], and colon [32].
Bone and soft-tissue sarcomas, although only accounting for 2.2% of all cancers, are associated with substantial morbidity and mortality [33]. These sarcomas are typically treated with a multidisciplinary approach to care, consisting of surgical treatment, radiotherapy, and/or systemic chemotherapy. Given the coordination between highly subspecialized team members, as well as the overall rarity of these diseases, providers have debated centralizing the care of these cancers at higher-volume facilities. Current evidence to support such a strategy is scant. Only recently did research studies seem to suggest that a higher facility volume is linked to improved survival of patients with soft-tissue sarcomas [1, 19, 22]. Although these studies had similar conclusions, they were limited by their arbitrary definitions of volume thresholds. In contrast, evidence regarding the volume-outcome relationship in malignant bone tumors is limited. Having a better understanding about the facility volume-outcome relationship in primary malignant bone tumors will help health policy makers in further discussion on ways to promote delivery of quality cancer care nationwide.
In the light of these observations, we used a validated national cancer dataset to answer our proposed questions: (1) Is there a difference in overall survival for patients with primary malignant bone tumors undergoing treatment at a high-volume facility (at least 20 patients per year) versus those treated at a low-volume facility (less than 20 patients per year)? (2) Do surgical treatment patterns (limb-salvage versus amputation) and margin status (positive versus negative) vary between high-volume and low-volume facilities?
Patients and Methods
Database and Patient Selection
This study was a retrospective study of patients with cancer registered in the National Cancer Database (NCDB). Owned by the American Cancer Society and the American College of Surgeons, the NCDB gathers diagnostic, treatment, and outcome data from more than 70% of cancer patients across 1500 cancer programs in the United States [4]. The data are de-identified and made available to prospective researchers from participating institutions. To maintain the Commission on Cancer accreditation, the database requires participants to ensure at least a 90% annual follow-up rate on reported patients. This database was preferred over other available national cancer registries (such as, the Surveillance, Epidemiology and End Results database) as it offers unique facility identification numbers that allow researchers to conduct facility-level analyses. Further details about the NCDB can be found on its official website (https://www.facs.org/quality-programs/cancer/ncdb). Because the database was de-identified, this study was exempt from institutional review board approval.
We queried the 2004 to 2015 NCDB using International Classification of Disease for Oncology topographical codes to identify all patients undergoing treatment (surgery, chemotherapy, and/or radiotherapy) for primary malignant bone tumors of the extremities (C40.0-C40.3, C40.8, and C40.9) or pelvis (C41.4). Primary malignant spinal osseous tumors were not included for two main reasons. First, primary osseous spinal neoplasms are very rare tumors, with only about one to two patients per center every year, and they are not uniformly represented throughout the nation. Not all centers had patients with spinal osseous neoplasms. This is because these cancers are often operated on by orthopaedic spine surgeons and/or neurological surgeons rather than orthopaedic oncologists. We do know of certain scenarios where the main treatment (for example, surgery) is done in private practice setting by an orthopaedic or neurological spine surgeon, and the individual undergoes remainder of the treatment in another setting. Our observations have been supported by recent reports showing an absence of patients with spine tumors logged by early-career orthopaedic oncologists [11, 24]. All of these factors prevented us from getting a more homogenous patient sample that underwent complete treatment in a single facility, and they were thus excluded. We used histologic codes to group the tumors into the following categories: osteosarcomas, Ewing’s sarcomas, chondrosarcomas, chordomas, and other. Patients with benign tumors were excluded. Pediatric patients (age 18 years or younger) and adult patients (age older than 18 years) were both included because malignant bone tumors occur in both age groups. Patients who did not receive any treatment in the reporting facility (class of case 00) were excluded. The one patient with missing or unknown status at the last follow-up examination was right-censored during survival analysis. The 674 patients who did not receive any treatment (surgery, chemotherapy, and/or radiotherapy) were also excluded. The median range of follow-up was 39.4 months [interquartile range 16.4 to 77.1].
Included Variables
Baseline patient demographics and characteristics included in the analysis were age (stratified into 18 years or younger, 19-50 years, 51-75 years, and older than 75 years), sex, race (white, black, Asian, other, and unknown), insurance status (private, Medicare, other government, Medicaid, uninsured, and unknown), median household income (USD), proportion of patients without a high school education, Charlson comorbidity score, and distance from the facility (based on tertiles or three equal groups of 0-13 miles, 13-49 miles, and further than 49 miles).
Tumor characteristics included location (upper extremity, lower extremity, pelvis, and not specified), histologic type (osteosarcoma, chondrosarcoma, Ewing’s sarcoma, chordomas, and other [see Appendix, Supplemental Digital Content 1, http://links.lww.com/CORR/A252]), tumor size (0-8 cm, greater than 8 cm, or unknown), metastases at diagnosis, grade (I: well-differentiated or low-grade; II: moderately differentiated or intermediate-grade; III: poorly differentiated or high-grade; and IV: undifferentiated or high-grade and unknown), and American Joint Committee on Cancer clinical stage (I, II, III, IV, and unknown) [5].
Treatment variables included surgical procedure (no surgical procedure received, local excision or partial resection, resection with limb salvage, amputation, unspecified operation, and unknown), surgical margins (negative: R0; positive: R1 or R2; and unknown), and whether individuals received adjuvant therapy, including radiation therapy and/or chemotherapy.
