Abstract
Aim:
The aim of this study was to determine the prevalence of periodontal disease in type 2 diabetes mellitus (T2DM) patients of North India.
Materials and Methods:
A total of 500 patients fulfilling the selection criteria were initially given a health questionnaire to gather information regarding their demographic characteristics, attitude for oral hygiene, and disease status. Based on eligibility 427 patients were finally recruited for statistical analysis. A partial-mouth periodontal examination (PMPE) protocol which assessed one maxillary quadrant and one mandibular quadrant was used to examine three fixed sites per tooth (mesiobuccal, midbuccal, and distobuccal). Gingival Index, Oral Hygiene Index-Simplified, Debris Index-Simplified, Calculus Index-Simplified (CI-S), probing pocket depth, and clinical attachment level were examined.
Results:
More than 90% (95.1%) of the total diabetic participants had some degree of periodontal destruction. Of the total population, 27.1% of participants had good oral hygiene, 68.8% had fair oral hygiene, and 3.9% had poor oral hygiene status. The prevalence of severe periodontitis in participants with good, fair, and poor oral hygiene status was reported as 0.8%, 17%, and 29.4%, respectively. The prevalence of severe periodontitis in participants with good, fair, and poor oral hygiene status with poor glycemic control (glycated hemoglobin ≥8%) was 2.5%, 28.1%, and 30.7%, respectively.
Conclusion:
This single-centered cross-sectional study represents that more than 95% of type 2 diabetic patients have some periodontal destruction. These results may act as baseline data to promote the collaborative integrated management of diabetes for reducing its burden on society.
Keywords: Glycemic control, periodontitis, type 2 diabetes mellitus
Introduction
Diabetes mellitus is well-predictable risk factor for periodontal disease, and in converse, periodontitis is thought to affect the systemic inflammatory condition, insulin resistance, and lipid and glucose metabolism. In 2000, India (31.7 million) topped the world with the maximum number of people with diabetes mellitus followed by China (20.8 million) with the United States (17.7 million) in the second and third place, respectively.[1] The prevalence of diabetes in India is diverse in different region of the country. The National Urban Survey conducted across the metropolitan cities of India reported 11.7% in Kolkata (East India), 6.1% in Kashmir Valley (North India),[2] 11.6% in New Delhi (North India), 16.6% in Hyderabad (South India), 13.5% in Chennai (South India), 9.3% in West India (Mumbai), and 12.4% in Bangalore (South India).[3] The existing prevalence of self-reported type 2 diabetes mellitus (T2DM) in Lucknow region (India) is 24.6%.[4] Periodontitis is responsible for increasing insulin resistance and poor glycemic control,[5] thus worsening the condition of diabetics, and conversely, improvement in glycemic control has been advocated in several studies after periodontal therapy.[6,7] Consequently, recording prevalence and severity of periodontitis in diabetic patients is need of the hour.
Therefore, the primary objective of the study was to determine the prevalence and correlation between severity of periodontal destruction, oral hygiene, and glycemic status in T2DM patients of Uttar Pradesh region (India).
Materials and Methods
This cross-sectional study was performed in the Department of Periodontology, Saraswati Dental College, Lucknow, in collaboration with Dr. Ram Manohar Lohia Combined Hospital, Gomti Nagar, Lucknow, from January 2015 to November 2016. The study population consisted of the known type 2 diabetic patients attending the outpatient department of the Department of Periodontology, Saraswati Dental College, Lucknow, and Dr. Ram Manohar Lohia Combined Hospital, Gomti Nagar, Lucknow. All eligible participants were thoroughly informed of the nature, potential risks, and benefits of their participation in the study.
Inclusion criteria
Patients, within the age group of 30–65 years, have been diagnosed as T2DM for at least the past 2 years based on criteria given by the WHO[8,9] (random blood sugar [RBS] level ≥200 mg/dl, fasting plasma glucose ≥126 mg/dl, and 2-h postprandial glucose ≥200 mg/dl)
Having not <20 remaining teeth in oral cavity
Nonsmoker, nonalcoholic, nonpregnant, and nonlactating must not be suffering from any disease other than T2DM.
Exclusion criteria
Patients taking any medication other than hypoglycemic agents
Females who were pregnant, lactating, and postmenopausal
Patients who have undergone periodontal treatment over the preceding 6 months
Based on clinical examination (tender on percussion/grossly decayed with pulp exposure), participants with suspected periapical pathology, orthodontic appliances, and multiple systemic complications of diabetes mellitus were also excluded from the study.
Sample size
Studies advocate 25%–98% prevalence of periodontal disease in type 2 diabetes mellitus patients. In the present study, expecting at least 50% prevalence of periodontal disease in type 2 diabetes mellitus patients of Uttar Pradesh region (India), with considering 5% margin of error (Type I error: α = 0.05) and 80% power (Type II error: 1-β = 0.80), the minimum sample size required will be 400 evaluated. Thus, the study required a minimum of 400 participants.[10]
Methodology
Minimum sample size recommended for this cross-sectional study was 400. To overcome sample attrition, a total of 500 patients were initially screened for the study, based on the above-mentioned inclusion and exclusion criteria [Figure 1]. Detailed medical and dental records were obtained. Patients fulfilling the selection criteria were initially given a health questionnaire to gather information regarding their demographic characteristics, attitude for oral hygiene, and diabetes status. The questionnaire and study protocol were approved by the Institutional Research and Development Committee of Saraswati Dental College, Lucknow, India (SDC/IRDC/2014/MDS-P/24). Investigator personally disseminated the questionnaire and got them completed by cross-checking through interview, thereby avoiding any obscurity pertaining to questionnaire. All participants provided written informed consent.
Figure 1.
