Abstract
BACKGROUND:
Regulatory agencies warn about the risk of acute kidney injury (AKI) after the initiation of sodium-glucose cotransporter-2 (SGLT2) inhibitors. Our objective was to quantify the 90-day risk of AKI in older adults after initiation of SGLT2 inhibitors in routine clinical practice.
METHODS:
We conducted a population-based retrospective cohort study in Ontario, Canada, involving adults with diabetes who were aged 66 years or older and who were newly dispensed either an SGLT2 inhibitor or a dipeptidyl peptidase-4 (DPP4) inhibitor in an outpatient setting between 2015 and 2017. We used inverse probability of treatment weighting based on a propensity score to balance the 2 groups on measured baseline characteristics. The primary outcome was 90-day risk of a hospital encounter (i.e., visit to the emergency department or admission to hospital) with AKI, which we defined by a 50% or greater increase in the concentration of serum creatinine from the baseline value or an absolute increase of at least 27 μmol/L after an SGLT2 or DDP4 inhibitor was dispensed. We obtained weighted risk ratios using modified Poisson regression and weighted risk differences using binomial regression.
RESULTS:
We included 39 094 patients with a median age of 70 (interquartile range 68–74) years in the study. Relative to new use of a DPP4 inhibitor, initiation of a SGLT2 inhibitor was associated with a lower 90-day risk of a hospital encounter with AKI: 216 events in 19 611 patients (1.10%) versus 388 events in 19 483 patients (1.99%); weighted risk ratio 0.79 (95% confidence interval 0.64–0.98).
INTERPRETATION:
In routine care of older adults, new use of SGLT2 inhibitors compared with use of DPP4 inhibitors was associated with a lower risk of AKI. Together with previous evidence, our findings suggest that regulatory warnings about AKI risk with SGLT2 inhibitors are unwarranted.
Sodium–glucose cotransporter-2 (SGLT2) inhibitors became available to treat type 2 diabetes in Canada in 2014. There are 4 SGLT2 inhibitors available: canagliflozin, dapagliflozin, empagliflozin and ertugliflozin.1 In addition to effectively lowering blood glucose levels, they also prevent adverse cardiovascular events.2–5
In October 2015 and June 2016 (summarized in Appendix 1A, available at www.cmaj.ca/lookup/suppl/doi:10.1503/cmaj.191283/-/DC1) Health Canada and the United States Food and Drug Administration issued safety warnings about the risk of acute kidney injury (AKI) after initiation of canagliflozin and dapagliflozin, based on evidence from case reports.6,7 These safety warnings led to changes in the drug product monographs to include information about the risk of AKI shortly after initiation. There is a plausible mechanism for AKI induced by SGLT2 inhibitors. By interfering with the co-uptake of glucose and sodium in the proximal nephron, SGLT2 inhibitors can increase sodium delivery to the distal nephron, which can result in afferent arteriole vasoconstriction and an associated reduction in estimated glomerular filtration rate (eGFR).8–12 Even so, recent clinical trials and results from 3 population-based studies suggest either no increase or a decrease in AKI risk after initiation of SGLT2 inhibitors (summarized in Appendix 1B).2–5,8,13–15
Current guidance for clinicians on appropriate use of SGLT2 inhibitors in routine clinical practice includes counselling patients not to take the drug during an acute illness.16 However, patients in routine clinical practice are generally monitored less often and have more comorbidity than patients in clinical trials.17 This may result in a potential underestimate of safety, as has been observed with limb amputation in some observational studies of SGLT2 inhibitors.15,18
We conducted this study to examine the 90-day risk of a hospital encounter (defined as a visit to the emergency department or admission to hospital) for AKI in older adults with diabetes who were newly dispensed an SGLT2 inhibitor or a dipeptidyl peptidase-4 (DPP4) inhibitor in an outpatient setting. We selected the DPP4 inhibitor as a comparator drug to reduce concerns about confounding by indication because DPP4 inhibitors are also frequently used in addition to insulin or metformin for diabetes care but, unlike SGLT2 inhibitors, they have no known risk of AKI.19,20
Methods
Study design, setting and population
We conducted a population-based retrospective cohort study of adults aged 66 years or older in Ontario, Canada, between July 1, 2015, and Sept. 30, 2017. We used linked health care databases at ICES, a not-for-profit research institute. At present, Ontario has more than 14 million residents, 17% of whom are aged 65 years or older.21 Ontario residents are covered by publicly funded health insurance for hospital and physician care (Ontario Health Insurance Plan [OHIP]). Those 65 years of age and older receive prescription drug coverage through the Ontario Drug Benefit program (about 2.4 million residents).21 We have used these data sources to study associations between other drugs and the risk of AKI.22–25 In this study we followed reporting guidelines for observational pharmacoepidemiology studies (Appendix 1C).26
Sources of data
Data sets used for this study are presented in Appendix 1D, and information about which variables came from each data set can be found in Appendix 1E. These data sets were linked using unique encoded identifiers and analyzed at ICES.
Our data sources were complete for all study variables except for prescriber specialty (< 10% missing), rural residence (< 0.5% missing) and neighbourhood income quintile (< 0.5% missing).
We classified missing prescriber specialty as “missing,” missing rural status as nonrural and imputed the third income quintile for missing income status. Emigration from Ontario is less than 0.1% per year and was the only reason for lost follow-up.27 We created a cohort of adults aged 66 years or older in Ontario who were newly dispensed an SGLT2 inhibitor (i.e., canagliflozin, empagliflozin or dapagliflozin) or a DPP4 inhibitor (i.e., saxagliptin, sitagliptin or linagliptin) between July 1, 2015 (the earliest date that SGLT2 inhibitors were available through the Ontario Drug Benefit Plan),28 and Sept. 30, 2017. All drugs were available as General Benefit products in Ontario during the period of study. The dispensing date of their first eligible prescription during the accrual period was considered the cohort entry or index date. We included those aged 66 years or older to establish complete medication history and ensure they were not in their first eligibility year for prescription drug coverage (age 65 years). Study participants were assigned to geographic hospital catchment areas with corresponding linked laboratory data, using previously published methods.29 We included only Ontarians who resided within these catchment areas to ensure accurate ascertainment of outcomes, as not all hospital-based laboratories started contributing to Ontario Laboratories Information System (OLIS) at the same time and, to date, not all are contributing.
To ascertain outcomes accurately for participants in our cohort, we ensured that participants resided within areas serviced by OLIS, so that they would receive serum creatinine tests in hospitals captured in our data source.29 In terms of accuracy and completeness, OLIS values for serum creatinine levels are preferable compared with diagnostic codes, and once a hospital begins contributing to OLIS, the database should have complete capture of all test results. For baseline serum creatinine measurement, we selected an outpatient value (measured by outpatient community or hospital laboratories) within the past year that was closest to their index date. Patients were excluded if their baseline eGFR value was below 45 mL/min per 1.73 m2, as SGLT2 inhibitors were contraindicated in Ontario for patients with a lower eGFR during the study period.30 To define new use, we required that patients be free of the drugs under study for at least 180 days before the index date and evaluated the first such exposure during the accrual period.