Defining Evidence-based Thresholds for Facility Volume
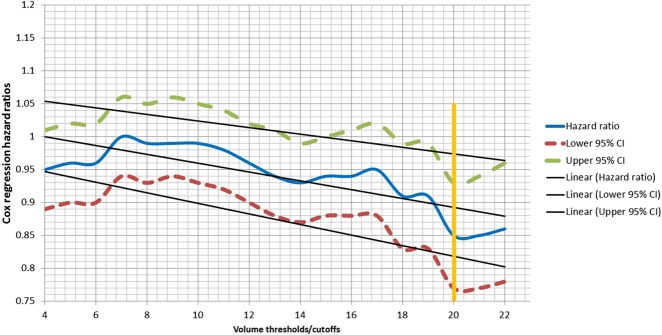
Facility volume was defined as the average annual number of malignant bone cancer patients treated in a facility throughout the study period (2004-2015). Unlike published reports investigating the volume-outcome relationship in surgical oncology [1, 9, 10, 22], we did not arbitrarily define volume thresholds for high-volume and low-volume facilities. Instead, we used an evidence-based approach to identify meaningful thresholds for high-volume and low-volume facilities. Similar to a spline regression analysis performed for categorical variable analyses [2, 7, 37], we performed a preliminary stratum-specific Cox regression hazard analysis assessing the incremental impact of increasing volume (four patients per year, five patients per year, six patients per year, and seven patients per year, up to 22 patients per year), while controlling for differences in baseline demographics, tumor characteristics, and treatment patterns. Adjusted hazard ratios and 95% CIs for each stratum (four patients per year, five patients per year, six patients per year, and seven patients per year, up to 22 patients per year) were plotted on a linear graph (Fig. 1). We identified a cutoff point at which there was a “dip” in the hazard ratio (HR)-volume curve (20 patients per year) and used it to divide facilities into two cohorts: high-volume (at least 20 patients per year) and low-volume (fewer than 20 patients per year) facilities. Similar evidence-based approaches to identifying meaningful cutoffs have been used for other elective orthopaedic procedures [27, 29, 36]. An evidence-based approach not only reduces variability induced by arbitrary or quartile-based definitions of volume cutoffs, but also allows health policy makers to better identify hospitals that could serve as centralization “hubs.”
Fig. 1.
This hazard ratio-volume linear curve shows a dip in overall mortality at 20 patients per year (HR 0.85 [95% CI 0.77 to 0.93]; p < 0.001).
Baseline Demographics, Tumor Characteristics, and Treatment Patterns
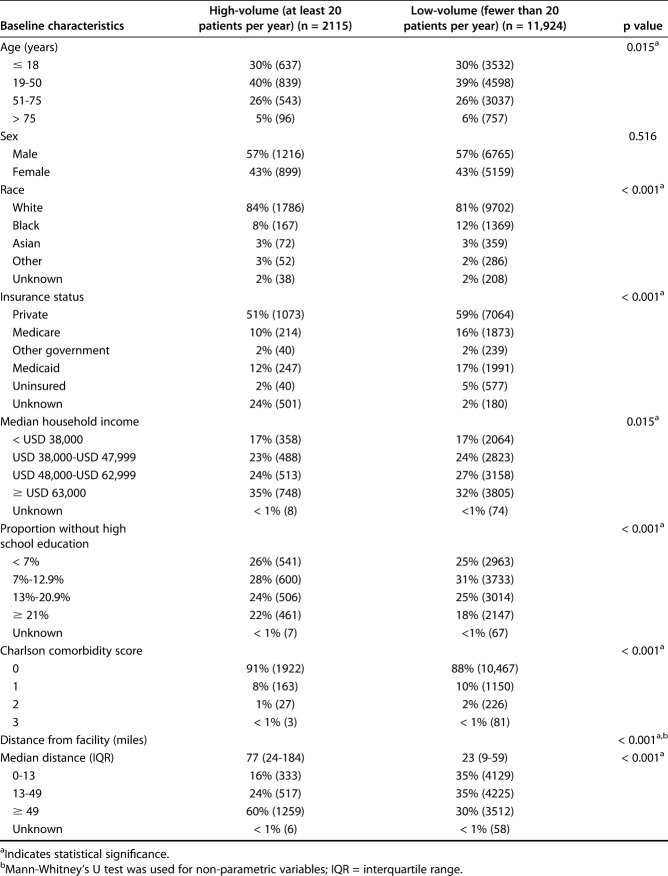
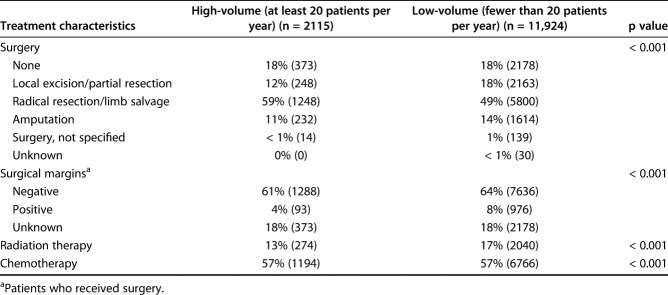
A total of 14,039 patients undergoing surgery in 840 hospitals were included, 15% (2115) of whom underwent treatment in at least one of six high-volume facilities; 85% of patients (11,924) patients were treated in a low-volume facility (n = 827 facilities) (Table 1). Patients undergoing treatment at a high-volume facility were more likely to be white, have tumors involving the pelvis, have a larger tumor size, and have a higher tumor grade at presentation than those who underwent treatment at a low-volume facility (Table 2). Limb-salvage surgery was more likely to be carried out in high-volume facilities (Table 3). High-volume facilities were also more likely to be located further away from the patient, at a median distance of 77 miles, than low-volume facilities, with a median distance of 22 miles.
