Abstract
Demand for agricultural crop continues to escalate in response to increasing population and damage of prime cropland for cultivation. Research interest is diverted to utilize soils with marginal plant production. Moisture stress has negative impact on crop growth and productivity. The plant growth promoting rhizobacteria (PGPR) and plant growth regulators (PGR) are vital for plant developmental process under moisture stress. The current study was carried out to investigate the effect of PGPR and PGRs (Salicylic acid and Putrescine) on the physiological activities of chickpea grown in sandy soil. The bacterial isolates were characterized based on biochemical characters including Gram-staining, P-solubilisation, antibacterial and antifungal activities and catalases and oxidases activities and were also screened for the production of indole-3-acetic acid (IAA), hydrogen cyanide (HCN) and ammonia (NH3). The bacterial strains were identified as Bacillus subtilis, Bacillus thuringiensis and Bacillus megaterium based on the results of 16S-rRNA gene sequencing. Chickpea seeds of two varieties (Punjab Noor-2009 and 93127) differing in sensitivity to drought were soaked for 3 h before sowing in fresh grown cultures of isolates. Both the PGRs were applied (150 mg/L), as foliar spray on 20 days old seedlings of chickpea. Moisture stress significantly reduced the physiological parameters but the inoculation of PGPR and PGR treatment effectively ameliorated the adverse effects of moisture stress. The result showed that chickpea plants treated with PGPR and PGR significantly enhanced the chlorophyll, protein and sugar contents. Shoot and root fresh (81%) and dry weights (77%) were also enhanced significantly in the treated plants. Leaf proline content, lipid peroxidation and antioxidant enzymes (CAT, APOX, POD and SOD) were increased in reaction to drought stress but decreased due to PGPR. The plant height (61%), grain weight (41%), number of nodules (78%) and pod (88%), plant yield (76%), pod weight (53%) and total biomass (54%) were higher in PGPR and PGR treated chickpea plants grown in sandy soil. It is concluded from the present study that the integrative use of PGPR and PGRs is a promising method and eco-friendly strategy for increasing drought tolerance in crop plants.
Introduction
Change in current climate resulted change in temperature and precipitation profiles, leading to intense drought condition. These fluctuation in ecological condition resulted an increase in global warming which in turn resulted an increase in demand for irrigation [1]. On the other hand rise in population resulted severe devastation of prime cropland due to increase in soil erosion and urbanization. Therefore, there is an inordinate need to utilize soils with minimal ability for crop growth and production [2]. Sandy soils are poorer in plant growth and have less ability for water passage from deeper layers of soils through capillary transport. These soils have loose structure and light in structure due to which they drain very quickly. However, the fertility status and yield capabilities of these soils can be improved with the application of compost leaf mould, manure or by the application of PGPR [3]. There is an increasing demand for improving tolerance in pulses especially in chickpea against drought, in order to fulfil the world food demand [4]. Thus, policies may be develop to accomplish crops plants against drought stress and to develop drought tolerance in crop plants [5].
Bacteria that live in the locality of plant roots and interact with plants and enhance their growth directly or indirectly are known as plant growth-promoting rhizobacteria (PGPR). PGPR improve the plant growth and increase their yield as they improve the root growth and thus enhance the accessibility of micro-nutrients to the roots of host plant [6]. Plant roots produces an array of organic compounds that secrete form the roots as exudates and attract soil microbes including PGPR, as they are efficient source of carbon inside soil [7, 8]. Soil bacteria, maintain mutualistic interactions with plant roots that enable plants to grow well and tolerate several abiotic stresses [9, 10]. Rhizobacteria service plants to preserve an encouraging water status under water deficit condition by improving the growth of the root system [11]. Plant roots also perform an imperative role in water use efficiency (WUE) and PGPR further augment the water absorption ability of roots under water scarcity [12]. Inoculation of plants with PGPR results an increase in growth rate, seedling emergence, and improve the responses of plants to various stimuli and plant pathogen. PGPR induce increase in the development and yield of crop has been confirmed in both green house and field trials [13, 14]. They were also stimulatory to the growth and yield of rice, radish, sugar beet, potato, apple, tomato, wheat, beans and in ornamental plants [15, 16]. Many mechanisms have been described for the action of PGPR [17]. Some of them produces different types of plant metabolites such as hydrogen cyanide (HCN), 2,4-diacetylphloroglucinol (DAPG) [18]; antibiotics, e.g. phenazine [19]; and volatile compounds that motivate plant growth [20]. Other strains produce siderophores, biofilm and plant hormones which influence plant physiological processes [21].
Plant growth regulators also perform a significant role in plant developmental process and thus modulate plant replies to abiotic stresses. They have been found to improve the damages caused by abiotic stresses. Salicylic acid (SA) is known for its defensive role when present in plants under appropriate concentration [22–23]. SA was shown to be responsible for drought tolerance in plants [24]. Foliar spray of SA repairs the negative effects of drought and increases the restoration process in plants [25–27]. Putrescine also play constructive role in decreasing the opposing effects of abiotic stresses on plants as it has acid neutralizing and cell wall stabilizing abilities [28]. Putrescine has the ability to develop tolerance in plants against drought, oxidative and salinity stress [29, 30] and also control plant developmental process [31]. The present study was therefore aimed to evaluate the effects of bacterial isolates, salicylic acid and putrescine alone or in combination on secondary metabolites, growth and yield of chickpea grown under sandy soil conditions.
Materials and methods
The experiments were carried out using chickpea plants grown under natural condition of field. The experiments were performed during the chickpea growing seasons 2014–15 and 2015–16. Seeds were grown in the sandy soil at Girot (soil moisture 6%), 20 km away from Khushab. Khushab is the driest and hot district with varied topographical condition, having arid hills of salt range with bushy vegetation in its north (soon sakesar valley) and central part have irrigated low land plains and southern part has hot dry desert with scarce vegetation. The temperature ranges from 24–50 oC in summer and 20–30 oC in winter with normal yearly precipitation of 370 millimetre. Seeds of two chickpea varieties i.e., Punjab Noor-2009 (drought sensitive) (Shah et al. 2016) and 93127 (drought tolerant) (Irshad et al. 2010), were obtained from Ayub Agriculture Research Institute, Faisalabad. Bacterial colonies were secluded from the rhizosphere of chickpea plants grown in sandy soil of Karak, Bhakkar and Cholistan (with 7%, 6% and 4% soil moisture contents) and were named as P1, P2 and P3. The experiment was carried out in a Randomized Complete Block Design (RCBD) with a plot size of 5 ×1. 5 m, with four replications.
The experiment had 11 treatments which are described below
T1- Seeds inoculated with Bacillus subtilis
T2- Treatment with Bacillus subtilis + 2 PGRs
T3- Inoculation of seeds with Bacillus subtilis and Bacillus thuringiensis
T4- Inoculation of seed with Bacillus subtilis and Bacillus thuringiensis + Plants Sprayed with both the PGRs
T5- Seeds inoculated with Bacillus subtilis, Bacillus thuringiensis and Bacillus megaterium.
T6- Combined treatment of all 3 PGPR and 2 PGRs
T7- Plants treated with SA
T8- Plants treated with Put
T9- combined treatment of SA and Put
T10- Untreated control
T11- Irrigated control
Collection of soil samples
Soil samples were collected at 6 inches from top soil from three rain-fed areas (Karak, Bhakkar and Cholistan) of Pakistan, with 7%, 6% and 4% of soil moisture contents. The method of McKeague [32] and McLean [33] was followed for determination of soil pH and electrical conductivity (EC).
Isolation and purification of PGPR strains
Bacterial strains were isolated from the rhizosphere of chickpea. Decimal dilutions were made from the supernatant of all soil samples and were spread (20 μl) on Luria-Bertani (LB) agar plates. The agar plates were incubated for 2 days. The appeared bacterial colonies agar plates were streaked 6–7 times till purification.
Sterilization of seeds
Before seed inoculation, they were sterilized with ethanol (70%) and clorox (10%) for 3 minutes and washed with autoclaved distilled water [34].
Seed inoculation with bacterial culture
The inoculated Luria Bertani (LB) broth was used for seeds inoculation before sowing.
Characterization of bacterial isolates for beneficial plant growth promoting traits
Morphology and colony of isolated PGPR
Bacterial isolates were grown on pikovskaya’s overnight and the isolates were placed on agar plates [35]. The color and shape of the colonies was recorded after 24 hours.
Gram staining
For gram staining, slides of bacterial strains were prepared, following the method of Vincent [36].
Oxidase and catalase test
The oxidase tests was performed following the method adopted by Steel [37] while, for the determination of oxidase activity, kovacs reagent [38] was used.
IAA production by selected PGPR strains
Indole-3-acetic acid (IAA) production by selected PGPR was determined by a colorimetric method using the Salkowski's reagent [39]. The optical density was recorded at 530 nm. IAA production was matched with YMD and LB media and YMD medium was also matched with and without tryptophan.
Hydrogen Cyanide (HCN) production by selected PGPR strains
Selected strains were screened for hydrogen cyanide production following the method of Lorck [40]. The whatman No. 1 filter paper was used for this purpose and change in filter paper color from yellow to light brown, brown or reddish brown was recorded for weak (+), moderate (++) and strong (+++) reaction respectively.
For quantitative analysis of HCN, bacterial cultures were grown in used King’s B broth augmented with glycine (4.4 g/ l) and its absorbance was read at 625 nm [41].
Ammonia (NH3) production by selected PGPR strains
The method of Cappuccino and Sherman [42] was adopted for NH3 production. The presence of NH3 was shown by alteration in color from yellow to brown.
Phosphate Solubization Index (PSI)
For PSI Pikovskaya’s media was inoculated with bacterial isolates and were incubated for 7 days (28°C). Solubilization index (SI) was determined by using the formula of Pikovskaya [34].
SI = diameter (cm) + halozone (cm)/ diameter (cm)
Extraction of bacterial DNA
A single colony of bacterial culture was used to inoculate tryptone yeast extract (TY) broth. The inoculated TY broth was incubated overnight in a shaker (Model: Excella E-24). The centrifugation was done twice by adding with 100% ethanol to clean the obtained DNA. The DNA was dissolved in distilled water. The purity of DNA was assessed through nanodrop spectrophotometry (260–280 nm) [43].
PCR-amplification and 16S rRNA sequence analysis
Weisburg et al. [44] method was used for amplification of genomic DNA. The obtained amplified PCR products were electrophoresed (1.2% w/v) agarose gel with a DNA ladder of 1 kb as molecular marker. Ethidium bromide (0.01 gm/ml) was applied to the gel and examined under UV trans-illuminator lamp. Approximately 1400 bp purified PCR products were sequenced by using primers 27FAgAgTTTgATCMTGGCTCAg, 1492RTACggYTACCTTgTTACgACTT, 518FCCA gCAgCCgCggTA ATA Cg, and 800R TAC CAgggT ATC TAA TCC.
Biochemical analyses of crop plants
Leaf chlorophyll content
The soil plant analysis development (SPAD) chlorophyll meter was used for the determination of chlorophyll content of plant leaves, Chlorophyll content of three leaves in each plant was measured.
Leaf proline content
Bates et al. [45] method was used for the estimation of proline content of leaves. The absorbance of upper layer of the solution was recorded at 520 nm and total proline was calculated as:
Proline μg/g = k value x dilution factor x absorbance/fresh sample wt.
K value = 17.52, Dilution factor = 2, Wt. of sample = 0.5 g
Leaf protein content
Lowery et al. [46] method was followed for the determination of protein content in the leaves of crop plants using BSA (Bovine Serum Albumen) as standard. The absorbance of each sample was determined at 650 nm along with the absorbance of different concentrations of bovine serum albumen (BSA). Protein concentration was calculated by using the below mentioned formula:
Protein content mg/g = K value × Dilution factor × Absorbance/sample wt.
K value = 19.6, Dilution factor = 2, Wt. of sample = 0.1 g
Sugar estimation
Sugar content was estimated, following the method of Dubois et al. [47]. The concentration of sugar in unknown sample was considered with reference to standard curve made from glucose:
Sugar content (mg/g) = K value × Dilution factor × Absorbance/Sample wt.
K value = 20, Dilution factor = 10, Weight of sample = 0.5 g.
Lipid peroxidation and total phenolic content
For determination of lipid peroxidation, the amount of malondialdehyde (MDA) formed by thiobarbituric acid (TBA) reaction was calculated as defined by Li [48] whereas, folin-Ciocalteu colorimetric method [49] was used for the estimation of total phenolic content.
Shoot fresh and dry weights
The fresh weight of five plants were measured with the help of an electronic balance. The shoots of these plants were than oven dried at 70 oC and their dry weight was measured.
