Abstract
With the ultimate goal of investigating boundary conditions for post-reactivation amnesia, we set out to replicate studies in which systemic, post-reactivation administration of midazolam, propranolol, or cycloheximide resulted in amnesia for contextual fear memories. Our experiments involved conceptual as well as exact replications of previously published studies. In most of our experiments, we adopted a procedure that conformed to the standard 3-day protocol typically used in the literature, with contextual fear conditioning on day 1, unreinforced re-exposure to the conditioning context followed by systemic injection of the amnestic drug on day 2, and a memory retention test on day 3. Given the plethora of successful studies with large effects sizes and the absence of any failed replications in the literature, we were surprised to find that we were generally unable to replicate those findings. Our results suggest that post-reactivation amnesia by systemic drug administration in rats is more difficult to obtain than what would be expected based on published empirical reports. At present, it remains unclear which conditions determine the success of this procedure.
Keywords: contextual fear memory, post-reactivation amnesia, reconsolidation, rats, midazolam, propranolol
1. Introduction
In a ground-breaking paper, Misanin, Miller, and Lewis (1968) showed that fear memory retention in rats was impaired when an electroconvulsive shock was given after a brief, unreinforced presentation of a previously conditioned cue. Interest in the phenomenon of reactivation-dependent amnesia was renewed around the turn of the century, when several publications reported impaired memory expression after post-reactivation administration of pharmacological agents such as anisomycin, MK-801 or propranolol (PROP) (Nader, Schafe, & Le Doux, 2000; Przybyslawski, Roullet, & Sara, 1999; Przybyslawski & Sara, 1997; Sara, 2000). Based on those results, it was hypothesized that adequate memory retention after reactivation required – at least partial – recapitulation of cellular events that occur during initial consolidation (such as protein synthesis and NMDA-dependent long-term potentiation) (Przybyslawski & Sara, 1997). Based on this analogy, the term ‘reconsolidation’ was proposed to describe the cascade of molecular events required for adequate retention of destabilized memories, and post-reactivation pharmacologically-induced amnesia was attributed to (partial) blockage of these molecular events.
The view of post-reactivation amnesia as resulting from reconsolidation interference has been challenged by observations of recovery from amnesia (e.g., after passage of time, reinstatement, or after a change in the internal context by re-administration of the amnestic agent) (DeVietti & Larson, 1971; Eisenberg & Dudai, 2004; Gisquet-Verrier et al., 2015; Gisquet-Verrier & Riccio, 2018; Lattal & Abel, 2004; Power, 2006; Trent, Barnes, Hall, & Thomas, 2015). Similar memory preservation despite initial amnesia has been observed when amnestic treatments were administered after initial learning (Ryan, Roy, Pignatelli, Arons, & Tonegawa, 2015). The fact that recovery from amnesia is sometimes observed after post-reactivation pharmacological interventions, suggests that different or multiple mechanisms might be at play when observing (temporary) post-reactivation amnesia. Elsey, Van Ast, and Kindt (2018) have provided a range of control conditions that should (minimally) be met in order to infer the occurrence of reconsolidation. In the remainder of the paper we use a terminology that refers to the expected behavioral outcome (i.e., ‘post-reactivation amnesia’) without committing to a specific underlying mechanism.
In the past 20 years, post-reactivation amnesia has been demonstrated for various types of memory and in a variety of species, indicating its ubiquitous nature. After initial observations of post-reactivation amnesia induction for Pavlovian fear memories, this procedure was successfully applied to other types of aversive memories (e.g., inhibitory avoidance and conditioned taste aversion) (Gisquet-Verrier et al., 2015; but see Muravieva & Alberini, 2010), as well as appetitive memories (Milton, Lee, Butler, Gardner, & Everitt, 2008). Apart from demonstrations in rodents, post-reactivation amnesia has been established in slugs, chicks, crabs, fish and humans (Kindt, Soeter, & Vervliet, 2009; Sara, 2000; but see Bos, Beckers, & Kindt, 2014; Hardwicke, Taqi, & Shanks, 2016; Schroyens, Beckers, & Kindt, 2017; Thome et al., 2016). Studies in clinical populations have provided mixed results, emphasizing the necessity of a deeper understanding regarding the underlying mechanisms and conditions required for post-reactivation amnesia induction (Beckers & Kindt, 2017; Soeter & Kindt, 2015; Wood et al., 2015). To gain a better insight into these conditions on a neurobiological and behavioral level, we set out to establish post-reactivation amnesia in rodent contextual and cued fear conditioning.
Amnesia for contextual or cued fear memories in rodents has been observed after post-reactivation (systemic, intra-amygdala, or intra-hippocampal) administration of various pharmacological agents, e.g., protein synthesis inhibitors (e.g., anisomycin, rapamycin, cycloheximide) (Duvarci & Nader, 2004; Haubrich et al., 2015; Hoffman et al., 2015; Nader et al., 2000), NMDA receptor antagonists (e.g., MK-801) (Cassini, Flavell, Amaral, & Lee, 2017; Merlo, Milton, & Everitt, 2018; Przybyslawski & Sara, 1997), propranolol (PROP, a β-adrenergic receptor antagonist) (Dębiec & Ledoux, 2004; Przybyslawski et al., 1999), and midazolam (MDZ, a positive allosteric modulator of the GABA-A receptor) (Bustos, Maldonado, & Molina, 2006; Espejo, Ortiz, Martijena, & Molina, 2017; Ortiz, Giachero, Espejo, Molina, & Martijena, 2015). Non-pharmacological interventions, such as electroconvulsive therapy (Misanin et al., 1968; Schneider & Sherman, 1968; but see Dawson & McGaugh, 1969), hypothermia (Mactutus, Ferek, George, & Riccio, 1982; Mactutus, Riccio, & Ferek, 1979), conducting extinction training (Ferrer Monti et al., 2017; Monfils, Cowansage, Klann, & LeDoux, 2009) or presenting appetitive information shortly after or during CS re-exposure (Ferrer Monti et al., 2016; Haubrich et al., 2015; Ortiz, Molina, & Martijena, 2016), have also been shown to induce amnesia for fear memories. While there have been several reports of failures to successfully replicate the post-reactivation extinction procedure in rodents (e.g., Chan, Leung, Westbrook, & McNally, 2010; Ishii et al., 2015; Luyten & Beckers, 2017; for a meta-analysis, see Kredlow, Unger, & Otto, 2016), the existing literature suggests that pharmacologically-mediated post-reactivation amnesia is a more consistent and robust finding.
With the ultimate goal of obtaining a robust protocol that could be used to investigate constraints on and opportunities of the clinical application of post-reactivation amnesia, we performed a series of experiments aiming to conceptually or exactly replicate published studies using systemic drug administration after unreinforced CS re-exposure in rats. The results of our replication attempts involving contextual fear memories are reported in the current paper. Those involving cued fear conditioning are reported elsewhere (Luyten et al., in prep). In order to induce post-reactivation amnesia for contextual fear memories, we used a standard behavioral protocol. At least 24 hours after conditioning, rats were briefly re-exposed to the conditioning context, followed by systemic administration of vehicle or amnestic agent(s). Fear memory retention was assessed 24 h later. Given that our original project aimed to focus on the clinical relevance of post-reactivation amnesia, we limited ourselves to systemic administration of commonly-used and non-invasive drugs that can be safely used in humans as well (except for one study, in which we also injected a drug that directly interferes with protein synthesis (i.e., cycloheximide)). Published studies from other labs have reported robust amnestic effects using similar or identical protocols and midazolam (see Table 1) or propranolol (see Table 2) are commonly used as amnestic agents. Although there are several reports of conditions in which post-reactivation amnesia for contextual fear memories does not occur (e.g., using strong training conditions, with stress induction prior to learning, or depending on the length of the reactivation session; Alfei, Ferrer Monti, Molina, Bueno, & Urcelay, 2015; Bustos, Maldonado, & Molina, 2009; Cassini et al., 2017; Espejo, Ortiz, Martijena, & Molina, 2016; Lee & Flavell, 2014), there are currently no publications of failures to replicate amnesia when using a standard contextual fear conditioning paradigm and drug injection/infusion after unreinforced re-exposure to the conditioned context in rodents.
Table 1. Twenty-nine experiments (until April 2019) from 15 different papers reporting amnestic effects of post-reactivation midazolam (MDZ) administration for contextual fear conditioning in adult rats (in chronological order based on publication date).
- IFEC-CONICET, Departamento de Farmacología, Facultad de Ciencias Químicas, Universidad Nacional de Córdoba, Córdoba, Argentina.
- School of Psychology, University of New South Wales, Sydney, Australia.
- Department of Pharmacology, Federal University of Santa Catarina, Florianópolis, Santa Catarina, Brazil.
- Laboratorio de Psicología Experimental, Facultad de Psicología, Universidad Nacional de Córdoba, Córdoba, Argentina.
- Department of Neuropsychopharmacology, National Institute of Mental Health, National Center of Neurology and Psychiatry, Tokyo, Japan.
| Publication | Lab | Exp. | Fig. | Duration reactivation session | MDZ dose | Ntotal | Effect size (Cohen’s d) |
|---|---|---|---|---|---|---|---|
| Bustos et al. (2006) | I | 1B | 2B | 90 s | 1 mg/kg | 16 | 2.23 |
| Bustos et al. (2006) | I | 2A | 3B | 90 s | 1 mg/kg | 18 | 2.75 |
| Bustos et al. (2006) | I | 3B | 7B | 90 s | 1 mg/kg | 16 | 4.87 |
| Zhang & Cranney (2008) | II | 1 | 1 | 90 s | 2 mg/kg | 18 | .96 |
| Zhang & Cranney (2008) | II | 3 | 3 | 90 s | 2 mg/kg | 16 | 1.83 |
| Bustos et al. (2009) | I | NA | 1C | 3 min | 1.5 mg/kg | 14 | 3.87 |
| Bustos et al. (2009) | I | NA | 1D | 5 min | 1.5 mg/kg | 20 | 3.08 |
| Bustos et al. (2009) | I | NA | 3B | 3 min | 1.5 mg/kg | 14 | 3.35 |
| Bustos et al. (2010) | I | 1 | 1B | 3 min | 1.5 mg/kg | 14 | 2.62 |
| Bustos et al. (2010) | I | 1 | 1B | 3 min | 3 mg/kg | 14 | 5.74 |
| Bustos et al. (2010) | I | 1 | 1C | 5 min | 1.5 mg/kg | 18 | 2.50 |
| Bustos et al. (2010) | I | 1 | 1C | 5 min | 3 mg/kg | 18 | 2.47 |
| Stern et al. (2012) | III | 1 | 1A | 3 min | 1,5 mg/kg | 14-24 | 1.49 |
| Piñeyro et al. (2013) | IV | 1 | 1C | 4 min | 3 mg/kg | 12 | 3.82 |
| Piñeyro et al. (2013) | IV | 1 | 1C | 5 min | 3 mg/kg | 12 | 3.21 |
| Alfei et al. (2015) | IV | 5 | 5A | 2 min | 3 mg/kg | 18 | 1.80 |
| Alfei et al. (2015) | IV | 6 | 6B | 5 min | 3 mg/kg | 14 | 2.77 |
| Ortiz et al. (2015) | I | 1 | 1C | 3 min | 3 mg/kg | 19 | 3.37 |
| Ortiz et al. (2015) | I | 1 | 1E | 5 min | 3 mg/kg | 12 | 4.46 |
| Ortiz et al. (2015) | I | 2 | 3C | 5 min | 3 mg/kg | 16 | 4.33 |
| Ferrer Monti et al. (2016) | IV | 1 | 1C | 4 min | 3 mg/kg | 14 | 2.98 |
| Espejo et al. (2016) | I | 1 | 1 | 5 min | 3 mg/kg | 15 | 4.05 |
| Espejo et al. (2016) | I | 2 | 2 | 5 min | 3 mg/kg | 22 | 3.03 |
| Saitoh et al. (2017) | V | NA | 2B | 3 min | 1 mg/kg | 24 | .88 |
| Ferrer Monti et al. (2017) | IV | 2 | 2B | 2 min | 3 mg/kg | 12 | 3.88 |
| Espejo et al. (2017) | I | 1 | 1 | 5 min | 3 mg/kg | 16 | 3.49 |
| Espejo et al. (2017) | I | 3 | 3 | 5 min | 3 mg/kg | 18 | 3.91 |
| Akagi et al. (2018) | V | NA | 2C | 3 min | 1 mg/kg | 30 | .87 |
| Franzen et al. (2019) | III | NA | 1B | 2 min | 3 mg/kg | 17 | 2.45 |
Table 2. Eight experiments (until April 2019) from 7 different papers reporting amnestic effects of post-reactivation propranolol (PROP) administration for contextual fear conditioning in adult rats (in chronological order based on publication date).