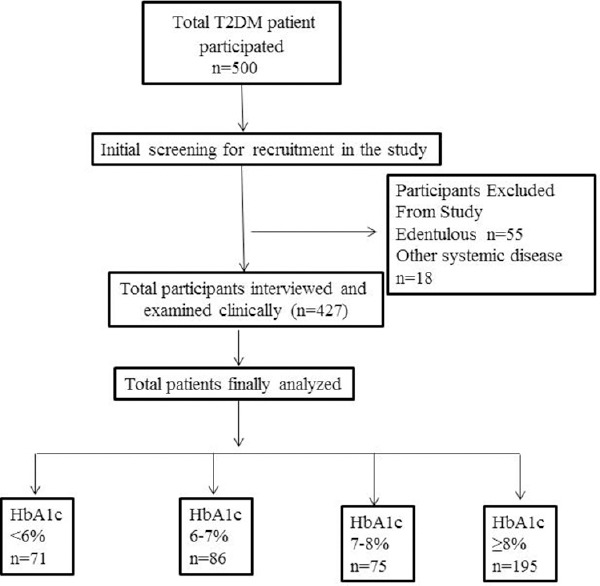
Flowchart representing division of stud
Questionnaire validity
To assess the validity of the questionnaire for assessing periodontal disease in T2DM patients, a pilot study was conducted on 10 randomly selected patients before conducting the study. From questionnaire, 4 randomly selected questions were assessed before and after 1 week from the same individuals and analyzed by kappa test (κ). The kappa test showed good-to-very good strength of agreement before and after response to questionnaire suggesting high validity of the questionnaire.
Systemic and health parameters
Patients fulfilling the requisite criteria were then examined and investigated for following systemic parameters to rule out any further systemic involvement other than T2DM. Investigation reports of all the patients were carefully analyzed before recruiting the participants for clinical examination. These are vital parameters such as blood pressure, pulse rate (beats/min), temperature and respiratory rate (breath/min), measured waist circumference, and body mass index (BMI). RBS as measured by chairside digital glucometer (Omron Blood Glucose Monitoring System, Model: HGM-11, Omron Healthcare India Pvt., Ltd.) and glycated hemoglobin (HbA1c) using nephelometry method using HbA1c kit (Agappe Diagnostics Ltd., Kerala, India).
Clinical parameters
All the participants (n = 427) received oral examination using diagnostic instruments. A partial-mouth periodontal examination (PMPE) protocol which assesses fewer sites yet still estimating the overall periodontal status except for Gingival Index (GI) and Oral Hygiene Index-Simplified (OHI-S) is used for this population-based study. Accordingly, one maxillary quadrant and one mandibular quadrant were selected and examined three fixed sites per tooth (mesiobuccal, midbuccal, and distobuccal).[11] To reduce the individual variability, all the clinical measurements were recorded by a single calibrated investigator throughout the study.
The following clinical parameters were assessed. GI,[12] OHI-S,[13] Debris Index-Simplified (DI-S), Calculus Index-Simplified, probing pocket depth (PPD), and clinical attachment loss (CAL).
Intra-observer reliability
To assess the reliability of periodontal parameters such as PPD and CAL in the present study, a pilot study was conducted on 10 randomly selected patients before conducting the study. The PPD and CAL were assessed before and after 24 h by the same observer in random order and analyzed by intraclass correlation (ICC) analysis and summarized in Table 1. The ICC showed a significant (P < 0.001) and positive correlation in both PPD (ICC = 0.988) and CAL (ICC = 0.999) between the two periods (before and after 24 h) indicating high intra-observer reliability of the periodontal parameters.
Table 1.
Intra-observer reliability of periodontal parameters between two periods by intraclass correlation coefficient analysis (n=10)
| Serial number | PPD (mm) | CAL (mm) | ||||
|---|---|---|---|---|---|---|
| Before | After 24 h | ICC value | Before | After 24 h | ICC value | |
| 1 | 1.72 | 1.66 | 0.988*** | 3.38 | 3.33 | 0.999*** |
| 2 | 1.44 | 1.55 | 1.44 | 1.55 | ||
| 3 | 1.38 | 1.33 | 0.44 | 0.38 | ||
| 4 | 1.44 | 1.38 | 2.11 | 2.05 | ||
| 5 | 1.38 | 1.38 | 3.72 | 3.72 | ||
| 6 | 2.27 | 2.27 | 6.66 | 6.66 | ||
| 7 | 2.88 | 2.77 | 1.88 | 1.83 | ||
| 8 | 1.33 | 1.33 | 0.77 | 0.77 | ||
| 9 | 2 | 1.94 | 2 | 1.94 | ||
| 10 | 1.22 | 1.05 | 1.66 | 1.5 | ||
***P<0.001. PPD: Probing pocket depth; CAL: Clinical attachment level; ICC: Intraclass correlation
Statistical analysis
Quantitative data were summarized as mean ± standard error while discreet (categorical) in number and percentages. Continuous groups were compared by independent Student's t-test while categorical groups were compared by Chi-square test. Spearman's rank-order correlation analysis was done to assess association between the variables. The intra-observer agreement was done by ICC coefficient analysis. Reliability of questionnaire was assessed by unweighted kappa (κ) test. Groups were compared by one-way analysis of variance, and the significance of mean difference between the groups was done by Tukey's honestly significant difference post hoc test. A two-tailed (α = 2) P < 0.05 was considered statistically significant. Analyses were performed on SPSS software, window version 17.0 (Chicago, Inc., USA).
Results
For the present study, a total of 500 patients were interviewed in a tertiary hospital of the North Indian city representing the broad range of population. Among 500 participants, 73 were excluded during the final data analysis. Out of 73 individuals, 55 patients were edentulous and 18 individual had other systemic diseases such as hypertension and hyperlipidemia. In the present study, the mean age of diabetic patients was 49.13 ± 0.49 years, with a range of 30-65 years [Table 2]. Most of the diabetic patients (30.7%) were between 40 and 50 years of age. The average age of patients with normal glycemic (HbA1c <6%), good glycemic control (HbA1c 6%–7%), moderate glycemic control (HbA1c 7%–8%), and poor glycemic control (HbA1c ≥8%) was 50.5, 48.6, 50.4, and 48.33 years, respectively. In the present study, the ratio of male-to-female patients was 1.06. The ratio of male-to-female patients in all the four categories of glycemic control was 1.53, 0.95, 0.82, and 1.07 for normal glycemic, good, moderate, and poor glycemic control, respectively. The average BMI of the participants was 24.31 ± 0.19, with a range of 13–36 kg/m2. The average BMI (with range) in all the four categories of glycemic control was 24.54 (16.6–36.5), 23.6 (15.6–36), 24.44 (17–33), and 24.51 (13.14–35.27) kg/m2 in normal, good, moderate, and poor glycemic control, respectively [Table 2]. More than 58.8% of the total diabetic patients had BMI of ≤25 kg/m2 and 41.2% had >25 kg/m2. Out of total patients who were overweight (>25 kg/m2), 49.2% had poor glycemic control.
