Abstract
Aim:
The aim of this study was to assess the effectiveness of two herbal mouthwashes in comparison with Chlorhexidine mouthwash on gingivitis.
Materials and Methods:
This is a triple-blind randomized controlled clinical trial, where in 60 patients were randomly allocated into three study groups: Triphala mouthwash (Group A), Aloe vera mouthwash (Group B), and Chlorhexidine mouthwash (Group C). All groups were treated with scaling and asked to rinse with respective mouthwashes twice daily for 1 month. Clinical parameters such as plaque index (PI), gingival index (GI), and bleeding index (BI) were recorded at baseline, 15 days, and 30 days, respectively.
Results:
Our results suggested that Triphala group effectively demonstrated a higher reduction in GI and BI index scores compared to A. vera group (P ≤ 0.005) and the effect is equivocal to the reduction seen with Chlorhexidine group. However, no statistically significant difference was observed between the mouthwashes in reduction of PI scores (P > 0.005).
Conclusion:
The results of our study evidenced Triphala to be superior in the reduction of plaque, gingival inflammation, and bleeding compared to that of A. vera. However, the results of our study also indicated that Triphala was as effective as chlorhexidine mouthwash in its ability in reducing plaque accumulation, gingival inflammation, and bleeding. Furthermore, Triphala is relatively free of side effects compared to that of chlorhexidine.
Keywords: Aloe vera, anti-inflammatory, chlorhexidine, gingivitis, triphala
Introduction
It is well established that periodontal disease is one of the major reasons for tooth loss. Among the various periodontal disease conditions, gingivitis is the most commonly occurring one. While gingivitis manifests itself as the inflammation of gums, periodontitis manifests as an inflammatory condition of periodontal tissues eventually resulting in tooth loss. It is very well evidenced that periodontal health plays a key role in the oral as well as in the overall health of an individual.
The prime culprit associated with this condition is the presence of plaque biofilm which is a nonspecific but highly variable structural entity, resulting from colonization and growth of microorganisms on the surfaces of natural teeth and prosthesis and plays a major role in the initiation and progression of periodontal disease.
Hence, elimination of plaque and its control plays a vital role in the maintenance of periodontal health.
Plaque control which determines the long-term success of any periodontal therapy is broadly categorized as mechanical and chemical plaque control. As mechanical plaque control helps in reduction of the rate of deposits, it is commonly executed on a regular basis. On the other hand, chemical plaque control can be considered as an adjunct to mechanical plaque control, and various agents have been successfully used for control and prevention of gingivitis.
Antimicrobial mouthrinses as adjuncts in the maintenance of oral hygiene have assumed greater importance, as they have been found to be very effective in reducing the oral microbial load. Among the innumerable antimicrobial rinses available, one of the gold standards in enhancing the periodontal health is the chlorhexidine mouthwash which has been considered to be the most efficient one till date.
However, it has its own inherent side effects such as staining of teeth, metallic taste, burning sensation, and altered taste perception.[1] Weighing the advantages over disadvantages, the paradigm shift toward herbal products that have been used for centuries in including oral health have also been identified in the literature.
Such products which are not only locally available but are also culturally acceptable and affordable to be a great extent include Aloe vera and Triphala to be named as few.
Triphala, which is a Rasayana Drug used in the Indian System of Medicine, has been used in Ayurveda, from time immemorial with many potential systemic benefits.[2] Triphala, which is a mixture of three herbal products, i.e., Terminalia chebula, Terminalia bellirica, and Embilica officinalis, has been shown to exhibit strong antioxidant, immunomodulatory, anti-inflammatory, analgesic, astringent, antimetastatic properties, etc., A. vera have been used therapeutically, certainly since Roman times and perhaps long before. The pharmacological actions of A. vera as studied in vitro or in animals include anti-inflammatory and anti-arthritic activity and antibacterial and hypoglycemic effects.[3]
These two products have elicited their beneficial effects on gingiva apart from their various systemic and immunological benefits. Hence, an attempt was made in this study to assess the efficacy of these two herbal products on the antiplaque and antigingivitis effects.
