Abstract
Background:
Despite their lower strength, glass ionomer cements (GICs) are widely used as restorative materials because of their anti-cariogenic properties, direct adhesion to tooth structure and good biocompatibility. Recently, the addition of nano-hydroxyapatite (nano-HA)-silica to conventional GIC (cGIC) has been shown to improve the strength of cGIC. However, the biocompatibility and cell attachment properties of this material are unknown.
Aims:
This study aims to evaluate and compare the cytotoxicity and cell attachment properties of cGIC and nano-HA-silica-GIC on dental pulp stem cells (DPSCs).
Methods and Materials:
Material extracts of nano-HA-silica-GIC and cGIC were prepared into seven serial dilutions and applied to 96 well plates seeded with DPSCs. After 72 h, the cell viability was determined using MTT assay. The DPSCs cell attachment properties were examined under scanning electron microscope (SEM) after 24 and 72 h. Kruskal–Wallis test was used to analyse the data for MTT assay (P < 0.05). SEM images of cell attachment properties were also described.
Results:
Nano-HA-silica-GIC and cGIC was shown to be slight to non-cytotoxic at all concentrations, except 200 mg/ml. Moderate cytotoxicity has been observed at 200 mg/ml concentration where nano-HA-silica-GIC and cGIC revealed cell viability values of 44.38 and 42.15%, respectively. Nano-HA-silica-GIC demonstrated better cell viability values than cGIC at all concentrations except for 6.25 and 12.5 mg/ml. Nevertheless, the results were not statistically significant (P > 0.05). SEM examination revealed the increasing numbers of DPSCs attached to both groups with prominent filopodia, especially after 72 h.
Conclusions:
Nano-HA-silica-GIC exhibited good biocompatibility which is comparable to cGIC and favoured the attachment of DPSCs.
Keywords: Cell attachment, cytotoxicity, dental pulp, glass ionomer cements, stem cells
Introduction
Biomaterials are native or synthetic polymers that act as scaffolds for tissue regeneration and have great value in root canal therapy, tooth repair, pulp therapy and dental surgery.[1,2] Certain basic requirement of biomaterials for these applications needs to satisfy some criteria such as biocompatibility, strength, fatigue, durability, non-toxicity, corrosion resistance and sometimes aesthetics.[3]
Glass ionomer cements (GICs) were invented in 1969 and their use was reported by Wilson in the early 1970s.[4] They are used as restorative materials in paediatric dentistry, as lining and base, fissure sealants and atraumatic restorative treatment (ART) materials.[5] GICs possess excellent properties such as biocompatibility, long-term release of fluoride which acts as an anti-cariogenic agent, elasticity similar to dentin and direct bonding to the tooth structure.[6–8] Therefore, they are one of the most popular dental materials in dentistry. Despite these advantages, they have some limitations such as brittle and mechanically weak.[9] These limitations have led to the restriction of their use as a filling material in high stress-bearing area such as on posterior teeth. As such, modifications have been made to overcome the limitations of conventional GIC (cGIC). These include the incorporation of alumina, zirconia, silicon carbide, hydroxyapatite (HA), glass fibre and bioactive glass into GICs.[10–14] Nevertheless, these efforts did not significantly improve their mechanical strength.
HA is a naturally occurring mineral form of calcium apatite. It has an excellent biological behaviour and its hardness is similar to the natural tooth and intrinsic radiopaque response.[15–17] In addition, nano-hydroxyapatite (n-HAp) crystals can favour remineralisation of enamel.[18,19] Due to these excellent properties, HA has been used in many fields of dentistry such as implant dentistry,[20] caries prevention,[21] bone void fillers,[22] restoration of periodontal defects,[23] alveolar ridge augmentation,[24] endodontic treatment,[25] repair of mechanical furcation perforations,[26] desensitising agent and remineralising agent in toothpastes.[27] Studies have been conducted to evaluate the effect of HA added to cGIC. It was shown that HA improves the physical properties of cGIC including enhanced release of fluoride, improves mechanical strength and bonding to tooth.[10,16,28]
Biocompatibility of dental materials is an important consideration for patients, clinicians, laboratory technician and manufacturer. Dental material that is used in the oral cavity should be harmless to oral tissues. Ideally, it should not contain toxic or leachable substance that could possibly release into the oral environment which may result in systemic toxic responses or an allergic reaction. Hence, the testing on the biocompatibility of any dental material is necessary to ensure the safety of the material. A wide range of in vitro cytotoxicity assays have been developed to evaluate the biocompatibility of various biomaterials. Among these are lactate dehydrogenase (LDH) leakage assay, protein assay, neutral red assay and MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay.[29] MTT assay is an in vitro assay, which is a sensitive, quantitative and reliable colorimetric assay that measures viability, proliferation and activation of cells.[30] It can be performed on material extracts or through direct contact and the results are reproducible. The basic principle of this assay is MTT, yellow tetrazole is reduced to purple formazan by succinate dehydrogenase. An acidified solution is added to dissolve the insoluble purple formazan product into a coloured solution. The absorbance of this coloured solution can be quantified by its measurement at a certain wavelength.[31]
Cell attachment is the ability of a single cell to stick to another cell or to an extracellular matrix (ECM). The interactions between cells and the ECM components allow signalling control for cell survival, proliferation and differentiation.[32,33] Besides MTT assay, scanning electron microscope (SEM) can be used to give some additional information related to biocompatibility. Asgary et al. in 2006 suggested that cell morphology and material–cell interaction can be obtained with SEM.[34] In this instance, it can offer the view of how the cells interact with the material of interest, in terms of its attachment and proliferation. As such, in contrast to an optical microscope, SEM is applied widely in many scientific applications as it enables a clearer observation of very small surface structures.
