Abstract
Background
Lumbosacral radicular pain (commonly called sciatica) is a syndrome involving patients who report radiating leg pain. Epidural corticosteroid injections deliver a corticosteroid dose into the epidural space, with the aim of reducing the local inflammatory process and, consequently, relieving the symptoms of lumbosacral radicular pain. This Cochrane Review is an update of a review published in Annals of Internal Medicine in 2012. Some placebo‐controlled trials have been published recently, which highlights the importance of updating the previous review.
Objectives
To investigate the efficacy and safety of epidural corticosteroid injections compared with placebo injection on pain and disability in patients with lumbosacral radicular pain.
Search methods
We searched the following databases without language limitations up to 25 September 2019: Cochrane Back and Neck group trial register, CENTRAL, MEDLINE, Embase, CINAHL, PsycINFO, International Pharmaceutical Abstracts, and two trial registers. We also performed citation tracking of included studies and relevant systematic reviews in the field.
Selection criteria
We included studies that compared epidural corticosteroid injections of any corticosteroid drug to placebo injections in patients with lumbosacral radicular pain. We accepted all three anatomical approaches (caudal, interlaminar, and transforaminal) to delivering corticosteroids into the epidural space. We considered trials that included a placebo treatment as delivery of an inert substance (i.e. one with no pharmacologic activity), an innocuous substance (e.g. normal saline solution), or a pharmacologically active substance but not one considered to provide sustained benefit (e.g. local anaesthetic), either into the epidural space (i.e. to mimic epidural corticosteroid injection) or adjacent spinal tissue (i.e. subcutaneous, intramuscular, or interspinous tissue). We also included trials in which a local anaesthetic with a short duration of action was used as a placebo and injected together with corticosteroid in the intervention group.
Data collection and analysis
Two authors independently performed the screening, data extraction, and 'Risk of bias' assessments. In case of insufficient information, we contacted the authors of the original studies or estimated the data. We grouped the outcome data into four time points of assessment: immediate (≤ 2 weeks), short term (> 2 weeks but ≤ 3 months), intermediate term (> 3 months but < 12 months), and long term (≥ 12 months). We assessed the overall quality of evidence for each outcome and time point using the GRADE approach.
Main results
We included 25 clinical trials (from 29 publications) investigating the effects of epidural corticosteroid injections compared to placebo in patients with lumbosacral radicular pain. The included studies provided data for a total of 2470 participants with a mean age ranging from 37.3 to 52.8 years. Seventeen studies included participants with lumbosacral radicular pain with a diagnosis based on clinical assessment and 15 studies included participants with mixed duration of symptoms. The included studies were conducted mainly in North America and Europe. Fifteen studies did not report funding sources, five studies reported not receiving funding, and five reported receiving funding from a non‐profit or government source. Eight trials reported data on pain intensity, 12 reported data on disability, and eight studies reported data on adverse events. The duration of the follow‐up assessments ranged from 12 hours to 1 year. We considered eight trials to be of high quality because we judged them as having low risk of bias in four out of the five bias domains. We identified one ongoing trial in a trial registry.
Epidural corticosteroid injections were probably slightly more effective compared to placebo in reducing leg pain at short‐term follow‐up (mean difference (MD) −4.93, 95% confidence interval (CI) −8.77 to –1.09 on a 0 to 100 scale; 8 trials, n = 949; moderate‐quality evidence (downgraded for risk of bias)). For disability, epidural corticosteroid injections were probably slightly more effective compared to placebo in reducing disability at short‐term follow‐up (MD −4.18, 95% CI −6.04 to −2.17, on a 0 to 100 scale; 12 trials, n = 1367; moderate‐quality evidence (downgraded for risk of bias)). The treatment effects are small, however, and may not be considered clinically important by patients and clinicians (i.e. MD lower than 10%).
Most trials provided insufficient information on how or when adverse events were assessed (immediate or short‐term follow‐up) and only reported adverse drug reactions — that is, adverse events that the trialists attributed to the study treatment. We are very uncertain that epidural corticosteroid injections make no difference compared to placebo injection in the frequency of minor adverse events (risk ratio (RR) 1.14, 95% CI 0.91 to 1.42; 8 trials, n = 877; very low quality evidence (downgraded for risk of bias, inconsistency and imprecision)). Minor adverse events included increased pain during or after the injection, non‐specific headache, post‐dural puncture headache, irregular periods, accidental dural puncture, thoracic pain, non‐local rash, sinusitis, vasovagal response, hypotension, nausea, and tinnitus. One study reported a major drug reaction for one patient on anticoagulant therapy who had a retroperitoneal haematoma as a complication of the corticosteroid injection.
Authors' conclusions
This study found that epidural corticosteroid injections probably slightly reduced leg pain and disability at short‐term follow‐up in people with lumbosacral radicular pain. In addition, no minor or major adverse events were reported at short‐term follow‐up after epidural corticosteroid injections or placebo injection. Although the current review identified additional clinical trials, the available evidence still provides only limited support for the use of epidural corticosteroid injections in people with lumbosacral radicular pain as the treatment effects are small, mainly evident at short‐term follow‐up and may not be considered clinically important by patients and clinicians (i.e. mean difference lower than 10%). According to GRADE, the quality of the evidence ranged from very low to moderate, suggesting that further studies are likely to play an important role in clarifying the efficacy and tolerability of this treatment. We recommend that further trials should attend to methodological features such as appropriate allocation concealment and blinding of care providers to minimise the potential for biased estimates of treatment and harmful effects.
Plain language summary
Corticosteroid injections for treatment of sciatica
What is sciatica?
Lumbosacral radicular pain, often referred to as sciatica, is a type of pain that arises from irritation or inflammation of a low back spinal nerve. People typically experience pain radiating down the leg, sometimes with altered sensation and weakness of leg muscles. In this plain language summary, the term 'sciatica' will be used to describe lumbosacral radicular pain.
How injections of anti‐inflammatory steroids directly into the spinal region might work
To relieve the sciatica symptoms, some practitioners treat their patients with an injection of a corticosteroid (anti‐inflammatory medicine) directly into the spinal region. The injections are believed to work by reducing inflammation around the spinal nerve. What was the aim of this review?
We aimed to investigate whether injections of anti‐inflammatory steroids into the lower spine are effective and safe compared to placebo injection (that is, injection of an inert (i.e. inactive) or innocuous substance (e.g. salt water)) in people with sciatica.
Search dates
This review includes all eligible studies up to 25 September 2019.
Study characteristics
We included 25 clinical trials (reported in 29 publications) enrolling a total of 2470 people with sciatica comparing injection of anti‐inflammatory steroids into the lower spine to placebo injection. We identified one ongoing trial in a registry of trial protocols. Most studies included participants with sciatica detected through clinical findings and most studies included participants with mixed duration of symptoms. The included studies were carried out mainly in North America and Europe. Fifteen studies did not report any information related to funding, five studies reported not receiving any funding for conducting the study, and five studies reported receiving funding from a non‐profit source (e.g. research institute, university) or from government sources. Eight trials reported data on leg pain, 12 trials reported data on disability, and eight studies reported data on adverse events. The duration of the follow‐up assessments ranged from 12 hours to 1 year. We considered only eight trials to be at low risk of bias.
Key messages
We provide a summary of the key results of the review in the 'Additional tables' section.
Injections of anti‐inflammatory steroids into the lower spine is probably slightly better than placebo in reducing leg pain and disability at short‐term follow‐up. However, the treatment effects were small and may not be considered clinically important by patients and clinicians (i.e. less than 10 points on a 0 to 100 scale).
Adverse events may occur after injection of anti‐inflammatory steroids into the lower spine for sciatica. Most studies provided insufficient information on how or when adverse events were assessed (immediate or short‐term follow‐up) and only reported adverse drug reactions (unexpected events that the authors attributed to the study treatment). We are very uncertain that the frequency of minor adverse events is different following injections of anti‐inflammatory steroids compared to placebo injection. Adverse events included increased pain during or after the injection, non‐specific headache, headache after accidental spinal puncture, irregular periods, accidental spinal puncture, thoracic pain, non‐local rash, sinusitis, vasovagal response (brief loss of consciousness), hypotension, nausea, and tinnitus. One study reported a major drug reaction: one patient on anticoagulant therapy had a retroperitoneal haematoma (bleeding in the abdominal space) as a complication of the injection of anti‐inflammatory steroids.
Although the current review identified additional trials, the available evidence still provides only limited support for the use of injections of anti‐inflammatory steroids into the lower spine for sciatica as the treatment benefits are small, mainly evident at short‐term follow‐up, and may not be considered clinically important by patients and clinicians.
Quality of evidence
The quality of evidence was at best moderate, suggesting that further studies may change our conclusions. Uncertainty was mostly due to problems with trial design and inconsistency.
Summary of findings
Summary of findings for the main comparison. Summary of findings ‐ short‐term follow‐up.
| Epidural corticosteroid injection compared to placebo injection for lumbosacral radicular pain | ||||||
|
Patient or population: lumbosacral radicular pain Setting: secondary and tertiary care Intervention: epidural corticosteroid injection Comparison: placebo injection | ||||||
| Outcomes | Illustrative comporative risks (95% CI) | Treatment effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk* | |||||
| Placebo injection | Epidural corticosteroid injections | |||||
|
Leg pain ‒short‐term follow‐up (> 2 weeks but ≤ 3 months) Leg pain reported using VAS (0 to 100) and NRS (0 to 10). Pain scores were converted to a common 0 to 100 scale. Higher scores indicate worse leg pain |
The mean leg pain across the placebo groups ranged from 15.0 to 55.0 points. | The mean leg pain in the epidural corticosteroid injection groups was 4.93 points lower (8.77 lower to 1.09 lower) than in the placebo group | Mean difference −4.93 (−8.77 to −1.09) | 949 participants (8 RCTs) | ⊕⊕⊕⊝ MODERATE 1 | |
|
Disability ‒short‐term (> 2 weeks but ≤ 3 months) Disability reported using multiple scales (RMDQ and ODI). Disability scores were converted to a common 0 to 100 scale. Higher scores indicate greater disability. |
The mean disability across the placebo groups ranged from 9.1 to 42.5 points | The mean disability in the epidural corticosteroid injection groups was 4.18 points lower (6.04 lower to 2.17 lower) | Mean difference −4.18 (−6.04 to −2.17) | 1367 participants (12 RCTs) | ⊕⊕⊕⊝ MODERATE 1 | We calculated the standardised mean difference for disability (SMD −0.27, 95% CI −0.39 to −0.14) and translated to unstandardised mean difference using the baseline SD from Karppinen 2001. |
|
Minor adverse events** ‒short‐term (> 2 weeks but ≤ 3 months) Minor adverse events provided as the proportion of patients reporting any untoward medical occurrence after an epidural injection without hospitalisations. |
128 of 484 (26 per 100) participants reported minor adverse events | 65 of 393 (16 per 100) participants reported minor adverse events. | RR 1.14 (0.91 to 1.42) | 877 participants (8 RCTs) | ⊕⊝⊝⊝ VERY LOW 1, 2, 3 | Adverse events included increased pain during or after the injection, non‐specific headache, post‐dural puncture headache injection, irregular periods, accidental dural puncture, thoracic pain, non‐local rash, sinusitis, vasovagal response, hypotension, nausea, and tinnitus. |
|
Major adverse events** ‒short‐term (> 2 weeks but ≤ 3 months) Major adverse events provided as the proportion of patients reporting any untoward medical occurrence after an epidural injection with hospitalizations. |
1 of 80 (1 per 100) reported major adverse events | 0 of 80 (0 per 100) reported major adverse events | ‐ | 160 participants (1 RCT) | ⊕⊕⊕⊝ Moderate1 | 1 study reported that 1 patient on anticoagulant therapy had a retroperitoneal haematoma as a complication of the injection. |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
|
GRADE Working Group grades of evidence
High quality: further research is very unlikely to change confidence in estimate of effect
Moderate quality: further research is likely to have an important impact on confidence in estimate of effect and may change the estimate
Low quality: further research is very likely to have an important impact on confidence in estimate of effect and is likely to change the estimate
Very low quality: very little confidence in the effect estimate. No evidence: no RCTs were identified that addressed this outcome | ||||||
1 Downgraded 1 level due to risk of bias (more than 25% of the participants were from studies judged as having high risk of bias)
2 Downgraded 1 level due to imprecision (number of participants lower than 400 for continuous outcomes or number of events lower than 300 for dichotomous outcomes)
3 Downgraded 1 level due to inconsistency (heterogeneity of pooled estimates greater than moderate (I² > 45%)
**Most trials provided insufficient information on how or when adverse events were assessed (immediate or short‐term follow‐up). In addition, most trials only reported adverse drug reactions, that is adverse events that the triallists attributed to the study treatment. 5 studies reported that there were no adverse events and 6 trials reported adverse drug reactions or adverse events without specifying which group the patients had been allocated.
Background
Description of the condition
Lumbosacral radicular pain (commonly called sciatica) is a condition characterised by radiating leg pain caused by a dysfunction in the spinal nerve root (Koes 2007). According to IASP 1994, the term “sciatica” should be avoided because it might suggest that the condition is a disorder of the sciatic nerve rather than a lumbosacral nerve root. Hence, we have used the term “lumbosacral radicular pain” in this review to align with the IASP name for this condition. The estimated one‐year prevalence of lumbosacral radicular pain ranges from 3% to 14% (Younes 2006 and Palmer 2003, respectively). While the prognosis for patients with acute lumbosacral radicular pain is favourable (Koes 2007; Valat 2010), more than a half of these patients still have pain after two years or more (Tubach 2004). Furthermore, patients with lumbosacral radicular pain may report higher disability and pain intensity (Frymoyer 1988), and poorer quality of life and higher rates of work absence compared to patients with axial low back pain (Hider 2015; Konstantinou 2013).
Lumbosacral radicular pain is diagnosed by history taking and physical examination including neurological testing (Koes 2007). Diagnostic imaging is not recommended by clinical practice guidelines unless the results may change management, such as when lumbosacral radicular pain symptoms are accompanied by severe or progressive neurological deficits, where there is suspected serious underlying medical pathology or a failure to respond to conservative management, with surgery being considered (Koes 2007; Valat 2010). Although the most commonly reported symptom of lumbosacral radicular pain is radiating leg pain below the knee, there are other clinical features associated with this condition, such as unilateral leg pain worse than spinal pain, a positive result on the straight leg raising test, pain and sensation loss (i.e. numbness or pins and needles) following a dermatomal distribution, or muscle weakness or reflex changes or both in a myotomal distribution. The criteria for the clinical diagnosis of lumbosacral radicular pain vary but a common approach is to require radiating leg pain below the knee in association with dermatomal leg pain or at least one neurological finding indicating neurological deficit (e.g. muscle weakness, reflex changes, sensory deficits) (Koes 2007; Valat 2010).
Description of the intervention
Therapeutic epidural injection of a corticosteroid is the most popular non‐surgical procedure for lumbosacral radicular pain worldwide (Manchikanti 2012b). The injection delivers medication in the epidural space. There are two main types of corticosteroid to be delivered into the epidural space: particulate corticosteroids (e.g. triamcinolone, methylprednisolone, metamethasone), and non‐particulate corticosteroids (e.g. dexamethasone). In addition, there are three anatomical approaches commonly used for administering epidural corticosteroids: caudal, interlaminar, and transforaminal approaches. The caudal approach is the earliest described technique, and delivers the medication into the epidural space from the sacrococcygeal ligament through the sacral hiatus. With this approach larger volumes of medication are required in order to reach the target site, but it is also considered the safest and easiest technique. With the interlaminar approach, the medication is delivered directly into the posterior epidural space. However, the medication may have difficulty transiting from the posterior to ventral epidural space due to the presence of epidural ligaments or scar tissue (Botwin 2004; Weil 2008). The transforaminal approach is the most recently proposed approach where the needle is inserted into the 'safe triangle' (i.e. pedicle at the superior border, lateral side of the vertebral body at lateral border, and the spinal nerve root at medial border) which delivers the medication at the target site of pathology (i.e. interspace between the spinal nerve and the herniated disk) (Lutz 1998). Imaging procedures have been used to provide anatomical precision and accurate needle placement to enhance the safety and efficacy of epidural injection (Chen 2004; Silbergleit 2001), with fluoroscopy recommended for all interlaminar and transforaminal injection by a multispecialty working group sponsored by the US Food and Drug Administration (Rathmell 2015). Although many pain medicine practitioners advocate transforaminal injection as being the most effective approach, the superiority of one epidural approach over another remains unproven (Chang‐Chien 2014; Liu 2016).
How the intervention might work
Lumbosacral radicular pain is mostly caused by nerve root dysfunction associated with a lumbar disc herniation of the nucleus pulposus (Valat 2010). Chemical mediators originating from the nucleus pulposus (Igarashi 2000; Olmarker 1998), in addition to mechanical factors due to disc herniation or excessive tissue proliferation, result in nerve compression and irritation. The corticosteroids stimulate an anti‐inflammatory process inhibiting the expression of pro‐inflammatory cytokines (Barnes 1993). Whereas the principal mechanism of action for epidural corticosteroid injections is believed to be a reduction in the inflammatory process at the connection between the herniated disk and epidural space (Lee 1998; McLain 2005), other possible mechanisms include the washout of inflammatory cytokines, dissolution of scar tissue, suppression of ectopic discharges from inflamed and injured dorsal root ganglia and nerve roots enhancing blood flow to ischemic nerve roots, and alterations in gene expression (Devor 1992; Fukusaki 1998). These other mechanisms may explain why some investigators have found epidural local anaesthetic or corticosteroids (or both) provide superior benefit compared to intramuscular injection (Bicket 2013).
Why it is important to do this review
This Cochrane Review is an update of a previous review published in Annals of Internal Medicine in 2012 (Pinto 2012). Our last review showed high‐quality evidence that epidural corticosteroid injections were more effective compared to placebo in reducing pain intensity (MD −6.20, 95% CI −9.40 to −3.00 on a 0 to 100 scale) and disability (MD −3.10, 95% CI −5.00 to −1.20 on a 0 to 100 scale) at short‐term follow‐up. Subsequent to our previous review some reviews also reported small effects at short‐term follow‐up (Shamliyan 2014; Chou 2015 ; Bhatia 2016). Nevertheless, some placebo‐controlled trials have been published recently, which highlights the importance of updating the previous review.
Objectives
To investigate the efficacy and safety of epidural corticosteroid injections compared with placebo injection on pain and disability in patients with lumbosacral radicular pain.
Methods
Criteria for considering studies for this review
Types of studies
We included only placebo‐controlled randomised trials.
Types of participants
We considered trials enrolling patients with lumbosacral radicular pain to be eligible. Diagnostic criteria for lumbosacral radicular pain vary in the literature. There are several typical clinical features of lumbosacral radicular pain such as radiating leg pain below the knee, leg pain worse than spinal pain, a positive result on the straight leg raising test in individuals with a lumbar herniated disc, sensation loss (i.e. numbness or pins and needles) following a dermatomal distribution, and muscle weakness or reflex changes, or both, in a myotomal distribution. Thus we included those studies using clinical assessments, regardless of the use of concordant imaging evidence, for diagnosis of lumbosacral radicular pain. In addition we considered the following as lumbosacral radicular pain synonyms: sciatica, radicular leg pain, radiculopathy, nerve root compromise, nerve root compression, lumbosacral radicular syndrome, radiculitis, nerve root pain and nerve root entrapment.
We placed no restriction regarding the duration of symptoms; however, we classified patients as acute (< 6 weeks), subacute (from 6 to 12 weeks), chronic (> 12 weeks), or mixed duration (i.e. trials including patients with a mix of acute, subacute, or chronic symptoms). We included trials with mixed populations (e.g. low back pain with or without lumbosacral radicular pain) if separate data for the participants with lumbosacral radicular pain were provided. We excluded studies of participants with previous surgery or patients with central spinal canal stenosis.
Types of interventions
We included studies that compared epidural corticosteroid injection to placebo injection. We permitted all three anatomical approaches (i.e. caudal, interlaminar, and transforaminal) to delivering corticosteroids into the epidural space; and we included any corticosteroid drugs administered via these approaches. We considered trials that included a placebo treatment as delivery of an inert substance (i.e. no pharmacologic activity), innocuous substance (e.g. normal saline solution) or pharmacologically active substance not considered to provide sustained benefit (e.g. local anaesthetic) either into the epidural space (i.e. to mimic epidural corticosteroid injection) or adjacent spinal tissue (i.e. subcutaneous, intramuscular, or interspinous). We also included trials in which a local anaesthetic with a short duration of action was used as a placebo and injected together with corticosteroid in the intervention group.
Types of outcome measures
Primary outcomes
The primary outcomes of this review were:
Leg pain intensity measured by a self‐reported scale (e.g. visual analogue scale or numerical rating scale); and
Disability measured by a self‐reported questionnaire (e.g. Oswestry Disability Index or Roland‒Morris Disability Questionnaire).
Secondary outcomes
Overall pain intensity measured by a self‐reported scale (e.g. visual analogue scale or numerical rating scale).
Back pain intensity measured by a self‐reported scale (e.g. visual analogue scale or numerical rating scale).
Pain intensity measured by the proportion of patients with pain relief from baseline.
Disability measured by the proportion of patients with disability reduction from baseline.
Adverse events measured by the proportion of patients reporting any untoward medical occurrence after an epidural corticosteroid injection, which did not necessarily have a causal relationship with the epidural injection procedure or the substance administered.
We grouped the outcome data into four time points of assessment after randomisation: immediate (≤ 2 weeks), short term (> 2 weeks but ≤ 3 months), intermediate term (> 3 months but < 12 months), and long term (≥ 12 months). If multiple time points fell into the same time window, we used the time point closest to one week for immediate follow‐up, eight weeks for short‐term, six months for intermediate, and twelve months for the long‐term follow‐up. If two time points in one time window also had similar length from the closest time point (e.g. 4 weeks and 12 weeks are equidistant to the short‐term follow‐up criterion of 8 weeks), we considered the time point closest to the end of the intervention.
Search methods for identification of studies
Electronic searches
We searched the following databases, without language restrictions, to 25 September 2019 to identify relevant articles.
Cochrane Back and Neck group trials register (through Cochrane Register of Studies (CRS web)); 25 September 2019
Cochrane Central Register of Controlled Trials (CENTRAL, in the Cochrane Library, CRS Web); 25 September 2019
MEDLINE (Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE(R) Daily and Ovid MEDLINE(R); Ovid SP, 1946 to 25 September 2019)
Embase (OvidSP, 1980 to 2019 Week 38); 25 September 2019
Cumulative Index to Nursing and Allied Health Literature (CINAHL, EBSCO, 1981 to 25 September 2019)
PsycINFO (OvidSP, 2002 to September Week 2 2019); 25 September 2019
International Pharmaceutical Abstracts (OvidSP, 1970 to August 2019); 25 September 2019
ClinicalTrials.gov; 25 September 2019
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP); 25 September 2019
We searched PubMed in 2015 for studies not in MEDLINE using the strategy recommended by Duffy 2014. In 2017 we began searching MEDLINE (Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE(R) Daily and Ovid MEDLINE(R)) as several MEDLINE databases can be searched through one interface. In 2017, we began searching CENTRAL in CRS Web; previously it was searched in CRS standalone.
The information specialist of the Cochrane Back and Neck group revised all search strategies since the original review. They can be found in Appendix 1.
Searching other resources
We performed citation tracking of the references of included studies and relevant systematic reviews in the field (Bhatia 2016; Chou 2015). In addition, we re‐assessed all studies included in the previous review — Pinto 2012 — in accordance with inclusion criteria adopted for this review.
Data collection and analysis
Selection of studies
Two independent reviewers (CBO and VCO) screened titles and abstracts to determine potentially eligible studies. Then, two independent reviewers (CBO and VCO) assessed the full texts of potentially eligible studies against the inclusion and exclusion criteria. In cases of disagreement, we consulted a third independent reviewer (CGM) to resolve the dispute.
Data extraction and management
Two reviewers (CBO and RZP) extracted the data using a standardised form independently of each other. In case of disagreement, we consulted a third reviewer (CGM) to arbitrate the disagreement. We extracted from each study the following information: participants’ characteristics (e.g. age, gender, source and duration of lumbosacral radicular pain), intervention and comparator (e.g. type of injected material, dose, injectate vehicle and anatomical approach), and study results for each primary outcome at the time point follow‐up assessments (e.g. immediate, short term, intermediate term, and long term).
For continuous outcomes, we extracted the change from baseline or final values (standard deviations (SDs)), and sample sizes. When both change from baseline and final values were available, we extracted data from change scores because these measures provide an estimate without the component of between‐person variability (Higgins 2011). However, we extracted the final values (SDs) and sample sizes for disability because included studies consistently reported this type of measure. For dichotomous outcomes, we extracted the proportion of the participants who had an improvement or adverse events and the sample sizes.
Assessment of risk of bias in included studies
We assessed the risk of bias of the included studies using the expanded criteria developed by Cochrane Back and Neck Review Group (Furlan 2015). Two reviewers (CBO and MLF/MH/RZP/PHF) independently assessed the risk of bias of each included trial and we consulted a third reviewer (CGM) to resolve any disagreements. First, we scored the included studies as having high, low or unclear risk of bias for each criterion (see Table 2 and Table 3). We considered all included studies using an epidural injection in the placebo group as having low risk of bias for blinding the participants. Given that in these studies the participants are blinded to the group allocation and that pain and disability are self‐reported measures, we also judged all studies as having low risk of bias for blinding the outcome assessor. Where the placebo injection was not given as an epidural injection (e.g. intramuscular) we judged the trial as having high risk of bias for blinding of clinicians, participants and outcome assessors. We adopted this approach for assessment of the blinding of participants because when the medication (placebo) is given 'epidurally' patients often do not feel something in their leg(s). However, patients receiving a non‐epidural injection would be more unlikely to feel a sensation in their leg(s). Therefore, unless the trial used an epidural injection as the placebo intervention, there might be a strong potential for the patient to be unblinded. In addition, we judged all studies as having low risk of bias for compliance of the intervention due to the type of intervention. After assessment of each individual item, we assessed the risk of bias domains considering the scores of specific items related to each domain. We considered a study as having low risk of bias if we judged four out of the five bias domains as having low risk of bias (Appendix 2).
1. Sources of risk of bias.
| Bias domain | Source of bias | Possible answers |
| Selection | (1) Was the method of randomisation adequate? | Yes/no/unsure |
| Selection | (2) Was the treatment allocation concealed? | Yes/no/unsure |
| Performance | (3) Was the patient blinded to the intervention? | Yes/no/unsure |
| Performance | (4) Was the care provider blinded to the intervention? | Yes/no/unsure |
| Detection | (5) Was the outcome assessor blinded to the intervention? | Yes/no/unsure |
| Attrition | (6) Was the drop‐out rate described and acceptable? | Yes/no/unsure |
| Attrition | (7) Were all randomised participants analysed in the group to which they were allocated? | Yes/no/unsure |
| Reporting | (8) Are reports of the study free of suggestion of selective outcome reporting? | Yes/no/unsure |
| Selection | (9) Were the groups similar at baseline regarding the most important prognostic indicators? | Yes/no/unsure |
| Performance | (10) Were co interventions avoided or similar? | Yes/no/unsure |
| Performance | (11) Was the compliance acceptable in all groups? | Yes/no/unsure |
| Detection | (12) Was the timing of the outcome assessment similar in all groups? | Yes/no/unsure |
| Other | (13) Are other sources of potential bias unlikely? | Yes/no/unsure |
2. Criteria for a judgment of 'yes' for the sources of risk of bias.
| 1 | A random (unpredictable) assignment sequence. Examples of adequate methods are coin toss (for studies with 2 groups), rolling a dice (for studies with 2 or more groups), drawing of balls of different colours, drawing of ballots with the study group labels from a dark bag, computer‐generated random sequence, preordered sealed envelopes, sequentially‐ordered vials, telephone call to a central office, and preordered list of treatment assignments. Examples of inadequate methods are: alternation, birth date, social insurance/security number, date in which they are invited to participate in the study, and hospital registration number. |
| 2 | Assignment generated by an independent person not responsible for determining the eligibility of the patients. This person has no information about the persons included in the trial and has no influence on the assignment sequence or on the decision about eligibility of the patient. |
| 3 | Index and control groups are indistinguishable for the patients or if the success of blinding was tested among the patients and it was successful. |
| 4 | Index and control groups are indistinguishable for the care providers or if the success of blinding was tested among the care providers and it was successful. |
| 5 | Adequacy of blinding should be assessed for each primary outcome separately. This item should be scored ʺyesʺ if the success of blinding was tested among the outcome assessors and it was successful or: ‐ for patient‐reported outcomes in which the patient is the outcome assessor (e.g., pain, disability): the blinding procedure is adequate for outcome assessors if participant blinding is scored ‘‘yes’’ ‐ for outcome criteria assessed during scheduled visit and that supposes a contact between participants and outcome assessors (e.g., clinical examination): the blinding procedure is adequate if patients are blinded, and the treatment or adverse effects of the treatment cannot be noticed during clinical examination ‐ for outcome criteria that do not suppose a contact with participants (e.g., radiography, magnetic resonance imaging): the blinding procedure is adequate if the treatment or adverse effects of the treatment cannot be noticed when assessing the main outcome ‐ for outcome criteria that are clinical or therapeutic events that will be determined by the interaction between patients and care providers (e.g., co‐interventions, hospitalisation length, treatment failure), in which the care provider is the outcome assessor: the blinding procedure is adequate for outcome assessors if item ‘‘4’’ (caregivers) is scored ‘‘yes’’ ‐ for outcome criteria that are assessed from data of the medical forms: the blinding procedure is adequate if the treatment or adverse effects of the treatment cannot be noticed on the extracted data |
| 6 | The number of participants who were included in the study but did not complete the observation period or were not included in the analysis must be described and reasons given. If the percentage of withdrawals and drop‐outs does not exceed 20% for short‐term follow‐up and 30% for long‐term follow‐up and does not lead to substantial bias a ‘‘yes’’ is scored. (N.B. these percentages are arbitrary, not supported by literature). |
| 7 | All randomised patients are reported/analysed in the group they were allocated to by randomizations for the most important moments of effect measurement (minus missing values) irrespective of noncompliance and cointerventions. |
| 8 | All the results from all prespecified outcomes have been adequately reported in the published report of the trial. This information is either obtained by comparing the protocol and the report, or in the absence of theprotocol, assessing that the published report includes enough information to make this judgment. |
| 9 | Groups have to be similar at baseline regarding demographic factors, duration and severity of complaints, percentage of patients with neurological symptoms, and value of main outcome measure(s). |
| 10 | If there were no co‐interventions or they were similar between the index and control groups. |
| 11 | The reviewer determines if the compliance with the interventions is acceptable, based on the reported intensity, duration, number and frequency of sessions for both the index intervention and control intervention(s). For example, physiotherapy treatment is usually administered for several sessions; therefore it is necessary to assess how many sessions each patient attended. For single‐session interventions (e.g., surgery), this item is irrelevant. |
| 12 | Timing of outcome assessment should be identical for all intervention groups and for all primary outcome measures. |
| 13 | Other types of biases. For example: ‐ When the outcome measures were not valid. There should be evidence from a previous or present scientific study that the primary outcome can be considered valid in the context of the present. ‐ Industry‐sponsored trials. The conflict of interest (COI) statement should explicitly state that the researchers have had full possession of the trial process from planning to reporting without funders with potential COI having any possibility to interfere in the process. If, for example, the statistical analyses have been done by a funder with a potential COI, usually ʺunsureʺ is scored. |
Measures of treatment effect
For pain intensity, we estimated weighted mean differences (MD) and 95% CIs using the change scores. If change scores were not available, we used the final scores. Given that the two pain measures most commonly used are the visual analogue scale (range, 0 to 100) and the numerical rating scale (range, 0 to 10), we converted the outcome measures to a common 0 to 100 scale to calculate the pooled effects. We adopted this strategy to harmonise the outcome to a common scale because these scales are highly correlated and can be used interchangeably when transformed (Hjermstad 2011). We defined three levels for the treatment effects considering the MD: small effect size (MD < 10% of the scale), medium effect size (MD 10% to 20% of the scale) or large effect size (MD > 20% of the scale) (Cohen 1988; Rubinstein 2011). For disability, we calculated standardised mean difference (SMD) and 95% CIs because trials used different instruments (i.e. Roland‒Morris Disability questionnaire and Oswestry Disability index). We estimated the SMD using only the final scores because included studies consistently reported this type of measure. To facilitate interpretation, however, we transformed the pooled effect measured as an SMD into an MD expressed on 0 to 100 scale (i.e. the same scale used in the interpretation of the Oswestry Disability index scores) by multiplying the pooled SMD by the standard deviation (SD) of the baseline of a representative study in the meta‐analysis (Higgins 2011). We considered the most representative study in the meta‐analysis the study reporting disability using the Oswestry Disability index (0 to 100) containing the highest weight with low risk of bias. Therefore, we used the SD of 15.5 from the baseline data of Karppinen 2001. Considering that the proposed thresholds for clinically important change vary greatly in the literature and there is no cut‐off for patients with lumbosacral radicular pain, we considered a clinically important difference for most people to be a mean between‐group difference greater than 10% of the scale (Dworkin 2008). We conducted the analyses using random‐effects models to account for between‐study variance. We used Review Manager 5 software to perform the analyses (Review Manager 2014).
For dichotomous outcomes, we calculated risk ratios (RRs) and 95% CIs to describe the treatment effects. Given that the definition of pain relief and disability reduction expressed as dichotomous outcomes varied greatly in the included studies we considered all definitions reported by the studies, including percentage of improvement from baseline, recovery considering pain or disability, and combination of percentage of improvement from baseline and another criterion (e.g. global recovery measured by a global perceived effect scale). We interpreted the magnitude of treatment effects using dichotomous outcomes as follows: small (RR < 1.25), moderate (RR 1.25 to 2.00), or large (RR > 2.0) (Ostelo 2008). When the dichotomous outcome showed significant treatment effects, we also calculated and reported the number needed to treat (with active rather than placebo treatment) for an additional beneficial outcome (NNTB) based on absolute risk difference.
Unit of analysis issues
We grouped similar trials according to assessment time points (i.e. immediate, short term, intermediate term, and long term) and outcomes (i.e. pain, disability, pain relief, and disability improvement). When trials presented more than one possible placebo intervention, we extracted data from the comparison group that most closely mimicked the epidural injection procedure using a substance that we considered most likely to be inert. When the studies reported more than one pain or disability outcome measure, we chose the outcome specified as the primary outcome in the trial report; or if the primary outcome was not specified, we selected the most commonly reported outcome in our pooled estimate.
Dealing with missing data
When there was insufficient information in trial reports, we first contacted the authors to request the missing data. When this method failed, we estimated data using methods recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Therefore, when SDs were not reported in tables or text we estimated them from graphs. If information regarding SDs was missing, we estimated them from 95% CIs or standard errors. However, if no measure of variability was presented anywhere in the trial report, we estimated the average SD from the most similar trial included in this review, considering the sample size and individual risk of bias.
Assessment of heterogeneity
To assess heterogeneity, we visually inspected the forest plots and calculated the I² statistic for each pooled analysis, as recommended by the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We assessed heterogeneity using the I² statistic and classified it as low (I² values < 40%), moderate to substantial (I² values from 40% to 75%), and high (I² values > than 75%) (Furlan 2015).
Assessment of reporting biases
We assessed reporting bias for each meta‐analysis with more than 10 trials by visual interpretation of the funnel plot asymmetry. If the funnel plots suggested publication bias, we downgraded the quality of the evidence by one level.
Data synthesis
We assessed the overall quality of evidence for each outcome and time‐point assessment using the GRADE approach, as recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), and adapted in the updated Cochrane Back and Neck Group guidelines (Furlan 2015). We downgraded the quality of evidence of the meta‐analysis by one level when each domain was not satisfied. For each meta‐analysis, we downgraded the quality of the evidence by one level, according to the performance against five domains: risk of bias, imprecision, inconsistency, indirectness, and publication bias. Appendix 2 describes the interpretation of each domain and quality of evidence.
'Summary of findings' tables
For the 'Summary of findings' tables, we included data for each primary outcome (i.e. leg pain intensity, disability) and adverse events at immediate, short‐term, intermediate and long‐term follow‐up separately in each table. We opted for providing the primary outcomes at short‐term follow‐up as the main comparison, due to the mechanism of action expected from epidural corticosteroid injection.
Subgroup analysis and investigation of heterogeneity
We performed subgroup analyses for the primary outcome (i.e. leg pain at short‐term follow‐up) to investigate the potential influence of trial characteristics. They were planned to investigate whether the efficacy of epidural corticosteroid injections varied by epidural injection approaches (i.e. caudal vs interlaminar vs transforaminal), use of imaging to guide the injection (i.e. use of imaging guiding vs no use of imaging guided), type of placebo (i.e. epidural anaesthetic vs epidural saline vs interspinous saline), and definition of lumbosacral radicular pain (i.e. clinical assessment vs required concordant imaging). We conducted the subgroup analyses using a random‐effects model.
Sensitivity analysis
We conducted sensitivity analyses to investigate the impact of risk of bias on estimates of treatment effect on leg pain at short‐term follow‐up. We performed a sensitivity analysis including only studies having low risk of bias, classified as studies having four out of five bias domains that we judged as having low risk of bias. We also performed a sensitivity analysis considering the three following individual criteria: allocation concealment (i.e. criterion 2 from the risk of bias tool); blinding of care provider (i.e. criterion 4 from the risk of bias tool); and intention‐to‐treat analysis (i.e. criterion 9 from the risk of bias tool). These criteria were selected considering previous studies showing that the lack of these domains may overestimate the treatment effect.
Results
Description of studies
The details of the studies are provided in the Characteristics of included studies, Characteristics of excluded studies and Characteristics of ongoing studies tables.
Results of the search
The updated literature searches from 2012 to 25 September 2019 identified 4724 records. After removing duplicates 3027 studies remained for title and abstract screening. From these, we identified 33 potentially eligible studies. Following full‐text assessment we considered six trials to be eligible: two studies reporting full results from preliminary trials included in the original review (Manchikanti 2012; Manchikanti 2014a); and four additional trials (Datta 2011; Ghai 2015; Manchikanti 2014b; Nandi 2017). In addition, nineteen trials were included from our earlier review (Arden 2005; Bush 1991; Carette 1997; Cohen 2012; Cuckler 1985; Dilke 1973; Ghahreman 2010; Helliwell 1985; Iversen 2011; Karppinen 2001; Klenerman 1984; Kraemer 1997; Mathews 1987; Ng 2005; Ridley 1988; Rogers 1992; Snoek 1977; Tafazal 2009; Valat 2003). In total, the current review includes 25 clinical trials (reported in 29 publications).
The search for ongoing and unpublished trials retrieved 419 registered trials. After removing duplicates and irrelevant titles, we assessed 27 trials for eligibility. Of these, we considered two ongoing trials (NTR4457; NCT03240783) to be eligible for this review (Characteristics of ongoing studies). We included one study (Vad 2002) from the previous review in the awaiting classification section because we are waiting for the authors to clarify the aspects of the randomisation process and another study (Abedini 2018) because we are also waiting more details regarding the study population (Characteristics of studies awaiting classification). The details of the selection of the studies are described in the study flow diagram (Figure 1).
1.

