ABSTRACT
Posttranslational modifications (PTMs) of histone proteins are important for various cellular processes including regulation of gene expression and chromatin structure, DNA damage response and chromosome segregation. Here we comprehensively review mitotic histone PTMs, in particular phosphorylations, and discuss their interplay and functions in the control of dynamic protein–protein interactions as well as their contribution to centromere and chromosome structure and function during cell division. Histone phosphorylations can create binding sites for mitotic regulators such as the chromosomal passenger complex, which is required for correction of erroneous spindle attachments and chromosome bi-orientation. Other histone PTMs can alter the structural properties of nucleosomes and the accessibility of chromatin. Epigenetic marks such as lysine methylations are maintained during mitosis and may also be important for mitotic transcription as well as bookmarking of transcriptional states to ensure the transmission of gene expression programs through cell division. Additionally, histone phosphorylation can dissociate readers of methylated histones without losing epigenetic information. Through all of these processes, mitotic histone PTMs play a functional role in priming the chromatin for faithful chromosome segregation and preventing genetic instability, one of the characteristic hallmarks of cancer cells.
KEYWORDS: Mitosis, histone modifications, phosphorylation, chromosome condensation, chromosome segregation, chromosomal stability
Introduction
Histone proteins are subject to various posttranslational modifications (PTMs) including phosphorylation, acetylation, methylation and ubiquitination [1]. Many histone PTMs contribute to the organization of higher-order chromatin structures to regulate gene expression and maintain different transcriptional states during the cell cycle. While some PTMs such as H3 lysine 4 di- and tri-methylation (H3K4me2/3) are largely confined to accessible euchromatin and actively transcribed regions, others including H3K27me3 characterize silenced heterochromatin. As histones can carry several different PTMs at the same time, a combinatorial effect or “histone code” was proposed that offers an additional level of information beyond the linear sequence of DNA [2].
Although mitosis-specific alterations are seen for most of the different types of PTMs, phosphorylation seems to be the most highly abundant and dynamic modification [3]. Increased phosphorylation is found not only on many spindle- or chromatin-associated proteins, but also on histones and histone variants. Phosphorylations occurring either alone or in dense clusters can have a great impact on many functions of the modified protein. Phosphorylation as such can affect the structure of the modified protein, due to the bulky nature of the phosphate group and its negative charge. In addition, phosphorylated residues can facilitate or prevent binding of other proteins, thus allowing a temporal and dynamic control of protein–protein interactions during mitosis. Other phosphorylation sites have a priming function by enabling the subsequent modification of further residues by other PTMs. In addition, it should be mentioned that not all phosphorylations must necessarily be functionally relevant. Interestingly, the majority of phosphorylation sites are not under stronger evolutionary constraints when compared to random residues. It may be that individual phosphorylation sites within functional multi-site clusters are under weak constraints that are difficult to detect. However, this observation has led to the suggestion that a substantial fraction of phosphorylations is nonfunctional and rather due to random encounters between protein kinases and degenerate recognition motifs in the crowded chromatin microenvironment [4]. Therefore, this review will not cover phosphorylations that are only recorded in databases and rather focus on PTMs that are characterized by scientific publications (as summarized in Table 1 and Figure 1).
Table 1.
List of mitotic histone PTMs (identified in humans) as well as their writers, erasers and proposed functions during mitosis.
| PTM site | Writer | Eraser | Cell cycle-dependent regulation & function |
|---|---|---|---|
| Histone H2A | |||
| H2AS1ph | unknown | Increased in S and M phase; required for histone deposition in S phase & chromatin condensation in M phase? [36] | |
| H2AT120ph | Bub1 | Increased at mitotic centromeres; required for recruitment of Sgo1 and Aurora B [35,63] | |
| Histone H2B | |||
| H2BS6ph | CDK1-cyclin B1 | PP1 | Increased at inner centromeres during mitosis; SET displacement; important for chromosome segregation [37] |
| Histone H3 | |||
| H3T3ph | Haspin | PP1γ-Repo-Man | Increased during mitosis; required for Aurora B recruitment; allows displacement of PHD finger proteins [27,30,104,168] |
| H3K4me2/3 | SET1, MLL1 | LSD1, JARID1A | Maintained during mitosis; required for centromeric transcription & bookmarking of active genes [5,57] |
| H3T6ph | unknown | Increased during mitosis; allows displacement of PHD finger proteins [30] | |
| H3K9me2/3 | SUV39H1/2 | JMJD2A | Increased at pericentromeres; required for heterochromatin formation & HP1 recruitment [107–109] |
| H3S10ph | Aurora B | PP1γ | Increased during mitosis; HP1 displacement [22,112,113] |
| H3T11ph | unknown | PP1γ | Increased during mitosis at pericentromeric heterochromatin [31] |
| H3K27me3 | EZH2 | UTX, JMJD3 | Maintained during mitosis; heterochromatin bookmarking [5] |
| H3S28ph | Aurora B | PP1γ | Increased during mitosis; displacement of Polycomb group proteins? [25,130] |
| H3K36me2/3 | NSD2, SETD2 | JMJD2A | Maintained during mitosis; required for centromeric transcription & bookmarking of active genes [5,57] |
| H3K79me2 | DOT1L | KDM2B | Increased during mitosis; important for chromosomal stability [45] |
| H3T80ph | unknown | Increased during mitosis; important for chromosomal stability [28] | |
| H3T118ph | Aurora A | Increased during mitosis; decreased interaction between DNA and histone core; regulation of condensin I and cohesin binding to chromatin [29] | |
| Histone H4 | |||
| H4S1ph | unknown | Increased in S and M phase; required for histone deposition in S phase & chromatin condensation in M phase? [36] | |
| H4K20me1 | PR-Set7 | PHF8 | Increased during mitosis; required for condensin II binding & CENP-A deposition [46,48,84] |
| Histone variants | |||
| H2A.XS121ph | Aurora B | Increased at mitotic centromeres; Aurora B auto-activation at inner centromeres [40] | |
| H2A.XS139ph (γH2A.X) | ATM, CHK2, DNA-PK | PP2A, PP4 | Detected during mitosis; marks unresolved DNA damage? [42–44] |
| H3.3S31ph | Aurora B | Increased during mitosis; p53 activation in response to chromosome missegregation [39,71,72] | |
| CENP-AG1me3 | NRMT1 | Recruitment of CENP-I and CENP-T [92] | |
| CENP-AS7ph | Aurora A, Aurora B | PP1γ? | Increased during mitosis; Aurora B activation; cohesin protection [41,65,66] |
| CENP-AS16ph | unknown | Important for proper chromosome segregation (together with S18) [68] | |
| CENP-AS18ph | CDK2-cyclin E | Regulation of CENP-A deposition [68,93] | |
| CENP-AS68ph | CDK1-cyclin B1 | PP1α | Inhibition of HJURP-mediated CENP-A deposition during mitosis [88] |
| CENP-AK124ub | CUL4A-RBX1-COPS8 | Mediates interaction with HJURP and deposition at centromeres [96] | |
| Linker histones | |||
| H1S/Tph (multiple sites) | CDKs | Increasing phosphorylation during cell cycle progression; involved in transcription, replication, chromatin compaction [38,161] | |
| H1.4T17ph/H1.5S17ph | CDKs | Increased in mitosis; increased H1 mobility & eviction from chromatin by SET [74,161] | |
| H1.4S27ph | Aurora B | Increased in mitosis; precludes HP1 binding; decreased H1 mobility [114,115] | |
| H1.4S35ph | PKA | Increased in mitosis; mediates H1.4 dissociation from chromatin [163] | |
| H1.5T10ph | GSK-3 | Increased in mitosis; increased H1.5 binding to chromatin [164] |
Figure 1.
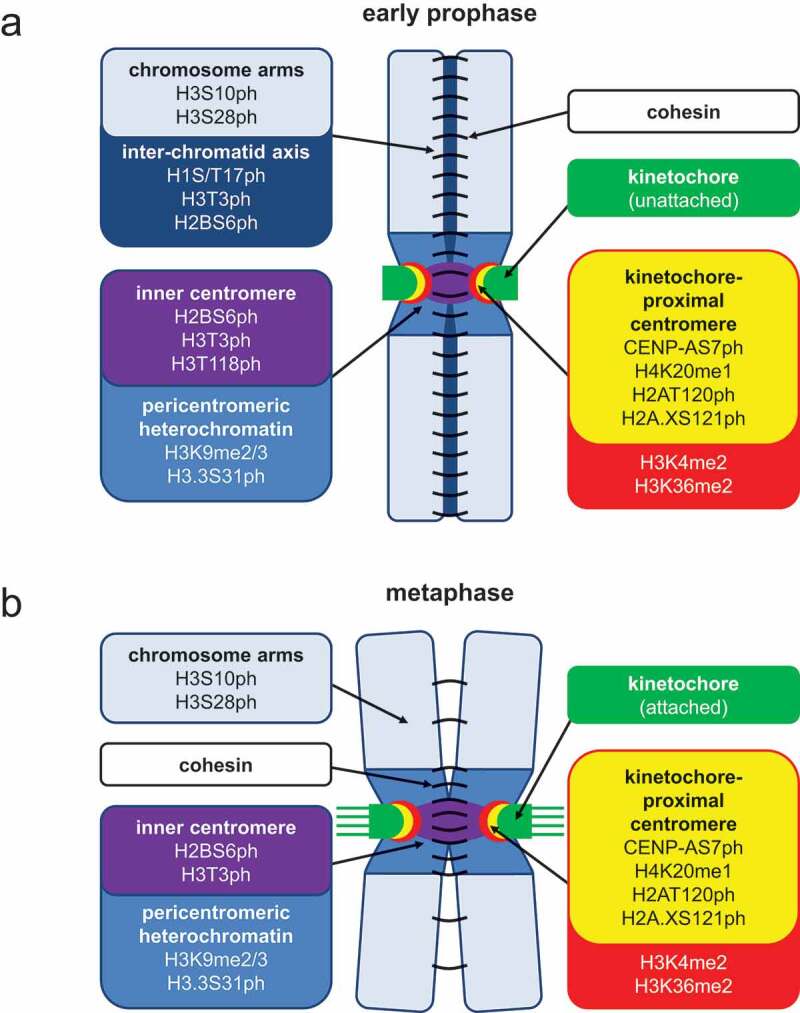
Localization of mitotic histone PTMs on chromosomes from early prophase (a) and metaphase (b). At the beginning of prophase, sister chromatids are connected by cohesin (black semicircles) and kinetochores (green) are assembled on centromeric chromatin. During prophase, cohesin is released from chromosome arms but retained at centromeres. Chromosome condensation further increases during progression to metaphase. Metaphase is reached when all sister kinetochores are attached by microtubules (green lines) and tension is created at centromeres. The localization of histone PTMs is indicated by arrows and colors (chromosome arms are shown in light blue, inter-chromatid axis in dark blue, pericentromeric heterochromatin in deep blue, inner centromeres in purple, and centromeric chromatin in red or yellow for H3 or CENP-A-containing nucleosomes, respectively).