Table 1.
Baseline patient demographics at high-volume and low-volume facilities
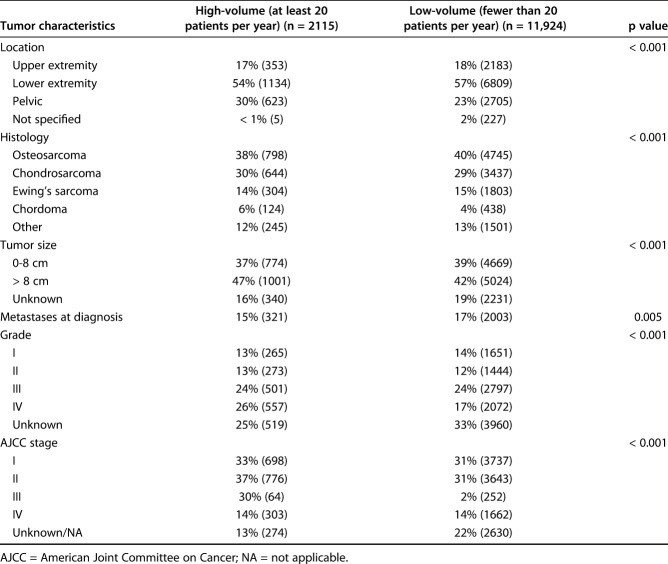
Table 2.
Differences in tumor characteristics between high-volume and low-volume facilities
Table 3.
Treatment characteristics of patients at high-volume and low-volume facilities
Statistical Analysis
We used Pearson’s chi-square tests to assess for unadjusted differences in baseline demographics, tumor characteristics, and treatment patterns between high-volume and low-volume facilities. A Kaplan-Meier survival analysis was used to compare 5-year overall survival rates between high-volume and low-volume facilities. It is important for readers to note that Kaplan-Meier survival analyses are unadjusted for any confounders, and therefore do not account for the variation in baseline demographics, tumor characteristics, and treatment between high-volume and low-volume facilities. Therefore, multivariate Cox regression analyses, adjusted for baseline demographics, tumor characteristics and treatment patterns, were used to assess whether undergoing treatment at a high-volume facility was associated with improved overall survival. We ran an additional sensitivity analysis using Cox regression, after excluding data from variables with at least 5% of missing/unknown information (insurance status, surgical margins, tumor size, grade and stage). We also used multivariate regression models to evaluate whether patients receiving surgical care in a high-volume facility were more likely to undergo resections with limb salvage surgery or an amputation, and whether facility volume was associated with a patient’s likelihood of having positive resection margins. All statistical analyses were performed using SPSS version 24 (IBM Corp, Armonk, NY, USA). The results of the Cox regression and multivariate logistic regression models are reported as adjusted HR and odds ratios, respectively, along with their 95% CIs. For all statistical analyses, a p value less than 0.05 was considered statistically significant.
Results
Association of Facility Volume with Overall Survival
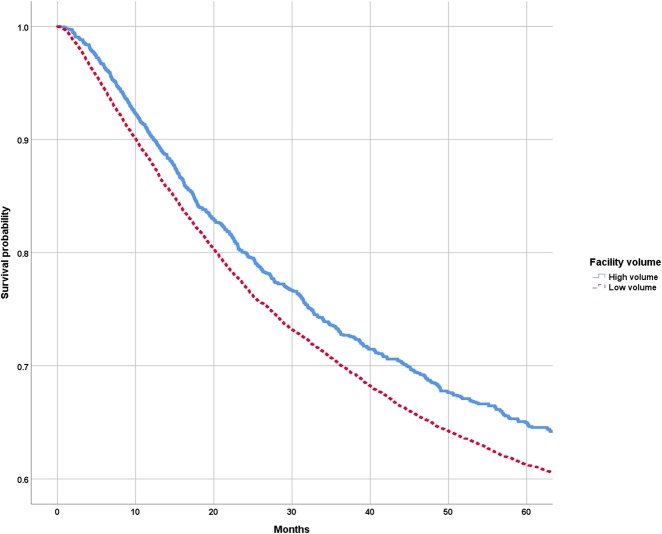
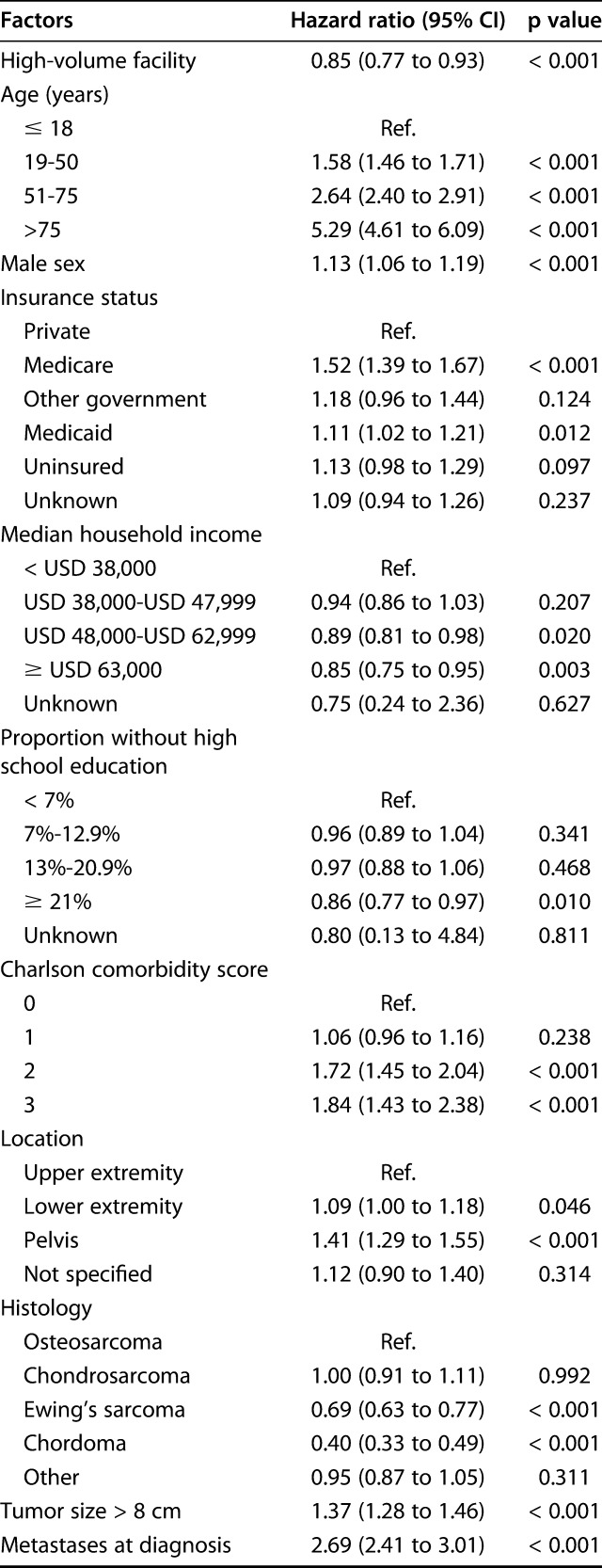
The Kaplan-Meier survival analysis showed that patients undergoing treatment in high-volume facilities had a longer mean survival time than those undergoing treatment at low-volume facilities (107.4 months [95% CI 103.9 to 110.8] versus 102.6 months [95% CI 101.1 to 104.1]; p < 0.001). Overall 5-year survival rates were also greater for high-volume facilities than for low-volume facilities (65% [standard error 1.2] versus 61% [standard error 0.5]; p = 0.003) (Fig. 2). After controlling for differences in patient demographics, tumor characteristics (including histologic type), and treatment factors (including the type of surgery, radiation therapy or chemotherapy) in the multivariate Cox regression model, we found that patients treated at high-volume facilities had a slightly lower overall risk of mortality than those undergoing treatment at low-volume facilities (HR 0.85 [95% CI 0.77 to 0.93]; p < 0.001) (Table 4). Other important risk factors associated with an increased risk of overall mortality were increasing age, higher Charlson comorbidity index score, pelvic or lower-extremity tumor location, tumor size greater than 8 cm, having metastases at diagnosis, increasing