Stem cells are unspecialised cells that are able to self-renew and differentiate into various types of specialised cells.[35] They can be identified in a number of adult tissues including dental pulp cells.[36] Huang and Chang (2002) highlighted that in vitro cytotoxicity tests should be performed using cells that are homologous to human tissues of ultimate concern.[37] Among the cells that are largely present in the dental pulp are dental pulp stem cells (DPSCs). Moderate to large size cavities normally end up being near to the pulp. As such, dental materials that is to be placed in the tooth should support the native functions of DPSCs, as these cells lie in close approximation to the area where the dental material is to be placed.[38] Apart from that, leaching of material substance, if present, should not be cytotoxic to the pulp tissue, especially to these cells. For that reasons, DPSCs were used as the cell of interests in this study to determine the materials' biocompatibility towards these cells.
Recently, the application of nano-sized particles for biomaterials is getting popular in dentistry.[39] A numbers of studies have suggested that the incorporation of nano-sized particles or 'nanoclusters' improve the mechanical properties of cGIC.[40,41,42] The production of nano-HA-silica by the one-pot sol-gel technique has been reported recently.[40,42] Researchers proclaimed that the addition of nano-HA-silica into cGIC improved the hardness of cGIC by 73% compared to cGIC alone.[40] Transmission electron microscope (TEM) and SEM micrographs further demonstrated good distribution of the elongated HA and spherical silica within the specimen.[43] Moreover, nano-HA-silica-GIC exhibited higher mechanical, physical and chemical properties compared to cGIC.[44,45,46] Despite many studies have been conducted to investigate their physical and mechanical properties, data with regards to biocompatibility study of nano-HA-silica-GIC are very limited. Moreover, no in vitro study has been conducted to evaluate the cell attachment properties of nano-HA-silica-GIC on DPSCs. Hence, the aim of this study is to evaluate and compare the cytotoxicity and cell attachment properties of HA-silica-GIC and cGIC on DPSCs, by means of MTT assay and SEM.
Methods and Materials
Cement preparation
The nano-HA-silica-GIC and commercially available cGIC Fuji IX GP (GC International, Japan) were used in this study. Nano-HA-silica-GIC was prepared by adding nano-HA-silica onto cGIC as described by Noorani et al.[47] In the meantime, cGIC was prepared according to the manufacturer's instructions.
Nano-HA-silica powder was synthesised using the one-pot sol-gel technique according to Ab Rahman et al.[41] About 100 mg of nano-HA-silica powder was weighed and added to 1900 mg of cGIC powder to obtain a 5% nano-HA-silica-GIC powder mixture. This 5% nano-HA-silica-GIC powder mixture was grounded manually using a mortar and pestle. The Fuji XI liquid was added into powder mixture at a powder/liquid ratio of 1:1 and mixed. The cement was then introduced into an acrylic mould with internal perforation dimension of 10 mm × 2 mm. The cement was left undisturbed for 24 h to allow setting.
In the meantime, cGIC was made by spatulation of the powder into the Fuji XI liquid at a powder/1iquid ratio of 1:1 and mixed. Similarly, they were introduced into an acrylic mould with internal perforation dimension of 10 mm × 2 mm and left undisturbed for 24 h to allow setting.
After 24 h of setting, the cements were removed from the moulds. They were weighed and sterilised under UV radiation for 30 min. Subsequently, they were introduced individually into centrifuge tube with the suitable amount of complete growth medium and standardised at 200 mg/ml. The medium containing the materials was incubated for 72 h at 37°C with 5% CO2 following the studies conducted by Ahmed et al.[45,48] After incubation, the material extracts were filtered into centrifuge tube, using a 0.22 μm syringe filter.
Cell culture
DPSCs purchased from AllCells, USA were used in this study. DPSCs were cultured in Alpha Minimum Essential Medium (α-MEM) (Gibco, Life Technologies, USA) supplemented with 10% (v/v) fetal bovine serum (FBS) (Gibco, Life Technologies, USA) and 1% (v/v) penicillin/streptomycin (Gibco, Life Technologies, USA). Cell cultures were grown in 75 cm2 tissue culture flasks (Thermo Fisher Scientific) and incubated in a humidified atmosphere at 37°C with 5% CO2. Media was changed every 2–3 days. Upon reaching 70–80% confluence, cell lines were passaged by trypsinisation.
MTT cell viability test
Cell viability experiments were performed with two experimental groups, cGIC and nano-HA-silica-GIC. The extracts of respective cement were exposed directly to DPSCs for 72 h to assess cytotoxicity. Untreated DPSCs (control) were included to calculate the percentage cell viability. The experiments were performed in triplicates.
The protocol for MTT assay was followed according to guidelines proposed by Mosmann.[31] MTT assay (Gibco, Life Technologies, USA) were performed in 96 well plates (Nunc™, Denmark). Cells were seeded into each well at a density of 10,000 cells/well. The plates were then incubated at 37°C and 5% CO2 for 24 h. For treatment groups, the material extracts of nano-HA-silica-GIC and cGIC were prepared at the concentration of 200, 100, 50, 25, 12.5, 6.25 and 3.125 mg/ml, which achieved by serial dilution before adding it to the cells. The media in the seeded 96 well plates were then replaced with the 200 μl of material extracts. For negative control, only complete growth medium were added into the wells seeded with cells. The plates were then incubated for 72 h. After that, 20 μl of MTT (5 mg/ml) was added into each well to a final concentration of 0.5 mg/ml and incubated for 4 h. Then, all the content of each well was discarded by pipetting. Following that, 100 μl of dimethyl sulphoxide (DMSO) (Merck, Germany) was then added immediately into each well and the plate was shaken gently to ensure that the DMSO was completely dissolved. The absorbance of each well was measured using the enzyme-linked immunosorbent assay (ELISA) reader (Sunrise, Tecan) at wavelength of 570 nm. Cell viability was scored according to Table 1.[49] The experiment was performed in triplicate to validate data obtained. The data were entered using the SPSS version 20 (IBM SPSS, 2013). Kruskal–Wallis test was used to analyse the data obtained and the level of significance was set at P < 0.05.
Table 1.