Root fresh and dry weights
The roots of five plants were weighed with the help of an electronic balance for measuring their fresh weights. The selected roots were then oven dried at 70 oC till constant weight for the determination of their dry weights.
Relative Water Content (RWC)
The Relative Water Content (RWC) of each leaf was calculated according to the formula of Weatherly [50].
RWC = [(fresh mass—dry mass)/ (saturated mass—dry mass)] × 100.
Antioxidant enzymes extraction
For determination of antioxidant enzymes activity, 0.5 g of leaves was crushed in 5 ml of 50 mM phosphate buffer while keeping on ice bath. The homogenate obtained after the procedure was centrifuged for 22 minutes at 13000 g at 4 oC. The supernatant obtained was used to study the antioxidant enzymes activity including POD [51–52], APOX [53], CAT [54] and SOD [55].
Yield and yield related parameters
Plant height
Five plants/replication was randomly selected and their height was recorded (in cm) at maturity from ground level to the base of the spike with the help of a meter rod.
Spike length
Five spikes were randomly selected in each treatment and length was measured (cm) in selected spikes from base to the tip of the spike with the help of a meter rod. The average length of 5 spikes was used for statistical analysis.
100-Grain weight
Hundred grains were counted after harvest and weighed for each replication. The mean value of four replication was used in figure.
100-Pod weight
Hundred pod were counted after harvest and weighed for each replication. The mean value of four replication was used in figure.
Total biomass
Dry weight of 20-plants was measured (in g) at maturity for each replication and used for statistical analysis.
Harvest index
Ratio between grain yield per 5-plants and biomass per 5-plants was calculated by using the following formula:
HI (%) = (Grain yield per 5-plants / biomass per 5-plants) × 100.
Data analysis
Experiments were repeated four times. The data analysis was carried by using software Statistics, version. 8.1. An ANOVA was performed to determine the effect of treatments and error associated with the experiment. To identify significant differences among treatments, a mean comparison of traits was carried out by using protected LSD (P = 0.05) test where error mean square was used to estimate the standard error of differences between mean.
Results
The experiment was conducted in two consective vegetation season (2014–15 and 2015–16). The patteren of response of grwoth parameters to various treatments was almost simillar during both growing years but the % increase in comparison to untreated uninocualted control was greater during second year (2015–16). All the treatments exhibited substantial affects over all the studied parameters.
Morphological and biochemical characteristics of isolated PGPR
All the isolated PGPR strains were categorised on the basis of their colony shape, cell motility, gram staining and oxidase and catalase activity. The selected strains were checked for P-solubilisation, antibacterial and antifungal activities, proline, IAA, HCN and ammonia production. All the PGPR strains were gram positive and were predominantly rod shaped, with colony color varied from white to off-white. All the isolates were found positive for oxidase and catalase activity (Table 1).
Table 1. Morphological, physiological and biochemical characteristics of isolated bacterial strains.
| S.NO | Reaction | Test | Bacillus Megaterium | Bacillus Thuringiensis | Bacillus subtilis |
|---|---|---|---|---|---|
| 1 | CS | COLONY SHAPE | Rod | Irregular | Rod |
| 2 | CM | CELL MOTILITY | Motile | Motile | Motile |
| 3 | GS | GRAM STAINING | + | + | + |
| 4 | OXID | OXIDASE | + | + | + |
| 5 | CAT | CATALASE | + | + | + |
| 6 | ONPG | ORTHO NITRO PHENYL GALACTOPYRANOSIDE | + | + | + |
| 7 | CIT | SODIUM CITRATE | - | + | - |
| 8 | MALO | SODIUM MELONATE | - | - | + |
| 9 | LDC | LYSINE DECASE | + | + | + |
| 10 | ADH | ARGININE DIHYDROLASE | - | - | - |
| 11 | ODC | ORNITHINE DECARBOXYLASE | - | + | + |
| 12 | H2S | H2S PRODUCTION | - | + | + |
| 13 | UREA | UREA HYDROLYSIS | + | + | - |
| 14 | TDA | TRYPHTOPHANE DEAMINASE | + | - | - |
| 15 | IND | INDOLE | - | + | + |
| 16 | VP | VOGES PROSKAUER | - | - | - |
| 17 | GEL | GELATIN HYDROLYSIS | + | - | - |
| 18 | GLU | ACID FROM GLUCOSE | + | + | + |
| 19 | NO3/N2 | + | + | + | |
| 20 | MALT | ACID FROM MALTOSE | + | + | |
| 21 | SUC | ACID FROM SUCROSE | + | + | + |
| 22 | MANN | ACID FROM MANNOSE | - | - | + |
| 23 | ARAB | ACID FROM ARABINOSE | + | - | + |
| 24 | RHAM | ACID FROM RHAMNOSE | + | + | |
| 25 | SORB | ACID FROM SORBITOL | - | - | |
| 26 | INOS | ACID FROM INOSITOL | - | + | |
| 27 | ADO | ACID FROM ADONITOL | - | - | |
| 28 | MEL | ACID FROM MELIBIOSE | + | + | |
| 29 | RAF | ACID FROM RAFFINOSE | - | - |
+ Present,—Absent
Phosphorus Solubilisation Index (PSI)
The three isolated PGPR strains i.e. Bacillus subtilis, Bacillus thuringiensis and Bacillus megaterium were phosphate solubilizers (Table 2). Bacillus subtilis was with the greatest potential for phosphorus solubilization with PSI of 2.822. The PSI for Bacillus megaterium and Bacillus thuringiensis were 2.621 and 2.411 respectively.
Table 2. P-solubilizing index of selected PGPR strains.
| S.No | Isolates | Halozone diameter (mm) | P-solubilisation index |
|---|---|---|---|
| 1 | Bacillus subtilis | 1.4 | 2.822 |
| 2 | Bacillus thuringiensis | 1.1 | 2.411 |
| 3 | Bacillus megaterium | 1.3 | 2.621 |
Proline, IAA, HCN and NH3 production by selected PGPR isolates
Maximum proline production (1.699 ug/mg) was recorded in Bacillus thuringiensis followed by Bacillus megaterium (1.671 ug/mg) whereas, Bacillus subtilis was more effective in producing indole 3-acetic acid (Table 3). All the 3-selected PGPR isolates were tested for the production of hydrogen cyanide and found that all of them (except B. subtilis) were adept to change the color of filter paper from yellow to orange or dark brown which indicated the presence of hydrogen cyanide. In quantitative analysis, Bacillus megaterium was found most effective with maximum O.D value of 0.097 followed by Bacillus thuringiensis (0.082), for hydrogen cyanide production. Similarly, all the strains were found positive for ammonia production.
Table 3. Proline, IAA, HCN production by Selected PGPR strains and detection of NH3.
| S.No | Selected PGPR Strains | Proline Production (μg/mg) | IAA Production (μg/ml) | HCN production | NH3 Detection | |
|---|---|---|---|---|---|---|
| Qualitative | Quantitative (OD readings) | |||||
| 1 | B. subtilis | 1.011 | 0.499 | - | 0.011 | + |
| 2 | B. thuringiensis | 1.699 | 0.442 | +++ | 0.082 | + |
| 3 | B. megaterium | 1.671 | 0.381 | +++ | 0.097 | + |
HCN production (based on intensity of color):—negative, +weak, ++ moderate, +++ strong
Alignment of 16S rRNA sequence
For the isolate P1, isolated from the rhizosphere of chickpea (at Karak, 7% soil moisture content), a total length of sequence with 1557 nucleotid was obtained. The evaluation of the nucleotide sequence with data nucleotide bank indicated 100% (1506/1506) similarity with Bacillus subtilis (Accession No. MF616407). For the isolate P2, obtained from the rhizosphere of chickpea (at Bhakkar, 6% soil moisture content), the total length of sequence with 1517 nucleotide was obtained. The evaluation of the nucleotide sequence with data nucleotide bank indicated sequence similarity of 99% (1514/1517) with Bacillus thuringiensis (Accession No. MF662971). For the isolate P3, isolated from the rhizosphere of chickpea (at Cholistan, 4% soil moisture content), the total length of sequence with 1474 nucleotide was obtained. The evaluation of the nucleotide sequence with data nucleotide bank showed maximum sequence similarity of 99% (1492/1498 bases) with Bacillus megaterium (Accession No. MF008110).
Biochemical characters
Chlorophyll content
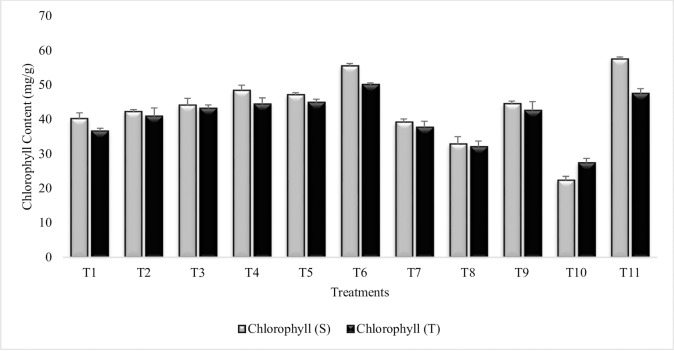
In comparison to untreated control plants grown in sandy soil (T10) chlorophyll content was improved in all the treatments in both the varieties (Fig 1). Maximum increase (59% and 45%) was noted in T6 (B. subtilis, B. thuringiensis and B. megaterium in combination with PGRs), and the increase in T6 was greater than irrigated control (T11) for tolerant variety. The sensitive variety had higher chlorophyll content then tolerant variety in all treatments except for stress control (T10). The least increase was recorded in Put treatment. Combined treatment of both PGRs (T9) was more effective than SA (T7) and Put (T8) alone. The performance of PGPR was higher than PGR particularly the Put treatment. Similar results were recorded for all treatments during succeeding year 2015–16 (S1 Table).
Fig 1. Chlorophyll content of chickpea grown in sandy soil.
Data are means of four replicates along with standard error bars (S-Sensitive Variety; T-Tolerant Variety). T1- Seeds inoculated with P1, T2- Seeds inoculated with P1 + Plants Sprayed with SA and Put, T3- Seeds inoculated with P2 and P3, T4- Seeds inoculated with P2 and P3+ Plants Sprayed with SA and Put, T5- Seeds inoculated with P1, P2 and P3, T6- Seeds inoculated with P1, P2 and P3 + Plants Sprayed with SA and Put, T7- Plants sprayed with SA, T8- Plants sprayed with Put, T9- Plants sprayed with SA and Put, T10- Untreated control plants grown in sandy soil, T11- Irrigated control plants.
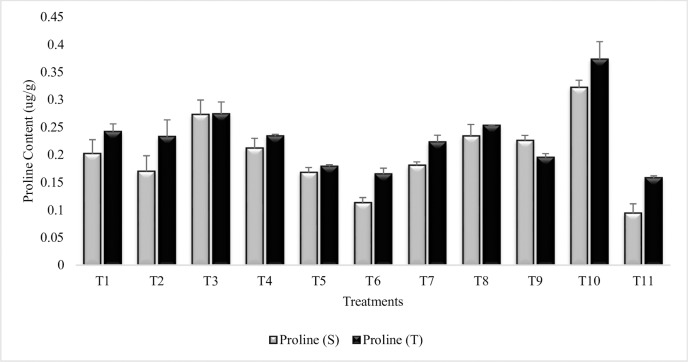
Leaf proline content
The result revealed significant decrease in leaf proline content as compared to stress control (T10), though the values were greater than irrigated control (T11) (Fig 2). T6 (Combined treatment of B. subtilis, B. thuringiensis and B. megaterium in combination with PGRs) had equally decreased the leaf proline content as compared to irrigated control in tolerant variety. The tolerant variety displayed enhanced proline accumulation over sensitive variety in most treatments. Treatment with B. subtilis and B. thuringiensis (T3) was at par in both the varieties and was least effective for decreasing proline content among all the treatments. The combined treatment of plant growth regulators (T9) was more effective in tolerant variety for the decrease in leaf proline content whereas, SA (T7) and Put (T8) resulted less proline accumulation in sensitive variety. Similar pattern of decrease in leaf proline content was recorded in the 2nd year (S2 Table).
Fig 2. Proline content of chickpea grown in sandy soil.
Data are means of four replicates along with standard error bars (S-Sensitive Variety; T-Tolerant Variety).
