Abstract
Background:
YKL-40 is a novel potential inflammatory marker in relation to both acute and chronic inflammation. It is secreted by activated neutrophils and macrophages in acute or chronic inflammation. It strongly binds to chitin and heparin. There remains paucity in information regarding the quantification of YKL-40 levels in serum and gingival crevicular fluid (GCF) in patients with periodontal disease.
Aim:
The aim of this study was to evaluate and estimate the YKL-40 levels in serum and GCF samples collected from patients with or without periodontitis and type 2 diabetes among the South Indian population.
Materials and Methods:
A total of 40 patients were included who were divided into four groups based on inclusion and exclusion criteria as follows: Group A comprised ten healthy individuals, Group B comprised ten patients diagnosed with chronic periodontitis without type 2 diabetes, Group C comprised ten patients diagnosed with chronic periodontitis with type 2 diabetes, and Group D comprised ten patients diagnosed with only type 2 diabetes. Gingival Index (GI), pocket depth (PD), and clinical attachment level (CAL) were recorded at baseline. Serum and GCF samples were collected at baseline which were subjected to enzyme-linked immunosorbent assay analysis for estimation of YKL-40 levels.
Results:
All the clinical parameters (GI, PD, and CAL) and the concentration of YKL-40 level in serum and GCF samples were statistically significant among the groups. The amount of YKL-40 in GCF and serum was found to be highly correlated with PD of patients in all the groups.
Conclusions:
Within the limitations of the sample size, the present study indicates that the measurement of YKL-40 in Serum and GCF samples shows potential as a quantitative indicator of periodontal disease extent.
Keywords: Acute-phase protein, chronic periodontitis, diabetes, gingival crevicular fluid, serum, YKL-40
Introduction
Periodontal disease is a chronic inflammatory disease initiated by microbial infections that leads to a host response resulting in inflammatory breakdown of tooth-supporting osseous and soft tissues. Periodontopathogens are the primary etiologic agents for disease initiation. The onset, progression, and severity of periodontal disease are influenced by the interaction between periodontal microorganisms and the host immune response. In response to bacterial endotoxins, mediators that cause tissue breakdown, including acute-phase proteins (APPs), cytokines, and prostaglandins, are produced as part of the host response.[1]
Periodontal disease is the most frequent oral complication of diabetes, as reported by Löe in 1993, who referred to it as “the sixth complication of diabetes mellitus (DM).” Patients with type 2 DM (T2DM) are 2.8 times more likely to have destructive periodontal disease and 4.2 times more likely to have significant alveolar bone loss compared to systemically healthy individuals.[2] In addition to this, studies have also examined how periodontal therapy can affect glycemic control in diabetic patients.
According to “Biomarkers Definitions Working Group,” a biomarker is defined as “a characteristic that is objectively measured and evaluated as an indicator of normal biological processes, pathogenic processes, or pharmacologic responses to a therapeutic intervention.[3] Gingival crevicular fluid (GCF) has been particularly attracted as a marker of the periodontal disease activity.[4]
GCF contains elevated levels of a vast array of biochemical factors which offers scope for proper diagnosis of disease activity. It contains many proteins and peptides which are possible markers for periodontitis and other diseases. Few among these components have been investigated as diagnostic markers for periodontitis in patients with diabetes.[5]
APPs are defined as proteins whose serum concentration is altered at least 25% in response to inflammation and include proteins of the complement, coagulation and fibrinolytic system, antiproteases, transport proteins, inflammatory mediators, and others. They can be broadly classified into Type I and Type II. They can be positive or negative APP depending on their response to inflammation.[6] They are sensitive markers for evaluating the status of inflammation and are usually associated with increased risk for cardiovascular events, periodontitis, etc.[7]
One such APP is YKL-40, which is a novel potential inflammatory marker in relation to both acute and chronic inflammation. It is a member of “mammalian chitinase-like proteins” secreted by activated neutrophils and macrophages in acute or chronic inflammation. It is a glycoprotein with a molecular weight of approximately 40 kDa and is named from three amino acids of tyrosine (Y), lysine (K), and leucine (L) at the N-terminal. YKL-40 is also called chitinase-3-like protein 1 (CHI3 L1) and human cartilage glycoprotein 39 (HC-gp39) and strongly binds to chitin and heparin but lacks chitinase activity.[1]
YKL-40 is also shown to be produced by vascular smooth muscle and endothelial cells, arthritic chondrocytes, cancer cells, and embryonic and fetal cells. It probably has a role in cell adhesion, migration, proliferation, and differentiation, inflammation, and protection from apoptosis. In addition, YKL-40 is a growth factor for connective tissue cells (fibroblasts, chondrocytes, and human synovial cells) and initiates a signaling cascade in these cells that lead to increased cell proliferation, play a central role primarily in pathologic conditions associated with the homeostasis.[8,9]
There remains paucity in information regarding the quantification of YKL-40 levels in serum and GCF samples of healthy and periodontally affected patients. Thus, this study was carried out to evaluate and estimate the YKL-40 levels in serum and GCF samples collected from patients with or without periodontitis and type 2 diabetes among the South Indian population. To the best of our knowledge, this is the first study of this kind to be conducted among the South Indian population.