We excluded participants as follows: patients with a prescription for more than 1 type of DPP4 or SGLT2 inhibitor on the index date to compare mutually exclusive groups; patients residing in long-term care residences, because these individuals are inherently different than the general population in terms of disease and medication management;31 patients discharged from a hospital in the 2 days before the index date, to ensure new outpatient prescriptions because patients who start treatment in hospital typically fill ongoing prescriptions on the discharge date or the day after; and patients with nonstandard daily doses of drugs for treatment of diabetes to ensure generalizability to usual prescribing; Appendix 1F).32
Outcomes
Our primary outcome was a hospital encounter (i.e., admission to hospital or presentation to the emergency department) with AKI, defined by thresholds from the 2012 Kidney Disease: Improving Global Outcomes (KDIGO) working group: a 50% or greater increase in the concentration of serum creatinine over baseline or an absolute increase of at least 27 μmol/L (0.3 mg/dL).33 We considered the baseline value to be the most recent value for serum creatinine in the outpatient setting within the past year. We compared this baseline value to the highest hospital-based value for serum creatinine in the 90 days after entry into the cohort. We chose a 90-day follow-up period based on previous evidence showing that SGLT2 inhibitors lead to a decline in eGFR soon after the drug is started.5,8
As secondary outcomes, we assessed admission to hospital with AKI and a hospital encounter with moderate to severe AKI (i.e., increase in serum creatinine level that meets the KDIGO threshold of stage 2 or more; Appendix G).33 We also examined evidence of AKI restricted to the outpatient setting and in any setting (i.e., outpatient, in hospital or emergency department).
We conducted 7 additional analyses to assess the robustness of our results: assessing for possible surveillance bias, assessing absolute and relative changes in serum levels of creatinine, conducting subgroup analyses to understand potential risks associated with SGLT2 inhibitors in patients at higher risk of AKI34–40 (Appendix 1H), analyzing the 90-day risk of a hospital encounter with AKI defined using database diagnostic codes, performing a survival analysis of the primary outcome within 365 days of follow-up, evaluating the 90-day risk of a hospital encounter with bowel obstruction as a negative control outcome and an E-value sensitivity analysis to assess the effect of unmeasured confounding.41,42
Statistical analysis
We conducted all analyses using SAS version 9.4 (SAS Institute). We used DPP4 inhibitors as the referent group for all analyses. We compared baseline characteristics between those participants who were newly dispensed SGLT2 and DPP4 inhibitors using standardized differences, for which a threshold of 10% or greater was considered a meaningful difference.43 We used inverse probability of treatment weighting based on propensity scores, using a multivariable logistic regression model with 97 baseline characteristics (including indications for SGLT2 inhibitor use and risk factors for AKI; Appendix 1I). We chose this weighting over propensity-score matching to achieve the largest possible study cohort.44 We used weights to estimate the average treatment effect in the treated group (SGLT2 inhibitor users). Patients in the reference group (DPP4 inhibitor users) were weighted as [propensity score/(1 – propensity score)]. This method produces a weighted pseudosample of patients in the reference group with the same distribution of measured covariates as the exposure group.44–47 We used modified Poisson regression for all primary and secondary outcomes to estimate weighted risk ratios (RRs) and 95% confidence intervals (CIs).48 We used binomial regression with an identity link function to estimate weighted risk differences (RDs) between the groups and 95% CIs. Interaction p values in the subgroup analyses were determined by including interaction terms in the modified Poisson regression models. We performed a survival analysis of the primary outcome within 365 days of follow-up using Cox proportional hazards regression, censoring on death and treating death as a competing risk. We considered 2-tailed p values less than 0.05 statistically significant for all outcomes.
Ethics approval
The use of data in this project was authorized under section 45 of Ontario’s Personal Health Information Protection Act, which does not require review by a Research Ethics Board.
Results
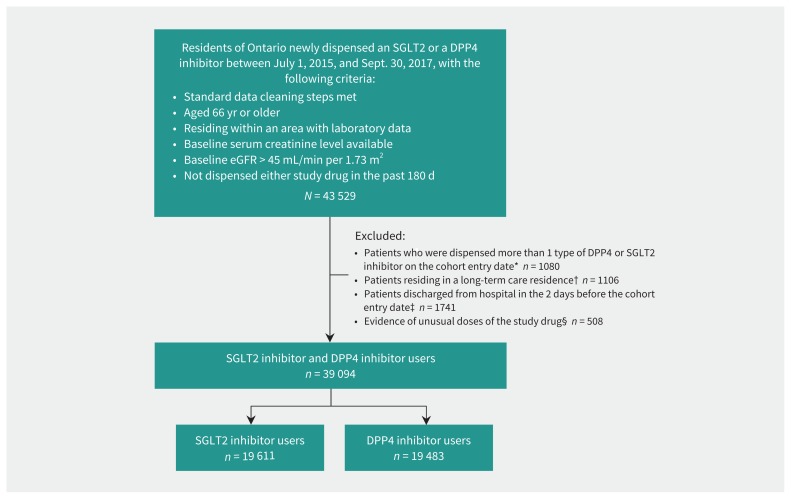
After exclusions, we had 19 611 SGLT2 inhibitor users and 19 483 DPP4 inhibitor users (Figure 1 shows the cohort assembly). Forty-eight percent (n = 9404) of SGLT2 inhibitor users were dispensed canagliflozin, 37.3% (n = 7311) empagliflozin and 14.8% (n = 2896) dapagliflozin. The median doses were 100 (interquartile range [IQR] 100–300) mg/d for canagliflozin, 10 (IQR 10–10) mg/d for empagliflozin and 10 (IQR 5–10) mg/d for dapagliflozin. For DPP4 inhibitor users, 67.2% (n = 13 086) were dispensed sitagliptin, 24.3% (n = 4726) linagliptin and 8.6% (n = 1671) saxagliptin. Mean age of participants was 71.4 (SD 4.9) years and 7903 (40.3%) were women. Baseline creatinine was measured a median of 28 (IQR 9-89) days before study entry in SGLT2 inhibitor users and a median of 23 (IQR 8–21) days before study entry in DPP4 inhibitor users. Table 1 provides selected baseline characteristics; the full list of baseline characteristics can be found in the table in Appendix 1J.
Figure 1:
Cohort assembly for patients in the sodium–glucose cotransporter-2 (SGLT2) inhibitor user group and the comparator dipeptidyl peptidase-4 (DPP4) inhibitor user group. Note: eGFR = estimated glomerular filtration rate. *To ensure 2 mutually exclusive groups. †Patients were inherently different than the general population in terms of medication management. ‡To ensure new outpatient prescriptions. §To ensure generalizability to usual prescribing.