Classification of the cell viability
| Cell viability classification | Percentage (%) |
|---|---|
| Severe | <30 |
| Moderate | 30-59 |
| Slight | 60-90 |
| Non-cytotoxic | >90 |
| Control | 100 |
Cell attachment properties
The cell attachment properties were examined as described by Ahmed et al.[50] Acrylic moulds were fabricated, sterilised and the cements were added after mixing. After 1 day of setting, each mould/cement assembly was sterilised using UV for 30 min in six-well plates (Nunc™, Denmark). Then, 250 μl of prepared medium having 100,000 cells was added on the top of the cement and left for 30 min. Subsequently, 5 ml of prepared medium was added slowly to each side of well and the plate was incubated for 24 and 72 h.
Following that, the samples were washed by sterile distilled water. After that, 2.5% glutaraldehyde (Merck, Germany) was added for 2 h. Subsequently, the samples were dehydrated in ethanol at five concentrations (30, 50, 70, 90 and 100%). The samples were rinsed with sterile distilled water and dried overnight at room temperature. The samples were fitted onto aluminium stubs via carbon double-sided tape, coated with gold using a sputter coating machine (Leica EM SCD005, Czech Republic) and then viewed under SEM (FEI, QUANTA FEG 450, Netherland).
Results
Cytotoxicity evaluation
Cell viability of DPSCs treated with nano-HA-silica-GIC and cGIC after 72 h is shown in Figure 1. The results demonstrated that the cell viability decreases when the concentration of nano-HA-silica-GIC and cGIC extracts increases. At 3.125 and 6.125 mg/ml concentration, nano-HA-silica-GIC was shown to be non-cytotoxic to the DPSC cells. In contrast, cGIC showed slight cytotoxicity and non-cytotoxic when the material extracts were at 3.125 and 6.25 mg/ml concentrations, respectively. In the meantime, both materials had exerted slight cytotoxicity effects to DPSCs at the concentrations of 12.5, 25, 50 and 100 mg/ml. Moderate cytotoxicity has been observed when maximum concentration of the materials extracts (200 mg/ml) were placed on DPSCs, with nano-HA-silica-GIC and cGIC revealed cell viability values of 44.38 and 42.15%, respectively. In general, nano-HA-silica-GIC demonstrated better cell viability values than cGIC at all the concentration except for 6.25 and 12.5 mg/ml. Nevertheless, the results were not statistically significant (P > 0.05) [Table 2].
Figure 1.
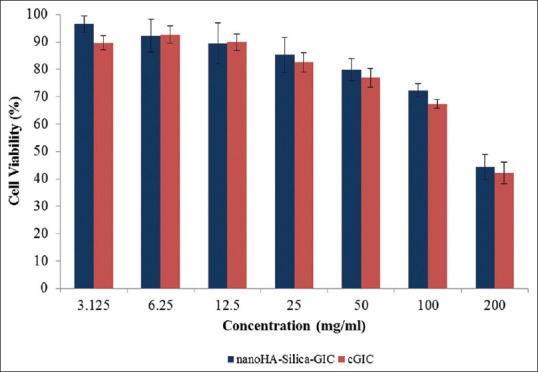
Cell viability of dental pulp stem cells treated with hydroxyapatitesilica-glass ionomer cement (GIC) and conventional GIC after 72 h
Table 2.
Kruskal-Wallis test results for 72 h incubation period variable
| Concentration (mg/ml) | Materials | Mean (SEM) | Median (IQR) | P |
|---|---|---|---|---|
| 3.125 | Nano-HA-silica-GIC | 96.57 (3.06) | 99.36 (9.43) | 0.127 |
| cGIC | 89.68 (2.58) | 87.82 (7.93) | ||
| 6.25 | Nano-HA-silica-GIC | 92.21 (5.98) | 97.81 (18.30) | 0.827 |
| cGIC | 92.65 (3.24) | 90.65 (10.68) | ||
| 12.5 | Nano-HA-silica-GIC | 89.47 (7.48) | 96.57 (22.80) | 0.513 |
| cGIC | 89.93 (2.97) | 88.41 (9.95) | ||
| 25 | Nano-HA-silica-GIC | 85.22 (6.42) | 90.12 (20.57) | 0.513 |
| cGIC | 82.61 (3.53) | 83.46 (12.14) | ||
| 50 | Nano-HA-silica-GIC | 79.79 (4.06) | 82.15 (13.47) | 0.513 |
| cGIC | 76.93 (3.44) | 77.42 (11.87) | ||
| 100 | Nano-HA-silica-GIC | 72.29 (2.53) | 73.69 (8.43) | 0.127 |
| cGIC | 67.32 (1.60) | 62.02 (5.07) | ||
| 200 | Nano-HA-silica-GIC | 44.38 (4.61) | 47.10 (15.25) | 0.275 |
| cGIC | 42.15 (3.86) | 36.56 (12.79) |
Cell attachment properties
DPSCs were cultured on surface of nano-HA-silica-GIC and cGIC as well as on the top of the mould. The cells were observed at 24 and 72 h. In general, analysis of results revealed that nano-HA-silica-GIC and cGIC favour the attachment of DPSCs.
a. 24 h of incubation
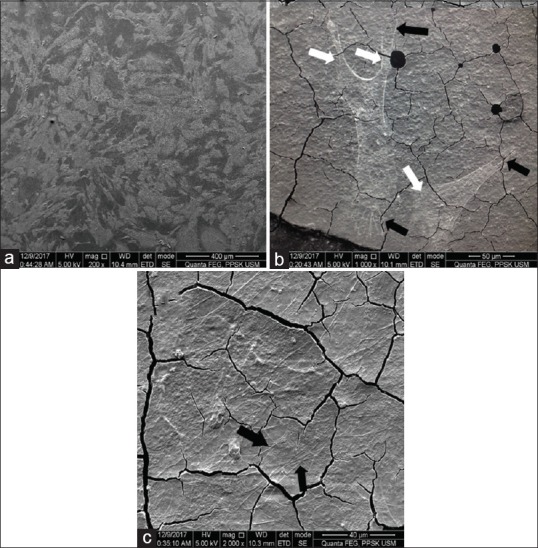
DPSCs adhered over the top surface of the mould and cGIC is shown in Figure 2. Meanwhile, DPSCs adhered over the top surface of the mould and nano-HA-silica-GIC was depicted in Figure 3. In cGIC, the cells exhibited fibroblast-like shape at 1000 × and round shape at 5000 × magnification. In the meantime, DPSCs showed fibroblast-like shape on nano-HA-silica-GIC. In addition, the lamellipodia (indicated by white arrow) and filopodia (indicated by black arrow) were observed for both groups. Apart from that, membrane ruffles was evident on cGIC samples at 5000 × magnification (indicated by black arrow head).
