ABSTRACT
Epstein-Barr virus (EBV) is a widely dispersed herpesvirus, transferred through close personal contact between susceptible individuals and asymptomatic shedders of the virus. The liver is often affected, and involvement is usually subclinical and self-limited. However, immunocompromised patients and, more rarely, immunocompetent individuals can develop a severe and potentially fatal acute liver injury. To differentiate EBV hepatitis from other conditions, such as autoimmune hepatitis, lymphoproliferative disorders, and drug-induced liver injury, correlation with clinical history, laboratory findings, and histopathologic features is crucial. We report a unique case of a man who developed acute liver injury from a severe EBV infection.
INTRODUCTION
Epstein-Barr virus (EBV) is the primary causative agent of infectious mononucleosis, an illness frequently seen in adolescents and young adults. Infectious mononucleosis is initially characterized by headache, malaise, and low-grade fever before the development of tonsillitis and/or pharyngitis, cervical lymphadenopathy and tenderness, and moderate to high fever.1–4 Affected individuals frequently experience severe fatigue, nausea, vomiting, and anorexia—perhaps reflecting the mild hepatitis observed in roughly 90% of those infected.
Infrequently, patients can present with liver injury characterized by acute cholestatic hepatitis.5–10 Early investigation for the virus in individuals with fever and abnormal liver function tests (LFTs) and exclusion of extrahepatic biliary obstruction through imaging can establish the diagnosis, thus avoiding the need for expensive or invasive procedures such as liver biopsy.5,7 The mainstay of treatment is supportive care. We report a unique case of acute liver injury due to a severe EBV infection.
CASE REPORT
A 25-year-old healthy white man was transferred from a community hospital to a tertiary care liver transplant center because of concern for acute liver failure. He had experienced dry mouth, fatigue, and dark urine for 6 days. He denied experiencing any additional symptoms recently. Of note, he worked in a police academy and regularly participated in rigorous physical training. He used multiple over-the-counter bodybuilding supplements, including clomiphene, creatine, and various testosterone-boosting dietary supplements. In addition, he would ingest 1,600–2,400 mg of ibuprofen in a day, roughly 12 days per month for general soreness from his workouts.
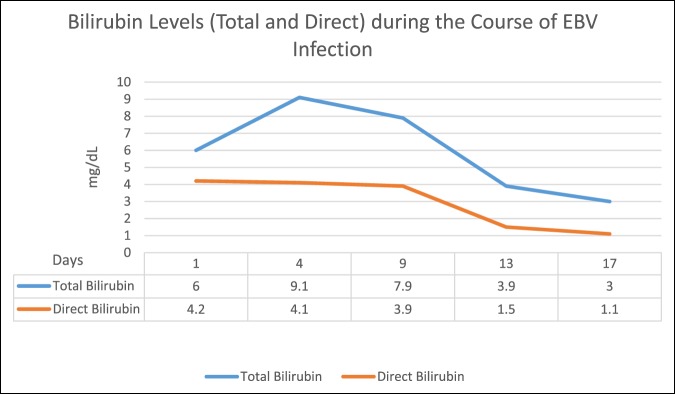
Initial LFTs showed aspartate aminotransferase 360 U/L, alanine aminotransferase 628 U/L, total bilirubin 6.0 mg/dL, direct bilirubin 4.2 mg/dL, alkaline phosphatase 342 U/L, and international normalized ratio 1.4 (Figure 1). The complete blood count showed white blood cell 13.4 × 103/uL and 72% lymphocytes. The serum electrolytes were within normal limits. The creatine kinase level was 182 U/L. He was admitted, and the supplements were discontinued. However, his LFTs continued to increase. He was then transferred for acute liver injury that, at the time, was suspected to be secondary to drug-induced liver injury (DILI).
Figure 1.
Total and direct bilirubin levels during the course of Epstein-Barr virus infection.
On further questioning, the patient reported that he drank no more than 2–3 cans of beer on weekends. Apart from his bodybuilding supplements, he denied taking any medications, complementary and alternative therapies, or herbal supplements. He denied any drug allergies. He denied a history of intravenous (IV) drug use, smoking, acquiring tattoos or piercings, receiving transfusions of blood or blood products, sexual promiscuity, sexually transmitted infections or rashes, occupational exposure to toxins, or previous liver diseases, or viral hepatitis. He denied any family history of liver disease.
The patient had intermittent low-grade fevers and was hemodynamically stable. The physical examination was remarkable for icteric sclera, generalized jaundice, a palpable liver edge, and a palpable spleen edge 5 cm below the left costal margin. He was alert and oriented 3 times with no asterixis noted. An abdominal ultrasound revealed hepatomegaly and splenomegaly.