- Laboratory of Learning and Memory, Department and Research Center of Physiology, School of Medicine, Semnan University of Medical Sciences, Semnan, Iran
- Department of Psychology, Swarthmore College, Swarthmore, USA
- IFEC-CONICET, Departamento de Farmacología, Facultad de Ciencias Químicas, Universidad Nacional de Córdoba, Córdoba, Argentina.
- Centre for Clinical Brain Sciences, Centre for Cognitive Ageing and Cognitive Epidemiology, University of Edinburgh, UK
| Publication | Lab | Exp. | Fig. | Duration reactivation session | PROP dose | Ntotal | Effect size (Cohen’s d) |
|---|---|---|---|---|---|---|---|
| Abrari et al. (2008) | I | 2 | 2B | 90 s | 5 mg/kg | 20 | .97 |
| Taherian et al. (2014) | I | 2 | 2B | 90 s | 10 mg/kg | 24 | 1.30 |
| Schneider et al. (2014) | II | 2 | 3 | 2 min | 10 mg/kg | 16 | 2.26 |
| Ortiz et al. (2015) | III | 1 | 1G | 5 min | 10 mg/kg | 19 | 4.05 |
| Ortiz et al. (2015) | III | 2 | 3E | 5 min | 10 mg/kg | 14 | 3.61 |
| Espejo et al. (2017) | III | 2B | 2F | 5 min | 10 mg/kg | 16 | 3.17 |
| Adbullahi et al. (2018) | I | NA | 5B | 90 s | 5 mg/kg | 12 | 1.19 |
| Wang (2018) | IV | 3 | 6E | 90 s | 10 mg/kg | 16 | 1.68 |
In a series of 25 conceptual replication attempts, we varied properties of the training and reactivation session and used several amnestic drugs (MDZ, PROP, and/or cycloheximide) and doses. In one experiment, one of the amnestic drugs, MDZ, was administered before the reactivation session. In some experiments, D-cycloserine was administered before the reactivation session in an attempt to boost memory destabilization (Bustos, Giachero, Maldonado, & Molina, 2010; Lee, Gardner, Butler, & Everitt, 2009). In addition, across experiments, there were variations in the rat strain, amount of handling prior to conditioning, use of cage enrichment, time interval between training and reactivation session, laboratory in which the experiment was performed, and researcher who performed the experiment. We also performed 6 exact replication attempts, in which the methodology of prior reports was followed as precisely as possible after detailed consultation with the authors of these studies (Alfei et al., 2015; Ferrer Monti et al., 2017; Stern, Gazarini, Takahashi, Guimarães, & Bertoglio, 2012).
2. Materials and Methods
2.1. Preregistration
For some of the current experiments, the adopted study protocols and performed statistical analyses were preregistered on aspredicted.org. The preregistration forms, as well as all raw data and results of preregistered analyses, can be found on the Open Science Framework (OSF) (Schroyens, Alfei, Luyten, & Beckers, 2019). For some studies (i.e., JA01-JA05, JA08), a larger sample size of 8 rats per group was preregistered but not reached given the absence of a promising trend in the first batch of 4-6 animals.
2.2. Subjects
Depending on the experiment, male Sprague-Dawley or Wistar rats (Janvier Labs, Le Genest-Saint-Isle, France) were used. An overview of the rat strain and sample size for each experiment is provided in Appendix A. All experiments were approved by the KU Leuven animal ethics committee (in accordance with the Belgian Royal Decree of 29/05/2013 and European Directive 2010/63/EU). Animals were housed in groups of 3-4 rats per cage with food and water available ad libitum on a 12 h/12 h day-night cycle (lights on at 8 am). Experiments were carried out between 9 am and 6 pm. Cage enrichment, in the form of a tunnel hanging from the top grid, was provided in most of the experiments.
2.3. Habituation
In most – but not all – experiments, rats were habituated to handling before the start of the experiment. The amount of handling ranged between 0 and 20 min, spread over 3 to 5 days (see Appendix A). In experiment NS11, rats were not handled before conditioning but they were habituated to the training context during a 3-min familiarization session on the day prior to conditioning, in line with Stern et al. (2012).
2.4. Drug Administration
Midazolam (MDZ, 1.5, 3, or 5 mg/ml, Accord Healthcare Limited or Mylan), propranolol (PROP, 10 mg/ml, Sigma-Aldrich), cycloheximide (CYCLO, 1.5 mg/ml, Sigma-Aldrich) and D-cycloserine (DCS, 15 mg/ml, Sigma-Aldrich) were diluted/dissolved in sterile saline (SAL, 0.9%, w/v) (Espejo et al., 2017). Drugs (or equivalent amounts of SAL) were injected intraperitoneally (IP) at a volume of 1 ml/kg, except for CYCLO, which was administered subcutaneously. A volume of 2 ml/kg MDZ (5 mg/ml) was injected when a higher dose was used (experiment NS21, 10 mg/kg). Rats were semi-randomly assigned to treatment groups, with the restrictions that (1) each home cage contained at least one rat of each group, (2) rats from one group were not always tested in the same of four identical boxes during the behavioral sessions, (3) average post-shock freezing (%) during the conditioning session was comparable between groups.
2.5. Apparatus
Four Med Associates boxes (30.5 cm (L) x 24.1 cm (W) x 21 cm (H)) with built-in ventilation fans (±67 dB) and illuminated by infrared and white light (level 5, 50 lux) were used. The experimental context consisted of a grid floor (19 rods of 4.8 mm diameter, 16 mm center to center) and a triangular-shaped black insert. Before and/or after each behavioral session, the contexts were cleaned with diluted cleaning product (5.55 % in water). The four boxes were used simultaneously for all rats of a given home cage. In experiment NS11, rats were run one by one in order to exactly replicate Stern et al. (2012).
2.6. Contextual fear conditioning
After introduction in the training context, one or multiple foot shocks were administered (inter-shock interval of 30 s). The duration of context exposure before administration of the first shock (i.e., pre-shock period), properties of the shock(s) (amount, intensity, duration) and post-shock period varied between experiments (see Appendix A).
2.7. Reactivation session
One day after training, rats were re-exposed to the training context without shock administration. The duration of the reactivation session varied between experiments (see Appendix A). In experiment NS10, there was an exceptional 72-h interval between the training and reactivation session, in line with Alfei et al. (2015) and Ferrer Monti et al. (2017). In experiments JA01 and JA02, shock administration during the reactivation session was manipulated between groups as outlined below in the procedure section.
2.8. Test session
One day after the reactivation session, rats were again exposed to the training context without shock administration. The duration of the test session varied between 5 and 30 min. In order to obtain a uniform overview of data from all studies, average % freezing during the first 5 min of the test session was considered in order to assess fear memory retention.
2.9. Procedure
Upon arrival in the lab, rats were left undisturbed in their home cages to acclimatize for 1 to 15 days prior to handling (or prior to the start of the fear conditioning protocol in case no handling was performed, see Appendix A). Depending on the experiment, rats were not, briefly, or extensively habituated to handling (see Appendix A). Below, experiments are grouped based on similarities in their design and/or research question.
2.9.1. Eighteen attempts to induce amnesia using a standard 3-day contextual fear conditioning protocol and drug injection after memory retrieval
Twenty-four hours after contextual fear conditioning (or 72 h in case of NS10), the fear memory was reactivated by brief non-reinforced re-exposure to the training context (experiments NS01A-NS07, NS10, NS13, NS14, NS21, and JA03-JA08). The amnestic agent (MDZ and/or PROP) or SAL was administered immediately after context re-exposure. Twenty-four hours later, fear memory retention was assessed during a 5-min exposure session to the training context. In experiment NS03, an additional group received no injection to examine the influence of the injection procedure itself.
2.9.2. Three attempts to induce post-reactivation amnesia using a protocol with multiple training days
The procedure of these experiments (NS08, NS17, and NS20) was similar to what was described in the previous paragraph, except that multiple sessions of contextual fear conditioning took place. The aim of including multiple training sessions was to establish a stronger context-shock association. We hypothesized that the implementation of multiple training episodes would lead to greater discrepancy between the training experiences and the non-reinforced reactivation session, resulting in a larger prediction error. In addition, we aimed to prevent the between-session decline in freezing that was observed in the SAL groups in most of our preceding experiments.
2.9.3. Six exact replication attempts of published studies reporting impaired contextual fear memory retention after post-reactivation MDZ administration
Experiments JA09, JA10, JA11, JA12, and NS09 were exact replications of studies reported by Alfei et al. (2015) and Ferrer Monti et al. (2017) (see Table A.2 (Appendix A)). Experiments JA11 and JA12 were performed in the same lab (i.e., Laboratorio de Psicología Experimental, Facultad de Psicología, Córdoba, Argentina), by the same researcher (i.e., JA) and using animals from the same breeding company as in previous successful studies. Experiment JA09 was performed by the same experimenter (i.e., JA) but in our lab at KU Leuven. To (partially) exclude the possibility that certain properties of our department’s lab environment or housing facilities prevented us from obtaining amnesia, experiment JA10 was carried out in a different lab at KU Leuven and no cage enrichment was used.
