Abstract
Aim and Objective:
The aim of this study is to compare the efficiency of subgingivally delivered 1% ornidazole and 0.25% chlorhexidine (CHX) gluconate (Ornigreat™ gel) and Aloe vera (AV) gel in the treatment of chronic periodontitis.
Materials and Methods:
Twenty chronic periodontitis patients with pocket depths ranging from 4 to 6 mm bilaterally at least in one site were included in the study. After a thorough nonsurgical periodontal therapy, 1% ornidazole and 0.25% CHX gluconate (Ornigreat™ gel) and AV gel were subgingivally delivered into the pocket sites, and the clinical parameters such as plaque index (PI), gingival index (GI), bleeding index (BI), and clinical attachment level (CAL) were evaluated at baseline and after 4 weeks.
Results:
In both the groups, a reduction in PI, GI, and probing depth readings was evidenced after 4 weeks. A significant improvement was noticed in the BI in the AV group when compared with that of Ornigreat™ group. Taking CAL into consideration, though improvement was there in both the groups, it was not appreciable.
Conclusion:
AV which is an herbal derivative when subgingivally delivered in the pocket site could be an equally effective and affordable substitute for Ornigreat™ gel.
Keywords: Aloe vera, chronic periodontitis, nonsurgical periodontal therapy, ornidazole
Introduction
Among the various diseases afflicting humanity, chronic periodontitis is an inflammatory condition affecting the supporting tissues of the periodontium with microbial plaque being the primary etiological agent.[1]
Although the microbial plaque is supposed to be the major etiology behind the periodontal disease, the host response also encompassing subgingival bacterial challenge exhibits an exuberant response resulting in the destruction of the periodontium.[2]
To combat periodontal disease, nonsurgical periodontal therapy (NSPT) is one of the gold standards besides a broad range of treatment modalities advocated to eliminate periodontal disease.[3]
On the other hand, a high rate of recurrence is associated with periodontal disease, primary reason being the pocket acting as a nidus for growth and proliferation of anaerobic pathogenic bacteria, thereby limiting the accessibility to these areas.[4] To overcome these limitations and to attain a disease-free periodontium, local drug delivery (LDD) is one of the treatment modalities that were advocated in the literature.
LDD is a treatment concept that was introduced by Max Goodson[5] that could direct a pharmacological compound into a localized site in a manner that can safely achieve its desired therapeutic effect.
Although numerous literature search has shown better results with the various LDD systems encompassing tetracycline fibers, minocycline ointments, chlorhexidine (CHX) chips, and doxycycline hyclate in an attempt to reduce the individuals exposure to systemic complications, newer agents are also upsurging in the market towards achieving periodontal health.[6,7,8]
In the recent times, ornidazole is one of the LDD agents that have shown a marked antibacterial and antiprotozoal activity which is active against most of the periodontal pathogens. Ornidazole, a member of the nitroimidazole class of antibiotics, specifically targets anaerobic microorganisms.[9]
However, in this era, a search for naturopathies is also on the rise in an attempt of reducing the effects of allopathic medication[9] in contrary to the conventional mechanical therapy.[10]
AV, a medicinal plant with immune therapeutic advantages widely known for its benefits of wound healing[11] and anti-inflammatory[12] effects, has been extensively studied for its oral applications.
However, very little literature on its role in the LDD is available and has not been substantiated.
Hence, an attempt was made in this study in comparing the efficacy of subgingivally delivered (Ornigreat™ gel) and Aloe vera (AV) gel for the treatment of chronic periodontitis after NSPT.
Materials and Methods
Source of the data
This is a randomized, single-blind, split-mouth study, wherein patients from the Department of Periodontics, Vishnu Dental College and hospital, were included in the study. A total of 20 patients comprising 13 female and 7 male patients, with age groups ranging from 25 to 65 years, who were diagnosed by mild-to-moderate chronic periodontitis and were willing to participate were voluntarily enrolled in the study. An ethical clearance for the study was obtained from the Institutional Ethical Committee and Review Board, Vishnu Dental College and Hospital, before the commencement of the study.
