Abstract
Brassica incana Ten. is an edible plant belonging to the Brassicaceae family. In this work, the phenolic composition and the antioxidant and cytotoxic properties of the hydroalcoholic extracts obtained from the leaves and the flowering tops of B. incana grown wild in Sicily (Italy) were studied for the first time. A total of 17 and 20 polyphenolic compounds were identified in the leaf and in the flowering top extracts, respectively, by HPLC-PDA-ESI-MS analysis. Brassica incana extracts showed in vitro antioxidant properties; the leaf extract displayed greater radical scavenging activity in the 2,2-diphenyl-1-picrylhydrazyl (DPPH) test than the flowering top extract (IC50 = 1.306 ± 0.049 mg/mL and 2.077 ± 0.011 mg/mL), which in turn had a stronger ferrous ion chelating ability than the other (IC50 = 0.232 ± 0.002 mg/mL and 1.147 ± 0.016 mg/mL). The cytotoxicity of the extracts against human colorectal adenocarcinoma (CaCo-2) and breast cancer (MCF-7) cell lines was evaluated through the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay and the lactic dehydrogenase (LDH) release determination. The extracts showed cytotoxic efficacy against Caco-2 cells, with the flowering top extract being the most effective (about 90% activity at the highest concentration tested). In the brine shrimp lethality bioassay, the extracts exhibited no toxicity, indicating their potential safety.
Keywords: Brassica incana Ten., phenolic compounds, antioxidant activity, cytotoxicity, Artemia salina Leach
1. Introduction
In recent decades, interest in new sources of health-promoting compounds has become a major research issue. Considerable attention has been paid to edible plants, especially those rich in bioactive phytochemicals. The Brassicaceae family includes 338 genera and 3709 species [1]. The genus Brassica is the most important one within the 51 genera and belongs to the subtribe Brassicinae, one of the nine subtribes of the Brassiceae tribe; the genus includes many species with economic and agricultural relevance [2,3]. Brassicaceae are recognized as rich sources of bioactive compounds, such as carotenoids, tocopherols, ascorbic acid, glucosinolates, and of phenolic compounds [4,5]. Strong epidemiological evidence demonstrated that these compounds may help to protect the human body against damage caused by reactive oxygen species and reduce the risk of chronic pathologies, including cardiovascular diseases and cancer [5].
In continuation of our earlier published studies on species belonging to the Brassicaceae family endemic to Sicily (Italy) [6,7,8], Brassica incana Ten. has been selected.
Brassica incana, a wild B. oleracea-related species, is a suffrutex growing up 100 cm high [3,9,10]. As reported in the Euro+Med PlantBase, B. incana is native to south-eastern Europe, including Albania, Bosnia-Herzegovina, Croatia, Greece, and Italy; the plant has also been introduced in Ukraine and Crimea [11]. In Italy, it grows in Tuscany, Lazio, Campania, Puglia, Basilicata, Calabria, and Sicily, where it mainly occurs on the cliffs and the calcareous rocky slopes, from sea level up to about 600-800 m of altitude [12,13].
B. incana is an edible plant [14]. Its use for the preparation of omelettes and of a typical Sicilian polenta, known as “Frascatula”, together with Brassica fruticulosa Cyr. and other wild herbs, is reported in Sicily.
Several species belonging to the Brassica genus have been the subject of numerous phytochemical investigations and studies on therapeutic potential in human and animal diseases [4,15,16,17]. Instead, concerning B. incana, very limited information is available. Indeed, to the best of our knowledge, only one article concerning the characterization of the volatile constituents of B. incana leaves and roots is present in the literature [18], whereas some research has been focused on the glucosinolates contained in leaves and seeds [19,20,21].
Based on the above considerations, the present work aimed to investigate the phenolic composition and certain biological properties of the hydroalcoholic extracts obtained from the leaves and the flowering tops of B. incana grown wild in Sicily (Italy). In particular, the study includes the quali–quantitative characterization of the phenolic constituents of the extracts by HPLC-PDA-ESI-MS analysis, and the evaluation of their antioxidant properties by in vitro assays and on Escherichia coli, which is used as biological substrate. The cytotoxic activity was assessed in both cell systems (human colorectal adenocarcinoma CaCo-2 and breast cancer MCF-7 cell lines) through the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay and the lactic dehydrogenase (LDH) release determination and in vivo by the brine shrimp (Artemia salina Leach) lethality bioassay.
2. Results
2.1. Phytochemical Investigations
2.1.1. Determination of Total Phenolic Content
The total phenolic content was found to be greater in the B. incana leaf extract than in the flowering top extract, with values of 37.20 ± 0.93 mg gallic acid equivalent (GAE)/g extract and 27.98 ± 0.32 mg GAE/g extract, respectively.
2.1.2. Identification of Phenolic Compounds by HPLC-PDA-ESI-MS
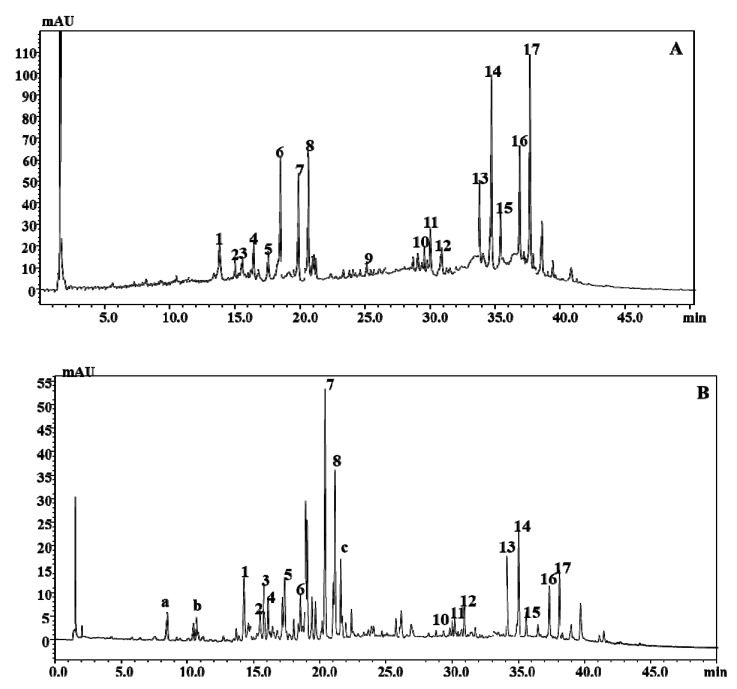
The determination of the polyphenolic content of B. incana extracts was performed by HPLC-PDA-ESI-MS. Regarding the chromatographic analysis, a superficially porous C18 stationary phase at 1.0 mL/min was used, whereas as far as detection is concerned, both PDA and MS detection were employed. A total of 17 and 20 polyphenolic compounds were positively identified in the leaf and in the flowering top extracts, respectively. So far, no work has been carried on the characterization of the polyphenolic content in B. incana. The results illustrated in Figure 1 and Table 1 show that the extracts contain derivatives of the flavonols quercetin, kaempferol, and isorhamnetin, and of the hydroxycinnamic acids sinapic acid and ferulic acid, which were found in conjugation with sugars or hydroxycinnamic acids.
Figure 1.
HPLC-PDA-ESI-MS polyphenolic fingerprint of B. incana leaf (A) and flowering top (B) hydroalcoholic extracts. Column: Ascentis Express C18, 15 cm × 4.6 mm, 2.7 μm d.p. (ESI, negative ionization mode). For peak identification, see Table 1.
Table 1.