Table 2.
Average values of various parameters and vitals
| Parameters | Mean±SE | Median (range) |
|---|---|---|
| Age (years) | 49.13±0.49 | 50 (30-65) |
| Blood pressure (mmHg) | ||
| Systolic | 123.04±0.28 | 122 (110-135) |
| Diastolic | 82.23±0.21 | 81 (76-92) |
| Temperature (°F) | 98.58±0.03 | 99 (97.5-99.9) |
| Respiratory rate (breath/min) | 15.44±0.08 | 16 (12-19) |
| Weight (kg) | 64.75±0.64 | 64 (30-111) |
| Height (cm) | 162.83±0.36 | 162 (150-185) |
| WC (cm) | 84.11±0.56 | 81 (66-114) |
| BMI (kg/m2) | 24.31±0.19 | 24 (13-36) |
| HbA1c (%) | 7.90±0.10 | 8 (4-15) |
| FPG (mg/dl) | 193.11±3.74 | 183 (71-548) |
| PP plasma glucose (mg/dl) | 278.62±4.43 | 280 (95-620) |
| RBS (mg/dl) | 241.34±5.10 | 217 (81-589) |
| OHI-S | 1.684±0.031 | 1.7 (0.33-3.83) |
| GI | 1.253±0.014 | 1.2 (0.54-2.66) |
| CAL (mm) | 3.386±0.067 | 3.4 (0.00-7.38) |
| PPD (mm) | 3.271±0.051 | 3.2 (1.00-9.29) |
WC: Waist circumference; BMI: Body mass index; HbA1c: Glycated hemoglobin; FPG: Fasting plasma glucose; PP: Postprandial; RBS: Random blood sugar; OHI-S: Oral Hygiene Index-Simplified; GI: Gingival Index; PPD: Probing pocket depth; CAL: Clinical attachment level; SE: Standard error
The result of the present study suggested that 95.1% of the diabetic participants examined had at least some amount of periodontal destruction. The prevalence of no periodontitis, mild periodontitis, moderate periodontitis, and severe periodontitis was 4.9%, 30.9%, 51.1%, and 13.1%, respectively, among diabetic patients, which has been observed in this study [Table 3]. Association of HbA1c with various periodontal health parameters is given in Table 4, association of periodontitis and HbA1c and oral hygiene is given in Table 5, whereas association of oral hygiene status with HbA1c of patients is given in Table 6.
Table 3.
Distribution of population for various parameters n (%)
| Parameter | Distribution, n (%) | Parameter | Distribution, n (%) |
|---|---|---|---|
| Duration of DM (years): ≤5 | 276 (64.6) | Consultations for oral problems with dentist | 244 (57.1) |
| Duration of DM (years) >5 | 151 (35.4) | Do not consult dentist for oral problems | 183 (42.9) |
| No periodontitis | 21 (4.9) | Frequency of daily brushing (at least once daily) | 427 (100.0) |
| Mild chronic periodontitis | 132 (30.9) | Oral hygiene aids for cleaning: Toothbrush and toothpaste | 402 (94.1) |
| Moderate chronic periodontitis | 218 (51.1) | Oral hygiene aids for cleaning: Finger and toothpaste | 7 (1.6) |
| Severe chronic periodontitis | 56 (13.1) | Oral hygiene aids for cleaning: Twigs or leaves | 18 (4.2) |
| Normal (HbA1c<6) | 71 (16.6) | Good oral hygiene | 116 (27.1) |
| Good controlled (6-7) | 86 (20.1) | Fair oral hygiene | 294 (68.8) |
| Moderate controlled (7-8) | 75 (17.6) | Poor oral hygiene | 17 (3) |
| Poor controlled (≥8) | 195 (45.7) |
DM: Diabetes mellitus; HbA1c: Glycated hemoglobin
Table 4.
Association of glycated hemoglobin with periodontal health parameters
| HbA1c (%) | n | Mean±SE | F | P | |
|---|---|---|---|---|---|
| OHI-S | Normal (HbA1c <6) | 71 | 1.698±0.077 | 0.56 | 0.645 |
| Good controlled (6-7) | 86 | 1.644±0.068 | |||
| Moderate controlled (7-8) | 75 | 1.622±0.067 | |||
| Poor controlled (≥8) | 195 | 1.721±0.049 | |||
| GI | Normal (HbA1c <6) | 71 | 1.190±0.029 | 3.82 | 0.010 |
| Good controlled (6-7) | 86 | 1.197±0.028 | |||
| Moderate controlled (7-8) | 75 | 1.306±0.036 | |||
| Poor controlled (≥8) | 195 | 1.280±0.021 | |||
| CAL (mm) | Normal (HbA1c <6) | 71 | 2.484±0.162 | 33.51 | <0.001 |
| Good controlled (6-7) | 86 | 2.772±0.142 | |||
| Moderate controlled (7-8) | 75 | 3.400±0.120 | |||
| Poor controlled (≥8) | 195 | 3.979±0.091 | |||
| PPD (mm) | Normal (HbA1c <6) | 71 | 2.405±0.097 | 47.47 | <0.001 |
| Good controlled (6-7) | 86 | 2.899±0.101 | |||
| Moderate controlled (7-8) | 75 | 3.150±0.081 | |||
| Poor controlled (≥8) | 195 | 3.797±0.073 |
HbA1c: Glycated hemoglobin; OHI-S: Oral Hygiene Index-Simplified; GI: Gingival Index; PPD: Probing pocket depth; CAL: Clinical attachment level; SE: Standard error
Table 5.
