Abstract
Hindbrain astrocytes are emerging as critical components in the regulation of homeostatic functions by either modulating synaptic activity or serving as primary detectors of physiological parameters. Recent studies have suggested that the glucose counter-regulation response (CRR), a critical defense against hypoglycemic emergencies, is dependent on glucoprivation-sensitive astrocytes in the hindbrain. This subpopulation of astrocytes produces a robust calcium signal in response to glucopenic stimuli. Both ex vivo and in vivo evidence suggest that low-glucose sensitive astrocytes utilize purinergic gliotransmission to activate catecholamine neurons in the hindbrain that are critical to the generation of the integrated CRR. Lastly, reports in the clinical literature suggest that an uncontrolled activation of CRR may as part of the pathology of severe traumatic injury. Work in our laboratory also suggests that this pathological hyperglycemia resulting from traumatic injury may be caused by the action of thrombin (generated by tissue trauma or bleeding) on hindbrain astrocytes. Similar to their glucopenia-sensitive neighbors, these hindbrain astrocytes may trigger hyperglycemic responses by their interactions with catecholaminergic neurons.
1. Astrocytes in homeostasis
There has been an historic shift in the appreciation of the astrocyte from passive supporter to active participant in the generation of brain activity [7,20,39,40,77,99]. The degree to which astrocytes are involved in regulating central nervous system-wide function is controversial [3,92]. However, the hindbrain astrocyte has emerged as a powerful component in homeostatic regulation [2,35,37,38,46,63,69,99]. There are several reports of astrocyte involvement in the regulation of hindbrain synaptic activity [1,99]; neural inputs regulating astrocyte function [70,78]; and astrocyte involvement as detectors of physiological parameters critical to the maintenance of homeostasis [1,35,37]. Here we review recent results supporting a special function of astrocytes as primary sensor in triggering the rapid, life-saving homeostatic adjustments to hypoglycemia together referred to as “counter-regulation”.
2. The counter-regulatory response to glucose deficit
Glucose deficits caused by acute food deprivation, catabolism, or medication errors can be life-threatening physiological emergencies. Dangerous reductions in circulating glucose levels are resisted by a series of unique autonomic and behavioral mechanisms critically de-pendent on circuitry in the hindbrain. These “counter-regulatory responses” (CRR; reviewed extensively [27,66,82,102]) specifically detect low CNS glucose levels and engage several defensive reactions to restore glucose homeostasis. Physiological and behavioral CRRs include: release of glucagon and epinephrine (to trigger glycogenolysis), release of corticosteroids (to shift dependence of non-neural tissues away from glucose toward fatty acids), initiation of glucoprivic feeding (to restore metabolic fuel) and a dramatic increase in gastrointestinal motility in anticipation of the arrival of food (to aid rapid digestion). These physiological responses are typically coupled with “sympathetic” sensations of sweating, shaking, weakness, fatigue, headache, and hunger, collectively serving as hypoglycemia “awareness” for the individual.
The accurate and timely initiation of CRR is critical to the survival of individuals with type-1 diabetes. A badly controlled patient with insulin-dependent diabetes may experience a couple of medication-induced hypoglycemic episodes per week. The CRR mechanism becomes desensitized by successive bouts of hypoglycemia. This desensitization occurs in both clinical populations as well as in normal experimental subjects [28,85]. The resultant hypoglycemia unawareness and hypoglycemia-associated autonomic failure (HAAF) can be lethal. It is not uncommon for a diabetic patient to suffer one such event annually that is serious enough to require the intervention by another for their survival. Approximately one in twenty individuals with insulin-dependent diabetes will die as a consequence of hypoglycemia and the failure of CRR [28].
3. CRR and the “Milieu Interieur”
Claude Bernard introduced the concept of autonomic control over glucose production in the mid-19th century. Bernard conducted de-tailed experimental analyses of the origin and synthesis of glucose appearing in the circulation of carbohydrate starved subjects. At the time, it was believed that only plants were capable of de-novo synthesis of glucose, so Bernard was held to a very high standard of proof. His careful, stepwise demonstration that the liver is capable of the synthesis, storage, and release of glucose became the essential foundation of his concept of the physiological defense of the “milieu interieur”. Part of this work included studies on the role of the brain as a stimulus for the release of hepatic glucose. His only available method for reliable “site-specific” CNS stimulation involved applying pressure with a needle to the hindbrain region containing the nucleus of the solitary tract (NST). This maneuver elicited a robust increase in glucose release from the liver; a “piqure diabetes”. Bernard later attributed the effect to descending sympathetic activation of hepatic glucose release [11–13]. Walter B. Cannon used Bernard’s seminal work to advance the concept of homeostatic regulation of circulating glucose. Specifically, Cannon identified the link between glucose deficit and the sympathetic activation of adrenal epinephrine and glucocorticoid release essential to hepatic glycogenolysis and gluconeogenesis; the basis for counter-regulatory control [22].
Considerable attention has been directed toward hypothalamic mechanisms of glucoregulatory and feeding control, yet, an unbroken chain of observations from Bernard to the present clearly associate the hindbrain with the hyperglycemia portion of CRR. Chandler Brooks (working first in the Cannon and Bard laboratories at Harvard and then independently at Princeton in 1931) showed that hypothalamic stimulation can produce an elevation in blood glucose. However, using the acute, decerebrate cat preparation, he also demonstrated that the critical circuitry necessary for electrical stimulation-driven hyperglycemia is the caudal medulla [17]. Nearly 50 years later, DiRocco and Grill [29] used the awake, chronic decerebrate rat to verify that the critical glucodetection and integration mechanisms necessary to evoke an increase in blood glucose in response to cytoglucopenia are located in the hindbrain. The evidence is now clear that glucopenia-induced adrenal hormone release, hepatic glucose production, increases in gastric motility, and feeding behavior are all dependent on the intact hindbrain. In particular, it is clear that the hindbrain is essential for the detection of the glucopenic state and the translation of that data into physiological action [66,82,102].
4. A contemporary view of CRR neurocircuitry
The CRR mechanism is dependent on two populations of catecholamine (CA) neurons in the hindbrain; the A2/C2 grouping in the NST and the A1/C1 group in the ventrolateral medulla (VLM) [82,102]. Localized glucoprivation (i.e., targeted microinjection of 2-deox-yglucose or 5-thioglucose) or site/phenotype-specific saporin lesions suggest that these neuron groups are necessary for glucoprivic feeding, as well as adrenal epinephrine and corticosterone secretion. That is, specific destruction of hindbrain CA neurons results in an inability to evoke CRR [60]. Thus, these CA neurons are a final common path for elicitation of these responses. Local glucoprivation of hindbrain CA regions drive sympathetically-mediated glucagon secretion [4]. How-ever, since a significant amount of pancreatic alpha cell activation is under local glycemic, paracrine, endocrine, and enteric control, removal of hindbrain CA circuits does not completely eliminate systemic hypoglycemia-mediated glucagon release [82]. While there is evidence for segregation of different CRR functions (i.e., glucoprivic feeding, corticosterone, glucagon, epinephrine secretion for the A1/C1 versus A2/C2 areas), there is also evidence for overlap in neurocircuitry involved in CRR function [82,102]. One exception may involve neurons in the dorsal vagal complex (DVC) which control glucoprivation-mediated increases in gastric motility. That is, this reflex control is mediated entirely by the DVC without any involvement of the VLM [44]. Nevertheless, at least some of the critical NST neurons regulating vago-vagal control of gastric function are also noradrenergic [44,88] not unlike other CRR circuits. Hindbrain CA neurons form highly divergent pathways that are in position to directly influence autonomic outflows controlling relevant hormone secretion, gastric motility, and feeding control [49,61,62,80,82]. These hindbrain CA neurons form the integrative core of the CRRs.
The CRR response elicited by hindbrain CA neurons is produced by a unique, “protected” circuitry that can function to mobilize glucose and drive feeding behavior in an emergency without significant modulation of, or by, other homeostatic systems. The advantage of such a system in the management of a physiological emergency is that it retains its sensitivity to the defended parameter under practically all other physiological circumstances [30,82]. This exclusivity of CRR circuitry can help identify CRR versus non-CRR metabolic and feeding control elements. For example, CRR circuit elements are probably not affected by leptin or α-melanocyte-stimulating hormone (α-MSH) inputs, while non-CRR regulatory feeding and metabolic control elements are certainly biased in their response to glucoprivic stimuli in the presence of signals corresponding to repletion state [30,72,82,102].
The specific cellular mechanisms connecting low glucose detection with any local neurons, even the CA neurons in the hindbrain, are not yet clear. A small percentage of hindbrain CA neurons may autonomously behave like glucosensors and may express the components of hypothetical glucodetectors (e.g., KATP channels, glucokinase, AMP kinase, etc. [66,82,102]. However, it has not yet been established with certainty whether any of these CA neurons are themselves sensitive to glucoprivation and are involved in CRR [82]. It is recognized that vagal afferents to and cells within the dorsal medulla can sense glucose. However, the specific involvement of these processes in CRR (as op-posed to circuits involved in the broader integrated control of metabolism [82]) is not clear [66,82,102]. Regardless of the details, until recently, it was assumed that the hindbrain cells responsible for sensing low glucose availability in CRR were neurons. However, several earlier papers [56,65,66,106] suggested that astrocytes may be the primary detectors of low glucose conditions.
5. Classic and contemporary views of astrocyte function in the brain
Classically, the astrocyte has been thought to play a subservient role to the neuron in the control of brain function. In this case, the astrocyte is seen to maintain the extracellular nutrient, metabolite, and ionic environment for the neuron, while also working to dispose of and re-cycle released neurotransmitters and their break-down products. Additionally, the astrocyte provides mechanical support, literally the “glue” that holds the nervous system together. This view is essentially the same as that proposed by Cajal in his “Histologie du système nerveux de l’homme & des vertébrés” of 1909 [21]. While the astrocyte is certainly responsible for these basic functions, the reputation of the glial cell as an active participant in CNS signaling and control has undergone a recent and dramatic revival [43,101].
The foundation for the glial-neuronal interaction controversy was laid over 100 years ago by two of the most famous antagonists in neuroscience: Camillio Golgi and Santiago Ramon y Cajal. Golgi developed his silver chromate “black reaction” stains in 1872 to specifically investigate glial morphology. Based on his observations, Golgi correctly concluded that astrocytes are connected in a syncytial fashion, but made the error of extending this argument to include neuronal interconnections [67]. Cajal modified Golgi’s procedure and used improved optical techniques to conclude correctly that neurons maintain synaptic relationships. Unfortunately, he also concluded, but in-correctly, that glial cells were unlikely to be involved in processes other than the insulation of neurons. Although both men won the Nobel Prize in 1906 for their respective work, the fallout from Cajal’s neuronal argument in Stockholm probably set glial-neural physiology back 100 years. The relatively recent discovery that glial-neural interactions could modify synaptic strength or initiate changes in neuronal excitability [40,76,77] was revolutionary, requiring a modern re-examination of the role of astrocytes in CNS function.