Polyphenolic determination of B. incana leaf (A) and flowering top (B) hydroalcoholic extracts by LC-PDA-MS/MS.
| Peak | Compound | tR (min) |
[M − H]− | mg/g ± %RSD (A) |
mg/g ± %RSD (B) |
|---|---|---|---|---|---|
| a | Kaempferol-3-O-diglucoside-7-O-glucoside | 8.4 | 773 (609) | - | 1.11 ± 1.44 |
| b | Quercetin-3-sophoroside-7-glucoside | 10.7 | 787 (625) | - | 1.32 ± 1.32 |
| 1 | Quercetin-3-hydroxyferuloylsophoroside-7-glucoside | 13.9 | 979 (625) | 1.91 ± 0.52 | 1.59 ± 0.57 |
| 2 | Quercetin-3-caffeoylsophoroside-7-glucoside | 15.2 | 949 (625) | 1.55 ± 1.23 | 1.22 ± 1.41 |
| 3 | Kaempferol-3-hydroxyferuloylsophoroside-7-glucoside | 15.6 | 963 (801) | 1.43 ± 1.21 | 1.41 ± 1.39 |
| 4 | Quercetin-3-sinapoyltriglucoside-7-glucoside | 16.3 | 1155 (831) | 1.22 ± 1.11 | 0.62 ± 1.87 |
| 5 | Quercetin-3-feruloyl-diglucoside-7-glucoside | 17.6 | 963 (801) | 1.52± 1.32 | 1.17 ± 1.11 |
| 6 | Kaempferol-3-sinapoylsophoroside-7-glucoside | 18.5 | 977 (817) | 2.84 ± 1.52 | 0.59 ± 1.98 |
| 7 | Kaempferol-3-feruloylsophoroside-7-glucoside | 19.9 | 947 (609) | 2.11 ± 0.98 | 2.14 ± 0.48 |
| 8 | Isorhamnetin-3-glucoside-7-glucoside | 20.7 | 639 (747) | 3.33 ± 0.77 | 1.79 ± 0.32 |
| c | Feruloylmalate | 21.3 | 309 | - | N.Q. |
| 9 | Sinapoylmalic acid | 25.2 | 339 (223) | N.Q. | N.Q. |
| 10 | Sinapoyl-hydroxyferuloyldiglycoside | 28.6 | 739 (515) | N.Q. | N.Q. |
| 11 | Isorhamnetinglycoside | 29.8 | 477 (315) | 1.28 ± 0.54 | 0.57 ± 2.01 |
| 12 | Kaempferolglycoside | 30.7 | 447 (285) | 0.64 ± 1.08 | 0.42 ± 1.94 |
| 13 | Disinapoylgentiobiose | 33.6 | 753 (529) | N.Q. | N.Q. |
| 14 | Sinapoylferuloylgentiobiose | 34.8 | 723 (529) | N.Q. | N.Q. |
| 15 | Diferuloyldiglucoside | 35.4 | 693 (499) | N.Q. | N.Q. |
| 16 | Trisinapoylgentiobiose | 36.7 | 959 (735, 529) | N.Q. | N.Q. |
| 17 | Feruloyldisinapoylgentiobiose | 37.7 | 929 (705, 511) | N.Q. | N.Q. |
N.Q.: Not quantified.
From a qualitative point of view, the polyphenolic profiles of the extracts are superimposable, except for the compounds kaempferol-3-O-diglucoside-7-O-glucoside, quercetin-3-sophoroside-7-glucoside, and feruloylmalate, which were identified exclusively in the flowering top extract.
Regarding quantification, since none of the compounds identified are commercially available, three selected reference standards were considered, namely quercetin-glucoside, kaempferol-glucoside, and isorhamentin-glucoside, for the determination of quercetin, kaempferol, and isorhamnetin derivates, respectively. In particular, for the leaf extract, isorhamnetin-3-glucoside-7-glucoside turned out to be the most abundant flavonoid (3.33 mg/g ± 0.54 % relative standard deviation (RSD)), followed by kaempferol-3-sinapoylsophoroside-7-glucoside (2.84 mg/g ± 0.77 %RSD) and kaempferol-3-feruloylsophoroside-7-glucoside (2.11 mg/g ± 0.98 %RSD); on the other hand, for the flowering top extract, kaempferol-3-feruloylsophoroside-7-glucoside (2.14 mg/g ± 0.48 %RSD) was the main flavonoid compound, followed by isorhamnetin-3-glucoside-7-glucoside (1.79 mg/g ± 0.32 %RSD) and quercetin-3-hydroxyferuloylsophoroside-7-glucoside (1.59 mg/g ± 0.57 %RSD).
2.2. Antioxidant Activity
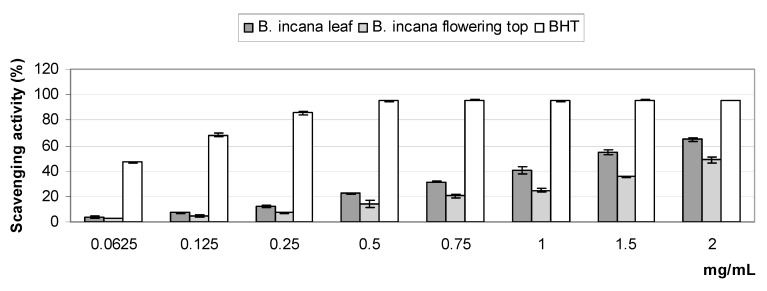
The results of the 2,2-diphenyl-1-picrylhydrazyl (DPPH) test are shown Figure 2. Brassica incana extracts exhibited radical scavenging activity, which increased with increasing amounts of the extracts. The leaf extract displayed higher activity than the flowering top extract, reaching about 65% and 50%, respectively, at the highest tested concentration. This was also confirmed by the IC50 values (1.306 ± 0.049 mg/mL and 2.077 ± 0.011 mg/mL, respectively). Compared to the standard BHT (IC50 = 0.065 ± 0.008 mg/mL), the activity of the extracts was moderate.
Figure 2.
Free radical scavenging activity (2,2-diphenyl-1-picrylhydrazyl (DPPH) test) of B. incana leaf and flowering top hydroalcoholic extracts. Values are expressed as the mean ± SD (n = 3).
In the reducing power assay, the activity of both the extracts was found to be weak in comparison to that of the BHT.
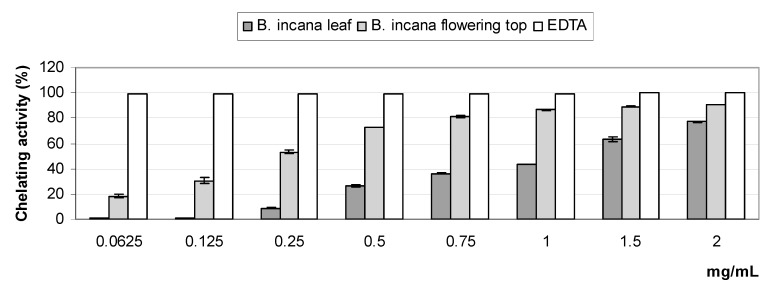
In the Fe2+ chelating activity assay, B. incana extracts exhibited relatively high and dose-dependent chelating properties (Figure 3). In this test, the flowering top extract was much more effective than the leaf one, reaching approximately 90% and 80% activity, respectively, at the highest tested concentration. The strongest efficacy of the flowering top extract was also confirmed by the calculated IC50 values (0.232 ± 0.002 mg/mL and 1.147 ± 0.016 mg/mL, respectively). However, the extracts were not as effective as the reference standard, EDTA (IC50 = 0.012 ± 3.546 × 10−5 mg/mL).
