Abstract
Objective
To provide family physicians with an updated approach to diagnosis and treatment of pharyngitis, detailing key symptoms, methods of investigation, and a summary of common causes.
Sources of information
The approach described is based on the authors’ clinical practice and peer-reviewed literature from 1989 to 2018.
Main message
Sore throat caused by pharyngitis is commonly seen in family medicine clinics and is caused by inflammation of the pharynx and surrounding tissues. Pharyngitis can be caused by viral, bacterial, or fungal infections. Viral causes are often self-limiting, while bacterial and fungal infections typically require antimicrobial therapy. Rapid antigen detection tests and throat cultures can be used with clinical findings to identify the inciting organism. Pharyngitis caused by Streptococcus pyogenes is among the most concerning owing to its associated severe complications such as acute rheumatic fever and glomerulonephritis. Hence, careful diagnosis of pharyngitis is necessary to provide targeted treatment.
Conclusion
A thorough history is key to diagnosing pharyngitis. Rapid antigen detection tests should be reserved for concerns about antibiotic initiation. Physicians should exercise restraint in antibiotic initiation for pharyngitis, as restraint does not delay recovery or increase the risk of S pyogenes infections.
Sore throat and pharyngitis represent more than 2% and 5% of all outpatient primary care visits for adult and pediatric populations, respectively.1 It is characterized by inflammation of the pharynx, nasopharynx, and tonsillar tissues.2 Incidence peaks between late winter and early spring. Eighty percent of cases are caused by viral agents, while the remaining are bacterial and, rarely, fungal infections3 (Table 1). Herein, we provide an updated clinical review of pharyngitis for Canadian family physicians.
Table 1.
Most common organisms causing pharyngitis
| VIRAL PHARYNGITIS | BACTERIAL PHARYNGITIS | FUNGAL PHARYNGITIS |
|---|---|---|
| • Rhinovirus | • Streptococcus pyogenes (GAS) | • Candida albicans |
| • Adenovirus | • Haemophilus influenzae | |
| • Coxsackievirus | • Chlamydophila pneumoniae | |
| • Coronavirus | • Mycoplasma pneumoniae | |
| • Respiratory syncytial virus -Parainfluenza |
• Arcanobacterium haemolyticum • Neisseria gonorrhoeae |
|
| • Epstein-Barr virus | • Treponema pallidum | |
| • Orthomyxoviridae |
GAS—group A streptococcus.
Case description
Ms Z. is an 18-year-old woman presenting to the family medicine clinic with a 3-day history of sore throat and odynophagia. She denies having a cough or runny nose but has been febrile with intermittent chills. She denies recent sick contacts and has not traveled in the past 2 months. She had similar symptoms a few years ago, which were treated with antibiotics. She is hoping to obtain an antibiotic prescription to alleviate her symptoms. Given Ms Z.’s symptoms and probable fever in the absence of cough and rhinorrhea, pharyngitis is suspected.
Sources of information
The approach described is based on the authors’ clinical practice along with research and clinical review articles from 1989 to 2018.
Main message
Although viral pharyngitis is typically self-limiting with minimal sequelae, bacterial and fungal infections are more severe. Streptococcus pyogenes—group A streptococcus (GAS)—infections (“strep throat”) occur in up to 30% and 15% of sore throats in pediatric and adult populations, respectively.2 Group A streptococcus infections can have life-threatening complications in less than 0.015% of pediatric and 0.05% of adult patients.4,5 These can be separated into nonsuppurative (acute rheumatic fever, glomerulonephritis, pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections) and suppurative (peritonsillar abscess, septic jugular-vein thrombophlebitis, Vincent angina) complications that warrant urgent medical or surgical intervention.2,6
Preventing complications requires antimicrobial treatment, but growing antibiotic resistance has placed emphasis on minimizing antibiotic use.7 Unfortunately, differentiating bacterial pharyngitis from other infections is difficult.
Signs and symptoms.
Clinical differentiation of viral, bacterial, and fungal pharyngitis is challenging owing to similarities in presentation. Sore throat, odynophagia, and fever are all common features. These symptoms typically peak within 3 to 5 days and resolve by day 10.8 Although some pathogen-specific symptoms have been reported, predictive values have only been formulated for GAS pharyngitis (Table 2).2,3,9–14
Table 2.