Association of periodontal status (clinical attachment level) with glycated hemoglobin and oral hygiene status of patients (n=427)
| Parameters | Periodontal status | χ2 | P | |||
|---|---|---|---|---|---|---|
| Normal (n=21), n (%) | Mild (n=132), n (%) | Moderate (n=218), n (%) | Severe (n=56), n (%) | |||
| Normal (HbA1c <6) | 8 (38.1) | 35 (26.5) | 24 (11.0) | 4 (7.1) | 75.12 | <0.001 |
| Good controlled (6-7) | 7 (33.3) | 40 (30.3) | 35 (16.1) | 4 (7.1) | ||
| Moderate controlled (7-8) | 2 (9.5) | 24 (18.2) | 46 (21.1) | 3 (5.4) | ||
| Poor controlled (≥8) | 4 (19.0) | 33 (25.0) | 113 (51.8) | 45 (80.4) | ||
| Good oral hygiene | 17 (81.0) | 64 (48.5) | 34 (15.6) | 1 (1.8) | 97.55 | <0.001 |
| Fair oral hygiene | 4 (19.0) | 67 (50.8) | 173 (79.4) | 50 (89.3) | ||
| Poor oral hygiene | 0 (0.0) | 1 (0.8) | 11 (5.0) | 5 (8.9) | ||
HbA1c: Glycated hemoglobin
Table 6.
Association of oral hygiene status with glycated hemoglobin of patients (n=427)
| HbA1c (%) | Oral hygiene status (OHI-S) | χ2 | P | ||
|---|---|---|---|---|---|
| Fair (n=294), n (%) | Good (n=116), n (%) | Poor (n=17), n (%) | |||
| Normal (<6) | 46 (15.6) | 24 (20.7) | 1 (5.9) | 14.26 | 0.027 |
| Good controlled (6-7) | 59 (20.1) | 25 (21.6) | 2 (11.8) | ||
| Moderate controlled (7-8) | 47 (16.0) | 27 (23.3) | 1 (5.9) | ||
| Poor controlled (≥8) | 142 (48.3) | 40 (34.5) | 13 (76.5) | ||
HbA1c: Glycated hemoglobin; OHI-S: Oral Hygiene Index-Simplified
Among the total participants with fair oral hygiene status (294), 15.6% had normal glycemic control, 20.1% had good glycemic control, 16% had moderate glycemic control, and 48.3% had poor glycemic control. While among the total participants with poor oral hygiene status,[14] 5.9% had normal glycemic control, 11.8% had good glycemic control, 5.9% had moderate glycemic control, and 76.5% had poor glycemic control. On correlating, significant association of oral hygiene status with HbA1c status (P = 0.027) was observed indicating that patients with poor glycemic control had poor oral hygiene status [Table 7].
Table 7.
Percentage distribution of periodontal status (clinical attachment level) and glycated hemoglobin with various oral hygiene status group
| HbA1c (%) | No periodontitis (%) | Mild periodontitis (%) | Moderate periodontitis (%) | Severe periodontitis (%) | χ2 (df) | P | |
|---|---|---|---|---|---|---|---|
| Good oral hygiene | Normal (<6) n=24 | 5 (20.8) | 15 (62.5) | 4 (16.6) | 0 | 16.07 (9) | 0.065 |
| Good control (6-7) n=25 | 6 (24) | 17 (68) | 2 (8) | 0 | |||
| Moderate control (7-8) n=27 | 2 (7.4) | 13 (48.1) | 12 (44.4) | 0 | |||
| Poor control (≥8) n=40 | 4 (10) | 19 (47.5) | 16 (40) | 1 (2.5) | |||
| Fair oral hygiene | Normal (<6) n=46 | 3 (6.5) | 20 (43.4) | 20 (43.4) | 3 (6.5) | 62.40 (9) | <0.001* |
| Good control (6-7) n=59 | 1 (1.6) | 23 (38.9) | 31 (52.5) | 4 (6.7) | |||
| Moderate control (7-8) n=47 | 0 | 11 (23.4) | 33 (70.2) | 3 (6.3) | |||
| Poor control (≥8) n=142 | 0 | 13 (9.1) | 89 (62.6) | 40 (28.1) | |||
| Poor oral hygiene | Normal (<6) n=1 | 0 | 0 | 0 | 1 (100) | 4.14 (6) | 0.658 |
| Good control (6-7) n=2 | 0 | 0 | 2 (100) | 0 | |||
| Moderate control (7-8) n=1 | 0 | 0 | 1 (100) | 0 | |||
| Poor control (≥8) n=13 | 0 | 1 (7.6) | 8 (61.5) | 4 (30.7) |
HbA1c: Glycated hemoglobin; df: Degree of freedom, *Highly significant
The present study demonstrated that 33.8% of population with normal glycemic values (among 71) had good oral hygiene, 64% had fair oral hygiene, and 1.4% had poor oral hygiene status. The prevalence of patients having good, fair, and poor oral hygiene was 29.06%, 68.6%, and 2.3%, respectively, among diabetic patients with good glycemic control. Among moderate glycemic control patient, the prevalence of good, fair, and poor oral hygiene status was 36%, 62.6%, and 1.3%, respectively. Whereas, 20.5% of population with poor glycemic control had good oral hygiene, 72.8% had fair oral hygiene, and 6.7% had poor oral hygiene. Mean GI score reported in the study for all the categories of glycemic control was almost similar that is 1.19 ± 0.029, 1.19 ± 0.028, 1.30 ± 0.036, and 1.28 ± 0.021 for normal, good, moderate, and poor glycemic control, respectively (P = 0.010).
The average PPD of the diabetic patients reported in the study was 3.27 ± 0.051 mm. In all the four categories of glycemic control, the mean PPD recorded was 2.40 ± 0.097, 2.89 ± 0.101, 3.15 ± 0.081, and 3.79 ± 0.073 mm in normal, good, moderate, and poor glycemic control, respectively (P < 0.001). The mean PPD found to be significantly different and higher in poor glycemic control groups as compared to normal glycemic group. The mean CAL of the diabetic patient reported in the study was 3.38 ± 0.067 mm. In all the four categories of glycemic control, the mean CAL recorded was 2.48 ± 0.16, 2.77 ± 0.14, 3.40 ± 0.12, and 3.97 ± 0.091 mm in normal, good, moderate, and poor glycemic control, respectively. There were significantly higher mean values for CAL in poor glycemic control as compared to normal and good glycemic control (P < 0.001).