Materials and Methods
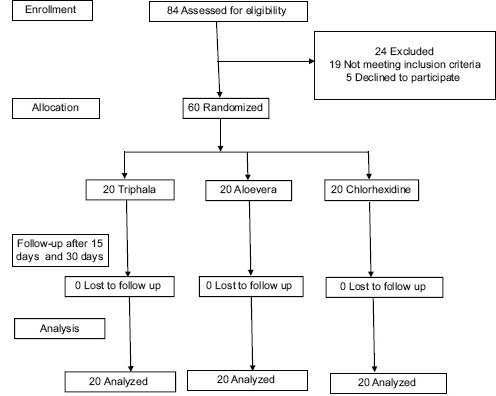
Sixty individuals (29 males and 31 females, aged 18–40 years; mean age: 29 years) with chronic generalized gingivitis were recruited from the Outpatient Department of Periodontics, Vishnu Dental College, Bhimavaram. The research protocol was approved by the Institute and all participants were recruited into the study, after a written informed consent was obtained.
Inclusion and exclusion criteria
After having a thorough clinical examination and medical history, systemically healthy individuals with previously untreated gingivitis were selected for the study with following inclusion criteria: Clinical parameters for inclusion were: age groups of 18–45 years with mild-to-moderate gingivitis, patients with a minimum of 20 teeth, and patients who have not undergone periodontal treatment in the past 6 months.
Exclusion criteria were: (1) use of anti-inflammatory drugs and antibiotics in previous 6 months; (2) individuals with prosthetic appliances or orthodontic appliances that would interfere with evaluation; (3) allergy to Triphala, A. vera, and Chlorhexidine used in the study; (4) smokers or users of tobacco in any form; (5) lactating females or pregnant women; and (6) mental-retarded patients.
Study design and treatment protocol
The current study was designed as a double-masked, randomized, placebo-controlled clinical trial. Out of 84 individuals who were assessed for eligibility, 60 individuals met inclusion criteria and were randomly assigned to one of three groups were randomly assigned to the participants of the study.
Group A: n = 20 was given Triphala mouthwash
Group B: n = 20 was given A. vera mouthwash
Group C: n = 20 was given Chlorhexidine mouthwash.
Method of preparation of Triphala mouthwash
TRP is available in a finely sieved powder form called churna. Churna form has a shelf life of 6 months. In the present study, 6% Triphala mouthwash was prepared. Sixty grams of pure Triphala churna was dissolved in 1 L of distilled water to obtain 6% of extract. To improve patient compliance, 2 mL of glycerin (sweetening agent) and 1 mL of Pudin Hara (flavoring agent) were added to the solution. The solution was brought to a boil for 10 min, then cooled and filtered.
TRP and placebo mouthwashes were prepared at Vishnu dental College, Bhimavaram, India. These medications were placed in brown-colored opaque bottles marked only with patient number by the study coordinator. Materials used for recording indices mouth mirror, explorer, periodontal probe, and tweezers. Baseline (B/L) examination was performed for individuals who agreed to participate in the study. Individuals were instructed to refrain from any oral hygiene methods (including chewing gum) for 8 h prior to B/L and follow-up examinations. Clinical parameters recorded were: (1) plaque index (PI), (2) gingival index (GI), and (3) bleeding index (BI), after which all individuals received scaling and polishing with fluoride-containing paste to remove plaque, calculus, and extrinsic stains. The brown-colored opaque bottles were distributed to individuals by the clinical examiner who was masked to packet contents. Individuals were instructed to use 15 mL mouthwash twice daily, 30–45 min after brushing, and further instructed not to rinse/eat anything for 30 min after its usage. Individuals were also instructed to refrain from any forms of oral hygiene aids, including dental floss and chewing gum, during the study.
Statistical analysis
All the data were entered in Microsoft Excel Version 2014 and is statistically analyzed using SPSS version 20 SPSS (IBM, Chicago, US). For intergroup comparisons, one-way ANOVA followed by Tukey's post hoc test was used. For intragroup comparisons, repeated-measures ANOVA followed by least significant difference Bonferroni test was used. Mean percentage reduction in index scores in comparison with the B/L scores was also calculated [Chart 1].
Chart 1.
A complete chart describing the statistical analysis of study
Results
The protocol of the study was followed by all the participants in our study. The mean plaque scores, gingival scores, bleeding scores at B/L, 15 days, and at 30 days were tabulated.
The mean values of PI, GI, and BI of three groups (Triphala, A. vera, and Chlorhexidine at different time intervals).
Plaque index
At B/L, PI values [Table 1] for the Triphala group, A. vera group, and Chlorhexidine group were 1.3685 ± 0.29623, 1.3715 ± 0.13963, and 1.3850 ± 0.30672, respectively. There was not any significant difference between all three groups at the B/L (P = 0.977).
Table 1.