Figure 2.
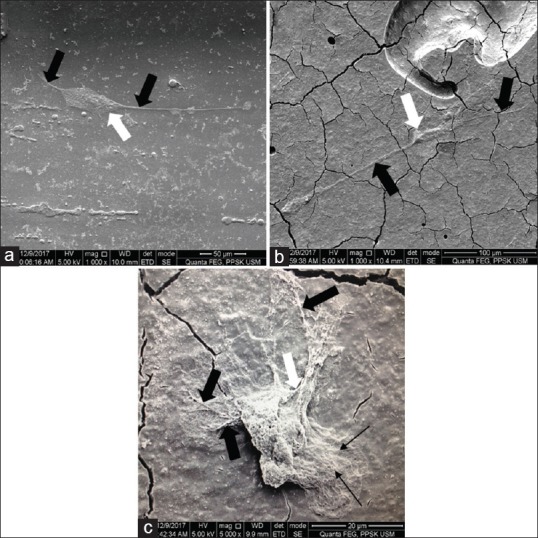
Scanning electron microscope images of conventional GIC (cGIC) group after 24 h incubation. (a) Top of the mould (1000 × magnification); (b) top of cGIC (1000 × magnification); (c) top of cGIC (5000 × magnification)
Figure 3.

Scanning electron microscope images of nano-hydroxyapatite -silica-glass ionomer cement (nano-HA-silica-GIC) group after 24 h incubation. (a) Top of the mould (2500×); (b) top of nano-HA-silica-GIC (1000 × magnification); (c) top of nano- HA-silica-GIC (5000 × magnification)
b. 72 h of incubation
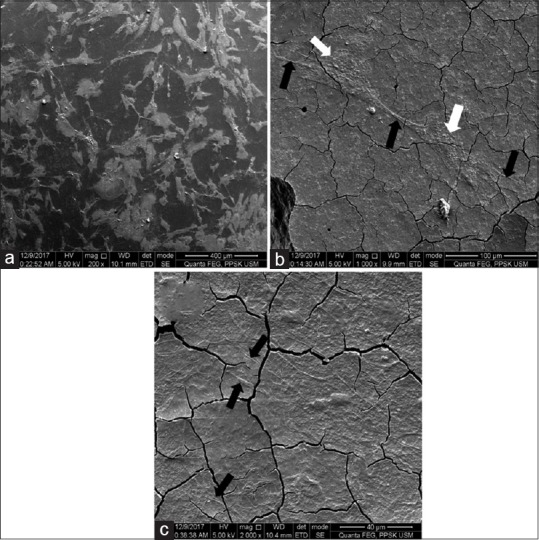
DPSCs adhered over the top surface of the mould and cGIC is demonstrated in Figure 4. In the meantime, DPSCs adhered over the top surface of the mould and nano-HA-silica-GIC is shown in Figure 5. After 72 h, the body of DPSCs appeared flattened in shaped, similar to a sheet like structure, which was present on both materials. In addition, there was an increase in the numbers of DPSCs with abundant filopodia (indicated by black arrow), which appeared to be in contact with the surface of test materials and interacting with neighbouring cells. It was noted that DPSCs were more confluent at the mould, compared to on the surface of both materials.
Figure 4.
Scanning electron microscope images of conventional glass ionomer cement (cGIC) group after 72 h incubation. (a) Top of the mould (200 × magnification); (b) top of cGIC (1000 × magnification); (c) top of cGIC (2000 × magnification)
Figure 5.
Scanning electron microscope images of nano- hydroxyapatite-silica-glass ionomer cement (nano-HA-silica-GIC) group after 72 h incubation. (a) Top of the mould (200×); (b) top of nano-HA-silica-GIC (1000 × magnification); (c) top of nano-HA-silica-GIC (2000 × magnification)
Discussion
Cytotoxic activity can be determined using a number of laboratory tests. The MTT assay has been used as an screening assay and regarded as the gold standard of cytotoxicity assays as it is highly sensitive.[31,51] It is based on the ability of mitochondrial dehydrogenase enzyme from viable cells to cleave the tetrazolium rings of the pale yellow MTT. The dark blue formazan crystals formed is largely impermeable to cell membrane, thus resulting in its accumulation within viable cells. The number of viable cells is directly proportional to the level of the formazan product created.[52]
Selection of an appropriate cell line is a very important part of the study during in vitro cytotoxicity assessments. The ISO 10993 standard, standardising in vitro studies, supports the use of permanent cell lines.[48,53,54] DPSCs were selected in this study because they act as target cells to simulate the clinical situation and represent important populations in the dental pulp tissue that are usually in contact with restorative materials. Besides that, DPSCs play significant role in the preparation processes of damaged pulp.
The extraction dilution method was selected in this study to examine the cytotoxic effects of leachable elements from nano-HA-silica-GIC and cGIC on cells that are distant to and in close contact with them. This method would also stimulate the clinical situation where toxic component of those materials may leach into the surrounding fluid and into the bone crypt.
