Abstract
Nonalcoholic fatty liver disease (NAFLD) is the most common chronic liver disease characterized with the spectrum of hepatic steatosis, inflammation and fibrosis. The etiology of NAFLD remains incompletely understood. Numerous studies have implied that the gut microbiota (GM) is involved in the development of NAFLD, as it particularly mediating the interaction between nutrient intake and the gut-liver function. Meanwhile, the omega-3 and omega-6 polyunsaturated fatty acids (n-3/n-6 PUFA) as essential fatty acids have been linked to NAFLD. Increasing studies in the past decades have indicated that there is a reciprocal interaction between GM and n-3/n-6 PUFA, which may be underlying at least in part, the pathogenesis of NAFLD. In this review, we will discuss: 1) How GM is linked to NAFLD by interacting with various nutrients; 2) How imbalanced dietary n-3/n-6 PUFA is linked to NAFLD; 3) How n-3/n-6 PUFA may affect the GM balance, leading to altered nutrients release to the liver and 4) How GM may modify ingested n-3/n-6 PUFA, alter their absorption, bioavailability, and biotransformation.
Keywords: Gut microbiota, NAFLD, nutrient, n-3/n-6 PUFA
Non-alcoholic Fatty Liver Disease (NAFLD)
NAFLD is a spectrum of chronic liver disease initiated from hepatic steatosis without significant alcohol intake. Overtime without treatment, about 10–30% of hepatic steatosis can further develop into non-alcoholic steatohepatitis (NASH), characterized by inflammation and fibrosis. NASH is a high-risk factor for liver cirrhosis as well as hepatocellular carcinoma. NAFLD is highly correlated with other metabolic diseases, especially obesity and diabetes. Over the past decades, NAFLD has been a pandemic, with generally 20–30% of the population in the developed countries are affected (1).
The mechanism underlying the development of NASH is hypothesized as a “double-hit” theory. The first hit is sensitization of liver through steatosis and insulin resistance which further causes steatohepatitis. The second hit could be a multifaceted process including inflammation, oxidative stress, lipid peroxidation, and mitochondrial dysfunction (2). Unfortunately, there is currently no FDA-approved pharmacotherapy is available for NAFLD/NASH, which is largely attributed to the incomplete understanding about their etiology.
GM Is Linked to NAFLD by Mediating the Nutrients-Liver Connection
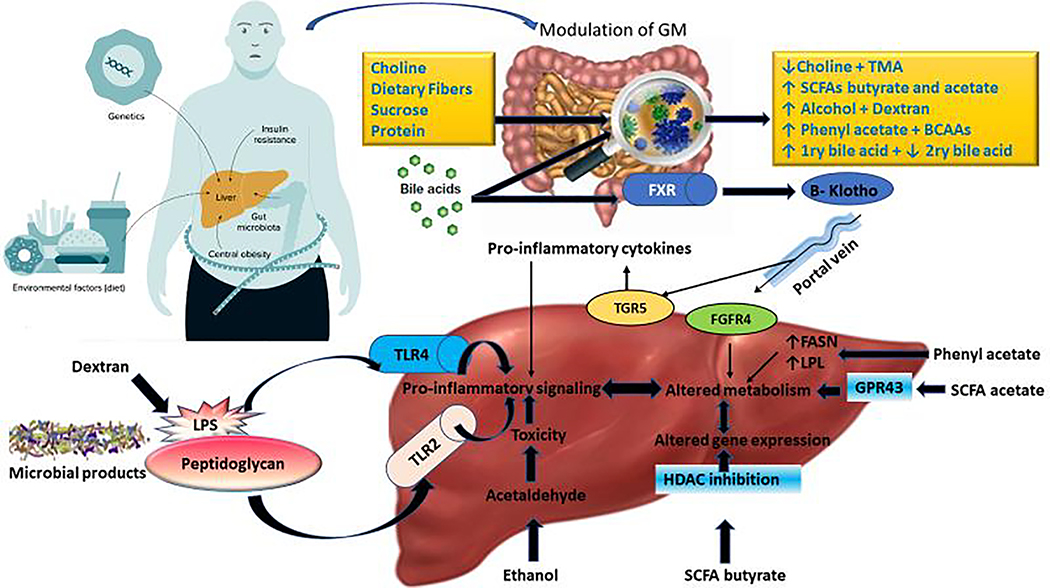
The gut microbiota (GM) is a distinct ecosystem consisting of a variety of microorganism such as bacteria, protozoa, archaea, fungi, and viruses, living in symbiosis with each other and with the human body as well. The role of GM on modulation of different exogenous and endogenous substrates can improve or worsen the progression of NAFLD/NASH. GM act upon these substrates releasing some metabolites affecting liver status subsequently (Fig.1). Exogenous substrates include, choline, carbohydrates, sucrose, protein and dietary fibers. Whereas, bile acid is one the endogenous substrates synthesized in liver and metabolized by GM. Interestingly, dextran a resultant from sucrose fermentation increased portal endotoxin lipopolysaccharide (LPS) levels and enhanced hepatic inflammation and fibrogenesis in experimental NASH by promoting