Astrocytes are the most abundant cells within the brain. A single astrocyte may contact tens to hundreds of thousands of synapses and, along with presynaptic terminals and postsynaptic neurons, will form what has been termed the “tripartite synapse” [7,20,39,40,77]. This intimate relationship creates an opportunity in which presynaptic terminals and synaptic efficacy as well as the post-synaptic responsiveness to afferent input and neuronal excitability may be regulated by astrocytes.
This revised view of the significance of the astrocyte includes the observation that these cells are subject to modulation by neuro-transmitters released from neuronal presynaptic terminals as well as gliotransmitters released by other astrocytes [43,70]. Additionally, astrocytic cytoplasmic calcium levels are increased in response to hormones, circulating factors (e.g. thrombin), changes in critical physiological parameters such as O2/CO2, pH, and reductions in glucose concentration or metabolic availability [85]. Calcium release from stores in the endoplasmic reticulum is the principal means by which afferent inputs are translated by the astrocyte into chemical transmission. This increase in astrocytic calcium is coupled to a release of “gliotransmitters” in processes similar to neurotransmission [6,39,40,77]. Currently recognized gliotransmitters include glutamate, ATP, adenosine and D-serine; there are likely to be many others [5,14,26,32,64,75].
6. Connecting astrocytes with CRR neurocircuitry
Our laboratory arrived at the concept of astrocytic regulation of CRR mechanisms through a circuitous route. One of our principal interests is in determining mechanistic explanations for autonomic failure to control gastric function in chronic disease or following traumatic injury [46,99]. For example, head injury, burns, and severe bleeding trauma have all been known to cause a dramatic suppression of gastric motility, resulting in a high degree of gastric feeding intolerance and predisposition toward nausea and emesis. While the effects of head injury to affect autonomic dysregulation might reasonably be blamed on increased intracranial pressure, this connection has been difficult to defend [36,58]. Furthermore, intracranial pressure changes cannot be responsible for the autonomic failures produced by burns or corporeal injury. Therefore, some other “product” of injury must be responsible for changes in CNS-autonomic control. We turned to thrombin, a pro-duct of traumatic injury, as a potential link between injury and autonomic dysfunction. Thrombin is a potent serine proteinase that triggers the initiation of fibrinogenesis in blood clotting. Additionally, this proteinase can act on an unusual class of G-protein-coupled receptors; proteinase-activated receptors (PARs). PAR type I is widely expressed in the brain [53,95]. Thrombin, generated as a consequence of head injury or peripheral trauma, can access these central receptors via the circulation [97]. Early studies suggested that the dorsal vagal complex (DVC) of the hindbrain contained a high density of PARs [103]. This dorsal hindbrain region regulates vago-vagal control of the stomach as well as other complex autonomic responses such as CRR.
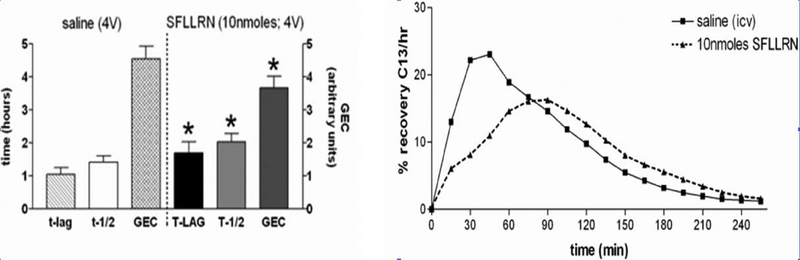
Originally, we presumed that traumatic injury and thrombin production were linked to the suppression of gastric motility via PAR receptors on neurons in the nucleus of the solitary tract (NST). Gastric-NST neurons are excited by vagal afferents from the stomach. These neurons, in turn, inhibit adjacent vagal motor neurons in the dorsal motor nucleus (DMN). Inhibition of gastric-DMN neurons withdraws a source of tonic parasympathetic excitation to the stomach; the result being a dramatic relaxation of the stomach and a suppression of motility. This “vago-vagal” reflex normally regulates gastric tone and motility based on the degree to which the stomach is filled (i.e., monitored by NST neurons) [84]. As predicted, PAR agonist peptides injected into the fourth ventricle produced a significant gastric stasis [46,84](Fig. 1).
Fig. 1.
Gastric emptying rate in an awake, freely moving animal can be monitored by determining the rate of appearance of 13C in respired CO2 that had been ingested as 13C-tagged sodium octanoate-doped meal. 13C in respired CO2 is indicative of the transit of the carbohydrate meal from the stomach to the duodenum. This 13C method captures the entire time course of the transit event (samples taken at 15 min intervals) and is displayed in the right-hand graphs. Specific parameters of this transit can be extracted for comparisons: Tlag corresponds with the time at which rate of excretion of 13CO2 is maximal, T1/2 is the gastric half-emptying time (time when half of the label that is to be excreted has been excreted) and the GEC (a global index of rate of emptying). Each animal served as its own control. That is, the first session the animal receives the control injection (saline either i.p. or 4 V, as appropriate); the next session the animal receives the test injection. Therefore, paired t-test comparisons could be made between the saline control and the agonist condition for each animal. Examples of such paired gastric transit experiments of individual animals are seen in the right-hand graphs. In the left-hand column are group averages for the gastric transit parameters under each condition; statistical comparisons were only made within each condition group. Microinjection of the PAR1-selective agonist SFLLRN (10 nmol) into the fourth ventricle caused a significant reduction in gastric transit as measured by Tlag and T1/2 as well as a slowed overall GEC compared with their respective saline controls. (Adapted from Hermann et al., J Neurosci, 2009, 29 [29]: 9292–9300).
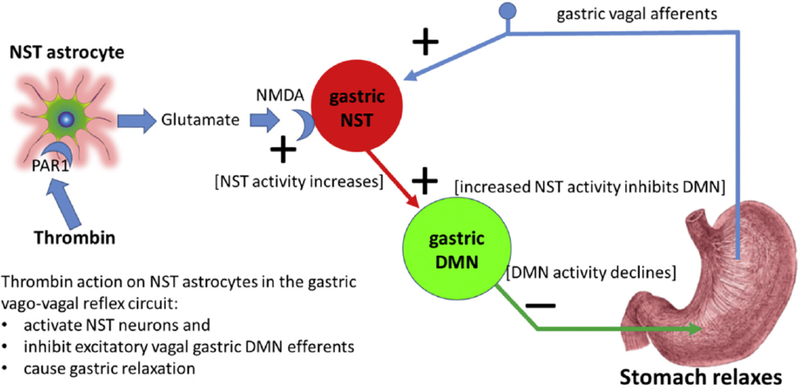
We were very surprised to find that PAR1 receptors in the DVC were located on astrocytes, rather than on neurons [46]. Activation of PAR’s on astrocytes in the NST produced a dramatic increase in cytoplasmic calcium signal in NST astrocytes that was translated, with a delay, into activation of neurons in the NST. Subsequent work showed that PAR activation of NST astrocytes produces a calcium-mediated gliotransmission of glutamate onto neuronal NST NMDA receptors [99]; an effect that would activate inhibitory vagal control of the stomach [84](Fig. 2).
Fig. 2.
Proposed circuitry explaining how thrombin effects on astrocytes can suppress gastric motility in response to traumatic injury.
Traumatic injury causes other failures of autonomic function. The association between severe trauma and hyperglycemia is clinically axiomatic [16,41]. This phenomenon is characterized by a catabolic profile including persistent hyperglycemia, functional insulin resistance and greatly elevated metabolic fuel use. The severity of the hyperglycemia is highly correlated with post-trauma morbidity and mortality [10,25].
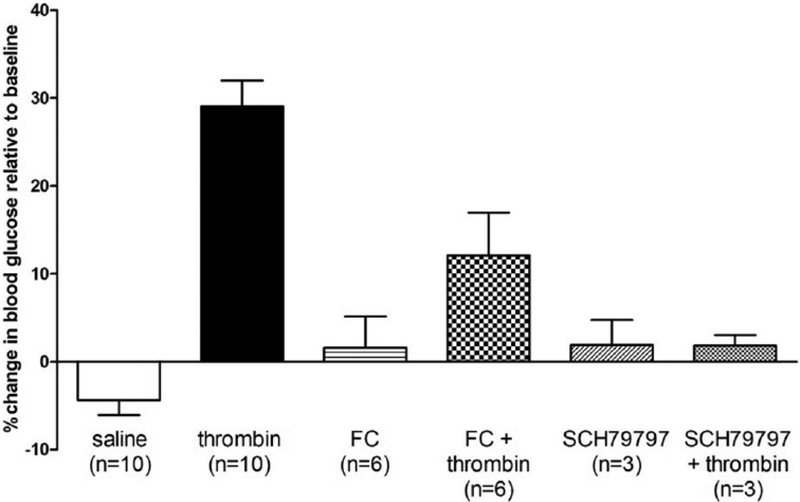
Our preliminary studies indicate that fourth ventricular application of thrombin in the anesthetized rat provokes a significant increase in blood glucose levels compared to control saline applications [45]. This thrombin effect on glycemia is blocked by pretreatment with either the PAR antagonist, SCH79797 [51], or the astrocyte calcium signaling inhibitor, fluorocitrate (FC) [15,42](Fig. 3). Thus, we have seen that the presence of thrombin in the hindbrain can evoke the gastric stasis and hyperglycemia associated with traumatic injury and both of these symptoms are dependent on functioning astrocytes.
Fig. 3.
Preliminary studies indicate that fourth ventricular application of thrombin in the thiobutabarbital anesthetized rat provokes a significant increase in blood glucose levels compared to control saline applications [45]. This thrombin effect on glycemia is blocked by pretreatment with either the PAR antagonist, SCH79797 or the astrocyte calcium signaling inhibitor, fluorocitrate (FC). Thus, the presence of thrombin in the hindbrain can evoke hyperglycemia and this effect is dependent on functioning astrocytes.