In the present study, the cell viability of both materials increased with decreasing concentration of the material extract. The cell viability for cGIC were >89% at lower concentration (3.125, 6.25 and 12.5 mg/ml) after 72 h incubation period, indicating that they were non-cytotoxic and slightly cytotoxic at these concentrations. At the maximum concentration (200 mg/ml), cGIC demonstrated moderate cytotoxicity to the cells (42.15%). However, our findings are contradicted with the other studies whereby their cGIC exhibited cell viability >50% at concentration of 200 mg/ml which indicate slight cytotoxicity.[47,48,55]
On the other hand, nano-HA-silica-GIC demonstrated non-cytotoxicity to the cells as the cell viability was >90% at lower concentrations (3.125 and 6.25 mg/ml). At the highest concentration, nano-HA-silica-GIC caused moderate cytotoxicity to the cells (44.38%). The findings are in disagreement with previously reported by Noorani et al.[47] who reported that nano-HA-silica-GIC demonstrated severe cytotoxicity (21.27%). These contradictory results might be related to the use of different types of media cultures in the studies. Noorani et al.[44] and Ahmed et al.[48] use mesenchymal stem cell (MSC) basal medium with supplement to culture their DPSCs. On the contrary, the current study uses complete α-MEM media instead of MSC basal medium. It has been agreed that complete α-MEM media can be used as a common media to test cytotoxicity for DPSCs and it has the advantage of being cheaper than MSC basal medium.[56,57]
In the current study, the moderate cytotoxicity of nano-HA-silica-GIC at maximum concentration of 200 mg/ml might be related to the formation of byproduct/component that may leaches out from cements into the liquid medium. Consequently, the released components may lead to a greater cytotoxic effect to the cells. Previous study reported that there was a presence of a high degree of cross linking of silyl species between the nanosilica and glass particles in the GIC matrix.[43] As a consequence, lesser glass particles are available to react with the polyacrylic acid (PAA) during the setting of nano-HA-silica-GIC, therefore causing more unreacted freely available PAA molecules to be present in the set nano-HA-silica-GIC matrix. These freely available PAA molecules may be released from nano-HA-silica-GIC into the liquid medium and cause cytotoxic to the cells.[43,47] However, confirmation regarding the exact components released from nano-HA-silica-GIC into the culture medium could not be verified as the chemical analysis of the released component was not carried out in this study. A study by Musa et al. in 2012[58] demonstrated that nano-HA-silica alone demonstrated moderate to low level of cytotoxicity at their highest concentration (100 mg/ml). Similarly, nano-HA-silica-GIC in the current study also showed slight cytotoxicity at this particular concentration.
Cell attachment onto biomaterials is one of the criteria for the evaluation of their biological properties. The biocompatibility of biomaterials is very closely related to cell behaviour that comes in contact with them. In particular, the attachment of cells to the material surfaces has been shown to participate in cell proliferation, migration and differentiation.[59] However, cell attachment is difficult to quantify as most materials are non-transparent and transmission microscopy could not be used for this purpose. Besides that, contrast in reflection microscopy is rather poor.[60] As such, SEM has been suggested to become a suitable form of cells attachment and viability evaluation as it can improve visualisation and provides information in establishing biocompatibility through observation of cell morphology and material–cell interactions.[50]
Basically, cell adhesion/attachment is involved in stimulating signals that regulate the cell cycle, differentiation, migration and survival of the cells.[61] Normally, the process of cell adhesion and spreading involves four events which are the attachment of cells at the point of contact with the substratum, centrifugal growth of filopodia, cytoplasmic webbing and flattening of the central mass. The cytoplasmic surface extensions formed by cultured cells can be filopodia, microvilli, lamellipodia or blebs.[62–64] On the contrary, the rounded cells with little or no spreading and vacuolisation of the cytoplasm indicate that the surface of the material may be toxic.[63,65,66]
In the present study, SEM examination on the both materials showed DPSCs demonstrated the fibroblastic phenotype, which is the typical MSC morphology. The numbers of cells increase after 72 h of incubation, indicating that both cements favour the attachment of the cells [Figures 4 and 5]. Yan et al. in 2000[67] has reported similar findings about the cell attachment properties on cGIC material. However, the study was performed using the human gingival fibroblast instead of DPSCs.[67]
In addition, SEM micrographs revealed that lamellipodia, filopodia and membrane ruffles were found on both nano-HA-silica-GIC and cGIC. Lamellipodia, filopodia and membrane ruffles are essential for cell motility, organisation of membrane domains, phagocytosis and the development of substrate adhesions.[68] Lamellipodia contains a quasi-two-dimensional actin mesh in which the whole structure propels the cell across a substrate.[69] It was speculated that the cytoskeletal protein actin projections on the leading edge of the cell in both materials in our study was the lamellipodia [Figures 2–5: white arrows]. On the other hand, filopodia are slender cytoplasmic projections that extend beyond the leading edge of lamellipodia in migrating cells. Filopodia have roles in sensing, migration and cell–cell interaction.[70] Based on the results of SEM study, there was an increased numbers of filipodia attached on both cements particularly after 72 h of incubation [Figures 2–5: black arrows]. The increased numbers of filipodia indicated that the cells are active and viable, and thus suggesting that the cell attachment and migration process were taken place. On top of that, membrane ruffling is the formation of a motile cell surface that contains a meshwork of newly polymerised actin filaments. At 24 h of incubation, the membrane ruffles was detected in cGIC [Figure 2c: black arrow head]. The result was in concordance with the fact reported by Ridley in 1994,[71] whereby the membrane ruffles is one of the earliest structural changes that can be observed in the cell. In summary, the surface of nano-HA-silica-GIC and cGIC promotes DPSCs to attach and proliferate.
Conclusions
Nano-HA-silica-GIC exhibited good biocompatibility which is comparable to cGIC. Moreover, both materials favoured the attachment and spreading of DPSCs with notable filopodia. Nevertheless, further studies need to be carried out to validate the potential use of nano-HA-silica-GIC in clinical applications.
Financial support and sponsorship
This research work was supported by the Malaysian Ministry of Higher Education under Fundamental Research Grant Scheme (FRGS/203/PPSG/6171173).
Conflicts of interest
There are no conflicts of interest.