Experiment NS11 aimed to exactly replicate part of the first experiment described by Stern et al. (2012). Detailed descriptions of the protocol used in the original study were kindly provided by the authors and allowed us to replicate the study as closely as possible. Contextual fear conditioning (day 1), reactivation (day 2), drug administration, and testing (day 3) were performed as similarly as possible to the original study (see Table A.2 (Appendix A)). Additionally, rats were tested one by one rather than in cohorts of 3 or 4, the light intensity of the testing lab in which the Med Associates boxes were located was reduced to 70 lux, the context was cleaned with a 10% ethanol-water solution, and cage enrichment was omitted during housing in our animal facility. All behavioral sessions took place between 2 pm and 4.30 pm. We performed a retention test of 5 min (as opposed to the 3-min test in the original study), allowing evaluation of % freezing during the first 3 min of this session (in line with Stern et al. (2012)) and during the complete 5-min session (in line with our other experiments). We pre-planned to perform an additional retention test in context B but only if we observed a group difference during the retention test in context A.
2.9.4. Variations on exact replication attempts: Modifying the reactivation duration and drug dose
Importantly, some of our studies (i.e., NS10, NS12-NS14, NS18, NS21) include variations on the procedures used by Alfei et al. (2015) and Ferrer Monti et al. (2017). Experiments NS10 and NS14 adopted the same training parameters as in the original studies, but included variations in reactivation duration, with longer (i.e., 3 min rather than 2 min) and shorter (i.e., 1 min) sessions, respectively (see Table A.1 (Appendix A)). In other studies, we adopted the same training and reactivation parameters as in the original studies, but injected a higher dose of MDZ (i.e., 10 mg/kg, NS21), or injected MDZ, SAL, or DCS before the reactivation session (NS18, NS12, NS13, see below).
2.9.5. The influence of pre-reactivation MDZ administration on fear memory retention
In experiment NS18, we investigated whether MDZ administration 20 min before the reactivation session influenced fear memory retention during a 5-min test 24 h later (versus pre-reactivation SAL administration). The previously-mentioned standard 3-day protocol was used in this experiment.
2.9.6. The influence of pre-reactivation DCS administration on fear memory malleability
Previous research has shown that administration of the partial NMDA agonist D-cycloserine (DCS) before the reactivation session can allow destabilization of otherwise insusceptible fear memories (Bustos et al., 2010; Espejo et al., 2016; Espejo et al., 2017; Gazarini, Stern, Piornedo, Takahashi, & Bertoglio, 2015; Ortiz et al., 2015). Using our standard 3-day protocol, we investigated whether DCS administered 30 min before context re-exposure could enhance memory sensitivity to post-reactivation MDZ administration (NS12). As a control, SAL was administered 30 min before context re-exposure (SAL-MDZ versus DCS-MDZ), a condition in which we did not expect an amnestic effect based on the results of prior, similar studies in our lab. However, a relatively strong decrease in freezing from the reactivation to the test session was observed in the SAL-MDZ group, while such a decrease was not observed in previous studies without pre-reactivation SAL administration in which the same training and reactivation parameters were used (e.g., NS09). Therefore, SAL was administered after the reactivation session as well (SAL-SAL) and compared to SAL-MDZ in an additional experiment (NS13). In order to overcome the limitations of experiments NS12 and NS13, the effect of pre-reactivation DCS administration was investigated in experiment NS20, in which a SAL-SAL control group was included (SAL-SAL, SAL-MDZ, DCS-MDZ) and we adopted a protocol that should yield stable freezing levels from the reactivation to the test session (as in NS17, using 3 training days).
2.9.7. Manipulating prediction error during reactivation to achieve memory destabilization
Memory destabilization does not occur each time a memory is retrieved, and one factor that determines the outcome of a CS re-exposure session is the extent to which the information provided during this session deviates from what is expected based on previous learning (i.e., the amount of prediction error, PE) (Alfei et al., 2015; Díaz-Mataix, Ruiz Martinez, Schafe, LeDoux, & Doyère, 2013; Exton-McGuinness, Lee, & Reichelt, 2015; Pedreira, Pérez-Cuesta, & Maldonado, 2004; Sevenster, Beckers, & Kindt, 2014). What constitutes an optimal degree of PE for inducing destabilization may differ for different types of memory (Alfei et al., 2015; Suzuki, 2004). For example, Díaz-Mataix et al. (2013) have shown that strong fear memories can be destabilized by changing the time point at which the shock is administered during retraining (compared to initial learning). While the conditions of other experiments reported here implicated a negative PE during the reactivation session (no shock was administered during the reactivation session), experiments JA01 and JA02 aimed to induce either negative (no shock during the reactivation session) or positive PE (altered shock characteristics during the reactivation session relative to the initial conditioning session). PROP was administered immediately after the reactivation session and fear memory retention was assessed 24 h later during a 5-min retention test. There was no saline control group in these experiments, but a ‘no PE’ control group in which we did not expect to observe an amnestic effect of propranolol based on previous research (Alfei et al., 2015; Díaz-Mataix et al., 2013).
In experiment JA01, rats were exposed to the conditioning context for 3 min, after which 2 shocks of .5 mA were administered. Twenty-four hours after conditioning, the animals were either retrained using the same parameters as during initial training (no PE) or using a higher shock intensity (1 mA, positive PE). In the third group, the shock was omitted during context re-exposure (negative PE). Duration of the reactivation or ‘retraining’ session was equal to training in all conditions.
Experiment JA02 was similar to JA01, with slight alterations in training and reactivation parameters. During initial training, only 1 shock was administered after 3 min, followed by a post-shock period of 30 s. Twenty-four hours after conditioning, the animals were either retrained using the same parameters as during initial training (no PE) or using 2 shocks (inter-shock interval and post-shock period = 15 s, positive PE). In the third group, the shock was omitted during context re-exposure (negative PE).
2.9.8. The effects of MDZ administration prior to fear conditioning and after extinction learning
Since post-reactivation MDZ administration did not affect fear memory retention in any of our experiments, we aimed to confirm the efficacy of this drug in interfering with other learning- or memory-related processes, such as extinction consolidation (Alfei et al., 2015; Bustos et al., 2009; Ferrer Monti et al., 2017) (NS14) and fear acquisition (NS15). In experiment NS14, MDZ was administered immediately after a 1- or 30-min reactivation session and fear memory retention was assessed 1 and 8 days later. In experiment NS15, MDZ was administered 20 min before contextual fear conditioning. The main aim of this study was to assess acute effects of MDZ on locomotor activity by using the motion index (MedAssociates software) during the 3-min baseline period (i.e., pre-shock period) of the training session. In addition, fear memory retention was assessed 24 h later.
2.10. Behavioral scoring
Percentage of time that the animals spent freezing was used as a measure of contextual fear. Freezing, a defensive response that is characterized by complete immobility apart from movements associated with breathing, was continuously scored from videos by one or two raters that were blinded to experimental conditions. The amount of freezing was expressed as a percentage of the total scoring period. Percentage freezing per minute was calculated as well in order to assess temporal patterns of contextual fear. For a subset of 8 experiments, average % freezing during the reactivation and test sessions was scored by two independent raters and interrater reliability was calculated (N = 220 observations). The obtained intraclass correlation coefficient (ICC; two-way mixed effects, single rater, absolute agreement) of .972, 95% CI [.964; .979] indicated excellent reliability (Koo & Li, 2016).
2.11. Statistical analyses
Statistical analyses reported in the current paper can deviate from preregistered analyses, because we chose to provide a consistent and encompassing overview of all experiments’ results. Nevertheless, preregistered analyses were performed as well and results can be found on OSF (Schroyens, Alfei, et al., 2019). Importantly, those analyses yielded the same general conclusions as the ones reported here. R (version 3.3.2) was used for statistical analyses and creation of the graphs (R Foundation, 2016).
Rats were excluded if freezing during the reactivation session was lower than 25%. This predefined criterion aimed to exclude rats that did not sufficiently acquire the context-shock association, because this could hamper the investigation of memory interference. In experiment NS11, rats showing less than 35% freezing during the reactivation session were excluded, in line with the original study by Stern and colleagues. All analyses were performed with the aforementioned exclusion criteria, as well as with all rats included.
One-sided t-tests were performed to investigate whether post-reactivation administration of the amnestic agent impaired fear memory retention, manifested as lower % freezing during the retention test compared to vehicle-treated rats. Two-sided t-tests were performed to assess whether freezing levels during the reactivation session were comparable between treatment groups (i.e., before the treatment was given). In case of between-group differences during the reactivation session, mixed ANOVAs with within-subjects factor Session (reactivation versus test) and between-subjects factor Treatment (amnestic agent versus SAL) were performed to assess whether the amnestic treatment affected the change in freezing from the reactivation to the test session.
Bayesian meta-analyses using t-values of the one-sided t-tests were performed for MDZ and PROP separately in order to quantify obtained support for the alternative hypothesis (HA, MDZ < SAL or PROP < SAL) or the null hypothesis (H0, MDZ = SAL or PROP = SAL) (Rouder & Morey, 2011). BF10 indicates how likely the obtained data are under HA, relative to H0. For example, while a BF10 of 2 indicates that the data are 2 times more likely to occur under HA than under H0, a BF10 of .5 indicates that the data are 2 times more likely to occur under H0 than under HA. Thus, if BF10 is larger than 1, the data provide evidence in favor of an effect (i.e., HA) and if BF10 is smaller than 1, the data provide evidence for the absence of an effect (i.e., H0). The BayesFactor package in R was used to calculate BFs, and the default Cauchy prior on the standardized effect size with a scale of .707 was adopted. BFs are interpreted using the labels proposed by Jeffreys (Jeffreys, 1961; Wetzels et al., 2011).
One-way ANOVAs were used in experiments JA01 and JA02 (factor ‘PE’), and experiment NS15 (factor ‘Treatment’). A two-way ANOVA with factors ‘Reactivation Duration’ (1 min versus 30 min) and ‘Treatment’ (post-reactivation SAL or MDZ) was used for experiment NS14. Significant ANOVAs were followed-up by Tukey’s HSD post-hoc tests.
3. Results
Appendix B contains an overview of descriptive statistics (sample size, mean, SD) and results of statistical analyses (t-value, p-value, Cohen’s d, and BF10) for each experiment in which MDZ or PROP was administered after re-exposure to the conditioning context (Table B.1 and Table B.2, respectively). Figures 1 and 2 provide an overview of freezing during the test session of all experiments in which we aimed to induce amnesia. Detailed graphical representations of all studies are shown in Appendix C (average % freezing during the reactivation and the test session) and Appendix D (% freezing per minute during the reactivation and test session).