Table 1:
Baseline characteristics of participants who were newly dispensed sodium–glucose cotransporter-2 inhibitors and dipeptidyl peptidase-4 inhibitors in Ontario (2015–2017)
| Characteristic* | Observed data | Weighted data† | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| No. (%) of participants‡ | Standardized difference,§ % | No. (%) of participants‡ | Standardized difference,§ % | |||
|
|
|
|||||
| Using SGLT2 inhibitors n = 19 611 | Using DPP4 inhibitors n = 19 483 | Using SGLT2 inhibitors n = 19 611 | Using DPP4 inhibitors n = 19 775 | |||
| Demographic | ||||||
|
| ||||||
| Age, yr; mean ± SD | 71.4 ± 4.9 | 74.1 ± 6.3 | 47 | 71.4 ± 4.9 | 71.4 ± 5.0 | 1 |
|
| ||||||
| Sex, female | 7903 (40.3) | 9325 (s47.9) | 15 | 7903 (40.3) | 8104 (41.0) | 1 |
|
| ||||||
| Rural residence¶ | 2192 (11.2) | 2088 (10.7) | 2 | 2192 (11.2) | 2423 (12.3) | 3 |
|
| ||||||
| Neighbourhood income quintile** | ||||||
|
| ||||||
| 1 (lowest) | 4350 (22.2) | 4566 (23.4) | 3 | 4350 (22.2) | 4397 (22.2) | 0 |
|
| ||||||
| 2 | 4236 (21.6) | 4390 (22.5) | 2 | 4236 (21.6) | 4328 (21.9) | 1 |
|
| ||||||
| 3 | 4011 (20.5) | 3953 (20.3) | 0 | 4044 (20.6) | 4047 (20.5) | 0 |
|
| ||||||
| 4 | 3679 (18.8) | 3513 (18.0) | 2 | 3679 (18.8) | 3683 (18.6) | 1 |
|
| ||||||
| 5 (highest) | 3302 (16.8) | 3043 (15.6) | 3 | 3302 (16.8) | 3321 (16.8) | 0 |
|
| ||||||
| Prescriber specialty | ||||||
|
| ||||||
| Cardiology | 413 (2.1) | 108 (0.6) | 13 | 413 (2.1) | 506 (2.6) | 3 |
|
| ||||||
| Endocrinology | 3786 (19.3) | 1475 (7.6) | 35 | 3786 (19.3) | 3574 (18.1) | 3 |
|
| ||||||
| Family medicine | 12 798 (65.3) | 15 685 (80.5) | 35 | 12 798 (65.3) | 12 927 (65.4) | 0 |
|
| ||||||
| Internal medicine | 1139 (5.8) | 540 (2.8) | 15 | 1139 (5.8) | 1232 (6.2) | 2 |
|
| ||||||
| Nephrology | 217 (1.1) | 97 (0.5) | 7 | 217 (1.1) | 234 (1.2) | 1 |
|
| ||||||
| Other | 167 (0.9) | 317 (1.6) | 6 | 167 (0.9) | 171 (0.9) | 0 |
|
| ||||||
| Missing | 1091 (5.6) | 1261 (6.5) | 4 | 1091 (5.6) | 1131 (5.7) | 0 |
|
| ||||||
| Comorbidity†† | ||||||
|
| ||||||
| Duration of diabetes, yr; mean ± SD | 13.8 ± 6.9 | 12.0 ± 7.2 | 25 | 13.8 ± 6.9 | 13.8 ± 7.1 | 1 |
|
| ||||||
| Diabetic retinopathy | 168 (0.9) | 140 (0.7) | 2 | 168 (0.9) | 172 (0.9) | 0 |
|
| ||||||
| Diabetic neuropathy | 231 (1.2) | 257 (1.3) | 1 | 231 (1.2) | 223 (1.1) | 1 |
|
| ||||||
| Hypoglycemia | 115 (0.6) | 185 (0.9) | 3 | 115 (0.6) | 127 (0.6) | 0 |
|
| ||||||
| Hyperglycemic emergency | 82 (0.4) | 47 (0.2) | 4 | 75 (0.4) | 47 (0.2) | 4 |
|
| ||||||
| Previous acute kidney injury | 351 (1.8) | 702 (3.6) | 11 | 351 (1.8) | 395 (2.0) | 1 |
|
| ||||||
| Previous acute urinary retention | 252 (1.3) | 452 (2.3) | 8 | 252 (1.3) | 237 (1.2) | 1 |
|
| ||||||
| Chronic lung disease | 3885 (19.8) | 3976 (20.4) | 1 | 3885 (19.8) | 4049 (20.5) | 2 |
|
| ||||||
| Cancer (including skin) | 5586 (28.5) | 5987 (30.7) | 5 | 5586 (28.5) | 5579 (28.2) | 1 |
|
| ||||||
| Stroke | 270 (1.4) | 556 (2.9) | 10 | 270 (1.4) | 256 (1.3) | 1 |
|
| ||||||
| Atrial fibrillation | 717 (3.7) | 930 (4.8) | 5 | 717 (3.7) | 702 (3.5) | 1 |
|
| ||||||
| Coronary artery bypass graft surgery | 513 (2.6) | 372 (1.9) | 5 | 513 (2.6) | 514 (2.6) | 0 |
|
| ||||||
| Percutaneous coronary intervention | 1051 (5.4) | 777 (4.0) | 7 | 1051 (5.4) | 1010 (5.1) | 1 |
|
| ||||||
| Pacemaker | 543 (2.8) | 561 (2.9) | 1 | 543 (2.8) | 518 (2.6) | 1 |
|
| ||||||
| Congestive heart failure | 1649 (8.4) | 1876 (9.6) | 4 | 1649 (8.4) | 1674 (8.5) | 0 |
|
| ||||||
| Chronic liver disease | 947 (4.8) | 978 (5.0) | 1 | 947 (4.8) | 916 (4.6) | 1 |
|
| ||||||
| Peripheral vascular disease | 202 (1.0) | 218 (1.1) | 1 | 202 (1.0) | 188 (1.0) | 0 |
|
| ||||||
| Hypertension | 15 302 (78.0) | 13 528 (69.4) | 20 | 15 302 (78.0) | 15 477 (78.3) | 1 |
|
| ||||||
| Previous urinary tract infection | 578 (2.9) | 1015 (5.2) | 12 | 578 (2.9) | 661 (3.3) | 2 |
|
| ||||||
| Charlson Comorbidity Index score‡‡ | ||||||
|
| ||||||
| 0 | 16 722 (85.3) | 15 676 (80.5) | 13 | 16 722 (85.3) | 16 998 (86.0) | 2 |
|
| ||||||
| 1 | 943 (4.8) | 1147 (5.9) | 5 | 943 (4.8) | 852 (4.3) | 2 |
|
| ||||||
| 2 | 862 (4.4) | 1044 (5.4) | 5 | 862 (4.4) | 862 (4.4) | 0 |
|
| ||||||
| 3 | 1084 (5.5) | 1616 (8.3) | 11 | 1084 (5.5) | 1063 (5.4) | 0 |
|
| ||||||
| Medication§§ | ||||||
|
| ||||||
| ACE inhibitor | 7155 (36.5) | 6128 (31.5) | 11 | 7155 (36.5) | 7271 (36.8) | 1 |
|
| ||||||
| ARB | 4754 (24.2) | 4095 (21.0) | 8 | 4754 (24.2) | 4856 (24.6) | 1 |
|
| ||||||
| ACE or ARB | 11 796 (60.1) | 10 124 (52.0) | 16 | 11 796 (60.1) | 12 008 (60.7) | 1 |
|
| ||||||
| ASA¶¶ | 436 (2.2) | 395 (2.0) | 1 | 436 (2.2) | 497 (2.5) | 2 |
|
| ||||||
| β-Blocker | 6427 (32.8) | 5679 (29.1) | 8 | 6427 (32.8) | 6442 (32.6) | 0 |
|
| ||||||
| Calcium-channel blocker | 6167 (31.4) | 5540 (28.4) | 7 | 6167 (31.4) | 6205 (31.4) | 0 |
|
| ||||||
| NSAID*** | 2076 (10.6) | 1684 (8.6) | 7 | 2076 (10.6) | 2144 (10.8) | 1 |
|
| ||||||
| Statin | 14 887 (75.9) | 12 257 (62.9) | 28 | 14 887 (75.9) | 15 031 (76.0) | 0 |
|
| ||||||
| Proton pump inhibitor | 4264 (21.7) | 4137 (21.2) | 1 | 4264 (21.7) | 4352 (22.0) | 1 |
|
| ||||||
| Any diuretic | 4240 (21.6) | 4231 (21.7) | 0 | 4240 (21.6) | 4460 (22.6) | 2 |
|
| ||||||
| Hypoglycemic medication§§ | ||||||
|
| ||||||
| Insulin | 5229 (26.7) | 2508 (12.9) | 35 | 5229 (26.7) | 5582 (28.2) | 3 |
|
| ||||||