proinflammatory gene expression (IL-1β, IL-17, TNF-α, etc.) (3). Recently, phenyl acetate and branched chain amino acids (BCAAs) (leucine, isoleucine and valine) released due to proteolytic cleavage of protein by Clostridium species are implicated in NASH pathogenesis (4). Phenyl acetate can upregulate expression of genes that are related to fat metabolism, which may lead to the accumulation of hepatic triglycerides, increasing the risk for liver steatosis (5). Gaggini et al, 2018 (6) observed a significant increase in level of BCAAs in patients with obesity and NAFLD. Particularly the BCAAs cause insulin resistance by reduction in rates of glycogen synthesis, where insulin resistance is one of the crucial factors for development of NASH (7). In contrast to this assumption, BCAAs attenuated hepatic steatosis and liver injury accompanied with NASH by inhibiting fatty acid synthase expression on gene and protein levels (8).
Figure 1:
Schematic explanation for the influence of different effectors on GM and its relation on the development of NAFLD. Microbiome-derived metabolites cause liver damage via several pathways including pro-inflammatory signaling, alteration in gene expression, and metabolic modification and toxicity. Bile acids interact with Farnesoid-X receptor (FXR) in the intestine releasing β-Klotho that enters liver via portal vein then binds FGFR4 on the surface of hepatocytes and leads to metabolic changes. Bile acids also stimulate the TGR5 receptor leading to the production of pro-inflammatory cytokines. Short-chain fatty acids (e.g. acetate) bind to their receptors (GPR43) and remodel metabolism; while butyrate inhibits histone deacetylase (HDAC) and regulates gene expression by changing overall chromatin state. Microbial products, i.e., peptidoglycan and LPS, cause pro-inflammatory signaling via Toll like receptors (TLR2 and TLR4). Phenylacetate increases gene expression of Fatty acid synthase (FASN) and lipoprotein lipase (LPL) by unknown receptors leading to metabolic changes. Ethanol is oxidized to acetaldehyde aggravates oxidative stress on the hepatocytes. Dextran increases gut permeability lead to over release of LPS to blood.
The Influence of PUFA on GM
Omega-3/omega-6 PUFA are essential fatty acids [namely, linoleic acid (n-6 C18:2) and alpha-linolenic acid (n-3 C18:3) as the precursors of long-chain PUFAs], relying on dietary intake in humans. While long-chain n-3 PUFAs [mainly eicosapentaenoic acid (EPA, n-3 C20:5) and docosahexaenoic acid (DHA, n-3 C22:6)] as well as their derived lipids, are mainly anti-inflammatory, long chain-6 PUFAs [mainly arachidonic acid (n-6 C20:4)] and their metabolites are pro-inflammatory. Therefore, a balanced intake of n-3/n-6 PUFA is essential for maintaining the health resilience. Over the past three decades, the ratio of n-6/n-3 PUFA has been dramatically altered particularly in the Modern Western diet, which leads to a significantly increase in the content of n-6 PUFAs (9). This change in the composition of PUFAs substantially alters the management of homeostasis and inflammation profile, increases the susceptibility to various metabolic disease, including NAFLD as well as overweight, obesity and insulin resistance which are contributing factors for NAFLD (Fig. 2).