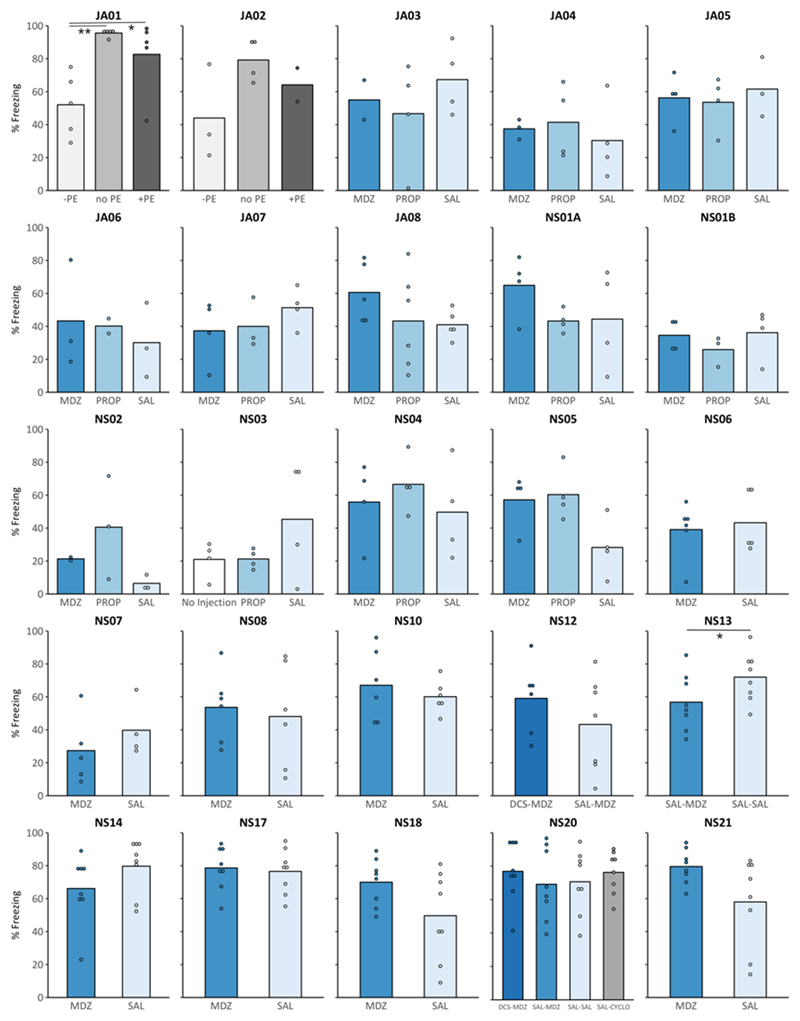
Figure 1. In a series of conceptual replication attempts, % freezing in rats treated with midazolam (MDZ, 3 or 10 mg/kg), propranolol (PROP, 10 mg/kg), or cycloheximide (CYCLO, 1.5 mg/kg) was not significantly lower than in the appropriate controls (% freezing at test).
In most experiments, control rats received saline (SAL) systemically after the reactivation session (or 20 min before in case of experiment NS18). In experiments JA01 and JA02, control rats received PROP after a reactivation session without prediction error (PE), while rats in the experimental groups received PROP after a reactivation session with positive or negative PE (*p < .05, BF10 < 3; **p = .004; BF = 27.32). In experiments NS12 and NS20, D-cycloserine (DCS, 15 mg/kg) or SAL was administered 30 min before the reactivation session. Exceptionally, in experiment NS13, freezing in MDZ rats was lower compared to SAL rats, but there was already a tendency of MDZ rats to freeze less during the reactivation session (see Appendix C for an overview of freezing scores during the reactivation and test session).
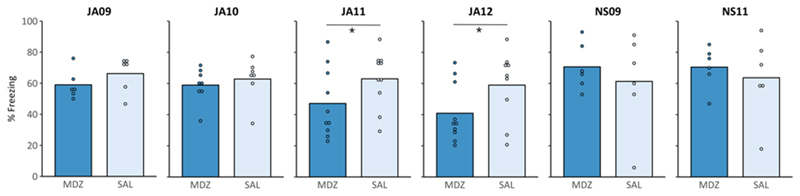
Figure 2. Six exact replication attempts provide no clear evidence for post-reactivation amnesia.
Experiments JA09-JA12 and NS09 followed the methodology of Alfei et al. (2015) and Ferrer Monti et al. (2017) as closely as possible, while experiment NS11 followed the methodology of Stern et al. (2012). When the experiments were performed by the same researcher, in the same lab space, and using rats from the same breeding company as in the original studies (JA11, JA12), % freezing in MDZ rats was significantly lower during the retention test (*p < .05, BF10 < 3, compared to SAL). However, the observed effect was smaller than in the original studies. On the other hand, when the same (JA09, JA10) or another (NS09) researcher performed the experiments in two other lab spaces using different equipment and rats from another breeding company, the results could not be replicated, as there was no evidence for a difference between SAL and MDZ rats at test (see Appendix C for an overview of freezing scores during the reactivation and test session).
3.1. Twenty-one failures to conceptually replicate amnesia for contextual fear memories by post-reactivation drug injection
In a series of 21 experiments, propranolol (PROP) and/or midazolam (MDZ) (versus saline, SAL) were administered after non-reinforced re-exposure to the conditioning context (Appendix C, Fig. C.1). MDZ was used as (one of) the amnestic agent(s) in 20 of these experiments (experiment NS03, in which PROP was compared to SAL or no injection, was the one exception). PROP was used in 12 of these experiments. One-sided t-tests (amnestic agent < SAL) suggested that there was no evidence for an amnestic effect of post-reactivation MDZ or PROP during the retention test in any of the performed experiments, except for experiment NS13 (Appendix B, Table B.1 and Table B2). Note that there already was a numerical difference in freezing between SAL rats (M = 82.19, SD = 8.95) and MDZ rats (M = 68.23, SD = 17.49) during the reactivation session in this experiment.
As shown in Table B.1 and B.2 (Appendix B), the sample sizes in the current studies were rather small (especially after applying the predefined exclusion criterion), varying from two to eight (typically four) rats per treatment condition. These small sample sizes were adopted to minimize the number of animals used in each of the experiments, and the initial (and pre-registered) goal was to increase sample size by repeating an experiment only if a promising trend was observed (see preregistration experiment NS01A). In addition, the effect sizes obtained in successful reports of post-reactivation amnesia for contextual fear are large (Cohen’s d ranging from .87 to 5.74) (Table 1 and 2), requiring 2 to 18 rats per group for sufficient power to statistically detect such an effect (calculated using G*Power, version 9.1.9.3; Faul, Erdfelder, Lang, & Buchner, 2007). Still, with such small sample sizes, failures of individual experiments may be relatively meaningless. To allow for more robust conclusions, we combined the data from comparable experiments (i.e., using the same parameters for the training and reactivation session, same drug, dose and time of administration), and exploratively performed statistical analyses on these samples. Combining the results of experiment JA07 and JA08 yielded a total of 9 SAL and 9 MDZ rats. A one-sided t-test showed no effect of treatment (t(16) = .58, p = .716, d = .27) and Bayesian analysis suggested substantial evidence for the absence of an amnestic effect (BF10 = .30). Likewise, in experiments JA05, JA06, and NS04 the same procedures were used (although these experiments were carried out by different researchers). Combining these 3 experiments yielded a total of 12 SAL and 12 MDZ rats, of which 2 SAL and 1 MDZ rats were excluded due to freezing levels of <25% during the reactivation session. Again, a one-sided t-test suggested no effect of treatment (t(19) = .50, p = .690, d = .22) and Bayesian analysis suggested substantial evidence for the absence of an amnestic effect (BF10 = .29).
To conclude, we obtained no evidence for the induction of amnesia by post-reactivation systemic MDZ or PROP administration in a series of 21 experiments, despite using a typical 3-day fear conditioning paradigm in most of these studies. These findings are surprising given the abundance of publications reporting amnesia for contextual fear memories after systemic post-reactivation administration of MDZ or PROP in rats (Table 1 and 2), and suggest that the success of this manipulation may depend on subtle and poorly understood variations in certain parameters.
3.2. Six attempts to exactly replicate post-reactivation amnesia induction for contextual fear memories
Apart from conceptual replication attempts, we performed several studies in which we followed the methodology of successful studies from other labs as closely as possible. In these experiments, none of the rats met the predefined exclusion criteria, so all rats were included.
First, experiments JA11 and JA12 constituted exact replications of the procedures reported in Alfei et al. (2015, experiment 5) and Ferrer Monti et al. (2017, experiment 2). These experiments were performed in the same lab, by the same researcher, and using animals from the same strain and breeding company as in previous successful studies (Fig. 2 and Appendix C, Fig. C2.D,E). A one-sided t-test (MDZ < SAL) revealed a significant effect on memory retention (JA11: t(18) = -1.76, p = 0.048, d = -0.79; JA12: t(17) = -1.91, p = 0.037, d = -0.88), and the Bayesian analogue of this test provided anecdotal evidence for the presence of an amnestic effect (JA11: BF10 = 2.07; JA12: BF10 = 2.50). On the other hand, a two-sided t-test, as used in the original papers, failed to provide significant evidence for a difference in memory retention between SAL- and MDZ-treated rats (JA11: t(18) = -1.76, p = 0.096; JA12: t(17) = -1.91, p = 0.074). Although the effect sizes obtained in the present experiments are conventionally classified as large, they are markedly smaller than those reported in published studies (the latter ranging between 1.80 and 3.88).
Second, in experiments JA09, JA10, and NS09 we tried to replicate the experiments of Alfei et al. (2015) and Ferrer Monti et al. (2017) in two different labs at KU Leuven. One-sided t-tests provided no evidence for an amnestic effect of MDZ in any of the experiments (Table B.3). Bayesian analogues of these tests suggested anecdotal to substantial evidence for the absence of an amnestic effect (JA10, NS09) or anecdotal evidence in favor of an amnestic effect (JA09) (Table B.3). Using the smallest effect size obtained in published studies (d = 1.80 in experiment 5 of Alfei and colleagues), the power of our current studies to detect such an effect was between .89 and .96 (n = 6 or 8 rats per group, respectively; calculated using G*Power). However, considering the smaller effect size that was obtained in our recently performed replication studies (i.e., JA11 and JA12, see previous paragraph), 21 rats per condition are required in order to detect such an effect with a power of .80 (G*Power). We again combined the results of these studies (i.e., JA09, JA10, and NS09, in which the same drug, training and reactivation parameters are used), and exploratively performed statistical analyses on this sample. It should be noted that there are some methodological differences between these studies, including the experimenter who performed the study, the use/omission of cage enrichment, and the lab environment. None of these factors substantially affected the amnestic influence of MDZ, as suggested by the absence of an Experiment x Treatment interaction (F(2,33) = .87, p = .430, η2p = .05). After combining these studies (including 19 SAL and 20 MDZ rats), statistical analysis suggested that there was no evidence for an amnestic effect of MDZ (t(37) = -0.19, p = .427, d= -.06, BF10 = .36).
Third, in experiment NS11, an exact replication of Stern et al. (2012), freezing scores observed during the reactivation session were consistent with those obtained in the original paper, suggesting successful and similar fear acquisition. However, visual inspection of the graphs (Fig. 2 and Appendix C, Fig. C.2F) shows that SAL rats showed lower freezing at test compared to MDZ rats, a pattern opposite to the findings reported in the original paper. The one-sided t-test (MDZ < SAL) provided no evidence for an amnestic effect of MDZ (t(10) = .57, p = .709, d = .33) (Table B.3). The Bayesian analogue of this t-test suggested anecdotal evidence for the absence of an amnestic effect (BF10 = .34). Based on the effect size obtained in the original paper (d = 1.49, estimated from reported graphs), the adopted sample size of 6 rats per group in the current experiment yielded a power of .78 to detect such an effect (calculated using G*Power, version 9.1.9.3). Similar analyses as reported in the original paper were performed (and preregistered) as well (results are available on OSF at osf.io/m2png) (Schroyens, Alfei, et al., 2019). Results of these analyses also confirmed that we failed to replicate the results of the original paper.