| Acarbose | 366 (1.9) | 141 (0.7) | 11 | 366 (1.9) | 447 (2.3) | 3 |
|
| ||||||
| Gliclazide | 6606 (33.7) | 4385 (22.5) | 25 | 6606 (33.7) | 6870 (34.7) | 2 |
|
| ||||||
| Glyburide | 719 (3.7) | 1004 (5.2) | 7 | 719 (3.7) | 740 (3.7) | 0 |
|
| ||||||
| Metformin | 15 765 (80.4) | 12 738 (65.4) | 34 | 15 765 (80.4) | 15 837 (80.1) | 1 |
|
| ||||||
| Repaglinide | 6 (0.0) | 10 (0.1) | 4 | 6 (0.0) | 23 (0.1) | 4 |
|
| ||||||
| Rosiglitazone maleate | 13 (0.1) | 16 (0.1) | 0 | 13 (0.1) | 12 (0.1) | 0 |
|
| ||||||
| Pioglitazine | 100 (0.5) | 104 (0.5) | 0 | 100 (0.5) | 108 (0.5) | 0 |
|
| ||||||
| Laboratory test result‡‡‡ | ||||||
|
| ||||||
| Baseline eGFR,§§§ mL/min/1.73 m2; mean ± SD | 77 ± 14 | 73 ± 16 | 26 | 77 ± 14 | 77 ± 16 | 0 |
|
| ||||||
| eGFR > 60 mL/min/1.73 m2 | 16 786 (86) | 14 405 (74) | 29 | 16 786 (86) | 16 009 (81) | 12 |
|
| ||||||
| eGFR = 45–60 mL/min/1.73 m2 | 2825 (14.4) | 5078 (25.9) | 29 | 2825 (14.4) | 3766 (19.0) | 12 |
|
| ||||||
| Baseline serum creatinine, μmol/L; mean ± SD | 79.6 ± 18.1 | 81.2 ± 20.2 | 8 | 79.6 ± 18.1 | 79.7 ± 20.3 | 0 |
|
| ||||||
| Potassium data available | 5556 (28.3) | 7072 (36.3) | 17 | 5556 (28.3) | 6110 (30.9) | 6 |
|
| ||||||
| Baseline potassium, mEq/L; mean ± SD | 4.5 ± 0.4 | 4.4 ± 0.5 | 13 | 4.5 ± 0.4 | 4.5 ± 0.4 | 7 |
|
| ||||||
| Glycosylated hemoglobin available | 6516 (33.2) | 8071 (41.4) | 17 | 6516 (33.2) | 7288 (36.9) | 8 |
|
| ||||||
| Glycosylated hemoglobin, %; mean ± SD | 7.8 ± 1.2 | 7.7 ± 1.3 | 12 | 7.8 ± 1.2 | 7.8 ± 1.2 | 2 |
|
| ||||||
| Urine ACR available | 14 637 (74.6) | 12 381 (63.5) | 24 | 14 637 (74.6) | 14 240 (72.0) | 6 |
|
| ||||||
| Baseline ACR category, mg/mmol | ||||||
|
| ||||||
| Undetected | 9424 (48.1) | 7903 (40.6) | 15 | 9424 (48.1) | 9129 (46.2) | 4 |
|
| ||||||
| 3–30 | 4263 (21.7) | 3729 (19.1) | 6 | 4263 (21.7) | 4288 (21.7) | 0 |
|
| ||||||
| > 30 | 950 (4.8) | 749 (3.8) | 5 | 950 (4.8) | 823 (4.2) | 3 |
Note: ACE = angiotensin-converting-enzyme, ACR = albumin-to-creatinine ratio, ARB = angiotensin receptor blocker, ASA = acetylsalicylic acid, DPP4 = dipeptidyl peptidase-4, ED = emergency department, eGFR = estimated glomerular filtration rate, NSAID = nonsteroidal anti-inflammatory drug, SD = standard deviation, SGLT2 = sodium–glucose cotransporter-2.
Unless otherwise specified, we assessed baseline characteristics on the date that the patient filled their prescription (the cohort entry date).
Weighted using inverse probability of treatment weighting based on propensity scores, using weights to estimate the average treatment effect in the treated group. Patients in the reference group were weighted as [propensity score/(1 – propensity score)]. This method produces a weighted pseudosample of patients in the reference group with the same distribution of measured covariates as the exposure group.44–46 When we evaluated plots of the distribution of propensity scores before and after weighting, there was sufficient overlap between the 2 groups before weighting (summarized in Appendices 1R and 1S, available at www.cmaj.ca/lookup/suppl/doi:10.1503/cmaj.191283/-/DC1).
Binary variables are presented as number and percentage, and continuous variables as mean ± SD (exception: health care use, median [IQR]).
The difference between the groups divided by the pooled SD: a value greater than 10% is interpreted as a meaningful difference.43
Rural residence was defined as a population of < 10 000 people. Residential information was not available for 33 (0.2%) SGLT2 inhibitor users and 18 (0.1%) DPP4 inhibitor users in the unweighted cohort. We reclassified missing values in the unweighted cohort into the “Not rural” category during weighting.
Income was categorized into fifths of average neighbourhood income on the cohort entry date.
The prevalence of comorbidities was defined using a 5-year look-back period before the cohort entry date.
We calculated the Charlson Comorbidity Index score49,50 using 5 years of data for admissions to hospital. “No admissions to hospital” received a score of 0.
Use of medications and hypoglycemic agents was evaluated in the 120-day period before the cohort entry date (the Ontario Drug Benefit program dispenses a maximum 100-day supply).
Includes dispensed ASA use only; value does not account for over-the-counter ASA use.
Excludes ASA.
Total number of health care visits in the 12-month period before the cohort entry date.
Most recent laboratory test results in the 1- to 365-day period before the cohort entry date.
eGFR was calculated using the Chronic Kidney Disease (CKD)-Epidemiology (EPI) equation.51
After weighting, groups were still imbalanced on eGFR categories, but there was no statistically or clinically meaningful difference when we assessed baseline eGFR as a continuous variable. Overall, 17% of the cohort had a weighted baseline eGFR between 45 and 60 mL/min per 1.73 m2. Otherwise, more than 120 measured baseline characteristics were similar between SGLT2 and DPP4 inhibitor users, including diabetes parameters, diabetes medications and health care use measures.
We determined that use of SGLT2 inhibitors was associated with a lower 90-day risk of a hospital encounter with AKI relative to use of DPP4 inhibitors (216 events in 19 611 participants [1.10%] versus 388 events in 19 483 participants [1.99%; weighted RR 0.79 [95% CI 0.64 to 0.98]; weighted RD −0.29% [95% CI −0.57% to −0.01%]; Table 2).