7. Astrocytes, CRR and gastric motility
Recall that neurocircuitry for CRR control shares this region of the hindbrain and one of the earliest physiological hallmarks of CRR involves a dramatic increase in gastric motility. While CRR is typically associated with autonomically-mediated increases in glucose release from the liver and increases in feeding behavior to make up for metabolic fuel deficits, it has been recognized for > 100 years that hypoglycemia and acute food deprivation dramatically increase gastric motility [18,23]. The increase in gastric motility in anticipation of glucoprivic feeding speeds digestion and metabolism and is an obligatory feature of CRR glucose homeostasis. Therefore, we extended our original hypothesis concerning the role of astrocytes in the hindbrain to include the regulation of gastric vago-vagal reflex control during low glucose availability. We predicted that some astrocytes in the dorsal vagal complex might be sensitive to reductions in the local concentration of glucose, and if so, would, ultimately, act to increase gastric motility.
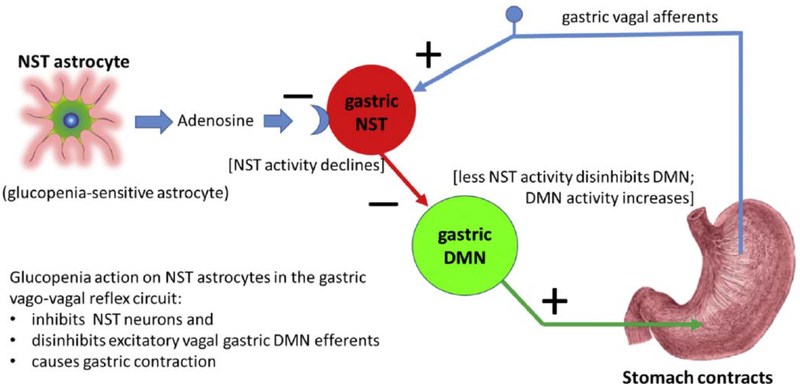
Fluorocitrate (FC) is an astrocyte-selective metabolic suppressor which blocks astrocyte metabotropic signaling while leaving neuronal function intact [15,42]. If astrocytes in the DVC are sensitive to low glucose availability, and, if they are involved in triggering a vagally-mediated increase in gastric motility, then local application (i.e., fourth ventricular; 4 V) of FC should block the increase in motility that is seen following localized hindbrain cytoglucopenia induced by 4 V 2-deox-yglucose (2DG; competitive antagonist of glycolysis). If these hindbrain astrocytes are involved in CRR, then 4 V-FC should also reduce gastric motility responses to systematic hypoglycemia produced by sub-cutaneous insulin. Indeed, FC applied to 4 V blocks the increase in gastric motility evoked by either central or peripheral cytoglucopenia [47]. Additionally, in vivo single unit recordings of physiologically-identified neurons in the DVC show that hypoglycemic conditions de-crease the sensitivity of both NST and DMN neurons responding to slight gastric distension. While FC, alone, has no effect on the sensitivity of either NST or DMN neurons responding to gastric distension, it is clear that interfering with astrocytic function via the pretreatment of FC prior to hypoglycemic conditions evoked an increase in DMN activity and, ultimately, an increase in gastric motility (Fig. 4; also refer to Figs. 2 and 4 in Hermann et al., 2014, J Neurosci, 34:10488–10496). Adenosine is the likely gliotransmitter involved in this astrocyte-NST neuron connection [31,33,71,87,91,98].
Fig. 4.
Proposed circuitry responsible for hypoglycemic effects on astrocytes to elicit an increase in gastric motility.
8. In vivo demonstration: Hindbrain astrocytes and CRR-triggered hyperglycemia
Earlier reports [56,65,66,106] had suggested that astrocytes may be the primary detectors of low glucose conditions, thus involved in counter-regulatory control. We investigated this possibility for CRR-induced hyperglycemia in thiobutabarbital-anesthetized rats [87]. Our data showed that 4 V exposure to 2DG in these anesthetized rats can elicit the expected elevation in blood glucose of CRR as has been previously reported in response to intracranial or peripheral challenges in the awake animal [24,30,49,83,102,105]. This centrally-induced CRR effect on glycemia was blocked by exposure of the 4 V to fluorocitrate, a selective blocker of astrocyte metabotropic signaling and gliotransmission [99](Fig. 5). Thus, these data are consistent with the hypothesis that hindbrain astrocytes are important components of CNS glucodetection circuits that drive multiple aspects of CRR [47,65,69].
Fig. 5.
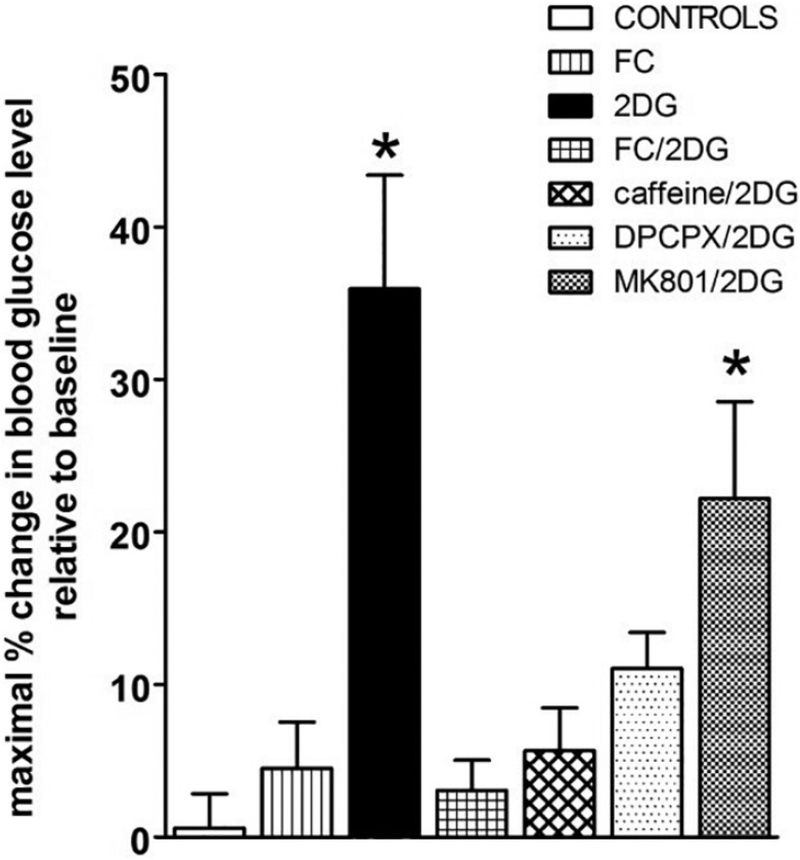
Fourth ventricle 2DG evokes a counter-regulatory hyperglycemia that is suppressed by FC and adenosine antagonists. Maximal percent change in blood glucose levels relative to baseline levels were averaged within each group. Maximal peak responses for the 2DG (alone) group averaged 36.0 ± 7.4%change. While 4 V application of vehicle controls (saline or 1:4 DMSO/saline) or FC (alone) or caffeine (alone; data not shown here) had no effect on glucose levels, pretreatment of the 4 V with FC blocked the effect of subsequent 2DG to increase blood glucose (FC + 2DG: 3.1 ± 2.0%). Similarly, pretreatment with caffeine or DPCPX suppressed the glycemic effects of 2DG (caffeine+2DG = 5.7 ± 2.8%; DPCPX+2DG = 11.1 ± 2.4%). In contrast, the NMDA antagonist, MK801, did not block the 2DG effect to increase blood glucose (MK801 + 2DG = 22.2 ± 6.4%). One way ANOVA F7,38 = 9.78, p < .0001; Dunnett’s post-hoc test * p < .05. These results are consistent with a counter-regulatory hyperglycemia triggered by astrocytes utilizing adenosine as a gliotransmitter. (Adapted from Rogers et al., 2016, Am J Physiol Regul Integr Comp Physiol 310: R1102–R1108).
Further, the 4 V application of adenosine receptor antagonists (caffeine and the A1 antagonist, DPCPX) also suppressed the hindbrain 2DG effect to initiate CRR; while glutamate receptor antagonist (MK801) was ineffective in suppressing the increase in glycemia. These data suggest that astrocytes are important sensors of low glucose availability (central 2DG-induced glycemia). In turn, astrocytes are probably signaling this “hypoglycemic condition” to appropriate neurons in the hindbrain neurocircuitry involved in CRR reflexes via purinergic gliotransmission. Fourth ventricular FC also suppressed the effect of systemic 2DG to provoke hyperglycemia (Fig. 6).
Fig. 6.
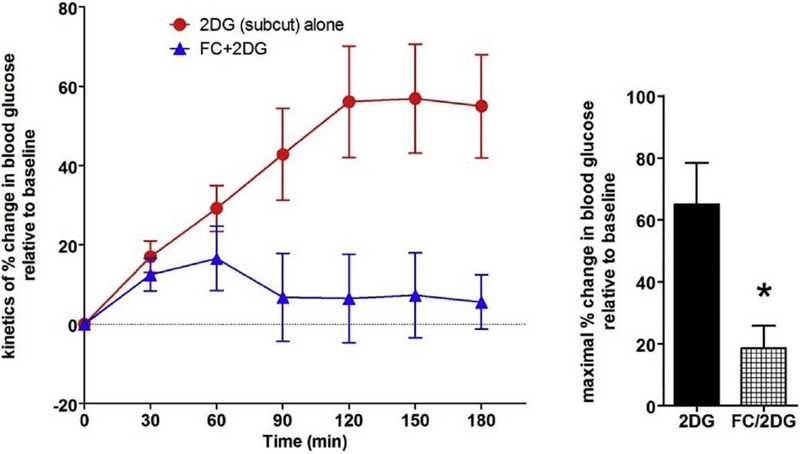
Subcutaneous 2DG (100 mg/kg/ml) in the thiobutabarbital-anesthetized rat produces a counter-regulatory increase in blood glucose. Pretreatment with 4 V FC (5nanomol) 30 min prior to subcutaneous delivery of 2DG significantly reduced the hyperglycemic effect of systemic 2DG (t = 3.074; p = .02). These results suggest that the principal mechanism connecting systemic glucopenia with counter-regulatory hyperglycemia involves hindbrain astrocytes. (Adapted from Rogers et al., 2016, Am J Physiol Regul Integr Comp Physiol 310: R1102–R1108).
9. Ex vivo imaging: physiological evidence for low-glucose-sensitive astrocytes in the hindbrain
While compelling, the data discussed so far concerning astrocyte low glucose detection were collected using indirect in vivo methods. Direct physiological study of astrocyte “activation” is difficult because these cells are not electrically excitable and produce no obvious electrical signatures of activation as do neuronal or beta cell glucosensors. Therefore, unlike neurons, they cannot be studied with direct electro-physiological methods [7]. Astrocyte signaling is based on calcium flux [6,99]. Astrocyte calcium signaling can be observed directly, in real time, under physiological conditions with the aid of exogenously applied calcium-sensitive fluorescent intracellular dyes (i.e., Calcium Green or Cal520) or genetic constructs such as GCaMP; a genetically encoded calcium indicator fused with green fluorescent protein. The intracellular dyes or constructs are illuminated and visualized with laser confocal microscope using ex vivo tissue slice recording methods. Our laboratory originally used these methods to determine that PAR signaling in the hindbrain operated through gliotransmission to neurons in the NST [46](Fig. 7).