Study flow diagram.
Included studies
Among the included 25 clinical trials, 14 were conducted in Europe (Arden 2005; Bush 1991; Dilke 1973; Iversen 2011; Karppinen 2001; Klenerman 1984; Kraemer 1997; Mathews 1987; Ng 2005; Ridley 1988; Rogers 1992; Snoek 1977; Tafazal 2009; Valat 2003); six in North America (Carette 1997; Cohen 2012; Cuckler 1985; Manchikanti 2012; Manchikanti 2014a; Manchikanti 2014b); three in Asia (Ghai 2015; Nandi 2017; Datta 2011); one in Oceania (Ghahreman 2010); and we could not identify the country or continent where Helliwell 1985 was undertaken. The included studies reported outcomes for a total of 2470 participants with mean ages ranging from 37.3 to 52.8 years. Of note: there was an increase of 509 participants in comparison with the original review which identified 21 trials reporting outcomes for a total of 1961 participants (Pinto 2012). Eight studies diagnosed lumbosacral radicular pain based upon clinical assessment and imaging findings (Carette 1997; Cohen 2012; Ghahreman 2010; Ghai 2015; Nandi 2017; Ng 2005; Snoek 1977; Tafazal 2009); and 17 studies formed the diagnosis based solely on the clinical assessment (Arden 2005; Bush 1991; Cuckler 1985; Datta 2011; Dilke 1973; Helliwell 1985; Iversen 2011; Karppinen 2001; Klenerman 1984; Kraemer 1997; Manchikanti 2012; Manchikanti 2014a; Manchikanti 2014b; Mathews 1987; Ridley 1988; Rogers 1992; Valat 2003). Fifteen trials involved patients with a mixed duration of symptoms (i.e. acute, subacute, or chronic) (Arden 2005; Carette 1997; Cohen 2012; Cuckler 1985; Datta 2011; Dilke 1973; Ghahreman 2010; Karppinen 2001; Klenerman 1984; Kraemer 1997; Nandi 2017; Ridley 1988; Rogers 1992; Snoek 1977; Valat 2003); one trial investigated patients with acute and subacute symptoms (Mathews 1987); and two trials included patients with subacute and chronic symptoms (Helliwell 1985; Ng 2005). One trial included only patients with acute symptoms (Bush 1991); and six trials included only patients with chronic symptoms (Ghai 2015; Iversen 2011; Manchikanti 2012; Manchikanti 2014a; Manchikanti 2014b; Tafazal 2009).
Considering the epidural injection approaches, seven trials used the caudal approach (Bush 1991; Datta 2011; Ghai 2015; Iversen 2011; Manchikanti 2012; Mathews 1987; Nandi 2017); six trials administered corticosteroids using the transforaminal approach (Cohen 2012; Ghahreman 2010; Karppinen 2001; Manchikanti 2014b; Ng 2005; Tafazal 2009); and 12 trials using the interlaminar approach (Arden 2005; Carette 1997; Cuckler 1985; Dilke 1973; Helliwell 1985; Klenerman 1984; Kraemer 1997; Manchikanti 2014a; Ridley 1988; Rogers 1992; Snoek 1977; Valat 2003). The corticosteroids investigated were methylprednisolone (Carette 1997; Cohen 2012; Cuckler 1985; Dilke 1973; Ghai 2015; Helliwell 1985; Karppinen 2001; Klenerman 1984; Mathews 1987; Nandi 2017; Ng 2005; Ridley 1988; Rogers 1992; Snoek 1977; Tafazal 2009); prednisone or prednisolone (Valat 2003); triamcinolone (Arden 2005; Bush 1991; Ghahreman 2010; Iversen 2011; Kraemer 1997); and betamethasone (Manchikanti 2014a; Manchikanti 2014b). One study administered methylprednisolone or betamethasone (Manchikanti 2012); and another study investigated the efficacy of three different types of corticosteroid: methylprednisolone, triamcinolone, and dexamethasone (Datta 2011).
The placebo interventions varied among included trials. Some trials used a less invasive placebo approach. Two studies used intramuscular injection of paravertebral muscles or injection at points of maximum tenderness on the back muscles or over the sacral hiatus (Kraemer 1997; Mathews 1987); and four studies used interspinous injection (Arden 2005; Dilke 1973; Helliwell 1985; Ridley 1988). Most trials investigated a placebo approach that was as invasive as the active approach, such as the epidural approach (Bush 1991; Carette 1997; Cohen 2012; Cuckler 1985; Ghahreman 2010; Iversen 2011; Karppinen 2001; Manchikanti 2012; Manchikanti 2014a; Manchikanti 2014b; Nandi 2017; Ng 2005; Rogers 1992; Snoek 1977; Tafazal 2009; Valat 2003). The substances used in the placebo injection were saline solution (Arden 2005; Bush 1991; Carette 1997; Cohen 2012; Dilke 1973; Helliwell 1985; Karppinen 2001; Nandi 2017; Ridley 1988; Snoek 1977; Valat 2003); anaesthetic (Datta 2011; Ghai 2015; Kraemer 1997; Manchikanti 2012; Manchikanti 2014a; Manchikanti 2014b; Mathews 1987; Ng 2005; Tafazal 2009); or a combination of saline and anaesthetic (Cuckler 1985; Rogers 1992). Three studies investigated two types of placebo administered via epidural route: saline solution alone and combining saline and anaesthetic solution (Ghahreman 2010; Iversen 2011; Klenerman 1984). For these studies, we considered the group receiving the most inert substance (i.e. saline solution alone) in the quantitative analysis (Ghahreman 2010; Iversen 2011; Klenerman 1984).
Excluded studies
We excluded 26 studies because they did not enrol patients with lumbosacral radicular pain (but conditions such as central canal stenosis and non‐specific lower back pain, for example) (Baek 2019; Friedly 2017; Friedly 2019; Glémarec 2018; Hauritz 2018; Kennedy 2018; Manchikanti 2008; Manchikanti 2015; Okmen 2017; Sayegh 2009); they were not placebo‐controlled trials (Ackerman 2007; Ahadian 2011; Becker 2007; Beliveau 1971; Buchner 2000; Burgher 2011; Cohen 2015; Dashfield 2005; Kamble 2016; Kim 2018; Lee 2009; Wilson‐MacDonald 2005; Yin 2018); they included patients with previous surgery (Breivik 1976; el Zahaar 1991); or they did not actually investigate epidural corticosteroid injection (Freeman 2013). In addition, we excluded one trial from the original review due to unclear information regarding the randomisation of the participants (Swerdlow 1970). We excluded 25 registered trials because they were either not placebo‐controlled (n = 22) or did not include patients with lumbosacral radicular pain (n = 3). We provide more details of excluded studies in the Characteristics of excluded studies table.
Risk of bias in included studies
We provide the 'Risk of bias' assessments for all included studies in Characteristics of included studies, Figure 2, and Figure 3. We considered eight trials to be of high quality because we judged them as having low risk of bias in four out of the five bias domains (Cohen 2012; Ghai 2015; Iversen 2011; Karppinen 2001; Manchikanti 2012; Ng 2005; Manchikanti 2014a; Manchikanti 2014b).
2.
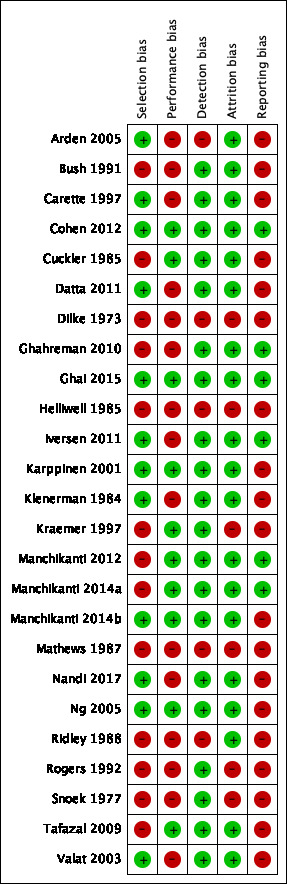
Risk of bias summary: review authors' judgements about each type of bias for each included study.
3.

Risk of bias graph: review authors' judgements about each type of bias presented as percentages across all included studies.
Allocation
We judged 18 studies as having low risk of bias for random sequence generation because they reported the use of an appropriate method to generate the allocation schedule. We judged five studies as having unclear risk of bias because they reported random allocation of the participants but failed to describe the methods (Bush 1991; Cuckler 1985; Helliwell 1985; Kraemer 1997; Mathews 1987). We judged one trial as having high risk of bias, because the eligible patients were randomised by one of the authors of the study (Snoek 1977). Twelve studies reported use of an appropriate method of allocation concealment (Arden 2005; Carette 1997; Cohen 2012; Datta 2011; Ghai 2015; Iversen 2011; Karppinen 2001; Klenerman 1984; Manchikanti 2014b; Ng 2005; Nandi 2017; Valat 2003). We judged 13 studies as having unclear risk of bias because they did not mention allocation concealment. We judged 12 studies as having low risk of bias for the selection bias domain because we judged them as having low risk of bias for random sequence generation and allocation concealment (Arden 2005; Carette 1997; Cohen 2012; Datta 2011; Ghai 2015; Iversen 2011; Karppinen 2001; Klenerman 1984; Manchikanti 2014b; Ng 2005; Nandi 2017; Valat 2003).
Blinding
Given that all included trials were placebo‐controlled and the outcomes of interest of this review were self‐report measures, we judged all studies using an epidural approach in the placebo group as having low risk of bias for blinding the participants and the outcome assessor. We judged five trials as having high risk of bias because they investigated other types of placebo (e.g. intramuscular and interspinous injections) (Arden 2005; Dilke 1973; Helliwell 1985; Mathews 1987; Ridley 1988). Ten trials reported blinding of care provider (Cohen 2012; Cuckler 1985; Ghai 2015; Karppinen 2001; Kraemer 1997; Manchikanti 2012; Ng 2005; Tafazal 2009; Manchikanti 2014a; Manchikanti 2014b). We judged two as having high risk of bias because they reported that the health care provider was aware of the treatments (Iversen 2011; Nandi 2017). We also judged the remaining trials as having high risk of bias because they clearly described different procedures for administering the interventions and did not report any attempts for blinding the health care provider. For the performance bias domain, we judged 10 studies as having low risk of bias because we judged them as having low risk of bias for blinding of participants and healthcare providers (Cohen 2012; Cuckler 1985; Ghai 2015; Karppinen 2001; Kraemer 1997; Manchikanti 2012; Ng 2005; Tafazal 2009; Manchikanti 2014a; Manchikanti 2014b). Regarding the detection bias domain, we judged 20 studies as having low risk of bias because we judged them as having low risk of bias for blinding of outcome assessors (Bush 1991; Carette 1997; Cohen 2012; Cuckler 1985; Datta 2011; Ghahreman 2010; Ghai 2015; Iversen 2011; Karppinen 2001; Klenerman 1984; Kraemer 1997; Manchikanti 2012; Manchikanti 2014a; Manchikanti 2014b; Nandi 2017; Ng 2005; Rogers 1992; Snoek 1977; Tafazal 2009; Valat 2003).
Incomplete outcome data
We judged 20 trials as having low risk of bias since they reported less than 20% loss to follow‐up at short‐term follow‐up or less than 30% loss at long‐term follow‐up. We judged one study as having high risk of bias because loss to short‐term follow‐up was above the threshold (Mathews 1987). We judged the remaining four studies as having unclear risk of bias because the reports contained insufficient information for assessing loss to follow‐up (Dilke 1973; Helliwell 1985; Kraemer 1997; Rogers 1992). We judged 20 studies as having low risk of bias for the attrition bias domain because we judged them as having low risk of bias for adequate follow‐up rate (Arden 2005; Bush 1991; Carette 1997; Cohen 2012; Cuckler 1985; Datta 2011; Ghahreman 2010; Ghai 2015; Iversen 2011; Karppinen 2001; Klenerman 1984; Manchikanti 2012; Manchikanti 2014a; Manchikanti 2014b; Nandi 2017; Ng 2005; Ridley 1988; Snoek 1977; Tafazal 2009; Valat 2003).
Selective reporting
Eight trials were previously registered (Arden 2005; Cohen 2012; Ghahreman 2010; Ghai 2015; Iversen 2011; Manchikanti 2012; Manchikanti 2014a; Manchikanti 2014b). We judged six trials as having low risk of bias because we did not identify any discrepancy between protocol and publication (Cohen 2012; Ghahreman 2010; Ghai 2015; Iversen 2011; Manchikanti 2012; Manchikanti 2014a). Arden 2005 did not provide any data regarding an outcome reported in the protocol (i.e. mood) and we judged it as having high risk of bias. Manchikanti 2014b reported pain and disability measured as continuous outcomes in the protocol, but also reported them as dichotomous outcomes in the publication; therefore, we judged it as having high risk of bias. We judged the remaining trials as having unclear risk of bias because they did not provide any information on trial registration. We judged six studies as having low risk of bias for the reporting bias domain because we judged them as having low risk of bias for selective reporting (Cohen 2012; Ghahreman 2010; Ghai 2015; Iversen 2011; Manchikanti 2012; Manchikanti 2014a).
Other potential sources of bias
A rule of thumb suggests that publication bias should be assessed solely in comparisons that include at least 10 studies (Furlan 2015). Thus, we assessed publication bias for overall pain and disability at short‐term follow‐up (Figure 4; Figure 5). Our visual inspection of the funnel plots revealed no asymmetry.
4.