Table 2:
Primary and secondary outcomes at 90-day follow-up in participants who used sodium–glucose cotransporter-2 inhibitors compared with those who used dipeptidyl peptidase-4 inhibitors
| Outcome | Observed | Weighted* | |||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| No. events (%) | No. events (%) | RD,† % (95% CI) | RR† (95% CI) | p value | |||
|
|
|
||||||
| Participants taking SGLT2 inhibitors n = 19 611 | Participants taking DPP4 inhibitors n = 19 483 | Participants taking SGLT2 inhibitors n = 19 611 | Participants taking DPP4 inhibitors n = 19 775 | ||||
| Primary | |||||||
|
| |||||||
| Hospital encounter with AKI‡ | 216 (1.10) | 388 (1.99) | 216 (1.10) | 275 (1.39) | −0.29 (−0.57 to −0.01) | 0.79 (0.64 to 0.98) | 0.04 |
|
| |||||||
| Secondary | |||||||
|
| |||||||
| Admission to hospital with AKI | 149 (0.76) | 291 (1.49) | 149 (0.76) | 206 (1.04) | −0.28 (−0.53 to −0.03) | 0.73 (0.56 to 0.95) | 0.02 |
|
| |||||||
| Hospital encounter with moderate-to-severe AKI§ | 44 (0.22) | 74 (0.38) | 44 (0.22) | 55 (0.28) | −0.05 (−0.18 to 0.08) | 0.81 (0.49 to 1.33) | 0.40 |
|
| |||||||
| AKI in outpatient setting only | 573 (2.92) | 609 (3.13) | 573 (2.92) | 513 (2.60) | 0.33 (−0.12 to 0.77) | 1.13 (0.95 to 1.33) | 0.16 |
|
| |||||||
| AKI in all settings | 716 (3.65) | 837 (4.30) | 716 (3.65) | 681 (3.44) | 0.21 (−0.28 to 0.70) | 1.06 (0.92 to 1.22) | 0.42 |
Note: AKI = acute kidney injury, CI = confidence interval, DPP4 = dipeptidyl peptidase-4, RD = risk difference, RR = risk ratio, SGLT2 = sodium-glucose cotransporter-2.
Weighted using inverse probability of treatment weighting based on propensity scores, using weights to estimate the average treatment effect in the treated.
Reference group: DPP4 inhibitor users.
Based on hospital presentation (emergency department or admission to hospital) and assessed using the results for serum creatinine level from the Ontario Laboratories Information System. We defined this using thresholds from the 2012 Kidney disease: improving global outcomes (KDIGO) working group: an increase in serum creatinine level of 50% or greater or an absolute increase of at least 27 μmol/L compared with baseline.33
We defined moderate-to-severe AKI according to KDIGO staging thresholds of stages 2 and 3 combined.33
Use of SGLT2 inhibitors was associated with a lower 90-day risk of admission to hospital with AKI (149 events in 19 611 participants [0.76%] versus 291 events in 19 483 participants [1.49%]; weighted RR 0.73 [95% CI 0.56 to 0.95]; weighted RD −0.28% [95% CI −0.53% to −0.03%]; Table 2). We found that the relative risk of a hospital encounter with moderate-to-severe AKI after use of SGLT2 inhibitors compared with use of DPP4 inhibitors was similar to the primary outcome analysis; with fewer events there was less precision in the estimate and the between-group difference was not significantly different (44 events in 19 611 participants [0.22%] versus 74 events in 19 483 participants [0.38%] events; weighted RR 0.81 [95% CI 0.49 to 1.33]). There was no significant difference in the risk of AKI in an outpatient setting (573 events in 19 611 participants [2.92%] versus 609 events in 19 483 participants [3.13%]; weighted RR 1.13 [95% CI 0.95 to 1.33]) and the risk of AKI in all settings (716 events in 19 611 participants [3.65%] versus 837 events in 19 483 participants [4.30%]; weighted RR 1.06 [95% CI 0.92 to 1.22]; Table 2).
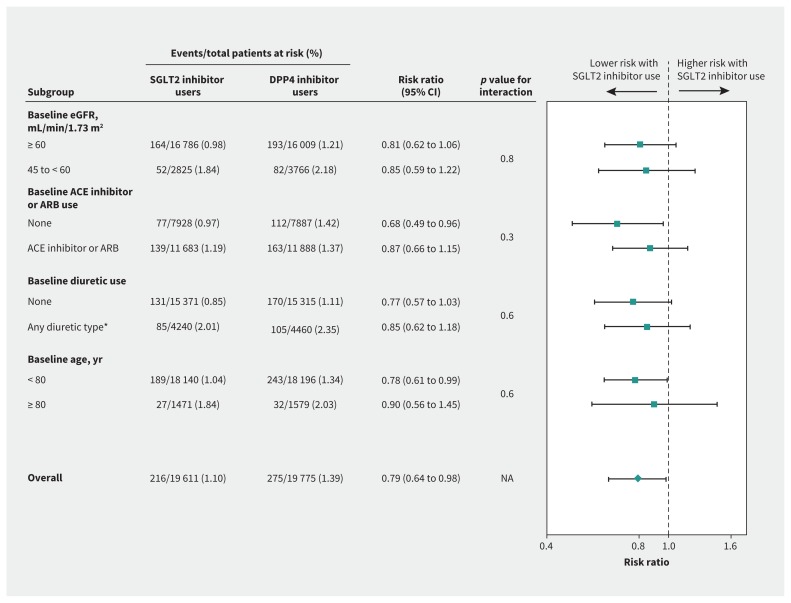
All 7 additional analyses showed that our main findings were robust (Appendices 1K–1Q; Figure 2). Of note, a significant difference in hospital encounters with bowel obstruction between SGLT2 inhibitor users and DPP4 inhibitor users was neither expected nor observed: 20 events in 19 611 participants taking a SGLT2 inhibitor (0.10%) versus 36 events in 19 483 participants taking a DPP4 inhibitor (0.18%); weighted RR 1.00 (95% CI 0.49 to 2.06) (Appendix 1P).
Figure 2:
Subgroup analyses for the outcome of AKI from sodium–glucose cotransporter-2 (SGLT2) inhibitor use. Note: ACE = angiotensin-converting–enzyme inhibitor, AKI = acute kidney injury, ARB = angiotensin receptor blocker, CI = confidence interval, DPP4 = dipeptidyl peptidase-4, eGFR = estimated glomerular filtration rate, NA = not applicable. *Types of diuretics included loop, potassium sparing and thiazide.
Interpretation
In this large population-based cohort study of older adults with diabetes, we observed that being newly dispensed an SGLT2 inhibitor was associated with a lower 90-day risk of a hospital encounter with AKI compared with being newly dispensed a DPP4 inhibitor. These findings provide reassurance about the safety of SGLT2 inhibitors as currently prescribed in routine care. An explanation of this observed effect may relate to the overall reno-protective benefits of SGLT2 inhibitors,52–54 which reduce the amount of albuminuria and risk of progressive chronic kidney disease.55,56 The kidney is dependent on good cardiac function, and the cardiovascular benefits of SGLT2 inhibitors may also result in renal benefits.
Our finding of a 21% lower relative risk of AKI is consistent with 3 published observational cohort studies.13–15 Two of these studies also used laboratory data to define AKI (albeit with smaller sample sizes) and both found that AKI risk was more than 50% lower with SGLT2 inhibitors compared with nonusers and DPP4 inhibitor users.13,14 The most recent observational study with the most comparable sample size to our study found use of SGLT2 inhibitors compared to use of GLP1 receptor agonists was associated with a 31% reduction in AKI risk, but the result was not statistically significant.15
Our results are also consistent with recent large randomized controlled trials (RCTs) where residual confounding was not a concern. In patients with type 2 diabetes and chronic kidney disease, the Effects of Canagliflozin on Renal and Cardiovascular Outcomes in Participants with Diabetic Nephropathy (CREDENCE) RCT found a trend toward lower risk of AKI in patients taking SGLT2 inhibitors compared with placebo.5 A 2019 systematic review and meta-analysis of RCTs that assessed cardiovascular or kidney outcomes, involving more than 38 723 participants, reported a statistically significant 25% reduction in risk of AKI with use of SGLT2 inhibitors versus placebo.56 Another 2019 systematic review and meta-analysis of RCTs involving more than 7000 patients with type 2 diabetes mellitus and chronic kidney disease found a 31% reduction in risk of AKI with use of SGLT2 inhibitors versus placebo that was not statistically significant.57 Another meta-analysis of 3 major efficacy trials of cardiovascular outcomes reported a 34% statistically significant relative risk reduction in the likelihood of AKI in participants who were randomly assigned to receive SGLT2 inhibitors versus placebo.58 The totality of this evidence suggests that regulatory warnings from agencies such as Health Canada and the US Food and Drug Administration about a higher risk of AKI with SGLT2 inhibitors is unwarranted. Some prescribing references also warn about a higher risk of AKI after use of SGLT2 inhibitors, which should be reconsidered.59,60
Our study has several strengths. It is a large population-based study that assessed the risk of a clinically important complication of SGLT2 inhibitor use among older adults. It also evaluated AKI risk in Canada in association with an important medication that is likely to be used more often in response to recent trials showing its benefits.2,4,5 We used laboratory values that are more accurate in assessing AKI compared with database diagnostic codes.61,62 We selected patients who filled a prescription for a different class of oral hypoglycemic agents as our comparator group to reduce confounding by indication bias that would arise if we used patients who were not taking SGLT2 inhibitors as the comparator group.