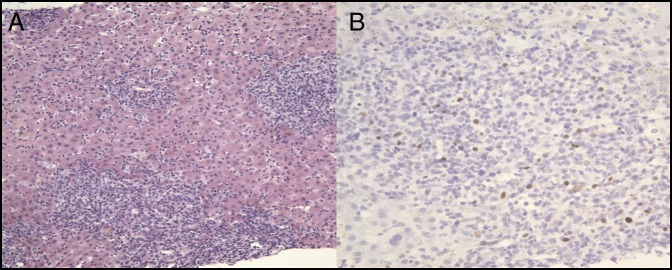
The patient was started on IV N-acetylcysteine (NAC). Workup for chronic liver diseases was significant for a positive EBV polymerase chain reaction (PCR) with 52,000 copies/mL. He underwent an interventional radiology-guided liver biopsy, which showed a portal and lobular inflammatory infiltrate and lymphocytes within the sinusoids (Figure 2). Strongly positive titers for EBV IgM were noted as well.
Figure 2.
Histopathologic images of the patient's acute liver injury from liver biopsy showing (A) there is severe acute hepatitis, consistent with an Epstein-Barr virus-driven hepatitis. There is a marked mixed portal and lobular inflammatory infiltrate showing numerous inflammatory cells in the hepatic sinusoids. Marked cholestasis is present, and apoptotic hepatocytes are present (hematoxylin & eosin stain 200×). (B) Highlighted scattered nuclear staining in the lymphocytes, which is compatible with Epstein-Barr virus infection (hematoxylin & eosin stain 400×).
During the hospital course, the patient's hemoglobin decreased to 6.0 g/dL with an unknown baseline. There were no signs of overt bleeding. Laboratory evaluation showed a peripheral blood smear with multiple nucleated red blood cells (RBCs), marked RBC agglutination, and microspherocytes, as well as total bilirubin 9.1 mg/dL, indirect bilirubin 5 mg/dL, lactate dehydrogenase 900 U/L, reticulocyte count 2.8%, and haptoglobin 18 mg/dL (Figure 1). The direct antiglobulin test or the Coombs test was positive for the complement component C3b.
The patient received 3 units of packed RBCs. His hemoglobin did not improve with the first 2 units. However, the last unit was warmed before transfusion, and there was adequate improvement in the hemoglobin. Testing for cold agglutinins in the blood later came back positive with an elevated titer of 1:512.
The clinical presentation was consistent with EBV-driven acute liver injury with possible predisposition to liver injury from taking potentially hepatotoxic bodybuilding supplements, along with a hemolytic anemia caused by cold agglutinin disease secondary to EBV infection. A triple-phase abdominal computed tomography scan was performed in anticipation of possible evaluation for liver transplantation (Figure 3). He received IV NAC for 5 days, and his liver enzymes improved. The patient was discharged home in improved and stable condition.
Figure 3.
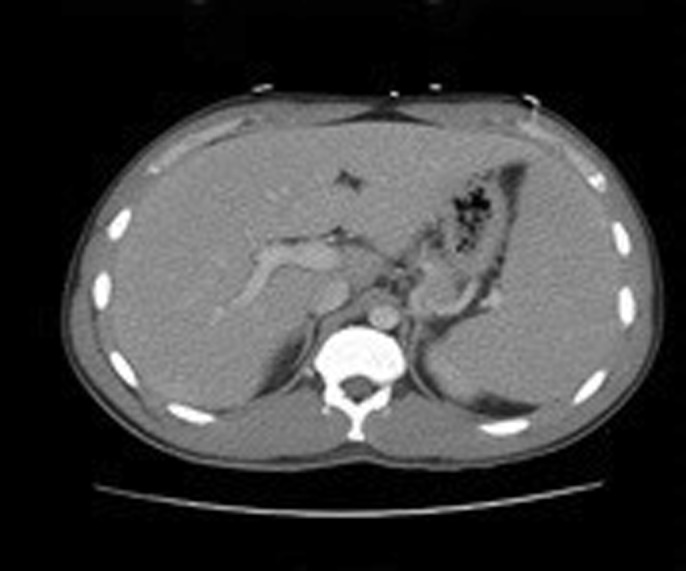
Abdominal computed tomography showing hepatomegaly and splenomegaly with good hepatic vascular anatomy.