Fig. 7.
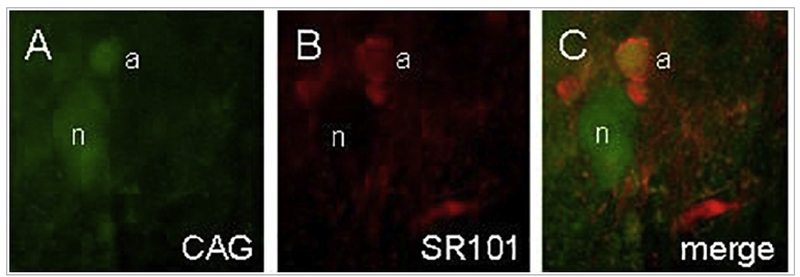
Identification of astrocytes and neurons during live cell imaging. A) Calcium Green (CAG), the Ca++ reporter dye, is taken up by both astrocytes (a) and neuronal cells (n). B) SR101 astrocyte-specific vital dye staining. C) Same field as both A and B; demonstration of ex vivo glial and neuronal identification. (Adapted from Hermann et al., J Neurosci, 2009, 29 [29]: 9292–9300).(For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Calcium signals can be generated by membrane channel activities, but as in other cell types, transmembrane calcium flux is usually coupled to mass calcium release from storage in the endoplasmic reticulum (ER) via calcium-induced calcium release (CICR) through ER ryanodine channels. However, mass release of calcium from ER storage can also occur through a completely separate but parallel, inositol trisphosphate (IP3) channel. This mechanism for ER calcium release is activated by G-protein receptors coupled to phospholipase C (PLC). When activated by Gq/11 type receptors (such as the PAR receptor), PLC cleaves the membrane phospholipid PIP2 into diacyl glycerol (DAG) and IP3. Then, IP3 activates ER calcium release through a receptor mechanism separate from ryanodine [100].
The “wave” of cytoplasmic calcium released from the ER due to membrane receptor signaling initiates a cascade of signal transduction events, most notably vesicle secretion from beta cells and glio-transmission from astrocytes [89,107]. The ER calcium-ATPase pumps are largely responsible for the restoration of cytoplasmic calcium to low levels after signaling events. Re-establishing the transmembrane and ER to cytoplasm concentration gradients are necessary for renewed calcium signaling. Modulation of the calcium ATPase pump and changes in the rate of removal of cytoplasmic calcium could alter the dynamics of the calcium signal as well [73].
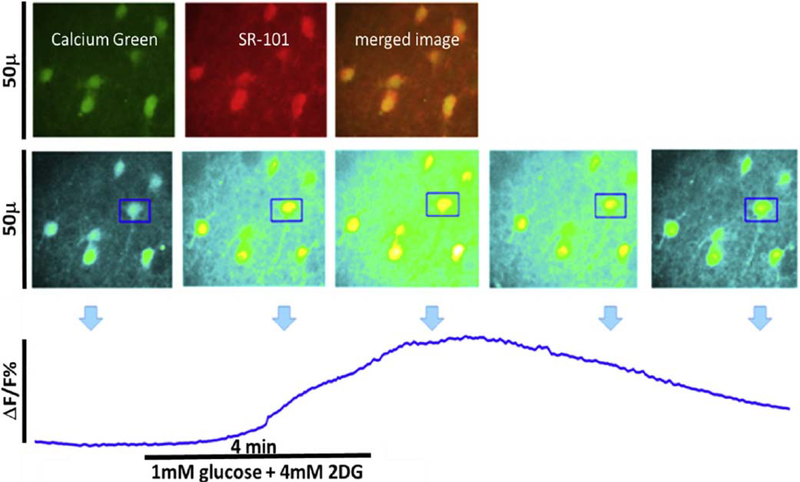
Using confocal live cell imaging methods, we have produced direct and unambiguous evidence that a population of astrocytes in the NST produce cytoplasmic calcium signals proportional to a reduction in glucose concentration or the intracellular store of utilizable glucose [69]. In these studies, astrocytes and neurons in the NST were labeled with Calcium Green 1-AM, an intracellular calcium reporter dye. As-trocytes were discriminated from neurons with the aid of an astrocyte-specific vital stain, SR101. Reductions of bath glucose concentrations from “normal” level (5 mM; 90 mg%) to hypoglycemic levels (1 mM; 18 mg%) produced a robust increase in calcium signal in approximately 50% of NST astrocytes that returned to basal levels in the presence of normal glucose Krebs solution. This effect was also seen with 2DG (in 5 mM glucose) or both stimuli together, demonstrating that astrocyte calcium signaling is related to changes in utilizable intracellular glucose (Fig. 8).
Fig. 8.
Live cell calcium imaging of NST astrocytes in response to glucoprivic challenge. Top panel: In vivo co-injection of the calcium indicator dye, Calcium Green (green) and the glial vital stain sulforhodamine 101 (SR101; red) allows for simple discrimination of astrocytes and neurons in the in situ brain stem slice preparation. Following a dual exposure utilizing the 488 nm and 561 nm laser lines, a composite image is obtained in which astrocytes are clearly labeled yellow, i.e. positive for both CAG and SR101, while neurons remain green. Middle panel: screen shots of field of astrocytes responding to glucoprivic challenge over time (time-lapse imaging with only the 488 nm laser line). Changes in intracellular calcium concentrations are indicated by a proportional change in the intensity of the fluorescence of the calcium green. One region of interest [ROI] was drawn around an individual astrocyte (blue box) for analysis of its time-lapse re-sponse to the challenge. Lower panel: plot of change in magnitude of fluorescence of individual astrocyte before, during, and after exposure to glucoprivic challenge. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Further, approximately 50% of the NST neurons also produced an increase in cytoplasmic calcium in response to decreases in glucose levels, but after a significant delay relative to astrocytic activation. Pharmacologic inactivation of neuronal excitability and synaptic transmission with tetrodotoxin (TTX) eliminated increases in calcium signal in neurons, but not in astrocytes. These results correspond well with those of Marty, et al. [65], and suggest that a subset of astrocytes in the NST are intrinsically responsive to reductions in metabolizable glucose [69].
Hindbrain catecholaminergic (CA) neurons are required for critical autonomic, endocrine, and behavioral counter-regulatory responses (CRRs) to hypoglycemia [81,82]. To test the proposition that hindbrain CA responses to glucoprivation are astrocyte dependent, we utilized transgenic mice in which the calcium reporter construct (GCaMP5) was expressed selectively in tyrosine hydroxylase neurons (TH-GCaMP5). We conducted live cell calcium-imaging studies on hindbrain slices containing the nucleus of the solitary tract (NST) or the ventrolateral medulla (VLM); critical CRR initiation sites.
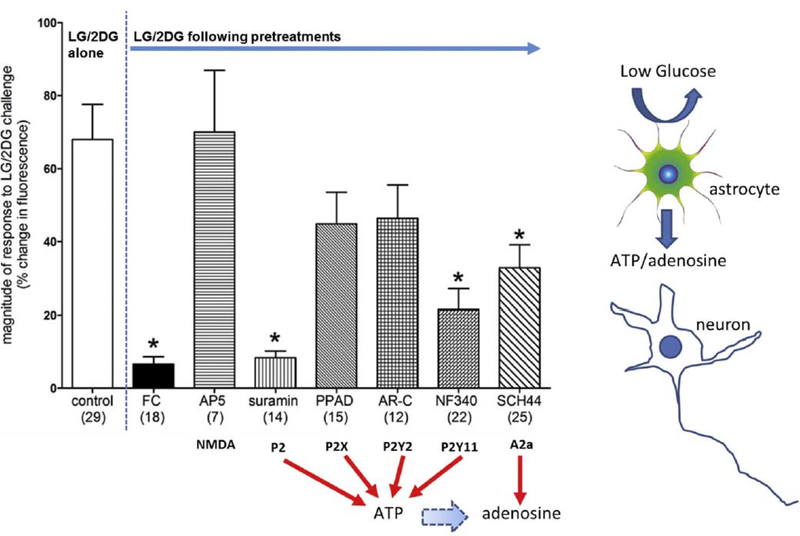
Our results [86] show that approximately 90% of the TH-GCaMP5 neurons (both NST and VLM) were robustly activated by a glucoprivic challenge and that this response was dependent on functional astrocytes. Pretreatment of hindbrain slices with fluorocitrate (an astrocytic metabolic suppressor) abolished TH-GCaMP5 neuronal re-sponses to glucoprivation. Pharmacologic results suggest that the astrocytic connection with hindbrain CA neurons is purinergic via P2 receptors (Fig. 9).
Fig. 9.
Magnitude of changes in fluorescence due to intracellular calcium fluxes in TH-GCaMP5 NST neurons in response to glucoprivic challenge after specific pretreatment conditions (Number of neurons studied per each group is noted in parentheses). Exposure of TH-GCaMP5 neurons in hindbrain slices to the various pretreatment conditions produced significant differences in response to subsequent glucoprivic challenge (ANOVA F(10,175) = 7.39; p < .0001). Similar to the responses seen in the general population of NST neurons, TH-GCaMP5 NST neurons were robustly activated by glucoprivation and that effect is essentially blocked by pre-treatment with FC (Dunnett’s post hoc test q = 6.415; *p < .05). The NMDA antagonist (AP5) had no effect to inhibit the TH-GCaMP5 NST neuron response to glucoprivation. However, the non-selective P2 antagonist (suramin) also blocked the re-sponses to low glucose/2DG (Dunnett’s post hoc test q = 5.715; *p < .05). Lastly, both NF 340 (P2Y11 antagonist) and SCH442416 (A2a antagonist) sup-pressed TH-GCaMP5 NST neuronal responses to glucoprivic conditions (Dunnett’s post hoc test q = 5.160 and 4.047, respectively; *p < .05). These data suggest that the catecholamine neurons critical to activating counter-regulation in response to central glucopenia depend on purinergic gliotransmission. (Adapted from Rogers et al., 2018, Am J Physiol Regul Integr Comp Physiol 315: R153–R164.)