Limitations
There are several limitations to our study. Given the observational study design, causality cannot be inferred. Although we chose an active comparator drug for the treatment of diabetes and achieved good balance on 97 potential confounders, confounding by indication could still be present. Some confounders could not be captured in our data sets, including smoking status, body mass index and oral water intake, which, when poor, may predispose to volume depletion.63–66 That said, several additional analyses were conducted, and all supported the main findings (Appendices 1K–1Q).
Selection bias could be present in our study because DPP4 inhibitors may have been used preferentially in patients with chronic kidney disease. When estimating eGFRs using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) creatinine equation,51 we had no information about ethnicity and for the purpose of the calculation, we assumed all patients to be white.67
We cannot determine whether strategies such as stopping SGLT2 inhibitors during acute illness altered the risk of AKI. We identified prescriptions dispensed but had no information about medication use or adherence. We included only older adults; however, our findings are consistent with those of other studies that included adults of all ages.14,15 The 2012 KDIGO guideline includes timing elements for when measurement of serum creatinine level needs to be taken to meet the definition of AKI, which we did not consider in our study.33 Measurements of serum creatinine level were done as per routine care and about half of the participants did not have an outpatient measurement of serum creatinine that was used in some secondary outcomes during the 90-day follow-up period. Although we observed a significant between-group difference in the likelihood of measurement of serum creatinine in follow-up, the absolute difference was not large, and we believe that the overall results were not affected. After initiation of SGLT2 inhibitors, clinicians may be more likely to check levels of serum creatinine, especially in patients at higher risk compared with our comparator group, which could lead to a greater risk of AKI associated with SGLT2 inhibitors. In addition, although the median continuous usage of medications for participants in both groups was over 90 days, we did not account for drug stoppages and switches during follow-up. Finally, our study population was “low risk” with respect to AKI, having well-preserved kidney function and minimal or no albuminuria. Extrapolation of the findings to higher risk patients should be done with caution.
Conclusion
We found that in older adults with diabetes in routine clinical practice new initiation of an SGLT2 inhibitor compared with use of a DPP4 inhibitor was associated with a lower 90-day risk of AKI.
See related article at www.cmaj.ca/lookup/doi/10.1503/cmaj.200426
Footnotes
Competing interests: David Cherney has received consulting fees or speaking honoraria or both from Bayer, Mitsubishi-Tanabe, Abbvie, BMS, Prometic, Novo-Nordisk, Janssen, Boehringer Ingelheim and Lilly, AstraZeneca, Merck and Sanofi, and has received operating funds from Sanofi, Novo-Nordisk, Janssen, Boehringer Ingelheim and Lilly, AstraZeneca and Merck Jacob Udell has received honoraria for advisory board participation from Amgen, AstraZeneca, Boehringer Ingelheim, Janssen, Merck, Novartis and Sanofi Pasteur, and grant support to his institutions from AstraZeneca. Kristin Clemens has received a Diabetes Canada Junior Investigator Award, sponsored by Astra Zeneca, and has also received speaker fees from Sutherland Global Services Canada ULC. She has attended conferences sponsored by Merck. Chirag Parikh has received consultant fees from Akebia Therapeutics and Genfit Biopharmaceutical, grants from the National Institute of Diabetes and Digestive and Kidney Diseases and the National Heart, Lung, and Blood Institute, and other support from Renalytix AI. Navdeep Tangri has received personal fees from AstraZeneca, Otsuka, Janssen, and Boehringer Ingelheim and Lilly, and Tricida, grants from AstraZeneca and Tricida, and other support from Tricida. No other competing interests were declared.
This article has been peer reviewed.
Contributors: Carina Iskander and Eric McArthur analyzed the data. Carina Iskander wrote the manuscript, and all of the other authors revised it for important intellectual content. All of the authors were involved in the design of the study, gave final approval of the version to be published and agreed to be accountable for all aspects of the work.
Funding: This study was supported by the ICES Western site. ICES is funded by an annual grant from the Ontario Ministry of Health and Long-Term Care (MOHLTC). Core funding for ICES Western is provided by the Academic Medical Organization of Southwestern Ontario (AMOSO), the Schulich School of Medicine and Dentistry (SSMD), Western University, and the Lawson Health Research Institute (LHRI). Parts of this material are based on data and/or information compiled and provided by the Canadian Institute for Health Information (CIHI). However, the analyses, conclusions, opinions and statements expressed in the material are those of the author(s), and not necessarily those of CIHI. The research was conducted by members of the ICES Kidney, Dialysis and Transplantation team at the ICES Western facility, who are supported by a grant from the Canadian Institutes of Health Research (CIHR). Carina Iskander’s training is supported by the CIHR Drug Safety and Effectiveness Cross-Disciplinary Training (DSECT) Program Scholarship. Amit Garg was supported by the Dr. Adam Linton Chair in Kidney Health Analytics and a CIHR Clinician Investigator Award. Jacob Udell was supported by a Heart and Stroke Foundation of Canada National New Investigator-Ontario Clinician Scientist Award; Ontario Ministry of Research, Innovation and Science Early Researcher Award, Women’s College Research Institute and Department of Medicine, Women’s College Hospital. The opinions, results and conclusions are those of the authors and are independent from the funding sources. No endorsement by ICES, AMOSO, SSMD, LHRI, CIHR or the MOHLTC is intended or should be inferred.
Data sharing: The data from this study are held securely in coded form at ICES. Although data-sharing agreements prohibit ICES from making the data publicly available, access may be granted to those who meet prespecified criteria for confidential access (available at www.ices.on.ca/DAS).
Disclaimer: The opinions, results and conclusions reported in this paper are those of the authors and are independent from the funding sources. No endorsement by ICES or the Ontario Ministry of Health and Long-Term Care is intended or should be inferred.