Phosphorylation as a predominant PTM during mitosis
During proliferation, cells have to copy and share their chromosomes to transmit their genetic information to their daughter cells. To this end, identical copies of DNA are produced by semi-conservative replication in S phase. Previous studies have shown that epigenetic marks such as histone methylation are transmitted to the replicated chromatin in order to maintain epigenetic states and reestablish transcription after cell division [5,6]. When cells enter mitosis, topological domains have to be resolved and higher-order structures further condense in order to allow chromosomes to compact and finally separate and segregate to the daughter cells [7]. This process not only requires the removal of cohesive elements from the chromatin, but also the stepwise association of condensin complexes at the beginning (condensin II) and end of prophase (condensin I) [8].
After DNA replication in S phase, sister chromatids remain connected by the presence of cohesin. Cohesin is a multi-protein complex that holds the sister chromatids together by forming a ring-like structure which entraps DNA from different strands [9]. During mitotic prophase in vertebrates, cohesin is largely removed from chromosome arms in a phosphorylation-dependent manner (so-called prophase pathway) and concentrates at centromeres in prometaphase [10,11]. Centromeric cohesion is retained by Shugoshin 1 (Sgo1) which inhibits the binding of the release factor Wapl to cohesin [12] and recruits protein phosphatase 2A (PP2A) to prevent phosphorylation-mediated release of cohesin [13,14]. Correct attachment of spindle microtubules to the kinetochores of sister chromatids is a trial-and-error process that involves both an error correction mechanism (involving the kinase Aurora B) and a surveillance mechanism called the spindle assembly checkpoint (SAC). In concert with error correction, the SAC monitors mitotic progression and maintains centromeric cohesion until all kinetochores are attached to microtubules from opposing poles of the mitotic spindle [15]. SAC signaling inhibits the activity of the anaphase promoting complex/cyclosomeCDC20 (APC/CCDC20), an E3 ubiquitin ligase complex that targets several mitotic regulators such as cyclin B1 or Securin for proteasomal degradation [16]. Once the SAC is satisfied and the APC/CCDC20 is active, proteolytic cleavage of centromeric cohesin subunit RAD21/Scc1 by Separase is followed by the segregation of chromosomes to the spindle poles [17,18]. This process ensures that the daughter cells share an identical set of chromosomes after cell division. Defects in chromosome segregation can lead to structural damage of the chromosomes or acquisition of aberrant copy numbers [19,20]. Chromosomally unstable cancer cells can tolerate these defects to a certain extent and thus gain, lose or re-arrange chromosomes during cell division. The resulting aneuploidy can further facilitate tumorigenesis and genetic instability is therefore considered as one of the hallmarks of cancer [21].
Mitosis-specific modifications of histones and histone variants
Probably the most extensively studied mitotic histone modification is phosphorylation of H3 at serine 10 (H3S10ph) which is conserved from yeast to humans and catalyzed by Aurora B [22,23]. H3S10ph can be detected at pericentromeres in late G2 phase and is found all over chromosomes in prophase [24]. Aurora B also phosphorylates H3S28 in mitosis, which is found within a similar A-R-K-S motif [25]. Although H3S10 and H3S28 were also shown to be phosphorylated during gene activation in interphase, the responsible kinases and functional consequences are distinct from mitotic phosphorylation [26]. However, other sites of H3 are also subject to mitotic phosphorylation. H3 threonine 3 (H3T3) is phosphorylated by the atypical kinase Haspin [27], and other mitosis-specific phosphorylations were also reported for H3T118 by Aurora A and H3T80 by an unknown kinase [28,29]. Moreover, phosphorylation of H3T6 and T11 is increased on mitotic chromatin [30,31] but, in contrast to their regulation and function during interphase, the roles of these phosphorylations in mitosis are not understood [32–34].
Mitotic phosphorylation is not limited to H3 and was also reported for all canonical histones as well as several histone variants and linker histones. H2AT120ph generated by the kinetochore-associated kinase Bub1 is important for centromeric cohesion by directly recruiting Sgo1 [35]. Phosphorylation of H2AS1 and H4S1 is increased during mitosis but the kinase and functional consequences of this modification are not known so far [36]. We recently identified H2BS6ph by cyclin-dependent kinase 1 (CDK1) as a histone modification which is required for faithful chromosome segregation [37]. Of note, although CDKs are well characterized as linker histone kinases, phosphorylation of canonical histones was not reported before. This might be due to the lack of the S/T-P-x-K consensus motif which is often used to identify CDK substrates. However, CDK-mediated phosphorylation of the N-terminal domain of H1 can also occur in the absence of a CDK consensus sequence [38], potentially extending the spectrum of CDK substrates. Furthermore, mitotic phosphorylation is also reported for histone variants CENP-A, H3.3 and H2A.X [39–41]. Phosphorylation of H2A.XS139, also known as γH2A.X, is detectable during mitosis, indicating the presence of unresolved DNA damage or ongoing repair processes [42–44].
In contrast to the mitosis-specific increase in phosphorylation, fewer changes are observed for other histone PTMs. Although histone methylation is more stable over the cell cycle and less affected by mitosis [5], some methylation marks such as H3K9me2/3, H3K79me2 and H4K20me1 increase during mitosis and are important for chromosomal stability [45–48]. Acetylation, which is typically associated with active transcription, is globally decreased during mitosis but retained at some promoter regions for rapid transcriptional re-activation at mitotic exit [5,49]. This is in line with the observation that transcription is generally decreased during mitosis with only a few exceptions such as centromeres [50,51]. Similar to acetylation, mono-ubiquitination, which was reported for H2AK119 and H2BK120 in interphase, was found to be reduced during mitosis [52,53].
Modifications of centromeric and pericentromeric histones
Centromeres play a crucial role during mitosis as they are hubs of kinetochore formation and sister chromatid cohesion [54]. Centromeres consist of a core domain surrounded by pericentromeric heterochromatin and are characterized by the enrichment of specific histone variants and their modifications (see Figure 1). H2A.Z and the H3 variants CENP-A and H3.3 are incorporated in centromeric chromatin in a replication-independent manner [55,56]. The centromeric core domain contains alternating clusters of nucleosomes containing the active H3K4me2 and H3K36me2 modifications or the H3 variant CENP-A [57,58]. The adjacent pericentromeric heterochromatin is, in contrast, enriched with repressive H3K9me2/3 marks [47,59]. CENP-A specifies the location of the constitutive centromere-associated network (CCAN), a complex of proteins assembled on the centromere core domain that provides the foundation for kinetochore formation. In mitosis, outer kinetochore proteins including those that bind microtubules and checkpoint proteins are recruited to this foundation [54]. Among these proteins is the histone kinase Bub1, which phosphorylates H2AT120 in the centromeric chromatin that immediately underlies the kinetochores during mitosis [35]. Recently, it was shown that acetylation of H2AK118 prevents T120ph on chromosome arms and that recruitment of histone deacetylase 1 (HDAC1) to centromeres by the chromatin remodeler RSF1 contributes to centromere-specific phosphorylation of H2AT120 [60].
The inner centromere lies between the CENP-A-containing regions of condensed sister chromatids in mitosis and is thought to be formed primarily from pericentromeric heterochromatin [58]. Several histone PTMs are enriched at centromeres and pericentromeric heterochromatin, and may be important for chromosome alignment at the metaphase plate and therefore chromosome segregation in anaphase. During prometaphase, H3T3ph becomes concentrated at inner centromeres by a positive feedback loop between Aurora B and the H3T3 kinases and phosphatases (see Figure 2) [61,62]. Both H2AT120ph and H3T3ph can recruit the chromosomal passenger complex (CPC) to centromeres [63], but whether they co-operate or recruit distinct CPC populations is yet to be fully determined. The CPC is a ternary complex consisting of Aurora B and its chromatin targeting factors inner centromere protein (INCENP), Survivin and Borealin [64]. Aurora B activity is required for the correction of erroneous kinetochore-microtubule attachments and maintaining SAC signaling to prevent chromosome missegregation [64]. In addition, as well as phosphorylating H3S10 and H3S28 (see above), Aurora B phosphorylates H2A.XS121 at mitotic centromeres, which in turn contributes to Aurora B auto-activation and Haspin recruitment [40]. After its activation at centromeres, Aurora B phosphorylates human CENP-AS7 [41]. Although it is predominately localized to mitotic spindle poles, Aurora A was also reported to produce CENP-AS7ph in early prophase. This may enhance Aurora B activity at kinetochores, and also protect centromeres against cohesion fatigue [65,66]. Furthermore, it has been reported that binding of 14-3-3 proteins to CENP-AS7ph contributes to CENP-C recruitment and kinetochore formation [67], although CENP-C primarily binds to centromeric chromatin by direct interaction with the C-terminal domain of CENP-A [54]. However, the importance of CENP-AS7ph and its different roles in centromere function are still under debate. Mouse CENP-A lacks an equivalent phosphorylatable residue, CENP-AS7ph occurs at low abundances that miss detection by mass spectrometry [68], and rescue experiments with CENP-AS7A mutants (after targeting the endogenous CENP-A locus) could not show long-term effects on cell viability [69]. In addition to catalyzing CENP-AS7ph, Aurora A has been reported to phosphorylate H3T118 at inner centromeres in prophase, and this modification is lost on aligned chromosomes in metaphase. H3T118ph alters the structure of nucleosomes by weakening the interaction between DNA and the histone core, which may limit the occupancy of condensin I and cohesin complexes. Consistent with this, overexpression of Aurora A or H3T118 phosphorylation-mimicking mutants results in loss of sister chromatid cohesion and chromosome missegregation [29], although it is unclear how this finding fits with the idea that Aurora A protects cohesion through CENP-AS7ph [65].
Figure 2.