Summary of common signs and symptoms of viral, bacterial, and fungal pharyngitis: Signs and symptoms of bacterial pharyngitis can overlap with those of streptococcal pharyngitis.
| PATHOGEN | SIGN OR SYMPTOM | POSITIVE LR (95% CI) | SPECIFICITY (95% CI) | SENSITIVITY (95% CI) |
|---|---|---|---|---|
| Viral | Cough3,9,10 | NA | NA | NA |
| Rhinorrhea3,9,10 | ||||
| Diarrhea3,9,10 | ||||
| Fatigue3,9,10 | ||||
| Conjunctivitis3,9,10 | ||||
| Tonsillar hypertrophy2 | ||||
| Oropharyngeal erythema or edema2 | ||||
| Pharyngeal “cobblestoning”2 | ||||
| Bacterial | Nausea and vomiting3,10,11 | NA | NA | NA |
| Headache3,10,11 | ||||
| Abdominal pain3,10,11 | ||||
| Group A streptococcus | Scarlatiniform rash3,10–12 | 3.91 (2.00–7.62) | 0.98 (0.95–0.99) | 0.08 (0.05–0.14) |
| Palatal petechiae11–13 | 2.69 (1.92–3.77) | 0.95 (0.91–0.97) | 0.15 (0.10–0.21) | |
| Tonsillar exudate11–13 | 1.53 (1.00–2.24) | 0.74 (0.64–0.83) | 0.38 (0.27–0.51) | |
| Arthralgia or myalgia11–13 | 1.42 (1.00–1.91) | 0.87 (0.70–0.95) | 0.18 (0.06–0.44) | |
| Cervical adenopathy11–13 | 1.40 (1.12–1.89) | 0.40 (0.23–0.61) | 0.82 (0.71–0.89) | |
| Fungal | Loss of taste14 | NA | NA | NA |
| Mouth numbness14 | ||||
| Oropharyngeal white curdlike plaques14 | ||||
| Oropharyngeal smooth red patches14 | ||||
| Angular cheilitis14 |
LR—likelihood ratio, NA—not available.
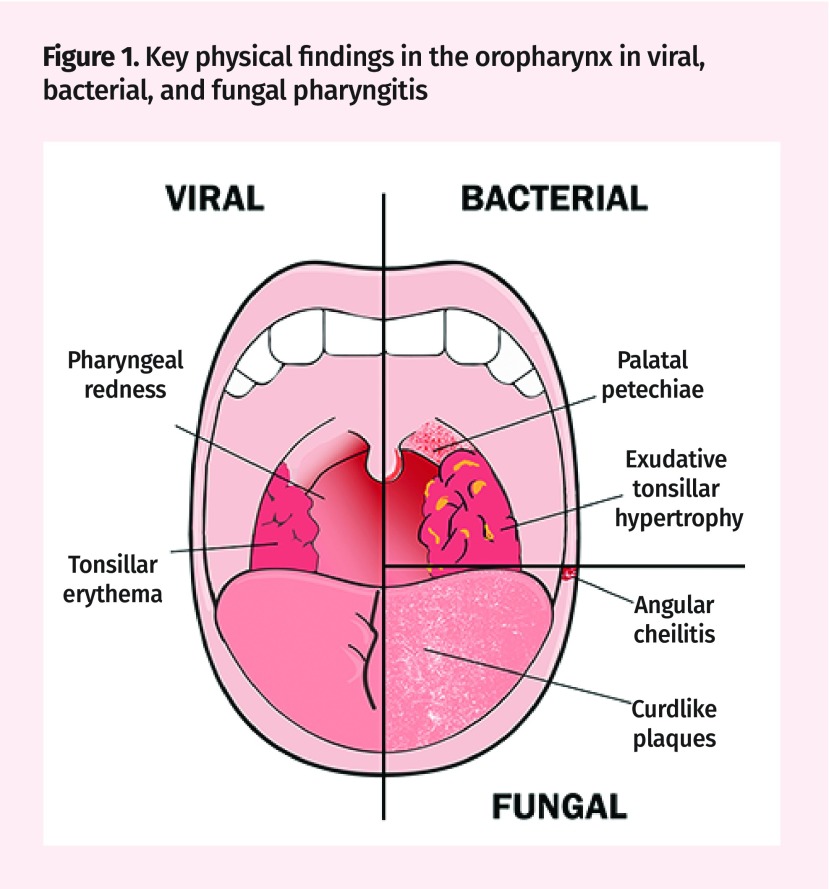
Physical findings can help guide diagnosis (Figure 1). Tonsillar hypertrophy, erythema, edema, or “cobblestoning” of the posterior pharynx suggest viral infections.2 Findings like upper-lip edema, splenomegaly, posterior cervical adenopathy, and polymorphic rashes increase suspicion for Epstein-Barr virus (EBV) infections.9,15 Bacterial pathogens might cause anterior cervical lymphadenopathy, sandpaperlike (scarlatiniform) rashes, tonsillar exudates, and palatal petechiae.16 Fungal pharyngitis presents with angular cheilitis and painful white curdlike plaques or smooth red patches within the oropharynx.14
Figure 1.