Parallel imaging studies on hindbrain slices of NST from wild-type C57BL/6J mice, in which astrocytes and neurons were prelabeled with a calcium reporter dye and an astrocytic vital dye, show that both cell types are activated by glucoprivation but astrocytes responded significantly sooner than neurons. Pretreatment of these hindbrain slices with P2 purinergic antagonists abolished neuronal responses to glucoprivation without interruption of astrocyte responses; pretreatment with fluorocitrate eliminated both astrocytic and neuronal responses [86]. Neither FC nor P2 antagonists blocked astrocytic or neuronal responses to glutamate demonstrating that under blockade both cell types are still viable and responsive to stimuli other than glucopenia. These results support earlier work in the rat [69] which suggested that the primary detection of glucoprivic signals by the hindbrain is mediated by astrocytes.
There is evidence for a separation of hindbrain influences over the hyperglycemic CRR responses based on the speed of onset of the hypoglycemic challenge [52]. Catecholaminergic neurons that receive input from visceral glucosensors in the portal circulation are especially tuned to a gradual onset of hypoglycemic challenges. This porto-mesenteric pathway produces glucoprotective responses which involve higher-order catecholaminergic hindbrain projections to hypothalamic structures that, in turn, invoke gradual changes in sympathoadrenal hormone release. Rapid onset CRR responses to abrupt hypoglycemia challenges are likewise dependent on hindbrain catecholamine neurons but not on those projecting to the forebrain [52]. Our imaging studies revealed that hindbrain astrocyte and neuron reactions to local cytoglucopenia are very rapid, starting within a few minutes after the onset of the challenge and peaking minutes later [69,86]. This high-speed astrocyte and neuron interaction is probably involved in driving a CRR response to rapid onset glycemic challenges. However, our in vivo data also show that the reflex hyperglycemia caused by slow-onset hypoglycemic challenges such as subcutaneous 2DG or systemic insulin are dependent on intact hindbrain astrocytes [87]. Together, these data suggest that astrocytes in the hindbrain are not only necessary for the detection of hindbrain cytoglucopenia but are also important for the accurate integration of cytoglucopenia information from the periphery [87].
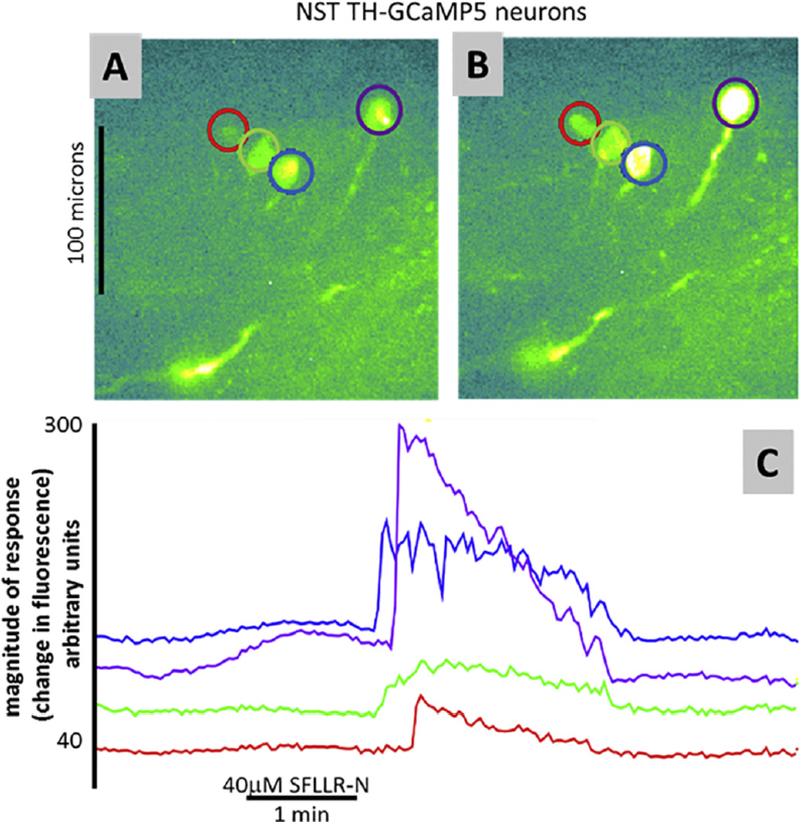
As an aside, preliminary ex vivo imaging studies using the TH-GCaMP5 transgenic mouse preparation have suggested that these identified catecholaminergic neurons are probably also involved in the hyperglycemia induced by thrombin. Not unlike previous imaging studies done in rats [99], the thrombin-receptor agonist, SFLLR-N, activated NST astrocytes first; NST neurons followed (Fig. 10). These studies reinforce the proposal that hindbrain catecholaminergic neurons are critical to producing hyperglycemia.
Fig. 10.
Preliminary ex vivo imaging studies using the TH-GCaMP5 transgenic preparation show that these identified catecholaminergic NST neurons are also responsive to the PAR1 agonist, SFLLRN. A) Identified catecholaminergic (TH-GCaMP5) cells at rest. B) Same cells as in (A) stimulated by perfusion of SFLLR-N. C) Individual response profiles of cells identified in (A) and (B).
Of the 28 TH-GCaMP5 neurons in the NST that were responsive to glutamate/ATP challenge (i.e., viability test), 15 (i.e., ~54%) were also responsive to SFLLR-N. These data suggest that hindbrain catecholaminergic neurons are also involved in the hyperglycemia induced by thrombin.
10. Astrocyte glucose detection; different from “classic” models
Glucose detection and coupling to mechanisms regulating cellular excitability have been elegantly described for the pancreatic beta cell and for some neurons presumed to be involved in physiological and nutrient homeostasis. The critical element of this mechanism is a specialized transmembrane glucose transporter, GLUT2 [56,65]. GLUT2 is a reversible carrier in that it will transport glucose down a concentration gradient in either direction across the cell membrane. Further, GLUT2 operates at a high relative volume but with a low affinity compared to other hexose transporters. That is, the KD for transport for GLUT2 is in the physiological range for glucoregulation of 1–10milli-molar range versus the high micromolar range for the high affinity but low volume GLUT1. Most important, however, is that the combination of the high volume of transport and low affinity characteristics makes the GLUT2 transporter the critical discriminator of physiological glucose concentrations [56]. The physiological consequence is that glucose concentrations are rapidly equalized between the extracellular and intracellular compartments for cells that possess a significant number of GLUT2 transporters.
In the beta cell, for example, increases in extracellular glucose concentrations are rapidly translated into elevated intracellular glucose. Newly available utilizable glucose is metabolized through glycolysis to produce lactate and ATP. The intracellular ATP concentration, in this instance, mirrors the supply of intracellular glucose. This in-formation is converted to proportional changes in excitation through the action of ATP on a specialized potassium ion channel, KATP.In glucose-detecting cells, this tonically open potassium conductance helps hold the resting membrane in an inactive, hyperpolarized state. ATP inactivates this conductance, causing a localized membrane de-polarization. In beta cells, this depolarization triggers the opening of voltage-gated calcium channels and calcium-induced calcium release (CICR). CICR is a process by which calcium entering the cytoplasm binds to ryanodine channels in the endoplasmic reticulum (ER), dis-charging large amounts of calcium into the cytoplasm. The resulting wave of cytoplasmic calcium, in turn, drives insulin secretion in pro-portion to the concentration of intracellular glucose [50]. In glucose sensitive neurons, the process is similar with the distinction that localized membrane depolarization couples to the voltage-dependent sodium conductance which generates action potentials in proportion to the amount of utilizable glucose [9]. Since the GLUT2 transporter is bi-directional, any reduction in extracellular glucose would be rapidly reflected in a reduction in intracellular glucose available for the synthesis of ATP, hence a reduction in beta cell insulin secretion and a reduction in neuronal glucosensor excitability [56].
The importance of GLUT2 to physiological glucose detection makes it a convenient marker for the identification of putative glucose-sensing cells. This logic propelled the work of Marty, et al. in a search for the cells necessary for the detection of low glucose and the initiation of the CRR [65,66]. A complex transgenic scheme was required to show that CNS astrocytes, probably localized to the hindbrain, are essential components of the CRR glucodetection mechanism. A global knockout of GLUT2 is not survivable given the dependence of normal beta cell insulin secretion on GLUT2, as discussed above. To solve this problem, GLUT2 knockouts were paired with an additional transgenic modification of beta cells such that these insulin secreting cells also ex-pressed the GLUT1 transporter. This manipulation produced the global GLUT2 deletion while leaving beta cells sufficiently permeable to glucose to allow normal basal insulin secretion. However, GLUT2 deletion eliminated the initiation of CNS hypoglycemia-triggered CRR. Surprisingly, rescue expression of GLUT2 in neurons had no effect to reverse the elimination of CRR. Even more surprising was the observation that re-expression of GLUT2 in CNS astrocytes, in fact, rescued CRR. The conclusion of this ground-breaking study is that astrocytes are key elements in the direct sensing of glucopenia [56]; a view supported by recent investigations from our laboratory [69] as well as suspected from the results of earlier studies [106].
While the GLUT2 glucodetection model for astrocytes may provide a dominant influence over CRR mechanisms, it is certainly not the only available glucosensor mechanism that can influence glucoregulatory mechanisms that parallel hindbrain CRR. For example, the sweet taste transduction mechanism used in the taste bud (i.e., T1R2 + T1R3 receptors), metabolic control of the K-ATPase (i.e., control of neuronal excitability) and nitric oxide production take part in hypothalamic circuits that can influence CRR [34,57,79,104].
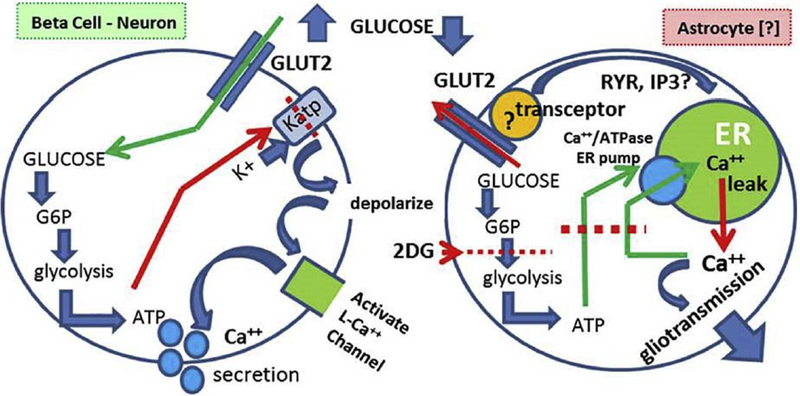
The mechanism by which a reduction in glucose availability signals an increase in cytoplasmic calcium (e.g., Fig. 11) in the NST-astrocyte remains under investigation. While astrocytic expression of GLUT2 appears necessary for proper glucose counter-regulation [65], the “classic” transduction mechanisms coupled to GLUT2 in beta cells and neurons produce changes in calcium that run in the opposite direction to that observed for astrocytes. For example, pancreatic beta cells respond with an increase in intracellular calcium levels in response to increasing extracellular glucose. As discussed earlier, glucose is transported through the GLUT2 transporter and metabolized via glycolysis producing ATP. Increased cellular ATP energy charge acts to phosphorylate and inactivate KATP channels, leading to depolarization, activation of voltage gated calcium channels and ending in excitation-secretion coupling in the beta cell [48,59,90].