Selection criteria
Patients with mild-to-moderate chronic periodontitis pocket depth (PD) 4–6 mm and clinical attachment loss 1–4 mm and no history of antibiotic or periodontal therapy in the past 6 months were included in the study. Patients under medication that can affect periodontal status, patients with known allergy to herbal medications, patients under systemic antimicrobial therapy and aggressive periodontitis, patients with adverse habits, and pregnancy and lactating females were excluded from the study.
According to split-mouth design, sites with probing depths of 4–6 mm were randomly divided into two parallel treatment arms by flip of a coin. Of the selected 40 sites, 20 were test sites and 20 were control sites, respectively. Group I comprised 20 sites wherein local delivery of AV gel was administered and Group II comprised 20 sites which was followed by the local delivery of ornidazole and CHX gluconate (Ornigreat™ gel).
Clinical parameters including assessment of plaque index (PI, Loe and Silness), gingival index (GI, Silness and Loe), bleeding index (BI, Muhlemann HR and Sons), probing PD, and clinical attachment loss were recorded at baseline and at 4 weeks after NSPT. UNC-15 (university of North Carolina) periodontal probe was used to measure PD and CAL.
Primary and secondary outcome measures
The primary outcome of the study was probing Pocket depth (PD). The secondary outcomes included PI, GI, BI, and clinical attachment level (CAL).
Formulation of Aloe vera in situ gel
The AV gel was developed at the Vishnu College of Pharmacy, Bhimavaram, Andhra Pradesh, India, following the procedure described by Velam et al.[13] The aloe leaves were collected and treated with distilled water, and then, central pulp from leaves of aloe was collected and washed repeatedly with water and then with 0.1 N NaOH. Then, with the help of blender, pulp was converted into juice, and using a cotton bed, juice was filtered and vacuum filtration was done until a clear liquid was obtained. Then, 1% w/w polymer and 0.5% w/w methylparaben were added and dispersed uniformly. Under alkaline conditions, polymer converts liquid to gel. A 0.5% NaOH solution was added dropwise and agitated until a gel was formed. The prepared AV gel was stored in air-tight containers in a dark room to prevent photo-oxidation.[14]
Both AV and Ornigreat™ gels are similar in color and consistency for blinding.
Local drug delivery
For standardization, a blunt cannula was advocated that could deliver the prepared AV gel and Ornigreat™ gel into the periodontal pocket at control and test sites respectively. After delivery, a periodontal dressing was placed over the test and control sites to prevent the escape of medicament from the pocket.
Statistical analysis
Power analysis calculations were performed before the study was initiated. To achieve 90% power and detect mean differences of the clinical parameters between groups, 20 sites in each group required. Continuous variables (PI, GI, BI, PD, and CAL) were expressed as the mean ± standard deviation. After completion of the clinical trial, data obtained from the sites were computed and put to statistical analysis. Paired t-test for differences within the group and ANOVA test for differences between the groups were performed. Statistical significance was defined as P < 0.05. Statistical analysis was performed with statistical software (SPSS version 10.5, SPSS, Chicago, IL, USA).
Results
Twenty patients completed the study. No patients were reported with any adverse reaction. There was a significant improvement in the PI, GI, and probing PD readings after 4 weeks in both the groups [Tables 1–3]. A significant improvement was noticed in BI in the AV group when compared with the Ornigreat™ group [Table 4]. Taking CAL into consideration, though improvement was there in both the groups, it was not statistically significant [Table 5]. On comparing, AV and Ornigreat™ group after NSPT results between the groups had no significant difference.
Table 1.