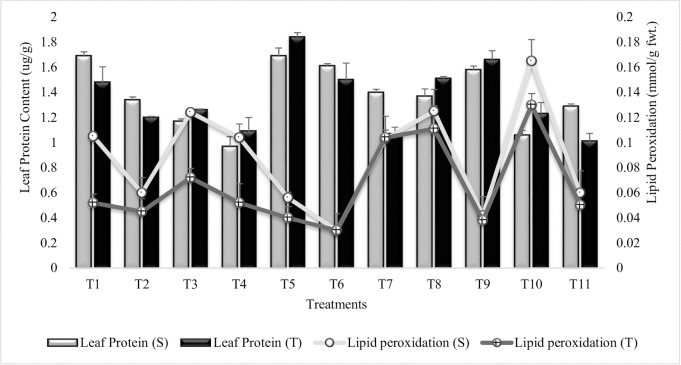
Leaf protein content
The result showed that T5 (Combined treatment of B. subtilis, B. thuringiensis and B. megaterium) had significantly (33% and 37%) enhanced the leaf protein content over uninoculated untreated plants grown in sandy soil (T10), followed by T1 (inoculation with Bacillus subtilis) in both the varieties. T1, T5, T6, T8 and T9 had higher protein accumulation, even the values were greater than irrigated control (T11) (Fig 3). Treatments T1, T6 and T8 were at par, for leaf protein accumulation in tolerant variety whereas, T1 = T5 and T6 = T9 in sensitive variety. T2, T4 and T6 had lower values as compared to T1, T3 and T5, demonstrating that PGR (SA and Put) had no or little role on accumulation of leaf protein content when applied in combination with PGPR. SA (T7) alone was more effective (23%) in sensitive variety than tolerant variety. SA in combination with Put (T9) was more effective than their separate application (T7 and T8). Put was more effective (9%) than SA (T7) in tolerant variety but was at par in sensitive variety. Similar pattern of increase was recorded for protein content in the succeeding year 2015–16 (S3 Table).
Fig 3. Leaf protein and lipid peroxidation contents of chickpea grown in sandy soil.
Data are means of four replicates along with standard error bars (S-Sensitive Variety; T-Tolerant Variety).
Lipid peroxidation
Substantial reduction in the content of lipid peroxidation was noted in all the treatments in comparison to control plants grown in sandy soil (T10) (Fig 3). The highly significant decrease (81% and 76%) in lipid peroxidation was noted in T6 (B. subtilis, B. thuringiensis and B. megaterium in combination with SA and Put) followed by T9 (SA and Put) for both the sensitive and tolerant varieties. In general, the rise in lipid peroxidation was greater in sensitive variety than tolerant variety, except for T6 and T7 (SA treatment), which were at par in both the varieties. Treatments T1, T4 and T7 had equal % decrease in lipid peroxidation for sensitive variety. Similar results were recorded for all the treatments during succeeding year 2015–16 (S4 Table).
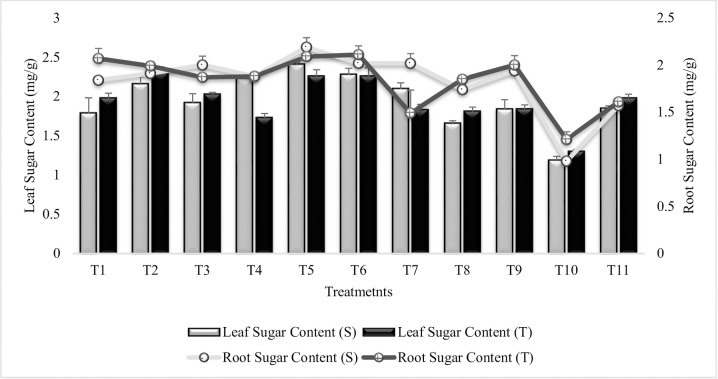
Leaf sugar content
As compared to control plants grown in sandy soil (T10), leaf sugar content was increased in all the treatments of both the varieties (Fig 4). Significant increase (50% and 42%) in leaf sugar content was recorded in T5 (Combined treatment of B. subtilis, B. thuringiensis and B. megaterium) followed by T6 (Combined treatment of B. subtilis, B. thuringiensis and B. megaterium in combination with SA and Put). Sensitive variety had higher values in T4, T5 and T7 than tolerant variety. SA (T7) was more effective for the increase in leaf sugar content than Put (T8) and combined treatment of SA and Put (T9). SA and Put had equal % increase in tolerant variety if applied alone or in combination. Similar results were recorded for all the treatments in the succeeding year 2015–2016 (S5 Table).
Fig 4. Leaf and root sugar contents of chickpea grown in sandy soil.
Data are means of four replicates along with standard error bars (S-Sensitive Variety; T-Tolerant Variety).
Root sugar content
The root sugar was lower in T7 (SA treatment) of tolerant variety as compared to irrigated control, all other treatments had significantly increased the root sugar content as compared to stress or irrigated control (T11) (Fig 4). T5 (Combined treatment of B. subtilis, B. thuringiensis and B. megaterium) showed maximum increase (55%) in root sugar content followed by T6 (Combined treatment of B. subtilis, B. thuringiensis and B. megaterium in combination with SA and Put) in sensitive variety whereas, in tolerant variety maximum increase (42%) was recorded in T6 followed by T5. T1, T5, T6 and T9 were at par in tolerant variety whereas, T6 = T4 for sensitive variety. T4 (Combined treatment of B. thuringiensis and B. megaterium in combination with SA and Put) had equal % increase in both the tolerant and sensitive varieties. Plant growth regulators, had no synergistic effects on root sugar content when applied in combination with PGPR however, PGR alone were more effective in enhancing root sugar content. All the treatments followed the same pattern of increase in the succeeding second year 2015–16 (S6 Table).
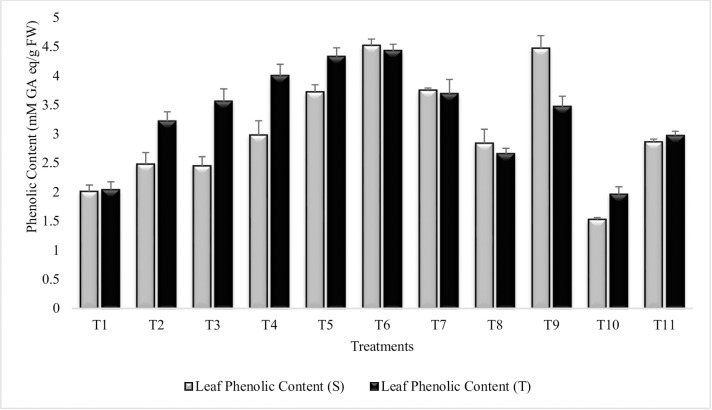
Phenolic content of leaves
The result revealed that all the treatments significantly increased the leaf phenolic content over the untreated plants grown in sandy soil (T10) (Fig 5). Maximum increase (66% and 55%) in phenolic content was recorded in T6 (Combined treatment of B. subtilis, B. thuringiensis and B. megaterium in combination with SA and Put) for both the tolerant and sensitive varieties and T6 = T9 for sensitive variety whereas, T6 = T5 for tolerant variety. Phenolic content was higher in combined treatment of SA and Put (T9) for sensitive variety over tolerant variety. T3 (treatment with Bacillus subtilis and Bacillus thuringiensis) had similar effect on leaf phenolic content of tolerant variety as compared to T9 (SA and Put) and T5 = T7 for sensitive variety. SA (T7), was more effective than Put (T8) in both the varieties. Combined treatment of SA and Put (T9), was more responsive than SA and Put alone in sensitive variety and significantly enhanced the leaf phenolic content over stressed control (T10) and irrigated control (T11). Similar findings were recorded during the succeeding year 2015–16 (S7 Table).
Fig 5. Leaf phenolic content of chickpea grown in sandy soil.
Data are means of four replicates along with standard error bar (S-Sensitive Variety; T-Tolerant Variety).
Antioxidant enzymes activity
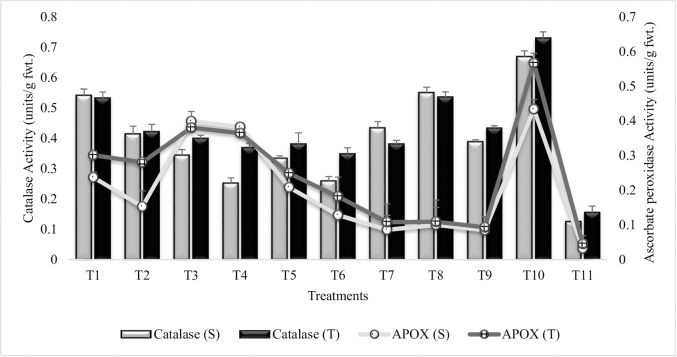
All the inoculated plants showed significant decrease in catalase activity as compared to untreated plants grown in sandy soil (T10), though the values were higher than irrigated control (T11) (Fig 6). The significant reduction (64% and 40%) in catalase activity was recorded in T4 (combined treatment of B. thuringiensis + B. megaterium in combination with SA and Put) and T6 (Combined treatment of B. subtilis, B. thuringiensis and B. megaterium in combination with SA and Put) for both the sensitive and tolerant varieties. Bacillus subtilis (T1) was less effective in reducing catalase activity and T1 (treatment with Bacillus subtilis) and T2 (treatment with Bacillus subtilis in combination with SA and Put) had similar values for catalase activity in both the drought tolerant and sensitive varieties. The plant growth regulator, Put had equal % decrease in both the varieties whereas, SA (T7) was more responsive in sensitive variety. The combined treatment of SA and Put (T9) had similar impact on the catalase activity in T4 and T6 for sensitive variety. Similar results were reported during second year experiment (S8 Table).
Fig 6. Catalase and ascorbate peroxidase activities of chickpea grown in sandy soil.
Data are means of four replicates along with standard error bars (S-Sensitive Variety; T-Tolerant Variety).
Ascorbate peroxidase activity was reduced in PGPR and PGR treated plants as compared to untreated plants grown under sandy soil (T10) (Fig 6). Maximum reduction (80% and 83%) in ascorbate peroxidase activity was recorded in T9 (combined treatment of SA and Put) followed by T7 (SA alone) in both the sensitive and tolerant varieties, respectively. Foliar applications of SA and Put was less effective in combination with Bacillus thuringiensis and Bacillus megaterium (T4). Tolerant variety had higher values for ascorbate peroxidase over sensitive variety in T1, T2, T5, T6, T7 and T10 whereas, T8 and T9 were at par for both the sensitive and tolerant varieties. Treatment T3 and T4 had equal % decrease in ascorbate peroxidase activity in both the varieties. These findings suggest, that PGPR or PGR alone or in combination significantly reduced the reactive oxygen species thus reducing antioxidant enzymes activity in chickpea under water deficit condition. Similar pattern of response was observed during second year (S9 Table).
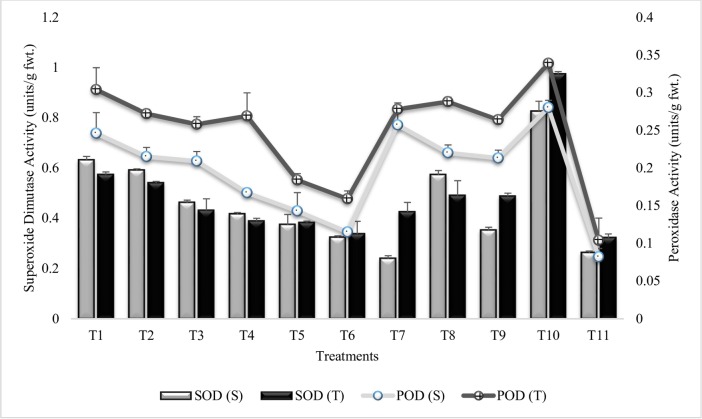
In general, the peroxidase activity was decreased in all the inoculated plants as compared to untreated control plants grown in sandy soil (T10) however, highly significant decrease (58% and 53%) in peroxidase activity was recorded in T6 (Combined treatment of B. subtilis, B. thuringiensis and B. megaterium in combination with SA and Put) followed by T5 (Combined treatment of B. subtilis, B. thuringiensis and B. megaterium), for both the sensitive and tolerant varieties over T10 (Fig 7). Treatments, T4, T7 and T8 were at par in tolerant variety. The peroxidase activity were decreased with the increase in number of PGPR (T1-T6). Bacillus subtilis (T1) alone was less effective than coinoculation of all three PGPR (T5). Plant growth regulators, were more effective in sensitive variety for reducing peroxidase activity when applied alone (T7 and T8) or in combination (T9). Significant reduction in SOD activity was noticed in all the treatments as compared to untreated control plants grown in sandy soil (T10). Maximum decrease (72%) was recoded in T7 (SA treatment) followed by T6 (60%) in sensitive variety whereas, in tolerant variety maximum decrease (65%) was recorded in T6 (Fig 7). Treatment T5 (Combined treatment of B. subtilis, B. thuringiensis and B. megaterium) and T6 (Combined treatment of B. subtilis, B. thuringiensis and B. megaterium in combination with SA and Put) were at par in both the tolerant and sensitive variety, whereas, T4 and T5 had equal % decrease in SOD activity in tolerant variety. Among the PGR treatments, T7 (SA) was more effective and significantly reduced the SOD activity notably, the reduction was even more than irrigated control (T11) in the sensitive variety. SA (T7) alone or in combination with Put (T9) was more responsive for reducing the SOD activity in sensitive variety than tolerant variety. Similar pattern of decrease was followed by all the treatments for POD and SOD activities in the second year (S10 and S11 Tables).