Fig. 11.
Fundamental differences in how the “classic model” (i.e., beta cell) of glucodetection must differ from the astrocyte mechanism:
1. Activating levels of glucose are opposite. That is, glucopenia activates astrocytes; while elevated glucose activates beta cells and glucose-sensitive neurons.
2. 2. The mechanism of glucose activation of beta cells is well known; ATP generated by glycolysis inhibits a dominant K+ conductance, causing depolarization. Depolarization opens voltage-gated cation channels triggering action potential generation and/or secretion.
3. Mechanisms for activating calcium entry/release after low glucose exposure are not known but may involve ATP “starvation” induced shut-down of ER calcium storage followed by calcium leakage from the ER. The role of “transceptors” attached to GLUT2 possibly mediating ER calcium release (via inhibition or activation) are common in yeast and may exist in vertebrates, but this is not yet clear. (Adapted from Rogers et al., 2017, Hindbrain Astrocyte Glucodetectors and Counterregulation. In: Appetite and Food Intake: Central Control, edited by Harris RBS, p. 205–228.
However, not unlike our observations, others have reported that glucose restriction in cultured astrocytes causes an increase in astrocytic cytoplasmic calcium signals (8, 54). Similar to our present studies, this increase in cytoplasmic calcium is reversed with the restoration of normal levels of glucose in the perfusion bath. Astrocytes are highly dependent on glycolysis for ATP production; removal of glucose or blocking glucose utilization may starve astrocytes of glucose for ATP production. With impaired glycolysis and subsequent low ATP following low extracellular glucose availability, the calcium-ATPase pump in the endoplasmic reticulum (ER) of astrocytes fails and ER calcium is released to the cytoplasm [8,54]. These results are consistent with our observations in the slice preparation. However, it is unlikely that this particular mechanism is responsible for the cytoplasmic calcium signal seen by our laboratory in in situ NST-astrocytes. One reason for doubt is that the response time for astrocytes in our NST slice preparation [69]is approximately one tenth that reported for cultured hippocampal astrocytes [8,54].
Rapid signaling of glucose status in intact hindbrain astrocytes may involve the action of a GLUT2 -glucose “transceptor”, i.e., a protein that functions as both a transporter and receptor [93,94](Fig. 11). A GLUT2-based transceptor could detect changes in extracellular glucose concentration and then rapidly initiate a calcium-based transduction event. This system would function somewhat like a membrane G-protein based receptor linked to ER calcium release. Transgenic mice generated to knock down a GLUT2-intracellular loop domain yielded animals that demonstrated an inability to detect glucose but left the GLUT2-dependent glucose transport unaffected [93,94]. More recent preliminary imaging results in our laboratory reinforce the idea that GLUT2 is a necessary component of the astrocyte low-glucose detection mechanism. Specifically, the potent and selective GLUT2 blocker, quercetin, blocks astrocyte responsiveness to glucoprivation. In contrast, the GLUT1/4 antagonist, fasentin, and the SGLT blocker, phlorizin, do not. It remains to be shown whether GLUT2 and its intracellular signaling domain are connected directly to intracellular calcium release transduction components such as phospholipase C (PLC) which drives ER calcium release [68]. If so, this astrocyte low glucose sensing mechanism would be roughly analogous to the yeast glucose sensor, Gpr1, and the mammalian sweet taste sensor in that all three are G-protein coupled and all invoke a rapid PLC-mediated increase in calcium when activated [55,96]. A unique feature of the astrocyte mechanism is its activation by a nutrient deficit in perhaps the same manner signaled by hepatic vagal glucose sensors [19,74].
11. Perspective
There is now good evidence that astrocytes in the hindbrain perform important functions as chemosensors. The anatomical relationships between the vascular supply, glial cells, and the neuropil certainly suggest that astrocytes occupy a “favored” (i.e., gateway) position from the perspective of monitoring material flux into the brain. Glial cells, including astrocytes, literally form vascular-neuropil and ventricular-neuropil diffusion barriers. But these cells also possess transporters that allow the penetration of even large-sized signal molecules, such as cytokines into the neuronal matrix. Additionally, small nutrient molecules, such as glucose, ions, and blood gases access the neuropil by passing through or around astrocytes. Thus, this arrangement places glial cells in an ideal position to detect the fluxes of physiologically critical solutes and exert early influence on adjacent neural systems dedicated to homeostatic regulation of these agents.
Work from 20 years ago [106] to the present suggest that astrocytes are important sensors of low glucose availability. While astrocytes are not the only source of chemosensory input modulating glucose CRR, hindbrain astrocytes are in a position to exert a dominant influence over the initiation of the CRR. Our in vivo physiological studies demonstrate that functional astrocytes are required for the initiation of the CRR in response to either peripheral of hindbrain glucopenic sti-muli. Calcium-imaging studies demonstrate a rapid communication between low-glucose sensitive hindbrain astrocytes and catecholaminergic neurons in response to glucoprivic conditions. Astrocytes apparently relay the glucopenic signal to catecholamine neurons in the hindbrain that organize CRR reflex responses via purinergic agonists, such as ATP or adenosine. While it is not yet clear how astrocytes transduce glucopenia into calcium-based gliotransmission, recent data point to a “transceptor” mechanism as a potential explanation.
Acknowledgements
We gratefully acknowledge funding support we received for the work described in this paper from: NIH NS60664, DK108765, and HL128454; The John S. McIlhenny Professorship; PBRC Botanical Research Center T32; Pennington/Louisiana NORC 2 P30 DK072476- 11A1; and the PBRC Botanicals and Metabolic Resiliency 2 P50 AT002776.
References
- [1].Accorsi-Mendonca D, Bonagamba LGH, Machado BH, ATP released by glia increases the excitatory neurotransmission onto NTS neurons related to the peripheral chemoreflex, Soc. Neurosci 824 (809) (2012). [Google Scholar]
- [2].Agulhon C, Boyt KM, Xie AX, Friocourt F, Roth BL, McCarthy KD, Modulation of the autonomic nervous system and behaviour by acute glial cell Gq protein-coupled receptor activation in vivo, J. Physiol. 591 (2013) 5599–5609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Agulhon C, Fiacco TA, McCarthy KD, Hippocampal short- and long-term plas- ticity are not modulated by astrocyte Ca2+ signaling, Science 327 (2010) 1250–1254. [DOI] [PubMed] [Google Scholar]
- [4].Andrew SF, Dinh TT, Ritter S, Localized glucoprivation of hindbrain sites elicits corticosterone and glucagon secretion, Am. J. Phys. Regul. Integr. Comp. Phys. 292 (2007) R1792–1798. [DOI] [PubMed] [Google Scholar]
- [5].Angulo MC, Kozlov AS, Charpak S, Audinat E, Glutamate released from glial cells synchronizes neuronal activity in the hippocampus, J. Neurosci. 24 (2004) 6920–6927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Araque A, Carmignoto G, Haydon PG, Dynamic signaling between astrocytes and neurons, Annu. Rev. Physiol. 63 (2001) 795–813. [DOI] [PubMed] [Google Scholar]
- [7].Araque A, Parpura V, Sanzgiri RP, Haydon PG, Tripartite synapses: glia, the unacknowledged partner, Trends Neurosci. 22 (1999) 208–215. [DOI] [PubMed] [Google Scholar]
- [8].Arnold S, Estrogen suppresses the impact of glucose deprivation on astrocytic calcium levels and signaling independently of the nuclear estrogen receptor, Neurobiol. Dis. 20 (2005) 82–92. [DOI] [PubMed] [Google Scholar]
- [9].Ashcroft FM, Adenosine 5′-triphosphate-sensitive potassium channels, Annu. Rev. Neurosci. 11 (1988) 97–118. [DOI] [PubMed] [Google Scholar]
- [10].Ballian N, Rabiee A, Andersen DK, Elahi D, Gibson BR, Glucose metabolism in burn patients: the role of insulin and other endocrine hormones, Burns 36 (2010) 599–605. [DOI] [PubMed] [Google Scholar]
- [11].Bernard C, De l’origine de sucre dans l’économie animale, Arch. Gén de Méd. 4e (1848) 303–319 http://www.claude-bernard.co.uk/page302.htm. [Google Scholar]
- [12].Bernard C, Leçons sur le diabète et la glycogenèse animale, (1877), p. 576 (Cours du Collège de France, rec: Mathias Duval.). Paris, Baillière: 576, http://www.claude-bernard.co.uk/page572.htm1877. [Google Scholar]
- [13].Bernard C Magendie annonce à l’Académie des Sciences que Bernard a achevé une augmentation de glucose dans le sang par une blessure d’un certain point du cerveau C. R. Hebd. Acad. Sci. t. 28: 393–394 http://www.claude-bernard.co.uk/page392.htm, 1849. [Google Scholar]
- [14].Bezzi P, Carmignoto G, Pasti L, Vesce S, Rossi D, Rizzini BL, Pozzan T, Volterra A, Prostaglandins stimulate calcium-dependent glutamate release in astrocytes,Nature 391 (1998) 281–285. [DOI] [PubMed] [Google Scholar]
- [15].Bonansco C, Couve A, Perea G, Ferradas CA, Roncagliolo M, Fuenzalida M, Glutamate released spontaneously from astrocytes sets the threshold for synaptic plasticity, Eur. J. Neurosci. 33 (2011) 1483–1492. [DOI] [PubMed] [Google Scholar]
- [16].Bosarge PL, Kerby JD, Stress-induced hyperglycemia: is it harmful following trauma? Adv. Surg. 47 (2013) 287–297. [DOI] [PubMed] [Google Scholar]
- [17].Brooks CM, A delimitation of the central nervous mechanism involved in reflex hyperglycemia, Am. J. Phys. 99 (1931) 64–76. [Google Scholar]