Plaque index values at the baseline and after 4 weeks
| Group | Baseline | 4 weeks | Intragroup (P) | Intergroup (P) |
|---|---|---|---|---|
| Ornigreat™ | 0.7965±0.28365 | 0.4720±0.22782 | <0.001 (S) | >0.001(NS) |
| Aloe vera | 0.8535±0.18149 | 0.5650±0.25326 | <0.001 (S) | |
| Baseline P value (intergroup) | 0.454 (NS) | |||
| 4-week P value (intergroup) | 0.230 (NS) | |||
NS: Not significant; S: Significant
Table 3.
Probing depth values at the baseline and after 4 weeks
| Group | Baseline | 4 weeks | Intragroup (P) | Intergroup (P) |
|---|---|---|---|---|
| Ornigreat™ | 5.2500±0.78640 | 4.0000±0.64889 | <0.001 (S) | >0.001(NS) |
| Aloe vera | 5.1000±0.71818 | 3.9500±0.82558 | <0.001 (S) | |
| Baseline P value (intergroup) | 0.533 (NS) | |||
| 4-week P value (intergroup) | 0.833 (NS) | |||
NS: Not significant; S: Significant
Table 4.
Bleeding index values at the baseline and after 4 weeks
| Group | Baseline | 4 weeks | Intragroup (P) | Intergroup (P) |
|---|---|---|---|---|
| Ornigreat™ | 1.0785±0.68574 | 0.5665±0.19151 | >0.001(NS) | >0.001(NS) |
| Aloe vera | 1.1035±0.42596 | 0.4290±0.21506 | <0.001(S) | |
| Baseline P value (intergroup) | 0.891 (NS) | |||
| 4-week P value (intergroup) | 0.39 (NS) | |||
NS: Not significant; S: Significant
Table 5.
Clinical attachment level values at the baseline and after 4 weeks
| Group | Baseline | 4 weeks | Intragroup (P) | Intergroup (P) |
|---|---|---|---|---|
| Ornigreat™ | 3.5315±0.66692 | 3.4910±0.79631 | >0.001 (NS) | >0.001 (NS) |
| Aloe vera | 3.9025±0.46155 | 3.6085±0.65802 | >0.001(NS) | |
| Baseline P value (intergroup) | 0.48 (NS) | |||
| 4-week P value (intergroup) | 0.614 (NS) | |||
NS: Not significant; S: Significant
Plaque index
At baseline, PI values [Table 1] for the Ornigreat™ group and AV group were 0.7965 ± 0.28365 and 0.8535 ± 0.18149, respectively. There was not any significant difference between both the groups at the baseline (P = 0.454).
After 4 weeks, in Ornigreat™ group, there was a statistically significant decrease in PI values from 0.7965 ± 0.28365 to 0.4720 ± 0.22782 (P < 0.001). In the AV group, there was also a statistically significant decrease in the plaque values from 0.8535 ± 0.18149 to 0.5650 ± 0.25326 (P < 0.001). In an intergroup comparison, though the Ornigreat™ group showed slightly better results than the AV group, the difference was not statistically significant (P = 0.230) [Table 1 and Figure 1].
Figure 1.
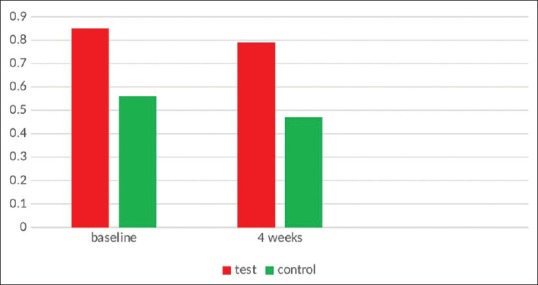
Plaque index
Gingival index
The GI values for the Ornigreat™ group and AV group were 0.7815 ± 0.33333 and 0.8535 ± 0.18149 at baseline, respectively. There was not any significant difference between both the groups at the baseline (P = 0.402).