Plaque index
| Group A | Group B | Group C | ANOVA (F) | P | |
|---|---|---|---|---|---|
| PI baseline | 1.3685 | 1.3715 | 1.3850 | 0.023 | 0.977 |
| PI 15 days | 0.6085 | 0.6270 | 0.5145 | 1.713 | 0.189 |
| PI 30 days | 0.4990 | 0.5050 | 0.4195 | 1.348 | 0.268 |
PI: Plaque index
At 15 days, in Triphala group, A. vera group, and Chlorhexidine group, the PI values were 0.6085 ± 0.23663, 0.6270 ± 0.12770, and 0.5145 ± 0.23482, respectively. There was not any significant difference between all the three groups at 15 days (P = 0.189).
At 30 days, in the Triphala group, A. vera group, and Chlorhexidine group evidenced the plaque values of 0.4990 ± 0.19633, 0.5050 ± 0.10952, and 0.4195 ± 0.22542, respectively. No significant difference between all the three groups at 30 days (P = 0.268) was noticed. However, greater reduction was seen in Chlorhexidine followed by Triphala and A. vera.
Gingival index
At B/L, GI values [Table 2] for the Triphala group, A. vera group, and Chlorhexidine group were 1.2380 ± 0.25554, 1.2625 ± 0.27484, and 1.2720 ± 0.37346, respectively. There was no significant difference between all the groups at B/L (P = 0.936).
Table 2.
Gingival index
| Group A | Group B | Group C | ANOVA (F) | P | |
|---|---|---|---|---|---|
| G I baseline | 1.2380 | 1.2625 | 1.2720 | 0.066 | 0.936 |
| G I 15 days | 0.5860 | 0.5926 | 0.4955 | 1.373 | 0.262 |
| G I 30 days | 0.1970 | 0.3355 | 0.2025 | 12.037 | 0.000 |
GI: Gingival index
At 15 days, the Triphala group, A. vera group, and Chlorhexidine group exhibited the GI values of 0.5860 ± 0.26033, 0.5926 ± 0.13573, and 0.4955 ± 0.20595, respectively. There was not any significant difference between all the three groups at 15 days (P = 0.262).
At 30 days, the Triphala group, A. vera group, and Chlorhexidine group exhibited the GI values of 0.1970 ± 0.07299, 0.3355 ± 0.09987, and 0.2025 ± 0.12392, respectively. In intergroup comparison, the values in the Triphala group were found to be statistically significant (P = 0.00).
Higher and similar reduction was seen in Triphala and Chlorhexidine groups which varied significantly with A. vera group.
Bleeding index
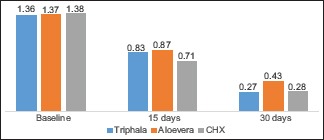
At B/L, BI values [Table 3] [Graph 1] for the Triphala group, A. vera group, and Chlorhexidine group were 1.3635 ± 0.28303, 1.3716 ± 0.24088, and 1.3800 ± 0.47197, respectively. There was no significant difference between all the groups at the B/L (P = 0.989).
Table 3.
Bleeding index
| Group A | Group B | Group C | ANOVA (F) | P | |
|---|---|---|---|---|---|
| BI baseline | 1.3635 | 1.3716 | 1.3800 | 0.011 | 0.989 |
| BI 15 days | 0.8330 | 0.8745 | 0.7135 | 3.769 | 0.029 |
| BI 30 days | 0.2710 | 0.4275 | 0.2845 | 5.859 | 0.005 |
BI: Bleeding index
Graph 1.
Mean bleeding index scores among the three group at baseline, 15, and 30 days
At 15 days, in Triphala group, A. vera group, and Chlorhexidine group, the BI values were 0.8330 ± 0.19375, 0.8745 ± 0.16869, and 0.7135 ± 0.21266, respectively. In an intergroup comparison, the Chlorhexidine group showed slightly better results than the other groups, wherein the difference was statistically significant (P = 0.029).
At 30 days, the values in Triphala group, A. vera group, and Chlorhexidine group were 0.2710 ± 0.12251, 0.4275 ± 0.19134, and 0.2845 ± 0.15936, respectively. In an intergroup comparison, triphala group exhibited values that were statistically significant (P = 0.05).
Higher and similar reduction was seen in Triphala and Chlorhexidine groups which varied significantly with A. vera.