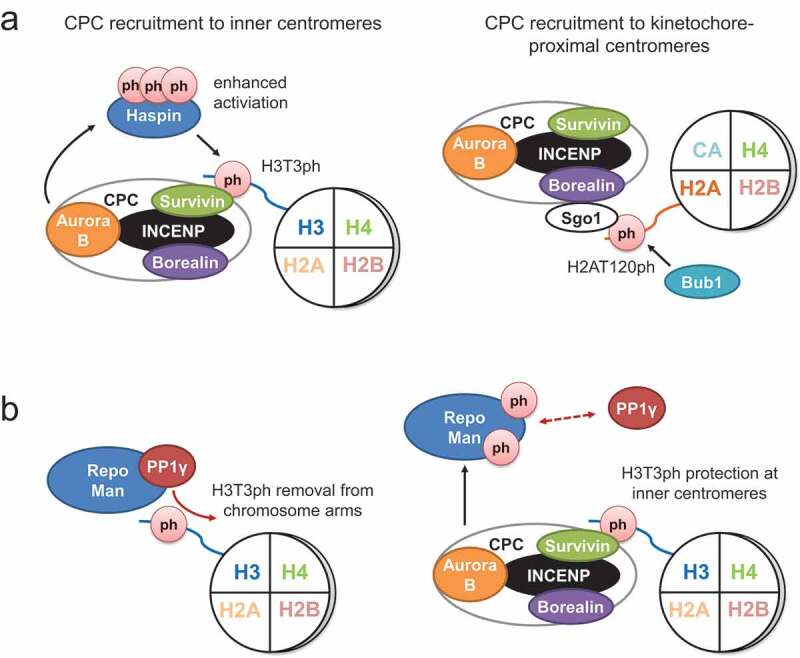
Histone modifications and feedback loops that promote accumulation of the chromosomal passenger complex (CPC) at inner centromeres. (a) Bub1 phosphorylates H2AT120 which is then recognized by Sgo1. The CPC is recruited to kinetochore-proximal centromeres via interaction between Borealin and Sgo1, and to inner centromeres by direct binding of Survivin to H3T3ph. Aurora B phosphorylates Haspin to enhance its kinase activity which, in turn, increases H3T3ph and CPC accumulation at inner centromeres. (b) Recruitment of PP1γ to chromatin by its targeting factor Repo-Man causes the removal of H3T3ph from chromosome arms during prometaphase. Inner centromeric H3T3ph is protected by CPC kinase Aurora B. Phosphorylation of Repo-Man by Aurora B precludes the interaction of Repo-Man with PP1γ and histones. The black arrows indicate phosphorylation (ph) and red arrows point out dephosphorylation. Inhibition of protein–protein interactions is shown by red arrows with dotted lines.
Pericentromeric phosphorylation is also observed for H3.3S31, but the responsible kinase is not certain since this modification can be carried out by checkpoint kinase 1 (CHK1) and also Aurora B in vitro [39,70,71]. H3.3S31ph increases on chromosomes that become spatially isolated from other chromosomes during mitosis and this hyperphosphorylation can persist and induce p53-dependent cell cycle arrest in the subsequent G1 phase [72]. The underlying mechanism for this process is not known but, in this way, H3.3 hyperphosphorylation could allow p53 to be directly activated in response to chromosome missegregation [73].
Recently, we discovered that H2BS6 is phosphorylated at inner centromeres and pericentromeres during early mitosis [37]. This modification can be first detected at the inter-chromatid axis in prophase and then strongly increases at inner centromeres during prophase. H2BS6ph is directly mediated by CDK1-cyclin B1. The spatiotemporal localization of H2BS6ph corresponds to CDK1-mediated phosphorylation of Sororin and H1 at the inter-chromosomal axis in prophase [10,74]. Furthermore, binding of the CDK1 partner cyclin B1 to unattached kinetochores might contribute to the increase in centromeric H2BS6ph in late prophase and prometaphase [75,76]. In cells, H2BS6ph also strongly depends on the activity of Aurora B, but Aurora B cannot detectably phosphorylate this site in vitro, indicating that it is not a direct H2BS6 kinase. When PP1 phosphatases were depleted from cells, H2BS6ph was only slightly reduced by Aurora B inhibition, suggesting that Aurora B sustains H2BS6ph by inhibition of phosphatase activity, as also previously shown for H3T3ph [61]. In this case, dephosphorylation of H3T3 is prevented by Aurora B-mediated phosphorylation of the PP1γ targeting protein Repo-Man, thus inhibiting its chromatin binding and maintaining H3T3ph at inner centromeres. Interestingly, Repo-Man can also bind to H2B [77], but a direct role of PP1γ-Repo-Man for H2B dephosphorylation has yet to be investigated. In addition, Aurora B-mediated restriction of phosphatases also involves phosphorylation of a conserved R-V-x-F interaction motif in various PP1 targeting factors, thus indirectly controlling phosphatase localization and activity at centromeres [78,79].
Histone modifications involved in centromeric transcription and kinetochore formation
Centromeres are defined epigenetically by the presence of CENP-A nucleosomes and, in many organisms, are also characterized by arrays of repetitive satellite DNA. Human α-satellites contain a sequence required for CENP-B recruitment (so-called CENP-B box) and can direct de novo kinetochore formation on human artificial chromosomes (HACs) [80]. However, functional centromeres can be established by CENP-A-containing chromatin independently of satellite DNA and CENP-B, as demonstrated by the formation of new centromeres (neocentromeres) at chromosomal region lacking the repetitive centromere sequences [81]. Nevertheless, centromeric and pericentromeric transcription during G2/M phase may play an important role for epigenetic maintenance and recruitment of kinetochore proteins. Expression of non-coding RNAs is required for reestablishment of pericentromeric heterochromatin after DNA replication in fission yeast [82]. In mammals, bulk transcription is generally turned down to very low levels during mitosis [51], but continuous transcription appears to occur specifically at the centromere, enabled by active H3K4me2 and H3K36me2 marks at the core domain [57,58]. Removal of H3K4me2 by targeting lysine-specific demethylase 1 (LSD1) to centromeres precludes transcription and inhibits CENP-A loading at mitotic exit resulting in gradual loss of centromere integrity [57]. Similar defects in CENP-A loading were observed after inhibition of RNA polymerase II (RNAPII) or targeted degradation of a long non-coding α-satellite RNA [83]. Other modifications associated with transcription such as acetylation are not enriched at mitotic centromeres. H2BK120 mono-ubiquitination (H2BK120ub1) is important for centromeric transcription but declines during M phase [45,53]. Nevertheless, inhibition of H2B ubiquitination by mutation of the lysine residue in yeast or knockdown of the responsible E3 ligase RNF20 in human cells causes defects in kinetochore formation and chromosome segregation with daughter cells exhibiting DNA damage and micronuclei [53]. Centromeric RNAPII activity might also affect chromatin cohesion and was reported to drive the re-localization of Sgo1 from H2AT120ph-modified chromatin to inner centromeric cohesin during early mitosis [50].
H4K20me1 is increased during mitosis [46,48] and this modification was identified in CENP-A-containing nucleosomes where it is important for recruitment of CCAN members CENP-H and CENP-T and kinetochore formation [84]. Several PTMs of CENP-A were reported to regulate CENP-A deposition and centromere integrity during cell division. Unlike canonical histones, which are integrated into chromatin during S phase in a replication-dependent manner, CENP-A is not deposited by its loading factor HJURP until the end of mitosis [85,86]. The CENP-A concentration in centromeric chromatin is diluted after S phase, and newly synthesized CENP-A is found in a pre-assembly complex with H4 acetylated at K5/K12 [87]. Instead, H3.3 is loaded into centromeric chromatin in S phase and serves as a placeholder for CENP-A during mitosis [55]. Integration of newly synthetized CENP-A into chromatin is inhibited by CDK1-mediated phosphorylation of CENP-AS68 [88]. Removal of CENP-AS68ph by PP1α at the end of mitosis allows the interaction of CENP-A with HJURP and its centromeric deposition in the next G1 phase. However, phosphorylation of HJURP and Mis18BP1 by CDK1 also interferes with HJURP recruitment to centromeres, suggesting that timely CENP-A deposition is controlled by multiple phosphorylations at various proteins [89–91].
Pre-nucleosomal CENP-A is also modified by tri-methylation of glycine 1 (CENP-AG1me3) as well as phosphorylation at S16/S18 and these modifications can also be detected in nucleosomal CENP-A [68]. CENP-AG1me3 is catalyzed by N-terminal RCC methyltransferase 1 (NRMT1) and is required for the recruitment of CCAN components CENP-I and CENP-T to centromeres during mitosis [92]. Phosphorylation of CENP-AS16/S18 is important for chromosome segregation, as mutation of both phosphorylation sites results in segregation defects with lagging chromosomes [68]. CENP-AS18ph also plays a role in CENP-A loading and S18 hyperphosphorylation by CDK2-cyclin E in FBW7-/- tumor cells leads to chromosomal instability due to defective CENP-A deposition [93]. Ubiquitination of CENP-AK124 by the CUL4A-RBX1-COPS8 complex was reported to facilitate the interaction with HJURP as well as centromere-specific CENP-A deposition and thus contribute to the maintenance of centromere identity [94–96]. However, these findings were not substantiated in another study where mutations of CENP-AS68 and K124 remained without a significant impact on centromeric CENP-A deposition [97]. At the G1/S transition, chromosomal CENP-AK164 is acetylated together with H4K79, which alters the properties of CENP-A nucleosomes and decreases accessibility to CENP-C binding [98]. CENP-AK164ac is removed and replaced by mono-methylation during S phase. Expression of a CENP-AK124Q mutant, which mimics acetylation, showed delayed centromere replication and mitotic defects with lagging chromosomes or multipolar spindles due to decreased CENP-C binding [98].
Mitotic histone PTMs as regulators of dynamic protein–protein interactions
While some histone marks can directly alter the structural properties of nucleosomes, others create a binding site for regulatory proteins or preclude the interaction with chromatin-associated factors. Phosphorylation of both H3T3 and H2AT120 was shown to be crucial for CPC recruitment to centromeres where it contributes to Aurora B-dependent correction of erroneous kinetochore-microtubule attachments [63], though this may not be the case in all organisms [99]. The H3T3 kinase Haspin associates with chromatin by interaction with the cohesin-associated factor Pds5B [63,100], and via a SUMO-interacting motif (SIM) that binds to SUMOylated proteins such as Topoisomerase IIα (TopoIIα) [101,102] as well as through HP1 (see below). H3T3ph is recognized by the CPC via the BIR domain of Survivin [63,103,104], and the cohesin protector Sgo1 directly interacts with Bub1-mediated H2AT120ph via its C-terminal basic SGO motif [35]. As the CPC subunit Borealin also binds to Sgo1 when it is phosphorylated by CDK1, H2AT120ph indirectly recruits Aurora B to mitotic centromeres [105].