Fig 7. Superoxide dismutase and peroxidase activities of chickpea grown in sandy soil.
Data are means of four replicates along with standard error bars (S-Sensitive Variety; T-Tolerant Variety).
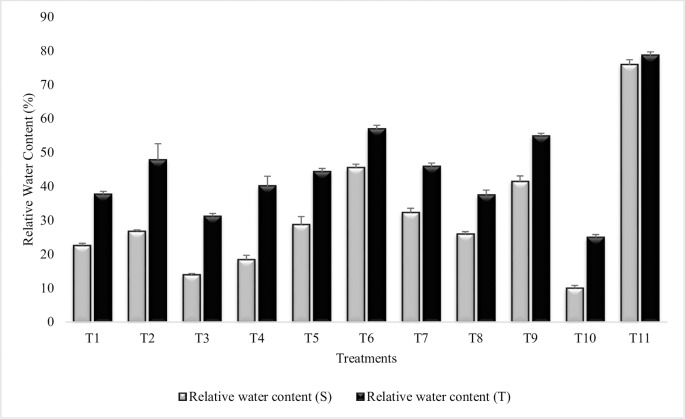
Relative water content
All the treatments significantly enhanced Relative Water Content as compared to untreated uninoculated plants grown in sandy soil (T10) though the values were lower than irrigated control (T11) (Fig 8). Highly significant increase (78% and 56%) in Relative Water Content was recorded in T6 (Combined treatment of B. subtilis, B. thuringiensis and B. megaterium in combination with SA and Put) for both the sensitive and tolerant varieties, respectively. In general, tolerant variety had higher values for Relative Water Content in all the treatments over sensitive variety. Treatments T1 (Bacillus subtilis) and T8 (Put treatment) were at par for Relative Water Content in tolerant variety. Treatment T3 (Combined treatment of B. thuringiensis and B. megaterium) was less effective than T1 (B. subtilis alone) and T2 (B. subtilis in combination with SA and Put). The Relative Water Content of tolerant variety was more (60%) than sensitive variety, in uninoculated untreated plants grown in sandy soil (T10). Plant growth regulators, had significantly enhanced the Relative Water Content when applied alone or in combination. Combined treatment of SA and Put (T9) was more effective than SA (T7) and Put (T8) alone. Similar results were reported for Relative Water Content during second year (S12 Table).
Fig 8. Relative water content of chickpea grown in sandy soil.
Data are means of four replicates along with standard error bars (S-Sensitive Variety; T-Tolerant Variety).
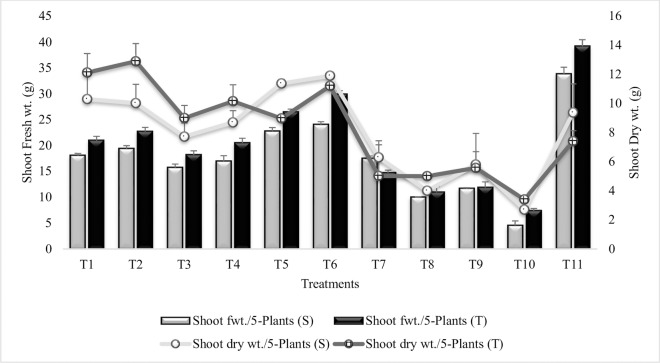
Shoot fresh weight
It in inferred from results that shoot fresh weight was significantly increased in all the treatments over untreated plants gown in sandy soil (T10) (Fig 9). Maximum increase (81% and 75%) in shoot fresh weight was recorded in T6 (Combined treatment of B. subtilis, B. thuringiensis and B. megaterium in combination with SA and Put) followed by T5 (Combined treatment of B. subtilis, B. thuringiensis and B. megaterium), in both the sensitive and tolerant varieties. T1 (B. subtilis treatment) and T4 (Combined treatment of B. thuringiensis and B. megaterium in combination with SA and Put) had equal % increase in shoot fresh weight for both the varieties. The values for shoot fresh weight was higher in tolerant variety over sensitive variety in all the treatments except for T7 (SA treatment). Among PGR treatments, SA was more effective for shoot fresh weight than Put (T8) or combined treatment of SA and Put (T9). Similar results were recorded during the succeeding year 2015–16 (S13 Table).
Fig 9. Shoot fresh and dry weights of chickpea grown in sandy soil.
Data are means of four replicates along with standard error bars (S-Sensitive Variety; T-Tolerant Variety).
Shoot dry weight
Shoot dry weight was highly significantly increased in all the inoculated treatments over untreated plants grown in sandy soil (T10) and over irrigated control (T11) (Fig 9). Maximum increase (77%) in shoot dry weight was recorded in T6 (Combined treatment of B. subtilis, B. thuringiensis and B. megaterium in combination with SA and Put) for the sensitive variety whereas, in tolerant variety maximum increase (71%) was recorded in T2 (P1 inoculation in combination with SA and Put). Treatments T5, T6, T7 and T11 had higher values in sensitive variety than tolerant variety. Bacillus subtilis alone (T1) or in combination with PGRs (T2) were more effective for shoot dry weight than B. thuringiensis and B. megaterium (T3) alone or in combination with SA and Put (T4). PGRs were less effective when applied in combination with PGPR. SA (T7) was more effective than Put (T8) while, combined treatment of SA and Put (T9) had similar effect on sensitive and tolerant variety. All the treatments followed similar pattern of response for shoot dry weight during second year except for T8 and T9 which were reduced (S14 Table).
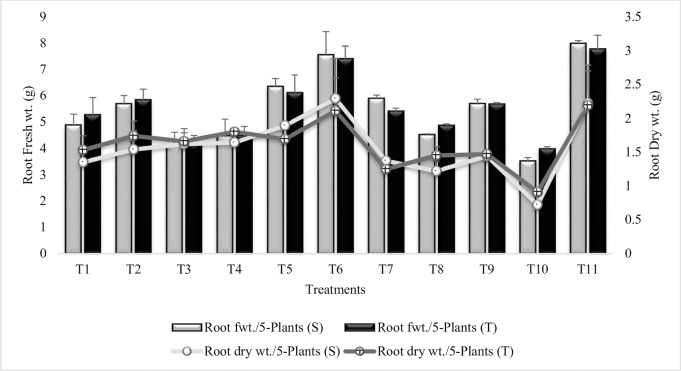
Root fresh weight
Root fresh weight was increased in all the treatments over untreated plants grown in sandy soil (T10) however, the increase was less than irrigated control (T11) (Fig 10). Maximum increase (68% and 56%) in root fresh weight was recorded in T6 (Combined treatment of B. subtilis, B. thuringiensis and B. megaterium in combination with SA and Put) in both the tolerant and sensitive varieties. Treatments T3 (Combined treatment of B. thuringiensis and B. megaterium) and T4 (Combined treatment of B. thuringiensis and B. megaterium in combination with SA and Put) were less effective than T1 (B. subtilis alone) and T2 (B. subtilis in combination with SA and Put). SA (T7) was more effective in sensitive variety whereas, Put (T8) was more effective in tolerant variety. Similar results were reported during the second year experiment (S15 Table).
Fig 10. Root fresh and dry weights of chickpea grown in sandy soil.
Data are means of four replicates along with standard error bars (S-Sensitive Variety; T-Tolerant Variety).
Root dry weight
Root dry weight was increased in all the treatments over T10 (Fig 10). Maximum increase in root dry weight was recorded in T6 (coinoculation of P1, P2 and P3 in combination with SA and Put) in both the sensitive and tolerant varieties. Treatments T5, T6 and T7 had greater values in sensitive variety over tolerant variety. T3 (Combined treatment of B. thuringiensis and B. megaterium) and T5 (Combined treatment of B. subtilis, B. thuringiensis and B. megaterium) had equal % increase in root dry weight for tolerant variety and T8 = T9 whereas, T3 and T4 showed equal % increase in sensitive variety. All the PGR treatments showed increase in root dry weight when applied alone (T7 and T8) or in combination (T9). SA was more effective in sensitive variety whereas, tolerant variety was more responsive to Put. Similar, results were recorded during second year (S16 Table).
Yield and yield contributing characters
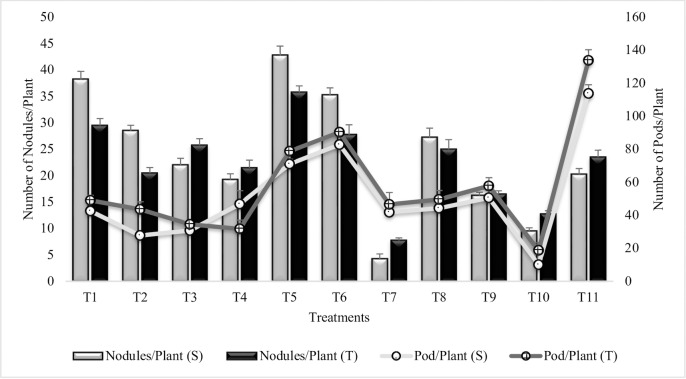
Number of nodules plant-1
All the treatments significantly enhanced the number of nodules per plant over untreated uninoculated plants grown in sandy soil (T10) (Fig 11). Maximum increase (78% and 64%) in number of nodules/plant was recorded in T5 (Combined treatment of B. subtilis, B. thuringiensis and B. megaterium) for both the sensitive and tolerant varieties. Bacillus subtilis alone (T1) instigated highly significant increase (75% and 56%) in number of nodules. In general, the PGPR inoculation was more responsive in sensitive variety than tolerant variety. Combined treatment of B. thuringiensis and B. megaterium (T3) and putrescine treatment (T8) had equal % increase in nodules/plant for tolerant variety whereas, T5 in tolerant variety was at par with T6 of sensitive variety, similarly T4 = T11 for sensitive variety. Notably, the values for T1, T3 and T4 in both the varieties were higher than T2, T4 and T6, suggesting the antagonistic effects of SA on number of nodules. SA (T7) significantly reduced (55%) the number of nodules but the combined treatment of SA and Put (T9) was stimulatory to the number of nodules. Similar results were reported during second year (S17 Table).
Fig 11. Number of nodules and pods per plant in chickpea grown in sandy soil.
Data are means of four replicates along with standard error bars (S-Sensitive Variety; T-Tolerant Variety).
Number of pods plant-1
The number of pods per plant were increased significantly in all the treatments over untreated uninoculated plants grown in sandy soil (T10) though the values were lower than irrigated control (T11) (Fig 11). Highly significant increase (88% and 79%) was recorded in T6 (Combined treatment of B. subtilis, B. thuringiensis and B. megaterium in combination with SA and Put) followed by T5 (Combined treatment of B. subtilis, B. thuringiensis and B. megaterium). Tolerant variety, had higher % increase than sensitive variety, except for T4 (Combined treatment of B. thuringiensis and B. megaterium in combination with SA and Put) which was more effective in sensitive variety. Bacillus subtilis alone (T1) had equal % increase in pods/plant as compared to T8 (Put treatment) in tolerant variety whereas, T4 = T8 in sensitive variety. PGR, enhanced the number of pods per plant when applied alone or in combination with PGPR except for T2. Combined treatment of SA and Put (T9) was more effective than SA (T7) and Put (T8) alone. Similar results were obtained during second year (S18 Table).
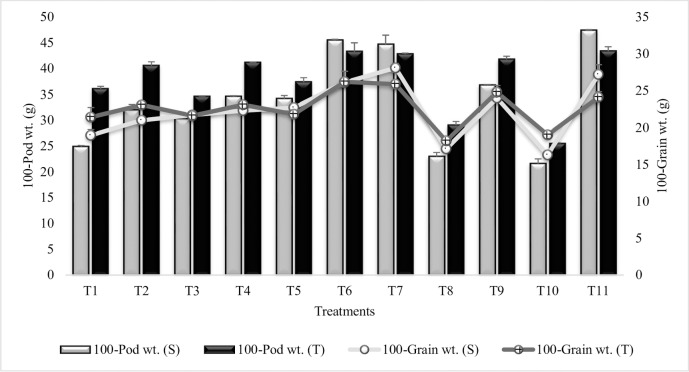
100-Pod weight
Pod weight was significantly increased in all the treatments as compared to plants grown in sandy soil (T10) (Fig 12). Maximum increase (53% and 41%) in 100-pod weight was recorded in T6 (Combined treatment of B. subtilis, B. thuringiensis and B. megaterium in combination with SA and Put) for both the sensitive and tolerant varieties, respectively. SA treatment (T7) had equal % increase in pod weight as compared to T6 and both T6 and T7 had greater values even more than irrigated control (T11) for tolerant variety, while in sensitive variety the % increase was similar to irrigated control. Sensitive variety showed maximum increase over tolerant variety in T6, T7 and T11. T4 and T9 were at par for 100-pod weight in tolerant variety. SA (T7) was more effective among all the PGR treatments and had significantly enhanced (51% and 40%) pod weight, both in sensitive and tolerant varieties as compared to T10, suggesting the dominant role of SA on weight of pods. Similar results were reported during second year (S19 Table).