Materials and Methods
This is a randomized clinical controlled trial conducted to quantify the YKL-40 levels in serum and GCF samples collected from patients with or without periodontitis and type 2 diabetes.
Patient selection
A total of 40 patients (age range: 35–65 years) were randomly allocated by using coin-toss method from the Department of Periodontology, Rajarajeswari Dental College and Hospital, Bengaluru. Participants were explained about the study and a written informed consent was obtained from all the patients. The study was approved by the Ethical Committee of the institution. The study was conducted in accordance with the Helsinki Declaration of 1975, as revised in 2013.
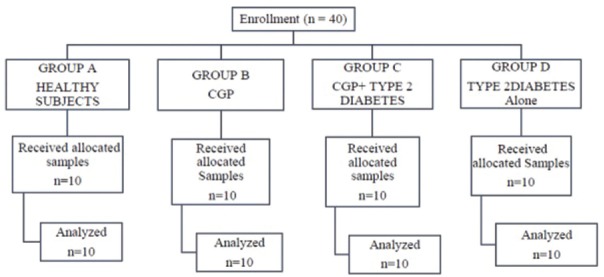
The patients were divided into four groups [Figure 1]:
Figure 1.
Consort flowchart of the study
Group A: Ten healthy individuals without chronic periodontitis and type 2 diabetes
Group B: Ten patients with chronic periodontitis and without type 2 diabetes
Group C: Ten patients with chronic periodontitis and type 2 diabetes
Group D: Ten patients with T2DM and without chronic periodontitis.
The inclusion criteria for the study were as follows:
For Group A: Healthy individuals without chronic periodontitis and type 2 diabetes.
(1) No sites with probing depth ≥4 mm or clinical attachment loss ≥1 mm, (2) h ealthy gingival condition with a GI score <1, and (3) patients without type 2 diabetes.
For Group B: Patients with chronic periodontitis and without type 2 diabetes.
(1) Untreated chronic periodontitis with a probing depth ≥5 mm. (2) Clinical attachment loss ≥3 mm. (3) Radiographic evidence of alveolar bone loss on at least two teeth per quadrant excluding the third molars.
For Group C: Patients with chronic periodontitis and type 2 diabetes.
(1) Untreated chronic periodontitis with a probing depth ≥5 mm. (2) Clinical attachment loss ≥3 mm. (3) Radiographic evidence of alveolar bone loss on at least two teeth per quadrant excluding the third molars. (4) Type 2 diabetes was defined by HbA1c of more than 6.5% National Glycol Hemoglobin Standardization Program (NGSP), American Diabetes Association 2014.
For Group D: Patients with T2DM and without chronic periodontitis.
(1) Type 2 diabetes was defined by HbA1c of more than 6.5% (NGSP), American Diabetes Association 2014. (2) No sites with probing depth ≥4 mm or clinical attachment loss ≥1 mm. (3) Healthy gingival condition with a GI score <1.
Participants were excluded if: (1) who suffered from any systemic condition (except DM) that could affect the progression of periodontal disease (for example, immunological disorders), smokers and former smokers, alcoholics and former alcoholics, obese, pregnant and lactating women, and those taking oral contraceptive drugs; (2) who had a presence of an active infection other than periodontitis; (3) who had an intake of antibiotics/corticosteroids and or nonsteroidal anti-inflammatory drugs during the previous 6 months; (4) who had received professional periodontal treatment during the 6 month period prior to the study; (5) who had used mouthrinses containing antimicrobials in the preceding 2 months; (6) who had periapical pathology, orthodontic treatment; (7) who had bone disease (e.g. osteoporosis, Paget's disease, osteopetrosis).