grade and stage, and presence of positive surgical margins (Table 4). A sensitivity analysis that excluded missing/unknown data for insurance status, surgical margins, tumor size, stage and grade, showed a consistent observation of treatment at high-volume facilities associated with a lower overall risk of mortality (HR 0.78 [95% CI 0.68 to 0.89]; p < 0.001). Regarding chondrosarcoma patients, a total of 1459 patients underwent treatment for well-differentiated chondrosarcomas. After excluding patients with well-differentiated chondrosarcomas from the analysis, we still noted that patients undergoing treatment at high-volume facility was associated with lower overall risk of mortality (HR 0.85 [95% CI 0.77 to 0.93]). This was essentially the same as our overall results, as grade was adjusted for as a confounder during prior analysis.
Fig. 2.
Kaplan-Meier survival curves show 5-year survival rates for patients undergoing treatment at high-volume and low-volume facilities.
Table 4.
Multivariate Cox regression hazards showing significant factors associated with a higher overall risk of mortality
Variation in Treatment Patterns and Margin Status Between High-volume and Low-volume Facilities
After controlling for patient demographics, tumor characteristics (including American Joint Committee on Cancer stage, tumor size, and tumor grade) and other treatments (radiation therapy or chemotherapy), we found patients treated at high-volume facilities were slightly more likely to undergo resection with limb salvage surgery than to undergo amputation (OR 1.34 [95% CI 1.14 to 1.59]; p = 0.001). Patients undergoing surgical treatment at high-volume facilities also had lower odds of having positive resected margins than those undergoing treatment at low-volume facilities (OR 0.56 [95% CI 0.44 to 0.72]; p < 0.001). With the numbers we had, we could not find differences in the use of radiation therapy (OR 0.94 [95% CI 0.78 to 1.13]; p = 0.519) and chemotherapy (OR 0.89 [95% CI 0.73 to 1.09]; p = 0.262) between high-volume and low-volume facilities.
Discussion
As the current healthcare system evolves toward implementing value-based approaches, there is an increased emphasis on improving the delivery of oncology care while controlling healthcare costs. With evidence consistently showing that higher facility volume is associated with better survival, centralization of cancer care at high-volume facilities or centers of excellence has long been advocated and, to some extent, has been successfully implemented for complex general surgical oncology [32]. Centralization efforts largely remain unheard of in the bone sarcoma community, either because of the relative dearth of reports supporting the presence of a volume-outcome relationship for these cancers or due to the complex logistical and financial issues associated with access to care at high-volume facilities. Using a national cancer dataset, we demonstrated that individuals with primary malignant bone tumors undergoing treatment at a high-volume facility were likely to have a slightly better overall survival than those receiving care at low-volume facilities. Furthermore, patients had a slightly higher risk-adjusted odds of undergoing limb salvage procedures versus amputations if treated in high-volume facilities and had lower rates of positive resection margins.