Pericentromeric tri-methylation of H3K9 by SUV39H1 is required for the recruitment of heterochromatin protein 1 (HP1) and influences activation of Aurora B and H3S10ph in G2 phase [59,106,107]. HP1 recognizes methylated H3K9 via its N-terminal chromodomain [108,109], and can directly recruit the CPC to chromatin prior to mitosis [110,111]. The mitotic localization of HP1 is, in turn, regulated by Aurora B as phosphorylation of the adjacent H3S10 interferes with the binding of HP1 to methylated H3K9 and may remove HP1 from chromosomes [112,113]. HP1 also binds to H1.4K26me and is released by Aurora B-dependent phosphorylation of H1.4S27 [114,115]. In addition, HP1 may recruit Sgo1 to chromatin, perhaps in G2 phase, which could contribute to centromeric cohesion [116,117]. However, the requirement of HP1 and its interaction with Sgo1 for centromeric cohesion in mammalian cells was challenged by other studies [118–120]. Some HP1 remains associated with centromeres during mitosis, but this is facilitated by a different mechanism: interaction of the chromo shadow domain of HP1 with P-x-V-x-L-motif-containing proteins such as INCENP [118,121]. This interaction modestly stimulates Aurora B activity [122], and centromeric HP1 (and its yeast homologue Swi6) was also shown to recruit Haspin to chromatin in mitosis, providing a mechanism to regulate cohesion [63,123].
Similar to the displacement of HP1 from H3S10 phosphorylated chromatin, a “phospho-methyl switch” has also been proposed for other chromatin modifications (see Figure 3a). Mass spectrometry analysis identified H3T3ph in a combinatorial pattern together with H3K4me3 and H3R8me2 [124]. During mitosis, H3K4me3 is maintained at transcription start sites, perhaps as a bookmark for re-activation of gene expression after cell division [5]. H3T3ph precludes the in vitro binding of the plant homeodomain (PHD) finger of the transcription initiation factor TFIID subunit 3 (TAF3) which recognizes H3K4me3, and may regulate TFIID function in cells [125]. Phosphorylation of H3T3, and to some degree of H3T6, generally interferes with the in vitro binding of PHD finger proteins such as DIDO3, RAG2, ING2 and MLL5 to H3K4me3 [126–128]. H3T3/T6ph also lowers the binding affinity of the double Tudor domain of histone demethylase JMJD2A [128]. In addition, other PTMs such as H3S28ph and H3.3S31ph can be detected simultaneously with methylated K27 and K36 during mitosis [129]. H3S28ph could serve to displace Polycomb group proteins from H3K27me3-marked heterochromatin in mitosis as well as interphase [25,130]. H3K36me3 is found in actively transcribed gene bodies and retained during mitosis [5]. Phosphorylation of the histone variant H3.3S31 interferes with the binding of the Tudor domain protein PHF1 to K36me3 [127]. This suggests that histone phosphorylation co-regulates the recruitment and release of methyl-lysine reader proteins.
Figure 3.
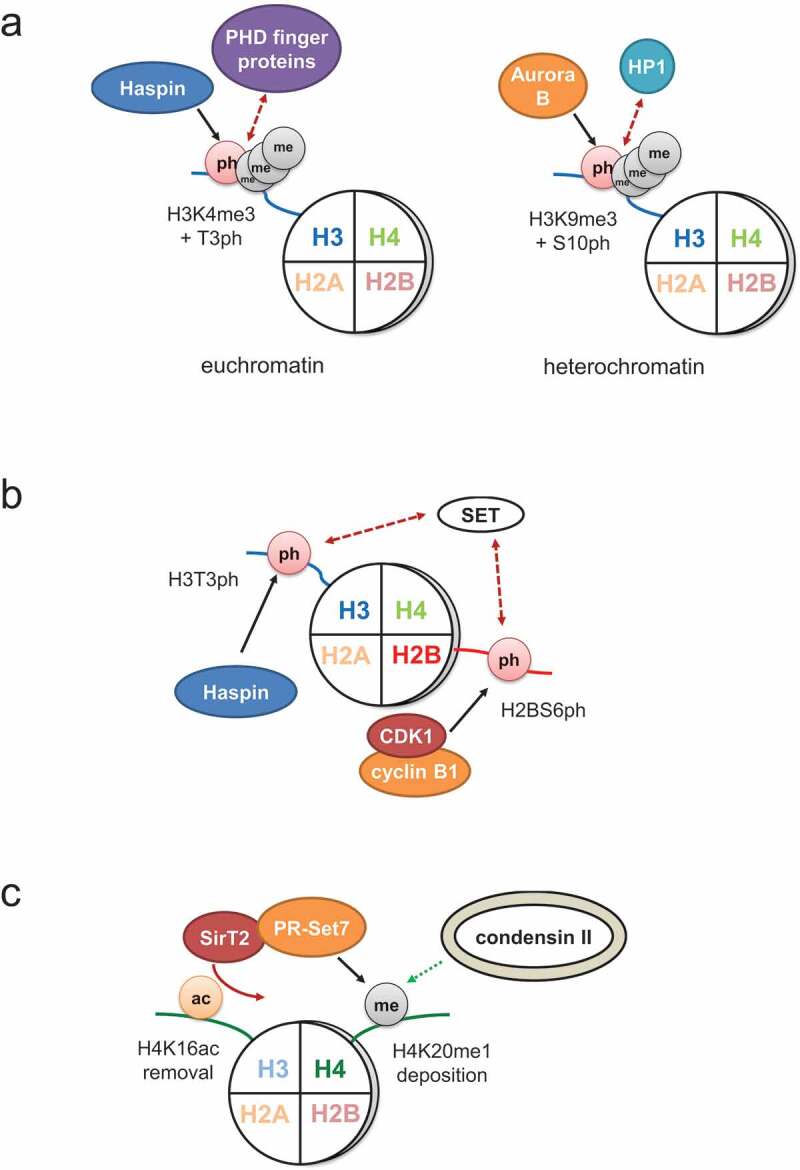
Functional interplay between mitotic histone PTMs. (a) Methylation of H3K4 and H3K9 is maintained during mitosis. Phosphorylation of H3T3 disrupts the interaction of proteins with plant homeodomains (PHD finger proteins) with H3K4me3. Similarly, co-modification of H3S10ph precludes the binding of HP1 to H3K9me3. (b) Increasing phosphorylation of H3T3 and H2BS6 by Haspin and CDK1-cyclin B1, respectively, interferes with the binding of SET to nucleosomes and might contribute to SET re-location from inner centromeres to kinetochore-proximal centromeres in metaphase. (c) Deacetylation of H4K16 and methylation of H4K20 are associated with chromosome condensation. SirT2 removes H4K16ac from mitotic chromatin and recruits PR-Set7 for H4K20 methylation. H4K20me1 can be directly bound by subunits of the condensin II complex to promote chromosome compaction. The black arrows indicate phosphorylation (ph) and methylation (me), respectively, and the red arrow points out deacetylation. Recruitment or dissociation of factors from chromatin is shown by green or red arrows with dotted lines, respectively.
The function of mitotic γH2A.X is not clear since γH2A.X foci are detectable during normal mitotic progression [42–44] but do not co-localize with p53-binding protein 1 (53BP1), a mediator of DNA damage response [131]. However, phosphorylation of H2A.X creates a binding site for reader proteins with a BRCA1 C-Terminal (BRCT) domain which recognizes a phospho-S/T-Q motif [132]. The BRCT domain protein MDC1 regulates the recruitment of DNA repair proteins to γH2A.X foci [133]. Recently it was shown that phosphorylated MDC1 interacts with Topoisomerase IIβ-binding protein 1 (TopBP1) during mitosis independently of 53BP1 [134]. The recruitment of TopBP1 to MDC1 foci was reported to stabilize DNA double-strand breaks (DSBs) by the formation of TopBP1 filaments to prevent genomic instability during chromosome segregation [134]. DSBs which are transmitted through mitosis or occurred during chromosome segregation then induce the formation of 53BP1 nuclear bodies and the DNA damage response in the next G1 phase [20,135]. Taken together, the recruitment of BRCT domain proteins to γH2A.X seems to play an important role in maintaining genomic stability during chromosome segregation and re-activation of DNA damage repair after cell division.
Phosphorylation of H2B at S6 and H3 at T3, S10, T11 and S28 precludes the interaction of the oncoprotein SET with the tails of the modified histones (see Figure 3b) [37,136,137]. SET is also known as inhibitor 2 of PP2A (I2PP2A) or template activating factor-Iβ (TAF-Iβ) and plays a role in different cellular processes. It is frequently upregulated in cancer cells, correlating with poor prognosis [138]. SET was shown to bind histones H2B and H3 as well as linker histones [74,139,140]. As part of the inhibitor of acetyltransferases (INHAT) complex, binding of SET to nucleosomes contributes to transcriptional repression by inhibiting the p300/CBP- and PCAF-mediated acetylation of H3 and H4 [141]. Furthermore, SET was identified as a potent inhibitor of PP2A phosphatase activity [142]. This function was suggested to be responsible for the finding that depletion of SET in oocytes prevents chromosome segregation during meiosis II and SET overexpression causes premature separation of sister chromatids in meiosis I [143,144]. In line with this, nucleosome assembly protein 1 (NAP1), a histone chaperone related to SET, mediates sister chromatid resolution by inhibiting PP2A in Drosophila S2 cells during mitosis [145]. Recent studies have shown that SET is also required for mitotic chromosome segregation in human cells [74,146–148]. Moreover, SET was identified as a direct interaction partner of Sgo1 and PP2A by mass spectrometry in several independent studies [13,74,147,149]. SET binds to inner centromeres in early mitosis and re-localizes to kinetochores during chromosome bi-orientation in metaphase [37,146,147]. Interestingly, Sgo1 shows a similar spatiotemporal re-localization from inner centromeric cohesin to kinetochore-proximal H2AT120ph [150]. Whereas the interaction with cohesin depends on Sgo1 phosphorylation by CDK1 [14], the release of Sgo1 from cohesin is governed by SET [147]. Furthermore, SET can be recruited to centromeric PP2A by interaction with Shugoshin 2 (Sgo2), which enhances Aurora B activity at kinetochore substrates by inhibiting PP2A [146]. Collectively, these data support a model where mitotic phosphorylation of H2A, H2B and H3 contributes to the spatiotemporal regulation of Shugoshin localization and PP2A activity, thus allowing sister chromatid separation and chromosome segregation in anaphase.