The following clinical parameters were assessed at six sites per tooth:
GI (Loe and Sillness, 1963) – using a mouth mirror and probe
Probing depth (PD) in mm – graduated William's periodontal probe [Figure 2]
Clinical attachment level (CAL) in mm – measured as the distance from the cementoenamel junction to the base of the gingival sulcus using the periodontal probe.
Figure 2.
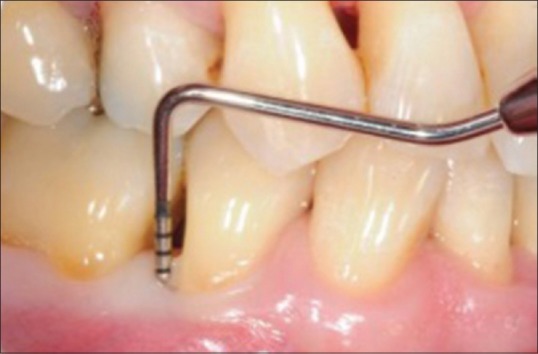
Probing pocket depth measurement
Sample collection and storage
Gingival crevicular fluid sample
The site with both the highest clinical signs of inflammation deepest periodontal PD and with radiographic bone loss was selected for GCF sampling. The area was dried gently. Then, the area was isolated by cotton rolls, to prevent saliva contamination GCF of approximately 3 μl was collected from all the groups by extracrevicular method and was collected using a graduated microcapillary pipette [Figures 3 and 4]. After GCF collection, the microcapillary pipettes were wrapped in a tin foil and placed in cryovial, sealed with parafilm, and stored at −700°C until analysis. The GCF samples were pipetted using a blower and the entire volume of collected GCF was transferred directly to the prepared microplate wells.
Figure 3.
Micropipette used for gingival crevicular fluid sample collection
Figure 4.
Collection of gingival crevicular fluid sample
Serum sample
The venous blood samples of 5 mL were collected from the antecubital fossa by a standard venipuncture method, and serum was separated by centrifugation at 1500 × g for 15 min within 30 min of blood collection [Figures 5 and 6]. The serum samples were then stored at-70°C and thawed immediately before biochemical analysis.
Figure 5.
Collection of blood sample
Figure 6.

Centrifugation unit and serum preparation
Biochemical analysis
Qualitative analysis of estimation of YKL-40 in Groups A, B, Group C, and Group D was done by using EK0974 Human CHI3L1/YKL-40 PicoKine enzyme-linked immunosorbent assay Kit [Figures 7 and 8].
Figure 7.
Gingival crevicular fluid and serum samples delivered into wells
Figure 8.

YKL-40 enzyme-linked immunosorbent assay Kit
Statistical analysis
Descriptive and inferential statistical analysis has been carried out in the present study. The results were analyzed by using SPSS version 18 (IBM Corporation, SPSS Inc., Chicago, IL, USA). Significance was assessed at 5% level of significance. Normality of data was assessed using the Shapiro–Wilk test. Chi-square test with Yate's correction, Kruskal–Wallis test, and Mann–Whitney U-test are used to find the significance of study parameters between the groups. Spearman's correlation is used to find the relationship between the parameters.
Results
The age of participants ranged between 22 and 27 in Group A (Healthy) with the mean age of 25.40 ± 1.58, 30–48; in Group B (Chronic periodontitis without type 2 diabetes) with mean age of 38.60 ± 6.53, 32–48; in Group C (Chronic periodontitis with type 2 diabetes) with mean age of 40.8 ± 4.69; and 35–54 in Group D (only type 2 diabetes) with mean age of 44.50 ± 7.04.
There were 4 male and 6 female in Group A (Healthy), 3 male and 7 female in Group B (Chronic periodontitis without type 2 diabetes), 8 males and 2 females in Group C (Chronic periodontitis with type 2 diabetes), and 4 males and 6 females in Group D (only type 2 diabetes), respectively.