Figure 3.
Ferrous ions chelating activity of B. incana leaf and flowering top hydroalcoholic extracts. Values are expressed as the mean ± SD (n = 3).
In the experimental model of oxidative stress induced by H2O2 in E. coli, the extracts did not show any protective effect on bacterial growth and survival.
2.3. Cytotoxic Activity
2.3.1. Cell Viability Assay on Human Colorectal Adenocarcinoma (CaCo-2) and Breast Cancer (MCF-7) Cells
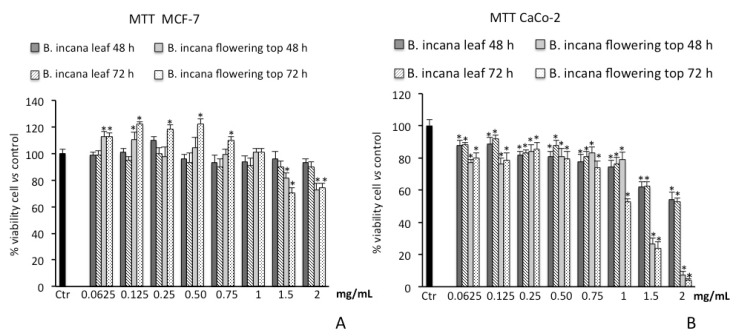
We firstly verified that both extracts do not induce toxicity in human foreskin fibroblast (HFF-1) cells. For a time of 72 h at the highest tested concentration of 2 mg/mL, cell viability was not affected and was similar to untreated control cells. The results of the MTT bioassay on cancer cell lines showed that B. incana leaf extract was not able to reduce cell viability in MCF-7 at all concentrations tested at both 48 h and 72 h of exposure. Surprisingly, at the lowest dosages, a slight increase in cell viability was observed for the treatments at 48 h and 72 h for the flowering top extract. The flowering top extract exerted a significant cytotoxic effect on MCF-7 cell line, starting from the concentration of 1.5 mg/mL, with a reduction of viability of about 30% at 72 h of exposure (Figure 4A).
Figure 4.
Cell viability in MCF-7 (A) and CaCo-2 (B) cells untreated and treated for 48 h and 72 h with B. incana leaf and flowering top hydroalcoholic extracts. Values are the mean ± SD of four experiments in triplicate. * Significant vs. untreated control cells: p < 0.001. MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide.
The treatment of CaCo-2 cells with different concentrations of B. incana extracts induced an inhibitory effect on succinate dehydrogenase activity at both 48 h and 72 h of exposure, resulting significant starting from 0.0625 mg/mL (Figure 4B). The flowering top extract was found to be the more effective, where the inhibitory effects reach a value of about 90% at the highest tested concentration (2 mg/mL).
The IC50 values for the cytotoxic activity of the flowering top extract on CaCo-2 cells are 1.25 ± 0.037 mg/mL and 1.1 ± 0.036 mg/mL at 48 h and 72 h, respectively.
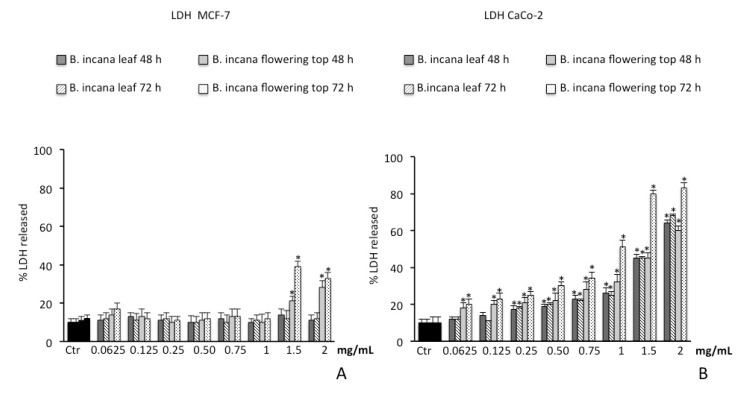
As shown in Figure 5A, consistent with the results obtained by MTT assay, after 48 h and 72 h of incubation with B. incana leaf extract, no appreciable LDH release was observed at all concentrations tested on MCF-7 cell line. A significant LDH release was spotted only for the B. incana flowering top extract at concentrations starting from 1.5 mg/mL.
Figure 5.
Lactic dehydrogenase (LDH) released in MCF-7 (A) and CaCo-2 (B) cells untreated and treated for 48 h and 72 h with B. incana leaf and flowering top hydroalcoholic extracts. Values are the mean ± SD of four experiments in triplicate. *Significant vs. untreated control cells: p < 0.001.
Conversely, on CaCo-2 cell lines, a time- and dose-dependent LDH release was pointed out by the experimental model for both extracts. In particular, the necrotic effect is more evident for the B. incana flowering top extract after 72 h of exposure, being significant from the concentration of 0.0625 mg/mL (Figure 5B).
2.3.2. Brine Shrimp Lethality Bioassay
The median lethal concentration values of B. incana extracts were found to be greater than 1000 μg/mL, indicating that they did not display any toxicity against brine shrimps.
3. Discussion
The presence of high amounts of phenolic compounds in Brassica spp. is well documented and it is recognized that the contribution of these secondary metabolites to the positive health effects of species belonging to this genus has generally been associated with their antioxidant capacity [4,22].
Herein, the phenolic profiles of B. incana leaf and flowering top hydroalcoholic extracts are characterized. The total phenolic content of the extracts, determined by the Folin–Ciocalteau method, was found to be higher than that previously reported for various hydroalcoholic extracts obtained from commonly consumed varieties of Brassica oleracea. Heimler et al. [23] evaluated the total phenolic content of 70% ethanol extracts obtained from edible parts of white cabbage (B. oleracea L. var. capitata L.), broccoli (B. oleracea L. conv. botrytis L. var. italica Plenk), Italian kale (B. oleracea L. var. acephala DC.), Savoy cabbage (B. oleracea L. var. sabauda L.), green cauliflower (B. oleracea L. conv. botrytis L. var. botrytis cv Verde di Macerata), cauliflower (B. oleracea L. conv. botrytis L. var. botrytis cv Snow ball), and Brussels sprouts (B. oleracea L. var. gemmifera Zencher), ranging from 4.30 to 13.80 mg GAE/g sample. A comparative study undertaken by Jaiswal et al. [24] to optimize the best solvents among 60% ethanol, acetone, and methanol for the extraction of polyphenols from Brassica vegetables showed that 60% methanolic extracts had the highest total phenolic content, which was 23.6, 20.4, and 18.7 mg GAE/g extract for broccoli, Brussels sprouts, and white cabbage, respectively.
HPLC-PDA-ESI-MS analysis showed that the extracts contain derivatives of the flavonols quercetin, kaempferol, and isorhamnetin, and of the hydroxycinnamic acids sinapic acid and ferulic acid, which are conjugated with sugar moieties or hydroxycinnamic acids. These types of compounds are very common in Brassica species [5,25,26,27,28,29,30]. Both flavonol glycosides and hydroxycinnamic esters have been reported to possess antioxidant activity [31,32].
Flavonoids and phenolic acids can act as hydrogen or electron donors, reducing agents, and metal ion chelators resulting from different conjugations and varying numbers of hydroxyl groups [33]. Thus, the antioxidant potential of B. incana extracts was investigated by means of in vitro assays based on these different mechanisms. The primary (chain-breaking) antioxidant properties were examined using two different tests: the DPPH test, which is based on a combination of hydrogen atom transfer (HAT) and single electron transfer (SET) reactions, and the reducing power assay, which is a SET-based method [34,35,36]. The secondary (preventive) antioxidant ability was determined by the Fe2+ chelating activity assay.