Fig 12. 100-pod and grain weight of chickpea grown in sandy soil.
Data are means of four replicates along with standard error bars (S-Sensitive Variety; T-Tolerant Variety).
100-Grain weight
Result revealed that maximum increase in 100-grain weight (41%) was due to T7 (SA treatment) followed by T6 (Combined treatment of B. subtilis, B. thuringiensis and B. megaterium in combination with SA and Put) in sensitive variety whereas, maximum increase (27%) in tolerant variety was recorded in T6 followed by T7 as compared to untreated plants grown in sandy soil (T10) (Fig 12). Notably, T7 had greater values than irrigated control (T11) in both the varieties whereas, T6 had greater values than irrigated control for the tolerant variety. T5, T7 and T11 had greater values for 100-grain weight in sensitive variety than tolerant variety. Combined treatment of B. thuringiensis and B. megaterium in combination with SA and Put (T4) and Combined treatment of B. subtilis, B. thuringiensis and B. megaterium (T5) had equal % increase in sensitive variety whereas, T6 and T7 had equal % increase in tolerant variety and T2 = T4. Put (T8) alone or in combination with SA (T9) was less effective than SA (T7) alone, indicating the synergistic effects of SA on 100-grain weight. These results were confirmed from second year data where similar pattern of increase was recorded for all treatments (S20 Table).
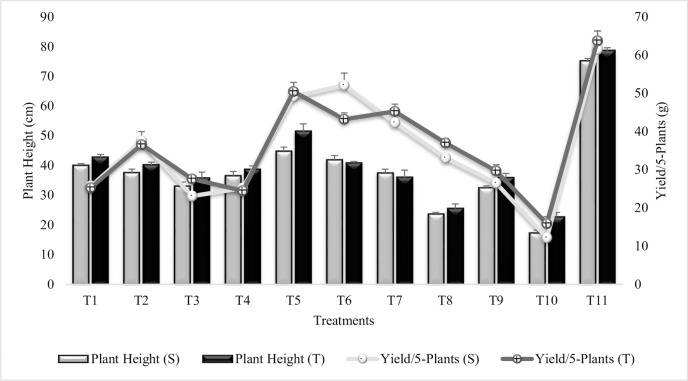
Plant height
All the treatments had significantly enhanced the plant height over untreated plants grown in sandy soil (T10), though the values were lower than irrigated control (T11) (Fig 13). Highly significant increase (61% and 56%) in plant height was recorded in T5 (Combined treatment of B. subtilis, B. thuringiensis and B. megaterium) for both the sensitive and tolerant varieties, respectively. Bacillus subtilis alone (T1) was more effective for increasing plant height than combined treatment of B. thuringiensis and B. megaterium (T3) and their combination with PGRs (T4). Treatments T6 and T7 had greater values in sensitive variety over tolerant variety. T7 (SA) alone or in combination with Put (T9) significantly enhanced the plant height over T10. T8 (Put) was less effective when applied alone but showed maximum increase when applied in combination with SA. Plant height followed similar pattern of increase during the succeeding year 2015–16 (S21 Table).
Fig 13. Plant height and yield per 5-plants of chickpea grown in sandy soil.
Data are means of four replicates along with standard error bars (S-Sensitive Variety; T-Tolerant Variety).
Yield per 5-plants
The result revealed that yield/5-plants had significantly enhanced in all the treatments over untreated plants grown in sandy soil (T10), (Fig 13). Maximum increase (76%) in Yield per 5-plants was recorded in T6 (Combined treatment of B. subtilis, B. thuringiensis and B. megaterium in combination with SA and Put) for sensitive variety followed by T5 (coinoculation of P1, P2 and P3) whereas, in tolerant variety, maximum increase (69%) was shown by T5 followed by T6. T6 had greater values for yield/5-plants in sensitive variety than tolerant variety and T1 = T4 for sensitive variety. Combined treatment of B. thuringiensis and B. megaterium (T3) was less effective than combined treatment of B. subtilis, B. thuringiensis and B. megaterium (T5). SA (T7) had significantly enhanced (71% and 64%) yield/5-plants in both sensitive and tolerant varieties as compared to T10. Combined treatment of SA and Put (T9) was less effective than SA (T7) and Put (T8) alone, similarly, Put alone (T8) was less effective than SA (T7). Similar results were recorded during succeeding year 2015–16 (S22 Table).
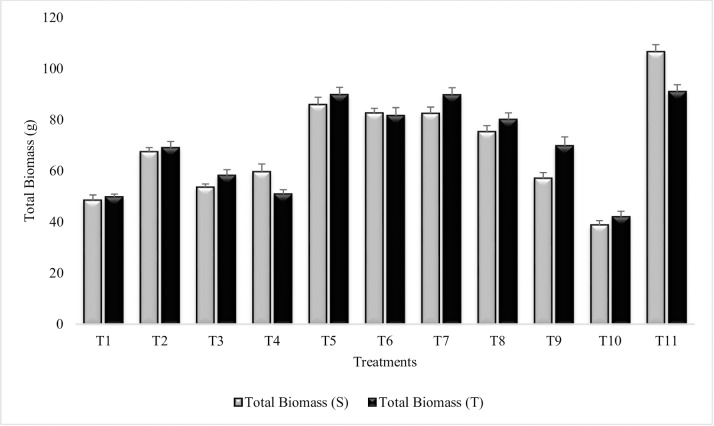
Total biomass
Results revealed significant increase in total biomass in treated plants over untreated control plants grown in sandy soil (T10). Maximum increase (54% and 53%) in total biomass was recorded in T5 (Combined treatment of B. subtilis, B. thuringiensis and B. megaterium) followed by T7 (SA treatment) for both the sensitive and tolerant varieties, respectively (Fig 14). Notably, T1 (B. subtilis alone), T2 (B. subtilis in combination with SA and Put) and T6 had equal % increase in both the varieties. Combined treatment of B. thuringiensis and B. megaterium in combination with SA and Put (T4) and irrigated C (T11) had greater values for total biomass in sensitive variety over tolerant variety. SA (T7) was more effective among all the PGR treatments and had significantly enhanced (52% and 53%) the total biomass. Combined treatment of SA and Put (T9) was less effective than SA (T7) and Put (T8) alone. Similar results were reported during succeeding year 2015–16 (S23 Table).
Fig 14. Total biomass of chickpea grown in sandy soil.
Data are means of four replicates along with standard error bars (S-Sensitive Variety; T-Tolerant Variety).
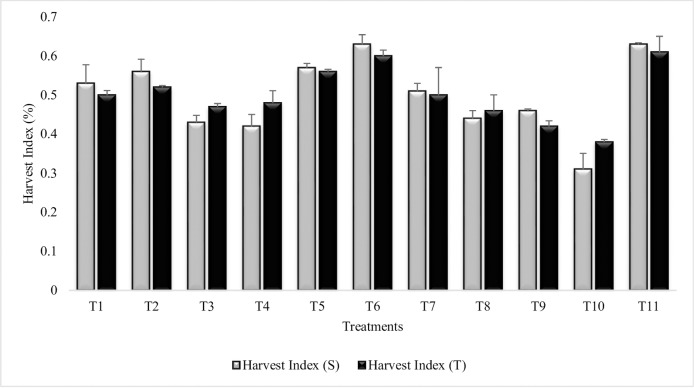
Harvest index
All the treatments had significantly enhanced harvest index over untreated plants grown in sandy soil (T10). Maximum increase in harvest index was recorded in T6 (Combined treatment of B. subtilis, B. thuringiensis and B. megaterium in combination with SA and Put) for both the sensitive and tolerant varieties (Fig 15). T6 was at par with irrigated control (T11). Sensitive variety showed maximum increase in T1, T2, T6, T9 and T11 over tolerant variety. Combined treatment of B. thuringiensis and B. megaterium (T3) and their combination with PGRs (T4) enhanced the harvest index for both the sensitive and tolerant varieties. SA alone (T7) was more effective than Put (T8) or combined treatment of SA and Put (T9). It was also inferred from results that combined treatment of SA and Put (T9) had antagonistic effects on tolerant variety. Similar results were reported during second year experiment (S24 Table).
Fig 15. Harvest index of chickpea grown in sandy soil.
Data are means of four replicates along with standard error bars (S-Sensitive Variety; T-Tolerant Variety).
Discussion
Moisture stress is one of the major restraint for agricultural crops that not only affect plant physiology but productivity [56, 57]. Approximately, 40% of the agricultural lands lies at arid and semi-arid regions of the world. Moisture stress effects the morphological and physiological characteristics, and also have negative impacts on fresh weights, relative water content and decrease nutrient diffusion. Plant growth promoting rhizobacteria (PGPR) and plant growth regulators (PGR) could play a significant role in alleviation of moisture stress in plants.
Chlorophyll content was increased in all the treatments but maximum increase was recorded in T6 and T5 for both the field and pot experiments. This increase may be due to the synergistic effects of PGPR consortia and PGR, salicylic acid (SA) + Putrescine (Put) which was at par to irrigated control. Thereby, suggesting that combined treatment of PGR and PGPR can effectively ameliorate the adverse effects of moisture stress. It had been reported previously that PGPR induce chlorophyll content in many plants grown under abiotic stress condition [58]. Kumar et al. [59] reported decrease in chlorophyll content in the leaves of chickpea due to drought stress however; inoculation with PGPR amended the adverse effects of drought on chlorophyll content. It had been previously reported that SA significantly enhanced the chlorophyll content in many crop plants [60, 61]. Previous studies suggest the significantly positive effects of Put on leaf chlorophyll content and was helpful in preventing the degradation of chlorophyll due to abiotic stresses [62, 63].
It is inferred from the results that proline production was decreased in the PGPR consortia inoculated plants which possibly demonstrate the mitigation ability of the osmotic stress and maintenance of bioenergetics of cell in moisture stress condition. Further decrease in proline content was obvious in the combined treatment (T6) of PGR and PGPR keeping it at par to the irrigated control. This indicating that PGR + PGPR effectively protect the Put from secondary stresses (osmotic stress) created by the moisture stress induced by sandy soil. Similar, results had also been reported by Jha et al. [64], that proline accumulation increased with salinity but decreased in plants inoculated with Pseudomonas pseudoalcaligenes and B. pumilus alone or in combination. The role of PGR on proline had also been reported earlier [65, 66]. Su and Bai [67] studied the accumulation of proline in soyabean leaves grown under stress condition and found a negative correlation between accumulation of proline and endogenous Put content; enhanced accumulation of Put lead to decrease in proline content.
The coinoculation of 3-PGPR namely, Bacillus subtilis, Bacillus thuringiensis and Bacillus megaterium had significantly enhanced the leaf protein content in drought tolerant and sensitive varieties. New proteins possibly appear to be synthesized in stressed plants grown in sandy soil (T10) and the tolerant variety had greater potential for this. However, sensitive variety was more effective under irrigated condition. Plants normally synthesize heat shock proteins, antioxidant enzymes and several plant hormones to cope with environmental stresses. Noteworthy, the PGPR (T5) significantly enhanced the protein content. Dashti et al. [68] indicated that co-inoculation of soybean with B. japonicum and Serratia species increased grain yield, protein yield, and total plant protein content. Afzal and Bano [69] reported PGPR induced increase in leaf protein content of wheat. Similar results were reported by Islam et al. [70] and Pérez-Montaño et al. [71] in cereal and leguminous plants. The additive effect of SA on leaf protein content had been reported previously by Neelam et al. [72]. Çanakci and Dursun, [73] reported increase in protein content in leaves of chickpea treated with SA. Put induced increase in protein content had also been reported previously by many authors [74, 75].
In present study, a significant change in leaf sugar content was obvious in inoculated plants. The maximum sugar accumulation in the leaves of T5 (coinoculation of P1, P2 and P3), demonstrated the better mechanism for osmo-adjustment. It was noted that the sensitive variety was more responsive to sugar accumulation. Plant growth regulators (SA and Put) had no or little affect when applied to inoculated plants but had significantly enhanced the sugar content when applied alone. Environmental stresses had significantly decreased the leaf sugar content thus causes, physiological and biochemical alterations as sugar preserve the structure of macromolecules and membranes during extreme dehydration [76]. It had been reported that PGPR-accumulated soluble sugars may lead to drought tolerance in plants [77]. Beneficial effects of PGPR on root sugar content had also been reported previously [78, 79]. It is supposed that SA treatment disturbs the enzymatic system of polysaccharide hydrolysis and thus lead to increase sugar level which may lead to osmotic adjustment under stress condition [80]. The role of Put in accumulation of sugar in plant leaves under stress condition had also been reported previously by many workers [81, 82].