The mean values of all the clinical parameters in all the groups and YKL-40 levels are shown in Table 1. The mean YKL-40 levels in GCF were found to be 42.92 ± 27.52 in Group A, 41.93 ± 12.89 in Group B, 61.42 ± 22.07 in Group C, and 48.83 ± 30 in Group D, respectively.
Table 1.
Mean±SD values of clinical parameters
| Parameters | Group A | Group B | Group C | Group D |
|---|---|---|---|---|
| GI | 1.16±0.81 | 2.16±0.47 | 1.72±0.57 | 1.47±1.03 |
| PD | 3.30±1.64 | 6.50±0.53 | 6.30±0.82 | 4.30±1.81 |
| CAL | 2.45±1.79 | 6.20±1.69 | 6.60±1.07 | 3.50±2.01 |
| YKL 40-GCF | 42.92±27.52 | 41.93±12.89 | 61.42±22.07 | 48.83±30.11 |
| YKL40- Serum | 4.74±5.50 | 7.39±7.62 | 11.67±14.98 | 8.07±6.12 |
Overall comparison between the groups for YKL-40 in GCF and serum samples was done by using Kruskal–Wallis test and found to be not statistically significant between the groups [Tables 2 and 3 and Figures 9 and 10].
Table 2.
Overall comparison between the groups for ykl 40-gcf (kruskal wallis test)
| Variable | n | Mean rank | Chi-square | df | P |
|---|---|---|---|---|---|
| Group A | 10 | 16.30 | 5.911 | 3 | 0.116 |
| Group B | 10 | 16.50 | |||
| Group C | 10 | 27.20 | |||
| Group D | 10 | 22.00 | |||
| Inference | Overall there is no statistically significant difference between the groups for mean CAL values | ||||
Table 3.
Overall comparison between the groups for ykl 40-serum (kruskal wallis test)
| Variable | n | Mean rank | Chi-square | df | P |
|---|---|---|---|---|---|
| GROUP A | 10 | 10.30 | 10.625 | 3 | 0.014* |
| GROUP B | 10 | 23.15 | |||
| GROUP C | 10 | 22.60 | |||
| GROUP D | 10 | 25.95 | |||
| Inference | Overall there is no statistically significant difference between the groups for mean YKL 40-Serum values | ||||
Figure 9.
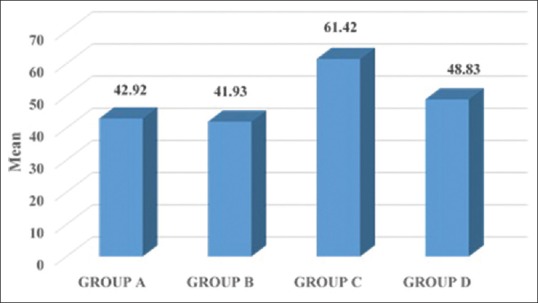
Mean values of YKL-40 gingival crevicular fluid among the groups
Figure 10.
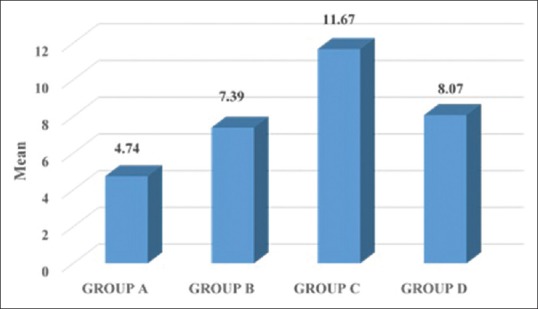
Mean values of YKL-40 serum among the groups
The amount of YKL-40 in GCF and serum was found to be highly correlated with PD of patients in all the groups [Table 4]. The correlation between serum YKL-40 levels and GCF YKL-40 levels between all the groups was done using Mann–Whitney U-test. There was a positively significant correlation seen among the groups [Table 5].
Table 4.
Correlation between different clinical parameters among groups (spearmans correlation)
| Group | GCF to SERUM | GCF to CAL | SERUM to CAL | GCF to PD | SERUM to PD | GCF to GI | SERUM to GI |
|---|---|---|---|---|---|---|---|
| A | 0.824** | 0.838** | 0.934** | 0.845** | 0.942** | 0.957** | 0.717** |
| B | 0.997** | 0.982** | 0.972** | 0.870** | 0.838** | 0.994** | 0.997** |
| C | 0.997** | 0.944** | 0.960** | 0.132 | 0.132 | 0.972** | 0.982** |
| D | 0.932** | 0.966** | 0.966** | 0.931** | 0.931** | 0.994** | 0.994** |
Table 5.