Thus, in this series of exact replication attempts performed in two different labs at KU Leuven, we obtained no evidence for the induction of amnesia by post-reactivation MDZ administration, despite following the methodology of previous, successful studies as precisely as possible. In two exact replication attempts that were performed in the same lab as the original studies, we did find statistically significant effects of MDZ (Cohen’s d of .79 and .88), but the obtained effect sizes were smaller than those reported in prior publications (ranging between 1.80 and 3.88).
3.3. Variations on exact replication attempts: Modifying the reactivation duration or drug dose did not enable post-reactivation amnesia induction
In two of our conceptual replication attempts, experiments NS10 and NS14, we adopted the same training parameters as Alfei et al. (2015) and Ferrer Monti et al. (2017), but included variations in reactivation duration, with longer and shorter sessions, respectively. In another experiment (NS21), the same training and reactivation parameters as in the original studies were used, but a higher dose of MDZ was injected. None of these studies provided evidence for an amnestic effect of MDZ (Fig. 1).
3.4. Bayesian meta-analysis suggests substantial support for the absence of an amnestic effect
All meta-analytic Bayes factors are provided in Table 3. A Bayesian meta-analysis including all aforementioned studies in which MDZ was administered after the reactivation session, (i.e., 26 studies; 20 conceptual and 6 exact replications; Table B.1 and B.3), provided substantial support in favor of the null hypothesis (i.e., no amnestic effect of MDZ). When considering only conceptual replication attempts, the Bayesian meta-analysis suggested strong support for the absence of an amnestic effect of MDZ. A similar analysis on the six exact replication attempts (including 2 ‘successful’ studies) suggested mere anecdotal support for the presence of an effect. A Bayesian meta-analysis including all studies in which PROP was administered after the reactivation session (i.e., 12 studies; Table B.2) again yielded substantial support in favor of the null hypothesis.
Table 3. Results of Bayesian meta-analyses indicate that our data mostly provide clear support for the absence of an amnestic effect.
First, all performed studies are included, afterwards subdivided in conceptual or exact replication attempts (with or without exclusion of subjects showing less than 25% freezing during reactivation). BF10 quantifies evidence in favor of the HA (i.e., presence of an amnestic effect), relative to the H0 (i.e., absence of an amnestic effect). For clarity, BF01, quantifying evidence in favor of the H0 relative to the HA (= 1/BF10), is also provided.
| Studies included | # Experiments | N | HA | Excluded subjects | BF10 | BF01 |
|---|---|---|---|---|---|---|
| All | 26 | 295 | MDZ<SAL | Freezing <25% during reactivation session | .19 | 5.28 |
| All | 26 | 308 | MDZ<SAL | None | .17 | 5.83 |
| Conceptual | 20 | 205 | MDZ<SAL | Freezing <25% during reactivation session | .10 | 10.31 |
| Conceptual | 20 | 218 | MDZ<SAL | None | .09 | 11.03 |
| Exact | 6 | 90 | MDZ<SAL | None | 1.93 | .52 |
| Conceptual | 12 | 91 | PROP<SAL | Freezing <25% during reactivation session | .20 | 5.07 |
| Conceptual | 12 | 98 | PROP<SAL | None | .23 | 4.37 |
3.5. No influence of pre-reactivation MDZ administration on fear memory retention
When MDZ was administered 20 min before the reactivation session (experiment NS18), an acute effect of MDZ on freezing during the reactivation session was observed (versus SAL; t(7.74) = 3.32, p = 0.011, d = .55, BF10 = 8.56). Observed freezing levels after MDZ administration were difficult to interpret because MDZ rats were heavily sedated and locomotor activity was impaired in these rats (see also section 3.9). Since freezing scores during the retention test were not subject to these acute locomotion-impairing effects of MDZ, we did not perform the preregistered mixed ANOVA to assess the influence of MDZ treatment on the change in freezing from the reactivation to the test session. Rather, a two-sided t-test was performed in order to investigate whether pre-reactivation MDZ administration affected fear memory retention one day later. Although % freezing during the retention test was higher in MDZ rats (Appendix C, Fig. C.3B), the Welch’s t-test provided no evidence for an effect of MDZ versus SAL (t(10.71) = 1.9, p = 0.084, d = .35, BF10 = 1.33).
3.6. No influence of pre-reactivation DCS administration on fear memory malleability
In experiment NS12, rats received an IP injection of SAL or D-cycloserine (DCS) 30 min prior to the reactivation session and all rats received MDZ immediately after the reactivation session (Fig. C.4, Appendix C). A one-sided t-test (DCS-MDZ < SAL-MDZ) provided no evidence for lower freezing in the DCS-MDZ group during the retention test (t(11) = 1.10, p = .852, d = .62) and Bayesian analysis suggested substantial evidence for the null hypothesis (BF10 = .27). In an additional experiment (i.e., NS13), there was no clear evidence for a difference in fear memory retention between SAL-MDZ or SAL-SAL rats (see results of experiment NS13 in section 3.1). In experiment NS20, there was no amnestic effect of post-reactivation MDZ, regardless of pre-reactivation DCS administration. One-sided t-tests suggested that post-reactivation MDZ administration did not impair fear memory retention (SAL-MDZ < SAL-SAL; t(14) = -0.15, p = 0.443, d = -0.07, BF10 = 0.47), and that DCS was not able to enhance memory susceptibility (DCS-MDZ < SAL-MDZ: t(14) = 0.77, p = 0.774, d = 0.39, BF10 = 0.28).
3.7. Inducing a positive prediction error during reactivation does not allow for the induction of post-reactivation amnesia
In experiment JA01, animals were either retrained using the same parameters as during initial training (no prediction error, ‘no PE’), retrained using a higher shock intensity (+PE), or re-exposed to the conditioning context without shock (-PE) (Fig. C.5, Appendix C). The ANOVA revealed that characteristics of the reactivation session (no PE, +PE, -PE) before PROP administration influenced fear memory retention 24 h later (F(2,12) = 8.30, p = .005, ηp2 = .58, BF10 = 8.93). Tukey’s post-hoc tests showed that a non-reinforced reactivation session (-PE) resulted in lower freezing during the retention test, compared to rats that experienced no PE (.5-mA shock) (q(12) = 3.97, p = .004, d = 3.19, BF10 = 27.32) or a positive PE (1-mA shock) (q(12) = 2.79, p = .040, d = 1.44, BF10 = 1.82) before PROP administration. Lower freezing in the -PE group might have resulted from the fact that these rats did not experience any shock during the reactivation session rather than reflecting a memory-interfering effect of PROP. The latter interpretation is supported by the observation that the experience of a positive PE before PROP administration did not induce an impairment in memory retention, compared to no PE (q(12) = 1.18, p = .487, d = .79, BF10 = .78).
In experiment JA02, 3 rats were excluded due to low freezing scores during the reactivation session on day 2 (<25%). The ANOVA provided no clear evidence for an effect of PE during the reactivation session on performance during the retention test (F(2,6) = 2.68, p = .147, ηp2 = .47, BF10 = 1.22). It should be noted that, when the complete sample was used, there was an effect of PE on % freezing during the retention test (F(2,9) = 5.17, p = .032, ηp2 = .53, BF10 = 2.65), and Tukey’s post-hoc tests showed that the difference between -PE and no PE was significant (q(9) = 3.17, p = .028, d = 1.92, BF10 = 2.38).
In any case, based on these 2 experiments, it can be concluded that the experience of a positive PE during the reactivation session (induced by altering the strength or number of shocks during the reactivation session, with respect to initial training) did not allow for memory-interfering effects of post-reactivation PROP administration.
3.8. No influence of MDZ on extinction consolidation
Visual inspection of Fig. C.6B (Appendix C) shows that the 30-min exposure session successfully induced extinction, but that MDZ did not affect fear memory retention when administered after either the brief or long reactivation session (experiment NS14). These observations were confirmed by a two-way ANOVA showing a main effect of Reactivation duration (F(1,28) = 26.83, p < .0001, ηp2 = .49, BF10 = 809.63), but no main effect of Treatment (F(1,28) = 3.73, p = .064, ηp2 = .12, BF10 = .72) and no Treatment x Reactivation duration interaction (F(1,28) = .01, p = .91, ηp2 = .0005, BF10 = 0.42).
3.9. Effects of MDZ on locomotion and contextual fear memory acquisition
Administration of MDZ prior to contextual fear conditioning reduced locomotor activity during the 3-min baseline period of the training session (Appendix C, Fig. C.7B) and resulted in lower fear memory expression during the retention test one day later (versus SAL, experiment NS15) (Appendix C, Fig. C.7C). The ANOVA showed an effect of Treatment (SAL, 1.5 mg/kg MDZ, or 3 mg/kg MDZ) on locomotor activity during the baseline period (F(2,21) = 7.60, p = .003, ηp2 = .42, BF10 = 13.25). Tukey’s post-hoc tests found a significant difference between SAL and MDZ3 (q(21) = 3.85, p = .003, d = 2.07, BF10 = 29.39), and a marginally significant difference between SAL and MDZ1.5 (q(21) = 2.47, p = .055, d = 1.41, BF10 = 4.17), but there was no evidence for a difference between MDZ1.5 and MDZ3 (q(21) = 1.38, p = .369, d = .59, BF10 = .68). These results indicate that MDZ induced an acute impairment in locomotor activity. Pre-training administration of MDZ also impaired freezing during a retention test performed 24 h after training (F(2,21) = 6.36, p = .007, ηp2 = .38, BF10 = 7.48). Tukey’s post-hoc tests revealed a difference between SAL and MDZ1.5 (q(21) = 2.55, p = .047, d = 1.34, BF10 = 3.46) or MDZ3 (q(21) = 3.44, p = .006, d = 1.96, BF10 = 21.03).
4. Discussion
The ultimate goal of our experiments was to establish a protocol that could be used to investigate (and overcome) boundary conditions for post-reactivation amnesia induction. To this aim, we set out to conceptually or directly replicate previous studies in which systemic, post-reactivation administration of midazolam (MDZ, see Table 1), propranolol (PROP, see Table 2), or cycloheximide (CYCLO; Haubrich et al., 2015) resulted in amnesia for contextual fear memories. In most of the experiments reported here, we adopted a procedure that conformed to the standard 3-day protocol typically used in rodent studies. Given the existing literature, which contains a plethora of successful studies with large effect sizes and not a single failure to replicate, it is surprising that we failed to obtain clear evidence for post-reactivation amnesia using systemic administration of drugs that have been shown to be effective for several forms of memory, including contextual fear memory (Dębiec & Ledoux, 2004; Flint & Marino, 2007; Haubrich et al., 2015; Milton, Lee, & Everitt, 2008; Robinson & Franklin, 2010; Taubenfeld, Riceberg, New, & Alberini, 2009). Of note, we administered all drugs systemically, and our findings should not be generalized to effects obtained with intracerebral drug infusions.
The absence of amnestic effects in our studies may result from one (or a combination) of several factors (Reichelt & Lee, 2013). First, the amnestic agents (i.e., MDZ, PROP, and CYLCO) might have been ineffective at interfering with the destabilized memory trace. Second, the (extensive set of) behavioral parameters that we adopted might have been inappropriate for achieving memory destabilization. Instead, the adopted reactivation parameters could have induced a different mnemonic process, such as mere retrieval or extinction. Third, the contextual fear memories that we installed might have been impervious to destabilization, or at least insensitive to destabilization under conditions that have been commonly used in the past. Each of these possible explanations is addressed below in the light of the experiments performed and the results observed.