References
- 1.Goldberg M, Smith AJ. Cells and extracellular matrices of dentin and pulp: A biological basis for repair and tissue engineering. Crit Rev Oral Biol Med. 2004;15:13–27. doi: 10.1177/154411130401500103. [DOI] [PubMed] [Google Scholar]
- 2.Mauth C, Huwig A, Graf-Hausner U, Roulet J. Restorative applications for dental pulp therapy. In: Ashammakhi N, Reis R, Chiellini E, editors. Topics in Tissue Engineering. Vol. 3. Tampere: Expertissues; 2007. pp. 1–32. [Google Scholar]
- 3.Yang J, Xiang HJ. A three-dimensional finite element study on the biomechanical behavior of an FGBM dental implant in surrounding bone. J Biomech. 2007;40:2377–85. doi: 10.1016/j.jbiomech.2006.11.019. [DOI] [PubMed] [Google Scholar]
- 4.Wilson A. Alumino-silicate polyacrylic acid and related cements. Polym Int. 1974;6:165–79. [Google Scholar]
- 5.Sidhu SK, Nicholson JW. A review of glass-ionomer cements for clinical dentistry. J Funct Biomater. 2016;7:16–31. doi: 10.3390/jfb7030016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kent BE, Lewis BG, Wilson AD. Glass ionomer cement formulations: I. The preparation of novel fluoroaluminosilicate glasses high in fluorine. J Dent Res. 1979;58:1607–19. doi: 10.1177/00220345790580061001. [DOI] [PubMed] [Google Scholar]
- 7.Smith D. Polyacrylic acid-based cements: Adhesion to enamel and dentin. Oper Dent. 1992;5:177–83. [PubMed] [Google Scholar]
- 8.Katsuyama S, Ishikawa T, Fuji B. Glass ionomer dental cement: The materials and their clinical use. In: Katsuyama S, Ishikawa T, Fuji B, editors. St. Louis: Ishiyaku EuroAmerica Inc; 1993. pp. 166–8. [Google Scholar]
- 9.Nicholson J. Glass ionomer dental cements: Update. Mater Technol. 2010;25:8–13. [Google Scholar]
- 10.Lucas ME, Arita K, Nishino M. Toughness, bonding and fluoride-release properties of hydroxyapatite-added glass ionomer cement. Biomaterials. 2003;24:3787–94. doi: 10.1016/s0142-9612(03)00260-6. [DOI] [PubMed] [Google Scholar]
- 11.Gu Y, Yap A, Cheang P, Khor K. Effects of incorporation of HA/ZrO2 into glass ionomer cement (GIC) Biomaterials. 2005;26:713–20. doi: 10.1016/j.biomaterials.2004.03.019. [DOI] [PubMed] [Google Scholar]
- 12.Yli-Urpo H, Narhi M, Narhi T. Compound changes and tooth mineralization effects of glass ionomer cements containing bioactive glass (S53P4), an in vivo study. Biomaterials. 2005;26:5934–41. doi: 10.1016/j.biomaterials.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 13.Yli-Urpo H, Lassila LV, Narhi T, Vallittu PK. Compressive strength and surface characterization of glass ionomer cements modified by particles of bioactive glass. Dent Mater. 2005;21:201–9. doi: 10.1016/j.dental.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 14.Hammouda IM. Reinforcement of conventional glass-ionomer restorative material with short glass fibers. J Mech Behav Biomed Mater. 2009;2:73–81. doi: 10.1016/j.jmbbm.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 15.Arcis RW, Lopez-Macipe A, Toledano M, Osorio E, Rodrriguez-Clemente R, Murtra J, et al. Mechanical properties of visible light-cured resins reinforced with hydroxyapatite for dental restoration. Dent Mater. 2002;18:49–57. doi: 10.1016/s0109-5641(01)00019-7. [DOI] [PubMed] [Google Scholar]
- 16.Arita K, Lucas ME, Nishino M. The effect of adding hydroxyapatite on the flexural strength of glass ionomer cement. Dent Mater J. 2003;22:126–36. doi: 10.4012/dmj.22.126. [DOI] [PubMed] [Google Scholar]
- 17.Wang J, Shaw LL. Nanocrystalline hydroxyapatite with simultaneous enhancements in hardness and toughness. Biomaterials. 2009;30:6565–72. doi: 10.1016/j.biomaterials.2009.08.048. [DOI] [PubMed] [Google Scholar]
- 18.Huang S, Gao S, Yu H. Effect of nano-hydroxyapatite concentration on remineralization of initial enamel lesion in vitro. Biomed Mater. 2009;4:034104. doi: 10.1088/1748-6041/4/3/034104. [DOI] [PubMed] [Google Scholar]
- 19.Najeeb S, Khurshid Z, Zafar MS, Khan AS, Zohaib S, Marti JM, et al. Modifications in glass ionomer cements: Nano-sized fillers and bioactive nanoceramics. Int J Mol Sci. 2016;17:1134. doi: 10.3390/ijms17071134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Javed F, Vohra F, Zafar S, Almas K. Significance of osteogenic surface coatings on implants to enhance osseointegration under osteoporotic-like conditions. Implant Dent. 2014;23:679–86. doi: 10.1097/ID.0000000000000161. [DOI] [PubMed] [Google Scholar]
- 21.Ong JL, Chan DC. Hydroxyapatite and their use as coatings in dental implants: A review. Crit Rev Biomed Eng. 2000;28:667–707. doi: 10.1615/critrevbiomedeng.v28.i56.10. [DOI] [PubMed] [Google Scholar]
- 22.Pryor LS, Gage E, Langevin CJ, Herrera F, Breithaupt AD, Gordon CR, et al. Review of bone substitutes. Craniomaxillofac Trauma Reconstr. 2009;2:151–60. doi: 10.1055/s-0029-1224777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaushick BT, Jayakumar N, Padmalatha O, Varghese S. Treatment of human periodontal infrabony defects with hydroxyapatite+ β tricalcium phosphate bone graft alone and in combination with platelet rich plasma: A randomized clinical trial. Indian J Dent Res. 2011;22:505–10. doi: 10.4103/0970-9290.90278. [DOI] [PubMed] [Google Scholar]