The present study participants who demonstrated good oral hygiene status represent moderate and severe periodontitis, respectively, in 29.3% and 0.8%. Individuals with, fair oral hygiene status showed 58.8% and 17% of moderate and severe periodontitis, respectively. While in particpants with poor oral hygiene status 64.7% and 29.4% were showing moderate and severe periodontitis respectively. In the present study, the prevalence of periodontitis in participants with normal glycemic control was 88.7%, good glycemic control was 91.8%, moderate glycemic control was 97.3%, poor glycemic control was 97.9%. Within the participants with good OHI status when CAL was related with normal, good, moderate, and poor glycemic control, it was seen that when glycemic control worsens from normal to poor, there was an increase in number of individuals with CAL.
Periodontal destruction was distributed according to glycemic control of normal, good, moderate, and poor as 38.1%, 33.3%, 9.5%, and 19.0% for no periodontitis; 26.5%, 30.3%, 18.2%, and 25.0% for mild periodontitis; 11.0%, 16.1%, 21.1%, and 51.8% for moderate periodontitis; and 7.1%, 7.1%, 5.4%, and 80.4% for severe periodontitis, clearly demonstrating that worsening of glycemic status had a significant influence (P < 0.001) in individuals irrespective of their oral hygiene status. In the present study, 64.6% of diabetic patients were ≤5 years and 35.4% were >5 years. Patients with duration of ≤5 years of diabetes mellitus had mean CAL of 3.15 mm ± 0.083 mm and with duration of >5 years of diabetes mellitus had mean CAL of 3.81 mm ± 0.10 mm, and the difference between the two is statistically significant (P < 0.001). Thus, the study showed positive association between the severities of periodontitis with duration of T2DM. Similarly, a prevalence of 95.1% was found in the present study supporting a strong association of periodontitis and T2DM. Furthermore, CAL significantly deteriorated with worsening of glycemic control when individuals of similar oral hygiene status were examined.
While correlating oral hygiene, glycemic control, and periodontal status, the present study demonstrated that in participants who had good oral hygiene status with normal glycemic as 20.8%, 62.5%, 16.6%, and 0% for no periodontitis, mild periodontitis, moderate periodontitis, and severe periodontitis, respectively. Participants with good glycemic control reported prevalence of periodontal status prevalence for no periodontitis, mild periodontitis, moderate periodontitis, and severe periodontitis, was 20.8%, 62.5%, 16.6%, and 0% respectively. For the patients with moderate glycemic control, the frequency of no periodontitis, mild periodontitis, moderate periodontitis, and severe periodontitis reported as 7.4%, 48.1%, 44.4%, and 0%, respectively. Patients with poor glycemic control demonstrated the prevalence of 10%, 47.5%, 40%, and 2.5% for no periodontitis, mild periodontitis, moderate periodontitis, and severe periodontitis, respectively. Thus, in the present study, it has been shown that the participants with good oral hygiene had severe periodontitis only in poor glycemic control patients.
In the present study T2DM participants with fair oral hygiene demonstrated that in normal glycemic group the frequency of no periodontitis, mild periodontitis, moderate periodontitis, and severe periodontitis as 6.5%, 43.4%, 43.45%, and 6.5%, respectively. For good glycemic control patients, the prevalence of periodontal destruction has been reported as 1.6%, 38.9%, 52.5%, and 6.7% for no periodontitis, mild periodontitis, moderate periodontitis, and severe periodontitis, respectively. For moderate control glycemic patients, the prevalence of 0%, 23.4%, 70.2% and 6.3% for no periodontitis, mild periodontitis, moderate periodontitis, and severe periodontitis, respectively, has been reported. For poor control glycemic patients, the prevalence of no periodontitis, mild periodontitis, moderate periodontitis, and severe periodontitis has been reported as 0%, 9.1%, 62.6%, and 28.1%, respectively. Therefore, the present study demonstrates that all the patients of moderate and poor control diabetic patients with fair oral hygiene had periodontal destruction, with the highest prevalence was of moderate periodontitis.
While correlating poor oral hygiene patients with periodontal status and glycemic control, the present study showed the frequency of 100% severe periodontitis in normal glycemic patients, 100% moderate periodontitis in good glycemic control patient, and 100% moderate periodontitis in moderate glycemic control patients. In poor glycemic control diabetic patients, the prevalence of periodontal status in the same oral hygiene group has been reported as 0%, 7.6%, 61.5%, and 30.7% for no periodontitis, mild periodontitis, moderate periodontitis, and severe periodontitis, respectively. While reading these results care must be taken that a total number of patients in poor oral hygiene group were 17 only and number of patients in normal, good and moderate glycemic control patients were 1, 2, and 1, respectively. Further, most of the T2DM population observed were having fair oral hygiene.
Discussion
Dentists have long been aware about the importance of diagnosis of diabetes in their patients. The present study was aimed to see the prevalence and severity of periodontitis in type 2 diabetics of Uttar Pradesh region. The data were analyzed and arranged to see the influence of OHI, GI, and glycemic control on severity of periodontitis and glycemic status and it was compared with the same oral hygiene status individuals by removing the confounding factor of oral hygiene. The prevalence of periodontitis in this study has been reported as 95.1% among diabetic patients. As in the present study, Kumar et al.[15] also reported prevalence of periodontitis 91.7% among diabetic participants in Bareilly region. Similar results were reported in the trial of Mansour and Abd-Al-Sada[16] and Zhang et al.[17] who reported prevalence of 95.9% and 96.7% of periodontitis in type 2 diabetic populations, respectively. Variation in the prevalence of periodontal disease may be attributed to the definition of periodontitis as diagnostic criteria. The present study more precisely recorded even the slight attachment loss, thus identifying the actual periodontal disease burden in diabetic population. Whereas, certain studies have reported lesser prevalence of periodontitis (13%) as it only recorded periodontitis when CAL exceeded 3 or 4 mm.[14]
Similar to Kumar et al.,[15] the present study includes following clinical parameters, i.e., OHI-S, GI, CAL, and PPD to assess the periodontal status. Radiographic examination was not done, which can be justified keeping in mind the large sample size under investigation. Periodontal examination was done by a single examiner to eliminate the inter-examiner variability. After periodontal examination of patients, appropriate periodontal treatment was advised.