Key physical findings in the oropharynx in viral, bacterial, and fungal pharyngitis
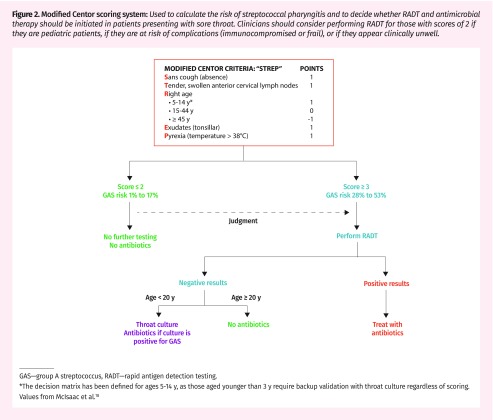
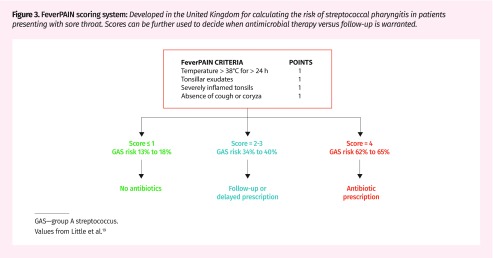
Patients can present with some or none of these signs and symptoms. Predictive algorithms have been developed to guide investigation and prevent antibiotic overprescribing by assigning signs and symptoms an aggregated pretest probability for bacterial pharyngitis.17 The modified Centor score (Figure 2) remains the most widely used method to work up streptococcal pharyngitis.18 Those with scores of 1 or less are at very low risk (< 10%), while those with scores of 4 or greater are at high risk (53%) of streptococcal infections. Alternatively, the FeverPAIN score (Figure 3) has gained popularity in the United Kingdom.19 It predicts strep throat based on acute symptom onset (< 3 days), recent (< 24 hours) fever, absence of cough or coryza, and purulent or inflamed tonsils. Scores below 2 to 3 have up to a 40% chance of streptococcal infection, and risk increases to up to 65% with a score of 4.19 This approach might be equivalent if not superior to the modified Centor score for reducing the need for diagnostic testing and antibiotics without negatively affecting patient outcomes.19
Figure 2.
Modified Centor scoring system: Used to calculate the risk of streptococcal pharyngitis and to decide whether RADT and antimicrobial therapy should be initiated in patients presenting with sore throat. Clinicians should consider performing RADT for those with scores of 2 if they are pediatric patients, if they are at risk of complications (immunocompromised or frail), or if they appear clinically unwell.
GAS—group A streptococcus, RADT—rapid antigen detection testing.
*The decision matrix has been defined for ages 5–14 y, as those aged younger than 3 y require backup validation with throat culture regardless of scoring. Values from McIsaac et al.18
Figure 3.
FeverPAIN scoring system: Developed in the United Kingdom for calculating the risk of streptococcal pharyngitis in patients presenting with sore throat. Scores can be further used to decide when antimicrobial therapy versus follow-up is warranted.
GAS—group A streptococcus.
Values from Little et al.19
Laboratory investigations.
Throat culture remains the criterion standard for bacterial pharyngitis diagnosis, with 97% to 100% specificity20 and 90% to 95% sensitivity.10 Unfortunately, culture of throat samples is difficult and can delay antibiotics.21 Cultures rarely influence antibiotic selection, as prescribing practices currently cover for GAS. Rather, they can rule out atypical infections such as non-GAS and fungal pharyngitis that require alternate antimicrobial regimens.22
Rapid antigen detection testing (RADT) affords same-visit diagnostics. These point-of-care tests detect bacterial and viral antigens from throat swabs taken from tonsillar exudates or the posterior oropharynx using dipsticks. Currently, they have been designed to rule in streptococcal infections, respiratory syncytial virus, and influenza.23–25 The specificity and sensitivity of RADT vary widely from 54% to 100% and 38% to 100%, respectively.2,23,25–27 Although results are immediate, each kit is pathogen-specific and cannot broadly differentiate between viral and bacterial pharyngitis. Hence, negative results cannot rule out non-GAS bacterial pharyngitis.
Antistreptolysin O titre tests are used for patients with suspected suppurative complications of GAS. However, they are not recommended in acute illness, as serologic markers peak 3 to 8 weeks after symptom onset.28,29
Individuals suspected of having EBV infections should receive mononucleosis spot testing. Despite having a sensitivity of 70% to 92% and specificity of 96% to 100%,30 there is a 25% false-negative rate when used in the first 10 days of presentation.31 Neisseria gonorrhoeae pharyngitis was traditionally diagnosed by oral swab culture; recently nucleic acid amplification tests for extragenital testing have been approved by Public Health Ontario, the Food and Drug Administration, and the Centers for Disease Control and Prevention.32,33
Clinical decision making.