Limitations
There are several important limitations to our study. First, we only investigated the impact of hospital volume on long-term outcomes and did not evaluate the effect of individual surgeon volumes. The NCDB does not contain individual surgeon identifiers that would allow an appropriate calculation of surgeon volume. As mentioned, malignant bone tumors are often treated by a multidisciplinary approach to care, involving input from and coordination between the surgical teams, radiologists, and medical and radiation oncologists. Although surgery is an important facet of the overall management and treatment of malignant bone tumors, radiation therapy and systematic chemotherapy play a critical role in determining the survival of most patients with bone tumors. Therefore, overall facility volume rather than surgeon volume may be a better metric for determining the volume-outcome relationship and evaluating the quality of oncology care. Regardless, musculoskeletal tumor societies need to advocate for the construction of registries that capture surgeon-level/physician-level data to allow researchers to further investigate the surgeon-volume outcome relationship.
Second, the NCDB does not report data on recurrence rates and/or disease-free survival, which would have been useful for further understanding the long-term impact of the volume-outcome relationship on patients with malignant bone tumors. The database also lacks data on the costs associated with the entire episode of care, which would have allowed us to better understand the financial ramifications of centralization policies. Although we included a comprehensive set of demographic, tumor, and treatment variables that are known to impact survival, the database does not contain functional outcome scores. We also did not have surgery-specific data on patients with pelvic, acetabular, or lower-extremity tumors who underwent reconstructive procedures (arthroplasty or rotationplasties). The NCDB also does not report whether facilities/surgeons adhered to guideline-driven care, which would have been an important metric for determining quality. Furthermore, the histologic grade, surgical margin, and American Joint Committee on Cancer stage was unknown for several patients. To prevent a selection bias and to maintain an appropriate study sample, we chose to include these patients and adjust for them in subsequent multivariate regression analyses. This approach to managing unknown data has been used in other studies [12, 16]. To further support our findings, we ran an additional sensitivity analysis that excluded missing data on insurance status, surgical margins, tumor size, stage and grade and noted a similar trend toward better survival when treatment was received at a high-volume facility compared with a low-volume facility. Regardless, the possibility of bias due to missing data should not be ruled out [20].
We also did not assess differences in the sequence of radiation therapy and chemotherapy because this was beyond the scope of this study. We did not analyze differences in chemotherapy regimens over time and/or participation in clinical trials between high-volume and low-volume facilities because this information is not available in the database. The NCDB only records the definitive surgical treatment if more than one procedure was performed. For instance, if a patient underwent a prior limb-salvage surgery, and then underwent a subsequent amputation, the database will only contain information about the latter operation We also did not include or adjust for the effect of facility type (academic or integrated cancer network or community cancer program) or hospital region on survival outcomes. This was because the NCDB purposely does not report data regarding facility type and region for individuals younger than 40 years. Nearly 56% of our study population was younger than 40 years, and an attempt to include facility type and region would have introduced a bias to our results. The NCDB only reports endpoint follow-up status as “alive” or “dead,” making it difficult to understand disease-specific survival rates. Finally, although the NCDB provided an adequate sample size of more than 14,000 malignant bone tumors, it only recorded data from more than 1500 hospitals across the United States and does not represent the entire national cohort.
Survival is Slightly Higher Among Patients Treated at High-volume Centers
After controlling for confounding variables (including baseline demographics, tumor characteristics and treatment patterns), we observed that undergoing treatment for malignant bone tumors at a high-volume facility was associated with better overall survival. Only one prior report, from Japan, has evaluated the volume-outcome relationship in malignant bone tumors undergoing surgical treatment [25]. The authors noted that higher hospital volume was associated with a lower rate of postoperative complications and in-hospital mortality; however, they did not evaluate long-term survival. Although no prior study has investigated the volume-survival association in malignant bone tumors, our findings are largely similar to previously reported volume-outcome relationships on soft-tissue sarcomas. Using the NCDB and an arbitrary definition of a high-volume facility of at least 10 patients per year, Abarca et al. [1] found that patients undergoing surgical treatment for non-metastatic soft-tissue sarcomas of the extremities at low-volume facilities had lower odds of overall survival at 5 years than those undergoing treatment at high-volume facilities. In a similar NCDB analysis, Lazarides et al. [22] used a high-volume facility cutoff of at least 20 patients per year and concluded that higher volume is associated with a lower odds of overall mortality. Though our findings add to prior reports showing the presence of a volume-outcome association in orthopaedic oncology, readers should interpret the results with caution. This is important given that there was only a slight improvement in survival when treatment was sought in a high-volume center, as evidenced by the lower end of the confidence interval of the hazard risk ratio reaching 1 (HR 0.85 [95% CI 0.77 to 0.93]). Health policy makers and surgeons should factor in the slight improvement in survival when considering centralization of bone sarcoma care, the challenges of which will be discussed in following paragraphs.