The results of the in vitro antioxidant tests highlighted that B. incana leaf and flowering top extracts have antioxidant properties; the former displays greater radical scavenging activity than the latter, which in turn has a stronger chelating ability.
In the reducing power assay, both the extracts were found to possess weak activity in the range of concentrations tested (0.0625–2 mg/mL). The in vitro antioxidant activities of the main Brassica crops have been studied by different authors; similarly to our results, some of these investigations have highlighted good radical scavenging activity and low reducing power in Brassica spp. extracts [37,38].
The observed radical scavenging properties could be mainly attributed to the phenolic compounds detected in the extracts. A few studies aimed at establishing the antioxidant properties of phenolic compounds isolated from Brassica species have been previously reported. Some authors have investigated the contribution of flavonoid glycosides and hydroxycinnamic acid derivatives to the antioxidant activity of Brassica oleracea var. sabellica (kale), by evaluating their ability to scavenge the ABTS radical in order to determine the structure–antioxidant-activity relationships [26,27]. In the work of Zietz and colleagues [26], the quercetin derivatives monoacylated with sinapic, hydroxyferulic, ferulic, and caffeic acids; along with those glycosidated with sophorose in position 3-O and glucose or diglucose in position 7-O, were found to display higher radical scavenging activity than the kaempferol ones, together with the hydroxycinnamic acid ester 1,2-disinapoyl-gentiobiose. Several research studies on the antioxidant potential of plant extracts highlighted a strong correlation between ABTS and DPPH assays [39,40,41]. Thus, it can be hypothesized that quercetin derivatives with the same structural features and 1,2-disinapoyl-gentiobiose contained in B. incana extracts are the main derivatives responsible for the observed free radical scavenging activity.
In a study carried out by Yokozawa et al. [42], isorhamnetin diglucoside isolated from the leaves of Brassica juncea (L.) Czern. was found to be inactive in the DPPH test. Therefore, this compound, which represents the main flavonoid detected in B. incana leaf extract, would not be involved in the scavenging effect.
Concerning the chelating properties of B. incana extracts, the activity of the flowering top extract was found to be about five time stronger than that of the leaf one, despite the lower phenolic content. This suggests that the phenolic compounds are only partially responsible for the observed chelating activity, and also that other polar phytochemicals contained in the extracts may contribute to this activity.
The cancer preventive properties of various Brassica species have been reported. A regular intake of vegetables such as broccoli, cabbage, and cauliflower is well-known to reduce the risk of cancer [4,43,44]. Although this effect has been mainly related to the glucosinolate compounds and their derivative products, flavonoids and other phenolics also contribute to it [5,15,16].
The cytotoxicity of B. incana extracts in MCF-7 and CaCo-2 cell lines was evaluated by the MTT assay and by determination of LDH release as an index of necrotic death. Necrosis is a type of cell death that is morphologically characterized by swelling and rupture of intracellular organelles, leading to the disruption of the plasma membrane and the release of intracellular contents. LDH is a soluble cytoplasmic enzyme that is present in almost all cells and is released into extracellular space due to the loss of membrane integrity in dying cells. Thus, the determination of LDH release can be utilized as a useful method for detection of necrosis, and therefore as a measure of cytotoxicity [45,46]. The capacity of a plant extract to induce necrotic cell death could potentially be a valuable adjunctive therapy in cancer treatment [47].
Brassica incana leaf and flowering top extracts showed different cytotoxic effects on the investigated cell lines. Both extracts showed no appreciable cytotoxic activity on MCF-7 cell line; only the flowering top extract showed a slight inhibitory action at higher dosages after 72 h of treatment, accompanied by necrotic cell death. This result is possibly due to the high drug resistance that MCF-7 breast cancer cells possess [48]. Conversely, both the extracts showed a cytotoxic effect in a dose dependent manner, decreasing cell viability of CaCo-2 cells. The different response of CaCo-2 cells in an in vitro model of colon cancer is probably related to the strong absorption capacity of this cell line, making it more sensitive to cytotoxic actions of the extracts [49].
The results highlighted that the flowering top extract exerted a higher cytotoxic action than the leaf one, despite the lower phenolic content and radical scavenging activity. The greater activity of the flowering top extract may depend on its strong ferrous ions chelating properties. Indeed, it has been shown that some metals, such as copper and iron, play a significant role in the rapid proliferation of cancer cells [50].
Further research should be carried out to better clarify the underlying mechanism responsible for the observed cytotoxic effect.
The cytotoxicity of the extracts was also assessed by the brine shrimp lethality bioassay. It represents a simple and low-cost technique for predicting the toxicity of plant extracts in order to consider their safety. It is also a useful system for testing plant extract bioactivity, which in most cases correlates reasonably well with cytotoxic and anti-tumor properties [51]. However, various studies did not highlight this relationship [6,52,53]. Despite the observed activity against tumor cell lines, in this experimental model B. incana extracts exhibited no toxicity against brine shrimp larvae, which indicated their potential safety.
4. Materials and Methods
4.1. Chemicals
LC-MS-grade acetonitrile (ACN) and water (H2O), kaempferol-3-O-glucoside, isorhamnetin-3-O-glucoside, and quercetin-3-O-glucopyranoside were obtained from Merck Life Science (Merck KGaA, Darmstadt, Germany). Methanol (MeOH) was purchased from Baker Analyzed Reagents. Ferrous chloride (FeCl2) was obtained from Carlo Erba (Milan, Italy). Luria–Bertani (LB) broth medium was supplied from Oxoid (Basingstoke, UK). Unless indicated otherwise, all chemicals were purchased from Sigma-Aldrich (Milan, Italy).
4.2. Plant Material and Extraction Procedure
Brassica incana Ten. was collected around Capo d’Orlando (Messina, Italy), with the leaves collected in November 2018 and the flowering tops in May 2019. The taxonomic identification was confirmed by Prof. S. Ragusa, Department of Health Sciences, University Magna Graecia of Catanzaro (Italy). A voucher specimen (1108/18) was deposited in the same department.
After harvesting, the plant material was washed, blended, frozen, and then lyophilized. Subsequently, it was subjected to a preventive maceration at 25 °C with 70% MeOH for an hour. The extraction was carried out with 70% MeOH in an ultrasonic bath at 50 °C for 15 min, repeated four times. The filtrates were combined and evaporated to dryness by rotavapor; the yields of the leaf and flowering top extracts, compared to 100 g of lyophilized plant material, were 26.47% and 33.16%, respectively.
4.3. Phytochemical Investigations
4.3.1. Determination of Total Phenolic Content
The total phenolic contents of B. incana extracts were determined by the Folin–Ciocalteau method compared to the calibration curve of gallic acid phenol compound used as a standard [54]. The extracts were dissolved in 70% MeOH; to 100 µL of each sample solution, 0.2 mL Folin–Ciocalteu reagent, 2 mL of H2O, and 1 mL of 15% Na2CO3 were added. After 2 h incubation at room temperature, the absorbance was measured at 765 nm with a UV-1601 spectrophotometer (Shimadzu, Milan, Italy). The total polyphenols were estimated as gallic acid equivalent (GAE) and expressed in mg GAE/g extract (dw) ± standard deviation (SD). The data were obtained from the average of three independent determinations.