In concurrence to Mansour and Abd-Al-Sada,[16] the present study used the principles of the National Health And Nutrition Examination Survey-IV protocol that recommend PMPE of one maxillary quadrant and one mandibular quadrant and three fixed sites per tooth (mesiobuccal, midbuccal, and distobuccal).[11] Because of the time, labor, and cost constraints, variations of a PMPE protocol using clinical assessments from samples of teeth and sites have been the clinical protocol of choice for large-scale surveillance of periodontitis.[18] Moderate periodontitis was found to be more prevalent (51.1%) in population under investigation. These findings were consistent with that of previous studies Kumar et al.[15] and Fernandes et al.[19] who reported higher prevalence of moderate periodontitis.
All the diabetic participants of the present study reported toothbrushing as oral hygiene method at least once a day. Aggarwal and Panat[20] reported 22% of diabetics brushed their teeth twice daily; Apoorva et al.[21] observed 89% diabetic patients brushed only once a day and 11% brushed twice daily. It has been found in the current study that male patients had 8.2% higher OHI-S scores as compared to female patients; thus, females had better oral hygiene status than males. Kumar et al.[15] reported that 30% of population with good glycemic control had good oral hygiene status, similar to the present study. However, in contrast to 20.5% population with good oral hygiene as revealed in the current study, Kumar et al.[15] reported only 8% of diabetics with good oral hygiene in poor glycemic control population.
Saito et al.[22] found an increase in mean pocket depth that was more closely associated with the development of glucose intolerance from normal status than the past glucose tolerance status itself. Similar to the present result, Awartani[23] also showed higher mean CAL for poor glycemic control, as compared to in good glycemic control patients. Kumar et al.[15] observed that an increased amount of local factors as assessed by OHI-S scores was positively associated with clinical attachment loss in type 2 diabetics. Further, these results are in concurrence with previous reports by Kumar et al.[15] and Rajhans et al.[24] who reported that the prevalence of periodontal disease increases as the level of glycated hemoglobin increases.
”Dose-response relation,” i.e., as glycemic control worsens the adverse effect of diabetes on periodontal health become greater,[25] was also revealed in the present study, and results reported that CAL was linearly and significantly associated with worsening of glycemic control. A total of 4.6% of diabetic population with good glycemic control had severe periodontitis which was increased to 23.07% of participants with poor glycemic control. The present epidemiological study was designed with aim to assess the prevalence and severity of periodontitis in type 2 diabetics.
In accordance with Kumar et al.[15] to nullify the confounding effect of oral hygiene status on clinical attachment loss, CAL was correlated with glycemic level within similar oral hygiene groups. A significant increase in CAL was seen, which was positively related with worsening of glycemic control similar to other studies.[15,26,27,28] Within the participants with good OHI status when CAL was related with normal, good, moderate, and poor diabetic control, it was seen that when glycemic control worsens from good to poor, there was an increase in number of individuals with CAL. Similar to the present study, Ueno et al.[29] also reported the highest proportion of participants with fair oral hygiene. From the above result, it can be inferred that in the similar oral hygiene patients, there was an increase in the periodontal destruction with worsening of glycemic status. Significantly more periodontal attachment and alveolar bone loss were in diabetic patients who had poor glycemic control than those who were well controlled or nondiabetic.[30]
The most striking changes in uncontrolled diabetes are the reduction in defense mechanism and the increased susceptibility to infections, leading to destructive periodontal disease. The glucose content of gingival fluid and blood is higher in individuals with diabetes than in those without diabetes, with similar plaque and GI scores.[31] The increased glucose in the gingival fluid and blood of diabetic patients could change the environment of the microflora, inducing qualitative changes in bacteria that could contribute to the severity of periodontal disease observed in those with poorly controlled diabetes. In patients with poorly controlled diabetes, the function of polymorphonuclear granulocytes (PMNs) and monocytes/macrophages is impaired,[32] and as a result, the primary defense (PMNs) against periodontal pathogens is diminished, and bacterial proliferation is more likely.
In the hyperglycemic state, numerous proteins and matrix molecules undergo a nonenzymatic glycosylation, resulting in formation of accumulated glycation end-products (AGEs). Collagen is cross-linked by AGE formation, making it less soluble and less likely to be normally repaired or replaced (i.e., collagen is not renewed at a normal rate).[33] As a result, collagen in the tissues of patients with poorly controlled diabetes is older and more susceptible to pathogenic breakdown (i.e., less resistant to destruction by periodontal infections). It has been postulated that AGE- RAGE (Receptors for Accumulated Glycation End-products) interaction induces an oxidant stress that may be responsible for monocytic upregulation, activation of nuclear factor-kappa B, and subsequent expression of mRNA and secretion of pro-inflammatory cytokines (tumor necrosis factor-alpha [TNF-α], interleukin [IL]-1β, and IL-6) by monocytic phagocytes involved in periodontal tissue inflammation and destruction.[34]
Further, AGEs induce osteoblast apoptosis through the MAP kinase pathway.[35] Diabetes also interferes with bone formation by reducing the expression of transcription factors that regulate osteoblast differentiation.[36] In addition, RAGE is expressed at higher levels in osteoblasts in diabetic conditions, thus rendering diabetic animals even more sensitive to the effects of AGEs.[37] Human studies of diabetes mellitus generally indicate that T2DM exhibits increased circulating levels of tartrate-resistant acid phosphatase, which is indicative of increased osteoclastic activity.[38] The cumulative effect of altered cellular response to local factors, impaired tissue integrity, and altered collagen metabolism undoubtedly plays a significant role in the susceptibility of diabetic patients to infections and destructive periodontal disease.
Current literature suggests two-way inter-relationships of diabetes mellitus and periodontitis.[39] It can be concluded from the present study that periodontitis is prevalent in 95.1% of diabetes mellitus patients. Moreover, since periodontitis–diabetes interaction may negatively influence glycemic control of the patients, therefore, management of highly prevalent periodontal disease in diabetic patients is beneficial in overall improvement of health status in diabetic patients. In country like India, where the current epidemiological trend [1] is alarming toward the prevalence of diabetes and its complications henceforth, it is of paramount importance for the clinician to control every focus of infection that may somehow increase the insulin resistance and worsen glycemic control.