Regulation of chromosome condensation by histone PTMs
Several histone PTMs are reported to recruit or dissociate chromatin factors to achieve chromosome condensation, but the role of core histone phosphorylation in mitotic chromosome condensation is less clear than commonly assumed. In budding yeast, hypercondensation of artificially elongated chromosomes, specifically in anaphase, is compromised when the Aurora B target site H3S10 is mutated [151]. However, the effects of Aurora B on chromosome condensation in other organisms have been ascribed to substrates other than histones [152,153]
H4K20 is increasingly methylated by PR-Set7 during mitosis [48]. H4K20me1 can be directly bound by condensin II-specific subunits CAP-D3 & CAP-G2 which contributes to chromosome condensation in prophase (see Figure 3c) [46]. Studies in budding yeast revealed that H3S10ph is recognized by the HDAC Hst2p which removes H4K16ac from mitotic chromatin [154]. The deacetylation of H4K16 then enables the interaction of the H4 N-terminus with the H2A-H2B dimer of the neighboring nucleosome and condensin-independent chromosome condensation. Interestingly, H4K16ac is largely removed by the mammalian Hst2p homologue SirT2 at the beginning of mitosis and SirT2 associates with PR-Set7 to promote H4K20me1 [155,156], but the role of these changes in condensation in mammalian cells remains to be fully explored.
In DT40 chicken lymphoma cells, depletion of condensin subunit SMC2 during mitosis resulted in loss of chromosomal architecture and anaphase failure with chromosomal bridges [157]. Maintenance of CDK1 activity in anaphase or the inhibition of Repo-Man-mediated targeting of PP1γ to chromatin could rescue the condensation and segregation defects, indicating a role for mitotic phosphorylation. Of note, in vitro reconstitution of DT40 chromatin with mitotic histones, including their PTMs, could bring about condensin-independent chromosome compaction which was not observed with histones from interphase chromatin [158]. Interestingly, in vitro competition experiments with reconstituted nucleosomes and chromatin from Xenopus egg extracts showed that the N-terminal domain of H2B but not of H3 or H4 was necessary for chromosome condensation [159]. It would be interesting to investigate if mitosis-specific modifications of the H2B tail such as H2BS6ph are involved in the regulation of chromosome condensation.
In addition, H1 linker histones are essential for chromosome condensation and segregation [160]. Isoform-specific phosphorylation of H1 proteins by CDKs occurs at different sites from S to M phase [38,161,162]. Mitotic H1 phosphorylation is also mediated by Aurora B, protein kinase A (PKA) and glycogen synthase kinase-3 (GSK-3) [115,163,164]. Phosphorylation by CDKs increases the mobility of linker histones by lowering their affinity to nucleosomes which is important for chromatin re-arrangement during DNA replication [165]. Some mitotic isoform-specific H1 phosphorylation sites are, in contrast, tightly associated with condensed chromatin during mitosis [166]. Moreover, Krishnan et al. reported that H1 phosphorylation at serine or threonine 17 (H1S/T17ph) allows the SET-dependent eviction of H1 from the inter-chromatid axis and separation of chromosome arms in prophase [74]. Interestingly, SET itself is a target of mitotic phosphorylation by CDK1 [148]. As CDK1 also phosphorylates the cohesin protectors Sororin and Sgo1 as well as H2B [10,14,37], CDK1 acts as a master regulator that balances the release of H1 and cohesin from chromosome arms and the protection of centromeric cohesion in order to support chromosome bi-orientation and faithful segregation.
Concluding remarks
Great progress has been made in the identification of mitotic histone modifications and ongoing progress in the sensitivity of mass spectrometry is continuously allowing the identification of new and also combinatorial histone modifications. Many of the modifications are important during several phases of mitosis, where they play distinct roles depending on the context of co-modifications and the availability and localization of readers and erasers. A large number of histone PTMs have also been detected in interphase cells, where they can play overlapping or even completely distinct roles. It remains notoriously difficult to assign specific functions to a particular modification site, as many conclusions have been drawn from the downregulation of the corresponding writer or eraser protein, thus necessarily affecting co-regulated modification sites. Analysis of PTM functions by the introduction of point mutations is possible for most histone variants, which are encoded by single or low copy numbers in the human genome. In contrast, mutation of canonical histones is currently only possible in model organisms such as Drosophila [167]. Recent progress in genome engineering might enable the manipulation of these genes in vertebrate cells in the future, perhaps in an inducible manner, a possibility that may revolutionize our understanding of histone modification in mitosis and beyond.
Funding Statement
This work was supported by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) - TRR81/3 - project number A07 [109546710-TRR81]; the Wellcome Trust and the Royal Society, UK.
Acknowledgments
We apologize to all authors whose relevant work was not cited due to space constraints. This work was funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) - TRR81/3- project number A07. JMGH is a Wellcome Trust Investigator and holds a Royal Society Wolfson Research Merit Award.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- [1].Kouzarides T. Chromatin modifications and their function. Cell. 2007;128:693–705. [DOI] [PubMed] [Google Scholar]
- [2].Jenuwein T, Allis CD.. Translating the histone code. Science. 2001;293:1074–1080. [DOI] [PubMed] [Google Scholar]
- [3].Olsen JV, Vermeulen M, Santamaria A, et al. Quantitative phosphoproteomics reveals widespread full phosphorylation site occupancy during mitosis. Sci Signal. 2010;3:ra3. [DOI] [PubMed] [Google Scholar]
- [4].Landry CR, Levy ED, Michnick SW. Weak functional constraints on phosphoproteomes. Trends Genet. 2009;25:193–197. [DOI] [PubMed] [Google Scholar]
- [5].Javasky E, Shamir I, Gandhi S, et al. Study of mitotic chromatin supports a model of bookmarking by histone modifications and reveals nucleosome deposition patterns. Genome Res. 2018;28:1455–1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Reveron-Gomez N, Gonzalez-Aguilera C, Stewart-Morgan KR, et al. Accurate recycling of parental histones reproduces the histone modification landscape during DNA replication. Mol Cell. 2018;72(239–249):e235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Gibcus JH, Samejima K, Goloborodko A, et al. A pathway for mitotic chromosome formation. Science. 2018;359:eaao6135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Hirota T, Gerlich D, Koch B, et al. Distinct functions of condensin I and II in mitotic chromosome assembly. J Cell Sci. 2004;117:6435–6445. [DOI] [PubMed] [Google Scholar]
- [9].Haarhuis JH, Elbatsh AM, Rowland BD. Cohesin and its regulation: on the logic of X-shaped chromosomes. Dev Cell. 2014;31:7–18. [DOI] [PubMed] [Google Scholar]
- [10].Nishiyama T, Sykora MM, Huis in ‘T Veld PJ, et al. Aurora B and Cdk1 mediate Wapl activation and release of acetylated cohesin from chromosomes by phosphorylating Sororin. Proc Natl Acad Sci U S A. 2013;110:13404–13409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Sumara I, Vorlaufer E, Stukenberg PT, et al. The dissociation of cohesin from chromosomes in prophase is regulated by Polo-like kinase. Mol Cell. 2002;9:515–525. [DOI] [PubMed] [Google Scholar]
- [12].Hara K, Zheng G, Qu Q, et al. Structure of cohesin subcomplex pinpoints direct shugoshin-Wapl antagonism in centromeric cohesion. Nat Struct Mol Biol. 2014;21:864–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Kitajima TS, Sakuno T, Ishiguro K, et al. Shugoshin collaborates with protein phosphatase 2A to protect cohesin. Nature. 2006;441:46–52. [DOI] [PubMed] [Google Scholar]
- [14].Liu H, Rankin S, Yu H. Phosphorylation-enabled binding of SGO1-PP2A to cohesin protects sororin and centromeric cohesion during mitosis. Nat Cell Biol. 2013b;15:40–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Jia L, Kim S, Yu H. Tracking spindle checkpoint signals from kinetochores to APC/C. Trends Biochem Sci. 2013;38:302–311. [DOI] [PubMed] [Google Scholar]
- [16].Sivakumar S, Gorbsky GJ. Spatiotemporal regulation of the anaphase-promoting complex in mitosis. Nat Rev Mol Cell Biol. 2015;16:82–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Oliveira RA, Hamilton RS, Pauli A, et al. Cohesin cleavage and Cdk inhibition trigger formation of daughter nuclei. Nat Cell Biol. 2010;12:185–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Uhlmann F, Lottspeich F, Nasmyth K. Sister-chromatid separation at anaphase onset is promoted by cleavage of the cohesin subunit Scc1. Nature. 1999;400:37–42. [DOI] [PubMed] [Google Scholar]
- [19].Crasta K, Ganem NJ, Dagher R, et al. DNA breaks and chromosome pulverization from errors in mitosis. Nature. 2012;482:53–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Janssen A, van der Burg M, Szuhai K, et al. Chromosome segregation errors as a cause of DNA damage and structural chromosome aberrations. Science. 2011;333:1895–1898. [DOI] [PubMed] [Google Scholar]
- [21].Santaguida S, Amon A. Short- and long-term effects of chromosome mis-segregation and aneuploidy. Nat Rev Mol Cell Biol. 2015;16:473–485. [DOI] [PubMed] [Google Scholar]
- [22].Crosio C, Fimia GM, Loury R, et al. Mitotic phosphorylation of histone H3: spatio-temporal regulation by mammalian Aurora kinases. Mol Cell Biol. 2002;22:874–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Hsu JY, Sun ZW, Li X, et al. Mitotic phosphorylation of histone H3 is governed by Ipl1/aurora kinase and Glc7/PP1 phosphatase in budding yeast and nematodes. Cell. 2000;102:279–291. [DOI] [PubMed] [Google Scholar]
- [24].Hendzel MJ, Wei Y, Mancini MA, et al. Mitosis-specific phosphorylation of histone H3 initiates primarily within pericentromeric heterochromatin during G2 and spreads in an ordered fashion coincident with mitotic chromosome condensation. Chromosoma. 1997;106:348–360. [DOI] [PubMed] [Google Scholar]