4.3.2. Identification of Phenolic Compounds by HPLC-PDA-ESI-MS
Instrumentation: The analyses were carried out using a Shimadzu HPLC system (Kyoto, Japan) equipped with a CBM-20A controller, two LC-20AD pumps, a DGU-20A3 degasser, a SIL-20AC autosampler, a SPD-M20A photo diode array detector (PDA), and a triple quad mass analyzer (LCMS-8050, Shimadzu, Kyoto Japan) equipped with an ESI interface in negative ionization mode. Data acquisition was performed by Shimadzu LabSolution software version 5.65 (Kyoto, Japan).
Samples Preparation: Ten milligrams of B. incana leaf or flowering top extracts was dissolved in 1 mL of MeOH.
Chromatographic conditions: Analyses were performed on a Ascentis Express C18, 15 cm × 4.6 mm internal diameter (I.D.), with a particle size of 2.7 μm (Merck Life Science, Merck KGaA, Darmstadt, Germany). The mobile phase was composed of water/formic acid (99.9:0.1) (solvent A) and ACN/formic acid (99.9:0.1) (solvent B), with the following gradient: 0 min, 0% B; 5 min, 5% B; 15 min, 10% B; 30 min, 20% B; 60 min, 50% B; 70 min, 100% B; 71 min, 0% B. The injection volume was 10 μL. The flow rate was 1 mL/min and it was split to 0.2 mL/min prior to MS detection.
PDA conditions: The wavelength range was 200–400 nm and the chromatograms were extracted at 280 nm. The time constant was 25 ms and the sample frequency was 40 Hz.
MS conditions: The MS acquisition was performed using ESI in negative mode under the following conditions: mass spectral range: 100–1400 m/z; scan speed: 2727 u/sec; event time: 0.5 sec; nebulizing gas (N2) flow: 3 L/min; interface temperature: 300 °C Heat block: 400 °C, desolvation line (DL) temperature: 250 °C; DL voltage −34 V; probe voltage 4.5 kV; array voltage: 1.0 V, RF voltage: 90 V; detection gain 1.0 kV. Construction of calibration curves: In absence of the reference materials for the quantification of the polyphenolic content, three standards were selected, which were representative of the chemical classes under study, namely kaempferol-3-O-glucoside (y = 17660x − 10681, R² = 0.9963, limit of detection (LOD) = 0.023, limit of quantification (LOQ) = 0.072), isorhamnetin-3-O-glucoside (y = 14948x − 2966.9, LOD = 0.032, LOQ = 0.098), and quercetin-3-O-glucopyranoside (y = 13424x + 898.59, R² = 0.9939, LOD = 0.013, LOQ = 0.043). Standard calibration curves were prepared in a concentration range of 0.1-100 mg/L, considering five different concentration levels. Triplicate injections were made for each level, and a linear regression was generated. The calibration curves with the external standards were obtained using their concentrations (mg/L) with respect to the area obtained from the integration of the PDA peaks at a wavelength of 330 nm. The amount of the compound was finally expressed in mg/g of extract.
4.4. Antioxidant Activity
4.4.1. Free Radical Scavenging Activity
The free radical scavenging activity of B. incana extracts was determined using the 2,2-diphenyl-1-picrylhydrazyl (DPPH) test [54]. Different amounts of each extract were dissolved in 70% MeOH to obtain final concentrations in the range of 0.0625–2 mg/mL. A volume of 0.5 mL of each sample was mixed with 3 mL of daily prepared methanol DPPH solution (0.1 mM) and incubated for 20 min at room temperature in the dark. Then, the optical density change at 517 nm was measured with a model UV-1601 spectrophotometer (Shimadzu). Butylated hydroxytoluene (BHT) was used as a reference compound. The scavenging activity was measured as the decrease in absorbance of the samples versus DPPH standard solution. The results were obtained from the average of three independent experiments, and are reported as mean radical scavenging activity percentage (%) ± SD and mean 50% inhibitory concentration (IC50) ± SD.
4.4.2. Measurement of Reducing Power
The reducing power of B. incana extracts was evaluated by Fe3+-Fe2+ transformation method [54]. Different amounts of each extract were dissolved in 70% MeOH to obtain final concentrations in the range of 0.0625–2 mg/mL. A volume of 1 mL of each sample was mixed with 2.5 mL of phosphate buffer (0.2 M, pH 6.6) and 2.5 mL of 1% potassium ferrycyanide [K3Fe(CN)6]. The mixture was incubated at 50 °C for 20 min, then it was cooled rapidly, mixed with 2.5 mL of 10% trichloroacetic acid, and centrifuged at 3000 rpm for 10 min. The resulting supernatant (2.5 mL) was mixed with 2.5 mL of distilled water and 0.5 mL of 0.1% fresh ferric chloride (FeCl3), and the absorbance was measured at 700 nm after 10 min of incubation at room temperature in the dark; the increased absorbance of the reaction mixture indicates an increase in reducing power. As blank, an equal volume (1 mL) of water was mixed with a solution prepared as described above. Ascorbic acid and BHT were used as reference. The results were obtained from the average of three independent experiments, and are expressed as mean absorbance values ± SD and ascorbic acid equivalent (ASE/mL) ± SD.
4.4.3. Ferrous Ion (Fe2+) Chelating Activity
The Fe2+ chelating activity of B. incana extracts was estimated by measuring the formation of the Fe2+-ferrozine complex [54]. Different amounts of each extract were dissolved in 70% MeOH to obtain final concentrations in the range of 0.0625–2 mg/mL. A volume of 1 mL of each sample was mixed with 0.5 mL of MeOH and 0.05 mL of 2 mM FeCl2. Then, 0.1 mL of 5 mM ferrozine was added to initiate the reaction; the mixture was shaken vigorously and incubated at room temperature in the dark for 10 min. The absorbance of the solution was measured spectrophotometrically at 562 nm. The control contained FeCl2 and ferrozine, which are complex formation molecules. Ethylenediaminetetraacetic acid (EDTA) was used as the reference standard The results were obtained from the average of three independent experiments and are reported as mean inhibition of the ferrozine-(Fe2+) complex formation (%) ± SD and IC50 ± SD.
4.4.4. Protective Effect on Escherichia coli Growth and Survival under Peroxide Stress
The protective effects of B. incana extracts on bacterial growth and survival from the oxidative stress induced by hydrogen peroxide (H2O2) were evaluated in Escherichia coli ATCC 25922 [54]. The strain was obtained from the Department of Chemical, Biological, Pharmaceutical and Environmental Sciences, University of Messina, with in-house culture collection (Messina, Italy). After overnight growth in LB medium, the bacterial suspension was centrifuged (10 min, 3500 rpm), resuspended in LB fresh medium to obtain a final optical density at 600 nm (OD600) = 0.1, and then grown aerobically at 37 °C under low shaking (150 rpm). When the growth reached the mid-log phase (OD600 = 0.6), the bacterial suspension was centrifuged and the OD600 adjusted to 0.2 value with fresh medium and aliquoted, then B. incana extracts (1 mg/mL) and the reference standard quercetin (0.2 mM) were added. To establish the protective effect of the extracts against E. coli growth inhibition induced from oxidative stress, when OD600 was equal to 0.4, bacteria were treated with 2 mM H2O2, and the growth was monitored every 20 min for 180 min.
For survival studies, the bacteria (OD600 = 0.4) were exposed to 10 mM H2O2 for 30 min, then an aliquot of each sample was diluted in 0.9% NaCl to obtain serial dilutions (1:10). Each sample was poured onto LB agar plates, and the cell survival was estimated after 24 h of incubation at 37 °C by counting the number of viable colonies.
The results were obtained from the average of three independent experiments and expressed as mean absorbance ± SD and survival (%) ± SD for protective effect on E. coli growth and survival, respectively.