Funnel plot of comparison: 1 Epidural corticosteroid injection versus placebo, outcome: 1.2 Disability at short‐term
5.
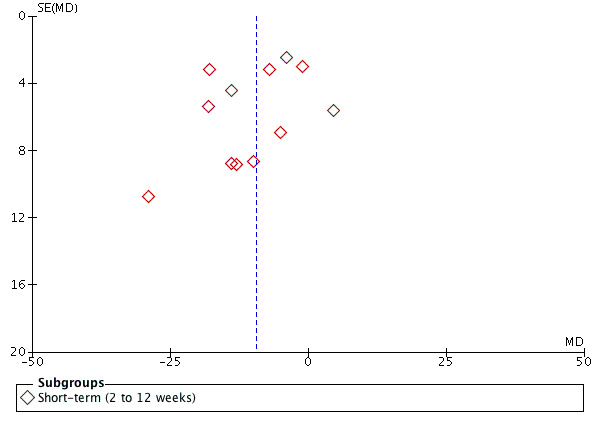
Funnel plot of epidural corticosteroid injection versus placebo. Outcome: 1.3 Overall pain at short‐term follow‐up.
Effects of interventions
See: Table 1
See Summary of findings table 1 for the main comparison and most clinically important time point – primary outcomes at short‐term follow‐up; Additional summary of findings Table 4 for the main comparison – primary outcomes at immediate follow‐up; Additional summary of findings Table 4Table 5 for the main comparison – primary outcomes at intermediate follow‐up; Additional summary of findings Table 6 for the main comparison – primary outcomes at long‐term follow‐up; and Summary of key results Table 7.
3. Additional summary of findings table ‐ immediate follow‐up.
| Epidural corticosteroid injection compared to placebo injection for lumbosacral radicular pain | ||||||
|
Patient or population: lumbosacral radicular pain Setting: secondary and tertiary care Intervention: epidural corticosteroid injection Comparison: placebo injection | ||||||
| Outcomes | Illustrative comparative risks (95% CI) | Treatment effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk* | |||||
| Placebo injection | Epidural corticosteroid injection | |||||
|
Leg pain ‒immediate follow‐up (≤ 2 weeks) Leg pain reported using VAS (0 to 100) and NRS (0 to 10). Pain scores were converted to a common 0 to 100 scale. Higher scores indicate worse leg pain |
The mean leg pain in the placebo group was 54.1 points. | The mean leg pain in the epidural corticosteroid injection groups was 15.0 points lower (25.88 lower to 4.12 lower) than in the placebo group | Mean difference −15.0 (−25.88 to −4.12) | 158 participants (1 RCTs) | ⊕⊕⊕⊝ MODERATE1 | |
|
Disability ‒immediate follow‐up (≤ 2 weeks) Disability reported using multiple scales (RMDQ and ODI). Disability scores were converted to a common 0 to 100 scale. Higher scores indicate greater disability. |
The mean disability across the placebo groups ranged from 12.8 to 15.8 points | The mean disability in the epidural corticosteroid injection groups was 1.24 points higher (2.63 lower to 5.11 higher) | Mean difference 1.24 (−2.63 to 5.11) | 243 participants (2 RCTs) | ⊕⊝⊝⊝ VERY LOW 1, 2, 3 | We calculated the standardised mean difference for disability (SMD 0.08, 95% CI −0.17 to 0.33) and translated to unstandardised mean difference using the baseline SD from Karppinen 2001. |
|
Minor adverse events** ‒immediate follow‐up (≤ 2 weeks) Minor adverse events provided as the proportion of patients reporting any untoward medical occurrence after an epidural injection without hospitalisations. |
128 of 484 (26 per 100) participants reported minor adverse events | 65 of 393 (16 per 100) participants reported minor adverse events. | RR 1.14 (0.91 to 1.42) | 877 participants (8 RCTs) | ⊕⊝⊝⊝ VERY LOW 1, 2, 3 | Adverse events included increased pain during or after the injection, non‐specific headache, post‐dural puncture headache injection, irregular periods, accidental dural puncture, thoracic pain, non‐local rash, sinusitis, vasovagal response, hypotension, nausea, and tinnitus. |
|
Major adverse events** immediate follow‐up (≤ 2 weeks) Major adverse events provided as the proportion of patients reporting any untoward medical occurrence after an epidural injection with hospitalizations. |
1 of 80 (1 per 100) reported major adverse events | 0 of 80 (0 per 100) reported major adverse events | ‐ | 160 participants (1 RCT) | ⊕⊕⊕⊝ Moderate1 | 1 study reported that 1 patient on anticoagulant therapy had a retroperitoneal haematoma as a complication of the injection. |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
|
GRADE Working Group grades of evidence
High quality: further research is very unlikely to change confidence in estimate of effect
Moderate quality: further research is likely to have an important impact on confidence in estimate of effect and may change the estimate
Low quality: further research is very likely to have an important impact on confidence in estimate of effect and is likely to change the estimate
Very low quality: very little confidence in the effect estimate. No evidence: no RCTs were identified that addressed this outcome | ||||||
1 Downgraded 1 level due to imprecision (number of participants lower than 400 for continuous outcomes or number of events lower than 300 for dichotomous outcomes)
2 Downgraded 1 level due to risk of bias (more than 25% of the participants were from studies judged as having high risk of bias)
3 Downgraded 1 level due to inconsistency (heterogeneity of pooled estimates greater than moderate (I² > 45%)
**Most trials provided insufficient information on how or when adverse events were assessed (immediate or short‐term follow‐up). In addition, most trials only reported adverse drug reactions, that is adverse events that the trialists attributed to the study treatment. 5 studies reported that there were no adverse events and 6 trials reported adverse drug reactions or adverse events without specifying which group the patients had been allocated.
4. Additional summary of findings table ‐ intermediate follow‐up.
| Epidural corticosteroid injection compared to placebo injection for lumbosacral radicular pain | ||||||
|
Patient or population: lumbosacral radicular pain Setting: secondary and tertiary care Intervention: epidural corticosteroid injection Comparison: placebo injection | ||||||
| Outcomes | Illustrative comporative risks (95% CI) | Treatment effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk* | |||||
| Placebo injection | Epidural corticosteroid injection | |||||
|
Leg pain ‒intermediate follow‐up (> 3 months but < 12 months) Leg pain reported using VAS (0 to 100) and NRS (0 to 10). Pain scores were converted to a common 0 to 100 scale. Higher scores indicate worse leg pain |
The mean leg pain in the placebo group was 21.6 points. | The mean leg pain in the epidural corticosteroid injection group was 9.10 points higher (1.44 lower to 19.64 higher) than in the placebo group | Mean difference 9.10 (−1.44 to 19.64) | 158 participants (1 RCTs) | ⊕⊕⊕⊝ MODERATE1 | |
|
Disability ‒intermediate follow‐up (> 3 months but < 12 months) Disability reported using multiple scales (RMDQ and ODI). Disability scores were converted to a common 0 to 100 scale. Higher scores indicate greater disability. |
The mean disability across the placebo groups ranged from 15.8 to 33.8 points | The mean disability in the epidural corticosteroid injection groups was 3.10 points lower (6.20 lower to 0.15 higher) | Mean Difference −3.10 (−6.20 to −0.15) | 866 participants (6 RCTs) | ⊕⊕⊝⊝ LOW 2, 3 | We calculated the standardised mean difference for disability (SMD −0.20, 95% CI −0.40 to −0.01) and translated to unstandardised mean difference using the the baseline SD from Karppinen 2001. |
|
Minor adverse events ‒intermediate follow‐up (> 3 months but < 12 months) Minor adverse events provided as the proportion of patients reporting any untoward medical occurrence after an epidural injection without hospitalisations. |
Not reported | |||||
|
Major adverse events intermediate follow‐up (> 3 months but < 12 months) Major adverse events provided as the proportion of patients reporting any untoward medical occurrence after an epidural injection with hospitalizations. |
Not reported | |||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
|
GRADE Working Group grades of evidence
High quality: further research is very unlikely to change confidence in estimate of effect
Moderate quality: further research is likely to have an important impact on confidence in estimate of effect and may change the estimate
Low quality: further research is very likely to have an important impact on confidence in estimate of effect and is likely to change the estimate
Very low quality: very little confidence in the effect estimate. No evidence: no RCTs were identified that addressed this outcome | ||||||
1 Downgraded 1 level due to imprecision (number of participants lower than 400 for continuous outcomes or number of events lower than 300 for dichotomous outcomes)
2 Downgraded 1 level due to risk of bias (more than 25% of the participants were from studies judged as having high risk of bias)
3 Downgraded 1 level due to inconsistency (heterogeneity of pooled estimates greater than moderate (I² > 45%))
5. Additional summary of findings table ‐ long‐term follow‐up.
| Epidural corticosteroid injection compared to placebo injection for lumbosacral radicular pain | ||||||
|
Patient or population: lumbosacral radicular pain Setting: secondary and tertiary care Intervention: epidural corticosteroid injection Comparison: placebo injection | ||||||
| Outcomes | Illustrative comporative risks (95% CI) | Treatment effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk* | |||||
| Placebo injection | Epidural corticosteroid injection | |||||
|
Leg pain ‒long‐term follow‐up (≥ 12 months) Leg pain reported using VAS (0 to 100) and NRS (0 to 10). Pain intensity scores were converted to a common 0 to 100 scale. Higher scores indicate worse leg pain |
The mean leg pain across the placebo groups ranged from 20.0 to 27.1 points. | The mean leg pain in the epidural corticosteroid injection groups was 0.35 points lower (6.23 lower to 5.53 higher) | Mean difference −0.35 (−6.23 to 5.53) | 453 participants (3 RCTs) | ⊕⊕⊕⊝ MODERATE 1 | |
|
Disability ‒long‐term follow‐up (≥ 12 months) Disability reported using multiple scales (RMDQ and ODI). Disability scores were converted to a common 0 to 100 scale. Higher scores indicate greater disability. |
The mean across the placebo groups ranged from 14.1 to 31.8 points | The mean disability in the epidural corticosteroid injection groups was 2.17 points lower (5.89 lower to 1.55 higher) | Mean difference −2.17 (−5.89 to 1.55) | 882 participants (7 RCTs) | ⊕⊕⊝⊝ LOW 1, 2 | We calculated the standardised mean difference for disability (SMD −0.14 (95% CI −0.38 to 0.10) and translated to unstandardised mean difference using the baseline SD from Karppinen 2001. |
|
Minor adverse events ‒long‐term follow‐up (≥ 12 months) Minor adverse events provided as the proportion of patients reporting any untoward medical occurrence after an epidural injection without hospitalizations. |
Not reported | |||||
|
Major adverse events ‒long‐term follow‐up (≥ 12 months) Major adverse events provided as the proportion of patients reporting any untoward medical occurrence after an epidural injection with hospitalizations. |
Not reported | |||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
|
GRADE Working Group grades of evidence
High quality: further research is very unlikely to change confidence in estimate of effect
Moderate quality: further research is likely to have an important impact on confidence in estimate of effect and may change the estimate
Low quality: further research is very likely to have an important impact on confidence in estimate of effect and is likely to change the estimate
Very low quality: very little confidence in the effect estimate. No evidence: no RCTs were identified that addressed this outcome | ||||||
1 Downgraded 1 level due to risk of bias (more than 25% of the participants were from studies judged as having high risk of bias)
2 Downgraded 1 level due to inconsistency (heterogeneity of pooled estimates greater than moderate (I² > 45%))
6. Summary of key results.
| Primary outcomes |
Immediate follow‐up |
Short‐term follow‐up |
Intermediate follow‐up |
Long‐term follow‐up |
| Leg pain intensity | Medium difference | Small difference | No difference | No difference |
| Disability | No difference | Small difference | Small difference | No difference |
| Adverse events | No difference | No difference | Not reported | Not reported |
| Note: comparison of epidural corticosteroid injection and placebo injection in patients with lumbosacral radicular pain. When there were significant differences, they were favouring the epidural corticosteroid injection. | ||||
Primary outcomes
Leg pain intensity
Eight trials reported leg pain data from 949 participants with lumbosacral radicular pain (Arden 2005; Carette 1997; Cohen 2012; Ghahreman 2010; Iversen 2011; Karppinen 2001; Ng 2005; Tafazal 2009). Epidural corticosteroid injections were probably slightly more effective compared to placebo injection for reducing leg pain at immediate follow‐up (MD −15.0, 95% CI −25.88 to –4.12 on a 0 to 100 scale; 1 trial, 158 participants; moderate‐quality evidence (downgraded for imprecision)). Epidural corticosteroid injections were probably slightly more effective compared to placebo injection for reducing leg pain at short‐term follow‐up (MD −4.93, 95% CI −8.77 to –1.09 on a 0 to 100 scale; 8 trials, 949 participants; moderate‐quality evidence (downgraded for risk of bias)). The effects of treatment are small, however, and may not be considered clinically important by patients and clinicians (i.e. MD lower than 10%). Epidural corticosteroid injections probably have no effect compared to placebo injection for reducing leg pain at intermediate follow‐up (MD 9.10, 95% CI −1.44 to 19.64 on a 0 to 100 scale; 1 trial, 158 participants; moderate‐quality evidence (downgraded for imprecision)); and at long‐term follow‐up (MD −0.35, 95% CI −6.23 to 5.53 on a 0 to 100 scale; 3 trials, 453 participants; moderate‐quality evidence (downgraded for risk of bias)) (Analysis 1.1).
Disability
Twelve studies reported disability outcomes from 1367 participants with lumbosacral radicular pain using the Roland‒Morris Disability Questionnaire (Valat 2003), Oswestry Disability Index (Arden 2005; Carette 1997; Cohen 2012; Ghai 2015; Iversen 2011; Karppinen 2001; Manchikanti 2012; Manchikanti 2014a; Manchikanti 2014b; Ng 2005) or both (Nandi 2017). Two trials reported insufficient information regarding the measure of variability (Arden 2005; Carette 1997). We estimated their SDs using the average baseline SDs from the most similar trial (Karppinen 2001).
We are very uncertain that epidural corticosteroid injections have no effect compared to placebo injection in reducing disability at immediate follow‐up (SMD 0.08, 95% CI −0.17 to 0.33; 2 trials, 243 participants; very low quality evidence (downgraded for risk of bias, imprecision, and inconsistency)). Epidural corticosteroid injections were probably slightly more effective compared to placebo injection for reducing disability at short‐term follow‐up (SMD −0.27, 95% CI −0.39 to −0.14; 12 trials, 1367 participants; moderate‐quality evidence (downgraded for risk of bias)). In addition, epidural corticosteroid injections may be slightly more effective compared to placebo injection for reducing disability at intermediate follow‐up (SMD −0.20, 95% CI −0.40 to −0.01; 6 trials, 866 participants; low‐quality evidence (downgraded for risk of bias and inconsistency)). Epidural corticosteroid injections may have no effect compared to placebo injection for reducing disability at long‐term follow‐up (SMD −0.14, 95% CI −0.38 to 0.10; 7 trials, 882 participants; low‐quality evidence (downgraded for risk of bias and inconsistency)) (Analysis 1.2). The SMD results of the pooled effect for disability translates to an unstandardised mean difference of 1.24 (95% CI −2.63 to 5.11, on a 0 to 100 scale) at immediate follow‐up; −4.18 (95% CI −6.04 to −2.17, on a 0 to 100 scale) at short‐term follow‐up; −3.10 (95% CI −6.20 to −0.15, on a 0 to 100 scale) at intermediate follow‐up; and −2.17 (95% CI −5.89 to 1.55, on a 0 to 100 scale) at long‐term follow‐up. Similarly to leg pain estimates the effects of treatment are small and may not be considered clinically important by patients and clinicians (i.e. MD lower than 10%).
Secondary outcomes
Overall pain intensity
Ten trials reported overall pain data from 911 participants with lumbosacral radicular pain (Bush 1991; Datta 2011; Ghai 2015; Helliwell 1985; Klenerman 1984; Manchikanti 2012; Manchikanti 2014a; Manchikanti 2014b; Nandi 2017; Valat 2003). Two trials reported insufficient information regarding the measure of variability (Datta 2011; Klenerman 1984): we estimated their SDs using the average SDs from the most similar trials (Arden 2005 and Helliwell 1985 respectively). In addition, one study investigated the efficacy of three different types of corticosteroid (Datta 2011); we therefore included each intervention group separately as separated study data in the meta‐analysis and adjusted the sample size of the placebo group accordingly (i.e. the placebo group sample size was divided by three) to avoid 'double‐counts' of participants.
Epidural corticosteroid injections may have no effect compared to placebo injection for reducing overall pain at immediate follow‐up (MD −4.71, 95% CI −12.92 to 3.50 on a 0 to 100 scale; 2 trials, 120 participants; low‐quality evidence (downgraded for risk of bias and imprecision)). Epidural corticosteroid injections may be slightly more effective compared to placebo injection for reducing overall pain at short‐term follow‐up (MD −9.35, 95% CI −14.05 to −4.65 on a 0 to 100 scale; 10 trials, 911 participants; low‐quality evidence (downgraded for risk of bias and inconsistency)). The effects of treatment are small, however, and may not be considered clinically important by patients and clinicians (i.e. MD lower than 10%). Epidural corticosteroid injections probably have no effect compared to placebo injection for reducing overall pain at intermediate follow‐up (MD −4.86, 95% CI –10.41 to 0.69 on a 0 to 100 scale; 4 trials, 429 participants; moderate‐quality evidence (downgraded for inconsistency)). Epidural corticosteroid injections were probably slightly more effective compared to placebo injection for reducing overall pain at long‐term follow‐up (MD −6.94, 95% CI −13.69 to −0.19 on a 0 to 100 scale; 5 trials, 452 participants; moderate‐quality evidence (downgraded for inconsistency)) (Analysis 1.3). Similarly, the effects of treatment are small and may not be considered clinically important by patients and clinicians (i.e. MD lower than 10%).
Back pain
Six studies reported back pain data from 728 participants with lumbosacral radicular pain (Arden 2005; Cohen 2012; Iversen 2011; Karppinen 2001; Ng 2005; Tafazal 2009). Epidural corticosteroid injections probably have no effect compared to placebo injection for reducing back pain at immediate follow‐up (MD −10.80, 95% CI −21.64 to 0.04 on a 0 to 100 scale; 1 trial, 158 participants; moderate‐quality evidence (downgraded for imprecision)), short‐term follow‐up (MD −2.01, 95% CI −6.36 to 2.34 on a 0 to 100 scale; 6 trials, 726 participants; moderate‐quality evidence (downgraded for risk of bias)), and intermediate follow‐up (MD 2.50, 95% CI −8.54 to 13.54 on a 0 to 100 scale; 1 trial, 158 participants; moderate‐quality evidence (downgraded for imprecision)). Epidural corticosteroid injections may have no effect compared to placebo injection for reducing back pain at long‐term follow‐up (MD 1.64, 95% CI −4.39 to 7.67 on a 0 to 100 scale; 3 trials, 453 participants; low‐quality evidence (downgraded for risk of bias and inconsistency)) (Analysis 1.4).
Pain relief
Nine trials reported pain relief data (i.e. pain intensity measured as dichotomous outcome) from 919 participants with lumbosacral radicular pain (Arden 2005; Cohen 2012; Ghahreman 2010; Ghai 2015; Manchikanti 2012; Manchikanti 2014a; Manchikanti 2014b; Mathews 1987; Ng 2005). Epidural corticosteroid injections may be slightly more effective compared to placebo injection for relieving pain at short‐term follow‐up (RR 1.17, 95% CI 1.02 to 1.35; 9 trials, 919 participants; low‐quality evidence (downgraded for risk of bias and inconsistency)). The effect was small (i.e. RR < 1.25) and the number needed to treat for an additional beneficial outcome (NNTB) was 10 patients based on an absolute risk difference of 0.10 indicating that 10 patients with lumbosacral radicular pain need to receive epidural corticosteroid injections for one patient to experience pain relief. Epidural corticosteroid injections may have no effect compared to placebo injection for relieving pain at intermediate follow‐up (RR 1.15, 95% CI 0.96 to 1.38; 4 trials, 429 participants; moderate‐quality evidence (downgraded for inconsistency)), and long‐term follow‐up (RR 1.13, 95% CI 0.95 to 1.34; 5 trials, 657 participants; low‐quality evidence (downgraded for risk of bias and inconsistency)) (Analysis 1.5).
Disability reduction
Seven trials reported disability improvement data (i.e. disability measured as dichotomous outcome) from 986 participants with lumbosacral radicular pain (Arden 2005; Carette 1997; Datta 2011; Manchikanti 2012; Manchikanti 2014a; Manchikanti 2014b; Ng 2005). Epidural corticosteroid injections may have no effect compared to placebo injection for disability reduction at short‐term follow‐up (RR 1.09, 95% CI 0.86 to 1.38; 7 trials, 986 participants; low‐quality evidence (downgraded for risk of bias and inconsistency)). We are very uncertain whether there is no effect of epidural corticosteroid injections compared to placebo for disability reduction at intermediate follow‐up (RR 1.09, 95% CI 0.89 to 1.34; 4 trials, 521 participants; very low quality evidence (downgraded for risk of bias, imprecision, and inconsistency)). Epidural corticosteroid injections may have no effect compared to placebo injection for disability reduction at long‐term follow‐up (RR 1.05, 95% CI 0.82 to 1.36; 4 trials, 453 participants; low‐quality evidence (downgraded for imprecision and inconsistency)) (Analysis 1.6).
Adverse events
We originally planned to report adverse events but we were limited by the reporting practices in the trials. Commonly trials only reported adverse drug reactions, that is adverse events that the trialists attributed to the study treatment e.g. "There were no complications attributable to the injections". This practice will necessarily under‐report adverse events. As well the trials often provided insufficient information on how or when adverse events were assessed. Nevertheless, we describe the adverse drug reactions or events narratively and provide a pooled estimate considering the studies reporting data about adverse events separately specific to each group without considering the time‐point assessment. These issues should be borne in mind when reading the results below.
Twenty‐one of the 25 trials reported data regarding adverse events. Five studies reported that there were no adverse events after the interventions. Of these, three studies reported adverse drug reactions (Ghahreman 2010; Klenerman 1984; Tafazal 2009), one study reported no adverse events (Manchikanti 2012), and one study provided insufficient information (Ng 2005). Six trials reported adverse drug reactions or adverse events without specifying to which group the patients had been allocated. Of these, four trials reported adverse drug reactions (Dilke 1973; Iversen 2011; Ridley 1988; Snoek 1977); and two trials reported the results for adverse events (Manchikanti 2014a; Manchikanti 2014b). Dilke 1973 reported that some patients (without specifying the number of events) had increased pain during the injection; and six patients (6%) had an inadvertent dural puncture (i.e. wet tap). Snoek 1977 reported that a few patients had increased pain after the injection. Ridley 1988 reported that one patient had a non‐specific headache (3%); and two patients (6%) had an accidental dural puncture at the time of injection. Iversen 2011 reported that six patients (5%) had increased pain and discontinued the intervention. Manchikanti 2014a reported that eleven patients (9%) had dural punctures; and Manchikanti 2014b reported that 28 patients (23%) had intravascular infiltrations and nine patients (7%) experienced nerve root irritation. In addition, one study reported that all patients in both groups had increased leg pain because the person who administered the epidural injection used this symptom to indicate correct needle placement (Helliwell 1985).
Ten trials reported adverse events from 1170 participants with lumbosacral radicular pain separately for treatment and placebo groups (Arden 2005; Bush 1991; Carette 1997; Cohen 2012; Datta 2011; Ghai 2015; Karppinen 2001; Kraemer 1997; Nandi 2017; Valat 2003). Of these, two studies reported adverse events (Cohen 2012; Ghai 2015); one study reported adverse drug reaction (Karppinen 2001); and the remaining studies provided insufficient information. We are very uncertain that epidural corticosteroid injections make no difference compared to placebo injection in the frequency of minor adverse events (RR 1.14, 95% CI 0.91 to 1.42; 8 trials, 877 participants; very low quality evidence (downgraded for risk of bias, inconsistency and imprecision)) (Analysis 1.7). The very nature of epidural injection means that there is a risk of an accidental dural puncture; and nerve root irritation or serious neurological complication, while very low and not significant, is higher with epidural steroid injection than with soft tissue placebo injection. Adverse events in the included studies were mainly considered to be minor or mild (i.e. without hospitalisation), except for Karppinen 2001, which reported a major drug reaction: one patient (1%) on anticoagulant therapy receiving a transforaminal injection of 40 mg of methylprednisolone had a retroperitoneal haematoma and discontinued participation in the study. Bush 1991 reported that one patient receiving caudal epidural injection of 80 mg of triamcinolone acetonide plus procaine hydrochloride (0.5%) plus saline solution experienced irregular periods over several months. Carette 1997 reported that 21 participants (28%) receiving an interlaminar epidural injection of 80 mg of methylprednisolone and 16 patients (21%) receiving an interlaminar injection with saline had a non‐specific headache and one patient in each group (1% in each group) had an accidental dural puncture during their epidural injection. Valat 2003 reported that two patients (5%) receiving an interlaminar injection of 50 mg of prednisolone experienced a non‐specific headache. In addition, two patients (5%) receiving the interlaminar injection of saline had a non‐specific headache one day after the injection and one patient (2%) had thoracic pain. Arden 2005 reported that four patients in each group receiving interlaminar injection of 80 mg of triamcinolone (3%) or interspinous injection of saline (4%) had a non‐specific headache after the injection, two patients in each group had post‐dural puncture headache and nausea (2% in each group), and five patients in each group (epidural corticosteroid injection 4% and placebo group 5%) had other adverse events. Cohen 2012 reported that one patient (3%) who received a transforaminal injection of 60 mg of methylprednisolone had a non‐local rash one week after the injection. Regarding patients receiving transforaminal saline injection, three (10%) had worsening pain. Of these, two cases were possibly related to the injection (one with and one without new neurologic findings), and one possibly related to the medication or the injection. Three patients reported sinusitis after one month of receiving transforaminal saline injection (10%), but it was considered unrelated to the treatment. Ghai 2015 reported that one patient in each group receiving either a caudal injection of 80 mg of methylprednisolone (3%) or saline solution (3%) had intravascular spread of contrast. In addition, one patient in the placebo group (3%) developed a vasovagal response during injection which was likely related to the injection. Nandi 2017 reported that two patients (4%) had low back pain and one patient (1%) had hypotension the day after the injection, all of which occurred in the group receiving the caudal injection of 80 mg of methylprednisolone. Datta 2011 reported high rates of adverse events across treatment groups. Among the 39 participants receiving a caudal injection of 80 mg of methylprednisolone, eight patients (20%) had local pain persisting more than a day, 15 patients (38%) experienced a non‐specific headache, one patient (2%) had tinnitus, and six had nausea (15%). Of 42 participants receiving a caudal injection of 80 mg triamcinolone, seven patients (17%) had local pain persisting more than a day, 16 patients (38%) had a non‐specific headache, four patients (9%) had tinnitus, seven patients (17%) had nausea, and one patient reported weight gain. Among the 40 participants receiving a caudal injection of 15 mg dexamethasone, four patients (10%) had local pain persisting more than a day, nine patients (22%) experienced a non‐specific headache, one patient (2%) had tinnitus, and eight patients (20%) had nausea. In the 42 participants receiving saline delivered by the caudal approach, three patients (7%) had local pain persisting more than a day, 13 patients (31%) had a non‐specific headache, three patients had tinnitus (3%), and seven patients had nausea (9%). Finally, Kraemer 1997 reported that 1.9%, 3.6% and 1% of the patients reported non‐specific headache after the interlaminar epidural injection, transforaminal epidural injection and the paravertebral injection, respectively. However, this article provided the results of two studies and reported the overall number of events without specifying the number of events in each study. Therefore, we did not include this data in the meta‐analysis.
Subgroup and sensitivity analysis
We conducted subgroup analyses considering the effects of epidural corticosteroid injections compared to placebo injection on reducing leg pain at short‐term follow‐up for different epidural approaches, types of placebo, imaging guidance use, and diagnosis criteria. Our subgroup analyses showed no difference in treatment effects between the epidural injection approaches: caudal (MD −5.30, 95% CI −16.68 to 6.08 on a 0 to 100 scale; 1 trial, 72 participants), interlaminar (MD −1.45, 95% CI −8.09 to 5.20 on a 0 to 100 scale; 2 trials, 384 participants), and transforaminal approaches (MD −6.97, 95% CI −12.14 to −1.79 on a 0 to 100 scale; 5 trials, 493 participants) (Analysis 2.1). In addition, there was no difference in treatment effects between types of placebo injection: epidural anaesthetic (MD −7.04, 95% CI −17.05 to 2.98 on a 0 to 100 scale; 4 trials, 342 participants), epidural saline (MD −7.63, 95% CI −13.10 to −2.16 on a 0 to 100 scale; 5 trials, 511 participants), and interspinous (MD −7.46, 95% CI −24.84 to 9.92 on a 0 to 100 scale; 2 trials, 284 participants) (Analysis 2.2). Similarly, our subgroup analyses showed no difference in treatment effects between studies using imaging to guide the injection (MD −9.50, 95% CI −17.22 to −1.78 on a 0 to 100 scale; 2 trials, 189 participants) and those studies that did not use imaging guidance (MD −3.43, 95% CI −7.86 to 1.01 on a 0 to 100 scale; 6 trials, 760 participants) (Analysis 2.3). Our subgroup analysis showed no difference in treatment effects between studies which required only clinical assessment (MD −3.23, 95% CI −8.97 to 2.51 on a 0 to 100 scale; 3 trials, 460 participants) and those trials which required concordant imaging evidence (MD −6.31, 95% CI −11.49 to −1.14 on a 0 to 100 scale; 5 trials, 489 participants) as the inclusion diagnosis criteria (Analysis 2.4).
We performed sensitivity analysis considering the effects of epidural corticosteroid injections compared to placebo injections on reducing leg pain at short‐term follow‐up for studies we judged as having low risk of bias (i.e. studies having four out of five bias domains we judged as having low risk of bias). In addition, we also performed sensitivity analyses considering studies we judged as having low risk of bias for the following criteria: allocation concealment, intention‐to‐treat analysis, blinding of care provider, and blinding of participants. Our sensitivity analysis demonstrated no difference in treatment effects between studies having low risk of bias (MD −5.01, 95% CI −10.96 to 0.94 on a 0 to 100 scale; 4 trials, 376 participants) and those studies having high risk of bias (MD −4.99, 95% CI −10.32 to 0.33 on a 0 to 100 scale; 4 trials, 573 participants) (Analysis 2.5). Similarly, our sensitivity analyses demonstrated no difference in treatment effects between studies performing allocation concealment (MD −3.43, 95% CI −7.86 to 1.01 on a 0 to 100 scale; 6 trials, 760 participants) and those studies not performing allocation concealment (MD −9.50, 95% CI −17.22 to −1.78 on a 0 to 100 scale; 2 trials, 189 participants) (Analysis 2.6) as well as studies performing intention‐to‐treat analysis (MD −2.74, 95% CI −7.58 to 2.09 on a 0 to 100 scale; 5 trials, 600 participants) and those studies which did not perform an intention‐to‐treat analysis (MD −8.68, 95% CI −15.01 to −2.35 on a 0 to 100 scale; 3 trials, 349 participants) (Analysis 2.7). In contrast, our sensitivity analysis showed significant differences, but not clinically important (i.e. MD < 10% of the scale) in treatment effects between studies that blinded the care provided (MD −5.84, 95% CI −11.41 to −0.26 on a 0 to 100 scale; 4 trials, 428 participants) and those studies which did not blind the care provider (MD −4.11, 95% CI −9.41 to 1.20 on a 0 to 100 scale; 4 trials, 521 participants) (Analysis 2.8). Regarding blinding participants, our sensitivity analysis showed no difference in treatment effects between studies that blinded the participants (MD −6.27, 95% CI −10.60 to −1.94 on a 0 to 100 scale; 7 trials, 721 participants) and those studies that did not blind the participants (MD 0.00, 95% CI −8.32 to 8.32 on a 0 to 100 scale; 1 trial, 228 participants) (Analysis 2.9).
Discussion
Summary of main results
We found moderate‐quality evidence that epidural corticosteroid injections were probably slightly more effective compared to placebo in reducing leg pain and disability at short‐term follow‐up. The treatment effects were small, however, and may not be considered clinically important by patients and clinicians. We identified mostly minor adverse events (e.g. headache, accidental dural punctures, worsening pain) after epidural corticosteroid injections and placebo injections and very low quality evidence that there is no difference between groups. The overall quality of evidence was at best moderate which implies that further studies are likely to change our estimates.
Overall completeness and applicability of evidence
The included studies were conducted in North America, Europe, and Asia. Regarding the epidural injection, most of the trials reported that the care provider was experienced in administering the injection. In addition, we found very low quality of evidence that there is no difference between epidural corticosteroid injections and placebo injection in frequency of minor adverse events at immediate follow‐up. While we are aware of other adverse events reported after epidural corticosteroid injections (e.g. fungal infections due to contaminated steroids in the USA (Smith 2013)), further high‐quality studies are warranted to clarify the safety of this intervention. We identified trials reporting data regarding both acute and chronic lumbosacral radicular pain; most of the studies did not, however, restrict the duration of symptoms. According to recent guidelines from an international panel of experts, clinical trials seeking to determine the efficacy of epidural corticosteroid injection should avoid enrolling individuals with less than 3 months or more than 24 months of radicular pain, given the high rate of resolution in individuals with acute pain, and the low likelihood of improvement in individuals with chronic pain (Bicket 2016; Rathmell 2015). Given the logistical and ethical concerns of repeating 'sham' procedures, this review cannot assess the duration of effect for multiple injections, which have been shown in some randomised trials to confer long‐term benefit (Riew 2006). Although previous studies have demonstrated significant differences across the volume of injected doses (Rabinovitch 2009), we could not compare the effects among them. We also could not assess the differences among types of corticosteroid (e.g. particulate vs nonparticulate); however, a previous review showed no differences among them and we would argue that these factors are unlikely to influence the findings of this review (Mehta 2017). Nevertheless, our findings might be generalisable for a large range of settings as well as symptom duration.
Quality of the evidence
The overall quality of evidence of this review was at best moderate, mostly downgraded due to risk of bias. These findings conflict with our earlier review — Pinto 2012 — which found high quality evidence for all pooled estimates. Some factors might have contributed to this discrepancy. In the current review, we assessed the overall quality of evidence using GRADE considering some different criteria compared to the previous review (Furlan 2015). For 'inconsistency', we included the assessment of heterogeneity using the I² statistic for downgrading the quality of evidence (i.e. I² > 40%) to the criterion used in the previous review (i.e. ≤ 75% of participants from studies with findings in the same direction). For 'imprecision', we downgraded the quality of evidence when the total number of participants was lower than 400 participants instead of lower than 300 participants adopted as a criterion in the previous review. For 'risk of bias', whereas our previous review considered a cut‐off based on the overall score of the PEDro scale (i.e. less than seven points out of 10), in the current review we considered specific trial aspects using the expanded criteria developed by the Cochrane Back and Neck Review Group (Furlan 2015). In the current review, we considered 17 out of 25 included trials (68%) as having high risk of bias because we judged them as having low risk of bias in less than four out of the five bias domains. Of note: the results of the sensitivity analyses considering only studies having low risk of bias (MD −5.01, 95% CI −10.96 to 0.94 on a 0 to 100 scale; 4 trials, 376 participants), studies judged as having low risk of bias for allocation concealment (MD −3.43, 95% CI −7.86 to 1.01 on a 0 to 100 scale; 6 trials, 760 participants), and intention‐to‐treat analysis (MD −2.74, 95% CI −7.58 to 2.09 on a 0 to 100 scale; 5 trials, 600 participants) showed that these aspects did not influence the size of treatment effects for leg pain at short‐term follow‐up. Hence, further studies should be conducted addressing these methodological flaws. Furthermore, considering that the adverse events were mostly reported at immediate follow‐up, future studies should investigate the occurrence of adverse events at longer time points.
Potential biases in the review process
Although our searches were carried out to identify all potentially eligible studies, we cannot exclude the possibility of missing studies. To increase the sensitivity of our searches, we also performed citation tracking of references of included studies and relevant systematic reviews in the field. One strength of this review was contacting the authors requesting missing data. Indeed, one study showed a discrepancy between the data displayed in graphs and findings described in the text (Ghai 2015). Although this discrepancy was resolved after contacting the authors, we do not discard the possibility of potential bias for this study. Furthermore, we also explored the treatment effects using sensitivity analyses considering the risk of bias of the studies and specific trial aspects, such as blinding of the care provider, allocation concealment, and intention‐to‐treat analysis. Of note: none of the aspects investigated clearly influenced the treatment effect size reported for leg pain at short‐term follow‐up.
Agreements and disagreements with other studies or reviews
One recent review investigated the efficacy of epidural corticosteroid injection in patients with lumbosacral radicular pain (Chou 2015). Chou 2015 included a slightly different set of trials (e.g. some studies included patients with back pain but without lumbosacral radicular pain (Sayegh 2009); or investigated the efficacy of epidural corticosteroid injection without comparing with a placebo (Buchner 2000). In addition, this review conducted the meta‐analysis using a combination of leg pain and overall pain (i.e. when leg pain was not reported). Although there are some differences in the methods, our results are similar to the previous review which consistently demonstrate that the treatment effects were small and may not be considered clinically important by patients and clinicians (i.e. MD lower than 10%) (Chou 2015).
The absence of a clinically meaningful difference between epidural corticosteroid injection and placebo injection should be viewed in the context of ongoing controversy surrounding what exactly constitutes a placebo. For example, a systematic review and meta‐analysis that compared epidural corticosteroid injection outcomes in randomised “controlled” trials found that those studies in which the placebo group received an epidural local anaesthetic and/or saline injection were more likely to be negative, or report a smaller effect size, than those studies in which the placebo group received an intramuscular or ligamentous injection (Bicket 2013). Based on an indirect analysis, the authors concluded that more than half of the benefit of epidural corticosteroid injection was due to the injection itself, rather than the corticosteroid. In fact, non‐steroidal mechanisms by which epidural injection may provide benefit include the washout of inflammatory cytokines, the dissolution of scar tissue, suppressing ectopic discharges from inflamed spinal nerve roots, enhancing blood flow to ischemic nerve roots, and favourably altering gene expression (Devor 1992; Fukusaki 1998). Nevertheless, our findings conflict with the results of Bicket 2013 due to the lack of the treatment effects between subgroups related to the types of placebo in the current review
Authors' conclusions
Implications for practice.
As a key part of a shared decision‐making approach we would encourage clinicians to inform patients of the average size of treatment effect. The evidence shows that epidural corticosteroid injections were probably slightly more effective compared to placebo injection in reducing leg pain and disability at short‐term follow‐up. We identified mostly minor adverse events (e.g. headache, accidental dural punctures, worsening pain) after epidural corticosteroid injections and placebo injections with no difference between groups. The available evidence still provides only limited support for the use of epidural corticosteroid injections in people with lumbosacral radicular pain as the treatment effects are small, mainly evident at short‐term follow‐up and may not be considered clinically important by patients and clinicians (i.e. mean difference lower than 10%).
Implications for research.
Considering that we found at best moderate quality evidence, the addition of further studies is likely to impact our treatment estimates. We identified only one ongoing study which might suggest that the field has stagnated. Further high‐quality trials are needed to reach definitive conclusions. Nevertheless, future studies should be conducted addressing trial aspects which may influence the treatment and harmful effects, such as allocation concealment, and blinding of care provider.
What's new
| Date | Event | Description |
|---|---|---|
| 25 September 2019 | New citation required and conclusions have changed | We identified 6 additional trials in the update searches resulting in a total of 25 included trials. The last review (Pinto 2012) showed that epidural corticosteroid injections were more effective compared to placebo in reducing pain intensity and disability at short‐term follow‐up. In the current review, we found that epidural corticosteroid injections were more effective in reducing leg pain at short‐term follow‐up and reducing disability at short‐term and intermediate‐term follow‐up. The quality of evidence changed from high quality in the last review to very low to moderate in the current review. |
| 25 September 2019 | New search has been performed | This Cochrane Review is an update of a previous review published in Annals of Internal Medicine in 2012 (Pinto 2012). We changed the name of the condition from "sciatica" to "lumbosacral radicular pain" because according to the IASP 1994 the term “sciatica” should be avoided because it might suggest that the condition is a disorder of the sciatic nerve rather than a lumbosacral nerve root. Hence, we have used the term “lumbosacral radicular pain” in this review to align with the IASP name for this condition. The methods were updated according to the Cochrane methods including risk of bias assessment (Furlan 2015) and rating the overall quality of the evidence using GRADE. We also included adverse events, overall pain intensity, back pain intensity, and pain intensity and disability measured as dichotomous outcomes as secondary outcomes. |
Acknowledgements
We credit Annals of Internal Medicine as the original source (Pinto RZ, Maher CG, Ferreira ML, Hancock M, Oliveira VC, McLachlan AJ, Ferreira PH. Epidural corticosteroid injections in the management of sciatica: a systematic review and meta‐analysis. Annals of Internal Medicine 2012;17:865‐77; annals.org/article.aspx?articleid=1390546).
Copyright notice: © 2012 by the American College of Physicians
We also thank Jill Hayden, Timothy Carey, and Anne Asher for peer review comments.
Appendices
Appendix 1. Search Strategies
CBN trials register
Last searched 25 September 2019
1 lumb* and corticosteroid* AND INREGISTER
2 lumb* and Glucocortic* AND INREGISTER
3 lumb* and Adrenal Cortex Hormone* AND INREGISTER
4 lumb* and Steroid* AND INREGISTER
5 lumb* and corticoid* AND INREGISTER
6 lumb* and prednis* AND INREGISTER
7 lumb* and cortisone* AND INREGISTER
8 lumb* and hydrocort* AND INREGISTER
9 lumb* and methylprednis* AND INREGISTER
10 lumb* and triamcinol* AND INREGISTER
11 lumb* and dexamet* AND INREGISTER
12 lumb* and betamet* AND INREGISTER
13 lumb* and beclomet* AND INREGISTER
14 lumb* and parametas* AND INREGISTER
15 lumb* and paramethas* AND INREGISTER
16 (sciatic* and corticosteroid*) or (radicul* and corticosteroid*) or (disc and corticosteroid*) or (disk and corticosteroid*) or (back pain and corticosteroid*) AND INREGISTER
17 (sciatic* and Glucocortic*) or (radicul* and Glucocortic*) or (disc and Glucocortic*) or (disk and Glucocortic*) or (back pain and Glucocortic*) AND INREGISTER
18 (sciatic* and Adrenal Cortex Hormone*) or (radicul* and Adrenal Cortex Hormone*) or (disc and Adrenal Cortex Hormone*) or (disk and Adrenal Cortex Hormone*) or (back pain and Adrenal Cortex Hormone*) AND INREGISTER
19 (sciatic* and Steroid*) or (radicul* and Steroid*) or (disc and Steroid*) or (disk and Steroid*) or (back pain and Steroid*) AND INREGISTER
20 (sciatic* and corticoid*) or (radicul* and corticoid*) or (disc and corticoid*) or (disk and corticoid*) or (back pain and corticoid*) AND INREGISTER
21 (sciatic* and prednis*) or (radicul* and prednis*) or (disc and prednis*) or (disk and prednis*) or (back pain and prednis*) AND INREGISTER
22 (sciatic* and cortisone*) or (radicul* and cortisone*) or (disc and cortisone*) or (disk and cortisone*) or (back pain and cortisone*) AND INREGISTER
23 (sciatic* and hydrocort*) or (radicul* and hydrocort*) or (disc and hydrocort*) or (disk and hydrocort*) or (back pain and hydrocort*) AND INREGISTER
24 (sciatic* and methylprednis*) or (radicul* and methylprednis*) or (disc and methylprednis*) or (disk and methylprednis*) or (back pain and methylprednis*) AND INREGISTER
25 (sciatic* and triamcinol*) or (radicul* and triamcinol*) or (disc and triamcinol*) or (disk and triamcinol*) or (back pain and triamcinol*) AND INREGISTER
26 (sciatic* and dexamet*) or (radicul* and dexamet*) or (disc and dexamet*) or (disk and dexamet*) or (back pain and dexamet*) AND INREGISTER
27 (sciatic* and betamet*) or (radicul* and betamet*) or (disc and betamet*) or (disk and betamet*) or (back pain and betamet*) AND INREGISTER
28 (sciatic* and beclomet*) or (radicul* and beclomet*) or (disc and beclomet*) or (disk and beclomet*) or (back pain and beclomet*) AND INREGISTER
29 (sciatic* and parametas*) or (radicul* and parametas*) or (disc and parametas*) or (disk and parametas*) or (back pain and parametas*) AND INREGISTER
30 (sciatic* and paramethas*) or (radicul* and paramethas*) or (disc and paramethas*) or (disk and paramethas*) or (back pain and paramethas*) AND INREGISTER
31 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30
32 (2018 OR 2019):YR AND INREGISTER
33 #32 AND #31
Searched 23 August 2018
#1 ((corticosteroid* or Glucocortic* or Adrenal Cortex Hormone* or Steroid* or corticoid* or prednis* or cortisone* or hydrocort* or methylprednis* or triamcinol* or dexamet* or betamet* or beclomet* or parametas* or paramethas*) AND (lumb* or sciatic* or radicul* or disc or disk or back pain) AND epidural) AND INREGISTER
#2 #63 AND (2017 TO 2018:YR)
Searched 8 October 2015
((corticosteroid* or Glucocortic* or Adrenal Cortex Hormone* or Steroid* or corticoid* or prednis* or cortisone* or hydrocort* or methylprednis* or triamcinol* or dexamet* or betamet* or beclomet* or parametas* or paramethas*) AND (lumb* or sciatic* or radicul* or disc or disk or back pain) AND epidural)
CENTRAL
Last searched 25 September 2019
1 MESH DESCRIPTOR Back Pain EXPLODE ALL AND CENTRAL:TARGET
2 ((lumb* NEAR3 pain) or (back NEAR3 pain)) AND CENTRAL:TARGET
3 dorsalgia or lumbago or ischialgia or coccydynia or backache* or back ache* AND CENTRAL:TARGET
4 back disorder* or spondylosis or coccyx AND CENTRAL:TARGET
5 MESH DESCRIPTOR Sciatic Neuropathy EXPLODE ALL AND CENTRAL:TARGET
6 MESH DESCRIPTOR Sciatica EXPLODE ALL AND CENTRAL:TARGET
7 MESH DESCRIPTOR Radiculopathy EXPLODE ALL AND CENTRAL:TARGET
8 MESH DESCRIPTOR Polyradiculopathy EXPLODE ALL AND CENTRAL:TARGET
9 sciatic* or radicul* or polyradicul* AND CENTRAL:TARGET
10 discitis or diskitis AND CENTRAL:TARGET
11 (disc* near degenerat*) or (disc* near prolapse*) or (disc* near herniat*) or (disk* near degenerat*) or (disk* near prolapse*) or (disk* near herniat*) AND CENTRAL:TARGET
12 MESH DESCRIPTOR Intervertebral Disc EXPLODE ALL AND CENTRAL:TARGET
13 MESH DESCRIPTOR Intervertebral Disc Displacement EXPLODE ALL AND CENTRAL:TARGET
14 MESH DESCRIPTOR Intervertebral Disc Degeneration EXPLODE ALL AND CENTRAL:TARGET
15 MESH DESCRIPTOR Lumbar Vertebrae EXPLODE ALL AND CENTRAL:TARGET
16 MESH DESCRIPTOR Nerve Compression Syndromes AND CENTRAL:TARGET
17 MESH DESCRIPTOR Spinal Osteophytosis EXPLODE ALL AND CENTRAL:TARGET
18 spinal osteophytosis AND CENTRAL:TARGET
19 MESH DESCRIPTOR Spinal Nerve Roots EXPLODE ALL AND CENTRAL:TARGET
20 arachnoiditis AND CENTRAL:TARGET
21 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #20 OR #19 AND CENTRAL:TARGET
22 MESH DESCRIPTOR Injections, Epidural EXPLODE ALL AND CENTRAL:TARGET
23 MESH DESCRIPTOR Injections, Spinal EXPLODE ALL AND CENTRAL:TARGET
24 MESH DESCRIPTOR Epidural Space EXPLODE ALL AND CENTRAL:TARGET
25 MESH DESCRIPTOR Anesthesia, Epidural EXPLODE ALL AND CENTRAL:TARGET
26 MESH DESCRIPTOR Analgesia, Epidural EXPLODE ALL AND CENTRAL:TARGET
27 epidural or extradural or peridural AND CENTRAL:TARGET
28 (spinal NEAR3 injection*) or (spinal NEAR3 analges*) or (spinal NEAR3 anaesthe*) or (spinal NEAR3 anesthe*) AND CENTRAL:TARGET
29 (local NEAR3 anesth*) or (local NEAR3 anaesthe*) or (local NEAR3 analges*) or (local NEAR3 injection*) AND CENTRAL:TARGET
30 MESH DESCRIPTOR Anesthetics, Local EXPLODE ALL AND CENTRAL:TARGET
31 MESH DESCRIPTOR Anesthesia, Local EXPLODE ALL AND CENTRAL:TARGET
32 TFESI or SNRB AND CENTRAL:TARGET
33 transforaminal epidural steroid injection AND CENTRAL:TARGET
34 selective nerve root block AND CENTRAL:TARGET
35 MESH DESCRIPTOR Nerve Block EXPLODE ALL AND CENTRAL:TARGET
36 #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35 AND CENTRAL:TARGET
37 MESH DESCRIPTOR Steroids EXPLODE ALL AND CENTRAL:TARGET
38 MESH DESCRIPTOR Glucocorticoids EXPLODE ALL AND CENTRAL:TARGET
39 MESH DESCRIPTOR Adrenal Cortex Hormones EXPLODE ALL AND CENTRAL:TARGET
40 MESH DESCRIPTOR Beclomethasone EXPLODE ALL AND CENTRAL:TARGET
41 MESH DESCRIPTOR Betamethasone EXPLODE ALL AND CENTRAL:TARGET
42 MESH DESCRIPTOR Clobetasol EXPLODE ALL AND CENTRAL:TARGET
43 MESH DESCRIPTOR Cortisone EXPLODE ALL AND CENTRAL:TARGET
44 MESH DESCRIPTOR Hydrocortisone EXPLODE ALL AND CENTRAL:TARGET
45 MESH DESCRIPTOR Desoximetasone EXPLODE ALL AND CENTRAL:TARGET
46 MESH DESCRIPTOR Dexamethasone EXPLODE ALL AND CENTRAL:TARGET
47 MESH DESCRIPTOR Diflucortolone EXPLODE ALL AND CENTRAL:TARGET
48 MESH DESCRIPTOR Flumethasone EXPLODE ALL AND CENTRAL:TARGET
49 fluocinolone AND CENTRAL:TARGET
50 MESH DESCRIPTOR Fluocinonide EXPLODE ALL AND CENTRAL:TARGET
51 MESH DESCRIPTOR Fluocortolone EXPLODE ALL AND CENTRAL:TARGET
52 MESH DESCRIPTOR Fluorometholone EXPLODE ALL AND CENTRAL:TARGET
53 MESH DESCRIPTOR Fluprednisolone EXPLODE ALL AND CENTRAL:TARGET
54 melengestrol AND CENTRAL:TARGET
55 MESH DESCRIPTOR Methylprednisolone EXPLODE ALL AND CENTRAL:TARGET
56 MESH DESCRIPTOR Paramethasone EXPLODE ALL AND CENTRAL:TARGET
57 MESH DESCRIPTOR Prednisolone EXPLODE ALL AND CENTRAL:TARGET
58 MESH DESCRIPTOR Prednisone EXPLODE ALL AND CENTRAL:TARGET
59 MESH DESCRIPTOR Triamcinolone EXPLODE ALL AND CENTRAL:TARGET
60 corticosteroid* or Glucocortic* or Adrenal Cortex Hormone* or Steroid* or corticoid* or prednis* or cortisone* or hydrocort* or methylprednis* or triamcinol* or dexamet* or betamet* or beclomet* or parametas* or paramethas* AND CENTRAL:TARGET
61 MESH DESCRIPTOR Flurandrenolone EXPLODE ALL AND CENTRAL:TARGET
62 #37 OR #38 OR #39 OR #40 OR #41 OR #42 OR #43 OR #44 OR #45 OR #46 OR #47 OR #48 OR #49 OR #50 OR #51 OR #52 OR #53 OR #54 OR #55 OR #56 OR #57 OR #58 OR #59 OR #60 OR #61 AND CENTRAL:TARGET
63 #21 AND #36 AND #62 AND CENTRAL:TARGET
64 (2018 OR 2019):YR AND CENTRAL:TARGET
65 #63 AND #64
Searched 23 August 2018
1 MESH DESCRIPTOR Back Pain EXPLODE ALL AND CENTRAL:TARGET
2 ((lumb* or back) near pain) AND CENTRAL:TARGET
3 dorsalgia or lumbago or ischialgia or coccydynia or backache* or back ache* AND CENTRAL:TARGET
4 back disorder* or spondylosis or coccyx AND CENTRAL:TARGET
5 MESH DESCRIPTOR Sciatic Neuropathy EXPLODE ALL AND CENTRAL:TARGET
6 MESH DESCRIPTOR Sciatica EXPLODE ALL AND CENTRAL:TARGET
7 MESH DESCRIPTOR Radiculopathy EXPLODE ALL AND CENTRAL:TARGET
8 MESH DESCRIPTOR Polyradiculopathy EXPLODE ALL AND CENTRAL:TARGET
9 sciatic* or radicul* or polyradicul* AND CENTRAL:TARGET
10 discitis or diskitis AND CENTRAL:TARGET
11 ((disc* or disk*) near (degenerat* or prolapse* or herniat*)) AND CENTRAL:TARGET
12 MESH DESCRIPTOR Intervertebral Disc EXPLODE ALL AND CENTRAL:TARGET
13 MESH DESCRIPTOR Intervertebral Disc Displacement EXPLODE ALL AND CENTRAL:TARGET
14 MESH DESCRIPTOR Intervertebral Disc Degeneration EXPLODE ALL AND CENTRAL:TARGET
15 MESH DESCRIPTOR Lumbar Vertebrae EXPLODE ALL AND CENTRAL:TARGET
16 MESH DESCRIPTOR Nerve Compression Syndromes AND CENTRAL:TARGET
17 MESH DESCRIPTOR Spinal Osteophytosis EXPLODE ALL AND CENTRAL:TARGET
18 spinal osteophytosis AND CENTRAL:TARGET
19 MESH DESCRIPTOR Spinal Nerve Roots EXPLODE ALL AND CENTRAL:TARGET
20 arachnoiditis AND CENTRAL:TARGET
21 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #20 OR #19 AND CENTRAL:TARGET
22 MESH DESCRIPTOR Injections, Epidural EXPLODE ALL AND CENTRAL:TARGET
23 MESH DESCRIPTOR Injections, Spinal EXPLODE ALL AND CENTRAL:TARGET
24 MESH DESCRIPTOR Epidural Space EXPLODE ALL AND CENTRAL:TARGET
25 MESH DESCRIPTOR Anesthesia, Epidural EXPLODE ALL AND CENTRAL:TARGET
26 MESH DESCRIPTOR Analgesia, Epidural EXPLODE ALL AND CENTRAL:TARGET
27 epidural or extradural or peridural AND CENTRAL:TARGET
28 (spinal NEAR3 (injection* or analges* or anaesthe* or anesthe*)) AND CENTRAL:TARGET
29 (local NEAR3 (anesthe* or anaesthe* or analges* or injection*)) AND CENTRAL:TARGET
30 MESH DESCRIPTOR Anesthetics, Local EXPLODE ALL AND CENTRAL:TARGET
31 MESH DESCRIPTOR Anesthesia, Local EXPLODE ALL AND CENTRAL:TARGET
32 TFESI or SNRB AND CENTRAL:TARGET
33 transforaminal epidural steroid injection AND CENTRAL:TARGET
34 selective nerve root block AND CENTRAL:TARGET
35 MESH DESCRIPTOR Nerve Block EXPLODE ALL AND CENTRAL:TARGET
36 #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35 AND CENTRAL:TARGET
37 MESH DESCRIPTOR Steroids EXPLODE ALL AND CENTRAL:TARGET
38 MESH DESCRIPTOR Glucocorticoids EXPLODE ALL AND CENTRAL:TARGET
39 MESH DESCRIPTOR Adrenal Cortex Hormones EXPLODE ALL AND CENTRAL:TARGET
40 MESH DESCRIPTOR Beclomethasone EXPLODE ALL AND CENTRAL:TARGET
41 MESH DESCRIPTOR Betamethasone EXPLODE ALL AND CENTRAL:TARGET
42 MESH DESCRIPTOR Clobetasol EXPLODE ALL AND CENTRAL:TARGET
43 MESH DESCRIPTOR Cortisone EXPLODE ALL AND CENTRAL:TARGET
44 MESH DESCRIPTOR Hydrocortisone EXPLODE ALL AND CENTRAL:TARGET
45 MESH DESCRIPTOR Desoximetasone EXPLODE ALL AND CENTRAL:TARGET
46 MESH DESCRIPTOR Dexamethasone EXPLODE ALL AND CENTRAL:TARGET
47 MESH DESCRIPTOR Diflucortolone EXPLODE ALL AND CENTRAL:TARGET
48 MESH DESCRIPTOR Flumethasone EXPLODE ALL AND CENTRAL:TARGET
49 fluocinolone AND CENTRAL:TARGET
50 MESH DESCRIPTOR Fluocinonide EXPLODE ALL AND CENTRAL:TARGET
51 MESH DESCRIPTOR Fluocortolone EXPLODE ALL AND CENTRAL:TARGET
52 MESH DESCRIPTOR Fluorometholone EXPLODE ALL AND CENTRAL:TARGET
53 MESH DESCRIPTOR Fluprednisolone EXPLODE ALL AND CENTRAL:TARGET
54 melengestrol AND CENTRAL:TARGET
55 MESH DESCRIPTOR Methylprednisolone EXPLODE ALL AND CENTRAL:TARGET
56 MESH DESCRIPTOR Paramethasone EXPLODE ALL AND CENTRAL:TARGET
57 MESH DESCRIPTOR Prednisolone EXPLODE ALL AND CENTRAL:TARGET
58 MESH DESCRIPTOR Prednisone EXPLODE ALL AND CENTRAL:TARGET
59 MESH DESCRIPTOR Triamcinolone EXPLODE ALL AND CENTRAL:TARGET
60 corticosteroid* or Glucocortic* or Adrenal Cortex Hormone* or Steroid* or corticoid* or prednis* or cortisone* or hydrocort* or methylprednis* or triamcinol* or dexamet* or betamet* or beclomet* or parametas* or paramethas* AND CENTRAL:TARGET
61 MESH DESCRIPTOR Flurandrenolone EXPLODE ALL AND CENTRAL:TARGET
62 #37 OR #38 OR #39 OR #40 OR #41 OR #42 OR #43 OR #44 OR #45 OR #46 OR #47 OR #48 OR #49 OR #50 OR #51 OR #52 OR #53 OR #54 OR #55 OR #56 OR #57 OR #58 OR #59 OR #60 OR #61 AND CENTRAL:TARGET
63 #21 AND #36 AND #62 AND CENTRAL:TARGET
64 #63 AND (2017 TO 2018:YR)
16 June 2017 strategy
#1 MESH DESCRIPTOR Back Pain EXPLODE ALL AND CENTRAL:TARGET
#2 ((lumb* or back) near pain) AND CENTRAL:TARGET
#3 dorsalgia or lumbago or ischialgia or coccydynia or backache* or back ache* AND CENTRAL:TARGET
#4 back disorder* or spondylosis or coccyx AND CENTRAL:TARGET
#5 MESH DESCRIPTOR Sciatic Neuropathy EXPLODE ALL AND CENTRAL:TARGET
#6 MESH DESCRIPTOR Sciatica EXPLODE ALL AND CENTRAL:TARGET
#7 MESH DESCRIPTOR Radiculopathy EXPLODE ALL AND CENTRAL:TARGET
#8 MESH DESCRIPTOR Polyradiculopathy EXPLODE ALL AND CENTRAL:TARGET
#9 sciatic* or radicul* or polyradicul* AND CENTRAL:TARGET
#10 discitis or diskitis AND CENTRAL:TARGET
#11 ((disc* or disk*) near (degenerat* or prolapse* or herniat*)) AND CENTRAL:TARGET
#12 MESH DESCRIPTOR Intervertebral Disc EXPLODE ALL AND CENTRAL:TARGET
#13 MESH DESCRIPTOR Intervertebral Disc Displacement EXPLODE ALL AND CENTRAL:TARGET
#14 MESH DESCRIPTOR Intervertebral Disc Degeneration EXPLODE ALL AND CENTRAL:TARGET
#15 MESH DESCRIPTOR Lumbar Vertebrae EXPLODE ALL AND CENTRAL:TARGET
#16 MESH DESCRIPTOR Nerve Compression Syndromes AND CENTRAL:TARGET
#17 MESH DESCRIPTOR Spinal Osteophytosis EXPLODE ALL AND CENTRAL:TARGET
#18 spinal osteophytosis AND CENTRAL:TARGET
#19 MESH DESCRIPTOR Spinal Nerve Roots EXPLODE ALL AND CENTRAL:TARGET
#20 arachnoiditis AND CENTRAL:TARGET
#21 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #20 OR #19
#22 MESH DESCRIPTOR Injections, Epidural EXPLODE ALL AND CENTRAL:TARGET
#23 MESH DESCRIPTOR Injections, Spinal EXPLODE ALL AND CENTRAL:TARGET
#24 MESH DESCRIPTOR Epidural Space EXPLODE ALL AND CENTRAL:TARGET
#25 MESH DESCRIPTOR Anesthesia, Epidural EXPLODE ALL AND CENTRAL:TARGET
#26 MESH DESCRIPTOR Analgesia, Epidural EXPLODE ALL AND CENTRAL:TARGET
#27 epidural or extradural or peridural AND CENTRAL:TARGET
#28 (spinal NEAR3 (injection* or analges* or anaesthe* or anesthe*)) AND CENTRAL:TARGET
#29 (local NEAR3 (anesthe* or anaesthe* or analges* or injection*)) AND CENTRAL:TARGET
#30 MESH DESCRIPTOR Anesthetics, Local EXPLODE ALL AND CENTRAL:TARGET
#31 MESH DESCRIPTOR Anesthesia, Local EXPLODE ALL AND CENTRAL:TARGET
#32 TFESI or SNRB AND CENTRAL:TARGET
#33 transforaminal epidural steroid injection AND CENTRAL:TARGET
#34 selective nerve root block AND CENTRAL:TARGET
#35 MESH DESCRIPTOR Nerve Block EXPLODE ALL AND CENTRAL:TARGET
#36 #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35
#37 MESH DESCRIPTOR Steroids EXPLODE ALL AND CENTRAL:TARGET
#38 MESH DESCRIPTOR Glucocorticoids EXPLODE ALL AND CENTRAL:TARGET
#39 MESH DESCRIPTOR Adrenal Cortex Hormones EXPLODE ALL AND CENTRAL:TARGET
#40 MESH DESCRIPTOR Beclomethasone EXPLODE ALL AND CENTRAL:TARGET
#41 MESH DESCRIPTOR Betamethasone EXPLODE ALL AND CENTRAL:TARGET
#42 MESH DESCRIPTOR Clobetasol EXPLODE ALL AND CENTRAL:TARGET
#43 MESH DESCRIPTOR Cortisone EXPLODE ALL AND CENTRAL:TARGET
#44 MESH DESCRIPTOR Hydrocortisone EXPLODE ALL AND CENTRAL:TARGET
#45 MESH DESCRIPTOR Desoximetasone EXPLODE ALL AND CENTRAL:TARGET
#46 MESH DESCRIPTOR Dexamethasone EXPLODE ALL AND CENTRAL:TARGET
#47 MESH DESCRIPTOR Diflucortolone EXPLODE ALL AND CENTRAL:TARGET
#48 MESH DESCRIPTOR Flumethasone EXPLODE ALL AND CENTRAL:TARGET
#49 fluocinolone AND CENTRAL:TARGET
#50 MESH DESCRIPTOR Fluocinonide EXPLODE ALL AND CENTRAL:TARGET
#51 MESH DESCRIPTOR Fluocortolone EXPLODE ALL AND CENTRAL:TARGET
#52 MESH DESCRIPTOR Fluorometholone EXPLODE ALL AND CENTRAL:TARGET
#53 MESH DESCRIPTOR Fluprednisolone EXPLODE ALL AND CENTRAL:TARGET
#54 melengestrol AND CENTRAL:TARGET
#55 MESH DESCRIPTOR Methylprednisolone EXPLODE ALL AND CENTRAL:TARGET
#56 MESH DESCRIPTOR Paramethasone EXPLODE ALL AND CENTRAL:TARGET
#57 MESH DESCRIPTOR Prednisolone EXPLODE ALL AND CENTRAL:TARGET
#58 MESH DESCRIPTOR Prednisone EXPLODE ALL AND CENTRAL:TARGET
#59 MESH DESCRIPTOR Triamcinolone EXPLODE ALL AND CENTRAL:TARGET
#60 corticosteroid* or Glucocortic* or Adrenal Cortex Hormone* or Steroid* or corticoid* or prednis* or cortisone* or hydrocort* or methylprednis* or triamcinol* or dexamet* or betamet* or beclomet* or parametas* or paramethas* AND CENTRAL:TARGET
#61 MESH DESCRIPTOR Flurandrenolone EXPLODE ALL AND CENTRAL:TARGET
#62 #37 OR #38 OR #39 OR #40 OR #41 OR #42 OR #43 OR #44 OR #45 OR #46 OR #47 OR #48 OR #49 OR #50 OR #51 OR #52 OR #53 OR #54 OR #55 OR #56 OR #57 OR #58 OR #59 OR #60 OR #61
#63 #21 AND #36 AND #62
#64 #63 AND (2015 TO 2017:YR)
#65 #63 AND (07/10/2015 TO 16/06/2017:CRSMODIFIED)
#66 #64 OR #65
June 2013 strategy
#1 (Radicul* or Sciatic*)
#2 ((systemic adj5 corticosteroid*) or Glucocorticoids or Adrenal Cortex Hormones or Steroids or glucocortic* or adrenal Cortex Horm* or prednisone* or prednisol* or cortisone* or hydrocort* or methylprednis* or triamcinol* or dexamethas* or betamethas* or beclomethas* or paramethas* or dexametas* or betametas* or beclometas* or parametas*)