There was a statistically significant decrease in GI values from 0.7815 ± 0.33333 to 0.3960 ± 0.15856 (P < 0.001) after 4 weeks in Ornigreat group. In the AV group, there was also a statistically significant decrease in the plaque values from 0.8535 ± 0.18149 to 0.3910 ± 0.13719 (P < 0.001). AV group showed slightly better results than the Ornigreat™ group in intergroup comparison although the difference was not statistically significant (P = 0.916) [Table 2 and Figure 2].
Table 2.
Gingival index values at the baseline and after 4 weeks
| Group | Baseline | 4 weeks | Intragroup (P) | Intergroup (P) |
|---|---|---|---|---|
| Ornigreat™ | 0.7815±0.33333 | 0.3960±0.15856 | <0.001 (S) | >0.001 (NS) |
| Aloe vera | 0.8535±0.18149 | 0.3910±0.13719 | <0.001 (S) | |
| Baseline P value (intergroup) | 0.402 (NS) | |||
| 4-week P value (intergroup) | 0.916 (NS) | |||
NS: Not significant; S: Significant
Figure 2.
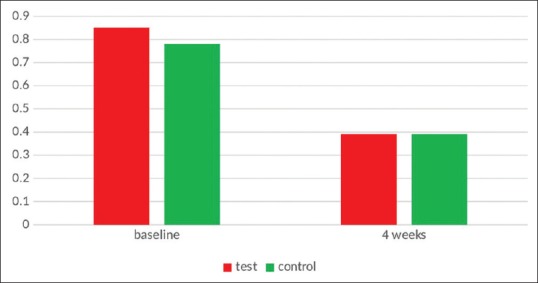
Gingival index
Bleeding index
At baseline, the BI values for the Ornigreat™ group and AV group were 1.0785 ± 0.68574 and 1.1035 ± 0.42596, respectively. The difference between both the groups at the baseline (P = 0.891) was not statistically significant.
After 4 weeks, the BI index values were reduced from 0.7815 ± 0.33333 to 0.3960 ± 0.15856 (P > 0.001) in the Ornigreat group which was not statistically significant. In the AV group, there was a statistically significant decrease in the BI values from 0.8535 ± 0.18149 to 0.3910 ± 0.13719 (P < 0.001). In an intergroup comparison, though the AV group showed slightly better results than the Ornigreat™ group, the difference was not statistically significant (P = 0.916) [Table 4 and Figure 3].
Figure 3.
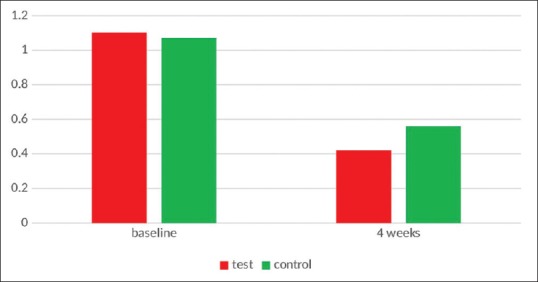
Bleeding index
Probing depth
At baseline, probing depth values for the Ornigreat™ group and AV group were 5.2500 ± 0.78640 and 5.1000 ± 0.71818, respectively, wherein the difference between both the groups at the baseline was not statistically significant (P = 0.533).
After 4 weeks, in Ornigreat™ group, there was a statistically significant decrease in probing depth values from 5.2500 ± 0.78640 to 4.0000 ± 0.64889 (P < 0.001). In the AV group, there was also a statistically significant decrease in the probing depth values from 0.8535 ± 0.18149 to 0.3910 ± 0.13719 (P < 0.001). Although the AV group showed slightly better results than the Ornigreat™ group, the difference was not statistically significant (P = 0.833) in an intergroup comparison though the AV group showed slightly better results than the Ornigreat group [Table 3 and Figure 4].
Figure 4.