Therefore, from the present study, we advocate that the inclusion of periodontal treatment may be an important part of diabetic patient management protocol.
Clinical significance
Results of the present study drew attention on the periodontal status of diabetes in population. Early diagnosis and prevention are of fundamental importance to avoid the irreversible tissue destruction that occurs in periodontitis. Periodontal therapy in patients with diabetes is associated with improvement in glycemic control that may be clinically relevant in the management of diabetes. Oral health should be promoted in people with diabetes as an integral component of their overall diabetes management. Closer collaboration between medical and dental clinical teams is necessary for the joint management of people with diabetes and periodontitis. Interaction with dentists is important after the diagnosis of diabetes, to seek active and continued care for their problems in order to have a satisfactory prognosis and an improved quality of life.
Conclusion
This single centered cross-sectional study examined 427 diabetic patients and revealed that more than 95 % of total T2DM patients finally recruited had some periodontal destruction. Therefore their periodontal management may be an important part of diabetic patient management protocol. The prevalence of severe periodontitis reported in T2DM participants with good, fair, and poor oral hygiene status was reported 0.8%, 17% and 24.9%, respectively. Similar to previous study, this study also observed dose-response relation revealing increasing CAL associated with worsening of glycemic control. These results may act as baseline data to promote the collaborative integrated management of diabetes for reducing its burden on society.
Limitation of the study
The mean PPD values in the present study were low as compared to other clinical studies. This may be due to the cross-sectional design of the study that underestimates the periodontal status during survey (because of the presence of calculus). Low sample size and unequal distribution of participants according to their glycemic control and single-centered observation were the further limitations of the study. Therefore, long-term multicenter longitudinal studies are recommended. Further, collection of the serum from the patients and estimation of inflammatory cytokines like TNF-α and reactive oxygen species generation by ELISA may be more helpful.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Kaveeshwar SA, Cornwall J. The current state of diabetes mellitus in India. Australas Med J. 2014;7:45–8. doi: 10.4066/AMJ.2013.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zargar AH, Khan AK, Masoodi SR, Laway BA, Wani AI, Bashir MI, et al. Prevalence of type 2 diabetes mellitus and impaired glucose tolerance in the Kashmir Valley of the Indian subcontinent. Diabetes Res Clin Pract. 2000;47:135–46. doi: 10.1016/s0168-8227(99)00110-2. [DOI] [PubMed] [Google Scholar]
- 3.Ramachandran A, Snehalatha C, Kapur A, Vijay V, Mohan V, Das AK, et al. High prevalence of diabetes and impaired glucose tolerance in India: National urban diabetes survey. Diabetologia. 2001;44:1094–101. doi: 10.1007/s001250100627. [DOI] [PubMed] [Google Scholar]
- 4.Yadav S, Boddula R, Genitta G, Bhatia V, Bansal B, Kongara S, et al. Prevalence and amp; risk factors of pre-hypertension and amp; hypertension in an affluent North Indian population. Indian J Med Res. 2008;128:712–20. [PubMed] [Google Scholar]
- 5.Taylor G. Periodontal infection and glycemic control in diabetes: Current evidence. Inside Dent. 2003;2:1–5. [Google Scholar]
- 6.Singh S, Kumar V, Kumar S, Subbappa A. The effect of periodontal therapy on the improvement of glycemic control in patients with type 2 diabetes mellitus: A randomized controlled clinical trial. Int J Diabetes Dev Ctries. 2008;28:38–44. doi: 10.4103/0973-3930.43097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Teshome A, Yitayeh A. The effect of periodontal therapy on glycemic control and fasting plasma glucose level in type 2 diabetic patients: Systematic review and meta-analysis. BMC Oral Health. 2016;17:31. doi: 10.1186/s12903-016-0249-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organization, Department of Non-communicable Disease Surveillance. Definition, Diagnosis and Classification of Diabetes Mellitus and its Complications. Geneva: World Health Organization, Department of Non-communicable Disease Surveillance; 1999. [Google Scholar]
- 9.WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363:157–63. doi: 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- 10.Snedecor GW, Cochran WG. Statistical Methods. 8th ed. Ames: Iowa State Press; 1989. [Google Scholar]
- 11.Tran DT, Gay I, Du XL, Fu Y, Bebermeyer RD, Neumann AS, et al. Assessing periodontitis in populations: A systematic review of the validity of partial-mouth examination protocols. J Clin Periodontol. 2013;40:1064–71. doi: 10.1111/jcpe.12165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Loe H, Silness J. Periodontal disease in pregnancy. Acta Odontol Scand. 1964;21:533–51. doi: 10.3109/00016356309011240. [DOI] [PubMed] [Google Scholar]
- 13.Greene JC, Vermillion JR. The simplified oral hygiene index. J Am Dent Assoc. 1964;68:7–13. doi: 10.14219/jada.archive.1964.0034. [DOI] [PubMed] [Google Scholar]
- 14.Morton AA, Williams RW, Watts TL. Initial study of periodontal status in non-insulin-dependent diabetics in Mauritius. J Dent. 1995;23:343–5. doi: 10.1016/0300-5712(94)00001-v. [DOI] [PubMed] [Google Scholar]
- 15.Kumar A, Pandey MK, Singh A, Mittra P, Kumar P. Prevalence and severity of periodontal disease in type 2 diabetes mellitus of Bareilly region (India) Int J Med Sci Public Health. 2013;2:77–83. [Google Scholar]