Differences in Limb Salvage and Margin Status Between High- and Low-volume Centers
We found a slightly greater likelihood of patients receiving limb-salvage surgery at high-volume facilities than low-volume facilities. Furthermore, we also observed that patients who underwent surgical treatment for malignant bone tumors at high-volume facilities had lower odds of having positive resected margins. Even though our analysis was adjusted for various confounders (including tumor characteristics, such as stage, size and/or location), as with most database studies, readers should be cautious about deriving causality from the findings. Due to the availability of resources, high-volume facilities may have more experienced surgeons and more readily available multidisciplinary surgical teams (such as plastic surgery) that make limb salvage a feasible option. In addition, patients undergoing limb salvage tend to benefit from specialized postoperative care, primarily intense rehabilitation, to ensure adequate functional outcomes. High-volume facilities may have structured rehabilitation programs that positively influence patient outcomes and bias providers and patients to elect limb salvage surgery. Similarly, high-volume facilities, due to a large pool of patients, will perform a greater number of radical resection/limb salvage procedures, and through the development of a learning curve/experience, should have a better survival in their patients. Our findings are somewhat similar to prior volume-outcome studies on soft-tissue sarcomas [1, 22]. Using the NCDB both Lazarides et al. [22] and Abarca et al. [1] observed that high-volume facilities had lower odds of positive resected margins, but there was no preferential bias towards choosing limb-salvage surgery over amputations.
Challenges of Centralization
In our analysis, six high-volume facilities delivered the highest quality care for only 2000 patients (15% of the entire sample). In contrast, nearly 85% of individuals received care in a low-volume facility, with approximately 34% of these patients receiving care in a facility that treated, on average, only five patients per year. Although centralization of bone sarcoma care may be an interesting avenue to improve delivery of quality care, we were only able to detect a slight improvement in overall survival when malignant bone tumors were treated at a high-volume facility, as evidenced by the lower end of the confidence interval nearing 1 (HR 0.85 [0.77 to 0.93]. As mentioned previously, the small improvement in overall survival should be interpreted with caution when translating the findings into health policy changes. This is particularly important given that the current healthcare system is undergoing a shift towards value-based care, with an emphasis to maintain quality of care while controlling/reducing costs.
A recently published study used a state-wide cancer registry to investigate the value of undergoing treatment a high-volume center to improve survival after pancreatic cancer [8]. The authors defined high-value care as care that resulted in overall survival of at least the fourth quintile (≥ 26 months) and cost of care not to exceed the second quintile (≤ USD 40,674). Interestingly, the study showed that even though undergoing care at a high-volume facility was associated with a slight improvement in survival (HR 0.78 [95% CI 0.61 to 0.99], the care did not increase survival or control costs enough to be defined as of high-value. Because we did not have access to cost-based data in the NCDB, the findings of the pancreatic cancer study highlight the need for a similar cost-based survival analysis of patients with musculoskeletal tumors to facilitate discussion on whether centralization will indeed drive value in orthopaedic oncology. Rather than addressing the logistical and financial issues fraught with expanding the capacity of high-volume facilities, we feel that improving/establishing referral policies at low-volume facilities (that is, five patients or fewer per year) to transfer patients to relatively higher volume facilities (that is, 15 or 16 patients per year) would eventually increase the pool of high-volume facilities and be a better way of centralizing care. Transferring patients with complex conditions (such as a greater comorbidity burden, larger tumor size, higher-grade tumor, and/or pelvic location) to higher-volume centers may also be a feasible approach to addressing centralization. However, geographic variation of high-volume cancer centers will ultimately be a major obstacle in the delivery of quality cancer care. In our study, nearly 60% of all high-volume facilities were located at least 49 miles from the patient’s home. Not all patients have sufficient means to travel to high-volume centers, even though prior research in esophageal cancers has shown that travel to high-volume centers may improve survival compared with undergoing treatment at a nearby low-volume center [30]. The study also went on to report that patients who traveled to high-volume centers for esophageal cancer treatment were typically younger, white individuals with lower comorbidity burden, private insurance, and a lower clinical stage at presentation [30].
In the light of latter observations, health-policy makers should consider that centralization may actually introduce barriers to access-of-care for vulnerable patient populations (such as, black individuals or uninsured and/or Medicaid patients). Such disparities are also reflected in our study, with black individuals more likely to undergo treatment in a low-volume versus high-volume facility (12% versus 8%). Furthermore, compared with low-volume facilities, high-volume facilities catered to a lower proportion of Medicaid (12% versus 17%) and uninsured individuals (2% versus 5%). As the federal government begins to address underlying racial and socioeconomic disparities in health care, health policy makers must ensure that centralization policies aim for a more uniform distribution of high-volume facilities to keep care equitable and accessible to all.
According to the 2017 American Society of Clinical Oncology report [6], only 6% of oncologists practice in rural areas. Revamping the infrastructure by ensuring providers are present in “oncology deserts” could be an effective way of improving access to care for vulnerable patients with malignant bone tumors. Unfortunately, a major obstacle to increasing the number of surgical providers for patients with malignant bone tumors is the low number of active, fellowship-trained orthopaedic oncologists in the United States [35]. Although there has been a relative increase in the number of orthopaedic oncology fellowships in the nation over the past decade, practice spectrum and employment opportunities are limited. Conversely, increasing the number of orthopaedic oncologists may also result in a division of cases, preventing young surgeons from gaining exposure and training expertise associated with a large-volume practice, leading to an unintended decentralization of cancer care. A recent editorial has raised issues regarding the need for reducing the number of fellowships [11], and/or concentrating training at the highest volume sarcoma centers to ensure that early-career orthopaedic oncologists have enough breadth of cases to build a healthy practice.