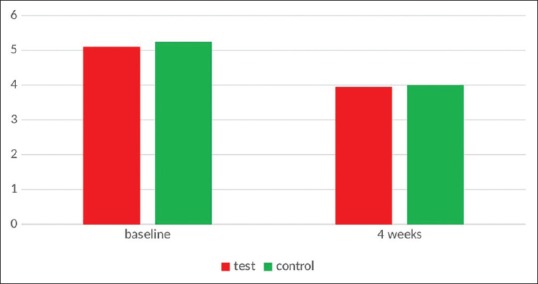
Probing pocket depth
Clinical attachment level
The values for the Ornigreat™ group and AV group were 3.5315 ± 0.66692 and 3.9025 ± 0.46155, respectively. There was not any significant difference between both the groups at the baseline (P = 0.48).
In Ornigreat™ group, there was not any statistically significant decrease in CAL index values and they reduced from 3.5315 ± 0.66692 to 3.4910 ± 0.79631 after 4 weeks (P > 0.001). In the AV group, there was also no statistically significant decrease in the CAL values from 3.9025 ± 0.46155 to 3.6085 ± 0.65802, which was not statistically significant (P > 0.001). In an intergroup comparison, though the AV group showed slightly better results than the Ornigreat™ group, the difference was not statistically significant (P = 0.614) [Table 5 and Figure 5].
Figure 5.
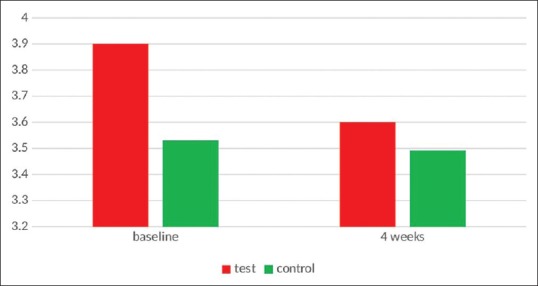
Clinical attachment level
Discussion
Periodontal diseases are the chronic infectious diseases resulting from complex host pathogenic interaction leading to destruction of periodontium and eventually tooth loss.[13]
In an attempt to combat periodontal diseases and attain periodontal health, various modalities of treatment have been advocated in the literature. Along with the conventional scaling and root planing, numerous antimicrobial agents have also been advocated to prevent periodontal disease progression.
LDD is one such treatment modalities that were introduced by Dr. Max Goodson in 1979 utilizing the concept of local delivery of therapeutic agents into the periodontal pocket with prolonged availability of the drug resulting in its sustainability and thereby attaining 100 folds of higher concentrations in the subgingival site. On the other hand, it also has various advantages of reducing the dangers such as adverse reactions[15] and resistant strains[16] compared to that of systemic administration which are likely to cause side effects.
Among the various antimicrobials used as LDD agents, ornidazole is the most recent one. Even though it belongs to nitroimidazole family, unlike metronidazole, it requires a very low minimum inhibitory concentration to inhibit the growth of periodontal pathogens as compared to that of metronidazole. Although the antimicrobial activity of ornidazole has been proposed to be due to the reduction of nitro group to a more reactive amine, thus attacking the microbial DNA, a combination of ornidazole and CHX on the other hand shows a prolonged antiplaque action, substantivity, and its ability to adsorb and desorb, thereby providing in effect, a timed release of the antimicrobial agent.[17,18,19]
Literature search, on the other hand, has proved AV which is a medicinal plant to have shown various applications in the treatment of lichen planus, submucous fibrosis, aphthous stomatitis, and radiation-induced oral mucositis.[20]
Although studies in the literature do exist evaluating the individual roles of ornidazole and AV, no study in the literature has compared the effects of Ornigreat™ and AV gels in the treatment of chronic periodontitis.
Hence, an attempt was made to evaluate periodontal parameters of both Ornigreat™ and AV gels at baseline and 4 weeks.