The suppressive effects of PGPR and more so by PGPR + PGR is noteworthy for reducing the lipid peroxidation as measured by the malondialdehyde (CMDA) content of the leaves. It can be inferred from the result that similar characteristic exist for PGR (SA + Put) both in terms of proline content and lipid peroxidation content of leaves. Lipid peroxidation act as biomarker for tissues and membrane damage under stress condition. Increase in lipid peroxidation is considered as indication for increase in oxidative damage. Singh and Jha et al. [83] recorded an increase in lipid peroxidation in wheat with the increase in salt concentration however, inoculation with PGPR significantly reduced the lipid peroxidation in salt treated plants. This decrease in lipid peroxidation with PGPR inoculation may be attributed to the fact that PGPR inoculation lower cell injuries caused by abiotic stresses and increase tolerance to environmental stresses. Coinoculation of Pseudomonas pseudoalcaligenes and Bacillus pumilus had significant adverse effects on lipid peroxidation in paddy grown under salt stress condition [64]. Put reduce oxidative damages by reducing lipid peroxidation had been reported earlier by Tang et al. [84].
Antioxidant enzymes play a critical role in detoxifying the harmful effects of reactive oxygen species, produced in response to environmental stresses. However, PGPR inoculation reduced the antioxidant enzyme activities in all inoculated treatments and the addition of PGR further reduced the antioxidant enzyme activities. This decrease in antioxidant enzyme activities with PGPR inoculation may be attributed to the fact that PGPR ameliorated the harmful effects of moisture stress hence, reducing the production of reactive oxygen species. It is inferred from results that inoculation of chickpea with PGPR could provide drought tolerance ability by reducing the harmful effects of reactive oxygen species. These results were in agreement with those reported by Jha and Subramanian [64] that the inoculation with PGPR strains reduced the lipid peroxidation and superoxide dismutase (SOD) activity in sensitive cultivars of Oryza sativa and endorsed resistance to salt stress. PGPR induced decrease in antioxidant enzyme activity had been reported previously in many plants including canola, cucumis, wheat and barley [85, 86]. It has been observed that exogenous application of SA and Put can regulate the activities of intracellular antioxidant enzymes such as SOD, ascorbate peroxidase (APOX) and increase plant tolerance to environmental stresses [87, 88].
Plant phenolics are secondary metabolites that play an imperative role in growth and reproduction and help plant to withstand under severe stress condition. It is inferred from result that phenolic content had been increased significantly in the leaves of inoculated plants. Coinoculation of all 3-PGPR and 2-PGR had significantly augmented phenolic content of both the sensitive and tolerant varieties and hence increase the plant tolerance to moisture stress. These results were in agreement with those reported by Bahadur et al. [89], who noted an increase in phenolic content in the leaves of pea plants inoculated with PGPR strains and with the results of Chakraborty et al. [90] who also reported PGPR induced increase in phenolic content. Combined treatment of SA and Put had significantly enhanced the phenolic content and was more responsive in sensitive variety, indicating the role of PGR in drought tolerance. These results were in agreement with the findings of War et al. [91], who reported increase in phenolic contents of chickpea sprayed with SA. Similar results had also been reported previously by many workers [72, 92]. Put increase, phenolic content of Gladiolus, Chamomilla and wheat had been documented earlier [93, 94].
Relative Water Content reflects a measure of plant water status, which in turn is used as an index for dehydration tolerance. Decrease in Relative Water Content in untreated uninoculated plants grown in sandy soil was obvious during the present research however, PGPR inoculation overcome the water deficit induced reduction in Relative Water Content. These results correspond to that of Casanovas et al. [95] in maize seedlings, inoculated with A. brasilense that lead to improved relative and absolute water contents compared to uninoculated plants grown under moisture stress. Inoculation with Pseudomonas putida improved plant biomass, Relative Water Content and leaf water potential in maize plants exposed to moisture stress [96]. PGPR induce increase in Relative Water Content had been studied by many other workers [97]. This increase in relative water content by PGPR inoculation was attributed to the PGPR induced product of plant hormones such as IAA by the bacteria that improved root growth and formation of lateral roots their by increasing uptake of water and nutrients under moisture stress [98].
The coinoculation of 3-PGPR (P1, P2 and P3) alone or in combination with PGR (SA and Put) had significantly enhanced shoot fresh and dry weights and these treatments were more responsive in sensitive variety than tolerant variety. These results were confirmed by Ahemad and Kibret [99] who reported enhanced shoot fresh and dry weights in Brassica plants inoculated with PGPR. Similar results were reported by Islam et al. [100] in cucumber and by Huang et al. [101] in corn, pepper and tomato. SA induced increase in shoot fresh and dry weights had been well documented in many plants including, Ocimum, lemongrass, sunflower, strawberry, wheat and maize [102]. Results of an experiment conducted by Ahmed et al. [103] indicated that Put application significantly enhanced shoot fresh and dry weights of cotton grown under abiotic stress condition.
In the present study root area was highly significantly enhanced in plants inoculated with consortium of 3 PGPR. The observed increase in root area in SA and Put treated plants may be attributed to SA and Put modulation of stimulating phytohormones IAA and cytokinin. Noteworthy, the sensitive variety was more responsive to both PGPR and PGR for root parameters. PGPR stimulated root growth because of their ability to produce IAA and IAA had long been known for its stimulatory effects on root parameters [104]. Erturk et al. [105] reported 47% increase in rooting ratios when plants were inoculated with PGPR strains. Gamalero et al. [106] reported the impact of two fluorescent pseudomonads and an arbuscular mycorrhizal fungus on the growth and root architecture of tomato plant and observed enhanced root growth in inoculated treatments. This increase in root parameters play an important role in developing tolerance to abiotic stresses especially to moisture stress because enhanced root length promote plant growth during stress condition [107]. Zhu et al. [108] pointed out the synergistic effects of Put on the shoot and root growth of chickpea and soyabean under stress condition.
Coinoculation of 3-PGPR significantly enhanced number of nodules per plant. However, P1 inoculation alone was much effective in increasing number of nodules per plant. This increase in number of nodules was due to the fact, that PGPR has positive effects on the symbiotic performance of rhizobia that cause more nodulation. Nodulation is of great importance for N-fixation, play an important role in plant growth and productivity and enhance tolerance to adverse environmental factors [109]. Co-inoculation of plant growth promoting rhizobacteria (PGPR) with Bradyrhizobium had been shown to increase legume nodulation and nitrogen fixation in alfalfa, soyabean and common bean [110]. Similarly, legume growth and yield have been shown to increase in inoculated plants. Bai et al. [111] reported that the co-inoculation of Bacillus strains in soybean plants with Bradyrhizobium japonicum showed the highest increase in nodule number, nodule weight, shoot and root weights, total biomass, total nitrogen, and grain yield. Put induced increase in nodules had been reported previously [112].
It is inferred from results that the number and weight of pods have been increased in inoculated plants. Combination of 3-PGPR and 2-PGR was more effective and significantly enhanced the pod number and weight. It has been reported that Pseudomonas fluorescens and Azospirillum lipoferum significantly increased pods per plant, weight of pod and total dry matter in Phaseolus vulgaris [113]. Dey et al. [114] reported the significant increase in number of pods and pod weight in peanut plants inoculated with seven different pseudomonas species. Combined inoculation of Escherichia coli, Pseudomonas fluorescens and Burkholderia sp. significantly enhanced the pod bearing branches, pod/plant and weight of pod in chickpea plants [115]. SA alone had significantly enhanced pod and grain weight and the pods matured late in SA treated plants. These results are in support by those of El-Hak et al. [116] who found that foliar application of SA significantly increased pod and grain weight in pea plant.
The combined treatments of SA and Put was more effective for increase in yield and yield related components (yield/5-plants, total biomass and harvest index) of chickpea grown in sandy soil. Several studies have currently exposed that inoculation with PGPR, increased leaf area, growth and yield in many plants including legumes [117, 118]. PGPR can enhance plant growth and yield either by increasing leaf area, nitrogen uptake, phytohormone production, minerals solubilisation or by chelation of iron [119]. Beside this, PGPR induced increase in yield had been studied in many other plants including sweet potato, apple, tomato, maize and peanut [120, 121].
Conclusion
The PGPR and/or PGR showed increase in all growth parameters but the magnitude of increase was higher in their combined treatment. The combined effect of PGR with PGPR was to decrease PGPR induced decrease in antioxidant enzymes, proline production and lipid per oxidation as measured by MDA content of leaves. Moisture stress significantly reduced the physiological parameters but the inoculation of PGPR and PGR treatment effectively ameliorated the adverse effects of moisture stress. Significant increase in the content of secondary metabolites was noted in the leaves of all treated plants but the content of antioxidant enzymes were decreased in reaction to PGPR and PGR treatments that lead to systematic acquired resistance. PGPR and/or PGR treatment was also helpful for increase in plant height, grain weight number of nodule and pods that lead to increase in plant yield. It is therefore, concluded that the integrative use of active PGPR strains (biotic elicitors) and PGRs seems to be a promising method and eco-friendly strategy that will help to reduce the harmful effects of moisture stress on crop plants cultivated in arid regions all over the world.
Supporting information
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Data Availability
All relevant data are within the manuscript and its Supporting Information files. Supplementary date are available from figshare at the following link: https://figshare.com/articles/SUPPLYMENTARY_TABLES_docx/12014892.
Funding Statement
The author(s) received no specific funding for this work.