In order to examine whether MDZ could have been (in)effective at memory interference, we assessed the influence of MDZ on other learning and memory processes. First, MDZ (1.5 or 3 mg/kg) was administered 20 min before contextual fear conditioning, resulting in lower locomotor activity during the 3-min pre-shock period of the training session, and lower fear memory expression during the retention test (compared to SAL). Second, MDZ was administered after extinction, in line with prior publications showing that MDZ can interfere with extinction consolidation (Alfei et al., 2015; Bustos et al., 2009; Ferrer Monti et al., 2017; Franzen, Giachero, & Bertoglio, 2019). We adopted training parameters from Alfei et al. (2015) and Ferrer Monti et al. (2017) and administered MDZ (or SAL) after a 30-min re-exposure session, but did not replicate their results, suggesting that MDZ did not affect extinction consolidation. These findings may suggest decreased memory sensitivity to MDZ in our animals. In line with this hypothesis, it has been shown that benzodiazepine binding to GABAA receptors can be altered by experiences during early life or adulthood (Mody & Maguire, 2012; Skilbeck, Johnston, & Hinton, 2010). Since subtle changes in the environment can affect the development of the GABAergic system, differences in these experience-induced changes between different laboratories may account for the discrepant findings concerning MDZ’s memory-interfering effects. On the other hand, we can presumably exclude the possibility that ineffectiveness of the drug can count as the sole explanation for our failure to induce post-reactivation amnesia, since we were also unable to observe post-reactivation amnesia when systemically administering other drugs that had been used successfully in the past (i.e., PROP or CYCLO), or using a dose of MDZ that was more than three times higher than the dose commonly used to induce amnesia. As indicated above, we found acute effects of MDZ on locomotor activity. In addition, other control experiments performed in our lab confirmed behavioral effects of PROP and CYCLO. More specifically, we found that injection of PROP impaired freezing to a conditioned tone (compared to saline), illustrating its acute effect on fear memory expression, and another study revealed that systemic injection of CYCLO after extinction of a cued fear memory could impair extinction retention. These control studies, illustrating the effectiveness of our drugs in disrupting other fear-related processes, were performed in the light of a series of studies aiming to induce post-reactivation amnesia for cued fear memories, and are described in detail elsewhere (Luyten et al., in prep).
Second, the current failures to induce post-reactivation amnesia could be attributed to the fact that the adopted behavioral parameters were inappropriate for destabilizing the reactivated memory trace. However, this explanation seems unlikely since we have adopted a wide variety of parameters that have been successful in the past. The current experiments used similar (and sometimes identical) training parameters as in previous successful studies and the observed freezing scores during the reactivation session are in line with those obtained in the original studies (except in experiments replicating Alfei et al. (2015), in which freezing during the reactivation session was around 70%, compared to around 50% in the original studies). In addition, we implemented a wide range of reactivation durations (including 1, 2, 3, 4, or 5 min), covering the reactivation durations used in previous reports (see Table 1 and Table 2).
In two of our experiments, we attempted to pharmacologically enhance memory destabilization. NMDA receptor activation plays a crucial role in memory destabilization: pre-reactivation administration of the NMDA receptor antagonist Ifenprodil can prevent the impairing effect of post-reactivation anisomycin (Mamou, Gamache, & Nader, 2006); conversely, administration of the NMDA partial agonist D-cycloserine (DCS) before the reactivation session allows destabilization of otherwise resistant contextual fear memories (Bustos et al., 2010; Espejo et al., 2016, 2017; Gazarini et al., 2015; Ortiz et al., 2015). We investigated whether DCS could boost destabilization and thereby enable memory interference with post-reactivation MDZ, but experiments NS12 and NS20 provided no evidence for an amnestic effect of MDZ when DCS was administered before the reactivation session.
A third potential explanation for our negative findings is that the fear memory trace was acquired or stored in such a way that it was impervious to destabilization, or that destabilization would require unusual reactivation parameters. Given that we have adopted a variety of behavioral parameters, covering the range of those successfully used by other labs but without apparent success in our hands, we previously hypothesized that the inability to replicate previous findings could result from factors that are beyond our control, such as the rats’ genetic background or experiences before conditioning (Schroyens, Bender, et al., 2019). Obvious differences between previous studies and our exact replication attempts constitute the vendor or breeding setting and the physical space in which the animals are housed and tested. Vendor-dependent aspects include rearing conditions and genotypic heterogeneity due to genetic drift (Theilmann et al., 2016), and laboratory-dependent differences include housing conditions and equipment. After a thorough comparison of rearing conditions at a number of the labs and breeders involved in experiments on post-reactivation amnesia, the use of cage enrichment appeared as a valid candidate factor in determining the success of post-reactivation interference. The role of cage enrichment in rendering fear memories insusceptible to post-reactivation MDZ administration was therefore investigated, and results of this study are reported elsewhere (Schroyens, Bender, et al., 2019). Unfortunately, we were unable to reproduce the amnestic effect of MDZ despite using the same behavioral parameters, pharmacological manipulation, and lab space as in prior successful studies (Espejo et al., 2016, 2017; Ortiz et al., 2015). These negative findings again underline the subtle nature of post-reactivation amnesia induction when using systemic MDZ administration, and moderators of the effectiveness of this manipulation remain elusive.
Overall, we hypothesized that the failure to induce post-reactivation amnesia could be attributed to (a combination of) the following factors: (1) the amnestic drugs were ineffective at inducing memory interference, (2) the behavioral parameters that we adopted were inappropriate for achieving memory destabilization, and/or (3) the acquired memory trace did not destabilize under standard conditions. Although we cannot entirely exclude the first possible explanation, this seems rather implausible given that we have used three different amnestic agents that had been shown effective in inducing post-reactivation amnesia in the past. Second, given that we adopted a wide variety of commonly used behavioral parameters that have been shown to be successful in other labs, the second possible explanation also seems unlikely. Rather, it seems that (unknown) conditions influenced fear memory malleability. Although we performed several studies to gain more insight into our negative findings, we were unable to identify which factor(s) might underlie the current results.
A possible limitation of most of the current experiments is that we observed a decrease in freezing from the reactivation session to the retention test in control rats (Appendix C) (see also Schroyens, Bender, et al., 2019), sometimes to an extent comparable to the magnitude of the amnestic effect observed in prior successful studies. The presence of such a decline in freezing in control rats might have prevented us from observing an amnestic effect in some studies, although in most of our experiments there was still room to observe an amnestic effect. Notably, other labs did find post-reactivation amnesia (with systemic MK-801 or MDZ) despite a significant between-session decrease in freezing in the control group (Cassini et al., 2017; Franzen et al., 2019). In addition, in several of our experiments there was still room to observe an amnestic effect despite the between-session decline in SAL rats, and in others (i.e., JA05, JA06, JA09, JA10, NS09, NS11, NS17, and NS21), there was no between-session decrease in freezing while amnesia was still not obtained, ruling out this possibility as the sole explanation for our null findings.
Although effect sizes of amnestic effects reported in the animal literature are large, another limitation of some of our experiments is the use of relatively small samples, implying that failures of these individual experiments may be relatively uninformative. To allow for more robust conclusions, we combined experiments in which the same amnestic drug and behavioral parameters were used (but carried out at different time points and sometimes by different researchers). These exploratory analyses, including 9 to 20 rats per treatment condition, provided no evidence for post-reactivation amnesia induction. In addition, two Bayesian meta-analyses that included all our studies involving post-reactivation administration of MDZ or PROP suggested substantial evidence for the absence of amnestic effects. Most remarkably, our exact replication attempts that should have had sufficient power according to sample size calculations based on the original papers, did not provide robust evidence for amnesia either.
We can conclude that our findings are in stark contrast with the existing literature on post-reactivation amnesia in rats, in which systemic administration of MDZ (Table 1), PROP (Table 2), or CYCLO (Haubrich et al., 2015) has been shown to induce amnesia for contextual fear memories or other types of memories (Briggs & Olson, 2013; Dębiec & Ledoux, 2004; Flint & Marino, 2007; Milton, Lee, & Everitt, 2008; Przybyslawski et al., 1999; Robinson & Franklin, 2010; Taubenfeld et al., 2009). These studies report large effect sizes, and no failures to replicate have been published when using methodologies similar to the ones adopted in the current experiments (i.e., using systemic drug administration after unreinforced re-exposure to the conditioned context in rats). On the other hand, our results are in line with the mixed findings in the human fear conditioning literature, in which systemic administration of PROP after CS exposure has not always been successful in impairing conditioned fear (Bos et al., 2014; Chalkia, Weermeijer, Van Oudenhove, & Beckers, 2019; Schroyens et al., 2017; Thome et al., 2016). Our results cannot readily be attributed to previously determined boundary conditions, such as the adopted behavioral parameters (e.g., strength of training, duration of the reactivation session, etc.), given that we performed several exact replications in which the behavioral parameters were kept the same as in prior successful studies. In addition, we extensively varied training and reactivation parameters throughout our studies. We do not want to make any inferences on the basis of the current results regarding amnestic effects of intracerebral drug infusions or non-pharmacological approaches that are often used in the animal literature, nor do we want to cast doubt on the veracity of the phenomenon of post-reactivation memory interference. Rather, our results - and therefore, their implications - only relate to amnesia for contextual fear memories using systemic drug administration after unreinforced context re-exposure, and using freezing as a measure of fear memory retention. We do think that our findings bear significant relevance for the clinical application of post-reactivation memory interventions, given that in clinical practice, patients would also receive amnestic treatment systemically. In addition, the current failures to replicate successful studies despite our efforts to follow their methodology as closely as possible illustrate that the success of this intervention depends on subtle (and unknown) parameters and suggests that the current literature may present an overestimation of the true effect size.
Supplementary Material
Acknowledgements
We would like to thank Ineke Pillet and Victoria A. Ossorio Salazar for their assistance with the experimental work and all the researchers who commented on the preprint version of the manuscript.
Funding sources
This work was supported by a Consolidator Grant of the European Research Council (ERC) [T. Beckers, grant number 648176] and a Doctoral Fellowship of the Research Foundation – Flanders (FWO) [N. Schroyens, grant number 1114018N].
Footnotes
Declarations of interest: none.
Color is not required for any figures in print
Contributor Information
Natalie Schroyens, Email: natalie.schroyens@kuleuven.be.
Joaquín Matias Alfei, Email: joaquin.alfei@kuleuven.be.
Anna Elisabeth Schnell, Email: annaelisabeth.schnell@kuleuven.be.
Laura Luyten, Email: laura.luyten@kuleuven.be.
Tom Beckers, Email: tom.beckers@kuleuven.be.