4.5. Cytotoxic Activity
4.5.1. Cell viability Assay on Human Colorectal Adenocarcinoma (CaCo-2) and Breast Cancer (MCF-7) Cells
Cell culture and treatments: Human colorectal carcinoma cells (CaCo-2), obtained from the American Type Culture Collection (Rockville, MD, USA), were cultured in Dulbecco’s modified Eagle’s medium (Gibco BRL, Life Technologies, Grand Island, NY, USA) supplemented with 10% fetal calf serum, 1 mmol/L sodium pyruvate, 2 mmol/L L-glutamine, streptomycin (50 mg/mL), and penicillin (50 U/mL) in 5% CO2 at 37 °C. MCF-7 breast cancer cells (ATCC cell bank, Rockville, MD, USA) were cultured in Roswell Park Memorial Institute (RPMI) medium containing 10% fetal bovine serum (FBS), 100 U/mL penicillin, and 100 μg/mL streptomycin in 5% CO2 at 37 °C. Human foreskin fibroblast (HFF-1, ATCCVR SCRC-1041TM) cells were cultured in Dulbecco’s modified Eagle’s medium, 4.5 g/L glucose, and penicillin or treptomycin (100 U/mL penicillin and 100 μg/mL streptomycin), with 15% fetal bovine serum.
HFF-1 cell line was exposed for 72 h at the highest concentration of 2 mg/mL of both extracts, while CaCo-2 and MCF-7 cell lines were exposed to the different concentrations of B. incana leaf and flowering top extracts for 48 h and 72 h. Extracts were dissolved in medium to the final concentrations, ranging from 0.0625 to 2 mg/mL of extract.
MTT bioassay: Cell viability was assessed by MTT assay on a 96-multiwell plate (8 × 103 cells/well), as previously described [55]. Inside the metabolically active cells, the tetrazolium salt is converted to yield-colored formazan. The amount of formazan is proportional to the number of living cells. The optical density was measured with a microplate spectrophotometer reader (Titertek Multiskan, Flow Laboratories, Helsinki, Finland) at λ = 570 nm. Results are expressed as percentage of cell viability with respect to control (untreated cells).
Lactic dehydrogenase release: The presence of LDH in a medium of cultured cells is a useful tool to evaluate cell necrosis as a result of cell membrane disruption. LDH activity was measured spectrophotometrically in the culture medium and in the cellular lysates at λ = 340 nm by measuring β-Nicotinamide-adenine dinucleotide (NADH) reduction [56]. LDH release was calculated as the percentage of the total amount, considered as the sum of the enzymatic activity present in the cellular lysate and in the culture medium. Results are expressed as percentage of LDH released.
One-way analysis of variance (ANOVA) followed by Bonferroni’s t-test was performed in order to estimate significant differences among groups. Data were reported as mean values ± SD and differences among groups were considered to be significant at p < 0.001.
4.5.2. Brine Shrimp Lethality Bioassay
The toxic potential of B. incana extracts was investigated in brine shrimp (Artemia salina Leach) [54]. Ten brine shrimp larvae, taken 48 h after initiation of hatching in artificial seawater, were transferred to each sample vial, then artificial seawater was added to obtain a final volume of 5 mL. Different concentrations of each extract were added (10–1000 µg/mL) and the brine shrimp larvae were incubated for 24 h at 25–28 °C. Then, the surviving larvae were counted using a magnifying glass. The assay was carried out in triplicate, and median lethal concentration (LC50) values were determined by the Litchfield and Wilcoxon’s method. Extracts giving LC50 values greater than 1000 μg/mL were considered non-toxic.
5. Conclusions
In the present work, the polyphenolic profile and the antioxidant and cytotoxic properties of B. incana leaves and flowering tops are reported for the first time.
Based on the data obtained from the different in vitro tests carried out to establish the antioxidant potential of the B. incana hydroalcoholic extracts, it can be stated that they have stronger secondary antioxidant properties than the primary ones. The chelating activity of the extract obtained from the flowering tops is particularly relevant.
Brassica incana extracts showed cytotoxic action against Caco-2 cells, probably through the activation of some biochemical pathways related to the necrotic cell death, and the flowering top extract was the most effective.
Overall, the present findings highlighted that Brassica incana represents a safe source of bioactive compounds, providing a valuable contribution to the knowledge of the phytochemical composition and the biological properties of this edible plant.
Acknowledgments
Emilia Cavò thanks the Antonio Imbesi Foundation for the fellowship. The authors are thankful to Shimadzu and Merck Life Science Corporations for the continuous support.
Author Contributions
N.M. and M.F.T. designed the study; N.M., F.C., R.A., G.A.M., and M.F.T. wrote the manuscript. N.M., F.C., L.M., R.A., G.A.M., A.M., and M.F.T. reviewed and edited the manuscript. F.C. and L.M. carried out the HPLC-PDA-ESI-MS analysis. N.M., E.C., M.R., L.D., M.D., and M.F.T. prepared the extracts and performed the antioxidant experiments and the brine shrimp lethality bioassay. R.A. and G.A.M. performed the cytotoxicity experiments on tumor cell lines. All authors have read and given approval for the final version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds are not available from the authors.
References
- 1.Katche E., Quezada-Martinez D., Katche E.I., Vasquez-Teuber P., Mason A.S. Interspecific hybridization for Brassica crop improvement. Crop Breed. Genet. Genom. 2019;1:e190007. doi: 10.20900/cbgg20190007. [DOI] [Google Scholar]
- 2.Rakow G. Species origin and economic importance of Brassica. In: Pua E.C., Douglas C.J., editors. Brassica. Biotechnology in Agriculture and Forestry. Volume 54. Springer; Berlin, Germany: 2004. pp. 3–11. [Google Scholar]
- 3.Branca F., Cartea E. Brassica. In: Kole C., editor. Wild Crop Relatives: Genomic and Breeding Resources, Oilseeds. Springer-Verlag; Berlin, Germany: 2011. pp. 17–36. [Google Scholar]
- 4.Jahangir M., Kim H.K., Choi Y.H., Verpoorte R. Health-affecting compounds in Brassicaceae. Compr. Rev. Food Sci. Food Saf. 2009;8:31–43. doi: 10.1111/j.1541-4337.2008.00065.x. [DOI] [Google Scholar]
- 5.Cartea M.E., Francisco M., Soengas P., Velasco P. Phenolic compounds in Brassica vegetables. Molecules. 2011;16:251–280. doi: 10.3390/molecules16010251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miceli N., Filocamo A., Ragusa S., Cacciola F., Dugo P., Mondello L., Celano M., Maggisano V., Taviano M.F. Chemical characterization and biological activities of phenolic-rich fraction from cauline leaves of Isatis tinctoria L. (Brassicaceae) growing in Sicily, Italy. Chem. Biodivers. 2017;14:e1700073. doi: 10.1002/cbdv.201700073. [DOI] [PubMed] [Google Scholar]
- 7.Taviano M.F., Filocamo A., Ragusa S., Cacciola F., Dugo P., Mondello L., Paterniti Mastrazzo G., De Rose R.F., Celano M., Lombardo G.E., et al. Phenolic profile, antioxidant and cytotoxic properties of polar extracts from leaves and flowers of Isatis tinctoria L. (Brassicaceae) growing in Sicily. Plant Biosyst. 2018;152:795–803. doi: 10.1080/11263504.2017.1338629. [DOI] [Google Scholar]
- 8.Miceli N., Cavò E., Ragusa S., Cacciola F., Dugo P., Mondello L., Marino A., Cincotta F., Condurso C., Taviano M.F. Phytochemical characterization and biological activities of a hydroalcoholic extract obtained from the aerial parts of Matthiola incana (L.) R. Br. Subsp. Incana (Brassicaceae) growing wild in Sicily (Italy) Chem. Biodivers. 2019;16:e1800677. doi: 10.1002/cbdv.201800677. [DOI] [PubMed] [Google Scholar]
- 9.Heywood V.H. Brassica L. In: Tutin T.G., Heywood V.H., Burges N.A., Moore D.M., Valentine D.H., Walters S.M., Webb D.A., editors. Flora Europaea. Volume I. Cambridge at the University Press; Cambridge, UK: 1964. pp. 335–339. [Google Scholar]
- 10.Heywood V.H., Zohary D. A catalogue of the wild relatives of cultivated plants native to Europe. Flora Mediterr. 1995;5:375–415. [Google Scholar]
- 11.Marhold K. Brassicaceae. [(accessed on 1 February 2020)];Euro+Med Plantbase-the Information Resource for Euro-Mediterranean Plant Diversity. Available online: http://www.emplantbase.org/home.html.