Management of pharyngitis focuses on deciding whether to watch and wait, provide symptomatic treatment, or initiate antimicrobial therapy. This relies on accurate differentiation between bacterial and viral infections. Cultures effectively identify pathogens but should not delay or guide initial treatment in atypical presentations, as results have a 5- to 10-day latency and fail to distinguish those with acute infections from carriers. Alternatively, RADT technology is specific but equally should not guide management in isolation, as its sensitivity can be variable and RADT lacks high-quality evidence in the pediatric population.23 Negative RADT results in patients aged 5 to 15 should be verified with a throat culture.34 Moreover, children younger than 3 should not be tested unless there is a high chance of GAS exposure, as incidence within this population is less than 14% and infection rarely causes acute rheumatic fever.35
Approximately 7% of pediatric and 20% of adult patients are asymptomatic and noninfectious carriers of GAS.36 Superfluous antibiotic use can lead to unnecessary side effects and increase health care costs. The Infectious Diseases Society of America 2012 guidelines suggest that the modified Centor score could guide laboratory testing and antimicrobial therapy.10,34 Symptomatic treatment is recommended for scores of 1 while antimicrobial treatment guided by RADT or culture is advised for scores of 2 to 3 (Figure 2).18 Unfortunately, this tool has a 54% specificity in patients aged 3 to 14.37 Clinicians should exercise caution when applying this schema within this population owing to limited diagnostic accuracy.12,37
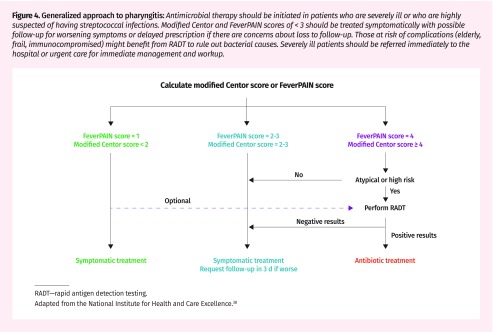
The National Institute for Health and Care Excellence endorses a combination of the modified Centor and FeverPAIN scores to guide follow-up and initiation of antimicrobial prescriptions (Figure 4).38 Low-risk patients are advised to receive symptomatic treatment with a 1-week follow-up. Delayed prescriptions with instructions to use if symptoms do not improve within 3 to 5 days are advised for patients with intermediate risk of GAS.38
Figure 4.
Generalized approach to pharyngitis: Antimicrobial therapy should be initiated in patients who are severely ill or who are highly suspected of having streptococcal infections. Modified Centor and FeverPAIN scores of < 3 should be treated symptomatically with possible follow-up for worsening symptoms or delayed prescription if there are concerns about loss to follow-up. Those at risk of complications (elderly, frail, immunocompromised) might benefit from RADT to rule out bacterial causes. Severely ill patients should be referred immediately to the hospital or urgent care for immediate management and workup.
RADT—rapid antigen detection testing.
Adapted from the National Institute for Health and Care Excellence.38
Traditionally, there has been a low threshold for treating pharyngitis owing to the risks of bacterial complications. There is arising evidence that delaying antimicrobial therapy by 3 days might not prolong illness recovery39,40 and that laboratory diagnostics cannot adequately differentiate subclinical bacterial carriers.41 The more conservative antimicrobial approach presented by the National Institute for Health and Care Excellence guidelines might be beneficial (Figure 4).38 With a focus on symptom management with close follow-up of cases with low pretest probability and delayed prescriptions in intermediate-risk groups, this strategy might decrease Canadian antibiotic use by as much as the 27% seen in the United Kingdom without increasing complication rates or mortality.41
These frameworks should guide, but not supersede, a physician’s clinical judgment. Testing and empirical treatment of the severely ill or those at increased risk of complications (eg, elderly, frail, or immunocompromised patients) should not be delayed. Physicians should also have a low threshold for suspecting suppurative complications, as they are life-threatening if untreated. These should be immediately treated along with urgent or emergency otolaryngologist consultation.
Treatment.
Clinical management depends on the inciting cause of pharyngitis but ultimately can be separated into symptomatic and antimicrobial therapy. Maintaining adequate hydration is critical, regardless of treatment strategy.