#3 #1 and #2 from 2012 to 2013, in Trials
MEDLINE and MEDLINE In‐Process & Other Non‐Indexed Citations
Last searched 25 September 2019
1 randomized controlled trial.pt.
2 controlled clinical trial.pt.
3 pragmatic clinical trial.pt.
4 comparative study.pt.
5 random*.ti,ab,kw.
6 placebo.ti,ab,kw.
7 control*.ti,ab,kw.
8 prospective*.ti,ab,kw.
9 compar*.ti,ab,kw.
10 trial.ti,ab,kw.
11 groups.ti,ab,kw.
12 or/1‐11
13 (animals not (humans and animals)).sh.
14 12 not 13
15 back disorder*.tw,kf.
16 dorsalgia.tw,kf.
17 exp Back Pain/
18 (backache* or back ache*).tw,kf.
19 ((lumb* adj3 pain) or (back adj3 pain)).tw,kf.
20 coccyx.tw,kf.
21 coccydynia.tw,kf.
22 sciatic*.tw,kf.
23 exp sciatic neuropathy/
24 spondylosis.tw,kf.
25 lumbago.tw,kf.
26 ischialgia.tw,kf.
27 (discitis or diskitis).tw,kf.
28 ((disc* adj3 degenerat*) or (disk* adj3 degenerat*)).tw,kf.
29 ((disc* adj3 prolapse*) or (disk* adj3 prolapse*)).tw,kf.
30 ((disc* adj3 herniat*) or (disk* adj3 herniat*)).tw,kf.
31 Intervertebral Disc/
32 exp Intervertebral Disk Displacement/
33 exp Intervertebral Disc Degeneration/
34 Lumbar Vertebrae/
35 Nerve Compression Syndromes/
36 Spinal Osteophytosis/
37 Radiculopathy/
38 Polyradiculopathy/
39 radicul*.tw,kf.
40 polyradicul*.tw,kf.
41 arachnoiditis.tw,kf.
42 or/15‐41
43 exp Spinal Nerve Roots/
44 (compress* or inflam* or pathol*).tw,kf.
45 43 and 44
46 42 or 45
47 exp Injections, Epidural/
48 exp Injections, Spinal/
49 exp Epidural Space/
50 exp Anesthesia, Epidural/
51 exp Analgesia, Epidural/
52 epidural.tw,kf.
53 extradural.tw,kf.
54 peridural.tw,kf.
55 ((spinal adj3 inject*) or (spinal adj3 analges*) or (spinal adj3 anaesthe*) or (spinal adj3 anesthe*)).tw,kf.
56 exp anesthetics, local/
57 exp anesthesia, local/
58 ((local adj3 anesthe*) or (local adj3 anaesthe*) or (local adj3 analges*) or (local adj3 injection*)).tw,kf.
59 TFESI.tw,kf.
60 SNRB.tw,kf.
61 "transforaminal epidural steroid injection".tw,kf.
62 "selective nerve root block".tw,kf.
63 exp Nerve Block/
64 or/47‐63
65 exp Glucocorticoids/
66 exp Adrenal Cortex Hormones/
67 exp Steroids/
68 exp Beclomethasone/
69 exp Betamethasone/
70 exp Clobetasol/
71 exp Cortisone/
72 exp Hydrocortisone/
73 exp Desoximetasone/
74 exp Dexamethasone/
75 exp Diflucortolone/
76 exp Flumethasone/
77 fluocinolone.tw,kf.
78 exp Fluocinonide/
79 exp Fluocortolone/
80 exp Fluorometholone/
81 exp Fluprednisolone/
82 exp Flurandrenolone/
83 melengestrol.tw,kf.
84 exp Methylprednisolone/
85 exp Paramethasone/
86 exp Prednisolone/
87 exp Prednisone/
88 exp Triamcinolone/
89 (corticosteroid* or Glucocortic* or Adrenal Cortex Hormone* or Steroid* or corticoid* or prednis* or cortisone* or hydrocort* or methylprednis* or triamcinol* or dexamet* or betamet* or beclomet* or parametas* or paramethas*).tw,kf.
90 or/65‐89
91 14 and 46 and 64 and 90
92 limit 91 to yr=2018‐2019
93 limit 91 to ed=20180823‐20190925
94 92 or 93
Searched 23 August 2018
1 randomized controlled trial.pt.
2 controlled clinical trial.pt.
3 pragmatic clinical trial.pt.
4 comparative study.pt.
5 random*.ti,ab,kw.
6 placebo.ti,ab,kw.
7 control*.ti,ab,kw.
8 prospective*.ti,ab,kw.
9 compar*.ti,ab,kw.
10 trial.ti,ab,kw.
11 groups.ti,ab,kw.
12 or/1‐11
13 (animals not (humans and animals)).sh.
14 12 not 13
15 back disorder*.tw,kf.
16 dorsalgia.tw,kf.
17 exp Back Pain/
18 (backache* or back ache*).tw,kf.
19 ((lumb* or back) adj3 pain).tw,kf.
20 coccyx.tw,kf.
21 coccydynia.tw,kf.
22 sciatic*.tw,kf.
23 exp sciatic neuropathy/
24 spondylosis.tw,kf.
25 lumbago.tw,kf.
26 ischialgia.tw,kf.
27 (discitis or diskitis).tw,kf.
28 ((disc* or disk*) adj3 degenerat*).tw,kf.
29 ((disc* or disk*) adj3 prolapse*).tw,kf.
30 ((disc* or disk*) adj3 herniat*).tw,kf.
31 Intervertebral Disc/
32 exp Intervertebral Disk Displacement/
33 exp Intervertebral Disc Degeneration/
34 Lumbar Vertebrae/
35 Nerve Compression Syndromes/
36 Spinal Osteophytosis/
37 Radiculopathy/
38 Polyradiculopathy/
39 radicul*.tw,kf.
40 polyradicul*.tw,kf.
41 arachnoiditis.tw,kf.
42 or/15‐41
43 exp Spinal Nerve Roots/
44 (compress* or inflam* or pathol*).tw,kf.
45 43 and 44
46 42 or 45
47 exp Injections, Epidural/
48 exp Injections, Spinal/
49 exp Epidural Space/
50 exp Anesthesia, Epidural/
51 exp Analgesia, Epidural/
52 epidural.tw,kf.
53 extradural.tw,kf.
54 peridural.tw,kf.
55 (spinal adj3 (inject* or analges* or anaesthe* or anesthe*)).tw,kf.
56 exp anesthetics, local/
57 exp anesthesia, local/
58 (local adj3 (anesthe* or anaesthe* or analges* or injection*)).tw,kf.
59 TFESI.tw,kf.
60 SNRB.tw,kf.
61 "transforaminal epidural steroid injection".tw,kf.
62 "selective nerve root block".tw,kf.
63 exp Nerve Block/
64 or/47‐63
65 exp Glucocorticoids/
66 exp Adrenal Cortex Hormones/
67 exp Steroids/
68 exp Beclomethasone/
69 exp Betamethasone/
70 exp Clobetasol/
71 exp Cortisone/
72 exp Hydrocortisone/
73 exp Desoximetasone/
74 exp Dexamethasone/
75 exp Diflucortolone/
76 exp Flumethasone/
77 fluocinolone.mp.
78 exp Fluocinonide/
79 exp Fluocortolone/
80 exp Fluorometholone/
81 exp Fluprednisolone/
82 exp Flurandrenolone/
83 melengestrol.tw,kf.
84 exp Methylprednisolone/
85 exp Paramethasone/
86 exp Prednisolone/
87 exp Prednisone/
88 exp Triamcinolone/
89 (corticosteroid* or Glucocortic* or Adrenal Cortex Hormone* or Steroid* or corticoid* or prednis* or cortisone* or hydrocort* or methylprednis* or triamcinol* or dexamet* or betamet* or beclomet* or parametas* or paramethas*).tw,kf.
90 or/65‐89
91 14 and 46 and 64 and 90
92 limit 91 to yr=2017‐2018
93 limit 91 to ed=20170616‐20180823
94 92 or 93
16 June 2017 strategy. The .mp.field for the disorder and intervention terms was changed to .tw,kf. The wildcard $ was replaced with *.
1 randomized controlled trial.pt.
2 controlled clinical trial.pt.
3 pragmatic clinical trial.pt.
4 comparative study.pt.
5 random*.ti,ab,kw.
6 placebo.ti,ab,kw.
7 control*.ti,ab,kw.
8 prospective*.ti,ab,kw.
9 compar*.ti,ab,kw.
10 trial.ti,ab,kw.
11 groups.ti,ab,kw.
12 or/1‐11
13 (animals not (humans and animals)).sh.
14 12 not 13
15 back disorder*.tw,kf.
16 dorsalgia.tw,kf.
17 exp Back Pain/
18 (backache* or back ache*).tw,kf.
19 ((lumb* or back) adj3 pain).tw,kf.
20 coccyx.tw,kf.
21 coccydynia.tw,kf.
22 sciatic*.tw,kf.
23 exp sciatic neuropathy/
24 spondylosis.tw,kf.
25 lumbago.tw,kf.
26 ischialgia.tw,kf.
27 (discitis or diskitis).tw,kf.
28 ((disc* or disk*) adj3 degenerat*).tw,kf.
29 ((disc* or disk*) adj3 prolapse*).tw,kf.
30 ((disc* or disk*) adj3 herniat*).tw,kf.
31 Intervertebral Disc/
32 exp Intervertebral Disk Displacement/
33 exp Intervertebral Disc Degeneration/
34 Lumbar Vertebrae/
35 Nerve Compression Syndromes/
36 Spinal Osteophytosis/
37 Radiculopathy/
38 Polyradiculopathy/
39 radicul*.tw,kf.
40 polyradicul*.tw,kf.
41 arachnoiditis.tw,kf.
42 or/15‐41
43 exp Spinal Nerve Roots/
44 (compress* or inflam* or pathol*).tw,kf.
45 43 and 44
46 42 or 45
47 exp Injections, Epidural/
48 exp Injections, Spinal/
49 exp Epidural Space/
50 exp Anesthesia, Epidural/
51 exp Analgesia, Epidural/
52 epidural.tw,kf.
53 extradural.tw,kf.
54 peridural.tw,kf.
55 (spinal adj3 (inject* or analges* or anaesthe* or anesthe*)).tw,kf.
56 exp anesthetics, local/
57 exp anesthesia, local/
58 (local adj3 (anesthe* or anaesthe* or analges* or injection*)).tw,kf.
59 TFESI.tw,kf.
60 SNRB.tw,kf.
61 "transforaminal epidural steroid injection".tw,kf.
62 "selective nerve root block".tw,kf.
63 exp Nerve Block/
64 or/47‐63
65 exp Glucocorticoids/
66 exp Adrenal Cortex Hormones/
67 exp Steroids/
68 exp Beclomethasone/
69 exp Betamethasone/
70 exp Clobetasol/
71 exp Cortisone/
72 exp Hydrocortisone/
73 exp Desoximetasone/
74 exp Dexamethasone/
75 exp Diflucortolone/
76 exp Flumethasone/
77 fluocinolone.mp.
78 exp Fluocinonide/
79 exp Fluocortolone/
80 exp Fluorometholone/
81 exp Fluprednisolone/
82 exp Flurandrenolone/
83 melengestrol.tw,kf.
84 exp Methylprednisolone/
85 exp Paramethasone/
86 exp Prednisolone/
87 exp Prednisone/
88 exp Triamcinolone/
89 (corticosteroid* or Glucocortic* or Adrenal Cortex Hormone* or Steroid* or corticoid* or prednis* or cortisone* or hydrocort* or methylprednis* or triamcinol* or dexamet* or betamet* or beclomet* or parametas* or paramethas*).tw,kf.
90 or/65‐89
91 14 and 46 and 64 and 90
92 limit 91 to yr=2015‐2017
93 limit 91 to ed=20151007‐20170616
94 92 or 93
Searched December 2015 in MEDLINE and MEDLINE In‐Process & Other Non‐Indexed Citations
1 randomized controlled trial.pt.
2 controlled clinical trial.pt.
3 pragmatic clinical trial.pt.
4 comparative study.pt.
5 random$.ti,ab,kw.
6 placebo.ti,ab,kw.
7 control$.ti,ab,kw.
8 prospective$.ti,ab,kw.
9 compar$.ti,ab,kw.
10 trial.ti,ab,kw.
11 groups.ti,ab,kw.
12 or/1‐11
13 (animals not (humans and animals)).sh.
14 12 not 13
15 back disorder$.mp.
16 dorsalgia.mp.
17 exp Back Pain/
18 (backache$ or back ache$).mp.
19 ((lumb$ or back) adj3 pain).mp.
20 coccyx.mp.
21 coccydynia.mp.
22 sciatic$.mp.
23 exp sciatic neuropathy/
24 spondylosis.mp.
25 lumbago.mp.
26 ischialgia.mp.
27 (discitis or diskitis).mp.
28 ((disc$ or disk$) adj3 degenerat$).mp.
29 ((disc$ or disk$) adj3 prolapse$).mp.
30 ((disc$ or disk$) adj3 herniat$).mp.
31 Intervertebral Disc/
32 exp Intervertebral Disk Displacement/
33 exp Intervertebral Disc Degeneration/
34 Lumbar Vertebrae/
35 Nerve Compression Syndromes/
36 Spinal Osteophytosis/
37 Radiculopathy/
38 Polyradiculopathy/
39 radicul$.mp.
40 polyradicul$.mp.
41 arachnoiditis.mp.
42 or/15‐41
43 exp Spinal Nerve Roots/
44 (compress$ or inflam$ or pathol$).mp.
45 43 and 44
46 42 or 45
47 exp Injections, Epidural/
48 exp Injections, Spinal/
49 exp Epidural Space/
50 exp Anesthesia, Epidural/
51 exp Analgesia, Epidural/
52 epidural.mp.
53 extradural.mp.
54 peridural.mp.
55 (spinal adj3 (inject$ or analges$ or anaesthe$ or anesthe$)).mp.
56 exp anesthetics, local/
57 exp anesthesia, local/
58 (local adj3 (anesthe$ or anaesthe$ or analges$ or injection$)).mp.
59 TFESI.mp.
60 SNRB.mp.
61 "transforaminal epidural steroid injection".mp.
62 "selective nerve root block".mp.
63 exp Nerve Block/
64 or/47‐63
65 exp Glucocorticoids/
66 exp Adrenal Cortex Hormones/
67 exp Steroids/
68 exp Beclomethasone/
69 exp Betamethasone/
70 exp Clobetasol/
71 exp Cortisone/
72 exp Hydrocortisone/
73 exp Desoximetasone/
74 exp Dexamethasone/
75 exp Diflucortolone/
76 exp Flumethasone/
77 fluocinolone.mp.
78 exp Fluocinonide/
79 exp Fluocortolone/
80 exp Fluorometholone/
81 exp Fluprednisolone/
82 exp Flurandrenolone/
83 melengestrol.mp.
84 exp Methylprednisolone/
85 exp Paramethasone/
86 exp Prednisolone/
87 exp Prednisone/
88 exp Triamcinolone/
89 (corticosteroid$ or Glucocortic$ or Adrenal Cortex Hormone$ or Steroid$ or corticoid$ or prednis$ or cortisone$ or hydrocort$ or methylprednis$ or triamcinol$ or dexamet$ or betamet$ or beclomet$ or parametas$ or paramethas$).mp.
90 or/65‐89
91 14 and 46 and 64 and 90
92 limit 91 to yr=2013‐2015
93 limit 91 to ed=20130627‐20151007
94 92 or 93
June 2013 strategy (Pinto 2012)
1 (randomized controlled trial or controlled clinical trial or randomized controlled trials or randomized controlled trials or random allocation or double‐blind method or single‐blind method or clinical trial or clinical trials or placebos or placebo$ or random$ or research design or comparative study or evaluation studies or follow‐up studies or prospective studies or cross‐over studies or control$ or prospective$ or volunteer$).mp.
2 animal/ not human/
3 1 not 2
4 (Radicul$ or Sciatic$).mp.
5 3 and 4
6 ((systemic adj5 corticosteroid$) or Glucocorticoids or Adrenal Cortex Hormones or Steroids or glucocortic$ or adrenal Cortex Horm$ or prednisone$ or prednisol$ or cortisone$ or hydrocort$ or methylprednis$ or triamcinol$ or dexamethas$ or betamethas$ or beclomethas$ or paramethas$ or dexametas$ or betametas$ or beclometas$ or parametas$).mp.
7 5 and 6
8 limit 7 to yr="2012 ‐ 2013"
9 limit 7 to ed=20120401‐20130627
10 8 or 9
The following strategy was also used for MEDLINE in June 2013
1 randomized controlled trial.pt.
2 controlled clinical trial.pt.
3 randomi#ed.ti,ab.
4 placebo.ti,ab.
5 randomly.ti,ab.
6 controlled.ti,ab.
7 prospective.ti,ab.
8 trial.ti,ab.
9 groups.ti,ab.
10 or/1‐9
11 (animals not (humans and animals)).sh.
12 10 not 11
13 back disorder$.ti,ab.
14 dorsalgia.ti,ab.
15 exp Back Pain/
16 backache.ti,ab.
17 (lumbar adj pain).ti,ab.
18 coccyx.ti,ab.
19 coccydynia.ti,ab.
20 sciatic$.ti,ab.
21 exp sciatic neuropathy/
22 spondylosis.ti,ab.
23 lumbago.ti,ab.
24 exp Spine/
25 discitis.ti,ab.
26 exp Spinal Diseases/
27 (disc adj degeneration).ti,ab.
28 (disc adj prolapse).ti,ab.
29 (disc adj herniation).ti,ab.
30 intervertebral disk.sh.
31 ischialgia.mp.
32 exp Intervertebral Disk Displacement/
33 Nerve Compression Syndromes/
34 Spinal Osteophytosis/
35 Radiculopathy/
36 Polyradiculopathy/
37 radicul$.mp.
38 polyradicul$.mp.
39 exp Spinal Nerve Roots/
40 (compress$ or inflamm$ or pathol).mp.
41 39 and 40
42 (or/13‐39) or 41
43 exp arachnoiditis/
44 42 or 43
45 exp Injections, Epidural/
46 exp Injections, Spinal/
47 exp Epidural Space/
48 exp Anesthesia, Epidural/
49 exp Analgesia, Epidural/
50 epidural.mp.
51 extradural.mp.
52 peridural.mp.
53 (spinal adj inject$).mp.
54 (spinal adj analges$).mp.
55 TFESI.mp.
56 SNRB.mp.
57 "transforaminal epidural steroid injection".mp.
58 "selective nerve root block".mp.
59 exp Nerve Block/
60 or/45‐59
61 exp Glucocorticoids/
62 Adrenal Cortex Hormones/
63 Steroids/
64 steroid$.mp.
65 corticosteroid$.mp.
66 glucocorticoid$.mp.
67 exp Beclomethasone/
68 exp Betamethasone/
69 exp Clobetasol/
70 exp Desoximetasone/
71 exp Dexamethasone/
72 exp Diflucortolone/
73 exp Flumethasone/
74 fluocinolone.mp.
75 exp Fluocinonide/
76 exp Fluocortolone/
77 exp Fluorometholone/
78 exp Fluprednisolone/
79 exp Flurandrenolone/
80 melengestrol.mp.
81 exp Methylprednisolone/
82 exp Paramethasone/
83 exp Prednisolone/
84 exp Prednisone/
85 exp Triamcinolone/
86 ((systemic adj5 corticosteroid$) or Glucocorticoids or Adrenal Cortex Hormones or Steroids or glucocortic$ or adrenal Cortex Horm$ or prednisone$ or prednisol$ or cortisone$ or hydrocort$ or methylprednis$ or triamcinol$ or dexamethas$ or betamethas$ or beclomethas$ or paramethas$ or dexametas$ or betametas$ or beclometas$ or parametas$).mp.
87 or/61‐86
88 12 and 44 and 60 and 87
89 limit 88 to yr="2012 ‐ 2013"
90 limit 88 to ed=20120401‐20130627
91 89 or 90
Embase
Last searched 25 September 2019
1 Randomized Controlled Trial/
2 exp Controlled clinical trial/
3 Controlled Study/
4 Double Blind Procedure/
5 Single Blind Procedure/
6 crossover procedure/
7 placebo/
8 random*.ti,ab.
9 placebo?.ti,ab.
10 allocat*.ti,ab.
11 assign*.ti,ab.
12 blind*.ti,ab.
13 (compare or compared or comparing or comparison or comparative).ti,ab.
14 ((controlled adj7 study) or controlled design).ti,ab.
15 (cross‐over or crossover).ti,ab.
16 ((singl* adj7 mask*) or (doubl* adj7 mask*) or (trebl* adj7 mask*) or (tripl* adj7 mask*)).ti,ab.
17 trial.ti,ab.
18 or/1‐17
19 exp animals/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/
20 human/ or normal human/ or human cell/
21 19 and 20
22 19 not 21
23 18 not 22
24 dorsalgia.tw,kw.
25 ((lumb* adj3 pain) or (back adj3 pain)).tw,kw.
26 exp BACKACHE/
27 coccyx.tw,kw.
28 coccydynia.tw,kw.
29 exp ISCHIALGIA/
30 spondylosis.tw,kw.
31 lumbago.tw,kw.
32 (discitis or diskitis).tw,kw.
33 ((disc* adj3 degenerat*) or (disc* adj3 prolapse*) or (disc* adj3 herniat) or (disk* adj3 degenerat*) or (disk* adj3 prolapse*) or (disk* adj3 herniat)).tw,kw.
34 intervertebral disk/ or exp intervertebral disk disease/ or exp Intervertebral Disk Hernia/
35 lumbar disk/ or lumbar spine/ or lumbar vertebra/ or lumbosacral spine/
36 exp RADICULOPATHY/
37 exp Radicular Pain/
38 radicul*.tw,kw.
39 polyradicul*.tw,kw.
40 exp sciatica/
41 sciatic*.tw,kw.
42 exp "Nerve Root Compression"/
43 spondylosis/
44 exp arachnoiditis/
45 or/24‐44
46 exp "Spinal Root"/
47 (compress* or inflam* or pathol*).tw,kw.
48 46 and 47
49 45 or 48
50 exp Epidural Drug Administration/
51 exp Epidural Anesthesia/
52 exp Epidural Space/
53 (epidural or extradural or peridural).tw,kw.
54 ((spinal adj3 inject*) or (spinal adj3 analges*) or (spinal adj3 anaesthe*) or (spinal adj3 anesthe*)).tw,kw.
55 exp Intraspinal Drug Administration/
56 local anesthesia/
57 local anesthetic agent/
58 ((local adj3 anesthe*) or (local adj3 anaesthe*) or (local adj3 analges*) or (local adj3 injection*)).tw,kw.
59 exp Nerve Block/
60 (TFESI or SNRB).tw,kw.
61 "transforaminal epidural steroid injection".tw,kw.
62 "selective nerve root block".tw,kw.
63 ei.fs. [epidural injection floating subject heading]
64 or/50‐63
65 exp antiinflammatory agent/
66 exp nonsteroid antiinflammatory agent/
67 65 not 66
68 exp STEROID/ or steroid therapy/
69 exp CORTICOSTEROID/ or "corticosteroid therapy"/
70 exp GLUCOCORTICOID/
71 exp Beclometasone/
72 exp betamethasone/
73 exp CLOBETASOL/
74 exp desoximetasone/
75 exp Dexamethasone/
76 exp Diflucortolone/
77 exp Flumetasone/
78 exp fluocinolone/
79 exp fluocinonide/
80 exp Fluocortolone/
81 exp Fluorometholone/
82 exp fluprednisolone/
83 exp fludroxycortide/
84 exp melengestrol/
85 exp methylprednisolone/
86 exp paramethasone/
87 exp Prednisolone/
88 exp Prednisone/
89 exp Triamcinolone/
90 (predniso* or cortisone* or hydrocort* or methylprednis* or triamcinol* or dexamet* or betamet* or beclomet* or parametas* or paramethas*).tw,kw.
91 (corticosteroid* or Glucocortic* or Steroid* or corticoid* or Adrenal cortex hormone* or Adrenal cortical hormone* or Adreno corticosteroid* or Adrenocortical hormone* or adrenocorticosteroid*).tw,kw.
92 or/67‐91
93 23 and 49 and 64 and 92
94 limit 93 to yr=2018‐2019
95 limit 93 to dd=20180823‐20190925
96 94 or 95
Searched 23 August 2018
1 Randomized Controlled Trial/
2 exp Controlled clinical trial/
3 Controlled Study/
4 Double Blind Procedure/
5 Single Blind Procedure/
6 crossover procedure/
7 placebo/
8 random*.ti,ab.
9 placebo?.ti,ab.
10 allocat*.ti,ab.
11 assign*.ti,ab.
12 blind*.ti,ab.
13 (compare or compared or comparing or comparison or comparative).ti,ab.
14 (controlled adj7 (study or design or trial)).ti,ab.
15 (cross‐over or crossover).ti,ab.
16 ((singl* or doubl* or trebl* or tripl*) adj7 (blind* or mask*)).ti,ab.
17 trial.ti,ab.
18 or/1‐17
19 exp animals/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/
20 human/ or normal human/ or human cell/
21 19 and 20
22 19 not 21
23 18 not 22
24 dorsalgia.tw,kw.
25 ((lumb$ or back) adj3 pain).tw,kw.
26 exp BACKACHE/
27 coccyx.tw,kw.
28 coccydynia.tw,kw.
29 exp ISCHIALGIA/
30 spondylosis.tw,kw.
31 lumbago.tw,kw.
32 (discitis or diskitis).tw,kw.
33 ((disc$ or disk$) adj3 (degenerat$ or prolapse$ or herniat$)).tw,kw.
34 intervertebral disk/ or exp intervertebral disk disease/ or exp Intervertebral Disk Hernia/
35 lumbar disk/ or lumbar spine/ or lumbar vertebra/ or lumbosacral spine/
36 exp RADICULOPATHY/
37 exp Radicular Pain/
38 radicul$.tw,kw.
39 polyradicul$.tw,kw.
40 exp sciatica/
41 sciatic$.tw,kw.
42 exp "Nerve Root Compression"/
43 spondylosis/
44 exp arachnoiditis/
45 or/24‐44
46 exp "Spinal Root"/
47 (compress$ or inflam$ or pathol$).tw,kw.
48 46 and 47
49 45 or 48
50 exp Epidural Drug Administration/
51 exp Epidural Anesthesia/
52 exp Epidural Space/
53 (epidural or extradural or peridural).tw,kw.
54 (spinal adj3 (inject$ or analges$ or anaesthe$ or anesthe$)).tw,kw.
55 exp Intraspinal Drug Administration/
56 local anesthesia/
57 local anesthetic agent/
58 (local adj3 (anesthe$ or anaesthe$ or analges$ or injection$)).tw,kw.
59 exp Nerve Block/
60 (TFESI or SNRB).tw,kw.
61 "transforaminal epidural steroid injection".tw,kw.
62 "selective nerve root block".tw,kw.
63 ei.fs. [epidural injection floating subject heading]
64 or/50‐63
65 exp antiinflammatory agent/ (1637151)
66 exp nonsteroid antiinflammatory agent/ (639665)
67 65 not 66 (997486)
68 exp STEROID/ or steroid therapy/
69 exp CORTICOSTEROID/ or "corticosteroid therapy"/
70 exp GLUCOCORTICOID/
71 exp Beclometasone/
72 exp betamethasone/
73 exp CLOBETASOL/
74 exp desoximetasone/
75 exp Dexamethasone/
76 exp Diflucortolone/
77 exp Flumetasone/
78 exp fluocinolone/
79 exp fluocinonide/
80 exp Fluocortolone/
81 exp Fluorometholone/
82 exp fluprednisolone/
83 exp fludroxycortide/
84 exp melengestrol/
85 exp methylprednisolone/
86 exp paramethasone/
87 exp Prednisolone/
88 exp Prednisone/
89 exp Triamcinolone/
90 (predniso$ or cortisone$ or hydrocort$ or methylprednis$ or triamcinol$ or dexamet$ or betamet$ or beclomet$ or parametas$ or paramethas$).tw,kw.
91 (corticosteroid$ or Glucocortic$ or Steroid$ or corticoid$ or Adrenal cortex hormone$ or Adrenal cortical hormone$ or Adreno corticosteroid$ or Adrenocortical hormone$ or adrenocorticosteroid$).tw,kw.
92 or/67‐91
93 23 and 49 and 64 and 92
94 limit 93 to yr=2017‐2018
95 limit 93 to dd=20170616‐20180823
96 94 or 95
16 June 2017 strategy. The study design filter was revised. The .mp.field for the disorder and intervention terms was changed to .tw,kw.
1 Randomized Controlled Trial/
2 exp Controlled clinical trial/
3 Controlled Study/
4 Double Blind Procedure/
5 Single Blind Procedure/
6 crossover procedure/
7 placebo/
8 random*.ti,ab.
9 placebo?.ti,ab.
10 allocat*.ti,ab.
11 assign*.ti,ab.
12 blind*.ti,ab.
13 (compare or compared or comparing or comparison or comparative).ti,ab.
14 (controlled adj7 (study or design or trial)).ti,ab.
15 (cross‐over or crossover).ti,ab.
16 ((singl* or doubl* or trebl* or tripl*) adj7 (blind* or mask*)).ti,ab.
17 trial.ti,ab.
18 or/1‐17
19 exp animals/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/
20 human/ or normal human/ or human cell/
21 19 and 20
22 19 not 21
23 18 not 22
24 dorsalgia.tw,kw.
25 ((lumb$ or back) adj3 pain).tw,kw.
26 exp BACKACHE/
27 coccyx.tw,kw.
28 coccydynia.tw,kw.
29 exp ISCHIALGIA/
30 spondylosis.tw,kw.
31 lumbago.tw,kw.
32 (discitis or diskitis).tw,kw.
33 ((disc$ or disk$) adj3 (degenerat$ or prolapse$ or herniat$)).tw,kw.
34 intervertebral disk/ or exp intervertebral disk disease/ or exp Intervertebral Disk Hernia/
35 lumbar disk/ or lumbar spine/ or lumbar vertebra/ or lumbosacral spine/
36 exp RADICULOPATHY/
37 exp Radicular Pain/
38 radicul$.tw,kw.
39 polyradicul$.tw,kw.
40 exp sciatica/
41 sciatic$.tw,kw.
42 exp "Nerve Root Compression"/
43 spondylosis/
44 exp arachnoiditis/
45 or/24‐44
46 exp "Spinal Root"/
47 (compress$ or inflam$ or pathol$).tw,kw.
48 46 and 47
49 45 or 48
50 exp Epidural Drug Administration/
51 exp Epidural Anesthesia/
52 exp Epidural Space/
53 (epidural or extradural or peridural).tw,kw.
54 (spinal adj3 (inject$ or analges$ or anaesthe$ or anesthe$)).tw,kw.
55 exp Intraspinal Drug Administration/
56 local anesthesia/
57 local anaesthetic agent/
58 (local adj3 (anesthe$ or anaesthe$ or analges$ or injection$)).tw,kw.
59 exp Nerve Block/
60 (TFESI or SNRB).tw,kw.
61 "transforaminal epidural steroid injection".tw,kw.
62 "selective nerve root block".tw,kw.
63 ei.fs. [epidural injection floating subject heading]
64 or/50‐63
65 exp antiinflammatory agent/
66 exp nonsteroid antiinflammatory agent/
67 65 not 66
68 exp STEROID/ or steroid therapy/
69 exp CORTICOSTEROID/ or "corticosteroid therapy"/
70 exp GLUCOCORTICOID/
71 exp Beclometasone/
72 exp betamethasone/
73 exp CLOBETASOL/
74 exp desoximetasone/
75 exp Dexamethasone/
76 exp Diflucortolone/
77 exp Flumetasone/
78 exp fluocinolone/
79 exp fluocinonide/
80 exp Fluocortolone/
81 exp Fluorometholone/
82 exp fluprednisolone/
83 exp fludroxycortide/
84 exp melengestrol/
85 exp methylprednisolone/
86 exp paramethasone/
87 exp Prednisolone/
88 exp Prednisone/
89 exp Triamcinolone/
90 (predniso$ or cortisone$ or hydrocort$ or methylprednis$ or triamcinol$ or dexamet$ or betamet$ or beclomet$ or parametas$ or paramethas$).tw,kw.
91 (corticosteroid$ or Glucocortic$ or Steroid$ or corticoid$ or Adrenal cortex hormone$ or Adrenal cortical hormone$ or Adreno corticosteroid$ or Adrenocortical hormone$ or adrenocorticosteroid$).tw,kw.
92 or/67‐91
93 23 and 49 and 64 and 92
94 limit 93 to yr=2015‐2017
95 limit 93 to dd=20151007‐20170616
96 94 or 95
8 October 2015 strategy
1 Randomized Controlled Trial/
2 Controlled Clinical Trial/
3 Controlled Study/
4 Double Blind Procedure/
5 Single Blind Procedure/
6 crossover procedure/
7 placebo/
8 allocat$.mp.
9 assign$.mp.
10 blind$.mp.
11 compar$.mp.
12 control$.mp.
13 (crossover or cross over or cross‐over).mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword]
14 factorial$.mp.
15 (follow‐up or followup).mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword]
16 prospectiv$.mp.
17 random$.mp.
18 placebo.mp.
19 (clinic$ adj25 (study or trial)).mp.
20 ((singl$ or doubl$ or trebl$ or tripl$) adj25 (blind$ or mask$)).mp.
21 or/1‐20
22 exp animals/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/
23 human/ or normal human/ or human cell/
24 22 and 23
25 22 not 24
26 21 not 25
27 dorsalgia.mp.
28 ((lumb$ or back) adj3 pain).mp.
29 exp BACKACHE/
30 coccyx.mp.
31 coccydynia.mp.
32 exp ISCHIALGIA/
33 spondylosis.mp.
34 lumbago.mp.
35 (discitis or diskitis).mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword]
36 ((disc$ or disk$) adj3 (degenerat$ or prolapse$ or herniat$)).mp.
37 intervertebral disk/ or exp intervertebral disk disease/ or exp Intervertebral Disk Hernia/
38 lumbar disk/ or lumbar spine/ or lumbar vertebra/ or lumbosacral spine/
39 exp RADICULOPATHY/
40 exp Radicular Pain/
41 radicul$.mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword]
42 polyradicul$.mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword]
43 exp sciatica/
44 sciatic$.mp.
45 exp "Nerve Root Compression"/
46 spondylosis/
47 exp arachnoiditis/
48 or/27‐47
49 exp "Spinal Root"/
50 (compress$ or inflam$ or pathol$).mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword]
51 49 and 50
52 48 or 51
53 exp Epidural Drug Administration/
54 exp Epidural Anesthesia/
55 exp Epidural Space/
56 (epidural or extradural or peridural).mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword]
57 (spinal adj3 (inject$ or analges$ or anaesthe$ or anesthe$)).mp.
58 exp Intraspinal Drug Administration/
59 local anesthesia/
60 local anaesthetic agent/
61 (local adj3 (anesthe$ or anaesthe$ or analges$ or injection$)).mp.
62 exp Nerve Block/
63 (TFESI or SNRB).mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword]
64 "transforaminal epidural steroid injection".mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword]
65 "selective nerve root block".mp.
66 ei.fs. [epidural injection floating subject heading]
67 or/53‐66
68 exp antiinflammatory agent/
69 exp nonsteroid antiinflammatory agent/
70 68 not 69
71 exp STEROID/ or steroid therapy/
72 exp CORTICOSTEROID/ or "corticosteroid therapy"/
73 exp GLUCOCORTICOID/
74 exp Beclometasone/
75 exp betamethasone/
76 exp CLOBETASOL/
77 exp desoximetasone/
78 exp Dexamethasone/
79 exp Diflucortolone/
80 exp Flumetasone/
81 exp fluocinolone/
82 exp fluocinonide/
83 exp Fluocortolone/
84 exp Fluorometholone/
85 exp fluprednisolone/
86 exp fludroxycortide/
87 exp melengestrol/
88 exp methylprednisolone/
89 exp paramethasone/
90 exp Prednisolone/
91 exp Prednisone/
92 exp Triamcinolone/
93 (predniso$ or cortisone$ or hydrocort$ or methylprednis$ or triamcinol$ or dexamet$ or betamet$ or beclomet$ or parametas$ or paramethas$).mp.
94 (corticosteroid$ or Glucocortic$ or Steroid$ or corticoid$ or Adrenal cortex hormone$ or Adrenal cortical hormone$ or Adreno corticosteroid$ or Adrenocortical hormone$ or adrenocorticosteroid$).mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword]
95 or/70‐94
96 26 and 52 and 67 and 95
97 limit 96 to yr=2013‐2015
98 limit 96 to em=201326‐201540
99 97 or 98
June 2013 strategy
1 Clinical Article/
2 exp Clinical Study/
3 Clinical Trial/
4 Controlled Study/
5 Randomized Controlled Trial/
6 Major Clinical Study/
7 Double Blind Procedure/
8 Multicenter Study/
9 Single Blind Procedure/
10 Phase 3 Clinical Trial/
11 Phase 4 Clinical Trial/
12 crossover procedure/
13 placebo/
14 or/1‐13
15 allocat$.mp.
16 assign$.mp.
17 blind$.mp.
18 (clinic$ adj25 (study or trial)).mp.
19 compar$.mp.
20 control$.mp.
21 cross?over.mp.
22 factorial$.mp.
23 follow?up.mp.
24 placebo$.mp.
25 prospectiv$.mp.
26 random$.mp.
27 ((singl$ or doubl$ or trebl$ or tripl$) adj25 (blind$ or mask$)).mp.
28 trial.mp.
29 (versus or vs).mp.
30 or/15‐29
31 14 and 30
32 exp animals/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/
33 human/ or normal human/ or human cell/
34 32 and 33
35 32 not 34
36 31 not 35
37 dorsalgia.mp.
38 back pain.mp.
39 exp BACKACHE/
40 (lumbar adj pain).mp.
41 coccyx.mp.
42 coccydynia.mp.
43 sciatica.mp.
44 exp ISCHIALGIA/
45 spondylosis.mp.
46 lumbago.mp.
47 exp SPINE/
48 discitis.mp.
49 exp Spine Disease/
50 (disc adj degeneration).mp.
51 (disc adj prolapse).mp.
52 (disc adj herniation).mp.
53 intervertebral disk.mp.
54 exp RADICULOPATHY/
55 exp Radicular Pain/
56 radicul$.mp.
57 polyradicul$.mp.
58 exp sciatica/
59 sciatic$.mp.
60 exp "Nerve Root Compression"/
61 exp Intervertebral Disk Hernia/
62 ostheophyt$.mp.
63 exp "Spinal Root"/
64 (compress$ or inflam$ or pathol$).mp
65 63 and 64
66 (or/37‐62) or 65
67 exp arachnoiditis/
68 67 or 66
69 exp Epidural Drug Administration/
70 exp Epidural Anesthesia/
71 exp Epidural Space/
72 epidural$.mp.
73 extradural$.mp.
74 peridural$.mp.
75 ((spine or spinal) adj2 inject$).mp
76 exp Intraspinal Drug Administration/
77 exp Nerve Block/
78 TFESI.mp.
79 "transforaminal epidural steroid injection".mp
80 SNRB.mp.
81 "selective nerve root block".mp.
82 or/69‐81
83 exp STEROID/
84 steroid$.mp.
85 exp CORTICOSTEROID/
86 exp GLUCOCORTICOID/
87 glucocorticoid$.mp.
88 beclomethasone.mp. or exp Beclometasone/
89 exp BETAMETHASONE/
90 exp CLOBETASOL/
91 exp DESOXIMETASONE/
92 exp DEXAMETHASONE/
93 exp DIFLUCORTOLONE/
94 flumethasone.mp. or exp Flumetasone/
95 exp FLUOCINOLONE/
96 exp FLUOCINONIDE/
97 exp FLUOCORTOLONE/
98 exp FLUOROMETHOLONE/
99 exp FLUPREDNISOLONE/
100 flurandrenolone.mp. or exp Fludroxycortide/
101 exp MELENGESTROL/
102 exp METHYLPREDNISOLONE/
103 exp PARAMETHASONE/
104 exp PREDNISOLONE/
105 exp PREDNISONE/
106 exp TRIAMCINOLONE/
107 or/83‐106
108 (prednisone$ or prednisol$ or cortisone$ or hydrocort$ or methylprednis$ or triamcinol$ or dexamethas$ or betamethas$ or beclomethas$ or paramethas$ or dexametas$ or betametas$ or beclometas$ or parametas$).mp
109 (Adrenal cortex hormone$ or Adrenal cortical hormone$ or Adreno corticosteroid or Adrenocortical hormone or adrenocorticosteroid).mp.
110 or/83‐109
111 37 or 38 or 39 or 40 or 41 or 42 or 43 or 44 or 45 or 46 or 48 or 50 or 51 or 52 or 53 or 54 or 55 or 56 or 57 or 58 or 59 or 60 or 61 or 62 or 65 or 67
112 36 and 110 and 111
113 36 and 82 and 110 and 111
114 limit 113 to yr="2012 ‐ 2014"
115 limit 113 to em=201212‐201326
116 114 or 115
CINAHL
Last searched 25 September 2019
S96 S94 OR S95
S95 S93 AND EM 20180823‐20190925
S94 S24 AND S53 AND S67 AND S92 Limiters ‐ Published Date: 20180801‐20190931
S93 S24 AND S53 AND S67 AND S92
S92 S68 OR S69 OR S70 OR S71 OR S72 OR S73 OR S74 OR S75 OR S76 OR S77 OR S78 OR S79 OR S80 OR S81 OR S82 OR S83 OR S84 OR S85 OR S86 OR S87 OR S88 OR S89 OR S90 OR S91
S91 corticosteroid* or Glucocortic* or Adrenal Cortex Hormone* or Steroid* or corticoid* or prednis* or cortisone* or hydrocort* or methylprednis* or triamcinol* or dexamet* or betamet* or beclomet* or parametas* or paramethas*
S90 (MH "Antiinflammatory Agents, Steroidal+")
S89 (MH "Triamcinolone")
S88 (MH "Prednisone")
S87 (MH "Prednisolone+")
S86 "Paramethasone"
S85 (MH "Methylprednisolone")
S84 "melengestrol"
S83 "Flurandrenolone"
S82 "Fluprednisolone"
S81 "Fluorometholone"
S80 "Fluocortolone"
S79 "Fluocinonide"
S78 "fluocinolone"
S77 "Flumethasone"
S76 "Diflucortolone"
S75 (MH "Dexamethasone")
S74 "Desoximetasone"
S73 "Clobetasol"
S72 (MH "Betamethasone")
S71 (MH "Beclomethasone")
S70 (MH "Adrenal Cortex Hormones+")
S69 (MH "Glucocorticoids+")
S68 (MH "Steroids+")
S67 S54 OR S55 OR S56 OR S57 OR S58 OR S59 OR S60 OR S61 OR S62 OR S63 OR S64 OR S65 OR S66
S66 (MH "Nerve Block")
S65 "selective nerve root block"
S64 "transforaminal epidural steroid injection"
S63 TFESI OR SNRB
S62 ((local N3 anesthe*) or (local N3 anaesthe*) or (local N3 analges*) or (local N3 injection*))
S61 ((spinal N2 inject*) or (spinal N2 analges*) or (spinal N2 anaesthe*) or (spinal N2 anesthe*))
S60 epidural or extradural or peridural
S59 (MH "Anesthetics, Local")
S58 (MH "Anesthesia, Local")
S57 (MH "Anesthesia, Epidural")
S56 (MH "Analgesia, Epidural")
S55 (MH "Injections, Intraspinal")
S54 (MH "Injections, Epidural")
S53 S49 OR S52
S52 S50 AND S51
S51 compress* or inflam* or pathol*
S50 (MH "Spinal Nerve Roots+")
S49 S25 OR S26 OR S27 OR S28 OR S29 OR S30 OR S31 OR S32 OR S33 OR S34 OR S35 OR S36 OR S37 OR S38 OR S39 OR S40 OR S41 OR S42 OR S43 OR S44 OR S45 OR S46 OR S47 OR S48
S48 "arachnoiditis"
S47 (MH "Nerve Compression Syndromes")
S46 (MH "Spinal Osteophytosis")
S45 (MH "Intervertebral Disk")
S44 (MH "Intervertebral Disk Displacement")
S43 ((disc* N2 prolapse*) or (disk* N2 prolapse*)
S42 ((disc* N2 herniat*) or (disk* N2 herniat*)
S41 ((disc* N2 degenerat*) or (disk* N2 degenerat*))
S40 discitis or diskitis
S39 lumbago
S38 (MH "Spondylosis")
S37 lumbar N2 vertebrae
S36 (MH "Lumbar Vertebrae")
S35 back disorder*
S34 coccydynia
S33 coccyx
S32 sciatica
S31 (MH "Sciatica")
S30 (MH "Polyradiculopathy") OR (MH "Radiculopathy")
S29 radicul* or polyradicul*
S28 (lumb* N2 pain) or (back N2 pain)
S27 backache* or back ache*
S26 (MH "Back Pain+")
S25 dorsalgia
S24 S22 not S23
S23 (MH "Animals+")
S22 S21 or S20 or S19 or S18 or S17 or S16 or S15 or S14 or S13 or S12 or S11 or S10 or S9 or S8 or S7 or S6 or S5 or S4 or S3 or S2 or S1
S21 volunteer*
S20 prospectiv*
S19 control*
S18 followup stud*
S17 follow‐up stud*
S16 (MH "Prospective Studies+")
S15 (MH "Evaluation Research+")
S14 (MH "Comparative Studies")
S13 latin square
S12 (MH "Study Design+")
S11 (MH "Random Sample+")
S10 random*
S9 placebo*
S8 (MH "Placebos")
S7 (MH "Placebo Effect")
S6 triple‐blind
S5 single‐blind
S4 double‐blind
S3 clinical W3 trial
S2 randomi?ed controlled trial*
S1 (MH "Clinical Trials+")
Searched 23 August 2018
S96 S94 OR S95
S95 S93 AND EM 20170616‐20180823
S94 S24 AND S49 AND S67 AND S92 Limiters ‐ Published Date: 20151001‐20170631
S93 S24 AND S49 AND S67 AND S92
S92 S68 OR S69 OR S70 OR S71 OR S72 OR S73 OR S74 OR S75 OR S76 OR S77 OR S78 OR S79 OR S80 OR S81 OR S82 OR S83 OR S84 OR S85 OR S86 OR S87 OR S88 OR S89 OR S90 OR S91
S91 corticosteroid* or Glucocortic* or Adrenal Cortex Hormone* or Steroid* or corticoid* or prednis* or cortisone* or hydrocort* or methylprednis* or triamcinol* or dexamet* or betamet* or beclomet* or parametas* or paramethas*
S90 (MH "Antiinflammatory Agents, Steroidal+")
S89 (MH "Triamcinolone")
S88 (MH "Prednisone")
S87 (MH "Prednisolone+")
S86 "Paramethasone"
S85 (MH "Methylprednisolone")
S84 "melengestrol"
S83 "Flurandrenolone"
S82 "Fluprednisolone"
S81 "Fluorometholone"
S80 "Fluocortolone"
S79 "Fluocinonide"
S78 "fluocinolone"
S77 "Flumethasone"
S76 "Diflucortolone"
S75 (MH "Dexamethasone")
S74 "Desoximetasone"
S73 "Clobetasol"
S72 (MH "Betamethasone")
S71 (MH "Beclomethasone")
S70 (MH "Adrenal Cortex Hormones+")
S69 (MH "Glucocorticoids+")
S68 (MH "Steroids+")
S67 S54 OR S55 OR S56 OR S57 OR S58 OR S59 OR S60 OR S61 OR S62 OR S63 OR S64 OR S65 OR S66
S66 (MH "Nerve Block")
S65 "selective nerve root block"
S64 "transforaminal epidural steroid injection"
S63 TFESI OR SNRB
S62 (local N3 (anesthe* or anaesthe* or analges* or injection*))
S61 (spinal N2 (inject* or analges* or anaesthe* or anesthe*))
S60 epidural or extradural or peridural
S59 (MH "Anesthetics, Local")
S58 (MH "Anesthesia, Local")
S57 (MH "Anesthesia, Epidural")
S56 (MH "Analgesia, Epidural")
S55 (MH "Injections, Intraspinal")
S54 (MH "Injections, Epidural")
S53 S49 OR S52
S52 S50 AND S51
S51 compress* or inflam* or pathol*
S50 (MH "Spinal Nerve Roots+")
S49 S25 OR S26 OR S27 OR S28 OR S29 OR S30 OR S31 OR S32 OR S33 OR S34 OR S35 OR S36 OR S37 OR S38 OR S39 OR S40 OR S41 OR S42 OR S43 OR S44 OR S45 OR S46 OR S47 OR S48
S48 "arachnoiditis"
S47 (MH "Nerve Compression Syndromes")
S46 (MH "Spinal Osteophytosis")
S45 (MH "Intervertebral Disk")
S44 (MH "Intervertebral Disk Displacement")
S43 ((disc* or disk*) N2 prolapse*)
S42 ((disc* or disk*) N2 herniat*)
S41 ((disc* or disk*) N2 degenerat*)
S40 discitis or diskitis
S39 lumbago
S38 (MH "Spondylosis")
S37 lumbar N2 vertebrae
S36 (MH "Lumbar Vertebrae")
S35 back disorder*
S34 coccydynia
S33 coccyx
S32 sciatica
S31 (MH "Sciatica")
S30 (MH "Polyradiculopathy") OR (MH "Radiculopathy")
S29 radicul* or polyradicul*
S28 (lumb* or back) N2 pain
S27 backache* or back ache*
S26 (MH "Back Pain+")
S25 dorsalgia
S24 S22 not S23
S23 (MH "Animals+")
S22 S21 or S20 or S19 or S18 or S17 or S16 or S15 or S14 or S13 or S12 or S11 or S10 or S9 or S8 or S7 or S6 or S5 or S4 or S3 or S2 or S1
S21 volunteer*
S20 prospectiv*
S19 control*
S18 followup stud*
S17 follow‐up stud*
S16 (MH "Prospective Studies+")
S15 (MH "Evaluation Research+")
S14 (MH "Comparative Studies")
S13 latin square
S12 (MH "Study Design+")
S11 (MH "Random Sample+")
S10 random*
S9 placebo*
S8 (MH "Placebos")
S7 (MH "Placebo Effect")
S6 triple‐blind
S5 single‐blind
S4 double‐blind
S3 clinical W3 trial
S2 randomi?ed controlled trial*
S1 (MH "Clinical Trials+")
16 June 2017 strategy
S96 S94 OR S95
S95 S93 AND EM 20151007‐20170616
S94 S93 Limiters ‐ Published Date: 20151001‐20170631
S93 S24 AND S53 AND S67 AND S92
S92 S68 OR S69 OR S70 OR S71 OR S72 OR S73 OR S74 OR S75 OR S76 OR S77 OR S78 OR S79 OR S80 OR S81 OR S82 OR S83 OR S84 OR S85 OR S86 OR S87 OR S88 OR S89 OR S90 OR S91
S91 corticosteroid* or Glucocortic* or Adrenal Cortex Hormone* or Steroid* or corticoid* or prednis* or cortisone* or hydrocort* or methylprednis* or triamcinol* or dexamet* or betamet* or beclomet* or parametas* or paramethas*
S90 (MH "Antiinflammatory Agents, Steroidal+")
S89 (MH "Triamcinolone")
S88 (MH "Prednisone")
S87 (MH "Prednisolone+")
S86 "Paramethasone"
S85 (MH "Methylprednisolone")
S84 "melengestrol"
S83 "Flurandrenolone"
S82 "Fluprednisolone"
S81 "Fluorometholone"
S80 "Fluocortolone"
S79 "Fluocinonide"
S78 "fluocinolone"
S77 "Flumethasone"
S76 "Diflucortolone"
S75 (MH "Dexamethasone")
S74 "Desoximetasone"
S73 "Clobetasol"
S72 (MH "Betamethasone")
S71 (MH "Beclomethasone")
S70 (MH "Adrenal Cortex Hormones+")
S69 (MH "Glucocorticoids+")
S68 (MH "Steroids+")
S67 S54 OR S55 OR S56 OR S57 OR S58 OR S59 OR S60 OR S61 OR S62 OR S63 OR S64 OR S65 OR S66
S66 (MH "Nerve Block")
S65 "selective nerve root block"
S64 "transforaminal epidural steroid injection"
S63 TFESI OR SNRB
S62 (local N3 (anesthe* or anaesthe* or analges* or injection*))
S61 (spinal N3 (inject* or analges* or anaesthe* or anesthe*))
S60 epidural or extradural or peridural
S59 (MH "Anesthetics, Local")
S58 (MH "Anesthesia, Local")
S57 (MH "Anesthesia, Epidural")
S56 (MH "Analgesia, Epidural")
S55 (MH "Injections, Intraspinal")
S54 (MH "Injections, Epidural")
S53 S49 OR S52
S52 S50 AND S51
S51 compress* or inflam* or pathol*
S50 (MH "Spinal Nerve Roots+")
S49 S25 OR S26 OR S27 OR S28 OR S29 OR S30 OR S31 OR S32 OR S33 OR S34 OR S35 OR S36 OR S37 OR S38 OR S39 OR S40 OR S41 OR S42 OR S43 OR S44 OR S45 OR S46 OR S47 OR S48
S48 "arachnoiditis"
S47 (MH "Nerve Compression Syndromes")
S46 (MH "Spinal Osteophytosis")
S45 (MH "Intervertebral Disk")
S44 (MH "Intervertebral Disk Displacement")
S43 ((disc* or disk*) N3 prolapse*)
S42 ((disc* or disk*) N3 herniat*)
S41 ((disc* or disk*) N3 degenerat*)
S40 discitis or diskitis
S39 lumbago
S38 (MH "Spondylosis")
S37 lumbar N2 vertebrae
S36 (MH "Lumbar Vertebrae")
S35 back disorder*
S34 coccydynia
S33 coccyx
S32 sciatic*
S31 (MH "Sciatica")
S30 (MH "Polyradiculopathy") OR (MH "Radiculopathy")
S29 radicul* or polyradicul*
S28 (lumb* or back) N3 pain
S27 backache* or back ache*
S26 (MH "Back Pain+")
S25 dorsalgia
S24 S22 not S23
S23 (MH "Animals+")
S22 S21 or S20 or S19 or S18 or S17 or S16 or S15 or S14 or S13 or S12 or S11 or S10 or S9 or S8 or S7 or S6 or S5 or S4 or S3 or S2 or S1
S21 volunteer*
S20 prospective*
S19 control*
S18 followup stud*
S17 follow‐up stud*
S16 (MH "Prospective Studies+")
S15 (MH "Evaluation Research+")
S14 (MH "Comparative Studies")
S13 latin square
S12 (MH "Study Design+")
S11 (MH "Random Sample+")
S10 random*
S9 placebo*
S8 (MH "Placebos")
S7 (MH "Placebo Effect")
S6 triple‐blind 95
S5 single‐blind
S4 double‐blind
S3 clinical W3 trial
S2 randomi?ed controlled trial*
S1 (MH "Clinical Trials+")
June 2013 strategy
S8 S7 Limiters ‐ Published Date from: 20120101‐20131231
S7 S5 AND S6
S6 corticosteroid$ or Glucocorticoids or (Adrenal Cortex Hormones) or Steroids or glucocortic* or (adrenal Cortex Horm*) or prednisone* or prednisol* or cortisone* or hydrocort* or methylprednis* or triamcinol* or dexamethas* or betamethas* or beclomethas* or paramethas* or dexametas* or betametas* or beclometas* or parametas*
S5 S3 AND S4
S4 “radicul*” OR “Sciatic*”
S3 S1 NOT S2
S2 (MH “Animals+”) not (MH “Human”)
S1 randomized controlled trial or controlled clinical trial or randomized controlled trials or randomized controlled trials or random allocation or double‐blind method or single‐blind method or clinical trial or clinical trials or placebos or placebo$ or random$ or research design or comparative study or evaluation studies or follow‐up studies or prospective studies or cross‐over studies or control$ or prospectiv$ or volunteer$ or (“volunteer$”) or (MH “Clinical Trials+”) or (MH “Cochrane Library”) or (MH “Random Assignment”) or (MH “Random Sample+”) or (MH “Double‐Blind Studies”) or (MH “Single‐Blind Studies”) or (MH “Triple‐Blind Studies”) or (MH “Placebos”) or (MH “Placebo Effect”) or (MH “Comparative Studies”) or (MH “Evaluation Research+”) or (MH “Concurrent Prospective Studies”) or (MH “Prospective Studies+”) or (MH “Crossover Design”)
PsycINFO
Last searched 25 September 2019
1 clinical trials/
2 control*.mp.
3 RCT.mp.
4 trial.mp.
5 (sing* adj2 blind*).mp.
6 (doub* adj2 blind*).mp.
7 exp Placebo/
8 placebo*.mp.
9 latin square.mp.
10 random*.mp.
11 prospective studies/
12 prospective*.mp.
13 compar*.mp.
14 treatment effectiveness evaluation/
15 evaluation.mp.
16 exp Posttreatment Followup/
17 (followup or follow up).mp.
18 or/1‐17
19 "Back (Anatomy)"/
20 exp back pain/
21 lumbar spinal cord/
22 spinal column/
23 ((lumb* adj3 pain) or (back adj3 pain)).mp.
24 dorsalgia.mp.
25 lumbago.mp.
26 spondylosis.mp.
27 coccyx.mp.
28 back disorder$.mp.
29 coccydynia.mp.
30 (backache or back ache).mp.
31 ischialgia.mp.
32 sciatic$.mp.
33 Spinal Nerves/
34 (radicul$ or polyradicul$).mp.
35 (discitis or diskitis).mp.
36 ((disc$ or disk$) adj3 (degenerat$ or herniat$ or prolapse$)).mp.
37 spinal osteophytosis.mp.
38 arachnoiditis.mp.
39 or/19‐38
40 injections/
41 (epidural or peridural or extradural).mp.
42 exp Local Anesthetics/
43 (spinal adj3 (inject$ or analges$ or anaesthe$ or anesthe$)).mp.
44 (local adj3 (anesthe$ or anaesthe$ or analges$ or injection$)).mp.
45 (TFESI or SNRB).mp.
46 "transforaminal epidural steroid injection".mp.
47 "selective nerve root block".mp.
48 (nerve adj2 block$).mp.
49 or/40‐48
50 exp Steroids/
51 exp Glucocorticoids/
52 exp Adrenal Cortex Hormones/
53 exp Corticosteroids/
54 clobetasol.mp.
55 Desoximetasone.mp.
56 Dexamethasone/
57 exp Cortisone/
58 exp Hydrocortisone/
59 Diflucortolone.mp.
60 Diflucortolone.mp.
61 Flumethasone.mp.
62 fluocinolone.mp.
63 Fluocinonide.mp.
64 Fluocortolone.mp.
65 Fluorometholone.mp.
66 Fluprednisolone.mp.
67 Flurandrenolone.mp.
68 melengestrol.mp.
69 Prednisolone/
70 (corticosteroid$ or Glucocortic$ or Adrenal Cortex Hormone$ or Steroid$ or corticoid$ or prednis$ or cortisone$ or hydrocort$ or methylprednis$ or triamcinol$ or dexamet$ or betamet$ or beclomet$ or parametas$ or paramethas$).mp.
71 or/50‐70
72 18 and 39 and 49 and 71
73 limit 72 to yr=2018‐2019
Searched 23 August 2018
1 clinical trials/
2 control$.ti,ab,kw.
3 RCT.ti,ab,kw.
4 (clin$ adj3 trial).ti,ab,kw.
5 (sing$ adj2 blind$).ti,ab,kw.
6 (doub$ adj2 blind$).ti,ab,kw.
7 exp Placebo/
8 placebo$.ti,ab,kw.
9 latin square.ti,ab,kw.
10 random$.ti,ab,kw.
11 prospective studies/
12 prospective$.ti,ab,kw.
13 compar$.ti,ab,kw.
14 treatment effectiveness evaluation/
15 evaluation.ti,ab,kw.
16 exp Posttreatment Followup/
17 (followup or follow up).ti,ab,kw.
18 or/1‐17
19 "Back (Anatomy)"/
20 exp back pain/
21 lumbar spinal cord/
22 spinal column/
23 ((lumb$ or back) adj3 pain).mp.
24 dorsalgia.mp.
25 lumbago.mp.
26 spondylosis.mp.
27 coccyx.mp.
28 back disorder$.mp.
29 coccydynia.mp.
30 (backache or back ache).mp.
31 ischialgia.mp.
32 sciatic$.mp.
33 Spinal Nerves/
34 (radicul$ or polyradicul$).mp.
35 (discitis or diskitis).mp.
36 ((disc$ or disk$) adj3 (degenerat$ or herniat$ or prolapse$)).mp.
37 spinal osteophytosis.mp.
38 arachnoiditis.mp.
39 or/19‐38
40 injections/
41 (epidural or peridural or extradural).mp.
42 exp Local Anesthetics/
43 (spinal adj3 (inject$ or analges$ or anaesthe$ or anesthe$)).mp.
44 (local adj3 (anesthe$ or anaesthe$ or analges$ or injection$)).mp.
45 (TFESI or SNRB).mp.
46 "transforaminal epidural steroid injection".mp.
47 "selective nerve root block".mp.
48 (nerve adj2 block$).mp.
49 or/40‐48
50 exp Steroids/
51 exp Glucocorticoids/
52 exp Adrenal Cortex Hormones/
53 exp Corticosteroids/
54 clobetasol.mp.
55 Desoximetasone.mp.
56 Dexamethasone/
57 exp Cortisone/
58 exp Hydrocortisone/
59 Diflucortolone.mp.
60 Diflucortolone.mp.
61 Flumethasone.mp.
62 fluocinolone.mp.
63 Fluocinonide.mp.
64 Fluocortolone.mp.
65 Fluorometholone.mp.
66 Fluprednisolone.mp.
67 Flurandrenolone.mp.
68 melengestrol.mp.
69 Prednisolone/
70 (corticosteroid$ or Glucocortic$ or Adrenal Cortex Hormone$ or Steroid$ or corticoid$ or prednis$ or cortisone$ or hydrocort$ or methylprednis$ or triamcinol$ or dexamet$ or betamet$ or beclomet$ or parametas$ or paramethas$).mp.
71 or/50‐70
72 18 and 39 and 49 and 71
73 limit 72 to yr=2017‐2018
16 June 2017 strategy
1 clinical trials/
2 control$.ti,ab,kw.
3 RCT.ti,ab,kw.
4 (clin$ adj3 trial).ti,ab,kw.
5 (sing$ adj2 blind$).ti,ab,kw.
6 (doub$ adj2 blind$).ti,ab,kw.
7 exp Placebo/
8 placebo$.ti,ab,kw.
9 latin square.ti,ab,kw.
10 random$.ti,ab,kw.
11 prospective studies/
12 prospective$.ti,ab,kw.
13 compar$.ti,ab,kw.
14 treatment effectiveness evaluation/
15 evaluation.ti,ab,kw.
16 exp Posttreatment Followup/
17 (followup or follow up).ti,ab,kw.
18 or/1‐17
19 "Back (Anatomy)"/
20 exp back pain/
21 lumbar spinal cord/
22 spinal column/
23 ((lumb$ or back) adj3 pain).mp.
24 dorsalgia.mp.
25 lumbago.mp.
26 spondylosis.mp.
27 coccyx.mp.
28 back disorder$.mp.
29 coccydynia.mp.
30 (backache or back ache).mp.
31 ischialgia.mp.
32 sciatic$.mp.
33 Spinal Nerves/
34 (radicul$ or polyradicul$).mp. [mp=title, abstract, heading word, table of contents, key concepts, original title, tests & measures]
35 (discitis or diskitis).mp. [mp=title, abstract, heading word, table of contents, key concepts, original title, tests & measures]
36 ((disc$ or disk$) adj3 (degenerat$ or herniat$ or prolapse$)).mp.
37 spinal osteophytosis.mp.
38 arachnoiditis.mp.
39 or/19‐38
40 injections/
41 (epidural or peridural or extradural).mp.
42 exp Local Anesthetics/
43 (spinal adj3 (inject$ or analges$ or anaesthe$ or anesthe$)).mp.
44 (local adj3 (anesthe$ or anaesthe$ or analges$ or injection$)).mp.
45 (TFESI or SNRB).mp. [mp=title, abstract, heading word, table of contents, key concepts, original title, tests & measures]
46 "transforaminal epidural steroid injection".mp.
47 "selective nerve root block".mp.
48 (nerve adj2 block$).mp.
49 or/40‐48
50 exp Steroids/
51 exp Glucocorticoids/
52 exp Adrenal Cortex Hormones/
53 exp Corticosteroids/
54 clobetasol.mp.
55 Desoximetasone.mp.
56 Dexamethasone/
57 exp Cortisone/
58 exp Hydrocortisone/
59 Diflucortolone.mp.
60 Diflucortolone.mp.
61 Flumethasone.mp.
62 fluocinolone.mp.
63 Fluocinonide.mp.
64 Fluocortolone.mp.
65 Fluorometholone.mp.
66 Fluprednisolone.mp.
67 Flurandrenolone.mp.
68 melengestrol.mp.
69 Prednisolone/
70 (corticosteroid$ or Glucocortic$ or Adrenal Cortex Hormone$ or Steroid$ or corticoid$ or prednis$ or cortisone$ or hydrocort$ or methylprednis$ or triamcinol$ or dexamet$ or betamet$ or beclomet$ or parametas$ or paramethas$).mp. [mp=title, abstract, heading word, table of contents, key concepts, original title, tests & measures]
71 or/50‐70
72 18 and 39 and 49 and 71
June 2013 strategy
1 (randomized controlled trial or controlled clinical trial or randomized controlled trials or randomized controlled trials or random allocation or double‐blind method or single‐blind method or clinical trial or clinical trials or placebos or placebo$ or random$ or research design or comparative study or evaluation studies or follow‐up studies or prospective studies or cross‐over studies or control$ or prospective$ or volunteer$).mp.
2 animal/ not human/
3 1 not 2
4 (Radicul$ or Sciatic$).mp.
5 3 and 4
6 ((systemic adj5 corticosteroid$) or Glucocorticoids or Adrenal Cortex Hormones or Steroids or glucocortic$ or adrenal Cortex Horm$ or prednisone$ or prednisol$ or cortisone$ or hydrocort$ or methylprednis$ or triamcinol$ or dexamethas$ or betamethas$ or beclomethas$ or paramethas$ or dexametas$ or betametas$ or beclometas$ or parametas$).mp.
7 5 and 6
8 limit 7 to yr="2012 ‐ 2013"
9 limit 7 to ed=20120401‐20130627
10 8 or 9
IPA
Last searched 25 September 2019
1 (random* or placebo or blind* or control* or prospective* or compar* or trial).mp. [mp=title, subject heading word, registry word, abstract, trade name/generic name]
2 (sciatic* or radicul* or polyradicul*).mp. [mp=title, subject heading word, registry word, abstract, trade name/generic name]
3 ((lumb* adj3 pain) or (back adj3 pain) or backache or back ache or lumbago or dorsalgia or coccydynia or ischialgia or back disorder*).mp. [mp=title, subject heading word, registry word, abstract, trade name/generic name]
4 (disc*adj3 degenerat* or (disc* adj3 herniat*) or (disc* adj3 prolapse*) or disk*adj3 degenerat* or (disk* adj3 herniat*) or (disk* adj3 prolapse*)).mp.
5 (spinal osteophytosis or arachnoiditis).mp. [mp=title, subject heading word, registry word, abstract, trade name/generic name]
6 or/2‐5
7 (epidural or extradural or peridural).mp. [mp=title, subject heading word, registry word, abstract, trade name/generic name]
8 ((spinal adj3 inject*) or (spinal adj3 analges*) or (spinal adj3 anaesthe*) or (spinal adj3 anesthe*)).mp.
9 ((local adj3 anesthe*) or (local adj3 anaesthe*) or (local adj3 analges*) or (local adj3 injection*)).mp.
10 (TFESI or SNRB).mp. [mp=title, subject heading word, registry word, abstract, trade name/generic name]
11 "transforaminal epidural steroid injection".mp.
12 "selective nerve root block".mp.
13 (nerve adj2 block$).mp.
14 or/7‐13
15 (corticosteroid* or Glucocortic* or Adrenal Cortex Hormone* or Steroid* or corticoid* or prednis* or cortisone* or hydrocort* or methylprednis* or triamcinol* or dexamet* or betamet* or beclomet* or parametas* or paramethas*).mp.
16 (clobetasol or desoximetasone or diflucortolone or flumethasone or fluocinolone or fluocinonide or fluocortolone or fluorometholone or fluprednisolone or flurandrenolone or melengestrol).mp. [mp=title, subject heading word, registry word, abstract, trade name/generic name]
17 or/15‐16
18 1 and 6 and 14 and 17
19 limit 18 to yr=2018‐2019
Searched 23 August 2018
1 (random$ or placebo or blind$ or control$ or prospective$ or compar$ or trial).mp. [mp=title, subject heading word, registry word, abstract, trade name/generic name]
2 (sciatic$ or radicul$ or polyradicul$).mp. [mp=title, subject heading word, registry word, abstract, trade name/generic name]
3 (((lumb$ or back) adj3 pain) or backache or back ache or lumbago or dorsalgia or coccydynia or ischialgia or back disorder$).mp. [mp=title, subject heading word, registry word, abstract, trade name/generic name]
4 ((disc$ or disk$) adj3 (degenerat$ or herniat$ or prolapse$)).mp.
5 (spinal osteophytosis or arachnoiditis).mp. [mp=title, subject heading word, registry word, abstract, trade name/generic name]
6 or/2‐5
7 (epidural or extradural or peridural).mp. [mp=title, subject heading word, registry word, abstract, trade name/generic name]
8 (spinal adj3 (inject$ or analges$ or anaesthe$ or anesthe$)).mp.
9 (local adj3 (anesthe$ or anaesthe$ or analges$ or injection$)).mp.
10 (TFESI or SNRB).mp. [mp=title, subject heading word, registry word, abstract, trade name/generic name]
11 "transforaminal epidural steroid injection".mp.
12 "selective nerve root block".mp.
13 (nerve adj2 block$).mp.
14 or/7‐13
15 (corticosteroid$ or Glucocortic$ or Adrenal Cortex Hormone$ or Steroid$ or corticoid$ or prednis$ or cortisone$ or hydrocort$ or methylprednis$ or triamcinol$ or dexamet$ or betamet$ or beclomet$ or parametas$ or paramethas$).mp.
16 (clobetasol or desoximetasone or diflucortolone or flumethasone or fluocinolone or fluocinonide or fluocortolone or fluorometholone or fluprednisolone or flurandrenolone or melengestrol).mp. [mp=title, subject heading word, registry word, abstract, trade name/generic name]
17 or/15‐16
18 1 and 6 and 14 and 17
19 limit 18 to yr=2017‐2018
7 October 7 2015 strategy
1 (random$ or placebo or blind$ or control$ or prospective$ or compar$ or trial).mp. [mp=title, subject heading word, registry word, abstract, trade name/generic name]
2 (sciatic$ or radicul$ or polyradicul$).mp. [mp=title, subject heading word, registry word, abstract, trade name/generic name]
3 (((lumb$ or back) adj3 pain) or backache or back ache or lumbago or dorsalgia or coccydynia or ischialgia or back disorder$).mp. [mp=title, subject heading word, registry word, abstract, trade name/generic name]
4 ((disc$ or disk$) adj3 (degenerat$ or herniat$ or prolapse$)).mp.
5 (spinal osteophytosis or arachnoiditis).mp. [mp=title, subject heading word, registry word, abstract, trade name/generic name]
6 or/2‐5
7 (epidural or extradural or peridural).mp. [mp=title, subject heading word, registry word, abstract, trade name/generic name]
8 (spinal adj3 (inject$ or analges$ or anaesthe$ or anesthe$)).mp.
9 (local adj3 (anesthe$ or anaesthe$ or analges$ or injection$)).mp.
10 (TFESI or SNRB).mp. [mp=title, subject heading word, registry word, abstract, trade name/generic name]
11 "transforaminal epidural steroid injection".mp.
12 "selective nerve root block".mp.
13 (nerve adj2 block$).mp.
14 or/7‐13
15 (corticosteroid$ or Glucocortic$ or Adrenal Cortex Hormone$ or Steroid$ or corticoid$ or prednis$ or cortisone$ or hydrocort$ or methylprednis$ or triamcinol$ or dexamet$ or betamet$ or beclomet$ or parametas$ or paramethas$).mp.
16 (clobetasol or desoximetasone or diflucortolone or flumethasone or fluocinolone or fluocinonide or fluocortolone or fluorometholone or fluprednisolone or flurandrenolone or melengestrol).mp. [mp=title, subject heading word, registry word, abstract, trade name/generic name]
17 or/15‐16
18 1 and 6 and 14 and 17
June 2013 strategy
1 (randomized controlled trial or controlled clinical trial or randomized controlled trials or randomized controlled trials or random allocation or double‐blind method or single‐blind method or clinical trial or clinical trials or placebos or placebo$ or random$ or research design or comparative study or evaluation studies or follow‐up studies or prospective studies or cross‐over studies or control$ or prospective$ or volunteer$).mp.
2 animal/ not human/
3 1 not 2
4 (Radicul$ or Sciatic$).mp.
5 3 and 4
6 ((systemic adj5 corticosteroid$) or Glucocorticoids or Adrenal Cortex Hormones or Steroids or glucocortic$ or adrenal Cortex Horm$ or prednisone$ or prednisol$ or cortisone$ or hydrocort$ or methylprednis$ or triamcinol$ or dexamethas$ or betamethas$ or beclomethas$ or paramethas$ or dexametas$ or betametas$ or beclometas$ or parametas$).mp.
7 5 and 6
8 limit 7 to yr="2012 ‐ 2013"
9 limit 7 to ed=20120401‐20130627
10 8 or 9
ClinicalTrials.gov
Last searched 25 September 2019
Other terms field: ((sciatica OR radicular OR radiculopathy OR disc OR back pain) AND (steroid OR corticosteroid OR glucocorticoid) AND epidural)
First posted: 08/23/2018 to 09/25/2019
8 October 2015 search
((sciatica OR radicular OR radiculopathy OR disc OR back pain) AND (steroid OR corticosteroid OR glucocorticoid) AND epidural)
21 August 21 2013 strategy
Condition: back pain AND Intervention: steroid
WHO ICTRP
Last searched 25 September 2019
sciatica AND steroid OR radicular AND steroid OR radiculopathy AND steroid OR disc AND steroid OR back AND steroid
21 August 2013 strategy
Condition: back pain
AND
Intervention: steroid
PubMed
Last searched 8 October 2015
((corticosteroid OR Glucocorticoid OR glucocorticosteroid OR steroid OR corticoid OR prednisone OR prednisolone OR cortisone OR hydrocortisone OR methylprednisone OR methylprednisolone OR triamcinolone OR dexamethasone OR dexametasone OR betamethasone OR betametasone OR beclomethasone OR beclometasone OR paramethasone OR parametasone) AND (sciatica OR radicular OR radiculopathy OR disc OR disk OR back pain OR lumbago OR dorsalgia OR coccydynia OR lumbar OR lumbosacral) AND epidural AND (pubstatusaheadofprint OR publisher[sb] or pubmednotmedline[sb]))
Appendix 2. The GRADE approach to evidence synthesis
The quality of evidence will be categorized as follows:
High (⊙⊙⊙⊙): further research is very unlikely to change the confidence in the estimate of effect.
Moderate (⊙⊙⊙○): further research is likely to have an important impact in the confidence in the estimate of effect.
Low (⊙⊙○○): further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very Low (⊙○○○): any estimate of effect is very uncertain.
The evidence available to answer each sub‐question will be graded on the domains in the following manner:
1. Study Design
We included only randomized controlled trials in this review.
2. Risk of bias
We downgraded the quality of evidence by one level when 25% of the participants were from studies judged as high risk of bias (i.e. one of the following criteria judged as having “high” or “unclear” risk of bias: random allocation, allocation concealment, blinding procedures, and adequate follow‐up) (Pinto 2012; Henschke 2010). We evaluated the included studies using the following criterion for each bias domain:
‐ Selection (random sequence generation, allocation concealment, group similarities at baseline): We scored this domain as low risk of bias if random sequence generation and allocation concealment were judged as having low risk. ‐ Performance (blinding of participants, blinding of healthcare providers, co‐interventions, and compliance with intervention). We scored this domain as low risk of bias if blinding of participants and healthcare providers were judged as having low risk. ‐ Attrition (dropouts and intention‐to‐treat analysis): We scored this domain as low risk of bias if adequate follow‐up rate was judged as having low risk. ‐ Detection (blinding of the outcome assessors and timing of outcome assessment): We scored this domain as low risk of bias if blinding of outcomes was judged as having low risk. ‐ Reporting bias (selective reporting): We will score this item as low risk of bias if it is defined as having low risk. We considered the studies as having a low risk of bias, if we judged four out of five bias domains as having low risk of bias.
3. Inconsistency
We downgraded the quality of evidence by one level when the heterogeneity of pooled estimates was higher than moderate (I2 > 40%) or ≤75% of participants from studies with findings in the same direction (Higgins 2011).
4. Indirectness
We downgraded the quality of evidence by one level when the participants were considered outside of those defined in the inclusion criteria. Indirectness is not relevant to this review because it involves a specific population, outcome measures, and comparisons.
5. Imprecision
We downgraded the quality of evidence by one level when the total of events was lower than 300 for dichotomous data and the total number of participants was lower than 400 for continuous data (Guyatt 2011).
6. Publication bias
We downgraded the quality of evidence by one level when visual inspection of the funnel plots suggested publication bias.
Data and analyses
Comparison 1. Epidural corticosteroid injection versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Leg pain intensity | 8 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Immediate (≤ 2 weeks) | 1 | 158 | Mean Difference (IV, Random, 95% CI) | ‐15.0 [‐25.88, ‐4.12] |
| 1.2 Short‐term (> 2 weeks but ≤ 3 months) | 8 | 949 | Mean Difference (IV, Random, 95% CI) | ‐4.93 [‐8.77, ‐1.09] |
| 1.3 Intermediate‐term (> 3 months but < 12 months) | 1 | 158 | Mean Difference (IV, Random, 95% CI) | 9.10 [‐1.44, 19.64] |
| 1.4 Long‐term (≥ 12 months) | 3 | 453 | Mean Difference (IV, Random, 95% CI) | ‐0.35 [‐6.23, 5.53] |
| 2 Disability | 12 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Immediate (≤ 2 weeks) | 2 | 243 | Std. Mean Difference (IV, Random, 95% CI) | 0.08 [‐0.17, 0.33] |
| 2.2 Short‐term (> 2 weeks but ≤ 3 months) | 12 | 1367 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.27 [‐0.39, ‐0.14] |
| 2.3 Intermediate‐term (> 3 months but < 12 months) | 6 | 866 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐0.40, ‐0.01] |
| 2.4 Long‐term (≥ 12 months) | 7 | 882 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.14 [‐0.38, 0.10] |
| 3 Overall pain | 10 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Immediate (≤ 2 weeks) | 2 | 120 | Mean Difference (IV, Random, 95% CI) | ‐4.71 [‐12.92, 3.50] |
| 3.2 Short‐term (> 2 weeks but ≤ 3 months) | 10 | 911 | Mean Difference (IV, Random, 95% CI) | ‐9.35 [‐14.05, ‐4.65] |
| 3.3 Intermediate‐term (> 3 months but < 12 months) | 4 | 429 | Mean Difference (IV, Random, 95% CI) | ‐4.86 [‐10.41, 0.69] |
| 3.4 Long‐term (≥ 12 months) | 5 | 452 | Mean Difference (IV, Random, 95% CI) | ‐6.94 [‐13.69, ‐0.19] |
| 4 Back pain | 6 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 Immediate (≤ 2 weeks) | 1 | 158 | Mean Difference (IV, Random, 95% CI) | ‐10.80 [‐21.64, 0.04] |
| 4.2 Short‐term (> 2 weeks but ≤ 3 months) | 6 | 726 | Mean Difference (IV, Random, 95% CI) | ‐2.01 [‐6.36, 2.34] |
| 4.3 Intermediate‐term (> 3 months but < 12 months) | 1 | 158 | Mean Difference (IV, Random, 95% CI) | 2.5 [‐8.54, 13.54] |
| 4.4 Long‐term (≥ 12 months) | 3 | 453 | Mean Difference (IV, Random, 95% CI) | 1.64 [‐4.39, 7.67] |
| 5 Pain relief | 9 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 Short‐term (> 2 weeks but ≤ 3 months) | 9 | 919 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [1.02, 1.35] |
| 5.2 Intermediate‐term (> 3 months but < 12 months) | 4 | 429 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.96, 1.38] |
| 5.3 Long‐term (≥ 12 months) | 5 | 657 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.95, 1.34] |
| 6 Disability reduction | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 Short‐term (> 2 weeks but ≤ 3 months) | 7 | 986 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.86, 1.38] |
| 6.2 Intermediate‐term (> 3 months but < 12 months) | 4 | 521 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.89, 1.34] |
| 6.3 Long‐term (≥ 12 months) | 4 | 453 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.82, 1.36] |
| 7 Minor adverse events | 8 | 877 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [0.91, 1.42] |
1.1. Analysis.