- 12.Castellano G., Bazan G. Aspetti ӱistributive e fitosociologici di Brassica incana (Brassicaceae, Magnoliophyta) in Sicilia. Quad. Bot. Amb. Appl. 2009;20:263–268. [Google Scholar]
- 13.Pignatti S. Brassica L. In: Edagricole-New Business Media, editor. Flora d’Italia. Volume 2. Edagricole; Milano, Italy: 2017. pp. 1016–1028. [Google Scholar]
- 14.Do Nurb A. In: Piante Spontanee d’uso Alimentare-Riconoscere, Raccogliere. Rifletto & Rifrango, editor. Lulu.com®; Rome, Italy: 2018. pp. 87–89. [Google Scholar]
- 15.Cartea M.E., Velasco P. Glucosinolates in Brassica foods: Bioavailability in food and significance for human health. Phytochem. Rev. 2008;7:213–229. doi: 10.1007/s11101-007-9072-2. [DOI] [Google Scholar]
- 16.Kumar S., Andy A. Health promoting bioactive phytochemicals from Brassica. Int. Food Res. J. 2012;19:141–152. [Google Scholar]
- 17.Sobrinho Santos E.M., Almeida A.C., Santos H.O., Cangussu A.R., Costa K.S., Alves J.N., Bertucci Barbosa L.C., Souza Aguiar R.W. Mechanism of Brassica oleracea performance in bovine infectious mastitis by bioinformatic analysis. Microb. Pathog. 2019;129:19–29. doi: 10.1016/j.micpath.2019.01.029. [DOI] [PubMed] [Google Scholar]
- 18.Tripodi G., Verzera A., Dima G., Condurso C., Ragusa S. Brassica fruticulosa Cyr. and Brassica incana Ten. (Brassicaceae) as Mediterranean traditional wild vegetables: A valuable source of bioactive compounds. J. Essent. Oil Res. 2012;24:539–545. doi: 10.1080/10412905.2012.730492. [DOI] [Google Scholar]
- 19.Horn P.J., Vaughan J.G. Seed glucosinolates of fourteen wild Brassica species. Phytochemistry. 1983;22:465–471. doi: 10.1016/0031-9422(83)83025-8. [DOI] [Google Scholar]
- 20.Heaney R.K., Fenwick G.R., Mithen R.F., Lewis B.G. Glucosinolates of wild and cultivated Brassica species. Phytochemistry. 1987;26:1969–1973. doi: 10.1016/S0031-9422(00)81740-9. [DOI] [Google Scholar]
- 21.Velasco L., Becker H.C. Variability for seed glucosinolates in a germplasm collection of the genus Brassica. Genet. Resour. Crop Evol. 2000;47:231–238. doi: 10.1023/A:1008793623395. [DOI] [Google Scholar]
- 22.Podsędek A. Natural antioxidants and antioxidant capacity of Brassica vegetables: A review. LWT-Food Sci. Technol. 2007;40:1–11. doi: 10.1016/j.lwt.2005.07.023. [DOI] [Google Scholar]
- 23.Heimler D., Vignolini P., Dini M.G., Vincieri F.F., Romani A. Antiradical activity and polyphenol composition of local Brassicaceae edible varieties. Food Chem. 2006;99:464–469. doi: 10.1016/j.foodchem.2005.07.057. [DOI] [Google Scholar]
- 24.Jaiswal A.K., Abu-Ghannam N., Gupta S. A comparative study on the polyphenolic content, antibacterial activity and antioxidant capacity of different solvent extracts of Brassica oleracea vegetables. Int. J. Food Sci. Technol. 2012;47:223–231. doi: 10.1111/j.1365-2621.2011.02829.x. [DOI] [Google Scholar]
- 25.Ferreres F., Sousa C., Vrchovská V., Valentão P., Pereira J.A., Seabra R.M., Andrade P.B. Chemical composition and antioxidant activity of tronchuda cabbage internal leaves. Eur. Food Res. Technol. 2006;222:88–98. doi: 10.1007/s00217-005-0104-0. [DOI] [Google Scholar]
- 26.Zietz M., Weckmüller A., Schmidt S., Rohn S., Schreiner M., Krumbein A., Kroh L.W. Genotypic and climatic influence on the antioxidant activity of flavonoids in Kale (Brassica oleracea var. sabellica) J. Agric. Food Chem. 2010;58:2123–2130. doi: 10.1021/jf9033909. [DOI] [PubMed] [Google Scholar]
- 27.Fiol M., Adermann S., Neugart S., Rohn S., Mügge C., Schreiner M., Krumbein A., Kroh L.W. Highly glycosylated and acylatedflavonols isolated from kale (Brassica oleracea var. sabellica)—Structure–antioxidant activity relationship. Food Res. Int. 2012;47:80–89. doi: 10.1016/j.foodres.2012.01.014. [DOI] [Google Scholar]
- 28.Nićiforović N., Abramovič H. Sinapic acid and its derivatives: Natural sources and bioactivity. Compr. Rev. Food Sci. Food Saf. 2014;13:34–51. doi: 10.1111/1541-4337.12041. [DOI] [PubMed] [Google Scholar]
- 29.Li Z., Lee H.W., Liang X., Liang D., Wang Q., Huang D., Ong C.N. Profiling of phenolic compounds and antioxidant activity of 12 cruciferous vegetables. Molecules. 2018;23:E1139. doi: 10.3390/molecules23051139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Groenbaek M., Tybirk E., Neugart S., Sundekilde U.K., Schreiner M., Kristensen H.L. Flavonoid glycosides and hydroxycinnamic acid derivatives in baby leaf rapeseed from white and yellow flowering cultivars with repeated harvest in a 2-years field study. Front. Plant Sci. 2019;10:355. doi: 10.3389/fpls.2019.00355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Plumb G.W., Price K.R., Rhodes M.J., Williamson G. Antioxidant properties of the major polyphenolic compounds in broccoli. Free Radic. Res. 1997;27:429–435. doi: 10.3109/10715769709065782. [DOI] [PubMed] [Google Scholar]
- 32.Braca A., Fico G., Morelli I., De Simone F., Tomé F., De Tommasi N. Antioxidant and free radical scavenging activity of flavonol glycosides from different Aconitum species. J. Ethnopharmacol. 2003;86:63–67. doi: 10.1016/S0378-8741(03)00043-6. [DOI] [PubMed] [Google Scholar]
- 33.Dai J., Mumper R.J. Plant phenolics: Extraction, analysis and their antioxidant and anticancer properties. Molecules. 2010;15:7313–7352. doi: 10.3390/molecules15107313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Prior R.L., Wu X., Schaich K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005;53:4290–4302. doi: 10.1021/jf0502698. [DOI] [PubMed] [Google Scholar]
- 35.Kasote D.M., Katyare S.S., Hegde M.V., Bae H. Significance of antioxidant potential of plants and its relevance to therapeutic applications. Int. J. Biol. Sci. 2015;11:982–991. doi: 10.7150/ijbs.12096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Csepregi K., Neugart S., Schreine M., Hideg É. Comparative evaluation of total antioxidant capacities of plant polyphenols. Molecules. 2016;21:E208. doi: 10.3390/molecules21020208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bidchol A.M., Wilfred A., Abhijna P., Harish R. Free radical scavenging activity of aqueous and ethanolic extract of Brassica oleracea L. var. italica. Food Bioprocess Tech. 2011;4:1137–1143. doi: 10.1007/s11947-009-0196-9. [DOI] [Google Scholar]