Viral pharyngitis: Treatment is conservative, as these infections are generally self-limiting. Oral corticosteroids for 1 to 2 days have been shown to reduce odynophagia (number needed to treat of 4) but they have no effect on the clinical course.6,42 Lozenges and benzocaine or lidocaine mouth rinses also provide mild pain relief by numbing the oropharynx.10 Nonsteroidal antiinflammatory drugs such as ibuprofen, along with acetaminophen, can be used to reduce pain and fever in adults and children.43 Acetylsalicylic acid is contraindicated in pediatric patients owing to the risk of Reye syndrome.10 Patients suspected of EBV infections should be advised to refrain from contact sports owing to the increased risk of splenic rupture secondary to EBV. Currently, there is no consensus on the length of restriction.44
Bacterial pharyngitis: Bacterial pharyngitis treatments focus on the eradication of GAS. A 6- to 10-day course of amoxicillin is the mainstay for candidates requiring antimicrobial therapy. A single intramuscular dose of benzathine penicillin G can alternatively be used if adherence is in question.3,27 The number needed to prevent 1 sore throat at 1 week using antibiotics in patients with a positive throat swab is 21. Historical data from before 1975 also suggest that antibiotics reduce the risk of rheumatic fever by 67%, but newer studies exploring this complication are required.45 Concurrent antibiotic-corticosteroid therapy is not indicated, as it does not improve pain and might delay recovery from bacterial pharyngitis.46
Patients with a type 4 penicillin or amoxicillin hypersensitivity (rash) requiring antibiotics should receive 10 days of cephalexin, clindamycin, or clarithromycin.3 Similarly, patients with β-lactamase type 1 hypersensitivity (anaphylaxis) can be prescribed a 5-day treatment of cefdinir or cefpodoxime.3 Cephalexin should be avoided in these patients, as there is a 2.5% risk of co-hypersensitivity to second-generation cephalosporins.47 Nonhypersensitivity maculopapular exanthems might appear in 70% of EBV-infected patients after amoxicillin, but do not require treatment.48 No statistical differences have been reported for symptom reduction between cephalosporin or macrolide treatments compared with penicillin.49
Atypical pharyngitis: Patients with infections refractory to first-line treatments can be treated for 72 hours with amoxicillin–clavulanic acid or clindamycin. If atypical bacteria such as N gonorrhoeae or Corynebacterium diphtheriae are suspected, patients should be started on ceftriaxone or erythromycin, respectively.3 Fungal pharyngitis should be suspected in immunocompromised patients and the elderly, for which fluconazole and miconazole treatments should be employed.14
Recurrent pharyngitis should be treated with penicillin-rifampin or cefpodoxime proxetil. Patients with recurrent episodes of streptococcal bacterial tonsillitis (> 7 in the past year, > 5 per year for the past 2 years, or > 3 per year for the past 3 years) can be referred to an otolaryngology–head and neck surgery specialist for consideration of tonsillectomy.35 Eradication for asymptomatic colonized carriers is currently not indicated.50 However, acute flares should be treated as concurrent infections requiring 10 days of clindamycin or penicillin-rifampin, or 1 dose of benzathine penicillin G and rifampin.51–54
Case resolution
Ms Z.’s lack of cough and rhinorrhea help to rule out sinusitis and postnasal drip. She appears to be distressed and in pain when swallowing but does not appear severely ill. She is febrile and examination reveals an enlarged cervical lymph node on her left side, along with bilateral tonsillar hypertrophy without exudates. Her pharynx appears red and inflamed. The modified Centor and FeverPAIN scores are both calculated to be 2. Viral pharyngitis is suspected, and RADT is not performed. Ms. Z is asked to take ibuprofen for pain and to maintain adequate hydration. She is instructed to return if her symptoms worsen over the next 3 days.
Conclusion
Pharyngitis is a common concern seen in primary care, caused by viral, bacterial, and fungal agents. The most concerning are S pyogenes infections, which can lead to suppurative and nonsuppurative complications. Diagnosis of the cause of pharyngitis is currently achieved through key clinical features seen in the modified Centor or FeverPAIN scoring systems in conjunction with RADT. Antibiotic stewardship and the low incidence of streptococcal pharyngitis complications suggest that treatments can be largely supportive. Empirical antibiotic use should be limited to patients who are severely ill, have a high risk of complications, or show no signs of improvement within 5 days of presentation.
Editor’s key points
▸ Sore throat and pharyngitis represent more than 2% and 5% of all outpatient primary care visits for adult and pediatric populations, respectively. Although viral pharyngitis is typically self-limiting with minimal sequelae, bacterial and fungal infections are more severe. The most concerning are Streptococcus pyogenes infections, which can lead to suppurative and nonsuppurative complications.
▸ Diagnosis of the cause of pharyngitis is primarily achieved using key clinical features seen in the modified Centor or FeverPAIN scoring systems and, sparingly, with rapid antigen detection testing.
▸ Antibiotic stewardship and the low incidence of streptococcal pharyngitis complications suggest that treatments can be largely supportive. Empirical antibiotic use should be limited to patients who are severely ill, have a high risk of complications, or show no signs of improvement within 5 days of presentation.
Footnotes
Contributors
Drs Sykes and Wu contributed equally to the literature review and its interpretation. All authors contributed to the interpretation of the literature and preparation of the manuscript for submission.
Competing interests
None declared
This article is eligible for Mainpro+ certified Self-Learning credits. To earn credits, go to www.cfp.ca and click on the Mainpro+ link.
This article has been peer reviewed.
La traduction en français de cet article se trouve à www.cfp.ca dans la table des matières du numéro d’avril 2020 à la page e119.