On intragroup comparison, all the groups have demonstrated similar and statistically significant reduction in PI, GI, and BI scores at the end of 30 days when compared with B/L (P ≤ 0.05). For PI at the end of 30 days, the values in Triphala group, A. vera group, and Chlorhexidine group have demonstrated a reduction of 63.5%, 63.5%, and 70.28%, respectively. For GI at the end of 30 days, the values in Triphala group, A. vera group, and Chlorhexidine group have demonstrated a reduction of 84.5%, 73.8%, and 84.25%, respectively. However, for BI at the end of 30 days, the values in Triphala group, A. vera group, and Chlorhexidine group have demonstrated a reduction of 80%, 69.34%, and 79.71%, respectively.
Discussion
In our study which is the first of its kind in comparing the efficacy of Triphala and A. vera mouthwashes with a Chlorhexidine mouthwash in reducing the gingival inflammation, plaque scores, and bleeding scores in patient with generalized gingivitis included a total sample of 60 participants divided into three equal groups. All the three parameters PI, GI and BI were evaluated at B/L, 15 days, and 30 days after the usage of respective mouthwash. All the three mouthwashes were effective in reducing the scores of PI, GI, and BI when compared to the B/L values in our study.
In our study after a revaluation of 15 days, the Chlorhexidine group showed better reduction in PIs, GIs, and BIs followed by reductions in Triphala group and A. vera group, respectively, and these results are similar to those of studies conducted by Irfan et al. for 7, 30, and 45 days, respectively.[4]
On the other hand, when the efficacy of three mouthwashes was evaluated after 1 month, Chlorhexidine showed superior results in PI, compared to that of other two groups, whereas the reduction in GIs and BIs was evident in Triphala group. These results are similar to those of studies conducted by Naiktari et al. and Bajaj and Tandon, wherein the efficacy of Chlorhexidine and Triphala were evaluated for 15 days.[5,6] These results are also similar to those of studies conducted by Mamgain P et al. where similar reduction in scores of PI, GI, and organoleptic scores was observed when Chlorhexidine was compared with Triphala and Ela decoction.[7]
In our study groups, the reduction of PI was effective in the Chlorhexidine group at the end of 15 days and 30 days, respectively, compared to the other two groups which are consistent to the results of Bhattacharjee and Nekkanti.[7]
Aloe vera mouthwash has shown its efficacy in reduction of plaque, which was in accordance with an in vitro study done by Lee et al. which demonstrated the anti-bacterial effects of Aloe vera on Streptococcus mutans, Streptococcus sanguis and A. viscous. Furthermore, a significant reduction in plaque scores was evidenced in a 4-day plaque regrowth model in studies conducted by Manasa and Aniruth (2014).
Chlorhexidine proved to be effective in reducing all the three indices when compared to Aloe Vera and the results are consistent with the results of Karim et al.[8] However, the results of our study have not evidenced the significant reduction in the GI and BI at the end of 15 days in the A. vera group.
On the contrary when the efficacy of chlorhexidine and A. vera were evaluated at 15 days and 30 days, respectively, in a study done by Vangipuram et al., both the groups were equally effective in reducing the clinical parameters.[9]
A study done by Chandrahas et al., Gupta et al., and Chhina et al. on the efficacy of aloevera mouthwash has shown a significant reduction in PIs, GIs and BIs at 7, 14, and 22 days interval which is in contrary to the findings of our study at 30 days.[10,11,12] However, the results of our study were also in accordance with the study of Rezaei et al., wherein there was a significant improvement in the GIs and BIs after 1 month usage of A. vera mouthwash.[1]
While these reductions in scores were maintained in all the three groups after 30 days reevaluation, there was a slight difference in all three variables. The reduction in plaque scores was again better in Chlorhexidine group when compared to Triphala and A. vera groups. However, the results of GIs and BIs were more consistent in Triphala group at the end of 30 days followed by Chlorhexidine and A. vera, respectively. Thus, from the observations of our study, it can very well be elicited that Triphala mouthrinse can be used with effective results over a period of 30 days.
However, there was no much statistically significant difference as all the compounds possessed anti-inflammatory and antibacterial properties. The results were comparable to a study done by Sharma et al., wherein no statistical difference in the results was observed when Triphala and Chlorhexidine were compared in 210 patients after 7 and 15 days.[13] Patients have also been highly motivated to maintain oral hygiene which might have caused reduction in inflammation. The usage of herbal products have proved to be safe and effective. However concrete statistical evidence regarding their additional benefit when compared to chemical agents needs to be further established. However, further studies with larger sample sizes are recommended with Triphala and A. vera to further evaluating their antiplaque and antigingivitis efficacy.