Pair-wise comparison between gcf and serum among the groups (mann-whitney u test)
| Variable | n | Mean rank | Sum of ranks | Mann-Whitney U | Z | P |
|---|---|---|---|---|---|---|
| GROUP A GCF | 10 | 15.50 | 155 | 0 | -3.780 | <0.001* |
| GROUP A SERUM | 10 | 5.50 | 55 | |||
| GROUP B GCF | 10 | 15.40 | 154 | 1 | -3.705 | <0.001* |
| GROUP B SERUM | 10 | 5.60 | 56 | |||
| GROUP C GCF | 10 | 15.20 | 152 | 3 | -3.554 | <0.001* |
| GROUP C SERUM | 10 | 5.80 | 58 | |||
| GROUP D GCF | 10 | 14.90 | 149 | 6 | -3.326 | <0.001* |
| GROUP D SERUM | 10 | 6.10 | 61 | |||
| Inference | Statistically significant difference is seen in all the groups. | |||||
Discussion
In the path of achieving an accurate diagnosis of periodontal disease, as it is a multifactorial condition research has focused mainly on objective measures such as biomarkers in assessing the current disease status and predicting the risk and outcome of the disease. Along with various inflammatory mediators that can be assessed in periodontitis, YKL-40 is one of the biomarkers that can be measured in inflammatory conditions.[10]
An in vivo study done by Johansen et al. stated that YKL-40 protein expression is found in a subpopulation of macrophages in different tissues with inflammation and extracellular matrix remodeling such as macrophages in atherosclerotic plaques, in inflamed synovial membranes of patients with rheumatoid arthritis. YKL-40 increases in patients by more than 25% following an inflammatory stimulus. Serum or plasma concentrations of YKL-40 are often elevated, compared to healthy individuals, in patients with diseases characterized by inflammation, which is in line with the results of the present study.[11]
A study conducted by Persson et al. stated that elevated YKL-40 levels are seen in patients with type 2 diabetes and also increase with increasing levels of albuminuria in type 1 diabetes. Significantly, an increase in the GCF YKL-40 levels and serum YKL-40 levels with severity of periodontal disease is observed, which indicates the results of the present study that serum and GCF YKL-40 levels can be used as a biomarker in inflammatory conditions.[12]
To the best of our knowledge, the present study is the first in its attempt to investigate, assess, and compare YKL-40 levels in serum and GCF samples collected from patients with or without periodontitis and type 2 diabetes among the South Indian population. The present study investigated the serum and GCF levels of YKL-40 in periodontal diseases and showed that the total amount of Serum and GCF YKL-40 levels clearly increased in patients with periodontal disease cored with healthy individuals. Moreover, both serum and GCF YKL-40 levels were found to be higher in the chronic periodontitis, chronic periodontitis with type 2 diabetes patients when compared with type 2 diabetes alone.
There is an increase in the body of evidence that shows increased serum or plasma YKL-40 levels in several diseases characterized by inflammation.[13] It is well known that the pathogenesis of rheumatoid arthritis and periodontal disease are substantially similar. Growing clinical evidence reveals that increased expression and secretion of YKL-40 levels contribute the pathogenesis of rheumatoid arthritis.[14] Data suggest that acute-phase reactants might be related to the inflammatory status of the periodontal tissues and that YKL-40 is released by a variety of cells, namely neutrophils and macrophages, that are significantly excessive during periodontal inflammation.[15]
Serum and GCF YKL-40 levels in patients with chronic periodontitis and type 2 diabetes were high compared with healthy individuals. In the present study, there was a slight difference in serum YKL-40 levels when compared between chronic periodontitis with type 2 diabetes and type 2 diabetes patients alone.