- 16.Mansour AA, Abd-Al-Sada N. Periodontal disease among diabetics in Iraq. MedGenMed. 2005;7:2. [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang JQ, Pan YP, Ma L, Tan LS, Liu JB, Wei JJ, et al. Asurvey on the periodontal status in type 2 diabetic patients. Zhonghua Kou Qiang Yi Xue Za Zhi. 2009;44:668–71. [PubMed] [Google Scholar]
- 18.Dye BA, Thornton-Evans G. A brief history of national surveillance efforts for periodontal disease in the United States. J Periodontol. 2007;78(Suppl 7S):1373–9. doi: 10.1902/jop.2007.060210. [DOI] [PubMed] [Google Scholar]
- 19.Fernandes JK, Wiegand RE, Salinas CF, Grossi SG, Sanders JJ, Lopes-Virella MF, et al. Periodontal disease status in Gullah African Americans with type 2 diabetes living in South Carolina. J Periodontol. 2009;80:1062–8. doi: 10.1902/jop.2009.080486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aggarwal A, Panat SR. Oral health behavior and hbA1c in Indian adults with type 2 diabetes. J Oral Sci. 2012;54:293–301. doi: 10.2334/josnusd.54.293. [DOI] [PubMed] [Google Scholar]
- 21.Apoorva SM, Sridhar N, Suchetha A. Prevalence and severity of periodontal disease in type 2 diabetes mellitus (non-insulin-dependent diabetes mellitus) patients in Bangalore city: An epidemiological study. J Indian Soc Periodontol. 2013;17:25–9. doi: 10.4103/0972-124X.107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saito T, Shimazaki Y, Kiyohara Y, Kato I, Kubo M, Iida M, et al. The severity of periodontal disease is associated with the development of glucose intolerance in non-diabetics: The Hisayama study. J Dent Res. 2004;83:485–90. doi: 10.1177/154405910408300610. [DOI] [PubMed] [Google Scholar]
- 23.Awartani FA. Evaluation of the relationship between type 2 diabetes and periodontal disease. Saudi Med J. 2009;30:902–6. [PubMed] [Google Scholar]
- 24.Rajhans NS, Kohad RM, Chaudhari VG, Mhaske NH. A clinical study of the relationship between diabetes mellitus and periodontal disease. J Indian Soc Periodontol. 2011;15:388–92. doi: 10.4103/0972-124X.92576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taylor GW. Bidirectional interrelationships between diabetes and periodontal diseases: An epidemiologic perspective. Ann Periodontol. 2001;6:99–112. doi: 10.1902/annals.2001.6.1.99. [DOI] [PubMed] [Google Scholar]
- 26.Snajder N, Carraro JJ, Rugna S, Sereday M. Periodontal findings in diabetic and non-diabetic patients. J Periodontol. 1978;49:445–8. doi: 10.1902/jop.1978.49.9.445. [DOI] [PubMed] [Google Scholar]
- 27.Negrato CA, Tarzia O, Jovanovič L, Chinellato LE. Periodontal disease and diabetes mellitus. J Appl Oral Sci. 2013;21:1–12. doi: 10.1590/1678-7757201302106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thaper S, Thaper T, Priya V, Thaper R, Thaper R. Prevalence of periodontitis in diabetic and non-diabetic patients. Asian J Pharm Clin Res. 2016;9:329–31. [Google Scholar]
- 29.Ueno M, Takeuchi S, Oshiro A, Shinada K, Ohara S, Kawaguchi Y, et al. Association between diabetes mellitus and oral health status in Japanese adults. Int J Oral Sci. 2010;2:82–9. doi: 10.4248/IJOS10025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tervonen T, Karjalainen K, Knuuttila M, Huumonen S. Alveolar bone loss in type 1 diabetic subjects. J Clin Periodontol. 2000;27:567–71. doi: 10.1034/j.1600-051x.2000.027008567.x. [DOI] [PubMed] [Google Scholar]
- 31.Ficara AJ, Levin MP, Grower MF, Kramer GD. A comparison of the glucose and protein content of gingival fluid from diabetics and nondiabetics. J Periodontal Res. 1975;10:171–5. doi: 10.1111/j.1600-0765.1975.tb00022.x. [DOI] [PubMed] [Google Scholar]
- 32.Iacopino AM. Periodontitis and diabetes interrelationships: Role of inflammation. Ann Periodontol. 2001;6:125–37. doi: 10.1902/annals.2001.6.1.125. [DOI] [PubMed] [Google Scholar]
- 33.Grossi SG, Zambon JJ, Ho AW, Koch G, Dunford RG, Machtei EE, et al. Assessment of risk for periodontal disease I Risk indicators for attachment loss. J Periodontol. 1994;65:260–7. doi: 10.1902/jop.1994.65.3.260. [DOI] [PubMed] [Google Scholar]
- 34.Taylor GW, Borgnakke WS. Periodontal disease: Associations with diabetes, glycemic control and complications. Oral Dis. 2008;14:191–203. doi: 10.1111/j.1601-0825.2008.01442.x. [DOI] [PubMed] [Google Scholar]
- 35.Alikhani M, Alikhani Z, Boyd C, MacLellan CM, Raptis M, Liu R, et al. Advanced glycation end products stimulate osteoblast apoptosis via the MAP kinase and cytosolic apoptotic pathways. Bone. 2007;40:345–53. doi: 10.1016/j.bone.2006.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lu H, Kraut D, Gerstenfeld LC, Graves DT. Diabetes interferes with the bone formation by affecting the expression of transcription factors that regulate osteoblast differentiation. Endocrinology. 2003;144:346–52. doi: 10.1210/en.2002-220072. [DOI] [PubMed] [Google Scholar]
- 37.Santana RB, Xu L, Chase HB, Amar S, Graves DT, Trackman PC, et al. Arole for advanced glycation end products in diminished bone healing in type 1 diabetes. Diabetes. 2003;52:1502–10. doi: 10.2337/diabetes.52.6.1502. [DOI] [PubMed] [Google Scholar]
- 38.Suzuki K, Kurose T, Takizawa M, Maruyama M, Ushikawa K, Kikuyama M, et al. Osteoclastic function is accelerated in male patients with type 2 diabetes mellitus: the prevention role of osteoclastogenesis inhibitory factor/osteoprotegrin (OCIF/OPG) on the decrease of bone mineral density. Diabetes Res Clin Pract. 2005;68:1506–12. doi: 10.1016/j.diabres.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 39.Preshaw PM, Alba AL, Herrera D, Jepsen S, Konstantinidis A, Makrilakis K, et al. Periodontitis and diabetes: A two-way relationship. Diabetologia. 2012;55:21–31. doi: 10.1007/s00125-011-2342-y. [DOI] [PMC free article] [PubMed] [Google Scholar]