References
- 1.Summary Safety Review — SGLT2 inhibitors (canagliflozin, dapagliflozin and empagliflozin) — Health Canada. Ottawa: Health Canada; modified 2019 Apr. 15. Available: https://hpr-rps.hres.ca/reg-content/summary-safety-review-detail.php?lang=en&linkID=SSR00204 (accessed 2019 Nov. 4). [Google Scholar]
- 2.Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015;373:2117–28. [DOI] [PubMed] [Google Scholar]
- 3.Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 2017;377:644–57. [DOI] [PubMed] [Google Scholar]
- 4.Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2019;380:347–57. [DOI] [PubMed] [Google Scholar]
- 5.Perkovic V, Jardine MJ, Neal B, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med 2019;380:2295–306. [DOI] [PubMed] [Google Scholar]
- 6.Summary Safety Review — sodium glucose cotransporter 2 (SGLT2) inhibitors INVOKANA (canagliflozin) and FORXIGA (dapagliflozin) — evaluation of a potential risk of acute kidney injury. Ottawa: Health Canada; 2015. Available: www.canada.ca/en/health-canada/services/drugs-health-products/medeffect-canada/safety-reviews/summary-safety-review-sodium-glucose-cotransporter-2-sglt2-inhibitors-invokana-canagliflozin-forxiga-dapagliflozinl-risk.html (accessed 2019 Apr. 3). [Google Scholar]
- 7.FDA Drug Safety Communication: FDA strengthens kidney warnings for diabetes medicines canagliflozin (Invokana, Invokamet) and dapagliflozin (Farxiga, Xigduo XR) [safety announcement]. Silver Spring (MD): U.S. Food and Drug Administration; 2016. June 14 Available: www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-strengthens-kidney-warnings-diabetes-medicines-canagliflozin (accessed 2019 Apr. 3). [Google Scholar]
- 8.Wanner C, Inzucchi SE, Lachin JM, et al. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med 2016;375:323–34. [DOI] [PubMed] [Google Scholar]
- 9.Lambers Heerspink HJ, de Zeeuw D, Wie L, et al. Dapagliflozin a glucose-regulating drug with diuretic properties in subjects with type 2 diabetes. Diabetes Obes Metab 2013;15:853–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ptaszynska A, Johnsson KM, Parikh SJ, et al. Safety profile of dapagliflozin for type 2 diabetes: pooled analysis of clinical studies for overall safety and rare events. Drug Saf 2014;37:815–29. [DOI] [PubMed] [Google Scholar]
- 11.Cefalu WT, Leiter LA, Yoon K-H, et al. Efficacy and safety of canagliflozin versus glimepiride in patients with type 2 diabetes inadequately controlled with metformin (CANTATA-SU): 52-week results from a randomised, double-blind, phase 3 non-inferiority trial. Lancet 2013;382:941–50. [DOI] [PubMed] [Google Scholar]
- 12.Heerspink HJL, Perkins BA, Fitchett DH, et al. Sodium glucose cotransporter 2 inhibitors in the treatment of diabetes mellitus: cardiovascular and kidney effects, potential mechanisms, and clinical applications. Circulation 2016;134:752–72. [DOI] [PubMed] [Google Scholar]
- 13.Nadkarni GN, Ferrandino R, Chang A, et al. Acute kidney injury in patients on SGLT2 inhibitors: a propensity-matched analysis. Diabetes Care 2017;40: 1479–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cahn A, Melzer-Cohen C, Pollack R, et al. Acute renal outcomes with sodium-glucose co-transporter-2 inhibitors: real-world data analysis. Diabetes Obes Metab 2019;21:340–8. [DOI] [PubMed] [Google Scholar]
- 15.Ueda P, Svanstrom H, Melbye M, et al. Sodium glucose cotransporter 2 inhibitors and risk of serious adverse events: nationwide register-based cohort study. BMJ 2018;363:k4365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cherney DZI, Udell JA. Use of sodium glucose cotransporter 2 inhibitors in the hands of cardiologists: with great power comes great responsibility. Circulation 2016;134:1915–7. [DOI] [PubMed] [Google Scholar]
- 17.Cohen AT, Goto S, Schreiber K, Torp-Pedersen C. Why do we need observational studies of everyday patients in the real-life setting? Eur Hear J Suppl 2015;17(Suppl D):D2–8. [Google Scholar]
- 18.Udell JA, Yuan Z, Rush T, et al. Cardiovascular outcomes and risks after initiation of a sodium glucose cotransporter 2 inhibitor: results from the EASEL population-based cohort study (Evidence for Cardiovascular outcomes with sodium glucose cotransporter 2 inhibitors in the real world). Circulation 2018;137:1450–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pendergrass M, Fenton C, Haffner SM, et al. Exenatide and sitagliptin are not associated with increased risk of acute renal failure: a retrospective claims analysis. Diabetes Obes Metab 2012;14:596–600. [DOI] [PubMed] [Google Scholar]
- 20.Lo Re V, Carbonari DM, Saine ME, et al. Postauthorization safety study of the DPP-4 inhibitor saxagliptin: a large-scale multinational family of cohort studies of five outcomes. BMJ Open Diabetes Res Care 2017;5:e000400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Population estimates on July 1st, by age and sex. Ottawa: Statistics Canada; 2018. Available: www150.statcan.gc.ca/t1/tbl1/en/tv.action?pid=1710000501&pickMembers%5B0%5D=1.7&pickMembers%5B1%5D=2.1 (accessed 2019 Apr. 8). [Google Scholar]
- 22.Yau K, Burneo JG, Jandoc R, et al. Population-based study of risk of AKI with levetiracetam. Clin J Am Soc Nephrol 2019;14:17–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hwang YJ, Dixon SN, Reiss JP, et al. Atypical antipsychotic drugs and the risk for acute kidney injury and other adverse outcomes in older adults: a population-based cohort study. Ann Intern Med 2014;161:242–8. [DOI] [PubMed] [Google Scholar]
- 24.Gandhi S, Fleet JL, Bailey DG, et al. Calcium-channel blocker–clarithromycin drug interactions and acute kidney injury. JAMA 2013;310:2544–53. [DOI] [PubMed] [Google Scholar]
- 25.Patel AM, Shariff S, Bailey DG, et al. Statin toxicity from macrolide antibiotic coprescription: a population-based cohort study. Ann Intern Med 2013;158: 869–76. [DOI] [PubMed] [Google Scholar]
- 26.Langan SM, Schmidt SA, Wing K, et al. The reporting of studies conducted using observational routinely collected health data statement for pharmacoepidemiology (RECORD-PE). BMJ 2018;363:k3532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ontario Demographic Quarterly: highlights of fourth quarter, 2018. Oshawa (ON): Ontario Ministry of Finance; modified 2019 Mar. 21. Available: www.fin.gov.on.ca/en/economy/demographics/quarterly/dhiq4.html (accessed 2019 Apr. 28). [Google Scholar]
- 28.Ontario Drug Benefit Formulary. Toronto: Ministry of Health and Long-Term Care; modified 2019 Nov. 29. Available: https://www.formulary.health.gov.on.ca/formulary/results.xhtml?q=flozin&type=1 Search Results (accessed 2019 Apr. 3). [Google Scholar]
- 29.Iskander C, McArthur E, Nash DM, et al. Identifying Ontario geographic regions to assess adults who present to hospital with laboratory-defined conditions: a descriptive study. CMAJ Open 2019;7:E624–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hsia DS, Grove O, Cefalu WT. An update on sodium-glucose co-transporter-2 inhibitors for the treatment of diabetes mellitus. Curr Opin Endocrinol Diabetes Obes 2017;24:73–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.This is long-term care 2019. Toronto: Ontario Long Term Care Association; 2019. Available: www.oltca.com/OLTCA/Documents/Reports/TILTC2019web.pdf (accessed 2019 June 5). [Google Scholar]
- 32.Drug Product Database online query. Ottawa: Health Canada; Available: https://health-products.canada.ca/dpd-bdpp/index-eng.jsp (accessed 2019 June 5). [Google Scholar]