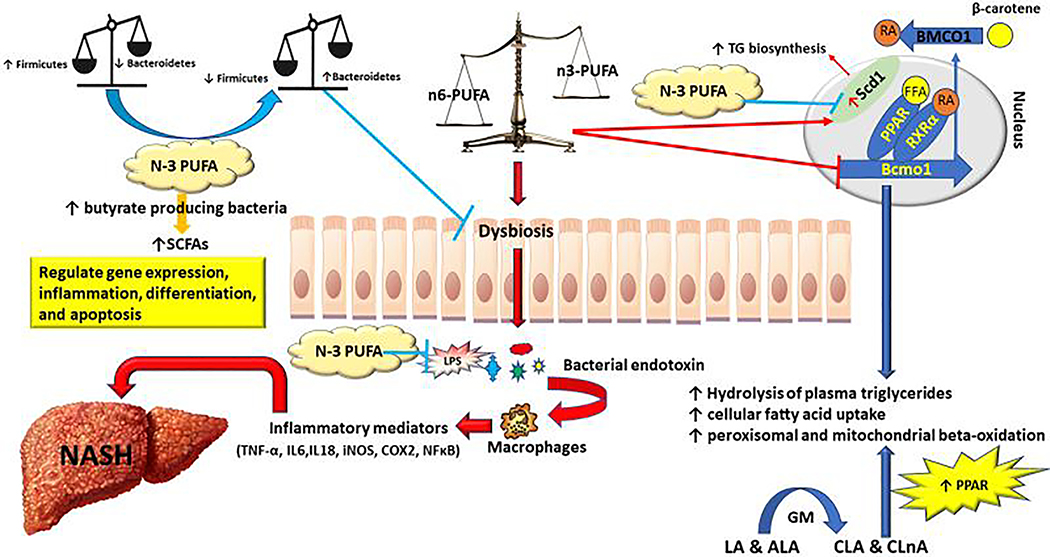
Figure 2:
Cross-link between PUFA and GM and their effect on development and progression of NASH. Abbreviations: GM, Gut microbiota; LA, Linoleic acid; ALA, α-linolenic acid; CLA, Conjugated linoleic acid; CALA, Conjugated α-linolenic acid; RA, Retinoic acid; FFA, Free fatty acid; RXRα, Retinoid X receptor alpha; PPAR, peroxisome proliferator-activated receptor; BCMO1, Beta-carotene oxygenase 1; SCFAs, Short chain fatty acids.
Besides the direct impact of n-3/n-6 PUFA on hepatic lipid metabolism, increased evidence has indicated that n-3/n-6 PUFA can also directly or indirectly influence the balance of GM, contributing to the development of NAFLD. Firmicutes and Bacteroidetes are two major phyla of the domain bacteria and dominant in human gut microbiota. It has been shown by multiple studies that the Firmicutes to Bacteroidetes (F/B ratio) is associated with obesity and other diseases. Overweight and obesity attributed to imbalanced n-3/n-6 PUFA intake can lead to GM dysbiosis especially a significant increase in F/B ratio. Beside this indirect effect that PUFA exerts through causing obesity, saturated fatty acid (SFA) and n-6 PUFAs may aggravate intestinal inflammation by creating dysbiosis via increasing F/B ratio, which can lead to increased activity of Toll like receptor (TLR) and nucleotide oligomerization domain receptors (NODs) pathways and increase intestinal permeability as a result of an alteration in tight junction protein. The increased intestinal permeability will in turn accumulate toxic bacterial products such as LPS and bacterial DNA in the hepatic portal circulation (10). Consequently, these products enhance liver expression of the pro-inflammatory cytokine through hepatic stellate cells (11). Studies have shown that n-3 PUFAs inhibit the expression of TLR4 (12) and NOD2 signaling by suppression of the release of NF-κB from MAPK (13). On the other hand, modified gut microbiome and improved dysbiosis through enrichment of Lactobacillus species and a reduction in bacteria of the Bacteroidaceae family after ingestion of n-3 PUFAs (14). N-3 PUFAs consumption leads to the repression of the production of LPS-induced proinflammatory cytokines in blood mononuclear cells in humans (15). Increased daily intake of 4g mixed n-3 PUFA (DHA and EPA) significantly increased the density of bacteria that is known to be butyrate producers (16). The butyrate producing bacteria plays a key role in maintaining human gut health by degradation of nonfermentable dietary fiber into short chain fatty acids (SCFAs) such as butyrate (Fig 1). Butyrate considered to be the fundamental source of energy to the colonic mucosa and control gene expression, inflammation, differentiation, and apoptosis in host cells (17). N-3 PUFA also alter the composition of cell membranes by replacing n-6 PUFAs, influencing lipid raft formation in cell signaling. Mice fed with high SFA diet (and high n-6 PUFA diet also observed an overflow of these fats to the distal part of the intestine. The increase of fats in intestine changed gut microbiota composition that complicates to obesity and hepatic steatosis via changes in particular lipid metabolism-related genes (18). Genetic changes include downregulation of Beta-carotene oxygenase 1 (BCMO-1) gene leads to triglycerides (TG) accumulation (19). On the other hand, there was upregulation of Stearoyl-CoA desaturase-1 (SCD1) a rate limiting enzyme in converting the SFAs to monounsaturated fatty acids (MUFAs). SCD1 plays a central role in the regulation of hepatic TG biosynthesis. Dietary supplementation with long-chain n-3 PUFA decreases SCD1 activity in liver microsomes and represses constitutive TG biosynthesis (20).