References
- 1.Sadeghirad B, Siemieniuk RAC, Brignardello-Petersen R, Papola D, Lytvyn L, Vandvik PO, et al. Corticosteroids for treatment of sore throat: systematic review and meta-analysis of randomised trials. BMJ. 2017;358:j3887. doi: 10.1136/bmj.j3887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.ESCMID Sore Throat Guideline Group. Pelucchi C, Grigoryan L, Galeone C, Esposito S, Huovinen P, et al. Guideline for management of acute sore throat. Clin Microbiol Infect. 2012;18(Suppl 1):1–28. doi: 10.1111/j.1469-0691.2012.03766.x. [DOI] [PubMed] [Google Scholar]
- 3.Yoon YK, Park CS, Kim JW, Hwang K, Lee SY, Kim TH, et al. Guidelines for the antibiotic use in adults with acute upper respiratory tract infections. Infect Chemother. 2017;49(4):326–52. doi: 10.3947/ic.2017.49.4.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cars T, Eriksson I, Granath A, Wettermark B, Hellman J, Norman C, et al. Antibiotic use and bacterial complications following upper respiratory tract infections: a population-based study. BMJ Open. 2017;7(11):e016221. doi: 10.1136/bmjopen-2017-016221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nakhoul GN, Hickner J. Management of adults with acute streptococcal pharyngitis: minimal value for backup strep testing and overuse of antibiotics. J Gen Intern Med. 2013;28(6):830–4. doi: 10.1007/s11606-012-2245-8. Epub 2012 Oct 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sigra S, Hesselmark E, Bejerot S. Treatment of PANDAS and PANS: a systematic review. Neurosci Biobehav Rev. 2018;86:51–65. doi: 10.1016/j.neubiorev.2018.01.001. Epub 2018 Jan 6. [DOI] [PubMed] [Google Scholar]
- 7.Linder JA. Editorial commentary: antibiotics for treatment of acute respiratory tract infections: decreasing benefit, increasing risk, and the irrelevance of antimicrobial resistance. Clin Infect Dis. 2008;47(6):744–6. doi: 10.1086/591149. [DOI] [PubMed] [Google Scholar]
- 8.Wolford RW, Schaefer TJ. Pharyngitis. Treasure Island, FL: StatPearls Publishing; 2018. [Google Scholar]
- 9.Cunha BA. A positive rapid strep test in a young adult with acute pharyngitis: be careful what you wish for! IDCases. 2017;10:58–9. doi: 10.1016/j.idcr.2017.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shulman ST, Bisno AL, Clegg HW, Gerber MA, Kaplan EL, Lee G, et al. Clinical practice guideline for the diagnosis and management of group A streptococcal pharyngitis: 2012 update by the Infectious Diseases Society of America. Clin Infect Dis. 2012;55(10):e86–102. doi: 10.1093/cid/cis629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thai TN, Dale AP, Ebell MH. Signs and symptoms of group A versus non-group A strep throat: a meta-analysis. Fam Pract. 2018;35(3):231–8. doi: 10.1093/fampra/cmx072. [DOI] [PubMed] [Google Scholar]
- 12.Shaikh N, Swaminathan N, Hooper EG. Accuracy and precision of the signs and symptoms of streptococcal pharyngitis in children: a systematic review. J Pediatr. 2012;160(3):487–93.e3. doi: 10.1016/j.jpeds.2011.09.011. Epub 2011 Nov 1. [DOI] [PubMed] [Google Scholar]
- 13.Ebell MH, Smith MA, Barry HC, Ives K, Carey M. The rational clinical examination. Does this patient have strep throat? JAMA. 2000;284(22):2912–8. doi: 10.1001/jama.284.22.2912. [DOI] [PubMed] [Google Scholar]
- 14.Pankhurst CL. Candidiasis (oropharyngeal) BMJ Clin Evid. 2013;2013:1304. [PMC free article] [PubMed] [Google Scholar]
- 15.Mohseni M, Boniface MP. Mononucleosis. Treasure Island, FL: StatPearls Publishing; 2018. [PubMed] [Google Scholar]
- 16.Anthony R, Flores MTC. Pharyngitis. In: Bennett JE, Dolin R, Blaser MJ, editors. Mandell, Douglas, and Bennett’s principles and practice of infectious disease. 8th ed. Philadelphia, PA: Elsevier Saunders; 2015. pp. 753–9. [Google Scholar]
- 17.Shapiro DJ, Lindgren CE, Neuman MI, Fine AM. Viral features and testing for streptococcal pharyngitis. Pediatrics. 2017;139(5):e20163403. doi: 10.1542/peds.2016-3403. [DOI] [PubMed] [Google Scholar]
- 18.McIsaac WJ, Kellner JD, Aufricht P. Empirical validation of guidelines for the management of pharyngitis in children and adults. JAMA. 2004;291(13):1587–95. doi: 10.1001/jama.291.13.1587. Erratum in: JAMA 2005;294(21):2700. [DOI] [PubMed] [Google Scholar]