DISCUSSION
Infrequently, individuals with primary EBV infection can present with an acute cholestatic hepatitis. Although the diagnosis is suggested by antibody testing, EBV PCR is a noninvasive laboratory test that helps in identifying primary EBV infection, especially in cases where the clinical presentation is atypical. In addition, in early stages of EBV infection, PCR is more sensitive than serological testing.11 The incidence of clinically significant hemolysis in EBV infection is low, and most such cases are associated with cold agglutinins.12,13 In this case, the cholestatic presentation was, in part, because of such hemolysis.
In this case, the findings on pathology pointed toward severe acute hepatitis and marked cholestasis patterns, secondary to EBV. In addition, a portion of the liver biopsy was stained for EBV and was compatible with EBV infection. Conversely, a liver biopsy in cases of DILI can show a variety of histological findings. Further complicating matters was the patient's use of multiple suspect agents, some of which are new and do not yet have clearly defined patterns of hepatotoxicity. We believe that the mechanism of liver injury was related more so to EBV infection, but that it was likely multifactorial with the patient's use of potentially hepatotoxic bodybuilding supplements contributing to the liver injury. Thus, IV NAC was given. Although current available evidence is limited regarding the role of NAC in nonacetaminophen DILI, it is frequently given for this purpose at our hospital. Additional clinical testing should be performed to exclude non-DILI etiologies suggested by liver biopsy.14
DISCLOSURES
Author contributions: J. Shah wrote the manuscript and is the article guarantor. V. Lingiah, N. Pyrsopoulos, and M. Galan revised the manuscript for intellectual content.
Financial disclosure: None to report.
Informed consent was obtained for this case report.
Previous presentation: This case was presented at the American College of Gastroenterology Annual Scientific Meeting, October 25-30, 2019; San Antonio, Texas.
REFERENCES
- 1.Dunmire SK, Verghese PS, Balfour HH., Jr Primary Epstein-Barr virus infection. J Clin Virol . 2018;102:84–92. [DOI] [PubMed] [Google Scholar]
- 2.Dunmire SK, Hogquist KA, Balfour HH. Infectious mononucleosis. Curr Top Microbiol Immunol. 2015;390(pt 1):211–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Luzuriaga K, Sullivan JL. Infectious mononucleosis. N Engl J Med. 2010;362(21):1993–2000. [DOI] [PubMed] [Google Scholar]
- 4.Evans AS. Infectious mononucleosis in University of Wisconsin students. Report of a five-year investigation. Am J Hyg. 1960;71:342–62. [DOI] [PubMed] [Google Scholar]
- 5.Khoo A. Acute cholestatic hepatitis induced by Epstein-Barr virus infection in an adult: A case report. J Med Case Rep. 2016;10:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Salva I, Silva IV, Cunha F. Epstein-Barr virus-associated cholestatic hepatitis. BMJ Case Rep. 2013;2013:bcr2013202213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Doğan I, Ergün M, Cindoruk M, et al. Acute hepatitis induced by Epstein-Barr virus infection: A case report. Turk J Gastroenterol. 2007;18(2):119–21. [PubMed] [Google Scholar]
- 8.Park MJ, Chung IK, Park YD, et al. A case of cholestatic hepatitis induced by Epstein-Barr virus infection [in Korean]. Korean J Hepatol. 2006;12(2):237–42. [PubMed] [Google Scholar]
- 9.Hinedi TB, Koff RS. Cholestatic hepatitis induced by Epstein-Barr virus infection in an adult. Dig Dis Sci. 2003;48(3):539–41. [DOI] [PubMed] [Google Scholar]
- 10.Crum NF. Epstein-Barr virus hepatitis: Case series and review. South Med J. 2006;99(5):544–7. [DOI] [PubMed] [Google Scholar]
- 11.Uluğ M, Celen MK, Ayaz C, et al. Acute hepatitis: A rare complication of Epstein-Barr virus (EBV) infection. J Infect Dev Ctries. 2010;4(10):668–73. [DOI] [PubMed] [Google Scholar]
- 12.Fadeyi EA, Simmons JH, Jones MR, et al. Fatal autoimmune hemolytic anemia due to immunoglobulin g autoantibody exacerbated by epstein-barr virus. Lab Med. 2015;46(1):55–9. [DOI] [PubMed] [Google Scholar]
- 13.Capra JD, Dowling P, Cook S, et al. An incomplete cold-reactive gamma G antibody with i specificity in infectious mononucleosis. Vox Sang. 1969;16(1):10–7. [DOI] [PubMed] [Google Scholar]
- 14.Foong S, Pendle S, Kwok R, et al. Acute Epstein-Barr virus hepatitis superimposed on drug induced liver injury causing severe hepatic dysfunction. Pathology. 2019;51(1):104–6. [DOI] [PubMed] [Google Scholar]