The Influence of GM on PUFA
Not only can n-3/n-6 PUFA affect the GM, but the GM can have direct impact on PUFA absorption, bioavailability, and biotransformation, which can further affect the PUFA intake imbalance as well as their function. It was found that gut microbes can produce PUFA-derived metabolites that could be a new kind of active metabolites. Several animal studies have shown that microbes play a crucial rule in biotransformation of PUFA. Some microbial species e.g. Butyrivibrio fibrisolvens, Clostridium proteoclasticum or Lactobacillus plantarum can convert the n-3/n-6 PUFA precursors ALA and LA into CLA (conjugated linoleic acids) and CALA (conjugated α-linolenic acids) which undergo further hydrogenation to saturated fatty acid (stearic acid, C18:0) (21), thus reducing the PUFA components. An abundance of some Clostridium species was observed in mice fed on high cholesterol containing diet and associated with the development of severe NASH (22). CLA plays an important role in lipid metabolism as a potent peroxisome proliferator-activated receptor (PPAR)-α agonist an important modulator for lipid metabolism. Indeed, CLA treatment increases the catabolism of lipids in the liver of rodents (23). The saturation of n-3/n-6 PUFA is catalyzed by the action of four enzymes which are CLA hydratase (CLA-HY), CLA dehydrogenase (CLA-DH), CLA isomerase (CLA-DC), and CLA enoate reductase (CLA-ER) These enzymes generate more saturated products e.g. trans-10-octadecenoic acid and oleic acid from linoleic acid). The genes that encode CLA-DH, CLA-DC, and CLA-ER form a gene cluster in L. plantarum. Furthermore, many species of lactic acid bacteria have one or more of these four genes (24). Moreover, the derivatives released during saturation processes such as 9-oxo-10,12-octadecadienoic acid (9-oxo-ODA) generally serves as a PPARα agonist. In mouse primary hepatocytes, 9-oxo-ODA enhanced fatty acid oxidation via PPARα activation and consequently inhibited triglycerides accumulation (23). Multiple studies have demonstrated that Lactobacillus rhamnosus GG (LGG) supplementation has been found to ameliorate non-alcoholic fatty liver disease (NAFLD) (25).
Future Perspective
Increased literature has indicated that n-3/n-6 PUFA and GM have a reciprocal interaction, which may be further involved in at least in part, the pathogenesis of NAFLD. Future study should be focused on the causal relationship between GM, n3/n-6 PUFA and NAFLD. This is of particularly importance on laying the foundation for developing precision treatment or dietary supplementation to treat or prevent NAFLD.
Acknowledgments
This work is supported in part by NIH/NIDDK R01 DK106540) (W.L.). S.S. is a visiting scholar supported by the Egyptian Ministry of Higher Education and the Egyptian Cultural Affairs and Missions Sector, Egypt.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- 1).Lazo M, Hernaez R, Eberhardt MS, et al. Prevalence of non-alcoholic fatty liver disease in the United States: The Third National Health and Nutrition Examination Survey, 1988–1994. Am J Epidemiol. 2013; 178:38–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2).Adams LA, Lymp JF, St. Sauver J, et al. The natural history of non-alcoholic fatty liver disease: a population based cohort study. Gastroenterology. 2005; 129:113–121. [DOI] [PubMed] [Google Scholar]
- 3).Gäbele E, Dostert K, Hofmann C, et al. DSS induced colitis increases portal LPS levels and enhances hepatic inflammation and fibrogenesis in experimental NASH. J Hepatol. 2011; 55: 1391–1399. [DOI] [PubMed] [Google Scholar]
- 4).Kolodziejczyk A, Zheng D, Shibolet O, et al. The role of the microbiome in NAFLD and NASH. Mol. Med. 2019; 11: e9302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5).Hoyles L, Fernández-Real J-M, Federici M, et al. Molecular phenomics and metagenomics of hepatic steatosis in non-diabetic obese women. Nat. Med. 2018; 24: 1070–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6).Gaggini M, Carli F, Rosso C, et al. Altered amino acid concentrations in NAFLD: impact of obesity and insulin resistance. Hepatology 2018; 67: 145–15.8 [DOI] [PubMed] [Google Scholar]
- 7).Krebs M, Krssak M, Bernroider E, et al. Mechanism of Amino Acid–Induced Skeletal Muscle Insulin Resistance in Humans. Diabetes 2002; 51: 600–605. [DOI] [PubMed] [Google Scholar]