References
- Abdullahi PR, Eskandarian S, Ghanbari A, Rashidy-Pour A. Oxytocin receptor antagonist atosiban impairs consolidation, but not reconsolidation of contextual fear memory in rats. Brain Research. 2018;1695:31–36. doi: 10.1016/j.brainres.2018.05.034. [DOI] [PubMed] [Google Scholar]
- Abrari K, Rashidy-Pour A, Semnanian S, Fathollahi Y. Administration of corticosterone after memory reactivation disrupts subsequent retrieval of a contextual conditioned fear memory: Dependence upon training intensity. Neurobiology of Learning and Memory. 2008;89(2):178–184. doi: 10.1016/j.nlm.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Akagi K, Yamada M, Saitoh A, Oka J-I, Yamada M. Post-reexposure administration of riluzole attenuates the reconsolidation of conditioned fear memory in rats. Neuropharmacology. 2018;131:1–10. doi: 10.1016/j.neuropharm.2017.12.009. [DOI] [PubMed] [Google Scholar]
- Alfei JM, Ferrer Monti RI, Molina VA, Bueno AM, Urcelay GP. Prediction error and trace dominance determine the fate of fear memories after post-training manipulations. Learning & Memory. 2015;22(8):385–400. doi: 10.1101/lm.038513.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckers T, Kindt M. Memory reconsolidation interference as an emerging treatment for emotional disorders: Strengths, limitations, challenges, and opportunities. Annual Review of Clinical Psychology. 2017;13:99–121. doi: 10.1146/annurev-clinpsy-032816-045209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos MGN, Beckers T, Kindt M. Noradrenergic blockade of memory reconsolidation: A failure to reduce conditioned fear responding. Frontiers in Behavioral Neuroscience. 2014;8 doi: 10.3389/fnbeh.2014.00412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs JF, Olson BP. Reexposure to the amnestic agent alleviates cycloheximide-induced retrograde amnesia for reactivated and extinction memories. Learning & Memory. 2013;20(5):285–288. doi: 10.1101/lm.030270.113. [DOI] [PubMed] [Google Scholar]
- Bustos SG, Giachero M, Maldonado H, Molina VA. Previous stress attenuates the susceptibility to midazolam’s disruptive effect on fear memory reconsolidation: Influence of pre-reactivation D-cycloserine administration. Neuropsychopharmacology. 2010;35(5):1097–1108. doi: 10.1038/npp.2009.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustos SG, Maldonado H, Molina VA. Midazolam disrupts fear memory reconsolidation. Neuroscience. 2006;139(3):831–842. doi: 10.1016/j.neuroscience.2005.12.064. [DOI] [PubMed] [Google Scholar]
- Bustos SG, Maldonado H, Molina VA. Disruptive effect of midazolam on fear memory reconsolidation: Decisive influence of reactivation time span and memory age. Neuropsychopharmacology. 2009;34(2):446–457. doi: 10.1038/npp.2008.75. [DOI] [PubMed] [Google Scholar]
- Cassini LF, Flavell CR, Amaral OB, Lee JLC. On the transition from reconsolidation to extinction of contextual fear memories. Learning & Memory. 2017;24(9):392–399. doi: 10.1101/lm.045724.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalkia A, Weermeijer J, Van Oudenhove L, Beckers T. Acute but not permanent effects of propranolol on fear memory expression in humans. Frontiers in Human Neuroscience. 2019;13 doi: 10.3389/fnhum.2019.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan WYM, Leung HT, Westbrook RF, McNally GP. Effects of recent exposure to a conditioned stimulus on extinction of Pavlovian fear conditioning. Learning & Memory. 2010;17(10):512–521. doi: 10.1101/lm.1912510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson RG, McGaugh JL. Electroconvulsive shock effects on a reactivated memory trace: Further examination. Science. 1969;166(3904):525–527. doi: 10.1126/science.166.3904.525. [DOI] [PubMed] [Google Scholar]
- Dębiec J, Ledoux JE. Disruption of reconsolidation but not consolidation of auditory fear conditioning by noradrenergic blockade in the amygdala. Neuroscience. 2004;129(2):267–272. doi: 10.1016/j.neuroscience.2004.08.018. [DOI] [PubMed] [Google Scholar]
- DeVietti TL, Larson RC. ECS effects: Evidence supporting state-dependent learning in rats. Journal of Comparative and Physiological Psychology. 1971;74(3):407–415. doi: 10.1037/h0030564. [DOI] [Google Scholar]
- Díaz-Mataix L, Ruiz Martinez RC, Schafe GE, LeDoux JE, Doyère V. Detection of a temporal error triggers reconsolidation of amygdala-dependent memories. Current Biology. 2013;23(6):467–472. doi: 10.1016/j.cub.2013.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvarci S, Nader K. Characterization of fear memory reconsolidation. The Journal of Neuroscience. 2004;24(42):9269–9275. doi: 10.1523/JNEUROSCI.2971-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg M, Dudai Y. Reconsolidation of fresh, remote, and extinguished fear memory in medaka: Old fears don’t die. European Journal of Neuroscience. 2004;20(12):3397–3403. doi: 10.1111/j.1460-9568.2004.03818.x. [DOI] [PubMed] [Google Scholar]
- Elsey JWB, Van Ast VA, Kindt M. Human memory reconsolidation: A guiding framework and critical review of the evidence. Psychological Bulletin. 2018;144(8):797–848. doi: 10.1037/bul0000152. [DOI] [PubMed] [Google Scholar]
- Espejo PJ, Ortiz V, Martijena ID, Molina VA. Stress-induced resistance to the fear memory labilization/reconsolidation process. Involvement of the basolateral amygdala complex. Neuropharmacology. 2016;109:349–356. doi: 10.1016/j.neuropharm.2016.06.033. [DOI] [PubMed] [Google Scholar]
- Espejo PJ, Ortiz V, Martijena ID, Molina VA. GABAergic signaling within the Basolateral Amygdala Complex modulates resistance to the labilization/reconsolidation process. Neurobiology of Learning and Memory. 2017;144:166–173. doi: 10.1016/j.nlm.2017.06.004. [DOI] [PubMed] [Google Scholar]
- Exton-McGuinness MTJ, Lee JLC, Reichelt AC. Updating memories--the role of prediction errors in memory reconsolidation. Behavioural Brain Research. 2015;278:375–384. doi: 10.1016/j.bbr.2014.10.011. [DOI] [PubMed] [Google Scholar]
- Faul F, Erdfelder E, Lang A-G, Buchner A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavior Research Methods. 2007;39(2):175–191. doi: 10.3758/BF03193146. [DOI] [PubMed] [Google Scholar]
- Ferrer Monti RI, Alfei JM, Mugnaini M, Bueno AM, Beckers T, Urcelay GP, Molina VA. A comparison of behavioral and pharmacological interventions to attenuate reactivated fear memories. Learning & Memory. 2017;24(8):369–374. doi: 10.1101/lm.045385.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrer Monti RI, Giachero M, Alfei JM, Bueno AM, Cuadra G, Molina VA. An appetitive experience after fear memory destabilization attenuates fear retention: Involvement GluN2B-NMDA receptors in the Basolateral Amygdala Complex. Learning & Memory. 2016;23(9):465–478. doi: 10.1101/lm.042564.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flint RW, Marino CL. Cycloheximide impairs reconsolidation of a contextually reactivated memory in a conditioned taste aversion paradigm. Behavioral Neuroscience. 2007;121(2):433–438. doi: 10.1037/0735-7044.121.2.433. [DOI] [PubMed] [Google Scholar]
- Franzen JM, Giachero M, Bertoglio LJ. Dissociating retrieval-dependent contextual aversive memory processes in female rats: Are there cycle-dependent differences? Neuroscience. 2019;406:542–553. doi: 10.1016/j.neuroscience.2019.03.035. [DOI] [PubMed] [Google Scholar]
- Gazarini L, Stern CAJ, Piornedo RR, Takahashi RN, Bertoglio LJ. PTSD-like memory generated through enhanced noradrenergic activity is mitigated by a dual step pharmacological intervention targeting its reconsolidation. International Journal of Neuropsychopharmacology. 2015;18(1) doi: 10.1093/ijnp/pyu026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gisquet-Verrier P, Lynch JF, Cutolo P, Toledano D, Ulmen A, Jasnow AM, Riccio DC. Integration of new information with active memory accounts for retrograde amnesia: A challenge to the consolidation/reconsolidation hypothesis? The Journal of Neuroscience. 2015;35(33):11623–11633. doi: 10.1523/JNEUROSCI.1386-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gisquet-Verrier P, Riccio DC. Memory integration: An alternative to the consolidation/reconsolidation hypothesis. Progress in Neurobiology. 2018;171:15–31. doi: 10.1016/j.pneurobio.2018.10.002. [DOI] [PubMed] [Google Scholar]
- Hardwicke TE, Taqi M, Shanks DR. Postretrieval new learning does not reliably induce human memory updating via reconsolidation. Proceedings of the National Academy of Sciences. 2016;113(19):5206–5211. doi: 10.1073/pnas.1601440113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haubrich J, Crestani AP, Cassini LF, Santana F, Sierra RO, Alvares L, de O, Quillfeldt JA. Reconsolidation allows fear memory to be updated to a less aversive level through the incorporation of appetitive information. Neuropsychopharmacology. 2015;40(2):315–326. doi: 10.1038/npp.2014.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman AN, Parga A, Paode PR, Watterson LR, Nikulina EM, Hammer RP, Conrad CD. Chronic stress enhanced fear memories are associated with increased amygdala zif268 mRNA expression and are resistant to reconsolidation. Neurobiology of Learning and Memory. 2015;120:61–68. doi: 10.1016/j.nlm.2015.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii D, Matsuzawa D, Matsuda S, Tomizawa H, Sutoh C, Shimizu E. An isolated retrieval trial before extinction session does not prevent the return of fear. Behavioural Brain Research. 2015;287:139–145. doi: 10.1016/j.bbr.2015.03.052. [DOI] [PubMed] [Google Scholar]
- Jeffreys H. Theory of probability. Oxford, UK: Oxford University Press; 1961. [Google Scholar]
- Kindt M, Soeter M, Vervliet B. Beyond extinction: Erasing human fear responses and preventing the return of fear. Nature Neuroscience. 2009;12(3):256–258. doi: 10.1038/nn.2271. [DOI] [PubMed] [Google Scholar]
- Koo TK, Li MY. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. Journal of Chiropractic Medicine. 2016;15(2):155–163. doi: 10.1016/j.jcm.2016.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kredlow MA, Unger LD, Otto MW. Harnessing reconsolidation to weaken fear and appetitive memories: A meta-analysis of post-retrieval extinction effects. Psychological Bulletin. 2016;142(3):314–336. doi: 10.1037/bul0000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lattal KM, Abel T. Behavioral impairments caused by injections of the protein synthesis inhibitor anisomycin after contextual retrieval reverse with time. Proceedings of the National Academy of Sciences. 2004;101(13):4667–4672. doi: 10.1073/pnas.0306546101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JLC, Flavell CR. Inhibition and enhancement of contextual fear memory destabilization. Frontiers in Behavioral Neuroscience. 2014;8 doi: 10.3389/fnbeh.2014.00144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JLC, Gardner RJ, Butler VJ, Everitt BJ. D-cycloserine potentiates the reconsolidation of cocaine-associated memories. Learning & Memory. 2009;16(1):82–85. doi: 10.1101/lm.1186609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luyten L, Beckers T. A preregistered, direct replication attempt of the retrieval-extinction effect in cued fear conditioning in rats. Neurobiology of Learning and Memory. 2017;144:208–215. doi: 10.1016/j.nlm.2017.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mactutus CF, Ferek JM, George CA, Riccio DC. Hypothermia-induced amnesia for newly acquired and old reactivated memories: Commonalities and distinctions. Physiological Psychology. 1982;10(1):79–95. doi: 10.3758/BF03327011. [DOI] [Google Scholar]