- [18].Bulatao E, Carlson A, Contributions to the physiology of the stomach: influence of experimental changes in blood sugar level on gastric hunger contractions, Am. J. Phys. 69 (1924) 107–115. [Google Scholar]
- [19].Burcelin R, Dolci W, Thorens B, Glucose sensing by the hepatoportal sensor is GLUT2-dependent: in vivo analysis in GLUT2-null mice, Diabetes 49 (2000) 1643–1648. [DOI] [PubMed] [Google Scholar]
- [20].Bushong EA, Martone ME, Jones YZ, Ellisman MH, Protoplasmic astrocytes in CA1 stratum radiatum occupy separate anatomical domains, J. Neurosci. 22 (2002) 183–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Cajal SRy, Histology of the Nervous System, Oxford University Press, New York, 1995. [Google Scholar]
- [22].Cannon WB, McIver MA, Bliss SW, Studies on the conditions of activity in endocrine glands, Am. J. Physiol. 69 (1924) 46–66. [Google Scholar]
- [23].Cannon WB, Washburn AL, An explanation of hunger, Am. J. Phys. 29 (1912) 441–454. [Google Scholar]
- [24].Chun S, Niijima A, Shima T, Okada M, Nagai K, Effect of infusion of vasoactive intestinal peptide (VIP)-antisense oligodeoxynucleotide into the third cerebral ventricle above the hypothalamic suprachiasmatic nucleus on the hyperglycemia caused by intracranial injection of 2-deoxy-D-glucose in rats, Neurosci. Lett. 257 (1998) 135–138. [DOI] [PubMed] [Google Scholar]
- [25].Cochran A, Scaife ER, Hansen KW, Downey EC, Hyperglycemia and outcomes from pediatric traumatic brain injury, J. Trauma 55 (2003) 1035–1038. [DOI] [PubMed] [Google Scholar]
- [26].Coco S, Calegari F, Pravettoni E, Pozzi D, Taverna E, Rosa P, Matteoli M, Verderio C, Storage and release of ATP from astrocytes in culture, J. Biol. Chem. 278 (2003) 1354–1362. [DOI] [PubMed] [Google Scholar]
- [27].Cryer PE, Glucose counterregulation: prevention and correction of hypoglycemia in humans, Am. J. Phys. 264 (1993) E149–155. [DOI] [PubMed] [Google Scholar]
- [28].Cryer PE, Davis SN, Shamoon H, Hypoglycemia in diabetes, Diabetes Care 26 (2003) 1902–1912. [DOI] [PubMed] [Google Scholar]
- [29].DiRocco RJ, Grill HJ, The forebrain is not essential for sympathoadrenal hyperglycemic response to glucoprivation, Science 204 (1979) 1112–1114. [DOI] [PubMed] [Google Scholar]
- [30].Donovan CM, Watts AG, Peripheral and central glucose sensing in hypoglycemic detection, Physiology (Bethesda) 29 (2014) 314–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Erlichman JS, Leiter JC, Gourine AV, ATP glia and central respiratory control, Respir. Physiol. Neurobiol 173 (2010) 305–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Fellin T, Carmignoto G, Neurone-to-astrocyte signalling in the brain represents a distinct multifunctional unit, J. Physiol. 559 (2004) 3–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Fellin T, Sul JY, D’Ascenzo M, Takano H, Pascual O, Haydon PG, Bidirectional astrocyte-neuron communication: the many roles of glutamate and ATP, Novartis Found. Symp. 276 (2006) 208–217 (discussion 217–221, 233–237, 275–281). [DOI] [PubMed] [Google Scholar]
- [34].Fioramonti X, Marsollier N, Song Z, Fakira KA, Patel RM, Brown S, Duparc T, Pica-Mendez A, Sanders NM, Knauf C, Valet P, McCrimmon RJ, Beuve A, Magnan C, Routh VH, Ventromedial hypothalamic nitric oxide production is necessary for hypoglycemia detection and counterregulation, Diabetes 59 (2010) 519–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Funk GD, The ‘connexin’ between astrocytes, ATP and central respiratory che- moreception, J. Physiol. 588 (2010) 4335–4337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Garrick T, Mulvihill S, Buack S, Maeda-Hagiwara M, Tache Y, Intracerebroventricular pressure inhibits gastric antral and duodenal contractility but not acid secretion in conscious rabbits, Gastroenterology 95 (1988) 26–31. [DOI] [PubMed] [Google Scholar]
- [37].Gourine AV, Kasparov S, Astrocytes as brain interoceptors, Exp. Physiol. 96 (2011) 411–416. [DOI] [PubMed] [Google Scholar]
- [38].Gourine AV, Kasymov V, Marina N, Tang F, Figueiredo MF, Lane S, Teschemacher AG, Spyer KM, Deisseroth K, Kasparov S, Astrocytes control breathing through pH-dependent release of ATP, Science 329 (2010) 571–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Halassa MM, Fellin T, Takano H, Dong JH, Haydon PG, Synaptic islands defined by the territory of a single astrocyte, J. Neurosci. 27 (2007) 6473–6477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Halassa MM, Haydon PG, Integrated brain circuits: astrocytic networks modulate neuronal activity and behavior, Annu. Rev. Physiol. 72 (2010) 335–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Hartl WH, Jauch KW, Metabolic self-destruction in critically ill patients: origins, mechanisms and therapeutic principles, Nutrition 30 (2014) 261–267. [DOI] [PubMed] [Google Scholar]
- [42].Hassel B, Paulsen RE, Johnsen A, Fonnum F, Selective inhibition of glial cell metabolism in vivo by fluorocitrate, Brain Res. 576 (1992) 120–124. [DOI] [PubMed] [Google Scholar]
- [43].Haydon PG, Carmignoto G, Astrocyte control of synaptic transmission and neurovascular coupling, Physiol. Rev. 86 (2006) 1009–1031. [DOI] [PubMed] [Google Scholar]
- [44].Hermann GE, Nasse JS, Rogers RC, Alpha-1 adrenergic input to solitary nucleus neurones: calcium oscillations, excitation and gastric reflex control, J. Physiol. 562 (2005) 553–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Hermann GE, Rogers RC, Thrombin action on NST astrocytes disrupts glycemic and respiratory control, FASEB J. 32 (2018) 731–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Hermann GE, Van Meter MJ, Rood JC, Rogers RC, Proteinase-activated receptors in the nucleus of the solitary tract: evidence for glial-neural interactions in autonomic control of the stomach, J. Neurosci. 29 (2009) 9292–9300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Hermann GE, Viard E, Rogers RC, Hindbrain glucoprivation effects on gastric vagal reflex circuits and gastric motility in the rat are suppressed by the astrocyte inhibitor fluorocitrate, J. Neurosci. 34 (2014) 10488–10496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Holsbeeks I, Lagatie O, Van Nuland A, Van de Velde S, Thevelein JM, The eukaryotic plasma membrane as a nutrient-sensing device, Trends Biochem. Sci. 29 (2004) 556–564. [DOI] [PubMed] [Google Scholar]
- [49].Hudson B, Ritter S, Hindbrain catecholamine neurons mediate consummatory responses to glucoprivation, Physiol. Behav. 82 (2004) 241–250. [DOI] [PubMed] [Google Scholar]
- [50].Islam MS, Calcium signaling in the islets, Adv. Exp. Med. Biol. 654 (2010) 235–259. [DOI] [PubMed] [Google Scholar]
- [51].Itzekson Z, Maggio N, Milman A, Shavit E, Pick CG, Chapman J, Reversal of trauma-induced amnesia in mice by a thrombin receptor antagonist, J. Mol. Neurosci. 53 (2014) 87–95. [DOI] [PubMed] [Google Scholar]
- [52].Jokiaho AJ, Donovan CM, Watts AG, The rate of fall of blood glucose determines the necessity of forebrain-projecting catecholaminergic neurons for male rat sympathoadrenal responses, Diabetes 63 (2014) 2854–2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Junge CE, Lee CJ, Hubbard KB, Zhang Z, Olson JJ, Hepler JR, Brat DJ, Traynelis SF, Protease-activated receptor-1 in human brain: localization and functional expression in astrocytes, Exp. Neurol. 188 (2004) 94–103. [DOI] [PubMed] [Google Scholar]
- [54].Kahlert S, Reiser G, Requirement of glycolytic and mitochondrial energy supply for loading of Ca(2+) stores and InsP(3)-mediated Ca(2+) signaling in rat hippocampus astrocytes, J. Neurosci. Res. 61 (2000) 409–420. [DOI] [PubMed] [Google Scholar]
- [55].Kinnamon SC, Taste receptor signaling- from tongues to lungs, Acta Physiol (Oxford) 204 (2012) 158–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Klip A, Hawkins M, Desperately seeking sugar: glial cells as hypoglycemia sensors, J. Clin. Invest.115 (2005) 3403–3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Kohno D, Koike M, Ninomiya Y, Kojima I, Kitamura T, Yada T, Sweet taste receptor serves to activate glucose- and leptin-responsive neurons in the hypothalamic arcuate nucleus and participates in glucose responsiveness, Front. Neurosci 10 (2016) 502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Larson GM, Koch S, O’Dorisio TM, Osadchey B, McGraw P, Richardson JD, Gastric response to severe head injury, Am. J. Surg. 147 (1984) 97–105. [DOI] [PubMed] [Google Scholar]
- [59].Levin BE, Routh VH, Kang L, Sanders NM, Dunn-Meynell AA, Neuronal glucosensing: what do we know after 50 years? Diabetes 53 (2004) 2521–2528. [DOI] [PubMed] [Google Scholar]
- [60].Li AJ, Wang Q, Dinh TT, Powers BR, Ritter S, Stimulation of feeding by three different glucose-sensing mechanisms requires hindbrain catecholamine neurons, Am. J. Phys. Regul. Integr. Comp. Phys. 306 (2014) R257–R264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Li AJ, Wang Q, Dinh TT, Ritter S, Simultaneous silencing of Npy and Dbh expression in hindbrain A1/C1 catecholamine cells suppresses glucoprivic feeding, J. Neurosci. 29 (2009) 280–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Li AJ, Wang Q, Dinh TT, Wiater MF, Eskelsen AK, Ritter S, Hindbrain ca- techolamine neurons control rapid switching of metabolic substrate use during glucoprivation in male rats, Endocrinology 154 (2013) 4570–4579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Lin LH, Moore SA, Jones SY, McGlashon J, Talman WT, Astrocytes in the rat nucleus tractus solitarii are critical for cardiovascular reflex control, J. Neurosci. 33 (2013) 18608–18617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Martineau M, Shi T, Puyal J, Knolhoff AM, Dulong J, Gasnier B, Klingauf J, Sweedler JV, Jahn R, Mothet JP, Storage and uptake of D-serine into astrocytic synaptic-like vesicles specify gliotransmission, J. Neurosci. 33 (2013) 3413–3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Marty N, Dallaporta M, Foretz M, Emery M, Tarussio D, Bady I, Binnert C, Beermann F, Thorens B, Regulation of glucagon secretion by glucose transporter type 2 (glut2) and astrocyte-dependent glucose sensors, J. Clin. Invest.115 (2005) 3545–3553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Marty N, Dallaporta M, Thorens B, Brain glucose sensing, counterregulation, and energy homeostasis, Physiology (Bethesda) 22 (2007) 241–251. [DOI] [PubMed] [Google Scholar]