References
- 1.Khan N, Bano A, Rahman MA, Rathinasabapathi B. UPLC‐HRMS‐based untargeted metabolic profiling reveals changes in chickpea (Cicer arietinum) metabolome following long‐term drought stress. Plant Cell Environ 2018; 10.1111/pce.13195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vymazal J. Removal of nutrients in various types of constructed wetlands. Sci Total Environ 2007; 380:48–65. 10.1016/j.scitotenv.2006.09.014 [DOI] [PubMed] [Google Scholar]
- 3.Tester CF. Organic amendment effects on physical and chemical properties of a sandy soil. Soil Sci Soc America J 1990;54:827–831. [Google Scholar]
- 4.Graham PH, Vance CP. Legumes: importance and constraints to greater use. Plant Physiol 2003;131:872–877. 10.1104/pp.017004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Venkateswarlu B, Shanker AK. Climate change and agriculture: adaptation and mitigation strategies Indian J Agron 2009;54:226–230. [Google Scholar]
- 6.Viveros OM, Jorquera MA, Crowley DE, Gajardo G. Mechanisms and practical considerations involved in plant growth promotion by rhizobacteria. J Soil Sci Plant Nut 2010;10:293–319. [Google Scholar]
- 7.Drogue B, Dore H, Borland S, Wisniewski-Dye F. Which specificity in cooperation between phytostimulating rhizobacteria and plants? Res Microbiol 2013;163:500–510. [DOI] [PubMed] [Google Scholar]
- 8.Pothier JF, Wisniewski-Dye F, Weiss-Gayet M, Moenne-Loccoz Y. Promoter trap identification of wheat seed extract induced genes in the plant growth promoting rhizobacterium Azospirillum brasilense Sp245. Microbiol 2007;153:3608–3622. [DOI] [PubMed] [Google Scholar]
- 9.Dimkpa C., Weinand T. and Asch F. Plant rhizobacteria interactions alleviate abiotic stress conditions. Plant Cell Environ 2009;32:1682–1694. 10.1111/j.1365-3040.2009.02028.x [DOI] [PubMed] [Google Scholar]
- 10.Yang J, Kloepper JW, Ryu CM. Rhizosphere bacteria help plants tolerate abiotic stress. Trends Plant Sci 2009;14:1–4. 10.1016/j.tplants.2008.10.004 [DOI] [PubMed] [Google Scholar]
- 11.Marulanda A, Barea JM, Azcon B. Stimulation of plant growth and drought tolerance by native microorganisms (AM fungi and bacteria) from dry environments: mechanisms related to bacterial effectiveness. J Plant Growth Reg 2009;28:115–124. [Google Scholar]
- 12.Sade N, Gebretsadik M, Seligmann R, Schwartz A. The role of tobacco Aquaporin1 in improving water use efficiency, hydraulic conductivity, and yield production under salt stress. Plant Physiol 2010;152:245–54. 10.1104/pp.109.145854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gravel V, Antoun H, Tweddell RJ. Growth stimulation and fruit yield improvement of greenhouse tomato plants by inoculation with Pseudomonas putida or Trichoderma atroviride: possible role of indole acetic acid (IAA). Soil Biol Biochem 2007;39:1968–1977. [Google Scholar]
- 14.Han HS, Lee KD. Physiological responses of soybean inoculation of Bradyrhizobium japonicum with PGPR in saline soil conditions. Res J Agri Biol Sci 2005;1:216–221. [Google Scholar]
- 15.Almaghrabi OA, Massoud SI, Abdelmoneim TS. Influence of inoculation with plant growth promoting rhizobacteria (PGPR) on tomato plant growth and nematode reproduction under greenhouse conditions. Saudi J Biol Sci 2013;20:57–61. 10.1016/j.sjbs.2012.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khalid A, Arshad M, Zahir ZA. Screening plant growth‐promoting rhizobacteria for improving growth and yield of wheat. J App Microbiol 2004;96:473–480. [DOI] [PubMed] [Google Scholar]
- 17.Beneduzi A, Ambrosini A, Passaglia LM. Plant growth-promoting rhizobacteria (PGPR): their potential as antagonists and biocontrol agents. Gen Molec Biol 2012;35:1044–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Compant S, Duffy B, Nowak J, Clement C. Use of plant growth promoting bacteria for biocontrol of plant diseases: principles, mechanisms of action, and future prospects. Appl Environ Microb 2005;71:4951–4959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chakraborty N, Ghosh R, Ghosh S, Narula K. Reduction of oxalate levels in tomato fruit and consequent metabolic remodeling following overexpression of a fungal oxalate decarboxylase. Plant Physiol 2013;162:364–78. 10.1104/pp.112.209197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ryu CM, Farag MA, Hu CH, Reddy MS. Bacterial volatiles induce systemic resistance in Arabidopsis. Plant Physiol 2004;134:1017–1026. 10.1104/pp.103.026583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kloepper JW, Rodriguez-Kabana R, Zehnder AW, Murphy JF. Plant root bacterial interactions in biological control of soilborne diseases and potential extension to systemic and foliar diseases. Aust Plant Pathol 1999; 28:21–26. [Google Scholar]
- 22.Purohit SS, editor. Hormonal regulation of plant growth and development. Springer Science & Business Media 2012. December 6. [Google Scholar]
- 23.Hara M, Furukawa J, Sato A, Mizoguchi T. Abiotic stress and role of salicylic acid in plants. InAbiotic Stress Responses in Plants 2012; 235–251. Springer, New York, NY. [Google Scholar]
- 24.Singh B, Usha K. Salicylic acid induced physiological and biochemical changes in wheat seedlings under water stress. Plant Growth Reg 2003;39:137–141. [Google Scholar]
- 25.Sakhabutdinova AR, Fatkhutdinova DR, Bezrukova MV, Shakirova FM. Salicylic acid prevents the damaging action of stress factors on wheat plants. Bulg J Plant Physiol 2003;21:314–9. [Google Scholar]
- 26.Bezrukova MV, Sakhabutdinova R, Fathutdinova RA, Kyldiarova I. The role of hormonal changes in protective action of salicylic acid on growth of wheat seedlings under water deficit. Agrochemiya (Russ) 2001;2:51–54. [Google Scholar]
- 27.Mishra A, Choudhuri MA. Effects of salicylic acid on heavy metal-induced membrane deterioration mediated by lipoxygenase in rice. Biol Plant 1999;42:409–415. [Google Scholar]
- 28.Zhao JL, Zhou LG, Wu JY. Effects of biotic and abiotic elicitors on cell growth and tanshinone accumulation in Salvia miltiorrhiza cell cultures. Appl Microbiol Biotech 2010;87:137–144. [DOI] [PubMed] [Google Scholar]
- 29.Duan JJ, Li J, Guo SR, Kang YY. Exogenous Spermidine affects polyamine metabolism in salinity-stressed Cucumis sativus roots and enhances short term salinity tolerance. J Plant Physiol 2008;165:1620–1635. 10.1016/j.jplph.2007.11.006 [DOI] [PubMed] [Google Scholar]
- 30.Rider JE, Hacker A, Mackintosh CA, Pegg AE. Spermine and spermidinemediate protection against oxidative damage caused by hydrogen peroxide. Amino Acids 2007;33:231–240. 10.1007/s00726-007-0513-4 [DOI] [PubMed] [Google Scholar]
- 31.Couee I, Hummel I, Sulmon C, Gouesbet G. Involvement of polyamines in root development. Plant Cell Tissue Organ Cult 2007;76:1–10. [Google Scholar]
- 32.McKeague JA. Manual on soil sampling and methods of analysis. Canad Soc Soil Sci 1978;30:66–68. [Google Scholar]
- 33.McLean EO. Soil pH and lime requirement. In Page A. L, Miller R. H. and Keeney D. R. (eds.) Methods of soil analysis. Part 2- Chemical and microbiological properties. Agron 1982;9:199–223. [Google Scholar]
- 34.Pikovskaya RI. Mobilization of phosphorous in soil in the connection with vital activity of some microbial species. Mikorobiologiya 1948;17:362–370. [Google Scholar]
- 35.Vincent JM. A manual for the practical study of the root-nodule bacteria. (IBP Handbuch No. 15 des International Biology Program, London). XI u. 164 S., 10 Abb., 17 Tab., 7 Taf. Oxford-Edinburgh: Blackwell Scientific Publisher; 1970. [Google Scholar]
- 36.Khan N, Bano A. Modulation of phytoremediation and plant growth by the treatment with PGPR, Ag nanoparticle and untreated municipal wastewater. Int J Phytorem 2016;18:1258–69. [DOI] [PubMed] [Google Scholar]
- 37.Steel KJ. The oxidase reaction as a toxic tool. J Gen Microbiol 1956;25:297–306. [Google Scholar]
- 38.Kovacs N. Identification of Pseudomonas pyocyanea by the oxidase reaction. Nature 1956;178:703 10.1038/178703a0 [DOI] [PubMed] [Google Scholar]
- 39.Forni C, Riov J, Caiola MG Tel-Or E. Indole-3-acetic acid (IAA) production by Arthrobacter species isolated from Azolla. Microbiol 1992;138:377–381. [DOI] [PubMed] [Google Scholar]
- 40.Lorck H. Production of hydrocyanic acid by bacteria. Physiol Plant 1948;1:142–146. [Google Scholar]
- 41.Sadasivam S, Manickam A. Page 246 in Biochemical Methods for Agricultural Sciences.
- 42.Cappuccino JG, Sherman N. Serial dilution agar plating procedure to quantitate viable cells. Microbiology: a laboratory manual, 3rd edn The Benjamin Cummings Publishing Co., Inc, Bedwood: 1992; 77–82. [Google Scholar]
- 43.Chen WP, Kuo TT. A simple and rapid method for the preparation of Gram–ve bacterial genomic DNA. Nucleic acid Res 1993;21:2260 10.1093/nar/21.9.2260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weisburg WG, Barns SM, Pelletier DA, Lane DJ. I6S-ribosomal DNA amplification for phylogenetic study. J Biotechnol 1991;173:16–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bates LS, Waldern TID. Rapid determination of free proline for water stress studies. Plant Soil 1983;39:205–297. [Google Scholar]
- 46.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem 1951;193:265–275. [PubMed] [Google Scholar]
- 47.Dubois M, Gilles KA, Hamilton JK, Rebers PT, Smith F. Colorimetric method for determination of sugars and related substances. Analyt Chem 1956;28:350–6. [Google Scholar]
- 48.Li QT, Yeo MH, Tan BK. Lipid peroxidation in small and large phospholipid unilamellar vesicles induced by water-soluble free radical sources. Biochem Biophy Res Commun 2000;273:72–76. [DOI] [PubMed] [Google Scholar]
- 49.Barka EA, Nowak J, Clément C. Enhancement of chilling resistance of inoculated grapevine plantlets with a plant growth-promoting rhizobacterium, Burkholderia phytofirmans strain PsJN. App Environ Microb 2006;72: 7246–7252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Weatherly PE. Studies in the water relations of the cotton plant. The field measurement of water deficits in leaves. New Phytol 1950:49:81–97. [Google Scholar]
- 51.Vetter JL, Steinberg MP, Nelson AI. Enzyme assay, quantitative determination of peroxidase in sweet corn. J Agri Food Chem 1958;6:39–41. [Google Scholar]
- 52.Gorin N, Heidema FT. Peroxidase activity in Golden Delicious apples as a possible parameter of ripening and senescence. J Agri Food Chem 1976;24:200–201. [DOI] [PubMed] [Google Scholar]
- 53.Asada K, Takahashi M. Production and scavenging of active oxygen in photosynthesis. In Kyle DJ, Osmond CB, and Arntzen CJ, editors. Photoinhibition Amsterdam: Elsevier; 1987;227–287. [Google Scholar]
- 54.Chandlee JM, Scandalios JG. Analysis of variants affecting the catalase developmental program in maize scutellum. Theor App Gene 1984;69:71–77. [DOI] [PubMed] [Google Scholar]
- 55.Beauchamp C, Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Analyt Biochem 1971;44:276–87. 10.1016/0003-2697(71)90370-8 [DOI] [PubMed] [Google Scholar]
- 56.Ghorbanpour M, Hatami M, Khavazi K. Role of plant growth promoting rhizobacteria on antioxidant enzyme activities and tropane alkaloid production of Hyoscyamus niger under water deficit stress. Turk J Biol 2013;37:350–60. [Google Scholar]
- 57.Rad Amir Hossein Shirani, and Zandi Peiman. "The effect of drought stress on qualitative and quantitative traits of spring rapeseed (Brassica napus L.) cultivars." Zemdirbyste-Agriculture 99 (2012): 47–54. [Google Scholar]
- 58.Habib SH, Kausar H, Saud HM. Plant growth-promoting rhizobacteria enhance salinity stress tolerance in okra through ROS-scavenging enzymes. BioMed Res Int 2016;21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kumar A, Bahadur I, Maurya BR, Raghuwanshi R. Does a plant growth promoting rhizobacteria enhance agricultural sustainability? J Pure App Microbiol 2015;9:715–724. [Google Scholar]
- 60.Czerpak R, Dobrzyn P, Krotke A, Kicinska E. The Effect of Auxins and Salicylic Acid on Chlorophyll and Carotenoid Contents. Polish J Environ Stud 2002;11:231–235. [Google Scholar]
- 61.Khandaker L, Masum Akond AS, Oba S. Foliar application of salicylic acid improved the growth, yield and leaf's bioactive compounds in red amaranth (amaranthus tricolor l.). Veget Crops Res Bull 2011;74:77–86. [Google Scholar]
- 62.Durmuş N, Bekircan T. Pretreatment with polyamines alleviate the deleterious effects of diuron in maize leaves Acta Biol Hung 2013;66:52–65. [DOI] [PubMed] [Google Scholar]