Conclusion
Chemical plaque control is considered to be corner stones of periodontal health maintenance in adjunct with mechanical plaque control. Although chlorohexidine is considered to be gold standard agent for chemical plaque, various herbal preparations are in demand to overcome the side effects associated with chlorohexidine. One of the limitations of our study attributes to the sample size and results cannot be generalized as the study population include patient attending to the dental college. Also further studies with sample sizes are recommended with two herbal products Triphala and Aloe Vera in evaluating their antiplaque and antigingivitis efficacy.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Rezaei S, Rezaei K, Mahboubi M, Jarahzadeh MH, Momeni E, Bagherinasab M, et al. Comparison the efficacy of herbal mouthwash with chlorhexidine on gingival index of intubated patients in intensive care unit. J Indian Soc Periodontol. 2016;20:404–8. doi: 10.4103/0972-124X.194269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gowda D, Ganesh M, Rohan D, Rangesh P. Phytochemical and pharmacological actions of triphala: Ayurvedic formulation-A review. Int J Pharm Sci Rev Res. 2012;15:61–5. [Google Scholar]
- 3.Ajmera N, Chatterjee A, Goyal V. Aloe vera: It's effect on gingivitis. J Indian Soc Periodontol. 2013;17:435–8. doi: 10.4103/0972-124X.118312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Irfan M, Kumar S, Amin V. Evaluation of efficacy of triphala mouth rinse as coadjuvant in the treatment of chronic generalized periodontitis: A randomized clinical trial. Mouth Teeth. 2017;1:4–6. [Google Scholar]
- 5.Naiktari RS, Gaonkar P, Gurav AN, Khiste SV. A randomized clinical trial to evaluate and compare the efficacy of triphala mouthwash with 0.2% chlorhexidine in hospitalized patients with periodontal diseases. J Periodontal Implant Sci. 2014;44:134–40. doi: 10.5051/jpis.2014.44.3.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bajaj N, Tandon S. The effect of triphala and chlorhexidine mouthwash on dental plaque, gingival inflammation, and microbial growth. Int J Ayurveda Res. 2011;2:29–36. doi: 10.4103/0974-7788.83188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mamgain P, Kandwal A. Mamgain RK Comparative Evaluation of Triphala and Ela Decoction With 0.2% Chlorhexidine as Mouthwash in the Treatment of Plaque-Induced Gingivitis and Halitosis:A Randomized Controlled Clinical Trial. J Evid Based Complementary Altern Med. 2017;22:468–472. doi: 10.1177/2156587216679532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karim B, Bhaskar DJ, Agali C, Gupta D, Gupta RK, Jain A, et al. Effect of Aloe vera mouthwash on periodontal health: Triple blind randomized control trial. Oral Health Dent Manag. 2014;13:14–9. [PubMed] [Google Scholar]
- 9.Vangipuram S, Jha A, Bhashyam M. Comparative efficacy of Aloe vera mouthwash and chlorhexidine on periodontal health: A randomized controlled trial. J Clin Exp Dent. 2016;8:e442–7. doi: 10.4317/jced.53033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chandrahas B, Jayakumar A, Naveen A, Butchibabu K, Reddy PK, Muralikrishna T. A randomized, double-blind clinical study to assess the antiplaque and antigingivitis efficacy of Aloe vera mouth rinse. J Indian Soc Periodontol. 2012;16:543–8. doi: 10.4103/0972-124X.106905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gupta RK, Gupta D, Bhaskar DJ, Yadav A, Obaid K, Mishra S. Preliminary antiplaque efficacy of Aloe vera mouthwash on 4 day plaque re-growth model: Randomized control trial. Ethiop J Health Sci. 2014;24:139–44. doi: 10.4314/ejhs.v24i2.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chhina S, Singh A, Menon I, Singh R, Sharma A, Aggarwal V. A randomized clinical study for comparative evaluation of Aloe vera and 0.2% chlorhexidine gluconate mouthwash efficacy on de-novo plaque formation. J Int Soc Prev Community Dent. 2016;6:251–5. doi: 10.4103/2231-0762.183109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sharma AR, Kannan KS, Sharma H, Amith P, Chandran DR, Sharma P. The effect of triphala and chlorhexidine mouthwash on dental plaque and gingival inflammation. Indian J Med Sci. 2018;70:1–6. [Google Scholar]


