As the powerhouse of cardiac myocytes mitochondria are responsible for generating most of the chemical energy in the form of ATP that is required for cardiac contractility and robust heart function1. Mitochondrial dysfunction and consequent reductions in ATP contribute to pathology in heart disease1. While numerous studies have shown that heart disease is associated with, if not caused by the misfolding of proteins outside mitochondria in cardiac myocytes2, little is known about the effects of protein misfolding in mitochondria, which could have dire effects on myocardial energy generation and cardiac function. In this issue of the Journal, Smyrnias et al examined the effects of left ventricular pressure overload on the misfolding of mitochondrial proteins3. They showed that pressure overload in mice activates an intracellular signaling program called the mitochondrial unfolded protein response (UPRmt), and that pharmacologic boosting of the UPRmt reduces cardiac pathology in this model. They also showed that hearts from patients with aortic stenosis, which is often associated with left ventricular overload4, exhibited increased expression of genes associated with the UPRmt. This study suggests that cardiac pathology causes the misfolding of mitochondrial proteins, which activates adaptive aspects of the UPRmt, and that enhancing the UPRmt might serve as a potential therapeutic strategy for treating heart disease.
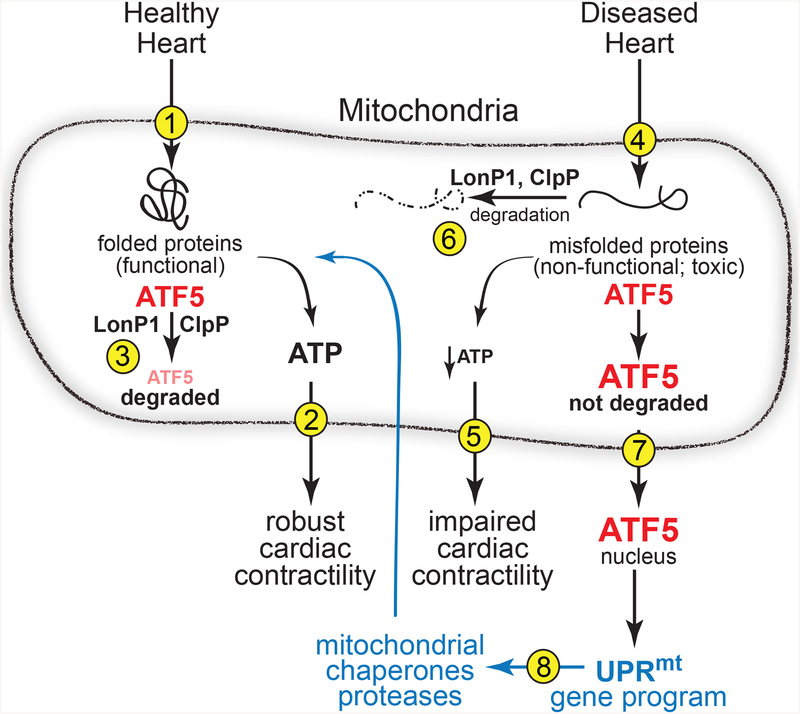
Studies of the UPRmt and other unfolded protein responses in C. elegans, yeast and mammalian cells provide important clues for our understanding of the pathological consequences in the heart of toxic misfolded proteins5,6. Quality control pathways, such as the unfolded protein responses, are designed to guard against accumulation of toxic misfolded proteins by detecting them and then reconfiguring the cellular machinery in ways that augment cellular protein-folding capacity while also increasing the degradation of misfolded proteins7. There are distinct unfolded protein responses that are activated by misfolded proteins in different organelles. For example, the UPRmt, which is activated by misfolded proteins in mitochondria, reconfigures the cellular machinery to remedy this impairment, which ensures the maintenance of optimal mitochondrial performance and, in the heart, robust cardiac myocyte contractility8 (Figure 1, steps 1 and 2). The UPRmt is involved in a major aspect of mitochondrial quality control, the removal of dysfunctional mitochondria by mitophagy. Mitophagy is impaired in the diseased heart in ways that challenge the maintenance of optimally functioning mitochondria, leading to increases in dysfunctional mitochondria, which due to reduced ATP generation, places cardiac myocyte contractility at risk. Impaired folding of mitochondrial proteins that are critical for mitochondrial function is an activator of mitophagy and the UPRmt 9. This co-activation of the UPRmt and mitophagy implicates the importance of the UPRmt as a regulator of mitochondrial quality control in the heart. Further underscoring the crucial nature of the UPRmt in the heart are studies in other cells and organs showing that the UPRmt is activated by imbalances in the levels of the proteins that comprise the electron transport chain, by decreased levels of mitochondrial chaperones, and by reactive oxygen species (ROS)5.
Figure 1 -.
Diagram of the roles for ATF5 in the mitochondrial unfolded protein response (UPRmt) in the healthy and diseased heart. Steps 1–8 are described in the text of this article.