The results of the present study demonstrated that the periodontal variables, namely mean GI, PD, and CAL scores were found to be statistically significant between all the groups (P < 0.001), which is similar to the results stated by Keles et al. in their study.[1] A recent study done by Kranti et al. stated that elevated GCF levels of interleukin-6 in patients with CP and CP with type 2 DM which support YKL-40 findings, including that patients were in an inflammatory state, and further confirm the role of this cytokine in the pathogenesis of periodontal disease. The composition of GCF is the result of interplay between the bacterial biofilm and cells of the periodontal tissues.[16]
In the present study, microcapillary pipettes were used for collection of GCF samples in this study to avoid nonspecific attachment of the analyte to filter paper fibers, ensuing in false reduction in the detectable YKL-40 levels that, in turn, can underestimate the correlation of levels to disease severity.
Our study supports the results of Keles et al. which showed that YKL-40 level was elevated in GCF from sites with periodontitis.[1] Although there is no statistically significant difference in the levels of YKL-40 in periodontitis and diabetic group, in a study by Kido et al., there is overexpression of YKL-40 in GCF of CP and CP with T2DM patients than healthy patients. Plasma and serum YKL-40 levels in patients with T2DM were high compared with those of non-DM individuals and correlated with fasting plasma glucose, insulin, and HOMA-IR, a marker of insulin resistance, suggesting that YKL-40 is associated with DM.[11,17,18]
In the light of above, it is plausible to suggest that total amount of YKL-40 was higher in periodontal tissue breakdown. YKL-40 levels in GCF samples from chronic periodontitis patients increased significantly compared with healthy individuals, suggesting that YKL-40 in GCF may reflect the inflammation of periodontitis and can be a useful biomarker for inflammation in periodontal diseases and T2DM.
Significance of the present study
It is the first in its attempt to investigate, assess, and compare YKL-40 levels in serum and GCF samples collected from patients with or without periodontitis and type 2 diabetes among the South Indian population. GCF YKL-40 may be a useful biomarker for inflammation in periodontal disease. Within the limitations of the sample size, the present study seems to indicate that measurement of YKL-40 in serum and GCF shows potential as a quantitative indicator of disease extent.
Limitations of the present study
The sample size was small, and hence, the results cannot be generalized to the entire population.
Future directions
Further studies with larger sample sizes must be performed in order to establish accurately the role of YKL-40 in chronic periodontitis. Additional studies should be conducted to further elucidate the role of YKL-40 in periodontal pathogenesis.
Conclusions
As an attempt to assess the role of serum and GCF YKL-40 levels in periodontitis, it was concluded that the levels significantly increase among patients with chronic periodontitis and type 2 diabetes. The present study indicated that the measurement of YKL-40 levels in serum and GCF shows potential as a quantitative indicator of disease extent in addition to being an inflammatory biomarker of periodontal disease presence. An increment in serum YKL-40 levels might be related in that periodontal disease, is a low-grade local infection, microorganisms, and their products are the principle etiologic agents, and it is associated with a moderate systemic inflammatory response. Examining serum and GCF YKL-40 levels might help in identifying patients with periodontal break down or those who are at risk for periodontal inflammation.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Keles ZP, Keles GC, Avci B, Cetinkaya BO, Emingil G. Analysis of YKL-40 acute-phase protein and interleukin-6 levels in periodontal disease. J Periodontol. 2014;85:1240–6. doi: 10.1902/jop.2014.130631. [DOI] [PubMed] [Google Scholar]