In the present study, when PI scores were evaluated, there was no statistically significant difference in the mean plaque scores between the two groups. These results correlate with the study done by Sato et al. 1993[21] and also correlated with the findings of a study done by Patel et al. 2014[22] in which ornidazole gel was used subgingivally after NSPT. On the other hand, in a study by Pradeep 2012,[23] where 1% metronidazole and 0.25% CHX gluconate (Metrohex™ gel) was used as an adjunct to NSPT in the treatment of gingivitis, a statistically significant difference in plaque scores was evident.
When the gingival scores were taken into consideration, there was no significant difference in the gingival scores between the two sites. These results are similar with the study done by Sato et al. 1993.[21] However, additional benefits can be obtained when antimicrobial gel is used as an adjunctive therapy as elicited by Pradeep 2012.[23] On the other hand, wherein 1% metronidazole and 0.25% CHX gluconate (Metrohex™ gel) was used as an adjunct to NSPT in the treatment of gingivitis, a statistically significant difference in PI, GI, and papillary BI was evidenced.
In a 6-month comparison study of the three local antimicrobial agents, wherein metronidazole, minocycline, and tetracycline were delivered into persistent periodontal pockets, all three showed better results than NSPT which correlated with the studies done by Radvar et al. 1996[24] and Kinane and Radvar 1999.[25]
The results of our study has clearly proposed the usage of aloevera to be an equally effective and an affordable substitute for Ornigreat.
The results of our study were also in accordance with the studies of Bhat et al.,[26] Virdi et al.,[27] Ajmera et al.,[28] and Chandrahas et al.,[29] wherein there was a statistically significant improvement in the PI, GI, and probing PD readings after 4 weeks in both the groups, and a statistically significant improvement was noticed in BI in the AV group when compared with that of Ornigreat™ group.
Taking CAL into consideration, though improvement was there in both the groups, it was not statistically significant. On comparing AV group and Ornigreat™ group after NSPT, there was no statistically significant difference between the groups. Studies in the literature do exist using 25% metronidazole[30] as a LDD agent in the treatment of chronic periodontitis wherein a statistically significant difference was elicited. Furthermore, metformin[31] when used as an agent locally to treat infrabony pockets, a gain in CAL, was observed.
In another study done by Jagadish et al.,[32] wherein CHX chip and varnish were used, a significant difference was found in CAL values.
However, ours was the first study wherein Ornigreat and AV gels were used to assess the CALs.
The herbal antimicrobial AV gel that was administered in the periodontal pockets in our study produced a similar improvement in clinical parameters compared to Ornigreat™ gel. However, the efficiency of this locally applied agent for chronic periodontitis on long-term basis needs further investigation.
Conclusion
Local application of combination of 1% ornidazole and 0.25% CHX (Ornigreat™) and AV gels after NSPT. When the results of both the test groups were compared, local application of AV can be an equally effective and affordable herbal substitute for Ornigreat™.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Kornman KS, Löe H. The role of local factors in the etiology of periodontal diseases. Periodontol. 2000;1993(2):83–97. doi: 10.1111/j.1600-0757.1993.tb00222.x. [DOI] [PubMed] [Google Scholar]