Conclusions
By analyzing evidence from a large national sample of patients with primary malignant bone tumors, our study suggests that there is a slight survival benefit associated with undergoing treatment at a high-volume facility. However, we also noted that most of the bone sarcoma care is provided in low-volume centers. One solution to this issue that has been proposed is to create centralized tumor centers in this country that could potentially improve sarcoma care, but given the challenges associated with centralization, there is a need for further prospective study using more granular clinical data to facilitate next-level discussions on whether centralization will indeed drive value in bone sarcoma care.
Acknowledgments
None.
Footnotes
Each author certifies that neither he, nor any member of his immediate family, has funding or commercial associations (consultancies, stock ownership, equity interest, patent/licensing arrangements, etc.) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research® editors and board members are on file with the publication and can be viewed on request.
Each author certifies that his institution waived approval for the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
This work was performed at the James Cancer Hospital and Solove Research Institute, The Ohio State University Wexner Medical Center, Columbus, OH, USA.
References
- 1.Abarca T, Gao Y, Monga V, Tanas MR, Milhem MM, Miller BJ. Improved survival for extremity soft tissue sarcoma treated in high-volume facilities. J Surg Oncol. 2018;117:1479-1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adam MA, Thomas S, Youngwirth L, Hyslop T, Reed SD, Scheri RP, Roman SA, Sosa JA. Is there a minimum number of thyroidectomies a surgeon should perform to optimize patient outcomes? Ann Surg. 2017;265:402-407. [DOI] [PubMed] [Google Scholar]
- 3.Adkins ZB, Malik AT, Jain N, Yu E, Kim J, Khan SN. Does hospital volume affect outcomes in spine surgeries? A systematic review. Clin Spine Surg. 2019;32:285-294. [DOI] [PubMed] [Google Scholar]
- 4.American College of Surgeons. National Cancer Database. 2019. Available at: https://www.facs.org/quality-programs/cancer/ncdb. Accessed September 3, 2019.
- 5.American Joint Comission on Cancer. Cancer Staging Manual. 2019. Available at: https://cancerstaging.org/references-tools/deskreferences/pages/default.aspx. Accessed September 3, 2019.
- 6.American Society of Clinical Oncology. Cancer Care by ZIP Code: Examining Geographic Health Disparities in the United States. 2019. Available at: https://connection.asco.org/magazine/features/cancer-care-zip-code-examining-geographic-health-disparities-united-states. Accessed September 4, 2019.
- 7.Anderson KL, Jr., Thomas SM, Adam MA, Pontius LN, Stang MT, Scheri RP, Roman SA, Sosa JA. Each procedure matters: threshold for surgeon volume to minimize complications and decrease cost associated with adrenalectomy. Surgery. 2018;163:157-164. [DOI] [PubMed] [Google Scholar]
- 8.Bateni SB, Gingrich AA, Hoch JS, Canter RJ, Bold RJ. Defining value for pancreatic surgery in early-stage pancreatic cancer. JAMA Surg. 2019:e193019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beal EW, Mehta R, Hyer JM, Paredes A, Merath K, Dillhoff ME, Cloyd J, Ejaz A, Pawlik TM. Association between travel distance, hospital volume, and outcomes following resection of cholangiocarcinoma. J Gastrointest Surg. 2019;23:944-952. [DOI] [PubMed] [Google Scholar]
- 10.Beal EW, Mehta R, Merath K, Tsilimigras DI, Hyer JM, Paredes A, Dillhoff ME, Cloyd J, Ejaz A, Pawlik TM. Outcomes after resection of hepatocellular carcinoma: Intersection of travel distance and hospital volume. J Gastrointest Surg. 2019. [DOI] [PubMed] [Google Scholar]
- 11.Biermann JS. CORR Insights(R): how much tumor surgery do early-career orthopaedic oncologists perform? Clin Orthop Relat Res. 2015;473:703-705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bovonratwet P, Bohl DD, Russo GS, Ondeck NT, Nam D, Della Valle CJ, Grauer JN. How common-and how serious-is Clostridium difficile colitis after geriatric hip fracture? Findings from the NSQIP Dataset. Clin Orthop Relat Res. 2018;476:453-462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buettner S, Gani F, Amini N, Spolverato G, Kim Y, Kilic A, Wagner D, Pawlik TM. The relative effect of hospital and surgeon volume on failure to rescue among patients undergoing liver resection for cancer. Surgery. 2016;159:1004-1012. [DOI] [PubMed] [Google Scholar]
- 14.Courtney PM, Frisch NB, Bohl DD, Della Valle CJ. Improving value in total hip and knee arthroplasty: The role of high volume hospitals. J Arthroplasty. 2018;33:1-5. [DOI] [PubMed] [Google Scholar]
- 15.Gani F, Johnston FM, Nelson-Williams H, Cerullo M, Dillhoff ME, Schmidt CR, Pawlik TM. Hospital volume and the costs associated with surgery for pancreatic cancer. J Gastrointest Surg. 2017;21:1411-1419. [DOI] [PubMed] [Google Scholar]
- 16.Gingrich AA, Bateni SB, Monjazeb AM, Thorpe SW, Kirane AR, Bold RJ, Canter RJ. Extremity soft tissue sarcoma in the elderly: Are we overtreating or undertreating this potentially vulnerable patient population? J Surg Oncol. 2019;119:1087-1098. [DOI] [PubMed] [Google Scholar]