- [25].Goto H, Yasui Y, Nigg EA, et al. Aurora-B phosphorylates Histone H3 at serine28 with regard to the mitotic chromosome condensation. Genes Cells. 2002;7:11–17. [DOI] [PubMed] [Google Scholar]
- [26].Nowak SJ, Corces VG. Phosphorylation of histone H3: a balancing act between chromosome condensation and transcriptional activation. Trends Genet. 2004;20:214–220. [DOI] [PubMed] [Google Scholar]
- [27].Dai J, Sultan S, Taylor SS, et al. The kinase haspin is required for mitotic histone H3 Thr 3 phosphorylation and normal metaphase chromosome alignment. Genes Dev. 2005;19:472–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Hammond SL, Byrum SD, Namjoshi S, et al. Mitotic phosphorylation of histone H3 threonine 80. Cell Cycle. 2014;13:440–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Wike CL, Graves HK, Hawkins R, et al. Aurora-A mediated histone H3 phosphorylation of threonine 118 controls condensin I and cohesin occupancy in mitosis. Elife. 2016;5:e11402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Gatchalian J, Gallardo CM, Shinsky SA, et al. Chromatin condensation and recruitment of PHD finger proteins to histone H3K4me3 are mutually exclusive. Nucleic Acids Res. 2016;44:6102–6112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Preuss U, Landsberg G, Scheidtmann KH. Novel mitosis-specific phosphorylation of histone H3 at Thr11 mediated by Dlk/ZIP kinase. Nucleic Acids Res. 2003;31:878–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Metzger E, Imhof A, Patel D, et al. Phosphorylation of histone H3T6 by PKCbeta(I) controls demethylation at histone H3K4. Nature. 2010;464:792–796. [DOI] [PubMed] [Google Scholar]
- [33].Metzger E, Yin N, Wissmann M, et al. Phosphorylation of histone H3 at threonine 11 establishes a novel chromatin mark for transcriptional regulation. Nat Cell Biol. 2008;10:53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Shimada M, Niida H, Zineldeen DH, et al. Chk1 is a histone H3 threonine 11 kinase that regulates DNA damage-induced transcriptional repression. Cell. 2008;132:221–232. [DOI] [PubMed] [Google Scholar]
- [35].Kawashima SA, Yamagishi Y, Honda T, et al. Phosphorylation of H2A by Bub1 prevents chromosomal instability through localizing shugoshin. Science. 2010;327:172–177. [DOI] [PubMed] [Google Scholar]
- [36].Barber CM, Turner FB, Wang Y, et al. The enhancement of histone H4 and H2A serine 1 phosphorylation during mitosis and S-phase is evolutionarily conserved. Chromosoma. 2004;112:360–371. [DOI] [PubMed] [Google Scholar]
- [37].Seibert M, Kruger M, Watson NA, et al. CDK1-mediated phosphorylation at H2B serine 6 is required for mitotic chromosome segregation. J Cell Biol. 2019;218:1164–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Swank RA, Th’ng JP, Guo XW, et al. Four distinct cyclin-dependent kinases phosphorylate histone H1 at all of its growth-related phosphorylation sites. Biochemistry. 1997;36:13761–13768. [DOI] [PubMed] [Google Scholar]
- [39].Li M, Dong Q, Zhu B. Aurora kinase B phosphorylates histone H3.3 at serine 31 during mitosis in mammalian cells. J Mol Biol. 2017;429:2042–2045. [DOI] [PubMed] [Google Scholar]
- [40].Shimada M, Goshima T, Matsuo H, et al. Essential role of autoactivation circuitry on Aurora B-mediated H2AX-pS121 in mitosis. Nat Commun. 2016;7:12059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Zeitlin SG, Shelby RD, Sullivan KF. CENP-A is phosphorylated by Aurora B kinase and plays an unexpected role in completion of cytokinesis. J Cell Biol. 2001;155:1147–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Martin M, Terradas M, Hernandez L, et al. gammaH2AX foci on apparently intact mitotic chromosomes: not signatures of misrejoining events but signals of unresolved DNA damage. Cell Cycle. 2014;13:3026–3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].McManus KJ, Hendzel MJ. ATM-dependent DNA damage-independent mitotic phosphorylation of H2AX in normally growing mammalian cells. Mol Biol Cell. 2005;16:5013–5025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Tu WZ, Li B, Huang B, et al. gammaH2AX foci formation in the absence of DNA damage: mitotic H2AX phosphorylation is mediated by the DNA-PKcs/CHK2 pathway. FEBS Lett. 2013;587:3437–3443. [DOI] [PubMed] [Google Scholar]
- [45].Guppy BJ, McManus KJ. Mitotic accumulation of dimethylated lysine 79 of histone H3 is important for maintaining genome integrity during mitosis in human cells. Genetics. 2015;199:423–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Liu W, Tanasa B, Tyurina OV, et al. PHF8 mediates histone H4 lysine 20 demethylation events involved in cell cycle progression. Nature. 2010b;466:508–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].McManus KJ, Biron VL, Heit R, et al. Dynamic changes in histone H3 lysine 9 methylations: identification of a mitosis-specific function for dynamic methylation in chromosome congression and segregation. J Biol Chem. 2006;281:8888–8897. [DOI] [PubMed] [Google Scholar]
- [48].Rice JC, Nishioka K, Sarma K, et al. Mitotic-specific methylation of histone H4 Lys 20 follows increased PR-Set7 expression and its localization to mitotic chromosomes. Genes Dev. 2002;16:2225–2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Zhao R, Nakamura T, Fu Y, et al. Gene bookmarking accelerates the kinetics of post-mitotic transcriptional re-activation. Nat Cell Biol. 2011;13:1295–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Liu H, Qu Q, Warrington R, et al. Mitotic transcription installs Sgo1 at centromeres to coordinate chromosome segregation. Mol Cell. 2015;59:426–436. [DOI] [PubMed] [Google Scholar]
- [51].Palozola KC, Donahue G, Liu H, et al. Mitotic transcription and waves of gene reactivation during mitotic exit. Science. 2017;358:119–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Joo HY, Zhai L, Yang C, et al. Regulation of cell cycle progression and gene expression by H2A deubiquitination. Nature. 2007;449:1068–1072. [DOI] [PubMed] [Google Scholar]
- [53].Sadeghi L, Siggens L, Svensson JP, et al. Centromeric histone H2B monoubiquitination promotes noncoding transcription and chromatin integrity. Nat Struct Mol Biol. 2014;21:236–243. [DOI] [PubMed] [Google Scholar]
- [54].Musacchio A, Desai A. A molecular view of kinetochore assembly and function. Biology (Basel). 2017;6:E5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Dunleavy EM, Almouzni G, Karpen GH. H3.3 is deposited at centromeres in S phase as a placeholder for newly assembled CENP-A in G(1) phase. Nucleus. 2011;2:146–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Greaves IK, Rangasamy D, Ridgway P, et al. H2A.Z contributes to the unique 3D structure of the centromere. Proc Natl Acad Sci U S A. 2007;104:525–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Bergmann JH, Rodriguez MG, Martins NM, et al. Epigenetic engineering shows H3K4me2 is required for HJURP targeting and CENP-A assembly on a synthetic human kinetochore. Embo J. 2011;30:328–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Sullivan BA, Karpen GH. Centromeric chromatin exhibits a histone modification pattern that is distinct from both euchromatin and heterochromatin. Nat Struct Mol Biol. 2004;11:1076–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Lehnertz B, Ueda Y, Derijck AA, et al. Suv39h-mediated histone H3 lysine 9 methylation directs DNA methylation to major satellite repeats at pericentric heterochromatin. Curr Biol. 2003;13:1192–1200. [DOI] [PubMed] [Google Scholar]
- [60].Lee HS, Lin Z, Chae S, et al. The chromatin remodeler RSF1 controls centromeric histone modifications to coordinate chromosome segregation. Nat Commun. 2018;9:3848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Qian J, Beullens M, Lesage B, et al. Aurora B defines its own chromosomal targeting by opposing the recruitment of the phosphatase scaffold Repo-Man. Curr Biol. 2013;23:1136–1143. [DOI] [PubMed] [Google Scholar]
- [62].Wang F, Ulyanova NP, van der Waal MS, et al. A positive feedback loop involving Haspin and Aurora B promotes CPC accumulation at centromeres in mitosis. Curr Biol. 2011;21:1061–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Yamagishi Y, Honda T, Tanno Y, et al. Two histone marks establish the inner centromere and chromosome bi-orientation. Science. 2010;330:239–243. [DOI] [PubMed] [Google Scholar]
- [64].Carmena M, Wheelock M, Funabiki H, et al. The chromosomal passenger complex (CPC): from easy rider to the godfather of mitosis. Nat Rev Mol Cell Biol. 2012;13:789–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Eot-Houllier G, Magnaghi-Jaulin L, Fulcrand G, et al. Aurora A-dependent CENP-A phosphorylation at inner centromeres protects bioriented chromosomes against cohesion fatigue. Nat Commun. 2018;9:1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Kunitoku N, Sasayama T, Marumoto T, et al. CENP-A phosphorylation by Aurora-A in prophase is required for enrichment of Aurora-B at inner centromeres and for kinetochore function. Dev Cell. 2003;5:853–864. [DOI] [PubMed] [Google Scholar]
- [67].Goutte-Gattat D, Shuaib M, Ouararhni K, et al. Phosphorylation of the CENP-A amino-terminus in mitotic centromeric chromatin is required for kinetochore function. Proc Natl Acad Sci U S A. 2013;110:8579–8584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Bailey AO, Panchenko T, Sathyan KM, et al. Posttranslational modification of CENP-A influences the conformation of centromeric chromatin. Proc Natl Acad Sci U S A. 2013;110:11827–11832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Barra V, Logsdon GA, Scelfo A, et al. Phosphorylation of CENP-A on serine 7 does not control centromere function. Nat Commun. 2019;10:175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Chang FT, Chan FL, JD RM, et al. CHK1-driven histone H3.3 serine 31 phosphorylation is important for chromatin maintenance and cell survival in human ALT cancer cells. Nucleic Acids Res. 2015;43:2603–2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Hake SB, Garcia BA, Kauer M, et al. Serine 31 phosphorylation of histone variant H3.3 is specific to regions bordering centromeres in metaphase chromosomes. Proc Natl Acad Sci U S A. 2005;102:6344–6349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Hinchcliffe EH, Day CA, Karanjeet KB, et al. Chromosome missegregation during anaphase triggers p53 cell cycle arrest through histone H3.3 Ser31 phosphorylation. Nat Cell Biol. 2016;18:668–675. [DOI] [PubMed] [Google Scholar]