- 2.Slots J. Subgingival microflora and periodontal disease. J Clin Periodontol. 1979;6:351–82. doi: 10.1111/j.1600-051x.1979.tb01935.x. [DOI] [PubMed] [Google Scholar]
- 3.Pandit N, Dahiya R, Gupta R, Bali D, Kathuria A. Comparative evaluation of locally delivered minocycline and metronidazole in the treatment of periodontitis. Contemp Clin Dent. 2013;4:48–53. doi: 10.4103/0976-237X.111615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sunil A, Venkatesh M, Udupa N. Controlled drug delivery systems for periodontitis. Pharm Rev. 2004:61–82. [Google Scholar]
- 5.Lindhe J, Heijl L, Max Goodson J, Socransky SS. Local tetracycline delivery using hollow fiber devices in periodontal therapy. J Clin Periodontol. 1979;6:141–9. doi: 10.1111/j.1600-051x.1979.tb02193.x. [DOI] [PubMed] [Google Scholar]
- 6.Killoy WJ. Assessing the effectiveness of locally delivered chlorhexidine in the treatment of periodontitis. J Am Dent Assoc. 1999;130:567–70. doi: 10.14219/jada.archive.1999.0253. [DOI] [PubMed] [Google Scholar]
- 7.Greenstein G, Polson A. The role of local drug delivery in the management of periodontal diseases: A comprehensive review. J Periodontol. 1998;69:507–20. doi: 10.1902/jop.1998.69.5.507. [DOI] [PubMed] [Google Scholar]
- 8.Stanford TW., Jr Local drug delivery in the treatment of periodontitis. Tex Dent J. 2001;118:978–83. [PubMed] [Google Scholar]
- 9.Edwards DI. Nitroimidazole drugs – Action and resistance mechanisms I. Mechanisms of action. J Antimicrob Chemother. 1993;31:9–20. doi: 10.1093/jac/31.1.9. [DOI] [PubMed] [Google Scholar]
- 10.Stambaugh RV, Dragoo M, Smith DM, Carasali L. The limits of subgingival scaling. Int J Periodontics Restorative Dent. 1981;1:30–41. [PubMed] [Google Scholar]
- 11.Dat AD, Poon F, Pham KB, Doust J. Aloe vera for treating acute and chronic wounds. Cochrane Database Syst Rev. 2012;2:CD008762. doi: 10.1002/14651858.CD008762.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davis RH, Rosenthal KY, Cesario LR, Rouw GA. Processed Aloe vera administered topically inhibits inflammation. J Am Podiatr Med Assoc. 1989;79:395–7. doi: 10.7547/87507315-79-8-395. [DOI] [PubMed] [Google Scholar]
- 13.Cosyn J, Wyn I, De Rouck T, Sabzevar MM. A chlorhexidine varnish implemented treatment strategy for chronic periodontitis: Short-term clinical observations. J Clin Periodontol. 2005;32:750–6. doi: 10.1111/j.1600-051X.2005.00751.x. [DOI] [PubMed] [Google Scholar]
- 14.Velam V, Yalavarthi PR, Sundaresan C, Vandana K, Dudala TB, Kodavatikanti H, et al. In vitro and in vivo assessment of piroxicam incorporated Aloe vera transgel. Int J Pharm Investig. 2013;3:212–6. doi: 10.4103/2230-973X.121303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walker CB. Selected antimicrobial agents: Mechanisms of action, side effects and drug interactions. Periodontol. 2000;1996(10):12–28. doi: 10.1111/j.1600-0757.1996.tb00066.x. [DOI] [PubMed] [Google Scholar]
- 16.Walker CB. The acquisition of antibiotic resistance in the periodontal microflora. Periodontol. 2000;1996(10):79–88. doi: 10.1111/j.1600-0757.1996.tb00069.x. [DOI] [PubMed] [Google Scholar]
- 17.Van Winkelhoff AJ, Van der Velden U, Clement M, De Graaff J. Intra-oral distribution of black-pigmented Bacteroides species in periodontitis patients. Oral Microbiol Immunol. 1988;3:83–5. doi: 10.1111/j.1399-302x.1988.tb00087.x. [DOI] [PubMed] [Google Scholar]