Much of what is known about the UPRmt has come from studies in C. elegans5,6 leading to many inferences about how the UPRmt might function in cardiac myocytes. A key regulator of the UPRmt is ATF510, a transcription factor that localizes to mitochondria when mitochondrial protein folding are optimal. Under these conditions mitochondrial proteases, such LonP1 and ClpP, degrade ATF5 so it does not function as a transcription factor (Figure 1, step 3). However, when misfolded proteins accumulate in mitochondria, which decreases ATP and impairs cardiac contractility (Figure 1, steps 4 and 5), LonP1 and ClpP are diverted to degrading those misfolded proteins to minimize their toxic effects on mitochondrial function (Figure 1, step 6). This diversion of mitochondrial proteases away from ATF5 allows the level of ATF5 to increase, which leads to its export from mitochondria into the cytosol and eventually to the nucleus (Figure 1, step 7). In the nucleus ATF5 binds to and increases the transcription of genes encoding proteins that improve mitochondrial protein folding, including mitochondrial chaperones and the mitochondrial proteases, LonP1 and ClpP8 (Figure 1, step 8); thus, increased levels of ATF5 and the genes it transcriptionally induces are indicators of UPRmt activation. Indeed, Smyrnias et al. showed that ATF5 and its target genes were induced in mouse hearts subjected to chronic pressure overload, as well as in the hearts of patients with aortic stenosis. They went on to demonstrate that treating mice with nicotinamide riboside, which is known to induce the UPRmt, decreased pathology and improved cardiac performance during pressure overload3.
Interestingly, UPRmt activation induces the C/EBP transcription factor, CHOP, which under these conditions induces many genes that encode adaptive proteins of the UPRmt 11. Consistent with this was the finding by Smyrnias et al that CHOP was also increased by pressure overload and aortic stenosis3. This finding raises the question of overlap between unfolded protein responses, since CHOP is also induced by the ER unfolded protein response, UPRER. The UPRER is analgous to the UPRmt, in that misfolded proteins lead to its activation, but differs from the UPRmt in that it is misfolded proteins in the ER that lead to activation of the UPRER. Further distinguishing the UPRmt and the UPRER are that the initiators of the two responses differ, consistent with their different subcellular locations5. However, even though they are initiated by protein misfolding in two different subcellular locations, and by different sensors of misfolded proteins, the UPRmt and the UPRER induce several common regulatory proteins, one of which is CHOP. However, in contrast to the UPRmt, where CHOP is adaptive10, in the case of the UPRER, CHOP initiates apoptotic death in cells where the adaptive aspects of the UPRER are insufficient for restoring protein folding in the ER12. Findings such as this highlight the fact that there is overlap between cellular unfolded proteins responses, such as the UPRmt and the UPRER, but at least in the case of CHOP, there must be some yet-to-be-discovered, stress-specific mechanisms involved that dictate the dramatically different functions of such overlapping regulatory proteins5.
While much remains to be learned about the diverse cellular unfolded protein responses, the study here by Smyrnias et al provides important new information about one of these responses, the UPRmt, demonstrating that key features of this important unfolded protein response are activated in pressure overload, and that in this setting, the UPRmt appears to serve an adaptive, protective role in the heart.
Footnotes
Conflicts of Interest: NONE
References
- 1.Dorn GW 2nd, Vega RB & Kelly DP Mitochondrial biogenesis and dynamics in the developing and diseased heart. Genes Dev 29, 1981–1991, doi: 10.1101/gad.269894.115 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McLendon PM & Robbins J Proteotoxicity and cardiac dysfunction. Circ Res 116, 1863–1882, doi: 10.1161/CIRCRESAHA.116.305372 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smyrnias. J Am Coll Cardiol (2018). [Google Scholar]
- 4.Rockman HA et al. Segregation of atrial-specific and inducible expression of an atrial natriuretic factor transgene in an in vivo murine model of cardiac hypertrophy. Proc Natl Acad Sci U S A 88, 8277–8281 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Qureshi MA, Haynes CM & Pellegrino MW The mitochondrial unfolded protein response: Signaling from the powerhouse. J Biol Chem 292, 13500–13506, doi: 10.1074/jbc.R117.791061 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Callegari S & Dennerlein S Sensing the Stress: A Role for the UPR(mt) and UPR(am) in the Quality Control of Mitochondria. Front Cell Dev Biol 6, 31, doi: 10.3389/fcell.2018.00031 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Walter P & Ron D The unfolded protein response: from stress pathway to homeostatic regulation. Science 334, 1081–1086, doi: 10.1126/science.1209038 (2011). [DOI] [PubMed] [Google Scholar]
- 8.Melber A & Haynes CM UPR(mt) regulation and output: a stress response mediated by mitochondrial-nuclear communication. Cell Res 28, 281–295, doi: 10.1038/cr.2018.16 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pickles S, Vigie P & Youle RJ Mitophagy and Quality Control Mechanisms in Mitochondrial Maintenance. Curr Biol 28, R170–R185, doi: 10.1016/j.cub.2018.01.004 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fiorese CJ et al. The Transcription Factor ATF5 Mediates a Mammalian Mitochondrial UPR. Curr Biol 26, 2037–2043, doi: 10.1016/j.cub.2016.06.002 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rainbolt TK, Saunders JM & Wiseman RL Stress-responsive regulation of mitochondria through the ER unfolded protein response. Trends Endocrinol Metab 25, 528–537, doi: 10.1016/j.tem.2014.06.007 (2014). [DOI] [PubMed] [Google Scholar]
- 12.Ma Y, Brewer JW, Diehl JA & Hendershot LM Two distinct stress signaling pathways converge upon the CHOP promoter during the mammalian unfolded protein response. J Mol Biol 318, 1351–1365 (2002). [DOI] [PubMed] [Google Scholar]