Comparison 1 Epidural corticosteroid injection versus placebo, Outcome 1 Leg pain intensity.
1.2. Analysis.
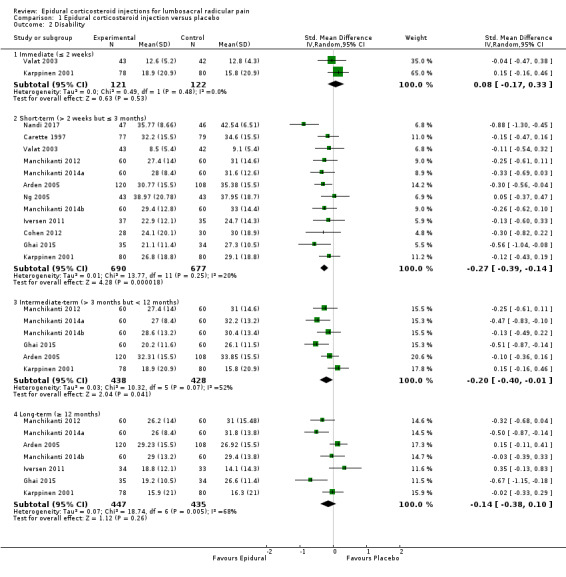
Comparison 1 Epidural corticosteroid injection versus placebo, Outcome 2 Disability.
1.3. Analysis.