- 38.Anwar F., Kalsoom U., Sultana B., Mushtaq M., Mehmood T., Arshad H.A. Effect of drying method and extraction solvent on the total phenolics and antioxidant activity of cauliflower (Brassica oleracea L.) extracts. Int. Food Res. J. 2013;20:653–659. [Google Scholar]
- 39.Dudonné S., Vitrac X., Coutière P., Woillez M., Mérillon J.M. Comparative study of antioxidant properties and total phenolic content of 30 plant extracts of industrial interest using DPPH, ABTS, FRAP, SOD, and ORAC assays. J. Agric. Food Chem. 2009;57:1768–1774. doi: 10.1021/jf803011r. [DOI] [PubMed] [Google Scholar]
- 40.Augusto T.R., Salinas E.S.S., Alencar S.M., D’arce M.A.B.R., de Camargo A.C., Vieira T.M.F.D.S. Phenolic compounds and antioxidant activity of hydroalcoholic extracts of wild and cultivated murtilla (Ugni molinae Turcz.) Food Sci. Technol. 2014;34:667–679. doi: 10.1590/1678-457X.6393. [DOI] [Google Scholar]
- 41.Han J.-H., Lee H.-J., Cho M.R., Chang N., Kim Y., Oh S.-Y., Kang M.-H. Total antioxidant capacity of the Korean diet. Nutr. Res. Pract. 2014;8:183–191. doi: 10.4162/nrp.2014.8.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yokozawa T., Kim H.Y., Cho E.J., Choi J.S., Chung H.Y. Antioxidant effects of isorhamnetin 3,7-di-O-beta-d-glucopyranoside isolated from mustard leaf (Brassica juncea) in rats with streptozotocin-induced diabetes. J. Agric. Food. Chem. 2002;50:5490–5495. doi: 10.1021/jf0202133. [DOI] [PubMed] [Google Scholar]
- 43.Anupama M., Murgan S.S., Murthy P.B. Broccoli flower head extract reduces mitomycin-C induced sister chromatid exchange in cultured human lymphocytes. Food Chem. Toxicol. 2008;46:3351–3353. doi: 10.1016/j.fct.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 44.Lam T.K., Gallicchio L., Lindsley K., Shiels M., Hammond E., Tao X.G., Chen L., Robinson K.A., Caulfield L.E., Herman J.G., et al. Cruciferous vegetable consumption and lung cancer risk: A systematic review. Cancer Epidemiol. Biomarkers. Prev. 2009;18:184–195. doi: 10.1158/1055-9965.EPI-08-0710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chan F.K., Moriwaki K., De Rosa M.J. Detection of necrosis by release of lactate dehydrogenase activity. Methods Mol. Biol. 2013;979:65–70. doi: 10.1007/978-1-62703-290-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Malfa G.A., Tomasello B., Acquaviva R., Genovese C., La Mantia A., Cammarata F.P., Ragusa M., Renis M., Di Giacomo C. Betula aetnensis Raf. (Betulaceae) extract induced HO-1 Expression and ferroptosis cell death in human colon cancer cells. Int. J. Mol. Sci. 2019;20:E2723. doi: 10.3390/ijms20112723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tadić V.M., Jeremic I., Dobric S., Isakovic A., Markovic I., Trajkovic V., Bojovic D., Arsic I. Anti-inflammatory, gastroprotective, and cytotoxic effects of Sideritis scardica extracts. Planta Med. 2012;78:415–427. doi: 10.1055/s-0031-1298172. [DOI] [PubMed] [Google Scholar]
- 48.Sargent J.M., Williamson C.J., Maliepaard M., Elgie A.W., Scheper R.J., Taylor C.G. Breast cancer resistance protein expression and resistance to daunorubicin in blast cells from patients with acute myeloid leukaemia. Br. J. Haematol. 2001;115:257–262. doi: 10.1046/j.1365-2141.2001.03122.x. [DOI] [PubMed] [Google Scholar]
- 49.Miret S., Abrahamse L., de Groene E.M. Comparison of in vitro models for the prediction of compound absorption across the human intestinal mucosa. J. Biomol. Screen. 2004;9:598–606. doi: 10.1177/1087057104267162. [DOI] [PubMed] [Google Scholar]
- 50.Gaur K., Vázquez-Salgado A.M., Duran-Camacho G., Dominguez-Martinez I., Benjamín-Rivera J.A., Fernández-Vega L., Carmona Sarabia L., Cruz García A., Pérez-Deliz F., Méndez Román J.A., et al. Iron and copper intracellular chelation as an anticancer drug strategy. Inorganics. 2018;6:126. doi: 10.3390/inorganics6040126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Veni T., Pushpanathan T. Comparison of the Artemia salina and Artemia fransiscana bioassays for toxicity of Indian medicinal plants. J. Coast. Life Med. 2014;2:453–457. doi: 10.12980/JCLM.2.201414J29. [DOI] [Google Scholar]
- 52.Vitali F., Pennisi C., Tomaino A., Bonina F., Pasquale A., Saija A., Tita B. Effect of a standardized extract of red orange juice on proliferation of human prostate cells in vitro. Fitoterapia. 2006;77:151–155. doi: 10.1016/j.fitote.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 53.Hong L.S., Ibrahim D., Kassim J. Assessment of in vivo and in vitro cytotoxic activity of hydrolysable tannin extracted from Rhizophora apiculata barks. World J. Microbiol. Biotechnol. 2011;27:2737–2740. doi: 10.1007/s11274-011-0727-1. [DOI] [Google Scholar]
- 54.Mohti H., Taviano M.F., Cacciola F., Dugo P., Mondello L., Zaid A., Cavò E., Miceli N. Silene vulgaris subsp. macrocarpa leaves and roots from Morocco: Assessment of the efficiency of different extraction techniques and solvents on their antioxidant capacity, brine shrimp toxicity and phenolic characterization. Plant Biosyst. 2019 doi: 10.1080/11263504.2019.1674404. [DOI] [Google Scholar]
- 55.Malfa G.A., Tomasello B., Sinatra F., Villaggio G., Amenta F., Avola R., Renis M. “Reactive” response evaluation of primary human astrocytes after methylmercury exposure. J. Neurosci. Res. 2014;92:95–103. doi: 10.1002/jnr.23290. [DOI] [PubMed] [Google Scholar]
- 56.Acquaviva R., Sorrenti V., Santangelo R., Cardile V., Tomasello B., Malfa G., Vanella L., Amodeo A., Genovese C., Mastrojeni S., et al. Effects of an extract of Celtis aetnensis (Tornab.) Strobl twigs on human colon cancer cell cultures. Oncol. Rep. 2016;36:2298–2304. doi: 10.3892/or.2016.5035. [DOI] [PubMed] [Google Scholar]