- 33.Kellum JA, Lameire N, Aspelin P, et al. Kidney disease: improving global outcomes (KDIGO) acute kidney injury work group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl 2012;2:1–138. [Google Scholar]
- 34.Fournier J-P, Sommet A, Durrieu G, et al. More on the “Triple Whammy”: anti-hypertensive drugs, non-steroidal anti-inflammatory agents and acute kidney injury — a case/non-case study in the French pharmacovigilance database. Ren Fail 2014;36:1166–8. [DOI] [PubMed] [Google Scholar]
- 35.Dreischulte T, Morales DR, Bell S, et al. Combined use of nonsteroidal anti-inflammatory drugs with diuretics and/or renin–angiotensin system inhibitors in the community increases the risk of acute kidney injury. Kidney Int 2015;88:396–403. [DOI] [PubMed] [Google Scholar]
- 36.Lombardi R, Ferreiro A. Risk factors profile for acute kidney injury after cardiac surgery is different according to the level of baseline renal function. Ren Fail 2008;30:155–60. [DOI] [PubMed] [Google Scholar]
- 37.Thakar CV, Arrigain S, Worley S, et al. A clinical score to predict acute renal failure after cardiac surgery. J Am Soc Nephrol 2005;16:162–8. [DOI] [PubMed] [Google Scholar]
- 38.Chawla LS, Eggers PW, Star RA, et al. Acute kidney injury and chronic kidney disease as interconnected syndromes. N Engl J Med 2014;371:58–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Palmer BF. Renal dysfunction complicating the treatment of hypertension. N Engl J Med 2002;347:1256–61. [DOI] [PubMed] [Google Scholar]
- 40.Chawla LS, Kimmel PL. Acute kidney injury and chronic kidney disease: an integrated clinical syndrome. Kidney Int 2012;82:516–24. [DOI] [PubMed] [Google Scholar]
- 41.Haneuse S, VanderWeele TJ, Arterburn D. Using the E-Value to assess the potential effect of unmeasured confounding in observational studies. JAMA 2019;321:602–3. [DOI] [PubMed] [Google Scholar]
- 42.VanderWeele TJ, Ding P. Sensitivity analysis in observational research: introducing the E-Value. Ann Intern Med 2017;167:268–74. [DOI] [PubMed] [Google Scholar]
- 43.Austin PC. Using the standardized difference to compare the prevalence of a binary variable between two groups in observational research. Commun Stat Comput 2009;38:1228–34. [Google Scholar]
- 44.Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res 2011;46:399–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sato T, Matsuyama Y. Marginal structural models as a tool for standardization. Epidemiology 2003;14:680–6. [DOI] [PubMed] [Google Scholar]
- 46.Brookhart MA, Wyss R, Layton JB, et al. Propensity score methods for confounding control in nonexperimental research. Circ Cardiovasc Qual Outcomes 2013; 6:604–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med 2015;34:3661–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol 2004;159:702–6. [DOI] [PubMed] [Google Scholar]
- 49.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373–83. [DOI] [PubMed] [Google Scholar]
- 50.Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care 2005;43:1130–9. [DOI] [PubMed] [Google Scholar]
- 51.Levey AS, Stevens LA. Estimating GFR using the CKD Epidemiology Collaboration (CKD-EPI) creatinine equation: more accurate GFR estimates, lower CKD prevalence estimates, and better risk predictions. Am J Kidney Dis 2010;55:622–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lapi F, Azoulay L, Yin H, et al. Concurrent use of diuretics, angiotensin converting enzyme inhibitors, and angiotensin receptor blockers with non-steroidal anti-inflammatory drugs and risk of acute kidney injury: nested case–control study. BMJ 2013;346:e8525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lim HJ, Lee HH, Kim AJ, et al. Renin-angiotensin-aldosterone system blockade in critically ill patients is associated with increased risk for acute kidney injury. Tohoku J Exp Med 2016;238:17–23. [DOI] [PubMed] [Google Scholar]
- 54.Heerspink HJL, Kosiborod M, Inzucchi SE, et al. Renoprotective effects of sodium-glucose cotransporter-2 inhibitors. Kidney Int 2018;94:26–39. [DOI] [PubMed] [Google Scholar]
- 55.Kelly MS, Lewis J, Huntsberry AM, et al. Efficacy and renal outcomes of SGLT2 inhibitors in patients with type 2 diabetes and chronic kidney disease. Post-grad Med 2019;131:31–42. [DOI] [PubMed] [Google Scholar]
- 56.Neuen BL, Young T, Heerspink HJL, et al. SGLT2 inhibitors for the prevention of kidney failure in patients with type 2 diabetes: a systematic review and meta-analysis. Lancet Diabetes Endocrinol 2019;7:845–54. [DOI] [PubMed] [Google Scholar]
- 57.Toyama T, Neuen BL, Jun M, et al. Effect of SGLT2 inhibitors on cardiovascular, renal and safety outcomes in patients with type 2 diabetes mellitus and chronic kidney disease: A systematic review and meta-analysis. Diabetes Obes Metab 2019;21:1237–50. [DOI] [PubMed] [Google Scholar]
- 58.Gilbert RE, Thorpe KE. Acute kidney injury with sodium-glucose co-transporter-2 inhibitors: A meta-analysis of cardiovascular outcome trials. Diabetes Obes Metab 2019;21:1996–2000. [DOI] [PubMed] [Google Scholar]
- 59.Diabetes Canada Clinical Practice Guidelines Expert Committee Houlden RL. Diabetes Canada 2018 Clinical Practice Guidelines for the Prevention and Management of Diabetes in Canada. Can J Diabetes 2018;42(Suppl 1):S1–5.29650079 [Google Scholar]
- 60.DeSantis A. Sodium-glucose co-transporter 2 inhibitors for the treatment of type 2 diabetes mellitus. UpToDate (Wolters Kluwer; ); updated 2020 Feb. 5. Available: www.uptodate.com/contents/sodium-glucose-co-transporter-2-inhibitors-for-the-treatment-of-type-2-diabetes-mellitus?search=sglt2inhibitors&source=search_result&selectedTitle=1~59&usage_type=default&display_rank=1 (accessed 2019 Apr. 3). [Google Scholar]
- 61.Hwang YJ, Shariff SZ, Gandhi S, et al. Validity of the International Classification of Diseases, 10th Revision code for acute kidney injury in elderly patients at presentation to the emergency department and at hospital admission. BMJ Open 2012;2pii e001821. 10.1136/bmjopen-2012-001821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fleet JL, Shariff SZ, Gandhi S, et al. Validity of the International Classification of Diseases 10th revision code for hyperkalaemia in elderly patients at presentation to an emergency department and at hospital admission. BMJ Open 2012;2 pii e002011. 10.1136/bmjopen-2012-002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mittalhenkle A, Stehman-Breen CO, Shlipak MG, et al. Cardiovascular risk factors and incident acute renal failure in older adults: the cardiovascular health study. Clin J Am Soc Nephrol 2008;3:450–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pedersen AB, Gammelager H, Kahlert J, et al. Impact of body mass index on risk of acute kidney injury and mortality in elderly patients undergoing hip fracture surgery. Osteoporos Int 2017;28:1087–97. [DOI] [PubMed] [Google Scholar]
- 65.Rahman M, Shad F, Smith MC. Acute kidney injury: a guide to diagnosis and management. Am Fam Physician 2012;86:631–9. [PubMed] [Google Scholar]
- 66.Naicker S, Aboud O, Gharbi MB. Epidemiology of acute kidney injury in Africa. Semin Nephrol 2008;28:348–53. [DOI] [PubMed] [Google Scholar]
- 67.National Household Survey 2011. Ottawa: Statistics Canada; 2011. Available: https://www12.statcan.gc.ca/nhs-enm/2011/dp-pd/prof/search-recherche/lst/page.cfm?Lang=E&TABID=1&GEOCODE=35 (accessed 2019 June 5). [Google Scholar]