- 2.Nathan DM, Buse JB, Davidson MB, Ferrannini E, Holman RR, Sherwin R, et al. Medical management of hyperglycemia in type 2 diabetes: A consensus algorithm for the initiation and adjustment of therapy: A consensus statement of the American Diabetes Association and the European Association for the study of diabetes. Diabetes Care. 2009;32:193–203. doi: 10.2337/dc08-9025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Biomarkers Definitions Working Group. Biomarkers and surrogate endpoints: Preferred definitions and conceptual framework. Clin Pharmacol Ther. 2001;69:89–95. doi: 10.1067/mcp.2001.113989. [DOI] [PubMed] [Google Scholar]
- 4.Kinney JS, Morelli T, Braun T, Ramseier CA, Herr AE, Sugai JV, et al. Saliva/pathogen biomarker signatures and periodontal disease progression. J Dent Res. 2011;90:752–8. doi: 10.1177/0022034511399908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Subrahmanyam MV, Sangeetha M. Gingival crevicular fluid a marker of the periodontal disease activity. Indian J Clin Biochem. 2003;18:5–7. doi: 10.1007/BF02867658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ide M, McPartlin D, Coward PY, Crook M, Lumb P, Wilson RF. Effect of treatment of chronic periodontitis on levels of serum markers of acute-phase inflammatory and vascular responses. J Clin Periodontol. 2003;30:334–40. doi: 10.1034/j.1600-051x.2003.00282.x. [DOI] [PubMed] [Google Scholar]
- 7.Ebersole JL, Cappelli D. Acute-phase reactants in infections and inflammatory diseases. Periodontol. 2000;2000(23):19–49. doi: 10.1034/j.1600-0757.2000.2230103.x. [DOI] [PubMed] [Google Scholar]
- 8.Ogawa H, Yoshihara A, Amarasena N, Hirotomi T, Miyazaki H. Association between serum albumin and periodontal disease in community-dwelling elderly. J Clin Periodontol. 2006;33:312–6. doi: 10.1111/j.1600-051X.2005.00901.x. [DOI] [PubMed] [Google Scholar]
- 9.Kido J, Bando Y, Bando M, Kajiura Y, Hiroshima Y, Inagaki Y, et al. YKL-40 level in gingival crevicular fluid from patients with periodontitis and type 2 diabetes. Oral Dis. 2015;21:667–73. doi: 10.1111/odi.12334. [DOI] [PubMed] [Google Scholar]
- 10.Murr C, Widner B, Wirleitner B, Fuchs D. Neopterin as a marker for immune system activation. Curr Drug Metab. 2002;3:175–87. doi: 10.2174/1389200024605082. [DOI] [PubMed] [Google Scholar]
- 11.Rathcke CN, Johansen JS, Vestergaard H. YKL-40, a biomarker of inflammation, is elevated in patients with type 2 diabetes and is related to insulin resistance. Inflamm Res. 2006;55:53–9. doi: 10.1007/s00011-005-0010-8. [DOI] [PubMed] [Google Scholar]
- 12.Persson F, Rathcke CN, Gall MA, Parving HH, Vestergaard H, Rossing P. High YKL-40 levels predict mortality in patients with type 2 diabetes. Diabetes Res Clin Pract. 2012;96:84–9. doi: 10.1016/j.diabres.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 13.Peltomaa R, Paimela L, Harvey S, Helve T, Leirisalo-Repo M. Increased level of YKL-40 in sera from patients with early rheumatoid arthritis: A new marker for disease activity. Rheumatol Int. 2001;20:192–6. doi: 10.1007/s002960100115. [DOI] [PubMed] [Google Scholar]
- 14.Johansen JS, Stoltenberg M, Hansen M, Florescu A, Hørslev-Petersen K, Lorenzen I, et al. Serum YKL-40 concentrations in patients with rheumatoid arthritis: Relation to disease activity. Rheumatology (Oxford) 1999;38:618–26. doi: 10.1093/rheumatology/38.7.618. [DOI] [PubMed] [Google Scholar]
- 15.Tüter G, Kurtis B, Serdar M. Evaluation of gingival crevicular fluid and serum levels of high-sensitivity C-reactive protein in chronic periodontitis patients with or without coronary artery disease. J Periodontol. 2007;78:2319–24. doi: 10.1902/jop.2007.070150. [DOI] [PubMed] [Google Scholar]
- 16.Kranti K, Pramod N, Ashwini S, Naik S. Levels of biomarker YKL40 and interleukin6 in gingival crevicular fluid in patients with chronic periodontitis and type 2 diabetes. Int J Recent Sci Res. 2017;8:171159. [Google Scholar]
- 17.Nielsen AR, Erikstrup C, Johansen JS, Fischer CP, Plomgaard P, Krogh-Madsen R, et al. Plasma YKL-40: A BMI-independent marker of type 2 diabetes. Diabetes. 2008;57:3078–82. doi: 10.2337/db08-0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schaller G, Brix JM, Placher-Sorko G, Höllerl F, Schernthaner GH, Schernthaner G. YKL-40 concentrations are not elevated in gestational diabetes. Eur J Clin Invest. 2010;40:339–43. doi: 10.1111/j.1365-2362.2010.02274.x. [DOI] [PubMed] [Google Scholar]