- 24.Singh A, Daing A, Anand V, Dixit J. Two dimensional alveolar ridge augmentation using particulate hydroxyapatite and collagen membrane: A case report. J Oral Biol Craniofac Res. 2014;4:151–4. doi: 10.1016/j.jobcr.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haghgoo R, Asgary S, Abbas FM, Hedeshi RM. Nano-hydroxyapatite and calcium-enriched mixture for pulp capping of sound primary teeth: A randomized clinical trial. Iran Endod J. 2015;10:107–11. [PMC free article] [PubMed] [Google Scholar]
- 26.Kakani AK, Veeramachaneni C, Majeti C, Tummala M, Khiyani L. A review on perforation repair materials. J Clin Diagn Res. 2015;9:09–13. doi: 10.7860/JCDR/2015/13854.6501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kantharia N, Naik S, Apte S, Kheur M, Kheur S, Kale B. Nano-hydroxyapatite and its contemporary applications. Bone. 2014;34:1–71. [Google Scholar]
- 28.Yamamoto Y. Study on hydroxyapatite-polyacrylic acid composite cement (hydroxyapatite-glass ionomer cement) Shika Zairyo Kikai. 1984;3:787–96. [PubMed] [Google Scholar]
- 29.Fotakis G, Timbrell JA. In vitro cytotoxicity assays: Comparison of LDH, neutral red, MTT and protein assay in hepatoma cell lines following exposure to cadmium chloride. Toxico Lett. 2006;160:171–7. doi: 10.1016/j.toxlet.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 30.Vega-Avila E, Pugsley MK. An overview of colorimetric assay methods used to assess survival or proliferation of mammalian cells. Proc West Pharmacol Soc. 2011;54:10–4. [PubMed] [Google Scholar]
- 31.Mosmann T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 32.Rosso F, Giordano A, Barbarisi M, Barbarisi A. From cell–ECM interactions to tissue engineering. J Cell Physiol. 2004;199:174–80. doi: 10.1002/jcp.10471. [DOI] [PubMed] [Google Scholar]
- 33.Spencer VA, Xu R, Bissell MJ. Extracellular matrix, nuclear and chromatin structure, and gene expression in normal tissues and malignant tumors: A work in progress. Adv Cancer Res. 2007;97:275–94. doi: 10.1016/S0065-230X(06)97012-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Asgary S, Parirokh M, Eghbal MJ, Ghoddusi J, Eskandarizadeh A. SEM evaluation of neodentinal bridging after direct pulp protection with mineral trioxide aggregate. Aust Endod J. 2006;32:26–30. doi: 10.1111/j.1747-4477.2006.00004.x. [DOI] [PubMed] [Google Scholar]
- 35.Potdar P, Deshpande S. Mesenchymal stem cell transplantation: New, avenues for stem cell therapies. J Transplant Technol Res. 2013;3:2161–0991. [Google Scholar]
- 36.Potdar P, Chaugule S. Establishment and molecular characterization of breast cancer mesenchymal stem cell line derived from human non-metastasis breast cancer tumor. SCD. 2011;1:21–8. [Google Scholar]
- 37.Huang F-M, Chang Y-C. Cytotoxicity of resin-based restorative materials on human pulp cell cultures. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;94:361–5. doi: 10.1067/moe.2002.126341. [DOI] [PubMed] [Google Scholar]
- 38.Vining KH, Scherba JC, Bever AM, Alexander MR, Celiz AD, Mooney DJ. Synthetic light-curable polymeric materials provide a supportive niche for dental pulp stem cells. Adv Mater. 2018:30. doi: 10.1002/adma.201704486. doi: 101002/adma 201704486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moheet IA, Luddin N, Rahman IA, Masudi SM, Kannan TP, Abd Ghani NR. Microleakage evaluation of novel nano-hydroxyapatite-silica glass ionomer cement. J Int Oral Health. 2019 DOI: 104103/jiohjioh_132_19. [Google Scholar]
- 40.Moshaverinia A, Ansari S, Moshaverinia M, Roohpour N, Darr JA, Rehman I. Effects of incorporation of hydroxyapatite and fluoroapatite nanobioceramics into conventional glass ionomer cements (GIC) Acta Biomater. 2008;4:432–40. doi: 10.1016/j.actbio.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 41.Ab Rahman I, Sam'an MM, Luddin N, Shiekh RA. One-pot synthesis of hydroxyapatite–silica nanopowder composite for hardness enhancement of glass ionomer cement (GIC) Bull Mater Sci. 2014;37:213–9. [Google Scholar]
- 42.Luddin N. Glass Ionomer Cement Incorporated Nano-Hydroxyapatite-Silica: The Road towards. Improvement Med Surg Ophthal Res. 1(5):1–2. MRD000524 2018. DOI: 1031031/MRD201801000524. [Google Scholar]
- 43.Ahmad Shiekh R, Ab Rahman I, Sam’an MM, Luddin N. Modification of glass ionomer cement by incorporating hydroxyapatite-silica nano-powder composite: Sol–gel synthesis and characterization. Ceram Int. 2014;40:3165–70. [Google Scholar]
- 44.Moheet IA, Luddin N, Ab Rahman I, Kannan TP, Ghani NR. Evaluation of mechanical properties and bond strength of nano-hydroxyapatite-silica added glass ionomer cement. Ceram Int. 2018;44:9899–906. [Google Scholar]
- 45.Moheet IA, Luddin N, Ab Rahman I, Kannan TP, Ghani NRNA, Masudi SM. Modifications of Glass Ionomer Cement Powder by Addition of Recently Fabricated Nano-Fillers and Their Effect on the Properties. A Review Eur J Dent. 2019 doi: 10.1055/s-0039-1693524. DOI: 101055/s-0039-1693524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moheet IA, Luddin N, Rahman IA, Masudi SAM, Kannan TP, Abd Ghani NRN. Novel nano-hydroxyapatite-silica–added glass ionomer cement for dental application: Evaluation of surface roughness and sol-sorption. Polym Polym Compos. 2019. https://doiorg/101177/0967391119874678 .