- 19.Little P, Hobbs FR, Moore M, Mant D, Williamson I, McNulty C, et al. PRImary care Streptococcal Management (PRISM) study: in vitro study, diagnostic cohorts and a pragmatic adaptive randomised controlled trial with nested qualitative study and cost-effectiveness study. Health Technol Assess. 2014;18(6):vii–xxv. 1–101. doi: 10.3310/hta18060. Erratum in: Health Technol Assess 2018;18(6):103–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Little P, Hobbs FD, Mant D, McNulty CA, Mullee M, PRISM investigators. Incidence and clinical variables associated with streptococcal throat infections: a prospective diagnostic cohort study. Br J Gen Pract. 2012;62(604):e787–94. doi: 10.3399/bjgp12X658322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tang S, Cowen U, Fader R, Duncan D, Quezada T, Walker K, et al. Diagnosis and antibiotic treatment of group a streptococcal pharyngitis in children in a primary care setting: impact of point-of-care polymerase chain reaction. BMC Pediatr. 2019;19(1):24. doi: 10.1186/s12887-019-1393-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kocoglu E, Karabay O, Yilmaz F, Ekerbicer H. The impact of incubating the throat culture for 72 h on the diagnosis of group A beta-hemolytic streptococci. Auris Nasus Larynx. 2006;33(3):311–3. doi: 10.1016/j.anl.2005.11.011. Epub 2006 Jan 23. [DOI] [PubMed] [Google Scholar]
- 23.Cheung L, Pattni V, Peacock P, Sood S, Gupta D. Throat swabs have no influence on the management of patients with sore throats. J Laryngol Otol. 2017;131(11):977–81. doi: 10.1017/S002221511700189X. Epub 2017 Sep 6. [DOI] [PubMed] [Google Scholar]
- 24.Cohen JF, Bertille N, Cohen R, Chalumeau M. Rapid antigen detection test for group A streptococcus in children with pharyngitis. Cochrane Database Syst Rev. 2016;(7):CD010502. doi: 10.1002/14651858.CD010502.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moesker FM, van Kampen JJA, Aron G, Schutten M, van de Vijver DA, Koopmans MP, et al. Diagnostic performance of influenza viruses and RSV rapid antigen detection tests in children in tertiary care. J Clin Virol. 2016;79:12–7. doi: 10.1016/j.jcv.2016.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hogan CA, Caya C, Papenburg J. Rapid and simple molecular tests for the detection of respiratory syncytial virus: a review. Expert Rev Mol Diagn. 2018;18(7):617–29. doi: 10.1080/14737159.2018.1487293. Epub 2018 Jun 29. [DOI] [PubMed] [Google Scholar]
- 27.Gonzalez RM, Varnum SM, Zangar RC. Sandwich ELISA microarrays: generating reliable and reproducible assays for high-throughput screens. In: Wang F, editor. Biomarker methods in drug discovery and development. Methods in pharmacology and toxicology. Totowa, NJ: Humana Press; 2008. pp. 273–90. [Google Scholar]
- 28.Cohen R, Haas H, Lorrot M, Biscardi S, Romain O, Vie Le Sage F, et al. Traitement antimicrobien des infections ORL. Arch Pediatr. 2017;24(12S):S9–16. doi: 10.1016/S0929-693X(17)30512-2. [DOI] [PubMed] [Google Scholar]
- 29.Stewart EH, Davis B, Clemans-Taylor BL, Littenberg B, Estrada CA, Centor RM. Rapid antigen group A streptococcus test to diagnose pharyngitis: a systematic review and meta-analysis. PLoS One. 2014;9(11):e111727. doi: 10.1371/journal.pone.0111727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matthys J, De Meyere M, Van Driel ML, De Sutter A. Differences among international pharyngitis guidelines: not just academic. Ann Fam Med. 2007;5(5):436–43. doi: 10.1370/afm.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Russell BL, Steele JC., Jr. Infectious mononucleosis testing at the point-of-care. Point Care. 2009;8(1):29–31. [Google Scholar]
- 32.Womack J, Jimenez M. Common questions about infectious mononucleosis. Am Fam Physician. 2015;91(6):372–6. [PubMed] [Google Scholar]
- 33.Public Health Agency of Canada. Canadian guidelines on sexually transmitted infections. Ottawa, ON: Government of Canada; 2016. [Google Scholar]
- 34.US Food and Drug Administration. FDA clears first diagnostic tests for extragenital testing for chlamydia and gonorrhea [news release] Silver Spring, MD: US Food and Drug Administration; 2019. Available from: https://www.fda.gov/news-events/press-announcements/fda-clears-first-diagnostic-tests-extragenital-testing-chlamydia-and-gonorrhea. Accessed 2020 Mar 6. [Google Scholar]
- 35.Workowski KA, Bolan GA, Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64(RR-03):1–137. Erratum in: MMWR Recomm Rep 2015;64(33):924. [PMC free article] [PubMed] [Google Scholar]
- 36.Randel A, Infectious Disease Society of America. IDSA updates guideline for managing group A streptococcal pharyngitis. Am Fam Physician. 2013;88(5):338–40. [PubMed] [Google Scholar]
- 37.Randel A. AAO-HNS guidelines for tonsillectomy in children and adolescents. Am Fam Physician. 2011;84(5):566–73. [PubMed] [Google Scholar]