- 17.Gooiker GA, van der Geest LG, Wouters MW, Vonk M, Karsten TM, Tollenaar RA, Bonsing BA. Quality improvement of pancreatic surgery by centralization in the western part of the Netherlands. Ann Surg Oncol. 2011;18:1821-1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Greenup RA, Obeng-Gyasi S, Thomas S, Houck K, Lane WO, Blitzblau RC, Hyslop T, Hwang ES. The effect of hospital volume on breast cancer mortality. Ann Surg. 2018;267:375-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gutierrez JC, Perez EA, Moffat FL, Livingstone AS, Franceschi D, Koniaris LG. Should soft tissue sarcomas be treated at high-volume centers? An analysis of 4205 patients. Ann Surg. 2007;245:952-958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoskin TL, Boughey JC, Day CN, Habermann EB. Lessons learned regarding missing clinical stage in the National Cancer Database. Ann Surg Oncol. 2019;26:739-745. [DOI] [PubMed] [Google Scholar]
- 21.Kim LK, Looser P, Swaminathan RV, Minutello RM, Wong SC, Girardi L, Feldman DN. Outcomes in patients undergoing coronary artery bypass graft surgery in the United States based on hospital volume, 2007 to 2011. J Thorac Cardiovasc Surg. 2016;151:1686-1692. [DOI] [PubMed] [Google Scholar]
- 22.Lazarides AL, Kerr DL, Nussbaum DP, Kreulen RT, Somarelli JA, 3rd BDG, Brigman BE, Eward WC. Soft tissue sarcoma of the extremities: What is the value of treating at high-volume centers? Clin Orthop Relat Res. 2019;477:718-727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Malik AT, Panni UY, Masri BA, Noordin S. The impact of surgeon volume and hospital volume on postoperative mortality and morbidity after hip fractures: A systematic review. Int J Surg. 2018;54:316-327. [DOI] [PubMed] [Google Scholar]
- 24.Miller BJ, Rajani R, Leddy L, Carmody Soni EE, White JR, Musculoskeletal oncology research I. How much tumor surgery do early-career orthopaedic oncologists perform? Clin Orthop Relat Res. 2015;473:695-702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ogura K, Yasunaga H, Horiguchi H, Ohe K, Shinoda Y, Tanaka S, Kawano H. Impact of hospital volume on postoperative complications and in-hospital mortality after musculoskeletal tumor surgery: analysis of a national administrative database. J Bone Joint Surg Am. 2013;95:1684-1691. [DOI] [PubMed] [Google Scholar]
- 26.Pamilo KJ, Peltola M, Paloneva J, Makela K, Hakkinen U, Remes V. Hospital volume affects outcome after total knee arthroplasty. Acta Orthop. 2015;86:41-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ramkumar PN, Navarro SM, Haeberle HS, Ricchetti ET, Iannotti JP. Evidence-based thresholds for the volume-value relationship in shoulder arthroplasty: outcomes and economies of scale. J Shoulder Elbow Surg. 2017;26:1399-1406. [DOI] [PubMed] [Google Scholar]
- 28.Ricciardi BF, Liu AY, Qiu B, Myers TG, Thirukumaran CP. What is the association between hospital volume and complications after revision total joint arthroplasty: A large-database study. Clin Orthop Relat Res. 2019;477:1221-1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schoenfeld AJ, Sturgeon DJ, Burns CB, Hunt TJ, Bono CM. Establishing benchmarks for the volume-outcome relationship for common lumbar spine surgical procedures. Spine J. 2018;18:22-28. [DOI] [PubMed] [Google Scholar]
- 30.Speicher PJ, Englum BR, Ganapathi AM, Wang X, Hartwig MG, D'Amico TA, Berry MF. Traveling to a high-volume center is associated with improved survival for patients with esophageal cancer. Ann Surg. 2017;265:743-749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stitzenberg KB, Meropol NJ. Trends in centralization of cancer surgery. Ann Surg Oncol. 2010;17:2824-2831. [DOI] [PubMed] [Google Scholar]
- 32.Stitzenberg KB, Sigurdson ER, Egleston BL, Starkey RB, Meropol NJ. Centralization of cancer surgery: implications for patient access to optimal care. J Clin Oncol. 2009;27:4671-4678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ward WG, Corey RM. The Burden of Musculoskeletal Diseases in the United States. 2019. Available at: https://www.boneandjointburden.org/2013-report/tumors/ixa Accessed September 4, 2019.
- 34.Weledji EP. Centralization of liver cancer surgery and impact on multidisciplinary teams working on stage IV colorectal cancer. Oncol Rev. 2017;11:331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.White J, Toy P, Gibbs P, Enneking W, Scarborough M. The current practice of orthopaedic oncology in North America. Clin Orthop Relat Res. 2010;468:2840-2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wilson S, Marx RG, Pan TJ, Lyman S. Meaningful thresholds for the volume-outcome relationship in total knee arthroplasty. J Bone Joint Surg Am. 2016;98:1683-1690. [DOI] [PubMed] [Google Scholar]
- 37.Yu TH, Chou YY, Tung YC. Should we pay attention to surgeon or hospital volume in total knee arthroplasty? Evidence from a nationwide population-based study. PLoS One. 2019;14:e0216667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang W, Lyman S, Boutin-Foster C, Parks ML, Pan TJ, Lan A, Ma Y. Racial and ethnic disparities in utilization rate, hospital volume, and perioperative outcomes after total knee arthroplasty. J Bone Joint Surg Am. 2016;98:1243-1252. [DOI] [PubMed] [Google Scholar]