- 63.Zhang Y, Zhu H, Zhang Q, Li M. Phospholipase Dα1 and phosphatidic acid regulate NADPH oxidase activity and production of reactive oxygen species in ABA-mediated stomatal closure in Arabidopsis. Plant Cell 2009;21:2357–2377. 10.1105/tpc.108.062992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jha Y, Subramanian RB. PGPR regulate caspase like activity, programmed cell death, and antioxidant enzyme activity in paddy under salinity. Physiol Molec Biol Plants 2014;20:201–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gupta B, Huang B. Mechanism of salinity tolerance in plants: physiological, biochemical, and molecular characterization. Int J Gen 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Khan MI, Iqbal N, Masood A, Per TS. Salicylic acid alleviates adverse effects of heat stress on photosynthesis through changes in proline production and ethylene formation. Plant Sig Behav 2013;8:26374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Su GX, Bai X. Contribution of putrescine degradation to proline accumulation in soybean leaves under salinity. Biol Plant 2008;52:796–9. [Google Scholar]
- 68.Dashti N, Zhang F, Hynes R, Smith DL. Application of plant growth promoting rhizobacteria to soybean (Glycine max L. Merr.) increases protein and dry matter yield under short season conditions. Plant Soil 1997;188:33–41. [Google Scholar]
- 69.Afzal Bano A. Rhizobium and phosphate solubilizing bacteria improve the yield and phosphorus uptake in wheat (Triticum aestivum). Int J Agri Biol Eng 2008;10:85–88. [Google Scholar]
- 70.Islam F, Yasmeen T, Ali Q, Ali S. Influence of Pseudomonas aeruginosa as PGPR on oxidative stress tolerance in wheat under Zn stress. Ecotox Environ Safety 2014;30:285–93. [DOI] [PubMed] [Google Scholar]
- 71.Perez-Montano F, Alias-Villegas C, Bellogin RA, Del Cerro P. Plant growth promotion in cereal and leguminous agricultural important plants: from microorganism capacities to crop production. Microbiol Res 2014;169:325–36. 10.1016/j.micres.2013.09.011 [DOI] [PubMed] [Google Scholar]
- 72.Neelam M, Rahul M, Ajiboye M, Kafayat Y. Salicylic Acid Alters Antioxidant and Phenolics Metabolism in Catharanthus roseus Grown Under Salinity Stress. African Journal of Traditional, Complem Altern Medic 2014;11:118–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Canakci S, Dursun B. Some physiological and biochemical responses to nickel in salicylic acid applied chickpea (Cicer arietinum L.) seedlings. Acta Biol Hung 2011;62:279–89. 10.1556/ABiol.62.2011.3.7 [DOI] [PubMed] [Google Scholar]
- 74.Anjum MA. Effect of exogenously applied spermidine on growth and physiology of citrus rootstock Troyer citrange under saline conditions. Turk J Agri Forest 2011;35:43–53. [Google Scholar]
- 75.Shu S, Yuan Y, Chen J, Sun J. The role of putrescine in the regulation of proteins and fatty acids of thylakoid membranes under salt stress. Sci Rep 2015;5:14390 10.1038/srep14390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Prado FE, Boero C, Gallardo M, Gonzalez JA. Effect of NaCl on germination, growth, and soluble sugar content in Chenopodium quinoa Willd. Seeds." Bot Bull Acad Sinica 2000;.41. [Google Scholar]
- 77.Hoekstra FA, Buitink J. Mechanisms of plant desiccation tolerance. Trend Plant Sci 2001;8: 431–438. [DOI] [PubMed] [Google Scholar]
- 78.Fernandez O, Theocharis A, Bordiec S, Feil R. Burkholderia phytofirmans PsJN acclimates grapevine to cold by modulating carbohydrate metabolism. Molec Plant Microbe Interact 2012;25:496–504. [DOI] [PubMed] [Google Scholar]
- 79.Vacheron J, Desbrosses G, Bouffaud ML, Touraine B. Plant growth-promoting rhizobacteria and root system functioning. Front Plant Sci 2013;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Khosravi S, Baghizadeh A, Nezami MT. The salicylic acid effect on the Salvia officianlis L. sugar, protein and proline contents under salinity (NaCl) stress. J Stress Physiol Biochem 2011;7:80–87. [Google Scholar]
- 81.Baniasadi F, Saffari VR, Moud AM. Effect of putrescine and salinity on morphological and biochemical traits and pigment content of marigold plant (Calendula officinalis L.). J Sci Tech Greenhouse Cult 2015;6(21). [Google Scholar]
- 82.Jiang W, Bai J, Yang X, Yu H. Exogenous application of abscisic acid, putrescine, or 2, 4-epibrassinolide at appropriate concentrations effectively alleviate damage to tomato seedlings from suboptimal temperature stress. Hort Technol 2012;22:137–44. [Google Scholar]
- 83.Singh RP, Jha PN. The multifarious PGPR Serratia marcescens CDP-13 augments induced systemic resistance and enhanced salinity tolerance of wheat (Triticum aestivum L.). PLos one 2016;11:e0155026 10.1371/journal.pone.0155026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tang W, Newton RJ. Polyamines reduce salt-induced oxidative damage by increasing the activities of antioxidant enzymes and decreasing lipid peroxidation in Virginia pine. Plant Growth Regul 2005;46:31–43. [Google Scholar]
- 85.Kang HM, Saltveit ME. Chilling tolerance of maize, cucumber and rice seedling leaves and roots are differentially affected by salicylic acid. Physiol Plant 2002;115:571–6. 10.1034/j.1399-3054.2002.1150411.x [DOI] [PubMed] [Google Scholar]
- 86.Turan M, Güllüce M, Çakmak R, Şahin F. Effect of plant growth-promoting rhizobacteria strain on freezing injury and antioxidant enzyme activity of wheat and barley. J Plant Nut 2013;36:731–48. [Google Scholar]
- 87.Sakhabutdinova AR, Fatkhutdinova DR, Shakirova FM. Effect of salicylic acid on the activity of antioxidant enzymes in wheat under conditions of salination. Appl BiochMicrobiol 2004;40:501–5. [PubMed] [Google Scholar]
- 88.Senaratna T, Touchell D, Bunn E, Dixon K. Acetyl salicylic acid (Aspirin) and salicylic acid induce multiple stress tolerance in bean and tomato plants. Plant Growth Reg 2000;30:157–61. [Google Scholar]
- 89.Bahadur A, Singh UP, Sarnia BK, Singh DP. Foliar application of plant growth-promoting rhizobacteria increases antifungal compounds in pea (Pisum sativum) against Erysiphe pisi. Mycobiol 2007;35:129–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chakraborty N, Ghosh R, Ghosh S, Narula K. Reduction of oxalate levels in tomato fruit and consequent metabolic remodeling following overexpression of a fungal oxalate decarboxylase. Plant physiol 2013;1:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.War AR, Paulraj MG, War MY, Ignacimuthu S. Role of salicylic acid in induction of plant defense system in chickpea (Cicer arietinum L.). Plant Sign Behav 2011;6:1787–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dihazi A, Jaiti F, Zouine J, El Hassni M. Effect of salicylic acid on phenolic compounds related to date palm resistance to Fusarium oxysporum f. sp. albedinis. Phytopathol Mediterr 2003;42:9–16. [Google Scholar]
- 93.Khorshidi M, Hamedi F. Effect of putrescine on lemon balm under salt stress. Int J Agri Crop Sci 2013;7:601. [Google Scholar]
- 94.Rahdari P, Hoseini SM. Role of poly amines (Spermidine and Putrescine) on protein, chlorophyll and phenolic compounds in wheat (Triticum aestivum L.) under salinity stress. J Sci Res Rep 2013;1:19–24. [Google Scholar]
- 95.Casanovas EM, Barassi CA, Sueldo RJ. Azospirillum inoculation mitigates water stress effects in maize seedlings. Cereal Res Commun 2002;1:343–50. [Google Scholar]
- 96.Sandhya VS, Ali SZ, Grover M, Reddy G. Effect of plant growth promoting Pseudomonas spp. on compatible solutes, antioxidant status and plant growth of maize under drought stress. Plant Growth Reg 2010;62:21–30. [Google Scholar]
- 97.Gou W, Tian L, Ruan Z, Zheng PE. Accumulation of choline and glycinebetaine and drought stress tolerance induced in maize (Zea mays) by three plant growth promoting rhizobacteria (PGPR) strains. Pak J Bot 2015;47:581–6. [Google Scholar]
- 98.Arzanesh MH, Alikhani HA, Khavazi K, Rahimian HA. Wheat (Triticum aestivum L.) growth enhancement by Azospirillum sp. under drought stress. World J Microbiol Biotech 2011;27:197–205. [Google Scholar]
- 99.Ahemad M, Kibret M. Mechanisms and applications of plant growth promoting rhizobacteria: current perspective. J King Saud University-Sci 2014;26:1–20. [Google Scholar]
- 100.Islam S, Akanda AM, Prova A, Islam MT. Isolation and identification of plant growth promoting rhizobacteria from cucumber rhizosphere and their effect on plant growth promotion and disease suppression. Front Microbiol 2016;6:1360 10.3389/fmicb.2015.01360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Huang P, de-Bashan L, Crocker T, Kloepper JW. Evidence that fresh weight measurement is imprecise for reporting the effect of plant growth-promoting (rhizo) bacteria on growth promotion of crop plants. Biol Fert Soils 2017;53:199–208. [Google Scholar]
- 102.Idrees M, Khan MM, Aftab T, Naeem M, Hashmi N. Salicylic acid-induced physiological and biochemical changes in lemongrass varieties under water stress. J Plant Interact 2010;5:293–303. [Google Scholar]
- 103.Ahmadi J, Asgharzadeh A, Bakhtiari S. The effect of microbial inoculants on physiological responses of two wheat cultivars under salt stress. Int J Adv Biol Biomed Res 2013;1:421–31. [Google Scholar]
- 104.Barazani OZ, Friedman J. Is IAA the major root growth factor secreted from plant-growth mediating bacteria? J Chem Ecol 1999;25:2397–406. [Google Scholar]
- 105.Erturk Y, Ercisli S, Haznedar A, Cakmakci R. Effects of plant growth promoting rhizobacteria (PGPR) on rooting and root growth of kiwifruit (Actinidia deliciosa) stem cuttings. Biol Res 2010;43:91–8. [PubMed] [Google Scholar]
- 106.Gamalero E, Trotta A, Massa N, Copetta A. Impact of two fluorescent pseudomonads and an arbuscular mycorrhizal fungus on tomato plant growth, root architecture and P acquisition. Mycorrhiza 2004;14:185–92. 10.1007/s00572-003-0256-3 [DOI] [PubMed] [Google Scholar]
- 107.Khodary SE. Effect of salicylic acid on the growth, photosynthesis and carbohydrate metabolism in salt stressed maize plants. Int J Agri Biol 2004;6:5–8. [Google Scholar]
- 108.Zhu XF, Wang B, Song WF, Zheng SJ. Putrescine alleviates iron deficiency via NO-dependent reutilization of root cell-wall Fe in Arabidopsis. Plant Physiol 2016;170:558–67. 10.1104/pp.15.01617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gresshoff PM. Molecular genetic analysis of nodulation genes in soybean. Plant Breed Rev 2010;11:275–318. [Google Scholar]
- 110.Korir H, Mungai NW, Thuita M, Hamba Y. Co-inoculation effect of rhizobia and plant growth promoting rhizobacteria on common bean growth in a low phosphorus soil. Front Plant Sci 2017;8:141 10.3389/fpls.2017.00141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bai Y, Zhou X, Smith DL Enhanced soybean plant growth resulting from coinoculation of Bacillus strains with Bradyrhizobium japonicum. Crop Sci 2003; 43:1774–1781. [Google Scholar]
- 112.Vassileva V, Ignatov G. Polyamine-induced changes in symbiotic parameters of the Galega orientalis Rhizobium galegae nitrogen-fixing system. Plant Soil 1999;210:83–91. [Google Scholar]
- 113.Yadegari M, Rahmani HA. Evaluation of bean (Phaseolus vulgaris) seeds inoculation with Rhizobium phaseoli and plant growth promoting Rhizobacteria (PGPR) on yield and yield components. African J Agri Res 2010;5:792–9. [DOI] [PubMed] [Google Scholar]
- 114.Dey RK, Pal KK, Bhatt DM, Chauhan SM. Growth promotion and yield enhancement of peanut (Arachis hypogaea L.) by application of plant growth-promoting rhizobacteria. Microbiol Res 2004;159:371–94. 10.1016/j.micres.2004.08.004 [DOI] [PubMed] [Google Scholar]
- 115.Dasgupta D, Ghati A, Sarkar A, Sengupta C. Application of plant growth promoting rhizobacteria (PGPR) isolated from the rhizosphere of Sesbania bispinosa on the growth of chickpea (Cicer arietinum L.). Int J Curr Microbiol App Sci 2015;4:1033–42. [Google Scholar]
- 116.El-Hak SG, Ahmed AM, Moustafa YM. Effect of foliar application with two antioxidants and humic acid on growth, yield and yield components of peas (Pisum sativum L.). J Hort Sci Ornam Plants 2012;4:318–28. [Google Scholar]
- 117.Pirlak LA, Kose MB. Effects of plant growth promoting rhizobacteria on yield and some fruit properties of strawberry. J Plant Nut 2009; 32:1173–1184. [Google Scholar]
- 118.Tilak KV, Ranganayaki N, Manoharachari C. Synergistic effects of plant‐growth promoting rhizobacteria and Rhizobium on nodulation and nitrogen fixation by pigeonpea (Cajanus cajan). Europ J Soil Sci 2006;57:67–71. [Google Scholar]
- 119.Egamberdieva D, Berg G, Lindström K, Räsänen LA. Co-inoculation of Pseudomonas spp. with Rhizobium improves growth and symbiotic performance of fodder galega (Galega orientalis Lam.). Europ J Soil Biol 2010;46:269–72. [Google Scholar]
- 120.Yasmin F, Othman R, Sijam K,. Saad MS. Effect of PGPR inoculation on growth and yield of sweetpotato. J Biol Sci 2007;7:421–4. [Google Scholar]
- 121.Karlidag H, Esitken A, Turan M, Sahin F. Effects of root inoculation of plant growth promoting rhizobacteria (PGPR) on yield, growth and nutrient element contents of leaves of apple. Sci Hort 2007;114:16–20. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files. Supplementary date are available from figshare at the following link: https://figshare.com/articles/SUPPLYMENTARY_TABLES_docx/12014892.