- [73].Thompson SL, Compton DA. Proliferation of aneuploid human cells is limited by a p53-dependent mechanism. J Cell Biol. 2010;188:369–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Krishnan S, Smits AH, Vermeulen M, et al. Phospho-H1 decorates the inter-chromatid axis and is evicted along with shugoshin by SET during mitosis. Mol Cell. 2017;67(579–593):e576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Bentley AM, Normand G, Hoyt J, et al. Distinct sequence elements of cyclin B1 promote localization to chromatin, centrosomes, and kinetochores during mitosis. Mol Biol Cell. 2007;18:4847–4858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Chen Q, Zhang X, Jiang Q, et al. Cyclin B1 is localized to unattached kinetochores and contributes to efficient microtubule attachment and proper chromosome alignment during mitosis. Cell Res. 2008;18:268–280. [DOI] [PubMed] [Google Scholar]
- [77].Vagnarelli P, Ribeiro S, Sennels L, et al. Repo-Man coordinates chromosomal reorganization with nuclear envelope reassembly during mitotic exit. Dev Cell. 2011;21:328–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Liu D, Vleugel M, Backer CB, et al. Regulated targeting of protein phosphatase 1 to the outer kinetochore by KNL1 opposes Aurora B kinase. J Cell Biol. 2010a;188:809–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Nasa I, Rusin SF, Kettenbach AN, et al. Aurora B opposes PP1 function in mitosis by phosphorylating the conserved PP1-binding RVxF motif in PP1 regulatory proteins. Sci Signal. 2018;11:eaai8669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Masumoto H, Nakano M, Ohzeki J. The role of CENP-B and alpha-satellite DNA: de novo assembly and epigenetic maintenance of human centromeres. Chromosome Res. 2004;12:543–556. [DOI] [PubMed] [Google Scholar]
- [81].Warburton PE. Epigenetic analysis of kinetochore assembly on variant human centromeres. Trends Genet. 2001;17:243–247. [DOI] [PubMed] [Google Scholar]
- [82].Grewal SI. RNAi-dependent formation of heterochromatin and its diverse functions. Curr Opin Genet Dev. 2010;20:134–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Quenet D, Dalal Y. A long non-coding RNA is required for targeting centromeric protein A to the human centromere. Elife. 2014;3:e03254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Hori T, Shang WH, Toyoda A, et al. Histone H4 Lys 20 monomethylation of the CENP-A nucleosome is essential for kinetochore assembly. Dev Cell. 2014;29:740–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Dunleavy EM, Roche D, Tagami H, et al. HJURP is a cell-cycle-dependent maintenance and deposition factor of CENP-A at centromeres. Cell. 2009;137:485–497. [DOI] [PubMed] [Google Scholar]
- [86].Foltz DR, Jansen LE, Bailey AO, et al. Centromere-specific assembly of CENP-a nucleosomes is mediated by HJURP. Cell. 2009;137:472–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Shang WH, Hori T, Westhorpe FG, et al. Acetylation of histone H4 lysine 5 and 12 is required for CENP-A deposition into centromeres. Nat Commun. 2016;7:13465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Yu Z, Zhou X, Wang W, et al. Dynamic phosphorylation of CENP-A at Ser68 orchestrates its cell-cycle-dependent deposition at centromeres. Dev Cell. 2015;32:68–81. [DOI] [PubMed] [Google Scholar]
- [89].Pan D, Klare K, Petrovic A, et al. CDK-regulated dimerization of M18BP1 on a Mis18 hexamer is necessary for CENP-A loading. Elife. 2017. 6. e23352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Spiller F, Medina-Pritchard B, Abad MA, et al. Molecular basis for Cdk1-regulated timing of Mis18 complex assembly and CENP-A deposition. EMBO Rep. 2017;18:894–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Wang J, Liu X, Dou Z, et al. Mitotic regulator Mis18beta interacts with and specifies the centromeric assembly of molecular chaperone holliday junction recognition protein (HJURP). J Biol Chem. 2014;289:8326–8336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Sathyan KM, Fachinetti D, Foltz DR. alpha-amino trimethylation of CENP-A by NRMT is required for full recruitment of the centromere. Nat Commun. 2017;8:14678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Takada M, Zhang W, Suzuki A, et al. FBW7 loss promotes chromosomal instability and tumorigenesis via cyclin E1/CDK2-mediated phosphorylation of CENP-A. Cancer Res. 2017;77:4881–4893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Niikura Y, Kitagawa R, Fang L, et al. CENP-A Ubiquitylation is indispensable to cell viability. Dev Cell. 2019;50:683–689 e686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Niikura Y, Kitagawa R, Kitagawa K. CENP-A ubiquitylation is inherited through dimerization between cell divisions. Cell Rep. 2016;15:61–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Niikura Y, Kitagawa R, Ogi H, et al. CENP-A K124 Ubiquitylation is required for CENP-A deposition at the centromere. Dev Cell. 2015;32:589–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Fachinetti D, Logsdon GA, Abdullah A, et al. CENP-A modifications on Ser68 and Lys124 are dispensable for establishment, maintenance, and long-term function of human centromeres. Dev Cell. 2017;40:104–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Bui M, Pitman M, Nuccio A, et al. Internal modifications in the CENP-A nucleosome modulate centromeric dynamics. Epigenetics Chromatin. 2017;10:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Campbell CS, Desai A. Tension sensing by Aurora B kinase is independent of survivin-based centromere localization. Nature. 2013;497:118–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Carretero M, Ruiz-Torres M, Rodriguez-Corsino M, et al. Pds5B is required for cohesion establishment and Aurora B accumulation at centromeres. Embo J. 2013;32:2938–2949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Edgerton H, Johansson M, Keifenheim D, et al. A noncatalytic function of the topoisomerase II CTD in Aurora B recruitment to inner centromeres during mitosis. J Cell Biol. 2016;213:651–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Yoshida MM, Ting L, Gygi SP, et al. SUMOylation of DNA topoisomerase IIalpha regulates histone H3 kinase Haspin and H3 phosphorylation in mitosis. J Cell Biol. 2016;213:665–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Kelly AE, Ghenoiu C, Xue JZ, et al. Survivin reads phosphorylated histone H3 threonine 3 to activate the mitotic kinase Aurora B. Science. 2010;330:235–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Wang F, Dai J, Daum JR, et al. Histone H3 Thr-3 phosphorylation by Haspin positions Aurora B at centromeres in mitosis. Science. 2010;330:231–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Tsukahara T, Tanno Y, Watanabe Y. Phosphorylation of the CPC by Cdk1 promotes chromosome bi-orientation. Nature. 2010;467:719–723. [DOI] [PubMed] [Google Scholar]
- [106].Melcher M, Schmid M, Aagaard L, et al. Structure-function analysis of SUV39H1 reveals a dominant role in heterochromatin organization, chromosome segregation, and mitotic progression. Mol Cell Biol. 2000;20:3728–3741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Peters AH, O’Carroll D, Scherthan H, et al. Loss of the Suv39h histone methyltransferases impairs mammalian heterochromatin and genome stability. Cell. 2001;107:323–337. [DOI] [PubMed] [Google Scholar]
- [108].Bannister AJ, Zegerman P, Partridge JF, et al. Selective recognition of methylated lysine 9 on histone H3 by the HP1 chromo domain. Nature. 2001;410:120–124. [DOI] [PubMed] [Google Scholar]
- [109].Lachner M, O’Carroll D, Rea S, et al. Methylation of histone H3 lysine 9 creates a binding site for HP1 proteins. Nature. 2001;410:116–120. [DOI] [PubMed] [Google Scholar]
- [110].Liu X, Song Z, Huo Y, et al. Chromatin protein HP1 interacts with the mitotic regulator borealin protein and specifies the centromere localization of the chromosomal passenger complex. J Biol Chem. 2014;289:20638–20649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Ruppert JG, Samejima K, Platani M, et al. HP1alpha targets the chromosomal passenger complex for activation at heterochromatin before mitotic entry. Embo J. 2018;37:e97677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Fischle W, Tseng BS, Dormann HL, et al. Regulation of HP1-chromatin binding by histone H3 methylation and phosphorylation. Nature. 2005;438:1116–1122. [DOI] [PubMed] [Google Scholar]
- [113].Hirota T, Lipp JJ, Toh BH, et al. Histone H3 serine 10 phosphorylation by Aurora B causes HP1 dissociation from heterochromatin. Nature. 2005;438:1176–1180. [DOI] [PubMed] [Google Scholar]
- [114].Daujat S, Zeissler U, Waldmann T, et al. HP1 binds specifically to Lys26-methylated histone H1.4, whereas simultaneous Ser27 phosphorylation blocks HP1 binding. J Biol Chem. 2005;280:38090–38095. [DOI] [PubMed] [Google Scholar]
- [115].Hergeth SP, Dundr M, Tropberger P, et al. Isoform-specific phosphorylation of human linker histone H1.4 in mitosis by the kinase Aurora B. J Cell Sci. 2011;124:1623–1628. [DOI] [PubMed] [Google Scholar]
- [116].Perera D, Taylor SS. Sgo1 establishes the centromeric cohesion protection mechanism in G2 before subsequent Bub1-dependent recruitment in mitosis. J Cell Sci. 2010;123:653–659. [DOI] [PubMed] [Google Scholar]
- [117].Yamagishi Y, Sakuno T, Shimura M, et al. Heterochromatin links to centromeric protection by recruiting shugoshin. Nature. 2008;455:251–255. [DOI] [PubMed] [Google Scholar]
- [118].Kang J, Chaudhary J, Dong H, et al. Mitotic centromeric targeting of HP1 and its binding to Sgo1 are dispensable for sister-chromatid cohesion in human cells. Mol Biol Cell. 2011;22:1181–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Koch B, Kueng S, Ruckenbauer C, et al. The Suv39h-HP1 histone methylation pathway is dispensable for enrichment and protection of cohesin at centromeres in mammalian cells. Chromosoma. 2008;117:199–210. [DOI] [PubMed] [Google Scholar]