- [67].Mazzarello P, Golgi: Biography of the Founder of Modern Neuroscience, Oxford University Press, New York, 2010. [Google Scholar]
- [68].McCudden CR, Hains MD, Kimple RJ, Siderovski DP, Willard FS, G-protein signaling: back to the future. Cellular and molecular life sciences, CMLS 62 (2005) 551–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].McDougal DH, Hermann GE, Rogers RC, Astrocytes in the nucleus of the solitary tract are activated by low glucose or glucoprivation: evidence for glial involvement in glucose homeostasis, Front. Neurosci 7 (2013) 249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].McDougal DH, Hermann GE, Rogers RC, Vagal afferent stimulation activates astrocytes in the nucleus of the solitary tract via AMPA receptors: evidence of an atypical neural-glial interaction in the brainstem, J. Neurosci. 31 (2011) 14037–14045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Meghji P, Tuttle JB, Rubio R, Adenosine formation and release by embryonic chick neurons and glia in cell culture, J. Neurochem. 53 (1989) 1852–1860. [DOI] [PubMed] [Google Scholar]
- [72].Mimee A, Ferguson AV, Glycemic state regulates melanocortin, but not nesfatin-1, responsiveness of glucose-sensing neurons in the nucleus of the solitary tract, Am. J. Phys. Regul. Integr. Comp. Phys. 308 (2015) R690–R699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Nett WJ, Oloff SH, McCarthy KD, Hippocampal astrocytes in situ exhibit calcium oscillations that occur independent of neuronal activity, J. Neurophysiol. 87 (2002) 528–537. [DOI] [PubMed] [Google Scholar]
- [74].Niijima A, The effect of D-glucose on the firing rate of glucose-sensitive vagal afferents in the liver in comparison with the effect of 2-deoxy-D-glucose, J. Auton. Nerv. Syst 10 (1984) 255–260. [DOI] [PubMed] [Google Scholar]
- [75].Parpura V, Haydon PG, Physiological astrocytic calcium levels stimulate glutamate release to modulate adjacent neurons, Proc. Natl. Acad. Sci. U. S. A. 97 (2000) 8629–8634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Pasti L, Volterra A, Pozzan T, Carmignoto G, Intracellular calcium oscillations in astrocytes: a highly plastic, bidirectional form of communication between neurons and astrocytes in situ, J. Neurosci. 17 (1997) 7817–7830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Perea G, Navarrete M, Araque A, Tripartite synapses: astrocytes process and control synaptic information, Trends Neurosci. 32 (2009) 421–431. [DOI] [PubMed] [Google Scholar]
- [78].Porter JT, McCarthy KD, Hippocampal astrocytes in situ respond to glutamate released from synaptic terminals, J. Neurosci. 16 (1996) 5073–5081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Ren X, Zhou L, Terwilliger R, Newton SS, de Araujo IE, Sweet taste signaling functions as a hypothalamic glucose sensor, Front. Integr. Neurosci. 3 (2009) 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Rinaman L, Hindbrain noradrenergic A2 neurons: diverse roles in autonomic, endocrine, cognitive, and behavioral functions, Am. J. Phys. Regul. Integr. Comp. Phys 300 (2011) R222–R235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Ritter S, Dinh TT, Li AJ, Hindbrain catecholamine neurons control multiple glucoregulatory responses, Physiol. Behav. 89 (2006) 490–500. [DOI] [PubMed] [Google Scholar]
- [82].Ritter S, Li AJ, Wang Q, Dinh TT, Minireview: the value of looking backward: the essential role of the hindbrain in counterregulatory responses to glucose deficit, Endocrinology 152 (2011) 4019–4032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Ritter S, Llewellyn-Smith I, Dinh TT, Subgroups of hindbrain catecholamine neurons are selectively activated by 2-deoxy-D-glucose induced metabolic challenge, Brain Res. 805 (1998) 41–54. [DOI] [PubMed] [Google Scholar]
- [84].Rogers RC, Hermann GE, Brainstem control of gastric function, in: Johnson LR (Ed.), Physiology of the Gastrointestinal Tract, fourth ed., Elsevier Academic Press, 2012, pp. 861–892. [Google Scholar]
- [85].Rogers RC, McDougal DH, Hermann GE, Hindbrain astrocyte glucodetectors and counterregulation, in: Harris RBS (Ed.), Appetite and Food Intake: Central Control, CRC Press/Taylor & Francis Group, LLC, Boca Raton (FL), 2017, pp. 205–228. [PubMed] [Google Scholar]
- [86].Rogers RC, McDougal DH, Ritter S, Qualls-Creekmore E, Hermann GE, Response of catecholaminergic neurons in the mouse hindbrain to glucoprivic stimuli is astrocyte dependent, Am. J. Phys. Regul. Integr. Comp. Phys. 315 (2018) R153–R164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Rogers RC, Ritter S, Hermann GE, Hindbrain cytoglucopenia-induced increases in systemic blood glucose levels by 2-deoxyglucose depend on intact astrocytes and adenosine release, Am. J. Phys. Regul. Integr. Comp. Phys. 310 (2016) R1102–R1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Rogers RC, Travagli RA, Hermann GE, Noradrenergic neurons in the rat solitary nucleus participate in the esophageal-gastric relaxation reflex, Am. J. Phys. Regul. Integr. Comp. Phys. 285 (2003) R479–R489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Satin LS, Localized calcium influx in pancreatic beta-cells: its significance for Ca2+−dependent insulin secretion from the islets of Langerhans, Endocrine 13 (2000) 251–262. [DOI] [PubMed] [Google Scholar]
- [90].Schuit FC, Huypens P, Heimberg H, Pipeleers DG, Glucose sensing in pancreatic beta-cells: a model for the study of other glucose-regulated cells in gut, pancreas, and hypothalamus, Diabetes 50 (2001) 1–11. [DOI] [PubMed] [Google Scholar]
- [91].Scislo TJ, O’Leary DS, Purinergic mechanisms of the nucleus of the solitary tract and neural cardiovascular control, Neurol. Res. 27 (2005) 182–194. [DOI] [PubMed] [Google Scholar]
- [92].Smith K, Neuroscience: settling the great glia debate, Nature 468 (2010) 160–162. [DOI] [PubMed] [Google Scholar]
- [93].Stolarczyk E, Guissard C, Michau A, Even PC, Grosfeld A, Serradas P, Lorsignol A, Penicaud L, Brot-Laroche E, Leturque A, Le Gall M, Detection of extracellular glucose by GLUT2 contributes to hypothalamic control of food in- take, Am. J. Physiol. Endocrinol. Metab 298 (2010) E1078–E1087. [DOI] [PubMed] [Google Scholar]
- [94].Stolarczyk E, Le Gall M, Even P, Houllier A, Serradas P, Brot-Laroche E, Leturque A, Loss of sugar detection by GLUT2 affects glucose homeostasis in mice, PLoS One 2 (2007) e1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Striggow F, Riek-Burchardt M, Kiesel A, Schmidt W, Henrich-Noack P, Breder J, Krug M, Reymann KG, Reiser G, Four different types of protease-activated receptors are widely expressed in the brain and are up-regulated in hippocampus by severe ischemia, Eur. J. Neurosci. 14 (2001) 595–608. [DOI] [PubMed] [Google Scholar]
- [96].Tisi R, Baldassa S, Belotti F, Martegani E, Phospholipase C is required for glucose-induced calcium influx in budding yeast, FEBS Lett. 520 (2002) 133–138. [DOI] [PubMed] [Google Scholar]
- [97].Traynelis SF, Trejo J, Protease-activated receptor signaling: new roles and regulatory mechanisms, Curr. Opin. Hematol 14 (2007) 230–235. [DOI] [PubMed] [Google Scholar]
- [98].Turner CP, Blackburn MR, Rivkees SA, A1 adenosine receptors mediate hypoglycemia-induced neuronal injury, J. Mol. Endocrinol. 32 (2004) 129–144. [DOI] [PubMed] [Google Scholar]
- [99].Vance KM, Rogers RC, Hermann GE, PAR1-activated astrocytes in the nucleus of the solitary tract stimulate adjacent neurons via NMDA receptors, J. Neurosci. 35 (2015) 776–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Verkhratsky A, Orkand RK, Kettenmann H, Glial calcium: homeostasis and signaling function, Physiol. Rev. 78 (1998) 99–141. [DOI] [PubMed] [Google Scholar]
- [101].Volterra A, Meldolesi J, Astrocytes, from brain glue to communication elements: the revolution continues, Nat. Rev. Neurosci. 6 (2005) 626–640. [DOI] [PubMed] [Google Scholar]
- [102].Watts AG, Donovan CM, Sweet talk in the brain: glucosensing, neural networks, and hypoglycemic counterregulation, Front. Neuroendocrinol. 31 (2010) 32–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Weinstein JR, Gold SJ, Cunningham DD, Gall CM, Cellular localization of thrombin receptor mRNA in rat brain: expression by mesencephalic dopaminergic neurons and codistribution with prothrombin mRNA, J. Neurosci. 15 (1995) 2906–2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Welcome MO, Mastorakis NE, Emerging concepts in brain glucose metabolic functions: from glucose sensing to how the sweet taste of glucose regulates its own metabolism in astrocytes and neurons, NeuroMolecular Med. 20 (2018) 281–300. [DOI] [PubMed] [Google Scholar]
- [105].Yamamoto H, Nagai K, Nakagawa H, Time-dependent involvement of autonomic nervous system in hyperglycemia due to 2-deoxy-D-glucose, Am. J. Phys. 255 (1988) E928–933. [DOI] [PubMed] [Google Scholar]
- [106].Young JK, Baker JH, Montes MI, The brain response to 2-deoxy glucose is blocked by a glial drug, Pharmacol. Biochem. Behav. 67 (2000) 233–239. [DOI] [PubMed] [Google Scholar]
- [107].Zorec R, Araque A, Carmignoto G, Haydon PG, Verkhratsky A, Parpura V, Astroglial excitability and gliotransmission: an appraisal of Ca2+ as a signalling route, ASN Neuro 4 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]