- Mactutus CF, Riccio DC, Ferek JM. Retrograde amnesia for old (reactivated) memory: Some anomalous characteristics. Science. 1979;204(4399):1319–1320. doi: 10.1126/science.572083. [DOI] [PubMed] [Google Scholar]
- Mamou CB, Gamache K, Nader K. NMDA receptors are critical for unleashing consolidated auditory fear memories. Nature Neuroscience. 2006;9(10):1237–1239. doi: 10.1038/nn1778. [DOI] [PubMed] [Google Scholar]
- Merlo E, Milton AL, Everitt BJ. A novel retrieval-dependent memory process revealed by the arrest of ERK1/2 activation in the basolateral amygdala. The Journal of Neuroscience. 2018;38(13):3199–3207. doi: 10.1523/JNEUROSCI.3273-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milton AL, Lee JLC, Butler VJ, Gardner R, Everitt BJ. Intra-amygdala and systemic antagonism of NMDA receptors prevents the reconsolidation of drug-associated memory and impairs subsequently both novel and previously acquired drug-seeking behaviors. Journal of Neuroscience. 2008;28(33):8230–8237. doi: 10.1523/JNEUROSCI.1723-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milton AL, Lee JLC, Everitt BJ. Reconsolidation of appetitive memories for both natural and drug reinforcement is dependent on beta-adrenergic receptors. Learning & Memory. 2008;15(2):88–92. doi: 10.1101/lm.825008. [DOI] [PubMed] [Google Scholar]
- Misanin JR, Miller RR, Lewis DJ. Retrograde amnesia produced by electroconvulsive shock after reactivation of a consolidated memory trace. Science. 1968;160(3827):554–555. doi: 10.1126/science.160.3827.554. [DOI] [PubMed] [Google Scholar]
- Mody I, Maguire J. The reciprocal regulation of stress hormones and GABAA receptors. Frontiers in Cellular Neuroscience. 2012;6 doi: 10.3389/fncel.2012.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monfils M-H, Cowansage KK, Klann E, LeDoux JE. Extinction-reconsolidation boundaries: Key to persistent attenuation of fear memories. Science. 2009;324(5929):951–955. doi: 10.1126/science.1167975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muravieva EV, Alberini CM. Limited efficacy of propranolol on the reconsolidation of fear memories. Learning & Memory. 2010;17(6):306–313. doi: 10.1101/lm.1794710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nader K, Schafe GE, Le Doux J. Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature. 2000;406:722–726. doi: 10.1038/35021052. [DOI] [PubMed] [Google Scholar]
- Ortiz V, Giachero M, Espejo PJ, Molina VA, Martijena ID. The effect of midazolam and propranolol on fear memory reconsolidation in ethanol-withdrawn rats: Influence of d-cycloserine. The International Journal of Neuropsychopharmacology. 2015;18(4) doi: 10.1093/ijnp/pyu082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz V, Molina VA, Martijena ID. Effect of a positive reinforcing stimulus on fear memory reconsolidation in ethanol withdrawn rats: Influence of d-cycloserine. Behavioural Brain Research. 2016;315:66–70. doi: 10.1016/j.bbr.2016.08.019. [DOI] [PubMed] [Google Scholar]
- Pedreira ME, Pérez-Cuesta LM, Maldonado H. Mismatch between what is expected and what actually occurs triggers memory reconsolidation or extinction. Learning & Memory. 2004;11(5):579–585. doi: 10.1101/lm.76904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piñeyro ME, Ferrer Monti RI, Alfei JM, Bueno AM, Urcelay GP. Memory destabilization is critical for the success of the reactivation-extinction procedure. Learning & Memory. 2013;21(1):785–793. doi: 10.1101/lm.032714.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power AE. Anisomycin infused into the hippocampus fails to block “reconsolidation” but impairs extinction: The role of re-exposure duration. Learning & Memory. 2006;13(1):27–34. doi: 10.1101/lm.91206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przybyslawski J, Roullet P, Sara SJ. Attenuation of emotional and nonemotional memories after their reactivation: Role of beta-adrenergic receptors. Journal of Neuroscience. 1999;19(15):6623–6628. doi: 10.1523/JNEUROSCI.19-15-06623.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przybyslawski J, Sara SJ. Reconsolidation of memory after its reactivation. Behavioural Brain Research. 1997;84(1–2):241–246. doi: 10.1016/S0166-4328(96)00153-2. [DOI] [PubMed] [Google Scholar]
- R Foundation. R (version 3.3.2) 2016 Retrieved from https://www.r-project.org.
- Reichelt AC, Lee JLC. Memory reconsolidation in aversive and appetitive settings. Frontiers in Behavioral Neuroscience. 2013;7 doi: 10.3389/fnbeh.2013.00118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson MJF, Franklin KBJ. Reconsolidation of a morphine place preference: Impact of the strength and age of memory on disruption by propranolol and midazolam. Behavioural Brain Research. 2010;213(2):201–207. doi: 10.1016/j.bbr.2010.04.056. [DOI] [PubMed] [Google Scholar]
- Rouder JN, Morey RD. A Bayes factor meta-analysis of Bem’s ESP claim. Psychonomic Bulletin & Review. 2011;18(4):682–689. doi: 10.3758/s13423-011-0088-7. [DOI] [PubMed] [Google Scholar]
- Ryan TJ, Roy DS, Pignatelli M, Arons A, Tonegawa S. Engram cells retain memory under retrograde amnesia. Science. 2015;348(6238):1007–1013. doi: 10.1126/science.aaa5542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sara SJ. Retrieval and reconsolidation: Toward a neurobiology of remembering. Learning & Memory. 2000;7(2):73–84. doi: 10.1101/lm.7.2.73. [DOI] [PubMed] [Google Scholar]
- Schneider AM, Simson PE, Daimon CM, Mrozewski J, Vogt NM, Keefe J, Kirby LG. Stress-dependent opioid and adrenergic modulation of newly retrieved fear memory. Neurobiology of Learning and Memory. 2014;109:1–6. doi: 10.1016/j.nlm.2013.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider AM, Sherman W. Amnesia: A Function of the temporal relation of footshock to electroconvulsive Shock. Science. 1968;159(3811):219–221. doi: 10.1126/science.159.3811.219. [DOI] [PubMed] [Google Scholar]
- Schroyens N, Alfei JM, Luyten L, Beckers T. Limited replicability of drug-induced amnesia after contextual fear memory retrieval in rats. 2019 Jun 26; doi: 10.1016/j.nlm.2019.107105. Retrieved from osf.io/m2png. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroyens N, Beckers T, Kindt M. In search for boundary conditions of reconsolidation: A failure of fear memory interference. Frontiers in Behavioral Neuroscience. 2017;11 doi: 10.3389/fnbeh.2017.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroyens N, Bender CL, Alfei JM, Molina VA, Luyten L, Beckers T. Post-weaning housing conditions influence freezing during contextual fear conditioning in adult rats. Behavioural Brain Research. 2019;359:172–180. doi: 10.1016/j.bbr.2018.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevenster D, Beckers T, Kindt M. Prediction error demarcates the transition from retrieval, to reconsolidation, to new learning. Learning & Memory. 2014;21(11):580–584. doi: 10.1101/lm.035493.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skilbeck KJ, Johnston GAR, Hinton T. Stress and GABA receptors. Journal of Neurochemistry. 2010;112(5):1115–1130. doi: 10.1111/j.1471-4159.2009.06539.x. [DOI] [PubMed] [Google Scholar]
- Soeter M, Kindt M. An abrupt transformation of phobic behavior after a post-retrieval amnesic agent. Biological Psychiatry. 2015;78(12):880–886. doi: 10.1016/j.biopsych.2015.04.006. [DOI] [PubMed] [Google Scholar]
- Stern CAJ, Gazarini L, Takahashi RN, Guimarães FS, Bertoglio LJ. On disruption of fear memory by reconsolidation blockade: Evidence from cannabidiol treatment. Neuropsychopharmacology. 2012;37(9):2132–2142. doi: 10.1038/npp.2012.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A. Memory reconsolidation and extinction have distinct temporal and biochemical signatures. Journal of Neuroscience. 2004;24(20):4787–4795. doi: 10.1523/JNEUROSCI.5491-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taherian F, Vafaei AA, Vaezi GH, Eskandarian S, Kashef A, Rashidy-Pour A. Propranolol–induced impairment of contextual fear memory reconsolidation in rats: A similar effect on weak and strong recent and remote memories. 2014;5(3):9. [PMC free article] [PubMed] [Google Scholar]
- Taubenfeld SM, Riceberg JS, New AS, Alberini CM. Preclinical assessment for selectively disrupting a traumatic memory via postretrieval inhibition of glucocorticoid receptors. Biological Psychiatry. 2009;65(3):249–257. doi: 10.1016/j.biopsych.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theilmann W, Kleimann A, Rhein M, Bleich S, Frieling H, Löscher W, Brandt C. Behavioral differences of male Wistar rats from different vendors in vulnerability and resilience to chronic mild stress are reflected in epigenetic regulation and expression of p11. Brain Research. 2016;1642:505–515. doi: 10.1016/j.brainres.2016.04.041. [DOI] [PubMed] [Google Scholar]
- Thome J, Koppe G, Hauschild S, Liebke L, Schmahl C, Lis S, Bohus M. Modification of fear memory by pharmacological and behavioural interventions during reconsolidation. PLOS ONE. 2016;11(8):e0161044. doi: 10.1371/journal.pone.0161044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trent S, Barnes P, Hall J, Thomas KL. Rescue of long-term memory after reconsolidation blockade. Nature Communications. 2015;6(1) doi: 10.1038/ncomms8897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S-H. Novelty enhances memory persistence and remediates propranolol-induced deficit via reconsolidation. Neuropharmacology. 2018;141:42–54. doi: 10.1016/j.neuropharm.2018.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetzels R, Matzke D, Lee MD, Rouder JN, Iverson GJ, Wagenmakers E-J. Statistical evidence in experimental psychology: An empirical comparison using 855 t Tests. Perspectives on Psychological Science. 2011;6(3):291–298. doi: 10.1177/1745691611406923. [DOI] [PubMed] [Google Scholar]
- Wood NE, Rosasco ML, Suris AM, Spring JD, Marin M-F, Lasko NB, et al. Pitman RK. Pharmacological blockade of memory reconsolidation in posttraumatic stress disorder: Three negative psychophysiological studies. Psychiatry Research. 2015;225(1–2):31–39. doi: 10.1016/j.psychres.2014.09.005. [DOI] [PubMed] [Google Scholar]
- Zhang S, Cranney J. The role of GABA and anxiety in the reconsolidation of conditioned fear. Behavioral Neuroscience. 2008;122(6):1295–1305. doi: 10.1037/a0013273. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.