- [120].Serrano A, Rodriguez-Corsino M, Losada A. Heterochromatin protein 1 (HP1) proteins do not drive pericentromeric cohesin enrichment in human cells. PLoS One. 2009;4:e5118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Chu L, Huo Y, Liu X, et al. The spatiotemporal dynamics of chromatin protein HP1alpha is essential for accurate chromosome segregation during cell division. J Biol Chem. 2014;289:26249–26262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Abe Y, Sako K, Takagaki K, et al. HP1-assisted aurora B kinase activity prevents chromosome segregation errors. Dev Cell. 2016;36:487–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Yi Q, Chen Q, Liang C, et al. HP1 links centromeric heterochromatin to centromere cohesion in mammals. EMBO Rep. 2018;19:e45484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Markaki Y, Christogianni A, Politou AS, et al. Phosphorylation of histone H3 at Thr3 is part of a combinatorial pattern that marks and configures mitotic chromatin. J Cell Sci. 2009;122:2809–2819. [DOI] [PubMed] [Google Scholar]
- [125].Varier RA, Outchkourov NS, de Graaf P, et al. A phospho/methyl switch at histone H3 regulates TFIID association with mitotic chromosomes. Embo J. 2010;29:3967–3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Ali M, Rincon-Arano H, Zhao W, et al. Molecular basis for chromatin binding and regulation of MLL5. Proc Natl Acad Sci U S A. 2013;110:11296–11301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Andrews FH, Gatchalian J, Krajewski K, et al. Regulation of methyllysine readers through phosphorylation. ACS Chem Biol. 2016;11:547–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Garske AL, Oliver SS, Wagner EK, et al. Combinatorial profiling of chromatin binding modules reveals multisite discrimination. Nat Chem Biol. 2010;6:283–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Garcia BA, Barber CM, Hake SB, et al. Modifications of human histone H3 variants during mitosis. Biochemistry. 2005;44:13202–13213. [DOI] [PubMed] [Google Scholar]
- [130].Gehani SS, Agrawal-Singh S, Dietrich N, et al. Polycomb group protein displacement and gene activation through MSK-dependent H3K27me3S28 phosphorylation. Mol Cell. 2010;39:886–900. [DOI] [PubMed] [Google Scholar]
- [131].Nelson G, Buhmann M, von Zglinicki T. DNA damage foci in mitosis are devoid of 53BP1. Cell Cycle. 2009;8:3379–3383. [DOI] [PubMed] [Google Scholar]
- [132].Manke IA, Lowery DM, Nguyen A, et al. BRCT repeats as phosphopeptide-binding modules involved in protein targeting. Science. 2003;302:636–639. [DOI] [PubMed] [Google Scholar]
- [133].Stucki M, Clapperton JA, Mohammad D, et al. MDC1 directly binds phosphorylated histone H2AX to regulate cellular responses to DNA double-strand breaks. Cell. 2005;123:1213–1226. [DOI] [PubMed] [Google Scholar]
- [134].Leimbacher PA, Jones SE, Shorrocks AK, et al. MDC1 interacts with TOPBP1 to maintain chromosomal stability during mitosis. Mol Cell. 2019;74:571–583.e578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Pedersen RT, Kruse T, Nilsson J, et al. TopBP1 is required at mitosis to reduce transmission of DNA damage to G1 daughter cells. J Cell Biol. 2015;210:565–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Kim JY, Kim KB, Son HJ, et al. H3K27 methylation and H3S28 phosphorylation-dependent transcriptional regulation by INHAT subunit SET/TAF-Ibeta. FEBS Lett. 2012;586:3159–3165. [DOI] [PubMed] [Google Scholar]
- [137].Schneider R, Bannister AJ, Weise C, et al. Direct binding of INHAT to H3 tails disrupted by modifications. J Biol Chem. 2004;279:23859–23862. [DOI] [PubMed] [Google Scholar]
- [138].Bayarkhangai B, Noureldin S, Yu L, et al. A comprehensive and perspective view of oncoprotein SET in cancer. Cancer Med. 2018;7:3084–3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Karetsou Z, Emmanouilidou A, Sanidas I, et al. Identification of distinct SET/TAF-Ibeta domains required for core histone binding and quantitative characterisation of the interaction. BMC Biochem. 2009;10:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Kato K, Okuwaki M, Nagata K. Role of Template Activating Factor-I as a chaperone in linker histone dynamics. J Cell Sci. 2011;124:3254–3265. [DOI] [PubMed] [Google Scholar]
- [141].Seo SB, McNamara P, Heo S, et al. Regulation of histone acetylation and transcription by INHAT, a human cellular complex containing the set oncoprotein. Cell. 2001;104:119–130. [DOI] [PubMed] [Google Scholar]
- [142].Li M, Makkinje A, Damuni Z. The myeloid leukemia-associated protein SET is a potent inhibitor of protein phosphatase 2A. J Biol Chem. 1996;271:11059–11062. [DOI] [PubMed] [Google Scholar]
- [143].Chambon JP, Touati SA, Berneau S, et al. The PP2A inhibitor I2PP2A is essential for sister chromatid segregation in oocyte meiosis II. Curr Biol. 2013;23:485–490. [DOI] [PubMed] [Google Scholar]
- [144].Qi ST, Wang ZB, Ouyang YC, et al. Overexpression of SETbeta, a protein localizing to centromeres, causes precocious separation of chromatids during the first meiosis of mouse oocytes. J Cell Sci. 2013;126:1595–1603. [DOI] [PubMed] [Google Scholar]
- [145].Moshkin YM, Doyen CM, Kan TW, et al. Histone chaperone NAP1 mediates sister chromatid resolution by counteracting protein phosphatase 2A. PLoS Genet. 2013;9:e1003719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Asai Y, Fukuchi K, Tanno Y, et al. Aurora B kinase activity is regulated by SET/TAF1 on Sgo2 at the inner centromere. J Cell Biol. 2019;218:3223–3236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Qu Q, Zhang Q, Yang L, et al. SET binding to Sgo1 inhibits Sgo1-cohesin interactions and promotes chromosome segregation. J Cell Biol. 2019;218:2514–2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Yin L, Zeng Y, Xiao Y, et al. Cyclin-dependent kinase 1-mediated phosphorylation of SET at serine 7 is essential for its oncogenic activity. Cell Death Dis. 2019;10:385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Herzog F, Kahraman A, Boehringer D, et al. Structural probing of a protein phosphatase 2A network by chemical cross-linking and mass spectrometry. Science. 2012;337:1348–1352. [DOI] [PubMed] [Google Scholar]
- [150].Liu H, Jia L, Yu H. Phospho-H2A and cohesin specify distinct tension-regulated Sgo1 pools at kinetochores and inner centromeres. Curr Biol. 2013a;23:1927–1933. [DOI] [PubMed] [Google Scholar]
- [151].Neurohr G, Naegeli A, Titos I, et al. A midzone-based ruler adjusts chromosome compaction to anaphase spindle length. Science. 2011;332:465–468. [DOI] [PubMed] [Google Scholar]
- [152].Mora-Bermudez F, Gerlich D, Ellenberg J. Maximal chromosome compaction occurs by axial shortening in anaphase and depends on Aurora kinase. Nat Cell Biol. 2007;9:822–831. [DOI] [PubMed] [Google Scholar]
- [153].Tada K, Susumu H, Sakuno T, et al. Condensin association with histone H2A shapes mitotic chromosomes. Nature. 2011;474:477–483. [DOI] [PubMed] [Google Scholar]
- [154].Wilkins BJ, Rall NA, Ostwal Y, et al. A cascade of histone modifications induces chromatin condensation in mitosis. Science. 2014;343:77–80. [DOI] [PubMed] [Google Scholar]
- [155].Serrano L, Martinez-Redondo P, Marazuela-Duque A, et al. The tumor suppressor SirT2 regulates cell cycle progression and genome stability by modulating the mitotic deposition of H4K20 methylation. Genes Dev. 2013;27:639–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Vaquero A, Scher MB, Lee DH, et al. SirT2 is a histone deacetylase with preference for histone H4 Lys 16 during mitosis. Genes Dev. 2006;20:1256–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Vagnarelli P, Hudson DF, Ribeiro SA, et al. Condensin and Repo-Man-PP1 co-operate in the regulation of chromosome architecture during mitosis. Nat Cell Biol. 2006;8:1133–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Zhiteneva A, Bonfiglio JJ, Makarov A, et al. Mitotic post-translational modifications of histones promote chromatin compaction in vitro. Open Biol. 2017;7:170076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].de la Barre AE, Angelov D, Molla A, et al. The N-terminus of histone H2B, but not that of histone H3 or its phosphorylation, is essential for chromosome condensation. Embo J. 2001;20:6383–6393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Maresca TJ, Freedman BS, Heald R. Histone H1 is essential for mitotic chromosome architecture and segregation in Xenopus laevis egg extracts. J Cell Biol. 2005;169:859–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Sarg B, Helliger W, Talasz H, et al. Histone H1 phosphorylation occurs site-specifically during interphase and mitosis: identification of a novel phosphorylation site on histone H1. J Biol Chem. 2006;281:6573–6580. [DOI] [PubMed] [Google Scholar]
- [162].Talasz H, Helliger W, Puschendorf B, et al. In vivo phosphorylation of histone H1 variants during the cell cycle. Biochemistry. 1996;35:1761–1767. [DOI] [PubMed] [Google Scholar]
- [163].Chu CS, Hsu PH, Lo PW, et al. Protein kinase A-mediated serine 35 phosphorylation dissociates histone H1.4 from mitotic chromosome. J Biol Chem. 2011;286:35843–35851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Happel N, Stoldt S, Schmidt B, et al. M phase-specific phosphorylation of histone H1.5 at threonine 10 by GSK-3. J Mol Biol. 2009;386:339–350. [DOI] [PubMed] [Google Scholar]
- [165].Contreras A, Hale TK, Stenoien DL, et al. The dynamic mobility of histone H1 is regulated by cyclin/CDK phosphorylation. Mol Cell Biol. 2003;23:8626–8636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Talasz H, Sarg B, Lindner HH. Site-specifically phosphorylated forms of H1.5 and H1.2 localized at distinct regions of the nucleus are related to different processes during the cell cycle. Chromosoma. 2009;118:693–709. [DOI] [PubMed] [Google Scholar]
- [167].Gunesdogan U, Jackle H, Herzig A. A genetic system to assess in vivo the functions of histones and histone modifications in higher eukaryotes. EMBO Rep. 2010;11:772–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Qian J, Lesage B, Beullens M, et al. PP1/Repo-man dephosphorylates mitotic histone H3 at T3 and regulates chromosomal aurora B targeting. Curr Biol. 2011;21:766–773. [DOI] [PubMed] [Google Scholar]