- 47.Noorani TY, Luddin N, Rahman IA, Sam’an MM. In vitro cytotoxicity evaluation of novel nano-hydroxyapatite-silica incorporated glass ionomer cement. J Clin Diagn Res. 2017;11:105–9. doi: 10.7860/JCDR/2017/24753.9739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ahmed HM, Omar NS, Luddin N, Saini R, Saini D. Cytotoxicity evaluation of a new fast set highly viscous conventional glass ionomer cement with L929 fibroblast cell line. J Conserv Dent. 2011;14:406–8. doi: 10.4103/0972-0707.87212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Silva EJNLd, Santos CC, Zaia AA. Long-term cytotoxic effects of contemporary root canal sealers. J Appl Oral Sci. 2013;21:43–7. doi: 10.1590/1678-7757201302304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ahmed HM, Luddin N, Kannan TP, Mokhtar KI, Ahmad A. Cell attachment properties of portland cement–based endodontic materials: Biological and methodological considerations. J Endod. 2014;40:1517–23. doi: 10.1016/j.joen.2014.06.013. [DOI] [PubMed] [Google Scholar]
- 51.Van Tonder A, Joubert AM, Cromarty AD. Limitations of the 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyl-2H-tetrazolium bromide (MTT) assay when compared to three commonly used cell enumeration assays. BMC Res Notes. 2015;8:47. doi: 10.1186/s13104-015-1000-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Freimoser FM, Jakob CA, Aebi M, Tuor U. The MTT [3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide] assay is a fast and reliable method for colorimetric determination of fungal cell densities. Appl Environ Microbiol. 1999;65:3727–9. doi: 10.1128/aem.65.8.3727-3729.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Murray PE, Garcia Godoy C, Garcia Godoy F. How is the biocompatibilty of dental biomaterials evaluated? Med Oral Patol Oral Cir Bucal. 2007;12:258–66. [PubMed] [Google Scholar]
- 54.Schmalz G. Concepts in biocompatibility testing of dental restorative materials. Clin Oral Investig. 1998;1:154–62. doi: 10.1007/s007840050027. [DOI] [PubMed] [Google Scholar]
- 55.de Souza Costa CA, Hebling J, Garcia-Godoy F, Hanks CT. In vitro cytotoxicity of five glass-ionomer cements. Biomaterials. 2003;24:3853–8. doi: 10.1016/s0142-9612(03)00253-9. [DOI] [PubMed] [Google Scholar]
- 56.Jo YY, Lee HJ, Kook SY, Choung HW, Park JY, Chung JH, et al. Isolation and characterization of postnatal stem cells from human dental tissues. Tissue Eng. 2007;13:767–73. doi: 10.1089/ten.2006.0192. [DOI] [PubMed] [Google Scholar]
- 57.Paduano F, Marrelli M, White LJ, Shakesheff KM, Tatullo M. Odontogenic differentiation of human dental pulp stem cells on hydrogel scaffolds derived from decellularized bone extracellular matrix and collagen type I. PloS One. 2016;11:e0148225. doi: 10.1371/journal.pone.0148225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Musa M, Kannan TP, Ab Rahman I. Assessment of DNA damage caused by locally produced hydroxyapatite-silica nanocomposite using Comet assay on human lung fibroblast cell line. Mol Cell Toxicol. 2012;8:53–60. [Google Scholar]
- 59.Shie MYHCC, Ding SJ. Effects of altering the Si/Ca molar ratio of a calcium silicate cement on in vitro cell attachment. Int Endod J. 2012;45:337–45. doi: 10.1111/j.1365-2591.2011.01981.x. [DOI] [PubMed] [Google Scholar]
- 60.Katsen-Globa A, Peter L, Zollner S, Dorge T, Daffertshofer M, Preckel H, et al. A novel approach for automated analysis of cell attachment and spreading based on backscattered electron imaging by scanning electron microscopy. Materials. 2009;2:1402–16. [Google Scholar]
- 61.Huang S, Ingber DE. The structural and mechanical complexity of cell-growth control. Nat Cell Biol. 1999;1:131–8. doi: 10.1038/13043. [DOI] [PubMed] [Google Scholar]
- 62.Al-Hiyasat AS, Al-Sa’Eed OR, Darmani H. Quality of cellular attachment to various root-end filling materials. J Appl Oral Sci. 2012;20:82–8. doi: 10.1590/S1678-77572012000100015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Balto HA. Attachment and morphological behavior of human periodontal ligament fibroblasts to mineral trioxide aggregate: A scanning electron microscope study. J Endod. 2004;30:25–9. doi: 10.1097/00004770-200401000-00005. [DOI] [PubMed] [Google Scholar]
- 64.Rajaraman R, Rounds DE, Yen SPS, Rembaum A. A scanning electron microscope study of cell adhesion and spreadingin vitro. Exp Cell Res. 1974;88:327–39. doi: 10.1016/0014-4827(74)90248-1. [DOI] [PubMed] [Google Scholar]
- 65.Zhu Q, Haglund R, Safavi KE, Spangberg LS. Adhesion of human osteoblasts on root-end filling materials. J Endod. 2000;26:404–6. doi: 10.1097/00004770-200007000-00006. [DOI] [PubMed] [Google Scholar]
- 66.Asgary S, Moosavi S, Yadegari Z, Shahriari S. Cytotoxic effect of MTA and CEM cement in human gingival fibroblast cells. Scanning electronic microscope evaluation. NY State Dent J. 2012;78:51–4. [PubMed] [Google Scholar]
- 67.Yan F, Xiao Y, Li H, Haase H, Bartold PM. A comparison of the effects of two kinds of glass-ionomer cement on human gingival fibroblast attachment, proliferation and morphology in vitro. J Int Acad Periodontol. 2002;2:14–8. [PubMed] [Google Scholar]
- 68.Small JV, Stradal T, Vignal E, Rottner K. The lamellipodium: Where motility begins. Trends Cell Biol. 2002;12:112–20. doi: 10.1016/s0962-8924(01)02237-1. [DOI] [PubMed] [Google Scholar]
- 69.Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P. Molecular biology of the cell. 4th ed. New York: Garland Science; 2002. pp. 351–2. [Google Scholar]
- 70.Mattila PK, Lappalainen P. Filopodia: Molecular architecture and cellular functions. Nat Rev Mol Cell Biol. 2008;9:446–54. doi: 10.1038/nrm2406. [DOI] [PubMed] [Google Scholar]
- 71.Ridley AJ. Membrane ruffling and signal transduction. Bioessays. 1994;16:321–7. doi: 10.1002/bies.950160506. [DOI] [PubMed] [Google Scholar]