- 38.Oliver J, Malliya Wadu E, Pierse N, Moreland NJ, Williamson DA, Baker MG, et al. Streptococcus pharyngitis and pharyngeal carriage: a meta-analysis. PLoS Negl Trop Dis. 2018;12(3):e0006335. doi: 10.1371/journal.pntd.0006335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cohen JF, Cohen R, Levy C, Thollot F, Benani M, Bidet P, et al. Selective testing strategies for diagnosing group A streptococcal infection in children with pharyngitis: a systematic review and prospective multicentre external validation study. CMAJ. 2015;187(1):23–32. doi: 10.1503/cmaj.140772. Epub 2014 Dec 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.National Institute for Health and Care Excellence. Sore throat (acute): antimicrobial prescribing. London, UK: National Institute for Health and Care Excellence; 2018. NICE guideline [NG84]. [Google Scholar]
- 41.Dumkow LE, Axford KL, Suda KJ, Draper HM, Brandt KL. Impact of a stewardship-focused culture follow-up initiative on the treatment of pharyngitis in the emergency department and urgent care settings. Diagn Microbiol Infect Dis. 2018;92(2):136–42. doi: 10.1016/j.diagmicrobio.2018.05.014. Epub 2018 May 28. [DOI] [PubMed] [Google Scholar]
- 42.Spurling GKP, Del Mar CN, Dooley L, Foxlee R, Farley R. Delayed antibiotic prescriptions for respiratory infections (review). Cochrane Database Syst Rev. 2017;(9):CD004417. doi: 10.1002/14651858.CD004417.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jaggi P, Leber A. Molecular testing for group A streptococcal pharyngitis: to test or not to test, that is the question. J Pediatric Infect Dis Soc. 2018 Apr 27; doi: 10.1093/jpids/pix106. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 44.Hayward G, Thompson MJ, Perera R, Glasziou PP, Del Mar CB, Heneghan CJ. Corticosteroids as standalone or add-on treatment for sore throat. Cochrane Database Syst Rev. 2012;(10):CD008268. doi: 10.1002/14651858.CD008268.pub2. [DOI] [PubMed] [Google Scholar]
- 45.Thomas M, Del Mar C, Glasziou P. How effective are treatments other than antibiotics for acute sore throat? Br J Gen Pract. 2000;50(459):817–20. [PMC free article] [PubMed] [Google Scholar]
- 46.O’Connor TE, Skinner LJ, Kiely P, Fenton JE. Return to contact sports following infectious mononucleosis: the role of serial ultrasonography. Ear Nose Throat J. 2011;90(8):E21–4. doi: 10.1177/014556131109000819. [DOI] [PubMed] [Google Scholar]
- 47.Spinks A, Glasziou PP, Del Mar CB. Antibiotics for sore throat. Cochrane Database Syst Rev. 2013;(11):CD000023. doi: 10.1002/14651858.CD000023.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Quintana EC. Effectiveness of oral dexamethasone in the treatment of moderate to severe pharyngitis in children. Ann Emerg Med. 2005;46(2):210. [Google Scholar]
- 49.Campagna JD, Bond MC, Schabelman E, Hayes BD. The use of cephalosporins in penicillin-allergic patients: a literature review. J Emerg Med. 2012;42(5):612–20. doi: 10.1016/j.jemermed.2011.05.035. Epub 2011 Jul 13. [DOI] [PubMed] [Google Scholar]
- 50.Ónodi-Nagy K, Kinyó Á, Meszes A, Garaczi E, Kemény L, Bata-Csörgő Z. Amoxicillin rash in patients with infectious mononucleosis: evidence of true drug sensitization. Allergy Asthma Clin Immunol. 2015;11(1):1. doi: 10.1186/1710-1492-11-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Van Driel ML, De Sutter AIM, Habraken H, Thorning S, Christiaens T. Different antibiotic treatments for group A streptococcal pharyngitis. Cochrane Database Syst Rev. 2016;(9):CD004406. doi: 10.1002/14651858.CD004406.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pontin IP, Sanchez DC, Di Francesco R. Asymptomatic group A streptococcus carriage in children with recurrent tonsillitis and tonsillar hypertrophy. Int J Pediatr Otorhinolaryngol. 2016;86:57–9. doi: 10.1016/j.ijporl.2016.03.044. Epub 2016 Apr 12. [DOI] [PubMed] [Google Scholar]
- 53.Tanz RR, Shulman ST, Barthel MJ, Willert C, Yogev R. Penicillin plus rifampin eradicates pharyngeal carriage of group A streptococci. J Pediatr. 1985;106(6):876–80. doi: 10.1016/s0022-3476(85)80229-8. [DOI] [PubMed] [Google Scholar]
- 54.Chaudhary S, Bilinsky SA, Hennessy JL, Soler SM, Wallace SE, Schacht CM, et al. Penicillin V and rifampin for the treatment of group A streptococcal pharyngitis: a randomized trial of 10 days penicillin vs 10 days penicillin with rifampin during the final 4 days of therapy. J Pediatr. 1985;106(3):481–6. doi: 10.1016/s0022-3476(85)80687-9. [DOI] [PubMed] [Google Scholar]