Comparison 1 Epidural corticosteroid injection versus placebo, Outcome 3 Overall pain.
1.4. Analysis.

Comparison 1 Epidural corticosteroid injection versus placebo, Outcome 4 Back pain.
1.5. Analysis.

Comparison 1 Epidural corticosteroid injection versus placebo, Outcome 5 Pain relief.
1.6. Analysis.
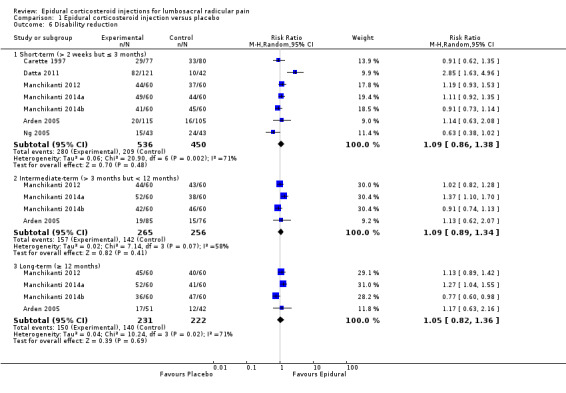
Comparison 1 Epidural corticosteroid injection versus placebo, Outcome 6 Disability reduction.
1.7. Analysis.

Comparison 1 Epidural corticosteroid injection versus placebo, Outcome 7 Minor adverse events.
Comparison 2. Epidural corticosteroid injections versus placebo ‐ subgroup and sensitivity analyses.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Leg pain short‐term ‐ epidural approaches | 8 | 949 | Mean Difference (IV, Random, 95% CI) | ‐4.93 [‐8.77, ‐1.09] |
| 1.1 Caudal | 1 | 72 | Mean Difference (IV, Random, 95% CI) | ‐5.30 [‐16.68, 6.08] |
| 1.2 Interlaminar | 2 | 384 | Mean Difference (IV, Random, 95% CI) | ‐1.45 [‐8.09, 5.20] |
| 1.3 Transforaminal | 5 | 493 | Mean Difference (IV, Random, 95% CI) | ‐6.97 [‐12.14, ‐1.79] |
| 2 Leg pain short‐term ‐ type of placebo | 8 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Epidural Anesthesic | 4 | 342 | Mean Difference (IV, Random, 95% CI) | ‐7.04 [‐17.05, 2.98] |
| 2.2 Epidural Saline | 5 | 511 | Mean Difference (IV, Random, 95% CI) | ‐7.63 [‐13.10, ‐2.16] |
| 2.3 Interspinous | 2 | 284 | Mean Difference (IV, Random, 95% CI) | ‐7.46 [‐24.84, 9.92] |
| 3 Leg pain short‐term ‐ imaging guidance | 8 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Fluoroscopic | 2 | 189 | Mean Difference (IV, Random, 95% CI) | ‐9.50 [‐17.22, ‐1.78] |
| 3.2 No guidance | 6 | 760 | Mean Difference (IV, Random, 95% CI) | ‐3.43 [‐7.86, 1.01] |
| 4 Leg pain short‐term ‐ inclusion diagnosis criteria | 8 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 Clinical assessment | 3 | 460 | Mean Difference (IV, Random, 95% CI) | ‐3.23 [‐8.97, 2.51] |
| 4.2 Required concordant imaging evidence | 5 | 489 | Mean Difference (IV, Random, 95% CI) | ‐6.31 [‐11.49, ‐1.14] |
| 5 Leg pain short‐term ‐ risk of bias | 8 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1 Studies judged as having low risk of bias | 4 | 376 | Mean Difference (IV, Random, 95% CI) | ‐5.01 [‐10.96, 0.94] |
| 5.2 Studies judged as having high risk of bias | 4 | 573 | Mean Difference (IV, Random, 95% CI) | ‐4.99 [‐10.32, 0.33] |
| 6 Leg pain short‐term ‐ concealment | 8 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6.1 Allocation concealment | 6 | 760 | Mean Difference (IV, Random, 95% CI) | ‐3.43 [‐7.86, 1.01] |
| 6.2 No allocation concealment | 2 | 189 | Mean Difference (IV, Random, 95% CI) | ‐9.50 [‐17.22, ‐1.78] |
| 7 Leg pain short‐term ‐ intention‐to‐treat analysis | 8 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 7.1 Intention‐to‐treat analysis | 5 | 600 | Mean Difference (IV, Random, 95% CI) | ‐2.74 [‐7.58, 2.09] |
| 7.2 No intention‐to‐treat analysis | 3 | 349 | Mean Difference (IV, Random, 95% CI) | ‐8.68 [‐15.01, ‐2.35] |
| 8 Leg pain short‐term ‐ blinding therapist | 8 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 8.1 Blinding care provider | 4 | 428 | Mean Difference (IV, Random, 95% CI) | ‐5.84 [‐11.41, ‐0.26] |
| 8.2 No blinding care provider | 4 | 521 | Mean Difference (IV, Random, 95% CI) | ‐4.11 [‐9.41, 1.20] |
| 9 Leg pain short‐term ‐ blinding participant | 8 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 9.1 Blinding participants | 7 | 721 | Mean Difference (IV, Random, 95% CI) | ‐6.27 [‐10.60, ‐1.94] |
| 9.2 No blinding participants | 1 | 228 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐8.32, 8.32] |
2.1. Analysis.

Comparison 2 Epidural corticosteroid injections versus placebo ‐ subgroup and sensitivity analyses, Outcome 1 Leg pain short‐term ‐ epidural approaches.
2.2. Analysis.

Comparison 2 Epidural corticosteroid injections versus placebo ‐ subgroup and sensitivity analyses, Outcome 2 Leg pain short‐term ‐ type of placebo.
2.3. Analysis.

Comparison 2 Epidural corticosteroid injections versus placebo ‐ subgroup and sensitivity analyses, Outcome 3 Leg pain short‐term ‐ imaging guidance.
2.4. Analysis.

Comparison 2 Epidural corticosteroid injections versus placebo ‐ subgroup and sensitivity analyses, Outcome 4 Leg pain short‐term ‐ inclusion diagnosis criteria.
2.5. Analysis.

Comparison 2 Epidural corticosteroid injections versus placebo ‐ subgroup and sensitivity analyses, Outcome 5 Leg pain short‐term ‐ risk of bias.
2.6. Analysis.

Comparison 2 Epidural corticosteroid injections versus placebo ‐ subgroup and sensitivity analyses, Outcome 6 Leg pain short‐term ‐ concealment.
2.7. Analysis.

Comparison 2 Epidural corticosteroid injections versus placebo ‐ subgroup and sensitivity analyses, Outcome 7 Leg pain short‐term ‐ intention‐to‐treat analysis.
2.8. Analysis.

Comparison 2 Epidural corticosteroid injections versus placebo ‐ subgroup and sensitivity analyses, Outcome 8 Leg pain short‐term ‐ blinding therapist.
2.9. Analysis.