- 18.Müller HP, Eickholz P, Heinecke A, Pohl S, Müller RF, Lange DE. Simultaneous isolation of Actinobacillus actinomycetemcomitans from subgingival and extracrevicular locations of the mouth. J Clin Periodontol. 1995;22:413–9. doi: 10.1111/j.1600-051x.1995.tb00169.x. [DOI] [PubMed] [Google Scholar]
- 19.Asikainen S, Chen C. Oral ecology and person-to-person transmission of Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis. Periodontol. 2000;1999(20):65–81. doi: 10.1111/j.1600-0757.1999.tb00158.x. [DOI] [PubMed] [Google Scholar]
- 20.Mangaiyarkarasi SP, Manigandan T, Elumalai M, Cholan PK, Kaur RP. Benefits of Aloe vera in dentistry. J Pharm Bioallied Sci. 2015;7:S255–9. doi: 10.4103/0975-7406.155943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sato K, Yoneyama T, Okamoto H, Dahlén G, Lindhe J. The effect of subgingival debridement on periodontal disease parameters and the subgingival microbiota. J Clin Periodontol. 1993;20:359–65. doi: 10.1111/j.1600-051x.1993.tb00373.x. [DOI] [PubMed] [Google Scholar]
- 22.Patel B, Shah S, Kumar S. Evaluation of ornidazole gel as an adjunct to the phase I therapy. Adv Hum Biol. 2014;4:21–5. [Google Scholar]
- 23.Pradeep AR, Kumari M, Priyanka N, Naik SB. Efficacy of chlorhexidine, metronidazole and combination gel in the treatment of gingivitis-a randomized clinical trial. J Int Acad Periodontol. 2012;14:91–6. [PubMed] [Google Scholar]
- 24.Radvar M, Pourtaghi N, Kinane DF. Comparison of 3 periodontal local antibiotic therapies in persistent periodontal pockets. J Periodontol. 1996;67:860–5. doi: 10.1902/jop.1996.67.9.860. [DOI] [PubMed] [Google Scholar]
- 25.Kinane DF, Radvar M. A six-month comparison of three periodontal local antimicrobial therapies in persistent periodontal pockets. J Periodontol. 1999;70:1–7. doi: 10.1902/jop.1999.70.1.1. [DOI] [PubMed] [Google Scholar]
- 26.Bhat G, Kudva P, Dodwad V. Aloe vera: Nature's soothing healer to periodontal disease. J Indian Soc Periodontol. 2011;15:205–9. doi: 10.4103/0972-124X.85661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Virdi HK, Jain S, Sharma S. Effect of locally delivered Aloe vera gel as an adjunct to scaling and root planing in the treatment of chronic periodontitis: A clinical study. Indian J Oral Sci. 2012;3:84–9. [Google Scholar]
- 28.Ajmera N, Chatterjee A, Goyal V. Aloe vera: It's effect on gingivitis. J Indian Soc Periodontol. 2013;17:435–8. doi: 10.4103/0972-124X.118312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chandrahas B, Jayakumar A, Naveen A, Butchibabu K, Reddy PK, Muralikrishna T, et al. Arandomized, double-blind clinical study to assess the antiplaque and antigingivitis efficacy of Aloe vera mouth rinse. J Indian Soc Periodontol. 2012;16:543–8. doi: 10.4103/0972-124X.106905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Riep B, Purucker P, Bernimoulin JP. Repeated local metronidazole-therapy as adjunct to scaling and root planing in maintenance patients. J Clin Periodontol. 1999;26:710–5. doi: 10.1034/j.1600-051x.1999.t01-2-261101.x. [DOI] [PubMed] [Google Scholar]
- 31.Pradeep AR, Rao NS, Naik SB, Kumari M. Efficacy of varying concentrations of subgingivally delivered metformin in the treatment of chronic periodontitis: A randomized controlled clinical trial. J Periodontol. 2013;84:212–20. doi: 10.1902/jop.2012.120025. [DOI] [PubMed] [Google Scholar]
- 32.Jagadish Pai BS, Rajan SA, Srinivas M, Padma R, Suragimath G, Walvekar A, et al. Comparison of the efficacy of chlorhexidine varnish and chip in the treatment of chronic periodontitis. Contemp Clin Dent. 2013;4:156–61. doi: 10.4103/0976-237X.114848. [DOI] [PMC free article] [PubMed] [Google Scholar]