- 8).Hondaa T, Ishigamia M, Ishigami M, et al. Branched-chain amino acids alleviate hepatic steatosis and liver injury in choline-deficient high-fat diet induced NASH mice. Metab Clin Exp. 2017; 69: 177–187. [DOI] [PubMed] [Google Scholar]
- 9).Patterson E, Wall R., Fitzgerald F et al. Health Implications of High Dietary Omega-6 Polyunsaturated Fatty Acids. J Nutr Metab. 2012; 2012: 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10).Lee JY, Plakidas A, Lee WH, et al. Differential modulation of Toll-like receptors by fatty acids: Preferential inhibition by n-3 polyunsaturated fatty acids. J. Lipid Res. 2003; 44: 479–486. [DOI] [PubMed] [Google Scholar]
- 11).Gupta G, Khadema F, Uzonnaab JE., et al. Role of hepatic stellate cell (HSC)-derived cytokines in hepatic inflammation and immunity. Cytokines 2019; 24:154542. [DOI] [PubMed] [Google Scholar]
- 12).Ibrahim A, Mbodji K, Hassan A, et al. Anti-inflammatory and anti-angiogenic effect of long chain n-3 polyunsaturated fatty acids in intestinal microvascular endothelium. Clin. Nutr. 2011; 30:678–687. [DOI] [PubMed] [Google Scholar]
- 13).Allam-Ndoul B, Guénard F, Barbier O, et al. Effect of n-3 fatty acids on the expression of inflammatory genes in THP-1 macrophages. Lipids Health Dis. 2016; 15: 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14).Prossomariti A, Scaioli E, Piazzi G, et al. Short-term treatment with eicosapentaenoic acid improves inflammation and affects colonic differentiation markers and microbiota in patients with ulcerative colitis. Sci. Rep. 2017; 7:7458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15).Scaioli E, Liverani E, and Belluzz A, et al. The Imbalance between n-6/n-3 Polyunsaturated Fatty Acids and Inflammatory Bowel Disease: A comprehensive review and future therapeutic perspectives. Int J Mol Sci. 2017; 18(12): 2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16).Watson H, Mitra S, Croden F, et al. A randomised trial of the effect of omega-3 polyunsaturated fatty acid supplements on the human intestinal microbiota. Gut 2018; 67:1974–1983. [DOI] [PubMed] [Google Scholar]
- 17).Cushing K, Alvarado D, Ciorba M. Butyrate and Mucosal Inflammation: New Scientific Evidence Supports Clinical Observation. Clin Transl Gastroenterol. 2015; 6(8): e108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18).De Wit N, Derrien M, Bosch-Vermeulen H, et al. Saturated fat stimulates obesity and hepatic steatosis and affects gut microbiota composition by an enhanced overflow of dietary fat to the distal intestine. Am J Physiol Gastrointest Liver Physiol. 2012; 303: G589–G599. [DOI] [PubMed] [Google Scholar]
- 19).Hessel S, Eichinger A, Isken A, et al. CMO1 deficiency abolishes vitamin A production from β-carotene and alters lipid metabolism in mice. J Biol Chem. 2007; 282: 33553–33561. [DOI] [PubMed] [Google Scholar]
- 20).Paton CM, Ntambi JM. Biochemical and physiological function of stearoyl-CoA desaturase. Am J Physiol Endocrinol Metab. 2009; 297: E28–E37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21).Jenkins TC, Wallace RJ, Moate PJ, et al. Board-invited review: Recent advances in biohydrogenation of unsaturated fatty acids within the rumen microbial ecosystem. J Anim Sci. 2008; 86:397–412. [DOI] [PubMed] [Google Scholar]
- 22).Yamada S, Kamada N, Amiya T, et al. Gut microbiota-mediated generation of saturated fatty acids elicits inflammation in the liver in murine high-fat diet-induced steatohepatitis. Gastroenterology 2017; 17:136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23).Kim Y, Hirai S, Goto T. Potent PPARa Activator Derived from Tomato Juice, 13-oxo-9,11-Octadecadienoic Acid, Decreases Plasma and Hepatic Triglyceride in Obese Diabetic Mice. PLoS ONE. 2012; 7(2): e31317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24).Kishino S, Takeuchi M, Park SB, et al. Polyunsaturated fatty acid saturation by gut lactic acid bacteria affecting host lipid composition. PNAS. 2013; 110 (44):17808–17813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25).Ritze Y, Bárdos G, Claus A, et al. Lactobacillus rhamnosus GG protects against non - alcoholic fatty Liver Disease in Mice. PLoS One. 2014; 9(1): e80169. [DOI] [PMC free article] [PubMed] [Google Scholar]