Comparison 2 Epidural corticosteroid injections versus placebo ‐ subgroup and sensitivity analyses, Outcome 9 Leg pain short‐term ‐ blinding participant.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Arden 2005.
| Methods | Placebo‐controlled trial Source: orthopaedic, rheumatology, and pain clinics at the participating hospitals. Country: UK |
|
| Participants | 228 patients randomised (Group 1 = 120, Group 2 = 108) Diagnosis: clinical assessment Mean age (SD): Group 1 = 43.0 years (12.0), Group 2 = 44.0 years (12.0) Duration of symptoms: mixed (4 weeks to 18 months) |
|
| Interventions | Group 1: epidural injection of triamcinolone acetonide (80 mg) plus bupivacaine (0.25%, 10 mL) Group 2: interspinous injection of saline (2 mL) | |
| Outcomes |
Pain intensity: leg pain and back pain (VAS) Disability: ODI Disability improvement: 75% of reduction in ODI from baseline Adverse events: proportion of participants experiencing adverse events Follow‐ups: 3, 6, 12, and 52 weeks |
|
| Notes |
Epidural approach: interlaminar without the use of imaging guidance. Participants received up to 3 injections at 0, 3, and 6 weeks, but injections at 3 and 6 weeks were omitted if ODI had improved by more than 75%. Anaesthetist's experience: "Anaesthetists experienced in the procedure, who were not involved in the patients’ further assessments, performed all injections" Co‐interventions: additional analgesics and NSAIDs allowed. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were randomized, using sealed envelopes, by random number generation, balanced after every 10 assignments and stratified by centre and by duration of symptoms". |
| Allocation concealment (selection bias) | Low risk | "Patients were randomized, using sealed envelopes, by random number generation, balanced after every 10 assignments and stratified by centre and by duration of symptoms". |
| Blinding of participants (performance bias) | High risk | The procedures are clearly different which confirms that the participants were not blinded. |
| Blinding of personnel/care providers (performance bias) All outcomes | High risk | The procedures are different which confirms that the care providers were not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Given that the participants were not blinded to the group allocation and the outcomes are self‐reported measures, we judged that the outcome assessors were not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Short‐term follow‐up = 10/220 (4.5%), intermediate follow‐up (13%) = 21/161 and long‐term follow‐up = 3/93 (3%). Loss to follow‐up at short‐term and long‐term follow‐up were below the proposed threshold. |
| Intention‐to‐treat analysis (attrition bias) | Low risk | "All analyses were performed using an intention‐to‐treat basis." |
| Selective reporting (reporting bias) | High risk | Mood was reported in the protocol, but not in the publication. |
| Group similarity at baseline (selection bias) | Low risk | "The ESI and placebo groups were well matched for baseline clinical characteristics." |
| Co‐interventions (performance bias) | Low risk | "All patients had received physiotherapy and analgesics for this episode of sciatica." |
| Compliance (performance bias) | Low risk | Compliance in both groups 100%. |
| Timing of outcome assessment (detection bias) | Low risk | "All important outcome assessments for both groups were measured at the same time". |
| Other bias | Low risk | Funding (non‐profit or government sources) and no conflict of interest were reported. |
Bush 1991.
| Methods | Placebo‐controlled trial Source: hospital rheumatology department Country: UK |
|
| Participants | 23 patients randomised (Group 1 = 12, Group 2 = 11); Diagnosis: clinical assessment Mean age (SD): Group 1 = 38.2 years (13.4), Group 2 = 37.3 years (8.0); Duration of symptoms: acute (< 1 month) |
|
| Interventions | Group 1: epidural injection of 25 mL containing triamcinolone acetonide (80 mg) plus procaine hydrochloride (0.5%) plus saline Group 2: epidural injection of saline (25 mL) | |
| Outcomes |
Pain intensity: overall pain (VAS); Adverse events: proportion of patients experiencing adverse events Follow‐ups: 4 and 52 weeks |
|
| Notes |
Epidural approach: caudal without the use of imaging guidance. Patients received 2 injections: first at admission to the trial and a second after 2 weeks. Anaesthetist's experience: not specified Co‐interventions: additional analgesics allowed. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "… the patients were randomised to receive either active or placebo treatment." |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned. |
| Blinding of participants (performance bias) | Low risk | Patients were blinded to the interventions (placebo‐controlled trial using epidural approach). |
| Blinding of personnel/care providers (performance bias) All outcomes | High risk | The procedures are clearly different which confirm that the care providers were not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Self‐report outcomes with patients blinded to the treatment allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 23 out of 28 (82%) completed the short‐term and long‐term follow‐up. Loss to follow‐up at short‐term and long‐term follow‐up were below the proposed threshold. |
| Intention‐to‐treat analysis (attrition bias) | Unclear risk | Not mentioned. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the trial was not registered by the authors. |
| Group similarity at baseline (selection bias) | High risk | "Statistical difference between the two groups in SLR ability". |
| Co‐interventions (performance bias) | Low risk | "Additional measures in the form of bed rest analgesics corsets and manipulation were allowed, only analgesics were permitted in the first four weeks." |
| Compliance (performance bias) | Low risk | Compliance in both groups 100%. |
| Timing of outcome assessment (detection bias) | Low risk | "Assessments were made at baseline 4 weeks and 1 year". |
| Other bias | Unclear risk | Funding and conflict of interest were not reported. |
Carette 1997.
| Methods | Placebo‐controlled trial Source: university hospital Country: Canada |
|
| Participants |
Sample size: 158 patients randomised (Group 1 = 78, Group 2 = 80); Diagnosis: clinical assessment with computed tomographic (CT) evidence of a herniated nucleus pulposus at a level corresponding to the symptoms and clinical findings. Mean age (SD): Group 1 = 39.0 years (9.3), Group 2 = 40.6 years (11.3); Duration of symptoms: mixed (> 4 weeks to < 1 year) |
|
| Interventions | Group 1: epidural injection of methylprednisolone acetate (80 mg, 2 mL) plus isotonic saline (8 mL) Group 2: epidural injection of saline (1 mL) | |
| Outcomes |
Pain intensity: leg pain (VAS) Disability: ODI Disability Improvement: ODI score less than 20 Adverse events: Proportion of patients experiencing adverse events Follow‐ups: 3 and 6 weeks, and 3 months |
|
| Notes |
Epidural approach: interlaminar without the use of imaging guidance. The injections were repeated after 3 and 6 weeks in the patients who continued to have ODI scores less than 20. Anaesthetist's experience: not specified Co‐interventions: acetaminophen tablets (325 mg) were given to all patients. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The assignment scheme was generated from a table of random numbers. Random assignments to the treatment groups were stratified according to study center and balanced after every four to six assignments." |
| Allocation concealment (selection bias) | Low risk | "The opaque pre‐numbered envelopes containing the assignments were kept by the hospital pharmacist." |
| Blinding of participants (performance bias) | Low risk | Patients were blinded to the interventions (placebo‐controlled trial using epidural approach). |
| Blinding of personnel/care providers (performance bias) All outcomes | High risk | The procedures are different which confirm that the care providers were not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Self‐report outcomes with patients blinded to the treatment allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 156/158 (98%). Loss to follow‐up were below the proposed threshold. |
| Intention‐to‐treat analysis (attrition bias) | Low risk | "The primary analysis was based on an intention‐to‐treat principle." |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the trial was not registered by the authors. |
| Group similarity at baseline (selection bias) | High risk | "The base‐line characteristics were similar in the two groups (Table 1), except that more of the patients were men, more were living with a partner, and the finger‐to‐floor distance was greater in the methylprednisolone group than in the placebo group." |
| Co‐interventions (performance bias) | Low risk | "In addition to the study treatment, patients were given a supply of acetaminophen tablets (325 mg) and a form on which to record each tablet taken." |
| Compliance (performance bias) | Low risk | Compliance in both groups 100%. |
| Timing of outcome assessment (detection bias) | Low risk | "The patients were reevaluated three weeks, six weeks, and three months after randomisation." |
| Other bias | Unclear risk | Funding and conflict of interest were not reported. |
Cohen 2012.
| Methods | Placebo‐controlled trial Source: 4 large military medical centres and 2 civilian hospitals Country: USA |
|
| Participants |
Sample size: 84 patients randomised (Group 1 = 28, Group 2 = 30, Group 3 = 26); Diagnosis: clinical assessment with evidence on magnetic resonance imaging of a pathologic disc condition (e.g. herniated disc or annular tear) Mean age (SD): Group 1 = 41.5 years (12.7), Group 2 = 42.3 years (10.7), Group 3 = 43.2 years (8.9); Duration of symptoms: mixed (> 4 weeks to < 6 months) |
|
| Interventions | Group 1: epidural injections of methylprednisolone (60 mg, 1.5 mL) plus saline (0.5 mL) Group 2: epidural injections of saline (2 mL) Group 3: epidural injections of etanercept (4 mg) in 2 mL of sterile water | |
| Outcomes |
Pain intensity: leg and back pain (NRS) Disability: ODI Pain relief: more than 50% of reduction in leg pain plus a positive global perceived effect (GPE) obviating the need for further interventions. Adverse events: proportion of patients experiencing adverse events Follow‐up: 1 month |
|
| Notes |
Epidural approach: transforaminal under fluoroscopic guidance. Anaesthetist's experience: "Each procedure was performed by a board‐certified pain medicine physician or at teaching hospitals (for example, Walter Reed Army Medical Center) by either the attending physician or a pain management fellow supervised by the attending physician." Co‐interventions: additional analgesics allowed. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The main research nurse performed randomisation schemes in groups of 6 through computer‐generated randomisation tables, stratified by study site." |
| Allocation concealment (selection bias) | Low risk | "An investigator‐physician enrolled the participants, and a research assistant divulged treatment allocation." |
| Blinding of participants (performance bias) | Low risk | Patients were blinded to the interventions (placebo‐controlled trial using epidural approach). |
| Blinding of personnel/care providers (performance bias) All outcomes | Low risk | Patients, treating physicians, and evaluators were blinded to treatment assignments. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Self‐report outcomes with patients blinded to the treatment allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | None of the patients was lost in the follow‐up. Only the patients with successful outcomes were followed in the follow‐ups of 3 and 6 months. However, this was a per‐protocol analysis. |
| Intention‐to‐treat analysis (attrition bias) | Low risk | "Data from all study participants were analysed for intention to treat." |
| Selective reporting (reporting bias) | Low risk | (ClinicalTrials.gov registration number: NCT00733096). Any discrepancies were identified between protocol and publication. |
| Group similarity at baseline (selection bias) | Low risk | There were no statistically significant baseline differences among treatment groups. |
| Co‐interventions (performance bias) | Low risk | "Participants received written instructions from the treating physician about how to increase or decrease analgesic medications based on their response to therapy. For patients with debilitating pain who required interval rescue analgesics, breakthrough opioids (if previously prescribed) could be temporarily increased, or a nonsteroidal antiinflammatory drug or tramadol was prescribed." |
| Compliance (performance bias) | Low risk | Compliance in both groups 100%. |
| Timing of outcome assessment (detection bias) | Low risk | "1 month and subsequent follow‐up visits took place 3 and 6 months after the final injection in patients who received a positive categorical outcome at the preceding visit." |
| Other bias | Low risk | Funding (research institutes, non‐profit or government sources) and no conflict of interest was reported. |
Cuckler 1985.
| Methods | Placebo‐controlled trial Source: private practices; Country: USA |
|
| Participants | 36 patients randomised (subgroup) (Group 1 = 22, Group 2 = 14); Diagnosis: clinical assessment Mean age (SD): Group 1 = 48.5 years (1.3), Group 2 = 49.5 years (2.8); Duration of symptoms: mixed (no restriction) |
|
| Interventions | Group 1: epidural injection of methylprednisolone (80 mg) plus procaine (5 mL, 1%) plus sterile water (2 mL) Group 2: epidural injection of procaine (5 mL, 1%) plus saline (2 mL) | |
| Outcomes | At least 75% of subjective improvement; Follow‐ups: 24 hours, 13 to 30 months |
|
| Notes |
Epidural approach: interlaminar without the use of imaging guidance. Second injection was allowed if patients showed 50% improvement 24 hours after the first injection. Anaesthetist's experience: "Ninety per cent of the epidural injections were performed by a single anaesthesiologist." Co‐intervention: not specified |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Either two millilitres of sterile water containing eighty milligrams of methylprednisolone acetate combined with five millilitres of I per cent procaine or two millilitres of saline combined with five millilitres of I per cent procaine was injected on a randomized basis." |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned. |
| Blinding of participants (performance bias) | Low risk | Patients were blinded to the interventions (placebo‐controlled trial using epidural approach). |
| Blinding of personnel/care providers (performance bias) All outcomes | Low risk | "Neither the treating physician nor the patient was informed of the contents of the initial injection until July 1981." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Self‐report outcomes with patients blinded to the treatment allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up were below the proposed threshold. |
| Intention‐to‐treat analysis (attrition bias) | Unclear risk | Not mentioned. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the trial was not registered by the authors. |
| Group similarity at baseline (selection bias) | Low risk | The authors reported significant differences on duration of symptoms based on Table 1, but it seems similar for acute herniated nucleus pulposus. |
| Co‐interventions (performance bias) | Low risk | "All patients were discharged twenty‐four hours after injection with instructions to continue activities as their symptoms permitted." |
| Compliance (performance bias) | Low risk | Compliance in both groups 100%. |
| Timing of outcome assessment (detection bias) | Low risk | "The results twenty‐four hours after the injection were evaluated independently by both the attending physician and a resident physician who asked each patient to quantity the percentage improvement in symptoms." |
| Other bias | Unclear risk | Funding and conflict of interest were not reported. |
Datta 2011.
| Methods | Placebo‐controlled trial Source: tertiary care service hospital Country: not specified |
|
| Participants | 207 patients randomised (Group 1 = 55, Group 2 = 50, Group 3 = 52, Group 4 = 50) Diagnosis: Clinical assessment with evidence of a herniated nucleus pulposus at a level corresponding of symptoms and clinical findings in the computed tomographic (CT) Mean age (range): Group 1 = 43.0 years (34 to 68 years), Group 2 = 40.0 years (38 to 58 years), Group 3 = 39.0 years (28 to 63 years), Group 4 = 42.0 years (27 to 70 years) Duration of symptoms: mixed (> 4 weeks to < 1 year) |
|
| Interventions |
Group 1: epidural injection of 80 mg methylprednisolone plus bupivacaine (10 ml to 15 ml, 0.125%).
Group 2: epidural injection of 80 mg triamcinolone plus bupivacaine (10 ml to 15 ml, 0.125%). Group 3: epidural injection of 15 mg dexamethasone plus bupivacaine (10 ml to 15 ml, 0.125%). Group 4: epidural injection of bupivacaine (10 ml to 15 ml, 0.125%). |
|
| Outcomes |
Pain intensity: overall pain (VAS) Disability: RMDQ Disability improvement: at least 50% of improvement in ODQ Adverse events: proportion of patients experiencing adverse events Follow‐ups: 3, 6, 12 weeks |
|
| Notes |
Epidural approach: caudal without the use of imaging guidance. Anaesthetist's experience: the anaesthesiologists making the assessments were not the same as those giving the injections. Co‐interventions: Diclofenac was allowed. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients fulfilling criteria were randomly allocated to the four groups using block randomisation sequence generated by the computer and instituted by the study nurse using sealed envelopes." |
| Allocation concealment (selection bias) | Low risk | "... instituted by the study nurse using sealed envelopes." |
| Blinding of participants (performance bias) | Low risk | Patients were blinded to the interventions (placebo‐controlled trial using epidural approach). |
| Blinding of personnel/care providers (performance bias) All outcomes | High risk | The procedures are different which confirms that the care providers were not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Self‐report outcomes with patients blinded to the treatment allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropout rate: 14/207 = 7%. Loss to follow‐up was below the proposed threshold. |
| Intention‐to‐treat analysis (attrition bias) | Low risk | "Data was analysed according to the intention‐to‐treat principle." |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the trial was not registered by the authors. |
| Group similarity at baseline (selection bias) | Low risk | No significant differences were identified at baseline assessment based on Table 3, 4 and 5. |
| Co‐interventions (performance bias) | Low risk | "Patients in all four groups were permitted to take Diclofenac (50 mg tablets, up to four times daily) at any time during the study and to change the frequency as necessary." |
| Compliance (performance bias) | Low risk | Compliance in both groups 100%. |
| Timing of outcome assessment (detection bias) | Low risk | "Patients were discharged after an hour of observation following ESI. Re‐evaluation was done after 1 week, 3, 6 and 12 weeks after the first injection." |
| Other bias | Low risk | Funding was not reported, but the authors reported no conflict of interest. |
Dilke 1973.
| Methods | Placebo‐controlled trial Source: hospital Country: UK |
|
| Participants | 99 patients randomised (Group 1 = 51, Group 2 = 48); Diagnosis: clinical assessment Mean age (range): Group 1 = 38.7 years (18 to 75 years), Group 2 = 42.3 years (18 to 66 years); Duration of symptoms: mixed (> 1 weeks to < 2 years) |
|
| Interventions | Group 1: epidural injection of methylprednisolone (80 mg) plus saline (10 mL) Group 2: interspinous injection of saline (1 mL) | |
| Outcomes | Pain relief considering the number and frequency of analgesics required, and a classification using a categorical scale (i.e. severe, not severe, and none) Follow‐up: 3 months |
|
| Notes |
Epidural approach: interlaminar without the use of imaging guidance. Anaesthetist's experience: all injections were performed by the same person. Co‐interventions: additional analgesics and skeletal muscle relaxant allowed. After an initial period of bed rest, all patients received hydrotherapy, postural exercises, and spinal mobilizing exercises. Second injection was allowed if the first injection was not satisfactory. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Were allocated at random to "treated" and "control" groups." |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned. |
| Blinding of participants (performance bias) | High risk | The procedures are different which confirms that the participants were not blinded. |
| Blinding of personnel/care providers (performance bias) All outcomes | High risk | The procedures are different which confirms that the care providers were not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Given that the participants were not blinded to the group allocation and the outcomes are self‐reported measures, we judged that the outcome assessors were not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not mentioned. |
| Intention‐to‐treat analysis (attrition bias) | Unclear risk | Not mentioned. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the trial was not registered by the authors. |
| Group similarity at baseline (selection bias) | Low risk | The authors stated that impulse pain, to be an adverse prognostic factor in sciatica, was more common in the treated group; however there were no large differences between groups. |
| Co‐interventions (performance bias) | Low risk | "Analgesics were offered four times a day, mefenamic acid being used routinely." |
| Compliance (performance bias) | Low risk | Compliance in both groups 100%. |
| Timing of outcome assessment (detection bias) | Low risk | "Assessment of progress was made by consideration of the following points." |
| Other bias | Unclear risk | Funding and conflict of interest were not reported. |
Ghahreman 2010.
| Methods | Placebo‐controlled trial Source: hospital Country: Australia |
|
| Participants | 150 patients randomised (Group 1 = 28, Group 2 = 27, Group 3 = 37, Group 4 = 28, Group 5 = 30); Diagnosis: clinical assessment with evidence of disc herniation computerised tomography (CT) or magnetic resonance imaging (MRI) Median age (IQR): Group 1 = 49 years (39 to 61 years), Group 2 = 43 years (35 to 66 years), Group 3 = 44 years (33 to 54 years), Group 4 = 49 years (38 to 62 years), Group 5 = 46 years (37 to 64 years); Duration of symptoms: mixed (no restriction) |
|
| Interventions | Group 1: epidural injection of triamcinolone (40 mg/mL, 1.75 mL) plus bupivacaine (0.75 mL, 0.5%) Group 2: epidural injection of bupivacaine (2 mL, 0.5%) Group 3: epidural injection of normal saline (2 mL) Group 4: intramuscular injection of steroids (1.75 mL, 40 mg/mL) Group 5: intramuscular injection of saline (2 mL) | |
| Outcomes |
Pain intensity: leg pain (NRS); Pain relief: at least 50% of pain relief. Follow‐up: 1 month |
|
| Notes |
Epidural approach: transforaminal using fluoroscopy imaging. Additional injections (maximum of 3 injections) were performed at discretion of the patient. Anaesthetist's experience: "At the primary site, various operators performed the injections, but the majority of patients were treated by either one of two operators. At the secondary site, all patients were treated by the same operator." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The randomisation schedule was based on a series of random numbers, allocated sequentially to patients as they enrolled." |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned. |
| Blinding of participants (performance bias) | Low risk | Patients were blinded to the interventions (placebo‐controlled trial using epidural approach in both groups). |
| Blinding of personnel/care providers (performance bias) All outcomes | High risk | The procedures are different which confirms that the care providers were not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Self‐report outcomes with patients blinded to the treatment allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 0%. "Prior to registering their response to treatment, no patient was lost to followup. In accordance with the ethics provisions, patients were not required to participate in follow‐up once they had registered failure to respond to either their allocated treatment or to rescue treatment. All patients who had a successful outcome, however, completed follow‐up until their relief lapsed. This analysis was defined per‐protocol." Loss to follow‐up were below the proposed threshold. |
| Intention‐to‐treat analysis (attrition bias) | Unclear risk | Not mentioned. |
| Selective reporting (reporting bias) | Low risk | (ACTRN 12608000401358). Any discrepancies were identified between protocol and publication. |
| Group similarity at baseline (selection bias) | Low risk | No significant differences between groups were identified based on Table 1 and 2. |
| Co‐interventions (performance bias) | Low risk | "Once they had registered their response and had completed the outcome instruments, they were entitled to pursue rescue therapy. According to the preference of the patient, this could be analgesics, surgery, or open‐label transforaminal injection of steroids." |
| Compliance (performance bias) | Low risk | Compliance in both groups 100%. |
| Timing of outcome assessment (detection bias) | Low risk | "Patients with continuing relief were assessed at 3, 6, and 12 months, or until relief of pain ceased." |
| Other bias | Unclear risk | Funding and conflict of interest were not reported. |
Ghai 2015.
| Methods | Placebo‐controlled trial Source: public tertiary care hospital Country: India |
|
| Participants | 69 patients randomised (Group 1 = 35, Group 2 = 34); Diagnosis: clinical assessment with evidence of disc herniation in the magnetic resonance imaging (MRI) Mean age (SD): Group 1 = 45.9 years (13.3), Group 2 = 44.7 years (10.5); Duration of symptoms: chronic (> 12 weeks) |
|
| Interventions | Group 1: epidural injection of methylprednisolone acetate (80 mg, 2 mL) plus lidocaine (6 mL, 0.5%) Group 2: epidural injections of lidocaine (8 mL, 0.5%) | |
| Outcomes |
Pain intensity: overall pain (NRS) Disability: ODI Pain relief: at least 50% of reduction in NRS Adverse events: proportion of patients experiencing adverse events Follow‐ups: 3, 6, and 12 months |
|
| Notes |
Epidural approach: interlaminar under fluoroscopic guidance. Additional injections were done when increased levels of pain were reported with deteriorating relief lower than 50%. Anaesthetist's experience: not specified Co‐interventions: all patients received conservative management including analgesics (adjuvant; pregabalin, amitriptyline, opioid, or non‐opioid) and/or exercise programme. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were randomized (block of six, software: Random‐Randomizer)". |
| Allocation concealment (selection bias) | Low risk | "Randomization codes were placed in sealed opaque envelopes and opened at the time of injection by an independent anaesthesiologist not involved in the study." |
| Blinding of participants (performance bias) | Low risk | Patients were blinded to the interventions (placebo‐controlled trial using epidural approach in both groups). |
| Blinding of personnel/care providers (performance bias) All outcomes | Low risk | "Patients, investigators including outcome assessor, and care providers were unaware of randomisation and group allocation." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Self‐report outcomes with patients blinded to the treatment allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 8/69 = 11% at short‐term; 13/69 = 19% at intermediate and 13/69 = 19% at long‐term. Loss to follow‐up was below the proposed threshold. |
| Intention‐to‐treat analysis (attrition bias) | Low risk | "The primary and secondary effectiveness analyses were performed on the intention‐to‐treat (ITT) population, defined as patients who received at least one injection and have one post‐procedure assessment. We analysed all patients according to the group to which they were allocated." |
| Selective reporting (reporting bias) | Low risk | (CTRI/2014/04/004572). Any discrepancies were identified between protocol and publication. |
| Group similarity at baseline (selection bias) | Low risk | Both groups were similar with respect to pre‐procedure demographic and clinical characteristics (Table 1). |
| Co‐interventions (performance bias) | Low risk | "All patients received conservative management including analgesics (adjuvant; pregabalin, mitriptyline, opioid, or non‐opioid) and/or exercise program during the study. Dose titration of analgesics was done as per patient requirement. Job attendance continued. All patients were encouraged to engage in physical activities. No additional occupation/physical therapy or any other interventions were offered beyond the protocol." |
| Compliance (performance bias) | Low risk | Compliance in both groups 100%. |
| Timing of outcome assessment (detection bias) | Low risk | "A blinded investigator followed the patients at 2 weeks, one, 2, 3, 6, 9, and 12 months post‐intervention." |
| Other bias | Low risk | Funding was not reported, but the authors reported no conflict of interest. |
Helliwell 1985.
| Methods | Placebo‐controlled trial Source: not specified Country: not specified |
|
| Participants | 39 patients randomised (Group 1 = 20, Group 2 = 19); Diagnosis: clinical assessment Mean age (range): Group 1 = 44.6 years (20 to 69 years), Group 2 = 47.4 years (23 to 68 years); Duration: subacute and chronic (> 2 months) |
|
| Interventions | Group 1: epidural injection of methylprednisolone (80 mg) plus saline (10 mL) Group 2: interspinous injection of saline (5 mL) | |
| Outcomes | Pain intensity: overall pain (VAS); Follow‐ups: 1 and 3 month | |
| Notes |
Epidural approach: interlaminar without imaging guidance. Anaesthetist's experience: not specified Co‐interventions: additional analgesics allowed. Patients already wearing lumbosacral supports were allowed to continue using if they wished. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "All patients had radiographs of the lumbar spine before being allocated at random to an EDI or a placebo." |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned. |
| Blinding of participants (performance bias) | High risk | The procedures are different which confirms that the participants were not blinded. |
| Blinding of personnel/care providers (performance bias) All outcomes | High risk | The procedures are different which confirms that the care providers were not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Given that the participants were not blinded to the group allocation and the outcomes are self‐reported measures, we judged that the outcome assessors were not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not mentioned. |
| Intention‐to‐treat analysis (attrition bias) | Unclear risk | Not mentioned. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the trial was not registered by the authors. |
| Group similarity at baseline (selection bias) | Unclear risk | Not reported data for pain and disability at baseline based on Table 1. |
| Co‐interventions (performance bias) | Low risk | "No other form of treatment was introduced in the 3 month follow‐up period although patients already wearing lumbo‐sacral supports were allowed to continue using these if they wished. Patients were also given the choice of reducing their analgesic consumption and returning to work or other full‐time activities." |
| Compliance (performance bias) | Low risk | Compliance in both groups 100%. |
| Timing of outcome assessment (detection bias) | Low risk | "Assessments were made prior to treatment and at 1 and 3 months." |
| Other bias | Unclear risk | Funding and conflict of interest were not reported. |
Iversen 2011.
| Methods | Placebo‐controlled trial Source: 2 university hospitals and 3 general hospitals. Country: Norway |
|
| Participants | 116 patients randomised (Group 1 = 37, Group 2 = 39, Group 3 = 40); Diagnosis: clinical assessment. The authors performed magnetic resonance imaging or computed tomography in all included patients, however inclusion in the study was not dependent on the results Mean age (SD): Group 1 = 40.1 years (10.0), Group 2 = 42.8 years (11.6), Group 3 = 42.8 years (9.2); Duration of symptoms: chronic (> 12 weeks) |
|
| Interventions | Group 1: epidural injections of triamcinolone (40 mg) plus saline (29 mL, 0.9%) Group 2: epidural injections of saline (30 mL, 0.9%) Group 3: subcutaneous sham injections of saline (2 mL, 0.9%) (superficial subcutaneous sham injections) | |
| Outcomes |
Pain intensity: leg and back pain (VAS) Disability: ODI Follow‐ups: 6, 12, and 52 weeks |
|
| Notes |
Epidural approach: caudal using ultrasound imaging. All patients received 2 injections with a 2‐week interval; the second injection was cancelled if spontaneous recovery had occurred between inclusion and the first intervention. Anaesthetist's experience: "An experienced anaesthesiologist gave the injections and followed a set template." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | " ... used a computer generated block scheme for randomisation, stratified by intervention hospital." |
| Allocation concealment (selection bias) | Low risk | "The centre was contacted by telephone on the day of intervention. The individuals undertaking the randomisation did not take any further part in the trial." |
| Blinding of participants (performance bias) | Low risk | Patients were blinded to the interventions (placebo‐controlled trial using epidural approach in both groups). |
| Blinding of personnel/care providers (performance bias) All outcomes | High risk | "The anaesthesiologist giving the injections was not blinded because inclusion of a subcutaneous sham group made this impossible." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Self‐report outcomes with patients blinded to the treatment allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 7/116 (6.0%) at short‐term and 17/116 (14.6%) at long‐term. Loss to follow‐up were below the proposed threshold. |
| Intention‐to‐treat analysis (attrition bias) | Low risk | "The analyses for all outcome measures used all available data on an intention to treat basis." |
| Selective reporting (reporting bias) | Low risk | ISRCTN No 12574253. Any discrepancies were identified between protocol and publication. |
| Group similarity at baseline (selection bias) | Low risk | We did not detect any significant differences between treatment groups. |
| Co‐interventions (performance bias) | Low risk | "Use of physiotherapy was recorded during follow‐up, but was not routinely offered to the patients." |
| Compliance (performance bias) | Low risk | Compliance in both groups 100%. |
| Timing of outcome assessment (detection bias) | Low risk | "A blinded physiotherapist and doctor followed up patients at 6, 12, and 52 weeks." |
| Other bias | Low risk | Funding (governmental sources) and no conflict of interest was reported |
Karppinen 2001.
| Methods | Placebo‐controlled trial Source: referred by general practitioners in catchment area of a university hospital; Country: Finland |
|
| Participants | 160 patients randomised (Group 1 = 80, Group 2 = 80); Diagnosis: clinical assessment Mean age (SD): Group 1 = 43.8 years (13.0), Group 2 = 43.7 years (13.0); Duration of symptoms: mixed (> 3 to < 28 weeks) |
|
| Interventions | Group 1: epidural injection of methylprednisolone (40 mg/mL) plus bupivacaine (5 mg/mL) Group 2: epidural injection of saline (0.9%) | |
| Outcomes |
Pain intensity: leg and back pain (VAS) Disability: ODI Adverse events: proportion of patients experiencing adverse events Follow‐ups: 2 and 4 weeks and 3, 6, and 12 months |
|
| Notes |
Epidural approach: transforaminal under fluoroscopic guidance (the volume of the injection was 2 mL for L4 and L5 blocks and 3 mL for S1 based on anatomical differences). Anaesthetist's experience: not specified Co‐interventions: all patients received “back school” instructions. Pain medication and physiotherapy were allowed for patients with persisting sciatic pain after injection. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The randomisation process was based on a published list of random permutations with a block size of 16." |
| Allocation concealment (selection bias) | Low risk | "A person uninvolved in the study placed the assignments in sealed envelopes with running numbers. The envelopes were used in the order provided." |
| Blinding of participants (performance bias) | Low risk | Patients were blinded to the interventions (placebo‐controlled trial using epidural approach in both groups). |
| Blinding of personnel/care providers (performance bias) All outcomes | Low risk | "The assignments were thus masked to the patient, the physicians, and the radiologist giving the injection." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Self‐report outcomes with patients blinded to the treatment allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2/160 (1.2%) at immediate, short‐term, intermediate and long‐term. Loss to follow‐up was below the proposed threshold. |
| Intention‐to‐treat analysis (attrition bias) | Unclear risk | Not mentioned. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the trial was not registered by the authors. |
| Group similarity at baseline (selection bias) | Low risk | No significant differences were identified based on Table 1. |
| Co‐interventions (performance bias) | Low risk | "Every patient was given back school instructions by the physiotherapist at the 2‐week follow‐up assessment." |
| Compliance (performance bias) | Low risk | Compliance in both groups 100%. |
| Timing of outcome assessment (detection bias) | Low risk | "At the follow‐up checks (2 weeks, 1 month, 3 months, 6 months, and 1 year after the intervention), the same questionnaires as those used at the baseline were completed." |
| Other bias | Low risk | Funding (non‐profit organisations) reported: "Conflict of interest category: 14." |
Klenerman 1984.
| Methods | Placebo‐controlled trial Source: day care unit; Country: UK |
|
| Participants | 51 patients randomised (Group 1 = 19, Group 2 = 16, Group 3 = 16); Diagnosis: clinical assessment Age: not specified; Duration of symptoms: mixed (< 6 months) |
|
| Interventions | Group 1: epidural injection of methylprednisolone (80 mg) plus saline (20 mL) Group 2: epidural injection of saline (20 mL) Group 3: epidural injection of bupivacaine (0.25%) plus saline (20 mL) | |
| Outcomes | Pain intensity: overall pain (VAS); Follow‐ups: 2 weeks, 2 months | |
| Notes |
Epidural approach: interlaminar without the use of imaging guidance. Anaesthetist's experience: "Injections were all given by the lumbar route by experienced anaesthetists who were meticulous in locating the epidural space" Co‐interventions: additional physiotherapy allowed for patients with severe symptoms at the first follow‐up point. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Treatments were allocated in groups containing one of each of the treatments which had been permuted into random order by a computer program." |
| Allocation concealment (selection bias) | Low risk | "The treatment followed for each patient was based on numbered envelopes containing the previously randomized variations of injection." |
| Blinding of participants (performance bias) | Low risk | Patients were blinded to the interventions (placebo‐controlled trial using epidural approach in both groups). |
| Blinding of personnel/care providers (performance bias) All outcomes | High risk | The procedures are different which confirms that the care providers were not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Self‐report outcomes with patients blinded to the treatment allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Follow‐up assessment for 1/74 = 1.3%. Loss to follow‐up was below the proposed threshold. |
| Intention‐to‐treat analysis (attrition bias) | Unclear risk | Not mentioned |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the trial was not registered by the authors |
| Group similarity at baseline (selection bias) | Unclear risk | Not mentioned. |
| Co‐interventions (performance bias) | Low risk | "The number of patients given this additional treatment did not show any statistically significant difference between the treatment groups." |
| Compliance (performance bias) | Low risk | Compliance in both groups 100%. |
| Timing of outcome assessment (detection bias) | Low risk | "The patients assessed by the same clinician where possible at two weeks and two months." |
| Other bias | Unclear risk | Funding and conflict of interest were not reported. |
Kraemer 1997.
| Methods | Placebo‐controlled trial Source: not specified Country: Germany |
|
| Participants | 133 patients randomised (Group 1 = 47, Group 2 = 40, Group 3 = 46); Diagnosis: clinical assessment Age: not specified Duration of symptoms: not specified |
|
| Interventions | Group 1: epidural injection of triamcinolone (10 mg) plus undisclosed volume of saline Group 2: epidural injection of triamcinolone (10 mg) plus undisclosed volume of saline Group 3: paravertebral local anaesthetic injection | |
| Outcomes | Proportion of patients classified as good, fair, poor, and surgery. Follow‐up: 3 months |
|
| Notes |
Epidural approach: interlaminar for G1 and transforaminal for G2. Anaesthetist's experience: "Our experience of this new epidural approach in over 600 cases over 3 years with good results and no severe complications." Co‐interventions: all patients received physiotherapy, “back school”, and a dynamic flexion orthosis. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "The first trial was a prospective randomized study to compare the new epidural perineural injection (47 patients) and the conventional epidural injection technique (40 patients)." |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned. |
| Blinding of participants (performance bias) | Low risk | Patients were blinded to the interventions (placebo‐controlled trial using epidural approach in both groups). |
| Blinding of personnel/care providers (performance bias) All outcomes | Low risk | "Study procedure and assessments for the second study were the same as for the first study: Neither patient nor doctor was aware of the injection content." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Self‐report outcomes with patients blinded to the treatment allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not mentioned. |
| Intention‐to‐treat analysis (attrition bias) | Unclear risk | Not mentioned. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the trial was not registered by the authors. |
| Group similarity at baseline (selection bias) | Unclear risk | "At baseline there was no statistically significant difference between the groups concerning age, sex, duration of symptoms or compression signs." However, the authors did not report baseline data for pain intensity and disability. |
| Co‐interventions (performance bias) | Unclear risk | "If patients did not sufficiently improve, they received epidural perineural injections containing triamcinolone." |
| Compliance (performance bias) | Low risk | Compliance in both groups 100%. |
| Timing of outcome assessment (detection bias) | Low risk | "Assessments, both subjective and objective, were made before treatment ‐ at baseline ‐ and again at 3 weeks and 3 months." |
| Other bias | Unclear risk | Funding and conflict of interest were not reported |
Manchikanti 2012.
| Methods | Placebo‐controlled trial Source: not specified Country: USA |
|
| Participants | 120 patients randomised (Group 1 = 60, Group 2 = 60) Diagnosis: clinical assessment Mean age (SD): Group 1 = 43.0 years (14.5), Group 2 = 48.7 years (14.1) Duration of symptoms: chronic (> 6 months) |
|
| Interventions | Group 1: epidural injection of brand name betamethasone (6 mg, 1 mL) or nonparticulate brand name betamethasone (6 mg, 1 mL) or methylprednisolone (40 mg, 1 mL) plus lignocaine (9 mL, 0.5%) plus saline (2 mL, 0.9%) Group 2: epidural injection of lignocaine (10 mL) 0.5% preservative‐free plus saline (2 mL, 0.9%) | |
| Outcomes |
Pain intensity: overall pain (NRS) Disability: ODI Pain relief: at least 50% of reduction in NRS Disability improvement: at least 50% of improvement in ODI Adverse events: Proportion of patients experiencing adverse events Follow‐ups: 3, 6, 12, 18 and 24 months |
|
| Notes |
Epidural approach: caudal under fluoroscopy guidance. Additional caudal epidurals were provided on the basis of the patient’s response. Anaesthetist's experience: "All caudal epidural procedures were performed by one physician in an ambulatory surgery setting" Co‐interventions: all patients continued previous exercise program, drug therapy, and work. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A computer generated random allocations sequence by simple randomisation was used for randomisation." |
| Allocation concealment (selection bias) | Unclear risk | "Based on randomisation, drugs were prepared by the operating room nurse assisting with the procedure." |
| Blinding of participants (performance bias) | Low risk | Patients were blinded to the interventions (placebo‐controlled trial using epidural approach in both groups). |
| Blinding of personnel/care providers (performance bias) All outcomes | Low risk | "Participants, those administering the interventions, and all others involved in patient care were blinded to the group assignments." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Self‐report outcomes with patients blinded to the treatment allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 21/120 = 17.5% loss to follow‐up was below the proposed threshold. |
| Intention‐to‐treat analysis (attrition bias) | Low risk | "An intent‐to‐treat analysis was performed." |
| Selective reporting (reporting bias) | Low risk | (NCT00370799). Any discrepancy was identified between protocol and publication suggesting selective reporting bias. |
| Group similarity at baseline (selection bias) | Low risk | We did not identify any large differences between groups with respect to the most prognostic factors. |
| Co‐interventions (performance bias) | Low risk | "There were no specific cointerventions or additional interventions. However, all patients continued previous exercise programs, drug therapy, and work." |
| Compliance (performance bias) | Low risk | Compliance in both groups 100%. |
| Timing of outcome assessment (detection bias) | Low risk | "The NRS pain scale (0‐10), the ODI disability scale on a 0 to 50 scale, employment status, and opioid intake in terms of morphine equivalents were assessed at 3, 6, 12, 18, and 24 months posttreatment." |
| Other bias | Low risk | No funding was received and no conflict of interest was reported |
Manchikanti 2014a.
| Methods | Placebo‐controlled trial Source: specialty referral centre (private practice setting) Country: USA |
|
| Participants | 120 patients randomised (Group 1 = 60, Group 2 = 60);
Mean age (SD): Group 1 = 40.6 years (13.4), Group 2 = 49 years (14.1); Duration of symptoms: chronic (> 6 months) |
|
| Interventions | Group 1: epidural injection of betamethasone (6 mg, 1 mL) plus lignocaine (5 mL, 0.5%) Group 2: epidural injections of lignocaine (6 mL) 0.5% preservative‐free | |
| Outcomes |
Pain intensity: overall pain (NRS) Disability: ODI Pain relief: at least 50% of reduction in NRS Disability improvement: at least 50% of improvement in ODI Adverse events: proportion of patients experiencing adverse events Follow‐ups: 3, 6, 12, 18 and 24 months |
|
| Notes |
Epidural approach: interlaminar under fluoroscopic guidance. Additional injections were done when increased levels of pain were reported with deteriorating relief lower than 50%. Anaesthetist's experience: "The procedures were performed by one physician (LM) using appropriate monitoring." Co‐interventions: additional opioid and non‐opioid analgesics, adjuvant analgesics, and therapeutic exercise programme allowed. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Simple randomisation was adopted with a computer‐generated random allocation sequence." |
| Allocation concealment (selection bias) | Unclear risk | "A study coordinator randomized the patients and an operating room nurse prepared the drugs appropriately." |
| Blinding of participants (performance bias) | Low risk | Patients were blinded to the interventions (placebo‐controlled trial using epidural approach in both groups). |
| Blinding of personnel/care providers (performance bias) All outcomes | Low risk | "All patients and all the medical personnel who administered the interventions were blinded. Injections used for both groups were clear and indistinguishable from each other." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Self‐report outcomes with patients blinded to the treatment allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Short‐term follow‐up = 7/70 (10%), intermediate follow‐up (8.5%) = 6/70 and long‐term follow‐up = 11/70 (15%). Loss to short‐term, intermediate and long‐term follow‐up were below the proposed threshold. |
| Intention‐to‐treat analysis (attrition bias) | Low risk | "The last follow‐up data or initial data were utilized in patients who dropped out of the study or for whom no other data were available for intent‐to‐treat analysis." |
| Selective reporting (reporting bias) | Low risk | (NCT00681447). Any discrepancy was identified between protocol and publication suggesting selective reporting bias. |
| Group similarity at baseline (selection bias) | High risk | We identified large differences with respect to gender and age between both groups. |
| Co‐interventions (performance bias) | Low risk | "All patients at their request continued drug therapy either with opioids or nonsteroidal antiinflammatory agents, generally at a lower level than initial doses." |
| Compliance (performance bias) | Low risk | Compliance in both groups: 100%. |
| Timing of outcome assessment (detection bias) | Low risk | "All patients were assessed with primary and secondary outcomes at pre‐defined intervals of 3, 6, 12, 18, and 24 months." |
| Other bias | Low risk | No funding was received and no conflict of interest was reported. |
Manchikanti 2014b.
| Methods | Placebo‐controlled trial Source: specialty referral centre (private practice setting) Country: USA |
|
| Participants | 120 patients randomised (Group 1 = 60, Group 2 = 60); Diagnosis: clinical assessment Mean age (SD): Group 1 = 42.6 years (11.2), Group 2 = 43.1 years (11.8); Duration of symptoms: chronic (> 6 months) |
|
| Interventions | Group 1: epidural injection of betamethasone either particulate or nonparticulate (3 mg) plus lidocaine (1%) preservative‐free Group 2: epidural injections of lidocaine (1.5 mL) 1% preservative‐free plus saline solution (0.5 ml) | |
| Outcomes |
Pain intensity: overall pain (NRS) Disability: ODI Pain relief: at least 50% of reduction in NRS Disability improvement: at least 50% of improvement in ODI Adverse events: proportion of patients experiencing adverse events Follow‐ups: 3, 6, 12, 18 and 24 months |
|
| Notes |
Epidural approach: transforaminal under fluoroscopic guidance. Anaesthetist's experience: "All procedures were performed by one physician with appropriate monitoring." Co‐interventions: additional drug therapy with opioids or nonsteroidal anti‐inflammatory drug and therapeutic exercise programme allowed. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A computer‐generated random allocation sequence randomized the patients." |
| Allocation concealment (selection bias) | Low risk | "In addition to the computer‐generated random allocation sequence with proper concealment ..." |
| Blinding of participants (performance bias) | Low risk | Patients were blinded to the interventions (placebo‐controlled trial using epidural approach in both groups). |
| Blinding of personnel/care providers (performance bias) All outcomes | Low risk | "Injections for both groups were clear and indistinguishable from each other (clear nonparticulate betamethasone was used) until September 2012. Because of reports of meningitis related to steroids from compounding pharmacies, commercial betamethasone was used after September 2012. During this period, after injecting local anaesthetic, the physician was provided either sodium chloride solution or betamethasone in opaque syringes." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Self‐report outcomes with patients blinded to the treatment allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Short‐term follow‐up = 21/120 (17%), intermediate follow‐up (12.5%) = 15/120 and long‐term follow‐up = 19/120 (16%). Loss to short‐term, intermediate and long‐term follow‐up were below the proposed threshold. |
| Intention‐to‐treat analysis (attrition bias) | Low risk | "An intention‐to‐treat analysis was performed wherever the data was missing or was unavailable." |
| Selective reporting (reporting bias) | High risk | The authors did not specify the dichotomous outcomes in the protocol, but reported in the publication (NCT 01052571). |
| Group similarity at baseline (selection bias) | Low risk | We did not identify any large differences between groups with respect to most prognostic factors. |
| Co‐interventions (performance bias) | Low risk | "Similar co‐interventions were provided for all patients, including a structured exercise program. Those employed continued working or returned to work when possible. All patients continued drug therapy with opioids or nonsteroidal anti‐inflammatory drugs, although generally, at a lower level than their initial doses." |
| Compliance (performance bias) | Low risk | Compliance in both groups: 100%. |
| Timing of outcome assessment (detection bias) | Low risk | "All patients were assessed for primary and secondary outcomes at predefined intervals of 3, 6, 12, 18, and 24 months." |
| Other bias | Low risk | No funding was received and no conflict of interest was reported. |
Mathews 1987.
| Methods | Placebo‐controlled trial Source: not specified Country: UK |
|
| Participants | 57 patients randomised (Group 1 = 23, Group 2 = 34); Diagnosis: clinical assessment Median age (range): Group 1 = 38 years (22–59 years), Group 2 = 41 years (18–58 years) Duration: acute and subacute (< 3 months) |
|
| Interventions | Group 1: epidural injection of methylprednisolone (80 mg, 2 mL) plus bupivacaine (0.125%, 20 mL) (up to 3 times as needed) Group 2: injection of lignocaine (2 mL) over sacral hiatus or into a tender spot | |
| Outcomes |
Pain relief: proportion of patients with no pain. Follow‐ups: 1, 3, and 12 months |
|
| Notes |
Epidural approach: caudal without the use of imaging guidance Anaesthetist's experience: not specified Co‐interventions: Additional analgesics, spinal corset, and education about posture and back care allowed. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Patients were allocated to the appropriate trial according to their symptoms and signs (Table I) and by a pre‐determined randomisation system to treatment (T) or control (C); groups within trials were stratified by age (under or over 45 years) and sex." |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned. |
| Blinding of participants (performance bias) | High risk | The procedures are different which confirms that the participants were not blinded. |
| Blinding of personnel/care providers (performance bias) All outcomes | High risk | The procedures are different which confirms that the care providers were not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Given that the participants were not blinded to the group allocation and the outcomes are self‐reported measures, we judged that the outcome assessors were not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 38/57 (67%) losses to follow‐up were greater than the proposed threshold. |
| Intention‐to‐treat analysis (attrition bias) | Unclear risk | Not mentioned. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the trial was not registered by the authors. |
| Group similarity at baseline (selection bias) | Unclear risk | Not reported data for pain and disability at baseline based on Table 3. |
| Co‐interventions (performance bias) | Low risk | "All patients were given a supply of paracetamol, 500 mg tablets with instructions to take two, 4‐hourly, as needed, and paracetamol‐dextropropoxyphene was available on request." |
| Compliance (performance bias) | Low risk | Compliance in both groups 100%. |
| Timing of outcome assessment (detection bias) | Low risk | "... 3 months assessment". |
| Other bias | Unclear risk | Funding and conflict of interest were not reported. |
Nandi 2017.
| Methods | Placebo‐controlled trial Source: tertiary care centre Country: India |
|
| Participants | 93 patients randomised (Group 1 = 47, Group 2 = 46) Diagnosis: clinical assessment with evidence of single or multiple lumbar disc prolapse confirmed by magnetic resonance imaging. Mean age (SD): Group 1 = 43.04 years (13.3), Group 2 = 42.85 years (13.0) Duration of symptoms: mixed (> 1 month to < 6 months) |
|
| Interventions | Group 1: epidural injection of methylprednisolone (80 mg, 2 mL) diluted in 18 ml of isotonic saline Group 2: epidural injection of isotonic saline (20 mL) | |
| Outcomes |
Pain intensity: leg pain (NRS) Disability: ODI and RMDQ Adverse events: proportion of patients experiencing adverse events Follow‐ups: 4 and 12 weeks |
|
| Notes |
Epidural approach: caudal without the use of imaging guidance. Additional caudal epidurals were provided on the basis of the patient’s response. Anaesthetist's experience: not specified. Co‐interventions: paracetamol, hot fomentation, local analgesic ointment and lumbar belts were allowed. NSAIDs were authorised only 4 weeks after the injection. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Before the study began the assessing doctor prepared two sets of 49 cards with either ‘A’ or ‘B’ written over it. The cards were randomly sealed inside opaque pre‐numbered envelopes." |
| Allocation concealment (selection bias) | Low risk | "Before the study began the assessing doctor prepared two sets of 49 cards with either ‘A’ or ‘B’ written over it. The cards were randomly sealed inside opaque pre‐numbered envelopes." |
| Blinding of participants (performance bias) | Low risk | Patients were blinded to the interventions (placebo‐controlled trial using epidural approach in both groups). |
| Blinding of personnel/care providers (performance bias) All outcomes | High risk | "The intervening doctor was aware about the treatment received." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Self‐report outcomes with patients blinded to the treatment allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 5/98 = 5%. Loss to follow‐up was below the proposed threshold. |
| Intention‐to‐treat analysis (attrition bias) | Unclear risk | Although the authors reported that the study protocol was approved, we did not identify any protocol registered in a trial register database. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the trial was not registered by the authors. |
| Group similarity at baseline (selection bias) | Low risk | No significant differences were identified at baseline assessment based on Table 2/Figure 2. |
| Co‐interventions (performance bias) | Low risk | "Paracetamol, hot fomentation, local analgesic ointment and lumbar belts were allowed." |
| Compliance (performance bias) | Low risk | Compliance in both groups 100%. |
| Timing of outcome assessment (detection bias) | Low risk | "Evaluation of participants was done at baseline, 4 week and 12 week by the assessing doctor." |
| Other bias | Low risk | No funding was received and no conflict of interest was reported. |
Ng 2005.
| Methods | Placebo‐controlled trial Source: spine specialist clinic at a university hospital Country: UK |
|
| Participants | 86 patients randomised (Group 1 = 43, Group 2 = 43); Diagnosis: clinical assessment with evidence of nerve root compression secondary to either LDH or foraminal stenosis in the magnetic resonance imaging (MRI). Mean age (SD): Group 1 = 51.2 years (14.5), Group 2 = 49.7 years (17.1); Duration of symptoms: subacute and chronic (> 6 weeks) |
|
| Interventions | Group 1: epidural injection of methylprednisolone (40 mg) plus bupivacaine (0.25%, 2 mL) Group 2: epidural injection of bupivacaine (0.25%, 2 mL) | |
| Outcomes |
Pain intensity: leg and back pain (VAS) Disability: ODI Follow‐ups: 6 and 12 weeks |
|
| Notes |
Epidural approach: transforaminal under fluoroscopic guidance. Anaesthetist's experience: "A senior surgeon performed all the procedures using a conventional technique as described by Lutz et al." Co‐interventions: additional analgesics allowed. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The randomisation was generated from a table of random numbers." |
| Allocation concealment (selection bias) | Low risk | "An independent theater staff uninvolved in the study placed the assignments in opaque, pre numbered envelopes." |
| Blinding of participants (performance bias) | Low risk | Patients were blinded to the interventions (placebo‐controlled trial using epidural approach in both groups). |
| Blinding of personnel/care providers (performance bias) All outcomes | Low risk | "The patient, the surgeon, and research fellow were therefore blinded from the assignment." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Self‐report outcomes with patients blinded to the treatment allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "Five patients (6%) 3 patients in the bupivacaine only group, 2 in the bupivacaine and steroids group) discontinued the trial at 6 weeks follow‐up due to worsening of the symptoms. At three months the follow up rate was 100%." Lost to short‐term follow‐up was below the proposed threshold. |
| Intention‐to‐treat analysis (attrition bias) | Low risk | "The results on intention‐to‐treat analysis including the 5 patients who had early termination from the trial are shown in Table 4." |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the trial was not registered by the authors reported. |
| Group similarity at baseline (selection bias) | Low risk | No significant differences were identified based on Table 1, 2 and Figure 1, 2, and 3. |
| Co‐interventions (performance bias) | Unclear risk | Not mentioned. |
| Compliance (performance bias) | Low risk | Compliance in both groups: 100%. |
| Timing of outcome assessment (detection bias) | Low risk | "The patients were observed up at 6 weeks and 12 weeks post injection." |
| Other bias | Low risk | No funding was received and no conflict of interest were reported. |
Ridley 1988.
| Methods | Placebo‐controlled trial Source: rheumatology clinic Country: UK |
|
| Participants | 35 patients randomised (Group 1 = 19, Group 2 = 16); Diagnosis: clinical assessments Mean age (SD): G1 = 40.0 years (9.0), G2 = 39.0 years (12.0); Duration: mixed (no restriction) |
|
| Interventions | Group 1: epidural injection of methylprednisolone (80 mg, 2 mL) plus saline (10 mL) Group 2: interspinous injection of saline (2 mL) | |
| Outcomes |
Pain relief: Proportion of any improvement in pain; Follow‐ups: 1, 2, and 4 weeks, 3 and 6 months |
|
| Notes |
Epidural approach: interlaminar without the use of imaging guidance. After 1 week, if there was little or no improvement, patients received another injection. Anaesthetist's experience: "All injections were performed by the same doctor" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were distributed to active or placebo injection according to random lists stratified for age and duration of symptoms." |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned. |
| Blinding of participants (performance bias) | High risk | The procedures are different which confirms that the participants were not blinded. |
| Blinding of personnel/care providers (performance bias) All outcomes | High risk | The procedures are different which confirms that the care providers were not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Given that the participants were not blinded to the group allocation and the outcomes are self‐reported measures, we judged that the outcome assessors were not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 5%. Loss to follow‐up was below the proposed threshold. |
| Intention‐to‐treat analysis (attrition bias) | Unclear risk | Not mentioned. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the trial was not registered by the authors. |
| Group similarity at baseline (selection bias) | Unclear risk | “The authors did not provide the baseline measures for clinical outcomes” |
| Co‐interventions (performance bias) | Unclear risk | Not mentioned. |
| Compliance (performance bias) | Low risk | Compliance in both groups 100%. |
| Timing of outcome assessment (detection bias) | Low risk | "Patients were examined at entry, and then after 1, 2 and 4 weeks." |
| Other bias | Unclear risk | Funding and conflict of interest were not reported. |
Rogers 1992.
| Methods | Placebo‐controlled trial Source: not specified Country: UK |
|
| Participants | Source: not specified
30 patients randomised (Group 1 = 15, Group 2 = 15) Diagnosis: clinical diagnosis Mean age (range): Group 1 = 42 years (22 to 61 years), Group 2 = 41 years (23 to 63 years) Duration of symptoms: mixed (> 1 month to < 24 months) |
|
| Interventions | Group 1: epidural injection of methylprednisolone acetate (80 mg) plus lignocaine (2%, 14 mL) plus aqueous suspension (2 mL) plus normal saline (4 mL) Group 2: epidural injection of lignocaine (2%, 14 mL) plus normal saline (6 mL) | |
| Outcomes | Overall pain (verbal rating scale with 5 categories); Follow‐up: 1 month |
|
| Notes |
Epidural approach: Interlaminar without the use of imaging guidance. Anaesthetist's experience: not specified Co‐interventions: additional analgesics allowed. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "the first patient of each pair being given either an epidural injection of local anaesthetic with added steroid or of local anaesthetic alone according to the toss of a coin." |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned. |
| Blinding of participants (performance bias) | Low risk | Patients were blinded to the interventions (placebo‐controlled trial using epidural approach in both groups). |
| Blinding of personnel/care providers (performance bias) All outcomes | High risk | The procedures were different which confirms that the care providers were not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Self‐report outcomes with patients blinded to the treatment allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not mentioned. |
| Intention‐to‐treat analysis (attrition bias) | Unclear risk | Not mentioned. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the trial was not registered by the authors. |
| Group similarity at baseline (selection bias) | Low risk | No significant differences were identified at baseline assessment based on Table 1, 2 and 3. |
| Co‐interventions (performance bias) | Unclear risk | Not mentioned. |
| Compliance (performance bias) | Low risk | Compliance in both groups 100%. |
| Timing of outcome assessment (detection bias) | Low risk | "Patients were compared within pairs by scoring at 1 month the change in each assessment category." |
| Other bias | Unclear risk | Funding and conflict of interest were not reported. |
Snoek 1977.
| Methods | Placebo‐controlled trial Source: hospital neurology department Country: Norway |
|
| Participants | 51 patients randomised (Group 1 = 27, Group 2 = 24); Diagnosis: clinical assessment with lumbar myelography findings at the appropriate level and side. Mean age (range): Group 1 = 43.8 years (26 to 59 years), Group 2 = 46.5 years (27 to 67 years); Duration of symptoms: mixed (12 days to 36 weeks) |
|
| Interventions | Group 1: epidural injection of methylprednisolone acetate (80 mg, 2 mL) Group 2: epidural injection of saline (2 mL) | |
| Outcomes | Proportion of improved patients; Follow‐ups: 12±10 hours and 48±24 hours |
|
| Notes |
Epidural approach: interlaminar without the use of imaging guidance. Anaesthetist's experience: all injections were performed by 1 of the authors. Co‐interventions: after 1 week, walking and physiotherapy (isometric training) were allowed. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | "The selected patients were randomly divided into two comparable groups (Table 1) by one of the authors (B.J.)" |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned. |
| Blinding of participants (performance bias) | Low risk | Patients were blinded to the interventions (placebo‐controlled trial using epidural approach in both groups). |
| Blinding of personnel/care providers (performance bias) All outcomes | High risk | The procedures are different which confirm that the care providers were not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Self‐report outcomes with patients blinded to the treatment allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "All patients included in this study were reviewed in December 1975." |
| Intention‐to‐treat analysis (attrition bias) | Unclear risk | Not mentioned. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the trial was not registered by the authors. |
| Group similarity at baseline (selection bias) | Low risk | No significant differences were identified at baseline assessment based on Table 1. |
| Co‐interventions (performance bias) | Low risk | "All patients were restricted to bed for the first 7 days of hospitalisation, but from the eighth day were allowed to walk about freely. Physiotherapy, mainly consisting of instruction and isometric training of the appropriate muscle groups, was identical for all." |
| Compliance (performance bias) | Low risk | Compliance in both groups 100%. |
| Timing of outcome assessment (detection bias) | High risk | “The follow‐up period ranged from 8 months for those admitted in April 1975 to nearly 20 months for those admitted in May 1974.” |
| Other bias | Unclear risk | Funding and conflict of interest were not reported. |
Tafazal 2009.
| Methods | Placebo‐controlled trial Source: specialist spine clinic Country: UK |
|
| Participants | 150 patients randomised (Group 1 = 74, Group 2 = 76); Diagnosis: clinical assessment with evidence of disc herniation of foraminal stenosis. Mean age: Group 1 = 51 years, Group 2 = 52.8 years; Duration of symptoms: mixed (not restricted) |
|
| Interventions | Group 1: epidural injection of methylprednisolone (40 mg) plus bupivacaine (0.25%, 2 mL) Group 2: epidural injection of bupivacaine (0.25%, 2 mL) | |
| Outcomes |
Pain intensity: leg and back pain (VAS) Disability: ODQ Pain relief: at least 20 mm change in the VAS Disability improvement: at least 10% of reduction in the ODI Adverse events: proportion of patients experiencing adverse events Follow‐ups: 6 and 12 weeks, and 1 year. |
|
| Notes |
Epidural approach: transforaminal without the use of imaging guidance. Anaesthetist's experience: "The same senior surgeon performed all of the procedures." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The randomisation was generated from a table of random numbers." |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned. |
| Blinding of participants (performance bias) | Low risk | Patients were blinded to the interventions (placebo‐controlled trial using epidural approach in both groups). |
| Blinding of personnel/care providers (performance bias) All outcomes | Low risk | "To ensure that the treatment agents were concealed from the surgeon performing the procedure the syringe with the treatment agents was wrapped with opaque tape." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Self‐report outcomes with patients blinded to the treatment allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "Therefore at 6 weeks follow‐up, data was available for 141 patients (94%). Sixteen patients did not attend for review at the 3 month follow‐up. Complete data at 3 months was therefore available for 124 patients (83%). At a minimum of 1 year follow‐up two patients had died and data was available for 129 patients (86%)." Loss to follow‐up was below the proposed threshold. |
| Intention‐to‐treat analysis (attrition bias) | Unclear risk | Not mentioned. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the trial was not registered by the authors. |
| Group similarity at baseline (selection bias) | Low risk | There was no statistically significant difference in the baseline characteristics for the 2 groups. |
| Co‐interventions (performance bias) | Low risk | "Patients enrolled into the trial agreed not to alter their oral analgesic medication during the follow‐up period and did not have any additional treatments such as physiotherapy during the trial." |
| Compliance (performance bias) | Low risk | Compliance in both groups 100%. |
| Timing of outcome assessment (detection bias) | Low risk | "Patients were assessed at enrolment and subsequently at 6 weeks, 12 weeks and finally at 1 year after the procedure." |
| Other bias | Unclear risk | Funding and conflict of interest were not reported. |
Valat 2003.
| Methods | Placebo‐controlled trial Source: 5 rheumatology departments from university hospitals Country: France |
|
| Participants | 85 patients randomised (Group 1 = 42, Group 2 = 43); Diagnosis: clinical assessment Mean age (SD): Group 1 = 43.5 years (11.8), Group 2 = 38.4 years (8.8); Duration of symptoms: mixed (15 days to 6 months) |
|
| Interventions | Group 1: epidural injections of prednisolone acetate (50 mg, 2 mL) Group 2: epidural injection of isotonic saline (2 mL) | |
| Outcomes |
Pain intensity: overall pain (VAS) Disability: (RMDQ) Adverse events: proportion of patients experiencing adverse events Follow‐ups: 5, 20, and 35 days |
|
| Notes |
Epidural approach: interlaminar without the use of imaging guidance. Patients received 3 injections at 2‐d interval. Anaesthetist's experience: not specified Co‐interventions: non‐opioid analgesics, NSAIDs, bed rest, mild lumbar traction, and lumbar belts were allowed. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The randomisation was generated from a table of random numbers, stratified according to study centres and balanced after every four. Opaque prenumbered envelopes contained the assignments." |
| Allocation concealment (selection bias) | Low risk | "Opaque prenumbered envelopes contained the assignments." |
| Blinding of participants (performance bias) | Low risk | Patients were blinded to the interventions (placebo‐controlled trial using epidural approach in both groups). |
| Blinding of personnel/care providers (performance bias) All outcomes | High risk | The procedures are different which confirm that the care providers were not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Self‐report outcomes with patients blinded to the treatment allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 5/85 (6%). Loss to follow‐up was below the proposed threshold. |
| Intention‐to‐treat analysis (attrition bias) | Low risk | "Data were analysed according to the intention to treat principle." |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the trial was not registered by the authors. |
| Group similarity at baseline (selection bias) | Low risk | None significant differences were identified at baseline assessment based on Table 1. |
| Co‐interventions (performance bias) | Low risk | "Lumbar exercises and other spinal injections were not authorised during the study." |
| Compliance (performance bias) | Low risk | Compliance in both groups 100%. |
| Timing of outcome assessment (detection bias) | Low risk | "The patients were re‐evaluated five days after inclusion (after the last injection, before leaving the hospital) and as outpatients 20 and 35 days after the first injection." |
| Other bias | Low risk | Funding (governmental source) was reported, but conflict of interest was not reported. |
Abbreviations: ESI, Epidural Steroid Injection; EDI, extra dural; LDH, Lumbar Disc Herniation; IQR, Interquartile Range; NSAIDs, Non‐steroidal anti‐inflammatory drugs; NRS, Numerical Rating Scale; ODI, Oswestry Disability Index, RMDQ; Roland Morris Questionnaire; SD, Standard Deviation; SLR, Straight Leg Raise; VAS, Visual Analogue Scale.
ESI (Arden 2005), EDI, ODI,
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ackerman 2007 | The study was not considered a placebo‐controlled trial. |
| Ahadian 2011 | The study was not considered a placebo‐controlled trial. |
| Baek 2019 | The study did not enrol participants with lumbosacral radicular pain. |
| Becker 2007 | The study was not considered a placebo‐controlled trial. |
| Beliveau 1971 | The study was not considered a placebo‐controlled trial. |
| Breivik 1976 | This study included patients with previous surgery. |
| Buchner 2000 | The study was not considered a placebo‐controlled trial. |
| Burgher 2011 | The study was not considered a placebo‐controlled trial. |
| Cohen 2015 | The study was not considered a placebo‐controlled trial. |
| Dashfield 2005 | The study was not considered a placebo‐controlled trial. |
| el Zahaar 1991 | This study included patients with previous surgery. |
| Freeman 2013 | This study did not investigate epidural corticosteroid injection. |
| Friedly 2017 | The study did not enrol participants with lumbosacral radicular pain. |
| Friedly 2019 | The study did not enrol participants with lumbosacral radicular pain. |
| Glémarec 2018 | The study did not enrol participants with lumbosacral radicular pain. |
| Hauritz 2018 | The study did not enrol participants with lumbosacral radicular pain. |
| Kamble 2016 | The study was not considered a placebo‐controlled trial. |
| Kennedy 2018 | The study did not enrol participants with lumbosacral radicular pain. |
| Kim 2018 | The study was not considered a placebo‐controlled trial. |
| Lee 2009 | The study was not considered a placebo‐controlled trial. |
| Manchikanti 2008 | The study did not enrol participants with lumbosacral radicular pain. |
| Manchikanti 2015 | The study did not enrol participants with lumbosacral radicular pain. |
| Okmen 2017 | The study did not enrol participants with lumbosacral radicular pain. |
| Sayegh 2009 | The study did not enrol participants with lumbosacral radicular pain. |
| Swerdlow 1970 | This study from the original review was excluded due to unclear information regarding the randomisation of the participants. |
| Wilson‐MacDonald 2005 | The study was not considered a placebo‐controlled trial. |
| Yin 2018 | The study was not considered a placebo‐controlled trial. |
Characteristics of studies awaiting assessment [ordered by study ID]
Abedini 2018.
| Methods | Placebo‐controlled trial Source: hospital affiliated with the university Country: Iran |
| Participants | 41 patients randomised (Group 1 = 11, Group 2 = 16, Group 3 = 14); Diagnosis: initial diagnosis of radicular chronic low back pain due to discal hernia (bulging, protrusion, etc) and radiological evidence or CT‐scan. Mean age (SD): Group 1 = 42.0 years, Group 2 = 45.6 years; Group 3 = 42.6 Duration of symptoms: acute (onset in the last 6 weeks) |
| Interventions |
Group 1: epidural injection of methylprednisolone (80 mg)
Group 2: epidural injection of bupivacaine (10cc) Group 3: epidural injection of saline. |
| Outcomes |
Pain intensity: overall pain (VAS) Disability: Oswestry Disability Index Questionnaire Follow‐ups: 1 and 2 weeks, 1, 2, and 3 months |
| Notes |
Epidural approach: not specified Anaesthetist's experience: not specified. Co‐interventions: oral medication (type was not specified) |
Vad 2002.
| Methods | Placebo‐controlled trial Source: private practice affiliated with the hospital. Country: UK |
| Participants | 48 patients randomised (Group 1 = 25, Group 2 = 23); Diagnosis: Clinical assessment with evidence of disc herniation estimated to have less than 50% intervertebral foraminal narrowing. Mean age (SD): Group 1 = 41.3 years, Group 2 = 42.1 years; Duration of symptoms: subacute and chronic (more than 6 weeks) |
| Interventions | Group 1: epidural injection of betamethasone (9 mg, 1.5 mL) xylocaine (2%, 1.5 mL) Group 2: injection of saline (3 mL) at points of maximal tenderness in the paravertebral muscles |
| Outcomes |
Pain intensity: overall pain (VNS) Disability: RMQ Follow‐ups: 3 and 6 weeks, 3, 6, and 12 months |
| Notes |
Epidural approach: transforaminal under fluoroscopic guidance. Patients in G1 and G2 received an average of 1.7 injections (range, 1 to 3) and 1.4 injections (range, 1 to 2), respectively. Anaesthetist's experience: not specified. Co‐interventions: all patients received a self‐directed home lumbar stabilizations exercises program and back cryobrace. |
Characteristics of ongoing studies [ordered by study ID]
NCT03240783.
| Trial name or title | A Comparative Effectiveness Randomised Placebo Controlled Pilot Trial of the Management of Acute Lumbar Radicular Pain (SCIATICA) |
| Methods | Pilot randomised controlled trial, 4 arms, double‐blind |
| Participants | Inclusion Criteria: Leg pain of any description with clinical findings consistent with single level radiculopathy Minimum symptom duration > 72hrs Maximum symptom duration < 3 weeks to ensure symptom duration at randomisation is ≤ 4 weeks No previous episode of same level radicular pain in the previous 6 months Pain intensity at >30 on the Oswestry Disability Index (ODI) Imaging (MRI and/or CT) indicating herniated disc or foraminal stenosis or both, concordant with the level indicated by history and physical examination Exclusion Criteria: Previous transforaminal epidural steroids at any level in the last 12 months Previous oral steroids in the last 12 months Any lumbar surgery at same level, or above or below the level at any time Previous lumbar surgery at any other level to that in (iii) within the last 12 months Pregnancy, or lactation/breastfeeding Direct indication for neurosurgery (e.g. cauda equina syndrome, or progressive motor loss i.e. less than or equal to 3/5 power) Inability to read or understand English Any serious medical or psychiatric condition that may interfere with participation or outcome assessment such as: need for uninterrupted anti‐coagulation, spinal fracture, active infection or metastatic disease suspected, active cancer, poorly controlled diabetes, or patients with diabetes on any insulin, uncontrolled hypertension (systolic blood pressure >180 or diastolic blood pressure >110 within 30 days of randomization date), active peptic ulcer disease, history of intolerance to steroid therapy, previous or current psychiatric history of bipolar disease, or secondary gain such as anticipated or ongoing legal proceedings, history of substance abuse |
| Interventions | Group 1: Selective CT fluoroscopic lumbosacral transforaminal epidural steroid with local anesthetic plus 15 days of oral placebo Group 2: Selective CT fluoroscopic lumbosacral transforaminal epidural saline with local anesthetic plus 15 days of oral placebo Group 3: Placebo CT fluoroscopic lumbosacral intramuscular injection plus 15 days of oral steroid Group 4: Placebo CT fluoroscopic lumbosacral intramuscular injection plus 15 days of oral placebo |
| Outcomes | Primary outcome: 1. Oswestry Disability Index (ODI) version 2.0 Secondary outcomes 1. Numerical Rating Scale for leg pain 2. Numerical Rating Scale for back pain 3. Pain DETECT Questionnaire 4. Short Form 36 (SF‐36) questionnaire for quality of life 5. Lumbosacral and lower limb musculoskeletal and neurological history and clinical examination 6. Work and health utilisation measures 7. Medications 8. Economic evaluation 9. Adverse events |
| Starting date | 8 July 2017 |
| Contact information | Name: Marissa N Lassere, MBBS PhD Email: m.lassere@unsw.edu.au Name: Sue Baker Email: Sue.Baker@health.nsw.gov.au |
| Notes |
NTR4457.
| Trial name or title | Transforaminal epidural corticosteroids versus short acting anaesthetic in patients with sciatica: a prospective randomised trial |
| Methods | Randomised controlled trial, 3 arms, single‐blind. |
| Participants | Individuals diagnosed with sciatica Inclusion criteria: 18 to 65 years of age, < 8 weeks sciatica, herniated disk on MRI as the cause of sciatica, correlation between symptoms and MRI findings, and VAS 40 or more despite pain medication Exclusion criteria: weakness graded MRC 3 or more, spine surgery at the same level, foraminal stenosis as the cause of symptoms, pregnancy, and severe comorbidity (e.g. cancer) Target sample size: 260 |
| Interventions | Group 1: epidural injection of steroids and local anaesthetics Group 2: epidural injections of local anaesthetic only Group 3: pain medication only |
| Outcomes | Primary outcomes: 1. Roland Disability Questionaire 2. Visual Analogue Scale (VAS) for back pain 3. Visual Analogue Scale (VAS) for leg pain 4. Global Perceived effect Secondary outcomes: 1. satisfaction 2. use of medication 3. absenteeism from work (yes‐ no) 4. number of operations The outcomes will be assessed at baseline, 3 weeks, 6 weeks, 3 months, and 6 months |
| Starting date | 01 June 2014 |
| Contact information | Name: Raymond Ostelo Postal address: EMGO Institute, VU University Medical Centre Amsterdam, van der Boechorststraat 7, 1081 BT, Amsterdan, The Netherlands. Email: r.ostelo@vumc.nl |
| Notes |
Differences between protocol and review
This Cochrane Review is an update of a previous review published in Annals of Internal Medicine in 2012 (Pinto 2012). We changed the name of the condition from "sciatica" to " lumbosacral radicular pain" because according to the IASP 1994 the term “sciatica” should be avoided because it might suggest that the condition is a disorder of the sciatica nerve rather than a lumbosacral nerve root. Hence, we have used the term “lumbosacral radicular pain” in this review to align with the IASP name for this condition. We updated the methods of our previous review according to Furlan 2015 method guidelines. Therefore, the criteria of some domains to assess the overall quality of evidence using GRADE approach as well as the risk of bias tool to assess the methodological quality of the included studies changed. In addition, the pooled estimates for leg pain was conducted in the last review using a combination of leg pain and overall pain intensity. In this review, we performed separated analyses for leg pain and overall pain intensity. We also included adverse events, overall pain intensity, back pain intensity, and pain intensity and disability measured as dichotomous outcomes as secondary outcomes.
Contributions of authors
Crystian Oliveira, Chris Maher and Rafael Pinto drafted the manuscript.
Crystian Oliveira, Vinicius Oliveira, Rafael Pinto, Manuela L Ferreira, Paulo H Ferreira, Mark Hancock and Chris Maher selected eligible studies from the systematic search, performed the data extraction, and performed ’Risk of bias’ assessments.
Crystian Oliveira, Rafael Pinto, Bart Koes and Andrew McLachlan performed the analyses.
Bart Koes reviewed the systematic review methods, Andrew McLachlan reviewed the handling of data on medicines and Steven Cohen reviewed the handling of data on epidural injections.
All review authors participated in the interpretation of the results, reading and approving the final manuscript.
Sources of support
Internal sources
Christopher Maher has a senior research fellowship by the National Health and Medical Research Council, Australia.
Crystian Oliveira was supported by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES ‐ Finance Code 001), Brazil.
External sources
No sources of support supplied
Declarations of interest
VO, RP, PF, BK have no known conflicts of interest.
CO was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior ‒ Brasil (CAPES) ‒ Finance Code 001 for conducting this study.
CM has received research grant and fellowship funding from Australia’s National Health and Medical Research Council; research grants from New South Wales Agency for Clinical Innovation, Medibank Health Research Fund, Sydney Health Partners, Sydney University, Arthritis Australia, Defence Health Foundation, WorkCover NSW, FAPESP (Sao Paulo Research Foundation); has had his travel expenses covered when presenting at scientific conferences; has received small gifts (e.g. bottle of wine) for giving lectures and talks and received Flexeze heat wraps (i.e. air activated heat patch which provides low‐level heat by means of controlled iron oxidation) for use in the SHaPED clinical trial.
CM is also on the Editorial Board of the Cochrane Back and Neck Review Group. Editors are required to conduct at least one Cochrane Review, which ensures that they are aware of the processes and commitment needed to conduct reviews.
MF has received research grant and fellowship funding from Australia’s National Health and Medical Research Council; research grants from Musculoskeletal Australia, Medibank Health Research Fund, Sydney Health Partners, Sydney University, Ramsay Research Foundation, Arthritis Australia, FAPESP (Sao Paulo Research Foundation), CAPES (Brazilian Research Council) , CNPq (Brazilian Government Research Agency), and has had her travel expenses covered when presenting at national and international scientific conferences.
MH has received research grant funding from Australia’s National Health and Medical Research Council, Arthritis NSW, Defence Health Foundation, WorkCover NSW, International Mechanical and Diagnosis Research Foundation, Physiotherapy Research Foundation; has had his travel expenses covered when presenting at scientific conferences; and has received small gifts (e.g. bottle of wine) for giving lectures and talks.
AM reports receiving research grant funding from Australia’s National Health and Medical Research Council and untied funding is provided to the Sydney Pharmacy School for a postgraduate research scholarship from GlaxoSmithKline Australia which supports a research student under his supervision. The GSK research scholarship funding was not received to conduct the review and it is for research in an area unrelated to the scope of the review.
AM reports receiving research grant funding from Australia’s National Health and Medical Research Council (NHMRC) and untied funding is provided to the Sydney Pharmacy School for the Peter Coates Postgraduate Scholarship in Ethnopharmacologyfrom GlaxoSmithKline (GSK) Australia which supports a research student under his supervision. AM and his co‐investigators received study medicines and in‐kind research support for a NHMRC‐funded clinical trial of paracetamol in acute low back pain (ACTRN12609000966291). AM also reports receiving in‐kind funding and study medicines from Pfizer for the PRECISE trial (ACTRN12613000530729) which was a NHMRC‐funded investigator‐initiated randomised controlled trial of pregabalin in sciatica. AM has received consultancy fees from the Australian Non‐Government Organisation NPS MedicineWise for developing and delivering educational material related to a range of medicines issues. He receives royalties from UnityHealth IMgateway for developing a Herb‐Drug Interaction database and the School of Pharmacy at the University of Sydney receives payment for a part‐time research assistant to maintain the herb‐drug interaction database.
PF has received research grant and fellowship funding from Australia’s National Health and Medical Research Council; research grants from Musculoskeletal Australia, Medibank Health Research Fund, Sydney Health Partners, Sydney University, Arthritis Australia, IMDTR USA, Murcia Foundation Spain, FAPESP (Sao Paulo Research Foundation), CAPES (Brazilian Research Council) , CNPq (Brazilian Government Research Agency), and has had her travel expenses covered when presenting at scientific conferences.
SC is member of Advisory Board of Scilex (formerly Semnur Pharmaceuticals), which is developing an injectable corticosteroid for epidural administration. SC received a payment from Semnur Pharmaceuticals to be a consultant for their phase 2 study investigating the efficacy and safety of the injectable corticosteroid developed for epidural administration. This payment has not been received to conduct this review. He has also served on advisory boards for Medtronic and Boston Scientific within the past three years, has consulted for and conducted research with SPR Therapeutics and Avanos within the past year, and does legal consultancy work.
New
References
References to studies included in this review
Arden 2005 {published data only}
- Arden NK, Price C, Reading I, Stubbing J, Hazelgrove J, Dunne C, et al. WEST Study Group. A multicentre randomized controlled trial of epidural corticosteroid injections for sciatica: the WEST study. Rheumatology (Oxford, England) 2005;44(11):1399‐406. [DOI] [PubMed] [Google Scholar]
- Price C, Arden N, Coglan L, Rogers P. Cost‐effectiveness and safety of epidural steroids in the management of sciatica. Health Technology Assessment 2005;9(33):iii‐46. [DOI] [PubMed] [Google Scholar]
Bush 1991 {published data only}
- Bush K, Hillier S. A controlled study of caudal epidural injections of triamcinolone plus procaine for the management of intractable sciatica. Spine 1991;16(5):572‐5. [DOI] [PubMed] [Google Scholar]
Carette 1997 {published data only}
- Carette S, Leclaire R, Marcoux S, Morin F, Blaise GA, St‐Pierre A, et al. Epidural corticosteroid injections for sciatica due to herniated nucleus pulposus. New England Journal of Medicine 1997;336(23):1634‐40. [DOI] [PubMed] [Google Scholar]
Cohen 2012 {published data only}
- Cohen SP, White RL, Kurihara C, Larkin TM, Chang A, Griffith SR, et al. Epidural steroids, etanercept, or saline in subacute sciatica a multicenter, randomized trial. Annals of Internal Medicine 2012;156(8):551‐9. [DOI] [PubMed] [Google Scholar]
Cuckler 1985 {published data only}
- Cuckler JM, Bernini PA, Wiesel SW, Booth RE Jr, Rothman RH, Pickens GT. The use of epidural steroids in the treatment of lumbar radicular pain. Journal of Bone and Joint Surgery. American Volume 1985;67(1):63‐6. [PubMed] [Google Scholar]
Datta 2011 {published data only}
- Datta R, Upadhyay KK. A randomized clinical trial of three different steroid agents for treatment of low backache through the caudal route. Medical Journal, Armed Forces India 2011;67(1):25‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dilke 1973 {published data only}
- Dilke TF, Burry HC, Grahame R. Extradural corticosteroid injection in management of lumbar nerve root compression. British Medical Journal 1973;2(5867):635‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ghahreman 2010 {published data only}
- Ghahreman A, Ferch R, Bogduk N. The efficacy of transforaminal injection of steroids for the treatment of lumbar radicular pain. Pain Medicine (Malden, Mass.) 2010;11(8):1149‐68. [DOI] [PubMed] [Google Scholar]
Ghai 2015 {published data only}
- Ghai B, Kumar K, Bansal D, Dhatt SS, Kanukula R, Batra YK. Effectiveness of parasagittal interlaminar epidural local anesthetic with or without steroid in chronic lumbosacral pain: a randomized, double‐blind clinical trial. Pain Physician 2015;18(3):237‐48. [PubMed] [Google Scholar]
Helliwell 1985 {published data only}
- Helliwell M, Robertson JC, Ellis RM. Outpatient treatment of low pack pain and sciatica by a single extradural corticosteroid injection. British Journal of Clinical Practice 1985;39(6):228‐31. [Google Scholar]
Iversen 2011 {published data only}
- Iversen T, Solberg TK, Romner B, Wilsgaard T, Twisk J, Anke A, et al. Effect of caudal epidural steroid or saline injection in chronic lumbar radiculopathy: multicentre, blinded, randomised controlled trial. BMJ (Clinical Research Ed.) 2011;343:d5278. [DOI] [PMC free article] [PubMed] [Google Scholar]
Karppinen 2001 {published data only}
- Karppinen J, Malmivaara A, Kurunlahti M, Kyllonen E, Pienimaki T, Nieminen P, et al. Periradicular infiltration for sciatica: a randomized controlled trial. Spine 2001;26(9):1059‐67. [DOI] [PubMed] [Google Scholar]
Klenerman 1984 {published data only}
- Klenerman L, Greenwood R, Davenport HT. Lumbar epidural injections in the treatment of sciatica. British Journal of Rheumatology 1984;23(1):35‐8. [DOI] [PubMed] [Google Scholar]
Kraemer 1997 {published data only}
- Kraemer J, Ludwig J, Bickert U, Owczarek V, Traupe M. Lumbar epidural perineural injection: a new technique. European Spine Journal 1997;6(5):357‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Manchikanti 2012 {published data only}
- Manchikanti L, Singh V, Cash KA, Pampati V, Damron KS, Boswell MV. A randomized, controlled, double‐blind trial of fluoroscopic caudal epidural injections in the treatment of lumbar disc herniation and radiculitis. Spine 2011;36(23):1897‐905. [DOI] [PubMed] [Google Scholar]
- Manchikanti L, Singh V, Cash KA, Pampati V, Damron KS, Boswell MV. Effect of fluoroscopically guided caudal epidural steroid or local anesthetic injections in the treatment of lumbar disc herniation and radiculitis: a randomized, controlled, double blind trial with a two‐year follow‐up. Pain Physician 2012;15(4):273‐86. [PubMed] [Google Scholar]
- Manchikanti L, Singh V, Cash KA, Pampati V, Damron KS, Boswell MV. Preliminary results of a randomized, equivalence trial of fluoroscopic caudal epidural injections in managing chronic low back pain: Part 2‐‐Disc herniation and radiculitis. Pain Physician 2008;11(6):801‐15. [PubMed] [Google Scholar]
Manchikanti 2014a {published data only}
- Manchikanti L, Singh V, Cash KA, Pampati V, Falco FJ. A randomized, double‐blind, active‐control trial of the effectiveness of lumbar interlaminar epidural injections in disc herniation. Pain Physician 2014;17(1):E61‐74. [PubMed] [Google Scholar]
- Manchikanti L, Singh V, Falco FJ, Cash KA, Pampati V. Evaluation of the effectiveness of lumbar interlaminar epidural injections in managing chronic pain of lumbar disc herniation or radiculitis: a randomized, double‐blind, controlled trial. Pain Physician 2010;13(4):343‐55. [PubMed] [Google Scholar]
Manchikanti 2014b {published data only}
- Manchikanti L, Cash KA, Pampati V, Falco FJ. Transforaminal epidural injections in chronic lumbar disc herniation: a randomized, double‐blind, active‐control trial. Pain Physician 2014;17(4):E489‐501. [PubMed] [Google Scholar]
Mathews 1987 {published data only}
- Mathews JA, Mills SB, Jenkins VM, Grimes SM, Morkel MJ, Mathews W, et al. Back pain and sciatica: Controlled trials of manipulation, traction, sclerosant and epidural injections. British Journal of Rheumatology 1987;26(6):416‐23. [DOI] [PubMed] [Google Scholar]
Nandi 2017 {published data only}
- Nandi J, Chowdhery A. A randomized controlled clinical trial to determine the effectiveness of caudal epidural steroid injection in lumbosacral sciatica. Journal of Clinical and Diagnostic Research : JCDR 2017;11(2):RC04‐RC08. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ng 2005 {published data only}
- Ng L, Chaudhary N, Sell P. The efficacy of corticosteroids in periradicular infiltration for chronic radicular pain: a randomized, double‐blind, controlled trial. Spine (Philadelphia, Pa. : 1986) 2005;30(8):857‐62. [DOI] [PubMed] [Google Scholar]
Ridley 1988 {published data only}
- Ridley MG, Kingsley GH, Gibson T, Grahame R. Outpatient lumbar epidural corticosteroid injection in the management of sciatica. British Journal of Rheumatology 1988;27(4):295‐9. [DOI] [PubMed] [Google Scholar]
Rogers 1992 {published data only}
- Rogers P, Nash T, Schiller D, Norman J. Epidural steroids for sciatica. Pain Clinic 1992;5(2):67‐72. [Google Scholar]
Snoek 1977 {published data only}
- Snoek W, Weber H, Jorgensen B. Double blind evaluation of extradural methyl prednisolone for herniated lumbar discs. Acta Orthopaedica Scandinavica 1977;48(6):635‐41. [DOI] [PubMed] [Google Scholar]
Tafazal 2009 {published data only}
- Tafazal S, Ng L, Chaudhary N, Sell P. Corticosteroids in peri‐radicular infiltration for radicular pain: A randomised double blind controlled trial ‐ One year results and subgroup analysis. European Spine Journal 2009;18(8):1220‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Valat 2003 {published data only}
- Valat JP, Giraudeau B, Rozenberg S, Goupille P, Bourgeois P, Micheau‐Beaugendre V, et al. Epidural corticosteroid injections for sciatica: a randomised, double blind, controlled clinical trial. Annals of the Rheumatic Diseases 2003;62(7):639‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Ackerman 2007 {published data only}
- Ackerman WE 3rd, Ahmad M. The efficacy of lumbar epidural steroid injections in patients with lumbar disc herniations. Anesthesia and Analgesia 2007;104(5):1217‐22. [DOI] [PubMed] [Google Scholar]
Ahadian 2011 {published data only}
- Ahadian FM, McGreevy K, Schulteis G. Lumbar transforaminal epidural dexamethasone: a prospective, randomized, double‐blind, dose‐response trial. Regional Anesthesia and Pain Medicine 2011;36(6):572‐8. [DOI] [PubMed] [Google Scholar]
Baek 2019 {published data only}
- Baek IC, Choi SY, Suh J, Kim SH. The Influence of Local Anesthesia Depth on Procedural Pain During Fluoroscopically Guided Lumbar Transforaminal Epidural Injections: A Randomized Clinical Trial. American Journal of Physical Medicine & Rehabilitation 2019;98(4):253‐257. [DOI] [PubMed] [Google Scholar]
Becker 2007 {published data only}
- Becker C, Heidersdorf S, Drewlo S, Rodriguez SZ, Kramer J, Willburger RE. Efficacy of epidural perineural injections with autologous conditioned serum for lumbar radicular compression: an investigator‐initiated, prospective, double‐blind, reference‐controlled study. Spine (Philadelphia, Pa. : 1986) 2007;32(17):1803‐8. [DOI] [PubMed] [Google Scholar]
Beliveau 1971 {published data only}
- Beliveau P. A comparison between epidural anaesthesia with and without corticosteroid in the treatment of sciatica. Rheumatology and Physical Medicine 1971;11(1):40‐3. [DOI] [PubMed] [Google Scholar]
Breivik 1976 {published data only}
- Breivik H, Hesla P, Molnar I, Lind B. Comparison of caudal epidural injections of bupivacaine and methylprednisolone with bupivacaine followed by saline. Advances in Pain Research and Therapy 1976;1:927‐32. [Google Scholar]
Buchner 2000 {published data only}
- Buchner M, Zeifang F, Brocai DR, Schiltenwolf M. Epidural corticosteroid injection in the conservative management of sciatica. Clinical Orthopaedics and Related Research 2000;375:149‐56. [DOI] [PubMed] [Google Scholar]
Burgher 2011 {published data only}
- Burgher AH, Hoelzer BC, Schroeder DR, Wilson GA, Huntoon MA. Transforaminal epidural clonidine versus corticosteroid for acute lumbosacral radiculopathy due to intervertebral disk herniation. Spine (Philadelphia, Pa. : 1986) 2011;36(5):E293‐E300. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cohen 2015 {published data only}
- Cohen SP, Hanling S, Bicket MC, White RL, Veizi E, Kurihara C, et al. Epidural steroid injections compared with gabapentin for lumbosacral radicular pain: multicenter randomized double blind comparative efficacy study. BMJ 2015;350:h1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dashfield 2005 {published data only}
- Dashfield AK, Taylor MB, Cleaver JS, Farrow D. Comparison of caudal steroid epidural with targeted steroid placement during spinal endoscopy for chronic sciatica: a prospective, randomized, double‐blind trial. British Journal of Anaesthesia 2005;94(4):514‐9. [DOI] [PubMed] [Google Scholar]
el Zahaar 1991 {published data only}
- Zahaar MS. The value of caudal epidural steroids in the treatment of lumbar neural compression syndromes. Journal of Neurological and Orthopaedic Medicine and Surgery 1991;12:181‐4. [Google Scholar]
Freeman 2013 {published data only}
- Freeman BJ, Ludbrook GL, Hall S, Cousins M, Mitchell B, Jaros M, et al. Randomized, double‐blind, placebo‐controlled, trial of transforaminal epidural etanercept for the treatment of symptomatic lumbar disc herniation. Spine (Philadelphia, Pa. : 1986) 2013;38(23):1986‐94. [DOI] [PubMed] [Google Scholar]
Friedly 2017 {published data only}
- Friedly JL, Comstock BA, Turner JA, Heagerty PJ, Deyo RA, Bauer Z, et al. Long‐term effects of repeated injections of local anesthetic with or without corticosteroid for lumbar spinal stenosis: a randomized trial. Archives of Physical Medicine and Rehabilitation 2017;98(8):1499‐1507. [DOI] [PubMed] [Google Scholar]
Friedly 2019 {published data only}
- Friedly JL, Comstock BA, Heagerty PJ, Bauer Z, Rothman MS, Suri P, Hansen R, Avins AL, Nedeljkovic SS, Nerenz DR, Akuthota V, Jarvik JG. Systemic effects of epidural steroid injections for spinal stenosis. Pain 2018;159(5):876‐883. [DOI] [PubMed] [Google Scholar]
Glémarec 2018 {published data only}
Hauritz 2018 {published data only}
- Hauritz RW, Hannig KE, Henriksen CW, Borglum J, Bjorn S, Bendtsen TF. The effect of perineural dexamethasone on duration of sciatic nerve blockade: a randomized, double‐blind study. Acta Anaesthesiologica Scandinavica 2018;62(4):548‐557. [DOI] [PubMed] [Google Scholar]
Kamble 2016 {published data only}
- Kamble PC, Sharma A, Singh V, Natraj B, Devani D, Khapane V. Outcome of single level disc prolapse treated with transforaminal steroid versus epidural steroid versus caudal steroids. European Spine Journal 2016;25(1):217‐21. [DOI] [PubMed] [Google Scholar]
Kennedy 2018 {published data only}
- Kennedy DJ, Huynh L, Wong J, Mattie R, Levin J, Smuck M, Schneider BJ. Corticosteroid Injections Into Lumbar Facet Joints: A Prospective, Randomized, Double‐Blind Placebo‐Controlled Trial. American Journal of Physical Medicine & Rehabilitation 2018;97(10):741‐746. [DOI] [PubMed] [Google Scholar]
Kim 2018 {published data only}
- Kim W‐J, Shin H‐Y, Yoo SH, Park HS. Comparison of Epidural Spreading Patterns and Clinical Outcomes of Transforaminal Epidural Steroid Injection with High‐Volume Injectate via the Subpedicular Versus the Retrodiscal Approach. Pain Physician 2018;21(3):269‐278. [PubMed] [Google Scholar]
Lee 2009 {published data only}
- Lee JH, An JH, Lee SH. Comparison of the effectiveness of interlaminar and bilateral transforaminal epidural steroid injections in treatment of patients with lumbosacral disc herniation and spinal stenosis. Clinical Journal of Pain 2009;25(3):206‐10. [DOI] [PubMed] [Google Scholar]
Manchikanti 2008 {published data only}
- Manchikanti L, Cash KA, McManus CD, Pampati V, Abdi S. Preliminary results of a randomized, equivalence trial of fluoroscopic caudal epidural injections in managing chronic low back pain: Part 4‐‐Spinal stenosis. Pain Physician 2008;11(6):833‐48. [PubMed] [Google Scholar]
Manchikanti 2015 {published data only}
- Manchikanti L, Cash KA, McManus CD, Damron KS, Pampati V, Falco FJ. A randomized, double‐blind controlled trial of lumbar interlaminar epidural injections in central spinal stenosis: 2‐year follow‐up.. Pain Physician 2015;18(1):79‐92. [PubMed] [Google Scholar]
Okmen 2017 {published data only}
- Okmen K, Okmen BM. The efficacy of interlaminar epidural steroid administration in multilevel intervertebral disc disease with chronic low back pain: a randomized, blinded, prospective study. Spine Journal 2017;17(2):168‐74. [DOI] [PubMed] [Google Scholar]
Sayegh 2009 {published data only}
- Sayegh FE, Kenanidis EI, Papavasiliou KA, Potoupnis ME, Kirkos JM, Kapetanos GA. Efficacy of steroid and nonsteroid caudal epidural injections for low back pain and sciatica: a prospective, randomized, double‐blind clinical trial. Spine (Philadelphia, Pa. : 1986) 2009;34(14):1441‐7. [DOI] [PubMed] [Google Scholar]
Swerdlow 1970 {published data only}
- Swerdlow M, Sayle‐Creer WS. A study of extradural medication in the relief of the lumbosciatic syndrome. Anaesthesia 1970;25(3):341‐5. [DOI] [PubMed] [Google Scholar]
Wilson‐MacDonald 2005 {published data only}
- Wilson‐MacDonald J, Burt G, Griffin D, Glynn C. Epidural steroid injection for nerve root compression. A randomised, controlled trial. Journal of Bone and Joint Surgery. British Volume 2005;87(3):352‐5. [DOI] [PubMed] [Google Scholar]
Yin 2018 {published data only}
- Yin M, Mo W, Wu H, Xu J, Ye J, Chen N, et al. Efficacy of caudal epidural steroid injection with targeted indwelling catheter and manipulation in managing patients with lumbar disk herniation and radiculopathy: a prospective, randomized, single‐blind controlled trial. World Neurosurgery 2018;114:e29‐e34. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Abedini 2018 {published data only}
- Abedini N, Pourfathi H, Eskandari M, Parish M. Comparison of epidural methylprednisolone, bupivacaine and normal saline injection in chronic low back pain due to discal hernia. Crescent Journal of Medical and Biological Sciences 2018;5(1):57–61. [Google Scholar]
Vad 2002 {published data only}
- Vad VB, Bhat AL, Lutz GE, Cammisa F. Transforaminal epidural steroid injections in lumbosacral radiculopathy: a prospective randomized study. Spine (Philadelphia, Pa. : 1986) 2002;27(1):11‐6. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
NCT03240783 {published data only}
- NCT03240783. A Comparative Effectiveness Randomised Placebo Controlled Pilot Trial of the Management of Acute Lumbar Radicular Pain (SCIATICA). https://clinicaltrials.gov/ct2/show/NCT03240783 (accessed 21 February 2020).
NTR4457 {published data only}
- NTR4457. Transforaminal epidural corticosteroids versus short acting anesthetic in patients with sciatica: a prospective randomized trial. www.trialregister.nl/trialreg/admin/rctview.asp?TC=4457 issue accessed 18 November 2017.
- Ter Meulen BC, Maas ET, Vyas A, Vegt M, Priester K, Boer MR, et al. Treatment of acute sciatica with transforaminal epidural corticosteroids and local anesthetic: design of a randomized controlled trial. BMC Musculoskeletal Disorders 2017;18(1):215. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Barnes 1993
- Barnes PJ, Adcock I. Anti‐inflammatory actions of steroids: molecular mechanisms. Trends in Pharmacological Sciences 1993;14(12):436‐41. [DOI] [PubMed] [Google Scholar]
Bhatia 2016
- Bhatia A, Flamer D, Shah PS, Cohen SP. Transforaminal epidural steroid injections for treating lumbosacral radicular pain from herniated intervertebral discs: a systematic review and meta‐analysis. Anesthesia and Analgesia 2016;122(3):857‐70. [DOI] [PubMed] [Google Scholar]
Bicket 2013
- Bicket MC, Gupta A, Brown CH 4th, Cohen SP. Epidural injections for spinal pain: a systematic review and meta‐analysis evaluating the "control" injections in randomized controlled trials. Anesthesiology 2013;119(4):907‐31. [DOI] [PubMed] [Google Scholar]
Bicket 2016
- Bicket MC, Hurley RW, Moon JY, et al. The development and validation of a quality assessment and rating of technique for injections of the spine (AQUARIUS). Regional Anesthesia and Pain Medicine 2016;41(1):80‐5. [DOI] [PubMed] [Google Scholar]
Botwin 2004
- Botwin KP, Natalicchio J, Hanna A. Fluoroscopic guided lumbar interlaminar epidural injections: a prospective evaluation of epidurography contrast patterns and anatomical review of the epidural space. Pain Physician 2004;7(1):77‐80. [PubMed] [Google Scholar]
Chang‐Chien 2014
- Chang‐Chien GC, Knezevic NN, McCormick Z, Chu SK, Trescot AM, Candido KD. Transforaminal versus interlaminar approaches to epidural steroid injections: a systematic review of comparative studies for lumbosacral radicular pain. Pain Physician 2014;17(4):E509‐24. [PubMed] [Google Scholar]
Chen 2004
- Chen CP, Tang SF, Hsu TC, Tsai WC, Liu HP, Chen MJ, et al. Ultrasound guidance in caudal epidural needle placement. Anesthesiology 2004;101(1):181‐4. [DOI] [PubMed] [Google Scholar]
Chou 2015
- Chou R, Hashimoto R, Friedly J, Fu R, Bougatsos C, Dana T, et al. Epidural corticosteroid injections for radiculopathy and spinal stenosis: a systematic review and meta‐analysis. Annals of Internal Medicine 2015;163(5):373‐81. [DOI] [PubMed] [Google Scholar]
Cohen 1988
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd Edition. Mahwah, NJ: Lawrence Erlbaum Associates, 1988. [Google Scholar]
Devor 1992
- Devor M, Wall PD, Catalan N. Systemic lidocaine silences ectopic neuroma and DRG discharge without blocking nerve conduction. Pain 1992;48(2):261‐8. [DOI] [PubMed] [Google Scholar]
Duffy 2014
- Duffy S, Misso K, Noake C, Ross J, Stirk L. Supplementary searches of PubMed to improve currency of MEDLINE and MEDLINE In‐Process searches via OvidSP [Kleijnen Systematic Reviews Ltd, York. Poster presented at the UK InterTASC Information Specialists' Sub‐Group (ISSG) Workshop; 9 July 2014; Exeter: UK (2014)]. Available from: medicine.exeter.ac.uk/media/universityofexeter/medicalschool/research/pentag/documents/Steven_Duffy_ISSG_Exeter_2014_poster_1.pdf (accessed 6 August 2014).
Dworkin 2008
- Dworkin RH, Turk DC, Wyrwich KW, Beaton D, Cleeland CS, Farrar JT, et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. Journal of Pain 2008;9(2):105‐21. [DOI] [PubMed] [Google Scholar]
Frymoyer 1988
- Frymoyer JW. Back pain and sciatica. New England Journal of Medicine 1988;318(5):291‐300. [DOI] [PubMed] [Google Scholar]
Fukusaki 1998
- Fukusaki M, Kobayashi I, Hara T, Sumikawa K. Symptoms of spinal stenosis do not improve after epidural steroid injection. Clinical Journal of Pain 1998;14(2):148‐51. [DOI] [PubMed] [Google Scholar]
Furlan 2015
- Furlan AD, Malmivaara A, Chou R, Maher CG, Deyo RA, Schoene M, et al. Editorial Board of the Cochrane Back, Neck Group. 2015 Updated Method Guideline for systematic reviews in the Cochrane Back and Neck Group. Spine (Philadelphia, Pa. : 1986) 2015;40(21):1660‐73. [DOI] [PubMed] [Google Scholar]
Guyatt 2011
- Guyatt GH, Oxman AD, Kunz R, Brozek J, Alonso‐Coello P, Rind D, et al. GRADE guidelines 6. Rating the quality of evidence‐‐imprecision. Journal of Clinical Epidemiology 2011;64(12):1283‐93. [DOI] [PubMed] [Google Scholar]
Henschke 2010
- Henschke N, Ostelo RW, Tulder MW, Vlaeyen JW, Morley S, Assendelft WJ, Main CJ. Behavioural treatment for chronic low‐back pain. Cochrane Database of Systematic Reviews 2010, Issue 7. [DOI: 10.1002/14651858.CD002014] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hider 2015
- Hider SL, Whitehurst DG, Thomas E, Foster NE. Pain location matters: the impact of leg pain on health care use, work disability and quality of life in patients with low back pain. European Spine Journal 2015;24(3):444‐51. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hjermstad 2011
- Hjermstad MJ, Fayers PM, Haugen DF, Caraceni A, Hanks GW, Loge JH, et al. European Palliative Care Research Collaborative (EPCRC). Studies comparing Numerical Rating Scales, Verbal Rating Scales, and Visual Analogue Scalesfor assessment of pain intensity in adults: a systematic literature review. Journal of Pain and Symptom Management 2011;41(6):1073‐93. [DOI] [PubMed] [Google Scholar]
IASP 1994
- IASP. Part I Topics and Codes. In: Mersky H, Bogduk N editor(s). Classification of Chronic Pain. 2nd Edition. Seattle, WA: IASP Press, 1994. [Google Scholar]
Igarashi 2000
- Igarashi T, Kikuchi S, Shubayev V, Myers RR. Exogenous Tumor Necrosis Factor‐Alpha Mimics Nucleus Pulposus‐Induced Neuropathology: Molecular, Histologic, and Behavioral Comparisons in Rats. Spine (Philadelphia, Pa. : 1986) 2000;25(23):2975‐80. [DOI] [PubMed] [Google Scholar]
Koes 2007
- Koes BW, Tulder MW, Peul WC. Diagnosis and treatment of sciatica. BMJ 2007;334(7607):1313‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Konstantinou 2013
- Konstantinou K, Hider SL, Jordan JL, Lewis M, Dunn KM, Hay EM. The impact of low back‐related leg pain on outcomes as compared with low back pain alone: a systematic review of the literature. Clinical Journal of Pain 2013;29(7):644‐54. [DOI] [PubMed] [Google Scholar]
Lee 1998
- Lee HM, Weinstein JN, Meller ST, Hayashi N, Spratt KF, Gebhart GF. The role of steroids and their effects on phospholipase A2. An animal model of radiculopathy. Spine (Philadelphia, Pa. : 1986) 1998;23(11):1191‐6. [DOI] [PubMed] [Google Scholar]
Liu 2016
- Liu J, Zhou H, Lu L, Li X, Jia J, Shi Z, et al. The effectiveness of transforaminal versus caudal routes for epidural steroid injections in managing lumbosacral radicular pain: a systematic review and meta‐analysis. Medicine (Baltimore) 2016;95(18):e3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lutz 1998
- Lutz GE, Vad VB, Wisneski RJ. Fluoroscopic transforaminal lumbar epidural steroids: an outcome study. Archives of Physical Medicine and Rehabilitation 1998;79(11):1362‐6. [DOI] [PubMed] [Google Scholar]
Manchikanti 2012b
- Manchikanti L, Falco FJ, Singh V, Pampati V, Parr AT, Benyamin RM, et al. Utilization of interventional techniques in managing chronic pain in the Medicare population: analysis of growth patterns from 2000 to 2011. Pain Physician 2012;15(6):E969‐82. [PubMed] [Google Scholar]
McLain 2005
- McLain RF, Kapural L, Mekhail NA. Epidural steroid therapy for back and leg pain: mechanisms of action and efficacy. Spine Journal 2005;5(2):191‐201. [DOI] [PubMed] [Google Scholar]
Mehta 2017
- Mehta P, Syrop I, Singh JR, Kirschner J. Systematic review of the efficacy of particulate versus nonparticulate corticosteroids in epidural injections. PM & R : the Journal of Injury, Function, and Rehabilitation 2017;9(5):502‐12. [DOI] [PubMed] [Google Scholar]
Olmarker 1998
- Olmarker K, Larsson K. Tumor necrosis factor alpha and nucleus‐pulposus‐induced nerve root injury. Spine (Philadelphia, Pa. : 1986) 1998;23(23):2538‐44. [DOI] [PubMed] [Google Scholar]
Ostelo 2008
- Ostelo RW, Deyo RA, Stratford P, Waddell G, Croft P, Korff M, et al. Interpreting change scores for pain and functional status in low back pain: towards international consensus regarding minimal important change. Spine (Philadelphia, Pa. : 1986) 2008;33(1):90‐4. [DOI] [PubMed] [Google Scholar]
Palmer 2003
- Palmer KT, Griffin MJ, Syddall HE, Pannett B, Cooper C, Coggon D. The relative importance of whole body vibration and occupational lifting as risk factors for low‐back pain. Occupational and Environmental Medicine 2003;60(10):715‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pinto 2012
- Pinto R, Maher CG, Ferreira ML, Hancock M, Oliveira VC, McLachlan AJ, et al. Epidural corticosteroid injections in the management of sciatica: a systematic review and meta‐analysis. Annals of Internal Medicine 2012;157(12):865‐77. [DOI] [PubMed] [Google Scholar]
Rabinovitch 2009
- Rabinovitch DL, Peliowski A, Furlan AD. Influence of lumbar epidural injection volume on pain relief for radicular leg pain and/or low back pain. Spine Journal 2009;9(6):509‐17. [DOI] [PubMed] [Google Scholar]
Rathmell 2015
- Rathmell JP, Benzon HT, Dreyfuss P, Huntoon M, Wallace M, Baker R, et al. Safeguards to prevent neurologic complications after epidural steroid injections: consensus opinions from a multidisciplinary working group and national organizations. Anesthesiology 2015;122(5):974‐84. [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Riew 2006
- Riew KD, Park JB, Cho YS, Gilula L, Patel A, Lenke LG, et al. Nerve root blocks in the treatment of lumbar radicular pain. A minimum five‐year follow‐up. Journal of Bone and Joint Surgery 2006;88(8):1722‐5. [DOI] [PubMed] [Google Scholar]
Rubinstein 2011
- Rubinstein SM, Middelkoop M, Assendelft WJ, Boer MR, Tulder MW. Spinal manipulative therapy for chronic low‐back pain. Cochrane Database of Systematic Reviews 2011, Issue 2. [DOI: 10.1002/14651858.CD008112.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Shamliyan 2014
- Shamliyan TA, Staal JB, Goldmann D, Sands‐Lincoln M. Epidural steroid injections for radicular lumbosacral pain: a systematic review. Physical Medicine and Rehabilitation Clinics of North America 2014;25(2):471‐89. [DOI] [PubMed] [Google Scholar]
Silbergleit 2001
- Silbergleit R, Mehta BA, Sanders WP, Talati SJ. Imaging‐guided injection techniques with fluoroscopy and CT for spinal pain management. Radiographics 2001;21(4):927‐39. [DOI] [PubMed] [Google Scholar]
Smith 2013
- Smith RM, Schaefer MK, Kainer MA, Wise M, Finks J, Duwve J, et al. Multistate Fungal Infection Outbreak Response Team. Fungal infections associated with contaminated methylprednisolone injections. New England Journal of Medicine 2013;369(17):1598‐609. [DOI] [PubMed] [Google Scholar]
Tubach 2004
- Tubach F, Beaute J, Leclerc A. Natural history and prognostic indicators of sciatica. Journal of Clinical Epidemiology 2004;57(2):174‐9. [DOI] [PubMed] [Google Scholar]
Valat 2010
- Valat JP, Genevay S, Marty M, Rozenberg S, Koes B. Sciatica. Best Practice & Research. Clinical Rheumatology 2010;24(2):241‐52. [DOI] [PubMed] [Google Scholar]
Weil 2008
- Weil L, Frauwirth NH, Amirdelfan K, Grant D, Rosenberg JA. Fluoroscopic analysis of lumbar epidural contrast spread after lumbar interlaminar injection. Archives of Physical Medicine and Rehabilitation 2008;89(3):413‐6. [DOI] [PubMed] [Google Scholar]
Younes 2006
- Younes M, Béjia I, Aguir Z, Letaief M, Hassen‐Zrour S, Touzi M, et al. Prevalence and risk factors of disk‐related sciatica in an urban population in Tunisia. Joint, Bone, Spine 2006;73(5):538‐42. [DOI] [PubMed] [Google Scholar]


